- Open access
- Published: 25 January 2024

Research trends in contemporary health economics: a scientometric analysis on collective content of specialty journals
- Clara C. Zwack ORCID: orcid.org/0000-0002-9866-6470 1 ,
- Milad Haghani 2 &
- Esther W. de Bekker-Grob 3
Health Economics Review volume 14 , Article number: 6 ( 2024 ) Cite this article
1382 Accesses
2 Altmetric
Metrics details
Introduction
Health economics is a thriving sub-discipline of economics. Applied health economics research is considered essential in the health care sector and is used extensively by public policy makers. For scholars, it is important to understand the history and status of health economics—when it emerged, the rate of research output, trending topics, and its temporal evolution—to ensure clarity and direction when formulating research questions.
Nearly 13,000 articles were analysed, which were found in the collective publications of the ten most specialised health economic journals. We explored this literature using patterns of term co-occurrence and document co-citation.
The research output in this field is growing exponentially. Five main research divisions were identified: (i) macroeconomic evaluation, (ii) microeconomic evaluation, (iii) measurement and valuation of outcomes, (iv) monitoring mechanisms (evaluation), and (v) guidance and appraisal. Document co-citation analysis revealed eighteen major research streams and identified variation in the magnitude of activities in each of the streams. A recent emergence of research activities in health economics was seen in the Medicaid Expansion stream. Established research streams that continue to show high levels of activity include Child Health, Health-related Quality of Life (HRQoL) and Cost-effectiveness. Conversely, Patient Preference, Health Care Expenditure and Economic Evaluation are now past their peak of activity in specialised health economic journals. Analysis also identified several streams that emerged in the past but are no longer active.
Conclusions
Health economics is a growing field, yet there is minimal evidence of creation of new research trends. Over the past 10 years, the average rate of annual increase in internationally collaborated publications is almost double that of domestic collaborations (8.4% vs 4.9%), but most of the top scholarly collaborations remain between six countries only.
Health economics, a discipline of economics that focuses on studying how resources are allocated, utilised, and distributed in the healthcare sector [ 1 ]. Health economists use various economic tools and techniques, such as cost-effectiveness analysis, cost-benefit analysis, econometric modelling, and microeconomic theory, to examine a wide range of healthcare issues [ 2 , 3 ]. The field has experienced rapid evolution, largely due to the decades of work of committed scholars. These scholars have not only built a foundation of knowledge, but also developed and refined a set of methodological tools to guide decision making by health care authorities [ 4 ]. Modern day health systems are constantly challenged by scarcity of resources, which is attributable to an aging population, diseases of prosperity, rapid urbanisation, technological advancement in the medical field and large scale migrations [ 4 ], not to mention the new threat of global pandemics [ 5 , 6 ]. Another contemporary issue is the rising out-of-pocket health spending that continues to threaten the affordability of medical care, even for some of the most advanced OECD countries [ 7 , 8 ]. These challenging and complex environments create strong drivers for the further development of health economics.
In 1963, Kenneth Arrow published “Uncertainty and the welfare economics of medical care” in The American Economic Review [ 9 ]. It became one of the most highly cited articles in health economics and was considered the article that established the field. From here, the term “health economics” increased rapidly in articles published in economics, however, it was not until the early 1980’s that saw the creation of specialised health economics journals.
The unprecedented surge in publications presents researchers with challenges in keeping up with the latest advancements in the field of health economics. Hence, consolidating research and its outcomes has gained even greater importance [ 10 ]. For scholars, it is important to understand the history and status of health economics—when it emerged, the rate of research output, trending topics, and its temporal evolution—to ensure clarity and direction when formulating research questions. The course of health economics has been charted previously [ 11 , 12 ], however, these analyses focus on bibliometric properties of the field. Whilst this is important to report, this paper will extend current knowledge by completing a scientometric analysis of contemporary health economics, using specialised sources and advanced analytical and clustering tools. In health economics, systematic reviews are considered the gold standard for measuring efficacy and effectiveness of a specific topic due to their rigorous nature. However, scientometrics can be utilised to complement systematic reviews to summarise the overall trends observed with a topic [ 10 , 13 ].
The main objectives of our study presented in this paper are to determine the patterns in regional distribution of relevant health economics publications, prominent author networks, the major divisions and research streams of health economics literature, and the variation of activity for each sub-area. This paper also reports on the trending topics and highlights, based on a multitude of objective metrics, the influential references of health economics literature that have shaped the formation of each research stream.
The dataset of references
To retrieve the data for this study, the Web of Science (WoS) Core Collection was accessed and searched in May 2022. A search query was formulated in consultation with an experienced health economist. The ten sources (i.e. scientific peer-reviewed journals) that predominantly publish articles relevant to health economics were included. A list of sources was initially identified if they were listed by the WoS in both categories of “Health Policy & Services” and “Economics”. From this list, the ten sources with the largest volume of content were selected for inclusion in the search. Keywords were not utilised in the search strategy due to the diversity of the terms being used across health economics along with the lack of distinctiveness across other fields (e.g. economics and medicine).
Search strategy
SO = (“Value in Health” OR “Health Economics” OR “Pharmacoeconomics” OR “Pharmacoeconomics Open” OR “International Journal of Health Economics and Management” OR “Journal of Health Economics” OR “Health Economics Review” OR “Applied Health Economics and Health Policy” OR “American Journal of Health Economics” OR “European Journal of Health Economics”).
Upon initial inspection of the 68,000 documents found by the search strategy, Value in Health journal has indexed 54,000 documents as meeting abstracts. These records did not display abstract or reference lists, which are essential for scientometric analysis. Hence, it was determined that for this analysis the inclusion criteria needed to be refined to articles and review articles only. No restrictions were set on other subcategories. The maximum year was set to December 31, 2021, with no restriction on the minimum. Full bibliographic details of the documents were exported from WoS as text files. Details include document title, authors, author affiliations, year of publication, source (journal) title, citation count, document type, abstract, author keywords, keywords plus, funding source, full list of document references and conference information, if relevant.
General findings
The estimated size of the literature, highly cited documents, prominent sources and author affiliations (i.e. country and institution) were analysed using the meta data extracted directly from WoS.
Semantic analysis
Title and abstract, and keyword analyses were conducted using VOSviewer 1.6.15. Keywords provide insight into the temporal shifts in research and scholarly focus. Clusters of terms extracted from the titles and abstracts are formed by the frequency they occur (set to a minimum of 15) in the articles to provide an objective overview of the structure and divisions within this research topic.
Networks of author collaboration
Analyses of author networks were conducted using VOSviewer 1.6.15. Each author is represented by a node and is connected to other authors via links. The number of co-authored documents is indicated by the thickness of the link between the two nodes.
Influential articles analysis
Document co-citation and citation burst analyses was completed using CiteSpace 5.7.R1 [ 14 ]. The concept of document co-citation, a methodology developed by Chen [ 15 ], was used to obtain an indication of the most influential studies within the field of health economics as well as the clusters of thematically similar references. The methodology identifies cohorts of references that are frequently co-cited in the reference lists of health economics papers, on the premise that such references are similar in subjects and represent the knowledge foundation of a certain topic in the field. Document co-citation analysis results in a new set of documents, which include valuable knowledge sources for health economics that are instrumental in the development of this literature but were not captured by the WoS search query.
From document co-citation we can find (i) references with the most local citations (citations from within the literature exclusively relevant to this topic), (ii) references with the strongest citation burst (heightened attention to an individual article within the field, representing a temporal component of the research topic) and, iii) references with the highest centrality (document co-citation across multiple clusters).
Temporal analysis
CiteSpace 5.7.R1 [ 14 ] was used to generate the dynamic visualisation, which shows insight into the emergence and activities of each research stream since 1990. Research streams are named using the titles of the citing articles (of each stream). Nouns and noun phrases are extracted from the titles. These nouns and noun phrases are each allocated a score depending on the frequency of appearance and the coverage of the citing article they are extracted from (coverage of a citing article refers to the number of cited references of the cluster that it cites). Heavier weighting is given to the noun phrases extracted from high coverage articles because they are more instrumental in the development of the cluster. These noun phrases are sorted based on this score and the top ones are used as a guide for the naming of the cluster. This means that labelling is done by the field expert but guided by an algorithmic determination. In the visualisation, parts of the network that have been most active during each year appear more striking, representing co-citation instances during that year. Influential references are identified using the three metrics (local citations, bursts, centrality). However, these metrics are measuring articles that may or may not be about health economics, so we must also look at the citing articles with the highest coverage to determine which articles related to health economics are citing the most references within the specific research stream.
The time period for the analysis was set for 1990–2021 (1-year intervals; look back years = 50 [reference lists published less than 50 years ago]). Each node represents an individual reference. The size of the node is proportional to the number of local citations identified to that reference, and the nodes are connected by links (indicating co-occurrence of co-citation) to create a network of major research streams, all contained within the field of health economics. Each stream has a descriptor based on the contents of the cluster. Furthermore, CiteSpace analysis also provides a timeline view of the evolution of research streams. The references of each stream are visualised and aligned across the timeline based on the year of publication from 1950–2021.
General findings and the history of health economics
The size of the specialised field of health economics is estimated to be 12,977 items, as of December 31, 2021. The first article published in a specialty journal ( Journal of Health Economics ) is ‘Effects of teaching on hospital costs’ in 1983 [ 16 ]. The following decade saw only a small number of documents published before a significant increase in research output was observed around the mid-1990s (Fig. 1 ). Since then, there has been an upwards trend, with post-2005 showing a sharp incline in the number of publications.
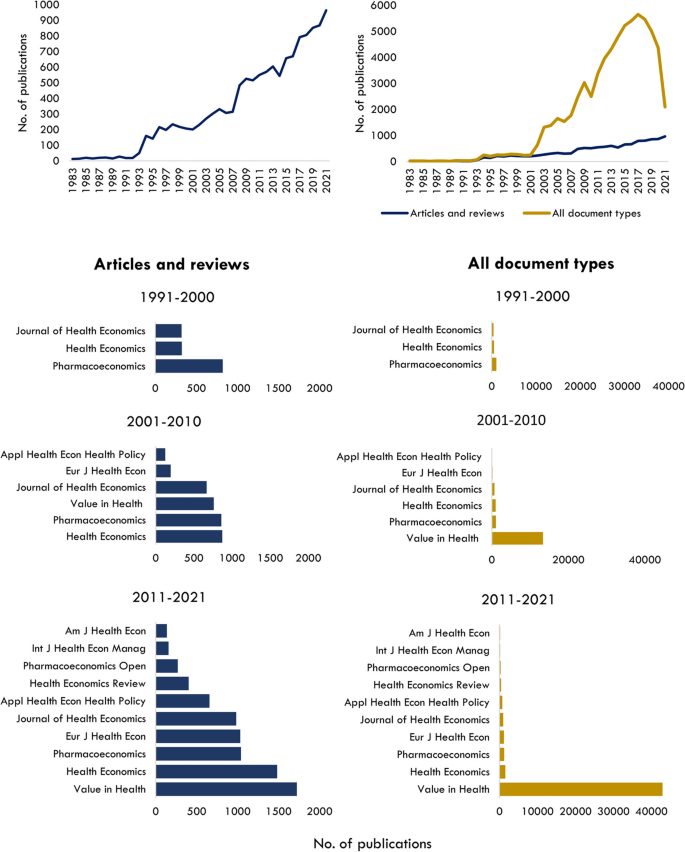
Above (L) Total number of articles and review articles in health economics specialty journals; Above (R) All document types versus total number of articles and reviews in health economics specialty journals; Bar graphs (L) Number of documents by journal source for articles and review articles. Bar graphs (R) Number of documents by journal source for all document types
If all document types were included in the field analysis, there would be nearly 70,000 items, with meeting abstracts published in Value in Health contributing to around 80% of documents (Fig. 1 ). Over the past three decades, the number of specialised health economics journals in this field has grown from three to ten, with Health Economics and Value in Health publishing the most literature in 2010–2021 (Fig. 1 ).
The onset of Covid-19 in early 2020 has not dampened publication of health economics articles and reviews, however, surprisingly only 72 published articles directly explore the topics related to the pandemic. Conversely, a large decline in meeting abstracts has occurred over the past 3 years, however, if and how the pandemic has contributed is unclear, as the decline started in 2019 (from 4,500 to 4000 in the years 18–19) and cannot be solely attributed to a reduction in organised conferences.
An overview of the articles specific subject areas was identified using WoS Categories . Unsurprisingly, all records are indexed in the disciplines of Economics and Health Policy Services (12,977 records, 100%). Other categories include Health Care Sciences Services (11,039 records, 85%), Pharmacology Pharmacy (2,992 records, 23%) and Business Finance (156 records, 1%).
Over 26,000 scholars have contributed to health economics research, of which 242 authors have published 15 or more documents related to this field. The top published authors include John Brazier ( n = 78 records), Werner Brouwer ( n = 64), Michael Drummond ( n = 55) and Maarten Postma ( n = 54). The top ranked academic institutions include the League of European Research Universities (7.5% of total publications), Erasmus University Rotterdam (5%), University of London (5%), University of York [UK] (4.5%) and Harvard University (3.5%).
The main body of research output in health economics is exclusive to six countries: USA, England, Netherlands, Canada, Australia, and Germany. More recently however, countries in Eastern Europe, Africa, Southeast Asia and the Middle East have become more prominent researchers in health economics. Over the previous three decades, the top five countries have remained mostly consistent (Fig. 2 ), except for Australia, where scholarly output in this area is growing extensively.
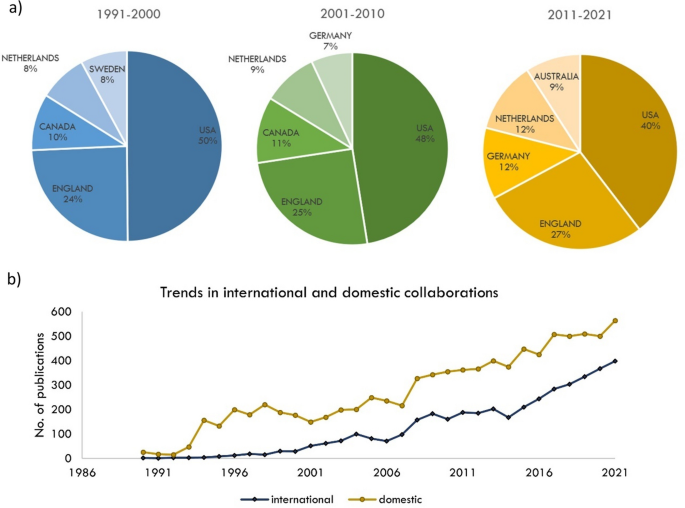
a Top five countries to contribute to health economics research output, by decade; b domestic versus international collaboration
Since 2015, international collaboration has been sharply on the rise (Fig. 2 ). The gap between domestic and international collaborated publications appears to be closing. Currently, domestic publications contribute to 58.6% of the scholarly output compared to 41.4% international publications, however, over the past 10 years, the average rate of annual increase in internationally collaborated publications is almost double that of domestic collaborations (8.4% vs 4.9%). The main six countries in health economics show patterns of strong international collaboration. Together, they have produced approximately one third of the research field (4,000 articles). The strongest links are between the USA and England, USA and Canada, and England and The Netherlands.
Semantic analysis; titles, abstracts and keywords
Five major divisions were identified in the field of health economics (Fig. 3 ). 1) Macro-economics, 2) Micro-economics, 3) Measurement and valuation of outcomes, 4) Monitoring mechanisms and 5) Guidance and appraisal. Division 3, measurements and valuation of outcomes is the most cited, and division 5, Guidance and appraisal has the most recent publications.

Major divisions of health economics. Below (L) divisions of bibliographic coupling; Below (R) average number of citations and average year of publication for each major division. Interactive version of the title and abstract map are available via this link: VOSviewer Online
Bibliographic coupling resulted in similar divisions of health economics research areas. Macro-economics (purple) and micro-economics (green) are the densest divisions, showing extensive overlap of references. Methods for measurement and valuation of patient outcomes, including Discrete Choice Experiments (DCEs), and the EQ-5Dto a lesser extent, are central to both macro- and micro-economics. Table 1 shows the top title and abstract terms of each major division in health economics.
The composition of the field of health economics research is dynamic. Keyword analysis across three decades shows there are common research themes including, cost effectiveness , QALYs and economic evaluation (Fig. 4 ). However, there is a distinct shift to health-related quality of life (HRQoL) in the early millennium, followed by the appearance of DCEs in the most recent decade. Unsurprisingly obesity , a global epidemic of the 21 st century, has also been a topic of focus for scholarly research since 2010.
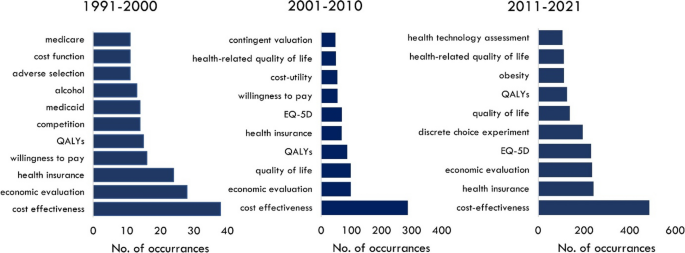
Top keywords in health economics, by decade
Influential references
This section acknowledges the most influential entities (authors and references) in health economics, aiming to pave the way for further interdisciplinary collaborations and advancements in the domain. These are the most influential entities in a subset of health economics journals. Although the analysis considered a large number of articles (approximately 13,000), it’s important to recognize that there may be other influential entities not represented in this paper.
The top ten globally cited articles have quite distinct topics (Appendix 1 ). The most cited article, according to WoS, is ‘The price of innovation: new estimates of drug development costs’, authored by DiMasie et al. and is published in Journal of Health Economics. The article has received 2,475 citations, and provides data used to estimate the average pre-tax of new drug development [ 17 ].
Influential articles relevant to health economics, ranked by local citation count, are listed in Appendix 2 . The most cited article specific to this research field is ‘Recommendations of the Panel on Cost-effectiveness in Health and Medicine’, published in JAMA in 1996 [ 18 ]. The authors recommended that if researchers follow a standard set of methods in cost-effectiveness analysis, the utility of studies can be much improved. Lastly, the articles that have had the strongest burst of citations since publication are shown in Appendix 3 . This article, published in 2016 and titled ‘Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses’ provides major changes to the recommendations made by Weinstein et al. in 1996 [ 19 , 20 ].
A major focus is on identifying temporal patterns of scholarly research in this field and the formation of its various research streams as well as the most influential entities within each stream. Document co-citation analysis revealed eighteen research streams. Figure 5 shows a bird’s-eye view of the field and Table 2 identifies the influential references that have shaped each stream. Two streams related to Economic Evaluation emerged, with slight variations. ‘Overall’ Economic Evaluation is broader and includes guidelines, applications of evaluation, reviews of evaluation studies, and articles reporting on willingness to pay studies. ‘Elements’ of Economic Evaluation includes steps involved in evaluation, criteria for evaluation and is mostly focussed on cost-effectiveness studies. These are both central to the field of health economics and are very active areas of research every year, as reflected in instances of article co-citation (Fig. 6 ). Economic Evaluation is closely related to the activities in Patient Preference and Health-related Quality of Life research (involving measurement tools such as DCEs and EQ-5D, respectively). Figure 7 shows the research streams in time-line format for clear observation of bursts of activity since 1950.
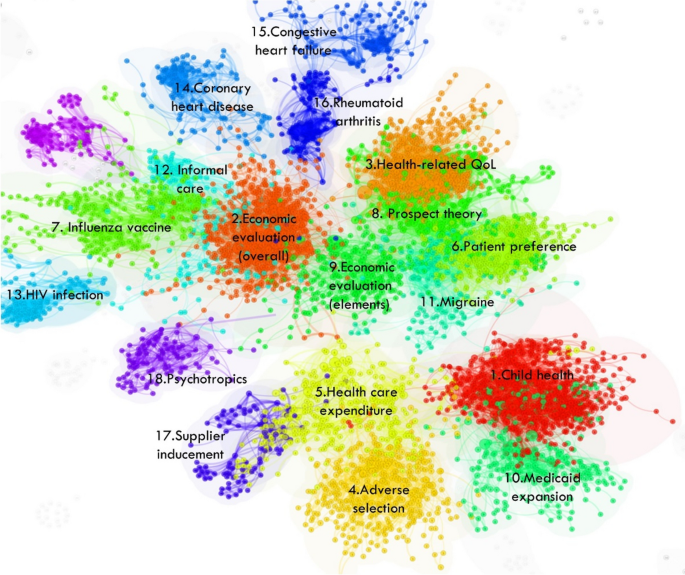
Bird’s-eye view of the major research streams in the field of health economics
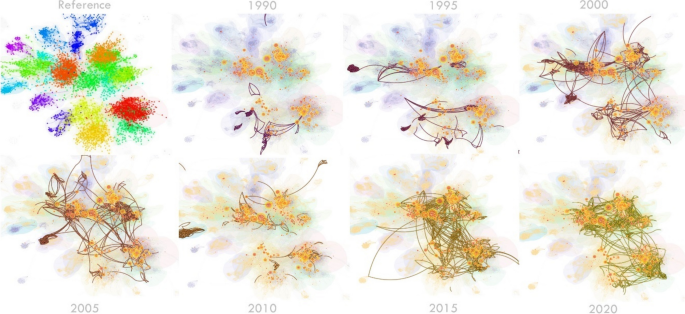
State of health economics literature during the last three decades of development. Salient parts of the map specify active areas of research during each year, as reflected in instances of article co-citation. A dynamic visualisation from 1990–2021 is available here https://unisyd-my.sharepoint.com/:v:/g/personal/clara_zwack_sydney_edu_au/EeT-KZTsqdJHuGzL6s-R9ksBzmQ0ln-2jjYJu5Cv7F0usg?e=pkaOqt

Timeline view of the major research streams in health economics
Co-citation also identified variation in the magnitude of activities in each of the streams (Fig. 8 ). A recent emergence of heightened research activities in health economics was only seen in the Medicaid Expansion stream. Medicaid expansion is an United States initiative with the goal to increase insurance coverage among low-income adults. It became effective in January 2014, which aligns with the clusters research activity increasing around 2015. Established research streams that continue to show high levels of activity include Child Health, HRQoL and Economic Evaluation (elements). Conversely, Patient Preference, Health care Expenditure and Economic Evaluation (overall) are now past their peak of activity and are slowing down in specialised health economic journals.
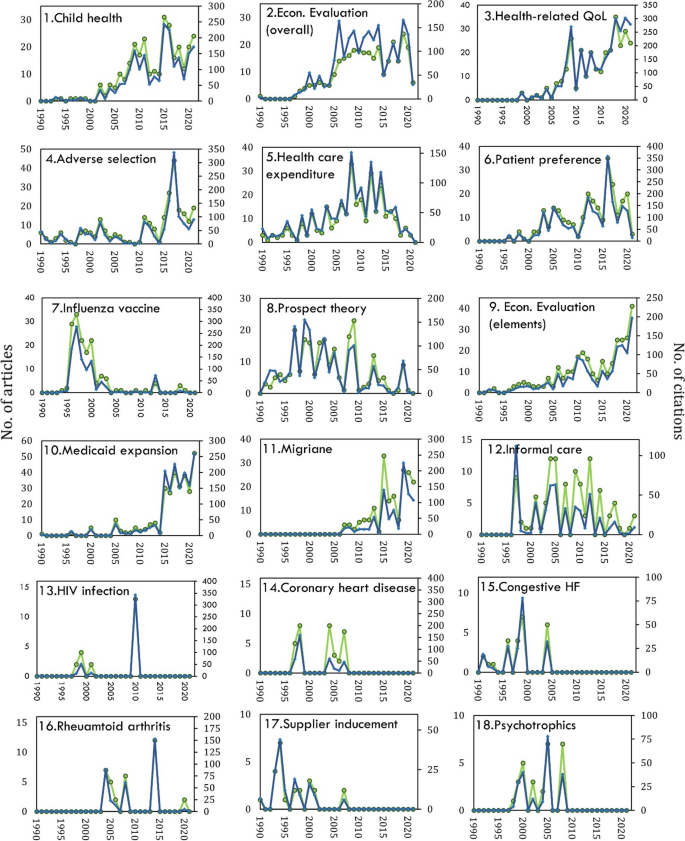
Number of citations (blue) and number of citing articles (green) for each research stream. Note: scale is different for each cluster. Y-axis is Number of articles and X-axis is Number of citations
Three streams show fluctuating patterns of activity: Adverse Selection (a phenomenon where individuals with higher risks or health issues are more likely to seek or retain health insurance coverage compared to individuals with lower risks), Migraine and Rheumatoid Arthritis. Analysis also identified several streams in this field that have transient peaks of activity and are currently not active. These include Influenza Vaccine, Prospect Theory, Coronary Heart Disease, Congestive Heart Failure, Supplied Inducement and Psychotropics. Lastly, HIV Infection had a very transient period of activity in the early 2000’s. It has since been mostly non-existent, aside from a distinct peak in 2010 where 13 citing articles gave a total coverage of around 140. The critical references were studies measuring the cost effectiveness of Darunavir/Ritonavir, a HIV antiviral drug [ 305 , 309 , 311 ].
This scientometric analysis presents an overview of health economics research exclusively from the top journals specific to the field. Evaluation of around 13,000 documents has revealed contemporary patterns of publication, authorship, and research activities. Five major divisions have been identified within the field using objective clustering methods. This includes macro-economics, micro-economics, measurement and valuation of outcomes, monitoring mechanisms (evaluation), and guidance and appraisal. Along with the major divisions, analysis of document co-citation revealed eighteen specific research streams, each showing varying levels of activity.
Interestingly, there are few ‘hot topics’ emerging in health economics. One possible reason for this could be that the pace of research in health economics could be to some degrees determined by the field of economics and advancement within that mother field, which is considered slow-moving in terms of establishment of new trends [ 401 ]. Economists tend to be cautious in recognising emerging areas of research, and instead prefer to use an established knowledge base when supporting their research with previous literature.
In a world where digital transformation is changing the face of every industry, including health care, it is surprising that economic evaluation of digital health innovations has not emerged as a trending research topic. However, there are examples in the literature highlighting the complexities of economic analysis for digital health innovations, which may be stalling the progression of this research area [ 402 , 403 , 404 ]. As the knowledge foundation for these freshly emerging areas develop, subsequent analyses of similar nature may be able to detect them as emerging divisions. This knowledge foundation could currently be scattered and not established. The emergence and progression of such area, however, could be detectable with a time lag once the health economics literature begins to converge on a specific cohort of references as the knowledge base in this area.
A sharp rise in scholarly output in health economics was observed around 2005. This is likely around the time that DCEs and patient preference surveys became trendy in healthcare [ 405 ]. After heightened research activity in this area for a decade (2005–2015), the Patient Preference research stream has now passed its peak in specialised health economic journals. However, this does not necessarily mean that it is no longer trendy. In fact, it is known that DCEs have now been more widely adopted to elicit preferences for health care products and programs across most medical fields [ 164 , 406 ]. Peer-reviewed articles are now likely being published in discipline-specific or broader health journals (e.g., British Medical Journal, Health Service Research Journal), rather than the health economics sources used in this analysis.
The main body of this literature has been produced by six countries in Europe, North America and Australia. Since the inception and rapid growth of health economics in the early 1990s, contribution to scholarly literature from these six countries has mostly been consistent, aligning with reports by Wagstaff and Culyer [ 12 ]. Few non-OECD countries are included in the top contributors to this research field. For example, China, which now surpassed the USA as the largest producer of scientific research in certain disciplines [ 407 ], is not a major contributor to health economics research. However, this may be because China’s primary research foci are technological fields and chemistry, and not social sciences. It is also promising to see recent health economic research output increasing in Low- and Middle-Income Countries. Internationally collaborated research output appears to be moving closer to the domestic output, a promising sign of a connected research field. However, the diversity of health care systems and unique public health issues will likely ensure that domestic research continues to thrive. Applications of new knowledge are often exclusive to a standalone health care system.
It should be noted that the conclusions of this study rely only on a sample of the literature of health economics, by analysing the collective content of ten mainstream health economics journals. While large enough to identify the research trends in the field, as the main motive of the study, the underlying dataset does not necessarily embody the entire literature of health economics. This limitation is simply due to the fact that an attempt for obtaining the entirety of health economics literature seems impossible without jeopardising the dataset with too many false positives. However, it should also be considered that the analytic methodology from which the core findings have been obtained has been chosen such that trends can be identified with minimal sensitivity to missing items in the dataset. The methodology of document co-citation analysis that has produced the core findings of the study is fairly robust to the effects of sampling and potential missing items. This is simply due to the fact that, in this methodology, influential references as well as trends are identified by referring to the reference lists of the articles in the dataset. In other words, the entities of analysis are items listed as the references of the papers in the dataset as opposed to the articles of the dataset itself (as in an article bibliographic coupling analysis for example [ 408 , 409 ]). In a document co-citation approach, the formation of a cluster on topic X does not rely capturing all citing articles that have contributed to the creation of stream/cluster X. If a large enough subset of such citing articles are captured in the data, then stream X as well as its temporal trends will still manifest. This is particularly the case in relation to the major streams (as opposed top smaller/minor clusters) whose sensitivity to the sample is minimal. For that reason, the analyses of this study were limited exclusively to interpreting the major streams only and minor clusters were excluded from an in-depth interpretation. For a typical cluster on a topic such as X, it is possible that papers outside the content of the ten specialty journals (i.e., the current dataset) are also identifiable, in addition to papers related to such topic and disseminated in mainstream specialty journals. But so long as enough of such papers do exist within the content of specialty journals, then the cohort of references co-cited by those papers will still form that stream and topic X along with the temporal patterns of its evolution is still captured by the sample. In summary, the coverage of the underlying data of this study can be improved, but at the same time, we believe that the sensitivity of the main findings to potential missing literature is rather minimal.
The current state of research in health economics has brought valuable insight into healthcare interventions, market dynamics and behavioural factors. Health economics is a growing field, yet there is minimal evidence of creation of new research trends. This doesn’t necessarily indicate that there are no ‘hot topics’ in health economics, but likely that the new research is being disseminated in sources beyond the speciality journals. Over the past 10 years, the average rate of annual increase in internationally collaborated publications is almost double that of domestic collaborations (8.4% vs 4.9%), but most of the top scholarly collaborations remain between six countries only.
Several avenues for future research exist to deepen our understanding and address the evolving challenges in this field. By considering broader societal perspectives, embracing technological advancements, and integrating behavioural insights, health economist researchers can contribute to evidence-based policy-making and drive improvements in healthcare outcomes, efficiency, and equity.
Availability of data and materials
All data is available upon reasonable request to the corresponding author.
Phelps CE. Health economics. New York: Routledge; 2017.
Brazier J, Ratcliffe J, Saloman J, Tsuchiya A. Measuring and valuing health benefits for economic evaluation. Oxford: Oxford University Press; 2017.
Weatherly H, Drummond M, Claxton K, Cookson R, Ferguson B, Godfrey C, Rice N, Sculpher M, Sowden A. Methods for assessing the cost-effectiveness of public health interventions: key challenges and recommendations. Health Policy. 2009;93(2–3):85–92.
Article PubMed Google Scholar
Jakovljevic M, Ogura S. Health economics at the crossroads of centuries – from the past to the future. Front Public Health. 2016;4:115.
Garg S, Norman GJ. Impact of COVID-19 on health economics and technology of diabetes care: use cases of real-time continuous glucose monitoring to transform health care during a global pandemic. Diabetes Technol Ther. 2021;23(S1):S-15.
Article PubMed Central Google Scholar
Hatswell AJ. Learnings for health economics from the early stages of the COVID-19 pandemic. Pharmacoecon Open. 2020;4(2):203–5.
Article PubMed PubMed Central Google Scholar
Callander EJ, Fox H, Lindsay D. Out-of-pocket healthcare expenditure in Australia: trends, inequalities and the impact on household living standards in a high-income country with a universal health care system. Heal Econ Rev. 2019;9(1):1–8.
Google Scholar
Rice T, Quentin W, Anell A, Barnes AJ, Rosenau P, Unruh LY, Van Ginneken E. Revisiting out-of-pocket requirements: trends in spending, financial access barriers, and policy in ten high-income countries. BMC Health Serv Res. 2018;18(1):1–18.
Article CAS Google Scholar
Arrow KJ. 21 - Uncertainty and the welfare economics of medical care. In: Diamond P, Rothschild M, editors. Uncertainty in economics. Pittsburgh: Academic Press; 1978. p. 345–75.
Haghani M. What makes an informative and publication-worthy scientometric analysis of literature: a guide for authors, reviewers and editors. Transp Res Interdiscip Perspect. 2023;22:100956.
Rubin RM, Chang CF. A bibliometric analysis of health economics articles in the economics literature: 1991–2000. Health Econ. 2003;12(5):403–14.
Wagstaff A, Culyer AJ. Four decades of health economics through a bibliometric lens. J Health Econ. 2012;31(2):406–39.
Zwack CC, Haghani M, Hollings M, Zhang L, Gauci S, Gallagher R, Redfern J. The evolution of digital health technologies in cardiovascular disease research. npj Digit Med. 2023;6(1):1.
Chen C. The citespace manual. Coll Comput Inform. 2014;1:1–84.
CAS Google Scholar
Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci. 2004;101(suppl 1):5303–10.
Article CAS PubMed PubMed Central Google Scholar
Sloan F, Feldman R, Steinwald AB. Effects of teaching on hospital costs. J Health Econ. 1983;2:1–28.
Article CAS PubMed Google Scholar
DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151–85.
Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–8.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, Salomon JA, Sculpher MJ, Trikalinos TA, Russell LB, Siegel JE, Ganiats TG. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses. JAMA. 2016;316(10):1093–103.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, Salomon JA, Sculpher MJ, Trikalinos TA, Russell LB, Siegel JE, Ganiats TG. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103.
Grossman M. The Demand for Health: A Theoretical and Empirical Investigation. Columbia University Press; 1972.
Grossman M. On the concept of health capital and the demand for health. J Polit Econ. 1972;80(2):223–55.
Article Google Scholar
Wooldridge JM. Econometric analysis of cross section and panel data. 2002.
Bound J, Jaeger DA, Baker RM. Problems with instrumental variables estimation when the correlation between the instruments and the endogeneous explanatory variable is weak. J Am Stat Assoc. 1995;90(430):443–50.
Cawley J. An economy of scales: a selective review of obesity’s economic causes, consequences, and solutions. J Health Econ. 2015;43:244–68.
Angrist JD. Mostly harmless econometrics: an empiricist’s companion. Princeton: Princeton University Press; 2009.
Book Google Scholar
vanDoorslaer E, Wagstaff A, Bleichrodt H, Calonge S, Gerdtham UG, Gerfin M, Geurts J, Gross L, Hakkinen U, Leu RE, Odonnell O, Propper C, Puffer F, Rodriguez M, Sundberg G, Winkelhake O. Income-related inequalities in health: some international comparisons. J Health Econ. 1997;16(1):93–112.
Barbaresco S, Courtemanche CJ, Qi Y. Impacts of the Affordable Care Act dependent coverage provision on health-related outcomes of young adults. J Health Econ. 2015;40:54–68.
Bound J. Self-reported versus objective measures of health in retirement models. J Hum Resour. 1991;26(1).
Baum CL, Ruhm CJ. Age, socioeconomic status and obesity growth. J Health Econ. 2009;28(3):635–48.
Becker GS, Murphy KM. A theory of rational addiction. J Polit Econ. 1988;96(4):675–700.
Wooldridge JM. Econometric analysis of cross section and panel data. 2nd ed. Massachusetts: MIT Press; 2010.
Garrouste C, Godard M. The lasting health impact of leaving school in a bad economy: Britons in the 1970s recession. Health Econ. 2016;25:70–92.
Staiger D, Stock JH. Instrumental variables regression with weak instruments. Econometrica. 1997;65(3):557–86.
Maddala GS. Limited-dependent and qualitative variables in econometrics. Cambridge: Cambridge University Press; 1983.
Arellano M, Bond S. Some tests of specification for panel data: Monte Carlo evidence and an application to employment equations. Rev Econ Stud. 1991;58(2):277–97.
Chuard C. Womb at work: the missing impact of maternal employment on newborn health. J Health Econ. 2020;73:102342.
Ruhm CJ. Are recessions good for your health? Q J Econ. 2000;115(2):617–50.
Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31(1):219–30.
Colmer J, Lin D, Liu S, Shimshack J. Why are pollution damages lower in developed countries? Insights from high-Income, high-particulate matter Hong Kong. J Health Econ. 2021;79:102511.
Chaloupka F. Rational addictive behavior and cigarette smoking. J Polit Econ. 1991;99(4):722–42.
Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;91(434):444–55.
Braakmann N. The causal relationship between education, health and health related behaviour: evidence from a natural experiment in England. J Health Econ. 2011;30(4):753–63.
Gerdtham U-G, Ruhm CJ. Deaths rise in good economic times: evidence from the OECD. Econ Hum Biol. 2006;4(3):298–316.
Gong J, Lu Y, Xie H. The average and distributional effects of teenage adversity on long-term health. J Health Econ. 2020;71:102288.
Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38(1):21–37.
Lee DS, Lemieux T. Regression discontinuity designs in economics. J Econ Lit. 2010;48(2):281–355.
Fleurbaey M, Schokkaert E. Unfair inequalities in health and health care. J Health Econ. 2009;28(1):73–90.
Ruhm CJ. Healthy living in hard times. J Health Econ. 2005;24(2):341–63.
Ásgeirsdóttir TL, Jóhannsdóttir HM. Income-related inequalities in diseases and health conditions over the business cycle. Health Econ Rev. 2017;7(1):12.
Case A, Lubotsky D, Paxson C. Economic status and health in childhood: the origins of the gradient. Am Econ Rev. 2002;92(5):1308–34.
Cleeren K, Lamey L, Meyer J-H, De Ruyter K. How business cycles affect the healthcare sector: a cross-country investigation. Health Econ. 2016;25(7):787–800.
Bago d’Uva T, Van Doorslaer E, Lindeboom M, O’Donnell O. Does reporting heterogeneity bias the measurement of health disparities? Health Econ. 2008;17(3):351–75.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programme. 3rd ed. Oxford: Oxford University Press; 2005.
Efron B, Tibshirani RJ. An introduction to the bootstrap. 1994.
Heather EM, Payne K, Harrison M, Symmons DPM. Including adverse drug events in economic evaluations of anti-tumour necrosis factor-α drugs for adult rheumatoid arthritis: a systematic review of economic decision analytic models. Pharmacoeconomics. 2013;32(2):109–34.
Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13(4):397–409.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–50.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. Consolidated health economic evaluation reporting standards (CHEERS) statement. Pharmacoeconomics. 2013;31(5):361–7.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. Consolidated health economic evaluation reporting standards (CHEERS) statement. Eur J Health Econ. 2013;14(3):367–72.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health. 2013;16(2):e1–5.
Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ. 1999;18(3):341–64.
Vanhout BA, Al MJ, Gordon GS, Ruten FFH. Costs, effects and c/e-ratios alongside a clinical-trial. Health Econ. 1994;3(5):309–19.
Ades AE, Claxton K, Sculpher M. Evidence synthesis, parameter correlation and probabilistic sensitivity analysis. Health Econ. 2006;15(4):373–81.
Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, Lu G. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics. 2006;24(1):1–19.
Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18(2 Suppl):S68-80.
Barton GR, Sach TH, Doherty M, Avery AJ, Jenkinson C, Muir KR. An assessment of the discriminative ability of the EQ-5Dindex, SF-6D, and EQ VAS, using sociodemographic factors and clinical conditions. Eur J Health Econ. 2007;9(3):237–49.
Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, Luce BR. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices-modeling studies. Value Health. 2003;6(1):9–17.
Vemer P, Corro Ramos I, van Voorn GA, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–61.
Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. a practical approach. Med Decis Making. 1985;5(2):157–77.
Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500.
O’Brien BJ, Drummond MF, Labelle RJ, William A. In search of power and significance: issues in the design and analysis of stochastic cost-effectiveness studies in health care. Med Care. 1994;32(2):150–63.
Coyle D, Lee KM, O’Brien BJ. The role of models within economic analysis. Pharmacoeconomics. 2002;20(Supplement 1):11–9.
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–38.
Barton P, Bryan S, Robinson S. Modelling in the economic evaluation of health care: selecting the appropriate approach. J Health Serv Res Policy. 2004;9(2):110–8.
Drummond M, Barbieri M, Cook J, Glick HA, Lis J, Malik F, Reed SD, Rutten F, Sculpher M, Severens J. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health. 2009;12(4):409–18.
Buxton MJ, Drummond MF, VanHout BA, Prince RL, Sheldon TA, Szucs T, Vray M. Modelling in economic evaluation: an unavoidable fact of life. Health Econ. 1997;6(3):217–27.
Birch S, Gafni A. Information created to evade reality (ICER). Pharmacoeconomics. 2006;24(11):1121–31.
Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10(8):779–87.
Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ. 1997;6(4):327–40.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–108.
Drummond MF, Sculpher MJ, Claxton K, Stoddart K, Torrance GW. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: Oxford University Press; 2015.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 2015.
Ware JE, Sherbourne CD. The MOS 36-ltem Short-Form Health Survey (SF-36). Med Care. 1992;30(6):473–83.
Golicki D, Jakubczyk M, Graczyk K, Niewada M. Valuation of EQ-5D-5L health states in Poland: the first EQ-VT-based study in central and eastern Europe. Pharmacoeconomics. 2019;37(9):1165–76.
Bleichrodt H. A new explanation for the difference between time trade-off utilities and standard gamble utilities. Health Econ. 2002;11(5):447–56.
Attema AE, Edelaar-Peeters Y, Versteegh MM, Stolk EA. Time trade-off: one methodology, different methods. Eur J Health Econ. 2013;14(S1):53–64.
Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–92.
Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36.
Feeny D, Furlong W, Boyle M, Torrance GW. Multi-attribute health status classification systems. Pharmacoeconomics. 1995;7(6):490–502.
Engel L, Bryan S, Whitehurst DGT. Conceptualising ‘benefits beyond health’ in the context of the quality-adjusted life-year: a critical interpretive synthesis. Pharmacoeconomics. 2021;39(12):1383–95.
Bansback N, Brazier J, Tsuchiya A, Anis A. Using a discrete choice experiment to estimate health state utility values. J Health Econ. 2012;31(1):306–18.
Brown CC, Tilford JM, Payakachat N, Williams DK, Kuhlthau KA, Pyne JM, Hoefman RJ, Brouwer WBF. Measuring health spillover effects in caregivers of children with autism spectrum disorder: a comparison of the EQ-5D-3L and SF-6D. Pharmacoeconomics. 2019;37(4):609–20.
Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72.
Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27(1):7–22.
Feeny D, Furlong W, Torrance GW, Goldsmith CH, Zhu Z, Depauw S, Denton M, Boyle M. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care. 2002;40(2):113–28.
Oppe M, Devlin NJ, van Hout B, Krabbe PFM, de Charro F. A program of methodological research to arrive at the new international EQ-5D-5L valuation protocol. Value Health. 2014;17(4):445–53.
Dolan P, Gudex C, Kind P, Williams A. The time trade-off method: results from a general population study. Health Econ. 1996;5(2):141–54.
Dolan P, Gudex C, Kind P, Williams A. Valuing health states: a comparison of methods. J Health Econ. 1996;15(2):209–31.
Barton GR, Sach TH, Avery AJ, Jenkinson C, Doherty M, Whynes DK, Muir KR. A comparison of the performance of the EQ-5D and SF-6D for individuals aged ≥ 45 years. Health Econ. 2008;17(7):815–32.
Versteegh MM, Vermeulen KM, Evers SMAA, de Wit GA, Prenger R, Stolk EA. Dutch tariff for the five-level version of EQ-5D. Value Health. 2016;19(4):343–52.
Jensen CE, Sørensen SS, Gudex C, Jensen MB, Pedersen KM, Ehlers LH. The Danish EQ-5D-5L value set: a hybrid model using cTTO and DCE data. Appl Health Econ Health Policy. 2021;19(4):579–91.
Al Shabasy SA, Abbassi MM, Finch AP, Baines D, Farid SF. RETRACTED ARTICLE: the EQ-5D-5L valuation study in Egypt. Pharmacoeconomics. 2021;39(5):549–61.
Brazier J. Measuring and valuing health benefits for economic evaluation. 2007.
Lipman SA, Brouwer WBF, Attema AE. The corrective approach: policy implications of recent developments in QALY measurement based on prospect theory. Value Health. 2019;22(7):816–21.
Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–9.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, Haes JCJMD, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Ntl Cancer Inst. 1993;85(5):365–76.
Arrow KJ. Uncertainty and the welfare economics of medical care. Am Econ Rev. 1963;53(5):941–73.
Layton TJ, Ellis RP, McGuire TG, van Kleef R. Measuring efficiency of health plan payment systems in managed competition health insurance markets. J Health Econ. 2017;56:237–55.
Ellis RP, McGuire TG. Provider behavior under prospective reimbursement. J Health Econ. 1986;5(2):129–51.
Clemens J, Gottlieb JD. Do physicians’ financial incentives affect medical treatment and patient health? Am Econ Rev. 2014;104(4):1320–49.
Blaug M. Where are we now in British health economics? Health Econ. 1998;7(S1):S63–78.
Rothschild M, Stiglitz J. Equilibrium in competitive insurance markets: an essay on the economics of imperfect information. Q J Econ. 1976;90(4);257–80.
Newhouse J. Reimbursing health plans and health providers: efficiency in production versus selection. J Econ Lit. 1996;34(3):1236–63.
Pilny A, Wübker A, Ziebarth NR. Introducing risk adjustment and free health plan choice in employer-based health insurance: evidence from Germany. J Health Econ. 2017;56:330–51.
Ma C-TA. Health care payment systems: cost and quality incentives. J Econ Manag Strategy. 1994;3(1):93–112.
Cooper Z, Gibbons S, Jones S, McGuire A. Does hospital competition save lives? Evidence from the English NHS patient choice reforms. Econ J. 2011;121(554):F228–60.
Decarolis F, Guglielmo A. Insurers’ response to selection risk: evidence from Medicare enrollment reforms. J Health Econ. 2017;56:383–96.
Kessler DP, McClellan MB. Is hospital competition socially wasteful? Q J Econ. 2000;115(2):577–615.
Dafny LS. How do hospitals respond to price changes? Am Econ Rev. 2005;95(5):1525–47.
Layton TJ. Imperfect risk adjustment, risk preferences, and sorting in competitive health insurance markets. J Health Econ. 2017;56:259–80.
Cutler DM, Reber SJ. Paying for health insurance: the trade-off between competition and adverse selection. Q J Econ. 1998;113(2):433–66.
Andrews DWK, Stock JH, Rothenberg TJ. Identification and inference for econometric models: essays in honor of Thomas Rothenberg. Cambridge; New York: Cambridge University Press; 2005.
Brosig-Koch J, Hehenkamp B, Kokot J. The effects of competition on medical service provision. Health Econ. 2017;26:6–20.
Gaynor M, Town RJ. Competition in Health Care Markets11We wish to thank participants at the Handbook of Health Economics meeting in Lisbon, Portugal, Pedro Pita Barros, Rein Halbersman, and Cory Capps for helpful comments and suggestions. Misja Mikkers, Rein Halbersma, and Ramsis Croes of the Netherlands Healthcare Authority graciously provided data on hospital and insurance market structure in the Netherlands. David Emmons kindly provided aggregates of the American Medical Association’s calculations of health insurance market structure. Leemore Dafny was kind enough to share her measures of market concentration for the large employer segment of the US health insurance market. All opinions expressed here and any errors are the sole responsibility of the authors. No endorsement or approval by any other individuals or institutions is implied or should be inferred. 2011. p. 499–637.
Selden TM. A model of capitation. J Health Econ. 1990;9(4):397–409.
van Kleef RC, McGuire TG, van Vliet RCJA, van de Ven WPPM. Improving risk equalization with constrained regression. Eur J Health Econ. 2016;18(9):1137–56.
McGuire TG. Physician agency. In: Culyer AJ, Newhouse JP, editors. Handbook of health economics, vol. 1. Elsevier; 2000. p. 461–536.
Gaynor M, Moreno-Serra R, Propper C. Death by market power: reform, competition, and patient outcomes in the National Health Service. Am Econ J Econ Pol. 2013;5(4):134–66.
Ellis RP, McGuire TG. Optimal payment systems for health services. J Health Econ. 1990;9(4):375–96.
Chalkley M, Malcomson JM. Contracting for health services when patient demand does not reflect quality. J Health Econ. 1998;17(1):1–19.
Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–94.
Duan N, Manning WG, Morris CN, Newhouse JP. A comparison of alternative models for the demand for medical care. J Bus Econ Stat. 1983;1(2):115–26.
Blough DK, Madden CW, Hornbrook MC. Modeling risk using generalized linear models. J Health Econ. 1999;18(2):153–71.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Greene WH. Econometric analysis. Boston: Prentice Hall; 2012.
Gaynor M, Anderson GF. Uncertain demand, the structure of hospital costs, and the cost of empty hospital beds. J Health Econ. 1995;14(3):291–317.
Duan N. Smearing estimate: a nonparametric retransformation method. J Am Stat Assoc. 1983;78(383):605–10.
Manning WG. The logged dependent variable, heteroscedasticity, and the retransformation problem. J Health Econ. 1998;17(3):283–95.
Tran-Duy A, Boonen A, Kievit W, van Riel PLCM, van de Laar MAFJ, Severens JL. Modelling outcomes of complex treatment strategies following a clinical guideline for treatment decisions in patients with rheumatoid arthritis. Pharmacoeconomics. 2014;32(10):1015–28.
Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47(1);153–61.
Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24(3):465–88.
Hausman JA. Specification tests in econometrics. Econometrica. 1978;46(6):1251–71.
Mullahy J. Much ado about two: reconsidering retransformation and the two-part model in health econometrics. J Health Econ. 1998;17(3):247–81.
Basu A, Rathouz PJ. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2004;6(1):93–109.
Vita MG. Exploring hospital production relationships with flexible functional forms. J Health Econ. 1990;9(1):1–21.
Breusch TS, Pagan AR. A simple test for heteroscedasticity and random coefficient variation. Econometrica. 1979;47(5):1287–94.
Moscone F, Tosetti E. Health expenditure and income in the United States. Health Econ. 2010;19(12):1385–403.
Cameron AC, Trivedi PK. Microeconometrics: methods and applications. Cambridge; New York: Cambridge University Press; 2005.
White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48(4):817–38.
Seshamani M, Gray A. Ageing and health-care expenditure: the red herring argument revisited. Health Econ. 2004;13(4):303–14.
Seshamani M, Gray AM. A longitudinal study of the effects of age and time to death on hospital costs. J Health Econ. 2004;23(2):217–35.
Auster R, Leveson I, Sarachek D. The production of health, an exploratory study. J Hum Resour. 1969;4(4):135–58.
Sloan FA, Hsieh CR. Health economics. 2012.
Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation? J Health Econ. 2004;23(3):525–42.
Martin S, Smith PC. Rationing by waiting lists: an empirical investigation. J Public Econ. 1999;71(1):141–64.
Balia S, Brau R. A country for old men? Long-term home care utilization in Europe. Health Econ. 2014;23(10):1185–212.
Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2010;20(8):897–916.
Louviere JJ, Hensher DA, Swait JD, Adamowicz W. Stated choice methods. 2010.
Diener A, O’Brien B, Gafni A. Health care contingent valuation studies: a review and classification of the literature. Health Econ. 1998;7(4):313–26.
Louviere JJ, Hensher DA, Swait JD. Stated choice methods: analysis and applications. Cambridge: Cambridge University Press; 2000.
Ryan M, Amaya-Amaya M. ‘Threats’ to and hopes for estimating benefits. Health Econ. 2005;14(6):609–19.
de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21(2):145–72.
Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, Bresnahan BW, Kanninen B, Bridges JFP. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13.
Mühlbacher AC, Kaczynski A, Zweifel P, Johnson FR. Experimental measurement of preferences in health and healthcare using best-worst scaling: an overview. Health Econ Rev. 2016;6(1):2.
Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making. Pharmacoeconomics. 2008;26(8):661–77.
Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. Pharmacoeconomics. 2014;32(9):883–902.
Ryan M, Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Policy. 2003;2(1):55–64.
PubMed Google Scholar
Grosse SD, Pike J, Soelaeman R, Tilford JM. Quantifying family spillover effects in economic evaluations: measurement and valuation of informal care time. Pharmacoeconomics. 2019;37(4):461–73.
Mühlbacher A, Johnson FR. Choice experiments to quantify preferences for health and healthcare: state of the practice. Appl Health Econ Health Policy. 2016;14(3):253–66.
Bridges JFP, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, Johnson FR, Mauskopf J. Conjoint analysis applications in health-a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–13.
Klose T. The contingent valuation method in health care. Health Policy. 1999;47(2):97–123.
Mühlbacher AC, Kaczynski A. Making good decisions in healthcare with multi-criteria decision analysis: the use, current research and future development of MCDA. Appl Health Econ Health Policy. 2015;14(1):29–40.
Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, Orlewska E, Penna P, Rodriguez Barrios J-M, Shau W-Y. Budget impact analysis—principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14.
Marsh K, Ijzerman M, Thokala P, Baltussen R, Boysen M, Kaló Z, Lönngren T, Mussen F, Peacock S, Watkins J, Devlin N. Multiple criteria decision analysis for health care decision making—emerging good practices: report 2 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health. 2016;19(2):125–37.
Ryan M, Hughes J. Using conjoint analysis to assess women’s preferences for miscarriage management. Health Econ. 1997;6(3):261–73.
Mühlbacher AC, Zweifel P, Kaczynski A, Johnson FR. Experimental measurement of preferences in health care using best-worst scaling (BWS): theoretical and statistical issues. Health Econ Rev. 2016;6(1):5.
Train K. Discrete choice methods with simulation. New York: Cambridge University Press; 2003.
Greene W. The econometric approach to efficiency analysis. In: Fried KLH, Schmidt S, editors. The measurement of productive efficiency. Oxford: Oxford University Press; 1993.
Cheung KL, Wijnen BFM, Hollin IL, Janssen EM, Bridges JF, Evers SMAA, Hiligsmann M. Using best-worst scaling to investigate preferences in health care. Pharmacoeconomics. 2016;34(12):1195–209.
Hensher DA, Rose JM, Greene WH. Applied choice analysis: a primer. Cambridge; New York: Cambridge University Press; 2005.
Mitchell RC, Carson RT. Using surveys to value public goods: the contingent valuation method. In: Resources for the future. 1989.
Hauber AB, González JM, Groothuis-Oudshoorn CGM, Prior T, Marshall DA, Cunningham C, Ijzerman MJ, Bridges JFP. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–15.
Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ. 1996;313(7052):275–83.
Greenberg PE, Stiglin LE, Finkelstein SN, Berndt ER. The economic burden of depression in 1990. J Clin Psychiatry. 1993;54(11):405–18.
CAS PubMed Google Scholar
Briggs A, Sculpher M, Buxton M. Uncertainty in the economic evaluation of health care technologies: the role of sensitivity analysis. Health Econ. 1994;3(2):95–104.
Henry JA, Rivas CA. Constraints on antidepressant prescribing and principles of cost-effective antidepressant use. Pharmacoeconomics. 1997;11(5):419–43.
Henry JA, Rivas CA. Constraints on antidepressant prescribing and principles of cost-effective antidepressant use. Pharmacoeconomics. 1997;11(6):515–37.
Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Sheldon TA. Problems of using modelling in the economic evaluation of health care. Health Econ. 1996;5(1):1–11.
Drummond M, Torrance G, Mason J. Cost-effectiveness league tables: more harm than good? Soc Sci Med. 1993;37(1):33–40.
Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 2000;9(3):235–51.
Siegel JE. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276(16):1339–41.
Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, Cook J, Glick H, Liljas B, Petitti D, Reed S. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health. 2005;8(5):521–33.
Detsky AS. Guidelines for economic analysis of pharmaceutical products. Pharmacoeconomics. 1993;3(5):354–61.
Birch S, Gafni A. Changing the problem to fit the solution - Johannesson and Weinstein (mis) application of economics to real-world problems. J Health Econ. 1993;12(4):469–76.
Drummond M. Cost-of-illness studies. Pharmacoeconomics. 1992;2(1):1–4.
Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3(4):419–58.
Torrance GW, Blaker D, Detsky A, Kennedy W, Schubert F, Menon D, Tugwell P, Konchak R, Hubbard E, Firestone T. Canadian guidelines for economic evaluation of pharmaceuticals. Pharmacoeconomics. 1996;9(6):535–59.
Jefferson T, Mugford M, Gray A, Demicheli V. An exercise on the feasibility of carrying out secondary economic analyses. Health Econ. 1996;5(2):155–65.
Jonsson B, Bebbington PE. What price depression? The cost of depression and the cost-effectiveness of pharmacological treatment. Br J Psychiatry. 1994;164(5):665–73.
Mason J. The generalisability of pharmacoeconomic studies. Pharmacoeconomics. 1997;11(6):503–14.
Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ. 1986;5(1):1–30.
Torrance GW. Measurement of health state utilities for economic appraisal - a review. J Health Econ. 1986;5(1):1–30.
Culyer AJ. The normative economics of health care finance and provision. Oxf Rev Econ Policy. 1989;5(1):34–58.
Berry C, McMurray J. A review of quality-of-life evaluations in patients with congestive heart failure. Pharmacoeconomics. 1999;16(3):247–71.
Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47(2):99–127.
Drummond M, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford; New York: Oxford University Press; 1987.
Labelle RJ, Hurley JE. Implications of basing health-care resource allocations on cost-utility analysis in the presence of externalities. J Health Econ. 1992;11(3):259–77.
Atkinson AB. On the measurement of inequality. J Econ Theory. 1970;2(3):244–63.
Green C, Brazier J, Deverill M. Valuing health-related quality of life. Pharmacoeconomics. 2000;17(2):151–65.
Torrance GW, Feeny D. Utilities and quality-adjusted life years. Int J Technol Assess Health Care. 1989;5(4):559–75.
Bleichrodt H, Pinto JL, Maria Abellan-Perpiñan J. A consistency test of the time trade-off. J Health Econ. 2003;22(6):1037–52.
Pliskin JS, Shepard DS, Weinstein MC. Utility functions for life years and health status. Oper Res. 1980;28(1):206–24.
Williams A. Intergenerational equity: an exploration of the ‘fair innings’ argument. Health Econ. 1997;6(2):117–32.
Birch S, Gafni A. Cost effectiveness/utility analyses. J Health Econ. 1992;11(3):279–96.
Wagstaff A. QALYs and the equity-efficiency trade-off. J Health Econ. 1991;10(1):21–41.
Dolan P. Valuing health-related quality of life. Pharmacoeconomics. 1999;15(2):119–27.
Dolan P. The measurement of individual utility and social welfare. J Health Econ. 1998;17(1):39–52.
Bleichrodt H, Johannesson M. Standard gamble, time trade-off and rating scale: experimental results on the ranking properties of QALYs. J Health Econ. 1997;16(2):155–75.
Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K, Martin PA. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13(2):89–102.
De Wit GA, Busschbach JJV, De Charro FT. Sensitivity and perspective in the valuation of health status: whose values count? Health Econ. 2000;9(2):109–26.
Williams A. Economics of coronary artery bypass grafting. BMJ. 1985;291(6491):326–9.
Gafni A, Birch S. QALYs and HYEs spotting the differences. J Health Econ. 1997;16(5):601–8.
Mehrez A, Gafni A. Quality-adjusted life years, utility theory, and healthy-years equivalents. Med Decis Making. 1989;9(2):142–9.
Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996.
Mauskopf J, Annemans L, Hill AM, Smets E. A review of economic evaluations of darunavir boosted by low-dose ritonavir in treatment-experienced persons living with HIV infection. Pharmacoeconomics. 2012;28(S1):1–16.
Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146(4):473–81.
CAS PubMed PubMed Central Google Scholar
Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, Devlin N, Smith PC, Sculpher M. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1–504.
Lavelle TA, D’Cruz BN, Mohit B, Ungar WJ, Prosser LA, Tsiplova K, Vera-Llonch M, Lin P-J. Family spillover effects in pediatric cost-utility analyses. Appl Health Econ Health Policy. 2018;17(2):163–74.
Garber AM, Phelps CE. Economic foundations of cost-effectiveness analysis. J Health Econ. 1997;16(1):1–31.
Gloria MAJ, Thavorncharoensap M, Chaikledkaew U, Youngkong S, Thakkinstian A, Culyer AJ. A systematic review of demand-side methods of estimating the societal monetary value of health gain. Value Health. 2021;24(10):1423–34.
Lakdawalla DN, Doshi JA, Garrison LP, Phelps CE, Basu A, Danzon PM. Defining elements of value in health care—a health economics approach: an ISPOR Special Task Force report [3]. Value Health. 2018;21(2):131–9.
Claxton K, Paulden M, Gravelle H, Brouwer W, Culyer AJ. Discounting and decision making in the economic evaluation of health-care technologies. Health Econ. 2011;20(1):2–15.
Bridges JFP, Onukwugha E, Mullins CD. Healthcare rationing by proxy. Pharmacoeconomics. 2010;28(3):175–84.
McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold. Pharmacoeconomics. 2008;26(9):733–44.
Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004;13(5):437–52.
Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness — the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7.
Martin S, Lomas J, Claxton K, Longo F. How effective is marginal healthcare expenditure? New evidence from England for 2003/04 to 2012/13. Appl Health Econ Health Policy. 2021;19(6):885–903.
Meltzer D. Accounting for future costs in medical cost-effectiveness analysis. J Health Econ. 1997;16(1):33–64.
Neumann PJ, Ganiats TG, Russell LB, Sanders GD, Siegel JE. Cost-effectiveness in health and medicine. 2016.
Brouwer WBF, Rutten FFH. The missing link: on the line between C and E. Health Econ. 2003;12(8):629–36.
Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296(13):716–21.
Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20(3):332–42.
Eichler H-G, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7(5):518–28.
Annemans L, Beutels P, Bloom DE, De Backer W, Ethgen O, Luyten J, Van Wilder P, Willem L, Simoens S. Economic evaluation of vaccines: Belgian reflections on the need for a broader perspective. Value Health. 2021;24(1):105–11.
Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ. 2004;329(7459):224–7.
Bertrand M, Duflo E, Mullainathan S. How much should we trust differences-in-differences estimates? Q J Econ. 2004;119(1):249–75.
Finkelstein A, Taubman S, Wright B, Bernstein M, Gruber J, Newhouse JP, Allen H, Baicker K. The Oregon health insurance experiment: evidence from the first year*. Q J Econ. 2012;127(3):1057–106.
Manning WG, Newhouse JP, Duan N, Keeler EB, Leibowitz A, Marquis MS. Health insurance and the demand for medical care: evidence from a randomized experiment. Am Econ Rev. 1987;77(3):251–77.
Colin Cameron A, Miller DL. A practitioner’s guide to cluster-robust inference. J Hum Resour. 2015;50(2):317–72.
Cheng L, Liu H, Zhang Y, Shen K, Zeng Y. The impact of health insurance on health outcomes and spending of the elderly: evidence from China’s new cooperative medical scheme. Health Econ. 2015;24(6):672–91.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55.
Cameron AC, Gelbach JB, Miller DL. Bootstrap-based improvements for inference with clustered errors. Rev Econ Stat. 2008;90(3):414–27.
Dillender M. Medicaid, family spending, and the financial implications of crowd-out. J Health Econ. 2017;53:1–16.
Card D, Dobkin C, Maestas N. The impact of nearly universal insurance coverage on health care utilization: evidence from Medicare. Am Econ Rev. 2008;98(5):2242–58.
Dunn A, Knepper M, Dauda S. Insurance expansions and hospital utilization: relabeling and reabling? J Health Econ. 2021;78:102482.
Card D, Dobkin C, Maestas N. Does Medicare save lives?*. Quart J Econ. 2009;124(2):597–636.
Maclean JC, Saloner B. Substance use treatment provider behavior and healthcare reform: evidence from Massachusetts. Health Econ. 2018;27(1):76–101.
Baicker K, Taubman SL, Allen HL, Bernstein M, Gruber JH, Newhouse JP, Schneider EC, Wright BJ, Zaslavsky AM, Finkelstein AN. The Oregon experiment — effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713–22.
Ghosh A, Simon K, Sommers BD. The effect of health insurance on prescription drug use among low-income adults: evidence from recent medicaid expansions. J Health Econ. 2019;63:64–80.
Kolstad JT, Kowalski AE. The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J Public Econ. 2012;96(11–12):909–29.
Ma Y, Nolan A. Public healthcare entitlements and healthcare utilisation among the older population in Ireland. Health Econ. 2017;26(11):1412–28.
Currie J, Gruber J. Health insurance eligibility, utilization of medical care, and child health. Q J Econ. 1996;111(2):431–66.
Abadie A, Diamond A, Hainmueller J. Synthetic control methods for comparative case studies: estimating the effect of California’s tobacco control program. J Am Stat Assoc. 2010;105(490):493–505.
Saloner B, Akosa Antwi Y, Maclean JC, Cook B. Access to health insurance and utilization of substance use disorder treatment: evidence from the Affordable Care Act dependent coverage provision. Health Econ. 2018;27(1):50–75.
Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27(3):531–43.
Bolin K, Lindgren B, Lundborg P. Informal and formal care among single-living elderly in Europe. Health Econ. 2008;17(3):393–409.
Hoefman RJ, van Exel J, Brouwer WBF. The monetary value of informal care: obtaining pure time valuations using a discrete choice experiment. Pharmacoeconomics. 2018;37(4):531–40.
Brouwer WBF, Culyer AJ, van Exel NJA, Rutten FFH. Welfarism vs. extra-welfarism. J Health Econ. 2008;27(2):325–38.
Greene WH. Econometric analysis. Upper Saddle River: Prentice Hall; 2000.
Wittenberg E, James LP, Prosser LA. Spillover effects on caregivers’ and family members’ utility: a systematic review of the literature. Pharmacoeconomics. 2019;37(4):475–99.
Gheorghe M, Hoefman RJ, Versteegh MM, van Exel J. Estimating informal caregiving time from patient EQ-5D data: the Informal CARE Effect (iCARE) Tool. Pharmacoeconomics. 2018;37(1):93–103.
Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JJ, Peters TJ. Valuing the ICECAP capability index for older people. Soc Sci Med. 2008;67(5):874–82.
van den Berg B, Brouwer WBF, Koopmanschap MA. Economic valuation of informal care. Eur J Health Econ. 2004;5(1):36–45.
Viscusi WK, Aldy JE. J Risk Uncertain. 2003;27(1):5–76.
Van Houtven CH, Norton EC. Informal care and health care use of older adults. J Health Econ. 2004;23(6):1159–80.
Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res. 2011;21(1):167–76.
Koopmanschap MA, van Exel JNA, van den Berg B, Brouwer WBF. An overview of methods and applications to value informal care in economic evaluations of healthcare. Pharmacoeconomics. 2008;26(4):269–80.
Hoefman RJ, van Exel J, Brouwer W. How to include informal care in economic evaluations. Pharmacoeconomics. 2013;31(12):1105–19.
Dixon P, Round J. Caring for carers: positive and normative challenges for future research on carer spillover effects in economic evaluation. Value Health. 2019;22(5):549–54.
Koopmanschap MA, Rutten FFH, van Ineveld BM, van Roijen L. The friction cost method for measuring indirect costs of disease. J Health Econ. 1995;14(2):171–89.
Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–7.
Finkler SA. The distinction between cost and charges. Ann Intern Med. 1982;96(1):102–9.
Heywood J, Bouchard J, Cortelli P, Dahlöf C, Jansen JP, Pham S, Hirsch J, Edwards CE, Adams J, Berto P, Brueggenjuergen B, Nyth AL, Lindsay P, Price KL. A multinational investigation of the impact of subcutaneous sumatriptan. Pharmacoeconomics. 1997;11(Supplement 1):11–23.
Brouwer WBF, Koopmanschap MA, Rutten FFH. Productivity costs measurement through quality of life? A response to the recommendation of the Washington Panel. Health Econ. 1997;6(3):253–9.
Coukell AJ, Lamb HM. Sumatriptan. Pharmacoeconomics. 1997;11(5):473–90.
Cortelli P, Dahlöf C, Bouchard J, Heywood J, Jansen JP, Pham S, Hirsch J, Adams J, Miller DW. A multinational investigation of the impact of subcutaneous sumatriptan. Pharmacoeconomics. 1997;11(Supplement 1):35–42.
Zhang W, Bansback N, Anis AH. Measuring and valuing productivity loss due to poor health: a critical review. Soc Sci Med. 2011;72(2):185–92.
Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States. Arch Intern Med. 1999;159(8):813–8.
Solomon GD, Price KL. Burden of migraine. Pharmacoeconomics. 1997;11(Supplement 1):1–10.
Koopmanschap MA, van Ineveld BM. Towards a new approach for estimating indirect costs of disease. Soc Sci Med. 1992;34(9):1005–10.
Dahlöf C, Bouchard J, Cortelli P, Heywood J, Jansen JP, Pham S, Hirsch J, Adams J, Miller DW. A multinational investigation of the impact of subcutaneous sumatriptan. Pharmacoeconomics. 1997;11(Supplement 1):24–34.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97.
Osterhaus JT, Gutterman DL, Plachetka JR. Healthcare resource and lost labour costs of migraine headache in the US. Pharmacoeconomics. 1992;2(1):67–76.
Bouchard J, Cortelli P, Dahlöf C, Heywood J, Jansen JP, Price KL, Pham S, Joseph A, Babiak L. A multinational investigation of the impact of subcutaneous sumatriptan. Pharmacoeconomics. 1997;11(Supplement 1):43–50.
Lofland JH, Nash DB. Oral serotonin receptor agonists. Pharmacoeconomics. 2005;23(3):259–74.
D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–81.
Thiry N, Beutels P, Van Damme P, Van Doorslaer E. Economic evaluations of varicella vaccination programmes. Pharmacoeconomics. 2003;21(1):13–38.
Solomon GD, Litaker DG. The impact of drug therapy on quality of life in headache and migraine. Pharmacoeconomics. 1997;11(4):334–42.
Adams ME, McCall NT, Gray DT, Orza MJ, Chalmers TC. Economic analysis in randomized control trials. Med Care. 1992;30(3):231–43.
Capri S, Ceci A, Terranova L, Merlo F, Mantovani L. Guidelines for economic evaluations in Italy: recommendations from the Italian group of pharmacoeconomic studies. Drug Inf J. 2001;35(1):189–201.
Moeremans K, Annemans L, Lothgren M, Allegri G, Wyffels V, Hemmet L, Caekelbergh K, Smets E. Cost effectiveness of darunavir/ritonavir 600/100 mg bid in protease inhibitor-experienced, HIV-1-infected adults in Belgium, Italy, Sweden and the UK. Pharmacoeconomics. 2010;28(Suppl 1):107–28.
Moeremans K, Hemmett L, Hjelmgren J, Allegri G, Smets E. Cost effectiveness of darunavir/ritonavir 600/100 mg bid in treatment-experienced, lopinavir-naive, protease inhibitor-resistant, HIV-infected adults in Belgium, Italy, Sweden and the UK. Pharmacoeconomics. 2010;28(Suppl 1):147–67.
Freedberg KA, Losina E, Weinstein MC, Paltiel AD, Cohen CJ, Seage GR, Craven DE, Zhang H, Kimmel AD, Goldie SJ. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344(11):824–31.
Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–60.
Hellinger FJ. The lifetime cost of treating a person with HIV. JAMA. 1993;270(4):474–8.
Brogan A, Mauskopf J, Talbird SE, Smets E. US cost effectiveness of darunavir/ritonavir 600/100 mg bid in treatment-experienced, HIV-infected adults with evidence of protease inhibitor resistance included in the TITAN Trial. Pharmacoeconomics. 2010;28(Suppl 1):129–46.
Mauskopf J, Annemans L, Hill AM, Smets E. A review of economic evaluations of darunavir boosted by low-dose ritonavir in treatment-experienced persons living with HIV infection. Pharmacoeconomics. 2010;28(Suppl 1):1–16.
Mauskopf J, Brogan A, Martin S, Smets E. Cost effectiveness of darunavir/ritonavir in highly treatment-experienced, HIV-1-infected adults in the USA. Pharmacoeconomics. 2010;28(Suppl 1):83–105.
Boyd MA, Hill AM. Clinical management of treatment-experienced, HIV/AIDS patients in the combination antiretroviral therapy era. Pharmacoeconomics. 2010;28(Suppl 1):17–34.
Clotet B, Bellos N, Molina J-M, Cooper D, Goffard J-C, Lazzarin A, Wöhrmann A, Katlama C, Wilkin T, Haubrich R, Cohen C, Farthing C, Jayaweera D, Markowitz M, Ruane P, Spinosa-Guzman S, Lefebvre E. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369(9568):1169–78.
Colin X, Lafuma A, Costagliola D, Smets E, Mauskopf J, Guillon P. Modelling the budget impact of darunavir in the treatment of highly treatment-experienced, HIV-infected adults in France. Pharmacoeconomics. 2010;28(Suppl 1):183–97.
Mouton Y, Alfandari S, Valette M, Cartier F, Dellamonica P, Humbert G, Lang JM, Massip P, Mechali D, Leclercq P, Modai J, Portier H. Impact of protease inhibitors on AIDS-defining events and hospitalizations in 10 French AIDS reference centres. AIDS. 1997;11(12):F101–5.
Madruga JV, Berger D, McMurchie M, Suter F, Banhegyi D, Ruxrungtham K, Norris D, Lefebvre E, de Béthune M-P, Tomaka F, De Pauw M, Vangeneugden T, Spinosa-Guzman S. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007;370(9581):49–58.
Levy A, Johnston K, Annemans L, Tramarin A, Montaner J. The impact of disease stage on direct medical costs of HIV management: a review of the international literature. Pharmacoeconomics. 2010;28(Suppl 1):35–47.
Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, Macfarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333(20):1301–8.
Szucs TD. Resource utilisation in the management of dyslipidaemia. Pharmacoeconomics. 1998;14(Supplement 3):11–8.
Brown WV. Impact of dyslipidaemia. Pharmacoeconomics. 1998;14(Supplement 3):1–9.
Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JMO, Wun C-C, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335(14):1001–9.
McKenney JM. The cost of treating dyslipidaemia using National Cholesterol Education Program (NCEP) guidelines. Pharmacoeconomics. 1998;14(Supplement 3):19–28.
Johannesson M, Jönsson B, Kjekshus J, Olsson AG, Pedersen TR, Wedel H. Cost effectiveness of simvastatin treatment to lower cholesterol levels in patients with coronary heart disease. N Engl J Med. 1997;336(5):332–6.
Cziraky M. Clinical positioning of HMG-CoA reductase inhibitors in lipid management protocols. Pharmacoeconomics. 1998;14(Supplement 3):29–38.
Anderson KM, Odell PM, Wilson PWF, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121(1):293–8.
Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto JAM, for the ATRG. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA. 1998;279(20):1615–22.
Goa KL, Barradell LB, McTavish D. Simvastatin. Pharmacoeconomics. 1997;11(1):89–110.
Expert Panel on Detection E, A. Treatment of High Blood Cholesterol in. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285(19):2486–97.
Coukell AJ, Wilde MI. Pravastatin. Pharmacoeconomics. 1998;14(2):217–36.
Caro J, Klittich W, McGuire A, Ford I, Norrie J, Pettitt D, McMurray J, Shepherd J. The West of Scotland coronary prevention study: economic benefit analysis of primary prevention with pravastatin. BMJ. 1997;315(7122):1577–82.
Koren MJ, Smith DG, Hunninghake DB, Davidson MH, McKenney JM, Weiss SR, Schrott HG, Henley RW, Tresh P, McLain RW, Bakker-Arkema RG, Black DM. The cost of reaching National Cholesterol Education Program (NCEP) goals in hypercholesterolaemic patients. Pharmacoeconomics. 1998;14(1):59–70.
Goldman L. Cost-effectiveness of HMG-CoA reductase inhibition for primary and secondary prevention of coronary heart disease. JAMA. 1991;265(9):1145–51.
Raftery J. NICE: faster access to modern treatments? Analysis of guidance on health technologies. BMJ. 2001;323(7324):1300–3.
Brown AD, Garber AM. Cost effectiveness of coronary heart disease prevention strategies in adults. Pharmacoeconomics. 1998;14(1):27–48.
Morris S. A comparison of economic modelling and clinical trials in the economic evaluation of cholesterol-modifying pharmacotherapy. Health Econ. 1997;6(6):589–601.
Jonsson B, Johannesson M, Kjekshus J, Olsson AG, Pedersen TR, Wedel H. Cost-effectiveness of cholesterol lowering. Results from the Scandinavian Simvastatin Survival Study (4S). Eur Heart J. 1996;17(7):1001–7.
Ashraf T, Hay JW, Pitt B, Wittels E, Crouse J, Davidson M, Furberg CD, Radican L. Cost-effectiveness of pravastatin in secondary prevention of coronary artery disease**This study was supported by Bristol-Myers Squibb, Plainsboro, New Jersey. Am J Cardiol. 1996;78(4):409–14.
Bergner M, Bobbitt RA, Carter WB, Gilson BS. The sickness impact profile: development and final revision of a health status measure. Med Care. 1981;19(8):787–805.
Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4.
Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M, Bhat G, Goldman S, Fletcher RD, Doherty J, Hughes CV, Carson P, Cintron G, Shabetai R, Haakenson C. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325(5):303–10.
Leidy NK, Rentz AM, Zyczynski TM. Evaluating health-related quality-of-life outcomes in patients with congestive heart failure. Pharmacoeconomics. 1999;15(1):19–46.
Johannesson M, Jönsson B, Borgquist L. Willingness to pay for antihypertensive therapy — results of a Swedish pilot study. J Health Econ. 1991;10(4):461–73.
Schadlich PK, Huppertz E, Brecht JG. Cost-effectiveness analysis of ramipril in heart failure after myocardial infarction. Economic evaluation of the Acute Infarction Ramipril Efficacy (AIRE) study for Germany from the perspective of Statutory Health Insurance. Pharmacoeconomics. 1998;14(6):653–69.
Coyle D, Tolley K. Discounting of health benefits in the pharmacoeconomic analysis of drug therapies. Pharmacoeconomics. 1992;2(2):153–62.
Amemiya T. Qualitative response models: a survey. J Econ Lit. 1981;19(4):1483–536.
Gerbrandt KR, Yedinak KC. Formulary management of ACE inhibitors. Pharmacoeconomics. 1996;10(6):594–613.
Gandjour A, Lauterbach KW. Review of quality-of-life evaluations in patients with angina pectoris. Pharmacoeconomics. 1999;16(2):141–52.
Wilson IB. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65.
Borch-Johnsen K. ACE inhibitors in patients with diabetes mellitus. Pharmacoeconomics. 1996;9(5):392–8.
DiMasi JA, Hansen RW, Grabowski HG, Lasagna L. Cost of innovation in the pharmaceutical industry. J Health Econ. 1991;10(2):107–42.
Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Möller J. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-4. Value Health. 2012;15(6):821–7.
Kobelt G, Eberhardt K, Jonsson L, Jonsson B. Economic consequences of the progression of rheumatoid arthritis in Sweden. Arthritis Rheum. 1999;42(2):347–56.
Scholz S, Mittendorf T. Modeling rheumatoid arthritis using different techniques - a review of model construction and results. Health Econ Rev. 2014;4(1):18.
Byford S. Economic note: cost of illness studies. BMJ. 2000;320(7245):1335–1335.
Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–45.
van Haalen HGM, Severens JL, Tran-Duy A, Boonen A. How to select the right cost-effectiveness model? Pharmacoeconomics. 2014;32(5):429–42.
Brennan A. Modelling the cost-effectiveness of etanercept in adults with rheumatoid arthritis in the UK. Rheumatology. 2004;43(1):62–72.
Lyseng-Williamson KA, Foster RH. Infliximab. Pharmacoeconomics. 2004;22(2):107–32.
Merkesdal S, Ruof J, Schoffski O, Bernitt K, Zeidler H, Mau W. Indirect medical costs in early rheumatoid arthritis: composition of and changes in indirect costs within the first three years of disease. Arthritis Rheum. 2001;44(3):528–34.
Lyseng-Williamson KA, Plosker GL. Etanercept. Pharmacoeconomics. 2004;22(16):1071–95.
Kobelt G, Lindgren P, Lindroth Y, Jacobson L, Eberhardt K. Modelling the effect of function and disease activity on costs and quality of life in rheumatoid arthritis. Rheumatology. 2005;44(9):1169–75.
Bansback N, Ara R, Karnon J, Anis A. Economic evaluations in rheumatoid arthritis. Pharmacoeconomics. 2008;26(5):395–408.
Kobelt G, Jönsson B. The burden of rheumatoid arthritis and access to treatment: outcome and cost-utility of treatments. Eur J Health Econ. 2007;8(S2):95–106.
Jönsson B. Patient access to rheumatoid arthritis treatments. Eur J Health Econ. 2007;8(S2):35–8.
Perlman M. The economics of health and medical care. 1974.
Cromwell J, Mitchell JB. Physician-induced demand for surgery. J Health Econ. 1986;5(4):293–313.
Birch S. The identification of supplier-inducement in a fixed price system of health care provision. J Health Econ. 1988;7(2):129–50.
Grytten J, Carlsen F, Sørensen R. Supplier inducement in a public health care system. J Health Econ. 1995;14(2):207–29.
Dranove D. Demand inducement and the physician/patient relationship. Econ Inq. 1988;26(2):281–98.
Evans RG. Strained mercy: the economics of Canadian health care. Toronto: Butterworths; 1984.
Scott A, Shiell A. Analysing the effect of competition on General Practitioners’ behaviour using a multilevel modelling framework. Health Econ. 1997;6(6):577–88.
Scott A, Shiell A. Do fee descriptors influence treatment choices in general practice? A multilevel discrete choice model. J Health Econ. 1997;16(3):323–42.
Reinhardt UE. The theory of physician-induced demand reflections after a decade. J Health Econ. 1985;4(2):187–93.
Schaafsma J. A new test for supplier-inducement and application to the Canadian market for dental care. J Health Econ. 1994;13(4):407–31.
Labelle R, Stoddart G, Rice T. A re-examination of the meaning and importance of supplier-induced demand. J Health Econ. 1994;13(3):347–68.
Matsaganis M, Glennerster H. The threat of ‘cream skimming’ in the post-reform NHS. J Health Econ. 1994;13(1):31–60.
Rice TH, Labelle RJ. Do physicians induce demand for medical services? J Health Polit Policy Law. 1989;14(3):587–600.
Dolan P, Cookson R, Ferguson B. Effect of discussion and deliberation on the public’s views of priority setting in health care: focus group study. BMJ. 1999;318(7188):916–9.
Dranove D, Wehner P. Physician-induced demand for childbirths. J Health Econ. 1994;13(1):61–73.
Fuchs VR. The supply of surgeons and the demand for operations. J Hum Resour. 1978;13 Suppl:35–56.
Folland S, Goodman AC, Stano M. The economics of health and health care. Upper Saddle River: Prentice Hall; 2001.
Bell CC. DSM-IV: diagnostic and statistical manual of mental disorders. JAMA. 1994;272(10):828–9.
American Psychiatric Association, American Psychiatric Association. Work Group to Revise DSM-III. Diagnostic and statistical manual of mental disorders: DSM-III-R. Washington, DC: American Psychiatric Association; 1987.
Edwards NC, Locklear JC, Rupnow MF, Diamond RJ. Cost effectiveness of long-acting risperidone injection versus alternative antipsychotic agents in patients with schizophrenia in the USA. Pharmacoeconomics. 2005;23(Suppl 1):75–89.
Edwards NC, Rupnow MF, Pashos CL, Botteman MF, Diamond RJ. Cost-effectiveness model of long-acting risperidone in schizophrenia in the US. Pharmacoeconomics. 2005;23(3):299–314.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23.
Laux G, Heeg BMS, van Hout BA, Mehnert A. Costs and effects of long-acting risperidone compared with oral atypical and conventional depot formulations in Germany. Pharmacoeconomics. 2012;23(S1):49–61.
Lehman AF, Steinwachs DM. Translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophr Bull. 1998;24(1):1–10.
Edgell ET, Andersen SW, Johnstone BM, Dulisse B, Revicki D, Breier A. Olanzapine versus risperidone. Pharmacoeconomics. 2000;18(6):567–79.
Rosenheck R. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. Am J Psychiatry. 2006;163(12):2080–9.
Albright PS, Livingstone S, Keegan DL, Ingham M, Shrikhande S, Le Lorier J. Reduction of healthcare resource utilisation and costs following the use of risperidone for patients with schizophrenia previously treated with standard antipsychotic therapy. Clin Drug Investig. 1996;11(5):289–99.
Foster RH, Goa KL. Olanzapine. Pharmacoeconomics. 1999;15(6):611–40.
Weiden PJ, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull. 1995;21(3):419–29.
Haycox A. Pharmacoeconomics of long-acting risperidone: results and validity of cost-effectiveness models. Pharmacoeconomics. 2005;23(Suppl 1):3–16.
Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002;346(1):16–22.
Lindstrom E, Bingefors K. Patient compliance with drug therapy in schizophrenia. Economic and clinical issues. Pharmacoeconomics. 2000;18(2):106–24.
Tollefson GD, Sanger TM. Negative symptoms: a path analytic approach to a double-blind, placebo- and haloperidol-controlled clinical trial with olanzapine. Am J Psychiatry. 1997;154(4):466–74.
Hamilton SH, Revicki DA, Edgell ET, Genduso LA, Tollefson G. Clinical and economic outcomes of olanzapine compared with haloperidol for schizophrenia. Pharmacoeconomics. 1999;15(5):469–80.
De Graeve D, Smet A, Mehnert A, Caleo S, Miadi-Fargier H, Mosqueda GJ, Lecompte D, Peuskens J. Long-acting risperidone compared with oral olanzapine and haloperidol depot in schizophrenia: a Belgian cost-effectiveness analysis. Pharmacoeconomics. 2005;23(Suppl 1):35–47.
Gilmer TP, Dolder CR, Lacro JP, Folsom DP, Lindamer L, Garcia P, Jeste DV. Adherence to treatment with antipsychotic medication and health care costs among medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692–9.
Ellison G. The slowdown of the economics publishing process. J Polit Econ. 2002;110(5):947–93.
McNamee P, Murray E, Kelly MP, Bojke L, Chilcott J, Fischer A, West R, Yardley L. Designing and undertaking a health economics study of digital health interventions. Am J Prev Med. 2016;51(5):852–60.
Mistry H, Garnvwa H, Oppong R. Critical appraisal of published systematic reviews assessing the cost-effectiveness of telemedicine studies. Telemed e-Health. 2014;20(7):609–18.
Murray E, Hekler EB, Andersson G, Collins LM, Doherty A, Hollis C, Rivera DE, West R, Wyatt JC. Evaluating digital health interventions: key questions and approaches. Elsevier. 2016;51:843–51.
Haghani M, Bliemer MCJ, Hensher DA. The landscape of econometric discrete choice modelling research. J Choice Model. 2021;40:100303.
Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–26.
Wu B. Superpowered science: charting China’s research rise. Nature. 2021;593(7860):S4–5.
Boyack KW, Klavans R. Co-citation analysis, bibliographic coupling, and direct citation: which citation approach represents the research front most accurately? J Am Soc Inform Sci Technol. 2010;61(12):2389–404.
Haghani M, Bliemer MCJ. Emerging trends and influential outsiders of transportation science. Transp Lett. 2023;15(5):386–422.
Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, Erikson P. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8(2):94–104.
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65.
DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33.
Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33(7):673–89.
Williams A. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. 2006.
National Institute for H. and E. Care. NICE process and methods guides. Guide to the methods of technology appraisal 2013. London: National Institute for Health and Care Excellence (NICE); 2013. Copyright © 2013 National Institute for Health and Clinical Excellence, unless otherwise stated. All rights reserved.
Download references
Acknowledgements
Not applicable.
None to declare.
Author information
Authors and affiliations.
Department of Nursing and Allied Health, School of Health Sciences, Swinburne University of Technology, Melbourne, VIC, Australia
Clara C. Zwack
School of Civil and Environmental Engineering, University of New South Wales, Sydney, NSW, Australia
Milad Haghani
Erasmus School of Health Policy & Management, Erasmus University Rotterdam, Rotterdam, The Netherlands
Esther W. de Bekker-Grob
You can also search for this author in PubMed Google Scholar
Contributions
CZ and MH led the conception of the study. CZ and MH developed the review protocol. CZ conducted the literature search. CZ conducted data extraction, with MH’s support in coordination. EWD provided support to refining the results. CZ drafted the manuscript, with MH’s support in the Methods section. All authors provided substantial suggestions and edits for the writing of the paper. All authors approved the submission of this paper.
Corresponding author
Correspondence to Clara C. Zwack .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
All authors provided consent for publication.
Competing interests
The Authors have no competing interests to declare.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zwack, C.C., Haghani, M. & de Bekker-Grob, E.W. Research trends in contemporary health economics: a scientometric analysis on collective content of specialty journals. Health Econ Rev 14 , 6 (2024). https://doi.org/10.1186/s13561-023-00471-6
Download citation
Received : 25 December 2022
Accepted : 28 November 2023
Published : 25 January 2024
DOI : https://doi.org/10.1186/s13561-023-00471-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health economics
- Cost-effectiveness
- Microeconomics
- Macroeconomics
- Scientometric analysis
Health Economics Review
ISSN: 2191-1991
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Healthcare Expenditure and Economic Development Dynamics in India: Experiences from COVID-19 Pandemic
- First Online: 04 February 2022
Cite this chapter

- Subrata Saha 5
Part of the book series: New Frontiers in Regional Science: Asian Perspectives ((NFRSASIPER,volume 55))
227 Accesses
3 Citations
The relationship between health and development is an important topic in the field of economics. India’s new Health Policy 2017 also acknowledges this relationship. The COVID-19 pandemic has exposed the chronic underinvestment in India’s healthcare system as India’s public healthcare system is facing a severe shortage of different resources and infrastructure. The present study aims to investigate the dynamics between healthcare expenditure- public health expenditure and private health expenditure and economic growth for the period from 1999 to 2018. The Johansen cointegration test exhibits that public health expenditures, private health expenditures, and economic growth are cointegrated, implying a long-run relationship between the variables concerned. The result of the Vector Error Correction Model represents that unidirectional causality between public health expenditure and economic growth and causality running from public health expenditure to economic growth exists and another unidirectional causal relationship which runs from economic growth to private health expenditure also exists for the sample period from 1999 to 2018. Block Exogeneity Wald Test has validated the results obtained from VECM. The impulse response functions and variance decomposition reveal weak causality (Granger) link between public health expenditure and economic growth and strong causal (Granger) relationship between Domestic private healthcare expenditure and economic growth during the study period. CUSUM and CUSUM-Square Test confirm that the parameters estimated by the model are stable for the period 1999 to 2018. The study concludes that the impact of public health expenditure on economic growth is relatively small, while economic growth on domestic private healthcare expenditure is relatively strong in India during the period of study.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others
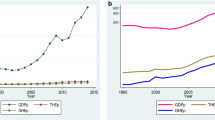
Healthcare expenditure and GDP in Ethiopia from 1995 to 2014: a time-series analysis
Analyzing the impact of fiscal conditions on private health expenditures in OECD countries: a quantile ARDL investigation

Health expenditure and growth dynamics in the SADC region: evidence from non-stationary panel data with cross section dependence and unobserved heterogeneity
Education in Article 21A of the constitution of India and Health in Article 21A of the constitution of India and Article 39,41,42, and 47.
World Bank data is available at www.data.worldbank.org
Situation Analyses: Backdrop to the National Health Policy-2017, Ministry of Health and Family Welfare. Government of India.
Atuahene SA, Yusheng K, Micah GB (2020) Health expenditure, co2 emissions and economic growth: China vs. India. Doi: https://doi.org/10.20944/preprints202009.0348.v1
Basumallik S (2017) Health and its impact on economic growth in India- an explanation. Int J Creat Res Thoughts 5(4):320–882
Google Scholar
Bedir S (2016) Healthcare expenditure and economic growth in developing countries. Adv Econ Business 4(2): 76-86. doi: https://doi.org/10.13189/aeb.2016.040202
Bhat R, Jain N (2004) Time series analysis of private healthcare expenditures and GDP; co integration results with structural breaks. Indian Institute of Management, Ahmedabad
Dincer H, Yuksel S (2019) Identifying the causality relationship between health expenditure and economic growth: an application on E7 countries. Journal of health systems and policies. 1: 157
Dolado JJ, Lütkepohl H (1996) Making Wald tests work for cointegrated VAR systems. Econ Rev 15:369–386
Article Google Scholar
Ehikioya IL, Mohammed I (2013) Determinants of public health care expenditure in Nigeria: an error correction mechanism approach. Int J Busi Soc Sci 4:13
Farahani M, Subramanian SV, David C (2010) Effects of state-level public spending on health on the mortality probability in India. Health Econ 19(11):1361–1376
Gangal VLN, Gupta H (2013) Public expenditure and economic growth: a case study of India. Glob J Mgmt Busi Stud 3(2):191–196
Granger CWJ (1969) Investigating causal relation by econometric models and cross-spectral methods. Econometrica 37:424–438
Gupta I, Mitra A (2004) Economic growth health and poverty: an exploratory study for India. Dev Policy Rev 22(2):193–206
Kulkarni L (2016) Health inputs, health outcomes and public health expenditure: evidence from BRICS countries. Int J Appl Econ 31(1):72–84
Maitra B, Mukhopadhyay CK (2012) Public spending on education, health care and economic growth in selected countries of Asia and the Pacific. Asia-Pacific development journal 9(2):14
Mohanty RK, Behera DK (2020) How effective is public health care expenditure in improving health outcome? An empirical evidence from the Indian states. NIPFP working paper no. 300
Mohapatra S (2019) Public health expenditure and its effect on health outcomes: a new methodological approach in the Indian context. Great Lakes Herald 13(1):148
Nyasha S, Odhiambo NM (2019) The impact of public expenditure on economic growth: a review of international literature. Folia Oeconomicstetinesia Sciendo 19(2):120. https://doi.org/10.2478/foli20190015
Pradhan RP (2010) The long run relation between health spending and economic growth in 11 OECD countries: evidence from panel cointegration. Int J Econ Perspect 4(2):427–438
Raghupathi V, Raghupathi W (2020) Healthcare expenditure and eco-nomic performance: insights from the United States data. Front Public Health 8:156
Rajeshkumar N, Nalraj P (2014) Public expenditure on health and economic growth in selected Indian states. Int J Sci Res. 3(3):13–204
Seshaiah SV, Koti Reddy T, Sarma IRS (2018) General government expenditure and economic growth in India: 1980-81 to 2015-16. Theor Econ Lett 8:728–740
Shafuda CP, De UK (2017) Upshot of public health expenditure on economic development, MPRA paper no. 101846
The World Bank Data (2018) Data.worldbank.org
Toda HY, Yamamoto T (1995) Statistical inference in vector auto regressions with possibly integrated processes. J Econ 66:225–250
Varkey RS, Joy J, Panda PK (2020) Health infrastructure, health outcome and economic growth: evidence from Indian major states. J Crit Rev 7(11):2394–5125
Verma CS, Usmani G (2019) Relationship between health and economic growth in India. Ind J Human Dev 2(2):1–13. https://doi.org/10.1177/0973703019887601
Zaman SB, Hossain N, Mehta V, Sharmin S, Mahmood SAI (2017) An association of total health expenditure with GDP and life expectancy. J Med Res Innov 1(2):27. https://doi.org/10.5281/zenodo.576546
Download references
Author information
Authors and affiliations.
Department of Economics, Raiganj University, Raiganj, West Bengal, India
Subrata Saha
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Department of Economics, Raiganj University, Uttar Dinajpur, West Bengal, India
Department of Geography, Dr. Meghnad Saha College, Uttar Dinajpur, West Bengal, India
Mukunda Mishra
Raiganj University, Uttar Dinajpur, West Bengal, India
Anil Bhuimali
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Saha, S. (2022). Healthcare Expenditure and Economic Development Dynamics in India: Experiences from COVID-19 Pandemic. In: Saha, S., Mishra, M., Bhuimali, A. (eds) Economic and Societal Transformation in Pandemic-Trapped India. New Frontiers in Regional Science: Asian Perspectives, vol 55. Springer, Singapore. https://doi.org/10.1007/978-981-16-5755-9_10
Download citation
DOI : https://doi.org/10.1007/978-981-16-5755-9_10
Published : 04 February 2022
Publisher Name : Springer, Singapore
Print ISBN : 978-981-16-5754-2
Online ISBN : 978-981-16-5755-9
eBook Packages : Economics and Finance Economics and Finance (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Evaluating and Pricing Health Insurance in Lower-income Countries: A Field Experiment in India
Universal health coverage is a widely shared goal across lower-income countries. We conducted a large-scale, 4-year trial that randomized premiums and subsidies for India’s first national, public hospital insurance program, RSBY. We find roughly 60% uptake even when consumers were charged premiums equal to the government’s cost for insurance. We also find substantial adverse selection into insurance at positive prices. Insurance enrollment increases insurance utilization, partly due to spillovers from use of insurance by neighbors. However, many enrollees attempted to use insurance but failed, suggesting that learning is critical to the success of public insurance. We find very few statistically significant impacts of insurance access or enrollment on health. Because there is substantial willingness-to-pay for insurance, and given how distortionary it is to raise revenue in the Indian context, we calculate that our sample population should be charged a premium for RSBY between INR 500-1000 rather than a zero premium to maximize the marginal value of public funds.
This study was funded by the Department for International Development in the UK Government; the Tata Trusts through the Tata Centre for Development at the University of Chicago; the MacLean Center, the Becker-Friedman Institute, the Neubauer Collegium, and the Law School at the University of Chicago; the Sloan Foundation; SRM University; Northwestern University and the International Growth Centre. The views expressed herein are those of the authors and do not necessarily reflect the views of the National Bureau of Economic Research.
MARC RIS BibTeΧ
Download Citation Data
- randomized controlled trials registry entry
- online appendix
Working Groups
More from nber.
In addition to working papers , the NBER disseminates affiliates’ latest findings through a range of free periodicals — the NBER Reporter , the NBER Digest , the Bulletin on Retirement and Disability , the Bulletin on Health , and the Bulletin on Entrepreneurship — as well as online conference reports , video lectures , and interviews .


COLLABORATORS
Lv prasad eye institute.

The L V Prasad Eye Institute (LVPEI) was established in 1987 at Hyderabad as a not-for-profit, non- government, public-spirited, comprehensive eye care institution
Indian Institute of Management

The Indian Institute of Management Ahmedabad (IIMA) was set up in 1961 by the Govt of India in collaboration with the Govt of Gujarat and the Indian industry
Indian Institute of Public Health

Indian Institute of Public Health, Hyderabad (IIPH) under the aegis of Public Health Foundation of India (PHFI) commenced its activities in 2008
NICE International

National Health Authority

National Health Authority was set up in 2019, to implement PM – JAY and formulation of various operational guidelines to ensure standardization and interoperability.
Join us at IHOPE

Research Areas

Public Health

Health Economics

Knowledge Exchange Seminar Series
A collaboration between nice international and ihope, ihope journal.

IHOPE Journal of Ophthalmology (official publication of IHOPE) is a quarterly, international journal. This peer-reviewed journal provides a forum for presentation and discussion of original research articles in the field of ophthalmology. Studies related to big data analysis, public health, health economics and outcomes research will remain its primary focus. The journal facilitates the dissemination of information through original research articles, review articles, editorials, letter to editor, meta-analysis and commentaries. The journal aims to develop, promote and exchange scientific knowledge and co-operation between clinicians, scientists, health economists and others involved in the field of ophthalmology and public health.
Be a part of the IHOPE Community
Interact with leading thinkers & doers in the field, economic growth and health, coping with omicron, supply chains for medical products, scientific team.
Meet the multi disciplinary principal investigators of the IHOPE Research Centre
Advisory Board
Extended team.
A diverse team of research associates, statisticians, economists & administrators
In The News

Research to Policy Conference 2022

Meeting with the Dr. C.S Pramesh, Director Tata Memorial Hospital

Meeting with the faculty of Brighton & Sussex Medical School

Quick Links
- Publications
Useful Links
- Quarterly Newsletter
- In the News
- Privacy Policy
- Terms & Conditions


Health Economics
| Course Status : | Completed |
| Course Type : | Elective |
| Duration : | 12 weeks |
| Category : | |
| Credit Points : | 3 | Postgraduate |
| Start Date : | 22 Jan 2024 |
| End Date : | 12 Apr 2024 |
| Enrollment Ends : | 05 Feb 2024 |
| Exam Registration Ends : | 16 Feb 2024 |
| Exam Date : | 21 Apr 2024 IST |
| Unlock the secrets of the microbial world without breaking the bank! Elevate your academic journey with affordable – because knowledge should be accessible to all students, regardless of budget constraints. |
Understanding ICMR Research Methodology
The success of ICMR’s research lies not only in its expansive scope but also in its rigorous methodology and ethical considerations. ICMR has established guidelines that researchers must adhere to, ensuring that studies funded by the council are not only scientifically sound but also ethically conducted.
This commitment to ethical research practices has been a cornerstone in building public trust and confidence in the findings generated by ICMR-funded studies.
100+ ICMR Research Topics For All Level Students
- Infectious Diseases: Emerging pathogens and control strategies.
- Non-Communicable Diseases (NCDs): Diabetes, cardiovascular research.
- Maternal and Child Health: Strategies for mortality reduction.
- Biomedical Research: Molecular insights into diseases.
- Cancer Research: Innovative approaches for treatment.
- Epidemiology: Studying disease patterns and trends.
- Vaccination Strategies: Enhancing immunization programs.
- Public Health Interventions: Effective community health measures.
- Antibiotic Resistance: Combating microbial resistance.
- Genetic Studies: Understanding genetic contributions to diseases.
- Neurological Disorders: Research on neurological conditions.
- Mental Health: Addressing mental health challenges.
- Nutrition and Health: Studying dietary impacts on health.
- Health Systems Research: Improving healthcare delivery.
- Ayurveda Research: Integrating traditional medicine practices.
- Environmental Health: Impact of environment on health.
- Emerging Technologies: Utilizing tech for healthcare innovations.
- Pharmacological Research: Advancements in drug discovery.
- Global Health Collaborations: International health partnerships.
- Waterborne Diseases: Prevention and control strategies.
- Health Policy Research: Shaping evidence-based policies.
- Health Economics: Studying economic aspects of healthcare.
- Telemedicine: Harnessing technology for remote healthcare.
- Rare Diseases: Understanding and treating rare disorders.
- Community Health: Promoting health at the grassroots level.
- HIV/AIDS Research: Advancements in HIV prevention and treatment.
- Aging and Health: Research on geriatric health issues.
- Cardiovascular Health: Preventive measures and treatments.
- Respiratory Diseases: Understanding lung-related conditions.
- Zoonotic Diseases: Investigating diseases transmitted from animals.
- Stem Cell Research: Applications in regenerative medicine.
- Yoga and Health: Studying the health benefits of yoga.
- Gender and Health: Research on gender-specific health issues.
- Oral Health: Preventive measures and treatments for oral diseases.
- Health Informatics: Utilizing data for healthcare improvements.
- Health Education: Promoting awareness for better health.
- Drug Resistance: Research on antimicrobial resistance.
- Hepatitis Research: Prevention and treatment strategies.
- Telehealth: Remote healthcare services and accessibility.
- Diabetes Management: Strategies for diabetes prevention and control.
- Tuberculosis Research: Advancements in TB diagnosis and treatment.
- Fertility Research: Understanding reproductive health issues.
- Artificial Intelligence in Healthcare: Integrating AI for diagnostics.
- Health Disparities: Addressing inequalities in healthcare access.
- Mental Health Stigma: Research on reducing stigma.
- Mobile Health (mHealth): Applications for mobile-based healthcare.
- Vector-Borne Diseases: Prevention and control measures.
- Nanotechnology in Medicine: Applications in healthcare.
- Occupational Health: Research on workplace health issues.
- Biobanking: Storing and utilizing biological samples for research.
- Telepsychiatry: Providing mental health services remotely.
- Health Equity: Promoting fairness in healthcare delivery.
- Community-Based Participatory Research: Engaging communities in research.
- E-health: Electronic methods for healthcare delivery.
- Sleep Disorders: Understanding and treating sleep-related conditions.
- Health Communication: Effective communication in healthcare.
- Global Burden of Disease: Research on disease prevalence and impact.
- Traditional Medicine: Studying traditional healing practices.
- Nutraceuticals: Research on health-promoting food components.
- Health Data Security: Ensuring privacy and security of health data.
- Regenerative Medicine: Advancements in tissue engineering.
- Social Determinants of Health: Studying social factors affecting health.
- Pharmacovigilance: Monitoring and ensuring drug safety.
- Gerontology: Research on aging and the elderly.
- Mobile Apps in Healthcare: Applications for health monitoring.
- Genetic Counseling: Supporting individuals with genetic conditions.
- Community Health Workers: Role in improving healthcare access.
- Health Behavior Change: Strategies for promoting healthier habits.
- Palliative Care Research: Enhancing end-of-life care.
- Nanomedicine: Applications of nanotechnology in medicine.
- Climate Change and Health: Impact on public health.
- Health Literacy: Promoting understanding of health information.
- Antibody Therapeutics: Advancements in antibody-based treatments.
- Digital Health Records: Electronic health record systems.
- Microbiome Research: Understanding the role of microorganisms in health.
- Disaster Preparedness: Research on health response during disasters.
- Food Safety and Health: Ensuring safe food consumption.
- Artificial Organs: Advancements in organ transplantation.
- Telepharmacy: Remote pharmaceutical services.
- Environmental Epidemiology: Studying the link between environment and health.
- E-mental Health: Digital tools for mental health support.
- Precision Medicine: Tailoring treatments based on individual characteristics.
- Health Impact Assessment: Evaluating the consequences of policies on health.
- Genome Editing: Applications in modifying genetic material.
- Mobile Clinics: Bringing healthcare to underserved areas.
- Telecardiology: Remote cardiac care services.
- Health Robotics: Utilizing robots in healthcare settings.
- Precision Agriculture and Health: Linking agriculture practices to health outcomes.
- Community-Based Rehabilitation: Supporting rehabilitation at the community level.
- Nanotoxicology: Studying the toxicological effects of nanomaterials.
- Community Mental Health: Strategies for promoting mental well-being.
- Health Financing: Research on funding models for healthcare.
- Augmented Reality in Healthcare: Applications in medical training and diagnostics.
- One Health Approach: Integrating human, animal, and environmental health.
- Disaster Mental Health: Addressing mental health issues after disasters.
- Mobile Laboratory Units: Rapid response in disease outbreaks.
- Health Impact Investing: Investing for positive health outcomes.
- Rehabilitation Robotics: Assisting in physical therapy.
- Human Microbiota: Understanding the microorganisms living in and on the human body.
- 3D Printing in Medicine: Applications in medical device manufacturing.
Success Stories from ICMR-Funded Research
Highlighting the impact of ICMR-funded research is essential in appreciating the council’s contribution to healthcare in India. From breakthrough discoveries to successful interventions, ICMR-supported studies have led to tangible improvements in health outcomes.
Case studies showcasing the journey from ICMR research topics and findings to real-world applications serve as inspiring examples of how scientific knowledge can translate into positive societal impacts.
Challenges and Opportunities in ICMR Research
While ICMR has achieved remarkable success in advancing health research, it is not without its challenges. Researchers face obstacles in conducting studies, ranging from resource constraints to logistical issues.
Acknowledging these challenges is crucial in finding solutions and optimizing the impact of ICMR-funded research. Additionally, there are opportunities for collaboration, both nationally and internationally, that can further enrich the research landscape and accelerate progress in addressing health challenges.
The Future of Health Research in India: ICMR’s Vision
Looking ahead, ICMR envisions a future where health research continues to play a central role in shaping the well-being of the nation. Strategic goals include harnessing the power of technology and innovation to drive research advancements, fostering interdisciplinary collaborations, and addressing emerging health challenges.
The vision extends beyond the laboratory, emphasizing the translation of research findings into practical solutions that can positively impact the lives of individuals and communities across India.
In conclusion, the Indian Council of Medical Research stands as a beacon in the realm of healthcare research, tirelessly working towards advancements that contribute to the well-being of the nation.
By exploring ICMR research topics, understanding its methodology, and reflecting on success stories, we gain insight into the transformative power of scientific inquiry.
As ICMR continues to forge ahead, the future of health research in India looks promising, guided by a vision of innovation, collaboration, and a steadfast commitment to improving the health of all citizens.
Related Posts

Step by Step Guide on The Best Way to Finance Car

The Best Way on How to Get Fund For Business to Grow it Efficiently

WELCOME TO IHEPA!
The Indian Health Economics and Policy Association (IHEPA) is a registered society under the Society’s Registration Act 1960. The office is located at International Institute for Population Sciences, Mumbai . IHEPA welcomes all those interested in issues relating to health economics and policy to be a part of this association.
IHEPA welcomes young scholars and researchers, grassroots practitioners, the private sector and community-based organizations to participate actively so that all views and experiences can be heard, debated, understood and absorbed.
11 th Annual Conference of the
Indian health economics and policy association (ihepa).
in collaboration with
FLAME University, Pune
Health policy: advancing research and communication.
Pre-conference workshop : January 17, 2024
Conference : January 18-19, 2024
Venue: FLAME University, Pune
Conference Brochure
Pre-conference Worskhop
Conference Programme
Students’ Interaction with Conference President
Conference Technical Sessions
RECENT EVENTS
IHEPA webinar series on Economics of Covid19: A Public Health Perspective Speaker Dr Jean-Frederic Levesque, MD, PhD, FRCP (Canada) and Associate Professor, Centre for Primary Care and Equity, University of New South Wales, Australia
View Abstract
9th IHEPA conference on Health System Response During the Pandemic COVID 19 pandemic has created several new challenges for health systems globally and in India. It has strained the already weak public health system, exposing vulnerabilities on various fronts…
View Conference Brochure | Book of Abstracts
OFFICE BEARERS
Udaya Shankar Mishra
Vice President
Manisha Karne
William Joe
Joint Secretary
Vijaya Lakshmi Hebbare Anuhya Korrapati
Vinod B Annigeri Sunil Nandaraj Godwin S K Rudra Narayan Mishra Ramna Thakur Subrata Mukherjee Salim Shah
MEMBERSHIP INFORMATION
IHEPA welcomes all to join and strengthen the Association to make it a vibrant and productive body of excellence in health research and policy.
Membership of the Association is open to all individuals and institutions engaged in health economics and related policy issues.
SECRETARIAT
IHEPA C/O PROF U S MISHRA International Institute For Population Sciences Govandi Station Road Opposite Sanjona Chamber Deonar, Mumbai 400088, Maharashtra
REGISTERED OFFICE
IHEPA Centre for Multi-Disciplinary Development Research (C.M.D.R), R.S.No.9A2, Plot No.82, Dr. B.R.Ambedkar Nagar, Lakamanahalli, Dharward, Karnataka – 580 004, India
IMPORTANT LINKS
© Copyright 2024 | All Rights Reserved | Powered by Snaps Technology
- MyU : For Students, Faculty, and Staff
Sylvester Zhang awarded Doctoral Dissertation Fellowship

MINNEAPOLIS / ST. PAUL (6/28/2024) – School of Mathematics PhD student Sylvester Zhang was recently awarded the Doctoral Dissertation Fellowship from the University of Minnesota. The Doctoral Dissertation Fellowship (DDF) gives the University's most accomplished Ph.D. candidates an opportunity to devote full-time effort to an outstanding research project by providing time to finalize and write their dissertation during the fellowship year.
Sylvester Zhang started the University of Minnesota Mathematics PhD program in Fall 2020, after completion of his undergraduate studies in Mathematics and Economics here at UMN. Zhang is interested in algebraic combinatorics. In particular he aims to explore topics like total positivity, cluster algebras, symmetric functions, and the flag manifold. Advised by Pavlo Pylyavskyy, Zhang is currently primarily focused on two distinct research topics: 1) an approach to Schubert polynomials using methods from mathematical physics, and 2) affine symmetric group and combinatorics of the affine flag variety. He says he is looking forward to continuing a career in academia and research after graduation.
The University of Minnesota DDF program aims to give the most accomplished Ph.D. candidates – those who have passed the written and oral preliminary examinations and their program coursework – an opportunity to devote full-time effort to an outstanding research project by providing time to finalize and write their dissertation during the fellowship year. The fellowship grants awardees a $25,000 stipend, academic year tuition, subsidized health insurance through the Graduate Assistant Health Plan for up to one calendar year, and a $1,000 conference grant.
Related news releases
- Alumni Profile: Jered Bright
- Five UMN graduate students win 24-hour data analytics challenge
- Professor Vladimir Sverak elected to the Academy of Arts and Sciences
- Lina Liu receives NSF Graduate Research Fellowship
- Professor Jasmine Foo receives Distinguished McKnight University Professorship
- Future undergraduate students
- Future transfer students
- Future graduate students
- Future international students
- Diversity and Inclusion Opportunities
- Learn abroad
- Living Learning Communities
- Mentor programs
- Programs for women
- Student groups
- Visit, Apply & Next Steps
- Information for current students
- Departments and majors overview
- Departments
- Undergraduate majors
- Graduate programs
- Integrated Degree Programs
- Additional degree-granting programs
- Online learning
- Academic Advising overview
- Academic Advising FAQ
- Academic Advising Blog
- Appointments and drop-ins
- Academic support
- Commencement
- Four-year plans
- Honors advising
- Policies, procedures, and forms
- Career Services overview
- Resumes and cover letters
- Jobs and internships
- Interviews and job offers
- CSE Career Fair
- Major and career exploration
- Graduate school
- Collegiate Life overview
- Scholarships
- Diversity & Inclusivity Alliance
- Anderson Student Innovation Labs
- Information for alumni
- Get engaged with CSE
- Upcoming events
- CSE Alumni Society Board
- Alumni volunteer interest form
- Golden Medallion Society Reunion
- 50-Year Reunion
- Alumni honors and awards
- Outstanding Achievement
- Alumni Service
- Distinguished Leadership
- Honorary Doctorate Degrees
- Nobel Laureates
- Alumni resources
- Alumni career resources
- Alumni news outlets
- CSE branded clothing
- International alumni resources
- Inventing Tomorrow magazine
- Update your info
- CSE giving overview
- Why give to CSE?
- College priorities
- Give online now
- External relations
- Giving priorities
- CSE Dean's Club
- Donor stories
- Impact of giving
- Ways to give to CSE
- Matching gifts
- CSE directories
- Invest in your company and the future
- Recruit our students
- Connect with researchers
- K-12 initiatives
- Diversity initiatives
- Research news
- Give to CSE
- CSE priorities
- Corporate relations
- Information for faculty and staff
- Administrative offices overview
- Office of the Dean
- Academic affairs
- Finance and Operations
- Communications
- Human resources
- Undergraduate programs and student services
- CSE Committees
- CSE policies overview
- Academic policies
- Faculty hiring and tenure policies
- Finance policies and information
- Graduate education policies
- Human resources policies
- Research policies
- Research overview
- Research centers and facilities
- Research proposal submission process
- Research safety
- Award-winning CSE faculty
- National academies
- University awards
- Honorary professorships
- Collegiate awards
- Other CSE honors and awards
- Staff awards
- Performance Management Process
- Work. With Flexibility in CSE
- K-12 outreach overview
- Summer camps
- Outreach events
- Enrichment programs
- Field trips and tours
- CSE K-12 Virtual Classroom Resources
- Educator development
- Sponsor an event
William A. Galston, Elaine Kamarck
June 28, 2024
Robin Brooks, Ben Harris
June 26, 2024
Yemi Osinbajo
Sofoklis Goulas
June 27, 2024
The Brookings Institution conducts independent research to improve policy and governance at the local, national, and global levels
We bring together leading experts in government, academia, and beyond to provide nonpartisan research and analysis on the most important issues in the world.
From deep-dive reports to brief explainers on trending topics, we analyze complicated problems and generate innovative solutions.
Brookings has been at the forefront of public policy for over 100 years, providing data and insights to inform critical decisions during some of the most important inflection points in recent history.
Subscribe to the Brookings Brief
Get a daily newsletter with our best research on top issues.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Already a subscriber? Manage your subscriptions
Support Our Work
Invest in solutions. Drive impact.
Business Insider highlights research by Jon Valant showing that Arizona's universal education savings accounts are primarily benefiting wealthy families.
Valerie Wirtschafter spoke to the Washington Post about her latest study finding that Russian state media are ramping up on TikTok in both Spanish and English.
Tony Pipa writes in the New York Times about what's necessary for rural communities to benefit from federal investments made in the IIJA, IRA, & CHIPs.
What does the death of Iran’s President really mean? Suzanne Maloney writes in Politico about a transition already underway.
America’s foreign policy: A conversation with Secretary of State Antony Blinken
Online Only
10:30 am - 11:15 am EDT
The Brookings Institution, Washington D.C.
10:00 am - 11:15 am EDT
10:00 am - 11:00 am EDT
AEI, Washington DC
12:45 pm - 1:45 pm EDT
Andre M. Perry Manann Donoghoe
Keesha Middlemass
Elizabeth Cox Chloe East Isabelle Pula
June 20, 2024
Daniel S. Hamilton
Zouera Youssoufou Zakari Momodu
Brookings Explains
Unpack critical policy issues through fact-based explainers.
Listen to informative discussions on important policy challenges.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Res Pharm Pract
- v.2(1); Jan-Mar 2013
The role of pharmacoeconomics in current Indian healthcare system
Akram ahmad.
1 Department of Pharmacy Practice, Annamalai University, Annamalai Nagar, Tamil Nadu, India
2 Department of Clinical, Social and Administrative Sciences, University of Michigan, Ann Arbor, MI, USA
Sundararajan Parimilakrishnan
Guru prasad mohanta, haechung chung.
3 Department of Public Health Sciences, The Pennsylvania State University, Pennsylvania, USA
Jongwha Chang
Phamacoeconomics can aid the policy makers and the healthcare providers in decision making in evaluating the affordability of and access to rational drug use. Efficiency is a key concept of pharmacoeconomics, and various strategies are suggested for buying the greatest amount of benefits for a given resource use. Phamacoeconomic evaluation techniques such as cost minimization analysis, cost effectiveness analysis, cost benefit analysis, and cost utilization analysis, which support identification and quantification of cost of drugs, are conducted in a similar way, but vary in measurement of value of health benefits and outcomes. This article provides a brief overview about pharmacoeconomics, its utility with respect to the Indian pharmaceutical industry, and the expanding insurance system in India. Pharmacoeconomic evidences can be utilized to support decisions on licensing, pricing, reimbursement, and maintenance of formulary procedure of pharmaceuticals. For the insurance companies to give better facility at minimum cost, India must develop the platform for pharmacoeconomics with a validating methodology and appropriate training. The role of clinical pharmacists including PharmD graduates are expected to be more beneficial than the conventional pharmacists, as they will be able to apply the principles of economics in daily basis practice in community and hospital pharmacy.
INTRODUCTION
Healthcare community is ever more sensitive to costs, as the overall health expenditures are escalating. Accordingly, appraisal of goods and services in healthcare goes beyond evaluation of safety and efficacy in which the economic impact of these goods and services on the cost of healthcare is also considered. As in economics, efficiency is the key concept in the pharmacoeconomics, and this principle helps one to design strategies for buying the greatest amount of benefits for a given resource use.[ 1 ]
Resources such as materials and equipments allocated for healthcare are scarce; nevertheless, their possible usages are infinite. Hence, it is a challenge for healthcare professionals to provide quality patient care with minimum cost. Given the limitations on healthcare resources, there is increased interest in assessing the value for money, or economic efficiency of healthcare treatments and programs. Economic evaluation, analyzing costs and outcomes of several alternative therapies can also be a useful approach; though can be very difficult to accomplish.[ 2 ]
In an environment where the cost of healthcare is sky rocketing, insurers are looking for evidence that can support decisions that determine purchasing, contracting, and inclusion of new medications in the formularies. The producers of medications therefore, have to assess the value of the drugs, both in terms of economic worth and clinical efficacy.[ 3 ] “Doctors prescribe, patients consume and, increasingly throughout the world, third purchasing parties (government insurance companies) pay the bill with money that they have obtained from increasingly reluctant healthy members of the public”.[ 4 ] Pharmacoeconomics identifies, measures, and compares the costs and consequences of pharmaceutical products and services.”[ 5 ] It involves economic evaluation of drug development, drug production, and drug marketing, i.e., all the steps that take place from the time the drug is manufactured to when it reaches the patients.[ 6 ]
Pharmacoeconomics
The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) defines pharmacoeconomics as “the field of study that evaluates the behavior of individuals, firms, and markets relevant to the use of pharmaceutical products, services, and programs, and which frequently focuses on the costs (inputs) and consequences (outcomes) of that use”.[ 7 ]
Genesis of pharmacoeconomics
Pharmacy was finally recognized as a clinical discipline within the healthcare system in the early 1960s. At this time, disciplines within the pharmaceutical sciences such as clinical pharmacy, drug information, and pharmacokinetics became an important part of pharmacy education and sciences. Pharmacoeconomics developed its roots in 1970s.[ 8 ] The first book on health economics was published in 1973 and in 1978, McGhan, Rowland, and Bootman from the University of Minnesota introduced the concept of cost-benefit and cost effectiveness analysis.[ 9 ] Utilizing sophisticated pharmacokinetic protocols, Bootman, et al .,[ 10 ] published an early pharmacy research article in 1979 in which cost-benefit analysis was employed to appraise the outcomes of individualizing aminoglycoside dosages to severely burned patients with gram-negative septicemia. In 1983, Ohio State University College of Pharmacy initiated a specialized pharmacy academic program with the objective of providing an overview of the application of cost benefit and cost effective analysis in healthcare, with emphasis on their application to the delivery of pharmaceutical care.
Initially, defined as “analysis of the costs of drug therapy to healthcare systems and society”,[ 8 ] the actual term “pharmacoeconomics” first appeared in the literature in 1986 when Townsend's work was published to highlight the need to develop research activities in this new discipline. In 1992, a journal named “Pharmacoeconomics” was launched.
Methods of pharmacoeconomic analysis
Pharmaeconomic studies compare costs, clinical, and humanistic outcomes associated with different therapies. The evaluation mechanisms delineated are often helpful in demonstrating the cost impact of innovative treatments, granting greater acceptance by healthcare providers, administrators, and the public.
There are four major types of pharmacoeconomic analysis:
A: Cost-minimization analysis
B: Cost-effectiveness analysis
C: Cost-benefit analysis
D: Cost-utility analysis
Major types of pharmacoeconomic analysis, formula and application given in Table 1 .[ 1 , 11 , 12 ]
Major types of pharmacoeconomic analysis-definition, formula and applications[ 1 , 11 , 12 ]

Healthcare and financing system in India
The Indian pharmaceutical industry is a hub where medications can be produced at a low price and still be of international quality. It witnessed a robust growth from the production turnover of about 1.14 billion dollars in 1990 to over 22.73 billion dollars in 2009-10, comprising about Rs, 14.1 billion dollars of domestic market and 9.58 billion dollars of exports.[ 13 ] In terms of production, the India pharma industry ranks 3 rd on a global scale, whereas in terms of turnover worth, it ranks 14 th . Medication prices are among the lowest prices in the world. However, the overall expenses associated with medications continue to soar in the country.[ 13 ] Although India is a producer of abundance of quality drug at low cost, only one third of its population has access to essential medicines.
More than 68% (Census 2011)[ 14 ] of the population lives in villages and work on farms or perform other menial jobs and are paid on a day to day basis. In rural areas comprised of villages and small towns, primary health-centers and community health-centers are put into service by the state government. On the breadline, the rural population heavily depend on the government funded hospitals for procuring healthcare.
In India, allopathic and alternative medicine healthcare practices (Ayurveda, Unani, Siddha, and Homeopathy) operate side by side. Many patients switch from one practice to another when relief is not adequate. The quality of healthcare services is much better in the urban areas compared with rural areas. Some rural areas might have very minimalistic healthcare. The practice of procuring private healthcare for many people is on the rise. The challenge that the Indian government faces is to make healthcare affordable for the majority of people in the country who cannot afford healthcare. Allopathic medications have a big market in India. In 2004, 5.2% of nominal GDP was spent on producing allopathic medications which is equivalent to US $34.9 billion. In 2005-2009, it grew by 12% per annum, i.e., 5.5% of nominal GDP which is equivalent to US $60.9 billion. As far as the ratio of doctors and nurses to the population is concerned, it is 5.9 doctors, 0.8 nurses, and 0.47 midwives for 1,000 people, which adds up to 1.86 health workers for every 1000 populace. The statistics provided by the Union Ministry of Health and Family Welfare's Health Information of India state that in 2004, there were 67,576 government doctors in India who provided healthcare to 15,980 people.[ 15 ] Out of the $24 million spent on healthcare in India, about 77% money is spent on private healthcare, i.e., US $18.643 million. Of the 77% money spend on private healthcare; about 86% is out of pocket expenditure. The public sector expenditure is 21%, i.e. US $5.04 million and the external aids amounts to the remaining 2%, i.e., US $0.48 million.[ 16 ] Limited number of people have health insurance in India. The major issues that govern insurance penetration are the extent and type of coverage. About 10% of the total population has insurance through health financing schemes. The ironic situation is that the insurers leave out the poor and the ill population as they cannot afford the prepayment schemes. The insurance that people purchase voluntarily accounts for Rs. 4 billion, i.e., US $86.3 million, and is estimated to grow at a very fast pace.[ 17 ]
Applications of pharmacoeconomics
Historically, the principles of pharmacoeconomics were applied in the field of hospital pharmacy activities. The cost effectiveness data were used to support the addition or deletion of a drug to or from a hospital formulary. At present, the pharmacoeconomic assessment of formulary actions has become a standardized part of many pharmacy and therapeutic committees.
Pharmacoeconomic studies find value in
- Fixing the price of a new drug and re-fixing the price of an existing drug
- Finalizing a drug formulary
- Creating data for promotional materials of medicines.
- Compliance of requirement for drug license.
- Including a drug in the medical/insurance reimbursement schemes.
- Introduction of new schemes and programs in hospital pharmacy and clinical pharmacy.
- Drug development and clinical trials.[ 12 ]
Pharmacoeconomics and drug development
In India, the estimate for the development of New Chemical Entity (NCE) is often quoted at US $90-100 million due to lower input costs. For every 10,000 NCE in discovery, ten enter preclinical development, five enter human trials, and only one might be approved.[ 18 ] Accordingly, large amount of money spent on pursuing a useless chemical entity is borne by the consumer. Pharmacoeconomic studies may be planned and conducted at the clinical development stages (phases 1 to 3) and post-marketing research stage (phase 4). Subsequently, studies may need to be conducted at several stages of pharmaceutical research.[ 12 ]
Phase 1 trials
The initial clinical trial endeavors to determine the toxicity profile of the drug of interest in humans. It is during this stage that cost of illness studies should be accomplished to aid in deciding whether to further develop the drug and gather background data for future pharmacoeconomic evaluations or not. Cost of illness data may also aid in the development of preliminary models to assess the clinical benefits that must be achieved to have a marketable product.
Phase 2 trials
In phase 2 trials, the drug is administered to a limited number of patients with the target disease. During this phase, cost of illness studies can begin or continue, as can preliminary development of quality of life and recourse utilization instruments. Models can be refined as more information is available about the clinical aspects of the drug.
Phase 3 trials
Cost of illness data can be an important factor that can determine the marketability of drugs. In the phase 3 clinical trials, the drugs are administered to the patients similarly as they would be when they are marketed. At this stage, the discussion, planning, and pharmacoeconomic studies are of prime importance. It is recommended that clinical studies presenting pharmacoeconomic evaluation be conducted along with efficacy evaluation of the drugs. Even though pharmacoeconomic evaluations might be time consuming and may delay the new drug application (NDA) process, they should be done unless the drug is very innovative and has no other alternatives.
Phase 4 trials
Phase 4 trials consist of the post-marketing phase. Pharmacoeconomics can be applied to retrospective and prospective studies involving the drug. Pharmacoeconomic evaluations provide information about cost and outcomes of drugs in real life settings unlike clinical trials that are conducted in controlled settings. Pharmacoeconomic evaluations conducted during clinical trials give information about the efficacy of drugs, which in turn provide an approximation to the real world. Pharmacoeconomic evaluations and clinical trials can be conducted in conjunction with each other in several ways:
- A clinical trial can be designed to test the safety and efficacy of a drug, followed by a pharmacoeconomic evaluation.
- A clinical trial can be designed to conduct a pharmacoeconomic evaluation.
- Clinical data collected prospectively in a clinical trial can be used to conduct a retrospective or prospective pharmacoeconomic evaluation.[ 12 ]
Compliance of requirement for drug license and pharmacoeconomics
Evidence about drug quality, efficacy, and safety is an essential requirement for drug licensing and regulation. Given the ever-increasing healthcare costs, this evidence needs to be backed by evidence of cost-effectiveness as well. In simple words, evidence comparing the effectiveness of available treatments for a particular disease condition and their related costs need to be presented to the federal body before they are introduced in the market.
Australia was the first country to form evidence based guidelines about medication reimbursement on the basis of cost-effectiveness research. Since 1993, the Australian Pharmaceutical Benefit Scheme enforces the production of evidence about economic evaluation of the drug before its introduction in the market.[ 19 ] The drug manufacturer provides the submission form to the Pharmaceutical Benefits Advisory Committee for inclusion of the drug in the reimbursement list who then verify the evidence provided by the manufacturer. The committee provides recommendations to the health ministry about drug inclusion in the reimbursement list on the basis of evidence about its cost effectiveness. The final decision making by the policy makers about the cost-effectiveness of the drug is determined by the following factors:
- The importance of the clinical area
- The availability of alternative treatments
- The likely effect of listing on the healthcare system and other therapeutic activities
- The investment of the sponsor in primary research.
The committee is willing to introduce a “breakthrough” medication which might be a bit pricey, provided the manufacturer has invested considerably in its development and production in contrast to ‘me-too drugs’ which have similar counterpart's existent in the market. In spite of this, relative cost effectiveness is an important criterion. So, many other countries like UK, Belgium, Finland, Norway, Portugal, Sweden and Hungary also follow a similar process such as Australia. The Netherlands also introduced a formal process of economic evaluation in 2005. Germany has an institution for economic evaluation research and Spain has regional centers that perform health technology assessment. In Denmark, France, and Italy, pharmaceutical companies provide data about economic evaluation on a voluntary basis. These data when provided are given importance and consideration.[ 20 ] Food and Drug Administration (FDA) in United States and Central Drug Standard Control Organization (CDSCO) in India do not require an economic analysis for Drug approval. A new drug has to be approved for a program based on pharmacoeconomic analysis.
The formulary system
Formulary creation involves preparing, updating, and using a list of essential medications with their detailed information (formulary manual) and standard treatment guidelines (STGs). A formulary list is an indicator of good pharmaceutical practice and rational drug usage. The formulary consists of appropriate therapies and cost-effective medications which are of a good quality. It is a precise list which makes the process of procuring, storing, distributing, and using the drugs very easy.[ 21 ] The medications that are part of a formulary, have the following advantages:
- Availability of cost contained quality drugs: When medications are purchased in bulk, there is more price competition and “economies of scale” for procuring, storing, and distributing the quality drugs. This makes it possible to provide drugs at subsidized rates to people who require them the most.
- Provision of quality care: Healthcare personnel can be better trained to provide cost effective medications. Usage of cost-effective drugs will also make the practitioners prescribe fewer drugs whose drug interactions and adverse reactions they are aware of. This in turn will improve the provision of quality care as the selection of medication is evidence based.
The formulary system, right from the national level to the institutional level, can be strengthened with the help of studies in the areas. It will also help for the rationalization of the drug procurement system in the country and for the practical implementation of the standard treatment protocols.
HEALTH INSURANCE AND PHARMACOECONOMICS IN INDIA
In the Indian health insurance system, mostly inpatient services are covered, so it is necessary to stay for a day in the hospital to claim the insurance. This, instead of saving costs leads to cost inflation. It is necessary to have some mechanism in place, whereby the insurers can strike a contract with healthcare providers and healthcare systems that can help in cost containment,[ 21 , 22 ] There is an added need for insurance systems that encourage consumer to contain costs by providing incentives as well as contain their health expenditure.[ 23 ] In case of members with multiple coverage, it is necessary that the benefits offered and liability achieved are coordinated and regulated. There needs to be further expansion of insurance services other than inpatient services, and more focus should be placed on preventive care and wellness programs.[ 24 ]
By implementing pharmacoeconomic principle in the hospital administration and treatment protocols, both the patients and the insurance industry will benefit. Patients will receive better quality healthcare at reduced costs, and the insurance companies will be able to provide enhanced care to their clients at minimum cost.
Indian pharmacy practice and pharmacoeconomics
As third largest producer of drugs by volume, Indian pharmaceutical industry has diversity of medicines; yet, brand name prescriptions are the rule of the day. Formulary system is very weak and treatment protocols exist only in theory. The resources are scarce and competing programs are plenty in healthcare. The concept of healthcare insurance is yet to be popularized in the country.[ 1 ] Given the issues prevalent in the Indian healthcare system, pharmacoeconomics has many applications. Pharmacoeconomics can aid in decision making when evaluating the affordability of and access to the right medication to the right patient at the right time, comparing two drugs in the same therapeutic class or drugs with similar mechanism of action, and in establishing accountability that the claims by a manufacturer regarding a drug are justified.
Practicing pharmacists in community, hospital, and clinical settings in India can benefit considerably from the application of the principles of pharmacoeconomic into their normal practice settings. Proper application of pharmacoeconomics will empower the pharmacy practitioners and administrators to make better and more informed decisions regarding products and services they provide. Pharmacotherapy decisions traditionally depended solely on clinical outcomes like safety and efficacy, but pharmacoeconomics teaches us that there are three basic outcomes to be considered clinical, economic, and humanistic in drug therapy. It is accepted by all that appropriate drug selection decisions could not be made today based on acquisition costs only. Applied pharmacoeconomics can help in decision making, in assessing the affordability of medicines to the patients, access to the medicines when needed, and comparing various products for treatment of a disease. It will provide evidence contraindicating the promotion of certain types of high-cost medicines and services.
Pharmacoeconomics has use in health policy decision making and can be applied by a number of healthcare professionals such as policy makers, primary healthcare providers, health-care administrators, and health managers. Available in large quantities, Indian primary care providers are often bombarded with many new drugs of the same category, in addition to the existing drugs. Introduction of new drugs can confuse the doctors and administrators for the judicious selection and rational use of medicines. When introducing new medications, its outcome should be equal or more effective compared to the existing drug and shall have some economic or related advantage.
Evidence about pharmacoeconomics can aid pharmacists and policy makers in the decision-making process about the use of medications and healthcare services. With clinical training about self-medication, Ayush physicians, i.e., Ayurvedic and Naturopathic physicians focus more on diagnostic rather than therapeutic skills, and they do not know much about the drugs, i.e., the brand name, the strength, the formulation, and the dose in specific conditions. Pharmacological and pharmacoeconomic knowledge is acquired and can be applied in practical prescribing skills. Conventional pharmacists also don’t know much about proper medication use. Present qualification of pharmacist in India is Diploma in pharmacy (2-year study, plus 500 h practical training in hospital) and B.Pharm 4-year degree program and its curriculum does not provide sufficient information, practice, and knowledge about pharmacoeconomics. To overcome such a dilemma, the government of India introduced a new program in pharmacy education named PharmD (2008), which highlights the principles of pharmacoeconomics in its syllabus. Consequently, we can expect the future M.pharm pharmacy practice and new generation of clinical pharmacists and PharmD graduates to be more beneficial than conventional pharmacist as they can be expected to implement the principles of economics in daily basis practice in community and hospital pharmacy.
With ever increasing healthcare costs, value added care provided to the patients by individual healthcare institution needs to be further researched. The development of pharamcoeconomics is at an infancy stage in India at the moment, despite the rapid growth of clinical research. India is an affordable destination for conducting clinical research for many western countries. The India Chapter of ISPOR has been formed, but it needs to develop the platform for pharmacoeconomics. We hope in India clinical pharmacists including PharmD graduates be more beneficial than conventional pharmacists as they can implement the principles of economics in daily basis practice in community and hospital pharmacy.
AUTHORS’ CONTRIBUTION
All authors have significantly contributed to the project in terms of conceptualization, writing and reviewing the entire document.
Source of Support: Nil
Conflict of Interest: None declared.
Advancing social justice, promoting decent work ILO is a specialized agency of the United Nations

Main Figures on Forced Labour
27.6 million
people are in forced labour.
US$ 236 billion
generated in illegal profits every year.
3.9 million
of them are in State-imposed form of forced labour.
of them are women and girls (4.9 million in forced commercial sexual exploitation, and 6 million in other economic sectors).
of them are children (3.3 million). More than half of these children are in commercial sexual exploitation.
3 times more
risk of forced labour for migrant workers.
- Victims of forced labour include 17.3 million exploited in the private sector; 6.3 million in forced commercial sexual exploitation, and 3.9 million in forced labour imposed by State.
- The Asia and the Pacific region has the highest number of people in forced labour (15.1 million) and the Arab States the highest prevalence (5.3 per thousand people).
- Addressing decent work deficits in the informal economy, as part of broader efforts towards economic formalization, is a priority for progress against forced labour.
Source: 2022 Global Estimates
Forced Labour Observatory
The Forced Labour Observatory (FLO) is a database that provides comprehensive global and country information on forced labour, including on international and national legal and institutional frameworks; enforcement, prevention and protection measures, as well as information related to access to justice; remedies, and cooperation.
Global Reports

2021 Global Estimates of Modern Slavery: Forced Labour and Forced Marriage
The latest estimates show that forced labour and forced marriage have increased significantly in the last five years, according to the International Labour Organization, Walk Free and the International Organisation for Migration.
- Full Report
- Executive Summary
- Press Release
- Third estimates: Modern Slavery (2017)
- Second estimates: Forced Labour (2012)
- First estimates: Forced Labour (2005)

Profits and Poverty: The Economics of Forced Labour (2024)
The study investigates the underlying factors that drive forced labour, of which a major one is illegal profits.
- Press release
- First edition of the report and second estimates (2014)
- First estimates of illegal profits from forced labour (2005)
Main Statistical Tools on Forced Labour
- Hard to See, Harder to Count: Guidelines for Forced Labour Surveys
- Ethical Guidelines for Research on Forced Labour
- Evidence Gap Map on Forced Labour
- Global Research Agenda (child labour, forced labour and human trafficking)
ICLS and forced labour

The International Conference of Labour Statisticians (ICLS) is the authoritative body to set global standards in labour statistics. During its 20th meeting, in October 2018, the ICLS adopted the "Guidelines concerning the measurement of forced labour". The intent of the guidelines is to facilitate the process of testing the measurement of forced labour in different national circumstances and/or measurement objectives.
- Guidelines concerning the measurement of forced labour (ICLS 2018)
- Measurement of forced labour: stocktaking and way forward (ICLS 2023 Room document 22)
- All ICLS documents






COMMENTS
The majority of health economics research in India are partnerships with scholars from other countries. In India, there is a dearth of understanding of the ideas and methodologies for performing pharmaco-economic analyses. In India, there is presence of a few practitioners of healthcare economic analysis working in education and professional ...
1. Introduction. Health economics is a growing subject in India. In the international scenario, health economics had its conceptual origin long back with the support of organizations like WHO and International Clinical Epidemiology Network. 1, 2, 3 During subsequent decades, the practical applications of this got conceived slowly in the industrialized countries.
Introduction Health economics is a thriving sub-discipline of economics. Applied health economics research is considered essential in the health care sector and is used extensively by public policy makers. For scholars, it is important to understand the history and status of health economics—when it emerged, the rate of research output, trending topics, and its temporal evolution—to ensure ...
Health Services Research, 38(6 Part 1), 1403-1421. Crossref. PubMed. Google Scholar. Hanson K., & Berman P. (1998). Private health care provision in developing countries: A preliminary analysis of levels and composition. ... Improving health outcomes and health care in India [Economics Department Working Papers No. 1184]. OECD Publishing ...
Healthcare is a growing industry in India and is valued at nearly $40 billion. The private sector accounts for more than 80% of the total healthcare spending, which is mostly out-of-pocket. Increasing population, longer life-expectancy, decline in infant mortality, more disposable income and therefore, ability to afford private healthcare ...
This brings forth the fact that sponsorship and motivation are the major factors in conducting such evaluations in India. In addition, we report on the diversity of other health economics and outcomes research studies from India, including QoL or patient reported outcomes studies.
Abstract. Health economics has long been neglected as a subset of the larger discipline of Economics and Finance. However, this could not be further from the truth. There is a large body of researchers and professionals alike that are of the consensus that extensive studying and working upon Healthcare Economics can help us avert the situation ...
The relationship between health and development is an important topic in the field of economics. India's new Health Policy 2017 also acknowledges this relationship. The COVID-19 pandemic has exposed the chronic underinvestment in India's healthcare system...
Universal health coverage is a widely shared goal across lower-income countries. We conducted a large-scale, 4-year trial that randomized premiums and subsidies for India's first national, public hospital insurance program, RSBY. We find roughly 60% uptake even when consumers were charged premiums equal to the government's cost for insurance.
Indian Health Outcomes, Public Health and Economics Research Centre (IHOPE) is an exciting collaborative and interdisciplinary research centre that has been set up with a 10 crore competitive research grant from the Wellcome Trust/ DBT India Alliance through collaborations between the L V Prasad Eye Institute, Indian Institute of Management, Ahmedabad (IIMA) and Indian Institute of Public ...
the sick and elevation of health. Health Economics is difficult to define in a few words because it encompasses such a broad range of concepts, theories, and topics. The Mosby Medical Encyclopedia (1992, p. 361) defines Health Economics as follows: Health economics studies the supply and demand of health care resources and the impact of health ...
Health Economics and Outcome Research journal aims to disseminate the latest information on the economics of healthcare delivery such that the care of the individuals and improvement in the individual quality of life is central to the economic policy development. The journal emphasizes customized healthcare service and strives to provide novel ...
Health Economics. ABOUT THE COURSE:The proposed course titled 'Health Economics' is unique in the NPTEL and other MOOC platforms. This has a coverage of latest nuances in healthcare emphasizing issues and policies of India and developing countries. This enables readers with a strong conceptual and theoretical explanations.
Research Methods. Recent studies in India and elsewhere that employed research methods such as observations of health care providers' performance, exit interviews of patients, vignette-based ...
Abstract. Background and objective: Economic evaluations are one of the important tools in policy making for rational allocation of resources. Given the very low public investment in the health sector in India, it is critical that resources are used wisely on interventions proven to yield best results. Hence, we undertook this study to assess ...
100+ ICMR Research Topics: Unlocking Health Insights. General / By Stat Analytica / 20th November 2023. The landscape of healthcare research in India has been significantly shaped by the endeavors of the Indian Council of Medical Research (ICMR). Established in 1911, the ICMR has played a pivotal role in advancing medical knowledge, informing ...
studies on personalized medicine. India will be the most populous country in the world. by 2030, and nearly 200 million Indians will be at least. 60 years of age by 2025. However, the growing ...
Public health expenditure in India, including that by both the central and state governments, was maintained constant at approximately 1.3% of GDP between 2008 and 2015 and increased marginally to 1.4% in 2016-17. The National Health Policy 2017 proposed to increase this figure to 2.5% by 2025.
WELCOME TO IHEPA! The Indian Health Economics and Policy Association (IHEPA) is a registered society under the Society's Registration Act 1960. The office is located at International Institute for Population Sciences, Mumbai. IHEPA welcomes all those interested in issues relating to health economics and policy to be a part of this association.
The Health Policy Research Unit (HPRU) was set up in 1998, to consolidate, continue, and significantly expand the research in the area of health economics and policy in IEG. Read More The objective of HPRU is to carry out research that is directly relevant to the changing health scenario in India, and the focus is on topics that either are ...
For the world as a whole public and. private expenditure on health servic es was about $2000 billion of 8% of total world product. The total annual spending ranged from less than $10 per person in ...
Though the spending on healthcare is. 6% of gross domestic product (GDP), the state. expenditure is only 0.9% of the total spending (4). Moreover, the available resources are not used efficiently ...
The Wire: The Wire News India, Latest News,News from India, Politics, External Affairs, Science, Economics, Gender and Culture
MINNEAPOLIS / ST. PAUL (6/28/2024) - School of Mathematics PhD student Sylvester Zhang was recently awarded the Doctoral Dissertation Fellowship from the University of Minnesota. The Doctoral Dissertation Fellowship (DDF) gives the University's most accomplished Ph.D. candidates an opportunity to devote full-time effort to an outstanding research project by providing time to finalize and ...
The Brookings Institution is a nonprofit public policy organization based in Washington, DC. Our mission is to conduct in-depth research that leads to new ideas for solving problems facing society ...
The first book on health economics was published in 1973 and in 1978, McGhan, ... The statistics provided by the Union Ministry of Health and Family Welfare's Health Information of India state that in 2004, ... despite the rapid growth of clinical research. India is an affordable destination for conducting clinical research for many western ...
Topics; Data and research on forced labour. Facebook Twitter ... 2022 Global Estimates. US$ 236 billion. generated in illegal profits every year. Profits and Poverty: The Economics of Forced Labour (2024) 3.9 million. of them are in State-imposed form of forced labour. ... Global Research Agenda (child labour, forced labour and human trafficking)