- Skip to Guides Search
- Skip to breadcrumb
- Skip to main content
- Skip to footer
- Skip to chat link
- Report accessibility issues and get help
- Go to Penn Libraries Home
- Go to Franklin catalog

Science and Engineering Libraries Course Guides: Information on Chemical Nomenclature
- Information on Chemical Nomenclature
- Information on Patents for Researchers
The International Union of Pure and Applied Chemistry (IUPAC) grew out of the international recognition of a need for standardization in chemistry. It is the recognized world authority on chemical nomenclature, terminology, symbols, units, atomic weights and related topics.
The IUPAC Home Page provides access to the full text of a number of IUPAC recommendations, including: IUPAC Glossary of Organic Class Names; Nomenclature of Amino Acids and Peptides; Steroid Nomenclature .
IUPAC have published a number of compilations of their rules on nomenclature, including:
Commission on the Nomenclature of Inorganic Chemistry "Red Book" Nomenclature of Inorganic Chemistry, Recommendations 1990, Blackwell Scientific Publications, 1990. Edited by G J Leigh. [Reference: QD 149.N66 1990]
Commission on the Nomenclature of Organic Chemistry "Blue Book" Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, Pergamon Press, 1979. Edited by J Rigaudy and S P Klesney. [Reference: QD 291.I57 1979]
A Guide to IUPAC Nomenclature of Organic Chemistry, Recommendations 1993, Blackwell Scientific Publications, 1993. Edited by R Panico, W H Powell and J C Richer. [Reference: QD 291.I57 1993]
Revisions or additions to the IUPAC recommendations appear in the journal Pure and Applied Chemistry . A list of these is available here
You can access the full text of many of the IUPAC recommendations from their old Web site, at http://old.iupac.org/publications/epub/index.html#nt .
CA Index Guide
The Index Guide to Chemical Abstracts is a useful tool for finding CA names for compounds when you know a common or trade name. For example: DDT See Benzene,1,1'-(2,2,2-trichloroethylidene)bis[4-chloro- [50-29-3]
It also indexes Enzyme Commission designations, for example: E.C.3.4.99.10 See Insulinase [9013-83-6]
Note: The numbers in square brackets are the CA Registry Numbers for these compounds. Registry Numbers uniquely identify a chemical substance, and should always be used in preference to names when searching computer databases such as SciFinder.
In 2010, Chemical Abstracts Service ceased publication of all printed works, including the Index Guide. The Chemistry Library owns the last available edition of the Index Guide, and it is housed in the library reading room. However, please note that it has not been updated in some years, and newer substances may not appear there.
As a chemist you will have a working knowledge of chemical nomenclature, but only a few experts will be so familiar with IUPAC rules that they are able to name (almost) any compound. It is nice to be able to draw the structure into a program and have the program name it for you.
Autonom was a windows-based program which generated IUPAC names from graphic structures. It worked from a library of about 7,000 entries, with an additional index of functional groups, claiming to have an 85% success rate in naming structures and giving a warning if a structure could not be named. Templates helped to simplify the drawing process. Autonom was very popular during the late 1990s and the early 2000s. It was packaged with Beilstein CrossFire, as well as contemporary versions of ChemDraw. However, the program is currently unavailable at the University of Pennsylvania, and its overall status is unknown.
Chemical Abstracts
The nomenclature used by CA has developed in parallel and generally in accordance with IUPAC rules. A major revision of CA index names was carried out in 1972, when most trivial names were dropped. The CA index names for most chemical substances have continued unchanged since that date. In the Chemical Substances Index, compounds are listed alphabetically by name. Ordering is based on the parent compound name, e.g. butane , plus a suffix to denote the principal function, e.g. sulfonic acid , and a locant e.g. 1- . Following a comma, the substituents are listed. e.g. 1-Butanesulfonic acid, 2,4-diamino-3-chloro-ethyl ester . A detailed explanation of names used in CA may be found in the Index Guide, Appendix IV Chemical Substance Index Names . The easiest way, currently, to locate a Chemical Abstracts index name is to search by structure on SciFinder. The first name given in the structure's record will be the index name. If a substance is novel, one can contact the experts at Chemical Abstracts Service ( http://cas.org ), and request that it be named, for a fee.
Ring Systems Handbook
The CA Ring Systems Handbook is very useful for finding CA names of any compounds containing rings. It consists of a catalog of over 91,000 structural diagrams, called the Ring Systems File . The entries are arranged firstly in order of the number of component rings, e.g. "3 RINGS" followed by the sizes of the component rings in ascending order, e.g. "5,6,7", followed by the elemental content of the rings, e.g. "C3N2-C6-C5NO". Substituents are not included in the handbook: it names the 'parent' compound. The RSH also contains an alphabetical listing of names, the Ring Name Index , which can be used to find the structure of any ring-containing parent compound listed in CA. The most recent edition of the RSH that the Chemistry Library owns is from 2003, and the last update supplement is from 2006. The RSH ceased publication in 2010, along with the rest of the Chemical Abstracts Service print resources. Currently, the only way to make use of the ring skeletons is to perform a screened search in the CAS REGISTRY on STN.
Reference Books
- << Previous: Home
- Next: Information on Patents for Researchers >>
- Last Updated: May 19, 2020 12:19 PM
- URL: https://guides.library.upenn.edu/Scienceandengineeringcourseguides

Nomenclature Notes
1 What is IUPAC Nomenclature?

In the first place, there is no monolithic construction called IUPAC Nomenclature. Nomenclature is a subject that has grown and changed over the years. The first widely accepted systematic nomenclature proposals arose in France amongst Lavoisier and his colleagues in the 1780s, and they were dealing with what today we recognise as inorganic compounds. Internationally-accepted systematic nomenclature may be reckoned to stem from the Geneva Congress of 1892. These nomenclatures attempted, and still attempt, to be systematic, but the systems they use are different. Consequently the methodologies employed in deriving the names of inorganic and organic compounds are generally different. [See box for examples.]
In the second place, there are more systems sheltered under the IUPAC umbrella, such as that for polymers, and also systems to deal with newer materials such as organometallic compounds, which may be regarded as falling into more than one category of compound, and may require the use of more than one system, or even a specially-modified system, to name them satisfactorily.
In the third place, studies related to chemistry, such as biochemistry, pharmacy, and cosmetics, have developed their own specific nomenclatures, which may be abbreviated and modified for commercial purposes, and these can also produce unequivocal names, but not names which necessarily convey the complete structural information usually required by IUPAC nomenclatures. However, IUPAC is usually involved jointly with other international bodies in the elaboration of these systems.
— {first published as ref 2}
This blogpost provides short notes and briefs about Principles of Chemical Nomenclature – A Guide to IUPAC Recommendations . Each section addresses a specific topic such as systematic nomenclature, the constructing of names, or the use of abbreviations. Nomenclature Note s were first published in Chem Int , from March 2012 to Dec 2013 < https://iupac.org/publications/ci/indexes/nomenclature-notes.html >
The author, Jeffery Leigh is the editor and contributing author of Principles of Chemical Nomenclature—A Guide to IUPAC Recommendations, 2011 Edition ( RSC 2011, ISBN 978-1-84973-007-5 ) [overview given in ref 1]. He has been an active contributor to IUPAC nomenclature since 1973.
2 On the Various Nomenclature Systems
In the first “Nomenclature Notes” (March-April 2012 CI ) [above], it was stated that IUPAC nomenclature is composed of a set of different nomenclatures, which do not necessarily use the same conventions and methods. IUPAC bodies such as the Interdivisional Committee on Terminology, Nomenclature and Symbols (ICTNS) are trying to remove these inconsistencies, but nevertheless, if you are trying to decipher an IUPAC name, or to construct one, it is necessary to realize which type of nomenclature is being used, which means recognizing the type of compound that is being treated. An ideal IUPAC name should be unique, but should also convey the structure of a given compound.
At the lowest level, compositional nomenclature simply lists the constituents of a compound in some prescribed order, generally alphabetical, and says nothing about the structure. It is really another way of presenting an entry in a formula index. Substitutive nomenclature is a principal IUPAC nomenclature system and the preferred method for naming organic compounds. This relies on selecting a suitable unsubstituted parent compound from which the compound at issue can be considered as ultimately derived, and then modifying the parent name in a series of formal operations. For example, a compound such as C 2 H 5 Cl would be considered to be derived from the parent compound, ethane, C 2 H 6 , which is then substituted by removing a hydrogen atom to produce C 2 H 5 •, an ethyl radical, and then adding a chlorine atom to yield C 2 H 5 Cl, called chloroethane.
This is a particularly simple case, but the principles used for naming in this way are all very similar, though as the complexity of the compound being treated increases, more and more complex parent compounds and many more rules are required.
An alternative name for chloroethane is ethyl chloride, and this is an example of binary nomenclature which developed from inorganic chemistry and its original systematization by Lavoisier and his colleagues. Binary nomenclature groups constituents of compounds into two classes, positive and negative. This works well for salts, such as sodium chloride, but more complex organic materials may require the employment of at least two classes of nomenclature, for example, substitutive to name a formal cation such as chloroethyl, and binary to name the related ester, chloroethyl acetate. Functional class nomenclature is a system used for organic compounds when substitutive nomenclature is less appropriate, for example with organic acids and anhydrides.
A more recent, though similarly historical, system is additive nomenclature , a second principal IUPAC nomenclature system, with its origins in inorganic coordination chemistry. In this approach, the name of a compound considered to be a coordination complex, such as Na 3 [CoCl 6 ], is derived by identifying the three cations and the single anion from which it is composed. The latter is a complex anion, and it is then divided formally into its constituent central cation and its associated six chloride anions or ligands. Finally, the individual names are then assembled to give the compound name trisodium hexachloridocobaltate. In this system, organic ligands would receive substitutive names. Then, to be used in an additive context, organometallic compounds almost always require such a nomenclature mixture. The additive system may be used to name some compounds which would not usually be considered to be coordination complexes.
The third major class of IUPAC nomenclature is polymer nomenclature. It is generally impossible to specify a polymer molecule exactly, as we attempt to do in the cases treated so far, because the chain lengths and end groups are rarely accurately known. One approach is to use a source-based system, because one generally knows the monomer from which the polymer was produced. This gives names such as poly(buta-1,3-diene), but it lacks structural information. A structure-based name for this material might be poly(1-vinylethane-1,2-diyl), but this represents only the units of the polymer chain, not the end groups, so it may be equally incomplete.
The 2011 edition of Principles of Chemical Nomenclature includes full descriptions of all these nomenclature systems, and of several others which are not necessarily due to IUPAC, with details of how to apply them. It also contains instructions for how to decipher IUPAC names to identify the structures that such names are intended to convey. Principles of Chemical Nomenclature also introduces what gives the promise of being a unique class of identifier applicable to a wide class of compound, the IUPAC International Chemical Identifier, or InChI.
{first published as ref 3}
3 Non-IUPAC Nomenclature Systems
In the second “Nomenclature Notes” (May-June 2012 CI , p. 28) [above] we alluded to various kinds of nomenclature that fall under the aegis of IUPAC. There are other nomenclatures in wide use, to some of which IUPAC contributes. For example, biochemical nomenclature is reviewed regularly by a committee which has joint membership from both IUPAC and IUBMB (International Union of Biochemistry and Molecular Biology). It is fair to say that biologists and biochemists are often not interested in systematic nomenclature in the way that chemists are. For them it may be sufficient that a given name is specific and defined so as to make the compound to which it refers unequivocally and universally-recognized within the discipline. The IUPAC requirement that a name should convey the structure of a compound is not necessary or even desirable, as long as all practitioners accept it. For example, the compound
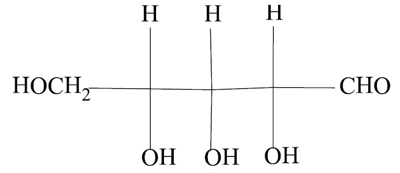
may receive a perfectly good IUPAC name, 2,3,4,5-tetrahydroxypentanal, but this might not be recognizable to biochemists unless the compound were named as a sugar, specifically an open-chain form of ribose. As this molecule also contains three chirality centers (asymmetric carbon atoms) to which are assigned the locants 2, 3, and 4, a more complete name would be (2 R *,3 R *,4 R *)-2,3,4,5-tetrahydroxypentanal. Biochemists would also prefer to know the absolute configuration at carbon atom 4, representing it by small capital letters, either D or L, and they would be very comfortable with a name such as D-ribose. When even more complex structures are considered, such as amino acids, steroids, and proteins, the use of defined but semisystematic names becomes necessary for ease of communication and comprehension in biochemical and biological contexts.
A system of nomenclature which is independent of IUPAC, but which is widely used by chemists, is due to the Chemical Abstracts Service (CAS) of the American Chemical Society. This has been developed to produce names for CAS use in both running text and indexes. Although nomenclaturists of CAS have contributed substantially to IUPAC, they cannot afford the leisurely contemplation of data in which IUPAC indulges. They have to make decisions rapidly to meet publication deadlines, and CAS names are designed to be adaptable for use in indexes. Because an index compiler would wish to locate all derivatives of, say, the parent compound butane, at the same place in the alphabetical index, CAS names are sometimes termed “inverted,” because, in this specific case in an index the parent name butane would always be cited first. In its Index Guide, CAS explicitly states that, outside of a CAS index, a name should be used in its uninverted form. In contrast, IUPAC nomenclature presents a name written in a continuous and linear fashion from left to right, and which contains prefixes and suffixes in a specific, linear order. The consequence is that IUPAC names for derivatives of a given parent compound would appear at different places in an index using IUPAC names because the initial letters of the names depend upon the specific prefixes employed. Two examples follow:may receive a perfectly good IUPAC name, 2,3,4,5-tetrahydroxypentanal, but this might not be recognizable to biochemists unless the compound were named as a sugar, specifically an open-chain form of ribose. As this molecule also contains three chirality centers (asymmetric carbon atoms) to which are assigned the locants 2, 3, and 4, a more complete name would be (2 R *,3 R *,4 R *)-2,3,4,5-tetrahydroxypentanal. Biochemists would also prefer to know the absolute configuration at carbon atom 4, representing it by small capital letters, either D or L, and they would be very comfortable with a name such as D-ribose. When even more complex structures are considered, such as amino acids, steroids, and proteins, the use of defined but semisystematic names becomes necessary for ease of communication and comprehension in biochemical and biological contexts.
IUPAC, 2-sulfanylethanol CAS, 2-mercaptoethanol CAS inverted, ethanol, 2-mercapto-
In a similar sequence, we have: 4-(methylsulfanyl)benzoic acid, 4-(methylthio)benzoic acid, and benzoic acid, 4-(methylthio)-
In addition to these nomenclatures, others are current in the literature. The Internatioanl Union of Biochemistry and Molecular Biology (IUBMB) categorizes and specifies and names of enzymes according to the types of reaction they catalyze. Considerations of enzyme chemical structure are secondary here. The World Health Organization (WHO) produces a list of nonproprietary names for drugs which are shaped so as to allow easy conversion to languages other than English and which indicate the action of the drug. Hence, the names of beta-blockers all carry the ending -olol. An example is propranolol, the structure of which is hardly reminiscent of that of propane, though the name was apparently suggested in part by the propane residues it may be considered to contain.

The International Organization for Standardization (ISO) lists names for pesticides, which bear a fleeting similarity to IUPAC names; for example, “trimesium” stands for “trimethylammonium.” There is also list of recommended names for cosmetic ingredients, the International Nomenclature of Cosmetic Ingredients. What characterizes these latter names is that they serve as specific identifiers for commercial and industrial materials which must be quickly-recognizable by users who are nonchemists, but they are usually inadequate for use by chemists. All these non-IUPAC nomenclatures are briefly described in the new edition of Principles, together with references allowing the reader to obtain more detailed information should it be required.
{first published as ref 4}
4 Systematic and Trivial Nomenclature
N omenclaturists recognize two general classes of nomenclature, systematic and trivial. Perhaps the use of the word trivial is unfortunate, because its usual meaning in every-day English according to the Oxford English Dictionary (OED) is “of small account, little esteemed, paltry, poor, trifling, inconsiderable, unimportant, slight.” However, the OED lists several other meanings, some derived from a Latin word implying “three.” A more general common meaning listed in the OED is “such as met with anywhere, common, commonplace, ordinary, trite.” The word trivial was adopted when nomenclature was in its infancy and when its use in the latter sense was more usual, and that is why it is still used in that sense today. It is not intended to be dismissive.
The traditional names of the elements are trivial in this sense. They are non-systematic and many have been adopted from alchemy and early chemistry. For example, the term mercury was applied to many plants, persons, and things as well as the metal itself, which was also called quicksilver, for obvious reasons. An alternative name, hydrargyrum, from which the symbol Hg was derived, is a compound word from Latin and Greek meaning liquid silver. The reason for such names is very evident, but that can hardly form the basis of a systematic nomenclature for all elements. However, most element names are so deeply embedded in many languages that even IUPAC has refrained from generally systematizing them. Nevertheless, during the 1990s it became clear that many scientists needed to write and speak about elements that had yet to be prepared, and that names and symbols were required. Hence, IUPAC developed names and symbols for such elements that are immediately recognizable and based upon their atomic numbers. These names are provisional and are replaced as soon as a given element is prepared and unequivocally characterized. Perhaps unfortunately, the unambiguous systematic name is then replaced by a trivial name suggested by the scientists who first prepared the element.
Trivial names for compounds are used by chemists everywhere, and such names are clearly useful for much exchange of information, especially within a given lab. However, IUPAC attempts to devise a fully systematic approach to the names of substances, which imply unequivocally their chemical constitutions. Such names should be used when an unambiguous identification of compounds is required, as in scientific documents, international treaties, patents, and legal definitions. This is why IUPAC nomenclature can sometimes appear to be so complicated.
There are other kinds of systematic nomenclature. The Chemical Abstracts Service of the American Chemical Society has its own systematic system, similar to IUPAC’s, and with similar aims, but not identical. Other nomenclatures may be systematic, but in a manner differing from IUPAC’s. For example, The International Organizarion for Standardization (ISO) lists approved names for pesticides, such as afidopyropen, which should be recognizable by professionally-qualified users rather than solely by chemists. Such names should be translatable into other scripts and into languages other than English. The entry for the pesticide afidopyropen (for details see < www.alanwood.net/pesticides >) also lists French and Russian versions of the name, a guide to (British) English pronunciation of the English name, the chemical structure, and full IUPAC and CAS names. The short ISO name is clearly preferable to the IUPAC systematic name for everyday commercial use, though the ISO listing also provides citations of relevant InChIKeys and InChIs.
Similarly, the World Health Organization (WHO) issues a list of international nonproprietary names for drugs (INNs), which are names devised and classified according to the pharmacological activity of the substance cited. This is also systematic, but is only loosely derived from IUPAC nomenclature. WHO lists proposed INN names together with a structural formula. Details of the listings can be found, for example, in WHO Drug Information , 23(2), 2009, which may be accessed on-line. The names are given in a form of Latin, and then in English, French, and Spanish, with an indication in each language of its claimed activity, its molecular formula, and its CAS registry number. The names listed here are proposed names, and recommended names are listed elsewhere.
These systems are a selection of those available. Many refer back to systematic IUPAC names, but are adapted to specific purposes. Principles offers a brief guide to some of these, together with suitable references for wider consultation.
{first published as ref 5}
5 InChIs and Registry Numbers
Constructing a systematic name of a chemical compound of known structure means that it is necessary for the reader to know the detailed nomenclature rules required to do this. Such a person must work within a particular system, of which IUPAC and the Chemical Abstracts Service (CAS) provide possibly the two most complete. These are both designed in the English language, but a person whose mother tongue is not English may face a further barrier to developing a name if another language, such as Russian or Japanese, should be the language of primary use. However, all trained chemists should be able to recognize a chemical structure displayed using atomic symbols and bond connections, etc., as these are independent of language, even if the basic chemical symbols, recognizable by all chemists, use the roman alphabet. IUPAC has recently developed a computer methodology for recognizing and codifying chemical structures, and, the converse, for reproducing the chemical structure from such a code. This code is called an InChI (IUPAC International Chemical Identifier), and there is a related, more abbreviated, version called an InChIKey.
Principles provides a brief introductory guide to InChIs, and InChIKeys, along with CAS Registry Numbers. These are quite distinct from each other. Registry Numbers are unique numbers used to identify a specific compound in the CAS database. The numbers are assigned to a compound the first time that it appears in Chemical Abstracts (CA) and can be used thereafter to find all references to this compound when it appears again in CA. However, it has no further significance, and does not contain structural information. The user of CA must be familiar with CA nomenclature. In contrast, the recently developed InChI and its related InChIKey are strings of numbers, letters, and other symbols that provide a complete description of the structure of a compound (see www.iupac.org/inchi ).

The strings are not comprehensible to a casual reader, though they are to a computer that is equipped with the necessary programs. The InChI system can now deal with many, but as yet not all, compounds that appear in the literature, but it is still under development. In theory, InChI software will eventually provide an InChI character string from structural data for any compound. InChIs are already being used by most of the major chemistry publishers and databases. The InChI Trust website (www.inchi-trust.org) lists some of the many organizations now using it.
The InChI software represents features of the compound structure as a sequence of levels and in strict order, starting with the formula and then dealing with various features, such as atom connectivity and stereochemistry. Production of an InChI is reversible, in that with the appropriate computer program it can be derived from a drawing of the structure and it can then be used to regenerate the structure from which it is derived. This is not true of the InChIKey. An InChI may contain many tens of characters. An InChIKey is an abbreviated form which contains only 27 characters. It cannot be used to regenerate its parent structure, but it is still unique and is designed primarily for searching databases. In that sense it is like a Registry Number, but unlike the Number, it derives ultimately from a compound’s structure.The strings are not comprehensible to a casual reader, though they are to a computer that is equipped with the necessary programs. The InChI system can now deal with many, but as yet not all, compounds that appear in the literature, but it is still under development. In theory, InChI software will eventually provide an InChI character string from structural data for any compound. InChIs are already being used by most of the major chemistry publishers and databases. The InChITrust website ( www.inchi-trust.org ) lists some of the many organizations now using it.
Of course, a primary requirement for someone to use both InChIs and InChIKeys is that they possess the programs that can construct and interpret them. Like all IUPAC products, these are freely available to the chemistry community. Principles contains enough detail and references for a beginner to obtain the programs and to start to use them.
{first published as ref 6}
6 Deciphering and Constructing Names
Someone attempting to construct a name using a specified kind of nomenclature, such as IUPAC nomenclature, or trying to discern the chemical structure implied by a name encountered in, say, an article, must first decide what kind of nomenclature is being used. Few chemists would have problems understanding a name such as sodium chloride. This is a venerable name, and the implications of the presence of the positive and negative entities of a salt are generally well understood. However, as we have seen in previous Notes, IUPAC promulgates several classes of nomenclature, sometimes specific to particular classes of compound, so that the chemist has first to decide which particular class of nomenclature should be used, or, if trying to decipher a name, which particular class has been used to construct the name under consideration.
The new edition of Principles , like the first edition, contains enormous amounts of information on how to construct names once the compound class has been recognized. This includes various classes of compound, such as organic, inorganic, organometallic, polymeric, and biochemical. However, a novel departure in the new edition is the incorporation of a new Chapter on deciphering (or deconstructing) names.
Upon encountering a new name, the chemist must first decide to which class of compound the name belongs. This is generally, but not always, straightforward. Then, for an organic compound, for example, it is recommended to decide first whether functional class or substitutive nomenclature is being used. Then, the chemist must deduce the identity of the parent hydride and hence its numbering scheme, recognize any suffixes to parent the name (there may not be any), and finally recognize the detachable prefixes and endings. These operations should enable the chemist to begin to write a structural formula. The names of biological compounds, mainly organic compounds, generally fall outside the scope of Principles , but information on the important groups of such compounds, such as sugars and nucleotides is given, and sources of more information are noted. Often the complete systematic names of such compounds may be so complicated and large that regular use requires alternative simpler, more compact names.
To name inorganic compounds, again the class of compound must be determined. A compound may span more than one class (e.g., it may be a salt and also contain a coordination entity) so that more than one system of nomenclature may need to be employed. To decipher the name of a coordination entity, the following steps are necessary: identify the central atom(s), identify the ligands (which may be organic compounds and be named using organic methods), and identify the coordination geometry and stereochemistry. The names of organometallic compounds reveal some specific aspects that need to be appreciated, and boron compounds often bear names that are derived using a different set of conventions.
The rules for constructing the names of polymeric compounds are different yet again. In decipherment, they are often easier to recognize because they contain the term “poly” at or near the beginning of the name. Then, the specific rules of the types of polymer nomenclature should be consulted to unravel the structure.
Constructing and deciphering chemical names are certainly not easy problems for a beginner, and are sometimes not easy even for the nomenclature expert. However, Principles provides a summary of methods employed in both name construction and name decipherment, replete with many examples of both kinds of process. Although the tasks of applying nomenclature rules may appear imposing, chemists should persist. A couple of old English sayings should always be remembered. One is that “practice makes perfect,” even though the most experienced nomenclaturists are still striving for perfection. None of us is ever likely to reach the stage where “familiarity breeds contempt.”
{first published as ref 7}
7 Drawing Chemical Structures
Although the drawing of chemical structures is not strictly a nomenclature matter as usually understood, it is a way of conveying the structure of a chemical compound just as is an IUPAC systematic name, though using a visual language rather than a verbal one. Consequently, it is necessary to use certain widely-accepted conventions when drawing a chemical structure, particularly if that structure is three-dimensional and the representation of that structure as drawn on a sheet of paper is necessarily in two dimensions. IUPAC has attempted to define preferable methods for achieving this, and the new edition of Principles , unlike its predecessor, contains a summary of what is currently considered to be best practice to this end.
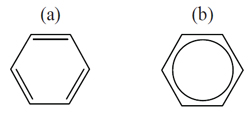
Certain recommendations are almost self-evident. For example, it is common, but not mandatory, to use the same font and font size in your structural diagrams as in your text. Principles uses Times New Roman, which was this editor’s choice. Some people prefer to use sans serif fonts such as Arial, though this can lead to minor confusion between symbols, such as l and 1 (Times New Roman) with l and 1 (Arial). Unusual abbreviations used in a diagram label should be defined somewhere in the article being written. It is not enough to assume that everyone will know what thf stands for, though this is probably acceptable for Ph. Bond lengths, thicknesses, and angles should be used consistently in all your diagrams. You should decide whether to represent aromatic rings as localized systems, as in (a) below, or as delocalized systems as in (b). Which you choose is not important, as long as your symbolism is clearly understood and is used consistently.
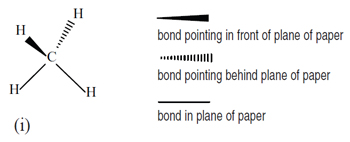
In the past, a variety of methods has been employed to portray a three-dimensional structure in two dimensions, for example, to show whether a bond which does not lie in the plane of the paper is pointing behind that plane or forwards towards the reader. In Principles , we have settled for conventional methods, as shown below in (i) for the tetrahedral molecule CH 4 .
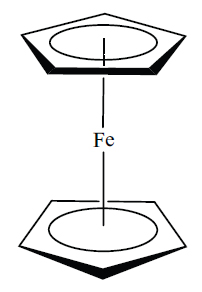
Principles also introduces adaptations of this convention, for example, in order to represent certain ring structures, as in the representation for the ferrocene molecule shown below (ii).
More complicated polyhedral shapes than tetrahedral are frequently encountered in chemistry, and these are also discussed in Principles , which introduces the most common three-dimensional structures found in coordination chemistry and also the most common projections used to represent the three-dimensional structures of organic molecules. For the beginner, these are sometimes not easy to understand.
The octahedron is a shape very often found in coordination chemistry, but the manner in which it is represented depends upon the circumstances. In the representation (iii) below of a coordination complex formally written as [Mabcdef], the bonds between the ligands (a, b, c, etc.) to the central metal [not specifically indicated in diagrams (iii)-(v)] are represented using the formalism described above in (iii), but in (iv) the principal plane of the octahedron is drawn, but only the bonds to ligands e and f. Finally, in (v), only the octahedron is delineated and no bonds. The edges of the solid octahedron invisible to a viewer are represented by dashed lines. Yet, all three are acceptable representations of the molecule [Mabcdef], and should be equally comprehensible to the informed reader. More complicated polyhedral shapes than tetrahedral are frequently encountered in chemistry, and these are also discussed in Principles , which introduces the most common three-dimensional structures found in coordination chemistry and also the most common projections used to represent the three-dimensional structures of organic molecules. For the beginner, these are sometimes not easy to understand.

Organic chemists have related problems when representing organic structures in three dimensions, and they use a variety of projections to do this, the principal ones being named after their inventors: Fischer, Haworth, and Newman. These particular projections are usually applied to specific classes of molecule. In a Fischer projection, the bonds to the carbon at the center of the tetrahedron are not represented as in the drawing of Cabcd (vi), but in a plane as in (vii), the convention being that bonds drawn vertically are pointing behind the plane of the paper, and the horizontal ones in front. The central carbon atom is not specifically represented. This type of projection is used primarily for carbohydrates and amino acids.
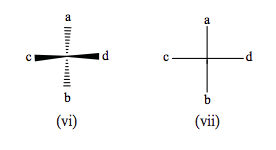
A Newman projection is employed to represent more complex molecules, such as an ethane-type molecule C 2 abcdef, where different conformations with respect to a selected carbon-carbon bond may be present. This is illustrated in (viii) and (ix) below. Finally, Haworth projections are often applied to compounds such as monosaccharides and polysaccharides.
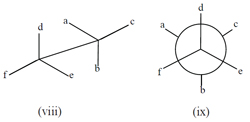
The use of all these devices and more, including various ways to represent conformations, are discussed in the new volume of Principles , together with appropriate examples and literature references.
{first published as ref 8}
8 Organometallic Nomenclature
The nomenclature of organometallic chemistry often poses a challenge to the chemist. For example, when constructing a systematic name for such a compound should one employ the methods of organic chemical nomenclature, or should one try to adapt the methods of inorganic nomenclature? The answer will depend upon the compound under consideration, with the addition that neither may be directly applicable because the compound presents a problem of assigning a structure with conventional electron-pair bonds. Principles goes some way to dealing with this poser.
Some organometallic compounds, primarily of main-group elements in Groups 13–16, are clearly formally so similar to their carbon analogs that they may be named in a rather like fashion, using an organic-type nomenclature. Hence, we can derive names such as methylalumane and trimethylsilanamine for the compounds AlH 2 Me and SiMe 3 NH 2 (Me = CH 3 ). The first name is derived from the name alumane, assigned to AlH 3 , and the second from silane, assigned to SiH 4 . More complex compounds, such as chain compounds may often be named by applying the methods of substitutive nomenclature to the names of the formal hydrocarbon parents. For example, skeletal replacement nomenclature can be used to develop a name for substances such as MeSiH 2 OPHOCH 2 Me. This may be considered to be derived from heptane, which, of course, contains a seven-carbon chain. Hence, the suggested name would be 3,5-dioxa-4-phospha-2-silaheptane. Although it actually contains only three carbon atoms in the chain, the name is unequivocal.
Although organometallic derivatives of Main Groups 1 and 2 may often also be conveniently named by using established additive nomenclature, originally developed to name transition-metal complexes such as [Co(NH 3 ) 6 ]Cl 3 , hexaamminecobalt(III) trichloride, organometallic derivatives of transition elements may often not be dealt with so easily because they exhibit not only metal-carbon single bonds, but features such as metal-carbon multiple bonds, and also bonds between a metal ion and unsaturated molecules and groups. Nevertheless, additive nomenclature has been adapted to name them too, though additional strategies have had to be devised.
To define the names of non-organometallic complex compounds which exhibit formally different possibilities for the donor atoms, it has been found useful to employ the so-called κ (kappa) convention, whereby the actual donors are specifically indicated. An example is shown below, where two modes of designating the binding of a nitrite ion (NO 2 ̶ ) to a metal ion, M, are exemplified.
M-NO 2 designated nitrito- κ N M-ONO designated nitrito- κ O
Compounds that contain a single metal-carbon bond present no new problems, but if such a bond is formally double or triple, the name of the carbon-donor must be altered by changing the termination –yl (as in methyl) to –ylidene or –ylidyne. However, the κ convention may also be applied to define which of more than one available carbon atom is a donor. Sometimes, two correct systematic names may be derived, as in the example below:
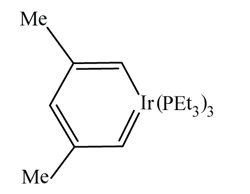
(2,4-dimethylpenta-2,4-diene-1,1,5-triido-κ 2 C 1 , C 5 )tris(triethylphosphane)iridium
(2,4-dimethylpenta-1,3-dien-1-yl-5-ylidene)tris(triethylphosphane)iridium
The carbon ligand is regarded as anionic in the first name and a radical in the second. Whether it is preferable to treat a specific carbon-donor ligand as an anion or as a radical is a matter still open for discussion, and Principles does not attempt to differentiate between the two approaches.
Sometimes contiguous atoms in a ligand act together as a donor group to a metal ion. This is particularly true of carbon-containing systems such as alkenes, alkynes, and various aromatic groups. To indicate which of the carbon atoms in a ligand is bonded to the metal ion, then the η (eta) or hapto system is applied, as in the examples below.
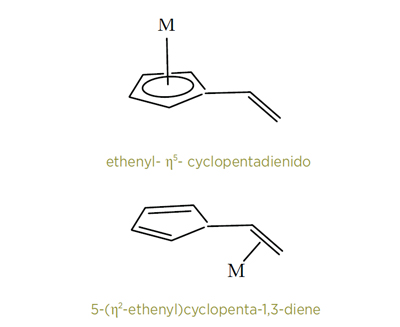
However, some compounds, notoriously those of boron, are even more difficult to name. They are sometimes termed electron-deficient and do not always obey the more usual rules governing compounds with two-atom localized electron-pair bonds. Researchers have developed a unique approach to naming these compounds, based upon the names for simple borane polyhedra and boron hydride anions. Often the numbers of hydrido-ligands combined with the polyboron skeleton need to be stated. The practitioners in this area also employ several specialized terms. For example, they may differentiate between heteraboranes and metallaboranes, and they employ the word “subrogation” where others might prefer to use the phrase “skeletal replacement.” A whole new chapter in the new Principles is devoted to these compounds alone.
The methodologies outlined here are described in the new version of Principles , together with guidance upon where to apply them and to which type of compound, with examples of such applications and, for those who require them, references to the more detailed literature.
{first published as ref 9}
9 Polymer Nomenclature
The aim of systematic IUPAC nomenclature is usually to introduce naming systems that define the structure of a molecule precisely, so that the reader can reproduce the exact structure of the molecule being discussed. The system works reasonably well for completely characterized small molecules, but cannot do so with the same level of precision for polymeric materials comprised of molecular chains (macromolecules), the structures of which are based on constitutional repeating units (CRUs). These repeat units may not be all of the same type and need not repeat in a regular fashion. A given polymer may consist of more than one chain and often of mixtures of different kinds of chain. In addition, there can be regular or irregular steric variations along the length of individual macromolecules and chains might be branched or linked to one another in diverse ways. For many polymers, the repetition may not be exactly regular, the material may consist of a mixture of chain macromolecules of different lengths, and the precise structure may not be known. Nevertheless, methods for naming such materials are necessary for general communication, and polymer chemists have been obliged to develop them. It is probably true that there is not yet a universal agreement among all polymer chemists as to how this should be done in every case, but there is a considerable consensus, and the new Principles presents its basic details.
A polymer is a substance composed of a collection of macromolecules of a range of molecular masses. As a consequence, it is characterized by an average molecular mass rather than a mass of a definite value, as typical of relatively small molecules. These macromolecules may consist of single strand, regular or irregular chains, or they may be double-stranded ladder-like structures or even sheets, the limit being a three-dimensional structure, which may be considered no longer to be within the province of polymers but better treated as a three-dimensional structure such as in a ceramic or glass. Finally, the polymers may be constituted of organic, organometallic, or even inorganic groups, including those of coordination type. Polymer nomenclature must attempt to describe all these types, and no satisfactory universal methodology has been developed.
Two basic methods have been developed to give names which are comprehensible and broadly consistent with the apparent structure. Neither method conveys all the details of the polymer structure, but one capable of doing so would probably be too long and complicated to be easily comprehensible, even to the informed reader. A shorter form is often adequate for many purposes.
Most polymers consisting of regular, single macromolecular chains may be named using structure-based nomenclature. Example (a) shows a generalized structure of such a polymer. A, B, C, and D represent groups of atoms comprising the main chain while E and R denote the chain end-groups and pendant groups, respectively. The CRU for this generalized structure, and the CRU and name and of a real polymer are also shown. Precise rules are necessary to govern the selection of the CRU.
Example (a)
Methods have also been developed to name irregular single-strand polymers and polymers of other structures, and these are also mentioned in Principles . Nevertheless, a precise structure-based name may be impossible to devise for a variety of reasons, such as lack of enough structural information. By far, the most widely used and easily implemented method of naming polymers is source-based nomenclature and example (b) shows three such names.
Example (b)
polyacrylonitrile polystyrene poly(dimethylstannanediyl)
The first two names are based on the names of the reagents (which may be monomers) from which the polymers were synthesized, acrylonitrile and styrene in these cases, but they convey little information about structure. However, this use of a reagent name may not be always applicable. For example, the source reagent to synthesize the third polymer cannot be simply dimethylstannanediyl, even though the polymer name itself is easily comprehensible. All three source-based names are organic style, but for the third, which describes an inorganic single-strand polymer, an inorganic-style name is also available:
catena -poly[dimethyltin]
These are only very simple examples, but they hint at some of the complexities involved in naming polymers, which differ from some of the methods used for naming small molecules. Principles also describes how more complicated polymer structures can be named, and also abbreviations for names that are commonly used both in academe and in industry. As usual, a list of basic references is also provided.
The recommendations and advice of Professor Richard G. Jones (University of Kent, Canterbury, and chair of the IUPAC Subcommittee on Polymer Terminology) during the preparation of this note are gratefully acknowledged.
{first published as ref 10}
10 The Special Case of Boron Hydrides
When writing down chemical structures, chemists feel happiest if they can depict how atoms are arranged in space and join them together appropriately with lines, each of which represents a two-electron bond. Unfortunately, though this type of model is adequate for many structures and compounds, it is not true for all. Organic chemists have developed methods that allow for the fact that aromatic compounds are not always adequately represented by names and structure based solely upon two-center two-electron bonds, and inorganic chemists have faced similar problems with certain classes of inorganic compound, such as boron hydrides. This edition of Principles carries a completely new chapter devoted to such compounds.
Like aromatic rings, boron hydrides are often not satisfactorily represented by structures consisting solely of two-center electron-pair bonds, though Nature still aims for full shells. The simplest boron hydride, B 2 H 6 , contains 12 valence electrons, and formally four pairs are localized in two-electron B-H bonds, with a further four in two three-center two electron B-H-B bonds, as in example (a). The complete name specifies both the number of boron atoms and the number of hydrogen atoms, which differs from organic practice, which assumes that the number of hydrogen atoms in the carbon analog of diborane would be obvious.

This method is extended to other boron hydrides as shown in example (b). All the apices represent B-H groups, and four three-center B-H-B bonds are designated. The name specifies the number of hydrogen atoms. In general, the polyboranes adopt the conformations of triangulated polyhedra, each of which has its own numbering convention.
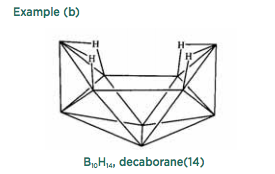
These polyboranes may formally lose hydrons to yield anions, of which Example (c) is typical.

Note that in this particular case, there are no bridging hydrogen atoms, and the number of hydrogen atoms is specified in the name.
The variations chemists can produce in these materials generate a wide range of different structures. A neutral borane can lose a one or two boron hydrides to yield so-called nido and arachno structures as depicted in example (d).

In addition to these variations, hydrogen atoms may be substituted. The names of resultant products must contain locants to specify at which skeletal positions substitutions have occurred.
Skeletal boron atoms may also be replaced by other atoms, operations termed subrogations by many boron chemists, yielding materials of which example (e) is an instance. There is an exo hydrogen atom, not shown, attached to each apical atom, carbon as well as boron.

Finally, the boranes may be considered to be similar to electronic delocalized aromatic systems, and like benzene and related derivatives they can also form sandwich compounds, as shown in example (f).
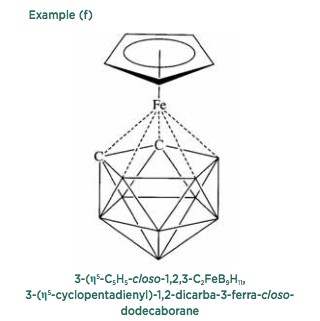
Sometimes all these variations may occur in the same structure, so that considerable care is required in determining the appropriate parent borane and the number of hydrogen atoms. Example (f) is derived from a parent dodecaborane, although there are only nine boron atoms in the actual structure, the three other apical positions being occupied by an iron atom and two carbon atoms. Consequently, an accurate structural diagram and the corresponding name can be rather large and complex. Principles summarizes all these structural types and the appropriate methods for naming them, together with references to the original literature.
{first published as ref 11}
11 Use of Abbreviations, Enclosing Marks, and Line-Breaks
The use of abbreviations sometimes causes more problems for a reader than is strictly necessary. The current free use of acronyms in texting has exaggerated these problems. In chemistry, care must always be taken to write in terms that are as clear as possible for any potential reader, and certain rules should always be followed in an attempt to achieve this. For the purposes of this post, the words abbreviation and acronym may be used interchangeably.
IUPAC has suggested a set of guidelines for the employment of abbreviations in chemistry texts (“The Use of Abbreviations in the Chemical Literature, Recommendations 1979,” PAC , 1980, 52(9), 2229– 2232). These recommendations suggest that “there are great advantages in defining all abbreviations . . . in a single conspicuous place in each paper . . . preferably near the beginning in a single list.” Included in these recommendations is a suggestion that no abbreviations should be used in titles or abstracts. The use of abbreviations in formulae is often preferable to the use of recommended names, but in such cases an accompanying definition may be absolutely necessary.
In English texts, there are certain abbreviations that are generally understood by all chemists, though thought should be given as to whether this will be true for speakers of other languages. Abbreviations such as thf (for tetrahydrofuran) may be self-evident to an English speaker but not to, say, a German or Hungarian speaker. Abbreviations for more complex organic groups should generally be defined. Generally accepted English abbreviations include those for organic substituent groups such as Ph, Me, Et. Pr, and Bu, though whether specific variants of qualified versions, such as t -Bu or Bu t , are preferred may be a matter of editorial style. Care should be exercised, because it is sometimes not evident whether an abbreviation such as Bz is meant to indicate benzyl or benzoyl or even benzene.
Inorganic chemists also generally have problems with abbreviations, especially for the names of ligands in the formulae of coordination complexes, because specific rules for producing abbreviations from systematic names are not generally available and lists of recommended abbreviations cannot be complete and comprehensive. The new Principles goes some way to deal with this by providing a long list containing the names of some of the commonest ligands, their recommended abbreviations, and the names from which the abbreviation was derived. For example, the abbreviation acac, derived from the non-standard name acetylacetonate, may be widely understood, though the current recommended IUPAC systematic name is 2,4-dioxopentan-3-ide. Some general principles for developing suitable abbreviations are also presented.
Polymers also have names that are often abbreviated, especially when the use is to define unequivocally a given material rather than to convey a detailed chemical structure. This is especially true in industry and commerce, names such as PTFE and PVC being common examples. Whereas IUPAC nomenclature methods can be used unequivocally to name specific polymers, the accepted abbreviations are often not based upon systematic names but upon trivial names, and many of the users of the abbreviations may not be chemists anyhow. The new Principles contains a discussion on polymer nomenclatures, including a list of the most widely used names and abbreviations. In addition, the subject of abbreviation is still a matter for discussion in particular areas, as demonstrated recently by Brimble et al. in “Rules for Abbreviation of Protecting Groups (IUPAC Technical Report),” PAC , 2013, 85(1), 307–313.
The IUPAC names of natural products are often rather long and complicated. For example, most people can identify what is meant by an acronym such as DNA, though each person probably understands its significance only in as much detail as is needed. Certainly the IUPAC name would only confuse most people, as well as consuming much time and space in presentation. In addition, IUPAC is not the only international body concerned with the nomenclature of materials such as DNA. Biochemical nomenclature is often based upon trivial names, and bodies such as the International Union of Biochemistry and Molecular Biology (IUBMB) are involved in developing and publishing recommendations, the latest IUBMB recommendations dating from 1992. There is a joint IUPAC/IUBMB committee that considers matters of interest to both Unions, including nomenclature problems. Amino acids, carbohydrates, and peptides, as well as nucleic acids, have generated their own specific nomenclatures, and all are dealt with in the new Principles , which provides references for those seeking more information.
Enclosing marks and line breaks are in common use throughout chemical literature. However, though their use may be defined quite clearly by nomenclaturists, their employment is often not consistent. The correct use is important when a sequence of enclosures is being used because these marks are employed in nomenclature as a hierarchy, dictating which set of marks enclose which. The principal enclosing marks are parentheses, ( ), sometimes simply called brackets or round brackets, curly brackets, { }, also often called braces in U.S. texts, and square brackets, [ ]. These are the principal marks used, and though some others may be found in specialized literature, these are those used by chemists. However, the order in which they are used depends upon the specific context.
In organic nomenclature generally and in inorganic names (but not formulae) the sequence to be employed is {[({[( )]})]} or ( ), [( )], {[( )]}, ({[( )]}), [({[( )]})}] {[({[( )]})]}. It will rarely be necessary to use a longer set than this. However, in formulae, and perhaps unfortunately, a different sequence is employed. One reason for this is the universal practice of enclosing the formulae of coordination entities, whether positively or negatively charged, or neutral, in square brackets. The sequence thus becomes [ ], [( )], [{( )}], [({( )})], [({({ })})], [({({( )})})], etc. This sequence, as printed, raises another question often posed when writing long names and formulae: Is the break at the end of the line in the fifth member of the last sequence simply an accident arising from the particular line and word length, or is it an intended break, so that the item is meant to read [({({ })})]? From the context, it is clearly the latter.
It is not possible here to describe all types of use of enclosing marks, and there may be some more specialized instances when minor variations to the above sequences are used. For example, polymer chemists employ an abbreviated hierarchy, which suffices for most general presentations of polymer formulae, namely {[( )]}, and there are other occasions when enclosing marks can help, even when their use is not mandatory. For example, simple parentheses may be used to distinguish terms such as trioxido, O 3 2- , from tri(oxido), (O 2- ) 3 . Such uses often amount to common sense. Clearly, the writer of names and formulae must be aware of the precise context in which the enclosing marks are being used, and select the appropriate sequence. All these matters are dealt with in Principles .
Principles also uses a specific device to deal with the problems sometimes posed by line-breaks. This device is not part of any IUPAC recommendation, but this writer has found it very useful and recommends it for consideration by the community as a whole. Many of the names, systematic or otherwise, employed by chemists contain hyphens to isolate and indicate distinct parts of the name. This is particularly common in names for organic compounds. They are often very long, and as written or printed contain a line-break, because it is not always possible or convenient to write a given name entirely on a single line. Since such names often contain hyphens anyhow, it may not be clear whether the hyphen at the end of a printed line is part of the name or simply indicates a line-break.
Take, for example, the following name: (1R*,3R*,5R*)-[(1S)- sec -Butoxy]-3-chloro-5-nitro- cyclohexane
Is the hyphen at the line end part of the name or should the final part read: nitrocyclohexane?
An inorganic example would be undecahydro-7,8- dicarba- nido -undecaborate(2-).
The hyphen at the end of the line poses a similar question.
In Principles these names would appear as follows, (1R*,3R*,5R*)-[(1S)-sec-Butoxy]-3-chloro-5-nitro-► cyclohexane and, in the inorganic example, undecahydro-7,8-► dicarba-nido-undecaborate(2-)
The symbol ► used as a line-break makes it clear that the hyphens are indeed part of the name and not imposed by typographical considerations. Principles contains many examples of the use of this device, and consideration of its adoption is recommended to the English-speaking chemical community. Whereas experienced chemists may not feel the need for such a device, the same will not be true for students, which is why it was employed in Principles. The use and value of such a device may vary from language to language and, as Bernardo Herold showed in CI , 2013, 35(3), 12–15 (ref 13), translations of chemistry texts and formulae between different languages raise all sorts of problems, for some of which this kind of device might also be useful.
{first published as ref 12}
- Nomenclature Notes, as published in Chem Int, from March 2012 to Dec 2013 - https://iupac.org/publications/ci/2012/3401/bw.html
- What is IUPAC Nomenclature? Chem. Int. Vol. 34, No. 2, Mar-Apr 2012 - https://iupac.org/publications/ci/2012/3402/nn.html
- On the Various Nomenclature Systems. Chem. Int. Vol. 34, No. 3, May-Jun 2012: - https://iupac.org/publications/ci/2012/3403/nn.html
- Non-IUPAC Nomenclature Systems. Chem. Int. Vol. 34, No. 4, July-Aug 2012 - https://iupac.org/publications/ci/2012/3404/nn.html
- Systematic and Trivial Nomenclature. Chem. Int. Vol. 34, No. 5, Sep-Oct 2012 - https://iupac.org/publications/ci/2012/3405/NN.html
- InChIs and Registry Numbers. Chem. Int. Vol. 34, No. 6, Nov-Dec 2012 - https://iupac.org/publications/ci/2012/3406/nn.html
- Deciphering and Constructing Names. Chem. Int. Vol. 35, No. 1, Jan-Feb 2013 - https://iupac.org/publications/ci/2013/3501/nn.html
- Drawing Chemical Structures. Chem. Int. Vol. 35, No. 2, Mar-Apr 2013 - https://iupac.org/publications/ci/2013/3502/nn.html
- Organometallic Nomenclature. Chem. Int. Vol. 35, No. 3, May-Jun 2013 - https://iupac.org/publications/ci/2013/3503/nn.html
- Polymer Nomenclature. Chem. Int. Vol. 35, No. 4, July-Aug 2013 - https://iupac.org/publications/ci/2013/3504/NN.html
- The Special Case of Boron Hydrides. Chem. Int. Vol. 35, No. 5, Sep-Oct 2013 - https://iupac.org/publications/ci/2013/3505/index.html
- Use of Abbreviations, Enclosing Marks, and Line-Breaks. Chem. Int. Vol. 35, No. 6, Nov-Dec 2013 - https://iupac.org/publications/ci/2013/3506/index.html
- Lost in Nomenclature Translation, Bernardo Herold, Chem. Int. Vol. 35, No. 3, May-Jun 2013, 12–15 - https://iupac.org/publications/ci/2013/3503/5_herold.html
Our Sponsors

Subscribe now
To be updated with the latest news and events from IUPAC
- ACS Publications
ACS Publishing Center
Author guidelines.
Last updated: December 29, 2023
Scope of the Journal
Manuscript types, submit with fast format, document templates and format, acceptable software, file designations, and tex/latex, cover letter, manuscript text components, supporting information, research data policy, data requirements, language and editing services, preparing graphics, figure and illustration services, prior publication policy, providing potential reviewer names, manuscript transfer, proofs via acs direct correct, publication date and patent dates, asap publication, post-publication policies, sharing your published article.
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained. Specifically, they may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis and semi-synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin. When new compounds are reported, manuscripts describing their biological activity are much preferred. Manuscripts that focus on biological properties of chemically complex extracts, mixtures, or essential oils are outside of the scope of the journal.
Manuscripts may be submitted as Articles, Notes, Reviews, or Perspectives. Authors should indicate in a cover letter accompanying the manuscript which category they intend for their submission.
All manuscripts will be submitted to a review process. Manuscripts will be considered for publication on the understanding that they have not been published or submitted for publication elsewhere.
Articles . Articles are comprehensive, critical accounts of work in the areas outlined above.
Notes. Notes are abbreviated papers presented in the same general style as Articles. Generally, studies that are narrower in scope than published in Articles are reported as Notes. When only one or two new compounds are reported in a manuscript, these substances must be of significant structural, biogenetic, and/or biological interest. The publication of known compounds will be considered only if they have been demonstrated to possess potentially important bioactivity. Manuscripts solely reporting NMR assignments or X-ray crystallographic data of known compounds will not be considered. For known compounds, authors should submit full experimental details of the isolation and identification data for consideration by the referees but not for publication. Full details of the isolation and identification procedures for known compounds may be made available to the reader as Supporting Information. (See the subsequent section on Supporting Information.)
Authors investigating the chemistry of a single species should aim to publish their results in a single manuscript rather than in a series of manuscripts describing each new compound as it is found. Manuscripts that report fragmentary parts of a larger study will be returned to the authors at the Editors’ discretion.
Reviews . Comprehensive reviews of topics within the scope of the journal and supported by significant literature should be submitted as reviews. Short reviews of recent literature that update a topic are also considered. The information in Reviews should be presented objectively, not limited to the contributions of the authors, and written with the intent of familiarizing the general reader with the broad current state of knowledge of a topic of active interest. The length of reviews should be commensurate with the information available; there are no formal limitations on length. Reviews are considered upon invitation, or after approval of presubmission inquiries outlining a synopsis of the manuscript sent to [email protected] .
Perspectives . Perspectives are personal reviews of subject or a topic in natural products, which should be focused rather than comprehensive. Perspective authors should assess the current status of the selected subject or topic, with an emphasis toward identifying important advances being made or those advances that are needed. Perspective reviews should present a forward-thinking approach in discussing the selected topic to be covered. The Journal of Natural Products Perspective should address the recent literature, including key contributors, aiming primarily to inspire and provide new insights to direct future research efforts. Perspectives authors will be invited by the Editor, or presubmission inquiries outlining a synopsis of the manuscript can be sent to [email protected] . Perspectives should be no more than 9,000 words, including the abstract, main text, and figure captions
While this document will provide basic information on how to prepare and submit the manuscript as well as other critical information about publishing, we also encourage authors to visit the ACS Publishing Center for additional information on everything that is needed to prepare (and review) manuscripts for ACS journals and partner journals, such as
- Mastering the Art of Scientific Publication , which shares editor tips about a variety of topics including making your paper scientifically effective, preparing excellent graphics, and writing cover letters.
- Resources on how to prepare and submit a manuscript to ACS Paragon Plus, ACS Publications’ manuscript submission and peer review environment, including details on selecting the applicable Journal Publishing Agreement .
- Sharing your research with the public through the ACS Publications open access program.
- ACS Reviewer Lab , a free online course covering best practices for peer review and related ethical considerations.
- ACS Author Lab , a free online course that empowers authors to prepare and submit strong manuscripts, avoiding errors that could lead to delays in the publication process.
- ACS Inclusivity Style Guide , a guide that helps researchers communicate in ways that recognize and respect diversity in all its forms.
Manuscript Preparation
All ACS journals and partner journals have simplified their formatting requirements in favor of a streamlined and standardized format for an initial manuscript submission. Read more about the requirements and the benefits these serves authors and reviewers here .
Manuscripts submitted for initial consideration must adhere to these standards:
- Submissions must be complete with clearly identified standard sections used to report original research, free of annotations or highlights, and include all numbered and labeled components.
- Figures, charts, tables, schemes, and equations should be embedded in the text at the point of relevance. Separate graphics can be supplied later at revision, if necessary.
- When required by a journal's structure or length limitations, manuscript templates should be used.
- References can be provided in any style, but they must be complete, including titles. For information about the required components of different reference types, please refer to the ACS Style Quick Guide .
- Supporting Information must be submitted as a separate file(s).
The templates facilitate the peer review process by allowing authors to place artwork and tables close to the point where they are discussed within the text. Learn more about document templates here .
General information on the preparation of manuscripts may also be found in the ACS Guide to Scholarly Communication .
See the list of Acceptable Software and appropriate File Designations to be sure your file types are compatible with ACS Paragon Plus. Information for manuscripts generated from TeX/LaTeX is also available.
A cover letter must accompany every manuscript submission. During the submission process, you may type it or paste it into the submission system, or you may attach it as a file.
To assist with the subsequent editorial process, it is preferred that manuscripts submitted to the Journal of Natural Products be prepared in single columns per page, double-spaced, with font size 12. The template is available for on the Information for Authors page. Use of a template is encouraged but not mandatory. The template facilitates the peer review process by allowing authors to place artwork and tables close to the point where they are discussed within the text.
Manuscripts may be submitted as Articles, Notes, Reviews (by invitation of presubmission inquiry), Perspectives (by invitation or presubmission inquiry), and Editorials (by invitation) (see “Scope and Editorial Policy” document). The manuscript title should appear on a separate page and should be followed by the author names and the institution name and address. The title, author name(s), and affiliations should all appear on their own respective line of text. Place an asterisk after the name of the author to whom enquiries regarding the paper should be directed and include that author’s telephone and fax numbers and e-mail address. Author affiliations should be footnoted using sequential lower-case letters, numbers, or symbols. Subdivisions (e.g., departments) of an institution should be grouped on the same line or lines. In article titles, the words “new” or “novel” (with the latter referring specifically to a compound based on an unprecedented carbon skeleton) should not be included, and the number of new substances obtained should not be specified. The title page and the rest of the manuscript should be typed in font size 12.
The abstract, detailing, in a single paragraph, the problem, experimental approach, major findings, and conclusions, should appear on the second page. It should be double spaced and should not exceed 200 words for Articles and Reviews or 100 words for Notes and Perspectives. Compounds mentioned in the abstract, and given as specific Arabic numerals that are bolded in the manuscript text, should also be accompanied in the abstract by the same bolded numerals. The abstract should be on a separate page and should be provided with the bolded and capitalized heading “ABSTRACT”.
Introduction
The manuscript should include an untitled introductory section stating the purpose of the investigation and relating the manuscript to similar research.
Results and Discussion
The “Results and Discussion” should be presented as a coherent whole section, in which the results are presented concisely. The discussion should interpret the results and relate them to existing knowledge in the field in as clear and brief a fashion as possible. Tables and figures should be designed to maximize the presentation and comprehension of the experimental data. Authors submitting a manuscript as a Note should omit the heading “Results and Discussion.” For Articles of unusual length, subheadings may be included within the “Results and Discussion” section. The major heading “Results and Discussion” should be bolded and capitalized, with the text starting on the line following. Subheadings are indented, followed by a period, and are a mix of uppercase and lowercase letters. The text follows on the same line as the subheading.
Bolded structural code numbers should only be used for new compounds and for those known compounds for which new biological data or spectroscopic values are being reported, and should be presented in the main text in ascending numerical order. Authors providing manuscripts focusing on the biological properties of two or fewer known natural products have the option of referring to the compound(s) concerned by name, rather than assigning each a bolded numerical code number. Other known compounds should be referred to in the text by name, wherever necessary. Sugar units in glycosides should not be inferred as D or L based solely on NMR data analysis, but should be determined by supporting experimental work such as measurement of their optical rotations following acid hydrolysis or by the preparation of chiral derivatives and comparison with standards using a chromatographic analytical method. If the aglycone of a glycoside is also a new compound, then it should be isolated and its physical constants and spectroscopic parameters stated. Authors are advised to use correctly the terms “relative and absolute configuration” instead of “relative and absolute stereochemistry”. In, for example, a carbocyclic compound, only a stereogenic carbon or a stereogenic element, such as an axis, possesses configuration. Substituents such as methyl groups are either alpha or beta oriented and are not alpha or beta configured. Care should be taken not to make erroneous configurational conclusions via NMR NOE associations from ring to side-chain protons of, for example, sterols and tetracyclic triterpenoids. The term “spectral” should be avoided in a structure elucidation discussion, when “spectroscopic” or “spectrometric” are meant instead. When describing mass spectrometric details, authors should not refer to the terms “pseudomolecular ion”, “quasimolecular ion”, or “protonated molecular ion" and should refer instead to, e.g., “a sodium adduct ion”, “a protonated molecule”, or a “deprotonated molecule” (see Pure Appl. Chem . 2013 , 85 , 1515–1609).
In manuscripts that present results of biological studies with tumor cell lines or animal-based tumor models, authors should pay special attention to the U.S. National Cancer Institute (NIH) guidelines for cancer drug discovery studies. Compounds that suppress the growth of, or kill, isolated tumor cell lines grown in culture should be referred to as either “cytostatic” or “cytotoxic”, as appropriate. Only compounds that inhibit the growth of tumors in animal-based models should be called “antitumor”. The term “anticancer” should be reserved for compounds that show specific activity in human-based clinical studies (see Suffness, M.; Douros, J. J. Nat . Prod . 1982 , 45 , 1–14). Some flexibility in this system is afforded in the description of compounds that show activity in molecular-targeted antitumor assays. Compounds should be compared against a suitable positive control substance and follow accepted guidelines when represented as “active”. For example, a cytotoxic pure substance when tested against a cancer cell line would exhibit an IC 50 value of <10 μ M (or 4–5 μ g/mL).
Experimental Section
The presentation of specific details about instruments used, sources of specialized chemicals, and related experimental details should be incorporated into the text of the Experimental Section as a paragraph headed General Experimental Procedures. The general order for inclusion should be as follows: melting points; optical rotations; UV spectra; ECD and/or VCD spectra; IR spectra; NMR spectra; mass spectra; and chromatographic and other techniques.
In a separate paragraph, experimental biological material should be reported as authenticated if cultivated or from a natural habitat, and the herbarium deposit site and voucher number should be recorded. The month and year when the organisms were collected should be stated, and it is recommended that the exact collection location be provided using a GPS navigation tool. All microorganisms used experimentally should bear a strain designation number and the culture collection in which they are deposited. The scientific name (genus, species, authority citation, and family) should be presented when first mentioned in the body of the manuscript. Thereafter, the authority should be eliminated, and the generic name should be reduced (except in tables and figure legends) to the first capital letter of the name (but avoid ambiguity, if two or more generic names have the same first letter). If the biological material has not been identified as to species, the manuscript will not be considered for publication unless a special protocol has been followed. Thus, a voucher specimen of the organism should be deposited with a recognized taxonomist for the particular group of organisms in question. The taxonomist should then assign to the specimen an identifying number unique to the organism so that any additional collections of the same organism would bear this same number. The number will be retained until the organism is completely identified. The taxonomist should write a brief taxonomic description to be included in the manuscript, which should state how the organism in question relates morphologically to known species. Contributors should use DNA sequence analysis to assist with the taxonomic identification of unknown microorganisms, and to deposit these data in GenBank . Photographs of incompletely identified organisms may be included as Supporting Information. Authors should be aware of the fact that the large-scale collection of marine or terrestrial organisms may have negative ecological effects. Therefore, authors describing an investigation derived from large-scale collections should thus include a statement in their manuscript (in the “Biological Material” paragraph of the Experimental Section) explaining why the collection had no significant adverse ecological effect or justifying such effect in terms of the benefit from the resulting work. When organisms are collected from a foreign country, the corresponding author must state in the cover letter with the submitted manuscript that formal collection permission was obtained. Authors who purchase dried “herbal remedies” or other materials from companies must make provision for their proper deposit in a herbarium or other permanent repository, for access by future workers. When a commercially available extract is obtained, the extraction procedure from the organism of origin must be specified. The identification of the extract should be supported by an HPLC trace of known secondary metabolite constituents of the organism, which should be included with the manuscript as Supporting Information.
When physical and spectroscopic data are presented in the body of the manuscript, the following general style must be used (with the various commonly used techniques presented in this same order):
Romucosine (1): colorless needles (CHCl 3 ); mp 152–153 °C; [a] 25 D –110 ( c 0.4, CHCl 3 ); UV (EtOH) λ max (log ε) 235 (4.23), 275 (4.18), 292 (sh) (3.52), 325 (3.41) nm; IR (Nujol) ν max 1680, 1040, 920 cm –1 ; 1 H NMR (CDCl 3 , 400 MHz) δ 8.11 (1H, d, J = 7.6 Hz, H-11), 7.54–7.28 (2H, m, H-9, H-10), 7.27 (1H, m, H-8), 6.59 (1H, s, H-3), 6.10, 5.97 (each 1H, d, J = 1.5 Hz, OC H 2 O), 4.86 (1H, dd, J = 13.7, 4.4 Hz, H-6a), 4.44 (1H, m, H-5a), 3.77 (3H, s, NCOOC H 3 ), 3.06 (1H, m, H-7a), 2.99 (1H, m, H-5b), 2.91 (1H, m, H-7b), 2.82 (1H, m, H-4a), 2.61 (1H, m, H-4b); 13 C NMR (CDCl 3 , 100 MHz) δ 155.8 (C, N C OOCH 3 ), 146.8 (C, C-2), 143.0 (C, C-1), 135.8 (C, C-7a), 130.7 (C, C-11a), 128.7 (CH, C-8), 127.79 (C, C-3a), 127.78 (CH, C-9), 127.2 (CH, C-10), 127.0 (CH, C-11), 125.6 (C, C-3b), 117.3 (C, C-1a), 107.6 (CH, C-3), 100.9 (CH2, OCH 2 O), 52.7 (CH3, NCOO C H 3 ), 51.7 (CH, C-6a), 39.2 (CH2, C-5), 34.5 (CH2, C-7), 30.4 (CH2, C-4); EIMS m / z 323 [M] + (98), 308 (28), 292 (5), 262 (20), 248 (21), 236 (81), 235 (100), 206 (17), 178 (27), 88 (17); HREIMS m / z 323.1152 (calcd for C 19 H 17 NO 4 , 323.1158).
The correct presentation of NMR spectroscopic data is shown in the table below.
Table 1. NMR Spectroscopic Data (400 MHz, C6D6) for Aurilides B ( 1 ) and C ( 2 )

The correct format to present elemental analysis data is: anal. C 72.87, H 11.13%, calcd for C 37 H 68 O 6 , C 73.02, H 11.18%. The structures of compounds are expected to be supported by high-resolution mass spectrometry (error limit 5 ppm or 0.003 m / z units) or elemental analysis. Melting point determinations should not be provided for compounds described as “amorphous solids.” The unit of concentration to be used for optical rotation measurements is grams per 100 mL. UV extinction coefficient data should be provided as log є values, to two places of decimals. In reporting 1 H NMR data of diastereotopic methylene protons, the deshielded one should be listed as the “a” proton and the shielded one as the “b” proton, as in “H-10a” and “H-10b”, respectively. If two proton or carbon signals in an NMR spectrum appear at the same chemical shift but are still distinguishable, an additional decimal place (three for 1 H NMR data and two for 13 C NMR data) may be used to designate the resonance in question. Carbon-13 NMR data should be reported to the nearest 0.1 ppm with the number of attached protons designated using the C, CH, CH 2 , and CH 3 notation.
Authors must emphasize any unexpected, new, and/or significant hazards or risks associated with the reported work. This information should be in the experimental details section of the Article, Note, or Rapid Communication.
Associated Content
This section has the bolded subheading Supporting Information and should contain a brief non- sentence description of each file deposited. (A full description of the requirements for the Supporting Information is provided later this document.)
Author Information
A section may be included, as needed, entitled “Author Notes” to provide pertinent information on the authors, such as the names of authors who contributed equally to the article.
Acknowledgments
The Acknowledgments section should include credits [initial(s) and last name] for technical assistance, financial support, and other appropriate recognition. During manuscript submission, the submitting author is asked to select funding sources from the list of agencies included in the FundRef Registry .
The References section should provide both citations to the literature and all notes, regardless of their nature, which should be numbered in order of appearance in the manuscript and cited in the text with superscript numbers. Each reference may have its own citation number, or alternatively, references referring to the same topic may be grouped under a common number using alphabetical subdesignations (e.g., 1a, 1b, 1c). Each note should be assigned its own number. References and notes should follow the format shown:
- Journal references can be provided in any style, as noted in the Review Ready Submission section, and titles must be included.
- Linington, R. G.; Williams, P. G.; MacMillan, J. B. Problems in Organic Structure Determination. A Practical Approach to NMR Spectroscopy ; CRC Press/Taylor and Francis Group: Boca Raton, FL, 2016.
- Harada, N.; Nakanishi, K.; Berova, N. In Comprehensive Chiroptical Spectroscopy, Vol. 2; Applications in Stereochemical Analysis of Synthetic Compounds, Natural Products, and Biomolecules ; Berova, N., Polavarapu, P. L., Nakanishi, K., Woody, R. W., Eds.; John Wiley & Sons: New York, 2012; pp 115–166.
- Zheng, G.; Kakisawa, H. Chin. Sci. Bull. 1990 , 35 , 1406–1407; Chem. Abstr. 1991 , 114 , 43213 m .
- Imai, A. Pharmacognosy of the Aerial Parts of Black Cohosh ( Cimicifuga racemosa ). Ph.D. Dissertation, University of Illinois at Chicago, Chicago, IL, 2013.
- Davis, R. U.S. Patent 5,708,591, 1998.
- Partial data for plakinic acid M were reported in the Supporting Information of Ref 5a, but a more complete listing is given here for comparative purposes.
- World Health Organization. Fact Sheet No. 94, 2015. http://www.who.int/mediacentre/factsheets/fs094/en/ (accessed October 1, 2015).
For additional information on the reference and note format to use, see The ACS Style Guide , 3rd ed. (2006) ( https://pubs.acs.org/page/styleguide ), available from Oxford University Press, Order Department, 2001 Evans Road, Cary, NC 27513 ( http://www.oup.com ).
The author is responsible for the accuracy and completeness of all references. In particular, authors must cite all of the references from their own work on a particular topic, such as all papers published or submitted on the constituents of a given organism under consideration. In addition to the citation, it should be explicitly indicated in the text if this is a continuing work for the same group. Because subscribers to the Web edition are now able to click on the “CAS” tag following each reference to retrieve the corresponding CAS abstract, reference accuracy is critical. Journal abbreviations should be those used by Chemical Abstracts [see Chemical Abstracts Service Source Index (CASSI) 1907 – 2004 ]. A list of journal abbreviations in the ACS Style Guide can also be accessed.
The author should supply the Editor-in-Chief with copies of related manuscripts that are cited as “in press” or “submitted” for use by the editors and the reviewers in evaluating the manuscript under consideration.
Nomenclature
It is the responsibility of the authors to provide correct nomenclature. All nomenclature must be consistent and unambiguous and should conform with current American usage. Insofar as possible, authors should use systematic names similar to those used by Chemical Abstracts Service, the International Union of Pure and Applied Chemistry, and the International Union of Biochemistry and Molecular Biology. For new natural products that are closely related structurally to known compounds, it is much preferred to assign the new compound as a derivative of the known compound, rather than introduce a completely new trivial name into the literature.
Chemical Abstracts ( CA ) nomenclature rules are described in Appendix IV of the Chemical Abstracts Index Guide . A list of ring systems, including names and numbering systems, is found in the Ring Systems Handbook , American Chemical Society, Columbus, OH, 2003, and its latest cumulative supplement. For CA nomenclature advice, consult the Manager of Nomenclature Services, Chemical Abstracts Service, P.O. Box 3012, Columbus, OH 43210-0012. A name generation service is available for a fee through CAS Client Services, 2540 Olentangy River Road, P.O. Box 3343, Columbus, OH 43210-0334; tel: (614) 447- 3870; fax: (614) 447-3747; or e-mail: [email protected].
For IUPAC rules, see:
- Nomenclature of Inorganic Chemistry, Recommendations, 1990 ; Blackwell Scientific Publications: Oxford, England, 1990.
- A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations, 1993 ; Blackwell Scientific Publications: Oxford, England, 1993.
- Nomenclature of Organic Chemistry, Sections A – F and H ; Pergamon Press: Elmsford, NY, 1979.
- Compendium of Macromolecular Nomenclature ; Blackwell Scientific Publications: Oxford, England, 1991.
- Biochemical Nomenclature and Related Documents , 2 nd ed.; Portland Press, Ltd.: London, England, 1992.
- Selected IUPAC recommendations can be found on the Web at http://www.chem.qmw.ac.uk/iupac/iupac.html .
- The ACS Web site has links to nomenclature recommendations: chemistry.org .
Abbreviations
Abbreviations are used without periods. Standard abbreviations should be used throughout the manuscript. All nonstandard abbreviations should be kept to a minimum and must be defined in the text following their first use. The preferred forms of some of the more commonly used abbreviations are mp, bp, °C, K, s, min, h, mL, μ L, kg, g, mg, μ g, cm, mm, nm, mol, mmol, μ mol, ppm, TLC, GC, NMR, MS, UV, ECD/VCD, and IR. For further information, refer to The ACS Style Guide (2006).
Authors should not provide a separate list of abbreviations in a manuscript; additional abbreviations should be spelled out in full the first time they are mentioned. Authors are discouraged from using abbreviations for terms that are included in the manuscript in only a few instances.
Figures, Schemes, and Charts are numbered with Arabic numerals. Blocks of chemical structures should not be designated as “Figures”. Each graphic must be identified outside the frame of the graphic. The quality of the illustrations depends on the quality of the originals provided. Graphics cannot be modified or enhanced by the journal production staff. The graphics must be submitted as part of the manuscript file and are used in the production of the Journal (material deposited as Supporting Information will not be published in the print edition). The preferred submission procedure is to embed graphics in a Word document. It may help to print the manuscript on a laser printer to ensure all artwork is clear and legible.
Additional acceptable file formats are TIFF, PDF, EPS (vector artwork), or CDX (ChemDraw file). Labeling of all figure parts should be present, and the parts should be assembled into a single graphic. (For EPS files, ensure all fonts are converted to outlines or embedded in the graphic file. The document settings should be in RGB mode.)
TIFF files should have the following minimum resolution requirements:
- Black and white line art: 1200 dpi
- Grayscale art: 600 dpi
- Color art (RGB mode): 300 dpi
Color graphics submitted in CMYK or at lower resolution may result in poor-quality images. Save graphic files at the final resolution and size using the program used to create the graphic. The inclusion of a color photograph is particularly recommended for manuscripts based on the constituents of organisms that are not identified beyond the genus level. Digital photographs are accepted. Photographs that are single or double column width so that they will not have to be reduced work best.
Layout. In preparing structures for publication, layout is critical. Figures, Schemes, Charts, and blocks of structures are presented in the Journal either in one-column or two-column format.
For efficient use of journal space, single-column illustrations are preferred.
- Maximum width:240 pts (3.33 in.)
- Maximum depth: 660 pts (9.16 in.)
- Minimum width: 300 pts (4.16 in.)
- Maximum width: 504 pts (7 in.)
Authors are advised that structural material labeled as a “Figure” is placed at the top or bottom of a page, as is all two-column material. All structural material that should immediately follow certain text must be designed to fit the one-column format, and its location in the text must be indicated in the manuscript. Structures, arrows, and compound designators should be arranged so as to make maximum use of the width afforded by the one-column or two-column format.
For best results, illustrations should be submitted in the actual size at which they should appear in the Journal. Consistently sized letters and labels in graphics throughout the manuscript will help ensure consistent graphic presentation for publication. Lettering should be no smaller than 4.5 points. (Helvetica or Arial type works well for lettering.) Lines should be no thinner than 0.5 point. Lettering and lines should be of uniform density. If artwork that should be reduced must be submitted, larger lettering and thicker lines should be used so that, when reduced, the artwork meets the above-mentioned parameters.
Complex textures and shading to achieve a three-dimensional effect should be avoided. To show a pattern, a simple cross-hatch design should be used.
Content. Abbreviations such as Me for CH 3 , Et for C 2 H 5 , and Ph (but not Φ ) for C 6 H 5 are acceptable. Make liberal use of “R and X groups” in equations, schemes, and structure blocks to avoid the repetition of similar structures. Do not repeat a structure; the number alone of an earlier structure can be used if a compound occurs several times. Within graphics, structures should be numbered with boldface Arabic numerals, consecutively from left to right, top to bottom, regardless of the order in which the compounds are discussed in the text. It is not necessary to give reagents and conditions in complete detail, since this detail is contained in the Experimental Section. Where needed, numbers such as NMR chemical shifts may be included directly on structural formulas.
Table of Contents/Abstract Graphic
A graphic must be included with each manuscript that will be used for both the abstract and the Table of Contents (TOC) of the Web edition of the Journal issue in which the Article, Note, Perspective, or Review will appear. This graphic should capture the reader’s attention and, in conjunction with the manuscript’s title, should give the reader a quick visual impression of the type of chemistry described and/or the biological results obtained; however it should not be too complex. Structures in the TOC graphic should be constructed as specified in the “Chemical Structures” section below. The TOC graphic should be submitted at the actual size to be used and should be no larger than 3.25 in. (8.5 cm) wide and 1.75 in. (4.45 cm) tall. (See detailed instructions at http://pubs.acs.org/page/4authors/submission/howtosubmit.html .) Text should be limited to labels for compounds, reaction arrows, and figures. The use of color to enhance the scientific value is highly encouraged. The TOC graphic should be inserted on a separate page at the end of the manuscript file. The title and author list will be added during production.
Chemical Structures
Structures should be produced with the use of a drawing program such as ChemDraw. Structure drawing requirements (preset in the ACS Stylesheet in ChemDraw) are as follows:
- chain angle, 120º
- bond spacing, 18% of width
- fixed length, 14.4 pt (0.508 cm, 0.2 in.)
- bold width, 2.0 pt (0.071 cm, 0.0278 in.)
- line width, 0.6 pt (0.021 cm, 0.0084 in.)
- margin width, 1.6 pt (0.056 cm, 0.0222 in.)
- hash spacing, 2.5 pt (0.088 cm, 0.0347 in.)
- font, Arial/Helvetica
- size, 10 pt
- units, points
- tolerances, 5 pixels
- paper, US Letter
- scale, 100%
- (5) Using the ChemDraw ruler or appropriate margin settings, create structure blocks, schemes, and equations having maximum widths of 11.3 cm (one-column format) or 23.6 cm (two-column format)
- (6) Embolden compound numbers, but not atom labels or captions.
- (7) Authors are urged to use only a single configurational descriptor when defining a stereocenter in a chemical structure. Atom numbering should be kept outside of rings wherever possible. Rather than rectangular solid and dashed lines, authors should use solid and dashed wedges to indicate configurations, as shown below. Dots at ring junctions intended to represent hydrogen atoms should not be used. Structures should be drawn in a neat manner ready for direct reproduction, and should not be cluttered or overlapping. Any arrows and numbering used for atoms in figures should not come into contact with bonds or ring systems. See an example of a prepared structure using ChemDraw with the specified preferences below. In molecules containing a chiral biphenyl axis, it is recommended that one of the aromatic rings be drawn in the plane of the paper and the second one be rotated out of the plane of the paper, to reflect the P or M conformation about the biphenyl bond (see below for example).

When the structure of a chiral compound is flipped horizontally, the stereodescriptors should be changed at every stereogenic carbon, otherwise the enantiomer of the relevant compound would be depicted. This is depicted below for the b-D-glucopyranoside of phenol. The 1 to 2 horizontal flip is incorrect since the depicted glucopyranosyl moiety belongs to the L-series of glucopyranoses. The 1 to 3 horizontal rotation through 180°/adjustment of the tetrahydropyran ring is correct and shows the descriptor changes required to retain the D-configuration of the glucopyranose moiety. Alternatively, in the “planar” presentations the 4 to 5 horizontal flip is incorrect and the 4 to 6 horizontal rotation is correct, showing the proper descriptor changes. Please note that presentations 4 and 6 are InChI (International Chemical Identifier) compliant, while 1 and 3 are not.

Authors using other drawing packages should, in as far as possible, modify their program’s parameters so that they reflect the above guidelines.
These should be numbered consecutively with Arabic numerals and should be placed as they should appear in the paper. Footnotes in tables should be given lowercase letter designations and be cited in the table by italic superscript letters. The sequence of letters should proceed by line rather than by column. If a footnote is cited both in the text and in a table, insert a lettered footnote in the table to refer to the numbered footnote in the text. Each table should be provided with a descriptive heading, which, together with the individual column headings, should make the table, as nearly as possible, self- explanatory. In setting up tabulations, authors are requested to keep in mind the type area of the journal page (17.8 × 25.4 cm) and the column width (8.5 cm), and to make tables conform to the limitations of these dimensions. Arrangements that leave many columns partially filled or that contain much blank space should be avoided.
Conflict of Interest Disclosure
A statement describing any financial conflicts of interest or lack thereof is published with each manuscript. During the submission process, the corresponding author must provide this statement on behalf of all authors of the manuscript. The statement should describe all potential sources of bias, including affiliations, funding sources, and financial or management relationships, that may constitute conflicts of interest (please see the ACS Ethical Guidelines to Publication of Chemical Research ). The statement will be published in the final article. If no conflict of interest is declared, the following statement will be published in the article: “The authors declare no competing financial interest.”
It is a mandatory requirement for authors to deposit copies of NMR spectra for all new compounds in the Supporting Information with at least the 1 H and 13 C NMR spectra included . A typical caption for a spectrum would be: “S1. 1 H NMR (400 MHz, CDCl 3 ) spectrum of the new compound xx ”. Supporting Information pages should be consecutively numbered and a table of contents for the Supporting Information should be included.
This information is provided to the reviewers during the peer-review process (for Review Only) and is available to readers of the published work (for Publication). Supporting Information must be submitted at the same time as the manuscript. See the list of Acceptable Software by File Designation and confirm that your Supporting Information is viewable .
If the manuscript is accompanied by any supporting information files for publication, these files will be made available free of charge to readers. A brief, nonsentence description of the actual contents of each file, including the file type extension, is required. This description should be labeled Supporting Information and should appear before the Acknowledgement and Reference sections. Examples of sufficient and insufficient descriptions are as follows:
Examples of sufficient descriptions: “Supporting Information: 1 H NMR spectra for all compounds (PDF)” or “Additional experimental details, materials, and methods, including photographs of experimental setup (DOC)”.
Examples of insufficient descriptions: “Supporting Information: Figures S1-S3” or “Additional figures as mentioned in the text”.
When including supporting information for review only, include copies of references that are unpublished or in-press. These files are available only to editors and reviewers.
All ACS journals strongly encourage authors to make the research data underlying their articles publicly available at the time of publication.
Research data is defined as materials and information used in the experiments that enable the validation of the conclusions drawn in the article, including primary data produced by the authors for the study being reported, secondary data reused or analyzed by the authors for the study, and any other materials necessary to reproduce or replicate the results.
The ACS Research Data Policy provides additional information on Data Availability Statements, Data Citation, and Data Repositories.
NMR Data Files
When submitting spectra, authors should adhere to the following guidelines:
A caption should be included on the spectrum, noting the nucleus being measured, the solvent (formula preferred, e.g., CDCl 3 ), and the field strength. A representation of the compound should be included on the spectrum; please use ChemDraw or a related program. The bold compound number used in the manuscript should be included. The largest peak in the 1 H NMR spectrum should normally arise from the compound, not the solvent. All peaks in the 1 H NMR spectrum should be integrated. Chemical shift values should be included. The solvent peak should be clearly labeled on the spectrum. All peaks should be visible on the spectrum. Insets are encouraged to show expanded regions. At minimum, the spectral window should be –1 ppm to 9 ppm for 1 H NMR and –10 ppm to 180 ppm for 13 C NMR. The font should be clear and large enough to read (minimum of 10 point). Horizontal orientation is preferred for spectra.
For every new compound, a copy of a well-resolved 1D proton NMR spectrum and a copy of a proton- decoupled 1D carbon spectrum (conventional, DEPT, DEPTQ, or PENDANT), should be included in the supporting information. In cases where structure assignments of complex molecules depend heavily on NMR data interpretation, including isolated and synthesized natural products, copies of the 2D spectra are requested. All original primary NMR data supporting a submission should be retained and provided if requested. Additionally, authors are strongly encouraged to furnish a folder of the primary (“raw”) NMR data files (free induction decay (FID) and 2D serial files) as additional Supporting Information. Authors reporting compounds of complex, unusual, or unexpected structure are encouraged to provide FID data. The FID data should be mentioned in the Supporting Information availability statement in the manuscript file. The FID and serial file data can also be deposited in a public repository that provides a permanent link, such as a DOI.
When preparing raw NMR data (FIDs):
- One folder (root folder) should be created for each compound
- The root folder should be named clearly, including the compound number and/or a unique identifier
- Establish subfolders for each spectrum and name them according to the type of nucleus measured and experiment performed: 1H, 13C, DEPT, COSY, etc.
- Include in each subfolder the actual FID or serial files, acquisition data and processing parameters for each experiment; depending on the spectrometer used, this can be several files or a combination of further folders and files
- In a text document with the same name as the root folder, include the name of the manufacturer of the spectrometer used to collect the data, the acquisition software and processing programs used to analyze the data and the operating frequency used to measure each nucleus (e.g. 300 MHz 1H or 75 MHz 13C)
- Include a structure file that shows the structure and compound identifier for each provided dataset. MolFile is the strongly preferred format
- Compress the entire root folder into a single zip archive file
Recommendations for Crystal Structure Papers
Although the results of crystal structure determinations are frequently of interest to readers of the Journal, details of crystal structure experiments are generally not. Results appropriate for the Journal are not, however, sufficient to allow referees to assess the quality of an X-ray structure determination. Thus, it is recommended that manuscripts involving such determinations be accompanied by material provided for the benefit of the reviewers only. Authors should submit the following minimum materials, in tabular form where possible, for each compound for which X-ray crystallographic supplementary data are available.

Published Manuscript:
- Crystal data, including chemical formula, formula weight, crystal system and space group, cell dimensions (with uncertainties), number of formulas per unit cell, calculated density, radiation used, and wavelength. When determined, the Flack and/or Hooft parameters should be included.
- Final fractional atomic coordinates. Hydrogen atom coordinates should be included only if they have been experimentally determined or refined. Calculated coordinates should be provided as reviewer’s material.
- A brief outline of procedures used for data collection and refinement, including the method used for intensity measurement, 0 limits, portion of the full sphere collected, handling of absorption (if applicable), method of refinement, number of reflections used in the refinement and criteria for their choice, treatment of hydrogen atoms, and final R factor.
- A perspective diagram (perhaps prepared by ORTEP, PLUTO, or similar programs) that gives the atom-numbering scheme if it is not unambiguous from the remainder of the paper. If the figure is a stereoview, it should be provided reduced to correct size, about 55–60 mm between images.
Besides a description of the structure, other information (important distances, torsion angles, results of best plane calculations, etc.) may be included if appropriate. A note should be cited at an appropriate place in the manuscript and included in the References and Notes Section: “Crystallographic data for the structure(s) reported in this paper have been deposited with the Cambridge Crystallographic Data Centre. Copies of the data can be obtained, free of charge, on application to the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-(0)1223-336033 or e-mail: [email protected] ) .”
Reviewer’s Material:
- Any calculated coordinate (e.g., hydrogen atoms).
- A full list of bond distances (and their uncertainties).
- A full list of bond angles (and their uncertainties).
All tables should be clearly legible, the contents nonredundant, and their interpretation immediately obvious. Authors must provide this information in the form of a Crystallographic Information File (CIF) for each compound for which X-ray crystallographic data are determined, with each CIF being separated from any other Supporting Information files.
Authors will deposit the tables of final fractional atomic coordinates and the full list of bond lengths and angles at the Cambridge Crystallographic Data Centre (CCDC) prior to the submission of their paper. The CCDC deposition number must be included in the submitted manuscript. A checklist of data items for deposition is available at www.ccdc.cam.ac.uk .
A well-written paper helps share your results most clearly. ACS Publications’ English Editing Service is designed to help scientists communicate their research effectively. Our subject-matter expert editors will edit your manuscript for grammar, spelling, and other language errors so your ideas are presented at their best.
The quality of illustrations in ACS journals and partner journals depends on the quality of the original files provided by the authors. Figures are not modified or enhanced by journal production staff. All graphics must be prepared and submitted in digital format.
Graphics should be inserted into the main body whenever possible. Please see Appendix 2 for additional information.
Any graphic (figure chart, scheme, or equation) that has appeared in an earlier publication should include a credit line citing the original source. Authors are responsible for obtaining written permission to re-use this material.
The impact of your research is not limited to what you can express with words. Tables and figures such as graphs, photographs, illustrations, diagrams, and other visuals can play a significant role in effectively communicating your findings. Our Artwork Editing and Graphical Abstract services generate publication-ready figures and Table of Contents (TOC) graphics that conform to your chosen journal’s specifications. For figures, this includes changes to file type, resolution, color space, font, scale, line weights, and layout (to improve readability and professional appearance). For TOC graphics, our illustrators can work with a rough sketch or concept or help extract the key findings of your manuscript directly for use as a visual summary of your paper.
Preparing for Submission
Manuscripts, graphics, supporting information, and required forms, as well as manuscript revisions, must all be submitted in digital format through ACS Paragon Plus , which requires an ACS ID to log in. Registering for an ACS ID is fast, free, and does not require an ACS membership. Please refer to Appendix 1 for additional information on preparing your submission
Journal of Natural Products authors are allowed to deposit an initial draft of their manuscript in a preprint service such as ChemRxiv , arXiv, or bioRxiv. A patent or a published patent application is not considered to be a prior "publication". Please note any use of a preprint server, patents, and dissertations in the cover letter, and as appropriate, state how the manuscript has been adjusted/updated between deposition and submission. All other prior/redundant publications are forbidden. Upon publication in Journal of Natural Products , authors are advised to add a link from the preprint to the published paper via the Digital Object Identifier (DOI).
Please suggest 4 reviewers. Authors are encouraged to avoid suggesting reviewers from the authors’ institutions. Do not suggest reviewers who may have a real or perceived conflict of interest . Whenever possible, suggest academic email addresses rather than personal email addresses.
If your submission is declined for publication by this journal, the editors might deem your work to be better suited for another ACS Publications journal or partner journal and suggest that the authors consider transferring the submission. Manuscript Transfer simplifies and shortens the process of submitting to another ACS journal or partner journal, as all the coauthors, suggested reviewers, manuscript files, and responses to submission questions are copied by ACS Paragon Plus to the new draft submission. Authors are free to accept or decline the transfer offer.
Note that each journal is editorially independent. Transferring a manuscript is not a guarantee that the manuscript will be accepted, as the final publication decision will belong to the editor of the next journal.
PRODUCTION AND PUBLICATION
Correction of the galley proofs is the responsibility of the Corresponding Author. The Corresponding Author of an accepted manuscript will receive e-mail notification and complete instructions when page proofs are available for review via ACS Direct Correct . Extensive or important changes on page proofs, including changes to the title or list of authors, are subject to review by the editor.
It is the responsibility of the Corresponding Author to ensure that all authors listed on the manuscript agree with the changes made on the proofs. Galley proofs should be returned within 48 hours in order to ensure timely publication of the manuscript.
Accepted manuscripts will be published on the ACS Publications Web site as soon as page proofs are corrected and all author concerns are resolved. The first date on which the document is published on the Web is considered the publication date.
Publication of manuscripts on the Web may occur weeks in advance of the cover date of the issue of publication. Authors should take this into account when planning their patent and intellectual property activities related to a document and should ensure that all patent information is available at the time of first publication, whether ASAP or issue publication.
All articles published ahead of print receive a unique Digital Object Identifier (DOI) number, which is used to cite the manuscript before and after the paper appears in an issue. Additionally, any supplemental information submitted along with the manuscript will automatically be assigned a DOI and hosted on Figshare to promote open data discoverability and use of your research outputs.
Manuscripts will be published on the “ASAP Articles” page on the web as soon as page proofs are corrected and all author concerns are resolved. ASAP publication usually occurs within a few working days of receipt of page proof corrections, which can be several weeks in advance of the cover date of the issue.
The American Chemical Society follows guidance from the Committee on Publication Ethics (COPE) when considering any ethical concerns regarding a published article, Retractions, and Expressions of Concern.
Additions and Corrections
Additions and Corrections may be requested by the author(s) or initiated by the Editor to address important issues or correct errors and omissions of consequence that arise after publication of an article. All Additions and Corrections are subject to approval by the Editor, and should bring new and directly relevant information and corrections that fix scientific facts. Minor corrections and additions will not be published. Readers who detect errors of consequence in the work of others should contact the corresponding author of that work.
Additions and Corrections must be submitted as new manuscripts via ACS Paragon Plus by the Corresponding Author for publication in the “Addition/Correction” section of the Journal. The corresponding author should obtain approval from all coauthors prior to submitting or provide evidence that such approval has been solicited. The manuscript should include the original article title and author list, citation including DOI, and details of the correction.
Retractions
Articles may be retracted for scientific or ethical reasons and may be requested by the article author(s) or by the journal Editor(s), but are ultimately published at the discretion of the Editor. Articles that contain seriously flawed or erroneous data such that their findings and conclusions cannot be relied upon may be retracted in order to correct the scientific record. When an article is retracted, a notice of Retraction will be published containing information about the reason for the Retraction. The originally published article will remain online except in extraordinary circumstances (e.g. where deemed legally necessary, or if the availability of the published content poses public health risks).
Expressions of Concern
Expressions of Concern may be issued at the discretion of the Editor if:
- there is inconclusive evidence of research or publication misconduct by the authors;
- there is evidence that the findings are unreliable but the authors’ institution will not investigate the case;
- an investigation into alleged misconduct related to the publication either has not been, or would not be, fair and impartial or conclusive;
- an investigation is underway but a judgment will not be available for a considerable time.
Upon completion of any related investigation, and when a final determination is made about the outcome of the article, the Expression of Concern may be replaced with a Retraction notice or Correction.
At ACS Publications, we know it is important for you to be able to share your peer reviewed, published work with colleagues in the global community of scientists. As sharing on sites known as scholarly collaboration networks (SCNs) is becoming increasingly prevalent in today’s scholarly research ecosystem, we would like to remind you of the many ways in which you, a valued ACS author, can share your published work .
Publishing open access makes it easy to share your work with friends, colleagues, and family members. In addition, ACS Publications makes it easy to share your newly published research with ACS Articles on Request (see below). Don’t forget to promote your research and related data on social media, at conferences, and through scholarly communication networks. Increase the impact of your research using the following resources: Altmetrics , Figshare , ACS Certified Deposit
When your article is published in an ACS journal or partner journal, corresponding authors are provided with a link that offers up to 50 free digital prints of the final published work. This link is valid for the first 12 months following online publication, and can be shared via email or an author’s website. After one year, the access restrictions to your article will be lifted, and you can share the Articles on Request URL on social media and other channels. To access all your Articles on Request links, log in to your ACS Publishing Center account and visit the “My Published Manuscripts” page.
Article , journal , and commercial reprints are available to order.
Appendix 1: PREPARING FOR SUBMISSION
We’ve developed ACS’ publishing and editorial policies in consultation with the research communities that we serve, including authors and librarians. Browse our policies below to learn more.
Ethical Guidelines
ACS editors have provided Ethical Guidelines for persons engaged in the publication of chemical research—specifically, for editors, authors, and reviewers. Each journal also has a specific policy on prior publication .
OFAC Compliance
As a U.S.-based non-profit organization, the American Chemical Society (ACS) is required to comply with U.S. sanctions laws and regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Control (OFAC). While these laws and regulations permit U.S.-based publishers like ACS to engage in publishing-related activities with authors located in sanctioned regions in many cases, ACS may be prohibited under U.S. law from engaging in publishing-related activities in some cases, including, but not limited to, instances where an author or the institution with which an author is affiliated is located in a particular sanctioned region or has been designated by OFAC as a Specially Designated National (SDN) pursuant to certain U.S. sanctions programs. ACS reserves the right to refrain from engaging in any publishing-related activities that ACS determines in its sole discretion may be in violation of U.S. law.
Safety Considerations
Authors must emphasize any unexpected, new, and/or significant hazards or risks associated with the reported work. This information should be in the Experimental Section of a full article and included in the main text of a letter. Statement examples can be found in the Safety Statement Style Sheet and additional information on communicating safety information from the ACS Guide to Scholarly Communication is freely available here .
A statement describing any financial conflicts of interest or lack thereof is published in each ACS journal and partner journal article.
During the submission process, the Corresponding Author must provide a statement on behalf of all authors of the manuscript, describing all potential sources of bias, including affiliations, funding sources, and financial or management relationships, that may constitute conflicts of interest. If the manuscript is accepted, the statement will be published in the final article.
If the manuscript is accepted and no conflict of interest has been declared, the following statement will be published in the final article: “The authors declare no competing financial interest.”
In publishing only original research, ACS is committed to deterring plagiarism, including self-plagiarism. ACS Publications uses CrossCheck's iThenticate software to screen submitted manuscripts for similarity to published material. Note that your manuscript may be screened during the submission process.
Further information about plagiarism can be found in Part B of the Ethical Guidelines to Publication of Chemical Research . See also the press release regarding ACS' participation in the CrossCheck initiative.
Authorship, Author List, and Coauthor Notification
Authors are required to obtain the consent of all their coauthors prior to submitting a manuscript. The submitting author accepts the responsibility of notifying all coauthors that the manuscript is being submitted.
During manuscript submission, the submitting author must provide contact information (full name, email address, institutional affiliation, and mailing address) for all of the coauthors. Because all of the author names are automatically imported into the electronic Journal Publishing Agreement , the names must be entered into ACS Paragon Plus. (Note that coauthors are not required to register in ACS Paragon Plus.) Author affiliation should reflect where the work was completed, even if the author has since left that institution. Authors may include a note with a current address if their institution has changed since the work was completed.
To expedite the processing of your manuscript, please format your author and affiliation information according the guidelines in this link: https://pubsapp.acs.org/paragonplus/submission/author-address-information.pdf .
Criteria for authorship can be found in Part B of the Ethical Guidelines to Publication of Chemical Research . Artificial intelligence (AI) tools do not qualify for authorship. The use of AI tools for text or image generation should be disclosed in the manuscript within the Acknowledgment section with a description of when and how the tools were used. For more substantial use cases or descriptions of AI tool use, authors should provide full details within the Methods or other appropriate section of the manuscript.
If any change in authorship is necessary after a manuscript has been submitted, confirmation is required that all of the authors (including those being added or removed) have been notified and have agreed to the change. To provide this confirmation, authors are asked to complete and sign an authorship change form and provide the completed form to the appropriate editorial office.
Authors with a single name: If you, or any of your coauthors, have only one name, please follow these steps for proper submission to ACS Paragon Plus:
- First (Given) Name Field: Enter an asterisk (*) into the "First (Given) Name" field.
- Last (Family) Name Field: Enter your single name into the "Last (Family) Name" field.
If your paper is accepted, the asterisk (*) will be removed from the published version of the paper.
Patent Activities and Intellectual Property
Authors are responsible for ensuring that all patent activities and intellectual property issues are satisfactorily resolved prior to first publication (ASAP or in issue). Acceptance and publication will not be delayed for pending or unresolved issues of this nature.
Open Researcher and Contributor ID (ORCID)
Authors submitting manuscript revisions are required to provide their own personal, validated ORCID iD before completing the submission, if an ORCID iD is not already associated with their ACS Paragon Plus user profiles. This ID may be provided during original manuscript submission or when submitting the manuscript revision. All authors are strongly encouraged to register for an ORCID iD, a unique researcher identifier. The ORCID iD will be displayed in the published article for any author on a manuscript who has a validated ORCID iD associated with ACS when the manuscript is accepted.
ORCID iDs should not be typed into the manuscript. ACS publishes only those ORCID iDs that have been properly verified and linked before the manuscript is accepted . After your ORCID iD is linked, it will be displayed automatically in all subsequently accepted manuscripts for any/all ACS journals. We do not publish ORCID iDs provided during proof review or via other communications after a manuscript is accepted for publication.
With an ORCID iD, you can create a profile of your research activities to distinguish yourself from other researchers with similar names, and make it easier for your colleagues to find your publications. If you do not yet have an ORCID iD, or you wish to associate your existing ORCID iD with your ACS Paragon Plus account, you may do so by clicking on “Edit Your Profile” from your ACS Paragon Plus account homepage and following the ORCID-related links. Learn more at www.orcid.org .
Copyright and Permissions
To obtain forms and guidelines for completing the Journal Publishing Agreement or obtaining permissions from copyright owners, and to explore a Copyright Learning Module for chemists, click here .
Funder Reporting Requirement
Authors are required to report funding sources and grant/award numbers. Enter ALL sources of funding for ALL authors in BOTH the Funder Registry Tool in ACS Paragon Plus and in your manuscript to meet this requirement.
Open Access Compliance
ACS offers options by which authors can fulfill the requirements for open access and deposition into repositories for funded research. Visit our ACS Open Science site to see how to fulfill requirements for specific funders and to find out if you are eligible to publish under a Read + Publish agreement between ACS and your institution. You can also find out more about Open Access Compliance and ACS Open Science initiatives .
Diversity and Inclusion Statement
During manuscript submission, ACS journal authors have the option to submit a statement sharing information related to diversity and inclusion that is relevant for their paper. If supplying a diversity and inclusion statement, the corresponding author must provide this on behalf of all authors of the manuscript during the submission process. These statements include but are not limited to analysis of citation diversity and acknowledgment of indigenous land on which research was conducted. Statements expressing political beliefs are not permitted and may be removed by the journal office. All statements are subject to final review by the Editor.
- Citation Diversity Statement: The citation diversity statement should appear in the Acknowledgements section of the manuscript. ACS recommends including the following: (1) the importance of citation diversity, (2) the proportion of citations by gender and race/ethnicity for the first and last authors, (3) the method used to determine those proportions and its limitations, and (4) steps taken to by the authors to improve citation diversity in the article. We recognize that one limitation of the current methods is that it cannot account for intersex, non-binary, and transgender people, or Indigenous and mixed-race authors. (Adapted from BMES/Springer Guidelines )
- Land acknowledgment: The land acknowledgment statement should appear in the Acknowledgements section of the manuscript. The statement should link to the institutions’ formal land acknowledgments on which the research took place, if possible. Further guidance for creating these statements can be found here: https://nativegov.org/news/a-guide-to-indigenous-land-acknowledgment/ .
Appendix 2: Preparing Graphics
Digital graphics pasted into manuscripts should have the following minimum resolutions:
- Black and white line art, 1200 dpi
- Grayscale art, 600 dpi
- Color art, 300 dpi
Graphics must fit a one- or two-column format. Single-column graphics can be sized up to 240 points wide (3.33 in.) and double-column graphics must be sized between 300 and 504 points (4.167 in. and 7 in.). The maximum depth for all graphics is 660 points (9.167 in.) including the caption (allow 12 pts. For each line of caption text). Lettering should be no smaller than 4.5 points in the final published format. The text should be legible when the graphic is viewed full-size. Helvetica or Arial fonts work well for lettering. Lines should be no thinner than 0.5 point.
Color may be used to enhance the clarity of complex structures, figures, spectra, and schemes, etc., and color reproduction of graphics is provided at no additional cost to the author. Graphics intended to appear in black and white or grayscale should not be submitted in color.
Type of Graphics
Table of contents (toc)/abstract graphic.
Consult the Guidelines for Table of Contents/Abstract Graphics for specifications.
Our team of subject-matter experts and graphical designers can also help generate a compelling TOC graphic to convey your key findings. Learn more about our Graphical Abstract service .
A caption giving the figure number and a brief description must be included below each figure. The caption should be understandable without reference to the text. It is preferable to place any key to symbols used in the artwork itself, not in the caption. Ensure that any symbols and abbreviations used in the text agree with those in the artwork.
Charts (groups of structures that do not show reactions) may have a brief caption describing their contents.
Each table must have a brief (one phrase or sentence) title that describes the contents. The title should be understandable without reference to the text. Details should be put in footnotes, not in the title. Tables should be used when the data cannot be presented clearly in the narrative, when many numbers must be presented, or when more meaningful inter-relationships can be conveyed by the tabular format. Tables should supplement, not duplicate, information presented in the text and figures. Tables should be simple and concise.
Each scheme (sequences of reactions) may have a brief caption describing its contents.
Chemical structures should be produced with the use of a drawing program such as ChemDraw.
Journal of Natural Products authors are encouraged to submit images to be considered for use on the journal’s front cover or Supplementary Covers at the time of the submission of their revised manuscript. If your article is accepted for publication, your suggestion may also be selected for use on one of the journal’s covers. If your art is selected for front cover, ACS will send you information about how to request one complimentary 18” by 24” printed poster featuring your work. Images chosen for the front cover will be published at no cost to the author.
Cover image submissions should be colorful and visually engaging, with minimal text. The cover image should not resemble a graphical abstract or data figure, but rather should be an artistic and scientifically accurate representation of the manuscript. Cover illustrations for the Journal of Natural Products generally are composed of a high-contrast photograph of an organism (e.g., higher or lower plant, microbe, or a marine animal), which is overlaid by the chemical structure of a constituent of significance from this organism. Representative past cover motifs are provided on the journal website .
Image files should be submitted as TIF, JPG, PNG, or EPS files (not PDF or PPT) with a resolution of at least 300 dpi for pixel-based images. Cover art should be 8.19 inches (20.8 cm) wide × 10 inches (25.4 cm) high at 300 ppi, and submission of “layered” artwork is encouraged. The journal’s logo will obscure the top 3 inches (7.62 cm) of the image. Authors should submit the cover image, along with a brief (<50-word) cover caption explaining the significance of the cover motif, inclusive of the citation of pertinent bibliography presented in the correct style for the journal. These items should be submitted as supplementary files to ACS Paragon Plus with the revised manuscript. Authors are responsible for providing signed permission forms as deemed necessary by the American Chemical Society.
If you wish to be considered only for the front cover, and not a paid supplementary cover, please respond NO accordingly to the Supplementary Cover Art question in ACS Paragon Plus. For more information on the Supplementary Covers program, please see this webpage .
All art submitted for consideration for a supplementary cover will also be considered for a front cover.
Web Enhanced Objects (WEO)
The Web editions of ACS journals allow readers to view multimedia attachments such as animations and movies that complement understanding of the research being reported.
WEOs should be uploaded in ACS Paragon Plus with ‘Web Enhanced Object’ selected as the file designation. Consult the list of compatible WEO formats .

1155 Sixteenth Street N.W. Washington, DC 20036
京ICP备13047075
Copyright © 2017 American Chemical Society
- Journals A–Z
- C&EN Archives
- ACS Legacy Archives
User Resources
- ACS Members
- Authors & Reviewers
- Website Demos
- Privacy Policy
- Mobile Site
- For Advertisers
- Institutional Sales
- Connect with ACS Publications

Naming and Indexing of Chemical Substances for Chemical AbstractsTM
2007 Edition
A publication of Chemical Abstracts Service
Published by the American Chemical Society Naming and Indexing of Chemical Substances for Chemical AbstractsTM
A publication of Chemical Abstracts Service Published by the American Chemical Society
Copyright © 2008 American Chemical Society All Rights Reserved. Printed in the USA
Inquiries concerning editorial content should be sent to: Editorial Office, Chemical Abstracts Service, 2540 Olentangy River Road, P.O. Box 3012, Columbus, Ohio 43210-0012 USA
SUBSCRIPTION INFORMATION
Questions about CAS products and services should be directed to:
United States and Canada: CAS Customer Care Phone: 800-753-4227 (North America) 2540 Olentangy River Road 614-447-3700 (worldwide) P.O. Box 3012 Fax: 614-447-3751 Columbus, Ohio 43210-0012 USA E-mail: [email protected]
Japan: JAICI (Japan Association for International Phone: 81-3-5978-3621 Chemical Information) Fax: 81-3-5978-3600 6-25-4 Honkomagome E-mail: [email protected] Bunkyo-ku, Tokyo Japan, 113-0021
Countries not named above: Contact CAS Customer Care, 2540 Olentangy River Road, P.O. Box 3012, Columbus, Ohio 43210-0012 USA; Telephone 614-447-3700; Fax 614-447-3751; E-mail [email protected] . For a list of toll-free numbers from outside North America, visit www.cas.org. 1 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 102
NAMING AND INDEXING OF CHEMICAL SUBSTANCES
101. Foreword. Although the account which follows describes in consid- zwitterions (inner salts , sydnones ). The changes for the Fourteenth (1997- erable detail the selection of substance names for Chemical Abstracts (CA) in- 2001) Collective Index period affect coordination nomenclature , stereochemi- dexes, it is not a nomenclature manual. It has the more restricted aim of cal practices, and stereoparents. The most recent changes (2007) involve lo- enabling a user of CA indexes to proceed from the structure of an individual cants, tautomers , and stereoparents. These changes, as well as the changes chemical compound to the place in the current Chemical Substance Index made in 1972, and in the Tenth (1977-1981) and Eleventh (1982-1986) Collec- where the particular index name and any associated index entries will be found. tive Index periods, are reviewed in Section G (¶¶ 225-293). The nomenclature This is the identical operation performed by a CA indexer when assigning an of fullerenes is more fully documented in ¶ 163A of Section B. index name to a new or previously unnamed substance. What follows, in fact, The arrangement of sections is as follows: is a comprehensive summary of CA substance indexing policies, which cover not only conventional organic and inorganic compounds but other completely A. Nomenclature Systems and General Principles (¶¶ 103-139) defined substances entered in the Chemical Substance Index and given CAS B. Molecular Skeletons (¶¶ 140-163A) Registry Number® identifiers . These substances include specific chemical ele- C. Principal Chemical Groups (Suffixes) (¶¶ 164-177) ments, alloys, minerals , mixtures , polymers , enzymes , polysaccharides , and el- D. Compound Classes (¶¶ 178-201) ementary particles . E. Stereochemistry and Stereoparents (¶¶ 202-212) The chemical nomenclature used by Chemical Abstracts Service (CAS) has F. Specialized Substances (¶¶ 213-224) developed in parallel and generally in accordance with the rules published by G. Chemical Substance Names for Retrospective Searches (¶¶ 225- the International Union of Pure and Applied Chemistry (IUPAC). Although 293A) these rules provide unambiguous text equivalents for the great majority of sub- H. Illustrative List of Substituent Prefixes (¶ 294) stances, equally acceptable alternative rules within the present IUPAC system J. Selective Bibliography of Nomenclature of Chemical Substances often lead to two or more unambiguous names. This causes no difficulty in nor- (¶¶ 295-308) mal scientific communication , but is totally unacceptable in a formal, rigidly K. Chemical Prefixes (¶¶ 309-311) controlled, alphabetic listing such as the Chemical Substance Index. Here the L. Chemical Structural Diagrams from CA Index Names (¶¶ 312-318) names must be not only unambiguous, unique, and totally reproducible, but se- M. Index lected so as to bring the names of structurally related substances into juxtapo- sition in the alphabetical listing. They must be equally derivable by index users The arrangement within each of these sections is indicated by a key at the be- searching for information about individual substances and by those who pre- ginning of the section. pare the index. It is also desirable that both should be able to use mechanical In the development of CAS policies for index names of chemical substanc- aids in name generation and retrieval. es, no new nomenclature systems have been devised. Adaptation of current IU- A major revision of CA index names was carried out in 1972 as the Ninth PAC rules to the specific needs of a highly ordered alphabetical index, not Collective Index period began. Most trivial names were dropped; exceptional arbitrary coinage of new terms, has been the approach taken. It continues to be treatment for various classes of substances was discontinued. Where, because recognized by CAS that, while a unique name is needed for an index, and that of the stereochemical complexity of a natural product name, a trivial name was this name, and the CAS Registry Number, are invaluable aids for substance retained as a “stereoparent” (see ¶ 202), diagrams were furnished in the Chem- identification, the use of this invariant index name for citation throughout every ical Substance Index to aid interpretation of index entries. The 1972 nomencla- context in the scientific community is neither practicable nor desirable. But in- ture revision and the reasons for its adoption are set forth in greater detail in the ternational agreement in chemical nomenclature, as embodied in the rules of Ninth Collective Index Guide and in a journal article (J. Chem. Doc. 1974, IUPAC, IUB, and other organizations, continues to be of the greatest impor- 14(1), 3-15). tance in restricting the arbitrary proliferation of substance names. References The preferred CA index names for most chemical substances have been to individual rules which have formed the basis of CAS policies recorded in the continued unchanged since that date. Changes in name-selection policies for sections that follow have not been cited, but the selective bibliography of the the Twelfth (1987-1991) and Thirteenth (1992-1996) Collective Index periods nomenclature of chemical substances which constitutes Section J contains a affect alloys, carbohydrates (lactams), coordination compounds, formazans, comprehensive list of current accepted rules. index name selection (multiplicative names), inorganic compounds (line for- 102. Acknowledgement. CAS acknowledges the large contribution made mulas of clusters, intermetallic compounds), molecular addition compounds by Cecil C. Langham in helping to develop and record CA name-selection pol- (common components; hydrates ), nitrilimines, onium compounds (free radi- icies for the Eighth Collective period (1967-1971) during the years immediate- cals), peptides, phosphonium ylides, phosphoryl halides and halogenoids, ly preceding his retirement in 1969. Dr. Langham’s work constituted an polymers ( block , graft, and hydrolytic), ring systems (list of common systems), invaluable starting point for the revised name-selection policies introduced in salts (lists of common anions), stereochemistry ( sign of optical rotation ), and 1972.
A. NOMENCLATURE SYSTEMS AND GENERAL PRINCIPLES
Introduction ¶ 103 Order of citation of derivative terms ¶ 113 Inversion of names 104 Locants 114 Name selection principles 105 for substituent suffixes 115 Order of precedence of compound classes 106 in substituent prefixes (radicals) 116 Spelling 107 for substituents on parents and parent radicals 117 Punctuation 108 in multiplicative nomenclature 118 Enclosing marks 109 for functional derivatives 119 Multiplicative prefixes 110 for indefinite compounds 120 “Mono” 111 Alphabetization 121 Functional derivatives 112 ¶ 103 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 2
Tautomeric compounds ¶¶ 122 Substituent suffixes 131 Additive nomenclature 123 Substituent prefixes 132 Conjunctive nomenclature 124 Compound radical names 133 Multiplicative nomenclature 125 Carbonyl radicals 134 Radiocofunctional nomenclature 126 Indicated hydrogen 135 Replacement (“a”) nomenclature 127 Added hydrogen 136 Replacement prefixes 128 Numbering of molecular skeletons 137 Replacement nomenclature for functions 129 Index name selection 138 Substitutive nomenclature 130 Subtractive nomenclature 139
103. Introduction. Many names may be employed in scientific publica- (i) Aldehydes , Thials, Selenals, Tellurals. tions for a single compound. Even so simple a compound as H2NCH2CH2OH (j) Ketones , Thiones, Selones , Tellones. can be named 2-Aminoethanol, 2-Aminoethyl alcohol , 2-Hydroxyethylamine, (k) Alcohols and Phenols (of equal rank), Thiols , Selenols , Tellurols. β-Hydroxyethylamine, 2-Hydroxyethanamine, 1-Amino-2-hydroxyethane etc., (l) Hydroperoxides. all of which describe it unambiguously; often, the less systematic (“trivial”) (m) Amines . name Ethanolamine may be encountered. For more complex compounds, the (n) Imines . (This is the lowest compound class expressed by a functional number of possible names increases exponentially. suffix; all the following classes are considered to be nonfunctional. For the In these circumstances, selection rules are essential if a single preferred ranking of nonfunctional cyclic and acyclic skeletons, see ¶ 138.) name for citation in an index of chemical names is to be determined for each (o) Nitrogen compounds: heterocyclic; acyclic (other than “a”-named identifiable substance; lacking such a single name, information regarding the chains; see ¶ 127), e.g., Triazane , Diazene, Hydrazine , Hydroxylamine , substance becomes scattered in the index. Beyond this, it is desirable that the Thiohydroxylamine. selection rules bring chemically related substances close together in the index, (p) Phosphorus compounds: heterocyclic, acyclic (other than “a”-named and that they should be as consistent and as free from exceptions as possible. chains; see ¶ 127), e.g., Diphosphine, Phosphine oxide , Phosphine sulfide , 104. Inversion of names. Ordering in the Chemical Substance Index is Phosphine imide , Phosphorane, Phosphine. based on the index heading parent (1), which is often made up of a basic skel- (q) Arsenic compounds (in similar order). eton name, e.g., “ Butane ,” plus a suffix denoting the principal function, e.g., (r) Antimony compounds (in similar order). “-sulfonic acid .” A locant, e.g., “1-,” fixing its position is also often necessary. (s) Bismuth compounds (in similar order). Following a comma (the comma of inversion) the substituents (2) are ex- (t) Boron compounds: carbapolyboranes, hetero polyboranes, polybo- pressed in alphabetical order, e.g., “2,4-diamino-3- chloro-,” and the modifi- ranes, heterocyclic, Borane . cation (3), now printed in boldface, completes the name by citing any (u) Silicon compounds: heterocyclic, acyclic (other than “a”-named derivatives of the principal function, e.g., “ethyl ester ”, and stereochemical in- chains; see ¶ 127), e.g., Disiloxane, Disilathiane, Trisilane , Disilane . Note formation (see ¶ 203) if appropriate. The uninverted name of the acid is 2,4- that the order is determined first by the total number of skeletal atoms , then by diamino-3-chloro-1-butanesulfonic acid, and of the ester, ethyl 2,4-diamino-3- the presence of oxygen , sulfur , etc; see ¶ 128. chloro-1-butanesulfonate. The latter appears in the Chemical Substance Index (v) Germanium compounds (in similar order). as follows: (w) Tin compounds (in similar order). (x) Lead compounds (in similar order). 1 2 (y) Oxygen compounds other than “a”-named chains (see ¶ 127): hetero- cyclic; acyclic polyoxides, e.g., Trioxide, Peroxide. (z) Sulfur compounds: heterocyclic; acyclic polysulfides and their ox- 1-Butanesulfonic acid, 2,4-diamino-3-chloro- ides, e.g., Trisulfone, Trisulfide, Disulfone, Disulfoxide, Disulfide . 1-ethyl ester (aa) Selenium and tellurium compounds (in similar order). (bb) Carbon compounds: carbocyclic 3 (cc) Silane (dd) Carbon compounds : acyclic Also appearing in the boldface headings of certain very well-known sub- 107. Spelling. CAS accepts Merriam-Webster’s Collegiate Dictionary2 stances are subdivision terms describing properties, etc., of the compound it- as the primary authority for spelling; e.g. sulfur (not sulphur); aluminum (not self, or classifying certain derivatives of it, e.g., reactions, esters . aluminium ). Random House Webster’s Unabridged Dictionary3 is used for 105. Name selection principles (see also ¶ 138). In choosing 1-Butane- words not found in the Collegiate Dictionary. Elision of vowels is often prac- sulfonic acid as the heading parent in the example above, rather than, for ex- ticed in combining the segments of names: e.g., in Butanone and disiloxanyl ample, 1,3-Butanediamine, an order of precedence of chemical functions and the final “e” of the basic skeleton name has been dropped; in Oxazepine an compound classes (¶ 106) was followed. In this hierarchy, sulfonic acids are “a” has been omitted twice, after “oxa” and before “ep”; and “a” is often omit- ranked higher than amines. In the example cited, so-called substitutive nomen- ted before a multiplied “ amine ” or “one” suffix, as in Benzenetetramine and clature (¶ 130) was the type of nomenclature used. Generally, a preferred index Cyclohexanehexone; the terminal “o” of acenaphtho, benzo, naphtho, and name is determined by proceeding as follows until a decision is reached: perylo, and the terminal “a” of cyclobuta, etc., are elided before vowels, e.g., (a) determine the most senior compound class; Benz[cd] indole , 5H-Cyclobut[f] indene . Other examples of elision are -imidic (b) determine the type of nomenclature that is appropriate; (not -imidoic) acids; -imidamides (not imidoamides); -thiones (not thioones); (c) determine the preferred index heading parent; and -hydrazonamides (not -hydrazonoamides). Examples will be found in later (d) name the remainder of the structure as substituents, and/or as func- paragraphs. In a few cases, the vowel “o” is added for euphony, e.g., Carbon- tional derivatives by modification phrases; othioic acid (not Carbonthioic acid). (e) choose between alternatives where more than one unambiguous name Elision of entire syllables is now uncommon. Remaining examples include is still possible. methoxy, ethoxy, propoxy, butoxy, phenoxy (not (methyloxy), etc.) radicals, The remainder of Section IVA is devoted to detailing the application of these and the thienyl (not thiophene -yl) radical. Carbamic acid is an elided form of rules. Carbonamidic acid (¶ 183); Sulfamic acid is used in place of Sulfuramidic 106. Order of precedence of compound classes, in descending order: acid, and Sulfamide instead of Sulfuramide or Sulfuric diamide. The suffix (a) Free radicals and compounds for which substituent prefixes are un- “- carboxylic acid ” undergoes various forms of elision in formation of replace- available, e.g., Sulfur diimide (¶ 200). ment names, e.g., “-carbothioamide.” (b) Cationic compounds: coordination cations, onium, aminium, ylium 108. Punctuation in chemical names is frequently of great importance in cations. removing ambiguities and in differentiating one substance from another. Low- (c) Neutral coordination compounds, including metallocenes . er case italic Roman letters are used in fusion prefixes (¶ 151) in ring system (d) Anionic compounds, e.g., Borate(1-). names, and in as- and s-Indacene; capital italics such as N-, O-, P-, S-, are lo- (e) Acids: peroxy acids, expressed as principal groups, in the order of the cants indicating substitution on these hetero atoms; H- denotes indicated or 1 parent acids; acids, expressed as principal groups, in the order carbon, sulfur, added hydrogen (¶¶ 135, 136); italic Arabic numerals are locants for atoms in selenium, tellurium; acids, expressed as functional parent compounds (¶ 130), abnormal valency states (¶ 158) and for “labeled” atoms (¶ 220); italic words in the order carbon (including Carbonic acid and Formic acid ; see ¶ 183), and syllables are used in modifications to express isomeric oxides , e.g., thiono- chalcogen , nitrogen, phosphorus, arsenic, antimony, silicon, and boron. oxide at a thio acid heading parent, and in stereochemical descriptors (¶ 203), (f) Acid halides and related species, first in the order of the parent acid e.g., erythro-, tetrahedro-. The small capitals D-, L-, and DL-, are configuration- (see (e), above); then, for each acid, in the order fluoride , chloride , bromide , al descriptors (¶ 203); like italic letters, they are disregarded in placing chemi- iodide, azide, isocyanate , isothiocyanate , isocyanide , cyanide (for non-carbon cal names in order until Roman letters have been alphabetized. acid residues only). The “comma of inversion” has been mentioned above (¶ 104). Other com- (g) Amides , in the same order as the parent acids (see (e), above). mas are used between individual locants in index heading parents, substituents, (h) Nitriles , in the same order as the parent acids (see (e), above).
2 Merriam-Webster’s Collegiate Dictionary,11th ed., Merriam-Webster, Springfield, MA, 2003. 1Acetic acid and Benzoic acid are ranked here as though they were named 3Random House Webster’s Unabridged Dictionary, 2nd ed., Random Ethanoic acid and Benzenecarboxylic acid, respectively. House, N.Y., 2001. 3 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 111 and modifications. Different types of functional derivatives are separated by are also employed around a ring-assembly name when it is followed by a prin- commas in the text of the modification. cipal- group suffix or forms part of a radical name. Examples: Examples:
(a) hydrazone phenylhydrazone [1,2′-Binaphthalene]-2-carboxylic acid [1,1′- biphenyl ]-4-yl (b) diethyl ester, sodium salt Brackets enclose structural features of bridges or component rings when the (c) ethyl methyl ester, hydrochloride enclosed locants are not applicable to the total system. Examples: Hyphens at the end of the set of substituents in the inverted part of a bold- face heading signify that no space is intended when the name is uninverted for 4a,9a-[2]Butenoanthracene (the “2” locates the double bond in use in textual matter . Conversely, absence of a hyphen after substituents at the buteno bridge) headings such as Disulfide, Hydroperoxide, Peroxide, indicates that a space appears at that point in the uninverted name. 4H-[1,3]Oxathiolo[5,4-g]benzo- (formed by fusion of 1,3-oxathio Examples: xazole with benzoxazole ; in the total ring system, the oxygen and Acetic acid , 2-chloro- (index name) sulfur atoms of the oxathiole ring 2- Chloroacetic acid (uninverted name) are in the 6- and 8-positions respectively) Disulfide, bis(2-chloroethyl) (index name) Bis(2-chloroethyl) disulfide (uninverted name) When a multiplicative index name is uninverted, brackets are placed around the heading parent. Hyphens separate locants from the words and syllables of a name; when used Examples: between locants, the intention is to indicate that such locants refer to different parts of the name; e.g., in Acetamide , N-2-naphthalenyl-, the “N-” places the Acetic acid, 2,2′-oxybis- (index name) 2-naphthalenyl substituent on the nitrogen of the heading parent, Acetamide. 2,2′-Oxybis[acetic acid] (uninverted name) Periods separate ring size descriptors in Von Baeyer and spiro names, e.g., Bicyclo[3.2.0] heptane . Colons separate sets of locants already related to one Benzoic acid, 4,4′-methylenebis[2-chloro- (index name) another; if a further step is called for, semicolons are employed. 4,4′-Methylenebis[2-chlorobenzoic acid] (uninverted name) Examples: Brackets are sometimes needed for functional terms in modifications, espe- 1,4:5,6-Dimethanonaphthalene cially following locants or multiplicative prefixes. ′′ ′′ ′′ ′′ ′ ′ ′ ′ ′ Benzo[1 ,2 :3,4;5 ,4 :3 ,4 ]dicyclobuta[1,2-a:1 ,2 -a ]diindene Examples:
109. Enclosing marks are placed around compound substituent radicals S-[(dodecylthio)methyl] ester and around and within complex radicals (¶ 162). Their presence or absence fre- bis[(2,4-dinitrophenyl)hydrazone] quently removes ambiguity , especially when locants are omitted through lack of precise structural information. 110. Multiplicative prefixes. Generally, prefixes derived from the Greek Examples: (di, tri-, etc.) are used, rather than the Latin (bi-, ter-, etc.); exceptions are nona- (not ennea-) for nine, and undeca- (not hendeca-) for eleven. (For lists of Latin MeSiH2Cl Silane, chloromethyl- and Greek prefixes, see ¶ 309.) The Latin prefixes bi-, ter-, etc., are used for ring assemblies. ClCH2SiH3 Silane, (chloromethyl)- The prefixes bis-, tris-, tetrakis-, etc., are used for compound and complex radicals and functional derivatives, and to avoid misunderstanding in other cas- CO2H es, especially with names beginning with replacement terms like “aza” or CO Benzoic acid, 3-(chlorobenzoyl)- “oxa”, fusion prefixes like “benzo” or “naphtho,” or compound fusion prefixes Cl like “cyclopentapyrido.” They are used always in multiplying a heading parent. Examples:
CO2H bis( methylene ) tris(decyl) Benzoic acid, (3-chlorobenzoyl)- CO bis(2-aminoethyl) tetrakis(1-aziridinyl) [1,2-ethanediylbis(oxymethylene)] bis(benz[a]anthracen-1-yl) ′ Cl bis(O-methyloxime) Benzo[1,2-c:3,4-c ]bis[1,2,5] bis(cyclohexaneacetate) oxadiazole tris(dihydrogen phosphate ) Biscyclopenta[5,6]pyrido[4,3- CO H 2 bis(aziridinyl) b:3′,4′-c] pyridine CO Benzoic acid, 3-(3-chlorobenzoyl)- bis(2,1-diazenediyl) Benzoic acid, 2,2′-silylenebis- bis([1,1′-biphenyl]-4-yl) Phosphonic acid, P,P′-1,4- Cl bis(bicyclo[2.2.1]hept-2-yl) phenylenebis-
Parentheses are placed around compound substituents like “(chlorometh- 111. “Mono” is only rarely employed in index heading parents (an exam- yl)”, above; in a case like (chloromethylamino), it is to be understood that both ple is Peroxymonosulfuric acid ) but is needed to express functional deriva- the chlorine atom and the unsubstituted methyl group are substituents of the tives of polyfunctional heading parents and inner salts when needed. It is not amino group, i.e., Cl(CH3)N-. The alternative structure, ClCH2NH- is named used if a locant is necessary, or when only one functional group is present. [(chloromethyl)amino], which is a complex substituent prefix. Parentheses are Mono is used for esters of polybasic mononuclear acids having only one type used around simple radicals when they are preceded by “bis,” “tris,” etc. (¶ of chalcogen atom, e.g. monoesters of carbonic acid, phosphonic acid, etc. The 110), e.g., bis(methylene), tris(decyl). They are used also to separate locants of term “hydrogen” in an uninverted ester name precludes the use of “mono.” the same kind which would otherwise be separated only by hyphens, to indicate Examples: the second atom involved in double-bond formation when it is not the next in the numbered pathway, to enclose parts of a heading parent, to set off added MeC( =NOH)Me 2−Propanone, hydrogen, and to enclose multiplied terms in modifications, ion terms that oxime would otherwise be ambiguous, Ewens-Bassett numbers (¶ 215), descriptive terms and ratios, and parts of synonym line formulas . MeOCO2H Carbonic acid, Examples: monomethyl ester Benzoic acid, 4-(2-naphthalenyl)- OH OAc Bicyclo[4.2.0]oct-1(6)-ene 1,2−Naphthalenediol, Butane(dithioic) acid (see ¶ 165) 2− acetate 1(2H)-Naphthalenone bis(inner salt) (disulfate) (from Disulfuric acid ) EtOP(O)(OMe)2 Phosphoric acid , iron (3+) salt ethyl dimethyl ester compd. with benzenamine (1:1) acetate (salt) + Thioperoxydiphosphoric acid ([(HO)2P(O)]2S2) S−Me 1,2,3-Oxadiazolium, Μe2Ν−Ν Me 2-(dimethylamino)-4- Brackets enclose complex substituent prefixes and derivative terms, as well N - (dimethylsulfonio)-5- as Von Baeyer and spiro ring size designations (already described above). They O O hydroxy-, mono(inner salt) ¶ 111 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 4
2,5-Cyclohexadiene-1,4-dione (f) other additive terms describing portions of the molecular structure 1-(phenylhydrazone), 4-oxime not covalently attached, e.g., compd. with..., hydrate , mixt. with..., polymer PhNHN NOH (previously mono(phenylhydrazone), with.... oxime, but now locants are used.) 114. Locants. When a choice is necessary, italic Roman letters are placed before Greek letters, and Arabic numerals are placed last, e.g., As, N, P, S, α, β, γ, 1, 2, 3. (For the Greek alphabet see ¶ 310.) EtOP(O)(OH)2 Phosphoric acid Unprimed locants are followed by primed locants, then by doubly primed monoethyl ester locants, etc., e.g., N, N′, S, α,1′,2,2′,2′′, 3. Low numbering of indices (su- (index name) perscript Arabic numbers) and application of primes are not considered until regular numerical locants have been chosen. Ethyl dihydrogen phosphate Examples: (uninverted name) ′ ′′ MeP( =NMe) (NHMe)2 Phosphonimidic diamide, N,N ,N ,P- tetramethyl- 112. Functional derivatives of the principal reactive chemical groups of systematically-named index heading parents are cited in the modification; these derivatives, as defined for indexing purposes, are restricted to acyclic an- Me CHMeNHMe Benzenemethanamine, N,α,4-tri- hydrides , esters, hydrazides , hydrazones , and oximes . Other derivatives, such methyl- as semicarbazones , azines, acetals, and cyclic esters, are named in other ways, e.g., substitutively at the highest functional heading parent, as substituted hy- 3 drazones, etc., or as heterocycles, as detailed in Section D, below. Cl NH 1,3-Benzenediamine, 4-chloro-N - Functional derivatives of subsidiary functions (those not expressed by the 2 methyl- (not 1,3- Benzenedi- suffix of the heading parent) are cited in the main boldface heading as com- amine, 6-chloro-N1-methyl-) pound or complex substituents. MeNH Examples: Benzoic acid, 4-(1-oxopropoxy)- 1,1′:4′,1′′-Terphenyl, 2′,2′′-dichloro- 4 1 ′ ′ ′′ EtCO CO Me methyl ester (not Benzoic acid, (not 1,1 :4 ,1 -Terphenyl, 2 2 ′ 4-hydroxy-, methyl ester, 2,3 -dichloro-) propanoate) Cl Cl Locants for unsaturation in compounds named as index heading parents are NNH2 Butanal, 3-hydrazono- (not always cited when the compound contains three or more skeletal atoms, except CH3 — C — CH2 — CHO Butanal, 3-oxo-, 3-hydrazone) for monocyclic hydrocarbons with one multiple bond and no suffix. 4321 Examples:
O O HN=NNH2 1- Triazene 13
CH2 — C — O — C — CH3 H2 C=C=CH2 1,2- Propadiene CH3OOC —CH2 — CH2 — CH — CH2 — CH2 — COOCH3 7 6 5 43 2 1 ≡ H 2C=CHC CH 1-Buten-3-yne Heptanedioic acid, 4-[2-(acetyloxy)- 14 2-oxoethyl]- 1,7-dimethyl ester (not Hep- H C= CH Ethene tanedioic acid, 4-(carboxymeth- 2 2 yl)-, 4-anhydride with acetic acid, dimethyl ester) Cyclohexene O CH3 + HO — P — O — CH2 — CH2 — N — CH3 1 2 1 − 5 1,3- Cyclopentadiene O CH3
Ethanaminium, N,N,N-trimethyl-2- (phosphonooxy)- Locants denoting ring junctions of ring assemblies are always cited except inner salt (not Ethanaminium, for two-component assemblies of cycloalkenes , cycloalkadienes, etc. 2-hydroxy-N,N,N-trimethyl-, Examples: dihydrogen phosphate (ester), inner salt) 11′ 1,1′-Biphenyl
NOH ′ 1 1 6′ Benzoic acid, 4-fluoro- 6 N N 4 1 ′ F CO2CH2CMe 2-(hydroxyimino)propyl ester 2,2 -Bipyrazine (not Benzoic acid, 4-fluoro-, N N 4 4′ 2-oxopropyl ester, oxime) 6 11′ Bi-2-cyclohexen-1-yl
4 1 Benzoic acid, 4-[(acetyloxy)- AcOSO CO Me 6′ 2 2 sulfonyl]- methyl ester (not Benzoic acid, 4-sulfo-, S-anhydride with 115. Locants for substituent suffixes of index heading parents are cited acetic acid, methyl ester) except for the following cases: mononuclear skeleton parents, a single substit- uent suffix on a homogeneous molecular skeleton with two skeletal atoms, or 113. Order of citation of derivative terms in modifications. The normal a single substituent suffix on benzene or a saturated homogeneous monocyclic order is: ring. Locants are not cited for Geneva suffixes which terminate a chain, e.g., (a) the term “inner salt”; “-oic acid’, “-dial”. (b) functional derivatives in the order: anhydrides, esters, hydrazides, Examples: hydrazones, oximes; multiplicative terms are cited before simple terms, e.g., 1,3-propanediyl dimethyl ester (with the appropriate locants), otherwise alpha- CN CN CN CN 1,3- Butadiene -1,1,2,3,4,4-hexa- || || carbonitrile betic order is followed; NC—C=C—C=C—CN (c) additive terms describing fragments covalently attached to the in- 1 234 dex heading compound, e.g., N-oxide; (d) ionic terms, e.g., ion(1-), radical ion(1-), then ionic terms derived H N from separate structural fragments, e.g., chloride, acetate, or an “amminium” O 6 O 1,3,5- Triazine -2,4,6(1H,3H,5H)- 1 trione heading; HN 5 3NH (e) metal salts, followed by other salts alphabetically, e.g., acetate, hy- drochloride; O 5 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 118
N O CO2 H 2-Pyrazinecarboxylic acid H2 Si Si cyclotrisiloxan-2-ylidene O O N Si H2 = HO2CN NCO2H 1,2-Diazenedicarboxylic acid O = 1,4-dioxan-2-yl H2C CHCO2H 2-Propenoic acid O
CH3CH2OCH2CH2OCH2CH2OCH2CH2OCH2CH2COOH 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 N 2-pyrazinyl 4,7,10,13-Tetraoxapentadecanoic N acid O O 2-oxiranyl 2-Cyclopropen-1-one
Locants are not cited for free valencies on charged heteroatoms in cationic het- 116. Locants in substituent prefixes (radicals). Locants are assigned for erocyclic rings containing only one heteroatom, e.g. oxiranio-. multiple bonds and for free valencies in all unsaturated radicals containing 117. Locants for substituents on index heading parents and parent three or more skeletal atoms. Locants are always cited for free valencies in- radicals are cited when the parent names possess locants for substituent suffix- volving more than one position of a skeleton. es, unsaturation, hetero atoms, indicated hydrogen, spiro or ring assembly Examples: junctions, or bridges (in fused systems). Locants are dispensed with for “hy- dro” prefixes in fully saturated ring systems unless ambiguity could result, e.g., because of a remaining etheno or other unsaturated bridge. Locants are not cit- − = − Η − 2-buten-1-yl H3C CH CH C 2 ed for a single substituent on a homogeneous monocyclic ring, e.g., benzene, methyl-. Similarly, locants are not cited for single substituents on homoge- neous parents, or parent radicals containing only two skeletal atoms, or for one HC≡CCH= 2-propyn-1-ylidene or more substituents on mononuclear radicals. 3 1 Examples:
O HN=NNH— 2-triazen-1-yl HO OH 2,3-Oxiranediol, 2,3-dichloro- 3 1 Cl Cl
O 2,4-cyclopentadien-1-ylidene Cl OH 2-Oxirenol, 3-chloro-
MeCOCOCH Cl 2,3-Butanedione, 1-chloro- —N=NNH— 1-triazene-1,3-diyl 2 13
—C≡CC≡C— 1,3-butadiyne-1,4-diyl H2NCONHMe Urea , N-methyl-
Me O Me 1,4-Dioxin, 2,3,5,6-tetramethyl- —CH2CH2— 1,2-ethanediyl Me O Me
= C C 1,2-ethenediylidene PhC≡CC≡C— (4-phenyl-1,3-butadiyn-1-yl)- 4 1 O 1,2-cyclopropanediyl 1,4-Naphthalenedione, octahydro-
O 1,4-phenylene When one or more locants are needed for substituents on a heading parent Locants are not cited for free valencies of radicals that have lost hydrogen or parent radical, all are cited. from one skeletal atom of a saturated homogeneous cyclic or acyclic molecular Examples: skeleton or an unsaturated molecular skeleton of two atoms. Locants are not Methanone, 1,1′-(1,4-phenylene)bis[1- cited for a phenyl radical. Locants are cited for free valencies of all other radi- − PhCO CO Ph phenyl- cals derived from cyclic parents. Locants are not cited for free valencies on acyclic radicals with only one possible site of attachment, e.g., acetyl, ethoxy, sulfonimidoyl, etc. 118. Locants in multiplicative nomenclature are always cited for the po- Examples: sitions of attachment on the heading parent, even for single atom parents. Skel- etal locants are also cited for parents that are multiplied by esterification, ethyl MeCH2— hydrazides, hydrazones or oximes. When a multiplied acid has more than one kind of chalcogen atom or has both carboxylic and carboperoxoic groups (or HN=N— diazenyl their chalcogen analogues), superscript skeletal locants are used in combina- tion with the heteroatom locants. Examples: HP − P — tetraphosphetanyl | | PhOPh Benzene, 1,1′-oxybis- HP − PH
′ PhCO− benzoyl F O F Benzene, 1,1 -oxybis[4-fluoro-
Acetic acid, 2,2′-(1,2-hydra- 5 HO CCH NHNHCH CO H 1 2-cyclopenten-1-yl 2 2 2 2 zinediyl)bis- ¶ 118 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 6
′ HO2 CNHSiH2 NHCO2H Carbamic acid, N,N -silylenebis- 120. Locants for indefinite compounds must often be omitted. In addi- tion, such compounds can sometimes be named only by departing from the re- gular name selection policies, e.g., by citation of the principal group in the MeSMe Methane , 1,1′-thiobis- modification instead of as a suffix of the heading parent, citation of a functional derivative in the modification rather than as a substituent, use of “mono” in- ′ HOSiH2CH2SiH2OH Silanol, 1,1 -methylenebis- stead of the (unknown) locant, replacement of a numerical locant by the indef- inite aromatic locant “ar-,” or inclusion of question marks in a set of otherwise known locants. Silanamine, 1,1′-methylenebis- H2NSiH2CH2SiH2NH2 Examples:
Acetic acid, 2-chloro-, ClCH2COO(CH2)2OCOCH2Cl Benzenediamine 1,1′-(1,2-ethanediyl) ester Benzoic acid, dichloro- Naphthalene , 2,2′-(1,4-phenylene)bis-, S S Phosphonothioic acid, P,P′-meth- disulfo deriv. − = − − = − P P P′ P′ Benzeneacetic acid, 2-carboxy-, MeO P CH2 P OMe ylenebis-, O ,O ,O ,O -tet- OMe− OMe− monomethyl ester ramethyl ester 1,2-Ethanediol, 1-phenyl-, monocarbamate Naphthalene, ar-chloro-1,2,3,4-tetrahydro- 119. Locants for functional derivatives are always cited when there is Benzenemethanol, ar-amino- more than one occurrence of the principal functional group. [1,1′-Biphenyl]-ar,ar′-dicarboxylic acid Examples: Benzene, 1,2,?-trimethyl-
1 The italic word “or” is used with substituent prefixes (but never with index CO Me 1,2-Benzenedicarboxylic acid, 2 3-methyl-, heading parents) when the number of alternative structures cannot be misinter- 1-methyl ester preted. Me CO2H Examples:
1 Quinoline , 2-chloro-3(or 4)-methyl- O Naphthalene, 1(or 2)-ethyl-2(or 1)-methyl- 2 || O a CH —O—C—CH 2 3 2,3-Furandimethanol, α3-chloro-, When one or more substituent prefixes are in known positions and the re- α2-acetate mainder in unknown positions, lowest locants are used for the former. a 3 CH—OH Example: | Cl Naphthalene, chloro-2-methyl- Diphosphoric acid, MeOP(O) (OH)OP(O) (OH) OMe An indefinite name like Piperidine , 2(or 4)-bromo-4(or 2)-chloro- cannot P p′ P,P′-dimethyl ester be used because this name could be held to include the 2-bromo-2-chloro- and 4-bromo-4-chloro- isomers ; in such cases locants are usually omitted. 121. Alphabetization of substituent prefixes affects the position in the in- HO2 C(CH2)2 CONHNH2 Butanedioic acid, 1- hydrazide dex where an inverted chemical substance name will be found. Simple prefixes are placed in alphabetic order according to their names; only then are multipli- cative prefixes (di-, tri-, etc.) placed in front of each as required, and locants MeC( =NOH)CMe = NNHPh 2,3-Butanedione, inserted; e.g., an index compound in which two nitro groups, three bromine at- 2-(phenylhydrazone), 3-oxime oms, and a chlorine atom are present receives the substituent name “tribromo- chlorodinitro,” and the substituents so arranged will be found together with an appropriate index parent, such as Naphthalene, alphabetized in accordance MeO−CO CO−OMe 1,4-Benzenedicarboxylic acid, with all the Roman letters in the complete name. The total name with locants, 1,4-dimethyl ester e.g., Naphthalene, 2,5,8-tribromo-3-chloro-1,6- dinitro-, will be preceded in the list of index entries by both Naphthalene, nitro- and Naphthalene, tetra- chloro-. Locants are cited for esters of compounds named at an alcohol parent. Compound and complex substituent prefixes (radicals) are constructed on Examples: similar alphabetic principles and then arranged by their first letters (which may have been derived from multiplicative prefixes within the radicals) in the total name. This name is then placed in the index as described above, all letters be- − H2N (CH2)2 OCOCH3 Ethanol , 2-amino-, 1-acetate ing alphabetized. When the letters are all identical, arrangement depends on lo- (the term ester is not cited cants. because a locant is present.) Examples:
PhCH2CH2OCOCH3 Benzeneethanol, 1-acetate (for a conjunctive name, the ring CO2H Benzoic acid, 3,4,5-trichloro-2,6- locant is used.) Et2N(CH2) 2 1 6 (CH2)2NEt 2 bis[2-(diethylamino)ethyl]- (letter "c" is placed before "d", the initial Locants are used in modifications for index headings that express the same letter of the complex radical) (or similar) functions in both the index parent and the substituents when the lat- Cl Cl ter are not derivatized. This avoids confusion with former CA index names. Cl Examples: CO H 2 1-Naphthalenecarboxylic acid, 4- CO Et Benzoic acid, 3-sulfo-, Br 1 2 1-ethyl ester 8a [bis(2-chloroethyl)amino]-6,7-di- bromo- (the multiplicative prefix
HO3 S "bis-" is part of the name of the com- Br 5 4 plex substituent prefix and this is therefore placed before "bromo") N[ (CH2)2Cl]2 CO H Benzoic acid, 3-(ethoxysulfonyl)- 2 (formerly Benzoic acid, 3-sulfo-, 3-ethyl ester) EtO S 3 NO Naphthalene, 2-(2-nitrophenyl)-7- 2 (3-nitrophenyl)- (the radicals are O2 N 1 8a placed in order, according to the lo- The locant for an additive term such as “oxide” is an Arabic number when cants they contain, before locants a nitrogen, phosphorus, sulfur, etc., atom of a ring in the index heading parent relating to the heading parent are in- is involved; otherwise a letter locant (N-, S-, etc.) is employed. 54 serted) Locants are not employed for ionic modification terms, e.g., salts such as “sodium salt,” “hydrochloride.” 7 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 122
122. Tautomeric compounds. Index names are normally based on the pre- A unique CA Chemical Substance Index name is selected for each com- cise structures shown or described in the author’s original document. Tautom- pound containing a normalized tautomeric structure by application of the rules erism (ready interconvertibility of isomers) in certain types of compounds that follow (see II, below). For tautomeric pyrazole derivatives and for tropo- causes a serious problem in index-name selection, the issue here being not lones, CA selects a single preferred structure and index name, and assigns a sin- which name to select for a given molecular structure but which diagram to gle CAS Registry Number, even though these systems do not conform to the name for a given tautomeric system if scattering at different names of informa- general equilibrium illustrated above and are not currently normalized by the tion about what is essentially a single substance is to be avoided. CAS Registry System. Lowest locants are employed successively in index par- Common (trivial) names for most nitrogenous tautomeric systems are ents and substituents in these cases. cross-referred to preferred systematic names. Examples: Examples: H 1 2 Me N N 1 Adenine. See 9H-Purin-6-amine N 2 Me Barbituric acid. See 2,4,6(1H,3H,5H)-Pyrimidinetrione 5 NH Caffeine . See 1H- Purine -2,6-dione, 3,7-dihydro-1,3,7-tri- 5 CH2 OH methyl- CH2 OH Carbostyril. See 2(1H)-Quinolinone 1H-Pyrazole-3- methanol , Cytosine. See 2(1H)-Pyrimidinone, 4-amino- 1H-Pyrazole-5-methanol, 5-methyl- NOT 3-methyl- Guanine. See 6H-Purin-6-one, 2-amino-1,9-dihydro- (principle: lowest locant Hydantoin. See 2,4-Imidazolidinedione for principal group) Melamine . See 1,3,5-Triazine-2,4,6-triamine Theophylline . See 1H-Purine-2,6-dione, 3,9-dihydro-1,3-dimethyl- Uracil. See 2,4(1H,3H)-Pyrimidinedione Uric acid . See 1H-Purine-2,6,8(3H)-trione, 7,9-dihydro- O OH 1 OH O Xanthine . See 1H-Purine-2,6-dione, 3,9-dihydro- 7 7 1 Br Br The necessity for many tautomeric structures to be redrawn to agree with selected CA index names is obviated by computer “normalization” algorithms 2,4,6-Cycloheptatrien- 2,4,6-Cycloheptatrien- in the CAS Registry System. In the normalization process, the different struc- 1-one, 3-bromo-2- 1-one, 2-bromo-7-hy- tural diagrams for a single tautomeric system (of one of the types expressed in hydroxy- NOT droxy- the cross-references above) are recognized as equivalent and stored in identical (principle: lowest machine - language representations. They share a single unique CAS Registry locants for substituents) Number and CA Chemical Substance Index name. The structural requirements for the normalization process and the rules for selecting unique CA Chemical Substance Index names are as follows: For phosphonic-phosphorous and phosphinic-phosphonous acid tautomers, see I. Requirements for normalization of structures. Tautomeric structures ¶ 197. represented by the following equilibrium: II. Rules for Choosing the Preferred Normalized Tautomer . These rules are used to select the particular structure of a tautomer from which the unique CA Chemical Substance Index name is then derived. They are applied M=Q−ZH HM−Q=Z in the order shown until a decision has been made. Rule 1: The Stereo Retention Rule are normalized, i.e., recognized as equivalent in the CAS Registry System, When the original document reports the isolation of a normalized tautomer when the following requirements are met: with an E or Z double bond connecting the central atom Q to a terminal N, the (a) Q = C, N, S, P, Sb, As, Se, Te, Br, Cl or I with any acceptable valency preferred tautomer retains the E or Z configuration. for the individual elements. (b) M and Z = any combination of trivalent N and/or bivalent O, S, Se or Z NNO2 H Te atoms. N N (c) The bonds involved in tautomerization may be in an acyclic chain or CH3 C E NH2 in a ring system or partly in both. OH (d) The end-points, M and Z, may be in adjacent rings of a fused ring sys- tem, but a nitrogen atom which occupies a fusion point in such a system cannot Ethanimidic acid, 2(1H)-Pyridinone, hydra- take part in tautomerization. N-nitro-, (1Z)- zone, (2E)- (e) The hydrogen atom of the tautomeric system may be replaced by deu- terium or tritium. (f) Two or more systems of the form shown above may be linked through a common atom, whereby a proton can be considered to migrate along the PhNH Me Z chain. N N Example: 1-Triazene, 1-methyl-3- phenyl-, (1Z)- ||| ||| —NH−C=N−C=N−C=N— —N=C−NH−C=N−C=N—
|| | | || Rule 2: The Oxo Rule —N=C−N=C−NH−C=N— —N=C−N=C−N=C−NH— For any tautomer mobile group, if M is a chalcogen and Z is N, the preferred tautomer has the mobile H on N; and if M and Z are both chalcogens , the pre- Replacement in the generalized formula above by specific elements affords ferred tautomer has the mobile H on the chalcogen of higher atomic number. normalized tautomeric systems such as the following: The overall order of priority for attachment of the mobile H is then
N > Te > Se > S > O || —NH−C=N— —N=C−ΝΗ— In other words, a double bond to oxygen is preferred above all others. ( amidine tautomerism)
− = = − H —NH N N— —N N NH— N O N NOT OH (diazoamino tautomerism) || —NH−C=O(S, Se, Te) —N=C−O(S, Se, Te)H 2(1H)-Pyridinone 2-Pyridinol
(lactam-lactim tautomerism) —NH−P=N— —N=P−NH— S SH NOT O OH || | Ph C NH2 Ph C NH − = —S NH2 —S NH || || Benzenecarbothioamide Benzenecarboximidothioic acid O O ¶ 122 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 8
H O O O OH O N NH N O O N N N O NOT HO P NH P OH NOT HO P N P OH
OH OH OH OH 2H-Pyrrol-2-one, 5,5′- 2H-Pyrrol-2-one, 1,5-dihydro- iminobis- 5-[(2-oxo-2H-pyrrol-5- Imidodiphosphoric acid Phosphorimidic acid, yl)imino]- N-phosphono- Rule 5: The Alternating-Bond Rule
O SeH HO Se For a cyclic tautomer mobile group in which M and Z are both N and Q lies at P P the junction of two rings, the preferred tautomer has one N in a ring with alter- NOT nating single and double bonds and the mobile H on the other N.
1H- Phosphole , 1-selenyl-, 1H-Phosphole, 1-hydroxy-, H H N O N O 1-oxide 1-selenide N N NOT N N N N H H Rule 3: The Unsubstituted Imino Rule 6(5H)-Pteridinone, 6(5H)-Pteridinone, For an acyclic tautomer mobile group, if M and Z are both N, the order of pri- 7,8-dihydro- 1,7-dihydro- ority for attachment of the mobile H is
NH2 NH2 R−N > T−N > D−N > H−N H H N N N HN O NOT O where R is any covalently attached atom or group other than H, D, or T. In oth- N N N N er words, an unsubstituted imino group is preferred to a substituted one, and an H unlabeled imino group is preferred to a labeled one. 8H-Purin-8-one, 6-amino- 8H-Purin-8-one, 6-amino- 7,9-dihydro- 1,7-dihydro-
N NOT N H H N N O N N O Et NH C NH Et N C NH2 NOT 1-Piperidinecarboximidamide, 1-Piperidinecarboximidamide, N N O N N O N-ethyl- N′-ethyl- H H
O O Pyrazino[2,3-b] pyrazine -2,3- Pyrazino[2,3-b]pyrazine-2,3- dione, 1,4-dihydro- dione, 5,8-dihydro- Ph S NH Ph NOT Ph S N Ph
NH NH2 Rule 6: The Tautomer CIP Rule For the isolated (n = 1) or extended (n > 1) tautomer Benzenesulfonimidamide, Benzenesulfonimidamide, N-phenyl- N′-phenyl- R NH Q N R′ n in which NH NH2 NOT a) R and R′ are any covalently attached atoms or groups, and Et C NHNH2 Et C NNH2 b) both terminal N’s (those attached to R and R′) are acyclic or both are in Propanimidic acid, Propanehydrazonamide a ring, hydrazide the preferred tautomer has the mobile H on the terminal N of higher priority based on the Cahn-Ingold-Prelog (CIP) sequence rule, the one used to assign absolute configuration [R. S. Cahn, C. K. Ingold, and V. Prelog, Angew. Chem. NH NH2 Int. Ed. Engl., 1966, 5, 385-415 (errata: 1966, 5, 511); V. Prelog and G. Helm- NOT chen, ibid., 1982, 21, 567-583. Et C NH D Et C N D For each tautomer, a tree diagram is constructed for the terminal N bearing the mobile H. Then the following CIP-based rules are used: Propanimidamide-N-d Propanimidamide-N′-d Subrule 6.1: The Element Subrule
Rule 4: The Amino/Hydrazinyl Rule Under subrule 6.1, at the first point of difference , the element with the higher atomic number has priority. The terminal N for that branch is then assigned the For a tautomer mobile group in which M and Z are both N, but one N is in a mobile H in the preferred tautomer. ring and the other is attached to the ring, the preferred tautomer has the mobile H on the acyclic N. N N HN N NOT N NH
N NHNH 2 H N NNH 2 NOT O2N NH2 O2N NH2
1H-1,2,3-Triazol-4-amine, 1H-1,2,3-Triazol-5-amine, Pyridine, 2-hydrazinyl- 2(1H)-Pyridinone, 5-nitro- 4-nitro- hydrazone
H Br N Br N H H NOT N NH2 N NH P NOT P N N N NH H 1H-Imidazol-2-amine 2H-Imidazol-2- imine , 1H-1,3,2-Benzodiazaphosphole, 1H-1,3,2-Benzodiazaphosphole, 1,3-dihydro- 6-bromo- 5-bromo- 9 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 124
NH2 NH2 unlike pair (R,S or S,R): H N N N NOT N CO2H Me CO2H Me
N N N N NH CH CH Et N CH CH Et H S S S S 9H-Purin-6-amine 7H-Purin-6-amine H C NOT H C R S R S N CH CH Et NH CH CH Et
CO2H Me CO2H Me HN N N NH NOT L- Isoleucine , N-[[[(1R,2S)-1- L-Isoleucine, N-[[[(1R,2S)-1- Me Me carboxy-2-methylbu- carboxy-2-methylbu- O Me O Me tyl]imino]methyl]- tyl]amino]methylene]- 4H-Imidazol-4-one, 3,5-dihydro- 4H-Imidazol-4-one, 1,5-di- 5,5-dimethyl- hydro-5,5-dimethyl- And finally, under subrule 6.5, an R chiral center has priority over an S one:
HN N NH2 H2N N NH HN N NHNH2 H2N N NNH2 NOT Cl Cl NOT Cl Cl S R S R H H H H 2H-Pyrrol-2-imine, 2H-Pyrrol-2-one, 5-amino-, 5-hydrazinyl- hydrazone 2H-Pyrrol-5-amine, 3,4- 2H-Pyrrol-5-amine, 3,4- dichloro-3,4-dihydro- dichloro-3,4-dihydro-2- 2-imino-, (3S,4R)- imino-, (3R,4S)-
HN N N NH NOT N N Cl CO2H Cl CO2H 123. Additive nomenclature embraces molecular structures whose several component parts are considered to be added together without replacement 1H-1,2,4- Triazole -3-carboxylic 1H-1,2,4-Triazole-5-carboxylic (substitution) of atoms (usually hydrogen). It includes coordination names (¶ acid, 5-chloro- acid, 3-chloro- 215), conjunctive nomenclature (¶ 124) and binary names of inorganic com- pounds (¶ 219). Examples:
Subrule 6.2: The Isotope Subrule Copper , dichlorobis(methanamine)- Benzeneethanol If a comparison of atomic numbers fails to reveal a difference in the tree dia- Sodium chloride (NaCl) grams, the CIP rule then compares mass numbers. Under subrule 6.2, at the first point of difference, the atom with the higher mass number has priority: The construction of additive names often involves indicated or added hy- drogen (¶¶ 135, 136) in that part of the molecule known as the heading parent, Me Me the addition of hydro “substituents”, or the use of additive terms such as “ox- ide” or “sulfide” in the modification. In a few cases, the additive term becomes 15 15 Ph NH C N Ph NOT Ph N C NH Ph a part of the heading parent. Examples: Ethanimidamide-N-15N, Ethanimidamide-N′-15N, N,N′-diphenyl- N,N′-diphenyl- 9(10H)-Anthracenone Naphthalene, 1,2,3,4-tetrahydro- Pyridine, 1-oxide Et Et Phosphine imide 13 NOT 13 CH NH C N Me CH N C NH Me 3 3 For salts and molecular addition compounds, see ¶¶ 192, 198. 124. Conjunctive nomenclature allows a cyclic molecular skeleton to be Propanimidamide, N′-methyl- Propanimidamide, N-methyl- included as a part of the heading parent name even though the principal chem- N-(methyl-13C)- N′-(methyl-13C)- ical group is separated from the ring by an acyclic chain. Larger molecules may be named thereby as heading parents and more compounds of similar structure can be collected at a given ring system name. Moreover, the major requirement of substitutive nomenclature, that the principal group be expressed in the head-
H2N N NH HN N NH2 ing parent as a suffix, is fulfilled. NOT A conjunctive name is employed when any ring system (including a poly- 35 35 SS SS hedral borane) is attached by single bonds to one or more saturated acyclic hy- drocarbon chains, each of which bears only one functional substituent 3H-1,2,4-Dithiazol-1-35S-5- 3H-1,2,4-Dithiazol-2-35S-5- corresponding to the principal chemical group of the compound. When a sec- amine, 3-imino- amine, 3-imino- ond or third such substituent is present on the chain, a conjunctive name may still sometimes be employed so long as the resulting index heading parent does not express more than a single function in each chain and other principles are not violated (see the final example below). It is always implied that the chem- Subrules 6.3-6.5: The Stereo Subrules ical functional group is at one end of the acyclic chain and the ring system is at the other. If a comparison of mass numbers also fails to reveal a difference, the CIP-based Examples rule then compares stereo elements. For example, under subrule 6.3, a Z dou- ble bond has priority over an E one if the structure is X−CH=CH−Y, where X and Y are any covalently attached atoms or groups: PhCH2 OH Benzenemethanol Z Z NH CH CH Me N CH CH Me
Ph C NOT Ph C E E CHMeCH2 OH β N CH CH Me NH CH CH Me βα Cyclopentaneethanol, -methyl-
Benzenecarboximidamide, Benzenecarboximidamide, N′-(1E)-1-propen-1-yl-N- N-(1E)-1-propen-1-yl-N′- (1Z)-1-propen-1-yl- (1Z)-1-propen-1-yl- [1,1′-Biphenyl]-4-acetic acid Ph CH2 CO2H Under subrule 6.4, a like pair of chiral centers (R,R or S,S) has priority over an ¶ 124 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 10
Pr Example: | 3 CHNH2 3-Pyridinemethanamine, α α -propyl- CO2 H CO2 H N 1 1′ 1 6′ Benzoic acid, 2,2′-thiobis- 6 S
3 In the example above, a simple one-part polyvalent radical, thio, was em- S CHClCH2 CH2 CONH2 γ β α N 1,3-Dithiolane-2-butanamide, ployed as a multiplier; other such radicals are oxy, -O-; methylene, -CH2-; 1,4- γ phenylene, -1,4-C6H4-; imino, -NH-; nitrilo, -N=; and 1,3-disiloxanediyl, -Si- S -chloro- 5 1 H2OSiH2-. In general, any simple multivalent radical may be used as a multi- plicative radical, and may itself be substituted; e.g., (methylimino), -N(CH3)-; (1-methyl-1,3-propanediyl), -CH(CH3)CH2CH2-. Multicomponent radicals may be used as multipliers if they contain a cen- α tral one-part multivalent radical (simple, compound, or complex) around which CHClCO2 H all other multivalent radicals are so arranged that the sequence of atoms and bonds in each path is identical as one proceeds outwards. There is no restriction 1,3-Benzenediacetic acid, α1,α3 in the number of components that may comprise the total multiplying radical, -dichloro- so long as their use results in an unambiguous total name. Examples of permissible multiplying radicals: CHClCO2 H α ′ —CH2OCH2— [oxybis(methylene)]
1H- Pyrrole -2-methanol, α−(2− − H OH CH2 CHCl— N | phenylethyl)- (the heading par- | [nitrilotris(1-chloro-2,1-ethanediyl)] —CHCl−CH −N−CH −CHCl— CHCH2 CH2 ent which expresses the pre- 2 2 α ferred ring system, not the preferred acyclic chain, is cho- [(1-chloro-1,2-ethanediyl)bis(oxy)] sen) —OCH2CHClO—
| SiH 2 (1-propanyl-3-ylidynetetrasilylene) —SiH CH CH C−SiH — Bicyclo[4.3.1]dec-7-ene-3,4-diacetal- 2 2 2 2 α3 SiH2 CHO dehyde, -(2-oxoethyl) (not Bicy- | | clo[4.3.1]dec-7-ene-3-propanal, β- 9 1 CHCH2CHO formyl-4-(2-oxoethyl)-; the pre- α 3 10 ferred heading parent expresses the maximum number of principal 6 CH CHO ([1,1′-biphenyl]-2,4′-diyldiimino) 2 groups and, because it also express- α 4 —NH es a ring system, is preferred to Bu- tanedial) NH—
The requirement for total symmetry in multiplication allows the use of A conjunctive name is not permissible under the following conditions and the “ylidene” in combination with other bivalent radicals. regular rules of substitutive nomenclature apply: 1) when a double bond joins the ring to the functional acyclic chain; 2) when a conjunctive index parent Η 2 would express two or more functional groups in a single acyclic chain; 3) when C the acyclic chain is unsaturated, or contains hetero atoms; and 4) when a con- (cyclobutylidenemethylene) H2C CH2 junctive name would fail to express the maximum number of principal chemi- C cal groups. || Examples: —C—
CHCO H Acetic acid, 2-cyclopropylidene- CH2 2 || (methylenesilylene) —Si—
O Enclosing marks are used to distinguish certain combinations of multivalent CH(OH)2 Methanediol, 1-(2-furanyl)- 5 1 radicals which would otherwise be ambiguous.
—C−O−C— [oxybis(cyclopropylidenemethylene)]
= O CH CHCH2NH2 2-Propen-1-amine, 3-(2-benzo- furanyl)- —CH2 —C—O—C—CH2 — [oxybis[(cyclopropylidene)(methyl- ene)]]
Examples of combinations of multivalent radicals which are not used as multipliers:
NHCO2 H Carbamic acid, N-cyclopropyl- = — CH2 NH — —CH2CH NCH2 CH=
—O S— —CHCl CH2PHCHCl CH2 — OH
CH2OH 1,4-Naphthalenediol, 2-(hy- — CH2OO — droxymethyl)- The naming of multiplying radicals is accomplished by citing first the cen- OH tral unit. This is followed by a prefix, e.g., “di” or “bis”, denoting the number of “radial” series generated by the central unit. The remaining terms of the 125. Multiplicative nomenclature employs polyvalent radicals by which name, in the form of radicals, are cited in appropriate enclosing marks as nec- a multiplicity of occurrences of an index heading parent in a compound may be essary. The entire multiplying radical is set off by further enclosing marks, expressed. 11 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 128 which are preceded by locants “placing” the radical at the proper point of at- Example: tachment on each index heading parent. Multivalent radicals other than central units are numbered (if there is a choice) from the heading parents towards the 1′ 2′ 21 HOCH2 CH2 OCH2 Si H2 OSi HOSi H2 CH2 OCH2 CH2 OH center of the complete radicals, and the locant relating to the position closest to | the heading parent is cited last. OSi H2 CH2 OCH2 CH2 OH Examples: Ethanol, 2,2′-[[3-[[[(2-hydroxethoxy) —CHCl CH2 OCH2CHCl— [oxybis(1-chloro-2,1-ethanediyl)] methyl]silyl]oxy]-1,5-trisiloxane- 12 21 diyl]bis(methyleneoxy)]bis- (not Ethanol, 2,2′,2′′-[silylidynetris(oxy- 1 1′ S—S (dithiodi-4,1-phenylene) silylenemethyleneoxy)]tris-) 6 6′
Η Η= = (selenodi-2- propene -3,1-diyl) 126. Radicofunctional nomenclature is used by CA in only a few cases, —C 2C CHSe CH CHCH2— 1 2 3 321 for disulfides , hydroperoxides, and peroxides (¶¶ 200, 196). Radicofunctional names express the compound type, e.g., “peroxide,” usually as a separate word. 1 1′ [methylenebis(1,3,5-triazine-6,2,4- When inverted, the substituents are not followed by a hyphen unless multipli- CH N 6 2 6′ N triyl)] (the 2- and 4-positions are cative nomenclature (¶ 125) is used with Hydroperoxide; e.g., Disulfide, eth- equally close to the heading parent yl methyl; Hydroperoxide, 1-methylethyl; Hydroperoxide, cyclohexylid- N N ′ 3N 5 5′N 3 and are therefore cited in normal se- enebis-. quence) 127. Replacement nomenclature (“a” nomenclature) is used for certain heterocyclic ring systems (¶ 146) and also, sometimes, for heteroorganic acy- clic compounds. This nomenclature is limited to cases in which carbon atoms Carbonyl groups which are part of carbon chains are expressed as oxo sub- have been replaced in chains and rings by nonmetals and/or elements of which stituents; chalcogen, imino, and hydrazono analogs are expressed as thioxo, se- the hydrides are CA index heading parents, i.e., P, As, Sb, Bi, Si, Ge, Sn, Pb, lenoxo, telluroxo, imino, and hydrazono substituents. and B. Examples: Requirements for its use in expressing acyclic chains are as follows: (a) A minimum of four hetero units must be present, none of which may be all or a part of a functional chemical group to be expressed in the index head- —CH2 NHCOCH2 CONHCH2 — [(1,3-dioxo-1,3-propanediyl)bis- 1 2 3 (iminomethylene)] ing parent as the preferred functional compound class (i.e., as a functional suf- fix or as a functional index compound such as Carbonic or Phosphonic acid). A hetero unit is defined as an isolated hetero atom or a series of consecutive —COCO— (1,2-dioxo-1,2-ethanediyl) hetero atoms, alike or different, that may be expressed as a unit, such as by a bivalent radical name. Examples: -S- (thio); -S-S- (dithio); -N=N- (1,2-diaz- = =ΝΗ) enediyl); -SiH -O-SiH -(1,3-disiloxanediyl); -SiH -NH-SiH -(1,3-disila- —CH2 C( NH) C( CH2 — 2 2 2 2 1 2 2′ 1′ zanediyl). The above are all single hetero units, but -HP-NH-, -S-O-, and -O- [1,4-phenylenebis(2-imino-2,1- SiH2-O-, are not. ethanediyl)] (b) The “a” name must not be lower in order of precedence than the name obtained by regular substitutive nomenclature, i.e., it must express at least as many principal functions of at least equal rank. When carbonyl groups and their analogs are not part of a larger acyclic car- (c) All hetero atoms must be in their standard valency state, or else the bon chain, the names carbonyl, carbonothioyl, carbonimidoyl, etc., are em- abnormal valency must be expressible unambiguously by use of “oxide,” etc., ployed. terms. Examples: (d) The chain may be terminated only by C, P, As, Sb, Bi, Si, Ge, Sn, Pb, or B. Acyclic “a” nomenclature is employed for organic chains containing silicon 1 1′ CO (carbonyldi-4,1-phenylene) or metal atoms, polyesters, anhydrides, amides, polyamides, polyalkylene gly- 6 6′ cols, and condensed carbonic acid derivatives. It is not used for peptides or polymers, or (if it can be avoided) for chains containing no carbon atoms. Oth- —NHC( = NH) NH— (carbonimidoyldiimino) erwise, if the above criteria are met, an “a” name is always selected. 128. Replacement prefixes for the elements most frequently found in car- bon chains are set out in descending order of precedence in Table I. (The order [1,4-phenylenebis(carbono- is the reverse of that shown in ¶ 215 for coordinated elements.) —NHCS CSNH— thioylimino)] TABLE I REPLACEMENT PREFIXES IN DESCENDING Valid multiplying radicals are used only when the entire compound is sym- ORDER OF PRECEDENCE metrical around the central unit of such a radical; i.e., the radical must be at- tached to the heading parent by bonds of the same type (single, double, or Element Substitutive Valence Prefix triple) and at equivalent positions, and this parent must be identical with regard Oxygen II Oxa to positions of principal groups (and their functional derivatives) and other sub- Sulfur II Thia stituents. Whether or not such other substituents are present, the terms “bis,” Selenium II Selena “tris,” etc., are employed after the multiplying radical, not “di,” “tri,” etc. If Tellurium II Tellura other substituents are present, they are cited as regular substitutive radicals af- Nitrogen III Aza ter an opening bracket (which, perforce, is left unclosed). Phosphorus III Phospha Examples: Arsenic III Arsa Antimony III Stiba 1 1′ Pyridine, 3,3′-thiobis[6-chloro- (not N N Pyridine, 5,5′-thiobis[2-chloro-; Bismuth III Bisma 6 6′ ClS Cl multiplicative radicals are given Silicon IV Sila lowest locants) (The uninverted Germanium IV Germa form of this name is 3,3′-Thio- Tin IV Stanna bis[6-chloropyridine].) Lead IV Plumba H N NH Boron III Bora 2 1′ 1 2 Me = The replacement prefixes are placed in descending order of precedence ′ C CH Me 6 6 ahead of the name of the carbon skeleton with locants to indicate the positions of the atoms replaced. Lowest locants are assigned to functional groups if ′ ′′ 1′′ Benzenamine, 3,3 ,3 -(1-ethen- present, otherwise to the hetero atoms. yl-2-ylidene)tris[6-methyl- ′′ Replacement nomenclature is also employed for acyclic substituent prefix- 6 NH2 es (radicals) when the above requirements are fulfilled. In this case, lowest lo- Me cants are assigned to free valency positions; i.e., the “a” names are based on the carbon chain radical names, but the free valency locant (“1”) is always cited. The principles of multiplicative nomenclature are applied only after the in- Examples: dex heading parent has been chosen, and after other principles, e.g., centrality
(¶ 138), have been applied. When more than one multiplicative name is possi- − − − − − − − ~ CH3 O CH2 CH2 O CH2 CH2 O— ~ ble, that one is used which multiplies the greatest number of index heading par- 14 13 12 11 10 9 8 7 ~ ents, and then, if a choice is still necessary, that one which appears earliest in ~ − − − − − Η − ~ —CH2 CH2 NH CH2 CH2 C 2 OH the alphabetic sequence of index entries. The number of occurrences of the par- ~ 65 4321 ent is not increased by arbitrary breaking of the skeleton from which the mul- tiplying radical is derived. 7,10,13-Trioxa-4-azatetradecan-1-ol ¶ 128 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 12
− − − − − − − ≡ (CH2)3 S (CH2)3 O CH2 CH2 CH2 SH er by N is denoted by nitrido. Peroxy acids are named by use of the affixes | 8 4 3 2 1 peroxo, (thioperoxo), and (dithioperoxo). O 12 These affixes are combined, in alphabetical order, with the functional suffix | 16 20 21 22 23 (CH ) −S−(CH ) −O−CH −CH=CH of compound names based on molecular skeletons. The systematic names Ben- 2 3 2 3 2 2 zenecarboxylic, Ethanoic, and Methanoic acids, not the trivial names Ben- 4,12,20-Trioxa-8,16-dithiatricos- zoic, Acetic, and Formic acids, are used as the parents for functional 22-ene-1- thiol replacement nomenclature. Examples:
CH −NH−CH −CH −NH−CH −CH −ΝΗ−CH 3 2 2 2 2 2 MeCS H Ethane (dithioic) acid (not Acetic 1 2 3 4 5 6 7 2 acid, dithio-) 14 13 12 11 10 9 8 − − − − − − − − CH3 NH CH2 CH2 NH CH2 CH2 NH CH2 3,6,9,12-Tetraazatetradecane- PhC( =NH) OH Benzenecarboximidic acid (the 1 14 1,14-diamine, N ,N -dimethyl- tautomeric Benzamide is pre- ferred, except for esters and anhydrides; see ¶ 122) OH O CH O CH O CH O CH NNH2 3 3 3 3 || | || | || | || | || | − − − − − − − − − − − − − − − CH2 S OH CH3 CH C O CH C O CH C O CH C O CH COOH || Benzenemethanesulfonohydra- 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 NH zonimidic acid (derived from 3,6,9,12-Tetraoxapentadecanoic the conjunctive name Benz- acid, 14-hydroxy-2,5,8,11- enemethanesulfonic acid) tetramethyl-4,7,10,13-tetraoxo- O || Ethane(dithioperoxoic) acid CH3CSSH O || − − − − − − − − − − − In replacement names from phosphorus and arsenic functional parent com- CH3 CH2 O (CH2 CH2 O)3 CH2 CH2 O C (CH2) 10 CH3 12 2 1 pounds (¶ 197), all the affixes except hydrazono may be employed as part of Dodecanoic acid the suffix. For Carbonic acid and its relatives (¶ 183) all but hydrazido and ni- 3,6,9,12-tetraoxatetradec-1-yl trido may be used. ester Examples: Phosphorochloridic acid Cl P(O)(OH)2 CH3 CH3 + − − − − − − − − MeAs (NH2) OH Arsonamidous acid, As-methyl- 1 CH2 NH CH2 NH CH2 NH CH2 NH CH2 1 + N 2 9 8 7 6 5 4 3 2 1 2 N − 2 I P(S)(NH2) 3 Phosphorothioic triamide 3 3 4 4 = (HO)2 C NNH2 Carbonohydrazonic acid Quinolinium, 2,2′-(2,4,6,8-tetra- azanonane-1,9-diyl)bis[1- (HOO)2 CO Carbonodiperoxoic acid methyl-, iodide (1:2) In replacement names from other mononuclear acids and from condensed nuclear acids (anhydrides), the affixes are used as nondetachable prefixes cited O || at the beginning of the heading parents. Because multiplicative prefixes are − − − − − − − − − − − C NH CH2 CH 2 S CH2 CH2 O CH2 CH2 NH COOH rarely used for thio and other chalcogen prefixes, ambiguity is resolved by syn- 12 11 10 9 8 7 6 5 4 3 2 1 onym line formulas in the boldface index headings. Examples: 5-Oxa-8-thia-2,11-diazadodecanoic acid, 12-oxo-12-phenyl- OOO || || || HO−P−NH−P−O−P−OH Imidotriphosphoric acid || | − − − − − − −Ο− − −Ο OH OH OH CH3 O CH2 O CH2 O CH2 CH2 CH2 COOH
HO2CNHCO2H Imidodicarbonic acid Benzoic acid, 4-(3,5,7,9-tetraoxa- dec-1-yloxy)- O || HO−S−SeH Selenosulfuric acid (H SO Se) || 2 3 O OO O CH O || || || 3 − − − − − − − − = S S P CH2 CH2 C NH CH2 NH C C CH2 CH O || || 3 HS−C−S−C−SH Thiodicarbonic acid ([(HS)C(S)]2S) CH O 3 − − − − − − − P−CH CH2 C NH CH2 NH C CH2 Nondetachable prefixes are used in a few other cases. || 2 || || O OO Examples: CH3 O Phosphonic acid, P,P′-(8-methylene- ClSO3H Chlorosulfuric acid 3,7,10,14-tetraoxo-4,6,11,13- = tetraazahexadecane-1,16-diyl)- S(O) ( NH) (NH2)2 Imidosulfamide bis-, P,P,P′,P′-tetramethyl ester (H2N)2CS Thiourea Thiohydroxylamine H2NSH 129. Replacement nomenclature for functions is a method of describing AcOSAc Thioperoxide, diacetyl the replacement of hydroxyl and oxo functional groups by nitrogen, chalco- Thiohydroperoxide, O-ethyl gens, halogens , or halogenoids such as isocyanato. The replacement may be EtOSH carried out in substituent suffixes, e.g., -thioic acid from -oic acid; in substitu- ent prefixes, e.g., carbonimidoyl (¶ 134) from carbonyl, and phosphinothioyl 130. Substitutive nomenclature, in which hydrogen atoms are replaced from phosphinyl (¶ 197); and in functional parent compounds (¶ 130), e.g., by other atoms or chemical groups, is of paramount importance among nomen- Phosphonimidodithioic acid from Phosphonic acid. clature systems because of its versatility. Substitutive parent compounds, Replacement of hydroxyl in compounds and radicals is denoted by the fol- which are real or hypothetical compounds whose names imply the presence of lowing affixes (the final “o” is often elided): amido (for -NH2), azido (for -N3), replaceable hydrogen atoms, are of two kinds: chlorido (for -Cl) (and similarly for other halo atoms), cyanatido (for -OCN), (a) Functional parent compounds have names which express a chemical hydrazido (for -NHNH2), isocyanatido (for -NCO), (isothiocyanatido) (for - function but are not based on a molecular skeleton. Substitutive examples NCS), and (thiocyanatido) (for -SCN). Seleno and telluro analogs are named include Arsonic acid, Imidodicarbonic acid, Carbamic acid and Phospho- analogously. namidic chloride. Their substituents are always expressed as prefixes, never Replacement of oxo is denoted by the affixes hydrazono (for =NNH2), im- as suffixes. ido (for =NH), thio (for =S), etc. Replacement of a hydroxyl and an oxo togeth- 13 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 133
(b) Molecular-skeleton parent compounds are nonfunctional. They are Examples: chains or rings of atoms with only hydrogen atoms attached and possessing names which express or imply the substitutive valency and bonding of the skel- Me(CH2)4O— (pentyloxy) NOT (pentylhydroxy) etal atoms. (Methane and monoatomic hydrides of the Group IVA elements and the Group VA elements (except nitrogen) are treated as molecular skele- ClCO— (chlorocarbonyl) NOT (chloroformyl) tons.) Examples include Ethane, Distannane, Diazene, 3,6,9,12,15-Pentaox- aheptadecane, Cyclohexane , Morpholine , Phosphine, and Stannane . They are transformed into index heading parents by appending, as a suffix, the sub- PhO2C— (phenoxycarbonyl) NOT (phenylcarboxy) stituent which represents the principal functional group of the compound; other substituents are expressed as prefixes. (MeO)2P(O)— (dimethoxyphosphinyl) NOT (dimethylphosphono) 131. Substituent suffixes of molecular-skeleton parent compounds are chosen to represent the principal chemical functional group (or groups) in ac- MeOCS— (methoxythioxomethyl) NOT (methoxycarbonothioyl) cordance with the order of precedence of compound classes (¶ 106). When no suffix is available to represent the preferred compound class, either a substitu- tive functional parent compound (¶ 130) is used as a heading parent, or another All compound radicals are enclosed in parentheses. Simple radicals are so system of nomenclature, e.g., coordination or radicofunctional, is adopted. enclosed when two locants of like type fall together; e.g., Benzoic acid, 3-(4- Examples: pyridinyl)-; for clarity, when one locant has been omitted in the name of an in- definite compound; e.g., 1,2-Propanediol, 3-(thienyl)-; and when “bis” or “tris” has been employed to remove ambiguity (see ¶ 110); e.g., tris(decyl), EtP(O) (OH)2 Phosphonic acid, P-ethyl-(Phos- phonic acid is a functional parent bis(benzanthracenyl), bis(azepinyl). Brackets are used in complex radicals, compound.) e.g., [2-(dimethylamino)ethoxy]. Spaces in a name often permit the dropping of one set of enclosing marks around radicals in substituents and modifications; e.g., Disulfide, 2-chloroethyl ethyl; Propanoic acid, 2-ethylbutyl ester. PhSF3 Sulfur, trifluorophenyl-, (T-4)- (a coordination name) 133. Compound radicals. Selection of names for compound radicals is usually simple, but, when chain branching is present, can sometimes become perplexing. The following rules are successively applied; ((a) through (e) lead F COOCF Peroxide, bis(trifluoromethyl) (a 3 3 radicofunctional name) to selection of the preferred parent radical, (f) through (h) to a particular occur- rence of this radical). (a) Greatest number of acyclic hetero atoms. The particular suffixes used for various classes of compounds in descend- (b) Greatest number of skeletal atoms. ing order of precedence from acids through imines (¶ 106) are described in the (c) Greatest number of most preferred acyclic hetero atoms (see Table sections of this Guide dealing with these classes. Only one class may be I, ¶ 128). expressed as a suffix in a single index heading parent; less preferred classes are (d) Greatest number of multiple bonds. denoted by prefixes. Multiplicative prefixes are employed to indicate the num- (e) Lowest locants in the simple radical for replacement atoms in “a” ber of principal groups present. names, then for multiple bonds of any kind, and finally for double bonds. Examples (in descending order of compound classes): (f) Greatest number of substituents attached to the simple radical. (g) Lowest locants for such substituents.
CO2 H (h) Earliest index position of the total radical as it appears within the Benzoic acid, 2-(chlorocarbonyl)- index name. COCl Examples (the italic letters on the left indicate the particular rule (above) that is exemplified):
EtCO H Propanoic acid 2 (a) CH −CH −CH | 2 2 3 MeCHMeSO3H 2-Propanesulfonic acid − − − − − − − − −Ο− − 1 2 CH3 O CH O CH2 CH2 O CH2 CH2 CH2 CH2— 12 11 10 9 8 7 6 5 4 3 2 1 (10-propyl-3,6,9,11-tetraoxadodec-1-yl) MeCOCH2CSMe 2-Pentanone, 4-thioxo- 15
(a) SiH3 HOSiMe OSiMe OH | (1-silyl-1-disiloxanyl) 2 2 1,3-Disiloxanediol, 1,1,3,3-tetra- − − H3 Si O SiH— methyl- 3 2 1 6 (b) 1 − = CH 2OH CH3 C O | (1-acetyl-2-buten-1-yl) Benzenemethanol, 2-hydroxy- − = − CH3 CH CH CH— OH 4 3 2 1
(b) CH 2 || − − (1-methylenepropyl) CH3 CH2 C— CH3CH2NH2 Ethanamine 3 2 1 [(1-propylpentyl)oxy](not (1-butyl- (b) − − CH2 CH2 CH3 | butoxy))(the selection principles − − − 132. Substituent prefixes (commonly called “radicals”) are employed to CH3 (CH2)3 CH O— are applied before elision is per- denote atoms and chemical groups attached to an index heading parent. The 5 4-2 1 formed; another example follows) following substituents are never expressed as suffixes; they may be termed (b) “compulsory” or “mandatory” prefixes: astato (At-), astatyl (AtO -), azido 2 ([1,1′-biphenyl]-4-yloxy)(not (4- (N -), bromo (Br-), chloro (Cl-), chlorosyl (OCl-), chloryl (O Cl-), diazo (N - — O 3 2 2 phenylphenoxy)) ), fluoro (F-), iodo (I-), iodosyl (OI-), iodyl (O2I-), isocyanato (OCN-), isocy- ano (CN-), nitro (O2N-), aci-nitro ((HO)(O)N=), nitroso (ON-), and perchloryl (O Cl-). In addition, all thio, sulfinyl, and sulfonyl radicals, (RS-), (RS(O)-), 3 (b) O ′ and (RS(O) -), and their seleno and telluro analogs, are mandatory substituent ([1,1 -biphenyl]-4-ylcarbonyl)- 2 || (not (4-phenylbenzoyl))(the prefixes; so are hydrocarbon radicals and other radicals derived from molecular C— skeletons, e.g., ethyl, furanyl, disiloxanediyl, when attached to a more simple radical benzoyl is retained preferred heading parent. for (phenylcarbonyl) only when Radicals may be simple, compound, or complex. A compound radical is the rules lead to this name) made up of two or more simple radicals, e.g., (chlorothio), (diaminomethyl). A complex radical is composed of a simple radical to which at least one com- (4-chlorobenzoyl)(not [(4-chloro- pound radical is attached; e.g., [(chloromethyl)amino], [1-(trichloromethyl)-2- (b) Cl CO— phenyl)carbonyl]) buten-1-yl]. In these examples, amino and 2-buten-1-yl are parent radicals, and methyl (in both cases) is a subsidiary radical. This procedure may be repeated indefinitely. (Chlorothio) is obtained by addition of the two components, (ami- nomethyl) by substitution of methyl by amino. Substitution is the preferred (b) method when a substitutive simple radical is available; e.g., (aminomethylene) 1 1′ 3′ 1′′ [1,1′:3′,1′′-terphenyl]-5′-yl is (NH CH=), not (NH CH -). Substitution in certain radicals, including the 2 2 2 6′ following, is not permitted: hydroxy, mercapto, selenyl, telluryl, hydroperoxy, sulfeno, formyl, carboxy, sulfo, phosphono, and carbonothioyl. ¶ 133 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 14
(c) − (2-aminoacetyl)(glycyl is permitted S SiH3 H2NCH2CO— | [1-(silylthio)-1-disiloxanyl] inpeptide nomenclature) − − H3Si O SiH— ClCOCO— (2-chloro-2-oxoacetyl) (c) − − −Ο− − S CH2 CH2 CH2 CH3 | AcNH— (acetylamino) − − − −Ο− − − − − − −Ο− − CH3 CH2 O CH2 CH2 CH CH2 O CH2 CH2 CH2 CH2— 14 13 12 11 10 9 8 7 6 5 4 3 2 1 EtCONH— [(1-oxopropyl)amino] [8-[(2-ethoxyethyl)thio]-3,6,10,12- tetraoxatetradec-1-yl] = (4-imino-4-phenyl-1-thioxobutyl) PhC( NH)(CH2)2CS— 4 1 (d) − = MeC(=NH)NH— [(1-iminoethyl)amino] CH3 C O | (1-acetyl-2-propen-1-yl) = − CH2 CH CH— Isolated carbonyl radicals, other than carboxy, are expressed as carbonyl (as (d) a doubling radical, or when both free valencies are attached to a single atom), − − ≡ − = CH 3 CH 2 C C CH CH formyl (if unsubstituted), benzoyl (if attached to a phenyl group which is not | − = − = − − itself attached to another phenyl), or as a compound radical in which carbonyl CH3 CH CH CH CH CH2 CH— 7 6 5 4 3 2 1 is the parent. [1-(1-hexen-3-yn-1-yl)-3,5-heptadi- Examples: en-1-yl] (double bonds are pre- ferred over an equal number of triple bonds) HO2CCO— (carboxycarbonyl) (e) (aminocarbonyl)(not carbamoyl) CH −O−CH −CH −O−SiH −Ο−CH −CH H2NCO— 3 2 2 2 2 | 2 − −Ο− − −Ο− −Ο− − − CH3 CH2 CH2 CH2 SiH2 CH2 CH CH2— ClCO— (chlorocarbonyl)(not chloroformyl) 11 10 9 8 7 6 5 4 3 2 1 [2-(3,5,8-trioxa-4-silanon-1-yl)- —NHCONH— (carbonyldiimino) 4,6,9-trioxa-5-silaundec-1-yl] (4-formylbenzoyl) HCO CO—
(e) − − ≡ − − = CH3 CH2 C C CH2 CH CH OC | − − − = − ≡ − − (3-carbonylcyclohexyl) CH3 CH2 CH2 CH CH C C CH CH2— 9 8 7 6 5 4 3 2 1 [2-(1-hepten-4-yn-1-yl)-5-nonen-3-yn-1-yl] Replacement analogs of isolated carbonyl groups (other than chalcogen an- alogs of carboxy) are named as thioxo, imino, etc., derivatives of methyl, un- (e) = − ≡ − CH2 CH C C CH2 less both free valencies are attached to a single atom, or the radical is being | ≡ − = − − [1-(4-penten-2-yn-1-yl)-3-hexen- used multiplicatively (¶ 125), in which case carbonimidoyl, carbonohydra- HC C CH CH CH2 CH— 6 5 4 3 2 1 5-yn-1-yl] zonoyl, carbonothioyl, etc., are employed. Examples:
(f) CF [3,3,3-trifluoro-2-methyl-2-(trifluo- HS2 CNH— [(dithiocarboxy)amino] | 3 − − romethyl)propyl] CH3 C CH2— | HCS CO— [4-(thioxomethyl)benzoyl] CF3 (f) − = CH3 CH2 HOC( NH) — (hydroxyiminomethyl) | (1-ethyl-3-phenylpropyl)(not [1-(2- − − CH2 CH2 CH— phenylethyl)propyl]) = H2NC( NH)NH — [(aminoiminomethyl)amino] (g) − − CH3 CH CH3 | [1-(2-methylpropyl)-2-oxobutyl] HN=C=N — (carbonimidoylamino) OCH2 (not [3-methyl-1-(1-oxo- || | − − − propyl)butyl]) CH3 CH2 C CH— 4 3 2 1 135. Indicated hydrogen is a designation comprising a locant followed immediately by an italic capital H placed before a ring system name to express (g) the position of each of the saturated atoms necessary for formation of a defin- CH | 3 [1-(2-bromoethyl)-2-methylpropyl] able, stable ring system. Thus, Pyrrole always has one saturated atom (an atom − − CH3 CH CH— (not [3-bromo-1-(1-methyleth- not connected to either of its neighbors by a double bond) and, according to the | − yl)propyl]) position of this atom, the compound is named as follows: BrCH2 CH2
H 1 1 (h) CH3 (1-acetyl-2-methylpropyl)(not [1- | N N N − − (1-methylethyl)-2-oxopropyl]) 5 5 CH3 CH CH— 1 5 | − = CH3 C O 134. Carbonyl radicals which form part of a carbon chain are expressed 1H-Pyrrole 2H-Pyrrole 3H-Pyrrole by oxo substituents on the chain; the only exceptions are carboxy (-C(O)OH) and acetyl (-C(O)CH3) radicals. The latter is used whenever (1-oxoethyl) would otherwise be called for. All chalcogen, imido and hydrazinylidene ana- logs of carbonyl in a chain are treated similarly by use of thioxo, selenoxo, tel- In the Chemical Substance Index only a single illustrative structural dia- luroxo, imido, and hydrazinylidene radicals, except for chalcogen analogs (but gram is provided for each ring system, viz., the diagram which shows the sat- not imido, etc., analogs) of carboxy; e.g., (HS(S)C-) is named (dithiocarboxy). urated center(s) in the lowest-numbered nonangular position(s). Replacement analogs of acetyl are named (1-iminoethyl), (1-thioxoethyl), etc. Tetrahydropyrrole has the trivial name Pyrrolidine : dihydropyrroles are Acyl radical names other than acetyl and benzoyl, e.g., propanoyl, are not used named as derivatives of that pyrrole which has indicated hydrogen at the lowest for substituents; neither are amido radicals, e.g., acetamido. numberedpositionconsistentwiththestructure.Othermonocyclicheterosys- Examples: tems are named in the same way. Hydrogen on a single ring atom between two bivalent hetero atoms is not indicated in the name. Examples: HO2CCH2— (carboxymethyl) H HCOCH2— (2-oxoethyl)(not (formylmethyl)) N 5 1 1H-Pyrrole, 2,5-dihydro- (not 2H-Pyrrole, 1,5-dihydro-) EtCO— (1-oxopropyl)
ClCOCH2— (2-chloro-2-oxoethyl) 15 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 136
1 1 7a O 2H- Pyran , tetrahydro- (not 4H- 6 Pyran, tetrahydro-) 3aH-Indene-3a-carboxylic 3 acid, 1,4-dihydro- 4 CO2H 1 S 4H-1,3-Dithiin (not 2H,4H-1,3- 6 9 10 1 Dithiin) O 10a O S3 4H,6H-Benzo[1,2-b:5,4-b′]dipyran- 6 4 4,6-dione, 2,3,7,8-tetrahydro- The lowest locants for nonangular positions of fused ring systems are nor- 5 mally cited for indicated hydrogen. O O Example: 1 4H-3a,7-Methanoazulene-4,9- 1 4H-Indene, 3a,5-dihydro- (not 8a 7 O dione, 1,2,3,7,8,8a-hexahydro- 7a 3aH-Indene, 4,5-dihydro- or (not 1H-3a,7-Methanoazulene-4,9- 9 3 5H-Indene, 3a,4-dihydro-) 3a (7H)-dione, 2,3,8,8a-tetrahydro-) 4 (the ketone function on the bridge O is ignored in choosing indicated hydrogen to accommodate the Indicated hydrogen is assigned to angular or nonangular positions when single remaining function) needed to accommodate structural features, e.g., a bridge, spiro junction or 1 ring-assembly junction, if that form of the ring system can exist. 8a S 2 Examples: NH (2,3-dihydro-4H-1,2,4-benzo 4 thiadiazin-4-yl)(not 2H-1,2,4- N H 5 benzothiadiazin-4(3H)-yl) 7a N 6 1 3a,6-Methano-3aH-indole, 8 1,4,5,6-tetrahydro- 3a O 1 8a O 2H-1- Benzopyran -4,5,8(3H)-trione, 1 3 5 4 6,7-dihydro- (not 4H-1-Benzo- 11a pyran-4,5,8-trione, 2,3,6,7-tetra- 11b 4 Spiro[7H-benz[de] anthracene - ′ O O hydro-) 7 7,1 -cyclohexane], 4,5,6,6a- 8 ′ 6 tetrahydro- 6 ′ 1 136. Added hydrogen is hydrogen which is added to a ring system in the same operation as, but in a position different from, hydrogen added to accom- H H ′ modate structural features of a ring system, e.g., bridges, or spiro or ring-as- 7a N 2 2′ N 7 a 1 Ν Ν 1′ 2,2′-Bi-2H- indazole , 1,1′,3,3′- sembly junctions, or principal groups of a heading parent, or free valencies of a parent radical, when indicated hydrogen (¶ 135) is either not needed for the ring 3 3′ tetrahydro- 4 4′ system itself or cannot be chosen to accommodate them. It differs from indi- cated hydrogen in being expressed as a locant and capital italic H in parenthe- ses immediately following the locant for the principal function or other When a bridge requires hydrogen to be added, but indicated hydrogen of the accommodated structural feature, e.g., “2(1H)-.” Use of added hydrogen per- parent system cannot be used for that purpose, the lowest locant, or a locant to mits expression of a principal function, etc., in a heading parent instead of as a accommodate a principal function, is chosen for the parent ring, and additional substituent. Thus, 1-Naphthalenone cannot exist without partial hydrogena- indicated hydrogen is cited in the name ahead of the bridge designation. tion of the naphthalene ring system; a name such as Naphthalene, 1,2-dihy- Example: dro-1-oxo- violates the rule that the principal function be expressed as a suffix. Therefore, two hydrogen atoms are added in one operation to provide the name 4H-3a,6-Methano-3H-1,2-benzox- 1(2H)-Naphthalenone, in which the “added” (or “extra”) hydrogen is at the 2- athiole, tetrahydro- (3aH-1,2- position. 1 Benzoxathiole cannot exist; the 7a O 6 O S 2 lowest available locant is there- 8 fore cited and the “extra” hydro- 8a 3a gen for the bridge cited as 1 additional indicated hydrogen, not in the “added” hydrogen 5 4 form (see below), 3a(4H),6- Methano....) When principal functions or free valencies require added hydrogen, it is as- signed to the lowest-numbered available angular or nonangular position; e.g., 1(2H)-Naphthalenone, 3,4-dihydro- (not 1(4H)-Naphthalenone, 2,3-dihydro-); After structural requirements have been met, indicated hydrogen is chosen 2(4aH)-Naphthalenone, 5,6,7,8-tetrahydro-. When the ring system requires to accommodate principal functions or (in a cyclic radical) free valencies, so indicated hydrogen and it cannot be assigned to accommodate a principal long as the number of indicated hydrogens cited equals or exceeds the number group or free radical, it has preference over added hydrogen for lowest locants. of principal groups or free valencies that must be accommodated. For the usual When a pair of principal groups, e.g., “-dione,” are expressed by a heading par- case of a ring which requires a single indicated hydrogen for its existence, a ent, added hydrogen is not cited unless necessary, it being understood that only single principal function or free valency is accommodated, but a polyfunctional sufficient hydrogen has been added to accommodate the functions. compound is named at the ring system with lowest nonangular indicated hydro- Examples: gen. (Functions on bridges are disregarded in applying this rule.) Examples: 1 8a 4a(2H)-Naphthalenecarboxylic acid, 1,3,4,5-tetrahydro- 1 O 4H-Pyran-4-one, tetrahydro- (a 4a(1H)- isomer cannot exist) 6 54
O H O N 1 6 4,6(1H,5H)-Pyrimidinedione, 9a 2 3NH dihydro- N(CH ) OH 2H-2-Benzazepine-2-ethanol, 2 2 1,3,4,5-tetrahydro- O 6 5 ¶ 136 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 16
H (3,4-dihydro-2(1H)-quinolinylidene) O 8a N 1 (not (1,4-dihydro-2(3H)-quinoli- nylidene)) (6-chloro-2,5-dioxo-3-cyclohexen- 1-yl) 5 4 Cl O 1 3 9a 1H-Benz[e]indene-3,5(2H,4H )- 9b = ≡ O dione, 3a,9b-dihydro- MeCH CHC CCF3 2-Hexen-4-yne, 6,6,6-trifluoro- 5 4 16 (double bond given preference 6 over triple bond) O OH
1 1 CO H O 8a 2 6 2H-Pyran-3(4H)-one, dihydro- (3H-Pyran cannot exist) 2-Azulenecarboxylic acid, 3 O 8-hydroxy-4,5-dimethyl- 4 Me Me O 8 1 9a 9 9,10-Anthracenedione (not 9,10- NHEt 10 (9H,10H)-Anthracenedione) 10a 4a 1 1 3 4 1,3-Benzenediamine, N -ethyl-N ,- 5 3 O N ,4-trimethyl- (not 1,3- 3 Benzenediamine, N3-ethyl- 1 1 NMe2 N ,N ,6-trimethyl-; see ¶ 114) O O 1H-Benz[e]indene-1,2(3H)-dione Me 1 (not 3H-Benz[e]indene-1,2-dione) 9b 9a 3 (low numbering of indicated hydrogen is observed, even if add- 4 ed hydrogen must then be cited) Br 6 5 Benzene, 1-bromo-3-nitro- (not Benzene, 3-bromo-1-nitro-) N 6 (2,3-dihydro-1,4-pyrazinediyl) (not 1 NO2 (1,4(2H,3H)-pyrazinediyl)) 4 N 138. Index name selection. Most organic compounds have names based on molecular skeletons, e.g., Propanoic acid (from propane ); 1,3-Dioxan-2- amine (from 1,3-dioxane). Procedures for selecting the preferred name of this Added hydrogen cited when hydrogen is required elsewhere for spiro and kind for index use are described in this section (see also ¶ 105). ring-assembly junctions is assigned (in descending order of preference) (a)to Selection of a heading parent name based on a molecular skeleton is made accommodate another spiro or ring-assembly junction, (b) to accommodate by successive application of the following principles until a decision is principal groups or free valencies, or (c) to lowest-numbered available posi- reached. tions. (a) Greatest number of the principal chemical functional group. 137. Numbering of molecular skeletons. Lowest locants for a set of prin- (b) Preferred atomic content of the molecular skeleton in accordance cipal groups, substituents, etc., are always preferred. The set, e.g., 5,6,1,2,1 is with the order of precedence of compound classes (¶ 106). The heading parent compared with another (alternative) set, e.g., 1,2,5,6,5, by rearranging them should express at least one occurrence of an atom appearing earliest in the fol- both in ascending numerical sequence: 1,1,2,5,6 and 1,2,5,5,6. The set which lowing list: N, P, As, Sb, Bi, B, Si, Ge, Sn, Pb, O, S, Se, Te. (This principle is contains the lowest locant at the first point of difference when all sets are com- used to decide between a cyclic and an acyclic parent, but is not applied to pared term by term is the lowest, i.e., 1,1,2,5,6 is lower than 1,2,5,5,6. choices between ring systems. When acyclic and cyclic skeletons of the same Example: compound class are present, a cyclic parent is preferred.) (c) Preferred ring system. The choice between ring systems for use as heading parents is based on the following criteria, applied successively until a NO 2 1 decision is reached. The senior ring system should: 8a Naphthalene, 5-bromo-6-chloro- 1,2-dihydro-1-nitro- (not Naphtha- (1) be a nitrogenous heterocycle; lene, 1-bromo-2-chloro-5,6-dihy- (2) be a heterocycle; dro-5-nitro-) (3) contain the largest number of rings; Cl 5 4 (4) be a cyclic system occurring earliest in the following list of sys- Br tems; spiro, bridged fused, bridged nonfused (Von Baeyer), fused; (5) contain the largest individual ring (applies to fused carbocyclic Lowest locants for various kinds of structural features in cyclic and acyclic systems); molecular skeletons are assigned, in order, to: (6) contain the greatest number of ring atoms; (a) hetero atoms (except for “a”-named radicals, see ¶¶ 127, 161); (7) contain the greater number of ring atoms common to two or more (b) indicated hydrogen; rings (applies to Von Baeyer ring systems); thus (c) principal groups or (for radicals) free valencies; (d) multiple bonds; (e) substituent prefixes; (f) the substituent prefix cited earliest in the name. is senior to Examples:
(8) contain lowest locants for bridges; CH −O−CH −CH −O−CH −CH −Ο−CH −CH −Ο−CH −CH −ΟH 3 2 2 2 2 2 2 2 2 (9) contain the largest number of hetero atoms; 13 12 11 10 9 8 7 6 5 4 3 2 1 (10) contain the most preferred hetero atom other than nitrogen, accord- 3,6,9,12-Tetraoxatridecan-1-ol ing to the order in Table I, ¶ 128, i.e., O, S, Se, Te, N, P, As, Sb, Bi, Si, Ge, Sn, Pb, B.
1 (11) possess the most linear arrangement of rings (thus, Anthracene is HO2 C O 2H-Pyran-6-carboxylic acid, 3,4- senior to Phenanthrene ); 6 dihydro- (not 4H-Pyran-2-carb- (12) possess the lowest locants for hetero atoms assigned according to oxylic acid, 5,6-dihydro-) the rules (¶¶ 146, 152); (13) express the lowest state of hydrogenation ; thus, Benzene is pre- ferred over Cyclohexane, Pyridine over Piperidine; (14) express the lowest locant for indicated hydrogen. O || 3-Buten-2-one (not 1-Buten-3-one) Note: These criteria differ from those employed in selecting base = − − CH2 CH C CH3 components for fused systems (¶ 150). 432 1 (d) Greatest number of acyclic hetero atoms. (e) Largest index heading parent. 17 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 138
(f) Greatest number of most preferred acyclic hetero atoms, according (e) to the order of precedence in ¶ 128, above; i.e., O, S, Se, Te, N, P, As, Sb, Bi, CH 2 Hexane , 3-methylene- (not 1-Pen- || Si, Ge, Sn, Pb, B. − − − − − tene, 2-ethyl-) CH3 CH2 CH2 C CH2 CH3 (g) Greatest number of multiple bonds. 6 5 4 3 2 1 (h) Lowest locants in the heading parent successively for hetero at- oms, principal groups (suffixes), all multiple bonds, double bonds. (e) If the preferred heading parent occurs more than once in the total com- HO CH(OH)CH2OH pound, further principles must be applied, as follows: β α 1,3-Benzenediethanol, β1, 5-dihy- (i) Centrality. For three or more occurrences of the heading parent, at droxy- (see ¶ 124) least one of which must be nonterminal, the basis of the name is the central oc- currence (or one of the central pair—both, when multiplicative nomenclature is permitted—if the total number is even) in the linear arrangement which com- (CH2)2OH prises all or part of the maximum number of occurrences. (f) (j) Maximum number of substituent prefixes. H3SiOSiH2SiH2SSiH3 3 2 1 Disiloxane, 1-disilathianyl- (k) Lowest locants for substituents on the heading parent. (l) Multiplication of heading parents; when there is a choice of multi- plicative names, that one is chosen which multiplies the largest number of oc- (g) CH3 currences of the index heading parent. | = − = (m) Earliest index position of the total name. CH2 C CH CH2 1,3-Butadiene, 2-methyl- Examples (the italic letters on the left indicate the particular rules 1 2 (above) that are exemplified): (g) (a) − − − − C≡C−CH −CH −OH OH CH2 CH2 CH2 CH2 CH3 2 2 | | | − − − − − − = − − − − = − − CH3 CH CH CH2 OH HO CH2 CH CH CH2 CH CH2 CH CH CH2 OH 4 3 2 1 1,3-Butanediol, 2-pentyl- 9 8 7 6 5 4 3 2 1 2,7-Nonadiene-1,9-diol, 5-(4-hy- (a) HO C CH(CO H)CH CO H droxy-1-butyn-1-yl)- (double 2 2 2 2 bonds are preferred over an equal Butanedioic acid, 2-(4-carboxy- number of triple bonds) phenyl)- (h) (b) 10 11 12 Hydrazinecarboxylic acid, 2-(4- − − HO C O CH2 CH3 2 NHNHCO2H carboxyphenyl)- | 2 1 − − − − − − − − − − − CH3 O CH2 CH O CH2 O CH2 O CH2 CH2 COOH 9 8 7 6 5 4 3 2 1 (b) O 4,6,8,10-Tetraoxadodecanoic SiH2SiH3 Disilane, 2-furanyl- acid, 9-(methoxymethyl)- (not 3,5,7,9-Tetraoxadodecan-12-oic Diazene, phenyl- (not Benzenamine, acid, 4-(methoxymethyl)-) (b) N-imino-) (homogeneous hetero PhN=NH chains are never broken to obtain a higher function or more (h) preferred parent) ΟΗ HO-CH2 -CH2 -CH2 (b) | | − − − − − − − − (CH ) −CH HO CH2 CH2 CH2 CH2 CH CH2 CH CH3 2 6 3 1 2 3 4 5 6 7 8 | α − Benzeneacetic acid, -heptyl- CH COOH (not Nonanoic acid, 2-phenyl-) 1,7-Octanediol, 5-(3-hydroxy- α propyl)- (not 1,8-Octanediol, 4-(2-hydroxypropyl)-) (b) 1 CO H 2-Pyridinecarboxylic acid, 5-(2- N 2 6 carboxyhydrazino)- (the number of preferred hetero atoms in the (h) − = − − cyclic and acyclic chains is disre- CH2 CH CH CH2 OH HO CNHNH | 2 garded) − − = − − − = − − − HO CH2 CH CH CH2 CH CH CH CH2 CH2 OH 1 2 3 4 5 6 7 8 9 − − −Ο− − (b) O CH2 CH2 CH2 CH3 2,6-Nonadiene-1,9-diol, 5-(4-hy- | −Ο− − −Ο− − −Ο− − − − − − droxy-2-buten-1-yl)- (not 2,7- CH3 CH2 CH2 CH2 CH2 CH CH2 NHNH CH2 CH2 CH3 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Nonadiene-1,9-diol, 5-(4-hy- droxy-1-butenyl)-) 2,5,8-Trioxa-11,12-diazapentade- cane, 9-(2-ethoxyethoxy)-
(c) NH N 3-Quinolinol, 2-(1,2-dihydro-2- (i) OH hydroxy-5-pyrimidinyl)- Benzene, 1,4-bis(phenylmethyl)- N PhCH2 CH2 Ph OH (d) − − CH2 CH2 CH2 | (i) −Ο− −Ο− −Ο− −Ο− −Ο− Cl2 CHCH2 O(CH2 )2O(CH2)2Cl CH3 CH CH2 CH2 CH2 CH3 Ethane, 1-(2-chloroethoxy)-2- 12 3 4 5 6 7 8 9 10 11 (2,2-dichloroethoxy)- 2,4,6,8,10-Pentaoxaundecane, 3- propyl- (i) NH2 (e) HCO (CH2) 8CHO Benzenenonanal, 4-formyl- (not Benzaldehyde , 4-(9-oxononyl)-) (e) H N N NH NH NH = − − 2 2 CH CH CH2 OH | − − − − − − = − − Η − HO CH2 CH2 CH2 CH2 CH CH CH CH2 C 2 OH 1 1 9 8 7 6 5 4 3 2 1 1,4-Benzenediamine, N ,N -bis(4- aminophenyl)-N4-[4-[(4-ami- 3- Nonene -1,9-diol, 5-(3-hydroxy-1- nophenyl)amino]phenyl]- propen-1-yl)- (not 2,5-Octadiene- 1,8-diol, 5-(4-hydroxybutyl)-) ¶ 138 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 18
HO2 C Cl Cl 1
CH3 6 O CO2 H
− CH 2 CH CH2 CH2 CH3 Benzoic acid, 3-(4-carboxy-2- | chlorophenoxy)-2-chloro- CH 3 CH3 CH3 Benzene, 2-methyl-1-[1-methyl-2- (l) [4-[(3,4,5-trimethylphenyl)- methyl]phenyl]ethyl]-4-(phe- − − − − nylmethyl)- (of the two central CH2 O CH O CH2 occurrences of the preferred heading parent, the one with the maximum number (three) of sub- ′ stituents is chosen; note that the Benzene, 1,1 -[(phenylmethyl- centrality principle prevents ene)bis(oxymethylene)]bis- choice of the tetrasubstituted par- (note that all occurrences of ben- ent on the right) zene are terminal and that the principle of centrality is therefore not applicable)
(i,l) (l,m)
Cl CH2 CH2 CH2 Cl
Benzene, 1,1′-methylenebis[4- C − O − C − S − C [(4-chlorophenyl)methyl]-
Benzene, 1,1′,1′′-[[[diphe- nyl(triphenylmethoxy)methyl] (i,l) thio]methylidyne]tris- (not Benzene, 1,1′,1′′-[[diphe- nyl[(triphenylmethyl)thio]meth- CH2 oxy]methylidynetris-)
CH CH CH 2 2 (m) Propanoic acid, 2,3,3,3-tetrafluo- F3CCF(CCl3)CO2H Benzene, 1,1′,1′′-methylidyne- 3 2 1 ro- 2-(trichloromethyl)- (not tris[4-( phenylmethyl)- (when Propanoic acid, 3,3,3-trichloro- multiplication of the heading par- 2-fluoro-2-(trifluoromethyl)-) ent is permitted, occurrences di- rectly attached to the central connecting radical are included in the same operation if possible) (m)
OO Cyclohexanone , 2-[2-(2-oxocyclo- (j) hexyl)ethylidene]- (not Cyclo- CHCH H N CH−CH −COOH 2 hexanone, 2-[2-(2-oxocyclo- 2 2 hexylidene)ethyl]-)
139. Subtractive nomenclature employs the suffixes “-ene” and “-yne” Benzenepropanoic acid, 4-amino- β to signify removal of hydrogen pairs from saturated molecular skeletons; re- -phenyl- moval of hydrogen atoms from an aromatic system is denoted by “dehydro.” Examples:
1 (j) 6 CH 7 Bicyclo[2.2.1]hepta-2,5- diene | 3 Propanoic acid, 3,3,3-trichloro- − − CCl3 C COOH 2-methyl-2-(trichloromethyl)- 4 3 | 2 1 (not Propanoic acid, 2,2- 1 CCl3 bis(trichloromethyl)-) (like N treatment of like groups is dis- 6 Pyridine, 2,3-didehydro- (not Pyri- continued ( ¶ 255)) dyne) (k) 6 1 NH Me Functional class names such as “anhydride,” “ester,” “oxime,” imply loss N of water and may strictly be regarded as subtractive terms. Prefixes such as Me Benzenamine, 2-methyl-N-(4- “deoxy,” “nor,” and “anhydro,” are sometimes employed in names for classes methylphenyl)- of stereoparents (see Section E), but never in general index nomenclature. 19 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 143
B. MOLECULAR SKELETONS
Introduction ¶ 140 Replacement (“a”) nomenclature for fused systems ¶ 153 Acyclic hydrocarbons 141 Bridged fused systems 154 Organic hetero chains 142 Von Baeyer nomenclature 155 Homogeneous hetero chains 143 Spiro systems 156 Heterogeneous hetero chains 144 Ring assemblies 157 Monocyclic hydrocarbons 145 Hetero atoms with abnormal valencies 158 Monocyclic hetero systems 146 Boron molecular skeletons 159 Polycyclic systems 147 “Hetero” polyboranes 160 Fundamental fused carbocycles 148 Substituent prefixes from molecular skeletons 161 Fundamental fused heterocycles 149 Compound and complex radicals 162 Selection of a base component 150 Molecular skeletons as index heading parents 163 Index names for fused systems 151 Fullerenes 163A Orientation and numbering of fused systems 152
140. Introduction. A molecular skeleton is defined for purposes of name set of multiple bonds; when there is a choice, double bonds are preferred over selection as a chain or ring of atoms in which the number of hydrogen atoms triple bonds. When principal groups (functional suffixes) are present, these and attached to each skeletal atom is (usually) implied or (occasionally) explicitly not the multiple bonds are preferred for low numbering. stated by citation of the substitutive valency and bonding of the skeletal atoms. Examples: Monoatomic hydrides of Group IVA and VA elements (except nitrogen) are also treated as molecular skeletons; so are boron hydrides, but because of their MeCH=CHCH2CH=CH2 1,4-Hexadiene (not 2,5-Hexadiene) unusual nature these are discussed separately (¶ 159). Examples: H2C=C=CH2 1,2-Propadiene CH4 Methane
≡ AsH5 Arsorane MeCH2C CCH2Me 3-Hexyne 61
H3SiOSiH2OSiH2 Trisiloxane HC≡ CC≡ CH 1,3-Butadiyne 14 H3SnSnH3 Distannane
HC≡CCH CH=CH 1-Penten-4-yne CH3-CH2-O-CH 2-CH2-O-CH2-CH2 -O -CH2 - CH2-O-CH2-CH3 2 2 14 13 12 11 10 9 8 7 6 5 4 3 2 1 51
3,6,9,12-Tetraoxatetradecane ≡ MeCH=CH(CH2)3C CH 6-Octen-1-yne (not 2-Octen-7-yne) 1
Benzene H2C=CHCH2OH 2-Propen-1-ol (not 1-Propen-3-ol)
142. Organic hetero chains containing at least four hetero units (¶ 127) H are given “a” names, i.e., replacement names based on the hydrocarbon N Pyrrolidine skeleton by use of “oxa,” “thia,” etc., prefixes. Lowest locants (¶ 137) are assigned to all hetero atoms regardless of type and then to preferred hetero atoms (Table I, ¶ 128). Locants for functional suffixes take precedence for low
8 9 1 numbering. 9a Examples: 9H- Fluorene
4b 4a CH3-CH2-O-CH2-CH2-O-CH2-CH2-O-CH2-CH2-O-CH3 13 12 11 10 9 8 7 6 5 4 3 2 1 2,5,8,11-Tetraoxatridecane Bicyclo[2.2.1]hept-2-ene −− −− −− −− −− −− −− CH3 O SiH2 CH2 CH2 SiH2 S CH3 1 2 3 4 5 6 7 8 2-Oxa-7-thia-3,6-disilaoctane (not 7-Oxa-2-thia-3,6-disilaoctane) 1 5 Spiro[ cyclopentane -1,1′-[1H ]- 7′a indene] 1′ CH3-CH2-SiH2-CH2-CH2-SiH2-CH2-CH2-SiH2-CH2-CH2-SiH2-CH2-CH3 1 2 3 4 5 6 7 8 9 10 11 12 13 14 3′ 4′ 3,6,9,12-Tetrasilatetradecane
6 1 4 S 7 S 7λ -[1,2]Dithiolo[1,5-b][1,2]di- H2C=CHCH2[O(CH2)2]3OMe 2,5,8,11-Tetraoxatetradec-13-ene S thiole 14 12 2 1 4 3 H2C=CH(OCH=CH)3OCH=CH2 3,6,9,12-Tetraoxatetradeca- 14 13 3 1 1,4,7,10,13-pentaene Molecular skeletons are nonfunctional substitutive parent compounds. Sub- stituents denoting a preferred compound class are generally cited as suffixes 143. Homogeneous hetero chains are considered to include the mononu- and other substituents as prefixes. Molecular skeletons, alone or in combina- clear hydrides Phosphine (PH ), Phosphorane (PH ), Arsine (AsH ), tion with a substituent suffix, are index heading parents. 3 5 3 Arsorane (AsH ), Stibine (SbH ), Bismuthine (BiH ), Silane (SiH ), This part of the manual deals with the formation of index names for 5 3 3 4 Germane (GeH ), Stannane (SnH ), and Plumbane (PbH ). Chains com- structures that consist solely of one or more molecular skeletons. 4 4 4 posed of two or more of these hydride residues are named by prefixing the hy- 141. Acyclic hydrocarbons. Saturated unbranched alkanes containing one dride name with “Di,” “Tri,” etc. through four carbon atoms are named Methane, Ethane, Propane, and Examples: Butane. Higher members of the class are named by adding the termination “-ane” to the appropriate multiplicative term, as, Nonane for C9H20, Hexade- H2BiBiH2 Dibismuthine cane for C16H34, Eicosane for C20H42, Heneicosane for C21H44, and Tritri- acontane for C H . 33 68 H PPH Diphosphorane Unsaturated unbranched acyclic hydrocarbons (unbranched alkenes , 4 4 alkadienes, alkynes , etc.) are named by replacing the ending “-ane” by “-ene” H SnSnH SnH Tristannane (for a single double bond), “-adiene” for two double bonds, “-yne” for a single 3 2 3 triple bond, etc. Combinations, e.g., “-enyne,” “-trienediyne,” are employed H Si(SiH ) SiH Pentasilane when both bond types are present. Low numbering (¶ 137) is employed for the 3 2 3 3 ¶ 143 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 20
Saturated nitrogen chains are named Hydrazine (not Diazane) for H2NNH2, Triazane for H2NNHNH2, Tetrazane for H2NNHNHNH2, etc. Unsaturation is denoted by use of the subtractive suffixes “-ene” and 1,3-Cyclopentadiene “-yne.” Locants are employed as for hydrocarbon chains (¶¶ 114, 141). Examples: Cyclohexene HAs=AsH Diarsene
H2Si=SiH2 Disilene
H NN=NNH 2- Tetrazene 1,3-Cyclohexadien-5-yne (not 2 2 Benzyne) HN=NN=NH 1,3-Tetrazadiene
146. Monocyclic hetero systems. 144. Heterogeneous hetero chains in which any one of the Group IVA (a) Rings of three through ten members containing nonmetallic hetero elements (silicon, germanium, tin, or lead) alternates with chalcogen are given atoms (except silicon) are named systematically by the (extended) Hantzsch- “oxane,” “thiane,” etc. names. Widman system or at trivial names. Table II supplies the stems for the system- Examples: atic names, which are completed by adding replacement prefixes for the hetero atoms in the order set out in Table I, ¶128, e.g., oxa, thia, aza, together with H3SiOSiH2OSiH3 Trisiloxane locants and multiplicative prefixes denoting the position and number of each. 1 2 3 4 5 A locant for a single hetero atom is not cited. The preferred hetero atom is num- bered “1.” This means the set of locants may not be the lowest possible, as de- H3GeSeGeH3 Digermaselenane fined in ¶137. The letter “a” of replacement prefixes is elided before another 1 2 3 vowel in Hantzsch-Widman names; e.g., Dioxazole, not Dioxaazole. Examples: H3SnSSnH2SSnH3 Tristannathiane 1 2 3 4 5 1 O Oxirene Chains of alternating atoms of a Group IVA element and nitrogen, e.g., SiH3NHSiH3, SnH3NHSnH3, are not named Disilazane, Distannazane, etc. 3 Instead, the amine function is recognized; thus, Disilazane is indexed at Silanamine, N-silyl-. However, substituent radicals derived from such hetero- H N geneous chains containing nitrogen, e.g., 1-disilazanyl for SiH3NHSiH2 -, are 1 Aziridine employed in the presence of higher functions (see ¶161). 3 “A” names for hetero chains are avoided unless carbon substituents can be included in the chain. Otherwise the preferred parent is a homogeneous hetero 1 P chain, hydride, or element name. 6 Examples: Phosphorin
H3GeOSiH2SPbH3 Silane, (germyloxy)(plumbylthio)- 1 O Oxazole (not 1,3-Oxazole, because the H3GeOPbH2SeMe 2-Oxa-4-selena-1-germa-3- 1 2 3 4 5 1,2-isomer has the trivial name plumbapentane Isoxazole ) N 3 145. Monocyclic hydrocarbons ( cycloalkanes , cycloalkenes, etc.) are 1 named by attaching the prefix “cyclo” to the name of the acyclic hydrocarbon O 1,2-Oxathiolane (all locants are with the same number of carbon atoms. Unsaturation is expressed by use of 5 S2 placed ahead of the name; cf. “-ene” and “-yne” in place of “-ane” as for the acyclic analogs. No locant is em- ¶146(c), below) ployed for a single multiple bond; lowest locants (¶ 137) are cited when two or more multiple bonds are present. The trivial name Benzene is used for 1,3,5- 2H-1,5,2-Dithiazine (not 1H-2,4,1- Cyclohexatriene. Dithiazine; not 6H-1,3,6-Dithiazine; Examples: 1 S 2 not 4H-1,5,4-Dithiazine) (The 6 NH numbering must begin with a sulfur 5 S atom and proceed in the direction Cyclohexane that gives lowest numbers to the remaining hetero atoms.) (The “2H” signifies indicated hydrogen (see ¶ 135)).
TABLE II HANTZSCH-WIDMAN STEMS FOR MONOCYCLIC HETERO SYSTEMS OF THREE THROUGH TEN MEMBERS1
No. of Rings containing nitrogen Rings containing no nitrogen members in the ring Unsaturated2 Saturated Unsaturated2 Saturated 3 -irine -iridine -irene -irane 4 -ete -etidine -ete -etane 5 -ole -olidine -ole -olane 6 -ine34-in3 -ane5,6 7 -epine 4 -epin -epane 8 -ocine 4 -ocin -ocane 9 -onine 4 -onin -onane 10 -ecine 4 -ecin -ecane
1The symbols denoting the ring sizes for 3, 4, 7, 8, 9, 10 members are de- 4Saturation is expressed by detachable prefixes such as “tetrahydro-,” rived from numerical prefixes as follows: “ri” from tri; “et” from tetra; “ep” “hexahydro-,” etc. The prefix “perhydro-” is not used. from hepta; “oc” from octa; “on” from nona; and “ec” from deca. 5This stem is not used for saturated hetero systems based on the elements 2Corresponds to the maximum number of noncumulative double bonds silicon, germanium, tin, or lead. Saturation of these rings is indicated by de- when the hetero atoms have the substituent valencies given in Table I, ¶ 128. tachable prefixes such as “tetrahydro-,” “-hexahydro-,” etc., when Hantzsch- 3When the Hantzsch-Widman prefixes “phospha,” “arsa,” or “stiba” are Widman names are used. immediately followed by the Hantzsch-Widman stems “-in” or “-ine,” they are 6Saturation of six-membered hetero systems based on the elements boron replaced by the prefixes “phosphor,” “arsen,” or “stibin,” respectively. or phosphorus is denoted by the stem “-inane.” 21 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 148
Table II indicates those cases in which special endings are employed for fully (c) Rings of more than ten members not containing silicon atoms are in- saturated monocyclic hetero systems: e.g., Azetidine , not Azete, tetrahydro-. dexed at organic replacement (“a”) names. Unsaturation is indicated by “-ene” Special names for partially saturated ring systems are discontinued. and “-yne” suffixes. Example: Examples:
H 1 4 11 N NH 1 Azete, 1,2-dihydro- (not 2-Azetine) Azacycloundecane
6 Certain five- and six-membered monocyclic hetero systems, both saturated and unsaturated, are indexed at trivial names. These names are set out in Table III H H P N H in the order of the corresponding Hantzsch-Widman names, which are not used 10 11 H N P for indexing. Fully hydrogenated five-membered rings are given “-olidine” HN 9 1 PH 12 2 1,3,5,7,9,11-Hexaaza-2,4,6,8,10,12- names, as for systematically named systems. Special names for partially hydro- 8 5 3 HP 4 NH hexaphosphacyclododecane genated five-membered rings e.g., 2-Pyrroline, were discontinued in 1972; 7 6 N N P H P they are now named as dihydro derivatives of the fully unsaturated rings. Indi- H cated hydrogen (see ¶135) is necessary in some rings to describe the location H H of the saturated skeletal atom, e.g., 1H-Pyrrole, 2H-Pyrrole, 3H-Pyrrole. O Presence of a triple bond in addition to the maximum number of noncumulative double bonds is expressed by the subtractive prefix “didehydro.” 1,6-Dioxacyclododec-3-yne Example: O
N (d) Rings containing silicon in general are indexed at replacement (“a”) names based on the cyclic hydrocarbons. Systems comprising only silicon at- Pyridine, 2,3-didehydro- oms or silicon atoms alternating with nitrogen or one of the chalcogens are giv- en “Cyclosila-” names. Examples:
H2 Si Silacyclopropane 1
TABLE III 3 MONOCYCLIC HETERO SYSTEMS H WITH TRIVIAL NAMES N 1 2 5 N 1,2,4-Triaza-3-silacyclopent-2-ene Hantzsch-Widman name Index name HN Si H Azine Pyridine 4 3 Azine, hexahydro- Piperidine H Azole Pyrrole (1H-, 2H-, or 3H-) Si Azolidine Pyrrolidine 6 1 Silabenzene 1,2-Diazine Pyridazine 1,3-Diazine Pyrimidine 1,4-Diazine Pyrazine 1,4-Diazine, hexahydro- Piperazine H2 5 Si 1,2-Diazole Pyrazole (1H-, 3H-, or 4H-) 1 2 H2Si Si H2 1,3-Diazole Imidazole (1H-, 2H-, or 4H-) Cyclopentasilane 1,2-Diazolidine Pyrazolidine H2 Si Si H2 1,3-Diazolidine Imidazolidine 4 3 2H-1,4-Oxazine, tetrahydro- Morpholine H 1,2-Oxazole Isoxazole N 4 12 Cyclodisilazane Oxin Pyran (2H-or 4H-) H2 Si SiH2 3 Oxole Furan N 1,2-Selenazole Isoselenazole H Selenole Selenophene H2 Tellurole Tellurophene Si O 1 10 H2 O 2H-1,4-Thiazine, tetrahydro- Thiomorpholine 11 Si 2 O9 SiH2 1,2- Thiazole Isothiazole 12 Cyclohexasiloxane Thiole Thiophene 8 H2Si O 4 O3 1,3,5,2,4,6-Triazatriborine, hexahydro- Borazine 7 6 5 Si O Si H2 1,3,5,2,4,6-Trioxatriborinane Boroxin H2 1,3,5,2,4,6-Trithiatriborinane Borthiin 147. Polycyclic systems may be subdivided into four classes as follows: (a) Fused systems contain at least two rings of five or more members and only “ortho-” or “ortho- and peri-” fusions (see below). (b) Bridged systems are monocyclic or fused systems with valence bonds, (b) Rings of three through ten members containing antimony, tin, lead, atoms, or chains connecting different parts of the structure. germanium or bismuth atoms in addition to carbon atoms are indexed at (c) Spiro systems have pairs of rings (or ring systems) with only one Hantzsch-Widman names. Partially saturated and fully saturated six-mem- common atom. bered ring systems of this type containing germanium, lead or tin are named on (d) Ring assemblies have pairs of rings (or ring systems) connected by the basis of the unsaturated rings. Heterocycles containing metallic atoms other single bonds. than the above five are indexed by coordination nomenclature (¶ 215). In the following sections, methods of naming ring systems of all these types Examples: will be described. The names of more complicated cases are built up from base components which may be described as “fundamental” systems. 1 148. Fundamental fused carbocycles with ortho-fusions only, e.g., O 2 6 SnH2 Naphthalene, have adjoining rings with only two atoms in common; they thus 4H-1,3,2-Dioxastannin, dihydro- have n common faces and 2n common atoms. An ortho- and peri-fused system O3 contains a ring which has two, and only two, atoms in common with each of two or more rings, the total system containing n common faces and fewer than 1 2n common atoms. O 5 2 Examples: H2Ge Ge H 2 Oxatetragermolane 4 1 H2 Ge Ge H2 1 10a 4 3 9 12c 5 3 12a 6 8 1 12b 10c 10b S 6 2 7 4 H 2Sn SnH2 1,3,5,2,4,6-Trithiatristannin 9 8 6 5 5S 4 S3 Sn An ortho-fused system An ortho- and peri-fused system H2 (3 common faces; 6 common atoms) (5 common faces; 6 common atoms) ¶ 148 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 22
Systems of five or more ortho-fused benzene rings are named by the Rubicene C5-C5-C6-C6-C6-C6-C6 “ acene ” system if the arrangement is linear, by the “phene” system if one Coronene C6-C6-C6-C6-C6-C6-C6 central angular site is present. Trinaphthylene C6-C6-C6-C6-C6-C6-C6 Examples: Heptaphene C6-C6-C6-C6-C6-C6-C6 Heptacene C6-C6-C6-C6-C6-C6-C6 11 12 13 14 1 14a Pyranthrene C6-C6-C6-C6-C6-C6-C6-C6 Pentacene Ovalene C6-C6-C6-C6-C6-C6-C6-C6-C6-C6
8 7 6 54 149. Fundamental fused heterocycles often have trivial names, e.g., 1 Cinnoline , Xanthene . Others belong to one or another semisystematic system. A linear set of three fused six-membered rings with the same hetero element in 20a 4 20 both unfused positions of the central ring are given “-anthrene” names. 5 Example: 19 15 16 17 18 18a Octaphene 6 9 10 1 18b B 10a 7 Boranthrene 12 11 10 9 8 6 B 4 Names ending in “-alene” are employed for bicyclic fused systems, and in 5 “-phenylene” for systems built up from benzene rings fused to alternate sides of a monocyclic hydrocarbon. (Analogous “-naphthylene” names have been A similar set with different hetero elements in these positions is given a used for corresponding 2,3-fusion systems of naphthalene.) “Pheno-” name containing the organic replacement terms in the usual order Examples: (Table I, ¶128) and the ending “-in” (or “-ine” if nitrogen, phosphorus or arsenic is included). 1 Example: 6a Pentalene 43 9 H 1 Si 10a 10 1 1H-Phenoxasilin
8 O 12a Triphenylene 5 4 12b 4a 8b 4b 8a An exception is Phenazine for the analog that contains nitrogen in both central positions. Arsenic and phosphorus analogs of fused nitrogen heterocycles (Indole, Table IV lists the names of trivially-named fundamental fused carbocycles Quinoline, etc.) are named as follows: Arsindole, Isoarsindole, Arsinoline, in ascending order of preference for adoption as base components in the nam- Isoarsinoline, Phosphindole, Isophosphindole, Phosphinoline, Isophosphi- ing of more complex fused hydrocarbon systems. Also included are some of noline, Arsanthridine, Acridarsine, Acridophosphine, Phenarsazine, the names discussed immediately above. (The order is based on the rules de- Phenophosphazine. scribed in ¶¶138, 150.) The ring analyses describe the number of component The replacement of the oxygen in Xanthene by sulfur or selenium has been rings and the number of atoms each ring contains. Diagrams of these rings, denoted by the appropriate chalcogen functional replacement prefix: Thioxan- which show the preferred orientation and numbering, are displayed in the Ring thene, Selenoxanthene. Systems Handbook. Diagrams justified by current entries are also provided in In the “benzo” system, bicyclic fused heterocyclic systems containing a the semiannual and collective Chemical Substance Indexes. benzene ring and a ring named by the Hantzsch-Widman system are indexed The following ring systems require citation of indicated hydrogen (¶ 135) by prefixing the latter name by “Benz-” or “Benzo-.” Indicated hydrogen, if to complete the name: Indene, Fluorene, Phenalene , Trindene. necessary, and locants are placed in front of the complete name. Similar names are used when benzene is fused to a monocycle with a trivial name (unless the bicyclic system itself has a trivial name). TABLE IV Examples: FUNDAMENTAL FUSED CARBOCYCLES IN 1 ASCENDING ORDER OF PRECEDENCE FOR 8a N USE AS BASE COMPONENTS IN FUSED 4H-3,1-Benzoxazine (not 4H-Benz- [d]oxazine) SYSTEMS O3 5 4 Name Ring analysis Pentalene C -C 1 5 5 Benzoxazole (locants are not cited; the Indene C -C 7a O 5 6 isomers are named 1,2- and 2,1- Naphthalene C6-C6 N Benzisoxazole ) Azulene C5-C7 3 4 Heptalene C7-C7 Biphenylene C4-C6-C6 as-Indacene C5-C5-C6 1 7a O s-Indacene C5-C5-C6 Benzofuran (the isomer is named Acenaphthylene C5-C6-C6 Isobenzofuran ) 3 Fluorene C5-C6-C6 4 Phenalene C6-C6-C6 Phenanthrene C6-C6-C6 Such “benzo” names are not usually adopted as base components of fused Anthracene C6-C6-C6 systems when only hydrocarbon rings are fused to the benzene portion. Trindene C5-C5-C5-C6 When benzene is fused to a heterocyclic ring containing more than ten Fluoranthene C5-C6-C6-C6 skeletal atoms, “Benzo-” or “Benz-” is placed ahead of the replacement (“a”) Acephenanthrylene C5-C6-C6-C6 name of the saturated ring and the ending changed to “-in” (or “-ine” if Aceanthrylene C5-C6-C6-C6 nitrogenous) to indicate the maximum number of noncumulative double bonds. Triphenylene C6-C6-C6-C6 (Saturated positions other than those occupied by indicated hydrogen are Pyrene C6-C6-C6-C6 denoted by hydro substituents.) Chrysene C6-C6-C6-C6 Example: Naphthacene C6-C6-C6-C6 Pleiadene C6-C6-C6-C7 Picene C6-C6-C6-C6-C6 1 Perylene C6-C6-C6-C6-C6 15a O 2H-1,11-Benzodioxacyclotridecin, Pentaphene C -C -C -C -C 6 6 6 6 6 6 5,6-dihydro- Pentacene C6-C6-C6-C6-C6 Tetraphenylene C -C -C -C -C O 6 6 6 6 8 12 11 Hexaphene C6-C6-C6-C6-C6-C6 Hexacene C6-C6-C6-C6-C6-C6 23 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 151
Trivially named fundamental fused hetero systems are listed in Table V. Triphenodioxazine C4NO-C4NO-C6-C6-C6 Also included are some of the systems discussed immediately above, as well Phenanthrazine C4N2-C6-C6-C6-C6-C6-C6 as a selection of monocyclic hetero systems to help illustrate the ascending Anthrazine C4N2-C6-C6-C6-C6-C6-C6 order of priority for adoption as base components in complex fused systems. Fused systems containing only silicon and carbon skeletal atoms are indexed at “Sila-” replacement names if the corresponding hydrocarbon has a fundamental name (Table IV, ¶148). 1The order of precedence is based first on the presence or absence of nitrogen, then upon the nature of the (other) hetero atoms (see Table I, ¶ 128). For fused heterocycles, this order (for base-component selection) is distinct from that used to determine seniority of a total ring system in an index name (¶ 138). 2Citation of indicated hydrogen (¶ 135), e.g., 1H-Pyrrole, 2H-Pyrrole,is TABLE V necessary when these component names are used alone. FUNDAMENTAL HETEROCYCLES IN 3Because of established usage, Quinolizine is favored over Isoquinoline ASCENDING ORDER OF PRECEDENCE FOR and Quinoline as a base component. USE AS BASE COMPONENTS IN FUSED 4Naphthyridine requires the locants 1,5-, 1,6-, 1,7-, 1,8-, 2,6-or2,7-to SYSTEMS1 define the position of the nitrogen atoms. 5Phenanthroline requires the locants 1,7-, 1,8-, 1,9-, 1,10-, 2,7-, 2,8-or Index Name Ring Analysis 2 2,9- to define the positions of the nitrogen atoms. Isoarsindole C4As-C6 2 Arsindole C4As-C6 Isoarsinoline C As-C 5 6 150. Selection of a base component is facilitated by use of Tables IV, Arsinoline C As-C 5 6 ¶ 148 and V, ¶149; the appropriate component listed latest in these Tables is Arsanthridine C As-C -C 5 6 6 used. The system must contain at least two rings of five or more atoms, Acridarsine C As-C -C 5 6 6 although such rings need not be directly fused to one another; i.e., they may be Arsanthrene C As -C -C 4 2 6 6 joined by a smaller ring, as in Cyclobutadicyclopentene. The criteria for base Isophosphindole2 C P-C 4 6 components differ markedly (in the case of heterocyclic systems) from those Phosphindole2 C P-C 4 6 described (¶ 138) for a preferred ring system in a compound containing more Isophosphinoline C P-C 5 6 than one. A base component of lower preference is used if the fusion procedure Phosphinoline C P-C 5 6 is not possible on the preferred component; as a last resource, an organic Tellurophene C Te 4 replacement (“a”) name based on the fused hydrocarbon is employed. Selenophene C Se 5 The preferred base component should: Selenanthrene C Se -C -C 4 2 6 6 (a) be a heterocycle; Thiophene C S 4 (b) be a nitrogenous heterocycle; Thianthrene C S -C -C 4 2 6 6 (c) be a nonnitrogenous heterocycle containing a hetero atom of highest Furan C O 4 precedence (see Table I, ¶ 128); Pyran2 C O 5 (d) contain the greatest number of rings; Isobenzofuran C O-C 4 6 (e) contain the largest individual ring; Benzindene (not Cyclopenta- Xanthene2 C O-C -C 5 6 6 naphthalene) is an exception based on established usage; Phenoxastibinin C OSb-C -C 4 6 6 (f) contain the greatest total number of hetero atoms; Phenoxarsine2 C AsO-C -C 4 6 6 (g) contain the greatest variety of hetero atoms, e.g., one nitrogen and one Phenoxaphosphine2 C OP-C -C 4 6 6 oxygen rather than two nitrogens ; Phenoxatellurin C OTe-C -C 4 6 6 (h) contain the greatest number of hetero atoms of highest precedence; Phenoxaselenin C OSe-C -C 4 6 6 (i) possess the most linear structure; Phenoxathiin C4OS-C6-C6 2 (j) have the lowest locants for hetero atoms (before fusion). Pyrrole C4N 2 Imidazole C2N2 Pyrazole2 C N 3 2 151. Index names for fused systems, other than fundamental systems Isothiazole C NS 3 which possess their own names (Tables IV, ¶ 148 and V, ¶149), are formulated Isoxazole C NO 3 from the names of the components. Cycloalkanes may be adopted as base Pyridine C N 5 components by invariant use of the “-ene” suffix. This denotes a maximum Pyrazine C N 4 2 number of noncumulative double bonds; e.g., Cyclooctene as part of a fused Pyridazine C N 4 2 system is not meant to imply the presence of a single double bond; instead, Pyrrolizine2 C N-C N 4 4 saturated carbon atoms are indicated by “hydro” prefixes. Fusion locants for Indolizine C N-C N 4 5 the base component comprise lower case italic letters assigned sequentially to Isoindole2 C N-C 4 6 all sides, beginning with the side “1,2” as denoted by the usual peripheral Indole2 C N-C 4 6 locants. (See the Ring Systems Handbook for a complete set of ring system Indazole2 C N -C 3 2 6 diagrams, including base components, complete with such locants.) If more Purine2 C N -C N 3 2 4 2 than twenty-six letters are required, subsequent alphabets of the form a , b , Isoquinoline3 C N-C 1 1 5 6 c ,... etc., are adopted. Locants for the fusion prefixes (derived from the less Quinoline3 C N-C 1 5 6 preferred fundamental ring systems) comprise the normal peripheral numerical Quinolizine3 C N-C N 5 5 locants. When a choice is possible, lowest alphabetic and numerical locants are Phthalazine C N -C 4 2 6 cited. When one or both types of locants are unnecessary they are usually Naphthyridine4 C N-C N 5 5 omitted. Numerical and letter locants are separated by a hyphen, and the locant Quinoxaline C N -C 4 2 6 set is bracketed. Quinazoline C N -C 4 2 6 Example: Cinnoline C4N2-C6 Pteridine C4N2-C4N2 2 Carbazole C4N-C6-C6 Benzene Phenanthridine C5N-C6-C6 Acridine C5N-C6-C6 2 Perimidine C4N2-C6-C6 5 + 1 Phenanthroline C5N-C5N-C6 11 12 Phenazine C4N2-C6-C6 1 4 j 12a Anthyridine C N-C N-C N k l m n a 12b 5 5 5 2 Phenarsazine C4AsN-C6-C6 i b 5 Phenophosphazine C4NP-C6-C6 2 Phenotellurazine C4NTe-C6-C6 h gf e d c 8 7 6 2 Phenoselenazine C4NSe-C6-C6 Anthracene (base 2 Benz[a]anthracene Phenothiazine C4NS-C6-C6 component) 2 Phenoxazine C4NO-C6-C6 Thebenidine C5N-C6-C6-C6 2 Quindoline C4N-C5N-C6-C6 2 In this example, a locant defining the fusion site on Benzene is unnecessary. Quinindoline C4N-C5N-C6-C6 2 The “1,2” side of Anthracene is lettered “a” and the lettering proceeds around Phthaloperine C5-C4N2-C6-C6-C6 Acrindoline2 C N-C N-C -C -C every side back to the 1-position. The fused system is then oriented (¶ 152) and 4 5 6 6 6 renumbered. Triphenodithiazine C4NS-C4NS-C6-C6-C6 ¶ 151 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 24
Angular positions of base components involved in fusion are not cited. Example: Cyclopenta[a]cyclopropa[4′,5′]- cyclopenta[1′,2′:2,3]cyclopropa- a Naphthalene [1,2-f ]naphthalene (not ′ ′ ′ ′ f Cyclopenta[a]cyclopropa[3 ,4 ]- 5 1 2 1 ′ ′ 2′ cyclopenta[2 ,1 :1,3]cyclopropa- 8 4′ 3 [2,1-f ]naphthalene.) (The locants a b 8a 4 1,2 in the preferred name are lower + NOT than 2,1 in the incorrect name. It c 1 should be noted that the use of 1′,2′ d 2 a requires that the cyclopentane ring e be numbered the long way around.) ′ ′ f 3 2 1 ′ ′ f 4 1 2 3
2′ 3′ 3 2 Perylene Naphtho[8,1,2-bcd]perylene ′′ 5 ′′ (not “[8,8a,1,2-bcd]”) a′ ′′ 1 a 4 2′′ ′ ′ 1 4 41 Benzo[1′′,2′′:3,4;5′′,4′′:3′,4′]dicy- When a hetero atom is shared by two or more rings, it is expressed in all the clobuta[1,2-a:1′,2′-a]diindene components. When the order of lettering of the base component proceeds in the (Note that a semicolon separates direction opposite to numbering in the fusion-prefix component, numerical locant sets which already contain locants for the latter are reversed. colons.) Example:
O 2 1 3 6 b Pyrrolo[2′′,3′′:4′ ,5′ ]pyrano- N 5 4 N ′ ′ ′ 1′′ 3 [2 ,3 :5,6]pyrano[4,3-b]pyrrole 2 Isoindolo[2,1-a ]quinoxaline 2′′ 4′ 2′ ′′ 5′ ′ N (not “[2,3-a]”; not “[1,2-c]”) 3 6′ 1 a O N 1 b 152. Orientation and numbering of fused systems. Fusion prefixes are placed in alphabetical order and the earliest cited prefix (a) Hydrocarbons. The component rings are normally drawn as regular is given preference for lowest letter locant. When two or more fusion prefixes polygons. The cyclopropane ring may point left or right, and cyclopentane and are identical, as in “Dibenzo-” systems, the letter locants are separated by cycloheptane rings may point up or down. The total system is oriented so that commas, e.g., “[a,j].” (a) a maximum number of rings are in a horizontal row, and (b) a maximum Example: number are above and to the right. If further choice is necessary, then (c) a min- imum number of rings should be in the lower left quadrant. Numbering begins at an atom not engaged in fusion in the most counterclockwise position of the uppermost ring furthest to the right. Angular positions are not counted; their locants, when needed, are derived from those of the preceding nonangular j a Benzo[a]cyclopent[j]anthracene positions by addition of the lower-case Roman letters, “a,” “b,” etc. Interior atoms are numbered last by addition of letters to the highest available numeri- cal locant in a continuous pathway, a clockwise route being followed whenever a choice presents itself; any remaining interior atoms are then numbered simi- larly from the next highest available numerical locant. Examples: A form of multiplicative name is employed for fused systems different from that described for general substitutive nomenclature. Multiplication proceeds 10 11 12 1 in steps, with “di,” “tri” repeated as necessary (not “bis,” “tris,” etc., except to 12a avoid ambiguity). Serially primed letters are used for fusion sites on the Naphthacene second, third, etc., base components and the locant sets are separated by colons. When a base component is fused to a central component and to another 7 6 5 4 component, lowest letters (when a choice must be made) relate to the central fusion site. 1 1 Examples: 10a 10a 9 9 5c 3 3 10b NOT 8 8 10c 5b O 4 4 5a c 1 Benzo[1,2-c:3,4-c′:5,6-c′′]trifuran 6 5 6 O c′′ 2 5 3 Pyrene 4 c′ O 4 4 Dinaphtho[1,2-d:1′,2′-d′]benzo- 1 1 ′ 5 5 [1,2-b:5,4-b ]dithiophene (not 16d 14 8d 1 4 2 1 16a Dinaphtho[2,1-b:2′,1′-b′]benzo- 13a 16a ′ 13b 16b 6 NOT 16b 8c d′ b b d [1,2-d:5,4-d′]dithiophene) 16c 6 13 8b 2′ S 5′ 1 S 2 7 7 8a 11 8 11 8 Ring systems fused to base components are designated primary compo- 10 9 10 9 nents; a ring system (other than a base component) fused only to a primary component is a secondary component, and primed numerical locants are used Naphtho[1′,8′:3,4,5]cyclohepta[1,2-c]phenanthrene to denote its fusion sites. Primed and unprimed locant sets are separated by colons. Lowest locants are used for the site closest to (or fused directly to) the When a further choice is needed for orientation and numbering, carbon base component. Doubly primed locants are needed (a) when the secondary atoms at angular positions are assigned lowest numbers. component is centrally located with identical primary and base components on Examples: both sides, and (b) when tertiary components are present. Examples: 12 13 8a 8a 3 NOT 4 N 8b 8b N ′ ′ 5 5 1 Pyrido[1 ,2 :1,2]imidazo[4,5-b]- b 4 2 quinoxaline 6 5 6 3 N N Acenaphthylene (Note: 2a,5a,8a,8b is lower than 3a,5a,8a,8b) 25 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 154
Indicated hydrogen is assigned the lowest locant. 153. Replacement (“a”) nomenclature for fused systems is employed Example: when fusion names fail to express all interfaces (fusion sites) between component systems. This occurs when two or more components that are ex- 1 pressed as prefixes are fused to one another as well as to the base component. 7a “A” names are also used for silicon replacement in a carbocycle that has a 1H-Indene (not 3H-Indene) trivial name. Indicated hydrogen of the parent carbocycle is ignored, but is 3 cited, if needed for the “a” name, ahead of the replacement prefixes. 4 Examples: The following fused carbocyclic compounds have special numbering 1O 2 6H−1,7−Dioxacyclopent[cd]indene systems: Anthracene, Phenanthrene, Cyclopenta[a]phenanthrene ( steroid 7a 7b (not 1,7-Dioxa-6H-cyclopent[cd]- numbering) and the Cyclopropacyclopenta[a] phenanthrenes . 7 O 3 indene) (b) Heterocycles. The ring systems are oriented as for hydrocarbons. When a choice is necessary, lowest locants are assigned to (a) all hetero atoms; (b) 4 most preferred hetero atom (Table I, ¶ 128); (c) carbon atoms common to two 5 or more rings; (d) positions bearing indicated hydrogen; (e) an angular rather than a nonangular atom of the same hetero element. The ring is then numbered 13 as for hydrocarbons, except that hetero atoms common to two or more rings are 9a 9b B counted. Interior atoms are numbered last, following the shortest path from the 4 9b-Boraphenalene highest previous number. Examples: 7 6
1 H 7a O 8a Si 1 Cyclopenta[b]pyran 1-Silanaphthalene, 3,4-dihydro- 5 4 5 4 Saturation of double bonds in fused systems is denoted by hydro prefixes 1 9a N which are given lowest locants; e.g., Naphthalene, 1,2,3,4(not 5,6,7,8)- 4,1-Benzothiazepine tetrahydro-. Triple bonds are indicated by “didehydro.” 154. Bridged fused systems are fused ring systems that possess atomic bridges or valence bonds which connect two or more parts of the system 6 S 5 4 without creating or extending a fused system. They are named by adding bridge prefixes (in alphabetical order if different types are present) to the fused system names. 6 1 − − − − − S 6a O Simple bivalent bridges include methano ( CH2 ), ethano ( CH2 CH2 ), − − − − − Thieno[2,3-b]furan etheno ( CH=CH ), propano ( CH2CH2CH2 ), 2-buteno ( CH2CH=- − − − − 4 3 CHCH2 ), and benzeno ( C6H4 ). Trivalent bridges, e.g., metheno ( CH=), − 1-propanyl-3-ylidene ( CH2CH2CH=), and tetravalent bridges are also em- 6 1 ployed; locants for positions of attachment on the fused system are cited in the N 6a O same order as free-valency locants of the radicals. Bridges from monocyclic 4H-1,3-Dioxolo[4,5-d]imidazole hydrocarbons other than benzene are named as for the fusion prefixes, except HN O 3 that “endo-” is used with them to avoid ambiguity, e.g., “endo-cyclopenta.” 4 Simple hetero bridges include epoxy (−O−), epithio (−S−), imino (−NH−), epidioxy (−O−O−), and -silano- (−SiH −). Heterocyclic rings may also be used 1 8 1 8 1 2 8 N N 8a N N 8a N as bridges. N NOT NOT Example: 5 N 4a N N N N 4 5 N 4 5 4 O 3,4-furano (cf. “furo” for the fusion Imidazo[1,2-b][1,2,4]triazine prefix)
When locants are used for the bridge itself, e.g., 2-buteno, 3,4-furano-, they are 1112 1 10 11 12 1 placed in brackets within the bridged system name. 12a 12a 12b 10a 10b Compound bridges are named by combination of simple bridges beginning NOT N at the terminal position which gives the preferred (a) cyclic bridge (¶ 138), (b) N 4a 6a 6 5a N 5a 5 N 7 5 4 hetero atom (Table I, ¶ 128), (c) chain, (d) alphabetic order. 6 Examples: Pyrrolo[1,2-a:5,4-b′]diindole − − NH(CH2)2 (iminoethano)
1 2 − − 8a 9 OSO (epoxythioxy) 7O 8b N 3 Furo[3,4-a]pyrrolo[2,1,5-cd]- indolizine 5b 5a O (epoxy[1,2]benzeno)
The following heterocyclic systems have special numbering systems: Indicated hydrogen of a fused system is cited, if possible, to accommodate Acridine, Carbazole, Purine, Xanthene, and Epoxy- and Epithiocyclopen- a bridge. When this is unnecessary or impossible, the lowest-numbered nonan- ta[a]phenanthrenes (steroid numbering). gular indicated hydrogen is cited for the fused system, and additional indicated Indicated hydrogen for fused carbocyclic and heterocyclic systems is hydrogen, when needed, is cited ahead of the bridge locants. normally cited, if there is a choice, at the lowest nonangular position, unless a Examples: saturated angular atom is required to accommodate a principal function or free valency (see ¶ 135). Indicated hydrogen of component systems is ignored in constructing a fused ring name, and is reassigned if it is still needed in the final 1 6a system. 1,4-Methanopentalene, 1,4-dihydro- Example: 7 4 3
1 5H-[2]Benzopyrano[3,4-b][1,4]- 12 11 O 12a 4 benzodioxin (Note: The locants 12b “2” and “1,4,” which relate to the 1 2 6,1,3-Ethanylylidenecyclopenta[cd] 5 components, are bracketed to 6a indicate that they do not conform pentalene O O 6 7 3 8 7 6 6b to the peripheral numbering of 8 the total system) 5 4 ¶ 154 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 26
21 22 1 15 1,7-Ethano-1H-pyrano[3,2-c]- 8a N 2 pyridazine, octahydro- (not 4,6- 7 NH 18 17 (Epoxymethano) -1H -pyrido[1,2- 16 9 10 11 12 13 14 1 b]pyridazine, octahydro-)(Thefused 14a O 6,13[2′,3′]-Naphthalenopentacene 5 4 ring system Pyranopyridazine is preferred to Pyridopyridazine; NOT see ¶ 138.) 8 7 6 5 4 H 9 N N 1 14 10 O11 12 11 8 1 ′ ′ 5 4 9 9a 9,10[1 ,2 ]-endo-Cyclobutanthracene
Fused carbocyclic and nonnitrogenous heterocyclic systems with simple 10a 4a 5 10 4 imino bridges are named by use of the termination “-imine” with appropriate locants. Such a name requires addition of a regular functional suffix when 6 1 appropriate. (Nitrogen heterocycles with imine bridges are named as bridged 6a O 2 3,2,6-(Epoxyethanylylidene)furo- fused heterocycles in the usual manner.) 7 [3,2-b]furan Example: 4 O 3 O 8 9 1 Br Naphthalen-4a,8a-imine-9 - 8a carboxylic acid, 2,3-dibromo- 1 3 octahydro- 9 NCO2H 10a 10b 10 4 4a 11 7,10-Ethenocyclohepta[de]- Br naphthalene 12 6 7 155. Von Baeyer nomenclature. This was first developed to name alicy- clic hydrocarbons containing two rings. It has been extended to all bridged sys- tems which cannot be treated as fused or bridged fused systems. Von Baeyer 1 8a O 5H-4a,7-Ethano-2H-1-benzopyran names for hydrocarbons are formed by prefixing to the name of the acyclic hy- 7 9 (4aH-1-Benzopyran cannot exist) drocarbon with the same number of carbon atoms “Bicyclo-,” “Tricyclo-,” etc., 10 terms, followed by a set of numerals, separated by periods and bracketed, 4a which describes in descending sequence the number of atoms in each bridge. The system is numbered from one bridgehead via the other bridgehead(s) and Numbering of bridged fused systems is based on the regular numbering of back, always choosing the longest route. The system is numbered along the the parent fused system. Lowest locants are assigned to bridgehead positions same route, ending with the smallest bridge, numbered from the bridgehead and the bridge atoms are numbered from the end nearest the highest numbered with the highest locant. position of the parent fused system. In cyclic bridges, e.g., benzeno, endo- Example: cyclobuta, the shorter bridge is first numbered, and then the rest of the ring in the same direction. If possible, hetero atoms in bridges are numbered low. 9 Examples: 6 1 Bicyclo[4.3.2] undecane
11 10 10 11 12 1 12a 14 1,4-Ethenonaphthacene 13 For tricyclo- and higher hydrocarbon systems, superscripts are employed to 7 6 5 4 indicate the positions of secondary bridges. Example: 1 8a 8a,4a-(Iminomethano)naphthalene 1 NH 2 9 (not 4a,8a-(Iminomethano)- 10 2,6 10 Tricyclo[5.3.1.1 ] dodecane naphthalene) 11 12 4a 7 6
15 When more than one Von Baeyer name is possible for a hydrocarbon, the 12 11 ′ ′ choice is determined by the following principles, applied successively until a 9 1 11H-5,10[1 ,2 ]-endo-Cyclopent- decision is reached. 1010a O 5H-oxazolo[3,2-b]isoquinoline (a) The main ring contains the maximum number of atoms, two of which N must serve as bridgeheads for the main bridge. 4 (b) The main bridge is as large as possible. 6 5 (c) The main ring is divided as symmetrically as possible by the main bridge. The exceptional numbering employed for bridged cyclopenta[a]- (d) Lowest superscripts (regardless of order of citation) are cited. phenanthrenes is shown in the following example. Steroid numbering is used Examples: for positions 1 through 17. When the methyl groups normally numbered 18 and 19 are transformed into methano bridges, their locants are retained. Other 1 bridges are numbered 20 and upward. 2 2,4 7 8 Tricyclo[3.2.2.0 ]nonane (not Tri− 2,3 17 9 cyclo[2.2.2.1 ]nonane) 11 4 19 13 1 9 18 5 15 10 8 20 O 7 2,7 5 Tricyclo[3.2.1.0 ] octane (not Tri- 1 2,6 8 cyclo[2.2.2.0 ]octane) 4 6 2 Criteria for the naming of bridged fused systems are applied successively as follows: 16 (a) The unbridged system contains the maximum number of (i) rings, (ii) 18 17 skeletal atoms. 14 1 1,14 (b) The bridges are as simple as possible; e.g., two simple bridges are 19 Tricyclo[12.2.2.1 ] nonadecane preferred to one compound bridge, and saturated bridges are preferred to 10 unsaturated ones. (c) The unbridged system has the highest precedence according to ¶ 138(c). 5 Examples: 10 1 1 Tricyclo[4.4.1.11,5] dodecane 8a 1,4-Methanonaphthalene, 1,2,3,4- 11 12 (not Tricyclo[5.3.1.11,6]dodecane) tetrahydro- (not 1,3-Ethano- 6 9 1H-indene, 2,3-dihydro-) 5 5 4 27 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 156
2,5 3,8 4,7 10 Pentacyclo[4.4.0.0 .0 .0 ] decane 156. Spiro systems contain pairs of rings or ring systems having only one 1 23 (not Pentacyclo[4.4.0.04,9.05,8.07,10] atom (a “spiro atom”) in common. In the simplest monospiro systems, in which 6 5 decane; not Pentacyclo- two alicyclic rings share an atom, the name is formed by prefixing to the acy- 8 4 [4.4.0.02,5.03,10.04,9]decane; not clic hydrocarbon name the term “Spiro” and numerals (separated by periods) 7 Pentacyclo[4.4.0.02,9.03,8.07,10] in ascending sequence to define the number of atoms in each ring linked to the decane) spiro atom. The numbering begins at the atom next to the spiro atom in the smaller ring and proceeds around that ring, through the spiro atom and around the larger ring. Unsaturation is denoted by “-ene” and “-yne” suffixes. A second locant in Example: parentheses is cited for a double bond at a bridgehead when it does not proceed to the next atom in the numbered path. Multiple-bond locants are determined 8 1 by the following criteria applied successively: 4 Spiro[3.4]octane (a) The numbering proceeds in a clockwise sequence. (b) The cases in which both locants for a double bond are cited are minimized. This system is extended to dispiro and higher systems. Numbering begins (c) Lowest locants are employed. next to a terminal spiro atom and proceeds in such a way as to give the spiro Examples: atoms lowest locants. Example: 8 2,8 Tricyclo[3.3.2.0 ]deca-3,6-diene 16 17 9 1 15 1 Dispiro[5.1.7.2] heptadecane (note 5 10 2 8 6 that the numbering path corresponds to the bracketed sequence)
81 Bicyclo[4.2.0]oct-6-ene (not Bicy- Heterocyclic analogs are named by “a” nomenclature. The hetero atoms are clo[4.2.0]oct-1(8)-ene) given locants as low as are compatible with the ring numbering. 6 Example:
1 4,8 4 Tricyclo[9.3.1.1 ]hexadeca-1- 10 1 14 (15),4,6,8(16),11,13-hexaene (not 6-Oxaspiro[4.5]decane 15 16 5 Tricyclo[9.3.1.14,8]hexadeca-4,6,8- 11 8 O (16),11(15),12,14-hexaene) 6
Von Baeyer names for heterocyclic systems are formed from the hydrocar- Unsaturation is expressed by “-ene” and “-yne” suffixes. bon names by use of replacement (oxa, thia, aza, etc.) prefixes and lowest Example: locants for hetero atoms in the order of Table I, ¶ 128. Unsaturation is denoted as for hydrocarbons. 10O N 12 5,10-Dioxa-12-azadispiro[3.1.3.3]- Examples: 9 6 4 1 dodec-11-ene O 5 3-Oxatricyclo[2.2.1.02,6]heptane 6 2,6 4 (not 5-Oxatricyclo[2.2.1.0 ]heptane; Saturated spiro systems containing only silicon atoms or silicon atoms 1 3,5 7 not 2-Oxatricyclo[2.2.1.0 ]heptane) alternating with nitrogen or one of the chalcogens are given Spirosilazane, O 3 2 (low numbering for bridge takes Spirosiloxane, etc., names. precedence over hetero atom) Example:
H2 2 11 Si 13 1 H2 1 S 6-Oxa-2-thia-4-azabicyclo[3.1.0]- O 12 O O Si hexane (not 6-Oxa-4-thia-2- 2 6 O azabicyclo[3.1.0]hexane) H2Si 10 Si 6 O3 Spiro[5.7]hexasiloxane NH 4 5 4 9O 8 O7 O Si Si 5 H2 H2 8 1
S 2,4-Dithia-3-stibabicyclo[3.3.1]- Monospiro systems containing at least one fused or bridged component are 2 named by placing the component names in brackets in alphabetical order and 9 nona-1(9),5,7-triene (not 2,4- 3 SbH Dithia-3-stibabicyclo[3.3.1]nona- prefacing them with “Spiro.” The position of the spiro atom is denoted by two S 5 1(8),5(9),6-triene) locants, separated by a comma, related to the two components. Primes are used 4 for the component cited second. Indicated hydrogen (¶ 135) is assigned, where Saturated bridged systems containing only silicon atoms, or silicon atoms possible, to accommodate the spiro unions. Locants related to the components alternating with nitrogen or one of the chalcogens, are given Bicyclosilazane, but not to the total spiro system are bracketed to avoid ambiguity. Tricyclosiloxane, etc., names. Regular Von Baeyer numbering is employed. Example: Examples:
5 Spiro[cyclopentane-1,1′-[1H]- H2 H2 1 7′a − 9 Si H Si 3 Bicyclo[4.4.0]decasilane 1′ indene] (not Spiro[cyclopentane 10 ′ ′ H2Si Si1 2 SiH2 1,1 -1 H-indene]) 3′ 6 H2 Si 7 Si 5 SiH2 4′ 8 Si H Si 4 H2 H2 6′ 1′ Spiro[9H−fluorene-9,3′-[2]thia- 7′ 8 H bicyclo[2.2.2]oct[5]ene] 1 8′ HN Si 4′ S2′ 2 8 3′ 1 H2Si NH Bicyclo[3.3.1]tetrasilazane 9 9a 7 NH HN Si H2 6 3 4b 4a Si 5 NH H 4 Added hydrogen (¶ 136) is cited in parentheses in the usual way, but with a 14 H primed locant if it does not relate to the component cited first. It is assigned the Si O 15 lowest available locant unless a different one can be used to accommodate a 16O principal group. 13 17 H 3,9 5,15 7,13 12 Pentacyclo[9.5.1.1 .1 .1 ]- HSi H O Si O18 Example: O 1 octasiloxane 11Si O2 H 1 O 3 4 N 10 9 Si H O H 5 ′ ′ 19O HSi O SiH Spiro[imidazolidine-4,2 (1 H)- 20 5 8′a N 4 1′ quinoxaline] 8 O 2′ NH 3 O HSi 6 7 N 5′ 4′ ¶ 156 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 28
1 1′ Monospiro systems containing identical fused components are given 8a 8′a “Spirobi-” names. The component name is bracketed if it is preceded by 2,2′-Binaphthalene locants or is itself made up of fusion components. Added hydrogen is cited in parentheses following the spiro locants. 5 4 4′ 5′ Examples:
1′ 1 1′ 6′ 6 5,5′-Bibicyclo[2.2.1]hept-2-ene (not 7′a N 7′ 7 ′ 4 2,2 -Bibicyclo[2.2.1]hept-5-ene) 3′ 3,3′-Spirobi[3H-indole] ′ 3 4 4 4′ N 7a 1 3′
1′ 1,1′:2′,1′′-Tercyclopropane 3′′ 1′′ 1
′ 8 a 5′ 1′ 3 1 8 2′ 4′ 2 2,2′(1H,1′H)-Spirobinaphthalene H H 2 N N 2′′ 1 5 5′′ 1′′ 54 HN NH 4,4′:4′,4′′-Terpyrazolidine Di-, tri-, etc., spiro systems containing at least one fused or bridged component are named by extension of these policies. If terminal components 5′ are identical, citation is determined by earliest index position of the complete HN NH name. Serially primed locants are used for successive components. 2′ 1′ “Branched” polyspiro systems in which a single component is surrounded by three or more identical components are named by citing the central 1′′′ component (which is assigned plain locants) first and multiplying the identical 1 5′ 5′′ (terminal) components. When two terminal components of a “branched” spiro 5 5′′′ system are identical, and one different, they are cited in alphabetical order (as 1′ 1′′ 3,3′:5′,3′′:5′′,3′′′-Quatercyclopentene usual) and the term “bis” is applied as appropriate. Examples: Indicated hydrogen (¶135) is assigned, where possible, to points of attach- 1 ment. When indicated hydrogen is cited in different positions for different 8 components, a ring-assembly name is not used. Added hydrogen (¶ 135) is Dispiro[bicyclo[3.3.1]nonane− cited immediately after the locant to which it relates. 9 3 1′ 3′ 1′′ 3,1′− cyclobutane −3′,1′′−cyclo− Example: butane] 4′ 4′′ 1 1′ 5 N 2 2′ N 5 N N 5′ 2,2′-Bi-2H-1,2,3-triazole 6′′′ 1′′ Trispiro[cyclohexane-1,1 ′-cyclo- 7′′a O N N 1′′′ − ′ ′′ ′′ − − ′ 6′′ pentane 3 ,3 (2 H) cyclopenta 3 3 ′′ [b]pyran−6′′(4′′H),1′′′−cyclo− 3 ′ 5′ 5′′ 3 hexane] 4′′ 1′ 1H-Benzotriazole, 1-(1,3−dihydro- 1 H H 2′ H N N 2H-benzotriazol-2-yl)-2,3-di- 6 N 23 NN1 hydro- (not 1H-Benzotriazole, 2-(2,3-dihydro-1H-benzotriazol-1- NH ′ 4′ yl)-2,3-dihydro-; not 1(3H),2 - 3′ Bi-2H-benzotriazole, 1′,3′ - O dihydro-) 2′ 1′′′ ′ 7′′′a O 1 O 7 a N 1′ ′ 2′′′ 3 4′′ 2(1H),4 -Biisoquinoline 2 O N 3′′ O 2′′ 3′′′′ 4′′′ O ′′ 1′′ 7 a Trispiro[cyclopropane-1,2′:2,2′′:3,- Linear benzene assemblies (polyphenyls) are named by prefacing “phenyl” 2′′′-tris[1,3]benzodioxole] with the appropriate term (Bi-, Ter-, etc.). Arabic numeral locants are cited in all cases for points of attachment. Two-component assemblies of monocyclic hydrocarbons and of hetero systems with “cyclo” names, e.g., Cyclopen- 5′′ 1′′ 3′′′ tastannane, Cyclotrisiloxane, and monocycles with one silicon using “sila” O O 5′′′ names, are named from the radicals, and locants for the points of attachment Trispiro[1,3-benzodioxole-2,1′- ′′ 2′′′ are cited only when the radical has no locant for the free valency. Two-compo- 3′′ 2 ′ ′′ ′ ′′′ O ′ ′ O cyclohexane-2 ,2 :4 ,2 -bis- 2 4 1′′′ nent ring-assembly names from unsaturated (“-enyl”) radicals are formed only 7a O [1,3] dioxolane ] 1 1′ when the unsaturation is symmetrical with respect to the points of attachment. 2 Examples: 6′ O3 4 6 11′ 157. Ring assemblies contain a multiplicity of the same cyclic system 1,1′-Biphenyl joined by single bonds, not necessarily in equivalent positions. They are 6′ treated as molecular skeletons in substitutive nomenclature and rank just above the component ring. Except for assemblies of benzene, and two-component assemblies of cycloalkanes, cycloalkenes, and hetero systems with “cyclo” ′′′′ ′′′ names (see below), they are named by prefixing the component names with the 6 6 6′′ 6′ terms Bi-, Ter-, Quater-, Quinque-, Sexi-, Septi-, Octi-, Novi-, Deci-, 1′′′′ 1′′′ 1′′ 1′ 1 Undeci-, etc. Locants are placed ahead of the name to define the points of attachment. 6 These locants are as low as possible, compatible with fixed numbering (ex- 1,1′:4′,1′′:4′′,1′′′:4′′′,1′′′′-Quinque- pressed or implied) of the components, including “-ene” and “-yne” suffixes. phenyl Examples:
6 1,1′- Bicyclohexyl (not Bicyclohex− 2,2′-Bipiperidine 11′ 6′ 1′ 1 6 1-yl) N N H H 6′ 29 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 158
6′ 1′ 1 Bi-2-cyclohexen-1-yl (not 1,1′-Bi- 2-cyclohexen-1-yl) Te 6′ Cl Cl
H2 1 1′ H 2 8′ λ4 Si O O Si 5H-5 -Dibenzotellurophene, H H 7 8 7′ 5,5-dichloro- O 2 Si Si 2′ O Bicyclotetrasiloxan-2-yl 6 3 3′ 6′ H2Si O O SiH2 4 4′ • A radical is formed according to the established rules–including the use of O Si Si O added hydrogen to accommodate an “ylidene” radical–when the free valence 5 H H 5′ 2 2 is on the nonstandard heteroatom. 3 Cyclohexene, 1-(3-cyclohexen-1- 11 yl)- (not Cyclohexene, 4-(1- cyclohexen-1-yl)- (lowest locant N SO2 Me 6 6 for the substituent prefix is preferred Me (¶ 138)) S
158. Nonstandard heteroatoms in CA ring nomenclature. Additive terms are used to describe oxides, sulfides , selenides, and tellurides of ring het- Me eroatoms: Benzenesulfonamide, O2 N-(3,4-dihydro-5,6-dimethyl- 7a S Benzo[b]thiophene, 3,5-dimethyl- 1λ4-thiopyran-1(2H)-ylidene)- 1 1,1-dioxide 3
H Me H N N O Acetic acid, 2-[(1-oxido-2-pyridinyl) P P N SCH2CO2H 6 1 thio]- N Me N H H 2λ5-1,3,2-Diazaphospholidine, For all other neutral ring heteroatoms with nonstandard bonding numbers 2-methyl-2-(2-methyl-2λ5-1,3,2- (hereafter referred to as nonstandard heteroatoms), the IUPAC lambda conven- diazaphospholidin-2-ylidene)- tion (Pure Appl. Chem. 1984, 56, 769-778) is used, regardless of the heteroat- om in question, its bonding number, or the kinds of bonds joining it to other skeletal atoms. N The rules are as follows: HP PH λ N N • The term is prefixed to the name for the ring parent, preceded by the locant P for the nonstandard heteroatom–in a fusion name, that locant is for the fused H2 ring system, not a component ring–and followed by a superscript denoting the nonstandard valence. (In the IUPAC recommendations, the λ is usually in- 2λ5,4λ5,6λ5-1,3,5,2,4,6-triazatriphosphorine-2,4-diyl serted within the ring name, after the locant for the nonstandard heteroatom.)
Me • After the established rules for low numbering have been applied–for heteroa- O toms, indicated hydrogen, an onium center, and so on–low numbering is as- Me S signed to a nonstandard heteroatom in preference to the same heteroatom O with the standard bonding number.
4 2λ -1,3,2-Dioxathiane, S 5 4 2,2-dimethyl- 6 3 1 2 H NEt S N 2 H P I 4 N 1λ -1,5-Benzodithiepin H NEt2 2λ5-1,3,2-Diazaphospholidine-2,2-diamine, • In a bridged fused ring system, the name of the bridge (including its locants N,N,N′,N′-tetraethyl-2-iodo- and any indicated hydrogen required by the bridge) is prefixed to the fusion name with its λ term.
F F S F I N O N S Me Me λ4 λ4 5 1,4-Methano-5,11-nitrilo-11H-5 ,11 - 1λ -1,2-Benziodoxole, dibenzo[c,f][1,5,2]dithiazepine 1,1,1-trifluoro-1,3-dihydro-3,3-dimethyl- • In a ring assembly or spiro name, the component name is always cited with H its own λ term. S 1 2 5 4 3 N Cl Et Me Cl N N N ′ Cl P 1 P P ′ 1 ′P Cl 1λ4-Pyrido[2,3-e]-1,3-thiazine 6 2 2 6 5 3 3′ 5′ N 4 N N 4′ N P P • For a ring parent with the maximum number of noncumulative double bonds, Cl Cl Cl Cl indicated hydrogen is used with a nonstandard heteroatom if that atom is joined to other skeletal atoms only by single bonds–unless the other atoms are 2,2′-Bi-2λ5,4λ5,6λ5-1,3,5,2,4,6-triazatriphosphorine, divalent, in which case indicated hydrogen is not used. Indicated hydrogen 4,4,4′,4′,6,6,6′,6′-octachloro-2-ethyl-2′-methyl- always precedes the λ term. ¶ 158 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 30
O N Until structures have been determined, author terms such as “iso” and “neo” 1 2 P 2′ are cited in index modifications to differentiate isomers. 4 3 1′ N N Numbering of polyboranes in CA indexing is based on “Nomenclature of Boron Compounds” adopted by the American Chemical Society, and Spiro[2λ5-1,3,2-benzoxazaphosphole-2,2′- “Nomenclature of Inorganic Boron Compounds”, published by the [2H-2λ5-1,3,2]diazaphosphole] International Union of Pure and Applied Chemistry,1 which may be consulted for further details. For “closed” polyboranes (those whose boron skeletons are S S polyhedra with triangular faces throughout) the numbering begins with the 1 3′ boron atom at the head of the largest axis of highest order, then proceeds 2 Te 2′ 3 1′ sequentially to the planes which intersect this axis. Boron atoms in each plane S S are numbered clockwise unless lowest locants for substituents demand 2,2′-Spirobi[2λ4-1,3,2-benzodithiatellurole] anticlockwise numbering. On succeeding planes, numbering begins at the boron atom immediately “below” the lowest-numbered one on the previous plane, or the one nearest to it in the direction of numbering. • In a replacement name for a saturated ring parent, both the lambda convention Example: and subtractive suffixes are used to describe a nonstandard heteroatom joined to cumulative double bonds. H B 1 S 5 6 1 2 B 2 H 4 BH 5 4 3 HB H B Si 9 2 6 H2 HB BH 3BH 1λ4-1-Thia-4-silacyclohexa-1,6-diene 5 1 3 H HB BH BH B HB 9 5 B • To describe all other nonstandard heteroatoms joined to cumulative double H BH 7 6 bonds, the lambda convention is used together with the IUPAC delta conven- HB BH tion (Pure Appl. Chem. 1988, 60, 1395-1401). The terms are combined into 8 7 a single expression, prefixed by the locant for the nonstandard heteroatom. B10 4 B H H The number of cumulative double bonds to the nonstandard heteroatom is Decaborane (10) planar projection specified by a superscript Arabic numeral after the δ. (Note that atom “6” (in the lower plane) is nearest to atom “2” in the S direction of numbering.) 1 2 N 3 N For “open” polyboranes (those with incomplete polyhedral boron 2 skeletons) the rules are more complex. A planar projection, as viewed from the 1λ4δ -1,2,3-Thiadiazole open side, is numbered so that interior boron atoms have lowest locants, beginning at the “center” or “apex.” Each atom set is numbered in the same direction. NS Examples: 65 7 4 N H2 B N 8 3 SH 1 Diborane (6) 12 H H SN 2 B H 2 2 1λ4δ ,3λ4-1,3,5,2,4,6,8-Trithiatetrazocine H B 1 H H B H S 2 N 5 2 1 2 H H H B B HBHB 5 B 3 4 3 S 1′ H 1 N H H 4 4 3 H H B B B H H H 6 2 6 H Spiro[3λ δ -1,3,2,4-benzodithiadiazine-3,1′-[1H-1λ ]thiophene] Pentaborane(9) planar projection
1 BH H 159. Boron molecular skeletons. Because the number of hydrogen atoms H H H B H in neutral and anionic boron hydrides often bears no simple relationship to the 2 number of boron atoms, borane names must express the number of both. (The 6 3 HB 1 BH single exception is Borane itself, which represents BH3.) The Ring Systems BH B H Handbook should be consulted for structural diagrams of the neutral 6 2 HB 5 4 3 BH H H polyboranes of established structure. Diagrams justified by current index HB BH 5 4 entries are displayed in the Chemical Substance Indexes. (In these diagrams, B B the lines do not represent electron -pair bonds but indicate the geometry of the H H H H structures.) Neutral boron hydrides, real or hypothetical, are treated as molecular skeletons in substitutive nomenclature, Borane and the diboranes as Hexaborane(10) planar projection heteroacyclic compounds, and the higher hydrides as heterocyclic compounds. Borane(1) is BH, Borane(2) is BH2. In higher boranes , the number of boron Some polyboranes can be named as derivatives of simpler polyboranes. atoms is expressed by multiplicative prefixes. Thus, a bimolecular polyborane, i.e., a two-component “ring” assembly in Examples: which both skeletons are identical, can be named as follows:
B2H4 Diborane(4) 1,1′-Bipentaborane(9)
B2H6 Diborane(6) When the various parts of the structure are not identical, the general principles of substitutive nomenclature are applied, and a polyborane radical is used for B3H7 Triborane(7) the less preferred skeleton, e.g., Decaborane(10), 2-octaboran(8)-1-yl-. Polyboranes joined along an edge, or with a triangular face in common, are B4H10 Tetraborane (10) named like fused ring systems; e.g., Decaborano(14)[5′,6′:5,6]decabo- rane(14), Undecaborano[2′,7′,11′:1,2,3]dodecaborane(17). B5H9 Pentaborane(9)
B6H10 Hexaborane(10) 1Inorg. Chem. 1968, 7(10), 1945-65; Pure Appl. Chem. 1972, 30(3-4), B10H14 Decaborane(14) 683-710. 31 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 161
160. “Hetero” polyboranes are boron hydride skeletons in which boron multiple) in one or two positions of an acyclic chain are always terminal, oth- atoms have been replaced by those of other elements, notably carbon. erwise a compound radical name is employed. When three or more positions Replacement prefixes, e.g., “carba,” “phospha,” are employed with the have free valencies, two of them must be terminal. polyborane name, and the number of hydrogen atoms attached to the skeleton Examples: expressed in parentheses after the name. Structural diagrams for compounds of − established structure can be found in the Ring Systems Handbook and, when MeCH2 ethyl justified by current entries, in the Chemical Substance Index. Numbering is as = − for the parent polyboranes, with lowest compatible locants assigned to the H2C CHCH2 2-propen-1-yl (not allyl) replacement atoms. Examples: EtCH= propylidene ≡ H PrC butylidyne C = = 1 H2C C ethenylidene BH = = HB 6 CH 1,2-Dicarbadodecaborane(12) H2C CHCH 2-propen-1-ylidene H H 5 B B 2 MeC≡CCH CH=CHCH= 2-hepten-5-yn-1-ylidene 4 3 2 − − H H (CH2)2 1,2-ethanediyl B B 10 11 − = HB 9 7 CH2CH 1-ethanyl-2-ylidene H 8 BH B − = − 12 CH CH 1,2-ethenediyl B − − H (CH2)3 1,3-propanediyl
=C=C=C= 1,2-propadiene-1,3-diylidene H B | 1 —CH2CHCH2— 1,2,3-propanetriyl H B || 6 —CH CCH — HB 5 2 CH 2,4-Dicarbaheptaborane(7) 2 2 1,3-propanediyl-2-ylidene H H C B 4 3 (c) Organic heteroacyclic (“a”) radicals are used when the requirements ( ¶127) are met. The numbering of the parent radical is retained. A single free
7 valency is hence always in the 1-position, and this locant is always cited. When B there is still a choice, lowest locants are assigned to hetero atoms, then to most H preferred hetero atoms (Table I, ¶ 128), then to unsaturation (with double bonds preferred). Examples: 161. Substituent prefixes (radicals) derived from molecular skeletons − are used very frequently in substitutive nomenclature. Their names are based Me[O(CH2)2]3 OCH2CH2 3,6,9,12-tetraoxatridec-1-yl (not on the skeleton names and may be classified accordingly as radicals from (a) [2-[2-[2-(2-methoxyethoxy)- monoatomic skeletons, (b) hydrocarbon chains, (c) organic hetero (“a”-named) ethoxyl]ethoxy]ethyl]) chains, (d) homogeneous hetero chains, (e) heterogeneous hetero chains, (f) = = carbocycles, (g) heterocycles, (h) ring assemblies, (i) polyboranes. Combina- MeSiH2CH2SiH2CH CHSiH2CH2SiH2CH tion of simple radicals to form compound and complex radicals is performed 10 9 7 4 2 1 2,4,7,9-tetrasiladec-5-en-1-ylidene by application of principles described earlier (¶ 133). (See also “Illustrative List of Substituent Prefixes,” which constitutes Section H (¶ 294). = —CH2CH2[S(CH2)2]2SCH CHNHCH2CH2- (a) Monoatomic radicals from borane, methane, silane, germane, stannane, 1 2 9 12 14 and plumbane are named by replacing “-ane” by “-yl,” “-ylene,” and 3,6,9-trithia-12-azatetradec-10-ene- “-ylidyne” to denote the loss of one, two, or three hydrogen atoms. The final 1,14-diyl “e” of the hydrides arsorane and phosphorane may be replaced by “-yl,” “-ylidene,” and “-ylidyne.” Stibine and bismuthine may have the “-ine” ending (d) Homogeneous heteroacyclic radicals are named analogously to acyclic replaced by “-ino,” “-ylene,” and “-ylidyne.” Phosphine and arsine may have hydrocarbon radicals, except that only the “e” of “ane” suffixes of heteroacy- the “-ine” replaced by “-ino,” “-inidene,” and “-inidyne.” The -tetrayl suffixes clic skeleton names is replaced by “-yl.” indicate loss of all hydrogen from Group IVA monoatomic hydrides. Examples: Examples: HN=N− diazenyl (also used for the substituted radical, rather than azo) − ≡ H2B boryl HC methylidyne − H2NNHNH triazanyl = ≡ H2C methylene HSi silylidyne HN=NNH− 2-triazen-1-yl =Sn= stannanetetrayl HAs= arsinidene = = − H2NN NN N 1,3-pentazadien-1-yl − ≡ H2P phosphino As arsinidyne H PPH− diphosphinyl − 2 HP= phosphinidene H2Sb stibino −PHPH− 1,2-diphosphinediyl P≡ phosphinidyne HSb= stibylene = = − ≡ AsAs 1,2-diarsinediylidene H4P phosphoranyl Sb stibylidyne
− −Sb=Sb− 1,2-distibenediyl H3P= phosphoranylidene H2Bi bismuthino
≡ H NNH− hydrazinyl (not hydrazino) H2P phosphoranylidyne HBi= bismuthylene 2
− ≡ = H2As arsino Bi bismuthylidyne H2NN hydrazinylidene (not hydrazono)
−NHN= 1-hydrazinyl-2-ylidene (b) Acyclic hydrocarbon radicals are named from the skeletons by replacing “-ane,” “-ene,” and “-yne” suffixes by “-yl,” “-enyl,” and “-ynyl,” −NHNH− 1,2-hydrazinediyl (not hydrazo or (for monovalent radicals); by “-diyl,” “-triyl,” “-enediyl,” “-ynediyl,” etc., for hydrazi) divalent radicals with hydrogen removed at more than one position; and by “-ylidene” and “-ylidyne” to indicate two or three hydrogen atoms lost at one −N=N− 1,2-diazenediyl (not azo or azi) position. (Methylene is an exception.) Locants are not cited for monovalent radicals (the free valency position is always “l”), but unsaturated positions are =NN= 1,2-hydrazinediylidene (not azino) always indicated for chains of three or more atoms. Free valencies (single or ¶ 161 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 32
(e) Heterogeneous heteroacyclic radicals are named analogously to 1 7a hydrocarbon radicals. 1H-inden-2-yl Examples: 3 4 − H3SiOSiH2 1-disiloxanyl
= H3SiOSiH 1-disiloxanylidene 1 7a 3aH-inden-3a-yl (a hydro derivative would be named, e.g., 2,3-dihydro- H SnOSn≡ 1-distannoxanylidyne 3 3 3aH-inden-3a-yl, not 2H-inden- 3a(3H)-yl) − − 4 SiH2NHSiH2 1,3-disilazanediyl
| 10 —SiH2OSiHOSiH2— 1,3,5-trisiloxanetriyl 9 1 11a 5H-dibenzo[a,d]cyclohepten-5-ylid- (f) Cyclic hydrocarbon radicals. Loss of one or two hydrogen atoms from ene a single cycloalkane carbon atom is denoted by replacement of “ane” of the 6 4 ring name by “-yl” and “-ylidene,” respectively. The implied locant (“l” in all cases) is not expressed. In radicals from cycloalkenes, cycloalkadienes, etc., only the final “e” is replaced by the radical suffix, and locants for unsaturation and the free valency (always “l”) are all cited. Loss of hydrogen at more than one position is expressed by the suffixes “-diyl” (not “-ylene”), “-diylidene,” 9 “-enylylidene,” etc. All locants are cited, and locants for free valencies are 8 10a 1 2,7-phenanthrenediyl assigned lowest locants. Examples: 4b 4a
1 cyclohexyl 6 8a 1 3-naphthalenyl-1(4H)-ylidene (not 2-naphthalenyl-4(1H)-ylidene)
1 2-cyclopropen-1-yl 3 Von Baeyer and spiro radicals follow similar principles. Free valency locants are assigned lowest locants compatible with ring-system numbering and are preferred over locants for unsaturation. Examples: 1 2,4-cyclopentadien-1-yl 5 bicyclo[2.2.2]oct-5-en-2-yl (not bi- cyclo[2.2.2]oct-2-en-5-yl)
1 6 1,5-cyclohexadien-3-yn-1-yl 8 1 bicyclo[3.3.1]nonane-2,3-diyl-4- 9 ylidene
1 6 2,4-cyclohexadien-1-ylidene 11 12 1 dispiro[4.1.4.1]dodec-2-yl 7 5
1 1,3-cyclopentanediyl (not 1,3-cyclo- spiro[bicyclo[2.2.1]hept-5-ene-2,1′- 5 pentylene) 6 1 [3,5]cyclohexadien]-2′-yl (note: 7 2 1′ lowest locants are assigned, in ′ order, to spiro atoms, free valencies, 4 6 all multiple bonds, double bonds)
(g) Heterocyclic radicals from ring systems not named by organic 1 1-cyclohexanyl-2-ylidene (not 1- 6 replacement (“a”) nomenclature are named analogously to fused-hydrocarbon cyclohexyl-2-ylidene) radicals (above). The shortened form thienyl is used for thiophene radicals and all their fused derivatives; selenophene-yl (not selenophenyl) is the name of the seleno analog. Examples: 1 6-cyclohexen-1-yl-2-ylidene 6 1 N 6 3-pyridinyl (not 3-pyridyl)
Radicals from benzene are named phenyl (C6H5-), 1,2-, 1,3-, and 1,4- phenylene (-C6H4-), 1,2,3-benzenetriyl, 1,2,3,4-benzenetetrayl, etc. Fused hydrocarbon radicals are assigned the lowest locants compatible with N 6 1 1(2H)-pyridinyl the fixed numbering of the ring system. Indicated hydrogen (¶ 135) necessary for the existence of the ring is assigned to the lowest nonangular position unless it can be located to accommodate a monovalent radical in an angular position or an “ylidene” radical in a nonangular position. Added hydrogen (¶ 136) is 1 O cited immediately after the radical locant and is assigned the lowest available 6 4-morpholinyl (not morpholino) angular or nonangular position. 4 Examples: N
1 8a 1 2-naphthalenyl (not 2-naphthyl) 7a S benzo[b]thien-2-yl (not benzo[b]- 5 4 3 thiophen-2-yl) 4 33 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 162
1 6′ 7a N 3H-indol-2-yl-3-ylidene (ahydro derivat would be named, e.g., 1,2-dihydro- 1′ 1 [bi-2,5-cyclohexadien-1-yl]-4,4′- 3 3H-indol-3-ylidene, not 1H-indol- diylidene 4 3(2H)-ylidene) 6 (i) Polyborane radicals are formed from polyborane and hetero polyborane 1 O names by citing the usual radical suffixes after the parenthetical designation of 5 2,5-furandiylidene the number of hydrogen atoms, with elision of the final “e” before “y.” Requirements for locant citation with diborane radicals are the same as for the analogous acyclic hydrocarbon radicals. Examples: 1 7a N 2H-benzimidazol-5-yl-2-ylidene diboran(4)yl 1-pentaboran(9)yl
N 3 4 diboran(6)yl 1,2-dicarbadodecaboran(12)-1-yl
1 diboran(4)ylidene 6,9-decaborane(10)diyl 6 O 2 HSi SiH cyclotrisiloxane-2,4,6-triyl 1,2-diborane(4)diyl
5 O 4 O 3 162. Compound and complex radicals from molecular skeletons are Si H named by principles described earlier (¶ 133). Examples:
− “A”-named cyclic radicals are named like the hydrocarbon parents from Me2CH (1-methylethyl) which they have been derived by atom replacement. The hetero atoms receive = − lowest locants (cited or implied), then the free valencies (cited just ahead of the H2C CMe (1-methylethenyl) (the unsaturated radical suffix). parent radical is preferred) Examples: = − ≡ − CH CH C C CH3 H | 2 − = − = − − = − = − Si CH3 CH CH CH CH CH CH CH CH CH CH2— 11 10 9 8 7 6 5 4 3 2 1 6 1 silacyclohex-2-yl (not 2-silacyclohexyl) [6-(1-penten-3-yn-1-yl)-2,4,7,9-unde- catetraen-1-yl]
PhCH=CH− (2-phenylethenyl) (not styryl)
1 3 3-oxabicyclo[3.1.0]hex-6-ylidene 6 O PhC≡ (phenylmethylidyne) (not benzylidyne)
5 Me (h) Ring-assembly radicals are derived by bracketing the assembly name, (2-methylphenyl) (not o-tolyl) eliding a final “e” if “y” is to follow, and appending the radical endings “-yl,” 1
“-ylidene,” “-diyl,” etc., as appropriate. The free valencies need not be on the 6 terminal rings of the assembly. Examples:
6′ 1 [1,1′-biphenyl]-4-yl (not 4-biphenylyl) 4-methyl-1,2-phenylene) (not 4- 11′ 6 methyl-o-phenylene)
6 Me 6 [1,1′-biphenyl]-2,4′-diyl (not 2,4′- ′ 11 biphenylylene) Radicals from branched polyphenyls are chosen by application of the
6′ following principles successively until a decision is reached: (a) longest chain of rings containing all of the free valencies, which need not be on terminal rings; 6′ (b) lowest locants in the radical name for (1) ring junctions, and (2) [1,1′-biphenyl]-3,4-diyl (not (4- 1′ 1 free valencies; phenyl-1,2-phenylene)) (c) maximum number of substituent prefixes; 6 (d) lowest locants for substituent prefixes; (e) earliest index position of the radical name. 6′ Examples (the letters on the left refer to the principles above): 1′ 1 [1,1′:3′,1′′-terphenyl]-4,4′-diyl (not (2-phenyl[1,1′-biphenyl]-4,4 -diyl))′ (a) (3′′,6′′-diphenyl[1,1′:4′,1′′:2′′,1′′′:- 6 6′′ ′′ 4′′′,1′′′′-quinquephenyl]-4′′,- 1 1′′′′ 6′′′′ 5′′-diyl) 1′′′ 6′′′ 1′′ 1′ 6′ 8′a 6′′ 1 [1,2′-binaphthalene]-4′,5-diyl 1′ 4 1 ′ 8a ′ 4 5 5 6
1′ 1 S S ′ (b) (1) ′ [5,5 -bithiazol]-2-yl (not [5-(5-thia- 6 5 5 zolyl)-2-thiazolyl]) 1 6′′ 1′ 6′ 3′ N N3 1′′
1′ 1 ′′′ N N 6 1 6′ [2,2′-bipyridine]-4,6-diyl (not [6-(2- ′′ ′ ′ ′ ′′ ′′ ′′′ ′′′ (4 ,5 -diphenyl[1,1 :2 ,1 :2 ,1 - pyridinyl)-2,4-pyridinediyl]) 6 quaterphenyl]-3′-yl) ¶ 162 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 34
(b) (2) ′ ′ ′ Example: 6 [6 -(4-methylphenyl)-4 ,5 -diphenyl- [1,1′:2′,1′′-terphenyl]-3′-yl] 1 ′′ ′′ 1′ 1 6 [1,1′:2′,1′′-Terphenyl]-3 ′-carboxylic 6′ Me acid, 4′′-bromo-3-fluoro-4′,6′- diphenyl- (a decision is reached by CO2H choosing lowest locants for the Terphenyl skeleton and then for the Br 1’’ principal group) 6’ 1’ 1 6’’ 6
(c) Cl [6′-(2-bromophenyl)-4-(3-chloro- F phenyl)-2′′,4′′-diiodo[1,1′:2′,1′′- terphenyl]-4-yl] 163A. Fullerenes. The even-numbered, closed spheroidal structures of 20 Br or more carbon atoms, in which every atom is bonded to three other atoms and 6′′ of which the C60 “ buckminsterfullerene ” is the prime example, as known gener- 1′′ 2,3 I ically as “fullerenes”. “Fullerene” has been adopted as the class name and to 6′ 1′ it are added ring sizes, number of carbon atoms, and point group symmetries to 1 name specific members of the class. For example, in the name [5,6]Fullerene- I 6 C60-Ih, “[5,6]” indicates the presence of ring sized 5 and 6, “C60” the number 4 of carbon atoms, and “Ih” the point group symmetry. When a fullerene is mod- ified (e.g., by addition, replacement, or deletion of atoms), the ring sizes, the number of carbon atoms, and the point group symmetry remain those of the par- ent fullerene. Incompletely described fullerenes, i.e., where ring sizes and/or point group symmetries are not known or disclosed are named with only the (d) ′′ ′ ′ number of carbon atoms, e.g., Fullerene-C60. Existing nomenclature practices [2 -chloro-6 -(4-chlorophenyl)-4 - are followed as closely as possible when naming derivatives. Anions, cations, phenyl[1,1′:2′,1′′-terphenyl]-4-yl] 6′′ protonated fullerenes, and free radicals are named as follows: 1′′ 6′ Cl [5,6]Fulleride(3-)-C60-Ih 1′ 1 [5,6]Fullerene-C60-Ih Cl 6 ion(1+)
[5,6]Fulleren-C60-Ih-1(2H)-ylium
[5,6}Fulleren-C60-Ih-1(2H)-yl
(e)[2′′-bromo-6′-(2−chlorophenyl)-4′- Metallofullerenes are compounds in which one or more metals are either phenyl[1,1′:2′,1′′-terphenyl]-4- trapped inside the fullerene or located outside without bonding directly to it. yl](not [6′-(2−bromophenyl)-2′′- They are given fullerene names, with the metals cited by name in alphabetical Cl chloro-4′-phenyl[1,1′:2′,1′′-ter- order 6′′ phenyl]-4-yl]) Example: 1′′ 6′ 1′ [5,6]Fullerene-C -I 1 60 h 6 compd. with potassium (1:1), ion(1+) Br Partial hydrogenation of a fullerene is described by terms such as “hexatria- contahydro-”, while full saturation is implied by the name “fullerane”, e.g., [5,6]Fullerane-C60-Ih. Fullerenes containing substituents require that hydrogen be added before the 5 163. Molecular skeletons as index heading parents. These two entities substituents can be named. For example, C60F60 is named [5,6]Fullerane- coincide when no suffix, expressing a principal group, is added to the skeleton C60-Ih, hexacontafluoro- (the hydrogen being part of the parent name). C60Br2 name, either because such a group is absent, or because it is attached to a hetero is named as a dibromodihydrofullerene, and C60H6Ph6 is named as a dodecahy- atom of the skeleton which changes it from a functional into a nonfunctional drohexaphenylfullerene. group. Addition of hydrogen is not necessary when a fullerene contains two func- Examples: tional groups, as in [5,6]Fullerene-C60-Ih-1,60-diamine. Modification of the fullerene network such that some carbon atoms no − H3C CH3 Ethane longer have a connectivity of 3 are named by using “homo”, “nor”, and “seco”, e.g., 1,2(2a)-Homo[5,6]fullerene-C60-Ih-2a-carboxylic acid. PhNO2 Benzene, nitro- (“nitro” is a man- Replacement of a carbon atom by a trivalent hetero atom such as boron or 5 datory prefix (¶ 132)) nitrogen results in a free radical, e.g., 1-Bora[5,6]fulleren-C60-Ih-2- yl.
OH Piperidine, 1-hydroxy- (“hydroxy” N is considered nonfunctional when attached to a hetero atom other than silicon)
Me3SiCOMe Ethanone,1-(trimethylsilyl)-
Choice of a preferred index heading parent has already been described (¶ 138). In the case of a branched polyphenyl, the criteria are the same as for 2 the derived radicals (¶ 162), except that principal groups replace free valencies. Science 1988, 242, 1017-22; 1139-1145. 3“Fullerene” has also been defined as a closed, hollow network of 12 pen- tagonal and m hexagonal faces for a C20+2m molecule (Science 1991, 254, 1768-1770), but CAS also includes structures with 3-, 4-, and 7- through 10- sided faces as fullerenes for purposes of naming. 4“Character Tables for Chemically Important Symmetry Groups”. In: F. A. Cotton, Chemical Applications of Group Theory. 3rd ed., John Wiley & Sons, 1990. Appendix IIA, pp. 426-435. 5International Union of Pure and Applied Chemistry, Nomenclature of Organic Chemistry , Sections A,B,C,D,E,F, and H, 1979 ed., Pergamon Press, Oxford (England), 1979. Rules C-0.1 and C-32. 35 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 165
C. PRINCIPAL CHEMICAL GROUPS (SUFFIXES)
Introduction ¶ 164 Amides ¶ 171 Acids expressed as substituent suffixes 165 Nitriles 172 Acyclic acids with cyclic substituents 166 Aldehydes 173 Order of precedence of acids 167 Ketones 174 Acid radicals 168 Alcohols (and phenols) 175 Functional derivatives of acids 169 Amines 176 Acid halides 170 Imines 177
164. Introduction. In substitutive nomenclature, a principal chemical H2PCO2H Phosphinecarboxylic acid (not group is that substituent of a molecular skeleton which is selected for expres- Formic acid, phosphino-) sion as a suffix. Only one kind of substituent may be cited as a suffix, viz., the most senior one as determined by the Order of Precedence of Compound Class- es (¶ 106); all other substituents are expressed as prefixes (radicals) which may HO2CSnH2OSnH2CO2H 1,3-Distannoxanedicarboxylic acid be simple, compound, or complex (¶¶ 132, 133). For functional compounds, 123 the molecular skeleton name together with its suffix constitutes an index head- ing parent. The locants for suffixes are placed in front of the heading parent H name unless locants for indicated hydrogen, hetero atoms (in “a” names), un- H B BHCO H Diborane(6)-1-carboxylic acid saturation, fusion sites, etc., are present, in which case they are placed just be- 2 2 fore the suffix. H In the following paragraphs, compound classes expressed as substituent suffixes are discussed in descending order of precedence. CO2H 2,6-Cyclohexadiene-1,2-dicarboxylic 1 165. Acids expressed as substituent suffixes on molecular skeletons in- CO2H acid (lowest locants for principal clude carboxylic, sulfonic, sulfinic, selenonic, and telluronic acids and their 6 groups are preferred) functional replacement analogs, such as peroxy, imidic and thio acids. For Carbonic acid and its relatives (including Carbamic acid and Formic acid) see ¶ 183. CO H (a) Carboxylic acids are named by the Geneva (“-oic”) or “-carboxylic” 2 8 9 1 system. The “-oic acid” suffix is employed for acyclic mono- and dicarboxylic 9a 1-Anthracenecarboxylic acid acids of carbon chains, including “a”-named acids; the “-carboxylic acid” suf- fix is used for acyclic polycarboxylic acids and compounds in which the car- 10a 4a boxyl group is attached to a ring, a monoatomic hydride, or a heteroacyclic 5 10 chain. The trivial names Acetic acid and Benzoic acid are retained for these 1 two acids and their substituted derivatives. (The amides, acid chlorides , etc., N are named similarly, but organic replacement analogs are named systematical- 8a ly, e.g., Benzenecarboximidic acid (not Benzimidic acid.) 4-Quinolinecarboxylic acid Examples: 5 4 MeCH2CO2H Propanoic acid (not Propionic acid ) CO2H
5 Pentanoic acid (not Valeric acid ) 1 Spiro[1,3-dioxolane-2,2′(1′H)- Me(CH2)3CO2H O 1′ ′ 5 1 2 8′a naphthalene]-5 -carboxylic acid 3 O
H2C=CHCO2H 2-Propenoic acid (not Acrylic acid ) 31 ′ 4′ 5
CO2H Me(CH2)4CH=CHCH2CH=CH(CH2)7CO2H 18 13 9 1 HO2C [1,1′-Biphenyl]-2,2′-dicarboxylic 9,12-Octadecadienoic acid 6 acid 1 1′
6′ HO2CCO2H Ethanedioic acid (not Oxalic acid ) 12 CO2H
Pentanedioic acid (not Glutaric acid) 1,4(4H)-Pyridinedicarboxylic acid HO2C(CH2)3CO2H CO2H 15 N (the added hydrogen is expressed 6 1 after the final locant but relates to − − − − − − − − − − − − − ~ H3C CH2 O CH2 CH2 O CH2 CH2 O CH2 CH2 O CH2 CH= ~ the 1-carboxyl group; see ¶ 136) 23 21 18 15 1211 10 ~
~ − − =CH (CH2) 7 COOH ~ 9 1 CO2H 12,15,18,21-Tetraoxatricos-9-enoic acid (lowest locants are assigned to 1 2 1,2-Dicarbadodecaborane(12)-1,2- functional suffixes, not replacement HO2C CO2H dicarboxylic acid (the 1,2-bond prefixes) B 10H 10 does not possess single-bond character) (b) Sulfonic, sulfinic and sulfenic acids and their selenium and tellurium COOH 1,2,3-Propanetricarboxylic acid analogs are expressed by appending the appropriate suffix to the name of the
− molecular skeleton. Mono- and diacids of these series, unlike “-oic acids,” HOOC−CH −CH−CH −COOH (numbering excludes the carboxyl 2 2 groups) above, do not need to occupy terminal positions on a chain. 123 Examples:
EtSO3H Ethanesulfonic acid (HO2C)2C=C(CO2H)2 1,1,2,2-Ethenetetracarboxylic acid
SO H − 3 1,2-Hydrazinedicarboxylic acid (not − − − − HO2CNHNHCO2H CH3 CH2 CH2 CH CH3 2-Pentanesulfonic acid 12 Bicarbamic acid) 54 321 ¶ 165 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 36
1 PhSO3H Benzenesulfonic acid N 3-Pyridinecarbohydrazonic acid 6 N 6 1 3-Pyridinesulfinic acid C(=NNH2)OH
SO2H 6 1 HOC(=NH) C(=NH)OH MeSOH Methanesulfenic acid 1,4-Benzenedicarboximidic acid
1 = = 1-Triazene-1-sulfonic acid 8a S( NH)O2H H2NN NSO3H 32 1 2-Naphthalenesulfonimidic acid
5 4 H2NNHSO2H Hydrazinesulfinic acid
− = EtS( NH)2OH Ethanesulfonodiimidic acid − − − − − − − − − − −Ο− Η − − CH3 CH2 O CH2 CH2 O CH2 CH2 O CH2 CH2 C 2 CH CH3 15 13 10 7 4 3 2 1
= =Ν 4,7,10,13-Tetraoxapentadecane- S( NH)( NH2)OH 2-sulfonic acid (principal groups are preferred over het- 2-Naphthalenesulfonohydrazonimidic eroatoms for lowest locants) acid
H2PSO3H Phosphinesulfonic acid EtS(=NH)OH Ethanesulfinimidic acid
PhSeO3H Benzeneselenonic acid = PhSe( NH)O2H Benzeneselenonimidic acid
SeO− 2H − − − − − CH3 CH2 CH2 CH2 CH CH3 2-Hexaneseleninic acid (d) Peroxy acids are named by use of the suffixes “-peroxoic acid” (from 654321 “-oic acid”) and “-carboperoxoic acid” (from “-carboxylic acid”). Acetic acid and Benzoic acid afford systematically named peroxy acids. Sulfonic, sulfinic, TeO H − 2 and sulfenic acids and their selenium and tellurium analogs are named by use − − − of the suffixes “-sulfonoperoxoic acid”, etc. Combinations with imidic and hy- CH3 CH2 CH CH3 2-Butanetellurinic acid 4321 drazonic suffixes are made, in alphabetic order, by the normal rules. Examples:
PhTeOH Benzenetellurenic acid Ο
= Ethaneperoxoic acid (not − − CH3 C OOH Peroxyacetic acid) (c) Imidic and hydrazonic acids. Names for these are formed from the parent carboxylic, sulfonic, sulfinic, selenonic, etc., acid names by functional replacement nomenclature (¶ 129). Some modification of the formal endings is Cyclohexanecarboperoxoic acid made; thus, an “-oic acid” becomes an “imidic acid”, not an “-imidoic acid”. CO3H Acetic acid affords systematically named Ethanoic acid replacement analogs, while Benzoic acid is treated as Benzenecarboxylic acid in a similar manner. The suffixes appended to the molecular skeleton name in each case are as fol- Butanediperoxoic acid lows: HO3C(CH2)2CO3H
Parent Acid Imidic acid Hydrazonic acid MeC(=NH)OOH Ethanimidoperoxoic acid (not -oic -imidic -hydrazonic Peroxyacetimidic acid) -carboxylic -carboximidic -carbohydrazonic -sulfonic -sulfonimidic -sulfonohydrazonic -sulfinic -sulfinimidic -sulfinohydrazonic PhC(=NH)OOH Benzenecarboximidoperoxoic acid (not Peroxybenzimidic acid)
The group -S(:NH)2OH is named by the suffix “-sulfonodiimidic acid,” and Peroxy analogs of acids expressed as heading parents, e.g., Carbonoperox- -S(:NH)(:NNH2)OH by “-sulfonohydrazonimidic acid.” Selenium and telluri- oic acid (¶ 183), Phosphoroperoxoic acid (¶ 197), are ranked with the acids, um acids are named analogously. e.g., Carbonic acid, Phosphoric acid, from which they are derived. Note: Imidic acids are tautomeric with amides; except for derivatives in (e) Thio acids derived from carboxylic, sulfonic, sulfinic, and sulfenic ac- which an acid proton has been replaced, e.g., esters and anhydrides, amides ids and their imidic, hydrazonic, and peroxy replacement analogs are named by are preferred for index entries; see ¶ 122. Hydrazonic acids are tautomeric incorporating “thio” (or “dithio”) into the suffixes of the oxygenated acid with hydrazides, which are preferred as index entries. names. The terms “seleno” and “telluro” are used similarly when appropriate. Examples: Selenonic, telluronic, etc., acids are handled like sulfonic acids. The names do not distinguish between replacement of oxygen in =O and -OH groups in the MeC(=NH)OH Ethanimidic acid (not Acetimidic acid) unesterified acids. This information is usually given in the ester name, or by a substituent prefix. Examples: PhC(=NH)OH Benzenecarboximidic acid (not Ο
Benzimidic acid) = Benzenecarbothioic acid, − C SCH3 S-methyl ester
= Me(CH2)4C( NH)OH Hexanimidic acid 6 1
S O = H = Butanoic acid, 4-ethoxy-4-thioxo- (for N C(=NH)OH 1H-Pyrrole-2-carboximidic acid CH CH O−CCH CH C−OH order of precedence of acid groups, 5 1 3 2 2 2 43 2 1 see ¶ 167)
When a specific name for a single form is imperative, an italicized element HOC(=NH)(CH ) C(=NH)OH Hexanediimidic acid symbol is used in the heading parent, e.g., Ethanethioic O-acid for CH3C(S)- 2 4 OH. Replacement by two sulfur atoms in a monocarboxylic acid named by the H NNHC(=NH)OH Hydrazinecarboximidic acid suffix “-oic acid” is denoted by the suffix “-(dithioic) acid” and by one sulfur 2 atom in each of the two groups in a “-dioic acid” by “-bis(thioic) acid.” Ambi- guity is absent from “-carboxylic” names, and parentheses are therefore not MeC(=NNH )OH Ethanehydrazonic acid employed for the analogous “-carbodithioic acid” and “-dicarbothioic acid” 2 suffixes. 37 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 167
Examples: 166. Acyclic acids with cyclic substituents may usually be named by con- junctive nomenclature (¶ 124). Examples: Me(CH2)2COSH Butanethioic acid
PhCH2CH2CO2H βα Benzenepropanoic acid (not Hydrocinnamic acid) = H2C CHCS2H 2-Propene(dithioic) acid 31 CH
= 2 Benzeneacetic acid, α-methylene- (not C-COOH α Atropic acid) PhCOSH Benzenecarbothioic acid (not Benzoic acid, thio-) Benzeneacetic acid, α-(phenylmethyl- CS2H ene)- (not Benzene-2-propenoic acid, N 1-Piperidinecarbodithioic acid α-phenyl-; conjunctive nomenclature is not used with unsaturated acyclic acids CH=C−COOH (¶ 124)) α Propanebis(thioic) acid HOSCCH2COSH CH3 − − − CH2 CH SO3H Benzeneethanesulfonic acid, α-methyl- = β α HS2CCH2CH CHCH2CS2H 3-Hexenebis(dithioic) acid 16
COSH CH2SO2H 1 1-Naphthalenemethanesulfinic acid 8a 1 COSH 1,2-Naphthalenedicarbothioic acid 8a
5 4 5 4 1,3-Benzenedisulfonothioic acid (CH ) CO H 9-Anthracenepropaneperoxoic acid HSSO2 SO2SH 2 2 3 8 1 9 9a
10a 4a MeCH[S(S)SH]Me 2-Propanesulfinodithioic acid 5 10 12 3
6 MeSSH Methanesulfenothioic acid 1 HO2C(CH2)2 (CH2)2CO2H
1 1,4-Benzenedipropanoic acid S = 2-Thiophenecarboximidothioic acid 5 C( NH)SH
When conjunctive names are impermissible, e.g., for unsaturated and poly- EtS(=NH)OSH Ethanesulfonimidothioic acid functional acyclic acids, acids attached to rings by a double bond, and acids of noncarbon chains, the cyclic group is expressed as a substituent. When different numbers of sulfur atoms replace oxygen in the functional Examples: groups of polyacids, the groups of higher sulfur content are expressed as sub- 1 stituent prefixes. N Hydrazinecarboxylic acid, 2-(2- NHNHCO2H Examples: 6 pyridinyl)-
ΗΟ Η SCC 2CH2CO2H Propanoic acid, 3-(thiocarboxy)-
CH2CO2H − EtOSCCO2H Acetic acid, 2-ethoxy-2-thioxo- CHCO2H 8 1 9a Butanedioic acid, 2-(9H-fluoren- Thio peroxy acids are named by similar principles; the replacement affixes 9 9-yl)- “(thioperoxo)” and “(dithioperoxo)” are placed, in alphabetic order with other terms such as “imido” and “hydrazono,” in the “-oic,” “carboxylic,” “sulfonic,” 4b 4a etc., suffixes of the appropriate parent acids. Epoxy derivatives of acids are named as oxirane and oxetane derivatives. Examples: Example: MeCOSSH Ethane(dithioperoxoic) acid
O 2,3-Oxiranedicarboxylic acid (not Butanedioic acid, 2,3-epoxy-) PhC(=NH)OSH Benzenecarboximido(thioperoxoic) acid HO2C CO2H
6 1,4-Piperazinedicarbo(dithioperoxo)- 167. Order of precedence of acids. Acid suffixes are the most preferred HS3CN 1 NCS3H thioic acid principal group of all non-cationic substituent suffixes (¶ 106), but only one type of acid suffix may be expressed in a heading parent. Less preferred acid functions are cited as substituent prefixes. The choice is made in accordance with the following hierarchy, listed in order of descending precedence: PhSO SSH Benzenesulfono(dithioperoxoic) acid 2 (a) Peroxy acids. (Among peroxy acids, the choice depends on the nature of the parent acid as described in (b) through (i), below.) (See also the separate SSSH N ranking of peroxy carbonic and peroxy phosphorus acids at ¶¶ 183, 197.) 6 1 1-Piperidinesulfeno(dithioperoxic) (b) Carboxylic acids, followed by thio, seleno, and telluro analogs, in that acid order. The preferred acid group contains the maximum number of preferred chalcogens, oxygen being the most preferred. (For Carbonic acid, Formic ac- id, etc., see ¶ 183.) (c) Carbohydrazonic acids, followed by chalcogen analogs (see (b)). MeCHMeSSOH 2-Propanesulfeno(thioperoxoic) acid 1 2 (d) Carboximidic acids, likewise. (e) Sulfonic acids, followed by chalcogen and nitrogen analogs in the or- Ethaneselenoic acid der of (b), (c), and (d). MeCSeOH (f) Sulfinic acids, likewise. (g) Sulfenic acids, likewise. Benzenecarboselenothioic acid (h) Selenonic, seleninic, and selenenic acids, as for sulfonic acids. PhCSeSH (i) Telluronic, tellurinic, and tellurenic acids, likewise. ¶ 167 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 38
Examples: HOC(=NH)C(=NH)— (2-hydroxy-1,2-diiminoethyl)
Benzenesulfonoperoxoic acid, 4- CO — HO2C SO2OOH carboxy- (2-naphthalenylcarbonyl)
S S = − − − − = − — HO C CH2 CH2 C SH Propanethioic acid, 3-(dithiocarboxy)- CO 12 3 4 (1,2-phenylenedicarbonyl) CO — CO2H 1 6 Benzoic acid, 3,4-disulfo- O = ([1,1′-biphenyl]-4-ylcarbonyl) (not — C — SO3H (4-phenylbenzoyl) (see ¶ 133))
NH − = − − Propanoic acid, 3-imino-3-phenoxy- (1,4-phenylenedicarbonothioyl) O C CH2 COOH — CS — — CS — 32 1
When two or more like acid groups are attached to different molecular skel- N etons, or when one or more are attached to a branched skeleton, the preferred (3,5-pyridinediyldicarbonimidoyl) index name is selected according to the usual rules (¶ 138). Examples: C(=NH) C(=NH)
6 Propanedioic acid, 2-(4-carboxyphe- PhSO — (phenylsulfonyl) 1 2 HO2C CH(CO2H)2 nyl)- (principle: maximum number of the principal group) PhSO— (phenylsulfinyl)
PhS(=NH)— (S-phenylsulfinimidoyl) HO2C NHNHCO2H Hydrazinecarboxylic acid, 2-(3-car- boxyphenyl)- (principle: hetero acyclic parent preferred) PhS — (phenylthio) 169. Functional derivatives of acids. In the absence of higher functions or more preferred compound classes (¶ 106), esters are indexed in the modifica- Benzeneacetic acid, α-(cyclopentyl- PhCH(CO H)CH tion, usually at the acid name, sometimes at the alcohol (see ¶ 185). Hydrazides 2 2 methyl)- (principle: preferred ring are likewise indexed at the acid name (¶ 189). Hydrazones, azines, and semi- system) carbazones are named at hydrazonic acid parents (RC(OH):NNH2) (¶ 165). Oximes of carboxylic acids are given N-hydroxy imidic acid names; hydrates 1 and acetals (ortho carboxylic acids and their diesters) are indexed as alcohols O (or thiols). 3 2-Oxiranebutanoic acid, 2-carboxy- (CH2)3CO2H Examples: Ph 3-phenyl- (principle: largest head- CO2H ing parent) MeC(OH)3 1,1,1-Ethanetriol (not Orthoacetic acid)
CH3 Propanoic acid, 3,3,3-trifluoro-2-me- − MeC(OMe) OH F C−C−COOH thyl-2-(trifluoromethyl)- 2 Ethanol, 1,1-dimethoxy- 3 2 3 − 1 principle: maximum number of CF 3 substituent prefixes) 170. Acid halides. In this category are now included the halogenides, in which the hydroxyl groups of acids are replaced by -NC, -NCO, -NCS, -NCTe, Aldehydic, amic, anilic, hydroxamic, hydroximic, nitrolic, and nitrosolic -N3, and (in acids other than carbon acids) -CN groups. They are named by acids are indexed as compounds of mixed function (see ¶ 228). So are trivially placing the halide (etc.) term in the heading as a separate word following an named hydroxy and oxo acids, e.g., Glycolic acid, Acetoacetic acid , and amino acid term which ends as follows for various acid classes: acids, e.g., Sulfanilic acid , other than those which are of biological significance (¶ 205). 168. Acid radicals derived, by removal of hydroxyl groups, from acids ex- Acid suffix Acid halide suffix pressed as suffixes are named as compound and complex radicals. Acyl radi- -carboxylic -carbonyl cals, e.g., propionyl, naphthoyl, acetimidoyl, are no longer used as substituent -carbohydrazonic -carbohydrazonoyl prefixes; the only exceptions in general index nomenclature are acetyl -carbothioic -carbothioyl (CH3CO-) and benzoyl (C6H5CO-). Amino acid radicals, e.g., glycyl, L-alanyl, -carboximidic -carboximidoyl are restricted to use in peptide and depsipeptide names (¶ 206). -oic -oyl Radicals derived from monocarboxylic acids are in general named as (1- -hydrazonic -hydrazonoyl oxoalkyl) or (arylcarbonyl); carboximidic acids afford (1-iminoalkyl) and -thioic -thioyl (aryliminomethyl) radicals; carbothioic acids give (1-thioxoalkyl) and (arylth- -imidic -imidoyl ioxomethyl) radicals (carbonimidoyl and carbonothioyl are used only as mul- -sulfonic -sulfonyl tiplicative radicals (¶ 125) and in cases where both bonds are attached to the -sulfonimidic -sulfonimidoyl same atom); sulfonic, sulfinic, and sulfenic acid radical names are based on the parent radicals “sulfonyl,” “sulfinyl,” and “thio.” Examples: Examples: EtCO— (1-oxopropyl) Me(CH2)3CONCS Pentanoyl isothiocyanate
MeCS— (1-thioxoethyl) (not thioacetyl) O − − − − − − − − − − − − − = − CH3 CH2 O CH2 CH2 O CH2 CH2 O CH2 CH2 O CH2 C F 14 13 12 11 10 98 7 65321 4 HN=CH— (iminomethyl) (not formimidoyl) 3,6,9,12-Tetraoxatetradecanoyl fluoride —COCOCO— (1,2,3-trioxo-1,3-propanediyl)
Hydrazinecarbonyl chloride H2NNHCOCl HO2C(CH2)2CO— (3-carboxy-1-oxopropyl) 39 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 171
= Benzoyl isocyanide Examples: — C−NC
MeCONH2 Acetamide 21
PhC(=NH)Br Benzenecarboximidoyl bromide = HN NHCONH2 Diazenecarboxamide
PhCSCl Benzenecarbothioyl chloride MeCSNH2 Ethanethioamide
H NCS(CH ) CSNH2 Hexanedithioamide − − − − − 2 2 4 CH3 CH2 CH2 CH2 SO2 NCO 1-Butanesulfonyl isocyanate 16 4 312
= = H2NC( NH)CH2C( NH)NH2 Propanediimidamide N1 N′1 2 3 N′3 N3 PhS(O)(=NH)Cl Benzenesulfonimidoyl chloride = PhC( NNH2)NH2 Benzenecarbohydrazonamide N′ N
= ClCO(CH2)2COCl Butanedioyl dichloride (not MeS( NH)2NH2 Methanesulfonodiimidamide 14 Butanedioyl chloride) SO2NHMe 1 8a 1,5-Naphthalenedisulfonamide, 1 5 OCNSO2CH2(CH2)4CH2SO2NCO 1,6-Hexanedisulfonyl diisocyanate N ,N -dimethyl- 1 6 5 4 MeNHSO2
PhCH2CH2CON3 Benzenepropanoyl azide PhCSNHPh Benzenecarbothioamide, N-phenyl- Halides, etc., of peroxy acids are generally indexed as anhydrides, anhydro- H sulfides, etc., with halogen or halogenoid “oxo” acids such as Hypochlorous 7a N 1H-Indole-3-ethanimidamide or Thiocyanic acid . Halides, etc., of (thioperoxy)sulfenic acids are indexed at 1
Disulfide. 3 Examples: 4 CH2C(=NH)NH2 α N′ N ClCOSCl Carbonochloridothioic acid, anhydrosulfide with thiohypo- MeCONHCOMe Acetamide, N-acetyl- (not Diacet- chlorous acid 21N amide )
PhSO2NHSO2Ph Benzenesulfonamide, N-(phenyl- S sulfonyl)- (not Dibenzenesulfon-
= amide) C−O−CN Benzenecarbothioic acid, anhydride with cyanic acid Amides of peroxy acids and thio peroxy acids are indexed as azanyl esters (¶ 193). Amide radicals are named as compound or complex radicals based on “ami- no” or “imino,” with acid radicals (¶ 168) as substituents. Examples: AcNH— SSCl Disulfide, chloro 2-naphthalenyl (acetylamino) (not acetamido)
BzN= (benzoylimino)
When more than one acid halide residue is present in a compound, only one type is named in the heading parent. This is chosen by consideration first of the Me(CH2)4CONH— [(1-oxohexyl)amino] (not hexanamido) hierarchy of the parent acids (¶ 167) and then, if a further choice is necessary, of the following list of halides and halogenides (in descending order of prece- [(phenylsulfonyl)amino] (not dence): -F, -Cl, -Br, -I, -N3, -NCO, -NCS, -NCSe, -NCTe, -NC, -CN. PhSO2NH— Examples: benzenesulfonamido)
FSO2 PhC(=NH)NH— [(iminophenylmethyl)amino] COCl Benzoyl chloride , 3,5-bis(fluoro- sulfonyl)-
FSO2 The radicals above are employed as substituents when a more preferred amide or a higher function, e.g., an acid or acid chloride, is present in part of Butanoyl fluoride, 4-iodo-4-oxo- FCO(CH2)2COI the molecule attached to the amide by way of the nitrogen atom. Other attach- ments call for use of amino and oxo, or (aminocarbonyl), (aminosulfonyl), etc., Functional derivatives of acid chlorides are indexed similarly to those of the radicals. parent acids (¶ 169). Examples: 171. Amides are named by modification of the parent acid suffixes, thus: H2NCO(CH2)2CO2H Butanoic acid, 4-amino-4-oxo- 41 -oic acid becomes -amide -carboxylic acid becomes -carboxamide 6 -carbohydrazonic acid becomes -carbohydrazonamide 1 CO2H -carbothioic acid becomes -carbothioamide Benzoic acid, 2-(aminocarbonyl)-
-carboximidic acid becomes -carboximidamide CONH2 -sulfonic acid becomes -sulfonamide 6 1 Benzoyl azide, 4-(aminosulfonyl)- Secondary and tertiary amides are named as primary amides with N-substit- H2NSO2 CON3 uents. Anilides, toluidides, etc., are indexed as N-aryl amides. ¶ 171 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 40
+ − H2NCSCH2CONHMe Propanamide, 3-amino-N-methyl- The zwitterionic nitrilimines (RC≡Ν−Ν−R′) are named as substituted hy- 31N 3-thioxo- drazinium inner salts (¶¶ 201, 267). Examples:
MeCN Acetonitrile AcNHSO2Ph Acetamide, N-(phenylsulfonyl)-
HC≡CCH=CHCN 2-Penten-4-ynenitrile Replacement (“a”) nomenclature is employed where applicable (¶ 127) for 5 1 naming polyamides, but not peptides (¶ 206). Example: NCCN Ethanedinitrile (not Cyanogen )
H2NCOCH2CONH(CH2)2NHCOCH2CONH(CH2)2NHCOCH2CONH2 H3GeCN Germanecarbonitrile 147 1114 17
4,7,11,14-Tetraazaheptadecanediamide, H2NNHCN Hydrazinecarbonitrile 3,8,10,15-tetraoxo-
(NC)2C=C(CN)2 1,1,2,2-Ethenetetracarbonitrile Amides incorporated in a ring system are indexed at the ring name and ranked according to the nature of the suffix, if any (¶ 106), not as amides. Examples: PhCN Benzonitrile
H − − CH2 CH2 CH3 Me 6 N O 1 2-Piperidinone, 6-methyl- (ranked as − Benzeneacetonitrile, α-propyl- CH−CN a ketone) α
O 2 1,2-Benzisothiazole, 2,3-dihydro- 7a S In the presence of higher functions, nitriles are always expressed as cyano 1,1-dioxide (ranked as a non- 2NH radicals. functional cyclic nitrogen skeleton) 3 Examples: 4 Acetamide, 2-cyano- (not NCCH2CONH2 OH 2 1 Propanamide, 3-nitrilo-) 1 1,3-Dioxolo[4,5-c]pyridin-4(3aH)- O Ph 7 one, tetrahydro-7-hydroxy-2- 2 phenyl- 5 HN 4 O MeCH(CN)COCl Propanoyl chloride, 2-cyano- 3 O CO2H Benzoic acid, 2-cyano- Amides of which only the nitrogen atom forms part of a ring, formerly re- ferred to as “unexpressed amides”, are named according to the highest function CN present. 173. Aldehydes, RCHO, are named from “-carboxylic” and “-oic” acids by Examples: use of “-carboxaldehyde” and “-al” suffixes, respectively. Examples: HO Methanone, (2-hydroxyphenyl)- 1 AcH(MeCHO) Acetaldehyde 1 O 4 NCO 4-morpholinyl-
6 6 MeCH=CHCHO 2-Butenal Ethanone, 1-(4-hydroxy-1-piperidinyl)- AcN1 OH ≡ 6 HCOCH2CH=CHC CCH2CHO 3-Octen-5-ynedial 1358
1 EtCON 1 H2P(O)CHO Phosphinecarboxaldehyde SO2NH2 5 oxide Benzenesulfonamide, 4-[1-(1-oxopropyl)- 6 1H-pyrrol-3-yl]- 1 Me CHO Benzaldehyde, 4-methyl-
Amides of amino acids with trivial names are named systematically except 6 in peptide nomenclature (¶ 206). 1 CHO 1,2-Benzenedicarboxaldehyde Example: CHO
H2NCH2CONH2 Acetamide, 2-amino- (not Glycin- Benzeneacetaldehyde amide) PhCH2CHO Chalcogen analogs of aldehydes are given “-thial,” “-selenal,” “-carbothio- Oximes of amides (amidoximes) (R-C(:NOH)NH2) are tautomeric with N- aldehyde ,” etc., names. hydroxy carboximidamides (R-C(:NH)NHOH) and are indexed at imidamide In the presence of more highly ranked compound classes (¶ 106) or more names: preferred aldehydes, the -CHO group is expressed by formyl (if it does not Example: form part of an acyclic carbon chain) or by a terminal oxo radical. For thio al- dehydes, the equivalent radicals are (thioxomethyl) and a terminal thioxo rad- ical. = Me(CH2)4C( NH)NHOH Hexanimidamide, N-hydroxy- Examples:
≡ 172. Nitriles (RC N) are indexed at names derived from “-carboxylic” and HCOCH2CO2H Propanoic acid, 3-oxo- “-oic” acid names by use of “-carbonitrile” and “- nitrile ” suffixes, respectively. 41 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 174
(CH2)2CHO Benzenepropanal, 3-formyl- O= − (principle: largest heading parent) C PH2 Methanone, phenylphosphino-
CHO Methanone, 1-piperidinyl-1H-pyrrol-1-yl- CN Benzonitrile, 2-(thioxomethyl)- NCON
TeCH(CH2)2CHS Butanethial, 4-telluroxo- EtCOSiH2OH 1-Propanone, 1-(hydroxysilyl)- Substitution of the aldehydic hydrogen atom is not permitted. Examples: H2P(S)CSPh Methanethione, phenylphosphinothioyl- MeC(=NOH)NO Ethanone, 1-nitroso-, oxime (not Acetonitrosolic acid) Cyclic ketones (including those with neighboring hetero atoms) are named by appending “-one,” “-thione,” etc., suffixes to the ring names. Indicated hy-
O= drogen (required to form the ring system itself) is often chosen (where possi- C−NO Methanone, nitrophenyl- 2 (not Benzaldehyde, α-nitro-) ble) to accommodate a single ketone group (¶ 135). In other cases, added hydrogen (¶ 136) is introduced at some other position of the ring system when the ketone suffix is attached. 174. Ketones, RC(:O)R′, and their chalcogen analogs are named by use of Examples: the characteristic suffixes -one, -thione, - selone , and -tellone. (The last two classes must be differentiated from selenones and tellurones, which contain the O 3-Cyclohexen-1-one noncarbon groups -SeO2- and -TeO2-, respectively.) The carbonyl group may be attached to carbon or other elements with the exception of hydrogen and 6 1 those that could form an acid, acid derivative, or amide name. Conjunctive names are not employed for acyclic ketones attached to ring systems; instead, the cyclic portion is expressed as a substituent of the acyclic ketone parent (in 1 8a O Ph 4H-1-Benzopyran-4-one, 2-phenyl- which the oxo group may occupy the 1-position). When an acyclic ketone with (not flavone) two cyclic substituents consists only of a single carbon atom with a chalcogen 4 attached, the heading parents Methanone, Methanethione, Methaneselone 5 and Methanetellone are employed. O Examples: 1 S 6 MeCOMe 2-Propanone (not Acetone ) 4H-Thiopyran-4-one, 2,3-dihydro- 1 2 3 4 (not 2H-Thiopyran-4(3H)-one)
O MeCH2OSiH2(CH2)2CO(CH2)2SiH2OCH2Me 134137 10 11 S
3,11-Dioxa-4,10-disilatridecan-7-one 7a 1 3 1H-Indene-1,3(2H)-dithione
MeCOCH2COCH=CH2 5- Hexene -2,4-dione (not 1-Hexene- 4 S 1 6 3,5-dione) 1 S 5 4-Thiazolidinone H C=C=O Ethenone (not Ketene ) 3 2 NH O
Me(CH2)3CH=C=O 1-Hexen-1-one 61 H 1H-Indole-5,6-dione, 2,3-dihydro- O 7a N (the two oxo substituents can be MeCSeCH2Me 2-Butaneselone 1 14 added as a pair without hydrogen being added elsewhere to the ring O 3 4 system) MeCOCH2CSCH2Me 2-Hexanone, 4-thioxo- (not 2,4- 16 Hexanedione, 4-thio-) O O 8 9a 1,9,10(2H)-Anthracenetrione, 3,4- CH2CH2COMe 2-Butanone, 4-cyclohexyl- 9 1 41 dihydro- (the added hydrogen is 10 cited in the lowest-numbered 10a 4a 5 available position) 1-Hexanone, 1-phenyl- (not Hexanal, PhCO(CH2)4Me O 1-phenyl-) In the presence of higher functions or more preferred ketones, oxo, thioxo, Methanone, cyclohexylidene- selenoxo, and telluroxo radicals, are used. The = CO group, when not part of C=O an acyclic chain or a ring, is expressed as carbonyl; the chalcogen analogs (car- bonothioyl, carbonoselenoyl, and carbonotelluroyl) are used when they are not part of a chain, and when, in addition, they are either bonded to a single atom Ph2CO Methanone, diphenyl- (not Benzophenone ) or used as multiplicative radicals. The trivially named radicals benzoyl, for (oxophenylmethyl), and acetyl, for (1-oxoethyl), are used when appropriate. Examples: ′ 6 1 1 6′ ′ ′ NN Methanone, 1,1 -[2,2 -bipyridine]- 5,5′-diylbis[1-phenyl- (a multipli- Butanoyl bromide, 3-oxo- PhCO — — COPh MeCOCH2COBr cative name (¶ 125)) (not Acetyl bromide , acetyl-)
MeCO COEt N N 1-Propanone, 1-(4-acetylphenyl)- — COCO — 1,2-Ethanedione, 1,2-di-2-pyridinyl-
S CO H 2H-Thiopyran-3-carboxylic acid, 2 3,4-dihydro-4-thioxo- AcN=NNH Ethanone, 1-(1-triazen-1-yl)- S ¶ 174 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 42
HCOCH2COCO2H Butanoic acid, 2,4-dioxo- (not 6 CH=CHCH OH Phenol , 2-(3-hydroxy-1-propen-1-yl)- 41 2 Propanoic acid, 3-formyl-2-oxo-) (not 2-Propen-1-ol, 3-(2- 1 OH hydroxyphenyl)-) Hydrazinecarboxylic acid, 2-(cyclo- CONHNHCO2H 41 propylcarbonyl)- O(CH2)9OH Phenol, 2-[(9-hydroxynonyl)oxy]- (principle: a cyclic parent is OH preferred (¶ 138))
N CN Benzonitrile, 3-(2-pyridinylthioxo- — CS — methyl)- (not Benzonitrile, 3-(2- HSCH2CH2OH Ethanol, 2-mercapto- pyridinylcarbonothioyl)-) 2 1
Hydroxy derivatives of thiophene, selenophene, and tellurophene are in- dexed at Thiophene-ol, etc., (the final “e” of the parents being left unelided to S=C CO2H Cyclopentanecarboxylic acid, 3- avoid confusion with chalcogen analogs of Phenol). The locant is placed im- carbonothioyl- mediately before the suffix. Example:
1 1 HTe 5 S 8a N 1,3-Butanedione, 4-(4-oxo-3(4H)- quinazolinyl)-1-phenyl- (princi- Thiophene-3-ol, 5-telluryl- 4 NCH2COCH2COPh ple: maximum number of the 41 OH 5 principal chemical functional O group (¶ 138)) Hydroxy, mercapto, selenyl, and telluryl groups attached to hetero atoms other than silicon are always expressed as prefixes (unless they form part of an acid functional parent compound (¶ 130)). “Esters” of such groups are also ex- 175. Alcohols (and phenols) and their chalcogen analogs (thiols, selenols, pressed as prefixes. and tellurols) are expressed by the suffixes -ol, -thiol, - selenol , and -tellurol, at- Examples: tached to a carbon or silicon atom of a molecular skeleton name. The only triv- ial name employed in CA indexes for a compound of this class is Phenol (for H2NNHSH Hydrazine, mercapto- Benzenol). Phenols as a class are treated precisely like alcohols, the choice of index name for a compond containing alcoholic and phenolic groups depend- ing on the usual rules (¶ 138). Alcoholic groups and their analogs are expressed Me2BOH Borinic acid, B,B-dimethyl- as hydroxy, mercapto, selenyl, and telluryl prefixes on more preferred heading parents. Examples: OH 8a N 1 3-Quinolinethiol, 1,2-dihydro-1- EtOH Ethanol (not Ethyl alcohol) hydroxy-
H2C=CHCH2OH 2-Propen-1-ol (not Allyl alcohol ) 31
HO− O = MeCH(OH)Me 2-Propanol (not Isopropyl alcohol ) P OH 2-Phosphorinol, 1,2-dihydro-1- 32 1 hydroxy-, 1-oxide H2C=CHCH2CH(SH)CH2Me 5-Hexene-3-thiol 6321
6 MeSiH2OH Silanol, 1-methyl- 1 NOAc Acetic acid, 1-piperidinyl ester (not Piperidine, 1-(acetyloxy)-)
Me(CH2)2CH2TeH 1-Butanetellurol 4 1 SSO Ph
− 2 CH2-(CH2)11-S-CH2-CH2-O-CH2-CH2-O-CH2-CH2-O-CH2-CH2-OH 9 1 24 12 11 10 9 8 7 6 5 4 3 2 1 As 10a Benzenesulfonothioic acid, S-10H- 10 phenoxarin-10-yl ester (not 3,6,9-Trioxa-12-thiatetracosan-1-ol 10H-Phenoxarsine, 10-[(phenyl- O 6 5 4 sulfonyl)thio]-) PhSeH Benzeneselenol O OH 1 1-Naphthalenol (not 1-Naphthol) 7a 8a 1 Propanedioic acid, 1-(1,3-di- NOCOCH2CO2H 3 hydro-1,3-dioxo-2H-isoindol- 2-yl) ester (not Propanoic acid, 5 4 4 O 3-[(1,3-dihydro-1,3-dioxo-2H- isoindol-2-yl)oxy]-3-oxo-) SH SH — — 2,3-Butanedithiol CH3-CH-CH-CH3 4321 176. Amines are always named as primary amines, RNH2, or their N-deriv- atives, by attaching the suffix “-amine” to the name of a molecular skeleton, cyclic or acyclic. Attachment may be at a carbon or hetero atom. Trivial names,
HO-SiH2-SiH2-OH 1,2-Disilanediol e.g., Aniline , and radicofunctional names, e.g., Methylamine , are not used. Examples:
OH MeNH Methanamine (not Methylamine) 1 2 6 1,3-Benzenediol
OH MeCH(NH2)Me 2-Propanamine (not Isopropylamine ) 12 3
H2C=CHCH(NH2)Me 3-Buten-2-amine 6 42 1 1 CH2OH Benzenemethanol, 2-hydroxy- (not Phenol, 2-(hydroxymethyl)-) OH (principle: largest parent) H3PbNH2 Plumbanamine 43 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 177
H2PPHNH2 Diphosphinamine H3SiNHSnH3 Silanamine, N-stannyl- (principle: preferred hetero atom)
PhNH2 Benzenamine (not Aniline) Cyclohexanamine, N-octyl- (principle: Me(CH2) 7NH2 1 a cyclic parent is preferred) NH2 6 Bicyclo[2.2.1]hept-5-en-2-amine 7 NH2 NH2 6 1 1,2,4-Benzenetriamine, N2-(3,5-di- 4 NH aminophenyl)- (principle: lowest locants for principal groups) H2NBiHNH2 Bismuthinediamine H2N NH2 1 O 5 ′ 2AsNH2 1,3,2-Dioxarsolan-2-amine Me2NONMe2 Methanamine, N,N -oxybis[N- methyl- (principle: multiplication) O3
H 1H-Pyrrol-2-amine N NH MeNHNH NH NHMe 5 1 2
1,4-Benzenediamine, N1,N4-bis- 1,2-Ethanediamine (not Ethylene - H NCH CH NH [4-(methylamino)phenyl]- 2 2 2 2 diamine) 21 (principle: centrality)
Replacement names are employed for acyclic secondary and tertiary amines H2NCH2CH(NH2)Me 1,2-Propanediamine 12 3 provided that the “-amine” suffix of the “aza” name expresses at least the same number of such groups as the conventional name and that other requirements 6 1 NH2 1,2-Benzenediamine (not 1,2-Phe- (¶ 127), e.g., the presence of a minimum of four hetero units in the molecular nylenediamine) skeleton, are satisfied. Examples: NH2 α PhCH2CHMeNH2 Benzeneethanamine, -methyl- EtNHCH2CH2NH[(CH2)2NH]3CH2CH2NHEt β α N1 13 13 14 N14 1 3,6,9,12-Tetraazatetradecane-1,14- N 6 5-Pyrimidinemethanamine diamine, N1,N14-diethyl-
N 3 H2NCH2 CH3-NH-CH2-CH2-NH-CH2-CH2-NH-CH2-CH2-NH-CH2-CH2-NH-CH3 N1 12N2 When higher functions (¶ 106) or more preferred amine parents are present, 1,2-Ethanediamine, N1-[2-(methyl- the prefix “amino” is employed. 2 Examples: amino)ethyl]-N -[2-[[2- (methyl- amino)ethyl]amino]ethyl]- (not 3,6,9-Triazaundecane-1,11- 1,2-Propanediamine, 3-(4-amino- diamine, N1,N11-dimethyl-; this H2NCH2CH(NH2)CH2NH2 phenyl)- (principle: maximum organic replacement name expresses number of the principal group) only three hetero units) (not 2,5,8,11,14-Pentaazapentadecane; this name does not express the H N(CH ) SiH NH Silanamine, 1-(5-aminopentyl)- amino groups as a substituent suffix) 2 2 5 2 2 (principle: hetero atom parent preferred) Schiff bases (anils, azomethine compounds) contain the -N=C- grouping 6 α and are therefore both amines and imines. They are indexed as amines in the 1 H2NCH2NH2 Benzenemethanamine, 4-amino- absence of higher functions. (principle: largest heading parent) Example:
NH2 NH2 NH2 PhN=CHPh Benzenamine, N-(phenylmethylene)- Me Me CH2 CH2 N-Hydroxy amines are named as such, not as hydroxylamine derivatives. Amine oxides are named by citation of the additive term “N-oxide” in the mod- Me Me ification. Benzenamine, 2,6-bis[(2-amino- Examples: 3,5-dimethylphenyl)methyl]- PhNHOH Benzenamine, N-hydroxy- (principle: centrality)
EtN(O)Et2 Ethanamine, N,N-diethyl-, Secondary and tertiary amines, RR′NH and RR′R′′N, are named as deriva- N-oxide tives of primary amines by application of the usual criteria (¶ 138). Examples: 177. Imines are ranked as the lowest compound class named by use of a functional suffix. The “-imine” suffix is attached to a cyclic or acyclic molec- MeCH2NHCH2Me Ethanamine, N-ethyl- (not Diethy- 2 1N ular skeleton (at a carbon or hetero atom). Indicated and added hydrogen (¶¶ lamine) 135, 136) for cyclic imines are assigned as for the analogous ketones (¶ 174). N- Alkyl , N-aryl, etc., imines are indexed as amines (¶ 176). Conjunctive no- menclature is used for imines when the molecular skeleton to which a single (Me CH) N 2 3 2-Propanamine, N,N-bis(1- function is attached is itself connected to a ring system by a single bond . methylethyl)- (not Examples: Triisopropylamine ) MeCH=NH Ethanimine (not Ethylideimine) 21 N O O 2-Furanamine, N-2-furanyl- NH MeC(=NH)Me 2-Propanimine 1 2 3
HP=NH H2N-CH2-CH2-CH2-NH-CH2-CH2-CH2-NH2 Phosphinimine 3 1 N 321 N NH 1,3-Propanediamine, N1-(3- aminopropyl)- 5 1 2,4-Cyclopentadien-1-imine ¶ 177 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 44
1 4H-Pyran-4-imine, tetrahydro- (not In the presence of any other chemical function expressible as a suffix, imi- O 6 2H-Pyran-4(3H)-imine, dihydro-) nes are described by substituent prefixes. The =NH group is named imino; the =C=NH group is expressed as carbonimidoyl in a multiplying radical or when attached to a single atom; the -CH=NH group is named (iminomethyl) (not formimidoyl) unless the methyl group is part of an acyclic carbon chain. NH Examples:
HN=C=NH Methanediimine (not Carbodiimide ) PhC(=NH) SO2NH2 Benzenesulfonamide, 3-(imino- phenylmethyl)-
= = Me2CHN C NCHMe2 2-Propanamine, N,N′-methanetet- raylbis- (not Methanediimine, N,N′-bis(1-methylethyl)-) HN=CHCH2CN Propanenitrile, 3-imino- 3 21
MeCH=N(O)Cl Ethanimine, N-chloro-, 21 N OHC CHO N-oxide C(=NH) C(=NH) Et N NH 6 1 2(1H)-Pyridinimine, 1-ethyl- Benzaldehyde, 3,3′-(1,4-phenyl- enedicarbonimidoyl)bis- 1 NH NH 7a O 8 1 9a 9,10-Anthracenediimine S 1,3-Benzoxathiol-4-ol, 2-imino- 9 3 4 10 10a 4a OH 5 NH EtC(=NH) OH Phenol, 3-(1-iminopropyl)- = = ′ Ph2C NSSN CPh2 Benzenemethanimine, N,N -dithio- bis[α-phenyl- (principle: multipli- cation of a conjunctive name) 45 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 179
D. COMPOUND CLASSES
Introduction ¶ 178 Hydrazones ¶ 190 Anhydrides 179 Imides 191 Anions 180 Molecular addition compounds 192 Antimony and Bismuth compounds 181 Nitrogen compounds 193 Boron compounds 182 Organometallic compounds 194 Carbonic acid and relatives 183 Oximes 195 Cations 184 Oxygen compounds 196 Esters 185 Phosphorus and Arsenic compounds 197 Ester-anhydrides 186 Salts 198 Free radicals 187 Silicon, Germanium, Tin, and Lead compounds 199 Halogen and Halogenoid compounds 188 Sulfur, Selenium, and Tellurium compounds 200 Hydrazides 189 Zwitterionic compounds 201
178. Introduction. Previous sections have dealt with general principles and Anhydrides of monobasic organic acids with polybasic acids are indexed at with the naming of specific compounds by combination of a molecular skele- the name of the preferred acid. The terms “di,” etc., are used to indicate the ton name with a suffix which describes the principal chemical function. The number of molecules of water lost in anhydride formation. Locants are cited for present section discusses nonfunctional compound classes (those that fall be- polybasic organic acids or when the parent is multiplied. A ratio is cited for an- low imines in order of precedence (¶ 106)), and classes of compounds such as hydrides of an unmultiplied monobasic acid with an unmultiplied mononuclear esters, free radicals, ions , addition compounds, oxo acids, and carbonic acid polybasic acid and for anhydrides of two different unmultiplied polybasic relatives, which are named by application of principles already discussed. The mononuclear acids. order is alphabetic by class. Examples: 179. Anhydrides of acid groups, at least one of which is expressed as a
functional suffix (“-oic acid,” “-carboxylic acid,” “-sulfonic acid,” etc.), are O= O= O= Propanedioic acid, − − − − − − indexed, if cyclic, at heterocycle names and, if acyclic, either at “a” names CH3 C O C CH2 C OH 1-anhydride with acetic acid (¶ 127) or at acid heading parents with the term “anhydride” in the modifica- tion. (Acyclic anhydrides of certain mononuclear “oxo” acids, e.g., Carbonic
acid, Phosphonic acid, are indexed at such headings as Dicarbonic acid, CH− 3 − − − − − − − Triphosphonic acid.) CH3 SO2 O SO2 CH CH2 CH2 SOH Replacement (“a”) names are used for acyclic anhydrides when the suffix 32 1 expresses no lower functionality than the heading parent of the regular substi- 1,3-Butanedisulfonic acid, tutive name and the other requirements (¶ 127), e.g., that the molecular skele- 3-anhydride with methane- ton contain at least four hetero units, are satisfied. sulfonic acid (not “3-mono- Example: anhydride with...”)
HO2CCH2COOSO2CH2SO2OCOCH2CO2H O O 134578 11 = = Benzoic acid, C−O−P−OH
4,8-Dioxa-5,7-dithiaundecanedioic − anhydride with phosphoric acid, 3,9-dioxo-, OH acid (1:1) 5,5,7,7-tetraoxide Symmetrical anhydrides of monobasic organic acids are indexed at the acid O O = = CH −CH −C−O−Β−O−B−O−C−CH −CH heading parent with the term “anhydride” in the modification. Locants are used 3 2 − − 2 3 when the parent is multiplied. Unsymmetrical anhydrides of monobasic acids, − − − − − − CH3 CH2 C= O O C= CH2 CH3 at least one of which is organic, are indexed at the name of the preferred acid O O (¶¶ 167, 138) with an “anhydride with” phrase in the modification. Anhydrides Propanoic acid, of Hydrazinecarboxylic acid and related compounds are given special treat- 1,1′,1′′,1′′′-tetraanhydride with ment (see the final example below). boric acid (H4B2O5) Examples: O O O O = = = =
O= O= Propanoic acid, − − − − − − − − − − − − Cl CH2 C O C CH2 O CH2 C O C CH2 Cl − − − − − − ′ 12 2′ 1′ CH3 CH2 C O C CH2 CH3 1,1 -anhydride (Locants are used because the Acetic acid, 2,2′-oxybis-, parent is multiplied.) 1,1′-dianhydride with 2-chloro- acetic acid (principle: centrality O O = = Benzoic acid, 4-chloro-, (¶ 138)) Cl 4 1 C−O−C Cl 1,1′-anhydride O = HOOC−CH −CH −C−O−CN Butanedioic acid, Benzoic acid, 2 2
O= O= 1-anhydride with cyanic acid C−O−C−CH anhydride with acetic acid 3 (The parent is not multiplied so there are no locants.) O = − − − HOOC 4 1 C O SO2 OH 1,4-Benzenedicarboxylic acid,
O= O= Acetic acid, 1-anhydride with sulfuric acid − − − = S O C CH3 anhydride with benzenesulfonic O acid Acyclic anhydrides of different polybasic acids require locants. Examples: O Acetic acid, = O O O − − = = = CH3 C ONO anhydride with nitrous acid HOOC 4 1 C−O−C−O−C COOH
O O Benzoic acid,
= = 1,4-Benzenedicarboxylic acid, − − − − anhydride with methyl hydrogen C O C O CH3 1,1′-dianhydride with carbonic carbonate (the ester name is acid given in its uninverted form (¶ 185); not Benzoic acid, anhydride with methoxyformic acid) O O = = HO−SO −O−C−CH −C−O−SO −OH Propanedioic acid, O O 2 2 2 = = 1,3-dianhydride with sulfuric − − Dicarbonic dihydrazide, 2-methyl- H2NNHC O CNHNHCH3 acid (see also ¶ 183) ¶ 179 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 46
OH COOH COOH 1,2-Benzenedicarboxylic acid, − −( ) − − −Β− − − −( ) − −
H C N− CH B− O − O B CH N CH 3 2 4 2 4 − 3
O= O= 4-methyl-, CH OH (CH ) 3 − 2 4 CH3 1′ C−O−C 2 ′ ′ 1 3 1,2 -anhydride − − 2 ′ H3C N CH3 4 (formerly bimol. 1,2 -monoan- Boronic acid , B-[4-(dimethy- hydride) CH3 lamino)butyl]-, B,B′:B′,B′′-, CH3 dianhydride (formerly Boronic acid, [4- (dimethylamino)butyl]- O O O O O O
======trimol. dianhydride) − −( ) − − − −( ) − − − −( ) − − Η HO C CH2 4 C O C CH2 4 C O C CH2 4 C O Unsymmetrical anhydrides of “oxo” acids in general are indexed by use of Hexanedioic acid, ′ ′ ′′ “anhydride with” phrases at the preferred acid component heading parents, but 1,1 :6 ,1 -dianhydride cyanic acid anhydrides with mononuclear arsenic and phosphorus acids are ex- (formerly trimol. dianhydride) pressed by means of “cyanatido” replacement affixes or by the class term “cy- anate.” Examples: When the acid group which has undergone anhydride formation would have been expressed (in its unmodified form) as a substituent of the preferred head- O = CH −P−O−CN ing parent, the anhydride is likewise expressed as a (more complex) substituent 3 − Phosphonocyanatidic acid, P-methyl- of the same parent. Anhydride formation of an acid group expressed as a sub- OH stituent in an “anhydride with” phrase is treated similarly. Examples: O = CH −P−O−CN Phosphinic cyanate , P,P-dimethyl- 3 −
O= Benzoic acid, 4-[(acetyloxy)- − − − H3C CH3 C O SO2 COOH sulfonyl]- Anhydrosulfides of “oxo” acids are generally named analogously by use of “anhydrosulfide” terms. Other chalcogens are treated similarly. When sulfur, Benzoic acid, etc., replaces oxygen in Diphosphonic acid and similar compounds, the non- O= O= O= detachable prefixes “Thio,” etc., are employed. The number of sulfur atoms is − − − − − − − anhydride with 2-[(acetyloxy)- C O C CH2 SO2 O C CH3 sulfonyl]acetic acid not indicated in the name; instead, a synonym line formula is always cited. Example:
Chalcogen analogs of acyclic anhydrides are indexed like anhydrides. = = Thiodiphosphonic acid H−P−S−P−H When the oxygen atom connecting the acid residues has been replaced, the − − ((HO)HP(O)SHP(S)SH) terms “anhydrosulfide,” “anhydroselenide,” and “anhydrotelluride” are used, OH SH and the sulfur, selenium, or tellurium is indicated in the names of both acid components. The peroxy analogs of this kind of “oxo” acid are indexed (with synonym line Examples: formulas) at such headings as Thioperoxydiarsonic acid ([(HO)HAs(S)]2S2). Anhydrides of mononuclear peroxy “oxo” acids are generally named at Perox- ide, Disulfide, etc.
S= S= − − Benzenecarbothioic acid, Examples: C O C 1,1′-anhydride (not “anhydrosul- fide”) O O
= = Peroxide, carboxy formyl HC−O−O−C−ΟH
O O = = H−P−S−S−S−ΟH Disulfide, hydroxyphosphinyl sulfo −Τ − Benzenesulfonotelluroic acid, − − SO2 e SO2 1,1′-anhydrotelluride OH OH Cyclic anhydrides, anhydrosulfides, etc., are indexed like other heterocy- clic compounds. Examples:
O= O= Benzenecarboselenoic acid, C−Se−C−CH 3 anhydroselenide with ethane- O 1,3-Isobenzofurandione (not 1,2- selenoic acid 7a 1 2O Benzenedicarboxylic acid, cyclic 3 anhydride) 4 O
O= Se= Propanethioic acid, CH −CH −C−S−P−CH −CH anhydrosulfide with O-ethyl 3 2 − 2 3 1 O−CH −CH hydrogen P-ethylphosphonosel- O S O 2 2 3 2 Naphth[1,2-c][1,2,5]oxadithiole, enothioate 9b 3 9a SO2 1,1,3,3-tetraoxide (not 1,2- Naphthalenedisulfonic acid, 4 cyclic anhydride) 6 5 MeC(=NOH)SCN Ethanimidothioic acid, N-hydroxy-, anhydrosulfide with thiocyanic acid O
7a 1 Benzo[c]thiophene-1,3-dione Symmetrical anhydrides of the monobasic “oxo” acids Formic, Phosphin- S 2 3 ic, Arsenic, Phosphinous, and Arsinous acids (and their substituted deriva- tives) are indexed by citation of the simple term “anhydride” in the 4 O modification. Symmetrical anhydrides of the dibasic “oxo” acids Phosphonic, Arsonic, Phosphonous, and Arsonous acids and their substituted derivatives 180. Anions. Index names for anions are required as sole entries when an- are indexed at Di-, Tri-, etc., acid headings. ions themselves are being studied, and as additional entries in the indexing of Example: salts (¶ 198). Anions are often expressed differently as modification terms at cationic heading parents. Anions from unsubstituted Ethyne, Arsine, Phosphine, Stibine, Silane MeAs(OH)OAs(OH)Me Diarsonous acid, dimethyl- − (Si4 only), and Hydrazine are named Acetylide , Arsenide , Phosphide , An- timonide, Silicide, and Hydrazide. Synonym line formulas are used, e.g., Acetylide (C 2−), except for Hydrazide (which is H NNH−) and Silicide, and Anhydrides of boron acids are named as anhydrides. 2 2 for Arsenide, Phosphide, and Antimonide when all hydrogens have been lost. Examples: Anions derived from compounds with names based on substitutive parent compounds (¶ 130) other than those just described are named at the heading PhBMeOBMePh Borinic acid, B-methyl-B-phenyl-, parent for the neutral compound with a modification term such as “ion(1−),” B,B′-anhydride “ion(2−),” or (if indefinite) “ion (neg).” Anions from esters of “oxo” acids are (not Borane, oxybis[methylphenyl-) named similarly. 47 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 181
Examples: Radical ions are named at the neutral compound heading with “radical ion” terms in the modification. 1 1,3-Cyclopentadiene, Examples: − − − HC ion(1 ) • Naphthalene, radical ion(1−)
− MeCH2CH2O 1-Propanol, 31 − ion(1 ) (not Propoxide) O • − 1 O 6 2,5-Cyclohexadiene-1,4-dione, − O2N 1 radical ion(1 ) 6 NO2 Phenol, 2,4,6-trinitro-, − ion(1 ) − O
NO At cationic index headings, e.g., Ethanaminium (see ¶¶ 184, 198), anions 2 are expressed by “-ide” or “-ate” terms, as described below, or by “salt with” phrases. The “salt with” phrase is followed by a ratio, e.g., (1:1). − 6 CO Modification terms for unsubstituted carbanions from acyclic and monocy- 1 2 1,2-Benzenedicarboxylic acid, − clic hydrocarbons are derived by adding “-ide” to the hydrocarbon name after ion(2 ) elision of the final “e,” e.g., “benzenide,” “cyclopentadienide.” Unsubstituted − CO2 acids expressed as principal groups (e.g., carboxylic and sulfonic acids) afford anions which are named by “-ate” terms in the modification, e.g., “acetate,” − “1,2-benzenedicarboxylate.” Phosphonic acid, Carbamic acid, and other Me As Arsine, dimethyl-, 2 ion(1−) substitutive functional parent acids, whether substituted or not, also provide anions which are named in modifications by means of “-ate” terms, e.g., “phos- phonate,” “dimethylcarbamate.” Similar terms are used for anions from partial − EtP(O)(OH)O Phosphonic acid, P-ethyl-, esters of polybasic “oxo” acids. ion(1−) Examples: O = − − CH −P−O P-methyl O-methylphosphonate PhOSO3 Sulfuric acid, 3 − − monophenyl ester, ion(1−) O CH3
− MeO3SCH2CH2SO3 1,2-Ethanedisulfonic acid, O= 1 2 − − − − O-methyl carbonothioate 1-methyl ester, ion(1−) CH3 O C S
− Certain resonance -stabilized anions and cations containing hetero atoms are CO2 indexed by CA at names corresponding to preferred canonical structures. In the 1-methyl 1,2-benzenedicarboxylate same manner as the analogous tautomeric compounds (¶ 122), anions are nor- malized, i.e., recognized as equivalent, by machine programs, regardless of CO2Me how the structures are shown in the original documents. Each ion is assigned a single CAS Registry Number and a unique CA index name. O = Resonance-stabilized anions of the general formula − CH −O−P−O 3 − P-methyl phosphate −− − M=Q−Z M−Q=Z O Anions from the unsubstituted alcohols and phenols Methanol, Ethanol, in which Q = C, N, S, P, Sb, As, Se, Te, Br, Cl, or I, and M and Z represent any 1-Propanol, 1-Butanol, and Phenol are named by “-oxide” terms, e.g., “pro- combination of trivalent N and/or bivalent O, S, Se, or Te atoms are normalized poxide,” “phenoxide”. Loss of hydrogen from the mercapto group of unsubsti- in this way. The formula is analogous to that for normalized tautomeric com- tuted Benzenethiol is expressed as “benzenethiolate”. pounds (¶ 122), with a negative charge replacing the hydrogen atom, and the In all other cases, anion names at cation headings are replaced by “salt with” requirements described for them apply equally to normalized anions. The phrases; it is to be understood that, in a complete salt name, a ratio would al- names are derived by the same structural rules, and are identical except for ad- − ways be added when known. dition of the index modification term “ion(1 )”. Examples: Examples:
− − salt with 4-methoxyphenol (not O O MeO O
= − − “4-methoxyphenoxide”) EtC−NH EtC=NH
Propanamide, NOT Propanimidic acid, − − ion(1 ) ion(1 ) PhNH— salt with benzenamine
3 1 4 NO2 N N 7a − − O NO N N 2 salt with 2,4,6-trinitrophenol (not 7a − Cl 1 Cl 4 3 “picrate”) NO2 1H- Benzimidazole , NOT 1H-Benzimidazole, 6-chloro-, 5-chloro-, ion(1−) ion(1−) 181. Antimony and Bismuth compounds, are conveniently discussed to- gether because of the close similarity in the indexing treatment of their deriva- tives. Antimony and bismuth are metals (¶ 215), and their salts are named as Negative ions from tautomeric pyrazole and tropolone systems are not normal- such, not as cyclic or acyclic molecular skeletons. (Prior to CA Volume 95 (see ized by the CAS Registry System; their preferred structures and names are ¶ 101), antimony was classed as a nonmetal for indexing purposes; now, it and based on nomenclature rules, such as low numbering for principal functional bismuth are treated alike.) groups, low numbering for substituents, etc. Hydrides of trivalent antimony and bismuth are named Stibine and Bis- muthine, respectively; polymolecular saturated and unsaturated hydride 1 2 chains have names such as Distibine, Distibene, Tribismuthine. The mono- Me N Me N − nuclear oxide heading parents Stibine oxide and Bismuthine oxide are em- N 2 − N 1 ployed, as are also the analogous names for the sulfides, etc., and imides. In 5 heterocyclic compounds the valency is understood to be three unless an abnor- CN CN mal valency can be expressed in the name (¶ 158). 1H-Pyrazole-3-car- NOT 1H-Pyrazole-5-car- Examples: bonitrile, 5-methyl-, bonitrile, 3-methyl-, − − Bismuthine, triethyl- ion(1 ) ion(1 ) Et3Bi ¶ 181 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 48
EtSbCl2 Stibine, dichloroethyl- Acyclic carbon chains containing boron atoms are given “a” names if the requirements (¶ 127) are met. Example: Ph2BiI Bismuthine, iododiphenyl- MeB(Me)CH2O(CH2)2OCH2B(Me)Me 4,7-Dioxa-2,9-diboradecane, 147102 9 2,9-dimethyl-
(F3C)2SbSbMe2 Distibine, 1,1-dimethyl-2,2-bis- 21 Heterocyclic boron compounds and their derivatives are named by the usual (trifluoromethyl)- procedures (¶ 146). Intramolecular coordination bonds between boron atoms and other hetero atoms are ignored in naming; thus, a zwitterionic ring bond between boron and phosphorus in the last example below is disregarded, and PhSb=SbPh Distibene, 1,2-diphenyl- the monocyclic system is named. (A monocycle entirely dependent on such a bond is named as an acyclic compound.) Examples: Ph2Sb(O)OH Stibine oxide, hydroxydiphenyl- H B 1H-Borole Stibinamine, 5 1 H2Sb(O)NH2 1N 1-oxide H 6 N 2 EtBiH2=NMe Bismuthine imide, 1-ethyl-N- HB 1 BH Borazine 1 N methyl- HN 4 NH H 5 B 3 Sb H 1 5 1H-Stibole 1 7a O 2 B(CH2)2CN 1 1,3,2-Benzodioxaborole-2-propan- S 7a 2 3 enitrile BiH 1,3,2-Benzodithiabismole O 4 S 3 8 4 1 P Heterocyclic antimony and bismuth compounds without functional suffixes 1,5-Phosphaborocine are ranked in accordance with the seniority of ring systems (¶ 138). Nonfunc- B tional acyclic antimony substitutive parent compounds follow arsenic com- 5 pounds in order of precedence (¶ 106) and are followed in turn by bismuth and Boron molecular skeletons fall between nonfunctional bismuth and silicon then boron parents. Within each class the order is determined by the number of compounds in the order of precedence of compound classes (¶ 106). Within the hetero atoms, then unsaturation, size and additive hetero atoms, as illustrated boron class, the descending order is carbapolyboranes, hetero polyboranes, po- by the following descending order of antimony compounds: Tristibine, Dist- lyboranes, heterocyclic boron compounds, and finally Borane. When more ibene, Distibine, Stibine oxide, Stibine sulfide, Stibine imide, Stibine. In the preferred groups or molecular skeletons are present, boron substituent prefixes presence of more preferred compound classes, the following substituent prefix- (¶ 161) are used. − − es are employed. (The substituent prefixes stiboso ( SbO), stibo ( SbO2), sti- Examples: − binico (=Sb(O)OH), and stibono ( Sb(O)(OH)2) were used prior to CA Volume 95 (see ¶ 101).) Me3B Borane, trimethyl- Substituent Substituent Prefix Prefix H2SbBH2 Stibine, boryl- − − SbH2 stibino BiH2 bismuthino
Me2BNHMe Boranamine, N,1,1-trimethyl- =SbH stibylene =BiH bismuthylene 1 N (principle: heteroatom molecular skeleton preferred) ≡Sb stibylidyne ≡Bi bismuthylidyne 3 1 1′ − − NBPhN Sb=Sb 1,2-distibenediyl 3′ Aziridine, 1,1′-(phenylborylene)bis- Examples: H Cl Acetic acid, 2-[[4-(diiodostibino)- H2B B Diborane(6), 1-chloro- H HO2CCH2S SbI2 phenyl]thio]- H When the position of substituent suffixes or prefixes cannot be related to the accepted numbering of polyboranes and hetero polyboranes (as illustrated in the Ring Systems Handbook and current Chemical Substance Index), no numer- PhN=BiI Benzenamine, N-(iodo- ical locants are used, but capital italic letters may be cited to denote substitution N bismuthylene)- on a “hetero” atom in a hetero polyborane. Trihydroxy and hydroxy oxo derivatives of Stibine, Bismuthine, Stibine Examples: oxide, Bismuthine oxide and their chalcogen analogs are given binary oxide, hydroxide , etc., names (with synonym line formulas) such as Antimony hy- 1,2-Dicarbadodecaborane(12), B10H9 B-(acetyloxy)- droxide (Sb(OH)3), Bismuth hydroxide (Bi(OH)3), Antimony hydroxide oxide (Sb(OH)O) and Bismuth hydroxide oxide (Bi(OH)O). (Antimonic AcO acid headings were used prior to CA Volume 95 (see ¶ 101).) Dicarbadodecaborane(12), C,C′- Halo, alkoxy, and aryloxy derivatives of Stibine, Bismuthine and their ox- B10C2Me2H10 ides are so named; amino derivatives are named at Stibinamine, Stibinedi- dimethyl- amine, Bismuthinamine, etc. Substitution of bridging hydrogen of a polyborane or hetero polyborane is Examples: indicated by the prefix “µ” (mu); when necessary, the locants of the boron- MeOSbCl Stibine, dichloromethoxy- atom bridgeheads are cited. 2 Examples: H (HO) Sb(O)NH Stibinamine, 1,1-dihydroxy-, µ 2 2 H2B BH2 Diborane(6), 1,2-[ -(phenylamino)]- 1-oxide N Ph H Arsenic compounds. See Phosphorus and Arsenic compounds (¶ 197). 182. Boron compounds. For the naming of neutral boron hydrides and re- H placement (“a”) analogs (hetero polyboranes) see ¶¶ 159, 160. Except for hy- H B NH2 1 droxyl groups attached to boron (¶ 175), principal groups on such hydrides are H2B 4 2 BH2 Tetraborane(10), 1,2-µ-amino- expressed as suffixes in the regular way, and conjunctive names are adopted H B H with those known to have closed polyhedral structures; e.g., Diborane(4)- 3 H 1,1,2,2-tetramine and 1,2-Dicarbadodecaborane(12)-1,2-diethanol. 49 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 183
Cyclic derivatives of polyboranes (other than Diborane(4)) and hetero po- H Me lyboranes formed by replacement of non-bridging hydrogen atoms by bivalent Borazine, 2,2,4,4,6,6-hexabromo- N radicals are named by citing such radicals as substituents. Br B BBr 1,2,3,4,5,6-hexahydro-1,3,5- Examples: 2 2 trimethyl- Me−N N−H H Diborane(6), 1,1-(1,4-butanediyl)- B H Br2 Me H2B 2 1 B H Ionic boron compounds are indexed by coordination nomenclature (see ¶ − 2− O 215) at such names as Borate(2 ), decahydrodeca- (for [B10H10] ), and Bo- 1+ 1,2-Dicarbadodecaborane(12), ron(1+), diamminedihydro- (for [BH2(NH3)2] ). Acidic polyboranes and − 1,2-[oxybis(methylene)]- hetero polyboranes are named as complex acids (¶ 215); e.g., Borate(2 ), 12 decahydrodeca-, hydrogen (1:2) (not Decaborane(12)). Prior to the Eleventh B 10H 10 Collective Index period, the special term “borata” denoted a tetrahedral borate anion attached to carbon atoms in a heterocyclic ring system. Now, compounds Hydroxy derivatives of Borane have acid names as follows: of this type are named by coordination nomenclature at such index headings as Borate(1−).
B(OH)3 Boric acid (H3BO3) 183. Carbonic acid and relatives, with a few trivially named exceptions, are indexed by the principles of replacement nomenclature for functions (¶ HB(OH)2 Boronic acid 129), based on the names Carbonic acid (for (HO)2C=O) and Formic acid (for HCO2H). Trivial names employed in CA indexes are: Formyl halides and H2B(OH) Borinic acid halogenides (except the cyanide, which is indexed at Acetonitrile, 2-oxo-), Formamide , Formaldehyde , Hydrocyanic acid, Urea, Guanidine , Cyanic acid, Thiocyanic acid, Selenocyanic acid, and Tellurocyanic acid. These Boronic and borinic acids have replaceable hydrogen atoms attached to boron trivially named compounds are ranked with the appropriate class (acid, amide, and are used as substitutive parent compounds. Their esters, anhydrides and etc.) as Carbonic acid derivatives, which fall below derivatives of acids salts are named in the usual way, but their acid halides, amides and hydrazides named as principal groups (carboxylic, sulfonic, etc.) and above inorganic are named as Borane, Boranamine, and Hydrazine derivatives, respectively. “oxo” acids ( Hypochlorous acid , Phosphonic acid, etc.) (see ¶ 106). Chalcogen analogs are named by use of afffixes thio, seleno, and telluro. Examples: Hydroxy derivatives of Diborane(4), are now indexed at that index heading parent. O = Formyl isocyanate Examples: H−C−NCO
O MeB(OMe)OEt Boronic acid, B-methyl-, = − − Formic acid, ethyl methyl ester H C OCN anhydride with cyanic acid PhBMeOH Borinic acid, B-methyl-B-phenyl- HCΟΝHMe Formamide, N-methyl-
PhB(SH)2 Boronodithioic acid, B-phenyl- Analogs (imidic, hydrazonic, peroxy, chalcogen) of Formic acid, Forma- mide, etc., are named systematically as methanoic acid analogs, but are ranked Acetic acid AcOBMe2 as compounds related to Formic acid. anhydride with B,B-dimethyl- Examples: borinic acid O = Methaneperoxoic acid ′ PhB(NHNH2)2 Hydrazine, 1,1 -(phenylborylene)bis- H−C−O−OH
S = Methane(dithioic) acid (not Formic − − (HO)2BB(OH)2 Diborane(4), 1,1,2,2-tetrahydroxy- H C SH acid, dithio-) (prior to CA Volume 95 the name Hypoboric acid HCSNH2 Methanethioamide (not Formamide, was used) thio-)
B(NH ) Boranetriamine 2 3 HC(=NH)NCO Methanimidoyl isocyanate
Boric acid (H3BO3) is not a substitutive parent. Esters and anhydrides are HCS2CN Methane(dithioic) acid, indexed as functional derivatives; hydrazides are indexed at Hydrazine. anhydrosulfide with thiocyanic Examples: acid S = Formic acid, 1-(thiocarboxy)- (HO)2BOPr Boric acid (H3BO3), HO−C−CO H monopropyl ester 2 (not Methanethioic acid, carboxy-) (H2NNH)3B Hydrazine, 1,1′,1′′-borylidynetris- Replacement of the nuclear hydrogen atom in formic acid compounds by Addition compounds of neutral boranes are named as molecular coordina- radicals derived from molecular skeletons leads to carboxylic acids, carbothio- tion compounds (see ¶ 215). (Prior to CA Volume 95 (see ¶ 101), they were in- amides, etc., expressed as suffixes on the skeleton names. dexed at the component names (¶ 192).) Formaldehyde analogs have systematic names based on Methane, e.g., Example: Methanimine (for CH2=NH) and Methanethial (for CH2=S). (Methaneth- ione (¶ 174) is employed only as the index heading parent for thio ketones with two cyclic substituents and for cyclic thio ketenes .) Replacement of hydrogen B(NMe3)H3 Boron, (N,N-dimethyl- methanamine)trihydro- in formaldehyde by carbon skeletons leads to larger aldehydes and to ketones; (T-4)- (preferred index name) they and their analogs are named by the usual principles of substitutive nomen- (formerly indexed at clature (¶¶ 173, 174). Replacement by nitrogen, halogen, etc., leads to com- Methanamine, N,N-dimethyl-, pounds which are often named as Formic or Carbonic acid derivatives. compd. with borane (1:1), and at Examples: Borane, compd. with N,N-di- methylmethanamine (1:1)) N 3-Pyridinecarboxaldehyde Oligomeric boranamines which are linear or unspecified are indexed at the CHO monomer name with “ dimer ,” “trimer,” etc., in the modification. Cyclic dimers are named as µ-derivatives of Diborane(6). Monocyclic trimers, tetramers, Ph2CO Methanone, diphenyl- etc., are given ring names in which the abnormal valencies of hetero atoms are expressed (see ¶ 158). PhC(=NH)Me Benzenemethanimine, α-methyl- Examples: HCONH2 Formamide Me2 N Diborane(6), bis[µ-(dimethyl amino)]tetrafluoro- H2NCOCl Carbamic chloride F2B BF2 N Cl2C=NH Carbonimidic dichloride Me2 ¶ 183 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 50
Carbonic acid analogs in which oxygen is replaced by halogen, halogenoid, Carbonic acid and its relatives are placed in the order of precedence of com- chalcogen, or nitrogen atoms or groups (¶ 129) (except cyano or a single hy- pound classes just below acids expressed as suffixes attached to molecular drazino) are given functional replacement names. The replacement of one hy- skeleton names, e.g., sulfonic acids (¶ 106). Within this subclass of acids, they droxyl by amino leads formally to Carbonamidic acid, H2NC(O)OH, but it and are ranked by the following criteria, applied successively until a decision is its analogs are named at the approved abbreviated forms Carbamic acid, Car- reached: (a) number of acid groups; (b) number of nuclear carbon atoms; (c) bamothioic acid, etc. precedence of atoms directly attached to nuclear carbon atoms (see Table I, ¶ Examples: 128); (d) number of most preferred hetero atoms directly attached to nuclear carbon atoms; (e) order of priority of other atoms or groups attached to nuclear (HO)2C=NH Carbonimidic acid (the tautomeric carbon atoms. A partial list in descending order is: Peroxydicarbonic acid, Di- Carbamic acid is preferred in carbonic acid, Imidodicarbonic acid, Carbonoperoxoic acid, Carbonic ac- indexing unless both acid hydrogen id, Carbonimidic acid, Carbonochloridic acid, Carbamic acid, Formic atoms have been replaced.) acid, Cyanic acid, and Thiocyanic acid. (Chalcogen analogs of each acid im- mediately follow it in descending order of increasing replacement of oxygen by sulfur, selenium, and tellurium.) Acid chlorides, amides, etc., are ranked H2NC(=NH)OH Carbamimidic acid (not Carbon- amidimidic acid) (Urea is preferred within their own classes in a similar order. in indexing in the absence of a Carbamic acid derivatives with cyclic substituents are not assigned con- covalent acid derivative.) junctive names. In the presence of higher functions, including any acid ex- pressed as a suffix, the carbamic acid residue is indicated by a (carboxyamino)
ClCO2H Carbonochloridic acid radical. Its replacement analogs are named in the usual manner. Its hydrazides are indexed at Hydrazinecarboxamide. NCCO2H Carbonocyanidic acid Examples:
(HOO) CO 2 Carbonodiperoxoic acid NHCO H 2 Carbamic acid, N-2-naphthalenyl-
H2NC(=NH)SSH Carbamo(dithioperox)imidic acid (not 2-Naphthalenecarbamic acid)
Cl2CS Carbonothioic dichloride Carbamic acid, N,N′-1,4- ClCOBr Carbonic bromide chloride HO2CNH NHCO2H phenylenebis- (not Carbamic acid, 1,4- phenylenebis- (see ¶ 118)) Carbonic diamide is named Urea, and its chalcogen analogs are indexed at Thiourea (not Urea, thio-), etc. The monohydrazide of carbonic acid is Hydra- zinecarboxylic acid, but the dihydrazide is named Carbonic dihydrazide. Me2NCS2H Carbamodithioic acid, N,N- The monocyanide of Carbonic acid is Acetonitrile, 2-oxo-; the dicyanide is dimethyl- Propanedinitrile, 2-oxo-; the trivial name Guanidine is employed for Car- bonimidic diamide. Examples: PrNHC(=NH)OEt Carbamimidic acid, N-propyl-, ethyl ester MeNHC(=NH)NHEt Guanidine, N-ethyl-N′-methyl- N′ N′′ N
Carbonic acid linear polyanhydrides are named at Dicarbonic acid, Tri- HO3S NHCO2H Benzenesulfonic acid, 4-(carboxy- carbonic acid, etc. Carbonimidic, carbonoperoxoic, and carbonimidoperoxoic amino)- acid anhydrides are named in the same manner. When all the anhydride oxy- gens have been replaced by -OO- or -NH- groups, nondetachable “peroxy-” and “imino-” prefixes are cited ahead of the name along with multiplicative Cyanic acid and its chalcogen analogs are treated as mononuclear “oxo” prefixes. Chalcogen analogs are treated similarly, except that synonym line acids; their esters and anhydrides are named in the usual way. Isocyanic acid formulas form part of the name, and multiplicative prefixes are not cited. When and its analogs are not used in general index nomenclature; their esters and an- both acid groups have been replaced by amide or acid halide functions, appro- hydrides are named like halogen compounds. The acids themselves, and their priate names are derived, but when only one hydroxyl group has been replaced, salts, are indexed at Cyanic acid, Thiocyanic acid, etc. The amide, H2NCN, or different functions are present, choice of a simpler parent is made. Longer is named Cyanamide . chains can often be indexed by replacement (“a”) nomenclature (¶ 127). Examples: Examples: O O MeOCN Cyanic acid, = = Dicarbonic acid HO−C−O−C−OH methyl ester
NH NH NH H CC(O)SCN = = = 3 Ethanethioic acid, HO−C−O−C−O−C−OH Tricarbonimidic acid anhydrosulfide with thiocyanic acid O O = = HO−O−C−O−C−O−OH Dicarbonodiperoxoic acid NCO CO2H Benzoic acid, 4-cyanato-
HO2COOCO2H Peroxydicarbonic acid PhNCO Benzene, isocyanato- (not Isocyanic NH NH NH = = = acid, phenyl ester) HO−C−NH−C−NH−C−OH Diimidotricarbonimidic acid
H2C=CHCONCO 2-Propenoyl isocyanate S S = = Thioperoxydicarbonic acid − − − − − HO C S S C OH ([(HO)C(S)]2S2) PhNHCN Cyanamide, N-phenyl- (not Benzene- O O = = carbamonitrile) Cl−C−O−C−Cl Dicarbonic dichloride Numerical and italic letter locants are used with carbonic acid relatives to O O = = place substituents. Locants are employed with monosubstituted Guanidine Cl−C−NH−C−Br Imidodicarbonic bromide chloride and Carbamimidic acid. Examples:
H2NNHCONHCONHNH2 Imidodicarbonic dihydrazide MeNHCONH2 Urea, N-methyl- N N′
ClCONHCO2H Carbamic acid, N-(chlorocarbonyl)- MeNHCSNHMe Thiourea, N,N′-dimethyl- NN′ O O O O O = = = = = NH −C−NH−C−NH−C−NH−C−NH−C−NH = 2 2 H2NNHC( NH)SEt Hydrazinecarboximidothioic 12 34 5 6 78 9 acid, 2,4,6,8-Tetraazanonanediamide, ethyl ester 3,5,7-trioxo- 51 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 184
H NNHCONHNH + + 1 1 1 2 2 2 2 ′ ′ 2 Carbonic dihydrazide Pr3N (CH2)2N Pr3 1,2-Ethanediaminium, N ,N ,N ,N ,N ,N - 21 1 2 hexapropyl- (principle: maximum number of the preferred substituent suffix) = H2NC( NH)NH2 Guanidine N N′′ N′
N′′ N′′′ MeC≡N+Me Methanaminium, N-ethylidyne- NH NH − = − − = − Imidodicarbonimidic diamide H2N C NH C NH2 Quaternary ammonium cations not included in the heading parent are ex- N 1N23′ + − + + ≡ pressed as substituted ammonio, NH3 , iminio, NH2=, and nitrilio, NH , radicals. N′′ N′′′ N′′′′ NH NH NH Example:
= = = Diimidotricarbonimidic diamide − − − − − − NH2 C NH C NH C NH2 N 1N2345′ CH CH CH − 3 − 3 − 3 + ++ H C−N−(CH ) −N−(CH ) −N−(CH ) −NH
3 − 2 6 − 2 3 − 2 6 2 184. Cations, including carbonium ions (which possess an electron-defi- CH CH CH cient, tricoordinate carbon atom), “ium” ions (defined for index nomenclature 3 3 3 purposes as resulting from addition of a proton to a saturated carbon atom or of 1,6-Hexanediaminium, N1-[3-[(6- one covalent substituent other than hydrogen to a fully substituted hetero at- aminohexyl)dimethylammonio]- om), and radical cations related to these two classes are described here. (For propyl]-N1,N1,N6,N6,N6-penta- salts of which they are components, see ¶ 198.) When a proton is added to a methyl- (principle: largest hetero atom, the resulting compound is named as a salt (if the anion is known) heading parent) or by such modification phrases as “ conjugate acid ” or “conjugate monoacid” at the neutral component followed by the appropriate ratio. Aminium and diaminium names may be derived from amines named by re- Examples: placement (“a”) nomenclature. In addition, cationic centers in the “a” name of a molecular skeleton may be expressed, if such a name is permitted (see ¶ 127), − MeOH • HF (not [MeOH2] + F ) Hydrofluoric acid , by use of the “azonia” replacement prefix. compd. with methanol (1:1) Example:
N CH3 CH3 CH3 CH3 CH3 CH3 + |||| | | •H Pyridine, − − −(+ − −(++− −( − −(++− −( − −+ − CH3 CH2 N CH2)6 N CH2)6 N CH2)6 N CH2)6 N |CH2)6 N CH2 CH3 conjugate acid (1:1) N|| 7| 14| 21 28 ||N′ CH3 CH3 CH3 CH3 CH3 CH3
Carbon cations formally derived by addition of a proton to a saturated car- 7,14,21,28-Tetraazoniatetratria- bon atom are named at the molecular skeleton name by use of a modification contane-1,34-diaminium, N1,N34- term such as “protonated”. diethyl-N1,N1,N34,N34,7,7,14,14,- Example: 21,21,28,28-dodecamethyl- Ethane, MeCH4+ Iminium names are employed when the quaternary nitrogen atom is at- protonated (1:1) tached to one or more bivalent radicals derived from a molecular skeleton, and analogous monovalent radicals are absent. The names are derived from those Orthodox carbonium compounds are named from the hydrocarbon (or other of the preferred imines (¶ 177). parent) radical by addition of “-ium.” Example: Examples: Benzenemethaniminium, N- Ph C=N+=CPh MeCH2+ Ethylium 2 2 (diphenylmethylene)-α-phenyl-
+ Me2CH Ethylium, 1-methyl- (not 2- − + Propylium Diazonium compounds contain the N2 group attached to a substitutive parent compound. When such a parent is a molecular skeleton, the suffix “-di- MeCO+ Ethylium, 1-oxo- (not Acetylium) azonium” is employed; otherwise Diazonium is the heading parent. The corre- 21 sponding substituent prefix is “diazonio.” Examples:
CO+ Methylium, cyclopropyloxo- (not + Cyclopropylcarbonylium) MeCOCH(N2 )COMe 3-Pentanediazonium, 2,4-dioxo- H 13 45
+ + Benzenediazonium, 4-chloro- Cl N2 O O 1,3-Dioxan-5-ylium
+ + 1,4-Benzenebis(diazonium) N2 N2 2+ Naphthalenediylium, dihydro- + MeNHN2 Diazonium, (methylamino)- Acyclic nitrogen cations derived from amines attached to parent molecular skeletons are named by converting the preferred “-amine” name (¶ 176) to Cl Benzenediazonium, 3-chloro-4- “-aminium” and expressing the remaining quaternizing groups as substituent + + [(4-diazoniophenyl)methyl- prefixes. N2 NMe N2 amino]- Examples:
+ Me4N Methanaminium, N,N,N-trimethyl- Cations from acyclic nitrogen molecular skeletons are named in a similar manner by use of the “-ium” suffix. Lowest locants are given to cationic centers when a choice is necessary. + Me2N O Methanaminium, N-methyl-N-oxo- Examples:
+ PhN Me2NH2 Hydrazinium, 1,1-dimethyl-1- CH3 CH3 2-Propanaminium, N,2-dimeth- phenyl- − + − − CH=N C CH3 yl-N-(phenylmethylene)- − (principle: largest amine parent) + CH H2NN Me2NH2 Triazanium, 2,2-dimethyl- 3 12 3
+ MeN=NN Me3 2-Triazenium, 1,1,1,3-tetramethyl- 321 O + 2-Furanethanaminium, N,N-di- (CH2)2N Et2Me ethyl-N-methyl- (from a + + conjunctively-named amine) Me3N N Me3 Hydrazinium, 1,1,1,2,2,2-hexamethyl- ¶ 184 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 52
Acyclic cations from nonnitrogenous hetero atoms are indexed at Phospho- In all the examples above, the charge of the cationic atom was derived from nium, Arsonium, Stibonium, Bismuthonium, Oxonium, Sulfonium, Iodo- an additional covalent substituent at a ring hetero atom already saturated to the nium, etc. The corresponding substituent prefixes are phosphonio, arsonio, etc. covalency limit. Similar names are used (for rings other than those with “a” Diphosphine,H2P-PH2, affords Diphosphinium. names) when the covalency limit is exceeded within the ring system itself, no Examples: “external” substitution being involved. The “-ium” name is derived from the uncharged heterocycle, indicated hydrogen, if present, being removed. If indi- + + ′ Me3P (CH2)2P Me3 Phosphonium, 1,1 -(1,2-ethanediyl)bis[1,1,1- cated hydrogen is needed for the cationic system, it is added at the lowest avail- trimethyl- (analogous compounds with able nonangular position. The trivial names Furylium, Pyrylium, Thio- one or more noncarbon attachments to pyrylium (and their benzo analogs), and Xanthylium (from Xanthene) are phosphorus are indexed at Phosphorus(1+) used. When other hetero atoms are present in the ring system, a locant before (see ¶ 215)) the “-ium” suffix defines the position of the cationic center. Examples: + Diphosphinium, 2,2-dichloro- Cl2PP Me3 1,1,1-trimethyl- 1 9a
Me As+ Quinolizinium 4 Arsonium, tetramethyl- +N 5 + Me3S Sulfonium, trimethyl- (the corresponding S-oxide is named Sulfoxonium, trimethyl-) 6 Pyrylium, 4-methoxy- (from 2H- MeO— + O 1 + or 4H-Pyran) Ph2I Iodonium, diphenyl- (the corresponding I-oxide is named Iodonium, diphenyl-, I-oxide) MeO OMe 1 9a 9b Cyclic cations from rings with “a” names are named by replacing the aza, + Dibenziodolium, 1,9-dimethoxy- oxa, thia, etc., terms by the cationic prefixes azonia, oxonia, thionia, etc. They I are cited in the same order as the corresponding neutral terms (Table I ¶ 128); 6 5 4 when the same element is present in neutral and cationic forms, alphabetic or- 1 der of the terms is followed, but the cationic center is preferred for lowest lo- O Me cants if a choice is available. 5 + 1,3-Dioxol-1-ium, 2-methyl- Examples: O 3 8 1 2 9 1O 1 9 +NEt N 1Oa 10 5-Aza-2-azoniabicyclo[4.2.2]de- 6 cane, 2,2-diethyl- Phenothiazin-5-ium, 3,7-bis(di- HN + methylamino)- 5 Me2N S NMe2 6 5 4 1 P 6 O O 2 2,6,7-Trioxa-1-phospha-4-phos- Cationic heterocyclic monospiro compounds, other than those from “a”- 7 O 8 phoniabicyclo[2.2.2]octane, named rings, have the “-ium” term placed in the name in accordance with the 4 4-methyl- nature of the component rings. When the cationic hetero atoms are in nonspiro + P Me positions, the “-ium” suffix is appended to the appropriate component. If the spiro and cationic centers coincide, or if both component rings contain such a 1 center, the “-ium” is attached only to the second component (if the components 7 are different). For “a”-named components, “azonia,” “oxonia,” etc., terms are + 8-Thioniabicyclo[3.2.1]octane, 8 SMe 8-methyl- used as appropriate; in addition, an “-ium” suffix is attached to the second com- ponent if the spiro atom is cationic and the second component does not have an 5 “a” name. Examples: 9 1 Me O Me 1-Oxa-4-azoniaspiro[4.4]nonane, 1 5 2,2,4,4-tetramethyl- Me 7a 4 S ′ N Spiro[benzothiazolium-4(5H),1 - + + cyclopentane], 6,7-dihydro-2,3- Me2 MeN 4 3 dimethyl- ′ 1′ 9 1 5 + P 5 5-Phosphoniaspiro[4.4]nonane 1′ 8′a 2′ Cationic monocyclic and fused ring systems, other than those with “a” 1 +NMe2 Spiro[1,3-dioxolane-2,6′(2′H)- O 6′ ′ ′ names, are indexed at heading parents derived from the heterocycle name by 5 2 isoquinolinium], 2 ,2 -dimethyl- ′ ′ addition of an “-ium” suffix. Lowest locants are preferred for indicated hydro- O 5 4 gen, e.g., 1H (never 3H)-Benzimidazolium; a cationic center is numbered 3 lower than a neutral hetero atom of the same element if a choice is available. Examples: 4′ 6′ 5′ N ′ +Me 8a + Spiro[isoquinoline-2(1H), 2 -[2H] N 2 N pyrido[1,2-a]pyrazinium] 6 1 9′a ′ Pyridinium, 1,3-dimethyl- 1 Me 5 4
1 9 1O 1 9a 9b N 1Oa Phenazinium, 3,7-dibromo-5- 5 5 Spirobi[5H-dibenzophospholium] methyl- (not Phenazinium, 2,8- 6 + 4 N ′ P ′ Br 6 + 4 Br 4 6 Me dibromo-10-methyl-) 5′
Me2 9′b 9′a +N 1′ 6 1 4 Piperazinium, 1,1,4,4-tetramethyl- +N 1′ Me2 1 + 4′a Spiro[3-azoniabicyclo[3.1.0]hexane- Me N 3 6 9′ 6,9′-[9H]fluorene], 3,3-dimethyl- Et 2 + 4′b O 5 1 Furanium, 1-ethyltetrahydro- 8′ 53 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 184
1 5′ Spiro[3-azoniabicyclo[3.2.2]no- Cationic prefixes are used when more preferred compound classes (¶ 106) + ′ nane-3,3′(2H)-oxazolium] are present, or when different cations, or additional occurrences of the same 8 N 3 7 3 O 1′ cation, cannot be included in the heading parent. Heteroacyclic cationic prefix- 9 es are based on “-onio” radicals such as ammonio and iminio (for singly and 5 doubly bound nitrogen, respectively), diazonio, sulfonio, phosphonio, and io- donio. Cyclic cationic radicals from rings, not named by “a” nomenclature, Dispiro compounds with other than “a”-named components and containing containing a single hetero atom at which the free valency is located are named two cationic spiro atoms have the “ium” suffix appended to the terminal, i.e., by changing the “-ium” ending of the cation name to “-io”; e.g., pyridinio, 2H- the first and third, component names. pyranio, phenanthridinio. In all other cases, heteroacyclic and heterocyclic cat- Several classes of resonance-stabilized cations containing nitrogen atoms ionic prefixes are derived from the cation name by adding “-yl,” “-diyl,” etc., are either normalized by the CAS Registry System or treated as though they to the “-ium” suffix with locants (low-numbered if there is a choice) to indicate were; i.e., to avoid scattering of information in CA indexes, each such cation, the points of attachment. regardless of how its structure is drawn, is assigned a unique CAS Registry Examples: Number and unique CA Chemical Substance Index name (cf. ¶¶ 122, 180). A normalized cation is any cyclic cation with the substructure Me + — — N + + 6 1 (1-methylpyridinium-2-yl) − = − − − − = − N C(or N) N− N C(or N) N− − − ′ ′ R R R R Me where (a) the terminal N’s are in the same ring or adjacent rings (but not at a + N ′ 6 ring junction) and (b) the bonds to R and R are not part of the ring. 1 (1,4-dimethylpiperazinium-1,4-diyl) Examples: 4 N + Me 4 Me Me 7a N 3 N 1 + + 9 1 NPh NPh N 10a 3 7a 4 1 10 (5-methyl-10H-phenothiazinium- 5 10-yl) 1H-Benzimidazolium, 1- NOT 1H-Benzimidazolium, 3- S 6 + 4 methyl-3-phenyl- methyl-1-phenyl- Me (principle: positive charge on the CIP-pre- = + − Me2NN NN Me2 (1,1,4,4-tetramethyl-2-tetrazenium- ferred nitrogen) 4 321 1-yl)
Me Me + N Me 6 7 Me 6 + 7 6 1 5 N 1 5 N 1 N 8 N 8 piperidinium-1-ylidene + 2 N 2 3 N O N 4 9 O N 4 9 3 Ph Ph 1 8 N+ 1H-Purinium, 2,7-di- NOT 1H-Purinium, 2,9-di- 1-azoniabicyclo[3.3.1]non-1-yl hydro-1,7-dimethyl- hydro-1,7-dimethyl- 9 2-oxo-9-phenyl- 2-oxo-9-phenyl- (principle: positive 5 charge on the CIP-pre- ferred nitrogen) Carbon cationic prefixes are named by addition of “-yl” to the cation name. Locants are cited (except for methane prefixes) for both the cationic center and For resonance-stabilized cations that are not normalized, the unique CA Chem- the point of attachment, with lowest locants for the latter. ical Substance Index name is derived by regular nomenclature rules (see Examples: ¶ 138). + H2C — methyliumyl CH CH CH CH CH CH 3 2− + − 3 3 2− + − 3 + N=CH−N N−CH=N MeC H — − − − − 1-ethylium-1-yl CH3CH2 CH3 CH3CH2 CH3
Ethanaminium, N-[(di- NOT Methanaminium, N-[(di- O 6 1 2 methylamino)methyl- ethylamino)methylene]- (tetrahydro-4H-pyran- ene]-N-ethyl- N-methyl- + 4-ylium-4-yl) (principle: preferred index parent) (octahydro-8a(1H)- Et Et 1 1 azulenylium-1-yl) 8a N 8a N 8a + 1 + + 3 N N 4 4 4 Imidazo[1,2-a]pyridinium, NOT 1H-Imidazo[1,2-a]pyridin- 1-ethyl- 4-ium, 1-ethyl- Cationic compounds are ranked in the following descending order of prece- (principle: the neutral dence of elements: C, N, P, As, Sb, Bi, O, S, Se, Te, F, Cl, Br, I. When more ring, quaternized by than one cationic center is present, the general rules of substitutive nomencla- substitution, is preferred) ture (¶ 138) are applied, the cationic centers being considered as principal chemical groups for this purpose. When at least one canonical form of a resonance-stabilized cation repre- Examples: sents a hydrogen atom as being attached to a positively charged nitrogen atom, it is named as a neutral compound with an index modification term such as Cycloheptatrienylium, (1-methyl- “conjugate acid” or (if the anion is known) as a salt such as “hydrochloride” + followed by the appropriate ratios. MeN + pyridinium-4-yl)- (principle: Example: carbon cation preferred)
Me Me + Me +N NHPh N NHPh N NPh − − •Cl •Cl • HCl + + Me3N (CH2)3S Me2 1-Propanaminium, 3-(dimethyl- CO2Me CO2Me CO2Me sulfonio)-N,N,N-trimethyl- (principle: nitrogen cation (The hydrochloride salt is the preferred structure.) preferred) ¶ 184 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 54
Me Me are modified for esters. The chemical functionality (¶ 106) of certain very com- + Pyrazinium, 1,4-dimethyl-2-[(1- N N mon acids (“Class I” acids) is disregarded for the purpose of naming their es- + 4 methylquinolinium-6-yl) methyl]- (principle: maximum ters, unless the “alcoholic” component is also very common, and the entry is 6 made instead at the uncommon alcohol, despite its lower functionality. The CH +N number of cationic centers) 2 Me “Class I” acids comprise: Acetic acid; Benzenesulfonic acid; Benzenesulfonic acid, 4-methyl-; Benzoic acid and its monoamino, mononitro, and dinitro de- rivatives; Boric acid (H BO ); Carbamic acid; Carbamic acid, N-methyl-; + 3 3 (CH ) NMe Carbamic acid, N-phenyl-; Carbonic acid; Formic acid; Methanesulfonic 2 2 3 Pyridinium, 1-[2-(trimethyl- acid; Nitric acid ; Phosphoric acid; Phosphorodithioic acid; Phosphorothio- +N ammonio)ethyl]- (principle: 6 1 ic acid; Phosphorous acid ; Propanoic acid; Sulfuric acid; and Sulfurous ac- ring parent preferred) id. All other acids, including isotopically labeled forms of “Class I” acids, belong to “Class II.” Esters of “Class I” monobasic acids with “Class II” alcohols and thiols are indexed at the latter. Esters of “Class I” acids with “Class I” alcohols and thiols Me 1 are indexed at the acids. “Class I” alcohols are: Benzeneethanol; Benzen- N (CH2)2 S 7a + emethanol; 1-Butanol; 1-Butanol, 2-ethyl-; 2-Butanol; Cyclohexanol ; 1- + Decanol; 1-Dodecanol; Ethanol; Ethanol, 2-(diethylamino)-; Ethanol, 2- Me MeN (dimethylamino)-; Ethenol; 1-Heptanol; 1-Hexanol; 1-Hexanol, 2-ethyl-; 3 4 Methanol; 1-Nonanol; 1-Octadecanol; 1-Octanol; 1-Pentanol; Phenol and Me Benzothiazolium, 3-methyl-2- its monochloro, monomethyl, and mononitro derivatives; 1-Propanol; 1-Pro- [2-(1,3,3-trimethyl-3H- panol, 2-methyl-; 2-Propanol; 2-Propanol, 2-methyl-; and 2-Propen-1-ol. The list of “Class I” thiols is completely analogous to the “Class I” alcohol list; indolium-2-yl)ethyl]- the individual index names are also analogous, e.g., Benzeneethanethiol; 2- (principle: preferred ring) Propanethiol, 2-methyl-; except that the Phenol analog is Benzenethiol. All selenols and tellurols belong to “Class II.” Cationic compounds of indefinite structure are named by use of alternative Esters formed from hydroxy derivatives of nitrogen, phosphorus, arsenic, antimony, bismuth, germanium, tin and lead are named as such. They are al- locants if possible, e.g., Quinazolinium, 1(or 3)-methyl-, iodide (1:1); other- ways indexed at the acid regardless of the type of acid. The acyclic nitrogen wise as molecular addition compounds of the neutral compounds with a term radicals used in ester names are azanyl, diazanyl, diazenyl, triazanyl, etc. such as “compd. with iodomethane (1:1)” (not “monomethiodide”) in the mod- Examples: ification.
Cationic free radicals from carbonium ions are named like the carbon cat- AcO(CH2)2Ph Acetic acid, ionic prefixes (above); e.g., Methyliumyl; 4-Cyclohexylium-1-yl. When the 2-phenylethyl ester free radical and cationic centers are separated, the “-yl” suffix is preceded by a locant, if known. When the free radical and cationic center are in different par- MeOSO3H Sulfuric acid, ent molecular skeletons, the free radical supplies the heading parent. monomethyl ester (the uninverted Examples: name is methyl hydrogen sulfate )
• Piperidinium-4-yl, PhSP(S)(OMe)OH Phosphorodithioic acid, 1,1-dimethyl- O-methyl S-phenyl ester + N O Acetic acid, Me Me − = − − CH3 C O NPh2 diphenylazanyl ester 2-Pyrrolidinyl, • + 2-[(1,1-dimethyl- In each of the examples above, a “Class I” acid is esterified by a “Class I” N CH2 N pyrrolidinium-2-yl)- alcohol or thiol. When the acid belongs to “Class I” and the alcohol or thiol to Me Me Me methyl]-1-methyl- “Class II,” the entry is found at the alcoholic component name, except when the acid is polybasic and the alcohols differ from one another, in which case the acid heading is chosen. When the ester is named at the alcohol heading a locant Delocalized radical cations are described at the index name of the corre- is always cited for the “ate” or “ite” term. This eliminates the need for the par- sponding neutral compound by the modification term “radical ion” followed by enthetical expression “(ester)”. a cationic Ewens-Bassett number in parentheses. Examples: Example: − − − −Ο • CH3 SO2 O CH2 CH2 H + Azulene, 12 radical ion(1+) 1,2-Ethanediol, 6 1-(4-methylbenzenesulfonate) 1 OAc Cationic free radicals formally derived by loss of an electron from a hetero 1,2-Cyclohexanediol, atom of a molecular skeleton or of an isolated chalcogen atom are named sim- OBz 1-acetate 2-benzoate ilarly (¶ 270). Example: 1,2-Ethanediol, HCO2CH2CH2O2CH 12 1,2-diformate (the uninverted name + Methanamine, N,N-dimethyl-, is 1,2-ethanediyl diformate) Me3N• radical ion(1+) O2CNHMe
185. Esters, other than cyclic esters, of principal functions are named as 1,3-Benzenediol, 5-nitro-, such for indexing purposes, usually at the acid component name. Cyclic esters O2N OH 1-(N-methylcarbamate) are named as heterocyclic ring system derivatives. Esters of hydroxy, mercap- to, carboxy, sulfo, etc., groups expressed as substituents of heading parents are expressed as compound or complex substituents, not as modification terms at AcO the same heading parent. To be recognized as an ester in CA indexing, a C-O, 1,3-Benzenediol, 4-amino-, C-S, C-Se, or C-Te bond must be present. Thus, ethyl cyanate and ethyl per- 1-acetate chlorate are considered to be esters, but ethyl azide, ethyl isocyanate, and ethyl OH chloride are not. NH2 Examples:
[Cl(CH2)2O]3P(O) Ethanol, 2-chloro-, EtOCN Cyanic acid, 1,1′,1′′-phosphate (3:1) (all the “Class II” ethyl ester alcoholic components are alike here; the ratio is necessary to indicate a neutral ester EtOClO3 Perchloric acid , of known composition; the mono- and ethyl ester diesters are named with “dihydrogen phosphate” and “hydrogen phosphate”, EtN3 Ethane, azido- respectively in the modification)
EtNCO Ethane, isocyanato- HO(CH2)2OP(OMe)2 Phosphorous acid, 2-hydroxyethyl dimethyl ester EtCl Ethane, chloro- (the entry is made at a “Class I” acid, not at the “Class II” alcohol 1,2-Ethanediol, be- To permit information on esterified alcohols and thiols to be more readily cause the alcoholic components are unlike) found in the Chemical Substance Index, the usual rules of index name selection 55 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 185
− − − − − − − − When an ester of the type illustrated immediately above has a more pre- HO CH2 CH2 O SO2 O CH2 CH2 OH ferred acid residue present in the molecule, the “Class I” polybasic acid be- 211′ 2′ comes a substituent. 1,2-Ethanediol, Example: 1,1′-sulfate O Acetic acid, 2-[[(2-hydroxyethoxy)- − − = − − − Esters are named by replacement (“a”) nomenclature if the requirements (¶ CH3 O P O CH2 COOH methoxyphosphinyl]oxy]- (not − 127) are met. O−CH −CH −OH Acetic acid, hydroxy-, 2-hydroxy- 2 2 ethyl methyl phosphate) Example:
Esterified substituents of the index heading parent are expressed as substit- HOCH2CH2OSO2O(CH2)2OSO2OCH2CH2OSO3H 12 10 9 8 5 4 3 1 uents, not as modification phrases. Examples: 3,5,8,10-Tetraoxa-4,9-dithiado- decane-1,12-diol, 1-(hydrogen sulfate), AcO 4,4,9,9-tetraoxide Benzenesulfonic acid, 3-(acetyloxy)-, SO3Me methyl ester (not Benzenesulfonic Multiplicative radicals in modifications are cited ahead of nonmultiplicative. acid, 3-hydroxy-, methyl ester Example: acetate)
Boric acid (H3BO3), (MeO)2BO(CH2)3OB(OMe)2 B,B′-(1,3-propanediyl) B,B,B′B′- O
= tetramethyl ester HOOC4′ O−C−Ο 4 1 COOH Although locants to differentiate between principal and subsidiary groups ′ are strictly not necessary in ester modifications (because esterification of the Benzoic acid, 4,4 -[carbonylbis- latter is not expressed in the modification), they are nevertheless cited to pre- (oxy)]bis- (not Benzoic acid, clude misinterpretation. 4-hydroxy-, carbonate (2:1)) Examples: The phrase “ester with” is usually avoided in general index nomenclature for compounds of known structure, even when the alcoholic component con- HO3S Benzoic acid, 3-sulfo-, tains a function higher than alcohol, but it is employed when an acid which re- 1-phenyl ester quires a line formula is cited in a modification; a ratio is included if the alcohol CO2Ph is monohydric and has a single occurrence, and locants are used if the alcohol is polyhydric or has multiple occurrences. Examples:
PhO3S ΟΗ − Ethanol, 2-chloro, Benzoic acid, 3-(phenoxysulfonyl)- − − − − − ester with boric acid CO2H Cl CH2 CH2 O B OH 2 1 (H3BO3) (1:1) Esterification of an alcoholic component cited in the index modification is ΟΗ expressed as a substituent of the esterifying radical.
− 1,2-Ethanediol, − − − − − − − 1,2-diester with boric acid Example: HO B O CH2 CH2 O B OH 12 (H3BO3) Ο Ο Ο Ο CH− 3 = = = = − − − − − − − − − − − − − CH3 CH C O CH2 C CH2 O C CH2 C O CH2 CH3 ΟΗ 3 21 − − − − − − − − − HO CH2 CH2 O B O CH2 CH2 OH 21 1′ 2′ Propanedioic acid, 1-ethyl 3-[3-(2-methyl-1-oxoprop- 1,2-Ethanediol, oxy)-2-oxopropyl] ester (not 1,1′-diester with boric acid Propanedioic acid, ethyl 3-hydroxy-2- (H3BO3) oxopropyl ester, 2-methylpropanoate)
The choice of a preferred index name for a complex ester depends on the The word “hydrogen,” with multiplicative prefixes if necessary, is used normal criteria (¶ 138), first on the preferred acid class (peroxoic, carboxylic, with an “-ate” (or “-ite”) term derived from a polybasic acid cited in a modifi- carboximidic, sulfonic, carbonic, etc.) then on the preferred heading parent and cation to denote unesterified acid groups. One or more “hydrogen” terms may the particular occurrence of such a parent if it occurs more than once. If there be replaced by radicals to denote further esterification not expressed in the are three or more occurrences, the principle of centrality is invoked; if there are heading parent. When all acid groups of a polybasic acid have been esterified, only two, often the preferred name is the one appearing earliest in index se- “hydrogen” cannot be cited; therefore, if the precise structure is known, a ratio quence. is placed after the “-ate” term. If more than one such term is necessary, the Example: complete “-ate” phrases are cited (without commas) in alphabetical order. O O =
Examples: = Br C−Ο−CΗ2 O−C 1 4 Br Ο Ο = = − −Ο− − − − − − − − HO P CH2 CH2 O P O CH2 CH2 OH Benzoic acid, 4-bromo-, − 1 2 − ΟΗ ΟΗ 4-[[(4-bromobenzoyl)oxy]- 1,2-Ethanediol, methyl]phenyl ester (not Benzoic 1-(dihydrogen phosphate) 2-(hy- acid, 4-bromo-, [4-[(4-bromo- droxyethyl hydrogen phosphate) benzoyl)oxy]phenyl]methyl ester) (principle: maximum number of functional derivatives) Ο When functions higher in precedence than acids are present, all esters are = expressed as substituents. − −Ο− − − − − HO P CH2 CH2 O SO2 OH Example:
− 1 2 ΟΗ 1,2-Ethanediol, Me 1-(dihydrogen phosphate) 2-(hy- − N 1 Cl Pyridinium, 3-(ethoxycarbonyl)- drogen sulfate) 6 + 1-methyl-, chloride (1:1) CO2Et ΟΗ 1,2,3-Propanetriol, − Peroxy acid esters and their chalcogen analogs are named in the usual way, − − − − − − 1-(hydrogen sulfate) HO CH2 CH CH2 O SO2 OH with “OO,” “OS,” “SeO,” “SS,” etc., locants used when necessary along with 3 2 1 the usual “O,” “S,” etc., locants. ¶ 185 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 56
Examples: O − = −( ) − O C CH2 16 CH3 O O Ethanediperoxoic acid, O O = = − − − − − − − −( ) − = − − − − − − = −( ) − Cl3C O O C C O O CCl3 1,2-bis(trichloromethyl) ester CH3 CH2 16 C O CH2 CH CH2 O C CH2 16 CH3 Octadecanoic acid, CH3 O CH3 CH3 O CH3 1,1′,1′′-(1,2,3-propanetriyl) ester − = − − = − CH −CH−O−C−O−O−C−CH −CH −C−O−O−C−O−CH−CH 3 − 2 2 − 3 O CH CH − − = −( ) − 3 3 CH2 O C CH2 16 CH3 O O
Carbonoperoxoic acid, = = c c′ −( ) − = −( ) − − − − − − −( ) − OO ,OO -(1,1,4,4-tetramethyl- CH3 CH2 7 CH CH CH2 7 C O CH CH2 O C CH2 16 CH3 1,4-butanediyl) Oc,Oc′-bis(1- 18 17−11 10 9 8−21 methylethyl) ester 9-Octadecenoic acid, 2-[(1-oxooctadecyl)oxy]-1- O = Benzenecarbo(thioperoxoic) acid, [[(1-oxooctadecyl)oxy]methyl]- C−Ο−S−CCl 3 OS-(trichloromethyl) ester ethyl ester (principle: unsaturated acid preferred)
EtSSOMe Ethanesulfeno(thioperoxoic) acid, Urethanes are esters of Carbamic acid and its derivatives. Xanthic acids SO-methyl ester are O-esters of Carbonodithioic acid. Examples:
− Carbono(dithioperox)imidic acid, PhNHCO2Et Carbamic acid, N-phenyl-, N CH3 = ethyl ester CH −CH −O−C−S−S−CCl N-methyl-, 3 2 3 O-ethyl SS-(trichloromethyl) ester PhOCS2H Carbonodithioic acid, O-phenyl ester Esters of boric acids and their chalcogen analogs are indexed regularly, ex- cept for those of Hypoboric acid and cyclic metaboric acids, (HBO2)n, which Cyclic esters and lactones are indexed as heterocycles. are named as derivatives of the molecular skeletons. Examples: Examples:
(EtO) BB(OMe) Diborane(4), 1,1-diethoxy-2,2- 1 1,3-Dioxane-4,6-dione, 2,2-di- 2 2 6 O Me dimethoxy- O methyl- 1 Me (not Propanedioic acid, cyclic 1- O 6 O 2 3 methylethylidene ester) MeOB BOMe O 5 3 Boroxin, 2,4,6-trimethoxy- O 4 O B OMe 1 O NH 2(5H)-Furanimine (not 2-Buten- (BuS) BOMe 5 2 Thioboric acid ((HO)(HS)2B), imidic acid, 4-hydroxy-, S,S-dibutyl O-methyl ester γ- lactone )
Esters of ortho acids and their peroxy and chalcogen analogs are indexed like ethers , sulfides, peroxides, hydroperoxides, alcohols, etc. Ortho acid names are not used as heading parents. 1 1,2-Oxaphosphorinane, 2-ethoxy-, O O 2-oxide (not Phosphonic acid, Examples: 6 2 P (4-hydroxybutyl)-, monoethyl ester, OEt δ MeC(OMe)3 Ethane, 1,1,1-trimethoxy- (not -lactone) Orthoacetic acid, trimethyl ester)
PhC(SMe)3 Benzene, [tris(methylthio)methyl]- 1 2H-1-Benzopyran-2-one (not MeC(OEt)2OH Ethanol, 1,1-diethoxy- 8a O O Coumarin ; not 2-Propenoic acid, 3-(2-hydroxyphenyl)-, δ-lactone) MeC(OEt)2OOCMe3 Peroxide, 1,1-diethoxyethyl 1,1- dimethylethyl 5 4 − O CH3 − − − − − − Methanesulfenic acid, trimethoxy-, CH3 O C S O CH3
− methyl ester − O CH3 186. Ester-anhydrides are named by combining the policies for anhy- drides and esters (¶¶ 179, 185). Cyclic anhydride and cyclic ester components Oxides of thio esters are named not as ester derivatives but at the preferred are named in accordance with heterocyclic nomenclature. Anhydride terms molecular skeleton or other heading parent with sulfinyl or sulfonyl prefixes. precede ester terms where this can be done unambiguously (but see the last ex- Examples: ample, below). Esters cited in modifications are named in the uninverted form unless the acid is one for which a synonym line formula is required. O O
= = Examples:
H C−OS1 4 −S O−CH 3 = 3 O
Benzene, 1-methoxy-4-[[(4-meth- O O oxyphenyl)sulfinyl]sulfonyl]- 7a O 1 O 2 3 α O CO (CH ) O C
= 2 2 2 2 O O C−S−CH 4 = 3 Methanone, (methylsulfinyl)phenyl-, N−OH oxime N 5-Isobenzofurancarboxylic acid, 1,3-dihydro-1,3-dioxo-, Glycerides, esters of 1,2,3-Propanetriol ( glycerol ), are indexed at the name 5,5′-(1,2-ethanediyl) ester of the preferred acid unless only “Class I” acids are present. Examples: O
= 4H-3,1-Benzoxathiin-4-one, − − O O C CH3 O 1 2-(cyclohexylimino)- (not = − = 8a S − − − − − − − − N CH3 C O CH2 CH CH2 O C CH3 Carbonimidothioic acid, 123 cyclohexyl-, anhydride with 4 O 1,2,3-Propanetriol, 3 2-mercaptobenzoic acid, cyclic 1,2,3-triacetate 5 O ester) 57 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 188
O Acetic acid, The heading parent Nitroxide is employed for the free radical H N−O•.
= 2 EtO−P−OAc anhydride with ethyl Example: H− hydrogen phosphonate Me2NO• Nitroxide, dimethyl O
CH −CH = O 2 3 − −
− O C CH = 3 − − − = − Free radicals from hydroxyl and hydroperoxy groups that are attached to a CH3 CH2 C C C O B molecular skeleton, including (acyloxy) radicals (see below), are indexed at “- − O−C−CH CH 3 3 = oxy” and “-dioxy” heading parents with systematically named nondetachable O prefixes. Chalcogen analogs are named similarly at “-thio” and “-dithio” head- ing parents. Acetic acid, Examples: anhydride with boric acid (H3BO3) mono(1-ethyl-2- methyl-3-oxo-1-penten-1-yl) MeO• Methoxy (not Oxy, methyl-) ester (2:1) O = O O HC−O• Methoxy, oxo- (not Formyloxy) − −Ο− = − − − = ClCH2 CH2 C CH2 CH2 C Butanedioic acid, − ′ ′ O
1,1 -anhydride, 4,4 -bis(2- = O O − −Ο
− CH C O• = chloroethyl) ester 3 Ethyldioxy, 1-oxo- − −Ο− − − − ClCH2 CH2 C CH2 CH2 C = O O − = − CH3 C O• Ethoxy, 1-oxo- (not Acetyloxy)
O O O Cl N=NO• Diazenyloxy, 2-(4-chlorophenyl)- −Ο− = − − − = −Ο− = − CH3 C CH2 CH2 C C CH3 43 2 1 Butanedioic acid, MeS• Methylthio 1-anhydride with acetic acid, 4-methyl ester Acyl free radicals (derived from aldehydes or acids) are named as α-oxo de- CH3
− rivatives of the alkyl parents. − − O O O CH CH3 Examples: = = Ο− − − −Ο− − − − C CH2 C B O CH3 O
= Ethyl, 1-oxo- (not Acetyl) − Propanedioic acid, CH3 C• 1-anhydride with boric acid Methyl, cyclopropyloxo- (not (H3BO3) monomethyl mono CO• (1-methylethyl) ester, 3-phenyl Cyclopropylcarbonyl) ester
H2NCO• Methyl, aminooxo- (not Amino- 187. Free radicals, highest in the order of precedence of compound classes carbonyl) (¶ 106) and functional groups, if present, are named as substituents. Most, but not all, free radical names coincide with the names of substituent prefixes (¶¶ Free radicals from sulfonic and sulfinic acids are named as derivatives of 132, 133). alkyl and aryl sulfonyl and sulfinyl radicals and the analogous oxy radicals. Examples: Examples: − − CH3 SO2 O• (Methylsulfonyl)oxy Et• Ethyl
Methyl, phenyl- PhCH2• Me S(O)• Phenylsulfinyl, 4-methyl-
′ •CH2 CH2• Methyl, 1,1 -(1,4-phenylene)bis- The following chalcogen hydride free radicals are employed: Hydroxyl, HO•; Hydroperoxo, HOO•; Mercapto, HS•; and Selenyl, HSe•. 188. Halogen and halogenoid compounds. (See also Acid halides, ¶ 170). • BrC• Methylidyne, bromo- The halogen and halogenoid “oxo” acids are hypohalous acids, HOX (e.g., Hy- pochlorous acid, HOCl); halous acids, HOXO; halic acids, HOXO2; perhalic acids, HOXO ; Cyanic acid, HOCN; and Fulminic acid , HONC. Their esters EtO2CCO• Ethyl, 2-ethoxy-1,2-dioxo- (not 3 21 Acetyl, ethoxyoxo-) and anhydrides are named in the regular way. They rank below acids, e.g., car- boxylic, sulfonic, expressed as suffixes on molecular skeleton names (¶ 106), Phosphino and in their presence are expressed as cyanato, (iodyloxy), (chloryloxy), etc., H2P• radicals. Examples: • Silylene, difluoro- F2Si• PhOF Hypofluorous acid , Ph2NN(CHO)• Hydrazinyl, 1-formyl-2,2-diphenyl- phenyl ester 21
MeCO(CH2)2OBrO2 Bromic acid , Free radicals that might be considered as derived from Borane are indexed 3-oxobutyl ester at Borane(1) and Borane(2). OCN Examples:
FHB• Borane(2), fluoro- (not Boryl, fluoro-) Cyanic acid, 3-methylphenyl ester Me FB• Borane(1), fluoro- (not Boron • fluoride (BF)) Benzoic acid, 4-chloro-, Cl CO2I Free radicals from ammonia , amides, or amines are named as Amidogen, anhydride with hypoiodous acid (not Benzoyl hypoiodite, 4-chloro-) H2N•, Imidogen , HN:, and their derivatives. O Propanethioic acid, − − = − − CH3 CH2 C S Cl anhydrosulfide with thiohypo- F2N• Amidogen, difluoro- (not Nitrogen chlorous acid (not Propanoyl fluoride (NF2)) thiohypochlorite)
• NCOCH CO H Acetic acid, 2-cyanato- PhCH(CO2H)N• Imidogen, (carboxyphenylmethyl)- 2 2 ¶ 188 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 58
Benzoic acid, 4-(chloryloxy)- 190. Hydrazones of ketone and aldehyde principal groups are expressed by O2ClO CO2H hydrazone terms in the index modification, except for hydrazones of carbonyl groups of acids, acid halides, and amides; these are usually indexed as hydra- − − − − − − − zonic acids (¶ 169) and their halides and amides. Azines are indexed as The groups X, XO, XO2, XO3, NC, NCO, N3, and their chalcogen analogs, e.g., −XSe; −NCS, are normally expressed as substituent prefixes “-ylidene” hydrazones; osazones as dihydrazones (of adjacent carbonyl when attached to a molecular skeleton; the radicals chloro, chlorosyl, chloryl, groups); and cyclic hydrazones as heterocycles. In the presence of compound perchloryl, isocyano, isocyanato, isothiocyanato, azido, etc., are used (see the classes more preferred than the aldehyde or ketone bearing the hydrazone, a Illustrative List of Substituent Prefixes in Section H (¶ 294)). hydrazono substituent is cited. Examples: Examples: = Methane, trichloro- (not Chloroform ) Me2C NNH2 2-Propanone, CHCl3 hydrazone
PhIO2 Benzene, iodyl- = = Ph(CH2)2CH NNH NHN CH(CH2)2Ph NC N Piperidine, 1-isocyano- 6 1 Benzenepropanal, 1,1′-[2,2′-(1,4-phenylene)dihydra- zone]
Me2Si(NCO)2 Silane, diisocyanatodimethyl- (not H2 H2 CH Isocyanic acid, dimethylsilylene C − C 3 = − = − −( − ester) H2C C N N C CH2)4 CH3 C − C − − H2 H2 Groups such as I(OH)2, ClBr2, are not expressed by (dihydroxyiodo) and Cyclohexanone, (dibromochloro) radicals; instead, coordination nomenclature (¶ 215) based on 2-(1-methylhexylidene)hydrazone the central halogen atom is employed. (not 2-Heptanone, azine with Example: cyclohexanone) (principle: ring preferred to chain) PhI(OH)2 Iodine , dihydroxyphenyl-

- University of Texas Libraries
- UT Libraries
- Chemical Abstracts
- For New Researchers
- Literature Tutorial
- Support the Library
- Science of Synthesis
- Landolt-Börnstein
- Gmelin Handbook
- Beilstein Handbook
- Textbooks & OER
- Conferences & Symposia
- Tech Reports
- Historical Information
- Free Resources
- Analytical Chemistry
- Biochemistry This link opens in a new window
- Crystallography
- Electrochemistry
- General Chemistry
- Inorganic Chemistry
- Organic Chemistry
- Physical Properties
- Polymers and Plastics
- Safety, Hazards, Environment
- Spectroscopy

- History of Chemical Abstracts ACS Chemical Landmark series.
- Organization
- CA Abstract Numbers
- For Librarians
Abstracts There were 26 weekly issues per semiannual "volume." Each Abstract issue was divided into 80 Subject Sections. An abstract appeared in just one section, based on the novelty of the process or substance being reported in the literature. Each weekly issue also contained indexes by author, subject keyword (not official headings), and patent number. The issue indexes were superseded first by a volume index published every six months, and then by the 5-year Collective Index. (The library did not retain the issue and volume indexes.)
Collective Indexes Every five years CAS published a Collective Index (CI). The 14th CI was published in 2002 and covers the years 1997-2001. The library has all Collective Indexes up to this point. They are divided into:
- Author Index , 1907-2001
- Subject Index 1907-71 (included chemical substance names through 1971)
- Chemical Substance Index , 1972-2001 (includes all CA Index Names used during the specific index period)
- General Subject Index , 1972-2001 (includes all subject and compound-class terms that are not systematic CA Index Names)
- Formula Index , 1920-2001
- Patent Index , 1907-2001
Index Guides The Index Guide (IG) for each Collective Index period provides cross-references from commonly used chemical names to official CA Index Names (with registry numbers) used in the corresponding Chemical Substance Index. It also serves as a thesaurus of all controlled-vocabulary subject headings used in the General Subject Index. The Index Guide should always be consulted before looking up a chemical name or subject term in the Collective Indexes.
Ring Systems Handbook The RSH leads you from a ring or cage structure to the CA Index Name and Registry Number of a ring parent compound, for searching in the Chemical Substance Index. Entries are in ring analysis order and are indexed by molecular formula and Index Name.
Registry Handbook The Registry Handbook - Number Section was a cumulative numerical listing of Registry Numbers assigned to chemical substances from 1965 to 1996. If you have only a registry number and need the CA Index Name for that compound, look it up here first and then use the name to consult the Chemical Substance Indexes. A corresponding Names Section issued on microfiche provided registry numbers for several hundred thousand of the most-indexed common names.
CASSI CASSI (Chemical Abstracts Service Source Index) is the comprehensive and retrospective list of publications that have been indexed by Chemical Abstracts since it began in 1907. It includes journals, books, conferences, and other series, arranged by CA abbreviation. This is the source you use to translate journal title abbreviations into full titles for searching in the library catalog and other finding aids. The last print edition of CASSI (1907-2004) is kept in the Librarian's office. It is also available in a somewhat limited form on the web:
Doing a manual search in printed Chemical Abstracts is a tedious, mutli-step process. This is how it was done.
- Author: Entries are arranged by last name, then by first and second initials (not by first name). Qualifying text is the title of the document. Coauthors are cross-referenced to first author.
- Formula: Entries contain only abstract numbers unless there is a large number of them, and no qualifying text. It's best to use the Formula Index to get the corresponding CA Index Name, then look up that name in the corresponding Chemical Substance or Subject (1907-71) index, where the entries are more detailed. Formulas are listed in Hill order: C, then H, then other elements in alphabetical order.
- Chemical Substance name: Start with the Index Guide to see if there's an entry for the name you have. If not, use the Formula Index or Ring Systems Handbook to get the name. In the CSI you must use only the specific CA Index Name for that CI period. There are no cross references to earlier or generic names. Names are arranged by "parent" (the structural skeleton) followed by substituents and modifications. Qualifying text in each entry indicates what the document is primarily about, followed by an abstract number. About 600 of the most frequently indexed compounds are called "Qualified Substances." Their document entries are grouped into seven categories: Analysis, Biological studies, Occurrence, Preparation, Properties, Reactions, Uses and miscellaneous.
- Subject term: Check the Index Guide first to find an appropriate term to look up in the Subject Index (1907-71) or General Subject Index (1972- ). Classes of compounds (e.g. Carcinogens), undefined compounds and mixtures (e.g. Gasoline), processes, plant/animal species, and other general topical terms are found in this index, along with cross references and scope notes.
- Patent number: Arranged by issuing country/organization, then by patent number. CA abstracts only the first member of a patent family, and links later equivalent patents to this parent patent. Equivalents are cross-referenced to the parent. Prior to 1981 the equivalents were listed in the Patent Concordance.
- Note Abstract Numbers from the entries of interest. Abstract numbers prefixed "R" indicate a review; "P" indicates a patent.
- Go to the corresponding Abstracts volume and look up the abstract by its number.
- Repeat this process for earlier or later index periods. Remember that Index Names and subject headings changed over time, so consult the Index Guide for each CI period.
For Librarians: Retention of Chemical Abstracts

- Here's the most important consideration: It's highly unlikely that any scientist born after the mid-1970s would have any experience using print CA (or any printed index for that matter), or even be aware of its existence. Therefore academic libraries should expect all potential use to be initiated and mediated by a library staff member or senior faculty member who has working knowledge of this tool. If no such persons remain on the campus, then print CA is almost certainly a waste of space. (Similarly, there is no longer any practical reason to teach students how to use it!)
- SciFinder is not identical to Chemical Abstracts. All (or nearly all) the metadata content of the latter is included in the CAPLUS file and robustly substance-indexed via the Registry file. But it is inaccurate to say that you can do everything in SciFinder that you could do in the print.
- The Collective subject/substance/formula indexes allow browsing of chemical names, formulas, and subject headings in a way that isn't possible in SciFinder. SciFinder is great for snapshots, but it provides only a limited view of the hierarchical structure of the CA database, or its indexing and nomenclature practices; nor does it allow easy browsing for derivatives, salts, and other variants of a parent structure. In other words, you can't browse online for nearby entries like you can in the print, which removes a serendipity factor. For some purposes, this is an important distinction. Browsing and searching CA indexing terms for concepts, chemical classes, and taxonomic vocabulary from the CAS Lexicon (thesaurus) is possible in SciFinder, as of 2023.
- When you can't figure out how CAS has defined the structure or formula of certain types of compounds, especially inorganic (salts, hydrates, ions, decimals, etc), coordination compounds, and multicomponent substances, SciFinder can be frustrating. Using the Index Guide and Chemical Substance Index can actually save some time, and when you find the Registry number then you can go back to SciFinder, locate the substance record and complete the literature search. (Of course, this method only works for compounds registered before your last Collective Index.)
- Pre-1967 CA abstract numbers are not searchable or displayed in SciFinder, and can only be looked up or verified in the print. These numbers were occasionally cited in the older literature, especially as stand-ins for obscure and foreign documents.
- Some older printed abstracts may contain structure graphics that aren't duplicated online.
- If you have bound any of the six-month volume indexes, and you have the equivalent Collectives and their Index Guides, the former are expendable and should be discarded to save space. And hopefully the indexes in the back of the weekly issues were sliced out and discarded before binding -- those are indeed useless and add a significant amount of linear footage.
- Production of printed CA ceased in 2009, and the hardcopy is now only applicable to historical searching. It is not a viable substitute for any form of current online searching.
- Even if you decide to discard the bulk of CA, consider retaining the most valuable parts, such as the Index Guides (potentially useful for finding contemporary index terms, synonyms, controlled vocabulary, Registry Numbers, etc.). If you wish to split the run by time period, collective wisdom suggests that the older (and smaller) pre-1967 portion of CA is more useful than the post-1967 volumes.
- If the facility lacks space and staff who can retrieve and consult CA volumes to mediate a reference question, stored CA can't be used as designed.
- If storage space is at a premium, it's difficult to justify the space CA would occupy there. (A complete set of CA with indexes can occupy as much as 1000 linear feet of shelving, depending on how a library has bound it.) The trend toward shared/consortial storage may allow multiple institutions to share a single print copy.
- If the item-specific metadata in your catalog don't include abstract number ranges -- as opposed to issue numbers, which are useless -- remote usage/retrieval of CA volumes becomes even more problematic and impractical.
- ACS does not require institutions to retain print CA for chemistry program approval. (There's no requirement for SciFinder either. See ACS Committee on Professional Training guidelines for more information.)
- Last Updated: May 6, 2024 9:41 AM
- URL: https://guides.lib.utexas.edu/chemistry

Chemical Abstracts Index Names: Automatic Generation
Cite this chapter.

- Shengang Yuan 3 ,
- Chongzhi Zheng 3 &
- Jianhua Yao 3
Part of the book series: Data and Knowledge in a Changing World ((DATAKNOWL))
120 Accesses
The complexity for assigning the systematic name (IUPAC system or CA index name) to a structure led to the development of computer software to support the automatic generation of nomenclature. The Chemical Abstracts (CA) index names are very useful for finding various information through CA. We designed a structure naming algorithm whose core is the unic Bridge-E_Block approach. This approach dissects the structure into E_Blocks and converts it into E_Block Bridge Tree (EBBT) in which each node corresponds to a namable structural fragment. This approach makes it easier to identify namable structural fragments, parent selection and substituent determination. Based on the approaches proposed in this paper a naming system with high performance could be developed.
La complexité de l’assignation d’un nom systématique (système IUPAC ou nom indexé CAS) à une structure a conduit au développement de logiciels pour aborder la génération automatique de la nomenclature. Les noms indexés du Chemical Abstracts (CA) sont très utiles pour chercher des informations variées dans CA. Nous avons conçu un algorithme de génération d’un nom structural dont le centre est du type unic Bridge-E_Block Cette approche dissèque la structure en Bloc E et la convertit en un arbre de Bridge-E_Block (EBBT) dans lequel chaque noeud correspond à un fragment structural qui peut recevoir un nom. Cette approche facilite l’identification des fragments structuraux à “nom”, la sélection de parents et de substituants. Basé sur les approches proposées dans cet article un système de nomenclature de haute performance pourrait être développé.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Unable to display preview. Download preview PDF.
Mockus J., Isenberg A.C., Vander Stouw G.G., J. Chem. Inf. Comput. Sci., 21, 183–195 (1981)
Article CAS Google Scholar
Meyer D.E., Gould, S.R., Am. Lab., 20(11), 92 (1988)
CAS Google Scholar
Wisniewski J.L., J. Chem. Inf. Comput. Sci, 30, 324 (1990)
Ullman J.R., J. Assoc. Comput. Mach., 23, 31–42 (1976)
Article Google Scholar
Brint A.T., Mitchell E., Willett, P., Substructure searching in files of three-dimensional chemical structures, in W.A. Warr (ed.) Chemical structure, The International Language of Chemistry.
Google Scholar
Rucker G., Rucker C., Chimia, 44, 116–120 (1990)
Van Binnendyk D., Mackay A.C., Can. J. Chem, 51, 718–723 (1973)
Balaban A.T., J. Comput. Chem., 6, 316–329 (1985)
Download references
Author information
Authors and affiliations.
Laboratory of Computer Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032, China
Shengang Yuan, Chongzhi Zheng & Jianhua Yao
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Institut de Topologie et de Dynamique des Systèmes (ITODYS), Université Denis Diderot Paris VII, 1 rue Guy de la Brosse, F - 75005, Paris, France
Jacques-Emile Dubois
The MITRE Corporation, 7525 Colshire Drive, McLean, VA, 22102, USA
Nahum Gershon
Rights and permissions
Reprints and permissions
Copyright information
© 1996 Springer-Verlag Berlin Heidelberg
About this chapter
Yuan, S., Zheng, C., Yao, J. (1996). Chemical Abstracts Index Names: Automatic Generation. In: Dubois, JE., Gershon, N. (eds) Industrial Information and Design Issues. Data and Knowledge in a Changing World. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-80286-7_31
Download citation
DOI : https://doi.org/10.1007/978-3-642-80286-7_31
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-642-80288-1
Online ISBN : 978-3-642-80286-7
eBook Packages : Springer Book Archive
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Architecture and Design
- Asian and Pacific Studies
- Business and Economics
- Classical and Ancient Near Eastern Studies
- Computer Sciences
- Cultural Studies
- Engineering
- General Interest
- Geosciences
- Industrial Chemistry
- Islamic and Middle Eastern Studies
- Jewish Studies
- Library and Information Science, Book Studies
- Life Sciences
- Linguistics and Semiotics
- Literary Studies
- Materials Sciences
- Mathematics
- Social Sciences
- Sports and Recreation
- Theology and Religion
- Publish your article
- The role of authors
- Promoting your article
- Abstracting & indexing
- Publishing Ethics
- Why publish with De Gruyter
- How to publish with De Gruyter
- Our book series
- Our subject areas
- Your digital product at De Gruyter
- Contribute to our reference works
- Product information
- Tools & resources
- Product Information
- Promotional Materials
- Orders and Inquiries
- FAQ for Library Suppliers and Book Sellers
- Repository Policy
- Free access policy
- Open Access agreements
- Database portals
- For Authors
- Customer service
- People + Culture
- Journal Management
- How to join us
- Working at De Gruyter
- Mission & Vision
- De Gruyter Foundation
- De Gruyter Ebound
- Our Responsibility
- Partner publishers

Your purchase has been completed. Your documents are now available to view.
Systematic Nomenclature of Organic, Organometallic and Coordination Chemistry. Chemical-Abstracts Guidelines with IUPAC Recommendations and Many Trivial Names
by Ursula Bünzli-Trepp
Logos Verlag Berlin 2021; 685 pp.
reviewed by Molly Strausbaugh, Edwin Constable, Andrey Yerin, and Ture Damhus, all of IUPAC Division VIII.

Accuracy and unambiguity are at the heart of good science communication, and the publication of the second edition of Ursula Bünzli-Trepp’s book on chemical nomenclature is a significant contribution toward achieving these standards. The title of the book is self-explanatory, and the author presents the topic in an encyclopedic manner. The book comprises six chapters: Directions for the use of this book , Fundamentals , Guide to name construction and name interpretation , Molecular-skeleton parents , Substituent prefixes , and Compound classes, together with appendices and tables. The coverage of organic compounds is more comprehensive than that of coordination and organometallic species, which is confined to 55 pages in the chapter entitled Compound classes . The text is an overview of the major nomenclature systems used in the Anglophone world but cannot be considered a complete guide to any of them. The liberal approach to nomenclature found in the primary literature is a justification for a book that shows what could (and could not) be considered an appropriate name.
The text usually emphasizes the differences between IUPAC and Chemical Abstracts Service (CAS) nomenclatures and occasionally gives value judgements of the approaches (see below on anionic ligands). Very often, the author lands on the side of the CAS name. Much of the book comprises loose paraphrases of IUPAC and CAS documents, making it a good reference work but does not necessarily enhance the readability. The organization of the sections might not be obvious to all readers, and it is not always simple to determine the hierarchical level of nomenclature to which a particular section belongs.
It is useful to consider what readers might expect to gain from this book:
1. An understanding of the basics of CAS and IUPAC nomenclature
2. The ability to convert an “official” CAS or IUPAC name to a structure
3. A feeling for the most appropriate nomenclature system to use for their compounds of choice
4. All of the above in an accessible and readable form that, at the same time, would serve as a single point of reference.
The structure of this review will center upon these four aspects.
In the first aspect, the author succeeds in giving a broad understanding of the principles of the two major systems of nomenclature, although the approach of dealing with them together has an inherent danger of leaving the reader confused and adopting hybrid nomenclature schemes (which probably is a fair assessment of the general approach to nomenclature by most practicing chemists). The author probably over-estimates the level of knowledge in the general community regarding the nuances and similarities of, as well as the differences in, the CAS and IUPAC systems. The 520 pages of main text are information-rich and contain numerous chemical structures which clearly illustrate the nomenclature principles. The author has had to base herself on the 2013 IUPAC Blue Book [ 1 ], which was, however, revised in the 2021 html online version [ 2 ]. The reader should refer also to the latter version. The present book fills a gap between the very short IUPAC publications, Brief Guide to Polymer Nomenclature [ 3 ] , Brief Guide to the Nomenclature of Organic Chemistry [ 4 ] and Brief Guide to the Nomenclature of Inorganic Chemistry [ 5 ], the broader Principles of Chemical Nomenclature: A Guide to IUPAC Recommendations (2011) [ 6 ], and the full documentation in the IUPAC Red (2005) [ 7 ], Purple (2008) [ 8 ] and Blue (2013, 2021) [ 1 , 2 ] Books and the CAS Index Guide [ 9 ].
The emphasis of the book is on the construction of accurate and unambiguous names for compounds of known structure. In reality, the opposite process is equally important for a chemist and the need to deconvolute a systematic, semi-systematic or trivial name in a chemical catalog or publication to a chemical structure is one of the most common ways in which nomenclature impinges on our everyday life. Despite the title of Chapter 3 mentioned above, this deconvolution aspect, as found in other general texts dealing with nomenclature, is not explicitly addressed in the current book. Although the process is relatively straightforward if IUPAC names are used, a degree of familiarity with the rules is essential. In a future edition, a flow chart illustrating this reverse procedure for both CAS and IUPAC names would considerably benefit the reader.
Although we like to think that chemists want to invest time in constructing accurate and unambiguous names for their compounds using one of the commonly accepted ( i.e. , CAS or IUPAC) systems, we know this is not the case. This book presents strategies for naming most of the types of compounds likely to be encountered in molecular chemistry. The author often prefers the CAS name over IUPAC, but usually gives justification for the choice. In many cases, the author only gives CAS names arguing that the CAS guidelines are simpler to apply than the IUPAC recommendations. This is a personal choice, and many readers may prefer the IUPAC system.
A particularly debatable subject is the naming of esters (Section 6.14). The functional class names preferred by IUPAC (“ethyl acetate” type) constitute a uniform system, while the CAS system is more complicated with different name constructions depending on the complexity of alcohol and acid components ( e.g., “acetic acid ethyl ester” but “2-chloroethanol acetate”), and the book requires considerable space to describe the system.
Another example could be the discussion of anionic ligands on p. 465 and pp. 476-477. The IUPAC selection of “ido” endings for anionic ligands, such as chlorido, oxido and cyanido, was made to ensure a simple general and pedagogical procedure for generating ligand names from anion names. These names also distinguish these species when acting as ligands rather than as substituents, when they are notated chloro, oxo and cyano. These aspects do not seem to be appreciated by the Author.
The final question relates to accessibility and readability and whether the book can serve as a single point of reference. The book is not entirely successful in this respect. The text is attractively presented, although the font is relatively small. Colors are used sensibly and will be helpful, except to readers who are color-blind. Molecular structures are nicely drawn, although the hashed lines representing bonds pointing below the plane are ambiguous and have been replaced with hashed wedges by both CAS and IUPAC. Structures are generally placed where they are named in the text; Table 6.4 with the structural diagrams on the opposite page from the table of ligand abbreviations is much more user-friendly than the combination of two tables needed in IUPAC’s Red Book 2005 [ 7 ].
There is a genuine question of whether a 680-page book can really be readable. It is certainly more reader-friendly than the Blue Book , but in this respect, Principles of Chemical Nomenclature mentioned above probably succeeds better, although with less comprehensive coverage. The real question is who the target audience is and whether it can serve as a single point of reference. This is very difficult to answer. On the one hand, the book is too detailed for doctoral and pre-doctoral workers and, on the other hand, does not contain enough detail for the established researcher who really wants to find the “correct” name for a compound. So, in this sense, the text successfully reaches the “normal” audience of chemists who want to use names that other scientists will understand without recourse to IUPAC’s color books or the CAS guidelines. Paradoxically, however, the concept of the book in presenting all of IUPAC, CAS and trivial names might deter this audience if they are seeking “ the name”.
The book does not properly address the future and pragmatic reality of nomenclature. We all know that in the laboratory, fully systematic names such as the book’s example 231 on p. 490, [1,1'-methylenebis[1,1-diphenylphosphine-κ P ]]bis(triphenylphosphine)palladium(2+) tetrafluoroborate(1–) (1:2), are not used, but rather short code-like names such as Pd-phosp or BJ219. Correct nomenclature is important for the precise identification of compounds in print and in electronic records. The future of nomenclature will increasingly involve machine-readable scripts, with a resultant drift away from plain text to precise code, which is not necessarily human-friendly. This omission from the book is all the more surprising considering that industry standard software such as ChemDraw can efficiently generate SMILES, InChI, and InChIKey descriptions for the vast majority of organic structures.
It would be surprising if a work of this length and with this large scope were completely free of errors, inaccuracies and omissions, but true typographical errors such as “bis(chlorate) (2 ClO 4 - )” on p. 477 seem to be rare.
There are issues of terminology, however. The introductory list of nomenclature terms under “Additive name” does not mention the use of additive procedures in coordination names and names of addition compounds—this is a significant omission. Under “Affix” in the same list, it says, “IUPAC uses the term affix as a collective term for prefixes and suffixes” (as if IUPAC does not include infixes). However, the wording in Section P-14.2 in the IUPAC Blue Book (1) is clear: “affixes (suffixes, infixes, and prefixes)”. Still further down, the definitions of “characteristic group” and “isolated group” are slightly surprising. For the less common term heterochains , one notes that there are homogeneous heterochains! A definition of “nonfunctional compound” is absent or at least difficult to find. The term “ending” is used differently from IUPAC, but the difference is explicitly acknowledged. In Table 3.2 and other places it seems that there is a seniority order of entire compounds of various kinds. It is not clear what this has to do with the choice of principal groups.
Bunzli-Trepp chooses to use “proton” generally instead of “hydron.” Just a matter of taste or convenience? The general name (from IUPAC) has officially been “hydron” since 1988, as opposed to “proton” specifically for 1 H + .
Being very brief when referring to alternative names may lead to inaccuracies. On p. 508 for Cp it says “IUPAC: cyclopentadienyl” as if IUPAC does not use cyclopenta-2,4-dien-1-yl (which IUPAC does, but cyclopentadienyl is given as an acceptable short form). We also note that the forms cyclopentadienido or cyclopenta-2,4-dien-1-ido are recommended by IUPAC in the names of coordination and organometallic entities.
The title of the book refers also to trivial names , i.e. , names containing no parts with a systematic meaning. Examples are given together with the definition of this term, but a couple of them (like D-glucose) contain stereodescriptors and may not be seen to fulfil the criterion. Further appearances of trivial names seem to be primarily those that have been adopted into systematics as names for parent hydrides or functional parents. Also, examples of various current systematic names are accompanied by notes giving alternatives, for example “trivially diethyl azodicarboxylate” or “trivially methylene chloride”. It may be debated whether these are true trivial names or just belong to older, more traditional, systematics.
The question is what expectations the title’s use of “trivial names” creates in the reader’s mind. One will not find a broad collection of daily-life trivial names like alum, borax, calomel, carborundum, caustic soda, ferroin, laughing gas, magnesia, nicotine, red lead, etc. , not to speak of common international non-proprietary names like amfetamine and triclosan or eponyms like Caro’s acid and Zeise’s salt or toponyms like Epsom salt. At least these names are not in the index, which appears to be otherwise very comprehensive, although IUPAC variants mentioned in small print in the main text may not be included in the index ( e.g. , borinine, arsinine, phosphinane).
The author frequently uses the heading “Instructions” to introduce the principles of naming a specific compound, although these are actually “Descriptions” rather than “Instructions.” There are several mistaken usages of “principals” for “principles.” Some classes of compounds are dealt with by what is called “Specialist nomenclature,” although compounds like carbohydrates and polymers are important to many of us who are not specialists within these classes.
In conclusion, despite the specifics discussed above and some inconsistencies, this book provides a comprehensive and detailed summary of current systematic CAS and IUPAC nomenclature systems. The inclusion of a great number of chemical structures with their various systematic and trivial names makes it a very useful handbook for the nomenclature of many classes of chemical compounds.
1. Favre, H.A.; Powell, W.H. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (IUPAC Blue Book); Royal Society of Chemistry Publishing, 2014. DOI: 10.1039/9781849733069 10.1039/9781849733069 Search in Google Scholar
2. Favre, H.A.; Powell, W.H. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (IUPAC Blue Book); html version with corrections as of 26 January 2022; IUPAC. https://iupac.qmul.ac.uk/BlueBook/ (accessed 2022-01-27). Search in Google Scholar
3. Hiorns, R.C.; et al . A brief guide to polymer nomenclature (IUPAC Technical Report). Pure Appl. Chem. 2012 , 84 (10), 2167-2169. DOI: 10.1351/PAC-REP-12-03-05 10.1351/PAC-REP-12-03-05 Search in Google Scholar
4. Hellwich, K-H.; et. al. Brief guide to the nomenclature of organic chemistry (IUPAC Technical Report). Pure Appl. Chem. 2020 , 92 (3), 527-539. DOI: 10.1515/pac-2019-0104 10.1515/pac-2019-0104 Search in Google Scholar
5. Hartshorn, R.M.; Hellwich, K-H.; Yerin, A.; Damhus, T.; Hutton, A.T. Brief guide to the nomenclature of inorganic chemistry (IUPAC Technical Report). Pure Appl. Chem. 2015 , 87 (9-10), 1039-1049. DOI: 10.1515/pac-2014-0718 10.1515/pac-2014-0718 Search in Google Scholar
6. Leigh, G.J. (Ed.). Principles of Chemical Nomenclature: A Guide to IUPAC Recommendations. Royal Society of Chemistry Publishing, 2011. 10.1039/9781839169021 Search in Google Scholar
7. Connelly, N.G.; et. al. Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005 ; Royal Society of Chemistry Publishing, 2005. Search in Google Scholar
8. Jones, R.G.; et. al Compendium of Polymer Terminology and Nomenclature: IUPAC Recommendations 2008 ; Royal Society of Chemistry Publishing, 2009. 10.1039/9781847559425 Search in Google Scholar
9. CAS, Naming and Indexing of Chemical Substances for Chemical Abstracts TM , 2007 Edition; CAS, American Chemical Society, 2008, https://www.cas.org/sites/default/files/documents/indexguideapp.pdf Search in Google Scholar
©2022 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
- X / Twitter
Supplementary Materials
Please login or register with De Gruyter to order this product.
Journal and Issue
Articles in the same issue.

IMAGES
VIDEO
COMMENTS
The chemical nomenclature used by Chemical Abstracts Service (CAS) has developed in parallel and generally in accordance with the rules published by the International Union of Pure and Applied Chemistry (IUPAC). Although these rules provide unambiguous text equivalents for the great majority of sub-
Chemical Abstracts is a periodical index that provides numerous tools such as SciFinder as well as tagged keywords, summaries, indexes of disclosures, and structures of compounds in recently published scientific documents. Approximately 8,000 journals, technical reports, dissertations, conference proceedings, and new books, available in at least 50 different languages, are monitored yearly, as ...
Chemical nomenclature is a set of rules to generate systematic names for chemical compounds. ... This requires adding more rules to the standard IUPAC system (the Chemical Abstracts Service system (CAS system) is the one used most commonly in this context), ...
CA Index Guide. The Index Guide to Chemical Abstracts is a useful tool for finding CA names for compounds when you know a common or trade name. For example: DDT See Benzene,1,1'- (2,2,2-trichloroethylidene)bis [4-chloro- [50-29-3] Note: The numbers in square brackets are the CA Registry Numbers for these compounds.
and the Chemical Abstracts Service (CAS) provide possibly the two most complete. These are both ... Jeffery Leigh is the editor and contributing author of Principles of Chemical Nomenclature—A Guide to IUPAC Recommendations, 2011 Edition (RSC 2011, ISBN 978-1-84973-007-5). Leigh is emeritus professor at the University of Sussex and has
Chemical Abstracts was not the first publication to abstract chemical information. Scientific abstracts first appeared in primary journals, which published abstracts of work reported in other sources in addition to original research. ... The indexing system had an important influence on chemical nomenclature. Over the years indexing assumed ...
The Index Guide to Chemical Abstracts is a useful tool for finding CA names for compounds when you know a common or trade name.For example: DDT See Benzene,1,1'-(2,2,2-trichloroethylidene)bis[4-chloro- [50-29-3]. It also indexes Enzyme Commission designations, for example: E.C.3.4.99.10 See Insulinase [9013-83-6]. Note: The numbers in square brackets are the CA Registry Numbers for these ...
A system of nomenclature which is independent of IUPAC, but which is widely used by chemists, is due to the Chemical Abstracts Service (CAS) of the American Chemical Society. This has been developed to produce names for CAS use in both running text and indexes.
Vander Stouw, G. G., Gustafson, C, Rule, J. D. and Watson, C. E. (1976) The Chemical Abstracts Service Chemical Registry System. IV. Use of the Registry System to support the preparation of index nomenclature. Journal of Chemical Information and Computer Sciences, 16 (4), 213-218. Google Scholar.
70 CHEMICAL NOMENCLATURE volume of the chemical literature. The system consisted of essentially three components: integrated, computer-assisted input of all information comprising Chemical Abstracts, computer databases to store and organize the information and a system to package the information in ways most useful to information users.
The rules of chemical nomenclature frequently change and each chemical substance is liable to have several names: i.e. trade or brand name(s); generic or common name(s); trivial or semisystematic name(s); and systematic or IUPAC name(s). ... Chemical Abstracts Index Guide. Columbus, OH: Chemical Abstracts Service, 1967-96 (ref QD 1 A51) ...
Chemical Abstracts (CA) nomenclature rules are described in Appendix IV of the Chemical Abstracts Index Guide. A list of ring systems, including names and numbering systems, is found in the Ring Systems Handbook, American Chemical Society, Columbus, OH, 2003, and its latest cumulative supplement. For CA nomenclature advice, consult the Manager ...
Stereochemistry and Stereoparents (¶¶ 202-212) The chemical nomenclature used by Chemical Abstracts Service (CAS) has F. Specialized Substances (¶¶ 213-224) developed in parallel and generally in accordance with the rules published by G. Chemical Substance Names for Retrospective Searches (¶¶ 225- the International Union of Pure and ...
nomenclature is discussed first in the following paragraphs, followed by mono-mer-based polymer nomenclature. Systematic (SRU) nomenclature for polymers has been adapted from the system developed by the Committee on Nomenclature of the Division of Poly-mer Chemistry of the American Chemical Society*. Names derived by this
generation of nomenclature. The Chemical Abstracts (CA) index names are very useful for finding various information through CA. We designed a structure naming algorithm whose core is the unic Bridge-E_Block approach. This approach dissects the structure into E _Blocks and converts it into E _Block Bridge Tree (EBBT) in which
chemical nomenclature and index term, which could now be done by the computer. This freed staff for the more intellectual tasks of analyzing the primary chemical literature. The CAS RegistrySM The mechanization of information yielded CAS' most significant and far-reaching innovation: The Chemical Registry System. G. Malcolm Dyson, hired
In this paper, we look at some of the chemicals described within the CWC and discuss how atoms and molecules, nomenclature and Chemical Abstracts Service (CAS) numbers, isomer enumeration, ring strain and stereochemistry influence the obligations of States to an international treaty. The material draws upon the authors' experiences from an ...
Its CA Index Name (the official name assigned to the structure according to Chemical Abstracts nomenclature rules) is: Ethanone, 1- (1 R, 6 R) - 9- azabicyclo [4.2.1] non- 2- en- 2- yl-. which doesn't exactly roll off the tongue! It's easy to see why people would rarely use this name in conversation, media, or even in a technical paper.
Chemical Abstracts - Chemistry - LibGuides at University of Texas at Austin. Chemical Abstracts was published in print from 1907 to 2009 by the American Chemical Society. In the decades before computer databases, large indexes like CA were the standard entry point into the scientific literature. Using them effectively was a skill that required ...
The Chemical Abstracts (CA) index names are very useful for finding various information through CA. We designed a structure naming algorithm whose core is the unic Bridge-E_Block approach. This approach dissects the structure into E_Blocks and converts it into E_Block Bridge Tree (EBBT) in which each node corresponds to a namable structural ...
IUPAC Polymer Nomenclature are standardized naming conventions for polymers set by the International Union of Pure and Applied Chemistry (IUPAC) and described in their publication "Compendium of Polymer Terminology and Nomenclature", which is also known as the "Purple Book". Both the IUPAC and Chemical Abstracts Service (CAS) make similar naming recommendations for the naming of polymers.
Article Systematic Nomenclature of Organic, Organometallic and Coordination Chemistry. Chemical-Abstracts Guidelines with IUPAC Recommendations and Many Trivial Names was published on July 1, 2022 in the journal Chemistry International (volume 44, issue 3).
Abstract The food enzyme sucrose phosphorylase (sucrose: ... IUBMB nomenclature: Sucrose phosphorylase: Systematic name: Sucrose:phosphate α-d-glucosyltransferase: ... 2.4.1.7: CAS no: 9074-06-0: EINECS no: 851-947-9: Abbreviations: CAS, Chemical Abstracts Service; EINECS, European Inventory of Existing Commercial Chemical Substances; IUBMB ...