- Conclusions
- Article Information
Evidence reviews for the USPSTF use an analytic framework to visually display the key questions that the review will address in order to allow the USPSTF to evaluate the effectiveness and safety of a preventative service. The questions are depicted by linkages that relate interventions and outcomes. A dashed line indicates a health outcome that immediately follows an intermediate outcome. For additional details see the US Preventive Services Task Force Procedure Manual. 13
Reasons for exclusion: Design: Study did not use an included design. Outcomes: Study did not have relevant outcomes or had incomplete outcomes. Comparator: Study used an excluded comparator. Intervention: Study used an excluded intervention/screening approach. Population: Study was not conducted in an average-risk population. Timing: Study only reported first (prevalence) round screening follow-up. Publication type: Study was published in non–English-language or only available in an abstract. Quality: Study did not meet criteria for fair or good quality. Setting: Study was not conducted in a setting relevant to US practice. KQ indicates key question.
DBT indicates digital breast tomosynthesis; DM, digital mammography; and RR, relative risk.
a From random-effects restricted maximum likelihood model.
eMethods. Literature Search Strategies for Primary Literature
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Quality Assessment Criteria
eTable 3. Included Studies and Their Ancillary Publications
eTable 4. Screen-Detected DCIS Diagnosed in Studies Comparing Digital Breast Tomosynthesis and Digital Mammography
eFigure 1. Pooled Analysis of Screen-Detected Invasive Cancers Diagnosed in Trials Comparing Digital Breast Tomosynthesis and Digital Mammography
eFigure 2. Pooled Analysis of Interval Cancers Diagnosed in Trials Comparing Digital Breast Tomosynthesis and Digital Mammography
eFigure 3. Cumulative Probability of False-Positive Biopsy in One NSRI Using BCSC Data Comparing Annual vs Biennial Screening with DBT or DM
eFigure 4. Cumulative Probability of False-Positive Recall in One NSRI Using BCSC Data Comparing Annual vs Biennial Screening with DBT or DM
eFigure 5. Cumulative Probability of False-Positive Recall or Biopsy in One NSRI Using BCSC Data Comparing Annual vs Biennial Screening with DBT or DM, among Women with Extremely Dense Breasts
- USPSTF Recommendation: Screening for Breast Cancer JAMA US Preventive Services Task Force April 30, 2024 This 2024 Recommendation Statement from the US Preventive Services Task Force recommends biennial screening mammography for women aged 40 to 74 years (B recommendation) and concludes that evidence is insufficient to assess the balance of benefits and harms of screening mammography in women 75 years or older (I statement) and of screening using ultrasonography or MRI in women with dense breasts on a negative mammogram (I statement). US Preventive Services Task Force; Wanda K. Nicholson, MD, MPH, MBA; Michael Silverstein, MD, MPH; John B. Wong, MD; Michael J. Barry, MD; David Chelmow, MD; Tumaini Rucker Coker, MD, MBA; Esa M. Davis, MD, MPH; Carlos Roberto Jaén, MD, PhD, MS; Marie Krousel-Wood, MD, MSPH; Sei Lee, MD, MAS; Li Li, MD, PhD, MPH; Carol M. Mangione, MD, MSPH; Goutham Rao, MD; John M. Ruiz, PhD; James J. Stevermer, MD, MSPH; Joel Tsevat, MD, MPH; Sandra Millon Underwood, PhD, RN; Sarah Wiehe, MD, MPH
- USPSTF Report: Collaborative Modeling to Compare Breast Cancer Screening Strategies JAMA US Preventive Services Task Force April 30, 2024 This modeling study uses Cancer Intervention and Surveillance Modeling Network models and national data on breast cancer incidence, mammography performance, treatment effects, and other-cause mortality in US women without previous cancer diagnoses to estimate outcomes of various mammography screening strategies. Amy Trentham-Dietz, PhD, MS; Christina Hunter Chapman, MD, MS; Jinani Jayasekera, PhD, MS; Kathryn P. Lowry, MD; Brandy M. Heckman-Stoddard, PhD, MPH; John M. Hampton, MS; Jennifer L. Caswell-Jin, MD; Ronald E. Gangnon, PhD; Ying Lu, PhD, MS; Hui Huang, MS; Sarah Stein, PhD; Liyang Sun, MS; Eugenio J. Gil Quessep, MS; Yuanliang Yang, MS; Yifan Lu, BASc; Juhee Song, PhD; Diego F. Muñoz, PhD; Yisheng Li, PhD, MS; Allison W. Kurian, MD, MSc; Karla Kerlikowske, MD; Ellen S. O’Meara, PhD; Brian L. Sprague, PhD; Anna N. A. Tosteson, ScD; Eric J. Feuer, PhD; Donald Berry, PhD; Sylvia K. Plevritis, PhD; Xuelin Huang, PhD; Harry J. de Koning, MD, PhD; Nicolien T. van Ravesteyn, PhD; Sandra J. Lee, ScD; Oguzhan Alagoz, PhD, MS; Clyde B. Schechter, MD, MA; Natasha K. Stout, PhD; Diana L. Miglioretti, PhD, ScM; Jeanne S. Mandelblatt, MD, MPH
- Toward More Equitable Breast Cancer Outcomes JAMA Editorial April 30, 2024 Joann G. Elmore, MD, MPH; Christoph I. Lee, MD, MS
- Screening for Breast Cancer JAMA JAMA Patient Page April 30, 2024 In this JAMA Patient Page, the US Preventive Services Task Force provides a guide to screening for breast cancer. US Preventive Services Task Force
- When Is It Best to Begin Mammograms, and How Often? JAMA Medical News & Perspectives May 3, 2024 This Medical News story discusses new USPSTF recommendations about the timing of screening mammograms. Rita Rubin, MA
- New Recommendations for Breast Cancer Screening—In Pursuit of Health Equity JAMA Network Open Editorial April 30, 2024 Lydia E. Pace, MD, MPH; Nancy L. Keating, MD, MPH
- USPSTF Breast Cancer Screening Guidelines Do Not Go Far Enough JAMA Oncology Editorial April 30, 2024 Wendie A. Berg, MD, PhD

See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
- CME & MOC
Henderson JT , Webber EM , Weyrich MS , Miller M , Melnikow J. Screening for Breast Cancer : Evidence Report and Systematic Review for the US Preventive Services Task Force . JAMA. Published online April 30, 2024. doi:10.1001/jama.2023.25844
Manage citations:
© 2024
- Permissions
Screening for Breast Cancer : Evidence Report and Systematic Review for the US Preventive Services Task Force
- 1 Kaiser Permanente Evidence-based Practice Center, Center for Health Research, Portland, Oregon
- 2 University of California Davis Center for Healthcare Policy and Research, Sacramento
- Editorial Toward More Equitable Breast Cancer Outcomes Joann G. Elmore, MD, MPH; Christoph I. Lee, MD, MS JAMA
- Editorial New Recommendations for Breast Cancer Screening—In Pursuit of Health Equity Lydia E. Pace, MD, MPH; Nancy L. Keating, MD, MPH JAMA Network Open
- Editorial USPSTF Breast Cancer Screening Guidelines Do Not Go Far Enough Wendie A. Berg, MD, PhD JAMA Oncology
- US Preventive Services Task Force USPSTF Recommendation: Screening for Breast Cancer US Preventive Services Task Force; Wanda K. Nicholson, MD, MPH, MBA; Michael Silverstein, MD, MPH; John B. Wong, MD; Michael J. Barry, MD; David Chelmow, MD; Tumaini Rucker Coker, MD, MBA; Esa M. Davis, MD, MPH; Carlos Roberto Jaén, MD, PhD, MS; Marie Krousel-Wood, MD, MSPH; Sei Lee, MD, MAS; Li Li, MD, PhD, MPH; Carol M. Mangione, MD, MSPH; Goutham Rao, MD; John M. Ruiz, PhD; James J. Stevermer, MD, MSPH; Joel Tsevat, MD, MPH; Sandra Millon Underwood, PhD, RN; Sarah Wiehe, MD, MPH JAMA
- US Preventive Services Task Force USPSTF Report: Collaborative Modeling to Compare Breast Cancer Screening Strategies Amy Trentham-Dietz, PhD, MS; Christina Hunter Chapman, MD, MS; Jinani Jayasekera, PhD, MS; Kathryn P. Lowry, MD; Brandy M. Heckman-Stoddard, PhD, MPH; John M. Hampton, MS; Jennifer L. Caswell-Jin, MD; Ronald E. Gangnon, PhD; Ying Lu, PhD, MS; Hui Huang, MS; Sarah Stein, PhD; Liyang Sun, MS; Eugenio J. Gil Quessep, MS; Yuanliang Yang, MS; Yifan Lu, BASc; Juhee Song, PhD; Diego F. Muñoz, PhD; Yisheng Li, PhD, MS; Allison W. Kurian, MD, MSc; Karla Kerlikowske, MD; Ellen S. O’Meara, PhD; Brian L. Sprague, PhD; Anna N. A. Tosteson, ScD; Eric J. Feuer, PhD; Donald Berry, PhD; Sylvia K. Plevritis, PhD; Xuelin Huang, PhD; Harry J. de Koning, MD, PhD; Nicolien T. van Ravesteyn, PhD; Sandra J. Lee, ScD; Oguzhan Alagoz, PhD, MS; Clyde B. Schechter, MD, MA; Natasha K. Stout, PhD; Diana L. Miglioretti, PhD, ScM; Jeanne S. Mandelblatt, MD, MPH JAMA
- JAMA Patient Page Screening for Breast Cancer US Preventive Services Task Force JAMA
- Medical News & Perspectives When Is It Best to Begin Mammograms, and How Often? Rita Rubin, MA JAMA
Importance Breast cancer is a leading cause of cancer mortality for US women. Trials have established that screening mammography can reduce mortality risk, but optimal screening ages, intervals, and modalities for population screening guidelines remain unclear.
Objective To review studies comparing different breast cancer screening strategies for the US Preventive Services Task Force.
Data Sources MEDLINE, Cochrane Library through August 22, 2022; literature surveillance through March 2024.
Study Selection English-language publications; randomized clinical trials and nonrandomized studies comparing screening strategies; expanded criteria for screening harms.
Data Extraction and Synthesis Two reviewers independently assessed study eligibility and quality; data extracted from fair- and good-quality studies.
Main Outcomes and Measures Mortality, morbidity, progression to advanced cancer, interval cancers, screening harms.
Results Seven randomized clinical trials and 13 nonrandomized studies were included; 2 nonrandomized studies reported mortality outcomes. A nonrandomized trial emulation study estimated no mortality difference for screening beyond age 74 years (adjusted hazard ratio, 1.00 [95% CI, 0.83 to 1.19]). Advanced cancer detection did not differ following annual or biennial screening intervals in a nonrandomized study. Three trials compared digital breast tomosynthesis (DBT) mammography screening with digital mammography alone. With DBT, more invasive cancers were detected at the first screening round than with digital mammography, but there were no statistically significant differences in interval cancers (pooled relative risk, 0.87 [95% CI, 0.64-1.17]; 3 studies [n = 130 196]; I 2 = 0%). Risk of advanced cancer (stage II or higher) at the subsequent screening round was not statistically significant for DBT vs digital mammography in the individual trials. Limited evidence from trials and nonrandomized studies suggested lower recall rates with DBT. An RCT randomizing individuals with dense breasts to invitations for supplemental screening with magnetic resonance imaging reported reduced interval cancer risk (relative risk, 0.47 [95% CI, 0.29-0.77]) and additional false-positive recalls and biopsy results with the intervention; no longer-term advanced breast cancer incidence or morbidity and mortality outcomes were available. One RCT and 1 nonrandomized study of supplemental ultrasound screening reported additional false-positives and no differences in interval cancers.
Conclusions and Relevance Evidence comparing the effectiveness of different breast cancer screening strategies is inconclusive because key studies have not yet been completed and few studies have reported the stage shift or mortality outcomes necessary to assess relative benefits.
Breast cancer is the second leading cause of cancer mortality for US women, despite a steady overall decline in breast-cancer mortality rates over the past 20 years. 1 The average age-adjusted rate for the years 2016-2020 was 19.6 per 100 000, with an estimated 43 170 deaths in 2023. 1 , 2 The majority of cases occur between the ages of 55 and 74 years, 1 and incidence is highest among women ages 70 to 74 (468.2 per 100 000). 3 Non-Hispanic White women have the highest breast cancer incidence, 4 but mortality is 40% higher for non-Hispanic Black women (27.6 per 100 000) compared with White women (19.7 per 100 000); non-Hispanic Black women experience lower 5-year survival regardless of the cancer subtype or stage at the time of detection. 1 , 5 - 7
Previous reviews of breast cancer screening effectiveness established the benefits and harms of mammography based primarily on large, long-term trials. 8 , 9 In 2016, the US Preventive Services Task Force (USPSTF) recommended screening for breast cancer in women starting at age 50 years every 2 years continuing through age 74 years (B recommendation) and that screening from ages 40 to 49 years should be based on clinical discussions of patient preferences and individual breast cancer risk (C recommendation). 10 This comparative effectiveness systematic review of breast cancer screening strategies was conducted concurrently with a separate decision modeling study. 11 Both informed the USPSTF updated breast cancer screening recommendations. 12
This review addressed 3 key questions (KQs) on the comparative effectiveness and harms of different screening strategies ( Figure 1 ). Methodological details including study selection, a list of excluded studies, detailed study-level results for all outcomes and for specific subpopulations, and contextual observations are available in the full evidence report. 14
Studies included in the 2016 USPSTF reviews 8 , 9 , 15 , 16 were evaluated for inclusion with eligibility criteria for the current review. In addition, database searches for relevant studies published between January 2014 and August 22, 2022, were conducted in MEDLINE, the Cochrane Central Register of Controlled Clinical Trials, and the Cochrane Database of Systematic Reviews (eMethods in the Supplement ). Reference lists of other systematic reviews were searched to identify additional relevant studies. ClinicalTrials.gov was searched for relevant ongoing trials. Ongoing surveillance to identify newly published studies was conducted through March 2024 to identify major studies published in the interim. Two new nonrandomized studies were identified 17 , 18 and are not further discussed, as they would not change interpretation of the review findings or conclusions.
Two independent reviewers screened titles, abstracts, and relevant full-text articles to ensure consistency with a priori inclusion and exclusion criteria (eTable 1 in the Supplement ). We included English-language studies of asymptomatic screening populations not at high risk for breast cancer. The eligible population for this review is adult females (sex assigned at birth). For consistency with the underlying evidence, the term “women” is used throughout this report; however, cancer registries and studies of breast cancer generally infer gender based on physiology and medical history rather than measuring self-reported gender. Included studies compared mammography screening modalities (mammography with or without digital breast tomosynthesis [DBT]), different screening strategies with respect to interval, age to start, age to stop, or supplemental screening strategies using ultrasound or magnetic resonance imaging (MRI) with mammography.
For KQ1, randomized clinical trials (RCTs) or nonrandomized studies of interventions with contemporaneous comparison groups that reported breast cancer morbidity, mortality, all-cause mortality, or quality of life were included. For KQ2, the primary outcome of interest was progression to advanced breast cancer, defined for this review as stage IIB or higher, which encompasses tumors with local lymph node involvement or distant metastases. 19 Study-defined advanced breast cancer outcomes were used when this outcome was not reported (eg, stage II or higher). Invasive breast cancer detection outcomes from multiple screening rounds can indicate whether a screening modality or strategy reduces the risk of advanced cancer by detecting early cancers that would otherwise have progressed (stage shift), thereby potentially reducing breast cancer morbidity and mortality. 20 - 23
For KQ3, RCTs and nonrandomized studies of interventions reporting adverse events, including psychological harms, radiation exposure, and interval invasive cancers (incident or missed due to false-negative screening) were included, regardless of the number of screening rounds reported. False-positive recall, false-positive biopsy recommendation, and false-positive biopsy rates (individuals who underwent a biopsy for a benign lesion) were obtained from included RCTs and from nonrandomized studies reporting cumulative rates of these potential harms of screening.
Two reviewers evaluated all articles that met inclusion criteria using prespecified quality criteria (eTable 2 in the Supplement ). Discordant quality ratings were resolved through discussion and input from a third reviewer. Risk-of-bias assessment was conducted using the USPSTF-specific criteria for randomized trials 13 and an adapted tool from the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I). 24 Studies determined to be at high risk of bias were excluded. One reviewer extracted key elements of included studies into standardized evidence tables in DistillerSR (Evidence Partners) and a second reviewer checked the data for accuracy. Limited evidence on sub-KQs is available in the full report. 14 When available, reported relative risks were provided in the tables, but we calculated and reported crude effect estimates and confidence intervals when studies did not provide them. For KQ2 intermediate detection outcomes, the definition of advanced cancer reported in the studies was used for synthesis; commonly this was stage II or later. Comparisons of prognostic characteristics or markers (eg, grade, tumor size, nodal involvement, receptor status) were included for comparisons as data allowed.
All quantitative analyses were conducted in Stata version 16 (StataCorp). The presence of statistical heterogeneity was assessed among pooled studies using the I 2 statistic. Where effects were sufficiently consistent and clinical and statistical heterogeneity low, random-effects meta-analyses were conducted using the restricted maximum likelihood; all tests were 2-sided, with P < .05 indicating statistical significance.
Aggregate strength of evidence (ie, high, moderate, or low) was assessed for each KQ and comparison using the approach described in the Methods Guide for the Effectiveness and Comparative Effectiveness Reviews, 25 based on consistency, precision, publication bias, and study quality.
Investigators reviewed 10 378 unique citations and 419 full-text articles for all KQs ( Figure 2 ). Twenty studies reported in 45 publications were included. 26 - 45 A full list of included studies by KQ is located in eTable 3 in the Supplement .
Key Question 1. What is the comparative effectiveness of different mammography-based breast cancer screening strategies (eg, by modality, interval, initiation and stopping age, use of supplemental imaging, or personalization based on risk factors) on breast cancer morbidity and mortality?
Two nonrandomized studies reported on the association of different screening programs with breast cancer morbidity and mortality. One study was designed to compare different ages to stop screening 30 and another compared annual and triennial screening intervals. 41
A fair-quality observational study (n = 1 058 013) on age to stop screening used an emulated trial methodology to analyze a random sample of US Medicare A and B claims data for enrollees aged 70 to 84 years (1999 to 2008), eligible for breast cancer screening, and with at least a 10-year estimated life expectancy. The study estimated the effect of stopping screening at ages 70, 75, and 80 years compared with continued annual screening. 30 , 46 Continuation of screening between the ages of 70 and 74 years was associated with reduced mortality risk based on survival analysis (hazard ratio, 0.78 [95% CI, 0.63 to 0.95]), but the absolute difference in the risk of death for the age group was small and the confidence interval included null (1.0 fewer deaths per 1000 screened [95% CI, −2.3 to 0.1]). These results indicate a difference in the cumulative incidence curves that approached a difference in the mortality risk for the age group. Conversely, continued screening vs no screening from ages 75 to 84 years did not result in statistically significant differences in the absolute risk of breast cancer mortality (0.07 fewer deaths per 1000 [95% CI, –0.93 to 1.3]) or the cumulative mortality incidence (hazard ratio, 1.00 [95% CI, 0.83 to 1.19]).
A fair-quality nonrandomized clinical study (n = 14 765) conducted in Finland during the years 1985 to 1995 assigned participants aged 40 to 49 years to annual or triennial screening invitations by alternating birth year. 41 The study reported no difference in breast cancer mortality: 20.3 deaths per 100 000 person-years with annual screening invitations and 17.9 deaths per 100 000 person-years with triennial screening invitations (relative risk [RR], 1.14 [95% CI, 0.59-1.27]).
Key Question 2. What is the comparative effectiveness of different mammography-based breast cancer screening strategies (eg, by modality, interval, initiation and stopping age, use of supplemental imaging, or personalization based on risk factors) on the incidence of and progression to advanced breast cancer?
No eligible studies of age to start or stop screening, supplemental screening, or personalized screening were included, because no RCTs or nonrandomized studies reported more than a single round of screening comparing screening strategies. For screening interval, 1 RCT 26 and 1 nonrandomized study, 41 and for comparisons of different screening modalities (DBT vs digital mammography) 3 RCTs 27 , 33 , 42 and 2 nonrandomized studies, 34 , 44 met eligibility criteria.
Two fair-quality studies addressed the effect of screening interval on the characteristics of detected cancers. A fair-quality United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) RCT comparing screening intervals was conducted as part of the UK National Breast Screening Program. The study randomized participants aged 50 to 62 years to annual (n = 37 530) or triennial (n = 38 492) breast cancer screening during the years 1989 to 1996. 26 After 3 years of screening (1 incidence screen in the triennial screening group), a similar number of cancers (screen-detected and interval) had been diagnosed in the annual and triennial screening groups (6.26 and 5.40 per 1000 screened, respectively; RR, 1.16 [95% CI, 0.96 to 1.40]). No statistically significant differences were found in the cancer characteristics (tumor size, nodal status, histological grade) between groups over the course of the study.
A fair-quality nonrandomized study using Breast Cancer Surveillance Consortium (BCSC) registry data (1996 to 2012) 39 found the relative risk of being diagnosed with a breast cancer with less favorable prognostic characteristics (stage IIB or higher, tumor size >15 mm, or node-positive) was not statistically different for women screened biennially compared with those screened annually for any age category (40-49, 50-59, 60-69, 70-85 years).
Three fair-quality RCTs 27 , 33 , 42 reported cancer detection over 2 rounds of screening, comparing the effects of screening with DBT and digital mammography on the presence of advanced cancer at subsequent screening rounds ( Table 1 ). Participants were randomized to the DBT intervention group or the digital mammography control group at a first round of screening, followed in 2 trials by a second round of screening with digital mammography for all second-round participants (Proteus Donna, 27 RETomo 42 ) and in 1 trial with DBT for all second-round participants (To-Be 33 ). The trials used an identical screening modality for both study groups at the second round because using the same instrument is a stronger design for detection of stage shift.
The RCTs reported increased detection of invasive cancer with DBT at the first round of screening (pooled RR, 1.41 [95% CI, 1.20 to 1.64]; 3 RCTs [n = 129 492]; I 2 = 7.6%) and no statistical difference in invasive cancer at the subsequent screening (pooled RR, 0.87 [95% CI, 0.73 to 1.05]; 3 RCTs [n = 105 064]; I 2 = 0%) (eFigure 1 in the Supplement ). 27 , 33 , 42 There was no statistically significant difference in the incidence of advanced cancers at the subsequent screening round (progression of cancers not found at prior screening that would indicate stage shift) in the individual trials ( Figure 3 ). Results were inconsistent and thus not pooled for the advanced cancer, larger tumor (>20 mm), and node-positive cancer outcomes. The results for histologic grade 3 cancer at the second screening were consistent (pooled RR, 0.97 [95% CI, 0.61-1.55]; 3 RCTs [n = 105 244]; I 2 = 0%) ( Figure 3 ). Due to the small number of cases, it was not possible to assess differences in the detection of cancers lacking hormone or growth factor receptors (ie, triple-negative cancers) that have the worst prognosis among breast cancer subtypes.
Two fair-quality nonrandomized studies of interventions (NRSIs), including a US study using BCSC data, compared breast cancer detection outcomes from screening over multiple rounds (≥2) with either DBT-based mammography or digital mammography alone. 34 , 44 The findings were generally consistent with the trial results for cancer detection and stage shift.
Key Question 3. What are the comparative harms of different mammography-based breast cancer screening strategies (modality, interval, initiation age, use of supplemental imaging, or personalization based on risk factors)?
No eligible studies of age to start screening or personalized screening were identified. For age to stop screening, 1 fair-quality nonrandomized study met eligibility criteria. 30 For comparisons of potential harms associated with different screening intervals, a fair-quality RCT 26 and 2 fair-quality nonrandomized studies 39 , 41 were included. For comparisons of different screening modalities (DBT vs digital mammography), 4 RCTs (3 good- and 1 fair-quality) 27 , 31 , 33 , 42 and 7 fair-quality nonrandomized studies were included. 28 , 32 , 34 - 36 , 43 , 44
In the NRSI using an emulated trial methodology to evaluate the age to stop screening, 30 the 8-year cumulative proportion of participants with a breast cancer diagnosis was higher among those who continued annual screening from ages 70 to 84 years (5.5%) compared with those who discontinued screening (3.9%) at age 70 years. Because fewer cancers were diagnosed among those who discontinued screening, there was a lower risk of undergoing cancer treatment and experiencing related morbidity. Notably, for participants aged 75 to 84 years, screening (and treatment) were not associated with lower breast cancer mortality (see KQ1 results).
The UKCCCR trial included for KQ2 26 reported fewer interval cancers (false-negative and incident cancers) diagnosed in the annual invitation group compared with triennial screening (1.84 vs 2.70 per 1000 women screened, respectively; RR, 0.68 [95% CI, 0.50 to 0.92]). The nonrandomized clinical trial conducted in Finland included for KQ1 41 also reported interval cancers diagnosed with annual vs triennial screening and found no statistical difference in incidence ( P = .22, data not reported). Data from 2 studies from the BCSC registry reported higher probabilities of false-positive recalls and biopsy recommendations with annual screening compared with biennial screening and no statistical difference in interval cancers in adjusted analyses. 32 , 39 , 44
Four RCTs (3 good-quality, 1 fair-quality) 27 , 31 , 33 , 42 and 7 fair-quality nonrandomized studies 28 , 32 , 34 - 36 , 43 , 44 reported outcomes related to potential screening harms associated with DBT-based screening compared with digital mammography–only screening, including interval cancer rates, round-specific and cumulative false-positive recalls and biopsies, and radiation exposure. Meta-analysis of 3 large trials did not show a statistically significant difference in rates of interval cancer after screening with DBT compared with digital mammography (pooled RR, 0.87 [95% CI, 0.64 to 1.17]; 3 RCTs [n = 130 196]; I 2 = 0%) (eFigure 2 in the Supplement ). 27 , 33 , 42
Data on interval cancers were also obtained from 7 nonrandomized studies. 28 , 32 , 34 - 36 , 43 , 44 The most recent BCSC analysis, reporting interval cancer rates across multiple screening rounds with either DBT or digital mammography, did not identify statistically significant differences in invasive or advanced interval cancers. 44
The effects of DBT screening on false-positive recall and false-positive biopsy rates varied across studies 27 , 33 , 42 and by screening round, with small or no statistical differences between study groups, not consistently favoring DBT-based mammography or digital mammography.
Evidence from 2 nonrandomized BCSC studies provided false-positive results across several screening rounds. 32 , 44 In 1 study, rates of false-positive recall and false-positive biopsy rates were lower with DBT in initial screening rounds, but differences were attenuated and not statistically significant compared with digital mammography only after additional rounds of screening ( Table 2 ). 44 The other study reported no statistical difference in 10-year cumulative false-positive biopsy recommendation rates between biennial DBT and digital mammography screening, but false-positive recall was slightly lower with DBT (eFigures 3 and 4 in the Supplement ); no differences by modality were identified for individuals with extremely dense breasts in stratified analyses (eFigure 5 in the Supplement ). 32
Four RCTs 27 , 31 , 33 , 42 and 1 NRSI 35 reported the mean, median, or relative radiation dose received in each study group at a single screening round. The 3 studies using DBT/digital mammography screening reported radiation exposure approximately 2 times higher in the intervention group compared with the digital mammography–only group. 27 , 35 , 42 Differences between study groups in radiation exposure were smaller in studies using DBT with synthetic digital mammography. 33 , 47
The Dense Tissue and Early Breast Neoplasm Screening (DENSE) trial, a good-quality RCT conducted in the Netherlands, randomized (1:4) participants aged 50 to 75 years with extremely dense breasts and negative mammography findings (2011-2015) (n = 40 373) to an invitation or no invitation for supplemental MRI screening. 45 (The RCT was not included for KQ2 because second round results in the control group were unavailable). Fifty-nine percent of those randomized to the invitation underwent an MRI examination (n = 4783). In intention-to-treat analysis, 2.2 per 1000 experienced interval breast cancer diagnoses in the supplemental screening invitation group, compared with 4.7 per 1000 screened in the digital mammography control group (RR, 0.47 [95% CI, 0.29 to 0.77]). Adverse events related to the supplemental MRI screening reported in the trial included 5 classified as serious adverse events (2 vasovagal reactions and 3 allergic reactions to the contrast agent) and 2 reports of extravasation (leaking) of the contrast agents and 1 shoulder subluxation. Twenty-seven participants (0.6% of the MRI group) reported a serious adverse event within 30 days of the MRI. Those who underwent supplemental MRI screening also experienced additional recalls (94.9 per 1000 screened), false-positive recalls (80.0 per 1000 screened), and false-positive biopsies (62.7 per 1000 screened).
A fair-quality nonrandomized study used claims data from commercially insured women (MarketScan database) aged 40 to 64 years who had received at least 1 bilateral screening breast MRI (n = 9208) or mammogram (n = 9208) between January 2017 and June 2018. 29 Following propensity score matching, those undergoing screening with MRI were more likely to have additional health care cascade events such as office visits and follow-up tests unrelated to breast conditions (adjusted difference between groups, 19.6 per 100 screened [95% CI, 8.6 to 30.7]) in the subsequent 6 months.
A fair-quality RCT, the Japan Strategic Anti-cancer Randomized Trial, randomly assigned asymptomatic women aged 40 to 49 years (2007-2011) to breast cancer screening with mammography plus handheld ultrasound (digital mammography/ultrasound) (n = 36 859) or mammography only (digital mammography) (n = 36 139). 40 The relative risk of invasive interval cancer was not statistically significantly different for digital mammography/ultrasound vs digital mammography only (RR, 0.58 [95% CI, 0.31 to 1.08]). This result differs from the statistically significant population-average effect reported in the study ( P = .03), which included interval ductal carcinoma in situ (proportion difference, −0.05% [95% CI, −0.09 to 0]). Those undergoing ultrasound in addition to digital mammography experienced 48.0 per 1000 additional false-positive recall results compared with those assigned to digital mammography screening only.
A fair-quality nonrandomized study using data from 2 BCSC registry sites compared screening outcomes for participants receiving ultrasonography on the same day as a screening mammogram (digital mammography/ultrasound) (n = 3386, contributing 6081 screens) compared with those that received only a mammogram (digital mammography) (n = 15 176, contributing 30 062 screens). 37 However, 31% of participants had a first-degree family history of breast cancer or previous breast biopsy. There was no statistical difference in interval cancer risk (adjusted RR, 0.67 [95% CI, 0.33 to 1.37]), and rates of false-positive biopsy were twice as high for the mammography/ultrasound group (adjusted RR, 2.23 [95% CI, 1.03 to 2.58]).
Prior screening effectiveness reviews based on large trials initiated in previous decades established a statistically significant mortality benefit for mammography screening of women aged 50 to 69 years. 8 , 9 , 15 The current review considered comparative effectiveness questions on the relative benefits and harms of different screening start and stop ages, intervals, and modalities for women at average breast cancer risk. Findings are summarized in Table 3 .
The evidence was insufficient for addressing the age to start or end screening. No eligible studies comparing different ages to start screening were identified. Limited evidence from 1 nonrandomized study, using an emulated trial study design, suggested that screening beyond age 74 years may not reduce breast cancer mortality. 30
Evidence was also insufficient for evaluating the effect of screening intervals on breast cancer morbidity and mortality. Two nonrandomized studies found no difference in breast cancer outcomes. 26 , 39 Moderate evidence supported longer screening intervals (eg, biennial) to reduce the cumulative risk of false-positive recall and biopsy. The observational studies of different screening intervals compared individuals who self-selected or were referred for different screening intervals, contributing to risk of bias in the results.
Results from 3 RCTs 27 , 33 , 42 and 2 nonrandomized studies 34 , 44 provided moderate evidence that DBT-based mammography does not reduce the risk of invasive interval cancer or advanced cancer at subsequent screening rounds. Additional rounds of screening and longer follow-up are needed to fully evaluate whether DBT reduces breast cancer morbidity and mortality. Consistent with trial findings, a nonrandomized BCSC study did not find reduced risks of advanced or interval cancers with DBT. 44 Limited evidence from trials on harms of screening with DBT 27 , 33 , 42 indicated similar false-positive recall and biopsy rates. An observational BCSC study did not show differences in the 10-year cumulative false-positive biopsy rates 32 ; lower false-positive recall and biopsy with DBT screening were attenuated after several screening rounds. 44 Additional research is needed to ascertain whether DBT-based screening would reduce false-positives over a lifetime of screening.
The evidence was not adequate to evaluate the benefits and harms of supplemental MRI screening for people with dense breasts. No eligible studies were identified that provide evidence on breast cancer morbidity or mortality outcomes with supplemental MRI screening compared with mammography alone among individuals with dense breasts. The DENSE trial 45 reported fewer interval cancers with 1 round of supplemental MRI screening, but results from a second screening round are not yet published. Evidence of higher advanced cancer incidence in the mammography-only group relative to the MRI group would be needed to anticipate effects on morbidity or mortality. Supplemental MRI led to additional false-positive recalls and biopsies, and uncommon but serious adverse events were observed. 45 Two recent systematic reviews of the test performance literature reported higher cancer detection with supplemental MRI screening along with substantially increased recall and biopsy rates among individuals without cancer. 48 , 49
Lack of a standardized and reliable assessment tool for measuring breast density and density variation across the lifespan pose challenges for research into the optimal screening strategy for persons with dense breasts. 16 Research is also needed to evaluate personalized risk-based screening, based on breast cancer risk factors and personal screening preferences. The ongoing WISDOM trial and My Personalized Breast Screening study (expected completion in 2025) may help to address these research gaps. 50 , 51
Breast cancer is an active area of research, yet few longitudinal RCTs comparing different screening strategies have been conducted following completion of the major trials that established the effectiveness of mammography for reducing breast cancer mortality for women aged 50 to 69 years. This review included 6 new randomized trials, 27 , 31 , 33 , 40 , 42 , 45 4 comparing DBT with digital mammography screening 27 , 31 , 33 , 42 and 2 on supplemental screening compared with mammography alone. 40 , 45 Three of these trials are ongoing 31 , 40 , 45 and have reported preliminary results only. Observational studies were also included, but few studies were available that followed up a screening population over time to compare the health outcomes associated with different screening approaches. These studies, while potentially more representative of a screening population, have higher risk of biased results due to confounding and selection.
Changes in population health, imaging technologies, and available treatments may limit the applicability of previous studies. Recent trials included in this review were conducted outside of the US and enrolled mostly White European populations. No studies evaluated screening outcomes for racial or ethnic groups in the US that experience health inequities and higher rates of breast cancer mortality. Black women are at highest risk of breast cancer mortality, 52 with lower 5-year survival than all other race and ethnicity groups. 7 Breast cancer mortality risk also increases at younger ages for Black women compared with White women. 53 This review did not address additional factors beyond screening that contribute to breast cancer mortality inequities. 54 Rigorous research is essential to understand and identify improvements needed along the pathway from screening to treatment 55 and to address inequities in follow-up time after a positive screening result, time to diagnosis, 56 - 60 and receipt of high-quality treatment and support services. 59 , 61 , 62
Evidence comparing outcomes for different screening intervals and ages to start and stop screening was limited or absent. Trials of personalized screening based on risk and patient preferences are in progress and may address evidence gaps related to optimal screening start ages and intervals. Research is needed to better characterize potential harms of screening, including patient perspectives on experiencing false-positive screening results. Women with false-positive screening results may be less likely to return for their next scheduled mammogram, as reported in a large US health system study. 55 , 63 Rigorous studies that enroll screening populations and report advanced cancer detection, morbidity, and mortality outcomes from multiple rounds of screening are needed to overcome persistent limitations in the evidence on breast cancer screening. Multiple screening rounds are essential to determine whether a screening modality or strategy reduces the risk of advanced cancer by detecting early cancers that would otherwise have progressed (stage shift), potentially reducing breast cancer morbidity and mortality. 20 - 23 , 64
The potential benefits of risk-stratified screening strategies, including the use of supplemental screening with ultrasound or MRI, have not been fully evaluated, although some harms are evident. Longer term follow-up on existing comparative effectiveness trials, complete results from ongoing RCTs of personalized screening programs, 65 , 66 and rigorous new studies are needed to further strengthen the evidence and optimize breast cancer screening strategies.
Evidence comparing the effectiveness of different breast cancer screening strategies is inconclusive because key studies have not yet been completed and few studies have reported the stage shift or mortality outcomes necessary to assess relative benefits.
Accepted for Publication: November 23, 2023.
Published Online: April 30, 2024. doi:10.1001/jama.2023.25844
Corresponding Author: Jillian T. Henderson, PhD, MPH, Kaiser Permanente Evidence-based Practice Center, Center for Health Research, Kaiser Permanente Northwest, 3800 N Interstate Ave, Portland, OR 97227 ( [email protected] ).
Author Contributions: Dr Henderson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: All authors.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical review of the manuscript for important intellectual content: Henderson, Weyrich, Miller.
Statistical analysis: Henderson.
Administrative, technical, or material support: Webber, Melnikow.
Supervision: Henderson.
Conflict of Interest Disclosures: None reported.
Funding/Support: This research was funded under contract number 75Q80120D00004, Task Order 75Q80121F32004, from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services.
Role of the Funder/Sponsor: Investigators worked with USPSTF members and AHRQ staff to develop the scope, analytic framework, and key questions for this review. AHRQ had no role in study selection, quality assessment, or synthesis. AHRQ staff provided project oversight, reviewed the report to ensure that the analysis met methodological standards, and distributed the draft for peer review. Otherwise, AHRQ had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript findings.
Disclaimer: The opinions expressed in this document are those of the authors and do not reflect the official position of AHRQ or the US Department of Health and Human Services.
Additional Contributions: The authors gratefully acknowledge the following individuals for their contributions to this project: Howard Tracer, MD (AHRQ); Heidi D. Nelson, MD, MPH, MACP (Kaiser Permanente Bernard J. Tyson School of Medicine); current and former members of the USPSTF who contributed to topic deliberations; and Evidence-based Practice Center staff members Melinda Davies, MA, Jill Pope, and Leslie A. Purdue, MPH, for technical and editorial assistance at the Kaiser Permanente Center for Health Research. USPSTF members, peer reviewers, and federal partner reviewers did not receive financial compensation for their contributions.
Additional Information: A draft version of this evidence report underwent external peer review from 5 content and methods experts (Nehmat Houssami, MBBS, MPH, Med, PhD [University of Sydney-Australia]; Patricia Ganz, MD [UCLA]; Gerald Gartlehner, MD, MPH [Cochrane Austria]; Karla Kerlikowske, MD [UC San Francisco]; Lisa Newman, MD, MPH [New York Presbyterian/Weill Cornell Medical Center]) and 4 scientific representatives from 3 federal partner organizations (Centers for Disease Control and Prevention; Office of Research on Women’s Health; National Institute on Minority Health and Health Disparities). Comments were presented to the USPSTF during its deliberation of the evidence and were considered in preparing the final evidence review.
Editorial Disclaimer: This evidence report is presented as a document in support of the accompanying USPSTF Recommendation Statement. It did not undergo additional peer review after submission to JAMA .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Cost-Effectiveness Analysis of Digital Breast Tomosynthesis and Mammography in Breast Cancer Screening: A Markov Modeling Study
Affiliations.
- 1 Department of Medical Imaging, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
- 2 Department of Medical Imaging, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
- 3 School of Global Health Management and Informatics, University of Central Florida, Orlando, USA.
- 4 Department of Healthcare Administration and Medical Informatics, Kaohsiung Medical University, No. 100, Tzyou 1st Road, Kaohsiung, Taiwan.
- 5 Department of Healthcare Administration and Medical Informatics, Kaohsiung Medical University, No. 100, Tzyou 1st Road, Kaohsiung, Taiwan. [email protected].
- 6 Department of Business Management, National Sun Yat-Sen University, Kaohsiung, Taiwan. [email protected].
- 7 Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan. [email protected].
- 8 Department of Medical Research, China Medical University Hospital, China Medical University, Taichung, Taiwan. [email protected].
- PMID: 38748377
- DOI: 10.1007/s44197-024-00239-z
Background: Mammography (MG) has demonstrated its effectiveness in diminishing mortality and advanced-stage breast cancer incidences in breast screening initiatives. Notably, research has accentuated the superior diagnostic efficacy and cost-effectiveness of digital breast tomosynthesis (DBT). However, the scope of evidence validating the cost-effectiveness of DBT remains limited, prompting a requisite for more comprehensive investigation. The present study aimed to rigorously evaluate the cost-effectiveness of DBT plus MG (DBT-MG) compared to MG alone within the framework of Taiwan's National Health Insurance program.
Methods: All parameters for the Markov decision tree model, encompassing event probabilities, costs, and utilities (quality-adjusted life years, QALYs), were sourced from reputable literature, expert opinions, and official records. With 10,000 iterations, a 2-year cycle length, a 30-year time horizon, and a 2% annual discount rate, the analysis determined the incremental cost-effectiveness ratio (ICER) to compare the cost-effectiveness of the two screening methods. Probabilistic and one-way sensitivity analyses were also conducted to demonstrate the robustness of findings.
Results: The ICER of DBT-MG compared to MG was US$5971.5764/QALYs. At a willingness-to-pay (WTP) threshold of US$33,004 (Gross Domestic Product of Taiwan in 2021) per QALY, more than 98% of the probabilistic simulations favored adopting DBT-MG versus MG. The one-way sensitivity analysis also shows that the ICER depended heavily on recall rates, biopsy rates, and positive predictive value (PPV2).
Conclusion: DBT-MG shows enhanced diagnostic efficacy, potentially diminishing recall costs. While exhibiting a higher biopsy rate, DBT-MG aids in the detection of early-stage breast cancers, reduces recall rates, and exhibits notably superior cost-effectiveness.
Keywords: Breast cancer screening; Cost-utility analysis; Digital breast tomosynthesis; Mammography; Markov model.
© 2024. The Author(s).
Grants and funding
- MOST 108-2410-H-037-006-SS3/Ministry of Science and Technology
- 111-2410-H-037-002-MY3/Ministry of Science and Technology
- Research article
- Open access
- Published: 22 October 2019
Knowledge, attitudes, and practices related to breast cancer screening among female health care professionals: a cross sectional study
- Humariya Heena ORCID: orcid.org/0000-0002-0493-1422 1 ,
- Sajid Durrani 2 ,
- Muhammad Riaz 3 ,
- Isamme AlFayyad 1 ,
- Rabeena Tabasim 4 ,
- Gazi Parvez 5 &
- Amani Abu-Shaheen 1
BMC Women's Health volume 19 , Article number: 122 ( 2019 ) Cite this article
28k Accesses
45 Citations
1 Altmetric
Metrics details
Incidence of breast cancer in the Kingdom of Saudi Arabia (KSA) has increased in recent years. Screening helps in early detection of cancer and early diagnosis and timely treatment of breast cancer lead to a better prognosis. Women in the healthcare profession can have a positive impact on the attitudes, beliefs, and practices of general public. Therefore, it is important that the healthcare workers themselves have adequate knowledge and positive attitudes. We conducted a study to assess the knowledge, attitudes, and practices related to breast cancer screening among female healthcare professionals.
A cross-sectional study was conducted on female health professional of KFMC (King Fahad Medical City). Data was collected using a pre-designed, tested, self-administered questionnaire. The questionnaire included specific sections to test the participants’ knowledge, attitude, and practices related to cervical cancer and its screening. Data analysis was done using descriptive statistics.
A total of 395 health care workers participated in this study. The mean age of the participants was 34.7 years. Participants included physicians ( n = 63, 16.0%), nurses ( n = 261, 66.1%), and allied health workers ( n = 71, 18.0%). Only 6 (1.5%) participants had a good level of knowledge of breast cancer and 104 (26.8%) participants demonstrated a fair level of knowledge. Overall, 370 (93.7%), 339 (85.8%), and 368 (93.2%) participants had heard of breast self-examination, clinical breast examination, and mammography, respectively. A total of 295 (74.7%) participants reported practicing breast self-examination, 95 (24.1%) had undergone clinical breast examination, and 74 (18.7%) had ever undergone mammography.
The knowledge, attitudes, and practices related to breast cancer screening were found to be lower than expected. Active steps are required to develop educational programs for the health care staff, which might empower them to spread the knowledge and positively influence the attitudes of female patients in the hospital.
Peer Review reports
Breast cancer is the most common cancer among women worldwide [ 1 ]. In 2018, over 2 million new cases of breast cancer were diagnosed globally accounting for 11.6% of all cancers. Breast cancer is also the most common cause of cancer-related deaths in women [ 1 ]. It is no longer prevalent only in the developed part of the world but is commonly reported in the developing countries as well. In Kingdom of Saudi Arabia incidence of breast cancer has been on the rise in recent years with number of cases increasing from 1152 per 100.000 inhabitants in 2008 to 1473 per 100.000 inhabitants in 2010, and 1826 per 100.000 inhabitants in 2014 [ 2 , 3 ]. The KSA health council 2014 cancer registry reported breast cancer to be the most common cancer in women accounting to 28.7% of all cancers while another study attributed 13.08% of all deaths to breast cancer 98% of which occurred in women and 12% in men [ 3 , 4 ].
Although there has been immense progress in the treatment of breast cancer, prognosis remains poor in developing countries including KSA [ 4 ]. An important reason for the poor prognosis could be a delay in diagnosis. When breast cancer is diagnosed at an early stage, prognosis is believed to be good with reduced morbidity and mortality [ 5 ]. Therefore, steps should be taken to ensure early detection and timely treatment. Two vital strategies for early detection include early diagnosis and screening [ 6 ]. An important aspect of early diagnosis includes increasing the awareness of early signs of cancer among physicians, nurses, other healthcare workers as well as the general population [ 7 ]. Screening, on the other hand, includes employing simple tests to identify individuals with cancer even before symptoms appear. Breast self-examination (BSE), clinical breast examination (CBE), and mammography are well recognized screening methods for breast cancer [ 6 , 7 ]. Although in recent international guidelines, which focus on developed countries, the timeframes for screening have been questioned, this may not apply to the developing countries including Saudi Arabia where the awareness is very low and patients routinely present at advanced stage of breast cancer [ 8 , 9 ].
Breast cancers in women from Arab populations have different characteristics and affected patients are at least a decade younger. Hence, the of Ministry of health in KSA guidelines in contrast to international guidelines recommend the use of screening strategies with mammography for the detection of breast cancer in women aged 40–49 years every 1 to 2 years. The indication that higher benefit on breast cancer mortality justifies a recommendation in favor of implementing breast cancer screening using mammography in this age group in this population.
Based on local cancer registry data, the incidence of breast cancer in the KSA for the age group 50–69 years is similar to the ones reported in the literature in other countries. Hence the Ministry suggests screening with mammography in women aged 50–69 years every 2 years and no screening with mammography for women aged 70–74 years, however a nationalized large scale screening program is yet to take off [ 10 ].
In KSA despite the healthcare facilities being free of cost, utilization of breast cancer screening methods, including mammography, is very low with one study reporting that out of women 50 years or older, 89% of them reported not having a clinical breast examination (CBE) and 92% of women reported never having a mammogram in the past year [ 11 ].
For effective screening and early diagnosis, adequate knowledge and awareness are of utmost importance. Women healthcare workers can bring about a significant change in the overall perspective of their female patients, regarding screening practices and positively influence their attitudes and beliefs [ 12 ]. They are also the first point of contact irrespective of their specialty of work for not only their female patients but also female relatives and friends for advice regarding breast cancer screening. Females usually feel embarrassed to talk about this issue with their male physicians. Consequently, measures are required to educate women and spread awareness. To achieve this, an important step would be to ensure that female healthcare professionals themselves possess adequate knowledge which they can transmit to their patients, relatives and acquaintances [ 13 ].
Several studies have been conducted in other developing countries to assess the knowledge and practices of breast cancer screening both in the general population as well as specifically in healthcare professionals [ 14 , 15 , 16 , 17 , 18 ]. In KSA, also several similar studies have been conducted on the general population [ 19 ]. However, the number of studies conducted on healthcare professionals in KSA have been limited. We, therefore, conducted this study to assess the knowledge, attitudes, and practices related to breast cancer screening among female healthcare professionals.
Study design and study population
A cross-sectional study was conducted on female healthcare workers (with at least 1 year of clinical experience) in 2018, including physicians, nurses, and allied health staff, at King Fahad Medical City (KFMC), Riyadh, Saudi Arabia.
Data collection
Data were collected using a pre-designed, pre-tested, and self-administered questionnaire. The questionnaire was developed from previous studies after an in depth literature review [ 14 , 15 , 16 , 17 , 18 , 20 , 21 , 22 ]. Before administering the questionnaire to the study population, the face validity of the questionnaire was ensured by a committee of experts in research methodology, obstetrics and gynecology, and oncology. A pilot study was conducted on 70 participants to ensure the clarity and reliability of the questionnaire. Cronbach’s alpha was used to evaluate the reliability which was found to be > 0.70. A trained research assistant randomly approached the subjects in each department and distributed the questionnaires. A survey cover sheet explaining the study was attached to the questionnaire for the participants to sign and complete. Complete anonymity was maintained to protect participants’ identity and to ensure confidentiality of data.
After an extensive literature search, the various survey questions were formulated and the questionnaire was divided into several sections. Some of the question were modified or deleted as per the recommendations of the expert committee since they were either off topic or not suitable for health care workers. The questionnaire included different parts.
First part elicited socio-demographic data on age, clinical experience, education, designation, department, marital status, age at marriage, number of pregnancies, number of children, history of breast cancer, and family history of breast cancer of each study participant.
Questions relating to knowledge of breast cancer were included in the second part. These questions were included under three categories: potential risk factors, signs and symptoms, and ways of screening/diagnosis of breast cancer including BSE, CBE, and mammography.
The respondents were requested to record their answers by choosing one of the three options: ‘Yes’, ‘No’, or ‘Don’t Know’. The scale was then dichotomized (Yes = 1 and No/Don’t Know = 0) and the total knowledge score for each participant was computed by adding up (maximum score of 30). The total score was then categorized as poor knowledge (score of 0–4), fair knowledge (score of 5–14), and good knowledge (score of 15–30).
Participants’ attitude regarding breast cancer was assessed in the third part by asking them to rate 10 specific statements on a 5-point Likert scale. Following 10 statements were included in the questionnaire: 1) Any woman is at risk for breast cancer; 2) Breast cancer can be prevented; 3) If I examine my breast myself, I cannot detect abnormalities in my breast; 4) There is no reason to examine my breasts; 5) If I knew the benefit of breast self-examination, I would have done it by now; 6) Women prefer female doctor for breast examination; 7) If there is no problem in the breasts, periodic breast examinations by a physician are not required; 8) Early detection methods have no effect on treatment; 9) Personal hygiene decreases breast cancer risk; 10) By early diagnosis of breast cancer, the person will have prolonged life. Participants were asked to choose one of the following options for each of the statements above: ‘strongly agree’, ‘agree’, ‘neither agree nor disagree’, ‘disagree’, or ‘strongly disagree’. For presenting results, ‘strongly agree’ and ‘agree’ were combined; similarly, ‘disagree’ and ‘strongly disagree’ were combined.
Participants’ practices were assessed through the last section asking specific questions about BSE, CBE, and mammography. Participants were asked whether they had heard of BSE, CBE, and mammography and whether they believed these tests were useful for early detection of breast cancer. Other questions under BSE enquired whether they had been taught BSE, whether they practice BSE, what age should BSE be done, how frequently should BSE be done, what is the best time to do BSE, what action must be taken when any abnormality is found in BSE, and what, according to them, are the benefits of BSE. Similarly, questions under CBE sought information on whether they had undergone CBE, how CBE is done (by whom, using what), and how often should CBE be done. Questions on mammography tested the participants’ knowledge on what age mammography should be started, how often should it be done, and whether they had undergone mammography.
Ethical considerations
An informed consent was obtained from each participant before enrolment and no compensation or incentive was paid to the participants for this study. The study was approved by the ethics committee at KFMC.
Sample size estimate
The study population was stratified according to their professions into three groups: physicians, nurses, and allied healthcare workers. To ensure appropriate representation from each group of healthcare professionals, the proportionate population sampling method in the form of 4:1:1 for nurses, physicians, and allied healthcare workers, respectively, was adopted. Hence, 260 nurses (out of 2400), 65 physicians (out of 600) and 65 allied health care workers (out of 700) were approached on a random basis from each department and the total sample size was determined to be 390.
Statistical analysis
The statistical package for social science (IBM SPSS statistics 22. Ink) was used for data analysis. Descriptive statistics (i.e., frequencies, percentages, mean [standard deviation, SD] /median [interquartile range, IQR]) were used to describe the demographic characteristics, knowledge, attitude, and practice of breast cancer screening.
Socio-demographic characteristics
A total of 420 questionnaires were distributed to the KFMC female employees of which 395 (94%) were returned and included in the analysis. The mean age (SD) of the participants was 34.7 (8.3) years. Majority of the participants were married ( n = 239, 60.5%). Respondents included 261 (66.1%) nurses, 63 (16.0%) physicians, and 71 (18.0%) other healthcare workers including pharmacists, dieticians, technicians, health educators, physiotherapists, and therapists. Majority of the participants were bachelor’s degree holders ( n = 272, 68.9%) and 52 (13.2%) had a postgraduate qualification. Average work experience was 10 [ 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 ] years. Nine (2.3%) participants reported having history of breast cancer and 40 (10.1%) participants reported having a first-degree relative with history of breast cancer (Table 1 ).
Participants’ knowledge about breast Cancer
The knowledge score achieved in this study is very low; the median score of (range) = 1(0–5). When ranked in order, the 75th percentile is =5 (it means knowledge of only 5 items on the scale). Therefore, in this study, we considered a score of [ 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 ] as fair and a score of (> = 15) as good. The total score was therefore categorized as poor knowledge (score of 0–4), fair knowledge (score of 5–14), and good knowledge (score of 15–30). About 14 to 26% of the participants responded ‘Yes’ to the following potential risk factors for breast cancer: high-fat diet, working-class women, alcohol consumption, first child at a late age, early onset of menarche, late menopause, obesity, and larger breast (Table 2 ).
For other risk factors listed in the questionnaire, less than 14% responded ‘Yes’. Under the section of signs and symptoms of breast cancer, 49 (12.4%) participants agreed that scaling/dry skin in the nipple region could be a sign of breast cancer and 40 (10.1%) participants knew that weight loss could also be a sign of breast cancer. Less than 10% of the participants responded ‘Yes’ to rest of the signs/symptoms. Similarly, a lower rate (< 10%) was observed for the methods of diagnosis of breast cancer. The median (IQR) total score of knowledge about breast cancer was 1 (0–5), and only 5 (1.3%) participants appeared to have good level of knowledge (score: 15–30), while 104 (26.3%) scored fair level knowledge (score:5–14).
Slightly higher proportion of the fair (score: 5–14) knowledge was achieved by other allied health workers 27 (38.6%) comparing to 13 (21.7%) by the physician, and 64 (24.6%) by the nurses, however, this was not significant statistically ( p = 0.113). When compared, there was no statistically significant difference of proportions of the fair (score: 5–14) knowledge among female physicians working under different specialties at KFMC ( p = 0.183).
Participants’ attitudes toward breast Cancer screening and self-examination
Table 3 shows the responses on the statements for attitudes toward breast cancer screening and self-examination. Only 20 (5.1%) participants believed that any woman is at risk of breast cancer and 37 (9.4%) believed that breast cancer can be prevented (Table 3 ). Also, 53.4% of the participants believed that they could not detect abnormalities in breast by self-examination.
Knowledge and practice of breast self-examination
Results for the knowledge and practice of BSE are presented in Table 4 . Overall, 370 (93.7%) participants were aware of BSE, and 358 (90.6%) agreed that it is a useful tool for early detection of breast cancer. A total of 336 (85.1%) participants had been taught about BSE and 295 (74.7%) participants reported to be practicing it. Overall, 170 (43.0%) participants chose that BSE should be started from puberty, and 91 (23.0%) chose the age of 20 years to start doing BSE. A total of 317 (80.3%) participants agreed that the best time for BSE is a week after period and 293 (74.2%) participants agreed that BSE should be done monthly. Overall, 362 (91.7%) participants agreed that BSE is a good practice.
Knowledge and practice of clinical breast examination
Results for the knowledge and practice of CBE are presented in Table 5 . A total of 345 (87.3%) participants believed that CBE is a useful tool for detection of breast cancer, but only 95 (24.1%) had undergone CBE. Also, 273 (60.0%) respondents chose that a physician should do CBE, 131 (33.2%) believed that mammography should be used in CBE, and 190 (48.1%) agreed that the examination should be conducted at an interval of 1 year.
Knowledge and use of mammography
Overall, 368 (93.2%) participants had heard about mammography. A total of 287 (72.7%) participants agreed that mammography should be started at 40 years of age and 183 (46.3%) participants believed that mammography should be done every year. Seventy-four (18.7%) participants had undergone mammography (Table 6 ). Out of these 18.7% women, 59.5% were aged above 41 years while 40.5% were either less than or equal to 41 years of age.
Under reasons for not undergoing mammography, 104 (33.2%) participants responded that they were not old enough and 75 (24.0%) didn’t believe there was any reason to undergo mammography.
Knowledge and awareness play a vital role in early detection and optimal treatment of breast cancer. The knowledge level of healthcare professionals and their attitudes towards screening methods for breast cancer are important determinants of the practice of these methods by their patients. This study was, therefore, conducted to evaluate the knowledge, attitudes, and practices of breast cancer screening in the female healthcare workers at KFMC. Our cohort demonstrated especially poor knowledge on risk factors, signs and symptoms, and methods of diagnosis. The knowledge related to breast cancer in our cohort appears to be lesser than that found in some other studies [ 20 , 21 , 22 ]. Our results for attitudes of participants towards breast cancer screening were also discouraging, which could be due to lack of knowledge in this study population.
With regard to BSE, the results appeared positive with most participants being aware of the importance of BSE. Their knowledge related to BSE was also satisfactory. Also, almost 75% of the participants reported practicing BSE. This is much higher than the rate for BSE seen in some other studies [ 19 , 20 , 23 ]. This is very encouraging indeed and also a little surprising considering the low level of knowledge and attitude in this cohort. The usefulness of breast self-examination as an appropriate method for early breast cancer detection has been debated in the recent past. Whereas, WHO states that there is no evidence of the effect of screening through BSE, although BSE can empower women and it can be used to create awareness some organizations/countries recommend against BSE altogether (e.g. Dutch guidelines), while others still promote it (ACS, Medscape). In KSA, breast self-examination role is important in regions where mammography may not be offered due to socio-cultural reasons. Besides, statistics indicate that 90% of breast lumps are discovered by women themselves. One of the aspects of screening is that women in developing country settings are more aware of the BSE as the information regarding BSE is transmitted more frequently and is readily acceptable than mammography given the specific cultural norms in KSA. Women would prefer to undergo BSE in the privacy of their homes than to reach out to health care services for mammography, which is also embarrassing and uncomfortable procedure. Also, most of the participants in our study had heard of CBE and believed that it is a useful tool. However, only quarter of the participants had undergone CBE. The results were similar for mammography as well with most being aware of mammography as a screening tool but only a few opting for it. Another important reason for the lower number of participants undergoing screening, especially mammography, could be that it is usually recommended after the age of 40 years and the average age of this cohort was younger [ 24 ]. However, the low knowledge of breast cancer is of concern and needs to be addressed. Poorly informed healthcare staff could be a concerning barrier in increasing the awareness of general population. Several other studies have been conducted in the KSA to assess the knowledge and practices of breast cancer screening [ 19 , 20 , 21 , 22 , 25 , 26 , 27 ]. The results of these studies were similar with knowledge and attitudes of women towards breast cancer screening below expectation, thus, emphasizing the need for appropriate steps to spread awareness.
As regards to the practice of screening methods, results from studies conducted in other developing countries have not been very encouraging either. An important barrier in other countries is financial constraints [ 16 , 18 , 28 ]. However, this is not a concern in the KSA where healthcare facilities are provided free of cost. Optimal utilization of these services is what needs to be targeted. Thus, proper education of the healthcare staff as well as general population appears to be the single most crucial step required.
Also, reservations that women may have about screening also need to be addressed. Recently, a study ( N = 816) was conducted by Abdel-Aziz et al. in the Al Hassa region of KSA to evaluate the perceived barriers for breast cancer screening. They found personal fears such as fear of physicians, fear of results, and fear of hospitals as the main barriers for not practicing screening for breast cancer [ 29 ]. Being healthcare professionals themselves, such fears were understandably less commonly seen in our cohort. Knowledgeable healthcare professionals with good communication skills and well-planned educational campaigns could make a difference in helping women overcome their fears and hesitations.
In a study ( N = 500) conducted in five primary healthcare centers in Najran, Saudi Arabia, 57% of the study participants were unaware of mammogram and BSE and 44% were unaware of CBE [ 20 ]. Thus, lack of awareness of the methods of screening was an important barrier in the general population in this region of KSA. A quarter of the patients reported not receiving CBE due to unavailability of female doctors. Although this is another important aspect that needs to be addressed however clinical breast examination as method for breast cancer screening should be used only when mammography is unavailable as per the latest recommendations of the Ministry of health in Saudi Arabia [ 20 ]. Interestingly, very few participants in our study believed that women prefer female doctors for breast examination. This could be because women may not be able to openly express this reservation to the healthcare staff and therefore healthcare workers are not aware of this fact. Also, it has been found in studies that women who have frequent contact with their physician are more likely to undergo screening further emphasizing the crucial role of healthcare staff. All this implies that some factors affect the rate of breast cancer screening in any community. These include the knowledge and attitude of the healthcare workers themselves, the educational programs for the healthcare workers and for general public, the faith of the women on their clinicians, and the extent of barriers and steps taken to overcome them [ 29 ].
One limitation of our study is that it was conducted at one center. Nevertheless, this study provides important insights on the current knowledge and practices of screening methods in female healthcare workers and emphasizes the need for educational programs for the healthcare staff at KFMC. The results also urge other hospitals in the KSA to conduct similar studies to evaluate gaps in the knowledge, attitudes, and practices in their staff. Moreover, we recommend further studies to validate the questionnaire through analysis as the questionnaire was compiled after in-depth review of several articles in the field of study and more extensive studies to be conducted to draw comparison between the differences in health care and non health care workers knowledge and attitude towards screening and practices.
Overall, the knowledge, attitudes, and practices of the staff related to breast cancer at KFMC were found to be lower than expected. However, the study population had fairly good awareness of the availability and the usefulness of the screening methods. The results from this study, conducted on women healthcare professionals at KFMC, highlight the need for well-planned and comprehensive educational programs for the hospital staff.
Although, the screening tools and resources are available and free of charge in KSA however there is lack of active educational programs and campaigns directed at healthcare workers. Hence, inadequate knowledge about methods of breast cancer screening and their benefits among them could be the reason for lower than expected results of the study. In addition, a nationalized education and screening program in the region, combined with considerations for social and cultural factors needs to be functional.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the KFMC research center through the corresponding author on reasonable request.
Abbreviations
American Cancer Society
- Breast self-examination
- Clinical breast examination
Fine Needle Aspiration Cytology
Interquartile range
King Fahad Medical City
Kingdom of Saudi Arabia,
Standard deviation
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Google Scholar
Saggu S, Rehman H, Abbas ZK, Ansari AA. Recent incidence and descriptive epidemiological survey of breast cancer in Saudi Arabia. Saudi Med J. 2015;36(10):1176–80.
Article Google Scholar
Kingdom of Saudi Arabia Saudi Health Council Saudi Cancer Registry. Cancer Incidence Report Saudi Arabia 2014. Available at: https://nhic.gov.sa/eServices/Documents/2014.pdf . Accessed 19 Nov 2018.
Alotaibi RM, Rezk HR, Juliana CI, Guure C. Breast cancer mortality in Saudi Arabia: Modelling observed and unobserved factors. PLoS One. 2018;13(10):e0206148.
Davidson A, Chia S, Olson R, Nichol A, Speers C, Coldman AJ, et al. Stage, treatment and outcomes for patients with breast cancer in British Columbia in 2002: a population-based cohort study. CMAJ Open. 2013;1(4):E134–41.
World Health Organisation. Breast cancer - Early diagnosis and screening. Available at: http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ . Updated in 2018. Accessed 19 Nov 2018.
World Health Organisation. Cancer. Screening and early detection. Available at: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/cancer/policy/screening-and-early-detection . Accessed 19 Nov 2018.
Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, et al. Cancer screening in the United States, 2017: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2017;67(2):100–21.
Chouchane L, Boussen H, Sastry KS. Breast cancer in Arab populations: molecular characteristics and disease management implications. Lancet Oncol. 2013;14(10):e417–24.
Article CAS Google Scholar
Use of Screening Strategies for Detection of Breast Cancer. Kingdom of Saudi Arabia: MoH, The Saudi Center for EBHC Clinical Practice Guideline; April 2014. Available from: https://www.moh.gov.sa/depten/TCP/Documents/88.%20Breast%20Cancer%20-%20Use%20of%20Screening%20Strategies%20for%20the%20Detection%20of%20Breast%20Cancer.pdf . Accessed 19 Dec 2018.
El Bcheraoui C, Basulaiman M, Wilson S, Daoud F, Tuffaha M, AlMazroa MA, et al. Breast cancer screening in Saudi Arabia: free but almost no takers. PLoS One. 2015;10(3):e0119051.
Coleman EA, Lord J, Heard J, Coon S, Cantrell M, Mohrmann C, et al. The Delta project: increasing breast cancer screening among rural minority and older women by targeting rural healthcare providers. Oncol Nurs Forum. 2003;30(4):669–77.
Mandal R, Basu P. Cancer screening and early diagnosis in low and middle income countries. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz. 2018;61(12):1505–12.
Akpinar YY, Baykan Z, Nacar M, Gun I, Cetinkaya F. Knowledge, attitude about breast cancer and practice of breast cancer screening among female health care professionals: a study from Turkey. Asian Pac J Cancer Prev. 2011;12(11):3063–8.
PubMed Google Scholar
Othman A, Ahram M, Al-Tarawneh MR, Shahrouri M. Knowledge, attitudes and practices of breast cancer screening among women in Jordan. Health Care Women Int. 2015;36(5):578–92.
Naqvi AA, Zehra F, Ahmad R, Ahmad R, Ahmad N, Yazdani N, et al. Awareness, knowledge and attitude towards breast cancer, breast screening and early detection techniques among women in Pakistan. J Pak Med Assoc. 2018;68(4):576–86.
Ibrahim NA, Odusanya OO. Knowledge of risk factors, beliefs and practices of female healthcare professionals towards breast cancer in a tertiary institution in Lagos, Nigeria. BMC Cancer. 2009;9:76.
Ramakant P, Singh KR, Jaiswal S, Singh S, Ranjan P, Rana C, et al. A survey on breast Cancer awareness among medical, paramedical, and general population in North India using self-designed questionnaire: a prospective study. Indian J Surg Oncol. 2018;9(3):323–7.
Mahfouz AA, Hassanein MH, Nahar S, Farheen A, Gaballah II, Mohamed A, et al. Breast cancer knowledge and related behaviors among women in Abha City, southwestern Saudi Arabia. J Cancer Educ. 2013;28(3):516–20.
Alshahrani M, Alhammam SY, Al Munyif HA, Alwadei AM, Alwadei AM, Alzamanan SS, Aljohani NS. Knowledge, Attitudes, and Practices of Breast Cancer Screening Methods Among Female Patients in Primary Healthcare Centers in Najran, Saudi Arabia. J Cancer Educ. 2018:1–6.
Dandash KF, Al-Mohaimeed A. Knowledge, attitudes, and practices surrounding breast cancer and screening in female teachers of Buraidah, Saudi Arabia. Int J Health Sci (Qassim). 2007;1(1):61–71.
Jahan S, Al-Saigul AM, Abdelgadir MH. Breast cancer. Knowledge, attitudes and practices of breast self examination among women in Qassim region of Saudi Arabia. Saudi Med J. 2006;27(11):1737–41.
Ravichandran K, Al-Hamdan NA, Mohamed G. Knowledge, attitude, and behavior among Saudis toward cancer preventive practice. J Family Community Med. 2011;18(3):135–42.
Bevers TB, Helvie M, Bonaccio E, Calhoun KE, Daly MB, Farrar WB, et al. Breast Cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2018;16(11):1362–89.
Milaat WA. Knowledge of secondary-school female students on breast cancer and breast self-examination in Jeddah, Saudi Arabia. East Mediterr Health J. 2000;6(2–3):338–44.
CAS PubMed Google Scholar
Hussein DM, Alorf SH, Al-Sogaih YS, Alorf SH, Alaskar RS, Al-Mahana AM, et al. Breast cancer awareness and breast self-examination in northern Saudi Arabia. A preliminary survey. Saudi Med J. 2013;34(7):681–8.
Al-Wassia RK, Farsi NJ, Merdad LA, Hagi SK. Patterns, knowledge, and barriers of mammography use among women in Saudi Arabia. Saudi Med J. 2017;38(9):913–21.
Mamdouh HM, El-Mansy H, Kharboush IF, Ismail HM, Tawfik MM, El-Baky MA, et al. Barriers to breast cancer screening among a sample of Egyptian females. J Family Community Med. 2014;21(2):119–24.
Abdel-Aziz SB, Amin TT, Al-Gadeeb MB, Alhassar AI, Al-Ramadan A, Al-Helal M, et al. Perceived barriers to breast cancer screening among Saudi women at primary care setting. J Prev Med Hyg. 2018;59(1):E20–E9.
CAS PubMed PubMed Central Google Scholar
Download references
Acknowledgements
Not applicable.
This work was supported by Intramural Research Fund (IRF No: 050–017) by Research Center at KFMC. The funding body was not involved in the design of the study; collection, analysis, and interpretation of data; and in writing the manuscript.
Author information
Authors and affiliations.
Research Center, King Fahad Medical City, Riyadh, Saudi Arabia
Humariya Heena, Isamme AlFayyad & Amani Abu-Shaheen
Comprehensive Cancer Center, King Fahad Medical City, Riyadh, Saudi Arabia
Sajid Durrani
Department of statistics, University of Malakand, Chakdara, Pakistan
Muhammad Riaz
Women’s Specialized Hospital, King Fahad Medical City, Riyadh, Saudi Arabia
Rabeena Tabasim
Department of Anaesthesia, Sheikh Khalifa Medical City, Ajman, United Arab Emirates
Gazi Parvez
You can also search for this author in PubMed Google Scholar
Contributions
HH conceived, designed the study and drafted the manuscript, MR, IA, RT, GP helped in data collection, questionnaire development, data interpretation, data analysis and drafting the manuscript. SD, AA revised the manuscript, providing valuable intellectual content. All authors commented on and approved the final manuscript.
Corresponding author
Correspondence to Humariya Heena .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Institutional Review Board (IRB) at KFMC with IRB Number: 17–159. All participants provided informed consent for the study after ensuring that the participants understood the consent form.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Heena, H., Durrani, S., Riaz, M. et al. Knowledge, attitudes, and practices related to breast cancer screening among female health care professionals: a cross sectional study. BMC Women's Health 19 , 122 (2019). https://doi.org/10.1186/s12905-019-0819-x
Download citation
Received : 24 December 2018
Accepted : 23 September 2019
Published : 22 October 2019
DOI : https://doi.org/10.1186/s12905-019-0819-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast Cancer
- Mammography
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]
What is the best age to start getting mammograms? The recommended age has lowered

Adam’s Journal
Like many employers, the Oklahoma Medical Research Foundation teams with a mobile mammography service to offer onsite breast cancer screening services to our employees.
An employee in her 20s has inquired about using the service. Of course, she could. But should she?
Dr. James Prescribes
Recently, recommendations for when women should begin regular screening for breast cancer have changed. Where guidelines had suggested waiting until age 50, the U.S. Preventive Services Task Force now counsels that women should get a mammogram every year beginning at age 40.
Not coincidentally, it is typically starting at this age — 40 — that insurance companies will pay for the procedure as a routine preventive test.
Health experts do not recommend that women under 40 who are at average risk for breast cancer undergo mammograms. That generally means that women in their 20s and 30s are too young to begin screening unless they have: (1) a known genetic mutation (like BRCA1 or BRCA2) that puts them at high risk for breast cancer; (2) an extremely strong family history of breast cancer; or (3) physical symptoms of the disease, such as a mass or other breast changes.
Young women have a low risk for breast cancer. Mammography involves radiation exposure, so the risk-benefit calculus — especially when one considers lifetime radiation exposure, a risk factor for many cancers — does not make sense for normal-risk patients under the age of 40. However, that risk-benefit calculus changes once women reach the age of 40, as about 1 in 6 breast cancers are diagnosed in this age group.
Mammograms for young women also carry a higher level of false positives. This can mean unnecessary anxiety, additional testing, and costs.
It is important that women of any age understand if they are at higher risk for breast cancer. This involves learning your family history, which could in some circumstances lead to testing for certain genetic variants like BRCA1and BRCA2.
For young women, instead of mammography, doctors suggest performing self-breast exams regularly beginning at the age of 20. These self-exams can help women know how their breasts should feel, and they will help them notice any changes.
If they do detect a lump or any abnormality, that’s the time to talk to a healthcare provider and seek a breast examination.
James is executive vice president and chief medical officer of the Oklahoma Medical Research Foundation. Cohen, a marathoner, is OMRF’s senior vice president and general counsel. Send your health questions to [email protected].
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-.

PDQ Cancer Information Summaries [Internet].
Breast cancer screening (pdq®).
PDQ Screening and Prevention Editorial Board .
Published online: March 28, 2024.
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about breast cancer screening. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
This summary is reviewed regularly and updated as necessary by the PDQ Screening and Prevention Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Note: The Overview section summarizes the published evidence on this topic. The rest of the summary describes the evidence in more detail.
Other PDQ summaries with information related to breast cancer screening include the following:
- Breast Cancer Prevention
- Breast Cancer Treatment
- Male Breast Cancer Treatment
- Breast Cancer Treatment During Pregnancy
Mammography is the most widely used screening modality for the detection of breast cancer. There is evidence that it decreases breast cancer mortality in women aged 50 to 69 years and that it is associated with harms, including the detection of clinically insignificant cancers that pose no threat to life (overdiagnosis). The benefit of mammography for women aged 40 to 49 years is uncertain.[ 1 , 2 ] Randomized trials in India, Iran, and Egypt have studied the use of clinical breast examination (CBE) as a screening test. Some of these studies suggested a shift in late-stage disease; however, there is still insufficient evidence to conclude a mortality benefit.[ 3 - 8 ] Breast self-examination has been shown to have no mortality benefit.
Technologies such as ultrasound, magnetic resonance imaging, and molecular breast imaging are being evaluated, usually as adjuncts to mammography. They are not primary screening tools in the average population.
Informed medical decision making is increasingly recommended for individuals who are considering cancer screening. Many different types and formats of decision aids have been studied. For more information, see Cancer Screening Overview .
Screening With Mammography
Randomized controlled trials (RCTs) initiated 50 years ago provide evidence that screening mammography reduces breast cancer–specific mortality for women aged 60 to 69 years (solid evidence) and women aged 50 to 59 years (fair evidence). Population-based studies done more recently raise questions about the benefits for populations who participate in screening for longer time periods.
Magnitude of Effect: Based on a meta-analysis of RCTs, the number of women needed to invite for screening to prevent one breast cancer death depends on the woman’s age: for women aged 39 to 49 years, 1,904 women needed (95% confidence interval [CI], 929–6,378); for women aged 50 to 59 years, 1,339 women needed (95% CI, 322–7,455); and for women aged 60 to 69 years, 377 women needed (95% CI, 230–1,050).[ 9 ]
- Study Design : RCTs, population-based evidence.
- Internal Validity : Variable, but meta-analysis of RCTs is good.
- Consistency : Poor.
- External Validity : Uncertain.
The validity of meta-analyses of RCT demonstrating a mortality benefit is limited by improvements in medical imaging and treatment in the decades since their completion. The 25-year follow-up from the Canadian National Breast Screening Study (CNBSS),[ 10 ] completed in 2014, showed no mortality benefit associated with screening mammograms.
Based on solid evidence, screening mammography may lead to the following harms:
- Long-term follow-up of RCTs of screening.
- The calculation of excess incidence in large screening programs.[ 11 , 12 ]
- Study Design : RCTs, descriptive, population-based comparisons, autopsy series, and series of mammary reduction specimens.
- Magnitude of Effect : In the United States, approximately 10% of women are recalled for further testing after a screening examination. However, only 0.5% of tested women have cancer. Thus, approximately 9.5% of tested women have a false-positive exam.[ 14 , 15 ] Approximately 50% of women screened annually for 10 years in the United States experience a false-positive exam; of these, 7% to 17% will undergo biopsies.[ 16 , 17 ] Additional testing is less likely when prior mammograms are available for comparison.
- Study Design : Descriptive, population-based.
- Magnitude of Effect : Invasive breast cancer is present but undetected by mammography (false-negative) in 6% to 46% of exams. False-negative exams are more likely for mucinous and lobular types of cancer and for rapidly growing interval tumors, which become detectable between regular mammograms and in dense breasts, which are common in younger women.[ 18 - 20 ]
- Magnitude of Effect : Theoretically, annual mammograms in women aged 40 to 80 years may cause up to one breast cancer per 1,000 women.[ 21 , 22 ]
For all of these conclusions regarding potential harms from screening mammography, internal validity, consistency, and external validity are good.
Clinical Breast Examination (CBE)
The CNBSS trial did not study the efficacy of CBE versus no screening. Ongoing randomized trials, two in India and one in Egypt, are designed to assess the efficacy of screening CBE but have not reported mortality data.[ 3 - 8 ] Thus, the efficacy of screening CBE cannot be assessed yet.
- Magnitude of Effect : The current evidence is insufficient to assess the additional benefits and harms of CBE. The single RCT comparing high-quality CBE with screening mammography showed equivalent benefit. CBE accuracy in the community setting might be lower than in the RCT.[ 3 - 6 ]
- Study Design : Single RCT, population cohort studies.
- Internal Validity : Good.
- Consistency and External Validity : Poor.
Screening by CBE may lead to the following harms:
- Magnitude of Effect : Specificity in women aged 50 to 59 years was 88% to 99%, yielding a false-positive rate of 1% to 12% for all women screened.[ 23 ]
- Study Design : Descriptive, population based.
- Internal Validity, Consistency, and External Validity : Good.
- Magnitude of Effect : Of women with cancer, 17% to 43% have a negative CBE. Sensitivity is higher with longer duration and higher quality of the examination by trained personnel.
- Internal and External Validity : Good.
- Consistency : Fair.
Breast Self-Examination (BSE)
BSE has been compared with no screening and has been shown to have no benefit in reducing breast cancer mortality.
- Magnitude of Effect : No effect.[ 24 , 25 ]
- Study Design : Two RCTs.
- Internal Validity and Consistency : Fair.
- External Validity : Poor.
There is solid evidence that formal instruction and encouragement to perform BSE leads to more breast biopsies and more diagnoses of benign breast lesions.
- Magnitude of Effects on Health Outcomes : Biopsy rate was 1.8% among the study population, compared with 1.0% among the control group.[ 24 ]
- Study Design : Two RCTs, cohort studies.
- Moss SM, Cuckle H, Evans A, et al.: Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years' follow-up: a randomised controlled trial. Lancet 368 (9552): 2053-60, 2006. [ PubMed : 17161727 ]
- Moss SM, Wale C, Smith R, et al.: Effect of mammographic screening from age 40 years on breast cancer mortality in the UK Age trial at 17 years' follow-up: a randomised controlled trial. Lancet Oncol 16 (9): 1123-32, 2015. [ PubMed : 26206144 ]
- Hassan LM, Mahmoud N, Miller AB, et al.: Evaluation of effect of self-examination and physical examination on breast cancer. Breast 24 (4): 487-90, 2015. [ PubMed : 25977176 ]
- Anderson BO, Bevers TB, Carlson RW: Clinical Breast Examination and Breast Cancer Screening Guideline. JAMA 315 (13): 1403-4, 2016. [ PubMed : 27046372 ]
- Yen AM, Tsau HS, Fann JC, et al.: Population-Based Breast Cancer Screening With Risk-Based and Universal Mammography Screening Compared With Clinical Breast Examination: A Propensity Score Analysis of 1 429 890 Taiwanese Women. JAMA Oncol 2 (7): 915-21, 2016. [ PubMed : 27030951 ]
- Myers ER, Moorman P, Gierisch JM, et al.: Benefits and Harms of Breast Cancer Screening: A Systematic Review. JAMA 314 (15): 1615-34, 2015. [ PubMed : 26501537 ]
- Mittra I, Mishra GA, Singh S, et al.: A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: methodology and interim results after three rounds of screening. Int J Cancer 126 (4): 976-84, 2010. [ PubMed : 19697326 ]
- Sankaranarayanan R, Ramadas K, Thara S, et al.: Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst 103 (19): 1476-80, 2011. [ PubMed : 21862730 ]
- Nelson HD, Tyne K, Naik A, et al.: Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 151 (10): 727-37, W237-42, 2009. [ PMC free article : PMC2972726 ] [ PubMed : 19920273 ]
- Miller AB, Wall C, Baines CJ, et al.: Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ 348: g366, 2014. [ PMC free article : PMC3921437 ] [ PubMed : 24519768 ]
- Welch HG, Black WC: Overdiagnosis in cancer. J Natl Cancer Inst 102 (9): 605-13, 2010. [ PubMed : 20413742 ]
- Bleyer A, Welch HG: Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 367 (21): 1998-2005, 2012. [ PubMed : 23171096 ]
- Yen MF, Tabár L, Vitak B, et al.: Quantifying the potential problem of overdiagnosis of ductal carcinoma in situ in breast cancer screening. Eur J Cancer 39 (12): 1746-54, 2003. [ PubMed : 12888370 ]
- Jørgensen KJ, Gøtzsche PC: Overdiagnosis in publicly organised mammography screening programmes: systematic review of incidence trends. BMJ 339: b2587, 2009. [ PMC free article : PMC2714679 ] [ PubMed : 19589821 ]
- Rosenberg RD, Yankaskas BC, Abraham LA, et al.: Performance benchmarks for screening mammography. Radiology 241 (1): 55-66, 2006. [ PubMed : 16990671 ]
- Elmore JG, Barton MB, Moceri VM, et al.: Ten-year risk of false positive screening mammograms and clinical breast examinations. N Engl J Med 338 (16): 1089-96, 1998. [ PubMed : 9545356 ]
- Hubbard RA, Kerlikowske K, Flowers CI, et al.: Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med 155 (8): 481-92, 2011. [ PMC free article : PMC3209800 ] [ PubMed : 22007042 ]
- Rosenberg RD, Hunt WC, Williamson MR, et al.: Effects of age, breast density, ethnicity, and estrogen replacement therapy on screening mammographic sensitivity and cancer stage at diagnosis: review of 183,134 screening mammograms in Albuquerque, New Mexico. Radiology 209 (2): 511-8, 1998. [ PubMed : 9807581 ]
- Kerlikowske K, Grady D, Barclay J, et al.: Likelihood ratios for modern screening mammography. Risk of breast cancer based on age and mammographic interpretation. JAMA 276 (1): 39-43, 1996. [ PubMed : 8667537 ]
- Porter PL, El-Bastawissi AY, Mandelson MT, et al.: Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 91 (23): 2020-8, 1999. [ PubMed : 10580027 ]
- Ronckers CM, Erdmann CA, Land CE: Radiation and breast cancer: a review of current evidence. Breast Cancer Res 7 (1): 21-32, 2005. [ PMC free article : PMC1064116 ] [ PubMed : 15642178 ]
- Goss PE, Sierra S: Current perspectives on radiation-induced breast cancer. J Clin Oncol 16 (1): 338-47, 1998. [ PubMed : 9440762 ]
- Fenton JJ, Rolnick SJ, Harris EL, et al.: Specificity of clinical breast examination in community practice. J Gen Intern Med 22 (3): 332-7, 2007. [ PMC free article : PMC1824753 ] [ PubMed : 17356964 ]
- Thomas DB, Gao DL, Ray RM, et al.: Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 94 (19): 1445-57, 2002. [ PubMed : 12359854 ]
- Semiglazov VF, Manikhas AG, Moiseenko VM, et al.: [Results of a prospective randomized investigation [Russia (St.Petersburg)/WHO] to evaluate the significance of self-examination for the early detection of breast cancer]. Vopr Onkol 49 (4): 434-41, 2003. [ PubMed : 14569932 ]
- Description of the Evidence
Breast Cancer Incidence and Mortality
Breast cancer is the most common noncutaneous cancer in U.S. women, with an estimated 310,720 cases of invasive disease, 56,500 cases of in situ disease, and 42,250 deaths expected in 2024.[ 1 ] Women with inherited risk, including BRCA1 and BRCA2 gene carriers, make up approximately 5% to 10% of breast cancer cases.[ 2 ] Men account for about 1% of breast cancer cases and breast cancer deaths.[ 1 ]
The biggest risk factor for breast cancer is being female followed by advancing age. Other risk factors include hormonal aspects (such as early menarche, late menopause, nulliparity, late first pregnancy, and postmenopausal hormone therapy use), alcohol consumption, and exposure to ionizing radiation.
Breast cancer incidence in White women is higher than in Black women, who also have a lower survival rate for every stage when diagnosed.[ 3 ] This may reflect differences in screening behavior and access to health care. Hispanic women, Asian or Pacific Islander women, and American Indian or Alaska Native women have lower incidence and mortality rates than White or Black women.[ 4 ]
Breast cancer incidence depends on reproductive issues (such as early vs. late pregnancy, multiparity, and breastfeeding), participation in screening, and postmenopausal hormone usage. The incidence of breast cancer (especially ductal carcinoma in situ [DCIS]) increased dramatically after mammography was widely adopted in the United States and the United Kingdom.[ 5 ] Widespread use of postmenopausal hormone therapy was associated with a dramatic increase in breast cancer incidence, a trend that reversed when its use decreased.[ 6 ]
In any population, the adoption of screening is not followed by a decline in the incidence of advanced-stage cancer.
Evaluation of Breast Symptoms
Women with breast symptoms undergo diagnostic mammography as opposed to screening mammography, which is done in asymptomatic women. In a 10-year study of breast symptoms prompting medical attention, a breast mass led to a cancer diagnosis in 10.7% of cases, whereas pain was associated with cancer in only 1.8% of cases.[ 7 ]
Pathological Evaluation of Breast Tissue
Invasive breast cancer.
Breast cancer can be diagnosed when breast tissue cells removed during a biopsy are studied microscopically. The breast tissue to be sampled can be identified by an abnormality on an imaging study or because it is palpable. Breast biopsies can be performed with a thin needle attached to a syringe (fine-needle aspirate), a larger needle (core biopsy), or by excision (excisional biopsy). Image guidance can improve accuracy. Needle biopsies sample an abnormal area large enough to make a diagnosis. Excisional biopsies aim to remove the entire region of abnormality.
Ductal carcinoma in situ (DCIS)
DCIS is a noninvasive condition that can be associated with, or evolve into, invasive cancer, with variable frequency and time course.[ 8 ] Some authors include DCIS with invasive breast cancer statistics, but others argue that it would be better if the term were replaced with ductal intraepithelial neoplasia, similar to the terminology used for cervical and prostate precursor lesions, and that excluding DCIS from breast cancer statistics should be considered.
DCIS is most often diagnosed by mammography. In the United States, only 4,900 women were diagnosed with DCIS in 1983 before the adoption of mammography screening, compared with approximately 56,500 women who are expected to be diagnosed in 2024.[ 1 , 8 , 9 ] The Canadian National Breast Screening Study-2, which evaluated women aged 50 to 59 years, found a fourfold increase in DCIS cases in women screened by clinical breast examination (CBE) plus mammography, compared with those screened by CBE alone, with no difference in breast cancer mortality.[ 10 ] For more information, see Breast Cancer Treatment .
The natural history of DCIS is poorly understood because nearly all DCIS cases are detected by screening and nearly all are treated. Development of breast cancer after treatment of DCIS depends on the pathological characteristics of the lesion and on the treatment. In a randomized trial, 13.4% of women whose DCIS was excised by lumpectomy developed ipsilateral invasive breast cancer within 90 months, compared with 3.9% of those treated by both lumpectomy and radiation.[ 11 ] Among women diagnosed and treated for DCIS, the percentage of women who died of breast cancer is lower than that for the age-matched population at large.[ 12 , 13 ] This favorable outcome may reflect the benign nature of the condition, the benefits of treatment, or the volunteer effect (i.e., women who undergo breast cancer screening are generally healthier than those who do not do so).
Atypia, which is a risk factor for breast cancer, is found in 4% to 10% of breast biopsies.[ 14 , 15 ] Atypia is a diagnostic classification with considerable variation among practicing pathologists.[ 16 ]
Variability of pathologists’ diagnoses on the interpretation of breast biopsy specimens
The range of pathologists' diagnoses of breast tissue includes benign without atypia, atypia, DCIS, and invasive breast cancer. The incidence of atypia and DCIS breast lesions has increased over the past three decades as a result of widespread mammography screening, although atypia is generally mammographically occult.[ 17 , 18 ] Misclassification of breast lesions may contribute to either overtreatment or undertreatment of lesions—with variability especially in the diagnoses of atypia and DCIS.[ 16 , 19 - 23 ]
The largest study on this topic, the B-Path study, involved 115 practicing U.S. pathologists who interpreted a single-breast biopsy slide per case, and it compared their interpretations with an expert consensus-derived reference diagnosis.[ 16 ] While the overall agreement between the individual pathologists’ interpretations and the expert reference diagnoses was highest for invasive carcinoma, there were markedly lower levels of agreement for DCIS and atypia.[ 16 ] As the B-Path study included higher proportions of cases of atypia and DCIS than typically seen in clinical practice, the authors expanded their work by applying Bayes’ theorem to estimate how diagnostic variability affects accuracy from the perspective of a U.S. woman aged 50 to 59 years having a breast biopsy.[ 19 ] At the U.S. population level, it is estimated that 92.3% (95% confidence interval [CI], 91.4%–93.1%) of breast biopsy diagnoses would be verified by an expert reference consensus diagnosis, with 4.6% (95% CI, 3.9%–5.3%) of initial breast biopsies estimated to be overinterpreted and 3.2% (95% CI, 2.7%–3.6%) under interpreted. Figure 1 shows the predicted outcomes per 100 breast biopsies, overall and by diagnostic category.

Figure 1. Predicted outcomes per 100 breast biopsies, overall and by diagnostic category. From Annals of Internal Medicine, Elmore JG, Nelson HD, Pepe MS, Longton GM, Tosteson AN, Geller B, Onega T, Carney PA, Jackson SL, Allison KH, Weaver DL, Variability in Pathologists' Interpretations of Individual Breast Biopsy Slides: A Population Perspective, Volume 164, Issue 10, Pages 649–55, Copyright © 2016 American College of Physicians. All Rights Reserved. Reprinted with the permission of American College of Physicians, Inc.
To address the high rates of discordance in breast tissue diagnosis, laboratory policies that require second opinions are becoming more common. A national survey of 252 breast pathologists participating in the B-Path study found that 65% of respondents reported having a laboratory policy that requires second opinions for all cases initially diagnosed as invasive disease. Additionally, 56% of respondents reported policies that require second opinions for initial diagnoses of DCIS, while 36% of respondents reported mandatory second opinion policies for cases initially diagnosed as atypical ductal hyperplasia.[ 24 ] In this same survey, pathologists overwhelmingly agreed that second opinions improved diagnostic accuracy (96%).
A simulation study that used B-Path study data evaluated 12 strategies for obtaining second opinions to improve interpretation of breast histopathology.[ 25 ] Accuracy improved significantly with all second-opinion strategies, except for the strategy limiting second opinions only to cases of invasive cancer. Accuracy improved regardless of the pathologists’ confidence in their diagnosis or their level of experience. While the second opinions improved accuracy, they did not completely eliminate diagnostic variability, especially in the challenging case of breast atypia.
Special Populations
Women at increased risk who may benefit more from screening, women with brca1 and brca2 genetic mutations.
Women with an increased risk of breast cancer caused by a BRCA1 or BRCA2 genetic mutation might benefit from increased screening. For more information, see BRCA1 and BRCA2: Cancer Risks and Management .
Recipients of thoracic radiation
Women with Hodgkin and non-Hodgkin lymphoma who were treated with mantle irradiation have an increased risk of breast cancer, starting 10 years after completing therapy and continuing life-long. Therefore, screening mammography has been advocated, even though it may begin at a relatively young age.[ 26 , 27 ]
Individuals who benefit less from screening
Women with limited life expectancy.
The potential benefits of screening mammography occur well after the examination, often many years later, whereas the harms occur immediately. Therefore, women with limited life expectancy and comorbidities who suffer harms may do so without benefit. Nonetheless, many of these women undergo screening mammography.[ 28 ] In one study, approximately 9% of women with advanced cancer underwent cancer screening tests.[ 29 ]
Older women
Screening mammography may yield cancer diagnoses in approximately 1% of women aged 66 to 79 years, but most of these cancers are low risk.[ 30 ] The question remains whether the diagnosis and treatment of localized breast cancer in older women is beneficial.
Young women
There is no evidence of benefit in performing screening mammography in average-risk women younger than 40 years.
Approximately 1% of all breast cancers occur in men.[ 31 ] Most cases are diagnosed during the evaluation of palpable lesions, which are generally easy to detect. Treatment consists of surgery, radiation, and systemic adjuvant hormone therapy or chemotherapy. For more information, see Male Breast Cancer Treatment . In this population, screening is unlikely to be beneficial.
- American Cancer Society: Cancer Facts and Figures 2024. American Cancer Society, 2024. Available online . Last accessed January 17, 2024.
- Kurian AW, Griffith KA, Hamilton AS, et al.: Genetic Testing and Counseling Among Patients With Newly Diagnosed Breast Cancer. JAMA 317 (5): 531-534, 2017. [ PMC free article : PMC5530866 ] [ PubMed : 28170472 ]
- Ellington TD, Henley SJ, Wilson RJ, et al.: Trends in breast cancer mortality by race/ethnicity, age, and US census region, United States─1999-2020. Cancer 129 (1): 32-38, 2023. [ PMC free article : PMC10128100 ] [ PubMed : 36309838 ]
- Surveillance Research Program, National Cancer Institute: SEER*Explorer: An interactive website for SEER cancer statistics. Bethesda, MD: National Cancer Institute. Available online . Last accessed March 6, 2024.
- Johnson A, Shekhdar J: Breast cancer incidence: what do the figures mean? J Eval Clin Pract 11 (1): 27-31, 2005. [ PubMed : 15660534 ]
- Haas JS, Kaplan CP, Gerstenberger EP, et al.: Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med 140 (3): 184-8, 2004. [ PubMed : 14757616 ]
- Barton MB, Elmore JG, Fletcher SW: Breast symptoms among women enrolled in a health maintenance organization: frequency, evaluation, and outcome. Ann Intern Med 130 (8): 651-7, 1999. [ PubMed : 10215561 ]
- Allegra CJ, Aberle DR, Ganschow P, et al.: National Institutes of Health State-of-the-Science Conference statement: Diagnosis and Management of Ductal Carcinoma In Situ September 22-24, 2009. J Natl Cancer Inst 102 (3): 161-9, 2010. [ PubMed : 20071686 ]
- Virnig BA, Tuttle TM, Shamliyan T, et al.: Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst 102 (3): 170-8, 2010. [ PubMed : 20071685 ]
- Miller AB, To T, Baines CJ, et al.: Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 92 (18): 1490-9, 2000. [ PubMed : 10995804 ]
- Fisher B, Dignam J, Wolmark N, et al.: Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol 16 (2): 441-52, 1998. [ PubMed : 9469327 ]
- Ernster VL, Barclay J, Kerlikowske K, et al.: Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med 160 (7): 953-8, 2000. [ PubMed : 10761960 ]
- Welch HG, Prorok PC, O'Malley AJ, et al.: Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N Engl J Med 375 (15): 1438-1447, 2016. [ PubMed : 27732805 ]
- Weaver DL, Rosenberg RD, Barlow WE, et al.: Pathologic findings from the Breast Cancer Surveillance Consortium: population-based outcomes in women undergoing biopsy after screening mammography. Cancer 106 (4): 732-42, 2006. [ PubMed : 16411214 ]
- Rubin E, Visscher DW, Alexander RW, et al.: Proliferative disease and atypia in biopsies performed for nonpalpable lesions detected mammographically. Cancer 61 (10): 2077-82, 1988. [ PubMed : 3359405 ]
- Elmore JG, Longton GM, Carney PA, et al.: Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA 313 (11): 1122-32, 2015. [ PMC free article : PMC4516388 ] [ PubMed : 25781441 ]
- Hall FM: Identification, biopsy, and treatment of poorly understood premalignant, in situ, and indolent low-grade cancers: are we becoming victims of our own success? Radiology 254 (3): 655-9, 2010. [ PubMed : 20177083 ]
- Elmore JG, Nelson HD, Pepe MS, et al.: Variability in Pathologists' Interpretations of Individual Breast Biopsy Slides: A Population Perspective. Ann Intern Med 164 (10): 649-55, 2016. [ PMC free article : PMC5064832 ] [ PubMed : 26999810 ]
- Rosai J: Borderline epithelial lesions of the breast. Am J Surg Pathol 15 (3): 209-21, 1991. [ PubMed : 1847606 ]
- Schnitt SJ, Connolly JL, Tavassoli FA, et al.: Interobserver reproducibility in the diagnosis of ductal proliferative breast lesions using standardized criteria. Am J Surg Pathol 16 (12): 1133-43, 1992. [ PubMed : 1463092 ]
- Wells WA, Carney PA, Eliassen MS, et al.: Statewide study of diagnostic agreement in breast pathology. J Natl Cancer Inst 90 (2): 142-5, 1998. [ PubMed : 9450574 ]
- Della Mea V, Puglisi F, Bonzanini M, et al.: Fine-needle aspiration cytology of the breast: a preliminary report on telepathology through Internet multimedia electronic mail. Mod Pathol 10 (6): 636-41, 1997. [ PubMed : 9195583 ]
- Geller BM, Nelson HD, Carney PA, et al.: Second opinion in breast pathology: policy, practice and perception. J Clin Pathol 67 (11): 955-60, 2014. [ PMC free article : PMC4521120 ] [ PubMed : 25053542 ]
- Elmore JG, Tosteson AN, Pepe MS, et al.: Evaluation of 12 strategies for obtaining second opinions to improve interpretation of breast histopathology: simulation study. BMJ 353: i3069, 2016. [ PMC free article : PMC4916777 ] [ PubMed : 27334105 ]
- Mariscotti G, Belli P, Bernardi D, et al.: Mammography and MRI for screening women who underwent chest radiation therapy (lymphoma survivors): recommendations for surveillance from the Italian College of Breast Radiologists by SIRM. Radiol Med 121 (11): 834-837, 2016. [ PubMed : 27406629 ]
- Allen SD, Wallis MG, Cooke R, et al.: Radiologic features of breast cancer after mantle radiation therapy for Hodgkin disease: a study of 230 cases. Radiology 272 (1): 73-8, 2014. [ PubMed : 24666474 ]
- Walter LC, Lindquist K, Covinsky KE: Relationship between health status and use of screening mammography and Papanicolaou smears among women older than 70 years of age. Ann Intern Med 140 (9): 681-8, 2004. [ PubMed : 15126251 ]
- Sima CS, Panageas KS, Schrag D: Cancer screening among patients with advanced cancer. JAMA 304 (14): 1584-91, 2010. [ PMC free article : PMC3728828 ] [ PubMed : 20940384 ]
- Smith-Bindman R, Kerlikowske K, Gebretsadik T, et al.: Is screening mammography effective in elderly women? Am J Med 108 (2): 112-9, 2000. [ PubMed : 11126304 ]
- Fentiman IS, Fourquet A, Hortobagyi GN: Male breast cancer. Lancet 367 (9510): 595-604, 2006. [ PubMed : 16488803 ]
- Mammography
Description and Background
Mammography uses ionizing radiation to image breast tissue. The examination is performed by compressing the breast firmly between two plates, which spreads out overlapping tissues and reduces the amount of radiation needed for the image. For routine screening in the United States, examinations are taken in both mediolateral oblique and craniocaudal projections.[ 1 ] Both views will include breast tissue from the nipple to the pectoral muscle. Radiation exposure is 4 to 24 mSv per standard two-view screening examination. Two-view examinations have a lower recall rate than single-view examinations because they reduce concern about abnormalities caused by superimposition of normal breast structures.[ 2 ] Two-view exams have lower interval cancer rates than single-view exams.[ 3 ]
Under the Mammography Quality Standards Act (MQSA) enacted by Congress in 1992, all U.S. facilities that perform mammography must be certified by the U.S. Food and Drug Administration (FDA) to ensure the use of standardized training for personnel and a standardized mammography technique utilizing a low radiation dose.[ 4 ] (See the FDA's web page on Mammography Facility Surveys, Mammography Equipment Evaluations, and Medical Physicist Qualification Requirement under MQSA .) The 1998 MQSA Reauthorization Act requires that patients receive a written lay-language summary of mammography results.
The following Breast Imaging Reporting and Data System (BI-RADS) categories are used for reporting mammographic results:[ 5 ]
- 0: Incomplete—needs additional image evaluation and/or prior mammograms for comparison.
- 1: Negative; the risk of cancer diagnosis within 1 year is 1%.
- 2: Benign; the risk of cancer diagnosis within 1 year is 1%.
- 3: Probably benign; the risk of cancer diagnosis within 1 year is 2%.
- 4a: 2%–10%.
- 4b: 10%–50%.
- 4c: 50%–95%.
- 5: Highly suggestive of malignancy; the risk of cancer diagnosis within 1 year is 95%.
- 6: Known biopsy—proven malignancy.
Most screening mammograms are interpreted as negative or benign (BI-RADS 1 or 2, respectively); about 10% of women in the United States are asked to return for additional evaluation.[ 6 ] The percentage of women asked to return for additional evaluation varies not only by the inherent characteristics of each woman but also by the mammography facility and radiologist.[ 7 ]
Tumor detection has not been validated as a proper surrogate outcome measure for breast cancer mortality, and novel screening methods that simply increase tumor detection rates may not necessarily reduce the risk of dying from breast cancer. Nonetheless, there are numerous studies demonstrating improvements in breast tumor detection rates with modern imaging technology, with the absence of mortality data. Between 1963 and 1990, screening mammography was assessed in nine randomized trials with breast cancer-specific mortality as the primary end point, and screening mammography recommendations were largely based on the results of these trials. However, in more recent years, novel breast screening technologies have often been assessed in clinical trials and observational studies with end points that have not been validated as proper surrogate outcome measures for breast cancer mortality.[ 8 ]
A systematic review of studies with a total of 488,099 patients compared digital breast tomosynthesis (DBT) alone, combined DBT and digital mammography (DM), and DM alone. DBT alone and combined DBT and DM were more sensitive than DM alone for breast cancer detection, but there appeared to be no significant difference in diagnostic accuracy between DBT alone and the combination of DBT and DM. A subsequent systematic review and meta-analysis by the same authors seemed to support the replacement of DM by synthetic 2-dimensional mammography (S2D) combined with DBT for breast cancer screening, as combining S2D and DBT improved tumor detection rates, and reduced recall rates, radiation dose, and overall costs.[ 8 - 10 ]
Digital Mammography and Computer-Aided Detection
DM is more expensive than screen-film mammography (SFM) but is more amenable to data storage and sharing. Performance of both SFM and DM for cancer detection rate, sensitivity, specificity, and positive predictive value (PPV) has been compared directly in several trials, with similar results in most patient groups.
The Digital Mammographic Imaging Screening Trial (DMIST) compared the findings of digital and film mammograms in 42,760 women at 33 U.S. centers. Although DM detected more cancers in women younger than 50 years (area under the curve [AUC] of 0.84 +/- 0.03 for digital; AUC of 0.69 +/- 0.05 for film; P = .002), there was no difference in breast cancer detection overall.[ 11 ] A second DMIST report found a trend toward higher AUC for film mammography than for DM in women aged 65 years and older.[ 12 ]
Another large U.S. cohort study [ 13 ] also found slightly better sensitivity for film mammography for women younger than 50 years with similar specificity.
A Dutch study compared the findings of 1.5 million digital versus 4.5 million screen-film screening mammograms performed between 2004 and 2010. A higher recall and cancer detection rate was observed for the digital screens.[ 14 ] A meta-analysis [ 15 ] of 10 studies, including the DMIST [ 11 , 12 ] and the U.S. cohort study,[ 13 ] compared DM and film mammography in 82,573 women who underwent both types of the exam. In a random-effects model, there was no statistically significant difference in cancer detection between the two types of mammography (AUC of 0.92 for film and AUC of 0.91 for digital). For women younger than 50 years, all studies found that sensitivity was higher for DM, but specificity was either the same or higher for film mammography.
Computer-aided detection (CAD) systems highlight suspicious regions, such as clustered microcalcifications and masses,[ 16 ] generally increasing sensitivity, decreasing specificity,[ 17 ] and increasing detection of ductal carcinoma in situ (DCIS).[ 18 ] Several CAD systems are in use. One large population-based study that compared recall rates and breast cancer detection rates before and after the introduction of CAD systems, found no change in either rate.[ 16 , 19 ] Another large study noted an increase in recall rate and increased DCIS detection but no improvement in invasive cancer detection rate.[ 18 , 20 ] Another study, using a large database and DM in women aged 40 to 89 years, found that CAD did not improve sensitivity, specificity, or detection of interval cancers, but it did detect more DCIS.[ 21 ]
The use of new screening mammography modalities by more than 270,000 women aged 65 years and older in two time periods, 2001 to 2002 and 2008 to 2009, was examined, relying on a Surveillance, Epidemiology, and End Results (SEER)–Medicare-linked database. DM increased from 2% to 30%, CAD increased from 3% to 33%, and spending increased from $660 million to $962 million. CAD was used in 74% of screening mammograms paid for by Medicare in 2008, almost twice as many screening mammograms as in 2004. There was no difference in detection rates of early-stage (DCIS or stage I) or late-stage (stage IV) tumors.[ 22 ]
Digital Breast Tomosynthesis
DBT is a mammographic technique, which was approved by the FDA (April 2018).[ 23 ] Like conventional mammography, DBT compresses the breast and uses x-rays to create images. In DBT, an x-ray tube moves in an arc around the compressed breast, taking multiple images at different angles, which are then reconstructed or synthesized into a set of 3-dimensional images by a computer. Some cancers are better seen with this method than on conventional DM or ultrasound.
DBT has rapidly become a prominent method of breast cancer screening in the United States, especially in higher-income regions with larger White populations. Use of DBT for breast cancer screening increased from 13% in 2015 to over 40% in 2017.[ 24 ] Seventy-three percent of facilities now report use of DBT.[ 23 ]
Observational data from eight screening facilities in Vermont compared the findings from 86,379 DBT and 97,378 full-field DM screening examinations performed between 2012 and 2016. Women were included if they had no history of breast cancer or breast implants. Demographic and risk factor information was obtained by questionnaire, and pathology for all biopsies was obtained through the Vermont Breast Cancer Surveillance System. Recall rate was lower with DBT than with DM (7.9% vs. 10.9%; odds ratio [OR], 0.81; 95% confidence interval [CI], 0.77–0.85), but there was no difference in the rates of biopsy or the detection of benign or malignant disease.[ 25 ]
The Oslo Tomosynthesis Screening Trial was conducted between November 2010 and December 2012 and included 24,301 women with 281 cancers. The trial compared the sensitivity of DM with DM plus DBT and with DM plus computer-aided detection and of DM plus DBT with synthesized 2-dimensional mammography plus DBT. Researchers report that DBT plus DM detected more breast cancers than DM alone (230 vs. 177, a 22.7% relative increase [95% CI, 17%–28.6%]). The trial also reported somewhat fewer false-positive findings on DBT plus DM compared with DM alone (2,081 vs. 2,466, a 0.8% relative reduction [95% CI, -1.03 to -0.57]), except in women with extremely dense breasts.[ 26 ] Difference between CAD plus DM and DM alone were not statistically significant.
The Tomosynthesis Trial in Bergen (To-Be) compared DBT plus synthesized mammography (SM) with conventional DM in population-based screening, including all women aged 50 to 69 years who were invited for breast cancer screening in Bergen, Norway. Screening was performed with two-view DBT plus SM or two-view conventional DM. A pool of eight radiologists independently double read the screening mammograms. Interim results from the first year of the trial showed:[ 27 ]
- Longer interpretation times for DBT plus SM (71 vs. 41 seconds).
- Equivalent mean glandular radiation dose.
- Lower overall recall rate for DBT plus SM (3.6% vs. 3.0%), despite an equivalent recall rate for women with dense breasts (3.6%).
The primary outcome results were published later.[ 28 ] The authors suggested explanations for the difference between these results and those from previous studies. First, SM may produce inferior quality images when compared with conventional DM, including poor visualization of microcalcifications. Second, the eight radiologists had wide variations in experience (ranging from 0–19 years) reading screen film and/or DM and DBT in population-based breast cancer screening.
Another study used three different Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer models and incorporated DBT screening performance data into the models to determine the cost and benefits of DBT versus DM. The study concluded that the use of DBT screening instead of DM reduced false-positives and recall rates and was projected to reduce breast cancer deaths (0–0.21 deaths per 1,000 women) and increased quality-adjusted life-years (QALYs) (1.97–3.27 per 1,000 women). However, these improvements were generally small and were associated with high costs relative to benefits: cost-effectiveness ratios ranged from $195,026 to $270,135 per QALY gained. These are greater than commonly accepted thresholds of $50,000 to $150,000 per QALY.[ 29 ]
An important limitation of the available studies and statistical modeling is lack of evidence of the clinical significance of the additional breast cancers detected by DBT (with or without DM) versus DM alone. The extent to which DBT may contribute to overdiagnosis of non–life-threatening lesions or lesions that would have still been detected in an asymptomatic woman at the time of a future DM is unknown. To date, there are no studies of DBT that show a reduction in metastatic disease or other late-stage disease.
Five ongoing randomized controlled trials with a combined recruitment of 430,000 women in Europe, the United Kingdom, and the United States are expected to provide information about clinical breast cancer outcomes of mammographic screening using DBT compared with DM.[ 26 , 30 ]
The randomized TOSYMA trial assessed DBT plus synthesized mammography versus digital screening mammography alone for the detection of breast cancer. The primary end points were detection of invasive breast cancer and the interval invasive cancer detection rate at 24 months. However, neither of these end points has been validated as proper surrogate outcome measures for mortality. The detection of greater numbers of early-stage cancers may confer no mortality benefit, as many of these cancers may fail to progress or progress so slowly that they pose no threat to the patient’s life (i.e., result in overdiagnosis). Moreover, if the detection of nonlethal cancers substantially increases, then the interval cancer detection rates may decrease with no subsequent reduction in mortality.[ 8 ]
A cohort study comparing DBT with DM found that the two modalities were not associated with a significant difference in risk of interval invasive cancer. However, DBT was associated with a significantly lower risk of advanced breast cancer among women with extremely dense breasts at high risk of developing breast cancer.[ 31 ] Better clarification on this issue may come from the ongoing Tomosynthesis Mammographic Imaging Screening Trial (TMIST), in which women are randomly assigned to either standard digital breast imaging or DBT, and the primary outcome is rate of advanced cancers, a composite end point that includes distant metastases.
Characteristics of Cancers Detected by Breast Imaging
Regardless of stage, nodal status, and tumor size, screen-detected cancers have a better prognosis than those diagnosed outside of screening.[ 2 ] This suggests that they are biologically less lethal (perhaps slower growing and less likely to invade locally and metastasize). This is consistent with the length bias effect associated with screening. That is, screening is more likely to detect indolent (i.e., slow-growing) breast cancers, while the more aggressive cancers are detected in the intervals between screening sessions.
A 10-year follow-up study of 1,983 Finnish women with invasive breast cancer demonstrated that the method of cancer detection is an independent prognostic variable. When controlled for age, nodal status, and tumor size, screen-detected cancers had a lower risk of relapse and better overall survival. For women whose cancers were detected outside of screening, the hazard ratio (HR) for death was 1.90 (95% CI, 1.15–3.11), even though they were more likely to receive adjuvant systemic therapy.[ 32 ]
Similarly, an examination of the breast cancers found in three randomized screening trials (Health Insurance Plan, National Breast Screening Study [NBSS]-1, and NBSS-2) accounted for stage, nodal status, and tumor size and determined that patients whose cancer was found via screening had a more favorable prognosis. The relative risks (RRs) for death were 1.53 (95% CI, 1.17–2.00) for interval and incident cancers, compared with screen-detected cancers; and 1.36 (95% CI, 1.10–1.68) for cancers in the control group, compared with screen-detected cancers.[ 33 ]
A third study compared the outcomes of 5,604 English women with screen-detected cancers to those with symptomatic breast cancers diagnosed between 1998 and 2003. After controlling for tumor size, nodal status, grade, and patient age, researchers found that the women with screen-detected cancers fared better. The HR for survival of the symptomatic women was 0.79 (95% CI, 0.63–0.99).[ 32 , 34 ]
The findings of these studies are also consistent with the evidence that some screen-detected cancers are low risk and represent overdiagnosis.
Screening biases–concepts
Numerous uncontrolled trials and retrospective series have documented the ability of mammography to diagnose small, early-stage breast cancers, which have a favorable clinical course.[ 35 ] Individuals whose cancer is detected by screening show a higher survival rate than those whose cancers are not detected by screening even when screening has not prolonged any lives. This concept is explained by the following four types of statistical bias:
- Lead-time bias : Cancer detected by screening earlier than the cancer would have been detected based on symptoms does nothing but advance the date of diagnosis. Earlier detection and treatment do not alter the natural disease progression. The 5-year survival rate from the time of diagnosis is longer for a cancer caught early even when the screening has made no difference in how long the person lives.
- Length bias : Screening mammography detects slowly growing cancers that have a better prognosis than cancers presenting clinically (detected by the doctor or the person when he or she gets ill). Adding these nonprogressive cancers to the life-threatening cancers (whose outcome is not affected by earlier treatment) increases the 5-year survival rate, even though screening has made no difference in how many lives are saved.
- Overdiagnosis bias : Screening detects cancers that would never cause symptoms or death and will increase survival rates without changing length of life.
- Healthy volunteer bias : Those who volunteer to participate in screening may be the healthiest, and the most health-conscious women in the general population. Therefore, their outcomes will be better than those of women who are neither healthy nor health-conscious, regardless of possible benefits of early diagnosis. One study identified that women who accept invitations to screening are more health-conscious, have better access to health care, and have lower mortality from causes other than breast cancer.[ 36 ]
The impact of these biases is not known. A new randomized controlled trial (RCT) with cause-specific mortality as the end point is needed to determine both survival benefit and impact of overdiagnosis, lead time, length time, and healthy volunteer biases. This is not achievable; randomly assigning patients to screen and nonscreen groups would be unethical, and at least three decades of follow-up would be needed, during which time changes in treatment and imaging technology would invalidate the results. Decisions must therefore be based on available RCTs, despite their limitations, and on ecological or cohort studies with adequate control groups and adjustment for confounding. For more information, see Cancer Screening Overview .
Assessment of performance and accuracy
Performance benchmarks for screening mammography in the United States are described on the Breast Cancer Surveillance Consortium (BCSC) website . For more information, see Cancer Screening Overview .

Sensitivity
The sensitivity of mammography is the percentage of women with breast cancers detected by mammographic screening. Sensitivity depends on tumor size, conspicuity, hormone sensitivity, breast tissue density, patient age, timing within the menstrual cycle, overall image quality, and interpretive skill of the radiologist. Overall sensitivity is approximately 79% but is lower in younger women and in those with dense breast tissue (see the BCSC website ).[ 37 - 39 ] Sensitivity is not the same as benefit because some woman with possible breast cancer are harmed by overdiagnosis. According to the Physician's Insurance Association of America (PIAA), delay in diagnosis of breast cancer and errors in diagnosis are common causes of medical malpractice litigation. PIAA data from 2002 through 2011 note that the largest total indemnity payments for breast cancer claims are for errors in diagnosis.[ 40 ]
Specificity and false-positive rate
The specificity of mammography is the percentage of all women without breast cancer whose mammograms are negative. The false-positive rate is the likelihood of a positive test in women without breast cancer. Low specificity and high rate of false-positives result in unnecessary follow-up examinations and procedures. Because specificity includes all women without cancer in the denominator, even a small percentage of false-positives turns out to be a large number in absolute terms. Thus—in screening—a good specificity must be very high. Even 95% specificity is quite low for a screening test.
Interval cancers
Interval cancers are cancers that are diagnosed in the interval between a normal screening examination and the anticipated date of the next screening mammogram. One study found interval cancers occurred more often in women younger than 50 years, and had mucinous or lobular histology, high histological grade, high proliferative activity with relatively benign mammographic features, and no calcifications. Conversely, screen-detected cancers often had tubular histology, small size, low stage, hormone sensitivity, and a major component of DCIS.[ 41 ] Overall, interval cancers have characteristics of rapid growth,[ 41 , 42 ] are diagnosed at an advanced stage, and carry a poor prognosis.[ 43 ]
Analysis of mammography screening length bias preferentially detects indolent cancers that grow more slowly (e.g., exist for a longer length of time in the preclinical phase). In contrast, the more aggressive cancers grow faster (e.g., spend a shorter length of time in the preclinical phase) and are often detected clinically in the intervals between screening sessions. For a more detailed explanation of length and lead-time bias in cancer screening, see Cancer Screening Overview .
In recent years, novel breast cancer screening technologies have been assessed in clinical trials with the interval cancer detection rate as the primary outcome of interest, and newer screening methods recommended on the basis of reductions in interval cancer detection rates. However, the interval cancer detection rate has not been validated as a proper surrogate for breast cancer mortality, and its use as a surrogate outcome measure in breast cancer screening trials remains controversial.
In breast cancer screening programs, screen-detected breast cancers tend to have a better prognosis than cancers detected during the intervals between screening sessions (interval breast cancers). This was confirmed in a registry-based cohort study from Manitoba in which interval breast cancers were more likely than were screen-detected breast cancers to be high-grade and estrogen receptor–negative, and associated with greater than a threefold increased risk of breast cancer death.[ 44 ]
The Nova Scotia Breast Screening Program defined missed cancers as those that were false-negatives on the previous screening exam, occurring less often than 1 per 1,000 women. It concluded that interval cancers occurred in approximately 1 per 1,000 women aged 40 to 49 years, and 3 per 1,000 women aged 50 to 59 years.[ 45 ]
Conversely, a larger trial found that interval cancers were more prevalent in women aged 40 to 49 years. Those appearing within 12 months of a negative screening mammogram were usually attributable to greater breast density. Those appearing within a 24-month interval were related to decreased mammographic sensitivity caused by greater breast density or to rapid tumor growth.[ 46 ]
Variables Associated With Accuracy
Patient characteristics.
The accuracy of mammography has been noted to vary with patient characteristics, such as a woman's age, breast density, whether it is her first or subsequent exam, and the time since her last mammogram. Younger women have lower sensitivity and higher false-positive rates than do older women.
The Million Women Study in the United Kingdom found decreased sensitivity and specificity in women aged 50 to 64 years if they used postmenopausal hormone therapy, had prior breast surgery, or had a body mass index below 25.[ 47 ] Increased time since the last mammogram increases sensitivity, recall rate, and cancer detection rate and decreases specificity.[ 48 ]
The United Kingdom Age Trial assessed the efficacy of mammography screening for women younger than 50 years. After a median follow-up of 22.8 years, there was no difference in breast cancer mortality between women randomly assigned to initiate screening at age 39 to 41 years until entry into the National Health Service (NHS) breast screening program at age 50 to 52 years, versus the group that did not initiate mammography screening until entry into the NHS breast screening program (RR, 0.98; 95% CI, 0.79–1.22; P = .86).[ 49 ]
Sensitivity may be improved by scheduling the exam after the initiation of menses or during an interruption from hormone therapy.[ 50 ] Obese women have more than a 20% increased risk of having false-positive mammography, although sensitivity is unchanged.[ 51 ]
Breast density
Dense breasts may obscure the detection of small masses on mammography, thereby reducing the sensitivity of mammography.[ 13 ] For women of all ages, high breast density is associated with 10% to 29% lower sensitivity.[ 38 ] High breast density is also associated with a modestly increased risk of developing breast cancer.[ 52 ] High breast density does not confer a higher risk of breast cancer death.
High breast density is an inherent trait, which can be inherited [ 53 , 54 ] or affected by age; endogenous [ 55 ] and exogenous [ 56 , 57 ] hormones;[ 58 ] selective estrogen receptor modulators, such as tamoxifen;[ 59 ] and diet.[ 60 ] Hormone therapy is associated with increased breast density, lower mammographic sensitivity, and an increased rate of interval cancers.[ 61 ]
Dense breast tissue is not abnormal. Breast density describes the proportion of dense versus fatty tissue in a mammographic image.[ 62 ] The American College of Radiology’s BI-RADS classifies breast density as follows:
- Almost entirely fatty.
- Scattered fibroglandular densities.
- Heterogeneously dense.
- Extremely dense.
The latter two categories are considered dense breast tissue , a description affecting 43% of women aged 40 to 74 years.[ 63 ] A radiologist's assignment of breast density is subjective and may vary over time in any woman.[ 63 , 64 ]
There is limited high-quality evidence to guide optimal breast cancer screening in individuals with dense breasts. For dense breasts, digital breast tomosynthesis has improved sensitivity and modestly lowers false-positive rates compared with conventional digital mammography.[ 65 ]
Supplemental imaging with ultrasonography or breast magnetic resonance imaging (MRI) has been suggested by some groups for screening women with dense breasts, but there are no data showing that this strategy results in lower breast cancer mortality. The potential harm of adding these supplemental screening tests is the likelihood of producing more false-positives, leading to additional imaging and breast biopsies, with resultant anxiety and cost.[ 66 ] Supplemental screening may also increase overdiagnosis of breast cancer with resultant overtreatment.
A study examining cancer detection end points in women with dense breasts undergoing supplemental screening (e.g., ultrasound, MRI, digital resources) showed higher breast cancer detection, but it is not known if that translates into cancer protection.[ 67 ] An RCT of supplemental MRI versus mammography only in 40,373 individuals aged 50 to 75 years with extremely dense breasts in the Netherlands was performed.[ 68 ] The study showed lower incidence of interval cancers at 2 years of follow-up in the MRI group (2.5 per 1,000 screenings in the group invited to receive MRI, 0.8 per 1,000 in the group that actually received MRI, and 5.0 per 1,000 in the group that received mammography only). This finding suggests that at least some of the excess cancers detected by MRI in the MRI group were earlier diagnoses of cancers that would have become clinically apparent. However, whether earlier diagnoses facilitated by MRI resulted in improved clinical outcomes has not been shown. As would be expected, cancers detected by MRI were more likely to have favorable tumor characteristics than interval cancers. MRI screening was associated with 79.8 false-positive results per 1,000 screenings.[ 68 ]
A prospective multicenter study, known as the Dense Breast Tomosynthesis Ultrasound Screening Trial (DBTUST), investigated whether ultrasound improved cancer detection after DBT in women with dense breasts.[ 69 ] Between December 2015 and June 2021, 6,179 women at three Pennsylvania locations underwent three rounds of annual screening with DBT and technologist-performed handheld ultrasounds. The images were interpreted by two radiologists at baseline, 12 months, and 24 months. The study concluded that technologist-performed ultrasound screening modestly improved detection of cancer in women with dense breasts by 1.3 cases per 1,000 in year 1 and by 1 case per 1,000 in years 2 to 3. This screening also increased the false-positive recall rate. In 3 years, 1,007 (16.3%) women had a false-positive recall based on DBT, and an additional 761 (12.3%) women had a false-positive recall based on ultrasound.
The FDA mandates that mammography facilities report breast density to patients and suggest that patients speak with their primary care clinician about supplemental screening.[ 70 ] However, limited evidence, inconsistent guidelines, and wording of breast density reports have generated confusion and anxiety among patients and health care providers.[ 71 ]
Tumor characteristics
Mucinous and lobular cancers are more easily detected by mammography. Rapidly growing cancers can sometimes be mistaken for normal breast tissue (e.g., medullary carcinomas, an uncommon type of invasive ductal breast cancer that is often associated with the BRCA1 mutation and aggressive characteristics, but that may demonstrate comparatively favorable responses to treatment).[ 41 , 72 ] Some other cancers associated with BRCA1/2 mutations, which may appear indolent, can also be missed.[ 73 , 74 ]
Physician characteristics
Radiologists’ performance is variable, affected by levels of experience and the volume of mammograms they interpret.[ 75 ] Biopsy recommendations of radiologists in academic settings have a higher positive PPV than do community radiologists.[ 76 ] Fellowship training in breast imaging may improve detection.[ 11 ]
Performance also varies by facility. Mammographic screening accuracy was higher at facilities offering only screening examinations than at those also performing diagnostic tests. Accuracy was also better at facilities with a breast imaging specialist on staff, performing single rather than double readings, and reviewing performance audits two or more times each year.[ 77 ]
False-positive rates are higher at facilities where concern about malpractice is high and at facilities serving vulnerable women (racial or ethnic minority women and women with less education, limited household income, or rural residence).[ 78 ] These populations may have a higher cancer prevalence and a lack of follow-up.[ 79 ]
Artificial intelligence algorithms
Artificial intelligence (AI) algorithms are being developed to interpret screening mammograms and breast biopsy specimens.[ 80 - 82 ] While such tools may improve interpretive speed and reproducibility in the future, it is unknown if they will exacerbate overdiagnosis [ 83 ] and how they might influence physicians’ final assessments.
International comparisons
International comparisons of screening mammography have found higher specificity in countries with more highly centralized screening systems and national quality assurance programs.[ 84 , 85 ]
The recall rate in the United States is twice that of the United Kingdom, with no difference in the rate of cancer detection.[ 84 ]
Prevalent versus subsequent examination and the interval between exams
The likelihood of diagnosing cancer is highest with the prevalent (first) screening examination, ranging from 9 to 26 cancers per 1,000 screens, depending on the woman’s age. The likelihood decreases for follow-up examinations, ranging from 1 to 3 cancers per 1,000 screens.[ 86 ]
The optimal interval between screening mammograms is unknown; there is little variability across the trials despite differences in protocols and screening intervals. A prospective U.K. trial randomly assigned women aged 50 to 62 years to receive mammograms annually or triennially. Although tumor grade and nodal status were similar in the two groups, more cancers of slightly smaller size were detected in the annual screening group than in the triennial screening group.[ 87 ]
A large observational study found a slightly increased risk of late-stage disease at diagnosis for women in their 40s who were adhering to a 2-year versus a 1-year schedule (28% vs. 21%; OR, 1.35; 95% CI, 1.01–1.81), but no difference was seen for women in their 50s or 60s based on schedule difference.[ 88 , 89 ]
A Finnish study of 14,765 women aged 40 to 49 years randomly assigned women to receive either annual screens or triennial screens. There were 18 deaths from breast cancer in 100,738 life-years in the triennial screening group and 18 deaths from breast cancer in 88,780 life-years in the annual screening group (HR, 0.88; 95% CI, 0.59–1.27).[ 90 ]
Benefit of Mammographic Screening on Breast Cancer Mortality
Randomized controlled trials (rcts).
RCTs that studied the effect of screening mammography on breast cancer mortality were performed between 1963 and 2015, with participation by over half-a-million women in four countries. One trial, the Canadian NBSS-2, compared mammography plus clinical breast examination (CBE) to CBE alone; the other trials compared screening mammography with or without CBE to usual care. For a detailed description of the trials, see the Appendix of Randomized Controlled Trials section.
The trials differed in design, recruitment of participants, interventions (both screening and treatment), management of the control group, compliance with assignment to screening and control groups, and analysis of outcomes. Some trials used individual randomization, while others used cluster randomization in which cohorts were identified and then offered screening; one trial used nonrandomized allocation by day of birth in any given month. Cluster randomization sometimes led to imbalances between the intervention and control groups. Age differences have been identified in several trials, although the differences had no major effect on the trial outcome.[ 91 ] In the Edinburgh Trial, socioeconomic status, which correlates with the risk of breast cancer mortality, differed markedly between the intervention and control groups, rendering the results uninterpretable.
Breast cancer mortality was the major outcome parameter for each of these trials, so the attribution of cause of death required scrupulous attention. The use of a blinded monitoring committee (New York) and a linkage to independent data sources, such as national mortality registries (Swedish trials), were incorporated but could not ensure impartial attributions of cancer death for women in the screening or control arms. Possible misclassification of breast cancer deaths in the Two-County Trial biasing the results in favor of screening has been suggested.[ 92 ]
There were also differences in the methodology used to analyze the results of these trials. Four of the five Swedish trials were designed to include a single screening mammogram in the control group and were timed to correspond with the end of the series of screening mammograms in the study group. The initial analysis of these trials used an evaluation analysis, tallying only the breast cancer deaths that occurred in women whose cancer was discovered at or before the last study mammogram. In some of the trials, a delay occurred in the performance of the end-of-study mammogram, resulting in more time for members of the control group to develop or be diagnosed with breast cancer. Other trials used a follow-up analysis, which counts all deaths attributed to breast cancer, regardless of the time of diagnosis. This type of analysis was used in a meta-analysis of four of the five Swedish trials as a response to concerns about the evaluation analyses.[ 92 ]
The accessibility of the data for international audits and verification also varied, with a formal audit having been undertaken only in the Canadian trials. Other trials have been audited to varying degrees, but with less rigor.[ 93 ]
All of these studies were designed to study breast cancer mortality rather than all-cause mortality because breast cancer deaths contribute only a small proportion of total mortality in any given population. When all-cause mortality in these trials was examined retrospectively, only the Edinburgh Trial showed a difference attributable to the previously noted socioeconomic differences in the study groups. The meta-analysis (follow-up methods) of the four Swedish trials also showed a small improvement in all-cause mortality.
The relative improvement in breast cancer mortality attributable to screening is approximately 15% to 20%, and the absolute improvement at the individual level is much less. The potential benefit of breast cancer screening can be expressed as the number of lives extended because of early breast cancer detection.[ 94 , 95 ]
The RCT results represent experiences in a defined period of regular examinations, but in practice, women undergo 20 to 30 years of screening throughout their lifetimes.[ 89 , 96 ]
There are several problems with using these RCTs that were performed up to 50 years ago to estimate the current benefits of screening on breast cancer mortality. These problems include the following:
- Improvements in mammography technology, with the ability to identify increasingly subtle abnormalities.
- Enhanced breast cancer awareness in the general population, with women seeking evaluation and treatment earlier.
- Changes in the risk factors for breast cancer in the population (including age at menarche, age at first pregnancy, obesity, and use of postmenopausal hormone treatment).
- Improvements in breast cancer treatment, such that larger, more advanced cancers have higher cure rates than in the past.
- Applying results of short-term RCTs (e.g., 5 to 10 years) to make estimates of lifetime effects of breast cancer screening.
For these reasons, estimates of the breast cancer mortality reduction resulting from current screening are based on well-conducted cohort and ecological studies in addition to the RCTs.
Effectiveness of population-based screening programs
An estimate of screening effectiveness can be obtained from nonrandomized controlled studies of screened versus nonscreened populations, case-control studies of screening in real communities, and modeling studies that examine the impact of screening on large populations. These studies must be designed to minimize or exclude the effects of unrelated trends influencing breast cancer mortality such as improved treatment and heightened awareness of breast cancer in the community.
Three population-based, observational studies from Sweden compared breast cancer mortality in the presence and absence of screening mammography programs. One study compared two adjacent time periods in 7 of the 25 counties in Sweden and found a statistically significant breast cancer mortality reduction of 18% to 32% attributable to screening.[ 97 ] The most important bias in this study is that the advent of screening in these counties occurred over a period during which dramatic improvements in the effectiveness of adjuvant breast cancer therapy were being made, changes that were not addressed by the study authors. The second study considered an 11-year period comparing seven counties with screening programs with five counties without them.[ 98 ] There was a trend in favor of screening, but again, the authors did not consider the effect of adjuvant therapy or differences in geography (urban vs. rural) that might affect treatment practices.
The third study attempted to account for the effects of treatment by using a detailed analysis by county. It found screening had little impact, a conclusion weakened by several flaws in design and analysis.[ 99 ]
In Nijmegen, the Netherlands, where a population-based screening program was undertaken in 1975, a case-cohort study found that screened women had decreased mortality compared with unscreened women (OR, 0.48).[ 100 ] However, a subsequent study comparing Nijmegen breast cancer mortality rates with neighboring Arnhem in the Netherlands, which had no screening program, showed no difference in breast cancer mortality.[ 101 ]
A community-based case-control study of screening in high-quality U.S. health care systems between 1983 and 1998 found no association between previous screening and reduced breast cancer mortality, but the mammography screening rates were generally low.[ 102 ]
A well-conducted ecological study compared three pairs of neighboring European countries that were matched on similarity in health care systems and population structure, one of which had started a national screening program some years earlier than the others. The investigators found that each country had experienced a reduction in breast cancer mortality, with no difference between matched pairs that could be attributed to screening. The authors suggested that improvements in breast cancer treatment and/or health care organizations were more likely responsible for the reduction in mortality than was screening.[ 103 ]
A systematic review of ecological and large cohort studies published through March 2011 compared breast cancer mortality in large populations of women, aged 50 to 69 years, who started breast cancer screening at different times. Seventeen studies met inclusion criteria, but all studies had methodological problems, including control group dissimilarities, insufficient adjustment for differences between areas in breast cancer risk and breast cancer treatment, and problems with similarity of measurement of breast cancer mortality between compared areas. There was great variation in results among the studies, with four studies finding a relative reduction in breast cancer mortality of 33% or more (with wide CIs) and five studies finding no reduction in breast cancer mortality. Because only a part of the overall reduction in breast cancer mortality could possibly be attributed to screening, the review concluded that any relative reduction in breast cancer mortality resulting from screening would likely be no more than 10%.[ 104 ]
A U.S. ecological analysis conducted between 1976 and 2008 examined the incidence of early-stage versus late-stage breast cancer for women aged 40 years and older. To assess a screening effect, the authors compared the magnitude of increase in early-stage cancer with the magnitude of an expected decrease in late-stage cancer. Over the study, the absolute increase in the incidence of early-stage cancer was 122 cancers per 100,000 women, while the absolute decrease in late-stage cancers was 8 cases per 100,000 women. After adjusting for changes in incidence resulting from hormone therapy and other undefined causes, the authors concluded (1) the benefit of screening on breast cancer mortality was small, (2) between 22% and 31% of diagnosed breast cancers represented overdiagnosis, and (3) the observed improvement in breast cancer mortality was probably attributable to improved treatment rather than screening.[ 105 ]
An analytic approach was used to approximate the contributions of screening versus treatment to breast cancer mortality reduction and the magnitude of overdiagnosis.[ 106 ] The shift in the size distribution of breast cancers in the United States (before the introduction of mammography) to 2012 (after its widespread dissemination), was investigated using SEER data in women aged 40 years and older. The rate of clinically meaningful breast cancer was assumed to be stable during this time. The authors documented a lower incidence of larger (≥2 cm) tumors as well as a reduction in breast cancer case fatality. The lower mortality for women with larger tumors was attributed to improvements in therapy. Two-thirds of the decline in size-specific case fatality was ascribed to improved treatment.
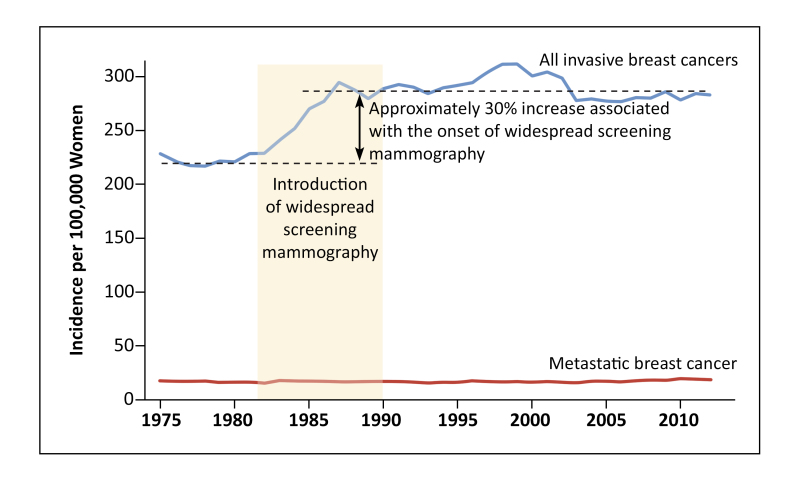
Figure 2. Screening mammography and increased incidence of invasive breast cancer. Shown are the incidences of overall invasive breast cancer and metastatic breast cancer among women 40 years of age or older at nine sites of the Surveillance, Epidemiology, and End Results (SEER) program, during the period from 1975 through 2012. From New England Journal of Medicine, Welch HG, Prorok PC, O'Malley AJ, Kramer BS, Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness, Volume 375, Issue 15, Pages 1438-47, Copyright © 2016 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
A prospective cohort study of community-based screening programs in the United States found that annual compared with biennial screening mammography did not reduce the proportion of unfavorable breast cancers detected in women aged 50 to 74 years or in women aged 40 to 49 years without extremely dense breasts. Women aged 40 to 49 years with extremely dense breasts did have a reduction in cancers larger than 2.0 cm with annual screening (OR, 2.39; 95% CI, 1.37–4.18).[ 107 ]
An observational study of women aged 40 to 74 years conducted in 7 of 12 Canadian screening programs compared breast cancer mortality in those participants screened at least once between 1990 and 2009 (85% of the population) with those not screened (15% of the population). The abstract reported a 40% average breast cancer mortality among participants; however, it was likely intended to report a 40% reduction in breast cancer mortality on the basis of language used in the Discussion section.[ 108 ]
Limitations of this study included the lack of all-cause mortality data, the extent of screening, screening outside of the study, screening prior to the study, the method used for calculating expected mortality and the referent rates of nonparticipants, nonparticipant survival, province-specific population differences, the extent to which limitations of the database prevented correcting for age and other differences between participants, the generalizability of the substudy data of a single province (British Columbia), and the potentially large impact of selection bias. Overall, the study lacked important data and had limitations in methodology and data analysis.
Statistical modeling of breast cancer incidence and mortality in the United States
The optimal screening interval has been addressed by modelers. Modeling makes assumptions that may not be correct; however, the credibility of modeling is greater when the model produces overall results that are consistent with randomized trials and when the model is used to interpolate or extrapolate. For example, if a model’s output agrees with RCT outcomes for annual screening, it has greater credibility to compare the relative effectiveness of biennial versus annual screening.
In 2000, the National Cancer Institute formed a consortium of modeling groups (Cancer Intervention and Surveillance Modeling Network [CISNET]) to address the relative contribution of screening and adjuvant therapy to the observed decline in breast cancer mortality in the United States.[ 109 ] These models predicted reductions in breast cancer mortality similar to those expected in the circumstances of the RCTs but updated to the use of modern adjuvant therapy. In 2009, CISNET modelers addressed several questions related to the harms and benefits of mammography, including comparing annual versus biennial screening.[ 89 ] Women aged 50 to 74 years received most of the mortality benefit of annual screening by having a mammogram every 2 years. The reduction in breast cancer deaths that was maintained because of the move from annual to biennial screening ranged across the six models from 72% to 95%, with a median of 80%.
Data are limited as to how much of the reduction in mortality, seen over time from 1990 onward, is attributable to advances in imaging techniques for screening and as to how much is the result of the improved effectiveness of therapy. In one CISNET study of six simulation models, about one-third of the decrease in breast cancer mortality in 2012 was attributable to screening, with the balance attributed to treatment.[ 110 ] In this CISNET study, the mean estimated reduction in overall breast cancer mortality rate was 49% (model range, 39%–58%), relative to the estimated baseline rate in 2012 if there was no screening or treatment; 37% (model range, 26%–51%) of this reduction was associated with screening, and 63% (model range, 49%–74%) of this reduction was associated with treatment.
Harms of Mammographic Screening
The negative effects of screening mammography are overdiagnosis (true positives that will not become clinically significant), false-positives (related to the specificity of the test), false-negatives (related to the sensitivity of the test), discomfort associated with the test, radiation risk, psychological harm, financial stress, and opportunity costs.
Table 1 provides an overview of the estimated benefits and harms of screening mammography for 10,000 women who underwent annual screening mammography over a 10-year period.[ 111 ]
Table 1. Estimated Benefits and Harms of Mammography Screening for 10,000 Women Who Underwent Annual Screening Mammography During a 10-Year Period a
View in own window
No. = number; CI = confidence interval; DCIS = ductal carcinoma in situ .
a Adapted from Pace and Keating.[ 111 ]
b Number of deaths averted are from Welch and Passow.[ 112 ] The lower bound represents breast cancer mortality reduction if the breast cancer mortality relative risk were 0.95 (based on minimal benefit from the Canadian trials [ 113 , 114 ]), and the upper bound represents the breast cancer mortality reduction if the relative risk were 0.64 (based on the Swedish 2-County Trial [ 115 ]).
c False-positive and biopsy estimates and 95% confidence intervals are 10-year cumulative risks reported in Hubbard et al. [ 116 ] and Braithwaite et al.[ 117 ]
d The number of overdiagnosed cases are calculated by Welch and Passow.[ 112 ] The lower bound represents overdiagnosis based on results from the Malmö trial,[ 118 ] whereas the upper bound represents the estimate from Bleyer and Welch.[ 105 ]
e The lower-bound estimate for overdiagnosis reported by Welch and Passow [ 112 ] came from the Malmö study.[ 118 ] The study did not enroll women younger than 50 years.
Overdiagnosis
Overdiagnosis occurs when screening procedures detect cancers that would never become clinically apparent in the absence of screening. It is a special concern because identification of the cancer does not benefit the individual, while the side effects of diagnostic procedures and cancer treatment may cause significant harm. The magnitude of overdiagnosis is debated, particularly regarding DCIS, a cancer precursor whose natural history is unknown. By reason of this inability to predict confidently the tumor behavior at time of diagnosis, standard treatment for invasive cancers and DCIS can cause overtreatment. The related harms include treatment-related side effects and the number of harms associated with a cancer diagnosis, which are immediate. Conversely, a mortality benefit would occur at an uncertain point in the future.
One approach to understanding overdiagnosis is to examine the prevalence of occult cancer in women who died of noncancer causes. In an overview of seven autopsy studies, the median prevalence of occult invasive breast cancer was 1.3% (range, 0%–1.8%) and of DCIS was 8.9% (range, 0%–14.7%).[ 119 , 120 ]
Overdiagnosis can be indirectly measured by comparing breast cancer incidence in screened versus unscreened populations. These comparisons can be confounded by differences in the populations, such as time, geography, health behaviors, and hormone usage. The calculations of overdiagnosis can vary in their adjustment for lead-time bias.[ 121 , 122 ] An overview of 29 studies found calculated rates of overdiagnosis to be 0%–54%, with rates from randomized studies between 11% and 22%.[ 123 ] In Denmark, where screened and unscreened populations existed concurrently, the rate of overdiagnosis of invasive cancer was calculated to be 14% and 39%, using two different methodologies. If DCIS cases were included, the overdiagnosis rates were 24% and 48%. The second methodology accounts for regional differences in women younger than the screening age and is likely more accurate.[ 124 ]
Theoretically, in a given population, the detection of more breast cancers at an early stage would result in a subsequent reduction in the incidence of advanced-stage cancers. This has not occurred in any of the populations studied to date. Thus, the detection of more early-stage cancers likely represents overdiagnosis. A population-based study in the Netherlands showed that about one-half of all screen-detected breast cancers, including DCIS, would represent overdiagnosis and is consistent with other studies, which showed substantial rates of overdiagnosis associated with screening.[ 125 ]
A cohort study in Norway compared the increase in cancer incidence in women who were eligible for screening with the cancer incidence in younger women who were not eligible for screening, eligibility was based on age and residence. Eligible women experienced a 60% increase in incidence of localized cancers (RR, 1.60; 95% CI, 1.42–1.79), while the incidence of advanced cancers remained similar in the two groups (RR, 1.08; 95% CI, 0.86–1.35).[ 126 ]
A population study that compared different counties in the United States showed that higher rates of screening mammography use were associated with higher rates of breast cancer diagnoses, yet there was no corresponding decrease in 10-year breast cancer mortality.[ 127 ] The strengths of this study include its very large size (16 million women) and the strength and consistency of correlation observed across counties. The limitations of this study include the self-reporting of mammograms, the use of a 2-year window to estimate screening prevalence, and the period of analysis (when menopausal hormone use was present).[ 127 ]
The extent of overdiagnosis has been estimated in the Canadian NBSS, a randomized clinical trial. At the end of the five screening rounds, 142 more invasive breast cancer cases were diagnosed in the mammography arm, compared with the control arm.[ 128 ] At 15 years, the excess number of cancer cases in the mammography arm versus the control arm was 106, representing an overdiagnosis rate of 22% for the 484 screen-detected invasive cancers.[ 128 ]
As a consequence of screening mammography, greater numbers of breast cancers with indolent behavior are now identified, resulting in potential overtreatment. In a secondary analysis of a randomized trial of tamoxifen versus no systemic therapy in patients with early breast cancer, the authors utilized the 70-gene MammaPrint assay and identified 15% of patients at ultra-low risk, with 20-year disease-specific survival rates of 97% in the tamoxifen group and 94% in the control group. Thus, these patients would likely have extremely good outcomes with surgery alone. The frequency of such ultra-low risk cancers in the screened population is likely around 25%. Tools such as the 70-gene MammaPrint assay might be utilized in the future to identify these cancers, and thereby, reduce the risk of overtreatment. However, additional studies are needed to confirm these findings.[ 129 ]
In 2016, the Canadian NBSS, a randomized screening trial with 25-year follow-up, re-estimated overdiagnosis of breast cancer from mammography screening by age group and concluded that approximately 30% of invasive screen-detected cancers in women aged 40 to 49 years and up to 20% of those detected in women aged 50 to 59 years were overdiagnosed. When in situ cancers are included, the estimated risks of overdiagnosis are 40% aged 40 to 49 years and 30% in women aged 50 to 59 years. Overdiagnosis was calculated as the persistent excess incidence in the screened arm versus the control arm divided by the number of screen-detected cases (excess incidence method). Requirements for adequate estimation of overdiagnosis utilizing this method included the following:
- Cessation of screening among participants in the screened arm when the trial screening protocol is completed.
- Follow-up after screening ceases needs to be as long as the longest lead time (the time between the identification of a screen-detected cancer until symptomatic diagnosis of that cancer in the absence of screening) among the screen-detected cases.
- The comparison population for the cancer incidence during screening and after screening cessation in the screened arm needs to comprise individuals with comparable cancer risk in the absence of screening, as in a randomized control arm.
- Compliance with screening is high in the screened arm during the trial protocol screening phase, and contamination (nonprotocol screening) in the control arm is low.
These conditions were largely met in the CNBSS because population-based screening did not become available throughout Canada until a minimum of 2 years later and in most instances 5 to 10 years later (thereby, allowing for cessation of screening after the trial screening period and follow-up longer than most estimates of lead time), because contamination is documented to have been minimal, and because individual randomization resulted in 44 almost identically distributed demographic factors and risk factors between the two trial arms.
Since the conclusion of the trial screening period in 1988, differences in screening quality, intensity, invited age range, and biopsy thresholds decrease the generalizability of these results. These factors and improved imaging technique/quality and low threshold for biopsy, likely contribute to lower estimates of overdiagnosis of in situ cancer than that of invasive cancer.[ 130 ]
Table 1 shows results from a 10-year period of screening 10,000 women, estimating the number of women with breast cancer or DCIS that would never become clinically important (overdiagnosis). There was likely no overdiagnosis in the Health Insurance Plan study, which used old-technology mammography and CBE. Overdiagnosis has become more prominent in the era of improved-technology mammography. The improved technology has not, however, been shown to make further reductions in mortality than the original technology. In summary, breast cancer overdiagnosis is a complex topic. Studies that used many different methods reported a wide range of estimates, and there is currently no way to assess whether new cancer cases are overdiagnosed or are of real harm to patients.[ 111 ]
False-positives leading to additional interventions
Because fewer than 5 per 1,000 women screened have breast cancer, most abnormal mammograms are false-positives, even given the 90% specificity of mammography (i.e., 90% of all women without breast cancer will have a negative mammogram).[ 86 ]
This high false-positive rate of mammography is underestimated and can seem counterintuitive because of a statistically based cognitive bias known as the base rate fallacy. Because the base rate of breast cancer is low, (5/1000), the false-positive rate vastly exceeds the true-positive rate, even when using a very accurate test.
Mammography’s true-positive rate of approximately 90% means that, of women with breast cancer, approximately 90% will test positive. The true-negative rate of 90% means that, of women without breast cancer, 90% will test negative. A 10% false-positive rate over 1,000 people means that there will be 100 false-positives in 1,000 people. If 5 in 1,000 women have breast cancer, then 4.5 women with breast cancer will have a positive test. In other words, there will approximately 100 false-positives for every 4.5 true positives.
Further, abnormal results from screening mammograms prompt additional tests and procedures, such as mammographic views of the region of concern, ultrasound, MRI, and tissue sampling (by fine-needle aspiration, core biopsy, or excisional biopsy). Overall, the harm from unnecessary tests and treatments must be weighed against the benefit of early detection.
A study of breast cancer screening in 2,400 women enrolled in a health maintenance organization found that over a decade, 88 cancers were diagnosed, 58 of which were identified by mammography. One-third of the women had an abnormal mammogram result that required additional testing: 539 additional mammograms, 186 ultrasound examinations, and 188 biopsies. The cumulative biopsy rate (the rate of true positives) resulting from mammographic findings was approximately 1 in 4 (23.6%). The PPV of an abnormal screening mammogram in this population was 6.3% for women aged 40 to 49 years, 6.6% for women aged 50 to 59 years, and 7.8% for women aged 60 to 69 years.[ 131 ] A subsequent analysis and modeling of data from the same cohort of women, estimated that the risk of having at least one false-positive mammogram was 7.4% (95% CI, 6.4%–8.5%) at the first mammogram, 26.0% (95% CI, 24.0%–28.2%) by the fifth mammogram, and 43.1% (95% CI, 36.6%–53.6%) by the ninth mammogram.[ 132 ] Cumulative risk of at least one false-positive result depended on four patient variables (younger age, higher number of previous breast biopsies, family history of breast cancer, and current estrogen use) and three radiologic variables (longer time between screenings, failure to compare the current and previous mammograms, and the individual radiologist’s tendency to interpret mammograms as abnormal). Overall, the factor most responsible for a false-positive mammogram was the individual radiologist’s tendency to read mammograms as abnormal.
A prospective cohort study of community-based screening found that a greater proportion of women undergoing annual screening had at least one false-positive screen after 10 years than did women undergoing biennial screening, regardless of breast density. For women with scattered fibroglandular densities, the difference was 68.9% (annual) versus 46.3% (biennial) for women in their 40s. For women aged 50 to 74 years, the difference for this density group was 49.8% (annual) versus 30.7% (biennial).[ 107 ]
As shown in Table 1 , the estimated number of women out of 10,000 who underwent annual screening mammography during a 10-year period with at least one false-positive test result is 6,130 for women aged 40 to 50 years and 4,970 for women aged 60 years. The number of women with a false-positive test that results in a biopsy is estimated to range from 700 to 980, depending on age.[ 111 ]
Relationship between prior screening results and subsequent breast cancer diagnosis
A longitudinal Norwegian study correlated benign abnormal screening results with long-term breast cancer outcomes. Women with any abnormal screening examination had an increased risk of subsequent breast cancer, despite a negative evaluation (see Table 2 ). The features of the subsequent breast cancer were more favorable for the women who had prior screening abnormalities, possibly because the preexisting breast abnormality was a marker for slow-growing premalignant disease.[ 133 ]
Table 2. Relationship Between Prior Screening Results and Subsequent Breast Cancer Diagnosis
False-negatives leading to a false sense of security.
The sensitivity of mammography ranges from 70% to 90%, depending on characteristics of the interpreting radiologist (level of experience) and characteristics of the woman (age, breast density, hormone status, and diet). Assuming an average sensitivity of 80%, mammograms will miss approximately 20% of the breast cancers that are present at the time of screening (false-negatives). Many of these missed cancers are high risk, with adverse biological characteristics. If a normal mammogram dissuades or postpones a woman or her doctor from evaluating breast symptoms, she may suffer adverse consequences. Thus, a negative mammogram should never dissuade a woman or her physician from additional evaluation of breast symptoms.
Positioning of the woman and breast compression reduce motion artifact and improve mammogram image quality. Pain and/or discomfort was reported by 90% of women undergoing mammography, with 12% of women rating the sensation as intense or intolerable.[ 134 ] A systematic review of 22 studies investigating mammography-associated pain and discomfort found wide variations, some of which were associated with menstrual cycle stage, anxiety, and premammography anticipation of pain.[ 135 ]
Radiation exposure
The major risk factors for radiation-associated breast cancer are young age at exposure and dose; however, rarely there are women with an inherited susceptibility to radiation-induced damage who must avoid radiation exposure at any age.[ 136 , 137 ] For many women older than 40 years, the likely benefits of screening mammography outweigh the risks.[ 138 , 136 , 139 ] Standard two-view screening mammography exposes the breasts to a mean dose of 4 mSv, and the whole body to 0.29 mSv.[ 137 , 140 ] Thus, up to one breast cancer may be induced per 1,000 women undergoing annual mammograms from ages 40 to 80 years. Such risk is doubled in women with large breasts who require increased radiation doses and in women with breast augmentation who require additional views. Radiation-induced breast cancers may be reduced fivefold for women who begin biennial screening at age 50 years rather than annually at age 40 years.[ 141 ]
Psychological harms of false-positives
A telephone survey of 308 women performed 3 months after screening mammography revealed that about one-fourth of the 68 women recalled for additional testing were still experiencing worry that affected their mood or functioning, even though that testing had ruled out cancer.[ 142 ] Research into whether the psychological impact of a false-positive test is long-standing yields mixed results. A cohort study in Spain in 2002 found immediate psychological impact to a woman after receiving a false-positive mammogram, but these results dissipated within a few months.[ 143 ] A cohort study in Denmark in 2013 that measured the psychological effects of a false-positive test result several years after the event found long-term negative psychological consequences.[ 144 ] Several studies have shown that the anxiety after evaluation of a false-positive test leads to increased participation in future screening examinations.[ 145 - 148 ]
Financial strain and opportunity costs
These potential harms of screening have not been well researched, but it is clear that they exist.
- Siu AL; U.S. Preventive Services Task Force: Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 164 (4): 279-96, 2016. [ PubMed : 26757170 ]
- Sickles EA: Findings at mammographic screening on only one standard projection: outcomes analysis. Radiology 208 (2): 471-5, 1998. [ PubMed : 9680578 ]
- Dibden A, Offman J, Parmar D, et al.: Reduction in interval cancer rates following the introduction of two-view mammography in the UK breast screening programme. Br J Cancer 110 (3): 560-4, 2014. [ PMC free article : PMC3915134 ] [ PubMed : 24366303 ]
- Lillie-Blanton M: Mammography Quality Standards Act : X-ray Quality Improved, Access Unaffected, but Impact on Health Outcomes Unknown: Testimony Before the Subcommittee on Health and the Environment, Committee on Commerce, House of Representatives. Washington, D.C.: Committee on Commerce, 1998. Available online . Last accessed January 16, 2024.
- D'Orsi CJ, Sickles EA, Mendelson EB, et al.: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. American College of Radiology, 2013. Also available online . Last accessed January 16, 2024.
- Sickles EA, D'Orsi CJ, Bassett LW, et al.: ACR BI-RADS Mammography. In: D'Orsi CJ, Sickles EA, Mendelson EB, et al.: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. American College of Radiology, 2013, pp 3-171. Also available online . Last accessed January 16, 2024.
- Jatoi I, Pinsky PF: Breast Cancer Screening Trials: Endpoints and Overdiagnosis. J Natl Cancer Inst 113 (9): 1131-1135, 2021. [ PMC free article : PMC8633447 ] [ PubMed : 32898241 ]
- Alabousi M, Zha N, Salameh JP, et al.: Digital breast tomosynthesis for breast cancer detection: a diagnostic test accuracy systematic review and meta-analysis. Eur Radiol 30 (4): 2058-2071, 2020. [ PubMed : 31900699 ]
- Alabousi M, Wadera A, Kashif Al-Ghita M, et al.: Performance of Digital Breast Tomosynthesis, Synthetic Mammography, and Digital Mammography in Breast Cancer Screening: A Systematic Review and Meta-Analysis. J Natl Cancer Inst 113 (6): 680-690, 2021. [ PMC free article : PMC8168096 ] [ PubMed : 33372954 ]
- Pisano ED, Gatsonis C, Hendrick E, et al.: Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 353 (17): 1773-83, 2005. [ PubMed : 16169887 ]
- Pisano ED, Hendrick RE, Yaffe MJ, et al.: Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST. Radiology 246 (2): 376-83, 2008. [ PMC free article : PMC2659550 ] [ PubMed : 18227537 ]
- Kerlikowske K, Hubbard RA, Miglioretti DL, et al.: Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med 155 (8): 493-502, 2011. [ PMC free article : PMC3726800 ] [ PubMed : 22007043 ]
- van Luijt PA, Fracheboud J, Heijnsdijk EA, et al.: Nation-wide data on screening performance during the transition to digital mammography: observations in 6 million screens. Eur J Cancer 49 (16): 3517-25, 2013. [ PubMed : 23871248 ]
- Souza FH, Wendland EM, Rosa MI, et al.: Is full-field digital mammography more accurate than screen-film mammography in overall population screening? A systematic review and meta-analysis. Breast 22 (3): 217-24, 2013. [ PubMed : 23489759 ]
- Gur D, Sumkin JH, Rockette HE, et al.: Changes in breast cancer detection and mammography recall rates after the introduction of a computer-aided detection system. J Natl Cancer Inst 96 (3): 185-90, 2004. [ PubMed : 14759985 ]
- Ciatto S, Del Turco MR, Risso G, et al.: Comparison of standard reading and computer aided detection (CAD) on a national proficiency test of screening mammography. Eur J Radiol 45 (2): 135-8, 2003. [ PubMed : 12536093 ]
- Fenton JJ, Taplin SH, Carney PA, et al.: Influence of computer-aided detection on performance of screening mammography. N Engl J Med 356 (14): 1399-409, 2007. [ PMC free article : PMC3182841 ] [ PubMed : 17409321 ]
- Elmore JG, Carney PA: Computer-aided detection of breast cancer: has promise outstripped performance? J Natl Cancer Inst 96 (3): 162-3, 2004. [ PubMed : 14759974 ]
- Fenton JJ, Xing G, Elmore JG, et al.: Short-term outcomes of screening mammography using computer-aided detection: a population-based study of medicare enrollees. Ann Intern Med 158 (8): 580-7, 2013. [ PMC free article : PMC3772716 ] [ PubMed : 23588746 ]
- Lehman CD, Wellman RD, Buist DS, et al.: Diagnostic Accuracy of Digital Screening Mammography With and Without Computer-Aided Detection. JAMA Intern Med 175 (11): 1828-37, 2015. [ PMC free article : PMC4836172 ] [ PubMed : 26414882 ]
- Killelea BK, Long JB, Chagpar AB, et al.: Evolution of breast cancer screening in the Medicare population: clinical and economic implications. J Natl Cancer Inst 106 (8): , 2014. [ PMC free article : PMC4155428 ] [ PubMed : 25031307 ]
- U.S. Food and Drug Administration: Mammography Quality Standards Act (MQSA) National Statistics. Silver Spring, Md: Food and Drug Administration, 2021. Available online . Last accessed January 16, 2024.
- Richman IB, Hoag JR, Xu X, et al.: Adoption of Digital Breast Tomosynthesis in Clinical Practice. JAMA Intern Med 179 (9): 1292-1295, 2019. [ PMC free article : PMC6593646 ] [ PubMed : 31233086 ]
- Fujii MH, Herschorn SD, Sowden M, et al.: Detection Rates for Benign and Malignant Diagnoses on Breast Cancer Screening With Digital Breast Tomosynthesis in a Statewide Mammography Registry Study. AJR Am J Roentgenol 212 (3): 706-711, 2019. [ PMC free article : PMC6386608 ] [ PubMed : 30673339 ]
- Østerås BH, Martinsen ACT, Gullien R, et al.: Digital Mammography versus Breast Tomosynthesis: Impact of Breast Density on Diagnostic Performance in Population-based Screening. Radiology 293 (1): 60-68, 2019. [ PubMed : 31407968 ]
- Aase HS, Holen ÅS, Pedersen K, et al.: A randomized controlled trial of digital breast tomosynthesis versus digital mammography in population-based screening in Bergen: interim analysis of performance indicators from the To-Be trial. Eur Radiol 29 (3): 1175-1186, 2019. [ PMC free article : PMC6510877 ] [ PubMed : 30159620 ]
- Hofvind S, Holen ÅS, Aase HS, et al.: Two-view digital breast tomosynthesis versus digital mammography in a population-based breast cancer screening programme (To-Be): a randomised, controlled trial. Lancet Oncol 20 (6): 795-805, 2019. [ PubMed : 31078459 ]
- Lowry KP, Trentham-Dietz A, Schechter CB, et al.: Long-Term Outcomes and Cost-Effectiveness of Breast Cancer Screening With Digital Breast Tomosynthesis in the United States. J Natl Cancer Inst 112 (6): 582-589, 2020. [ PMC free article : PMC7301096 ] [ PubMed : 31503283 ]
- Melnikow J, Fenton JJ: Digital Breast Tomosynthesis-Diffusion Into Practice Preceding Evidence. JAMA Intern Med 179 (9): 1295-1296, 2019. [ PubMed : 31233084 ]
- Kerlikowske K, Su YR, Sprague BL, et al.: Association of Screening With Digital Breast Tomosynthesis vs Digital Mammography With Risk of Interval Invasive and Advanced Breast Cancer. JAMA 327 (22): 2220-2230, 2022. [ PMC free article : PMC9198754 ] [ PubMed : 35699706 ]
- Joensuu H, Lehtimäki T, Holli K, et al.: Risk for distant recurrence of breast cancer detected by mammography screening or other methods. JAMA 292 (9): 1064-73, 2004. [ PubMed : 15339900 ]
- Shen Y, Yang Y, Inoue LY, et al.: Role of detection method in predicting breast cancer survival: analysis of randomized screening trials. J Natl Cancer Inst 97 (16): 1195-203, 2005. [ PubMed : 16106024 ]
- Wishart GC, Greenberg DC, Britton PD, et al.: Screen-detected vs symptomatic breast cancer: is improved survival due to stage migration alone? Br J Cancer 98 (11): 1741-4, 2008. [ PMC free article : PMC2410118 ] [ PubMed : 18506175 ]
- Moody-Ayers SY, Wells CK, Feinstein AR: "Benign" tumors and "early detection" in mammography-screened patients of a natural cohort with breast cancer. Arch Intern Med 160 (8): 1109-15, 2000. [ PubMed : 10789603 ]
- Walpole E, Dunn N, Youl P, et al.: Nonbreast cancer incidence, treatment received and outcomes: Are there differences in breast screening attendees versus nonattendees? Int J Cancer 147 (3): 856-865, 2020. [ PubMed : 31808149 ]
- Carney PA, Miglioretti DL, Yankaskas BC, et al.: Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med 138 (3): 168-75, 2003. [ PubMed : 12558355 ]
- Lee MV, Konstantinoff K, Gegios A, et al.: Breast cancer malpractice litigation: A 10-year analysis and update in trends. Clin Imaging 60 (1): 26-32, 2020. [ PubMed : 31864196 ]
- Hakama M, Holli K, Isola J, et al.: Aggressiveness of screen-detected breast cancers. Lancet 345 (8944): 221-4, 1995. [ PubMed : 7741862 ]
- Tabár L, Faberberg G, Day NE, et al.: What is the optimum interval between mammographic screening examinations? An analysis based on the latest results of the Swedish two-county breast cancer screening trial. Br J Cancer 55 (5): 547-51, 1987. [ PMC free article : PMC2001715 ] [ PubMed : 3606947 ]
- Niraula S, Biswanger N, Hu P, et al.: Incidence, Characteristics, and Outcomes of Interval Breast Cancers Compared With Screening-Detected Breast Cancers. JAMA Netw Open 3 (9): e2018179, 2020. [ PMC free article : PMC7519419 ] [ PubMed : 32975573 ]
- Payne JI, Caines JS, Gallant J, et al.: A review of interval breast cancers diagnosed among participants of the Nova Scotia Breast Screening Program. Radiology 266 (1): 96-103, 2013. [ PubMed : 23169791 ]
- Buist DS, Porter PL, Lehman C, et al.: Factors contributing to mammography failure in women aged 40-49 years. J Natl Cancer Inst 96 (19): 1432-40, 2004. [ PubMed : 15467032 ]
- Banks E, Reeves G, Beral V, et al.: Influence of personal characteristics of individual women on sensitivity and specificity of mammography in the Million Women Study: cohort study. BMJ 329 (7464): 477, 2004. [ PMC free article : PMC515195 ] [ PubMed : 15331472 ]
- Yankaskas BC, Taplin SH, Ichikawa L, et al.: Association between mammography timing and measures of screening performance in the United States. Radiology 234 (2): 363-73, 2005. [ PubMed : 15670994 ]
- Duffy SW, Vulkan D, Cuckle H, et al.: Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol 21 (9): 1165-1172, 2020. [ PMC free article : PMC7491203 ] [ PubMed : 32800099 ]
- American Cancer Society: Breast Density and Your Mammogram Report. Atlanta, Ga: American Cancer Society, 2017. Available online . Last accessed date January 16, 2024.
- Elmore JG, Carney PA, Abraham LA, et al.: The association between obesity and screening mammography accuracy. Arch Intern Med 164 (10): 1140-7, 2004. [ PMC free article : PMC3143016 ] [ PubMed : 15159273 ]
- McCormack VA, dos Santos Silva I: Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15 (6): 1159-69, 2006. [ PubMed : 16775176 ]
- Pankow JS, Vachon CM, Kuni CC, et al.: Genetic analysis of mammographic breast density in adult women: evidence of a gene effect. J Natl Cancer Inst 89 (8): 549-56, 1997. [ PubMed : 9106643 ]
- Boyd NF, Dite GS, Stone J, et al.: Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med 347 (12): 886-94, 2002. [ PubMed : 12239257 ]
- White E, Velentgas P, Mandelson MT, et al.: Variation in mammographic breast density by time in menstrual cycle among women aged 40-49 years. J Natl Cancer Inst 90 (12): 906-10, 1998. [ PubMed : 9637139 ]
- Harvey JA, Pinkerton JV, Herman CR: Short-term cessation of hormone replacement therapy and improvement of mammographic specificity. J Natl Cancer Inst 89 (21): 1623-5, 1997. [ PubMed : 9362162 ]
- Laya MB, Larson EB, Taplin SH, et al.: Effect of estrogen replacement therapy on the specificity and sensitivity of screening mammography. J Natl Cancer Inst 88 (10): 643-9, 1996. [ PubMed : 8627640 ]
- Baines CJ, Dayan R: A tangled web: factors likely to affect the efficacy of screening mammography. J Natl Cancer Inst 91 (10): 833-8, 1999. [ PubMed : 10340902 ]
- Brisson J, Brisson B, Coté G, et al.: Tamoxifen and mammographic breast densities. Cancer Epidemiol Biomarkers Prev 9 (9): 911-5, 2000. [ PubMed : 11008908 ]
- Boyd NF, Greenberg C, Lockwood G, et al.: Effects at two years of a low-fat, high-carbohydrate diet on radiologic features of the breast: results from a randomized trial. Canadian Diet and Breast Cancer Prevention Study Group. J Natl Cancer Inst 89 (7): 488-96, 1997. [ PubMed : 9086005 ]
- Crouchley K, Wylie E, Khong E: Hormone replacement therapy and mammographic screening outcomes in Western Australia. J Med Screen 13 (2): 93-7, 2006. [ PubMed : 16792833 ]
- Melnikow J, Fenton JJ, Whitlock EP, et al.: Supplemental Screening for Breast Cancer in Women With Dense Breasts: A Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med 164 (4): 268-78, 2016. [ PMC free article : PMC5100826 ] [ PubMed : 26757021 ]
- Sprague BL, Gangnon RE, Burt V, et al.: Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst 106 (10): , 2014. [ PMC free article : PMC4200066 ] [ PubMed : 25217577 ]
- Ho JM, Jafferjee N, Covarrubias GM, et al.: Dense breasts: a review of reporting legislation and available supplemental screening options. AJR Am J Roentgenol 203 (2): 449-56, 2014. [ PubMed : 25055284 ]
- Ho TH, Bissell MCS, Kerlikowske K, et al.: Cumulative Probability of False-Positive Results After 10 Years of Screening With Digital Breast Tomosynthesis vs Digital Mammography. JAMA Netw Open 5 (3): e222440, 2022. [ PMC free article : PMC8956976 ] [ PubMed : 35333365 ]
- Smetana GW, Elmore JG, Lee CI, et al.: Should This Woman With Dense Breasts Receive Supplemental Breast Cancer Screening?: Grand Rounds Discussion From Beth Israel Deaconess Medical Center. Ann Intern Med 169 (7): 474-484, 2018. [ PubMed : 30285208 ]
- Comstock CE, Gatsonis C, Newstead GM, et al.: Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection Among Women With Dense Breasts Undergoing Screening. JAMA 323 (8): 746-756, 2020. [ PMC free article : PMC7276668 ] [ PubMed : 32096852 ]
- Bakker MF, de Lange SV, Pijnappel RM, et al.: Supplemental MRI Screening for Women with Extremely Dense Breast Tissue. N Engl J Med 381 (22): 2091-2102, 2019. [ PubMed : 31774954 ]
- Berg WA, Zuley ML, Chang TS, et al.: Prospective Multicenter Diagnostic Performance of Technologist-Performed Screening Breast Ultrasound After Tomosynthesis in Women With Dense Breasts (the DBTUST). J Clin Oncol 41 (13): 2403-2415, 2023. [ PMC free article : PMC10150890 ] [ PubMed : 36626696 ]
- U.S. Food and Drug Administration: Mammography Quality Standards Act. Food and Drug Administration, 2023. Available online . Last accessed January 2, 2024.
- DenseBreast-info: Legislation and Regulations for Dense Breast [News]. Deer Park, NY: DenseBreast-info, Inc., 2019. Available online . May 6, 2019.
- Wallis MG, Walsh MT, Lee JR: A review of false negative mammography in a symptomatic population. Clin Radiol 44 (1): 13-5, 1991. [ PubMed : 1873944 ]
- Tilanus-Linthorst M, Verhoog L, Obdeijn IM, et al.: A BRCA1/2 mutation, high breast density and prominent pushing margins of a tumor independently contribute to a frequent false-negative mammography. Int J Cancer 102 (1): 91-5, 2002. [ PubMed : 12353239 ]
- Ganott MA, Harris KM, Klaman HM, et al.: Analysis of False-Negative Cancer Cases Identified with a Mammography Audit. Breast J 5 (3): 166-175, 1999. [ PubMed : 11348280 ]
- Elmore JG, Jackson SL, Abraham L, et al.: Variability in interpretive performance at screening mammography and radiologists' characteristics associated with accuracy. Radiology 253 (3): 641-51, 2009. [ PMC free article : PMC2786197 ] [ PubMed : 19864507 ]
- Meyer JE, Eberlein TJ, Stomper PC, et al.: Biopsy of occult breast lesions. Analysis of 1261 abnormalities. JAMA 263 (17): 2341-3, 1990. [ PubMed : 2157903 ]
- Taplin S, Abraham L, Barlow WE, et al.: Mammography facility characteristics associated with interpretive accuracy of screening mammography. J Natl Cancer Inst 100 (12): 876-87, 2008. [ PMC free article : PMC2430588 ] [ PubMed : 18544742 ]
- Jackson SL, Taplin SH, Sickles EA, et al.: Variability of interpretive accuracy among diagnostic mammography facilities. J Natl Cancer Inst 101 (11): 814-27, 2009. [ PMC free article : PMC2689871 ] [ PubMed : 19470953 ]
- Goldman LE, Walker R, Miglioretti DL, et al.: Accuracy of diagnostic mammography at facilities serving vulnerable women. Med Care 49 (1): 67-75, 2011. [ PMC free article : PMC3689881 ] [ PubMed : 20966780 ]
- Schaffter T, Buist DSM, Lee CI, et al.: Evaluation of Combined Artificial Intelligence and Radiologist Assessment to Interpret Screening Mammograms. JAMA Netw Open 3 (3): e200265, 2020. [ PMC free article : PMC7052735 ] [ PubMed : 32119094 ]
- McKinney SM, Sieniek M, Godbole V, et al.: International evaluation of an AI system for breast cancer screening. Nature 577 (7788): 89-94, 2020. [ PubMed : 31894144 ]
- Mercan E, Mehta S, Bartlett J, et al.: Assessment of Machine Learning of Breast Pathology Structures for Automated Differentiation of Breast Cancer and High-Risk Proliferative Lesions. JAMA Netw Open 2 (8): e198777, 2019. [ PMC free article : PMC6692690 ] [ PubMed : 31397859 ]
- Adamson AS, Welch HG: Machine Learning and the Cancer-Diagnosis Problem - No Gold Standard. N Engl J Med 381 (24): 2285-2287, 2019. [ PubMed : 31826337 ]
- Smith-Bindman R, Chu PW, Miglioretti DL, et al.: Comparison of screening mammography in the United States and the United kingdom. JAMA 290 (16): 2129-37, 2003. [ PubMed : 14570948 ]
- Elmore JG, Nakano CY, Koepsell TD, et al.: International variation in screening mammography interpretations in community-based programs. J Natl Cancer Inst 95 (18): 1384-93, 2003. [ PMC free article : PMC3146363 ] [ PubMed : 13130114 ]
- Kerlikowske K, Grady D, Barclay J, et al.: Positive predictive value of screening mammography by age and family history of breast cancer. JAMA 270 (20): 2444-50, 1993. [ PubMed : 8230621 ]
- The Breast Screening Frequency Trial Group: The frequency of breast cancer screening: results from the UKCCCR Randomised Trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer 38 (11): 1458-64, 2002. [ PubMed : 12110490 ]
- White E, Miglioretti DL, Yankaskas BC, et al.: Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst 96 (24): 1832-9, 2004. [ PubMed : 15601639 ]
- Mandelblatt JS, Cronin KA, Bailey S, et al.: Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med 151 (10): 738-47, 2009. [ PMC free article : PMC3515682 ] [ PubMed : 19920274 ]
- Parvinen I, Chiu S, Pylkkänen L, et al.: Effects of annual vs triennial mammography interval on breast cancer incidence and mortality in ages 40-49 in Finland. Br J Cancer 105 (9): 1388-91, 2011. [ PMC free article : PMC3241549 ] [ PubMed : 21934688 ]
- Gøtzsche PC, Olsen O: Is screening for breast cancer with mammography justifiable? Lancet 355 (9198): 129-34, 2000. [ PubMed : 10675181 ]
- Gøtzsche PC, Nielsen M: Screening for breast cancer with mammography. Cochrane Database Syst Rev (4): CD001877, 2006. [ PubMed : 17054145 ]
- Nyström L, Andersson I, Bjurstam N, et al.: Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet 359 (9310): 909-19, 2002. [ PubMed : 11918907 ]
- Kerlikowske K: Efficacy of screening mammography among women aged 40 to 49 years and 50 to 69 years: comparison of relative and absolute benefit. J Natl Cancer Inst Monogr (22): 79-86, 1997. [ PubMed : 9709281 ]
- Glasziou PP, Woodward AJ, Mahon CM: Mammographic screening trials for women aged under 50. A quality assessment and meta-analysis. Med J Aust 162 (12): 625-9, 1995. [ PubMed : 7603372 ]
- Duffy SW, Tabár L, Chen HH, et al.: The impact of organized mammography service screening on breast carcinoma mortality in seven Swedish counties. Cancer 95 (3): 458-69, 2002. [ PubMed : 12209737 ]
- Jonsson H, Nyström L, Törnberg S, et al.: Service screening with mammography of women aged 50-69 years in Sweden: effects on mortality from breast cancer. J Med Screen 8 (3): 152-60, 2001. [ PubMed : 11678556 ]
- Autier P, Koechlin A, Smans M, et al.: Mammography screening and breast cancer mortality in Sweden. J Natl Cancer Inst 104 (14): 1080-93, 2012. [ PubMed : 22811439 ]
- Broeders MJ, Peer PG, Straatman H, et al.: Diverging breast cancer mortality rates in relation to screening? A comparison of Nijmegen to Arnhem and the Netherlands, 1969-1997. Int J Cancer 92 (2): 303-8, 2001. [ PubMed : 11291061 ]
- Verbeek AL, Hendriks JH, Holland R, et al.: Reduction of breast cancer mortality through mass screening with modern mammography. First results of the Nijmegen project, 1975-1981. Lancet 1 (8388): 1222-4, 1984. [ PubMed : 6144933 ]
- Elmore JG, Reisch LM, Barton MB, et al.: Efficacy of breast cancer screening in the community according to risk level. J Natl Cancer Inst 97 (14): 1035-43, 2005. [ PubMed : 16030301 ]
- Autier P, Boniol M, Gavin A, et al.: Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database. BMJ 343: d4411, 2011. [ PMC free article : PMC3145837 ] [ PubMed : 21798968 ]
- Harris R, Yeatts J, Kinsinger L: Breast cancer screening for women ages 50 to 69 years a systematic review of observational evidence. Prev Med 53 (3): 108-14, 2011. [ PubMed : 21820465 ]
- Kerlikowske K, Zhu W, Hubbard RA, et al.: Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med 173 (9): 807-16, 2013. [ PMC free article : PMC3699693 ] [ PubMed : 23552817 ]
- Coldman A, Phillips N, Wilson C, et al.: Pan-Canadian study of mammography screening and mortality from breast cancer. J Natl Cancer Inst 106 (11): , 2014. [ PubMed : 25274578 ]
- Berry DA, Cronin KA, Plevritis SK, et al.: Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353 (17): 1784-92, 2005. [ PubMed : 16251534 ]
- Plevritis SK, Munoz D, Kurian AW, et al.: Association of Screening and Treatment With Breast Cancer Mortality by Molecular Subtype in US Women, 2000-2012. JAMA 319 (2): 154-164, 2018. [ PMC free article : PMC5833658 ] [ PubMed : 29318276 ]
- Pace LE, Keating NL: A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 311 (13): 1327-35, 2014. [ PubMed : 24691608 ]
- Welch HG, Passow HJ: Quantifying the benefits and harms of screening mammography. JAMA Intern Med 174 (3): 448-54, 2014. [ PubMed : 24380095 ]
- Miller AB, To T, Baines CJ, et al.: The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up. A randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med 137 (5 Part 1): 305-12, 2002. [ PubMed : 12204013 ]
- Tabár L, Vitak B, Chen TH, et al.: Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 260 (3): 658-63, 2011. [ PubMed : 21712474 ]
- Braithwaite D, Zhu W, Hubbard RA, et al.: Screening outcomes in older US women undergoing multiple mammograms in community practice: does interval, age, or comorbidity score affect tumor characteristics or false positive rates? J Natl Cancer Inst 105 (5): 334-41, 2013. [ PMC free article : PMC3589257 ] [ PubMed : 23385442 ]
- Zackrisson S, Andersson I, Janzon L, et al.: Rate of over-diagnosis of breast cancer 15 years after end of Malmö mammographic screening trial: follow-up study. BMJ 332 (7543): 689-92, 2006. [ PMC free article : PMC1410836 ] [ PubMed : 16517548 ]
- Welch HG, Black WC: Using autopsy series to estimate the disease "reservoir" for ductal carcinoma in situ of the breast: how much more breast cancer can we find? Ann Intern Med 127 (11): 1023-8, 1997. [ PubMed : 9412284 ]
- Black WC, Welch HG: Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med 328 (17): 1237-43, 1993. [ PubMed : 8464435 ]
- Duffy SW, Lynge E, Jonsson H, et al.: Complexities in the estimation of overdiagnosis in breast cancer screening. Br J Cancer 99 (7): 1176-8, 2008. [ PMC free article : PMC2567065 ] [ PubMed : 18766185 ]
- Gøtzsche PC, Jørgensen KJ, Maehlen J, et al.: Estimation of lead time and overdiagnosis in breast cancer screening. Br J Cancer 100 (1): 219; author reply 220, 2009. [ PMC free article : PMC2634697 ] [ PubMed : 19127274 ]
- Nelson HD, Pappas M, Cantor A, et al.: Harms of Breast Cancer Screening: Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med 164 (4): 256-67, 2016. [ PubMed : 26756737 ]
- Jørgensen KJ, Gøtzsche PC, Kalager M, et al.: Breast Cancer Screening in Denmark: A Cohort Study of Tumor Size and Overdiagnosis. Ann Intern Med 166 (5): 313-323, 2017. [ PubMed : 28114661 ]
- Autier P, Boniol M, Koechlin A, et al.: Effectiveness of and overdiagnosis from mammography screening in the Netherlands: population based study. BMJ 359: j5224, 2017. [ PMC free article : PMC5712859 ] [ PubMed : 29208760 ]
- Lousdal ML, Kristiansen IS, Møller B, et al.: Effect of organised mammography screening on stage-specific incidence in Norway: population study. Br J Cancer 114 (5): 590-6, 2016. [ PMC free article : PMC4782212 ] [ PubMed : 26835975 ]
- Harding C, Pompei F, Burmistrov D, et al.: Breast Cancer Screening, Incidence, and Mortality Across US Counties. JAMA Intern Med 175 (9): 1483-9, 2015. [ PubMed : 26147578 ]
- Esserman LJ, Yau C, Thompson CK, et al.: Use of Molecular Tools to Identify Patients With Indolent Breast Cancers With Ultralow Risk Over 2 Decades. JAMA Oncol 3 (11): 1503-1510, 2017. [ PMC free article : PMC5710197 ] [ PubMed : 28662222 ]
- Baines CJ, To T, Miller AB: Revised estimates of overdiagnosis from the Canadian National Breast Screening Study. Prev Med 90: 66-71, 2016. [ PubMed : 27374944 ]
- Christiansen CL, Wang F, Barton MB, et al.: Predicting the cumulative risk of false-positive mammograms. J Natl Cancer Inst 92 (20): 1657-66, 2000. [ PubMed : 11036111 ]
- Lilleborge M, Falk RS, Russnes H, et al.: Risk of breast cancer by prior screening results among women participating in BreastScreen Norway. Cancer 125 (19): 3330-3337, 2019. [ PubMed : 31206638 ]
- Freitas R, Fiori WF, Ramos FJ, et al.: [Discomfort and pain during mammography]. Rev Assoc Med Bras 52 (5): 333-6, 2006 Sep-Oct. [ PubMed : 17160308 ]
- Armstrong K, Moye E, Williams S, et al.: Screening mammography in women 40 to 49 years of age: a systematic review for the American College of Physicians. Ann Intern Med 146 (7): 516-26, 2007. [ PubMed : 17404354 ]
- Swift M, Morrell D, Massey RB, et al.: Incidence of cancer in 161 families affected by ataxia-telangiectasia. N Engl J Med 325 (26): 1831-6, 1991. [ PubMed : 1961222 ]
- Kopans DB: Mammography and radiation risk. In: Janower ML, Linton OW, eds.: Radiation Risk: a Primer. American College of Radiology, 1996, pp 21-22.
- Feig SA, Ehrlich SM: Estimation of radiation risk from screening mammography: recent trends and comparison with expected benefits. Radiology 174 (3 Pt 1): 638-47, 1990. [ PubMed : 2305043 ]
- Helzlsouer KJ, Harris EL, Parshad R, et al.: Familial clustering of breast cancer: possible interaction between DNA repair proficiency and radiation exposure in the development of breast cancer. Int J Cancer 64 (1): 14-7, 1995. [ PubMed : 7665242 ]
- Suleiman OH, Spelic DC, McCrohan JL, et al.: Mammography in the 1990s: the United States and Canada. Radiology 210 (2): 345-51, 1999. [ PubMed : 10207413 ]
- Miglioretti DL, Lange J, van den Broek JJ, et al.: Radiation-Induced Breast Cancer Incidence and Mortality From Digital Mammography Screening: A Modeling Study. Ann Intern Med 164 (4): 205-14, 2016. [ PMC free article : PMC4878445 ] [ PubMed : 26756460 ]
- Lerman C, Trock B, Rimer BK, et al.: Psychological side effects of breast cancer screening. Health Psychol 10 (4): 259-67, 1991. [ PubMed : 1915212 ]
- Sandin B, Chorot P, Valiente RM, et al.: Adverse psychological effects in women attending a second-stage breast cancer screening. J Psychosom Res 52 (5): 303-9, 2002. [ PubMed : 12023127 ]
- Brodersen J, Siersma VD: Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med 11 (2): 106-15, 2013 Mar-Apr. [ PMC free article : PMC3601385 ] [ PubMed : 23508596 ]
- Gram IT, Lund E, Slenker SE: Quality of life following a false positive mammogram. Br J Cancer 62 (6): 1018-22, 1990. [ PMC free article : PMC1971570 ] [ PubMed : 2257206 ]
- Burman ML, Taplin SH, Herta DF, et al.: Effect of false-positive mammograms on interval breast cancer screening in a health maintenance organization. Ann Intern Med 131 (1): 1-6, 1999. [ PubMed : 10391809 ]
- Pisano ED, Earp J, Schell M, et al.: Screening behavior of women after a false-positive mammogram. Radiology 208 (1): 245-9, 1998. [ PubMed : 9646820 ]
- Brewer NT, Salz T, Lillie SE: Systematic review: the long-term effects of false-positive mammograms. Ann Intern Med 146 (7): 502-10, 2007. [ PubMed : 17404352 ]
- Other Imaging Modalities: Ultrasound, Magnetic Resonance Imaging (MRI), and Thermography
Ultrasound is used for the diagnostic evaluation of palpable or mammographically identified masses, rather than serving as a primary screening modality. A review of the literature and expert opinion by the European Group for Breast Cancer Screening concluded that “there is little evidence to support the use of ultrasound in population breast cancer screening at any age.”[ 1 ] The Japan Strategic Anti-cancer Randomized Trial (J-START) is a screening trial that randomly assigned women aged 40 to 49 years to either mammography and ultrasound screening (intervention group) or mammography screening alone (control group). The initial results of this trial indicated that supplemental screening with ultrasound (i.e., mammography + ultrasound versus mammography alone) increased the detection rate of early-stage breast cancers, but its effect on mortality is not clear at this time.[ 2 ]
Breast MRI is used in women for diagnostic evaluation, including evaluating the integrity of silicone breast implants, assessing palpable masses after surgery or radiation therapy, detecting mammographically and sonographically occult breast cancer in patients with axillary nodal metastasis, and preoperative planning for some patients with known breast cancer. There is no ionizing radiation exposure with this procedure. MRI has been promoted as a screening test for breast cancer among women at elevated risk of breast cancer based on BRCA1/2 mutation carriers, a strong family history of breast cancer, or several genetic syndromes, such as Li-Fraumeni syndrome or Cowden disease.[ 3 - 5 ] Breast MRI is more sensitive but less specific than screening mammography [ 6 , 7 ] and is up to 35 times as expensive.[ 8 - 12 ]
Thermography
Using infrared imaging techniques, thermography of the breast identifies temperature changes in the skin as a possible indicator of an underlying tumor, displaying these changes in color patterns. Thermographic devices have been approved by the U.S. Food and Drug Administration under the 510(k) process, but no randomized trials have compared thermography to other screening modalities. Small cohort studies do not suggest any additional benefit for the use of thermography as an adjunct modality.[ 13 , 14 ]
- Teh W, Wilson AR: The role of ultrasound in breast cancer screening. A consensus statement by the European Group for Breast Cancer Screening. Eur J Cancer 34 (4): 449-50, 1998. [ PubMed : 9713292 ]
- Ohuchi N, Suzuki A, Sobue T, et al.: Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet 387 (10016): 341-348, 2016. [ PubMed : 26547101 ]
- Warner E, Plewes DB, Hill KA, et al.: Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 292 (11): 1317-25, 2004. [ PubMed : 15367553 ]
- Kriege M, Brekelmans CT, Boetes C, et al.: Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 351 (5): 427-37, 2004. [ PubMed : 15282350 ]
- Warner E, Hill K, Causer P, et al.: Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol 29 (13): 1664-9, 2011. [ PMC free article : PMC4874196 ] [ PubMed : 21444874 ]
- Lord SJ, Lei W, Craft P, et al.: A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer 43 (13): 1905-17, 2007. [ PubMed : 17681781 ]
- Lehman CD, Gatsonis C, Kuhl CK, et al.: MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 356 (13): 1295-303, 2007. [ PubMed : 17392300 ]
- Pataky R, Armstrong L, Chia S, et al.: Cost-effectiveness of MRI for breast cancer screening in BRCA1/2 mutation carriers. BMC Cancer 13: 339, 2013. [ PMC free article : PMC3711845 ] [ PubMed : 23837641 ]
- Saadatmand S, Tilanus-Linthorst MM, Rutgers EJ, et al.: Cost-effectiveness of screening women with familial risk for breast cancer with magnetic resonance imaging. J Natl Cancer Inst 105 (17): 1314-21, 2013. [ PubMed : 23940285 ]
- Ahern CH, Shih YC, Dong W, et al.: Cost-effectiveness of alternative strategies for integrating MRI into breast cancer screening for women at high risk. Br J Cancer 111 (8): 1542-51, 2014. [ PMC free article : PMC4200098 ] [ PubMed : 25137022 ]
- Pistolese CA, Ciarrapico AM, della Gatta F, et al.: Inappropriateness of breast imaging: cost analysis. Radiol Med 118 (6): 984-94, 2013. [ PubMed : 23801396 ]
- Cott Chubiz JE, Lee JM, Gilmore ME, et al.: Cost-effectiveness of alternating magnetic resonance imaging and digital mammography screening in BRCA1 and BRCA2 gene mutation carriers. Cancer 119 (6): 1266-76, 2013. [ PMC free article : PMC3586945 ] [ PubMed : 23184400 ]
- Wishart GC, Campisi M, Boswell M, et al.: The accuracy of digital infrared imaging for breast cancer detection in women undergoing breast biopsy. Eur J Surg Oncol 36 (6): 535-40, 2010. [ PubMed : 20452740 ]
- Arora N, Martins D, Ruggerio D, et al.: Effectiveness of a noninvasive digital infrared thermal imaging system in the detection of breast cancer. Am J Surg 196 (4): 523-6, 2008. [ PubMed : 18809055 ]
- Nonimaging Screening Modalities
Clinical Breast Examination
The effect of screening clinical breast examination (CBE) on breast cancer mortality has not been fully established. The Canadian National Breast Screening Study (CNBSS) compared high-quality CBE plus mammography with CBE alone in women aged 50 to 59 years. CBE, lasting 5 to 10 minutes per breast, was conducted by trained health professionals, with periodic evaluations of performance quality. The frequency of cancer diagnosis, stage, interval cancers, and breast cancer mortality were similar in the two groups and similar to outcomes with mammography alone.[ 1 ] With a mean follow-up of 13 years, breast cancer mortality was similar in the two groups (mortality rate ratio, 1.02; 95% confidence interval [CI], 0.78–1.33).[ 2 ] The investigators estimated the operating characteristics for CBE alone; for 19,965 women aged 50 to 59 years, sensitivity was 83%, 71%, 57%, 83%, and 77% for years 1, 2, 3, 4, and 5 of the trial, respectively; specificity ranged between 88% and 96%. Positive predictive value (PPV), which is the proportion of cancers detected per abnormal examination, was estimated to be 3% to 4%. For 25,620 women aged 40 to 49 years who were examined only at entry, the estimated sensitivity was 71%, specificity was 84%, and PPV was 1.5%.[ 3 ]
In clinical trials involving community clinicians, CBE-type screening had higher specificity (97%–99%) [ 4 ] and lower sensitivity (22%–36%) than that experienced by examiners.[ 5 - 8 ] A study of screening in women with a positive family history of breast cancer showed that, after a normal initial evaluation, the patient herself, or her clinician performing a CBE, identified more cancers than did mammography.[ 9 ]
Another study examined the usefulness of adding CBE to screening mammography; among 61,688 women older than 40 years and screened by mammography and CBE, sensitivity for mammography was 78%, and combined mammography-CBE sensitivity was 82%. Specificity was lower for women undergoing both screening modalities than it was for women undergoing mammography alone (97% vs. 99%).[ 10 ] Another study reported the results of a large cluster randomized controlled trial in India that assessed the efficacy of screening with CBE versus no screening on breast cancer mortality.[ 11 ] This trial recruited 151,538 women aged 35 to 64 years with no history of breast cancer. After 20 years of follow-up, there was an overall statistically nonsignificant 15% reduction in breast cancer mortality in the screening with CBE arm versus the control arm, but a post hoc subset analysis demonstrated a statistically significant 30% relative reduction in mortality attributable to screening with CBE for women older than 50 years. However, the results of the subset analysis should be interpreted with caution, as this was a cluster randomized trial with only 20 clusters, which raises concerns about potential imbalances between the control and study arms of the trial. Other international trials of CBE are under way, one in India and one in Egypt.
Monthly BSE has been promoted, but there is no evidence that it reduces breast cancer mortality.[ 12 , 13 ] The only large, randomized clinical trial of BSE assigned 266,064 female Shanghai factory workers to either BSE instruction with reinforcement and encouragement, or instruction on the prevention of lower back pain. Neither group underwent any other breast cancer screening. After 10 to 11 years of follow-up, 135 breast cancer deaths occurred in the instruction group, and 131 cancer deaths occurred in the control group (relative risk [RR], 1.04; 95% CI, 0.82–1.33). Although the number of invasive breast cancers diagnosed in the two groups was about the same, women in the instruction group had more breast biopsies and more benign lesions diagnosed than did women in the control group.[ 14 ]
Other research results on BSE come from three trials. First, more than 100,000 Leningrad women were assigned to BSE training or control by cluster randomization; the BSE group training had more breast biopsies without improved breast cancer mortality.[ 15 ] Second, in the United Kingdom Trial of Early Detection of Breast Cancer, more than 63,500 women aged 45 to 64 years were invited to educational sessions about BSE. After 10 years of follow-up, breast cancer mortality rates were similar to the rates in centers without organized BSE education (RR, 1.07; 95% CI, 0.93–1.22).[ 16 ] Thirdly, in contrast, a case-control study nested within the CNBSS compared self-reported BSE frequency before enrollment with breast cancer mortality. Women who examined their breasts visually, used their finger pads for palpation, and used their three middle fingers had a lower breast cancer mortality rate.[ 17 ]
Tissue Sampling (Fine-Needle Aspiration, Nipple Aspirate, Ductal Lavage)
Various methods to analyze breast tissue for malignancy have been proposed to screen for breast cancer, but none have been associated with mortality reduction.
- Baines CJ: The Canadian National Breast Screening Study: a perspective on criticisms. Ann Intern Med 120 (4): 326-34, 1994. [ PubMed : 8291826 ]
- Baines CJ, Miller AB, Bassett AA: Physical examination. Its role as a single screening modality in the Canadian National Breast Screening Study. Cancer 63 (9): 1816-22, 1989. [ PubMed : 2702588 ]
- Fenton JJ, Barton MB, Geiger AM, et al.: Screening clinical breast examination: how often does it miss lethal breast cancer? J Natl Cancer Inst Monogr (35): 67-71, 2005. [ PubMed : 16287888 ]
- Bobo JK, Lee NC, Thames SF: Findings from 752,081 clinical breast examinations reported to a national screening program from 1995 through 1998. J Natl Cancer Inst 92 (12): 971-6, 2000. [ PubMed : 10861308 ]
- Oestreicher N, White E, Lehman CD, et al.: Predictors of sensitivity of clinical breast examination (CBE). Breast Cancer Res Treat 76 (1): 73-81, 2002. [ PubMed : 12408378 ]
- Kolb TM, Lichy J, Newhouse JH: Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 225 (1): 165-75, 2002. [ PubMed : 12355001 ]
- Gui GP, Hogben RK, Walsh G, et al.: The incidence of breast cancer from screening women according to predicted family history risk: Does annual clinical examination add to mammography? Eur J Cancer 37 (13): 1668-73, 2001. [ PubMed : 11527694 ]
- Oestreicher N, Lehman CD, Seger DJ, et al.: The incremental contribution of clinical breast examination to invasive cancer detection in a mammography screening program. AJR Am J Roentgenol 184 (2): 428-32, 2005. [ PubMed : 15671358 ]
- Mittra I, Mishra GA, Dikshit RP, et al.: Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai. BMJ 372: n256, 2021. [ PMC free article : PMC7903383 ] [ PubMed : 33627312 ]
- Baxter N; Canadian Task Force on Preventive Health Care: Preventive health care, 2001 update: should women be routinely taught breast self-examination to screen for breast cancer? CMAJ 164 (13): 1837-46, 2001. [ PMC free article : PMC81191 ] [ PubMed : 11450279 ]
- Humphrey LL, Helfand M, Chan BK, et al.: Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 137 (5 Part 1): 347-60, 2002. [ PubMed : 12204020 ]
- Semiglazov VF, Moiseyenko VM, Bavli JL, et al.: The role of breast self-examination in early breast cancer detection (results of the 5-years USSR/WHO randomized study in Leningrad). Eur J Epidemiol 8 (4): 498-502, 1992. [ PubMed : 1397215 ]
- Ellman R, Moss SM, Coleman D, et al.: Breast cancer mortality after 10 years in the UK trial of early detection of breast cancer. UK Trial of Early Detection of Breast Cancer Group. The Breast 2 (1): 13-20, 1993.
- Harvey BJ, Miller AB, Baines CJ, et al.: Effect of breast self-examination techniques on the risk of death from breast cancer. CMAJ 157 (9): 1205-12, 1997. [ PMC free article : PMC1228347 ] [ PubMed : 9361639 ]
- Appendix of Randomized Controlled Trials
Health Insurance Plan, United States 1963 [ 1 , 2 ]
- Age at entry: 40 to 64 years.
- Randomization: Individual, but with significant imbalances in the distribution of women between assigned arms, as evidenced by menopausal status ( P < .0001) and education ( P = .05).
- Sample size: 30,000 to 31,092 in study group and 30,565 to 30,765 in control group.
- Consistency of reports: Variation in sample size reports.
- Intervention: Annual two-view mammography (MMG) and clinical breast examination (CBE) for 3 years.
- Control: Usual care.
- Compliance: Nonattenders to first screening (35% of the screened population) were not reinvited.
- Contamination: Screening MMG was not available outside the trial; frequency of CBE performance among control women is unknown.
- Cause of death attribution: Women who died of breast cancer that had been diagnosed before entry into the study were excluded from the comparison between the screening and control groups. However, these exclusions were determined differently within the two groups. Women in the screening group were excluded based on determinations made during the study period at their initial screening visits. These women were dropped from all further consideration in the study. By design, controls did not have regular clinic visits, so the prestudy cancer status of control patients was not determined. When a control patient died and her cause of death was determined to be breast cancer, a retrospective examination was made to determine the date of diagnosis of her disease. If the date preceded the study period, the control patient was excluded from the analysis. This difference in methodology has the potential for a substantial bias when comparing breast cancer mortality between the two groups, and this bias is likely to favor screening.
- Analysis: Follow-up.
- External audit: No.
- Follow-up duration: 18 years.
- Relative risk of breast cancer death, screening versus control (95% confidence interval [CI]): 0.71 (0.55–0.93) at 10 years and 0.77 (0.61–0.97) at 15 years.
- Comments: The MMGs were of poor quality compared with those of later trials, because of outdated equipment and techniques. The intervention consisted of both MMG and CBE. Major concerns about trial performance are the validity of the initial randomization and the differential exclusion of women with a prior history of breast cancer.
Malmo, Sweden 1976 [ 3 , 4 ]
- Age at entry: 45 to 69 years.
- Randomization: Individual, within each birth-year cohort for the first phase, MMG screening trial (MMST I). Individual for the entire birth cohort 1933 to 1945 for MMST II but with variations imposed by limited resources. Validation by analysis of age in both groups shows no significant difference.
- Exclusions: In a Swedish meta-analysis, there were 393 women with preexisting breast cancer excluded from the intervention group and 412 from the control group. Overall, however, 86 more women were excluded from the intervention group than from the control group.
- Sample size: 21,088 study and 21,195 control.
- Consistency of reports: No variation in patient numbers.
- Intervention: Two-view MMG every 18 to 24 months × 5.
- Control: Usual care, with MMG at study end.
- Compliance: Participants migrating from Malmo (2% per year) were not followed. The participation rate of study women was 74% for the first round and 70% for subsequent rounds.
- Contamination: 24% of all control women had at least one MMG, as did 35% of the control women aged 45 to 49 years.
- Cause of death attribution: 76% autopsy rate in early report, lower rate later. Cause of death assessment blinded for women with a breast cancer diagnosis. Linked to Swedish Cause of Death Registry.
- Analysis: Evaluation, initially. Follow-up analysis, as part of the Swedish meta-analysis.[ 5 ]
- Follow-up duration: 12 years.
- Relative risk of breast cancer death, screening versus control (95% CI): 0.81 (0.62–1.07).
- Comments: Evaluation analysis required a correction factor for the delay in the performance of MMG in the control group. The two Malmo trials, MMST I and MMST II, have been combined for most analyses.
Östergötland (County E of Two-County Trial), Sweden 1977 [ 6 - 8 ]
- Age at entry: 40 to 74 years.
- Randomization: Geographic cluster, with stratification for residence (urban or rural), socioeconomic factors, and size. Baseline breast cancer incidence and mortality were comparable between the randomly assigned geographic clusters. The study women were older than the control women, P < .0001, which would not have had a major effect on the outcome of the trial.
- Exclusions: Women with preexisting breast cancer were excluded from both groups, but the numbers were reported differently in different publications. The Swedish meta-analysis excluded all women with a prior breast cancer diagnosis, regardless of group assignment.
- Sample size: Variably reported, ranging from 38,405 to 39,034 in the study and from 37,145 to 37,936 in the control.
- Consistency of reports: Variable.
- Intervention: Three single-view MMGs every 2 years for women younger than 50 years and every 33 months for women 50 years and older.
- Compliance: 89% screened.
- Contamination: 13% of women in the Two-County trial had MMG as part of routine care, mostly in 1983 and 1984.
- Cause of death attribution: Determined by a team of local physicians. When results were recalculated in the Swedish meta-analysis, using data from the Swedish Cause of Death Registry, there was less benefit for screening than had been previously reported.
- Analysis: Evaluation initially, with correction for delay in control group MMG. Follow-up analysis, as part of the Swedish meta-analysis.[ 5 ]
- External audit: No. However, breast cancer cases and deaths were adjudicated by a Swedish panel that included the trial's investigators.[ 9 ]
- Relative risk of breast cancer death, screening versus control (95% CI): 0.82 (0.64–1.05), Östergötland.
- Comments: Concerns were raised about the randomization methodology and the evaluation analysis, which required a correction for late performance of the control group MMG. The Swedish meta-analysis resolved these questions appropriately.
Kopparberg (County W of Two-County Trial), Sweden 1977 [ 6 - 8 ]
- Randomization: Geographic cluster, with stratification for residence (urban or rural), socioeconomic factors, and size. The process for randomization has not been described. The study women were older than the control women, P < .0001, but this would not have had a major effect on the outcome of the trial.
- Exclusions: Women with preexisting breast cancer were excluded from both groups, but the numbers were reported differently in different publications.
- Sample size: Variably reported, ranging from 38,562 to 39,051 in intervention and from 18,478 to 18,846 in control.
- Intervention: Three single-view MMGs every 2 years for women younger than 50 years and every 33 months for women aged 50 years and older.
- Compliance: 89% participation.
- Contamination: 13% of women in the Two-County trial had MMG as part of routine care, mostly between 1983 and 1984.
- Cause of death attribution: Determined by a team of local physicians (see Östergötland).
- Analysis: Evaluation.
- Relative risk of breast cancer death, screening versus control (95% CI): 0.68 (0.52–0.89).
Edinburgh, United Kingdom 1976 [ 10 ]
- Age at entry: 45 to 64 years.
- Randomization: Cluster by physician practices, though many randomization assignments were changed after study start. Within each practice, there was inconsistent recruitment of women, according to the physician’s judgment about each woman’s suitability for the trial. Large differences in socioeconomic status between practices were not recognized until after the study end.
- Exclusions: More women (338) with preexisting breast cancer were excluded from the intervention group than from the control group (177).
- Sample size: 23,226 study and 21,904 control.
- Consistency of reports: Good.
- Intervention: Initially, two-view MMG and CBE; then annual CBE, with single-view MMG in years 3, 5, and 7.
- Compliance: 61% screened.
- Contamination: None.
- Cause of death attribution: Cancer Registry Data.
- Follow-up duration: 10 years.
- Relative risk of breast cancer death, screening versus control (95% CI): 0.84 (0.63–1.12).
- Comments: Randomization process was flawed. Socioeconomic differences between study and control groups probably account for the higher all-cause mortality in control women compared with screened women. This difference in all-cause mortality was four times greater than the breast cancer mortality in the control group, and therefore, may account for the higher breast cancer mortality in the control group compared with screened women. Although a correction factor was used in the final analysis, this may not adjust the analysis sufficiently.
The study design and conduct make these results difficult to assess or combine with the results of other trials.
National Breast Screening Study (NBSS)-1, Canada 1980 [ 11 ]
- Age at entry: 40 to 49 years.
- Randomization: Individual volunteers, with names entered successively on allocation lists. Although criticisms of the randomization procedure have been made, a thorough independent review found no evidence of subversion and that subversion on a scale large enough to affect the results was unlikely.[ 12 ]
- Exclusions: Few, balanced between groups.
- Sample size: 25,214 study (100% screened after entry CBE) and 25,216 control.
- Intervention: Annual two-view MMG and CBE for 4 to 5 years.
- Compliance: Initially 100%, decreased to 85.5% by screen five.
- Contamination: 26.4% in usual care group.
- Cause of death attribution: Death certificates, with review of questionable cases by a blinded review panel. Also linked with the Canadian Mortality Data Base, Statistics Canada.
- External audit: Yes. Independent, with analysis of data by several reviewers.
- Follow-up duration: 25 years.
- Relative risk of breast cancer death, screening versus control (95% CI): 1.09 (0.80–1.49).
- Comments: This is the only trial specifically designed to study women aged 40 to 49 years. Cancers diagnosed at entry in both study and control groups were included. Concerns were expressed before the completion of the trial about the technical adequacy of the MMGs, the training of the radiologists, and the standardization of the equipment, which prompted an independent external review. The primary deficiency identified by this review was the use of the mediolateral view from 1980 to 1985 instead of the mediolateral oblique view, which was used after 1985.[ 13 ] Subsequent analyses found the size and stage of the cancers detected mammographically in this trial to be equivalent to those of other trials.[ 14 ] This trial and NBSS-2 differ from the other randomized controlled trials (RCTs) in the consistent use of adjuvant hormone therapy and chemotherapy following local breast cancer therapy in women with axillary node-positive disease.
NBSS-2, Canada 1980 [ 15 ]
- Age at entry: 50 to 59 years.
- Randomization: Individual volunteer (see NBSS-1 ).
- Sample size: 19,711 study (100% screened after entry CBE) and 19,694 control.
- Intervention: Annual two-view MMG and CBE.
- Control: Annual CBE.
- Compliance: Initially 100%, decreased to 86.7% by screen five in the MMG and CBE group. Initially 100%, decreased to 85.4% by screen five in the CBE only group.
- Contamination: 16.9% of the CBE only group.
- External audit: Yes. Independent with analysis of data by several reviewers.
- Relative risk of breast cancer death, screening versus control: 1.02 (95% CI, 0.77–1.36)
- Comments: This trial is unique in that it compares one screening modality to another and does not include an unscreened control. Regarding criticisms and comments about this trial, see NBSS-1 .
Stockholm, Sweden 1981 [ 16 ]
- Randomization: Cluster by birth date. There were two subtrials with balanced randomization in the first and a significant imbalance in the second, with 508 more women in the screened group than the control.
- Exclusions: Inconsistently reported.
- Sample size: Between published reports, the size declined from 40,318 to 38,525 in the intervention group and rose from 19,943 to 20,978 in the control group.
- Intervention: Single-view MMG every 28 months × 2.
- Control: MMG at year 5.
- Compliance: 82% screened.
- Contamination: 25% of women entering the study had MMG in the 3 years before entry.
- Cause of death attribution: Linked to Swedish Cause of Death Registry.
- Analysis: Evaluation, with 1-year delay in the post-trial MMG in the control group. Follow-up analysis as part of the Swedish meta-analysis.[ 5 ]
- Follow-up duration: 8 years.
- Relative risk of breast cancer death, screening versus control (95% CI): 0.80 (0.53–1.22).
- Comments: Concerns exist about randomization, especially in the second subtrial, exclusions, and the delay in control group MMG. Inclusion of these data in the Swedish meta-analysis resolves many of these questions.
Gothenburg, Sweden 1982
- Age at entry: 39 to 59 years.
- Randomization: Complex; cluster randomly assigned within birth year by day of birth for older group (aged 50–59 years) and by individual for younger group (aged 39–49 years); ratio of study to control varied by year depending on MMG availability (randomization took place, 1982–1984).
- Exclusions: A similar proportion of women were excluded from both groups for prior breast cancer diagnosis (1.2% each).
- Sample size: Most recent publication: 21,650 invited; 29,961 controls.
- Intervention: Initial two-view MMG, then single-view MMG every 18 months × 4. Single-read first three rounds, then double-read.
- Control: Control group received one screening exam approximately 3 to 8 months after the final screen in study group.
- Cause of death attribution: Linked to Swedish Cause of Death Registry; also used an independent end point committee.
- Analysis: Both evaluation and follow-up methods.[ 5 ]
- Follow-up duration: 12 to 14 years.
- Relative risk of breast cancer death, screening versus control (95% CI): Aged 39 to 59 years: 0.79 (0.58–1.08) [evaluation]; 0.77 (0.60–1.00) [follow-up].
- Comments: No reduction for women aged 50 to 54 years, but similar reductions for other 5-year age groups.
- Conclusions: Delay in the performance of MMG in the control group and unequal numbers of women in invited and control groups (complex randomization process) complicates interpretation.
AGE Trial [ 17 , 18 ]
- Age at entry: 39 to 41 years.
- Randomization: Individuals from lists of general practitioners in geographically defined areas of England, Wales, and Scotland; allocation was concealed.
- Exclusions: Small (n = 30 in invited group and n = 51 in not invited group) number excluded in each group because individuals could not be located or were deceased.
- Sample size: 160,921 (53,884 invited; 106,956 not invited).
- Consistency of reports: Not applicable.
- Intervention: Invited group aged 48 years and younger were offered annual screening by MMG (double-view first screen, then single mediolateral oblique view thereafter); 68% accepted first screening and 69% to 70% were reinvited (81% attended at least one screen).
- Control: Those who were not invited received usual medical care, unaware of their participation, and few were screened before randomization.
- Cause of death attribution: From the National Health Service (NHS) central register, death certificate code accepted.
- Analysis: Follow-up method was intention-to-treat (although all women aged 50 years would be offered screening by NHS).
- External audit: None.
- Follow-up duration: 10.7 years.
- Relative risk of breast cancer death, screening versus control (95% CI): 0.83 (0.66–1.04).
- Conclusions: Not a statistically significant result but fits with other studies.
- Follow-up duration: Restricted to 10 years from randomization.
- Relative risk of breast cancer death, screening versus control (95% CI): 0.75 (0.58–0.97).
- Conclusions: A statistically significant result.
- Follow-up duration: Median 17.7 years.
- Relative risk of breast cancer death, screening versus control (95% CI): 0.88 (0.74–1.04).
- Conclusions: Not a statistically significant result.
- Relative risk of all-cause mortality, screening versus control (95% CI): 0.98 (0.93–1.03).
The United Kingdom Age Trial, a large RCT, compared the effect of mammographic screening on breast cancer mortality in women invited for annual mammography aged 40 years and older when compared with NHS screening programs that began at age 50 years. The primary end point of the AGE Trial was mortality from breast cancer diagnosed during the intervention period until immediately before participants’ first NHS screening. This trial remains the only trial designed specifically to study the effect of mammographic screening starting at age 40 years and is one of three RCTs, which the Cochrane group’s 2013 meta-analysis deemed adequately randomized.
In 2006, the AGE Trial published results of breast cancer mortality at a mean follow-up at 10.7 years: a reduction in breast cancer mortality in the intervention group, which did not reach statistical significance (105 breast cancer deaths in intervention group vs. 251 breast cancer death in control group).
In 2015, the AGE Trial published results of breast cancer mortality at a median follow-up of 17.7 years: no statistically significant reduction after more than 10 years of follow-up and no statistically significant decrease in all-cause mortality. At this time, it also published results of a reanalysis of the original data set: a small, transient, statistically significant reduction in breast cancer mortality in the intervention group during the first 10 years after randomization (83 breast cancer deaths in intervention group vs. 219 breast cancer death in control group).
In 2020, the AGE Trial published final results based on median follow-up of 22.9 years including:
- Positive effect in the first 10 years after randomization. The absolute difference in breast cancer mortality was -0.6 deaths per 1,667 women in the 40 to 49 years age group; 1,150 women would need to be screened to prevent one breast cancer death in this age group. A post hoc analysis showed that years of life lost caused by breast cancer mortality were 67.4 out of 1,000 women in the intervention group versus 78.9 out of 1,000 women in the control group; this is equivalent to 11.5 years of life saved per 1,000 women invited to screening in the intervention group and a total of 620 years of life saved.
- There was no statistically significant reduction in breast cancer mortality or all-cause mortality in the intervention group compared with the control group.
- In the intervention group, 18.1% of women had at least one false-positive result.
This evidence is inadequate to support the conclusion of a clinically significant breast cancer mortality reduction attributable to initiation of screening mammography among women aged 39 to 49 years. The reported mortality reduction is a small, transient reduction in breast cancer mortality based on post hoc, subset analysis, nonstandard imaging protocol, and nonstandard threshold for biopsy (microcalcifications were not biopsied). In absolute terms, the difference in breast cancer mortality was -0.6 deaths per 1,667 women in the 40 to 49 years age group based on a reanalysis of the original data set, which was not statistically significant, and the recalculation of breast cancer mortality in a subgroup restricted to 10 years of follow-up. At a median follow-up of 22.9 years, there was no statistically significant decrease in risk of breast cancer or all-cause mortality.[ 18 ]
This evidence is inadequate to make a clear determination of the magnitude of overdiagnosis. Because the evidence is based on subgroup analysis and nonstandard imaging schedule, nonstandard imaging protocol, and a nonstandard threshold for biopsy (microcalcifications were not biopsied) with uncertain relevance to the general population, it does not support the investigators' conclusion of “at worst a small amount of overdiagnosis."[ 18 ]
- Shapiro S, Venet W, Strax P, et al.: Ten- to fourteen-year effect of screening on breast cancer mortality. J Natl Cancer Inst 69 (2): 349-55, 1982. [ PubMed : 6955542 ]
- Shapiro S: Periodic screening for breast cancer: the Health Insurance Plan project and its sequelae, 1963-1986. Johns Hopkins University Press, 1988.
- Andersson I, Aspegren K, Janzon L, et al.: Mammographic screening and mortality from breast cancer: the Malmö mammographic screening trial. BMJ 297 (6654): 943-8, 1988. [ PMC free article : PMC1834636 ] [ PubMed : 3142562 ]
- Nyström L, Rutqvist LE, Wall S, et al.: Breast cancer screening with mammography: overview of Swedish randomised trials. Lancet 341 (8851): 973-8, 1993. [ PubMed : 8096941 ]
- Tabár L, Fagerberg CJ, Gad A, et al.: Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet 1 (8433): 829-32, 1985. [ PubMed : 2858707 ]
- Tabàr L, Fagerberg G, Duffy SW, et al.: Update of the Swedish two-county program of mammographic screening for breast cancer. Radiol Clin North Am 30 (1): 187-210, 1992. [ PubMed : 1732926 ]
- Tabar L, Fagerberg G, Duffy SW, et al.: The Swedish two county trial of mammographic screening for breast cancer: recent results and calculation of benefit. J Epidemiol Community Health 43 (2): 107-14, 1989. [ PMC free article : PMC1052811 ] [ PubMed : 2512366 ]
- Holmberg L, Duffy SW, Yen AM, et al.: Differences in endpoints between the Swedish W-E (two county) trial of mammographic screening and the Swedish overview: methodological consequences. J Med Screen 16 (2): 73-80, 2009. [ PubMed : 19564519 ]
- Roberts MM, Alexander FE, Anderson TJ, et al.: Edinburgh trial of screening for breast cancer: mortality at seven years. Lancet 335 (8684): 241-6, 1990. [ PubMed : 1967717 ]
- Bailar JC, MacMahon B: Randomization in the Canadian National Breast Screening Study: a review for evidence of subversion. CMAJ 156 (2): 193-9, 1997. [ PMC free article : PMC1226907 ] [ PubMed : 9012720 ]
- Baines CJ, Miller AB, Kopans DB, et al.: Canadian National Breast Screening Study: assessment of technical quality by external review. AJR Am J Roentgenol 155 (4): 743-7; discussion 748-9, 1990. [ PubMed : 2119103 ]
- Fletcher SW, Black W, Harris R, et al.: Report of the International Workshop on Screening for Breast Cancer. J Natl Cancer Inst 85 (20): 1644-56, 1993. [ PubMed : 8105098 ]
- Miller AB, Baines CJ, To T, et al.: Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. CMAJ 147 (10): 1477-88, 1992. [ PMC free article : PMC1336544 ] [ PubMed : 1423088 ]
- Frisell J, Eklund G, Hellström L, et al.: Randomized study of mammography screening--preliminary report on mortality in the Stockholm trial. Breast Cancer Res Treat 18 (1): 49-56, 1991. [ PubMed : 1854979 ]
- Latest Updates to This Summary (03/28/2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Updated statistics with estimated new cases and deaths for 2024 (cited American Cancer Society as reference 1).
Added text about a prospective multicenter study, known as the Dense Breast Tomosynthesis Ultrasound Screening Trial or DBTUST, that investigated whether ultrasound improved cancer detection after digital breast tomosynthesis in women with dense breasts (cited Berg et al. as reference 69). The study concluded that technologist-performed ultrasound screening modestly improved detection of cancer and also increased the false-positive recall rate in women with dense breasts.
This summary is written and maintained by the PDQ Screening and Prevention Editorial Board , which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
- About This PDQ Summary
Purpose of This Summary
Reviewers and updates.
This summary is reviewed regularly and updated as necessary by the PDQ Screening and Prevention Editorial Board , which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us . Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Screening and Prevention Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Screening and Prevention Editorial Board. PDQ Breast Cancer Screening. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/breast/hp/breast-screening-pdq . Accessed <MM/DD/YYYY>. [PMID: 26389344]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online , a collection of over 2,000 scientific images.
The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us .
- Cite this Page PDQ Screening and Prevention Editorial Board. Breast Cancer Screening (PDQ®): Health Professional Version. 2024 Mar 28. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-.
Version History
- NBK65906.40 March 28, 2024 (Displayed Version)
- NBK65906.39 March 6, 2024
- NBK65906.38 January 19, 2024
- NBK65906.37 June 7, 2023
- NBK65906.36 December 20, 2022
- NBK65906.35 October 7, 2022
- NBK65906.34 February 10, 2022
- NBK65906.33 September 13, 2021
- NBK65906.32 September 9, 2021
- NBK65906.31 June 17, 2021
- NBK65906.30 May 7, 2021
- NBK65906.29 August 27, 2020
- NBK65906.28 April 29, 2020
- NBK65906.27 March 11, 2020
- NBK65906.26 December 18, 2019
- NBK65906.25 October 30, 2019
- NBK65906.24 September 26, 2019
- NBK65906.23 August 2, 2019
- NBK65906.22 June 6, 2019
- NBK65906.21 March 14, 2019
- NBK65906.20 October 8, 2018
- NBK65906.19 June 1, 2018
- NBK65906.18 February 7, 2018
- NBK65906.17 December 20, 2017
- NBK65906.16 December 11, 2017
- NBK65906.15 October 3, 2017
- NBK65906.14 June 19, 2017
- NBK65906.13 April 27, 2017
- NBK65906.12 April 7, 2017
- NBK65906.11 February 22, 2017
- NBK65906.10 December 1, 2016
- NBK65906.9 October 10, 2016
- NBK65906.8 July 29, 2016
- NBK65906.7 June 9, 2016
- NBK65906.6 March 4, 2016
- NBK65906.5 January 8, 2016
- NBK65906.4 December 11, 2015
- NBK65906.3 October 9, 2015
- NBK65906.2 September 18, 2015
- NBK65906.1 July 14, 2015
In this Page
Related publications.
- Patient Version
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Breast Cancer Prevention (PDQ®): Health Professional Version. [PDQ Cancer Information Summari...] Review Breast Cancer Prevention (PDQ®): Health Professional Version. PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Cancer Screening Overview (PDQ®): Health Professional Version. [PDQ Cancer Information Summari...] Review Cancer Screening Overview (PDQ®): Health Professional Version. PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Neuroblastoma Screening (PDQ®): Health Professional Version. [PDQ Cancer Information Summari...] Review Neuroblastoma Screening (PDQ®): Health Professional Version. PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Thyroid Cancer Screening (PDQ®): Health Professional Version. [PDQ Cancer Information Summari...] Review Thyroid Cancer Screening (PDQ®): Health Professional Version. PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Testicular Cancer Screening (PDQ®): Health Professional Version. [PDQ Cancer Information Summari...] Review Testicular Cancer Screening (PDQ®): Health Professional Version. PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
Recent Activity
- Breast Cancer Screening (PDQ®) - PDQ Cancer Information Summaries Breast Cancer Screening (PDQ®) - PDQ Cancer Information Summaries
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Debate Over Breast Cancer Screening: Age 40 or 50 for Mammograms?
T here’s a growing belief that regular mammograms for breast cancer screening should begin at age 40 rather than the previously recommended age of 50.
The U.S. Preventive Services Task Force (USPSTF) has stated that women between the ages of 40 and 74 should have a mammogram every two years, based on an analysis of seven randomized clinical trials and 13 non-randomized clinical trials carried out by Meta Platforms, Inc.
According to the existing breast cancer screening guidelines in effect since 2016, having a mammogram every two years from the age of 50 to 74 is most effective for women. For women under 50, it is recommended that they decide whether to have screening tests based on their risk level.
The USPSTF stated that starting mammograms from age 40 could save 20% more lives than the existing guidelines. They further explained that having a mammogram every year could increase false-positive results, unnecessary biopsies, and inaccurate diagnoses, so women at average risk of breast cancer should have it every two years.
The USPSTF also added that regular biennial mammograms are unnecessary for women over 75, as current knowledge suggests that mammograms do not increase survival rates in this age group.
The USPSTF guidelines apply to women at moderate risk of breast cancer, women with a family history of breast cancer, and women with dense breasts.
On the other hand, the USPSTF guidelines do not apply to women with a history of breast cancer, those at high risk due to genetic factors, those with a history of high-dose chest radiation at a young age, or those who have had lesions found in previous biopsies. These women should discuss the frequency of mammograms with their healthcare professionals.
Editor's Pick
- Depression More Likely to Occur During Menopause Transition
- Feeling Tired This Spring? Here’s Why and What to Do About It
- Green Tea vs. Hot Water: Which Boosts Brainpower Better?
- Can the Keto Diet Help Prevent Alzheimer’s? New Study Says Yes!
Read more: How to Bake Perfectly Crispy Chocolate Chip Cookies Every Time
The USPSTF recommends that women with dense breasts have a mammogram every two years, although it’s uncertain whether additional screening tests would be helpful.
Some experts countered this by saying that MRI scans can help reduce false positives and lower the risk of cancer in women with dense breasts. They argued that the USPSTF’s claims lack sufficient evidence to establish a guideline and are not significantly different from existing breast cancer screening recommendations.
Last year’s study using data from the Cancer Intervention and Surveillance Modeling Network (CISNET) found that annual mammogram screenings for women aged 40 to 74 were most effective in reducing breast cancer mortality rates.
In other words, the study suggested that performing mammograms on women aged 40 to 74 every year could reduce breast cancer mortality rates by about 40%, which is superior to the 30% reduction achieved by following the 2023 USPSTF guidelines of biennial screenings for the same age group.
Therefore, experts argue that it’s effective for women at moderate risk of breast cancer to start having mammograms annually from the age of 40.
The research team agreed that there hasn’t been enough research on mammograms to establish detailed breast cancer screening guidelines. They suggested that each patient should decide on the best screening method with their healthcare professionals, considering their risk of breast cancer.
Most Viewed in ViewusGlobal
- 10 Science Behind the Struggle to Quit Smoking
- How to Bake Perfectly Crispy Chocolate Chip Cookies Every Time
- Why Oatmeal Bread Is the New Must-Try Recipe of the Year

Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Breast cancer awareness, risk factors and screening practices among future health professionals in Ghana: A cross-sectional study
Roles Data curation, Investigation, Methodology, Project administration, Writing – review & editing
Affiliation Department of Physician Assistantship, School of Medicine, University of Health and Allied Sciences, Ho, Ghana
Roles Supervision, Writing – review & editing
Affiliation Department of Psychological Medicine and Mental Health, School of Medicine, University of Health and Allied Sciences, Ho, Ghana
Roles Formal analysis, Software, Validation, Writing – review & editing
Affiliation Department of Epidemiology and Biostatistics, School of Public Health, University of Health and Allied Sciences, Ho, Ghana
Roles Writing – review & editing
Affiliation Department of Population and Behavioural Sciences, School of Public Health, University of Health and Allied Sciences, Ho, Ghana
Roles Resources, Supervision, Writing – review & editing
Affiliation Directorate of Human Resource, University of Cape Coast, Cape Coast, Ghana
Roles Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Department of Population and Behavioural Sciences, School of Public Health, University of Health and Allied Sciences, Ho, Ghana, Department of Public Health Graduate School, Yonsei University, Seoul, Republic of Korea
- Sandra Osei-Afriyie,
- Albert Kwesi Addae,
- Samuel Oppong,
- Hubert Amu,
- Emmanuel Ampofo,

- Published: June 24, 2021
- https://doi.org/10.1371/journal.pone.0253373
- Reader Comments
Like many other women in the developing world, the practice of breast cancer screening among Ghanaian women is unsatisfactory. As a result, many cases are diagnosed at advanced stages leading to poor outcomes including mortalities. An understanding of the awareness and predictors of breast examination is an important first step that may guide the design of interventions aimed at raising awareness across the general population. This study aimed to explore the awareness, risk factors, and self-reported screening practices of breast cancer among female undergraduate students at the University of Health and Allied Sciences.
This cross-sectional study was conducted among 385 female undergraduate students using a pre-tested questionnaire. Data were analysed using Stata Version 13.1 and presented using descriptive and inferential statistics comprising frequency, percentage, chi-square, and binary logistic regression. Odds ratios and 95% confidence intervals were computed to quantify the association between regular Breast-Self Examination (BSE) and socio-demographic characteristics of respondents.
Seventy-three per cent of the students were aware of breast cancer, with social media being the most important source of information (64.4%). The prevalence of breast cancer risk factors varied from 1% of having a personal history of breast cancer to 14.3% for positive family history of breast cancer. Current use of oral pills/injectable contraceptives was confirmed by 13.2% of participants; 20% were current alcohol users and10.1% were physically inactive. Regarding breast examination, 42.6% performed BSE; 10.1% had Clinical Breast Examination (CBE), while 2.3% had undergone mammography in the three years preceding the study. Women who did not believe to be susceptible to breast cancer (AOR: 0.04; 95%CI: 0.02–0.09) and those who did not know their risk status (AOR: 0.02; 95%CI: 0.005–0.57) were less likely to perform regular BSE compared to those who displayed pessimism. Further, women with no religious affiliation had 0.11 (95%CI: 0.02–0.55) odds of examining their breast regularly compared to Christians.
This study demonstrated moderate awareness of the modalities of breast cancer screening and the risk factors of breast cancer among the students. However, there exists a gap between awareness and practice of breast cancer screening, which was influenced by optimism in breast cancer risk perception and religion. Awareness campaigns and education should be intensified in the University to bridge this gap.
Citation: Osei-Afriyie S, Addae AK, Oppong S, Amu H, Ampofo E, Osei E (2021) Breast cancer awareness, risk factors and screening practices among future health professionals in Ghana: A cross-sectional study. PLoS ONE 16(6): e0253373. https://doi.org/10.1371/journal.pone.0253373
Editor: Antonio Simone Laganà, University of Insubria, ITALY
Received: July 4, 2020; Accepted: June 4, 2021; Published: June 24, 2021
Copyright: © 2021 Osei-Afriyie et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its S1 Data , S1 Questionnaire files.
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Breast cancer is the most prevalent cancer in women and generally the second most common cancer globally, with approximately 1.4 million cases diagnosed annually [ 1 ]. In 2018, 2.1 million incident cases of breast cancer were diagnosed worldwide (second most common cancer overall after lung cancer) representing nearly 12% of all incident cancer cases and an estimated 627,000 deaths were expected to occur globally [ 2 , 3 ]. Generally, the breast cancer rate is higher in the developed world than the developing countries, which may be as a result of certain lifestyles and reproductive factors that are more common in the developed world. The difference may be exaggerated due to relatively low awareness, screening practices, and diagnoses in the developing countries, though the rates are increasing rapidly in many developing countries [ 4 ].
In Ghana, breast cancer is becoming a great public health challenge among women. With about 2,900 incident cases occurring annually, and one-eighth of them dying from it, the disease has become the most common cancer-related death among Ghanaian women [ 5 , 6 ]. Previous studies have revealed an increased breast cancer burden in Ghana over the past decade. Clegg-Lamptey et al. [ 7 ] reported in 2009 that breast cancer now accounts for about 16% of all cancers and the most common cancer female cancer in Ghana. Naku et al. [ 8 ] estimated the incidence of breast cancer to be 76 per 100 000 Ghanaian women.
It is anticipated that the incidence of breast cancer will increase as Ghana’s population ages and women adopt western lifestyles [ 9 ]. Studies have shown that breast cancer is increasingly becoming common among younger Ghanaian women, and they present at a more advanced stage of the disease [ 5 , 7 , 10 ]. The advance stage at diagnosis is due to patients’ delay in seeking healthcare, which can be up to 10 months after the onset of symptoms, as a result of lack of awareness of the disease at its early stage and stigma associated with the disease [ 8 , 11 , 12 ].
Like most cancers, the primary risk factor for breast cancer in women is older age. Many other risk factors alter the exposure of breast tissues to reproductive hormones [ 4 ]. Some of these are modifiable, and include weight gain or being overweight/obese, long term use of the postmenopausal hormone, alcohol consumption, and physical inactivity [ 4 , 13 ]. Long menstrual history (younger age at menstruation and/or end later age at onset of menopause), never given birth, having one’s first child after age 30, and current use of hormonal contraceptives are other reproductive factors that influence the risk of breast cancer [ 11 , 12 ]. Personal medical or family history of breast cancer are also known to increase one’s risk for breast cancer [ 4 ].
Early diagnosis of breast cancer can increase the chance of early case detection and favourable outcomes, resulting in improved survival rates and quality of life of women and is therefore important public health strategy at all settings [ 2 ]. However, studies have demonstrated that factors related to women´s awareness, knowledge and perceptions about the disease may contribute significantly to health-seeking behaviours [ 14 , 15 ].
Notwithstanding that mammography is known to be the most effective screening tool for early detection of breast cancer [ 16 ], Breast-Self Examination (BSE) may be useful in resource-limited countries to detect any abnormalities in the breast as it provides an opportunity for women to be familiar with their breasts and promptly report any changes [ 17 ]. Additionally, clinical encounters by women provide the opportunity for women to have a number of important clinical activities such as breast cancer risk assessment, education about lifestyle, counselling, and clinical breast examination (CBE) that may not otherwise be done [ 17 ]. There are no current national breast cancer screening protocols in Ghana or countrywide literacy initiatives, and there is limited availability of mammography or ultrasound machines [ 5 ]. Although the effectiveness of BSE and CBE in detecting breast cancer is debatable [ 16 ], they have been campaigned as major screening tools given their availability at little to no cost among limited-resource settings in most developing countries.
Women who practice regular breast screening have been known to have a lower risk of developing an advanced form of breast cancer than those who do not. Reports have shown low uptake of breast cancer screening among women in many sub-Saharan African countries and this results in late detection and increased mortality in affected women [ 18 ].
Even though the incidence and mortality of breast cancer have been on the increase, there is a paucity of the empirical literature on student’s awareness, risk factors and screening practices in Ghana. This population is especially important because they have abundant access to health information as health students and they are potential sources of information for non-health students and the general population. College students have a significant influence on colleague students and also, findings regarding this target group have implications for their capacity in the role of promoting screening for breast cancer as potential health professionals. This study, therefore, explored breast cancer awareness, selected risk factors, and screening practices among female undergraduate students, to provide information for the control, prevention, and early treatment of the disease.
Materials and methods
Study site and design.
We conducted a cross-sectional study among female undergraduate students at the University of Health and Allied Sciences (UHAS), Ghana in February 2019. Ghana has a projected population of 30,280,482 in 275 districts distributed among 16 administrative regions as of June 2019 and is divided into some 75 ethnic groups with the Akans forming the majority. UHAS is among the newest public Universities in Ghana which was established in 2011 and is located at Ho in the Volta Region. UHAS is so far the only public University solely dedicated to the training of varied health professionals such as medical doctors, nurses, midwives, pharmacists, public health professionals etc. in Ghana. The University has two main campuses; the main campus, which is located at Ho (where this study was carried out), and the Hohoe campus, which has the School of Public Health. There are currently six schools/colleges in the University, with a current student population of about 3,752. Among the programmes offered by the University are Medicine, Physician Assistantship, Nursing, Midwifery, Physiotherapy, Pharmacy, Dietetics, Speech and Language Therapy, and Public Health [ 19 ].
Sample size determination
We obtained the required sample size for this study using Cochran’s single proportion formula; n = z 2 pq/d 2 , where n = sample size, z = z-score at 95% confidence level, p = estimated proportion of an attribute that is present in the population, q = 1-p, d = margin of error. This study assumed a margin of error(d) of 0.05 at 95% confidence level and an estimated proportion of 60.9% women with regular breast screening practice [ 20 ]. Adjusting for a non-response rate of 5%, 385 students were sampled and participated in the study. Thus, we sampled 385 students of which all participated.
Sampling procedure
We used two levels of stratified sampling method to select the study participants from to ensure representativeness. First, students were stratified according to faculty (school), defined as one of the 5 constituent schools in the University, namely, School of Allied Health Sciences; School of Basic and Biomedical Sciences; School of Medicine; School of Nursing and Midwifery; and School of Pharmacy. For each faculty, students were stratified according to their level (year) of study, where simple random sampling was used to select the required number of students separately from each faculty and academic year. This was done by obtaining names of all female students according to their faculty and level of study from the University’s administration. The names of eligible students were arranged and numbered alphabetically separately for each faculty and level in a Microsoft excel sheet, where random numbers were generated, and the corresponding students selected to take part of the study. The number of students selected from each academic year and faculty was proportional to the students’ population. Selected students were approached by the lead author who was also a student in the University in January 2019 and after explaining the study’s objectives and procedures to them, all (100%) agreed to participate in the study. Students who were under 18 years of age were excluded from this study.
Data collection tools and procedure
A structured pre-tested questionnaire was used to collect data from participants. The questionnaire contained closed-ended questions adapted from previously published studies that adapted validated questions and had the following sections: demographic information, breast screening practices, risk factors of breast cancer (Behavioural, reproductive, and hormonal factors), and awareness of breast cancer.
Consenting participants were handed printed copies of the questionnaire and were given 24 hours to fill their responses and return them anonymously to the researchers. The objectives of the study were explained to all participants before the questionnaires were given out. Filled questionnaires were checked daily by the researchers for consistency and omissions before collection. Where there were inconsistencies, participants were made to correct them in the presence of the researchers before they were taken.
Definition of variables.
Table 1 presents the definitions of some of the study variables measured in this study.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0253373.t001
Data analysis
All analyses were carried out using STATA (Stata Corp, College Station) statistical package Version 13. Descriptive statistics were used to describe the study population in relation to relevant variables. Bivariable and multivariable logistic regression models were used to identify significant predictors of regular BSE defined as having examined the breast at least once every month. First, the association of regular BSE with each variable of interest (Participant’s age, religion, ethnicity, faculty of study, academic year, family history of breast cancer, having known or seen a breast cancer patient, perceived risk for breast cancer, physical activity, current Alcohol use and current use of contraception) was examined. Second, variables with p-value <0.20 in the first model were considered for inclusion to construct a model with risk factors independently associated with the outcome variable. The degree of association between dependent and independent variables was assessed using odds ratios (OR) with 95% confidence intervals (CI).
Ethical issues
The study approval by the University of Health and Allied Sciences’ Ethics Review Committee with reference UHAS-REC A.8 [ 56 ] 18–19. Written informed consent was obtained from each respondent after explaining the study’s procedure and potential risk and benefits to them. Participants’ identifiers such as name and address were not collected.
General characteristics of respondents
All 385 participants returned their completed questionnaire. The mean age of the study population was 22 ± 2.78. The majority (55.8%) were between 20 and 24 years old, while 1.3% were 30 years and above; 16.4% were currently married and 83.1% were single; 74.0% were Christians and 18.9% were Muslims. Regarding ethnicity, Akans were the majority (38.4%), followed by Ewes (30.9%), while 14.3% belonged to the Ga-Dangbe ethnic group. Respondents constituting 26.2% (n = 101) were in their first year of study, 21.8% were in the second year, 30.2% in the third year and 21.8% were fourth-year students. The comparative majority (52.5%) were studying Nursing and midwifery, 22.3% were Medical students, and 19.7% were Allied Health Sciences students ( Table 2 ).
https://doi.org/10.1371/journal.pone.0253373.t002
Breast cancer awareness and risk perception
Regarding breast cancer awareness, 281 (73.0%) of the respondents reported having ever heard of breast cancer. The remaining items in Table 2 were asked of the 281 women who had heard of breast cancer. The social media remained the most important source of information on breast cancer (181;64.4%), followed by teachers (173; 62%) and the electronic media (172; 61%). As presented in Table 2 , the majority (200; 71.1%) of respondents were aware of mammography as a screening method for breast cancer; 196 (69.8%) were aware of BSE; while 28 (10.0%) of them did not know of any the screening methods. Family history of breast cancer (n = 236, 83.9%), genetics (n = 229, 81.7%) female sex (n = 173, 71.9%) and individual lifestyle (n = 176, 62.6%) were the most frequently indexed risk factors for breast cancer. Meanwhile, nulliparity (n = 100, 35.6%), early menses/menopause (n = 116,41.3%), and Obesity (n = 159, 56.6%) were the least known risk factors. Putting money in the brassiere was implicated as a potential risk factor for breast cancer by more than a third (n = 106, 37.7%) of study participants. The most common presentation of breast cancer aware of was a lump in the breast (n = 259, 92.2%), followed by nipple discharge (n = 219, 77.9%), and lymph node in the armpit (n = 191, 68%) while pulling off a nipple (n = 47, 16.7%), nipple itch (n = 55, 19.6), and swollen nipple (n = 97, 34.5) were the least known sign/symptom of breast cancer.
Among students who were aware of breast cancer, 129 (45.9%) thought that they do not have the chance of getting breast cancer, while 46 (16.4%) did not know whether they were at risk or not ( Table 3 ).
https://doi.org/10.1371/journal.pone.0253373.t003
Breast cancer risk factors
Among the 385 study participants, menarche occurred below age 12 in 48 (12.5%) of them, while 72 (18.7%) were more than 14 years at menarche. The prevalence of current oral pill/injectable contraceptive use was 13.2% (n = 51). The occurrence of breast cancer in the family was confirmed in 55 (14.3%) of the respondents; 14 (4%) had their first-degree relatives (Mother, Sister) affected with the disease, while 41 (11%) were second-degree relatives. Four (1%) of the students had a personal history of breast cancer. Regarding behavioural factors, 39 (10.1%) did not engage in physical activity and 77 (20%) currently use at least one standard of alcohol per day ( Table 4 ).
https://doi.org/10.1371/journal.pone.0253373.t004
Breast cancer screening practices
Of the 385 participants, 212 (55.1%) ever applied at least one breast cancer screening method. Of these, 164 (42.6%) practised BSE, 39(10.2%) had undergone CBE, and 9 (2.3%) were screened for breast cancer via mammography. Of those who performed BSE, 136 (82.9%) did it at least once in every month; 6 (3.7%) examine their breast yearly, and 22 (13.4%) did it at random; 115 (70.1%) of them learned BSE skills from their school-teachers, 112 (68.3%) from the media, 37 (22.6%) were taught by their friends, and 26 (15.9%) learned it from their mothers. The most common (28.1%) reason for not practising BSE was “1 do not know how to perform it” followed by “I have no family history of breast cancer” (23.1%) and “I am not at risk of breast cancer” (17.2%), while 63 (28.5%) had no reason for not doing BSE ( Table 5 ).
https://doi.org/10.1371/journal.pone.0253373.t005
Predictors of breast self-examination
Table 6 shows the results of bivariable and multivariable logistic regression analyses aimed at identifying variables associated with the odds of performing regular BSE. After adjusting for confounding effect of the variables, women who were between 25 and 29 years old were 5.13 (95%CI: 1.18–22.26; P = 0.03) times more likely to perform regular BSE compared to those less than 20 years. Further, women with no religious affiliation had 60% less odds of performing regular BSE compared to Christians. Women who expressed optimism regarding breast cancer risk (AOR: 0.04; 95%CI: 0.02–0.09; p<0.001) and those who did not know their risk level (AOR: 0.02; 95%CI: 0.005–0.57; p<0.001) were less likely to perform regular BSE compared to those who were pessimistic about breast cancer risk.
https://doi.org/10.1371/journal.pone.0253373.t006
This study though has demonstrated considerable awareness about the existence of breast cancer, insufficient knowledge, and misconceptions regarding its risk factors and causes and disease presentation also existed among participants. Less than three-quarters of our study participants had heard about breast cancer. This is unexpectedly far lower than the 100% observed in medical students in Harar, Ethiopia [ 23 ], 98.7% among University of Ibadan female students [ 24 ], 95% previously reported among female students of Faculty of Health and Medical Sciences in Ghana [ 25 ], and 88.1% among Teacher Training college students in Cameroon [ 26 ]. The difference in the awareness rate found in this study and that of the aforementioned studies cannot directly be explained. However, breast cancer has been adopted in the curriculum of the first two aforementioned studies in an attempt to create awareness among students and might have had a positive impact on the students’ awareness about the disease. Ours finding further show unsatisfactory levels of awareness and understanding of breast cancer risk factors and disease presentation. More than one-third of participants were not recognised increasing age, nulliparity, obesity, and early menstruation and late menopause as potential risk factors for breast cancer, while 5% did not know any risk factor for breast cancer. This finding confirms reports from previous studies in Ethiopia [ 27 ], Nigeria [ 28 ], Egypt [ 29 ], and Angola [ 30 ] where general knowledge and risk factors of breast cancer were found to be low among female college students. Knowledge gaps have also been identified among the general population elsewhere [ 31 , 32 ]. About one-third of our respondents held the belief that putting money in the brassiere can result in breast cancer. This is in line with other studies among women in the general population [ 33 , 34 ] as well as university students [ 24 ] that suggest that women still have misperceptions about the cause of breast cancer with some attributing it to the spiritual origins. Additionally, as reported by previous studies elsewhere, [ 23 , 26 ] awareness of other clinical manifestations of breast cancer other than breast lump was worryingly low. These existing knowledge gaps and misconceptions may impact health-seeking behaviours and uptake of breast screening resulting in late diagnosis which in turn may lead to complications and death. Thus, the need for health education programmes aiming to increase awareness about the causes, risk factors and clinical presentation of breast cancer is warranted.
Predictors of breast cancer risk are varied, including individual lifestyle, reproductive status, and genetics. This study attempted to identify some of these risk factors among the students. First menstruation at an early age (early menarche) is known to be associated with increased levels of endogenous hormones (estrogen and progesterone) in a woman’s lifetime which increases the risk of breast cancer [ 17 ]. In this study, about 13% of participants had their menarche at an age younger than 12 years. In the Lublin region of Poland, menarche occurred at 11 years in only about 3% of women attending screening a programme [ 35 ].
Among the most extensively researched risk factors of breast cancer is the use of exogenous hormones in the form of oral contraceptives and hormone replacement therapy (HRT) [ 36 ]. Lina et al. [ 37 ] in their study to assess the association between the use of hormonal contraception and the risk of invasive breast cancer among 1.8 million Danish women demonstrated that current and recent users of hormonal contraception had 20% increased risk of breast cancer compared with those who had never used hormonal contraception. We found in this study that, about 13% of women were current users of oral pills/injectables. None of them was however on HRT.
A positive family history of breast cancer in a first-degree relative is the most commonly known risk factor for the disease [ 38 ]. Women with a family history of breast cancer in a mother or sister have up to a 3-fold increase in the risk of developing breast cancer [ 38 ]. In this present study, about 14% of the participants had a positive family history of breast cancer, among whom 4% occurred in first degree relatives (mother and sister), and hence have increased risk of the disease. Our result is higher than that reported in the previous study in Ghana, where 1.4% of women had a first-degree family history of breast cancer [ 8 ]. and another study among university students in Ajman, UAE, where a positive family history of breast cancer was found in 9% of students and about 1% with the first-degree relative affected with breast cancer [ 39 ]. Researchers have devoted much attention to understanding the role that genes play in the development of breast cancer. This has helped in recognizing that some women could be at increased risk as a result of inherited predisposition. It must be acknowledged however that other than genes, families also share other factors such as cultural background and environmental exposures, which are themselves potential predictors of breast cancer [ 39 ].
The relationship between alcohol consumption and an increased risk of developing breast cancer has been the subject of many studies. Compared with non-alcohol drinkers, women who drink even in small amounts, have increased risk of breast cancer. The risk increases with an increased amount of alcohol consumed per day [ 40 ]. In many traditional African societies including Ghana, alcohol consumption is not common among females and the youth and people usually frown on alcohol intoxication [ 41 ]. The prevalence of alcohol consumption in African women varies from 1% in Malawi to 30% in Burkina Faso with about 81% of women in Africa reporting lifetime abstinence [ 42 ]. The prevalence of current alcohol consumption (20%) among female undergraduates found in this study is similar to levels (19.6%) reported among women in the WHO African regions in 2012 [ 43 ]. Martinize et al. [ 42 ] reported that about two-thirds of Ghanaian women abstain from alcohol in their lifetime. Kofi Adesi Kyei and colleagues found in their previous 5-year retrospective review to identify predominant lifestyle risk factors of breast cancer among Ghanaian women that alcohol contributed to about 19% to the disease with respect to preventable risk factors [ 44 ] and concluded that alcohol is not the most important preventable risk factor for breast cancer in Ghana. However, having 2 in 10 future female health professionals drinking alcohol is significant enough to warrant public health action due to the health consequences of alcohol on women.
Many studies have reported an inverse relationship between regular physical activity and breast cancer risk. Several biologic mechanisms have supported the protective effect of physical activity on breast cancer which includes effect on immune, endogenous sex steroid hormone production, and antioxidant system [ 44 – 46 ]. In this present study, 1 in 10 women was not physically active. Of those who were, the majority engaged in brisk walking. Comparing the most active and least active women, most studies estimate the risk reduction of breast cancer to be between 20 and 40% and recognise the dose-response relationship with risk increase levels of risk reduction [ 47 – 49 ]. Several mechanisms might be the cause of this inverse association with physical activity. Increased levels of activity are known to reduce body weight, thus reducing the risk of breast cancer. Physical activity may also influence the production, metabolism, and excretion of endogenous hormones that can result in lower levels of bioactive oestrogen, insulin, and other growth factors [ 50 ].
Accurate and early diagnosis of breast cancer depends mainly on the "opportunist approach". Given the challenges faced by resource-limited countries, improving breast cancer awareness and the application of screening methods remains a practical option for early detection and treatment of breast cancer. Similar to reports from previous studies done in different parts of the world including Ghana at different times, the performance of breast cancer examination is generally low [ 51 – 56 ]. Notably in this study, less than half of the participants performed BSE, 10% had CBE, and an even lower percentage (2.3%) had Mammography. This is a worrying phenomenon owing to the fact that breast cancer is increasingly becoming common in this part of the world, hence women and young ladies need to frequently subject themselves to screening for early detection and treatment to avoid complications and death. The low coverage of breast cancer screening among our study population could perhaps be explained by their young age as breast cancer has been known to be common among older women.
While the effectiveness of BSE to detect breast malignant tumour remains debatable, its importance in breast self-awareness creation in resource-limited countries with non-existent population screening programmes cannot be overemphasis, thus deserves consideration. The level of BSE practice in this study is, however, higher than that reported among over 10,000 undergraduate students from 24 countries across Africa, Asia and America. (9.1%) [ 57 ], and that of other studies among students in Ethiopia (39.4%), Cameroon (3%), Libya (23.5%) [ 53 , 58 , 59 ]. The rising trends in the incidence of breast cancer in Africa may be explained by the lack of health consciousness of young women to examine themselves for the timely identification of any breast abnormality. In this study, lack of skills, no family history of breast cancer and pessimism about the risk of the disease were the most important reasons for not performing BSE. This is a worrying finding since these are future healthcare professionals who are required to educate others in the community about the disease and the need for primary prevention. University students are among the well-informed group of women in Ghana. Their lack of skills in performing breast screening and the need for periodic screening, therefore, is indicative of a greater lack of skills and awareness among the general population of less-educated women. Similar, in Ethiopia, being healthy and lack of knowledge were the overriding factors given [ 53 ]. In a previous study among Presbyterian university college students in Ghana, varied reasons including, lack of time, forgetfulness, procrastination, and fear were the reasons for not performing BSE [ 25 ]. The social media could be an important tool that can be harnessed to educate women on the need for regular and correct practice of BSE since it remained the most important source of education for women who performed BSE in this study.
Expectedly, this study demonstrates that risk perception, an important component of behavioural change paradigm, is a sufficient enough variable that is capable of affecting women’s breast cancer screening behaviour. Women who did not believe to be vulnerable to breast cancer and those who did not know their risk status were less likely to practice BSE regularly compared to those who were pessimistic about their risk of the disease. Our result confirms the assertion of the Health Belief model that women who with a higher risk for breast cancer, perceive breast cancer as a serious threat, have a lower perception barrier, and who hold a higher perception of the benefits are more likely to perform regular BSE [ 60 ]. Erbil and Bolukbas also suggest that women’s health beliefs and attitudes remain the predominant factors that influence whether or not they will get themselves screened for breast cancer [ 61 ]. Among Korean women, those with lower perceived comparative risk were more likely to have no intention of getting a mammogram [ 62 ]. Further, this study demonstrated that who were not affiliated to any religion were less likely to examine their breast regularly compared to their counterparts who belonged to the Christianity religion perhaps due to exposure to breast cancer information in churches.
It is interesting to note that other risk variables such as physical activity, Alcohol consumption, and contraception use, and positive family history did not influence breast BSE in this present study. This is a worrying finding because women with risk factors are expected to perform regular breast examinations to detect the disease at its early stage to prevent complications and death. It can be suggested that university women do not practice BSE even though they have some risk factors of breast cancer.
There are three limitations of our study worth mentioning. First, the findings cannot be generalised beyond the study population since they are young and well-educated women. University students are not representative of young adults in general, and the risk perception, risk factors, and breast cancer screening practice may differ from that of the general population. Secondary, all data were self-reported with no objective measures to assess the accuracy of these reports. Lastly, the instrument used was not tested for its validity, however, questions used were taken from validated tools from previous studies at other settings. Nevertheless, the results of this study provide some understanding regarding perceived risk and the practice of breast screening among future health professionals, which can be useful for directed health promotion and education.
The research concludes that the awareness of breast cancer and its causes, risk factors, and disease manifestation was generally unsatisfactorily low. Additionally, even though some students possess some important risk factors of the disease, the practice of BSE coverage which was influenced by risk perception and religion was low. Improved methods of risk communication are recommended to ensure that women have appropriate risk information to make informed choices about risk management options and preventative interventions. Social media can also be a reliable tool for health education on breast cancer.
Supporting information
S1 data. anonymised data set used for this study..
https://doi.org/10.1371/journal.pone.0253373.s001
S1 Questionnaire. Assessment of breast cancer risk factors and screening practices among university female students, Ghana.
https://doi.org/10.1371/journal.pone.0253373.s002
Acknowledgments
SOA acknowledge the support provided to her by all faculty in the department of Physician Assistantship, University of Health and Allied Sciences, Ho, Ghana. Sincere thanks go to all study participants.
- View Article
- PubMed/NCBI
- Google Scholar
- 2. World Health Organization, “Cancer,” retrieved from http://www.who.int/topics/cancer/en/ on February 10, 2019.
- 16. American Cancer Society, “American Cancer Society recommendations for the early detection of breast cancer”. https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html . On February 12 2020.
- 17. Bevers T, El-Serag H, Hanash S, Thrift AP, Tsai K, Maresso KC, et al. Screening and early detection. In Abellof’s Clinical Oncology, sixth edition; 2020. Elsevier. ISBN 978-0-323-47674-4.
- 19. The University of Health and Allied Sciences, Ghana. http://www.uhas.du.gh .
- 21. World Health Organization. International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva: WHO; 2000.
- 22. Global Physical Activity Questionnaire (GPAQ) Analysis Guide. https://www.who.int/ncds/surveillance/steps/resources/GPAQ_Analysis_Guide.pdf (accessed April 20, 2020).
- 38. National Breast and Ovarian Cancer Centre. Breast cancer risk factors: a review of the evidence. National Breast and Ovarian Cancer Centre, Surry Hills, NSW, 2009.
- 41. Oshodin OG. Nigeria. In Heath B. D. (Ed.), International handbook on alcohol and culture (First ed., pp. 213–223). Westport: Greenwood Press. 1995.
- 49. Vainio H, Bianchini F. IARC Working group on the evaluation of cancer-preventive agents. Weight control and physical activity. Lyon, France: International Agency for Research on Cancer (IARC), 2002.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- About the Campaign
- Campaign Home
- Campaign Materials
About the Right to Know Campaign
At a glance.
Women with disabilities are less likely to have been screened for breast cancer within the recommended guidelines. The "Right to Know" campaign materials increase awareness of the importance of breast cancer screening among women with disabilities.

Why it's needed
Breast cancer is a major public health concern for all women, including women with disabilities. However, women with disablities are significantly less likely to have been screened within the recommended guidelines . 1 The public health community has used health communication messages and campaigns to increase breast cancer awareness and encourage women to take steps to help prevent breast cancer, yet few communication messages exist for women with disabilities.
Several studies have explored the barriers to breast cancer screening for women who have physical disabilities. 2 3 4 The barriers include:
- Thinking, "It won't happen to me"
- Focusing on other health issues
- Difficulty with positioning while getting a mammogram
- Not knowing where to go for accessible screening
- Inaccessible facilities and equipment
- Healthcare provider's knowledge and attitudes
Women with disabilities also identified the lack of health promotion messages and materials that reflect their needs as a problem and requested that CDC address this issue.
As a result of this study, CDC developed health promotion materials (e.g., print advertisements, posters, fliers, tip sheets, audio files and transcripts) to increase awareness of breast cancer among women with physical disabilities and encourage these women to get screened. Materials share the tagline "Breast Cancer Screening. The Right To Know," and feature four women with physical disabilities who have survived breast cancer .
- Courtney-Long E, Armour B, Frammartino B, Miller J. Factors associated with self-reported mammography use for women with and women without a disability. Journal of Women's Health . 2011; 20:1279-1286.
- Magasi, Susan, et al. ScreenABLE: Breast Cancer Screening among Women with Disabilities from Community Identified Challenges to Community-Based Programs. Progress in Community Health Partnerships: Research, Education, and Action , vol. 13 no. 5, 2019; p. 61-69. Project MUSE .
- Todd A, Stuifbergen A. Breast cancer screening barriers and disability. Rehabil Nurs. 2012 Mar-Apr;37(2):74-9. doi: 10.1002/RNJ.00013. Erratum in: Rehabil Nurs. 2012 Sep-Oct;37(5):266. PMID: 22434617; PMCID: PMC4521895.
- Thierry, JM (2004). Barriers to breast cancer screening among women aged 40 years and older who have physical disabilities. (Doctoral dissertation) Retrieved from https://getd.libs.uga.edu/pdfs/thierry_joann_m_200412_phd.pdf
Breast Cancer Screening — The Right to Know Campaign
Doctors warn of rise in rate of cancer among young people
by Holly Menino, KOMO News Anchor

SEATTLE — It seems like people are getting diagnosed with cancer at younger and younger ages. Doctors confirm that is indeed happening.
"The rate of cancer in young adults worldwide has been going up at a rate of about 1% to 2% per year over at least the last three decades," said Dr. Donald Karcher, president of the College of American Pathologists.
Karcher said there's probably no single cause, but a combination of things, that's leading to the increase, including diet, lifestyle factors and things young people are exposed to.
"We know that a high fat, low fiber diet or a diet high in ultra processed foods, things like cold cuts and diet sodas, that's associated now with cancer, lack of exercise, obesity," Karcher said. "That's associated with certain cancer types. Smoking, we know, is associated with lung cancer, but it may also be associated with at least eight other types of cancer. Alcohol consumption, exposure to sunlight because of skin cancer."
Even things like over exposure to certain medications can take a toll.
"Some we know are associated with cancer," Karcher said. "Some we didn't suspect, like antibiotics, might be contributing to this because of the effect that they have on the microorganisms that normally live in our gut."
Karcher says from breast cancer to colon cancer, even kidney and pancreatic cancer, doctors are seeing an increase in all cancers in young adults. He says to watch out of symptoms.
"Things like a swelling or a lump or unexplained pain or unexplained weight loss, persistent fatigue, change in bowel habits, blood in the stool or in the urine, chronic cough or chronic hoarseness, even a change in a skin lesion like a mole," Karcher said.
The other important thing to do is screening tests, he says. For women with an average risk of breast cancer, they should now start having mammograms at age 40, he added.
For young women with a higher-than-average risk of breast cancer, according to Karcher, they should start even earlier. For cervical cancer, screening with pap tests and or HPV screening tests should start at age 21. Colon cancer screenings should start at age 45, unless you have a higher-than-average risk, then those screenings should start even earlier. These are screenings that could save your life, Karcher said.

IMAGES
COMMENTS
Breast cancer is the second leading cause of cancer mortality for US women, despite a steady overall decline in breast-cancer mortality rates over the past 20 years. 1 The average age-adjusted rate for the years 2016-2020 was 19.6 per 100 000, with an estimated 43 170 deaths in 2023. 1,2 The majority of cases occur between the ages of 55 and 74 ...
The variation in breast cancer incidence rates across different regions may reflect disparities in breast cancer screening (BCS) practices. Understanding the factors associated with these screening behaviors is crucial for identifying modifiable elements amenable to intervention. This systematic review aims to identify common factors influencing BCS behaviors among women globally.
The low coverage of breast cancer screening among our study population could perhaps be explained by their young age as breast cancer has been known to be common among older women. While the effectiveness of BSE to detect breast malignant tumour remains debatable, its importance in breast self-awareness creation in resource-limited countries ...
Recent case-control studies on the effectiveness of population-based breast cancer screening show differences in the magnitude of breast cancer mortality reduction. We investigated the role played by aspects of the case-control study design on these differences, e.g. the definition of cases and exposure to screening.
Introduction. Breast cancer (BC) was allocated 11.7% of all kinds of cancers in 2020.[] To date, the new cases of the disease were beyond two million, forecasted to reach more than 3 million cases with more than one million deaths by 2040.[2,3]BC was imposed a considerable economic burden on countries as well as public health problems[4,5] while it can be prevented by early detection ...
Background: In 2009, the U.S. Preventive Services Task Force recommended biennial mammography screening for women aged 50 to 74 years and selective screening for those aged 40 to 49 years. Purpose: To review studies of the effectiveness of breast cancer screening in average-risk women. Data sources: MEDLINE and Cochrane databases to 4 June 2015.
Background: Mammography (MG) has demonstrated its effectiveness in diminishing mortality and advanced-stage breast cancer incidences in breast screening initiatives. Notably, research has accentuated the superior diagnostic efficacy and cost-effectiveness of digital breast tomosynthesis (DBT). However, the scope of evidence validating the cost ...
In a literature review, Leonard Fass (2008) and Safarpour Lima and colleagues (2019) found that cancer care is dependent on imaging through screening [ 11, 14 ]. Breast cancer can be detected early using imaging tools [ 6 ]. The sensitivity and specificity of various techniques, however, vary [ 15, 16 ]. Integrated imaging techniques, according ...
The National BreastScreen Australia Program, a population-based breast cancer screening program, invites women aged 50 to 74 to have a free mammogram every two years [].The program is widely promoted by delegated state and territory breast cancer screen promotion organisations [], and eligible women receive invitation and reminder letters at the address registered on the electoral roll.
(HealthDay, 2010). As such, death rates caused by breast cancer are higher among Hispanic/Latino women (HealthDay, 2010). Numerous studies have established a statistically significant correlation between fatalism and diminished use of breast cancer screening services (HealthyDay, 2010). Improving breast cancer diagnosis, screening
Mammography can detect breast cancer at the asymptomatic phase with around 85% sensitivity and around 95% specificity (19). Since 2009 the U.S. Preventive Services Task Force recommends breast cancer screening with biennial mammograms for women age 50 to 74 years old (18, 20).
In 1996, the age-adjusted. mortality rate for breast cancer was 24.3 per 100,000 women, a slight decrease from 26.9% in. 1973 (Ries et al., 1999). It is important to note that this represents the first sustained decline in. breast cancer mortality rates since 1973 when SEER surveillance for breast cancer was.
Background In Ghana, breast cancer is a major public health concern and the most common type of cancer among women in terms of mortality and incidence. This study determined the factors influencing breast cancer screening among women of reproductive age in Nandom Municipality, Ghana using the Health Belief Model as the conceptual model. Methods The study was cross-sectional in design. A ...
Most breast cancer mortality occurs in women diagnosed in late stages of the disease due to lack of knowledge, cultural and religious beliefs, and other barriers to regular breast cancer screening (World Health Organization [WHO], 2017). American Cancer Society (2016) estimated that 3.1 million U.S. women survived breast cancer in 2015.
new cases of breast cancer is registered and deaths were 464.454 fallowed by other types of cancer (WHO, 2008). Health care authorities put a lot of efforts to overcome this merciless disease. One of these efforts is screening. By screening the breast cancer can be detected in early stages and thus the treatment can be more effective.
Background Incidence of breast cancer in the Kingdom of Saudi Arabia (KSA) has increased in recent years. Screening helps in early detection of cancer and early diagnosis and timely treatment of breast cancer lead to a better prognosis. Women in the healthcare profession can have a positive impact on the attitudes, beliefs, and practices of general public. Therefore, it is important that the ...
This cross-sectional study examines knowledge, attitudes, and practices surrounding breast cancer awareness and screening among women residents in Qatar. Females, >18 years old, registered with the Primary Health Care Corporation were invited to complete an Arabic or English online survey using a modified version of the Breast Cancer Awareness Module. Of the 9008 participants, 69% report ...
There are three screening methods for early detection of breast cancer including mammography, clinical breast examination, and breast self-examination, in the order of importance [15, 16]. Breast self-examination is a screening method that does not require specialized equipment and staff due to its simplicity, cost-effectiveness, and efficiency ...
Benign and malignant lesions presenting as retro- aerolar lumps can occur, although male breast cancer is rare: < 1% of all breast cancers occur in men and <0.5%. of deaths in men can be ...
prevalent cases of cancer and about 35,000 new cases are added to this every year. Based on the. (18.5%) it ranks second to cervical cancer. The burden of breast cancer is increasing in both. rate ...
Breast cancer and disability. In the United States in 2021, the percentage of women with disabilities aged 50-74 that received a mammogram during the past 2 years was lower than the percentage of women without disabilities of the same ages who received a mammogram during the past 2 years (73.5% vs. 80.4%). 1 Studies also show higher rates of death related to breast cancer among women with ...
The Breast Cancer Screening Change Package is part of the Cancer Screening Change Packages Toolkit. The tools and resources in the Breast Cancer Screening Change Package are for women who: Are ages 40 to 74 years. Do not have signs or symptoms of breast cancer. Have not been diagnosed with high-risk breast lesions, breast cancer, or underlying ...
Provided cervical cancer screening and diagnostic services to 121,197 women and diagnosed 99 invasive cervical cancers and 5,732 premalignant cervical lesions, of which 35% were high-grade. During 2015 to 2017, about 5.7% of US women were eligible for NBCCEDP cervical cancer screening services, and the program served 6.8% of those eligible ...
Health experts do not recommend that women under 40 who are at average risk for breast cancer undergo mammograms. That generally means that women in their 20s and 30s are too young to begin screening unless they have: (1) a known genetic mutation (like BRCA1 or BRCA2) that puts them at high risk for breast cancer; (2) an extremely strong family ...
Published online: March 28, 2024. This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about breast cancer screening. It is intended as a resource to inform and assist clinicians in the care of their patients.
According to the existing breast cancer screening guidelines in effect since 2016, having a mammogram every two years from the age of 50 to 74 is most effective for women. For women under 50, it ...
Background Like many other women in the developing world, the practice of breast cancer screening among Ghanaian women is unsatisfactory. As a result, many cases are diagnosed at advanced stages leading to poor outcomes including mortalities. An understanding of the awareness and predictors of breast examination is an important first step that may guide the design of interventions aimed at ...
The American College of Obstetricians and Gynecologists, for instance, recommends screening every one or two years starting at age 40 and continuing until at least age 75. The American Cancer ...
Several studies have explored the barriers to breast cancer screening for women who have physical disabilities. 2 3 4 The barriers include: Thinking, "It won't happen to me". Focusing on other health issues. Difficulty with positioning while getting a mammogram. Not knowing where to go for accessible screening.
For young women with a higher-than-average risk of breast cancer, according to Karcher, they should start even earlier. For cervical cancer, screening with pap tests and or HPV screening tests ...