
UCL Research Ethics
- Advice on writing an information sheet and consent form


Writing a Participant Information Sheet and Consent Form
Recruitment documents help people make informed choices about whether to participate in a research study. Find out how to write a Participant Information Sheet, example forms and further guidance.
Writing a Participant Information Sheet
Participant Information Sheets must be designed to assist participants to make informed choices. Potential recruits must be given sufficient information to allow them to decide whether or not they want to take part. The process of obtaining consent and the accompanying documentation must be approved by a research ethics committee and, where only verbal consent to research is contemplated include consideration of an appropriate process for witnessing the consent.
Researchers must take the steps necessary to ensure that all participants in the research understand the process in which they are to be engaged, including why their participation is necessary, how it will be used, and how and to whom it will be reported so that the prospective participant can make an informed decision about whether they really do want to take part.
It is highly recommended that the information provided is presented on headed paper and is accurate, clear and simple so that someone with a reading age of 8 would understand the contents (use short words, sentences and paragraphs). The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon and abbreviations, bias, coercion or any inappropriate inducements.
What should the Participant Information Sheet include:
- A friendly invitation to participate.
- A brief and simple explanation of the purposes of the research and a statement explaining how the participant was chosen and how many other participants will be involved in the study.
- A statement that participation is voluntary; refusal to participate will involve no penalty or loss of benefits to which the participant is otherwise entitled; and the participant may discontinue participation at any time without penalty or loss of benefits.
- A thorough explanation of the expected duration of participation in the research and the procedures to be followed.
- A description of any reasonably foreseeable risks or discomforts and any benefits to the participant. For research involving more than minimal risk, an explanation as to whether any compensation or any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained.
- A statement describing the extent, if any, to which confidentiality of records identifying the participant will be maintained.
- It is considered good practice for researchers to debrief participants at the conclusion of the research and to provide them with copies of any reports or other publications arising from their participation.
- If appropriate, a statement indicating that the data might be used for additional or subsequent research.
- An explanation of who to contact for answers to pertinent questions about the research and the rights of the participant and who to contact in the event of a research-related injury to the participant.
- If applicable, a statement declaring that each researcher who may have access to children (aged under 18) or vulnerable adults has undergone a satisfactory criminal records check.
- Remember to thank your participant for considering taking part in the study and include a statement indicating that the research study has been approved by the UCL Research Ethics Committee.
Language and layout
It is highly recommended that the information provided is presented on headed paper and is accurate, clear, and simple. The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon, and abbreviations, bias, coercion, or any inappropriate inducements.
The following points should be considered when writing an information sheet:
- Use clear, non-technical language. We recommend that you refer to the Plain English Campaign
- Use appropriate language for the target audience. For example, consider the different ways needed to communicate with primary school children as opposed to their teachers, or people with expertise in the area of study as opposed to people with no such knowledge
- Divide the text into paragraphs for ease of reading
- Consider using sub-headings for clarity, such as questions and answers
- Make sure the font and font size are legible.
Ask someone else to review your information sheet before it is circulated.
- Template Participant Information Sheet (Word)
- Template Consent Form (Word)
- Guidance on obtaining consent from research participants online (for online and in-person study designs)
Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL
- Recording & Obtaining Consent
UCL Research Ethics Committee Guidance Note 2: Extract from Nuffield Council on Bioethics website
Page last updated: April 2023

- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search
IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults

Informed Consent and Consent Forms for Research Participants
Informed consent is a communication process by which researchers reach agreement with people about whether they wish to participate in research. Confusing informed consent with a signed consent form may violate the ethical intent of informed consent, which is to communicate clearly and respectfully, to foster trust, comprehension, and good decision making, and to ensure that participation is voluntary.
Consent forms are often written in “legalese” and are long, complex, and often inappropriate to the culture or language of the potential subject, insulting, and virtually impossible for most people to comprehend. They convey to some the impression that signing such a formal-looking document commits them to participation. Among subjects who willingly sign documents, most sign the consent form without reading it.
How has this come to pass? Early concern with ethics of human research was about biomedical research and focused on the necessity of obtaining informed consent. Over the decades, the elements of informed consent have grown in number, as has the idea that informed consent is a form that is to be signed by the subject. According to the Federal Regulation of Human Research 46.117(a):
Except as provided in paragraph (c) of this section, informed consent shall be documented by the use of a written consent form approved by the IRB and signed by the subject or the subject’s legally authorized representative. A copy shall be given to the person signing the form.
However, many researchers and the Institutional Review Boards that govern their research fail to recognize that 46.117(c) provides for a waiver of signed consent forms:
(c) An IRB may waive the requirement for the investigator to obtain a signed consent form for some or all subjects if it finds either: (1) That the only record linking the subject and the research would be the consent document and the principal risk would be potential harm resulting from a breach of confidentiality. Each subject will be asked whether the subject wants documentation linking the subject with the research, and the subject’s wishes will govern, or (2) That the research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside of the research context.
The reason for obtaining a signed consent form has always been much more to protect the researcher and the institution than to serve the interests of the research subject. In case the subject claims later that consent was inadequate or omitted, the researcher can counter by showing the form. Recently, the Office of Human Research Protection has imposed highly publicized and costly sanctions against a few research institutions. Understandably, IRBs and research administrators consider it in their self-interest to make highly conservative decisions. Since IRBs must take steps to justify waiving documentation of informed consent by deeming the research to be minimal risk, many consider it safer not to do so, fearing that such an action might leave them open to questions by the OHRP. Thus, the reason for obtaining a signed consent form is typically to protect the institution, not the subject. Researchers, science, institutions, subjects, and IRBs would all be better off if they made intelligent interpretations of the requirements of the Common Rule.
The Social and Behavioral Sciences Working Group has made various recommendations based on the Common Rule, designed to guide social and behavioral researchers and IRBs out of such conundrums. The authors, both members of the Working Group, developed recommendations concerning informed consent, some of which are summarized here:
1. Informed consent should take the form of an open, easily understood communication process. Typically, this means a friendly verbal exchange between researcher and subject, with a written summary of the information for the subject to keep, as appropriate. (The copy for the subject to keep would be inappropriate if the written record of the subject’s participation could be damaging to the subject, as when the research is about domestic violence, or illegal behavior). Both the verbal and written discussion should be brief, and simply phrased at such a level that all of the subjects can understand it.
2. Subjects must receive enough easily understood, accurate information to judge whether the risk or inconvenience involved is at a level they can accept. The responsibility rests with the investigator to describe any risks accurately and understandably. There are many kinds of minor or everyday risks or inconveniences that most persons would gladly undertake if it were their choice to do so, but which they would not wish to have imposed upon them unilaterally. However, some may make a rational decision that the experience would be too stressful, risky, or unpleasant for some idiosyncratic reason that applies to them and not to other subjects.
3. Especially when the research procedure is long and complex, the researcher must make it quite clear that the subject is free to ask questions at any time. Informed consent, as a conversation (not a form), needs to be available throughout the research, as subjects do not necessarily develop questions or concerns about their participation until they are well into the research experience. For example, a discussion of confidentiality may not capture subjects’ attention or comprehension until they are asked some quite personal questions in the ensuing research experience.
4. When subjects can readily refuse to participate by hanging up the phone or tossing out a mailed survey, the informed consent can be extremely brief (a sentence or two). Courtesy and professionalism require that the identity of the researcher and research institution be mentioned, along with the nature and purpose of the research. However, if there are no risks, benefits, or confidentiality issues involved, these topics and the right to refuse to participate need not be mentioned, as such details would be gratuitous and might decrease participation by implying greater risk that actually exists. If the researcher has any connection with the institution at which the subjects receive health care or other essential services, it is necessary to mention the right of the research subject to refuse or withdraw without prejudice. Such rights may be honored implicitly by making it clear that you are asking their permission to involve them as research subjects.
5. Verbal informed consent need not be detailed and written consent is not appropriate when the research is not concerned with sensitive personal information and when subjects are peers or superiors of the researcher.
6. The cultural norms and life-styles of subjects should be considered when deciding how to approach informed consent. For example, research on homeless injection drug users should probably be preceded by a several week-long process of “hanging out” and talking with them. The resulting informal communication will raise issues they wish to discuss with the researcher. The conditions under which the research is conducted can then be negotiated orally between the researcher and the community members, as appropriate. Written documents and signed forms would expose subjects to risk of arrest and serve no redeeming purpose.
7. A wide range of media are appropriate for administering informed consent. Video tapes, brochures, group discussions, web sites, community newsletters, and the “grape vine” can be more appropriate ways of communicating with potential subjects than the potentially confusing formal consent forms that often are used.
8. When written or signed consent places subjects at risk, it must be waived. There are times when the written record is the only evidence that the subject has participated in a study in which there is acknowledgement or appearance of situations that would place the subject at risk.
9. When it is important to have some record of the informed consent but when written or signed consent would place the subject at risk or be difficult for the subject to read, one useful procedure is to have a trusted colleague witness the verbal consent.
10. Community consultation, or meeting with community leaders of the potential subjects, is a useful way to plan research that is likely to raise sensitive questions among those to be studied and members of their community. This is not a substitute for individual informed consent, but often clears the way for potential subjects to be ready to decide whether to participate.
11. In certain circumstances, persons are not in a position to decide whether to consent until immediately after their participation, e.g., in brief sidewalk interviews, which persons are likely to welcome.
12. Some research cannot validly be conducted if all details are disclosed at the outset. The alternatives to outright deception of subjects are to a) obtain permission to provide only a description of what the subject will experience, with an agreement that the full details of the study will be disclosed afterward; b) obtain permission to engage in concealment or deception with the understanding that pilot research has shown that peers of the subject do not find such concealment or deception objectionable and that a full explanation will follow their participation, c) explain that the subject might be enrolled in one of several possible conditions and to gain permission to disclose in which of these the subject was actually enrolled after his or her participation is completed.
Author’s Note: The Social and Behavioral Sciences Working Group (formerly a part of the National Human Research Protections Advisory Council but now an independent body) chaired by Felice Levine helped to develop these ideas.
Reference Melton, G., Levine, R. J., Koocher, G., Rosethal, R., & Thompson, W. (1988). Community Consultation in Socially Sensitive Research: Lessons from Clinical Trials on Treatments for AIDS. American Psychologist , 43, 573-581.
APS regularly opens certain online articles for discussion on our website. Effective February 2021, you must be a logged-in APS member to post comments. By posting a comment, you agree to our Community Guidelines and the display of your profile information, including your name and affiliation. Any opinions, findings, conclusions, or recommendations present in article comments are those of the writers and do not necessarily reflect the views of APS or the article’s author. For more information, please see our Community Guidelines .
Please login with your APS account to comment.
About the Authors
Joan Sieber is professor of psychology at California State University, Hayward. She received her bachelor's, master's, and doctorate from the University of Delaware. Robert J. Levine is professor of medicine and co-chair of the interdisciplinary bioethics project at Yale University. He is also the founding editor of IRB: A Review of Human Subjects Research.

Making a Career Choice: Follow in Your Own Footsteps
In a guest column, APS Fellow Barbara Wanchisen shares observations and ideas on broadening career opportunities for psychological scientists.

Student Notebook: Finding Your Path in Psychological Science
Feeling unsure or overwhelmed as an early-career psychology student? Second-year graduate student Mariel Barnett shares advice to quell uncertainties.

Matching Psychology Training to Job Market Realities
APS President Wendy Wood discusses how graduate programs can change the habit of focusing on academic-career preparation.
Privacy Overview
Template participant consent form and participant information sheet
Providing information about the research to participants, and gaining consent from participants before their involvement is a critical part of conducting ethical research.
Participant information sheet
All participants need to be provided access to an information sheet, and to understand the full details of the research, and how they will be involved. Please use the following template:
- Template participant information sheet
- Template participant information sheet veterinary research
Participant consent form
Before research begins, it is important to first obtain participant’s consent on the basis of their full and proven understanding of what the research will entail. Please use the following template:
- Template participant consent form
These templates should be followed as far as possible, as these have been developed using national guidance and expert input from Committee members and Lay Members. However, there may be times when it is appropriate to deviate from the templates in order to meet the needs of a specific research population.
The Health Research Authority and UK Research and Innovation webpages contain further guidance and templates on good practice when consenting participants.
Good practice in consenting participants
You should consider innovative ways of providing consent that are appropriate to your research population, for example, in addition to participant information and consent forms, could you provide the information using visual methods, such as a recorded video, or a study leaflet. Could you develop your forms in partnership with the communities who will take part in the study?
Back to: Research

Study at Cambridge
About the university, research at cambridge.
- Undergraduate courses
- Events and open days
- Fees and finance
- Postgraduate courses
- How to apply
- Postgraduate events
- Fees and funding
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Term dates and calendars
- Visiting the University
- Annual reports
- Equality and diversity
- A global university
- Public engagement
- Give to Cambridge
- For Cambridge students
- For our researchers
- Business and enterprise
- Colleges & departments
- Email & phone search
- Museums & collections
- Cambridge Psychology Research Ethics Committee
- School of the Biological Sciences
- About the School
- School Leadership
- Meet the School Office staff
- Spotlight on...
- Public Engagement overview
- ‘Changing Pathogens in a Changing World’ at the Cambridge Festival 2023
- Council of the School of the Biological Sciences overview
- Sub-committees overview
- G C Grindley Fund
- Cambridge Human Biology Research Ethics Committee overview
- Application form
- Cambridge Psychology Research Ethics Committee overview
- Participant information sheets and consent forms
- Ethical review process: outline
- Terms of reference
- Does my study need ethical review?
- Faculty of Biology
- Postgraduate School of Life Sciences
- Departments overview
- Department of Biochemistry
- Department of Genetics
- The Gurdon Institute
- MRC Toxicology Unit
- Department of Pathology
- Department of Pharmacology
- Department of Physiology, Development and Neuroscience
- Department of Plant Sciences
- Department of Psychology
- Sainsbury Laboratory
- Department of Veterinary Medicine
- Department of Zoology
- Wellcome - MRC Cambridge Stem Cell Institute
- Undergraduate Study
- Postgraduate Study
- MPhil in Biological Sciences overview
- Developmental Biology - Course Structure
- Developmental Biology - Participating Research Groups
- Student Testimonials
- Widening Participation
- Research overview
- Research Themes overview
- Microbiome research for human and planetary health
- Grand Challenge Topics
- Molecules and Cells: The Building Blocks of Life
- Infection and Immunity overview
- Changing pathogens in a changing world
- Seeing infection through a new lens
- Neuroscience, Psychology and Behaviour overview
- Mental health and illness across scales, species, and society
- Functional and Evolutionary Genomics overview
- Network of Life
- Reproduction, Development and Lifelong Health overview
- Complex tissue regeneration across scales and systems
- Extending healthy lifespan
- Organisms, Evolution and Ecology overview
- Life at the Extremes
- Molecular Biology for Climate Change
- Participatory Research
- Research Fellows: Support and Development overview
- Parke Davis Exchange Fellowship
- Training and development opportunities
- Research Policies and Ethics
- Bioscience Impact
- Facilities overview
- Bioinformatics and Computing
- Biological Sciences Libraries
- Collections
- Flow Cytometry
- Structural Biology and Biophysics
- Trace Element Analysis
- 'Facility Fridays' Seminar Series
- Culture and Inclusion overview
- Research Culture overview
- Research Culture Champions
- Equality, Diversity and Inclusion overview
- Equality, Diversity and Inclusion Champions
Athena Swan
- Roving Researcher Scheme
- Biological Sciences Early PI Network
- Support for Research Fellows
- Inclusion Networks and Support Initiatives
- Mentoring Schemes
- Public Engagement
- For Professional Services
- Council of the School of the Biological Sciences
- Cambridge Human Biology Research Ethics Committee
Participant Information Sheets and Consent Forms are important aspects to the organisation and conduct of a study. The participant Information Sheet gives potential participants the necessary understanding for the motivation and procedures of the study and sources of information to answer any further questions to allow them to give informed consent. A Consent Form essentially reprises this information to ensure the key points are understood and then records this understanding, usually with a signature. Consent may also be recorded electronically, for example through web-forms by clicking a button. More than one Consent Form may be needed (for adults and children separately, for example). Consent Forms are usually in addition to Participant Information Sheets.
Participants aged 18 years and under generally require consent from parents or carers, and therefore Participant Information Sheets and Consent Forms are addressed to them in these circumstances. However, it might be appropriate to make a Participant Information Sheet and Assent Form for child participants using age-appropriate language.
Participant Information Sheets
The Participant Information Sheet (PIS) should be a clear and simple document, on headed paper with the University Crest (or equivalent for other institutions), that would be easily understood by those to whom it is aimed; for example, it should be age-appropriate. It should be a concise document; the length and design should encourage a potential participant to read it in full.
There is no set format, however investigators may like to consider including the following sections:
Use a simplified title if the original title would be too technical
Invitation paragraph
A brief introduction; for example: Before you decide to take part in this study it is important for you to understand why the research is being done and what it will involve. Please take time to read the following information carefully and discuss it with others if you wish. A member of the team can be contacted if there is anything that is not clear or if you would like more information. Take time to decide whether or not you wish to take part.
Purpose of the study
State the background and aim of the study. When will the study be completed?
Why have I been chosen?
Explain why the potential participant has been approached.
Do I have to take part?
Explain that taking part is entirely voluntary and that refusal or withdrawal will involve no penalty or loss, now or in the future.
What will happen to me if I take part?
Say where the assessments will take place, how many will there be, how long the assessments will be each time and what exactly will happen. What are the participant’s responsibilities? Set down clearly what you expect of them.
What do I have to do?
Make clear if there are any lifestyle restrictions as a result of participating.
Make clear if video or audio taping will be used and if so, say when they will be destroyed. For example: Tapes will be identified only by a code, and will not be used or made available for any purposes other than the research project. These tapes will be destroyed at the end of the study.
Are there possible disadvantages and/or risks in taking part?
Describe any reasonably foreseeable discomforts, disadvantages and risks.
What are the possible benefits of taking part?
Any benefits to the participants that can reasonably be expected should be stated. However, where there is no intended benefit to the participant from taking part in the project this should be stated clearly. It is important not to exaggerate the possible benefits to the particular participant during the course of the project. This could be seen as coercive.
Will my taking part in this project be kept confidential?
The participant’s permission will be needed to allow restricted access to information collected about them in the course of the project. You should explain that all information collected about them will be kept strictly confidential and briefly described how this will be ensured. For example: All data will be identified only by a code, with personal details kept in a locked file or secure computer with access only by the immediate research team.
Please include the following link to general information about how the University uses personal data: https://www.information-compliance.admin.cam.ac.uk/data-protection/research-participant-data .
Bear in mind that investigators are responsible for ensuring that when collecting or using data, they are not contravening legal or regulatory requirements.
What will happen to the results of the research project?
You should be able to tell the participants what will happen to the results of the research. When are the results likely to be published? Where can they obtain a copy of the published results? Will they be told which arm of the project they were in? You might add that they will not be identified in any report or publication. For example : Results will be presented at conferences and written up in journals. Results are normally presented in terms of groups of individuals. If any individual data are presented, the data will be totally anonymous, without any means of identifying the individuals involved.
Depending on the nature of your proposed project, you may need to include a statement indicating that the data collected during the course of the project might be used for additional or subsequent research.
Who is organising and funding the research?
Name the organisation or company sponsoring or funding the research.
Ethical review of the study
Example text: The project has been reviewed by the University of Cambridge Psychology Research Ethics Committee.
Contact for further information
You should give contact details of a named investigator for further information and what to do next should they want to take part.
Consent Forms
A Consent Form should be no longer than one side of A4. It should be formatted on headed paper with the University Crest (or equivalent for other institutions) and clearly stating the title of the study.
The Consent Form concisely covers the main points of the Participant Information Sheet phrased as statements with which potential participants can agree or disagree. You could add a space for initials or yes/no deletions.
Some example statements include:
- I confirm that I have read and understand the Participant Information Sheet
- I have had the opportunity to ask questions and had them answered
- I understand that all personal information will remain confidential and that all efforts will be made to ensure I cannot be identified (except as might be required by law)
- I agree that data gathered in this study may be stored anonymously and securely, and may be used for future research
- I understand that my participation is voluntary and that I am free to withdraw at any time without giving a reason.
- I agree to take part in this study
Participant’s signature
Make a space for the participant to sign, print their name and date. If parents or carers are consenting for a child, then a space to print the child’s name is also needed.
Spaces for the investigator taking consent to sign, print their name and date can also be included.
Postal Address: School of the Biological Sciences 17 Mill Lane Cambridge CB2 1RX Information provided by: [email protected]
About this site
Privacy policy
The School holds an Athena Swan Bronze Award
athena_swan_bronze_award_logo_for_website_footer.jpg

Connect with us
x_for_website_50_x_50.png
© 2024 University of Cambridge
- Contact the University
- Accessibility
- Freedom of information
- Privacy policy and cookies
- Statement on Modern Slavery
- Terms and conditions
- University A-Z
- Undergraduate
- Postgraduate
- Research news
- About research at Cambridge
Template documents
The University has developed template participant documents based on key ethical issues and legal requirements.
These outline the key information which must be relayed to participants for all research involving human participants, their data or samples. They provide a template for how this information can be presented when using a document format (see the guidance on consent for information on alternative formats).
These templates (or the information, if presented in an alternative format) should be tailored to your study, participants and their accessibility requirements (see 'accessibility resources' below) - modifiable content is in red text, and guidance is in blue text.
In the templates there are three types of text:
- Red text - text which you should adjust to suit your study
- Blue text - provides guidance on using the template
- Black text - core information which must usually be included.
If you need to adjust the black text you must provide details and justification in Q31 of the ethical review application form (Word) .
Participant advertisement
The participant advertisement (Word) or an alternative containing the same information must be used for projects where adverts will be used to recruit participants (displayed physically, or sent via post or email). Also consider the University's guidance on branding .
Participant information sheet (PIS)
The participant information sheet (PIS) (Word) , or an alternative containing the same information, must be used for all research involving living human participants.
- provides key information about the project
- helps to ensure consent is fully informed
- helps to ensure consent is freely given
- contains statements on use of participants’ data in compliance with data protection law.
It is essential that participants are given:
- a copy of the PIS that they can retain such as a hardcopy or downloadable PDF (if participants can read)
- time to consider the participant information and decide whether to take part.
There may be some instances where a PIS and consent form (in any format) are not appropriate, for example when accessing large external datasets via a 'gatekeeper'. If you feel this applies to you, discuss this with your School ethics committee and provide justification in Question 31 of the ethical review application form.
Anonymous participants
If you are conducting a study where the participants will be anonymous from the outset (such as an online questionnaire) you can use the Participant information sheet (anonymous) (Word)
Consent form
The consent form (Word) , or an equally robust consent process, must be used for all research projects involving living human participants.
Written consent is considered the 'gold standard'. In limited circumstances an alternative such as oral consent may be appropriate but this must be justified in the ethics application - see the guidance on consent .
Using the consent template ensures and evidences that participants have explicitly provided consent:
- to participate in your project
- in full knowledge of the nature of the project.
It also ensures and provides evidence that participants have consented explicitly to audiovisual recordings or photographs, future contact from researchers, or other optional components. If possible, you should allow individuals to participate even if they decline optional components.
The consent form contains sections on the use of participant data, ensuring compliance with data protection law.
There may be some instances where a participant information sheet and consent form (in any format) are not appropriate, for example when accessing large external datasets via a 'gatekeeper'. If you feel this applies to you, discuss this with your School ethics committee and provide justification in Question 31 of the ethical review application form.
The participant debrief (Word) , or an equivalent debriefing process, must be used for projects that involve:
- withholding of information
- where the participant is likely to experience distress.
You must give the contact details of any support services or organisations that the participant may find helpful. The debrief should usually be in a format that participants can retain, such as a hardcopy or pdf but may be supplied in an alternative format if literacy or accessibility needs require.
For other projects, the participant debrief (Word) is not mandatory but can be helpful to:
- ensure participants know that their contribution is valued
- remind participants of the aims of the research
- remind participants of the contact details of the researcher
- detail how participants can access the findings of the research when the project has completed.
Participant information and consent for child participants
When your research involves child participants, please use the age-appropriate versions of the participant information sheet (PIS) and consent forms as indicated below (PG indicates 'parent or guardian'). Consider the guidance above on 'normal' templates and alternative formats.
Both parent (or guardian) and child should be given information about the study and provide consent (parent and older children) plus ongoing assent (children). Assent may be verbal for younger children. For very young or non-verbal children researchers must describe in their ethics application how they will ensure ongoing assent and identify any signs of discomfort or a child's wish to discontinue participation.
These age groups are provided for guidance; however, researchers should consider which participant information and consent options are most appropriate for their participants (for example, due to literacy or comprehension levels).
Researchers working with child participants should seek guidance from the Child Panel representative, Barbara Dritschel ( [email protected] ).
Child aged 0 to 8 years
Participant information:
- Parents or guardians should be given the PIS - PG (0-12y) (Word) .
- The child should be given information verbally.
- Parents or guardians should be given the consent form - PG (0-12y) (Word) .
- The child’s ongoing assent should be ensured throughout the research activity.
Child aged 8 to 12 years
- The child should be either given the information verbally, or using the PIS - child (12-16y) (Word) , depending on their literacy and comprehension levels.
Child aged 12 to 16 years
- Parents or guardians should be given the PIS - PG (12-16y) (Word) .
- The child should be given the PIS - child (12-16y) (Word) .
- Parents or guardians should be given the consent form - PG (12-16y) (Word) .
- The child should be given the consent form - child (12-16y) (Word) and their ongoing assent ensured throughout the research activity.
Letter to school
The template letters to schools must be used when planning research within a school, such as with pupils.
There are two template letters to reflect the process of gaining approval:
- The letter to school - agreement in principle (Word) is to establish with the school whether they agree, in principle, to participate. You will need this to apply for ethical and Local Education Authority (LEA) approval.
- The letter to school - study commencing (Word) should be used, once you have obtained ethical and LEA approval, to confirm that the school is still willing to participate, and to arrange the practicalities of the research.
Both letters include summary information about the project and what the participants, and the school, will be required to do.
Letter to parent or guardian
The letter to parent or guardian (Word) , or an alternative format if needed due to accessibility or literacy requirements, must be used when planning research with participants under the age of 16 years.
The letter includes summary information about the project and what the participant, and the parent if appropriate, will be required to do.
Accessibility resources
Researchers may find some of the following resources useful when considering accessibility and readability of participant documents.
Researchers should also consider the sections above on participant information sheets and consent forms for children. If researchers need to use a simplified version of the legal basis statement for the participant information sheet, they should use the version included in the template PIS - child (12-16y) (Word) as this was developed in collaboration with the University's Data Protection Officer to ensure it meets the University's obligations regarding GDPR.
University pages
Equality, diversity and inclusion
Diversity online training
Digital standards (this contains a variety of resources on accessibility, content standards and house style that are primarily aimed at University web pages and digital content but which can also be useful when thinking about participant information or where participants will be recruited via a web page).
Tools to assess readability
Hemingway Editor
SMOG (simplified measure of gobbledygook) calculator
Microsoft Word - get your document's readability and level statistics
Readability Formulas
Resources for suggested wording, styles and formatting
The British Dyslexia Association style guide
The Plain English Campaign
Microsoft Word - make your Word documents accessible to people with disabilities
Other tools and resources
Microsoft Office 365 - accessibility checker
We Are Colorblind - resources for colour blind friendly designs
KnightLab at Northwestern University - blog on colour blind friendly graphics for reporting data
Our websites may use cookies to personalize and enhance your experience. By continuing without changing your cookie settings, you agree to this collection. For more information, please see our University Websites Privacy Notice .
Neag School of Education
Educational Research Basics by Del Siegle
Research ethics and informed consent.
As researchers, we are bound by rules of ethics. For example, we usually cannot collect data from minors without parental or guardian permission. All research participants must give their permission to be part of a study and they must be given pertinent information to make an “informed” consent to participate. This means you have provided your research participants with everything they need to know about the study to make an “informed” decision about participating in your research. Researchers must obtain a participant’s (and parents’ if the participant is a minor) permission before interacting with the participant or if the participant is the focus of the study. Generally, this permission is given in writing; however, there are cases where the research participant’s completion of a task (such as a survey) constitutes giving informed consent. Research participants have the right to refuse to participate without penalty if they wish. Each university that receives federal funds (and most do) must have an Institutional Review Board (IRB) that reviews all research conducted at the university. Therefore, anyone doing research associated with the university must submit and receive IRB approval before beginning research. Even if the research is exempt from a full review by the IRB, an Exemption Form must be filed and approved by the Department chair and submitted and reviewed by the IRB.
Researchers are bound by a code of ethics that includes the following protections for subjects
- Protected from physical or psychological harm (including loss of dignity, loss of autonomy, and loss of self-esteem)
- Protection of privacy and confidentiality
- Protection against unjustifiable deception
- The research participant must give voluntary informed consent to participate in research. Guardians must give consent for minors to participate. In addition to guardian consent, minors over age 7 (the age may vary) must also give their consent to participate.
NOTE: Voluntary informed consent means that the person involved should have legal capacity to give consent; should be so situated as to be able to exercise free power of choice, without the intervention of any element of force, fraud, deceit, duress, over reaching, or other ulterior form of constraints or coercion; and should have sufficient knowledge and comprehension of the elements of the subject matter involved as to enable them to make an understanding and enlightened decision. This latter element requires that before the acceptance of an affirmation decision by the participant there should be made known to them the nature, duration, and purpose of the experiment; the method and means by which it is to be conducted; all inconveniences and hazards reasonably to be expected; and the effects upon their health or person which may possibly come from their participation in the study.
The consent form that study participants sign should cover the following main points:
- It should tell the participants what they are being asked to do, by whom, and for what purpose. Participants must know the identity of the researcher, his or her affiliations if any, and whom to contact for information if they have problems with the research process. This not only includes contact information for the researcher, but also contact information for the university IRB.
- It should inform the participants of any risks they might be taking by participating in the research.
- It should inform the participants what rights they have in the process, particularly the right of review of material and the right to withdraw from the process.
- It should indicate whether or not participants’ names will be used in the study, whether any other names will be used, or whether pseudonyms will be substituted.
- It should indicate how the results of the study will be disseminated and whether participants can expect to benefit in any way, monetarily or otherwise, from participating in the study.
- It should indicate that participants are free to participate or not participate in the research without prejudice to them.
- In the case of children, it must be signed by the child’s legal guardian. Children cannot be expected to give total informed consent.
- The consent form should be written in the second person (e.g., “You have the right to …”) and in easy to understand language.
THE UNIVERSITY OF CONNECTICUT gives assurance that it will comply with the Department of Health and Human Services (DHHS) regulations for the protection of human research subjects and has set up an Institutional Review Board to review all research associated with the University. Researchers (including student researchers) are required to file a IRB prior to conducting research. Certain types of studies qualify for exempt or expedited review. Research involving minors SELDOM qualifies for exempt status . Even if a study qualifies for exempt status, the researcher must still file a IRB with the Department Head and submit it to the IRB.
Note: Exempt and expedited studies that are not DoJ-funded or subject to FDA regulations must complete a short study status report every year. Full board review and expedited studies that are DoJ-funded or subject to FDA regulations must complete the continuing review process. Therefore, if a research project extends beyond one year, the project must be reviewed each year by the institutional review board as long as data are being collected. If a researcher changes any aspect of a study (including adding or changing questions on a survey) an amendment must be filed and approved by the IRB before using the survey. If a researcher plans to enroll more participants than he or she indicated in the initial IRB, an amendment must be filed and approved by the IRB before enrolling the additional participants. If a researcher wishes to change any of the research procedures that were approved in the approved IRB, and amendment must be filed and approved by the IRB before those changes are made. If a researcher has completed data collection and is only analyzing data and writing the research results, then IRB renewals are no longer required. Informed consent must also be given for interviews. Informed consent can be given verbally, provided there is a witness.
The following criteria are often considered by Institutional Review Boards for the Protection of Human Subjects:
- Are risks greater than “minimal risk”*?
- Are risks minimized?
- Are risks reasonable in relation to the benefits?
- Is subject selection equitable (e.g., subject population included or excluded; risk of coercion in recruitment, etc.)
- Is the process for obtaining consent appropriate?
- Is informed consent appropriately documented?
- Is there adequate provision for monitoring the data collection to insure safety of the subjects?
- Are the provisions for protecting privacy adequate?
- Are the provisions for maintaining confidentiality adequate?
- Have additional safeguards for subjects vulnerable to coercion or undue influence been included?
- Is “annual” continuing review sufficient?
- Do the research staff/investigators have appropriate expertise to perform their responsibilities in the study?
*”minimal risk” means that the probability and magnitude of harm or discomfort anticipated in the research are no greater in and of themselves from those ordinarily encountered in daily life or during the performance of routine physical or psychological examination or tests.
Heightened Awareness of Problems with Unethical Research
In 1966 Dr. Henry Beecher, an anesthesiologist, wrote an article for the June 16, 1966 New England Journal of Medicine called “Ethics and Clinical Research”. In it he described 22 examples of research studies with controversial ethics that had been conducted by reputable researchers and published in major journals. He noted that “unethical or questionable ethical procedures are not uncommon.” Beecher’s article played an important role in heightening the awareness of researchers, the public, and the press to the problem of unethical human subjects research.
Establishment of the National Research Act The publicizing of the Public Health Service Syphilis Study at Tuskeegee (1932-1971) led to the establishment of the National Research Act of 1974 which created a national commission that ultimately issued the Belmont Report (1979). The Belmont Report outlined three basic ethical principles. The TUSKEGEE SYPHILIS STUDY involved 399 African-American men with latent syphilis who were not told by researchers there was a cure for the disease. This was done so the researchers could study the long-term effects of the disease. The Tuskegee syphilis study, coupled with abuses reported in the NUREMBERG TRIALS indicated that researchers and the research they conduct needed to be monitored.
Three Basic Ethical Principles Outlined in the Belmont Report
Respect for Persons (Treat individuals as autonomous human beings, capable of making their own decisions and choices, and do not use people as a means to an end) Three Requirements Based on Respect for Persons
– obtain and document informed consent – respect the privacy interests of research participants – consider additional protection when conducting research on individuals with limited autonomy
Beneficence (Minimize the risks of harm and maximize the potential benefits) Five Requirements Based on Beneficence
– use procedures that present the least risk to participants consistent with answering the scientific question – gather data from procedures or activities that are already being performed for non-research reasons – risks to subjects should be reasonable in relation to both the potential benefits to the participants and the importance of the knowledge expected to result – maintain promises of confidentiality – monitor the data to ensure the safety of participants
Justice (Treat people fairly and design research so that its burdens and benefits are shared equitably) Two Requirements Based on Justice
– select participants equitably – avoid exploitation of vulnerable populations or populations of convenience
Rationale for an Institutional Review Board (IRB) The ethical principles and federal regulation generated by the Belmont Report provide a framework for IRBs to evaluate research involving human subjects. An objective review of research is necessary because
- highly motivated people tend to focus on their goals and may unintentionally overlook other implications or aspects of their work and
- no one can be totally objective about his or her work.
The IRB review system is designed to provide an independent, objective review of research involving human subjects so that the privilege of conducting human subjects research may be maintained.
Only activities that meet the definition of research with human subjects need review by an Institutional Review Board (IRB).
Research is a
- systematic investigation (this might range from applying scientific methodology involving independent and dependent variables to an ethnographic study of a community)
- including research development, testing, and evaluation (this also includes pilot studies, feasibility studies, and other preliminary studies)
- designed to develop or contribute to generalizable knowledge (An essential consideration is whether it is the intention of the investigator to contribute to generalizable knowledge).
Some activities that involve interactions with humans and data gathering may not fit the definition of research with human subjects, since they are designed to accomplish something else, such as in-house quality improvement. For example, a survey of college students about their university’s counseling services may be designed to improve the service delivery for students on campus. Publication of the results is sometimes used as a measure of whether research is generalizable, but this is too narrow a measure for two reasons. First, not every study will produce results worthy of publication. Second, there are other ways that results can be made available to others. They may be presented at a conference. They may be shared with colleagues through the Internet, appear in a dissertation, provided to Board members in a project report, or archived for future research).
A human subject is a “living individual about whom an investigator (whether professional or student) conducting research obtains:
- Data through intervention or interaction (does not need to be face-to-face, could be via email or a participant observation) with the individual or
- Identifiable private information” (a) information about behaviors that occur in a context where the individual can reasonably expect that no observations or recording is taking place or b) information that is provided for a specific purpose and for which the individual can reasonably expect will not be made public).
Once the IRB approves a protocol, it must be reviewed at least annually (every 12 months) until data collection is complete, although IRBs may specify a shorter review period. The extent of the yearly review will vary depending on the research. Amendments and changes to approved protocols must be approved prior to their implementation. An exception could occur if a revision to approved procedures was in response to an unanticipated risk and had to be implemented immediately for the health or well-being of the subjects. Such revisions must be reported promptly to the IRB, not when the research is completed.
The federal regulations governing research with human subjects, which have been adopted by numerous federal departments and agencies, are often referred to as the Common Rule, which is modified from time to time. They were first written by the Department of Health and Human Services (DHHS). The DHHS regulations are often referred to as 45 CFR 46. Pregnant women, fetuses, neonates, children, and prisoners are considered vulnerable populations and are provided additional protection in the DHHS regulations . The DHHS regulations do not have specific additional protections for the elderly, for mentally disabled persons, or for persons whose decision-making capabilities are impaired. Investigators may consider and the IRB may require additional safeguards for these populations. The Common Rule does not include requirements for formatting protocols for IRB review. Formatting requirements are institution specific. Most institutions decide to apply the Common Rule to all research with human subjects, regardless of the funding source.
Research is eligible for expedited review when it poses no more than minimal risk (minimal risk means that “the probability and magnitude of harm or discomfort anticipated in the research are not greater…than those ordinarily encountered in daily life…”) to the participants and when all the activities fall within the categories identified as eligible.
Research is only eligible for exemption if all the activity associated with the research fall into one of six categories of activities described in federal regulations. Three of these are frequently used by social and behavioral scientists:
- Research conducted in established or commonly accepted educational settings, involving normal educational practices
- Research involving survey procedures, interview procedures, or observations of public behavior providing that any disclosure of identifiable information outside the research setting would not place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation (Note: By institution choice, interviews with children and participant observation with children may not be exempt).
- Research involving the collection or study of existing data (collected prior to the research for purposes other than the research) if the data is publicly available or recorded by the investigator in such a manner that the subjects cannot be identified.
The regulations do allow some research with children to be exempt (although institutional policy may not). The duration of the study and the experience of the investigator are not criteria for determining eligibility for exemption.
IRBs must either have sufficient expertise among their members or seek expertise through consultation if the members are not familiar with a methodology or population under consideration. They must have the expertise and professional competence to evaluate research activities commonly conducted by their institution. The primary purpose of the IRB is to protect the rights and welfare of research subjects. An IRB must exercise all of its authorities in order to do so, including monitoring research when appropriate. Although IRBs serve their institutions, they do not represent the interests of their institutions. According to federal regulations, institutional officials may not override an IRB disapproval of a protocol.
While physical risks are minimal in social and behavioral science research, risks associated with participation in social and behavioral science research are often more elusive and less predictable. Every interaction in a research context is a communication of some sort, and communications can go awry. For example, notification by mail to set up a follow-up appointment for a participant in a research study may result in an inadvertent breach of confidentiality.
Risks in social and behavioral sciences generally fall into three categories (in rare circumstances the risk may be physical such as a study of victims of domestic violence who may become the victims of retaliatory violence):
- Invasion of Privacy – This can occur if personal information is accessed or collected without the subjects’ knowledge or consent. The subjects’ participation may be revealed without their knowledge (e.g., e-mail communications with a subject about recovering from sexual assault might be read by family members).
- Breach of Confidentiality — The primary source of risk in the social and behavioral sciences is that information obtained by researchers could harm subjects if disclosed outside the research setting. This could include unintended disclosure of subject’s HIV status resulting in loss of health insurance coverage; revelation of sexual preferences that results in discrimination; disclosure of employee attitudes about their employer resulting in job lose; revealing information about illegal activities or status (drug use or immigrant status) resulting in legal consequences.
- Study Procedures — In some cases, simply participating in the research can cause social or psychological harm. Subjects who experienced abuse as children may experience emotional or psychological distress by participating in a study.
When assessing risk associated with participation in a research study, there are two distinct elements of risk that need to be considered.
- The Probability of Harm — The likelihood that a specific harm might occur
- The Magnitude of Such Harm
Risks in research participation are specific to time, situation, and culture. Thus, what may be a socially sensitive issue or topic at one time or place may not be so at another time or place. Risks in social and behavioral science research are mostly culturally determined. For example, asking women if they have had abortions would carry very different risk in cultures where abortion is a routine medical practice, a country where it is illegal, and a country where it is legal but fraught with religious and political controversy. Risks will differ according to the subject population. A survey about sexually transmitted disease would carry different risks for middle class suburban men, Catholic clergy, and gang members (who in one study claimed to have STD’s when they did not). The risk of emotional distress cannot be managed by anonymizing data, but rather by developing a plan to respond to the distress should it occur.
Risks and Benefits — Researchers tend to underestimate risks involved in activities with which they are familiar and to overestimate the benefits of things that are important to them. Thus, an independent assessment of risk is critical. One function of Institutional Review Boards is to provide this independent assessment. When potential outcomes are severe, people tend to overestimate their probability, regardless of the true probability. And when potential outcomes are less severe, such as embarrassment, people tend to underestimate their probability. Federal regulations, based on the ethical principle of beneficence, require that risks associated with research be reasonable in relation to the anticipated benefits. A great deal of research in the social and behavioral sciences offers little potential for direct benefits to the subjects themselves. The benefits of the research often lie in the importance of the knowledge to be gained. Most research in the social and behavioral sciences poses little or no risk to the subject.
A Certificate of Confidentiality protects sensitive information provided by research subjects from civil, criminal, or administrative subpoena. This protects identifiable research information from forced disclosure. The Certificates are issued by the National Institute of Health (NIH) and may be secured for any research (even non-NIH research), regardless of the source of funding or even for un-funded research. Certificates of Confidentiality may be granted for studies collecting information that, if disclosed, could have adverse consequences for subjects or damage their financial standing, employability, insurability, or reputation. Substance abuse or other illegal behaviors; sexual attitudes, preferences, or practices; genetic information; and psychological well-being are kinds of information that can be protected.
If the only identifier collected in the course of a study would be the signature on the consent document and the principal source of harm would be a breach of confidentiality, a waiver of documentation of informed consent should be sought. A waiver of documentation of informed consent is helpful when the consent form is the only document that links the subject to the study.
Note: Some of the material provided here was adapted from material available in CITI (Course in The Protection of Human Research Subjects).
Last updated on January 16, 2023
- Privacy Policy

Home » Informed Consent in Research – Types, Templates and Examples
Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________
Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.
Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.
Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author

Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples
Sample consent and permission forms
General consent form to participate in research (DOC)
Two stage project consent form (DOC)
Parent permission form for research with child (DOC)
Child assent form (DOC)
Multiple consent form including audio-recording and quotations (DOC)
Photo and video consent form (DOC)
Video-recording consent form (DOC)
Re-contact agreement form (DOC)
Post-debriefing consent form (DOC)
University Ethics Committee Information sheet & consent form
The UEC has drafted templates for researchers to use as a starting point when developing a Participant Information Sheet and Consent Form for their projects.
It is important that you tailor the Participant Information Sheet and Consent Form for your participant group and project. For example, use appropriate language for the age and understanding of your participant group, remove paragraphs related to risk where not relevant and only use the options on the Consent Form related to audio recording, human tissue etc., if these apply to your project.
If your project is within the remit of your Department or School Ehtics Committee you should use your Department or School Ethics Committee details as the contact point in the Participant Information Sheet and not the Secretary of the University Ethics Committee as set out in the template.
These templates will not be appropriate for NHS studies and the NHS template should be used if you are making an IRAS application.
Download PIS and Consent Form Template
Data protection
In order to comply with its responsibilities under data protection legislation, the University of Strathclyde is also required to provide important information regarding how we use research participants’ personal information and their rights under the legislation.
Please ensure that whenever personal information is being processed for research purposes, each participant is provided with a Privacy Notice. This Privacy Notice should be used in conjunction with the above PIS consent form template, to ensure all necessary information is provided to participants.
Download the Privacy Notice for Participants v0.8
University Branding
All participant documentation must have the correct University branding.
View University branding guidelines
Digital Accessibility
Digital accessibility is about making sure that everyone can access our information. In order to have informed consent, it's important that online or digital content is developed in a way that allows people to use it no matter how they choose to access it (e.g. with assistive technologies). There are many helpful resources that can help you to make your participant documentation more accessible.
- A guide to understanding what makes a typeface accessible – The Readability Group
- Captioning Key and Description Key – Described and Captioned Media Programme
- Making audio and video media accessible – W3C
- Create and verify PDF accessibility (Acrobat Pro) – Adobe
- PAC 2021 Free PDF Accessibility Checker – PDF/UA Foundation
The University also has guidance and training on digital accessibility:
- tips for creating accessible content .
- MyPlace course: Introduction to Digital Accessibility
- MyPlace course: Microsoft Office Accessibility
Some additional links:
- Understanding the Web Accessibility Guidelines ( WCAG 2.2 )
- Accessibility Maze Game
- WebAIM Contrast Checker
- Tanaguru Contrast Finder
- Colour Contrast Matrix
- Dyslexia example page
- POET Alt Text training tool
Our faculties & departments
Engineering.
- Faculty of Engineering
- Architecture
- Biomedical Engineering
- Chemical & Process Engineering
- Civil & Environmental Engineering
- Design, Manufacturing & Engineering Management
- Electronic & Electrical Engineering
- Mechanical & Aerospace Engineering
- Naval Architecture, Ocean & Marine Engineering
Humanities & Social Sciences
- Faculty of Humanities & Social Sciences
- Centre for Lifelong Learning
- Government & Public Policy
- Psychological Sciences & Health
- Social Work & Social Policy
- Faculty of Science
- Computer & Information Sciences
- Mathematics & Statistics
- Pure & Applied Chemistry
- Strathclyde Institute of Pharmacy & Biomedical Sciences
- Strathclyde Business School
- Accounting & Finance
- Hunter Centre for Entrepreneurship
- Management Science
- MBA & General Management
- Strathclyde Executive Education & Development
- Work, Employment & Organisation

Research Ethics: Tools and Guides
- Institutional Review Board (IRB): Research Ethics
- Office Hours
- Research Ethics Review Process by IRB
- Tools and Guides
- Frequently Asked Questions
- Walden Research Ethics and Compliance Policies
- Walden University Participant Pool
- Red Flag Issues
- International Research
- Conducting Doctoral Research in One's Own Work Setting
- Educational Settings
- Clinical and Intervention Settings
- Collecting Data about Obligatory Referral Topics
- Guidance on Masking Partner Organizations in Walden Capstone Documents
- Previous Page: Research Ethics Review Process by IRB
- Next Page: Frequently Asked Questions
Research Ethics Guides and Manuals
- Research Ethics Approval Checklist (worksheet format that mirrors the ethics self-check researchers will be asked to complete if they are collecting new data)
- Tips for Ethically Maximizing Participant Response Rate (YouTube)
- Should I work with a partner organization? (YouTube)
- Manual for Low-Risk Work-Related Interviews
- Manual for Low-Risk Anonymous Surveys
- Red flag issues that should trigger an IRB consultation
- Conducting Doctoral Research in One's Own Work Setting
- Collecting Data About Obligatory Referral Topics such as Bullying, Trauma, Depression, Illegal Activities, Etc.
- Masking Partner Organizations in Walden Capstone
- Maximizing Participant Response Rate Power Point
Sample Documents
- Consent Form Template (for participants aged 18 and over)
- Parent Consent Form Template
- Assent Form Template (for participants under 18)
- Invitation Templates for Email, Print, and Social Media
- Sample Data Collection Logs
Reporting Issues to the IRB
- Request for a Change in Procedures
- Adverse Event Reporting
Walden University IRB and Research Policies
- Read the IRB Policy in the Walden Student Handbook
Research Ethics Resources
- CITI Program Human Subjects Protection Training Module We can accept other training certificates but this non-expiring training is recommended and the university has a subscription covering all Walden researchers if they register under Walden.
- International Compilation of Human Research Standards
- National Institutes of Health (NIH) Kiosk for Federal Certificates of Confidentiality
- View More Information on Using Protected Health Information in Research
- U.S. Office for Human Research Protections
- View U.S. Federal Regulations
IRB Process Tools and Guides Research Ethics Office Hours Frequently Asked Questions Meet the Staff Email Research Ethics
Social Media
Connect with the office of research and doctoral services.
- Office of Student Disability Services
Walden Resources
Departments.
- Academic Residencies
- Academic Skills
- Career Planning and Development
- Customer Care Team
- Field Experience
- Military Services
- Student Success Advising
- Writing Skills
Centers and Offices
- Center for Social Change
- Office of Academic Support and Instructional Services
- Office of Degree Acceleration
- Office of Research and Doctoral Services
- Office of Student Affairs
Student Resources
- Doctoral Writing Assessment
- Form & Style Review
- Quick Answers
- ScholarWorks
- SKIL Courses and Workshops
- Walden Bookstore
- Walden Catalog & Student Handbook
- Student Safety/Title IX
- Legal & Consumer Information
- Website Terms and Conditions
- Cookie Policy
- Accessibility
- Accreditation
- State Authorization
- Net Price Calculator
- Contact Walden
Walden University is a member of Adtalem Global Education, Inc. www.adtalem.com Walden University is certified to operate by SCHEV © 2024 Walden University LLC. All rights reserved.

Academic Advising
Meet your advisor.
Appointments are required to discuss the following:
- Review degree program requirements
- Track progress in the program
- Review graduation procedures and deadlines
- Clarify university and department policies and procedures
- Leave of absence
Book an advising appointment
Department Consent Form Use this form to request an override into PHY 792, 795, and/or 799. Please have the form signed by the professor who will be giving you a grade at the end of the semester. Submit the form to the Graduate Coordinator when complete.
Maintain Continuous Enrollment Form
Use this form if you anticipate not enrolling for one or more semesters. Graduate students must have their leave of absence approved in advance.
Supervisory Committee Nomination Form
Advisor must be chosen and committee must be formed no later than end of semester after oral comprehensive is passed or TA support is jeopardized.
Report of Doctoral Comprehensive Exams This form is required on the day of your comprehensive exams. When complete, please submit to the Graduate Coordinator.
Report of Dissertation Prospectus This form is required on the day you deliver your prospectus. When complete, please submit to the Graduate Coordinator.
Forms required by Graduate Education are found here: https://graduate.asu.edu/forms/index.html
Student Handbooks, Policies, and Procedures
Physics PhD Student Handbook 2023
Graduate Policies and Procedures
Master of Natural Science (MNS) Graduate Student Handbook
Physics PhD Graduate Student Handbook
ASU TA/RA Handbook
How to submit an iPOS
Teaching Assistant Application
The teaching assistant application is available ONLY for students enrolled in the Physics PhD program. Students will be notified by email when the application is available. Students seeking TA support must apply every fall, spring and summer semesters.
MNS Resources
Course descriptions.
MNS courses include Modeling Workshops, and are typically offered in summer sessions so as to be compatible with full-time appointments in the high school system nationwide. The program is designed for summer only attendance.
Download a detailed list of courses and descriptions here . View the summer schedule at that same page.
4-year course rotation (tentative)
Summer Housing
Affordable housing is available! In 2019, it cost about $750 for 3 weeks, including about $25 for linens (optional) for a private bedroom and shared bath.
Please contact Jane Jackson for more information.
1. How many credit hours should I enroll in? Students enrolled in the Physics PhD program must enroll in a minimum of 9 credit hours every fall and spring semester. Per International Students & Scholar's Office, international students are required to enroll in 9 credit hours.
2. How do I enroll in research or dissertation credit? When enrolling in research or dissertation, students must submit a Department Consent Form. This form is found under the Forms section of this page. The instructor who you will enroll with (typically your research advisor) is required to sign this form. This helps the department keep track of who is involved in research, and with who.
3. How can I find deadlines for dropping and adding courses? All deadlines related to academics can be found of the Academic Calendar .
4. What does it mean to Audit a course? Will an Audit course affect my GPA?
- Auditing a course is when a student registers for a course, but does not receive credit for the course, and receives a grade of “X.”
- An audit course will not go on your Plan of Study (iPOS), nor will it affect your GPA.
- You will choose to Audit a course when enrolling
5. What is a Plan of Study (iPOS)? The Plan of Study (iPOS) functions as a contract between the student, the academic unit, and Graduate Education.
- Students must submit thier iPOS by the time they have enrolled for 50 percent of the minimum credit hours required for the program.
- The iPOS contains degree requirements such as coursework, committee, comprehensive exams, graduation deadlin.
- An approved iPOS MUST be on file before a student can take the comprehensive exams
- A "How to Guide" for submitting the iPOS is found here https://graduate.asu.edu/sites/default/files/how-to-ipos.pdf
- UB Directory
- Office of the Provost >
- Resources >
- Research Roundtable: ClinicalTrials.gov FAQ — Results Reporting (Part 2)
Research Roundtable: ClinicalTrials.gov FAQ — Results Reporting (Part 2)

Published May 13, 2024
Part two in a series of articles highlighting frequently asked questions to comply with results reporting for ClinicalTrials.gov includes guidance on the Quality Control review process, protocol and statistical analysis plans, and appendices. (See part one here .)
ClinicalTrials.gov has an extensive FAQ section ranging from general to investigation-specific. Many of these questions frequently arise not only at the University at Buffalo but also at many other institutions that are part of the Clinical Trials Registration and Results Reporting Taskforce . See additional ClinicalTrials.gov FAQs here .
How long does the Quality Control review process take?
After the Responsible Party releases (submits) information to ClinicalTrials.gov, the information undergoes a manual quality control review to identify possible errors, deficiencies, or inconsistencies that are not detected automatically during data entry. The Responsible Party will be notified of any issues that need correction, usually within a few days after release of the protocol information. Review of records with results is prioritized for the studies that appear to be Applicable Clinical Trials (ACT) or are NIH-funded and can take up to 30 days or longer. The review process for non-ACTs and studies that are not funded by NIH can be lengthier.
Is a protocol and statistical analysis plan (SAP) required to be submitted?
The regulations require a copy of the protocol and SAP (if not included in the protocol) to be submitted as part of clinical trial results information. The Responsible Party may redact names, addresses, and other personally identifiable information, as well as any trade secrets and/or confidential commercial information.
If there is more than one IRB-approved version of a consent form, how many must be posted?
The Revised Common Rule requires that the version of the consent document that is posted must a) be IRB-approved and b) have been used to enroll a participant in the clinical trial. The version of the consent that should be posted is the most recent IRB-approved version that was used to enroll a participant.
Are appendices required to be included in the uploaded study protocol?
ClinicalTrials.gov considers the protocol appendices that contain a “description of the clinical trial, including objective(s), design, and methods,” and any “relevant scientific background and statistical considerations,” to be part of the full protocol and as such they must be included with the uploaded protocol.
When posting a protocol with many add-on sub-studies, which version of the protocol should be posted?
Post the version of the protocol that was originally approved and is relevant to the study at hand. If sub-studies answer the same research questions, register under the originally approved protocol. If sub-studies are clearly different from the main study, each sub-study should be registered separately in ClinicalTrials.gov.
How can I get ClinicalTrials.gov help at UB?
For UB assistance with ClinicalTrials.gov registration and reporting requirements, contact the UB ClinicalTrials.gov team ( [email protected] ) or ClinicalTrials.gov Protocol Registration and Results System (PRS) Administrators Lynn Jagodzinski ( [email protected] ), CTSI Clinical Research Regulatory Administrator, and Urmo “Mo” Jaanimägi ( [email protected] ), CTSI Quality Assurance Specialist.
“Research Roundtable” is a section in the University at Buffalo Clinical and Translational Science Institute (CTSI) Translational Spotlight newsletter. Add your email to the newsletter mailing list here .
Do you have questions or comments for the Office of the Provost? Let us know your thoughts and we’ll be happy to get back to you.
PhD Excellence Initiative
A campus-wide, student-centric effort to ensure that UB’s PhD programs remain among the strongest in the world.
Recent University News
- 5/16/24 NASA taps UB researchers’ team to advance satellite observation of climate change
- 5/16/24 UB School of Management community poses for ‘Human 100’
- 5/16/24 EDI Collaboration Builds Off Feltri’s MS Drug Research
- 5/16/24 38 UB School of Management MBAs earn LeaderCORE certification
- 5/16/24 Krupski recognized for excellence in teaching
- Open access
- Published: 08 May 2024
Measurement and analysis of change in research scholars’ knowledge and attitudes toward statistics after PhD coursework
- Mariyamma Philip 1
BMC Medical Education volume 24 , Article number: 512 ( 2024 ) Cite this article
190 Accesses
Metrics details
Knowledge of statistics is highly important for research scholars, as they are expected to submit a thesis based on original research as part of a PhD program. As statistics play a major role in the analysis and interpretation of scientific data, intensive training at the beginning of a PhD programme is essential. PhD coursework is mandatory in universities and higher education institutes in India. This study aimed to compare the scores of knowledge in statistics and attitudes towards statistics among the research scholars of an institute of medical higher education in South India at different time points of their PhD (i.e., before, soon after and 2–3 years after the coursework) to determine whether intensive training programs such as PhD coursework can change their knowledge or attitudes toward statistics.
One hundred and thirty research scholars who had completed PhD coursework in the last three years were invited by e-mail to be part of the study. Knowledge and attitudes toward statistics before and soon after the coursework were already assessed as part of the coursework module. Knowledge and attitudes towards statistics 2–3 years after the coursework were assessed using Google forms. Participation was voluntary, and informed consent was also sought.
Knowledge and attitude scores improved significantly subsequent to the coursework (i.e., soon after, percentage of change: 77%, 43% respectively). However, there was significant reduction in knowledge and attitude scores 2–3 years after coursework compared to the scores soon after coursework; knowledge and attitude scores have decreased by 10%, 37% respectively.
The study concluded that the coursework program was beneficial for improving research scholars’ knowledge and attitudes toward statistics. A refresher program 2–3 years after the coursework would greatly benefit the research scholars. Statistics educators must be empathetic to understanding scholars’ anxiety and attitudes toward statistics and its influence on learning outcomes.
Peer Review reports
A PhD degree is a research degree, and research scholars submit a thesis based on original research in their chosen field. Doctor of Philosophy (PhD) degrees are awarded in a wide range of academic disciplines, and the PhD students are usually referred as research scholars. A comprehensive understanding of statistics allows research scholars to add rigour to their research. This approach helps them evaluate the current practices and draw informed conclusions from studies that were undertaken to generate their own hypotheses and to design, analyse and interpret complex clinical decisions. Therefore, intensive training at the beginning of the PhD journey is essential, as intensive training in research methodology and statistics in the early stages of research helps scholars design and plan their studies efficiently.
The University Grants Commission of India has taken various initiatives to introduce academic reforms to higher education institutions in India and mandated in 2009 that coursework be treated as a prerequisite for PhD preparation and that a minimum of four credits be assigned to one or more courses on research methodology, which could cover areas such as quantitative methods, computer applications, and research ethics. UGC also clearly states that all candidates admitted to PhD programmes shall be required to complete the prescribed coursework during the initial two semesters [ 1 ]. National Institute of Mental Health and Neurosciences (NIMHANS) at Bangalore, a tertiary care hospital and medical higher education institute in South India, that trains students in higher education in clinical fields, also introduced coursework in the PhD program for research scholars from various backgrounds, such as basic, behavioral and neurosciences, as per the UGC mandate. Research scholars undertake coursework programs soon after admission, which consist of several modules that include research methodology and statistical software training, among others.
Most scholars approach a course in statistics with the prejudice that statistics is uninteresting, demanding, complex or involve much mathematics and, most importantly, it is not relevant to their career goals. They approach statistics with considerable apprehension and negative attitudes, probably because of their inability to grasp the relevance of the application of the methods in their fields of study. This could be resolved by providing sufficient and relevant examples of the application of statistical techniques from various fields of medical research and by providing hands-on experience to learn how these techniques are applied and interpreted on real data. Hence, research methodology and statistical methods and the application of statistical methods using software have been given much importance and are taught as two modules, named Research Methodology and Statistics and Statistical Software Training, at this institute of medical higher education that trains research scholars in fields as diverse as basic, behavioural and neurosciences. Approximately 50% of the coursework curriculum focused on these two modules. Research scholars were thus given an opportunity to understand the theoretical aspects of the research methodology and statistical methods. They were also given hands-on training on statistical software to analyse the data using these methods and to interpret the findings. The coursework program was designed in this specific manner, as this intensive training would enable the research scholars to design their research studies more effectively and analyse their data in a better manner.
It is important to study attitudes toward statistics because attitudes are known to impact the learning process. Also, most importantly, these scholars are expected to utilize the skills in statistics and research methods to design research projects or guide postgraduate students and research scholars in the near future. Several authors have assessed attitudes toward statistics among various students and examined how attitudes affect academic achievement, how attitudes are correlated with knowledge in statistics and how attitudes change after a training program. There are studies on attitudes toward statistics among graduate [ 2 , 3 , 4 ] and postgraduate [ 5 ] medical students, politics, sociology, ( 6 – 7 ) psychology [ 8 , 9 , 10 ], social work [ 11 ], and management students [ 12 ]. However, there is a dearth of related literature on research scholars, and there are only two studies on the attitudes of research scholars. In their study of doctoral students in education-related fields, Cook & Catanzaro (2022) investigated the factors that contribute to statistics anxiety and attitudes toward statistics and how anxiety, attitudes and plans for future research use are connected among doctoral students [ 13 ]. Another study by Sohrabi et al. (2018) on research scholars assessed the change in knowledge and attitude towards teaching and educational design of basic science PhD students at a Medical University after a two-day workshop on empowerment and familiarity with the teaching and learning principles [ 14 ]. There were no studies that assessed changes in the attitudes or knowledge of research scholars across the PhD training period or after intensive training programmes such as PhD coursework. Even though PhD coursework has been established in institutes of higher education in India for more than a decade, there are no published research on the effectiveness of coursework from Indian universities or institutes of higher education.
This study aimed to determine the effectiveness of PhD coursework and whether intensive training programs such as PhD coursework can influence the knowledge and attitudes toward statistics of research scholars. Additionally, it would be interesting to know if the acquired knowledge could be retained longer, especially 2–3 years after the coursework, the crucial time of PhD data analysis. Hence, this study compares the scores of knowledge in statistics and attitude toward statistics of the research scholars at different time points of their PhD training, i.e., before, soon after and 2–3 years after the coursework.
Participants
This is an observational study of single group with repeated assessments. The institute offers a three-month coursework program consisting of seven modules, the first module is ethics; the fifth is research methodology and statistics; and the last is neurosciences. The study was conducted in January 2020. All research scholars of the institute who had completed PhD coursework in the last three years were considered for this study ( n = 130). Knowledge and attitudes toward statistics before and soon after the coursework module were assessed as part of the coursework program. They were collected on the first and last day of the program respectively. The author who was also the coordinator of the research methodology and statistics module of the coursework have obtained the necessary permission to use the data for this study. The scholars invited to be part of the study by e-mail. Knowledge and attitude towards statistics 2–3 years after the coursework were assessed online using Google forms. They were also administered a semi structured questionnaire to elicit details about the usefulness of coursework. Participation was voluntary, and consent was also sought online. The confidentiality of the data was assured. Data were not collected from research scholars of Biostatistics or from research scholars who had more than a decade of experience or who had been working in the institute as faculty, assuming that their scores could be higher and could bias the findings. This non funded study was reviewed and approved by the Institute Ethics Committee.
Instruments
Knowledge in Statistics was assessed by a questionnaire prepared by the author and was used as part of the coursework evaluation. The survey included 25 questions that assessed the knowledge of statistics on areas such as descriptive statistics, sampling methods, study design, parametric and nonparametric tests and multivariate analyses. Right answers were assigned a score of 1, and wrong answers were assigned a score of 0. Total scores ranged from 0 to 25. Statistics attitudes were assessed by the Survey of Attitudes toward Statistics (SATS) scale. The SATS is a 36-item scale that measures 6 domains of attitudes towards statistics. The possible range of scores for each item is between 1 and 7. The total score was calculated by dividing the summed score by the number of items. Higher scores indicate more positive attitudes. The SAT-36 is a copyrighted scale, and researchers are allowed to use it only with prior permission. ( 15 – 16 ) The author obtained permission for use in the coursework evaluation and this study. A semi structured questionnaire was also used to elicit details about the usefulness of coursework.
Statistical analysis
Descriptive statistics such as mean, standard deviation, number and percentages were used to describe the socio-demographic data. General Linear Model Repeated Measures of Analysis of variance was used to compare knowledge and attitude scores across assessments. Categorical data from the semi structured questionnaire are presented as percentages. All the statistical tests were two-tailed, and a p value < 0.05 was set a priori as the threshold for statistical significance. IBM SPSS (28.0) was used to analyse the data.
One hundred and thirty research scholars who had completed coursework (CW) in the last 2–3 years were considered for the study. These scholars were sent Google forms to assess their knowledge and attitudes 2–3 years after coursework. 81 scholars responded (62%), and 4 scholars did not consent to participate in the study. The data of 77 scholars were merged with the data obtained during the coursework program (before and soon after CW). Socio-demographic characteristics of the scholars are presented in Table 1 .
The age of the respondents ranged from 23 to 36 years, with an average of 28.7 years (3.01), and the majority of the respondents were females (65%). Years of experience (i.e., after masters) before joining a PhD programme ranged from 0.5 to 9 years, and half of them had less than three years of experience before joining the PhD programme (median-3). More than half of those who responded were research scholars from the behavioural sciences (55%), while approximately 30% were from the basic sciences (29%).
General Linear Model Repeated Measures of Analysis of variance was used to compare the knowledge and attitude scores of scholars before, soon after and 2–3 after the coursework (will now be referred as “later the CW”), and the results are presented below (Table 2 ; Fig. 1 ).
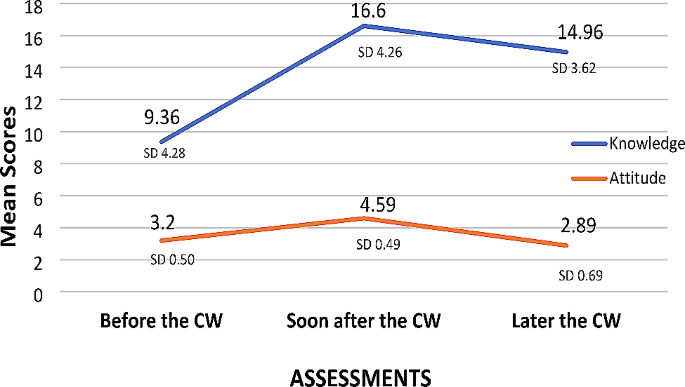
Comparison of knowledge and attitude scores across the assessments. Later the CW – 2–3 years after the coursework
The scores for knowledge and attitude differed significantly across time. Scores of knowledge and attitude increased soon after the coursework; the percentage of change was 77% and 43% respectively. However, significant reductions in knowledge and attitude scores were observed 2–3 years after the coursework compared to scores soon after the coursework. The reduction was higher for attitude scores; knowledge and attitude scores have decreased by 10% and 37% respectively. The change in scores across assessments is evident from the graph, and clearly the effect size is higher for attitude than knowledge.
The scores of knowledge or attitude before the coursework did not significantly differ with respect to gender or age or were not correlated with years of experience. Hence, they were not considered as covariates in the above analysis.
A semi structured questionnaire with open ended questions was also administered to elicit in-depth information about the usefulness of the coursework programme, in which they were also asked to self- rate their knowledge. The data were mostly categorical or narratives. Research scholars’ self-rated knowledge scores (on a scale of 0–10) also showed similar changes; knowledge improved significantly and was retained even after the training (Fig. 2 ).
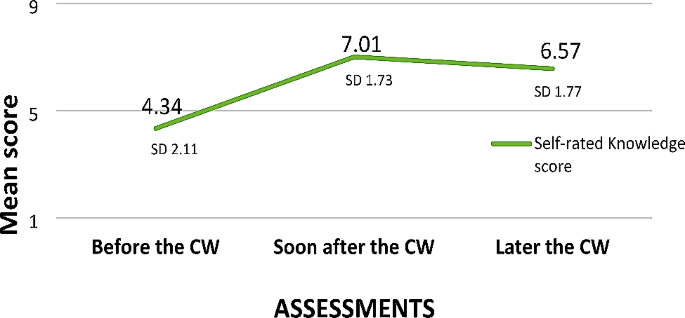
Self-rated knowledge scores of research scholars over time. Later the CW – 2–3 years after the coursework
The response to the question “ How has coursework changed your attitude toward statistics?”, is presented in Fig. 3 . The responses were Yes, positively, Yes - Negatively, No change – still apprehensive, No change – still appreciate, No change – still hate statistics. The majority of the scholars (70%) reported a positive change in their attitude toward statistics. Moreover, none of the scholars reported negative changes. Approximately 9% of the scholars reported that they were still apprehensive about statistics or hate statistics after the coursework.
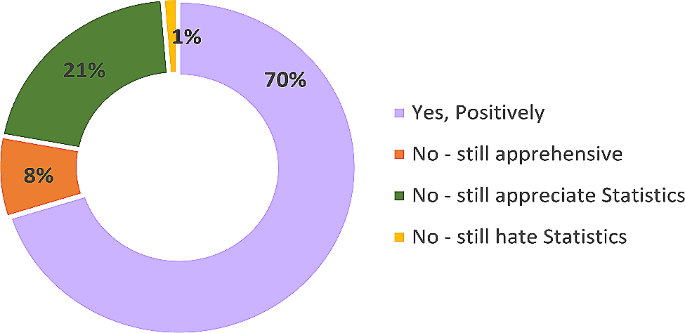
How has coursework changed your attitude toward statistics?
Those scholars who reported that they were apprehensive about statistics or hate statistics noted the complexity of the subject, lack of clarity, improper instructions and fear of mathematics as major reasons for their attitude. Some responses are listed below.
“The statistical concepts were not taught in an understandable manner from the UG level” , “I am weak in mathematical concepts. The equations and formulae in statistics scare me”. “Lack of knowledge about the importance of statistics and fear of mathematical equations”. “The preconceived notion that Statistics is difficult to learn” . “In most of the places, it is not taught properly and conceptual clarity is not focused on, and because of this an avoidance builds up, which might be a reason for the negative attitude”.
Majority of the scholars (92%) felt that coursework has helped them in their PhD, and they were happy to recommend it for other research scholars (97%). The responses of the scholars to the question “ How was coursework helpful in your PhD journey ?”, are listed below.
“Course work gave a fair idea on various things related to research as well as statistics” . “Creating the best design while planning methodology, which is learnt form course work, will increase efficiency in completing the thesis, thereby making it faster”. “Course work give better idea of how to proceed in many areas like literature search, referencing, choosing statistical methods, and learning about research procedures”. “Course work gave a good idea of research methodology, biostatistics and ethics. This would help in writing a better protocol and a better thesis”. “It helps us to plan our research well and to formulate, collect and plan for analysis”. “It makes people to plan their statistical analysis well in advance” .
This study evaluated the effectiveness of the existing coursework programme in an institution of higher medical education, and investigated whether the coursework programme benefits research scholars by improving their knowledge of statistics and attitudes towards statistics. The study concluded that the coursework program was beneficial for improving scholars’ knowledge about statistics and attitudes toward statistics.
Unlike other studies that have assessed attitudes toward statistics, the study participants in this study were research scholars. Research scholars need extensive training in statistics, as they need to apply statistical tests and use statistical reasoning in their research thesis, and in their profession to design research projects or their future student dissertations. Notably, no studies have assessed the attitudes or knowledge of research scholars in statistics either across the PhD training period or after intensive statistics training programs. However, the findings of this study are consistent with the findings of a study that compared the knowledge and attitudes toward teaching and education design of PhD students after a two-day educational course and instructional design workshop [ 14 ].
Statistics educators need not only impart knowledge but they should also motivate the learners to appreciate the role of statistics and to continue to learn the quantitative skills that is needed in their professional lives. Therefore, the role of learners’ attitudes toward statistics requires special attention. Since PhD coursework is possibly a major contributor to creating a statistically literate research community, scholars’ attitudes toward statistics need to be considered important and given special attention. Passionate and engaging statistics educators who have adequate experience in illustrating relatable examples could help scholars feel less anxious and build competence and better attitudes toward statistics. Statistics educators should be aware of scholars’ anxiety, fears and attitudes toward statistics and about its influence on learning outcomes and further interest in the subject.
Strengths and limitations
Analysis of changes in knowledge and attitudes scores across various time points of PhD training is the major strength of the study. Additionally, this study evaluates the effectiveness of intensive statistical courses for research scholars in terms of changes in knowledge and attitudes. This study has its own limitations: the data were collected through online platforms, and the nonresponse rate was about 38%. Ability in mathematics or prior learning experience in statistics, interest in the subject, statistics anxiety or performance in coursework were not assessed; hence, their influence could not be studied. The reliability and validity of the knowledge questionnaire have not been established at the time of this study. However, author who had prepared the questionnaire had ensured questions from different areas of statistics that were covered during the coursework, it has also been used as part of the coursework evaluation. Despite these limitations, this study highlights the changes in attitudes and knowledge following an intensive training program. Future research could investigate the roles of age, sex, mathematical ability, achievement or performance outcomes and statistics anxiety.
The study concluded that a rigorous and intensive training program such as PhD coursework was beneficial for improving knowledge about statistics and attitudes toward statistics. However, the significant reduction in attitude and knowledge scores after 2–3 years of coursework indicates that a refresher program might be helpful for research scholars as they approach the analysis stage of their thesis. Statistics educators must develop innovative methods to teach research scholars from nonstatistical backgrounds. They also must be empathetic to understanding scholars’ anxiety, fears and attitudes toward statistics and to understand its influence on learning outcomes and further interest in the subject.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
UGC Regulations on Minimum Standards and Procedure for the award of, M.Phil/Ph D, Degree R. 2009. Ugc.ac.in. [cited 2023 Oct 26]. https://www.ugc.ac.in/oldpdf/regulations/mphilphdclarification.pdf .
Althubaiti A. Attitudes of medical students toward statistics in medical research: Evidence from Saudi Arabia. J Stat Data Sci Educ [Internet]. 2021;29(1):115–21. https://doi.org/10.1080/10691898.2020.1850220 .
Hannigan A, Hegarty AC, McGrath D. Attitudes towards statistics of graduate entry medical students: the role of prior learning experiences. BMC Med Educ [Internet]. 2014;14(1):70. https://doi.org/10.1186/1472-6920-14-70 .
Hasabo EA, Ahmed GEM, Alkhalifa RM, Mahmoud MD, Emad S, Albashir RB et al. Statistics for undergraduate medical students in Sudan: associated factors for using statistical analysis software and attitude toward statistics among undergraduate medical students in Sudan. BMC Med Educ [Internet]. 2022;22(1):889. https://doi.org/10.1186/s12909-022-03960-0 .
Zhang Y, Shang L, Wang R, Zhao Q, Li C, Xu Y et al. Attitudes toward statistics in medical postgraduates: measuring, evaluating and monitoring. BMC Med Educ [Internet]. 2012;12(1):117. https://doi.org/10.1186/1472-6920-12-117 .
Bechrakis T, Gialamas V, Barkatsas A. Survey of attitudes towards statistics (SATS): an investigation of its construct validity and its factor structure invariance by gender. Int J Theoretical Educational Pract. 2011;1(1):1–15.
Google Scholar
Khavenson T, Orel E, Tryakshina M. Adaptation of survey of attitudes towards statistics (SATS 36) for Russian sample. Procedia Soc Behav Sci [Internet]. 2012; 46:2126–9. https://doi.org/10.1016/j.sbspro.2012.05.440 .
Coetzee S, Van Der Merwe P. Industrial psychology students’ attitudes towards statistics. J Industrial Psychol. 2010;36(1):843–51.
Francesca C, Primi C. Assessing statistics attitudes among College Students: Psychometric properties of the Italian version of the Survey of attitudes toward statistics (SATS). Learn Individual Differences. 2009;2:309–13.
Counsell A, Cribbie RA. Students’ attitudes toward learning statistics with R. Psychol Teach Rev [Internet]. 2020;26(2):36–56. https://doi.org/10.53841/bpsptr.2020.26.2.36 .
Yoon E, Lee J. Attitudes toward learning statistics among social work students: Predictors for future professional use of statistics. J Teach Soc Work [Internet]. 2022;42(1):65–81. https://doi.org/10.1080/08841233.2021.2014018 .
Melad AF. Students’ attitude and academic achievement in statistics: a Correlational Study. J Posit School Psychol. 2022;6(2):4640–6.
Cook KD, Catanzaro BA. Constantly Working on My Attitude Towards Statistics! Education Doctoral Students’ Experiences with and Motivations for Learning Statistics. Innov High Educ. 2023; 48:257–84. https://doi.org/10.1007/s10755-022-09621-w .
Sohrabi Z, Koohestani HR, Nahardani SZ, Keshavarzi MH. Data on the knowledge, attitude, and performance of Ph.D. students attending an educational course (Tehran, Iran). Data Brief [Internet]. 2018; 21:1325–8. https://doi.org/10.1016/j.dib.2018.08.081 .
Chau C, Stevens J, Dauphine T. Del V. A: The development and validation of the survey of attitudes toward statistics. Educ Psychol Meas. 1995;(5):868–75.
Student attitude surveys. and online educational consulting [Internet]. Evaluationandstatistics.com. [cited 2023 Oct 26]. https://www.evaluationandstatistics.com/ .
Download references
Acknowledgements
The author would like to thank the participants of the study and peers and experts who examined the content of the questionnaire for their time and effort.
This research did not receive any grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Biostatistics, Dr. M.V. Govindaswamy Centre, National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, 560 029, India
Mariyamma Philip
You can also search for this author in PubMed Google Scholar
Contributions
Mariyamma Philip: Conceptualization, Methodology, Validation, Investigation, Writing- Original draft, Reviewing and Editing.
Corresponding author
Correspondence to Mariyamma Philip .
Ethics declarations
Ethics approval and consent to participate.
This study used data already collected data (before and soon after coursework). The data pertaining to knowledge and attitude towards statistics 2–3 years after coursework were collected from research scholars through the online survey platform Google forms. The participants were invited to participate in the survey through e-mail. The study was explained in detail, and participation in the study was completely voluntary. Informed consent was obtained online in the form of a statement of consent. The confidentiality of the data was assured, even though identifiable personal information was not collected. This non-funded study was reviewed and approved by NIMHANS Institute Ethics Committee (No. NIMHANS/21st IEC (BS&NS Div.)
Consent for publication
Not applicable because there is no personal information or images that could lead to the identification of a study participant.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Philip, M. Measurement and analysis of change in research scholars’ knowledge and attitudes toward statistics after PhD coursework. BMC Med Educ 24 , 512 (2024). https://doi.org/10.1186/s12909-024-05487-y
Download citation
Received : 27 October 2023
Accepted : 29 April 2024
Published : 08 May 2024
DOI : https://doi.org/10.1186/s12909-024-05487-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Knowledge of statistics
- Attitude towards statistics
- PhD coursework
- Research scholars
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
'Annotated' Template Example Consent Form. Template Consent Form (Word) Further Guidance. Guidance on obtaining consent from research participants online (for online and in-person study designs) Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL. Recording & Obtaining Consent
permission, adult consent, teacher consent, screening consent, etc.). • In this template, "we" refers to the researchers. If there is only one researcher, edit as appropriate. If the PI is a student, always use "we" to include the faculty advisor. • Submit consent documents in MS Word whenever possible. The iMedRIS comparison tool for
A Consent Form is read by the participant, signed and handed back to the researcher and should include the following features: 1. Use University of Wollongong/AHS letterhead. 2. Provide the title of the research project, the researcher(s) name, supervisor's name (for student research), the Unit in which the researcher is based and the name of the
Informed Consent for Human Subjects Research. Purpose of Primer series: To help bridge the gaps between health services researchers, policy makers, managers, and clinicians in an effort to improve the quality and cost-effectiveness of health care for veterans. The Primer series is part of a larger set of dissemination initiatives developed by ...
2023-04-10. Assent Form Ages 7-14. 2023-06-27. Consent Addendum for Unencrypted Communication. 2020-10-26. Information or Fact Sheet. 2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information.
General Consent Form Templates. Social and Behavioral Research Projects (last updated 03/16/2023) Biomedical Research Projects (last updated 07/18/2022) Consent Form Templates for Specific Biomedical Procedures. MRI and fMRI. Blood Collection by Finger Stick. Blood Collection by Venipuncture. Oral Consent Template. Guidance for Protocols ...
The Research Ethics Board must approve any changes to the consent form before the research begins. Changes to an approved study and its documents are done via an Amendment. Your application will be sent back, and approval delayed, if a complete consent form or consent document is not submitted with your application.
However, many researchers and the Institutional Review Boards that govern their research fail to recognize that 46.117(c) provides for a waiver of signed consent forms: (c) An IRB may waive the requirement for the investigator to obtain a signed consent form for some or all subjects if it finds either:
The use of "you" throughout this document refers to the research participant. It also refers to the person authorized to give consent for the subject's participation in this research study. Consent to Participate in a Research Study You are being asked to participate in a research study. All research is voluntary.
Participant consent form. Before research begins, it is important to first obtain participant's consent on the basis of their full and proven understanding of what the research will entail. Please use the following template: These templates should be followed as far as possible, as these have been developed using national guidance and expert ...
The participant Information Sheet gives potential participants the necessary understanding for the motivation and procedures of the study and sources of information to answer any further questions to allow them to give informed consent. A Consent Form essentially reprises this information to ensure the key points are understood and then records ...
Participant Consent Form This template is designed primarily for those doing qualitative interviews with adults from non-vulnerable populations and dealing with non-sensitive topics. The form would be different in the case of focus groups or quantitative research. If conducting research with vulnerable populations and / or sensitive topics please
The consent form (Word), or an equally robust consent process, must be used for all research projects involving living human participants.. Written consent is considered the 'gold standard'. In limited circumstances an alternative such as oral consent may be appropriate but this must be justified in the ethics application - see the guidance on consent.
The informed consent form should be accompanied by an information sheet that describes: 1. General information about the research and the collected research data • Purpose of the research • Type of research intervention, e.g. questionnaire, interview, etc. • Voluntary nature of participation • Benefits and risks of participating
Researchers are bound by a code of ethics that includes the following protections for subjects. Protected from physical or psychological harm (including loss of dignity, loss of autonomy, and loss of self-esteem) Protection of privacy and confidentiality. Protection against unjustifiable deception. The research participant must give voluntary ...
Informed Consent in Research. Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of ...
Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
The HHS regulations at 45 CFR part 46 for the protection of human subjects in research require that an investigator obtain the legally effective informed consent of the subject or the subject's legally authorized representative, unless (1) the research is exempt under 45 CFR 46.101(b); (2) the IRB finds and documents that informed consent can be waived (45 CFR 46.116(c) or (d)); or (3) the ...
Download PIS and Consent Form Template. Data protection . In order to comply with its responsibilities under data protection legislation, the University of Strathclyde is also required to provide important information regarding how we use research participants' personal information and their rights under the legislation.
Participant Information Leaflets and Consent Forms - Guidance for Researchers. Informed consent is a key issue in the conduct of ethical research. In order for consent to be informed, participants should be given comprehensive information regarding the nature, purpose and consequences of the research project. Where possible this should be ...
FORM FOR CONSENT OF PH.D. GUIDE & PH.D. STUDENT CONSENT OF PH.D. GUIDE With reference to the above-mentioned subject, I wish to inform you that I am willing to accept Mr./Ms. _____as my Ph.D. student, and for guiding his/her research work leading to Ph.D. degree of The Assam Royal Global University, Guwahati.
Research Ethics Resources. CITI Program Human Subjects Protection Training Module. We can accept other training certificates but this non-expiring training is recommended and the university has a subscription covering all Walden researchers if they register under Walden. International Compilation of Human Research Standards.
When enrolling in research or dissertation, students must submit a Department Consent Form. This form is found under the Forms section of this page. The instructor who you will enroll with (typically your research advisor) is required to sign this form. This helps the department keep track of who is involved in research, and with who. 3.
The version of the consent that should be posted is the most recent IRB-approved version that was used to enroll a participant. ... "Research Roundtable" is a section in the University at Buffalo Clinical and Translational ... student-centric effort to ensure that UB's PhD programs remain among the strongest in the world. Learn more about ...
One hundred and thirty research scholars who had completed coursework (CW) in the last 2-3 years were considered for the study. These scholars were sent Google forms to assess their knowledge and attitudes 2-3 years after coursework. 81 scholars responded (62%), and 4 scholars did not consent to participate in the study.
06.01.2023 [Insert consent form title] mm/dd/yyyy Version 1.0 Version x.x Page 1 of 2 Title: TEMPLATE_Consent_Short_Spanish_v1.0_2023.06.01 Author: Karen Allen Keywords: #Consent # Last modified by: Amanda Created Date: 12/27/2022 5:56:00 PM Company: UC Irvine Other titles: