

Sample Consent Forms
Consent form templates.
These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find instructions here .
Summary of Changes to the Regulations for Informed Consent: Revised Common Rule Changes to Informed Consent and Waiver Requirements
Summary of Changes to Consent Documents:
- Informed Consent Documents – Version 2.0 Summary of Changes
- Informed Consent Documents – Version 2.1 Summary of Changes
- Informed Consent Documents – 10/26/2020 Summary of Changes
- Informed Consent Documents – 4/10/2023 Summary of Changes
Concise Summary examples can be found here .
Guidance on the use of plain language in consent forms:
- Clinical Research Glossary
- Webinar: The Promise of Plain Language: Launching a Glossary to Support Participant Understanding of Clinical Research – Recording & Slides
There are a few additional forms that are not provided online and may be accessed below. As needed, these should be completed and uploaded to your IRB application.
Foreign Language Consent Forms
COVID-19 Related Forms:
- Spanish-IRB-COVID Information Sheet
- Spanish COVID Consent Letter v2
- Spanish COVID Informational Sheet Translation Certificate
Informed Consent Short Form (for a single subject who may be illiterate, or otherwise unable to read the consent form — used when full consent form has to be read or translated for subject).
- Informed Consent Short Form Guidance
- Simplified Chinese
HIPAA Templates
- Sample HIPAA Authorization Template
- Sample HIPAA Authorization Template in Spanish ( Certification )

Institutional Review Board (IRB): Consent
- Institutional Policies
- Get Started with IRB
- Archival/Secondary Data
- Research with Adults
- Research with Minors
- Research at a Site
- US Government or Military Research
- Research with American Indian or Alaska Native Populations
- International Research
- Research on Sensitive or Triggering Topics
- Special Guidance
- CITI Training
- Confidentiality Agreements
- Data Collection Instruments
- Data Use Agreements
- Eligibility Criteria
- Information Letters
- Online Surveys & Questionnaires
- Parental Permission and Minor Assent
- Recruitment Materials
- Site Permissions
- Group Writing Sessions
- Office Hours
- IRB Help Materials
- IRB Newsletters
- IRB Webinars
- Application Submission Instructions
- IRB Determinations
- Modify Your Study
- Close Your Study
- Faculty Resources
Consent Materials Templates & Resources
- Consent Online Anonymous Survey or Questionnaire
- Consent Form Guidelines and Template
- Consent Form Examples
Contact the IRB
When do I need a consent material?
You are required to write consent material/s for your NU IRB application if all of the following are true:
- You plan to conduct human subjects research
- You will be recruiting participants who are age 18 or older
- You are not researching a normal educational setting
If your research involves minors (17 years of age or younger), see the information regarding Parental Permission and Minor Assent . If your research involves normal education activities in everyday educational settings (adults or minors), see the information regarding Information Letters .
Introduction to Consent
Using one of the Consent material templates, you will write a consent material so that potential participants have all the information about your study in one place and can make an informed decision as to whether they want to participate in your study. Consent material is just one part of the informed consent process. Informed consent is an ongoing process that begins during recruitment and continues throughout the study.
How to gain Consent from potential participants
If you are engaging with adult participants in real-time (interviews, focus groups, observations, etc.) as your first or only activity, you will send potential participants a copy of the Consent Form before you meet with them. You then must do the following:
- Set aside time to review the consent form with each participant. As you review the consent form, remind participants that they do not have to participate if they do not want to, they can skip any question they do not want to answer, they can skip any activity they do not want to participate in, and they can stop participating at any time.
- Give participants an opportunity to ask questions about your study.
- Ask participants for verbal consent (e.g., "Do you still want to participate in this study?"). For Exempt and Expedited studies, the NU IRB does not require you to collect participant signatures or any written documentation of consent (if you are using a site IRB , they may require participant signatures).
The suggested verbal consent process
Email the consent form before the interview/focus group/observation with enough time for the participant to review it, and ask these questions at the beginning of the first data collection activity:
- Did you receive the consent form I emailed?
- Did you have time to review the consent form?
- Do you have any questions about the research or the consent form?
- Do you consent to participate in this research?
You need a 'yes' to questions 1, 2, & 4.
Adding the Online Consent to my survey/questionnaire
If you are distributing an Online Survey or Questionnaire , as your first or only activity, you will do the following:
You will paste your Online Consent into your survey/questionnaire as the first page. Please use the following steps (in Qualtrics).
- Open your survey/questionnaire for editing.
- Click on "Add Block" text below any block.
- Drag and drop the block so that it is the first block. This will place it above the default question block.
- Rename the block to Consent .
- Deselect the checkmark to the left of the question number. You do not need to have a question number on the consent.
- Click on the Add new question button.
- Click Text/Graphic from the list of question types.
- Copy and paste your consent into the text box.
- Review the headers to ensure they are bold, and follow them with a space. This will help your headings stand out by adding some space around them.
- Ensure that your entire consent was pasted into the text box and that is readable. You should paste the consent without the MS Word formatting as the hidden characters in MS Word can interfere with proper display and screen reader functionality.
- Add the following text to the bottom of the consent: "By clicking the next button and completing the survey you indicate that you have consented to participate in this research. If you do not want to participate, please close the browser."
- Save your work.
- Preview your survey/questionnaire and revise as needed.
When do I collect consent?
Only after you have received IRB approval can you consent participants. You cannot consent participants or collect data until you have IRB approval.
Consent is only collected once, before the first research activity.
Writing Consent
You are required to use the NU IRB consent template, unless you are working with an IRB/ HRPP that is requiring you to use their template.
Do I need to reword the template to avoid plagiarism?
No! Using a required template is not plagiarism. The consent template has language that is required by federal regulations. You should not change or revise any of the template language.
How do I write my consent if I have multiple participant groups?
If you have multiple participant groups, you will use a separate consent for each group.
Receive feedback on your consent
The NU IRB recommends that you receive feedback on your consent before you submit your IRB application:
- Attend a Group Writing Session
Submitting Your Consent material
The consent material you upload to your IRB application should be ready to send to the participants. Double-check the following:
- Don't change or reword any of the required template language
- Remove all blue highlighting, template instructions, and optional sections that aren't applicable to your study
- Remove any Track Changes or comments from your chair or the IRB
- Do not include any labeling or formatting from your dissertation (Appendix, etc.)
- << Previous: Confidentiality Agreements
- Next: Data Collection Instruments >>
- Last Updated: May 20, 2024 5:55 PM
- URL: https://resources.nu.edu/irb

© Copyright 2024 National University. All Rights Reserved.
Privacy Policy | Consumer Information
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
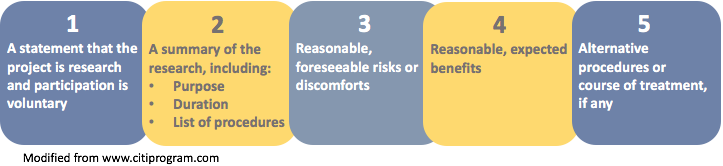
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
Informed consent guidance.
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Informed Consent Templates (2018 Common Rule)
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 04/10/2024.
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 04/10/2024
Other Templates
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects. Last updated 4/17/24
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools. Last updated 4/10/24
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
- Brief protocol for exempt research including data management and security questionnaire
Child Assent and Parental Permission
- Child assent ages 3-6
- Child assent 7-11
- Parent permission
- Child assent 12-14
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections

- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search
IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture

Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults
Template participant consent form and participant information sheet
Providing information about the research to participants, and gaining consent from participants before their involvement is a critical part of conducting ethical research.
Participant information sheet
All participants need to be provided access to an information sheet, and to understand the full details of the research, and how they will be involved. Please use the following template:
- Template participant information sheet
- Template participant information sheet veterinary research
Participant consent form
Before research begins, it is important to first obtain participant’s consent on the basis of their full and proven understanding of what the research will entail. Please use the following template:
- Template participant consent form
These templates should be followed as far as possible, as these have been developed using national guidance and expert input from Committee members and Lay Members. However, there may be times when it is appropriate to deviate from the templates in order to meet the needs of a specific research population.
The Health Research Authority and UK Research and Innovation webpages contain further guidance and templates on good practice when consenting participants.
Good practice in consenting participants
You should consider innovative ways of providing consent that are appropriate to your research population, for example, in addition to participant information and consent forms, could you provide the information using visual methods, such as a recorded video, or a study leaflet. Could you develop your forms in partnership with the communities who will take part in the study? Please see the 'informed consent' section of the research ethics handbook for further guidance.
Back to: Research
Dissertation surveys: Questions, examples, and best practices
Collect data for your dissertation with little effort and great results.
Dissertation surveys are one of the most powerful tools to get valuable insights and data for the culmination of your research. However, it’s one of the most stressful and time-consuming tasks you need to do. You want useful data from a representative sample that you can analyze and present as part of your dissertation. At SurveyPlanet, we’re committed to making it as easy and stress-free as possible to get the most out of your study.
With an intuitive and user-friendly design, our templates and premade questions can be your allies while creating a survey for your dissertation. Explore all the options we offer by simply signing up for an account—and leave the stress behind.
How to write dissertation survey questions
The first thing to do is to figure out which group of people is relevant for your study. When you know that, you’ll also be able to adjust the survey and write questions that will get the best results.
The next step is to write down the goal of your research and define it properly. Online surveys are one of the best and most inexpensive ways to reach respondents and achieve your goal.
Before writing any questions, think about how you’ll analyze the results. You don’t want to write and distribute a survey without keeping how to report your findings in mind. When your thesis questionnaire is out in the real world, it’s too late to conclude that the data you’re collecting might not be any good for assessment. Because of that, you need to create questions with analysis in mind.
You may find our five survey analysis tips for better insights helpful. We recommend reading it before analyzing your results.
Once you understand the parameters of your representative sample, goals, and analysis methodology, then it’s time to think about distribution. Survey distribution may feel like a headache, but you’ll find that many people will gladly participate.
Find communities where your targeted group hangs out and share the link to your survey with them. If you’re not sure how large your research sample should be, gauge it easily with the survey sample size calculator.
Need help with writing survey questions? Read our guide on well-written examples of good survey questions .
Dissertation survey examples
Whatever field you’re studying, we’re sure the following questions will prove useful when crafting your own.
At the beginning of every questionnaire, inform respondents of your topic and provide a consent form. After that, start with questions like:
- Please select your gender:
- What is the highest educational level you’ve completed?
- High school
- Bachelor degree
- Master’s degree
- On a scale of 1-7, how satisfied are you with your current job?
- Please rate the following statements:
- I always wait for people to text me first.
- Strongly Disagree
- Neither agree nor disagree
- Strongly agree
- My friends always complain that I never invite them anywhere.
- I prefer spending time alone.
- Rank which personality traits are most important when choosing a partner. Rank 1 - 7, where 1 is the most and 7 is the least important.
- Flexibility
- Independence
- How openly do you share feelings with your partner?
- Almost never
- Almost always
- In the last two weeks, how often did you experience headaches?
Dissertation survey best practices
There are a lot of DOs and DON’Ts you should keep in mind when conducting any survey, especially for your dissertation. To get valuable data from your targeted sample, follow these best practices:
Use the consent form.
The consent form is a must when distributing a research questionnaire. A respondent has to know how you’ll use their answers and that the survey is anonymous.
Avoid leading and double-barreled questions
Leading and double-barreled questions will produce inconclusive results—and you don’t want that. A question such as: “Do you like to watch TV and play video games?” is double-barreled because it has two variables.
On the other hand, leading questions such as “On a scale from 1-10 how would you rate the amazing experience with our customer support?” influence respondents to answer in a certain way, which produces biased results.
Use easy and straightforward language and questions
Don’t use terms and professional jargon that respondents won’t understand. Take into consideration their educational level and demographic traits and use easy-to-understand language when writing questions.
Mix close-ended and open-ended questions
Too many open-ended questions will annoy respondents. Also, analyzing the responses is harder. Use more close-ended questions for the best results and only a few open-ended ones.
Strategically use different types of responses
Likert scale, multiple-choice, and ranking are all types of responses you can use to collect data. But some response types suit some questions better. Make sure to strategically fit questions with response types.
Ensure that data privacy is a priority
Make sure to use an online survey tool that has SSL encryption and secure data processing. You don’t want to risk all your hard work going to waste because of poorly managed data security. Ensure that you only collect data that’s relevant to your dissertation survey and leave out any questions (such as name) that can identify the respondents.
Create dissertation questionnaires with SurveyPlanet
Overall, survey methodology is a great way to find research participants for your research study. You have all the tools required for creating a survey for a dissertation with SurveyPlanet—you only need to sign up . With powerful features like question branching, custom formatting, multiple languages, image choice questions, and easy export you will find everything needed to create, distribute, and analyze a dissertation survey.
Happy data gathering!
Sign up now
Free unlimited surveys, questions and responses.
Sample consent and permission forms
General consent form to participate in research (DOC)
Two stage project consent form (DOC)
Parent permission form for research with child (DOC)
Child assent form (DOC)
Multiple consent form including audio-recording and quotations (DOC)
Photo and video consent form (DOC)
Video-recording consent form (DOC)
Re-contact agreement form (DOC)
Post-debriefing consent form (DOC)
Our Courses
- Undergraduate Courses
- Postgraduate Courses
- Online Distance Learning
- Degree Apprenticeships
- CPD Short Courses
- Architecture and Construction
- Business and Law
- Creative Industries
- Digital and Cyber
- Health and Social Care
- Teaching and Education
- Courses A-Z
- Order a Prospectus
- How to apply
- Ask about a course
- Accounting, Finance and Economics
- Architecture
- Business and Management
- Computer Science
- Film, Media and Screen
- Pharmacy, Pharmaceutical Science and Pharmacology
- Public Health
- Social Work and Social Care
- Sport and Physical Activity
- Civil Engineering and Built Environment
- Cyber Security
- Health Sciences
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/240531-Sports-Awards-Resized.jpg)
Game Changers: Sporting elite take centre stage at annual sports awards
University Life
- Living in Wolverhampton
- Living in Walsall
- Living in Telford
- Make a Course Enquiry
- Opening Times
- Student Memberships
- Bus Stop Locations
- Humans of WLV
- Disability Support
- Mature Students Support
- Part-time work
- Student Safety
- How do I apply?
- City Campus
- Walsall Campus
- Telford Campus
- Springfield Campus
- Our Facilities
- Virtual Tour
- News and Events
- Find the right course for you
- Making your application
- After you've applied
- Scholarships
- Costs and Funding
- Repayment Options
- Contact the Gateway
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-18-19/220325-Engineers_teach_thumbail.jpg)
Looking for Bright Sparks: Engineers to teach Physics in new project
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/240524-Deaf-Studies-Resized.jpg)
Podcast launch celebrates 30 years of Sign Language Interpreter education
International
- Entry Requirements
- English Entry Requirements
- Apply Direct
- International Fees
- Prospective Students
- New Students
- Current Students
- Who to Contact
- Pre-Arrival Form
- Accommodation
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/240320-Uzbekistan-Resized.jpg)
New partnership agreement extends reach to Uzbekistan
Our Schools
- School of Creative Industries
- School of Social Science and Humanities
- University of Wolverhampton Business School
- University of Wolverhampton Law School
- School of Allied Health and Midwifery
- School of Education
- School of Nursing
- School of Psychology
- School of Health and Society
- School of Sport
- School of Architecture & Built Environment
- School of Engineering, Computing and Mathematical Sciences
- School of Life Sciences
- School of Pharmacy
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/240229-The-Link-Resized.jpg)
West Midlands Mayor cuts ribbon on new employment and skills hub in Walsall
Work With Us
- National Brownfield Institute
- University of Wolverhampton Science Park
- e-Innovation Centre
- Business and Technology Centre
- Conference Booking
- Dining & Refreshments
- Apprenticeship Courses
- Make an Enquiry
- Adopt green processes (EnTRESS)
- Knowledge Transfer Partnerships
- University Procurement
- University Jobs
- Executive Education Programmes and Courses
- Accessing our graduate talent pool
- General Higher Education sessions
- Partnerships
- Aspire to HE
- Supplying the University
- Our History
- Governance
- Academic Calendar
- Equality & Diversity
- Contacts & Maps
- Corporate Information
- Security Services
- Safeguarding
- Applicant Day
- Campus Tours
- Latest News
- Media Relations
- Follow Our Socials
- Teaching and Research
- Human Resources
- Training & Development Opportunities
- eLearning Portal
- Digital Print Services
- Staff Wellbeing Hub
- Comms Toolkit
- Careers Enterprise and The Workplace
- Student Support
- Academic calendar
- Course Guides
- Student Voice
- Stay in touch – Update your details
- Benefits & Services
- Transcripts & Certificates
- Volunteer Opportunities
- Make a donation
- Search Library Resources
- Using the library
- Subject Resources
- Skills for learning
- Digital Campus
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/240531-Rail-Alliance-Sponsored-Room-Opening.jpg)
Newly sponsored teaching room on track to provide opportunities for students
Information Sheets and Consent Forms
Potential recruits to your research study must be given sufficient information to allow them to decide whether or not they want to take part. An Information Sheet can take many forms though one of the most user friendly is the question and answer format. The information sheet should contain information that answers each question and tells participants about the study and what it involves.
The Information Sheet for participants and the consent/assent form are vital and must be completed carefully following the guidelines given below. The Information Sheet(s) and consent form should always be included with your application for ethical approval in order that the reviewing panel can see how you are presenting the project to potential participants.
You must provide separate Information Sheets where the differences between participants or different sections of the research require it (e.g. an Information Sheet for a child and a separate one for his/her parents/guardians, or an Information Sheet for those filling in a questionnaire and a different one for participants being interviewed).
The writer should include:
- simple words, sentences and paragraphs (take care to use language that is appropriate for the participant group)
- requests rather than commands
- the active voice (e.g. we will book) rather than the passive voice (e.g. appointments will be booked)
- a personal approach (e.g. we, you, your baby) rather than the impersonal (e.g. student, subject, they, those, he or she).
- A tick or initial box beside each statement
Jargon and acronyms should be avoided or accompanied by a clear explanation in everyday language. The whole Information Sheet should be understandable to the participant group e.g. a lay person, age appropriate or appropriate for people with a learning disability.
Things to include:
- Title of the Study (this should be in lay language)
- You should begin by making it clear that this is a study in which the volunteer is being requested to participate and that such participation is entirely voluntary.
- Explain - in straightforward, lay language - the nature and aims of the research project and describe any possible benefits.
- Where the project is being funded by a body/organisation other than the University of Wolverhampton, (such as a charity, organisation, research council or by a private company), identify who the funder is.
- As part of ensuring informed consent is obtained, your anticipated plans for dissemination/publication should be described.
- Who you are recruiting (who should, and who should not, take part).
- What will happen if the participant agrees to take part (when, where, etc) and how long their involvement will last for. If extensive/complicated schedule of procedures a flow chart of what will happen and when is visually helpful. Pictures of equipment help too e.g. what Scanners look like or monitors
- Any risks (e.g. the limits to confidentiality, the circumstances where they may be a need for disclosure of information to a third party, possibility for distress) or inconveniences/discomfort that may be reasonably anticipated.
- Possible benefits (it is good practice to offer participants a copy of the final report and in such cases you should indicate how participants can access this). Any other benefits stated must be honest and realistic.
- State the arrangements for ensuring anonymity and confidentiality and how far these can be realistically guaranteed (if there are possible limits to this). If you are giving participants a choice of full anonymity, partial identification or full identification, this should be made clear. To ensure compliance with the UK Data Protection Act 1998 participants must be informed of what information will be held about them and who will have access to it (this relates to information that is identifiable or could potentially be linked back to an individual).
- Explain that the participant can withdraw themselves from participation at any time and that they can also withdraw their data (including any limitations e.g. after anonymisation or statistical analysis). If limitations apply you should state a date beyond which withdrawal of data is no longer possible.
- Give the name, telephone number, university email address and Departmental/Faculty postal address of who to contact to obtain further details about the study (this should usually be the researcher) and who to contact in an emergency. Please be aware that the use of a researcher’s personal contact details may cause problems.
- State what compensation arrangements are available in the event of injury. In the case of drug company - sponsored research, the Information Sheet should state that the company concerned has agreed to compensate participants who may be harmed without them having to prove negligence.
Additional Notes
- When an outside agency is being used to transcribe data, you must inform participants of this.
- Where necessary, explain research procedures such as randomised control trial, anonymisation etc. in non-technical terms.
- Some researchers may find it helpful to include all information about a study in one letter to potential participants. In such cases the researcher must ensure that the required components for an Information Sheet are present within the letter.
- If the study is self-completion questionnaire based but is not seeking sensitive personal information it should still be stated on the Information Sheet that submission of a completed questionnaire implies consent to participate
- For questionnaire studies using online data collection methods, such as Survey Monkey, where it is possible for the researcher to access data from partially completed questionnaires (where the individual has navigated away from the questionnaire web page prior to reaching the end of the questionnaire and pressing the ‘submit’ button) the wording used regarding the consent process must be carefully considered, making it clear what data will be accessible to the researcher, what data will be used towards the study, and what the procedures are in terms of data withdrawal.
- For studies using online data collection methods, ensure it is clear to participants what sort of data the website administrators/owners will have access to.
- Where recruitment documents are to be translated into the participant’s own language, you will not be required to submit copies of the translated documents for review, only the English language versions. However it is the responsibility of the researcher to ensure that appropriate and accurate translations of the English language documents which are then approved, are used throughout the study once it formally commences. The researcher must provide assurances to the reviewers that the translated version will be an accurate translation of the one approved/submitted as part of the application.
- Explicit consent for data processing is required when collecting information classified as ‘sensitive personal data’* which is identifiable or could potentially be traced back to an individual. (‘Explicit consent’ is normally considered to be specific, written consent). As such, participants will need to give explicit consent for researchers to process sensitive data and permission for this should be sought on the consent form. In the case of self-completion questionnaires where no consent form is to be used the following statement should be included in the body of the questionnaire:
‘I consent to the processing of my personal information for the purposes of this research study. I understand that such information will be treated as strictly confidential and handled in accordance with the provisions of the Data Protection Act 2018.’
*The Data Protection Act, 2018 classifies sensitive personal data as consisting of: (a) the race or ethnic background of the data subject, (b) political opinions, (c) religious beliefs or other beliefs of a similar nature, (d) trade union membership (e) genetics, (f) biometrics, (g) health (h) sex life or orientation ( https://www.gov.uk/data-protection ).
Online surveys or questionnaires
This page contains information on selecting an appropriate platform for an online survey or questionnaire and how to ensure that:
- participants are given appropriate project information, including legally required data protection information, in a form they can retain indefinitely
- participants have the opportunity to ask questions should they wish
- participants' consent is obtained.
Selecting a platform
The University recommends Qualtrics as an online survey and questionnaire platform for research and encourages researchers to use it. The University has a licence for Qualtrics, meaning it is free to use for all those with a University login.
Current staff and students can access their account by going to the University of St Andrews Qualtrics site and using their University login details. Within Qualtrics, a range of training materials on how to use the software are provided.
Use of Microsoft Forms (via your University Office365 account) is also acceptable.
You must check with the University's Data Protection team before using any other platforms to process participants' personal data - email [email protected] .
Participant information and consent
Before participating in a project, participants should always be provided with a participant information sheet that they can keep and provided with an opportunity to ask questions about the project should they wish. You must obtain their consent to participate. When using online surveys or questionnaires to collect data, researchers should:
- this is important so that participants are given the legally required data protection information in a form that they can retain.
- Provide a final page containing a ‘Submit’ button, prefaced by a statement reminding the participant that clicking the final ‘Submit’ button of the survey at the end will constitute the participant providing consent to participate, in full knowledge of the information in the participant information sheet.
Example statements for both of these pages is provided below. The template participant information sheet is provided on the template documents web page .
Partial completion
Researchers should consider what will happen with participants data should they only partially complete the survey and fail to complete the final 'submit' phase that indicates consent. If partial data is of value and would not compromise the integrity of the dataset it may be worthwhile:
- ensuring it is not a requirement for submission that all questions be answered
- including a statement to let participants know that they can exit the survey at any time by skipping to the final page
Online survey or questionnaire - template first page opening statement
(Note: replace the text inside the square brackets with text appropriate for your project)
You are being invited to participate in a research study titled [project title]. This study is being done by [researcher name] from the [School/Department name] at the University of St Andrews.
[Provide the information contained in the Participant Information Sheet, indicating the aim of the project, a brief description of what the participant will be asked to do, and how long it will take. If there is a reward associated with any participation, please provide brief details].
If you are interested in taking part, please download a copy of the participant information sheet here [provide download link] and retain this for your records before starting the [survey or questionnaire]. If you have any questions, please email or call [me or us] at [researcher’s University email address] or [researcher’s University phone number].
Your participation is entirely voluntary, and you can withdraw at any time. You are free to omit any question. [Make sure your survey/questionnaire allows for the omission of questions].
[Start button]
Online survey or questionnaire - template final page closing statement
By clicking the ‘Submit’ button below, you are consenting to participate in this study, as it is described in the participant information sheet, which you can download here [provide download link]. If you did not yet download and keep a copy of this document for your records, we recommend you do that now.
[Submit button]

IMAGES
VIDEO
COMMENTS
Participant Consent Form This template is designed primarily for those doing qualitative interviews with adults from non-vulnerable populations and dealing with non-sensitive topics. The form would be different in the case of focus groups or quantitative research. If conducting research with vulnerable populations and / or sensitive topics please
The following is a sample consent form for a research project. It is a research project on faculty life on campus, carried out by the principle investigator (PI) of this project from the fake-named Century University. The interviewer (the investigator) should have the interviewee read this form carefully and ask any questions the interviewee ...
2023-04-10. Assent Form Ages 7-14. 2023-06-27. Consent Addendum for Unencrypted Communication. 2020-10-26. Information or Fact Sheet. 2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information.
This is a template to assist researchers in the design of their informed consent form. You must adapt this template to the requirements of your particular study, using the notes and suggestions provided. Before using this template, check whether your organisation provides a template consent form and if so, incorporate their requirements into ...
permission, adult consent, teacher consent, screening consent, etc.). • In this template, "we" refers to the researchers. If there is only one researcher, edit as appropriate. If the PI is a student, always use "we" to include the faculty advisor. • Submit consent documents in MS Word whenever possible. The iMedRIS comparison tool for
'Annotated' Template Example Consent Form. Template Consent Form (Word) Further Guidance. Guidance on obtaining consent from research participants online (for online and in-person study designs) Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL. Recording & Obtaining Consent
A Consent Form is read by the participant, signed and handed back to the researcher and should include the following features: 1. Use University of Wollongong/AHS letterhead. 2. Provide the title of the research project, the researcher(s) name, supervisor's name (for ... (eg thesis, journal publication, etc), and I consent for it to be used in ...
You are required to write consent material/s for your NU IRB application if all of the following are true: If your research involves minors (17 years of age or younger), see the information regarding Parental Permission and Minor Assent. If your research involves normal education activities in everyday educational settings (adults or minors ...
questionnaire, you are giving your consent. Summary I volunteer to take part in this PhD research questionnaire. I understand that the research aims to collect data on user requirements for security in humanitarian operations. The data collected in this questionnaire will be used in a PhD thesis and help expand the knowledge base of security ...
Whenever you are proposing research with human participants you must provide a form, known as an Informed Consent Form (ICF), with each proposal to indicate that the research participant has decided to take part in the research of her/his own free will. If the research involves more than one group of individuals, for example healthcare users ...
Template - Informed Consent for Anonymous Surveys Use if your research involves anonymous surveys online, in -person, via email, etc. Avoid Common Problems with Consent Forms. Read these tips! 1. Customize this template to reflect the specifics of your study and participant population. • Text in [brackets]
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS) Phone: (734) 936-0933. Fax: (734) 936-1852. [email protected]. Defines the term "informed consent process" and provides tips and other information to craft an appropriate informed consent document for a human subjects study and Univeristy of Michigan IRB review.
Oral Consent Template. Guidance for Protocols Involving Oral Consent. Debriefing Template. Guidance and Template for Debriefing Participants. Studies Involving Children (Assent/Permission Forms) Parent-Guardian Permission for Studies Involving Children. Sample Parental Notification Form. Sample Child Assent Form. Performance Release for Minors ...
An Ethics Consent Form is not always required for dissertation research at the undergraduate or master's level. It will depend on: (a) whether it is feasible to get informed consent, which varies according to the type of dissertation research you are performing; and (b) the expectations of your supervisor, department and/or university.
The following templates and samples are provided for investigators who are designing consent, assent, or permission forms for research with human participants. Theses forms are not intended as boilerplate text. Revise bracketed and example-specific text in the forms as appropriate to your project, keeping in mind best practices for informed ...
Participant consent form. Before research begins, it is important to first obtain participant's consent on the basis of their full and proven understanding of what the research will entail. Please use the following template: These templates should be followed as far as possible, as these have been developed using national guidance and expert ...
First you will be asked to fill a consent form. Then, if you consent to take part in the study, you will be asked to answer 38 short questions. All data will be collected and stored in accordance with the Data Protection Act 2018 and the General Data Protection Regulation. 6. What are the possible disadvantages and risks of taking part?
Dissertation survey best practices. There are a lot of DOs and DON'Ts you should keep in mind when conducting any survey, especially for your dissertation. To get valuable data from your targeted sample, follow these best practices: Use the consent form. The consent form is a must when distributing a research questionnaire.
Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
This consent form is necessary for us to ensure that you understand the purpose of your involvement and that you agree to the conditions of your participation. Would you therefore read the accompanying information sheet and then sign this form to certify that you approve the following: the interview will be recorded and a transcript will be ...
The Information Sheet (s) and consent form should always be included with your application for ethical approval in order that the reviewing panel can see how you are presenting the project to potential participants. You must provide separate Information Sheets where the differences between participants or different sections of the research ...
Selecting a platform. The University recommends Qualtrics as an online survey and questionnaire platform for research and encourages researchers to use it. The University has a licence for Qualtrics, meaning it is free to use for all those with a University login. Current staff and students can access their account by going to the University of ...
Appendix 1 is a data file for the Questionnaire Consent Form used for the mini study on the intricacies of teacher monitoring ... Join ResearchGate to discover and stay up-to-date with the latest ...