An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Med Princ Pract
- v.30(2); 2021 Apr


Management of Type 2 Diabetes: Current Strategies, Unfocussed Aspects, Challenges, and Alternatives
Swapnil p. borse.
a AYUSH-Center of Excellence, Center for Complementary and Integrative Health (CCIH), Interdisciplinary School of Health Sciences, Savitribai Phule Pune University (SPPU), Pune, India
b Department of Pharmacology and Toxicology, B. V. Patel Pharmaceutical Education and Research Development (PERD) Centre, Thaltej, India
Abu Sufiyan Chhipa
c Institute of Pharmacy, Nirma University, Ahmedabad, India
Vipin Sharma
d Translational Health Science and Technology Institute, Faridabad, India
Devendra Pratap Singh
e Sun Pharmaceutical Industries Ltd., Vadodara, India
Manish Nivsarkar
Type 2 diabetes mellitus (T2DM) accounts for >90% of the cases of diabetes in adults. Resistance to insulin action is the major cause that leads to chronic hyperglycemia in diabetic patients. T2DM is the consequence of activation of multiple pathways and factors involved in insulin resistance and β-cell dysfunction. Also, the etiology of T2DM involves the complex interplay between genetics and environmental factors. This interplay can be governed efficiently by lifestyle modifications to achieve better management of diabetes. The present review aims at discussing the major factors involved in the development of T2DM that remain unfocussed during the anti-diabetic therapy. The review also focuses on lifestyle modifications that are warranted for the successful management of T2DM. In addition, it attempts to explain flaws in current strategies to combat diabetes. The employability of phytoconstituents as multitargeting molecules and their potential use as effective therapeutic adjuvants to first line hypoglycemic agents to prevent side effects caused by the synthetic drugs are also discussed.
- Type 2 diabetes is a multifactorial disorder that leads to a disturbed glucose homeostasis.
- Lifestyle management along with pharmacological approaches is crucial to achieve a successful management of diabetes.
- Complex interplays between genetics and environmental factors play important roles in the development of diabetes.
- Combinational therapies employed after failure of monotherapy result in comorbidities.
- Phytoconstituents are better alternatives owing to their multitargeting capability.
Introduction
Insulin resistance and β-cell dysfunction are the 2 major hallmarks of type 2 diabetes mellitus (T2DM) that appear as the result of disturbed homeostasis [ 1 ]. Failure of β-cells (∼80% of their β-cell function) and insulin resistance in muscles and the liver is a vicious triumvirate responsible for the core physiological defects. However, T2DM is classically viewed as a disorder of insulin deficiency and resistance, and further insights into the pathophysiology of T2DM suggest the role of other key players in insulin deficiency and its functional inability. Pancreatic islets are composed of insulin-releasing β-cells (48–59%), glucagon-releasing α-cells (33–46%), somatostatin (SsT)-releasing δ-cells, and F cells that release polypeptides (PPs) in similar proportion [ 2 ]. Moreover, paracrine interactions occur in the sequence from β-cell to α-cells followed by δ-cells and PP-cells/F-cells [ 3 ]. While the β-cell interactions are emphasized at present, the interaction of other cells in pancreas is of crucial importance that needs to be explored further to understand their roles in glucose homeostasis [ 2 ]. Also, the development of glucose resistance in T2DM is largely influenced by fat cells (accelerated lipolysis), gastrointestinal tract (incretin deficiency/resistance), α-cells (hyperglucagonemia), kidneys (increased glucose reabsorption) and brain (insulin resistance), and complex interactions that occur between these factors and T2DM associated genes [ 4 ]. Changes in the lifestyle of T2DM patients are crucial along with pharmacological interventions to improve the overall health status of the patient. The present review discusses our current understanding of the pathogenesis of T2DM and attempts to emphasize on generally unfocused aspects of T2DM pathogenesis and treatment that may contribute significantly to treatment approaches and patient-related outcomes.
Understanding the Diabetes Machinery: The Unfocused Aspects
Amylin proteins and pancreatic β-cell function.
β-Cells are the most extensively studied pancreatic cells for their roles in glucose homeostasis in T2DM. Islet amyloid PP (amylin) is a β-cell peptide hormone that is secreted along with insulin in the ratio of approximately 100:1. Its secretion is also altered in diabetic patients. Amylin functions as an inhibitor of glucagon secretion and delays gastric emptying thus acting as a satiety agent [ 5 ]. Amylin action is executed through an area postrema (glucose-sensitive part of the brain stem) that collectively aims to reduce the demand of total insulin [ 6 ]. Besides these functions, amylin also plays roles in the destruction of β-cell via the formation of amyloid aggregates and fibers [ 7 ]. Findings from histopathology have shown the accumulation of extracellular amyloid proteins, hyperphosphorylated tau, ubiquitin, apolipoprotein E, apolipoprotein (a), c-Jun N-terminal kinases (JNK1), and islet-brain 1/JNK1 interacting protein-1 (IB1/JIP-1) as the characteristic feature of pancreatic islets in T2DM individuals, suggesting that amylin in association with endocrine system plays important roles in physiology, pathology, and progression of T2DM [ 8 ].
α-Cells
α-cells are known to play crucial roles in the pathophysiology of T2DM. The secretion of glucagon from α-cell is regulated by glucose, hormones, and other substrates that work in unison. Any abnormality in α-cells is reflected in altered glucose homeostasis [ 9 ]. In T2DM, a relative elevated secretion of glucagon takes place in fasting and postprandial states during normal and increased glucose levels along with altered hypoglycemic response [ 10 ]. According to the bi-hormonal hypothesis, T2DM is the consequence of insulin resistance/deficiency with a relative excess glucagon secretion, leading to a rate of hepatic glucose production that is much higher than the rates of glucose utilization. This consequently results in hyperglycemia. The hypothesis is supported by a plethora of clinical and experimental investigations [ 11 , 12 ]. Reduced suppression of glucagon release under hyperglycemic conditions is a contributing factor to postprandial hyperglycemia [ 13 ]. Interestingly, α-cells do not show this behavior in the presence of adequate insulin levels, suggesting that impairment in insulin machinery also cause the abnormalities in glucagon release in T2DM [ 14 ]. In addition to this, hypoglycemia is remarkably influenced by glucagon secretion in T2DM patients treated with insulin. In such patients, the secretory response of α-cells to low-glucose concentrations is compromised, which further aggravates the risks of severe hypoglycemia [ 15 ]. The deficiency of glucagon action in response to hypoglycemia is linked with multiple failures in α-cell regulation [ 16 ]. Even in the situation of islet allotransplantation that helps diabetes patients to remain independent to insulin for a long time, the retarded response of α-cell response to hypoglycemia usually remains unaffected, indicating that the procedure does not completely restore the physiological functions of α-cells [ 17 ]. Collectively, defects in α-cell regulation and glucagon secretion lead to defective glucose sensing, loss of β-cell function, and insulin resistance.
δ-Cells, SsT, and Pancreatic PP Cells (F-Cells)
The δ-cells are located in the stomach, intestine, neuroendocrine cells, and pancreas. They secrete SsT in a pulsatile manner in response to fluctuations in glucose levels [ 18 ]. SsT regulates the endocrine functions and also plays an important role in the gut-brain axis. The receptors of SsT are present on α- and β-cells where they act as inhibitory receptors for the secretion of insulin and glucagon. SsT exerts a tonic inhibitory effect on the secretion of insulin and glucagon and facilitates the islet response to cholinergic activation. In addition, SsT is also involved in the suppression of nutrient-induced glucagon secretion [ 19 ]. Further, SsT significantly alters the normal glucose homeostasis and feedback loops [ 20 ].
F-cells of the pancreas release pancreatic PP after the food intake. It exerts inhibitory postprandial effects on gastric emptying, intestinal motility, exocrine pancreatic secretion, hepatic glucose production, and gallbladder contraction. Functional abilities of PP significantly affect food intake and energy metabolism [ 21 ]. When administered through intracerebroventricular route, PP exerts an orexigenic (appetite stimulating) effect in the brain. On contrary, intraperitoneal administration of PP reduces the food intake and lowers body weight by enhancing energy expenditure [ 22 , 23 ]. Increased plasma levels of PP are implicated in obesity and diabetes.
Adipose Tissue and Resistin
Adipose tissue consists of adipocytes, connective tissue matrix, nerve tissue, stromovascular cells, and immune cells. The role of adipose tissue as an endocrine organ is well established [ 24 ]. It releases leptin, cytokines, adiponectin, complement components, plasminogen activator inhibitor-1, proteins of the renin-angiotensin system, and resistin. Apart from secreting factors/hormones, adipose tissue also functions in coordination with other hormone systems and the central nervous system. Typically, adipose tissues serve as a store house for fat under normal conditions, while they also release free fatty acids (FFAs) in metabolic disorders. Consistent decline in the function of β-cell in normal individuals has been shown to be associated with progressive secretion of FFAs and insulin resistance in adipose tissue [ 25 ]. Resistin or adipose tissue-specific secretory factor released from adipose tissue is largely implicated in the progression and development of T2DM [ 24 ]. It acts as an inhibitory hormone that causes resistance to insulin [ 26 ]. Levels of circulating resistin increase in T2DM, resulting in oxidative stress, insulin resistance, and platelet activation [ 27 ]. Expression of the resistin gene is also observed in the pancreatic islets, pituitary, and hypothalamus [ 28 ]. Although resistin is primarily secreted by macrophages in humans [ 29 ] where it is involved in the recruitment of immune cells and pro-inflammatory factors, the involvement of resistin is also seen in hyperglycemia and insulin resistance [ 30 , 31 ]. Resistin-induced hyperglycemia and obesity are induced through the activation of AMP-protein kinase and decreased expression of gluconeogenic enzymes in the liver. Induction of insulin resistance is also evident in rodents after the administration of recombinant resistin that reverses with the immune neutralization [ 32 ].
T2DM is notorious for being “the geneticist's nightmare.” Occurring due to the combined contribution of genetic and environmental factors, leading to multiple gene alterations [ 33 ]. Multiple mechanisms act either directly or in association with other factors to influence the development and progression of T2DM. These include defects in pancreatic angiogenesis, innervation, and modification of parental imprinting [ 34 ]. The pathogenesis of T2DM depends on the intensity of both maternal and paternal insulin resistivity and/or insulin sensitivity [ 35 ]. According to one study, the first-degree relatives of T2DM patients live at a higher risk of developing T2DM and have a strong genetic predisposition to β-cell failure [ 36 ]. Moreover, β-cell dysfunction, autosomal dominance, and heterozygous mutations in β-cell transcription factors are some of the major causes leading to early onset of T2DM. The identified genes responsible for the early-onset T2DM include insulin promoter factor-1, hepatocyte nuclear factor (HNF)-4α, NeuroD1/BETA2, HNF-1α, and HNF-1β [ 37 ]. A hyperglycemic intrauterine environment has also been implicated in T2DM or pre-diabetes in the offspring of women suffering from gestational diabetes [ 38 ]. Also, during gestational diabetes, the expression of insulin receptor-β, PI3K (phosphatidylinositol 3-kinase) with its subunit p85α and GLUT-4 decreases with a compensatory elevation in the expression of GLUT-1 mRNA in placental tissues [ 39 ]. Polymorphism in resistin gene 299 (G>A) and increase in serum resistin is also known to be a contributing factor to increased insulin resistance with a subsequent higher risk of T2DM in offspring. Moreover, offspring carrying AA and combined GA + AA genotypes tend to be at higher risk [ 40 ]. On the other hand, diabetes also has the capacity to make genetic alterations leading to associated comorbidities. For instance, alterations in genes involved in vitamin synthesis leads to lowering of levels of riboflavin and glycemia, microalbumineria, and altered levels of uric acid in T2DM individuals and development of insulin resistance due to vitamin D deficiency [ 41 , 42 , 43 , 44 , 45 , 46 ]. Importantly, the genes of vitamin D receptor and its binding protein along with CYP1α show polymorphisms in diabetics [ 42 , 43 , 44 ].
The gut serves as a prominent link between the brain and the enteric nervous system [ 47 ]. The secretion of gastrointestinal hormones (incretin, glucagon-like peptide-1 [GLP-1], and glucose-dependent insulinotropic polypeptide [GIP]) increases after food intake. These hormones assist insulin and glucagon in maintaining glucose homeostasis and improve α-cell glucose sensing. GLP-1 promotes assimilation of ingested nutrients through glucose-stimulated insulin secretion and evidently improves β-cell sensitivity to glucose [ 48 ]. Moreover, GLP-1 also suppresses glucose-dependent glucagon secretion, retards gastric emptying, and promotes satiety [ 49 ]. In the pancreas, β-cell proliferation and inhibition of apoptosis are promoted by GIP and GLP-1 that ultimately expand pancreatic β-cell mass. In addition, fat deposition is also facilitated by GIP. In the brain, GIP and GLP-1 are involved in appetite control. GIP also decreases gastric acid secretion, while GLP-1 decreases the duration of gastric emptying. Moreover, the insulinotropic effects of GIP and GLP-1 differ in T2DM patients such that GLP-1 secretion is impaired, while the secretion of GIP remains unaffected [ 50 ]. Alterations in incretin functioning and the associated pathways result in increased gastrointestinal permeability in T2DM and form one of the basic underlying mechanisms responsible for diabetic comorbidities in the latter phase [ 48 , 49 , 51 ].
The gut also releases other hormones which are involved in multiple signaling cascades. These include (but not limited to) ghrelin, galanin, cholecystokinin (CCK or pancreozymin) and leptin [ 52 ]. The enteroendocrine cells (I cells of the duodenum and jejunum) and neurons synthesize and release CCK in response to meals and induce pancreatic acinar cells to secrete pancreatic digestive enzymes. CCK also reduces gastric emptying and enhances the digestion process [ 53 ]. Vagus stimulation causes trypsin release from pancreas that hydrolyzes CCK to maintain homeostasis through the feedback mechanism. CCK is positively associated with leptin and insulin levels resulting in disrupted glucose homeostasis and diabetic complications in T2DM [ 53 , 54 ].
Gut Microbiota
Diabetes is considered as a disease of the intestine where gut microbiota plays a crucial role [ 55 , 56 ]. The concentration of microflora distally increases along the length of the gastrointestinal tract [ 57 ]. The flora of the upper intestine generally accounts for <10 5 cfu/mL of the total microflora content. The concentration of microflora increases in the mid-ileum to 10 7 cfu/mL and ultimately populates the colon heavily [ 57 , 58 ]. Commonly populating bacteria in humans are (a) Firmicutes (60–80%): Ruminiococcus , Clostridium , and Lactobacillus ; (b) Bacteroidetes (20–30%): Bacteroides , Prevotella , and Xylanibacter ; (c) Actinobacteria (<10%): Bifidobacterium ; (d) Proteobacteria (<1%): Escherichia and Enterobacteriaceae ; and (e) yeast Saccharomyces boulardi [ 59 ]. Obesity/adiposity is undoubtedly a pivotal contributing factor in T2DM. Interestingly, the level of Staphylococcus , Enterobacteriaceae , Faecalibacterium prausnitzii , and E. coli increases during obese conditions, while Bacteroides concentration decreases [ 60 ]. Moreover, in T2DM, Firmicutes , Lactobacillus gasseri , Streptococcus mutans , and E. coli are increased, while proteobacteria, butyrate-producing bacteria, Bacteroidetes , Roseburia , Eubacterium halii , and Faecalibacterium prauznitzii are decreased considerably [ 59 ]. Changes in gut microbiota/gut-brain microbiota result in insulin resistance and disease/metabolic syndrome [ 59 , 61 ]. Also, low-grade inflammation is remarkably influenced by obesity in association with alteration of gut-brain-microbiota interactions that render T2DM as an inflammatory disorder [ 62 ]. An increased intestinal permeability due to inflammation is evident in obesity and diabetes that may reach to leak gut conditions to facilitate the entry of gut microbes into circulation. This increases circulating LPS and thereby activates inflammasome formation [ 63 ]. Moreover, vagal control is significantly compromised in diabetes in association with chronic hyperglycemia, damaged interstitial cells of Cajal and gastroparesis (5–12% diabetic patients) [ 64 ]. Increase in mucosal surface area, intestinal weight, and number of goblet cells per villus leads to disrupted esophagus peristalsis and lower sphincter tone [ 65 ]. The overall disturbances in intestinal motor functions lead to stasis and bacterial outgrowth; thus, possibly disturbing the intestinal barrier and affecting permeability to allow the entry of microbes [ 63 , 64 , 65 ]. Moreover, circulating LPS are involved in the insulin resistance and diabetes progression toward comorbidities [ 63 , 65 , 66 ]. Gut microbes influence the metabolic and immune networks of the host to cause obesity and diabetes through enhanced nutrient absorption from the diet, cellular uptake of circulating triglycerides, prolonged intestinal transit time, altered bile acid enterohepatic cycle, enhanced de novo lipogenesis, reduced FFA oxidation, altered tissue composition of biologically active polyunsaturated fatty acid, chronic low-grade inflammation triggered by the endotoxin TLR-4 axis, and altered intestinal barrier function [ 67 ].
Lifestyle Modifications, Environmental Factors, and Management of T2DM
The pharmacological approach to treat T2DM can be only partly effective in the long-term management of diabetes. Major modifications in the lifestyle of patients along with the interventions through pharmacological approaches are crucial to ensure an effective management of the disease. These include changes in physical activity, dietary modifications, management of stress or associated factors, and improved sleeping patterns. The next few sections of this review will discuss and explore the potential of these factors in the management of diabetes when followed in parallel with the pharmacological management of the disease.
Physical Activity
Physical activity is positively associated with controlled glycemic levels among T2DM patients. Moderate but daily physical activity has been found to be an effective way to control the long-term manifestations of diabetes. These include walking, gardening, and performing common household chores. Walking is the most effective physical activity in T2DM, as it allows significant glycemic control with limited physical burden in patients who are already physically weak [ 68 ]. Moreover, a much warranted lifestyle alteration in T2DM patients are changes in sedentary patterns. Sedentary behavior leads to considerably low expenditure of energy. An extended sedentary period in T2DM patients is also associated with uncontrolled glycemic levels. A reduced sedentary time, therefore, is crucial in diabetes patients, which can be achieved by increasing the physical work [ 69 ]. In addition, regular aerobic exercise is acknowledged to improve HbA1c levels in patients with diabetes [ 70 ]. Aerobic exercise tends to improve health outcomes in patients through multiple mechanisms that include the manifold increase in mitochondrial densities, improved sensitivity to insulin, improved compliance of blood vessels, and lung functions with enhanced cardiac output [ 71 ].
Dietary Changes and Medical Nutrition Therapy
Insulin resistance and subsequent appearance of T2DM are closely linked with high intake of sugars, fried food, and red meat [ 72 ]. On the contrary, reduced risk of T2DM development is observed in case of intake of vegetables having high content of antioxidants, fiber, and other nutrients [ 73 , 74 ]. The average energy intake of diabetes patients differs with their obesity status. Usually, for a nonobese diabetic patient, an average energy intake of 1,500–2,500 calories per day is recommended, while for obese patients, the average calorie intake is reduced to 800–1,500 calories per day. Limited intake of refined sugars is highly recommended in T2DM patients. Non-nutritive sweeteners (aspartame, saccharine, etc.) can be the good substitutes for sugar in such patients. Moreover, the restricted intake of food rich in saturated fats and cholesterol and its replacement with food rich in polysaturated fats is also recommended. In addition, changes in eating patterns, such as dividing meals into small fractions over the day rather than taking 1 or 2 large meals can prevent vigorous postprandial peaks in blood glucose levels [ 75 ]. Strict adherence to controlled diet with sufficient physical activity is largely associated with lower incidence of diabetes [ 76 ]. Incorporation of Paleolithic diet (a diet rich in lean meat, fish, fruits, and vegetables) in the daily routine of diabetic patients results in marked improvement in glucose handling [ 77 ]. The employment of nutritional therapy in the management of diabetes is also widely suggested. Nutritional therapy is an approach to treat a disease through the modifications in food and nutrition intake. The application of evidence-based nutrition care therapy in diseased patients by a qualified and registered dietician is termed as medical nutrition therapy [ 78 ]. Reduced reliance on oral hypoglycemic therapy is evident in diabetes patients receiving nutritional therapy [ 79 ]. Also, considerable improvements in clinical outcomes are observed in diabetes patients receiving intensive nutritional education by registered dietician in comparison to patients receiving basic nutrition information (BE) [ 80 ]. Taken together, simple but profound changes in dietary pattern in diabetic patients is a potential approach to curb the long-term implications of diabetes. Moreover, successful application of nutritional therapy in individuals with diabetic conditions can be a lucrative approach to achieve a better management of diabetes with improved health outcomes.
Increased levels of stress are associated with poor treatment adherence and glycemic control in T2DM patients [ 81 ]. In a longitudinal study, moderate/high levels of stress were found to be accountable for multifold increase in the incidences of diabetes [ 82 ]. Moreover, consistent exposure to stressors, compromised mental health, and psychological stress are highly implicated in increasing risk of T2DM development [ 83 ]. Allostatic load (wear and tear in the body occurring as a result of chronic exposure to psychological stress) is assumed to be the major factor responsible for this increased risk of T2DM in such individuals [ 84 ]. In addition, consistent stress is also implicated in worsening of clinical outcomes in T2DM patients. Chronic stress is associated with dysregulated glucose metabolism and neuroendocrine function accompanied with low-grade inflammation. A majority of factors that are implicated in T2DM are largely influenced by psychological stress including the release of glucose (and lipids) in circulation, expression of inflammatory cytokines, and elevated blood pressure [ 85 ]. In one study, in type 2 diabetes patients when exposed to acute stress during the postprandial period, considerable increases in blood glucose levels were observed [ 86 ]. Apparently, treatment strategies, including stress management interventions, are a promising approach in effectively preventing or controlling the incidence of type 2 diabetes.
Sleep Patterns and Chronopharmacology
Although physical activity and maintained dietary pattern result in considerable improvements in the management of T2DM, they cannot be envisioned as the sole contributors to the worsening of diabetes incidences. Sleep is another modifiable lifestyle behavior that has proven roles in influencing metabolic health and energy status. Optimization of sleeping patterns is crucial in diabetes control [ 87 ]. A population-based study suggests that short sleep (<5 h) or insomnia is associated with increased risk of T2DM [ 88 ]. In similar studies, poor sleep was associated with higher HbA1c levels (>7%) and insulin resistance in T2DM patients [ 88 ]. Disturbed circadian rhythms and sleep-wake patterns also result in significant effect on onset, development, and management of diabetes [ 89 ]. Shift workers tend to remain much prone to metabolic disorders due to consistent sleep loss and disrupted circadian rhythm [ 90 ]. In addition, developed propensity of napping as a consequence of poor or insufficient nocturnal sleep is also associated with high risk of T2DM [ 91 ]. In one study, experimental manipulation of sleep and circadian pattern resulted in significant reduction in insulin response to standardized meal which could be recovered with restored sleeping patterns [ 92 ]. Changes in hormones that regulate appetite (leptin and ghrelin) are observed to be associated with short sleep causing an increased urge for carbohydrate-rich food and increased calorie intake [ 89 , 93 ]. Moreover, lack of sleep also results in oxidative stress and release of orexin or hypocretin, a neuropeptide that regulates sleep and appetite and causes the stimulation of sympathetic nervous system and increased release of cortisol with simultaneous decrease in growth hormone secretion, all leading to considerable hyperglycemia [ 89 , 94 ].
Pharmacokinetics and pharmacodynamics (PK-PD) are markedly influenced by daily rhythms in physiology. This phenomenon is termed chronopharmacology [ 95 ]. Indeed, the pathogenesis of diabetes largely depends on hormonal and body homeostasis. Chronopharmacology should be considered as part of treatment strategies for diabetes. The failing β-cells in T2DM do not lose all their capability to respond to glucose. Insulin secretion in response to stimulation through amino acids or other hormones such as glucagon-like peptide 1 (GLP-1), remains preserved [ 96 ]. The levels of leptin (satiety hormone) in blood generally remain higher between midnight and early morning, conceivably to suppress appetite during the night [ 97 ]. Moreover, the levels of ghrelin increase with increase in the duration of sleep [ 93 ]. In addition, the time dependency in GLUT4-mediated glucose uptake is also a function of circadian variation [ 98 ]. Furthermore, meal timings can modify the diurnal rhythm of blood leptin levels [ 99 ]. Both ghrelin and leptin work with other hormones and HPA axis through feedback loops to indirectly affect the psychophysiological satisfaction in diabetic patients [ 100 ]. Chronopharmacology, therefore, may considerably affect diabetic pathophysiology and PK-PD of administered drugs.
Interplay of Genetics, Gut Microbiota, Lifestyle, and Environmental Factors
Multiple epidemiological investigations have suggested that the effects of multiple T2DM-associated loci can be attenuated by improving lifestyle, dietary patterns, and other associated environmental factors. For instance, the Ala12 variant of PPARγ is associated with improved insulin sensitivity. Apparently, the Ala12 carriers are more responsive to unsaturated fat and less responsive to saturated fat. On contrary, the Pro12 variant carriers of PPARγ are more responsive to the deleterious effects of saturated fat and altered glucose homeostasis. Seemingly, unsaturated fat interacts with PPARγ Ala12 variant and upregulates the activity of latter [ 101 ]. Potential gene-environment (G × E) interactions also occur between TCF7L2 risk-variant (rs7903146) and lifestyle modifications (physical activity, MNT, and dietary changes). Decreased insulin resistance and reduced risk in TCF7L2 risk-variant carriers is significantly affected by lifestyle modifications [ 102 , 103 ]. A common SNP in fat mass and obesity associated gene (FTO rs9939609) is associated with increased risk of T2DM. Increased physical activity reduces the FTO rs9939609-induced obesity and associated risk of T2DM [ 104 ]. SNP in glucokinase regulatory protein gene results in an insulin-raising allele, GCKRrs780094. Its interaction with the whole grain (increased whole grain intake) results in reduced fasting insulin in the carriers [ 105 ]. The potassium voltage-gated channel subfamily Q member 1 (KCNQ1) is a susceptible gene in T2DM. Mutations in KCNQ1 are associated with decreased insulin secretion. Reduced expression of noncoding RNA Kcnq1ot1 in Kcnq1 genetic region leads to increase in cyclin-dependent kinase inhibitor 1C (Cdkn1c) expression, resulting in reduced pancreatic β-cell mass and insulin release. The CCAAT sequence in the promoter region of Cdkn1c gene serves as the binding site for transcription factor C/EBP that increases the further expression of Cdkn1c. Evidently, the expression of C/EBPβ results in endoplasmic reticulum stress to cause dysfunctions in β-cells. The accumulation of C/EBPβ in pancreatic β-cells increases in the presence of high fat diet, thereby potentiating the β-cells dysfunction in the vulnerable population [ 106 ]. Collectively, the emerging investigations to explore the interactions between gene and environmental factors suggest a high influence of dietary patterns, physical exercise, and other lifestyle interventions on the expression of genes that are peculiar to the development of T2DM.
Apart from gene expression, environmental factors also tend to exert a potential impact on gut microbiota. The gut environment is affected by a number of factors including the diet, pH, and nutrient absorption. While the presence of Firmicutes and Proteobacteria increases under the influence of carbohydrates and simple sugar-rich diet, saturated fats, and animal protein-rich diet encourages the proliferation of Bacteroidetes and Actinobacteria [ 107 ]. Moreover, a high −at diet is also accountable for significant alterations in intestinal flora, including the Bifidobacterium and Bacteroides (increased Gram-negative/Gram-positive bacteria ratio). This allowed and increased secretion of LPS, fat content, body weight, and inflammatory reactions associated with T2DM [ 108 ]. Reduction in butyrate is largely responsible for the loss of tight intestinal barrier. An intestinal pH of 5.5 favors the proliferation of butyrate-producing Phytophthora which starts to diminish with a pH value of 6.5 [ 109 ]. In addition, the hypoglycemic agents utilized for the antidiabetic therapy also pose a remarkable influence on the gut microbiota. Metformin and acarbose are known to increase the proliferation of lactobacilli, Akkermansia, and several other bacteria that are acknowledged to exert beneficial effects in diabetes [ 110 ].
Gut microbiota composition also affects the regulation of expression of different genes in T2DM. Although reports are limited in terms of potential interactions between gut microbes and T2DM associated gene variants, existing reports on the influence of gut microbes in the expression genes that are crucial in T2DM are highly suggestive of a complex gene-microbes interplay in the etiology of T2DM. Also, microbiome plays a crucial role in the epigenetic regulation of genes by the modification of DNA methylation [ 111 ]. F. prausnitzii , a short-chain fatty acid-producing bacteria was found crucial in epigenetic regulation of FFA receptor gene in patients of T2DM. A significant reduced presence of F. prausnitzii was evident in such patients. As a result, a considerably low methylation in the promoter region of FFA receptor gene is observed in these individuals [ 112 ]. Increased release of pro-inflammatory cytokines is a key event in T2DM. Microbes are largely known to be associated with increased release of inflammatory cytokines by producing the products such as LPS that promote low-grade inflammation and endotoxemia. On contrary, several microbes are known to induce the expression of anti-inflammatory cytokines, including the IL-10 and IL-22, that have proven roles in improving the insulin sensitivity Roseburia intestinalis , Bacteroides fragilis , Akkermansia muciniphila , Lactobacillus plantarum , and Lactobacillus casei [ 113 ]. Two other beneficial microbes − Bacteroides vulgatus and Bacteroides dorei − are observed to increase the expression of tight junction genes in T2DM to compensate with the compromised gut permeability (leaky gut) [ 114 ]. A major contribution of probiotics is observed in the case of glucose metabolism and homeostasis. For instance, L. gasseri BNR17 is known to increase the expression of GLUT-4 transporter gene [ 115 ]. Another gut microbe, L. casei is witnessed to increase the expression of multiple T2DM-related genes, including ClC1-7, GlyRα1, SLC26A3, SLC26A6, GABAAα1, Bestrophin-3, and CFTR, thus resulting in a significant reduction in hyperglycemia [ 116 ]. It appears to be of vital importance to consider the potential interplay between various T2DM-related genes and these microbes. Undoubtedly, the absence of these microbes among the gut microbiota can be largely responsible for the altered regulation of different genes in T2DM patients. Also, exploring the interactions between different T2DM-associated gene variants and gut microbiota is warranted to further understand the complex interactions between environmental factors, gut microbiota, and genetics in the development of T2DM.
Current Approaches for Diabetes Management: What Are We Missing?
The guidelines for the pharmacological management of diabetes provided by American Diabetes Association suggest that metformin be prescribed as the initial intervention to T2DM patients. However, the same guideline also indicates that vitamin B 12 deficiency is a prominent side effect observed in metformin consumers and a periodic vitamin B 12 measurement is required in such patients [ 117 , 118 ]. Furthermore, metformin is also notorious for causing lactic acidosis, especially in patients with kidney disease, liver injury, or other CVS complications that create a low level of oxygen in circulation [ 119 ]. For T2DM patients with cardiovascular or CKDs, the guidelines recommend adding sodium-glucose cotransporter 2 (SGTL2) inhibitors and/or glucagon-like peptide 1 receptor agonists along with hypoglycemic agents [ 118 ]. The employability of SGTL2 inhibitors with almost all classes of hypoglycemic agents makes them ideal candidates to be combined when dual and triple combination therapies are warranted [ 120 ]. In an ideal scenario, a drug used in combination should be able to reverse the pathology with an improved overall health status of the patient and ensure that no new complications arise due to the existing management strategies. In case of T2DM, drug combination should not only be able to just merely reduce the glycosylated hemoglobin levels (HbA1C) but also an improved overall metabolic condition of the patient is expected through such interventions [ 120 ]. The combination of SGTL2 inhibitors with metformin may have proved beneficial in curbing hyperglycemia that cannot be controlled by metformin alone [ 120 ], but the adverse effects associated with the SGTL2 inhibitors still remain unresolved. Genital infections caused by SGTL2 inhibitors due to high glycosuria still remain an unfocussed aspect while prescribing such combinations. In addition, during the event of excessive osmotic diuresis caused by SGTL2 inhibitors, a low extracellular fluid volume and subsequent hypotension is another complication that may arise [ 121 ]. Multiple reports have also raised concerns regarding the use of SGTL2 inhibitors in diabetes due to their substantial involvement in causing diabetic ketoacidosis [ 122 ]. Two separate reports published in 2015 claimed that canagliflozin, an SGTL2 inhibitor is implicated in pancreatitis in T2DM patients [ 123 , 124 ]. GLP-1 agonists are also a preferred class of adjuvant hypoglycemic agents that are combined with first-line hypoglycemics [ 125 ]. Apart from gastrointestinal disorders (nausea, vomiting, and constipation), infections and acute renal injury, a major raising concern regarding the use of GLP-1 agonists is their association with pancreatitis [ 125 , 126 ]. Cases of acute pancreatitis are reported with the use of liraglutide and exenatide [ 127 , 128 ]. More importantly, recent reports also raise concerns regarding the long-term reliance on incretin-based therapies due to frequently reported cases of their association with pancreatitis and pancreatic cancer [ 129 ]. Studies based on FDA Adverse Events Reporting System demonstrated that incretin-based therapies are associated with the increased incidences of pancreatic and thyroid cancer [ 130 , 131 ]. Exenatide use is also positively associated with the incidences of bone fractures [ 132 ].
Alternatives: Phytoconstituents
Failure of monotherapy in diabetes is simply managed by the dual or triple drug combination therapies that involve the addition of supportive hypoglycemic agents with the first-line drugs. However, adding the supportive or second-line drugs in combination seldom includes the assessment of risk factors associated with these new additions. The sole aim of these therapies remains to be a controlled glycemic condition. Unfortunately, in the pursuit of maintaining normal blood glucose levels, the occurrence of new complications is largely taken for granted. Monotherapies supplemented with herbal extracts or phytoconstituents have showed appreciable improvements in the blood glucose levels in diabetic patients. Chemical constituents from plants have also proved to be promising alternatives. Table Table1 1 represents the known effects of different phytoconstituents in diabetes exerted through multiple targets. As a result, unlike in the case of conventional single target therapy where chances of treatment failures are high, therapy failures with multi-targeting approach are rare.
Multiple targets of different phytoconstituents in the management of T2DM and their possible outcomes [133–140]
T2DM, type 2 diabetes mellitus; G6Pase, glucose-6-phosphatase; PEPCK, phosphoenolpyruvate carboxykinase.
Conclusions
Diabetes is a metabolic disorder that is influenced by a variety of factors. Recent insights into the pathogenesis of diabetes have unraveled newer pathways and factors that contribute substantially in disease development and progression. Insulin resistance and β-cell dysfunction are the 2 major events that are largely responsible for the onset of diabetes. A major objective of this review is to focus on the unfocused aspects of diabetes to develop better strategies for diabetes treatment. In this review, we have discussed the factors that have played crucial roles in the etiology of T2DM but have not received adequate attention. We have also discussed the efficiency of existing approaches in the treatment of T2DM. Lifestyle modifications that favor the improvement of management of diabetes and their complex interplays with genetics and gut environment is a crucial factor that warrants further research in the development of more efficient and individualized therapy approaches for disease treatment. The use of multidrug combination therapy in diabetes may have improved health outcomes in T2DM patients and also result in additional complications that need serious consideration. Moreover, more attention is required toward the developing comorbidities during diabetes. The diabetic milieu accelerates the formation of advanced glycation end products that may encourage the development of diabetic complications and even cancer in diabetic patients. Multiple pathways are involved in diabetes that can contribute to the manifestation of comorbidities that are largely neglected during disease treatment.
Multitargeting is a promising approach for the treatment of T2DM as it includes multiple pathways. The failure of single target approaches is the major challenge faced in T2DM treatment. Phytoconstituents are promising as they interact with multiple pathways simultaneously. However, the reluctance to rely on phytoconstituents as the main therapy still remains as a limiting factor for such drugs to serve as mainstream interventions.
Conflict of Interest Statement
All authors have read the journal's policy on disclosure of potential conflicts of interest and have none to declare.
Acknowledgement
The authors are thankful to B. V. Patel Pharmaceutical Education and Research Development (PERD) Center, Ahmedabad, and AYUSH − Center of Excellence, Center for Complimentary and Integrative Health (CCIH), Interdisciplinary School of Health Sciences, Savitribai Phule Pune University, Pune, for providing facilities for the successful completion of the work. The authors are also thankful to those colleagues whose work could not be cited directly owing to space constraints.
Swapnil P. Borse and Abu Sufiyan Chhipa contributed equally; Vipin Sharma and Devendra Pratap Singh contributed equally.
The Paradox of How We Treat Diabetes

Understanding diabetes today requires holding two conflicting realities in your head simultaneously.
First, diabetes therapy has been revolutionized by a world of new drugs that have become available since the turn of the century—most notably, drugs of the same class as Wegovy and Ozempic that began their existence as diabetes medications and are now hailed as wonder drugs for treating obesity. These drugs do the best job yet of controlling blood sugar and, of course, body weight, which is critical for those Type 2 diabetes, the common form of the disease that constitutes over 90 percent of cases and is associated with age and obesity. For type 1 diabetes, the acute condition that typically strikes in childhood and adolescence, new devices—continuous blood sugar monitors and automated insulin delivery systems—make blood sugar control easier than ever. Still more advanced devices and better drugs are in the pipeline.
But then there’s the flip-side. It’s why the pharmaceutical industry has invested so heavily in new therapies: Once a relatively rare condition, diabetes is now so common that drugstores dedicate entire aisles to it and television commercials for diabetic medications are common fare. In 1960, when the first concerted federal surveys were quantifying prevalence, two million Americans were living with a diabetes diagnosis. Today that number is 30 million; almost nine million more have diabetes but don’t yet know it. Each year, 1.4 million new cases are diagnosed and at ever younger ages.
Diabetes puts all of these individuals at increased risk of heart disease, strokes, cancer, blindness, kidney failure, nerve damage, gangrene, and lower limb amputation. It increases cognitive impairment and dementia risk as patients age. Living with diabetes still comes with a decrease in life expectancy of six years .
For those with Type 1 diabetes, despite the remarkable new drugs and devices, blood sugar control is seemingly getting worse, on average, not better. As of 2018, fewer than one in five individuals diagnosed with Type 1 diabetes were achieving even the relatively generous blood-sugar goals set by the American Diabetes Association (ADA); this was a smaller proportion than a decade earlier.
More From TIME
Despite the remarkable advances in therapy, both Type 1 and Type 2 diabetes are still considered progressive chronic diseases, meaning the patient’s condition is expected inevitably to deteriorate as they live with the disease. The greatest challenge to better therapy, as one recent analysis suggested , is the hesitation of physicians to continue prescribing more or newer drugs and increasing dosages as the diseases progress.
All of this comes with a staggering financial burden. In November, the ADA estimated that the total annual cost of diabetes in the U.S. is over $400 billion; over $300 billion is direct medical costs. This was up $80 billion from 2017 when an editorial commenting on a similar accounting characterized these costs as the “elephant in the room” of the diabetes epidemic. Patients with diabetes are likely to spend over $12,000 a year just for medical care, almost three times that of healthy individuals of equivalent age. It does not help that the drugs themselves—whether insulin or Ozempic and its ilk —are expensive, costing many thousands of dollars a year. One in every four health care dollars spent in America goes to treating diabetic patients.
And the U.S. is by no means unique. The World Health Organization estimates that diabetes prevalence worldwide increased four-fold between 1980 and 2014, from 108 million to over 400 million, with the greatest rise coming, paradoxically, in the poorest countries. In 2016, Margaret Chan, then WHO director general, described the situation as a “ slow-motion disaster” and predicted with near absolute certainty that these numbers would only get worse. They have.
So how do we reconcile these conflicting realities: Unprecedented advances in medical therapies for an out-of-control disease epidemic in which patients, at least in general, are doing poorly and can expect to do worse as time goes on? Confronted with such a dismal state of affairs shouldn’t we be asking how we got to this point? Were mistakes made in how we think about this disease? Were questionable assumptions treated as facts, and could those assumptions be wrong?
Asking the Right Questions
These are the kinds of questions you would hope health organizations worldwide would be asking, but surprisingly they have no mechanisms or protocols to do so. Diabetes associations like the ADA will regularly convene expert panels to address revisions in the latest standard of care guidelines to accommodate the latest research, but not whether the guiding principles underlying those guidelines should be rethought entirely. Independent investigators are not recruited to analyze and to provide an unbiased assessment of where progress might have gone off the rails. That job instead has been left to physicians in their clinics, those confronted with ever more diabetic patients and willing to take the risk of thinking independently, and to investigative journalists like myself, whose obligation when confronted with such conflicting realities is to ask just these kinds of questions.
Among the revolutions that changed medical practice over the past half century, one in particular is very relevant here. Beginning in the 1970s, health-care analysts began to confront quite how little physicians really knew about the risks and benefits of what they were doing for their patients. Not only had clinical trials demonstrated that some standard medical practices resulted in far more harm than good—the surgical procedure known as a radical mastectomy, most infamously, for breast cancer—but researchers were documenting wide variations in medical practices from physician to physician, hospital to hospital and state to state. This, in turn, resulted in a wide variation of benefits, harms and costs to the patients, depending on which physicians they might visit, and so which treatments they might get.
Read More: Should We End Obesity?
The revolution that followed became known as the Evidence-Based Medicine (EBM) movement, founded on the principle that medical interventions should be rigorously tested in clinical trials— double-blind, randomized, placebo-controlled—before they be used or prescribed. This would be necessary whenever physicians were faced with a choice between multiple options, and whenever the harms of an intervention might outweigh the benefits. David Sackett of McMaster University, a founder of the movement, would describe the EBM process as beginning with the fact that half of what aspiring doctors learn in medical school is “dead wrong,” and then trying to establish thoughtfully and critically which half that is. David Eddy of Duke University, another EBM pioneer, later described his motivation and that of his colleagues as the revelation that “medical decision making was not built on a bedrock of evidence or formal analysis, but was standing on Jell-O.”
It would be nice to think that this situation has been widely resolved by evidence-based guidelines, but that’s not the case. Journalists or physicians looking for the evidence base in decision making about diabetes therapies, will likely find themselves, as I did, with the same revelation. Clearly it, too, was standing on Jello-O in the 1970s, but the problem neither began nor ended there. A remarkable history emerges, with three clear observations.
First, we’ve been here before. We have had miracle drugs for diabetes. Most notably, the hormone insulin itself, when University of Toronto researchers led by Frederick Banting and Charles Best purified it and put it to use in 1922 treating patients with severe cases of diabetes. We then had better insulins, slower-acting and longer-lasting, and then, in the post-World War 2 years, drugs (oral hypoglycemic agents) that could lower blood sugar without having to be injected, as insulin did. We have had revolutionary advances in diabetes technology, beginning in the 1970s with devices that allowed patients to monitor their own blood sugar, and then insulin pumps that automated the process of insulin therapy. All contributed to easing the day-to-day burden of diabetes. None had any influence in controlling the epidemic, nor did they eradicate or meaningfully reduce the long-term complications of the disease. Put simply: diabetes plus drug therapy and devices, even the best drug therapy and devices, does not equate to health.
Secondly, diabetes researchers have not been averse to testing their fundamental assumptions. They‘ve done so in ever more ambitious clinical trials. But a disconcerting proportion of those trials failed to confirm the assumptions, despite the fact that it was these assumptions that constituted the rationale for therapeutic approaches. The $200 million Look AHEAD Trial, for example, tested a foundational belief in the field: that weight loss in those with Type 2 diabetes would lengthen lives. The trial was ended for “futility” in 2012 . ”We have to have an adult conversation about this,” as David Nathan, a Harvard diabetes specialist, said to The New York Times . The 10,000-patient ACCORD trial had also been ended prematurely just four years earlier. “Halted After Deaths,” in the words of The New York Times headline. “Medical experts were stunned,” the 2008 article said. ACCORD was one of three trials testing the assumption that intensive blood sugar control by medications would reduce the macrovascular complications of Type 2 diabetes—particularly heart disease—and premature death. All three trials failed to confirm it.
Third, the remarkable aspect of all these trials is that they all assumed an approach to dietary therapy that itself had never been tested. This is the “standing on Jell-O” problem. For well over a century, diabetes textbooks and chapters in medical texts invariably included some variation on the statement that diet is the cornerstone of treatment. The most recent guidelines from the ADA refer to dieting as “medical nutrition therapy” (MNT) and say MNT is “integral” to therapy.
But what constitutes MNT—the dietary advice given—has been determined not by any meaningful research comparing different dietary approaches. Rather it has been assumed that individuals with diabetes should eat the same “healthful eating pattern” that health organizations recommend for all of us—“non-starchy vegetables, fruits, legumes, dairy, lean sources of protein… nuts, seeds, and whole grains”—albeit with the expectation, if weight control is necessary, that they should eat fewer calories.
Read More: Are Weight Loss Drugs From Compounding Pharmacies Safe?
Controlling the symptoms and complications of the disease is left to insulin and the pharmacopeia of drugs that work to maintain blood sugar levels near enough normal that the specter of diabetic complications may be reduced as well. Diabetes associations have assumed that this approach is easiest on the patients, allowing them to balance the burden of insulin injections or multi-drug therapy, against the joy of eating as their non-diabetic friends and family do. But this assumption has never been tested to see if it is true, nor whether a better approach exists that might truly minimize the disease burden of diabetes, extend lives and make the trade-off of restrictive eating vs. health worthwhile.
History of Diet and Diabetes
This is where understanding the history of the diet-diabetes relationship can be vitally important. What has been known for certain about diabetes since the 19 th century is that it is characterized by the inability to safely metabolize the carbohydrates in our diet. This observation led to two divergent approaches/philosophies to dietary therapy. Beginning in 1797, when a British physician named John Rollo wrote about curing a diabetic patient using a diet of fatty (rancid) meat and green vegetables, through the early 1900s, diabetes therapy was based on the assumption that since individuals with diabetes could not safely metabolize the sugary and starchy foods in their diet, they should abstain from eating them. In this pre-insulin era, the only meaningful advice physicians could give their patients was dietary, variations on Rollo’s approach: sugars, grains, starches, even legumes were prohibited because they are carbohydrate-rich: meats, ideally as fatty as possible, butter and eggs, along with green leafy vegetables (boiled three times to remove the digestible carbohydrates) could be eaten to satiety.
Throughout Europe and America, this was known was “the animal diet,” endorsed by virtually every major diabetes specialist of the 19th Century. Physicians believed that the more calories their diabetic patients consumed, and ideally the more fat (because protein is composed of amino acids, some of which the liver converts to carbohydrates), the healthier they would be. “Patients were always urged to take more fat,” is how this was described in 1930 by the Harvard physician Elliot Joslin, who was then, far and away, the most influential diabetes authority worldwide. “At one time my patients put fat in their soup, their coffee and matched their eggs with portions of fat of equal size. The carbohydrate was kept extraordinarily low….”
This thinking only changed in the years before World War I, when Joslin embraced and disseminated the idea promoted by a Harvard colleague, Frederick Allen, that diabetic patients, still without insulin, were best served if they were semi-starved—avoiding carbohydrates and fat. In short, patients suffering from a disease in which one characteristic symptom is ravenous hunger would be treated by making them go even hungrier than otherwise. The approach was unsurprisingly controversial. Joslin and others, though, came to believe they could keep their young Type 1 patients alive longer with Allen’s starvation therapy, even while the high fat, animal-based diet seemed more than adequate for their older Type 2 patients. Allen’s starvation therapy was in turn challenged between 1920 and 1923, when University of Michigan physicians Louis Newburgh and Robert Marsh reported in a series of articles that it was simply unnecessary, that even young patients with severe diabetes could thrive on the high-fat, carbohydrate-abstention approach if properly administered. By then, though, it was too late.
Insulin therapy had arrived in the winter of 1922. It launched what medical historians would call a “therapeutic revolution,” as close as medicine had ever come, and maybe ever has, to a miracle. Patients, often children, on the brink of death, horribly emaciated by the disease and the starvation therapy, would recover their health in weeks, if not days on insulin therapy. They were resurrected, to use the biblical terminology, which physicians of the era often did.
Diabetes specialists realized that insulin therapy was not a cure of the disease, but it allowed their patients to metabolize carbohydrates and held the promise of allowing them to eat whatever and however they wanted. “Were I a diabetic patient,” wrote Frederick Banting in 1930, by then a Nobel Laureate. “I would go to the doctor and tell him what I was going to eat and relieve myself of the worry by demanding of him a proper dose of insulin.”
That thinking, for better or worse, has governed diabetes therapy ever since.
While diabetes specialists still had no conception of the long-term complications of living with diabetes—the damage to large and small blood vessels that results in heart disease, strokes, kidney disease, neuropathy, amputations, blindness, dementia—they would advocate for ever more liberal carbohydrate diets and ever higher insulin doses to cover them. Patients would be taught to count the carbohydrate content of each meal, but only so they could properly dose their insulin. Diets would be prescribed, and still are, to allow for the drugs to be used freely, not to minimize their use. Patients, in turn, were allowed to eat anything, which physicians assumed they would do anyway.

Whether the patients lived longer, healthier lives because of it, would never be tested. As diabetes specialists began to understand the burden of the disease they were treating, the wave of microvascular and macrovascular complications that set in after 10 or 20 years, they would rarely, if ever, ask the question, whether these complications were mitigated by their dietary approach or perhaps exacerbated by it. They would only test drug therapy.
In 1971, the American Diabetes Association institutionalized this philosophy with dietary guidelines that would commit the organization to this approach ever after: diabetic patients would be told to restrict dietary fat—by then thought to cause heart disease—rather than carbohydrates, the one macronutrient they could not metabolize safely without pharmaceutical help. “Medical Group, in a Major Change, Urges a Normal Carbohydrate Diet for Diabetics,” was the headline in The New York Times . By taking the ADA’s advice, diabetic patients would trade off blood sugar control for cholesterol, assuming this would prevent heart disease and lengthen their lives. While the guidelines explicitly acknowledged that the ADA authorities had no idea if this was the right thing to do, the advice would be given anyway.
Read More: Why You're Not Losing Weight
By 1986, the ADA was recommending diabetic patients get “ideally up to 55-60% of total calories” from carbohydrates, while researchers led by the Stanford endocrinologist Gerald Reaven had established that such a diet was almost assuredly doing more harm than good. That same year, the NIH held a “consensus conference” on diet and exercise in Type 2 diabetes. The assembled authorities concluded that, at best, the nature of a healthy diet for diabetes remained unknown. The conference chairman, Robert Silverman of the NIH, summed the state of affairs up this way: “High protein levels can be bad for the kidneys. High fat is bad for your heart. Now Reaven is saying not to eat high carbohydrates. We have to eat something.” And then he added, “Sometimes we wish it would go away, because nobody knows how to deal with it.”
The modern era of the diabetes-diet relationship began 25 years ago, with the awareness that the nation was in the midst of an obesity epidemic. Physicians, confronted with ever more obese and diabetic patients and the apparent failure of conventional advice—eat less, exercise more—suggested instead the only obvious options, the approaches suggested by popular diet books. Many of these— Dr. Atkins’ Diet Revolution, Protein Power, Sugar Busters —were touting modern incarnations of Rollo’s animal diet.
The Diet Trials
The result was a series of small, independent clinical trials, comparing, for the first time, the conflicting dietary philosophies of a century before. Is it better for patients with Type 2 diabetes, specifically, to avoid dietary fat and, if they’re gaining weight, restrict total calories (both carbohydrates and fat), or will they do better by avoiding carbohydrate-rich foods alone and perhaps entirely? The earliest trials focused on treating obesity, but many of the participants also struggled with Type 2 diabetes. In 2003, physicians at the Philadelphia VA Medical Center published the results from the first of such trials in the New England Journal of Medicine : patients with both obesity and diabetes counseled to eat as much food as they desired but to avoid carbohydrates, became both leaner and healthier than patients counseled to eat the low-fat, carbohydrate-rich, calorie-restricted diet prescribed by both the American Heart Association and ADA. The numerous trials since then have concluded much the same.
Among the profound assumptions about Type 2 diabetes that these trials have now challenged is that it is, indeed, a progressive, degenerative disorder. This may only be true in the context of the carbohydrate-rich diets that the ADA has recommended. In 2019, researchers led by the late Sarah Hallberg of the University of Indiana, working with a healthcare start-up called Virta Health, reported that more than half of the participants in their clinical trial were able to reverse their type 2 diabetes by eating what amounts to a 21 st century version of Rollo’s animal diet or the Newburgh and Marsh approach. They were able to discontinue their insulin therapy and all but the most benign of their diabetes medications (known as metformin) while achieving healthy blood sugar control. A third of these patients remained in remission, with no sign of their disease, for the five years , so far, that their progress has been tracked.
As for Type 1 diabetes, in 2018, a collaboration led by the Harvard endocrinologists Belinda Lennerz and David Ludwig reported on a survey of members of a Facebook Group called TypeOneGrit dedicated to using the dietary therapy promoted by Dr. Richard Bernstein in his book Dr. Bernstein’s Diabetes Solution . Bernstein’s approach requires patients to self-experiment until they find the diet that provides stable healthy levels of blood sugar with the smallest doses of insulin. Such a diet, invariably, is very low in carbohydrates with more fat than either the ADA or AHA would deem healthy. Both youth and adults in the Harvard survey maintained near-normal blood sugar with surprisingly few signs of the kind of complications—including very low blood sugar, known as hypoglycemia—that make the life of a patient with Type 1 diabetes so burdensome. The TypeOneGrit survey, Lennerz said , revealed “a finding that was thought to not exist. No one thought it possible that people with type one diabetes could have [blood sugar levels] in the healthy range.” This does not mean that such diets are benign. They may still have the potential to cause significant harm, as Lennerz and Ludwig and their colleagues made clear. That, again, has never been tested.
One consequence of the diabetes associations embracing and prescribing a dietary philosophy in 1971 that has only recently been tested is that we’re back to the kind of situation that led to the evidence based medicine movement to begin with: enormous variation in therapeutic options from physician to physician and clinic to clinic with potentially enormous variations in benefits, harms and costs.
Even the ADA advice itself varies from document to document and expert panel to expert panel. In 2019, for instance, the ADA published two consensus reports on lifestyle therapy for diabetes. The first was the association’s consensus report on the standard of car e for patients with diabetes. The authors were physicians; their report repeated the conventional dietary wisdom about eating “vegetables, fruits, legumes, whole grains….” It emphasized “healthful eating patterns”, with “less focus on specific nutrients,” and singled out Mediterranean diets, Dietary Approaches to Stop Hypertension (known as the DASH diet) and plant-based diets as examples that could be offered to patients. This ADA report still argued for the benefits of low-fat and so carbohydrate-rich diets, while suggesting that the “challenges with long-term sustainability” of carbohydrate-restricted eating plans made them of limited use.
Three months later, the ADA released a five-year update on nutrition therapy . This was authored by a 14-member committee of physicians, dietitians and nutritionists. Among the conclusions was that the diets recommended as examples of healthful eating patterns in the lifestyle management report—low-fat diets, Mediterranean diets, plant-based diets and the DASH diet—were supported by surprisingly little evidence. In the few short-term clinical trials that had been done, the results had been inconsistent. As for carbohydrate-restricted high fat eating patterns, they were now “among the most studied eating patterns for Type 2 diabetes,” and the only diets for which the results had been consistent. “Reducing overall carbohydrate intake for individuals with diabetes,” this ADA report stated, “has demonstrated the most evidence for improving glycemia [high blood sugar] and may be applied in a variety of eating patterns that meet individual needs and preferences.”
Physician awarenessof the potential benefits of carbohydrate-restriction for Type 2 diabetes, meanwhile, still often comes from their patients, not their professional organizations. In the United Kingdom, for instance, David Unwin, a senior partner in a medium-sized practice began suggesting carbohydrate-restricted high fat diets to his patients in 2011, after seeing the results in one such patient who chose to do it on her own and lost 50 pounds. When results of her blood tests came back, says Unwin, they both realized that she was no longer suffering from diabetes. Both the weight loss and the reversal of diabetes were unique in Unwin’s experience. After reading up on the burgeoning literature on carbohydrate restriction, Unwin began counseling his diabetic patients to follow a very-low-carbohydrate, high-fat eating pattern. In 2017, the UK’s National Health Service awarded Unwin its “innovator of the year” award for applying a 200-year-old approach to diabetes therapy, as Unwin says, that “was routine until 1923.” Unwin has now published two papers documenting the experience in his medical practice. As of last year, 20 percent of the clinic’s diabetic patients— 94 in total —had chosen to follow this restricted dietary approach and put their Type 2 diabetes into remission.
If the diabetes community is to solve the formidable problems confronting it, even as drug therapies get ever more sophisticated, it will have to accept that some of its fundamental preconceptions about diabetes and diet may indeed be wrong. As it does so, it will have to provide support for those living with diabetes who decide that what theyhave been doing is not working. Some patients, when confronted with the choice between following a restricted eating pattern that seemingly maximizes their health and wellbeing or eating whatever they want and treating the symptoms and complications with drug therapy, will prefer the former. For those who do, the informed guidance of their physicians and diabetes educators will be invaluable.
When I interviewed individuals living with Type 1 diabetes, among the most poignant comments I heard was from a nutrition consultant diagnosed in 1977 when she was eight years old. She told me that she finally had faith she could manage her blood sugar and live with her disease when she met a physician who said to her “What can I do to help you?” That’s what changed her life, as much as any technology or medical intervention. In the context of the dietary therapies we’re discussing, that requires practitioners who are themselves open-minded and willing to spend the necessary time and effort to truly understand an approach to controlling diabetes that is, by definition, unconventional and, in Type 1 diabetes, still lacking clinical trials that test (or testify to) its safety and efficacy. Easy as it is for physicians to continue believing that what they should be doing is what they have been doing, they do not serve their patients best by doing so.
Adapted from Gary Taubes' new book Rethinking Diabetes: What Science Reveals About Diet, Insulin and Successful Treatments
More Must-Reads from TIME
- The New Face of Doctor Who
- Putin’s Enemies Are Struggling to Unite
- Women Say They Were Pressured Into Long-Term Birth Control
- Scientists Are Finding Out Just How Toxic Your Stuff Is
- Boredom Makes Us Human
- John Mulaney Has What Late Night Needs
- The 100 Most Influential People of 2024
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at [email protected]

- Previous Article
- Next Article
Acknowledgments
Connected content.
In a special series of the ADA Journals' podcast Diabetes Core Update , host Dr. Neil Skolnik interviews special guests and authors of this clinical compendium issue. Listen now at Special Podcast Series: Focus on Diabetes or view the interviews on YouTube at A Practice Guide to Diabetes-Related Eye Care .
Summary and Conclusion
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Thomas W. Gardner; Summary and Conclusion. ADA Clinical Compendia 1 July 2022; 2022 (3): 20. https://doi.org/10.2337/db20223-20
Download citation file:
- Ris (Zotero)
- Reference Manager
Diabetes is a multifactorial disease process, and its long-term management requires the active involvement of people with diabetes and their families, as well as a large multidisciplinary care team to ensure optimal health, quality of life, and productivity. Keeping up with new medications, emerging technology, and evolving treatment recommendations can be challenging, and the language and care processes commonly used by practitioners in one discipline may be less familiar to other diabetes care professionals.
In the realm of diabetes-related eye care, our ability to prevent the progression of diabetes-related retinal disease and thereby preserve vision has never been greater. However, far too many people with diabetes still are not receiving appropriate screening to identify eye disease early and ensure its timely treatment.
It is our hope that this compendium has provided information and guidance to improve communication and encourage collaboration between eye care professionals and other diabetes health care professionals and allow them to more effectively cooperate to reduce barriers to care and improve both the ocular and systemic health of their shared patients.
Editorial and project management services were provided by Debbie Kendall of Kendall Editorial in Richmond, VA.
Dualities of Interest
B.A.C. is a consultant for Genentech and Regeneron. S.A.R. is a speaker for Allergan, Inc., and VSP Vision Care. No other potential conflicts of interest relevant to this compendium were reported.
Author Contributions
All authors researched and wrote their respective sections. Lead author T.W.G. reviewed all content and is the guarantor of this work.
The opinions expressed are those of the authors and do not necessarily reflect those of VSP Vision Care, Regeneron, or the American Diabetes Association. The content was developed by the authors and does not represent the policy or position of the American Diabetes Association, any of its boards or committees, or any of its journals or their editors or editorial boards.
Email alerts
- Online ISSN 2771-6880
- Print ISSN 2771-6872
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Appointments at Mayo Clinic
Diabetes treatment: using insulin to manage blood sugar.
Learning how insulin affects your blood sugar can help you better manage your condition.
Insulin therapy often is an important part of diabetes treatment. It helps keep blood sugar under control and prevents diabetes complications. It works like the hormone insulin that the body usually makes.
The role of insulin in the body
Insulin comes from an organ in the stomach area called the pancreas. The main role of insulin is to ensure that sugar from nutrients in food is correctly used or stored in the body.
If your body can make enough insulin, you don't have diabetes. In people who don't have diabetes, insulin helps:
Control blood sugar levels. After you eat, your body breaks down nutrients called carbohydrates into a sugar called glucose. Glucose is the body's main source of energy. It's also called blood sugar. Blood sugar goes up after you eat.
When glucose enters the bloodstream, the pancreas responds by making insulin. Then insulin allows glucose to enter the body's cells to give them energy.
Store extra glucose for energy. After you eat, insulin levels are high. Extra glucose is stored in the liver. This stored glucose is called glycogen.
Between meals, insulin levels are low. During that time, the liver releases glycogen into the bloodstream in the form of glucose. This keeps blood sugar levels within a narrow range.
If you have diabetes:
Your blood sugar levels keep rising after you eat. That's because there's not enough insulin to move the glucose into your body's cells. With type 1 diabetes, the pancreas stops making insulin. With type 2 diabetes, the pancreas doesn't make enough insulin. And in some people with diabetes, insulin does not work well.
If you don't get treatment for diabetes, high blood sugar can lead to health problems over time. These conditions include:
- Heart attack or stroke.
- Kidney disease leading to kidney failure.
- Eye problems, including blindness.
- Nerve damage with nerve pain or numbness, called diabetic neuropathy.
- Foot problems that may lead to surgery to remove the foot.
- Dental issues.
Goals of insulin therapy
Insulin therapy keeps your blood sugar within your target range. It helps prevent serious complications.
If you have type 1 diabetes, you need insulin therapy to stay healthy. It replaces the insulin your body doesn't make.
If you have type 2 diabetes, insulin therapy might be part of your treatment. It's needed when healthy-lifestyle changes and other diabetes treatments don't control your blood sugar well enough.
Insulin therapy also is sometimes needed to treat a type of diabetes that happens during pregnancy. This is called gestational diabetes. If you have gestational diabetes, you might need insulin therapy if healthy habits and other diabetes treatments don't help enough.
Types of insulin
Any types of insulin help treat diabetes. Each type varies in how quickly and how long it controls blood sugar. You may need to take more than one kind of insulin. Factors that help determine which types of insulin you need and how much you need include:
- The type of diabetes you have.
- Your blood sugar levels.
- How much your blood sugar levels change during the day.
- Your lifestyle.
The main types of insulin therapy include:
Long-acting, ultralong-acting or intermediate-acting insulins. When you're not eating, your liver releases glucose so your body has energy. Long-, ultralong- or intermediate-acting insulin prevents blood sugar levels from rising without eating.
Examples of these insulins are glargine (Lantus, Basaglar, others), detemir (Levemir), degludec (Tresiba) and NPH (Humulin N, Novolin N, others). Intermediate-acting insulin lasts about 12 to 18 hours. Long-acting insulin works for about 24 hours. And ultralong-acting insulin lasts about 36 hours or longer.
Rapid-acting or short-acting insulins. These insulins are ideal for use before meals. If taken with a meal, they can help bring blood sugar back down to the baseline. They also blunt the sugar spikes after you eat. They start to work much faster than long-acting or intermediate-acting insulins do. Sometimes, rapid-acting insulins begin working in as few as 5 to 15 minutes. But they work for a much shorter time. Rapid-acting insulin lasts about 2 to 3 hours. Short-acting insulin lasts about 3 to 6 hours.
Examples of these insulins include ultrafast-acting aspart (Fiasp) and lispro (Lyumjev); rapid-acting aspart (NovoLog), glulisine (Apidra) and lispro (Humalog, Admelog); and short-acting, regular (Humulin R, Novolin R).
Sometimes, insulin-makers combine two types of insulin. This is called pre-mixed insulin. It can be helpful for people who have trouble using more than one type of insulin. Pre-mixed insulin often starts to work in 5 to 60 minutes. It can keep working for 10 to 16 hours.
Be aware that different preparations of insulin vary in terms of when they start working and how long they last. Be sure to read the instructions that come with your insulin. And follow any directions from your health care team.
Ways to take insulin
Insulin doesn't come in pill form. The digestive system would break the pill down before it had a chance to work. But there are other ways to take insulin. Your health care team can help you decide which method fits best for you.
Choices include:
- Shots or pens. You can inject insulin into the fat just below the skin with a syringe and needle. Or you can inject it with a pen-like device. Both types of devices hold insulin with a needle attached. How often you need to use an insulin pen or shot depends on the type of diabetes you have. It also depends on your blood sugar levels and how often you eat and exercise. You may need to take insulin shots or use insulin pens multiple times a day.
- Insulin pump. An insulin pump gives you small, steady amounts of rapid-acting insulin throughout the day. This works like using a shot of long-acting insulin. A pump also can give a rapid burst of insulin, often taken with food. This works like using a shot of rapid-acting insulin. The pump pushes the insulin into a thin tube placed beneath the skin. Several different kinds of insulin pumps are available.
- Inhaled insulin (Afrezza). This type of insulin is rapid acting. You breathe it in through a device that goes in your mouth, called an inhaler. You take this type of insulin at the start of each meal. People who smoke should not use inhaled insulin. Nor should people who have lung problems such as asthma or chronic obstructive pulmonary disease.
Sometimes, using insulin therapy can be a challenge. But it's an effective way to lower blood sugar. Talk to a member of your health care team if you have any trouble with your insulin routine. Ask for help right away if at-home glucose tests show that you have very low or very high blood sugar. Your insulin or other diabetes medicines may need to be adjusted. With time, you can find an insulin routine that fits your needs and lifestyle. And that can help you lead an active, healthy life.
If you take many doses of insulin a day, ask your health care provider if there's a way to make the routine simpler. Adding noninsulin medicines to your treatment plan might lower the number of insulin shots you need each day. And if you take fewer insulin shots, you'll need to check your blood sugar less often. Certain noninsulin medicines have other health benefits too. Some can help control weight and lower the chances of heart attack or stroke, heart failure, and kidney failure. Some people with type 2 diabetes can stop taking insulin completely after they start taking noninsulin medicines. But it's important to keep taking your insulin as prescribed until your health care provider tells you it's OK to stop.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Insulin basics. American Diabetes Association. https://diabetes.org/healthy-living/medication-treatments/insulin-other-injectables/insulin-basics. Accessed March 8, 2023.
- Mantzoros C, et al. Insulin action. https://www.uptodate.com/contents/search. Accessed March 8, 2023.
- What is diabetes? Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/basics/diabetes.html. Accessed March 8, 2023.
- Insulin, medicines & other diabetes treatments. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/insulin-medicines-treatments. Accessed March 8, 2023.
- Weinstock RS. General principles of insulin therapy in diabetes mellitus. https://www.uptodate.com/contents/search. Accessed March 8, 2023.
- What is diabetes? National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes. Accessed March 8, 2023.
- Afrezza (prescribing information). MannKind Corp.; 2023. https://afrezza.com/. Accessed March 8, 2023.
- Insulin routines. American Diabetes Association. https://diabetes.org/healthy-living/medication-treatments/insulin-other-injectables/insulin-routines. Accessed March 8, 2023.
- Types of insulin. Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/basics/type-1-types-of-insulin.html. Accessed March 9, 2023.
- Diabetes and nerve damage. Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/library/features/diabetes-nerve-damage.html. Accessed March 28, 2023.
- Diabetes and your feet. Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/library/features/healthy-feet.html. Accessed March 28, 2023.
- Shah P (expert opinion). Mayo Clinic. March 28, 2023.
- Castro MR. Mayo Clinic The Essential Diabetes Book. 3rd ed. Mayo Clinic Press; 2022.
- Wu J, et al. Reasons for discontinuing insulin and factors associated with insulin discontinuation in patients with type 2 diabetes mellitus: A real-world evidence study. Clinical Diabetes and Endocrinology. 2021; doi:10.1186/s40842-020-00115-2.
Products and Services
- The Mayo Clinic Diet Online
- A Book: The Essential Diabetes Book
- Medication-free hypertension control
- Alcohol: Does it affect blood pressure?
- Alpha blockers
- Amputation and diabetes
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers
- Anxiety: A cause of high blood pressure?
- Artificial sweeteners: Any effect on blood sugar?
- Bariatric surgery
- Beta blockers
- Beta blockers: Do they cause weight gain?
- Beta blockers: How do they affect exercise?
- Blood glucose meters
- Blood glucose monitors
- Blood pressure: Can it be higher in one arm?
- Blood pressure chart
- Blood pressure cuff: Does size matter?
- Blood pressure: Does it have a daily pattern?
- Blood pressure: Is it affected by cold weather?
- Blood pressure medication: Still necessary if I lose weight?
- Blood pressure medications: Can they raise my triglycerides?
- Blood pressure readings: Why higher at home?
- Blood pressure tip: Get more potassium
- Blood sugar levels can fluctuate for many reasons
- Blood sugar testing: Why, when and how
- Bone and joint problems associated with diabetes
- Pancreas transplant animation
- Caffeine and hypertension
- Calcium channel blockers
- Calcium supplements: Do they interfere with blood pressure drugs?
- Can whole-grain foods lower blood pressure?
- Central-acting agents
- Choosing blood pressure medicines
- COVID-19: Who's at higher risk of serious symptoms?
- Diabetes and depression: Coping with the two conditions
- Diabetes and exercise: When to monitor your blood sugar
- Diabetes and heat
- 10 ways to avoid diabetes complications
- Diabetes diet: Should I avoid sweet fruits?
- Diabetes diet: Create your healthy-eating plan
- Diabetes foods: Can I substitute honey for sugar?
- Diabetes and liver
- Diabetes management: How lifestyle, daily routine affect blood sugar
- Diabetes symptoms
- Diabetes treatment: Can cinnamon lower blood sugar?
- Diabetic Gastroparesis
- Diuretics: A cause of low potassium?
- Erectile dysfunction and diabetes
- High blood pressure and exercise
- Exercise and chronic disease
- Free blood pressure machines: Are they accurate?
- Frequent urination
- Home blood pressure monitoring
- Glucose tolerance test
- Glycemic index: A helpful tool for diabetes?
- Hemochromatosis
- High blood pressure (hypertension)
- High blood pressure and cold remedies: Which are safe?
- High blood pressure and sex
- High blood pressure dangers
- What is hypertension? A Mayo Clinic expert explains.
- Hypertension FAQs
- Hypertensive crisis: What are the symptoms?
- Insulin and weight gain
- Isolated systolic hypertension: A health concern?
- Kidney disease FAQs
- L-arginine: Does it lower blood pressure?
- Late-night eating: OK if you have diabetes?
- Low-phosphorus diet: Helpful for kidney disease?
- Medications and supplements that can raise your blood pressure
- Menopause and high blood pressure: What's the connection?
- Infographic: Pancreas Kidney Transplant
- Pancreas transplant
- Pulse pressure: An indicator of heart health?
- Reactive hypoglycemia: What can I do?
- Resperate: Can it help reduce blood pressure?
- Sleep deprivation: A cause of high blood pressure?
- Stress and high blood pressure
- The dawn phenomenon: What can you do?
- Unexplained weight loss
- Vasodilators
- Vegetarian diet: Can it help me control my diabetes?
- How to measure blood pressure using a manual monitor
- How to measure blood pressure using an automatic monitor
- What is blood pressure?
- Can a lack of vitamin D cause high blood pressure?
- Weight Loss Surgery Options
- White coat hypertension
- Wrist blood pressure monitors: Are they accurate?
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Diabetes treatment Using insulin to manage blood sugar
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
bestessayhelp.com
Diabetes Treatment and Prevention – Essay Sample
Diabetes may seem to be a serious obstacle in ones everyday life. However, there are certain ways to treat this illness, or at least, lessen its negative impact on the life of a person. In essence, there are two major ways of treating diabetes: home treatment and medical treatment. In practice diabetes is most effectively treated with the help of medications. Various drugs are used for general healing and specialized healing of different processes. For instance, sulfonylureas are used to stimulate pancreas for producing more insulin and, thus, maintaining normal blood sugars level. Thiazolidinediones are used to increase sensitivity of the cells towards insulin and to maintain effect written above. If slow insulin production is observed D-phenylalanine derivatives are consumed in order to increase the amount of insulin produced. Insulin injections help to maintain appropriate level of insulin in blood and cause the least allergic reactions (Mathur 2008). Home treatment of diabetes may include strict adherence to a specific diet, physical exercises, restriction of alcohol consumption, and constant testing of sugar levels. A well-balanced diet rich in fiber and low in fat and sweets, a relatively same amount of calories consumed at the same period of time can help a person to acquire a steady knowledge of his body responses to certain food, keep the blood level at a certain level, and, what is even more important, can assist the doctor to prescribe correct doses of medication. It is also beneficial to get a consultation on the variety and amount of daily exercise that would be useful for people who suffer from diabetes. Exercises in any form may reduce the risk of having kidney and other complications caused by the harmful effect of diabetes. It is vital to reduce the consumption of alcohol as part of a daily diet. Excessive intake of beer, wine, of liquor may result in neuritis and type 2 diabetes. Smoking can also be extremely dangerous for people with this illness. Smoking damages the blood vessels and has a negative impact on ones heart, consequently increasing the possibility of a heart disease or stroke. Any person sick with diabetes must schedule the blood level checks several times during the day, as far as this is a good way to control and acknowledge any changes occurring in a system.
Another way to contribute to the welfare of a person diagnosed with diabetes is to attend special educational classes in a hospital. One may learn important information on how to control the level of sugar in your blood, get emotionally boosted by the fact that people all over the world suffer from the disease but find ways to face and deal with the problem on their own and with the help of professional treatment, find new ways of adjusting to the state of the illness and more. A constant check at the nearest healthcare center is a must that cannot be omitted. Moreover, one has to learn the symptoms of low sugar levels and be ready to react in the most effective and timely manner. Family members can be a great helping hand in assisting the people sick with diabetes to feel normally and not panic in case of an emergency (Mathur 2008).
Diabetes has become one of the major deceases US citizens face. Though it can cause severe complications there are a lot of ways to control the flow of the illness. Insulin injections and other types of drugs are created in order to reduce negative impact of the decease on the person’s health. Proper medication and heath care can not only reduce those complications, but also prevent person from getting sick with diabetes mellitus. One needs to understand that this illness is very fast flowing. Therefore, immediate actions should be performed in order to stop its development and save person’s health. Taking diabetes tests is quite essential procedure, as it may help in detecting diabetes mellitus during the incubation period. Neglecting symptoms of the decease may cause very harmful consequences, which can only be treated, but not cured completely.

The road to success is easy with a little help. Let's get your assignment out of the way.
- Open access
- Published: 08 May 2024
Advances and challenges of the cell-based therapies among diabetic patients
- Ramin Raoufinia 1 , 2 ,
- Hamid Reza Rahimi 2 ,
- Ehsan Saburi 2 &
- Meysam Moghbeli ORCID: orcid.org/0000-0001-9680-0309 2
Journal of Translational Medicine volume 22 , Article number: 435 ( 2024 ) Cite this article
511 Accesses
Metrics details
Diabetes mellitus is a significant global public health challenge, with a rising prevalence and associated morbidity and mortality. Cell therapy has evolved over time and holds great potential in diabetes treatment. In the present review, we discussed the recent progresses in cell-based therapies for diabetes that provides an overview of islet and stem cell transplantation technologies used in clinical settings, highlighting their strengths and limitations. We also discussed immunomodulatory strategies employed in cell therapies. Therefore, this review highlights key progresses that pave the way to design transformative treatments to improve the life quality among diabetic patients.
Diabetes mellitus poses a formidable global public health challenge due to its rapid growing prevalence and associated morbidity, disability, and mortality [ 1 ]. According to the International Diabetes Federation, over 537 million adults aged 20–79 had diabetes worldwide in 2021 that is expected to rise to around 783 million cases by 2045 [ 2 ]. Obesity, unhealthy diets, physical inactivity as well as genetic and epigenetic predispositions are important risk factors of diabetes [ 3 , 4 , 5 ]. Diabetes is typically classified into type 1 diabetes mellitus (T1DM), gestational diabetes mellitus (GDM), and type 2 diabetes mellitus (T2DM) [ 2 ]. T1DM primarily arises from autoimmune-related damage of insulin-secreting beta cells, resulting in severe hyperglycemia and ketoacidosis [ 6 ]. In contrast, T2DM generally has a more gradual onset characterized by insulin resistance along with diminished compensatory insulin secretion from pancreatic beta cell dysfunction [ 7 ]. Diabetes is associated with macrovascular complications such as heart disease and stroke, as well as microvascular issues in eyes, kidneys, and nervous system [ 8 ]. Cancer is also a leading cause of diabetes-related death, and dementia-associated mortality has risen in recent decades [ 9 , 10 , 11 , 12 ]. Cell therapy involves transferring autologous or allogenic cellular material into patients [ 13 ]. The global market size of cell therapy is estimated to grow from $9.5 billion in 2021 to $23 billion by 2028 [ 14 ]. It combines stem and non-stem cell therapies consisting of unicellular or multicellular preparations. Cell therapies typically use autologous or allogenic cells via injection and infusion [ 15 ]. In the present review, we discussed the recent advances in cell-based therapy of diabetes, from foundational islet transplantation to regenerative strategies to highlight key developments that improve the effective treatments for diabetic patients.
Cell replacement therapy for diabetes
Pancreatic transplantation was firstly used in 1966 to treat type 1 diabetes using whole organ transplants. During the 1970s–80s, segmental pancreatic grafts were combined with techniques to divert digestive secretions away from transplanted cells. Three main techniques emerged; simultaneous pancreas-kidney transplants, pancreas transplants following kidney transplants, and pancreatic transplants. International collaboration on tracking outcomes began in 1980 with the formation of several pancreatic transplant registries and associations. However, whole organ transplantation was faced with several challenges including organ rejection, vascular complications, limited organ availability, and the effects of lifelong immunosuppression [ 16 , 17 ]. Islet cell transplantation was explored as an alternative, however isolating and transplanting pancreatic islets proved difficult due to donor availability, rejection, and immunosuppression side effects. Recent research has focused on stem cell sources that could reconstitute immune tolerance and preserve beta cell function such as mesenchymal stem cells, bone marrow cells, and embryonic stem cells [ 18 ]. A novel stem cell therapy called VX-880 was developed using proprietary technology to grow insulin-producing beta cells from allogeneic stem cells. Clinical trials began in 2021 after FDA approval to deliver the cells intrahepatically under immune suppression. A second approach called VX-264 encapsulates the same cells, avoiding immunosuppression but requiring surgical implantation [ 17 ]. In 2023, FDA approved the first allogeneic pancreatic islet cell therapy called Lantidra for adults with type 1 diabetes experiencing severe hypoglycemia. Approval was based on two studies where 21–30% of participants no longer required insulin one year post-treatment, with benefits lasting over five years in some cases. However, this treatment have mild and serious adverse events that are associated with treatment dose and the methods of islet cell infusion [ 19 , 20 ].
Emerging strategies for cell delivery via microencapsulation and biological devices in clinical trials
Alginate capsules as cell delivery systems.
A seminal investigation conducted in 1994 demonstrated the successful transplantation of alginate-encapsulated islets into the peritoneum of kidney transplant patients who were receiving immunosuppression therapy. Remarkably, these patients achieved insulin independence for up to nine months [ 21 ]. However, subsequent trials conducted without immunosuppression yielded inconsistent outcomes. In a study conducted in 2006, islets were encapsulated in triple-layer alginate capsules and implanted intraperitoneally in type 1 diabetes (T1D) patients. There was a positive correlation between the encapsulation and insulin production that reduced exogenous insulin requirements during one year. Despite this progress, the entry of cytokines remained a potential concern [ 22 ]. Another study employed the single-layer barium-alginate capsules that sustained insulin production for up to 2.5 years [ 23 ]. It has been reported that the microneedle, comprising a calcium alginate frame with polydopamine-coated poly-lactic-co-glycolic acid microspheres encapsulating insulin, enables light-triggered insulin release. Microneedle provided a suitable insulin dose to maintain blood glucose levels in line with daily fluctuations. These results established the efficacy and safety of the developed microneedle for diabetes treatment [ 24 ]. Another therapeutic approach explored the encapsulation of pancreatic islets with mesenchymal stem cells (MSCs) and decellularized pancreatic extracellular matrix (ECM). ECM derived from the pancreas supported islet cell growth and maintenance to enhance insulin expression [ 25 ]. Sodium alginate and hyaluronic acid were incorporated due to their roles in collagen production, wound healing, and physical crosslinking. The 3D porous membranes allowed optimal water and oxygen transfer while diverting excess exudate from diabetic wounds. Hydrogel accelerated re-epithelization, while decreased inflammation, indicating potential as the diabetic wound dressings [ 26 ]. Additionally, the incorporation of specific ECM components, such as collagen IV and RGD, into alginate-based microcapsules significantly improved the survival, insulin secretion, and longevity of microencapsulated islets [ 27 ].
Encaptra® device from ViaCyte
In contrast to microencapsulation techniques, ViaCyte developed a semipermeable pouch method named Encaptra, which contains pancreatic precursor cells derived from the embryonic stem cells [ 28 ]. In the initial trial conducted in 2014, the “VC-01” device was implanted in T1D individuals without the use of immunosuppression [ 29 ]. The trial confirmed the safety of the device; however, the occurrence of hypoxia induced cellular necrosis [ 30 ]. The device was modified as “VC-02” with larger pores, and two trials (NCT03162926, NCT03163511) demonstrated promising outcomes, including increased fasting C-peptide levels and a 20% reduction in insulin requirements during one year in the majority of participants [ 31 ]. In order to eliminate the necessity for immunosuppressants, ViaCyte collaborated with Gore to develop an expanded polytetrafluoroethylene (ePTFE) device with both immuno-isolating and pro-angiogenic properties [ 32 ]. This device (NCT04678557) aimed to prevent immune cell attachment and T-cell activation [ 33 ]. Additionally, ViaCyte is exploring the integration of CRISPR technology to modify stem cells, specifically by eliminating β2-microglobulin expression and PD-L1 up regulation. It is hypothesized that these genetic modifications will further hinder immune cell attachment and T-cell activation [ 30 , 34 ].
Semipermeable device from Semma therapeutics
Semma Therapeutics, which has been acquired by Vertex, pioneered the utilization of differentiated stem cell-derived islet cell clusters in clinical trials. Semma houses these cells between two semipermeable polyvinylidene fluoride membranes and is designed for subcutaneous implantation (NCT04786262) [ 31 , 35 ]. Vertex reported a significant breakthrough by infusing differentiated beta cells via the portal vein in a participant who was receiving immunosuppressants. This approach led to substantial C-peptide production and improved glycemic control during 90 days [ 36 ].
βAir device from Beta O2
Beta O2’s innovative βAir device utilizes an alginate-PTFE membrane complex to encapsulate islets, providing partial immunoisolation while ensuring a continuous supply of oxygen, which is crucial for optimal islet function [ 37 , 38 ]. The βAir device that was seeded with human islets was subcutaneously implanted in T1D individuals (NCT02064309). Although, low insulin levels were produced for up to eight weeks, there was not any reduction in the required exogenous insulin [ 37 ]. While, increasing the number of islets could potentially enhance their function, it is important to note that the continuous reliance on oxygen poses a risk of infection, despite efforts to optimize the survival of encapsulated islets [ 39 , 40 ].
Cell pouch™ device from Sernova
Sernova has developed the Cell Pouch device, which offers pre-vascularized polypropylene chambers for islet transplantation without the need for immunoprotection. The device consists of multiple cylindrical chambers that are prefilled with PTFE plugs, which are then removed after implantation to create the empty space [ 41 ]. In a 2012 trial (NCT01652911), islets were placed in the vascularized pouches of three recipients who were also receiving immunosuppression that resulted in a transient increase in C-peptide levels [ 41 ]. In a 2018 trial (NCT03513939), immunosuppression was administered after implantation and islet introduction. This trial reported sustained C-peptide production for up to nine months in two recipients, along with improved glycemic control [ 42 ]. Regarding the limitations of immunosuppression, Sernova is exploring the possibility of encapsulating islets in hydrogel as an alternative approach [ 43 ].
Shielded living therapeutics™ from Sigilon Therapeutics
Sigilon has developed the Shielded Living Therapeutics sphere, which consists of cell clusters enclosed within an alginate-TMTD coating [ 44 ]. Preclinical studies demonstrated that murine islet transplants encapsulated within these spheres maintained normoglycemia for a period of six months [ 45 ]. In a 2020 trial conducted for hemophilia (NCT04541628), the spheres were evaluated for their ability to express Factor VIII [ 46 ]. However, the trial was paused due to the development of antibodies in the third recipient receiving the highest cell doses. While, preclinical studies have shown promising efficacy, there are safety concerns regarding the TMTD coating that need to be addressed before these spheres can be used for human islet transplantation as a treatment for diabetes [ 31 ]. Emerging technologies have been investigated in clinical trials for delivering insulin-producing islets or stem cell-derived beta cells via microencapsulation or use of implantable biological devices (Table 1). Optimizing encapsulation and developing alternative implantable devices moves the field toward delivering safe and effective islet replacement without chronic immunosuppression dependency that represented an important new frontier for the cell-based treatment of diabetes. However, continued refining will be required to fully realize this promising vision and using these preclinical concepts in clinic.
Immunoengineering strategies: biomaterials for modulating immune responses
Islet encapsulation aims to prevent immune responses toward transplant antigens. However, foreign body response (FBR) against biomaterials induces inflammation around encapsulated islets that obstructs oxygen/nutrient access and causes graft failure [ 31 ]. Extensive research revealed biomaterial properties profoundly influence FBR severity, with high purity/biocompatibility moderating inflammation [ 47 ]. Deeper understanding of biomaterial immunobiology enabled developing immune-modulating constructs to steer host interactions. By altering topology/chemistry to hinder nonspecific binding and cell adhesion, these “immune-evasive biomaterials” intended to attenuate xenograft rejection at inception [ 44 ]. Both innate and adaptive immune responses have crucial roles in the context of pancreatic islet transplantation. These responses encompass the activation of tissue macrophages and neutrophils following injury, leading to the release of inflammatory cytokines that subsequently activate antigen-presenting cells (APCs), CD8 + T cells, CD4 + T cells, and cytotoxic T lymphocytes (Fig. 1 ). Zwitterionic polymers conferred anti-fouling attributes but crosslinking limitations constrained their application [ 48 ]. Novel mild zwitterionization introduced alginate modifications that prolonged prevention of fibrotic overgrowth by mitigating initial responses [ 49 , 50 , 51 ]. The prevention of graft rejection following islet cell transplantation necessitates the systemic administration of immunosuppressive agents. While, these agents effectively suppress immune responses, their continuous use exposes patients to an increased risk of infection and cancer. To mitigate these concerns, an alternative approach involving the localized delivery of immunosuppressants at the transplantation site has emerged. This localized delivery system offers several advantages, including targeted drug delivery, reduced systemic exposure, and potentially reduces the immunosuppressants doses [ 52 ]. Polymeric carriers dispersed cyclosporine A continuously at the graft site to dynamically tamp down proinflammatory cascades and T-cell activation [ 53 , 54 ]. TGF-β/IL-10 co-delivery at the microencapsulation interface hindered innate antigen presentation, obstructing adaptive response priming [ 55 , 56 ]. Regulatory T-cells emerged as the potent immunomodulators when coated on islets to improve insulin production in vitro [ 57 ]. Similarly, recombinant Jagged-1 surface patterning increased regulatory lymphocytes in vitro while enhancing glycemic oversight in vivo [ 58 ]. Targeting proinflammatory effector T-cells or presenting their Fas ligand death receptor improved long-term viability when combined with rapamycin prophylaxis [ 52 , 59 ]. Immobilizing thrombomodulin or urokinase mitigated local inflammation, with the latter conferring lifelong xenotransplant survival [ 60 ]. Peptides recognizing IL-1 receptors provided robust protection from destabilizing proinflammatory cytokines [ 61 ]. Leukemia inhibiting factor improved islet performance over polyethylene glycol encapsulation alone by inducing regulatory T-cell lineages [ 62 ]. Silk scaffolds facilitated IL-4/dexamethasone emancipation that meaningfully decreased immune reactions to grafts [ 63 ]. Therefore, the localized delivery of immunosuppressants at the transplantation site represents a promising strategy for islet cell transplantation. Compared to systemic administration, local delivery can achieve targeted immune modulation only at the graft location while reducing drug exposure throughout the body. This localized approach aims to sufficiently suppress the immune response to prevent rejection, while limiting negative side effects that may occur from systemic immunosuppression. A variety of biomaterials and surface modification strategies have been developed and investigated for the local delivery of immunosuppressive agents and immunomodulatory cytokines [ 64 , 65 , 66 ]. Understanding how biomaterial properties influence the immune response is critical to design biomaterials that can modulate inflammation and improve islet graft survival through localized immunomodulation.
Cell-based therapy through the integration of additive manufacturing techniques
Additive manufacturing utilizes computer modeling to fabricate complex 3D structures on-site with minimal post-processing. Common methods for the biomedical application are fused filament fabrication (FFF), stereolithography (SLA), and bioprinting [ 67 ]. FFF is a layer-by-layer technique that extrudes heated thermoplastics [ 68 ]. Commonly used feedstocks include acrylonitrile butadiene styrene (ABS) and polylactic acid (PLA). Other thermoplastics that have been utilized with FDM include thermoplastic polyurethane (TPU), polycarbonate (PC), polystyrene (PS), polyetherimide (PEI), polycaprolactone (PCL), polyaryletherketone (PAEK), and polyetheretherketone (PEEK), with the latter demonstrating high strength and heat tolerance. A major advantage of FDM is its ability to fabricate multi-material objects through continuous printing and alteration of the build material. In addition to typical polymers like PC and polystyrene (PS), FDM can print composites reinforced with glass, metals, ceramics, and bioresorbable polymers via integration of the constituent powders with a binding matrix. This enables enhanced control over the experimental component fabrication. While, ceramic and metal filaments traditionally contain the corresponding powder mixed with a binder, FDM provides versatility in the functional prototype construction from a wide range of thermoplastic feedstocks using precise and additive layer manufacture [ 68 , 69 , 70 , 71 , 72 ]. It provides geometric reproducibility and reduced variability compared to traditional techniques. FFF prints served as scaffolds for the transplanted cells [ 67 ]. However, minimum feature size is limited to ? ∼ 250 μm by nozzle diameter [ 68 ]. SLA employs light-curable liquid resins and achieves higher 50–150 μm resolution than FFF but with restricted material choices. Bone grafts and surgical guides are common applications [ 67 ]. Incorporating biomaterials like hydroxyapatite has expanded utility, though processing is required to mitigate cytotoxicity. Additive manufacturing can address limitations in oxygen transport, cell/material placement control and vasculature formation, and clinically translatable insulin-secreting implants [ 67 ]. Therefore, additive manufacturing technologies have the potential to enhance various aspects of the cell-based transplant design, from improving nutrient transport through optimized implant geometry to achieving precision integration of therapeutic agents (Table 2).
Enhancing nutrient transport through optimization of implant geometry
Tissue engineering for the islet transplantation requires maximizing nutrient transport [ 73 , 74 ]. Traditional scaffold fabrication introduces macroporosity but lacks precision that results in inflammation [ 67 ]. Cell encapsulation provides immunoprotection by limiting interactions between transplanted cells and the host immune system. However, this protective barrier also poses challenges for the efficient transport of essential nutrients, including oxygen, to the encapsulated cells. Modifying the geometries of encapsulation devices using conventional methods to enhance oxygen delivery has proven to be inconsistently challenging [ 67 ], so that novel approaches are required to address these challenges. Additive manufacturing allows customizing biomaterial scaffolds with defined geometries and micropore sizes to improve transport [ 75 , 76 , 77 , 78 , 79 ]. The 3D printed PLA scaffolds with islets have successful vascularization and cellular survival after subcutaneous transplantation [ 80 , 81 ]. Interlocking toroidal hydrogel-elastomer constructs also increased surface area and cell viability [ 82 , 83 , 84 ].
Enhancing vascularization and engraftment
Rich host vascularization of transplant devices is essential to support long-term islet survival through efficient nutrient delivery and insulin kinetics. Early platforms modified bulk material properties to promote vessel infiltration and anastomoses [ 85 , 86 , 87 , 88 , 89 ]. Additive manufacturing can further optimize microscale geometry to both accelerate host vessel connections and control intra-device vasculature homogeneity beyond traditional fabrication. Initial work reproduced macroscale vessels but scales were diverged from cell-based therapies [ 73 , 90 , 91 , 92 ]. Leveraging Additive manufacturing designed structures guided vessel formation in vitro and in vivo [ 80 , 89 , 93 ]. Shifting to bioprinting complex branching conduits in supportive hydrogels facilitated clinical translation for diverse cell therapies [ 94 , 95 , 96 , 97 , 98 ]. Researchers focused on developing a 3D scaffold platform to improve the transplantation outcomes of islet cells in T1D. The scaffold featured a heparinized surface and immobilized vascular endothelial growth factor (VEGF) to enhance vascularization. Scaffold effectively promoted angiogenesis and facilitated the growth of new blood vessels. Additionally, encapsulated islets within the scaffold had functional responses to glucose stimuli. These findings suggested that the developed scaffold platform holds potential for successful extra-hepatic islet transplantation, offering new possibilities for T1D treatment [ 99 ]. Research on vascularization of islets via additive manufacturing techniques has primarily focused on the fundamental discoveries. In one study, engineered pseudo islets (EPIs) were created by combining the mouse insulin-secreting beta cells with rat heart microvascular endothelial cells. EPIs demonstrated extensive outgrowth of capillaries into the surrounding matrix. Although, EPIs containing both cell types that underwent capillarization maintained viability and function over time in culture, non-vascularized EPIs lacking endothelial cells could not sustain viability or functionality long-term. This supported the potential for inducing angiogenesis within bioengineered islet constructs. Future work may combine patient-specific stem cell-derived human beta cells with endothelial cells using this approach to promote long-term graft survival for treating type 1 diabetes [ 98 ]. While, large-scale 3D printed vascularized structures are currently limited for the islet transplantation, advancements in leveraging additive manufacturing for the optimization vascularization conditions through the pore sizes and material choices, may facilitate translation to β-cell therapy in type 1 diabetes.
Precision placement of cells and matrix for enhanced control
Beyond distributing biomaterials, additive manufacturing enables micro-level cell and protein control. For islet transplantation, optimal cellular distribution and supportive extracellular matrix niche reduce rapid dysfunction and apoptosis [ 100 , 101 , 102 ]. Traditional techniques heterogeneously load cells after fabrication or struggle with incomplete encapsulation [ 103 , 104 ]. Bioprinting allows in situ encapsulation and printing of multiple cell types and matrix components while dictating 3D placement and dimensions [ 105 , 106 ]. Islet transplant research prints hydrogel-encapsulated clusters surrounded by supportive cells and doped with immune modulators to improve the transplant environment [ 107 ]. Progress in bioprinting offers consistency and defines physical/chemical graft properties beyond traditional fabrication.
Achieving controlled integration of therapeutic agents for enhanced efficacy
In addition to the cell and matrix placement, additive manufacturing enables precision therapeutic integration. Incorporating therapeutics aims to recapitulate the in vivo environment through angiogenesis, islet health promotion, and immunomodulation [ 67 , 108 ]. Growth factors promote vessel formation and insulin secretion while decrease apoptosis [ 108 , 109 , 110 , 111 ]. Local immunomodulators regulate the immune system in a specific site of the body. They decrease inflammation and promote the successful integration of transplanted cells or tissues by minimizing the need for widespread immune suppression in whole body [ 67 ]. Traditional homogeneous delivery methods restrict the ability to customize the spatial distribution of substances and pose a risk of harmful effects on transplants or hosts [ 112 ]. The use of discreet gradients in bioprinting can offer precise physiological signals. By combining traditional drug release methods with AM, it becomes possible to create tissues that exhibit distinct therapeutic localization. Bioprinted composites have the ability to release factors with gradients throughout the entire construct that enables a more comprehensive and targeted approach in tissue engineering [ 112 , 113 , 114 ].
Cell based gene therapy
Gene therapy holds great promise for diabetes management, offering innovative approaches to deliver and manipulate the insulin gene in various tissues. Viral methods, such as lentivirus, adenovirus, and adeno-associated virus (AAV), along with non-viral techniques like liposomes and naked DNA, have been utilized to deliver the insulin gene to target tissues [ 115 ]. This section aims to provide an overview of important studies in the field of gene therapy for diabetes management, emphasizing advancements in insulin gene delivery and manipulation (Table 3).
Enteroendocrine K-cells and pancreatic β-cells
Enteroendocrine K-cells in the intestines and pancreatic β-cells share similarities in their production of glucose-dependent insulinotropic polypeptide (GIP) and their regulatory mechanisms. Understanding these similarities offers insights into T2D management and improving glucose homeostasis. However, attempts to reverse diabetes effectively through K-cell transplantation have been unsuccessful. Nevertheless, research on gene editing techniques has shown promising results in management of the diabetes mellitus [ 116 , 117 ]. AAV vectors have been employed to co-express insulin and glucokinase genes in skeletal muscles, demonstrating long-term effectiveness in achieving normo-glycemia without exogenous insulin [ 118 , 119 ].
Gene editing techniques
Gene editing techniques using AAV vectors effectively improved normo-glycemia in animal models. Co-expression of insulin and glucokinase in transgenic mice increased glucose absorption and regulated insulin production. Duodenal homeobox 1 (PDX1) gene transfer via AAV2 in a humanized liver mouse model also led to insulin secretion and glycemic control [ 120 ]. Adenovirus-mediated transfection of hepatic cells with neurogenin 3 (NGN3) resulted in insulin production and trans-differentiation of oval cell populations [ 121 , 122 ]. Targeting specific promoters in liver cells such as phosphoenolpyruvate carboxykinase (PEPCK), glucose 6-phosphatase (G6Pase), albumin, and insulin-like growth factor binding protein-1 (IGFBP-1) enhanced hepatic insulin gene therapy [ 123 , 124 ]. AAV-mediated overexpression of SIRT1 reduced inflammation, hypoxia, apoptosis and improved neural function in the retina of diabetic db/db mice [ 125 ]. Another study developed a plasmid expressing a single-strand insulin analogue for intramuscular injection using a specialized gene delivery technique. A single administration provided sustained insulin expression for 1.5 months and effectively regulated blood glucose levels without immune responses or tissue damage in diabetic mice.
Non-viral gene delivery methods
Non-viral approaches have also key roles in achieving glycemic control. The combination of insulin fragments with DNA plasmid, administered via intravenous injection improved normo-glycemia for extended periods. DNA transposon facilitated gene integration into the host chromosome that addressed the short-term liver expression. Additionally, the co-injection of DNA plasmid containing insulin with furin significantly enhanced insulin production within muscles [ 126 ]. Non-viral plasmids were engineered to carry proinsulin and pancreatic regenerating genes to ameliorate streptozotocin-induced T1DM [ 127 ]. The pVAX plasmid vectors prolonged therapeutic effects in achieving normo-glycemia without the need for further treatment [ 127 ]. Bioreducible cationic polymers, such as poly-(cystamine bisacrylamide-diamino hexane) (p(CBA-DAH)), have been employed to deliver RAE-1 to pancreatic islets, resulting in improved insulin levels [ 128 ]. Furthermore, ex vivo gene transfer and autologous grafts have shown promising outcomes in animal models. The introduction of the human insulin gene into pancreatic or liver cells followed by autologous grafts improved insulin secretion, glycemic control, and alleviated the diabetic complications in pigs. However, gene silencing eventually occurred, necessitating a deeper understanding of the underlying mechanisms [ 128 , 129 ].
Stem cell based therapy in diabetes
Efforts are ongoing to develop standardized processes for donor and recipient selection/allocation to increase pancreas utilization [ 130 , 131 , 132 , 133 ]. Techniques for isolating pancreatic islets are being optimized to become more standardized and consistent. Noninvasive imaging technologies allow the monitoring of the transplanted islets without surgery [ 134 , 135 ]. Biomarkers could also evaluate how immunomodulation strategies are working [ 136 , 137 , 138 ]. Researchers are also exploring alternative transplant sites in the body beyond just the liver, to see if the other locations may better support islet graft survival and function. Together, these areas of refinement aim to improve the safety and reliability of islet transplantation procedures as a potential therapy for diabetes [ 139 ]. Bioengineering approaches are being developed to optimize the islet transplantation microenvironment using biomaterials which enhance islet engraftment and function through engineered extracellular niches [ 140 , 141 ]. For example, encapsulation techniques aim to protect pancreatic islets against immune reponse by enclosing them within semipermeable hydrogel polymer capsules [ 142 , 143 ]. This localized immunoisolation strategy utilizes biomaterials like alginate to create a physical barrier preventing immune cell contact while still allowing nutrient and oxygen diffusion. Researchers concurrently seek alternative unlimited cellular sources to address limited islet availability. Mesenchymal stem cells possess immunomodulatory properties and their adjuvant delivery, either early in disease onset or simultaneously with islet transplantation, has shown promising signs of improving outcomes in preclinical investigations. By dampening inflammatory responses and favoring regenerative processes, stem cells may help to establish a more tolerogenic transplant environment. These bioengineering and cell therapy approaches offer potential pathways towards eliminating the exogenous insulin requirement [ 144 , 145 ]. A variety of stem cell types have therapeutic potential for diabetes (Fig. 2 ). Pluripotent stem cells possess immense promise for overcoming the limitations of islet transplantation. Human embryonic stem cells and induced pluripotent stem cells are especially attractive candidates due to their unique ability to both self-renew indefinitely and differentiate into any cell type. This makes them an ideal source of replacement pancreatic beta cells. Significant research effort across academic and industrial laboratories has led to advancement in differentiation protocols that can convert pluripotent stem cells into functional beta-like cells in vitro. However, establishing consistent, well-characterized cellular production methods that comply with stringent safety and efficacy standards remains a priority for clinical translation. Ongoing work aims to generate therapeutic stem cell-derived beta cell replacements exhibiting stable, glucose-responsive insulin secretion comparable to primary islets. Although, technological and regulatory hurdles still must be cleared, pluripotent stem cells have the greatest potential to finally solve the problem of limited cell availability and provide an unlimited source of transplantable tissue suitable for widespread treatment of diabetes [ 145 , 146 , 147 , 148 ]. There are currently six registered clinical trials evaluating the use of human pluripotent stem cells for the T1D treatment. All trials except one use PEC-01 cells, which consist of a mixture of pancreatic endoderm and polyhormonal cell population derived from CyT49 stem cells that are fully committed to endocrine differentiation upon implantation [ 149 ]. The initial trial implanted PEC-01 cells within an encapsulation device, hypothesizing no need for immunosuppression. While, well-tolerated with minor adverse effects, insufficient engraftment occurred due to foreign body responses that eliminated the cells [ 150 ]. The trial transitioned in 2017 to use an open encapsulation device that required immunosuppression. Subcutaneous engraftment, differentiation of cells into islet-like clusters, and glucose-responsive insulin production provided the first evidence that pancreatic progenitor cells can survive, mature, and function as the endocrine cells in humans. Potential benefits on stimulated C-peptide levels and glycemic control were observed in one patient [ 151 , 152 ]. Two reports in late 2021 described results in 17 patients receiving PEC-01 cells in an open device. Engraftment and insulin expression occurred in the majority, glucose-responsive secretion in over one-third, and various glycemic improvements were observed at six months. Explanted tissues contained heterogeneous pancreatic compositions including mature beta cells, with no teratoma formation and mild adverse effects related to surgery/immunosuppression. VX-880 uses fully differentiated insulin-producing stem cell-derived islet cells in phase 1/2 trial evaluating portal infusion and different doses requiring immunosuppression. Preliminary results suggest early engraftment and insulin secretion. The manin challenge was controlling immune rejection without systemic immunosuppression [ 149 ]. Several strategies are being explored to address the challenges of immune rejection in stem cell therapies for diabetes. They include generating stem cell lines that are universally compatible through HLA silencing, developing milder regimens of immunosuppression, and refining encapsulation and containment approaches to protect transplanted cells toward immune response. Establishing standardized stem cell banks is also an area of investigation [ 153 , 154 ]. Xenotransplantation using gene-edited porcine islets remains an exciting avenue of research given advances to improve engraftment and reduce immunogenicity in preclinical studies [ 155 ]. Novel approaches continue to emerge as well, such as decellularization techniques, 3D bioprinting of tissue constructs, and creating interspecies chimeras. Rapid evolution of cell-based therapies across both academic and commercial sectors is promising to restore normoglycemic control in diabetic cases. Refinement of existing methods and development of new strategies hold potential to perform a safe and effective cell replacement without reliance on systemic immunosuppression. Stem cell and regenerative therapies may ultimately manage diabetes through restored endogenous insulin production [ 156 ]. Recently a meta analysis evaluated the safety and efficacy of MSC-based therapy for diabetes in humans. This comprehensive analysis was conducted on 262 patients across six trials that met the inclusion criteria within the last five years. The results reveal that treatment with MSCs significantly reduced the dosage of anti-diabetic drugs over a 12-months. Following treatment, HbAc1 levels decreased by an average of 32%, fasting blood glucose levels decreased by an average of 45%, and C-peptide levels showed a decrease of 38% in two trials and an increase of 36% in four trials. Notably, no severe adverse events were reported across all trials. Therefore, it can be concluded that MSC therapy for type 2 diabetes is safe and effective [ 157 ].
Advances in islet transplantation and stem cell-derived Beta cells
Limited number of the islet transplantation donors highlights the importance of cell therapy in diabetes. Although, higher islet numbers from multiple donors increase the success, limited pancreas availability restricts widespread use [ 158 ]. Using multiple donors also increases rejection risk, while isolation of the islets can cause tissue damage [ 159 ]. To overcome these challenges, researchers have explored the differentiation of stem cells into beta cells in vitro to generate an unlimited supply of insulin-producing cells with standardized and characterized products. Genetic engineering techniques have also been investigated to confer advantages such as stress resistance or immune evasion [ 158 ]. ViaCyte has developed a stem cell-derived pancreatic progenitor called PEC-01, which has the ability to mature into endocrine cells in rodent models. To protect the transplanted cells from immune response, retrieval encapsulation devices were also created [ 160 , 161 , 162 ]. In an initial human clinical trial conducted in 2014 (NCT02239354), the Encaptra device was utilized with the aim of providing complete immunoprotection of transplanted cells through the use of a cell-impermeable membrane. Although, the PEC-Encap product showed reliable tolerance and minimal adverse effects, the trial was stopped due to the inadequate engraftment of functional products. While, a few endocrine cells were observed, fibrosis around the capsule led to graft loss and supression of the insulin secretion. To address this challenge, a more recent development called the PEC-Direct device was introduced, which featured openings in the membrane to facilitate vascularization, thereby improving nutrient exchange and supporting cell viability. However, since host cells could infiltrate the device, immunosuppression was necessary following the transplantation [ 163 , 164 , 165 ]. Protocols were developed to generate clusters of stem cell-derived beta cells that secreted glucose-responsive insulin. These clusters, referred to SC-islets, also contained other endocrine cells, including glucagon-producing cells. SC-islets improved glycemic control in diabetic mice and nonhuman primates [ 146 , 166 , 167 , 168 ]. In a trial conducted in 2017 (NCT03163511), the transplantation of progenitor cells resulted in the maturation of endocrine cells, and glucose-responsive C-peptide secretion was observed 6–9 months post-transplantation. Notably, the majority of these mature endocrine cells exhibited glucagon-positive characteristics. The porous regions housing the endocrine cells allowed for the infiltration of host vessels to facilitate vascularization. However, non-cellular regions were isolated by the presence of fibrosis [ 164 , 165 ]. Although, there was not a sufficient levels of circulating C-peptide in these trials, the findings underscored the significance of promoting vascularization and minimizing fibrotic reactions [ 164 , 169 ]. Vertex conducted a human trial in 2021 (NCT04786262) involving the transplantation of half-dose VX-880 cells (SC-islets) without a device to avoid previous problems, which necessitated immunosuppression. Preliminary results reported improved glycemic control, although it took longer to achieve the same outcome compared to rodent models [ 158 ]. Overall, progresses in islet transplantation and stem cell-derived beta cells pave the way for overcoming the limitations of traditional approaches. Further research and refinements are also required to achieve consistent and clinically significant outcomes in the treatment of diabetes.
Chalenges and limitations
Cell-based therapies have been significantly progressed for diabetes; however, there are still several challenges that need to be overcome. Clinical trials investigating encapsulation devices and islet transplantation techniques have provided valuable insights but face several obstacles including oxygenation, host immune responses, and insufficient long-term engraftment success. Immunoengineering of biomaterials and additive manufacturing for the development of 3D islet structures aim to modulate inflammation and promote graft revascularization. Nevertheless, achieving consistent normalization of blood glucose levels without exogenous insulin remains a challenge in human studies. In the field of gene therapy and stem cell differentiation, research focuses on genetically-modified or progenitor-derived insulin-secreting β-like cells to optimize protocols that ensure safety and functionality. The main challenge is to establish stable and functional cells capable of permanently restoring normoglycemia without the need for external intervention. One major barrier is the immune response, which targets allogeneic and xenogeneic islet grafts. Although, local immunotherapy minimizes the systemic effects, evading graft destruction through biomaterials without the requirement of immune suppression remains a significant challenge. The translation of precision 3D islet constructs and genetically reprogrammed cells also necessitates scalable manufacturing processes to ensure consistent function and long-term safety across batches. When critically appraising progress in the field of cell-based diabetes treatments, it is imperative to consider the regulatory, ethical, economic, and safety factors that shape translational applications. At the regulatory level, oversight bodies play a pivotal role in establishing standards to ensure patient welfare while enabling therapeutic innovation. FDA oversees clinical trials and product approvals in the United States (US), while in Europe the EMA provides parallel regulatory guidance. Within the US, organizations like the United Network for Organ Sharing (UNOS) and Organ Procurement and Transplantation Network (OPTN) govern organ and cell allocation protocols [ 17 , 170 ]. However, as regenerative approaches diverge from traditional organ transplantation, regulatory pathways require ongoing harmonization between the agencies and jurisdictions. Continual dialogue between researchers, oversight boards, and policymakers will be crucial to streamline guidelines in a patient-centric manner that balances safety, efficacy, and timely access to cutting-edge therapies. For instance, as stem cell-derived beta cells and 3D bioprinted tissue constructs emerge, traditional drug and device frameworks may not adequately address product characterization and manufacturing complexities for these advanced therapeutic products [ 67 ]. Within clinics, maintaining compliance with evolving regulations impacts research directives and ultimately patients’ access to the novel treatments. Addressing informed consent, clinical trial design, and privacy protections for sensitive health data are also paramount from an ethical perspective [ 128 , 129 ]. Autonomy and agency of research participants in decision-making related to experimental therapies demand prudency. Equitable accessibility of new treatment options also warrants attention to avoid certain populations facing undue barriers. Cell sourcing presents ethical issues depending on derivation from embryonic, fetal or adult tissues. Logistical matters like shipping and processing stem cell-derived islets prior to transplantation necessitate scrutiny. Tumorigenic potential of the undifferentiated pluripotent stem cells should be optimized through rigorous preclinical testing. Transitioning therapies between animal and early human investigations necessitates well-characterized cellular products showing consistent safety and glucose-responsive insulin secretion profiles comparable to pancreatic islets. Long-term animal model data substantiating lack of malignant transformation following transplantation aids allaying ethical safety concerns as the therapies progress clinically. Researchers carefully screen new concepts to prevent side effects in participants while pursuing curative goals. In terms of economic costs, islet and stem cell transplant procedures remain prohibitively expensive for broad applicability despite promising clinical signals. The field requires sustained study to validate techniques, track long-term outcomes, assess healthcare costs offsets from mitigating diabetes’ debilitating complications, and establish cost-benefit ratios for national reimbursement paradigms. Public-private partnerships may accelerate large, interventional trials and longitudinal research to precisely quantify the cellular therapies’ safety profiles and real-world efficacies compared to intensive management versus costs of intensive diabetes care. Ongoing developments like 3D bioprinting offer catalytic manufacturing potential fundamentally recalibrating economics by enhancing yields, standardizing procedures, and reducing costs through scale. By thoroughly and sensitively examining regulatory frameworks, informed consent processes, risks and benefits, as well as financial considerations at both micro and macro levels, researchers, oversight boards and broader stakeholder networks can advance cell-based therapies towards delivering life-changing benefits for all communities. A multidisciplinary, conscientious approach balances progress against patient welfare. A combination of multiple strategies may help to overcome these limitations. For instance, gene-modified islets integrated within vascularized biomaterial implants or sequenced therapies have promising results to prime grafts in pro-regenerative environments before transplantation. Collaboration across disciplines offers hope that refined individualized therapies may eventually achieve durable insulin independence through functional pancreatic cell or tissue engraftment, not only for diabetes but also for chronic pancreatitis. Regarding, ongoing progresses in unraveling these barriers, cell replacement approaches have the potential to improve diabetes management.
Conclusions
This review provides a comprehensive overview of the advances, challenges, and future directions in various cell-based therapeutic approaches for the treatment of diabetes. Significant progresses have been achieved in microencapsulation design, immunomodulation, tissue constructs, genetic and cellular reprogramming techniques, as well as initial clinical translation. However, the complete restoration of normoglycemia without the need for lifelong immunosuppression is still considered as a significant therapeutic challenge. Therefore, addressing the transplant environment of the hostile nature, developing minimally invasive delivery methods, and overcoming limitations in engraftment efficiency and longevity are crucial issues for the future researches. Through the sustained multidisciplinary efforts for the improvement of existing strategies and establishing novel paradigms, achieving durable insulin independence can be a realistic goal for all diabetic cases through the personalized cell replacement or regeneration.
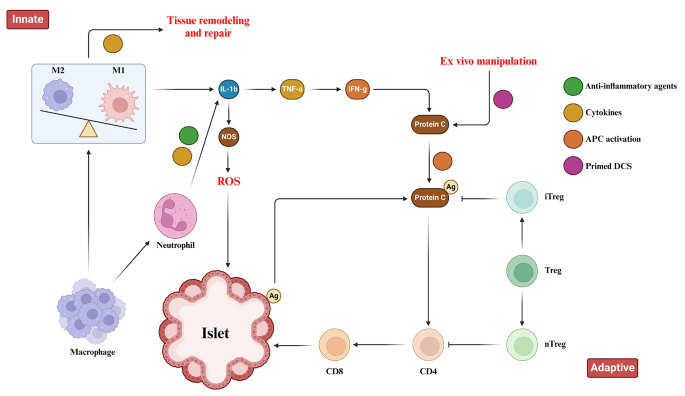
Immune Responses toward pancreatic islets following transplantation. This figure illustrates the immune responses, including the innate and adaptive immunity that are triggered upon pancreatic islet transplantation. Immune response begins with the activation of tissue macrophages and neutrophils in response to injury. Subsequent, release of inflammatory cytokines stimulates antigen-presenting cells (APCs), CD4 + T cells, CD8 + T cells, and cytotoxic T lymphocytes to orchestrate the immune response
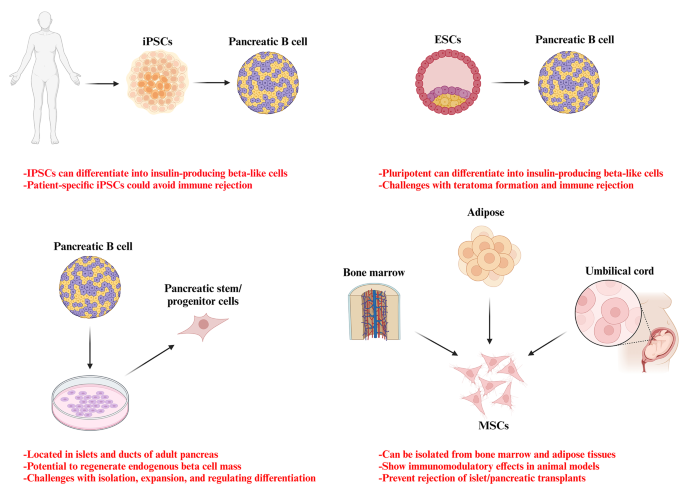
Potential stem cell sources for the treatment of diabetes
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Acrylonitrile butadiene styrene
Activate antigen-presenting cells
Adeno-associated virus
Duodenal homeobox 1
Engineered pseudo islets
Expanded polytetrafluoroethylene
Extracellular matrix
Foreign body response
Fused filament fabrication
Gestational diabetes mellitus
Glucose 6-phosphatase
Insulin-like growth factor binding protein-1
Mesenchymal stem cells
Neurogenin 3
Organ Procurement and Transplantation Network
Phosphoenolpyruvate carboxykinase
Polyaryletherketone
Polycaprolactone
Polycarbonate
Polyetheretherketone
Polyetherimide
Poly-lactic acid
Polystyrene
Stereolithography
Thermoplastic polyurethane
Type 1 diabetes
Type 1 diabetes mellitus
Type 2 diabetes mellitus
United Network for Organ Sharing
United States
Vascular endothelial growth factor
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
Article Google Scholar
Cho NH, Shaw J, Karuranga S, Huang Y, da Rocha Fernandes J, Ohlrogge A, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Article CAS PubMed Google Scholar
Moghbeli M, Naghibzadeh B, Ghahraman M, Fatemi S, Taghavi M, Vakili R, et al. Mutations in HNF1A gene are not a Common cause of familial young-onset diabetes in Iran. Indian J Clin Biochem. 2018;33(1):91–5.
Akhlaghipour I, Bina AR, Mogharrabi MR, Fanoodi A, Ebrahimian AR, Khojasteh Kaffash S, et al. Single-nucleotide polymorphisms as important risk factors of diabetes among Middle East population. Hum Genomics. 2022;16(1):11.
Article CAS PubMed PubMed Central Google Scholar
Moghbeli M, Khedmatgozar H, Yadegari M, Avan A, Ferns GA, Ghayour Mobarhan M. Cytokines and the immune response in obesity-related disorders. Adv Clin Chem. 2021;101:135–68.
Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Reviews Endocrinol. 2020;16(7):349–62.
Article CAS Google Scholar
Siqueira ISLd, Alves Guimarães R, Mamed SN, Santos TAP, Rocha SD, Pagotto V, et al. Prevalence and risk factors for self-report diabetes mellitus: a population-based study. Int J Environ Res Public Health. 2020;17(18):6497.
Free radical research.
Zhu B, Qu S. The relationship between diabetes mellitus and cancers and its underlying mechanisms. Front Endocrinol. 2022;13:800995.
Mojarrad M, Moghbeli M. Genetic and molecular biology of bladder cancer among Iranian patients. Mol Genet Genomic Med. 2020;8(6):e1233.
Article PubMed PubMed Central Google Scholar
Moghbeli M. Genetic and molecular biology of breast cancer among Iranian patients. J Transl Med. 2019;17(1):218.
Abbaszadegan MR, Moghbeli M. Genetic and molecular origins of colorectal Cancer among the iranians: an update. Diagn Pathol. 2018;13(1):97.
Kim I. A brief overview of cell therapy and its product. J Korean Association Oral Maxillofacial Surg. 2013;39(5):201.
Mount NM, Ward SJ, Kefalas P, Hyllner J. Cell-based therapy technology classifications and translational challenges. Philosophical Trans Royal Soc B: Biol Sci. 2015;370(1680):20150017.
El-Kadiry AE-H, Rafei M, Shammaa R. Cell therapy: types, regulation, and clinical benefits. Front Med. 2021;8:756029.
Squifflet J-P, Gruessner R, Sutherland D. The history of pancreas transplantation: past, present and future. Acta Chir Belg. 2008;108(3):367–78.
Article PubMed Google Scholar
Parums DV. First Regulatory approval for allogeneic pancreatic islet Beta cell infusion for adult patients with type 1 diabetes Mellitus. Med Sci Monitor: Int Med J Experimental Clin Res. 2023;29:e941918–1.
Yang L, Hu Z-M, Jiang F-X, Wang W. Stem cell therapy for insulin-dependent diabetes: are we still on the road? World J Stem Cells. 2022;14(7):503.
Affan M, Dar MS. Donislecel-the first approved pancreatic islet cell therapy medication for type 1 diabetes: a letter to the editor. Ir J Med Sci (1971-). 2023:1–2.
Harris E. FDA greenlights first cell therapy for adults with type 1 diabetes. JAMA. 2023.
Soon-Shiong P, Heintz R, Merideth N, Yao Q, Yao Z, Zheng T, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet (London England). 1994;343(8903):950–1.
Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29(1):137–8.
Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32(10):1887–9.
Weng L, Wang X, Liu H, Yu Z, Liu S. Light-responsive microneedle array with tunable insulin release function for painless and on-demand anti-diabetic therapy. Mater Lett. 2023:135684.
Okcu A, Yazir Y, Şimşek T, Mert S, Duruksu G, Öztürk A, et al. Investigation of the effect of pancreatic decellularized matrix on encapsulated islets of Langerhans with mesenchymal stem cells. Tissue Cell. 2023;82:102110.
Khaliq T, Sohail M, Minhas MU, Mahmood A, Munir A, Qalawlus AHM, et al. Hyaluronic acid/alginate-based biomimetic hydrogel membranes for accelerated diabetic wound repair. Int J Pharm. 2023;643:123244.
Kuwabara R, Qin T, Llacua LA, Hu S, Boekschoten MV, de Haan BJ, et al. Extracellular matrix inclusion in immunoisolating alginate-based microcapsules promotes longevity, reduces fibrosis, and supports function of islet allografts in vivo. Acta Biomater. 2023;158:151–62.
Kirk K, Hao E, Lahmy R, Itkin-Ansari P. Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem cell Res. 2014;12(3):807–14.
Dufrane D, van Steenberghe M, Goebbels R-M, Saliez A, Guiot Y, Gianello P. The influence of implantation site on the biocompatibility and survival of alginate encapsulated pig islets in rats. Biomaterials. 2006;27(17):3201–8.
Pullen LC. Stem cell–derived pancreatic progenitor cells have now been transplanted into patients: report from IPITA 2018. Wiley Online Library; 2018. pp. 1581–2.
Dang HP, Chen H, Dargaville TR, Tuch BE. Cell delivery systems: toward the next generation of cell therapies for type 1 diabetes. J Cell Mol Med. 2022;26(18):4756–67.
Viacyte. ViaCyte and gore enter clinical phase agreement based on novel membrane technology for PEC-encap product candidate. 2020.
Viacyte. viacyte announces initiation of phase 2 study of encapsulated cell therapy for type 1 diabetes patients 2021 2021. https://viacyte.com/press-releases/viacyte‐announces‐initiation‐of‐phase‐2‐study‐of‐encapsulated‐cell‐ther‐apy‐for‐type‐1‐diabetes‐patients/ .
Hodgson J. Drug pipeline 3Q23—ERT, bispecifics and CRISPR in sickle cell disease. Nat Biotechnol. 2023;41(11):1498–500.
Pagliuca F. Pre-clinical proof-of-Concept in two lead programs in type 1 diabetes. International Socety for Stem Cell Research; 2019.
Jones PM, Persaud SJ. β-cell replacement therapy for type 1 diabetes: closer and closer. Diabet Med. 2022;39(6).
Carlsson P-O, Espes D, Sedigh A, Rotem A, Zimerman B, Grinberg H, et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am J Transplant. 2018;18(7):1735–44.
Ludwig B, Zimerman B, Steffen A, Yavriants K, Azarov D, Reichel A, et al. A novel device for islet transplantation providing immune protection and oxygen supply. Horm Metab Res. 2010;42(13):918–22.
Evron Y, Colton CK, Ludwig B, Weir GC, Zimermann B, Maimon S, et al. Long-term viability and function of transplanted islets macroencapsulated at high density are achieved by enhanced oxygen supply. Sci Rep. 2018;8(1):6508.
Cao R, Avgoustiniatos E, Papas K, de Vos P, Lakey JR. Mathematical predictions of oxygen availability in micro-and macro‐encapsulated human and porcine pancreatic islets. J Biomedical Mater Res Part B: Appl Biomaterials. 2020;108(2):343–52.
Gala-Lopez B, Pepper A, Dinyari P, Malcolm A, Kin T, Pawlick L, et al. Subcutaneous clinical islet transplantation in a prevascularized subcutaneous pouch–preliminary experience. CellR4. 2016;4(5):e2132.
Google Scholar
Sernova Corp Presents Positive Preliminary. Safety and Efficacy Data in its Phase I/II Clinical Trial for Type-1 Diabetes: Biospace. https://www.biospace.com/article/sernova‐corp‐presents‐positive‐preliminary‐safety‐and‐efficacy‐data‐in‐its‐phase‐i‐ii‐clinical‐trial‐for‐type‐1‐diabetes/ .
Bachul PJ, Perez-Gutierrez A, Juengel B, Golab K, Basto L, Perea L et al. 306-OR: modified approach for improved isllotransplantation into prevascularized sernova cell pouch device: preliminary results of the phase i/ii clinical trial at University of Chicago. Diabetes. 2022;71(Supplement_1).
Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol. 2016;34(3):345–52.
Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, et al. Long-term glycemic control using polymer-encapsulated human stem cell–derived beta cells in immune-competent mice. Nat Med. 2016;22(3):306–11.
Shapiro AD, Konkle BA, Croteau SE, Miesbach WA, Hay CRM, Kazmi R, et al. First-in-human phase 1/2 clinical trial of SIG-001, an innovative shielded cell therapy platform, for hemophilia Α. Blood. 2020;136:8.
Taraballi F, Sushnitha M, Tsao C, Bauza G, Liverani C, Shi A, et al. Biomimetic tissue engineering: tuning the immune and inflammatory response to implantable biomaterials. Adv Healthc Mater. 2018;7(17):1800490.
Yesilyurt V, Veiseh O, Doloff JC, Li J, Bose S, Xie X, et al. A facile and versatile method to endow biomaterial devices with zwitterionic surface coatings. Adv Healthc Mater. 2017;6(4):1601091.
Liu Q, Chiu A, Wang L-H, An D, Zhong M, Smink AM, et al. Zwitterionically modified alginates mitigate cellular overgrowth for cell encapsulation. Nat Commun. 2019;10(1):5262.
Noverraz F, Montanari E, Pimenta J, Szabó L, Ortiz D, Gonelle-Gispert C, et al. Antifibrotic effect of ketoprofen-grafted alginate microcapsules in the transplantation of insulin producing cells. Bioconjug Chem. 2018;29(6):1932–41.
Jeon SI, Jeong J-H, Kim JE, Haque MR, Kim J, Byun Y, et al. Synthesis of PEG-dendron for surface modification of pancreatic islets and suppression of the immune response. J Mater Chem B. 2021;9(11):2631–40.
Derakhshankhah H, Sajadimajd S, Jahanshahi F, Samsonchi Z, Karimi H, Hajizadeh-Saffar E, et al. Immunoengineering Biomaterials in Cell-based therapy for type 1 diabetes. Tissue Eng Part B: Reviews. 2022;28(5):1053–66.
Piemonti L, Maffi P, Nano R, Bertuzzi F, Melzi R, Mercalli A, et al. Treating diabetes with islet transplantation: lessons from the Milan experience. Transplantation, Bioengineering, and regeneration of the endocrine pancreas. Elsevier; 2020. pp. 645–58.
Azzi J, Tang L, Moore R, Tong R, El Haddad N, Akiyoshi T, et al. Polylactide-cyclosporin A nanoparticles for targeted immunosuppression. FASEB J. 2010;24(10):3927.
Chen X, Liu H, Li H, Cheng Y, Yang L, Liu Y. In vitro expansion and differentiation of rat pancreatic duct-derived stem cells into insulin secreting cells using a dynamic three-dimensional cell culture system. Genet Mol Res. 2016;15(2).
Becker MW, Simonovich JA, Phelps EA. Engineered microenvironments and microdevices for modeling the pathophysiology of type 1 diabetes. Biomaterials. 2019;198:49–62.
Graham JG, Zhang X, Goodman A, Pothoven K, Houlihan J, Wang S, et al. PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng Part A. 2013;19(11–12):1465–75.
Izadi Z, Hajizadeh-Saffar E, Hadjati J, Habibi-Anbouhi M, Ghanian MH, Sadeghi-Abandansari H, et al. Tolerance induction by surface immobilization of Jagged-1 for immunoprotection of pancreatic islets. Biomaterials. 2018;182:191–201.
McHugh MD, Park J, Uhrich R, Gao W, Horwitz DA, Fahmy TM. Paracrine co-delivery of TGF-β and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells. Biomaterials. 2015;59:172–81.
Chen H, Teramura Y, Iwata H. Co-immobilization of urokinase and thrombomodulin on islet surfaces by poly (ethylene glycol)-conjugated phospholipid. J Controlled Release. 2011;150(2):229–34.
Su J, Hu B-H, Lowe WL Jr, Kaufman DB, Messersmith PB. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31(2):308–14.
Dong H, Fahmy TM, Metcalfe SM, Morton SL, Dong X, Inverardi L, et al. Immuno-isolation of pancreatic islet allografts using pegylated nanotherapy leads to long-term normoglycemia in full MHC mismatch recipient mice. PLoS ONE. 2012;7(12):e50265.
Kumar M, Nandi SK, Kaplan DL, Mandal BB. Localized immunomodulatory silk macrocapsules for islet-like spheroid formation and sustained insulin production. ACS Biomaterials Sci Eng. 2017;3(10):2443–56.
Hotaling NA, Tang L, Irvine DJ, Babensee JE. Biomaterial Strategies for Immunomodulation. Annu Rev Biomed Eng. 2015;17:317–49.
Shi Y, Zhao YZ, Jiang Z, Wang Z, Wang Q, Kou L, et al. Immune-Protective formulations and process strategies for improved survival and function of transplanted islets. Front Immunol. 2022;13:923241.
Zhang S, Yang H, Wang M, Mantovani D, Yang K, Witte F, et al. Immunomodulatory biomaterials against bacterial infections: Progress, challenges, and future perspectives. Innovation. 2023;4(6):100503.
CAS PubMed PubMed Central Google Scholar
Accolla RP, Simmons AM, Stabler CL. Integrating Additive Manufacturing techniques to improve cell-based implants for the treatment of type 1 diabetes. Adv Healthc Mater. 2022;11(13):e2200243.
Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. ACS; 2014.
Bol RJ, Šavija B. Micromechanical models for FDM 3D-Printed polymers: a review. Polymers. 2023;15(23):4497.
Paul S. Finite element analysis in fused deposition modeling research: a literature review. Measurement. 2021;178:109320.
Monaldo E, Ricci M, Marfia S. Mechanical properties of 3D printed polylactic acid elements: experimental and numerical insights. Mech Mater. 2023;177:104551.
Anoop M, Senthil P. Microscale representative volume element based numerical analysis on mechanical properties of fused deposition modelling components. Materials Today: Proceedings. 2021;39:563 – 71.
McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proceedings of the National Academy of Sciences. 2006;103(31):11461-6.
Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proceedings of the National Academy of Sciences. 2012;109(11):4245-50.
Espona-Noguera A, Ciriza J, Cañibano-Hernández A, Orive G, Hernández RM, del Saenz L, et al. Review of advanced hydrogel-based cell encapsulation systems for insulin delivery in type 1 diabetes mellitus. Pharmaceutics. 2019;11(11):597.
Dimitrioglou N, Kanelli M, Papageorgiou E, Karatzas T, Hatziavramidis D. Paving the way for successful islet encapsulation. Drug Discovery Today. 2019;24(3):737–48.
Omer A, Duvivier-Kali V, Fernandes J, Tchipashvili V, Colton CK, Weir GC. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation. 2005;79(1):52–8.
Song S, Roy S. Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: cells, biomaterials, and devices. Biotechnol Bioeng. 2016;113(7):1381–402.
Zhi ZL, Kerby A, King AJF, Jones PM, Pickup JC. Nano-scale encapsulation enhances allograft survival and function of islets transplanted in a mouse model of diabetes. Diabetologia. 2012;55(4):1081–90.
Farina M, Chua CYX, Ballerini A, Thekkedath U, Alexander JF, Rhudy JR, et al. Transcutaneously refillable, 3D-printed biopolymeric encapsulation system for the transplantation of endocrine cells. Biomaterials. 2018;177:125–38.
Farina M, Ballerini A, Fraga DW, Nicolov E, Hogan M, Demarchi D et al. 3D printed vascularized device for Subcutaneous Transplantation of Human islets. Biotechnol J. 2017;12(9).
Lei D, Yang Y, Liu Z, Yang B, Gong W, Chen S, et al. 3D printing of biomimetic vasculature for tissue regeneration. Mater Horiz. 2019;6(6):1197–206.
Melchels FP, Domingos MA, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW. Additive manufacturing of tissues and organs. Prog Polym Sci. 2012;37(8):1079–104.
Ernst AU, Wang LH, Ma M. Interconnected toroidal hydrogels for islet encapsulation. Adv Healthc Mater. 2019;8(12):1900423.
Liang J-P, Accolla RP, Jiang K, Li Y, Stabler CL. Controlled release of anti-inflammatory and proangiogenic factors from macroporous scaffolds. Tissue Eng Part A. 2021;27(19–20):1275–89.
Pedraza E, Brady A-C, Fraker CA, Molano RD, Sukert S, Berman DM, et al. Macroporous three-dimensional PDMS scaffolds for extrahepatic islet transplantation. Cell Transplant. 2013;22(7):1123–35.
Chiu Y-C, Cheng M-H, Engel H, Kao S-W, Larson JC, Gupta S, et al. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials. 2011;32(26):6045–51.
Kuss MA, Wu S, Wang Y, Untrauer JB, Li W, Lim JY, et al. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J Biomedical Mater Res Part B: Appl Biomaterials. 2018;106(5):1788–98.
Liu X, Jakus AE, Kural M, Qian H, Engler A, Ghaedi M, et al. Vascularization of natural and synthetic bone scaffolds. Cell Transplant. 2018;27(8):1269–80.
Costa-Almeida R, Gomez-Lazaro M, Ramalho C, Granja PL, Soares R, Guerreiro SG. Fibroblast-endothelial partners for vascularization strategies in tissue engineering. Tissue Eng Part A. 2015;21(5–6):1055–65.
Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell. 2011;22(20):3791–800.
Vlahos AE, Cober N, Sefton MV. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proceedings of the National Academy of Sciences. 2017;114(35):9337-42.
Farina M, Ballerini A, Fraga DW, Nicolov E, Hogan M, Demarchi D, et al. 3D printed vascularized device for subcutaneous transplantation of human islets. Biotechnol J. 2017;12(9):1700169.
Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14(13):2202–11.
Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68.
Gao Q, Liu Z, Lin Z, Qiu J, Liu Y, Liu A, et al. 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomaterials Sci Eng. 2017;3(3):399–408.
Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci. 2019;6(11):1900344.
Hospodiuk M, Dey M, Ayan B, Sosnoski D, Moncal KK, Wu Y, et al. Sprouting angiogenesis in engineered pseudo islets. Biofabrication. 2018;10(3):035003.
Marchioli G, Luca AD, de Koning E, Engelse M, Van Blitterswijk CA, Karperien M, et al. Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de novo vessel formation for the transplantation of islets of Langerhans. Adv Healthc Mater. 2016;5(13):1606–16.
Dionne KE, Colton CK, Lyarmush M. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12–21.
de Groot M, Schuurs TA, Keizer PP, Fekken S, Leuvenink HG, Van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12(8):867–75.
Thomas F, Wu J, Contreras JL, Smyth C, Bilbao G, He J, et al. A tripartite anoikis-like mechanism causes early isolated islet apoptosis. Surgery. 2001;130(2):333–8.
Barkai U, Rotem A, de Vos P. Survival of encapsulated islets: more than a membrane story. World J Transplantation. 2016;6(1):69.
Jiang K, Chaimov D, Patel SN, Liang JP, Wiggins SC, Samojlik MM, et al. 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials. 2019;198:37–48.
Pati F, Jang J, Ha D, Won Kim S, Rhie J, Shim J, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935.
Kim BS, Kwon YW, Kong J-S, Park GT, Gao G, Han W, et al. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53.
Hu S, Martinez-Garcia FD, Moeun BN, Burgess JK, Harmsen MC, Hoesli C, et al. An immune regulatory 3D-printed alginate-pectin construct for immunoisolation of insulin producing β-cells. Mater Sci Engineering: C. 2021;123:112009.
Phelps EA, Templeman KL, Thulé PM, García AJ. Engineered VEGF-releasing PEG–MAL hydrogel for pancreatic islet vascularization. Drug Delivery Translational Res. 2015;5:125–36.
Kooptiwut S, Kaewin S, Semprasert N, Sujjitjoon J, Junking M, Suksri K, et al. Estradiol prevents high glucose-induced β-cell apoptosis by decreased BTG2 expression. Sci Rep. 2018;8(1):12256.
Dang TT, Thai AV, Cohen J, Slosberg JE, Siniakowicz K, Doloff JC, et al. Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug. Biomaterials. 2013;34(23):5792–801.
Wang Y, He D, Ni C, Zhou H, Wu S, Xue Z, et al. Vitamin D induces autophagy of pancreatic β-cells and enhances insulin secretion. Mol Med Rep. 2016;14(3):2644–50.
Tarafder S, Koch A, Jun Y, Chou C, Awadallah MR, Lee CH. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. 2016;8(2):025003.
Liu YY, Yu HC, Liu Y, Liang G, Zhang T, Hu QX. Dual drug spatiotemporal release from functional gradient scaffolds prepared using 3 D bioprinting and electrospinning. Polym Eng Sci. 2016;56(2):170–7.
Freeman FE, Pitacco P, van Dommelen LH, Nulty J, Browe DC, Shin J-Y, et al. 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci Adv. 2020;6(33):eabb5093.
Wong MS, Hawthorne WJ, Manolios N. Gene therapy in diabetes. Self Nonself. 2010;1(3):165.
Ahmad Z, Rasouli M, Azman AZF, Omar AR. Evaluation of insulin expression and secretion in genetically engineered gut K and L-cells. BMC Biotechnol. 2012;12:1–9.
Tudurí E, Bruin JE, Kieffer TJ. Restoring insulin production for type 1 diabetes. J Diabetes. 2012;4(4):319–31.
Romer AI, Sussel L. Pancreatic islet cell development and regeneration. Current opinion in endocrinology, diabetes, and obesity. 2015;22(4):255.
Jaén ML, Vilà L, Elias I, Jimenez V, Rodó J, Maggioni L, et al. Long-term efficacy and safety of insulin and glucokinase gene therapy for diabetes: 8-year follow-up in dogs. Mol therapy-methods Clin Dev. 2017;6:1–7.
Li H, Li X, Lam KS, Tam S, Xiao W, Xu R. Adeno-associated virus-mediated pancreatic and duodenal homeobox gene-1 expression enhanced differentiation of hepatic oval stem cells to insulin-producing cells in diabetic rats. J Biomed Sci. 2008;15:487–97.
Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127(16):3533–42.
Abed A, Critchlow C, Flatt PR, McClenaghan NH, Kelly C. Directed differentiation of progenitor cells towards an islet-cell phenotype. Am J Stem Cells. 2012;1(3):196.
PubMed PubMed Central Google Scholar
Zhao M, Amiel SA, Ajami S, Jiang J, Rela M, Heaton N, et al. Amelioration of streptozotocin-induced diabetes in mice with cells derived from human marrow stromal cells. PLoS ONE. 2008;3(7):e2666.
Handorf AM, Sollinger HW, Alam T. Genetic engineering of surrogate β cells for treatment of type 1 diabetes mellitus. J Diabetes Mellitus. 2015;5(04):295–312.
Grant MB, Adu-Agyeiwaah Y, Vieira CP, Asare-Bediako B, Hammer SS, Calzi SL, et al. Intravitreal administration of AAV2-SIRT1 reverses diabetic retinopathy (DR) in a murine model of type 2 diabetes (T2D). Investig Ophthalmol Vis Sci. 2022;63(7):2310.
Yoon J-W, Jun H-S. Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol Med. 2002;8(2):62–8.
Hou W-R, Xie S-N, Wang H-J, Su Y-Y, Lu J-L, Li L-L, et al. Intramuscular delivery of a naked DNA plasmid encoding proinsulin and pancreatic regenerating III protein ameliorates type 1 diabetes mellitus. Pharmacol Res. 2011;63(4):320–7.
Joo WS, Jeong JH, Nam K, Blevins KS, Salama ME, Kim SW. Polymeric delivery of therapeutic RAE-1 plasmid to the pancreatic islets for the prevention of type 1 diabetes. J Controlled Release. 2012;162(3):606–11.
Dezashibi HM, Shabani A. A Mini-review of Current Treatment approaches and Gene Therapy as potential interventions for diabetes Mellitus types 1. Adv Biomed Res. 2023;12:219.
Vantyghem M-C, de Koning EJ, Pattou F, Rickels MR. Advances in β-cell replacement therapy for the treatment of type 1 diabetes. Lancet. 2019;394(10205):1274–85.
Hudson A, Bradbury L, Johnson R, Fuggle S, Shaw J, Casey J, et al. The UK pancreas allocation scheme for whole organ and islet transplantation. Am J Transplant. 2015;15(9):2443–55.
Cornateanu SM, O’Neill S, Dholakia S, Counter CJ, Sherif AE, Casey JJ, et al. Pancreas utilization rates in the UK–an 11-year analysis. Transpl Int. 2021;34(7):1306–18.
Nordheim E, Lindahl JP, Carlsen RK, Åsberg A, Birkeland KI, Horneland R, et al. Patient selection for islet or solid organ pancreas transplantation: experiences from a multidisciplinary outpatient-clinic approach. Endocr Connections. 2021;10(2):230–9.
Arifin DR, Bulte JW. In vivo imaging of pancreatic islet grafts in diabetes treatment. Front Endocrinol. 2021;12:640117.
Murakami T, Fujimoto H, Inagaki N. Non-invasive beta-cell imaging: visualization, quantification, and beyond. Front Endocrinol. 2021;12:714348.
Piemonti L, Everly MJ, Maffi P, Scavini M, Poli F, Nano R, et al. Alloantibody and autoantibody monitoring predicts islet transplantation outcome in human type 1 diabetes. Diabetes. 2013;62(5):1656–64.
Anteby R, Lucander A, Bachul PJ, Pyda J, Grybowski D, Basto L, et al. Evaluating the prognostic value of islet autoantibody monitoring in islet transplant recipients with long-standing type 1 diabetes mellitus. J Clin Med. 2021;10(12):2708.
Buron F, Reffet S, Badet L, Morelon E, Thaunat O. Immunological monitoring in beta cell replacement: towards a pathophysiology-guided implementation of biomarkers. Curr Diab Rep. 2021;21:1–11.
Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep. 2011;11:364–74.
Tremmel DM, Odorico JS. Rebuilding a better home for transplanted islets. Organogenesis. 2018;14(4):163–8.
Citro A, Moser PT, Dugnani E, Rajab TK, Ren X, Evangelista-Leite D, et al. Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials. 2019;199:40–51.
Basta G, Montanucci P, Calafiore R. Microencapsulation of cells and molecular therapy of type 1 diabetes mellitus: the actual state and future perspectives between promise and progress. J Diabetes Invest. 2021;12(3):301–9.
Samojlik MM, Stabler CL. Designing biomaterials for the modulation of allogeneic and autoimmune responses to cellular implants in type 1 diabetes. Acta Biomater. 2021;133:87–101.
Carlsson P-O, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587–92.
Madani S, Setudeh A, Aghayan HR, Alavi-Moghadam S, Rouhifard M, Rezaei N, et al. Placenta derived mesenchymal stem cells transplantation in type 1 diabetes: preliminary report of phase 1 clinical trial. J Diabetes Metabolic Disorders. 2021;20:1179–89.
Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–39.
Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34(13):1759–72.
Sambathkumar R, Migliorini A, Nostro MC. Pluripotent stem cell-derived pancreatic progenitors and β-like cells for type 1 diabetes treatment. Physiology. 2018;33(6):394–402.
Sordi V, Monaco L, Piemonti L. Cell therapy for type 1 diabetes: from islet transplantation to stem cells. Hormone Res Paediatrics. 2022;96(6):658–69.
Henry RR, Pettus J, Wilensky J, SHAPIRO AJ, Senior PA, Roep B et al. Initial clinical evaluation of VC-01TM combination product—a stem cell–derived islet replacement for type 1 diabetes (T1D). Diabetes. 2018;67(Supplement_1).
Shapiro A, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus JH et al. Insulin expression and glucose-responsive circulating C-peptide in type 1 diabetes patients implanted subcutaneously with pluripotent stem cell-derived pancreatic endoderm cells in a macro-device. David and Donner, Thomas W and Bellin, Melena D and Hsueh, Willa and Pettus, Jeremy H and Wilensky, Jon S and Daniels, Mark and Wang, Richard M and Kroon, Evert J and Brandon, Eugene Paul and D’Amour, Kevin A and Foyt, Howard, Insulin Expression and Glucose-Responsive Circulating C-Peptide in Type. 2019;1.
Keymeulen B, Jacobs-Tulleneers-Thevissen D, Kroon EJ, Jaiman MS, Daniels M, Wang R et al. 196-LB: stem cell–derived islet replacement therapy (VC-02) demonstrates production of C-peptide in patients with type 1 diabetes (T1D) and hypoglycemia unawareness. Diabetes. 2021;70(Supplement_1).
Piemonti L. Felix dies natalis, insulin… ceterum autem censeo beta is better. Acta Diabetol. 2021;58(10):1287–306.
Sordi V, Pellegrini S, Piemonti L. Immunological issues after stem cell-based β cell replacement. Curr Diab Rep. 2017;17:1–8.
Coe TM, Markmann JF, Rickert CG. Current status of porcine islet xenotransplantation. Curr Opin Organ Transpl. 2020;25(5):449–56.
Edgar L, Pu T, Porter B, Aziz J, La Pointe C, Asthana A, et al. Regenerative medicine, organ bioengineering and transplantation. J Br Surg. 2020;107(7):793–800.
Mathur A, Taurin S, Alshammary S. The safety and efficacy of mesenchymal stem cells in the treatment of type 2 Diabetes- A literature review. Diabetes Metab Syndr Obes. 2023;16:769–77.
Hogrebe NJ, Ishahak M, Millman JR. Developments in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell. 2023;30(5):530–48.
Paraskevas S, Maysinger D, Wang R, Duguid WP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20(3):270–6.
Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29(8):750–6.
Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, et al. Maturation of human embryonic stem cell–derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–29.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52.
Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, et al. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Translational Med. 2015;4(10):1214–22.
Ramzy A, Thompson DM, Ward-Hartstonge KA, Ivison S, Cook L, Garcia RV, et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell. 2021;28(12):2047–61. e5.
Dolgin E, Diabetes. Encapsulating the problem. Nature. 2016;540(7632):S60–2.
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–33.
Hogrebe NJ, Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat Biotechnol. 2020;38(4):460–70.
Nair GG, Liu JS, Russ HA, Tran S, Saxton MS, Chen R, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat Cell Biol. 2019;21(2):263–74.
Shapiro AJ, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med. 2021;2(12).
Witkowski P, Anteby R, Olaitan OK, Forbes RC, Niederhaus S, Ricordi C, et al. Pancreatic islets Quality and Potency cannot be verified as required for drugs: reflection on the FDA Review of a biological license application for human islets. Transplantation. 2021;105(12):e409–10.
Download references
Acknowledgements
Author information, authors and affiliations.
Noncommunicable Diseases Research Center, Neyshabur University of Medical Sciences, Neyshabur, Iran
Ramin Raoufinia
Department of Medical Genetics and Molecular Medicine, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Ramin Raoufinia, Hamid Reza Rahimi, Ehsan Saburi & Meysam Moghbeli
You can also search for this author in PubMed Google Scholar
Contributions
RR, HRR, and ES were involved in search strategy and drafting. MM revised, designed, and supervised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Meysam Moghbeli .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Raoufinia, R., Rahimi, H.R., Saburi, E. et al. Advances and challenges of the cell-based therapies among diabetic patients. J Transl Med 22 , 435 (2024). https://doi.org/10.1186/s12967-024-05226-3
Download citation
Received : 14 February 2024
Accepted : 22 April 2024
Published : 08 May 2024
DOI : https://doi.org/10.1186/s12967-024-05226-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cell therapy
- Translplantation
- Immunosuppression
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Diabetes, heart problems and kidney disease are closely linked
Uc expert discusses prevention in new york times article.

Heart disease, diabetes and kidney disease, among the most common chronic illnesses in the United States, are all closely connected.
People with diabetes are twice as likely to have heart disease or a stroke and are at risk of developing kidney disease, while heart disease is more likely for those with kidney problems as the heart works harder to pump blood to the kidneys.
The New York Times reports people should pay attention to shared risk factors for these illnesses, including excess body fat, uncontrolled blood sugar, high blood pressure and high cholesterol.
The University of Cincinnati's Estrelita Dixon, MD, commented in the New York Times article on the importance of prevention.
Preventive measures can include adding more fiber, fruit and vegetables to your diet to regulate blood sugar and lower blood pressure and increasing muscle mass through strength training to help with insulin resistance. Just moving in general can be beneficial, and experts recommend aiming for 150 minutes of exercise each week, but Dixon noted gradual steps can still make a difference.
“Don’t think in terms of all or nothing,” said Dixon, division chief and associate professor in the Department of Internal Medicine in UC's College of Medicine.
R ead the New York Times story. (Note: Subscription may be required to access full article.)
Featured image at top of diabetes testing supplies. Photo/David Moruzzi/Unsplash.
- Faculty Staff
- In The News
- Internal Medicine
- College of Medicine
Related Stories
Dermatology times features uc research of nail squamous cell carcinoma treatment.
June 9, 2022
Dermatology Times featured recent research by University of Cincinnati researchers that showed a low recurrence rate of nail squamous cell carcinoma when treated with surgical approaches other than amputation.
Local 12, Business Courier highlight Blood Cancer Healing Center
February 19, 2024
Local 12 and the Cincinnati Business Courier highlighted the University of Cincinnati Cancer Center's Blood Cancer Healing Center, a comprehensive all-in-one facility dedicated solely to advancing research, treatment and wellness for blood cancer patients, opening this summer.
MedPage Today: Combination treatment promising for certain leukemia patients
May 22, 2023
The University of Cincinnati Cancer Center's Emily Curran recently provided commentary, highlighted by MedPage Today, on a study that showed a combination of antibody-drug conjugate and chemotherapy treatments were promising for older patients with B-cell acute lymphocytic leukemia.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Risk Factors
- Providing Care
- Living with Diabetes
- Clinical Guidance
- DSMES for Health Care Providers
- Prevent Type 2 Diabetes: Talking to Your Patients About Lifestyle Change
- Employers and Insurers
- Community-based Organizations (CBOs)
- Toolkits for Diabetes Educators and Community Health Workers
- National Diabetes Statistics Report
- Reports and Publications
- Current Research Projects
- National Diabetes Prevention Program
- State, Local, and National Partner Diabetes Programs for Public Health
- Diabetes Self-Management Education and Support (DSMES) Toolkit
Diabetes and Polycystic Ovary Syndrome (PCOS)
- Polycystic ovary syndrome (PCOS) is a condition that can impact fertility, and increase the risk of other chronic health conditions.
- More than half of people with PCOS develop type 2 diabetes by age 40.
- Find out the signs of PCOS, and what to do if you have it.

About PCOS and diabetes
PCOS is a condition where cysts (small sacs of fluid) develop on the ovaries. PCOS can cause irregular menstruation (periods), and is a common cause of infertility, affecting as many as 5 million people. In addition to infertility, it is a lifelong condition that can have other impacts.
People with PCOS often have insulin resistance . This is when their bodies make insulin, a key hormone in balancing blood sugar, but they can't use it effectively. Insulin resistance increases the risk of type 2 diabetes.
People with PCOS can develop serious health problems, especially if they have overweight, including:
- Type 2 diabetes
- Gestational diabetes (diabetes when pregnant)
- Heart disease —people with PCOS have a higher risk, which increases with age
- High blood pressure
- High LDL ("bad") cholesterol and low HDL ("good") cholesterol—increase the risk for heart disease
- Sleep apnea —a disorder that causes breathing to stop during sleep
PCOS is also linked to depression and anxiety, though the connection is not fully understood.
Symptoms of PCOS
The exact causes of PCOS aren't yet known. Imbalances in androgen levels (male reproductive hormones) may play an important part in PCOS. Family history of PCOS and overweight may also contribute.
Someone with PCOS may have few symptoms, while others may have them all. It's common for people to not find out they have PCOS until they are trying to get pregnant. PCOS often develops as young as age 11 or 12, around first menstruation. Symptoms include:
- Hair growth.
- Darkening of the skin in body creases, known as acanthosis nigricans.
- Irregular periods.
- Weight gain.
See your health care provider if you have these symptoms. Some people can have ovarian cysts without having PCOS.
If you're told you have PCOS, ask about getting tested for type 2 diabetes and how to manage the condition if you have it. Making healthy changes such as losing weight if you have overweight and increasing physical activity can lower your risk for type 2 diabetes . These behavior changes can also help you better manage diabetes if you have it to prevent or delay other health problems.
There are medicines that can help you ovulate, as well as reduce acne and hair growth. Make sure to talk with your health care provider about all your treatment options.
Diabetes is a chronic disease that affects how your body turns food into energy. About 1 in 10 Americans has diabetes.
For Everyone
Health care providers, public health.
Type 2 Diabetes and Treatment Approaches Essay
In order to treat patients with type 2 diabetes, a comprehensive approach is essential. It involves educating the patient, assessing them for related issues, achieving near-normal blood glucose levels, and reducing the risk of cardiovascular problems through appropriate medications (Wexler & Nathan, 2022). The goals for blood sugar control must be tailored to each patient, simultaneously considering the possibility of hypoglycemia and other side effects and the expected decrease in microvascular complications over time. Generally, an A1C of ≤7 percent is a reasonable target, though it may be increased for elderly adults and those with other conditions or limited life expectancy (Wexler & Nathan, 2022). Better regulation of sugar levels in the blood has a protective effect against microvascular complications in type 2 diabetes and a beneficial effect on macrovascular outcomes.
In a clinical setting, this information can apply to patients with newly diagnosed type 2 diabetes. Healthcare providers can use this information to develop a personalized treatment plan tailored to the individual patient’s needs, goals, and health status. It is crucial to consider the patient’s age, comorbidities, and life expectancy when setting treatment goals for glycemic management. Moreover, the healthcare professional should prioritize managing cardiovascular risks, including helping people quit smoking and recommending aspirin for those with atherosclerotic cardiovascular disease or after discussing the potential benefits and risks with the patient.
Healthcare practitioners must consider the ethical, cultural, and societal consequences when managing those with type 2 diabetes. One relevant moral issue is the possibility of hypoglycemia and other unwanted treatment effects, which must be balanced with the advantages of glycemic control. Additionally, caregivers must acknowledge cultural and social aspects, like the patient’s dietary inclinations and lifestyle habits, when developing a tailored care plan. Furthermore, nurses should be mindful of disparities in healthcare access and understanding, which may significantly affect specific groups of patients. To sum up, healthcare providers must treat type 2 diabetes with consideration and awareness of each patient’s unique needs and circumstances.
The key points of treating the diabetes 2 include the following:
- Mindfulness-based interventions can improve nurse well-being by reducing stress, anxiety, and burnout while also increasing job satisfaction.
- Mindfulness-based interventions can lead to better patient outcomes such as reduced pain, anxiety, and length of hospital stay.
- The use of mindfulness-based interventions in a clinical setting should consider ethical, cultural, and social implications, as well as accessibility for nurses from diverse backgrounds.
Wexler, D. J. (2022). Initial management of hyperglycemia in adults with type 2 diabetes mellitus . UpToDate. Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 16). Type 2 Diabetes and Treatment Approaches. https://ivypanda.com/essays/type-2-diabetes-and-treatment-approaches/
"Type 2 Diabetes and Treatment Approaches." IvyPanda , 16 Feb. 2024, ivypanda.com/essays/type-2-diabetes-and-treatment-approaches/.
IvyPanda . (2024) 'Type 2 Diabetes and Treatment Approaches'. 16 February.
IvyPanda . 2024. "Type 2 Diabetes and Treatment Approaches." February 16, 2024. https://ivypanda.com/essays/type-2-diabetes-and-treatment-approaches/.
1. IvyPanda . "Type 2 Diabetes and Treatment Approaches." February 16, 2024. https://ivypanda.com/essays/type-2-diabetes-and-treatment-approaches/.
Bibliography
IvyPanda . "Type 2 Diabetes and Treatment Approaches." February 16, 2024. https://ivypanda.com/essays/type-2-diabetes-and-treatment-approaches/.
- Mindfulness and Improvement of Life
- Nursing Questions and Evidence-Based Approach
- Hypoglycemia in Diabetic Patients
- Type 2 Diabetes Mellitus and Its Implications
- Development of Comprehensive Inpatient and Outpatient Programs for Diabetes
- Reflection on the Analysis of Process Recording
- Improving Glycemic Control in Black Patients with Type 2 Diabetes
- Managing Obesity as a Strategy for Addressing Type 2 Diabetes

IMAGES
VIDEO
COMMENTS
In one study, in type 2 diabetes patients when exposed to acute stress during the postprandial period, considerable increases in blood glucose levels were observed . Apparently, treatment strategies, including stress management interventions, are a promising approach in effectively preventing or controlling the incidence of type 2 diabetes.
Diabetes puts all of these individuals at increased risk of heart disease, strokes, cancer, blindness, kidney failure, nerve damage, gangrene, and lower limb amputation. It increases cognitive ...
However, type 2 diabetes makes up more than 90% of diagnosed diabetes cases in the United States. 35 Thus, our findings largely reflect risk-factor treatment and control in those with type 2 diabetes.
Diabetes, often referred to by doctors as diabetes mellitus, describes a group of metabolic diseases in which the person has high blood glucose (blood sugar), either because insulin production is ...
Conclusion. The DCCT, the DPP, and the Look AHEAD are three interventions, which have proved that nutrition and physical activity are central in the treatment and management of diabetes among the population. The DCCT intervention aims at aiding a diabetic patient to understand how to manage body weight and blood glucose levels.
Type 2 diabetes is more prevalent than type 1 diabetes. This essay discusses some of the most frequently asked questions about type 2 diabetes through a sample dialogue between a patient and a doctor. ... Another treatment for type 2 diabetes is weight loss surgery that is recommended for obese people. This treatment has been proved effective ...
357 Diabetes Essay Topics & Examples. Updated: Feb 25th, 2024. 26 min. When you write about the science behind nutrition, heart diseases, and alternative medicine, checking titles for diabetes research papers can be quite beneficial. Below, our experts have gathered original ideas and examples for the task. We will write.
Summary and Conclusion. Diabetes is a multifactorial disease process, and its long-term management requires the active involvement of people with diabetes and their families, as well as a large multidisciplinary care team to ensure optimal health, quality of life, and productivity. Keeping up with new medications, emerging technology, and ...
With type 1 diabetes, the pancreas stops making insulin. With type 2 diabetes, the pancreas doesn't make enough insulin. And in some people with diabetes, insulin does not work well. If you don't get treatment for diabetes, high blood sugar can lead to health problems over time. These conditions include: Heart attack or stroke.
Type 2 diabetes, formerly known as non-insulin dependent diabetes mellitus, is a serious and progressive disease. It is chronic in nature and has no known cure. It is the fourth most common cause of death in most developed countries (UK Prospective Diabetes Study Group, 1998a).
An Essay On The Treatment Of Diabetes. Diabetes is an increasingly common metabolic disorder distinguished by lack of production or dysfunction of the insulin hormone, resulting in raised blood glucose levels, known as hyperglycaemia (Bailey 2015). In 2015, it was recorded that approximately 415 million individuals had diabetes worldwide, which ...
With aggressive care, there are strict guidelines to keep diabetes in check. The goal of the intensive treatment is to lower the blood glucose level by administering glucose checks by pricking fingers multiple times a day. Get Access. Free Essay: Public health emphasizes the importance of prevention and proactively taking care of one's body.
Type 2 diabetes can alter people's life drastically, changing their diet, lifestyle, activities, and health. For example, untreated diabetes can lead to many other issues such as cardiovascular problems, hypertension, kidney damage, vision impairment, hearing problems, and others (ADA, 2015). Short-term changes are also visible as type 2 ...
Diabetes Treatment and Prevention - Essay Sample. Diabetes may seem to be a serious obstacle in ones everyday life. However, there are certain ways to treat this illness, or at least, lessen its negative impact on the life of a person. In essence, there are two major ways of treating diabetes: home treatment and medical treatment.
Diabetes mellitus is a significant global public health challenge, with a rising prevalence and associated morbidity and mortality. Cell therapy has evolved over time and holds great potential in diabetes treatment. In the present review, we discussed the recent progresses in cell-based therapies for diabetes that provides an overview of islet and stem cell transplantation technologies used in ...
Life expectancy is known as the number of years a person is expected to live. At age 50, life expectancy is 6 years shorter for people with type 2 diabetes than for people without diabetes. By meeting type 2 diabetes treatment goals, life expectancy can increase by 3 years, or for some, as much as 10 years.
The 15-15 rule. If your blood sugar is low, follow the 15-15 rule: Have 15 grams of carbs, then wait 15 minutes. Check your blood sugar again. If it's still less than 70 mg/dL, repeat this process. Keep repeating these steps until your blood sugar is back up in your target range. After treating your low blood sugar, eat a balanced snack or meal ...
Prices for a month's supply of different GLP-1 drugs range from $936 to $1,349 before insurance coverage, according to the Peterson-KFF Health System Tracker. Medicare spending for three popular ...
Heart disease, diabetes and kidney disease, among the most common chronic illnesses in the United States, are all closely connected. People with diabetes are twice as likely to have heart disease or a stroke and are at risk of developing kidney disease, while heart disease is more likely for those with kidney problems as the heart works harder to pump blood to the kidneys.
Treating Diabetes. Certainly, the treatment of diabetes starts with the discovery of the condition in one's body. Once the symptoms have been discovered, treatment should follow immediately to control the disease. Until recent times, people with diabetes could only use diet, exercise, or weight control for treatment.
and morbidity. Therefore, assessing adherence is the need of the hour to tide over this crisis and hence present study was conducted Objectives: : 1. To assess the prevalence of treatment adherence among Type 2 DM patients >18years attending tertiary care hospital, Madurai 2. To identify the factors associated with treatment adherence among the study population Methodology: A cross-sectional ...
Demand for Wegovy and other GLP-1 drugs used to treat obesity and diabetes has skyrocketed, but a new report suggests that many people may not be sticking with their weight-loss treatment long enough.
Treatment. If you're told you have PCOS, ask about getting tested for type 2 diabetes and how to manage the condition if you have it. Making healthy changes such as losing weight if you have overweight and increasing physical activity can lower your risk for type 2 diabetes.These behavior changes can also help you better manage diabetes if you have it to prevent or delay other health problems.
Type 2 Diabetes and Treatment Approaches Essay. In order to treat patients with type 2 diabetes, a comprehensive approach is essential. It involves educating the patient, assessing them for related issues, achieving near-normal blood glucose levels, and reducing the risk of cardiovascular problems through appropriate medications (Wexler ...