- Research article
- Open access
- Published: 04 June 2021

Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
16k Accesses
29 Citations
13 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
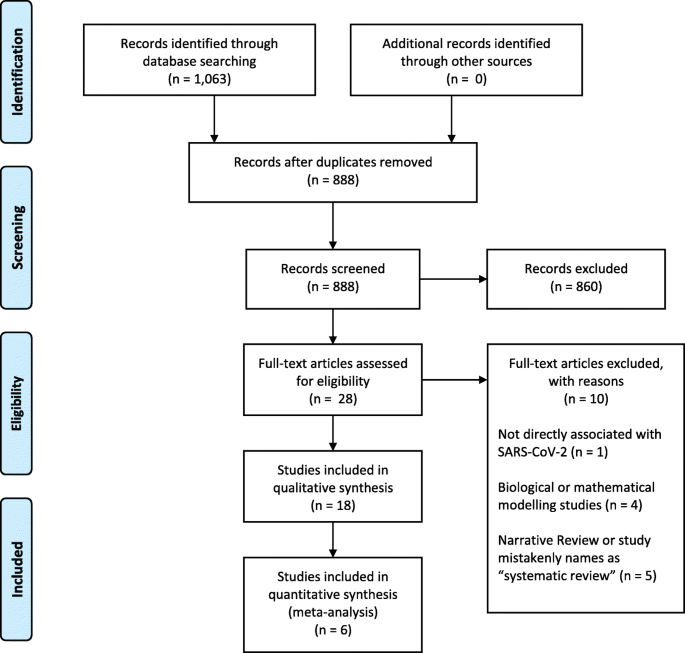
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
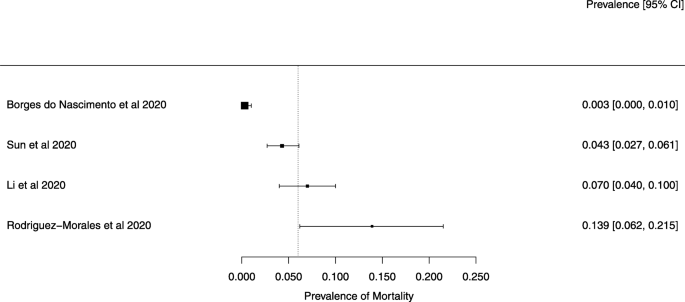
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
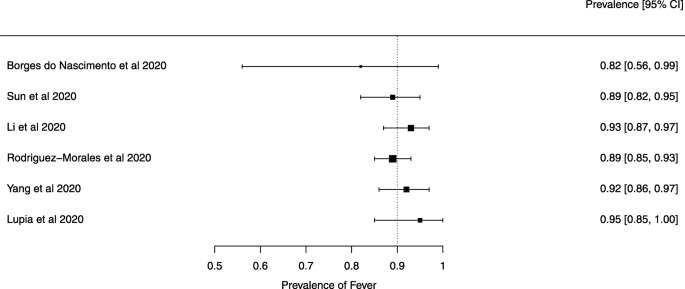
A meta-analysis of the prevalence of fever
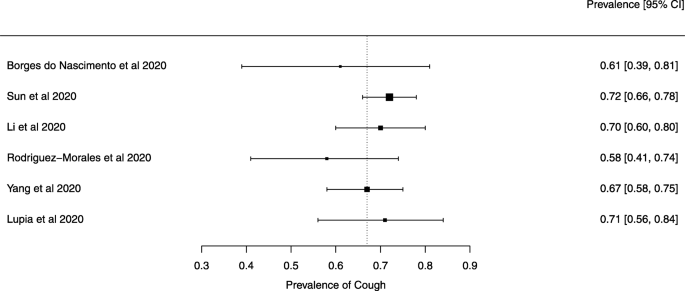
A meta-analysis of the prevalence of cough
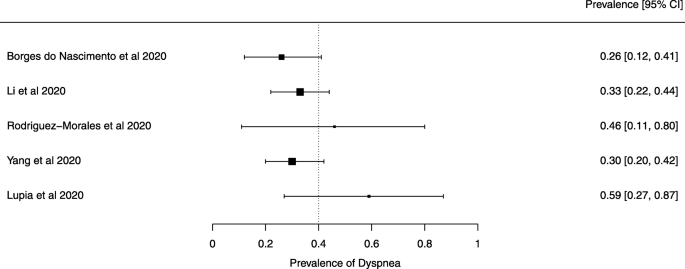
A meta-analysis of the prevalence of dyspnea
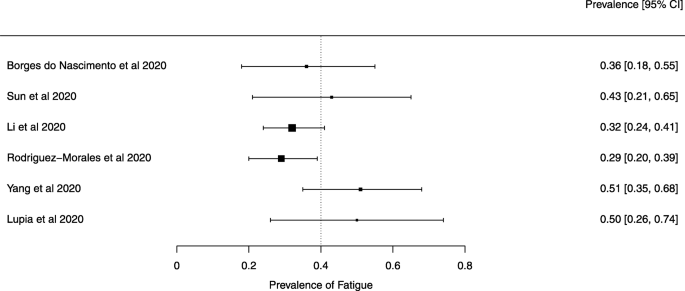
A meta-analysis of the prevalence of fatigue or myalgia
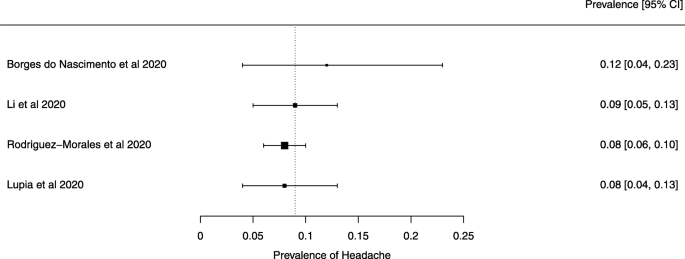
A meta-analysis of the prevalence of headache
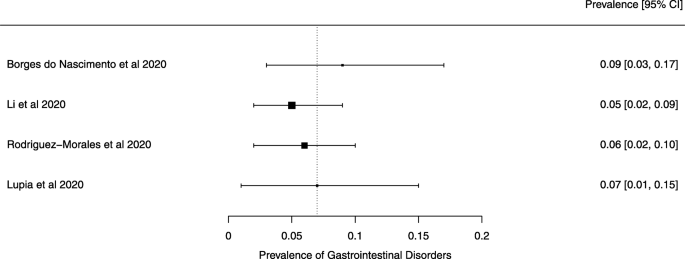
A meta-analysis of the prevalence of gastrointestinal disorders
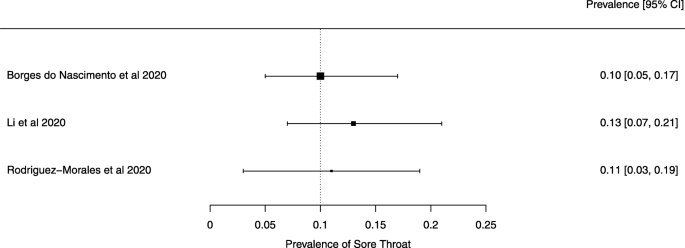
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
REVIEW article
Coronavirus disease (covid-19): comprehensive review of clinical presentation.

- 1 Department of Medicine, King Edward Medical University/ Mayo Hospital, Lahore, Pakistan
- 2 Department of Anesthesia and Intensive Care, Post-Graduate Medical Institute/LGH, Lahore, Pakistan
- 3 Rajarshee Chhatrapati Shahu Maharaj Government Medical College, Kolhapur, India
- 4 Department of Medicine, Faculty of Medicine, University of Tlemcen, Tlemcen, Algeria
- 5 School of Tropical Medicine and Global Health, Nagasaki University, Nagasaki, Japan
- 6 Institute of Research and Development, Duy Tan University, Da Nang, Vietnam
COVID-19 is a rapidly growing pandemic with its first case identified during December 2019 in Wuhan, Hubei Province, China. Due to the rampant rise in the number of cases in China and globally, WHO declared COVID-19 as a pandemic on 11th March 2020. The disease is transmitted via respiratory droplets of infected patients during coughing or sneezing and affects primarily the lung parenchyma. The spectrum of clinical manifestations can be seen in COVID-19 patients ranging from asymptomatic infections to severe disease resulting in mortality. Although respiratory involvement is most common in COVID-19 patients, the virus can affect other organ systems as well. The systemic inflammation induced by the disease along with multisystem expression of Angiotensin Converting Enzyme 2 (ACE2), a receptor which allows viral entry into cells, explains the manifestation of extra-pulmonary symptoms affecting the gastrointestinal, cardiovascular, hematological, renal, musculoskeletal, and endocrine system. Here, we have reviewed the extensive literature available on COVID-19 about various clinical presentations based on the organ system involved as well as clinical presentation in specific population including children, pregnant women, and immunocompromised patients. We have also briefly discussed about the Multisystemic Inflammatory Syndrome occurring in children and adults with COVID-19. Understanding the various clinical presentations can help clinicians diagnose COVID-19 in an early stage and ensure appropriate measures to be undertaken in order to prevent further spread of the disease.
Introduction
COVID-19 is a growing pandemic with initial cases identified in Wuhan, Hubei province, China toward the end of December 2019. Labeled as Novel Coronavirus 2019 (2019-nCoV) initially by the Chinese Center for Disease Control and Prevention (CDC) which was subsequently renamed as Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) due to its homology with SARS-CoV by the International Committee on Taxonomy of Viruses (ICTV) ( 1 , 2 ). The World Health Organization (WHO) later renamed the disease caused by SARS-CoV-2 as Coronavirus Disease-2019 (COVID-19) ( 3 ). COVID-19 is primarily a disease of the respiratory system affecting lung parenchyma with fever, cough, and shortness of breath as the predominant symptoms. Recent studies have shown that it can affect multiple organ systems and cause development of extra-pulmonary symptoms. Presence of extra-pulmonary symptoms can often lead to late diagnosis and sometimes even mis-diagnosis of COVID-19 which can be detrimental to patients. As researchers globally continue to understand COVID-19 and its implications on the human body, knowledge about the various clinical presentations of COVID-19 is paramount in early diagnosing and treatment in order to decrease the morbidity and mortality caused by the disease.
Epidemiology and Pathophysiology
While studying the early transmission dynamics of COVID-19 outbreak in Wuhan, many cases were found to be linked to the Huanan wholesale seafood market. Further investigation revealed <10% of the total cases could be linked to the market which led to the conclusion of human-to-human transmission of the virus occurring through respiratory droplets and contact transmission contributing to the rise in the number of affected individuals ( 4 ). The exponential rise in the number of cases in China and reporting of cases outside China in multiple countries led WHO to declare COVID-19 as a pandemic on 11th March 2020 ( 5 ).
SARS-CoV-2 tends to infect all age groups and is transmitted via direct contact or respiratory droplets generated during coughing or sneezing by the infected patient during both symptomatic or pre-symptomatic phase of infection. Other routes of transmission include fecal-oral route and fomites along with small risk of vertical transmission from mother to child if infection occurs during third trimester of pregnancy ( 6 , 7 ). There has also been evidence of asymptomatic transmission of COVID-19 ( 8 ). The concept of super spreaders in relation to COVID-19 is emerging where a single individual either symptomatic or asymptomatic can infect a disproportionately large number of individuals in an appropriate super spreading conditions such as mass gathering due to production of large number of infectious agent for prolonged duration of time ( 9 ). As per the literature, the incubation period of COVID-19 ranges from 2 to 14 days with a mean incubation period of 3 days ( 10 ). The basic Reproduction number (Ro) of SARS-CoV-2 is 2–2.5. Each individual infected with COVID-19 can infect 2–2.5 other individuals in a naïve population which also explains the exponential growth in the number of cases ( 10 ). The disease tends to be of mild to moderate severity in roughly 80% of patients, and severe disease is associated with infants, elderly patients above 65 years, and patients with other comorbidities such as diabetes mellitus, hypertension, coronary artery disease, and other chronic conditions ( 1 , 2 ). COVID-19 has also been found to be more severe in males than in females with a case fatality rate of 2.8% in males and 1.7% in females ( 11 ). The major organ system affected by the virus is the respiratory system, but it can affect other organ systems either directly or by the effect of host immune response. SARS-CoV-2, the causative agent of COVID-19, after entering the human host initially replicates in the epithelial mucosa of the upper respiratory tract (nose and pharynx) followed by migration to the lungs where further replication of virus occurs causing transient viraemia. The virus uses Angiotensin Converting Enzyme 2 (ACE2) receptor as a primary entry to cells. ACE2 is found abundantly in the mucosal lining of the respiratory tract, vascular endothelial cells, heart, intestine, and kidney. Thus, the virus has potential for replication in all these organs. After entry into cells, the virus undergoes further rapid replication within the target cells and induces extensive epithelial and endothelial dysfunction leading to exponential inflammatory response with the production of a large amount of proinflammatory cytokines and chemokines. Activation of proinflammatory cytokines and chemokines leads to neutrophil activation and migrations and results in the characteristic cytokine storm. The immunological downregulation of ACE2 by the virus contributes to acute lung injury in COVID-19. ACE2 also regulates the renin angiotensin system (RAS); thus, downregulation of ACE2 also causes dysfunction of RAS which contributes to enhanced inflammation ( 2 , 11 – 15 ). These entire factors contribute to symptoms of COVID-19 with sepsis, multi-organ dysfunction, acute respiratory distress syndrome (ARDS), and prothrombotic state leading to an exacerbation of organ dysfunction.
Clinical Manifestation
We review here the system based clinical features of COVID-19.
Respiratory
According to report from WHO-China-Joint Mission on COVID-19, 55,924 laboratories confirmed cases of COVID-19 had fever (87.9%), dry cough (67.7%), fatigue (38.1%), sputum production (33.4%), difficulty breathing (18.6%), sore throat (13.9%), chills (11.4%), nasal congestion (4.8%), and hemoptysis (0.9%) ( 1 ).
Some patients may rapidly progress to acute lung injury and ARDS with septic shock. The median interval between the onset of initial symptoms to development of dyspnea, hospital admission, and ARDS was 5, 7, and 8 days respectively ( 10 ). Some patients with COVID-19 may have reduced oxygen saturation in blood (≤ 93%) with oxygen saturation down to 50 or 60% but remained stable without significant distress, and as such, were termed as salient hypoxia or happy hypoxia ( 16 , 17 ). Trial of oxygen therapy, prone positioning, high flow continuous positive airway pressure, non-re-breathable mask alongside trial of anticoagulation are often used to manage these patients ( 16 , 17 ). However, further study is required to define the role of these strategies in management.
The most frequent radiological abnormality among 975 patients with COVID-19 in computed tomography (CT) scan of chest was ground glass opacity (56.4%) and bilateral patchy shadowing (51.8%) ( 18 ). A scientific review of 2,814 patients have shown that the most common chest CT finding in COVID-19 patients was ground glass opacity followed by consolidation. However, the findings can vary in different patients and at various stages of diseases. Other CT findings include interlobular septal thickening, reticular pattern, crazy paving, etc. Atypical findings like air bronchogram, bronchial wall thickening, nodule, pleural effusion, and lymphadenopathy have also been noted in some studies ( 19 ). A study showed that among 877 patients with non-severe diseases and 173 patients with severe diseases, 17.9 and 2.9% of the patients did not have any detectable radiological abnormalities, respectively ( 18 ).
ENT (Ear, Nose, and Throat)
ENT manifestations are one of the most frequent symptoms encountered by physicians in COVID-19. A peculiar clinical presentation in some COVID-19 patients includes the deterioration of sense, taste (dysgeusia), and loss of smell (anosmia). A systematic review and meta-analysis of 10 studies with 1,627 participants surveyed for olfactory deterioration and 9 studies with 1,390 participants examined for gustatory symptoms demonstrated prevalence of 52.73 and 43.93% of these symptoms among COVID-19 patients, respectively. These clinical features may often present at earlier stages of the disease ( 20 ). Additionally, sore throat, rhinorrhea, nasal congestion, tonsil edema, and enlarged cervical lymph nodes are commonly seen among otolaryngological dysfunctions in patients ( 21 ). A large observational study of 1,099 COVID-19 patients reported tonsils swelling in 23 patients (2.1%), throat congestion in 19 patients (1.7%) and enlarged lymph nodes in 2 patients (0.2%) ( 18 ). This can be explained by the fact that there is a high expression of ACE2 receptors on the epithelial cells of the oral and nasal mucosa including the tongue. It has been known that the novel coronavirus has a strong binding affinity to ACE2 receptors through which it invades host cells ( 22 ). This theory may explain the exhibition of extra-respiratory symptoms including ENT manifestations as part of COVID-19 symptoms.
Cardiovascular
Cardiac manifestation in patient with COVID-19 can occur due to cardiac strain secondary to hypoxia and respiratory failure, direct effect of SARS-CoV-2 on heart or secondary to inflammation and cytokine storm, metabolic derangements, rupture of plaque and coronary occlusion by thrombus, and consequences of drugs used for treatment ( 23 – 25 ). The need for intensive care admission, non-invasive ventilation (46.3 vs. 3.9%), and invasive mechanical ventilation (22 vs. 4.2%) were higher among patients with cardiac ailments as compared to those without cardiac involvement as well as higher hospital mortality than those without myocardial involvement (51.2 vs. 4.5%) ( 26 ). These patients tend to have electrocardiographic (ECG) changes as well as elevations in high sensitivity cardiac troponin (hsCTn) and N- terminal pro-B-type natriuretic peptide (NT proBNP) which corresponded to raised inflammatory markers. Hypertension, acute and fulminant myocarditis, ventricular arrhythmias, atrial fibrillation, stress cardiomyopathy, hypotension and heart failure, acute coronary syndrome (ACS) with ST elevation or depression MI with normal coronaries have been reported ( 23 , 27 ). In a Chinese cohort of 138 patients, 16.7% had arrhythmias with risk higher among those needing ICU care with no mention of the type of arrhythmia that was present ( 28 ). Less frequently, cardiac symptoms like chest pain or tightness and palpitation can be the initial presenting features without fever producing a diagnostic dilemma. Some of these patients eventually go on to develop respiratory symptoms as diseases progress ( 29 ). Patients who have recovered from acute illness may develop arrhythmias as a result of myocardial scar and need future monitoring ( 27 ). One important point to note is use of Renin Angiotensin Aldosterone System (RAAS) modulators in patients with COVID-19. Guidelines from ACC/AHA/HFSA recommends continuing them in high risk patient based on goal directed therapy approach supported by a recent systematic review and meta-analysis conducted by Hasan et. Al. which demonstrated use of ACEI/ARB in COVID-19 patients is associated with lower odds/ hazards of mortality and development of severe/critical diseases as compared to no use of ACEI/ARB ( 30 , 31 ).
Gastrointestinal
In the initial cohort of patients from China, nausea or vomiting and diarrhea were present in 5 and 3.7% of patients ( 1 ). Review of data from 2,023 patients showed anorexia to be the most frequently occurring gastrointestinal symptom in adults. Diarrhea was the most common presenting gastrointestinal symptom in both adults and children while vomiting was found to be more common in children ( 32 ). Other rare symptoms included nausea, abdominal pain, and gastrointestinal bleeding. There have been few instances where COVID-19 patients presented with only gastrointestinal symptoms without the development of fever or respiratory symptoms at the onset and during disease progression ( 33 ). In a smaller cohort of 204 patients, 50.5% had some form of intestinal symptoms and of those, 5.8% had only intestinal symptoms while the remaining patients developed respiratory symptoms subsequently. The most common symptoms reported among them was anorexia (78.64%), non-dehydrating diarrhea (34%), vomiting (3.9%), and abdominal pain (1.94%) ( 34 ). In addition, those with GI symptoms tend to have a longer interval between symptom onset and hospital admission (9 vs. 7.3 days) possibly due to lack of clinical suspicion and delay in diagnosis. Patients with gastrointestinal symptoms tend to have higher elevation in AST and ALT indicating coexistent liver injury ( 34 ). The mechanism behind GI illness is not clearly known but could be due to direct invasion of virus via ACE2 receptor in the intestinal mucosa. This can be supported by the fact that viral RNA can be detected in stool samples of COVID-19 patients which may also hint toward possible fecal-oral transmission ( 35 ). Liver dysfunction is likely secondary to the use of hepatotoxic drugs, hypoxia induced liver injury, systemic inflammation, and multi organ failure ( 36 ).
Renal manifestation in patients with COVID-19 can occur due to direct invasion of podocytes and proximal tubular cells by SARS-CoV-2 virus, secondary endothelial dysfunction causing effacement of foot process with vacuolation and detachment of podocytes, and acute proximal tubular dysfunction ( 37 ). Furthermore, hypoxia, cytokine storm, rhabdomyolysis, nephrotoxic drugs, and overlying infections can all exacerbate renal injury ( 38 ). Based on initial reports, prevalence of Acute Kidney Injury (AKI) among COVID-19 hospitalized patients range from 0.5 to 29%. In a cohort of 701 patients, proteinuria (43.9%), hematuria (26.7%), elevated creatinine (14.4%), elevated blood urea nitrogen (13.1%), and low glomerular filtration rate (≤ 60 ml/min/1.73 m 2 ) (13.1%) were present at the time of hospital admission with 5.1% developing AKI during the illness. AKI was more prevalent among those with baseline renal impairment ( 39 ). In another large cohort of 5,449 patients, 36.6% had AKI with prevalence higher among mechanically ventilated patients compared to non-ventilated patients (89.7 vs. 21.7%) ( 40 ). Patients developing renal impairment are prone to have higher mortality within the hospital. Another point to highlight is the presentation of COVID-19 in renal transplant recipients. Due to immunosuppression, these patients are likely to have low fever at presentation with swift clinical decline and requirement for mechanical ventilation with high mortality as compared to the general population ( 41 ).
Neurological
Most patients with COVID-19 develop neurological symptoms along with respiratory symptoms during the course of illness; however, several case reports in review of literature document patient presentation of neurological dysfunction without typical symptoms of fever, cough, and difficulty breathing ( 42 ). There is a 2.5-fold enhanced risk of severe illness and increased death in patients with a history of previous stroke with similar findings among those with Parkinson's diseases. The prevalence of neurological features ranges from 6 to 36% along with hypoxic ischemic encephalopathy up to 20% in some series of patients ( 43 ). Neurological symptoms tend to occur early in the course of illness (median 1–2 days) with most common neurological features being headache, confusion, delirium, anosmia or hyposmia, dysgeusia or ageusia, altered mental status, ataxia, and seizures ( 44 ). Among patients admitted with COVID-19, the prevalence of ischemic stroke ranges from 2.5 to 5% despite receiving prophylaxis for venous thromboembolism. Patients prone to have established cardiovascular risk factors are likely to have a more severe diseases ( 43 ). Other presentations include viral encephalitis, acute necrotizing encephalopathy (ANE), infectious toxic encephalopathy, meningitis, Guillain Barre Syndrome (GBS), Miller Fisher syndrome, and polyneuritis cranialis with GBS being the first feature of COVID-19 in few cases ( 42 , 43 , 45 ). In COVID-19 patients, CNS features are possibly due to direct invasion of neurons and glial cells by SARS-CoV-2 as well as by endothelial dysfunction of blood brain barrier (BBB). Virus can gain access to CNS via hematogenous spread or retrograde movement across the olfactory bulb. The virus can be detected in CSF by RT-PCR and on brain parenchyma during autopsy. The fact that most patients develop anosmia or hyposmia during illness support this theory ( 45 ). After entry, the virus can cause reactive gliosis with activation of the inflammatory cascade. The combination of systemic inflammation, cytokine storm, and coagulation dysfunction can impair BBB function and alter brain equilibrium causing neuronal death ( 42 ).
Ocular manifestations can vary from conjunctival injection to frank conjunctivitis. In a Chinese cohort of 38 patients, 31.6% had ocular symptoms consisting primarily of conjunctivitis while conjunctival hyperemia, foreign body sensation in eye, chemosis, tearing or epiphora were more common among severe COVID-19 patients. Among them SARS-CoV-2 can be demonstrated in conjunctival as well as nasopharyngeal swab in 5.2% of patients, indicating a potential route for viral transmission ( 46 ). Conjunctivitis or tearing can be the initial presenting symptoms of COVID-19. Despite this fact, there is no documented case of severe ocular features relating to COVID-19.
Similar to other viral infections, SARS-CoV-2 can also produce varied dermatological features. A study of 88 patients from Italy showed that about 20.4% had some form of skin manifestations with 44.4% developing features at onset and duration of the disease progression ( 47 ). Maculopapular exanthem (36.1%) was identified as most common dermatological features followed by papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis (2.8%), and petechiae (1.4%) ( 48 ). A study of 375 COVID-19 cases in Spain identified five different patterns of cutaneous manifestations in patients: acral areas of erythema with vesicles or pustules (pseudo-chilblain) (19%), other vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedo or necrosis (6%) ( 49 ). Majority of patients had lesions on the trunk with some experiencing lesions on hands and feet. There are case reports of COVID-19 associated with erythema multiforme and Kawasaki Disease in children ( 50 , 51 ). Pathogenesis behind skin involvement remains unclear with some features explained by small vessel vasculitis, thrombotic events like DIC, hyaline thrombus formation, acral ischemia, or the direct effect of the virus like other viral illnesses ( 52 ).
Musculoskeletal
The initial report from China revealed 14.8% of patients had myalgia or arthralgia among 55,924 COVID-19 patients. A review article reports that of 12,046 patients, fatigue was identified in 25.6% and myalgia and/or arthralgia in 15.5% with most patients reporting symptoms from the start of illness ( 53 ). There are reports suggesting myositis and rhabdomyolysis with markedly elevated creatinine kinase can occur during COVID-19 illness especially in patients with severe diseases and multi organ failure. Additionally, in some patients, rhabdomyolysis has been documented as the initial presentation of COVID-19 illness without typical respiratory symptoms ( 54 , 55 ). A case series of four patients developing acute arthritis during hospital admission for COVID-19 has been reported with exacerbation of crystal arthropathy (gout and calcium pyrophosphate diseases) but negative for SARS-CoV-2 RT-PCR in synovial fluid ( 56 ). Treatment with steroids and colchicine was used in all four cases. An important consideration to note was that all four patients developed arthritis despite previous treatment with immunomodulatory therapy (hydroxychloroquine, tocilizumab, and pulse methylprednisolone).
Hematological
As stated, COVID-19 is a systemic disease inducing systemic inflammation and occasionally cytokine storm. This can significantly impact the process of hematopoiesis and hemostasis. During early disease, normal or decreased leukocyte and lymphocyte counts were documented with marked lymphopenia as the diseases progressed, especially in those with cytokine storms and severe disease. In a study of 1,099 patients, lymphopenia, thrombocytopenia, and leukopenia were present in 83.2, 36.2, and 33.7%, respectively, with findings more marked in those with severe diseases ( 18 ). Leukocytosis in COVID-19 patients might suggest a bacterial infection or a superinfection with leukocytosis found more commonly in severe cases (11.4%) as compared to mild and moderate cases (4.8%) ( 18 ). Similarly, thrombocytopenia has been found to be more common (57.7%) in severe cases in contrast to mild and moderate cases (31.6%) ( 18 ). Lymphopenia was also linked with an increased necessity for ICU admission and the risk of ARDS. Thrombocytosis with elevated platelet to lymphocyte ratio may indicate a more marked cytokine storm ( 57 ).
Also, coagulation abnormality can manifest in the form of thrombocytopenia, prolonged prothrombin time (PT), low serum fibrinogen level, and raised D-dimer suggesting Disseminated Intravascular Coagulation (DIC) with these changes more marked in those with severe diseases ( 58 ). Raised lactate dehydrogenase (LDH) and serum ferritin were also present and correlated with the degree of systemic inflammation. In a study of 426 COVID-19 patients, C-Reactive Protein (CRP) was noted to be increased in 75–93% of patients, more commonly in patients with severe disease. Serum procalcitonin levels might not be altered at admission, but progressive increase in its value can suggest a worsening prognosis. Severe disease is linked to increased ALT, bilirubin, serum urea, creatinine, and lowered serum albumin ( 59 ). A study of 1,426 patients showed that Interleukin-6 (IL-6) were raised more in patients with severe COVID-19 than non-severe COVID-19 with progressive rise indicating an increased risk of mortality. Thus, its levels could be regarded as an important prognostic indicator for the extensive inflammation and cytokine storm in COVID-19 patients ( 60 ). Other plasma cytokines and chemokines like IL1B, IFNγ, IP10, MCP, etc. have also been found to be elevated in patients with COVID-19 both in severe and non-severe diseases. Additionally, GCSF, IP10, IL2, IL7, IL10, MCP1, MIP1A, and TNFα were increased in patients who require ICU admission which indicates that cytokine storm is associated with a severe disease ( 61 ).
Endocrine and Reproductive
From the available literature there is no doubt that diabetes mellitus is an important risk factor for COVID-19 illness and is associated with increased risk of development of severe disease. Additionally, there are case reports of subacute thyroiditis linked to SARS-CoV-2 infection ( 62 , 63 ). Based on the statement released from European Society of Endocrinology, patients with primary adrenal failure and congenital adrenal hyperplasia may have theoretically increased susceptibility to infection with higher risk of complications and ultimately mortality but there is no current evidence to support this ( 64 ). The dose of steroids may need to be doubled if there is a clinical suspicion of infection in these patients.
Several claims have been made regarding the impact of COVID-19 on male reproductive function, hypothesizing that COVID-19 can cause potential testicular damage either by binding directly to testicular ACE2 receptors, which are highly expressed in the testicles or by damaging the testis indirectly by exciting local immune system ( 65 ). A study comparing 81 male COVID-19 patients with 100 age matched healthy adults highlighted the presence of low testosterone levels, high levels of luteinizing hormone (LH), low testosterone/LH ratios, low Follicle stimulating hormone (FSH) to LH ratio, and raised serum prolactin. This may suggest a potential COVID-19 testicular damage affecting the Leydig cells in the testis ( 66 ). COVID-19 infected male patients may have reduced sperm count and decreased motility leading to diminished male fertility for 3 months post-infection ( 67 ).
Clinical Presentation in Specific Population
In children.
A case series of 72,314 cases published by the Chinese Center for Disease Control and Prevention reported that 0.9% of the total patients were between 0 and 9 years of age, and 1.2% of the total patients were between 10 and 19 years of age ( 68 ). The most common symptoms found in children are fever, (59%), cough (46%), few cases (12%) of gastrointestinal symptoms, and some cases (26%) showed no specific symptoms initially with patchy consolidation and ground glass opacities in CT chest findings ( 69 ). Chilblain-like acral eruptions, purpuric, and erythema multiforme-like lesions have been found to be more common in children and young adult patients mainly with asymptomatic or mild disease ( 70 ). Lymphopenia in children is relatively less common which is in direct contrast in cases of SARS in children where lymphopenia was more commonly noted ( 69 ).
Multisystem inflammatory syndrome (MIS) is another feared complication of Covid-19 seen in children. Abrams et al. systematically summarized the clinical evidence of 8 studies reporting MIS in 440 children. The median age of patients ranged from 7.3 to 10 years with 59% of all patients being male. The greatest proportion of patients had gastrointestinal symptoms (87%) followed by mucocutaneous symptoms (73%) and cardiovascular symptoms (71%) while fewer patients reported respiratory (47%), neurologic (22%), and musculoskeletal (21%) symptoms. Ferritin and d-dimer were elevated in 50% of patients, and C-reactive protein, interleukin-6, and fibrinogen were elevated in at least 75% of patients. Additionally, 100% of children with cardiovascular involvement reported elevated cardiac-damage markers such as Troponin. Although respiratory manifestation is most frequently expressed in adults, children with MIS exhibited less pulmonary symptoms and more of the other manifestations ( 71 ).
In Pregnant Women
The most common symptoms reported in pregnant women are fever (61.96%), cough (38.04%), malaise (30.49%), myalgia (21.43%), sore throat (12%), and dyspnea (12.05%). Other symptoms found in pregnant women are diarrhea and nasal congestion ( 72 ). In a systematic review including 92 patients, 67.4% manifested diseases at presentation with 31.7% having negative RT-PCR though they had features of viral pneumonia. Only one patient required admission to intensive care and 0% mortality. Fetal outcomes were reported as: 63.8% preterm delivery, 61.1% fetal distress, 80% Cesarean section delivery, 76.92% neonatal intensive care admission, 42.8% low birth weight, and 66.67% had lymphopenia ( 72 ). There was no evidence of vertical transmission. A study of 41 pregnant women with COVID-19 showed that consolidation was more commonly found in CT of pregnant women in contrast to ground-glass opacities in CT of non-pregnant adults ( 73 ). WHO also recommends encouraging lactating mothers with confirmed or suspected COVID-19 to begin or continue breastfeeding including 24-h rooming in, skin to skin contact, and kangaroo mother care especially in immediate postnatal period ( 74 ). On July 14th, 2020, Vivanti et al. published the first case of transplacental transmission of COVID-19 from a 23-years-old pregnant woman to her baby ( 75 ). Thereafter, more studies reported the possibility of the vertical transmission of COVID-19. In this context, Kotlayer et al. published a systematic review of 38 studies. Out of 936 neonates from COVID-19 mothers, 27 tested positive for the virus indicating a pooled proportion of 3.2% (2.2–4.3) for vertical transmission ( 7 ).
In Immuno-Compromised Population
Due to their impaired immune response, it is not surprising that immunocompromised patients with COVID-19 infection might be at greater risk of developing severe forms of the disease and co-infections in comparison to normal populations. Nevertheless, recent studies showed the association between cytokine storm syndrome and the overreaction of the immune system with COVID-19 raising the possibility that immunodeficient states might alleviate the overexpression of the host immune system and thereby prevent deadly forms of the disease ( 76 ). After the RECOVERY trial ( 77 ) that showed the efficacy of dexamethasone in lowering the mortality in severe forms of the disease, many questions were raised regarding whether immunocompromised patients have a greater or lower risk of developing severe forms of the disease. In order to address these questions, Minotti et al. recently published a systematic review that included 16 studies with 110 patients presenting mostly with cancer along with transplantation and immunodeficiency. Out of the 110 patients, 72 (65.5%) recovered without being admitted to the intensive care unit while 23 (20.9%) died ( 76 ). The authors concluded that immunosuppression in both children and adults seem to have a better disease course in comparison to normal population. One of the limitations of this study is that the conclusion was made only based on qualitative synthesis and no meta-analysis was performed. On the other hand, Gao et al. performed a meta-analysis on 8 relevant studies with 4,007 patients. The study showed that immunosuppression and immunodeficiency were associated with non-statistically significant increased risk of severe COVID-19 disease ( 78 ). Additionally, Mirzaei et al. summarized the clinical evidence of 252 HIV positive patients co-infected with COVID-19. The clinical manifestation did not differ from that of the general population. However, out of the 252 patients, 204 (80.9%) were male. Low CD4 count (<200 cells/mm 3 ) were reported for 23 of 176 patients (13.1%). COVID-19 symptoms were present in 223 patients with the most common symptoms of fever in 165 (74.0%) patients, cough in 130 (58.3%), headache in 44 (19.7%), arthralgia and myalgia in 33 (14.8%), gastrointestinal symptoms in 29 (13.0%) followed by sore throat in 18 (8.1%) patients ( 79 ). The number of deaths accounted for 36 (14.3%). Similar to the general population, immunocompromised, and HIV patients were no different in terms of clinical manifestation or severity. However, the results from these studies should be interpreted with caution and more studies are recommended to establish the link between this particular group of patients with severity of the disease.
Multisystem Involvement in COVID-19
As evident from the discussion above, SARS-CoV-2 can affect multiple organ systems and produce a wide array of clinical presentation of COVID-19. Certain studies conducted in Europe and United States have shown that COVID-19 can also have a multi-systemic presentation in individuals in form of a multi-system inflammatory syndrome (MIS) which has been found in both children and adults and is known as MIS-C and MIS-A, respectively ( 80 – 83 ).
According to a recent CDC report about MIS-A, it was found that only half of the patients with MIS-A had preceding respiratory symptoms of COVID-19 ~2–5 weeks before ( 80 ). The most common clinical signs and symptoms included fever, chest pain, palpitations, diarrhea, abdominal pain, vomiting, skin rash, etc. Nearly all patients had electro-cardiological abnormalities like arrythmias, elevated troponin levels, and electrocardiography evidence of left or right ventricular dysfunction. Even though most patients had minimal respiratory symptoms, chest imaging had features of ground glass opacity and pleural effusion. All patients had signs of elevated laboratory markers of inflammation, coagulation markers, and lymphopenia ( 80 ).
MIS-C can clinically mimic Kawasaki Disease ( 81 ). By the end of July, about 570 cases of MIS-C with COVID-19 were found in the United States ( 81 ). In MIS-C, there is involvement of at least four organ systems, most commonly the gastrointestinal system followed by cardiovascular and dermatological systems ( 81 ). Prominent signs and symptoms found in children with MIS-C were abdominal pain, vomiting, skin rash, diarrhea, hypotension, and conjunctival injection. The majority of the children needed ICU admission due to the development of severe complications including cardiac dysfunction, shock, myocarditis, coronary artery aneurysm, and acute kidney injury ( 81 ).
Association Between Clinical Presentations, COVID-19 Severity and Prognosis
Evaluation of 55,924 laboratory confirmed COVID-19 cases in China, the presence of dyspnea, respiratory rate ≥ 30/min, blood saturation levels ≤ 93%, PaO2/FiO2 ratio ≤ 300, lung infiltrates ≥ 50% of the lung fields between 12 and 48 h were associated with severe COVID-19 infection ( 1 ). Clinical signs suggestive of respiratory failure, septic shock, or multiple organ dysfunction/failure were associated with critical disease and poor prognosis ( 1 ). Individuals at highest risk of severe disease and deaths were patients with age > 80 years and associated co-morbidities such as underlying cardiovascular disease, diabetes, hypertension, chronic respiratory disease, and cancer ( 1 ). Another study done with 418 patients in Catalonia (Spain) showed that dyspnea was an important predictor of severe disease while confusion was an important predictor of death, and the presence of cough was strongly associated with good prognosis ( 84 ). Advanced age, male sex, and obesity were independent markers of poor prognosis while eosinophilia was a marker of less severe disease ( 84 ). The mortality was lower in patients with symptoms of diarrhea, arthromyalgia, headache, and loss of smell and taste sensations while low oxygen saturation, high CRP levels, and higher number of lung quadrants affected on Xray were found to be associated with severe disease and death ( 84 ).
COVID-19 is a viral illness which can cause multi-systemic manifestations. Review of existing literature concludes that SARS-CoV-2 can affect any organ system either directly or indirectly leading to a myriad of clinical presentation. The most commonly affected system is the respiratory system with presenting symptoms of fever, cough, and shortness of breath, etc. Other systems which can be affected in COVID-19 include ENT (sore throat, loss of taste, smell, and sensations, and rhinorrhea), cardiovascular system (chest pain, chest tightness, palpitations, and arrhythmias), gastrointestinal system (anorexia, diarrhea, vomiting, nausea, and abdominal pain), renal (proteinuria, hematuria, and acute kidney injury), neurological (headache, confusion, delirium, and altered mental status), ocular (conjunctival hyperemia, foreign body sensation in the eye, chemosis, and tearing), cutaneous (rash, papules, and urticaria), musculoskeletal system (myalgia and arthralgia), hematological (lymphopenia, thrombocytopenia, leukopenia, elevated inflammatory markers, and elevated coagulation markers), endocrine (low testosterone, low FSH, and high LH) and reproductive system (decreased sperm count and decreased sperm motility). Clinical presentation in specific populations like children, pregnant women, and immunocompromised people may vary which emphasizes the importance of further investigation in order to avoid late diagnosis of COVID-19. Severe multi-systemic involvement in COVID-19 in the form of MIS-C and MIS-A can cause significant morbidity and mortality if undiagnosed. The clinical presentations of respiratory failure, acute kidney injury, septic shock, cardiovascular arrest is associated with severe COVID-19 disease and can result in poor prognosis. In the light of exponentially growing pandemic, every patient presenting to hospital must be tested for SARS-CoV-2 by RT-PCR if resources are available to detect early presentations of diseases even if the features are atypical. Understanding of the various clinical presentations of COVID-19 will help the clinicians in early detection, treatment, and isolation of patients in order to contain the virus and slow down the pandemic.
Author Contributions
All authors have contributed equally to the work, and all agreed to be accountable for the content of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Ms. Sairah Zia (American University of Caribbean, School of Medicine, Sint Maarten), a native speaker of English, for proofreading the manuscript.
Abbreviations
ACC/AHA/HFSA, American College of Cardiology/American Heart Association/Heart Failure Society of America; IL1B, Interleukin 1B; IFNγ, Interferon Gamma; IP10, Interferon-inducible Protein 10; MCP1, Monocyte Chemoattractant Protein 1; GCSF, Granulocyte Colony Stimulating Factor; IL2, Interleukin 2; IL7, Interleukin 7; IL10, Interleukin 10; MIP1A, Macrophage Inflammatory Protein-1 alpha; TNFα, Tumor Necrosis Factor alpha.
1. Who-China-Joint-Mission-on-Covid-19-Final-Report.pdf . Available online at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed June 1, 2020).
2. Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. (2020) 12:372. doi: 10.3390/v12040372
CrossRef Full Text | Google Scholar
3. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes it . (2020). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed June 7, 2020).
Google Scholar
4. Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. (2020) 13:667–73. doi: 10.1016/j.jiph.2020.03.019
PubMed Abstract | CrossRef Full Text | Google Scholar
5. WHO Director-General's opening remarks at the media briefing on COVID-19 . (2020). Available online at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-−11-march-2020 (accessed June 7, 2020).
6. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. (2020) 109:102433. doi: 10.1016/j.jaut.2020.102433
7. Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. (2020). doi: 10.1016/j.ajog.2020.07.049. [Epub ahead of print].
8. Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. (2020) 80:401–6. doi: 10.1016/j.jinf.2020.02.018
9. Cave E. COVID-19 super-spreaders: definitional quandaries and implications. Asian Bioeth Rev. (2020) 12:235–42. doi: 10.1007/s41649-020-00118-2
10. Kakodkar P, Kaka N, Baig M. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19). Cureus. (2020) 12:e7560. doi: 10.7759/cureus.7560
11. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. (2020) 20:363–74. doi: 10.1038/s41577-020-0311-8
PubMed Abstract | CrossRef Full Text
12. Veerdonk F, van de Netea MG, Deuren M, van Meer JWM, van der Mast Q, de Bruggemann RJ, et al. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. eLife. (2020) 9:e57555. doi: 10.7554/eLife.57555
13. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. (2020) 80:607–13. doi: 10.1016/j.jinf.2020.03.037
14. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. (2020) 220, 1–13. doi: 10.1016/j.trsl.2020.04.007
15. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. (2020) 9:727–32. doi: 10.1080/22221751.2020.1746199
16. Ottestad W, Seim M, Mæhlen JO. COVID-19 with silent hypoxemia. Tidsskr Den NorLegeforening. (2020) 140. doi: 10.4045/tidsskr.20.0299
17. Couzin-Frankel J. The mystery of the pandemic's “happy hypoxia.” Science . (2020) 368:455–6. doi: 10.1126/science.368.6490.455
18. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
19. Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. (2020) 30:4381–9. doi: 10.1007/s00330-020-06801-0
20. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Neck Surg. (2020) 163:3–11. doi: 10.1177/0194599820926473
CrossRef Full Text
21. Krajewska J, Krajewski W, Zub K, Zatoński T. COVID-19 in otolaryngologist practice: a review of current knowledge. Eur Arch Otorhinolaryngol. (2020) 277:1885–97. doi: 10.1007/s00405-020-05968-y
22. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. (2020) 12:8. doi: 10.1038/s41368-020-0074-x
23. Akhmerov A, Marbán E. COVID-19 and the Heart. Circ Res. (2020) 126:1443–55. doi: 10.1161/CIRCRESAHA.120.317055
24. Clerkin Kevin J, Fried Justin A, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. COVID-19 and cardiovascular disease. Circulation. (2020) 141:1648–55. doi: 10.1161/CIRCULATIONAHA.120.046941
25. Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr Clin Res Rev. (2020) 14:247–50. doi: 10.1016/j.dsx.2020.03.013
26. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. doi: 10.1001/jamacardio.2020.0950
27. Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. (2020) 31:1003–8. doi: 10.1111/jce.14479
28. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
29. Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. (2020) 17:259–60. doi: 10.1038/s41569-020-0360-5
30. Patients Taking ACE-i and ARBs who Contract COVID-19 Should Continue Treatment Unless Otherwise Advised by Their Physician . American Heart Association (2020). Available online at: https://newsroom.heart.org/news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician (accessed June 27, 2020).
31. Hasan SS, Kow CS, Hadi MA, Zaidi STR, Merchant HA. Mortality and disease severity among COVID-19 patients receiving renin-angiotensin system inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. (2020). doi: 10.22541/au.158880148.84250526. [Epub ahead of print].
32. Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. (2020) 51:843–51. doi: 10.1111/apt.15731
33. An P, Chen H, Jiang X, Su J, Xiao Y, Ding Y, et al. Clinical Features of 2019 Novel Coronavirus Pneumonia Presented Gastrointestinal Symptoms But Without Fever Onset . Rochester, NY: Social Science Research Network (2020). Available online at: https://papers.ssrn.com/abstract=3532530 (accessed June 27, 2020). doi: 10.2139/ssrn.3532530
34. Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of covid-19 patients with digestive symptoms in hubei, china: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. (2020) 115, 766–73. doi: 10.14309/ajg.0000000000000620
35. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. (2020) 158:1831–3.e3. doi: 10.1053/j.gastro.2020.02.055
36. Feng G, Zheng KI, Yan Q-Q, Rios RS, Targher G, Byrne CD, et al. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. (2020) 8:18–24. doi: 10.14218/JCTH.2020.00018
37. Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. (2020) 98:219–27. doi: 10.1016/j.kint.2020.04.003
38. Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. (2020) 16:308–10. doi: 10.1038/s41581-020-0284-7
39. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. (2020) 97:829–38. doi: 10.1016/j.kint.2020.03.005
40. Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. (2020) 98:209–18. doi: 10.1016/j.kint.2020.05.006
41. Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. Covid-19 and kidney transplantation. N Engl J Med. (2020) 382:2475–7. doi: 10.1056/NEJMc2011117
42. Sheraton M, Deo N, Kashyap R, Surani S. A review of neurological complications of COVID-19. Cureus. (2020) 12:e8192. doi: 10.7759/cureus.8192
43. Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. (2020) 38:1549.e3–7. doi: 10.1016/j.ajem.2020.05.024
44. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:683–90. doi: 10.1001/jamaneurol.2020.1127
45. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. (2020). doi: 10.21203/rs.3.rs-31183/v1. [Epub ahead of print].
46. Dockery DM, Rowe SG, Murphy MA, Krzystolik MG. The ocular manifestations and transmission of COVID-19; recommendations for prevention. J Emerg Med. (2020) 59:137–40. doi: 10.1016/j.jemermed.2020.04.060
47. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. (2020) 34:e212–3. doi: 10.1111/jdv.16387
48. Sachdeva M, Gianotti R, Shah M, Lucia B, Tosi D, Veraldi S, et al. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J Dermatol Sci. (2020) 98:75–81. doi: 10.1016/j.jdermsci.2020.04.011
49. Casas CG, Català A, Hernández GC, Rodríguez-Jiménez P, Fernández-Nieto D, Lario AR-V, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. (2020) 183:71–7. doi: 10.1111/bjd.19163
50. Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, et al. COVID-19 and kawasaki disease: novel virus and novel case. Hosp Pediatr. (2020) 10:537–40. doi: 10.1542/hpeds.2020-0123
51. Labé P, Ly A, Sin C, Nasser M, Chapelon-Fromont E, Saïd PB, et al. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J Eur Acad Dermatol Venereol. (2020) 34:e539–41. doi: 10.1111/jdv.16666
52. Tang K, Wang Y, Zhang H, Zheng Q, Fang R, Sun Q. Cutaneous manifestations of the Coronavirus Disease 2019 (COVID-19): a brief review. Dermatol Ther. (2020) 33:e13528. doi: 10.1111/dth.13528
53. Cipollaro L, Giordano L, Padulo J, Oliva F, Maffulli N. Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. J Orthop Surg. (2020) 15:178. doi: 10.1186/s13018-020-01702-w
54. Suwanwongse K, Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus. (2020) 12:e7561. doi: 10.7759/cureus.7561
55. Chan KH, Farouji I, Hanoud AA, Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19). Am J Emerg Med. (2020) 38:1548.e1–3. doi: 10.1016/j.ajem.2020.05.015
56. López-González M-C, Peral-Garrido ML, Calabuig I, Tovar-Sugrañes E, Jovani V, Bernabeu P, et al. Case series of acute arthritis during COVID-19 admission. Ann Rheum Dis. (2020) doi: 10.1136/annrheumdis-2020-217914. [Epub ahead of print].
57. Qu R, Ling Y, Zhang Y, Wei L, Chen X, Li X, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. (2020) 92:1533–41. doi: 10.1002/jmv.25767
58. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. (2020) 7:e438–40. doi: 10.1016/S2352-3026(20)30145-9
59. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med CCLM. (2020) 58:1131–4. doi: 10.1515/cclm-2020-0198
60. Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. (2020) 92:2283–5. doi: 10.1002/jmv.25948
61. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
62. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab. (2020) 105:dgaa276. doi: 10.1210/clinem/dgaa276
63. AsfurogluKalkan E, Ates I. A case of subacute thyroiditis associated with Covid-19 infection. J Endocrinol Invest. (2020) 43:1173–4. doi: 10.1007/s40618-020-01316-3
64. Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases a statement from the European society of endocrinology. Endocrine. (2020) 68:2–5. doi: 10.1007/s12020-020-02294-5
65. Illiano E, Trama F, Costantini E. Could COVID-19 have an impact on male fertility? Andrologia. (2020) 52:e13654. doi: 10.1111/and.13654
66. Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, et al. Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.21.20037267
67. Segars J, Katler Q, McQueen DB, Kotlyar A, Glenn T, Knight Z, et al. Prior and novel coronaviruses, Coronavirus disease 2019 (COVID-19), and human reproduction: what is known? FertilSteril. (2020) 113:1140–9. doi: 10.1016/j.fertnstert.2020.04.025
68. CDC Weekly C, The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Wkly . (2020) 2:113–22. doi: 10.46234/ccdcw2020.032
69. Chang T-H, Wu J-L, Chang L-Y. Clinical characteristics and diagnostic challenges of pediatric COVID-19: a systematic review and meta-analysis. J Formos Med Assoc. (2020) 119:982–9. doi: 10.1016/j.jfma.2020.04.007
70. Wollina U, Karadag AS, Rowland-Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. (2020) 33:e13549. doi: 10.1111/dth.13549
71. Abrams JY, Godfred-Cato SE, Oster ME, Chow EJ, Koumans EH, Bryant B, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. (2020) 226:45–54.e1. doi: 10.1016/j.jpeds.2020.08.003
72. Smith V, Seo D, Warty R, Payne O, Salih M, Chin KL, et al. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS ONE. (2020) 15:e0234187. doi: 10.1371/journal.pone.0234187
73. Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J Infect. (2020) 80:e7–13. doi: 10.1016/j.jinf.2020.03.007
74. Breastfeeding and COVID-19 . (2020). Available online at: https://www.who.int/news-room/commentaries/detail/breastfeeding-and-covid-19 (accessed Jun 25, 2020).
75. Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. (2020) 11:3572. doi: 10.1038/s41467-020-17436-6
76. Minotti C, Tirelli F, Barbieri E, Giaquinto C, Donà D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? a systematic review. J Infect. (2020) 81:e61–6. doi: 10.1016/j.jinf.2020.04.026
77. Europe PMC . (2020). Available online at: https://europepmc.org/articles/pmc7383595/bin/nejmoa2021436_appendix.pdf (accessed November 20, 2020).
78. Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect. (2020) 81:e93–5. doi: 10.1016/j.jinf.2020.05.017
79. Mirzaei H, McFarland W, Karamouzian M, Sharifi H. COVID-19 among people living with HIV: a systematic review. AIDS Behav. (2020) 1–8. doi: 10.1007/s10461-020-02983-2. [Epub ahead of print].
80. Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection — United Kingdom and United States, March–August 2020. Morb Mortal Wkly Rep. (2020) 69:1450–6. doi: 10.15585/mmwr.mm6940e1
81. Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, et al. COVID-19–Associated Multisystem Inflammatory Syndrome in Children — United States, March–July 2020. Morb Mortal Wkly Rep. (2020) 69:1074–80. doi: 10.15585/mmwr.mm6932e2
82. Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May (2020. Eurosurveillance. (2020) 25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010
83. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) 324:259. doi: 10.1001/jama.2020.10369
84. Rodríguez-Molinero A, Gálvez-Barrón C, Miñarro A, Macho O, López GF, Robles MT, et al. Association between COVID-19 prognosis and disease presentation, comorbidities and chronic treatment of hospitalized patients. PLoS ONE. (2020) 15:e0239571. doi: 10.1371/journal.pone.0239571
Keywords: SARS-CoV-2, Covid-19, symptomatology, clinical presentation, signs and symptoms, clinical features, coronavirus
Citation: Mehta OP, Bhandari P, Raut A, Kacimi SEO and Huy NT (2021) Coronavirus Disease (COVID-19): Comprehensive Review of Clinical Presentation. Front. Public Health 8:582932. doi: 10.3389/fpubh.2020.582932
Received: 13 July 2020; Accepted: 15 December 2020; Published: 15 January 2021.
Reviewed by:
Copyright © 2021 Mehta, Bhandari, Raut, Kacimi and Huy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nguyen Tien Huy, tienhuy@nagasaki-u.ac.jp
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 11 February 2021
Methodological quality of COVID-19 clinical research
- Richard G. Jung ORCID: orcid.org/0000-0002-8570-6736 1 , 2 , 3 na1 ,
- Pietro Di Santo 1 , 2 , 4 , 5 na1 ,
- Cole Clifford 6 ,
- Graeme Prosperi-Porta 7 ,
- Stephanie Skanes 6 ,
- Annie Hung 8 ,
- Simon Parlow 4 ,
- Sarah Visintini ORCID: orcid.org/0000-0001-6966-1753 9 ,
- F. Daniel Ramirez ORCID: orcid.org/0000-0002-4350-1652 1 , 4 , 10 , 11 ,
- Trevor Simard 1 , 2 , 3 , 4 , 12 &
- Benjamin Hibbert ORCID: orcid.org/0000-0003-0906-1363 2 , 3 , 4
Nature Communications volume 12 , Article number: 943 ( 2021 ) Cite this article
13k Accesses
95 Citations
238 Altmetric
Metrics details
- Infectious diseases
- Public health
The COVID-19 pandemic began in early 2020 with major health consequences. While a need to disseminate information to the medical community and general public was paramount, concerns have been raised regarding the scientific rigor in published reports. We performed a systematic review to evaluate the methodological quality of currently available COVID-19 studies compared to historical controls. A total of 9895 titles and abstracts were screened and 686 COVID-19 articles were included in the final analysis. Comparative analysis of COVID-19 to historical articles reveals a shorter time to acceptance (13.0[IQR, 5.0–25.0] days vs. 110.0[IQR, 71.0–156.0] days in COVID-19 and control articles, respectively; p < 0.0001). Furthermore, methodological quality scores are lower in COVID-19 articles across all study designs. COVID-19 clinical studies have a shorter time to publication and have lower methodological quality scores than control studies in the same journal. These studies should be revisited with the emergence of stronger evidence.
Similar content being viewed by others
A meta-analysis on global change drivers and the risk of infectious disease

Long COVID: major findings, mechanisms and recommendations

Causal machine learning for predicting treatment outcomes
Introduction.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic spread globally in early 2020 with substantial health and economic consequences. This was associated with an exponential increase in scientific publications related to the coronavirus disease 2019 (COVID-19) in order to rapidly elucidate the natural history and identify diagnostic and therapeutic tools 1 .
While a need to rapidly disseminate information to the medical community, governmental agencies, and general public was paramount—major concerns have been raised regarding the scientific rigor in the literature 2 . Poorly conducted studies may originate from failure at any of the four consecutive research stages: (1) choice of research question relevant to patient care, (2) quality of research design 3 , (3) adequacy of publication, and (4) quality of research reports. Furthermore, evidence-based medicine relies on a hierarchy of evidence, ranging from the highest level of randomized controlled trials (RCT) to the lowest level of case series and case reports 4 .
Given the implications for clinical care, policy decision making, and concerns regarding methodological and peer-review standards for COVID-19 research 5 , we performed a formal evaluation of the methodological quality of published COVID-19 literature. Specifically, we undertook a systematic review to identify COVID-19 clinical literature and matched them to historical controls to formally evaluate the following: (1) the methodological quality of COVID-19 studies using established quality tools and checklists, (2) the methodological quality of COVID-19 studies, stratified by median time to acceptance, geographical regions, and journal impact factor and (3) a comparison of COVID-19 methodological quality to matched controls.
Herein, we show that COVID-19 articles are associated with lower methodological quality scores. Moreover, in a matched cohort analysis with control articles from the same journal, we reveal that COVID-19 articles are associated with lower quality scores and shorter time from submission to acceptance. Ultimately, COVID-19 clinical studies should be revisited with the emergence of stronger evidence.
Article selection
A total of 14787 COVID-19 papers were identified as of May 14, 2020 and 4892 duplicate articles were removed. In total, 9895 titles and abstracts were screened, and 9101 articles were excluded due to the study being pre-clinical in nature, case report, case series <5 patients, in a language other than English, reviews (including systematic reviews), study protocols or methods, and other coronavirus variants with an overall inter-rater study inclusion agreement of 96.7% ( κ = 0.81; 95% CI, 0.79–0.83). A total number of 794 full texts were reviewed for eligibility. Over 108 articles were excluded for ineligible study design or publication type (such as letter to the editors, editorials, case reports or case series <5 patients), wrong patient population, non-English language, duplicate articles, wrong outcomes and publication in a non-peer-reviewed journal. Ultimately, 686 articles were identified with an inter-rater agreement of 86.5% ( κ = 0.68; 95% CI, 0.67–0.70) (Fig. 1 ).
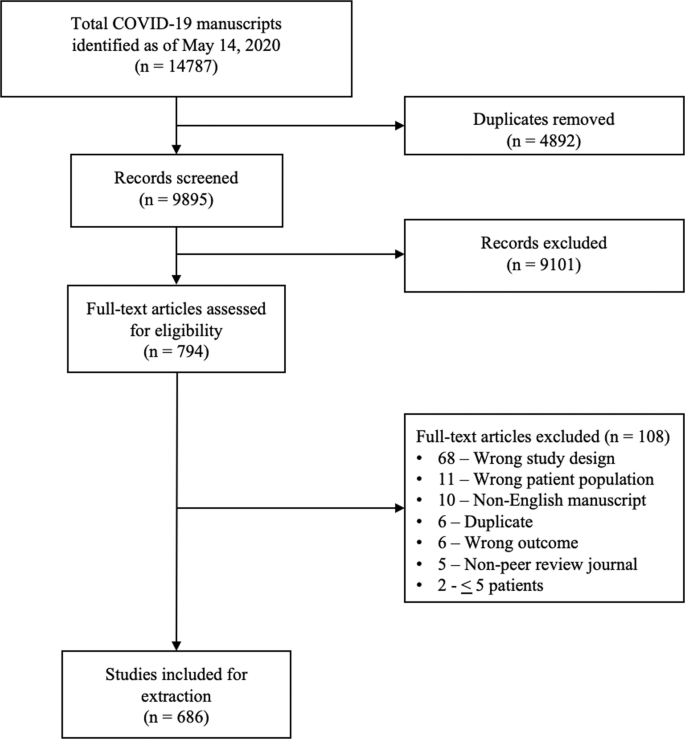
A total of 14787 articles were identified and 4892 duplicate articles were removed. Overall, 9895 articles were screened by title and abstract leaving 794 articles for full-text screening. Over 108 articles were excluded, leaving a total of 686 articles that underwent methodological quality assessment.
COVID-19 literature methodological quality
Most studies originated from Asia/Oceania with 469 (68.4%) studies followed by Europe with 139 (20.3%) studies, and the Americas with 78 (11.4%) studies. Of included studies, 380 (55.4%) were case series, 199 (29.0%) were cohort, 63 (9.2%) were diagnostic, 38 (5.5%) were case–control, and 6 (0.9%) were RCTs. Most studies (590, 86.0%) were retrospective in nature, 620 (90.4%) reported the sex of patients, and 7 (2.3%) studies excluding case series calculated their sample size a priori. The method of SARS-CoV-2 diagnosis was reported in 558 studies (81.3%) and ethics approval was obtained in 556 studies (81.0%). Finally, journal impact factor of COVID-19 manuscripts was 4.7 (IQR, 2.9–7.6) with a time to acceptance of 13.0 (IQR, 5.0–25.0) days (Table 1 ).
Overall, when COVID-19 articles were stratified by study design, a mean case series score (out of 5) (SD) of 3.3 (1.1), mean NOS cohort study score (out of 8) of 5.8 (1.5), mean NOS case–control study score (out of 8) of 5.5 (1.9), and low bias present in 4 (6.4%) diagnostic studies was observed (Table 2 and Fig. 2 ). Furthermore, in the 6 RCTs in the COVID-19 literature, there was a high risk of bias with little consideration for sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting (Table 2 ).
A Distribution of COVID-19 case series studies scored using the Murad tool ( n = 380). B Distribution of COVID-19 cohort studies scored using the Newcastle–Ottawa Scale ( n = 199). C Distribution of COVID-19 case–control studies scored using the Newcastle–Ottawa Scale ( n = 38). D Distribution of COVID-19 diagnostic studies scored using the QUADAS-2 tool ( n = 63). In panel D , blue represents low risk of bias and orange represents high risk of bias.
For secondary outcomes, rapid time from submission to acceptance (stratified by median time of acceptance of <13.0 days) was associated with lower methodological quality scores for case series and cohort study designs but not for case–control nor diagnostic studies (Fig. 3A–D ). Low journal impact factor (<10) was associated with lower methodological quality scores for case series, cohort, and case–control designs (Fig. 3E–H ). Finally, studies originating from different geographical regions had no differences in methodological quality scores with the exception of cohort studies (Fig. 3I–L ). When dichotomized by high vs. low methodological quality scores, a similar trend was observed with rapid time from submission to acceptance (34.4% vs. 46.3%, p = 0.01, Supplementary Fig. 1B ), low impact factor journals (<10) was associated with lower methodological quality score (38.8% vs. 68.0%, p < 0.0001, Supplementary Fig. 1C ). Finally, studies originating in either Americas or Asia/Oceania was associated with higher methodological quality scores than Europe (Supplementary Fig. 1D ).
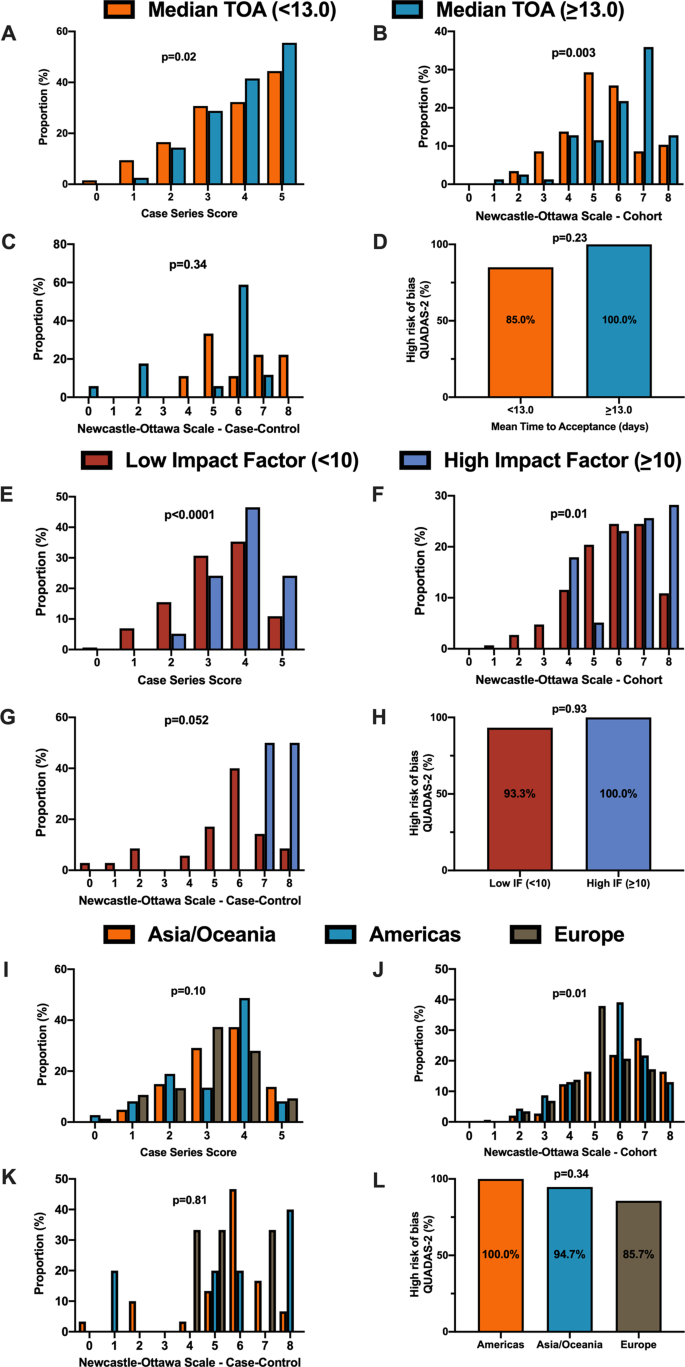
A When stratified by time of acceptance (13.0 days), increased time of acceptance was associated with higher case series score ( n = 186 for <13 days and n = 193 for >=13 days; p = 0.02). B Increased time of acceptance was associated with higher NOS cohort score ( n = 112 for <13 days and n = 144 for >=13 days; p = 0.003). C No difference in time of acceptance and case–control score was observed ( n = 18 for <13 days and n = 27 for >=13 days; p = 0.34). D No difference in time of acceptance and diagnostic risk of bias (QUADAS-2) was observed ( n = 43 for <13 days and n = 33 for >=13 days; p = 0.23). E When stratified by impact factor (IF ≥10), high IF was associated with higher case series score ( n = 466 for low IF and n = 60 for high IF; p < 0.0001). F High IF was associated with higher NOS cohort score ( n = 262 for low IF and n = 68 for high IF; p = 0.01). G No difference in IF and case–control score was observed ( n = 62 for low IF and n = 2 for high IF; p = 0.052). H No difference in IF and QUADAS-2 was observed ( n = 101 for low IF and n = 2 for high IF; p = 0.93). I When stratified by geographical region, no difference in geographical region and case series score was observed ( n = 276 Asia/Oceania, n = 135 Americas, and n = 143 Europe/Africa; p = 0.10). J Geographical region was associated with differences in cohort score ( n = 177 Asia/Oceania, n = 81 Americas, and n = 89 Europe/Africa; p = 0.01). K No difference in geographical region and case–control score was observed ( n = 37 Asia/Oceania, n = 13 Americas, and n = 14 Europe/Africa; p = 0.81). L No difference in geographical region and QUADAS-2 was observed ( n = 49 Asia/Oceania, n = 28 Americas, and n = 28 Europe/Africa; p = 0.34). In panels A – D , orange represents lower median time of acceptance and blue represents high median time of acceptance. In panels E – H , red is low impact factor and blue is high impact factor. In panels I – L , orange represents Asia/Oceania, blue represents Americas, and brown represents Europe. Differences in distributions were analysed by two-sided Kruskal–Wallis test. Differences in diagnostic risk of bias were quantified by Chi-squares test. p < 0.05 was considered statistically significant.
Methodological quality score differences in COVID-19 versus historical control
We matched 539 historical control articles to COVID-19 articles from the same journal with identical study designs in the previous year for a final analysis of 1078 articles (Table 1 ). Overall, 554 (51.4%) case series, 348 (32.3%) cohort, 64 (5.9%) case–control, 106 (9.8%) diagnostic and 6 (0.6%) RCTs were identified from the 1078 total articles. Differences exist between COVID-19 and historical control articles in geographical region of publication, retrospective study design, and sample size calculation (Table 1 ). Time of acceptance was 13.0 (IQR, 5.0–25.0) days in COVID-19 articles vs. 110.0 (IQR, 71.0–156.0) days in control articles (Table 1 and Fig. 4A , p < 0.0001). Case-series methodological quality score was lower in COVID-19 articles compared to the historical control (3.3 (1.1) vs. 4.3 (0.8); n = 554; p < 0.0001; Table 2 and Fig. 4B ). Furthermore, NOS score was lower in COVID-19 cohort studies (5.8 (1.6) vs. 7.1 (1.0); n = 348; p < 0.0001; Table 2 and Fig. 4C ) and case–control studies (5.4 (1.9) vs. 6.6 (1.0); n = 64; p = 0.003; Table 2 and Fig. 4D ). Finally, lower risk of bias in diagnostic studies was in 12 COVID-19 articles (23%; n = 53) compared to 24 control articles (45%; n = 53; p = 0.02; Table 2 and Fig. 4E ). A similar trend was observed between COVID-19 and historical control articles when dichotomized by good vs. low methodological quality scores (Supplementary Fig. 2 ).
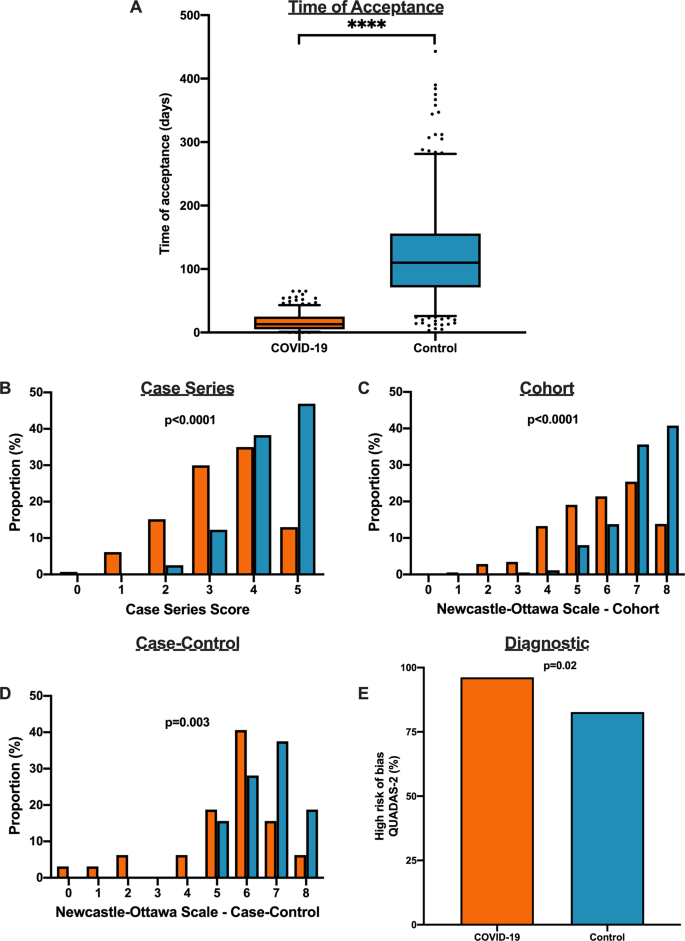
A Time to acceptance was reduced in COVID-19 articles compared to control articles (13.0 [IQR, 5.0–25.0] days vs. 110.0 [IQR, 71.0–156.0] days, n = 347 for COVID-19 and n = 414 for controls; p < 0.0001). B When compared to historical control articles, COVID-19 articles were associated with lower case series score ( n = 277 for COVID-19 and n = 277 for controls; p < 0.0001). C COVID-19 articles were associated with lower NOS cohort score compared to historical control articles ( n = 174 for COVID-19 and n = 174 for controls; p < 0.0001). D COVID-19 articles were associated with lower NOS case–control score compared to historical control articles ( n = 32 for COVID-19 and n = 32 for controls; p = 0.003). E COVID-19 articles were associated with higher diagnostic risk of bias (QUADAS-2) compared to historical control articles ( n = 53 for COVID-19 and n = 53 for controls; p = 0.02). For panel A , boxplot captures 5, 25, 50, 75 and 95% from the first to last whisker. Orange represents COVID-19 articles and blue represents control articles. Two-sided Mann–Whitney U-test was conducted to evaluate differences in time to acceptance between COVID-19 and control articles. Differences in study quality scores were evaluated by two-sided Kruskal–Wallis test. Differences in diagnostic risk of bias were quantified by Chi-squares test. p < 0.05 was considered statistically significant.
In this systematic evaluation of methodological quality, COVID-19 clinical research was primarily observational in nature with modest methodological quality scores. Not only were the study designs low in the hierarchy of scientific evidence, we found that COVID-19 articles were associated with a lower methodological quality scores when published with a shorter time of publication and in lower impact factor journals. Furthermore, in a matched cohort analysis with historical control articles identified from the same journal of the same study design, we demonstrated that COVID-19 articles were associated with lower quality scores and shorter time from submission to acceptance.
The present study demonstrates comparative differences in methodological quality scores between COVID-19 literature and historical control articles. Overall, the accelerated publication of COVID-19 research was associated with lower study quality scores compared to previously published historical control studies. Our research highlights major differences in study quality between COVID-19 and control articles, possibly driven in part by a combination of more thorough editorial and/or peer-review process as suggested by the time to publication, and robust study design with questions which are pertinent for clinicians and patient management 3 , 6 , 7 , 8 , 9 , 10 , 11 .
In the early stages of the COVID-19 pandemic, we speculate that an urgent need for scientific data to inform clinical, social and economic decisions led to shorter time to publication and explosion in publication of COVID-19 studies in both traditional peer-reviewed journals and preprint servers 1 , 12 . The accelerated scientific process in the COVID-19 pandemic allowed a rapid understanding of natural history of COVID-19 symptomology and prognosis, identification of tools including RT-PCR to diagnose SARS-CoV-2 13 , and identification of potential therapeutic options such as tocilizumab and convalescent plasma which laid the foundation for future RCTs 14 , 15 , 16 . A delay in publication of COVID-19 articles due to a slower peer-review process may potentially delay dissemination of pertinent information against the pandemic. Despite concerns of slow peer review, major landmark trials (i.e. RECOVERY and ACTT-1 trial) 17 , 18 published their findings in preprint servers and media releases to allow for rapid dissemination. Importantly, the data obtained in these initial studies should be revisited as stronger data emerges as lower quality studies may fundamentally risk patient safety, resource allocation and future scientific research 19 .
Unfortunately, poor evidence begets poor clinical decisions 20 . Furthermore, lower quality scientific evidence potentially undermines the public’s trust in science during this time and has been evident through misleading information and high-profile retractions 12 , 21 , 22 , 23 . For example, the benefits of hydroxychloroquine, which were touted early in the pandemic based on limited data, have subsequently failed to be replicated in multiple observational studies and RCTs 5 , 24 , 25 , 26 , 27 , 28 , 29 , 30 . One poorly designed study combined with rapid publication led to considerable investment of both the scientific and medical community—akin to quinine being sold to the public as a miracle drug during the 1918 Spanish Influenza 31 , 32 . Moreover, as of June 30, 2020, ClinicalTrials.gov listed an astonishing 230 COVID-19 trials with hydroxychloroquine/plaquenil, and a recent living systematic review of observational studies and RCTs of hydroxychloroquine or chloroquine for COVID-19 demonstrated no evidence of benefit nor harm with concerns of severe methodological flaws in the included studies 33 .
Our study has important limitations. We evaluated the methodological quality of existing studies using established checklists and tools. While it is tempting to associate methodological quality scores with reproducibility or causal inferences of the intervention, it is not possible to ascertain the impact on the study design and conduct of research nor results or conclusions in the identified reports 34 . Second, although the methodological quality scales and checklists used for the manuscript are commonly used for quality assessment in systematic reviews and meta-analyses 35 , 36 , 37 , 38 , they can only assess the methodology without consideration for causal language and are prone to limitations 39 , 40 . Other tools such as the ROBINS-I and GRADE exist to evaluate methodological quality of identified manuscripts, although no consensus currently exists for critical appraisal of non-randomized studies 41 , 42 , 43 . Furthermore, other considerations of quality such as sample size calculation, sex reporting or ethics approval are not considered in these quality scores. As such, the quality scores measured using these checklists only reflect the patient selection, comparability, diagnostic reference standard and methods to ascertain the outcome of the study. Third, the 1:1 ratio to identify our historical control articles may affect the precision estimates of our findings. Interestingly, a simulation of an increase from 1:1 to 1:4 control ratio tightened the precision estimates but did not significantly alter the point estimate 44 . Furthermore, the decision for 1:1 ratio in our study exists due to limitations of available historical control articles from the identical journal in the restricted time period combined with a large effect size and sample size in the analysis. Finally, our analysis includes early publications on COVID-19 and there is likely to be an improvement in quality of related studies and study design as the field matures and higher-quality studies. Accordingly, our findings are limited to the early body of research as it pertains to the pandemic and it is likely that over time research quality will improve over time.
In summary, the early body of peer-reviewed COVID-19 literature was composed primarily of observational studies that underwent shorter peer-review evaluation and were associated with lower methodological quality scores than comparable studies. COVID-19 clinical studies should be revisited with the emergence of stronger evidence.
A systematic literature search was conducted on May 14, 2020 (registered on June 3, 2020 at PROSPERO: CRD42020187318) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Furthermore, the cohort study was reported according to the Strengthening The Reporting of Observational Studies in Epidemiology checklist. The data supporting the findings of this study is available as Supplementary Data 1 – 2 .
Data sources and searches
The search was created in MEDLINE by a medical librarian with expertise in systematic reviews (S.V.) using a combination of key terms and index headings related to COVID-19 and translated to the remaining bibliographic databases (Supplementary Tables 1 – 3 ). The searches were conducted in MEDLINE (Ovid MEDLINE(R) ALL 1946–), Embase (Ovid Embase Classic + Embase 1947–) and the Cochrane Central Register of Controlled Trials (from inception). Search results were limited to English-only publications, and a publication date limit of January 1, 2019 to present was applied. In addition, a Canadian Agency for Drugs and Technologies in Health search filter was applied in MEDLINE and Embase to remove animal studies, and commentary, newspaper article, editorial, letter and note publication types were also eliminated. Search results were exported to Covidence (Veritas Health Innovation, Melbourne, Australia) and duplicates were eliminated using the platform’s duplicate identification feature.

Study selection, data extraction and methodological quality assessment
We included all types of COVID-19 clinical studies, including case series, observational studies, diagnostic studies and RCTs. For diagnostic studies, the reference standard for COVID-19 diagnosis was defined as a nasopharyngeal swab followed by reverse transcriptase-polymerase chain reaction in order to detect SARS-CoV-2. We excluded studies that were exploratory or pre-clinical in nature (i.e. in vitro or animal studies), case reports or case series of <5 patients, studies published in a language other than English, reviews, methods or protocols, and other coronavirus variants such as the Middle East respiratory syndrome.
The review team consisted of trained research staff with expertise in systematic reviews and one trainee. Title and abstracts were evaluated by two independent reviewers using Covidence and all discrepancies were resolved by consensus. Articles that were selected for full review were independently evaluated by two reviewers for quality assessment using a standardized case report form following the completion of a training period where all reviewers were trained with the original manuscripts which derived the tools or checklists along with examples for what were deemed high scores 35 , 36 , 37 , 38 . Following this, reviewers completed thirty full-text extractions and the two reviewers had to reach consensus and the process was repeated for the remaining manuscripts independently. When two independent reviewers were not able reach consensus, a third reviewer (principal investigator) provided oversight in the process to resolve the conflicted scores.
First and corresponding author names, date of publication, title of manuscript and journal of publication were collected for all included full-text articles. Journal impact factor was obtained from the 2018 InCites Journal Citation Reports from Clarivate Analytics. Submission and acceptance dates were collected in manuscripts when available. Other information such as study type, prospective or retrospective study, sex reporting, sample size calculation, method of SARS-CoV-2 diagnosis and ethics approval was collected by the authors. Methodological quality assessment was conducted using the Newcastle–Ottawa Scale (NOS) for case–control and cohort studies 37 , QUADAS-2 tool for diagnostic studies 38 , Cochrane risk of bias for RCTs 35 and a score derived by Murad et al. for case series studies 36 .
Identification of historical control from identified COVID-19 articles
Following the completion of full-text extraction of COVID-19 articles, we obtained a historical control group by identifying reports matched in a 1:1 fashion. From the eligible COVID-19 article, historical controls were identified by searching the same journal in a systematic fashion by matching the same study design (“case series”, “cohort”, “case control” or “diagnostic”) starting in the journal edition 12 months prior to the COVID-19 article publication on the publisher website (i.e. COVID-19 article published on April 2020, going backwards to April 2019) and proceeding forward (or backward if a specific article type was not identified) in a temporal fashion until the first matched study was identified following abstract screening by two independent reviewers. If no comparison article was found by either reviewers, the corresponding COVID-19 article was excluded from the comparison analysis. Following the identification of the historical control, data extraction and quality assessment was conducted on the identified articles using the standardized case report forms by two independent reviewers and conflicts resolved by consensus. The full dataset has been made available as Supplementary Data 1 – 2 .
Data synthesis and statistical analysis
Continuous variables were reported as mean (SD) or median (IQR) as appropriate, and categorical variables were reported as proportions (%). Continuous variables were compared using Student t -test or Mann–Whitney U-test and categorical variables including quality scores were compared by χ 2 , Fisher’s exact test, or Kruskal–Wallis test.
The primary outcome of interest was to evaluate the methodological quality of COVID-19 clinical literature by study design using the Newcastle–Ottawa Scale (NOS) for case–control and cohort studies, QUADAS-2 tool for diagnostic studies 38 , Cochrane risk of bias for RCTs 35 , and a score derived by Murad et al. for case series studies 36 . Pre-specified secondary outcomes were comparison of methodological quality scores of COVID-19 articles by (i) median time to acceptance, (ii) impact factor, (iii) geographical region and (iv) historical comparator. Time of acceptance was defined as the time between submission to acceptance which captures peer review and editorial decisions. Geographical region was stratified into continents including Asia/Oceania, Europe/Africa and Americas (North and South America). Post hoc comparison analysis between COVID-19 and historical control article quality scores were evaluated using Kruskal–Wallis test. Furthermore, good quality of NOS was defined as 3+ on selection and 1+ on comparability, and 2+ on outcome/exposure domains and high-quality case series scores was defined as a score ≥3.5. Due to a small sample size of identified RCTs, they were not included in the comparison analysis.
The finalized dataset was collected on Microsoft Excel v16.44. All statistical analyses were performed using SAS v9.4 (SAS Institute, Inc., Cary, NC, USA). Statistical significance was defined as P < 0.05. All figures were generated using GraphPad Prism v8 (GraphPad Software, La Jolla, CA, USA).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The authors can confirm that all relevant data are included in the paper and in Supplementary Data 1 – 2 . The original search was conducted on MEDLINE, Embase and Cochrane Central Register of Controlled Trials.
Chen, Q., Allot, A. & Lu, Z. Keep up with the latest coronavirus research. Nature 579 , 193 (2020).
Article ADS CAS Google Scholar
Mahase, E. Covid-19: 146 researchers raise concerns over chloroquine study that halted WHO trial. BMJ https://doi.org/10.1136/bmj.m2197 (2020).
Chalmers, I. & Glasziou, P. Avoidable waste in the production and reporting of research evidence. Lancet 374 , 86–89 (2009).
Article Google Scholar
Burns, P. B., Rohrich, R. J. & Chung, K. C. The levels of evidence and their role in evidence-based medicine. Plast. Reconstr. Surg. 128 , 305–310 (2011).
Article CAS Google Scholar
Alexander, P. E. et al. COVID-19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J. Clin. Epidemiol. 123 , 120–126 (2020).
Barakat, A. F., Shokr, M., Ibrahim, J., Mandrola, J. & Elgendy, I. Y. Timeline from receipt to online publication of COVID-19 original research articles. Preprint at medRxiv https://doi.org/10.1101/2020.06.22.20137653 (2020).
Chan, A.-W. et al. Increasing value and reducing waste: addressing inaccessible research. Lancet 383 , 257–266 (2014).
Ioannidis, J. P. A. et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 383 , 166–175 (2014).
Chalmers, I. et al. How to increase value and reduce waste when research priorities are set. Lancet 383 , 156–165 (2014).
Salman, R. A.-S. et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet 383 , 176–185 (2014).
Glasziou, P. et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 383 , 267–276 (2014).
Bauchner, H. The rush to publication: an editorial and scientific mistake. JAMA 318 , 1109–1110 (2017).
He, X. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 26 , 672–675 (2020).
Guaraldi, G. et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2 , e474–e484 (2020).
Duan, K. et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl Acad. Sci. USA 117 , 9490–9496 (2020).
Shen, C. et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA 323 , 1582–1589 (2020).
Beigel, J. H. et al. Remdesivir for the treatment of covid-19—final report. N. Engl. J. Med. 383 , 1813–1826 (2020).
Group, R. C. et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2021436 (2020).
Ramirez, F. D. et al. Methodological rigor in preclinical cardiovascular studies: targets to enhance reproducibility and promote research translation. Circ. Res 120 , 1916–1926 (2017).
Heneghan, C. et al. Evidence based medicine manifesto for better healthcare. BMJ 357 , j2973 (2017).
Mehra, M. R., Desai, S. S., Ruschitzka, F. & Patel, A. N. RETRACTED: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet https://doi.org/10.1016/S0140-6736(20)31180-6 (2020).
Servick, K. & Enserink, M. The pandemic’s first major research scandal erupts. Science 368 , 1041–1042 (2020).
Mehra, M. R., Desai, S. S., Kuy, S., Henry, T. D. & Patel, A. N. Retraction: Cardiovascular disease, drug therapy, and mortality in Covid-19. N. Engl. J. Med. 382 , 2582–2582, https://doi.org/10.1056/NEJMoa2007621. (2020).
Article PubMed Google Scholar
Boulware, D. R. et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N. Engl. J. Med. 383 , 517–525 (2020).
Gautret, P. et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med. Infect. Dis. 34 , 101663–101663 (2020).
Geleris, J. et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 382 , 2411–2418 (2020).
Borba, M. G. S. et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw. Open 3 , e208857–e208857 (2020).
Mercuro, N. J. et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5 , 1036–1041 (2020).
Molina, J. M. et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Médecine et. Maladies Infectieuses 50 , 384 (2020).
Group, R. C. et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med . 383, 2030–2040 (2020).
Shors, T. & McFadden, S. H. 1918 influenza: a Winnebago County, Wisconsin perspective. Clin. Med. Res. 7 , 147–156 (2009).
Stolberg, S. A Mad Scramble to Stock Millions of Malaria Pills, Likely for Nothing (The New York Times, 2020).
Hernandez, A. V., Roman, Y. M., Pasupuleti, V., Barboza, J. J. & White, C. M. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann. Int. Med. 173 , 287–296 (2020).
Glasziou, P. & Chalmers, I. Research waste is still a scandal—an essay by Paul Glasziou and Iain Chalmers. BMJ 363 , k4645 (2018).
Higgins, J. P. T. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343 , d5928 (2011).
Murad, M. H., Sultan, S., Haffar, S. & Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 23 , 60–63 (2018).
Wells, G. S. B. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. http://wwwohrica/programs/clinical_epidemiology/oxfordasp (2004).
Whiting, P. F. et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155 , 529–536 (2011).
Sanderson, S., Tatt, I. D. & Higgins, J. P. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int. J. Epidemiol. 36 , 666–676 (2007).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 , 603–605 (2010).
Guyatt, G. et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 64 , 383–394 (2011).
Quigley, J. M., Thompson, J. C., Halfpenny, N. J. & Scott, D. A. Critical appraisal of nonrandomized studies-A review of recommended and commonly used tools. J. Evaluation Clin. Pract. 25 , 44–52 (2019).
Sterne, J. A. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355 , i4919 (2016).
Hamajima, N. et al. Case-control studies: matched controls or all available controls? J. Clin. Epidemiol. 47 , 971–975 (1994).
Download references
Acknowledgements
This study received no specific funding or grant from any agency in the public, commercial, or not-for-profit sectors. R.G.J. was supported by the Vanier CIHR Canada Graduate Scholarship. F.D.R. was supported by a CIHR Banting Postdoctoral Fellowship and a Royal College of Physicians and Surgeons of Canada Detweiler Travelling Fellowship. The funder/sponsor(s) had no role in design and conduct of the study, collection, analysis and interpretation of the data.
Author information
These authors contributed equally: Richard G. Jung, Pietro Di Santo.
Authors and Affiliations
CAPITAL Research Group, University of Ottawa Heart Institute, Ottawa, Ontario, Canada
Richard G. Jung, Pietro Di Santo, F. Daniel Ramirez & Trevor Simard
Vascular Biology and Experimental Medicine Laboratory, University of Ottawa Heart Institute, Ottawa, Ontario, Canada
Richard G. Jung, Pietro Di Santo, Trevor Simard & Benjamin Hibbert
Department of Cellular and Molecular Medicine, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada
Richard G. Jung, Trevor Simard & Benjamin Hibbert
Division of Cardiology, University of Ottawa Heart Institute, Ottawa, Ontario, Canada
Pietro Di Santo, Simon Parlow, F. Daniel Ramirez, Trevor Simard & Benjamin Hibbert
School of Epidemiology and Public Health, University of Ottawa, Ottawa, Ontario, Canada
Pietro Di Santo
Faculty of Medicine, University of Ottawa, Ontario, Canada
Cole Clifford & Stephanie Skanes
Department of Medicine, Cumming School of Medicine, Calgary, Alberta, Canada
Graeme Prosperi-Porta
Division of Internal Medicine, The Ottawa Hospital, Ottawa, Ontario, Canada
Berkman Library, University of Ottawa Heart Institute, Ottawa, Ontario, Canada
Sarah Visintini
Hôpital Cardiologique du Haut-Lévêque, CHU Bordeaux, Bordeaux-Pessac, France
F. Daniel Ramirez
L’Institut de Rythmologie et Modélisation Cardiaque (LIRYC), University of Bordeaux, Bordeaux, France
Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA
Trevor Simard
You can also search for this author in PubMed Google Scholar
Contributions
R.G.J., P.D.S., S.V., F.D.R., T.S. and B.H. participated in the study conception and design. Data acquisition, analysis and interpretation were performed by R.G.J., P.D.S., C.C., G.P.P., S.P., S.S., A.H., F.D.R., T.S. and B.H. Statistical analysis was performed by R.G.J., P.D.S. and B.H. The manuscript was drafted by R.G.J., P.D.S., F.D.R., T.S. and B.H. All authors approved the final version of the manuscript and agree to be accountable to all aspects of the work.
Corresponding author
Correspondence to Benjamin Hibbert .
Ethics declarations
Competing interests.
B.H. reports funding as a clinical trial investigator from Abbott, Boston Scientific and Edwards Lifesciences outside of the submitted work. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Communications Ian White and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary files, supplementary data 1, supplementary data 2, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Jung, R.G., Di Santo, P., Clifford, C. et al. Methodological quality of COVID-19 clinical research. Nat Commun 12 , 943 (2021). https://doi.org/10.1038/s41467-021-21220-5
Download citation
Received : 16 July 2020
Accepted : 13 January 2021
Published : 11 February 2021
DOI : https://doi.org/10.1038/s41467-021-21220-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The quality of covid-19 systematic reviews during the coronavirus 2019 pandemic: an exploratory comparison.
- Kevin T. McDermott
- Jos Kleijnen
Systematic Reviews (2024)
Gatekeeping should be conserved in the open science era
- Hugh Desmond
Synthese (2024)
Exploring COVID-19 research credibility among Spanish scientists
- Eduardo Garcia-Garzon
- Ariadna Angulo-Brunet
- Guido Corradi
Current Psychology (2024)
Primary health care research in COVID-19: analysis of the protocols reviewed by the ethics committee of IDIAPJGol, Catalonia
- Anna Moleras-Serra
- Rosa Morros-Pedros
- Ainhoa Gómez-Lumbreras
BMC Primary Care (2023)
Identifying patterns of reported findings on long-term cardiac complications of COVID-19: a systematic review and meta-analysis
- Chenya Zhao
BMC Medicine (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Confused about COVID? Here’s how to read a research paper
Senior Lecturer in Evidence-Based Healthcare and University Ethics Advisor, University of Portsmouth
Disclosure statement
Simon Kolstoe does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
University of Portsmouth provides funding as a member of The Conversation UK.
View all partners
Scientific evidence can be difficult to understand. Normally we can rely on experts to interpret it for us, or the media to accurately report any interesting new discoveries, but the pandemic has challenged this.
Almost daily we are faced with contradictory views claiming to be “based on the scientific evidence”. But if you’re not an academic, how can you go about checking the evidence for yourself?
Scientific research is communicated in the form of “research papers” published in professional journals. To ensure accuracy, each paper is carefully checked by both editors and outside academic experts in a process called “peer review”. Although peer review is not perfect , it does tend to ensure articles are more reliable compared with those produced in other types of publishing .
Therefore, to judge the scientific evidence for yourself, you need to read and understand peer-reviewed papers. This can be daunting, but if you approach research papers with the right strategy they can be easier to digest.
1. Find the research paper
Following the publication of new research, the results are often summarised by the media. Frustratingly, these summaries seldom provide a link to the original peer-reviewed paper itself.
To find the original paper, one good strategy is to track down the original press release from the university or company releasing the research. You can also use an academic search engine like Google scholar or PubMed to search for recent papers published by the authors, who are normally (although not always) named by journalists.
Historically readers have had to pay to read academic papers, but increasingly research papers are free to readers through “ open access ” arrangements. Unfortunately, if a paper is not open access, there is not much you can do to read it without paying a fee to the publisher.

2. Read the abstract and look at the pictures
Research papers are long and dense with a very different structure compared with articles in the normal media. Media articles start with the most important information in the first few lines and then add background or contextual information as the article progresses.
Research papers start off with an introduction describing the background, then sections describing the methods and results, a discussion (highlighting strengths and weaknesses of the research), and finally the conclusion – often only in the very last few sentences. However, to help speed up reading, a summary or “abstract” is always provided at the beginning.
The abstract is the best place to start (and is almost always available for free). If you are not an expert in the subject area, make sure you look up any words you do not understand, because everything mentioned in the abstract will be key to understanding the paper as a whole.
After reading the abstract you may find you have gathered all the information you need about the research, but if after reading it you still would like to find out more, have a quick look at the pictures, figures and diagrams (if available) to get a better idea of the experiments being reported.
3. Determine how good the journal is and who wrote the paper
After reading the abstract I normally look at who the authors are, what university or company they work for, and how good the journal publishing the paper is.
Academics with a track record of producing high-quality research are a good sign. The first and last authors listed in research papers are often the most important , so look them up to see what else they have produced.

Having the research published in a good journal is also important, because the better journals are able to access more experienced peer reviewers and editors. Here the “impact factor” of a journal is often quoted, which relates to how many other researchers refer to the papers published in it.
However, in recent years impact factors have been strongly criticised as a way of judging journals, even though it’s still true that the best research is published in a fairly small number of journals. One alternative to relying on the impact factor is to simply look up the journal title online to see what researchers say about it. As researchers spend a lot of time discussing which journals are best, this should allow you to find out fairly quickly whether the journal you’re looking at is a reputable one.
4. Read the discussion
If you have got this far you are probably convinced that the research paper is interesting and worth a bit more effort to read. So next, find the part of the paper that discusses the results (often called the discussion) and read through this carefully, flicking back to the methods or results sections if you need to understand in more detail how the experiments were done. Again, look up any terms you do not understand.
5. Read the introduction and check out some of the references
Once you have a good idea of what the paper is reporting, finish off by reading the introduction – this normally provides an overview of why the experiments were conducted in the first place. You should now have a very good idea of what the paper is reporting and some of the wider context.
If you are particularly interested in the topic, look too at some of the key references that the paper quotes. If the paper isn’t brand new, go back to an academic search engine to see whether others have since referenced (or cited) it, and what they are saying about the research.
6. When a paper is not a paper
A word of warning: not every article published in a journal reports new research. Journals also contain news articles, opinion pieces and reviews. These are seldom peer reviewed, and although still written for a professional audience, are not considered primary research.
Another thing to watch out for are versions of research papers that are made available online in advance of being checked by peer reviewers, in a form called “preprints”. Preprints can be very useful for finding out about new results quickly because the peer review and journal publication process can take up to a year. This has been necessary during the pandemic, for example. These preprints are normally clearly labelled, just as a warning that the information in them should not be relied upon in the same way as a full, peer-reviewed research paper.
- Coronavirus
- Academic research
- Coronavirus insights

Compliance Lead

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health

Lecturer/Senior Lecturer, Earth System Science (School of Science)
- Author info
- Editorial board
- Submit manuscript
- Manuscript login
- Current issue
- Volume 20; 2024
- Volume 19; 2023
- Volume 18; 2022
- Volume 17; 2021
- Volume 16; 2020
- Advance articles
- Cover images
- Index & coverage
- Cover suggestion
- Special issues
Theranostics
International Journal of Medical Sciences
Nanotheranostics
Journal of Cancer
Journal of Genomics
Int J Biol Sci 2020; 16(10):1753-1766. doi:10.7150/ijbs.45134 This issue Cite
COVID-19: what has been learned and to be learned about the novel coronavirus disease
Ye Yi, Philip N.P. Lagniton, Sen Ye, Enqin Li, Ren-He Xu ✉
Institute of Translational Medicine, and Centre of Reproduction, Development and Aging, Faculty of Health Sciences, University of Macau, Taipa, Macau, China.
The outbreak of Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2), has thus far killed over 3,000 people and infected over 80,000 in China and elsewhere in the world, resulting in catastrophe for humans. Similar to its homologous virus, SARS-CoV, which caused SARS in thousands of people in 2003, SARS-CoV-2 might also be transmitted from the bats and causes similar symptoms through a similar mechanism. However, COVID-19 has lower severity and mortality than SARS but is much more transmissive and affects more elderly individuals than youth and more men than women. In response to the rapidly increasing number of publications on the emerging disease, this article attempts to provide a timely and comprehensive review of the swiftly developing research subject. We will cover the basics about the epidemiology, etiology, virology, diagnosis, treatment, prognosis, and prevention of the disease. Although many questions still require answers, we hope that this review helps in the understanding and eradication of the threatening disease.
Keywords : Coronavirus, pneumonia, outbreak, SARS-CoV-2, COVID-19
Citation styles
©2024 Ivyspring International Publisher . Terms of use

Coronavirus (COVID-19)
How to publish in coronavirus (covid-19).
- The work is original. The manuscript (or substantial parts of it) must not have been published previously, or be under consideration or review by another journal. Manuscripts that were previously posted on a preprint server such as ArXiv or BioRxiv are welcome.
- At least one author must be formally affiliated with funding from Wellcome (for more details, see our Publication criteria ).
- The reported study meets all applicable research and publication standards . We strongly recommend that you consult our editorial policies for more detail on reporting guidelines and ethical requirements.
- All methodological details and relevant data are made available to allow others to replicate the study, and that the manuscript adheres to appropriate reporting guidelines and community standards. For more detail, please see our policies and Data preparation guidelines .
- All authors have understood Wellcome Open Research’s policies for article publication and its publishing model .
- Your manuscript includes full author and affiliation information, and a conflict of interest statement.

- ORCID allows identification beyond names. Globally, names can be very common, they can change, they can be transliterated into other alphabets and so reliably linking researchers with their research and organizations can be difficult - this is solved through a unique ORCID iD.
- An ORCID iD also allows you to keep a constantly updated digital curriculum vitae. Individuals decide to register, which research activities to connect to their ID, which organizations to allow access, what information to make publicly available, what to share with trusted parties, and what to keep private. Individuals can control their profiles and can change these settings and permissions at any time.
- we collect and store authenticated ORCID iDs for authors and reviewers
- we publicly display the iDs with the iD icon for those authors and reviewers, linked to their ORCID account
- we connect to the user's ORCID record and update it with new published works

Are you a Wellcome-funded researcher?
If you are a previous or current Wellcome grant holder, sign up for information about developments, publishing and publications from Wellcome Open Research.
We'll keep you updated on any major new updates to Wellcome Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here .
If you still need help with your Google account password, please click here .
You registered with F1000 via Facebook, so we cannot reset your password.
If you still need help with your Facebook account password, please click here .
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.

COVID-19 and Higher Education
This RAPID award is made by the Improving Undergraduate STEM Education program in the Division of Undergraduate Education (Education and Human Resources Directorate), using funds from the Coronavirus Aid, Relief, and Economic Security (CARES) Act.

Project Abstract
How do institutions communicate about COVID-19? How do COVID-19 changes impact faculty and students? What resources are most important for the demonstration of resilience across undergraduate education? In order to answer these questions, we are randomly sampling institutions across the United States using the Postsecondary Education Data System (IPEDS) to represent various school sizes and types. Our hope is that this method of sampling will be inclusive and best represent experiences across the United States. We will be collecting crisis communication documents from the universities as well as conducting three waves of surveys and follow-up interviews. Through such a rigorous process we might be able to better measure experiences, reactions and how these unfold over time. This research is unique as we are studying the pandemic while it unfolds.
This research offers an opportunity to help the greater academic community that has been significantly uprooted and disrupted by COVID-19. There are dozens of other studies going on at the same time to unlock the secrets of this unprecedented event. Our approach is specifically designed in a way that will help us contribute a substantial piece of the puzzle. By opening a channel for the participating institutions, faculty, and students to systematically share their experience and their voices, we hope to contribute to a stronger, more resilient system of Higher Education that will emerge from this experience with the information and lessons needed to thrive in the future.
This project will examine the experiences faced by faculty and students in higher education during the COVID-19 pandemic. To understand the complexity of the situation we combine theory and best practices from the fields of I/O Psychology, Crisis Communication, Disaster Management, and Higher Education. Essentially, better understanding faculty and student perspectives will help us understand what sorts of directional changes Higher Ed might take in future adverse situations.
The majority of our data collection will take place in the summer and fall of 2020 and we will be working with the data for about one year.
Our recruitment process ensures that results will be generalizable to all higher education institutions in the United States, no matter whether they are public or private, large or small, located in urban or rural settings, or minority-serving.
COVID-19 Vaccine: A comprehensive status report
Affiliations.
- 1 Department of Microbiology, Ram Lal Anand College, University of Delhi, Benito Juarez Road, New Delhi 110021, India.
- 2 Department of Microbiology, Ram Lal Anand College, University of Delhi, Benito Juarez Road, New Delhi 110021, India. Electronic address: [email protected].
- PMID: 32800805
- PMCID: PMC7423510
- DOI: 10.1016/j.virusres.2020.198114
The current COVID-19 pandemic has urged the scientific community internationally to find answers in terms of therapeutics and vaccines to control SARS-CoV-2. Published investigations mostly on SARS-CoV and to some extent on MERS has taught lessons on vaccination strategies to this novel coronavirus. This is attributed to the fact that SARS-CoV-2 uses the same receptor as SARS-CoV on the host cell i.e. human Angiotensin Converting Enzyme 2 (hACE2) and is approximately 79% similar genetically to SARS-CoV. Though the efforts on COVID-19 vaccines started very early, initially in China, as soon as the outbreak of novel coronavirus erupted and then world-over as the disease was declared a pandemic by WHO. But we will not be having an effective COVID-19 vaccine before September, 2020 as per very optimistic estimates. This is because a successful COVID-19 vaccine will require a cautious validation of efficacy and adverse reactivity as the target vaccinee population include high-risk individuals over the age of 60, particularly those with chronic co-morbid conditions, frontline healthcare workers and those involved in essentials industries. Various platforms for vaccine development are available namely: virus vectored vaccines, protein subunit vaccines, genetic vaccines, and monoclonal antibodies for passive immunization which are under evaluations for SARS-CoV-2, with each having discrete benefits and hindrances. The COVID-19 pandemic which probably is the most devastating one in the last 100 years after Spanish flu mandates the speedy evaluation of the multiple approaches for competence to elicit protective immunity and safety to curtail unwanted immune-potentiation which plays an important role in the pathogenesis of this virus. This review is aimed at providing an overview of the efforts dedicated to an effective vaccine for this novel coronavirus which has crippled the world in terms of economy, human health and life.
Keywords: COVID-19; Clinical Trials; Convalescent Plasma Therapy; Monoclonal Antibodies; SARS-CoV-2; Vaccine.
Copyright © 2020 Elsevier B.V. All rights reserved.
Publication types
- Angiotensin-Converting Enzyme 2
- Antibodies, Viral / biosynthesis*
- Betacoronavirus / drug effects
- Betacoronavirus / immunology*
- Betacoronavirus / pathogenicity
- COVID-19 Serotherapy
- COVID-19 Vaccines
- Clinical Trials as Topic
- Coronavirus Infections / epidemiology
- Coronavirus Infections / immunology
- Coronavirus Infections / prevention & control*
- Coronavirus Infections / therapy
- Coronavirus Infections / virology
- Genetic Vectors / chemistry
- Genetic Vectors / immunology
- Immunity, Innate / drug effects
- Immunization, Passive / methods
- Immunogenicity, Vaccine
- Pandemics / prevention & control*
- Patient Safety
- Peptidyl-Dipeptidase A / genetics
- Peptidyl-Dipeptidase A / immunology
- Peptidyl-Dipeptidase A / metabolism
- Pneumonia, Viral / epidemiology
- Pneumonia, Viral / immunology
- Pneumonia, Viral / prevention & control*
- Pneumonia, Viral / virology
- Receptors, Virus / genetics
- Receptors, Virus / immunology
- Receptors, Virus / metabolism
- Vaccines, Attenuated
- Vaccines, DNA
- Vaccines, Subunit
- Vaccines, Virus-Like Particle / administration & dosage
- Vaccines, Virus-Like Particle / biosynthesis
- Vaccines, Virus-Like Particle / immunology
- Viral Vaccines / administration & dosage
- Viral Vaccines / biosynthesis
- Viral Vaccines / immunology*
- Antibodies, Viral
- Receptors, Virus
- Vaccines, Virus-Like Particle
- Viral Vaccines
- Peptidyl-Dipeptidase A
- ACE2 protein, human
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

COVID-19 Vaccine: A comprehensive status report
The current COVID-19 pandemic has urged the scientific community internationally to find answers in terms of therapeutics and vaccines to control SARS-CoV-2. Published investigations mostly on SARS-CoV and to some extent on MERS has taught lessons on vaccination strategies to this novel coronavirus. This is attributed to the fact that SARS-CoV-2 uses the same receptor as SARS-CoV on the host cell i.e. human Angiotensin Converting Enzyme 2 (hACE2) and is approximately 79% similar genetically to SARS-CoV. Though the efforts on COVID-19 vaccines started very early, initially in China, as soon as the outbreak of novel coronavirus erupted and then world-over as the disease was declared a pandemic by WHO. But we will not be having an effective COVID-19 vaccine before September, 2020 as per very optimistic estimates. This is because a successful COVID-19 vaccine will require a cautious validation of efficacy and adverse reactivity as the target vaccinee population include high-risk individuals over the age of 60, particularly those with chronic co-morbid conditions, frontline healthcare workers and those involved in essentials industries. Various platforms for vaccine development are available namely: virus vectored vaccines, protein subunit vaccines, genetic vaccines, and monoclonal antibodies for passive immunization which are under evaluations for SARS-CoV-2, with each having discrete benefits and hindrances. The COVID-19 pandemic which probably is the most devastating one in the last 100 years after Spanish flu mandates the speedy evaluation of the multiple approaches for competence to elicit protective immunity and safety to curtail unwanted immune-potentiation which plays an important role in the pathogenesis of this virus. This review is aimed at providing an overview of the efforts dedicated to an effective vaccine for this novel coronavirus which has crippled the world in terms of economy, human health and life.
1. Introduction
The novel beta-coronavirus SARS-CoV-2 is believed to have emerged last year in 2019 in Wuhan from Bats. Crossing the species barrier it entered human beings with furtherance of infection through human to human transmission. The beta-coronaviruses have jumped between the species and have caused three zoonotic outbreaks namely, SARS CoV (2002-03), MERS-CoV (2012), and SARS-CoV-2 (2019- till date) in the last 2 decades. The existence of a myriad of coronaviruses in bats, including many SARS-related CoV (Severe Acute Respiratory Syndrome related Coronaviruses) and the sporadic crossing over of the species barriers of the coronaviruses to humans, suggest that the future occurrences of zoonotic transmission events may sustain ( Ou et al., 2020 ).
Since its emergence in Nov 2019, it has spread to 188 countries and 25 territories around the globe, despite elaborate efforts by WHO and Governments to contain the infection, primarily owing to the highly infectious nature of this virus ( Anon, 2020a ; Anon, 2020b ). As of 2 July 2020, 10,533,779 cases have been reported globally with 512,842 deaths ( (WHO) World Health Organisation, 2020 ). There has been a monumental increase in the number of infected patients, with a 7-day moving average of 210,209 cases per day, as of 2 July 2020 ( Anon, 2020a ). SARS-CoV-2, a highly contagious virus, tends to spread by the inhalation of the respiratory aerosols, direct human contact, and via fomites. Social distancing, personal hygiene, frequent hand washing or sanitizing using the alcohol (61-70%) based hand-sanitizers, and disinfection of the surfaces are some steps which can protect the individuals from getting infected ( (CDC), Centers for Disease Control and Prevention, 2020 ). R 0 is an epidemiological scale; used to measure the contagiousness of an infectious agent. Its magnitude depends upon various biological, environmental, and socio-behavioral factors. It can be defined as “the average number of secondary cases one would produce in a completely susceptible population in the absence of any deliberate intervention in disease transmission ( Delamater et al., 2019 ).” SARS-CoV-2 has an R 0 value range of 2-3 ( Park, 2020 ) which is significantly higher in comparison to Spanish flu for which the R 0 was recorded at 0.9-2.1 ( Pyrek, 2018 ). According to WHO, people living with non-communicable diseases (co-morbid conditions) are prone to severe illness due to COVID-19 infection. The incubation period of the virus ranges from 2-14 days with a median of 5.1 days ( Lauer et al., 2020 ). The symptoms include fever, dry cough, fatigue, shortness of breath, chills, muscles pain, headache, gastric disturbances and weight loss ( CDC, 2020 ). Some patients may have lymphopenia and bilateral ground-glass opacity changes in the chest CT scans. The histological examinations of the lungs’ biopsy samples have shown a bilaterally diffused alveolar damage with cellular fibromyxoid exudates. A few interstitial mononuclear inflammatory infiltrates were observed both in the liver and the heart specimens ( Xu et al., 2020 ). However, a large population of the infected patients have no or mild symptoms and remain asymptomatic ( Shang et al., 2020 ).
Structurally coronaviruses are pleomorphic, enveloped viruses with a characteristic fringe of projections composed of S protein on their surface. These viruses are equipped with a positive sense ssRNA genome, which is complexed with the nucleocapsid (N) protein forming helical nucleocapsids. The genome is both capped and polyadenylated ( Carter and Saunders, 2007 ). The genetic analysis of SARS-CoV-2 and SARS-CoV has revealed 79% similarity with a total of 380 amino acid substitutions condensed mainly within the NSP genes. Out of these substitutions, there are 27 amino acid replacements in the immune-dominant S protein while 102 and 61 amino acid substitutions are found in the NSP3 and NSP2. Whereas, NSP7, NSP13, E protein, and some accessory proteins are devoid of any amino acid substitutions ( Wu et al., 2020 ). SARS-CoV and SARS-CoV-2 bind a common host receptor, hACE2, to gain entry into the cell but SARS-CoV-2 binds the receptor with a higher affinity than the SARS-CoV. MERS-CoV uses an entirely different receptor that is, Dipeptidyl Peptidase 4 (DPP4) ( Wan et al., 2020 ) and the virus is distantly related to SARS-CoV-2 with around 50% similarity as per the sequence analysis of the two viruses ( Prof Roujian et al., 2020 ).
The genome of SARS-CoV-2 is transcribed in at least 10 Open Reading Frames (ORFs). ORF1ab translates into a polyprotein which is processed into 16 non-structural proteins (NSPs) ( Yoshimoto, 2020 ). The NSPs perform various functions like genome replication, inducing the cleavage of host mRNA, membrane rearrangement, generation of the autophagosome, cleavage of the NSP polyprotein, capping, tailing, methylation, unwinding of the RNA duplex, etc. which are essential for the viral life cycle ( da Silva et al., 2020 ). Besides, the SARS-CoV-2 virus contains four structural proteins namely, spike (S), nucleocapsid (N), envelope (E), and membrane (M) proteins which are encoded by the 3’-end of the viral genome ( Wrapp et al., 2020 ). Amongst the 4 structural proteins the S glycoprotein, being a large multi-functional trans-membrane protein, plays the vital role of viral attachment, fusion, and entry into the host cell ( Wrapp et al., 2020 ). The S protein consists of S1 and S2 subunits, which are further split into different functional domains. The S1 subunit has two functional domains viz. N-terminal Domain (NTD) and Receptor Binding Domain (RBD) and the latter contains conserved receptor binding motif (RBM) ( Jiang et al., 2020 ). The alignment studies have revealed that the region of RBD sequence lies between the residues 331 and 524 of the S protein ( Tai et al., 2020 ). Whereas, the S2 subunit has three operational domains namely, fusion peptide (FP), heptad repeat (HR) 1, and 2. The S1 protein trimer aligns itself at the top of the trimeric S2 stalk to form the immune-dominant S protein ( Jiang et al., 2020 ). Interestingly, a furin cleavage site is observed within the spike protein of SARS-CoV-2 while it is absent in the SARS-CoV which may be a possible explanation of the variation in the pathogenicity of the virus ( Walls et al., 2020 ). A host trans-membrane protease serine 2, (TMPRSS2) is responsible for the initial priming of the spike protein. The virus can utilize both TMPRSS2 and endosomal cysteine proteases cathepsin B and L (CatB/L) to initiate entry into the cell. The TMPRSS2 is responsible for the cleavage of the S protein to expose the FP region of the S2 subunit which is responsible for the initiation of the endosome mediated entry into the host cell. This indicates that TMPRSS2 is a host factor that is essential for viral entry; therefore, the drugs approved for the inhibition of this protease (like camostatmesylate) could be used for therapeutic purposes ( Hoffmann and Kleine-Weber, 2020 ). SARS-CoV-2 uses the human angiotensin-converting enzyme 2 (hACE2) receptor to seize the target cell through the spike glycoprotein (S-Protein), . It has been suggested that the coronaviruses exercise the use of conformational masking and glycan shielding of the spike protein to circumvent the host immune cells. The Cryo-EM structures have revealed the presence of two distinct: closed and open conformations of the S-Protein ectodomain trimer, as a consequence of the opening of the structure at the trimer apex. This conformational diversification is necessary for the receptor binding as the trimer opening exposes the RBM which is present at the interface between the protomers in the closed trimers ( Walls, 2020 ).
The E protein that forms E channels (called the viroporins), and is involved in a myriad of functions in the viral replication cycle involving assembly, release, pathogenesis, etc. ( Gralinski and Menachery, 2020 ). These reprobate ion channels exist in the form of homo-pentamers with each subunit containing 50-120 amino acids. E channels contain at least one trans-membrane domain (TMD) which facilitates the linkage in host cell membranes. SARS CoVs generally contain three categories of ion channels namely: E, 8a, and 3a. The E and 8a ion channels contain the PDZ (Post Synaptic Density Protein; Disc Large Tumor Suppressor; Zonula Occludens-1 Protein) Domain Binding Motif (PBM) which is responsible for the over-expression of the inflammatory cytokines which may result in the cytokine storm ( Pharmaceutical Targeting the Envelope Protein of SARS-CoV-2: the Screening for Inhibitors in Approved Drugs, 2020 ). From the sequence alignment study of the E protein, it was observed that a negatively charged glutamate residue (E69) in SARS-CoV corresponds to a positively charged arginine residue (R69) in SARS-CoV-2 ( Yoshimoto, 2020 ). However, this mutation is remote from the inhibitor binding site; therefore, E protein can be used as a pharmaceutical target ( Pharmaceutical Targeting the Envelope Protein of SARS-CoV-2: the Screening for Inhibitors in Approved Drugs, 2020 ).
M protein, the central organizer of CoV assembly, is most abundantly expressed in the virus particle. It functions crucially in the morphogenesis and assembly of the SARS-CoV-2 by interacting with the essential structural proteins ( Conserved Protein Domain Family: SARS-like-CoV_M, 2020 ). The binding of the M and N protein stabilizes the N protein and RNA complex, and the internal core of the virus. In case of SARS-CoV, the M protein has also been shown to induce the process of apoptosis in the host cell ( Yoshimoto, 2020 ).
In addition to stabilizing the ssRNA genome of the virus particle, the N protein is an antagonist of the antiviral RNAi. It is responsible for the inhibition of the cell cycle of the host cell as it can inhibit the entry of the cell into the S-phase ( Yoshimoto, 2020 ).
Immunotherapy is considered as an effective method for the prophylaxis and treatment of various infectious diseases and cancers, which involves the artificial triggering of the immune system to elicit the immune response ( Masihi, 2001 ). A vaccine that elicits the production of S protein neutralizing antibodies in the vaccinated subjects is the primary aim of all the programs for COVID-19 vaccines. Studies have revealed that there is a limited to no cross-neutralization between the sera of SARS-CoV and SARS-CoV-2, indicating that recovery from one infection may not shield against the other ( Ou et al., 2020 ). Furthermore, a database of approximately 5500 full-length genomes of SARS-CoV-2 isolated from various countries is now available at NCBI which facilitates delineating the polymorphisms in S protein and other important proteins of the virus concerning vaccine development. The rationale for writing this review is to gather all the information about the COVID-19 vaccine development programs and give the readers and researchers insight into types of vaccines being worked upon and the current status of the clinical trials of these vaccines for ready reference.
2. Vaccination strategies
Many efforts have been directed towards the development of the vaccines against COVID-19, to avert the pandemic and most of the developing vaccine candidates have been using the S-protein of SARS-CoV-2 ( Dhama et al., 2020 ). As of July 2, 2020, the worldwide SARS-CoV-2 vaccine landscape includes 158 vaccine candidates, out of which 135 are in the preclinical or the exploratory stage of their development. Currently, mRNA-1273 (Moderna), Ad5-nCoV (CanSino Biologicals), INO-4800 (Inovio, Inc.), LV-SMENP-DC, Pathogen-specific aAPC (ShinzenGeno-Immune Medical Institute), and ChAdOx1 (University of Oxford) have entered the phase I/II clinical trials ( WHO, 2020 ). The vaccines which are in the conduit are based upon inactivated or live attenuated viruses, protein sub-unit, virus-like particles (VLP), viral vector (replicating and non- replicating), DNA, RNA, nanoparticles, etc. with each exhibiting unique advantages and hindarances ( Table 1 ) ( Ning et al., 2020 ). COVID-19 vaccine landscape with percentage share of different types of vaccine is represented in Fig. 1 . To enhance the immunogenicity, various adjuvant technologies like AS03 (GSK), MF-59 (Novartis), CpG 1018 (Dynavax), etc. are now accessible to the researchers for the vaccine development ( Le et al., 2020 ). The immuno-informatics approach is also used for the epitope identification for the SARS-CoV-2 vaccine candidates. It can be used to identify the significant cytotoxic T cell and B-cell epitopes in the viral proteins ( Gupta et al., 2006 ; Baruah and Bose, 2020 ).
Outline of the vaccine production platforms for SARS-CoV-2 and their advantages and limitations

Pie Chart showing the different categories of SARS-CoV-2 vaccines under research ( Anon, 2020c ).
2.1. Protein Sub-unit vaccine
A subunit vaccine is the one which is based on the synthetic peptides or recombinant antigenic proteins, which are necessary for invigorating long-lasting protective and/or therapeutic immune response ( Ning et al., 2020 ). The subunit vaccine, however, exhibits low immunogenicity and requires auxiliary support of an adjuvant to potentiate the vaccine-induced immune responses. An adjuvant may enhance the biological half-life of the antigenic material, or it may ameliorate the immunomodulatory cytokine response. The addition of an adjuvant, therefore, helps in overcoming the shortcomings of the protein subunit vaccines ( Cao et al., 2018 ). The S protein of the SARS-CoV-2 is the most suitable antigen to induce the neutralizing antibodies against the pathogen. The S Protein consists of two subunits. The S1 subunit has the NTD, RBD, and RBM domains while the S2 subunit comprises of FP, HR 1, &2 ( Ou et al., 2020 ). The virus enters into the cell via endocytosis by utilizing the S-Protein mediated binding to the hACE2 receptor. Therefore, the S-Protein and its antigenic fragments are the prime targets for the institution of the subunit vaccine ( Ning et al., 2020 ). The S glycoprotein is a dynamic protein, possessing two conformational states i.e. pre-fusion and post-fusion state. Therefore, the antigen must maintain its surface chemistry and profile of the original pre-fusion spike protein to preserve the epitopes for igniting good quality antibody responses ( Graham, 2020 ). Moreover, means to target the masked RBM as an antigen will enhance the neutralizing antibody response and improve the overall efficacy of the vaccine.
2.1.1. NVX-CoV2373 (Novavax, Inc.| Emergent BioSolutions)
NVX-CoV2373 is a nano-particle based immunogenic vaccine which is based upon the recombinant expression of the stable pre-fusion, coronavirus S-Protein ( Coleman et al., 2020 ). The protein was stably expressed in the Baculovirus system ( Tu et al., 2020 ). The company plans to use the Matrix-M adjuvant to enhance the immune response against SARS-CoV-2 spike protein by the induction of high levels of neutralizing antibodies. In the animal models, a single immunization resulted in the high level of anti-spike protein antibodies which blocked the hACE2 receptor binding domain and could elicit SARS-CoV-2 wild type virus-neutralizing antibodies ( Novavax covid 19 vaccine trial, 2020 ).
2.1.2. Molecular Clamp Stabilized spike protein vaccine candidate
It is being developed by the University of Queensland in collaboration with GSK and Dynavax. The University will have access to vaccine adjuvant platform technology (AS03 Adjuvant system), which is believed to strengthen the vaccine response and minimize the amount of vaccine required per dose ( Lee, 2020 ). The University is developing a stabilized pre-fusion, recombinant viral protein sub-unit vaccine which is based upon the Molecular Clamp technology. This technology has been proved to induce the production of the neutralizing antibodies ( Tu et al., 2020 )
2.1.3. PittCoVacc (University of Pittsburgh)
It is a Micro-Needle Array (MNA) based recombinant SARS-CoV-2 vaccine which involves the administration of rSARS-CoV-2 S1 and rSARS-CoV-2-S1fRS09 (recombinant immunogens). A substantial increase in the antigen specific antibodies with a statistical significance was observed in the pre-clinical trials at the end of two weeks in the mice models. Furthermore, the immunogenicity of the vaccine was maintained even after the sterilization using gamma radiation. The statistically significant titers of antibodies at the early stages and also before boosting, support the feasibility of the MNA-SARS-CoV-2 vaccine ( Kim et al., 2020 ).
2.1.4. Triple Antigen Vaccine (Premas Biotech, India)
It is a multi-antigenic VLP vaccine prototype wherein the recombinant spike, membrane, and envelope protein of SARS-CoV-2 have been co-expressed in an engineered Saccharomyces cerevisiae expression platform (D-Crypt™). The proteins then undergo self-assembly as the VLP. The TEM and allied analytical data simultaneously furnished the biophysical characterization of the VLP. This prototype has the potential to enter the pre-clinical trials as a vaccine candidate after further research and development. Furthermore, it is thought to be safe and easy to manufacture on a mass scale, in a cost-effective manner ( Arora and Rastogi, 2020 ).
2.2. Viral Vectored vaccines
A vaccine based on viral vectors is a promising prophylactic solution against a pathogen. These vaccines are highly specific in delivering the genes to the target cells, highly efficient in the gene transduction, and efficiently induce the immune response, ( Ura et al., 2014 ). They offer a long term and high level of antigenic protein expression and therefore, have a great potential for prophylactic use as these vaccines trigger and prime the cytotoxic T cells (CTL) which ultimately leads to the elimination of the virus infected cells ( Le et al., 2020 ).
2.2.1. Ad5-nCoV (CanSino Biologics Inc | Beijing Institute of Biotechnology)
It is a recombinant, replication defective adenovirus type-5 vector (Ad5) expressing the recombinant spike protein of SARS-CoV-2. It was prepared by cloning an optimized full-length gene of the S Protein along with the plasminogen activator signal peptide gene in the Ad5 vector devoid of E1 and E3 genes. The vaccine was constructed using the Admax system from the Microbix Biosystem ( Zhu et al., 2020 ). The phase I clinical trials have established a positive antibody response or seroconversion. A four-fold increase in the RBD and S protein-specific neutralizing antibodies was noted within 14 days of immunization and peaked at day 28, post-vaccination. Furthermore, the CD4 + T cells and CD8 + T cells response peaked at day 14 post-vaccination. However, the pre-existing anti-Ad5 immunity partly limited both the antibody and the T cell responses ( Zhu et al., 2020 ). The study will further evaluate antibody response in the recipients who are between the age of 18 and 60, and received one of three study doses, with follow-up taking place at 3- and 6-months post-vaccination ( Anon, 2020d ).
2.2.2. Coroflu (University of Wisconsin-Madison | FluGen | Bharat Biotech)
M2SR, a self-limiting version of the influenza virus, which is modified by insertion of the SARS-CoV-2 gene sequence of the spike protein. Furthermore, the vaccine expresses the hemagglutinin protein of the influenza virus, thereby inducing immune response against both the viruses. The M2SR is self-limiting and does not undergo replication as it lacks the M2 gene. It is able to enter into the cell, thereby inducing the immunity against the virus. It shall be administered intra-nasally, mimicking the natural route of viral infection. This route activates several modes of the immune system and has higher immunogenicity as compared to the intramuscular injections ( Anon, 2020e ).
2.2.3. LV-SMENP-DC (Shenzhen Geno-Immune Medical Institute)
The LV-SMENP-DC vaccine is prepared by engineering the dendritic cells (DC) with the lentiviral vector expressing the conserved domains of the SARS-CoV-2 structural proteins and the protease using the SMENP minigenes. The subcutaneous inoculation of the vaccine presents the antigens on antigen presenting cells (APCs), that ultimately activate the Cytotoxic T cells and generate the immune response ( Le et al., 2020 ).
2.2.4. ChAdOx1 (University of Oxford)
ChAdOx1 recombinant adenovirus vaccine was developed using codon optimized S glycoprotein and synthesized with the tissue plasminogen activator (tPA) leader sequence at 5’ end. The sequence of SARS-CoV-2 coding for amino acids (2 to 1273) and the tPA leader and was propagated in the shuttle plasmid. This shuttle plasmid is responsible for encoding the major immediate early genes of the human cytomegalovirus (IE CMV) along with tetracycline operator (TetO) sites and polyadenylation signal from bovine growth hormone (BGH) between the Gateway® recombination cloning site. The Adenovirus vector genome is constructed in the Bacterial Artificial Chromosome by inserting the SARS-CoV-2 S gene into the E1 locus of ChAdOx1 adenovirus genome. The virus was then allowed to reproduce in the T-Rex 293 HEK (Human Embryonic Kidney 293) cell lines and purified by the CsCl gradient ultracentrifugation. The absence of any sub-genomic RNA (sgRNA) in the intra-muscularly vaccinated animals from the pre-clinical trials is indicative of the escalated immunity against the virus ( Doremalen et al., 2020 ). The previous studies have suggested that a single shot should marshal the immune response ( Ou et al., 2020 ). The vaccine has entered phase II clinical trials, where it shall be evaluated in a large sample of the population ( Anon, 2020f ).
2.3. mRNA Vaccine
mRNA is an emerging, non-infectious, and a non-integrating platform with almost no potential risk of insertional mutagenesis. Currently, the non-replicating RNA and the virus derived self-replicating RNAs are being studied. The immunogenicity of the mRNA can be minimized, and alterations can be made to increase the stability of these vaccines. Furthermore, the anti-vector immunity is also avoided as the mRNA is the minimally immunogenic genetic vector, allowing repeated administration of the vaccine ( Cuiling et al., 2020 ). This platform has empowered the rapid vaccine development program due to its flexibility and ability to mimic the antigen structure and expression as seen in the course of a natural infection ( Mulligan and Lyke, 2020 ).
2.3.1. mRNA-1273 (Moderna TX, Inc)
It is a vaccine composed of synthetic mRNA encapsulated in Lipid nanoparticle (LNP) which codes for the full-length, pre-fusion stabilized spike protein (S) of SARS-CoV-2. It has the potential to elicit a highly S-protein specific antiviral response. Furthermore, it is considered to be relatively safe as it is neither made up of the inactivated pathogen nor the sub-units of the live pathogen ( Tu et al., 2020 ). The vaccine has got a fast-track approval from FDA, to conduct the Phase II trials ( Anon, 2020g ).The company has released the interim phase I antibody data of eight participants who received various dose levels. The participants of the 25 μg dose group gave results comparable to the convalescent sera. Whereas, in participants who received the 100 μg dose, the levels of nAb essentially surpassed the levels found in convalescent sera. The vaccine was found to be predominantly safe and well tolerated in the 25 μg and 100 μg dose cohorts, while three participants experienced grade 3 systemic symptoms after the administration of the second dose of 250 μg dose levels ( Anon, 2020h ).
2.3.2. BNT162b1 (BioNTech| FosunPharma| Pfizer)
BNT162b1 is a codon-optimized mRNA vaccine that encodes for the trimerized SARS-CoV-2 RBD, a critical target of the virus nAb. The vaccine portrays an increased immunogenicity due to the addition of T4 fibritin-derived foldon trimerization domain to the RBD antigen. The mRNA is encapsulated in 80 nm ionizable cationic lipid nanoparticles, which ensures its efficient delivery. The Phase 1/2 clinical trials have revealed elevated RBD-specific IgG antibodies levels with a geometric mean concentration to be as high as 8 to 46.3 times titer of convalescent serum. Whereas, the geometric mean titers of the SARS-CoV-2 neutralizing antibodies were found to be 1.8 to 2.8 times the convalescent serum panel. Moderate and transient local reactions and systemic events were observed with no adverse effect. However, the data analysis did not evaluate the safety and immune responses beyond 2 weeks following the administration of the second dose ( Mulligan and Lyke, 2020 ).
2.4. DNA Vaccines
The most revolutionary approach to vaccination is the introduction of the DNA vaccine which encodes for the antigen and an adjuvant which induces the adaptive immune response. The transfected cells express the transgene which provides a steady supply of the transgene specific proteins which is quite similar to the live virus. Furthermore, the antigenic material is endocytosed by the immature Dendritic Cells which ultimately present the antigen to the CD4+ and CD8+ T cells in association with MHC 2 and MHC 1 antigens on the cell surface hence stimulating effective humoral as well as cell-mediated immune responses ( Hobernik and Bros, 2018 ).
2.4.1. INO-4800 (Inovio Pharmaceuticals)
It is a prophylactic DNA vaccine against SARS-CoV-2 ( Anon, 2020i ). It uses codon optimized S protein sequence of SARS-CoV-2 to which an IgE leader sequence is affixed. The SARS-CoV-2 IgE-spike sequence was synthesized and digested using BamHI and XhoI . The digested DNA was incorporated into the expression plasmid pGX0001 under the governance of IE CMV, and BGH polyadenylation signal. The presence of functional antibodies and T cell response in the preclinical trials suggest that the vaccine can produce an effective immune response within 7 days post-vaccination ( Smith et al., 2020 ). The vaccine has entered the Phase I clinical trials (Phase I: {"type":"clinical-trial","attrs":{"text":"NCT04336410","term_id":"NCT04336410"}} NCT04336410 ) and it is estimated to complete this phase of clinical trials by July, wherein the participants received 1.0 mg of INO-4800 by electroporation using CELLECTRA® 2000 device per dosing visit. The trial will evaluate the immunological profile, safety, and tolerability of the vaccine candidate upon intradermal injection and the electroporation in healthy human adults ( Anon, 2020i ).
2.5. Live Attenuated Vaccines
2.5.1. delns1-sars-cov2-rbd (university of hong kong).
This LAV is influenza-based vaccine strain with a deletion in the NS1 gene. It is re-organized to express the RBD domain of SARS-CoV-2 spike protein on its surface and, is cultivated in the chick embryo and/or Madin Darby Canine Kidney Cells (MDCK) cells. It is potentially more immunogenic than the wild type influenza virus and can be administered as a nasal spray ( Anon, 2020j ).
2.6. Others
The revelation of the structure and genome of the SARS-CoV-2 has led to the rapid development of various vaccine candidates with potential immunogenicity but also adverse reactogenicities. The task of vaccine development is long and cumbersome which requires evaluation in some long-lasting clinical trials. Various Biotech ventures are using different technologies for the development of their vaccine candidates; British and American Tobacco Company (BAT) recently unfolded the COVID-19 vaccine using their new, and fast-growing tobacco plant technology ( Anon, 2020k ), while Tianjin University has developed an oral vaccine which has successfully employed Saccharomyces cerevisiae to carry the S protein. The GRAS (Generally Regarded As Safe) status of the yeast provides high scalability, robustness, and cost-effective production of cosmic dosages required to fight off this pandemic ( Zhai et al., 2020 ). Furthermore, in silico studies, using various databases like VaxiJen, have revealed that the epitope sequences WTAGAAAYY and YDPLQPEL can be employed for the formulation of epitope-based peptide vaccines ( Garg et al., 2020 ).
2.6.1. Self Assembling Vaccine (HaloVax)
The vaccine uses a heat shock protein (hsp) to activate the immune system. It is composed of a fusion protein sandwiched between an hsp and Avidin. Biotinylated immunogenic peptides are also incorporated to customize the vaccine ( Voltron Therapeutics, Inc., 2020 ) Table 2 , Table 3 .
Rapidly progressing Anti COVID-19 vaccines. This table contains the information of rapidly developing vaccine candidates only, the list of all vaccine candidates in the pipeline can be accessed from: https://airtable.com/shrSAi6t5WFwqo3GM/tblEzPQS5fnc0FHYR/viweyymxOAtNvo7yH?blocks=bip
Legend: CCHF: Crimean-Congo Hemorrhagic Fever; CHIKV: Chikungunya Virus; DengV: Dengue Virus; FMD: Foot and Mouth Disease; EBOV: Ebola Virus; HAV: Hepatitis A Virus; HBV: Hepatitis B Virus; HIV: Human Immunodeficiency Virus; HPV: Human Papilloma Virus; Inf: Influenza; LASV: Lassa Fever Virus; MenB: Meningitis B; NIPV: Nipah Virus; NORV: Norovirus; RABV: Rabies Virus; RVF: Rift Valley Fever; SARS: Severe Acute Respiratory Syndrome; SIV: Simian Immunodeficiency Virus; TB: Tuberculosis; VEE: Venezuelan Equine; Encephalitis Virus; VZV: Varicella Vaccine (Chickenpox); YFV: Yellow Fever Virus; ZIKV: Zika Virus.
Latest developments in the status of the promising SARS-CoV-2 vaccines
3. Passive Immunization/adoptive immunity
It is the use of preformed antibodies in therapeutics of various diseases. It can be achieved by use of sera from convalescent patients, polyclonal serum raised in other animals such as horse, neutralizing monoclonal antibodies produced by hybridoma technology or humanized antibodies.
3.1. Convalescent Plasma therapy
To date, no distinct treatment has been proven to be efficacious against the COVID-19. Convalescent plasma (CP) therapy has been approved as an empirical treatment during the outbreaks ( (WHO), World Health Organisation, 2014 ). It is considered as the archetypal immunotherapy which has been used for the treatment and prevention of various viral diseases in the past such as SARS, MERS, H1N1 pandemic, measles, mumps, etc. ( Kai et al., 2020 ). A possible explanation for the efficacy of this classic adoptive immunotherapy is that the neutralizing immune-globulins from CP may conquer viremia, block new infection, and accelerate clearance of the infected cells.
Various studies conducted to evaluate therapeutic potential of CP have convincingly shown that administration of the neutralizing antibodies in the critically ill patients led to the amelioration of the clinical status in all patients without any deaths ( Kai et al., 2020 ; Shen et al., 2020a ; Ahn et al., 2020a ; Anon, 2020C ). The dosage prescribed for the CP therapy has not been standardized yet and needs Randomised Clinical Trials not only to eliminate the effect of other medicines but also to evaluate the efficacy and safety of CP therapy. ( Zhang et al., 2020 ). The patients who were considered critically ill with some of them having co-morbid conditions like hypertension, cardiovascular diseases, cerebrovascular diseases, chronic renal failure, etc. were included in the study. They were all admitted to the ICUs and were receiving either mechanical ventilation, high-flow nasal cannula oxygenation, or the low-flow nasal cannula oxygenation. All the patients in these studies were receiving antiviral or antibacterial or antifungal drugs for the treatment of co-infections ( Kai et al., 2020 ). Compared to the control group, the CP treatment group showed no notable differences in the baseline characteristics but exhibited a sizable difference in the clinical outcomes (i.e. normalization of the body temperature, absorption of pulmonary lesions, resolution of ARDS, weaning off the mechanical ventilators, etc.), and the death rates. The patients were tested negative for the viral loads after 7-37 days of CP infusion ( Shen et al., 2020b ). A reduction in the net quantity of inflammatory biomarkers CRP, procalcitonin, and Interleukin 6 (IL-6) in the trial group was observed along with a significant increase in the antibody titers (RBD specific IgM and IgG) post-convalescent plasma therapy ( Ahn et al., 2020b ). However, these uncontrolled and non-randomized trials for the CP therapy impede the researchers to come to a conclusive statement about the prospective potency of this treatment, and these observations require further evaluation which is ongoing in the clinical trials ( Yan, 2020 ).
3.2. Monoclonal Antibody
The monoclonal antibodies (mAb) or therapeutic antibodies, created in the laboratory are the clones of a unique parent which can bind to a single epitope, that is, they have a monovalent affinity ( Gelboin et al., 1999 ). The use of mAb in the prevention and treatment of infectious diseases can overcome various drawbacks which are cognate with the convalescent plasma therapy in terms of specificity, safety, low risk of blood-borne infection, purity, and other factors. A wide array of monoclonal antibodies have already been developed which are implemented in the anti-tumor, anti-platelet, or antiviral therapy ( Breedveld, 2000 ).
A SARS-CoV specific human mAb CR3022 has been found to bind with the RBD of the S protein of SARS-CoV-2, stipulating it as a prospective therapeutic agent, which can either be used alone or in combination therapy for the management of COVID-19 ( Tian et al., 2020 ). To achieve higher efficiency of disease prevention and treatment, a combinatorial effect of monoclonal antibodies recognizing different epitopes of the viral surface can be considered for the neutralization of the virus as it may prove to be more effective and prevent the viral escape ( Tian et al., 2020 ).
There are over 61 patents which claim to have prepared the SARS-specific, MERS-specific, and the diagnostic antibodies. Another group of 38 patents claims to have developed the antibodies that target the host proteins like IL-6/IL-6R, TLR3, CD16, ITAM (immune-receptor tyrosine-based activation motif), DC-SIGN (dendritic cell-specific intercellular adhesion molecule-grabbing non-integrin), ICAM-3 (intercellular adhesion molecule 3), or IP-10/CXCL10 (interferon γ-inducible protein 10). These antibodies can be used to counteract against the cytokine storm that has been reported to harmonize with the SARS-CoV-2 infection ( Liu et al., 2020 ). Tocilizumab, an anti-IL 6 receptor antibody is likely to control the hyper-inflammatory pulmonary symptoms which are coupled with the cytokine storm involving the chemokine dysregulation and various interleukins. Tocilizumab has been reported to block the cytokine axis IL6 hence inhibiting the inflammatory cascade. However, further clinical trials are essential to establish the effectiveness of the mAb ( Michot et al., 2020 ). Israel Institute for Biological Research (IIBR) claims to have successfully developed the mAb against SARS-CoV-2. The institute is in the process of patenting it which may soon be commercialized ( Upadhyay, 2020 ). A group led by Professor Vijay Chaudhary at the University of Delhi, Centre for Innovation in Infectious Disease Research, Education and Training (UDSC-CIIDRET), is isolating the genes encoding the antibodies responsible for the neutralization of the SARS-CoV-2. These genes will be employed to foster the recombinant Ab by exploiting the pre-existing in-house antibody library and a library fabricated from the cells of convalescent COVID-19 patients ( PIB, Delhi, 2020 ).
4. Limitations
The duration of clinical trials poses a sizable amount of hindrance to swift vaccine development. According to the norms laid down by the US Food and Drug Administration (FDA), and WHO, a vaccine candidate has to pass through at least three phases of placebo-controlled clinical trials for the validation of its safety and efficacy, which can take years to complete. Considering the severity of the pandemic, which has forced a complete shut-down of the global economy, speedy vaccine development is necessary. Some authors suggest that the controlled human challenge studies may be conducted to suitably divert the Phase 3 testing, and allow the rapid licensure of the immunogenic vaccines. However, in the expanded field study participants will be monitored constantly to look for any long-term implications posed by the vaccine. Furthermore, the safety trials for the special groups including, children and pregnant women, and immuno-compromised patients can be conducted before the extension of the vaccination to these groups ( Eyal et al., 2020 ).
The testing and development of safe and effective vaccines rely upon laboratory animal models. These animal models must show a similar course of the disease as in human beings. However, the standard inbred strains of mice are not susceptible to the COVID-19 infection, due to the difference between the humans and mice ACE2 receptors ( Anon, 2020D ). This calls for the development of transgenic mice, expressing the hACE2 receptor. Two animal models (hACE2 transgenic mice model and another, primate Macaques model) were previously developed for the SARS-CoV but the current situation requires steady breeding and distribution of these animal models to meet demands of the researchers around the globe ( Mice and Bao, 2020 ). The SARS-CoV-2 virus isolates can efficiently replicate in the lungs of the Syrian hamsters. The lungs of infected hamsters exhibit the pathological lesions analogous to the COVID-19 patients with pneumonia. Moreover, the nAb response exhibited by the infected hamster demonstrated immunity against the succeeding re-challenge studies. Furthermore, the transfusion of convalescent sera into the naïve hamsters mounted the antibody response and hence hindered the viral replication in the lungs. The assemblage of these experiments have illustrated the Syrian hamster may be a perfect model for comprehending SARS-CoV-2 pathogenesis, and evaluating antiviral drugs, and the immunotherapies ( Imai and Iwatsuki-Horimoto, 2020 ). Nevertheless, the assessment of the vaccine dependent immune enhancement cannot be extrapolated from the animal models and requires a legitimate survey from stage III human trials or the human challenge studies.
The Antibody dependent enhancement (ADE) is exploited by various viruses like Dengue, HIV, animal coronaviruses, etc. as an alternative method of infecting a variety of host cells. The virus-antibody complex can bind to the Fc receptors, activate the complement system, or induce a conformational change in the glycoprotein of the viral envelope ( Yip et al., 2016 ). This mechanism is observed when the vaccine-induced antibodies are either non-neutralizing or they are present in inadequate concentrations. This process triggers the viral entry into the cell due to the intensified binding efficiency of the virus-antibody complexes to FcR bearing cells. The clinical and preclinical trials of SARS-CoV vaccine candidates have demonstrated the aggravation of the disease due to ADE. Vaccine Associated Enhanced Respiratory Disease (VAERD) can also be induced by virus-antibody immune complex and T H 2-biased responses ( Graham, 2020 ).
The viral genome is vulnerable to mutations and can undergo the antigenic shift and the antigenic drift, as it continues to spread from one population to the next. The mutations may vary according to the environmental conditions of a geographical area, and the population density. By screening the 7500 samples of the infected patients, the scientists were able to figure out 198 mutations that may have materialized independently which may indicate the evolution of the virus inside the human host. These mutations may lead to different subtypes which may allow the virus to escape the immune system even after the administration of the vaccine ( Dorp et al., 2020 ).
5. Conclusion
SARS-CoV-2 has been the matter of the moment from the date it was declared as a pandemic, it has led to the termination of economic activities universally. Scientists across the continents are joining hands for the innovative tie-ups with both the pharmaceutical giants and the medical start-ups to repurpose drugs, develop vaccines, and devices to impede the progress of this overwhelming pandemic. A large number of COVID-19 vaccine candidates based upon various platforms have already been identified. Despite the undergoing efforts, a definitive answer does not exist. The process of vaccine development is quite laborious with various stages, including the pre-clinical stage, and clinical development which is a three-phase process. However, if sufficient data is already available, it has been recommended to skip a few stages, to accelerate the attainment of a vaccine faster with a quick regulatory review, approval, manufacturing, and quality control. This novel Coronavirus has therefore forced the scientific community to use unconventional approaches to accelerate the process of vaccine development. According to WHO: “vaccine must provide a highly favorable benefit-risk contour; with high efficacy, only mild or transient adverse effects and no serious ailments.” The vaccine must be suitable for all ages, pregnant, and lactating women and should provide a rapid onset of protection with a single dose and confer safety for at least up to one year of administration.
The use of novel technologies for vaccine development requires extensive testing for the safety and efficacy of a vaccine. The scientific community needs to construct various processes and capacities for the largescale manufacturing and administration of the coronavirus vaccines. The Coalition for Epidemic Preparedness Innovation (CEPI), an international non-governmental organization, which is funded by the Wellcome Trust, the European Commission, the Bill and Melinda Gates Foundation, and eight countries, is subsidizing the development of a large number of pandemic vaccine candidates around the globe. Moderna and the Vaccine Research Centre are co-developing an mRNA based vaccine candidate, wherein the mRNA is encapsulated in the lipid nanoparticles while Codagenix in collaboration with the Serum Institute of India is currently focused on developing the live attenuated viral vaccine. The pharmaceutical giants like Novavax, Sichuan Clover Biopharmaceuticals, iBio, and the University of Queensland are in the preclinical stage of the recombinant S glycoprotein vaccines. Additional strategies like the viral vector-based vaccines, targeting the S glycoprotein are being developed by the University of Oxford and CanSino Biologics, and other companies, Inovio and the Applied DNA Sciences are currently developing the DNA based vaccine candidates against the SARS-CoV-2 S Protein. Some of these vaccine candidates are at least months, away from being ready for human use, while others may take longer if at all approved for final use.
In India alone, six biotech ventures i.e. Serum Institute of India, ZydusCadila, Biological E, Indian Immunologicals, Bharat Biotech, and Mynvax are working in collaboration with various international vaccine developers. They are working on DNA vaccines, live attenuated recombinant measles vaccines, inactivated viral vaccines, subunit vaccines, and the vaccines developed by codon-optimization ( Coronavirus, 2020 ). Furthermore, the academic institutes like National Institute of Immunology (NII), Indian Institute of Science (IISc), International Center for Genetic Engineering and Biotechnology (ICGEB) New Delhi, Translational Health Science and Technology Institute (THSTI), etc. are attempting to develop the vaccines, and therapies, and the SARS-CoV-2 animal models to restrain the pandemic shortly ( Nandi, 2020 ).
The need of the hour is to develop a safe and effective COVID-19 vaccine which can induce an appropriate immune response to terminate this pandemic. It is the universal priority to spot the international funding mechanisms to support the development, manufacturing, and stockpiling of the coronavirus vaccines. This pandemic should serve as the guidepost to the international research community to not only acknowledge the outbreak but also indurate the following coronavirus crossing into mammals. A pan-coronavirus vaccine is urgently needed as the delay of vaccine rollout even by one week will accompany millions of deaths. Furthermore, it appears to be a scientifically feasible task if sufficient resources are made available in due time.
Funding Information
This work received no specific grant from any funding agency.
Declaration of Competing Interest
The author(s) declare that there are no conflicts of interest.
- Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., Jeong S.J., Kim J.H., Ku N.S., Yeom J.S., Roh J., Ahn M.Y., Chin B.S., Kim Y.S., Lee H., Yong D., Kim H.O., Kim S., Choi J.Y. Use of Convalescent Plasma Therapy in Two COVID-19 Patients with Acute Respiratory Distress Syndrome in Korea. J Korean Med Sci. 2020; 35 (14, April) doi: 10.3346/jkms.2020.35.e149. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahn J.Y., Sohn Y., Lee S.H., et al. Use of convalescent plasma therapy in two covid‐19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020 doi: 10.3346/jkms.2020.35.e149. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anon . 2020. Countries where COVID-19 has spread. www.worldometers.info. [Online] July 30, 2020. [Cited: July 31, 2020.] https://www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread/ . [ Google Scholar ]
- Anon . 2020. Coronavirus Resource Center. https://coronavirus.jhu.edu/. [Online] Johns Hopkins University. [Cited: August 05, 2020.] https://coronavirus.jhu.edu/map.html . [ Google Scholar ]
- Anon . 2020. COVID-19 Treatment and Vaccine Tracker. https://airtable.com/shrSAi6t5WFwqo3GM/tblEzPQS5fnc0FHYR/viweyymxOAtNvo7yH?blocks=bipZFzhJ7wHPv7x9z https://airtable.com/. [Online] Milken Institute. [ Google Scholar ]
- Anon . 2020. A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated Novel Coronavirus Pneumonia vaccine (Vero cells) Registration no.: ChiCTR2000031809. http://www.chictr.org.cn/showprojen.aspx?proj=52227 http://www.chictr.org.cn. [Online] [ Google Scholar ]
- Anon . 2020. UW–Madison, FluGen, Bharat Biotech to develop CoroFlu, a coronavirus vaccine. https://www.businesswire.com/news/home/20200402005666/en/UW%E2%80%93Madison-FluGen-Bharat-Biotech-develop-CoroFlu-coronavirus https://www.businesswire.com. [Online] April 02. [ Google Scholar ]
- Anon . 2020. A Study of a Candidate COVID-19 Vaccine (COV001)https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04324606","term_id":"NCT04324606"}} NCT04324606 ?term=vaccine&cond=covid-19&draw=2 https://clinicaltrials.gov. [Online]. [Cited: June 8, 2020.] [ Google Scholar ]
- Anon . 2020. Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19)https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04283461","term_id":"NCT04283461"}} NCT04283461 ?term=vaccine&cond=covid-19&draw=2 https://clinicaltrials.gov/. [Online] [ Google Scholar ]
- Anon . 2020. Moderna Announces Positive Interim Phase 1 Data for its mRNA Vaccine (mRNA-1273) Against Novel Coronavirus. https://investors.modernatx.com/. [Online] Moderna, Inc., May 18, 2020. [Cited: June 15, 2020.] https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-positive-interim-phase-1-data-its-mrna-vaccine. [ Google Scholar ]
- Anon . 2020. Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04336410","term_id":"NCT04336410"}} NCT04336410 ?term=inovio&cond=covid-19&draw=2&rank=1 https://clinicaltrials.gov/. [Online] 2020. 1. [ Google Scholar ]
- Anon . The University of Hong Kong; 2020. HKU joins global partnership to develop COVID-19 vaccine. https://fightcovid19.hku.hk/hku-state-key-laboratory-for-emerging-infectious-diseases-joins-global-effort-to-develop-covid-19-vaccine/ https://fightcovid19.hku.hk/. [Online], March 18, 2020. [ Google Scholar ]
- Anon . 2020. (BAT), British and American Tobacco Company. Potential COVID-19 vaccine – BAT in the news. https://www.bat.com/group/sites/UK__9D9KCY.nsf/vwPagesWebLive/DOBNHBWR https://www.bat.com/. [Online] 2020. [Cited: June 1, 2020.] [ Google Scholar ]
- Anon . 2020. Immunity and Safety of Covid-19 Synthetic Minigene Vaccine.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04276896","term_id":"NCT04276896"}} NCT04276896 https://clinicaltrials.gov. [Online] [ Google Scholar ]
- Anon . 2020. Safety and Immunity of Covid-19 aAPC Vaccine.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04299724","term_id":"NCT04299724"}} NCT04299724 https://clinicaltrials.gov/. [Online] [ Google Scholar ]
- Anon . 2020. Sinovac gets regulatory approval to assess Covid-19 vaccine. https://www.clinicaltrialsarena.com/news/sinovac-covid-19-vaccine-trial-approval/ https://www.clinicaltrialsarena.com. [Online] April 15. [ Google Scholar ]
- Anon . 2020. Sinovac reports positive data from Phase I/II trials of CoronaVac. https://www.clinicaltrialsarena.com/news/sinovac-coronavac-data/ https://www.clinicaltrialsarena.com/. [Online] June 15, 2020. [Cited: June 20, 2020.] [ Google Scholar ]
- Anon . 2020. An Open Study of the Safety, Tolerability and Immunogenicity of the Drug "Gam-COVID-Vac" Vaccine Against COVID-19.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04436471","term_id":"NCT04436471"}} NCT04436471 ?term=vaccine&cond=covid-19&draw=4 https://clinicaltrials.gov/. [Online] June 22, 2020. [Cited: June 22, 2020.]. NCT04436471. [ Google Scholar ]
- Anon . 2020. An Open Study of the Safety, Tolerability and Immunogenicity of "Gam-COVID-Vac Lyo" Vaccine Against COVID-19.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04437875","term_id":"NCT04437875"}} NCT04437875 https://clinicaltrials.gov/. [Online] June 22, 2020. [Cited: June 22, 2020.]. NCT04437875. [ Google Scholar ]
- Anon . 2020. Vaxart Announces Positive Pre-Clinical Data for its Oral COVID-19 Vaccine Program. https://investors.vaxart.com/. [Online] Vaxart Inc., April 21, https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-positive-pre-clinical-data-its-oral-covid-19. [ Google Scholar ]
- Anon . 2020. Zydus Cadila looks to expedite Covid-19 vaccine development. https://www.pharmaceutical-technology.com/news/zydus-cadila-covid-19-vaccine/ https://www.pharmaceutical-technology.com. [Online] 17 February, [ Google Scholar ]
- Anon . 2020. Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines https://www.who.int/. [Online] June 22, 2020. [Cited: June 23, 2020.] [ Google Scholar ]
- Anon . 2020. Clinical trial to assess the safety of a coronavirus vaccine in healthy men and women. http://www.isrctn.com/ISRCTN17072692 http://www.isrctn.com/ [Online] June 17, 2020. [Cited: June 22, 2020.]. ISRCTN17072692. [ Google Scholar ]
- Anon . 2020. A Trial Investigating the Safety and Effects of Four BNT162 Vaccines Against COVID-2019 in Healthy Adults.https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04380701","term_id":"NCT04380701"}} NCT04380701 https://clinicaltrials.gov/. [Online] May 8. [ Google Scholar ]
- Anon . BIOCAD Biotechnology Company; 2020. BIOCAD started working on mRNA vaccine against coronavirus. https://biocadglobal.com/index.php?posts&post=45 https://biocadglobal.com/. [Online], March 19. [ Google Scholar ]
- Anon . 2020. Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine With/Without Matrix-M Adjuvant.https://clinicaltrials.gov/ct2/show/record/ {"type":"clinical-trial","attrs":{"text":"NCT04368988","term_id":"NCT04368988"}} NCT04368988 https://clinicaltrials.gov/. [Online] May 27, 2020. [Cited: June 15, 2020.] [ Google Scholar ]
- Anon . 2020. Sanofi joins forces with U.S. Department of Health and Human Services to advance a novel coronavirus vaccine. http://www.news.sanofi.us/2020-02-18-Sanofi-joins-forces-with-U-S-Department-of-Health-and-Human-Services-to-advance-a-novel-coronavirus-vaccine http://www.news.sanofi.us/. [Online] Sanofi U.S., February 18, 2020. [ Google Scholar ]
- Anon . Medigaco Inc.; 2020. COVID-19 Vaccine Development Program. https://www.medicago.com/en/covid-19-programs/ https://www.medicago.com/. [Online] [ Google Scholar ]
- 2020. A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated Novel Coronavirus Pneumonia vaccine (Vero cells) http://www.chictr.org.cn/showprojen.aspx?proj=52227 http://www.chictr.org.cn. [Online] [ Google Scholar ]
- Anon . Sinovac Biotech Limited; 2020. Sinovac COVID-19 Vaccine Collaboration with Butantan Receives Approval from Brazilian Regulator for Phase III Trial. http://www.sinovac.com/?optionid=754&auto_id=907 http://www.sinovac.com/. [Online]July 06, 2020. [Cited: August 01, 2020.] [ Google Scholar ]
- Anon Treatment With Convalescent Plasma for Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Chest. 2020;(March) doi: 10.1016/j.chest.2020.03.039. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anon . 2020. COVID-19 / SARS-CoV-2. http://www.animalresearch.info/en/medical-advances/diseases-research/sars-cov-2/ http://www.animalresearch.info/. [Online] April 30, 2020. [Cited: June 11, 2020.] [ Google Scholar ]
- Arora Kajal, Rastogi Ruchir, et al. Multi-Antigenic Virus-like Particle of SARS CoV-2 produced in Saccharomyces cerevisiae as a vaccine candidate. Gurugram : s.n. bioRxiv. 2020;(May 19) doi: 10.1101/2020.05.18.099234. [ CrossRef ] [ Google Scholar ]
- Baruah V., Bose S. Immunoinformatics‐aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019‐nCoV. J Med Virol. 2020:495–500. doi: 10.1002/jmv.25698. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Breedveld F.C. Therapeutic monoclonal antibodies. The Lancet. 2000:735–740. doi: 10.1016/S0140-6736(00)01034-5. PMID 10703815. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- BROOK, STONY . 2020. Applied DNA Sciences Subsidiary, LineaRx, and Takis Biotech Collaborate for Development of a Linear DNA Vaccine Candidate Against Wuhan Coronavirus 2019-nCoV. https://adnas.com/coronoavirus-applied-dna-linearx-takis-biotech-vaccine/ https://adnas.com/. [Online] Applied DNA Sciences, February 07. [ Google Scholar ]
- Campbell Molly. 2020. Current Efforts in COVID-19 Vaccine Development. https://www.technologynetworks.com/biopharma/articles/current-efforts-in-covid-19-vaccine-development-332429 https://www.technologynetworks.com/. [Online] Technology Networks, March 23. [ Google Scholar ]
- Cao Y., Zhu X., Hossen M.N., et al. Augmentation of vaccine-induced humoral and cellular immunity by a physical radiofrequency adjuvant. Nat Commun. 2018 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carter John, Saunders Venitia. Wiley; Hoboken, New Jersey: 2007. Virology: Principles and Applications; p. 382. ISBN: (Paperback) 9780470023877 [ Google Scholar ]
- (CDC), Centers for Disease Control and Prevention . CDC; 2020. How COVID-19 Spreads. [Online] 2020. [Cited: June 1, 2020.] https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html? CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fprepare%2Ftransmission.html. [ Google Scholar ]
- CDC Coronavirus Disease 2019 (COVID-19)- Symptoms of Covid-19. Centres for Disease Control and Prevention. 2020 https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html [Online]. [ Google Scholar ]
- Coleman Christopher M., Liu Ye V., Mu Haiyan, Taylor Justin K., Massare Michael, Flyer David C., Glenn Gregory M., Smith Gale E., Frieman Matthew B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2020:3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Conserved Protein Domain Family: SARS-like-CoV_M . 2020. NCBI. https://www.ncbi.nlm.nih.gov/Structure/cdd/cd21569 [Online]. [Cited: June 1, 2020.] [ Google Scholar ]
- Coronavirus . 2020. Around 30 Indian attempts at COVID-19 vaccine, says Principal Scientific Adviser. https://www.thehindu.com/ [Online] The Hindu, May 11, 2020. [Cited: June 11, 2020.] thehindu.com/sci-tech/health/coronavirus-around-30-indian-attempts-at-covid-19-vaccine-says-principal-scientific-adviser/article31560932.ece. [ Google Scholar ]
- CTRI/2020/07/026352. 2020 http://ctri.nic.in/. [Online] July 31, 2020. [Cited: August 01, 2020.] http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=45306&EncHid=&userName=Zydus.
- Cuiling Zhang, Giulietta Maruggi, Hu Shan, Junwei Li. Advances in mRNA Vaccines for Infectious Diseases. Frontiers in Immunology. 2020:594. DOI=10.3389/fimmu.2019.00594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- da Silva S., da Silva C., Mendes R., Pena L. Role of Nonstructural Proteins in the Pathogenesis of SARS-CoV-2. Journal of medical virology. 2020 doi: 10.1002/jmv.25858. 10.1002/jmv.25858 p. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Delamater P.L., Street E.J., Leslie T.F., Yang Y., Jacobsen K.H. Complexity of the Basic Reproduction Number (R0) s.l. : Emerging Infectious Diseases. 2019; 25 doi: 10.3201/eid2501.171901. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020 doi: 10.1080/21645515.2020.1735227. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doremalen Neeltje van, Lambe Teresa, Spencer Alexandra, Belij-Rammerstorfer Sandra, Purushotham Jyothi N., Port Julia R., Avanzato Victoria, Bushmaker Trenton, Flaxman Amy, Ulaszewska Marta, Feldmann Friederike, Allen Elizabeth R., Sharpe Hannah. Jonathan ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. s.l. : bioRxiv. 2020 doi: 10.1101/2020.05.13.093195. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dorp Lucy van, Acman Mislav, Richard Damien, Shaw Liam P., Ford Charlotte E., Ormond Louise, Owen Christopher J., Pang Juanita, Tan Cedric C.S., Boshier Florencia A.T., Ortiz Arturo Torres, Balloux François. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. s.l. : Infection, Genetics and Evolution. 2020; 104351 doi: 10.1016/j.meegid.2020.104351. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eyal N., Lipsitch M., Smith P.G. Human Challenge Studies to Accelerate Coronavirus Vaccine Licensure. s.l. : The Journal of infectious diseases. 2020:1752–1756. doi: 10.1093/infdis/jiaa152. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Folegatti Pedro M, Ewer Katie J, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet. 2020;(July) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Garg P., Srivastava N., Srivastava P. An Integrated In-Silico Approach to Develop Epitope-Based Peptide Vaccine against SARS-CoV-2. s.l. : Preprints. 2020 doi: 10.20944/preprints202005.0401.v1). [ CrossRef ] [ Google Scholar ]
- Gelboin Harry V, et al. Inhibitory monoclonal antibodies to human cytochrome P450 enzymes: a new avenue for drug discovery. Trends in Pharmacological Sciences. 1999:432–438. doi: 10.1016/S01. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gralinski L.E., Menachery V.D. Return of the Coronavirus: 2019-nCoV. Viruses. 2020:135. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;(May 29):945–946. [ PubMed ] [ Google Scholar ]
- Gupta V., Tabiin T.M., Sun K., Chandrashekaran A., Anwar A., Yang K., Chikhlikar P., Salmon J., Brusic V., Marques E.T.A., Kellathur S.N., August T.J. SARS coronavirus nucleocapsid immunodominant T-cell epitope cluster is common to both exogenous recombinant and endogenous DNA-encoded immunogens. Virology. 2006; 347 (1):127–139. (impact factor: 2.657) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Heat Biologics’ COVID-19 Vaccine Program . 2020. Heat Biologics. https://www.heatbio.com/product-pipeline/covid-19-vaccine https://www.heatbio.com/. [Online] [ Google Scholar ]
- Hobernik D., Bros M. DNA Vaccines-How Far From Clinical Use? Int J Mol Sci. 2018:3605. doi: 10.3390/ijms19113605. PMID: 30445702; PMCID: PMC6274812. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoffmann Markus, Kleine-Weber Hannah, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020:271–280. doi: 10.1016/j.cell.2020.02.052. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Imai Masaki, Iwatsuki-Horimoto Kiyoko, et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proceedings of the National Academy of Sciences. 2020;(May):16587–16595. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jackson Lisa A., Anderson, Evan J., et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N Engl J Med. 2020;(July) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jiang S., Du L., Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerging microbes & infections. 2020:275–277. doi: 10.1080/22221751.2020.1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Johnson & Johnson Announces a Lead Vaccine Candidate for COVID-19 . 2020. Landmark New Partnership with U.S. Department of Health & Human Services; and Commitment to Supply One Billion Vaccines Worldwide for Emergency Pandemic Use. https://www.prnewswire.com/news-releases/johnson--johnson-announces-a-lead-vaccine-candidate-for-covid-19-landmark-new-partnership-with-us-department-of-health--human-services-and-commitment-to-supply-one-billion-vaccines-worldwide-for-emergency-pandemic- https://www.prnewswire.com/. [Online] [ Google Scholar ]
- Kai Duan, Liu Bende, Li Cesheng, Zhang Huajun, Yu Ting, Qu Jieming, Zhou Min, Chen Li, Meng Shengli, Hu Yong, Peng Cheng, Yuan Mingchao, Huang Jinyan, Wang Zejun, Yu Jianhong, Gao Xiaoxiao, Wang Dan, Yu Xiaoqi, Li Li., Zhang Jiayou, Wu Xiao, Li Bei, Yanpin Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proceedings of the National Academy of Sciences. 2020;(April) doi: 10.1073/pnas.2004168117. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim E., et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102743. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lauer S.A., Grantz K.H., Bi Q., et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020:577–582. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Le Tung Thanh, Andreadakis Zacharias, Kumar Arun, Román Raúl Gómez, Tollefsen Stig, Saville Melanie, Mayhew Stephen. The COVID-19 vaccine development landscape. Nature Reviews Drug Discovery. 2020; 19 (5):305–306. [ PubMed ] [ Google Scholar ]
- Lee Jaimy. These 23 companies are working on coronavirus treatments or vaccines — here’s where things stand. Market watch. 2020 https://www.marketwatch.com/story/these-nine-companies-are-working-on-coronavirus-treatments-or-vaccines-heres-where-things-stand-2020-03-06 [Online] May 6, 2020. [Cited: June 1, 2020.] [ Google Scholar ]
- Liu Cynthia, Zhou Qiongqiong, Li Yingzhu, Garner Linda V., Watkins Steve P., Carter Linda J., Smoot Jeffrey, Gregg Anne C., Daniels Angela D., Jervey Susan, Albaiu Dana. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Central Science. 2020:315–331. doi: 10.1021/acscentsci.0c00272. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Masihi K Noel. Fighting infection using immunomodulatory agents. Expert Opinion on Biological Therapy. 2001:641–653. doi: 10.1517/14712598.1.4.641. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mice L., Bao, et al. The Pathogenicity of 2019 Novel Coronavirus in hACE2 Transgenic. s.l. : bioRxiv. 2020 doi: 10.1101/2020.02.07.939389. preprint. [ CrossRef ] [ Google Scholar ]
- Michot Jean-Marie, Albiges Laurence, Chaput Nathalie, Saada Veronique, Fanny Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Annals of Oncology. 2020 doi: 10.1016/j.annonc.2020.03.300. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mulligan Mark J., Lyke Kirsten E., et al. Phase 1/2 Study to Describe the Safety and Immunogenicity of a COVID-19 RNA Vaccine Candidate (BNT162b1) in Adults 18 to 55 Years of Age: Interim Report; preprint. s.l. : medRxiv. 2020 06.30.20142570, 2020, medRxiv. [ Google Scholar ]
- Myupchar . 2020. Race for COVID-19 vaccine: Covaxin and ZyCoV-D begin human trials in India, Moderna publishes preliminary data from phase 1. https://www.firstpost.com/health/race-for-covid-19-vaccine-covaxin-and-zycov-d-begin-human-trials-in-india-moderna-publishes-preliminary-data-from-phase-1-8600211.html/amp https://www.firstpost.com/. [Online] July 15, 2020. [Cited: August 01, 2020.] [ Google Scholar ]
- Nandi Jayashree. 2020. Top Indian scientists join global fight against coronavirus. https://www.hindustantimes.com/india-news/top-indian-scientists-join-global-fight-against-virus/story-CKRGfycsjBJD2ypLcbJPHM.html https://www.hindustantimes.com. [Online] Hindustan Times, New Delhi, March 30, 2020. [Cited: June 9, 2020.] [ Google Scholar ]
- Ning Wang, Jian Shang, Shibo Jiang, Lanying Du. Subunit Vaccines Against Emerging Pathogenic Human Coronaviruses. Frontiers in Microbiology. 2020 doi: 10.3389/fmicb.2020.00298. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Novavax covid 19 vaccine trial . 2020. Clinical Trials Arena. https://www.clinicaltrialsarena.com/news/novavax-covid-19-vaccine-trial/ [Online] [ Google Scholar ]
- Ou X., Liu Y., Lei X., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020; 1620 :11. doi: 10.1038/s41467-020-15562-9. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Park Su Eun. Epidemiology, virology, and clinical features of severe acute respiratory syndrome -coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19) Clin Exp Pediatr. 2020:119–124. doi: 10.3345/cep.2020.00493. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pharmaceutical Targeting the Envelope Protein of SARS-CoV-2: the Screening for Inhibitors in Approved Drugs . XR Pharmaceuticals Ltd.; Cambridge, New Zealand: 2020. Chernyshev, Anatoly. [ Google Scholar ]
- PIB, Delhi . 2020. DBT/ Anti-COVID consortium- Efforts underway to produce therapeutic antibodies against COVID-19: Isolating genes encoding antibodies for neutralising the SARS-CoV-2, COVID-19. https://pib.gov.in/. [Online] April 12, 2020. [Cited: June 07, 2020.] https://pib.gov.in/PressReleaseIframePage.aspx? PRID=1613531. [ Google Scholar ]
- Prof Roujian Lu, Zhao Xiang, Li Juan, Niu Peihua, Bo Yang, Honglong Wu, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020:565–574. doi: 10.1016/S0140-6736(20)30251-8. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pyrek Kelly M. 2018. 100 Years after the Spanish Flu: Lessons Learned and Challenges for the Future. https://www.infectioncontroltoday.com/ [Online] October 11, 2018. https://www.infectioncontroltoday.com/public-health/100-years-after-spanish-flu-lessons-learned-and-challenges-future. [ Google Scholar ]
- Shang W., Yang Y., Rao Y., et al. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 2020 doi: 10.1038/s41541-020-0170-0. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shen Chenguang, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020; 323 (16):1582–1589. doi: 10.1001/jama.2020.4783. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shen C., Wang Z., Zhao F., et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020; 323 (16):1582–1589. doi: 10.1001/jama.2020.4783. 2020. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smith T.R.F., Patel A., Ramos S., et al. Immunogenicity of a DNA vaccine candidate for COVID-19. 2601. s.l. : Nat Commun. 2020; 11 doi: 10.1038/s41467-020-16505-0. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tai W., He L., Zhang X., et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020 doi: 10.1038/s41. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- TNX-1800 (Coronavirus Vaccine) 2020. Online] Tonix Pharmaceuticals. https://www.tonixpharma.com/pipeline/tnx-1800-coronavirus-vaccine https://www.tonixpharma.com. [ Google Scholar ]
- Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific. Emerg Microbes Infect. 2020:382–385. doi: 10.1080/22221751.2020.1729069. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tu Y.-F., Chien C.-S., Yarmishyn A.A., Lin Y.-Y., Luo Y.-H., Lin Y.-Y., Lai W.-Y., Yang D.-M., Chou S.-J., Yang Y.-P., Wang M.-L., Chiou S.-H. A Review of SARS-CoV-2 and the Ongoing Clinical Trials. International Journal of Molecular Sciences. 2020:2657. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Upadhyay Lipi. 2020. Italy claims to have developed the first COVID-19 vaccine: Here is what we know about all the potential coronavirus vaccines. https://timesofindia.indiatimes.com/life-style/health-fitness/health-news/italy-claims-to-develop-first-covid-19-vaccine-here-is-the-current-status-of-all-the-potential-coronavirus-vaccines/photostory/75575319.cms https://timesofindia.indiatimes.com/. [Online] May 8, 2020. [Cited: May 31, 2020.] [ Google Scholar ]
- Ura T., Okuda K., Shimada M. Developments in Viral Vector-Based Vaccines. Vaccines (Basel) 2014:624–641. doi: 10.3390/vaccines2030624. PMID: 26344749; PMCID: PMC4494222. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Voltron Therapeutics, Inc . 2020. Enters into Sponsored Research Agreement with The Vaccine & Immunotherapy Center at the Massachusetts General Hospital to Develop Potential COVID-19 Vaccine. https://www.prnewswire.com/news-releases/voltron-therapeutics-inc-enters-into-sponsored-research-agreement-with-the-vaccine--immunotherapy-center-at-the-massachusetts-general-hospital-to-develop-potential-covid-19-vaccine-301034225.html https://www.prnewswire.com/. [Online] April 02, 2020. [ Google Scholar ]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Vessler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020:281–292. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Walls Structure, Function, and Antigenicity of the SARSCoV-2 Spike Glycoprotein. Cell. 2020:281–292. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020 doi: 10.1128/JVI.00127-20. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- (WHO), World Health Organisation . s.l. : World Health Organisation; 2014. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease. September [ Google Scholar ]
- (WHO) World Health Organisation . s.l.: World Health Organisation; 2020. Coronavirus disease (Covid-19) Situation Report- 164. [ Google Scholar ]
- WHO . s.l. : World Health Organisation; 2020. Draft landscape of COVID-19 candidate vaccines. [ Google Scholar ]
- Wrapp Daniel, Wang Nianshuang, Corbett Kizzmekia S., Goldsmith Jory A., Hsieh Ching-Lin, Abiona Olubukola, Graham Barney S., Jason S. Mclellan Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;(13 March):1260–1263. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., et al. Commentary genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020:325–328. doi: 10.1016/j.chom.2020.02.001. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yan, Xuebing. 2020 http://www.chictr.org.cn/showprojen.aspx?proj=49544. http://www.chictr.org.cn. [Online] February 2020.
- Yip M.S., Leung H.L., Li P.H., Cheung C.Y., Dutry I., Li D., Daëron M., Bruzzone R., Peiris J.S.M., Jaume M. Hong Kong Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS: s.n. Hong Kong medical journal = Xianggang yi xue za zhi / Hong Kong Academy of Medicine. 2016:25–31. [ PubMed ] [ Google Scholar ]
- Yoshimoto F.K. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or n-COV19), the Cause of COVID-19. The protein journal. 2020:198–216. doi: 10.1007/s10930-020-09901-4. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. International Journal of Antimicrobial Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105955. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang B., Liu S., Tan T., et al. Treatment with convalescent plasma for critically ill patients with SARS‐CoV‐2 infection. Chest. 2020; 2020 :30571–30577. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhu Feng-Cai, Li Yu-Hua, Guan Xu-Hua, Hou Li-Hua, Wang Wen-Juan, Li Jing-Xin, Wu Shi-Po, Wang Bu-Sen, Wang Zhao, Wang Lei, Jia Si-Yue, Jiang Hu-Dachuan, Wang Ling, Jiang Tao, Hu Yi, Gou Jin-Bo, Xu Sha-Bei, Xu Jun-Jie, Wang Xue-Wen, Wang Wei, Chen Wei. Safety, tolerability, and immunogenicity of a recombinan tadenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet. 2020 doi: 10.1016/S0140-6736(20)31208-3. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhu Feng-Cai, Guan Xu-Hua, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet. 2020;(July) [ PMC free article ] [ PubMed ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Abstract. Since its emergence in December 2019, corona virus disease 2019 (COVID-19) has impacted several countries, affecting more than 90 thousand patients and making it a global public threat. The routes of transmission are direct contact, and droplet and possible aerosol transmissions. Due to the unique nature of dentistry, most dental ...
Abstract. In early December 2019, an outbreak of coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan City, Hubei Province, China. On January 30, 2020 the World Health Organization declared the outbreak as a Public Health Emergency of International Concern.
Abstract. In early December 2019, an outbreak of coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan City, Hubei Province, China. On January 30, 2020 the World Health Organization declared the outbreak as a Public Health Emergency of International Concern.
A novel coronavirus (CoV) named '2019-nCoV' or '2019 novel coronavirus' or 'COVID-19' by the World Health Organization (WHO) is in charge of the current outbreak of pneumonia that began at the beginning of December 2019 near in Wuhan City, Hubei Province, China [1-4]. COVID-19 is a pathogenic virus. From the phylogenetic analysis ...
The spread of the "Severe Acute Respiratory Coronavirus 2" (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [].The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [], causing massive economic strain ...
There have been around 96,000 reported cases of coronavirus disease 2019 (COVID-2019) and 3300 reported deaths to date (05/03/2020). The disease is transmitted by inhalation or contact with infected droplets and the incubation period ranges from 2 to 14 d. The symptoms are usually fever, cough, sore throat, breathlessness, fatigue, malaise ...
ABSTRACT In order to understand the impact of the COVID-19 pandemic on higher education, we surveyed ... COVID-19, we can back out the subjective treatment e ect of COVID-19 on academic performance. The credibility of our approach depends on: (1) students having well-formed beliefs about outcomes in the
Introduction. COVID-19 is a growing pandemic with initial cases identified in Wuhan, Hubei province, China toward the end of December 2019. Labeled as Novel Coronavirus 2019 (2019-nCoV) initially by the Chinese Center for Disease Control and Prevention (CDC) which was subsequently renamed as Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) due to its homology with SARS-CoV by the ...
Abstract Send to Citation Mgr. Add to Favorites. Email to a Friend ... COVID-19 and the need for global critical care training. Why ventilators alone are not the answer. ATS Sch 2020;2:13-18. A university health system deployed a tele-ICU system that benefited local clinicians as well as residents and fellows, who learned about critical care ...
We performed a systematic review to evaluate the methodological quality of currently available COVID-19 studies compared to historical controls. A total of 9895 titles and abstracts were screened ...
The published literature on COVID now exceeds 211,000 papers, books, and documents, which include: 22,866 observational studies, 19,591 reviews, 1496 meta-analyses and 781 randomized control trials. These publications comprise the backdrop for our research and writing. The project began in the spring of 2020 based on a limited source of cumulative COVID-19 data and has broadened considerably ...
AN ANALYSIS OF THE COVID-19 PANDEMIC iii ABSTRACT An Analysis of the COVID-19 Pandemic on Students at The University of South Dakota Alexandra Buss Director: Shane Nordyke Ph.D. The COVID-19 pandemic rapidly took over the United States (US) in the beginning of 2020. Nationally, damages to finances, housing, and mental health have impacted many.
2. Read the abstract and look at the pictures. Research papers are long and dense with a very different structure compared with articles in the normal media. Media articles start with the most ...
COVID-19: Insights on the Pandemic's Traumatic Effects and Global Implications. Special issue of the APA journal Psychological Trauma: Theory, Research, Practice, and Policy that presents early findings from Wuhan, China, and raises essential questions about the massive social experiment of mandated lockdown.
Abstract. The outbreak of Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2), has thus far killed over 3,000 people and infected over 80,000 in China and elsewhere in the world, resulting in catastrophe for humans. Similar to its homologous virus, SARS-CoV, which caused SARS in ...
Abstract. Coronavirus (COVID-19) endemic is growing exponentially in the whole world. Researchers, technologists, doctors and other healthcare workers are working day and night on the development of vaccine and medicinesto control and treat this virus. SARS-CoV-2 is the name of the virus responsible for causing COVID-19 disease, which is highly ...
An abstract that has been previously published or presented at a national, regional or international meeting can be submitted to the IAS COVID-19 Conference: Prevention only if there are new methods, findings, updated information or other valid reasons for resubmitting. If preliminary or partial data have been published or presented previously, the submitting author will be required to provide ...
Submit your Research. Articles are published rapidly as soon as they are accepted, after passing a series of prepublication checks to assess originality, readability, author eligibility, and compliance with Wellcome Open Research's policies and ethical guidelines. Peer review by invited experts takes place openly after publication.
The marginal impact of COVID-19 vaccination on. excess deaths at 80 percent vaccination rate is approximately 1/10th the marginal impact at the. beginning of the vaccination campaign. Figure 3 juxtaposes estimates from the above model with actual data to illustrate our.
This project will examine the experiences faced by faculty and students in higher education during the COVID-19 pandemic. To understand the complexity of the situation we combine theory and best practices from the fields of I/O Psychology, Crisis Communication, Disaster Management, and Higher Education. Essentially, better understanding faculty ...
Coronavirus (SARS-CoV-2), the cause of COVID-19, a fatal disease emerged from Wuhan, a large city in the Chinese province of Hubei in December 2019. Main body of abstract The World Health Organization declared COVID-19 as a pandemic due to its spread to other countries inside and outside Asia.
Abstract. The current COVID-19 pandemic has urged the scientific community internationally to find answers in terms of therapeutics and vaccines to control SARS-CoV-2. Published investigations mostly on SARS-CoV and to some extent on MERS has taught lessons on vaccination strategies to this novel coronavirus. This is attributed to the fact that ...
Without the availability of a vaccine (at the time of writing) to control the spread of the COVID-19 virus, ... Abstract. Without the availability of a vaccine (at the time of writing) to control the spread of the COVID-19 virus, countries must rely largely on lockdown measures to limit population movement and community spread. ...
Request PDF | Sustained Use of Data-Based Writing Instruction Before and During the COVID-19 Pandemic | For research-based academic interventions to continue to have positive effects on the ...
Introduction. The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there ...
Abstract. The COVID-19 pandemic highlighted the importance of cultivating a pedagogy of care and strengthening community for all postsecondary courses, especially those employing the high-impact practice of teaching with writing. ... In this mixed methods study, we found that instructors of Writing Intensive courses at a Midwestern university ...
Abstract. The current COVID-19 pandemic has urged the scientific community internationally to find answers in terms of therapeutics and vaccines to control SARS-CoV-2. ... The rationale for writing this review is to gather all the information about the COVID-19 vaccine development programs and give the readers and researchers insight into types ...