

Important Tips for Writing an Effective Conference Abstract
Academic conferences are an important part of graduate work. They offer researchers an opportunity to present their work and network with other researchers. So, how does a researcher get invited to present their work at an academic conference ? The first step is to write and submit an abstract of your research paper .
The purpose of a conference abstract is to summarize the main points of your paper that you will present in the academic conference. In it, you need to convince conference organizers that you have something important and valuable to add to the conference. Therefore, it needs to be focused and clear in explaining your topic and the main points of research that you will share with the audience.
The Main Points of a Conference Abstract
There are some general formulas for creating a conference abstract .
Formula : topic + title + motivation + problem statement + approach + results + conclusions = conference abstract
Here are the main points that you need to include.
The title needs to grab people’s attention. Most importantly, it needs to state your topic clearly and develop interest. This will give organizers an idea of how your paper fits the focus of the conference.
Problem Statement
You should state the specific problem that you are trying to solve.
The abstract needs to illustrate the purpose of your work. This is the point that will help the conference organizer determine whether or not to include your paper in a conference session.
You have a problem before you: What approach did you take towards solving the problem? You can include how you organized this study and the research that you used.
Important Things to Know When Developing Your Abstract
Do your research on the conference.
You need to know the deadline for abstract submissions. And, you should submit your abstract as early as possible.
Do some research on the conference to see what the focus is and how your topic fits. This includes looking at the range of sessions that will be at the conference. This will help you see which specific session would be the best fit for your paper.
Select Your Keywords Carefully
Keywords play a vital role in increasing the discoverability of your article. Use the keywords that most appropriately reflect the content of your article.
Once you are clear on the topic of the conference, you can tailor your abstract to fit specific sessions.
An important part of keeping your focus is knowing the word limit for the abstract. Most word limits are around 250-300 words. So, be concise.
Use Example Abstracts as a Guide
Looking at examples of abstracts is always a big help. Look at general examples of abstracts and examples of abstracts in your field. Take notes to understand the main points that make an abstract effective.
Avoid Fillers and Jargon
As stated earlier, abstracts are supposed to be concise, yet informative. Avoid using words or phrases that do not add any specific value to your research. Keep the sentences short and crisp to convey just as much information as needed.
Edit with a Fresh Mind
After you write your abstract, step away from it. Then, look it over with a fresh mind. This will help you edit it to improve its effectiveness. In addition, you can also take the help of professional editing services that offer quick deliveries.
Remain Focused and Establish Your Ideas
The main point of an abstract is to catch the attention of the conference organizers. So, you need to be focused in developing the importance of your work. You want to establish the importance of your ideas in as little as 250-300 words.
Have you attended a conference as a student? What experiences do you have with conference abstracts? Please share your ideas in the comments. You can also visit our Q&A forum for frequently asked questions related to different aspects of research writing, presenting, and publishing answered by our team that comprises subject-matter experts, eminent researchers, and publication experts.
best article to write a good abstract
Excellent guidelines for writing a good abstract.
Rate this article Cancel Reply
Your email address will not be published.

Enago Academy's Most Popular Articles

- AI in Academia
AI vs. AI: How to detect image manipulation and avoid academic misconduct
The scientific community is facing a new frontier of controversy as artificial intelligence (AI) is…

- Diversity and Inclusion
Need for Diversifying Academic Curricula: Embracing missing voices and marginalized perspectives
In classrooms worldwide, a single narrative often dominates, leaving many students feeling lost. These stories,…

- Career Corner
- Trending Now
Recognizing the signs: A guide to overcoming academic burnout
As the sun set over the campus, casting long shadows through the library windows, Alex…

Reassessing the Lab Environment to Create an Equitable and Inclusive Space
The pursuit of scientific discovery has long been fueled by diverse minds and perspectives. Yet…

- Reporting Research
How to Improve Lab Report Writing: Best practices to follow with and without AI-assistance
Imagine you’re a scientist who just made a ground-breaking discovery! You want to share your…
7 Steps of Writing an Excellent Academic Book Chapter
When Your Thesis Advisor Asks You to Quit
Virtual Defense: Top 5 Online Thesis Defense Tips

Sign-up to read more
Subscribe for free to get unrestricted access to all our resources on research writing and academic publishing including:
- 2000+ blog articles
- 50+ Webinars
- 10+ Expert podcasts
- 50+ Infographics
- 10+ Checklists
- Research Guides
We hate spam too. We promise to protect your privacy and never spam you.
I am looking for Editing/ Proofreading services for my manuscript Tentative date of next journal submission:

As a researcher, what do you consider most when choosing an image manipulation detector?

How to Write a Conference Abstract
- Finding Conferences
General Advice
Things to keep in mind when preparing your abstract, where to get writing inspiration, example resources.
- How to Write a Scientific or Research Abstract
- How to Write a Case Report Abstract
- How to Write a Quality Improvement Project Abstract
- Writing Tips
- Reasons for Rejection
This page provides general guidance on how to prepare for the common types of conference abstracts. Use tabs for more specific information on writing Scientific, Case Report, and Quality Improvement conference abstracts.
Submitting a conference abstract is competitive
Know the rules:
- Follow the rules for abstract submission, don't give the reviewers any reason to toss out your abstract
- Pay attention to word count limits, section headings, how to format your name, etc.
Know the timeline and deadline:
- Get your writing goal calendar together at the start to reduce stress
- Know what is expected at each step of writing
- Give yourself enough time for writing and re-writing
- There can be a range of turnaround times
Look for an archive of past abstracts from the conference to keep your abstract on track.
- Web of Science This link opens in a new window Includes a database called Conference Proceedings Citation Index. It includes conference proceedings from 1994 to the present. Nearly 400,000 conference proceedings are added to the index each year. You can search by topic, author, conference, year, and use various filters to focus in what you are searching for.
Here are some examples of what abstract submission guidelines look like:
- General abstracts submission guidelines
- General abstract submission FAQs
- Abstract submission checklist
- << Previous: Finding Conferences
- Next: How to Write a Scientific or Research Abstract >>
- Last Updated: Feb 14, 2024 8:15 AM
- URL: https://guides.temple.edu/howtowriteaconferenceabstract
Temple University
University libraries.
See all library locations
- Library Directory
- Locations and Directions
- Frequently Called Numbers

Need help? Email us at [email protected]
ACS Student Magazine
How To Write a Professional, Polished Scientific Abstract

In the science world, you make your first impression long before you meet anyone in person. How? Through an abstract—that brief, powerful paragraph that describes your research.
Whether it’s for a conference presentation, journal article, grant proposal, or dissertation, the abstract—as well as the title and the author listing—is the first window into the scope and purpose of your work. It tells the reader about the content of your research and the results of your experimentation. And it tells the reviewer or editor which session or journal you belong in, so potential collaborators can find you.
To show that your research is relevant and worth learning more about, you need to write a polished and professional abstract. Here’s how to write titles, author listings, and text for abstracts that are informative and professional in any presentation format.
Keep your title short and descriptive. Don’t oversell or sensationalize your work; simply state what it is. If you absolutely must give detail, you can add a subtitle, using a colon to separate it from the main title.
Reviewers start with the title to make sure they have you in the right session. For example, “C–H bond functionalization in long-chain alkanes” will be placed in an Organic Chemistry session, whereas “Iron oxide catalysts for hydrocarbon C–H bond functionalization” will be placed in an Inorganic Chemistry session.
Scientists use titles to see whether your work is relevant to them. If you write “Curing genetic diseases through molecular modeling”,
you had better have the clinical trial data to back up your claim.
“Molecular basis of multiple mitochondrial dysfunctions syndrome: Impact of substitution on the protein NFU1” will be of interest to other biochemists studying protein functionality.
Here are some additional technical tips for titles of scientific abstracts:
- Start with an adjective, a noun, or a verb. Never start with an article (e.g., “The”, “A”, or “An”).
- Do not end with punctuation; your title is not a sentence.
- Use plain text (no bold, italics, or special fonts).
- Use sentence case. The only words that need to be capitalized are the first word of the title, the first word after a colon, and any proper nouns, acronyms (e.g., FT-IR), or element symbols in formulas (e.g., NaOH).
The authors and affiliations
List the names and affiliations of everyone who contributed to the work, starting with you. If you are submitting the abstract for an oral or poster presentation, you are the presenting author and your name goes first. If you are submitting an article to a journal, your name will either go first or be highlighted in some fashion. (It depends on the protocols of the journal and the preferences of your research advisor.)
You also need to include your research advisor. With the exception of some very unusual circumstances, your project is part of a larger body of research that is coordinated by your advisor. Your advisor helped you to develop your project, guided your interpretation of the results, and provided you with laboratory space and supplies, and your results will be incorporated into your advisor’s overall body of research. So your advisor gets credit.
You should include anyone else who contributed significantly to the research, such as a labmate who performed some of the work or a colleague in another lab who assisted you with analyses.
Include your affiliation as well as that of your coauthors. Your affiliation is your school. For clarity, be sure to cite the complete name of your school, not an abbreviation or short form (e.g., use “California Institute of Technology”, not “Caltech”). Unless you are collaborating with a group outside of your department or doing research at another institution, your coauthors will have the same affiliation as you.
Abstracts are high-level summaries. They are typically no longer than 2000 characters (preferably shorter than 1000 characters). Using complete sentences, describe why the work was done, what types of experiments were completed, and the results. You are writing for other scientists, so you do not need to explain common scientific terms, only techniques specific to your research. This guideline will help you stay within the character limit.
Keep it simple. Experimental parameters, data, graphs, references, and extensive discussion of the results are for your presentation or article. If you find yourself trying to include these details, you are saying too much. Some abstract submission systems, like the ACS Meeting Abstracts Programming System (MAPS), do not accept graphs, figures, or references, so you run the risk of being rejected from a symposium. There are instances when a graph or figure is necessary, but they are the exception.
Here are some key elements to keep in mind as you are writing:
- Why is your research important?
- What problem does your work attempt to solve?
- What specific approaches did you take or methods did you use?
- What were the results?
- How does your research add to the body of knowledge?
Keeping the abstract general helps with the challenge: having to submit your abstract before you complete your research. This is especially common if you are presenting at a technical conference, like an ACS national meeting, where submissions are due six to seven months before the conference. In this case, you can submit a short abstract of the work you are planning to do, and end with, “The results of this work will be presented.”
Write in the third person using passive voice (e.g., “Microporous silicates were synthesized” rather than “I synthesized a series of microporous silicates”). In the scientific community, this is the more professional way to present research.
Finally, proofread, proofread, and proofread again. Make sure that your sentences are clear and error-free. Have a peer, grad student, or experienced labmate (or two) review your abstract for clarity, grammar, and punctuation. Also, have your research advisor review and approve it. This work represents your advisor’s lab, so your advisor should have a say in what you report.
Writing abstracts is a skill that is essential to both the research world and the business world (where it’s called an “executive summary”). Start developing this skill now to set yourself up for success later.
ABSTRACT CHALLENGE
See if you can spot the errors in this online quiz.
Anatomy of an Awesome Abstract
A professional abstract summarizes the scope of your research and shows why your study is worth learning more about. Check out the infographic below for a breakdown of what should be included in your abstract, and click on the image to access the PDF.

How to Write an Undergraduate Abstract, by Elzbieta Cook. www.acs.org/content/acs/en/meetings/national-meeting/agenda/student-program/undergraduate-abstract.html
About the Author

Blake Aronson is a program manager for ACS Student Communities. She works with undergraduate programs at two- and four-year institutions, as well as the SCI Scholars Program and other ACS initiatives.
More Careers Articles
From marine biology to chemical manufacturing, we talked to nanoscientists about how they tackle climate challenges with big innovations using the tiniest tools.

Past ACS President Katie Hunt shares her insights on industry vs. academic careers, sustainability in industry, and the most important question you can ask.

How do you know if you have good interpersonal skills? And how do you prove to a potential employer that years of group work were not in vain? Find out.

What started as a passion for inspiring students from underrepresented backgrounds turned this teacher into a kitchen-chemistry household name. Learn about his surprising career.
Subscribe to our Newsletter
American Society for Microbiology
Tips for writing your first conference abstract.
July 17, 2019
Communicating your research is an important part of being a scientist. While submitting an abstract to a national conference can seem overwhelming, it is one of the best ways to communicate your science and get your research in front of a large audience.
Students interested in learning how to write an abstract should review these helpful tips that describe components of a competitive abstract . Also review the below suggestions from ASM education specialist, Dr. Christopher Skipwith, on best practices for preparing your first abstract for a scientific conference.
Read the Instructions
Although this may be obvious, many times, abstracts are rejected because they are missing key components. Be sure to familiarize yourself with the submission requirements before starting your abstract.
Understand the Target Audience
Who will be reading your abstract? Whether you are submitting to a field-specific or general conference, make sure the language you use can be easily understood by your target audience. Field-specific language may be appropriate for specialized conferences, however plain language must be used for conferences that cover a wide range of fields.
When using acronyms, remember to always provide the full phrase on the first mention accompanied by the acronym in parenthesis.
Clearly State the Hypothesis/Statement of Purpose
Not every project has a hypothesis, but all projects have a purpose. Figure out the research questions you are trying to answer and include the hypothesis or purpose of your research in the abstract. It is imperative to communicate your hypothesis/statement of purpose clearly so the reader can get a better context of your research goals and understand the importance of your research.
Tie Results and Conclusions Back to the Hypothesis/Statement of Purpose
After you have written your results and conclusions, go back to your hypothesis/statement of purpose. Do the results and conclusions clearly support your research purpose? What did the results say about your hypothesis? Make sure the links between these components are obvious to the reader.
Review, Then Review Again
Re-read your abstract against a copy of the abstract guidelines. As you go through, check off each guideline to ensure your abstract is complete. Remember that even if it has all the requirements, a poorly written abstract will not be accepted. Then have multiple people, including your Principal Investigator, read it over. Incorporate their feedback to ensure a strong submission.
The Annual Biomedical Research Conference for Minority Students (ABRCMS) provides poster and oral presentation opportunities for undergraduates, postbaccalaureates, and masters students.
- Undergraduate Student
- Communicating Science
- Graduate Student
- Professional Development
- Scientific Writing
Author: Christopher Skipwith, Ph.D.

ASM Microbe 2024 Registration Now Open!
Discover asm membership, get published in an asm journal.
How to Structure a Scientific Article, Conference Poster and Presentation
- First Online: 22 April 2022
Cite this chapter

- Sarah Cuschieri 2
480 Accesses
9 Altmetric
The main aim of any research endeavor is to disseminate research findings among the scientific community and enrich scientific knowledge. The two most common scientific dissemination modes are journal articles and conference proceedings. Whichever route you decide to follow, the scientific writing that needs to be partaken typically follows the IMRaD format, i.e., Introduction, Methods, Results, and Discussion-Conclusion. This format is applicable for abstracts (unless an unstructured abstract is specifically required), scientific research articles, and conference poster or oral presentations. Understanding the importance of each IMRaD section along with the role of a clear and informative title is your road to success. This also applies to proper citation of other people’s work, ideas, and thoughts. This chapter will guide you, through a stepwise approach, to understand the various elements that make up a successful scientific article, an abstract, a conference poster, and an oral presentation.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Chan A-W, Tetzlaff JM, Altman DG et al (2013) SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 158:200
Article Google Scholar
Gagnier JJ, Kienle G, Altman DG et al (2013) The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Heal Med 2:38
Grech V (2018a) WASP (Write a Scientific Paper): preparing a poster. Early Hum Dev 125:57–59. https://doi.org/10.1016/j.earlhumdev.2018.06.007
Article PubMed Google Scholar
Grech V (2018b) WASP (Write a Scientific Paper): optimisation of PowerPoint presentations and skills. Early Hum Dev 125:53–56. https://doi.org/10.1016/j.earlhumdev.2018.06.006
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Moher D, Hopewell S, Schulz KF et al (2010) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 63:e1–e37
O’Brien BC, Harris IB, Beckman TJ et al (2014) Standards for reporting qualitative research. Acad Med 89:1245–1251
von Elm E, Altman DG, Egger M et al (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806
Download references
Author information
Authors and affiliations.
Department of Anatomy, Faculty of Medicine and Surgery, University of Malta, Msida, Malta
Sarah Cuschieri
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cuschieri, S. (2022). How to Structure a Scientific Article, Conference Poster and Presentation. In: A Roadmap to Successful Scientific Publishing. Springer, Cham. https://doi.org/10.1007/978-3-030-99295-8_3
Download citation
DOI : https://doi.org/10.1007/978-3-030-99295-8_3
Published : 22 April 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-99294-1
Online ISBN : 978-3-030-99295-8
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

How To Write A Scientific Abstract (11 Tips)
You put all this hard work into preparing your scientific paper.
But if you want your peers, colleagues, or students to read it, you need to put some more effort into crafting an effective abstract. Let’s find out how to do it.
What is a scientific abstract? A definition.
It’s a summary of a scientific paper, intended to summarize the research project, its purpose, achieved results, and conclusions. It gives a good idea of what’s inside the paper, but you have to read the whole thing. A more formal definition from Wikipedia: An abstract is a summary of a research article, thesis, review, conference proceeding, or any in-depth analysis of a particular subject and is often used to help the reader quickly find out the paper’s purpose.
A good abstract has the following qualities:
- 1. Summarize the findings in your paper
- 2. Persuades the reader to download and read the full article
- 3. If it’s prepared for a conference, it gets you selected for a talk and makes the audience curious about your subject
- 4. It presents the exact results of your research, not only a list of topics
- 5. It’s composed of an introduction, a body, and a conclusion
- 6. Usually, it’s no longer than 250 words (but may go up to 500), and it’s written in a 12-size font
- 7. It should be accessible to a general reader (you go into the nitty-gritty in the paper itself)
- 8. It communicates the main point of your research, why it matters, and what you concluded
- 9. If you co-authored the paper with someone else, you mention them in the abstract
- 10. If you’ve been mentored by any members of your faculty while doing research, you mention them as well
- 11. In it, you share the methods you used, and the results you achieved, and finish with a conclusion
“Somewhere, something incredible is waiting to be known.” – Carl Sagan
Types of abstracts for different uses:
- For an article in a scientific publication
- For a conference presentation (academic poster)
- Graphical abstract, video abstract (they include visuals but follow the same basic format shown below).
Here’s a simple example of an abstract:
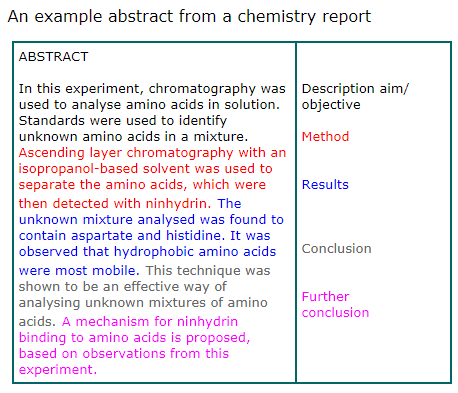
Writing an abstract for an article in a scientific publication
Here are a couple of rules to follow if you’re preparing to submit your work for publication:
- The title of the paper should be clear but enticing
- The inclusion of data is acceptable (but only in summary)
- Try not to use citations or URLs in the abstract. Leave that for the paper itself
- Use clear language, don’t overcomplicate, and be straightforward
- Before submitting, give it one more final edit
- Check the spelling, grammar, and syntax. You can use Grammarly for that.
- After the title and the introduction, say why the reader should care about this research (motivation)
- Make sure you include the right keywords at the bottom of the abstract. They will make your paper more searchable in the science database, which makes it easier for it to get found and receive citations. Answer this question when thinking about keywords: what would someone type in the search engine to find your paper?
Follow a proven format:
- The problem, challenge, or a question
- Why should you care
- Methods used
- Results achieved
- Conclusion and steps forward
- (Usually around 250 words)
Writing an abstract for a conference presentation (poster)
A good abstract can go a long way in advancing your scientific career. The organizers of the event will review it in every detail and select your article for a presentation based on its quality. It all depends on the caliber of the event, but for the well-attended ones, your abstract will make or break your chances of presenting to a big audience. People who approve articles for presentation want the audience to be engaged and will accept only the highest quality material .
Here are a few guidelines for preparing a solid conference abstract:
- People in the audience won’t have access to your full article, so you only have the abstract to present your research succinctly.
- Usually, there’s a strict limit of words you can use, so you need to plan how to pique the interest of the audience with a limited amount of space.
- The abstract is like a business card or a short persuasive letter that will determine if you get the spotlight or not. A perfect match of scientific understanding and persuasive skills is required.
- Ask about the exact format specifications and check examples of abstracts from previous years.
- Sometimes you’ll have to submit the abstract months before the actual event. You’ll still need to come up with something to make it sound interesting. And then make sure you send the updated version before the conference.
Here you can find six examples of scientific abstracts written for a presentation. And here you can find a great article, about abstracts for a presentation, written by a Ph.D. with a 90% acceptance rate. And if you need to create a poster out of your abstract, here’s a great guide you can check as well as some amazing examples.
Here’s another good example from humanities:
Pagel, J. F.; Vann, B. H. The effects of dreaming on awake behavior. Dreaming: Journal of the Association for the Study of Dreams. Vol 2(4)229-237, Dec 1992. Abstract: Reports of the incorporation of dream mentation into a spectrum of awake behaviors were obtained from a heterogeneous awake population group through the utilization of self-reporting questionnaires (N=265). Results were analyzed to determine associations between age, gender, race, and the dream use variables. Significantly higher dream use was found in females for a majority of behaviors, and a negative correlation was found between increasing age and all dream questions studied. No significant racial/ethnic variation was found in the responses of the sample. These findings suggest that such a sociological approach to the study of the effects of dream mentation on awake behavior can provide insight into the sleep/dream states. Keywords: dream; dream use; behavior; age; gender; race; sleep; questionnaire. Here are more examples of abstracts from the University of Wisconsin. And from another scientific paper about dreams (with the actual paper included below).
As you can see, writing a solid summary of your scientific paper is straightforward. I hope that now you have a better understanding of the process, and will accomplish great things in the scientific community. Next up, you may want to explore a list of the top educational book publishers .

Get your free PDF report: Download your guide to 100+ AI marketing tools and learn how to thrive as a marketer in the digital era.

Rafal Reyzer
Hey there, welcome to my blog! I'm a full-time entrepreneur building two companies, a digital marketer, and a content creator with 10+ years of experience. I started RafalReyzer.com to provide you with great tools and strategies you can use to become a proficient digital marketer and achieve freedom through online creativity. My site is a one-stop shop for digital marketers, and content enthusiasts who want to be independent, earn more money, and create beautiful things. Explore my journey here , and don't miss out on my AI Marketing Mastery online course.
How to write a good abstract for a scientific paper or conference presentation
Affiliation.
- 1 Department of Psychopharmacology, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
- PMID: 21772657
- PMCID: PMC3136027
- DOI: 10.4103/0019-5545.82558
s of scientific papers are sometimes poorly written, often lack important information, and occasionally convey a biased picture. This paper provides detailed suggestions, with examples, for writing the background, methods, results, and conclusions sections of a good abstract. The primary target of this paper is the young researcher; however, authors with all levels of experience may find useful ideas in the paper.
Keywords: Abstract; preparing a manuscript; writing skills.
Writing an Abstract for a Conference
January 2, 2022 8 min read
January 2, 2022 | 8 min read
Writing Abstracts for a Conference
An "abstract" for an academic conference is a short summary of the scientific research you are involved in. While abstracts generally have a standard format and include more or less the same information and in a similar layout, each conference may have its unique requirements. It is, therefore, essential that you make yourself aware of that conference's specific requirements when planning to submit an abstract for a conference.
Abstracts are submitted to the conference organizers by or on behalf of one of the research authors. This person is called the "presenting author". The presenting author submits the abstract because they wish to present their work at the conference. The conference then has a committee that decides and selects the abstracts that most fit the topic and purpose of the conference. These chosen abstracts are then scheduled into the conference.
Presenting at a conference is a privilege; so typically, the presenter registration fees are not waived. On the contrary, many conferences will not review an abstract if the person who has submitted it is not registered to attend the conference or has not paid an abstract submission fee.
How to Write a Research Abstract for a Conference.
Conferences are essential academic activities pursued by researchers worldwide. They drive the advancement of knowledge through presentations and discussions among their participants. They also help researchers from different regions and backgrounds to connect, thereby enabling future research cooperation.
The Benefits of Presenting at an Academic Conference
Researchers who present their research at conferences open the door to multiple opportunities to advance their research. They receive direct feedback, new ideas, and advice from influential scientific community members and colleagues.
On both a personal and professional level, presenters receive attention from influential members of the community that can benefit them in the future. In addition to this, presenters gain the opportunity to build their reputation and to add colleagues, future employers, and future collaborators to their network.
Participating in an international conference can be expensive. To present at a conference, participants must have ways to fund their conference participation, including travel and accommodation expenses. Unfortunately, the conference organizers usually will not cover presenters' costs and will not even exempt presenters from the conference registration free. However, presenters can apply for grants from any academic institution they are affiliated with. Associations may also have funds to help members present in conferences. In addition, many organizations will generally fund conference participation for their employees.
To begin with, you need to prepare and submit an abstract of your research.
1. What is a Conference Abstract?
As mentioned above, a conference abstract is a limited-length outline of an oral presentation or poster that you intend to present at a conference.
A conference abstract includes:
Article abstract vs. conference abstract.
Article abstracts are submitted alongside the full article or paper and are therefore evaluated alongside the full paper. In the case of academic journals, if the abstract is not perfect, but the editors liked the article, they can request that the author fix the abstract. However, this is not the case with a conference; a conference abstract is submitted by itself and judged by itself.
On the other hand, many conferences will accept poor abstracts because they need to fill slots to make their conference bigger. In a conference, the quality of your abstract as evaluated by the organizer will affect the type of presentation (live or poster) and the scheduling of the presentation provided to you.
2. Processing and Reviewing Abstracts in Conferences.

Conference abstracts are processed and reviewed in several steps. These are listed below:
- Conference name.
- Conference date and location.
- Conference topics.
- Abstract submission guidelines.
- Abstract submission deadlines.
- Abstract processing fees.
- Potential speakers submit their abstracts.
- The conference secretariat receives the abstracts. They then ensure the abstract is valid, complete, and follows the guidelines
The secretariat is responsible for assigning the abstract for review by one or more reviewers. The secretariat or the Abstract Management System will select the reviewers based on the abstract topic and rules defined by the conference organizers and the conference chairperson.
In small conferences, the chairperson will review all the abstracts and decide how to include them in the conference agenda.
In other conferences, a group of reviewers (known as the scientific committee) will review and give a grade to each abstract. Each reviewer will grade each abstract independently. Depending on the specific conference, each reviewer may also suggest filing the abstract under a different conference topic, recommend the presentation type (poster or oral), or ask the author to revise the abstract (revise and resubmit).
There are two main types of review processes:
- After all reviewers complete their review, the abstract management system will calculate the average score of each abstract. The chairperson will then make the final decision regarding the abstracts.
- The secretariat will communicate this decision to the abstract submitters and will guide them about the next steps they should take.
- The conference chairperson, along with the organizers, will schedule the accepted abstracts to a conference session.
Abstract review criteria.
Most conferences aks reviewers to review and grade abstracts based on similar criteria.
Common abstract grading factors:
- Relevance of the abstract to the conference.
- Originality.
- Significance.
- Adherence to abstract submission guidelines.
Conference organizers may have additional goals, so they may consider additional factors.
Example of additional abstract selection factors:
- Encouraging young researchers
- Ethnic diversity
- Author reputation
3. Challenges in Writing a Conference Abstract.
Writing a conference abstract is challenging since it is a limited-length text that needs to appeal to all the different groups of people involved in the conference. In addition, each group has somewhat other interests.
The main groups are the conference organizers, reviewers, and conference attendees. Organizers decide if the abstract is good enough before assigning it to the reviewers, and after the abstract is accepted, they choose when to schedule it. Reviewers score the abstract based on conference criteria such as fitting the conference topics and scientific significance. Attendees need to have an interest in attending the presentation after reading the abstract.

4. Getting Ready to Write the Abstract.
Before writing your abstract, check if a preliminary conference agenda has been published. There may be a list of sessions that you can aim to present and topics that get more time on the agenda.
How many users enter the website, where they are from, the browser they use, how many pages they visit, the time they spend on each page, and more.
Remember to check the conference's abstract submissions guidelines.
Things to note:
- Submission deadlines.
- Topic list.
- Abstract length limit.
- Are tables and figures permitted?
- Review criteria.
Check for scientific committee members and chairpersons.
Search Abstract Examples
Check abstracts submitted to the conference over the last years can help get an idea of what is required in the abstract.
If previous year abstracts are not available online, ask your colleges if they have a copy of the conference abstracts book from previous years. Attempt to figure out what made each one work.
5. Writing the Abstract Title.
The title is one sentence that describes your research and presentation. It is probably the most important sentence in your abstract because:
- It is the first impression of people reviewing your abstract.
- It will appear in the conference agenda with a possible link to your abstract.
- More people will only read the title than read the abstract or attend the presentation.
- People remember and recite your article by its title.
A good title is a clear, easily understood, and attention-grabbing sentence that describes your research and highlights its importance. A good title attracts attendees to read the full abstract or attend the oral presentation.
To make your title clear, straightforward, and short:
- Keep it under 14 words.
- Avoid using obvious words such as "Research on", "Results of ", "Investigation", "Role of".
- Remove unnecessary words such as "the".
- Remove words that give no information to the readers.
- Avoid special symbols and units.
- Avoid complicated words, uncommon abbreviations, and too much jargon.
Writing the abstract title step by step
- Explain what your research and presentation are about in two or three sentences. Do not reveal the conclusions.
- Shorten and combine the sentences into one title.
- Remove unnecessary words.
- Review and refine the title.
- Make sure that it is informative, clear, and interesting.
6. Writing the Abstract Body.
The abstract body is the main part of the abstract and typically has 200 to 500 words.
General tips:
- Concentrate on the research Objective, Methods, Results, and Consultation (OMRC).
- Keep sentences brief and concise.
- References are not required in the abstract.
- Keep background information to a minimum.
- Do extensively referring to other works.
- Do not define terms.
- Avoid asking questions and not answering them.
- Make sure your abstract is error-free before submitting it.
An abstract body typically has four parts abbreviated as OMRC.
Abstract body parts (OMRC):
- Conclusions
Let us have a look at the main parts of the abstract:
Part 1 - Objective and Purpose
This part is typically two to four sentences and covers: background information, the reason for doing the research, the problems or questions the research aims to solve, and the overall topic of the research. It also outlines why your research is important and how difficult it is.
Typically, this part of the body will end with a sentence that describes the purpose of the research. For example, "The purpose of this study was to _____."
Examples of abstract purpose:
- Examining a new topic. Remember to outline why you are examining this new topic.
- Filling a gap in previous research.
- Applying new methods to take a fresh look at existing ideas or data.
- Resolving a dispute within the literature.
Part 2 - Methods
When doing the research, what research methods were used? How extensive was the investigation? Remember to explain who the participants are, what the researchers measured, and what tools they used. Was the research empirical or theoretical? What sources of information did the research rely on?
This section should not include what the researchers expected to find.
Part 3 - Results
This section describes the research findings.
In the case that the research does not have results yet, you should describe the preliminary data or results with some statistical work. If you expect to have results before the conference, the abstract can include a note that a finalized version of the abstract will be updated at a later date before the conference.
Part 4 - Conclusion
This section explains the meaning of the findings, the importance of the findings, and their implications.
An abstract that does not include a conclusion or result section is called a descriptive abstract. If the abstract has a conclusion, it is called an informative abstract.
Participating in an international academic conference potentially brings multiple opportunities. Presenting at a conference adds a significant boost to these opportunities and can also help fund participation. Writing a good abstract is key to making this possible.
Please share your tips with us on Twitter. Did you find any part of this article helpful? Please share it with your colleagues and friends.
Read more about the Eventact Software for abstracts submission and review here.
Review Management
Event Websites
Event Professionals

- About the LSE Impact Blog
- Comments Policy
- Popular Posts
- Recent Posts
- Subscribe to the Impact Blog
- Write for us
- LSE comment
January 27th, 2015
How to write a killer conference abstract: the first step towards an engaging presentation..
34 comments | 132 shares
Estimated reading time: 6 minutes
Helen Kara responds to our previously published guide to writing abstracts and elaborates specifically on the differences for conference abstracts. She offers tips for writing an enticing abstract for conference organisers and an engaging conference presentation. Written grammar is different from spoken grammar. Remember that conference organisers are trying to create as interesting and stimulating an event as they can, and variety is crucial.
Enjoying this blogpost? 📨 Sign up to our mailing list and receive all the latest LSE Impact Blog news direct to your inbox.
The Impact blog has an ‘essential ‘how-to’ guide to writing good abstracts’ . While this post makes some excellent points, its title and first sentence don’t differentiate between article and conference abstracts. The standfirst talks about article abstracts, but then the first sentence is, ‘Abstracts tend to be rather casually written, perhaps at the beginning of writing when authors don’t yet really know what they want to say, or perhaps as a rushed afterthought just before submission to a journal or a conference.’ This, coming so soon after the title, gives the impression that the post is about both article and conference abstracts.
I think there are some fundamental differences between the two. For example:
- Article abstracts are presented to journal editors along with the article concerned. Conference abstracts are presented alone to conference organisers. This means that journal editors or peer reviewers can say e.g. ‘great article but the abstract needs work’, while a poor abstract submitted to a conference organiser is very unlikely to be accepted.
- Articles are typically 4,000-8,000 words long. Conference presentation slots usually allow 20 minutes so, given that – for good listening comprehension – presenters should speak at around 125 words per minute, a conference presentation should be around 2,500 words long.
- Articles are written to be read from the page, while conference presentations are presented in person. Written grammar is different from spoken grammar, and there is nothing so tedious for a conference audience than the old-skool approach of reading your written presentation from the page. Fewer people do this now – but still, too many. It’s unethical to bore people! You need to engage your audience, and conference organisers will like to know how you intend to hold their interest.
Image credit: allanfernancato ( Pixabay, CC0 Public Domain )
The competition for getting a conference abstract accepted is rarely as fierce as the competition for getting an article accepted. Some conferences don’t even receive as many abstracts as they have presentation slots. But even then, they’re more likely to re-arrange their programme than to accept a poor quality abstract. And you can’t take it for granted that your abstract won’t face much competition. I’ve recently read over 90 abstracts submitted for the Creative Research Methods conference in May – for 24 presentation slots. As a result, I have four useful tips to share with you about how to write a killer conference abstract.
First , your conference abstract is a sales tool: you are selling your ideas, first to the conference organisers, and then to the conference delegates. You need to make your abstract as fascinating and enticing as possible. And that means making it different. So take a little time to think through some key questions:
- What kinds of presentations is this conference most likely to attract? How can you make yours different?
- What are the fashionable areas in your field right now? Are you working in one of these areas? If so, how can you make your presentation different from others doing the same? If not, how can you make your presentation appealing?
There may be clues in the call for papers, so study this carefully. For example, we knew that the Creative Research Methods conference , like all general methods conferences, was likely to receive a majority of abstracts covering data collection methods. So we stated up front, in the call for papers, that we knew this was likely, and encouraged potential presenters to offer creative methods of planning research, reviewing literature, analysing data, writing research, and so on. Even so, around three-quarters of the abstracts we received focused on data collection. This meant that each of those abstracts was less likely to be accepted than an abstract focusing on a different aspect of the research process, because we wanted to offer delegates a good balance of presentations.
Currently fashionable areas in the field of research methods include research using social media and autoethnography/ embodiment. We received quite a few abstracts addressing these, but again, in the interests of balance, were only likely to accept one (at most) in each area. Remember that conference organisers are trying to create as interesting and stimulating an event as they can, and variety is crucial.
Second , write your abstract well. Unless your abstract is for a highly academic and theoretical conference, wear your learning lightly. Engaging concepts in plain English, with a sprinkling of references for context, is much more appealing to conference organisers wading through sheaves of abstracts than complicated sentences with lots of long words, definitions of terms, and several dozen references. Conference organisers are not looking for evidence that you can do really clever writing (save that for your article abstracts), they are looking for evidence that you can give an entertaining presentation.
Third , conference abstracts written in the future tense are off-putting for conference organisers, because they don’t make it clear that the potential presenter knows what they’ll be talking about. I was surprised by how many potential presenters did this. If your presentation will include information about work you’ll be doing in between the call for papers and the conference itself (which is entirely reasonable as this can be a period of six months or more), then make that clear. So, for example, don’t say, ‘This presentation will cover the problems I encounter when I analyse data with homeless young people, and how I solve those problems’, say, ‘I will be analysing data with homeless young people over the next three months, and in the following three months I will prepare a presentation about the problems we encountered while doing this and how we tackled those problems’.
Fourth , of course you need to tell conference organisers about your research: its context, method, and findings. It will also help enormously if you can take a sentence or three to explain what you intend to include in the presentation itself. So, perhaps something like, ‘I will briefly outline the process of participatory data analysis we developed, supported by slides. I will then show a two-minute video which will illustrate both the process in action and some of the problems encountered. After that, again using slides, I will outline each of the problems and how we tackled them in practice.’ This will give conference organisers some confidence that you can actually put together and deliver an engaging presentation.
So, to summarise, to maximise your chances of success when submitting conference abstracts:
- Make your abstract fascinating, enticing, and different.
- Write your abstract well, using plain English wherever possible.
- Don’t write in the future tense if you can help it – and, if you must, specify clearly what you will do and when.
- Explain your research, and also give an explanation of what you intend to include in the presentation.
While that won’t guarantee success, it will massively increase your chances. Best of luck!
This post originally appeared on the author’s personal blog and is reposted with permission.
Note: This article gives the views of the author, and not the position of the Impact of Social Science blog, nor of the London School of Economics. Please review our Comments Policy if you have any concerns on posting a comment below.
About the Author
Dr Helen Kara has been an independent social researcher in social care and health since 1999, and is an Associate Research Fellow at the Third Sector Research Centre , University of Birmingham. She is on the Board of the UK’s Social Research Association , with lead responsibility for research ethics. She also teaches research methods to practitioners and students, and writes on research methods. Helen is the author of Research and Evaluation for Busy Practitioners (2012) and Creative Research Methods in the Social Sciences (April 2015) , both published by Policy Press . She did her first degree in Social Psychology at the LSE.

About the author

Dr Helen Kara has been an independent researcher since 1999 and also teaches research methods and ethics. She is not, and never has been, an academic, though she has learned to speak the language. In 2015 Helen was the first fully independent researcher to be conferred as a Fellow of the Academy of Social Sciences. She is also an Honorary Senior Research Fellow at the Cathie Marsh Institute for Social Research, University of Manchester. She has written widely on research methods and ethics, including Research Ethics in the Real World: Euro-Western and Indigenous Perspectives (2018, Policy Press).
34 Comments
Personally, I’d rather not see reading a presentation written off so easily, for three off the cuff reasons:
1) Reading can be done really well, especially if the paper was written to be read.
2) It seems to be well suited to certain kinds of qualitative studies, particularly those that are narrative driven.
3) It seems to require a different kind of focus or concentration — one that requires more intensive listening (as opposed to following an outline driven presentation that’s supplemented with visuals, i.e., slides).
Admittedly, I’ve read some papers before, and writing them to be read can be a rewarding process, too. I had to pay attention to details differently: structure, tone, story, etc. It can be an insightful process, especially for works in progress.
Sean, thanks for your comment, which I think is a really useful addition to the discussion. I’ve sat through so many turgid not-written-to-be-read presentations that it never occurred to me they could be done well until I heard your thoughts. What you say makes a great deal of sense to me, particularly with presentations that are consciously ‘written to be read’ out loud. I think where they can get tedious is where a paper written for the page is read out loud instead, because for me that really doesn’t work. But I love to listen to stories, and I think of some of the quality storytelling that is broadcast on radio, and of audiobooks that work well (again, in my experience, they don’t all), and I do entirely see your point.
Helen, I appreciate your encouraging me remark on such a minor part of your post(!), which I enjoyed reading and will share. And thank you for the reply and the exchange on Twitter.
Very much enjoyed your post Helen. And your subsequent comments Sean. On the subject of the reading of a presentation. I agree that some people can write a paper specifically to be read and this can be done well. But I would think that this is a dying art. Perhaps in the humanities it might survive longer. Reading through the rest of your post I love the advice. I’m presenting at my first LIS conference next month and had I read your post first I probably would have written it differently. Advice for the future for me.
Martin – and Sean – thank you so much for your kind comments. Maybe there are steps we can take to keep the art alive; advocates for it, such as Sean, will no doubt help. And, Martin, if you’re presenting next month, you must have done perfectly well all by yourself! Congratulations on the acceptance, and best of luck for the presentation.
Great article! Obvious at it may seem, a point zero may be added before the other four: which _are_ your ideas?
A scientific writing coach told me she often runs a little exercise with her students. She tells them to put away their (journal) abstract and then asks them to summarize the bottom line in three statements. After some thinking, the students come up with an answer. Then the coach tells the students to reach for the abstract, read it and look for the bottom line they just summarised. Very often, they find that their own main observations and/or conclusions are not clearly expressed in the abstract.
PS I love the line “It’s unethical to bore people!” 🙂
Thanks for your comment, Olle – that’s a great point. I think something happens to us when we’re writing, in which we become so clear about what we want to say that we think we’ve said it even when we haven’t. Your friend’s exercise sounds like a great trick for finding out when we’ve done that. And thanks for the compliments, too!
- Pingback: How to write a conference abstract | Blog @HEC Paris Library
- Pingback: Writer’s Paralysis | Helen Kara
- Pingback: Weekend reads: - Retraction Watch at Retraction Watch
- Pingback: The Weekly Roundup | The Graduate School
- Pingback: My Top 10 Abstract Writing tips | Jon Rainford's Blog
- Pingback: Review of the Year 2015 | Helen Kara
- Pingback: Impact of Social Sciences – 2015 Year-In-Review: LSE Impact Blog’s Most Popular Posts
Thank you very much for the tips, they are really helpful. I have actually been accepted to present a PuchaKucha presentation in an educational interdisciplinary conference at my university. my presentation would be about the challenges faced by women in my country. So, it would be just a review of the literature. from what I’ve been reading, conferences are about new research and your new ideas… Is what I’m doing wrong??? that’s my first conference I’ll be speaking in and I’m afraid to ruin it!!! I will be really grateful about any advice ^_^
First of all: you’re not going to ruin the conference, even if you think you made a bad presentation. You should always remember that people are not very concerned about you–they are mostly concerned about themselves. Take comfort in that thought!
Here are some notes: • If it is a Pecha Kucha night, you stand in front of a mixed audience. Remember that scientists understand layman’s stuff, but laymen don’t understand scientists stuff. • Pecha Kucha is also very VISUAL! Remember that you can’t control the flow of slides – they change every 20 seconds. • Make your main messages clear. You can use either one of these templates.
A. Which are the THREE most important observations, conclusions, implications or messages from your study?
B. Inform them! (LOGOS) Engage them! (PATHOS) Make an impression! (ETHOS)
C. What do you do as a scientist/is a study about? What problem(s) do you address? How is your research different? Why should I care?
Good luck and remember to focus on (1) the audience, (2) your mission, (3) your stuff and (4) yourself, in that order.
- Pingback: How to choose a conference then write an abstract that gets you noticed | The Research Companion
- Pingback: Impact of Social Sciences – Impact Community Insights: Five things we learned from our Reader Survey and Google Analytics.
- Pingback: Giving Us The Space To Think Things Through… | Research Into Practice Group
- Pingback: The Scholar-Practitioner Paradox for Academic Writing [@BreakDrink Episode No. 8] – techKNOWtools
I don’t know whether it’s just me or if perhaps everybody else encountering problems with your site. It appears as if some of the text in your content are running off the screen. Can someone else please comment and let me know if this is happening to them as well? This could be a issue with my browser because I’ve had this happen before. Thank you
- Pingback: Exhibition, abstracts and a letter from Prince Harry – EAHIL 2018
- Pingback: How to write a great abstract | DEWNR-NRM-Science-Conference
- Pingback: Abstracting and postering for conferences – ECRAG
Thank you Dr Kara for the great guide on creating killer abstracts for conferences. I am preparing to write an abstract for my first conference presentation and this has been educative and insightful. ‘ I choose to be ethical and not bore my audience’.
Thank you Judy for your kind comment. I wish you luck with your abstract and your presentation. Helen
- Pingback: Tips to Write a Strong Abstract for a Conference Paper – Research Synergy Institute
Dear Dr. Helen Kara, Can there be an abstract for a topic presentation? I need to present a topic in a conference.I searched in the net and couldnt find anything like an abstract for a topic presentation but only found abstract for article presentation. Urgent.Help!
Dear Rekha Sthapit, I think it would be the same – but if in doubt, you could ask the conference organisers to clarify what they mean by ‘topic presentation’. Good luck!
- Pingback: 2020: The Top Posts of the Decade | Impact of Social Sciences
- Pingback: Capturing the abstract: what ARE conference abstracts and what are they FOR? (James Burford & Emily F. Henderson) – Conference Inference
- Pingback: LSEUPR 2022 Conference | LSE Undergraduate Political Review
- Pingback: New Occupational Therapy Research Conference: Integrating research into your role – Glasgow Caledonian University Occupational Therapy Blog
Leave a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Notify me of follow-up comments by email.
Related Posts

‘It could be effective…’: Uncertainty and over-promotion in the abstracts of COVID-19 preprints
September 30th, 2021.
How to design an award-winning conference poster
May 11th, 2018.

Are scientific findings exaggerated? Study finds steady increase of superlatives in PubMed abstracts.
January 26th, 2016.

An antidote to futility: Why academics (and students) should take blogging / social media seriously
October 26th, 2015.

Visit our sister blog LSE Review of Books
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Rev Bras Ter Intensiva
- v.25(2); Apr-Jun 2013
Language: English | Portuguese
How to prepare and submit abstracts for scientific meetings
Como elaborar e submeter resumos de trabalhos científicos para congressos.
The presentation of study results is a key step in scientific research, and submitting an abstract to a meeting is often the first form of public communication. Meeting abstracts have a defined structure that is similar to abstracts for scientific articles, with an introduction, the objective, methods, results and conclusions. However, abstracts for meetings are not presented as part of a full article and, therefore, must contain the necessary and most relevant data. In this article, we detail their structure and include tips to make them technically correct.
A apresentação dos resultados de um trabalho é ponto crucial da metodologia científica, e o envio de resumo para congressos é frequentemente sua primeira forma de comunicação pública. O resumo contém estrutura definida e é semelhante aos resumos de artigos científicos, com introdução, objetivo, métodos, resultados e conclusões. No entanto, o resumo para congresso não é apresentado como parte de artigo completo e, por isso, ele deve conter as informações necessárias e mais relevantes. Neste artigo, detalhamos sua estrutura e algumas dicas para torná-lo tecnicamente correto.
INTRODUCTION
Submitting abstracts for meetings is useful for communicating the first results of a new study, just as submitting scientific articles to journals for publication is the best way of communicating the final results of a study. The submission of abstracts describing scientific studies for professional meetings encompasses a variety of goals. The study authors may be partly or fully evaluated by their peers, i.e., other professionals in the same field may provide feedback and suggestions to refine the method and results presented. ( 1 ) This is also an excellent way of pre-reporting a study, whether it is an observational or intervention study, promoting interaction between researchers interested in the same topic. ( 1 )
Abstracts should only include the most relevant data from the study, with the goal of enabling the reviewer to assess whether the rationale and scientific context are appropriate to evaluate the topic. ( 2 ) Authors commonly clarify the details of the study, although this may result in a confusing and poorly structured abstract. An abstract must have sufficient impact to draw the reviewers' and readers' attention, i.e., maintain their interest while reading the text.
WRITING STYLE AND LANGUAGE
First, the instructions for writing the abstract and the deadline for its submission should be checked. The rules regarding the font type and size should be followed. Abstracts have word or character limits (including or excluding spaces) that are often 250 to 300 words. Prior knowledge of this limit is important when writing the introduction and method sections of the abstract because these sections are more flexible and may be adapted to remain within the length limit. Clear and concise language is necessary for each section of the abstract. The use of abbreviations is usually not allowed, despite the necessary economy of words. Abbreviations may be used in very special cases that require the repetition of long terms. They should be written in full the first time the abbreviation appears in the text. Another tip is to avoid using adjectives or adverbs, maintaining strictly scientific language; articles (mostly the indefinite) may eventually be omitted. The use of the first person plural has become increasingly common and is now often the most appropriate form for scientific texts. ( 3 ) Overuse of the passive voice renders the text boring, repetitive and impersonal. Traditional thinking regarding the use of the first person as petulant is countered by the argument that researchers are indeed the ones performing the actions they describe and that they should be responsible for them. This new approach may be used in abstract writing, although it applies primarily to the article, and the use of passive voice is more common in abstracts, given the necessary economy of words. ( 3 )
Misspellings should also be corrected because abstracts are frequently published in the annals of meetings without editing after submission. An up-to-date spell checker and word processing program should be used to correct grammatical errors and to count words and characters. The word count tools of the most common word processing programs, including Microsoft Word ® , use different counting rules from most electronic submission sites. Thus, the count performed in word processing software often exceeds the limit on the website. Therefore, although the writing may be mainly accomplished using a software program, final adjustments should be performed directly on the submission website.
Title, authors and affiliations
The title, authors and their affiliations must be included, regardless of the form of submission, electronic or otherwise. The title should be catchy and self-explanatory. All unnecessary words should be deleted. There are essentially two standards: one in which the title asks a question relating to the study objectives and one in which the main finding of the study is given. ( 4 ) The latter format has recently become more popular. Ideally, the title should also provide information on the mechanism and the population to which it applies. Thus, "Effects of early use of antibiotics" sounds less interesting than when phrased in the form of a question, such as "Do antibiotics alter the outcomes of sepsis?", although both describe the objective of the study. Conversely, "Early use of antibiotics reduces mortality in patients with shock, but not in those with sepsis" is much more appealing and descriptive.
The format of the authors' names should comply with the rules of the meeting. Ideally, the full name should always be provided, without abbreviations, to avoid ambiguity or errors in the author indexing process, when the abstract is published in an indexed journal. However, writing a name with an abbreviated middle name or the author's last name first may be required. The format of affiliations should also be standardized, and the rules of the meeting should be followed. Usually, the name of the institution should be written out in full, indicating the city, state and country. Including all authors' e-mail addresses is commonly required in electronic submission systems.
ABSTRACT STRUCTURE
Abstracts may have different structures, depending on the rules established by the scientific committee of a meeting. They may be continuous or structured. ( 2 ) Usually, review articles and reports of clinical cases use unstructured abstracts, i.e., the text is not divided into sections and is written as a single block. All key parts are included, and the flow of the text is maintained. Usually, structured abstracts are divided into the following sections: introduction or rationale, methods, results and conclusions. Structuring abstracts in this form is advised so that they comply with the rules of the event, as the use of other sections may result in automatic rejection. For example, one of the most frequent errors is the use of an introduction section when only objectives section is required.
Introduction and objectives
The introduction or study rationale description is the first part of most abstracts for meetings. An introductory sentence on the general topic is welcome, especially if it describes something that is general knowledge (e.g., "Maintenance of blood pressure at very low levels has a negative impact on cerebral ischemia"). Next, the topic or question that the study will address is given (e.g., "The use of nimodipine in the treatment of cerebral ischemia reduces the effects of oxidation in neurons, although it may lead to hypotension").
The study objectives should be cited next. The objective(s) should be described as specifically and concisely as possible. ( 2 ) Try to avoid citing too many objectives, as in a scientific article, because the goal of the abstract is to inform the reader of only the main points of the study. As already mentioned, there may be no room for introducing the topic. In this case, the proper formulation of the objectives is even more critical.
The methodology should be based on a few main points: the study design; the study setting (intensive care unit, emergency unit or ward); the inclusion and exclusion criteria; the intervention applied (or the data observed) and the outcomes to be analyzed. How the study objective was developed, the topics of observation or intervention in the patients studied, and the methods of data analysis should be clarified. "Prospective observational study included patients over 18 years of age, admitted to the ICU and under mechanical ventilation for 48 hours, after signing the consent form" and "Patients in the period following thoracic surgery were excluded" are appropriate sentences. Note that the exclusion criteria are included in the universe of inclusion, e.g., for the first case above, there is no need to mention excluding those below 18 years of age. The intervention applied or the data collected to address the objective should be mandatorily described. There is no need mention all the data collected in detail, e.g., demographic data may be cited instead of age, gender and race, among others. The numerical form in which the data are shown, e.g., mean and standard deviation or percentage, and the main statistical tests used should be reported if there is enough space. The statistical analysis may be summarized or omitted if there are not enough words/characters available; the reviewers will likely assume that the statistical analysis was properly performed. Eventually, the authors should report more specific statistical analyses, including regressions and propensity scores. The most common error in this section is the inclusion of results, e.g., data or the number of patients included. Although a statement of the approval of the study by an ethics committee is mandatory in the body of the full article, it is not usually required in meeting abstracts due to a lack of space. As a result, all ethical rules are presumably properly followed. Some systems require the responsible author state that these precepts were fully met during submission of the abstract.
The results constitute the main summary of the study, and the author(s) should save more words for that section. ( 3 ) The initial description of the population studied, followed by the analysis addressing the main objective, is the essential part of the results. The abstract must report the number of patients included because this information is necessary to judge the validity of the results presented. Tables or figures may be included in the abstracts for some meetings. Note that these tables and figures must be small and only show the most representative results, as abstracts are compact forms of publication. Large tables and complex figures can be difficult to read and comprehend. Finally, there is no room in this section for discussing and comparing the results with those of other studies.
Conclusions
The conclusions should be concise and impactful. The author(s) should include the answer(s) to the given objective(s) in one or two sentences. ( 1 ) The conclusion is the section that will be read most frequently, after the study title. Here, there is no room for discussing the results, which is a fairly frequent error. The most common error in the conclusion is to extrapolate the data evaluated by the study, which may result in the immediate rejection of the abstract, with no opportunity to resubmit.
The citation of references is recommended, although this may be difficult due to the inclusion of the number of words/characters in the references or the total word/character count, which is already limited.
ABSTRACT SUBMISSION
The abstract submission process also has steps that must be completed. Nowadays, most events use an electronic submission system, which facilitates the management of hundreds of submitted abstracts. From the authors' standpoint, these systems are also beneficial because they are usually self-explanatory and reduce the chance of inappropriate submissions.
Some additional considerations should be addressed. Choose the subject area that is most appropriate for your abstract; this will ensure that it is presented and discussed alongside studies of the same topic, which will benefit the author(s). Another point to be considered is that abstracts are presented individually and may be grouped in a session relating to the secondary objectives of the initial study. Reviewing committees may reject abstracts that discuss topics that are not included in the main study. Many meetings do not accept case reports and literature reviews, even in the form of systematic reviews and meta-analyses, because the original themes are given preference by the scientific committees. Other meetings accept these types of abstracts, but have different rules for them.
FINAL CONSIDERATIONS
The presentation of results from scientific studies at meetings is a key step in communicating science to those for whom the results are relevant. Furthermore, it greatly contributes to improving the quality of publications in their final format. Within this context, the development of a good abstract, according to the rules of good scientific writing, is essential. The tips outlined in table 1 may assist in this process.
Tips for preparing abstracts for scientific meetings
ICU - intensive care unit.
Conflicts of interest: None.
- Open access
- Published: 05 June 2019
Good Practice for Conference Abstracts and Presentations: GPCAP
- Cate Foster ORCID: orcid.org/0000-0001-6236-5580 1 ,
- Elizabeth Wager ORCID: orcid.org/0000-0002-4202-7813 2 , 3 ,
- Jackie Marchington ORCID: orcid.org/0000-0001-8482-3028 4 ,
- Mina Patel ORCID: orcid.org/0000-0001-9357-1707 5 ,
- Steve Banner ORCID: orcid.org/0000-0002-7852-9284 6 ,
- Nina C. Kennard ORCID: orcid.org/0000-0002-8480-7033 7 ,
- Antonia Panayi ORCID: orcid.org/0000-0002-1997-3705 8 ,
- Rianne Stacey ORCID: orcid.org/0000-0002-6516-3172 9 &
the GPCAP Working Group
Research Integrity and Peer Review volume 4 , Article number: 11 ( 2019 ) Cite this article
147k Accesses
23 Citations
54 Altmetric
Metrics details
Research that has been sponsored by pharmaceutical, medical device and biotechnology companies is often presented at scientific and medical conferences. However, practices vary between organizations and it can be difficult to follow both individual conference requirements and good publication practice guidelines. Until now, no specific guidelines or recommendations have been available to describe best practice for conference presentations.
This document was developed by a working group of publication professionals and uploaded to PeerJ Preprints for consultation prior to publication; an additional 67 medical societies, medical conference sites and conference companies were also asked to comment. The resulting recommendations aim to complement current good publication practice and authorship guidelines, outline the general principles of best practice for conference presentations and provide recommendations around authorship, contributorship, financial transparency, prior publication and copyright, to conference organizers, authors and industry professionals.
While the authors of this document recognize that individual conference guidelines should be respected, they urge organizers to consider authorship criteria and data transparency when designing submission sites and setting parameters around word/character count and content for abstracts. It is also important to recognize that conference presentations have different limitations to full journal publications, for example, in the case of limited audiences that necessitate refocused abstracts, or where lead authors do not speak the local language, and these have been acknowledged accordingly. The authors also recognize the need for further clarity regarding copyright of previously published abstracts and have made recommendations to assist with best practice.
By following Good Practice for Conference Abstracts and Presentations: GPCAP recommendations, industry professionals, authors and conference organizers will improve consistency, transparency and integrity of publications submitted to conferences worldwide.
Peer Review reports
Note on terminology
Company refers to any medical commercial organization involved with research, such as pharmaceutical or biotechnology companies and medical device manufacturers.
Company-sponsored refers to all types of research (preclinical and clinical, pre- and post-marketing) that is directly sponsored and/or funded by a company. While this classification does not necessarily include research performed under other types of funding arrangement, such as investigator-sponsored or investigator-initiated trials or research (where companies are not involved with conference presentations or publications), those involved in submitting investigator-initiated study material to conferences are encouraged to consider following these recommendations.
Conference is used to refer to meetings, often organized by academic societies, that invite submissions (usually as abstracts) presenting research findings on an aspect of medicine or science. Such conferences have a scientific (or programme) committee that reviews and selects presentations to be given at the meeting from the submitted abstracts.
Abstract refers to those submitted for consideration to scientific and medical conferences (see above).
Presentation refers to posters or slides developed from abstracts accepted for presentation at such conferences.
Lead author refers to the person who normally presents study findings at a conference and is usually listed as the first author. This is often the Principal Investigator.
Society sponsor refers to a member of the society that is holding the conference, who acts as sponsor (or guarantor) of a submitted abstract.
Presenting author refers to the person on the author list who attends the conference and presents the poster or abstract.
Non-author presenter or local presenter refers to a person who presents on behalf of the author group, but who is not listed as an author.
Introduction
Research that has been sponsored (see the ‘Note on terminology’ section for precise definitions of these terms) by commercial organizations (e.g. pharmaceutical, medical device and biotechnology companies) is often presented at scientific and medical conferences. These conferences are pivotal for the presentation of data from ongoing research projects and clinical trials to the relevant audience and are often the first opportunity to disclose and discuss potentially practice-changing data. They facilitate early communication of data long before publication of a full manuscript and also provide the opportunity to present results of additional analyses such as secondary and/or exploratory endpoints and post hoc analyses. However, while abstracts submitted to conferences are reviewed by a scientific committee for suitability and interest to the audience prior to acceptance, it is important to note that they are not considered peer-reviewed as they are not subject to the same rigorous peer-review process as are journal articles. Poster and oral presentations based upon accepted abstracts are rarely, if ever reviewed. Furthermore, a recent systematic review showed that less than 50% of all studies accepted as abstracts went on to be published in full following presentation at a conference [ 1 ]. While it is desirable to strive for full publication after a conference presentation to ensure transparency and allow healthcare professionals to make appropriate informed decisions based on the peer-reviewed literature, this is not always practical and/or achievable. Therefore, it is important that abstracts and conference presentations, particularly for company-sponsored research, are developed with as rigorous a process as that of a full publication, because these may ultimately become the only source for a particular analysis.
While there are recommendations on the preparation of journal articles and qualification for authorship [ 2 ], and guidelines for best practices in the publication of company-sponsored research [ 3 , 4 , 5 ], until now, no specific guidelines have been available to describe good practice and best principles for conference presentations. This has resulted in diverse practices and a lack of standard expectations for transparency and ethical approaches. Although some aspects of good practice in Good Publication Practice (GPP) [ 5 ] and in reporting guidelines such as CONSORT and PRISMA for Abstracts [ 6 , 7 ] can be applied to conference presentations, the most widely cited recommendations on authorship from the International Committee of Medical Journal Editors (ICMJE) relate exclusively to publications in peer-reviewed journals [ 3 ]. These recommendations were not designed for, and therefore are not fully applicable to, abstract submissions and conference presentations and are challenging to implement in practice. Building on the acceptance and recognition of the GPP guidelines (first published as GPP for Pharmaceutical Companies in 2003 [ 3 ], updated in 2010 [ 4 ] and most recently published as GPP3 in 2015 [ 5 ]), this article endeavours to extend their principles and to address challenges relating to the presentation of company-sponsored research at academic meetings. These recommendations, on Good Practice for Conference Abstracts and Presentations (GPCAP), focus on company-sponsored research (see the ‘Note on terminology’ section). However, they do not cover other company activities that may be linked to conferences (e.g. satellite symposia organized alongside scientific conferences, medical education and marketing activities) because these are governed by regional and national legislation or codes (e.g. EFPIA code of practice [ 8 ], FDA regulations [ 9 ]). As with the GPP guidelines, GPCAP focuses on the presentation of all types of company-sponsored research and the specific challenges surrounding this, rather than investigator-sponsored or investigator-initiated trials or research (where companies have no role in their presentation or publication), although many of the principles also apply to the presentation of other types of research at scientific meetings. The aim of GPCAP is therefore to provide guidance on good submission and presentation practice for scientific and medical congresses, specifically addressing certain aspects where current publication guidelines are inadequate.
These recommendations were developed after informal discussions among a group of individuals who have wide experience of working with authors to develop abstracts, posters and slides for oral presentations reporting company-sponsored research. The main impetus for this article arose from a meeting regarding GPP3 updates (with which some of the authors had been involved). Prior to this meeting, two authors had noted that even the revised GPP3 guidelines contained limited advice for conference abstracts and presentations. Meeting participants discussed the requirement for clearer guidance and formed a working group to address this gap. At this point, invitations to join the group were extended to potential authors known to have previously presented relevant research at meetings of the International Society of Medical Publication Professionals (ISMPP) or had a known interest in conference presentations. This also ensured a broader global representation and improved the balance between pharmaceutical and medical communication agency representation. The authors all work or have worked for pharmaceutical companies and/or medical communication agencies (see the ‘Competing interest’ section for specific details). After a search for recommendations and guidelines on this topic revealed nothing specific (either in ICMJE or in a search on EQUATOR), the authors developed an initial outline for this article; individuals worked on pre-agreed sections and then a collective review of the full draft, comprising all sections was completed (see ‘Authors’ contributions’ for specific details). The resulting article was posted as a preprint on PeerJ [ 10 ] on 19 October 2017 for open comment. All comments received (and their responses) can be seen with the preprint on the PeerJ website. These comments were used to revise the recommendations. Some authors invited informal consultation from colleagues, and a courtesy legal review, as appropriate, was completed to ensure compliance with employee company policies. The copyright section was reviewed specifically for appropriate interpretation of copyright law. In addition to the preprint, 65 medical societies and medical conference sites, and two for-profit companies that run conferences on behalf of societies, were contacted for comment via contact emails listed on their websites or via ‘contact us’ options found on their websites. The societies and conferences and conference service companies were selected by recommendation from within the author group, to ensure balance across therapeutic areas, geography and variety of website submission sophistication. Only one of these societies/companies responded. All comments received on the preprint by 10 July 2018 were collated and discussed, and this final version was generated. The preprint was viewed by 2769 unique visitors and downloaded 3300 times between 19 October 2017 and 25 March 2019.
The recommendations are given here by topic, and so there is some overlap by intention, to ensure that all the key elements for any given topic appear together and allow readers to browse by topic.
Recommendations
The following principles aim to cover the key areas relevant for submissions to any research-based conference.
Author listings should reflect those who did the research and can take accountability for its conduct, and for the analysis and interpretation of the findings. Criteria for authorship of conference abstracts and presentations should generally be the same as those for full publications, although there can be occasions where local presenters may be included as authors, for example, where a conference requires a presenter to be listed as an author.
All authors should be involved in the development, and approve the final version, of any abstract, poster or slides that bears their names. For studies involving large numbers of researchers it may be most efficient for a subgroup of those involved in the studies to develop conference abstracts and presentations (similar to the use of a writing group to develop publications from large studies).
Posters and slides should list key contributors and describe their contributions to the research and development of the presentation.
Study registration numbers (e.g. ClinicalTrials.gov , EudraCT, PROSPERO) should be included on abstracts, posters and slides.
All sources of funding for the research and its presentation, and any author conflicts of interest, should be disclosed on posters and slides, on the conference submission site, and if space permits, on abstracts.
Any medical writing support and associated funding should be acknowledged on posters and slides, on the conference submission site, and if space permits, on abstracts.
These recommendations are mapped against the development of an abstract and subsequent conference presentation workflow in Fig. 1 , referenced by section number.
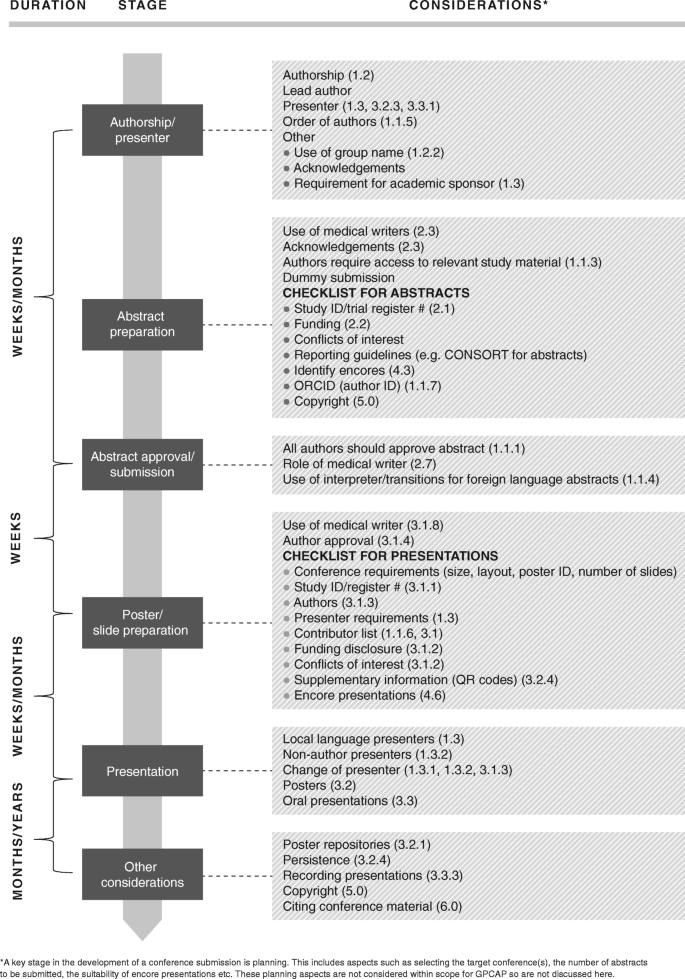
Roadmap of recommendations following abstract and presentation development stages
Recommendations for conference organizers
Conference organizers should:
encourage the inclusion of contributor lists on posters and slides;
include a field for trial registration details on abstract forms (outside the word or character limit) and publish this information with the abstract;
include a field for sponsor information on abstract forms (outside the word or character limit) and publish this information with the abstract;
include a field for disclosing medical writing support on abstract forms (outside the word or character limit) and publish this information with the abstract;
use ORCID identifiers (individual researcher identifiers [ 11 ]) to identify authors and presenters;
not set arbitrary limits on the number of authors, and permit the use of study group names; and
distinguish between authors (meeting the ICMJE criteria) and any additional individuals (who are not authors or contributors) included in the submission, for example, as a result of a requirement for a society member to sponsor submissions. With limited space in any printed book of abstracts, this information might be restricted to appearing with the online version of the abstract.
1.0 Authorship
1.1 authors.
1.1.1 The author listing on conference abstracts and presentations should reflect the people who did the research or contributed substantially to the design of the study or to the interpretation of the results, and who were involved in the development of the presentation and who are willing to take responsibility for the findings. Authorship and author order should be agreed by all authors (see 1.1.5 for factors to consider). While the authorship criteria recommended by the ICMJE are widely used for journal articles [ 2 ], GPP3 recognizes that it may be necessary to adopt slightly different criteria for conference abstracts and presentations [ 5 ]. For example, while all named authors should review (at least once), approve the content of abstracts and presentations and be willing to take responsibility for the findings, it may be impractical to expect all authors to contribute to drafting and critically revising abstracts in the same way as for full manuscripts, because of the abstract brevity, time constraints, etc. There is an argument for limiting the authors to a number that can meaningfully comment and review an abstract (see 1.2.1) and using a study group to identify others involved in the wider study. Our collective past experience indicates that it becomes impractical for everyone to be involved in a group with more than 10 authors, which is also the maximum number suggested by GPP3 [ 5 ].
1.1.2 Authorship criteria for all anticipated journal articles and primary conference presentations should, ideally, be agreed at the start of the research, and author listings for subsequent secondary abstracts and presentations should be finalized well before work starts on the secondary material [ 12 ]. As with journal publications, whatever criteria are used to determine authorship should be applied equally to all authors, regardless of whether they are company employees, contractors, independent clinicians, researchers or consultants.
1.1.3 Authors and contributors should have access to all relevant study materials and data to permit them to understand the research findings. Abstracts may need to be developed soon after results are analysed and before a final clinical study report is available. In such cases, authors should always have access to the protocol, statistical tables and any other information necessary to discuss and develop the planned abstract and presentation.
1.1.4 If individuals are authors on abstracts and presentations written in languages in which they are not proficient, companies should work with them and offer whatever reasonable assistance is required to permit them to discuss and review material effectively (e.g. to provide translations for the authors, or a discussion with an interpreter or local investigator/presenter who can read and explain the text). Authors may also choose not to be listed for such a conference abstract and presentation (see also 1.1.6).
1.1.5 Whatever convention is (or will be) used to determine the order of authors on the related full publications in journals should generally also be used to determine the order of listing on conference abstracts and presentations. The final order should be agreed by all authors; however, conference requirements (e.g. listing the presenting author first) must be respected. In cases where first or last co-authorship is requested, the conference organizers should be contacted for guidance.
1.1.6 While the authorship of conference abstracts and presentations should accurately reflect those who were involved in the research, individuals who meet the ICMJE authorship criteria (and may be listed on a subsequent full publication) may choose not to be listed for a conference abstract and presentation (e.g. if they are unable to review and/or approve the material within the deadline). While this individual choice should be respected, significant contributions to the research should be acknowledged where possible; that is, in a contributor list included on the presentation.
1.1.7 Conference organizers should encourage the use of ORCID identifiers to identify authors on abstracts and presentations, to avoid ambiguity between authors with similar or identical names. (Note: many journals and institutions now require authors to include their ORCID identifier at manuscript submission.)
1.2 Contributors/study groups
1.2.1 We encourage conferences (and company sponsors) not to limit the number of authors (or contributors) who may be listed on an abstract or presentation, because this practice may prevent the author list from accurately reflecting who did the work. However, named authors should be limited to those who have actively participated in the development of the abstract (see 1.1.1). GPP3 recommends an author group of fewer than 10 [ 5 ]; above this number, naming a study group may be a more practical approach. Likewise, if the source data come from a study, and the authors involved in that study meet authorship criteria, then the use of a study group name is strongly recommended.
1.2.2 Study group names may be helpful to acknowledge contributions to projects involving a large number of people, in addition to named authors who have contributed both to the research and to developing the presentation. Inclusion of a study name, either in the title or by including a study group in the author listing, will facilitate linkage of conference abstracts and presentations with journal publications. However, this should not be a substitute for including a unique study identifier such as a registration number for clinical trials (e.g. ClinTrials.gov or EudraCT numbers), which is a more reliable linkage method because these can be used as search terms in relevant databases. Provision should be made for study group membership details to be added during abstract submission and made available via the conference website once an abstract has been accepted.
1.3 Presenters and society sponsors
1.3.1 While the ICMJE criteria are a useful starting point for determining authorship, they were not designed for conference abstracts and presentations. Therefore, in certain circumstances, and if all authors agree, it is permissible for somebody who does not (or will not) meet the ICMJE authorship criteria for a journal article to present findings at a conference. For example, a local presenter may be included (preferably in a contributor list and not as an author) if the authors of the conference presentation will not attend a particular meeting, do not speak the language required or are not members of the academic society hosting the meeting. This local presenter, for example, could be an investigator who recruited patients but did not contribute to the study design or interpretation of data and will not be involved in developing journal articles. In the contributor list, this person should be designated as ‘presenter’ to clarify their role. However, if the conference requires that only authors can present, then the new presenter will need to be added to the author list.
1.3.2 Abstract authors (including company authors) attending a conference should always be preferred as presenters over non-author presenters. In cases where an author is not available to present, and the conference acquiesces to a non-author covering the presentation, the non-author presenter should be familiar with the research design and findings and have a good knowledge of the subject area in order to respond to questions about the presentation even if, unlike the authors, they cannot take direct responsibility for the research. An appropriately qualified individual from the sponsoring company (e.g. Medical Director) could present study findings if authors are not available; however, individuals with a commercial role in the sponsoring company (i.e. sales or marketing) should not act as non-author presenters.
1.3.3 All those listed as authors on an abstract or presentation must be able to take accountability for the research (following the spirit of the ICMJE recommendations). Therefore, if conferences require a society member to sponsor a submission, and none of the authors or study investigators is a member, this sponsorship role should be distinguished from that of the study authors if the sponsor/member was not involved with the research. If an existing author happened to be a society member, then no such distinction would be necessary. If the conference wishes to list the society sponsor, then this role should be indicated on the abstract (e.g. by an asterisk) and in a contributor list (not the author list) on the presentation.
Figure 2 illustrates some scenarios to differentiate between authors and non-author presenters.
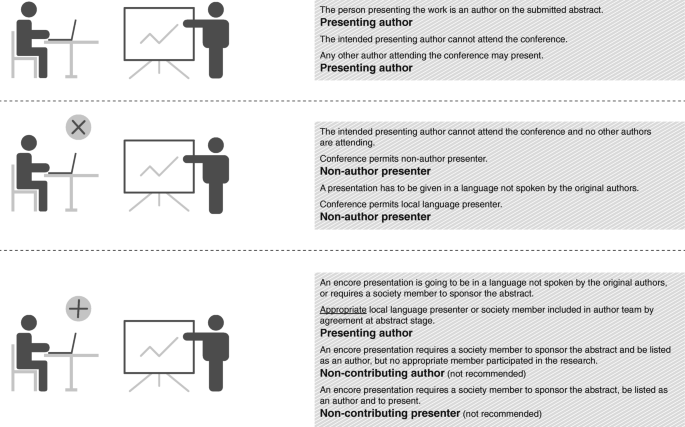
When is a presenter not an author? Different roles possible for authors and presenters of conference presentations
2.0 Conference abstracts
2.1 To facilitate linkage between conference abstracts and presentations, and subsequent publications, abstracts should include a study identifier such as a registration number (for clinical trials), study name, protocol number or grant number. To encourage this, conference organizers should require this information in a specific field on the submission form and publish it with the abstract.
2.2 Abstracts describing company-sponsored research should always name the sponsor and all funding sources (if more than the sponsor).
2.3 Authors or sponsoring companies may involve professional medical writers to support authors in the drafting of abstracts. All authors should agree to these arrangements and work closely with any writers and approve the final version. Space limitations on abstract submission sites usually preclude writing support acknowledgement. Conference organizers should consider requesting this information and publishing it with the abstract.
2.4 We encourage conference organizers to consider the requirements of reporting guidelines when setting limits on the length of abstracts. For example, CONSORT for Abstracts suggests that around 300 words may be needed to adequately report randomized clinical trials [ 7 ].
2.5 We also encourage conference organizers to maximize the available space for content in abstracts by not counting authors, affiliations, trial registration numbers and sponsor acknowledgments towards the word or character limit.
2.6 Most conferences will not consider reports of findings that have already been published in full (i.e. in a peer-reviewed journal). This requirement must be respected and, even if permitted, presenting findings after their full publication should be avoided. However, abstracts presenting findings or novel analyses that are not included in a full publication may be submitted if the conference permits this. In situations where a journal article is in preparation at the same time as abstract submission, subsequent submission of the article may overtake the abstract in acceptance, at which point the conference needs to be advised, and the journal also, to avoid issues of prior data release. It may be necessary to withdraw the abstract, or it might be possible for the journal and conference to come to a mutually acceptable arrangement regarding either delay of the article or amendment to the intended presentation. Posting summary results on a trial register (e.g. ClinicalTrials.gov , EudraCT) or a clinical study report to meet regulatory requirements is not regarded as full publication by the ICMJE [ 2 ] and should not prevent subsequent presentation at conferences.
2.7 As conference submission requirements become more detailed (and therefore labour-intensive), conference organizers should acknowledge that it is acceptable for the abstract submission process to be completed by a third party (e.g. a medical communications company) on behalf of the submitting author, with that author’s permission. Where feasible, the submission might be checked by the submitting author prior to the actual submission; however, there are some sites where submission has to be completed in one sitting, and on other occasions, time differences (and time pressures) may make this impractical.
3.0 Conference presentations (posters and slides)
3.1 general considerations.
3.1.1 Study identifiers (e.g., trial registration numbers) should be included on presentations to improve linkage between conference presentations and subsequent publications (see also Section 4).
3.1.2 All funding sources for the research, any assistance with the presentation (e.g. medical writing support, editorial assistance or design) or support for conference attendance and authors’ conflicts of interest should be clearly disclosed on posters and slides. For posters and slides, such disclosures should be clearly legible (i.e. not significantly smaller or lighter-coloured than the main text).
3.1.3 Author listing and order on posters and slides should be the same as that on the abstract. Authors should not be added to a presentation after the abstract is accepted. However, if an author is unavailable to work on a presentation after abstract acceptance, their name may be removed from the author list but their contribution (to the study and/or publication) should be acknowledged. If an author other than the first-named author is to present, this should be indicated without changing the author order. The principle is to retain the same information about authors as on the abstract for ease of identifying the related presentation. Similarly, the title of the presentation should not be changed after submission; thus, the titles of the abstract and poster or slides should be identical. [If someone not on the author list is to present, and this is known in time for poster preparation, the relevant name could be added as a footnote, or close to the author list thus: (Presenter: J. Doe, ABC Institute, City, Country).]
3.1.4 All named authors should contribute to the development of, and approve, the presentation (see 1.1.1). Authors should be given sufficient time for presentation development and review. Making significant changes to posters or slides after all-author approval should be avoided. If changes must be made after approval, the actual final version must be sent to all authors. As with journal articles, for large studies, it may be most efficient for a subgroup to coordinate the development of a presentation (similar to a writing group for an article). This should be considered when deciding authorship.
3.1.5 Each author’s contributions to the study and to the development of the presentation should be listed.
3.1.6 Conference presentations should include a list of contributors who have made a significant contribution to the research or the presentation, regardless of whether they are listed as authors or attending the meeting. Ideally, permission for such acknowledgment should be sought in writing.
3.1.7 Because abstracts are usually submitted several months before a conference, they may contain interim or preliminary findings. Therefore, by the time of the conference presentation, some details may have changed. If research findings change substantially between abstract submission and conference presentation and affect the conclusions of the research, we recommend that authors alert the conference to this discrepancy. This is particularly pertinent in the case of oral presentations (because abstracts are typically selected for oral presentations based on the impact of the findings). Regardless of whether the new data change the conclusions of the research, we recommend indicating (e.g. in a footnote) any data that are different from those on the accepted abstract.
3.1.8 Authors or sponsoring companies may involve professional medical writers in the production of posters and slides. Authors should agree to these arrangements and work closely with any writers, editors and/or designers throughout the development of the presentation. Such support should be disclosed on the presentation, along with source(s) of funding (see also 3.1.2).
3.2 Posters
3.2.1 While there are platforms where posters can be made permanently available (e.g. on conference websites or platforms such as F1000 Research), some journals regard this as prior publication which may jeopardize full publication. Authors should therefore check the policies of their target journal(s) and of the sponsor or funder before agreeing to a poster being publicly posted.
3.2.2 Posters are not peer-reviewed by conferences and may not describe all aspects of the research. Posters should therefore not be viewed as a substitute for a full article in a peer-reviewed journal. However, if a poster is publicly available (and, ideally, searchable via an indexing system or DOI), it may be cited until the full publication is available, although some journals consider citation of posters as unpublished information rather than full citations. See Section 6 for citation best practice.
3.2.3 The lead author should be given the first option to attend the poster session(s), but this role may be taken by other authors or a local presenter (if no author can attend or if no authors can present in the language of the conference). The poster presenter should ideally be agreed before the abstract is submitted, although it is understood that circumstances may change by the time of the actual conference (see 1.3.1).
3.2.4 While disclosures, funding sources, acknowledgements and contributions should be clearly noted on the main poster, supplementary sources can be used to expand on these if there is not enough room for detailed information, and may be accessed via a QR code (or similar link). Such content should normally be available until the research is published, in full, in a journal (at which point the link should be deactivated). If QR codes (or similar technology) are used to provide copies of the poster or to link to other scientific content, these should only be available to conference attendees, unless the conference elects to make the posters freely available after the conference. Links for the QR codes may be time-limited to close once the conference is finished. Supplementary materials may include translations. Supplementary material should be provided under the same usage conditions as the poster and indicate who is the copyright holder or licensee.
3.3 Slides for oral presentations
3.3.1 While the lead author is normally expected to present study findings at conferences (and is given the first option to do so), this may not be possible due to local language requirements, availability to travel, or personal circumstances, etc. If the lead author chooses not to present study findings, another author may give the oral presentation. If none of the named authors is available or able to give the presentation, a non-author presenter may present the findings if all authors agree to this and the conference permits it (see also 1.3.1 and 1.3.2). The presenter should be agreed before the abstract is submitted (and only changed if that person becomes unavailable). The lead author should discuss the contents of the presentation and the interpretation of the findings with the presenter (and co-authors, if possible) before the conference to ensure the authors’ views are correctly represented.
3.3.2 If a non-author presenter gives a presentation on behalf of the named authors (or study group), this should be indicated at the beginning of the presentation. The presenter’s conflicts of interest should be noted on the disclosure slide.
3.3.3 Recordings of oral presentations may be posted online by conference organizers but, as with posters, care should be taken to ensure this does not jeopardize full publication in a peer-reviewed journal. Slides alone (without the accompanying talk or speaker notes) may be hard to interpret and not provide full context, so care should be taken if these are made publicly available. As with posters (see 3.2.4), online sources may also be considered to host supplementary materials for presentations if they are made available after the presentation. If slides are made publicly available, this should not occur until after the presentation has been given and should only occur with the agreement of all authors and sponsors, who will need to consider any restrictions around the posting of the data and possible ‘prior publication’ concerns for later use (see 6.1.2).
3.3.4 Some scientific meetings offer Continuing Medical Education (CME) credit for attendance at oral presentations. Local regulations and requirements of the accreditation body for this must be respected.
4.0 Encore abstracts and presentations
4.1. It is permissible to present the same research findings at more than one conference if both the first and subsequent conferences allow this. This practice may be referred to as an ‘encore’ (or more specifically an encore abstract or encore presentation). However, presentations of the same findings to the same audience should be avoided.
4.2 Although encore abstracts are not considered to be redundant publications (unlike publication of the same findings in more than one journal), some conferences elect only to accept findings that have not been presented at other conferences, and such requirements must be respected.
4.3 When considering encore abstracts, the authors and sponsoring company should decide whether it is most appropriate to submit identical abstracts to multiple conferences or whether it is better to emphasize different aspects of a trial (e.g. those of interest to different audiences). Use of study identifiers can help identify that multiple conference abstracts and presentations are from a single study. However, to avoid any confusion, we recommend that encores should be specifically identified as such (e.g. by stating that the presentation is an ‘encore’ and listing where previous abstracts of all or some of the findings were presented) (see also 4.4 and 4.6). We also recommend that previous presentations should be listed on the presentation, if accepted.
4.4 Conference organizers should consider including a means of identifying encore abstracts (e.g. including details of prior presentations) on the abstract submission form. This information should not be included in the abstract word or character count.
4.5 Addition of new data to a previously accepted abstract may not necessarily constitute a new abstract: conference guidelines should be consulted to confirm if this is acceptable. If no specific guidelines are provided, then as a general guide, if the new iteration adds any new data other than an update on analyses already contained in a previous abstract, then the new iteration should be regarded as a new abstract.
4.6 Where encore abstracts, or updated abstracts that include previously presented data, are accepted, their presentations should indicate that this is not the first time of presentation, for example, by a statement on the poster or slides such as “Data/some data first presented at [conference name and date]”.
4.7 Encore checklist: When deciding whether to submit an encore abstract to a conference to reach different audiences, authors and study sponsors should consider the following points.
What is the overlap, if any, with the audience of the earlier conference (e.g. in terms of region, specialism or profession)?
Are there any differences in the licensing status of any products mentioned in the presentation between the first and subsequent conference locations? For example, if the first presentation occurred in a region where a product is licensed, but later presentation(s) will take place in a region where it is not yet licensed, this fact may need to be reflected. For international meetings, remember that participants will attend from several regions, so the licensing status in different countries should be clarified.
Presentation at multiple meetings might delay and/or potentially jeopardize the full publication of research in a peer-reviewed journal. Companies should consider whether resources would therefore be better spent on ensuring a timely submission to a journal rather than preparing several encore abstracts and presentations.
5.0 Copyright considerations
5.1 Copyright transfer or publishing licence agreements that are executed during the abstract submission process are common when abstracts are to be formally published (e.g. in a conference-specific journal issue). These agreements relate only to the abstract, not to any subsequent presentation, unless explicitly agreed otherwise.
5.2 Copyright in a presentation is normally held by the authors, unless they have assigned it either to the conference or the sponsoring company. Re-use of a poster (at a subsequent meeting or in another format, such as a poster book or handout) normally requires permission from the copyright holder(s). It may therefore be simplest for authors to assign usage rights to the sponsor company if encore presentations or other types of re-use are planned. If a company author is included, then the copyright for that individual’s contribution rests with the company (not the employee).
5.3 If a conference wishes to acquire usage rights for abstracts, slides, or posters, we recommend that the conference offers an open access option under a Creative Commons (CC) licence. We encourage the use of the least restrictive CC-BY licence, which will allow authors and sponsoring companies the usage rights for subsequent presentations, as well as future publications. If presentations contain third-party material to which the authors do not hold copyright, it should be the responsibility of the conference organizers to clear rights for any further usage. The authors cannot be expected to anticipate the future use of materials by the conference organizers.
5.4 As for any publication, permission must be sought for use of third-party copyrighted material (e.g. a figure) in a presentation (and again for any encore presentations). Material should not be altered simply to avoid having to obtain permission from the copyright holder.
5.5 Peer-to-peer presentation at a scholarly conference by a researcher is generally considered to be fair dealing (UK) [ 13 ] or fair use (USA) [ 14 ], which does not require copyright permission. Any other use of a presentation by a company outside the conference will most likely be considered commercial use, for which permission from the rights holder(s) will be necessary.
6.0 Citing conference material
6.1 References (or citations) in scientific texts provide readers with source or background material and are used to justify or support statements. To be useable, the referenced material must be both permanently accessible and reliable; therefore, citations to full publications in journals that apply rigorous peer review are the ideal. However, if citations are needed for research that has not yet been fully published in a peer-reviewed journal, abstracts that have undergone scientific review (and on the basis of that have been accepted for presentation by a conference) may be cited, especially if they have also been published in a journal and are therefore permanently accessible and discoverable. Abstracts should not be cited after the full (primary) publication has been accepted by a journal.
6.2 Posters and slides are not peer-reviewed by conferences and are often not permanently or widely accessible or discoverable. Citations to posters or slides should therefore be avoided (see 6.1). However, if a poster or slide set is publicly available (and, ideally, discoverable via an indexing system or DOI), it may be cited until the full publication is available (although some journals consider citation of posters or slides as unpublished information rather than full citations). Authors and sponsor companies should ensure that publishing posters or slides online does not jeopardize full publication in a peer-reviewed journal.
6.3 To avoid citing conference posters or slides, companies should consider other dissemination routes such as listing findings as ‘Data on File’ (i.e. an unpublished data package held by the pharmaceutical company, which then should be supplied to anyone requesting those data).
6.4 If specific findings that were presented at a conference are omitted from a journal article (e.g. because of space constraints), they could be made accessible as supplementary material.
These recommendations summarize the authors’ collective experience with a view to outlining the underlying principles for best practice and providing guidance on the practicalities for navigating conference requirements. We did consider whether some of our recommendations could be accomplished by amendments to company–author agreements, but decided that such recommendations for ‘good practice for author agreements’ were beyond the remit and scope of this article and that GPP3 [ 5 ] adequately covers this aspect of author–sponsor relationship. Many of these recommendations are drawn from the working group’s experience across a variety of disease areas and conferences. However, this is also a limitation, in that by the nature of the authors’ work, their experience lies in conferences and conference submission systems with strong industry involvement. We believe that these recommendations could be applied to any type of scientific/medical conference and are as relevant to academic research as to company-sponsored research. Conferences maintain their value to the scientific community by covering the latest research and providing a forum for discussion: this value must not be lost due to lack of transparency or ethics in the preparation and presentation of the new data. By following these recommendations, industry professionals, authors and conference organizers will improve consistency, transparency and integrity of publications submitted to conferences worldwide.
It is earnestly hoped that future input from conference organizers and societies, as well anyone involved in submitting research to conferences, will augment and strengthen these recommendations. We therefore welcome feedback via the website ( https://gpcap.org ).
Scherer RW, Meerpohl JJ, Pfeifer N, Schmucker C, Schwarzer G, von Elm E. Full publication of results initially presented in abstracts. Cochrane Database Syst Rev. 2018;11:MR000005.
Google Scholar
ICMJE Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. Available at www.icmje.org . Accessed 14 May 2019.
Wager E, Field EA, Grossman L. Good publication practice for pharmaceutical companies. Curr Med Res Opinion. 2003;19:149–54.
Article Google Scholar
Graf C, Battisti WP, Bridges D, Bruce-Winkler V, Conaty JM, Ellison JM, Field EA, Gurr JA, Marx ME, Patel M, Sanes-Miller C, Yarker YE. Good publication practice for communicating company sponsored medical research: the GPP2 guidelines. BMJ. 2009;339:b4330.
Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, Citrome L, Gurr JA, Mooney LA, Moore BJ, Peña T, Sanes-Miller CH, Veitch K, Woolley KL, Yarker YE. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163:461–4.
Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, Schulz KF, The CONSORT group. CONSORT for reporting randomized controlled trials in journal and conference abstracts. Lancet. 2008;371:281–3.
Beller EM, Glasziou PP, Altman DG, et al. PRISMA for Abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10(4):e1001419. https://doi.org/10.1371/journal.pmed.1001419 .
EFPIA Code of Ethics for Pharmaceutical Marketing. http://www.efpia-e4ethics.eu/usd/e4ethics.nsf/_/AFB7FD98CFCD31D5C125806E0043F1FE/%24File/farma_110039.pdf . Accessed 14 May 2019.
US Food & Drug Administration. Laws, regulations, guidances and enforcement actions. Available at https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/DrugMarketingAdvertisingandCommunications/ucm081617.htm . Accessed 14 May 2019.
Foster C, Wager E, Marchington J, Patel M, Banner S, Kennard NC, Panayi A, Stacey R. Good practice for conference abstracts and presentations: GP-CAP. PeerJ Preprints. 2017;5:e3356v1 https://doi.org/10.7287/peerj.preprints.3356v1 .
Open Researcher and Contributor ID (ORCID). Available at https://orcid.org/ . Accessed 14 May 2019.
MPIP Five-step authorship framework. Available at https://www.mpip-initiative.org/5-step-framework.html . Accessed 14 May 2019.
Copyright, Designs and Patents Act 1988, s 29(1); s 30(1). Available at https://www.legislation.gov.uk/ukpga/1988/48/contents . Accessed 14 May 2019.
Copyright Law of the United States | U.S. Copyright Office. Available at https://www.copyright.gov/title17/ . Accessed 14 May 2019.
Download references
Acknowledgements
Special thanks go to Peter Llewellyn of Network Pharma, for hosting the meeting on GPP3 that acted as a catalyst for getting these recommendations underway.
No author has received payment specifically for the development of this article.
Availability of data and materials
Not applicable.
Author information
Authors and affiliations.
Watermeadow Medical, Ashfield Healthcare Communications, Witney, UK
Cate Foster
Sideview, Princes Risborough, UK
Elizabeth Wager
University of Split, Split, Croatia
Caudex, a McCann Health Company, Oxford, UK
Jackie Marchington
Alexion Pharmaceuticals Inc, New Haven, CT, USA
Darwin Grey Communications Ltd, Oxford, UK
Steve Banner
Cello Health MedErgy, a Cello Health PLC Company, Farnham, UK
Nina C. Kennard
Shire International GmbH (now part of Takeda), Global Medical Affairs, Zug, Switzerland
Antonia Panayi
iMed Comms, Ashfield Healthcare Communications, Witney, UK
Rianne Stacey
You can also search for this author in PubMed Google Scholar
Contributions
CF raised the initial suggestion for guidelines, co-developed preliminary sections of text for the initial draft and discussed comments and revisions, reviewed all versions and approved the submitted version. EW drafted the Principles section and other portions of the text, discussed comments and revisions, reviewed all versions and approved the submitted version. JM consulted on the initial suggestion for these guidelines, drafted the Copyright section and other portions of the text, discussed comments and revisions, reviewed all versions and approved the submitted version. MP co-developed the foundation of the Encore Presentations section, discussed comments and revisions, reviewed all versions and approved the submitted version. SB consulted on the initial suggestion for these guidelines, assisted in the development of the initial draft, reviewed all subsequent drafts and approved the submitted version. NK drafted the abstract and other portions of the text, discussed comments and revisions, reviewed all versions and approved the submitted version. AP developed several sections with the author group, discussed comments and revisions, reviewed all versions and approved the submitted version. RS consulted on the initial suggestion for these guidelines, co-developed preliminary sections of text for the initial draft, discussed comments and revisions, incorporated feedback on the pre-print version, reviewed all versions and approved the submitted version. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Cate Foster .
Ethics declarations
Authors’ information.
Cate Foster, Jackie Marchington, Steve Banner and Nina C Kennard work for medical communication agencies that provide professional medical writing or editing services to not-for-profit and for-profit clients.
Elizabeth Wager is self-employed and provides training, consultancy and editing services on medical publishing and publication ethics for pharmaceutical companies, medical communication agencies, publishers, universities and academic societies.
Mina Patel and Antonia Panayi work in Global Medical Affairs functions within the pharmaceutical industry.
Rianne Stacey worked for a medical communication agency (see above) during the majority of the time the work was done and now works at Jazz Pharmaceuticals, Oxford, UK, in a Global Medical Affairs function within the pharmaceutical industry.
The content and opinions expressed in the article are the authors’ and do not necessarily reflect the policies or practices of their employers.
Ethics approval and consent to participate
Not applicable
Consent for publication
Competing interests.
The authors declare that they have no competing interests. Commercial affiliations are noted in the ‘Authors’ information’ section.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Foster, C., Wager, E., Marchington, J. et al. Good Practice for Conference Abstracts and Presentations: GPCAP. Res Integr Peer Rev 4 , 11 (2019). https://doi.org/10.1186/s41073-019-0070-x
Download citation
Received : 27 July 2018
Accepted : 26 April 2019
Published : 05 June 2019
DOI : https://doi.org/10.1186/s41073-019-0070-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Research Integrity and Peer Review
ISSN: 2058-8615
- Submission enquiries: [email protected]
- General enquiries: [email protected]
May 14, 2024
Expert reaction to conference abstract about bmi cut-off for over 40s.
A conference abstract presented at the European Congress on Obesity looks at BMI thresholds for obesity in over 40s.
Dr Katarina Kos, Senior Lecturer in Diabetes and Obesity, University of Exeter, said:
“Whilst awareness of unhealthy fat accumulation leading to obesity is important, BMI does neither capture muscle content nor the location of fat deposition. One way to measure unhealthy fat/abdominal fat is waist circumference which gives a good indication of heart disease risk though clinically less used. Some individuals may want some personalisation and fine tuning of their body composition related risk if at the threshold between being overweight and obese, and this study addresses the age factor. The authors indicate that as we grow older this fine tuning may be more important, the bigger question is in what makes us as the public more inclined to act upon our surplus weight. Are we more likely to change if we are being labelled with obesity at a lower BMI? And this other than being a statistic as a person with obesity. The number of people with obesity worldwide increases drastically with the proposed BMI threshold. Or are more of us simply going to feel stigmatised?”
Dr Adam Collins, Associate Professor of Nutrition, University of Surrey, said:
“Interesting idea and something that harks back to the flaws in BMI as a proxy for excess adiposity. Given that BMI is simply weight adjusted for body size (i.e. height). And assumes that higher weight is directly proportional to higher body fat.
“This is a good sample size with robust measures of adiposity (DXA). But as the authors state, this also needs to be looked at across different ethnicities– e.g. South Asians, who may have a propensity for higher visceral fat at a given BMI.
“The problem with BMI has always been the grey area between 25 and 30. Or the “overweight” category. When comparing with actual measures of adiposity (i.e. body fat), some individuals will be misclassified due to low body fat and higher muscle mass, whilst others can have as high or higher levels of body fat than those of greater BMI.
“Hence the sensitivity (how consistently it can detect true adiposity) and specificity (to only detect those with true adiposity) of BMI is problematic <30.
“The notion that this sensitivity and specificity is dependent on age makes sense, and translates as more of a potential issue in older people below BMI 27 rather than below BMI 30. Clinically, this is important in terms of a threshold for health concern.
“However, it is important to note that total adiposity (i.e. % body fat) is not, in itself, a direct marker of health – there is a strong association between adiposity and health, but you can still be at risk with low total adiposity (e.g. visceral fat /ectopic fat) compared to those with high total adiposity but low visceral/ectopic fat. It would be more appropriate to relate to central (abdominal) or visceral adiposity; in this regard, waist circumference could be a useful additional marker. More specifically, visceral and ectopic fat (fat where it shouldn’t be) are better markers of adiposity risk. For example, liver fat or intramuscular fat. Closely linked to insulin resistance, diabetes risk and cardiovascular disease. Admittedly this would only be measurable via MRI imaging.
“Of course, triangulating this with other markers of risk, e.g. Blood Pressure and blood parameters, would be helpful to fully establish risk. But triaging individuals for this assessment based on these lower BMI thresholds would be clinically very useful.”
Abstract title: ‘New BMI Cut-Off Points for Obesity in Middle-Aged and Older Adults in Nutrition Settings in Italy’ by Marwan El Ghoch et al.
This was presented at the European Congress on Obesity and was under embargo until 23:01 UK time on Tuesday 14 May 2024.
There is no full paper.
Declared interests
Dr Katarina Kos: “I have no conflict of interest.”
Dr Adam Collins : “No conflict of interest with this.”
in this section
Filter roundups by year, search by tag, privacy overview.

COMMENTS
What is an Abstract? •"The abstract is a brief, clear summary of the information in your presentation. A well-prepared abstract enables readers to identify the basic content quickly and accurately, to determine its relevance to their interests or purpose and then to decide whether they want to listen to the presentation in its entirety."
The abstract of a paper is the only part of the paper that is published in conference proceedings. The abstract is the only part of the paper that a potential referee sees when he is invited by an editor to review a manuscript. The abstract is the only part of the paper that readers see when they search through electronic databases such as PubMed.
A conference will state a set of guidelines for anything beyond the basics. This will include format, their minimum and maximum word count, word choice, and even specific details to include in the content. Note: The following are specifications for an abstract in APA style, used in the social sciences, such as psychology or anthropology.
Writing good abstracts is not an art, but a learned skill. Developing such a skill takes practice. Here is an exercise to help you develop this skill. Pick a scientific article in your field. Read the paper with the abstract covered. Then try to write an abstract based on your reading. Compare your abstract to the author's.
The first step is to write and submit an abstract of your research paper. The purpose of a conference abstract is to summarize the main points of your paper that you will present in the academic conference. In it, you need to convince conference organizers that you have something important and valuable to add to the conference.
As we have returned from the successful XXXIX Brazilian Thoracic Society Conference in Goiânia, Brazil-where more than 600 abstracts have been presented-and prepare for the American Thoracic Society International Conference deadline for submitting abstracts by November this year, we would like to emphasize the importance of presenting high-quality scientific abstracts at such conferences.
Here are some of the benefits of submitting a conference abstract: It's a good addition to your CV and resume. It may be published in the conference proceedings. It could be a basis for future publication. Garners recognition from colleagues online and through social media. Helps you make connections through networking at the conference.
Things to Keep in Mind When Preparing Your Abstract. Submitting a conference abstract is competitive. Know the rules: Follow the rules for abstract submission, don't give the reviewers any reason to toss out your abstract. Pay attention to word count limits, section headings, how to format your name, etc. Know the timeline and deadline:
How to Write a Scientific Abstract. Scientific publications are an important source of information and knowledge in Academics, Research and development. When articles are submitted for publication, the 1st part that comes across and causes an impact on the minds of the readers is the abstract. It is a concise summary of the paper and must ...
How to write a good abstract for a scientific paper or conference presentation. Indian Journal of Psychiatry, 53, 172-175. 10.4103/0019-5545.82558. ... Hitting the target! A no tears approach to writing an abstract for a conference presentation. International Journal of Mental Health Nursing, 16, 447-452. 10.1111/j.1447-0349.2007.00501.x.
Here are some additional technical tips for titles of scientific abstracts: Start with an adjective, a noun, or a verb. Never start with an article (e.g., "The", "A", or "An"). Do not end with punctuation; your title is not a sentence. Use plain text (no bold, italics, or special fonts). Use sentence case.
In this edition of Doing Science, we will address abstract writing, with a focus on conference abstracts. By providing an opportunity for discussing your work with your peers in specialised meetings, writing and submitting an abstract is often the very first step when you want to show the world the results of your work; be it your research, a clinical case or a review of the literature [1].
While submitting an abstract to a national conference can seem overwhelming, it is one of the best ways to communicate your science and get your research in front of a large audience. Students interested in learning how to write an abstract should review these helpful tips that describe components of a competitive abstract .
The abstract plays a very important role in both a scientific publication and a scientific conference. Indeed, the abstract is your "sales pitch" to get your work noticed and accepted. It is usually written after finishing off the whole article. The abstract represents an abbreviated version of your whole article or study.
1. Summarize the findings in your paper. 2. Persuades the reader to download and read the full article. 3. If it's prepared for a conference, it gets you selected for a talk and makes the audience curious about your subject. 4. It presents the exact results of your research, not only a list of topics. 5.
This paper provides detailed suggestions, with examples, for writing the background, methods, results, and conclusions sections of a good abstract. The primary target of this paper is the young researcher; however, authors with all levels of experience may find useful ideas in the paper. Keywords: Abstract; preparing a manuscript; writing skills.
Concisely describe how your results pertain to your study aim or hypothesis. Statements such as "to be completed" or "to be presented" are not acceptable. Remember to report non-significant differences too. Usually the longest section, 3-8 sentences.
Writing Abstracts for a Conference. An "abstract" for an academic conference is a short summary of the scientific research you are involved in. While abstracts generally have a standard format and include more or less the same information and in a similar layout, each conference may have its unique requirements.
Wri ng an Abstract f or a Scien c Conference. Kathmandu Univ Med J 2013;43 (3):262-265. ABSTRACT. For most students and junior researchers, wri ng an abstract for a poster or oral. presenta on at ...
Conference abstracts are presented alone to conference organisers. This means that journal editors or peer reviewers can say e.g. 'great article but the abstract needs work', while a poor abstract submitted to a conference organiser is very unlikely to be accepted. Articles are typically 4,000-8,000 words long.
Tips for preparing abstracts for scientific meetings. 1. Ideas based on experimental research, day-to-day practices at the ICU or a combination of both (translational) 2. The structure of abstracts is quite rigid; ensure that you add a variety of new information in a small amount of text. 3.
Conference is used to refer to meetings, often organized by academic societies, that invite submissions (usually as abstracts) presenting research findings on an aspect of medicine or science. Such conferences have a scientific (or programme) committee that reviews and selects presentations to be given at the meeting from the submitted abstracts.
Poster presentations at scientific conferences can provide early-career researchers with valuable opportunities to practice their communication skills, receive feedback on their research, and expand their network. ... Instead of including an abstract, use the space you save to make your results big and clear. Make sure that the text and labels ...
1. Bornmann L Mutz R Growth rates of modern science: a bibliometric analysis based on the number of publications and cited references J. Am. Soc. Inf. Sci. 2015 66 11 2215 2222 Google Scholar Digital Library; 2. Dernoncourt, F., Lee, J.Y.: Pubmed 200k rct: a dataset for sequential sentence classification in medical abstracts. In: Proceedings of the Eighth International Joint Conference on ...
The ECSACOP (East, Central, and Southern Africa College of Physicians) in conjunction with the Association of Physicians of Uganda (APU) is excited to announce the call for abstracts for the ECSACOP Conference 2024. The ECSACOP conference is scheduled to take place from 29th to 31st August 2024 at Mestil Hotel & Residences, Kampala, Uganda.This conference aims to bring together healthcare ...
Covalent organic frameworks (COFs) constitute a class of highly functional porous materials composed of lightweight elements interconnected by covalent bonds, characterized by structural order, high crystallinity, and large specific surface area. The integration of naturally occurring porphyrin molecules, renowned 33rd Annual Conference of the European Society for Biomaterials
A conference abstract presented at the European Congress on Obesity looks at BMI thresholds for obesity in over 40s. Dr Katarina Kos, Senior Lecturer in Diabetes and Obesity, University of Exeter, said: "Whilst awareness of unhealthy fat accumulation leading to obesity is important, BMI does neither capture muscle content nor the location of ...
Main Conference: Oct. 22-23, 2024 Extended Abstracts. Extended abstracts must be maximum of 2 pages length, including figures, tables, and references. Selected extended abstracts will be invited to give a 20-minute talk at the conference in extended abstract sessions. The authors will need to register for the conference for giving their talk.
The average emissions per delegate for each conference were 1.3 metric tons of carbon dioxide. This figure can be compared to the level of annual emissions per person we need to align with to ...