- ASQ® CQA Exam
- ASQ® CQE Exam
- ASQ® CSQP Exam
- ASQ® CSSYB Exam
- ASQ® CSSGB Exam
- ASQ® CSSBB Exam
- ASQ® CMQ/OE Exam
- ASQ® CQT Exam
- ASQ® CQPA Exam
- ASQ® CQIA Exam
- 7 Quality Tools
- Quality Gurus
- ISO 9001:2015
- Quality Cost
- Six Sigma Basics
- Risk Management
- Lean Manufacturing
- Design of Experiments
- Quality Acronyms
- Quality Awareness
- Quality Circles
- Acceptance Sampling
- Measurement System
- APQP + PPAP
- GD&T Symbols
- Project Quality (PMP)
- Full List of Quizzes >>
- Reliability Engineering
- Statistics with Excel
- Statistics with Minitab
- Multiple Regression
- Quality Function Deployment
- Benchmarking
- Statistical Process Control
- Quality Talks >> New
- Six Sigma White Belt
- Six Sigma Yellow Belt
- Six Sigma Green Belt
- Six Sigma Black Belt
- Minitab 17 for Six Sigma
- Casio fx-991MS Calculator
- CSSYB/LSSYB Mock Exam
- CSSGB/LSSGB Mock Exam
- CSSBB/LSSBB Mock Exam
- ASQ® CQA Preparation
- ASQ® CQE Preparation
- ASQ® CQPA Preparation
- ASQ® CQIA Preparation
- CQE Mock Exams
- CMQ/OE Mock Exams
- CQA Mock Exams
- CQIA Mock Exams
- CQPA Mock Exam
- CQT Mock Exam
- CQI Mock Exam
- CSQP Mock Exam
- Design of Experiments (DoE)
- Measurement System Analysis
- Statistics Using R
- Data Visualization with R
- Statistics Using Python
- Data Visualization with Python
- Regression with Minitab
- Logistic Regression
- Data Analysis Using Excel
- The Git Mindset
- Statistics Quiz
- Root Cause Analysis
- Kano Analysis
- Lean Management
- QMS Lead Auditor
- Quality Management
- ISO 9001:2015 Transition
- Project Quality Manager
- गुणवत्ता.org
- Summary Sheets
- Practice Tests
- QG Hall of Fame
- Testimonials – ASQ Exams Preparation
Blogs , ISO 9001
- Quality Control: Understanding Its Importance, Benefits, Approaches and Key Strategies
** Unlock Your Full Potential **

Maintaining high-quality products and services is crucial for success in today's competitive business environment. Quality control (QC) plays a critical role in ensuring that your company consistently meets customer expectations and regulatory requirements. This comprehensive guide will explore the importance of quality control, its benefits, and key strategies, with industry examples to illustrate its practical applications.

What is Quality Control?
Quality control refers to the systematic process of identifying, monitoring and correcting potential defects or deviations in products or services. This process ensures that the final output meets the established quality standards and customer requirements. QC is an essential part of the overall quality management system ( QMS ) and involves regular inspections, testing, and monitoring of various production stages.
ISO 9001:2015 defines Quality Control as “a part of quality management focused on fulfilling quality requirements.” It includes activities such as the inspection and testing of incoming raw materials, in-process products, and finished goods.
History of Quality Control
Quality control has evolved over time to keep pace with the increasing complexity and scale of production processes. Let's take a brief look at the key milestones in the history of quality control:
Craftsmanship Era (Pre-Industrial Revolution): Before the Industrial Revolution, craftsmen were responsible for producing goods and often had a personal relationship with their customers. Quality was maintained by the craftsman's reputation, skill, and pride in their work.
Industrial Revolution (Late 18th Century to Mid-19th Century): With the advent of mass production, the responsibility for quality control shifted from individual craftsmen to factory managers. Inspectors were employed to identify and segregate defective products, but the focus was on finding and fixing defects rather than preventing them.
Scientific Management (Early 20th Century): The introduction of scientific management principles by Frederick Winslow Taylor marked a significant shift in quality control. Taylor's ideas laid the groundwork for more systematic and data-driven approaches to managing production processes, paving the way for modern quality control methods.
Statistical Quality Control (Mid-20th Century): Walter A. Shewhart introduced the concept of statistical process control (SPC) in the 1920s. SPC allowed manufacturers to monitor and control production processes using statistical methods, enabling them to detect and correct defects more efficiently. During World War II, the U.S. military adopted statistical quality control techniques to improve the production of munitions and other equipment.
Total Quality Management (Post-WWII): After World War II, quality management pioneers such as W. Edwards Deming and Joseph M. Juran helped spread the concept of Total Quality Management ( TQM ). TQM emphasized continuous improvement, customer satisfaction, and employee involvement, transforming how companies approached quality control.
ISO 9001 and Modern Quality Control (Late 20th Century to Present): In 1987, the International Organization for Standardization (ISO) introduced the ISO 9000 quality management standards, including ISO 9001. These standards provided a global framework for implementing effective quality management systems. Today, quality has evolved to encompass a wide range of methodologies and tools, such as Six Sigma and Lean Manufacturing, helping businesses achieve higher levels of quality and efficiency.
The history of quality control shows how the concept has evolved and adapted to the changing needs of production processes and market demands. Understanding this history can help businesses appreciate the value of quality control and implement more effective systems to ensure long-term success.
Benefits and Importance of Quality Control
- Customer Satisfaction: Consistently delivering high-quality products and services helps build customer trust and loyalty, increasing the likelihood of repeat business and positive word-of-mouth marketing.
- Regulatory Compliance: QC processes help companies adhere to industry-specific regulations and standards, preventing costly fines or sanctions.
- Brand Reputation: A strong commitment to quality control enhances a company's reputation for producing reliable, high-quality products or services.
- Cost Savings: Identifying and correcting defects early in production minimizes waste and reduces the need for expensive rework or recalls.
- Competitive Advantage: Companies with robust QC systems are better positioned to differentiate themselves from competitors and capture market share.
Key Strategies for Effective Quality Control
- Establish Clear Quality Standards: Define and communicate the specific quality criteria for each product or service, ensuring all team members understand the expectations.
- Implement Regular Inspections and Testing: Conduct routine checks at various stages of production to identify defects and deviations from quality standards.
- Invest in Employee Training: Provide ongoing training to equip employees with the necessary skills and knowledge to maintain high-quality standards.
- Utilize Statistical Process Control ( SPC ): SPC techniques can help identify trends and patterns in production data, enabling companies to predict and prevent quality issues.
- Embrace Continuous Improvement: Encourage a culture that values ongoing learning and improvement and proactively empowers employees to identify and address quality concerns.
Quality Control Approaches
Different industries and organizations may adopt various approaches to quality, depending on their specific needs and goals. Some popular QC methodologies include:
- Total Quality Management ( TQM ): A holistic approach to quality management focuses on continuous improvement, customer satisfaction, and employee involvement. It aims to integrate quality principles into all aspects of a company's operations.
- Six Sigma: Six Sigma is a data-driven quality management methodology seeking to reduce defects and process variation. The goal is to achieve a defect rate of 3.4 per million opportunities, ensuring near-perfect quality.
- Lean Manufacturing: Lean focuses on eliminating waste and optimizing processes to deliver maximum value to customers. Although not explicitly a quality control approach, Lean principles can significantly contribute to improving product quality by enhancing efficiency and reducing defects.
- ISO 9001 : This international standard sets out the criteria for a quality management system . Achieving ISO 9001 certification demonstrates a company's commitment to maintaining consistent quality standards and continuously improving its processes.
Conclusion:
Quality control plays a crucial role in ensuring that businesses deliver high-quality products and services, meeting customer expectations and regulatory requirements. Companies can develop and implement effective QC systems that contribute to long-term success by understanding its importance, benefits, and key strategies.
Similar Posts:
May 8, 2021
Supplier Development and Remediation: The Key to Sustained Supplier Success
January 21, 2018
How many mandatory documents does ISO 9001-2015 require?
December 15, 2021
Criteria to Audit Against

49 Courses on SALE!
- Professional Scrum Product Owner (PSPO)
- SAFe for Government
- Professional Scrum Master (PSM)
- Certified ScrumMaster
- PMI-ACP Exam Prep
- Leading SAFe® 6.0 Certification
- SAFe Scrum Master
- Certified Scrum Product Owner (CSPO)
- SAFe for Teams
- Agile Scrum Foundation
- AgilePM Foundation and Practitioner Certification
- Agile Scrum Master (ASM)
- Kanban Training
- Scrum Fundamentals
- PMP Certification
- Project Management Fundamentals
- CAPM Exam Prep
- Change Management Foundation and Practitioner Certification
- PRINCE2 Foundation & Practitioner Certification (7th Edition)
- PRINCE2 Agile Foundation & Practitioner Certification
- Business Analysis Foundation and Practitioner Certification
- Microsoft Project Training
- JIRA Certification Training
- Lean Project Management
- ITIL 4 Foundation
- VeriSM™ Foundation
- SIAM Foundation
- SIAM Professional
- 7 QC Tools Training
- Minitab Essentials
- Lean Six Sigma Yellow Belt
- Six Sigma Awareness
- Lean Six Sigma Green Belt
- Design for Six Sigma
- Lean Six Sigma Black Belt
- Lean Fundamentals
- Value Stream Mapping
- Quality by Design
- Quality Function Deployment
- BPM and Six Sigma
- RCA through Six Sigma
- DevOps Foundation
- DevOps Master
- DevOps Professional
- Continuous Delivery Architecture
- COBIT 5 Certification
- Corporate Group Training
- 1-to-1 Training
- Join as a Trainer

- Top Blogs on Quality Management
What is Quality Control (QC)?

In the modern era, we often need to pay more attention to the consistency and excellence of the products and services we use daily. This was not the case at the beginning of the 20th century when quality control in manufacturing was far from reliable.
Today, thanks to the pioneering work in business problem-solving and analytical frameworks, businesses can achieve and maintain high-quality standards.
This blog explores how these Quality Control practices have evolved and impacted today’s industries.
Table of Contents:
Major Components of Quality Control
Quality control types, importance and benefits of quality control, quality control vs. quality assurance.
- Examples of Quality Control (QC)?
Quality Control Careers
- FAQs on Quality Control (QC)
Quality Control (QC) is vital in ensuring that products and services meet a set quality standard. It involves testing and measuring these products or services to confirm they align with the desired level of excellence.
The concept of ‘quality’ might vary, but in QC, it’s all about meeting established standards and providing value. This process enables businesses to assess, maintain, and enhance the quality of their offerings, ensuring customer satisfaction and trust.
The primary goal of Quality Control is twofold. Firstly, it aims to ensure products are as consistent as possible. Uniformity is key to maintaining a reliable brand image and customer experience. Secondly, QC strives to minimize errors and inconsistencies.
This is achieved by monitoring and inspecting products or services at different stages of production or delivery. QC isn’t just about fixing problems; it’s also focused on preventing defects by implementing control measures and improving processes, thus ensuring a higher standard of product or service delivery.
Quality Control (QC) is integral to maintaining high standards in product and service delivery. It encompasses a variety of components, each playing a crucial role in ensuring that the final output meets or exceeds the expected quality.
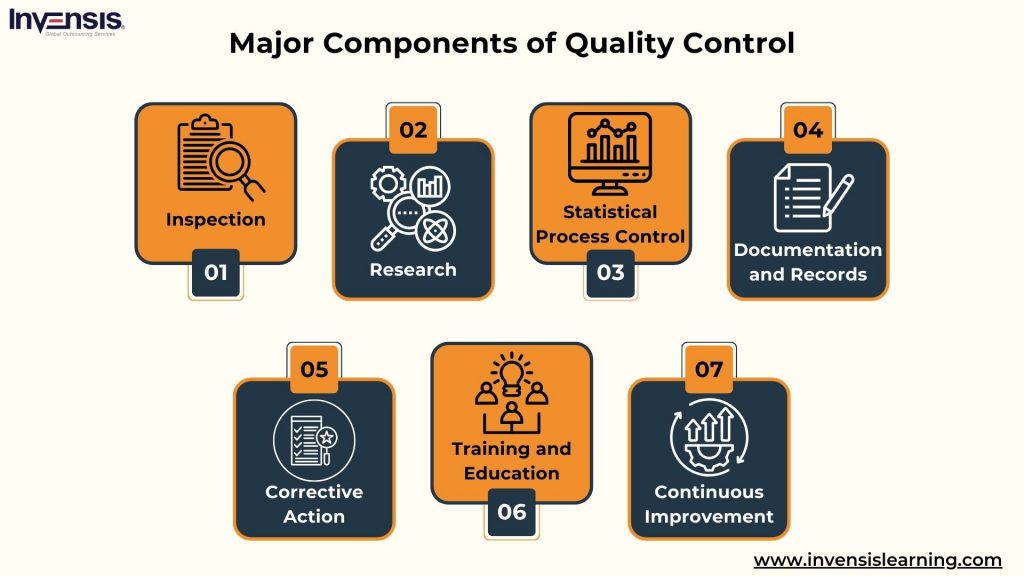
The components include:
- Inspection : This involves regularly examining products, materials, or services to identify any defects, non-compliance, or deviations from the established quality standards
- Testing : Various tests assess performance, functionality, or other characteristics, ensuring products or services meet quality expectations
- Statistical Process Control (SPC) : This employs statistical methods to monitor and control production processes, keeping them within acceptable quality limits
- Documentation and Records : Keeping detailed and accurate records of inspections, tests, and corrective actions is essential for maintaining traceability and accountability
- Corrective Action : Whenever quality issues are identified, appropriate measures are taken to rectify them and prevent their recurrence
- Training and Education : Empowering employees with the necessary skills and knowledge is vital for effectively maintaining quality standards
- Continuous Improvement involves analyzing data and feedback to refine and enhance the quality management system
Quality Control is closely intertwined with Quality Assurance (QA). While QC is focused on identifying and correcting defects, QA is about preventing these defects by establishing robust processes and procedures. Together, they form the backbone of an organization’s approach to quality management, crucial for meeting customer expectations and regulatory standards.
Quality Control (QC) is a diverse and dynamic field, with its methods varying significantly across different industries. The type of QC employed often depends on the specific requirements and risks associated with a particular sector.
For instance, industries like food and pharmaceuticals, where consumer safety is paramount, might lean towards more scientific and rigorous QC methods.
In contrast, fields like education or coaching might adopt a more qualitative approach, focusing on holistic improvement. Regardless of the industry, QC is fundamentally about meticulous attention to detail and robust research methodology.
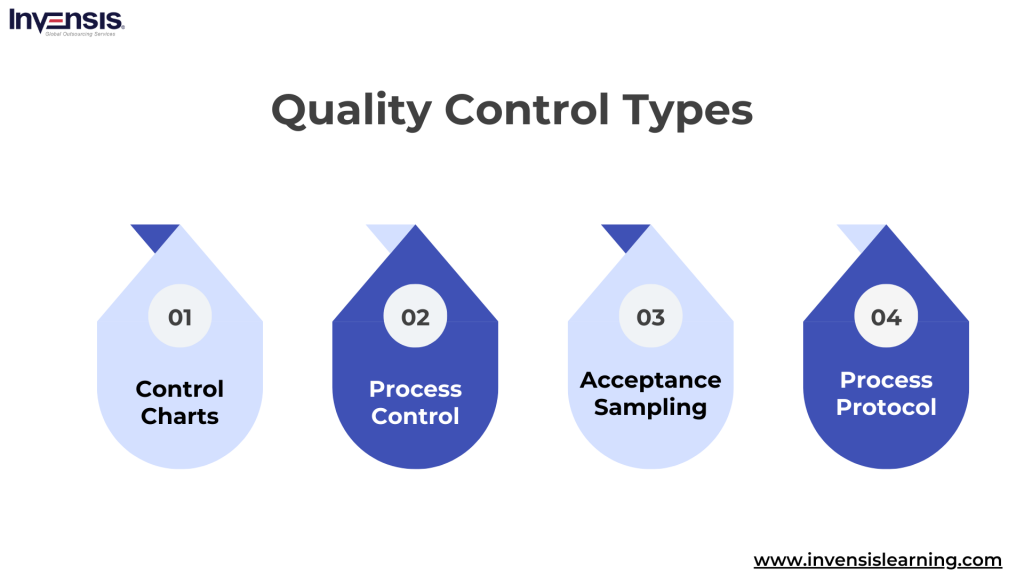
Several key types of Quality Control methods are commonly used across various industries:
Control Charts
Control charts are graphical representations that track process changes over time. By statistical analysis, businesses can determine whether their manufacturing or service processes are within the control limits.
Process Control
This involves continuous monitoring and adjusting processes to ensure consistent quality and improved performance. It often includes technical methods like feedback loops and industrial controls, especially in manufacturing.
Acceptance Sampling
This method uses statistical sampling to decide whether a batch of products meets the overall manufacturing standards, helping make informed decisions about product quality.
Process Protocol
This approach maps out the design and implementation processes, setting evaluative indicators for each step to ensure that each production or service delivery phase meets quality standards.
Beyond these, the approach to QC can also vary in terms of internal versus external monitoring.
Some companies establish internal QC divisions to oversee their products and services continuously. In contrast, others may rely on external bodies for quality assessment, especially in industries like food and pharmaceuticals with stringent regulations and safety.
Quality Control (QC) is more than just a set of procedures; it’s a pivotal aspect of business operations that brings numerous benefits to the company and its customers. By enforcing QC measures, organizations can enhance their product and service quality and gain a competitive edge in the market.
Here are some key benefits of implementing Quality Control:
- Customer Satisfaction : QC ensures products and services consistently meet or exceed customer expectations, fostering higher satisfaction and loyalty. This leads to repeat business and positive word-of-mouth referrals.
- Defect Prevention : Early identification and correction of issues in the QC process prevent defects, reducing costly recalls or rework. This also enhances the overall reliability of the product or service.
- Cost Reduction : Implementing QC measures leads to reduced waste, lower production costs, and improved efficiency, contributing to significant cost savings. This financial efficiency can be reinvested into further improvements or innovation.
- Compliance and Regulations : Adherence to industry standards and regulatory requirements through QC helps avoid legal issues and penalties, ensuring smooth business operations and maintaining corporate integrity.
- Brand Reputation : High-quality outputs build a positive brand image, enhancing reputation and market competitiveness. A strong reputation is invaluable for long-term business growth and success.
- Increased Efficiency : QC optimizes processes, leading to higher productivity and streamlined operations, faster delivery times, and increased capacity.
- Risk Mitigation : Through rigorous testing and inspections, QC helps identify potential risks and hazards, allowing businesses to address them proactively and maintain a safe environment for employees and customers.
- Continuous Improvement : A focus on QC encourages ongoing enhancement of products, services, and processes, fostering a culture of innovation and adaptability within the organization
- International Competitiveness : High-quality products enabled by QC can facilitate entry into global markets, increasing a company’s competitiveness on an international scale and opening up new revenue streams
- Customer Retention and Loyalty : Satisfied customers, as a result of high-quality products or services, are more likely to stay loyal and recommend the brand, contributing to the long-term success and sustainability of the business
Quality Control is crucial for maintaining high standards, minimizing risks, and ensuring a sustainable competitive advantage in today’s rapidly evolving business landscape. It is the foundation upon which superior products and services are delivered, ensuring customer satisfaction and fostering brand loyalty.
While often used interchangeably, Quality Control (QC) and Quality Assurance (QA) are distinct concepts in quality management.
Quality Control primarily focuses on the end product or service, ensuring it meets specific quality criteria and complies with necessary specifications. This involves direct actions like inspection, testing, and correcting defects in products or services. QC is about identifying and addressing problems after they occur.
On the other hand, Quality Assurance encompasses all the processes and actions necessary to provide confidence that quality requirements will be fulfilled. It’s more about managing and improving processes to prevent defects in the first place. QA is a proactive approach, emphasizing establishing a comprehensive quality management system and continuous improvement.
The relationship between the two is that QC is a part of the broader QA process. QA sets the standards and procedures to ensure quality, while QC involves the operational techniques to fulfill these standards.
As professionals grow in their careers, they may shift focus from QC to QA, understanding that ensuring quality is not just about fixing problems but also about creating systems that prevent them.
QA programs and departments are crucial for management, customers, and inspectors to guarantee that products meet all quality requirements and safety regulations.
Example of Quality Control (QC)
To illustrate Quality Control (QC) in action, consider a stuffed toy manufacturer focusing on a teddy bear product. The company has identified eight key parameters to control quality, aiming for consistency in look and feel across all teddy bears.
The QC team, which may vary in size depending on the scale of operations, is responsible for ensuring each teddy bear meets these standards.
Their tasks would typically include:
- Material Quality : Checking the fabric and stuffing material for consistency and quality.
- Size and Shape : Ensuring each teddy bear matches predefined size and shape specifications.
- Color : Verifying the color of the fabric and any additional elements like ribbons or buttons.
- Stitching Quality : Inspecting the stitching for durability and uniformity.
- Safety Standards : Ensure the toy meets safety regulations, especially if intended for young children.
- Softness and Texture : Testing the teddy bears for the right level of softness and texture.
- Overall Aesthetic : Assessing the appearance to ensure it meets the desired design criteria.
- Functionality : If the teddy bear includes additional features like sound or movement, these are tested for proper function.
In this example, the QC team plays a crucial role in maintaining the quality of the teddy bears, which directly impacts customer satisfaction and brand reputation. This scenario showcases how meticulous and comprehensive QC processes are essential in delivering a high-quality product.
Quality Control (QC) offers a rewarding career path for example Quality Control Inspector , Quality Control manager, etc. for those who enjoy working with others, presenting results, and striving for improvements and safety. The qualifications and skills needed for a career in QC vary depending on the industry, but here are some general guidelines:
Educational Requirements
- Entry-level QC positions typically require at least a high school diploma
- A bachelor’s degree may be necessary for more advanced roles, depending on the industry
- Industry-specific background knowledge is often important
Licenses and Certifications
- Some businesses and sectors may require specific licenses and certifications
- Professional development courses and certifications, such as Six Sigma or a Certified Quality Inspector designation, can be beneficial
Skills Needed
- Attention to detail is crucial in QC roles
- Mathematical and mechanical skills are often required
- Physical strength can be important, especially in manufacturing environments
- Technical knowledge relevant to the specific industry is essential
- The ability to perform well under pressure
Career Path
- The career trajectory in QC can vary by industry, but it generally involves gaining years of professional experience in your field
- Initially, you might start as a quality assurance or control associate
- With experience, you could advance to a senior specialist role and lead teams of QC specialists
A career in Quality Control is dynamic and requires a blend of education, specific skills, and continuous learning. It offers opportunities for growth and specialization, particularly for those passionate about ensuring the quality and safety of products and services.
Quality Control (QC) is an indispensable component in product and service delivery, playing a critical role in ensuring customer satisfaction, maintaining brand reputation, and enhancing overall business performance. The commitment to maintaining high-quality standards is essential in today’s competitive market, where customer expectations and industry regulations constantly evolve.
Understanding and implementing effective QC practices can significantly improve operational efficiency, cost reduction, and risk mitigation. If you want to deepen your understanding or implement quality control in your business operations, consider taking quality management courses or exploring our additional resources for more insights and guidance.
FAQs on Quality Control
Who is responsible for quality control in a company.
A dedicated QC department typically manages Quality Control, but it also involves employees across various levels of the organization. Depending on the industry and company size, QC responsibilities can include technicians, engineers, managers, and even frontline workers.
How does Quality Control differ in manufacturing and services?
QC often focuses on physical product inspections and testing against defined specifications in manufacturing. QC might evaluate the delivery process, customer satisfaction, and service consistency.
Can Quality Control impact a company’s financial performance?
Yes, effective QC can significantly impact a company’s financial performance. It can reduce costs associated with defects, improve customer satisfaction and loyalty, and enhance brand reputation, leading to better sales and profitability.
How do technology and automation influence Quality Control?
Technology and automation have revolutionized QC by introducing more accurate and efficient methods for testing and monitoring. This includes using AI, machine learning, and automated inspection systems, which can detect defects more reliably and quickly than manual methods.
What role does customer feedback play in Quality Control?
Customer feedback is crucial in QC, providing real-world insights into product performance and customer satisfaction. This feedback can be used to make continuous improvements in product quality.
Are there any global standards for Quality Control?
Several global standards for QC, like ISO 9001 , provide frameworks for quality management systems and are widely recognized across industries.
How often should Quality Control processes be reviewed and updated?
QC processes should be reviewed and updated regularly to adapt to new technologies, changes in consumer expectations, and evolving industry standards. This ensures that the QC processes remain effective and relevant.
What is the impact of Quality Control on product development?
QC significantly impacts product development by ensuring that new products meet quality standards before they reach the market. This can prevent costly recalls and enhance customer trust in new products.
Lean Six Sigma Yellow Belt Certification Training
Lean Six Sigma Green Belt Certification Training
Lean Six Sigma Black Belt Certification Training
Lean Fundamentals Certification Training
Lean IT Certification Training
RCA Through Six Sigma Certification Training
7QC Tools Certification Training
Value Stream Mapping Certification Training
RELATED ARTICLES MORE FROM AUTHOR

What is Cost of Poor Quality (COPQ)?

Top 6 Quality Management Trends to Follow in 2024
What is the Plan Do Check Act (PDCA) Cycle?
Leave a reply.
Save my name, email, and website in this browser for the next time I comment.
- 14,519 Likes
- 444 Followers
- 96,800 Subscribers
- 2,170 Followers
Related Articles

The Roles & Responsibilities of A Quality Management Team

How to Measure Project Success Using Business KPIs

Top 15 Six Sigma Interview Questions with Detailed Answers 2024

How to Bring Vision to Your Projects and Excel as a...

A Brief Comparison Between Six Sigma and PRINCE2
Popular posts.

The Project Management Life Cycle Explained

Roles and Responsibilities of a Quality Control Inspector

7 Cs of Effective Communication with Example

Top 5 Factors for Project Success

Quality Analyst Job Role and Responsibilities- Explained!
Suggested posts.
- 7 Cs of Effective Communication with Examples
- Project Management Lifecycle
- Project Success Factors
- Quality Control Inspector Job Description
- Risk Management Examples
- QA Manager Job Description
- Quality Management Team Roles and Responsibilities
- Risk Management Tools & Techniques
- Quality Analyst Job Description
- What is Business Value
- Who are Project Stakeholders
- Importance of Project Management
- What is Project Management
- Project Management Skills
- Project Manager Job Description
- Agile Project Manager Interview Questions
- Risk and Compliance Manager Job Description
- Risk Management Process
- Project Scope Management
- Healthcare Project Manager Job Description
- Six Sigma Project Examples
- Risk Analysis Methods
- ITIL Service Lifecycle
- Risk Manager Job Description
POPULAR CATEGORIES
- Best Project Management Blogs 265
- Top Agile Blog Posts 158
- Top Blogs on Quality Management 126
- Latest IT Service Management Blogs 108
- Trending Articles on DevOps 65
- Popular Blogs on IT Security and Governance 55
- Top Blogs on Professional Development 33
- Top Infographics Collection 8
Download E-book Blog
Thank You for submitting your enquiry. One of our training consultants will get in touch with you shortly.
50+ Training and Certification Programs - Upskill Today Learn more about our training programs.

What is Quality Control Inspection?
Feb 18, 2024 | Quality Control

I. Introduction to Quality Control Inspection
Quality Control Inspection is about maintaining standards and product quality . It’s a systematic process where professionals scrutinize various aspects of a product or service to ensure they meet specific criteria. These criteria could range from safety standards, functionality, to customer satisfaction. The goal of quality inspections ? To deliver a product or service that not only meets but often exceeds customer expectations.
A. How Does Quality Control Inspection Work?
QCI works in stages. First, there’s the planning phase where quality parameters are defined. These are the benchmarks that products or services must meet. Then comes the execution phase. Here, quality inspectors get down to the nitty-gritty. They examine materials, processes, and finished products using a range of techniques from visual inspections to complex testing methods.
The process is rigorous. Quality control Inspectors might use statistical sampling – where they test just a part of a batch to draw conclusions about the whole. Or, they may go for 100% inspection, scrutinizing every single unit. Whatever the method, the aim is to identify defects or deviations from the set standards.
The Role of Quality Control Inspectors Quality Control Inspectors are the champions of this process. They’re trained to spot issues that might escape an untrained eye. They use specialized equipment, like calipers, gauges, and even computerized testing systems, to measure and test products. Their keen observation and analytical skills ensure that only the best-quality products make it to the market.
Why is Quality Control Inspection Crucial? The benefits of QCI are manifold. For businesses, it’s a way to maintain reputation and customer trust. A single faulty product can tarnish a brand’s image, but regular inspections minimize this risk. For customers, QCI is a reassurance that what they’re buying is safe and worth their money. In industries like pharmaceuticals or automotive, where safety is paramount, QCI is non-negotiable.
B. Quality Control Inspection Definitions
Definition 1 Quality Control as defined by ASQ (American Society for Quality): It is a part of quality management that focuses on fulfilling quality requirements. This aspect of quality management primarily deals with the inspection side of the process, involving operational techniques and activities used to meet the quality standards. Definition 2 “Quality Control Inspection is the systematic process of checking products to ensure they meet specified standards of quality and compliance. It is a vital part of the manufacturing process that involves evaluating and testing products for defects, non-conformities, and overall performance.” Definition 3 “Quality Control Inspection refers to the detailed examination of goods or services against predefined quality criteria. It encompasses various techniques and procedures aimed at identifying and correcting issues before the product reaches the final customer, thereby ensuring customer satisfaction and adherence to industry standards.”
In the following sections, we will uncover the nuances of this process, its methodologies, the challenges faced, and the standards that guide it.
II. The Purpose and Objectives of Quality Control Inspection
A. ensure product quality and consistency.
The primary objective of Quality Control Inspection is to ensure product quality and consistency. This aspect is crucial in today’s market, where the slightest deviation in quality can lead to customer dissatisfaction and, subsequently, a tarnished brand reputation. QCI acts as a bulwark, ensuring that every product aligns with the specifications and expectations set forth at the onset. It is not just about catching defects; it is about creating a culture of excellence where the highest standards are the norm.
B. Compliance with Standards and Regulations
Another essential objective is compliance with industry standards and regulations. Various industries are governed by specific standards and legal requirements, often designed to ensure safety, reliability, and efficiency. QCI ensure that products are not just appealing but are also safe, reliable, and compliant with these regulatory frameworks. This compliance is not just a legal obligation; it is a moral one, ensuring that the products contribute positively to the lives of the consumers and the environment.
C. Compliance with Standards and Regulations
QCI serve as an early warning system, identifying potential issues before they escalate into major problems. This proactive approach not only saves time and resources but also reinforces the commitment to quality and customer satisfaction.
D. Process Improvement
By consistently monitoring and evaluating products and processes, quality inspections provides valuable insights into areas that require refinement. This continuous feedback loop is instrumental in driving innovation and efficiency, leading to improved processes, reduced waste, and enhanced product quality.
E. Supplier Quality Management
Quality Inspection also extends its reach to supplier quality management. In the globalized supply chain, the quality of raw materials and components significantly impacts the final product’s quality. Through rigorous inspection processes, businesses can ensure that their suppliers adhere to the same high standards of quality, creating a unified front in the pursuit of excellence.
III. Types of Quality Control Inspections

The term ‘Quality Control Inspection’ is not a one-size-fits-all concept. Rather, it unfolds into a spectrum of diverse types, each tailored to specific stages of a product’s lifecycle. Understanding these various types of QCI is crucial for anyone involved in the production process, from designers and engineers to quality assurance professionals.
A. Initial Production Check (IPC)
Conducted at the very beginning of production, Initial Production Check is the critical first step in ensuring that the manufacturing process can produce an item that meets design specifications and requirements. It involves a comprehensive and detailed examination of the first sample produced, serving as a prototype for subsequent production. The objective here is to verify that the production methods, materials, and tooling can deliver a product that matches the blueprint, both in terms of quality and functionality.
B. During Production Inspection (DUPRO)
As the name suggests, this inspection occurs throughout the production process. It’s a proactive approach, aiming to identify and rectify defects or deviations from quality standards as they happen. DUPRO are systematic and continuous, often employing statistical methods to monitor quality. They play a key role in ensuring that each step of the manufacturing process aligns with the set standards, thereby maintaining a consistent quality level throughout production.
C. Pre-Shipment Inspection (PSI)
Another critical type, comes into play in the final phase before the products are dispatched to customers or retailers. This type of inspection is particularly significant in international trade, where the buyer might not have the opportunity to inspect the goods before receiving them. Pre-Shipment Inspections are thorough examinations of product quantity, quality, packaging, and labeling. They ensure that the products being shipped are in accordance with the purchase order specifications and are packed and labeled correctly, ready for safe transportation to their final destination.
Each of these inspection types is a cog in the quality control machine, functioning to ensure that every aspect of a product, from its inception to its arrival in the customer’s hands, is subjected to rigorous and systematic scrutiny. They are not isolated processes but are interconnected, creating a comprehensive and dynamic quality control system that underpins the manufacturing process.
IV. Quality Control Inspection Standards

Standards and certifications are not just formalities; they are the backbone of a robust quality management system. These standards serve as the guiding principles and benchmarks that shape the practices and processes of quality control. Understanding these standards and the certification processes associated with them is pivotal for any business striving for excellence in product quality and reliability.
A. ISO 2859 – Sampling Procedures for Inspection by Attributes
A cornerstone in the realm of Quality Control Inspections, ISO 2859 is pivotal for its role in providing sampling procedures. This standard outlines methods for inspecting a sample batch of products to determine if the entire batch meets the specified quality criteria. It’s particularly relevant in situations where inspecting every single item is not feasible due to time or cost constraints. ISO 2859 helps in determining the sample size, inspection levels, and acceptable quality levels (AQLs) – crucial factors in deciding whether a batch passes or fails the inspection.
B. ANSI/ASQ Z1.4 – Sampling Procedures and Tables for Inspection
Similar to ISO 2859, the ANSI/ASQ Z1.4 standard is widely used for determining sample sizes and setting quality levels. This standard is essential for Quality Control Inspectors in deciding the number of units to inspect from a large batch and in assessing the maximum number of defects acceptable. It plays a significant role in ensuring that the inspection process is both efficient and effective.
C. ISO 9000 Series – Quality Management Principles
While ISO 9000 is typically associated with Quality Management, its principles are directly applicable to Quality Control Inspections. This series provides a set of standards that help organizations ensure they meet customer and other stakeholder needs within statutory and regulatory requirements related to a product or service. Although not specifically a Quality Control standard, ISO 9000 provides the foundational principles that guide the overall quality ethos, influencing the approach and methodology of quality control inspections.
D. AQL (Acceptable Quality Limit) Standards
AQL Standards are critical in Pre-Shipment Inspections. They define the quality threshold level that is considered acceptable. In simple terms, AQL is the maximum number of defective items that could be considered acceptable during the random sampling of an inspection. These standards are vital in decision-making processes during pre-shipment inspections, aiding inspectors in determining whether a product batch should be accepted or rejected.
V. Challenges in Quality Control Inspection
The challenges are as diverse and complex as the processes and products they aim to perfect. For professionals in this field, understanding and overcoming these hurdles is not just part of the job; it’s essential for ensuring the consistent delivery of high-quality products.
A. Product designs and materials
As technology advances, products become more complex, integrating new materials and sophisticated components. This evolution demands Quality Control Inspections to be continuously updated and refined. Inspectors must stay abreast of these changes, adapting their techniques and tools to effectively evaluate new products. This requires ongoing training and development, ensuring that the inspection team is always equipped with the latest knowledge and skills.
B. Globalization of supply chains
Today’s products often have components sourced from different parts of the world, each with its own set of standards and quality practices. Managing this global supply chain, ensuring consistency and quality across various suppliers, and navigating the logistical complexities present a formidable task. This calls for a robust quality management system that can integrate and standardize inspection practices across diverse geographical locations.
C. Balancing quality with cost and efficiency
QCI, by nature, are detailed and time-consuming. In a market where time is money, striking the right balance between thorough inspections and efficient production is crucial. Companies must invest in efficient quality control processes that do not compromise the thoroughness of inspections. This often requires leveraging advanced technologies and methodologies to streamline inspection processes without diluting their effectiveness.
D. Regulatory changes and standards
Regulations and standards are continually evolving, driven by technological advancements, changing market demands, and emerging safety and environmental concerns. Companies must ensure that their Quality Control Inspections are not just compliant with current regulations but are also forward-thinking, anticipating future changes and trends.
E. Human factors
The reliance on human judgment and skill means that there is always a risk of human error. Training and maintaining a skilled workforce, ensuring consistency in inspections, and managing human factors like fatigue and motivation are ongoing challenges in this field.

VI. Conclusion – Quality Control Inspection
As we draw the curtains on our exploration of Quality Control Inspection, it becomes evident that this process is much more than a mere checkpoint in the production cycle. It is a vital cog in the machinery of manufacturing and service industries, ensuring that the wheels of quality and excellence keep turning smoothly.
QCI stands as a testament to a company’s commitment to excellence. It is a promise of reliability and safety to customers, a pledge that every product that leaves the production line is not just good enough, but the best it can be. Through rigorous inspections, adherence to standards, and overcoming challenges, Quality Inspection plays a pivotal role in building and maintaining the trust that customers place in products and services.
This journey through the various facets of inspections – from its objectives and types to the standards, certification processes, and challenges – reveals its complexity and significance. It’s a process that requires precision, expertise, and an unwavering dedication to quality. It’s a process that, though often behind the scenes, is fundamental in shaping the face of a product and, by extension, the reputation of a company.
Mars Quality is a global quality control company providing factory audits, quality inspections, product sorting, and engineering recruitment services to a large variety of industries. We qualify suppliers, control product quality and recruit highly skilled specialists in over 100 countries. Contact us at [email protected]
- Inspiration
- Quality Control
- Recruitment
- Supplier Audit
Privacy Overview
- ERP Services

Next Chapter:
The Importance of Quality Control in the Industrial Sector

The comprehensive inventory management system has become a part of technological advancement. If you work on the production part, of course, it is familiar with the term quality control (QC). What’s quality control? Quality control (QC) is an important process that any company or business must undertake, especially if the production is in the form of products or services.
Running a QC can be easier with the help of inventory management software. A fully integrated inventory management system enables businesses to monitor stock transfers and quality control. Not doing a thorough QC will cause errors that could later harm the business.
Many companies are implementing the best inventory management software to automate the QC process. Therefore quality control has an important role, as all goods will go through the inspection process before delivery to know whether they meet the company’s standard or not.

Table of Content
What is Quality Control?
Quality control or QC is very necessary for the industrial sector, ranging from manufacturing, and production activities to the results of production. Quality control is a process of checking and testing conducted by a business or a company. Measuring and ensuring that the quality of the product meets the standard.
This process can make an entity in quality assessment of various factors involved in production activities. Generally, those doing QC duty are training personnel who make product quality benchmarks and check visuals in the tests of products that can last before, during, and after production.
Even so, this process is the responsibility of all employees. These tests can be done manually or with the assistance of technology. Moreover, this process could make the quality of the product better before its release to the public.
One of the few experts Dr. K. Ishikawa claims that quality control is an activity to research, develop, design, satisfy consumer satisfaction, and provide good service.
This procedure is vital because it helps improve and control product quality before it is sold, and it helps a corporation reach its goals. The main purpose of QC is to ensure that products are sent to clients in good condition and meet quality standards.
Also read: Production Process in Business: Definition, Types, and Characteristics

Difference between Quality Control and Quality Assurance
After learning what quality control is, then what is quality assurance and what is the difference? The fundamental difference between the two is that the QA checks into every system and the software needs to run properly.
Quality assurance also focuses more s on disability prevention. QC leads to software testing to increase product quality and meet requirements, thus the emphasis on spotting errors.
Quality assurance (QA) is a proactive process, unlike QC, which is a reactive process. From a different standpoint, if quality assurance has a cost-based approach, quality control is a by-product.
QA involves the process of handling quality issues, while QC verifies the quality of the product itself. Quality assurance is the quality of the audit and for example, the quality control in the inspection process and testing of a product.

The Quality Control Elements
The first element is controlled in management which should be present at QC. Such control is like the management of a job, defined and well-managed processes, integrity and performance criteria, and a record identification.
Competition
In maintaining the quality of the product there must be competition such as qualification, experience, and skill.
The purpose of this closeness is the existence of quality relationships such as staffing, HR management , integrity, trust, organizational culture, motivation, and team spirit.
How Quality Control Works

There must be some internal methods or procedures put in place when performing quality control. In general, the QC supervisor will ensure the product meets the company’s standards.
They will test, examine, research, and analyze a product’s quality according to company standards and consumer worthiness. Here’s how the quality control process works:
Set a standards
The first procedure is to define the standard. Need to consider what standards should be met before the product is at the release stage.
Does the examination have to be one at a time? Or can you check just a few percent of the production in one batch? Doing this is very important at the beginning, for it will determine the effectiveness of the production and preparation of a product or service.
Aligning the company’s vision and mission
Next is to align the vision and mission of the company with its employees. This is important when running quality control because the main goal is to maintain the quality of the product or service offered.
To achieve optimal results, management and employees must agree on maintaining product quality and standards. To align that vision and mission, the company can train its employees and set standards as in the previous points.
Improve the products offered
After determining the company’s standards (benchmarks) and aligning the vision of the mission of management and employees, the next step is to run the QC. In this quality control procedure, finding out how many products fail is one of the things you should do. Then, the responsible team can fix the product to fit the standards.
By doing QC, the percentage of products that fail can continue to decrease. So what if that hope is not achieved? What if too many products fail or services that don’t satisfy customers? If it is already at that point, the company must make a plan to improve the production process.
In essence, this process will continue to rotate, to ensure there are no errors or at least minimize errors.
Benefits of Quality Control
Not only about the quality of the product, but quality control also has other benefits that can support the sustainability of the company and survive the market competition. Among others, as follows:
1. Product consistency
For products that go through the QC process, of course, the consistency will be maintained, even starting from raw materials before production. This is the final quality of the product. When running quality control, you must have set the standard at the beginning.
As a result, every individual ensures that the product meets quality standards, — in other words, that there are no defects before the product or service is on the release stage.
2. Increase efficiency
The purpose of this efficiency is in terms of the production process, power, and time. Because, if you do not do a QC, there could be errors or defects in the product after release. Of course, you have to fix it, and it will take time, cost, and effort.
Therefore, it is better to check it from the beginning to avoid damage, so that costs, time, and energy can focus more on other things such as quality development or maybe business expansion.
Also read: Discover 10 Effective Business Expansion Strategies
3. Maintaining customer satisfaction
Implementing QC can reduce and avoid fatal undetected errors. Because if you ignore this process, it can make customers not want to buy products or use your services again.
Of course, there will be customer expectations when producing a product that is already in use. By building good standards that previously customer satisfaction will be maintained because it is one aspect that can affect customer loyalty.
Quality Control Responsibility
- Document inspections and tests on a company’s products or services.
- Monitor, analyze, and also test, and research all products.
- You can recommend reprocessing for any low-quality products to the company.
- Monitoring the process in the manufacture of products and monitoring their development.
- Verify the quality of the product.
- Ensuring all goods produced have a quality that meets the standards that the company has set.
Also read: ERP Software Application to Increase Business Performance
That’s the complete explanation of quality control ranging from understanding, differences with quality assurance, elements, how it works, benefits, to responsibility.
So the conclusion is that QC is an important aspect of producing products or services, in order to ensure quality in accordance with company standards so that it can meet consumer expectations.
The difference between QC and QA lies in their focus. QA focuses on disability prevention, while QC focuses on identifying or finding defects.
In addition, there are also other elements in the QC that are also important for you to know such as control, competition, and proximity. Quality control is closely related to inventory control that can optimize storage in your business goods inventory.
Not only that reducing the risk of losses due to inventory recording errors can be resolved with the Inventory Management System from HashMicro which has been integrated with inventory features and can create inventory reports and track them automatically.
If you want to feel the greatness of many other features in facilitating the management of your business, we also provide cloud-based ERP Software with the most complete system and can adjust to your business needs. So, wait no more and try the free demo today!
Interest in getting savvy tips for improving your business efficiency?

RELATED ARTICLES
5 extra ways to improve online help desk management, how to sell product effectively, a complete guide to real estate management, looking for software system to improve your business efficiency.
HashMicro is Singapore's ERP solution provider with the most complete software suite for various industries, customizable to unique needs of any business.
ERP SOLUTION
- Construction
- Engineering
- Manufacturing
Get HashMicro’s Software with 50% Off EDG !
Contact us via WhatsApp for a faster response!
© BusinessTech by Hashmicro

Alex Typically replies within an hour
See how HashMicro can improve your business
Growing and expanding the business is what every company is trying to achieve. However, this can add stress to the management due to increasing complexity. For that reason, we continuously develop products that can streamline business processes in all industrial sectors, no matter how big.
Dipercaya oleh

What is Quality Control? A Detailed Guide
Last Updated on April 1, 2024 by Owen McGab Enaohwo
Start your free 14-day trial of SweetProcess No credit card needed. Cancel anytime. Click Here To Try it for Free.
When buying products, customers expect quality all the time. Therefore as a business, it’s your responsibility to deliver products without defects. However, this is not the case in most companies, thanks to the absence of standardized procedures.
Lacking a proper quality control process affects the company’s output and puts your consumer at risk. If your products and services are of poor quality, you’ll spend more time and money reworking them, which eats into your profits in the long run. In addition, your customers won’t be satisfied with your products and will stop doing business with you. As word spreads about your products and services, your reputation will be affected.
This is why quality control procedures are a crucial function in any organization. Implementing a standardized process in your operations is the only way your company will deliver consistent and quality products and services to your customers.
Quality control comes in handy because it spots and removes the sub-standard output at three separate stages:
- When you receive raw materials for production.
- When products are going through the production process.
- Before finished products are dispatched to the customers.
If you are looking to implement a quality control process in your organization or improve the existing procedure, this article will cover all the steps you can use to create and enhance the process, the requirements, benefits, and methods to use as a guide. By the end of the article, you’ll have a clear understanding of why quality control is a must-have in your company.
Chapter 1: What is Quality Control
Chapter 2: quality control methods, chapter 3: requirements for efficient quality control procedures, chapter 4: benefits of quality control, chapter 5: quality control examples, chapter 6: differences between quality control and quality assurance (qc vs. qa), chapter 7: steps to creating a quality control process, chapter 8: steps to improving quality control procedures.
Chapter 9: Frequently Asked Questions on Quality Control
Chapter 10: Win in Quality Control With SweetProcess

Definition and Overview
Quality control (QC) is an approach of quality management that businesses use to ensure that the manufactured product or service adheres to a standardized quality criteria and meets the client’s requirements.
Organizations use a set of procedures and benchmarks to ensure that the quality of a product is maintained or improved and errors are reduced or eliminated. Quality control requires inspection and sampling to test whether the final product adheres to the set specifications.
Quality control testing is done at the various steps of manufacturing to identify the problems and help prevent them in the future.
Quality control is a core process in industries that rely on products. For instance, the food and drug manufacturing industries are quite sensitive because the products can cause the consumers to get sick. Companies in this sector perform chemical testing of samples to ensure the final product is free from contaminants to avoid such issues.
Other industries such as automotive and electrical companies also use quality control on their products. The procedures are essential because they help in boosting the safety of the manufactured parts.
Quality control was introduced following the industrial revolution in the 19th century. The rise of mass production prompted the establishment of processes to create parts with identical designs and dimensions. However, the consumers were not satisfied with the results because the processes were not uniform; therefore, quality control was introduced.
During this period, quality control involved using a sketch of the desired product. However, this process became too costly and challenging for most manufacturers. In 1840, companies introduced tolerance limits, and with this method, a design would pass the testing if the parts aligned with the set limits. Manufacturers used devices such as ring gauges and plug gauges to define quality.
The method did not address the issue with defective items; therefore, several methods of quality control were introduced. Here are the different approaches that have been developed over the years.
- Statistical quality control: 1930s
- Total quality control: 1956
- Statistical process control: 1960s
- Company-wide quality control: 1968
- Total quality management: 1985
- Six Sigma: 1986
- Lean Six Sigma: 2001

Once you introduce quality control in your organization, you need to measure its performance. Each company will adopt different standard operating procedures depending on their industry, business size, and nature of business. Here are the common methods used by most corporations.
Six Sigma is a set of quality control tools created by a scientist in the 1980s. This method is a data-driven process that was developed to reduce the defects and variations from set specifications. In statistics, Sigma is the standard deviation from the mean.
With this method, a process needs to have no more than 3.4 defects for every one million events or units. Originally Six Sigma was developed as a management method to enable companies to work faster with fewer mistakes, and has grown to become an industry standard that provides certifications. Companies use this model to boost their profits.
Over the years, Six Sigma has evolved to become a part of business management. Organizations use this method to meet customer requirements, improve customer retention, and sustain the business products and services.
Lean, or lean Six Sigma, is one of the latest quality control methods. This team-focused managerial approach identifies and eliminates waste in the production process to improve efficiency in the organization. In this case, “waste” is the tasks or steps that are deemed unnecessary in the company; therefore, they need to be discontinued.
Companies that use the lean model are focused on eliminating the following:
- Inefficient transportation of materials
- Overproduction that exceeds customer demands
- Too much waiting
- Inefficient processing standards
- Excess inventory levels
- Inefficient work execution by workers and machines
- Work spent fixing production defects
Kaizen in Japanese means change for good; however, it’s loosely translated to “continuous improvement.” This quality control process is quite different because it includes the entire organization, from the top management to the rank and file. If you implement this method in your company, it means that every employee has the opportunity to improve daily.
Organizations that use the Kaizen model also use these implementation tools.
- Gemba Walk: Management and top executives walk around the production floor or manufacturing plant to observe the work, ask questions, and identify areas of opportunity. This model enables the management to know exactly what’s happening.
- SIPOC: Supplies, inputs, process, outputs, and customers (SIPOC) is a process mapping tool that provides clarity in the process workflows. With this tool, the overall process gets optimized, and unnecessary tasks are discontinued.
- 8D Report: Also known as the eight disciplines model, this problem-solving tool resolves and prevents issues identified by quality control personnel. This method identifies and eliminates the root cause of the problem to improve the quality.
X-Bar Chart
Organizations also use quality control charts to measure whether the sampled processes or products are meeting the set specifications. With the X-bar chart method, randomly selected products are tested for the attributes the chart is tracking. On this chart, the Y-axis tracks the variation, while the X-axis monitors the sample tested.
You can use the pattern of variance from the chart to determine whether the facts are systematic or random. Using the results, you can plan on how to improve your quality control processes.
100 Percent Inspection
This quality control method involves examining and assessing all parts of a product. Companies use this method to rule out defects on a product; therefore, it’s common in organizations that deal in produce and metals. Quality control inspectors will need the data on the entire manufacturing process and an inventory analysis.
However, this method comes with some disadvantages. Checking every item that makes up the product is expensive and time-consuming. In addition, if the product is delicate, such as fruits, the inspection process could render it unusable, leading to massive company losses.
Unlike other quality control methods, the Taguchi model focuses on product design, development, and research. Developed by Genichi Taguchi, a Japanese statistician, it seeks to eliminate variances during production before they happen. Companies that use this method aim to reduce the occurrence of defects in their products.
This Taguchi method considers product design to be more important than the manufacturing process. Therefore, the focus is on using research and design to ensure every product will match the set specifications.

For your products to be of high quality, you have to have a strategy in place. A successful and efficient business has effective quality control techniques and procedures to help maintain the consistency of the products across all their locations. If you are unsure about where to start, here are some of the key things you need to build an efficient process.
Create a Dedicated Team for Quality Control
The first step is hiring a dedicated quality control team for the company. According to statistics, more than 122,528 quality control managers are employed in the U.S. Therefore, when searching for personnel, you need to recruit the most qualified candidate and with the right credentials to handle the job.
If your business is a startup with limited capital, it’s still advisable to hire a quality control team to help track and monitor your products’ quality. While you may need to source for extra funds to pay their salaries, the returns are worth it. If your products maintain the quality, you’ll generate more sales and revenue.
When putting together a team, consider their knowledge about the specific product or service they’ll be handling. In addition, you should confirm whether they have worked in this industry before coming to your company.
Consider recruits who understand what the customer wants and the product you are offering so that the quality control process is efficient. You should also recruit top performers who are diligent in their work. Having the right team will streamline your quality analysis procedures and maintain and improve the quality of your products in the long run.
Have a Specific Performance Goal
While this might sound obvious, some quality control teams work without a plan. This is counterproductive and will not meet the company objectives. To build efficient procedures, define success, and take all the steps necessary to improve the quality of your products and services.
During the initial phase, quality control teams need to track the existing procedures and identify any problems that affect the quality of processes. Once this step is complete, the next step is to eliminate all the tasks and steps that interfere with the ultimate goals.
For instance, if you identify some defects in your product, you need to develop a roadmap detailing the root cause of the problem and how to eliminate it. With this type of strategy, you’ll improve the consistency of your services and products while meeting customer needs.
Select the Best Quality Control Method for Your Organization
We have identified the different types of quality control methods above. Depending on your business and the industry you are in, you can choose between Six Sigma, lean, Kaizen, Taguchi, X-bar chart, or 100 percent inspection as the method for your organization.
Each of these procedures can be used to improve the overall quality, but you need to use the right tools to boost their effectiveness. If you select one and it doesn’t give you the expected results, you can choose another option until you find the most ideal for your organization.

Implementing a quality control procedure in your organization comes with the following benefits.
Earn More Money
Quality control ensures that your company delivers high-quality products without defects. This process in turn contributes to customer satisfaction and more sales. If your clients are assured of quality products and services, they will shop from your business which earns your company money in the long run.
Increased Customer Loyalty and Referrals
Customers are more likely to refer you to their friends and family members if your products are of a high quality. Statistics show that 82 percent of small businesses say referrals are their main source of revenue; therefore, it’s important to build that reputation. In addition, your customers become loyal to your brand because of consistency. For this reason, you should have an efficient quality control procedure in place.
Enjoy a Better Brand Image
When you have a quality control procedure for your company, all your products will be uniform and meet the same high standard. This plays a big role in building public confidence in your product. Consumers who have tried your products will have positive reviews about your brand, significantly boosting your image.
Reduce Liability Risks
Quality control is implemented to identify defects in your products and services. If you don’t test your finished products, you’ll be putting your consumers at risk. In addition, your company might get into legal trouble in case the products harm your customers. For instance, the company Takata Corporation had an airbag recall because of increased cases of airbag explosions during deployment leading to injuries and death. These types of liabilities can be costly to your organization if you don’t have quality control.
Meet Regulatory Requirements
Quality control is also critical for your business because it keeps your operations within the regulatory requirements. Organizations are checked against the ISO 9000 set of standards, ensuring that their operation meets stakeholder and customer needs under the statutory regulations. ISO 9000 standards are fundamentals of quality management systems; therefore, they are used by organizations globally to document the quality elements. Quality control is a part of these standards—they play a big role in ensuring that the company is not breaking the law.
Boost Organizational Safety
One of the benefits of introducing quality control in your company is its impact on your employees. Since they know that quality is important, they become keener and monitor all the procedures carefully. This effect will also trickle down to all the areas of business. Employees will become efficient during production to reduce the chances of error and accidents on the production floor.
Gain Competitive Advantage
If your customers are happy with your products, you’ll get more sales. As they become more loyal, you’ll gain a competitive advantage over the other companies in the same industry. Consumers tend to source products and services from organizations with a good reputation. Therefore, if you want to outsmart your competitors, invest in quality control protocols for your products.

Some of the most successful brands in the world have incorporated quality control procedures in their operations. Let’s look at some of the best examples.
How Toyota Used Kaizen
Kaizen, or “continuous improvement,” is one of the major principles in The Toyota Production System . The company uses this philosophy to ensure that its products are of high quality while eliminating waste and improving efficiency in their work procedures.
By following the principles of Kaizen, they have improvements in their standardized work. These standards involve following the set procedures consistently, making it easier for employees to identify defects.
Since this method involves all the employees, from the management to assembly line workers, the Toyota Production System is humanized. Every member of staff can identify areas that need improvement and suggest solutions. According to the company, every staff member has the responsibility to adopt the standard procedures and eliminate waste.
Motorola’s Use of Six Sigma
Six Sigma was introduced in 1986 by a scientist working at Motorola. This methodology was incorporated into the company procedures to reduce mistakes while documenting the manufacturing procedures.
Motorola introduced this quality control method during this period because they faced fierce competition from similar organizations overseas, especially in the Japanese market. Additionally, at this time, the customer complaints about their products were pretty high due to product defects and customer support; therefore, they had to take action.
To deal with this problem, the company introduced Six Sigma, which improved the company’s performance significantly. Between 1986 and 1991, Motorola had hit every target for improvement in different business functions.
Microsoft Using Six Sigma
As one of the largest software producers in the world, Microsoft has tons of customers. To improve the reliability of their network, the company had to use Six Sigma techniques. This method also helped them eradicate defects in their data centers and systems and reduce the failure incidences in their infrastructure.
Microsoft collected data based on past high-priority incidents, server issues, and customer recommendations. This historical data was used to develop a road map on addressing these problems and improving the quality of their services.
Once the company had all the data, they identified the defects and solutions for each of them. The company prioritized all the issues depending on how severe the disruption was. Using the Six Sigma method, the organization had improved the defects by about 40% within the first year. Furthermore, the server failure period improved from 18 days to 125 days. Thanks to these procedures, Microsoft improved the performance of their services, increasing customer satisfaction.
Lean Six Sigma at Dell
Lean Six Sigma had a significant role in Dell’s success. In the early 2000s, the company adopted this technique of quality control to improve its product.
Dell enjoyed massive success between 1997–1999 and increased its revenue from $7.8 billion to $18.2 billion. Once the company implemented the lean Six Sigma model, it saved up to $1.55 billion and achieved the highest profitability by 2004.
With this process in place, the organization increased its efficiency; therefore, customers enjoyed better service and received their computers much faster. At the same time, Dell cut down wastage and turn-around time and started listening to their customers. This quality control tool boosted Dell to become one of the most profitable companies globally.
Kaizen in TOTO Ltd
Just like with Toyota, Kaizen was also implemented at TOTO Ltd. TOTO is a Japanese manufacturer that specializes in plumbing fixtures. The company adopted the Kaizen philosophy to create a comfortable environment for employees and people with disabilities.
Thanks to this quality control technique, the company rearranged supplies for easier handling and better transportation. In addition, the executives introduced new procedures, including using flexible cords to stabilize hanging screwdrivers, which eliminated more than three working hours per month. This saved the company money on salaries.

Quality control (QC) and quality assurance (QA) are mostly used interchangeably. However, that should not be the case since they are quite distinct. Here are the major differences between the two.
Quality Control
Quality control is the process of inspecting products to ensure that they meet the required standards. Quality control is based on inspection and aims to take the defects out.
Quality control was introduced during the industrial revolution in the 20th century following the rise of mass production. Businesses needed a system to check the quality of their products.
Quality Assurance
On the other hand, QA is based on processes and aims to build in the quality without inspecting. These are the processes that a company puts in place to ensure the production quality aligns with the customer’s needs.
Businesses perform quality assurance to design how a product or service will be delivered and eliminate the chances that it will be sub-standard. The focus of QA is during the product development phase, making it easier to build in the quality. Therefore, if the production process is done well, there will be no need to inspect or do quality control on the finished product.
Quality assurance was first introduced in the Middle Ages. Guilds were formed to ensure a standard of quality was maintained. QA continued during the industrial revolution. Over the years, the process has evolved and led to the introduction of statistical control, total quality management, and ISO standards.
List of Differences
Here are the differences between quality control and quality assurance that you need to know.
- Quality control is a process that fulfills the quality request.
- Quality control aims to identify and improve defects.
- It verifies quality.
- The testing team is responsible for quality control.
- It involves executing the planned process.
- It ensures the results align with your expectations.
- This process verifies the deliverables.
- It ensures that the standards are followed when developing a product.
- The statistical technique on quality control is referred to as statistical quality control (SQC).
- QA is a process that aims to provide confidence that quality requirements will be fulfilled.
- QA aims to prevent product defects.
- This technique manages quality.
- All the team members are responsible for quality assurance.
- QA is not involved in executing the program.
- The process ensures the company is doing the right thing.
- QA creates the deliverables.
- QA defines the methodologies and standards to be followed to meet customer needs.
- The statistical technique used on QA is referred to as statistical process control (SPC).
How They Work Together
Quality control and quality assurance work together in an organization as part of the quality management system (QMS). QMS incorporates policies, planning, improvement initiatives, and quality control and QA procedures to boost business efficiency.
These two procedures work together in the following areas.
Customer Focus
Both quality control and QA are essential in meeting customer needs and are implemented to exceed expectations. Once your team understands customers’ current and future needs, it becomes easier to succeed. This strategy leads to increased customer value, loyalty, and satisfaction.
Employee Engagement
To deliver value to your customers, you need to engage all the employees at all organization levels. Quality control and QA techniques can be used to involve all the people in the company. This leads to increased motivation, enhanced personal development, and encourages more involvement in company activities.
When QA and quality control procedures are in place, leaders provide direction and create conditions for all employees to be engaged.
Organization Improvement
Organizations with an efficient QA and quality control process focus on improving all the business functions. This allows the business to maintain its high performance, prevent errors, and take corrective actions.
Relationship Management
Quality management processes in an organization help manage relationships with other parties such as suppliers. With proper relationship management, your company will enhance its performance and create a common understanding of the goals and values.
Process Approach
Companies need efficient QA and quality control techniques to produce consistent and predictable products and services. These processes come in handy in the company to help optimize the system and its performance.

If you are looking to create a quality control process in your company, here are the steps you can use as a guide.
Decide on Your Quality Standards and What You’ll Focus On
You need to set quality standards that you plan to follow in each department. Fortunately, some industries have guidelines created by an external organization or regulatory body. In this case, you have to follow these standards. However, if your industry does not have any external procedures, you’ll need to create your own.
When developing these standards, ensure that every department has quality control procedures that are measurable. This will help you track and monitor any issues and areas for improvement.
In addition, you can choose to focus on the important measures that have a significant effect on customer experience and your profits. This way, your business can get results quickly without overwhelming your team. Focusing on quality standards that are needed by your customers is the best way to improve their experience and ensure satisfaction.
Create Processes That Will Ensure You Deliver Quality
You’ll deliver high-quality products and services if you have a well-designed quality control process. You’ll need to continually measure the success of your quality control procedure and implement improvements to make your products and services better. For instance, you can start with the critical operations in the business and create a guide with benchmarks.
Additionally, these processes should be incorporated in every department. If you deliver quality, your customers will keep coming back because of the high-quality products and services.
Evaluate Results
The only way to know how your quality control process is performing is by reviewing the results. Use your business software, customer relationship management (CRM) tools, financial apps, and customer service tools to customize your information and collect data. These reviews will provide information on whether the company is meeting its standards.
Failure to evaluate your results will lead to poor quality products and services. If a quality control process is not working properly, you might miss the indicators that something needs to be improved.
Ask for Further Feedback
Apart from your company tools, you should also get external feedback from online ratings and reviews and customer surveys. This form of feedback is critical because it provides real-time information from the consumers on the quality of your products and services. You can use this information to improve your products.
Customer feedback provides first-hand information on the effectiveness of your quality control system. Always pay attention to these reviews to help you improve your operations.
Improve Your Processes Based on the Results
Even if you are meeting your quality standards, you should strive to do better. Once you get feedback on your quality standards, you should not stop there. Find ways to improve your quality and delivery of services to keep the customers satisfied.
When your processes are improved, your quality of products and services will become better. Otherwise, if you are stagnant, your bottomline will be affected.

Quality control processes are important because they help your business meet customer expectations. If you consistently improve your company processes, you’ll enjoy continued success and deliver quality products that align with the industry standards. Here are some tips you can use to enhance your company procedures.
Involve All Employees
For the quality control procedure to be effective, you need to engage all employees. This encourages them to adhere and commit to the set standards. You can enforce these procedures easily by training new hires during the onboarding period. Additionally, you can conduct refresher training every year to remind the employees that quality control is everyone’s responsibility.
If your company has a top-down approach, you need to transform it into a bottom-up approach to reach employees in all levels. Your quality control system will become better when everyone is working on a common goal.
Be Flexible and Proactive
During audits, ensure that you reinforce quality procedures and have regular checks to identify non-conforming employees. Typically, a company will have one external audit every year. However, others have several internal audits to keep track of company performance, either quarterly or twice a year.
After these audits, keep employees updated on the new quality control processes. Encourage them to share their feedback and suggestions on how the quality can be improved further.
Automate Records as Much as Possible
Paper-based record-keeping can be tiresome and time-consuming for any business. Ensure that you automate most of your records to make it easier to monitor and track quality standards and procedures. Digital recordkeeping saves time and helps in consistently recording internal checks.
In addition, you should automate all reporting systems from the employees. This way, they can send suggestions and comments in real-time about the progress of the quality control processes.
Take Advantage of All Improvement Tools and Opportunities
During reviews, use all the information you get from surveys, past mistakes, incident reports, and customer reviews to improve your quality control procedures. It would be best if you also stayed up to date with the latest industry best practices that can be used to improve your processes.
You can use internal and external tools to improve your systems. Ensure that every employee understands how the tools work to make system improvement much easier.
Maximize the Use of Technology
New technologies save time spent inspecting products and services. Invest in digital tools that make it easier to automate monitoring. You can find new ways to improve your quality control processes with these resources. Research the current industry trends and incorporate some of the technologies being used by your competitors.
Chapter 9: Frequently Asked Questions about Quality Control

What is quality control?
Quality control is a process that involves measuring the product quality against set standards to identify defects. It can be implemented by either testing products, creating benchmarks, or reviewing the manufacturing process.
What is the main difference between quality control and quality assurance?
While quality control involves the delivery of products and services, QA relates to each process involved in the production and delivery of goods and services.
How do you know your business has a quality control issue?
You’ll know if your business has quality control issues if you are underachieving when it comes to delivering products and services. Watch out for errors and defects in your business.
What are the main types of quality control?
The different quality control methods include Six Sigma, lean, X-bar chart, 100% inspection, Taguchi, and Kaizen.
Does a small business need a quality control program?
Yes! Whether you are running a small or large business, you need quality control. The success of your business depends on the quality of services and products.
What are examples of quality control?
In the medical industry, quality control is done on medication by checking the composition of the drugs to sanitary conditions to ensure that they don’t have any contamination that could be harmful to the human body.
What are the main objectives of quality control?
Companies use quality control procedures to ensure they don’t deliver defective products to the consumer. Therefore, they hire quality control employees to inspect and keep track of all the products and confirm that they are safe.

SweetProcess is a software that helps companies manage all their tasks and processes in one place. You can use it for various functions, from managing tasks to documenting company information to implementing quality control procedures. Lack of proper quality control procedures will affect the quality of your products and services and prevent you from meeting customer expectations.
However, if you adopt the SweetProcess platform in your operations, you can document your procedures, capture all information, and involve your team members in suggesting improvements. If you want to sign up, SweetProcess is available on the web, iOS, and Android.
Kevin Trapp, the director of operations at Forensic Analytical Consulting Services Inc , is responsible for ensuring consistency and efficiency in the organization. However, as the company expanded to multiple locations, it became challenging to maintain consistency. Although the company has standard operating procedures (SOPs), employees did not use them because they were buried in PDF files on the servers.
Employees who needed information would rather consult Kevin instead of checking the documented processes. This dependency was a setback in his productivity. He started looking for a solution and discovered the SweetProcess platform to solve this problem.
According to Kevin, SweetProcess was robust enough to complete tasks and simple enough; therefore, it didn’t overwhelm any of the users. They can now document SOPs that are more comprehensive and streamline their business operations.
David Brannen, the founder and managing lawyer at Resolute Legal , started his law firm to help people who had an unpleasant experience with the law in areas such as compensation. However, the business did not kick-off as expected. Since he started the business all by himself, David did not have a structure that prevented him from scaling his business.
The lack of procedures affected the quality of his output because he juggled all the tasks. To bring organization into the business, David discovered SweetProcess. Once he got on the platform, he started hiring other lawyers and staff, created a knowledge base for employees, and introduced quality control procedures.
Quality control helped the law firm maintain consistency in its services, which is essential in building customer loyalty. SweetProcess has made it easier to deliver quality services.
Florinela Serban, the head of operations at Onogo , was responsible for streamlining the company’s processes. The company was growing, and there were a lot of requirements from customers and partners. However, the business was experiencing challenges in keeping up with customer needs due to a lack of structure.
Florinela and her team decided to become proactive and document the company’s procedures to equip new hires. She took up the SweetProcess tool to help with the documentation, employee onboarding, and creating a knowledge base.
In addition, the Sweet Process platform helped the organization in establishing quality control and quality assurance procedures. There’s uniformity in the duties and increased collaboration between workers. Because of this, the company can meet its organizational goals much faster.
Click here to sign up for a free trial of SweetProcess now!
Every organization needs an efficient quality control procedure. This process ensures that you deliver quality products and services to your customers.
Companies with a quality control process can easily identify defects in their products and find ways to improve their delivery. You need to invest in a quality control process and team for your business to succeed. If you want to start your journey to create a new process, or improve the existing quality control process, sign up for a free trial of SweetProcess today. No credit card required .

Related Posts:
![What Is the Difference Between Quality Assurance and Quality Control? [+ Examples] quality-assurance-vs-quality-control](https://www.sweetprocess.com/wp-content/uploads/2024/03/quality-assurance-vs-quality-control-150x150.png)
Get Your Free Systemization Checklist

2 responses to “What is Quality Control? A Detailed Guide”
Clear and concise guide on Quality Control! Your breakdown of key concepts and methods makes it a valuable resource. Understanding the importance of QC is crucial for maintaining high standards. Great read!
Thanks for your feedback.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Table of Contents
What is quality control (qc), key components of quality control, types of quality control, why is quality control important what are the benefits, quality control roles and responsibilities, quality control vs. quality assurance, quality control careers, what does quality control mean, what are 3 examples of quality control, train to assess quality with lean today.

In today’s world, it’s not uncommon that we take the reliability and quality of products and services for granted. At the start of the 20th century, however, quality control in manufacturing was not exactly a reliable process.
Now, decades after early pioneers created business problem-solving processes and analysis frameworks to determine and control consistency and value, it’s possible more than ever for a business to implement and scale best practices.
Quality does not have a singular definition. Despite the relative meaning of “value,” quality control is the process by which products/services are tested and measured to ensure they meet a standard. Through this process, a business can evaluate, maintain, and improve product quality. The primary objective of Quality Control is to identify and correct any deviations from the established quality standards. This process involves monitoring and inspecting products or services at various stages of production or delivery to ensure that they meet the desired level of quality. QC is also concerned with preventing defects or errors from occurring in the first place by implementing measures to control and improve the production or service delivery processes.
Ultimately, there are two crucial goals of quality control: (1) to ensure that products are as uniform as possible and (2), to minimize errors and inconsistencies within them.
Key components of Quality Control may include:
Inspection: Regularly examining products, materials, or services to identify defects, non-compliance, or deviations from quality standards.
Testing: Conducting various tests and measurements to assess the performance, functionality, or characteristics of products or services.
Statistical Process Control (SPC): Employing statistical techniques to monitor and control the production processes, ensuring that they remain within acceptable quality limits.
Documentation and Records: Keeping detailed records of inspections, tests, and corrective actions taken to maintain traceability and accountability.
Corrective Action: Implementing appropriate measures to address any identified quality issues and prevent their recurrence.
Training and Education: Providing employees with the necessary skills and knowledge to maintain quality standards effectively.
Continuous Improvement: Constantly analyzing data and feedback to identify areas for improvement and enhancing the overall quality management system.
Quality Control is closely related to another quality management concept called Quality Assurance (QA). While QC focuses on detecting and correcting defects, QA concentrates on preventing them from occurring in the first place by setting up robust processes and procedures.
Together, QC and QA form the backbone of an organization's quality management system, helping to ensure that products and services consistently meet or exceed customer expectations and regulatory requirements.
Quality Control Process
Normally, quality testing is part of every stage of a manufacturing or business process. Employees frequently begin testing using samples collected from the production line, finished products, and raw materials. Testing during various production phases can help identify the cause of a production problem and the necessary corrective actions to prevent it from happening again.
Customer service reviews, questionnaires, surveys, inspections, and audits are a few examples of quality testing procedures that can be used in non-manufacturing businesses. A company can use any procedure or technique to ensure that the final product or service is safe, compliant, and meets consumer demands.
QC Is Different by Industry
Quality Control (QC) is an indispensable aspect of various industries, ensuring that products and services adhere to predefined standards. In the manufacturing sector, QC involves rigorous inspection and testing of raw materials, intermediate components, and final products to maintain consistent quality and minimize defects. In the food industry, QC guarantees the safety and integrity of consumables through thorough testing for contaminants and adherence to health regulations. In the pharmaceutical sector, QC plays a critical role in verifying the potency and purity of drugs, ensuring they are safe for consumption. Additionally, in the software industry, QC involves extensive testing of applications and programs to identify bugs and errors before release, guaranteeing a smooth user experience. Across all industries, QC is a fundamental process that enhances customer satisfaction, boosts efficiency, and fosters a reputation for reliability.
Just as quality is a relative word with many interpretations, quality control itself doesn’t have a uniform, universal process. Some methods depend on the industry. Take food and drug products, for instance, where errors can put people at risk and create significant liability. These industries may rely more heavily on scientific measures, whereas others (such as education or coaching) may require a more holistic, qualitative method.
At its core, quality control requires attention to detail and research methodology.
So, what is quality control? There are a wide range of quality control methods , including:
Control Charts:
A graph or chart is used to study how processes are changing over time. Using statistics, the business and manufacturing processes are analyzed for being “in control.”
Process Control:
Processes are monitored and adjusted to ensure quality and improve performance. This is typically a technical process using feedback loops, industrial-level controls, and chemical processes to achieve consistency.
Acceptance Sampling:
A statistical measure is used to determine if a batch or sample of products meets the overall manufacturing standard.
Process Protocol:
A mapping methodology that improves the design and implementation processes by creating evaluative indicators for each step.
There are other quality control factors to consider when selecting a method in addition to types of processes.
Some companies establish internal quality control divisions when defining what is quality control. They do this to monitor products and services, while others rely on external bodies to track products and performance. These controls may be largely dependent on the industry of the business. Due to the strict nature of food inspections, for example, it may be in a company’s best interest to sample products internally and verify these results in a third-party lab.
Become a Quality Management Professional
- 10% Growth In Jobs Of Quality Managers Profiles By 2025
- 11% Revenue Growth For Organisations Improving Quality
Certified Lean Six Sigma Green Belt
- 4 hands-on projects to perfect the skills learnt
- 4 simulation test papers for self-assessment
Lean Six Sigma Expert
- IASSC® Lean Six Sigma Green Belt and Black Belt certification
- 13 Projects, 12 Simulation exams, 18 Case Studies & 114 PDUs
Here's what learners are saying regarding our programs:
Xueting Liu
Mechanical engineer student at sargents pty. ltd. ,.
A great training and proper exercise with step-by-step guide! I'll give a rating of 10 out of 10 for this training.
Abdus Salam
I have completed the Lean Six Sigma Expert Master’s Program from Simplilearn. And after the course, I could take up new projects and perform better. My average pay rate for a research position increased by 21%.
Quality Control (QC) is essential for various reasons, and its importance lies in the numerous benefits it brings to both businesses and consumers. Here are some key reasons why QC is crucial:
Customer Satisfaction: QC ensures that products and services meet or exceed customer expectations, leading to higher satisfaction levels and increased customer loyalty.
Defect Prevention: By identifying and correcting issues early in the production or service delivery process, QC helps prevent defects, reducing the likelihood of expensive recalls or rework.
Cost Reduction: Implementing QC measures can lead to reduced waste, lower production costs, and improved operational efficiency, contributing to overall cost savings.
Compliance and Regulations: QC ensures that products and services adhere to industry standards and regulatory requirements, avoiding legal issues and penalties.
Brand Reputation: Consistent high-quality products or services build a positive brand image, enhancing the company's reputation and competitiveness in the market.
Increased Efficiency: QC optimizes processes and identifies areas for improvement, leading to increased productivity and streamlined operations.
Risk Mitigation: Through rigorous testing and inspections, QC helps identify potential risks and hazards, enabling businesses to address them proactively.
Continuous Improvement: QC encourages a culture of continuous improvement, where organizations strive to enhance their products, services, and processes constantly.
International Competitiveness: High-quality products can open doors to global markets, increasing a company's competitiveness on an international scale.
Customer Retention and Loyalty: Satisfied customers are more likely to remain loyal and recommend the brand to others, contributing to long-term business success.
Overall, Quality Control is crucial for maintaining high standards, minimizing risks, and fostering a competitive advantage in today's dynamic and demanding business environment. It serves as the foundation for delivering superior products and services while ensuring customer satisfaction and loyalty.
When answering what is quality control, it is critical to understand that it consists of multifaceted responsibilities and roles. Moreover, it shouldn’t be confused with quality assurance. Whereas quality assurance looks at the processes used to prevent defects, quality control is focused specifically on the measurement and analysis processes involved with determining product quality.
Quality control uses specific research tools to accomplish fact-finding processes and conduct analyses. A quality control professional is tasked with analyzing these measurements against some sort of standard determined by the quality management department, company policies, and industries or regulatory bodies. Based on this evidence-gathering, quality control will recommend changes.
We can see from this roadmap, too, how quality assurance and quality control differ. Quality assurance looks at the holistic picture to prevent a product from becoming defective. Quality control, on the other hand, later determines if a product is, in fact, defective or not. Both roles fit under the broad umbrella of quality management.
Thus, an individual in quality control is tasked with communicating results to stakeholders and significant parties. A good quality control specialist will be able to disseminate scientific and research-based thinking to a business community and assist with the problem-solving process. These specialists are a key component of a product’s design process, as they determine whether a company’s creation is truly acceptable for the market.
Even though the terms quality control and quality assurance are sometimes used interchangeably, they have some key differences. Quality criteria, such as ensuring an item complies with specifications, are the main emphasis of quality control. Quality assurance is the sum of all processes and actions necessary to demonstrate that the requirements for quality are satisfied.
Quality control can be a fulfilling job if you enjoy dealing with people, talking, presenting results, and trying to make things better and safer. Depending on the sector, you may need the following qualifications to work as a quality control inspector:
- Entry-level positions require a high school diploma.
- Depending on the business, a bachelor's degree
- A background in the industry
- Certain businesses and sectors require licenses and certifications.
Additional characteristics required by quality control specialists include:
- Observation of details
- Talents in math and mechanics
- Physical prowess and power
- Technical expertise
- Pressured performance
Career Path
There may be discrepancies because the path to quality assurance and control job varies by industry. However, you'll typically require a number of years of professional expertise in your field. After completing the necessary educational qualifications and gaining the necessary work experience, you are often hired as a quality assurance or control associate.
As you gain job experience, you can advance to the position of senior specialist and start leading groups of quality control specialists.
Your employer may require you to take professional development classes or obtain certifications like Six Sigma . A professional designation like Certified Quality Inspector may also be required.
Quality Control Salaries
Depending on the role, expertise, and industry, quality control specialists make a variety of salaries. As you get more expertise and advance into management positions, your pay rises. The average wage, as reported by the Bureau of Labor Statistics, is:
- Services rendered by experts in science and technology: $46,280
- Production: $40,020
- Trade in bulk: $37,800
- $30,070 for office supplies and support services
Quality control refers to a company's methods for assessing product quality and, if necessary, improving it. There are various ways to perform quality control, including benchmarking, examining manufacturing procedures, and testing products. All of this is done to keep track of significant product differences.
Three examples of quality control in the food sector are monitoring ingredient standards, verifying supplier lists, and making sure the manufacturing facility is hygienic.
Learn how to define what is quality control with Simplilearn’s Post Graduate Program in Lean Six Sigma , offered in partnership with the University of Massachusetts Amherst. This Lean Six Sigma green belt certification program will help you gain key skills to lead tranformational projects by improving overall quality and delivering the best results.
With the Six Sigma Black Belt certification , you'll be equipped to mentor Green Belts, guide projects, and drive substantial ROI for your organization. Elevate your career with this program's in-depth curriculum, designed to mold you into a proficient Six Sigma Black Belt capable of orchestrating impactful change and delivering excellence.
This course focuses on two important management methodologies — Lean practices and Six Sigma — that will enable you to accelerate business improvement.
1. What is Quality Control (QC)?
Quality control is the process by which services/products are measured and tested to ensure they are as uniform as possible and meet a standard. It helps businesses minimize inconsistencies and improve product quality.
2. What are the four types of Quality Control?
The four types of quality control are process control, control charts, acceptance sampling, and product quality control. While a control chart helps study changing processes over time, process control and product quality control help monitor and adjust products as per the standards. Acceptance sampling is a unique type that involves a statistical measure to determine whether a batch or sample of products satisfies the standards.
3. Why is Quality Control important?
Quality control is important to safeguard the company’s reputation, prevent products from being unreliable, and increase trust on the side of consumers. It ensures that the company looks at evidence-based data and research rather than anecdotal observations to ensure that the services/products live up to the standards. It reduces cost and maximizes profit, operational efficiency, and customer satisfaction.
4. What are three examples of Quality Control?
Some examples of quality control are: a high-speed car manufacturer runs thorough tests for every component, including manual and automated verifications; websites study the average response time per page for customer interactions and generate tickets when the service gets unacceptably slow; retail store owners employ secret shoppers to test the customer service of their stores.
5. What are the four steps of Quality Control?
The first step for quality control is to set your quality standards and decide which ones to focus on. Secondly, you must establish operational processes to deliver optimal quality and implement them. The third step is to review your results and identify gaps. Lastly, get feedback and make improvisations.
6. What are quality control techniques?
Inspection and Statistical quality control (SQC) are the two major techniques of Quality Control. Inspection checks the performance of items as per the pre-decided specifications. It involves periodic checking before, during and on completion of the process. It can be categorized into two types: Centralized and Floor Inspection. Statistical Quality Control relies on laws of probability. It controls the production quality within tolerance limits via sample procedure.
7. What is the difference QA and QC?
Quality Assurance (QA) focuses on preventing defects and maintaining the overall quality management system through process implementation and improvement. It ensures that proper processes are in place to avoid issues. On the other hand, Quality Control (QC) involves detecting and correcting defects through inspections and testing. QC ensures that products or services meet specific quality standards. While QA is proactive, emphasizing prevention, QC is reactive, emphasizing identification and correction of issues after they occur.
Our Quality Management Courses Duration And Fees
Explore our top Quality Management Courses and take the first step towards career success
Get Free Certifications with free video courses
Quality Management
Lean Management
Learn from Industry Experts with free Masterclasses
Digital marketing.
The Top 10 AI Tools You Need to Master Marketing in 2024
SEO vs. PPC: Which Digital Marketing Career Path Fits You Best in 2024?
Unlock Digital Marketing Career Success Secrets for 2024 with Purdue University
Recommended Reads
Free eBook: Quality Management Professionals Salary Report
Quality Control Manager Job Description
The Ultimate Guide to Understand Everything on Control Statements in C
Free eBook: Top 25 Interview Questions and Answers: Quality Management
Project Quality Management: Perform Quality Assurance Vs Perform Quality Control
What is Version Control and What Are Its Benefits?
Get Affiliated Certifications with Live Class programs
- PMP, PMI, PMBOK, CAPM, PgMP, PfMP, ACP, PBA, RMP, SP, and OPM3 are registered marks of the Project Management Institute, Inc.
Quality Management in a Lean Health Care Environment by Melissa Mannon, Daniel Collins
Get full access to Quality Management in a Lean Health Care Environment and 60K+ other titles, with a free 10-day trial of O'Reilly.
There are also live events, courses curated by job role, and more.
A quality management strategy for an organization that is seeking to or has already implemented a continuous improvement program must include respect for people, a transparent and open culture, employee empowerment, and actively engaged leaders. These are the elements that build an organization that values people, and when an organization puts the highest value on people, then quality efforts can focus on improving outcomes for people. There are more tangible and measurable components and tools that accompany a lean quality management system, such as root cause analyses, improvement slips, checklists, and observations. What cannot be stressed enough is that to unlock high-quality health care outcomes for patients and for ...
Get Quality Management in a Lean Health Care Environment now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.
Don’t leave empty-handed
Get Mark Richards’s Software Architecture Patterns ebook to better understand how to design components—and how they should interact.
It’s yours, free.

Check it out now on O’Reilly
Dive in for free with a 10-day trial of the O’Reilly learning platform—then explore all the other resources our members count on to build skills and solve problems every day.


HIGHER ED & K12 SOLUTIONS
Technology solutions, digital content transformation, cloud solutions.

Case Studies
How to guides, whitepapers, point of view, infographics, press releases.

Quality Assurance

Quality Control Process: Definition, Approaches, and Steps
When it comes to retaining loyal customers, companies have just one tool that’s powerful enough to appeal to every type of customer/audience they’re targeting. Yes, we’re talking about quality.
A product or service that meets the quality expectations of buyers will always keep them happy and coming back for more. But how can companies manage quality and keep it consistently high at all times? The answer lies in the quality control process.
What is Quality Control?
“Quality” refers to the degree of excellence or superiority that something possesses compared to things of a similar nature.
“Quality control ” refers to the set of activities, processes, and operations that companies have in place to maintain and improve the quality of the product/service they are offering.
Factors that Determine Quality
Quality is a subjective experience and differs based on industry and the type of product/service purchased. But ultimately, the following factors work together to increase quality in the eyes of the customer:
Type of Raw Materials Used
The type of raw materials used to create the product/service should be more long-lasting, functional, aesthetic, sustainable, and easy to obtain in the long run.
Functionality/Usefulness
This defines how useful the product/service is to the buyer after the purchase. Does the product/service offer additional value to their life by either solving an existing problem or filling a gap they have?
The type of features and their sophistication, device optimization, accessibility, variety, etc., all affect how useful a product/service can be to a buyer.
These features will determine whether the company’s offering is a better investment compared to other similar products.
Affordability is one of the key determinants of quality, especially in terms of the price buyers pay for the features they receive.
Adherence to Benchmarked Standards and Quality Compliances
Products/services that hold labels such as ISO, ISI, AGMARK, BIS, GI, GDPR, ADA, etc., indicate that the product/service conforms to stringent global compliance regulations, automatically improving their quality.
These days, companies must focus on creating products and experiences that have the least ecological impact and can meet Governments’ measures for anti-pollution and climate change reversal.
Variety and Accessibility
Even within one target group, there are often differences in tastes between customers. More variations of the product/service can make it more accessible and enjoyable to all your customers.
In the eyes of buyers, the safer a product/service is to use or experience, the better its quality. Similarly, safety also refers to protection against tampering by unauthorized individuals.
Reliability and Longevity
The longer the uninterrupted, seamless service a product/service offers and the higher its shelf life, the better it is considered by buyers.

Approaches to the Quality Control Process
In order to maintain high quality always and to consistently keep improving the quality of the product/service being provided, companies need to have a powerful quality control process in place.
Depending on their needs, a company might use one of these approaches to quality control:
Random Selection
Here, a random sample of the product/service is taken to measure select attributes (such as product durability, accessibility, or safety) to identify the item’s quality.
This approach helps the company determine if defects exist across the board or if they are isolated to specific attributes/batches/production cycles.
Taguchi Method
In this method, the focus is on the role of R&D and product design and development in product quality.
The foundational operations such as research, methodology design, and technology selection are made with greater care to reduce failures/errors in the later stages of product/service production.
100% Inspection
This approach is a comprehensive, 360-degree quality check process. Here, every stage of production is monitored and controlled to meet quality requirements. This takes a lot of time, but it is an approach that offers excellent quality results.
Quality assurance takes quality control a step further. It is a mindset adopted by the entire company, which aligns every activity, thought, and decision towards always striving for the best quality possible.
Here, quality is built into every single action, process, and technology that is related to the product/service in any way (including functions outside R&D and production).
Steps Involved in Quality Control
The fact is companies often lose 10%-15% of their operations cost or 15%-20% of their sales revenue due to poor quality .
A responsive and customized quality control and assurance process can help companies create products/services that always adhere to their benchmarked standards.
The quality control process typically looks like this:
Incoming Materials Quality Control
The first step is monitoring the quality of incoming materials. These materials could be physical materials to make a physical product/service or digital materials to offer an online experience.
The materials are inspected to make sure they meet the firm’s quality standards and requirements. Those that don’t are either discarded or returned for modification.
In-Production/Process Quality Control
This second step oversees quality management during the production/processing of the product/service. This could be testing the tool/technology being used to produce the physical item.
Alternatively, it could be testing the safety and accessibility of the digital environment in which the online product/service will operate.
At this stage, the company also checks its adherence to global compliance requirements. Additionally, any client-given specification becomes one of the main points for quality management.
Final/Outgoing Quality Control
In this third stage, the quality of the finished product/service is checked to ensure no defects or deficits exist. The product/service is implemented, and it is examined to make sure it operates seamlessly.
How the product/service looks, whether all features are operational, and if it can be easily used (i.e., downloaded, consumed, shared, etc.) are checked. If there’s something wrong, it is sent back to the production stage for revisions .
Adherence to client specifications is again checked, any necessary labels/marks/tags are added, and the product/service is sent to the desired location. This could be a physical distributor’s warehouse or uploading onto a digital platform.
Even after sale/distribution, the product/service is continually monitored and improved upon as time passes. The best quality control tool gives the company the flexibility to keep improving the quality offered through responsive changes.
Choose Hurix to Maintain and Boost the Quality of Your Online Education and Training Programs
The e-publishing and online training industry also must meet stringent quality requirements in order to provide the best quality education to their audience.
One way to effortlessly manage your quality control process is to use robust, cutting-edge digital content transformation software like Hurix.
Hurix has over two decades of experience providing content authoring, content quality control, and content distribution services to a variety of clients, such as K-12 schools and corporates.
With Hurix, you can:
- Create content in diverse formats, including text, video, audio, memes, slides, quizzes, polls, etc., using in-built content authoring tools and personalized production services.
- Integrate quality into content production through superior pre-editing and editorial services such as manuscript digitization, formatting, typesetting, XML conversion, illustrations, and more.
- Check content quality through powerful AI quality automation tools that enable various testing for copy, API, performance , mobile app, animation, portal security, payments, and accessibility.
- Securely upload your content onto a scalable cloud that is encrypted and accessed only through identity verification.
If this sounds like the perfect tool for your content creation and quality assurance needs , try Hurix today .

A highly enthusiastic and motivated sales professional with over twenty five years of experience in solution selling of training-related applications and services. Maintains an assertive and dynamic style that generates results. Ability to establish long-term relationships with clients built on trust, quality of service and strategic vision. Specializes in financial services, higher ed, publishing and government in the areas of learning and development.
Related Posts
- Why Localization Testing Must Be Your Top Priority?
- Differences Between Quality Assurance and Quality Control
- Penetration Testing – Types, Stages and Vulnerabilities
- Types Of Installation Testing and How To Do It?
- Why is Quality Control Important for a Business?
Request A Quote
Let's Collaborate & Succeed Together
Connect with us.
First Name *
Last Name *
Work Email *
HurixDigital – where innovation meets education, and digital solutions meet real-world challenges
- Workforce Learning
- Digital Content Transformation
- Higher Ed & K12 Solutions
- Technology Solutions
Quick Links
- Research & Innovation

All You Need to Know about the European Accessibility Act 2025 and WCAG

What is Prepress Workflow? Know These 5 Ways to Streamline Your Prepress Workflow!
Copyright © 2024 Hurix | All Rights Reserved.

- Privacy Policy
- Cookie Policy
- Get In Touch

- Contract Management
Supplier Management
Savings Management
- Data & Security
FAQ’s
oboloo Articles
Quality control in manufacturing: a guide to quality management in production.
Welcome to our blog, where we dive into the world of manufacturing and explore the critical aspect of quality control. In today’s fast-paced and competitive market, ensuring that your products meet or exceed customer expectations is essential for success. That’s where quality control in manufacturing comes into play – a process that ensures every product leaving your production line is flawless.
In this comprehensive guide, we will take you through the ins and outs of quality management in production. We’ll discuss its importance, the key principles behind it, essential tools and techniques to implement, as well as common challenges faced by manufacturers. So if you’re ready to enhance your manufacturing process and deliver top-notch products consistently, let’s get started!
The Importance of Quality Control in Manufacturing
In the manufacturing world, quality control is like a superhero that swoops in to save the day. It ensures that every product rolling off the assembly line meets stringent standards and satisfies customer expectations. Without proper quality control measures in place, manufacturers risk delivering defective products that can tarnish their reputation and lead to financial losses.
Quality control plays a crucial role in maintaining consistency. By closely monitoring each step of the production process, manufacturers can identify any deviations or defects early on and rectify them promptly. This helps ensure that every unit produced adheres to established specifications, leading to uniformity across batches.
Implementing robust quality control practices enhances customer satisfaction. When consumers purchase a product with confidence knowing it has been thoroughly checked for flaws or defects, they are more likely to become loyal customers who endorse your brand through word-of-mouth recommendations.
Moreover, effective quality control contributes significantly to cost reduction. Detecting and resolving issues early prevents costly recalls or warranty claims down the line. By minimizing rework and waste while maximizing efficiency during production, companies can optimize their resources effectively.
Furthermore, adhering to high-quality standards instills trust not only among customers but also among business partners and regulators alike. Demonstrating commitment towards producing reliable products fosters strong relationships with suppliers and other stakeholders in your industry.
Embracing quality control sets manufacturers apart from competitors by positioning them as leaders in their field. By prioritizing excellence throughout all aspects of production – from raw material sourcing to final inspection – companies demonstrate a dedication towards delivering superior products consistently.
To thrive amidst fierce competition in today’s global marketplace, investing time and effort into implementing an effective quality control system is non-negotiable for manufacturers seeking long-term success.
Understanding Quality Management in Production
Quality management is an essential aspect of manufacturing that ensures products meet or exceed customer expectations. It involves a systematic approach to monitoring and improving all processes involved in production, from raw material sourcing to final product inspection. By implementing effective quality management practices, manufacturers can enhance customer satisfaction, reduce waste and rework, and ultimately increase profitability.
One key component of quality management is establishing clear quality objectives and standards. This helps set the benchmark for product performance and guides the entire manufacturing process. Manufacturers must also implement robust quality control measures at each stage of production to identify any defects or deviations from the established standards.
Another important aspect of quality management is employee involvement. Engaging employees in the continuous improvement process fosters a sense of ownership and responsibility for product quality. Training programs should be provided to ensure that employees understand their roles in maintaining high-quality standards.
Effective communication within the organization is crucial for successful quality management. This includes sharing information about customer feedback, production issues, and improvement initiatives across different departments. Collaboration between teams allows for timely identification and resolution of potential quality problems.
Continuous monitoring through data collection and analysis plays a vital role in quality management. Various tools such as statistical process control charts can help identify trends or patterns indicating potential issues before they become significant problems.
In addition to internal controls, manufacturers may also implement external audits or certifications to validate their adherence to international standards such as ISO 9001:2015. These external evaluations provide customers with confidence in the manufacturer’s commitment to producing high-quality products.
Understanding quality management in production requires a comprehensive approach that encompasses clear objectives, employee involvement, effective communication channels, continuous monitoring through data analysis, and external validation through audits or certifications.
By embracing these principles of quality management, manufacturers can consistently deliver products that meet or exceed customer expectations while enhancing their competitiveness within the industry.
The 6 Key Principles of Quality Control
When it comes to quality control in manufacturing, there are six key principles that serve as the foundation for effective quality management. These principles guide manufacturers in ensuring that their products meet the highest standards and consistently deliver customer satisfaction.
1. Customer Focus: The first principle emphasizes the importance of understanding and meeting customer requirements. By putting the customer at the center of all decision-making processes, manufacturers can ensure that their products not only meet but exceed expectations.
2. Leadership: Strong leadership is crucial for driving a culture of quality within an organization. Leaders must set clear goals, provide resources, and empower employees to take ownership of quality control initiatives.
3. Involvement of People: Quality control is not solely the responsibility of a designated team or department; it is a collaborative effort involving every individual within an organization. Engaging employees at all levels ensures that everyone understands their role in maintaining high-quality standards.
4. Process Approach: Adopting a process-driven approach helps identify potential bottlenecks or areas for improvement in production workflows. By continuously monitoring and analyzing processes, manufacturers can make data-driven decisions to enhance efficiency and eliminate defects.
5. Continuous Improvement: Quality control should be an ongoing journey rather than a one-time event. Manufacturers must constantly seek ways to refine their practices through continuous improvement methodologies such as Six Sigma or Lean Manufacturing.
6. Evidence-based Decision Making: Data plays a vital role in effective quality control management by providing objective insights into product performance and identifying trends or patterns over time. Using data analytics tools allows manufacturers to make informed decisions based on evidence rather than intuition alone.
By adhering to these six key principles, manufacturers can establish robust quality management systems that drive organizational success and build trust with customers.
Tools and Techniques for Quality Management
Tools and techniques play a crucial role in ensuring effective quality management in manufacturing. By utilizing the right tools and employing appropriate techniques, manufacturers can identify and address potential quality issues before they impact the final product.
One commonly used tool is statistical process control (SPC). SPC involves monitoring key variables during production to detect any variations that may indicate an issue. This allows manufacturers to take corrective actions promptly, preventing defects from arising.
Another important technique is failure mode and effects analysis (FMEA). FMEA helps manufacturers proactively identify potential failures or defects in their processes or products. By analyzing possible failure modes and their impacts, manufacturers can implement preventive measures to mitigate these risks.
Other tools include Six Sigma methodologies, which focus on reducing variability and improving process efficiency, as well as lean manufacturing techniques that aim to eliminate waste throughout the production process.
Furthermore, technology plays a significant role in quality management. Manufacturers can use advanced software systems for data collection, analysis, and tracking of quality metrics. Automation technologies such as robotics can also enhance precision and reduce human error.
In addition to these tools and techniques, it is essential for manufacturers to establish clear documentation procedures outlining standard operating procedures (SOPs) for each stage of production. Regular audits should be conducted to ensure compliance with these SOPs.
By implementing robust tools and techniques along with proper documentation practices, manufacturers can achieve consistent quality levels while minimizing defects and improving overall customer satisfaction.
Implementing Quality Control in Your Manufacturing Process
When it comes to manufacturing, ensuring the quality of your products is crucial. Implementing an effective quality control system can help you achieve this goal. Here are some steps you can take to implement quality control in your manufacturing process.
Establish clear quality standards and guidelines that align with customer expectations and industry regulations. These standards will serve as a benchmark for evaluating the quality of your products.
Next, train your employees on quality control procedures and provide them with the necessary tools and resources to carry out their tasks effectively. This includes conducting regular training sessions, providing feedback, and encouraging open communication about any potential issues or improvements.
In addition to employee training, invest in appropriate technology and equipment that can aid in monitoring and measuring product quality. This could include automated inspection systems, statistical process control software, or data analytics tools.
To ensure consistency in product quality, implement a robust documentation system that records all aspects of the manufacturing process. This includes recording raw material specifications, production parameters, inspection results, and any corrective actions taken.
Regularly conduct audits and inspections throughout each stage of the manufacturing process to identify any deviations from established standards or potential areas for improvement. Use these findings to make informed decisions about process adjustments or modifications.
Lastly but importantly foster a culture of continuous improvement within your organization by encouraging collaboration among different departments involved in the manufacturing process. Regularly review performance metrics related to product quality and use this information to drive ongoing improvement initiatives.
By implementing these strategies for quality control in your manufacturing process , you can enhance customer satisfaction , minimize defects , optimize production efficiency ,and position yourself as a trusted provider within your industry .
Common Challenges and Solutions in Quality Management
Quality management is an integral part of any manufacturing process, but it’s not without its challenges. One common challenge faced by manufacturers is ensuring consistency in product quality across different batches or production lines. This can be addressed by implementing standardized processes and procedures, conducting regular audits, and providing training to employees.
Another challenge is detecting defects or non-conformities early on in the production process. To overcome this, manufacturers can utilize various inspection techniques such as statistical process control (SPC) charts, sampling plans, and automated vision systems. These tools help identify deviations from the desired specifications and enable prompt corrective actions.
Maintaining a strong supplier network is also crucial for quality management. Suppliers play a vital role in delivering raw materials that meet the required standards. Establishing clear communication channels with suppliers helps ensure that they understand your quality expectations and are committed to meeting them.
Effective documentation and record-keeping are essential for maintaining quality standards over time. Documenting standard operating procedures (SOPs), test results, equipment maintenance records, and customer feedback allows for traceability and facilitates continuous improvement efforts.
One ongoing challenge faced by manufacturers today is keeping up with rapidly evolving technologies used in production processes. Embracing automation solutions like robotics or artificial intelligence can enhance efficiency while reducing human errors associated with manual tasks.
In conclusion…
Quality management in manufacturing requires addressing these common challenges through proactive measures such as standardization of processes, effective communication with suppliers, leveraging advanced inspection techniques/tools,and embracing technological advancements.
The Future of Quality Control in Manufacturing
As technology continues to advance at a rapid pace, the future of quality control in manufacturing looks promising. With innovations such as artificial intelligence (AI), machine learning, and Internet of Things (IoT), manufacturers have access to a wide range of tools and techniques that can revolutionize quality management.
One exciting development is the use of AI-powered systems for real-time monitoring and predictive analytics. These systems can analyze vast amounts of data collected from sensors embedded in machines, allowing manufacturers to identify potential issues before they occur. This proactive approach not only helps prevent defects but also improves overall efficiency and reduces costs.
Another trend on the horizon is the integration of IoT devices into the manufacturing process. By connecting various components and equipment, manufacturers can gather real-time data on performance, temperature, vibration levels, and more. This enables them to monitor production processes closely and make adjustments as needed to maintain high-quality standards.
Additionally, advancements in robotics are transforming quality control practices. Robots equipped with cameras and sophisticated algorithms can perform intricate inspections with precision far beyond human capabilities. They can quickly detect even minor flaws or deviations from desired specifications, ensuring consistent product quality.
Furthermore, virtual reality (VR) and augmented reality (AR) technologies are being utilized for training purposes in quality control. Manufacturers can simulate real-world scenarios virtually, allowing employees to practice identifying defects without any risk involved. AR overlays digital information onto physical objects during inspections or repairs for enhanced accuracy.
In conclusion,
With these technological advancements shaping the future landscape of manufacturing industry – accompanied by increased automation – it is clear that quality control will continue evolving towards higher levels of efficiency and accuracy. As companies embrace these innovations, they will be able to streamline their operations while maintaining stringent quality standards—an essential factor in gaining a competitive edge in today’s global market.
Conclusion: Improving Your Manufacturing Process with Quality Control
Improving Your Manufacturing Process with Quality Control
Quality control plays a crucial role in the success of any manufacturing operation. By implementing effective quality management practices, businesses can ensure that their products meet or exceed customer expectations while reducing waste and improving efficiency.
To recap, we have explored the importance of quality control in manufacturing and how it contributes to overall business success. We have discussed the principles of quality control and examined various tools and techniques that can be employed to achieve optimal results. Additionally, we have delved into common challenges faced by manufacturers in maintaining high-quality standards and provided potential solutions to overcome them.
Looking ahead, the future of quality control in manufacturing is promising. Advances in technology will continue to revolutionize this field, enabling real-time monitoring, analysis, and predictive maintenance. Automation and artificial intelligence will enhance accuracy, speed up processes, and minimize human error.
Manufacturers must remain proactive in adapting to these changes by continuously evaluating their quality management systems and embracing new technologies as they emerge. By doing so, companies can stay competitive while meeting evolving customer demands for superior product quality.
In conclusion, integrating robust quality control measures into your manufacturing process is essential for achieving sustainable growth. Embrace the six key principles outlined earlier – customer focus, leadership commitment, employee involvement, continuous improvement mindset, data-driven decision-making approach, and supplier collaboration – as a foundation for your approach to quality management.
Remember: Quality should never be an afterthought; it should be at the heart of every step you take on your journey toward excellence.
Want to find out more about procurement?
Access more blogs, articles and FAQ's relating to procurement
The smarter way to have full visibility & control of your suppliers
Contract Management
Partnerships
Charities/Non-Profits
Service Status
Release Notes
Feel free to contact us here. Our support team will get back to you as soon as possible
Sustainability

- Project Management Concepts
- Project Management Home
- Activity Based Costing
- Agile Project Management
- Basic Management Skills
- Basic Quality Tools
- Benchmarking Process
- Cause and Effect Diagram
- Change Management Process
- Communication Blockers
- Communication Channels
- Communication Methods
- Communication Models
- Communications Management
- Conflict Management
- Crisis Management
- Critical Chain Scheduling
- Critical Path Method
- Decision Making Process
- Design of Experiment
- Effective Communication Skills
- Effective Presentation Skills
- Enterprise Resource Planning
- Event Chain Methodology
- Extreme Project Management
- Gantt Chart Tool
- Just-In-Time Manufacturing
- Knowledge Management
- Leads, Lags and Floats
- Management Best Practices
- Management Styles
- Management by Objectives
- Monte Carlo Analysis
- Motivation Theories
- Negotiation Skills
- Organizational Structures
- PERT Estimation Technique
- PRINCE2 Project Methodology
- Pareto Chart Tool
- Powerful Leadership Skills
- Process Based Management
- Procurement Documents
- Procurement Management
- Project Activity Diagram
- Project Charter
- Project Contract Types
- Project Cost Control
- Project Kick-off Meeting
- Project Lessons Learned
- Project Management Methodologies
- Project Management Office
- Project Management Processes
- Project Management Tools
- Project Management Triangle
- Project Manager Goals
- Project Portfolio Management
- Project Quality Plan
- Project Records Management
- Project Risk Categories
- Project Risk Management
- Project Scope Definition
- Project Selection Method
- Project Success Criteria
- Project Time Management
- Project Workforce Management
- Project Management Softwares
- QC and QA Processes
- RACI Chart Tool
- Recognition and Rewards
- Requirement Collection
- Resource Leveling
- Staffing Management Plan
- Stakeholder Management
- Statement of Work (SOW)
- Stress Management Techniques
- Structured Brainstorming
- Succession Planning
- Supply Chain Management
- Team Building Program
- Team Motivation
- The Balanced Scorecard
- The Halo Effect
- The Make or Buy Decision
- The Rule of Seven
- The Virtual Team
- Total Productive Maintenance
- Total Quality Management
- Traditional Project Management
- Work Breakdown Structure
- Useful Resource
- Management Concepts - Quick Guide
- Management Concepts - Resources
- Management Concepts - Discussion
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
Quality Control & Quality Assurance
Introduction.
Quality is an important factor when it comes to any product or service. With the high market competition, quality has become the market differentiator for almost all products and services.
Therefore, all manufacturers and service providers out there constantly look for enhancing their product or the service quality.
In order to maintain or enhance the quality of the offerings, manufacturers use two techniques, quality control and quality assurance. These two practices make sure that the end product or the service meets the quality requirements and standards defined for the product or the service.
There are many methods followed by organizations to achieve and maintain required level of quality. Some organizations believe in the concepts of Total Quality Management (TQM) and some others believe in internal and external standards.
The standards usually define the processes and procedures for organizational activities and assist to maintain the quality in every aspect of organizational functioning.
When it comes to standards for quality, there are many. ISO (International Standards Organization) is one of the prominent bodies for defining quality standards for different industries.
Therefore, many organizations try to adhere to the quality requirements of ISO. In addition to that, there are many other standards that are specific to various industries.
As an example, SEI-CMMi is one such standard followed in the field of software development.
Since standards have become a symbol for products and service quality, the customers are now keen on buying their product or the service from a certified manufacturer or a service provider.
Therefore, complying with standards such as ISO has become a necessity when it comes to attracting the customers.
Quality Control
Many people get confused between quality control (QC) and quality assurance (QA). Let's take a look at quality control function in high-level.
As we have already discussed, organizations can define their own internal quality standards, processes and procedures; the organization will develop these over time and then relevant stakeholders will be required to adhere by them.
The process of making sure that the stakeholders are adhered to the defined standards and procedures is called quality control. In quality control, a verification process takes place.
Certain activities and products are verified against a defined set of rules or standards.
Every organization that practices QC needs to have a Quality Manual. The quality manual outlines the quality focus and the objectives in the organization.
The quality manual gives the quality guidance to different departments and functions. Therefore, everyone in the organization needs to be aware of his or her responsibilities mentioned in the quality manual.
Quality Assurance
Quality Assurance is a broad practice used for assuring the quality of products or services. There are many differences between quality control and quality assurance.
In quality assurance, a constant effort is made to enhance the quality practices in the organization.
Therefore, continuous improvements are expected in quality functions in the company. For this, there is a dedicated quality assurance team commissioned.
Sometimes, in larger organizations, a 'Process' team is also allocated for enhancing the processes and procedures in addition to the quality assurance team.
Quality assurance team of the organization has many responsibilities. First and foremost responsibility is to define a process for achieving and improving quality.
Some organizations come up with their own process and others adopt a standard processes such as ISO or CMMi. Processes such as CMMi allow the organizations to define their own internal processes and adhere by them.
Quality assurance function of an organization uses a number of tools for enhancing the quality practices. These tools vary from simple techniques to sophisticated software systems.
The quality assurance professionals also should go through formal industrial trainings and get them certified. This is especially applicable for quality assurance functions in software development houses.
Since quality is a relative term, there is plenty of opportunity to enhance the quality of products and services.
The quality assurance teams of organizations constantly work to enhance the existing quality of products and services by optimizing the existing production processes and introducing new processes.
When it comes to our focus, we understand that quality control is a product-oriented process. When it comes to quality assurance, it is a process-oriented practice.
When quality control makes sure the end product meets the quality requirements, quality assurance makes sure that the process of manufacturing the product does adhere to standards.
Therefore, quality assurance can be identified as a proactive process, while quality control can be noted as a reactive process.
To Continue Learning Please Login
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Quality Control and Assurance - An Ancient Greek Term Re-Mastered
The Evolution of Quality Concepts and the Related Quality Management
Submitted: 29 April 2016 Reviewed: 12 December 2016 Published: 22 February 2017
DOI: 10.5772/67211
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Quality Control and Assurance - An Ancient Greek Term Re-Mastered
Edited by Leo D. Kounis
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
5,241 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Overall attention for this chapters
Enterprises usually adopt some quality practices to control the product quality during the manufacturing process in order to assure the delivery of qualitative good products to customers. The quality practices or quality management systems adopted by industries will further evolve due to the changes of quality concepts as time goes by. This chapter discusses the change of quality concepts and the related revolution of quality management systems in the past century. The quality concepts were gradually changed from the achievement of quality standards, satisfaction of customer needs, and expectations to customer delight. Since merely satisfying customers is not enough to ensure customer loyalty, the enterprises gradually focus on customers’ emotional responses and their delight in order to pursue their loyalty. The emotion of “delight” is composed of “joy” and “surprise,” which can be achieved as the customers’ latent requirements are satisfied. Thus, the concept of “customer delight” and the means to provide the innovative quality so as to meet the unsatisfied customers’ latent needs are elaborated on. Finally, a framework of innovation creation is developed that is based on the mining of customer's latent requirements. This outline will manifest the essential elements of the related operation steps.
- quality concept
- customer satisfaction
- customer delight
- customers’ latent requirement
- innovation creation system
Author Information
Ching-chow yang *.
- Department of Industrial and Systems Engineering, Chung Yuan Christian University, Taiwan, Republic of China
*Address all correspondence to: [email protected]
1. Introduction
It is widely recognized that consumers only buy the goods with good quality, desired functions, and accepted price [ 1 ]. Therefore, industries always adopt several management practices, or even develop some management systems to design and produce the products so as to meet customers’ needs and expectations showcasing good quality and lower costs. But the management practices or systems adopted by the industries are always changed due to the changes of the quality concepts as time goes by. This occurs as several quality gurus give the pragmatic definitions of “quality” over different time periods, thus causing industries to implement different practices or systems to control overall product quality [ 2 ].
In the first three decades of the last century, quality was defined as “conforming to the standards and specifications of a product” [ 3 ]. Thus, the commonly adopted quality practices by industries were the standardization of quality, inspection, and rework. Deming emphasized that “quality is to fulfill the requirements of customers and satisfy them” [ 4 ]. Hence, the meaning of quality was gradually changed to a “customer‐focused” perspective. Enterprises, therefore, committed themselves to satisfy customers’ needs and expectations. Their aim was to pursue customer's satisfaction and loyalty [ 5 , 6 ]. Companies also developed a number of methods to find out customers’ needs and expectations. To this end, in‐depth interviews with customers were performed, customer surveys conducted, and market research was done. But, when Apple announced several innovative products, and their sales were increasing, it became apparent that only satisfying customers’ requirements are not enough [ 7 ]. As a matter thereof the identification and fulfillment of customers’ unsatisfied latent needs was gauged in conjunction with their emotional responses [ 8 ].
Entering the new century, several studies have suggested that merely satisfying customers is not enough to ensure their loyalty [ 9 – 11 ]. The industries need to focus on the customer's emotional responses and provide the products with attractive quality in order to pursue customers’ delight [ 11 ]. It is worth mentioning at this point that Apple is regarded as a future trend setter, as it successfully created several innovative products such as iPod, iPhone, and iPad, triggering increased sales. A strong customer service department equally assisted in causing customers’ delight experiences [ 12 ].
In view of the above, quality concepts have changed. Terms such as “customer delight” are deemed as forming the crucial elements of quality concepts, which are coined as “attractive quality” and “innovative quality” [ 13 ]. The conceptualization of delight is that the emotion of “delight” is composed of “joy” and “surprise,” which can be achieved as the customers’ latent requirements are satisfied. The provision of innovative quality products is the strategic tool to meet the unsatisfied customers’ latent needs and their curiosity. These changes of quality concepts will lead the enterprises to reengineer their existing quality system, in order to develop the innovative quality attributes of products and services alike so as to retain and attract customers. This scenario causes the quality professionals and researchers to further develop and expand the new quality system beyond TQM. Based on performed research it is the author's view that research should focus on developing an effective new quality system.
During the past century, several quality gurus had led the changes of quality concepts. This chapter will discuss the changes of quality concepts during different time periods over the past century. To this end, it will also state the reflected revolutions of quality management systems with respect to the changing quality concepts. These are arranged in Sections 2 and 3, respectively. The development of “total quality management” (TQM) philosophy in the 1980s was an important landmark, which caused the change of new quality concepts and the reengineering of the quality management system, as introduced in Section 4. Based on the performance evaluation of the TQM implementation of TQM, it is worth noting at this point that the “business excellence model” had been proposed during the 1990s. This is elaborated in Section 5. This chapter also introduces the development of an integral model of a business excellence system beyond TQM, based on the realization of TQM.
Section 6 expands on the changes of new quality concepts pertaining to “customer delight” and “innovative quality” based on the investigation of customers’ latent needs in the new century. This chapter includes a system, which may assist and cater for identifying and foster upon customers’ undersatisfied latent needs and delights, enhancing innovative quality, which is addressed in Section 7. The conclusion of this chapter is listed in the final section.
2. The early quality concepts and the reflected quality management system
Since the early twentieth century, manufacturing processes and activities initiated the control practices to assure the product quality [ 14 ]. Manufacturing companies focused on the related productivity and manufacturing costs. As such, the quality concept and control were product‐focused.
2.1. The quality concept of “standard” and the “inspection” control
In 1913 Ford Motor Company, FMC, created the assembly line in their newly opened factory in Highland Park, Michigan due to the influence of the scientific management of Frederick W. Taylor. This resulted in Ford increasing its manufacturing volume [ 15 ]. Ford's assembly line was copied by many manufacturing companies. However, companies turned their attention to control product‐related quality issues. Since manufacturers were more “product‐focused” in that time, the quality concept was, therefore, aimed at “conforming to the standards and specifications of a product” [ 3 ]. This in turn, impelled quality engineers in manufacturing industries to implement the method of “inspection” so as to control the quality of a manufactured product.
Product designers and process engineers designed the standards and specifications of the products, which were based on their critical attributes. They also set up the standards of the manufacturing process and the standards of operations (SOPs). In doing so, the involved workforce was requested to perform the tasks according to the developed SOPs. The quality inspectors checked the dimensions and characteristics of products, detected the errors and failures, and took the necessary actions to improve the product quality.
2.2. The development of process quality control
In order to ensure product quality, companies needed to utilize the “full inspection” method. This was costly, since it required much time and labor efforts, and resulted in high internal quality costs [ 16 ]. Walter Shewhart, thus, created the control chart, a quality technique tool that he pioneered in Bell Laboratories working as a quality control engineer [ 17 ]. He proposed the use of a sampling inspection method instead of a 100% inspection to reduce the overall amount of inspection. The control chart was utilized to monitor the quality performance concerning the critical aspects of a process, whereas the attributes of the product were identified by means of sampling methods [ 18 ]. This enhanced overall effectiveness and also reduced the associated costs.
Sampling inspection and control charts use many statistic tools such as probability theory, the methods of random sample, analyses of sample mean and deviations to name but a few, as means for improving quality levels. Hence, the method of quality control suggested by Shewhart was called “statistical process control” (SPC) or “statistical quality control” (SQC). Sampling inspection may not always ensure product quality, as it might cause fewer defective products to be shipped to customers [ 19 ]. As a result, thereof, it leads to extra outside quality costs. Shewhart, however, had argued that if the missed number of defects was small, then the savings in inspection costs made it worthwhile [ 20 ].
3. The quality concepts and the reflected quality management system in the mid‐twentieth century
More often than not, quality and the associated price of the goods are primary factors that are considered by customers prior to them materializing a purchase [ 21 ]. As such, manufacturing companies mainly emphasis on the control of quality and costs during the manufacturing process, especially the ones that trigger poor quality.
3.1. The development of “quality costs”
In essence, Juran propounded the concept of “quality costs” in his book “ Quality Control Handbook ” in 1951 [ 22 ]. Juran subdivided the quality costs into prevention, appraisal, internal failure, and external failure costs. The performed literature review indicates that the losses due to the production of defects and manufacturing failures are more than the costs of quality control; in particular, the costs caused by internal and external failures. In view of the above, the implementation of SPC could not effectively control the quality costs.
prevention costs
appraisal costs
internal failure costs
external failure costs
extra resultant costs and
estimated hidden costs
Figure 1 depicts the new definition of quality costs.

Figure 1.
Definition of categories of quality costs.
3.2. The quality concept “quality assurance” and the related TQC‐system
After Juran and several quality experts emphasized the quality cost issue, the concept of quality costs was widely accepted by industry. Meanwhile, another quality concept appeared gradually, namely “quality assurance.” However, a large number of experts in the area of quality control, including Feigenbaum, asserted that implementing an SPC system on its own could not effectively control quality costs [ 23 ]. It is worth mentioning that the concept of “quality assurance” was “users‐oriented,” implying that “product possesses the fitness for purpose of use based on its functions” and hence “quality is zero defects and meeting the specifications 100%” [ 24 ].
product design,
incoming quality approval,
process quality control,
product reliability,
delivery, and
customer service.
Actually, Feigenbaum's quality concept and ideas were similar to those described by Deming, Juran, and Crosby [ 25 ].
The concepts and approaches of SPC, TQC, and “costs of quality” were introduced in Japan during 1960 by Deming and Juran. The Union of Japanese Scientists and Engineers (JUSE), which was formed in 1946, synthesized the concepts, principles, and approaches of statistical process control and total quality control [ 26 ]. JUSE promoted the practices of TQC and the quality concepts pursuing the zero defect culture and executing the task right first time.
4. The age of total quality management
While Japanese industries adopted the TQC practices, they emphasized the education and training of quality for all employees and the cultivation of a quality culture. Therefore, the implementation of TQC in Japanese industries was very different from the original TQC.
4.1. The emerging of the Japanese company‐wide quality control, CWQC
customer‐focused and quality‐first as the quality policies,
full participation and teamwork,
education and training of quality for all employees,
realization of “do the right thing first time,”
concept and materialization of a “zero defect” culture,
”continuous improvement” as the key quality activity,
everyone is responsible for achieving high quality levels,
emphasizing on the prevention activities and quality assurance,
cultivating a quality culture environment.
Based on aforementioned critical characteristics, the Japanese TQC was acknowledged as company‐wide quality control (CWQC).
The implication of CWQC in conjunction with Japanese industrial competitiveness and strategic advantages facilitated their entrance into western markets. Japanese enterprises enjoyed an increase in global market share by providing the customers with high‐quality products at lower prices [ 27 ]. This in turn, resulted in western companies facing an increased amount of competition from Japanese and other Asian manufacturers.
4.2. The development of total quality management
“customer‐focused” management,
“continuous improvement” as the key quality activity,
top management's promise persistence for pursuing quality,
education and training of quality for employees,
employees’ good quality concept,
quality leadership,
long‐term supplier relationship,
implementation of quality management system,
cultivation of quality culture.
It is the author's view that the TQM was well developed and suitable for western organizations, as depicted in Figure 2 . Thus, it was widely adopted by industries and nonprofit organizations around the world.

Figure 2.
The framework of TQM.
The development of TQM was also influenced by the western quality experts, namely Deming, Juran, and Crosby [ 28 ]. As already stated Deming's quality concept was that “quality is to fulfill the requirements of customers and satisfy them” [ 4 ]. Crosby's definition of quality was also similar, as he defined quality as “conformance to customers’ requirements.” Juran also defined quality as being “fitness for purpose of use, …, it is judged by the users, not the manufacturers, or the merchants” [ 22 ]. TQM was thus an integrated model of management philosophies, quality concepts, and a set of practices, which were also influenced by Deming's 14 points and Juran's quality trilogy. However, to implement the TQM successfully it is necessary to integrate the so‐called “hard side” elements (that is, statistical methods, quality control tools, process standardization, and improvement, etc.) with the “soft side” aspects (that is, quality concept, employees’ participation, education and training, and quality culture, etc.) [ 29 ].
From the mid‐1980s onward, several important quality programs were being launched. Besides the development of TQM, the ISO 9000 system and the Six‐Sigma program (which was initiated by Motorola) commenced in 1987. It is worth noting that up to date, the ISO system has had four revisions in 1994, 2000, 2008, and 2015, respectively. The Six‐Sigma quality scheme was being widely imitated by GE in 1995 [ 30 ]. The successful implementation of Six‐Sigma by Motorola, GE, and several other multinational companies caused the Six‐Sigma philosophy to be globally adopted by a number of industries and organizations [ 31 ].
5. From Malcolm Baldrige National Quality Award to the Business Excellence Model
From the late 1990s to present, the traditional quality concept has seen a number of changes. In the United States of America, the Department of Commerce introduced in 1987 the Malcolm Baldrige National Quality Award (MBNQA) by benchmarking the Japanese Deming Award [ 32 ] to promote the implementation of TQM in industries and nonprofit organizations.
5.1. The national quality award and TQM
The main aim of TQM implementation is to achieve customers’ satisfaction [ 33 ]. This is turn results in a company improving its financial performances [ 34 – 36 ]. The benefits may be seen in areas such as cost reduction, increased market share and profit, and enhanced business competitiveness [ 37 ]. As a result, MBNQA specifically emphasizes business excellence. Besides the implementation of TQM, MBNQA also considers the strategic management, and information management and analyses.
The European Foundation for Quality Management (EFQA) also launched in 1992 a European Quality Award (EQA) [ 38 ]. Based on the performed research, more than 80 countries have established National Quality Awards (NQAs). These NQAs are largely based on the MBNQA, EQA, and the Deming Prize [ 39 , 40 ], and are generally considered to be an effective way of pursuing business excellence, including customer loyalty and better long‐term financial performances. Most of these national quality awards may form part of the TQM system namely, strategic management, performance evaluation, human resource management, and even IT system and innovation.
5.2. Prospects of the business excellence model beyond TQM
strategic management system,
performance evaluation, and
innovation ( Figure 3 ).

Figure 3.
The framework of the proposed integral model of a business‐excellence system.
Meanwhile, the concept of “sustainable development” was also widely adopted in industry [ 42 , 43 ]. Zairi asserted that “sustainable development is based on a perceived need to address environmental deterioration and to maintain the vital functions of natural systems for the well‐being of present and future generations” [ 44 ]. He thus, proposed an integrated model that incorporates the sustainable development into a TQM system. Yang et al. developed a framework of the system of environmental management, which constitutes of 30 activity items themselves belonging to 12 initiatives [ 45 ].
6. The concepts of “customer delight” and “innovative quality” in the new century
A critical strategy of pursuing business excellence is to raise customers’ loyalty, which cannot be assured by grossly satisfying customers. Indeed, even satisfied customers would defect at a significant rate in many industries. It is recognized that customers’ satisfaction is the result of the fulfillment of their explicit needs. As such, satisfaction only of their needs does not directly result in customer loyalty, since the competitors would also fulfill these explicit needs with better customer‐valued products [ 46 ].
Several studies have revealed that the degree of loyalty depends on whether the customers are “totally satisfied” or merely “satisfied” [ 47 , 48 ]. The totally satisfied customers are actually about five times more likely to repurchase a product or service than those who are merely “satisfied” [ 49 , 50 ]. This suggests that enterprises must strive for “total customer satisfaction,” or even “customer's delight” [ 51 ].
“Customer delight” is a new quality concept. A conceptualization of “delight” is the customer's emotional response, which is composed of “joy” and “surprise”—both encountered in the providing process of goods/services [ 52 ]. In order to delight customers, the enterprises need to provide the goods/services with attractive and innovative quality incentives. The strategic actions are to identify customers’ latent needs and to create customer value by developing the innovative products and attractive services in order to fulfill these.
application innovation,
product innovation,
process innovation,
experience innovation,
marketing innovation,
business model innovation,
structural innovation, and
disruptive innovation.
These kinds of innovations, especially the first four types, will result in significant effects on the fulfilment of the customers’ latent needs and their delight experiences.
7. The development of the innovation system
In order to raise the customers’ loyalty, the enterprises should develop the core capabilities with an innovation system. In view of the above, the prerequisite for developing such a system is been described. As such, this chapter proposes a systematic innovation development system based on the product/service value chain.
7.1. The framework of the innovation system
At first, the differences among improvement, reengineering, and innovation are established as shown in Figure 4 . Improvement is implemented on the existing processes, as some problems may have been encountered during the manufacturing process or the service delivery process. Continuous improvement is the key activity of the TQM system, its aim is to improve the quality of products/services and all the observed and or experienced bottlenecks on the processes. The final objective is to raise customer satisfaction [ 55 ].

Figure 4.
The framework of the innovation system.
Hammer and Champy asserted that improving solely the production system or service delivery processes may not result in significant business performance [ 56 ]. Sometimes it is needed to reengineer the critical processes, or to redevelop the provision systems based on customer voices. The latter applies in particular to key customer values which are difficult to fulfill by adopting the improvement actions, as shown in Figure 4 . Thus, the aim of reengineering is to create customers’ values, which are the critical factors of raising customer loyalty. Besides, reengineering also reaps the benefits including shorter delivery times of products/services (including lead time and production time), reduction of costs, and the effective utilization of resources.
Most companies develop and implement enterprise resources planning (ERP), customer relationship management (CRM), and supply chain management (SCM) simultaneously [ 57 ]. By integrating these systems, enterprises can exert excellent business’ performance and customer's loyalty. In order to effectively utilize these networks and their integrated systems, it is the author's view that companies should proceed with the necessary processes of reengineering. This in turn, may have an impact on the critical functional activities on a timely basis.
The utilization of high‐tech functions and the rapid application of the internet resulted in the change of customers’ purchasing behavior [ 58 , 59 ]. Customers now focus not only on the evaluations of price and functions; rather they also integrate quality with the perceived value [ 60 ]. It is the author's view that business systems ought to change and pursue innovation, speed, and quality if they want to satisfy the overall needs of customers and create new value.
Customer satisfaction may be termed as fulfilling their requirements and expectations. This, however, is not enough for delighting them and raising their loyalty. Performed research indicates that a number of customer's latent needs are never discovered and satisfied by enterprises [ 61 ]. Thus, the latter's competitive force is to probe the unmet customers’ latent needs. There are some new methodologies proposed by several researchers to pinpoint customers’ latent needs [ 62 ]. Once the latter are identified, companies ought to overcome bottlenecks and aim at developing and providing innovative products/services to satisfy customers’ latent needs and curiosity.
Eventually the customer will be set at the center of innovation. Thomke and von Hippel asserted that “tapping into customer innovation can certainly generate tremendous value, but capturing customer value is hardly a simple or straightforward work” [ 63 ]. The term “innovation” is usually considered as a process or a capability to create new ideas, and then develop the innovative products/services in order to meet customers’ unsatisfied latent needs [ 64 , 65 ]. In essence, the realization of “innovation” depends on the implementation of an innovation system, as shown in Figure 4 . This innovation system starts at the “mind” mining of the customer's latent needs and ends in the customer's value and response, and vice versa.
7.2. The transfer loop of innovation system
technology innovation,
innovative products,
customer value, and
product functions.

Figure 5.
The transfer loop of the innovation system.
technology development,
development of new material,
product content, and
innovation process.
creativity,
core capability,
professional knowledge,
experience share,
resource inputs, and
cooperation.
integrated multifunctions,
curiosity satisfaction,
easy to use, and
good quality at an affordable price.
Each of the other three constructs also contains several items. These constructs and their involving items are shown in Figure 5 .
The features of “innovative products” will satisfy the “customer value” which is accentuated by good quality, delight, good experience, and extra value‐added features. In order for the companies to fulfill these characteristics, it is hereby suggested to use “mind mining” methods to identify customers’ unsatisfied and latent needs. They also ought to use methods and means to eliminate the inconvenient utilization, and to integrate product functions and quality. These findings and results may be hereby included into the quality functions. For realizing these quality functions, the drivers are the capability of “technology innovation.” As such, the four constructs form a “transfer loop of the innovation system,” as shown in Figure 5 .
8. Conclusion
In a highly globalized economy, it is widely recognized that “innovation” has become the key force for achieving business excellence, which in turn reflects as competitive advantages, growth, and development [ 66 ]. Only the pursuit of product quality and service quality is not enough to achieve the business objectives. The enterprises should alter their quality concepts from the narrow definition of quality to include customer value and innovation. In order to realize the innovation performance, this study has proposed a framework of the innovation system and the related transfer loop of the system. However, the proposed system is a conceptual model, empirical studies ought to be conducted to identify potential causalities among the critical constructs and their practices.
- 1. Ulwick, Anthony W. What Customers Want. 2005; New York: McGraw‐Hill Inc.
- 2. Yang, C.‐C. The Integration of TQM and Six‐Sigma. In the book “ Total Quality Management and Six Sigma ”, edited by Tauseef Aized, ISBN 978-953-51-0688-3, InTech Ltd, August 8, 2012; 219–246.
- 3. Edvard, C. D. The meaning of quality. Quality Progress . 1968; 1(10), 36–39.
- 4. Deming, W. E. Out of Crisis. 1986; Cambridge, MA: MIT Press.
- 5. Gorst, J., Kanji, G., & Wallage, W. Providing customer satisfaction. Total Quality Management , 1998; 9(4–5), 100–103.
- 6. Sirohi, N., McLaughlin, E.W., & Wittink, D. R. A model of customer perception and store loyalty intentions for a supermarket retailer. Journal of Retailing , 1998; 74(2), 223–245.
- 7. Teece, D. J. Business model, business strategy and innovation. Long Range Planning , 2010; 43, 172–194.
- 8. Rindova, V. P., & Petkova, A. P. When is a new thing a good thing? Technological change, product form design, and perceptions of value for product innovations. Organization Science , 2007; 18(2), 217–232.
- 9. Reichheld, F. F. Loyalty and renaissance of marketing. Marketing Management , 1994; 2(4), 10–21.
- 10. Schneider, B., & Bowen, D. E. Understanding customer delight and outrage. Sloan Management Review , 1999; 41(1), 35–45.
- 11. Kumar, A., Olshavsky, R. W., & King, M. F. Exploring alternative antecedents of customer delight. Journal of Customer Satisfaction, Dissatisfaction and Complaining Behaviour , 2001; 14, 14–26.
- 12. Gallo, C. The Apple Experience. 2012; New York: McGraw‐Hill Inc.
- 13. Yang, C.‐C. The identification of customer delight for quality attributes and its applications. Total Quality Management & Business Excellence , 2011; 22(1–2), 83–98.
- 14. Rao, A., Carr, L. P., Dambolena, I., Kopp, R. J., Martin, J., Rafii, F., & Schlesinger, P. F. Total Quality Management: A Cross Functional Perspective. 1996; New York: John Wiley & Sons.
- 15. Zokaei, K., & Simons, D. Performance improvements through implementation of lean practices: A study of the U.K. Red Meat Industry. International Food and Agribusiness Management Review , 2006; 9(2), 30–53.
- 16. Yang, C.‐C. Improving the definition and quantification of quality costs. Total Quality Management & Business Excellence , 2008; 19(3–4), 175–191.
- 17. Mitra A. Fundamentals of Quality Control and Improvement Second edition. 1998; London: Prentice‐Hall International (UK) Limited.
- 18. Xie, M., Goh, T. N., & Ranjan, P. Some effective control chart procedures for reliability monitoring. Reliability Engineering & System Safety , 2002; 77(2), 143–150.
- 19. Lavin, M. Inspection efficiency and sampling inspection plans. Journal of the American Statistical Association , 1946; 41(236), 432–438.
- 20. Giakatis, G., Enkawa, T. & Washitani, K. Hidden quality costs and the distinction between quality cost and quality loss, Total Quality Management, 2001; 12(2), 179–190.
- 21. Oakland, J. S. Total Quality Management. 2nd ed. 2000; Jordan Hill, Oxford: Butter-worth‐Heinemann.
- 22. Juran, J. M. (1974). Quality Control Handbook. 3rd ed. New York: McGraw‐Hill.
- 23. Feigenbaum, A. V. Total Quality Control. Harvard Business Review , 1956; 34 (6), 93–101.
- 24. Crosby, P. Quality is Free . 1979; New York: McGraw‐Hill.
- 25. Dotchin, J. A., & Oakland, J. S. Theories and concepts in total quality management. Total Quality Management , 1992; 3(2), 133–145.
- 26. Powell, T. C. Total quality management as competitive advantage: A review and empirical study. Strategic Management Journal , 1995; 16, 15–37.
- 27. Elshennawy, A. K., Maytubby, V. J., & Aly, N. A. Concepts and attributes of total quality management. Total Quality Management , 1991; 2(1), 75–97.
- 28. Yang, C.‐C. An integrated model of TQM and Six‐Sigma. International Journal of Six Sigma and Competitive Advantage, 2004; 1(1), 97–111.
- 29. Sila, I., & Ebrahimpour, M. An investigation of the total quality management survey based on research published between 1989 and 2000. Journal of Quality and Reliability International , 2002; 19(6–7), 902–970.
- 30. Pande, P. S., Neuman R. P., & Cavanach R. R. The Six Sigma Way . 2000; New York: McGraw‐Hill.
- 31. Antony, J., & Banuelas, R. Key ingredients for the effective implementation of Six Sigma program, Measuring Business Excellence , 2002; 6(4), 20–27.
- 32. Bell, R. & Keys, B. A conversation with Curt W. Reimann on the background and future of the Baldrige award. Organizational Dynamics , 1998; 26(4), 51–61.
- 33. Chin, K. S., Pun, K. F., & Hua, H. M. Consolidation of China's quality transformation efforts: A review. International Journal of Quality and Reliability Management , 2001; 18(8), 836–853.
- 34. Hendricks, K. B., & Singhal, V. R. Quality awards and the market value of the firms: an empirical investigation. Management Science , 1996; 42(3), 415–436.
- 35. Gunasekaran, A. Enablers of total quality management implementation on manufacturing: A case study. Total Quality Management , 1999; 10(7), 987–996.
- 36. Hansson, J., & Eriksson, H. The Impact of TQM on financial performance, Measuring Business Excellence , 2002; 6(4), 44–54.
- 37. Youssef, M. A., Boyd, J., & Williams, E. The impact of total quality management on firms responsiveness: An empirical analysis. Total Quality Management , 1996; 7(1), 127–144.
- 38. EFQM. The European Model for Total Quality Management , 1992; Brussels: European Foundation for Quality Management.
- 39. Tan, K. C., Wong, M. F., Mehta, T., & Khoo, H. H. Factors affecting the development of national quality awards. Measuring Business Excellence , 2003; 7(3), 37–45.
- 40. Cauchick Miguel, P. A., Morini, C., & Pires, S. R. I. An application case of the Brazilian National Quality Award. The TQM Magazine, 2004; 16(3), 186–193.
- 41. Yang, C.‐C. Development of an integrated model of a business excellence system. Total Quality Management & Business Excellence , 2009; 20(9), 931–944.
- 42. Kanji, G. K. Architecture of business excellence in the public and service sectors. Total Quality Management and Business Excellence , 2008; 19(3–4), 399–415.
- 43. Okumura, S., Morikuni, T., & Okino, N. Environmental effects of physical life span of a reusable unit following and physical failures in a remanufacturing system. International Journal of Production Research, 2003; 43(16), 3667–3687.
- 44. Zairi, M. Beyond TQM implementation: the new paradigm of TQM sustainability. Total Quality Management & Business Excellence , 2002; 13(8), 1161–1172.
- 45. Yang, C.‐C. Exploration strategies and key activities for the system of environmental management. Total Quality Management & Business Excellence, 2011; 22(11), 1179–1194.
- 46. Matzler, K., & Hinterhuber, H. H. How to make product development projects more successful by integrating Kano's model of customer satisfaction into quality function development. Technovation , 1998; 18(1), 25–38.
- 47. Finkelman, D. P., & Goland, A. R. How not to satisfy your customers. The McKinsey Quarterly , 1990; 26(4), 2–12.
- 48. Heskett, J. L., Jones, T.O., Loveman, G.W., Sasser, W. E., & Schlesinger, L.A. Putting the service‐profit chain to work. Harvard Business Review , 1994; 72(2), 164–174.
- 49. Jones, T. O., & Sasser Jr., W. E. Why satisfied customers defect. Harvard Business Review , 1995; 73(6), 88–99.
- 50. Johnson, R., Retail isn't broken Stores are. Harvard Business Review , 2011; 89(12), 78–82.
- 51. Johann, F., & Kurt, M. Customer delight and market segmentation: An application of three‐factor theory of customer satisfaction on lift style groups. Tourism Management , 2008; 29(1), 116–126.
- 52. Torres, E. N., & Shery, k. From customer satisfaction to customer delight: Creating a new standard of service for the hotel industry. International Journal of Contemporary Hospitality Management , 2013; 25(5), 642–659.
- 53. Rigby, D. K., Gruver, K., & Allen, J. Innovation in turbulent times, Harvard Business Review , 2009; 87(6), 79–86.
- 54. Moore, G. A., Innovating within established enterprises. Harvard Business Review , 2004; 82(7–8), 87–92.
- 55. Yang, C.‐C., The establishment of a TQM system for the health care industry. The TQM Magazine , 2003; 15(2), 93–98.
- 56. Hammer, M., & Champy, J. Reengineering the Corporation. 1993; New York: Harper Collins.
- 57. Hendricks, K. B., Singhai, V. R. & Stratman, J. K. The impact of enterprise systems on corporate performance: A study of ERP, SCM, and CRM system implementations. Journal of Operations Management , 2007; 25(1), 65–82.
- 58. Bill, G. Business @ the Speed of Thought: Using a Digital Nervous System. 1999; New York: Warner Books, Inc.
- 59. Chuck, M. Get Future . 1999; New York: McGraw‐Hill, Inc.
- 60. Feigenbaum, A. V. The new quality for the twenty‐first century. The TQM Magazine , 1999; 11(6), 376–383.
- 61. Ulwick, A. W. Turn customer input into innovation. Harvard Business Review , 2002; 80(1), 91–97.
- 62. Yang, C.‐C. An analytical methodology for identifying the latent needs of customers. Total Quality Management & Business Excellence , 2013; 24(11–12), 1332–1346.
- 63. Thomke, S., & von Hippel, E. Customers as Innovators: A new way to create value. Harvard Business Review, 2002; 80(4), 74–81.
- 64. Hurley, R. F., & Hult, G. T. M. Innovation, market orientation and organizational learning: an integration and empirical examination. Journal of Marketing , 1998; 62(3), 42–54.
- 65. Hult, G. T. M., Hurley, R. F., & Knight, G. A., Innovativeness: Its antecedents and impact on business performance. Industrial Marketing Management, 2004; 33, 429–438.
- 66. Sohel‐Uz‐Zaman, A. S. M. D., & Anjalin, U. Evolution of service: Importance, competitiveness and sustainability in the new circumstances. Journal of Service Science and Management , 2011; 4, 253–260.
© 2017 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Quality control and assurance.
Edited by Leo Kounis
Published: 22 February 2017
By Rodrigo S. Neves, Daniel P. Da Silva, Carlos E. C....
3190 downloads
By Julia Martín, Nieves Velázquez and Agustin G. Asue...
2739 downloads
By Joseph J. Valadez and Lusine Mirzoyan
1740 downloads
- Trending Blogs
- Geeksforgeeks NEWS
- Geeksforgeeks Blogs
- Tips & Tricks
- Website & Apps
- ChatGPT Blogs
- ChatGPT News
- ChatGPT Tutorial
What is Quality Control (QC)?
- What is Data Quality Management?
- What is Qunit ?
- What is the full form of QA?
- What is a Quality Management System?
- What is Stateful Inspection?
- Control Variables in Statistics
- Quality and Customer Satisfaction Metrics
- What is Project Quality Management and Why is it Important?
- What is System Integrity Check?
- Quality Assurance (QA) vs Quality Control (QC)
- Statistical Process Control (SPC)
- How EPOS help Stock Control
- Error Control in TCP
- What is QAOps?
- Quality of Service (QoS) in ATM
- How Software Inspection improves Software Quality ?
- McCall's Quality Model
- Version Control in Project
- Boehm's Software Quality Model
Quality control is like a silent hero in our daily lives . It’s all about making sure the stuff we use – like food , gadgets , or even the roads we drive on – are really good . It checks everything from how things are made to how they work , to be sure they meet super high standards . So, even though we might not always realize it, quality control is always working behind the scenes to make sure everything is top-notch .
To understand the essence of Quality Control, its significance, the various types of Quality Control, and the numerous career prospects within this field. Check out the article given below:
Table of Content
What is Quality Control?
Types of quality control, quality control tools, quality control: processes and principles, significance of quality control, quality control vs. quality assurance, quality control: careers.
In simple words, Quality control is a process that makes sure things are good enough to use . It involves checking products and services to make sure they meet certain standards . This could mean making sure a toy is safe for kids to play with, or checking that a car is built correctly and works properly . Quality control helps make sure things are made well and are safe for people to use. It’s like having a team of inspectors who make sure everything is up to scratch before it gets to you.
Imagine you make clothes . You want each piece to be really good – no holes , the right size , and the colors just right. So, you check them carefully before they’re sold. That’s Quality Control (QC) . It’s like a special check to make sure things are good enough before they’re sold.
There are several types of Quality Control (QC) methods used to ensure that products and services meet specific standards. Some common types include:
1. Statistical Quality Control (SQC)
Statistical Quality Control (SQC) is a way of keeping track of quality using numbers and stats . It helps businesses make sure their products or services are good by monitoring them with statistical tools . SQC helps to catch any problems early by watching how things are made and comparing them to set standards . It’s like having a team of detectives who use math to spot any issues in the production process before they become big problems .
2. Acceptance Sampling
Acceptance Sampling is a quality control method where random samples are inspected from a batch to determine its quality . Instead of checking every item, only a portion is tested. If the sampled items pass inspection , the entire batch is accepted ; otherwise, further action may be taken. It’s a practical way to ensure quality without examining every single item.
3. Total Quality Control (TQC)
Total Quality Control (TQC) means everyone in a company works together to make sure everything is really good . It’s about always trying to make things better and keeping customers happy . TQC involves everyone , from bosses to regular workers , in finding and fixing problems , making sure things work well , and making the company better overall. It’s like a team effort to make sure everything is top-notch .
These tools are essential for analyzing and improving processes, identifying potential issues, and ensuring high-quality standards are maintained throughout production
These processes and principles serve as essential frameworks for ensuring consistent quality, minimizing defects, and continuously improving operations to meet customer expectations effectively.
The significance of Quality Control lies in its ability to ensure that products and services meet high standards before reaching customers . By implementing Quality Control measures, businesses can:
- Ensure Customer Satisfaction : By delivering high-quality products, businesses can satisfy their customers, leading to customer loyalty and positive word-of-mouth .
- Maintain Reputation : Consistently delivering products that meet or exceed customer expectations helps businesses maintain a positive reputation in the market.
- Reduce Costs : Quality Control helps identify and correct defects early in the production process, reducing the need for rework or waste , which can ultimately save businesses money.
- Comply with Regulations : Many industries have regulatory requirements regarding product quality and safety. Quality Control ensures that businesses meet these standards and avoid penalties or legal issues.
- Drive Continuous Improvement : Quality Control processes provide valuable feedback that can be used to identify areas for improvement in production processes, leading to ongoing enhancements and efficiency gains.
Overall, Quality Control plays a crucial role in ensuring that businesses deliver products and services that meet or exceed customer expectations, ultimately contributing to their success and sustainability in the market.
Quality Control (QC) is like checking your homework to make sure all the answers are right before you hand it in. It’s about finding mistakes and fixing them.
Quality Assurance (QA) is like studying and practicing so you’re really good at your subjects and don’t make mistakes in your homework in the first place. It’s about making sure you’re doing things right from the start to avoid errors.
Learn More – Quality Assurance (QA) vs Quality Control (QC)
Working in quality control can be rewarding if you have an interest in interacting with people, communicating effectively, sharing results, and striving for improvement and safety. Qualifications for a quality control inspector vary depending on the industry:
- Entry-level roles typically require a high school diploma.
- Some businesses may prefer candidates with a bachelor’s degree.
- Industry-specific background knowledge is often beneficial.
- Certain sectors mandate licenses or certifications.
Key qualities for quality control specialists include:
- Attention to detail
- Proficiency in mathematics and mechanics
- Physical stamina and strength
- Technical proficiency
- Ability to perform under pressure
In conclusion , quality control is crucial for ensuring that products and services meet high standards before they reach customers . It helps maintain customer satisfaction , uphold a positive reputation , reduce costs , comply with regulations , and drive continuous improvement . Quality control careers offer opportunities for individuals with various qualifications and qualities , making it a rewarding field for those interested in ensuring product excellence and customer satisfaction .
Knowing its core values , kinds , hurdles , and what’s next helps businesses make sense of today’s complex world. This knowledge helps them to stick to top-notch standards. As we look to the future, the ongoing growth of quality control brings cool chances for new ideas and being the best we can be.
What is Quality Control (QC)? – FAQs
Quality control refers to a company’s methods for assessing product quality and, if necessary, improving it. There are various ways to perform quality control, including benchmarking, examining manufacturing procedures, and testing products. All of this is done to keep track of significant product differences.
What are the 4 types of quality control?
The four types of quality control are process control, acceptance sampling, control charts, and product quality control.
What is difference between QA and QC?
QA primarily focuses on the processes and procedures that improve quality, including training, documentation, monitoring and audits. QC focuses on the product to find defects that remain after development. QC professionals find these issues in a variety of ways, including software testing and beta or canary testing.
What are the 7 objectives of quality control?
The 7 key quality management principles—customer focus, leadership, engagement of people, process approach, improvement, evidence-based decision making and relationship management.
Please Login to comment...
Similar reads.
- tech-updates
- Software Testing
Improve your Coding Skills with Practice
What kind of Experience do you want to share?

- Onsite training
3,000,000+ delegates
15,000+ clients
1,000+ locations
- KnowledgePass
- Log a ticket
01344203999 Available 24/7
Quality Assurance vs Quality Control : Key Differences
Explore this insightful blog's critical distinctions and seamless integration of Quality Assurance vs Quality Control. From understanding the processes and nuances of each to exploring the key differences, dive into the world of Quality Assurance vs Quality Control. Unlock insights into fostering a holistic approach to quality management, ensuring top-notch product quality.

Exclusive 40% OFF
Training Outcomes Within Your Budget!
We ensure quality, budget-alignment, and timely delivery by our expert instructors.
Share this Resource
- Supplier Quality Management Training
- ISO 10001 Quality Management System Training
- ISO 10002 Quality Management Training
- Logistics Management Training
- Supply Chain Management Training

Table of Contents
1) What is Quality Assurance?
2) Quality Assurance process
3) Quality Control process
4) What is Quality Control?
5) Difference between Quality Assurance and Quality Control
6) The integration of Quality Assurance and Quality Control
7) Conclusion
What is Quality Assurance?
Quality Assurance (QA) is a systematic approach that ensures that the processes used to create and deliver a product or service are well-defined, consistent, and capable of meeting the specified requirements. It is like setting up a recipe for baking a cake and ensuring that you follow that recipe strictly every time. The goal of QA is to prevent mistakes or defects from occurring in the first place. Quality Assurance involves various elements, such as the following:
a) Adhering to standardised processes and procedures
b) Training employees to be skilled and knowledgeable
c) Conducting regular audits and assessments
These are done to ensure that everything is going as per the plan of the organisation. It helps to produce high-quality products or services consistently. As a result, it leads to happier customers and a strong reputation for the organisation.

Quality Assurance process
Discussed below are some of the key elements of the Quality Assurance process:
a) Process standardisation: QA teams work to define, document, and standardise the procedures, workflows, and best practices involved in production or service delivery. Standardisation gives clarity and consistency, reducing the chances of errors and variations.
b) Documentation and training: QA involves the creation and maintenance of comprehensive documentation that gives an idea of processes, guidelines, and quality standards. Employees are trained to read and follow these documented procedures. It ensures that all are on the same page when it comes to quality expectations.
c) Auditing and assessment: Regular audits and assessments are conducted to evaluate the extent to which processes adhere to established standards. Auditors review the documentation, observe the execution of tasks, and collect data to assess process effectiveness and identify areas for improvement.
d) Root cause analysis: When deviations from quality standards are identified, QA processes delve into root cause analysis. This means identifying the basic reasons for quality issues and taking necessary actions to address these issues at their source.
e) Customer feedback integration: Customer feedback plays a vital role in QA processes. Customer complaints and suggestions are analysed and integrated into the process.
f) Risk management: QA also involves identifying and managing risks that could impact the quality of the product or service. By proactively addressing potential risks, QA helps prevent quality issues.
What is Quality Control?
Quality Control, often abbreviated as QC, is a systematic and methodical process. It ensures that the products or services an organisation produces meet specific quality standards. It involves the following:
a) Examining
b) Testing
c) Inspecting
Its primary goal is to ensure that what the customer receives is of the highest possible quality, free from any flaws or defects.
Quality Control process
Quality Control process involves a series of systematic steps and methods to identify and rectify defects or deviations. These are discussed below:
1) Inspection: Inspection is a fundamental step in QC, where products or services are carefully examined to identify any visible defects from established standards. This step ensures that what is being delivered is in good condition and meets quality expectations.
2) Testing: Testing involves subjecting the product or service to various assessments to verify its functionality, performance, and adherence to specific requirements. Testing may include performing the following:
a) Functionality tests
b) Stress tests
c) Performance tests
3) Statistical Process Control (SPC): SPC is a method used to monitor and control processes by collecting and analysing data during production. This data helps identify variations and trends that could lead to quality issues, allowing for timely corrective action.
4) Sampling: Sampling is a technique where a sample of products or services is selected from a larger batch or production run for inspection or testing. This approach is often used to assess the quality of a large number of items efficiently.
5) Quality data collection : During the QC process, data related to the quality of products or services is collected and documented. This data can include defect rates, measurements, and any other relevant information that helps evaluate quality.
Corrective action: Once defects or quality issues are identified, appropriate corrective measures are taken to address them. This may involve repairing or reworking the product, modifying the service, or other necessary actions to ensure quality compliance.
Elevate your managerial capability with our Supplier Quality Management Training – empower your team, enhance efficiency, and drive excellence!
Difference between Quality Assurance and Quality Control
Quality Assurance vs Quality Control are two critical components of Quality Management, each with distinct roles and objectives. So, let’s look at the key differences between both in detail:

Quality Assurance is primarily concerned with optimising the processes and systems responsible for producing products or services. Its main objective is the prevention of defects and errors by ensuring that these processes are clearly defined, standardised, and consistently capable of delivering high-quality results.
QA achieves this by establishing guidelines, best practices, and standards to enhance the overall efficiency and effectiveness of operations. It is a proactive approach, actively addressing potential issues before they manifest during production, thereby reducing the likelihood of defects.
By focusing on continuous improvement and process refinement, it minimises the need for extensive corrective measures after production. This makes QA an integral part of ensuring consistent quality throughout the production process.
In contrast, Quality Control is centred on the final products or services. It is a reactive approach focused on detecting and addressing defects in the end results. QC involves the inspection, testing, and measurement of individual products to ensure they meet established quality standards. Unlike QA, QC's emphasis is on identifying and correcting defects rather than preventing them.
QC occurs after the production process and is the last line of defence to ensure that the product or service meets the required quality level. While it is essential for identifying and rectifying issues, it can be more resource-intensive and costly compared to QA. It is costly as it involves dealing with problems that have already occurred.
Thus, the key distinction lies in their roles. QA prevents defects by improving processes, while QC detects defects in the final product or service.
Master Quality Assurance with our comprehensive Quality Assurance Masterclass - join today!
The integration of Quality Assurance and Quality Control
The integration of Quality Assurance and Quality Control is an approach that organisations must use to build a robust Quality Management framework. It involves combining the strengths of both QA and QC to ensure that products or services not only meet predefined quality standards but also consistently exceed customer expectations. Here's how the integration of QA and QC works:

a) Setting quality standards: The integration begins by establishing clear quality standards that define what constitutes a high-quality product or service. These standards serve as a common reference point for both QA and QC.
b) Prevention and detection: QA focuses on preventing defects before they occur by optimising processes, while QC is primarily concerned with detecting defects in the final product or service. Integrating these approaches means actively working to prevent issues while also having robust systems in place to address any issues.
c) Documentation and training: The integration ensures that the documentation developed and training given to the employees in QA are effectively utilised in QC. QC teams follow the documented procedures, ensuring that everyone understands and follows the quality expectations.
d) Process optimisation: QA identifies areas where processes can be improved, standardised, and made more efficient. These enhancements reduce the likelihood of defects, lowering the need for extensive corrective actions in QC.
e) Data sharing: QA and QC processes generate valuable data that can be shared and analysed together. This data sharing allows organisations to identify trends, predict potential issues, and take proactive measures.
Conclusion
We hope this blog helped answer the question: Quality Assurance vs Quality Control – which one is better? Their differences highlight that they are two different processes essential for ensuring the quality of a product or service. Quality Assurance prevents quality issues through defined processes and continuous improvement, while Quality Control detects and rectifies defects in final output. Integration of both these processes helps in creating customer satisfaction.
Elevate your Management skills with our Management Training - sign up today to take your career forward!
Frequently Asked Questions
Upcoming business skills resources batches & dates.
Fri 9th Aug 2024
Fri 20th Dec 2024
Get A Quote
WHO WILL BE FUNDING THE COURSE?
My employer
By submitting your details you agree to be contacted in order to respond to your enquiry
- Business Analysis
- Lean Six Sigma Certification
Share this course
Our biggest spring sale.

We cannot process your enquiry without contacting you, please tick to confirm your consent to us for contacting you about your enquiry.
By submitting your details you agree to be contacted in order to respond to your enquiry.
We may not have the course you’re looking for. If you enquire or give us a call on 01344203999 and speak to our training experts, we may still be able to help with your training requirements.
Or select from our popular topics
- ITIL® Certification
- Scrum Certification
- Change Management Certification
- Business Analysis Courses
- Microsoft Azure Certification
- Microsoft Excel Courses
- Microsoft Project
- Explore more courses
Press esc to close
Fill out your contact details below and our training experts will be in touch.
Fill out your contact details below
Thank you for your enquiry!
One of our training experts will be in touch shortly to go over your training requirements.
Back to Course Information
Fill out your contact details below so we can get in touch with you regarding your training requirements.
* WHO WILL BE FUNDING THE COURSE?
Preferred Contact Method
No preference
Back to course information
Fill out your training details below
Fill out your training details below so we have a better idea of what your training requirements are.
HOW MANY DELEGATES NEED TRAINING?
HOW DO YOU WANT THE COURSE DELIVERED?
Online Instructor-led
Online Self-paced
WHEN WOULD YOU LIKE TO TAKE THIS COURSE?
Next 2 - 4 months
WHAT IS YOUR REASON FOR ENQUIRING?
Looking for some information
Looking for a discount
I want to book but have questions
One of our training experts will be in touch shortly to go overy your training requirements.
Your privacy & cookies!
Like many websites we use cookies. We care about your data and experience, so to give you the best possible experience using our site, we store a very limited amount of your data. Continuing to use this site or clicking “Accept & close” means that you agree to our use of cookies. Learn more about our privacy policy and cookie policy cookie policy .
We use cookies that are essential for our site to work. Please visit our cookie policy for more information. To accept all cookies click 'Accept & close'.
GuidelinePharma
- Expiration dating as per USFDA CFR 211.137 USFDA CFR
- Check Weigher Quality Assurance
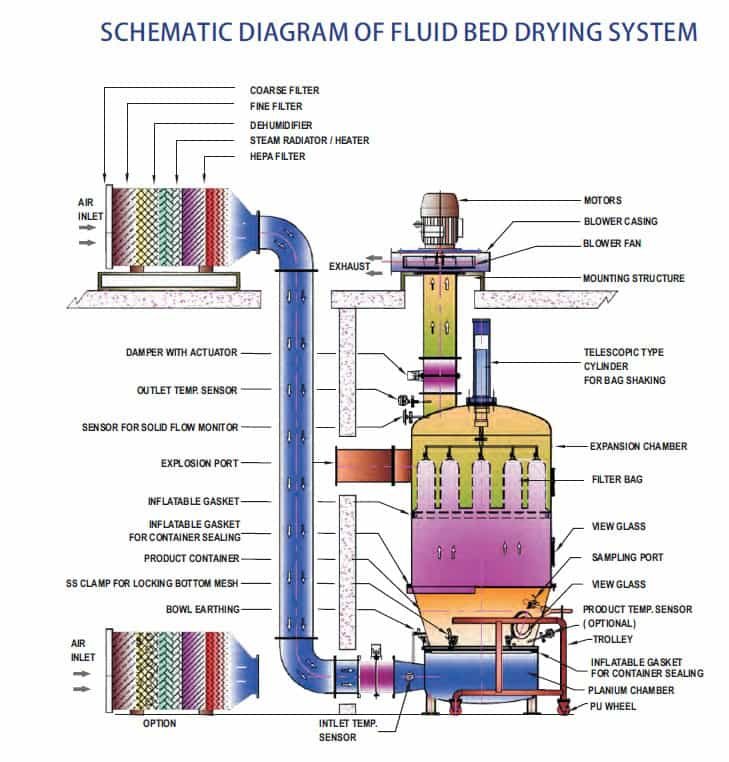
- Inspection of incoming foil Warehouse
- Market Complaints in the Pharmaceutical Industry: Ensuring Customer Satisfaction and Product Safety Quality Assurance

- Auto cartonator (CP-120) Quality Assurance

Quality Control in Pharmaceuticals: Ensuring Product Safety and Efficacy
Quality control is a critical component of the pharmaceutical industry, focused on ensuring that pharmaceutical products meet established quality standards, are safe for consumption, and provide the intended therapeutic effect. Quality control measures encompass a range of testing, analysis, and inspection procedures that are performed throughout the entire pharmaceutical product lifecycle. The main objective of quality control in pharmaceuticals is to deliver safe, effective, and consistent medications to patients worldwide. In this comprehensive blog post, we will delve into the world of quality control in pharmaceuticals, exploring its importance, key principles, analytical techniques, regulatory guidelines, challenges, and best practices. Join us on this journey to understand how robust quality control practices contribute to the success of pharmaceutical companies and the well-being of patients.
Understanding Quality Control in Pharmaceuticals
Definition and significance of quality control:.
Define quality control in the context of the pharmaceutical industry and its role in ensuring product quality and patient safety.
Discuss the importance of quality control in meeting regulatory requirements and maintaining the reputation of pharmaceutical companies.
- Key Principles of Quality Control:
Quality Control Procedures: Explain the principles of implementing comprehensive quality control procedures to ensure the accuracy and reliability of test results.
Statistical Process Control (SPC): Discuss the application of statistical tools in quality control to monitor and control manufacturing processes.
Role of Quality Control in the Pharmaceutical Lifecycle :
Raw Material Testing: Explain the importance of quality control in testing raw materials to ensure their suitability for pharmaceutical manufacturing.
In-Process Control: Discuss the role of quality control in monitoring critical parameters during the manufacturing process to maintain product quality.
Analytical Techniques in Pharmaceutical Quality Control
Physical and chemical testing:.
Physical Tests: Discuss various physical tests, such as hardness, friability, and disintegration, used to assess the physical characteristics of pharmaceutical products.
Chemical Tests: Explore the use of chemical tests, including titration, spectrophotometry, and chromatography, to determine the chemical composition and purity of drugs.
Microbiological Testing:
Microbial Limits Testing: Explain the testing of pharmaceutical products for microbial contamination and the establishment of microbial limits.
Sterility Testing: Discuss the importance of sterility testing for injectable and ophthalmic products to ensure they are free from viable microorganisms.
Dissolution Testing:
Purpose of Dissolution Testing: Explain the significance of dissolution testing in evaluating the rate at which a drug substance is released from a dosage form.
Dissolution Apparatus: Discuss the different types of dissolution apparatus and their relevance in dissolution testing.
Stability Testing:
Stability Study Design: Explore the design of stability studies to assess the shelf life and storage conditions of pharmaceutical products.
ICH Guidelines for Stability Testing: Discuss the International Council for Harmonization (ICH) guidelines on stability testing.
III. Regulatory Requirements for Quality Control in Pharmaceuticals
FDA Requirements for Quality Control:
cGMP Regulations: Explore how current Good Manufacturing Practices (cGMP) regulations by the FDA mandate quality control as a crucial aspect of pharmaceutical manufacturing.
FDA Guidance Documents: Discuss relevant FDA guidance documents related to quality control, such as ICH Q2(R1) – Validation of Analytical Procedures.
ICH Guidelines for Quality Control:
International Council for Harmonisation (ICH): Explain the role of ICH in harmonizing global regulatory requirements and guidelines for quality control in pharmaceuticals.
ICH Q2(R1) – Validation of Analytical Procedures: Explore the ICH guideline on analytical method validation.
Other Global Regulatory Authorities:
European Medicines Agency (EMA): Discuss EMA’s requirements and guidelines for quality control in pharmaceutical manufacturing and distribution.
Health Canada and Other Regulatory Authorities: Highlight the expectations of other regulatory authorities, such as Health Canada, PMDA (Japan), and TGA (Australia), regarding quality control.
Best Practices in Pharmaceutical Quality Control
Analytical method validation:.
Importance of Analytical Method Validation: Discuss the criticality of validating analytical methods to ensure the accuracy, reliability, and precision of test results.
Method Validation Parameters: Explain the parameters considered in analytical method validation, such as specificity, accuracy, precision, linearity, and robustness.
Quality Control Sample Handling and Management:
Sample Collection and Preservation: Discuss the proper handling, storage, and preservation of samples to ensure accurate and reliable test results.
Sample Retention: Explain the importance of retaining samples for future reference and regulatory inspections.
Good Documentation Practices (GDP) in Quality Control:
GDP Guidelines: Explore the guidelines for good documentation practices in pharmaceutical quality control to ensure data integrity and traceability.
Data Management Systems: Discuss the implementation of data management systems to facilitate data storage and retrieval.
Training and Competency of Quality Control Personnel:
Importance of Training: Discuss the critical role of training in maintaining a competent and skilled workforce in pharmaceutical quality control.
Continuous Learning and Skill Development: Explain the need for continuous learning and skill development to keep up with evolving analytical techniques.
Challenges in Pharmaceutical Quality Control
Analytical challenges:.
Complex Analytical Techniques: Discuss the complexities of using advanced analytical techniques, such as mass spectrometry and NMR, for pharmaceutical analysis.
Method Transfer and Harmonization: Explore the challenges associated with method transfer and harmonization between different laboratories.
Data Integrity and Compliance:
Data Integrity Challenges: Discuss the significance of data integrity in pharmaceutical quality control to ensure the accuracy and reliability of test results.
Compliance with Regulatory Requirements: Explain the challenges of meeting regulatory requirements and guidelines for data integrity.
Quality Control for Biopharmaceuticals
Biopharmaceutical analytical testing:.
Unique Challenges: Discuss the unique challenges of analytical testing for biopharmaceuticals, including protein characterization and post-translational modifications.
Bioassays: Explain the use of bioassays to assess the biological activity of biopharmaceutical products.
Quality Control of Vaccines and Cell-Based Therapies:
Vaccine Testing: Discuss the extensive quality control testing required for vaccines to ensure safety and efficacy.
Cell-Based Therapy Testing: Explore the quality control measures for cell-based therapies, such as CAR-T cell therapies.
VII. Quality Control for Generics and Biosimilar
Comparative testing:.
Comparative Analysis of Generics: Discuss the importance of comparative testing for generic drugs to demonstrate bioequivalence to the reference product.
Biosimilar Comparability Studies: Explain the need for comparability studies to establish bio similarity between a biosimilar and its reference product.
Quality Control Challenges for Complex Products:
Complex Formulations: Discuss the quality control challenges associated with complex formulations, such as liposomal and nanoparticle-based products.
Bio comparability: Explore the testing required to establish bio comparability for complex products.
VIII. Data Integrity and Quality Control
Data integrity in quality control:.
Importance of Data Integrity: Discuss the significance of data integrity in pharmaceutical quality control to ensure accurate and reliable test results.
Data Integrity Controls: Explain the implementation of data integrity controls, such as electronic signatures and audit trails, to prevent data manipulation.
Future Trends in Pharmaceutical Quality Control
Advanced Analytical Techniques:
Mass Spectrometry: Discuss the increasing application of mass spectrometry in pharmaceutical quality control for advanced characterization and impurity profiling.
NMR Spectroscopy: Explore the potential of nuclear magnetic resonance (NMR) spectroscopy for structural analysis and identification of drug substances.
Automation and Robotics in Quality Control:
Automated Sample Handling: Discuss the implementation of automation and robotics in sample handling and preparation to improve efficiency and reduce human errors.
High-Throughput Screening: Explore the use of high-throughput screening techniques for rapid analysis of large numbers of samples.
In conclusion, quality control in pharmaceuticals is a crucial aspect of ensuring the safety, efficacy, and consistency of medications delivered to patients worldwide. By adhering to stringent regulatory requirements, implementing robust analytical techniques, and embracing best practices, pharmaceutical companies can maintain product quality, compliance, and patient trust. The commitment to quality control, from raw material testing to finished product release, plays a pivotal role in safeguarding public health and improving global healthcare outcomes. As the pharmaceutical industry continues to evolve, the principles of quality control remain constant, reinforcing the commitment to delivering safe and effective medications to those in need. Through continuous improvement and the adoption of advanced analytical technologies, pharmaceutical companies can further enhance their quality control practices and contribute to the advancement of pharmaceutical science and patient care.
Quality Assurance in Pharmaceuticals: Ensuring Safe and Effective Medications
Related Posts
- CALIBRATION RECORD OF INFRARED MOISTURE BALANCE Quality Control
- SAMPLING, RELEASE, AND RETENTION SAMPLES OF RAW MATERIAL Quality Control
- Procedure for sampling of material for microbiological analysis Quality Control
- Procedure of the control samples keeping Quality Control
Do Not Sell My Personal Information
Privacy overview.
- HR & Payroll

Quality Control in Food Manufacturing: What You Need to Know

Food manufacturing is an important industry that employs millions of people around the world. The process of creating food products begins with harvesting crops, vegetables and fruits, which are then processed into foods like bread, cereal, pasta, meat products and dairy goods.
Food manufacturing is crucial to the healthy functioning of our economy and relies on strict quality control measures to ensure safety and consistency.
Factory-produced food is absolutely safe to eat. In fact, it's often more regulated and inspected than food produced by small farmers. The reason for this is simple: factory food is industrialized and standardized, which makes it easier to keep track of and eliminate any potential contamination. Every step in the production process from growing to packaging is scrutinized meticulously to ensure quality and safety.
In today’s guide, we’ll learn about quality control in food manufacturing and its related concepts. Let’s take a look at the table of content below:
Quality Control in Food Manufacturing
Significance of quality control in food manufacturing, goals of quality control in food manufacturing, important characteristics of food quality control system, quality control and compliance, key food quality control procedures, when to perform food quality control, tools and techniques for quality control in food manufacturing, challenges in quality control in food manufacturing, benefits of quality control in food manufacturing, case studies, final conclusion, how deskera can assist you.
Quality control in food manufacturing is a process that ensures that food products meet specific quality standards and regulatory requirements before they are released to the market.
It further involves monitoring and testing raw materials, production processes, and finished products to ensure that they meet certain specifications and are safe for consumption.
Quality control in food manufacturing is crucial for maintaining product consistency, ensuring food safety, and meeting consumer expectations.
The significance of quality control in food manufacturing cannot be overstated. It plays a crucial role in ensuring that food products are safe, of high quality, and meet regulatory requirements. Some of the key reasons why quality control is significant in food manufacturing are:
- Food safety : Quality control is essential for preventing foodborne illnesses and ensuring that food products are safe for consumption. By identifying and controlling potential hazards in the production process, quality control can help prevent contamination of food products.
- Compliance with regulations: Quality control helps ensure that food products comply with local and international regulations and standards. Failure to comply with these regulations can result in fines, product recalls, and reputational damage.
- Product consistency: Quality control is important for maintaining product consistency and ensuring that customers receive the same quality product every time they purchase it. This helps build brand loyalty and trust.
- Cost savings: Quality control can help identify and eliminate non-conforming products, which can result in cost savings for the company. It can also help identify areas for process improvement, which can lead to increased efficiency and reduced waste.
- Reputation: Quality control is essential for protecting the reputation of the company. A single incident of contaminated or unsafe food can have a significant negative impact on the company's reputation and bottom line.
Consequently, quality control in food manufacturing is crucial for protecting public health, complying with regulations, maintaining product consistency, reducing costs, and protecting the reputation of the company.
The goals of quality control in food manufacturing are to ensure that food products meet certain standards of quality, safety, and consistency.
The specific goals may vary depending on the type of food product and the regulatory requirements in the region. However, some common goals of quality control in food manufacturing include:
- Ensuring that the food products meet regulatory requirements and comply with food safety regulations.
- Preventing contamination of food products by identifying and controlling potential hazards in the production process.
- Maintaining product consistency and quality by monitoring production processes, raw materials, and finished products.
- Improving efficiency and reducing waste by identifying and eliminating non-conforming products.
- Enhancing customer satisfaction by providing safe and high-quality food products.
- Protecting the reputation of the company by avoiding product recalls and negative publicity.
- Continuously improving the quality control process by implementing feedback and making necessary improvements.
Following, we’ve discussed important characteristics of food quality control system. Let’s learn:
Proactive (Preventive) Quality Control
Proactive quality control, also known as preventive quality control, is a proactive approach to quality control that focuses on preventing quality issues before they occur.
Furthermore, it is a systematic and continuous process of identifying potential quality problems, analyzing their root causes, and taking preventive actions to eliminate them.
Proactive quality control is based on the principle that prevention is better than correction, and that it is more efficient and cost-effective to prevent quality issues than to correct them after they have occurred.
Some of the key elements of proactive quality control include:
- Risk assessment: Identifying potential risks and hazards in the production process and supply chain, and assessing their likelihood and severity.
- Root cause analysis: Identifying the root causes of quality issues and taking corrective and preventive actions to eliminate them.
- Continuous improvement: Continuously reviewing and improving the quality control process based on feedback and data analysis.
- Training and education: Providing training and education to personnel on quality control procedures, best practices, and regulatory requirements.
- Standardization: Developing and implementing standardized procedures and processes to ensure consistency and quality.
- Collaboration: Encouraging collaboration and communication between different departments and stakeholders to identify and prevent quality issues.
Food industry may prevent quality problems and guarantee the production of safe and high-quality goods by practicing proactive quality control. The chance of product recalls, damage to their reputation, and legal responsibility can be decreased by food industry by recognizing potential risks and hazards and implementing preventive measures to eliminate them.
Reactive Quality Control
Reactive quality control, also known as corrective quality control, is a reactive approach to quality control that focuses on correcting quality issues after they have occurred.
It further involves detecting and addressing quality issues through inspection, testing, and analysis of non-conforming products, customer complaints, and other quality-related incidents.
Reactive quality control is often used as a backup to preventive quality control, as it is not always possible to prevent all quality issues from occurring.
Some of the key elements of reactive quality control include:
- Inspection and testing: Inspecting and testing non-conforming products and analyzing the results to identify quality issues.
- Documentation and tracking: Documenting quality issues, actions taken, and outcomes, and tracking the effectiveness of corrective actions.
- Communication and collaboration: Communicating quality issues and corrective actions to relevant stakeholders, such as production personnel, suppliers, and customers, and collaborating to address quality issues.
While reactive quality control can help food industries address quality issues and prevent them from recurring, it is generally less effective and more costly than proactive quality control.
This is because reactive quality control involves the cost of analyzing non-conforming products, taking corrective actions, and potentially recalling or disposing of non-conforming products.
As such, food industries should aim to prioritize preventive quality control and minimize the need for reactive quality control.
Quality Control Culture
Quality control culture refers to the values, attitudes, and behaviors that prioritize and support quality control in a food manufacturing organization. A strong quality control culture involves a shared commitment to producing safe and high-quality products, and a willingness to invest time, resources, and effort into maintaining and improving quality control processes.
Some of the key characteristics of a quality control culture include:
- Leadership commitment: Top management demonstrates a commitment to quality control by providing the necessary resources, setting quality goals, and actively participating in quality control activities.
- Employee engagement: All employees are involved in quality control activities and encouraged to contribute to continuous improvement efforts.
- Accountability: All employees are held accountable for maintaining and improving quality control processes and are empowered to take action to prevent quality issues.
- Continuous improvement: The organization continuously seeks to improve quality control processes and encourages innovation and experimentation.
- Communication and collaboration : The organization fosters open communication and collaboration between different departments and stakeholders to identify and address quality issues.
- Training and education: The organization provides training and education to employees on quality control procedures, best practices, and regulatory requirements.
- Data-driven decision-making: The organization collects and analyzes data on quality control processes and uses this information to make informed decisions and drive continuous improvement.
A strong quality control culture is essential for food manufacturing organizations to produce safe and high-quality products, maintain customer satisfaction, and comply with regulatory requirements.
- Organizations can ensure that all staff members are dedicated to upholding and enhancing quality control procedures by establishing a culture of quality control. This will also help to ensure that quality control becomes an intrinsic part of the organization's entire strategy and operations.
Quality control and compliance are essential aspects of food manufacturing, as they ensure that food products are safe, of high quality, and meet regulatory requirements.
Furthermore, quality control focuses on monitoring and improving the quality of products and processes, while compliance involves meeting legal and regulatory requirements.
Some of the key regulations and standards that food industries need to comply with include:
- Food safety regulations: Food industries need to comply with food safety regulations such as the Food Safety Modernization Act (FSMA) in the United States, the European Union's General Food Law, and the Codex Alimentarius food safety standards.
- Quality management standards: Quality management standards such as ISO 9001 provide a framework for implementing and maintaining quality control processes.
- Good Manufacturing Practices (GMPs): GMPs provide guidelines for ensuring that food products are manufactured, processed, and packaged under sanitary conditions and meet quality standards.
- Hazard Analysis and Critical Control Points (HACCP): HACCP is a systematic approach to identifying and controlling potential hazards in the food production process.
For the food industry to guarantee the safety and quality of their products, uphold consumer confidence, and stay out of legal and regulatory trouble, effective quality control and compliance are essential.
Food industries may make sure that their goods are safe, of high quality, and satisfy customer and regulatory needs by putting in place effective quality control methods and adhering to rules and standards.
Even if they don't cover all that has to be monitored, the following are some of the most important quality control techniques that every facility that produces food should have:
Ingredient Specifications:
- Ingredient specifications are written documents that provide detailed information about the ingredients used in food products. These specifications include information such as the name of the ingredient, its source, quality standards, physical and chemical properties, and any restrictions or limitations on its use.
Ultimately, ingredient specifications are essential to ensure that only high-quality ingredients are used in food products and that they are used in the correct amounts.
Approved Supplier List:
An approved supplier list is a list of suppliers who have been approved to provide ingredients or other materials to a food manufacturing company. Suppliers are typically approved based on their ability to meet quality standards and regulatory requirements. Maintaining an approved supplier list is critical to ensure that only high-quality materials are used in food products.
Product Formulation/Recipe:
- A product formulation or recipe is a detailed document that outlines the ingredients, quantities, and processing steps needed to manufacture a specific food product. The formulation or recipe is typically developed by food technologists or product development teams and is critical to ensure consistency in product quality and to prevent errors in ingredient selection and processing.
Manufacturing Procedures:
Manufacturing procedures are written documents that provide detailed instructions for how to manufacture a specific food product. These procedures include information such as the sequence of processing steps, equipment specifications, and quality control checks. Manufacturing procedures are critical to ensure that products are manufactured consistently and to a high standard of quality.
In-Process Records:
In-process records are documents that record information about the processing of a food product during production. These records include information such as processing times, temperatures, and other key process parameters.
Furthermore, in-process records are critical to ensure that products are manufactured to the desired specifications and to identify and correct any quality issues that arise during production.
Packaging and Labeling:
Packaging and labeling are critical aspects of food quality control as they provide important information to customers, including ingredients, nutritional information, and handling and storage instructions. Packaging and labeling must meet regulatory requirements and be consistent with the product formulation and manufacturing procedures.
Environmental Monitoring:
Environmental monitoring involves testing the production environment for potential sources of contamination, such as microorganisms, allergens, and foreign materials. Environmental monitoring is critical to prevent contamination of food products and to identify and correct any potential sources of contamination.
Implementing these key food quality control procedures is critical to ensure that food products are safe, of high quality, and meet customer and regulatory requirements.
By implementing effective quality control procedures, food industries can prevent quality issues, minimize the risk of product recalls, and maintain customer trust and satisfaction.
Food quality control should be done throughout the entire production process, from receiving raw materials to shipping finished products. This includes:
- Receiving: Raw materials should be inspected upon arrival to ensure that they meet the required specifications and are free from contamination.
- Storage: Raw materials and finished products should be stored under appropriate conditions to maintain their quality and prevent contamination.
- Preparation: Food products should be prepared according to the approved formulations and manufacturing procedures.
- Processing: During processing, in-process controls should be in place to monitor critical process parameters and ensure that the products meet the required specifications.
- Packaging: Finished products should be packaged in appropriate packaging materials to maintain their quality and prevent contamination.
- Labeling: The labeling of the finished product should be reviewed to ensure that it contains accurate and appropriate information.
- Shipping: Finished products should be shipped under appropriate conditions to maintain their quality and prevent contamination.
In summary, quality control should be a continuous process throughout the entire production cycle to ensure that the products meet the desired specifications and are safe for consumption.
Following, we’ve discussed some crucial tools and techniques for quality control in food manufacturing. Let’s discuss:
Inspection and Testing:
Inspection and testing are essential tools for quality control in food manufacturing. Inspection involves the examination of the food product, raw materials, packaging, equipment, and the entire manufacturing process to ensure that they meet the required standards.
Testing involves the use of scientific methods to analyze food samples for various parameters such as nutritional content, microbiological safety, and sensory quality.
The inspection and testing process should be comprehensive and cover all stages of food production, from the receiving of raw materials to the packaging and shipping of the finished products. Food manufacturers should have a well-defined testing program that includes the selection of appropriate testing methods, sampling procedures, and acceptance criteria.
Sampling Plans:
Sampling plans are used to determine the number of samples that should be taken from a batch or lot of food products for testing purposes. A well-designed sampling plan ensures that representative samples are taken and tested, and that the results are reliable and accurate.
There are several samplings plans that food manufacturers can use, including random sampling, systematic sampling, and stratified sampling. The choice of sampling plan will depend on the characteristics of the food product, the testing requirements, and the level of confidence required in the results.
Quality Assurance Programs:
Quality assurance programs are systems that ensure that the food products meet the required standards and specifications. The program should cover all aspects of food production, from the selection of raw materials to the shipping of the finished product.
A comprehensive quality assurance program includes policies, procedures, and guidelines that guide all aspects of food production. It also includes training programs for employees, documentation requirements, and regular audits and inspections to ensure compliance with the program.
Quality Control Software:
Quality control software is a computer program that helps food manufacturers manage their quality control processes. The software can automate the sampling process, track test results, and provide real-time monitoring of the manufacturing process.
Quality control software can also generate reports, trend analysis, and alerts for non-conformances, enabling manufacturers to take corrective actions promptly. The software can also integrate with other systems, such as inventory management and logistics, to provide a comprehensive view of the manufacturing process.
In conclusion, important tools for quality control in food processing include inspection and testing, sampling strategies, quality assurance plans, and quality control software. A thorough quality control system should be put in place by food producers to guarantee that their goods fulfil the necessary requirements for standards and specifications as well as legal requirements for food safety.
Following, we’ve discussed challenges that emerges when it comes to quality control in food manufacturing. Let’s discuss:
Food Safety Regulations and Compliance:
One of the major challenges in quality control in food manufacturing is ensuring compliance with food safety regulations. Regulations vary by country and can be complex, making it difficult for manufacturers to keep up with the latest requirements. Failure to comply with regulations can result in fines, recalls, and damage to the company's reputation.
Food manufacturers need to stay up to date with regulatory changes and have systems in place to ensure compliance. This may involve investing in specialized software, hiring regulatory experts, and implementing regular audits and inspections.
Supply Chain Management:
Supply chain management is another challenge in quality control in food manufacturing. Manufacturers need to ensure that their suppliers meet their standards for quality, safety, and sustainability . This involves conducting regular audits of suppliers and monitoring their performance to ensure they meet requirements.
Manufacturers also need to ensure that their products are transported and stored under the appropriate conditions to maintain quality and safety. This can be challenging, especially when dealing with complex supply chains involving multiple countries and suppliers.
Automation and Technology:
Automation and technology offer opportunities for improving quality control in food manufacturing, but they also present challenges. Implementing new technologies can be costly, and staff may require additional training to use them effectively.
There is also a risk of overreliance on technology, leading to a decrease in human oversight and attention to detail. Manufacturers need to ensure that technology is used appropriately and that it enhances, rather than replaces, human decision-making and problem-solving.
Staff Training and Development:
The success of quality control in food manufacturing depends on the knowledge and skills of staff. However, turnover can be high in the food industry, and training new staff can be time-consuming and costly.
Manufacturers need to invest in ongoing training and development programs for staff to ensure that they have the knowledge and skills to maintain quality control standards. This may involve providing training on new technologies, regulatory compliance, and best practices for quality control.
Consequently, quality control in food manufacturing is critical for ensuring food safety, maintaining quality, and complying with regulations. However, it is not without its challenges. Food manufacturers need to stay up to date with regulatory changes, manage their supply chains effectively, implement technology appropriately, and invest in staff training and development to overcome these challenges and ensure the success of their quality control programs.
Following, we’ve discussed crucial benefits of quality control in food manufacturing. Let’s discuss:
Improved Product Quality:
Quality control is essential for improving the quality of food products. By monitoring and controlling the production process, manufacturers can ensure that products meet the required standards for safety, nutritional value, and sensory quality.
This leads to products that are consistently high in quality and meet customer expectations, resulting in increased customer satisfaction and loyalty.
Increased Efficiency and Productivity:
- Quality control can also lead to increased efficiency and productivity in food manufacturing. By identifying and addressing quality issues early in the production process, manufacturers can reduce the amount of time and resources required to correct problems.
This further results in a more streamlined production process and can lead to increased productivity, reducing production costs and increasing profitability.
Enhanced Brand Reputation:
Quality control can enhance a food manufacturer's brand reputation by ensuring that products meet the highest standards for quality and safety.
This can lead to increased consumer trust and loyalty, as well as positive word-of-mouth recommendations. A strong brand reputation can also help food manufacturers differentiate themselves from competitors and increase market share.
Reduced Costs and Waste:
Quality control can help food manufacturers reduce costs and waste by identifying and addressing quality issues early in the production process. By reducing the amount of rework and scrap, manufacturers can reduce production costs and increase profitability.
Additionally, by ensuring that products meet quality standards, manufacturers can reduce the risk of recalls and associated costs, such as fines and legal fees.
To sum up, quality control is essential to the achievement of food manufacturing activities. Food producers may produce high-quality products that satisfy consumer expectations and generate profits for the company through enhancing brand reputation, enhancing productivity and efficiency, decreasing costs, and minimizing waste.
Following, we’ve discussed some real-world examples of successful quality control implementation in food manufacturing. Let’s learn:
Case Study 1: Nestle Purina PetCare
Nestle Purina PetCare is a global leader in the production of pet food products. The company has implemented a robust quality control program to ensure that its products meet the highest standards for safety and quality.
The program includes regular testing of raw materials, finished products, and packaging materials, as well as audits of suppliers and manufacturing facilities. Nestle Purina PetCare also uses advanced technology, such as X-ray machines and metal detectors, to detect foreign objects in its products.
As a result of its quality control program, Nestle Purina PetCare has a low rate of product recalls and has built a strong reputation for producing high-quality pet food products.
Case Study 2: Chobani
Chobani is a leading producer of Greek yogurt and other dairy products. The company has implemented a comprehensive quality control program to ensure the safety and quality of its products.
The program includes regular testing of raw materials and finished products, as well as audits of suppliers and manufacturing facilities. Chobani also uses advanced technology, such as a microbial monitoring system, to detect any potential contaminants in its products.
In addition to its quality control program, Chobani has also implemented a system of continuous improvement, which involves regularly reviewing and analyzing data to identify opportunities for improving the quality of its products.
As a result of its quality control program and continuous improvement efforts, Chobani has built a strong reputation for producing high-quality dairy products and has experienced significant growth in the market.
Case Study 3: Mars Petcare
Mars Petcare is a global leader in the production of pet food products. The company has implemented a comprehensive quality control program to ensure the safety and quality of its products.
The program includes regular testing of raw materials, finished products, and packaging materials, as well as audits of suppliers and manufacturing facilities. Mars Petcare also uses advanced technology, such as high-pressure processing, to ensure the safety of its products.
In addition to its quality control program, Mars Petcare has also implemented a system of continuous improvement, which involves regularly reviewing and analyzing data to identify opportunities for improving the quality of its products.
As a result of its quality control program and continuous improvement efforts, Mars Petcare has a low rate of product recalls and has built a strong reputation for producing high-quality pet food products. The company has also been recognized for its commitment to sustainability and social responsibility in its operations.
In conclusion, quality control is a critical process in food manufacturing that helps ensure the safety and quality of food products. Through the use of various tools and techniques, including inspection and testing, sampling plans, quality assurance programs, and quality control software, manufacturers can monitor and control the production process to ensure that products meet the required standards for safety, nutritional value, and sensory quality.
While there are challenges in implementing quality control in food manufacturing, such as complying with food safety regulations and managing the supply chain, the benefits are significant. These benefits include improved product quality, increased efficiency and productivity, enhanced brand reputation, and reduced costs and waste.
By implementing a comprehensive quality control program, manufacturers can build a strong reputation for producing high-quality products and drive growth in the market. Ultimately, quality control is essential for ensuring that food products are safe and meet customer expectations for quality and taste, and for maintaining the trust of consumers in the food industry.
Deskera 's integrated financial planning tools allow investors to better plan their investments and track their progress. It can help investors make decisions faster and more accurately. Deskera Books enables you to manage your accounts and finances more effectively. Maintain sound accounting practices by automating accounting operations such as billing, invoicing, and payment processing.
Deskera CRM is a strong solution that manages your sales and assists you in closing agreements quickly. It not only allows you to do critical duties such as lead generation via email, but it also provides you with a comprehensive view of your sales funnel.
Deskera People is a simple tool for taking control of your human resource management functions. The technology not only speeds up payroll processing but also allows you to manage all other activities such as overtime, benefits, bonuses, training programs, and much more. This is your chance to grow your business, increase earnings, and improve the efficiency of the entire production process.
Final Takeaways
We've arrived at the last section of this guide. Let's have a look at some of the most important points to remember:
- Quality control is essential for preventing foodborne illnesses and ensuring that food products are safe for consumption. By identifying and controlling potential hazards in the production process, quality control can help prevent contamination of food products.
- Proactive quality control, also known as preventive quality control, is a proactive approach to quality control that focuses on preventing quality issues before they occur. Furthermore, it is a systematic and continuous process of identifying potential quality problems, analyzing their root causes, and taking preventive actions to eliminate them.
- Reactive quality control, also known as corrective quality control, is a reactive approach to quality control that focuses on correcting quality issues after they have occurred. It further involves detecting and addressing quality issues through inspection, testing, and analysis of non-conforming products, customer complaints, and other quality-related incidents.
Related Articles
How Plastics Manufacturing Can Innovate and Collaborate to Thrive

Will the Dog Days of Summer Slow Down US Manufacturing in 2024?

Quality Control and Assurance in Contract Manufacturing
Hey! Try Deskera Now!
Everything to Run Your Business
Get Accounting, CRM & Payroll in one integrated package with Deskera All-in-One .
Significance of quality control and quality assurance in pharmaceutical Industries
Quality control (QC) and quality assurance (QA) are two crucial components in the pharmaceutical industry that play a vital role in ensuring the safety, efficacy, and quality of pharmaceutical products. These processes are integral to meeting regulatory requirements, maintaining consumer trust, and ultimately contributing to public health. In this comprehensive exploration, we will delve into the significance of quality control and quality assurance in the pharmaceutical industry, examining their respective roles, key principles, regulatory frameworks, and emerging trends.
Table of Contents
Quality Control (QC):
1. definition and scope:.
Quality control in the pharmaceutical industry involves a set of activities and techniques used to evaluate the quality of raw materials, intermediate products, and finished pharmaceutical products. It encompasses both physical and chemical testing, as well as the monitoring of manufacturing processes to ensure compliance with predefined quality standards.
2. Role in Ensuring Product Quality:
QC is instrumental in ensuring that pharmaceutical products meet established specifications and are free from defects or contamination. Through rigorous testing and analysis, QC helps identify and rectify deviations from quality standards, preventing the release of substandard or unsafe products into the market.
3. Types of QC Tests:
- Chemical Testing: Involves analyzing the chemical composition of raw materials and finished products to ensure they meet predetermined specifications.
- Microbiological Testing: Focuses on detecting and controlling microbial contamination, critical in pharmaceutical products where sterility is often paramount.
- Physical Testing: Includes assessments of product appearance, stability, dissolution, and other physical characteristics.
4. Regulatory Compliance:
- Good Manufacturing Practice (GMP): QC activities must adhere to GMP regulations, which outline the minimum requirements for the methods, facilities, and controls used in the manufacturing process.
- Regulatory Agencies: Organizations like the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency) set stringent guidelines for QC processes to ensure product safety and efficacy.
5. Challenges and Advances:
- Technological Advances: Implementation of cutting-edge technologies, such as chromatography, spectroscopy, and automation, enhances the efficiency and accuracy of QC testing.
- Globalization Challenges: As pharmaceutical supply chains become more global, QC faces challenges in maintaining consistency and uniformity across diverse manufacturing facilities.
Quality Assurance (QA):
Quality assurance in the pharmaceutical industry is a comprehensive system of processes, procedures, and audits designed to ensure that the entire pharmaceutical development and manufacturing process consistently meets predefined quality standards.
You May Like: Overview on Green Chemistry
QA is a proactive approach aimed at preventing defects and deviations before they occur, ensuring that the overall quality management system is robust and effective. It involves continuous monitoring and improvement of processes.
3. Key Principles of QA:
- Risk Management: Identifying potential risks and implementing measures to mitigate or eliminate them is a central aspect of QA.
- Documentation and Record Keeping: Thorough documentation of processes, procedures, and outcomes is essential for traceability and accountability.
- Training and Education: Ensuring that personnel are well-trained and knowledgeable is crucial for maintaining consistent quality.
- ICH Guidelines: International Conference on Harmonisation (ICH) guidelines provide a global standard for the development, registration, and post-approval of pharmaceutical products, emphasizing the importance of QA.
- Audits and Inspections: Regulatory bodies conduct regular audits to assess a company’s compliance with GMP and other quality standards.
- Complex Supply Chains: QA faces challenges in ensuring the quality of pharmaceuticals with the globalization of supply chains. Advanced tracking and traceability technologies help address these challenges.
- Data Integrity: Ensuring the integrity of data is critical for QA. Advances in data management systems and technologies contribute to maintaining data integrity.
Significance of QC and QA in Pharmaceutical Industries:
1. patient safety and efficacy:.
- Ensuring that pharmaceutical products meet stringent quality standards is fundamental to patient safety and the efficacy of medical treatments.
- Rigorous QC testing detects impurities, contaminants, or deviations that could compromise the safety of the end-user.
2. Regulatory Compliance:
- Adhering to QC and QA practices is mandatory for regulatory compliance, as outlined by agencies such as the FDA, EMA, and other global regulatory bodies.
- Non-compliance can result in severe consequences, including product recalls, legal actions, and damage to a company’s reputation.
3. Consumer Trust:
- QC and QA contribute to building and maintaining consumer trust. Patients and healthcare providers rely on the pharmaceutical industry to deliver safe and effective products.
- A strong commitment to quality enhances the reputation of pharmaceutical companies and fosters trust among stakeholders.
4. Cost-Effectiveness:
- Implementing robust QC and QA processes early in the product development lifecycle can prevent costly errors and deviations.
- A proactive QA approach minimizes the need for corrective actions, reducing the risk of production delays and financial losses.
5. Continuous Improvement:
- QA emphasizes a culture of continuous improvement, encouraging companies to assess and enhance their processes continually.
- Regular QC testing and QA audits identify areas for improvement, leading to more efficient and effective manufacturing processes.
6. Globalization and Supply Chain Management:
- As pharmaceutical supply chains become more global, QC and QA play a crucial role in maintaining consistency and quality across diverse manufacturing sites.
- Harmonization of quality standards, as promoted by ICH guidelines, facilitates global collaboration and ensures uniformity in pharmaceutical quality.
7. Technological Advancements:
- The integration of advanced technologies in QC, such as real-time monitoring, data analytics, and artificial intelligence, improves the accuracy and efficiency of quality assessments.
- Automated processes reduce the likelihood of human errors and enhance the reliability of QC results.
8. Data Integrity and Transparency:
- Ensuring data integrity in both QC testing and QA processes is essential for transparency and accountability.
- Advanced information systems and blockchain technology contribute to maintaining the integrity of data throughout the pharmaceutical lifecycle.
Conclusion:
In conclusion, quality control and quality assurance are indispensable components of the pharmaceutical industry, collectively ensuring the safety, efficacy, and quality of pharmaceutical products. QC focuses on rigorous testing and analysis to detect and rectify deviations from established quality standards, while QA takes a proactive approach to prevent defects and ensure the overall robustness of the quality management system.
The significance of QC and QA extends beyond regulatory compliance; it directly impacts patient safety, consumer trust, and the overall success of pharmaceutical companies. Embracing technological advancements, addressing challenges posed by globalization, and fostering a culture of continuous improvement are essential for pharmaceutical companies to navigate the evolving landscape of QC and QA in the 21st century.
As the pharmaceutical industry continues to advance, QC and QA will remain at the forefront, driving innovation, ensuring compliance, and ultimately contributing to the development of safe and effective medications for global populations.
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

Five Ways to Improve Quality Control in Manufacturing
- By Cory Kyle
- May 10, 2024
Quality control matters. And there’s no other industry where this is more clear than manufacturing.

Quality control matters. And there’s no other industry where this is more clear than manufacturing. Other businesses may care about quality too. However, few businesses are as close to the results of their products as manufacturing. That means that the quality of their products is the difference between success and failure.
Why quality control matters
Manufacturing is a highly competitive industry. As a result, the only way to get customers and keep their business is through the quality of your products. This means making sure that what you produce is better than your competitors. Easier said than done. Your competitors are also doing everything possible to produce outstanding products. If you want to keep up, your business needs to be able to quickly identify product weaknesses and repeatedly produce high-quality products. That’s where quality control enters the picture. Quality control does more than just ensure you produce high-quality products. It gives you the control you need to keep making high-quality products.
What is quality control?
But what actually is quality control? It’s easy to think of it as nothing more than a way to ensure your products meet a certain level of standard. However, that’s only one way to make use of quality control. Quality control also allows you to deeply study your manufacturing chain. Knowing where things are breaking down means being able to fix the problems. More importantly, it means being able to improve the manufacturing chain. These minor improvements can easily serve as a strong foundation for future improvements.
Five ways to improve quality control in manufacturing
1. Study your manufacturing chain. Your manufacturing chain is the heart of your company. To have an effective quality control system in place, you need to know every aspect of how you assemble your products. Understanding this process is essential in being able to optimize and improve the final product. Likewise, having a deep understanding of the process allows you to find ways to streamline and raise the level of quality of your manufacturing chain. The only way to ensure quality control does its job is by having a strong foundation already in place. 2. Properly train your employees. No matter how effective your quality control system is, it’s useless without skilled employees. Properly training your employees is essential in manufacturing. Things are no different when it comes to quality control. Your employees need to be able to identify and resolve the problems that your quality control system exposes. Not only that, they also need to understand why it’s important to focus on quality control. And the closer your employees are to the actual production chain, the more important it becomes. Furthermore, having properly trained employees makes it a lot easier to change things in the future. 3. Make use of statistics. One of the strongest strengths of quality control is the versatility of how you can implement different systems. Using statistics, for example, is a great way of improving the final result of your products. Acceptance sampling or quality control charts, for example, are easy to implement and provide a strong form of quality control. These and other methods that utilize statistics are usually cheaper and easier to implement than more traditional inspection methods. What makes statistic-based quality control great, however, is that there are methods that focus on the end product as well as the actual processes. 4. Regularly test your systems. One of the best ways of improving quality control is to make sure that your equipment is performing effectively and optimally. One of the most useful ways to check that your equipment is working at 100% is through randomized testing. By holding unscheduled tests, quality control can be measured, as well as how effectively employees are following safety protocols. As a result, the importance of employee safety can be reinforced throughout your workforce. Aside from improving the safety of your employees, these random tests also help to improve how well your employees can efficiently do their jobs. 5. Understand which methods work best. Because quality control contains many different methods, it’s essential that you understand which method is best for your specific manufacturing process. Perishables like food, for example, require a 100% inspection method in order to comply with many stringent regulations. However, the nature of this method makes it highly expensive. Knowing which quality control method is best suited to your business is essential.
Better quality control leads to better products
When it comes to manufacturing, the quality of the final product is the cornerstone of any business. By improving your quality control, you ensure that the products you produce are worth your customer’s time and money. By spending the extra resources necessary to improve your quality control, you open up the path to continued success.
About The Author
Cory Kyle is the director of Marketing at BST North America.
Did you enjoy this great article?
Check out our free e-newsletters to read more great articles..

- > Statistics
What is Statistical Quality Control?
- Neelam Tyagi
- Aug 26, 2021

Basic Terminologies
Statistics: Statistics means data, a good amount of data. Or simply, the collaborative study of accumulation, analysis, interpretation and presentation of massive volumes of data.
“Statistics are valuable representations of data that assist in the analysis and decision making process”
Statistical tools: Applications of statistical methods in order to visualize, interpret and anticipate outcomes over collected data.
Quality “ a characteristic of fitness for purpose at lowest cost” , or “degree of perfection that suffices the customer requirements”. Quality can be defined as “the entirety of features and characteristics for products and services satisfying implicit and explicit demands of customers.
Control: An approach of measuring and inspecting a certain phenomenon for a product or a service, control suggests when to inspect, and how much to inspect. The system includes feedback to understand the causes for poor quality and necessary corrective steps. The control system basically determines the quality characteristics of an item, correlates the same with predefined quality standards and distinguishes between defective items from non-defectives ones.
Quality control: Quality control is one of the most important tools deployed to check the definite level of quality of products, or services. In todays’ highly competitive business environment, quality control has evolved as a prominent tool and a critical factor via any successful industry to ensure standard quality. In 1982, Peters and Waterman recognized quality as a crucial element in the virtue of excellence.
(Related blog: Types of statistical analysis )
Therefore, quality control is the employment of appropriate techniques and activities in order to accomplish, sustain and upgrade the quality of products and services and to satisfy customer’s needs in terms of price, safety, availability, reliability, usability, etc.
The method employs statistical techniques based on probability theory to establish standards of quality and uphold it in the most economical manner. ( From )
Let’s understand the technique of applying statistical methods for quality control systems.
Statistical Quality Control (SQC)
Employing a number of statistical methods, SQC validates the quality of premium goods and services. In 1924, Walter A. Shewhart produced the basic ideas for statistical quality control, since after the area of SQC has been scattered its foundation with extensive work of researchers, quality controlled philosophers and statisticians.
Making use of statistical tools and techniques in order to monitor and manage product quality across various industries including food, pharmaceutical and manufacturing units, the process is named as Statistical Quality Control. The method can be conducted as
A part of production process,
A part of last-minute quality control check
A part of eventual check by quality control department
“Statistical quality control can be simply defined as an economic & effective system of maintaining & improving the quality of outputs throughout the whole operating process of specification, production & inspection based on continuous testing with random samples.” -YA LUN CHOU
Statistical quality control techniques are extremely important for operating the estimable variations embedded in almost all manufacturing processes. Such variations arise due to raw material, consistency of product elements, processing machines, techniques deployed and packaging applications. Moreover, any of these factors or combination of two can impact the eventual quality of finished product.
The method incorporates legislation allowing manufacturing units to make sure that the finished product must contain the net quantity mentioned in packaging. Any overfilled quantity can lead to financial loss for the manufacturer and therefore must be avoided. Fill control, validating weight and weight variation are hugely deployed statistical quality control techniques that make use of weights of individual products in the statistical data analysis .
In case of pharmaceutical goods, such as tablets, pills, capsules, syrups etc, the standard weight must not be exceeded the upper limit that saves consumers from taking high doses of active ingredients that might result in severe consequences. At the same time, the weight shouldn’t be too less, if not the drug might not be effective. In this case, the weight variation based statistical quality control test is used to ensure the consistency of the dosage unit, and also to support product identity, reliability and quality.
Another example would be, in the production of food and beverages, it is required to inspect the weight of packages rendering quick confirmation such that filled quantities fulfil the legal necessities. Any deviation from standard value signifies errors in the production process, imprecise ingredient-quantities leading to impactful consequences.
In addition to this, while confirming consumer satisfaction, safety and compliance with regulations, SQC with weight determination is highly important. Though, it is recommended to employ actual balances or measuring scales and software suitable for particular applications.
(Must read: Statistical data distribution models )
For example, SQC serves as a medium allowing manufacturers to attain maximum benefits by following controlled testing of manufactured products. Using this procedure, a manufacturing team can investigate the range of products with certain values that can be expected to reside under some existing conditions. The information is precisely validated for a number of similar products and be informed to the producer and the purchaser.
In addition to this, the information determines compliance with specifications and looks at whether the manufacturing process/unit is capable of producing products within its unit. Also, if existing specifications are unable to meet the end outcome or economically unacceptable, then quality control data is helpful in providing minimum criteria for developing the improved standards.
(Suggested blog: Sampling distribution )
Advantages of Statistical Quality Control
One of the excellent scientific tools, SQC has the following advantages;
Cost reduction: In this method, only a fragmentary output is inspected to ensure the quality of product, therefore probe cost would be reduced greatly.
Huge efficiency: Inspection of a fractional portion requires lesser time and tedium in comparison to holistic investigation leading to huge escalation in efficiency and production.
Easier to use: Pitching SQC not only reduces process variability but also makes the process of production-in-control. Even, it is much to apply by an individual without having such extensive specialized guidance.
Authentic anticipation: SQC is the most preeminent approach that can accurately predict future production. To ensure the degree of perfection and product performance, SQC provides a great predictability.
Prior fault detection: Any deviation from standard control limits depicts signs of danger in the underlying production process that invites necessary corrective measurement to be taken earlier. SQC is helpful in early detection of faults.
While in holistic inspection, unnecessary fluctuations under quality control process would be detected in the final stage, but for the time being numerous defective items have already been produced.
In such conditions, SQC (using chart controls) enables a pictorial view of how the production process is performing and where curative steps must be accounted for for smooth functioning of the process. ( Source )
SQC vs SPC
Both SQC and SPC support smooth operations in order to escalate efficiencies, desired output and optimized performance while playing a key role in overall success in operations, but in different ways. Lets’ understand the difference;
SPC: is the procedure of collecting and computing parameters of a process such as speed, pressure, vernier caliper etc with respect to standard values using various statistical methods validating values must reside within limits while aiming to minimize variation and execute to achieve desired/optimum targets.
SQC: is the process of compiling and determining data on the subject of particular specifications regarding a product and to meet requirements, for example, size, weight, texture etc. while aiming at validating process outcomes to meet the user requirements or the next stage of the manufacturing process.
SPC is responsible for reduction of variation in processes and run efficiently, in contrast to this, SQC facilitates manufacturers to accomplish user requirements.
For example, in food and beverage manufacturing there are various numbers of different products being produced, SPC monitors that operations are executing effectively at their entirety, SQC controls measurable quality characteristics used during production so that finished products must live up with customer requirements/expectations.
(Read also: Data types in statistics )
“Statistical quality control should be viewed as a kit of tools which may influence decisions to the functions of specification, production or inspection. -EUGENE L. GRANT
SQC has turned out to be a vital platform as a business operation that is deployed to enhance productivity and sustain competitive advantages. The method reflects a systematic approach of efficient statistically-oriented experimentation particularly in terms of characterization, optimization, sample acceptance along with ensuring active determination/inspection of deployment process with respect to real-world applications.
Share Blog :
Be a part of our Instagram community
Trending blogs
5 Factors Influencing Consumer Behavior
Elasticity of Demand and its Types
What is PESTLE Analysis? Everything you need to know about it
An Overview of Descriptive Analysis
What is Managerial Economics? Definition, Types, Nature, Principles, and Scope
5 Factors Affecting the Price Elasticity of Demand (PED)
6 Major Branches of Artificial Intelligence (AI)
Scope of Managerial Economics
Dijkstra’s Algorithm: The Shortest Path Algorithm
Different Types of Research Methods
Latest Comments
- ERP Software /
- Cyrus Biz ERP /
How ERP Helps with Manufacturing Planning and Control
Table of Contents
Related Posts
- How Does ERP Help Improve Business Operations
- 7 Ways How ERP Solutions Facilitate Digital Transformation Initiatives
- What is ERP Finance Module: Features, Benefits & Example
- Tips to Improve the Academic Process of Your School with School ERP
- What Is ERP (Enterprise Resource Planning) & Why Do You Need It

Summary: If you remain constantly irked by rising costs and production hold-ups, let ERP take charge! Discover in detail within this blog how ERP software holds the key to reshaping the game for manufacturers like you. Learn how ERP streamlines data management, automates processes, and offers immediate insights to enhance production planning, reduce waste, and guarantee punctual deliveries.
In the hectic world of manufacturing planning and control, the pressure to deliver products on time is endless! You often end up sinking knee-deep into production chaos. Deadlines loom, your team scrambles for materials, and that nagging feeling of “there has to be a better way” just won’t quit. That sounds quite familiar, right?
The fact is that we have all been there! But what if you came across a solution that could streamline your workflow, minimize waste, and even help you sleep better at night? That’s ERP, your secret weapon to excel in manufacturing planning & control.
You might have this question in mind: Can ERP help manufacturers with production planning and control with ease, indeed? Here, you should realize that ERP isn’t some ordinary piece of technology taking over your factory.
It’s more like a super-powered organizing hero – a digital filing cabinet on steroid that keeps everything you need in one place. Experience the convenience of accessing real-time information on materials, orders, and production with just a click.
No more panicky searches for lost spreadsheets or relying solely on memory. The system empowers you to make informed and smart decisions, optimize production runs, and finally get those deadlines under control.
So, be ready to bid farewell to the production headache and embrace a smoother, more efficient way of working. This blog will be your guide to the world of ERP, showing you how it can transform your manufacturing life for good. Let’s explore how ERP software can turn your production woes into a thing of the past!
What is the Meaning and Significance of ERP?

If you have a feeling that you’re conducting an orchestra of manufacturing chaos—with inventory disappearing faster than smoke rings, orders piling up like a skyscraper about to collapse, and deadlines whooshing by like a runaway freight train—fear not, fellow manufacturer!
There’s a powerful tool waiting to bring harmony to your operations: ERP, or Enterprise Resource Planning. Think of ERP as your digital maestro. It doesn’t wield a baton, but it conducts a symphony of information, integrating crucial aspects such as inventory, production, and customer orders. It’s like having real-time data right at your fingertips—a transparent view of your entire operation.
Suppose you need to know if you have enough of a certain material to fulfill a new order; ERP can tell you that in a flash. And if you are struggling to figure out how to schedule production runs without creating bottlenecks, ERP production planning can help you optimize that too.
Here’s how the magic works: ERP streamlines communication, eliminates information silos, and empowers you to make informed and smart decisions. This translates to reduced waste, smoother production flows, and ultimately, a sigh of relief.
All in all, it’s a powerful tool that can give you a significant edge in the competitive world of manufacturing production planning and control.
ERP, or enterprise resource planning, can be best defined as the central nervous system of all your manufacturing operations. It’s a desirable piece of software that connects all the crucial aspects of your business—inventory, production schedules, customer orders—on a central platform.
You can also name it a digital filing cabinet on steroids that stores real-time data pertaining to everything you need to make informed and viable decisions and keep your production running without a hitch.
Here’s how ERP’s potential can help you conquer the production chaos and become a master of manufacturing planning and control:
1. Crystal Ball Planning
No more flying blind! Renowned ERP solutions like Cyrus Biz let you see into the future. By analyzing historical data on things such as sales trends and production times, this software helps you forecast demand and plan production accordingly.

Cyrus Biz ERP
Starting Price
Price on Request
This means you can simply avoid costly stock-outs or overproduction and ensure you have the right material on hand to meet customer needs.
2. The Efficiency Enhancer
Production bottlenecks can slow down your entire operation like a clogged drain. Cyrus Biz ERP production planning solutions help you identify these bottlenecks by analyzing real-time data on machine utilization and worker schedules.
With this special knowledge, you can optimize production runs, streamline workflows, and get the most out of your resources.
3. Material Matchmaker
You may feel like you are playing inventory whack-a-mole. That’s when Cyrus Biz ERP takes the guesswork out of material management. With its real-time inventory tracking, you can easily determine the precise quantity of each material you currently possess.
This helps you avoid running low on crucial components and prevents costly delays. ERP can even generate automatic purchase orders when the stock dips below a certain level, ensuring a smooth flow of materials.
4. The Deadline Do-Gooder
Deadlines are the greatest bane of any manufacturer’s life. Cyrus Biz helps you stay on top of tight deadlines by providing real-time visibility into your production schedule. You have visibility into the progress of every order, can spot potential delays, and make real-time adjustments as needed.
This transparency allows you to communicate realistic delivery times to customers and avoid those dreaded last-minute scrambles.
5. Quality King
Needless to mention, it’s essential to keep product quality consistent. One of the best ERP solutions , Cyrus Biz, can integrate with quality control systems, allowing you to track defects and identify areas for improvement.
This proactive approach helps you prevent quality issues before they become problems, ensuring your products meet customer expectations.
There is no need to settle for manufacturing disorders any longer! Top-notch ERP production planning solutions, such as Cyrus Biz ERP, can be your secret weapon for transforming your production floor from a chaotic orchestra into a well-rehearsed melody.
The smart system functions as your digital conductor, ensuring a seamless flow of information between inventory, production, and customer orders. It will give you real-time data at your fingertips, allowing you to forecast demand, optimize production runs, and eliminate costly bottlenecks.
ERP empowers you to make informed and smart decisions, minimize waste, and finally conquer those looming deadlines. So it’s time to seize the opportunity for smoother, more efficient manufacturing planning & control. Say goodbye to your production worries once and for all!
The Techjockey content team is a passionate group of writers and editors dedicated to helping businesses make informed software buying decisions. We have a deep understanding of the Indian software market and the challenges that businesses face when choosing the right software for their needs. We are committed... Read more
Related Question and Answers
QuickBooks is not an ERP system, it's accounting software. The most widely used ERP is SAP.
- Write Answer
Sap ERP is considered as the best ERP software for CA (chartered accountant).
Yes, Wrkplan is a good software. It can be beneficial for businesses as it offers HR and payroll management, leave and attendance tracking, and other features.
To generate a barcode in Odoo form view you can use two modules, i.e., manual generation and automatic generation. Follow the given steps to generate a barcode:
Steps for manual generation:
- Go to the barcode rule option
- Set a rule accordingly
- Set the base for the same
- Click on the generate option
- Click Enter
Steps for auto generation:
- Set up a rule accordingly
- Go to the generate base option, set a base value
- Click on the generate barcode option to generate
- Then, click enter
NetSuite, an ERP system, has barcode generation capabilities. You can also track the information or transaction status by generating barcodes.
Still Have a Question in Mind?
Get answered by real users or software experts
Recommended Products

DataNote ERP
Safal Infosoft Pvt. Ltd.

Dynamics 365 Business Central
Microsoft Corporation

Deskera All in One

Focus Softnet

Calotropis Software

SourcePro ERP
SourcePro Infotech

Orion 11s ERP
3i Infotech

Compserv Consultants Pvt.Ltd

Eazy Business

Gway Tech Solutions

Nippon Data

HostBooks ERP

d'Katia Career Book ERP

Royalsoft ERP
Royalsoft Solutions

Intelligic ERP software
Intelligic Software

Mummy Software Corporation
Trending Posts
21 Best Free Online Typing Software and App in 2024
February 7, 2024

Top 14 Free Bulk SMS Apps for Marketing in 2024
August 29, 2023

10 Best Open Source and Free Library Management Software
March 28, 2024

20 Top Free Bulk WhatsApp Sender Tools Online in India 2024

21 Best Technical Analysis Software for Stock Trading in India 2024
April 18, 2024

Top 27 Gaming Websites for PC, Android & iOS – Download Free Games Online 2024
September 18, 2023

16 Best Stock Screeners in India for Day Trading 2024
January 17, 2024

12 Best Hidden Call Recorder Apps for Android & iPhone in 2024
April 16, 2024
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Health Department HAI/AR Programs
- Cleaning in Global Healthcare Settings
- Infection Control Guidance
- Introduction to the Patient Notification Toolkit
- HAI Prevention and Control for Healthcare
- Public Health and Policy Strategies
- HAI Prevention, Control and Outbreak Response for Public Health and Healthcare
- Prevention Epicenters
- Healthcare-Associated Infections - Community Interface Activity (HAIC)
- HAIs: Reports and Data
- Preventing MDROs
- Laboratory Resources
- Antibiotic Prescribing and Use
- Antimicrobial Resistance
- Infection Control for Healthcare Providers
- Safe Injection Practices and Your Health
- Methicillin-resistant Staphylococcus aureus (MRSA) Basics
Conclusion & Further Reading
At a glance.
Concluding the CDC's best practices for environmental cleaning procedures in global healthcare settings.
The materials on this page were created for use in global healthcare facilities with limited resources, particularly in low- and middle-income countries. Environmental cleaning resources designed for U.S. healthcare facilities can be found at Healthcare Environment Infection Prevention .
The importance of environmental cleaning as a fundamental IPC intervention cannot be overstated. Environmental contamination plays a role in transmission of HAIs, which are a significant burden globally and disproportionately affect those in resource-limited settings.
The best practices contained in this document provide the framework for implementing effective environmental cleaning procedures and programs in healthcare facilities in resource-limited settings. While they are structured to be most relevant for resource-limited settings, implementing all the best practices for cleaning supplies and equipment, cleaning procedures, and, most importantly, for cleaning programs will require a strong and sustained commitment, including dedicated staff time and resources. Strong leadership support for environmental cleaning and recognition of the important role that it plays in IPC is a critical prerequisite to implementing these best practices.
It is important that environmental cleaning is implemented within the framework of a functional IPC program, while ensuring that a multi-sectorial approach is taken to enable engagement and coordination across the various sectors (e.g., WASH) that have a role to play to ensure a functional and effective cleaning program.
A toolkit for guiding the implementation of these best practices is currently under development. It will use the step-wise approach that IPC improvement programs use extensively. It will also address the need to prioritize actions that target the highest transmission risk based on environmental contamination and patient vulnerability, as well as the foundational program elements which are needed first in order to build an effective and robust environmental cleaning program over time.
Further Reading
Best practices from high-resource settings.
- Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for Environmental Infection Control in Health-Care Facilities . 2003.
- Ontario Agency for Health Protection and Promotion (Public Health Ontario), Provincial Infectious Diseases Advisory Committee. Best practices for environmental cleaning for prevention and control of infections in all health care settings. 3rd ed. [PDF – 250 pages] . Toronto, ON: Queen's Printer for Ontario; 2018.
- The Provincial Infection Control Network of British Columbia (PICNet). British Columbia Best Practices for Environmental Cleaning for Prevention and Control of Infection in All Healthcare Settings and Programs [PDF – 158 pages] . 2016.
- National Patient Safety Agency (England and Wales) – The revised health care cleaning manual [PDF – 174 pages] . 2009.
- Government of South Australia. Cleaning Standards for Healthcare Facilities [PDF – 48 pages] . 2017.
- Targeted training package for cleaning staff, generated for resource-limited settings. Soapbox Collaborative, UK. TEACH CLEAN . 2019.
- Allegranzi B, Begheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, Pittet D. 2011. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. The Lancet; 377:9761.
- Weber DJ, Rutala WA, Miller MB, et al. 2010. Role of hospital surfaces in the transmission of emerging healthcare-associated pathogens: norovirus, Clostridium difficile , and Acinetobacter species. Am J Infect Control 38:S25–S33.
- Otter JA, Yezlli S, Salkeld J, French G. 2013. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. American Journal of Infection Control; 41: S6-S11.
- Huang SS, Datta R, Platt R. 2006. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Archs Intern Med; 166:1945-1951.
- Drees M, Snydman DR, Schmid CH, et al. 2008. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clin Infect Dis; 46:678-685.
- Nseir S, Blazejewski C, Lubret R, Wallet F, Courcol R, Durocher A. 2011. Risk of acquiring multidrug-resistant Gram-negative bacilli from prior room occupants in the intensive care unit. Clin Microbiol Infect; 17:1201-1208.
- Datta R, Platt R, Yokoe DS, Huang SS. 2011. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Archs Intern Med; 171:491-494.
- Shaughnessy MK, Micielli RL, DePestel DD, et al. 2011. Evaluation of hospital room assignment and acquisition of Clostridium difficile Infect Control Hosp Epidemiol; 32:201-206.
- Ajao AO, Johnson K, Harris AD, et al. 2013. Risk of acquiring extended spectrum b-lactamase-producing Klebsiella species and Escherichia coli from prior room occupants in the intensive care unit. Infect Control Hosp Epidemiol; 34:453-458.
- Mitchell BG, Digney W, Ferguson JK. 2014. Prior room occupancy increases risk of methicillin-resistant Staphylococcus aureus acquisition. Healthcare Infect; 19:135-140.
- Kramer A, Schwebke I, Kampf G. 2006. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis; 6:130.
- Dancer SJ. 2014. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev; 27:665-690.
- Falk PS, Winnike J, Woodmansee C, Desai M, Mayhall CG. 2000. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol 21:575-82.
- Rampling A, Wiseman S, Davis L, Hyett AP, Walbridge AN, Payne GC, et al. 2001. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 49:109-16.
- Wilcox M., Fawley W., Wigglesworth N., Parnell P., Verity P., Freeman J. (2003) Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile J Hosp Infect 54: 109–114.
- Denton M, Wilcox MH, Parnell P, Green D, Keer V, Hawkey PM, et al. 2004. Role of environmental cleaning in controlling an outbreak of Acinetobacter baumannii on a neurosurgical intensive care unit. J Hosp Infect 56:106-10.
- Hayden MK, Bonten MJ, Blom DW, Lyle EA, van de Vijver DA, Weinstein RA. 2006. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 42:1552-60.
- McMullen K., Zack J., Coopersmith C., Kollef M., Dubberke E., Warren D. (2007) Use of hypochlorite solution to decrease rates of Clostridium difficile -associated diarrhea. Infect Control Hosp Epidemiol 28: 205–207.
- Dancer SJ, White LF, Lamb J, Girvan EK, Robertson C. 2009. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC Med 7:28.
- Wilson AP, Smyth D, Moore G, Singleton J, Jackson R, Gant V, et al. 2011. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 39:651-8.
- Grabsch EA, Mahony AA, Cameron DR, Martin RD, Heland M, Davey P, et al. 2012. Significant reduction in vancomycin-resistant Enterococcus colonization and bacteraemia after introduction of a bleach-based cleaning-disinfection programme. J Hosp Infect 82:234-42.
- Mitchell BG, Hall L, White N, Barnett AG, Halton K, Paterson DL, Riley TV, Gardner A, Page K, Farrington A, Gericke CA, Graves N. 2019. An environmental cleaning bundle and health-care-associated infections in hospitals (REACH): a multicenter, randomized trial . The Lancet Infectious Diseases.
- WHO/UNICEF Joint Monitoring Programme for Water Supply, Sanitation and Hygiene (JMP), 2019. WASH in Health Care Facilities: Global Baseline Report 2019 . WHO:Geneva.
- Rutala WA, Weber DJ. 2016. Monitoring and improving the effectiveness of surface cleaning and disinfection. American Journal of Infection Control 44: e69-e76
HAIs are associated with medical devices, complications following surgery, transmission between patients and healthcare workers, antibiotic overuse, and more.
For Everyone
Health care providers, public health.

Predicting local control of brain metastases after stereotactic radiosurgery with clinical, radiomics and deep learning features
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected] [email protected]
- Info/History
- Preview PDF
Background and purpose Timely identification of Local Failure (LF) after stereotactic radiosurgery offers the opportunity for appropriate treatment modifications that may result in improved treatment outcomes, patient survival, and quality of life. Previous studies showed that the addition of either radiomics or deep learning features to clinical features increased the accuracy of the models in predicting Local Control (LC) of brain metastases after stereotactic radiosurgery. To date, however, no study combined both radiomics and deep learning features together with clinical features to develop machine learning algorithms to predict LC of brain metastases. In this study, we examined whether a model trained with a combination of all these features could predict LC better than models trained with only a subset of these features.
Materials and methods Pre-treatment brain MRIs and clinical data were collected retrospectively for 129 patients at the Gamma Knife Center of Elisabeth-TweeSteden Hospital (ETZ), Tilburg, The Netherlands. The patients were split into 103 patients for training and 26 patients for testing. The segment-based radiomics features were extracted using the radiomics feature extractor of the python radiomics package. The deep learning features were extracted using a fine-tuned 3D ResNet model and then combined with the clinical and radiomics features. A Random Forest classifier was trained with the training data set and then tested with the test data set. The performance was compared across 4 different models trained with clinical features only, clinical and radiomics features, clinical and deep learning features, and clinical, radiomics and deep learning features.
Results The prediction model with only clinical variables provided an Area Under the receiver operating characteristic Curve (AUC) of 0.82 and an accuracy of 75.6%. The prediction model with the combination of clinical and radiomics features demonstrated an AUC of 0.880 and an accuracy of 83.3% whereas the prediction model with the combination of clinical and deep learning features demonstrated an AUC of 0.863 and an accuracy of 78.3%. The best prediction performance was associated with the model that combined the clinical, radiomics and deep learning features with an AUC of 0.886 and 87% accuracy.
Conclusion Machine learning models trained on radiomics features and deep learning features combined with patient characteristics show good potential to predict LC after stereotactic radiosurgery with high accuracy. The promising findings from this study demonstrate the potential for early prediction of stereotactic radiosurgery outcome for brain metastasis prior to treatment initiation and might offer the opportunity for appropriate treatment modifications that may result in improved treatment outcomes, patient survival, and quality of life.
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
This research is supported by KWF Kankerbestrijding and NWO Domain AES, as part of their joint strategic research programme: Technology for Oncology IL. The collaboration project is co-funded by the PPP Allowance made available by Health Holland, Top Sector Life Sciences & Health, to stimulate public-private partnerships.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
This study was approved by the ETZ science office and by the Ethics Review Board at Tilburg University.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability
The data used for this study is available at ETZ and is accessible after approval from the ETZ Science office.
List of abbreviations
View the discussion thread.
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Radiology and Imaging
- Addiction Medicine (324)
- Allergy and Immunology (627)
- Anesthesia (163)
- Cardiovascular Medicine (2372)
- Dentistry and Oral Medicine (289)
- Dermatology (206)
- Emergency Medicine (379)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (836)
- Epidemiology (11769)
- Forensic Medicine (10)
- Gastroenterology (702)
- Genetic and Genomic Medicine (3738)
- Geriatric Medicine (350)
- Health Economics (633)
- Health Informatics (2395)
- Health Policy (932)
- Health Systems and Quality Improvement (896)
- Hematology (341)
- HIV/AIDS (782)
- Infectious Diseases (except HIV/AIDS) (13308)
- Intensive Care and Critical Care Medicine (767)
- Medical Education (365)
- Medical Ethics (104)
- Nephrology (398)
- Neurology (3501)
- Nursing (198)
- Nutrition (525)
- Obstetrics and Gynecology (674)
- Occupational and Environmental Health (663)
- Oncology (1823)
- Ophthalmology (537)
- Orthopedics (219)
- Otolaryngology (287)
- Pain Medicine (232)
- Palliative Medicine (66)
- Pathology (446)
- Pediatrics (1033)
- Pharmacology and Therapeutics (426)
- Primary Care Research (420)
- Psychiatry and Clinical Psychology (3175)
- Public and Global Health (6139)
- Radiology and Imaging (1280)
- Rehabilitation Medicine and Physical Therapy (747)
- Respiratory Medicine (826)
- Rheumatology (379)
- Sexual and Reproductive Health (372)
- Sports Medicine (323)
- Surgery (402)
- Toxicology (50)
- Transplantation (172)
- Urology (145)

IMAGES
VIDEO
COMMENTS
Conclusion: Quality control plays a crucial role in ensuring that businesses deliver high-quality products and services, meeting customer expectations and regulatory requirements. Companies can develop and implement effective QC systems that contribute to long-term success by understanding its importance, benefits, and key strategies. ...
Conclusion. Quality Control (QC) is an indispensable component in product and service delivery, playing a critical role in ensuring customer satisfaction, maintaining brand reputation, and enhancing overall business performance. The commitment to maintaining high-quality standards is essential in today's competitive market, where customer ...
Conclusion - Quality Control Inspection . As we draw the curtains on our exploration of Quality Control Inspection, it becomes evident that this process is much more than a mere checkpoint in the production cycle. It is a vital cog in the machinery of manufacturing and service industries, ensuring that the wheels of quality and excellence ...
Quality control (QC) is an important process that any company or business must undertake, especially if the production is in the form of products or services. Running a QC can be easier with the help of inventory management software. A fully integrated inventory management system enables businesses to monitor stock transfers and quality control.
Conclusion. Quality Control (QC) in marketing and business constitutes a meticulously designed set of systematic processes and practices aimed at ensuring that products, services, or processes ...
Quality Control. Quality control is the process of inspecting products to ensure that they meet the required standards. Quality control is based on inspection and aims to take the defects out. Quality control was introduced during the industrial revolution in the 20th century following the rise of mass production.
Quality has to be delivered by the study personnel and the QA Unit has to assure the quality through an umbrella function. Each staff member of an organization has QC tasks, which are part of the processes to ensure the validity of data. The QA tasks are linked to the QA program, which is independent of the processes.
Quality Control (QC) is an indispensable aspect of various industries, ensuring that products and services adhere to predefined standards. In the manufacturing sector, QC involves rigorous inspection and testing of raw materials, intermediate components, and final products to maintain consistent quality and minimize defects.
(d) Addressing multipleissues related to engagement quality control reviews (e.g., the selection of the engagement quality control reviewer and their independence from the engagement team, the professional skepticism exercised by the reviewer and the objective, extent, timing, and documentation of the review). 3.
Fundamentals of quality control and improvement / Amitava Mitra. - 3rd ed. p. cm. Includes index. ISBN 978--470-22653-7 (cloth) 1. Quality control - Statistical methods. I. Title. TS156.M54 2008 658.4'0.13-dc22 2007036433 Printed in the United States of America 109 8 7 65
72 Laboratory Quality Management System Role in quality management system What is QC? 6-1: Introduction QC is the part of quality management focused on fulfi lling quality requirements (ISO 9000:2000 [3.2.10]). Simply put, it is examining "control" materials of known substances along with patient samples to monitor the accuracy and ...
Start your free trial. Chapter 4 Conclusion A quality management strategy for an organization that is seeking to or has already implemented a continuous improvement program must include respect for people, a transparent and …. - Selection from Quality Management in a Lean Health Care Environment [Book]
A responsive and customized quality control and assurance process can help companies create products/services that always adhere to their benchmarked standards. The quality control process typically looks like this: Incoming Materials Quality Control; The first step is monitoring the quality of incoming materials.
This means that we will continue to seek new methods applicable to the various control techniques, such as operations research, value engineering, creative techniques, management, and economics. In addition, we will initiate their application to quality control. The Osaka office of the Japanese Union of Scientists and Engineers has established ...
In conclusion, integrating robust quality control measures into your manufacturing process is essential for achieving sustainable growth. Embrace the six key principles outlined earlier - customer focus, leadership commitment, employee involvement, continuous improvement mindset, data-driven decision-making approach, and supplier ...
Conclusion. When it comes to our focus, we understand that quality control is a product-oriented process. ... When quality control makes sure the end product meets the quality requirements, quality assurance makes sure that the process of manufacturing the product does adhere to standards. Therefore, quality assurance can be identified as a ...
Enterprises usually adopt some quality practices to control the product quality during the manufacturing process in order to assure the delivery of qualitative good products to customers. The quality practices or quality management systems adopted by industries will further evolve due to the changes of quality concepts as time goes by. This chapter discusses the change of quality concepts and ...
Quality control is a process that makes sure things are good enough to use. It involves checking products and services to make sure they meet certain standards. This could mean making sure a toy is safe for kids to play with, or checking that a car is built correctly and works properly. Quality control helps make sure things are made well and ...
Quality Control process . Quality Control process involves a series of systematic steps and methods to identify and rectify defects or deviations. These are discussed below: 1) Inspection: Inspection is a fundamental step in QC, where products or services are carefully examined to identify any visible defects from established standards. This ...
Conclusion. In conclusion, quality control in pharmaceuticals is a crucial aspect of ensuring the safety, efficacy, and consistency of medications delivered to patients worldwide. By adhering to stringent regulatory requirements, implementing robust analytical techniques, and embracing best practices, pharmaceutical companies can maintain ...
Final Conclusion. In conclusion, quality control is a critical process in food manufacturing that helps ensure the safety and quality of food products. Through the use of various tools and techniques, including inspection and testing, sampling plans, quality assurance programs, and quality control software, manufacturers can monitor and control ...
In conclusion, quality control and quality assurance are indispensable components of the pharmaceutical industry, collectively ensuring the safety, efficacy, and quality of pharmaceutical products. QC focuses on rigorous testing and analysis to detect and rectify deviations from established quality standards, while QA takes a proactive approach ...
Five ways to improve quality control in manufacturing. 1. Study your manufacturing chain. Your manufacturing chain is the heart of your company. To have an effective quality control system in place, you need to know every aspect of how you assemble your products. Understanding this process is essential in being able to optimize and improve the ...
Conclusion "Statistical quality control should be viewed as a kit of tools which may influence decisions to the functions of specification, production or inspection. -EUGENE L. GRANT . SQC has turned out to be a vital platform as a business operation that is deployed to enhance productivity and sustain competitive advantages. The method ...
QA is a proactive approach that controls defects before they occur. Whereas QC is a reactive approach that detects deficiencies that may have slipped through QA. QA is more process-oriented and enables the prevention of quality issues during manufacturing. QC is product-oriented and identifies problems within the final product to ensure ...
By separating quality control from other auditing standards as a specific area of focus, the Sarbanes-Oxley Act acknowledged that a robust system of quality control is critical to audit quality. ... Conclusion. The road to this adoption began well in advance of the Concept Release in December 2019. So much has changed in the profession since ...
Controlling air pollution can bring significant benefits to human health, visibility, agriculture and forestry, construction materials and natural ecosystems. 22 Air quality control initiatives face uncertainties about the sources of pollution and their contribution to air pollutions levels in a given city. 23 Air quality management strategies ...
One of the best ERP solutions, Cyrus Biz, can integrate with quality control systems, allowing you to track defects and identify areas for improvement. ... Conclusion. There is no need to settle for manufacturing disorders any longer! Top-notch ERP production planning solutions, such as Cyrus Biz ERP, can be your secret weapon for transforming ...
The importance of environmental cleaning as a fundamental IPC intervention cannot be overstated. Environmental contamination plays a role in transmission of HAIs, which are a significant burden globally and disproportionately affect those in resource-limited settings. The best practices contained in this document provide the framework for ...
Background and purpose: Timely identification of Local Failure (LF) after stereotactic radiosurgery offers the opportunity for appropriate treatment modifications that may result in improved treatment outcomes, patient survival, and quality of life. Previous studies showed that the addition of either radiomics or deep learning features to clinical features increased the accuracy of the models ...