Watch CBS News

FDA approves new Alzheimer's treatment, donanemab from Eli Lilly
By Alexander Tin
Edited By Paula Cohen
Updated on: July 3, 2024 / 12:22 PM EDT / CBS News
The Food and Drug Administration approved a new Alzheimer's treatment called donanemab on Tuesday, clearing the way for the third addition to a new class of drugs aimed at slowing the brain's decline in patients facing the early stages of the disease.
Branded as Kisunla by drugmaker Eli Lilly, donanemab's approval follows years of setbacks and delays in getting the experimental Alzheimer's treatment to market, despite promising clinical trial results .
Eli Lilly says the drug will be available within weeks following the approval.
"Kisunla demonstrated very meaningful results for people with early symptomatic Alzheimer's disease, who urgently need effective treatment options. We know these medicines have the greatest potential benefit when people are treated earlier in their disease, and we are working hard in partnership with others to improve detection and diagnosis," Anne White, president of Eli Lilly's neuroscience arm, said in a news release .
CBS News chief medical correspondent Dr. Jon LaPook described it as "an incremental advance" that may slow the decline of cognitive function for people in the early stages of the disease.
"We're not reversing it — it's not a cure. But if you can slow the decline, I think that's important," LaPook said on "CBS Mornings."
The FDA previously rebuffed Eli Lilly's request for accelerated approval last year, citing concerns about its long-term safety data. After Eli Lilly submitted more data to the FDA, the company said it expected the agency would decide on approval by the end of March.
That decision was delayed after the FDA scheduled an advisory committee to wrestle with questions over the drug's safety issues and how effectiveness was measured in its trials. The panel ultimately voted unanimously last month in favor of the drug's benefits outweighing its risks, for patients in the early stages of Alzheimer's disease.
How does donanemab work?
Donanemab is part of a class of Alzheimer's treatments called anti-amyloid monoclonal antibodies, which work to combat the buildup of a protein in the brain called amyloid plaque that has been linked to Alzheimer's disease.
The antibody in donanemab targets amyloid plaques that have built up in patients by binding to and removing them from the brain.
Patients in Eli Lilly's trials were given intravenous donanemab infusions for around half an hour, every four weeks. Depending on brain scans measuring amyloid levels in the brain, patients were able to stop taking the drug after as early as six months.
In its trials , the company says almost half of patients were able to meaningfully clear out amyloid after around a year after taking the drug. Patients saw no "rebound of amyloid plaque" in the year after treatment wrapped up.
The only other Alzheimer's treatment that works in a similar way on the market is lecanemab, branded as Leqembi by drugmakers Eisai and Biogen. An earlier drug called aducanumab ( marketed as Aduhelm ) from Biogen was discontinued in January.
Beyond effectiveness, Eli Lilly has also touted a handful of other reasons that patients might choose their drug instead of lecanemab.
Donanemab infusions are shorter and less frequent. Trial participants were also able to stop using the drug after amyloid plaque was removed, "which can result in lower treatment costs and fewer infusions," a company spokesperson said.
How much will the treatment cost?
Eli Lilly says it will launch with a list price that adds up to $32,000 for 12 months of treatment, though the actual cost will depend on how long patients take the drug. Some patients in the clinical trials were able to stop the treatment after six months, based on results from brain scans, while others took it for 18 months.
Last year, Eisai defended its list price of $26,500 per year when it launched sales of Leqembi.
But most patients also do not pay the full list price for prescription drugs. For patients with Medicare Part B, the Centers for Medicare and Medicaid Services said donanemab will be covered in the same way it covers lecanemab (Leqembi), with patients paying a 20% coinsurance after they meet their deductible. These patients will need to get the drug from doctors enrolled in a study gathering data tracking its effectiveness.
"CMS is committed to helping people get timely access to treatments and improving care for people with Alzheimer's disease and their families," a CMS spokesperson said.
Eli Lilly noted in a statement: "The potential to complete treatment after a limited-duration course of therapy, along with 30-minute infusions once per month, could result in lower patient out-of-pocket treatment costs and fewer infusions compared to other amyloid-targeting therapies."
How effective was the treatment for Alzheimer's symptoms?
Eli Lilly measured donanemab's effectiveness primarily through rating scales designed to measure the cognitive and functional decline caused by dementia symptoms in patients with early stages of Alzhiemer's.
Compared with patients who received only a placebo, Eli Lilly said those who got the drug saw their decline slow. The gap widened over time, slowing by 22% overall at 76 weeks after first starting the donanemab infusions.
"Importantly, the magnitude of impact on these clinical endpoints meets, and in several respects exceeds prior approvals for demonstration of clinical benefit and effectiveness," the company said of the results in a briefing document given to the FDA panel.
The company says this translated to effectively prolonging how long it took until patients stepped down into the next stage of Alzheimer's disease.
What are the side effects of donanemab?
The labels for all of the anti-amyloid treatments greenlighted by the FDA to date for Alzheimer's already carry a boxed warning about "amyloid-related imaging abnormalities" that can show up on MRI scans.
While these abnormalities generally result in no symptoms, they have been linked to rare but serious issues in some patients like brain function issues and seizures.
These abnormalities were seen in around a quarter of participants in Eli Lilly's trials of donanemab. At least five deaths were reported in donanemab recipients in patients with these kinds of abnormalities, mostly from hemorrhages in the brain.
Eli Lilly says their trials of donanemab tested the drug in harder to treat patients than other treatments studied around the same time. That means the trial included older trial participants as well as those with a gene called APOE ε4 that can increase the risk of Alzheimer's as well as these abnormalities.
Close to 1 in 10 trial participants who took donanemab also experienced a reaction to the infusion, compared to 0.5% of placebo participants. The most common symptoms included chills, skin reddening, nausea, shortness of breath, headache and chest pain.
Approximately 3% of donanemab-treated participants developed hypersensitivity to the infusion, including 0.3% who had a severe allergic reaction.
- Alzheimer's Disease
Alexander Tin is a digital reporter for CBS News based in the Washington, D.C. bureau. He covers the Biden administration's public health agencies, including the federal response to infectious disease outbreaks like COVID-19.
More from CBS News

FDA bans ingredient found in some citrus-flavored sodas

Fake therapist fooled hundreds online until she died, state records say

Defense witnesses in Sen. Bob Menendez's bribery trial begin testimony

How drug shortages can make or break a patient's recovery
MIT Technology Review
- Newsletters
New breakthroughs on Alzheimer’s
MIT scientists have pinpointed the first brain cells to show signs of neurodegeneration in the disorder and identified a peptide that holds potential as a treatment.
- Anne Trafton archive page
Neuronal hyperactivity and the gradual loss of neuron function are key features of Alzheimer’s disease. Now researchers led by Li-Huei Tsai, director of MIT’s Picower Institute for Learning and Memory, have identified the cells most susceptible to this damage, suggesting a good target for treatment. Even more exciting, Tsai and her colleagues have found a way to reverse neurodegeneration and other symptoms by interfering with an enzyme that is typically overactive in the brains of Alzheimer’s patients.
In one study , the researchers used single-cell RNA sequencing to distinguish two populations of neurons in the mammillary bodies, a pair of structures in the hypothalamus that play a role in memory and are affected early in the disease. Previous work by Tsai’s lab found that they had the highest density of amyloid beta plaques, abnormal clumps of protein that are thought to cause many Alzheimer’s symptoms.
The researchers found that neurons in the lateral mammillary body showed much more hyperactivity and degeneration than those in the larger medial mamillary body. They also found that this damage led to memory impairments in mice and that they could reverse those impairments with a drug used to treat epilepsy.
In the other study , the researchers treated mice with a peptide that blocks a hyperactive version of an enzyme called CDK5, which plays an important role in development of the central nervous system. They found dramatic reductions in neurodegeneration and DNA damage in the brain, and the mice got better at tasks such as learning to navigate a water maze.
CDK5 is activated by a smaller protein known as P35, allowing it to add a phosphate molecule to its targets. However, in Alzheimer’s and other neurodegenerative diseases, P35 breaks down into a smaller protein called P25, which allows CDK5 to phosphorylate other molecules—including the Tau protein, leading to the Tau tangles that are another characteristic of Alzheimer’s.
Pharmaceutical companies have tried to target P25 with small-molecule drugs, but these drugs also interfere with other essential enzymes. The MIT team instead used a peptide—a string of amino acids, in this case a sequence matching that of a CDK5 segment that is critical to binding P25.
In tests on neurons in a lab dish, the researchers found that treatment with the peptide moderately reduced CDK5 activity. But in a mouse model that has hyperactive CDK5, they saw myriad beneficial effects, including reductions in DNA damage, neural inflammation, and neuron loss.
The treatment also produced dramatic improvements in a different mouse model of Alzheimer’s, which has a mutant form of the Tau protein. Tsai hypothesizes that the peptide might confer resilience to cognitive impairment in the brains of people with Tau tangles.
“We found that the effect of this peptide is just remarkable,” she says. “We saw wonderful effects in terms of reducing neurodegeneration and neuroinflammatory responses, and even rescuing behavior deficits.”
The researchers hope the peptide could eventually be used as a treatment not only for Alzheimer’s but for frontotemporal dementia, HIV-induced dementia, diabetes-linked cognitive impairment, and other conditions.
Keep Reading
Most popular, this grim but revolutionary dna technology is changing how we respond to mass disasters.
After hundreds went missing in Maui’s deadly fires, rapid DNA analysis helped identify victims within just a few hours and bring families some closure more quickly than ever before. But it also previews a dark future marked by increasingly frequent catastrophic events.
- Erika Hayasaki archive page
What’s next for bird flu vaccines
If we want our vaccine production process to be more robust and faster, we’ll have to stop relying on chicken eggs.
- Cassandra Willyard archive page
Google helped make an exquisitely detailed map of a tiny piece of the human brain
A small brain sample was sliced into 5,000 pieces, and machine learning helped stitch it back together.
Why Google’s AI Overviews gets things wrong
Google’s new AI search feature is a mess. So why is it telling us to eat rocks and gluey pizza, and can it be fixed?
- Rhiannon Williams archive page
Stay connected
Get the latest updates from mit technology review.
Discover special offers, top stories, upcoming events, and more.
Thank you for submitting your email!
It looks like something went wrong.
We’re having trouble saving your preferences. Try refreshing this page and updating them one more time. If you continue to get this message, reach out to us at [email protected] with a list of newsletters you’d like to receive.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Should colon cancer screening start at 40?

Stroke risk higher for the chronically lonely
Testing fitness of aging brain
Start of new era for alzheimer’s treatment.
Alvin Powell
Harvard Staff Writer
Expert discusses recent lecanemab trial, why it appears to offer hope for those with deadly disease
Researchers say we appear to be at the start of a new era for Alzheimer’s treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady, called amyloid-beta, and removing it from the body. Experts say the results offer hope that the slow, inexorable loss of memory and eventual death brought by Alzheimer’s may one day be a thing of the past.
The Gazette spoke with Scott McGinnis , an assistant professor of neurology at Harvard Medical School and Alzheimer’s disease expert at Brigham and Women’s Hospital , about the results and a new clinical trial testing whether the same drug given even earlier can prevent its progression.
Scott McGinnis
GAZETTE: The results of the Clarity AD trial have some saying we’ve entered a new era in Alzheimer’s treatment. Do you agree?
McGINNIS: It’s appropriate to consider it a new era in Alzheimer’s treatment. Until we obtained the results of this study, trials suggested that the only mode of treatment was what we would call a “symptomatic therapeutic.” That might give a modest boost to cognitive performance — to memory and thinking and performance in usual daily activities. But a symptomatic drug does not act on the fundamental pathophysiology, the mechanisms, of the disease. The Clarity AD study was the first that unambiguously suggested a disease-modifying effect with clear clinical benefit. A couple of weeks ago, we also learned a study with a second drug, donanemab, yielded similar results.
GAZETTE: Hasn’t amyloid beta, which forms Alzheimer’s characteristic plaques in the brain and which was the target in this study, been a target in previous trials that have not been effective?
McGINNIS: That’s true. Amyloid beta removal has been the most widely studied mechanism in the field. Over the last 15 to 20 years, we’ve been trying to lower beta amyloid, and we’ve been uncertain about the benefits until this point. Unfavorable results in study after study contributed to a debate in the field about how important beta amyloid is in the disease process. To be fair, this debate is not completely settled, and the results of Clarity AD do not suggest that lecanemab is a cure for the disease. The results do, however, provide enough evidence to support the hypothesis that there is a disease-modifying effect via amyloid removal.
GAZETTE: Do we know how much of the decline in Alzheimer’s is due to beta amyloid?
McGINNIS: There are two proteins that define Alzheimer’s disease. The gold standard for diagnosing Alzheimer’s disease is identifying amyloid beta plaques and tau neurofibrillary tangles. We know that amyloid beta plaques form in the brain early, prior to accumulation of tau and prior to changes in memory and thinking. In fact, the levels and locations of tau accumulation correlate much better with symptoms than the levels and locations of amyloid. But amyloid might directly “fuel the fire” to accelerated changes in tau and other downstream mechanisms, a hypothesis supported by basic science research and the findings in Clarity AD that treatment with lecanemab lowered levels of not just amyloid beta but also levels of tau and neurodegeneration in the blood and cerebrospinal fluid.
GAZETTE: In the Clarity AD trial, what’s the magnitude of the effect they saw?
McGINNIS: The relevant standards in the trial — set by the FDA and others — were to see two clinical benefits for the drug to be considered effective. One was a benefit on tests of memory and thinking, a cognitive benefit. The other was a benefit in terms of the performance in usual daily activities, a functional benefit. Lecanemab met both of these standards by slowing the rate of decline by approximately 25 to 35 percent compared to placebo on measures of cognitive and functional decline over the 18-month studies.

“In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal.”
Steven M. Smith
GAZETTE: What are the key questions that remain?
McGINNIS: An important question relates to the stages at which the interventions were done. The study was done in subjects with mild cognitive impairment and mild Alzheimer dementia. People who have mild cognitive impairment have retained their independence in instrumental activities of daily living — for example, driving, taking medications, managing finances, errands, chores — but have cognitive and memory changes beyond what we would attribute to normal aging. When people transition to mild dementia, they’re a bit further along. The study was for people within that spectrum but there’s some reason to believe that intervening even earlier might be more effective, as is the case with many other medical conditions.
We’re doing a study here called the AHEAD study that is investigating the effects of lecanemab when administered earlier, in cognitively normal individuals who have elevated brain amyloid, to see whether we see a preventative benefit. The hope is that we would at least see a delay to onset of cognitive impairment and a favorable effect not only on amyloid biomarkers, but other biomarkers that might capture progression of the disease.
GAZETTE: Is anybody in that study treatment yet or are you still enrolling?
McGINNIS: There’s a rolling enrollment, so there are people who are in the double-blind phase of treatment, receiving either the drug or the placebo. But the enrollment target hasn’t been reached yet so we’re still accepting new participants.
GAZETTE: Is it likely that we may see drug cocktails that go after tau and amyloid? Is that a future approach?
More like this

Newly identified genetic variant protects against Alzheimer’s

Using AI to target Alzheimer’s

Excessive napping and Alzheimer’s linked in study
McGINNIS: It has not yet been tried, but those of us in the field are very excited at the prospect of these studies. There’s been a lot of work in recent years on developing therapeutics that target tau, and I think we’re on the cusp of some important breakthroughs. This is key, considering evidence that spreading of tau from cell to cell might contribute to progression of the disease. Additionally, for some time, we’ve had a suspicion that we will likely have to target multiple different aspects of the disease process, as is the case with most types of cancer treatment. Many in our field believe that we will obtain the most success when we identify the most pertinent mechanisms for subgroups of people with Alzheimer’s disease and then specifically target those mechanisms. Examples might include metabolic dysfunction, inflammation, and problems with elements of cellular processing, including mitochondrial functioning and processing old or damaged proteins. Multi-drug trials represent a natural next step.
GAZETTE: What about side effects from this drug?
McGINNIS: We’ve known for a long time that drugs in this class, antibodies that harness the power of the immune system to remove amyloid, carry a risk of causing swelling in the brain. In most cases, it’s asymptomatic and just detected by MRI scan. In Clarity AD, while 12 to 13 percent of participants receiving lecanemab had some level of swelling detected by MRI, it was symptomatic in only about 3 percent of participants and mild in most of those cases.
Another potential side effect is bleeding in the brain or on the surface of the brain. When we see bleeding, it’s usually very small, pinpoint areas of bleeding in the brain that are also asymptomatic. A subset of people with Alzheimer’s disease who don’t receive any treatment are going to have these because they have amyloid in their blood vessels, and it’s important that we screen for this with an MRI scan before a person receives treatment. In Clarity AD, we saw a rate of 9 percent in the placebo group and about 17 percent in the treatment group, many of those cases in conjunction with swelling and mostly asymptomatic.
The scenario that everybody worries about is a hemorrhagic stroke, a larger area of bleeding. That was much less common in this study, less than 1 percent of people. Unlike similar studies, this study allowed subjects to be on anticoagulation medications, which thin the blood to prevent or treat clots. The rate of macro hemorrhage — larger bleeds — was between 2 and 3 percent in the anticoagulated participants. There were some highly publicized cases including a patient who had a stroke, presented for treatment, received a medication to dissolve clots, then had a pretty bad hemorrhage. If the drug gets full FDA approval, is covered by insurance, and becomes clinically available, most physicians are probably not going to give it to people who are on anticoagulation. These are questions that we’ll have to work out as we learn more about the drug from ongoing research.
GAZETTE: Has this study, and these recent developments in the field, had an effect on patients?
McGINNIS: It has had a considerable impact. There’s a lot of interest in the possibility of receiving this drug or a similar drug, but our patients and their family members understand that this is not a cure. They understand that we’re talking about slowing down a rate of decline. In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal. So there’s hope. There’s optimism. Our patients, particularly patients who are at earlier stages of the disease, have their lives to live and are really interested in living life fully. Anything that can help them do that for a longer period of time is welcome.
Share this article
You might like.

Amid surging early onset rates, Harvard experts say cost, effectiveness, equity must be considered, along with other ways to evaluate

Study of adults over 50 examines how feelings boost threat over time

Most voters back cognitive exams for older politicians. What do they measure?
When should Harvard speak out?
Institutional Voice Working Group provides a roadmap in new report
Had a bad experience meditating? You're not alone.
Altered states of consciousness through yoga, mindfulness more common than thought and mostly beneficial, study finds — though clinicians ill-equipped to help those who struggle
College sees strong yield for students accepted to Class of 2028
Financial aid was a critical factor, dean says

FDA approves new Alzheimer's disease drug donanemab in the US
Our Director of Research responds to news that the FDA has approved new Alzheimer's disease drug donanemab in the US.
The Food and Drug Administration (FDA) has announced its approval in the US of the drug donanemab, a ground-breaking new treatment for early Alzheimer’s disease .
Fiona Carragher, Director of Research and Influencing at Alzheimer’s Society, said: “Donanemab is now the third drug aiming to slow down Alzheimer’s disease to be approved in the US, marking another step forward in the fight against dementia, the biggest health and social care challenge of our time.
We’re proud that Alzheimer’s Society funded research over thirty years ago which identified amyloid as important in the development of Alzheimer’s disease and has led to this moment today.
What does this mean for people in the UK?
“People affected by Alzheimer's disease in the UK will wonder what this means for them - particularly as regulators did not approve aducanumab, which was also approved in the US a few years ago.
There are still many hurdles before donanemab could be available on the NHS.
"We now need to wait for the UK regulatory authority, the Medicines and Healthcare products Regulatory Agency (MHRA), to conduct its review of the donanemab data, and for The National Institute for Health and Care Excellenc (NICE )to evaluate its cost-effectiveness.
“This drug is only suitable for people in the early stages of Alzheimer’s disease, and until we fix dementia diagnosis in the UK, only small numbers of people will get the early, accurate diagnosis needed to access the treatment if it’s approved here.
"Right now, a third of people with dementia in the UK do not receive a diagnosis at all, so we need to see urgent investment into the specialist workforce and equipment needed to diagnose as many people as possible."
A call to the NHS
“Donanemab is just one of nearly 130 potential treatments for Alzheimer’s disease being tested in trials, so it’s paramount we ramp up preparation for their arrival and roll-out.
“Alzheimer’s Society is urgently calling on the NHS to publish plans for how they intend to improve early diagnosis and deliver new treatments to the people who desperately need them."
What is donanemab?
Find out more about donanemab, the new Alzheimer's disease drug approved in the US.
- Email this page to a friend.
Further reading
What kind of information would you like to read? Use the button below to choose between help, advice and real stories.
Choose one or more options
- Information
- Real stories
- Dementia directory
Donald Trump, Joe Biden and dementia: Why not to diagnose from a distance

Norfolk and Suffolk Dementia Conference
The Norfolk and Suffolk Dementia Conference will bring together key decision makers, practitioners, researchers, carers and experts by experience – people with lived experience of dementia – who understand the urgent necessity of tackling the disease, from across Norfolk and Suffolk.
Dementia Healthcare Inequalities Initiative funding call
We know that people with dementia and their carers face numerous inequalities from diagnosis through to the end of life, and something needs to be done.
Read about our new research initiative which aims to give people living with dementia a fairer deal throughout their dementia journeys.

Alzheimer’s Society Dementia Research Nurses
There is an urgent need for widespread, accelerated progress in dementia research. To help with lifechanging discoveries, the dementia clinical trial landscape needs to change. Alzheimer’s Society is committed to making the hope of new treatment breakthroughs a reality.

UK organisations release new dementia manifesto ahead of General Election
UK dementia organisations have joined forces to publish a new dementia manifesto ahead of the General Election on 4 July 2024.

How much does dementia care cost?

Research call to improve end of life experience for people with dementia
Alzheimer's Society has partnered with Marie Curie to fund £1 million for new, impact-focused research that can improve the end-of-life experience for people with dementia and those who care for and support them.
Sign up for dementia support by email
Our regular support email includes the latest dementia advice, resources, real stories and more.
You can change what you receive at any time and we will never sell your details to third parties. Here’s our Privacy Policy .

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Seven recent papers amplify advances in Alzheimer’s research
Alzheimer's Disease Biomarkers Dementias Neuroscience

AMP AD uses an open-science research model that makes all data and methods rapidly available to the research community at large through the data sharing infrastructure, the AD Knowledge Portal. Since the Portal’s launch in 2015, more than 3,000 researchers world wide from the academic, biotech, and pharmaceutical industry sectors have used the data resources for research on Alzheimer’s and related dementias.
Alzheimer’s is a complex disease, and as it slowly develops, many normal biological processes in the brain and the body go awry, from inflammation, to blood vessels damage and neuronal death. Seven recent AMP AD reports showcase research advances related to the discovery of new drug candidate targets, identification of molecular subtypes of the disease, and new potential biomarkers that can serve as the basis for a precision medicine approach to therapy development.
Identifying ATP6VA1 gene as a candidate target for treatment: Researchers at the Icahn School of Medicine at Mount Sinai in New York generated several types of molecular data from 364 brain donors at different stages of Alzheimer’s. Using network modeling, a way to show data and its relationships, the team identified large sets of genes associated with the disease. Among the thousands of molecular changes associated with Alzheimer’s, the expression of a set of neuronal genes (neuronal network) was the most disrupted. Their analyses identified ATP6VA1 as a master regulator gene of this neuronal network and demonstrated that increasing its expression genetically, or by using a pharmacologic agent, led to improving neuronal function in cultured cells and in flies. These findings were published in Neuron and pave the way for new drug discovery efforts targeting ATP6VA1 .
Finding and validating VGF gene as key regulator of Alzheimer’s: Another AMP AD study led by researchers at Icahn School of Medicine identified the VGF gene and protein as having a key role in protecting the brain against Alzheimer’s. This discovery was made possible by combining computational analyses that integrate large human Alzheimer’s molecular datasets, clinical features of Alzheimer’s, DNA variation, and data on gene- and protein expression with experimental studies in mouse models. The findings provide a new target for researchers seeking to develop drugs to treat or prevent Alzheimer’s. The report of the discovery of this gene as a key driver and its validation in mouse studies was published in Nature Communications .
Identifying different types of microglia associated with Alzheimer’s: An AMP AD research team at Columbia University conducted a study that measured the expression of genes in individual microglial cells purified from human brain samples obtained at autopsy and during neurosurgical procedures. This single cell profiling technology identified several molecular subtypes of microglia based on the pattern of gene expression. Follow-up validation studies in post mortem brain tissue showed that this microglia subtype was less abundant in Alzheimer's brains compared to control brains. These results, published in Nature Communications , will help design larger, more specific studies of the role of microglia subtypes in Alzheimer’s.
Using data to unfold and predict disease process: An AMP AD team led by researchers at Sage Bionetworks in Seattle used innovative computational approaches to make predictions about the sequence of molecular changes that lead to Alzheimer’s. The team used RNA sequencing data collected from a large collection of post-mortem tissue from Alzheimer’s and control brains. This modeling method, called the manifold learning method, predicted early-stage disease processes, such as RNA-splicing, mitochondrial function, and protein transport. Additionally, the method predicted several distinct molecular subtypes of late-onset Alzheimer’s. These predictions speak to the complex nature of the disease and the need to verify these observations in longitudinal studies where molecular signatures can be linked to different clinical features of the disease. These findings were published in Nature Communications .
Network modeling identifies molecular subtypes of Alzheimer’s: Using a large collection of human brain samples from different studies, a team led by researchers at Icahn School of Medicine also analyzed RNA sequencing data and identified three major molecular subtypes of Alzheimer’s. The subtypes, which are independent of age and disease stage, and are replicated across multiple brain regions, show how different combinations of biological pathways lead to brain degeneration. With further research and validation in larger groups, these molecular subtypes may help reveal how Alzheimer’s progresses and potential ways to slow or stop it. Their findings were published in Science Advances .
Identifying new biomarkers in spinal fluid: AMP AD researchers at Emory University identified groups of proteins (protein panels) associated with Alzheimer’s that could be identified in both brain and spinal fluid. These overlapping protein panels detected in the spinal fluid reflected changes in multiple biological process in the brain. The researchers found this by measuring 3,500 proteins in spinal fluid, and 12,000 proteins in a collection of postmortem brain samples, from patients with Alzheimer’s and cognitively normal study participants. The study also showed that these changes in the protein expression pattern were specific for Alzheimer’s. This work lays the foundation for the discovery of new fluid biomarkers for Alzheimer’s. These findings were published in Science Advances .
Investigating how being female may increase risk of Alzheimer’s: Duke University researchers and members of the Alzheimer’s Disease Metabolomic Consortium (ADMC) participating in the AMP AD program, analyzed the changes in the levels of 180 metabolites in the blood from more than 1,500 people who took part in the NIA-supported Alzheimer’s Disease Neuroimaging Initiative . The researchers reported that there are differences in a subset of blood metabolites associated with Alzheimer's based on sex and ApoE4 status. ApoE4 is the strongest Alzheimer's risk factor gene. Women with Alzheimer’s who carry the ApoE4 gene have a distinct metabolic pattern in blood. These metabolic changes suggest that females have a greater impairment of brain energy production than males. Dissecting metabolic differences in Alzheimer’s can identify specific pathways within specific patient subgroups and guide the way to personalized medicine.
The data and methods from the above studies are available and can be accessed by researchers across the world through the AD Knowledge Portal . The portal is the data repository for the AMP AD Target Discovery Program, and other NIA-supported team-science programs operating under open-science principles. Now in its sixth year, AMP AD is demonstrating the power of open science to enable the scientific community to investigate difficult scientific questions and jumpstart new drug discovery projects.
The AMP AD research teams are funded by NIA grants U01AG046152, U01AG046170, U01AG046139, U01AG046161, R01AG046171, R01AG046174, U19AG010483, U01AG042791, U01AG061357, U01AG061359, U01AG061835, and U24AG061340.
The studies outlined here were also supported by the following NIA grants (in order of appearance):
- ATP6VA1: NIA grants U01AG046170, RF1AG054014, RF1AG057440, R01AG057907, U01AG052411, R01AG062355, U01AG058635, and R01AG068030
- VGF: NIA grants U01AG046170, R01AG046170, RF1AG054014, RF1AG057440, R01AG057907, R01AG055501, U01AG046161, P50AG025688, 5R01AG053960, and 5R01AG062355
- Microglia: NIA grants U01AG046152, R01AG036836, R01AG048015, and RF1AG057473
- Disease process: NIA grants U54AG054345, RF1AG057443, P30AG10161, R01AG15819, R01AG17917, R01AG30146, R01AG36836, U01AG32984, U01AG46152, P50AG016574, R01AG032990, U01AG046139, R01AG018023, U01AG006576, U01AG006786, R01 AG025711, R01AG017216, and R01AG003949
- Subtypes: U01AG046170, RF1AG054014, RF1AG057440, R01AG057907, U01AG052411, R01AG062355, U01AG058635, R01AG068030, P30AG10161, R01AG15819, R01AG17917, R01AG30146, R01AG36836, U01AG32984, U01AG46152, U01AG52411, K01AG062683, and U01AG058635
- Spinal fluid biomarkers: NIA grants R01AG053960, R01AG057911, R01AG061800, RF1AG057471, RF1AG057470, R01AG061800, R01AG057911, R01AG057339, U01AG046161, and U01AG061357
- Female risk: NIA grants U01AG024904, P30AG10161, R01AG15819, R01AG17917, U01AG46152, U01AG61356, R01AG059093, R01AG046171, RF1AG051550, and U01AG024904, RF1AG058942, R01AG057452, R03AG054936, and RF1AG061872
These AMP AD activities relate to NIH’s AD+ADRD Research Implementation Milestone 2.A , “Create new research programs that use data-driven, systems-based approaches to integrate the study of fundamental biology of aging with neurobiology of aging and research on neurodegeneration, AD and AD-related dementias to better understand the mechanism(s) of vulnerability and resilience in AD across all levels of biologic complexity (from cellular to population level) and to gain a deeper understanding of the complex biology and integrative physiology of healthy and pathologic brain aging;” Milestone 9.B , "Accelerate the development of the next generation CNS imaging ligands and biofluid molecular signatures targeting a variety of disease processes (neuroinflammation, bioenergetic/metabolic compromise, oxidative stress, synaptic pathology) that can be used as research tools or developed into diagnostic, prognostic, theragnostic or target engagement biomarkers;" and Milestone 9.F , “Initiate studies to develop minimally invasive biomarkers for detection of cerebral amyloidosis, AD and AD-related dementias pathophysiology.”
References:
Wang M, et al. Transformative network modeling of multi-omics data reveals detailed circuits, key regulators, and potential therapeutics for Alzheimer's disease . Neuron . 2021;109(2):257-272.e14. doi:10.1016/j.neuron.2020.11.002.
Beckmann ND, et al. Multiscale causal networks identify VGF as a key regulator of Alzheimer's disease . Nature Communications. 2020;11(1): 3942. doi:10.1038/s41467-020-17405-z.
Olah M, et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer's disease . Nature Communications . 2020;11(1):6129. doi:10.1038/s41467-020-19737-2.
Mukherjee S, et al. Molecular estimation of neurodegeneration pseudotime in older brains . Nature Communications . 2020;11(1):5781. doi:10.1038/s41467-020-19622-y.
Neff RA, et al. Molecular subtyping of Alzheimer’s disease using RNA sequencing data reveals novel mechanisms and targets . Science Advances . 2021;7(2):eabb5398. doi: 10.1126/sciadv.abb5398.
Higginbotham L, et al. Integrated proteomics reveals brain-based cerebrospinal fluid biomarkers in asymptomatic and symptomatic Alzheimer's disease . Science Advances . 2020;6(43):eaaz9360. doi:10.1126/sciadv.aaz9360.
Arnold M, et al. Sex and APOE ε4 genotype modify the Alzheimer's disease serum metabolome . Nature Communications . 2020;11(1): 1148. doi:10.1038/s41467-020-14959-w.
nia.nih.gov
An official website of the National Institutes of Health

- July 2, 2024 | Scientists Uncover Brain-Boosting Potential of Vitamin B6
- July 2, 2024 | Scientists Crack the Code on Broccoli’s Health Benefits
- July 2, 2024 | Carbon Cataclysm: Scientists Shed New Light on Ancient Apocalypse That Affected the Entire Planet
- July 2, 2024 | Orion Spacecraft to Take a Test Spin in the Vacuum of Space Without Leaving Earth
- July 2, 2024 | Warning From the Deep: Ancient Extinction Holds Clues to Today’s Climate Crisis
Redefining Dementia Treatment: Berkeley Scientists Unveil Promising New Breakthrough
By University of California - Berkeley February 28, 2024
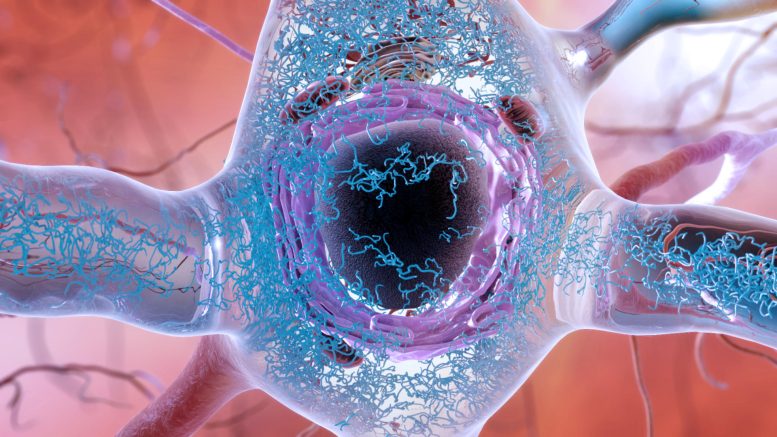
An illustration of a brain cell in a person with Alzheimer’s disease, showing the accumulation and clumping of tau proteins (blue squiggles) in the cytoplasm of a brain cell. Protein clumps, also known as aggregates, are thought to lead to cell death and dementia. New research suggests that such clumps may not cause brain cell death directly, but rather throw the cell’s response to stress off balance so that it never gets switched off. Credit: National Institute on Aging, National Institutes of Health
Research from UC Berkeley indicates that ongoing stress caused by protein aggregation is leading to the death of brain cells.
Numerous neurodegenerative conditions, including Alzheimer’s and Parkinson’s, involve the buildup of protein clusters, known as aggregates, within the brain. This phenomenon has prompted researchers to hypothesize that these protein masses are responsible for the death of brain cells. Despite this, efforts to develop treatments that break up and remove these tangled proteins have had little success.
But a new discovery by University of California, Berkeley , researchers suggests that the accumulation of aggregated proteins isn’t what kills brain cells. Rather, it’s the body’s failure to turn off these cells’ stress response.
In a study recently published in the journal Nature , the researchers reported that delivering a drug that forces the stress response to shut down saves cells that mimic a type of neurodegenerative disease known as early-onset dementia.
The Role of the SIFI Complex in Neurodegeneration
According to lead researcher Michael Rapé, the finding could offer clinicians another option for treatment for some neurodegenerative diseases, at least for those caused by mutations in the protein that switches off the cellular stress response. These include inherited diseases that lead to ataxia, or loss of muscle control, and early-onset dementia.
In addition, Rapé noted that other neurodegenerative diseases, including Mohr–Tranebjærg syndrome, childhood ataxia, and Leigh syndrome, are also characterized by stress responses in overdrive and have symptoms similar to those of the early onset dementia mimicked in the new study.
“We always thought that protein clumps directly kill neurons, for example by puncturing membrane structures within these cells. Yet, we now found that aggregates prevent the silencing of a stress response that cells originally mount to cope with bad proteins. The stress response is always on, and that’s what kills the cells,” said Rapé, head of the new division of molecular therapeutics in UC Berkeley’s Department of Molecular and Cell Biology and a Howard Hughes Medical Institute investigator. “We think that the same mechanisms may underlie more common pathologies that also show widespread aggregation, such as Alzheimer’s disease or frontotemporal dementia, but more work is needed to investigate the role of stress signaling in these diseases.”
Key to the discoveries by Rapé’s lab was the researchers’ finding that stress responses need to be turned off once a brain cell has successfully addressed a difficult situation. Rapé explained this finding to his son in simple terms: You not only need to clean up your room, but also turn out the light before going to bed. If you don’t turn off the light, you can’t fall asleep, but if you turn it off before you cleaned up your room, you would stumble if you had to get up in the dark.
Similarly, a cell has to clean up protein aggregates before turning off the stress response. If it doesn’t turn off the stress response, the cell will ultimately die.
“Aggregates don’t kill cells directly. They kill cells because they keep the light on,” he said. “But that means that you can treat these diseases, or at least the dozen or so neurodegenerative diseases that we found have kept their stress responses on. You treat them with an inhibitor that turns off the light. You don’t have to worry about completely getting rid of large aggregates, which changes how we think about treating neurodegenerative diseases. And most importantly, it makes this really doable.”
In their paper, Rapé and his colleagues describe a very large protein complex they discovered called SIFI (SIlencing Factor of the Integrated stress response). This machine serves two purposes: It cleans up aggregates and, afterward, turns off the stress response triggered by the aggregated proteins. The stress response controlled by SIFI is switched on to deal with specific intracellular problems — the abnormal accumulation of proteins that end up at the wrong location in the cell. If components of SIFI are mutated, the cell will accumulate protein clumps and experience an active stress response. But it is the stress response signaling that kills the cells.
“The SIFI complex would normally clear out the aggregating proteins. When there are aggregates around, SIFI is diverted from the stress response, and the signaling continues. When aggregates have been cleared — the room has been cleaned up before bedtime — then the SIFI is not diverted away anymore, and it can turn off the stress response,” he said. “Aggregates kind of hijack that natural stress response-silencing mechanism, interfere with it, stall it. And so that’s why silencing never happens when you have aggregates, and that’s why cells die.”
A future treatment, Rapé said, would likely involve the administration of a drug to turn off the stress response and a drug to keep SIFI turned on to clean up the aggregate mess.
Rapé, who is also the Dr. K. Peter Hirth Chair of Cancer Biology, studies the role of ubiquitin — a ubiquitous protein in the body that targets proteins for degradation — in regulating normal and disease processes in humans. In 2017, he discovered that a protein called UBR4 assembles a specific ubiquitin signal that was required for the elimination of proteins that tend to aggregate inside cells.
Only later did other researchers find that mutations in UBR4 are found in some inherited types of neurodegeneration. This discovery led Rapé to team up with colleagues at Stanford University to find out how UBR4 causes these diseases.
“This was a unique opportunity: We had an enzyme that makes an anti-aggregation signal, and when it’s mutated, it causes aggregation disease,” he said. “You put these two things together and you can say, ‘If you figure out how this UBR4 allows sustained cell survival, that probably tells you how aggregates kill cells.'”
They found that UBR4 is actually part of a much larger protein complex, which Rapé dubbed SIFI, and they found that this SIFI machinery was needed when a cell couldn’t sort proteins into its mitochondria. Such proteins that end up at the wrong location in cells tend to clump and, in turn, cause neurodegeneration.
“Surprisingly, though, we found that the core substrates of the SIFI complex were two proteins, one of which senses when proteins don’t make it into mitochondria. That protein detects that something is wrong, and it then activates a kinase that shuts down most of new protein synthesis as part of a stress response, giving the cell time to correct its problem with bringing proteins to the right location,” he said.
This kinase is also degraded through SIFI. A kinase is an enzyme that adds a phosphate group to another molecule, in this case, a protein, to regulate important activities in the cell. By helping degrade these two proteins, the SIFI complex turns off the stress response that is caused by clumpy proteins accumulating at the wrong location.
“That’s the very first time that we’ve seen a stress response turned off in an active manner by an enzyme — SIFI — that happens to be mutated in neurodegeneration,” Rapé said.
While investigating how SIFI can turn off the stress response at the right time — only after the room had been cleaned up — the researchers found that SIFI recognizes a short protein segment that acts as a kind of ZIP code that allows proteins or protein precursors to get into the mitochondria, where they are processed. When they are prevented from getting in, they accumulate in the cytoplasm, but SIFI homes in on that ZIP code to eliminate them. The ZIP code looks just like the light switch.
“When you have aggregates accumulating in the cytoplasm, now the ZIP code is still in the cytoplasm, and there’s a lot of it there,” he said. “And it’s the same signal as you would have in the proteins that you want to turn off. So it basically diverts the SIFI complex from the light switch back to the mess. SIFI tries to clean up the mess first, and it cannot turn off the light. And so when you have an aggregate in the cell, the light is always on. And if the light is always on, if stress signaling is always on, the cell will die. And that’s a problem.”
Implications for Treatment and Future Research
Rapé suspects that many intracellular protein aggregates characteristic of neurodegenerative diseases have similar consequences and may prevent the cell from switching off the stress response. If so, the fact that a drug can turn off the response and rescue brain cells bodes well for the development of treatments for potentially many neurodegenerative diseases.
Already, another stress response inhibitor, a drug called ISRIB discovered at UCSF in 2013, has been shown to improve memory in mice and reduce age-related cognitive decline.
“That means there is the prospect that by manipulating stress silencing, by turning off the light with chemicals, you might target other neurodegenerative diseases, as well,” he said. “At the very least, it’s another way we could help patients with these diseases. In the best possible way, I think it will change how we treat neurodegenerative diseases. That’s why this is a really important story, why I think it’s very exciting.”
Rapé, already a co-founder of two startups, Nurix Therapeutics Inc., and Lyterian Therapeutics, is now looking to develop therapies to silence the stress response while maintaining the cell’s cleanup of protein aggregates.
Reference: “Stress response silencing by an E3 ligase mutated in neurodegeneration” by Diane L. Haakonsen, Michael Heider, Andrew J. Ingersoll, Kayla Vodehnal, Samuel R. Witus, Takeshi Uenaka, Marius Wernig and Michael Rapé, 31 January 2024, Nature . DOI: 10.1038/s41586-023-06985-7
Co-authors with Rapé are postdoctoral fellows Diane Haakonsen, Michael Heider, and Samuel Witus and graduate student Andrew Ingersoll, all of UC Berkeley, and Kayla Vodehnal, Takeshi Uenaka, and Marius Wernig of Stanford. The work was supported primarily by the Stinehart–Reed Foundation and the National Institutes of Health (RF1 AG048131, T32MH020016-25).
More on SciTechDaily

Precision and Peril: Stunning New Image of Intuitive Machines Odysseus Landing on Moon

Challenging “Rule Breakers” – Children Will Confront Their Peers, but How They Do So Varies Across Cultures
Climate change and extreme weather will have complex effects on infectious disease transmission.

“Unusual” Findings Overturn Current Battery Wisdom

600 Million-Year-Old Time Capsule – New Discoveries From the Himalayas Shed Light on Earth’s Past

Billion Year Old Surface Water Found in Oceanic Plates
Beware the Splice of Life: This Killer Protein Causes Pancreatic Cancer

Reviving Ancient Skills to Solve Prehistoric Puzzles
Be the first to comment on "redefining dementia treatment: berkeley scientists unveil promising new breakthrough", leave a comment cancel reply.
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
- Earlier Diagnosis
- Part the Cloud
- Research Momentum
- Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
- Can Alzheimer's Disease Be Prevented?
- Brain Donation
- Navigating Treatment Options
- Aducanumab Discontinued as Alzheimer's Treatment
- Donanemab Approved for Treatment of Early Alzheimer's Disease
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Medicare Treatment Coverage
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
- Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
- Any Given Moment
- New IDEAS Study
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- RFI Amyloid PET Depletion Following Treatment
- Criteria for Diagnosis and Staging
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Zenith Fellow Awards
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Annual Conference: AAIC
- Professional Society: ISTAART
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donate Gold & Sterling Silver
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
- Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council

Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association
As the largest nonprofit funder of Alzheimer's research, the Association is committed to accelerating the global progress of new treatments, preventions and, ultimately, a cure.
Information for Researchers
Research we fund, apply for a grant, find a clinical trial, efforts we lead, the first survivor of alzheimer's is out there, but we won't get there without you., learn how alzheimer’s disease affects the brain..
Take the Brain Tour
Don't just hope for a cure. Help us find one.
Keep up with alzheimer’s news and events.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Dementia articles from across Nature Portfolio
Dementia is a syndrome that involves severe loss of cognitive abilities as a result of disease or injury. Dementia caused by traumatic brain injury is often static, whereas dementia caused by neurodegenerative disorders, such as Alzheimer's disease, is usually progressive and can eventually be fatal.
Related Subjects
- Alzheimer's disease
Latest Research and Reviews
Prolonged ventricular repolarization associated with mild cognitive impairment and white matter hyperintensities: a cross-sectional study
- Chengxuan Qiu
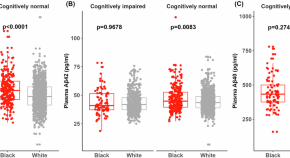
Baseline levels and longitudinal changes in plasma Aβ42/40 among Black and white individuals
Plasma samples from 324 Black and 1,547 white participants underwent analysis with C2N Diagnostics’ Precivity AD test for Aβ42 and Aβ40. Compared to white individuals, Black individuals had higher average plasma Aβ42/40 levels at baseline, consistent with a lower average level of amyloid pathology.
- Chengjie Xiong
- Jingqin Luo
- Suzanne E. Schindler
Changes in expression of VGF , SPECC1L , HLA-DRA and RANBP3L act with APOE E4 to alter risk for late onset Alzheimer’s disease
- Sergio Branciamore
- Grigoriy Gogoshin
- Amanda J. Myers
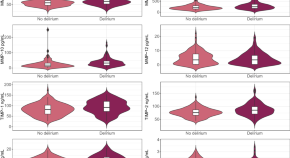
Differences in metalloproteinases and their tissue inhibitors in the cerebrospinal fluid are associated with delirium
Aksnes et al. evaluate the association between matrix metalloproteinases and their tissue inhibitors with delirium. Multivariate regression analyses find that while most associations are explained by acute trauma or pre-existing cognitive impairment, low TIMP-4 could be directly linked to delirium.
- Mari Aksnes
- Mari Haavig Schibstad
- Leiv Otto Watne
Incident traumatic spinal cord injury and risk of Alzheimer’s disease and related dementia: longitudinal case and control cohort study
- Neil Kamdar
- Elham Mahmoudi
Functional locus coeruleus imaging to investigate an ageing noradrenergic system
Functional locus coeruleus (LC) imaging revealed that older adults exhibited increased LC activation compared to younger adults, indicating possible compensatory overactivation of a structurally declining LC in ageing.
- Mareike Ludwig
- Dorothea Hämmerer
News and Comment
Non-invasive stimulation for treating cognitive impairment in alzheimer disease.
The use of non-invasive brain stimulation techniques to treat mild cognitive impairment and dementia in Alzheimer disease is expanding. Trials have produced varying results depending on the differing stimulation techniques, targeted brain regions and degrees of cognitive impairment among the treated cohorts.
- Irena Rektorová
APOE α4 homozygosity — a genetic form of Alzheimer disease?
Severe infections as a gateway to dementia
A study in Nature Aging on electronic health records from 1.7 million people in New Zealand reveals that most patients with dementia have a history of hospital-treated infection. In a dementia-free population, individuals with a severe infection were at a threefold-higher risk of dementia even 25 years later.
- Mika Kivimäki
- Keenan A. Walker
Genetic protection against Alzheimer disease
Neuronal activity drives glymphatic waste clearance.
Two new studies show that clearance of waste, including pathogenic amyloid, through the glymphatic system is driven by synchronized neuronal activity.
Five biomarkers from one cerebrospinal fluid sample to stage Alzheimer’s disease
Staging Alzheimer’s disease on the basis of the disease’s biological underpinnings might help with stratification and prognostication, both in the clinical setting and in clinical trials. We propose a staging model based on only five biomarkers, which are related to amyloid-β and tau pathologies in different ways and can be measured with a single sample of cerebrospinal fluid.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
Shots - Health News
Alzheimer's researchers are looking beyond plaques and tangles for new treatments.

Jon Hamilton

Scientists say research into Alzheimer's needs to take a broader view of how the disease affects the brain — whether that's changes in the cortex or the role of inflammation. Matt York/AP hide caption
Scientists say research into Alzheimer's needs to take a broader view of how the disease affects the brain — whether that's changes in the cortex or the role of inflammation.
The field of Alzheimer's research is branching out.
After decades of focusing on the sticky amyloid plaques and tangled tau fibers associated with the disease, brain researchers are searching for other potential causes of impaired memory and thinking.
That search is on full display this week at the Alzheimer's Association International Conference in San Diego, where sessions are exploring factors including genes, brain injury, clogged arteries and inflammation.
A group of researchers from Seattle even unveiled a highly detailed atlas showing how different types of brain cells change in Alzheimer's. The goal is to help scientists identify new approaches to treatment.
"Certainly, plaques and tangles are a hallmark," says Maria Carrillo , chief science officer of the Alzheimer's Association. "It doesn't mean plaques are the cause of cell death."
Plaques are clumps of a protein called beta-amyloid that appear in the spaces between neurons. Tangles are made up of a protein called tau that appears inside a neuron.
Both proteins tend to accumulate in the brains of people with Alzheimer's. But their role in killing brain cells is still unclear.
Carrillo says the Alzheimer's field needs to look to cancer research where a deeper understanding of the disease has led to better treatments.
The shift comes after a series of experimental drugs have succeeded in removing amyloid plaques and tau tangles from the brain, but failed to halt the disease.
The Food and Drug Administration has approved one amyloid drug, Aduhelm, but is still evaluating whether it actually helps patients.
An Alzheimer's Atlas
The study that produced the atlas is emblematic of how researchers are recalibrating.
"What we're trying to do with this study is to look at cell vulnerability early on in disease, before [people] have plaques and tangles, before they have cognitive impairment," says Dr. C. Dirk Keene , a neuropathologist at the University of Washington.
To create the atlas, Keene and a team of researches analyzed more than a million cells from 84 brains donated by people who'd signed up for Alzheimer's research projects run by the University of Washington and Kaiser Permanente Washington Research Institute.
The brains came from donors "at all different stages of disease" Keene says, "so we can pinpoint what's happening from the earliest levels all the way through to people with advanced disease."
The effort is funded by the National Institute on Aging and grew out of the federal BRAIN initiative launched by President Obama in 2013.
The atlas came from the realization that "If we want to treat diseases of an extremely complex cellular organ, you need to understand that organ much better than we do," says Ed Lein , a senior investigator at the Allen Institute for Brain Science, which played a key role in analyzing the brain tissue.
So the team spent years studying cells in healthy brains before looking at brains affected by Alzheimer's.
"We've defined what a normal adult brain looks like," Lein says, "and now we can use that knowledge and look for changes that are happening in specific kinds of cells."

Future Alzheimer's Treatments Aim To Do More Than Clear Plaques From The Brain
Finding vulnerable brain cells.
At the Alzheimer's meeting, the team described changes they saw in more than 100 types of cells taken from the cortex — an area of the brain which is important to memory and thinking.
One finding was that neurons that make connections within the cortex itself were much more likely to die than those that connect to distant areas of the brain.
"What we're seeing is a profound effect on cortical circuitry that very plausibly is the reason we have cognitive decline," Lein says.
If so, a treatment designed to protect those vulnerable neurons might prevent declines in memory and thinking linked to Alzheimer's.
The team also found a proliferation of brain cells that contribute to inflammation. These included certain immune cells and a type of cell that responds to injury.
"So while the neurons are lost, the non-neuronal cells are actually increasing and changing" Lein says.
The finding supports the idea that inflammation plays an important role in Alzheimer's, and that anti-inflammatory drugs might help protect the brain.
The Seattle team hopes other scientists will use the brain cell atlas to come up with new treatments for Alzheimer's.
"We've created an open-access resource where the whole community can come and look at this data," Lein says. "They can mine it to speed up progress in the field as a whole."
Speeding up progress is one reason Kyle Travaglini , a researcher at the Allen Institute, jumped at the chance to work on the Alzheimer's project.
"My grandmother started developing Alzheimer's disease when I was just going off to college," says Travaglini, who received his PhD in 2021.
Travaglini says the atlas project is appealing because it isn't based on a preconceived idea about what causes Alzheimer's.
"It's like looking at the same disease that everyone has been looking at but in an entirely different way," he says.

A substance found in young spinal fluid helps old mice remember

Scientists look to people with Down syndrome to test Alzheimer's drugs
- Share full article
Advertisement
Supported by
Embattled Alzheimer’s Researcher Is Charged With Fraud
Hoau-Yan Wang, a professor at City College, published studies supporting simufilam, now in advanced clinical trials.

By Apoorva Mandavilli
A scientist whose research has been at the center of controversy over an Alzheimer’s drug candidate has been charged with fraud.
A federal grand jury on Thursday indicted Hoau-Yan Wang, a professor at the City College of New York, on charges of falsifying data to obtain grants totaling roughly $16 million from the National Institutes of Health.
Dr. Wang’s studies underpinned research into a diagnostic test for Alzheimer’s disease and simufilam, a drug in advanced clinical trials. Simufilam’s manufacturer, Cassava Sciences, a pharmaceutical company based in Texas, has said the drug improves cognition in Alzheimer’s patients.
Alzheimer’s disease affects roughly six million Americans — a number that is expected to double by 2050 — and promising treatments generate tremendous excitement. Cassava’s stock soared after each round of reported results from its trials.
But some scientists had publicly disparaged the drug, saying its mechanism of action and purported results were implausible . Some went further and accused the company and Dr. Wang, its scientific consultant, of manipulating results. Several journals retracted or attached statements of concern to publications by Dr. Wang and a co-author at Cassava.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
Scientist behind Alzheimer’s drug in late-stage trials is indicted on charges of research fraud

The Summary
- A neuroscientist whose work helped pave the way for an Alzheimer’s drug candidate was indicted on charges of fraud.
- The charges are related to the alleged fabrication of research images and data that the scientist may have used to secure grants.
- The manipulation of research images is a growing concern in the scientific community.
A neuroscientist whose work helped pave the way for an Alzheimer’s drug candidate was indicted by a federal grand jury on Thursday on charges of fraud.
The indictment, announced Friday by the Justice Department , brings additional scrutiny to the work of Hoau-Yan Wang , who has had multiple studies retracted and faced an investigation by the City University of New York, his employer, that was later halted .
The charges in the indictment are related to the alleged fabrication of research images and data that Wang may have used to secure federal grants from the National Institutes of Health.
Wang, a medical professor at the City University of New York , collaborated with Cassava Sciences, a pharmaceutical company based in Austin, Texas, as it investigated an Alzheimer’s drug candidate called simufilam. He was awarded some $16 million in grants for early-stage drug development in collaboration with Cassava, according to the indictment.
The indictment charges Wang with one count of fraud against the United States, two counts of wire fraud and one count of false statements. It accuses Wang of manipulating or adding to images of Western blots, a laboratory method that researchers use to identify proteins, in order to bolster evidence and help secure grants.
The indictment also suggests that Wang may have lied to scientific journals to substantiate his research, which contributed to the early development of simufilam.
The drug is currently in a late-stage clinical trial, and some 735 patients had participated as of May 2024, according to a news release from Cassava last month.
Wang did not immediately respond to a request for comment. In 2023, he told The Wall Street Journal that a CUNY investigation made “no conclusive findings of data manipulation, consistent with what I’ve been saying for two years.”
Cassava said in a release on Friday that Wang had not participated in its most recent trial.
In a news release, the company said: “Wang’s work under these grants was related to the early development phases of the Company’s drug candidate and diagnostic test and how these were intended to work.”
Cassava added that Wang “had no involvement in the Company’s Phase 3 clinical trials of simufilam.”
A Cassava spokesperson also pointed to a news release the company issued September 2023, which said academic researchers outside of CUNY had found evidence that the drug could affect signaling pathways with suspected involvement in Alzheimer’s.
CUNY learned of the indictment on Friday, a spokesperson said in an email, adding: “The University has and will continue to cooperate to the fullest degree with the federal government’s investigation until the matter is resolved.”
The indictment doesn’t specifically name the university, drug or company, listing them instead as “University 1,” “Drug A” and “Company 1,” respectively.
Still, Cassava’s shares fell nearly 35% on Friday in a rapid plunge that triggered multiple trading halts.
Overall, the manipulation of research images and the handling of allegations of research misconduct is a growing concern in the scientific community.
The issue gained particular attention last summer, when then-Stanford President Marc Tessier-Lavigne stepped down from his post after allegations arose that images had been manipulated within his lab. Tessier-Lavigne said he never submitted papers he didn’t think were accurate and noted that a panel investigating his work did not find that he knew of misconduct within his lab.
Then in January, an amateur science sleuth made allegations of research image manipulation by top scientists at the Dana-Farber Cancer Institute , which led to subsequent retractions. Dana-Farber said it took decisive action to correct the scientific record.
Wang’s work has faced questioning for some time, as the journal Science has reported . The journal obtained a report by CUNY that found evidence suggesting research misconduct. The university halted its investigation after Science published the report .
Multiple journal articles on which Wang was an author have been retracted , according to the website Retraction Watch.
Evan Bush is a science reporter for NBC News. He can be reached at [email protected].

IMAGES
COMMENTS
FDA approves new Alzheimer's medication 01:21. The Food and Drug Administration approved a new Alzheimer's treatment called donanemab on Tuesday, clearing the way for the third addition to a new ...
Innovative research from Mount Sinai has also identified new pathways for research. Researchers at the Icahn School of Medicine at Mount Sinai have achieved a major breakthrough in Alzheimer's disease research. Their study identifies a promising method that could potentially slow or even stop the progression of the disease.
On Tuesday, the F.D.A. approved donanemab, a new Alzheimer's medication. We asked experts how the rollout of a similar drug has gone. By Dana G. Smith Over the last three years, a new class of ...
Anne Trafton. June 27, 2023. A pair of structures in the hypothalamus called the mammillary bodies (highlighted in green) are among the first brain regions to show neurodegeneration in Alzheimer ...
Raket says he has been approached by several people in the pharmaceutical industry and academia, and some are working with him to apply the concept to their research. At the 2023 Alzheimer's ...
Researchers say we appear to be at the start of a new era for Alzheimer's treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady ...
Alzheimer's disease is a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by mood swings, disorientation and eventually delirium. It is the ...
November 8, 2022. Alzheimer's Disease. NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug ...
Monday 17 July 2023. Dr Richard Oakley, Associate Director of Research at Alzheimer's Society, has called breakthrough Alzheimer's drug donanemab, 'a turning point', as the full trial results were revealed. Full results about the Alzheimer's disease drug donanemab have been released today, supporting earlier trial results that suggested the ...
Journal of Neurology (2024) Alzheimer disease (AD) is the most common contributor to dementia in the world, but strategies that slow or prevent its clinical progression have largely remained ...
The Food and Drug Administration (FDA) has announced its approval in the US of the drug donanemab, a ground-breaking new treatment for early Alzheimer's disease.. Fiona Carragher, Director of Research and Influencing at Alzheimer's Society, said: "Donanemab is now the third drug aiming to slow down Alzheimer's disease to be approved in the US, marking another step forward in the fight ...
Christopher van Dyck, MD, director of Yale's Alzheimer's Disease Research Unit, was the lead author of a study published in the Jan. 5 issue of The New England Journal of Medicine that shared results of a Phase III clinical trial of lecanemab. (Dr. van Dyck is also a paid consultant for the pharmaceutical company Eisai, which funded the ...
The research team — located at Emory University School of Medicine, part of the Accelerating Medicines Partnership® Program for Alzheimer's Disease (AMP®-AD) Consortium — used advanced automated techniques to compare the levels of both proteins and RNA molecules in more than 1,000 brain tissue samples. The samples came from the ...
Getty Images. The first drug to slow the destruction of the brain in Alzheimer's has been heralded as momentous. The research breakthrough ends decades of failure and shows a new era of drugs to ...
Under new draft guidelines for the diagnosis of Alzheimer's Disease, unveiled on Sunday at a large international gathering of experts, memory tests would take a backseat to biomarkers—proteins ...
New findings from big-data and open-science research are revealing clues about the molecular mechanisms of Alzheimer's disease and new ways to discover potential therapeutic targets and biomarkers. These new discoveries were made by six research teams participating in the Accelerating Medicines Partnership Alzheimer's Disease (AMP AD) program.
Research from UC Berkeley indicates that ongoing stress caused by protein aggregation is leading to the death of brain cells. Numerous neurodegenerative conditions, including Alzheimer's and Parkinson's, involve the buildup of protein clusters, known as aggregates, within the brain. This phenomenon has prompted researchers to hypothesize that these protein masses are responsible for the ...
December 20, 2023. PHILADELPHIA - A "chaperone" molecule that slows the formation of certain proteins reversed disease signs, including memory impairment, in a mouse model of Alzheimer's disease, according to a study from researchers at the Perelman School of Medicine at the University of Pennsylvania. In the study, published in Aging ...
Potential new target for early treatment of Alzheimer's disease Date: July 2, 2024 Source: Penn State Summary: A class of proteins that regulates cell repair and enhances cell growth-signaling ...
In the study, published in Advanced Science on May 21, 2024, the researchers used a new technique for studying single, living brain cells affected by Alzheimer's disease. By measuring the ...
Alzheimer's and dementia research - find the latest information on research funding, grants, clinical trials and global research news. Get information and resources for Alzheimer's and other dementias from the Alzheimer's Association. Call our 24 hours, seven days a week helpline at 800.272.3900.
By Mayo Clinic Staff. Current Alzheimer's treatments temporarily improve symptoms of memory loss and problems with thinking and reasoning. These Alzheimer's treatments boost the performance of chemicals in the brain that carry information from one brain cell to another. They include cholinesterase inhibitors and the medicine memantine (Namenda).
Alzheimer's researchers are trying new treatment approaches, including trying to boost the immune system, remove toxic tangles of protein and stimulate brain waves with light and sound.
Alzheimer's disease; Latest Research and Reviews. Changes in expression of VGF, SPECC1L, HLA-DRA and RANBP3L act with APOE E4 to alter risk for late onset Alzheimer's disease.
A group of researchers from Seattle even unveiled a highly detailed atlas showing how different types of brain cells change in Alzheimer's. The goal is to help scientists identify new approaches ...
The new criteria - from the leading patient advocacy organization for Alzheimer's - were developed by a 20-member working group, many of whom reported financial ties to many of the companies ...
Evan Seigerman, BMO Capital Markets senior research analyst, joins 'Squawk Box' to discuss the FDA's approval of Eli Lilly's Alzheimer's drug donanemab, how much revenue it could add to Elil LIlly ...
A scientist whose research has been at the center of controversy over an Alzheimer's drug candidate has been charged with fraud. A federal grand jury on Thursday indicted Hoau-Yan Wang, a ...
Wang, a medical professor at the City University of New York, collaborated with Cassava Sciences, a pharmaceutical company based in Austin, Texas, as it investigated an Alzheimer's drug ...