Get science-backed answers as you write with Paperpal's Research feature

How to Write a Conclusion for Research Papers (with Examples)

The conclusion of a research paper is a crucial section that plays a significant role in the overall impact and effectiveness of your research paper. However, this is also the section that typically receives less attention compared to the introduction and the body of the paper. The conclusion serves to provide a concise summary of the key findings, their significance, their implications, and a sense of closure to the study. Discussing how can the findings be applied in real-world scenarios or inform policy, practice, or decision-making is especially valuable to practitioners and policymakers. The research paper conclusion also provides researchers with clear insights and valuable information for their own work, which they can then build on and contribute to the advancement of knowledge in the field.
The research paper conclusion should explain the significance of your findings within the broader context of your field. It restates how your results contribute to the existing body of knowledge and whether they confirm or challenge existing theories or hypotheses. Also, by identifying unanswered questions or areas requiring further investigation, your awareness of the broader research landscape can be demonstrated.
Remember to tailor the research paper conclusion to the specific needs and interests of your intended audience, which may include researchers, practitioners, policymakers, or a combination of these.
Table of Contents
What is a conclusion in a research paper, summarizing conclusion, editorial conclusion, externalizing conclusion, importance of a good research paper conclusion, how to write a conclusion for your research paper, research paper conclusion examples.
- How to write a research paper conclusion with Paperpal?
Frequently Asked Questions
A conclusion in a research paper is the final section where you summarize and wrap up your research, presenting the key findings and insights derived from your study. The research paper conclusion is not the place to introduce new information or data that was not discussed in the main body of the paper. When working on how to conclude a research paper, remember to stick to summarizing and interpreting existing content. The research paper conclusion serves the following purposes: 1
- Warn readers of the possible consequences of not attending to the problem.
- Recommend specific course(s) of action.
- Restate key ideas to drive home the ultimate point of your research paper.
- Provide a “take-home” message that you want the readers to remember about your study.

Types of conclusions for research papers
In research papers, the conclusion provides closure to the reader. The type of research paper conclusion you choose depends on the nature of your study, your goals, and your target audience. I provide you with three common types of conclusions:
A summarizing conclusion is the most common type of conclusion in research papers. It involves summarizing the main points, reiterating the research question, and restating the significance of the findings. This common type of research paper conclusion is used across different disciplines.
An editorial conclusion is less common but can be used in research papers that are focused on proposing or advocating for a particular viewpoint or policy. It involves presenting a strong editorial or opinion based on the research findings and offering recommendations or calls to action.
An externalizing conclusion is a type of conclusion that extends the research beyond the scope of the paper by suggesting potential future research directions or discussing the broader implications of the findings. This type of conclusion is often used in more theoretical or exploratory research papers.
Align your conclusion’s tone with the rest of your research paper. Start Writing with Paperpal Now!
The conclusion in a research paper serves several important purposes:
- Offers Implications and Recommendations : Your research paper conclusion is an excellent place to discuss the broader implications of your research and suggest potential areas for further study. It’s also an opportunity to offer practical recommendations based on your findings.
- Provides Closure : A good research paper conclusion provides a sense of closure to your paper. It should leave the reader with a feeling that they have reached the end of a well-structured and thought-provoking research project.
- Leaves a Lasting Impression : Writing a well-crafted research paper conclusion leaves a lasting impression on your readers. It’s your final opportunity to leave them with a new idea, a call to action, or a memorable quote.

Writing a strong conclusion for your research paper is essential to leave a lasting impression on your readers. Here’s a step-by-step process to help you create and know what to put in the conclusion of a research paper: 2
- Research Statement : Begin your research paper conclusion by restating your research statement. This reminds the reader of the main point you’ve been trying to prove throughout your paper. Keep it concise and clear.
- Key Points : Summarize the main arguments and key points you’ve made in your paper. Avoid introducing new information in the research paper conclusion. Instead, provide a concise overview of what you’ve discussed in the body of your paper.
- Address the Research Questions : If your research paper is based on specific research questions or hypotheses, briefly address whether you’ve answered them or achieved your research goals. Discuss the significance of your findings in this context.
- Significance : Highlight the importance of your research and its relevance in the broader context. Explain why your findings matter and how they contribute to the existing knowledge in your field.
- Implications : Explore the practical or theoretical implications of your research. How might your findings impact future research, policy, or real-world applications? Consider the “so what?” question.
- Future Research : Offer suggestions for future research in your area. What questions or aspects remain unanswered or warrant further investigation? This shows that your work opens the door for future exploration.
- Closing Thought : Conclude your research paper conclusion with a thought-provoking or memorable statement. This can leave a lasting impression on your readers and wrap up your paper effectively. Avoid introducing new information or arguments here.
- Proofread and Revise : Carefully proofread your conclusion for grammar, spelling, and clarity. Ensure that your ideas flow smoothly and that your conclusion is coherent and well-structured.
Write your research paper conclusion 2x faster with Paperpal. Try it now!
Remember that a well-crafted research paper conclusion is a reflection of the strength of your research and your ability to communicate its significance effectively. It should leave a lasting impression on your readers and tie together all the threads of your paper. Now you know how to start the conclusion of a research paper and what elements to include to make it impactful, let’s look at a research paper conclusion sample.

How to write a research paper conclusion with Paperpal?
A research paper conclusion is not just a summary of your study, but a synthesis of the key findings that ties the research together and places it in a broader context. A research paper conclusion should be concise, typically around one paragraph in length. However, some complex topics may require a longer conclusion to ensure the reader is left with a clear understanding of the study’s significance. Paperpal, an AI writing assistant trusted by over 800,000 academics globally, can help you write a well-structured conclusion for your research paper.
- Sign Up or Log In: Create a new Paperpal account or login with your details.
- Navigate to Features : Once logged in, head over to the features’ side navigation pane. Click on Templates and you’ll find a suite of generative AI features to help you write better, faster.
- Generate an outline: Under Templates, select ‘Outlines’. Choose ‘Research article’ as your document type.
- Select your section: Since you’re focusing on the conclusion, select this section when prompted.
- Choose your field of study: Identifying your field of study allows Paperpal to provide more targeted suggestions, ensuring the relevance of your conclusion to your specific area of research.
- Provide a brief description of your study: Enter details about your research topic and findings. This information helps Paperpal generate a tailored outline that aligns with your paper’s content.
- Generate the conclusion outline: After entering all necessary details, click on ‘generate’. Paperpal will then create a structured outline for your conclusion, to help you start writing and build upon the outline.
- Write your conclusion: Use the generated outline to build your conclusion. The outline serves as a guide, ensuring you cover all critical aspects of a strong conclusion, from summarizing key findings to highlighting the research’s implications.
- Refine and enhance: Paperpal’s ‘Make Academic’ feature can be particularly useful in the final stages. Select any paragraph of your conclusion and use this feature to elevate the academic tone, ensuring your writing is aligned to the academic journal standards.
By following these steps, Paperpal not only simplifies the process of writing a research paper conclusion but also ensures it is impactful, concise, and aligned with academic standards. Sign up with Paperpal today and write your research paper conclusion 2x faster .
The research paper conclusion is a crucial part of your paper as it provides the final opportunity to leave a strong impression on your readers. In the research paper conclusion, summarize the main points of your research paper by restating your research statement, highlighting the most important findings, addressing the research questions or objectives, explaining the broader context of the study, discussing the significance of your findings, providing recommendations if applicable, and emphasizing the takeaway message. The main purpose of the conclusion is to remind the reader of the main point or argument of your paper and to provide a clear and concise summary of the key findings and their implications. All these elements should feature on your list of what to put in the conclusion of a research paper to create a strong final statement for your work.
A strong conclusion is a critical component of a research paper, as it provides an opportunity to wrap up your arguments, reiterate your main points, and leave a lasting impression on your readers. Here are the key elements of a strong research paper conclusion: 1. Conciseness : A research paper conclusion should be concise and to the point. It should not introduce new information or ideas that were not discussed in the body of the paper. 2. Summarization : The research paper conclusion should be comprehensive enough to give the reader a clear understanding of the research’s main contributions. 3 . Relevance : Ensure that the information included in the research paper conclusion is directly relevant to the research paper’s main topic and objectives; avoid unnecessary details. 4 . Connection to the Introduction : A well-structured research paper conclusion often revisits the key points made in the introduction and shows how the research has addressed the initial questions or objectives. 5. Emphasis : Highlight the significance and implications of your research. Why is your study important? What are the broader implications or applications of your findings? 6 . Call to Action : Include a call to action or a recommendation for future research or action based on your findings.
The length of a research paper conclusion can vary depending on several factors, including the overall length of the paper, the complexity of the research, and the specific journal requirements. While there is no strict rule for the length of a conclusion, but it’s generally advisable to keep it relatively short. A typical research paper conclusion might be around 5-10% of the paper’s total length. For example, if your paper is 10 pages long, the conclusion might be roughly half a page to one page in length.
In general, you do not need to include citations in the research paper conclusion. Citations are typically reserved for the body of the paper to support your arguments and provide evidence for your claims. However, there may be some exceptions to this rule: 1. If you are drawing a direct quote or paraphrasing a specific source in your research paper conclusion, you should include a citation to give proper credit to the original author. 2. If your conclusion refers to or discusses specific research, data, or sources that are crucial to the overall argument, citations can be included to reinforce your conclusion’s validity.
The conclusion of a research paper serves several important purposes: 1. Summarize the Key Points 2. Reinforce the Main Argument 3. Provide Closure 4. Offer Insights or Implications 5. Engage the Reader. 6. Reflect on Limitations
Remember that the primary purpose of the research paper conclusion is to leave a lasting impression on the reader, reinforcing the key points and providing closure to your research. It’s often the last part of the paper that the reader will see, so it should be strong and well-crafted.
- Makar, G., Foltz, C., Lendner, M., & Vaccaro, A. R. (2018). How to write effective discussion and conclusion sections. Clinical spine surgery, 31(8), 345-346.
- Bunton, D. (2005). The structure of PhD conclusion chapters. Journal of English for academic purposes , 4 (3), 207-224.
Paperpal is a comprehensive AI writing toolkit that helps students and researchers achieve 2x the writing in half the time. It leverages 21+ years of STM experience and insights from millions of research articles to provide in-depth academic writing, language editing, and submission readiness support to help you write better, faster.
Get accurate academic translations, rewriting support, grammar checks, vocabulary suggestions, and generative AI assistance that delivers human precision at machine speed. Try for free or upgrade to Paperpal Prime starting at US$19 a month to access premium features, including consistency, plagiarism, and 30+ submission readiness checks to help you succeed.
Experience the future of academic writing – Sign up to Paperpal and start writing for free!
Related Reads:
- 5 Reasons for Rejection After Peer Review
- Ethical Research Practices For Research with Human Subjects
7 Ways to Improve Your Academic Writing Process
- Paraphrasing in Academic Writing: Answering Top Author Queries
Preflight For Editorial Desk: The Perfect Hybrid (AI + Human) Assistance Against Compromised Manuscripts
You may also like, how to write a high-quality conference paper, academic editing: how to self-edit academic text with..., measuring academic success: definition & strategies for excellence, phd qualifying exam: tips for success , ai in education: it’s time to change the..., is it ethical to use ai-generated abstracts without..., what are journal guidelines on using generative ai..., quillbot review: features, pricing, and free alternatives, what is an academic paper types and elements , should you use ai tools like chatgpt for....
- USC Libraries
- Research Guides
Organizing Your Social Sciences Research Paper
- 9. The Conclusion
- Purpose of Guide
- Design Flaws to Avoid
- Independent and Dependent Variables
- Glossary of Research Terms
- Reading Research Effectively
- Narrowing a Topic Idea
- Broadening a Topic Idea
- Extending the Timeliness of a Topic Idea
- Academic Writing Style
- Applying Critical Thinking
- Choosing a Title
- Making an Outline
- Paragraph Development
- Research Process Video Series
- Executive Summary
- The C.A.R.S. Model
- Background Information
- The Research Problem/Question
- Theoretical Framework
- Citation Tracking
- Content Alert Services
- Evaluating Sources
- Primary Sources
- Secondary Sources
- Tiertiary Sources
- Scholarly vs. Popular Publications
- Qualitative Methods
- Quantitative Methods
- Insiderness
- Using Non-Textual Elements
- Limitations of the Study
- Common Grammar Mistakes
- Writing Concisely
- Avoiding Plagiarism
- Footnotes or Endnotes?
- Further Readings
- Generative AI and Writing
- USC Libraries Tutorials and Other Guides
- Bibliography
The conclusion is intended to help the reader understand why your research should matter to them after they have finished reading the paper. A conclusion is not merely a summary of the main topics covered or a re-statement of your research problem, but a synthesis of key points derived from the findings of your study and, if applicable, where you recommend new areas for future research. For most college-level research papers, two or three well-developed paragraphs is sufficient for a conclusion, although in some cases, more paragraphs may be required in describing the key findings and their significance.
Conclusions. The Writing Center. University of North Carolina; Conclusions. The Writing Lab and The OWL. Purdue University.
Importance of a Good Conclusion
A well-written conclusion provides you with important opportunities to demonstrate to the reader your understanding of the research problem. These include:
- Presenting the last word on the issues you raised in your paper . Just as the introduction gives a first impression to your reader, the conclusion offers a chance to leave a lasting impression. Do this, for example, by highlighting key findings in your analysis that advance new understanding about the research problem, that are unusual or unexpected, or that have important implications applied to practice.
- Summarizing your thoughts and conveying the larger significance of your study . The conclusion is an opportunity to succinctly re-emphasize your answer to the "So What?" question by placing the study within the context of how your research advances past research about the topic.
- Identifying how a gap in the literature has been addressed . The conclusion can be where you describe how a previously identified gap in the literature [first identified in your literature review section] has been addressed by your research and why this contribution is significant.
- Demonstrating the importance of your ideas . Don't be shy. The conclusion offers an opportunity to elaborate on the impact and significance of your findings. This is particularly important if your study approached examining the research problem from an unusual or innovative perspective.
- Introducing possible new or expanded ways of thinking about the research problem . This does not refer to introducing new information [which should be avoided], but to offer new insight and creative approaches for framing or contextualizing the research problem based on the results of your study.
Bunton, David. “The Structure of PhD Conclusion Chapters.” Journal of English for Academic Purposes 4 (July 2005): 207–224; Conclusions. The Writing Center. University of North Carolina; Kretchmer, Paul. Twelve Steps to Writing an Effective Conclusion. San Francisco Edit, 2003-2008; Conclusions. The Writing Lab and The OWL. Purdue University; Assan, Joseph. "Writing the Conclusion Chapter: The Good, the Bad and the Missing." Liverpool: Development Studies Association (2009): 1-8.
Structure and Writing Style
I. General Rules
The general function of your paper's conclusion is to restate the main argument . It reminds the reader of the strengths of your main argument(s) and reiterates the most important evidence supporting those argument(s). Do this by clearly summarizing the context, background, and necessity of pursuing the research problem you investigated in relation to an issue, controversy, or a gap found in the literature. However, make sure that your conclusion is not simply a repetitive summary of the findings. This reduces the impact of the argument(s) you have developed in your paper.
When writing the conclusion to your paper, follow these general rules:
- Present your conclusions in clear, concise language. Re-state the purpose of your study, then describe how your findings differ or support those of other studies and why [i.e., what were the unique, new, or crucial contributions your study made to the overall research about your topic?].
- Do not simply reiterate your findings or the discussion of your results. Provide a synthesis of arguments presented in the paper to show how these converge to address the research problem and the overall objectives of your study.
- Indicate opportunities for future research if you haven't already done so in the discussion section of your paper. Highlighting the need for further research provides the reader with evidence that you have an in-depth awareness of the research problem but that further investigations should take place beyond the scope of your investigation.
Consider the following points to help ensure your conclusion is presented well:
- If the argument or purpose of your paper is complex, you may need to summarize the argument for your reader.
- If, prior to your conclusion, you have not yet explained the significance of your findings or if you are proceeding inductively, use the end of your paper to describe your main points and explain their significance.
- Move from a detailed to a general level of consideration that returns the topic to the context provided by the introduction or within a new context that emerges from the data [this is opposite of the introduction, which begins with general discussion of the context and ends with a detailed description of the research problem].
The conclusion also provides a place for you to persuasively and succinctly restate the research problem, given that the reader has now been presented with all the information about the topic . Depending on the discipline you are writing in, the concluding paragraph may contain your reflections on the evidence presented. However, the nature of being introspective about the research you have conducted will depend on the topic and whether your professor wants you to express your observations in this way. If asked to think introspectively about the topics, do not delve into idle speculation. Being introspective means looking within yourself as an author to try and understand an issue more deeply, not to guess at possible outcomes or make up scenarios not supported by the evidence.
II. Developing a Compelling Conclusion
Although an effective conclusion needs to be clear and succinct, it does not need to be written passively or lack a compelling narrative. Strategies to help you move beyond merely summarizing the key points of your research paper may include any of the following:
- If your essay deals with a critical, contemporary problem, warn readers of the possible consequences of not attending to the problem proactively.
- Recommend a specific course or courses of action that, if adopted, could address a specific problem in practice or in the development of new knowledge leading to positive change.
- Cite a relevant quotation or expert opinion already noted in your paper in order to lend authority and support to the conclusion(s) you have reached [a good source would be from your literature review].
- Explain the consequences of your research in a way that elicits action or demonstrates urgency in seeking change.
- Restate a key statistic, fact, or visual image to emphasize the most important finding of your paper.
- If your discipline encourages personal reflection, illustrate your concluding point by drawing from your own life experiences.
- Return to an anecdote, an example, or a quotation that you presented in your introduction, but add further insight derived from the findings of your study; use your interpretation of results from your study to recast it in new or important ways.
- Provide a "take-home" message in the form of a succinct, declarative statement that you want the reader to remember about your study.
III. Problems to Avoid
Failure to be concise Your conclusion section should be concise and to the point. Conclusions that are too lengthy often have unnecessary information in them. The conclusion is not the place for details about your methodology or results. Although you should give a summary of what was learned from your research, this summary should be relatively brief, since the emphasis in the conclusion is on the implications, evaluations, insights, and other forms of analysis that you make. Strategies for writing concisely can be found here .
Failure to comment on larger, more significant issues In the introduction, your task was to move from the general [the field of study] to the specific [the research problem]. However, in the conclusion, your task is to move from a specific discussion [your research problem] back to a general discussion framed around the implications and significance of your findings [i.e., how your research contributes new understanding or fills an important gap in the literature]. In short, the conclusion is where you should place your research within a larger context [visualize your paper as an hourglass--start with a broad introduction and review of the literature, move to the specific analysis and discussion, conclude with a broad summary of the study's implications and significance].
Failure to reveal problems and negative results Negative aspects of the research process should never be ignored. These are problems, deficiencies, or challenges encountered during your study. They should be summarized as a way of qualifying your overall conclusions. If you encountered negative or unintended results [i.e., findings that are validated outside the research context in which they were generated], you must report them in the results section and discuss their implications in the discussion section of your paper. In the conclusion, use negative results as an opportunity to explain their possible significance and/or how they may form the basis for future research.
Failure to provide a clear summary of what was learned In order to be able to discuss how your research fits within your field of study [and possibly the world at large], you need to summarize briefly and succinctly how it contributes to new knowledge or a new understanding about the research problem. This element of your conclusion may be only a few sentences long.
Failure to match the objectives of your research Often research objectives in the social and behavioral sciences change while the research is being carried out. This is not a problem unless you forget to go back and refine the original objectives in your introduction. As these changes emerge they must be documented so that they accurately reflect what you were trying to accomplish in your research [not what you thought you might accomplish when you began].
Resist the urge to apologize If you've immersed yourself in studying the research problem, you presumably should know a good deal about it [perhaps even more than your professor!]. Nevertheless, by the time you have finished writing, you may be having some doubts about what you have produced. Repress those doubts! Don't undermine your authority as a researcher by saying something like, "This is just one approach to examining this problem; there may be other, much better approaches that...." The overall tone of your conclusion should convey confidence to the reader about the study's validity and realiability.
Assan, Joseph. "Writing the Conclusion Chapter: The Good, the Bad and the Missing." Liverpool: Development Studies Association (2009): 1-8; Concluding Paragraphs. College Writing Center at Meramec. St. Louis Community College; Conclusions. The Writing Center. University of North Carolina; Conclusions. The Writing Lab and The OWL. Purdue University; Freedman, Leora and Jerry Plotnick. Introductions and Conclusions. The Lab Report. University College Writing Centre. University of Toronto; Leibensperger, Summer. Draft Your Conclusion. Academic Center, the University of Houston-Victoria, 2003; Make Your Last Words Count. The Writer’s Handbook. Writing Center. University of Wisconsin Madison; Miquel, Fuster-Marquez and Carmen Gregori-Signes. “Chapter Six: ‘Last but Not Least:’ Writing the Conclusion of Your Paper.” In Writing an Applied Linguistics Thesis or Dissertation: A Guide to Presenting Empirical Research . John Bitchener, editor. (Basingstoke,UK: Palgrave Macmillan, 2010), pp. 93-105; Tips for Writing a Good Conclusion. Writing@CSU. Colorado State University; Kretchmer, Paul. Twelve Steps to Writing an Effective Conclusion. San Francisco Edit, 2003-2008; Writing Conclusions. Writing Tutorial Services, Center for Innovative Teaching and Learning. Indiana University; Writing: Considering Structure and Organization. Institute for Writing Rhetoric. Dartmouth College.
Writing Tip
Don't Belabor the Obvious!
Avoid phrases like "in conclusion...," "in summary...," or "in closing...." These phrases can be useful, even welcome, in oral presentations. But readers can see by the tell-tale section heading and number of pages remaining that they are reaching the end of your paper. You'll irritate your readers if you belabor the obvious.
Assan, Joseph. "Writing the Conclusion Chapter: The Good, the Bad and the Missing." Liverpool: Development Studies Association (2009): 1-8.
Another Writing Tip
New Insight, Not New Information!
Don't surprise the reader with new information in your conclusion that was never referenced anywhere else in the paper. This why the conclusion rarely has citations to sources. If you have new information to present, add it to the discussion or other appropriate section of the paper. Note that, although no new information is introduced, the conclusion, along with the discussion section, is where you offer your most "original" contributions in the paper; the conclusion is where you describe the value of your research, demonstrate that you understand the material that you’ve presented, and position your findings within the larger context of scholarship on the topic, including describing how your research contributes new insights to that scholarship.
Assan, Joseph. "Writing the Conclusion Chapter: The Good, the Bad and the Missing." Liverpool: Development Studies Association (2009): 1-8; Conclusions. The Writing Center. University of North Carolina.
- << Previous: Limitations of the Study
- Next: Appendices >>
- Last Updated: May 15, 2024 9:53 AM
- URL: https://libguides.usc.edu/writingguide
How To Write The Conclusion Chapter
A Simple Explainer With Examples + Free Template
By: Jenna Crossley (PhD) | Reviewed By: Dr. Eunice Rautenbach | September 2021
So, you’ve wrapped up your results and discussion chapters, and you’re finally on the home stretch – the conclusion chapter . In this post, we’ll discuss everything you need to know to craft a high-quality conclusion chapter for your dissertation or thesis project.
Overview: The Conclusion Chapter
- What the thesis/dissertation conclusion chapter is
- What to include in your conclusion
- How to structure and write up your conclusion
- A few tips to help you ace the chapter
- FREE conclusion template
What is the conclusion chapter?
The conclusion chapter is typically the final major chapter of a dissertation or thesis. As such, it serves as a concluding summary of your research findings and wraps up the document. While some publications such as journal articles and research reports combine the discussion and conclusion sections, these are typically separate chapters in a dissertation or thesis. As always, be sure to check what your university’s structural preference is before you start writing up these chapters.
So, what’s the difference between the discussion and the conclusion chapter?
Well, the two chapters are quite similar , as they both discuss the key findings of the study. However, the conclusion chapter is typically more general and high-level in nature. In your discussion chapter, you’ll typically discuss the intricate details of your study, but in your conclusion chapter, you’ll take a broader perspective, reporting on the main research outcomes and how these addressed your research aim (or aims) .
A core function of the conclusion chapter is to synthesise all major points covered in your study and to tell the reader what they should take away from your work. Basically, you need to tell them what you found , why it’s valuable , how it can be applied , and what further research can be done.
Whatever you do, don’t just copy and paste what you’ve written in your discussion chapter! The conclusion chapter should not be a simple rehash of the discussion chapter. While the two chapters are similar, they have distinctly different functions.

What should I include in the conclusion chapter?
To understand what needs to go into your conclusion chapter, it’s useful to understand what the chapter needs to achieve. In general, a good dissertation conclusion chapter should achieve the following:
- Summarise the key findings of the study
- Explicitly answer the research question(s) and address the research aims
- Inform the reader of the study’s main contributions
- Discuss any limitations or weaknesses of the study
- Present recommendations for future research
Therefore, your conclusion chapter needs to cover these core components. Importantly, you need to be careful not to include any new findings or data points. Your conclusion chapter should be based purely on data and analysis findings that you’ve already presented in the earlier chapters. If there’s a new point you want to introduce, you’ll need to go back to your results and discussion chapters to weave the foundation in there.
In many cases, readers will jump from the introduction chapter directly to the conclusions chapter to get a quick overview of the study’s purpose and key findings. Therefore, when you write up your conclusion chapter, it’s useful to assume that the reader hasn’t consumed the inner chapters of your dissertation or thesis. In other words, craft your conclusion chapter such that there’s a strong connection and smooth flow between the introduction and conclusion chapters, even though they’re on opposite ends of your document.
Need a helping hand?
How to write the conclusion chapter
Now that you have a clearer view of what the conclusion chapter is about, let’s break down the structure of this chapter so that you can get writing. Keep in mind that this is merely a typical structure – it’s not set in stone or universal. Some universities will prefer that you cover some of these points in the discussion chapter , or that you cover the points at different levels in different chapters.
Step 1: Craft a brief introduction section
As with all chapters in your dissertation or thesis, the conclusions chapter needs to start with a brief introduction. In this introductory section, you’ll want to tell the reader what they can expect to find in the chapter, and in what order . Here’s an example of what this might look like:
This chapter will conclude the study by summarising the key research findings in relation to the research aims and questions and discussing the value and contribution thereof. It will also review the limitations of the study and propose opportunities for future research.
Importantly, the objective here is just to give the reader a taste of what’s to come (a roadmap of sorts), not a summary of the chapter. So, keep it short and sweet – a paragraph or two should be ample.
Step 2: Discuss the overall findings in relation to the research aims
The next step in writing your conclusions chapter is to discuss the overall findings of your study , as they relate to the research aims and research questions . You would have likely covered similar ground in the discussion chapter, so it’s important to zoom out a little bit here and focus on the broader findings – specifically, how these help address the research aims .
In practical terms, it’s useful to start this section by reminding your reader of your research aims and research questions, so that the findings are well contextualised. In this section, phrases such as, “This study aimed to…” and “the results indicate that…” will likely come in handy. For example, you could say something like the following:
This study aimed to investigate the feeding habits of the naked mole-rat. The results indicate that naked mole rats feed on underground roots and tubers. Further findings show that these creatures eat only a part of the plant, leaving essential parts to ensure long-term food stability.
Be careful not to make overly bold claims here. Avoid claims such as “this study proves that” or “the findings disprove existing the existing theory”. It’s seldom the case that a single study can prove or disprove something. Typically, this is achieved by a broader body of research, not a single study – especially not a dissertation or thesis which will inherently have significant limitations . We’ll discuss those limitations a little later.

Step 3: Discuss how your study contributes to the field
Next, you’ll need to discuss how your research has contributed to the field – both in terms of theory and practice . This involves talking about what you achieved in your study, highlighting why this is important and valuable, and how it can be used or applied.
In this section you’ll want to:
- Mention any research outputs created as a result of your study (e.g., articles, publications, etc.)
- Inform the reader on just how your research solves your research problem , and why that matters
- Reflect on gaps in the existing research and discuss how your study contributes towards addressing these gaps
- Discuss your study in relation to relevant theories . For example, does it confirm these theories or constructively challenge them?
- Discuss how your research findings can be applied in the real world . For example, what specific actions can practitioners take, based on your findings?
Be careful to strike a careful balance between being firm but humble in your arguments here. It’s unlikely that your one study will fundamentally change paradigms or shake up the discipline, so making claims to this effect will be frowned upon . At the same time though, you need to present your arguments with confidence, firmly asserting the contribution your research has made, however small that contribution may be. Simply put, you need to keep it balanced .
Step 4: Reflect on the limitations of your study
Now that you’ve pumped your research up, the next step is to critically reflect on the limitations and potential shortcomings of your study. You may have already covered this in the discussion chapter, depending on your university’s structural preferences, so be careful not to repeat yourself unnecessarily.
There are many potential limitations that can apply to any given study. Some common ones include:
- Sampling issues that reduce the generalisability of the findings (e.g., non-probability sampling )
- Insufficient sample size (e.g., not getting enough survey responses ) or limited data access
- Low-resolution data collection or analysis techniques
- Researcher bias or lack of experience
- Lack of access to research equipment
- Time constraints that limit the methodology (e.g. cross-sectional vs longitudinal time horizon)
- Budget constraints that limit various aspects of the study
Discussing the limitations of your research may feel self-defeating (no one wants to highlight their weaknesses, right), but it’s a critical component of high-quality research. It’s important to appreciate that all studies have limitations (even well-funded studies by expert researchers) – therefore acknowledging these limitations adds credibility to your research by showing that you understand the limitations of your research design .
That being said, keep an eye on your wording and make sure that you don’t undermine your research . It’s important to strike a balance between recognising the limitations, but also highlighting the value of your research despite those limitations. Show the reader that you understand the limitations, that these were justified given your constraints, and that you know how they can be improved upon – this will get you marks.

Next, you’ll need to make recommendations for future studies. This will largely be built on the limitations you just discussed. For example, if one of your study’s weaknesses was related to a specific data collection or analysis method, you can make a recommendation that future researchers undertake similar research using a more sophisticated method.
Another potential source of future research recommendations is any data points or analysis findings that were interesting or surprising , but not directly related to your study’s research aims and research questions. So, if you observed anything that “stood out” in your analysis, but you didn’t explore it in your discussion (due to a lack of relevance to your research aims), you can earmark that for further exploration in this section.
Essentially, this section is an opportunity to outline how other researchers can build on your study to take the research further and help develop the body of knowledge. So, think carefully about the new questions that your study has raised, and clearly outline these for future researchers to pick up on.
Step 6: Wrap up with a closing summary
Tips for a top-notch conclusion chapter
Now that we’ve covered the what , why and how of the conclusion chapter, here are some quick tips and suggestions to help you craft a rock-solid conclusion.
- Don’t ramble . The conclusion chapter usually consumes 5-7% of the total word count (although this will vary between universities), so you need to be concise. Edit this chapter thoroughly with a focus on brevity and clarity.
- Be very careful about the claims you make in terms of your study’s contribution. Nothing will make the marker’s eyes roll back faster than exaggerated or unfounded claims. Be humble but firm in your claim-making.
- Use clear and simple language that can be easily understood by an intelligent layman. Remember that not every reader will be an expert in your field, so it’s important to make your writing accessible. Bear in mind that no one knows your research better than you do, so it’s important to spell things out clearly for readers.
Hopefully, this post has given you some direction and confidence to take on the conclusion chapter of your dissertation or thesis with confidence. If you’re still feeling a little shaky and need a helping hand, consider booking a free initial consultation with a friendly Grad Coach to discuss how we can help you with hands-on, private coaching.

Psst... there’s more!
This post was based on one of our popular Research Bootcamps . If you're working on a research project, you'll definitely want to check this out ...
You Might Also Like:

17 Comments
Really you team are doing great!
Your guide on writing the concluding chapter of a research is really informative especially to the beginners who really do not know where to start. Im now ready to start. Keep it up guys
Really your team are doing great!
Very helpful guidelines, timely saved. Thanks so much for the tips.
This post was very helpful and informative. Thank you team.
A very enjoyable, understandable and crisp presentation on how to write a conclusion chapter. I thoroughly enjoyed it. Thanks Jenna.
This was a very helpful article which really gave me practical pointers for my concluding chapter. Keep doing what you are doing! It meant a lot to me to be able to have this guide. Thank you so much.
Nice content dealing with the conclusion chapter, it’s a relief after the streneous task of completing discussion part.Thanks for valuable guidance
Thanks for your guidance
I get all my doubts clarified regarding the conclusion chapter. It’s really amazing. Many thanks.
Very helpful tips. Thanks so much for the guidance
Thank you very much for this piece. It offers a very helpful starting point in writing the conclusion chapter of my thesis.
It’s awesome! Most useful and timely too. Thanks a million times
Bundle of thanks for your guidance. It was greatly helpful.
Wonderful, clear, practical guidance. So grateful to read this as I conclude my research. Thank you.
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly

Conclusions
What this handout is about.
This handout will explain the functions of conclusions, offer strategies for writing effective ones, help you evaluate conclusions you’ve drafted, and suggest approaches to avoid.
About conclusions
Introductions and conclusions can be difficult to write, but they’re worth investing time in. They can have a significant influence on a reader’s experience of your paper.
Just as your introduction acts as a bridge that transports your readers from their own lives into the “place” of your analysis, your conclusion can provide a bridge to help your readers make the transition back to their daily lives. Such a conclusion will help them see why all your analysis and information should matter to them after they put the paper down.
Your conclusion is your chance to have the last word on the subject. The conclusion allows you to have the final say on the issues you have raised in your paper, to synthesize your thoughts, to demonstrate the importance of your ideas, and to propel your reader to a new view of the subject. It is also your opportunity to make a good final impression and to end on a positive note.
Your conclusion can go beyond the confines of the assignment. The conclusion pushes beyond the boundaries of the prompt and allows you to consider broader issues, make new connections, and elaborate on the significance of your findings.
Your conclusion should make your readers glad they read your paper. Your conclusion gives your reader something to take away that will help them see things differently or appreciate your topic in personally relevant ways. It can suggest broader implications that will not only interest your reader, but also enrich your reader’s life in some way. It is your gift to the reader.
Strategies for writing an effective conclusion
One or more of the following strategies may help you write an effective conclusion:
- Play the “So What” Game. If you’re stuck and feel like your conclusion isn’t saying anything new or interesting, ask a friend to read it with you. Whenever you make a statement from your conclusion, ask the friend to say, “So what?” or “Why should anybody care?” Then ponder that question and answer it. Here’s how it might go: You: Basically, I’m just saying that education was important to Douglass. Friend: So what? You: Well, it was important because it was a key to him feeling like a free and equal citizen. Friend: Why should anybody care? You: That’s important because plantation owners tried to keep slaves from being educated so that they could maintain control. When Douglass obtained an education, he undermined that control personally. You can also use this strategy on your own, asking yourself “So What?” as you develop your ideas or your draft.
- Return to the theme or themes in the introduction. This strategy brings the reader full circle. For example, if you begin by describing a scenario, you can end with the same scenario as proof that your essay is helpful in creating a new understanding. You may also refer to the introductory paragraph by using key words or parallel concepts and images that you also used in the introduction.
- Synthesize, don’t summarize. Include a brief summary of the paper’s main points, but don’t simply repeat things that were in your paper. Instead, show your reader how the points you made and the support and examples you used fit together. Pull it all together.
- Include a provocative insight or quotation from the research or reading you did for your paper.
- Propose a course of action, a solution to an issue, or questions for further study. This can redirect your reader’s thought process and help them to apply your info and ideas to their own life or to see the broader implications.
- Point to broader implications. For example, if your paper examines the Greensboro sit-ins or another event in the Civil Rights Movement, you could point out its impact on the Civil Rights Movement as a whole. A paper about the style of writer Virginia Woolf could point to her influence on other writers or on later feminists.
Strategies to avoid
- Beginning with an unnecessary, overused phrase such as “in conclusion,” “in summary,” or “in closing.” Although these phrases can work in speeches, they come across as wooden and trite in writing.
- Stating the thesis for the very first time in the conclusion.
- Introducing a new idea or subtopic in your conclusion.
- Ending with a rephrased thesis statement without any substantive changes.
- Making sentimental, emotional appeals that are out of character with the rest of an analytical paper.
- Including evidence (quotations, statistics, etc.) that should be in the body of the paper.
Four kinds of ineffective conclusions
- The “That’s My Story and I’m Sticking to It” Conclusion. This conclusion just restates the thesis and is usually painfully short. It does not push the ideas forward. People write this kind of conclusion when they can’t think of anything else to say. Example: In conclusion, Frederick Douglass was, as we have seen, a pioneer in American education, proving that education was a major force for social change with regard to slavery.
- The “Sherlock Holmes” Conclusion. Sometimes writers will state the thesis for the very first time in the conclusion. You might be tempted to use this strategy if you don’t want to give everything away too early in your paper. You may think it would be more dramatic to keep the reader in the dark until the end and then “wow” them with your main idea, as in a Sherlock Holmes mystery. The reader, however, does not expect a mystery, but an analytical discussion of your topic in an academic style, with the main argument (thesis) stated up front. Example: (After a paper that lists numerous incidents from the book but never says what these incidents reveal about Douglass and his views on education): So, as the evidence above demonstrates, Douglass saw education as a way to undermine the slaveholders’ power and also an important step toward freedom.
- The “America the Beautiful”/”I Am Woman”/”We Shall Overcome” Conclusion. This kind of conclusion usually draws on emotion to make its appeal, but while this emotion and even sentimentality may be very heartfelt, it is usually out of character with the rest of an analytical paper. A more sophisticated commentary, rather than emotional praise, would be a more fitting tribute to the topic. Example: Because of the efforts of fine Americans like Frederick Douglass, countless others have seen the shining beacon of light that is education. His example was a torch that lit the way for others. Frederick Douglass was truly an American hero.
- The “Grab Bag” Conclusion. This kind of conclusion includes extra information that the writer found or thought of but couldn’t integrate into the main paper. You may find it hard to leave out details that you discovered after hours of research and thought, but adding random facts and bits of evidence at the end of an otherwise-well-organized essay can just create confusion. Example: In addition to being an educational pioneer, Frederick Douglass provides an interesting case study for masculinity in the American South. He also offers historians an interesting glimpse into slave resistance when he confronts Covey, the overseer. His relationships with female relatives reveal the importance of family in the slave community.
Works consulted
We consulted these works while writing this handout. This is not a comprehensive list of resources on the handout’s topic, and we encourage you to do your own research to find additional publications. Please do not use this list as a model for the format of your own reference list, as it may not match the citation style you are using. For guidance on formatting citations, please see the UNC Libraries citation tutorial . We revise these tips periodically and welcome feedback.
Douglass, Frederick. 1995. Narrative of the Life of Frederick Douglass, an American Slave, Written by Himself. New York: Dover.
Hamilton College. n.d. “Conclusions.” Writing Center. Accessed June 14, 2019. https://www.hamilton.edu//academics/centers/writing/writing-resources/conclusions .
Holewa, Randa. 2004. “Strategies for Writing a Conclusion.” LEO: Literacy Education Online. Last updated February 19, 2004. https://leo.stcloudstate.edu/acadwrite/conclude.html.
You may reproduce it for non-commercial use if you use the entire handout and attribute the source: The Writing Center, University of North Carolina at Chapel Hill
Make a Gift
When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections
- How to Write Discussions and Conclusions

The discussion section contains the results and outcomes of a study. An effective discussion informs readers what can be learned from your experiment and provides context for the results.
What makes an effective discussion?
When you’re ready to write your discussion, you’ve already introduced the purpose of your study and provided an in-depth description of the methodology. The discussion informs readers about the larger implications of your study based on the results. Highlighting these implications while not overstating the findings can be challenging, especially when you’re submitting to a journal that selects articles based on novelty or potential impact. Regardless of what journal you are submitting to, the discussion section always serves the same purpose: concluding what your study results actually mean.
A successful discussion section puts your findings in context. It should include:
- the results of your research,
- a discussion of related research, and
- a comparison between your results and initial hypothesis.
Tip: Not all journals share the same naming conventions.
You can apply the advice in this article to the conclusion, results or discussion sections of your manuscript.
Our Early Career Researcher community tells us that the conclusion is often considered the most difficult aspect of a manuscript to write. To help, this guide provides questions to ask yourself, a basic structure to model your discussion off of and examples from published manuscripts.
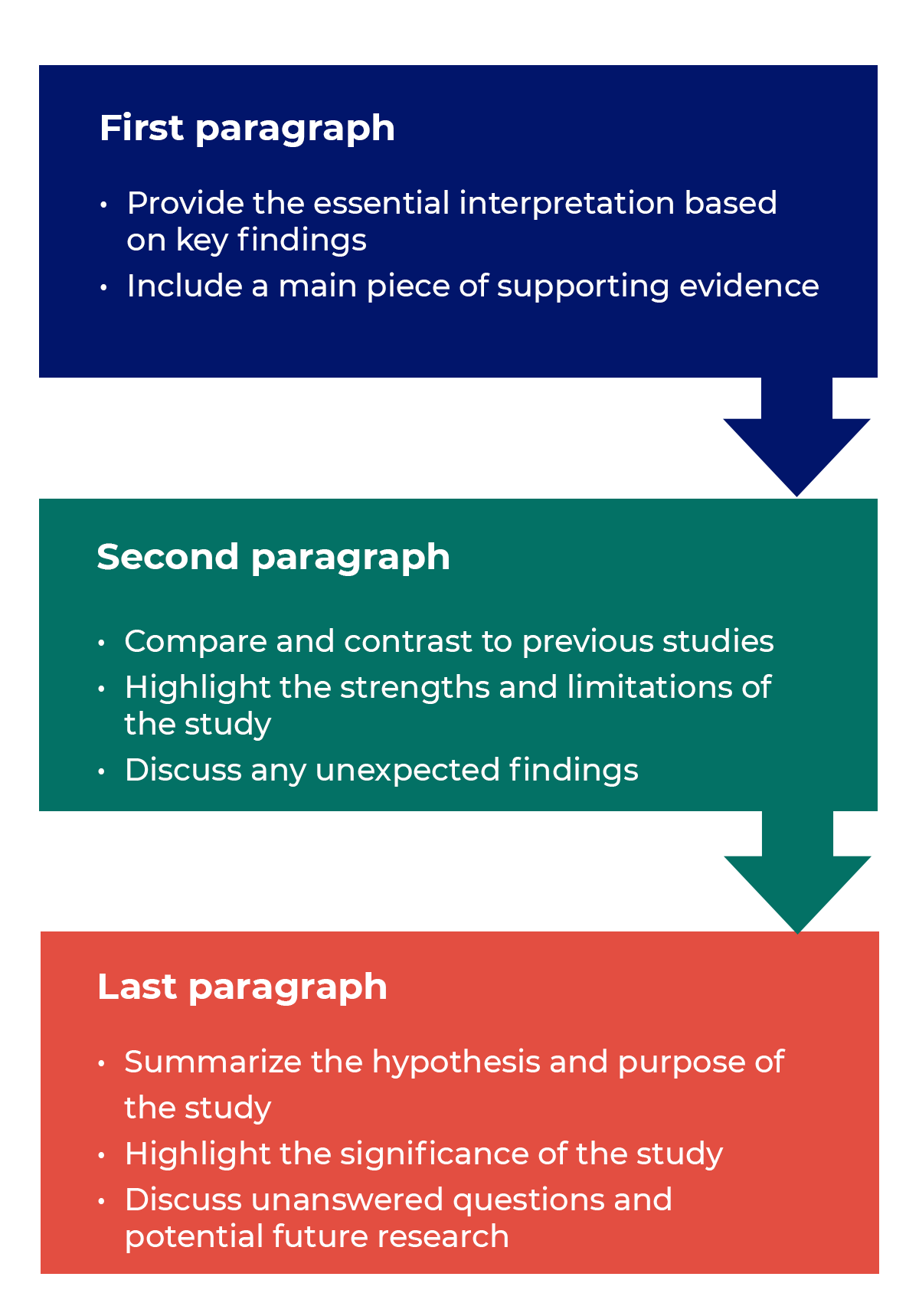
Questions to ask yourself:
- Was my hypothesis correct?
- If my hypothesis is partially correct or entirely different, what can be learned from the results?
- How do the conclusions reshape or add onto the existing knowledge in the field? What does previous research say about the topic?
- Why are the results important or relevant to your audience? Do they add further evidence to a scientific consensus or disprove prior studies?
- How can future research build on these observations? What are the key experiments that must be done?
- What is the “take-home” message you want your reader to leave with?
How to structure a discussion
Trying to fit a complete discussion into a single paragraph can add unnecessary stress to the writing process. If possible, you’ll want to give yourself two or three paragraphs to give the reader a comprehensive understanding of your study as a whole. Here’s one way to structure an effective discussion:
Writing Tips
While the above sections can help you brainstorm and structure your discussion, there are many common mistakes that writers revert to when having difficulties with their paper. Writing a discussion can be a delicate balance between summarizing your results, providing proper context for your research and avoiding introducing new information. Remember that your paper should be both confident and honest about the results!

- Read the journal’s guidelines on the discussion and conclusion sections. If possible, learn about the guidelines before writing the discussion to ensure you’re writing to meet their expectations.
- Begin with a clear statement of the principal findings. This will reinforce the main take-away for the reader and set up the rest of the discussion.
- Explain why the outcomes of your study are important to the reader. Discuss the implications of your findings realistically based on previous literature, highlighting both the strengths and limitations of the research.
- State whether the results prove or disprove your hypothesis. If your hypothesis was disproved, what might be the reasons?
- Introduce new or expanded ways to think about the research question. Indicate what next steps can be taken to further pursue any unresolved questions.
- If dealing with a contemporary or ongoing problem, such as climate change, discuss possible consequences if the problem is avoided.
- Be concise. Adding unnecessary detail can distract from the main findings.

Don’t
- Rewrite your abstract. Statements with “we investigated” or “we studied” generally do not belong in the discussion.
- Include new arguments or evidence not previously discussed. Necessary information and evidence should be introduced in the main body of the paper.
- Apologize. Even if your research contains significant limitations, don’t undermine your authority by including statements that doubt your methodology or execution.
- Shy away from speaking on limitations or negative results. Including limitations and negative results will give readers a complete understanding of the presented research. Potential limitations include sources of potential bias, threats to internal or external validity, barriers to implementing an intervention and other issues inherent to the study design.
- Overstate the importance of your findings. Making grand statements about how a study will fully resolve large questions can lead readers to doubt the success of the research.
Snippets of Effective Discussions:
Consumer-based actions to reduce plastic pollution in rivers: A multi-criteria decision analysis approach
Identifying reliable indicators of fitness in polar bears
- How to Write a Great Title
- How to Write an Abstract
- How to Write Your Methods
- How to Report Statistics
- How to Edit Your Work
The contents of the Peer Review Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
The contents of the Writing Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
There’s a lot to consider when deciding where to submit your work. Learn how to choose a journal that will help your study reach its audience, while reflecting your values as a researcher…
- +254728776317
- info@masomomsingi.com
Masomo Msingi Publishers Mobile App

MASOMO MSINGI PUBLISHERS
KASNEB|KNEC|KISM
SUMMARY OF FINDINGS, CONCLUSIONS AND RECOMMENDATIONS
This chapter summarizes the whole research process. It first provides a brief summary of the whole study with particular reference to the research problem, research methodology, results, the main contributions of the research and recommendations for future work. It provides a summary of the main findings of the study, conclusions and recommendations. This chapter should be reasonably short.
The readers would want to know whether the objectives of the study were achieved, and whether the work has contributed to knowledge. Therefore, when compiling this chapter, a researcher should focus on answering these questions.
Any conclusions drawn should be those resulting from the study. A researcher should make relevant references to chapters that support the listed findings and may also refer to the work of others for comparison. However, one should not discuss the stu1y’s results here.
Summary of the Main Findings
In summarizing, a researcher should identify the findings of the study and discuss them briefly. In addition, the methodological problems encountered should be outlined so that future/other researchers may take the relevant precautions. The researcher should clearly pinpoint if the study objectives were achieved or not. An effective summary has the following qualities:
- It bases on results from the study.
- It is brief, all statements are concise, and pinpoint to the contributions that the researcher has made.
Recommendations
- All statements are factual.
One way to present the summary is to use one paragraph for each idea. Alternatively, the researcher can use a point-by-point format.
The Conclusion section should be very brief, about half a page. It should indicate what the study results reaffirm. It should also briefly discuss some of the strategies highlighted by the respondents. In this section, the researcher should clearly state how the study has contributed to knowledge.
The recommendations section is important in research. This section often exposes further problems and introduces more questions. As a researcher, there is a time limit to the research project, so it is unlikely that the study would have solved all the problems associated with the area of study. The researcher is therefore expected to make suggestions about how his/her work can be improved, and also based on the study findings, point out whether there are areas that deserve further investigation. This section will indicate whether a researcher has a firm appreciation of his/her work, and whether he/ she has given sufficient thought to its implications, not only within the narrow confines of the research topic but to related fields. This section reflects the researcher’s foresightedness and creativity.
Written by MJ
- Privacy Policy

Home » Research Findings – Types Examples and Writing Guide
Research Findings – Types Examples and Writing Guide
Table of Contents

Research Findings
Definition:
Research findings refer to the results obtained from a study or investigation conducted through a systematic and scientific approach. These findings are the outcomes of the data analysis, interpretation, and evaluation carried out during the research process.
Types of Research Findings
There are two main types of research findings:
Qualitative Findings
Qualitative research is an exploratory research method used to understand the complexities of human behavior and experiences. Qualitative findings are non-numerical and descriptive data that describe the meaning and interpretation of the data collected. Examples of qualitative findings include quotes from participants, themes that emerge from the data, and descriptions of experiences and phenomena.
Quantitative Findings
Quantitative research is a research method that uses numerical data and statistical analysis to measure and quantify a phenomenon or behavior. Quantitative findings include numerical data such as mean, median, and mode, as well as statistical analyses such as t-tests, ANOVA, and regression analysis. These findings are often presented in tables, graphs, or charts.
Both qualitative and quantitative findings are important in research and can provide different insights into a research question or problem. Combining both types of findings can provide a more comprehensive understanding of a phenomenon and improve the validity and reliability of research results.
Parts of Research Findings
Research findings typically consist of several parts, including:
- Introduction: This section provides an overview of the research topic and the purpose of the study.
- Literature Review: This section summarizes previous research studies and findings that are relevant to the current study.
- Methodology : This section describes the research design, methods, and procedures used in the study, including details on the sample, data collection, and data analysis.
- Results : This section presents the findings of the study, including statistical analyses and data visualizations.
- Discussion : This section interprets the results and explains what they mean in relation to the research question(s) and hypotheses. It may also compare and contrast the current findings with previous research studies and explore any implications or limitations of the study.
- Conclusion : This section provides a summary of the key findings and the main conclusions of the study.
- Recommendations: This section suggests areas for further research and potential applications or implications of the study’s findings.
How to Write Research Findings
Writing research findings requires careful planning and attention to detail. Here are some general steps to follow when writing research findings:
- Organize your findings: Before you begin writing, it’s essential to organize your findings logically. Consider creating an outline or a flowchart that outlines the main points you want to make and how they relate to one another.
- Use clear and concise language : When presenting your findings, be sure to use clear and concise language that is easy to understand. Avoid using jargon or technical terms unless they are necessary to convey your meaning.
- Use visual aids : Visual aids such as tables, charts, and graphs can be helpful in presenting your findings. Be sure to label and title your visual aids clearly, and make sure they are easy to read.
- Use headings and subheadings: Using headings and subheadings can help organize your findings and make them easier to read. Make sure your headings and subheadings are clear and descriptive.
- Interpret your findings : When presenting your findings, it’s important to provide some interpretation of what the results mean. This can include discussing how your findings relate to the existing literature, identifying any limitations of your study, and suggesting areas for future research.
- Be precise and accurate : When presenting your findings, be sure to use precise and accurate language. Avoid making generalizations or overstatements and be careful not to misrepresent your data.
- Edit and revise: Once you have written your research findings, be sure to edit and revise them carefully. Check for grammar and spelling errors, make sure your formatting is consistent, and ensure that your writing is clear and concise.
Research Findings Example
Following is a Research Findings Example sample for students:
Title: The Effects of Exercise on Mental Health
Sample : 500 participants, both men and women, between the ages of 18-45.
Methodology : Participants were divided into two groups. The first group engaged in 30 minutes of moderate intensity exercise five times a week for eight weeks. The second group did not exercise during the study period. Participants in both groups completed a questionnaire that assessed their mental health before and after the study period.
Findings : The group that engaged in regular exercise reported a significant improvement in mental health compared to the control group. Specifically, they reported lower levels of anxiety and depression, improved mood, and increased self-esteem.
Conclusion : Regular exercise can have a positive impact on mental health and may be an effective intervention for individuals experiencing symptoms of anxiety or depression.
Applications of Research Findings
Research findings can be applied in various fields to improve processes, products, services, and outcomes. Here are some examples:
- Healthcare : Research findings in medicine and healthcare can be applied to improve patient outcomes, reduce morbidity and mortality rates, and develop new treatments for various diseases.
- Education : Research findings in education can be used to develop effective teaching methods, improve learning outcomes, and design new educational programs.
- Technology : Research findings in technology can be applied to develop new products, improve existing products, and enhance user experiences.
- Business : Research findings in business can be applied to develop new strategies, improve operations, and increase profitability.
- Public Policy: Research findings can be used to inform public policy decisions on issues such as environmental protection, social welfare, and economic development.
- Social Sciences: Research findings in social sciences can be used to improve understanding of human behavior and social phenomena, inform public policy decisions, and develop interventions to address social issues.
- Agriculture: Research findings in agriculture can be applied to improve crop yields, develop new farming techniques, and enhance food security.
- Sports : Research findings in sports can be applied to improve athlete performance, reduce injuries, and develop new training programs.
When to use Research Findings
Research findings can be used in a variety of situations, depending on the context and the purpose. Here are some examples of when research findings may be useful:
- Decision-making : Research findings can be used to inform decisions in various fields, such as business, education, healthcare, and public policy. For example, a business may use market research findings to make decisions about new product development or marketing strategies.
- Problem-solving : Research findings can be used to solve problems or challenges in various fields, such as healthcare, engineering, and social sciences. For example, medical researchers may use findings from clinical trials to develop new treatments for diseases.
- Policy development : Research findings can be used to inform the development of policies in various fields, such as environmental protection, social welfare, and economic development. For example, policymakers may use research findings to develop policies aimed at reducing greenhouse gas emissions.
- Program evaluation: Research findings can be used to evaluate the effectiveness of programs or interventions in various fields, such as education, healthcare, and social services. For example, educational researchers may use findings from evaluations of educational programs to improve teaching and learning outcomes.
- Innovation: Research findings can be used to inspire or guide innovation in various fields, such as technology and engineering. For example, engineers may use research findings on materials science to develop new and innovative products.
Purpose of Research Findings
The purpose of research findings is to contribute to the knowledge and understanding of a particular topic or issue. Research findings are the result of a systematic and rigorous investigation of a research question or hypothesis, using appropriate research methods and techniques.
The main purposes of research findings are:
- To generate new knowledge : Research findings contribute to the body of knowledge on a particular topic, by adding new information, insights, and understanding to the existing knowledge base.
- To test hypotheses or theories : Research findings can be used to test hypotheses or theories that have been proposed in a particular field or discipline. This helps to determine the validity and reliability of the hypotheses or theories, and to refine or develop new ones.
- To inform practice: Research findings can be used to inform practice in various fields, such as healthcare, education, and business. By identifying best practices and evidence-based interventions, research findings can help practitioners to make informed decisions and improve outcomes.
- To identify gaps in knowledge: Research findings can help to identify gaps in knowledge and understanding of a particular topic, which can then be addressed by further research.
- To contribute to policy development: Research findings can be used to inform policy development in various fields, such as environmental protection, social welfare, and economic development. By providing evidence-based recommendations, research findings can help policymakers to develop effective policies that address societal challenges.
Characteristics of Research Findings
Research findings have several key characteristics that distinguish them from other types of information or knowledge. Here are some of the main characteristics of research findings:
- Objective : Research findings are based on a systematic and rigorous investigation of a research question or hypothesis, using appropriate research methods and techniques. As such, they are generally considered to be more objective and reliable than other types of information.
- Empirical : Research findings are based on empirical evidence, which means that they are derived from observations or measurements of the real world. This gives them a high degree of credibility and validity.
- Generalizable : Research findings are often intended to be generalizable to a larger population or context beyond the specific study. This means that the findings can be applied to other situations or populations with similar characteristics.
- Transparent : Research findings are typically reported in a transparent manner, with a clear description of the research methods and data analysis techniques used. This allows others to assess the credibility and reliability of the findings.
- Peer-reviewed: Research findings are often subject to a rigorous peer-review process, in which experts in the field review the research methods, data analysis, and conclusions of the study. This helps to ensure the validity and reliability of the findings.
- Reproducible : Research findings are often designed to be reproducible, meaning that other researchers can replicate the study using the same methods and obtain similar results. This helps to ensure the validity and reliability of the findings.
Advantages of Research Findings
Research findings have many advantages, which make them valuable sources of knowledge and information. Here are some of the main advantages of research findings:
- Evidence-based: Research findings are based on empirical evidence, which means that they are grounded in data and observations from the real world. This makes them a reliable and credible source of information.
- Inform decision-making: Research findings can be used to inform decision-making in various fields, such as healthcare, education, and business. By identifying best practices and evidence-based interventions, research findings can help practitioners and policymakers to make informed decisions and improve outcomes.
- Identify gaps in knowledge: Research findings can help to identify gaps in knowledge and understanding of a particular topic, which can then be addressed by further research. This contributes to the ongoing development of knowledge in various fields.
- Improve outcomes : Research findings can be used to develop and implement evidence-based practices and interventions, which have been shown to improve outcomes in various fields, such as healthcare, education, and social services.
- Foster innovation: Research findings can inspire or guide innovation in various fields, such as technology and engineering. By providing new information and understanding of a particular topic, research findings can stimulate new ideas and approaches to problem-solving.
- Enhance credibility: Research findings are generally considered to be more credible and reliable than other types of information, as they are based on rigorous research methods and are subject to peer-review processes.
Limitations of Research Findings
While research findings have many advantages, they also have some limitations. Here are some of the main limitations of research findings:
- Limited scope: Research findings are typically based on a particular study or set of studies, which may have a limited scope or focus. This means that they may not be applicable to other contexts or populations.
- Potential for bias : Research findings can be influenced by various sources of bias, such as researcher bias, selection bias, or measurement bias. This can affect the validity and reliability of the findings.
- Ethical considerations: Research findings can raise ethical considerations, particularly in studies involving human subjects. Researchers must ensure that their studies are conducted in an ethical and responsible manner, with appropriate measures to protect the welfare and privacy of participants.
- Time and resource constraints : Research studies can be time-consuming and require significant resources, which can limit the number and scope of studies that are conducted. This can lead to gaps in knowledge or a lack of research on certain topics.
- Complexity: Some research findings can be complex and difficult to interpret, particularly in fields such as science or medicine. This can make it challenging for practitioners and policymakers to apply the findings to their work.
- Lack of generalizability : While research findings are intended to be generalizable to larger populations or contexts, there may be factors that limit their generalizability. For example, cultural or environmental factors may influence how a particular intervention or treatment works in different populations or contexts.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples

Institutional Review Board – Application Sample...

Evaluating Research – Process, Examples and...
- Key Differences
Know the Differences & Comparisons
Difference Between Summary and Conclusion

In the absence of conclusion, the research paper might seem incomplete. It is often contrasted with a summary, but there are subtle differences between the two. A summary is nothing but a short and clear account of the text, covering the main points, facts or elements only.
Content: Summary Vs Conclusion
Comparison chart, definition of summary.
A summary is the compact account of the main text, i.e. an article, essay, drama, or some other form of literature. It gives an overview of the key points of the piece of writing. Moreover, one can also summarize anything which he/she has seen and heard, like speech, movie or lecture, etc.
It is typically about 5% to 15% of the original work, i.e. it may extend up to one to three paragraphs, which is around 100 to 300 words. It simply depends on the length of the text which is summarized. Its aim is to describe a piece of writing while including considerably less content than its original.
Ideal Summary
- An ideal summary is one that objectively highlights the entire form of literature.
- It should cover the focal point of every paragraph and the evidence supporting it.
- It should exclude all the irrelevant examples, details and information.
- It can make use of the keywords used in the original work, but should not use the same sentences and phrases, except if quotation marks are used.
- It must express the sense of the original work while using your own words and sentences.
Definition of Conclsuion
Conclusion refers to the epilogue which is given at the end of something, to deduce the findings. It forms part of the thought process, which combines all the points discussed, so as to reach a comprehensive idea or statement.
It is the final step in the process of reasoning, in which judgement, decision or opinion is formed after complete investigation and consideration. To conclude something, different types of perspectives are considered. It is only 10% of the research paper, which has two segments – summary and final thought .
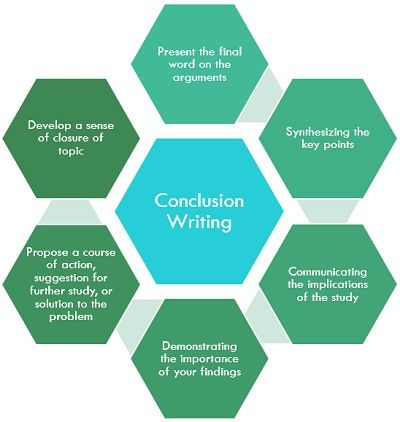
Ideal Conclusion
- The conclusion is said to be ideal when it gives an interesting insight and should end on a positive note.
- Highlights the main argument presented in the piece of writing.
- Sums up the answer to the question, often stated in the introduction.
- Refer back the questions, states the key points and findings, and wind up the discussion with the final observation.
- Reinforces the primary theme of the study.
- Makes a strong and long-lasting impression on the reader.
- It should never introduce new points.
Key Differences Between Summary and Conclusion
The points stated below discuss the differences between summary and conclusion:
- A summary is an abridgement of the work of literature, which covers the key points succinctly. On the contrary, conclusion refers to the final part of the discourse which sums up the argument and gives a statement of opinion or judgement.
- A summary is written to provide the reader with a precise and objective narrative of the central ideas and aspects of the original text. Conversely, conclusion paragraph wraps up the text and presents the reader that you have accomplished, what you have set forth in the beginning.
- While a summary restates the facts and elements, which are discussed in the original text, conclusion tends to synthesize all the points and wrap up the discussion. It helps the reader understand the importance of the research.
- Ideally, the length of the summary is 5% to 15%, whereas the conclusion constitutes only 10% of the original work.
- A summary often demonstrates the central ideas of the text clearly and concisely. In contrast, the conclusion introduces a new outlook, proposes a course of actions, provides a solution to the problem, makes suggestions for further study, and makes deductions on the basis of the argument.
- A summary only includes the ideas of the original text. One should not insert their opinion, criticism, comments or interpretations. As against, the conclusion can include the researcher’s or writer’s views, ideas and criticisms at the end.
In a nutshell, a summary condenses the material as well as it informs the reader about the vital points. Contrastingly, a conclusion gives the reader the sense of completeness of the argument or topic, with a reason or final thought. It focuses on the final outcome of the argumentation or research.
You Might Also Like:

essay ordering says
September 23, 2022 at 10:59 pm
Irresistible! Thank you a lot for this type and precise carrier.your offerings is higher than higher.
September 25, 2022 at 2:50 am
I will recommend my students to visit and read the explanation you give here. Thank you.
Endalkachew Sisay says
January 24, 2023 at 2:09 pm
I really appreciate the points you have addressed for us. Keep up the good work!!!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Jump to navigation

Cochrane Training
Chapter 15: interpreting results and drawing conclusions.
Holger J Schünemann, Gunn E Vist, Julian PT Higgins, Nancy Santesso, Jonathan J Deeks, Paul Glasziou, Elie A Akl, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group
Key Points:
- This chapter provides guidance on interpreting the results of synthesis in order to communicate the conclusions of the review effectively.
- Methods are presented for computing, presenting and interpreting relative and absolute effects for dichotomous outcome data, including the number needed to treat (NNT).
- For continuous outcome measures, review authors can present summary results for studies using natural units of measurement or as minimal important differences when all studies use the same scale. When studies measure the same construct but with different scales, review authors will need to find a way to interpret the standardized mean difference, or to use an alternative effect measure for the meta-analysis such as the ratio of means.
- Review authors should not describe results as ‘statistically significant’, ‘not statistically significant’ or ‘non-significant’ or unduly rely on thresholds for P values, but report the confidence interval together with the exact P value.
- Review authors should not make recommendations about healthcare decisions, but they can – after describing the certainty of evidence and the balance of benefits and harms – highlight different actions that might be consistent with particular patterns of values and preferences and other factors that determine a decision such as cost.
Cite this chapter as: Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, Akl EA, Guyatt GH. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook .
15.1 Introduction
The purpose of Cochrane Reviews is to facilitate healthcare decisions by patients and the general public, clinicians, guideline developers, administrators and policy makers. They also inform future research. A clear statement of findings, a considered discussion and a clear presentation of the authors’ conclusions are, therefore, important parts of the review. In particular, the following issues can help people make better informed decisions and increase the usability of Cochrane Reviews:
- information on all important outcomes, including adverse outcomes;
- the certainty of the evidence for each of these outcomes, as it applies to specific populations and specific interventions; and
- clarification of the manner in which particular values and preferences may bear on the desirable and undesirable consequences of the intervention.
A ‘Summary of findings’ table, described in Chapter 14 , Section 14.1 , provides key pieces of information about health benefits and harms in a quick and accessible format. It is highly desirable that review authors include a ‘Summary of findings’ table in Cochrane Reviews alongside a sufficient description of the studies and meta-analyses to support its contents. This description includes the rating of the certainty of evidence, also called the quality of the evidence or confidence in the estimates of the effects, which is expected in all Cochrane Reviews.
‘Summary of findings’ tables are usually supported by full evidence profiles which include the detailed ratings of the evidence (Guyatt et al 2011a, Guyatt et al 2013a, Guyatt et al 2013b, Santesso et al 2016). The Discussion section of the text of the review provides space to reflect and consider the implications of these aspects of the review’s findings. Cochrane Reviews include five standard subheadings to ensure the Discussion section places the review in an appropriate context: ‘Summary of main results (benefits and harms)’; ‘Potential biases in the review process’; ‘Overall completeness and applicability of evidence’; ‘Certainty of the evidence’; and ‘Agreements and disagreements with other studies or reviews’. Following the Discussion, the Authors’ conclusions section is divided into two standard subsections: ‘Implications for practice’ and ‘Implications for research’. The assessment of the certainty of evidence facilitates a structured description of the implications for practice and research.
Because Cochrane Reviews have an international audience, the Discussion and Authors’ conclusions should, so far as possible, assume a broad international perspective and provide guidance for how the results could be applied in different settings, rather than being restricted to specific national or local circumstances. Cultural differences and economic differences may both play an important role in determining the best course of action based on the results of a Cochrane Review. Furthermore, individuals within societies have widely varying values and preferences regarding health states, and use of societal resources to achieve particular health states. For all these reasons, and because information that goes beyond that included in a Cochrane Review is required to make fully informed decisions, different people will often make different decisions based on the same evidence presented in a review.
Thus, review authors should avoid specific recommendations that inevitably depend on assumptions about available resources, values and preferences, and other factors such as equity considerations, feasibility and acceptability of an intervention. The purpose of the review should be to present information and aid interpretation rather than to offer recommendations. The discussion and conclusions should help people understand the implications of the evidence in relation to practical decisions and apply the results to their specific situation. Review authors can aid this understanding of the implications by laying out different scenarios that describe certain value structures.
In this chapter, we address first one of the key aspects of interpreting findings that is also fundamental in completing a ‘Summary of findings’ table: the certainty of evidence related to each of the outcomes. We then provide a more detailed consideration of issues around applicability and around interpretation of numerical results, and provide suggestions for presenting authors’ conclusions.
15.2 Issues of indirectness and applicability
15.2.1 the role of the review author.
“A leap of faith is always required when applying any study findings to the population at large” or to a specific person. “In making that jump, one must always strike a balance between making justifiable broad generalizations and being too conservative in one’s conclusions” (Friedman et al 1985). In addition to issues about risk of bias and other domains determining the certainty of evidence, this leap of faith is related to how well the identified body of evidence matches the posed PICO ( Population, Intervention, Comparator(s) and Outcome ) question. As to the population, no individual can be entirely matched to the population included in research studies. At the time of decision, there will always be differences between the study population and the person or population to whom the evidence is applied; sometimes these differences are slight, sometimes large.
The terms applicability, generalizability, external validity and transferability are related, sometimes used interchangeably and have in common that they lack a clear and consistent definition in the classic epidemiological literature (Schünemann et al 2013). However, all of the terms describe one overarching theme: whether or not available research evidence can be directly used to answer the health and healthcare question at hand, ideally supported by a judgement about the degree of confidence in this use (Schünemann et al 2013). GRADE’s certainty domains include a judgement about ‘indirectness’ to describe all of these aspects including the concept of direct versus indirect comparisons of different interventions (Atkins et al 2004, Guyatt et al 2008, Guyatt et al 2011b).
To address adequately the extent to which a review is relevant for the purpose to which it is being put, there are certain things the review author must do, and certain things the user of the review must do to assess the degree of indirectness. Cochrane and the GRADE Working Group suggest using a very structured framework to address indirectness. We discuss here and in Chapter 14 what the review author can do to help the user. Cochrane Review authors must be extremely clear on the population, intervention and outcomes that they intend to address. Chapter 14, Section 14.1.2 , also emphasizes a crucial step: the specification of all patient-important outcomes relevant to the intervention strategies under comparison.
In considering whether the effect of an intervention applies equally to all participants, and whether different variations on the intervention have similar effects, review authors need to make a priori hypotheses about possible effect modifiers, and then examine those hypotheses (see Chapter 10, Section 10.10 and Section 10.11 ). If they find apparent subgroup effects, they must ultimately decide whether or not these effects are credible (Sun et al 2012). Differences between subgroups, particularly those that correspond to differences between studies, should be interpreted cautiously. Some chance variation between subgroups is inevitable so, unless there is good reason to believe that there is an interaction, review authors should not assume that the subgroup effect exists. If, despite due caution, review authors judge subgroup effects in terms of relative effect estimates as credible (i.e. the effects differ credibly), they should conduct separate meta-analyses for the relevant subgroups, and produce separate ‘Summary of findings’ tables for those subgroups.
The user of the review will be challenged with ‘individualization’ of the findings, whether they seek to apply the findings to an individual patient or a policy decision in a specific context. For example, even if relative effects are similar across subgroups, absolute effects will differ according to baseline risk. Review authors can help provide this information by identifying identifiable groups of people with varying baseline risks in the ‘Summary of findings’ tables, as discussed in Chapter 14, Section 14.1.3 . Users can then identify their specific case or population as belonging to a particular risk group, if relevant, and assess their likely magnitude of benefit or harm accordingly. A description of the identifying prognostic or baseline risk factors in a brief scenario (e.g. age or gender) will help users of a review further.
Another decision users must make is whether their individual case or population of interest is so different from those included in the studies that they cannot use the results of the systematic review and meta-analysis at all. Rather than rigidly applying the inclusion and exclusion criteria of studies, it is better to ask whether or not there are compelling reasons why the evidence should not be applied to a particular patient. Review authors can sometimes help decision makers by identifying important variation where divergence might limit the applicability of results (Rothwell 2005, Schünemann et al 2006, Guyatt et al 2011b, Schünemann et al 2013), including biologic and cultural variation, and variation in adherence to an intervention.
In addressing these issues, review authors cannot be aware of, or address, the myriad of differences in circumstances around the world. They can, however, address differences of known importance to many people and, importantly, they should avoid assuming that other people’s circumstances are the same as their own in discussing the results and drawing conclusions.
15.2.2 Biological variation
Issues of biological variation that may affect the applicability of a result to a reader or population include divergence in pathophysiology (e.g. biological differences between women and men that may affect responsiveness to an intervention) and divergence in a causative agent (e.g. for infectious diseases such as malaria, which may be caused by several different parasites). The discussion of the results in the review should make clear whether the included studies addressed all or only some of these groups, and whether any important subgroup effects were found.
15.2.3 Variation in context
Some interventions, particularly non-pharmacological interventions, may work in some contexts but not in others; the situation has been described as program by context interaction (Hawe et al 2004). Contextual factors might pertain to the host organization in which an intervention is offered, such as the expertise, experience and morale of the staff expected to carry out the intervention, the competing priorities for the clinician’s or staff’s attention, the local resources such as service and facilities made available to the program and the status or importance given to the program by the host organization. Broader context issues might include aspects of the system within which the host organization operates, such as the fee or payment structure for healthcare providers and the local insurance system. Some interventions, in particular complex interventions (see Chapter 17 ), can be only partially implemented in some contexts, and this requires judgements about indirectness of the intervention and its components for readers in that context (Schünemann 2013).
Contextual factors may also pertain to the characteristics of the target group or population, such as cultural and linguistic diversity, socio-economic position, rural/urban setting. These factors may mean that a particular style of care or relationship evolves between service providers and consumers that may or may not match the values and technology of the program.
For many years these aspects have been acknowledged when decision makers have argued that results of evidence reviews from other countries do not apply in their own country or setting. Whilst some programmes/interventions have been successfully transferred from one context to another, others have not (Resnicow et al 1993, Lumley et al 2004, Coleman et al 2015). Review authors should be cautious when making generalizations from one context to another. They should report on the presence (or otherwise) of context-related information in intervention studies, where this information is available.
15.2.4 Variation in adherence
Variation in the adherence of the recipients and providers of care can limit the certainty in the applicability of results. Predictable differences in adherence can be due to divergence in how recipients of care perceive the intervention (e.g. the importance of side effects), economic conditions or attitudes that make some forms of care inaccessible in some settings, such as in low-income countries (Dans et al 2007). It should not be assumed that high levels of adherence in closely monitored randomized trials will translate into similar levels of adherence in normal practice.
15.2.5 Variation in values and preferences
Decisions about healthcare management strategies and options involve trading off health benefits and harms. The right choice may differ for people with different values and preferences (i.e. the importance people place on the outcomes and interventions), and it is important that decision makers ensure that decisions are consistent with a patient or population’s values and preferences. The importance placed on outcomes, together with other factors, will influence whether the recipients of care will or will not accept an option that is offered (Alonso-Coello et al 2016) and, thus, can be one factor influencing adherence. In Section 15.6 , we describe how the review author can help this process and the limits of supporting decision making based on intervention reviews.
15.3 Interpreting results of statistical analyses
15.3.1 confidence intervals.
Results for both individual studies and meta-analyses are reported with a point estimate together with an associated confidence interval. For example, ‘The odds ratio was 0.75 with a 95% confidence interval of 0.70 to 0.80’. The point estimate (0.75) is the best estimate of the magnitude and direction of the experimental intervention’s effect compared with the comparator intervention. The confidence interval describes the uncertainty inherent in any estimate, and describes a range of values within which we can be reasonably sure that the true effect actually lies. If the confidence interval is relatively narrow (e.g. 0.70 to 0.80), the effect size is known precisely. If the interval is wider (e.g. 0.60 to 0.93) the uncertainty is greater, although there may still be enough precision to make decisions about the utility of the intervention. Intervals that are very wide (e.g. 0.50 to 1.10) indicate that we have little knowledge about the effect and this imprecision affects our certainty in the evidence, and that further information would be needed before we could draw a more certain conclusion.
A 95% confidence interval is often interpreted as indicating a range within which we can be 95% certain that the true effect lies. This statement is a loose interpretation, but is useful as a rough guide. The strictly correct interpretation of a confidence interval is based on the hypothetical notion of considering the results that would be obtained if the study were repeated many times. If a study were repeated infinitely often, and on each occasion a 95% confidence interval calculated, then 95% of these intervals would contain the true effect (see Section 15.3.3 for further explanation).
The width of the confidence interval for an individual study depends to a large extent on the sample size. Larger studies tend to give more precise estimates of effects (and hence have narrower confidence intervals) than smaller studies. For continuous outcomes, precision depends also on the variability in the outcome measurements (i.e. how widely individual results vary between people in the study, measured as the standard deviation); for dichotomous outcomes it depends on the risk of the event (more frequent events allow more precision, and narrower confidence intervals), and for time-to-event outcomes it also depends on the number of events observed. All these quantities are used in computation of the standard errors of effect estimates from which the confidence interval is derived.
The width of a confidence interval for a meta-analysis depends on the precision of the individual study estimates and on the number of studies combined. In addition, for random-effects models, precision will decrease with increasing heterogeneity and confidence intervals will widen correspondingly (see Chapter 10, Section 10.10.4 ). As more studies are added to a meta-analysis the width of the confidence interval usually decreases. However, if the additional studies increase the heterogeneity in the meta-analysis and a random-effects model is used, it is possible that the confidence interval width will increase.
Confidence intervals and point estimates have different interpretations in fixed-effect and random-effects models. While the fixed-effect estimate and its confidence interval address the question ‘what is the best (single) estimate of the effect?’, the random-effects estimate assumes there to be a distribution of effects, and the estimate and its confidence interval address the question ‘what is the best estimate of the average effect?’ A confidence interval may be reported for any level of confidence (although they are most commonly reported for 95%, and sometimes 90% or 99%). For example, the odds ratio of 0.80 could be reported with an 80% confidence interval of 0.73 to 0.88; a 90% interval of 0.72 to 0.89; and a 95% interval of 0.70 to 0.92. As the confidence level increases, the confidence interval widens.
There is logical correspondence between the confidence interval and the P value (see Section 15.3.3 ). The 95% confidence interval for an effect will exclude the null value (such as an odds ratio of 1.0 or a risk difference of 0) if and only if the test of significance yields a P value of less than 0.05. If the P value is exactly 0.05, then either the upper or lower limit of the 95% confidence interval will be at the null value. Similarly, the 99% confidence interval will exclude the null if and only if the test of significance yields a P value of less than 0.01.
Together, the point estimate and confidence interval provide information to assess the effects of the intervention on the outcome. For example, suppose that we are evaluating an intervention that reduces the risk of an event and we decide that it would be useful only if it reduced the risk of an event from 30% by at least 5 percentage points to 25% (these values will depend on the specific clinical scenario and outcomes, including the anticipated harms). If the meta-analysis yielded an effect estimate of a reduction of 10 percentage points with a tight 95% confidence interval, say, from 7% to 13%, we would be able to conclude that the intervention was useful since both the point estimate and the entire range of the interval exceed our criterion of a reduction of 5% for net health benefit. However, if the meta-analysis reported the same risk reduction of 10% but with a wider interval, say, from 2% to 18%, although we would still conclude that our best estimate of the intervention effect is that it provides net benefit, we could not be so confident as we still entertain the possibility that the effect could be between 2% and 5%. If the confidence interval was wider still, and included the null value of a difference of 0%, we would still consider the possibility that the intervention has no effect on the outcome whatsoever, and would need to be even more sceptical in our conclusions.
Review authors may use the same general approach to conclude that an intervention is not useful. Continuing with the above example where the criterion for an important difference that should be achieved to provide more benefit than harm is a 5% risk difference, an effect estimate of 2% with a 95% confidence interval of 1% to 4% suggests that the intervention does not provide net health benefit.
15.3.2 P values and statistical significance
A P value is the standard result of a statistical test, and is the probability of obtaining the observed effect (or larger) under a ‘null hypothesis’. In the context of Cochrane Reviews there are two commonly used statistical tests. The first is a test of overall effect (a Z-test), and its null hypothesis is that there is no overall effect of the experimental intervention compared with the comparator on the outcome of interest. The second is the (Chi 2 ) test for heterogeneity, and its null hypothesis is that there are no differences in the intervention effects across studies.
A P value that is very small indicates that the observed effect is very unlikely to have arisen purely by chance, and therefore provides evidence against the null hypothesis. It has been common practice to interpret a P value by examining whether it is smaller than particular threshold values. In particular, P values less than 0.05 are often reported as ‘statistically significant’, and interpreted as being small enough to justify rejection of the null hypothesis. However, the 0.05 threshold is an arbitrary one that became commonly used in medical and psychological research largely because P values were determined by comparing the test statistic against tabulations of specific percentage points of statistical distributions. If review authors decide to present a P value with the results of a meta-analysis, they should report a precise P value (as calculated by most statistical software), together with the 95% confidence interval. Review authors should not describe results as ‘statistically significant’, ‘not statistically significant’ or ‘non-significant’ or unduly rely on thresholds for P values , but report the confidence interval together with the exact P value (see MECIR Box 15.3.a ).
We discuss interpretation of the test for heterogeneity in Chapter 10, Section 10.10.2 ; the remainder of this section refers mainly to tests for an overall effect. For tests of an overall effect, the computation of P involves both the effect estimate and precision of the effect estimate (driven largely by sample size). As precision increases, the range of plausible effects that could occur by chance is reduced. Correspondingly, the statistical significance of an effect of a particular magnitude will usually be greater (the P value will be smaller) in a larger study than in a smaller study.
P values are commonly misinterpreted in two ways. First, a moderate or large P value (e.g. greater than 0.05) may be misinterpreted as evidence that the intervention has no effect on the outcome. There is an important difference between this statement and the correct interpretation that there is a high probability that the observed effect on the outcome is due to chance alone. To avoid such a misinterpretation, review authors should always examine the effect estimate and its 95% confidence interval.
The second misinterpretation is to assume that a result with a small P value for the summary effect estimate implies that an experimental intervention has an important benefit. Such a misinterpretation is more likely to occur in large studies and meta-analyses that accumulate data over dozens of studies and thousands of participants. The P value addresses the question of whether the experimental intervention effect is precisely nil; it does not examine whether the effect is of a magnitude of importance to potential recipients of the intervention. In a large study, a small P value may represent the detection of a trivial effect that may not lead to net health benefit when compared with the potential harms (i.e. harmful effects on other important outcomes). Again, inspection of the point estimate and confidence interval helps correct interpretations (see Section 15.3.1 ).
MECIR Box 15.3.a Relevant expectations for conduct of intervention reviews
15.3.3 Relation between confidence intervals, statistical significance and certainty of evidence
The confidence interval (and imprecision) is only one domain that influences overall uncertainty about effect estimates. Uncertainty resulting from imprecision (i.e. statistical uncertainty) may be no less important than uncertainty from indirectness, or any other GRADE domain, in the context of decision making (Schünemann 2016). Thus, the extent to which interpretations of the confidence interval described in Sections 15.3.1 and 15.3.2 correspond to conclusions about overall certainty of the evidence for the outcome of interest depends on these other domains. If there are no concerns about other domains that determine the certainty of the evidence (i.e. risk of bias, inconsistency, indirectness or publication bias), then the interpretation in Sections 15.3.1 and 15.3.2 . about the relation of the confidence interval to the true effect may be carried forward to the overall certainty. However, if there are concerns about the other domains that affect the certainty of the evidence, the interpretation about the true effect needs to be seen in the context of further uncertainty resulting from those concerns.
For example, nine randomized controlled trials in almost 6000 cancer patients indicated that the administration of heparin reduces the risk of venous thromboembolism (VTE), with a risk ratio of 43% (95% CI 19% to 60%) (Akl et al 2011a). For patients with a plausible baseline risk of approximately 4.6% per year, this relative effect suggests that heparin leads to an absolute risk reduction of 20 fewer VTEs (95% CI 9 fewer to 27 fewer) per 1000 people per year (Akl et al 2011a). Now consider that the review authors or those applying the evidence in a guideline have lowered the certainty in the evidence as a result of indirectness. While the confidence intervals would remain unchanged, the certainty in that confidence interval and in the point estimate as reflecting the truth for the question of interest will be lowered. In fact, the certainty range will have unknown width so there will be unknown likelihood of a result within that range because of this indirectness. The lower the certainty in the evidence, the less we know about the width of the certainty range, although methods for quantifying risk of bias and understanding potential direction of bias may offer insight when lowered certainty is due to risk of bias. Nevertheless, decision makers must consider this uncertainty, and must do so in relation to the effect measure that is being evaluated (e.g. a relative or absolute measure). We will describe the impact on interpretations for dichotomous outcomes in Section 15.4 .
15.4 Interpreting results from dichotomous outcomes (including numbers needed to treat)
15.4.1 relative and absolute risk reductions.
Clinicians may be more inclined to prescribe an intervention that reduces the relative risk of death by 25% than one that reduces the risk of death by 1 percentage point, although both presentations of the evidence may relate to the same benefit (i.e. a reduction in risk from 4% to 3%). The former refers to the relative reduction in risk and the latter to the absolute reduction in risk. As described in Chapter 6, Section 6.4.1 , there are several measures for comparing dichotomous outcomes in two groups. Meta-analyses are usually undertaken using risk ratios (RR), odds ratios (OR) or risk differences (RD), but there are several alternative ways of expressing results.
Relative risk reduction (RRR) is a convenient way of re-expressing a risk ratio as a percentage reduction:
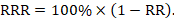
For example, a risk ratio of 0.75 translates to a relative risk reduction of 25%, as in the example above.
The risk difference is often referred to as the absolute risk reduction (ARR) or absolute risk increase (ARI), and may be presented as a percentage (e.g. 1%), as a decimal (e.g. 0.01), or as account (e.g. 10 out of 1000). We consider different choices for presenting absolute effects in Section 15.4.3 . We then describe computations for obtaining these numbers from the results of individual studies and of meta-analyses in Section 15.4.4 .
15.4.2 Number needed to treat (NNT)
The number needed to treat (NNT) is a common alternative way of presenting information on the effect of an intervention. The NNT is defined as the expected number of people who need to receive the experimental rather than the comparator intervention for one additional person to either incur or avoid an event (depending on the direction of the result) in a given time frame. Thus, for example, an NNT of 10 can be interpreted as ‘it is expected that one additional (or less) person will incur an event for every 10 participants receiving the experimental intervention rather than comparator over a given time frame’. It is important to be clear that:
- since the NNT is derived from the risk difference, it is still a comparative measure of effect (experimental versus a specific comparator) and not a general property of a single intervention; and
- the NNT gives an ‘expected value’. For example, NNT = 10 does not imply that one additional event will occur in each and every group of 10 people.
NNTs can be computed for both beneficial and detrimental events, and for interventions that cause both improvements and deteriorations in outcomes. In all instances NNTs are expressed as positive whole numbers. Some authors use the term ‘number needed to harm’ (NNH) when an intervention leads to an adverse outcome, or a decrease in a positive outcome, rather than improvement. However, this phrase can be misleading (most notably, it can easily be read to imply the number of people who will experience a harmful outcome if given the intervention), and it is strongly recommended that ‘number needed to harm’ and ‘NNH’ are avoided. The preferred alternative is to use phrases such as ‘number needed to treat for an additional beneficial outcome’ (NNTB) and ‘number needed to treat for an additional harmful outcome’ (NNTH) to indicate direction of effect.
As NNTs refer to events, their interpretation needs to be worded carefully when the binary outcome is a dichotomization of a scale-based outcome. For example, if the outcome is pain measured on a ‘none, mild, moderate or severe’ scale it may have been dichotomized as ‘none or mild’ versus ‘moderate or severe’. It would be inappropriate for an NNT from these data to be referred to as an ‘NNT for pain’. It is an ‘NNT for moderate or severe pain’.
We consider different choices for presenting absolute effects in Section 15.4.3 . We then describe computations for obtaining these numbers from the results of individual studies and of meta-analyses in Section 15.4.4 .
15.4.3 Expressing risk differences
Users of reviews are liable to be influenced by the choice of statistical presentations of the evidence. Hoffrage and colleagues suggest that physicians’ inferences about statistical outcomes are more appropriate when they deal with ‘natural frequencies’ – whole numbers of people, both treated and untreated (e.g. treatment results in a drop from 20 out of 1000 to 10 out of 1000 women having breast cancer) – than when effects are presented as percentages (e.g. 1% absolute reduction in breast cancer risk) (Hoffrage et al 2000). Probabilities may be more difficult to understand than frequencies, particularly when events are rare. While standardization may be important in improving the presentation of research evidence (and participation in healthcare decisions), current evidence suggests that the presentation of natural frequencies for expressing differences in absolute risk is best understood by consumers of healthcare information (Akl et al 2011b). This evidence provides the rationale for presenting absolute risks in ‘Summary of findings’ tables as numbers of people with events per 1000 people receiving the intervention (see Chapter 14 ).
RRs and RRRs remain crucial because relative effects tend to be substantially more stable across risk groups than absolute effects (see Chapter 10, Section 10.4.3 ). Review authors can use their own data to study this consistency (Cates 1999, Smeeth et al 1999). Risk differences from studies are least likely to be consistent across baseline event rates; thus, they are rarely appropriate for computing numbers needed to treat in systematic reviews. If a relative effect measure (OR or RR) is chosen for meta-analysis, then a comparator group risk needs to be specified as part of the calculation of an RD or NNT. In addition, if there are several different groups of participants with different levels of risk, it is crucial to express absolute benefit for each clinically identifiable risk group, clarifying the time period to which this applies. Studies in patients with differing severity of disease, or studies with different lengths of follow-up will almost certainly have different comparator group risks. In these cases, different comparator group risks lead to different RDs and NNTs (except when the intervention has no effect). A recommended approach is to re-express an odds ratio or a risk ratio as a variety of RD or NNTs across a range of assumed comparator risks (ACRs) (McQuay and Moore 1997, Smeeth et al 1999). Review authors should bear these considerations in mind not only when constructing their ‘Summary of findings’ table, but also in the text of their review.
For example, a review of oral anticoagulants to prevent stroke presented information to users by describing absolute benefits for various baseline risks (Aguilar and Hart 2005, Aguilar et al 2007). They presented their principal findings as “The inherent risk of stroke should be considered in the decision to use oral anticoagulants in atrial fibrillation patients, selecting those who stand to benefit most for this therapy” (Aguilar and Hart 2005). Among high-risk atrial fibrillation patients with prior stroke or transient ischaemic attack who have stroke rates of about 12% (120 per 1000) per year, warfarin prevents about 70 strokes yearly per 1000 patients, whereas for low-risk atrial fibrillation patients (with a stroke rate of about 2% per year or 20 per 1000), warfarin prevents only 12 strokes. This presentation helps users to understand the important impact that typical baseline risks have on the absolute benefit that they can expect.
15.4.4 Computations
Direct computation of risk difference (RD) or a number needed to treat (NNT) depends on the summary statistic (odds ratio, risk ratio or risk differences) available from the study or meta-analysis. When expressing results of meta-analyses, review authors should use, in the computations, whatever statistic they determined to be the most appropriate summary for meta-analysis (see Chapter 10, Section 10.4.3 ). Here we present calculations to obtain RD as a reduction in the number of participants per 1000. For example, a risk difference of –0.133 corresponds to 133 fewer participants with the event per 1000.
RDs and NNTs should not be computed from the aggregated total numbers of participants and events across the trials. This approach ignores the randomization within studies, and may produce seriously misleading results if there is unbalanced randomization in any of the studies. Using the pooled result of a meta-analysis is more appropriate. When computing NNTs, the values obtained are by convention always rounded up to the next whole number.
15.4.4.1 Computing NNT from a risk difference (RD)
A NNT may be computed from a risk difference as
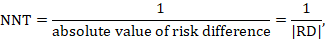
where the vertical bars (‘absolute value of’) in the denominator indicate that any minus sign should be ignored. It is convention to round the NNT up to the nearest whole number. For example, if the risk difference is –0.12 the NNT is 9; if the risk difference is –0.22 the NNT is 5. Cochrane Review authors should qualify the NNT as referring to benefit (improvement) or harm by denoting the NNT as NNTB or NNTH. Note that this approach, although feasible, should be used only for the results of a meta-analysis of risk differences. In most cases meta-analyses will be undertaken using a relative measure of effect (RR or OR), and those statistics should be used to calculate the NNT (see Section 15.4.4.2 and 15.4.4.3 ).
15.4.4.2 Computing risk differences or NNT from a risk ratio
To aid interpretation of the results of a meta-analysis of risk ratios, review authors may compute an absolute risk reduction or NNT. In order to do this, an assumed comparator risk (ACR) (otherwise known as a baseline risk, or risk that the outcome of interest would occur with the comparator intervention) is required. It will usually be appropriate to do this for a range of different ACRs. The computation proceeds as follows:
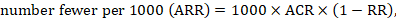
As an example, suppose the risk ratio is RR = 0.92, and an ACR = 0.3 (300 per 1000) is assumed. Then the effect on risk is 24 fewer per 1000:
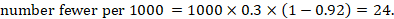
The NNT is 42:
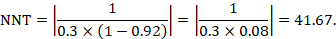
15.4.4.3 Computing risk differences or NNT from an odds ratio
Review authors may wish to compute a risk difference or NNT from the results of a meta-analysis of odds ratios. In order to do this, an ACR is required. It will usually be appropriate to do this for a range of different ACRs. The computation proceeds as follows:
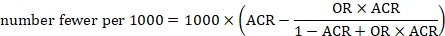
As an example, suppose the odds ratio is OR = 0.73, and a comparator risk of ACR = 0.3 is assumed. Then the effect on risk is 62 fewer per 1000:
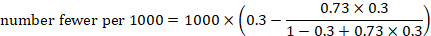
The NNT is 17:
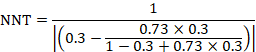
15.4.4.4 Computing risk ratio from an odds ratio
Because risk ratios are easier to interpret than odds ratios, but odds ratios have favourable mathematical properties, a review author may decide to undertake a meta-analysis based on odds ratios, but to express the result as a summary risk ratio (or relative risk reduction). This requires an ACR. Then
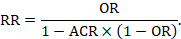
It will often be reasonable to perform this transformation using the median comparator group risk from the studies in the meta-analysis.
15.4.4.5 Computing confidence limits
Confidence limits for RDs and NNTs may be calculated by applying the above formulae to the upper and lower confidence limits for the summary statistic (RD, RR or OR) (Altman 1998). Note that this confidence interval does not incorporate uncertainty around the ACR.
If the 95% confidence interval of OR or RR includes the value 1, one of the confidence limits will indicate benefit and the other harm. Thus, appropriate use of the words ‘fewer’ and ‘more’ is required for each limit when presenting results in terms of events. For NNTs, the two confidence limits should be labelled as NNTB and NNTH to indicate the direction of effect in each case. The confidence interval for the NNT will include a ‘discontinuity’, because increasingly smaller risk differences that approach zero will lead to NNTs approaching infinity. Thus, the confidence interval will include both an infinitely large NNTB and an infinitely large NNTH.
15.5 Interpreting results from continuous outcomes (including standardized mean differences)
15.5.1 meta-analyses with continuous outcomes.
Review authors should describe in the study protocol how they plan to interpret results for continuous outcomes. When outcomes are continuous, review authors have a number of options to present summary results. These options differ if studies report the same measure that is familiar to the target audiences, studies report the same or very similar measures that are less familiar to the target audiences, or studies report different measures.
15.5.2 Meta-analyses with continuous outcomes using the same measure
If all studies have used the same familiar units, for instance, results are expressed as durations of events, such as symptoms for conditions including diarrhoea, sore throat, otitis media, influenza or duration of hospitalization, a meta-analysis may generate a summary estimate in those units, as a difference in mean response (see, for instance, the row summarizing results for duration of diarrhoea in Chapter 14, Figure 14.1.b and the row summarizing oedema in Chapter 14, Figure 14.1.a ). For such outcomes, the ‘Summary of findings’ table should include a difference of means between the two interventions. However, when units of such outcomes may be difficult to interpret, particularly when they relate to rating scales (again, see the oedema row of Chapter 14, Figure 14.1.a ). ‘Summary of findings’ tables should include the minimum and maximum of the scale of measurement, and the direction. Knowledge of the smallest change in instrument score that patients perceive is important – the minimal important difference (MID) – and can greatly facilitate the interpretation of results (Guyatt et al 1998, Schünemann and Guyatt 2005). Knowing the MID allows review authors and users to place results in context. Review authors should state the MID – if known – in the Comments column of their ‘Summary of findings’ table. For example, the chronic respiratory questionnaire has possible scores in health-related quality of life ranging from 1 to 7 and 0.5 represents a well-established MID (Jaeschke et al 1989, Schünemann et al 2005).
15.5.3 Meta-analyses with continuous outcomes using different measures
When studies have used different instruments to measure the same construct, a standardized mean difference (SMD) may be used in meta-analysis for combining continuous data. Without guidance, clinicians and patients may have little idea how to interpret results presented as SMDs. Review authors should therefore consider issues of interpretability when planning their analysis at the protocol stage and should consider whether there will be suitable ways to re-express the SMD or whether alternative effect measures, such as a ratio of means, or possibly as minimal important difference units (Guyatt et al 2013b) should be used. Table 15.5.a and the following sections describe these options.
Table 15.5.a Approaches and their implications to presenting results of continuous variables when primary studies have used different instruments to measure the same construct. Adapted from Guyatt et al (2013b)
15.5.3.1 Presenting and interpreting SMDs using generic effect size estimates
The SMD expresses the intervention effect in standard units rather than the original units of measurement. The SMD is the difference in mean effects between the experimental and comparator groups divided by the pooled standard deviation of participants’ outcomes, or external SDs when studies are very small (see Chapter 6, Section 6.5.1.2 ). The value of a SMD thus depends on both the size of the effect (the difference between means) and the standard deviation of the outcomes (the inherent variability among participants or based on an external SD).
If review authors use the SMD, they might choose to present the results directly as SMDs (row 1a, Table 15.5.a and Table 15.5.b ). However, absolute values of the intervention and comparison groups are typically not useful because studies have used different measurement instruments with different units. Guiding rules for interpreting SMDs (or ‘Cohen’s effect sizes’) exist, and have arisen mainly from researchers in the social sciences (Cohen 1988). One example is as follows: 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 1988). Variations exist (e.g. <0.40=small, 0.40 to 0.70=moderate, >0.70=large). Review authors might consider including such a guiding rule in interpreting the SMD in the text of the review, and in summary versions such as the Comments column of a ‘Summary of findings’ table. However, some methodologists believe that such interpretations are problematic because patient importance of a finding is context-dependent and not amenable to generic statements.
15.5.3.2 Re-expressing SMDs using a familiar instrument
The second possibility for interpreting the SMD is to express it in the units of one or more of the specific measurement instruments used by the included studies (row 1b, Table 15.5.a and Table 15.5.b ). The approach is to calculate an absolute difference in means by multiplying the SMD by an estimate of the SD associated with the most familiar instrument. To obtain this SD, a reasonable option is to calculate a weighted average across all intervention groups of all studies that used the selected instrument (preferably a pre-intervention or post-intervention SD as discussed in Chapter 10, Section 10.5.2 ). To better reflect among-person variation in practice, or to use an instrument not represented in the meta-analysis, it may be preferable to use a standard deviation from a representative observational study. The summary effect is thus re-expressed in the original units of that particular instrument and the clinical relevance and impact of the intervention effect can be interpreted using that familiar instrument.
The same approach of re-expressing the results for a familiar instrument can also be used for other standardized effect measures such as when standardizing by MIDs (Guyatt et al 2013b): see Section 15.5.3.5 .
Table 15.5.b Application of approaches when studies have used different measures: effects of dexamethasone for pain after laparoscopic cholecystectomy (Karanicolas et al 2008). Reproduced with permission of Wolters Kluwer
1 Certainty rated according to GRADE from very low to high certainty. 2 Substantial unexplained heterogeneity in study results. 3 Imprecision due to wide confidence intervals. 4 The 20% comes from the proportion in the control group requiring rescue analgesia. 5 Crude (arithmetic) means of the post-operative pain mean responses across all five trials when transformed to a 100-point scale.
15.5.3.3 Re-expressing SMDs through dichotomization and transformation to relative and absolute measures
A third approach (row 1c, Table 15.5.a and Table 15.5.b ) relies on converting the continuous measure into a dichotomy and thus allows calculation of relative and absolute effects on a binary scale. A transformation of a SMD to a (log) odds ratio is available, based on the assumption that an underlying continuous variable has a logistic distribution with equal standard deviation in the two intervention groups, as discussed in Chapter 10, Section 10.6 (Furukawa 1999, Guyatt et al 2013b). The assumption is unlikely to hold exactly and the results must be regarded as an approximation. The log odds ratio is estimated as
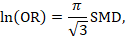
(or approximately 1.81✕SMD). The resulting odds ratio can then be presented as normal, and in a ‘Summary of findings’ table, combined with an assumed comparator group risk to be expressed as an absolute risk difference. The comparator group risk in this case would refer to the proportion of people who have achieved a specific value of the continuous outcome. In randomized trials this can be interpreted as the proportion who have improved by some (specified) amount (responders), for instance by 5 points on a 0 to 100 scale. Table 15.5.c shows some illustrative results from this method. The risk differences can then be converted to NNTs or to people per thousand using methods described in Section 15.4.4 .
Table 15.5.c Risk difference derived for specific SMDs for various given ‘proportions improved’ in the comparator group (Furukawa 1999, Guyatt et al 2013b). Reproduced with permission of Elsevier
15.5.3.4 Ratio of means
A more frequently used approach is based on calculation of a ratio of means between the intervention and comparator groups (Friedrich et al 2008) as discussed in Chapter 6, Section 6.5.1.3 . Interpretational advantages of this approach include the ability to pool studies with outcomes expressed in different units directly, to avoid the vulnerability of heterogeneous populations that limits approaches that rely on SD units, and for ease of clinical interpretation (row 2, Table 15.5.a and Table 15.5.b ). This method is currently designed for post-intervention scores only. However, it is possible to calculate a ratio of change scores if both intervention and comparator groups change in the same direction in each relevant study, and this ratio may sometimes be informative.
Limitations to this approach include its limited applicability to change scores (since it is unlikely that both intervention and comparator group changes are in the same direction in all studies) and the possibility of misleading results if the comparator group mean is very small, in which case even a modest difference from the intervention group will yield a large and therefore misleading ratio of means. It also requires that separate ratios of means be calculated for each included study, and then entered into a generic inverse variance meta-analysis (see Chapter 10, Section 10.3 ).
The ratio of means approach illustrated in Table 15.5.b suggests a relative reduction in pain of only 13%, meaning that those receiving steroids have a pain severity 87% of those in the comparator group, an effect that might be considered modest.
15.5.3.5 Presenting continuous results as minimally important difference units
To express results in MID units, review authors have two options. First, they can be combined across studies in the same way as the SMD, but instead of dividing the mean difference of each study by its SD, review authors divide by the MID associated with that outcome (Johnston et al 2010, Guyatt et al 2013b). Instead of SD units, the pooled results represent MID units (row 3, Table 15.5.a and Table 15.5.b ), and may be more easily interpretable. This approach avoids the problem of varying SDs across studies that may distort estimates of effect in approaches that rely on the SMD. The approach, however, relies on having well-established MIDs. The approach is also risky in that a difference less than the MID may be interpreted as trivial when a substantial proportion of patients may have achieved an important benefit.
The other approach makes a simple conversion (not shown in Table 15.5.b ), before undertaking the meta-analysis, of the means and SDs from each study to means and SDs on the scale of a particular familiar instrument whose MID is known. For example, one can rescale the mean and SD of other chronic respiratory disease instruments (e.g. rescaling a 0 to 100 score of an instrument) to a the 1 to 7 score in Chronic Respiratory Disease Questionnaire (CRQ) units (by assuming 0 equals 1 and 100 equals 7 on the CRQ). Given the MID of the CRQ of 0.5, a mean difference in change of 0.71 after rescaling of all studies suggests a substantial effect of the intervention (Guyatt et al 2013b). This approach, presenting in units of the most familiar instrument, may be the most desirable when the target audiences have extensive experience with that instrument, particularly if the MID is well established.
15.6 Drawing conclusions
15.6.1 conclusions sections of a cochrane review.
Authors’ conclusions in a Cochrane Review are divided into implications for practice and implications for research. While Cochrane Reviews about interventions can provide meaningful information and guidance for practice, decisions about the desirable and undesirable consequences of healthcare options require evidence and judgements for criteria that most Cochrane Reviews do not provide (Alonso-Coello et al 2016). In describing the implications for practice and the development of recommendations, however, review authors may consider the certainty of the evidence, the balance of benefits and harms, and assumed values and preferences.
15.6.2 Implications for practice
Drawing conclusions about the practical usefulness of an intervention entails making trade-offs, either implicitly or explicitly, between the estimated benefits, harms and the values and preferences. Making such trade-offs, and thus making specific recommendations for an action in a specific context, goes beyond a Cochrane Review and requires additional evidence and informed judgements that most Cochrane Reviews do not provide (Alonso-Coello et al 2016). Such judgements are typically the domain of clinical practice guideline developers for which Cochrane Reviews will provide crucial information (Graham et al 2011, Schünemann et al 2014, Zhang et al 2018a). Thus, authors of Cochrane Reviews should not make recommendations.
If review authors feel compelled to lay out actions that clinicians and patients could take, they should – after describing the certainty of evidence and the balance of benefits and harms – highlight different actions that might be consistent with particular patterns of values and preferences. Other factors that might influence a decision should also be highlighted, including any known factors that would be expected to modify the effects of the intervention, the baseline risk or status of the patient, costs and who bears those costs, and the availability of resources. Review authors should ensure they consider all patient-important outcomes, including those for which limited data may be available. In the context of public health reviews the focus may be on population-important outcomes as the target may be an entire (non-diseased) population and include outcomes that are not measured in the population receiving an intervention (e.g. a reduction of transmission of infections from those receiving an intervention). This process implies a high level of explicitness in judgements about values or preferences attached to different outcomes and the certainty of the related evidence (Zhang et al 2018b, Zhang et al 2018c); this and a full cost-effectiveness analysis is beyond the scope of most Cochrane Reviews (although they might well be used for such analyses; see Chapter 20 ).
A review on the use of anticoagulation in cancer patients to increase survival (Akl et al 2011a) provides an example for laying out clinical implications for situations where there are important trade-offs between desirable and undesirable effects of the intervention: “The decision for a patient with cancer to start heparin therapy for survival benefit should balance the benefits and downsides and integrate the patient’s values and preferences. Patients with a high preference for a potential survival prolongation, limited aversion to potential bleeding, and who do not consider heparin (both UFH or LMWH) therapy a burden may opt to use heparin, while those with aversion to bleeding may not.”
15.6.3 Implications for research
The second category for authors’ conclusions in a Cochrane Review is implications for research. To help people make well-informed decisions about future healthcare research, the ‘Implications for research’ section should comment on the need for further research, and the nature of the further research that would be most desirable. It is helpful to consider the population, intervention, comparison and outcomes that could be addressed, or addressed more effectively in the future, in the context of the certainty of the evidence in the current review (Brown et al 2006):
- P (Population): diagnosis, disease stage, comorbidity, risk factor, sex, age, ethnic group, specific inclusion or exclusion criteria, clinical setting;
- I (Intervention): type, frequency, dose, duration, prognostic factor;
- C (Comparison): placebo, routine care, alternative treatment/management;
- O (Outcome): which clinical or patient-related outcomes will the researcher need to measure, improve, influence or accomplish? Which methods of measurement should be used?
While Cochrane Review authors will find the PICO domains helpful, the domains of the GRADE certainty framework further support understanding and describing what additional research will improve the certainty in the available evidence. Note that as the certainty of the evidence is likely to vary by outcome, these implications will be specific to certain outcomes in the review. Table 15.6.a shows how review authors may be aided in their interpretation of the body of evidence and drawing conclusions about future research and practice.
Table 15.6.a Implications for research and practice suggested by individual GRADE domains
The review of compression stockings for prevention of deep vein thrombosis (DVT) in airline passengers described in Chapter 14 provides an example where there is some convincing evidence of a benefit of the intervention: “This review shows that the question of the effects on symptomless DVT of wearing versus not wearing compression stockings in the types of people studied in these trials should now be regarded as answered. Further research may be justified to investigate the relative effects of different strengths of stockings or of stockings compared to other preventative strategies. Further randomised trials to address the remaining uncertainty about the effects of wearing versus not wearing compression stockings on outcomes such as death, pulmonary embolism and symptomatic DVT would need to be large.” (Clarke et al 2016).
A review of therapeutic touch for anxiety disorder provides an example of the implications for research when no eligible studies had been found: “This review highlights the need for randomized controlled trials to evaluate the effectiveness of therapeutic touch in reducing anxiety symptoms in people diagnosed with anxiety disorders. Future trials need to be rigorous in design and delivery, with subsequent reporting to include high quality descriptions of all aspects of methodology to enable appraisal and interpretation of results.” (Robinson et al 2007).
15.6.4 Reaching conclusions
A common mistake is to confuse ‘no evidence of an effect’ with ‘evidence of no effect’. When the confidence intervals are too wide (e.g. including no effect), it is wrong to claim that the experimental intervention has ‘no effect’ or is ‘no different’ from the comparator intervention. Review authors may also incorrectly ‘positively’ frame results for some effects but not others. For example, when the effect estimate is positive for a beneficial outcome but confidence intervals are wide, review authors may describe the effect as promising. However, when the effect estimate is negative for an outcome that is considered harmful but the confidence intervals include no effect, review authors report no effect. Another mistake is to frame the conclusion in wishful terms. For example, review authors might write, “there were too few people in the analysis to detect a reduction in mortality” when the included studies showed a reduction or even increase in mortality that was not ‘statistically significant’. One way of avoiding errors such as these is to consider the results blinded; that is, consider how the results would be presented and framed in the conclusions if the direction of the results was reversed. If the confidence interval for the estimate of the difference in the effects of the interventions overlaps with no effect, the analysis is compatible with both a true beneficial effect and a true harmful effect. If one of the possibilities is mentioned in the conclusion, the other possibility should be mentioned as well. Table 15.6.b suggests narrative statements for drawing conclusions based on the effect estimate from the meta-analysis and the certainty of the evidence.
Table 15.6.b Suggested narrative statements for phrasing conclusions
Another common mistake is to reach conclusions that go beyond the evidence. Often this is done implicitly, without referring to the additional information or judgements that are used in reaching conclusions about the implications of a review for practice. Even when additional information and explicit judgements support conclusions about the implications of a review for practice, review authors rarely conduct systematic reviews of the additional information. Furthermore, implications for practice are often dependent on specific circumstances and values that must be taken into consideration. As we have noted, review authors should always be cautious when drawing conclusions about implications for practice and they should not make recommendations.
15.7 Chapter information
Authors: Holger J Schünemann, Gunn E Vist, Julian PT Higgins, Nancy Santesso, Jonathan J Deeks, Paul Glasziou, Elie Akl, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group
Acknowledgements: Andrew Oxman, Jonathan Sterne, Michael Borenstein and Rob Scholten contributed text to earlier versions of this chapter.
Funding: This work was in part supported by funding from the Michael G DeGroote Cochrane Canada Centre and the Ontario Ministry of Health. JJD receives support from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. JPTH receives support from the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
15.8 References
Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database of Systematic Reviews 2005; 3 : CD001927.
Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database of Systematic Reviews 2007; 3 : CD006186.
Akl EA, Gunukula S, Barba M, Yosuico VE, van Doormaal FF, Kuipers S, Middeldorp S, Dickinson HO, Bryant A, Schünemann H. Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database of Systematic Reviews 2011a; 1 : CD006652.
Akl EA, Oxman AD, Herrin J, Vist GE, Terrenato I, Sperati F, Costiniuk C, Blank D, Schünemann H. Using alternative statistical formats for presenting risks and risk reductions. Cochrane Database of Systematic Reviews 2011b; 3 : CD006776.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD, Group GW. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016; 353 : i2016.
Altman DG. Confidence intervals for the number needed to treat. BMJ 1998; 317 : 1309-1312.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW, Jr., Zaza S. Grading quality of evidence and strength of recommendations. BMJ 2004; 328 : 1490.
Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, Forbes C, Glanville J, Hicks NJ, Moody J, Twaddle S, Timimi H, Young P. How to formulate research recommendations. BMJ 2006; 333 : 804-806.
Cates C. Confidence intervals for the number needed to treat: Pooling numbers needed to treat may not be reliable. BMJ 1999; 318 : 1764-1765.
Clarke MJ, Broderick C, Hopewell S, Juszczak E, Eisinga A. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database of Systematic Reviews 2016; 9 : CD004002.
Cohen J. Statistical Power Analysis in the Behavioral Sciences . 2nd edition ed. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc.; 1988.
Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2015; 12 : CD010078.
Dans AM, Dans L, Oxman AD, Robinson V, Acuin J, Tugwell P, Dennis R, Kang D. Assessing equity in clinical practice guidelines. Journal of Clinical Epidemiology 2007; 60 : 540-546.
Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials . 2nd edition ed. Littleton (MA): John Wright PSG, Inc.; 1985.
Friedrich JO, Adhikari NK, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Medical Research Methodology 2008; 8 : 32.
Furukawa T. From effect size into number needed to treat. Lancet 1999; 353 : 1680.
Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E. Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Board on Health Care Services: Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011a; 64 : 383-394.
Guyatt GH, Juniper EF, Walter SD, Griffith LE, Goldstein RS. Interpreting treatment effects in randomised trials. BMJ 1998; 316 : 690-693.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336 : 924-926.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G, Akl EA, Post PN, Norris S, Meerpohl J, Shukla VK, Nasser M, Schünemann HJ. GRADE guidelines: 8. Rating the quality of evidence--indirectness. Journal of Clinical Epidemiology 2011b; 64 : 1303-1310.
Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, Brozek J, Norris S, Meerpohl J, Djulbegovic B, Alonso-Coello P, Post PN, Busse JW, Glasziou P, Christensen R, Schünemann HJ. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. Journal of Clinical Epidemiology 2013a; 66 : 158-172.
Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, Johnston BC, Karanicolas P, Akl EA, Vist G, Kunz R, Brozek J, Kupper LL, Martin SL, Meerpohl JJ, Alonso-Coello P, Christensen R, Schünemann HJ. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. Journal of Clinical Epidemiology 2013b; 66 : 173-183.
Hawe P, Shiell A, Riley T, Gold L. Methods for exploring implementation variation and local context within a cluster randomised community intervention trial. Journal of Epidemiology and Community Health 2004; 58 : 788-793.
Hoffrage U, Lindsey S, Hertwig R, Gigerenzer G. Medicine. Communicating statistical information. Science 2000; 290 : 2261-2262.
Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989; 10 : 407-415.
Johnston B, Thorlund K, Schünemann H, Xie F, Murad M, Montori V, Guyatt G. Improving the interpretation of health-related quality of life evidence in meta-analysis: The application of minimal important difference units. . Health Outcomes and Qualithy of Life 2010; 11 : 116.
Karanicolas PJ, Smith SE, Kanbur B, Davies E, Guyatt GH. The impact of prophylactic dexamethasone on nausea and vomiting after laparoscopic cholecystectomy: a systematic review and meta-analysis. Annals of Surgery 2008; 248 : 751-762.
Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2004; 4 : CD001055.
McQuay HJ, Moore RA. Using numerical results from systematic reviews in clinical practice. Annals of Internal Medicine 1997; 126 : 712-720.
Resnicow K, Cross D, Wynder E. The Know Your Body program: a review of evaluation studies. Bulletin of the New York Academy of Medicine 1993; 70 : 188-207.
Robinson J, Biley FC, Dolk H. Therapeutic touch for anxiety disorders. Cochrane Database of Systematic Reviews 2007; 3 : CD006240.
Rothwell PM. External validity of randomised controlled trials: "to whom do the results of this trial apply?". Lancet 2005; 365 : 82-93.
Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, Lasserson T, Opiyo N, Kunnamo I, Sinclair D, Garner P, Treweek S, Tovey D, Akl EA, Tugwell P, Brozek JL, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. Journal of Clinical Epidemiology 2016; 74 : 28-39.
Schünemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the Chronic respiratory disease questionnaire (CRQ). COPD: Journal of Chronic Obstructive Pulmonary Disease 2005; 2 : 81-89.
Schünemann HJ, Guyatt GH. Commentary--goodbye M(C)ID! Hello MID, where do you come from? Health Services Research 2005; 40 : 593-597.
Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 13. Applicability, transferability and adaptation. Health Research Policy and Systems 2006; 4 : 25.
Schünemann HJ. Methodological idiosyncracies, frameworks and challenges of non-pharmaceutical and non-technical treatment interventions. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2013; 107 : 214-220.
Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, Shea B, Wells G, Helfand M. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013; 4 : 49-62.
Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, Ventresca M, Brignardello-Petersen R, Laisaar KT, Kowalski S, Baldeh T, Zhang Y, Raid U, Neumann I, Norris SL, Thornton J, Harbour R, Treweek S, Guyatt G, Alonso-Coello P, Reinap M, Brozek J, Oxman A, Akl EA. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ: Canadian Medical Association Journal 2014; 186 : E123-142.
Schünemann HJ. Interpreting GRADE's levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision? Journal of Clinical Epidemiology 2016; 75 : 6-15.
Smeeth L, Haines A, Ebrahim S. Numbers needed to treat derived from meta-analyses--sometimes informative, usually misleading. BMJ 1999; 318 : 1548-1551.
Sun X, Briel M, Busse JW, You JJ, Akl EA, Mejza F, Bala MM, Bassler D, Mertz D, Diaz-Granados N, Vandvik PO, Malaga G, Srinathan SK, Dahm P, Johnston BC, Alonso-Coello P, Hassouneh B, Walter SD, Heels-Ansdell D, Bhatnagar N, Altman DG, Guyatt GH. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ 2012; 344 : e1553.
Zhang Y, Akl EA, Schünemann HJ. Using systematic reviews in guideline development: the GRADE approach. Research Synthesis Methods 2018a: doi: 10.1002/jrsm.1313.
Zhang Y, Alonso-Coello P, Guyatt GH, Yepes-Nunez JJ, Akl EA, Hazlewood G, Pardo-Hernandez H, Etxeandia-Ikobaltzeta I, Qaseem A, Williams JW, Jr., Tugwell P, Flottorp S, Chang Y, Zhang Y, Mustafa RA, Rojas MX, Schünemann HJ. GRADE Guidelines: 19. Assessing the certainty of evidence in the importance of outcomes or values and preferences-Risk of bias and indirectness. Journal of Clinical Epidemiology 2018b: doi: 10.1016/j.jclinepi.2018.1001.1013.
Zhang Y, Alonso Coello P, Guyatt G, Yepes-Nunez JJ, Akl EA, Hazlewood G, Pardo-Hernandez H, Etxeandia-Ikobaltzeta I, Qaseem A, Williams JW, Jr., Tugwell P, Flottorp S, Chang Y, Zhang Y, Mustafa RA, Rojas MX, Xie F, Schünemann HJ. GRADE Guidelines: 20. Assessing the certainty of evidence in the importance of outcomes or values and preferences - Inconsistency, Imprecision, and other Domains. Journal of Clinical Epidemiology 2018c: doi: 10.1016/j.jclinepi.2018.1005.1011.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.
- Open access
- Published: 13 May 2024
Sexual and reproductive health implementation research in humanitarian contexts: a scoping review
- Alexandra Norton 1 &
- Hannah Tappis 2
Reproductive Health volume 21 , Article number: 64 ( 2024 ) Cite this article
85 Accesses
Metrics details
Meeting the health needs of crisis-affected populations is a growing challenge, with 339 million people globally in need of humanitarian assistance in 2023. Given one in four people living in humanitarian contexts are women and girls of reproductive age, sexual and reproductive health care is considered as essential health service and minimum standard for humanitarian response. Despite growing calls for increased investment in implementation research in humanitarian settings, guidance on appropriate methods and analytical frameworks is limited.
A scoping review was conducted to examine the extent to which implementation research frameworks have been used to evaluate sexual and reproductive health interventions in humanitarian settings. Peer-reviewed papers published from 2013 to 2022 were identified through relevant systematic reviews and a literature search of Pubmed, Embase, PsycInfo, CINAHL and Global Health databases. Papers that presented primary quantitative or qualitative data pertaining to a sexual and reproductive health intervention in a humanitarian setting were included.
Seven thousand thirty-six unique records were screened for inclusion, and 69 papers met inclusion criteria. Of these, six papers explicitly described the use of an implementation research framework, three citing use of the Consolidated Framework for Implementation Research. Three additional papers referenced other types of frameworks used in their evaluation. Factors cited across all included studies as helping the intervention in their presence or hindering in their absence were synthesized into the following Consolidated Framework for Implementation Research domains: Characteristics of Systems, Outer Setting, Inner Setting, Characteristics of Individuals, Intervention Characteristics, and Process.
This review found a wide range of methodologies and only six of 69 studies using an implementation research framework, highlighting an opportunity for standardization to better inform the evidence for and delivery of sexual and reproductive health interventions in humanitarian settings. Increased use of implementation research frameworks such as a modified Consolidated Framework for Implementation Research could work toward both expanding the evidence base and increasing standardization.
Plain English summary
Three hundred thirty-nine million people globally were in need of humanitarian assistance in 2023, and meeting the health needs of crisis-affected populations is a growing challenge. One in four people living in humanitarian contexts are women and girls of reproductive age, and provision of sexual and reproductive health care is considered to be essential within a humanitarian response. Implementation research can help to better understand how real-world contexts affect health improvement efforts. Despite growing calls for increased investment in implementation research in humanitarian settings, guidance on how best to do so is limited. This scoping review was conducted to examine the extent to which implementation research frameworks have been used to evaluate sexual and reproductive health interventions in humanitarian settings. Of 69 papers that met inclusion criteria for the review, six of them explicitly described the use of an implementation research framework. Three used the Consolidated Framework for Implementation Research, a theory-based framework that can guide implementation research. Three additional papers referenced other types of frameworks used in their evaluation. This review summarizes how factors relevant to different aspects of implementation within the included papers could have been organized using the Consolidated Framework for Implementation Research. The findings from this review highlight an opportunity for standardization to better inform the evidence for and delivery of sexual and reproductive health interventions in humanitarian settings. Increased use of implementation research frameworks such as a modified Consolidated Framework for Implementation Research could work toward both expanding the evidence base and increasing standardization.
Peer Review reports
Over the past few decades, the field of public health implementation research (IR) has grown as a means by which the real-world conditions affecting health improvement efforts can be better understood. Peters et al. put forward the following broad definition of IR for health: “IR is the scientific inquiry into questions concerning implementation – the act of carrying an intention into effect, which in health research can be policies, programmes, or individual practices (collectively called interventions)” [ 1 ].
As IR emphasizes real-world circumstances, the context within which a health intervention is delivered is a core consideration. However, much IR implemented to date has focused on higher-resource settings, with many proposed frameworks developed with particular utility for a higher-income setting [ 2 ]. In recognition of IR’s potential to increase evidence across a range of settings, there have been numerous reviews of the use of IR in lower-resource settings as well as calls for broader use [ 3 , 4 ]. There have also been more focused efforts to modify various approaches and frameworks to strengthen the relevance of IR to low- and middle-income country settings (LMICs), such as the work by Means et al. to adapt a specific IR framework for increased utility in LMICs [ 2 ].
Within LMIC settings, the centrality of context to a health intervention’s impact is of particular relevance in humanitarian settings, which present a set of distinct implementation challenges [ 5 ]. Humanitarian responses to crisis situations operate with limited resources, under potential security concerns, and often under pressure to relieve acute suffering and need [ 6 ]. Given these factors, successful implementation of a particular health intervention may require different qualities than those that optimize intervention impact under more stable circumstances [ 7 ]. Despite increasing recognition of the need for expanded evidence of health interventions in humanitarian settings, the evidence base remains limited [ 8 ]. Furthermore, despite its potential utility, there is not standardized guidance on IR in humanitarian settings, nor are there widely endorsed recommendations for the frameworks best suited to analyze implementation in these settings.
Sexual and reproductive health (SRH) is a core aspect of the health sector response in humanitarian settings [ 9 ]. Yet, progress in addressing SRH needs has lagged far behind other services because of challenges related to culture and ideology, financing constraints, lack of data and competing priorities [ 10 ]. The Minimum Initial Service Package (MISP) for SRH in Crisis Situations is the international standard for the minimum set of SRH services that should be implemented in all crisis situations [ 11 ]. However, as in other areas of health, there is need for expanded evidence for planning and implementation of SRH interventions in humanitarian settings. Recent systematic reviews of SRH in humanitarian settings have focused on the effectiveness of interventions and service delivery strategies, as well as factors affecting utilization, but have not detailed whether IR frameworks were used [ 12 , 13 , 14 , 15 ]. There have also been recent reviews examining IR frameworks used in various settings and research areas, but none have explicitly focused on humanitarian settings [ 2 , 16 ].
Given the need for an expanded evidence base for SRH interventions in humanitarian settings and the potential for IR to be used to expand the available evidence, a scoping review was undertaken. This scoping review sought to identify IR approaches that have been used in the last ten years to evaluate SRH interventions in humanitarian settings.
This review also sought to shed light on whether there is a need for a common framework to guide research design, analysis, and reporting for SRH interventions in humanitarian settings and if so, if there are any established frameworks already in use that would be fit-for-purpose or could be tailored to meet this need.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping reviews was utilized to guide the elements of this review [ 17 ]. The review protocol was retrospectively registered with the Open Science Framework ( https://osf.io/b5qtz ).
Search strategy
A two-fold search strategy was undertaken for this review, which covered the last 10 years (2013–2022). First, recent systematic reviews pertaining to research or evaluation of SRH interventions in humanitarian settings were identified through keyword searches on PubMed and Google Scholar. Four relevant systematic reviews were identified [ 12 , 13 , 14 , 15 ] Table 1 .
Second, a literature search mirroring these reviews was conducted to identify relevant papers published since the completion of searches for the most recent review (April 2017). Additional file 1 includes the search terms that were used in the literature search [see Additional file 1 ].
The literature search was conducted for papers published from April 2017 to December 2022 in the databases that were searched in one or more of the systematic reviews: PubMed, Embase, PsycInfo, CINAHL and Global Health. Searches were completed in January 2023 Table 2 .
Two reviewers screened each identified study for alignment with inclusion criteria. Studies in the four systematic reviews identified were considered potentially eligible if published during the last 10 years. These papers then underwent full-text review to confirm satisfaction of all inclusion criteria, as inclusion criteria were similar but not fully aligned across the four reviews.
Literature search results were exported into a citation manager (Covidence), duplicates were removed, and a step-wise screening process for inclusion was applied. First, all papers underwent title and abstract screening. The remaining papers after abstract screening then underwent full-text review to confirm satisfaction of all inclusion criteria. Title and abstract screening as well as full-text review was conducted independently by both authors; disagreements after full-text review were resolved by consensus.
Data extraction and synthesis
The following content areas were summarized in Microsoft Excel for each paper that met inclusion criteria: publication details including author, year, country, setting [rural, urban, camp, settlement], population [refugees, internally displaced persons, general crisis-affected], crisis type [armed conflict, natural disaster], crisis stage [acute, chronic], study design, research methods, SRH intervention, and intervention target population [specific beneficiaries of the intervention within the broader population]; the use of an IR framework; details regarding the IR framework, how it was used, and any rationale given for the framework used; factors cited as impacting SRH interventions, either positively or negatively; and other key findings deemed relevant to this review.
As the focus of this review was on the approach taken for SRH intervention research and evaluation, the quality of the studies themselves was not assessed.
Twenty papers underwent full-text review due to their inclusion in one or more of the four systematic reviews and meeting publication date inclusion criteria. The literature search identified 7,016 unique papers. After full-text screening, 69 met all inclusion criteria and were included in the review. Figure 1 illustrates the search strategy and screening process.
Flow chart of paper identification
Papers published in each of the 10 years of the review timeframe (2013–2022) were included. 29% of the papers originated from the first five years of the time frame considered for this review, with the remaining 71% papers coming from the second half. Characteristics of included publications, including geographic location, type of humanitarian crisis, and type of SRH intervention, are presented in Table 3 .
A wide range of study designs and methods were used across the papers, with both qualitative and quantitative studies well represented. Twenty-six papers were quantitative evaluations [ 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 ], 17 were qualitative [ 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 ], and 26 used mixed methods [ 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 ]. Within the quantitative evaluations, 15 were observational, while five were quasi-experimental, five were randomized controlled trials, and one was an economic evaluation. Study designs as classified by the authors of this review are summarized in Table 4 .
Six papers (9%) explicitly cited use of an IR framework. Three of these papers utilized the Consolidated Framework for Implementation Research (CFIR) [ 51 , 65 , 70 ]. The CFIR is a commonly used determinant framework that—in its originally proposed form in 2009—is comprised of five domains, each of which has constructs to further categorize factors that impact implementation. The CFIR domains were identified as core content areas influencing the effectiveness of implementation, and the constructs within each domain are intended to provide a range of options for researchers to select from to “guide diagnostic assessments of implementation context, evaluate implementation progress, and help explain findings.” [ 87 ] To allow for consistent terminology throughout this review, the original 2009 CFIR domains and constructs are used.
Guan et al. conducted a mixed methods study to assess the feasibility and effectiveness of a neonatal hepatitis B immunization program in a conflict-affected rural region of Myanmar. Guan et al. report mapping data onto the CFIR as a secondary analysis step. They describe that “CFIR was used as a comprehensive meta-theoretical framework to examine the implementation of the Hepatitis B Virus vaccination program,” and implementation themes from multiple study data sources (interviews, observations, examination of monitoring materials) were mapped onto CFIR constructs. They report their results in two phases – Pre-implementation training and community education, and Implementation – with both anchored in themes that they had mapped onto CFIR domains and constructs. All but six constructs were included in their analysis, with a majority summarized in a table and key themes explored further in the narrative text. They specify that most concerns were identified within the Outer Setting and Process domains, while elements identified within the Inner Setting domain provided strength to the intervention and helped mitigate against barriers [ 70 ].
Sarker et al. conducted a qualitative study to assess provision of maternal, newborn and child health services to Rohingya refugees residing in camps in Cox’s Bazar, Bangladesh. They cite using CFIR as a guide for thematic analysis, applying it after a process of inductive and deductive coding to index these codes into the CFIR domains. They utilized three of the five CFIR domains (Outer Setting, Inner Setting, and Process), stating that the remaining two domains (Intervention Characteristics and Characteristics of Individuals) were not relevant to their analysis. They then proposed two additional CFIR domains, Context and Security, for use in humanitarian contexts. In contrast to Guan et al., CFIR constructs are not used nor mentioned by Sarker et al., with content under each domain instead synthesized as challenges and potential solutions. Regarding the CFIR, Sarker et al. write, “The CFIR guided us for interpretative coding and creating the challenges and possible solutions into groups for further clarification of the issues related to program delivery in a humanitarian crisis setting.” [ 51 ]
Sami et al. conducted a mixed methods case study to assess the implementation of a package of neonatal interventions at health facilities within refugee and internally displaced persons camps in South Sudan. They reference use of the CFIR earlier in the study than Sarker et al., basing their guides for semi-structured focus group discussions on the CFIR framework. They similarly reference a general use of the CFIR framework as they conducted thematic analysis. Constructs are referenced once, but they do not specify whether their application of the CFIR framework included use of domains, constructs, or both. This may be in part because they then applied an additional framework, the World Health Organization (WHO) Health System Framework, to present their findings. They describe a nested approach to their use of these frameworks: “Exploring these [CFIR] constructs within the WHO Health Systems Framework can identify specific entry points to improve the implementation of newborn interventions at critical health system building blocks.” [ 65 ]
Three papers cite use of different IR frameworks. Bolan et al. utilized the Theoretical Domains Framework in their mixed methods feasibility study and pilot cluster randomized trial evaluating pilot use of the Safe Delivery App by maternal and newborn health workers providing basic emergency obstetric and newborn care in facilities in the conflict-affected Maniema province of the Democratic Republic of the Congo (DRC). They used the Theroetical Domains Framework in designing interview questions, and further used it as the coding framework for their analysis. Similar to the CFIR, the Theoretical Domains Framework is a determinant framework that consists of domains, each of which then includes constructs. Bolan et al. utilized the Theoretical Domains Framework at the construct level in interview question development and at the domain level in their analysis, mapping interview responses to eight of the 14 domains [ 83 ]. Berg et al. report using an “exploratory design guided by the principles of an evaluation framework” developed by the Medical Research Council to analyze the implementation process, mechanisms of impact, and outcomes of a three-pillar training intervention to improve maternal and neonatal healthcare in the conflict-affected South Kivu province of the DRC [ 67 , 88 ]. Select components of this evaluation framework were used to guide deductive analysis of focus group discussions and in-depth interviews [ 67 ]. In their study of health workers’ knowledge and attitudes toward newborn health interventions in South Sudan, before and after training and supply provision, Sami et al. report use of the Conceptual Framework of the Role of Attitudes in Evidence-Based Practice Implementation in their analysis process. The framework was used to group codes following initial inductive coding analysis of in-depth interviews [ 72 ].
Three other papers cite use of specific frameworks in their intervention evaluation [ 19 , 44 , 76 ]. As a characteristic of IR is the use of an explicit framework to guide the research, the use of the frameworks in these three papers meets the intention of IR and serves the purpose that an IR framework would have in strengthening the analytical rigor. Castle et al. cite use of their program’s theory of change as a framework for a mixed methods evaluation of the provision of family planning services and more specifically uptake of long-acting reversible contraception use in the DRC. They describe use of the theory of change to “enhance effectiveness of [long-acting reversible contraception] access and uptake.” [ 76 ] Thommesen et al. cite use of the AAAQ (Availability, Accessibility, Acceptability and Quality) framework in their qualitative study assessing midwifery services provided to pregnant women in Afghanistan. This framework is focused on the “underlying elements needed for attainment of optimum standard of health care,” but the authors used it in this paper to evaluate facilitators and barriers to women accessing midwifery services [ 44 ]. Jarrett et al. cite use of the Centers for Disease Control and Prevention’s (CDC) Guidelines for Evaluating Public Health Surveillance Systems to explore the characteristics of a population mobility, mortality and birth surveillance system in South Kivu, DRC. Use of these CDC guidelines is cited as one of four study objectives, and commentary is included in the Results section pertaining to each criteria within these guidelines, although more detail regarding use of these guidelines or the authors’ experience with their use in the study is not provided [ 19 ].
Overall, 22 of the 69 papers either explicitly or implicitly identified IR as relevant to their work. Nineteen papers include a focus on feasibility (seven of which did not otherwise identify the importance of exploring questions concerning implementation), touching on a common outcome of interest in implementation research [ 89 ].
While a majority of papers did not explicitly or implicitly use an IR framework to evaluate their SRH intervention of focus, most identified factors that facilitated implementation when they were present or served as a barrier when absent. Sixty cite factors that served as facilitators and 49 cite factors that served as barriers, with just three not citing either. Fifty-nine distinct factors were identified across the papers.
Three of the six studies that explicitly used an IR framework used the CFIR, and the CFIR is the only IR framework that was used by multiple studies. As previously mentioned, Means et al. put forth an adaptation of the CFIR to increase its relevance in LMIC settings, proposing a sixth domain (Characteristics of Systems) and 11 additional constructs [ 2 ]. Using the expanded domains and constructs as proposed by Means et al., the 59 factors cited by papers in this review were thematically grouped into the six domains: Characteristics of Systems, Outer Setting, Inner Setting, Characteristics of Individuals, Intervention Characteristics, and Process. Within each domain, alignment with CFIR constructs was assessed for, and alignment was found with 29 constructs: eight of Means et al.’s 11 constructs, and 21 of the 39 standard CFIR constructs. Three factors did not align with any construct (all fitting within the Outer Setting domain), and 14 aligned with a construct label but not the associated definition. Table 5 synthesizes the mapping of factors affecting SRH intervention implementation to CFIR domains and constructs, with the construct appearing in italics if it is considered to align with that factor by label but not by definition.
Table 6 lists the CFIR constructs that were not found to have alignment with any factor cited by the papers in this review.
This scoping review sought to assess how IR frameworks have been used to bolster the evidence base for SRH interventions in humanitarian settings, and it revealed that IR frameworks, or an explicit IR approach, are rarely used. All four of the systematic reviews identified with a focus on SRH in humanitarian settings articulate the need for more research examining the effectiveness of SRH interventions in humanitarian settings, with two specifically citing a need for implementation research/science [ 12 , 13 ]. The distribution of papers across the timeframe included in this review does suggest that more research on SRH interventions for crisis-affected populations is taking place, as a majority of relevant papers were published in the second half of the review period. The papers included a wide range of methodologies, which reflect the differing research questions and contexts being evaluated. However, it also invites the question of whether there should be more standardization of outcomes measured or frameworks used to guide analysis and to facilitate increased comparison, synthesis and application across settings.
Three of the six papers that used an IR framework utilized the CFIR. Guan et al. used the CFIR at both a domain and construct level, Sarker et al. used the CFIR at the domain level, and Sami et al. did not specify which CFIR elements were used in informing the focus group discussion guide [ 51 , 65 , 70 ]. It is challenging to draw strong conclusions about the applicability of CFIR in humanitarian settings based on the minimal use of CFIR and IR frameworks within the papers reviewed, although Guan et al. provides a helpful model for how analysis can be structured around CFIR domains and constructs. It is worth considering that the minimal use of IR frameworks, and more specifically CFIR constructs, could be in part because that level of prescriptive categorization does not allow for enough fluidity in humanitarian settings. It also raises questions about the appropriate degree of standardization to pursue for research done in these settings.
The mapping of factors affecting SRH intervention implementation provides an example of how a modified CFIR framework could be used for IR in humanitarian contexts. This mapping exercise found factors that mapped to all five of the original CFIR domains as well as the sixth domain proposed by Means et al. All factors fit well within the definition for the selected domain, indicating an appropriate degree of fit between these existing domains and the factors identified as impacting SRH interventions in humanitarian settings. On a construct level, however, the findings were more variable, with one-quarter of factors not fully aligning with any construct. Furthermore, over 40% of the CFIR constructs (including the additional constructs from Means et al.) were not found to align with any factors cited by the papers in this review, also demonstrating some disconnect between the parameters posed by the CFIR constructs and the factors cited as relevant in a humanitarian context.
It is worth noting that while the CFIR as proposed in 2009 was used in this assessment, as well as in the included papers which used the CFIR, an update was published in 2022. Following a review of CFIR use since its publication, the authors provide updates to construct names and definitions to “make the framework more applicable across a range of innovations and settings.” New constructs and subconstructs were also added, for a total of 48 constructs and 19 subconstructs across the five domains [ 90 ]. A CFIR Outcomes Addendum was also published in 2022, based on recommendations for the CFIR to add outcomes and intended to be used as a complement to the CFIR determinants framework [ 91 ]. These expansions to the CFIR framework may improve applicability of the CFIR in humanitarian settings. Several constructs added to the Outer Setting domain could be of particular utility – critical incidents, local attitudes, and local conditions, each of which could help account for unique challenges faced in contexts of crisis. Sub-constructs added within the Inner Setting domain that seek to clarify structural characteristics and available resources would also be of high utility based on mapping of the factors identified in this review to the original CFIR constructs. As outcomes were not formally included in the CFIR until the 2022 addendum, a separate assessment of implementation outcomes was not undertaken in this review. However, analysis of the factors cited by papers in this review as affecting implementation was derived from the full text of the papers and thus captures content relevant to implementation determinants that is contained within the outcomes.
Given the demonstrated need for additional flexibility within an IR framework for humanitarian contexts, while not a focus of this review, it is worth considering whether a different framework could provide a better fit than the CFIR. Other frameworks have differing points of emphasis that would create different opportunities for flexibility but that do not seem to resolve the challenges experienced in applying the CFIR to a humanitarian context. As one example, the EPIS (Exploration, Preparation, Implementation, Sustainment) Framework considers the impact of inner and outer context on each of four implementation phases; while the constructs within this framework are broader than the CFIR, an emphasis on the intervention characteristics is missing, a domain where stronger alignment within the CFIR is also needed [ 92 ]. Alternatively, the PRISM (Practical, Robust Implementation and Sustainability Model) framework is a determinant and evaluation framework that adds consideration of context factors to the RE-AIM (Reach, Effectiveness, Adoption, Implementation, Maintenance) outcomes framework. It has a stronger emphasis on intervention aspects, with sub-domains to account for both organization and patient perspectives within the intervention. While PRISM does include aspects of context, external environment considerations are less robust and intentionally less comprehensive in scope, which would not provide the degree of alignment possible between the Characteristics of Systems and Outer Setting CFIR domains for the considerations unique to humanitarian environments [ 93 ].
Reflecting on their experience with the CFIR, Sarker et al. indicate that it can be a “great asset” in both evaluating current work and developing future interventions. They also encourage future research of humanitarian health interventions to utilize the CFIR [ 51 ]. The other papers that used the CFIR do not specifically reflect on their experience utilizing it, referring more generally to having felt that it was a useful tool [ 65 , 70 ]. On their use of an evaluation framework, Berg et al. reflected that it lent useful structure and helped to identify aspects affecting implementation that otherwise would have gone un-noticed [ 67 ]. The remaining studies that utilized an IR framework did not specifically comment on their experience with its use [ 72 , 83 ]. While a formal IR framework was not engaged by other studies, a number cite a desire for IR to contribute further detail to their findings [ 21 , 37 ].
In their recommendations for strengthening the evidence base for humanitarian health interventions, Ager et al. speak to the need for “methodologic innovation” to develop methodologies with particular applicability in humanitarian settings [ 7 ]. As IR is not yet routinized for SRH interventions, this could be opportune timing for the use of a standardized IR framework to gauge its utility. Using an IR framework to assess factors influencing implementation of the MISP in initial stages of a humanitarian response, and interventions to support more comprehensive SRH service delivery in protracted crises, could lend further rigor and standardization to SRH evaluations, as well as inform strategies to improve MISP implementation over time. Based on categorizing factors identified by these papers as relevant for intervention evaluation, there does seem to be utility to a modified CFIR approach. Given the paucity of formal IR framework use within SRH literature, it would be worth conducting similar scoping exercises to assess for explicit use of IR frameworks within the evidence base for other health service delivery areas in humanitarian settings. In the interim, the recommended approach from this review for future IR on humanitarian health interventions would be a modified CFIR approach with domain-level standardization and flexibility for constructs that may standardize over time with more use. This would enable use of a common analytical framework and vocabulary at the domain level for stakeholders to describe interventions and the factors influencing the effectiveness of implementation, with constructs available to use and customize as most appropriate for specific contexts and interventions.
This review had a number of limitations. As this was a scoping review and a two-part search strategy was used, the papers summarized here may not be comprehensive of those written pertaining to SRH interventions over the past 10 years. Papers from 2013 to 2017 that would have met this scoping review’s inclusion criteria may have been omitted due to being excluded from the systematic reviews. The review was limited to papers available in English. Furthermore, this review did not assess the quality of the papers included or seek to assess the methodology used beyond examination of the use of an IR framework. It does, however, serve as a first step in assessing the extent to which calls for implementation research have been addressed, and identify entry points for strengthening the science and practice of SRH research in humanitarian settings.
With one in 23 people worldwide in need of humanitarian assistance, and financing required for response plans at an all-time high, the need for evidence to guide resource allocation and programming for SRH in humanitarian settings is as important as ever [ 94 ]. Recent research agenda setting initiatives and strategies to advance health in humanitarian settings call for increased investment in implementation research—with priorities ranging from research on effective strategies for expanding access to a full range of contraceptive options to integrating mental health and psychosocial support into SRH programming to capturing accurate and actionable data on maternal and perinatal mortality in a wide range of acute and protracted emergency contexts [ 95 , 96 ]. To truly advance guidance in these areas, implementation research will need to be conducted across diverse humanitarian settings, with clear and consistent documentation of both intervention characteristics and outcomes, as well as contextual and programmatic factors affecting implementation.
Conclusions
Implementation research has potential to increase impact of health interventions particularly in crisis-affected settings where flexibility, adaptability and context-responsive approaches are highlighted as cornerstones of effective programming. There remains significant opportunity for standardization of research in the humanitarian space, with one such opportunity occurring through increased utilization of IR frameworks such as a modified CFIR approach. Investing in more robust sexual and reproductive health research in humanitarian contexts can enrich insights available to guide programming and increase transferability of learning across settings.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Availability, Accessibility, Acceptability and Quality
Centers for Disease Control and Prevention
Consolidated Framework for Implementation Research
Democratic Republic of the Congo
Exploration, Preparation, Implementation, Sustainment
- Implementation research
Low and middle income country
Minimum Initial Service Package
Practical, Robust Implementation and Sustainability Model
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Reach, Effectiveness, Adoption, Implementation, Maintenance
- Sexual and reproductive health
World Health Organization
Peters DH, et al. Implementation research: what it is and how to do it. RESEARCH METHODS. 2013;347:7.
Means AR, et al. Evaluating and optimizing the consolidated framework for implementation research (CFIR) for use in low- and middle-income countries: a systematic review. Implement Sci. 2020;15(1):17.
Article PubMed PubMed Central Google Scholar
Alonge O, et al. How is implementation research applied to advance health in low-income and middle-income countries? BMJ Glob Health. 2019;4(2):e001257.
Ridde V, Pérez D, Robert E. Using implementation science theories and frameworks in global health. BMJ Glob Health. 2020;5(4):e002269.
Gaffey MF, et al. Delivering health and nutrition interventions for women and children in different conflict contexts: a framework for decision making on what, when, and how. Lancet (London, England). 2021;397(10273):543–54.
Article PubMed Google Scholar
Singh NS, et al. Delivering health interventions to women, children, and adolescents in conflict settings: what have we learned from ten country case studies? The Lancet. 2021;397(10273):533–42.
Article Google Scholar
Ager A, et al. Strengthening the evidence base for health programming in humanitarian crises. Science. 2014;345(6202):1290–2.
Article CAS PubMed Google Scholar
Blanchet K, et al. Evidence on public health interventions in humanitarian crises. The Lancet. 2017;390(10109):2287–96.
Sphere A. The Sphere Handbook | Standards for quality humanitarian response. 2018.
Google Scholar
Barot S. In a State of Crisis: Meeting the Sexual and Reproductive Health Needs of Women in Humanitarian Situations. Guttmacher Policy Rev. 2017;20:7.
Crisis, I.-A.W.G.f.R.H.i., Minimum Initial Service Package. 2020: https://www.unfpa.org/resources/minimum-initial-service-package-misp-srh-crisis-situations .
Casey SE. Evaluations of reproductive health programs in humanitarian settings: a systematic review. Confl Heal. 2015;9(1):S1.
Singh NS, et al. A long way to go: a systematic review to assess the utilisation of sexual and reproductive health services during humanitarian crises. BMJ Glob Health. 2018;3(2):e000682.
Singh NS, et al. Evaluating the effectiveness of sexual and reproductive health services during humanitarian crises: A systematic review. PLoS ONE. 2018;13(7):e0199300.
Warren E, et al. Systematic review of the evidence on the effectiveness of sexual and reproductive health interventions in humanitarian crises. BMJ Open. 2015;5(12):e008226.
Dadich A, Piper A, Coates D. Implementation science in maternity care: a scoping review. Implement Sci. 2021;16(1):16.
Tricco AC, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73.
Devine A, et al. Strategies for the prevention of perinatal hepatitis B transmission in a marginalized population on the Thailand-Myanmar border: a cost-effectiveness analysis. BMC Infect Dis. 2017;17(1):552.
Jarrett P, et al. Evaluation of a population mobility, mortality, and birth surveillance system in South Kivu. Democratic Republic of the Congo Disasters. 2020;44(2):390–407.
PubMed Google Scholar
Logie CH, et al. A Psycho-Educational HIV/STI Prevention Intervention for Internally Displaced Women in Leogane, Haiti: Results from a Non-Randomized Cohort Pilot Study. PLoS ONE. 2014;9(2):e89836.
O’Laughlin KN, et al. A cohort study to assess a communication intervention to improve linkage to HIV care in Nakivale Refugee Settlement. Uganda Glob Public Health. 2021;16(12):1848–55.
Adam I. The influence of maternal health education on the place of delivery in conflict settings of Darfur. Sudan Conflict and Health. 2015;9:31.
Adam IF, et al. Relationship between implementing interpersonal communication and mass education campaigns in emergency settings and use of reproductive healthcare services: evidence from Darfur, Sudan. BMJ Open. 2015;5(9):e008285.
Edmond K, et al. Mobile outreach health services for mothers and children in conflict-affected and remote areas: a population-based study from Afghanistan. Arch Dis Child. 2020;105(1):18–25.
Nasir S, et al. Dissemination and implementation of the e-MCHHandbook, UNRWA’s newly released maternal and child health mobile application: a cross-sectional study. BMJ Open. 2020;10(3):e034885.
O’Laughlin KN, et al. Feasibility and acceptability of home-based HIV testing among refugees: a pilot study in Nakivale refugee settlement in southwestern Uganda. BMC Infect Dis. 2018;18(1):332.
Adam I. Evidence from cluster surveys on the association between home-based counseling and use of family planning in conflict-affected Darfur. Int J Gynecol Obstet. 2016;133(2):221–5.
Casey S, et al. Availability of long-acting and permanent family-planning methods leads to increase in use in conflict-affected northern Uganda: Evidence from cross-sectional baseline and endline cluster surveys. Glob Public Health. 2013;8(3):284–97.
Corna F, et al. Supporting maternal mental health of Rohingya refugee women during the perinatal period to promote child health and wellbeing: a field study in Cox’s Bazar. Intervention, the Journal of Mental Health & Psychosocial Support in Conflict Affected Areas. 2019;17(2):160–8.
Glass N, et al. Effectiveness of the Communities Care programme on change in social norms associated with gender-based violence (GBV) with residents in intervention compared with control districts in Mogadishu, Somalia. BMJ Open. 2019;9(3):e023819.
James LE, et al. Development and Testing of a Community-Based Intervention to Address Intimate Partner Violence among Rohingya and Syrian Refugees: A Social Norms-Based Mental Health-Integrated Approach. Int J Environ Res Public Health. 2021;18(21):11674.
Le Roux E, et al. Engaging with faith groups to prevent VAWG in conflict-affected communities: results from two community surveys in the DRC. BMC Int Health Hum Rights. 2020;20(1):27.
Morris CN, et al. When political solutions for acute conflict in Yemen seem distant, demand for reproductive health services is immediate: a programme model for resilient family planning and post-abortion care services. Sex Reprod Health Matters. 2019;27(2):1610279.
Anibueze AU, et al. Impact of counseling visual multimedia on use of family planning methods among displaced Nigerian families. Health Promot Int. 2022;37(3):daac060.
Doocy S, et al. Cash-based assistance and the nutrition status of pregnant and lactating women in the Somalia food crisis: A comparison of two transfer modalities. PLoS ONE. 2020;15(4):e0230989.
Article CAS PubMed PubMed Central Google Scholar
Draiko CV, et al. The effect of umbilical cord cleansing with chlorhexidine gel on neonatal mortality among the community births in South Sudan: a quasi-experimental study. Pan Afr Med J. 2021;38:78.
Edmond KM, et al. Can community health worker home visiting improve care-seeking and maternal and newborn care practices in fragile states such as Afghanistan? A population-based intervention study. BMC Med. 2018;16(1):106.
Edmond KM, et al. Conditional cash transfers to improve use of health facilities by mothers and newborns in conflict affected countries, a prospective population based intervention study from Afghanistan. BMC Pregnancy Childbirth. 2019;19(1):193.
Bakesiima R, et al. Effect of peer counselling on acceptance of modern contraceptives among female refugee adolescents in northern Uganda: A randomised controlled trial. PLoS ONE. 2021;16(9):e0256479.
Greene MC, et al. Evaluation of an integrated intervention to reduce psychological distress and intimate partner violence in refugees: Results from the Nguvu cluster randomized feasibility trial. PLoS ONE. 2021;16(6):e0252982.
Gupta J, et al. Gender norms and economic empowerment intervention to reduce intimate partner violence against women in rural Côte d’Ivoire: a randomized controlled pilot study. BMC Int Health Hum Rights. 2013;13(1):46.
Hossain M, et al. Working with men to prevent intimate partner violence in a conflict-affected setting: a pilot cluster randomized controlled trial in rural Côte d’Ivoire. BMC Public Health. 2014;14(1):339.
Vaillant J, et al. Engaging men to transform inequitable gender attitudes and prevent intimate partner violence: a cluster randomised controlled trial in North and South Kivu, Democratic Republic of Congo. BMJ Glob Health. 2020;5(5):e002223.
Thommesen T, et al. “The midwife helped me … otherwise I could have died”: women’s experience of professional midwifery services in rural Afghanistan - a qualitative study in the provinces Kunar and Laghman. BMC Pregnancy Childbirth. 2020;20(1):140.
Awasom-Fru A, et al. Doctors’ experiences providing sexual and reproductive health care at Catholic Hospitals in the conflict-affected North-West region of Cameroon: a qualitative study. Reprod Health. 2022;19(1):126.
Kabakian-Khasholian T, Makhoul J, Ghusayni A. “A person who does not have money does not enter”: a qualitative study on refugee women’s experiences of respectful maternity care. BMC Pregnancy and Childbirth. 2022;22(1):748.
Lilleston P, et al. Evaluation of a mobile approach to gender-based violence service delivery among Syrian refugees in Lebanon. Health Policy Plan. 2018;33(7):767–76.
Mugo NS, et al. Barriers Faced by the Health Workers to Deliver Maternal Care Services and Their Perceptions of the Factors Preventing Their Clients from Receiving the Services: A Qualitative Study in South Sudan. Matern Child Health J. 2018;22(11):1598–606.
Persson M, et al. A qualitative study on health care providers’ experiences of providing comprehensive abortion care in Cox’s Bazar, Bangladesh. Conflict and Health. 2021;15(1):6.
Phanwichatkul T, et al. The perceptions and practices of Thai health professionals providing maternity care for migrant Burmese women: An ethnographic study. Women Birth. 2022;35(4):e356–68.
Sarker M, et al. Effective maternal, newborn and child health programming among Rohingya refugees in Cox’s Bazar, Bangladesh: Implementation challenges and potential solutions. PLoS ONE. 2020;15(3):e0230732.
Tousaw E, et al. “Without this program, women can lose their lives”: migrant women’s experiences with the Safe Abortion Referral Programme in Chiang Mai. Thailand Reprod Health Matters. 2017;25(51):58–68.
Tousaw E, et al. “It is just like having a period with back pain”: exploring women’s experiences with community-based distribution of misoprostol for early abortion on the Thailand-Burma border. Contraception. 2018;97(2):122–9.
West L, et al. Factors in use of family planning services by Syrian women in a refugee camp in Jordan. Journal of Family Planning and Reproductive Health Care. 2017;43(2):96–102.
O’Connell KA, et al. Meeting the Sexual and Reproductive Health Needs of Internally Displaced Persons in Ethiopia’s Somali Region: A Qualitative Process Evaluation. Glob Health Sci Pract. 2022;10(5):e2100818.
Orya E, et al. Strengthening close to community provision of maternal health services in fragile settings: an exploration of the changing roles of TBAs in Sierra Leone and Somaliland. BMC Health Serv Res. 2017;17(1):460.
Perera SM, et al. Barriers to seeking post-abortion care in Paktika Province, Afghanistan: a qualitative study of clients and community members. BMC Womens Health. 2021;21(1):390.
Tanabe M, et al. Piloting community-based medical care for survivors of sexual assault in conflict-affected Karen State of eastern Burma. Confl Heal. 2013;7(1):12.
Tran NT, et al. Clinical outreach refresher trainings in crisis settings (S-CORT): clinical management of sexual violence survivors and manual vacuum aspiration in Burkina Faso, Nepal, and South Sudan. Reprod Health Matters. 2017;25(51):103–13.
Yankah E, et al. Feasibility and acceptability of mobile phone platforms to deliver interventions to address gender-based violence among Syrian adolescent girls and young women in Izmir. Turkey Vulnerable Children and Youth Studies. 2020;15(2):133–43.
Muuo S, et al. Barriers and facilitators to care-seeking among survivors of gender-based violence in the Dadaab refugee complex. Sex Reprod Health Matters. 2020;28(1):1722404.
Amsalu R, et al. Essential newborn care practice at four primary health facilities in conflict affected areas of Bossaso, Somalia: a cross-sectional study. Conflict and Health. 2019;13(13):27.
Myers A, et al. Facilitators and barriers in implementing the Minimum Initial Services Package (MISP) for reproductive health in Nepal post-earthquake. Conflict and Health. 2018;12:35.
Santo L.C.d, et al. Feasibility and acceptability of a video library tool to support community health worker counseling in rural Afghan districts: a cross-sectional assessment. Conflict and Health. 2020;14:56.
Sami S, et al. Understanding health systems to improve community and facility level newborn care among displaced populations in South Sudan: a mixed methods case study. BMC Pregnancy Childbirth. 2018;18(1):325.
Amsalu R, et al. Effectiveness of clinical training on improving essential newborn care practices in Bossaso, Somalia: a pre and postintervention study. BMC Pediatr. 2020;20(1):215.
Berg M, Mwambali SN, Bogren M. Implementation of a three-pillar training intervention to improve maternal and neonatal healthcare in the Democratic Republic Of Congo: a process evaluation study in an urban health zone. Glob Health Action. 2022;15(1):2019391.
Castillo M, et al. Turning Disaster into an Opportunity for Quality Improvement in Essential Intrapartum and Newborn Care Services in the Philippines: Pre- to Posttraining Assessments. Biomed Res Int. 2016;2016:1–9.
Foster AM, Arnott G, Hobstetter M. Community-based distribution of misoprostol for early abortion: evaluation of a program along the Thailand-Burma border. Contraception. 2017;96(4):242–7.
Guan TH, et al. Implementation of a neonatal hepatitis B immunization program in rural Karenni State, Myanmar: A mixed-methods study. PLoS ONE. 2021;16(12):e0261470.
Logie, C.H., et al., Mixed-methods findings from the Ngutulu Kagwero (agents of change) participatory comic pilot study on post-rape clinical care and sexual violence prevention with refugee youth in a humanitarian setting in Uganda. Global Public Health, 2022((Logie C.H., [email protected]) Factor-Inwentash Faculty of Social Work, University of Toronto, Toronto, Canada(Logie C.H., [email protected]) Women’s College Research Institute, Women’s College Hospital, Toronto, Canada(Logie C.H., carmen.l).
Sami S, et al. “You have to take action”: changing knowledge and attitudes towards newborn care practices during crisis in South Sudan. Reprod Health Matters. 2017;25(51):124–39.
Smith JR, et al. Clinical care for sexual assault survivors multimedia training: a mixed-methods study of effect on healthcare providers’ attitudes, knowledge, confidence, and practice in humanitarian settings. Confl Heal. 2013;7(1):14.
Stevens A, et al. Folate supplementation to prevent birth abnormalities: evaluating a community-based participatory action plan for refugees and migrant workers on the Thailand-Myanmar border. Public Health. 2018;161:83–9.
Nguyen Toan T, et al. Strengthening healthcare providers’ capacity for safe abortion and postabortion care services in humanitarian settings: lessons learned from the clinical outreach refresher training model (S-CORT) in Uganda, Nigeria, and the Democratic Republic of Congo. Conflict and Health. 2021;15(1):20.
Castle S, et al. Successful programmatic approaches to facilitating IUD uptake: CARE’s experience in DRC. BMC Womens Health. 2019;19(1):104.
Deitch J, et al. “They Love Their Patients”: Client Perceptions of Quality of Postabortion Care in North and South Kivu, the Democratic Republic of the Congo. Global health, science and practice. 2019;7(Suppl 2):S285–98.
Ferreyra C, et al. Evaluation of a community-based HIV test and start program in a conflict affected rural area of Yambio County, South Sudan. PLoS ONE. 2021;16(7):e0254331.
Ho LS, Wheeler E. Using Program Data to Improve Access to Family Planning and Enhance the Method Mix in Conflict-Affected Areas of the Democratic Republic of the Congo. Glob Health Sci Pract. 2018;6(1):161–77.
Klabbers RE, et al. Health Worker Perspectives on Barriers and Facilitators of Assisted Partner Notification for HIV for Refugees and Ugandan Nationals: A Mixed Methods Study in West Nile Uganda. AIDS Behav. 2021;25(10):3206–22.
Turner C, et al. Neonatal Intensive Care in a Karen Refugee Camp: A 4 Year Descriptive Study. PLoS ONE. 2013;8(8):e72721.
Vries Id, et al. Key lessons from a mixed-method evaluation of a postnatal home visit programme in the humanitarian setting of Gaza. Eastern Mediterr Health J. 2021;27(6):546–52.
Bolan NE, et al. mLearning in the Democratic Republic of the Congo: A Mixed-Methods Feasibility and Pilot Cluster Randomized Trial Using the Safe Delivery App. Global health, science and practice. 2018;6(4):693–710.
Khan MN, et al. Evaluating feasibility and acceptability of a local psycho-educational intervention for pregnant women with common mental problems affected by armed conflict in Swat, Pakistan: A parallel randomized controlled feasibility trial. Int J Soc Psychiatry. 2017;63(8):724–35.
Hynes M, et al. Using a quality improvement approach to improve maternal and neonatal care in North Kivu, Democratic Republic of Congo. Reprod Health Matters. 2017;25(51):140–50.
Gibbs A, et al. The impacts of combined social and economic empowerment training on intimate partner violence, depression, gender norms and livelihoods among women: an individually randomised controlled trial and qualitative study in Afghanistan. BMJ Glob Health. 2020;5(3):e001946.
Damschroder L, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation science: IS; 2009.
Moore GF, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015;350:h1258.
Proctor E, et al. Outcomes for Implementation Research: Conceptual Distinctions, Measurement Challenges, and Research Agenda. Adm Policy Ment Health. 2011;38(2):65–76.
Damschroder LJ, et al. The updated Consolidated Framework for Implementation Research based on user feedback. Implement Sci. 2022;17(1):75.
Damschroder LJ, et al. Conceptualizing outcomes for use with the Consolidated Framework for Implementation Research (CFIR): the CFIR Outcomes Addendum. Implement Sci. 2022;17(1):7.
Aarons GA, Hurlburt M, Horwitz SM. Advancing a Conceptual Model of Evidence-Based Practice Implementation in Public Service Sectors. Administration and Policy in Mental Health and Mental Health Services Research. 2011;38(1):4–23.
Feldstein AC, Glasgow RE. A Practical, Robust Implementation and Sustainability Model (PRISM) for Integrating Research Findings into Practice. The Joint Commission Journal on Quality and Patient Safety. 2008;34(4):228–43.
OCHA. Global Humanitarian Overview 2023. 2022 [cited 2023 8/3/2023]; Available from: https://humanitarianaction.info/node/13073/article/glance-0 . Accessed 8 Mar 2023.
Kobeissi L, et al. Setting research priorities for sexual, reproductive, maternal, newborn, child and adolescent health in humanitarian settings. Confl Heal. 2021;15(1):16.
Save the, C., et al. Roadmap to Accelerate Progress for Every Newborn in Humanitarian Settings 2020 – 2024. 2020. p. 52.
Inter-Agency Working Group on Reproductive Health in, C. Inter-Agency Field Manual on Reproductive Health in Humanitarian Settings. 2018.
Download references
Acknowledgements
Not applicable.
The authors received no funding for this study.
Author information
Authors and affiliations.
Duke University School of Medicine, 40 Duke Medicine Circle, Durham, NC, 27710, USA
Alexandra Norton
Johns Hopkins Bloomberg School of Public Health, 615 N. Wolfe St, Baltimore, MD, 21205, USA
Hannah Tappis
You can also search for this author in PubMed Google Scholar
Contributions
AN and HT designed the scoping review. AN conducted the literature search. AN and HT screened records for inclusion. AN extracted data from included studies. Both authors contributed to synthesis of results. AN drafted the manuscript and both authors contributed to editorial changes.

Corresponding author
Correspondence to Alexandra Norton .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
. Literature search terms: Exact search terms used in literature search, with additional detail on the methodology to determine search terms and definitions used for each component of the search
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Norton, A., Tappis, H. Sexual and reproductive health implementation research in humanitarian contexts: a scoping review. Reprod Health 21 , 64 (2024). https://doi.org/10.1186/s12978-024-01793-2
Download citation
Received : 06 November 2023
Accepted : 12 April 2024
Published : 13 May 2024
DOI : https://doi.org/10.1186/s12978-024-01793-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Humanitarian settings
Reproductive Health
ISSN: 1742-4755
- General enquiries: [email protected]
Cookies on the NHS England website
We’ve put some small files called cookies on your device to make our site work.
We’d also like to use analytics cookies. These send information about how our site is used to a service called Google Analytics. We use this information to improve our site.
Let us know if this is OK. We’ll use a cookie to save your choice. You can read more about our cookies before you choose.
Change my preferences I'm OK with analytics cookies
Summary of South East region virtual wards evaluation
Introduction.
Virtual ward services (also known as hospital at home) allow patients of all ages to safely and conveniently receive acute-level care at their usual place of residence, including care homes. These services aim to improve patient experience and outcomes, and narrow the gap between demand and capacity for hospital beds, by either preventing avoidable attendances and admissions, or reducing length of stay through early discharge.
This independent evaluation from PPL was commissioned by NHS England South East region and is a pioneering effort to fill the critical large-scale evidence gap on virtual wards, offering actionable insights for healthcare providers, policymakers, and researchers. The evaluation analysed a total of 29 virtual ward pathways in the South East, which equated to 49% of their overall capacity at the time of analysis.
This is a short summary of the evaluation. The full report is available on the PPL website . Participating integrated care systems and providers have also received individual reports to support localised learning and improvement.
Key findings
- Virtual wards in South East England are associated with a positive impact on avoided non-elective (NEL) hospital activity – on average 1 NEL admission ‘avoided’ was shown to be correlated with 2.5 virtual ward admission, with some more mature virtual wards achieving a 1:1 association between the ‘avoided’ NEL admissions and virtual ward activity.
- There is evidence of positive net financial benefits associated with the regional virtual ward provision – overall total annualised net benefit of £10.4 million, for the virtual wards analysed.
- It is clear that the longer they run, the more likely virtual wards are to show impact , as volumes of admissions going through virtual wards increase, and costs per admission start to fall.
- Black and minority ethnic people are consistently underrepresented in virtual ward patient cohorts. However, there are significant gaps in ethnicity data recorded in patient level data.
- Core-20 representation in virtual ward patient cohorts is more mixed , however it is more consistently reported.
Virtual wards in the South East
The South East region is responsible for delivering 1,939 virtual ward beds that collectively provide health services for approximately 9.4 million people. This represents 24.3 virtual ward ‘beds’ per 100,000 population (as reported in the 26th February 2024 situation report ).
Figure 1: map showing geography of six integrated care systems in the South East region
The above map shows the geography of six integrated care systems in the South East region. This include Buckinghamshire, Oxfordshire and Berkshire West, Hampshire and Isle of Wight, Frimley Health and Care, Surrey Heartlands Sussex, and Kent and Medway.
The region includes six integrated care systems, 32 NHS trusts delivering acute, community and ambulance services. Providers are responsible for delivering 1,939 admission avoidance and early supported discharge virtual ward beds across 76 virtual wards with 52% of this bed capacity reported to be technologically enabled.
Virtual ward bed capacity in the region has grown 20% over the past six-months whilst the proportion of technologically enabled beds has also increased by 10% and occupancy increased 10% over the same period. This suggests not only the ongoing creation of new virtual ward services but the continued integration of technology to support service provision across the region. Current bed capacity consists of approximately:
- 31% frailty
- 9% respiratory
- 42% mixed (any combination of frailty, respiratory and other specialities)
How this evaluation helps to bridge the evidence gap
Why is this evaluation needed.
Evidence gap: as noted by the Health Foundation’s February 2024 paper , there is a very limited published evidence on the system level consequences (such as patient flow and capacity) of virtual wards.
Limitations of previous evaluations: to date, there has not been a large-scale (recent evaluations have focused on hundreds of admissions), comprehensive evaluation examining multiple conditions across providers and integrated care systems.
Policy and practice implications: with healthcare systems under increasing pressure, virtual wards offer a promising solution but require solid evidence to guide widespread implementation and investment.
Innovating care delivery: by providing detailed insights into the operation and outcomes of virtual wards, this evaluation supports the evolution of healthcare towards more personalised, efficient, and accessible services.
The uniqueness of this evaluation
Comprehensive approach: the Treasury’s Magenta Book 3-stage evaluation approach encompasses a wide array of metrics including clinical outcomes, patient satisfaction, cost effectiveness, and system impacts.
Scale of the evaluation: the evaluation has analysed over 26,000 virtual ward admission avoidance attendances and has been enabled by a large patient-level dataset. Our analysis
incorporates 29 virtual wards which cover 64% of all South East region virtual ward admissions as of February 2024.
Advanced analytical techniques: PPL used robust data science methodologies, including predictive modelling to accurately assess the efficacy and efficiency of virtual wards.
Stakeholder engagement: collaborating with healthcare professionals, patients, and policymakers to ensure a multifaceted understanding of virtual ward impact.
Evaluation approach
The evaluation independently assesses virtual wards’ effectiveness, employing a structured methodology to cover six key areas specified in the invitation to tender (ITT), in alignment with the Treasury’s Magenta Book 3-stage evaluation guidelines.
Process evaluation
To understand the context within which the virtual wards have been implemented and support the development of a deep understanding of the core components of each virtual ward and the variation in the models.
- P1 – are virtual wards being delivered as local providers intended?
P2 – How have contextual and external factors influenced the delivery and functioning of virtual wards?
P3 – what can be learned from the delivery of virtual wards so far.
- P4 – How have patients, carers, and staff experienced virtual wards?
Impact evaluation
To demonstrate quantitative and qualitative impact, with a focus on admission avoidance, provision of equitable access and outcomes, and inequalities.
- IM1 – Has the implementation of virtual wards been associated with its intended impact of reducing hospital activity so far?
- IM2 – How might differences across virtual wards drive differences in impact?
- IM3 – To what extent have different groups at risk of inequalities (including ethnicity, deprivation, gender) seen differences in impact and why?
Cost-benefit evaluation
System cost benefit analysis, with a focus on admission avoidance.
- C1 – Have virtual wards been cost-effective so far?
- C2 – Is the intervention the best use of resources?
Key findings: impact and cost-benefit evaluation
This section highlights the most important findings of the evaluation, highlighting significant data points, trends, and any unexpected results organised around the evaluation’s key questions or objectives.
Headline figures
Key conclusions.
- Virtual wards in South East England are associated with a positive impact on non-elective (NEL) hospital activity – on average 1 NEL admission ‘avoided’ was shown to be correlated with 2.5 virtual ward admissions , with some more mature virtual wards achieving a 1:1 association between the ‘avoided’ NEL admissions and virtual ward activity.
- Black and minority ethnic people are consistently underrepresented in virtual ward patient cohorts. However, there is are significant gaps in ethnicity data recorded in patient level data.
The impact evidenced in this evaluation varies greatly between geographies and pathways – with our qualitative evaluation understanding reasons driving this variation.
Key findings: process evaluation
P1 – are virtual wards being delivered as local providers intended.
- They are being delivered as local providers intended to some extent. Context-specific variation drives how effectively virtual ward services are being delivered.
- Virtual wards adopting a flexible implementation approach and building upon existing services more frequently reported effective implementation.
- Having a pre-existing service engaged in delivering aspects of acute care and remote monitoring in the community is a significant theme amongst staff that felt their virtual wards were delivered as intended. Those services were able to draw on established standard operating procedures, professional relationships and an incumbent skilled workforce.
- In some cases, funding limitations in integrated care boards alter virtual ward delivery plans away from original intentions and have meant providers draw from other budgets and their existing workforce to staff new services. Misaligned strategies and expectations can undermine collaborative efforts to develop integrated services.
- Successful patient identification strategies demonstrate the reach of virtual wards; however, opportunities remain to ensure that the model effectively mitigates the influence of underlying health inequalities that might preclude some groups from presenting to the service.
- Seasonal service demands (peaking between October and February) drive virtual ward activity through increased patient volumes and acuity.
- Large or rural geographies can prove challenging to a single, centralised virtual ward team, but some services mitigated this issue by spreading a larger team across multiple localities with representation from the full multidisciplinary team.
- Digital integration, if done well, leads to more effective tech enabled virtual wards that improves information sharing processes within and across healthcare organisations. Misaligned digital strategies and technical incompatibilities across healthcare providers and GPs can hinder effectiveness.
- A shared workforce can support operational resilience by prioritising focus across co-located services in response to demand.
- Healthcare organisations adjacent to virtual ward services play a fundamental role in supporting the delivery of holistic patient-centred care. A range of factors are responsible for determining the extent of operational integration and collaboration between complimentary services which ultimately influence the effectiveness of virtual ward activity.
- Virtual ward clinicians and managers frequently felt that positive examples of virtual ward delivery championed patient-centred care and achieved success with the support of strong clinical leadership that advocated for the experience of patients and clinicians at a system-level.
- The capability of virtual ward services to effectively meet acute patients’ needs in the community is a common challenge as services can lack the equipment, skills or clinical governance to deliver the required interventions (such as intravenous fluids). This can sometimes result in the need for a hospital attendance despite virtual ward admission.
- Virtual ward clinicians felt that the complexity and time required to provide care is not necessarily reflected within current measures of acuity such as NEWS2 or the clinical frailty score.
P4 – How have patients, carers, and staff experienced virtual wards
- Patient experience of virtual ward services has generally been positive. Patients articulate an appreciation for home-based care, being closer to family and more comfortable than in an acute hospital setting
- Carers recognised the benefits of patients being treated in their own home and having more independence. However, carers did acknowledge the increased burden of care.
- Staff viewed virtual wards generally positively and saw value in the model of care. Some virtual ward staff feel patients recover more quickly as a result. Additionally, virtual ward staff recognised that they enjoyed working in a new and developing services that enabled them to develop new skills. However, some staff did feel operational pressures relating to virtual wards.
- Some staff observed inequalities in access driven by the requirements for virtual ward services to be able to deliver safe care such as a means of verbal communication and fixed address. The patient groups accessing virtual ward services are influenced by those most likely to present to the healthcare system. This was sometimes felt to be not representative of the wider patient population. In some areas, virtual ward outreach activities to engage black and minority ethnic communities have been planned to educate and raise the profile of virtual ward services.
Key findings: what a good virtual ward looks like
Based on findings, the below sets out what the data suggests, and what virtual ward managers and clinicians told PPL, are characteristics more likely to lead to virtual wards which impact on reduced hospital usage, and function effectively.
Timing and scale
Typical success criteria.
1. The longer virtual wards run, the more likely they are to show impact on non-elective admissions. Primarily due to them being larger; and being able to spread set-up, staff, and digital costs across a larger pool of admissions but also due to having time to embed the some of the elements below.
Barriers to success
1. Acknowledgement that virtual wards take time to demonstrate impact given the time needed to scale up, but also the time needed to build and embed collaboration and ways of working.
Staffing and resourcing
- Strong clinical leadership – advocating for the experience of patients and clinicians.
- Collaborative working, focusing on the patient, with strong links between acute, community, and primary care settings (for example carrying out daily multidisciplinary team ward rounds).
- Well-resourced and experienced teams.
- Fragmented clinical leadership.
- Teams not joined up across different services, and staff feeling under-confident with new ways of working if not properly implemented.
- Lack of proper funding can lead to recruitment challenges, or overworked staff.
- Digital integration, if done well, leads to more effective tech enabled virtual wards.
- Referrals received through a single point of access or via an urgent community response service.
- If there are misaligned digital strategies across healthcare providers and primary care.
- Insufficient data support and inefficient manual data collection processes.
Conclusions
Key conclusions from the independent evaluation are presented below. These are subject to the stated caveats (see page 13 of full evaluation report ):
- Virtual Wards in South-East England are associated with a positive impact on non-elective (NEL) hospital activity – on average 1 NEL admission ‘avoided’ was shown to be correlated with 2.5 virtual ward admissions, with some more mature virtual wards achieving a 1:1 association between the ‘avoided’ non-elective admissions and virtual ward activity.
- There is evidence of positive net financial benefits associated with the regional virtual wards provision – the majority of virtual wards analysed generated an estimated positive net benefit.
- Black and minority ethnic people are consistently underrepresented in virtual ward patient cohorts. However, there are significant gaps in ethnicity data recorded in patient level data. Respondents have identified several ways the system can better support these groups access virtual wards – which we recommend are taken forward immediately.
- The impact evidenced in this evaluation varies greatly between geographies and pathways. with our qualitative evaluation understanding reasons driving this variation.
- It is clear that the longer they run, the more likely virtual wards are to show impact – this is through a combination of higher volumes going through the wards, costs per admission typically falling over time, and the benefit per admission increasing.
- The evaluation has identified a clear set of enablers (including having sufficient funding, experienced staff, collaborative working, and strong clinical leadership) and barriers (inadequate resourcing, fragmented leadership, mis-aligned digital strategies) to effective virtual ward working.
- This evaluation is the starting point – the South East needs to build on the evidence gathered and lessons learned in this evaluation, and to work closely with individual pathways to support continuous improvement of the virtual ward offering in the South East.
Publication reference: PRN01291
- Open access
- Published: 15 May 2024
Novel frontiers in urogenital cancers: from molecular bases to preclinical models to tailor personalized treatments in ovarian and prostate cancer patients
- Giada De Lazzari ORCID: orcid.org/0000-0002-5217-8201 1 ,
- Alena Opattova ORCID: orcid.org/0000-0002-4508-6560 1 &
- Sabrina Arena ORCID: orcid.org/0000-0002-1318-2494 1 , 2
Journal of Experimental & Clinical Cancer Research volume 43 , Article number: 146 ( 2024 ) Cite this article
1 Altmetric
Metrics details
Over the last few decades, the incidence of urogenital cancers has exhibited diverse trends influenced by screening programs and geographical variations. Among women, there has been a consistent or even increased occurrence of endometrial and ovarian cancers; conversely, prostate cancer remains one of the most diagnosed malignancies, with a rise in reported cases, partly due to enhanced and improved screening efforts.
Simultaneously, the landscape of cancer therapeutics has undergone a remarkable evolution, encompassing the introduction of targeted therapies and significant advancements in traditional chemotherapy. Modern targeted treatments aim to selectively address the molecular aberrations driving cancer, minimizing adverse effects on normal cells. However, traditional chemotherapy retains its crucial role, offering a broad-spectrum approach that, despite its wider range of side effects, remains indispensable in the treatment of various cancers, often working synergistically with targeted therapies to enhance overall efficacy.
For urogenital cancers, especially ovarian and prostate cancers, DNA damage response inhibitors, such as PARP inhibitors, have emerged as promising therapeutic avenues. In BRCA -mutated ovarian cancer, PARP inhibitors like olaparib and niraparib have demonstrated efficacy, leading to their approval for specific indications. Similarly, patients with DNA damage response mutations have shown sensitivity to these agents in prostate cancer, heralding a new frontier in disease management. Furthermore, the progression of ovarian and prostate cancer is intricately linked to hormonal regulation. Ovarian cancer development has also been associated with prolonged exposure to estrogen, while testosterone and its metabolite dihydrotestosterone, can fuel the growth of prostate cancer cells. Thus, understanding the interplay between hormones, DNA damage and repair mechanisms can hold promise for exploring novel targeted therapies for ovarian and prostate tumors.
In addition, it is of primary importance the use of preclinical models that mirror as close as possible the biological and genetic features of patients’ tumors in order to effectively translate novel therapeutic findings “from the bench to the bedside”.
In summary, the complex landscape of urogenital cancers underscores the need for innovative approaches. Targeted therapy tailored to DNA repair mechanisms and hormone regulation might offer promising avenues for improving the management and outcomes for patients affected by ovarian and prostate cancers.
In this review, we aimed to explore the complex landscape of urogenital cancers, with a specific focus on the current therapeutic approaches available, particularly for ovarian and prostate cancer. We highlight the pivotal roles played by genomic instability and DNA repair mechanisms in both the development and treatment of these malignancies. We emphasize the crucial impact of mutations in DNA repair genes, which have paved the way for targeted therapeutic interventions. Furthermore, we underscore the intricate interplay between hormonal dysregulation and DNA damage, suggesting potential for new treatment modalities. Finally, we shed light on the importance of advanced models as genetically engineered mouse models, patient-derived xenografts and organoids. These models not only mimic human cancers more accurately, but also serve as indispensable tools in guiding the development of tailored therapies in the frame of a precision medicine approach in the battle against urogenital cancers.
Urogenital cancers: insights from ovarian and prostate tumorigenesis
Urogenital cancers encompass a diverse array of malignancies affecting the urinary and reproductive systems, arising from organs like the kidneys, bladder, prostate, testicles, ovaries, uterus, and related structures [ 1 ]. Each cancer type within this spectrum possesses unique characteristics, risk factors, and treatment approaches [ 2 ]. Therefore, early detection, accurate diagnosis, and timely intervention are crucial for improving outcomes in individuals diagnosed with these cancers [ 1 ]. A fundamental aspect of cancer development lies in the role of DNA repair mechanisms. In healthy cells, DNA repair mechanisms accurately fix genetic damage, preserving genomic stability. However, compromised repair systems lead to the accumulation of DNA damage, resulting in accumulation of mutations and genomic instability, which are key hallmarks of cancer [ 3 , 4 ]. Inherited defects in DNA repair genes, such as BRCA1/2 in breast, ovarian and prostate cancers, significantly increase the risk of tumor development [ 3 ]. Tumors exploiting deficient repair pathways become reliant on alternative mechanisms, driving genomic instability and cancer progression. This understanding of repair deficiency in cancer cells has led to the identification of specific therapeutic targets [ 5 ]. For instance, as demonstrated by González-Martín and colleagues, cancers with impaired homologous recombination (HR) are particularly sensitive to PARP inhibitors (PARPi) and the authors demonstrate the effectiveness of niraparib as specific therapeutic agent against HR in treating patients with ovarian cancer [ 6 ]. Given the critical role of DNA repair pathways in cancer progression, in this review we will delve these mechanisms focusing on two main subtypes of urogenital cancer, ovarian and prostate tumors.
Both these cancers, while distinct in their manifestation and impact on different genders, share common ground in the molecular dysregulation of cellular processes, including DNA repair pathways and common mutation in genes such as BRCA1/2 [ 7 , 8 ].
Ovarian cancer (OC), often termed as the “silent killer,” is the sixth most common cancer and the fifth for mortality in women and it poses unique challenges due to its asymptomatic nature in early stages [ 2 , 9 ]. Globally, the incidence and mortality rates of OC exhibit considerable geographical variability: higher incidence is shown in Northern Europe and the United States and lower in Japan while its mortality has exhibited a notable decrease from 2017 through 2020 [ 2 , 10 ]. The etiology of OC is multifaceted, implicating a range of risk factors. Advanced age emerges as a significant contributor, with the majority of cases diagnosed in postmenopausal women [ 9 , 11 ]. The pathophysiology of OC involves the dysregulation of key cellular processes, including uncontrolled cell proliferation and evasion of apoptosis, often leading to the formation of epithelial tumors [ 12 , 13 ]. Diagnostic strategies for OC encompass protein and imaging diagnostics, along with preoperative assessments, employing methods like different index assays as described in the work of Liberto and colleagues [ 14 ]. As pointed out in this and other works [ 14 , 15 , 16 ] a panel of four marker for OC diagnosis including CA125, CA72-4, CA15-3, and MCSF can help in increasing the sensitivity of the technology. Together with protein markers also imaging diagnostics have evolved; imaging techniques, such as ultrasound and magnetic resonance imaging (MRI), help in visualizing tumors and assessing their extent [ 17 , 18 ]. The complexity of OC is also reflected on treatment modalities since surgical interventions, including hysterectomy and oophorectomy are often employed as first line treatment [ 19 ]. The surgical approach is often reinforced by chemotherapy, with agents like cisplatin, carboplatin and taxanes (e.g. paclitaxel) and targeted therapies such as PARPi in specific genetically altered tumors [ 19 , 20 ]. Preventive strategies and screening programs are integral components of the comprehensive approach to urogenital cancers. Risk-reducing measures, such as prophylactic surgery for individuals with high-risk genetic mutations, offer a preventive option for OC [ 21 , 22 ]. However, challenges persist in developing effective screening methods for OC due to its often asymptomatic nature in early stages [ 21 , 22 ]. Moreover, OC distinctly highlights how genetic and molecular dysregulations in the urogenital tract can lead to malignancy. Genetic mutations, notably in the BRCA1 and BRCA2 genes but also in TP53 , KRAS and PIK3CA , are central to understand this type of tumor since they highlight broader tumorigenic processes across OC [ 23 , 24 ]. In fact, beyond their known role in double-strand DNA break repair pathways and in particular in the regulation of HR, these mutations also have other functions such as being a regulator of oxidative stress and cell cycle progression ( BRCA1 ) or being involved in transcriptional regulation ( BRCA1/2 ) [ 25 , 26 ]. In this context, starting from the main function of these genes, researchers have increasingly emphasized the analysis of the link between their dysregulation and tumorigenesis and consequently the study of homologous recombination repair (HRR) deficiencies which has led to significant therapeutic advancements on urogenital cancers [ 26 , 27 , 28 ]. Moreover, the observed heterogeneity in ovarian tumor cells, including variations in the tumor microenvironment and metabolic pathways, offers a deeper understanding of tumorigenesis. The intricate interactions within the ovarian tumor microenvironment, involving stromal cells, immune evasion mechanisms, and angiogenesis, further elucidate the complexities of tumorigenesis in the urogenital system. This understanding is pivotal in developing targeted therapeutic strategies, as it reveals how cancer cells manipulate their surroundings for survival and growth. Moreover, the metabolic adaptations seen in OC cells provide insights into potential vulnerabilities that could be therapeutically exploited, indicating how metabolic dysregulation in the urogenital tract can contribute to cancer development [ 29 ].
Prostate cancer (PC) is the most common type of solid cancer and the second cause of cancer-related death in men [ 2 ]. The etiology of PC includes different types of risk factors such as age, race, family history, and germline mutations ( BRCA1/2, CHEK2, ATM ) [ 30 ]; in addition, metabolic syndrome, obesity, and smoking have been identified as possible risk factors [ 31 ]. PC is characterized by different stages, from intraepithelial neoplasia and localized PC, to the advanced prostate adenocarcinoma with local invasion. The most advanced stage, metastatic PC (mPC), is characterized by the invasion of other different organs and tissues in the body. For the grading of PC, the Gleason grading system is used [ 32 ]. Early detection is crucial for successfully treating PC. Various screening methods aim to improve cancer detection in its early stages, with the prostate-specific antigen (PSA) test being the most widely promoted and FDA-approved method since 1986. PSA, typically found at low levels in the blood, becomes elevated in the presence of prostatic disease due to disruption in organ microarchitecture [ 33 ]. However, the low specificity of the PSA test necessitates additional measures to reduce unnecessary prostate biopsies, leading to the development of the prostate health index (PHI) blood test. This test combines free and total PSA with the (− 2) pro-PSA isoform (p2PSA) to enhance accuracy [ 34 ]. Recent studies showed Prostate Cancer Antigen 3 (PCA3) as overexpressed in 95% of PC cases, leading to the development of a non-invasive urine PCA3 test for screening [ 35 ]. Usually, the screening starts for 50-year-old men, but for high-risk individuals (germline mutations in BRCA1, BRCA2, ATM, CHEK2 ; family history of PC) the screening should commence as early as age 40 [ 36 ]. Diagnostic strategies for PC include MRI combined with dynamic contrast-enhanced MRI and more specific Prostate-Specific Membrane Antigen (PSMA) positron emission tomography PET/CT [ 37 ].
PC is well known by high morphological and genetic heterogeneity [ 38 ]. The main genetic alterations in PC affect androgen receptor (AR), Phosphatidylinositol-3-kinase/ Phosphatase and tensin homolog (PIK3CA–PTEN ), WNT , and genes involved in DNA repair signaling pathways ( BRCA1, BRCA2, ATM, CHEK2 ) [ 39 ].Treatment options for PC depend on the stage of the disease. For localized disease, active surveillance, radical prostatectomy, or ablative radiotherapy are employed. Patients with localized disease show a favourable outcome if the disease is early detected and treated. For the advanced stages, radiotherapy and/or androgen deprivation therapy are used. For the mPC, AR-targeted agents, chemotherapy (taxanes), and radionuclides are used [ 40 ]. As we already mentioned, PC is characterized by the presence of DNA repair mutations, which increases in the metastatic setting of the PC. Therefore, the PARPi olaparib has been approved for use in patients with BRCA2 mutations [ 41 ]. However, after an initial response, PC can progress in developing castration resistance (CRPC), posing ongoing challenges in disease management.
In this first section of this review, we aimed to explore urogenital cancers tumorigenesis which helps our understanding of these particular type of cancers but also provides critical insights into the mechanisms of cancer development. Both tumors share notable similarities for example in DNA damage and repair mechanisms, hormonal regulation and key tumor characteristics. They both frequently exhibit defects in DNA damage repair (DDR) pathways, such as homologous recombination (common mutations in BRCA genes) [ 42 ], and hormone regulation plays a significant role in both, with estrogen receptor signaling influencing OC and androgen receptor pathways being pivotal in PC [ 43 ]. Additionally, both cancers often develop resistance to hormone-based therapies and may respond to PARPi, highlighting shared therapeutic vulnerabilities [ 44 ]. Due to these similarities, from now on this review will be mainly focused on mechanisms of DNA damage and repair and hormonal regulation in the context of OC and PC by evaluating the currently available therapeutic strategies and preclinical available models for both cancers.
Deconvolution of urogenital cancer complexity
Exploring the role of OC and PC in the urogenital tract tumorigenesis lays the groundwork for understanding cancer’s broader complexities. This exploration extends to the fundamental framework of the hallmarks of cancer, delving into genetic instability and synthetic lethality, which are pivotal in comprehending the multifaceted nature of cancer. In this scenario, cancer research underwent a paradigm shift with the introduction of the “Hallmarks of Cancer” by Hanahan and Weinberg in their 2000 publication [ 45 ]. This concept delineates a set of mechanisms acquired by human cells during their transition from normal to neoplastic states, crucial for malignant tumor development [ 45 ]. Initially, Hanahan and Weinberg outlined six biological capabilities acquired during the multistep development of human tumors, such as insensitivity to antigrowth signals, evasion of apoptosis, sustained angiogenesis, limitless replicative potential, tissue invasion and self-sufficiency in growth signals [ 46 ]. Subsequently, this list was expanded to eight hallmarks and two enabling characteristics by incorporating tumor-promoting inflammation, genome instability and mutation and the ability of cancer cells to often undergo changes in their metabolism and to avoid immune system destruction [ 47 ]. Among these hallmarks, “Genome Instability and Mutation” holds a central position, driving the acquisition of other hallmarks.
Genomic instability, defined as an increased susceptibility of a cell's genome to acquire mutations, stems from defects in DNA repair mechanisms, replication errors, exposure to mutagenic agents, or other genetic or environmental factors, leading to high mutation rate and resulting in a heterogeneous tumor population with diverse genetic compositions [ 48 , 49 ].
In the context of OC, one of the most significant implications of genomic instability is the development of resistance both primary and secondary to platinum-based chemotherapy, a cornerstone of its treatment [ 50 , 51 , 52 ]. Primary resistance occurs when cancer cells exhibit intrinsic resistance to therapeutic agents, while secondary (acquired) resistance develops over time, likely due to adaptation to treatment selection pressure [ 50 , 53 ] For instance, alterations in the BRCA1/2 genes, which are crucial for HRR, are common in OC and can confer initial sensitivity to platinum-based therapies. During treatment, the occurrence of reversion mutations in these genes can restore lost repair function, leading to drug resistance [ 51 , 54 ]. Furthermore, other recent studies have identified additional genetic alterations that contribute to platinum resistance in OC, such as mutations in RAD51C and RAD51D , which further complicate the treatment landscape [ 55 , 56 ]. Additionally, the high degree of genomic instability in OC can correlate with tumor heterogeneity, as demonstrated by Bashashati and colleagues [ 57 ], revealing distinct genetic profiles among tumor subclones that may respond differently to the therapy In line with this, researchers start to explored the implications of intratumor heterogeneity in OC prognosis, emphasizing the need for personalized treatment approaches [ 58 ]. Liquid biopsy technologies offer dynamic and precise monitoring of these genetic variations, aiding in the assessment of treatment response and disease progression [ 58 , 59 , 60 ]. The genomic instability of both OC and PC has also opened new avenues for targeted therapy. PARPi, for example, exploit the concept of synthetic lethality in cancer cells deficient in HRR as seen in BRCA -mutated OC and PC [ 61 , 62 ]. Recent advancements in this area have shown promising results in the use of PARPi in prolonging progression-free survival especially in patients carrying BRCA mutation and HRD-positive status [ 63 , 64 ]. However, the adaptive capacity of cancer cells due to genomic instability presents an ongoing challenge. This adaptive nature of cancer due to its genomic instability, not only leads to challenges like chemoresistance and tumor heterogeneity, but also paves the way for innovative therapeutic strategies, such as those exploiting synthetic lethality [ 65 ]. In a synthetically lethal relationship, the simultaneous impairment of two genes or pathways leads to cell death, whereas the disruption of either alone is tolerable to the cell. This concept is particularly relevant in cancer cells, which often harbour specific genetic mutations making them susceptible to targeted therapies that exploit their inherent genetic weaknesses [ 65 ]. PC and OC are a prime candidate for therapies based on synthetic lethality; indeed, BRCA mutations impair the HR DNA repair pathway, making the cancer cells more dependent on alternative repair mechanisms [ 66 ]. This dependency creates an opportunity for targeted therapy as we have already discussed. More recent studies have expanded on these findings exploring the broader implications of synthetic lethality focusing especially on the combination therapies that integrate synthetic lethality concepts. Lord and Ashworth investigated the synergistic effects of combining PARPi with other targeted agents, offering novel strategies to overcome resistance mechanisms that OC cells develop in response to monotherapy [ 5 ]. While genomic instability poses significant challenges in the form of chemoresistance and tumor heterogeneity, it also provides opportunities for developing innovative targeted therapy strategies. The latest studies in the field reinforce the potential of synthetic lethality in offering effective, personalized treatment options for OC, catering to its adaptive nature and genetic diversity.
DNA damage: DNA repair mechanisms in ovarian and prostate tumors
As highlighted in the previous section, the ongoing research in genomic instability and synthetic lethality in OC and PC treatment sparks discussion about the intricate interplay among genomic instability, DNA damage, and repair mechanisms. Understanding these mechanisms is pivotal in this context where DDR plays a significant role in disease development and progression. A wealth of literature has been published on this topic and here we aim to provide a concise overview of the key concepts, primarily focusing on urogenital tumors.
DNA damage can be broadly categorized into two groups: single-strand breaks (SSBs) and double-strand breaks (DSBs). SSBs are the most common and are generally less harmful as the complementary DNA strand remains intact, serving as a template for repair. In contrast, DSBs are more critical and can lead to significant genomic instability if not appropriately repaired [ 47 ]. This distinction is crucial in the context of urogenital cancers, where genetic material integrity is paramount for cell function [ 67 ].
Cells have evolved several mechanisms to repair damaged DNA, each tailored to specific types of damage. These includes Nucleotide Excision Repair (NER), which is primarily responsible for repairing bulky DNA lesions caused by UV radiation and certain chemicals; Base Excision Repair (BER) which corrects small, non-helix-distorting base lesions caused by oxidation or methylation. In addition, Mismatch Repair (MMR) corrects errors that occur during DNA replication. Defects in MMR are known to contribute to the development of certain types of cancers, including urogenital cancers [ 47 ]. Finally, HR and Non-Homologous End Joining (NHEJ) are two critical pathways for repairing DSBs. HR is an error-free repair process utilizing a sister chromatid as a template for repair, while NHEJ is an error-prone process directly joining broken end [ 49 ]. These cancers often exhibit inherent defects in DNA repair pathways, particularly in HR [ 68 ]. DNA damage and repair mechanisms are critically linked to the therapeutic potential of DDR inhibitors (DDRi). These inhibitors, such as PARPi, target mechanisms that cancer cells rely on for survival and proliferation exploiting the concept of synthetic lethality [ 46 ]. As discussed in the previous paragraph, BRCA1 and BRCA2 mutations impair HR repair in OC and make OC cells particularly vulnerable to PARPi. By inhibiting PARP enzymes, which play a crucial role in single-strand break repair, these drugs exacerbate DNA damage in cells already compromised in their ability to repair double-strand breaks, leading to cell death. For this reason, this approach is often use therapeutically [ 69 ] and clinical trials with PARPi in OC are extensively reported in different studies [ 70 , 71 , 72 ]. However, the scope of DDRi extends beyond PARPi and BRCA mutations. Recent studies have shown that other DDR pathways and inhibitors are also clinically significant; indeed, we will focus on other DDRi such as ATRi, CHK1i, WEE1i and DNA-PKi. For instance, inhibitors targeting the ATR-CHK1-WEE1 axis, which are key components of the DDR involved in the cell cycle checkpoint regulation, have shown promise in preclinical models of OC [ 73 ]. These inhibitors can enhance the effects of DNA-damaging chemotherapy and radiation therapy, offering a potential combinatorial approach to cancer treatment. For this reason, several clinical trials based on ATR-CHK1-WEE1 axis are further exploring this avenue (Table 1 ) [ 47 ]. Addressing this challenge requires a deeper understanding of resistance mechanisms and the development of next-generation DDR inhibitors able to overcome it [ 74 ]. When developing new DDRi, tailoring treatments based on individual genetic profiles is imperative. As suggested in the work of Foster and colleagues, genomic sequencing can identify specific DNA repair deficiencies in tumors, guiding the selection of appropriate DDR inhibitors [ 75 ]. This precision medicine approach ensures that patients receive the most effective treatment tailored to their unique cancer biology.
Comprehensive molecular characterization of PC has revealed a significant inter-patient genomic heterogeneity and phenotypic diversity. The most prominently altered pathways include androgen signaling (50%), PI3K signaling (40%), the cell cycle (24%), WNT/beta-catenin signaling (19%), RAS pathway (8%) [ 76 , 77 ] along with DDR pathways (27%) [ 78 ]. Recent studies have indicated that germline mutations in DDR genes are associated with a higher risk of developing PC and worse clinical outcomes as well as with aggressive phenotype with increased probability to develop metastasis [ 79 ]. Approximately 10–19% of primary PCs exhibit somatic alterations in DDR genes, with this number increasing to 23–27% in the metastatic setting. Mateo and colleagues showed differences in AR, TP53, RB1 , and PI3K/AKT mutational status between matched hormone-naive and metastatic castration-resistant prostate cancer (mCRPC) biopsies [ 80 ]. Furthermore, multicentric study on a cohort of 150 mCRPC showed increased aberrations of BRCA2, BRCA1 and ATM (19.3%) compared to primary PCs [ 81 ]. Taken together this introduces important prognostic value of DDR mutations. Current studies and clinical trials indicated that alterations in DDR genes also contribute to disease progression and therapy response in PC [ 41 ]. Initially identified mutations in DDR genes were BRCA1 and BRCA2 genes, followed by discoveries of germline or somatic mutations also in other DDR genes e.g.: ATM, CDK12, FANCA, RAD51B, and RAD51C, CHEK2 in PC [ 76 , 82 ] . Inactivating mutations in these tumor suppressor genes increase predisposition to PC. Moreover, loss-of-function mutations of DDR-associated genes leads to a deficiency in error-free HR repair. DSBs are then repaired by alternative repair pathways that are more error-prone, e.g. NHEJ. Consequently, these lead to the genetic instability of the tumor. Despite this, these genes present potential therapeutic targets in PC [ 41 ]. Increasing evidence suggests that other DNA repair pathways, such as a MMR and BER, may play an important role in PC. Approximately 4% of PC tumors and 6% of metastatic PCs (mPC) had alterations in MSH2 and MSH6 , with clinical implications such as resistance to immune checkpoint inhibitors (ICIs) noted in MMR-deficient patients [ 83 ]. Vasquez and colleagues showed that upregulation of BER related genes is associated with poor survival in PC patients, with inhibition of BER by natamycin significantly impaired PC cells proliferation in androgen depleted PC [ 84 ]. As pointed out before, genome instability is one of the important hallmarks of cancer [ 4 ] and DDR is responsible for the maintenance of genome integrity. In PC, cancer cells frequently harbour DDR gene deficiencies, providing a potential avenue for targeting DDR to induce cancer cell death. The PARPi olaparib was initially approved for the treatment of advanced ovarian and breast cancers associated with germline BRCA1 or BRCA2 mutations [ 85 ]. Clinical trials such as the TOPARP have demonstrated high response rates to PARPi in patients with DDR gene defects [ 39 ]. The clinical trial TOPARP-B studied the antitumour activity of olaparib against mCRPC with DDR gene aberrations [ 86 ]. Similar results have been obtained in clinical trials with rucaparib [ 87 ]. Based on these studies PARPi were approved by FDA for PC treatment in 2020 and the importance of these pathways in PC therapy response is also confirmed by the number of clinical trials already performed or currently ongoing [ 88 ]. Additionally, ongoing trials focusing on components like the ATR-CHK1-WEE1 axis suggest potential novel therapeutic options, as single agents or combinations, for PC. Drapela and colleagues showed synergistic effect of CHK1 inhibitor MU380 with gemcitabine in in vitro model of CRPC [ 89 ]. ATR inhibition led to the destabilization of PD-L1 protein in vitro. This indicates potential possibility to use of ATRi in combination with immune checkpoint blockade as a novel therapy option [ 90 ]. Examples of ongoing clinical studies focused on ATR-CHK1-WEE1 are summarized in the table below (Table 2 ).
How could the combination of PARPi and immune checkpoint inhibitors (ICI) affect “cold” tumor treatment?
Immunotherapy has emerged as novel approach in the oncological landscape and among the most promising strategies in this field are immune checkpoint inhibitors (ICIs), which have revolutionized cancer treatment by promoting the body's immune system to recognize and combat tumor cells. In particular, ICIs efficacy has recently seen a relevant improvement in tumors such as ovarian ad prostate ones, that are generally considered as immunologically "cold" due to their low mutation burden and reduced immunogenicity [ 91 , 92 ]. The most involved checkpoints pathways include the programmed cell death protein 1 (PD-1), PD-L1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) which modulate T cell function. In the PD-1/PD-L1 pathway, PD-1, a receptor expressed on T cells, binds to PD-L1, which is expressed on tumor cells and some immune cells. This interaction results in the inhibition of T cell activation and proliferation, thereby dampening the immune response against cancer cells. CTLA-4, on the other hand, reduces the activation of T cells, further downregulating the immune response [ 93 , 94 ]. These immune checkpoint pathways have emerged as promising targets for cancer immunotherapy, with the development of monoclonal antibodies against PD-1 (e.g. nivolumab, pembrolizumab), PD-L1 (e.g. avelumab, atezolizumab, durvalumab) and CTLA-4 (e.g. ipilimumab) showing clinical efficacy in the treatment of various cancers [ 93 , 95 ]. By inhibiting checkpoint molecules, ICIs are also beginning to show promise in overcoming the immune resistance often encountered in OC and PC treatment. Recent advancements have aimed to overcome these challenges by combining ICIs with other therapies such as chemotherapy, targeted therapy, and PARPi, which may affect the tumor microenvironment to enhance immune response [ 88 , 96 , 97 ]. In particular, the combination of PARPi and ICIs is being actively explored in clinical trials. In OC the main ICIs approved and used in clinical trials are pembrolizumab, nivolumab and ipilumab and they are used either alone (NCT02674061, NCT01611558 and NCT02728830) or in combination with chemotherapeutic agents such as paclitaxel (NCT03394885 and NCT02440425) and carboplatin (NCT03029598) or with PARPi such as rucaparib (NCT03824704, ARIES study) or niraparib (NCT02657889, TOPACIO study). From these clinical trials of note for their results are the TOPACIO/Keynote-162 study, the MEDIOLA study and the NCT2484404 study [ 98 ]. The TOPACIO study evaluated the combination of pembrolizumab and niraparib in recurrent platinum-resistant epithelial OC patients. The preliminary results of this study appear promising, being 4/8 evaluable OC patients responsive and the other 4 patients achieving SD, highlighting the importance of this combinatorial approach especially for OC and also other tumors with poor response to immunotherapy alone [ 99 ]. The MEDIOLA study evaluated the effect of the combination of olaparib and durvalumab (anti-PD-L1) in PARPi and ICI naïve BRCA mutant OC patients. As preliminary results, the combination has shown a high objective response rate (92%) in germline mutant BRCA patients, while the combination of olaparib, durvalumab and bevacizumab resulted as the best treatment for BRCA wild-type patients [ 100 ]. The results obtained from the MEDIOLA study were also confirmed by the NCT2484404 study in which the combination of olaparib and durvalumab was evaluated in patients with recurrent OC , showing also in this case a good tolerability for this treatment [ 101 ]. The encouraging results observed from this combinatorial treatment approach is fostering the design of novel clinical trial that might improve the response of OC to PD-1/PD-L1 and CTLA-4 inhibitors OC [ 102 ].
In PC, pembrolizumab has been approved only for patients with high microsatellite instability and deficient mismatch repair, which occur in 2–4% of cases [ 103 ]. There are several clinical studies to evaluate the effect of pembrolizumab alone [ 104 , 105 ] and in combination with enzalutamide [ 106 , 107 ] docetaxel [ 108 ] and olaparib [ 105 ] in PC. Initial data showed that only a minor subset of heavily pretreated patients can benefit from pembrolizumab therapy [ 104 ]. For example, in the Keynote 028 study, 23 patients with mCRPC positive for PD-L1 expression were enrolled and received pembrolizumab treatment, only four patients responded positively [ 104 ]. Keynote199 study showed that pembrolizumab as a monotherapy has antitumor activity in the bone-predominant mCRPC previously treated with docetaxel and targeted endocrine therapy (enzalutamide and abiraterone). This study also showed that 12% of the patients had aberrations in BRCA1/2 or ATM , and 10 (7%) had alterations in 12 or more other HRR genes. None of the six patients who experienced a response with evaluable genomic data had microsatellite instability. Taken together, responders with BRCA1/2 or ATM mutations had a longer response duration than responders without HRR aberrations [ 109 ]. The effect of the combination of olaparib and PD-1 has been published in several types of tumors [ 110 ]. In case of PC results of the combinational treatment with pembrolizumab and olaparib showed limited efficacy. Moreover, the efficacy was independent of HRR status and PD-L1 status [ 111 ]. When in combination, pembrolizumab plus enzalutamide in mCRPC previously treated with abiraterone showed limited antitumor activity. The phase 1b or 2 KEYNOTE-365 trial study included molecularly unselected docetaxel-treated mCRPC patients.
Recent studies indicate that ICIs alone and in combinations have only moderate effects in PC, but accurate predictive biomarkers have yet to be established for PC. Moreover, all the studies were performed on heavily pretreated and molecularly not selected patients. On a base of recent findings about pembrolizumab therapy and HRR [ 109 ], ICIs may be more effective in specific groups of molecularly selected PC patients carrying HRR defects. For example, as we have already mention above, the combination of ATR inhibition and anti-PD-L1 treatment resulted in synergistic, antitumor activity in PC [ 90 ]. This potent combination has already been tested in early-phase clinical trials in advanced malignancies (NCT04266912 and NCT04095273).
Hormonal regulation and its implications for DNA damage and repair
DNA damage in urogenital cancers is often pervasive, resulting from both endogenous metabolic processes and exogenous factors like radiation or chemotherapy [ 112 ]. Internally, DNA damage may arise from errors in DNA replication, reactive oxygen species (ROS) generated during cellular metabolism and natural cellular processes like hormone metabolism, particularly relevant in urogenital cancers [ 113 , 114 ]. While the previous paragraph addressed errors in DNA replication, we now aim to delve into hormonal regulation and its implication for DNA damage and repair in OC and PC, two hormone-regulated malignancies (Fig. 1 ).

Interplay between hormones and DNA Repair in BRCA -deficient cancers. The figure indicates the intersection of hormone therapy with the concept of 'BRCAness' in the context of ovarian (left side) and prostate (right side) cancer. In the nucleus, the DNA carrying BRCA1/2 mutations undergoes damage that can’t be repaired by the homologous recombination-based system. The inhibition of PARP, a key enzyme in the repair of single-strand DNA breaks, leads to synthetic lethality in these mutated cells, resulting in cell death. Modulation of estrogen (E), androgen (A) and progesterone (P) can influence the therapeutic landscape. Once the hormones enter inside the cells, they bind to their respective receptor (R) and might interact with different pathways and translocate to the nucleus to activate transcription of targeted genes. Inhibition of AR and ER blocks receptor translocation and might exert synthetic lethality with DNA damage response inhibitors, while the effect promoted by PR regulation through PR modulators (PRMs) remains still unclear
Hormonal regulation plays a significant role in the pathophysiology of OC. Ovarian hormones, primarily estrogen and progesterone, have been shown to affect cell proliferation, apoptosis, and DNA repair mechanisms [ 115 ]. A list of the main hormonal therapy and the respective clinical trials is presented below (Table 3 ).
Estrogen receptors (ER), primarily ERα and ERβ, are nuclear hormone receptors that mediate the effects of estrogen in target tissues. ERα is commonly associated with proliferative responses, while ERβ is thought to counteract these effects and is often linked with protective roles in cancer [ 116 ]. The mechanism of action of ERs involves the binding of estrogen, which facilitates their dimerization and subsequent binding to estrogen response elements (EREs) in the DNA. This binding initiates transcriptional regulation of various genes involved in cell growth, survival and differentiation [ 116 ]. In OC, the expression and activity of these receptors can significantly influence tumor behavior and patient prognosis. Recent studies, have highlighted the complex role of ERs in OC, demonstrating how ERα and ERβ can differentially regulate gene expression and contribute to cancer progression [ 117 , 118 , 119 ]. The link between ERs and DNA damage and repair mechanisms is an area of growing interest. Estrogen, through ER-mediated signaling, can influence the expression and activity of genes involved in DNA repair pathways, including HR and NHEJ [ 120 , 121 ]. While a considerable amount of literature has explored the relationship between hormonal regulation and DNA repair pathways, only a few studies have delved deeply into this area [ 122 ]. Some of them have shown that estrogen-induced ER activation can modulate the expression of key DNA repair proteins, such as BRCA1 and RAD51 ; this modulation can affect the efficiency of DNA repair mechanisms, influencing the sensitivity of OC cells to DNA-damaging agents [ 123 ]. Moreover, estrogen itself can be a source of DNA damage. Its metabolism can generate ROS and genotoxic metabolites, leading to DNA adducts and mutations and further implicating ER signaling in genomic instability [ 124 ]. Dysregulation of ERs, either through overexpression, mutation, or altered signaling pathways, can have significant implications in cancers, including OC [ 69 ]. Overexpression of ERα has been associated with increased tumor proliferation and poor prognosis. Conversely, loss or reduced expression of ERβ is often observed in OC and is thought to contribute to tumor aggressiveness and resistance to therapy [ 125 ]. Some researchers also highlighted the impact of ER dysregulation on the efficacy of hormonal therapies in BRCA mutant cancers showing that alterations in ER expression or function could lead to resistance to agents like selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) [ 126 , 127 ]. On the other hand, progesterone has been shown to exert a protective effect against the development of OC. Progesterone receptors (PR), existing in two main isoforms PR-A and PR-B, are expressed in ovarian tissue and influence various cellular processes. While PR-B is typically associated with progesterone's classical reproductive actions, PR-A can act as a dominant negative inhibitor of both PR-B and ERs [ 128 ]. Progesterone receptors, which exist in two isoforms, upon binding progesterone, undergo conformational changes, dimerize, and translocate to the nucleus where they bind to progesterone response elements (PREs) in the DNA. This binding initiates the transcription of various genes involved in cell proliferation, differentiation and survival. The mechanism is tightly regulated and is subject to modulation by various co-factors and cellular contexts [ 129 ]. These mechanisms have been explored in different studies in which it was demonstrate that PR signaling can influence tumor behavior and response to therapy [ 129 ]. Currently, different clinical trials are focusing on PR signaling, especially evaluating the therapeutic potential of progesterone receptor modulators (PRMs), a new class of synthetic compounds, such as mifepristone (NCT02014337, NCT02046421). PRMs compete in the binding sites of the PR and can act both as agonist or antagonists respectively by inducing or inhibiting transcriptional activation of the PR making them more clinically relevant [ 130 ]. Of note, interest in studying the relationship between PR signaling and DNA damage and repair mechanisms is increasingly emerging. Progesterone has been shown to impact the expression of genes involved in DNA repair pathways, potentially influencing genomic stability, but the mechanism remains still unknown [ 131 ]. Some work suggests that progesterone-activated PRs may modulate the expression of key DNA repair proteins and influence the cellular response to DNA damage [ 132 ]. This modulation may have critical implications in the context of OC, where DNA repair capacity can significantly affect tumor behavior and treatment response. Dysregulation of PR signaling, either through altered receptor expression, mutations, or changes in ligand availability, can significantly affect OC since the overexpression or constitutive activation of PRs can lead to abnormal stimulation of target genes, contributing to tumorigenesis and progression [ 133 ]. Conversely, loss of PR expression or function has been associated with a more aggressive tumor phenotype and poorer prognosis in OC [ 132 ]. In OC, also AR can play a critical role despite its pivotal role in other malignancies such as PC. In the work by Chung and colleagues, the researchers point out that AR can contribute to tumorigenesis, metastasis and chemoresistance [ 134 ]. Although OC is more traditionally associated with estrogen and progesterone receptors, different other studies have highlighted AR involvement in OC. AR expression has been observed in various subtypes of OC and its activation has been linked to tumor growth and poor prognosis suggesting that targeting AR signaling, especially with AR antagonists such as enzalutamide, might represent a potential therapeutic strategy for OC [ 134 , 135 , 136 ]. In this context, abiraterone, a potent inhibitor of the enzyme CYP17A1, plays a crucial role in androgen biosynthesis and has been explored as a therapeutic agent in AR-driven cancers. The CORAL (Cancer of the OvaRy Abiraterone triaL) study (NCT04476030) was designed to evaluate the clinical activity of abiraterone in epithelial OC and it is the only one currently available in the literature. In this trial a subset of patients derived sustained clinical benefit providing important information regarding the role of AR-mediated signaling inhibition in patients with recurrent, advanced epithelial OC (EOC) [ 137 ]. This trial represents a significant effort to target the AR pathway in OC, potentially offering a new therapeutic avenue for patients with AR-positive tumors.
The intricate relationship between hormonal influences and DNA repair processes in OC offers insights into novel therapeutic strategies, including the use of hormonal therapies for which many clinical trials exist. These therapies aim to modulate or block hormonal effects, particularly those of estrogen [ 132 ]. SERMs, AIs and Gonadotropin hormone-releasing hormone (GnRH) analogs are among the primary classes of hormonal therapies used [ 138 ]. SERMs, such as tamoxifen, function by competitively binding to estrogen receptors, thereby inhibiting estrogen-mediated signaling in cancer cells. In different clinical trials were evaluated the effect of different hormones; for example tamoxifen showing promising results in patients with resistant OC (NCT02728622). Aromatase inhibitors, including drugs like letrozole and anastrozole work by inhibiting the aromatase enzyme responsible for estrogen synthesis. Even in this case some trials assess the effectiveness of letrozole in advanced OC resistant or not to platinum therapy (NCT04720807, NCT04421547), demonstrating its potential. Finally, GnRH analogues, used primarily in premenopausal women, suppress ovarian function, thus reducing estrogen production [ 139 ].
Despite the potential of hormonal therapies, several challenges exist in their clinical application. Recent clinical trials have been instrumental in advancing our understanding of hormonal therapies in OC. As describe above, there are different clinical trials already focusing on SERMs or aromatase inhibitors, but fewer on the use of hormonal therapy in combination with other treatments, such as PARPi or other targeted therapies aiming to enhance efficacy and overcome resistance. As demonstrated in the work by Hao and colleagues, the intricate interplay between non-classical estrogen signaling and HRR deficiency in OC underscores the pivotal role of ERα in this process. In this study they provide evidence that ERα can exert a repressive effect on HRR activity identifying HR as an ERα target, thereby leading to an increased chemosensitivity of OC cells. [ 140 ] This work highlights the potential benefits of hormone replacement therapy in ameliorating the outcomes of OC treatment which can maybe be enhanced by combinatorial treatment with DDRi. Targeting the effects of estrogen and progesterone offers several advantages in the treatment of OC. One of the primary advantages of hormonal therapy is its targeted approach as we described before, since it allows targeting the hormonal key players in the proliferation and survival of OC cells. Compared to traditional chemotherapy, hormonal therapies generally present a more favourable toxicity profile. They are associated with fewer and less severe side effects, making them a more tolerable treatment option for many patients. Finally, hormonal therapies have also shown particular efficacy in certain subtypes of OC, such as estrogen receptor-positive (ER +) or low-grade serous carcinomas [ 141 ]. Despite these advantages, one of the major challenges with hormonal therapy is the development of resistance. Over time, OC cells can adapt to these therapies, altering their receptor expression or activating alternative signaling pathways, but there are only a few review articles in which this type of resistance is investigated and no research works are available [ 118 , 142 ]. Moreover, hormonal therapies are not universally effective across all OC subtypes. For example, high-grade serous OC (HGSOC), the most common and aggressive subtype, often does not effectively respond to hormonal therapy [ 118 ]. In summary, hormonal therapy in OC offers a targeted, less toxic alternative to traditional chemotherapy, with particular efficacy in certain cancer subtypes. However, challenges such as resistance development, limited efficacy in certain subtypes, and side effects cannot be overlooked. Thus, ongoing clinical trials and preclinical research are essential in addressing these challenges, improving therapeutic outcomes, finding alternatives to hormone therapy resistance and advancing personalized medicine approaches in the treatment of OC.
In PC, the AR is a member of the steroid hormone receptor family. AR signaling plays a fundamental role in physiological prostate development and function as well as in male morphologic development and configuration of the central neurons system [ 143 ]. The AR gene, located on the X chromosome, encodes 110 kDa protein composed of conserved DNA-binding domain and androgen-binding domain and a less conserved N-terminal transactivation domain [ 144 ]. AR influences transcription of androgen responsive genes. Recent findings showed the role of AR in PC growth and progression. In PC, AR can regulate cell proliferation, apoptosis, migration, invasion and cell differentiation [ 145 ]. Some studies also showed prognostic value of AR determined by immunohistochemistry, but the results are inconsistent and need to be verified [ 146 ]. PC development is dependent on androgens and androgen deprivation therapy (ADT) introduces an important therapeutic opportunity. ADT such as long-acting GnRH agonists (goserelin, histrelin, leuprolide, and triptorelin) or GnRH antagonists (degarelix), second-generation nonsteroidal AR antagonists (enzalutamide, apalutamide, and darolutamide) and the androgen biosynthesis inhibitor abiraterone are the first line therapy for patients with metastatic disease [ 147 ]. A list of the main hormonal therapy and the respective clinical trials is presented below (Table 4 ).
In 1% of primary PC cases, mutations and amplifications of the AR are observed, with this rate increasing to approximately 60% in metastatic tumors [ 148 ]. These mutations predominantly occur in the androgen-building domain of AR, resulting in antiandrogens (e.g. bicalutamide, hydroxyflutamide, enzalutamide, and apalutamide) functioning as AR agonists. This enables cancer progression and contributes to PC resistance to androgen deprivation therapy. Cai and colleagues showed that the T878A mutation has been associated with resistance to abiraterone in a xenograft PC model [ 149 ]. Moreover, mutant AR has been identified in circulating cell-free DNA [ 150 ]. Splicing variants of AR have also been detected in PCs, with AR-V7 splice variant also detected at the protein level [ 151 ]. AR-V7 is frequently detected in CRPC (around 75% of cases) [ 152 ]. Armstrong and collaborators in the prospective multicentric study (The PROPHECY Study) showed that AR-V7 detected in the blood of mCRPC was associated with shorter PFS and OS after abiraterone or enzalutamide treatment [ 153 ] On the other hand, in circulating tumor cells (CTCs) from AR-V7-positive PC, taxanes are more effective, while in AR-V7-negative PC, the effect is comparable [ 154 ]. In recent years, there is emerging evidence that AR signaling and the DDR pathways are related. Goodwin and collaborators showed that DNA damage induces AR activity, and active AR induces cell survival after DNA damage, indicating reciprocal regulation between AR and DDR. The study also revealed the impact of AR on the expression of DNA repair genes, identifying DNA PKcs as a key target of AR after DNA damage [ 155 ]. Furthermore, combining ADT with radiotherapy has been standard care approach for PC. RNAseq and Chipseq analysis on the xenograft model of castration-resistant PC LNCaP-AR, treated by enzalutamide, revealed downregulation of DNA repair genes. Further analysis defined 32 direct targets for AR, including RAD51C, MRE11A, CHEK1, LIG3 . AR signaling promotes double-strand DNA break repair and regulates the transcriptional program of DNA repair genes that promotes PC radio-resistance both in vitro and in vivo [ 156 ]. Previous studies showed that AR deprivation therapy enhances the effect of ionizing radiation by impairing NHEJ. However, AR signaling can also regulate HR genes. Asim and colleagues investigated the functional link between AR and HR, demonstrating decreased numbers of ionizing radiation-induced RAD51 foci in isogenic cells with low AR expression. Additionally, AR is required for effective ATM signaling mediated by MRE11. AR directly regulates HR activity, and androgen inhibition activates PARP signaling. Therefore, inhibition of AR is synthetically lethal with PARP inhibition in PC [ 157 ]. Furthermore, in PC, HR genes are frequently mutated, especially in mCRPC setting, offering potent therapeutic opportunities. The androgen inhibitor enzalutamide can suppress the expression of the HR genes, causing HR deficiency and BRCAness. This explains why enzalutamide and olaparib combination is effective in mCRPC patients and proves that also pharmaceutically induced BRCAness may expand the clinical use of PARPi [ 158 ]. A recent study showed that AR recruitment can be blocked by antineoplastic antibiotic mithramycin (MTM). MTM treatment caused the downregulation of AR target genes, including DDR genes. The study of Wang et al. discovered that MTM impaired DDR and enhanced effectiveness of the ionizing radiation and radiomimetic agent bleomycin [ 159 ]. Combining PARPi with AR inhibitors presents a powerful treatment option, as evidenced by several ongoing clinical studies. A phase 3 study is currently evaluating the PARPi niraparib in combination with apalutamide or abiraterone acetate plus prednisone in mCRPC [ 160 ]. Additionally, ongoing clinical studies are investigating combinations of enzalutamide with nanoparticle-based drugs [ 161 ] and I-131–1095 radiotherapy [ 162 ]. There is an increasing evidence about the role of progesterone and estrogen in the PC [ 163 ]. Recent findings indicated the potential oncogenic effects of progesterone in PC, with elevated progesterone levels associated with poor clinical outcomes in both castration-resistant and hormone-sensitive PC patients (HSPC). An increase in progesterone levels in the plasma of CRPC and HSPC patients was associated with poor clinical outcomes. Progesterone can activate canonical and non-canonical AR target genes, and inhibition of 3b-hydroxysteroid dehydrogenase 1 (3bHSD1) can suppress the oncogenic effects of progesterone [ 164 ]. Prostate tissues express both ERα and ERβ [ 165 ] and PC development depends also on estrogen signaling. Estrogen can increase the occurrence of androgen-induced PC [ 166 ]. Ricke and colleagues showed on a mice model that prostates from ERβ-knockout (βERKO) mice underwent carcinogenesis and the prostates from ER alpha-knockout mice remained free of disease [ 167 ]. Taking together ERβ is a tumor suppressor, and its inhibition leads to the prostate hyperplasia and tumor development. Therefore anti-estrogens and SERMS may reduce the risk of PC development in cases with high levels of ERβ [ 168 ]. ERα is also associated with the invasion and migration of PC cells [ 169 ]. Lombardi and colleagues demonstrated that PC3 cells express ERα and ERβ, with activation of ERβ influencing the expression of β-catenin and promoting proliferation of PC3 cells. Treatment with PKF 118–310, a drug that disrupts the β-catenin/TCF/LEF (T-cell-specific transcription factor/lymphoid enhancer-binding factor) complex, blocked the effect of ER-β [ 170 ].
Preclinical models for studying DNA damage and repair triggered by chemo-, targeted- and hormonal- agents
Thus far, we have recognized the significance of investigating DNA damage and repair alongside hormonal regulation in urogenital cancers, particularly in tumors like OC and PC. To dig deeper into these mechanisms, comprehensive studies necessitate various preclinical models. These range from traditional methods like cell culture and animal models to computational simulations and ex vivo models. Additionally, advanced translational platforms such as organoids, microfluidics, and organ-on-a-chip systems are invaluable tools in elucidating these intricate processes (Fig. 2 ).

Innovative therapeutic strategies and models in ovarian and prostate cancer: from bench to bedside. The figure encapsulates the multifaceted approach to cancer research and treatment, specifically for ovarian and prostate cancer. On the left side, two primary therapeutic targets for these tumors are indicated: the DNA damage response (DDR) pathway, which can be inhibited by DDR inhibitors and hormone therapy, which involves the modulation of androgens, estrogens and progesterone levels. On the right side, the available research models for studying these targets are indicated basing on their complexity: on the top part 2D in vitro models, on the middle part more complex 3D ex vivo models, such as organoids, microfluidic systems, and organ-on-a-chip technologies, on the bottom part animal models including genetically engineered mouse (GEMMs) and patient-derived xenograft (PDX) models
Investigating chemotherapy response using in vitro cell line studies
In vitro models, particularly cell line models, offer a simplified and controlled setting to study cancer biology, drug responses and genetic manipulations. We have extensively discussed how DDR pathways, particularly those involving HR and NHEJ, as well as hormonal regulation are often compromised especially in OC and PC. In this section we will delve into the main in-vitro models outlined in the literature, categorizing them based on the type of treatment and the development of resistance: chemotherapy, targeted therapy and hormone-based therapy both for ovary and prostate tumors.
In vitro chemotherapy-based studies
Despite advancements in research that introduce new therapeutic options, chemotherapy remains one of the primary treatments for OC and PC. Unfortunately, after the initial response, patients often develop resistance, highlighting the need for in vitro models to elucidate the mechanisms associated with these processes.
Cancer cell lines have been extensively utilized to investigate mechanisms of resistance to therapy, particularly in response to chemotherapy, which poses a significant challenge in treating OC and PC. To explore potential novel therapeutic strategies to overcome resistance, researchers have developed several cellular models with acquired resistance. By continuous exposure of cancer cell lines to the drug, researchers can observe the emergence of resistance and possibly identify the molecular changes that occur [ 49 , 171 , 172 ]. Consequently, several studies have focused on understanding the effects of chemotherapy alone or in combination with other treatments to elucidate the underlying mechanisms [ 74 ]. For instance, Bicaku and colleagues analyzed the response to carboplatin, cisplatin, and paclitaxel in OC survival. They treated 36 OC cell lines with these drugs, quantified IC50 levels and performed pre-treatment gene expression analyses correlating it with the IC50 levels biological pathway analysis. Results showed that cell line sensitivity to carboplatin, cisplatin, paclitaxel and their combination was associated with the expression of 77, 68, 64, and 25 biological pathways, respectively. From these results the study identified the Transcription/CREB pathway as one to be noted and that was associated with OC overall survival and cell line platinum sensitivity [ 74 ]. Similarly, Blanc-Durand and colleagues developed an assay to study HR in a chemotherapy treatment context. Their study found that HR deficiency, identified through a RAD51 functional assay, was associated with higher response rates to neoadjuvant platinum chemotherapy and longer progression-free survival in OC [ 173 ]. Another study by Acland et al. aimed to identify molecular features specific to chemoresistance in OC using carboplatin-resistant OVCAR5 and CaOV3 cell line models. The results of this study revealed enhanced migratory and invasive potential in the chemoresistant lines compared to the parental ones. Moreover, through mass spectrometry analysis they found distinct metabolic and signaling perturbations in chemoresistant lines, including dysregulation in cytokine and type 1 interferon signaling. This shared feature between cell lines and patient-derived primary cells indicates a common molecular aspect of chemoresistance, providing insights for future research on molecular mechanisms of chemoresistance and related phenotypes [ 46 ].
In PC, cell lines with acquired resistance to taxanes were developed by cultivation with increasing concentration of the drug [ 174 ]. Lima and colleagues identified multiple mechanisms associated with docetaxel resistance such as ABCB1, an ATP-binding cassette transporter overexpression, moreover increased expression of the genes associated with androgen signaling, cell survival, and overexpression of non-coding RNAs [ 175 ]. ABCB1 overexpression was also identified as a main player of cabazitaxel cross resistance with docetaxel [ 176 ]. Furthermore, DNA-PKc, a crucial component of the DDR, was found to promote taxane resistance in mCRPC [ 177 ]. According published evidence there are several mechanisms contributed in docetaxel resistance development as P-glycoprotein which was overexpressed in cell lines resistant to docetaxel (DU-145R and 22Rv1R). Inhibition of P-glycoprotein with elacridar (a P-glycoprotein inhibitor) reversed the presence of resistant phenotype [ 178 ]. Mumenthaler and colleagues used a pharmacological inhibitor targeting the Pim kinase (SGI-1776), to evaluate the effect of Pim kinase activity on PC cell survival and resistance. They exploited a paclitaxel-resistant 22Rv1 cell line, showing that inhibition of Pim kinase activity sensitized taxane chemoresistant cells to apoptosis, indicating its potential as a therapeutic target in overcoming docetaxel resistance [ 179 ].
In vitro targeted therapy-based studies
HR alterations are prevalent in both OC and PC, presenting potential and novel therapeutic targets for both diseases. However, to improve therapy response and advance personalized medicine, there is a critical need to develop accurate in vitro models.
For OC, A2780, OVCAR-3 and SKOV-3 cell lines are among the most frequently utilized to investigate the effects of targeted therapy, given their well-established profiles regarding BRCA1/2 mutations and other DDR-related genes [ 180 ]. For instance, numerous studies have employed these OC cell lines to elucidate the role of PARPi and/or ATM/ATR kinases in DNA repair processes [ 181 ]. Biegala and colleagues sought to understand olaparib resistance in OC and enhance its efficacy by investigating the cellular mechanisms of resistance. A key finding of their work was the development of an olaparib-resistant OC cell line (PEO1-OR) from BRCA2 mutated PEO1 cells. The study revealed that PEO1-OR cells acquired resistance through BRCA2 secondary mutations, upregulating HR repair-promoting factors and PARP1. Additionally, olaparib-resistant cells exhibited reduced sensitivity to ATR/CHK1 inhibitors, suggesting that combination therapy might resensitize them to PARPi, offering a potential strategy to overcome acquired resistance to PARPi in OC [ 182 ]. In another study, Fleury and colleagues investigated the sensitivity of HGSOC cell lines to PARPi, specifically olaparib. While PARPi sensitivity is commonly linked to HR deficiency, this study reveals a more complex scenario by demonstrating that downregulation of genes in the NER and MMR pathways also increases PARPi response. The highest sensitivity was observed when HR deficiency was concurrent with downregulation of either NER or MMR pathways, proposing a novel model for predicting PARPi sensitivity in patients [ 183 ].
In PC, LNCaP and C4-2B resistant to olaparib also exhibited resistance to other clinically relevant PARPi (rucaparib, niraparib, talazoparib). These olaparib-resistant cell lines accumulated DNA damage compared to parental cells, suggesting potential mechanisms underlying resistance [ 184 ]. On a base of current treatment strategies, it is clinically relevant to study cross resistance between current PC therapies i.e. (taxanes) and olaparib. There is increasing evidence that cells with acquired chemoresistance to docetaxel report cross-resistance to olaparib. DU-145 with acquired resistance to docetaxel showed ABCB1 overexpression-mediated cross-resistance to olaparib [ 184 ]. Schaaf and colleagues obtained similar results regarding cross-resistance between taxanes and olaparib; in addition, they show that cells resistant to docetaxel retain sensitivity to enzalutamide and vice versa [ 185 ].
In vitro hormone therapy-based studies
Given the significance of hormonal regulation in OC and PC, the following section will focus on the in vitro models that elucidate the mechanism of action, therapy response and chemoresistance of therapies targeted to hormonal regulation.
In OC, the majority of the studies is focused on estrogen-based therapy. For instance, Chao and colleagues investigated estrogen impact on OC cell growth and survival, focusing on alterations in cell-cycle regulatory proteins. They treated ovarian adenocarcinoma cell lines, OC-117-VGH (estrogen receptor-deficient) and OVCAR3 (estrogen receptor-positive), with different estrogen concentrations and observed differential effects on cell-cycle regulatory proteins. While there were no significant changes in cyclin D1 and E expression, p16/INK4a and p27/KIP1 expression was higher in OC-117-VGH than in OVCAR3. This suggests that estrogen-mediated inhibitory effects on OC might be mediated through different pathways in ER-positive and ER-negative cell lines [ 139 ]. Similarly, Li and colleagues explored estrogen role in EOC proliferation. They found that estrogen stimulation increased OC cell proliferation and invasion, with higher expression of transient receptor potential channel C3 (TRPC3) observed in OC tissue compared to normal tissue, suggesting TRPC3 as a potential therapeutic target [ 186 ]. In the study by Lima and colleagues, the impact of sex hormones on ADAMTS 1 and 4 expression in OC cells was evaluated. Progesterone was found to significantly increase ADAMTS protein and mRNA levels, particularly in ES-2 cells, with this effect reversed by the progesterone receptor antagonist RU486. This study concluded that progesterone, through its receptor, modulates ADAMTS 1 and 4 levels in OC cell lines, thereby influencing cancer features [ 187 ]. Additionally, Pedernera and colleagues assessed the effect of sexual steroids, including progesterone, on cell survival in primary cultures of ovarian carcinoma. From the analysis of samples from 35 patients with various subtypes of epithelial OC, they found a significant reduction in cell survival after progesterone treatment, particularly in endometrioid ovarian carcinoma. This effect was notably pronounced in cells positive for PR, suggesting a crucial role for progesterone and its receptor in reducing the progression of endometrioid ovarian carcinoma [ 188 ]. Furthermore, Limaye and colleagues evaluated the effectiveness of AR inhibition in managing HGSOC with recurrent cases. This study focused on a patient with HGSOC who experienced multiple relapses, but achieved excellent disease control through AR inhibition by using bicalutamide. The results of this study support the potential of targeting AR signaling in the treatment of OC, especially in patients with recurrent disease after initial treatments [ 189 ].
Androgen deprivation therapies are crucial for inhibiting PC progression. It is known that enzalutamide treatment decreases the expression of HR associated genes. Therapeutical approach where enzalutamide is followed by the olaparib showed significantly increased PC cell apoptosis [ 158 ]. Long-term culture in the presence of enzalutamide generated four genetically distinct enzalutamide-resistant AR-positive and AR-pathway dependent PC cell lines (CWR-R1, LAPC-4, LNCaP, VCaP). The transcriptomic characterization revealed deregulation in AR-associated and non-associated genes e.g. TMEFF2 (Transmembrane protein with EGF-like and two follistatin-like domains-2), β-catenin ( CTNNB1 ) pathways, MT2A (Metallothionein 2A) [ 190 ]. Additionally, studies by Liu and colleagues and Xu and colleagues demonstrated cross-resistance between enzalutamide and abiraterone in enzalutamide-resistant cells, with AR-V7 splicing variant identified as responsible for resistance to abiraterone. Inhibition of AR-V7 by niclosamide and enhancement of enzalutamide treatment by a novel HSP70 allosteric inhibitor, JG98, showed potential therapeutic benefits [ 191 , 192 ]. Moreover, enzalutamide resistant cells remain sensitive to olaparib [ 193 ], which provides interesting therapeutical option for therapy resistant patients. On the other hand, van Soest and colleagues published abiraterone and enzalutamide cross-resistance with taxanes. Notably, docetaxel and cabazitaxel inhibit AR translocation to the nucleus [ 194 ].
The role of DNA repair in enzalutamide treatment response was proved by study Zhang and colleagues. In this study they used CRISP/Cas9 knockout (GeCKO) library to identify the DNA-damaging agent idarubicin responsible to overcame abiraterone and enzalutamide resistance in PC in vitro. Idarubicin can fight enzalutamide and abiraterone resistance by inhibition of XPA expression [ 195 ]. In addition to in vitro models, also in silico models are employed in biological research. These computational models are based on algorithms and simulations to analyze biological data and predict outcomes starting from molecular simulations to whole-genome analyses. They are particularly useful to analyze large datasets, such as genomic sequences, and to identify patterns, mutations, gene expression changes, response to certain treatments and they can even support personalized medicine by predicting the most effective treatment strategies based on individual patients’ genetic profiles [ 196 ].
In vivo mouse models: from GEMMs to PDXs
Animal models serve as crucial systems for studying cancer mechanisms, with genetically engineered mouse models (GEMMs) and patient-derived xenografts (PDXs) offering significant insights into tumor growth, metastasis, and therapeutic responses in an in vivo context. In the first case, GEMMs, featuring specific mutations in DDR genes, provide insights into the role of these genes in cancer development and progression. In the context of OC, different reviews focused their attention on these models highlighting their advantages in managing specific gene mutations and consequently being helpful in understanding the efficacy of a treatment especially for targeted therapies potentially leading to better clinical outcomes [ 197 , 198 , 199 ]. For example, Shi and colleagues demonstrate that the inactivation of multiple genes like PTEN, TRP53, and RB1 in the ovarian surface epithelium of mice led to the development of type I low-grade OC, further emphasizing the utility of GEMMs in modelling the disease and its progression [ 200 ].
When studying PC, there are numerous GEMMs based on different genomic alterations relevant for PC and expressing different stages of the disease progression (for reviews see [ 201 ] and [ 202 ]). Ding and colleagues reported GEMMs by targeting PTEN and TP53 to develop model with metastatic PC and genomic instability [ 203 ]. Downregulation of CHK1 , which correlates with ERG expression in PTEN ± mice model, promoted high-grade prostatic intraepithelial neoplasia into invasive carcinoma [ 204 ].
On the other hand, PDXs generated by engrafting fresh human tumor fragments into immunodeficient mice, reflect patients' tissue histological and genetic characteristics [ 205 ]. The success rate of establishing PDXs depends on mouse origin and cancer tissue type, with higher rates observed in advanced or metastatic tumors. Indeed, the growth of PDX from primary tumors is around 2–10% while for advanced or metastatic tumors it is around 25–30% [ 206 ]. These models have been widely used in different tumors, for example animal models were used to study the effect of BRCA1/2 mutations in OC [ 207 ].
In OC research, PDX models are established by transplanting fresh patient tumor tissues into mice, often at orthotopic sites, to mimic the tumor's original environment and preserve its heterogeneity and genetic landscape [ 208 ]. PDXs have been instrumental in assessing the efficacy of PARPi in OC: Chen and colleagues in 2022 were able not only to replicate in PDX the results of clinical trials such as NOVA (NCT01847274), PRIMA (NCT02655016), and SOLO I, suggesting the utility of these models in mimicking clinical responses, but they predict also PARPi efficacy better than BRCA mutational status or platinum sensitivity. Key findings include high KRAS expression correlating with PARPi sensitivity, AKT1 enrichment indicating resistance, and low CA125 levels as potential PARPi efficacy indicators [ 209 ]. Additionally, Serra and colleagues investigated WEE1 and ATR inhibitors' efficacy in overcoming PARPi resistance in breast and ovarian cancers. Using patient-derived xeno-implant models, the study found that WEE1i response was associated with replication stress markers like STK11/RB1 and phospho-RPA, while ATRi response was associated to ATM mutations. The results suggest that targeting the replication stress response, particularly by WEE1i, can be an effective strategy to overcome PARPi resistance, even in tumors without homologous recombination repair deficiency. This approach provides important results and is under active testing in clinical trials [ 210 ].
In PC, there are several fundamental collection of PDX models like the MURAL collection [ 211 ] and the MD Anderson Prostate Cancer Patient-derived Xenograft Series (MDA PCa PDX) [ 212 ]. PDX models have demonstrated the antitumor activity of cabazitaxel in docetaxel- and enzalutamide-resistant tumors [ 177 ]. Therapy resistance is one of the biggest obstacles in PC therapy. Karkampouna et al. proposed a novel therapeutic strategy using multikinase inhibitors as ponatinib, sunitinib and sorafenib to overcome resistance to main PC therapies based on an androgen-dependent PCa PDX model [ 213 ].
Thus, differently from cell line models, PDX offers a platform for personalized medicine able also to recapitulate tumor heterogenity, crucial in studying the varied responses of different tumor cells to DNA damage and the efficacy of repair mechanisms. As far as PDXs present more advantages compare to cell lines, they also present several limitations: the establishment of PDX models is time-consuming, costly and resource-intensive since the growth rate of human tumors in mice can be slow, and not all patient samples successfully engraft and it requires specialized facilities [ 214 ]. Moreover, while PDX models maintain many aspects of the original tumor microenvironment, the immune component is significantly altered due to the immunodeficient nature of the host mice and this limitation can affect the study of immunological aspects of DDR. In addition, the use of animals in research brings ethical considerations and requires strict adherence to regulatory guidelines. Finally, while PDXs are valuable for preclinical studies, translating findings from these models to clinical outcomes can be challenging.
While animal models (syngeneic models) are widely used for the study of PARPi or other targeted therapies, we acknowledge that only few studies testing ICI in OC and PC are available, and even less works if considering possible combinatorial treatment with other drugs. Grabosch and colleagues assessed in vivo the response to anti-PD-L1 antibody and cisplatin either as single agents or in combination in EOC. The present study revealed that anti-PD-L1 targeted immunotherapy, when administered alone, exhibits remarkable efficacy against most aggressive models, even if this effect is tumor-dependent. It is important to note that cisplatin alone has the ability to modulate the immune microenvironment. Nevertheless, the combination of cisplatin with immune therapy appeared as the key for increasing mice survival rates in models of aggressive tumors and recurrent disease [ 215 ]. Also in the more recent work by Meng and colleagues, syngeneic mouse models were used to evaluate the therapeutic response of anti-PD-L1 therapy in OC, confirming how the effect of immunotherapy alone is limited, while the possible combination with PARPi such as niraparib, can improve the outcome [ 216 ]. Similarly to OC, for PC only few studies can be found [ 217 ]. Czernin and colleagues studied the synergistic effect of 225 Ac-PSMA617 and anti-PD-1 antibody on a model of C57BL/6-mice bearing syngeneic RM1-PGLS tumors. The results of the study demonstrate synergic antitumor effect of PSMA RNT plus PD-1 blockade [ 217 ]. Eximond and colleagues tested also the triple combination anti-CTLA-4 + anti-PD-1 + RT in the model of syngeneic CRPC mouse. Their study showed that two ICIs in combination with RT had a stronger effect in comparison with monotherapy [ 218 ]. In general ICI therapy has only a moderate effect in PC. But there is an evidence that ADT might sensitize tumors to the checkpoint blockade by enhancing CD8 T cell function in mice model. Study on mouse implanted with PD-1 resistant tumors showed that enzalutamide is able to sensitize these mice to anti-PD-L1 antibody therapy by direct effect of androgen deprivation on immune cells in the tumor [ 219 ].
Overall these works suggest that combinatorial strategies for ICI, including both chemotherapy or targeted therapies, should be taken into considerations both for OC and PC to increase ICI effect.
Patient-derived 3D models: organoids, microfluidics and organ-on-a-chip
While previous models have contributed significantly to our understanding of ovarian and PCs, they fall short in fully capturing the complexity of human tumor microenvironment. To bridge this gap, translational models like organoids, microfluidics, and organ-on-a-chip systems have emerged as pivotal tools in cancer research. These models represent a significant milestone, particularly microfluidics and organ-on-a-chip systems, which integrate living human cells within a micro-engineered environment, simulating the physiology and mechanics of human organs. In details, microfluidics involves the manipulation of fluids at a microscale in channels with dimensions of tens to hundreds of micrometres, allowing precise control of the cellular microenvironment and facilitating the study of cellular responses under various physiological conditions [ 220 ]. Organ-on-a-chip systems, an extension of microfluidic technology, integrate cell cultures in a micro-engineered environment to mimic the structure and function of human organs. These systems can replicate key aspects of an organ’s microarchitecture and biomechanical properties, providing a more physiologically relevant model for studying disease processes [ 221 ]. The use of the microfluidic models has been instrumental in studying OCs, replicating tumor microenvironment and providing insights into tumor invasion and drug testing [ 222 ]. Despite their advantages, these systems are not without limitations. First, the design and fabrication of microfluidic and organ-on-a-chip systems can be complex and costly; they are optimized for small-scale experiments and the translation to clinical applications is challenging and not immediate. Finally, these systems often involve intricate techniques and precise control of experimental conditions [ 223 ].
OC and PC research has been hampered by the lack of suitable in vitro model systems. The most noteworthy translational model is the organoid one, as a self-organizing three-dimensional cell cultures generated from isolated pluripotent stem cells or progenitor cells of a patient’s tumor or non-tumor tissue [ 224 ]. Organoids closely mimic the architecture, functionality and genetic landscape of the original tissue, bridging the gap between traditional in vitro models and in vivo studies, becoming an indispensable tool in both basic research and clinical applications [ 225 ]. The genesis of organoid technology is largely attributed to the pioneering work of Hans Clevers, who has opened new avenues in studying a wide array of organs. Clevers and his team first demonstrated the potential of organoids in modeling the gut, showing that a single Lgr5 + stem cell from the adult mouse intestine could grow into a self-organizing structure that recapitulates the intestinal epithelium in vitro [ 226 ]. This revolutionary work illuminated the path for organoid research across various organ systems including the brain, gut, liver, prostate and ovaries [ 227 ], providing moreover new models for drug testing and for understanding disease mechanisms at a cellular level.
Focusing in particular on OC, due to the high degree of heterogeneity, organoid establishment and maintenance in culture was not easy. In this context, the literature is plenty of studies focusing on their establishment and different protocols were published and are still improving [ 228 , 229 , 230 ]. Moreover, there are also many works in which these organoids provide a means to investigate the unique tumor microenvironment of OC, including the study of tumor initiation, progression, metastasis and drug resistance [ 231 ]. One of the first relevant study in this field is the one from Kopper and colleagues in which OC organoids have been used to model different subtypes of the disease, including HGSOC being thus crucial in studying subtype-specific characteristics and responses to treatment [ 232 ]. The primary objective of their research was to establish a diverse panel of OC organoids that accurately reflect the various subtypes of OC, including HGSOC, which is the most common and aggressive form of the disease. These organoids were developed from tumor samples of patients with different OC subtypes, ensuring that the models encompassed a wide range of genetic and histological variations seen in actual patient tumors. A critical aspect of their study was the successful maintenance of the histopathological and genetic characteristics of the original tumors in the organoids demonstrating that they retained key features of OC, including specific genetic mutations, gene expression profiles, and histological structures, making them highly representative of the in vivo condition. The second main point of this work is that ovarian organoids were also employed to evaluate responses to various chemotherapeutic agents and targeted therapies showing that organoids' responses to these treatments mirrored clinical outcomes, demonstrating their potential as predictive models for personalized medicine. In other studies, organoids have been employed in high-throughput drug screening to identify potential therapeutics for OC. Nanki and colleagues developed expandable OC organoids and, after demonstrating their ability to model various subtypes of OC and to reflect the heterogeneity of the disease, employed them for drug sensitivity and resistance testing [ 233 ]. Of note, in this work they successful developed organoids in less than 3 weeks, capturing the characteristics of different histological cancer subtypes and replicating the primary tumors' mutational landscape. Furthermore, one organoid with a BRCA1 pathogenic variant, showed higher sensitivity olaparib and platinum drugs and an organoid derived from clear cell OC exhibited resistance to conventional drugs, including platinum drugs, paclitaxel, and olaparib [ 233 ]. The potential of organoids in the evaluation of the molecular mechanism underlying OC was also demonstrated in the work from Wang and collaborators, where RNA sequencing of cisplatin-resistant and -sensitive OC organoids revealed higher FBN1 expression in resistant samples. From further investigations they found that FBN1 's is involved in energy stress, angiogenesis, and chemoresistance and thanks to these results, they were able to identify the FBN1/VEGFR2/STAT2 signaling axis as a key mediator in these processes, suggesting potential therapeutic strategies targeting FBN1 combined with antiangiogenic drugs for OC treatment [ 234 ]. Overall, these works suggest that organoids can accurately mirror the biology of the tumor of origin and can be exploited for high-throughput drug screening, identifying potential therapeutics and elucidating drug resistance mechanisms [ 225 , 232 ].
It is well known that cells with stem-like potential represent a potential source to create patient-derived organoids (PDOs); in the case of PC, mainly basal cells, that show high proliferation and self-renewal and CD133 and CD44 phenotype compared to luminal cells, contribute to organoid establishment [ 235 ]. In the first PDO models, only basal cells reconstitute a prostate gland. In 2014 Karthaus and colleagues described the development of an R-spondin1-based culture method. This method admits a long-term propagation of murine and human prostate epithelium consisting of fully differentiated CK5 + basal and CK8 + luminal cells [ 236 ]. These protocols allowed cultured PDOs from prostate tissues, but they did not recapitulate AR signaling, which is essential for prostate development and also for PC progression and therapy. By adding Epidermal Growth Factor (EGF), Noggin, and R-spondin 1 to the growth medium, Drost and colleagues were able to generate long term growing organoids that functionally recapitulate AR signaling [ 237 ].
In general, successful generation of PDOs cultures struggles with many pitfalls. Organoid cultures from PC biopsies have variable growth rates caused probably by the high heterogeneity of the disease [ 238 ]. PC PDOs also show low efficiency in their establishment (15–20%) [ 239 ]. Servant and collaborators generated organoids from 81 PC patient samples. The success rate was around 44% for tissues from metastatic prostatectomy and around 28% for tissues from transurethral resection of the prostate [ 240 ]. In the study of Puca and colleagues, organoids from metastatic tissue of 25 PC patients were generated with a success rate of only 16% and the organoids were classified as neuroendocrine PC [ 241 ]. There is a strong evidence that PC organoids grow at different rates depending on the tissue of origin and clinico-pathological features of the patients’ tissue [ 240 ]. Because of their slow growth and low success rate, there is a need to optimize the protocol for generation of PDOs. Gao and colleagues established in 2014 for the first time the long term fully characterized cultured PC organoid platform derived from advanced and metastatic PC tissues, which recapitulates molecular diversity of PC and showed TMPRSS2-ERG fusion, SPOP mutation, SPINK1 overexpression, and CHD1 loss as well as mutations in DNA repair pathway. These PDOs showed common features for advanced PC such as TP53 and RB loss, AR signaling, while mirroring the tumor of origin both at the genetic and phenotypic levels [ 242 ].
In conclusion, the versatility of organoids extends beyond disease modeling to regenerative medicine. Organoids offer a promising avenue for tissue regeneration and personalized medicine, including the potential for organ transplantation and the development of patient-specific treatment plans. Their ability to mimic patient-specific disease phenotypes makes them ideal for precision medicine applications, revolutionizing our ability to model human diseases and test therapeutic interventions with unprecedented precision and relevance.
Conclusions and perspectives
In this review, we emphasize the significance of genomic instability, DNA damage and repair mechanisms, synthetic lethality, and hormonal regulation in OC and PC as well as the importance to use precise in vivo and in vitro models to study these signaling pathways. Understanding these factors is essential for improving diagnosis, treatment and outcomes in patients with urogenital cancers. One key aspect we delves into is the hormonal regulation and its implication for urogenital cancers treatment and resistance especially in the context of DNA damage and repair due to its significant impact on both the development and progression of these hormonal-related diseases. Since hormones such as estrogen, progesterone and androgens play important roles in urogenital function and pathology, their dysregulation can lead to enhanced proliferation of cancer cells and contribute to carcinogenesis. Thus, it is imperative to understand the interplay between hormonal pathways and DNA damage repair mechanisms in the context of OC and PC. Deciphering the role of hormones could facilitate the development of personalized medicine by identifying novel tailored treatments that might effectively circumvent the onset of resistance based on each patient's distinct cancer profile.
In this review we also discuss two of the main challenges for cancer therapy: therapy response and chemoresistance development, both involving DDR. Advances in understanding DDR mechanisms have led to the development of targeted therapies, such as PARPi, and to foster the design of novel therapeutic approaches to overcome acquired resistance. This focus on DNA damage and repair mechanisms is crucial for advancing research in precision medicine and understanding individual variations in DDR pathways in urogenital cancers could help adapting therapies to specific genetic profiles and optimizing therapeutic outcomes. In addition to this point, we have also emphasised the role of ICIs both in OC and PC, especially showing how their effect can be increased when in combination with other agents like ATR inhibitors, which could yield synergistic antitumor effects in patients with limited response to conventional therapies. Thus, further exploration and optimization of combination therapies could extend the benefits of ICIs to a broader patient population.
Lastly, we highlight the importance of utilizing advanced models to study these mechanisms, as they provide important insights into molecular pathways. Animal models and 3D ex vivo models have provided significant advancements in the field of OC and PC research. Starting from animal models, we highlighted how GEMMS and PDXs play pivotal roles in cancer research by providing in vivo systems that closely mirror human tumor biology allowing researchers to study the molecular and cellular mechanisms of cancer and following tumor progression and drug response. Transitioning to 3D technologies, we highlight microfluidics and organ-on-a-chip, which replicate structure and function while enabling fluid manipulation, providing a physiologically relevant model for disease processes. In this context, we wanted to shed light especially on the organoids, which have emerged as a critical bridge between in vitro and in vivo studies. Organoids can mimic the architecture and genetic landscape of the source tissue, enabling the study of disease mechanisms, drug responses and tumor evolution with a level of precision and relevance that was previously unattainable. In OC, organoids effectively tackle disease heterogeneity, modeling different subtypes, like HGSOC and facilitating studies on tumor characteristics and treatment responses. Diverse platforms of OC organoids that retain the genetic and histopathological features of the original tumors have been created, making them suitable for personalized medicine approaches, being used for drug sensitivity testing and elucidating molecular mechanisms underlying cancer. Also PC research has benefited from organoid technology, utilizing basal cells with stem-like potential to establish PC organoids, that mimic AR signaling—a key factor in PC progression. Despite the low establishment efficiency and variable growth rates, significant strides have been made in fully characterizing cultured prostate organoids. Thus, these systems offer detailed insights into tumor biology and are instrumental for therapeutic efficacy and toxicity assessments. In the quest for new cancer treatments, organoids serve as a powerful tool, allowing for more personalized therapy development and reducing reliance on animal testing, thereby expediting translation from bench to bedside. Nonetheless, challenges regarding complexity, cost, and scalability for clinical applications persist and are in constant development and improvement.
By integrating advanced technologies and more reliable models, we might advance our understanding on the interplay between response to DNA damage and hormonal regulation in urogenital cancers and develop more effective and personalized therapeutic options. Overall, the integration of multidisciplinary approaches will be essential for addressing the challenges posed by these complex diseases to improve patient care in the era of personalized medicine.
Availability of data and materials
Not applicable.
Abbreviations
3b-hydroxysteroid Dehydrogenase 1
Aromatase Inhibitor
Androgen Deprivation Therapies
Androgen Receptor
Androgen Receptor splice variant-7
Base Excision Repair
Castration Resistance Prostate cancer
Circulating Tumor Cells
Cytotoxic T-lymphocyte-associated protein 4
DNA Damage Response
DNA Damage Response Inhibitor
Deoxyribonucleic Acid
Double strand Breaks
Epidermal Growth Factor
Estrogen Receptor
Estrogen Response Elements
Genetically engineered mouse models
Gonadotropin hormone-Releasing Hormone
High-Grade Serous Ovarian Cancer
Hormone Sensitive Prostate cancer
Homologous Recombination
Homologous Recombination Repair
Metastatic Castration Resistant Prostate cancer
Metastatic Prostate cancer
Mismatch Repair
Magnetic Resonance Imaging
Mithramycin
Non-Homologous End Joining
Nucleotide Excision Repair
- Ovarian cancer
- Prostate cancer
Poly-ADP Ribose Polymerase
Poly-ADP Ribose Polymerase inhibitor
Prostate cancer Antigen-3
Programmed cell death protein 1
Programmed cell death-ligand 1
Patient-derived xenografts
Patient Derived Organoids
Positron Emisssion Tomography
Prostate Health Index
Prostate Specif Antigen
Prostate Specific Membrane Antigen
Progesterone Receptor
Progesterone Response Elements
Progesterone Receptor Modulators
Reactive Oxygen Species
Selective Estrogen receptor modulators
Single strand Breaks
Transient receptor potential channel C3
Holtedahl K, et al. Symptoms and signs of urogenital cancer in primary care. BMC Prim Care. 2023;24(1):107.
Article PubMed PubMed Central Google Scholar
Siegel RL, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Article PubMed Google Scholar
Helleday T, Eshtad S, Nik-Zainal S. Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet. 2014;15(9):585–98.
Article CAS PubMed PubMed Central Google Scholar
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46.
Article CAS PubMed Google Scholar
Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–20.
González-Martín A, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391–402.
Mateo J, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30(9):1437–47.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Ali AT, Al-Ani O, Al-Ani F. Epidemiology and risk factors for ovarian cancer. Prz Menopauzalny. 2023;22(2):93–104.
CAS PubMed PubMed Central Google Scholar
Gaona-Luviano P, Medina-Gaona LA, Magaña-Pérez K. Epidemiology of ovarian cancer. Chin Clin Oncol. 2020;9(4):47.
Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55(1):3–23.
Walker M, Jacobson M, Sobel M. Management of ovarian cancer risk in women with. CMAJ. 2019;191(32):E886–93.
Budiana ING, Angelina M, Pemayun TGA. Ovarian cancer: pathogenesis and current recommendations for prophylactic surgery. J Turk Ger Gynecol Assoc. 2019;20(1):47–54.
Liberto JM, et al. Current and emerging methods for ovarian cancer screening and diagnostics: a comprehensive review. Cancers (Basel). 2022;14(12):2885.
Matsas A, et al. Tumor markers and their diagnostic significance in ovarian cancer. Life (Basel). 2023;13(8):1689.
CAS PubMed Google Scholar
Dochez V, et al. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28.
Dochez V, et al. Efficacy of HE4, CA125, risk of malignancy index and risk of ovarian malignancy index to detect ovarian cancer in women with presumed benign ovarian tumours: a prospective, multicentre trial. J Clin Med. 2019;8(11):1784.
Mathieu KB, et al. Screening for ovarian cancer: imaging challenges and opportunities for improvement. Ultrasound Obstet Gynecol. 2018;51(3):293–303.
Ledermann JA. First-line treatment of ovarian cancer: questions and controversies to address. Ther Adv Med Oncol. 2018;10:1758835918768232.
Raja FA, Chopra N, Ledermann JA. Optimal first-line treatment in ovarian cancer. Ann Oncol. 2012;23 Suppl 10:x118–27.
Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131(5):909–27.
Chien J, Poole EM. Ovarian cancer prevention, screening, and early detection: report from the 11th biennial ovarian cancer research symposium. Int J Gynecol Cancer. 2017;27(9S Suppl 5):S20–2.
Guo T, et al. Cellular mechanism of gene mutations and potential therapeutic targets in ovarian cancer. Cancer Manag Res. 2021;13:3081–100.
Maioru OV, et al. Developments in genetics: better management of ovarian cancer patients. Int J Mol Sci. 2023;24(21):15987.
Ramus SJ, Gayther SA. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol Oncol. 2009;3(2):138–50.
Gorodetska I, Kozeretska I, Dubrovska A. Genes: the role in genome stability, cancer stemness and therapy resistance. J Cancer. 2019;10(9):2109–27.
Stewart MD, et al. Homologous recombination deficiency: concepts, definitions, and assays. Oncologist. 2022;27(3):167–74.
Mangogna A, et al. Homologous recombination deficiency in ovarian cancer: from the biological rationale to current diagnostic approaches. J Pers Med. 2023;13(2):284.
Lin Y, et al. Metabolic reprogramming of the tumor immune microenvironment in ovarian cancer: a novel orientation for immunotherapy. Front Immunol. 2022;13:1030831.
Rajwa P, et al. Prostate cancer risk, screening and management in patients with germline BRCA1/2 mutations. Nat Rev Urol. 2023;20(4):205–16.
Gandaglia G, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol. 2021;4(6):877–92.
Wang G, et al. Genetics and biology of prostate cancer. Genes Dev. 2018;32(17–18):1105–40.
David MK, Leslie SW. Prostate Specific Antigen. 2022 Nov 10. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2024.
Lepor A, Catalona WJ, Loeb S. The prostate health index: its utility in prostate cancer detection. Urol Clin North Am. 2016;43(1):1–6.
Cui Y, et al. Evaluation of prostate cancer antigen 3 for detecting prostate cancer: a systematic review and meta-analysis. Sci Rep. 2016;6:25776.
Shah S, et al. BRCA mutations in prostate cancer: assessment, implications and treatment considerations. Int J Mol Sci. 2021;22(23):12628.
Hofman MS, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208–16.
Tolkach Y, Kristiansen G. The heterogeneity of prostate cancer: a practical approach. Pathobiology. 2018;85(1–2):108–16.
Mateo J, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697–708.
Rebello RJ, et al. Prostate cancer. Nat Rev Dis Primers. 2021;7(1):9.
de Bono J, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–102.
Boussios S, et al. BRCA mutations in ovarian and prostate cancer: bench to bedside. Cancers (Basel). 2022;14(16):3888.
Rajan A, et al. Deregulated estrogen receptor signaling and DNA damage response in breast tumorigenesis. Biochim Biophys Acta Rev Cancer. 2021;1875(1):188482.
Prados-Carvajal R, et al. Preventing and overcoming resistance to PARP inhibitors: a focus on the clinical landscape. Cancers (Basel). 2021;14(1):44.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Acland M, et al. Chemoresistant cancer cell lines are characterized by migratory, amino acid metabolism, protein catabolism and IFN1 signalling perturbations. Cancers (Basel). 2022;14(11):2763.
Huang R, Zhou PK. DNA damage repair: historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct Target Ther. 2021;6(1):254.
Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability–an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11(3):220–8.
Alatise KL, Gardner S, Alexander-Bryant A. Mechanisms of drug resistance in ovarian cancer and associated gene targets. Cancers (Basel). 2022;14(24):6246.
Sharma P, et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–23.
Patch AM, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–94.
Imyanitov E, Sokolenko A. Mechanisms of acquired resistance of BRCA1/2-driven tumors to platinum compounds and PARP inhibitors. World J Clin Oncol. 2021;12(7):544–56.
Aldea M, et al. Overcoming resistance to tumor-targeted and immune-targeted therapies. Cancer Discov. 2021;11(4):874–99.
Kondrashova O, et al. Secondary somatic mutations restoring. Cancer Discov. 2017;7(9):984–98.
Hurley RM, et al. Characterization of a RAD51C-silenced high-grade serous ovarian cancer model during development of PARP inhibitor resistance. NAR Cancer. 2021;3(3):zcab028.
Xu J, et al. RAD51D secondary mutation-mediated resistance to PARP-inhibitor-based therapy in HGSOC. Int J Mol Sci. 2023;24(19):14476.
Bashashati A, et al. Distinct evolutionary trajectories of primary high-grade serous ovarian cancers revealed through spatial mutational profiling. J Pathol. 2013;231(1):21–34.
Karimi F, et al. Liquid biopsy in ovarian cancer: advantages and limitations for prognosis and diagnosis. Med Oncol. 2023;40(9):265.
Pereira E, et al. Personalized circulating tumor DNA biomarkers dynamically predict treatment response and survival in gynecologic cancers. PLoS ONE. 2015;10(12):e0145754.
Alahdal M, et al. Current advances of liquid biopsies in prostate cancer: molecular biomarkers. Mol Ther Oncolytics. 2023;30:27–38.
Patel M, et al. The role of poly(ADP-ribose) polymerase inhibitors in the treatment of cancer and methods to overcome resistance: a review. Cell Biosci. 2020;10:35.
Abida W, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. 2017;2017:PO.17.00029.
PubMed Google Scholar
Huang XZ, et al. Efficacy and prognostic factors for PARP inhibitors in patients with ovarian cancer. Front Oncol. 2020;10:958.
Alameddine Z, et al. A Meta-analysis of randomized clinical trials assessing the efficacy of PARP inhibitors in metastatic castration-resistant prostate cancer. Curr Oncol. 2023;30(10):9262–75.
Chandrasekaran A, Elias KM. Synthetic lethality in ovarian cancer. Mol Cancer Ther. 2021;20(11):2117–28.
Neiger HE, Siegler EL, Shi Y. Breast cancer predisposition genes and synthetic lethality. Int J Mol Sci. 2021;22(11):5614.
Hopkins JL, Lan L, Zou L. DNA repair defects in cancer and therapeutic opportunities. Genes Dev. 2022;36(5–6):278–93.
Alenezi WM, et al. Genetic analyses of DNA repair pathway associated genes implicate new candidate cancer predisposing genes in ancestrally defined ovarian cancer cases. Front Oncol. 2023;13:1111191.
Omoike OE, et al. A cross-sectional study of the association between perfluorinated chemical exposure and cancers related to deregulation of estrogen receptors. Environ Res. 2021;196:110329.
Wu Y, et al. Clinical application of PARP inhibitors in ovarian cancer: from molecular mechanisms to the current status. J Ovarian Res. 2023;16(1):6.
Miller RE, El-Shakankery KH, Lee JY. PARP inhibitors in ovarian cancer: overcoming resistance with combination strategies. J Gynecol Oncol. 2022;33(3):e44.
Konstantinopoulos PA, Lheureux S, Moore KN. PARP inhibitors for ovarian cancer: current indications, future combinations, and novel assets in development to target DNA damage repair. Am Soc Clin Oncol Educ Book. 2020;40:1–16.
Alayev A, et al. Estrogen induces RAD51C expression and localization to sites of DNA damage. Cell Cycle. 2016;15(23):3230–9.
Bicaku E, et al. In vitro analysis of ovarian cancer response to cisplatin, carboplatin, and paclitaxel identifies common pathways that are also associated with overall patient survival. Br J Cancer. 2012;106(12):1967–75.
Foster KI, et al. Clinical implications of tumor-based next-generation sequencing in high-grade epithelial ovarian cancer. Cancer. 2023;129(11):1672–80.
Abida W, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A. 2019;116(23):11428–36.
Lukashchuk N, et al. Impact of DNA damage repair alterations on prostate cancer progression and metastasis. Front Oncol. 2023;13:1162644.
CrestaMorgado P, Mateo J. Clinical implications of homologous recombination repair mutations in prostate cancer. Prostate. 2022;82 Suppl 1:S45–59.
Google Scholar
Zhang W, et al. Role of the DNA damage response in prostate cancer formation, progression and treatment. Prostate Cancer Prostatic Dis. 2020;23(1):24–37.
Mateo J, et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J Clin Invest. 2020;130(4):1743–51.
Robinson D, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–28.
Pritchard CC, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–53.
Risdon EN, et al. PARP inhibitors and prostate cancer: to infinity and beyond BRCA. Oncologist. 2021;26(1):e115–29.
Vasquez JL, et al. Inhibition of base excision repair by natamycin suppresses prostate cancer cell proliferation. Biochimie. 2020;168:241–50.
Kaufman B, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–50.
Mateo J, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):162–74.
Abida W, et al. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the phase II TRITON2 study. Clin Cancer Res. 2020;26(11):2487–96.
Taylor AK, et al. PARP inhibitors in metastatic prostate cancer. Front Oncol. 2023;13:1159557.
Drápela S, et al. The CHK1 inhibitor MU380 significantly increases the sensitivity of human docetaxel-resistant prostate cancer cells to gemcitabine through the induction of mitotic catastrophe. Mol Oncol. 2020;14(10):2487–503.
Tang Z, et al. ATR inhibition induces CDK1-SPOP signaling and enhances Anti-PD-L1 cytotoxicity in prostate cancer. Clin Cancer Res. 2021;27(17):4898–909.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Tan S, Li D, Zhu X. Cancer immunotherapy: pros, cons and beyond. Biomed Pharmacother. 2020;124:109821.
Zhang H, et al. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer Res. 2021;40(1):184.
Pandey P, et al. Revolutionization in cancer therapeutics via targeting major immune checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals (Basel). 2022;15(3):335.
Wojtukiewicz MZ, et al. Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021;40(3):949–82.
Indini A, et al. Immune-checkpoint inhibitors in platinum-resistant ovarian cancer. Cancers (Basel). 2021;13(7):1663.
Bogani G, et al. Immunotherapy for platinum-resistant ovarian cancer. Gynecol Oncol. 2020;158(2):484–8.
Turinetto M, et al. The role of PARP inhibitors in the ovarian cancer microenvironment: moving forward from synthetic lethality. Front Oncol. 2021;11:689829.
Konstantinopoulos PA, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5(8):1141–9.
Drew Y, et al. Olaparib plus durvalumab, with or without bevacizumab, as treatment in PARP inhibitor-naïve platinum-sensitive relapsed ovarian cancer: a Phase II multi-cohort study. Clin Cancer Res. 2024;30(1):50–62.
Lee JM, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (ADP-Ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1–3 inhibitor cediranib in women’s cancers: a dose-escalation phase I study. J Clin Oncol. 2017;35(19):2193–202.
Zhu J, Yan L, Wang Q. Efficacy of PD-1/PD-L1 inhibitors in ovarian cancer: a single-arm meta-analysis. J Ovarian Res. 2021;14(1):112.
Sena LA, et al. Tumor frameshift mutation proportion predicts response to immunotherapy in mismatch repair-deficient prostate cancer. Oncologist. 2021;26(2):e270–8.
Hansen AR, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29(8):1807–13.
Antonarakis ES, et al. Pembrolizumab plus olaparib for patients with previously treated and biomarker-unselected metastatic castration-resistant prostate cancer: the randomized, open-label, phase III KEYLYNK-010 trial. J Clin Oncol. 2023;41(22):3839–50.
Graff JN, et al. KEYNOTE-641: a Phase III study of pembrolizumab plus enzalutamide for metastatic castration-resistant prostate cancer. Future Oncol. 2021;17(23):3017–26.
Gratzke C, et al. KEYNOTE-991: pembrolizumab plus enzalutamide and androgen deprivation for metastatic hormone-sensitive prostate cancer. Future Oncol. 2023.
Petrylak DP, et al. KEYNOTE-921: phase III study of pembrolizumab plus docetaxel for metastatic castration-resistant prostate cancer. Future Oncol. 2021;17(25):3291–9.
Antonarakis ES, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38(5):395–405.
Appleton KM, et al. PD-1/PD-L1 checkpoint inhibitors in combination with olaparib display antitumor activity in ovarian cancer patient-derived three-dimensional spheroid cultures. Cancer Immunol Immunother. 2021;70(3):843–56.
Yu EY, et al. Pembrolizumab plus olaparib in patients with metastatic castration-resistant prostate cancer: long-term results from the phase 1b/2 KEYNOTE-365 cohort a study. Eur Urol. 2023;83(1):15–26.
Moretton A, Loizou JI. Interplay between cellular metabolism and the DNA damage response in cancer. Cancers (Basel). 2020;12(8):2051.
Ding DN, et al. Insights into the role of oxidative stress in ovarian cancer. Oxid Med Cell Longev. 2021;2021:8388258.
Cucchi D, Gibson A, Martin SA. The emerging relationship between metabolism and DNA repair. Cell Cycle. 2021;20(10):943–59.
Li H, et al. Hormone therapy for ovarian cancer: emphasis on mechanisms and applications (Review). Oncol Rep. 2021;46(4):223.
Shen Z, et al. Correlation between estrogen receptor expression and prognosis in epithelial ovarian cancer: a meta-analysis. Oncotarget. 2017;8(37):62400–13.
Langdon SP, et al. Estrogen signaling and its potential as a target for therapy in ovarian cancer. Cancers (Basel). 2020;12(6):1647.
Simpkins F, Garcia-Soto A, Slingerland J. New insights on the role of hormonal therapy in ovarian cancer. Steroids. 2013;78(6):530–7.
Faratian D, et al. Trastuzumab and pertuzumab produce changes in morphology and estrogen receptor signaling in ovarian cancer xenografts revealing new treatment strategies. Clin Cancer Res. 2011;17(13):4451–61.
Song L, et al. Cell type-specific genotoxicity in estrogen-exposed ovarian and fallopian epithelium. BMC Cancer. 2020;20(1):1020.
Vernier M, et al. Estrogen-related receptors are targetable ROS sensors. Genes Dev. 2020;34(7–8):544–59.
Borella F, et al. Hormone receptors and epithelial ovarian cancer: recent advances in biology and treatment options. Biomedicines. 2023;11(8):2157.
Thasni KA, et al. Estrogen-dependent cell signaling and apoptosis in BRCA1-blocked BG1 ovarian cancer cells in response to plumbagin and other chemotherapeutic agents. Ann Oncol. 2008;19(4):696–705.
Maleki J, et al. 17β-Estradiol stimulates generation of reactive species oxygen and nitric oxide in ovarian adenocarcinoma cells (OVCAR 3). Iran J Cancer Prev. 2015;8(3):e2332.
Bogush TA, et al. Estrogen receptors alpha and beta in ovarian cancer: expression level and prognosis. Dokl Biochem Biophys. 2018;482(1):249–51.
Schuster EF, et al. Molecular profiling of aromatase inhibitor sensitive and resistant ER+HER2- postmenopausal breast cancers. Nat Commun. 2023;14(1):4017.
Patel HK, Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther. 2018;186:1–24.
Jacobsen BM, Horwitz KB. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol Cell Endocrinol. 2012;357(1–2):18–29.
Mauro LJ, et al. Progesterone receptors promote quiescence and ovarian cancer cell phenotypes via DREAM in p53-mutant fallopian tube models. J Clin Endocrinol Metab. 2021;106(7):1929–55.
Islam MS, et al. Selective progesterone receptor modulators-mechanisms and therapeutic utility. Endocr Rev. 2020;41(5):bnaa012.
Rangsrikitphoti P, et al. Sex steroid hormones and DNA repair regulation: Implications on cancer treatment responses. J Steroid Biochem Mol Biol. 2023;227:106230.
Kim O, et al. Targeting progesterone signaling prevents metastatic ovarian cancer. Proc Natl Acad Sci U S A. 2020;117(50):31993–2004.
Pu H, et al. Regulation of progesterone receptor expression in endometriosis, endometrial cancer, and breast cancer by estrogen, polymorphisms, transcription factors, epigenetic alterations, and ubiquitin-proteasome system. J Steroid Biochem Mol Biol. 2023;227:106199.
Chung WM, et al. Androgen/androgen receptor signaling in ovarian cancer: molecular regulation and therapeutic potentials. Int J Mol Sci. 2021;22(14):7748.
Manning-Geist BL, et al. Phase II study of enzalutamide in androgen receptor positive, recurrent, high- and low-grade serous ovarian cancer. Gynecol Oncol. 2022;164(1):12–7.
Calvillo-Robledo A, et al. Simultaneous expression of steroid sulfatase and androgen receptor reduced overall survival of patients with epithelial ovarian tumors. J Ovarian Res. 2021;14(1):98.
Banerjee S, et al. Abiraterone in patients with recurrent epithelial ovarian cancer: principal results of the phase II Cancer of the Ovary Abiraterone (CORAL) trial (CRUK - A16037). Ther Adv Med Oncol. 2020;12:1758835920975352.
Gadducci A, Cosio S, Genazzani AR. Old and new perspectives in the pharmacological treatment of advanced or recurrent endometrial cancer: Hormonal therapy, chemotherapy and molecularly targeted therapies. Crit Rev Oncol Hematol. 2006;58(3):242–56.
Chao KC, et al. The role of estrogen in the survival of ovarian tumors–a study of the human ovarian adenocarcinoma cell lines OC-117-VGH and OVCAR3. J Chin Med Assoc. 2013;76(2):63–70.
Hao D, et al. Non-classical estrogen signaling in ovarian cancer improves chemo-sensitivity and patients outcome. Theranostics. 2019;9(13):3952–65.
Mitra S, et al. Hormonal therapy for gynecological cancers: how far has science progressed toward clinical applications? Cancers (Basel). 2022;14(3):759.
Sarwar S, et al. Insights into the role of epigenetic factors determining the estrogen response in estrogen-positive ovarian cancer and prospects of combining epi-drugs with endocrine therapy. Front Genet. 2022;13:812077.
Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20(13):3001–15.
Aurilio G, et al. Androgen receptor signaling pathway in prostate cancer: from genetics to clinical applications. Cells. 2020;9(12):2653.
Culig Z, Santer FR. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev. 2014;33(2–3):413–27.
Tamburrino L, et al. Androgen receptor (AR) expression in prostate cancer and progression of the tumor: lessons from cell lines, animal models and human specimens. Steroids. 2012;77(10):996–1001.
Desai K, McManus JM, Sharifi N. Hormonal therapy for prostate cancer. Endocr Rev. 2021;42(3):354–73.
Kumar A, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. 2016;22(4):369–78.
Cai C, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71(20):6503–13.
Lallous N, et al. Functional analysis of androgen receptor mutations that confer anti-androgen resistance identified in circulating cell-free DNA from prostate cancer patients. Genome Biol. 2016;17:10.
Sowalsky AG, et al. Assessment of androgen receptor splice variant-7 as a biomarker of clinical response in castration-sensitive prostate cancer. Clin Cancer Res. 2022;28(16):3509–25.
Sharp A, et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J Clin Invest. 2019;129(1):192–208.
Armstrong AJ, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37(13):1120–9.
Antonarakis ES, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1(5):582–91.
Goodwin JF, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3(11):1254–71.
Polkinghorn WR, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3(11):1245–53.
Asim M, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. 2017;8(1):374.
Li L, et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10(480):eaam7479.
Wang S, et al. Mithramycin suppresses DNA damage repair via targeting androgen receptor in prostate cancer. Cancer Lett. 2020;488:40–9.
Saad F, et al. Niraparib with androgen receptor-axis-targeted therapy in patients with metastatic castration-resistant prostate cancer: safety and pharmacokinetic results from a phase 1b study (BEDIVERE). Cancer Chemother Pharmacol. 2021;88(1):25–37.
Schmidt KT, et al. A single-arm phase II study combining NLG207, a nanoparticle camptothecin, with enzalutamide in advanced metastatic castration-resistant prostate cancer post-enzalutamide. Oncologist. 2022;27(9):718–e694.
Afshar-Oromieh A, et al. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with. Eur J Nucl Med Mol Imaging. 2017;44(6):950–9.
Liao W, et al. Trends in estrogen and progesterone receptors in prostate cancer: a bibliometric analysis. Front Oncol. 2023;13:1111296.
Hou Z, et al. Inhibiting 3βHSD1 to eliminate the oncogenic effects of progesterone in prostate cancer. Cell Rep Med. 2022;3(3):100561.
Belluti S, Imbriano C, Casarini L. Nuclear estrogen receptors in prostate cancer: from genes to function. Cancers (Basel). 2023;15(18):4653.
Bonkhoff H, Berges R. The evolving role of oestrogens and their receptors in the development and progression of prostate cancer. Eur Urol. 2009;55(3):533–42.
Ricke WA, et al. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J. 2008;22(5):1512–20.
Steiner MS, Raghow S. Antiestrogens and selective estrogen receptor modulators reduce prostate cancer risk. World J Urol. 2003;21(1):31–6.
Kowalska K, et al. Estrogen receptor α is crucial in zearalenone-induced invasion and migration of prostate cancer cells. Toxins (Basel). 2018;10(3):98.
Lombardi APG, Vicente CM, Porto CS. Estrogen receptors promote migration, invasion and colony formation of the androgen-independent prostate cancer cells PC-3 through beta-catenin pathway. Front Endocrinol (Lausanne). 2020;11:184.
Compadre AJ, et al. RAD51 Foci as a biomarker predictive of platinum chemotherapy response in ovarian cancer. Clin Cancer Res. 2023;29(13):2466–79.
Godwin AK, et al. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci U S A. 1992;89(7):3070–4.
Blanc-Durand F, et al. A RAD51 functional assay as a candidate test for homologous recombination deficiency in ovarian cancer. Gynecol Oncol. 2023;171:106–13.
Mohr L, et al. Generation of prostate cancer cell models of resistance to the anti-mitotic agent Docetaxel. J Vis Exp. 2017;127:56327.
Lima TS, et al. Molecular profiling of docetaxel-resistant prostate cancer cells identifies multiple mechanisms of therapeutic resistance. Cancers (Basel). 2021;13(6):1290.
Lombard AP, et al. ABCB1 mediates cabazitaxel-docetaxel cross-resistance in advanced prostate cancer. Mol Cancer Ther. 2017;16(10):2257–66.
Chao OS, Goodman OB. DNA-PKc inhibition overcomes taxane resistance by promoting taxane-induced DNA damage in prostate cancer cells. Prostate. 2021;81(14):1032–48.
O’Neill AJ, et al. Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol Cancer. 2011;10:126.
Mumenthaler SM, et al. Pharmacologic inhibition of Pim kinases alters prostate cancer cell growth and resensitizes chemoresistant cells to taxanes. Mol Cancer Ther. 2009;8(10):2882–93.
Xu J, et al. Arsenic compound sensitizes homologous recombination proficient ovarian cancer to PARP inhibitors. Cell Death Discov. 2021;7(1):259.
Pillay N, et al. DNA replication vulnerabilities render ovarian cancer cells sensitive to poly(ADP-Ribose) glycohydrolase inhibitors. Cancer Cell. 2019;35(3):519–533.e8.
Biegała Ł, et al. PARP inhibitor resistance in ovarian cancer: underlying mechanisms and therapeutic approaches targeting the ATR/CHK1 pathway. Biochim Biophys Acta Rev Cancer. 2021;1876(2):188633.
Fleury H, et al. Cumulative defects in DNA repair pathways drive the PARP inhibitor response in high-grade serous epithelial ovarian cancer cell lines. Oncotarget. 2017;8(25):40152–68.
Lombard AP, et al. Olaparib-induced senescence is bypassed through G2-M checkpoint override in olaparib-resistant prostate cancer. Mol Cancer Ther. 2022;21(4):677–85.
Schaaf ZA, et al. Therapeutic resistance models and treatment sequencing in advanced prostate cancer. Cancers (Basel). 2023;15(21):5273.
Li S, et al. Estrogen enhances the proliferation and migration of ovarian cancer cells by activating transient receptor potential channel C3. J Ovarian Res. 2020;13(1):20.
Lima MA, da Silva SV, Freitas VM. Progesterone acts via the progesterone receptor to induce adamts proteases in ovarian cancer cells. J Ovarian Res. 2016;9:9.
Pedernera E, et al. Progesterone reduces cell survival in primary cultures of endometrioid ovarian cancer. J Ovarian Res. 2019;12(1):15.
Limaye S, et al. A case report of androgen receptor inhibitor therapy in recurrent high-grade serous ovarian cancer. Oncotarget. 2020;11(46):4358–63.
Kregel S, et al. Acquired resistance to the second-generation androgen receptor antagonist enzalutamide in castration-resistant prostate cancer. Oncotarget. 2016;7(18):26259–74.
Liu C, et al. Niclosamide enhances abiraterone treatment via inhibition of androgen receptor variants in castration resistant prostate cancer. Oncotarget. 2016;7(22):32210–20.
Xu P, et al. Allosteric inhibition of HSP70 in collaboration with STUB1 augments enzalutamide efficacy in antiandrogen resistant prostate tumor and patient-derived models. Pharmacol Res. 2023;189:106692.
Handle F, et al. Drivers of AR indifferent anti-androgen resistance in prostate cancer cells. Sci Rep. 2019;9(1):13786.
van Soest RJ, et al. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur J Cancer. 2013;49(18):3821–30.
Zhang Y, et al. Idarubicin combats abiraterone and enzalutamide resistance in prostate cells via targeting XPA protein. Cell Death Dis. 2022;13(12):1034.
Zhou Z, et al. The combination of cell cultured technology and in silico model to inform the drug development. Pharmaceutics. 2021;13(5):704.
Zakarya R, Howell VM, Colvin EK. Modelling epithelial ovarian cancer in mice: classical and emerging approaches. Int J Mol Sci. 2020;21(13):4806.
Tsang SI, et al. Experimental models for ovarian cancer research. Exp Cell Res. 2022;416(1):113150.
Hasan N, Ohman AW, Dinulescu DM. The promise and challenge of ovarian cancer models. Transl Cancer Res. 2015;4(1):14–28.
Shi M, et al. Inactivation of TRP53, PTEN, RB1, and/or CDH1 in the ovarian surface epithelium induces ovarian cancer transformation and metastasis. Biol Reprod. 2020;102(5):1055–64.
Adamiecki R, et al. In vivo models for prostate cancer research. Cancers (Basel). 2022;14(21):5321.
Arriaga JM, Abate-Shen C. Genetically engineered mouse models of prostate cancer in the postgenomic era. Cold Spring Harb Perspect Med. 2019;9(2):a030528.
Ding Z, et al. Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell. 2012;148(5):896–907.
Lunardi A, et al. Suppression of CHK1 by ETS family members promotes DNA damage response bypass and tumorigenesis. Cancer Discov. 2015;5(5):550–63.
Pompili L, et al. Patient-derived xenografts: a relevant preclinical model for drug development. J Exp Clin Cancer Res. 2016;35(1):189.
Slade D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020;34(5–6):360–94.
Sun C, et al. MiR-509-3 augments the synthetic lethality of PARPi by regulating HR repair in PDX model of HGSOC. J Hematol Oncol. 2020;13(1):9.
Hidalgo M, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013.
Chen J, et al. Using Patient-Derived Xenograft (PDX) models as a “Black Box” to identify more applicable Patients for ADP-Ribose Polymerase Inhibitor (PARPi) treatment in ovarian cancer: searching for novel molecular and clinical biomarkers and performing a prospective preclinical trial. Cancers (Basel). 2022;14(19):4649.
Serra V, et al. Identification of a molecularly-defined subset of breast and ovarian cancer models that respond to WEE1 or ATR inhibition, overcoming PARP inhibitor resistance. Clin Cancer Res. 2022;28(20):4536–50.
Risbridger GP, et al. The MURAL collection of prostate cancer patient-derived xenografts enables discovery through preclinical models of uro-oncology. Nat Commun. 2021;12(1):5049.
Palanisamy N, et al. The MD anderson prostate cancer patient-derived xenograft series (MDA PCa PDX) captures the molecular landscape of prostate cancer and facilitates marker-driven therapy development. Clin Cancer Res. 2020;26(18):4933–46.
Karkampouna S, et al. Patient-derived xenografts and organoids model therapy response in prostate cancer. Nat Commun. 2021;12(1):1117.
Jin J, et al. Challenges and prospects of patient-derived xenografts for cancer research. Cancers (Basel). 2023;15(17):4352.
Grabosch S, et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene. 2019;38(13):2380–93.
Meng J, et al. Niraparib exhibits a synergistic anti-tumor effect with PD-L1 blockade by inducing an immune response in ovarian cancer. J Transl Med. 2021;19(1):415.
Czernin J, et al. Immune-checkpoint blockade enhances 225Ac-PSMA617 efficacy in a mouse model of prostate cancerl. J Nucl Med. 2021;62(2):228–31.
Eximond M, Wang J, Kirschner A. Dual immune checkpoint therapy combined with radiotherapy treats castration-resistant prostate cancer. Int J Radiat Oncol Biol Phys. 2023;117(2):E229–E229.
Article Google Scholar
Guan X, et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature. 2022;606(7915):791–6.
Halldorsson S, et al. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron. 2015;63:218–31.
Danku AE, et al. Organ-on-a-chip: a survey of technical results and problems. Front Bioeng Biotechnol. 2022;10:840674.
Dadgar N, et al. A microfluidic platform for cultivating ovarian cancer spheroids and testing their responses to chemotherapies. Microsyst Nanoeng. 2020;6:93.
Sood A, et al. Translational nanomedicines across human reproductive organs modeling on microfluidic chips: state-of-the-art and future prospects. ACS Biomater Sci Eng. 2023;9(1):62–84.
Durinikova E, Buzo K, Arena S. Preclinical models as patients’ avatars for precision medicine in colorectal cancer: past and future challenges. J Exp Clin Cancer Res. 2021;40(1):185.
Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18(7):407–18.
Clevers H. Modeling development and disease with organoids. Cell. 2016;165(7):1586–97.
Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5.
Maenhoudt N, Vankelecom H. Protocol for establishing organoids from human ovarian cancer biopsies. STAR Protoc. 2021;2(2):100429.
Trillsch F, et al. Protocol to optimize the biobanking of ovarian cancer organoids by accommodating patient-specific differences in stemness potential. STAR Protoc. 2023;4(3):102484.
Graham O, et al. Generation and culturing of high-grade serous ovarian cancer patient-derived organoids . J Vis Exp. 2023;(191):10.3791/64878.
Raghavan S, et al. Microenvironment drives cell state, plasticity, and drug response in pancreatic cancer. Cell. 2021;184(25):6119–6137.e26.
Kopper O, et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25(5):838–49.
Nanki Y, et al. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci Rep. 2020;10(1):12581.
Wang Z, et al. The Fibrillin-1/VEGFR2/STAT2 signaling axis promotes chemoresistance via modulating glycolysis and angiogenesis in ovarian cancer organoids and cells. Cancer Commun (Lond). 2022;42(3):245–65.
Maitland NJ, Collins AT. Prostate cancer stem cells: a new target for therapy. J Clin Oncol. 2008;26(17):2862–70.
Karthaus WR, et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014;159(1):163–75.
Drost J, et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc. 2016;11(2):347–58.
Beshiri M, et al. Prostate organoids: emerging experimental tools for translational research. J Clin Invest. 2023;133(10):e169616.
Horst EN, et al. Personalized models of heterogeneous 3D epithelial tumor microenvironments: ovarian cancer as a model. Acta Biomater. 2021;132:401–20.
Servant R, et al. Prostate cancer patient-derived organoids: detailed outcome from a prospective cohort of 81 clinical specimens. J Pathol. 2021;254(5):543–55.
Puca L, et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat Commun. 2018;9(1):2404.
Gao D, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159(1):176–87.
Download references
Acknowledgements
Submission declaration and verification.
This manuscript neither is under consideration for publication elsewhere nor had been published previously.
This work is supported by FPRC 5 × 1000 MIUR 2018 INSIDE and FPRC 5 × 1000 Ministero della Salute 2021 EmaGen to S. Arena; Ricerca Locale 2022 and 2023 (premialità pubblicazioni) from Department of Oncology, University of Torino to S.Arena; Italian Ministry of Health, Ricerca Corrente 2024 to S.Arena; A.Opattova was supported by Fondazione Umberto Veronesi.
Author information
Authors and affiliations.
Candiolo Cancer Institute, FPO - IRCCS, Laboratory of Translational Cancer Genetics, Strada Provinciale 142, Km 3.95, Candiolo, TO, ZIP 10060, Italy
Giada De Lazzari, Alena Opattova & Sabrina Arena
Department of Oncology, University of Torino, Strada Provinciale 142, Km 3.95, Candiolo, TO, ZIP 10060, Italy
Sabrina Arena
You can also search for this author in PubMed Google Scholar
Contributions
GDL, AO and SA discussed and developed the concept; GDL and AO developed figures and tables; GDL, AO and SA wrote the manuscript; SA coordinated the effort and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Sabrina Arena .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
All authors provide consent for the publication of the manuscript detailed above.
Competing interests
All authors declare to have no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
De Lazzari, G., Opattova, A. & Arena, S. Novel frontiers in urogenital cancers: from molecular bases to preclinical models to tailor personalized treatments in ovarian and prostate cancer patients. J Exp Clin Cancer Res 43 , 146 (2024). https://doi.org/10.1186/s13046-024-03065-0
Download citation
Received : 31 January 2024
Accepted : 08 May 2024
Published : 15 May 2024
DOI : https://doi.org/10.1186/s13046-024-03065-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Genomic instability
- Targeted-therapies
- Chemotherapies
- DNA damage response
- Hormonal regulation
- Androgen receptor
- Preclinical models
Journal of Experimental & Clinical Cancer Research
ISSN: 1756-9966
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Diagnostic performance of F-18 FDG PET/CT in differentiating autoimmune pancreatitis from pancreatic cancer: a systemic review and meta-analysis
- Original Article
- Published: 15 May 2024
Cite this article

- Deepanksha Datta ORCID: orcid.org/0000-0001-6420-8415 1 ,
- B. Selvakumar ORCID: orcid.org/0000-0002-2280-3862 2 ,
- Akhil Dhanesh Goel ORCID: orcid.org/0000-0002-6156-7903 3 ,
- Sanskriti Chhibber ORCID: orcid.org/0009-0006-0735-4196 4 ,
- Vaibhav Kumar Varshney ORCID: orcid.org/0000-0003-1771-2787 2 &
- Rajesh Kumar ORCID: orcid.org/0000-0002-1889-4095 1
This study aims to evaluate the utility of F-18 FDG PET/CT in the non-invasive diagnosis of autoimmune pancreatitis (AIP) and differentiating it from pancreatic cancer (CaP) based on the amount and pattern of FDG uptake, as well as involvement of extra-pancreatic sites.
A systematic search was conducted using PubMed, Scopus, Cochrane Library and Google Scholar. Only those studies that compared the findings of F-18 FDG PET/CT in terms of SUVmax, pattern of FDG uptake and presence of FDG-avid extra-pancreatic sites in both AIP and CaP were included. Studies were qualitatively assessed for risk of bias and publication bias. The diagnostic performance of parameters on PET/CT was examined through pooled sensitivity, specificity, diagnostic odd’s ratio (DOR) and summary receiver operator characteristic (SROC) curve analysis.
Six studies were included with a total of 580 patients. 178 patients had AIP (Age 18–90 years, male, M: female, F ratio—8.4:1) and 402 patients had CaP (Age 22–88 years, M:F ratio-1.5:1). Type of AIP was reported in only 3 studies, with the included cases predominantly being type 1 AIP. All studies were retrospective with heterogeneity and a risk on patient selection and index test. The FDG uptake, expressed as SUVmax, was lower in AIP with a weighted mean difference of −3.11 (95% confidence interval, CI: −5.28 to −0.94). To diagnose AIP, the pooled sensitivity, specificity and DOR of diffuse pattern of FDG uptake were 0.59 (95% CI: 0.51–0.66), 0.89 (95% CI: 0.86–0.92) and 21.07 (95% CI: 5.07–88.32), respectively, with an area under curve (AUC) of 0.717 on SROC analysis. The pooled sensitivity, specificity and DOR of FDG-avid extra pancreatic sites were 0.55 (95% CI: 0.45–0.65), 0.58 (95% CI: 0.52–0.64) and 2.33 (95% CI: 1.40–3.89), respectively, with an AUC of 0.632.
On F-18 FDG PET/CT, a pancreatic lesion of AIP has a lower SUVmax value than CaP. A diffuse pattern of FDG uptake and presence of an extra-pancreatic FDG-avid site are nearly 21 times and twice more likely in AIP than CaP, respectively.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Abbreviations
- Autoimmune pancreatitis
- Pancreatic cancer
Area under the curve
Summary receiver operator curve
Diagnostic odd’s ratio
Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40(3):352–8.
Article PubMed Google Scholar
Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43(6):403–8.
Article CAS PubMed Google Scholar
Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40(6):809–14.
Hardacre JM, Iacobuzio-Donahue CA, Sohn TA, Abraham SC, Yeo CJ, Lillemoe KD, et al. Results of pancreaticoduodenectomy for lymphoplasmacytic sclerosing pancreatitis. Ann Surg. 2003;237(6):853–9.
Article PubMed PubMed Central Google Scholar
Meng Q, Xin L, Liu W, Lin H, Tian B, Wang L, et al. Diagnosis and treatment of autoimmune pancreatitis in China: a systematic review. PLoS ONE. 2015;10(6): e0130466.
Wu L, Li W. An analysis of clinical characteristics of autoimmune pancreatitis. Zhonghua Nei Ke Za Zhi. 2010;49(11):943–6.
PubMed Google Scholar
Kumar R, Chauhan A, Zhuang H, Alavi A. Assessment of therapy response by fluorine-18 fluorodeoxyglucose PET in infection and inflammation. PET Clin. 2006;1(2):191–8.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Devillé WL, Buntinx F, Bouter LM, Montori VM, de Vet HCW, van der Windt DAWM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–93.
Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31.
Lee TY, Kim MH, Park DH, Seo DW, Lee SK, Kim JS, et al. Utility of 18 F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. Am J Roentgenol. 2009;193(2):343–8.
Article Google Scholar
Shigekawa M, Yamao K, Sawaki A, Hara K, Takagi T, Bhatia V, et al. Is 18 F-fluorodeoxyglucose positron emission tomography meaningful for estimating the efficacy of corticosteroid therapy in patients with autoimmune pancreatitis? J Hepatobiliary Pancreat Sci. 2010;17(3):269–74.
Zhang J, Jia G, Zuo C, Jia N, Wang H. 18 F-FDG PET/CT helps differentiate autoimmune pancreatitis from pancreatic cancer. BMC Cancer. 2017;17:695.
Cheng MF, Guo YL, Yen RF, Chen YC, Ko CL, Tien YW, et al. Clinical utility of FDG PET/CT in patients with autoimmune pancreatitis: a case-control study. Sci Rep. 2018;8(1):3651.
Ohtani M, Ofuji K, Akazawa Y, Saito Y, Nosaka T, Ozaki Y, et al. Clinical usefulness of [18F]-fluoro-2-deoxy- d -glucose–positron emission tomography/computed tomography for distinguishing between autoimmune pancreatitis and pancreatic cancer. Pancreas. 2021;50(7):1014–9.
Liu Z, Li M, Zuo C, Yang Z, Yang X, Ren S, et al. Radiomics model of dual-time 2-[18F]FDG PET/CT imaging to distinguish between pancreatic ductal adenocarcinoma and autoimmune pancreatitis. Eur Radiol. 2021;31(9):6983–91.
Law R, Bronner M, Vogt D, Stevens T. Autoimmune pancreatitis: a mimic of pancreatic cancer. Cleve Clin J Med. 2009;76(10):607–15.
Vijayakumar A, Vijayakumar A. Imaging of focal autoimmune pancreatitis and differentiating it from pancreatic cancer. ISRN Radiol. 2013;2013: 569489.
Irie H, Honda H, Baba S, Kuroiwa T, Yoshimitsu K, Tajima T, et al. Autoimmune pancreatitis: CT and MR characteristics. Am J Roentgenol. 1998;170(5):1323–7.
Article CAS Google Scholar
Takahashi N, Fletcher JG, Hough DM, Fidler JL, Kawashima A, Mandrekar JN, et al. Autoimmune pancreatitis: differentiation from pancreatic carcinoma and normal pancreas on the basis of enhancement characteristics at dual-phase CT. Am J Roentgenol. 2009;193(2):479–84.
Sugiyama Y, Fujinaga Y, Kadoya M, Ueda K, Kurozumi M, Hamano H, et al. Characteristic magnetic resonance features of focal autoimmune pancreatitis useful for differentiation from pancreatic cancer. Jpn J Radiol. 2012;30(4):296–309.
Kamisawa T, Takuma K, Anjiki H, Egawa N, Hata T, Kurata M, et al. Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol. 2010;105(8):1870–5.
Wolske KM, Ponnatapura J, Kolokythas O, Burke LMB, Tappouni R, Lalwani N. Chronic pancreatitis or pancreatic tumor? A problem-solving approach. Radiographics. 2019;39(7):1965–82.
Basu S, Hess S, Nielsen Braad PE, Olsen BB, Inglev S, Høilund-Carlsen PF. The basic principles of FDG-PET/CT imaging. PET Clin. 2014;9(4):355–70, v.
Zhuang H, Alavi A. 18-Fluorodeoxyglucose positron emission tomographic imaging in the detection and monitoring of infection and inflammation. Semin Nucl Med. 2002;32(1):47–59.
Pu Y, Wang C, Zhao S, Xie R, Zhao L, Li K, et al. The clinical application of 18 F-FDG PET/CT in pancreatic cancer: a narrative review. Transl Cancer Res. 2021;10(7):3560–75.
Article CAS PubMed PubMed Central Google Scholar
Wang Z, Chen JQ, Liu JL, Qin XG, Huang Y. FDG-PET in diagnosis, staging and prognosis of pancreatic carcinoma: A meta-analysis. World J Gastroenterol WJG. 2013;19(29):4808–17.
Rauscher I, Eiber M, Algül H, Siveke JT, Weirich G, Schlitter AM, et al. Multiparametric 18 F-FDG PET/MR follow-up in a patient with autoimmune pancreatitis. Eur J Hybrid Imaging. 2017;1(1):11.
Shou Y, Xue Q, Yuan J, Zhao J. 68Ga-FAPI-04 PET/MR is helpful in differential diagnosis of pancreatitis from pancreatic malignancy compared to 18 F-FDG PET/CT: a case report. Eur J Hybrid Imaging. 2021;5(1):12.
Luo Y, Pan Q, Yang H, Peng L, Zhang W, Li F. Fibroblast Activation protein-targeted PET/CT with 68Ga-FAPI for imaging IgG4-related disease: comparison to 18 F-FDG PET/CT. J Nucl Med Off Publ Soc Nucl Med. 2021;62(2):266–71.
CAS Google Scholar
Nakajo M, Jinnouchi S, Fukukura Y, Tanabe H, Tateno R, Nakajo M. The efficacy of whole-body FDG-PET or PET/CT for autoimmune pancreatitis and associated extrapancreatic autoimmune lesions. Eur J Nucl Med Mol Imaging. 2007;34(12):2088–95.
Nakamoto Y, Saga T, Ishimori T, Higashi T, Mamede M, Okazaki K, et al. FDG-PET of autoimmune-related pancreatitis: preliminary results. Eur J Nucl Med. 2000;27(12):1835–8.
Ozaki Y, Oguchi K, Hamano H, Arakura N, Muraki T, Kiyosawa K, et al. Differentiation of autoimmune pancreatitis from suspected pancreatic cancer by fluorine-18 fluorodeoxyglucose positron emission tomography. J Gastroenterol. 2008;43(2):144–51.
Sa R, Zhao HG, Dai YY, Guan F. The role of dual time point PET/CT for distinguishing malignant from benign focal 18 F-FDG uptake duodenal lesions. Medicine (Baltimore). 2018;97(38): e12521.
Nakajo M, Jinguji M, Aoki M, Tani A, Sato M, Yoshiura T. The clinical value of texture analysis of dual-time-point 18 F-FDG-PET/CT imaging to differentiate between 18 F-FDG-avid benign and malignant pulmonary lesions. Eur Radiol. 2020;30(3):1759–69.
Hofman MS, Hicks RJ. How we read oncologic FDG PET/CT. Cancer Imaging. 2016;16(1):35.
Zhang Y, Cheng C, Liu Z, Wang L, Pan G, Sun G, et al. Radiomics analysis for the differentiation of autoimmune pancreatitis and pancreatic ductal adenocarcinoma in 18 F-FDG PET/CT. Med Phys. 2019;46(10):4520–30.
Download references
Author information
Authors and affiliations.
Department of Nuclear Medicine, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India
Deepanksha Datta & Rajesh Kumar
Department of Surgical Gastroenterology, All India Institute of Medical Sciences, Basni Industrial Area Phase 2, Jodhpur, Rajasthan, 342005, India
B. Selvakumar & Vaibhav Kumar Varshney
Department of Community and Family Medicine, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India
Akhil Dhanesh Goel
Freelance Editor-Biological Sciences Journal, New Delhi, India
Sanskriti Chhibber
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to B. Selvakumar .
Ethics declarations
Conflict of interest.
The authors declare that they have no potential conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 16 KB)
Supplementary file2 (docx 14 kb), supplementary file3 (docx 14 kb), rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Datta, D., Selvakumar, B., Goel, A.D. et al. Diagnostic performance of F-18 FDG PET/CT in differentiating autoimmune pancreatitis from pancreatic cancer: a systemic review and meta-analysis. Ann Nucl Med (2024). https://doi.org/10.1007/s12149-024-01934-4
Download citation
Received : 28 January 2024
Accepted : 18 April 2024
Published : 15 May 2024
DOI : https://doi.org/10.1007/s12149-024-01934-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- F-18 FDG PET
- Diffuse uptake
- Extra pancreatic sites
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 08 May 2024
Fluorinated hydroxyapatite conditions a favorable osteo-immune microenvironment via triggering metabolic shift from glycolysis to oxidative phosphorylation
- Kaidi Chen 1 , 2 , 3 na1 ,
- Seongmin Ha 1 , 2 , 3 na1 ,
- Leyao Xu 1 , 2 , 3 ,
- Chengwu Liu 1 , 2 , 3 ,
- Yuanxiang Liu 1 , 2 , 3 ,
- Xiayi Wu 1 , 2 , 3 ,
- Zhipeng Li 1 , 2 , 3 ,
- Shiyu Wu 1 , 2 , 3 ,
- Bo Yang 1 , 2 , 3 &
- Zhuofan Chen ORCID: orcid.org/0000-0002-3341-1485 1 , 2 , 3
Journal of Translational Medicine volume 22 , Article number: 437 ( 2024 ) Cite this article
281 Accesses
Metrics details
Biological-derived hydroxyapatite is widely used as a bone substitute for addressing bone defects, but its limited osteoconductive properties necessitate further improvement. The osteo-immunomodulatory properties hold crucial promise in maintaining bone homeostasis, and precise modulation of macrophage polarization is essential in this process. Metabolism serves as a guiding force for immunity, and fluoride modification represents a promising strategy for modulating the osteoimmunological environment by regulating immunometabolism. In this context, we synthesized fluorinated porcine hydroxyapatite (FPHA), and has demonstrated its enhanced biological properties and osteogenic capacity. However, it remains unknown whether and how FPHA affects the immune microenvironment of the bone defects.
FPHA was synthesized and its composition and structural properties were confirmed. Macrophages were cultured with FPHA extract to investigate the effects of FPHA on their polarization and the related osteo-immune microenvironment. Furthermore, total RNA of these macrophages was extracted, and RNA-seq analysis was performed to explore the underlying mechanisms associated with the observed changes in macrophages. The metabolic states were evaluated with a Seahorse analyzer. Additionally, immunohistochemical staining was performed to evaluate the macrophages response after implantation of the novel bone substitutes in critical size calvarial defects in SD rats.
The incorporation of fluoride ions in FPHA was validated. FPHA promoted macrophage proliferation and enhanced the expression of M2 markers while suppressing the expression of M1 markers. Additionally, FPHA inhibited the expression of inflammatory factors and upregulated the expression of osteogenic factors, thereby enhancing the osteogenic differentiation capacity of the rBMSCs. RNA-seq analysis suggested that the polarization-regulating function of FPHA may be related to changes in cellular metabolism. Further experiments confirmed that FPHA enhanced mitochondrial function and promoted the metabolic shift of macrophages from glycolysis to oxidative phosphorylation. Moreover, in vivo experiments validated the above results in the calvarial defect model in SD rats.
In summary, our study reveals that FPHA induces a metabolic shift in macrophages from glycolysis to oxidative phosphorylation. This shift leads to an increased tendency toward M2 polarization in macrophages, consequently creating a favorable osteo-immune microenvironment. These findings provide valuable insights into the impact of incorporating an appropriate concentration of fluoride on immunometabolism and macrophage mitochondrial function, which have important implications for the development of fluoride-modified immunometabolism-based bone regenerative biomaterials and the clinical application of FPHA or other fluoride-containing materials.
Graphical Abstract. FPHA was successfully prepared through the chemical and thermal process. The immunomodulatory effects of FPHA were investigated through in vitro and in vivo studies, revealing its ability to induce a metabolic shift in macrophages from glycolysis to mitochondrial oxidative phosphorylation (OxPhos). This metabolic remodeling resulted in a notable suppression of M1 macrophage polarization and promotion of M2 macrophage polarization. Furthermore, FPHA was found to enhance osteogenic differentiation and facilitate bone repair. These findings underscore the promising potential of FPHA as a biomaterial for bone regenerative applications, providing valuable insights for the development of bioactive materials with metabolic-immunoregulatory properties
Graphical abstract.

Bone defects, a serious clinical problem caused by tumors, trauma, inflammation, etc., trigger the development of smart biomaterials as bone substitutes for regeneration [ 1 , 2 ]. The osteo-immunomodulatory properties of biomaterials have emerged as crucial factors in maintaining bone homeostasis and have garnered significant attention [ 3 ]. Among immune cells, macrophages play a vital role [ 3 , 4 ], and the functional polarization of macrophages is pivotal in determining the quality and efficacy of injury repair [ 5 ]. Macrophage polarization involves two main states: M1 macrophages promote an inflammatory response, while M2 macrophages possess the ability to suppress inflammation and regulate tissue repair and regeneration [ 5 , 6 ]. Following the implantation of biomaterials, the incipient acute inflammation was triggered, leading to increased release of cytokines and reactive oxygen species (ROS). The elevated inflammation levels and excessive ROS can result in phagocytose and degrade the implanted biomaterials [ 7 ], potentially leading to broader detrimental effects [ 8 , 9 , 10 ]. Hence, precise regulation of macrophage activation is essential for tissue homeostasis maintenance and effectively reducing the ROS and inflammatory impact caused by material implantation. In this context, immunometabolism has been a subject of extensive research in recent years, as different macrophage activation states exhibit distinct metabolic profiles [ 5 , 11 ]. M1 polarization is characterized by increased glycolysis and pentose phosphate pathway (PPP) activity, coupled with reduced oxidative phosphorylation (OxPhos), and in contrast, M2 macrophages display effective OxPhos and reduced PPP activity [ 6 , 12 ]. Meanwhile, glycolysis and OxPhos uniquely regulate macrophage phenotype and function [ 13 , 14 , 15 ]. Therefore, manipulating factors that influence macrophage metabolism could potentially be utilized to modulate macrophage polarization [ 16 ].
Fluoride plays a crucial role in the regulation of bone tissue regeneration, repair, and remodeling [ 14 , 17 ]. Notably, fluoride exerts its effects in a concentration-dependent manner within the human body [ 18 ], which shows diverse effects on the metabolism and function of immune cells [ 14 , 19 , 20 ]. Appropriate concentration of fluoride ions was demonstrated to enhanced M2 macrophage polarization [ 21 ], via a potential metabolic pathway including the mitochondrial tricarboxylic acid (TCA) cycle and OxPhos [ 14 , 22 ]. Taken together, fluoride modification may be a promising strategy for modulating the osteoimmunology environment via regulating immunometabolism.
In our previous studies, FPHA was successfully synthesized using a chemical and thermal method [ 23 ]. FPHA displayed superior physicochemical and biological properties compared to PHA, particularly when prepared from a 0.25 M NaF aqueous solution [ 24 ]. Additionally, fluoride incorporation enhances the osteogenic activity of both BMSCs and MG63 cells [ 21 , 25 , 26 ]. In vivo experiments [ 26 , 27 ] demonstrated that fluoride incorporation exhibited a greater osteogenic ability. Moreover, clinical trials have been conducted to evaluate the efficacy and safety of FPHA. However, the underlying mechanism related to fluoride regulating immunometabolism remains unveiled.
Therefore, the objective of this study was to investigate the impact of FPHA on macrophage polarization and the underlying mechanism related to immunometabolism. In this research, we proved that FPHA can actively enhance macrophage mitochondrial function and promote a metabolic shift from glycolysis to mitochondrial oxidative phosphorylation. Consequently, FPHA induced M2 polarization and suppressed M1 polarization, creating a favorable osteo-immune microenvironment. These findings may provide valuable insights into the impact of incorporating an appropriate concentration of fluoride on immunometabolism and macrophage mitochondrial function, which have important implications for development of immunometabolism-based fluoride-containing biomaterials.
FPHA preparation, characterization and extract preparation
PHA and FPHA were synthesized using a chemical and thermal process previously established by our research group [ 25 , 26 , 27 ]. The surface morphology of PHA and FPHA was observed using scanning electron microscope (SEM, MIRA LM, Tescan, Czech Republic). Additionally, elemental characterization and distribution analysis were conducted using energy-dispersive X-ray spectroscopy (EDS) mode. Fourier-transform infrared spectroscopy (FTIR, Nicolet NXR 9650, Thermo-Fisher, USA) were utilized to identify crystal phases and specific chemical groups present in PHA and FPHA. The changes in crystallinity of PHA and FPHA were investigated using X-ray diffraction (XRD, Empyrean, Malvern Panalytical, Netherlands).
Extract were prepared following the ISO/EN 10993-5 standard, as previously described in another study [ 26 ]. The sterilized samples were then placed into serum-free DMEM (Gibco, USA) in a ratio of 100 mg to 1 ml. To ensure a more uniform interaction between the DMEM and the materials, the centrifuge tubes were positioned horizontally and incubated at 37 °C for 24 h. Subsequently, the supernatant was collected following centrifugation and filtrated through a 0.22 μm filter membrane (Merck Millipore, USA). The concentration of F ions in the solution was determined using a fluorine selective electrode (PF-202-C; Leici, China) connected to an ion analyzer (Origin Dual Star; Thermo Scientific, USA). For subsequent experiments, the PHA and FPHA extract was supplemented with 10% fetal bovine serum (FBS, A115-500, Nobimpex, Germany) and 1% penicillin/streptomycin.
Cell proliferation and viability
The biocompatibility of FPHA was evaluated by examining the proliferation and viability of RAW264.7 macrophages. The cells were seeded in 24-well plates at a density of 1 × 10 5 cells per well. Subsequently, they were cultured with DMEM, PHA extract, or FPHA extract for 1, 2, and 3 days. The proliferation of macrophages was assessed using the cell counting kit-8 (CCK-8, Dojindo Laboratories, Japan) assay, while the viability of macrophages was evaluated using the Calcein-AM/PI Live-Dead Cell Staining Kit (Solarbio, China).
In vitro macrophage responses following culture with PHA and FPHA extract
Cell culture and macrophage activation.
RAW264.7 macrophages (TCM-13) were obtained from the Cell Bank at the Shanghai Institute of Biochemistry and Cell Biology, China. The cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin (Gibco, USA), and maintained in a 5% CO 2 humidified atmosphere at 37 °C. Before using the FBS, it was inactivated by heating at 56 °C for 30 min. When the cells reached approximately 80% confluence, they were passaged by scraping.
To investigate the modulatory effects of FPHA on macrophages, LPS (L2630, Merck Millipore, USA) was used to activate the macrophages. The culture medium was replaced with medium containing 1000 ng/mL LPS and the cells were stimulated for 6 h. A previous study [ 28 ] demonstrated that macrophages exhibit rapid responsiveness to LPS stimulation, with the 6-hour time point being optimal. Stimulation period shorter than 6 h may fail to distinguish the intensity of different stimulators, while stimulation time longer than 6 h may lead to misjudgment of some highly potent stimulators [ 28 ]. Afterwards, the LPS-activated RAW264.7 macrophages were cultured with 1 ml DMEM, PHA extract, and FPHA extract in 6-well plates for subsequent experiments. Cells cultured with DMEM were served as a control. Building upon the findings of the previous study [ 28 ] and pre-experimental results, the peak hour of pro-inflammatory gene expression was 6 h. And considering the delayed protein expression, 24 h is a better time point for intracellular protein assessment, while 48 h may be a more suitable time for evaluating secretory protein levels.
To investigate the effects of the extract on macrophages at the gene level, qRT-PCR was performed. After culturing the macrophages with the extract for 6 h and 1 day, total RNA was isolated using the RNA Purification Kit (Esunbio, China). The quality and quantity of the total RNA were assessed using a spectrophotometer (Nanodrop One, Thermo Fisher Scientific, USA). Then, cDNA synthesis was performed using a reverse transcription kit (Hifair® III 1st Strand cDNA Synthesis SuperMix, Yeasen, China) with 500ng of RNA as the starting material, following the manufacturer’s instructions. The qRT-PCR analysis was conducted using the qPCR SYBR Mix (Hieff® qPCR SYBR Green Master Mix No Rox, Yeasen, China) with a 10 µl reaction volume, employing the LightCycler® 96 instrument (Roche, Switzerland). The primer sequences used for qRT-PCR are listed in Table S1 .
Western blot
To investigate the effects of the extract on macrophages at the protein level, Western blot analysis was performed. After culturing the macrophages with the extract for 1 day, the cells were lysed using RIPA lysis buffer. The protein concentration in the lysate was determined using the BCA Protein Assay Kit (CW0014, Cwbio, China). Subsequently, equal amounts of protein from each sample were loaded onto SDS-PAGE gels (SurePAGE™, GenScript, China) and separated by electrophoresis. The separated proteins were then transferred to a PVDF membrane (Merck Millipore, USA). To block non-specific binding sites, the membrane was incubated in 5% w/v skim milk (1172, Biofroxx, Germany) for 90 min. Next, the membrane was incubated overnight at 4 °C with primary antibodies diluted in appropriate concentrations. The primary antibodies used in this study included anti-iNOS antibody (1:1000, ab178945, Abcam, UK), anti-Arg antibody (1:100, 93668T, CST, USA), anti-IL10 antibody (1:500, Affinity, China), anti-MMP9 antibody (1:1000, Proteintech, USA) and anti-β-tubulin antibody (1:2500, #2128S, CST, USA). After washing, the membrane was incubated with a Goat anti-Rabbit secondary antibody (1:8000; #7074s, CST, USA). The protein bands were visualized using the Immobilon Western HRP Substrate (WBKLS0100, Merck Millipore, USA) and captured using a ChemiDoc XRS System (BioRad, Hercules, USA).
Enzyme linked immunosorbent assay (ELISA) of cytokines
To determine the levels of TNFα and TGFβ1, two secreted proteins, in the culture media supernatant after a 48-hour incubation, ELISA kits were utilized. The ELISA kits used in this study were for TNFα (Cusabio, China) and TGFβ1 (Mlbio, China). The protocols provided by the manufacturers were followed to measure the concentrations of TNFα and TGFβ1 in the supernatant samples. It is worth mentioning that prior to TGFβ1 protein detection, the test samples should be activated with HCl and NaOH according to the instructions, which facilitates the release and subsequent detection of TGFβ1 in the samples.
Nitric oxide (NO) release detection
After culturing the cells for 1 day, the production of nitrate and nitrite anions in the culture media supernatant was assessed using the Griess method. This was done using the Total Nitric Oxide Assay Kit (Beyotime Biotechnology, S0023) according to the manufacture’s protocol.
In vitro effects of macrophage-conditioned medium on osteogenesis
Isolation and culture of rat bone marrow-derived mesenchymal stem cells (rbmscs).
The rBMSCs were isolated from the bone marrow of three-week-old male Sprague-Dawley rats, following a previously described protocol [ 26 ]. Briefly, the bone marrow cells were obtained by flushing the tibias and femurs with complete medium with minimum essential medium alpha (α-MEM, Gibco, USA) supplemented with 10% FBS and 1% penicillin-streptomycin. The collected bone marrow cells were centrifuged at 1200 rpm for 5 min and then resuspended in 10 mL of complete medium. The suspended cells were seeded into 75 cm 2 culture flasks (Corning, USA). The cells were cultured in a humidified atmosphere with 5% CO 2 at 37 °C. After 48 h, the adherent cells, referred to as passage 0, were collected and further cultured. For subsequent experiments, the cells were passaged using TrypLE™ Express (Gibco, USA) when they reached approximately 80% confluence. The passage 3 to 5 cells were used in the subsequent experiments.
Macrophage-conditioned medium preparation
Macrophages were cultured with three types of culture media: DMEM, PHA extract, and FPHA extract. After 1 day of incubation, the culture supernatants were extracted and collected. The collected supernatants were subjected to centrifugation at 1500 rpm for 10 min at 4 °C and stored at -80 °C for subsequent experiments.
Osteogenic differentiation
The rBMSCs (1.0 × 10 5 cells per well) were cultured in 24-well plate with co-stimulation medium consisting of osteogenic induction medium and macrophage-conditioned medium with different treatments at a ratio of 1:1. The rBMSCs cultured for 7 days were stained with Alkaline Phosphatase Color Development Kit (C3206, Beyotime, China). Alizarin red S (ARS, Pricella, China) staining was performed on day 14 to detect mineral nodules. These staining techniques were employed to monitor and assess the osteogenic differentiation of the rBMSCs over the course of the experiment.
RNA-seq and data analysis
After 6 h of culturing with DMEM, PHA or FPHA extract, the macrophages were collected and total RNA was extracted as described before. The RNA sequencing was performed using the extracted total RNA by BGI Genomics, China. The thresholds for differentially expressed transcripts were set at P < 0.05 and logFC = 0.585. To gain insights into the biological functions and signaling pathways impacted by these differentially expressed transcripts, several analytical tools were employed. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed, which provided information on gene functions and the involvement of specific pathways. Furthermore, Gene Set Enrichment Analysis (GSEA, v4.3.2), Cytoscape (v3.9.1), ClueGo (v2.5.9), and STRING (a database for protein-protein interactions, v11.5) were used to analyze the differences between the PHA and FPHA groups within pre-defined gene sets.
Metabolism investigation of macrophages following culture with PHA or FPHA extract
Mitochondrial membrane potential (δψm).
The ΔΨm of macrophages was assessed using the JC-1 Assay Kit (C2003S, Beyotime Biotechnology, China) following the manufacturer’s protocol. After a 6-hour and 1-day culture with PHA extract and FPHA extract, macrophages were rinsed twice with PBS and then incubated with the JC-1 staining working solution at 37 °C. Following a 20-minute incubation, the cells were rinsed twice with JC-1 dilution buffer and immediately observed using a fluorescent inverted microscope (Axio Vert.A1, Zeiss, Germany) for qualitative analysis of mitochondrial membrane potential. Additionally, flow cytometry (BD LSRFortessa, USA) was utilized for quantitative analysis of mitochondrial membrane potential.
Reactive oxygen species (ROS)
The intracellular ROS level was measured using the reactive oxygen species assay kit (C1300-1, Solelybio, China) following the manufacturer’s instructions. Macrophages were activated and cultured for 6 h and 1 day. Subsequently, the cells were rinsed twice with PBS and incubated with serum-free culture medium supplemented with 10 µM DCFH-DA probe. Following a 30-minute incubation at 37 °C in the dark, the cells were rinsed twice with PBS. For qualitative analysis, the cells were immediately observed using a fluorescent inverted microscope. For quantitative analysis, the cells were promptly collected for flow cytometry analysis. A positive control group involving direct H 2 O 2 addition as a ROS source was omitted, as LPS stimulation effectively triggers ROS production [ 29 ].
Macrophage metabolism states
To assess the functional activity of macrophages in terms of oxidative phosphorylation and glycolysis, the real-time mitochondrial oxygen consumption rates (OCRs) and the real-time extracellular acidification rates (ECARs) were measured using the XF96 Seahorse analyzer (Agilent Technologies, USA) according to the manufacturer’s instructions. Macrophages were cultured in XF96 pro microplates at a density of 3.0 × 10 4 cells per well. Following activation as previously described, the macrophages were cultured for 6 h and 1 day with DMEM, PHA extract, or FPHA extract.
To measure OCRs, the cell culture medium was replaced with Seahorse XF DMEM supplemented with 10 mM glucose, 1 mM pyruvate, and 2 mM glutamine. Subsequently, the following compounds were injected into their respective ports to achieve a final concentration of 1.5 µM oligomycin (port A), 1.0 µM carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP, port B), and 0.5 µM rotenone and antimycin-A (port C).
For the assessment of ECARs, the cell culture medium was replaced with Seahorse XF DMEM containing 2 mM glutamine. Additionally, 10 mM glucose, 1.0 µM oligomycin and 50 mM 2-deoxy-D-glucose (2-DG) were added to each well.
In vivo immunomodulatory behavior of FPHA
Animal surgery.
In this study, male Sprague-Dawley rats aged 6 to 8 weeks were used to investigate the immune-modulatory process of FPHA. All animal surgical procedures were conducted following the approved protocols of the Institutional Animal Care and Use Committee of Sun Yat-sen University (approval number: IACUC-DB-16-0103). To create the critical size calvarial defect model, the rats were anesthetized and a 1.5 cm sagittal incision was made on the scalp. The calvarium was then exposed through blunt dissection. Using a 5 mm diameter trephine bur, two bilateral calvarial bone defects were created. The insertion of bone substitutes into the bilateral defects was done randomly according to allocation. After 7 days, all animals survived the procedure without any signs of diseases or complications. The grafts and surrounding tissue were carefully dissected. The harvested specimens were fixed by immersion in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) for 24 h and were decalcified in a 10% EDTA solution for four weeks.
Immunohistochemical staining
For immunohistochemical staining, after antigen retrieval, the slides were blocked with a 5% BSA solution for 1 hour and incubated with mouse anti-CD68 (1:100, Invitrogen, USA), anti-iNOS (1:100, Abcam, UK), anti-CD163 (1:1000, Proteintech, USA), anti-TNFα (1:100, ZenBio, China), anti-IL1β (1:100, Proteintech, USA), anti-IL10 (1:100, Proteintech, USA), anti-TGFβ1 (1:100, Proteintech, USA), anti-MMP9 (1:100, Proteintech, USA), anti-OCN (1:100, Affinity, China), anti-HIF1α (1:100, Proteintech, USA) and anti-SDHB (1:100, Proteintech, USA) at 4 °C overnight. Goat anti-rabbit IgG (1:100, Servicebio, China) was used as a secondary antibody and the nuclei were stained using 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI, Servicebio, China). Images were captured using Aperio AT2 (Leica, German).
Statistical analysis
In the study, all experiments were conducted at least three times to ensure reproducibility. The Shapiro-Wilk test was used to assess the normality of the data. If the data was found to be normally distributed, one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test was employed to determine statistical significance between more than two groups. The statistical analyses were conducted using GraphPad Prism software (version 9.0). The data are presented as the mean ± standard deviation (SD), and a p -value less than 0.05 was considered statistically significant.
Characterization and biocompatibility of PHA and FPHA
PHA and FPHA were synthesized using a chemical and thermal method (Fig. 1 A). SEM analysis demonstrated a uniform distribution of crystals in both PHA and FPHA. PHA crystals displayed a spherical shape (left), whereas more rectangular crystals were observed in FPHA (right) (Fig. 1 B). The EDS results indicated that the major chemical elements in PHA and FPHA were oxygen, calcium, carbon, and phosphorus, with sodium and magnesium detected at relatively low levels (Tables 1 and Fig. S1 ).
In the FTIR spectra, the vibrational bands corresponding to functional groups such as PO 4 3− (1058 cm − 1 and 569 cm − 1 ), hydroxyls (OH, 631 cm − 1 and 3573 cm − 1 ), and CO 3 2− (1415 cm − 1 and 1477 cm − 1 ) exhibited relatively consistent patterns for both PHA and FPHA. However, the intensity of the OH characteristic band at 3573 cm − 1 became weakened, while the band associated with OH-F or OH-F-HO became stronger at 3542 cm − 1 in FPHA. Another significant change was the disappearance of the absorption band around 634 cm − 1 attributed to OH, which was replaced by a new band around 740 cm − 1 after fluoridation (Fig. 1 C).
The XRD patterns of both PHA and FPHA were consistent with the stoichiometric reference pattern of hydroxyapatite (JCPDS72-1243), indicating that both materials were crystallized in a pure phase. However, the reflection peaks shifted towards higher diffraction angles, indicating structural changes induced by the incorporation of fluoride (Fig. 1 D).
In the cell culture experiments, the fluoride ion concentration in the medium was measured to be 0.19 ± 0.01 mg/L. In the CCK-8 assay, the cell viability of macrophages cultured with FPHA was significantly higher on day 1 and day 2 compared to the control group (Fig. 1 E). This indicates that FPHA promote cell vitality and proliferation in the early stages of culture. The live/dead staining assay further confirmed the cytocompatibility of PHA and FPHA. During the 3-day culture period, the majority of cells exhibited green fluorescence. Additionally, the number of live cells was higher when cultured with the FPHA extract compared to the control and PHA (Fig. 1 F). This suggests that FPHA may provide a more favorable microenvironment for cell survival and proliferation compared to PHA. This is an important prerequisite for a bone substitute, as its ability to support cell viability and proliferation is crucial for successful tissue integration and regeneration.
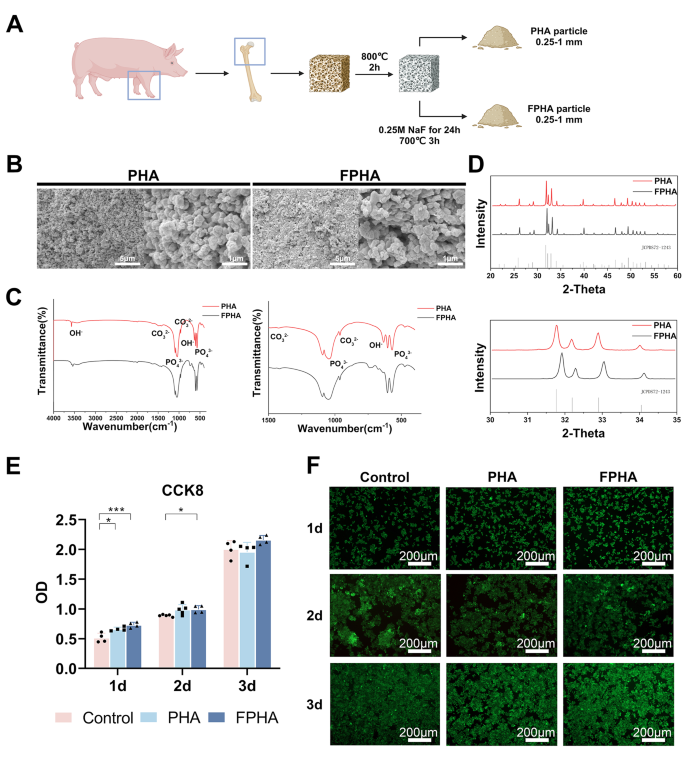
Characterization of fluorinated porcine hydroxyapatite. ( A ) The schematic diagram showed the workflow for the fabrication of FPHA. ( B ) SEM images displayed a spherical-shaped crystal of PHA (left) and a more rectangular crystal in FPHA (right). ( C ) FTIR spectra showed weakened intensity of the OH characteristic band at 3573 cm − 1 and 634 cm − 1 in FPHA. Instead, the band associated with OH-F or OH-F-HO became stronger at 3542 cm − 1 and 740 cm − 1 , respectively. ( D ) XRD patterns demonstrated shifted reflection peaks towards higher diffraction angles after fluoridation. ( E ) Cell viability of macrophages cultured with PHA and FPHA extract for 1 to 3 days showed enhanced cell vitality and proliferation in the early stages of culture. ( F ) The live/dead staining assay showed a higher number of cells remained viable in the FPHA group
FPHA promoted osteogenic differentiation via mediating macrophage polarization phenotype and conditioning an anti-inflammatory osteo-immune microenvironment
Fpha extract suppressed m1 polarization and induced macrophages m2 polarization.
The qRT-PCR analysis showed that culturing with FPHA extract significantly decreased the expression levels of M1 polarization-related genes, including iNOS and CD86 , at 6 h and 1 day (Fig. 2 A). On the other hand, the expression level of the M2 polarization-related gene arginase ( Arg ) increased after culturing with PHA or FPHA extract for 1 day, but no significant differences were found between the two groups (Fig. 2 A).
Further western blot analysis demonstrated that the expression of iNOS was inhibited, while the expression of Arg was enhanced in macrophages cultured with FPHA extract compared to those cultured with PHA extract (Fig. 2 B).
Additionally, a NO release detection assay revealed that a significantly lower level of NO was released after culturing with FPHA compared to PHA (Fig. 2 C), which indicated M1 polarization suppression in macrophages [ 30 ].
These findings collectively confirmed that FPHA suppressed M1 polarization and induced macrophages toward M2 polarization, even with LPS pre-stimulation, further supporting the immunomodulatory effects of FPHA in promoting an anti-inflammatory macrophage polarization phenotype.
FPHA extract promoted a favorable osteo-immune microenvironment
Osteo-immune microenvironment plays a key role in bone regeneration [ 3 ]. The qRT-PCR analysis showed that both FPHA and PHA extract reduced the expression of inflammatory factors compared to control group, such as TNFα, IL1α , and IL1β . Culturing with FPHA extract resulted in lower expression levels of Tnfαip8l1 , and inhibited expression of IL1β at 1 day in macrophages compared to PHA (Fig. 2 D). Increased expression levels of anti-inflammatory genes, including IL1RN and TGFβ1 , were observed in macrophages cultured with FPHA extract (Fig. 2 D). Furthermore, the expression of the osteogenesis-related gene OCN was highly upregulated in cells cultured with FPHA extract, indicating enhanced osteogenesis. Conversely, the expression of the osteoclast-specific gene MMP9 was relatively low (Fig. 2 E).
Western blot analysis revealed that the anti-inflammatory factor IL10 was upregulated, while the osteoclast-specific factor MMP9 was downregulated in the FPHA group (Fig. 2 F). For secretory proteins, a lower expression level of TNFα protein was detected in the supernatant of cell culture media after culturing with FPHA extract for 48 h (Fig. 2 G). And the anti-inflammatory factor TGFβ1 was significantly upregulated in the FPHA group (Fig. 2 G). It is worth noting that the expression of certain genes and proteins may not always perfectly correlate due to the involvement of multiple complex processes in the pathways from genes to proteins [ 31 ]. However, these findings collectively suggest that FPHA exhibits a enhanced osteoconductive and osteo-immunomodulatory capacity, promoting a favorable osteo-immune microenvironment. This implies that FPHA has the potential to enhance bone regeneration by regulating inflammation and facilitating osteogenesis.
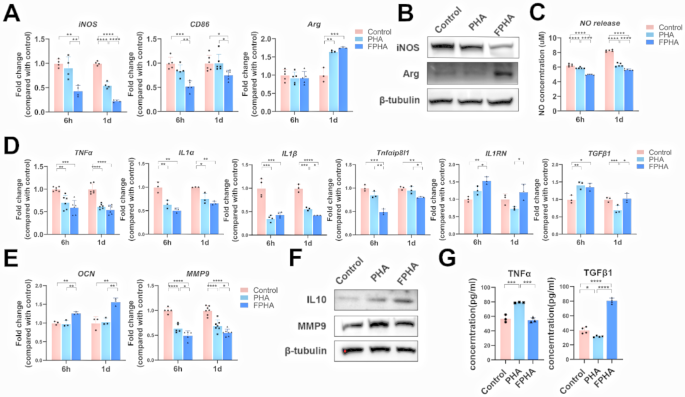
Macrophage response to FPHA. ( A ) The qRT-PCR analysis demonstrated that culturing with FPHA extract resulted in lower levels of M1 polarization-related genes ( iNOS and CD86 ) and higher levels of M2 polarization-related gene ( Arg ). ( B ) Western blot analysis showed that cells cultured with FPHA extract exhibited inhibited expression of iNOS and enhanced expression of Arg. ( C ) NO release detection indicating lower levels of NO in cells cultured with FPHA extract. ( D ) The qRT-PCR analysis demonstrated inhibited expression of inflammatory genes and enhanced expression of anti-inflammatory genes in the FPHA group. ( E ) The qRT-PCR analysis presented downregulation of osteoclast-specific genes and upregulation of osteogenesis-related gene expression in the FPHA group. ( F ) Western blot analysis proved that cells cultured with FPHA extract exhibited inhibited expression of MMP9 and enhanced expression of IL10. ( G ) ELISA assay showed increased level of TGFβ1 and decreased level of TNFα in cells cultured with FPHA extract. (The data are shown as the mean ± SD ( n ≥ 3); Statistical analysis was performed using ANOVA and multiple comparisons post-hoc tests (Tukey HSD). * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001)
FPHA extract promoted osteogenic differentiation via modulating macrophage polarization
To further elucidate the effect of FPHA on osteogenesis, we cultured rBMSCs in a co-stimulation medium (Fig. 3 A). The results of ALP and ARS staining demonstrated a noticeable enhancement of osteogenic differentiation in the FPHA group (Fig. 3 B).
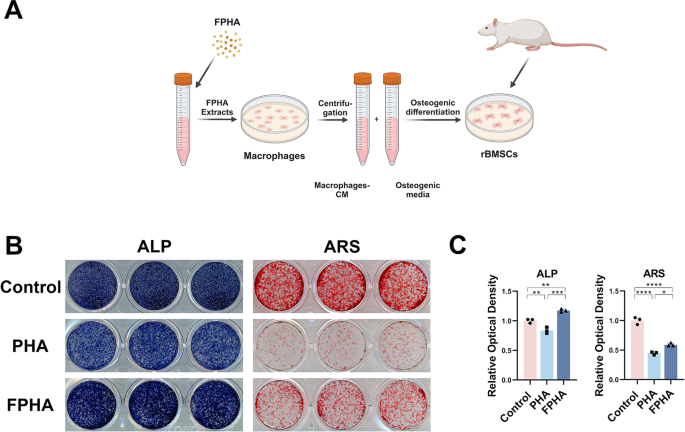
In vitro effects of FPHA on osteogenesis of rBMSCs. ( A ) Schematic representation of RAW264.7 macrophage conditioned medium extraction and preparation of osteogenic media for rBMSCs culture. ( B ) ALP and ARS staining showed a significant enhancement of osteogenic differentiation in the FPHA group. ( C ) Statistical analysis of ALP and ARS staining. (* p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001)
RNA-seq analysis indicated FPHA might mediate macrophage polarization through reprogramming cell metabolism
Based on the aforementioned findings, we can tentatively infer that FPHA suppressed M1 polarization and promoted M2 polarization of macrophages, thereby modulating the osteo-immune microenvironment and facilitating osteogenic differentiation. To further elucidate the underlying biological mechanisms, we conducted RNA sequencing analysis to investigate gene expression in macrophages cultured with FPHA extract.
The differential gene expression analysis revealed distinct patterns, as depicted in the volcano plot (Fig. 4 A). GO enrichment analysis highlighted the enrichment of differentially expressed genes primarily associated with the cell membrane, as observed in the cellular component category (Fig. 4 B). At the molecular function level, these genes were associated with activities such as oxidoreductase activity, transmembrane transporter activity, and growth factor activity. In terms of biological processes, the enriched genes were involved in the regulation of TGFβ receptor signaling pathway and the negative regulation of nitric-oxide synthase biosynthetic process, corroborating our earlier observations regarding the macrophage response to FPHA. Moreover, the KEGG enrichment analysis suggested that these differentially expressed genes may play active roles in the regulation of metabolic pathways (Fig. 4 C). To further investigate the specific metabolic pathways contributing to FPHA-mediated changes in macrophage polarization, we performed GSEA analysis. The results indicated upregulation of gene expression related to oxidative phosphorylation, and glycolysis and gluconeogenesis in the FPHA group (Fig. 4 D), with the core enrichment genes suggesting the involvement of FPHA in ATP synthesis and gluconeogenesis. In general, gluconeogenesis is an anabolic pathway that reverses and does not occur simultaneously with the glycolytic pathway [ 32 ]. It is well-documented that macrophage polarization states are largely determined by mitochondrial functions and metabolic cascades [ 6 , 12 , 33 ]. M2 macrophages exhibit metabolic shift towards oxidative phosphorylation, while M1 macrophages rely on glycolysis and increased production of reactive oxygen species [ 12 ]. Hence, these findings suggest that FPHA may induce a metabolic shift from glycolysis to oxidative phosphorylation, facilitating the reprogramming of M1 macrophages into the M2 phenotype.
Moreover, pathway enrichment analysis conducted using Cytoscape + ClueGo identified that FPHA downregulated the nitric-oxide synthase biosynthetic process and the inflammatory response to wounding (Fig. 4 E). Additionally, protein-protein interaction network analysis performed using STRING revealed that the associated proteins mainly localized to the cell membrane and may potentially influence macrophage polarization through nitric oxide synthesis (Fig. S2 ).
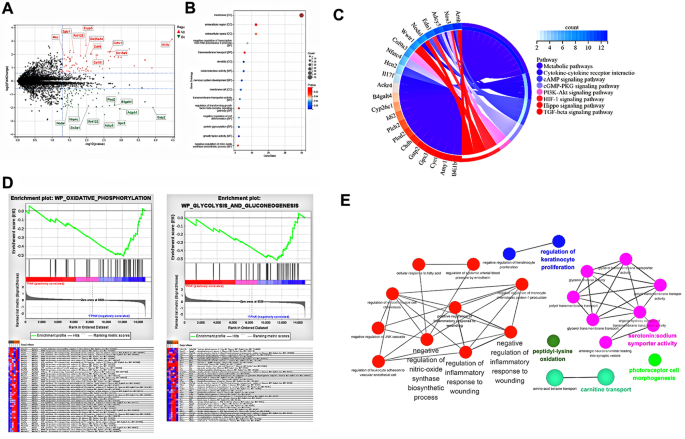
Culturing with FPHA extract enhanced OxPhos (associated with M2 Polarization) while attenuated glycolysis (associated with M1 Polarization) of macrophages, and downregulated the inflammatory response through RNA-seq analysis. ( A ) Volcano plot of RNA-seq analysis showed differentially expressed genes, with red representing upregulated genes and green representing downregulated genes in FPHA group. ( B ) GO enrichment analysis highlighted the enrichment of differentially expressed genes within the cellular component (CC), molecular function (MF), and biological processes (BP) categories. ( C ) The KEGG enrichment analysis demonstrated the potential active involvement of differentially expressed genes in the regulation of metabolic pathways. ( D ) GSEA presented that cells cultured with FPHA extract upregulated the oxidative phosphorylation pathway and the gluconeogenesis pathway. ( E ) Pathway enrichment analysis conducted using Cytoscape + ClueGo identified that FPHA downregulated the nitric-oxide synthase biosynthetic process and the inflammatory response to wounding
FPHA remodeled macrophage metabolism from glycolysis to OxPhos
Fpha extract strengthen macrophage mitochondrial function.
A decrease in ΔΨm is considered a characteristic of mitochondrial dysfunction and early apoptosis, leading to reduced energy synthesis [ 34 ]. To assess ΔΨm in macrophages, JC-1 staining was employed. Cells with high ΔΨm display red fluorescence due to the formation of JC-1 aggregates, while cells with low ΔΨm exhibit green fluorescence as JC-1 remains in its monomeric form. In validation experiments conducted at both 6 h and 1 day, cells treated with FPHA extract exhibited higher intensity of red fluorescence and lower intensity of green fluorescence, particularly at the 1-day time point (Fig. 5 A). This observation was further confirmed by flow cytometry analysis (Fig. 5 B).
Mitochondria are a major source of the ROS, and it is worth noting that ΔΨm plays a crucial role in governing ROS production [ 35 ]. To evaluate intracellular ROS levels, we utilized the DCFH-DA probe. The FPHA group displayed a significant decrease in the percentage of DCF-labeled cells (green fluorescence) compared to both control and PHA groups (Fig. 5 C), indicating a smaller amount of ROS. Further flow cytometry analysis also confirmed that FPHA effectively reduced intracellular ROS levels (Fig. 5 D).
The aforementioned data collectively suggest that FPHA extract can enhance ΔΨm and reduce ROS production in macrophages. Thus, FPHA demonstrates a robust ability to strengthen macrophage mitochondrial function.
Enhanced mitochondrial function in cells cultured with FPHA extract. ( A ) Evaluation of mitochondrial membrane potential using JC-1 probe revealed increased formation of JC-1 aggregates in cells treated with FPHA extract, leading to higher intensity of red fluorescence and lower intensity of green fluorescence (JC-1 monomers). ( B ) Flow cytometry analysis further supported the enhancement effect of FPHA on mitochondrial energy synthesis. ( C ) The FPHA-treated group exhibited a significant decrease in the percentage of DCF-labeled cells (green fluorescence) compared to both the control and PHA groups. ( D ) Flow cytometry analysis further confirmed the inhibitory effect of FPHA on ROS production
FPHA extract enhanced OxPhos and suppressed glycolysis
Different metabolic strategies can redirect macrophages polarization, for example, increasing oxidative phosphorylation allow M2 reprogramming [ 12 ]. Previous RNA-seq results indicated that FPHA upregulated OxPhos pathways and downregulated glycolysis pathways.
To validate these effects, we first recorded the real-time OCRs of macrophages treated with FPHA extract after sequential treatment with oligomycin, FCCP, rotenone, and antimycin A. These compounds allowed the analysis of OxPhos parameters in the macrophages. After a 6-hour culture with FPHA extract (Fig. 6 A), the macrophages exhibited significantly higher levels of basal respiration, maximal respiration, ATP production, and ATP spare respiratory capacity% (Fig. 6 B). However, the OxPhos levels were similar among the three groups after 1 day of culturing (Fig. 6 C and D).
In parallel, we also measured the real-time ECARs in response to sequential injection of glucose, oligomycin, and 2-DG to analyze the glycolytic parameters of the macrophages. Although glycolysis was similar at 6 h among the different groups (Fig. 6 E and F), macrophages cultured with FPHA extract showed significantly lower glycolysis and glycolytic capacity after 1 day of culturing (Fig. 6 G and H).
Enhanced OxPhos and suppressed glycolysis in cells cultured with FPHA extract. ( A ) Real-time OCARs of macrophages after 6 h of culturing, in response to the sequential injection of Oligo, FCCP, and rotenone (cell mitochondrial stress test). ( B ) Quantification of basal respiration, maximal respiration, ATP production and ATP spare respiratory capacity in macrophage mitochondria after 6 h of culturing. The FPHA group exhibited significantly higher levels of basal respiration, maximal respiration, ATP production, and ATP spare respiratory capacity compared to the control and PHA groups. ( C ) Real-time ECARs of macrophages after 6 h of culturing, in response to the sequential injection of glucose, Oligo and 2-DG (glycolysis stress test). ( D ) Quantification of glycolysis and glycolytic capacity after 6 h of culturing. ( E ) Real-time OCARs and ( F ) real-time ECARs of macrophages after 1 day of culturing. ( G ) Quantification analysis of OxPhos and ( H ) glycolysis parameters for cells cultured for 1 day. The FPHA group exhibited significantly higher levels of basal respiration, maximal respiration, ATP production, and ATP spare respiratory capacity compared to the control and PHA groups. (The data are shown as the mean ± SD ( n ≥ 3); Statistical analysis was performed using ANOVA and multiple comparisons post-hoc tests (Tukey HSD). * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001)
FPHA fostered a favorable osteo-immune microenvironment in rat critical size calvarial defects
Firstly, we investigated the response of macrophages to FPHA. Immunohistochemical staining results indicated that the expression level of the macrophage marker CD68 was higher in the FPHA group (Fig. 7 A), compared to PHA. In addition, FPHA was found to significantly reduce the expression of the M1 polarization marker iNOS and enhance the expression of the M2 polarization marker CD163 compared to PHA (Fig. 7 A), suggesting that FPHA had a greater ability to recruit macrophages and promote their polarization towards the M2 phenotype.
Additionally, we investigated the impact of FPHA on macrophage polarization and its subsequent effects on osteo-immunomodulation. The expression levels of the inflammation marker IL1β were notably decreased in the FPHA group, while both the FPHA and PHA groups showed an increase in expression levels of TNFα. Additionally, the FPHA group demonstrated increased expression of anti-inflammatory markers, including TGFβ1 and IL10 (Fig. 7 B). However, no significant differences were observed between the PHA and FPHA groups regarding the expression of the osteoclast-specific factor MMP9 and the osteogenesis-related factor OCN (Fig. 7 B). Furthermore, based on the aforementioned RNA-seq analysis, we assessed the expression of HIF1α and SDHB. It was observed that HIF1α was upregulated in both PHA and FPHA groups (Fig. 7 C), and the expression of SDHB was significantly enhanced in the FPHA group (Fig. 7 C).
Fluorinated porcine hydroxyapatite (FPHA) fostered a favorable osteo-immune microenvironment in rat critical size calvarial defects. ( A ) Immunohistochemistry analysis demonstrated the presence of CD68 + , iNOS + and CD163 + macrophages on day 7. ( B ) Immunohistochemistry analysis revealed the presence of IL1β + , TNFα + , TGFβ1 + , IL10 + , MMP9 + and OCN + cells on day 7. ( C ) Immunohistochemistry analysis showed the presence of HIF-1α + and SDHB + cells on day 7. (The data are shown as the mean ± SD ( n ≥ 3); Statistical analysis was performed using ANOVA and multiple comparisons post-hoc tests (Tukey HSD). * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001)
The development of smart biomaterials as bone substitutes for regeneration has garnered significant attention in recent years due to their osteo-immunomodulatory properties. Macrophage polarization plays a fundamental role in the quality and effectiveness of injury repair [ 5 ]. Fluoride exhibits diverse effects on immune cell metabolism and function [ 14 , 19 , 20 ] and has been proposed as a potential regulator of bone tissue regeneration. Thus, the modification of biomaterials with fluoride may be a promising strategy for modulating the osteoimmunology environment by regulating immunometabolism. In our previous studies [ 21 , 23 , 24 , 25 , 26 , 27 ], FPHA was synthesized and demonstrated enhanced physicochemical and biological properties. In the present study, we synthesized the optimum concentration of FPHA accordingly [ 24 ], and evaluated its physicochemical properties to confirm the incorporation of fluoride ions. Additionally, we explored the potential underlying mechanisms contributing to these improvements.
Fluoride plays a crucial role in the regulation of bone tissue regeneration and exerts its effects in a concentration-dependent manner within the human body [ 18 ]. The fluoride ion concentration in the medium was measured to be 0.19 ± 0.01 mg/L in the above cell culture experiment, which falls within the range of fluoride ion concentrations demonstrated to promote macrophage M2 polarization based on previous study [ 36 ]. Moreover, it has been demonstrated that FPHA has the ability to continuously release fluoride ions [ 25 ]. These findings suggested that FPHA has the potential to modulate macrophage polarization through the release of fluoride ions, providing a continuous immunomodulatory effect.
The aforementioned findings from the present study suggested that FPHA had the ability to suppress the M1 polarization of macrophages while promoting their polarization toward the M2 phenotype, leading to the creation of a favorable osteo-immune microenvironment. Bone remodeling is intricately and spatiotemporally regulated by the balance between osteoclast-mediated resorption and osteoblast-mediated formation [ 37 ]. Therefore, we cultured rBMSCs in a co-stimulation medium to evaluated the effect of FPHA on osteogenesis. Importantly, it should be noted that PHA showed inhibitory effects on osteogenic differentiation as observed through ALP and ARS staining. Additionally, cells cultured with PHA extract exhibited higher expression levels of TNFα and MMP9 proteins, while the expression of TGFβ1 was suppressed. These observations suggested that although PHA, as a bone substitute intended to enhance bone regeneration, seemed to induce a heightened inflammatory reaction and had a detrimental impact on bone remodeling at the early stage. Referring to the previous studies [ 38 , 39 ], it is postulated that the inflammatory response elicited by PHA may result from the release of small calcium phosphate (CaP) particles that are not efficiently filtered by a 0.22 μm filter. Importantly, CaP particles, particularly amorphous calcium phosphate (ACP) nanoparticles, have been shown to activate inflammatory cells and induce the release of inflammatory mediators [ 38 ]. Moreover, it has been demonstrated that the inflammatory response to CaP particles is influenced by their physicochemical properties [ 40 ]. The exciting result of our study was the significant attenuation of the inflammatory effects triggered by CaP particles through the application of FPHA, ultimately promoting a favorable osteo-immune microenvironment and facilitating osteogenic differentiation.
The RNA-seq results suggested that the aforementioned changes in osteo-immune microenvironment may be related to cellular metabolism. Mitochondria, often referred to as the “powerhouse of the cell,” are essential organelles involved in OxPhos and are a primary source of ROS [ 41 ]. Changes in cellular metabolism can influence mitochondrial functions [ 42 ]. The effect of fluoride ions is time dependent [ 19 , 22 ]. To further explore the metabolic changes, we conducted measurements at both 6 h and 1 day to obtain a more comprehensive understanding of the temporal effects of FPHA extract on macrophage mitochondrial function. Based on the mitochondrial metabolism states, it can be concluded that FPHA may have a temporal effect on the metabolic shift of macrophages. After a 6-hour culturing, FPHA promoted OxPhos and related parameters. However, the effects on OxPhos were no longer significant after 1 day. Additionally, FPHA reduced glycolysis and the glycolytic capacity of macrophages after 1 day. The ratio of oxidative phosphorylation to glycolysis was found to be upregulated at both 6 h and 1 day, indicating increased tendency towards M2 polarization in macrophages.
In our previous studies, we have already showcased the superior efficacy of FPHA over PHA in enhancing bone repair within critical size calvarial defects in SD rats [ 21 , 26 ] at 6 weeks and 12 weeks. Additionally, FPHA promoted bone regeneration in the mandible defects in Beagle dogs, with significant improvements observed at 12 weeks [ 27 ]. Building upon these findings, we carried out in vivo experiments using SD rat calvarial defects to further validate the observed outcomes from in vitro experiments in the present study. Based on the mechanism of bone healing, the period between days 5–10 during bone defect repair is when metabolism, inflammation, and the aggregation of osteogenesis-related cells converge [ 43 , 44 ]. The implanted bone substitutes are infiltrated by migrating mesenchymal stem cells, which undergo differentiation over a course of approximately 7 days into chondroblasts and chondrocytes, and subsequently into osteoblasts and osteoclasts, leading to the gradual resorption of cartilage and deposition of new bone [ 45 ]. Therefore, we detected the surrounding tissues on day 7 to capture this critical stage.
The changes in macrophage response and osteo-immune microenvironment in vivo were consistent with those in the in vitro experiments. Therefore, we further investigated the alterations in cellular metabolism. In the aforementioned RNA-seq analysis, KEGG enrichment analysis revealed that differentially expressed genes were associated with the HIF-1 signaling pathway. Previous studies have demonstrated that elevated levels of ROS activated hypoxia-inducible factor 1 alpha (HIF1α), a pivotal transcription factor involved in the regulation of proinflammatory gene expression [ 46 , 47 ]. Thus, we assessed the expression of HIF1α and it was observed that both PHA and FPHA groups exhibited upregulation of HIF1α. Hypoxia-inducible factors (HIFs) are recognized as principal regulators in the response to hypoxic conditions, with particular emphasis on the role of HIF1α [ 48 ]. Given the compromised blood supply, the provisional callus initially experiences a hypoxic microenvironment [ 49 ]. Despite the potential of HIF1α to induce inflammation during the initial inflammatory phase, it can also adapt to the hypoxic conditions by facilitating VEGF production, thereby promoting callus angiogenesis [ 49 ]. In the control group, the defect was in an aerobic environment with soft tissue containing blood supply through the vessels originating from the surrounding tissue. However, in both the PHA and FPHA groups, the bone defects were filled with bone substitutes, resulting in insufficient blood supply. Consequently, the upregulation of HIF1α facilitated vascular invasion and supported bone regeneration. It is worth noting that although HIF1a expression was upregulated, its downstream inflammatory factor IL1β [ 50 ] was significantly downregulated both in vitro and in vivo in the FPHA group. This finding suggested that FPHA did not induce an increase in inflammatory levels.
OxPhos is a highly efficient energy-generating process involving electron flow through five main respiratory chain complexes (complexes I, II, III, IV, and V) located in the inner mitochondrial membrane [ 46 , 51 ]. Among these complexes, succinate dehydrogenase (SDH) is unique as it participates in both the TCA cycle and the electron transport chain (ETC) [ 33 , 52 , 53 ], both of which are crucial for OxPhos. SDH catalyzes the conversion of succinate to fumarate in the TCA cycle, while also facilitating electron transfer, thus promoting respiratory chain activity [ 46 , 53 ]. SDHB is a secondary electron transfer subunit that tightly binds to SDHA and interacts with SDHC and SDHD to form a stable subunit complex [ 54 , 55 ]. Additionally, succinate, an inflammatory metabolite, accumulates during macrophage activation and affects HIF1α activity [ 50 ] and ROS levels [ 53 ]. To validate the in vivo impact of FPHA on mitochondrial function, we performed immunohistochemical staining analysis on SDHB expression in the FPHA group. The results demonstrated upregulation of SDHB, confirming that FPHA may enhance OxPhos through SDHB.
Taken together, the present study supported the notion that FPHA might upregulate SDHB. This promotion, on one hand, enhanced the TCA cycle and the electron transport chain, thereby facilitating OxPhos and suppressing the expression of ROS. On the other hand, the oxidation of succinic acid by SDHB reduced intracellular succinate accumulation, leading to reduced cellular inflammation. Consequently, FPHA contributed to the polarization of macrophages towards the M2 phenotype and created a favorable osteogenic microenvironment.
Inflammation is a universal response. Regardless of the location of bone defects, bone tissue regeneration progresses through three continuing and overlapping phases: inflammation, regeneration and remodeling [ 56 ]. Although appropriate inflammation is vital for bone defect healing and essential for normal tissue repair, heightened inflammatory state hinder the pro-regenerative environment and thereby impede bone tissue repair [ 57 ]. In clinical practice, excessive bone defects, local inflammation such as periodontitis, and systemic disease such as diabetes, may contribute to further elevated inflammation levels during bone defect repair. In such instances, macrophages are more inclined to M1 polarization, leading to a surge in glycolysis and a suppression of OxPhos. Of the various materials available for bone defect repair in medical therapy, biological-derived hydroxyapatite is the most commonly used material because their composition mimics the mineral bone phase [ 58 ]. However, the currently clinically used materials primarily act as scaffolds, with relatively insufficient osteo-immune regulatory properties. At this time, FPHA exhibits a certain targeted therapeutic potential. Through its metabolic-immunoregulatory properties, FPHA prompts surrounding macrophages to shift from glycolysis to OxPhos, timely eliminating the pro-inflammatory stage following the acute inflammation. This not only prevents the progression of acute inflammation, triggered by the implantation of bone replacement materials, into chronic inflammation, but also fosters a regenerative environment conducive to bone formation.
Currently, there are many other modification methods available to enhance the osteo-immune regulatory properties of materials, such as piezoelectric stimulation [ 15 ], addition of stem cells [ 59 ] and green chemical method [ 60 ]. Among these, nanobiomaterials, especially green nanomaterials have attracted considerable attention [ 61 , 62 ]. The previous study [ 63 ] has indicated that PLGA/nanofluorohydroxyapatite can better promote cell viability and enhance mechanical properties. It is widely recognized that the retention of carbonate in biological-derived hydroxyapatite is crucial for maintaining its biological and physicochemical properties [ 64 ]. The low-temperature green synthesis approach favors the retention of carbonate [ 65 ] and provides valuable insights for further improving FPHA.
Our research also has some limitations. Due to the particulate nature of the bone substitute material, it was challenging to maintain stable cell culture conditions [ 66 ]. Therefore, we utilized cell culture with the extract of the material following the ISO/EN 10993-5 standard [ 67 ]. Despite its limitations, this approach is widely accepted for assessing particulate bone substitutes due to its ability to mimic the effects of materials in the in vivo environment, following the release of soluble components [ 26 , 68 , 69 ]. This allows for the assessment of the modulation of the osteo-immune microenvironment by a variety of trace element ions released by the materials.
Additionally, the immunohistochemical results obtained in vivo did not entirely correspond with the findings from our in vitro experiments. The in vivo TNFα expression level was increased in all material groups. This may be due to the fact that the implantation of materials triggers acute inflammation, leading to an upregulation of TNFα expression. However, FPHA suppresses inflammation by upregulating SDHB and related metabolic pathways, which is primarily mediated through the regulation of IL1β expression levels [ 6 , 13 ], rather than TNFα. Both in vitro and in vivo experiments have indicated a downregulation of IL1β expression, with a more pronounced decrease observed in vivo. Therefore, this downregulation ensures that the anti-inflammatory effects of FPHA remain unaffected. Meanwhile, the in vivo expression level of MMP9 was not inhibited, and there was no statistical difference among the three groups. These discrepancies may be attributed to the immune response elicited by the bone substitute material as a foreign body in vivo, which may not solely involve macrophages. However, overall results still suggest that FPHA can influence macrophage polarization through the regulation of metabolic shift.
In light of the aforementioned limitations, further investigation is needed to delve deeper into the molecular mechanisms through which FPHA affects SDHB expression and induces its immunomodulatory effects. Different models could be used to explore efficacy of FPHA in various pathological conditions, such as periodontitis. Additionally, preliminary radiographic results of the ongoing clinical trials indicate that the effectiveness of FPHA in the repair of alveolar bone defects is comparable to that of bovine bone materials commonly used in clinical practice. However, the long-term clinical bone maintenance necessitates continuous observation and evaluate.
In summary, our study revealed that FPHA induced a metabolic shift in macrophages from glycolysis to OxPhos. This metabolic shift led to the suppression of ROS levels, potentially mediated by upregulated SDHB expression. As a result, FPHA lead to significant suppression of M1 macrophage polarization and promotion of M2 macrophage polarization. This resulted in the creation of an anti-inflammatory and osteogenic microenvironment, facilitating rBMSCs osteogenic differentiation. The findings of this study offer valuable insights into the impact of incorporating an optimal concentration of fluoride on immunometabolism and macrophage mitochondrial function. These results have important implications for the development of fluoride-modified bone regenerative biomaterials that leverage immunometabolism as a mechanism for enhanced bone regeneration.
Data availability
The data presented in the current study will be available from the corresponding author on reasonable request.
Abbreviations
Fluorinated porcine hydroxyapatite
Reactive oxygen species
Pentose phosphate pathway
Oxidative phosphorylation
Tricarboxylic acid
Nitric oxide
Rat bone marrow-derived mesenchymal stem cells
Alkaline phosphatase
Alizarin red S
Mitochondrial membrane potential
Oxygen consumption rates
Extracellular acidification rates
Carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone
2-deoxy-D-glucose
Calcium phosphate
Amorphous calcium phosphate
Succinate dehydrogenase
Electron transport chain
Dalfino S, Savadori P, Piazzoni M, Connelly ST, Gianni AB, Del Fabbro M, Tartaglia GM, Moroni L. Regeneration of critical-sized mandibular defects using 3D-Printed Composite scaffolds: a quantitative evaluation of new bone formation in in vivo studies. Adv Healthc Mater. 2023;e2300128. https://doi.org/10.1002/adhm.202300128 .
Ramanauskaite A, Becker K, Cafferata EA, Schwarz F. Clinical efficacy of guided bone regeneration in peri-implantitis defects. A network meta-analysis. Periodontology 2000 2023. https://doi.org/10.1111/prd.12510 .
Chen ZT, Klein T, Murray RZ, Crawford R, Chang J, Wu CT, Xiao Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater Today. 2016;19:304–21. https://doi.org/10.1016/j.mattod.2015.11.004 .
Article CAS Google Scholar
Wang YH, Zhao CZ, Wang RY, Du QX, Liu JY, Pan J. The crosstalk between macrophages and bone marrow mesenchymal stem cells in bone healing. Stem Cell Res Ther. 2022;13:511. https://doi.org/10.1186/s13287-022-03199-y .
Article PubMed PubMed Central Google Scholar
Chen SZ, Saeed AFUH, Liu Q, Jiang Q, Xu HZ, Xiao GG, Rao L, Duo YH. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Therapy. 2023;8:207. https://doi.org/10.1038/s41392-023-01452-1 .
Article Google Scholar
Viola A, Munari F, Sanchez-Rodriguez R, Scolaro T, Castegna A. The metabolic signature of macrophage responses. Front Immunol. 2019;10:1462. https://doi.org/10.3389/fimmu.2019.01462 .
Article CAS PubMed PubMed Central Google Scholar
Davenport Huyer L, Pascual-Gil S, Wang Y, Mandla S, Yee B, Radisic M. Advanced strategies for modulation of the material–macrophage interface. Adv Funct Mater. 2020;30. https://doi.org/10.1002/adfm.201909331 .
Eftekhari A, Hasanzadeh A, Khalilov R, Hosainzadegan H, Ahmadian E, Eghbal MA. Hepatoprotective role of berberine against paraquat-induced liver toxicity in rat. Environ Sci Pollut Res Int. 2020;27:4969–75. https://doi.org/10.1007/s11356-019-07232-1 .
Article CAS PubMed Google Scholar
Amrahov N, Allahverdiyeva RMS, Aliyev E, Alizada S, Aghazada G, Ojagverdiyeva S, Mammadov Z, Author C. Effect of indole-3-butyric acid on the antioxidant enzymes, no and chlorophyll content of agdash-3 and AP-317 genotypes of upland cotton (Gossypium hirsutum L). Adv Biology Earth Sci 2023.
Huseynov E. Novel nanomaterials for Hepatobiliary diseases treatment and future perspectives. Adv Biology Earth Sci. 2024;9:81–91. https://doi.org/10.62476/abes9s81 .
Li MY, Yang YH, Xiong LT, Jiang P, Wang JJ, Li CX. Metabolism, metabolites, and macrophages in cancer. J Hematol Oncol. 2023;16:80. https://doi.org/10.1186/s13045-023-01478-6 .
De Santa F, Vitiello L, Torcinaro A, Ferraro E. The role of metabolic remodeling in macrophage polarization and its effect on skeletal muscle regeneration. Antioxid Redox Signal. 2019;30:1553–98. https://doi.org/10.1089/ars.2017.7420 .
Van den Bossche J, O’Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406. https://doi.org/10.1016/j.it.2017.03.001 .
Araujo TT, Barbosa Silva Pereira HA, Dionizio A, Sanchez CDC, de Souza Carvalho T, da Silva Fernandes M, Rabelo Buzalaf MA. Changes in energy metabolism induced by fluoride: insights from inside the mitochondria. Chemosphere. 2019;236:124357. https://doi.org/10.1016/j.chemosphere.2019.124357 .
Wu H, Dong H, Tang Z, Chen Y, Liu YC, Wang M, Wei XH, Wang N, Bao SS, Yu DM, Wu ZG, Yang ZD, Li XK, Guo Z, Shi L. Electrical stimulation of piezoelectric BaTiO3 coated Ti6Al4V scaffolds promotes anti-inflammatory polarization of macrophages and bone repair via MAPK/JNK inhibition and OXPHOS activation. Biomaterials. 2023;293. https://doi.org/10.1016/j.biomaterials.2022.121990 .
He X-T, Li X, Zhang M, Tian B-M, Sun L-J, Bi C-S, Deng D-K, Zhou H, Qu H-L, Wu C, Chen F-M. Role of molybdenum in material immunomodulation and periodontal wound healing: targeting immunometabolism and mitochondrial function for macrophage modulation. Biomaterials. 2022;283. https://doi.org/10.1016/j.biomaterials.2022.121439 .
Birgani ZT, Gharraee N, Malhotra A, van Blitterswijk CA, Habibovic P. Combinatorial incorporation of fluoride and cobalt ions into calcium phosphates to stimulate osteogenesis and angiogenesis. Biomed Mater. 2016;11. https://doi.org/10.1088/1748-6041/11/1/015020 .
Pal P, Jha NK, Pal D, Jha SK, Anand U, Gopalakrishnan AV, Dey A, Mukhopadhyay PK. Molecular basis of fluoride toxicities: beyond benefits and implications in human disorders. Genes Dis. 2023;10:1470–93. https://doi.org/10.1016/j.gendis.2022.09.004 .
Pereira HABD, Dionizio AS, Araujo TT, Fernandes MD, Iano FG, Buzalaf MAR. Proposed mechanism for understanding the dose-and time-dependency of the effects of fluoride in the liver. Toxicol Appl Pharmcol. 2018;358:68–75. https://doi.org/10.1016/j.taap.2018.09.010 .
Yue BJ, Zhang XH, Li WP, Wang JD, Sun ZL, Niu RY. Fluoride exposure altered metabolomic profile in rat serum. Chemosphere. 2020;258:127387. https://doi.org/10.1016/j.chemosphere.2020.127387 .
Qiao W, Liu RH, Li ZP, Luo X, Huang BX, Liu Q, Chen ZT, Tsoi JKH, Su YX, Cheung KMC, Matinlinna JP, Yeung KWK, Chen ZF. Contribution of the in situ release of endogenous cations from xenograft bone driven by fluoride incorporation toward enhanced bone regeneration. Biomaterials Sci. 2018;6:2951–64. https://doi.org/10.1039/c8bm00910d .
Trevizol JS, Buzalaf NR, Dionizio A, Delgado AQ, Cestari TM, Bosqueiro JR, Magalhaes AC, Buzalaf MAR. Effects of low-level fluoride exposure on glucose homeostasis in female NOD mice. Chemosphere. 2020;254:126602. https://doi.org/10.1016/j.chemosphere.2020.126602 .
Liu Q, Chen ZT, Gu HJ, Chen ZF. Preparation and characterization of fluorinated porcine hydroxyapatite. Dent Mater J. 2012;31:742–50. https://doi.org/10.4012/dmj.2012-052 .
Qiao W, Liu Q, Li ZP, Zhang HQ, Chen ZF. Changes in physicochemical and biological properties of porcine bone derived hydroxyapatite induced by the incorporation of fluoride. Sci Technol Adv Mater. 2017;18:110–21. https://doi.org/10.1080/14686996.2016.1263140 .
Li ZP, Huang BX, Mai S, Wu XY, Zhang HQ, Qiao W, Luo X, Chen ZF. Effects of fluoridation of porcine hydroxyapatite on osteoblastic activity of human MG63 cells. Sci Technol Adv Mater. 2015;16:035006. https://doi.org/10.1088/1468-6996/16/3/035006 .
Liu RH, Qiao W, Huang BX, Chen ZT, Fang JH, Li ZP, Chen ZF. Fluorination enhances the osteogenic capacity of Porcine Hydroxyapatite. Tissue Eng Part A. 2018;24:1207–17. https://doi.org/10.1089/ten.tea.2017.0381 .
Liu YX, Liu CW, Huang RX, Chen KD, Huang BX, Liu Q, Chen ZF, Li ZP. Effects of fluorinated porcine hydroxyapatite on lateral ridge augmentation: an experimental study in the canine mandible. Am J Translational Res. 2020;12:2473–87.
CAS Google Scholar
Han SW, Chen ZT, Han PP, Hu QG, Xiao Y. Activation of macrophages by Lipopolysaccharide for assessing the Immunomodulatory Property of Biomaterials. Tissue Eng Part A. 2017;23:1100–9. https://doi.org/10.1089/ten.tea.2016.0501 .
Zhang P, Wang Y, Yang W, Yin Y, Li C, Ma X, Shi L, Li R, Tao K. 4-Octyl itaconate regulates immune balance by activating Nrf2 and negatively regulating PD-L1 in a mouse model of sepsis. Int J Biol Sci. 2022;18:6189–209. https://doi.org/10.7150/ijbs.74456 .
Liu YC, Teng LL, Lyu Y, Song GS, Zhang XB, Tan WH. Ratiometric afterglow luminescent nanoplatform enables reliable quantification and molecular imaging. Nat Commun. 2022;13:2216. https://doi.org/10.1038/s41467-022-29894-1 .
Kindler S, Wang HD, Richter D, Tiedge H. RNA transport and local control of translation. Annu Rev Cell Dev Biol. 2005;21:223–45. https://doi.org/10.1146/annurev.cellbio.21.122303.120653 .
Bian XL, Jiang HF, Meng Y, Li YP, Fang J, Lu ZM. Regulation of gene expression by glycolytic and gluconeogenic enzymes. Trends Cell Biol. 2022;32:786–99. https://doi.org/10.1016/j.tcb.2022.02.003 .
Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Dabritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP. O’Neill LA. Succinate dehydrogenase supports metabolic repurposing of Mitochondria to Drive Inflammatory macrophages. Cell. 2016;167:457–70. https://doi.org/10.1016/j.cell.2016.08.064 .
Popgeorgiev N, Gil C, Berthenet K, Bertolin G, Ichim G. Shedding light on mitochondrial outer-membrane permeabilization and membrane potential: state of the art methods and biosensors. Semin Cell Dev Biol. 2023;S1084–9521:141–6. https://doi.org/10.1016/j.semcdb.2023.07.003 .
Kuznetsov AV, Margreiter R, Ausserlechner MJ, Hagenbuchner J. The Complex Interplay between Mitochondria, ROS and Entire Cellular Metabolism. Antioxidants 2022; 11:1995. https://doi.org/10.3390/antiox11101995 .
Wu SY, Xia BB, Mai S, Feng ZC, Wang XS, Liu YD, Liu RH, Li ZP, Xiao Y, Chen ZF, Chen ZT. Sodium Fluoride under Dose Range of 2.4–24 µM, a Promising Osteoimmunomodulatory Agent for vascularized bone formation. ACS Biomaterials Sci Eng. 2018;5:817–30. https://doi.org/10.1021/acsbiomaterials.8b00570 .
Xu H, Wang WT, Liu X, Huang W, Zhu C, Xu YZ, Yang HL, Bai JX, Geng DC. Targeting strategies for bone diseases: signaling pathways and clinical studies. Signal Transduct Target Therapy. 2023;8:202. https://doi.org/10.1038/s41392-023-01467-8 .
Velard F, Braux J, Amedee J, Laquerriere P. Inflammatory cell response to calcium phosphate biomaterial particles: an overview. Acta Biomater. 2013;9:4956–63. https://doi.org/10.1016/j.actbio.2012.09.035 .
Ghanaati S, Barbeck M, Orth C, Willershausen I, Thimm BW, Hoffmann C, Rasic A, Sader RA, Unger RE, Peters F, Kirkpatrick CJ. Influence of beta-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010;6:4476–87. https://doi.org/10.1016/j.actbio.2010.07.006 .
Chen LJ, Qiao PY, Liu HC, Shao LQ. Amorphous calcium phosphate NPs mediate the macrophage response and modulate BMSC Osteogenesis. Inflammation. 2020;44:278–96. https://doi.org/10.1007/s10753-020-01331-9 .
Liu YE, Sun YH, Guo YD, Shi XY, Chen X, Feng WF, Wu LL, Zhang J, Yu SB, Wang Y, Shi YF. An overview: the Diversified Role of Mitochondria in Cancer Metabolism. Int J Biol Sci. 2023;19:897–915. https://doi.org/10.7150/ijbs.81609 .
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. https://doi.org/10.1126/science.1160809 .
Wang WH, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioactive Mater. 2017;2:224–47. https://doi.org/10.1016/j.bioactmat.2017.05.007 .
Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11:45–54. https://doi.org/10.1038/nrrheum.2014.164 .
Article PubMed Google Scholar
Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–34. https://doi.org/10.1126/science.3201241 .
Yin M, O’Neill LAJ. The role of the electron transport chain in immunity. Faseb J. 2021;35:e21974. https://doi.org/10.1096/fj.202101161R .
Movafagh S, Crook S, Vo K. Regulation of Hypoxia-Inducible Factor-1a by reactive oxygen species: New Developments in an old debate. J Cell Biochem. 2015;116:696–703. https://doi.org/10.1002/jcb.25074 .
Cowman SJ, Koh MY. Revisiting the HIF switch in the tumor and its immune microenvironment. Trends Cancer. 2022;8:28–42. https://doi.org/10.1016/j.trecan.2021.10.004 .
Xie C, Ye JC, Liang RJ, Yao XD, Wu XY, Koh YW, Wei W, Zhang XZ, Ouyang HW. Advanced strategies of Biomimetic tissue-Engineered grafts for bone regeneration. Adv Healthc Mater. 2021;10:e2100408. https://doi.org/10.1002/adhm.202100408 .
Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LAJ. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–42. https://doi.org/10.1038/nature11986 .
Liu LJ, Nam M, Fan W, Akie TE, Hoaglin DC, Gao GP, Keaney JF, Cooper MP. Nutrient sensing by the mitochondrial transcription machinery dictates oxidative phosphorylation. J Clin Invest. 2014;124:768–84. https://doi.org/10.1172/Jci69413 .
Tabebi M, Dutta RK, Skoglund C, Soderkvist P, Gimm O. Loss of SDHB induces a metabolic switch in the hPheo1 cell line toward enhanced OXPHOS. Int J Mol Sci. 2022;23:560. https://doi.org/10.3390/ijms23010560 .
Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–4. https://doi.org/10.1126/science.1079605 .
Kuo CC, Wu JY, Wu KK. Cancer-derived extracellular succinate: a driver of cancer metastasis. J Biomed Sci. 2022;29:93. https://doi.org/10.1186/s12929-022-00878-z .
Cao K, Xu J, Cao WL, Wang XQ, Lv WQ, Zeng MQ, Zou X, Liu JK, Feng ZH. Assembly of mitochondrial succinate dehydrogenase in human health and disease. Free Radic Biol Med. 2023;207:247–59. https://doi.org/10.1016/j.freeradbiomed.2023.07.023 .
Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–5. https://doi.org/10.1016/j.injury.2011.03.031 .
Newman H, Shih YV, Varghese S. Resolution of inflammation in bone regeneration: from understandings to therapeutic applications. Biomaterials. 2021;277:121114. https://doi.org/10.1016/j.biomaterials.2021.121114 .
Spennato P, Canella V, Aliberti F, Russo C, Ruggiero C, Nataloni A, Lombardo M, Cinalli G. Hydroxyapatite ceramic implants for cranioplasty in children: a retrospective evaluation of clinical outcome and osteointegration. Childs Nerv Syst. 2020;36:551–8. https://doi.org/10.1007/s00381-019-04423-6 .
Lee YC, Chan YH, Hsieh SC, Lew WZ, Feng SW. Comparing the osteogenic potentials and bone regeneration capacities of bone marrow and Dental Pulp Mesenchymal Stem Cells in a rabbit calvarial bone defect model. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20205015 .
Jahangirian H, Lemraski EG, Rafiee-Moghaddam R, Webster TJ. A review of using green chemistry methods for biomaterials in tissue engineering. Int J Nanomed. 2018;13:5953–69. https://doi.org/10.2147/IJN.S163399 .
Khalilov RK. Future prospects of Biomaterials in Nanomedicine. Adv Biology Earth Sci. 2024;9:5–10. https://doi.org/10.62476/abes.9s5 .
Rosic G. Cancer signaling, cell/gene therapy, diagnosis and role of nanobiomaterials. Adv Biology Earth Sci. 2024;9:11–34. https://doi.org/10.62476/abes9s11 .
Tahriri M, Moztarzadeh F, Tahriri A, Eslami H, Khoshroo K, Jazayeri HE, Tayebi L. Evaluation of the in vitro biodegradation and biological behavior of poly(lactic-co-glycolic acid)/nano-fluorhydroxyapatite composite microsphere-sintered scaffold for bone tissue engineering. J Bioactive Compatible Polym. 2017;33:146–59. https://doi.org/10.1177/0883911517720814 .
Taylor EA, Mileti CJ, Ganesan S, Kim JH, Donnelly E. Measures of bone Mineral Carbonate Content and Mineral Maturity/Crystallinity for FT-IR and Raman Spectroscopic Imaging differentially relate to Physical-Chemical properties of Carbonate-substituted hydroxyapatite. Calcif Tissue Int. 2021;109:77–91. https://doi.org/10.1007/s00223-021-00825-4 .
Cooper LF, Zhou Y, Takebe J, Guo J, Abron A, Holmen A, Ellingsen JE. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials. 2006;27:926–36. https://doi.org/10.1016/j.biomaterials.2005.07.009 .
Yao X, Peng R, Ding J. Cell–material interactions revealed via material techniques of surface patterning. Adv Mater. 2013;25:5257–86. https://doi.org/10.1002/adma.201301762 .
Wang Y, Bian Y, Zhou L, Feng B, Weng X, Liang R. Biological evaluation of bone substitute. Clin Chim Acta. 2020;510:544–55. https://doi.org/10.1016/j.cca.2020.08.017 .
Klara J, Hinz A, Bzowska M, Horak W, Lewandowska-Łańcucka J. In vitro/ex vivo evaluation of multifunctional collagen/chitosan/hyaluronic acid hydrogel-based alendronate delivery systems. Int J Biol Macromol. 2024;262. https://doi.org/10.1016/j.ijbiomac.2024.130142 .
Mu Y, Du Z, Gao W, Xiao L, Crawford R, Xiao Y. The effect of a bionic bone ionic environment on osteogenesis, osteoimmunology, and in situ bone tissue engineering. Biomaterials. 2024;304. https://doi.org/10.1016/j.biomaterials.2023.122410 .
Download references
Acknowledgements
BioRender.com kindly provided helps for figure plotting.
This study was supported by the National Natural Science Foundation of China (82001109, 81970975, 82001095), Guangdong Basic and Applied Basic Research Foundation (2023A1515010205), the State Key Laboratory of Oral Diseases (SKLOD) Open Fund (SKLOD2022OF06), Science and Technology Program of Guangzhou (SL2022A04J01709) and China Postdoctoral Science Foundation (2020M683131).
Author information
Kaidi Chen and Seongmin Ha contributed equally to this work and should be considered co-first authors.
Authors and Affiliations
Hospital of Stomatology, Sun Yat-sen University, Guangzhou, China
Kaidi Chen, Seongmin Ha, Leyao Xu, Chengwu Liu, Yuanxiang Liu, Xiayi Wu, Zhipeng Li, Shiyu Wu, Bo Yang & Zhuofan Chen
Guangdong Provincial Key Laboratory of Stomatology, Guangzhou, China
Guanghua School of Stomatology, Sun Yat-sen University, Guangzhou, China
You can also search for this author in PubMed Google Scholar
Contributions
ZC, BY, SW and KC contributed to the conception of the present study; KC, SH and LX contributed to the design of the work; KC and SH contributed to the acquisition and analysis of data; KC, LX, CL, XW, YL and ZL contributed to interpretation of data; XW, YL, BY and ZC contributed to the funding acquisition. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding authors
Correspondence to Shiyu Wu , Bo Yang or Zhuofan Chen .
Ethics declarations
Ethics approval and consent to participate.
All animal surgical procedures were conducted following the approved protocols of the Institutional Animal Care and Use Committee of Sun Yat-sen University (approval number: IACUC-DB-16-0103).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chen, K., Ha, S., Xu, L. et al. Fluorinated hydroxyapatite conditions a favorable osteo-immune microenvironment via triggering metabolic shift from glycolysis to oxidative phosphorylation. J Transl Med 22 , 437 (2024). https://doi.org/10.1186/s12967-024-05261-0
Download citation
Received : 16 December 2023
Accepted : 29 April 2024
Published : 08 May 2024
DOI : https://doi.org/10.1186/s12967-024-05261-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Metabolic shift
- Osteo-immunomodulation
- Bone regeneration
- Macrophages
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
The conclusion serves to provide a concise summary of the key findings, their significance, their implications, and a sense of closure to the study. Discussing how can the findings be applied in real-world scenarios or inform policy, practice, or decision-making is especially valuable to practitioners and policymakers.
Research Summary. Definition: A research summary is a brief and concise overview of a research project or study that highlights its key findings, main points, and conclusions. It typically includes a description of the research problem, the research methods used, the results obtained, and the implications or significance of the findings.
Argumentative paper summary. The evidence is clear: To create a truly futureproof agricultural sector, Dutch farmers must be incentivized to transition from livestock farming to sustainable vegetable farming. ... Having summed up your key arguments or findings, the conclusion ends by considering the broader implications of your research. This ...
While deciding the contents of your research summary, you must include a section on its importance as a whole, the techniques, and the tools that were used to formulate the conclusion. Additionally, there needs to be a short but thorough explanation of how the findings of the research paper have a significance.
The conclusion is intended to help the reader understand why your research should matter to them after they have finished reading the paper. A conclusion is not merely a summary of the main topics covered or a re-statement of your research problem, but a synthesis of key points derived from the findings of your study and, if applicable, where you recommend new areas for future research.
The conclusion should begin by restating the thesis statement from the introduction in a different way. This helps to remind the reader of the main argument or purpose of the research. Summary of Key Findings. The conclusion should summarize the main findings of the research, highlighting the most important results and conclusions.
Some universities will prefer that you cover some of these points in the discussion chapter, or that you cover the points at different levels in different chapters. Step 1: Craft a brief introduction section. As with all chapters in your dissertation or thesis, the conclusions chapter needs to start with a brief introduction.
Step 1: Answer your research question. Step 2: Summarize and reflect on your research. Step 3: Make future recommendations. Step 4: Emphasize your contributions to your field. Step 5: Wrap up your thesis or dissertation. Full conclusion example. Conclusion checklist. Other interesting articles.
The conclusion pushes beyond the boundaries of the prompt and allows you to consider broader issues, make new connections, and elaborate on the significance of your findings. Your conclusion should make your readers glad they read your paper. Your conclusion gives your reader something to take away that will help them see things differently or ...
Draft Summary of Findings: Draft a paragraph or two of discussion for each finding in your study. Assert the finding. Tell the reader how the finding is important or relevant to your studies aim and focus. Compare your finding to the literature. Be specific in the use of the literature. The link or connection should be clear to the reader.
Read the journal's guidelines on the discussion and conclusion sections. If possible, learn about the guidelines before writing the discussion to ensure you're writing to meet their expectations. Begin with a clear statement of the principal findings. This will reinforce the main take-away for the reader and set up the rest of the discussion.
This video explains ways on how to write your thesis Chapter 5 - Summary of Findings, Conclusions and Recommendations. Samples and tips are given as well.
A research article usually has seven major sections: Title, Abstract, Introduction, Method, Results, Discussion, and References. The first thing you should do is to decide why you need to summarize the article. If the purpose of the summary is to take notes to later remind yourself about the article you may want to write a longer summary ...
The conclusions are as stated below: i. Students' use of language in the oral sessions depicted their beliefs and values. based on their intentions. The oral sessions prompted the students to be ...
An effective summary has the following qualities: It bases on results from the study. It is brief, all statements are concise, and pinpoint to the contributions that the researcher has made. Recommendations. All statements are factual. One way to present the summary is to use one paragraph for each idea. Alternatively, the researcher can use a ...
Conclusion: This section provides a summary of the key findings and the main conclusions of the study. Recommendations: This section suggests areas for further research and potential applications or implications of the study's findings. How to Write Research Findings. Writing research findings requires careful planning and attention to detail.
A summary is an abridgement of the work of literature, which covers the key points succinctly. On the contrary, conclusion refers to the final part of the discourse which sums up the argument and gives a statement of opinion or judgement. A summary is written to provide the reader with a precise and objective narrative of the central ideas and ...
A 'Summary of findings' table, described in Chapter 14, Section 14.1, provides key pieces of information about health benefits and harms in a quick and accessible format. It is highly desirable that review authors include a 'Summary of findings' table in Cochrane Reviews alongside a sufficient description of the studies and meta ...
Table of contents. When to write a summary. Step 1: Read the text. Step 2: Break the text down into sections. Step 3: Identify the key points in each section. Step 4: Write the summary. Step 5: Check the summary against the article. Other interesting articles. Frequently asked questions about summarizing.
findings is an interpretation of practical analysis, While Conclusion as says Dr. Serrat, synthesizes and interprets the finding and makes a reasoned judgment that corresponds to the finding. Cite ...
This chapter contains the restatement of the problem, the summary of the findings, conclusions, and recommendations. Discover the world's research 25+ million members
The presses publish research monographs, undergraduate texts, school textbooks, professional books, trade books, reference works and research journals. The main publishing categories are undergraduate textbooks and research monographs. Table 5.4 gives the summary of categories published by each press. 5. Are they actually sold, if so in what ...
The findings from this review highlight an opportunity for standardization to better inform the evidence for and delivery of sexual and reproductive health interventions in humanitarian settings. ... Plain English summary. ... It is challenging to draw strong conclusions about the applicability of CFIR in humanitarian settings based on the ...
Conclusions and Relevance: In summary, our findings shed light on the contributions of CNVs to interindividual variability in complex traits related to neurocognitive development and child psychopathology. ### Competing Interest Statement AFA-B receives consulting income from Octave Bioscience.
Conclusions. Key conclusions from the independent evaluation are presented below. These are subject to the stated caveats (see page 13 of full evaluation report):. Virtual Wards in South-East England are associated with a positive impact on non-elective (NEL) hospital activity - on average 1 NEL admission 'avoided' was shown to be correlated with 2.5 virtual ward admissions, with some ...
Over the last few decades, the incidence of urogenital cancers has exhibited diverse trends influenced by screening programs and geographical variations. Among women, there has been a consistent or even increased occurrence of endometrial and ovarian cancers; conversely, prostate cancer remains one of the most diagnosed malignancies, with a rise in reported cases, partly due to enhanced and ...
Background: The Liver Imaging Reporting and Data System (LI-RADS) combines standardized terminology with a classification system for imaging findings in patients with HCC, therefore rendering diagnostic biopsy unnecessary in many cases. This retrospective study included 23 patients with a biopsy diagnosis of HCC, performed either before or after local interventional procedures, in order to ...
Objectives This study aims to evaluate the utility of F-18 FDG PET/CT in the non-invasive diagnosis of autoimmune pancreatitis (AIP) and differentiating it from pancreatic cancer (CaP) based on the amount and pattern of FDG uptake, as well as involvement of extra-pancreatic sites. Methods A systematic search was conducted using PubMed, Scopus, Cochrane Library and Google Scholar. Only those ...
Background Biological-derived hydroxyapatite is widely used as a bone substitute for addressing bone defects, but its limited osteoconductive properties necessitate further improvement. The osteo-immunomodulatory properties hold crucial promise in maintaining bone homeostasis, and precise modulation of macrophage polarization is essential in this process. Metabolism serves as a guiding force ...
Executive Summary In October 2023, the Adams' Administration announced that it would implement a new policy limiting shelter stays for newly arrived families with children to 60 days.[1] Previously, families with children in the shelter system had not faced time limits for eviction from shelter. In a press release issued...