Identifiers
Linking ISSN (ISSN-L): 2472-0712
URL https://www.xiahepublishing.com/journal/erhm/archive
URL http://xandhpublishing.com/currentissue.aspx?sia=2&id=2
Google https://www.google.com/search?q=ISSN+%222472-0712%22
Bing https://www.bing.com/search?q=ISSN+%222472-0712%22
Yahoo https://search.yahoo.com/search?p=ISSN%20%222472-0712%22
Pubmed https://pubmed.ncbi.nlm.nih.gov/?term=%222472-0712%22%5BJournal%5D&sort=
Library of Congress https://catalog.loc.gov/vwebv/search?searchCode=STNO&searchArg=2472-0712&searchType=1&limitTo=none&fromYear=&toYear=&limitTo=LOCA%3Dall&limitTo=PLAC%3Dall&limitTo=TYPE%3Dall&limitTo=LANG%3Dall&recCount=25

Resource information
Archival status.

Title proper: Exploratory research and hypothesis in medicine.
Other variant title: ERHM
Country: United States
Medium: Online
| Status | Publisher | Keeper | From | To | Updated | Extent of archive |
|---|---|---|---|---|---|---|
| Preserved | Xia & He Publishing Inc. | Portico | 2022 | 2023 | 01/07/2024 | |
Record information
Last modification date: 03/08/2023
Type of record: Confirmed
ISSN Center responsible of the record: ISSN National Centre for the USA Please contact this ISSN Centre by clicking on it for any request or query concerning the publication
downloads requested
Discover all the features of the complete ISSN records
Display mode x.
Labelled view
MARC21 view
UNIMARC view
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(1); 2023 Jan

Clinical Research: A Review of Study Designs, Hypotheses, Errors, Sampling Types, Ethics, and Informed Consent
Addanki purna singh.
1 Physiology, Department of Biomedical Sciences, Saint James School of Medicine, The Quarter, AIA
Sabitha Vadakedath
2 Biochemistry, Prathima Institute of Medical Sciences, Karimnagar, IND
Venkataramana Kandi
3 Clinical Microbiology, Prathima Institute of Medical Sciences, Karimnagar, IND
Recently, we have been noticing an increase in the emergence and re-emergence of microbial infectious diseases. In the previous 100 years, there were several incidences of pandemics caused by different microbial species like the influenza virus , human immunodeficiency virus (HIV), dengue virus , severe acute respiratory syndrome Coronavirus (SARS-CoV), middle east respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV-2 that were responsible for severe morbidity and mortality among humans. Moreover, non-communicable diseases, including malignancies, diabetes, heart, liver, kidney, and lung diseases, have been on the rise. The medical fraternity, people, and governments all need to improve their preparedness to effectively tackle health emergencies. Clinical research, therefore, assumes increased significance in the current world and may potentially be applied to manage human health-related problems. In the current review, we describe the critical aspects of clinical research that include research designs, types of study hypotheses, errors, types of sampling, ethical concerns, and informed consent.
Introduction and background
To conduct successful and credible research, scientists/researchers should understand the key elements of clinical research like neutrality (unbiased), reliability, validity, and generalizability. Moreover, results from clinical studies are applied in the real world to benefit human health. As a result, researchers must understand the various types of research designs [ 1 ]. Before choosing a research design, the researchers must work out the aims and objectives of the study, identify the study population, and address the ethical concerns associated with the clinical study. Another significant aspect of clinical studies is the research methodology and the statistical applications that are employed to process the data and draw conclusions. There are primarily two types of research designs: observational studies and experimental studies [ 2 ]. Observational studies do not involve any interventions and are therefore considered inferior to experimental designs. The experimental studies include the clinical trials that are carried out among a selected group of participants who are given a drug to assess its safety and efficacy in treating and managing the disease. However, in the absence of a study group, a single-case experimental design (SCED) was suggested as an alternative methodology that is equally reliable as a randomization study [ 3 ]. The single case study designs are called N-of-1 type clinical trials [ 4 , 5 ]. The N-of-1 study design is being increasingly applied in healthcare-related research. Experimental studies are complex and are generally performed by pharmaceutical industries as a part of research and development activities during the discovery of a therapeutic drug/device. Also, clinical trials are undertaken by individual researchers or a consortium. In a recent study, the researchers were cautioned about the consequences of a faulty research design [ 6 ]. It was noted that clinical studies on the effect of the gut microbiome and its relationship with the feed could potentially be influenced by the choice of the experimental design, controls, and comparison groups included in the study. Moreover, clinical studies can be affected by sampling errors and biases [ 7 ]. In the present review, we briefly discuss the types of clinical study designs, study hypotheses, sampling errors, and the ethical issues associated with clinical research.
Research design
A research design is a systematic elucidation of the whole research process that includes methods and techniques, starting from the planning of research, execution (data collection), analysis, and drawing a logical conclusion based on the results obtained. A research design is a framework developed by a research team to find an answer/solution to a problem. The research designs are of several types that include descriptive research, surveys, correlation type, experimental, review (systematic/literature), and meta-analysis. The choice of research design is determined by the type of research question that is opted for. Both the research design and the research question are interdependent. For every research question, a complementary/appropriate research design must have been chosen. The choice of research design influences the research credibility, reliability, and accuracy of the data collected. A well-defined research design would contain certain elements that include a specific purpose of the research, methods to be applied while collecting and analyzing the data, the research methodology used to interpret the collected data, research infrastructure, limitations, and most importantly, the time required to complete the research. The research design can broadly be categorized into two types: qualitative and quantitative designs. In a qualitative research method, the collected data are measured and evaluated using mathematical and statistical applications. Whereas in quantitative research, a larger sample size is selected, and the results derived from statistics can benefit society. The various types of research designs are shown in Figure Figure1 1 [ 8 ].

Types of research studies
There are various types of research study designs. The researcher who aims to take up the study determines the type of study design to choose among the available ones. The choice of study design depends on many factors that include but are not limited to the research question, the aim of the study, the available funds, manpower, and infrastructure, among others. The research study designs include systematic reviews, meta-analyses, randomized controlled trials, cross-sectional studies, case-control studies, cohort studies, case reports/studies, animal experiments, and other in vitro studies, as shown in Figure Figure2 2 [ 9 ].

Systematic Reviews
In these studies, the researcher makes an elaborate and up-to-date search of the available literature. By doing a systematic review of a selected topic, the researcher collects the data, analyses it, and critically evaluates it to evolve with impactful conclusions. Systematic reviews could equip healthcare professionals with more than adequate evidence with respect to the decisions to be taken during improved patient management that may include diagnosis, interventions, prognosis, and others [ 10 ]. A recent systematic research study evaluated the role of socioeconomic conditions on the knowledge of risk factors for stroke in the World Health Organization (WHO) European region. This study collected data from PubMed, Embase, Web of Science (WoS), and other sources and finally included 20 studies and 67,309 subjects. This study concluded that the high socioeconomic group had better knowledge of risk factors and warning signs of stroke and suggested improved public awareness programs to better address the issue [ 11 ].
Meta-Analysis
Meta-analysis is like a systematic review, but this type of research design uses quantitative tools that include statistical methods to draw conclusions. Such a research method is therefore considered both equal and superior to the original research studies. Both the systematic review and the meta-analyses follow a similar research process that includes the research question, preparation of a protocol, registration of the study, devising study methods using inclusion and exclusion criteria, an extensive literature survey, selection of studies, assessing the quality of the evidence, data collection, analysis, assessment of the evidence, and finally the interpretation/drawing the conclusions [ 12 ]. A recent research study, using a meta-analytical study design, evaluated the quality of life (QoL) among patients suffering from chronic pulmonary obstructive disease (COPD). This study used WoS to collect the studies, and STATA to analyze and interpret the data. The study concluded that non-therapeutic mental health and multidisciplinary approaches were used to improve QoL along with increased support from high-income countries to low and middle-income countries [ 13 ].
Cross-Sectional Studies
These studies undertake the observation of a select population group at a single point in time, wherein the subjects included in the studies are evaluated for exposure and outcome simultaneously. These are probably the most common types of studies undertaken by students pursuing postgraduation. A recent study evaluated the activities of thyroid hormones among the pre- and post-menopausal women attending a tertiary care teaching hospital. The results of this study demonstrated that there was no significant difference in the activities of thyroid hormones in the study groups [ 14 ].
Cohort Studies
Cohort studies use participant groups called cohorts, which are followed up for a certain period and assess the exposure to the outcome. They are used for epidemiological observations to improve public health. Although cohort studies are laborious, financially burdensome, and difficult to undertake as they require a large population group, such study designs are frequently used to conduct clinical studies and are only second to randomized control studies in terms of their significance [ 15 ]. Also, cohort studies can be undertaken both retrospectively and prospectively. A retrospective study assessed the effect of alcohol intake among human immunodeficiency virus (HIV)-infected persons under the national program of the United States of America (USA) for HIV care. This study, which included more than 30,000 HIV patients under the HIV care continuum program, revealed that excessive alcohol use among the participants affected HIV care, including treatment [ 16 ].
Case-Control Study
The case-control studies use a single point of observation among two population groups that are categorized based on the outcome. Those who had an outcome are termed as cases, and the ones who did not develop the disease are called control groups. This type of study design is easy to perform and is extensively undertaken as a part of medical research. Such studies are frequently used to assess the efficacy of vaccines among the population [ 17 ]. A previous study evaluated the activities of zinc among patients suffering from beta-thalassemia and compared it with the control group. This study concluded that the patients with beta-thalassemia are prone to hypozincaemia and had low concentrations of zinc as compared to the control group [ 18 ].
Case Studies
Such types of studies are especially important from the perspective of patient management. Although these studies are just observations of single or multiple cases, they may prove to be particularly important in the management of patients suffering from unusual diseases or patients presenting with unusual presentations of a common disease. Listeria is a bacterium that generally affects humans in the form of food poisoning and neonatal meningitis. Such an organism was reported to cause breast abscesses [ 19 ].
Randomized Control Trial
This is probably the most trusted research design that is frequently used to evaluate the efficacy of a novel pharmacological drug or a medical device. This type of study has a negligible bias, and the results obtained from such studies are considered accurate. The randomized controlled studies use two groups, wherein the treatment group receives the trial drug and the other group, called the placebo group, receives a blank drug that appears remarkably like the trial drug but without the pharmacological element. This can be a single-blind study (only the investigator knows who gets the trial drug and who is given a placebo) or a double-blind study (both the investigator and the study participant have no idea what is being given). A recent study (clinical trial registration number: {"type":"clinical-trial","attrs":{"text":"NCT04308668","term_id":"NCT04308668"}} NCT04308668 ) concluded that post-exposure prophylaxis with hydroxychloroquine does not protect against Coronavirus disease-19 (COVID-19) after a high and moderate risk exposure when the treatment was initiated within four days of potential exposure [ 20 ].
Factors that affect study designs
Among the different factors that affect a study's design is the recruitment of study participants. It is not yet clear as to what is the optimal method to increase participant participation in clinical studies. A previous study had identified that the language barrier and the long study intervals could potentially hamper the recruitment of subjects for clinical trials [ 21 ]. It was noted that patient recruitment for a new drug trial is more difficult than for a novel diagnostic study [ 22 ].
Reproducibility is an important factor that affects a research design. The study designs must be developed in such a way that they are replicable by others. Only those studies that can be re-done by others to generate the same/similar results are considered credible [ 23 ]. Choosing an appropriate study design to answer a research question is probably the most important factor that could affect the research result [ 24 ]. This can be addressed by clearly understanding various study designs and their applications before selecting a more relevant design.
Retention is another significant aspect of the study design. It is hard to hold the participants of a study until it is completed. Loss of follow-up among the study participants will influence the study results and the credibility of the study. Other factors that considerably influence the research design are the availability of a source of funding, the necessary infrastructure, and the skills of the investigators and clinical trial personnel.
Synthesizing a research question or a hypothesis
A research question is at the core of research and is the point from which a clinical study is initiated. It should be well-thought-out, clear, and concise, with an arguable element that requires the conduction of well-designed research to answer it. A research question should generally be a topic of curiosity in the researcher's mind, and he/she must be passionate enough about it to do all that is possible to answer it [ 25 ].
A research question must be generated/framed only after a preliminary literature search, choosing an appropriate topic, identifying the audience, self-questioning, and brainstorming for its clarity, feasibility, and reproducibility.
A recent study suggested a stepwise process to frame the research question. The research question is developed to address a phenomenon, describe a case, establish a relationship for comparison, and identify causality, among others. A better research question is one that describes the statement of the problem, points out the study area, puts focus on the study aspects, and guides data collection, analysis, and interpretation. The aspects of a good research question are shown in Figure Figure3 3 [ 26 ].

Research questions may be framed to prove the existence of a phenomenon, describe and classify a condition, elaborate the composition of a disease condition, evaluate the relationship between variables, describe and compare disease conditions, establish causality, and compare the variables resulting in causality. Some examples of the research questions include: (i) Does the coronavirus mutate when it jumps from one organism to another?; (ii) What is the therapeutic efficacy of vitamin C and dexamethasone among patients infected with COVID-19?; (iii) Is there any relationship between COPD and the complications of COVID-19?; (iv) Is Remdesivir alone or in combination with vitamin supplements improve the outcome of COVID-19?; (v) Are males more prone to complications from COVID-19 than females?
The research hypothesis is remarkably like a research question except for the fact that in a hypothesis the researcher assumes either positively or negatively about a causality, relation, correlation, and association. An example of a research hypothesis: overweight and obesity are risk factors for cardiovascular disease.
Types of errors in hypothesis testing
An assumption or a preliminary observation made by the researcher about the potential outcome of research that is being envisaged may be called a hypothesis. There are different types of hypotheses, including simple hypotheses, complex hypotheses, empirical hypotheses, statistical hypotheses, null hypotheses, and alternative hypotheses. However, the null hypothesis (H0) and the alternative hypothesis (HA) are commonly practiced. The H0 is where the researcher assumes that there is no relation/causality/effect, and the HA is when the researcher believes/assumes that there is a relationship/effect [ 27 , 28 ].
Hypothesis testing is affected by two types of errors that include the type I error (α) and the type II error (β). The type I error (α) occurs when the investigator contradicts the null hypothesis despite it being true, which is considered a false positive error. The type II error (β) happens when the researcher considers/accepts the null hypothesis despite it being false, which is termed a false negative error [ 28 , 29 ].
The reasons for errors in the hypothesis testing may be due to bias and other causes. Therefore, the researchers set the standards for studies to rule out errors. A 5% deviation (α=0.05; range: 0.01-0.10) in the case of a type I error and up to a 20% probability (β=0.20; range: 0.05-0.20) for type II errors are generally accepted [ 28 , 29 ]. The features of a reasonable hypothesis include simplicity and specificity, and the hypothesis is generally determined by the researcher before the initiation of the study and during the preparation of the study proposal/protocol [ 28 , 29 ].
The applications of hypothesis testing
A hypothesis is tested by assessing the samples, where appropriate statistics are applied to the collected data and an inference is drawn from it. It was noted that a hypothesis can be made based on the observations of physicians using anatomical characteristics and other physiological attributes [ 28 , 30 ]. The hypothesis may also be tested by employing proper statistical techniques. Hypothesis testing is carried out on the sample data to affirm the null hypothesis or otherwise.
An investigator needs to believe the null hypothesis or accept that the alternate hypothesis is true based on the data collected from the samples. Interestingly, most of the time, a study that is carried out has only a 50% chance of either the null hypothesis or the alternative hypothesis coming true [ 28 , 31 ].
Hypothesis testing is a step-by-step strategy that is initiated by the assumption and followed by the measures applied to interpret the results, analysis, and conclusion. The margin of error and the level of significance (95% free of type I error and 80% free of type II error) are initially fixed. This enables the chance for the study results to be reproduced by other researchers [ 32 ].
Ethics in health research
Ethical concerns are an important aspect of civilized societies. Moreover, ethics in medical research and practice assumes increased significance as most health-related research is undertaken to find a cure or discover a medical device/diagnostic tool that can either diagnose or cure the disease. Because such research involves human participants, and due to the fact that people approach doctors to find cures for their diseased condition, ethics, and ethical concerns take center stage in public health-related clinical/medical practice and research.
The local and international authorities like the Drugs Controller General of India (DCGI), and the Food and Drug Administration (FDA) make sure that health-related research is carried out following all ethical concerns and good clinical practice (GCP) guidelines. The ethics guidelines are prescribed by both national and international bodies like the Indian Council of Medical Research (ICMR) and the World Medical Association (WMA) Declaration of Helsinki guidelines for ethical principles for medical research involving human subjects [ 33 ].
Ethical conduct is more significant during clinical practice, medical education, and research. It is recommended that medical practitioners embark on self-regulation of the medical profession. Becoming proactive in terms of ethical practices will enhance the social image of a medical practitioner/researcher. Moreover, such behavior will allow people to comprehend that this profession is not for trade/money but for the benefit of the patients and the public at large. Administrations should promote ethical practitioners and penalize unethical practitioners and clinical research organizations. It is suggested that the medical curriculum should incorporate ethics as a module and ethics-related training must be delivered to all medical personnel. It should be noted that a tiny seed grows into an exceptionally gigantic tree if adequately watered and taken care of [ 33 ]. It is therefore inevitable to address the ethical concerns in medical education, research and practice to make more promising medical practitioners and acceptable medical educators and researchers as shown in Figure Figure4 4 .

Sampling in health research
Sampling is the procedure of picking a precise number of individuals from a defined group to accomplish a research study. This sample is a true representative subset of individuals who potentially share the same characteristics as a large population, and the results of the research can be generalized [ 34 , 35 ]. Sampling is a prerogative because it is almost impossible to include all the individuals who want to partake in a research investigation. A sample identified from a representative population can be depicted in Figure Figure5 5 .

Sampling methods are of different types and are broadly classified into probability sampling and non-probability sampling. In a probability sampling method, which is routinely employed in quantitative research, each individual in the representative population is provided with an equivalent likelihood of being included in the study [ 35 ]. Probability sampling can be separated into four types that include simple random sampling, systematic sampling, stratified sampling, and cluster sampling, as shown in Figure Figure6 6 .

Simple Random Sample
In the simple random sampling method, every person in the representative population is given an equal chance of being selected. It may use a random number generator for selecting the study participants. To study the employees’ perceptions of government policies, a researcher initially assigns a number to each employee [ 35 ]. After this, the researcher randomly chooses the required number of samples. In this type of sampling method, each one has an equal chance of being selected.
Systematic Sample
In this sampling method, the researcher selects the study participants depending on a pre-defined order (1, 3, 5, 7, 9…), wherein the researcher assigns a serial number (1-100 (n)) to volunteers [ 35 ]. The researcher in this type of sample selects a number from 1 to 10 and later applies a systematic pattern to select the sample like 2, 12, 22, 32, etc.
Stratified Sample
The stratified sampling method is applied when the people from whom the sample must be taken have mixed features. In this type of sampling, the representative population is divided into clusters/strata based on attributes like age, sex, and other factors. Subsequently, a simple random or systematic sampling method is applied to select the samples from each group. Initially, different age groups, sexes, and other characters were selected as a group [ 35 ]. The investigator finds his/her sample from each group using simple or systematic random sampling methods.
Cluster Sample
This sampling method is used to create clusters of the representative population with mixed qualities. Because such groups have mixed features, each one can be regarded as a sample. Conversely, a sample can be developed by using simple random/systematic sampling approaches. The cluster sampling method is similar to stratified sampling but differs in the group characteristics, wherein each group has representatives of varied ages, different sexes, and other mixed characters [ 35 ]. Although each group appears as a sample, the researcher again applies a simple or systematic random sampling method to choose the sample.
Non-probability Sample
In this type of sampling method, the participants are chosen based on non-random criteria. In a non-probability sampling method, the volunteers do not have an identical opportunity to get selected. This method, although it appears to be reasonable and effortless to do, is plagued by selection bias. The non-probability sampling method is routinely used in experimental and qualitative research. It is suitable to perform a pilot study that is carried out to comprehend the qualities of a representative population [ 35 ]. The non-probability sampling is of four types, including convenience sampling, voluntary response sampling, purposive sampling, and snowball sampling, as shown in Figure Figure7 7 .

Convenience Sample
In the convenience sampling method, there are no pre-defined criteria, and only those volunteers who are readily obtainable to the investigator are included. Despite it being an inexpensive method, the results yielded from studies that apply convenience sampling may not reflect the qualities of the population, and therefore, the results cannot be generalized [ 35 ]. The best example of this type of sampling is when the researcher invites people from his/her own work area (company, school, city, etc.).
Voluntary Response Sample
In the voluntary response sampling method, the participants volunteer to partake in the study. This sampling method is similar to convenience sampling and therefore leaves sufficient room for bias [ 35 ]. The researcher waits for the participants who volunteer in the study in a voluntary response sampling method.
Purposive Sample/Judgment Sample
In the purposive or judgemental sampling method, the investigator chooses the participants based on his/her judgment/discretion. In this type of sampling method, the attributes (opinions/experiences) of the precise population group can be achieved [ 35 ]. An example of such a sampling method is the handicapped group's opinion on the facilities at an educational institute.
Snowball Sample
In the snowball sampling method, suitable study participants are found based on the recommendations and suggestions made by the participating subjects [ 36 ]. In this type, the individual/sample recruited by the investigator in turn invites/recruits other participants.
Significance of informed consent and confidentiality in health research
Informed consent is a document that confirms the fact that the study participants are recruited only after being thoroughly informed about the research process, risks, and benefits, along with other important details of the study like the time of research. The informed consent is generally drafted in the language known to the participants. The essential contents of informed consent include the aim of research in a way that is easily understood even by a layman. It must also brief the person as to what is expected from participation in the study. The informed consent contains information such as that the participant must be willing to share demographic characteristics, participate in the clinical and diagnostic procedures, and have the liberty to withdraw from the study at any time during the research. The informed consent must also have a statement that confirms the confidentiality of the participant and the protection of privacy of information and identity [ 37 ].
Health research is so complex that there may be several occasions when a researcher wants to re-visit a medical record to investigate a specific clinical condition, which also requires informed consent [ 38 ]. Awareness of biomedical research and the importance of human participation in research studies is a key element in the individual’s knowledge that may contribute to participation or otherwise in the research study [ 39 ]. In the era of information technology, the patient’s medical data are stored as electronic health records. Research that attempts to use such records is associated with ethical, legal, and social concerns [ 40 , 41 ]. Improved technological advances and the availability of medical devices to treat, diagnose, and prevent diseases have thrown a new challenge at healthcare professionals. Medical devices are used for interventions only after being sure of the potential benefit to the patients, and at any cost, they must never affect the health of the patient and only improve the outcome [ 42 ]. Even in such cases, the medical persons must ensure informed consent from the patients.
Conclusions
Clinical research is an essential component of healthcare that enables physicians, patients, and governments to tackle health-related problems. Increased incidences of both communicable and non-communicable diseases warrant improved therapeutic interventions to treat, control, and manage diseases. Several illnesses do not have a treatment, and for many others, the treatment, although available, is plagued by drug-related adverse effects. For many other infections, like dengue, we require preventive vaccines. Therefore, clinical research studies must be carried out to find solutions to the existing problems. Moreover, the knowledge of clinical research, as discussed briefly in this review, is required to carry out research and enhance preparedness to counter conceivable public health emergencies in the future.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The potential of working hypotheses for deductive exploratory research
Affiliations.
- 1 Department of Political and Social Sciences, University of Bologna, Strada Maggiore 45, 40125 Bologna, Italy.
- 2 Texas State University, San Marcos, TX USA.
- PMID: 33311812
- PMCID: PMC7722257
- DOI: 10.1007/s11135-020-01072-9
While hypotheses frame explanatory studies and provide guidance for measurement and statistical tests, deductive, exploratory research does not have a framing device like the hypothesis. To this purpose, this article examines the landscape of deductive, exploratory research and offers the working hypothesis as a flexible, useful framework that can guide and bring coherence across the steps in the research process. The working hypothesis conceptual framework is introduced, placed in a philosophical context, defined, and applied to public administration and comparative public policy. Doing so, this article explains: the philosophical underpinning of exploratory, deductive research; how the working hypothesis informs the methodologies and evidence collection of deductive, explorative research; the nature of micro-conceptual frameworks for deductive exploratory research; and, how the working hypothesis informs data analysis when exploratory research is deductive.
Keywords: Deductive qualitative research; Exploratory research; Pragmatism; Working hypothesis.
© The Author(s) 2020.
PubMed Disclaimer
Conflict of interest statement
Conflict of interestNo potential conflict of interest was reported by the author.
A Common structure used in…
A Common structure used in the development of working hypotheses
Similar articles
- Rapid versus traditional qualitative analysis using the Consolidated Framework for Implementation Research (CFIR). Nevedal AL, Reardon CM, Opra Widerquist MA, Jackson GL, Cutrona SL, White BS, Damschroder LJ. Nevedal AL, et al. Implement Sci. 2021 Jul 2;16(1):67. doi: 10.1186/s13012-021-01111-5. Implement Sci. 2021. PMID: 34215286 Free PMC article.
- Clinical reasoning and hypothesis generation in expert clinical swallowing examinations. McAllister S, Tedesco H, Kruger S, Ward EC, Marsh C, Doeltgen SH. McAllister S, et al. Int J Lang Commun Disord. 2020 Jul;55(4):480-492. doi: 10.1111/1460-6984.12531. Epub 2020 Mar 17. Int J Lang Commun Disord. 2020. PMID: 32185861
- Avoiding and identifying errors in health technology assessment models: qualitative study and methodological review. Chilcott J, Tappenden P, Rawdin A, Johnson M, Kaltenthaler E, Paisley S, Papaioannou D, Shippam A. Chilcott J, et al. Health Technol Assess. 2010 May;14(25):iii-iv, ix-xii, 1-107. doi: 10.3310/hta14250. Health Technol Assess. 2010. PMID: 20501062 Review.
- Are ecological and evolutionary theories scientific? Murray BG Jr. Murray BG Jr. Biol Rev Camb Philos Soc. 2001 May;76(2):255-89. doi: 10.1017/s146479310100567x. Biol Rev Camb Philos Soc. 2001. PMID: 11396849 Review.
- Effectiveness of Positive Hypothesis Testing in Inductive and Deductive Rule Learning. Laughlin PR, Bonner BL, Altermatt TW. Laughlin PR, et al. Organ Behav Hum Decis Process. 1999 Feb;77(2):130-146. doi: 10.1006/obhd.1998.2815. Organ Behav Hum Decis Process. 1999. PMID: 10069943
- "Trust people you've never worked with" - A social network visualization of teamwork, cohesion, social support, and mental health in NHS Covid personnel. Schilling S, Armaou M, Morrison Z, Carding P, Bricknell M, Connelly V. Schilling S, et al. Front Psychol. 2024 Feb 20;15:1293171. doi: 10.3389/fpsyg.2024.1293171. eCollection 2024. Front Psychol. 2024. PMID: 38445057 Free PMC article.
- Correlation of Cognitive Reappraisal and the Microstructural Properties of the Forceps Minor: A Deductive Exploratory Diffusion Tensor Imaging Study. Porcu M, Cocco L, Cau R, Suri JS, Mannelli L, Manchia M, Puig J, Qi Y, Saba L. Porcu M, et al. Brain Topogr. 2024 Jan;37(1):63-74. doi: 10.1007/s10548-023-01020-4. Epub 2023 Dec 7. Brain Topogr. 2024. PMID: 38062326
- The use of the Capability-Opportunity- Motivation Behavior (COM-B) model to identify barriers to medication adherence and the application of mobile health technology in adults with coronary heart disease: A qualitative study. Park LG, Ng F, Handley MA. Park LG, et al. PEC Innov. 2023 Sep 7;3:100209. doi: 10.1016/j.pecinn.2023.100209. eCollection 2023 Dec 15. PEC Innov. 2023. PMID: 37753273 Free PMC article.
- Instructor experiences with online guitar lessons during the Covid-19 pandemic in Turkey. Ayyıldız EB, Zahal O. Ayyıldız EB, et al. Int J Music Educ. 2023 Aug;41(3):484-496. doi: 10.1177/02557614221123078. Epub 2022 Sep 27. Int J Music Educ. 2023. PMID: 37431490 Free PMC article.
- Exploring the conceptual framework of a health-promotion faculty from the perspective of members: a qualitative study. Sedghi F, Mahdizadeh M, Vahedian-Shahroodi M, Gholian-Aval M. Sedghi F, et al. BMJ Open. 2023 May 11;13(5):e073059. doi: 10.1136/bmjopen-2023-073059. BMJ Open. 2023. PMID: 37169494 Free PMC article.
- Adler E, Clark R. How It’s Done: An Invitation to Social Research. 3. Belmont: Thompson-Wadsworth; 2008.
- Arnold RW. Multiple working hypothesis in soil genesis. Soil Sci. Soc. Am. J. 1965;29(6):717–724. doi: 10.2136/sssaj1965.03615995002900060034x. - DOI
- Atieno O. An analysis of the strengths and limitation of qualitative and quantitative research paradigms. Probl. Educ. 21st Century. 2009;13:13–18.
- Babbie E. The Practice of Social Research. 11. Belmont: Thompson-Wadsworth; 2007.
- Biddle C, Schafft KA. Axiology and anomaly in the practice of mixed methods work: pragmatism, valuation, and the transformative paradigm. J. Mixed Methods Res. 2015;9(4):320–334. doi: 10.1177/1558689814533157. - DOI
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

Periodical (Journal)
- Periodicals
Journal Exploratory Research and Hypothesis in Medicine
- Overview
- Issue
- Retractions, EoC, and External CQC Notices 1
Aim & Scope
Exploratory Research and Hypothesis in Medicine (ERHM) publishes original exploratory research articles and state-of-the-art reviews that focus on novel findings and the most recent scientific advances that support new hypotheses in medicine. The journal accepts a wide range of topics, including innovative diagnostic and therapeutic modalities as well as insightful theories related to the practice of medicine. The exploratory research published in ERHM does not necessarily need to be comprehensive and conclusive, but the study design must be solid, the methodologies must be reliable, the results must be true, and the hypothesis must be rational and justifiable with evidence. ERHM welcomes and encourages article submission of original exploratory research as well as novel and timely state-of-the-art full reviews, mini-reviews, and opinion articles from leading scientists and scholars around the world. Topics of particular interest include but are not limited to: Drug mechanism of action and functions; New methods and concepts in the diagnosis, treatment, or prevention of disease; Novel discoveries in all diseases and disease mechanisms; Associations among diseases; Interesting findings in signal transduction; Changes in clinical paradigms and treatment approaches; Cutting edge technologies that can help improve scientific understanding; Preliminary findings that may change current thinking in a particular field. [ 1 ]
Go to Website
Placental Malaria and Its Relationship with Neonatal Birth Weight among Primigravidae: An Analytical Cross-sectional Study
K Oranuka , C Chama , I Adogu , ... , A Eke
10.14218/erhm.2023.00015

A Clinician’s View on Pre-chronic Obstructive Pulmonary Disease/Pre-non-obstructive Chronic Bronchitis: A Quest for More Research on Early Diagnosis and Treatment
T Hausen , C Corrigan
10.14218/erhm.2023.00090
Expansion of Intact Sensory-motor Territories in Proximal Neuraxial Lesions using Peripheral Nerve-muscle Weeding Interventions
10.14218/erhm.2023.00020
Thyroid Hormone Levels in Mothers and Cord Blood at Delivery in Crude Oil Producing Community in Delta State, Nigeria
M Emokpae , L Ogana
10.14218/erhm.2023.00085
Study on the Ideas and Methods of Bloodletting Therapy in the Treatment of Heat Stroke
Y Li , C Zhou , T Liu , Q Xu
10.14218/erhm.2023.00088
Implementing Pharmacogenomic and Genetic Testing into Prostate Cancer Clinics: A Literature Review of Current Trends and Applications
J Germany , J Martin
10.14218/erhm.2023.00087
Immunotherapy in Colorectal Cancer: A Review
No authors listed.
10.14218/erhm.2023.00008
Expanding Role of Epigenetics in Human Health and Disease
10.14218/erhm.2023.00086
Editorial Retractions, Expressions of Concern and External Notices
The Combination of LPS and Melatonin Induces M2 Macrophage Apoptosis to Prevent Lung Cancer
F Gao , Y Lin , M Zhang , ... , G Du
PubPeer Comment
Actinopolyspora Biskrensis
PubPeer 2023 - VOLUME 2023, ISSUE 5
Exploratory Research and Hypothesis in Medicine Latest Publications
Total documents, published by xia & he publishing.
- Latest Documents
- Most Cited Documents
- Contributed Authors
- Related Sources
- Related Keywords
The Effectiveness of Combined Extracorporeal Shockwave Therapy and Exercise for Plantar Heel Pain: A Systematic Review
Influence of possible natural and artificial collective immunity on new covid-19 pandemic waves in ukraine and israel, fructose metabolism and acute myeloid leukemia, mir-34a/sirt1 axis plays a critical role in regulating chondrocyte senescence in type 2 diabetes mellitus, arthroscopic treatment of atypical synovial osteochondromatosis of infrapatellar fat pad: a 4-year follow-up case report and literature review, be alert to the risk of adverse cardiovascular events after covid-19 vaccination, antioxidant, phytochemical and enzymatic characteristics of selected medicinal plants from the republic of korea: a commentary, effects of insulin pathway on glucose and lipid metabolism disorder in different pathological types of colorectal adenomas, production of sars-cov-2 specific ifn-γ/il-10 co-producing cd4 t cells from convalescent donors to treat covid-19: a hypothesis, the pathogenic potential of runx2, export citation format, share document.

Exploratory Research and Hypothesis in Medicine

eISSN 2472-0712 A Biomedical Open Access Journal
EXPLORATORY RESEARCH AND HYPOTHESIS IN MEDICINE
Volume 5 Issue 1, March 2020
XIA & HE PUBLISHING INC. EXPLORATORY RESEARCH AND HYPOTHESIS IN MEDICINE
CONTENTS 2020 5(1):1–38
Editorials The Coronavirus Disease 2019 Epidemic Situation in China Jin Wang, Zhihui Li, Jiahai Lu ...... 1 The Ongoing Outbreak and Challenges of Novel Coronavirus (COVID-19) in China Bohao Chen ...... 3
Opinions Blind Spots in Fighting the Outbreak of Coronavirus Disease 2019 Lanjing Zhang ...... 6. . . . . Characteristics of COVID-19 During the Onset Stage and Considerations for Disease Control Lili Wang ...... 8 . . . . .
Original Article Echocardiography Effectiveness in Improving Diagnosis of Rheumatic Heart Disease in North Dar- fur: A Hospital-based Study Mohammed Elmujtba Adam Essa Adam, Sherihan Mohammed Elkundi Osman, Daralsalam Ishag Ateem Abdalrasoul, Ibrahim Adam Osman Yagoup, Mustafa Mohamed Ali Hussein, Mutwaly Defealla Yousif Haron, Ziryab Imad Taha Mahmoud, Abdelkareem A . Ahmed ...... 11
Review Articles Microbial Biomarkers for Colorectal Cancer Identified with Random Forest Model Weili Sun, Lili Wang, Qiuyue Zhang, Quanjiang Dong ...... 19 The Effect of Honey as a Treatment for Oral Ulcerative Lesions: A Systematic Review Maddison Hunter, Jane Kellett, Nathan M . D’Cunha, Kellie Toohey, Andrew McKune, Nenad Naumovski . . . .27
Corrigendum Corrigendum: Acute Soft Skull Syndrome in an Adult Male with Sickle Cell Anemia in Sudan: A Case Report Ziryab Imad Taha, Sulafa Eisa Mohammed, Mohammed Elmujtba Adam Essa, Walaa Mohamed Elsid, Mustafa Mohamed Ali Hussein, Sherihan Mohammed Elkundi Osman, Hussein Osman Ahmed, Mutwaly Defealla Yousif, Abdelkareem A . Ahmed ...... 38 Editorial
The Coronavirus Disease 2019 Epidemic Situation in China
Jin Wang, Zhihui Li and Jiahai Lu*
Department of Epidemiology, School of Public Health, Sun Yat-sen University, Guangzhou, Guangdong Province, 510080, China
It has been 17 years since the outbreak of severe acute respiratory is a β CoV of group 2B, sharing 79.5% of genetic sequence with syndrome (SARS) in 2003, which causes at least 8096 infections SARS-CoV and has 96.2% homology to a bat coronavirus.9,10 and 774 deaths worldwide. History repeats itself! In Dec 2019, However, the possibility of direct transmission from bats to hu- China reported to the World Health Organization (WHO) a cluster mans is small, and an intermediate host is often needed to mediate of patients with pneumonia of unknown etiology in Wuhan , a ma- zoonoses from natural reservoir to terminal hosts. It is speculated jor hub of transportation with a population of 11 million people. that SARS-CoV-2 may come from a certain wild animal and spread On Jan 9, 2020, a novel coronavirus (officially named as SARS- rapidly in the Huanan Seafood Wholesale Market in Wuhan, where CoV-2 on Feb 11) was identified as the cause of this outbreak. the viruses were originally isolated. Homology modelling analy- The number of infected cases increased sharply, and the epidemic sis showed structural similarity between the receptor-binding do- rapidly spread throughout the country. The WHO on Jan 30 de- mains of SARS-CoV and SARS-CoV-2 to the human angiotensin- clared the outbreak a Public Health Emergency of International converting enzyme-2 (ACE-2) receptor.10 SARS-CoV-2 has been Concern. By Feb 15, 2020, the cumulative number of confirmed considered to link to game consumption, a habit obsessive by some cases had reached 66,577, far exceeding the number of SARS Chinese people.11 After the outbreak, the Chinese Government cases in 2003. According to the released news, the case rate fatal- has closed the Huanan Seafood Wholesale Market in Wuhan and ity is 2.3% (1,524/66,577). Geographically, the disease has spread banned all forms of wild animal transaction. beyond China to over 30 countries, with Japan having the most Since the outbreak, geographical spread of the disease raises confirmed cases outside China. grave concerns around the world. The Chinese health authorities The SARS-CoV-2 is mainly transmitted through respiratory have taken unprecedented measures to control the source of infec- droplets. After infection , patients showed lymphopenia and bilat- tion.12 From Jan 23, Chinese authorities have imposed travel bans eral ground-glass opacity or consolidation in chest CT scans, along on Wuhan and several cities near Wuhan. Individuals are popular- with common symptoms that include fever, dry cough, and short- ized the knowledge to take self-protection measures, such as good ness of breath, at the onset of illness.1,2 In severe cases, dyspnea, personal hygiene, fitted masks, ventilation and avoiding crowded respiratory distress syndrome or septic shock may develop.2 Early places. There is likelihood that some mild or asymptomatic pa- observation of infections of health-care workers as well as fam- tients do not seek health care but as a source transmitting the virus ily members has suggested that human-to-human transmission has to other humans, which may complicate or delay the effectiveness occurred among close contacts.3,4 The epidemic doubled in size of infection-control measures.13 At present, there are no specific every 6.4–7.4 days in its early stage, with the basic reproductive antivirals or vaccines although clinical trial of Lopinavir/ritonavir 3,5,6 number (R0) estimated to be 2.2–2.68. has been launched for SARS-CoV-2 (ChiCTR2000029308), and Coronavirus is an enveloped, positive-strand RNA virus, be- the management of infection is largely supportive. Thus, molecular longing to Coronavirinae subfamily within Coronaviridae family. diagnostic platforms for rapidly identifying SARS-CoV-2 as well The coronaviruses can infect human, livestock, avian, bat, mouse as effective vaccine strategies are urgently needed to develop. and other wild animals. They can cause respiratory, gastrointesti- In this century, the coronaviruses attacked China twice, each nal, hepatic and central nervous system diseases of varying sever- time causing public panic, heavy deaths and huge economic losses. ity, sometimes fatal. The two types of coronaviruses that we are It seems that people have not yet established a rapid, effective re- currently familiar with are SARS-CoV in 2003 and Middle East sponse to public health threats. Although scientific research on the respiratory syndrome coronavirus (MERS-CoV) broken out in etiology and structural features of unknown pathogens is impor- 2012. The SARS-CoV-2 is the seventh known coronavirus that can tant, it is more practical to establish a strong and effective public infect humans.7 This virus seems to have greater infectivity (e.g., a health system that can assure the nation's health and safety. The higher R0) but a lower case fatality rate.8 investment in core public-health systems and infrastructure will Bats might be the original host of this virus.9 The SARS-CoV-2 no doubt be critical for preventing or controlling this kind of bios- ecurity. Local health departments need to strengthen their ability to mount an effective health emergency response, including iden- Abbreviations: ACE-2, angiotensin-converting enzyme-2; CoV, coronavirus; COV- tification of suspicious cases as well as individuals with high-risk ID-19, coronavirus disease 2019; CT, computed tomography; MERS-CoV, Middle East respiratory syndrome coronavirus; SARS, severe acute respiratory syndrome; exposures at the early stage, even in a sudden outbreak of unknown WHO, World Health Organization. pathogens. Coordination and collaboration among surveillance Received: February 17, 2020; Revised: March 03, 2020; Accepted: March 04, 2020 departments, health sectors and laboratories are particularly im- *Correspondence to: Jiahai Lu, Department of Epidemiology, School of Public portant. In addition, both MERS and SARS nosocomial outbreaks Health, Sun Yat-sen University, Guangzhou, Guangdong Province, 510080, China. are characterized by early nosocomial super-spreading events. Al- Tel: +86-20 87332438, E-mail: [email protected] though so far no evidence indicates a super transmission event in How to cite this article: Wang J, Li Z, Lu J. The Coronavirus Disease 2019 Epidemic Situation in China. Exploratory Research and Hypothesis in Medicine 2020;5(1):1–2. the COVID-19 outbreak, the situation of nosocomial infection is doi: 10.14218/ERHM.2020.00009. still serious. As of Feb 11, 1,716 medical staffs had been infected
Exploratory Research and Hypothesis in Medicine 2020 vol. 5 | 1–2
Copyright: © 2020 Authors. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Explor Res Hypothesis Med Wang J. et al: COVID-19 epidemic situation in China in China, with 5 of them died. At present, it is still unclear how contributed to the manuscript and approved the submitted version. many patients were cross infected when they waited for diagnosis or treatment in fever clinic. Nosocomial infection may be a driving factor for the epidemic of infectious diseases. From this point of References view, strict control of nosocomial infection is also significant in fighting the spread of epidemic diseases. [1] Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiologi- So far, the spread of the epidemic has begun to slow down. cal and clinical characteristics of 99 cases of 2019 novel corona- Many questions remain unknown, including animal reservoir, virus pneumonia in Wuhan, China: a descriptive study. Lancet pathogenesis, epidemiology of SARS-CoV-2 as well as risks for 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7. globe transmission. There are some important things we need to [2] Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of pa- think about, in particular, how to change from passive response tients infected with 2019 novel coronavirus in Wuhan, China. Lancet to active surveillance before public health threats appear. At pre- 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5. [3] Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission sent, almost no surveillance system can effectively integrate hu- Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumo- man and animal disease information, which leads to the inability nia. N Engl J Med 2020. doi:10.1056/NEJMoa2001316. to detect new zoonotic diseases and evaluate the risk of spill-over [4] Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster from animals to humans. Thus, the establishment of an effective of pneumonia associated with the 2019 novel coronavirus indicat- zoonotic disease surveillance system may be a crucial step helpful ing person-to-person transmission: a study of a family cluster. Lancet for spotting the emergence of a pandemic virus and warning the 2020;395(10223):514–523. doi:10.1016/S0140-6736(20)30154-9. early spread of cross-species pathogens. [5] Wu JT, Leung K, Leung GM. Nowcasting and forecasting the po- tential domestic and international spread of the 2019-nCoV- out break originating in Wuhan, China: a modelling study. Lancet Acknowledgments 2020;395(10225):689–697. doi:10.1016/S0140-6736(20)30260-9. [6] Riou J, Althaus CL. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to None. January 2020. Euro Surveill 2020;25(4). doi:10.2807/1560-7917. ES.2020.25.4.2000058. [7] Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coro- Funding navirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020;382:727–733. doi:10.1056/NEJMoa2001017. [8] del Rio C, Malani PN. 2019 Novel Coronavirus-Important Information This work was funded by the Key-Area Research and Development for Clinicians. JAMA 2020. doi:10.1001/jama.2020.1490. Program of Guangdong Province (2018B020241002), the National [9] Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneu- Science and Technology Major Project (No. 2018ZX10101002- monia outbreak associated with a new coronavirus of probable bat 001-001) and the Guangdong Provincial Science and Technology origin. Nature 2020;579:270–273. doi:10.1038/s41586-020-2012-7. Project (2020B111112003). [10] Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisa- tion and epidemiology of 2019 novel coronavirus: implications for vi- rus origins and receptor binding. Lancet 2020;395(10224):565–574. Conflict of interest doi:10.1016/S0140-6736(20)30251-8. [11] Li J, Li JJ, Xie X, Cai X, Huang J, Tian X, et al. Game consumption and the 2019 novel coronavirus. Lancet Infect Dis 2020;20(3):275–276. The authors declare that they have no conflict of interest. doi:10.1016/S1473-3099(20)30063-3. [12] Nkengasong J. China's response to a novel coronavirus stands in stark contrast to the 2002 SARS outbreak response. Nat Med 2020;26:310– Author contributions 311. doi:10.1038/s41591-020-0771-1. [13] Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A Novel Coronavirus Emerging in China - Key Questions for Impact JW and ZL wrote the first draft of the manuscript; JL summarized Assessment. N Engl J Med 2020;382:692–694. doi:10.1056/NEJMp viewpoints of the article and corrected the manuscript. All authors 2000929.
2 DOI: 10.14218/ERHM.2020.00009 | Volume 5 Issue 1, March 2020 Editorial
The Ongoing Outbreak and Challenges of Novel Coronavirus (COVID-19) in China
Bohao Chen*
Section of Pulmonary and Critical Care Medicine, Department of Medicine, University of Chicago, Chicago, IL, USA
In December 2019, a cluster of unexplained cases of pneumonia two infect avian species. The genus Alphacoronavirus contains the was first reported by the Health Commission of Hubei Province, human viruses HCoV-229E, HCoV-NL63, and many other animal China. Most of cases were epidemiologically linked to a seafood viruses. The genus Betacoronavirus includes the human viruses and wet animal wholesale market in Wuhan, Hubei Province, HCoV-OC43 and HCoV-HKU1 along with a number of animal China, which was officially closed on January 1, 1 2020. As of coronaviruses. Some coronaviruses that infect animals can evolve January 3, the national authorities in China had reported a total to acquire efficient human transmission, making people sick and of 44 patients with pneumonia to WHO. On January 8, virologists becoming a new human coronavirus, such as SARS-CoV, MERS- and other public health investigators identified a new coronavirus, CoV, and now COVID-19.4 In the 2003 SARS pandemic, investi- now known as COVID-19, as the cause of the pneumonia clusters gators found SARS-CoV viral RNA in both palm civets and rac- and posted the genetic sequence of COVID-19 on January 12.2 coon dogs in wet markets. It is very likely that SARS-CoV was The clusters of new cases among families and the infection of 16 transmitted from bats to those animals which served as interme- healthcare professionals indicate human-to-human transmission diary reservoirs and then finally infected humans.5 MERS-CoV of the virus.3 Chinese public health authorities have quarantined is also a zoonotic virus with possible origins in bats and camels. travel from Wuhan to limit the spread of the virus since January The ongoing outbreak of COVID-19 is associated with the Wuhan 23, and since then, more Chinese cities have also been isolated. Huanan Seafood Market where snakes, birds, marmots, pangolin, As new cases and death toll from COVID-19 in China escalate and other small mammals were sold, suggesting animal-to-person rapidly and with COVID-19 spreading to other countries, WHO spread. However, as the outbreak progressed, most cases were con- Director-General Tedros Adhanom Ghebreyesus declared the firmed without exposure to animal markets, and cases were identi- COVID-19 outbreak a public health emergency of international fied among healthcare professionals and other contacts of patients concern on January 30, noting the potential spread of the virus to with COVID-19 infections, indicating either human to human countries with weak health systems. The Trump administration transmission or a more widespread animal source.3 COVID-19 is announced on January 31 that it would temporarily bar foreigners highly contagious and the current epidemic has become a serious from entering the US if they had been to China within the past 14 public health threat to China as well as to other countries. It re- days. Compared to prior SARS-CoV in 2003 and MERS-CoV in quires governments to take emergency and mandatory measures 2013, the ongoing outbreak of COVID-19 presents an urgent and to treat the patients, isolate the suspicious cases, protect healthcare huge health threat since it is far more transmittable and far more professionals, track the spread of the virus, and advise individuals damaging economically and socially. Therefore, governments for public health. and partners should take immediate actions in response to the So far, the source of COVID-19 has not been identified yet. COVID-19 outbreak and bring the scientific community together Since the sequencing of COVID-19 genome by Dr. Zhang’s group to accelerate research and innovation against the global epidemic at Fudan University was published on January 12, 44 COVID-19 of COVID-19. sequences have been deposited in the NCBI Virus website. Ge- Coronaviruses are single-stranded positive-sense RNA viruses nome comparison of all these strains found that they are almost and classified into four main sub-groupings, known as alpha, beta, identical with >99% sequence conservation. The low variability gamma, and delta, based on antigenic relationships and viral ge- suggests a likely single emergence of epidemic viruses from a netic phylogeny. The former two genera primarily infect the res- common animal reservoir. The COVID-19 genome (MN908947.3) piratory and gastrointestinal tract of mammals, whereas the latter shows similarities to Bat SARS-like coronavirus (CoVZC45) with sequence identity of 89.12% and to Bat SARS-like coronavirus (CoVZXC21) with sequence identity of 88.68%. However, COV- Abbreviations: ACE2, angiotensin-converting enzyme 2; COVID-19, novel corona- ID-19 is genetically distinct from the human SARS genome with virus; FDA, Food and Drug Administration; HCoV, human coronavirus; MERS-CoV, 82.3% sequence identity, supporting the claim that human COV- Middle East Respiratory Syndrome Coronavirus; RBD, receptor binding domain; ID-19 is a zoonotic virus with possible origins from bats to hu- SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; WHO, World Health man.6 However, no bats were sold at the Huanan seafood market, Organization. Received: February 17, 2020; Revised: February 21, 2020; Accepted: February 22, suggesting that another yet-to-be-identified animal acted as an in- 2020 termediate to transmit the virus to humans. It is urgently requested *Correspondence to: Bohao Chen, Section of Pulmonary and Critical Care Medi- that researchers clarify the source of 2019-nCoV including pos- cine, Department of Medicine, University of Chicago, 5841 S. Maryland Ave., Chi- sible intermediate animal vectors in order to end the COVID-19 cago, IL 60637, USA. Tel: 001-773-834-7476, Fax: 001-773-702-4736, spread. E-mail: [email protected] How to cite this article: Chen B. The Ongoing Outbreak and Challenges of Novel Coronaviruses encode five structural proteins in their genomes. Coronavirus (COVID-19) in China. Exploratory Research and Hypothesis in Medi- These are the spike surface glycoprotein (S), membrane protein cine 2020;5(1):3–5. doi: 10.14218/ERHM.2020.00008. (M), nucleocapsid protein (N), small envelope protein (E), and
Exploratory Research and Hypothesis in Medicine 2020 vol. 5 | 3–5
Copyright: © 2020 Authors. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Explor Res Hypothesis Med Chen B.: Outbreak and challenges of COVID-19 in China
Hemagglutinin Esterase protein (HE). All envelope proteins and peutics, including antibodies, nucleoside analogues, and protease N protein are present in all virions, but HE is only present in some inhibitors for repurposing under FDA Emergency Use Authori- beta coronaviruses. The spike surface glycoprotein plays an essen- zation. Chinese experts are considering a flexible design to offer tial role in engaging with the host cell receptor ACE2 and mediat- investigational therapeutics to those infected with COVID-19 and ing fusion between the viral and host cell membranes. The align- evaluate the safety and efficacy of these drugs on an emergency ment analysis found S protein of COVID-19 shares 97.41% of basis. The government and the private sectors should provide amino acid identity with Bat coronavirus (QHR63300.1), 80.32% more financial assistance for the scientific community to design or with Bat SARS-like coronavirus (AVP78042.1), and 76.12% with develop novel and specific antiviral countermeasures for emerg- SARS coronavirus (PC4-137 AAV49720.1). The modeling and ing coronaviruses. subsequent reports provide strong evidence that the S protein of The COVID-19 epidemic is still going on in China and is COVID-19 has sufficient affinity with the SARS ACE2 receptor far from over. As of February 15, China had reported more than to infect bronchial epithelial cells and type II pneumocytes.7 How- 68,594 confirmed cases, 1,716 of which are medical profession- ever, there is much more to learn about the association of affinity als. The death toll from COVID-19 had risen to 1,667 with 11,272 of the ACE2 receptor with the transmissibility and pathogenesis of critically ill patients. Under the transportation shutdown of Wuhan COVID-19, and more investigations will need to be done to block City and severe shortages of medical resources, Chinese authori- the virus invasion. ties face daunting challenges to provide appropriate medical treat- For SARS-CoV, the S protein is the main antigenic component ment for more than 68,000 patients, protect thousands of health- that is responsible for inducing host immune responses, neutraliz- care professionals from nosocomial infection, quarantine millions ing antibodies and/or protective immunity against virus infection. of people, and deal with the socioeconomic impacts of the epi- S protein has therefore been selected as a major target for vaccine demic. This problem requires international engagement and coop- development and anti-viral therapy. Such efforts include using full- eration against possible COVID-19 global pandemic. length (Novavax, Phase III) or recombinant S protein (Vaxine Pty Ltd, Australia, Phase I) to induce effective neutralizing-antibodies and protective immunity, RBD-ACE2 blockers to block RBD- Acknowledgments ACE2 binding and S protein-mediated infection, small interfering RNAs to reduce virus replication and/or silence S gene expression, inactivated or live attenuated vaccine, etc.8 Although the feasibil- The author thanks all investigators and clinicians who have con- ity of using the above approaches is partially limited by their low tributed to our understanding of COVID-19 infections. antiviral potency, studies and clinical trials on the SARS vaccine provide important information for designing novel strategies for Funding prophylaxis and therapies against emerging infections caused by COVID-19.7 We urge the government and private industry to grant additional funding to advance anti-coronavirus vaccines into clini- None to declare. cal trials. The symptoms and signs of COVID-19 infection are recent- ly described in multiple clinical observations and include fe- Conflict of interest ver, cough, nasal congestion, fatigue, dyspnea, and significant changes visible through chest X-rays and computer tomography The author has no conflict of interests related to this publication. techniques (ground glass abnormalities, patchy consolidation, al- veolar exudates, and interlobular involvement). Cases with gas- trointestinal symptoms and asymptomatic infections have also References been reported recently, especially among young children.9 Sta- tistical analysis showed that the highest incidence of COVID-19 [1] Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of pa- was observed among adults older than 50 years, with the lowest tients infected with 2019 novel coronavirus in Wuhan, China. Lancet incidence in the age group younger than 20 years. Furthermore, 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5. males experienced a higher incidence than females. The deaths [2] Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coro- also were concentrated in male patients over 60 years of age with navirus from Patients with Pneumonia in China, 2019. N Engl J Med severe pneumonia at diagnosis.10 It is unclear yet why some in- 2020;382(8):727–733. doi:10.1056/NEJMoa2001017. fections have no symptoms while others show severe pneumonia [3] Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, et al. and even death. More research should be conducted on the inter- Importation and Human-to-Human Transmission of a Novel Corona- virus in Vietnam. N Engl J Med 2020;382(9):872–874. doi:10.1056/ actions among viral virulence determinants, the density and the NEJMc2001272. affinity of ACE2 receptor on cell surface, and the host’s immune [4] Wang Q, Qi J, Yuan Y, Xuan Y, Han P, Wan Y, et al. Bat origins of response. MERS-CoV supported by bat coronavirus HKU4 usage of human re- Clinical treatment of infections with COVID-19 is still based ceptor CD26. Cell Host Microbe 2014;16(3):328–337. doi:10.1016/j. on supportive care, like oxygen therapy and fluid management, chom.2014.08.009. which has proven to be very effective. Encouraging preliminary [5] Kan B, Wang M, Jing H, Xu H, Jiang X, Yan M, et al. Molecular evolu- trials indicate that infusions of blood plasma from people who tion analysis and geographic investigation of severe acute respiratory have recovered from COVID-19 could help the critically ill pa- syndrome coronavirus-like virus in palm civets at an animal mar- tients improve clinical symptoms. Although antiviral drugs have ket and on farms. J Virol 2005;79(18):11892–11900. doi:10.1128/ JVI.79.18.11892-11900.2005. made great progress in recent years, currently there are no thera- [6] Gralinski LE, Menachery VD. Return of the Coronavirus: 2019-nCoV. peutic agents licensed and available for COVID-19. In theory, Viruses 2020;12(2):E135. doi:10.3390/v12020135. some anti-SARS-CoV and anti-Ebola virus drugs in clinical trials [7] Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by should have activity against COVID-19. In the current outbreak novel coronavirus from Wuhan: An analysis based on decade-long of COVID-19, the WHO prioritized a panel of candidate thera- structural studies of SARS. J Virol 2020;JVI.00127-20. Epub 2020 Jan
4 DOI: 10.14218/ERHM.2020.00008 | Volume 5 Issue 1, March 2020 Chen B.: Outbreak and challenges of COVID-19 in China Explor Res Hypothesis Med
29. doi:10.1128/JVI.00127-20. Wuhan, China. Radiology 2020;200269. Epub Feb 7. doi:10.1148/ra- [8] Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS- diol.2020200269. CoV—a target for vaccine and therapeutic development. Nat Rev Mi- [10] Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiologi- crobiol 2009;7(3):226–236. doi:10.1038/nrmicro2090. cal and clinical characteristics of 99 cases of 2019 novel corona- [9] Shi H, Han X, Zheng C. Evolution of CT Manifestations in a Patient virus pneumonia in Wuhan, China: a descriptive study. Lancet Recovered from 2019 Novel Coronavirus (2019-nCoV) Pneumonia in 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7.
DOI: 10.14218/ERHM.2020.00008 | Volume 5 Issue 1, March 2020 5 Opinion
Blind Spots in Fighting the Outbreak of Coronavirus Disease 2019
Lanjing Zhang*
Department of Pathology, Princeton Medical Center, Plainsboro, NJ, USA; Department of Biological Sciences, Rutgers University, Newark, NJ, USA; Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, USA; Department of Chemical Biology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, NJ, USA
The recent outbreak of 2019 novel coronavirus infection has involved more than 110,000 people and 105 coun- tries. Many efforts have been made to prevent, contain and treat the related disease (named as coronavirus disease 2019). However, many blind spots might not yet receive needed attention. I here discuss eight blind spots that may interest related parties. If these issues remain outstanding, they will likely lead to many severe harms to the public, healthcare providers and the economy. Additional research is therefore needed to better understand and address these blind spots in fighting the outbreak of coronavirus disease 2019.
Recently, the outbreak of 2019 novel coronavirus infection in- lance, prevention and control of COVID-19 on the global scale. volved more than 113,702 people and 109 countries in the word, Several key questions have been asked regarding the patho- and has led to a public health emergency of international concern.1,2 genicity and transmissibility characteristics of COVID-19.11 Many Many efforts were rightly and timely made to fight the outbreak of works have been focused on the trends and characteristics of the 2019 novel coronavirus in China, which was recently named as disease/viral infection.8,12,13 However, several blind spots in my Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) view are noteworthy during the fight against the outbreak of COV- by the Coronavirus Study Group,3 and Coronavirus disease 2019 ID-19. If not prevented or insufficiently prepared for, those blind (COVID-19) by the World Health Organization (WHO).4 A group spots might cause unexpected long-lasting harms, and consume of virologists in China also proposed to name the virus as human significant amount of resources. coronavirus 2019 (HCoV-19).5 Despite the differences in name, Eight issues might be of our particular interest and concern: we observed orchestral actions from governments, health care pro- 1. Distrust and isolated actions among (potentially) involved viders, non-profit organizations and individual citizens in China countries. Due to reasonable worries about the international and the other part of the world. As a result, the daily incidence of spread of the COVID-19, there is significant disruption of inter- infection has been decreasing since February 1, 2020 according to national trades, academic/educational exchanges and political the official websites6,7 and a trend analysis on the epidemiology collaborations.14,15 Such disruption may escalate in the near fu- of COVID-19.8,9 On the other hand, the rising incidence in Japan, ture if the spread of COVID-19 continues involving more coun- Korea and Iran is alarming.2 A modelling study also shows that tries. However, it is probably also the time when international Africa might be vulnerable to the pandemic of COVID-19.10 In- collaborations are urgently and most needed. The hard-learned deed, there were 10,566 COVID-19 cases in WHO member states lessons and practical experiences in China in my view are of outside of China on March 3, 2020, which was developed within ultimate values in fighting this novel virus around the world. the past 2 weeks.2 On March 3 alone, 8 new member states of the Probably more importantly, proper coordination among all (in- WHO (namely Andorra, Jordan, Latvia, Morocco, Portugal, Saudi volved) countries should be encouraged and could result in bet- Arabia, Senegal, and Tunisia) reported their first confirmed new ter utilization of available resources at the global scale. case.2 The daily incidence of COVID-19 outside of China has been 2. Performance of the diagnostic tests. We would need tests like HIV tests, which include a screening test of high sensitivity and higher than that in China since February 26, 2020 (459 versus 412). 16,17 Thus, we should continue our undivided attention on the surveil- a confirmatory test of high specificity. 3. Changes and updates of the diagnostic criteria might be neces- sary as we better understand the dynamic and characteristics of Keywords: COVID-19; Viral infection; Epidemic; Coronavirus; Epidemiology. COVID-19. But these changes and update should be kept as few Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute as possible for the continuity of disease surveillance, and con- respiratory syndrome coronavirus 2; WHO, World Health Organization. sistency in treatment protocols and outcome. One recent change Received: February 26, 2020; Revised: March 04, 2020; Accepted: March 05, 2020 of including clinically diagnosed cases in confirmed cases led *Correspondence to: Lanjing Zhang, Department of Pathology, Princeton Medical to a significant increase in daily incidence of COVID-19 in Wu- Center, Plainsboro, NJ, USA. Tel: +1-609-853-6833, Fax: +1-609-853-6841, han City and Hubei Province.6,7,9 It caused much confusion and E-mail: [email protected] anxiety among the health care providers and the public. How to cite this article: Zhang L. Blind Spots in Fighting the Outbreak of Coronavi- rus Disease 2019. Exploratory Research and Hypothesis in Medicine 2020;5(1):6–7. 4. Cautions in rushing into approving drugs for 2019-nCoV. We doi: 10.14218/ERHM.2020.00012. should follow the preset stand protocols in assessing and approv-
Exploratory Research and Hypothesis in Medicine 2020 vol. 5 | 6–7
Copyright: © 2020 Authors. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Zhang L.: Blind spots in fighting COVID-19 Explor Res Hypothesis Med
ing new drugs. The due diligence must not be jeopardized despite References the urgent needs for these drugs because an approval of such drug will have long-lasting and profound effects in the world. [1] Zarocostas J. What next for the coronavirus response? Lancet 5. The care for non-infectious diseases may be deprived of needed 2020;395(10222):401. doi:10.1016/S0140-6736(20)30292-0. resources. Largely due to lack of manpower and resources, one [2] WHO. Coronavirus disease (COVID-2019) situation reports. Available should anticipate and prepare for a significant rise in the deaths from: https://www.who.int/emergencies/diseases/novel-coronavi- due to cardiovascular, pulmonary and other chronic diseases. rus-2019/situation-reports/. Accessed Feb. 26, 2020. 6. Psychologic issues of the general public, patients, patient-fami- [3] Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva lies, local officials and healthcare providers. A recent comment AA, et al. Severe acute respiratory syndrome-related coronavirus: 18 The species and its viruses – a statement of the Coronavirus Study rightfully raise such a concern. If not timely and properly Group. bioRxiv 2020. doi:10.1101/2020.02.07.937862. addressed, such a psychological issue will exhaust healthcare [4] WHO. Coronavirus disease (COVID-19) outbreak. Available from: htt- manpower, decrease morale and cause long-term psychological ps://www.who.int/emergencies/diseases/novel-coronavirus-2019. diseases among impacted individuals including patients, fami- Accessed February 23, 2020. lies and health care providers. [5] Jiang S, Shi Z, Shu Y, Song J, Gao GF, Tan W, et al. A distinct name is 7. Documentation of related policies, implemented approaches needed for the new coronavirus. Lancet 2020. doi:10.1016/s0140- and observed outcomes. This practice will be critical for track- 6736(20)30419-0. ing records, learning the lessons, enforce accountability and [6] Wuhan Municipal Health Commission. Briefings on the viral disease improving efficiency. [Chinese]. Available from: http://wjw.wuhan.gov.cn/front/web/lis- t3rd/no/802. Accessed Feb. 23, 2020. 8. Information overload and errors in reporting. It is inevitable that [7] National Health Commission of China. Briefings on the viral outbreak the conventional media and social media will pour unprecedent [Chinese]. Available from: http://www.nhc.gov.cn/xcs/yqtb/list_gzbd. amount of information to the public, who are eager to better shtml. Accessed Feb. 24, 2020. understand the disease and current situation. The important and [8] Xu J, Cheng Y, Yuan X, Li WV, Zhang L. Trends in epidemiology of 2019 accountable information, however, might be buried in the infor- novel coronavirus. Available from: https://github.com/thezhanglab/ mation overload. Related parties should proactively reach out coronavirus. Accessed Feb. 23, 2020. to the public to “advertise” credible information through new [9] Xu J, Cheng Y, Yuan X, Li WV, Zhang L. Trends and prediction in daily media. Errors in reporting or rumors are very difficult to iden- incidence of novel coronavirus infection in China, Hubei Province and Wuhan City: an application of Farr law. medRxiv 2020:20025148. tify and handle since they could be proven wrong only upon doi:10.1101/2020.02.19.20025148. rigorous, long and time-consuming validation studies. [10] Gilbert M, Pullano G, Pinotti F, Valdano E, Poletto C, Boëlle PY, et al. Another question of significant consequences is how to prop- Preparedness and vulnerability of African countries against impor- erly deal with the aftermath of the COVID-19 outbreak. Hopefully, tations of COVID-19: a modelling study. Lancet 2020. doi:10.1016/ the end of the COVID-19 outbreak will soon come. Many issues S0140-6736(20)30411-6. will emerge. Thus, experts in public health, socioeconomical, geo- [11] Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. political and biomedical studies should work together to proac- A Novel Coronavirus Emerging in China - Key Questions for Impact tively articulate the related issues, discuss possible solutions and Assessment. N Engl J Med 2020;382(8):692–694. doi:10.1056/NE- formulate feasible, yet impactful, action plans. JMp2000929. [12] Zhang R, Liu H, Li F, Zhang B, Liu Q, Li X, et al. Transmission and epidemi- In summary, the eight blind spots may interest related parties. ological characteristics of Novel Coronavirus (2019-nCoV) Pneumonia If these issues remain outstanding, they will likely lead to many (NCP): preliminary evidence obtained in comparison with 2003-SARS. severe harms to the public, healthcare providers and the economy. medRxiv 2020:20019836(v3). doi:10.1101/2020.01.30.20019836. Additional research is therefore needed to better understand and [13] Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission address these blind spots in fighting the outbreak of coronavirus Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumo- disease 2019. nia. N Engl J Med 2020. doi:10.1056/NEJMoa2001316. [14] DHS. 2020 Travel/Visa Restrictions. Available from: https://www.dhs. gov/news/2020/01/31/2020-travelvisa-restrictions. Accessed March Acknowledgments 3, 2020. [15] Government UK. Policy paper: Coronavirus action plan: a guide to what you can expect across the UK. Available from: https://www.gov. The opinions expressed here are solely those of the author, and not uk/government/publications/coronavirus-action-plan/coronavirus- of his affiliations. action-plan-a-guide-to-what-you-can-expect-across-the-uk. Accessed March 3, 2020. [16] Branson BM. HIV Diagnostics: Current Recommendations and Oppor- Funding tunities for Improvement. Infect Dis Clin North Am 2019;33(3):611– 628. doi:10.1016/j.idc.2019.04.001. [17] Chen DJ, Yao JD. Comparison of turnaround time and total cost of None. HIV testing before and after implementation of the 2014 CDC/APHL Laboratory Testing Algorithm for diagnosis of HIV infection. J Clin Vi- rol 2017;91:69–72. doi:10.1016/j.jcv.2017.04.004. Conflict of interest [18] Xiang YT, Yang Y, Li W, Zhang L, Zhang Q, Cheung T, et al. Timely men- tal health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry 2020;7(3):228–229. doi:10.1016/S2215- The author has no conflict of interest related to this publication. 0366(20)30046-8.
DOI: 10.14218/ERHM.2020.00012 | Volume 5 Issue 1, March 2020 7 Opinion
Characteristics of COVID-19 During the Onset Stage and Considerations for Disease Control
Department of Medicine, Division of Infectious Diseases, Icahn School of Medicine at Mount Sinai, New York, NY, 10029, USA
COVID-19 outbreak in December 2019 has evolved into a world level pandemic. To cope with an emerging patho- gen like SARS-CoV-2, there is no vaccine or specific drug available to treat the disease. However, get to know the characters of the onset stage of the new disease may help us to control the spread of COVID-19. This short article aims to introduce COVID-19 briefly, getting you to know the clinical character, the treatment, public procedurals and researches on COVID-19 quickly but comprehensively.
The first pneumonia case of unknown origins was identified in Epidemiological characteristics Wuhan, China in early December 2019.1 The symptoms of it re- minded people of severe acute respiratory syndrome coronavirus According to the first published descriptive study based on 99 pa- (SARS-CoV), which had shocked China 17 years ago in 2003. tients from Jinyintan Hospital in Wuhan,4 49% of the 2019-nCoV High-throughput sequencing confirmed the new virus is the sev- infected patients had a history of exposure to Huanan Seafood enth member of enveloped RNA coronavirus (subgenus sarbeco- Wholesale Market. 2019-nCoV infection was of clustering onset, virus, Orthocoronavirinae subfamily). Currently, the new virus was more likely to affect older men with comorbidities, and could was named as severe acute respiratory syndrome coronavirus 2, result in severe and even fatal respiratory diseases such as acute SARS-CoV-2), which was also called 2019 novel coronavirus respiratory distress syndrome (ARDS). A later study published on (2019-nCoV) earlier. And the disease caused by it was named cor- Jan 29th with a sample size of 425 cases indicates the majority of onavirus disease in 2019 (COVID-19).2,3 Until now, COVID-19 cases (55%) with onset before Jan 1st, 2020 were linked to the Hua- outbreaks emerged in Wuhan have caused more than 80,000 con- nan Seafood wholesale Market, while after that date only 8.6%. firmed infections; including more than 2,800 deaths (December The mean incubation period was 5.2 days (95% confidence inter- 1st, 2019-Feb 29th 2020, 3 months). On January 30, 2020, the val, 4.1 to7.0) and the basic reproductive number was estimated to International Health Regulations Emergency Committee of the be 2.2 (95% confidence interval, 1.4 to 3.9).5 These data indicate World Health Organization declared the outbreak a “public health human-to-human transmission has occurred among close contacts emergency of international concern”. To cope with an emerging since the middle of December 2019. pathogen with serious outcomes, the first thing to do is get to know the enemy. Scientists and doctors in the frontier responded quick- ly to this emergency and provided valuable epidemiological and Clinical characteristics clinical data sharing with the rest of the world before its further spread. Knowing what happened during the onset of the diseases, Early reports from Huang et al. on Jan 24, 2020, provided clinical and what test method, diagnosis and treatment was performed is characteristics of 41 patients confirmed with 2019-nCoV infection important for us to assess the procedurals, and further evaluate and in Wuhan Jinyintan Hospital. These data indicated that patients’ control the disease. clinical manifestations included fever, nonproductive cough, dysp- nea, myalgia, fatigue, Lymphopenia, and radiographic evidence of pneumonia. Organ dysfunction (e.g., shock, acute respiratory dis- Keywords: COVID-19; SARS-CoV-2. tress syndrome (ARDS), acute cardiac injury, and acute kidney in- Abbreviations: SARS-CoV, severe acute respiratory syndrome coronavirus; 2019- jury) and death can occur in severe cases.1 138 patients confirmed nCoV, 2019 novel coronavirus; COVID-19, coronavirus disease in 2019; SARS- with 2019-nCoV infection from Zhongnan Hospital of Wuhan CoV-2, severe acute respiratory syndrome coronavirus 2; ARDS, acute respiratory distress syndrome. University showed similar clinical characteristics based on the re- 6 Received: February 14, 2020; Revised: February 29, 2020; Accepted: March 02, port on Feb 7 from Wang et al. In this report, one thing to notice is 2020 that infection for affected health professionals and hospitalized pa- *Correspondence to: Lili Wang, Department of Medicine, Division of Infectious tients take 29% and 12.3% respectively. This suggests among the Diseases, Icahn School of Medicine at Mount Sinai, New York, NY, 10029, USA. Tel: 138 patients, 41.3% got infected inside the hospital. Case reports +1 212-241-2503, E-mail: [email protected] confirmed human-to-human transmission of 2019-nCoV.7,8 On How to cite this article: Wang L. Characteristics of COVID-19 During the Onset Stage and Considerations for Disease Control. Exploratory Research and Hypothesis the national scale, data of 1,099 patients from 552 hospitals in 31 in Medicine 2020;5(1):8–10. doi: 10.14218/ERHM.2020.00005. provinces were assessed.9 Based on this study preprinted online on
Exploratory Research and Hypothesis in Medicine 2020 vol. 5 | 8–10
Copyright: © 2020 Authors. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Wang L.: COVID-19 onset Explor Res Hypothesis Med
Table 1. The summary of the most commonly seen symptom from studies Sample size Median age Fever Fatigue Dry cough Dyspnea Lymphopenia Jinyintan Hospital1 41 49 98% 44% 31% 22% 63% Zhongnan Hospital6 138 56 98.6% 69.6% 59.4% 31.2% 70.3% National9 1,099 47 87.9% 38.1% 67.7% 18.6% 82.1%
Feb 6, 31.30% of the patients had been to Wuhan and 71.80% had COVID-19. The vaccines are still under development. A cryo-EM contact people from Wuhan. This paper also indicated the median study on 2019-nCoV spike protein revealed the prefusion con- incubation period was 3.0 days (range, 0 to 24.0 days). The most formation of spike glycoprotein of 2019-nCoV and found it can commonly seen symptom from these studies was summarized in bind to the receptor ACE2 with higher affinity than its SARS-CoV Table 1.1,6,9 According to the 1,099 people study, 18.6% of the counterpart.14 As the spike glycoprotein is a key target for vac- patients were severe cases (based on American Thoracic Society cines, therapeutic antibodies and diagnostics, the discoveries in guideline), and 5% of them were treated in ICU. The death rate is the details of spike glycoprotein structure will contribute to the around 2% based on these reports. drug and vaccine development. Drugs against coronavirus RNA- dependent RNA polymerase is also under clinical trial. Due to the high infectivity of SARS-CoV-2, the optimal strategies against Treatment spreading remains to be early detection and early quarantine. The early detection relies on a quick and accurate detection method, As the SARS-CoV-2 is a novel coronavirus that just been identi- which is also under clinical test. Until Feb 29, 2020, the number fied, no specific drug has been found to treat COVID-19. Based of newly diagnosed people in China has declined for over a week, on the experiences gained on SARS-CoV infection, the early pa- which is a good sign of COVID-19 under control. However, new tients were treated with antibiotics and oseltamivir. Corticosteroid patients number confirmed in other countries is increasing.15 Rigid therapy was given as a combined regimen if severe pneumonia control by the government would be crucial under these public was diagnosed. Oxygen support was administered to patients ac- emergency circumstances. In this article, I hope all the endeavors cording to the severity of hypoxemia.1,6,9 In vitro studies on anti- we made will shorten the course of this modern plague, with the SARS-CoV-2 drugs have shown high efficacy of remdesivir.10 The least spreading to the other part of the world. first confirmed COVID patient in the U.S. was cured by remdesi- vir.11 At present at least two phase-3 clinical trials on remdesivir were carried out from early February 2020. With more patients Acknowledgments recovered from COVID-19 and released from the hospital, donat- ed blood plasma from recovered patients with antibodies against I thank the colleges from Icahn School of Medicine for meaningful SARS-CoV-2 is also used to treat severe cases. Other remedies are discussion. also performed to reduce the damage of vital organs by cytokine storm induced by the immune response.12 Funding Comments This article is not fund-supported. A confirmed case with COVID-19 was defined as a positive result to real-time reverse-transcriptase polymerase-chain-reaction (RT- Conflict of interest PCR) assay or high-throughput sequencing for nasal and pharyn- geal swab specimens.1 However, when an individual was infected but with low viral load, the test result could be negative due to The author has no conflict of interest related to this publication. the relatively high false-negative rate of the PCR based tests. This might potentially lead to the spread of SARS-CoV-2. There are References cases with negative results in the early days of COVID-19 when the symptom is not severe but later confirmed as positive SARS- CoV-2 infection.13 Self-quarantine for 14 days when has suspi- [1] Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of pa- cious syndrome is a safe strategy. SARS-CoV-2 spread rapidly tients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5. through human-to-human routes, accumulating tens of thousands [2] Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisa- of patients within a single month in Wuhan. The huge number of tion and epidemiology of 2019 novel coronavirus: implications for vi- patients that emerged during such a short time saturated the medi- rus origins and receptor binding. Lancet 2020;395(10224):565–574. cal resources. How to properly isolate and treat patients has be- doi:10.1016/S0140-6736(20)30251-8. come a great challenge to the public health system. [3] Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coro- navirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020;382:727–733. doi:10.1056/NEJMoa2001017. Perspectives [4] Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiologi- cal and clinical characteristics of 99 cases of 2019 novel corona- virus pneumonia in Wuhan, China: a descriptive study. Lancet Although several promising trials are carried out in China and 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7. America, at present no specific drugs have been confirmed to cure [5] Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission
DOI: 10.14218/ERHM.2020.00005 | Volume 5 Issue 1, March 2020 9 Explor Res Hypothesis Med Wang L.: COVID-19 onset
Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumo- virus (2019-nCoV) in vitro. Cell Res 2020;30:269–271. doi:10.1038/ nia. N Engl J Med 2020. doi:10.1056/NEJMoa2001316. s41422-020-0282-0. [6] Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Character- [11] Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et istics of 138 Hospitalized Patients With 2019 Novel Coronavirus- al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Infected Pneumonia in Wuhan, China. JAMA 2020. doi:10.1001/ Med 2020;382:929–936. doi:10.1056/NEJMoa2001191. jama.2020.1585. [12] Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological [7] Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster findings of COVID-19 associated with acute respiratory distress syn- of pneumonia associated with the 2019 novel coronavirus indicat- drome. Lancet Respir Med 2020. doi:10.1016/S2213-2600(20)30076- ing person-to-person transmission: a study of a family cluster. Lancet X. 2020;395(10223):514–523. doi:10.1016/S0140-6736(20)30154-9. [13] Zhou Y, Yang L, Han M, Huang M, Sun X, Zhen W, et al. Clinical Reports [8] Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, et al. on Early Diagnosis of Novel Coronavirus (2019-nCoV) Pneumonia in Importation and Human-to-Human Transmission of a Novel Coro- Stealth Infected Patients. Preprints 2020:2020020156. doi:10.20944/ navirus in Vietnam. N Engl J Med 2020;382:872–874. doi:10.1056/ preprints202002.0156.v1. NEJMc2001272. [14] Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et [9] Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Charac- al. Cryo-EM structure of the 2019-nCoV spike in the prefusion con- teristics of Coronavirus Disease 2019 in China. N Engl J Med 2020. formation. Science 2020. doi:10.1126/science.abb2507. doi:10.1056/NEJMoa2002032. [15] Hodcroft EB. Preliminary case report on the SARS-CoV-2 cluster in [10] Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and the UK, France, and Spain. Swiss Med Wkly 2020;150:w20212. chloroquine effectively inhibit the recently emerged novel corona- doi:10.4414/smw.2020.20212.
10 DOI: 10.14218/ERHM.2020.00005 | Volume 5 Issue 1, March 2020 Original Article
Echocardiography Effectiveness in Improving Diagnosis of Rheumatic Heart Disease in North Darfur: A Hospital-based Study
Mohammed Elmujtba Adam Essa Adam1,2*, Sherihan Mohammed Elkundi Osman3, Daralsalam Ishag Ateem Abdalrasoul1, Ibrahim Adam Osman Yagoup1,2, Mustafa Mohamed Ali Hussein1,2, Mutwaly Defealla Yousif Haron1,2, Ziryab Imad Taha Mahmoud4,2 and Abdelkareem A. Ahmed2,5*
1Department of Medicine, Faculty of Medicine, Al-Fashir University, Al-Fashir, Sudan; 2Department of Clinical Medicine, Medical and Cancer Research Institute (MCRI), Nyala, Sudan; 3Department of Molecular Medicine, Institute of Endemic Diseases, University of Khar- toum, Khartoum, Sudan; 4Department of Internal Medicine, Faculty of Medicine, University of Bahri, Khartoum, Sudan; 5Department of Physiology and Biochemistry, Faculty of Veterinary Science, University of Nyala, Nyala, Sudan
Background: Acute rheumatic fever (ARF) is an inflammatory disease caused by autoimmune responses to bacte- rial infection. Rheumatic heart disease (RHD) damages one or more heart valves through recurrent episodes of ARF. We aimed to determine the changes in sensitivity, specificity and predictive values in RHD Jones diagnostic guidelines following the inclusion of echocardiograph as an additional diagnostic tool for RHD.
Methods: This is a retrospective cross-sectional study done in the echocardiography center of Al-Fashir teach- ing hospital. We included a total of 1,103 patients who presented at our hospital and had a diagnosis of RHD, ischemic heart disease or congestive heart disease during 2011–2017.
Results: Among the RHD patients, screening with echocardiography was associated with increases of the sensitivity value, positive predictive value and specificity value by 18.1%, 8.1% and 1%, as compared to their initial diagnoses by Jones criteria alone, which were primarily based on clinical presentations. Mitral stenosis was the most common RHD abnormality, followed by aortic and tricuspid valve regurgitation. North Darfur state was found to have the low- est prevalence of RHD in all geographical parts of Sudan that have been studied. The female to male ratio was 3:1.
Conclusions: Our data highlight the important role of echocardiography in diagnosing RHD complications through improved diagnostic sensitivity, positive predictive value and specificity.
Introduction
Keywords: Early diagnosis; Echocardiography; Mitral stenosis; North Darfur state; Acute rheumatic fever (ARF) is a type of disease caused by bac- Rheumatic heart disease. Abbreviations: ARF, acute rheumatic fever; RHD, rheumatic heart disease; RF, rheu- terial infection, commonly involving groups with a β hemolytic matic fever. Streptococci and leading to an autoimmune inflammatory response. Received: August 27, 2019; Revised: October 16, 2019; Accepted: November 07, Most of the body’s organs, such as the heart, brain, joints and skin, 2019 can also be affected by the disease. The recurrent episodic valve *Correspondence to: Abdelkareem Abdallah Ahmed, Department of Physiology and damage to the heart in ARF are known as rheumatic heart disease Biochemistry, Faculty of Veterinary Science, University of Nyala, Nyala, PO Box: 1,2 155 Nyala, Sudan. Fax: +249711833123; E-mail: [email protected] ; Moham- (RHD). Both diseases are common in low socioeconomic com- 3 med Elmujtba Adam Essa Adam, Faculty of Medicine, Al-Fashir University, Al-Fa- munities. More than 0.5% of school-age children are suffering shir, Sudan. Tel: +249907009378; E-mail: [email protected] from RHD across the globe, and the incidence is increased in the How to cite this article: Adam MEAE, Osman SME, Abdalrasoul DIA, Yagoup IAO, sub-Saharan region of Africa by nearly half a million patients an- Hussein MMA, Haron MDY, Mahmoud ZIT, Ahmed AA. Echocardiography Effec- nually.4–6 This disease causes damage to the heart valves and the tiveness in Improving Diagnosis of Rheumatic Heart Disease in North Darfur: A Hos- 7,8 pital-based Study. Exploratory Research and Hypothesis in Medicine 2020;5(1):11–18. most common consequent abnormality is mitral regurgitation. doi: 10.14218/ERHM.2019.00020. There is a growing concern for patients with severe conditions
Exploratory Research and Hypothesis in Medicine 2020 vol. 5 | 11–18
Copyright: © 2020 Authors. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Explor Res Hypothesis Med Adam MEAE. et al: Echocardiography in improving diagnosis of rheumatic heart disease without access to cardiac surgery, which could lead to a potential were confirmed by echocardiography (SusKo, CH5-2Transducer; risk of death being imminent. Reportedly, 20% of patients die be- Siemens), which established the diagnosis. The severity was deter- fore the age of 5 years and 80% before the age of 25 years.9 mined by the American Society of Echocardiography (commonly In regard to the pathology and pathogenesis of RHD, the calci- known as ASE) modified criteria, and the valve lesion was defined fication in the mitral valve is not merely an inactive “dystrophic” according to the jet width and camper to the jet ratio (5 mm and process but it is a regulated inflammatory process with osteoblast more to the left atrium ratio of 20% was considered severe mitral and neo-angiogenesis markers’ expression.10 The World Health regurgitation; and if the jet-valve ratio with the jet width increased Organization (commonly referred of as WHO) Consultant’s Re- severity of the valve became more intense, a 3 mm jet width with port stated that any patient diagnosed by echocardiography with 25% left ventricular outflow tract as confirmed by aortic regurgita- a silent rheumatic valve should be considered and treated as RHD tion echocardiography). The Doppler mean gradient and valve ap- until other proof is available.11 Accurate diagnosis is important pearance were both used to calculate the mitral stenosis. The clini- to avoid long-term treatment for those who have been diagnosed cal examinations included several processes, and careful cardiac and also to prevent further heart damage and the need for surgi- examination was performed in supine and left lateral decubitus po- cal intervention (for those who are underdiagnosed). Currently, sition by an experienced physician. Patients with organic murmur there is no diagnostic test for RHD, and the diagnosis is based were detected clinically and confirmed by echocardiography, then solely on the clinical recognition of the major and minor criteria classified as patients with clinically-detected RHD. of Jones (guidelines published in 1944 by Dr T. Duckett Jones, modified in 2015) with evidence of Streptococcal throat infec- Statistical analysis tion.12 The estimated risk for developing rheumatic fever (RF) after a streptococcal pharyngitis episode is 0.3–3%,13 and a small per- The statistical software package SPSS (version 17.0) was used to centage of RF develops as RHD. The primary risk factor is Strep- analyze all the collected data of RHD as percentages, categories tococcal throat infection. Other types of infections, such as of and p-values for statistical significance testing. Evaluation data group A Streptococci, may lead to RF, as in pyoderma (skin infec- were analyzed by one-way analysis of variance (referred to as tion); however, age, sex, urban residence, overcrowding, poverty, ANOVA). The level of statistical significance was set as p<0.05. offspring of working mothers, and mother illiteracy are also poten- tial risk factors.14 The objective of this study was to determine the incidence, prevalence and risk factors of RHD among children and Results adults in North Darfur State, and to estimate the specificities, sen- sitivities, and positive predictive values of adding echocardiograph The study included in total 1,103 patients who were confirmed to the routine of diagnosing RHD patients. by echocardiography from January 2011 to December 2017. All patients who were screened by echocardiography were confirmed with the following diagnoses: RHD, ischemic heart disease, con- Methods gestive heart disease and cardiomyopathy, (35%, 29.2%, 20% and 15.8% respectively) (Fig. 1). We found that the most affected age Study area groups among all the patients were those above 25 years, and that both the age groups between 15–25 years and under 15 years have North Darfur State is one of the five States composing the Darfu- an equal incidence of RHD (38%, 31%,31% respectively) (Fig. rian region, with an area of 296,420 Km2 and a total population 2). The incidence of RHD was found to be higher in females than of 2,296,068 in 2017 (Central Statistical Agency of Sudan, 2017 males, with a ratio of 3:1 (Fig. 3) (Table 1). report). Al-Fashir, its capital, had 264.734 residents in 2006; how- Most of the patients in the year 2011 had three-valve involve- ever, that number decreased nearly to half in the last 5 years due to ments, double valve involvements and an isolated valve (55.9%, conflict in the area. 26.41% and 14.71% respectively). This contrasted the following years, which showed a semi-steady decrease of the three-valve involvements in the RHD patients and that those patients with Study design three-valve lesions became the fewest among the collective pa- tients with different valves lesions, followed by the isolated valve This is a retrospective study; the data was collected from Al-Fashir involvement patients and dominated by the double valve involve- Teaching Hospital. Scientific and ethical approvals were granted ment patients (4%, 38.8% and 57.2% respectively in 2015). In by the Scientific and Ethics Committees of the Institute ofEn- total, double valve involvement had the greatest incidence, fol- demic Diseases, University of Khartoum. Addition approvals were lowed by an isolated valve then the three-valve involvement obtained from the North Darfur State Ministry of Health and Al- (40.9%, 35.6% and 23.5% respectively) (Table 2). The peak inci- Fashir Teaching Hospital Management Board. The data covered dence of the three-valve involvement was the year 2011 (55.9%), the period from January 2011 to December 2017. The data collect- and the lowest incidence year was 2015 (4.1%) (Fig. 4). The most ed from the hospital files included the clinical records of patients affected valve in our RHD patients was found to be the mitral with confirmed RHD, RF and other cardiac diseases. Demographic valve, followed by the aortic then the tricuspid valve (56.9%, and clinical data were extracted along with each patient’s age, sex, 27.2% and 15.9% respectively) (Table 3). The highest incidences address, clinical presentations, valve involvement, and final diag- of the mitral valve were in 2014 and 2017, in which 100% of nosis. All the studied patients had been selected to be confirmed by patients had mitral valve lesion with or without other affected echocardiography after eliciting clinical suspicion. valves (Table 4). The steps of the disease diagnosis begun from the outpatient When comparing the same patients, who were diagnosed by yard, where the disease history and clinical examinations were per- Jones criteria before and after the screened echocardiography to formed for all patients. After diagnostic suspicion, all of the patients confirm the diagnosis, we found that the echocardiography screen-
12 DOI: 10.14218/ERHM.2019.00020 | Volume 5 Issue 1, March 2020 Adam MEAE. et al: Echocardiography in improving diagnosis of rheumatic heart disease Explor Res Hypothesis Med
Fig. 1. Jones criteria for diagnosing rheumatic fever. Data are hospital diagnoses from the period of 2011–2017.
ing adds better sensitivity, positive predictive value and specifi- cities (18.1%, 8.1% and 1%) (Table 5). The peak incidence year with RHD patients was 2011 (17.6%) and the least affected year was 2017 (11.6%). The years 2012, 2013, 2014, 2015 and 2016 had a fluctuating range of incidence (14.7%, 16.3%, 13.3%, 12.7% and 13.9%) (Table 6, Fig. 5). We also found that mitral stenosis is more common than mitral regurgitation, by a 12.4% difference. The mitral stenosis represents 56.2% of all the mitral involvement patients, in comparison to 43.8% represented by the mitral regur-
Table 1. Male, female differences with rheumatic heart disease
Frequency % Valid % p-value Valid Male 127 32.8 32.8 <0.05 Fig. 2. Age groups of rheumatic heart disease in North Darfur State pa- tients. Female 260 67.2 67.2 <0.05 Total 387 100 100
Table 2. Number and percentage of valve involvement in rheumatic heart disease patients from 2011–2017
Year Isolated valve Double valve Three valves 2011 14.71% 26.41% 55.88% 2012 35.10% 28% 36.90% 2013 44.40% 44.40% 11.20% 2014 53% 39.20% 7.80% 2015 38.80% 57.20% 4% 2016 37.10% 50% 12.90% 2017 31.20% 42.20% 26.60% Fig. 3. Incidence rate between male/female patients with rheumatic heart disease. Total 35.60% 40.90% 23.50%
DOI: 10.14218/ERHM.2019.00020 | Volume 5 Issue 1, March 2020 13 Explor Res Hypothesis Med Adam MEAE. et al: Echocardiography in improving diagnosis of rheumatic heart disease
Fig. 4. Prevalence of valvular abnormality in patients with rheumatic heart disease. gitation. Discussion The most common aortic type of lesion has been aortic regur- gitation, found in 84.7% of the patients with aortic valve involve- RF was first detected by Wig in 1935 and since then most of the ment, and the least common type of aortic involvement has been studies conducted in ARF and RHD patients have been identified aortic stenosis. We also found all the patients with tricuspid lesion by many risk factors associated with the disease,12 such as poverty had tricuspid regurgitation with zero tricuspid stenosis. The most and low health access.13–20 common clinical presentation of RHD patients has been arthritis, Most global studies show an inconsistent association of the male followed by carditis, arthralgia, chorea and erythema marginatum to female ratio. The epidemiological data indicate there is the same (62%, 41.1%, 33.5%, 11.4% and 1%). Most of the patients were prevalence of RHD in both sexes,21 which is in contrast to our study, diagnosed with more than one feature (Fig. 6). which demonstrated a higher incidence in females than males (being Table 3. Accumulative percentages and frequencies of the three-valve Table 5. Sensitivity, specificity and positive predictive value changes be- involvements in all the rheumatic heart disease patients fore and after addition of echocardiograph to rheumatic heart disease diagnosis Frequency Percent Valid Percent p-value Valid Jones criteria Jones criteria with alone echocardiography Mitral 377 56.9 56.9 <0.05 Sensitivity 51.2% 69.3% Aortic 180 27.2 27.2 <0.05 Specificity 97% 98% Tricuspid 105 15.9 15.9 <0.05 Positive predictive value 88.3% 96.4% Total 662 100 100 p-value <0.05 <0.05 Table 4. Percentages of cardiac valve lesions’ involvement in rheumatic heart disease patients from 2011–2017 Table 6. Percentage of rheumatic heart disease from 2011–2017
Year Mitral Aortic Tricuspid Year Frequency Percent Valid percent p-value 2011 91.1% 51.4% 39.7% Valid 2012 98.2% 47.3% 14% 2011 68 17.6 17.6 <0.05 2013 98.4% 44.4% 23.8% 2012 57 14.7 14.7 <0.05 2014 100% 37.2% 17.6% 2013 63 16.3 16.3 <0.05 2015 98% 53% 14.2% 2014 51 13.2 13.2 <0.05 2016 98.1% 40.7% 37% 2015 49 12.7 12.7 <0.05 2017 100% 51.1% 42.22% 2016 54 14 14 <0.05
. The table demonstrates the percentage of each valve involvement annually. As the 2017 45 11.6 11.6 <0.05 patients can have all three valves lesions at the same time, the percentage are over- lapping. Total 387 100 100
14 DOI: 10.14218/ERHM.2019.00020 | Volume 5 Issue 1, March 2020 Adam MEAE. et al: Echocardiography in improving diagnosis of rheumatic heart disease Explor Res Hypothesis Med
Fig. 5. Prevalence of rheumatic heart disease in Al-Fashir Teaching Hospital from 2011–2017.
3:1). Our findings are in line with previous studies conducted in Su- The incidence of RHD in North Darfur was found in our study dan and some parts of Asia, such as India and Pakistan, which gave to be less than in any other area in Sudan which had been studied, similar results but showed a lower female to male ratio.21–23 This such as South Darfur, which showed 3.3% in Nyala camps during could be explained by women seeking medical care more than men, the year 2016. Khartoum, being the capital city of North Darfur, especially during the antenatal care period, or the opposite, where was estimated to have 0.03%, and that prevalence are dominated women are less likely to seek medical care when they have a strep by the North Kordofan state (with 6.1% of RHD).23–25 On the oth- throat infection; however, no clear explanation has been identified. er hand, our study revealed that North Darfur has only 0.03% of In addition, there is an elevated infection risk of group A strepto- RHD, which reduced to 0.019% in 2017 (the population density of coccal infection, and RHD may develop if not treated properly; this North Darfur was obtained from the Central Statistical Organiza- constitutes 60% of heart disease during pregnancies.24,25 tion report). Our study found a higher rate of sensitivity and specificity in Another study showed the age groups from 11–30 years to be using the echocardiography to diagnose RHD compared by tradi- the most affected with RHD.23 Similarly, our study confirmed that tional means of screening, such as auscultation. There have been age groups above 25 years have higher incidence. This may be re- several studies in the literature that produced the same finding.26,27 lated to the lack of health services, timely diagnosis and treatment. Other studies have also detected the sensitivity rate of 73% and the There are general agreements on mitral valve, which has been the positive predictive value of 92% in using echocardiography align- valve disease most associated with RHD, and the mitral regurgi- ing with the reference criteria.28 tation, as the most common type of lesion in the valve.24,29 Our
Fig. 6. Clinical presentation of the rheumatic heart disease patients.
DOI: 10.14218/ERHM.2019.00020 | Volume 5 Issue 1, March 2020 15 Explor Res Hypothesis Med Adam MEAE. et al: Echocardiography in improving diagnosis of rheumatic heart disease
Fig. 7. Projected decrease in age-specific mortality from rheumatic heart disease based on country trends from 2000 to 2015. The x-axis demonstrates the total percentage reduction in mortality for those aged below 25 years (WHF) target between 2013 and the year 2025, if 2000 to 2015 trends continue. The y-axis illustrates all percent reduction in mortality for those aged 30–69 years (Sustainable Development Goal 3 [SDG3] target) between 2015 and 2030, if 2000 to 2015 trends continue. analyses also revealed the mitral valve to be the highest involved (Fig. 7).37 but also found mitral stenosis to be more common when compared There are some hypotheses about the role of RHD in being a to mitral regurgitation. The inconsistency of those findings may risk factor for some neurological diseases, such as with stroke, as indicate the higher prevalence of elderly patients, as mitral stenosis it has been detected that patients with mitral stenosis and atrial occurs in advanced ARF.30 fibrillation have increased 4% liability to have stroke.38 Yet, only To control both RHD and RF, there are many factors that should a few studies are being done in this matter, but future studies will be considered, starting with benzathine penicillin G administra- likely clarify the exact role. tion,31 vaccine availability, primordial, overcrowding, and im- proving primary and secondary prevention against the disease, according to the WHO.31,32 The secondary prevention to RF is the Conclusions continuous provision of some type of antibiotic to patients with an 31 exposed history of ARF and documented RHD, with considera- This was a retrospective hospital-based study which concluded tion to treatment availability and no surgical access. In valvular 33,34 that females were more vulnerable to RHD. Moreover, over 25 disease patients this is particularly important. The last expan- years-old is the most affected age group, and the incidence of valve sion of the echocardiography screening programs led to a reduc- involvement in RHD decreases with time. The sensitivity, specific- tion in the burden of RHD and it will continue to better identify ity and positive predictive value have improved after the addition patients while they are asymptomatic, like in silent RHD. The con- of echocardiography to the Jones criteria. tinuous data provided will help in estimating the prevalence of the diseases and their complications with annual bases. The study presented herein carries some limitations, such as Acknowledgments having no updated population census in Sudan, no available data in regard to patient follow-up, and so on. The authors are very grateful to the Al-Fashir Hospital administra- tion staff for their support in conducting this research. Future research directions, prospectives, predictions and hy- pothesis Ethics approval and consent to participate In 2013, the World Heart Federation called for a 25% reduction in RHD mortality amid individuals aged <25 years before the year Obtained. 2025.35 Recently, the United Nations Sustainable Development Goal 3 (referred to as SDG3) prospected a 1/3 reduction in prema- ture death due to non-communicable disease by the year 2030.36 Consent for publication Predicting that trends in mortality over the last 15 years hold, many endemic regions are in line to achieve either one of or both targets Not applicable.
16 DOI: 10.14218/ERHM.2019.00020 | Volume 5 Issue 1, March 2020 Adam MEAE. et al: Echocardiography in improving diagnosis of rheumatic heart disease Explor Res Hypothesis Med
Funding [15] Kumar R, Vohra H, Chakraborty A, Sharma YP, Bandhopadhya S, Dhanda V, et al. Epidemiology of group A streptococcal pharyngitis & impetigo: across-sectional & follow up study in a rural community of No funds have been received in relation to this study or its publica- northern India. Indian J Med Res 2009;130(6):765–771. tion. [16] Carapetis JR, Currie BJ, Kaplan EL. Epidemiology and prevention of group A streptococcal infections: acute respiratory tract infections, skin infections, and their sequelae at the close of the twentieth cen- Conflict of interest tury. Clin Infect Dis 1999;28(2):205–210. doi:10.1086/515114. [17] Nordet P, Lopez R, Duenas A, Sarmiento L. Prevention and control of rheumatic fever and rheumatic heart disease: the Cuban experience All authors declare that they have no conflicts of interest. (1986-1996-2002). Cardiovasc J Afr 2008;19(3):135–140. [18] Allen LB, Allen M, Lesa RF, Richardson GE, Eggett DL. Rheumat- ic fever in Samoa: education as prevention. Pac Health Dialog Author contributions 2011;17(1):107–118. [19] Orün UA, Ceylan O, Bilici M, Karademir S, Ocal B, Senocak F, et al. Acute rheumatic fever in the Central Anatolia Region of Turkey: a Performing the cardiovascular examination, echocardiography 30-year experience in a single center. Eur J Pediatr 2012;171(2):361– screening and confirming the diagnosis (DIAA), scientific writing 368. doi:10.1007/s00431-011-1555-x. and data analysis (MEAEA, SMEO, ZITM, MDYH), collecting [20] Carapetis JR. Rheumatic heart disease in developing countries. N the data from the hospital records (IAOY, MMAH), supervising Engl J Med 2007;357(5):439–441. doi:10.1056/NEJMp078039. the study (AAA). [21] Rizvi SF, Khan MA, Kundi A, Marsh DR, Samad A, Pasha O. Status of rheumatic heart disease in rural Pakistan. Heart 2004;90(4):394– 399. doi:10.1136/hrt.2003.025981. References [22] Agarwal AK, Yunus M, Ahmad J, Khan A. Rheumatic heart disease in India. J R Soc Health 1995;115(5):303–309. doi:10.1177/1466 42409511500509. [1] Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart [23] Ali S, Domi SB, Elfaki AM, Talib KA, Abdelrahman MH, Adam MS, et disease. Lancet 2012;379(9819):953–964. doi:10.1016/S0140- al. The echocardiographic prevalence of rheumatic heart disease in 6736(11)61171-9. North Kordofan and initiation of a control program. Sudan Medical [2] Kaplan MH, Bolande R, Rakita L, Blair J. Presence of Bound Immuno- Journal 2017;53(2):63–68. globulins and Complement in the Myocardium in AcuteRheumatic [24] Manjunath CN, Srinivas P, Ravindranath KS, Dhanalakshmi C. Inci- Fever. Association with Cardiac Failure. N Engl J Med 1964;271:637– dence and patterns of valvular heart disease in a tertiary care high- 645. doi:10.1056/NEJM196409242711301. volume cardiac center: a single center experience. Indian Heart J [3] Watkins DA, Zuhlke LJ, Engel ME, Mayosi BM. Rheumatic fe- 2014;66(3):320–326. doi:10.1016/j.ihj.2014.03.010. ver: neglected again. Science 2009;324(5923):37. doi:10.1126/ [25] Ahmed K, Elfil A, Hamid HM, Saeed A, Ali A. Epidemiology of cardiac science.324.5923.37b. disease during pregnancy in Khartoum Hospital, Sudan. J Women’s [4] Gordis L. The virtual disappearance of rheumatic fever in the United Health Care 2015;4(2):227. doi:10.4172/2167-0420.1000227. States: lessons in the rise and fall of disease. T. Duckett Jones me- [26] Marijon E, Celermajer DS, Tafflet M, El-Haou S, Jani DN, Ferreira B, morial lecture. Circulation 1985;72(6):1155–1162. doi:10.1161/01. et al. Rheumatic heart disease screening by echocardiography: the cir.72.6.1155. inadequacy of World Health Organization criteria for optimizing the [5] Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet diagnosis of subclinical disease. Circulation 2009;120(8):663–668. 2005;366(9480):155–168. doi:10.1016/S0140-6736(05)66874-2. doi:10.1161/CIRCULATIONAHA.109.849190. [6] Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden [27] Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, et of group A streptococcal diseases. Lancet Infect Dis 2005;5(11):685– al. Prevalence of rheumatic heart disease detected byechocardio- 694. doi:10.1016/S1473-3099(05)70267-X. graphic screening. N Engl J Med 2007;357(5):470–476. doi:10.1056/ [7] Chockalingam A, Gnanavelu G, Elangovan S, Chockalingam V. Clinical NEJMoa065085. spectrum of chronic rheumatic heart disease in India. J Heart Valve [28] Mirabel M, Celermajer DS, Ferreira B, Tafflet M, Perier MC, Karam N, Dis 2003;12(5):577–581. et al. Screening for rheumatic heart disease: evaluation of a simpli- [8] Tissier R, Chetboul V, Moraillon R, Nicolle A, Carlos C, Enriquez B, et fied echocardiography-based approach. Eur Heart J Cardiovasc Imag- al. Increased mitral valve regurgitation and myocardial hypertrophy ing 2012;13(12):1024–1029. doi:10.1093/ehjci/jes077. in two dogs with long-term pimobendan therapy. Cardiovasc Toxicol [29] Olson LJ, Subramanian R, Ackermann DM, Orszulak TA, Edwards WD. 2005;5(1):43–51. doi:10.1385/ct:5:1:043. Surgical pathology of the mitral valve: a study of 712cases spanning [9] Remenyi B, Carapetis J, Wyber R, Taubert K, Mayosi BM, World 21 years. Mayo Clin Proc 1987;62(1):22–34. doi:10.1016/s0025- Heart F. Position statement of the World Heart Federation on the 6196(12)61522-5. prevention and control of rheumatic heart disease. Nat Rev Cardiol [30] Hall P, Biorck G. The natural history of rheumatic valvular heart 2013;10(5):284–292. doi:10.1038/nrcardio.2013.34. disease and its bearing upon the results of surgery for mitral ste- [10] Viswanathan V. Acute rheumatic fever. Indian Journal of Rheumatol- nosis. Acta Rheumatol Scand 1958;4(2):70–78. doi:10.3109/ ogy 2012;7(5):36–43. doi:10.1016/S0973-3698(12)60027-2. rhe1.1958.4.issue-1-4.09. [11] Guilherme L, Kalil J. Rheumatic fever: How streptococcal throat in- [31] Moser M, Leo O. Key concepts in immunology. Vaccine 2010;28(Sup- fection triggers an autoimmune disease. In: Shoenfeld Y, Rose NR. pl 3):C2–C13. doi:10.1016/j.vaccine.2010.07.022. Infection and autoimmunity. Amsterdam, Elsevier. 2004:321–330. [32] Guilherme L, Kohler KF, Postol E, Kalil J. Genes, autoimmunity and [12] Gewitz MH, Baltimore RS, Tani LY, Sable CA, Shulman ST, Carape- pathogenesis of rheumatic heart disease. Ann Pediatr Cardiol tis J, et al. Revision of the Jones criteria for the diagnosis of acute 2011;4(1):13–21. doi:10.4103/0974-2069.79617. rheumatic fever in the era of Doppler echocardiography: ascien- [33] Cilliers AM. Rheumatic fever and its management. BMJ tific statement from the American Heart Association. Circulation 2006;333(7579):1153–1156. doi:10.1136/bmj.39031.420637.BE. 2015;131(20):1806–1818. doi:10.1161/CIR.0000000000000205. [34] Essa MEA, Ahmed AA, Osman SME. PREVALENCE OF PNEUMONIA [13] Kumar RK, Tandon R. Rheumatic fever & rheumatic heart disease: the IN SOUTH DARFUR STATE-SUDAN DATA COMPARED FROM 2009 last 50 years. Indian J Med Res 2013;137(4):643–658. THROUGH 2013. Eur J Biomed Pharm Sci 2017;4(3):32–35. [14] Kasmaei P, Atrkar-Roushan Z, Majlesi F, Joukar F. Mothers’ knowl- [35] Remenyi B, Carapetis J, Wyber R, Taubert K, Mayosi BM, World edge about acute rheumatic fever. Paediatr Nurs 2008;20(9):32–34. Heart F. Position statement of the World Heart Federation on the doi:10.7748/paed2008.11.20.9.32.c6825. prevention and control of rheumatic heart disease. Nat Rev Cardiol
DOI: 10.14218/ERHM.2019.00020 | Volume 5 Issue 1, March 2020 17 Explor Res Hypothesis Med Adam MEAE. et al: Echocardiography in improving diagnosis of rheumatic heart disease
2013;10(5):284–292. doi:10.1038/nrcardio.2013.34. doi:10.1016/j.jacc.2018.06.063. [36] United Nations. Sustainable Development Goals: 17 Goals to Trans- [38] Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, form Our World. Goal 3: Ensure Healthy Lives and Promote Well- Pais P, et al. Variations in cause and management of atrial fibrilla- Being For All at All Ages. New York, United Nations. 2016. tion in a prospective registry of 15,400 emergency department [37] Watkins DA, Beaton AZ, Carapetis JR, Karthikeyan G, Mayosi BM, patients in 46 countries: the RE-LY Atrial Fibrillation Registry.- Cir Wyber R, et al. Rheumatic heart disease worldwide: JACC sci- culation 2014;129(15):1568–1576. doi:10.1161/CIRCULATIONA- entific expert panel. J Am Coll Cardiol 2018;72(12):1397–1416. HA.113.005451.
18 DOI: 10.14218/ERHM.2019.00020 | Volume 5 Issue 1, March 2020 Review Article
Microbial Biomarkers for Colorectal Cancer Identified with Random Forest Model
Weili Sun1, Lili Wang2, Qiuyue Zhang2 and Quanjiang Dong1,2*
1The Affiliated Hospital of Dalian Medical University, Shandong, China;2 Department of Central Laboratories and Gastroenterology, Qingdao Municipal Hospital Affiliated to Qingdao University, Shandong, China
Colorectal cancer (CRC) is one of the most common cancers and a leading cause of cancer-related death. Gut mi- crobiota are part of a complex microbe-based ecosystem of the human body, and changes in the microbiota can lead to a variety of diseases. All currently used CRC detection methods, including endoscopy, guaiac-based fecal occult blood test and fecal immunochemical test, have many limitations. Therefore, establishing novel screening methods which are accurate, inexpensive and non-invasive is indicated. Random forest models, as a superiority machine learning model, are increasingly used in research to select biomarkers. In this review, we summarized progressions of the diagnoses of CRC based on the random forest model of gut microbiota. We concluded that some cancer-associated bacteria in gut microbiota could be used as biomarkers for detecting early CRC. We also aimed to discuss how to select possible markers of colorectal diseases based on gut microbiota using the random forest model.
Introduction agnosis. Colonoscopy, the gold standard for the diagnosis of CRC, can facilitate early prevention through adenoma resection; how- Colorectal cancer (CRC) is one of the most common cancers and ever, it is an invasive endoscopy, which causes patient discomfort and reduces their compliance to clinical examinations, relies on a leading cause of cancer-related death. The global annual inci- 5,6 dence of CRC is approximately 1.2 million, and the annual death endoscopist skill, and requires patient bowel cleansing. The tra- ditional guaiac-based FOBT, which is inexpensive and noninva- toll reaches 600,000.1 CRC is characterized by a long preclinical sive, is the first-choice screening test for CRC. Both guaiac-based phase, progressing over years from early adenoma to invasive FOBT and fecal immunochemical test (FIT) can improve the pos- cancer.2,3 Diagnosis of early-stage CRC can significantly improve itive rate of diagnosis by repeated detection over a long period patient prognosis, with a survival rate higher than 80%,4 which of time. However, cancer bleeding is usually intermittent, which makes early diagnosis of CRC more critical for better patient out- leads to lower sensitivity, specificity, and false-negative results comes. with these two tests.7 As the abovementioned methods have many Screening methods for CRC, including fecal occult blood test limitations, it is necessary to establish novel screening methods (referred to as FOBT), colonoscopy, determination of genetic that are accurate, inexpensive, and noninvasive. mutation and gut microbiota tests, have received widespread at- The human microbiota is a very large and complex microbial tention; although, these methods significantly differ in specificity, system. Gut microbiota includes bacteria, viruses, archaea, and accuracy, convenience, and universality with regard to clinical di- fungi, but the main component is bacteria; thus, most studies of the microbiota focus on bacteria, as it is composed of approximately 1014 bacteria, which equates to 10 times the total number of hu- Keywords: Gut microbiota; Colorectal cancer; Colorectal adenoma; Random forest 8 model. man cells. The gut microbiota contains nearly 11,500 common 9 Abbreviations: AUC, area under the curve; CRA, colorectal adenoma; CRC, colo- bacteria. The total number of bacterial genes in the gut microbiota rectal cancer; CEA, carcinoembryonic antigen; FIT, fecal immunochemical tests; is 150 times the total number of human genes.10–12 Coincidentally, FOBT, fecal occult blood test; OTU, operational taxonomic unit; RF, random forest; the gut microbiota has been called a “forgotten organ”.10–12 TNM, tumor-node-metastasis. Dysbacteriosis plays an important role in the pathogenesis of Received: October 24, 2019; Revised: November 21, 2019; Accepted: December 25, 2019 several diseases. For example, the gut microbiota is associated *Correspondence to: Quanjiang Dong, Department of Central Laboratories and Gas- with many diseases, such as obesity, type 2 diabetes, and athero- troenterology, Qingdao Municipal Hospital Affiliated to Dalian Medical University, sclerosis.13–17 Moreover, it is well-known that genomic alterations Shandong 266071, China; Present Address: Donghai Zhong Road 5, Qingdao, Shan- of the APC/Wnt pathway can potentially lead to carcinoma.18 Re- dong, China. Tel: +86-05328890589; E-mail: [email protected] cent studies have increasingly indicated that the gut microbiota is How to cite this article: Sun W, Wang L, Zhang Q, Dong Q. Microbial Biomarkers 19 for Colorectal Cancer Identified with Random Forest Model. Exploratory Research closely related to CRC, with many differences in gut microbiota and Hypothesis in Medicine 2020;5(1):19–26. doi: 10.14218/ERHM.2019.00026. between healthy people and patients with CRC. Studies have also
Exploratory Research and Hypothesis in Medicine 2020 vol. 5 | 19–26
Copyright: © 2020 Authors. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Explor Res Hypothesis Med Sun W. et al: Microbial biomarkers of CRC shown that biomarkers from gut microbiota may be used for the cellular and physiologic features. In the proximal colon, Bacte- diagnosis of CRC.20–23 This review focuses on the relationship roides, Actinomyces, Pseudomonas, and Enterobacteriaceae show between gut microbiota and CRC as well as the possibility that differential abundance between the lumen and mucosa.42 Simi- gut microbiotarelated markers may be novel biological screening larly, Enterobacteriaceae, Bacteroides, and Pseudomonas enrich markers for CRC. the proximal colonic mucosa, whereas there is increased relative abundance of Finegoldia, Murdochiella, Peptoniphilus, Porphy- romonas, and Anaerococcus in the distal colon.42 The abundance Random forest model of Turicibacter, Finegoldia, Peptoniphilus, and Anaerococcus was found to be different between the lumen and mucosa microbiota 42 Gut microbiota can be used as a marker to screen for CRC, but in the distal colon. Furthermore, there are differences in the mi- the screening capacity of a single flora is limited, and multibacte- crobiota compositions between different anatomical parts of the 42 rial models are needed to increase the screening capacity. If too colon. many bacterial species are present, this model will be difficult to Colorectal adenoma (CRA) confers a high risk for the develop- apply in the clinical setting; therefore, we need to screen for the ment of CRC but the gut microbiota is necessary for the formation most discriminating bacteria. As an integrated machine-learning of intestinal adenoma, and healthy gut microbiota are associated algorithm, random forest (RF) is a classifier with multiple decision with a reduced risk of advanced CRA.43 In cases of CRA, the rela- trees that are established through randomly repeated sampling, and tive abundance of two bacterial genera (Enterococcus and Strep- a final voting result is obtained.24 The current classifier algorithms tococcus) increased, whereas that of three genera (Clostridium, mainly include, Bayes net, simple logistic, TB-line (TB pedigree), Roseburia, and Eubacterium) decreased.44 Another study found machine learning, sequential minimum optimization algorithm for that some species belonging to Ruminococcaceae, Clostridium, support vector, and RF.25–32 Pseudomonas, and Porphyromonadaceae showed increased In many applications, the RF algorithm, by far, has the best ac- numbers in patients with CRA, whereas other species belonging curacy. Moreover, compared to other technologies, RF has many to Bacteroides, Lachnospiraceae, Clostridiales, and Clostridium advantages, such as anti-interference, simple optimization, and ef- decreased.20 Analyses of fecal microbiota from 95 patients with ficient parallel processing, in processing highly nonlinear biologi- CRA revealed substantial changes in the microbiota compositions. cal data.33,34 The RF model considers the interaction between non- In CRA, the Proteobacteria phylum was found to have enriched linear data and characteristics of the model, and can also carry out the microbiota. These bacteria are associated with precancerous internal cross-validation within the model to prevent overfitting.35 lesions.45 Lachnospiraceae, a potentially beneficial bacteria genus, A recent article reported that an RF model successfully screened was depleted, and the relative abundance of this genus had high microbial markers for early cirrhotic liver cancer.36 Another study accuracy in differentiating patients with CRA from normal indi- on hepatocellular carcinoma used the same method to obtain pre- viduals.23,46 dictive biomarkers for advanced hepatocellular carcinoma.37 An Dysbiosis of the gut microbiota and its substantial composition- RF model based on intestinal gut microbiota was established to al alterations are closely related to the development of CRC.47 The successfully predict the variation of Bifidobacteria after probiotic microbial communities in tumor microhabitat are different from treatment, and it revealed the effects of probiotics on intestinal those in tumor-adjacent healthy tissue.48 Moreover, antibiotic in- flora.38 Thus, RF models, as a superior machine-learning model, tervention in the microbiota can significantly reduce the burden are increasingly used in research for the selection of biomarkers. of colonic tumors.19 Virulence-associated genes in tumors may Therefore, we reviewed the recent progress in the diagnosis of potentially depend upon the genomes Fusobacterium and Provi- colorectal diseases by using an RF model based on gut microbiota dencia.48 A study on fecal microbiota from 120 patients with CRC as well as the selection of possible predictive markers of colorectal showed the increased abundance of a number of species, includ- diseases. ing Pophyromonas assaccharolytica, Fusobacterium nucleatum, Parvimonas micra, Peptostreptococcus stomatis, Gemella spp., and Prevotella spp.23 Furthermore, in a mouse model of CRC, the Dysbiosis of gut microbiota in CRC abundance of Lactobacillus negatively correlated with the num- ber of colonic tumors.49 Moreover, fecal microbiota from patients The gut microbiota and its hosts coparticipate in the establish- with CRC can promote tumorigenesis in both germ-free mice and 50 ment of a symbiotic relationship to maintain homeostasis in the conventional mice. Taking these findings together, it appears that digestive system. In healthy individuals, at the phyla level, the some cancer-associated bacteria in gut microbiota can serve as bio- Firmicutes and Bacteroidetes phyla are predominant in the gut markers to detect CRC. microbiota, despite remarkable interindividual differences.39 At the genus level, a meta-analysis indicated that a high abundance of the genera Barnesiella, Ruminococcaceae UCG-005, Alistipes, RF model for identification of microbial biomarkers for CRC Christensenellaceae R-7 group, and an unclassified member of the Lachnospiraceae family correlated with the healthy state in their Alterations in the relative abundance of bacteria in CRC indicate subjects.40 However, the most abundant bacterial genera in the gut they are potential predictive or diagnostic biomarkers for CRC or microbiota include Prevotella, Bacteroides, and Ruminococcus. CRA. Escherichia coli, Bacteroides fragilis, and Fusobacterium Based on genus compositional variations, the gut microbiota could nucleatum have been shown to directly influence tumor develop- be classified into different enterotypes, namely the Prevotella pre- ment in the colon.19 A recent small-sample study on the quantifi- dominant enterotype, Bacteroides predominant, and Ruminococ- cation of this bacterium in fecal samples found a great increase cus-related enterotype, respectively. Simultaneously, this research in the number of these bacteria in CRC; the conclusive findings shows that the human intestinal microbiota has commonalities.41 of the study supported the use of Fusobacterium nucleatum as As usual, the features of bacterial populations are specified by biomarker of CRC as well as a marker of early CRC.51–53 The su- tissue, colonic lumen, and feces, which themselves have different pernatant of a Fusobacterium nucleatum culture exhibited strong
20 DOI: 10.14218/ERHM.2019.00026 | Volume 5 Issue 1, March 2020 Sun W. et al: Microbial biomarkers of CRC Explor Res Hypothesis Med bactericidal activity against some probiotics, such as Faecalibac- (OTU87), Lachnospiraceae (OTU60), Lachnospiraceae (OTU32), terium prausnitzii and Bifidobacterium strains, that may cause Lachnospiraceae (OTU88), Lachnospiraceae (OTU44), Lachno- disease.54 spiraceae (OTU14), Bacteroides (OTU7), Bacteroides (OTU3), Bac- A study found a stepwise increase in the abundance of teroides (OTU2), Ruminococcus (OTU11), Ruminococcus (OTU16), Clostridium from normal tissues to adenoma and, finally, colonic Ruminococcaceae (OTU29), Blautia (OTU13), Blautia (OTU9), cancer.55 Therefore, Clostridium symbiosum can be used singly as Collinsella (OTU19), Firmicutes (OTU282), Enterobacteriaceae a biomarker for detecting CRC. The results showed that a stepped (OTU28), Parabacteroides (OTU49), Roseburia (OTU5), Clostridi- increase in Clostridium abundance outperformed all other con- ales (OTU10), Faecalibacterium (OTU6), Anaerostipes (OTU8), ventional screening methods, such as carcinoembryonic antigen Porphyromonas (OTU105), and FIT with a 100 ng/mL cutoff. We (referred to as CEA) and FIT, both of which were known to have infer that 16 of these were members of the Firmicutes phylum.23 The greater sensitivity (area under the curve (AUC) = 0.73 vs. 0.38– multitarget microbiota test detected 91.7% of cancers and 45.5% of 0.54 for other methods). In combination with FIT, the predicting adenomas, compared to 75.0% and 15.7% by FIT, respectively.23 accuracy of Clostridium symbiosum increased significantly, with Thus, screening methods for colorectal lesions need to be continu- an AUC of 0.803. Moreover, with the combination of Clostridium ally optimized to find the optimal screening program. symbiosum and Fusobacterium nucleatum, FIT (200 ng/mL) and A recent study analyzed a total of 969 fecal mate genomes, in- CEA (3.3 ng/mL) achieved a performance an AUC of 0.876.55 cluding 5 publicly available data sets, 2 new cohorts, and 2 valida- A meta-analysis of a publicly available dataset showed that the tion cohorts.58 Twenty-four species with high RF accuracy features depletion of Faecalibacterium, Bacteroides, and Romboutsis could were selected; these were: Actinomyces graevenitzii, Alistipes spp., be a potential biomarker for CRC.52 A Chinese study supported the Anaertuncus colihominis, Bifidobacterium longum, Clostridium identification of CRC and differentiation from the healthy group via hathewayi, Clostridium leptum, Clostridium symbiosum, Dialis- 76 fecal potential biomarkers; the CRC group was enriched with ter invisus, Eubacterium eligens, Escherichia coli, Fusobacterium 18 operational taxonomic units (OTUs); moreover, fecal metabo- nuleatum, Gemella morbillorum, Lachnospiaceae 3157FAA CT1, lites in healthy patients and cancer groups are different.56 Another Lachnospiaceae 8157FAA, Lachnospiaceae5163FAA, Parvimonas meta-analysis of eight studies from different countries and regions spp., Peptostreptococcus stomatis, Porphyromonas assccharo- identified 29 species as biomarkers of CRC.57 Furthermore, the mi- lyica, Pravimonas micra, Prevotella copri, Ruminococcus gnavus, crobial species can predict taxonomic and functional microbiome Subdoligranulum spp., Streptococcus parasanguinis, and Strepto- CRC signatures as a basis for future diagnostics. (These data are coccus salivarius. The predictive microbiome signatures trained summarized in Table 1.)49,52–55,57–59 on different data sets consistently showed high accuracy. Nonethe- There have been attempts to explore whether a combination of less, it appears their model has lower sensitivity and specificity bacterial markers could increase the AUC value in predicting CRC. values for predicting CRA.58 Zackular et al.20 analyzed fecal microbiota from healthy subjects Despite significant advances in the study of the effects of gut and patients with CRA or CRC; they found substantial alterations microbiota on colorectal lesions, few studies have investigated in the gut microbiome of patients with CRA or CRC compared to the gut microbiota after the treatment of patients with colorectal healthy controls, with a classification accuracy for CRC of 0.798 lesions. The tumor-node-metastasis (commonly known as TNM) AUC. Therefore, combining microbial markers with known clini- international staging system has always been considered the gold cal risk factors can significantly improve the differentiation ability standard to determine CRC prognosis. In addition, findings of ane- of the tests.21 uploidy, tumor-infiltrating lymphocytes, allelic loss inDCC , TP53, By using a LASSO logistic regression classifier, a model con- APC and MCC genes, TP53 gene mutations, CD44 protein expres- structed with fecal microbiota could predict CRC with an accura- sion, high levels of thymidylate synthetase, microsatellite instabili- cy of 0.82.22 Another study from China demonstrated that a model ty, and gene studies of both RAS and BRAF are independent, strong constructed with microbiota showed better value than FOBT.60 prognostic factors. In addition, C-reactive protein, overexpression Sze et al.49 constructed an RF classification model using 8 taxa of the CEA in tumors, and circulating free DNA are considered based on significant odds ratios obtained in a meta-analysis of to be associated with the prognosis of patients with CRC.19 Ai et 14 studies from various geographical regions. Their analysis in- al.59 analyzed the composition of fecal microbiota in 124 samples cluded 1,737 fecal samples and 492 tissue samples. These encom- from France and 99 samples from Austria. They excluded unre- passed Fusobacterium, Parvimonas, Porphyromonas, Peptostrep- lated and redundant features during feature selection by mutual tococcus, Clostridium XI, Enterobacteriaceae, Ruminococcus, information, and trained an RF classifier on a large mate genomic and Escherichia. The combined model had an AUC of 0.75 based data set of patients with CRC and healthy individuals. The RF clas- on fecal samples. Similarly, the AUC was 0.77 in tissue samples sifier assembled from published reports as well as extracted and used in a combined model trained by Dorea, Blautia, and Weis- analyzed information from learned decision trees. Porphyromonas sella.49 Their model could successfully classify CRC with high asaccharolytica, Peptostreptococcus stomatis, Fusobacterium, accuracy when models trained using one data set were tested on Parvimonas spp., Streptococcus vestibularis, and Flavonifractor other data sets.49 plautii were determined to be key microbial species associated Nonetheless, a test that objectively reflects the early gut changes with CRCs.59 in CRA or CRC fully is needed. New noninvasive screening meth- By using an RF model based on fecal microbiota, Sze et al.61 ods are needed to increase the sensitivity and specificity for CRC found significant differences between the pre- and post-treatment detection. Baxter et al.23 established an RF classification model by samples of 67 individuals, including those with adenoma (n = 22), using the relative abundance of gut microbiota and FIT from stool advanced adenoma (n = 19), and carcinoma (n = 26). Fusobac- samples of 490 patients. They observed that the sensitivity and speci- terium, Porphyromonas, and Parvimonas were significantly de- ficity of a combination model of bacterial abundance and FIT, which creased in the post-treatment samples.61 Furthermore, in a mouse they obtained by incorporating data on hemoglobin concentration model, interventions of microbiota with antibiotics led to a dra- (determined by FIT), and bacterial relative abundances (multitarget matic decrease in the tumor burden in the colon.19 In addition, as microbiota test) for CRC and CRA were better than those with FIT an important probiotic, Bifidobacterium has been shown to enrich alone.23 Their model used 23 OTUs, including Lachnospiraceae the gut microbiome in healthy individuals.61 Moreover, studies
DOI: 10.14218/ERHM.2019.00026 | Volume 5 Issue 1, March 2020 21 Explor Res Hypothesis Med Sun W. et al: Microbial biomarkers of CRC
Table 1. Characteristics of the bacteria species as potential biomarkers for CRC Phyla Genus Abundance Reference Firmicutes Clostridiales Increased Xie YH (2017)55 Unknown Clostridiales Increased Wirbel J et al. (2019)57 Clostridium bolteae/clostridioforme Increased Wirbel J et al. (2019)57 Clostridium symbiosum Increased Wirbel J et al. (2019)57; Thomas AM et al. (2019)58 Clostridium leptum Increased Thomas AM et al. (2019)58 Clostridium hathewayi Increased Thomas AM et al. (2019)58 Unknown Clostridiales Increased Wirbel J et al. (2019)57 Subdoligranulum spp. Decreased Thomas AM et al. (2019)58 Unknown Peptostreptococcaceae Increased Wirbel J et al. (2019)57 Peptostreptococcus stomatis Increased Thomas AM et al. (2019)58; Ai D et al. (2019)59 Anaerococcusobesiensis/vaginalis Increased Wirbel J et al. (2019)57 Anaertuncus colihominis Increased Thomas AM et al. (2019)58 Gemella morbillorum Increased Wirbel J et al. (2019)57; Thomas AM et al. (2019)58 Unknown Dialister Increased Wirbel J et al. (2019)57 Hungatellahathewayi Increased Wirbel J et al. (2019)57 Parvimonas species Increased Wirbel J et al. (2019)57; Ai D et al. (2019)59 Parvimonas spp. Increased Thomas AM et al. (2019)58 Pravimonas micra Increased Thomas AM et al. (2019)58 Ruminococcus torques Increased Wirbel J et al. (2019)57 Ruminococcus gnavus Decreased Thomas AM et al. (2019)58 Uubdoligranulum species Increased Wirbel J et al. (2019)57 Lachnospiaceae 3157FAA CT1 Increased Thomas AM et al. (2019)58 Lachnospiaceae 8157FAA Decreased Thomas AM et al. (2019)58 Lachnospiaceae5163FAA Decreased Thomas AM et al. (2019)58 Alistipes spp. Decreased Sze MA et al. (2018)49 Dialister invisus Decreased Thomas AM et al. (2019)58 Eubacterium eligens Decreased Thomas AM et al. (2019)58 Streptococcus parasanguinis Increased Thomas AM et al. (2019)58 Streptococcus salivarius Decreased Thomas AM et al. (2019)58 Streptococcus vestibularis Increased Ai D et al. (2019)59 Bacteriodetes Bacteroides Decreased Mangifesta M et al. (2018)53 Unknown Porphyromonas Increased Wirbel J et al. (2019)57 Porphyromonas uenonis Increased Wirbel J et al. (2019)57 Porphyromonas somerae Increased Wirbel J et al. (2019)57 Porphyromonas asaccharolytica Increased Ai D et al. (2019)59; Thomas AM et al. (2019)58 Prevotella intermedia Increased Wirbel J et al. (2019)57 Prevotellan igrescens Increased Wirbel J et al. (2019)57 Prevotella copri Increased Thomas AM et al. (2019)58 Flavonifractor Increased Ai D et al. (2019)59 plautii Proteobacteria Faecalibacterium Decreased Mangifesta M et al. (2018)53 Escherichia coli Increased Thomas AM et al. (2019)58
22 DOI: 10.14218/ERHM.2019.00026 | Volume 5 Issue 1, March 2020 Sun W. et al: Microbial biomarkers of CRC Explor Res Hypothesis Med
Table 1. Characteristics of the bacteria species as potential biomarkers for CRC - (continued) Phyla Genus Abundance Reference Fusobacteria Fusobacterium nucleatum Increased Tunsjø HS et al. (2019)52; Mangifesta M et al. (2018)53; Xie YH (2017)55; Bullman S et al. (2017)54; Thomas AM et al. (2019)58; Ai D et al. (2019)59 F. nucleatum subspecies animalis Increased Wirbel J et al. (2019)57 F. nucleatum subspecies nucleatum Increased Wirbel J et al. (2019)57 F. nucleatum subspecies vincentii Increased Wirbel J et al. (2019)57 Fusobacterium species Increased Wirbel J et al. (2019)57 oral taxon 370 Actinobacteria Actinomyces graevenitzii Increased Thomas AM et al. (2019)58 Bifidobacterium longum Decreased Thomas AM et al. (2019)58 Tenericutes Solobacterium moorei Increased Wirbel J et al. (2019)57 have shown that Bifidobacterium can inhibit the growth of intes- spite the close relation of bacteria to colorectal lesions, gut mi- tinal carcinogenic bacteria and protect intestinal mucosa, which crobes can interact with each other. Through RF modeling, Hanni- makes it an important probiotic for clinical application (Table gan et al.63 found that viruses indirectly affect cancer progression 2).20,23,42,44,45,49,59,61,62 by altering bacterial host communities. Nakatsu et al.64 conducted The gut microbiota includes bacteria, viruses, and fungi. De- a study on the survival prediction of CRC by viruses. Their study
Table 2. Important studies in dysbiosis of gut microbiota in CRC, bacterial features, detection methods and models Sample Bacteria Participants Models Bacteria, genus sources features Flynn et al. Healthy (n = 20) Mucosal, Random forest Enterobacteriaceae, Bacteroides Enriched in (2018)42 feces and classification and Pseudomonas the proximal luminal models contents Finegoldia, Murdochiella, Peptoniphilus, Colonic mucous Porphyromonas and Anaerococcus increased in the distal colon Chen et al. Healthy (n = 344), Feces NA Enterococcus and Streptococcus Increased (2013)44 A-CRA groups (n = 344) in A-CRA; Clostridium, Roseburia, and Eubacterium Decreased in A-CRA Zackular et Healthy (n = 30), Feces NA Ruminococcaceae, Clostridium, Increase in al. (2014)20 colonic adenoma (n Pseudomonas, and Porphyromonadaceae CRA patients = 30), and colonic adenocarcinoma (n = 30) Bacteroides, Lachnospiraceae, Decreased in Clostridiales, and Clostridium CRA patients Goedert et Normal patients (n Feces Random forest Proteobacteria Enriched in CRA al. (2015)45 = 24), CRA (n = 20), CRC (n = 2), and other conditions (n = 15) Ai D et al. France (n = 124); Feces Random forest Porphyromonas asaccharolytica, Enriched in CRC (2019)59 Austria (n = 99) Eubacterium hallii, Parvimonas spp., Fusobacterium 7, Prevotella melaninogenica, Streptococcus vestibularis, Prevotellacopri, Peptostreptococcus stomatis, Fusobacterium nucleatum, Parvimonas micra, Gemella morbillorum, Flavonifractor plautii, Fusobacterium, Clostridium SS2
DOI: 10.14218/ERHM.2019.00026 | Volume 5 Issue 1, March 2020 23 Explor Res Hypothesis Med Sun W. et al: Microbial biomarkers of CRC
Table 2. Important studies in dysbiosis of gut microbiota in CRC, bacterial features, detection methods and models- (continued) Sample Bacteria Participants Models Bacteria, genus sources features Baxter et CRC (n = 120), CRA Feces Random forest Pophyromonas assaccharolytica, Increased in CRC al. (2016)23 (n = 198), no colonic Fusobacterium nucleatum, Parvimonas lesions (n = 172) micra, Peptostreptococcus stomatis, Gemella spp. and Prevotella spp. Sze MA et Control (n = 1145), CRA Feces and Random forest Fusobacterium, Parvimonas, Significant ORs al. (2018)49 (n = 521), CRC (n = 536) tissue Porphyromonas, Peptostreptococcus, Clostridium XI, Enterobacteriaceae, Ruminococcus and Escherichia, Dorea, Blautia and Weissella Sze MA et Adenoma (n = 22), Feces Random forest Fusobacterium, Porphyromonas, Decrease in al. (2017)61 advanced adenoma Parvimonasare post-treatment (n = 19), CRC (n = 26)
CRC, colorectal cancer; CRA, colorectal adenoma; NA, not available; OR, odds ratio. found a combination of four classification markers that reduce Funding patient survival in CRC.43 Further research on CRC-related viral group characteristics could lead to the development of new tools to This work was supported by funding from the National Natural identify individuals with CRC or to predict outcomes. Science Foundation of China (No. 81602144, 31870777) There are some limitations of our meta-analysis. Despite the available data on CRC detection through the gut microbiome, there is a lack of consensus on which features are most informative. Conflict of interest The contradictory reports from some studies could be attributed to differences inherent among study populations, procedures for fecal collection and storage, DNA extraction and amplification, The authors have no financial interests or any conflict of interests sequencing, and bioinformatics processing methods. Moreover, to disclose. recent studies used their models only to differentiate CRC from CRA or healthy individuals. Further studies are required to identify methods that can differentiate CRC from other colonic diseases, Author contributions such as inflammatory bowel diseases. Manuscript writing (WLS, QJD); critical revision of the manuscript for important intellectual content (WLS, LLW, QYZ); administra- Future research directions tive, technical, or material support, study supervision (QJD).
Our analysis of recent studies on CRC biomarkers and the list of References related genera showed the absence of an accepted biomarker for CRC. The RF model has features of anti-interference, simple op- timization and efficient parallel processing, all of which imply it [1] Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61(2):69–90. doi:10.3322/ may be the best choice for screening biomarkers. Future research caac.20107. to develop a kit to accurately screen for CRA and CRC biomark- [2] Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, ers through an RF model for fecal microbiota could accurately, Haug U. Risk of progression of advanced adenomas to colorectal can- quickly and conveniently improve early detection of these condi- cer by age and sex: estimates based on 840,149 screening colonos- tions. copies. Gut 2007;56(11):1585–1589. doi:10.1136/gut.2007.122739. [3] Kuntz KM, Lansdorp-Vogelaar I, Rutter CM, Knudsen AB, van Balle- gooijen M, Savarino JE, et al. A systematic comparison of microsimu- Conclusion lation models of colorectal cancer: the role of assumptions about adenoma progression. Med Decis Making 2011;31(4):530–539. doi :10.1177/0272989X11408730. In recent years, several studies have demonstrated that the gut mi- [4] O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with crobiota in CRC patients differs substantially from that in healthy the new American Joint Committee on Cancer sixth edition staging. J individuals. Fecal microbial markers have the potential to provide Natl Cancer Inst 2004;96(19):1420–1425. doi:10.1093/jnci/djh275. a noninvasive alternative method to diagnose CRC. RF models [5] Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, or other statistical models based on a collection of bacteria in the Didkowska J, et al. Quality indicators for colonoscopy and the risk of gut microbiota could help identify CRC with high accuracy. When interval cancer. N Engl J Med 2010;362(19):1795–1803. doi:10.1056/ NEJMcoa0907667. combined with other conventional screening markers and clinical [6] Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. risk factors, the predictive accuracy for CRC increases dramati- The impact of suboptimal bowel preparation on adenoma miss rates cally. The findings in our review provide a new approach to iden- and the factors associated with early repeat colonoscopy. Gastroin- tify powerful biomarkers in the gut microbiota. This will facilitate test Endosc 2011;73(6):1207–1214. doi:10.1016/j.gie.2011.01.051. clinician decision-making for early intervention in CRC. [7] Kuipers EJ, Rösch T. Colorectal cancer screening—optimizing current
24 DOI: 10.14218/ERHM.2019.00026 | Volume 5 Issue 1, March 2020 Sun W. et al: Microbial biomarkers of CRC Explor Res Hypothesis Med
strategies and new directions. Nat Rev Clin Oncol 2013;10:130–142. Health Sci 2015;7(5):304–310. doi:10.5539/gjhs.v7n5p304. doi:10.1038/nrclinonc.2013.12. [30] Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. [8] Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diver- Machine learning applications in cancer prognosis and predic- sity, stability and resilience of the human gut microbiota. Nature tion. Comput Struct Biotechnol J 2014;13:8–17. doi:10.1016/j. 2012;489(7415):220–230. doi:10.1038/nature11550. csbj.2014.11.005. [9] Morgan XC, Segata N, Huttenhower C. Biodiversity and functional [31] Lebedev AV, Westman E, Van Westen GJ, Kramberger MG, Lunder- genomics in the human microbiome. Trends Genet 2013;29(1):51– vold A, Aarsland D, et al. Random Forest ensembles for detection 58. doi:10.1016/j.tig.2012.09.005. and prediction of Alzheimer’s disease with a good between co- [10] Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. hort robustness. Neuroimage Clin 2014;6:115–125. doi:10.1016/j. A human gut microbial gene catalogue established by metagenomic nicl.2014.08.023. sequencing. Nature 2010;464:59–65. doi:10.1038/nature08821. [32] Takahashi N, Guo J, Nishi T. Global convergence of SMO algorithm for [11] O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO support vector regression. IEEE Trans Neural Netw 2008;19(6):971– Rep 2006;7:688–693. doi:10.1038/sj.embor.7400731. 982. doi:10.1109/TNN.2007.915116. [12] Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. [33] De Bruyn T, van Westen GJ, Ijzerman AP, Stieger B, de Witte P, Au- Metagenomic analysis of the human distal gut microbiome. Science gustijns PF, et al. Structure-based identification of OATP1B1/3 2006;312:1355–1359. doi:10.1126/science.1124234. inhibitors. Mol Pharmacol 2013;83(6):1257–1267. doi:10.1124/ [13] Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fager- mol.112.084152. berg B, et al. Gut meta genome in European women with nor- [34] Menze BH, Kelm BM, Masuch R, Himmelreich U, Bachert P, Petrich mal, impaired and diabetic glucose control. Nature 498:99–103. W, et al. A comparison of random forest and its Gini importance doi:10.1038/nature12198. with standard che-mometric methods for the feature selection and [14] Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. classification of spectral data. BMC Bioinformatics 2009;10:213. Richness of human gut microbiome correlates with metabolic mark- doi:10.1186/1471-2105-10-213. ers. Nature 2013;500(7464):541–546. doi:10.1038/nature12506. [35] Liaw A, Wiener M. Classification and regression by random Forest. R [15] Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT,et al. Intesti- News 2002;2:18–22. nal microbiota metabolism of l-carnitine, a nutrient in red meat, pro- [36] Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, et al. Gut microbiome analy- mote satherosclerosis. Nat Med 2013;19(5):576–585. doi:10.1038/ sis as a tool towards targeted non-invasive biomarkers for early nm.3145. hepatocellular carcinoma. Gut 2019;68(6):1014–1023. doi:10.1136/ [16] Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide gutjnl-2017-315084. association study of gut microbiota in type 2 diabetes. Nature [37] Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A,et al. Gut mi- 2012;490(7418):55–60. doi:10.1038/nature11450. crobiome-based metagenomic signature for non-invasive detection [17] Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon of advanced fibrosis in human nonalcoholic fatty liver disease. Cell JI. An obesity-associated gut microbiome with increased capacity for Metab 2017;25(5):1054–1062.e5. doi:10.1016/j.cmet.2017.04.001. energy harvest. Nature 2006;444(7122):1027–1031. doi:10.1038/na- [38] Luo YM, Liu FT, Chen MX, Tang WL, Yang YL, et al. A machine learning ture05414. model based on initial gut microbiome data for predicting changes of [18] Cancer Genome Atlas Network. Comprehensive molecular charac- Bifidobacterium after prebiotics consumption. Nan Fang Yi Ke Da Xue terization of human colon and rectal cancer. Nature 2012;487(7407): Xue Bao 2018;38(3):251–260. 330–337. doi:10.1038/nature11252. [39] Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbi- [19] Zackular JP, Baxter NT, Chen GY, Schloss PD. Manipulation of the ome. Genome Med 2016;8(1):51. doi:10.1186/s13073-016-0307-y. gut microbiota reveals role in colon tumorigenesis. mSphere [40] Mancabelli L, Milani C, Lugli GA, Turroni F, Cocconi D, van Sinderen D, 2015;1(1):e00001–15. doi:10.1128/mSphere.00001-15. et al. Identification of universal gut microbial biomarkers of common [20] Zackular JP, Rogers MA, Ruffin MT, Schloss PD. The human gut mi- human intestinal diseases by meta-analysis. FEMS Microbiol Ecol crobiome as a screening tool for colorectal cancer. Cancer Prev Res 2017;93(12):fix153. doi:10.1093/femsec/fix153. 2014;7:1112–1121. doi:10.1158/1940-6207.CAPR-14-0129. [41] Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende [21] Zeller G, Tap J, Voigt AY, Sunagawa S, Kultima JR, Costea PI, et al. Po- DR, et al. Enterotypes of the human gut microbiome. Nature tential of fecal microbiota for early-stage detection of colorectal can- 2011;473(7346):174–180. doi:10.1038/nature09944. cer. Mol Syst Biol 2014;10:766. doi:10.15252/msb.20145645. [42] Flynn KJ, Ruffin MT4th, Turgeon DK, Schloss PD. Spatial Variation [22] Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, et al. Metagen- of the Native Colon Microbiota in Healthy Adults. Cancer Prev Res omic analysis of faecal microbiome as a tool towards targeted non- (Phila) 2018;11(7):393–402. doi:10.1158/1940-6207.CAPR-17-0370. invasive biomarkers for colorectal cancer. Gut 2017;66(1):70–78. [43] Dove WF, Clipson L, Gould KA, Loungo C, Marshall DJ, Moser AR, et doi:10.1136/gutjnl-2015-309800. al. Intestinal neoplasia in the ApcMin mouse: independence from [23] Baxter NT, Ruffin MT, Rogers MA, Schloss PD. Microbiota-based mod- the microbial and natural killer (beige locus) status. Cancer Res el improves the sensitivity of fecal immunochemical test for detect- 1997;57(5):812–814. ing colonic lesions. Genome Med 2016;8(1):37. doi:10.1186/s13073- [44] Chen HM, Yu YN, Wang JL, Lin YW, Kong X, Yang CQ, et al. De- 016-0290-3. creased dietary fiber intake and structural alteration of gut micro- [24] Breiman L. Random forests. Mach Learn 2001;45:5–32. biota in patients with advanced colorectal adenoma. Am J Clin Nutr [25] Nassif H, Wu Y, Page D, Burnside E. Logical Differential Prediction 2013;97(5):1044–1052. doi:10.3945/ajcn.112.046607. Bayes Net, improving breast cancer diagnosis for older women. [45] Goedert JJ, Gong Y, Hua X, Zhong H, He Y, Peng P, et al. Fecal microbi- AMIA Annu Symp Proc 2012;2012:1330–1339. ota characteristics of patients with colorectal adenoma detected by [26] Gevaert O, De Smet F, Timmerman D, Moreau Y, De Moor B. Predict- screening: a population-based study. EBioMedicine 2015;2(6):597– ing the prognosis of breast cancer by integrating clinical and microar- 603. doi:10.1016/j.ebiom.2015.04.010. ray data with Bayesian networks. Bioinformatics 2006;22(14):e184– [46] Lepage P, Häsler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun e190. doi:10.1093/bioinformatics/btl230. A, et al. Twin study indicates loss of interaction between microbiota [27] Xue X, Zeng N, Gao Z, Du MQ. Diffuse large B-cell lymphoma: sub- and mucosa of patients with ulcerative colitis. Gastroenterology classification by massive parallel quantitative RT-PCR. Lab Invest 2011;141(1):227–236. doi:10.1053/j.gastro.2011.04.011. 2015;95:113–120. doi:10.1038/labinvest.2014.136. [47] Arthur JC, Gharaibeh RZ, Mühlbauer M, Perez-Chanona E, Uronis JM, [28] Shabbeer A, Cowan LS, Ozcaglar C, Rastogi N, Vandenberg SL, Yener McCafferty J, et al. Microbial genomic analysis reveals the essential B, et al. TB-Lineage: an online tool for classification and analysis of role of inflammation in bacteria-induced colorectal cancer. Nat Com- strains of Mycobacterium tuberculosis complex. Infect Genet Evol mun 2014;5:4724. doi:10.1038/ncomms5724. 2012;12(4):789–797. doi:10.1016/j.meegid.2012.02.010. [48] Burns MB, Lynch J, Starr TK, Knights D, Blekhman R. Virulence genes [29] Habibi S, Ahmadi M, Alizadeh S. Type 2 diabetes mellitus screening are a signature of the microbiome in the colorectal tumor microen- and risk factors using decision tree: results of data mining. Glob J vironment. Genome Med 2015;7(1):55. doi:10.1186/s13073-015-
DOI: 10.14218/ERHM.2019.00026 | Volume 5 Issue 1, March 2020 25 Explor Res Hypothesis Med Sun W. et al: Microbial biomarkers of CRC
0177-8. [57] Wirbel J, Pyl PT, Kartal E, Zych K, Kashani Al, Milanese A, et al. Meta- [49] Sze MA, Schloss PD. Leveraging existing 16S rRNA gene surveys to analysis of fecal meta genomes reveals global microbial signatures identify reproducible biomarkers in individuals with colorectal tu- that are specific for colorectal cancer. Nat Med 2019;25(4):679–689. mors. mBio 2018;9(3):e00630–18. doi:10.1128/mBio.00630-18. doi:10.1038/s41591-019-0406-6. [50] Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of fe- [58] Thomas AM, Manghi P, Asnicar F, Pasolli E, Armanini F, Zolfo M, et al. cal samples from patients with colorectal cancer promotes intestinal Metagenomic analysis of colorectal cancer datasets identifies cross- carcinogenesis in germ-free and conventional mice. Gastroenterol- cohort microbial diagnostic signatures and a link with choline deg- ogy 2017;153(6):1621–1633.e6. doi:10.1053/j.gastro.2017.08.022. radation. Nat Med 2019;25(4):667–678. doi:10.1038/s41591-019- [51] Guo SH, Li LF, Xu BL, Li MH, Zeng QY, Xiao H, et al. A simple and novel 0405-7. fecal biomarker for colorectal cancer: ratio of to probiotics popula- [59] Ai D, Pan H, Han R, Li X, Liu G, Xia LC. Using decision tree aggrega- tions, based on their antagonistic effect. Clin Chem 2018;64(9):1327– tion with random forest model to identify gut microbes associated 1337. doi:10.1373/clinchem.2018.289728. with colorectal cancer. Genes (Basel) 2019;10(2):E112. doi:10.3390/ [52] Tunsjø HS, Gundersen G, Rangnes F, Noone JC, Endres A, Bemanian genes10020112. V. Detection ofFusobacterium nucleatum in stool and colonic tissues [60] Ai L, Tian H, Chen Z, Chen H, Xu J, Fang JY. Systematic evaluation of from Norwegian colorectal cancer patients. Eur J Clin Microbiol Infect supervised classifiers for fecal microbiota-based prediction of colo- Dis 2019;38(7):1367–1376. doi:10.1007/s10096-019-03562-7. rectal cancer. Oncotarget 2017;8(6):9546–9556. doi:10.18632/onco- [53] Mangifesta M, Mancabelli L, Milani C, Gaiani F, de’Angelis N, target.14488. de’Angelis GL, et al. Mucosal microbiota of intestinal polyps reveals [61] Sze MA, Baxter NT, Ruffin MT4th, Rogers MAM, Schloss PD, et al. putative biomarkers of colorectal cancer. Sci Rep 2018;8(1):13974. Normalization of the microbiota in patients after treatment for co- doi:10.1038/s41598-018-32413-2. lonic lesions. Microbiome 2017;5(1):150. doi:10.1186/s40168-017- [54] Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et 0366-3. al. Analysis of Fusobacterium persistence and antibiotic response in [62] Pinzone MR, Celesia BM, Di Rosa M, Cacopardo B, Nunnari G. colorectal cancer. Science 2017;358(6369):1443–1448. doi:10.1126/ Microbial translocation in chroni liver diseases. Int J Microbiol science.aal5240. 2012;2012:694629. doi:10.1155/2012/694629. [55] Xie YH, Gao QY, Cai GX, Sun XM, Zou TH, Chen HM, et al. Fecal [63] Hannigan GD, Duhaime MB, Ruffin MT4th, Koumpouras CC, Schloss Clostridiumsym biosum for noninvasive detection of early and ad- PD. Diagnostic potential and interactive dynamics of -the colo vanced colorectal cancer: test and validation studies. EBio Medicine rectal cancer virome. mBio 2018;9(6):e02248–18. doi:10.1128/ 2017;25:32–40. doi:10.1016/j.ebiom.2017.10.005. mBio.02248-18. [56] Yang Y, Misra BB, Liang L, Bi D, Weng W, Wu W, et al. Integrated [64] Nakatsu G, Zhou H, Wu WKK, Wong SH, Coker OO, Dai Z, et al. Al- microbiome and metabolome analysis reveals a novel interplay be- terations in enteric virome are associated with colorectal cancer tween commensal bacteria and metabolites in colorectal cancer. and survival outcomes. Gastroenterology 2018;155(2):529–541.e5. Theranostics 2019;9(14):4101–4114. doi:10.7150/thno.35186. doi:10.1053/j.gastro.2018.04.018.
26 DOI: 10.14218/ERHM.2019.00026 | Volume 5 Issue 1, March 2020 Review Article
The Effect of Honey as a Treatment for Oral Ulcerative Lesions: A Systematic Review
Maddison Hunter1,2, Jane Kellett1, Nathan M. D’Cunha1, Kellie Toohey1,2,3, Andrew McKune1,3,4 and Nenad Naumovski1*
1Faculty of Health, University of Canberra, Canberra, Australia; 2Prehabilitation, Cancer, Exercise and Survivorship (PACES) Research Group, University of Canberra, Canberra, Australia; 3Research Institute for Sport and Exercise (UCRISE), Faculty of Health, University of Canberra, Canberra ACT, Australia; 4Discipline of Biokinetics, Exercise and Leisure Sciences, School of Health Sciences, University of KwaZulu-Natal, Durban, KwaZulu-Natal, South Africa
Background and objectives: A healthy oral environment features a rapid turnover rate of epithelium cells capable of regeneration and repair, with the oral epithelium contributing as a physical barrier and immune defense. How- ever, the oral cavity can be subjected to unique damage, such as ulcerations. Honey is reported as a therapeutic agent for wound healing, due to its antioxidant, antibacterial and anti-inflammatory properties.
Methods: A systematic review was performed following the PRISMA 2015 Guidelines, to assess the efficacy and safety of the therapeutic use of honey in the oral cavity. Four electronic databases were searched (PubMed, Cochrane Library, Scopus, and Web of Science) for randomized controlled trials examining the effect of honey on oral cavity conditions.
Results: In total, 2,832 records were identified, and after applying exclusion criteria, 13 studies were included. Honey was applied topically throughout, for chemotherapy or radiotherapy-induced oral mucositis (n = 11), den- tal wounds (n = 1), and recurrent aphthous stomatitis (n = 1), all of which are ulcerations with different patholo- gies. In the majority of studies (12/13), honey reduced the severity and/or duration of the condition compared with control groups (all p<0.05). However, a group treated with Manuka honey (n = 1) experienced adverse effects and considerable participant attrition.
Conclusions: Honey is an effective treatment for a range of oral ulcerative conditions. Future research should focus on compositional analysis of honeys to determine those with optimal beneficial properties, and whether Manuka honey is safe to use in the oral cavity.
Introduction teeth, tongue, gingiva, and hard and soft palettes, with the latter be- ing composed of stratified squamous cells.1 A healthy oral environ- ment is characterized by a rapid cellular turnover rate, with the soft The oral cavity consists of a range of structures, including the and hard palettes averaging between 14 and 24 days, respectively.2 One of the main purposes of the rapid turnover of the oral epithe- Keywords: Honey; Oral health; Ulcer; Oral cavity. lium is as a defense mechanism, reducing the rate of pathogenic 2 Abbreviations: ROS, reactive oxygen species; PRISMA, preferred reporting items microbial colonization by removing the region they bind to. It for systematic reviews and meta-analyses; PICOS, population, intervention, compara- also serves multiple additional functions, such as a physical barrier tor, outcomes and setting; OM, oral mucositis; RAS, recurrent aphthous stomatitis; and immune defense to potential pathogens and toxins and in pro- MGO, methylglyoxal; GSH, glutathione. viding an environment that is optimal for the established healthy Received: November 01, 2019; Revised: December 10, 2019; Accepted: December 1 16, 2019 microbiome. *Correspondence to: Nenad Naumovski, Faculty of Health, University of Canberra, The turnover of the mucosal cells in the oral cavity is dependent P.O. Box 5018, Bruce, ACT 2617, Australia. Tel: +61-(0)2-6206-8719; E-mail: nenad. on a balance between cell differentiation and desquamation, which [email protected] primarily acts as a pathogen defense mechanism.3 However, this How to cite this article: Hunter M, Kellett J, D’Cunha NM, Toohey K, McKune A, can become unbalanced and lead to the development of several Naumovski N. The Effect of Honey as a Treatment for Oral Ulcerative Lesions: A Sys- tematic Review. Exploratory Research and Hypothesis in Medicine 2020;5(1):27–37. conditions, including hyperplasia and dysplasia, and a reduced doi: 10.14218/ERHM.2019.00029. rate of proliferation, which can lead to the development of ulcera-
Exploratory Research and Hypothesis in Medicine 2020 vol. 5 | 27–37
Copyright: © 2020 Authors. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Explor Res Hypothesis Med Hunter M. et al: Honey as a treatment for oral ulcerations tions.2 Additionally, the oral mucosa is subject to masticatory and Methods abrasive damage, with this oral epithelium disruption having the 1 possibility of leading to the development of oral diseases ; some This systematic review has been completed in accordance with of these common oral conditions include dental caries, denture 4 the Preferred Reporting Items for Systematic Reviews and Meta- wounds, and ulcerations. Analyses (PRISMA) 201520 Guidelines and was registered in the The treatment of adverse health conditions in the oral cavity International Prospective Register of Systematic Reviews (PROS- is focused on therapies that simultaneously eradicate pathogenic PERO Number: CRD42019128480). The systematic review was microbial growth, stimulate the wound healing process, and reduce completed following the Population, Intervention, Comparator, the sensation of pain. One possible treatment option that fulfils Outcomes and Setting (PICOS) approach as follows: these criteria is honey, which has been demonstrated to be effective 5 Population: Human populations with an existing oral health in the treatment of a range of wounds, such as burns and ulcers. condition, or at risk of developing an oral health condition. These potentially beneficial effects can be attributed to its antioxi- 5–7 Intervention: Randomized controlled trials utilizing topically- dant, antibacterial, and anti-inflammatory properties. applied undiluted honey from any floral source as a treatment or The composition of honey, in particular the exact proportions preventative agent without the concurrent use of other treatments. of each of its constituents, is dependent on the floral source of the 8 Only trials containing sample sizes over 12 participants were in- pollen used to produce the honey. Overall, honey is a mixture of cluded; however, there was no time limit placed on study duration. several different categories of compounds, including polyphenols, Comparator: Human comparators being treated with a placebo, ascorbic acid, carotenoids, organic acids, enzymes and other pro- control or routine standard oral care. 9 teins, all of which contribute to honey being a viable nutritional Outcomes: Effects of honey treatment on severity of oral health 10 source of antioxidants. Honey displays strong antioxidant activity conditions, improvements in healing time, and any adverse effects and has the ability to reduce the effects of oxidative reactions, which to participants caused by the use of honey during and following the 8 produce free radicals and reactive oxygen species (ROS). In addi- intervention protocol. tion, honey is also reported to exhibit strong antibacterial effects, Setting: Any. which can be attributed to its physicochemical properties, such as high osmolarity (due to high sugar levels)5 and relatively low pH11 due to the presence of several organic acids. Additionally, activation Search strategy of glucose oxidase during the dilution of honey causes the produc- tion of hydrogen peroxide from glucose metabolism, providing an The following four electronic databases were searched; Scopus, Pub- undesirable environment for growth and proliferation of bacterial Med, Cochrane Library and Web of Science, for manuscripts pub- cells12 and potentially mediating the wound healing process.13 lished since journal inception until June 2019. Furthermore, searches The concomitant effects of the antioxidant and antibacterial were also conducted to identify any new publications prior to sub- properties of honey also contribute to its anti-inflammatory ef- mission of this manuscript (end of October 2019). The searches were fects of reducing excessive inflammation, possibly resulting in a limited to studies that were conducted on humans, were randomized wound-healing effect.6 As the presence of ROS has been deter- controlled trials, and were published in peer-reviewed journals in the mined to lead to the production of inflammation,14 the antioxidant English language. The reference lists of included articles were also activity of honey can also contribute to a reduction of an excessive searched to locate any additional articles relating to the topic. The inflammatory response.7 Moreover, honey’s capability to prevent search terminology used included “honey*” and “oral”, “mouth”, “in- the development of bacterial infections (through providing an en- traoral”, “cavity”, “bucc*”, “tooth”, “orthodont*”, “saliv*”, and “dis- vironment that cannot support bacterial growth and proliferation) ease”, “condition”, “lesion”, “wound”, “carie*”, “plaque”, “ulcer”, can further assist with the inhibition of inflammation.5 Further- “bacter*”, “micro”, “count”, with the terms AND and OR between more, the application of honey to a wound has been demonstrated every term. to stimulate the production and release of pro-inflammatory cy- tokines that assist in the wound healing process, such as interleu- kin-1 and tumor necrosis factor-alpha.5 The topical application of Selection criteria honey to various injured tissues has also been shown to stimulate wound repair through the stimulation of growth of epithelial cells, Studies were included if they were original research, randomized reduction of edema, and wound debridement.6,7 controlled trials published in English and in peer-reviewed jour- It is well established that an individual’s oral health impacts their nals. Articles were only considered if they utilized undiluted quality of life.4,15,16 Poor oral health is shown to affect physical honey without any other interventions as a treatment against a and psychological wellbeing through condition-related reductions pre-existing condition of the mouth or as a preventative agent in a in functionality, including the inability to consume adequate nutri- group more susceptible to developing an oral condition. Further- tion and communicate, undesirable effects on physical appearance, more, articles were required to have a control or placebo group in addition to causing pain.15 These conditions, particularly those as a comparator. Any articles that did not meet these criteria were related to the soft and hard palette such as ulcerations, have been excluded from this review. There were no time limitations posed reported as treated using various types of honey in several different on the duration of the studies, as oral conditions have different clinical populations.17–19 These collective data suggest honey as a durations and pathological severities. For the purpose of this sys- reliable food product, being both financially affordable and readily tematic review, a condition of the mouth was defined as a symp- available, that could be used in the treatment of inflammatory and tomatic problem unique to the oral cavity, such as ulcers or dental microbial damage of the epithelial lining of the oral cavity. There- wounds. fore, this systematic review aimed to assess the efficacy of honey as a therapeutic agent used for the treatment of epithelial damage due to the inflammatory and microbial response in the oral cavity. Data extraction Additionally, this review will aim to evaluate the safety of honey in different clinical populations. All articles identified in the initial electronic search were imported
28 DOI: 10.14218/ERHM.2019.00029 | Volume 5 Issue 1, March 2020 Hunter M. et al: Honey as a treatment for oral ulcerations Explor Res Hypothesis Med into an EndNote library (vX8.2; Clarivate Analytics, USA) and Study characteristics duplicates removed. We excluded articles that did not investigate honey or oral health conditions. Following this, articles were ex- The characteristics of the included studies are presented in Table cluded based on study design, and only if pure undiluted honey 1.22–34 The total number of participants recruited in the included was utilized in the absence of any other treatments, with the excep- studies was 634, with sample sizes ranging from 20 to 106 par- tion of patient-required analgesics. ticipants. Of these, 11 studies investigated oral mucositis (OM) Two authors (MH and JK) independently screened each of the in 579 cancer patients,22–32 1 investigated 35 participants with articles based on the selection criteria. The titles were screened denture-induced ulcers,33 and 1 investigated 20 participants with to determine relevance, followed by screening of abstracts to de- recurrent aphthous stomatitis (RAS).34 Although the selection termine studies suitable for full-text analysis. Any disagreements criteria of this review was designed to include a broad range of regarding the selection criteria were resolved through discussion oral health conditions, the intervention was common across stud- until consensus was reached or in consultation with a third author ies, with pure, undiluted honey being applied topically to affected (NN). At this stage, the reference lists of the articles considered for areas in the mouths of patients in the treatment groups. The type full-text review were searched for additional articles. The extrac- of honey and their floral sources used in the included studies was tion of relevant data was completed by two authors (MH and JK) largely unspecified26,28,30,33,34; however, two studies stated that the working independently in predesigned tables including the infor- honey was sourced from a commercial supermarket.23,27 Five of mation on author name, year of publication, condition assessed, the studies identified the local flora that was used in the production honey application protocol, control or placebo, number of partici- of the honey used,22,24,29,31,32 and one study specified their use of pants, and outcomes. Manuka honey.25 In the included articles, participants were instructed to main- Assessment of bias tain good oral hygiene (n = 6)22,24–26,31,32 or prescribed the use of antibacterial, antifungal or analgesic solutions (n = 3).22,23,32 However, this was not specified in six studies.27–30,33,34 For trials The risk of bias was independently assessed according to the investigating OM (n = 11), the studies investigated head and neck Cochrane Collaboration’s Tool for Assessing Bias, which utilizes cancer patients in adult populations24–32 and pediatric patients re- the domains of selection bias through random sequence genera- ceiving treatment for a range of cancers, including leukemia.22,23 tion and allocation concealment, performance bias through blind- Participants with radiotherapy-induced OM were included in six ing of participants and personnel, detection bias through blinding studies,24,25,27–29,31 with 1 study having investigated chemother- of outcome assessment; attrition bias through the addressing of apy-induced OM22 and 4 having determined the effect of honey any incomplete data, reporting bias through selective reporting, on concurrent chemo-radiotherapy-induced OM.23,26,30,32 These and any other sources of bias.21 The risk of bias was assessed studies utilized routine oral care,23,24,30,32 saline rinses of differ- independently by two authors (MH and JK), with a third author ent concentration ranges (0.9%,27 0.09%,31 and unspecified26), (NN) introduced in the case of any disagreements, where appro- 28 priate. water, and a variety of anesthetic and analgesic solutions (7.5% benzocaine gel,22 15% benzydamine hydrochloride,27 lignocaine gel29) serving as controls, while only one study utilized a placebo Data analysis gel25 and another developed their own mixture.22
Due to the broad inclusion criteria used for this review to include Interventions a number of ulcerative lesions, which includes a variety of popula- tion groups and intervention protocols, this review consists of con- 24 siderable heterogeneity. As such, a meta-analysis was not deemed In a study by Biswal et al., patients receiving radiotherapy for appropriate. head and neck cancer were provided with 20 mL of honey to smear on the inside of their mouths, and instructed to swallow it slowly in order to coat both the oral and pharyngeal mucosa, with ap- Results plication occurring 15 min before and after they received radio- therapy and 6 h after receiving radiotherapy, for the duration of the treatment cycle. This study design was also replicated by other Study selection studies included in this review,27,29–32 with Jayalekshmi et al.28 and Howlader et al.26 with slight modifications, namely use of 15 The initial search of the electronic databases (Fig. 120) resulted mL of honey28 and consumption of additional honey to potentially in 2,838 articles, with the secondary searches identifying 1 contribute to serum antioxidant levels.26 article. Following removal of articles due to duplicates (n = The remaining studies investigating OM utilized study designs 1,181), study design (n = 115), absence of honey investigation similar to those above, topically coating the oral cavity with hon- (n = 1,416) and absence of oral health intervention (n = 80), ey; however, the amounts of honey used and timing of applica- 46 studies were subjected to full text-analysis. Articles were tion were different. In trials by Al Jaouni et al.23 and Hawley et further excluded due to use of diluted honey with or without al.25 durations were similar of treatments used to Biswal et al.24 the use of additional therapies (n = 14), unsuitable oral health and participants were followed throughout the duration of their conditions (n = 12) and study design (n = 8), with 13 studies oncology treatments. In addition, Hawley et al.25 maintained the fitting the inclusion criteria.22–34 An article investigating the use intervention for 7 days following final radiotherapy treatment, with of honey to assist in the recovery of dental extraction surgery35 all studies applying the honey intervention four times daily. The was considered but it was determined that since this wound is volumes of honey and subsequent saline rinse were not specified due to surgery, and not a condition unique to the oral cavity, this by Al Jaouni et al.23 but Hawley et al.25 specified the prescription article was excluded. of 5 mL of applied honey followed by a fluoride rinse. Abdulrhman
DOI: 10.14218/ERHM.2019.00029 | Volume 5 Issue 1, March 2020 29 Explor Res Hypothesis Med Hunter M. et al: Honey as a treatment for oral ulcerations
Fig. 1. Search strategy and article selection process according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines.20 et al.22 included participants with established OM using undiluted honey in the treatment of RAS in healthy adults. Following an ini- honey (0.5 g/kg) and having two control groups (receiving a cus- tial assessment, participants in the treatment group (n = 10) were tom-made mixture (placebo) or an analgesic control). All groups given honey and control groups (n = 10) were given salicylate gel, applied their mixtures three times daily for 10 days or until healing a standard prescribed treatment for RAS, to apply to their ulcers 3 of ulceration. times daily for 5 days. Ulcer size was determined using calipers, The study by Ceylan et al.33 investigated the use of honey in and a visual analog score was used to assess pain before and after the treatment of traumatic ulcers that were caused by newly fit- the intervention. ted dentures in 35 healthy, previously edentulous participants. Of these, 25 participants were provided with honey and instructed to smear directly onto the ulcers three times daily for 3 days follow- Study results ing denture fitting. The control group (n = 10) were provided with a saline rinse to use via the same protocol as the treatment group. The majority of studies investigating the use of honey in the treat- The ulcer areas were measured in all participants using planimetry ment of OM in an oncology population were successful in alleviat- on the first and third day following initial denture fitting. ing negative symptoms, including reducing the severity or duration The study by Halim et al.34 investigated the effectiveness of of the condition (all p<0.05) (Table 1).22–24,26–29,31,32 In these stud-
30 DOI: 10.14218/ERHM.2019.00029 | Volume 5 Issue 1, March 2020 Hunter M. et al: Honey as a treatment for oral ulcerations Explor Res Hypothesis Med p 0.05); and ulcer size pain score for gel in pain and ulcer size improvement observed 3 ( p = 0.77) for OM Grade groups between No difference nausea; 57% to and placebo contributed Honey rate and 52% placebo dropout treatment 0.05) in mucositis grade weeks weeks grade ( p > 0.05) in mucositis No difference improvements administered); 1–4 (radiotherapy in 4 n = 14 n = 30 n = 30 n = 20 n = 20 n = 20 15 mL water rinsed 15 min before and rinsed 15 min before 15 mL water radiotherapy; after and 6 h after, Routine oral care; care; oral Routine Comparator Group 2: 15mL 0.15% benzydamine 2: 15mL 0.15% benzydamine Group rinsed 15 min hydrochloride after and 6 h and after, before 5 min; n = 20 for radiotherapy 3: 20 mL 0.9% saline rinsed 15 Group after and 6 h and after, min before 5 min; n = 20 for radiotherapy Salt water rinse three times daily; n = 10 rinse three Salt water applied three gel Salicylate n = 10 for 5 days; times daily times applied four 5mL placebo gel saline rinse; fluoride daily following bedtime; n = 52 at treatment Group 2: 0.25 g/kgGroup of 4:2:1 mixture extract oil-propolis olive of honey, (HOPE) applied topically and beeswax for healing or times daily until three care; oral and routine 10 days care; oral Routine Saline rinsed in mouth 15 min before radiotherapy; and after Group 3: benzocaine 7.5% gel 7.5% gel 3: benzocaine Group times three mucosa oral applied to care; oral daily and routine n = 14 n = 20 n = 20 n = 20 n = 20 n = 30 Intervention Group 1: 20 mL honey 1: 20 mL honey Group rinsed 15 min before after and 6 h and after, radiotherapy; rinsed 15 min 15 mL honey and 6 h and after, before radiotherapy; after Group 1: 0.5 g/kgGroup applied the affected to topically times three mucosa oral healing or daily until and routine 10 days for care; oral applied topically Honey followed mucosa, oral to saline rinse alkaline by six times daily and to four care; oral routine applied to 20 mL honey immediately mouth before, after and 6 h after, radiotherapy; applied to Honey 3 times daily wound n = 25 3 days; for times applied three Honey n = 10 5 days; daily for applied four 5 mL honey saline following times daily rinse; fluoride treatment bedtime; n = 54 at applied to 20 mL honey and mouth 15 min before after and 6 hours after, radiotherapy; n = 60 n = 28 n = 40 n = 106 n = 40 Population Oral cancer cancer Oral receiving patients radiotherapy; Head and neck cancer receiving patients radiotherapy; Lymphoblastic Lymphoblastic receiving leukemia Grade chemotherapy; 2 and 3 OM; n = 90 cancer Pediatric chemo/ receiving radiotherapy; Head neck cancer receiving patients radiotherapy n = 40 denture New n = 35 wearers; Minor recurrent stomatitis aphthous n = 20 patients; Head and neck cancer receiving patients radiotherapy; Head and neck patients cancer chemo/ receiving radiotherapy; et
et 28 23 25 26 33 et al. et et al. et 24 34 et al. et (2012) (2012) (2017) (2014) (2019) (2016) (2003) Biswal Biswal Ceylan Ceylan (2010)* Al Jaouni al. Jayachandran Jayachandran al. et Study Abdulrhman al. et Halim (2013) et Hawley al. et Howlader al. Jayalekshmi Jayalekshmi al. Table 1. Summary of included study characteristics 1. Summary of included study Table
DOI: 10.14218/ERHM.2019.00029 | Volume 5 Issue 1, March 2020 31 Explor Res Hypothesis Med Hunter M. et al: Honey as a treatment for oral ulcerations
ies, OM was assessed using a grading scale, with Grade 1 repre- senting less severe cases and Grade 4 being the most severe. In these studies, the onset of OM was delayed in the honey-treatment group compared to the control group, and fewer participants in the honey group of each study developed higher gradings of the condi- tion. One OM study did not report any significant changes from p < 0.05) pre- to post-treatment nor any difference between the two groups (treatment and control); however, there was a self-reported ob- served difference in the OM.30 All of these studies utilized standard honey from no particular floral source, with no adverse reactions reported throughout the duration of the honey intervention, except- ing one study31 that reported the exclusion of participants who re- ported a stinging sensation, though the number of exclusions and effect on the study was not disclosed. A single study by Hawley et al.25 utilized Manuka honey and reported no significant difference 0.001) in treatment group; p < 0.001) in treatment in the severity or duration of OM between the honey and placebo groups (p = 0.77); however, this study experienced considerable dropout rate (57% in the honey group, 52% in the placebo group), mainly attributed to the sensation of nausea caused by the applica- tion of the honey. It was determined that honey significantly (p value unreported) Honey delayed onset in about 80%; 63% onset delayed Honey OM had severe group in control control. arm, 11/27 in 3 OM: 5/28 in intervention Grade control arm, 5/27 in 4: 1/28 in intervention Grade 4 3/20 Grade group: in honey severity Reduced arm) ( 4 (control arm), 9/20 Grade (honey Honey associated with less severe OM ( p < 0.0001) with less severe associated Honey OM intolerable developed 15/20 2: Group 1/20; 1: Group interruptions had treatment group Control ( lower OM score symptoms OM develop not did group intervention in 20% Results relieved the symptoms of the denture-induced ulcers earlier than in the control group, and (as assessed by a visual analogue score) re- duced pain, with no adverse effects.33 Finally, while no participants with RAS reported adverse effects, no significant difference was found between the honey group and control group of a currently utilized treatment for ulcer size (p = 0.879) and pain (p = 0.514); however, self-reported improvements were observed.34 n = 20
n = 27 n = 20 Risk of bias in included studies n = 20
The risk of bias was assessed according to the Cochrane Risk of Bias Tool and is outlined in Table 2.22–34 The risk of selection bias through allocation concealment was high for the included studies, which was due to placebo products being utilized by 2/13 22,25 28 20 mL lignocaine gel rinsed 15 gel 20 mL lignocaine radiotherapy, and after min before bedtime; and at care; oral Standard 20 mL 0.09% saline rinsed before radiotherapy; and after care; oral Standard Comparator studies. Additionally, Jayalekshmi et al. reported the use of similar bottles for both their treatment and control groups, result- ing in a low risk of selection bias. Similarly, the performance bias was high or unclear, which was attributed to the common
n = 20 n = 20 utilization of controls rather than a placebo. The detection bias for the included studies was relatively unclear but there was an overall low risk of attrition and reporting bias (Table 2). No other bias was detected. n = 20 n = 28
- (continued) Discussion Intervention 20 mL honey rinsed 15 20 mL honey and after min before and at radiotherapy, bedtime; rinsed 15 20 mL honey and after min before and at radiotherapy, bedtime; rinsed 15 min 20 mL honey and 6 h and after, before radiotherapy; after rinsed 15 min 20 mL honey and 6 h and after, before radiotherapy; after Effectiveness of honey
The primary aim of this review was to determine the effectiveness n = 40 n = 55 n = 40 n = 40 of topically-applied honey in the treatment of a range of oral health conditions. Of the 13 studies included in this review, 10 reported statistically significant reductions in the severity of the oral health condition compared to a control group, including 9 studies inves- tigating OM22–24,26–29,31,32 and 1 investigating denture wounds.33 Population Oral cancer cancer Oral receiving patients radiotherapy; Head and neck patients cancer chemo/ receiving radiotherapy; Head and neck cancer receiving patients radiotherapy; Head and neck patients cancer chemo/ receiving radiotherapy; While one study did not report any significance30 and another re- 34
31 ported no significant difference in the improvement of RAS, the
32 oral condition was improved in both studies, demonstrating that et 30 et al. et
29 honey was just as effective as the standard prescribed treatments et al. et (2008) for these conditions. Only one study, by Hawley et al.,25 did not (2009) report any improvements in oral condition. Motallebnejad Motallebnejad al. et (2012)* al. Maiti Rashad Study Khanal (2010)
Table 1. Summary of included study characteristics characteristics 1. Summary of included study Table mucositis. OM, oral provided. *No p value It has been proposed that honey possesses wound healing abili-
32 DOI: 10.14218/ERHM.2019.00029 | Volume 5 Issue 1, March 2020 Hunter M. et al: Honey as a treatment for oral ulcerations Explor Res Hypothesis Med
ties due to its antioxidant and antibacterial properties,5–7 which in- cludes the ability to heal ulcerations. All conditions included in this review culminated in the development of an oral ulcer, which had formed due to the loss and necrosis of epithelial tissue and result- Unclear Unclear Unclear Unclear Unclear Unclear Unclear Unclear Unclear Unclear Unclear Unclear Unclear Other bias ing in breaks in the skin or mucous membrane36; each condition
- formed due to different pathologies. Oral mucositis is a condition that develops as a toxicity during oncology treatments (chemother- apy and radiotherapy) and is characterized by inflammation, ery- thema, microbial colonization, and ulceration.37 Similarly, RAS is a condition of unknown cause that affects the lining of the mouth, causing erythema, burning and ulceration,34 and denture-induced Low Low Low Low Low Low Low Low Low Low Low Low Low Selective report Selective ing bias) (reporting ulcers are caused by the introduction of ill-fitting dentures causing abrasive damage to the lining of the mouth, resulting in ulcerative lesions.33 The antibacterial and antioxidant properties of honey contribute to honey’s wound-healing abilities. In the case of OM, the ini- tiation phase is characterized by the production of ROS,37 which could potentially cause further damage to the affected areas. A study investigating participants with RAS determined that those Low Low Low Low Low Low Low Low Low Low Low Low Low Incomplete data data Incomplete addressed bias) (attrition with the condition had significantly lower salivary antioxidant levels compared to healthy controls,38 demonstrating that the ul-
- cerations potentially produced ROS, causing a reduction in the antioxidant compounds present in the mouth. This highlights a possible mechanism for the effectiveness of honey as a treatment for the ulcerations discussed in this review. Potentially, the pres- ence of the antioxidant compounds provided by the topically-ap- plied honey can reduce the effects of ROS activity during the de- Unclear Unclear Unclear Unclear Unclear Low Low Unclear Unclear Low Unclear Low Unclear Blinding of out assessment come bias) (detection velopment of ulcerations. Furthermore, the consumption of honey increases total systemic plasma antioxidant levels, and swallow- - ing of the topically-applied honey may contribute to longer lasting antioxidant effects,10 further contributing to the wound healing process.
Adverse effects Unclear High Unclear Unclear Unclear Low High Unclear High High Unclear Low High Blinding of partici pants and personnel and personnel pants bias) (performance The secondary aim of this systematic review was to determine any
- adverse effects during the topically-applied honey interventions. Only one of the included studies reported an adverse effect due to the honey intervention.25 Namely, Hawley et al.25 reported con- siderable dropouts due to nausea, which could be attributed to the treatments being associated with radiotherapy; however, it was Low High High High High Low High High High High High High High Allocation con Allocation cealment (selection bias) reported that the Manuka honey that was used worsened nausea symptoms and encouraged a retching motion. A study by Bardy et al.39 also used Manuka honey and reported a low compliance rate due to the honey being difficult to use, along with an undesir- able taste and texture. A study by Parsons et al.40 initially had to be discontinued due to the Manuka honey not being well-tolerat- ed by participants, and in that study all participants withdrew due to a perceived stinging pain and nausea following application. In- terestingly, Abdulrhman et al.22 reported no adverse side effects Low Unclear Low Unclear Unclear Low Low Unclear Low Low Unclear Unclear Unclear Random sequence generation (selection bias) in the honey intervention arm of their study, which used honey from a different floral source to Manuka honey; however, in their 31 22 27 comparative group using their own formulation, discomfort was 28 reported, which was proposed to be due to the addition of propo- 26
(2008) lis. The studies investigating denture wounds and RAS did 25 32 29 (2012) 33 (2012) 24 34 30
(2016) not report any adverse effects. This further supports evidence that (2019) et al. et the application of honey to the inside of the oral cavity is effec- (2017) et al. et (2014) (2009) et al. et (2010) (2010)
(2003) tive in reducing the pathogenic bacteria that cause dental caries, (2013) (2012) 17,41,42
et al. et and has a positive effect on plaque levels and gingivitis. et al. et et al. et et al. et et al. et
et al. et These findings demonstrate that the use of honey in future studies et al. et is safe for the dental health of participants. It was also proposed that similar trials comparing two intervention groups of Manuka Study Al Jaouni Biswal Ceylan Halim al. et Hawley al. et Howlader al. et Jayachandran Jayalekshmi Khanal Maiti Motallebnejad Rashad Abdulrhuman 18 Table 2. Assessment of risk bias in included studies. 2. Assessment Table and conventional honey should be completed ; however, given
DOI: 10.14218/ERHM.2019.00029 | Volume 5 Issue 1, March 2020 33 Explor Res Hypothesis Med Hunter M. et al: Honey as a treatment for oral ulcerations the evidence of the adverse effects of Manuka honey, this may served beneficial health effects of honey, are largely unexplored. not be feasible. Four of the included studies, all investigating OM, completed in vitro analysis of the potential wound healing properties, including pH,22,24,31,32 moisture content,22,24,31,32 phytochemical profile,24,32 Issues with use and development of placebo and microbiological analysis.24,31 However, none of the studies utilized these results in their discussion on the effectiveness of The majority of the studies included in this review (n = 12) uti- honey as a treatment. A trial of this nature should be completed to lized a control or ‘standard’ prescribed care in the place of a pla- determine the individual in vitro wound healing properties of dif- cebo. Due to the nature and progression of oral health conditions ferent types of honey, so that recommendations can be offered to included in this review, particularly OM in oncology patients, it participants experiencing these oral health conditions about what would be unethical to provide no treatment to the control group, type of honey should be used to provide optimal benefits. Due to resulting in most studies in this review receiving standard care or the overall lack of analysis of the honey utilized in these trials, it a commonly-used treatment. This is also partially reflected by the is difficult to draw conclusions about the best type/s of honey and reported bias (Table 2), where many studies were graded as having the properties that are most appropriate for use in the treatment of a “high risk” of selection and performance bias, with the exception these oral conditions. of the study by Jayalekshmi et al.28 who utilized similar packaging for their honey treatment and control. If a placebo were to be used in future studies to assess the effec- Limitations tiveness of honey as a topical treatment, it would be best practice to use products which share certain physicochemical properties The inclusion criteria for this review evaluated only articles that with honey to support undetectability by participants, such as its utilized pure, undiluted honey as a treatment, as the concurrent properties of osmolarity and acidity.31 The use of such a placebo utilization of other compounds or medications has the possibility could have confounding effects, as the physicochemical properties to contribute to any potential beneficial effect provided by just the of honey, including its osmolarity, contribute to honeys wound- honey in isolation. As a result, studies that utilized honey and no healing abilities.19 One such example is brought forth by the study other treatments were included, though the use of standard care was by Bardy et al.,39 which had been excluded (during full-text re- allowed. It would be unethical to request that patients discontinue view) from our review due to not having used undiluted honey. their normal oral care routines for the duration of an intervention, In that study, golden syrup which has a similar appearance and due to the potential impact on their dental health. Additionally, the texture to honey, was assigned as a placebo alongside a Manuka conditions investigated in this review can cause considerable pain, honey treatment for OM. While there was no significant differ- which could lead to the requirement of the additional analgesics. ence observed in the severity or duration of the OM between the This may provide a reduction in the effectiveness of the honey; treatment and control groups (p > 0.05), the pathogenic bacteria however, the use of honey in isolation to routine oral care in in- present in both groups was consistent with baseline throughout the terventions such as the ones included in this review is impractical. study period, demonstrating that both honey and the placebo con- trolled the bacterial growth. This further supports the notion of the importance of osmolarity action (shared by the honey and golden Future research proposals syrup) on the antibacterial activity of honey. The only study in this review that utilized a placebo was com- pleted by Hawley et al.,25 where the product was developed to look The adverse effects caused by Manuka honey highlighted in this re- like honey. Similar to the study by Bardy et al.,39 this study did not view should be taken into consideration of future research for oral result in a significant reduction in the severity or duration of the health conditions. Manuka honey contains relatively high levels of OM experienced by participants; however, this could be attributed methylglyoxal (MGO), which has been identified to be unique to to the possible non-suitability of Manuka honey for this type of this type of honey and directly contributes to Manuka honey’s an- treatment, rather than the activity of the placebo. Additionally, a tibacterial abilities.43,44 This compound also occurs in humans as a study by Abdulrhman et al.22 developed a honey-containing prod- by-product to metabolic processes, such as glycolysis45; however, uct, which they assessed as a treatment for OM alongside a treat- it has been shown to exhibit potentially toxic side effects, includ- ment group that received pure, undiluted honey, in addition to the ing the ability to modify DNA and other macromolecules.46 Addi- use of a standard care control (Table 1). tionally, in healthy human cells, the tripeptide glutathione (GSH) is It is evident that the utilization of a placebo in studies of this capable of suppressing the activity of MGO47; however, once a cell nature is not practical, and so further care should be taken in future becomes damaged, such as during the oral conditions discussed in studies to ensure that both participants and researchers are blinded this review, there is a reduction in the expression of GSH.48 This to the allocation of honey or control. This could potentially be can result in further damage, with GSH depletion leading to MGO achieved through disclosing that the intervention is designed to accumulation.49 This is depicted in Figure 2, where, in a healthy improve the oral health condition, while not specifying that honey oral cavity, GSH is able to suppress the activity of MGO, thereby is the treatment. preventing any cellular modifications. Although, when Manuka honey is applied to the damaged oral cavity, the reduced levels of GSH allow the levels of MGO to increase, and to potentially cause Compositional properties of used honeys further cellular damage. As previously discussed, one study included in this review It is well established that several honey properties can contribute reported considerable adverse effects following the topical appli- to its wound healing effects, including a combination of antioxi- cation of Manuka honey.25 Studies of similar interventions also dant, physicochemical and antimicrobial properties. However, the reported adverse effects,39,40 which could potentially be due to the optimal levels of compounds or categories of compounds, includ- proposed interactions between MGO and reduced levels of GSH. ing their interaction with each other, that can contribute to the ob- These adverse reactions were not observed in studies utilizing hon-
34 DOI: 10.14218/ERHM.2019.00029 | Volume 5 Issue 1, March 2020 Hunter M. et al: Honey as a treatment for oral ulcerations Explor Res Hypothesis Med
Fig. 2. Effect of honey on the oral cavity. (a) GSH present in the healthy oral cavity suppresses the activity of MGO from Manuka honey. (b) The use of honey does not cause adverse effects in a healthy oral cavity, and is effective in treating a damaged oral cavity. (c) Reduced levels of GSH in the damaged oral- cav ity do not suppress the activity of MGO from Manuka honey, with the MGO potentially causing further damage, and Manuka honey not being an effective treatment. GSH, tripeptide glutathione; MGO, methylglyoxal. ey from other floral sources, with the topical application of honey be completed to determine optimal levels of MGO that can be tol- to a damaged oral cavity being demonstrated to result in improve- erated. ments to the oral condition (Fig. 2). While Manuka honeys have demonstrated substantial potential wound healing abilities in vitro and in vivo,11,44,50 there is currently Conclusion a lack of relevant clinical trials utilizing this product for wound healing treatments. Due to its compositional properties, it should The use of honey as a therapeutic treatment of oral conditions has be considered that Manuka honey be utilized in clinical trials for been identified to be effective in reducing the duration and sever- site-specific topical application in wound sites other than the oral ity of conditions in comparison to standard treatments and con- cavity, such as a laceration or burn on the skin to test for effective- trols. Specifically, it has been demonstrated that honey is effective ness and tolerability. Similarly, further trials in the treatment of against a range of oral ulcers with multiple pathologies and, in oral conditions or investigating alternative application sites could particular, in the treatment of oral mucositis. Any adverse reactions
DOI: 10.14218/ERHM.2019.00029 | Volume 5 Issue 1, March 2020 35 Explor Res Hypothesis Med Hunter M. et al: Honey as a treatment for oral ulcerations that were reported throughout the duration of the included studies foodchem.2013.02.015. could be attributed to the type of honey used and were not due to [9] Escuredo O, Seijo MC, Salvador J, González-Martín MI. Near infra- the type of oral health condition, demonstrating that the topical red spectroscopy for prediction of antioxidant compounds in the application of honey, other than Manuka honey, is relatively safe honey. Food Chem 2013;141(4):3409–3414. doi:10.1016/j.food- for use across a range of oral health conditions. The use of honey chem.2013.06.066. [10] Schramm DD, Karim M, Schrader HR, Holt RR, Cardetti M, Keen CL. for the treatment of oral mucositis should be considered for fur- Honey with high levels of antioxidants can provide protection to ther research; however, control over honey composition should be healthy human subjects. J Agric Food Chem 2003;51(6):1732–1735. taken into consideration, particularly phytochemical composition. doi:10.1021/jf025928k. The use of Manuka honey in the treatment of oral cavity conditions [11] Alvarez-Suarez JM, Giampieri F, Cordero M, Gasparrini M, Forbes- should be carefully considered before use, with future investiga- Hernández TY, Mazzoni L, et al. Activation of AMPK/Nrf2 signalling tions required to determine its safety. by Manuka honey protects human dermal fibroblasts against oxida- tive damage by improving antioxidant response and mitochondrial function promoting wound healing. J Funct Foods 2016;25:38–49. Acknowledgments doi:10.1016/j.jff.2016.05.008. [12] Bang LM, Buntting C, Molan P. The effect of dilution on therate of hydrogen peroxide production in honey and its implications for None wound healing. J Altern Complement Med 2003;9(2):267–273. doi:10.1089/10755530360623383. [13] Martinotti S, Laforenza U, Patrone M, Moccia F, Ranzato E. Honey- mediated wound healing: H Funding 2O2 entry through AQP3 determines extracellular Ca2+ influx. Int J Mol Sci 2019;20(3):764. doi:10.3390/ ijms20030764. M. Hunter is supported by an Australian Government Research [14] Safi SZ, Batumalaie K, Qvist R, Mohd Yusof K, Ismail IS. Gelam hon- Training Program Scholarship. N. M. D’Cunha is supported by ey attenuates the oxidative stress-induced inflammatory pathways a Dementia Australia Research Foundation PhD Scholarship. All in pancreatic hamster cells. Evid Based Complement Alternat Med other authors have no sources of funding to declare. 2016;2016:5843615. doi:10.1155/2016/5843615. [15] Halvari AEM, Halvari H, Deci EL. Dental anxiety, oral health-related quality of life, and general well-being: a self-determination theory perspective. J Appl Soc Psychol 2019;49(5):295–306. doi:10.1111/ Conflict of interest jasp.12583. [16] Barkokebas A, Silva IHM, de Andrade SC, Carvalho AAT, Gueiros All authors declare no conflict of interests. LAM, Paiva SM, et al. Impact of oral mucositis on oral-health-related quality of life of patients diagnosed with cancer. J Oral Pathol Med 2015;44(9):746–751. doi:10.1111/jop.12282. [17] Atwa ALDA, AbuShahba RY, Mostafa M, Hashem MI. Effect of honey in Author contributions preventing gingivitis and dental caries in patients undergoing ortho- dontic treatment. Saudi Dent J 2014;26(3):108–114. doi:10.1016/j. Study concept and design (MH, NN); data acquisition (MH, JK); sdentj.2014.03.001. data analysis (MH, JK, NN); drafting of the manuscript (MH); [18] Münstedt K, Momm F, Hübner J. Honey in the management of side critical revision of the manuscript (MH, JK, NMD, KT, AM, NN); effects of radiotherapy- or radio/chemotherapy-induced oral mucosi- tis. a systematic review. Complement Ther Clin Pract 2019;34:145– administrative, technical, and study supervision (NN). 152. doi:10.1016/j.ctcp.2018.11.016. [19] Al-Waili N, Salom K, Al-Ghamdi AA. Honey for wound healing, ul- cers, and burns; data supporting its use in clinical practice. Scienti- References ficWorldJournal 2011;11:766–787. doi:10.1100/tsw.2011.78. [20] Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred [1] Wang SS, Tang YL, Pang X, Zheng M, Tang YJ, Liang XH. The main- reporting items for systematic reviews and meta-analyses: the PRIS- tenance of an oral epithelial barrier. Life Sci 2019;227:129–136. MA statement. PLoS Med 2009;6(7):e1000097. doi:10.1371/journal. doi:10.1016/j.lfs.2019.04.029. pmed.1000097. [2] Qin R, Steel A, Fazel N. Oral mucosa biology and salivary biomark- [21] Higgins J, Green S (editor). Cochrane Handbook for Systematic Re- ers. Clin Dermatol 2017;35(5):477–483. doi:10.1016/j.clinderma- views of Interventions 2011. Version 5.1.0 [updated March 2011]. tol.2017.06.005. Available from www.handbook.cochrane.org. [3] Squier CA, Kremer MJ. Biology of oral mucosa and esophagus. J Natl [22] Abdulrhman M, Elbarbary NS, Ahmed Amin D, Saeid Ebrahim R. Hon- Cancer Inst Monogr 2001;2001(29):7–15. doi:10.1093/oxfordjour- ey and a mixture of honey, beeswax, and olive oil-propolis extract nals.jncimonographs.a003443. in treatment of chemotherapy-induced oral mucositis: a randomized [4] Haag DG, Peres KG, Balasubramanian M, Brennan DS. Oral condi- controlled pilot study. Pediatr Hematol Oncol 2012;29(3):285–292. d tions and health-related quality of life: a systematic review. J Dent oi:10.3109/08880018.2012.669026. Res 2017;96(8):864–874. doi:10.1177/0022034517709737. [23] Al Jaouni SK, Al Muhayawi MS, Hussein A, Elfiki I, Al-Raddadi R, Al [5] Molan PC, Rhodes T. Honey: a biologic wound dressing. Wounds Muhayawi SM, et al. Effects of honey on oral mucositis among pedi- 2015;27(6):141–151. atric cancer patients undergoing chemo/radiotherapy treatment [6] Hadagali MD, Chua LS. The anti-inflammatory and wound healing at King Abdulaziz University Hospital in Jeddah, Kingdom of Saudi properties of honey. Eur Food Res Technol 2014;239(6):1003–1014. Arabia. Evid Based Complement Alternat Med 2017;2017:5861024. doi:10.1007/s00217-014-2297-6. doi:10.1155/2017/5861024. [7] Yaghoobi R, Kazerouni A, Kazerouni O. Evidence for clinical use of [24] Biswal BM, Zakaria A, Ahmad NM. Topical application of honey in honey in wound healing as an anti-bacterial, anti-inflammatory anti- the management of radiation mucositis: a preliminary study. Support oxidant and anti-viral agent: A review. Jundishapur J Nat Pharm Prod Care Cancer 2003;11(4):242–248. doi:10.1007/s00520-003-0443-y. 2013;8(3):100–104. doi:10.17795/jjnpp-9487. [25] Hawley P, Hovan A, McGahan CE, Saunders D. A randomized placebo- [8] Liu JR, Ye YL, Lin TY, Wang YW, Peng CC. Effect of floral sources on the controlled trial of manuka honey for radiation-induced oral mucosi- antioxidant, antimicrobial, and anti-inflammatory activities of hon- tis. Support Care Cancer 2014;22(3):751–761. doi:10.1007/s00520- eys in Taiwan. Food Chem 2013;139(1-4):938–943. doi:10.1016/j. 013-2031-0.
36 DOI: 10.14218/ERHM.2019.00029 | Volume 5 Issue 1, March 2020 Hunter M. et al: Honey as a treatment for oral ulcerations Explor Res Hypothesis Med
[26] Howlader D, Singh V, Mohammad S, Gupta S, Pal U, Pal M. Effect of double-blind, placebo-controlled, randomised trial of active ma- topical application of pure honey in chemo-radiation-induced mu- nuka honey and standard oral care for radiation-induced oral mu- cositis and its clinical benefits in improving quality of life in patients of cositis. Br J Oral Maxillofac Surg 2012;50(3):221–226. doi:10.1016/j. oral squamous cell carcinoma. J Maxillofac Oral Surg 2019;18(1):73– bjoms.2011.03.005. 79. doi:10.1007/s12663-017-1077-9. [40] Parsons E, Begley A, Herst P. Manuka honey mouthwash does [27] Jayachandran S, Balaji N. Evaluating the effectiveness of topical appli- not affect oral mucositis in head and neck cancer patients in New cation of natural honey and benzydamine hydrochloride in the man- Zealand. J Radiother Pract 2012;11(4):249–256. doi:10.1017/ agement of radiation mucositis. Indian J Palliat Care 2012;18(3):190– S1460396911000410. 195. doi:10.4103/0973-1075.105689. [41] Jain A, Bhaskar DJ, Gupta D, Agali C, Gupta V, Gupta RK, et al. Com- [28] Jayalekshmi JL, Lakshmi R, Mukerji A. Honey on oral mucositis: a ran- parative evaluation of honey, chlorhexidine gluconate (0.2%) and domized controlled trial. Gulf J Oncolog 2016;1(20):30–37. combination of xylitol and chlorhexidine mouthwash (0.2%) on the [29] Khanal B, Baliga M, Uppal N. Effect of topical honey on limita- clinical level of dental plaque: a 30 days randomized control trial. Per- tion of radiation-induced oral mucositis: an intervention study. spect Clin Res 2015;6(1):53–57. doi:10.4103/2229-3485.148819. Int J Oral Maxillofac Surg 2010;39(12):1181–1185. doi:10.1016/j. [42] Singhal R, Siddibhavi M, Sankeshwari R, Patil P, Jalihal S, Ankola A. Ef- ijom.2010.05.014. fectiveness of three mouthwashes - Manuka honey, Raw honey, and [30] Maiti P, Ray A, Mitra TN, Jana U, Bhattacharya J, Ganguly S. The effect Chlorhexidine on plaque and gingival scores of 12-15-year-old school of honey on mucositis induced by chemoradiation in head and neck children: a randomized controlled field trial. J Indian Soc Periodontol cancer. J Indian Med Assoc 2012;110(7):453–456. 2018;22(1):34–39. [31] Motallebnejad M, Akram S, Moghadamnia A, Moulana Z, Omidi [43] Mavric E, Wittmann S, Barth G, Henle T. Identification and quantifi- S. The effect of topical application of pure honey on radiation-in- cation of methylglyoxal as the dominant antibacterial constituent of duced mucositis: a randomized clinical trial. J Contemp Dent Pract Manuka (Leptospermum scoparium) honeys from New Zealand. Mol 2008;9(3):40–47. doi:10.5005/jcdp-9-3-40. Nutr Food Res 2008;52(4):483–489. doi:10.1002/mnfr.200700282. [32] Rashad UM, Al-Gezawy SM, El-Gezawy E, Azzaz AN. Honey as topical [44] Deng J, Liu R, Lu Q, Hao P, Xu A, Zhang J, et al. Biochemical properties, prophylaxis against radiochemotherapy-induced mucositis in head antibacterial and cellular antioxidant activities of buckwheat honey and neck cancer. J Laryngol Otol 2009;123(2):223–228. doi:10.1017/ in comparison to manuka honey. Food Chem 2018;252:243–249. S0022215108002478. doi:10.1016/j.foodchem.2018.01.115. [33] Ceylan G, Yılmaz N, Nisbet HÖ, Nisbet C, Dede DÖ, Hoşgör F, et al. [45] Allaman I, Bélanger M, Magistretti PJ. Methylglyoxal, the dark side of Honey in wound care in complete denture wearers: a pilot study. glycolysis. Front Neurosci 2015;9:23. doi:10.3389/fnins.2015.00023. Mater Res Innov 2010;14(3):268–270. doi:10.1179/14330751 [46] Meeprom A, Sompong W, Suantawee T, Thilavech T, Chan CB, Adi- 0X12719005364945. sakwattana S. Isoferulic acid prevents methylglyoxal-induced protein [34] Halim DS, Mahanani ES, Saini R, Omar M, bt Ibrahi NR, Alam MK. A glycation and DNA damage by free radical scavenging activity. BMC comparison study on the effectiveness of local honey and salicylate Complement Altern Med 2015;15:346. doi:10.1186/s12906-015- gel for treatment of minor recurrent aphtous stomatitis. Int Med J 0874-2. 2013;20(6):770–772. [47] Hossain MA, Piyatida P, da Silva JAT, Fujita M. Molecular mecha- [35] Al-Khanati NM, Al-Moudallal Y. Effect of intrasocket application of nism of heavy metal toxicity and tolerance in plants: central role of manuka honey on postsurgical pain of impacted mandibular third glutathione in detoxification of reactive oxygen species and meth- molars surgery: split-mouth randomized controlled trial. J Maxillofac ylglyoxal and in heavy metal chelation. J Bot 2012;2012:872875. Oral Surg 2019;18(1):147–152. doi:10.1007/s12663-018-1142-z. doi:10.1155/2012/872875. [36] Lim YS, Kwon SK, Park JH, Cho CG, Park SW, Kim WK. Enhanced mu- [48] Kurahashi T, Fujii J. Roles of antioxidative enzymes in wound healing. cosal healing with curcumin in animal oral ulcer model. Laryngo- J Dev Biol 2015;3(2):57–70. doi:10.3390/jdb3020057. scope 2016;126(2):E68–73. doi:10.1002/lary.25649. [49] Choi CH, Park SJ, Jeong SY, Yim HS, Kang SO. Methylglyoxal accumu- [37] Sonis ST. Pathobiology of oral mucositis: novel insights and opportu- lation by glutathione depletion leads to cell cycle arrest in Dictyos- nities. J Support Oncol 2007;5(9 Suppl 4):3–11. telium. Mol Microbiol 2008;70(5):1293–1304. doi:10.1111/j.1365- [38] Babaee N, Hosseinkazemi H, Pouramir M, Khakbaz Baboli O, Salehi 2958.2008.06497.x. M, Khadir F, et al. Salivary oxidant/antioxidant status and hemato- [50] Almasaudi SB, El-Shitany NA, Abbas AT, Abdel-dayem UA, Ali SS, Al logical parameters in patients with recurrent aphthous stomatitis. Jaouni SK, et al. Antioxidant, anti-inflammatory, and antiulcer poten- Caspian J Intern Med 2016;7(1):13–18. tial of manuka honey against gastric ulcer in rats. Oxid Med Cell Lon- [39] Bardy J, Molassiotis A, Ryder WD, Mais K, Sykes A, Yap B, et al. A gev 2016;2016:3643824. doi:10.1155/2016/3643824.
DOI: 10.14218/ERHM.2019.00029 | Volume 5 Issue 1, March 2020 37 Corrigendum
Corrigendum: Acute Soft Skull Syndrome in an Adult Male with Sickle Cell Anemia in Sudan: A Case Report
Ziryab Imad Taha1,2*, Sulafa Eisa Mohammed3, Mohammed Elmujtba Adam Essa1,4*, Walaa Mohamed Elsid2, Mustafa Mohamed Ali Hussein1,4, Sherihan Mohammed Elkundi Osman4,5, Hussein Osman Ahmed4, Mutwaly Defealla Yousif1,4 and Abdelkareem A. Ahmed1,6*
1Department of Clinical Medicine, Medical and Cancer Research Institute (MCRI), Nyala, Sudan; 2Department of Internal Medicine, Faculty of Medicine, University of Bahri, Khartoum, Sudan; 3Department of Internal Medicine, National Ribat University, Khartoum, Sudan; 4Department of Internal Medicine, Faculty of Medicine, AlFashir University, AlFashir, Sudan; 5Department of Molecular Medi- cine, Institute of Endemic Diseases, University of Khartoum, Khartoum, Sudan; 6Department of Physiology and Biochemistry, Faculty of Veterinary Science, University of Nyala, Nyala, Sudan
Corrigendum on: Taha ZI, Mohammed SE, Essa MEA, Elsid WM, Hussein MMA, Osman SME, et al. Acute Soft Skull Syndrome in an Adult Male with Sickle Cell Anemia in Sudan: A Case Report. Explor Res Hypothesis Med. 2019;4(4):90–93. doi: 10.14218/ERHM.2019.00024.
The original version of the article contained an error in the last line of Table 1. It should appear as: Date Investigation Result Reference value 21/5/2019 Creatinine 1.5 mg/dL 0.7–1.4 mg/dL Instead of: Date Investigation Result Reference value 21/5/2019 Creatinine 0.5 mg/dL 0.7–1.4 mg/dL
The authors apologize for this error and state that the update does not change the results and conclusions originally reported. The original article has been updated.
*Correspondence to: Mohammed Elmujtba Adam Essa Adam, Medical and Cancer Research Institute (MCRI), Department of Clinical Medicine, Faculty of Medicine, AlFashir University, AlFashir, Sudan. Tel: +249907009378, E-mail: Awadali818@ya- hoo.com; Ziryab Imad Taha, Department of Internal Medicine, Faculty of Medicine, University of Bahri, Khartoum, Sudan, Tel: +249912129921, E-mail: Ziryab2008@ yahoo.com; Abdelkareem Abdallah Ahmed, Department of Physiology and Biochem- istry, Faculty of Veterinary Science, University of Nyala, Nyala, P.O. Box: 155 Nyala, Sudan. Fax: +249711833123, E-mail: [email protected] How to cite this article: Taha ZI, Mohammed SE, Essa MEA, Elsid WM, Hussein MMA, Osman SME, Ahmed HO, Yousif MD, Ahmed AA. Corrigendum: Acute Soft Skull Syndrome in an Adult Male with Sickle Cell Anemia in Sudan: A Case Re- port. Exploratory Research and Hypothesis in Medicine 2020;5(1):38. doi: 10.14218/ ERHM.2019.00024C.
Exploratory Research and Hypothesis in Medicine 2020 vol. 5 | 38
Copyright: © 2020 Authors. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.

| Loading… |
Publications > Journals > Exploratory Research and Hypothesis in Medicine > Article Full Text
Pericytes and the Neurovascular Unit: The Critical Nexus of Alzheimer Disease Pathogenesis?doi: 10.14218/ERHM.2020.00062 Alzheimer disease (AD) has been viewed as the quintessential neurodegenerative disorder, and has defied decades of extensive research to find safe and effective disease-modifying treatment approaches. However, over the last 15–20 years, a new focus has developed on the role of vascular dysfunction in AD. Key to this approach is the consideration of the non-neuronal cells and other structural elements comprising the neurovascular unit (NVU), in particular pericytes. This review will examine the role of pericytes and the NVU in AD pathogenesis and the manner in which they interact with traditional factors, such as neuroinflammation, amyloid-beta, and apolipoprotein E. Based on the emerging evidence of the unique properties of pericytes, these “forgotten cells” might represent a crucial nexus for solving the mysteries of AD.
Alzheimer disease (AD) is the pre-eminent enigma in clinical neuroscience. The disorder was first described in 1907 by the German psychiatrist and neuropathologist Alois Alzheimer. 1,2 To date, AD has resisted sustained efforts to develop effective disease-modifying therapies. From the first-generation cholinesterase inhibitor, tacrine, 3 to second-generation agents, including donepezil 4 and memantine, 5 to the development of monoclonal antibodies, such as solanezumab 6 and aducanumab, 7 investigators have failed to achieve satisfactory clinical endpoints. Some therapies have proved to be dangerous and serious adverse events have been observed, such as hepatotoxicity, 8 meningoencephalitis, 9 and cerebral edema. 10 From its initial description, AD has been conceptualized as representing the quintessential, pure neurodegenerative disorder. However, this perspective of AD belies its complexities and represents one of the false dichotomies that remain in the neurosciences. The most notable of these might be the outdated (yet still widely taught in medical schools and residency programs) distinction between “organic” versus “functional” disorders in neurology and psychiatry. As neuroscience researchers have expanded the knowledge base, the array of disordered functions at the molecular, cellular, behavioral, and cognitive levels has been revealed. The previous view of AD pits it against vascular dementia (VD). Diagnostic classification systems of dementia syndromes have traditionally assumed that a clear and reliable distinction exists between AD and VD. In recognition of its own complexities, the conceptualization of VD has undergone revision. The original terminology—multi-infarct dementia 11 —was changed to its current and more inclusive nosology, which reflects the fact that small-vessel disease contributes more frequently to cognitive decline than the accrual of large-vessel infarctions. 12 Throughout these changes in thinking and terminology, VD has continued to be viewed as the prime example of dementia caused by vascular pathology. Accumulating evidence has compelled researchers and clinicians to reconcile these findings and develop more refined and comprehensive hypotheses. It is now understood that the pathophysiology of AD is not limited to the classical view of neuronal degeneration. It also involves the full complement of glia, and cells that comprise the cerebral vasculature and other structural components, which are collectively referred to as the neurovascular unit (NVU). This review presents the evolution of knowledge on AD pathogenesis, beginning with the initial identification of the principal risk factor for sporadic AD, the discovery of a range of autosomal dominant mutations that cause familial AD, and the insights obtained from those rare cases. Then, it will discuss the increased prominence of the role of vascular mediators for AD and consider the specific processes through which the new data on the blood-brain barrier (BBB), pericytes, and other components of the NVU could provide a basis to integrate previously disparate factors into a comprehensive modern viewpoint. The classical view of Alzheimer disease pathophysiology: background and contextFactors that were initially identified as principal contributors to AD pathophysiology were amyloid-beta (Aβ) protein, tau protein, and apolipoprotein E (APOE). Each will be considered individually. APOE was the first established risk-mediating variable associated with sporadic AD. 13 In particular, the ε4 allele of APOE (APOE4) confers an elevated risk of sporadic AD. APOE4 operates dose-dependently. Heterozygosity for ε4 results in a 2–5 fold increased risk for the development of AD; ε4 homozygosity increases AD risk by approximately 5–10 fold. 13–15 In contrast, the APOE ε2 allele is protective, whereas the ε3 allele is neutral for AD risk. Despite the evidence that APOE confers substantial risk of AD, questions remained regarding the nature of its contribution to AD pathogenesis until recently. Ultimately, investigators determined that APOE interacts with Aβ by functioning as a chaperone or catalytic protein. APOE2 inhibits the polymerization of Aβ monomers to form toxic oligomeric species but APOE4 promotes β polymerization, 16,17 findings that are consistent with epidemiological studies of AD risk. In addition, APOE is an important component that regulates the clearance of Aβ from the extracellular space. The APOE4 variant is the least efficient at clearing Aβ. 18 Around the time that the importance of APOE was recognized, advances in genetic sequencing analyses allowed for the rapid identification and characterization of mutations associated with clinical presentations of early-onset familial AD (EOFAD). The first was identified in 1991, affecting amyloid precursor protein (APP), and accounts for approximately 15%–20% of all EOFAD cases. 19 The gene for APP resides on chromosome 21. Overexpression of APP explains the high incidence of AD among individuals with trisomy 21 — Down syndrome—due to the extra chromosome. 20 Symptom onset typically occurs in the late fourth to fifth decade of life among people affected by Down syndrome, which reflects the dose-dependent nature of APP translation. APP is a single-pass transmembrane protein that is involved in synapse production and maintenance and cellular signaling. 21 APP undergoes sequential cleavage by the gamma (γ)-secretase complex, which yields a specific set of polypeptide products. 22 The most important is Aβ, one of the principal markers of AD pathology. When APP undergoes γ-secretase processing, Aβ monomers are released primarily into the extracellular space. Aggregation of Aβ begins when amyloidogenic monomers attract and attach to additional colocated Aβ monomers to form oligomers. Aβ oligomers appear to be the most toxic form of Aβ, particularly the production of 40- and 42-residue isoforms via the amyloidogenic pathway. In addition to their propensity to aggregate, Aβ oligomers exert direct neurotoxic effects. 23 Accumulation of oligomers leads progressively to the production of insoluble Aβ fibrils, which self-propagate to form plaques 24 that are highly resistant to proteolysis and clearance. Regions of Aβ seeding then continue to propagate along interconnected neuroanatomic pathways, 25 in a manner similar to the process that occurs in prion diseases. 26 Aβ fibrils and mature plaques induce an inflammatory response that is mediated by astrocytes and activated microglia, which generate reactive oxygen species (ROS) and leads to oxidative stress and apoptosis. 27 In addition to increased production, dysfunctional clearance of Aβ has been implicated in AD pathophysiology. 28 Aβ clearance occurs via several mechanisms: glial endocytosis, proteolytic enzymatic degradation, transport across the BBB mediated by low-density lipoprotein family receptors, activation of the complement arm of the immune response, and passive drainage through interstitial perivascular spaces and specialized lymphatic vessels, referred to as the glymphatic system. 29,30 Events subsequent to Aβ deposition and aggregation—neuroinflammation, generation of ROS, excitotoxicity, tau hyperphosphorylation, microtubule disruption, and ultimately neuronal apoptosis—are referred to as the amyloid cascade hypothesis (ACH). The ACH initially came under criticism when studies showed that the correlation between cerebral Aβ burden and cognitive function was weak 31 and when evidence of neuronal injury was observed to precede Aβ deposition, such as in a murine model that over-expresses Aβ. 32 These findings raised doubts that the ACH was sufficient to account for AD pathophysiology. Accordingly, increased attention turned toward the other major marker of neuropathology in AD, tau protein. Microtubules are composed principally of the protein tubulin; together with tau, these proteins form a critical structural component of neurons. Tau is a soluble phosphoprotein that acts to stabilize microtubules, 33 and performs several other important functions which include providing intracellular axonal transport, regulating synaptic plasticity, and supporting the structural integrity of intraneuronal signaling pathways. 34 Under pathological conditions, tau becomes hyperphosphorylated, which decreases the affinity of tau toward tubulin and leads to the dissociation of tau from microtubules, thereby resulting in their destabilization and the formation of insoluble tau aggregates. These events contribute to structural degradation and lead to neuronal death. 35 The characteristic pathological marker of tau dysfunction is the flame-shaped neurofibrillary tangle that, when co-located with aggregations of Aβ, are referred to as neuritic plaques. In contrast to the weak relationship between Aβ levels and cognitive impairment, tau density is strongly correlated with dementia staging. 36 Subsequent research revealed that, although tau protein is an important mediator of AD pathogenesis, Aβ was a necessary condition for the development of the cognitive syndrome of AD. 37 For example, when Aβ was absent, there was no association between tau binding and hippocampal volume. In contrast, in the presence of Aβ, tau binding was greater and was associated with lower hippocampal volume. 38 Apart from modifications to the ACH, investigators believed that other more fundamental factors contributing to AD pathogenesis were likely to play an important role.
The early conceptualization of AD focused heavily on the neurodegenerative aspects of its pathophysiology. In addition, cerebrovascular disease (CVD) and related dementia syndromes, as exemplified by VD, were viewed as occupying two poles of a single spectrum, with neurodegeneration at one end and vascular pathology at the other. However, overlaps between AD and CVD have been uncovered; among the earliest research to demonstrate an overlap was the Rotterdam study. 39 Of note, Alois Alzheimer found 1 that neurofibrillary tangles co-existed with cerebral microvascular arteriosclerotic disease, 40 the first clue into the role of vascular pathology in neurodegeneration. Several factors identified as promoting risk for CVD have been shown to confer risk for AD. These include hypertension, hyperlipidemia, type 2 diabetes, and cigarette smoking. 41–45 These shared risk variables raised new questions about the processes that underlie the generation of AD pathology. This view coincided with an increased awareness of the relevance of changes that involve the cerebral microvasculature in AD, 42,46–47 and suggested that rather than existing as discrete entities, neurodegeneration and microvascular disease constituted two ends of a common spectrum of pathology. 48 Therefore, research into factors that mediate vascular damage in AD began. The most direct relationship between AD-type pathology and CVD manifests as an intramural deposition of Aβ within cerebral arteries, arterioles, and capillaries, as well as meninges, a condition that is called cerebral amyloid angiopathy (CAA). 49 The functional effect of Aβ infiltration is a weakening of vessel walls, which makes them susceptible to leakage, results in microhemorrhage, 50 and less frequently, rupture causing large-vessel hemorrhage. Accordingly, CAA is a major risk factor for stroke. 51 Although CAA and AD have overlapping features, they are considered distinct diagnostic entities. As with AD, most CAA cases are sporadic. Increased production and diminished clearance of Aβ have been implicated as underlying mechanisms of sporadic CAA. 29 CAA and AD share APOE4 as the primary risk factor for sporadic disease. However, there are important distinctions between these diseases. 52 Aβ40 is the predominant species deposited in CAA as opposed to Aβ42, which dominates in AD. In addition, APOE2 is protective in AD, but it is associated with an enhanced risk of blood vessel breakdown in CAA. Familial, or hereditary CAA (HCAA) is caused by a variety of rare autosomal dominant mutations. Six HCAA variants have been identified to date, and only the Dutch-type is caused by a mutation to APP on chromosome 21, as in familial AD. All other HCAA variants consist of mutations that code for amyloid proteins other than Aβ.
The BBB is composed of specialized endothelial cells that form the walls of all cerebral vessels. These cells are interconnected by tight junctions that strictly limit permeability bidirectionally and serve to compartmentalize the parenchyma from the blood. The BBB is a component of a larger, integrated set of elements that comprise the NVU, which will be discussed in the following sections. Dogma once held that the brain was immune-privileged; that is, it was assumed that innate and adaptive immune system components were sequestered from the brain by the BBB. However, the BBB is neither impenetrable nor impervious as previously understood. B lymphocytes migrate from the periphery across the BBB, 53 where they become activated and perform immune regulating functions within the central nervous system (CNS). 54 These activated microglia secrete interleukin-6 (IL-6) within the CNS compartment. 55 In addition to local cytokine production, circulating IL-1α and tumor necrosis factor-alpha (TNF-α) 46,56 enter the CNS via active transport. Complex links between neuroinflammation and BBB function have been discovered. Endothelial cells secrete pro-inflammatory cytokines. When exposed to Aβ40 species in vitro , cultured human brain endothelial cells respond by up-regulating gene expression for inflammatory cytokines IL-1β and IL-6. 57 Endothelial cells are primary regulators of Aβ influx into the brain 58 via receptors for advanced glycation end products (RAGE), 59 a process that contributes to the propagation of the inflammatory response. Pro-inflammatory cytokines IL-2 and IL-6, 60,61 regulate BBB permeability. Evidence indicates that age-related BBB disruption is more pronounced among individuals with cognitive dysfunction. 62 Similarly, levels of IL-1β, IL-6, and TNF-α in endothelial cells are higher in AD patients than in cognitively intact individuals. 63
The NVU consists of three major components: neurons, glia, and vascular cells. The vascular cells and their interactions with the other components have been the target of recent research. In addition to the endothelial cells, the basal lamina (also referred to as the basement membrane), microvascular smooth muscle cells, and pericytes comprise the vascular component of the NVU. The endothelium forms the first structural layer of the NVU and is in direct contact with plasma and other blood components. The second cellular layer of the NVU consists of the end feet of astrocytes, the basal membrane, which is an extension of astrocyte end feet, and the pericytes. Perictyes envelop cerebral capillaries, and precapillary arterioles and venules, and make anatomical contact with endothelial cells ( Fig. 1 ). 64 A host of disturbances that involve pericytes have been shown to occur during physiological aging. Disturbances of pericyte function might represent a unifying feature of disparate lines of evidence that accounts for age-related neuronal loss, neurodegeneration, and the pathophysiology of AD ( Fig. 2 ). 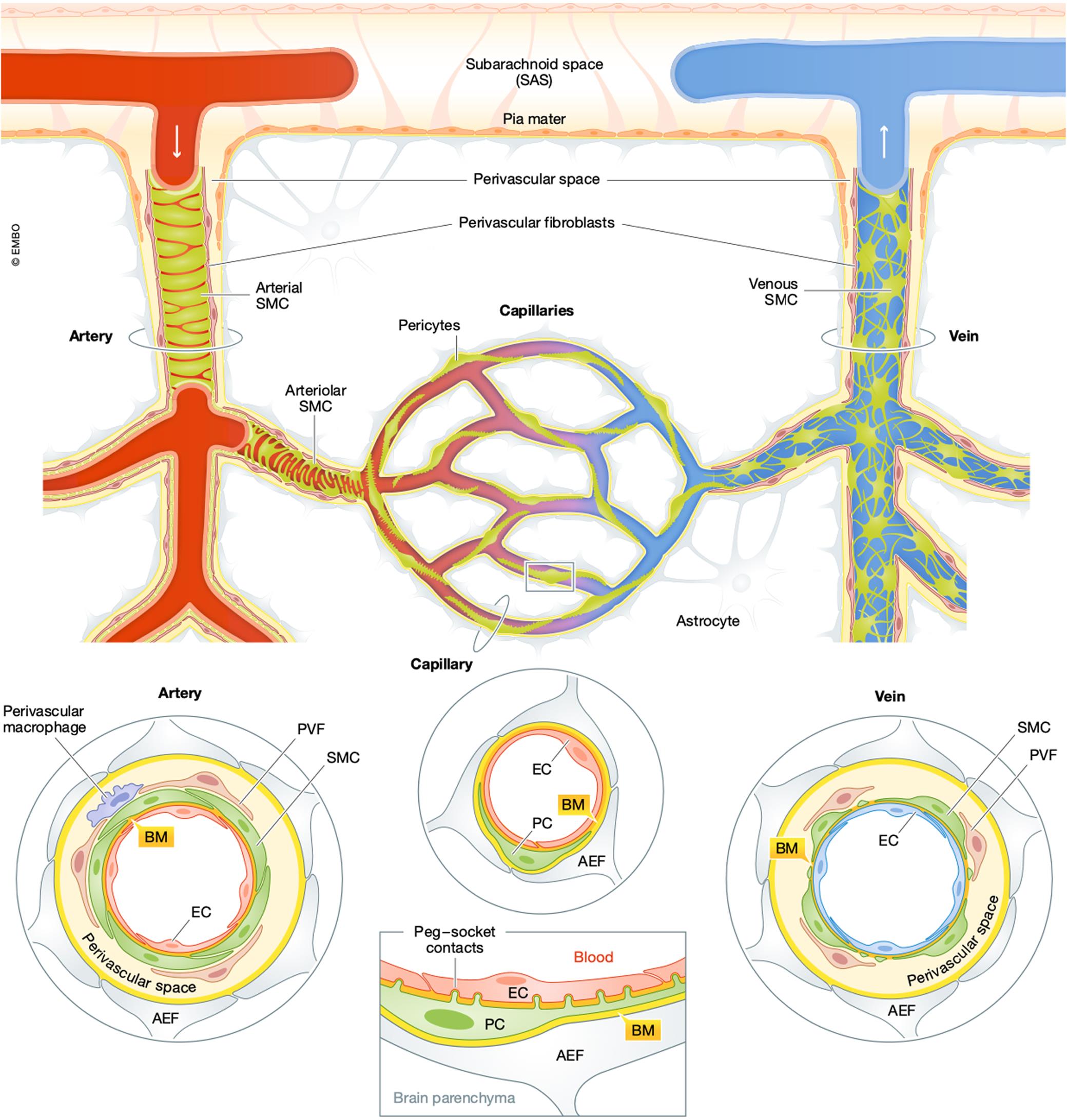 Penetrating arteriole branches into arterioles and capillaries, which then drain via venules into veins, returning to subarachnoid space. Schematic cross sections of arterial, capillary, and venous levels are shown, each with its vessel-associated cell types. The box depicts how pericytes establish direct connections with endothelial cells through peg-socket contacts. AEF, astrocyte endfoot; BM, basement membrane; EC, endothelial cell; PC, pericyte; PVF, perivascular fibroblast; SMC, smooth muscle cell. Source: Lendahl et al. 2019, Figure © EMBO. Reproduced under the terms of the Creative Commons Attribution 4.0 License which permits use, distribution, and reproduction in any medium, provided the original work is properly cited. 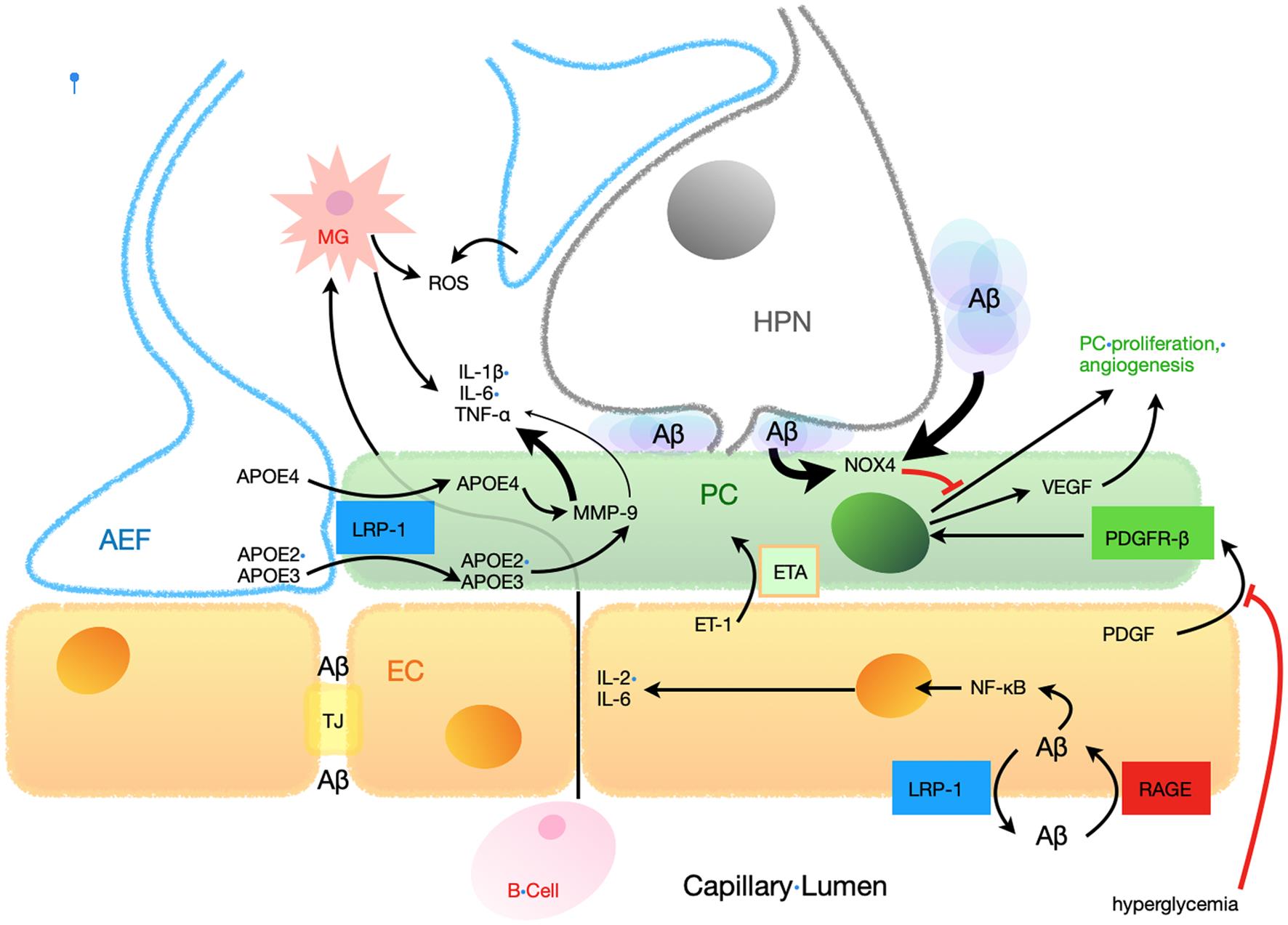 Microglia release ROS and inflammatory factors in the presence of Aβ aggregates. Accumulating extracellular Aβ is transported into endothelial cells and perictyes via RAGE, triggering transcription factors, such as NF-κB and MMP-9 to produce IL-x and TNF-α, setting up a feed-forward loop of accelerating Aβ production and neuroinflammation. APOE4 secreted by astrocytes is taken up by perictyes via LRP-1 and further promotes release of inflammatory factors, but APOE2 and APOE3 inhibit that pathway. Aβ induces a significant increase in NOX4 that inhibits pericyte proliferation and downstream angiogenesis. Aβ interferes directly with tight junction proteins, increasing BBB permeability. ET-1 secreted by endothelial cells binds to ETA receptors on perictyes, causing them to contract, leading to capillary constriction. Hyperglycemic states inhibit the interaction between PDGF secreted by endothelial cells and its receptor PGDFR-β found on perictyes, contributing to pericyte apoptosis, endothelial proliferation, capillary remodeling and BBB breakdown. Aβ, amyloid beta; AEF, astrocyte endfoot; APOEx, apolipoprotein Ex; BBB, blood-brain barrier; EC, endothelial cell; ET-1, endothelin-1; ETA, ET-1 type A receptor; HPN, hippocampal pyramidal neuron; IL-x, interleukin-x, LPR-1, low-density lipoprotein receptor-related protein-1; MG, microglial cell; MMP-9, matrix metalloproteinase-9 complex; NF-κB, nuclear-factor kappa B; NOX4, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4; PC, pericyte; PDGF, platelet-derived growth factor; PDGFR-β, platelet-derived growth factor receptor-beta; RAGE, receptor for advanced glycation end products; ROS, reactive oxygen species; TJ, tight junction; TNF-α, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor. Figure © Steven P. Cercy. Pericytes and agingOne consequence of physiological aging is the increased production and diminished scavenging of various ROS, in particular, the free radical superoxide anion (O 2 − ). Increased ROS levels affect many organ systems, including the cerebral microvasculature. The accumulation of ROS species gradually leads to mitochondrial dysfunction, DNA damage, and apoptosis. In addition, O 2 − breaks down nitric oxide (NO), which is a regulator of vascular tone in its role as a vasodilator. Aging is associated with reduced bioavailability of NO in multiple organ systems. 65 Perictytes are among the cells that are subject to the cumulative deleterious effects of age-associated ROS overexpression. Aging pericytes develop certain deviations of their ultrastructural elements; these include intracellular inclusions, pinocytotic vesicles, enlarged lipid granules, and mitochondrial abnormalities, all of which suggest cellular dysfunction, degeneration, or both. 66 Alterations of desmin protein filaments in pericytes have been observed, 67 which suggests a disturbance of their cellular structure. In human elders, pericytes become depopulated 68 and show a significant reduction in their area of capillary coverage. 69 The precise roles that pericytes play under optimal physiological conditions and during normal aging have not yet been fully elucidated. Research over the past 10–15 years, however, has revealed several key functions that these cells perform during the development and maintenance of cerebral microcirculation. Pericytes control microvascular blood flow directly via contraction, which causes capillary constriction 70 and, under pathological conditions, ischemia which might lead to localized hypoxemia. 71 They play an important role in angiogenesis 72 ; associated properties include migration and variability in phenotype, alignment, and endothelial cell contact. Pericytes guide and determine the direction and branching of newly formed blood vessels, 73 prevent vessel regression, 74 and promote endothelial cell survival. 75 Pericytes in the hypothalamus serve an important role in the regulation of glucose levels through insulin signaling. In a murine model, perictyes increased insulin sensitivity in hypothalamic neurons in a dose-dependent fashion; neither astrocytes nor vascular smooth muscle cells contributed to that process. 76 This finding suggests that hypothalamic pericyte loss might be implicated specifically in the dysregulation of insulin sensitivity, which is a fundamental aspect of diabetes. The molecular mechanisms that drive the formation and maintenance of the cerebral microvasculature are centered on intracellular signaling. Platelet-derived growth factor (PDGF) is secreted by endothelial cells and binds to platelet-derived growth factor receptor-beta (PDGFR-β), which is expressed by pericytes. This ligand-receptor complex activates signal transduction pathways that regulate migration and proliferation of pericytes toward endothelial cells that compose the vascular wall. 77 Experimental models of diabetic retinopathy that used pericyte-deficient mice revealed that chronic hyperglycemia resulted in diminished PDGFR-β signaling, which leads to pericyte apoptosis. 78 Pericyte loss induces endothelial cell proliferation with increased numbers of abnormal acellular capillaries, 74,79 rather than endothelial apoptosis. 80 Downstream events that are related to pericyte changes include significant capillary remodeling, which is characterized by vessel dilation and tortuosity, as well as basal lamina hypertrophy. 67,81 Subsequent studies revealed the particular importance of pericytes in microvascular regulation; they are the only cells of the NVU that express PDGFR-β to enable a response to PDGF. 80 Moreover, soluble PDGFR-β has been identified as a specific marker of pericyte injury. 82 In addition to PDGF, vascular endothelial growth factor is secreted by pericytes under hypoxic conditions, which stimulates proliferation and migration of additional pericytes. 83 Nuclear factor-kappa B (NF-κB), an important transcription factor that mediates the inflammatory response, is activated in a subpopulation of pericytes in response to exercise, which promotes angiogenesis. 84 In concert with their angiogenic properties, pericytes are crucial to maintain the integrity of the BBB. Vascular remodeling that occurs with pericyte apoptosis contributes to capillary destabilization, 67 which causes the breakdown of the BBB. Pericytes regulate the formation of endothelial tight junctions that modulate vascular permeability. Pericyte structural deterioration and apoptosis via any mechanism results in reduced contact with and coverage of endothelial cells 67,85 and the breakdown of the BBB, consequently permitting the influx and accumulation of serum proteins including the key coagulation macromolecules thrombin, fibrinogen, and fibrin—which thrombin cleaves from fibrinogen—as well as plasmin and hemoglobin. 80 Elevated thrombin in AD 86 contributes to neuronal loss, vascular injury, and cognitive impairment. 87 Fibrinogen exacerbates the neurotoxic effects of Aβ. 88 Moreover, fibrinogen leakage from injured cerebral microvessels activates resident microglia bearing CD-11b receptors, which then prune neuronal dendritic spines and whole dendrites. 89 Protein influx from all sources contributes to cerebral edema that might cause further capillary compression. 90 The NF-κB signaling pathway is a fundamental link between thrombosis and inflammation. 91 Free iron from degraded hemoglobin generates ROS. 92 All of these aberrant processes contribute to secondary neuronal degeneration. 90,93 Pericytes and AD pathophysiologyBased on the foregoing research findings, the issues to be determined are to what extent pericyte dysfunction contributes to or interacts with classical AD pathophysiological processes, and by what mechanisms those interactions occur. Pericyte degeneration begins early in AD, particularly in hippocampal neurons, which are among the first cells involved in AD pathology. Reductions in pericyte coverage correlate inversely with evidence of BBB permeability, 62 as measured by hippocampal levels of plasma proteins, including immunoglobulin G and fibrin. 94 Elevated soluble PDGFR-β in cerebrospinal fluid is an early biomarker of cognitive dysfunction, which is independent of Aβ and tau levels. 82,95 This shows that pericyte-specific dysfunction, which is characterized by vascular regression and disrupted vascular permeability, is associated with key attributes of AD pathology. 93 Pericytes, APOE and AβAs APOE and Aβ functions have been further elucidated, their relationships with pericytes and other elements of the NVU have come into focus. APOE2 and APOE3 are secreted by astrocytes and taken up by pericytes via low-density lipoprotein receptor-related protein-1 (LRP-1), where they inhibit a key pro-inflammatory pathway, the cyclophilin A-nuclear factor B-matrix metalloproteinase 9 complex. However, APOE4 promotes that pathway, 66,96 which directly increases pericyte injury 97 and impairs the formation of basement membranes. 98 Thus, APOE4 accelerates pericyte loss relative to carriers of APOE2 and APOE3 alleles. It is well known that in AD, cerebral capillary constriction is provoked by Aβ via the production of ROS. 99 However, the mechanisms of this and locus of action remained unclear. Aβ enhances the activity of nicotinamide adenine dinucleotide phosphate oxidases (NOXs) to form reactive superoxides. NOX4 is found in pericytes and endothelial cells. In a transgenic mouse model, Aβ increased NOX4 levels sevenfold, and the blockade of NOX4 prevented capillary constriction following the application of Aβ. In contrast, NOX2, which is found in macrophages, increased by only twofold in the presence of Aβ, and blocking NOX2 produced a substantially attenuated effect on capillary relaxation. Similar findings were elicited using human brain slices treated using comparable methods. 89 RAGE, which is expressed by neurons, glia, vascular smooth muscle cells, endothelial cells, and pericytes, has a crucial role in the influx of peripheral Aβ into the parenchyma. RAGEs bind to Aβ and a broad range of compounds that are referred to as advanced glycation end products (AGEs), also known as glycotoxins. These compounds are produced as a normal consequence of lipid and protein metabolism and are present in foods that are cooked at high temperatures. During physiological aging, AGEs accumulate progressively within all cells. However, in AD and diabetes, this normal process is accelerated. AGEs are present within Aβ plaques and neurofibrillary tangles of patients with AD. Accumulated AGEs contribute to the induction of oxidative stress by glial cells. 100 Increases in RAGE protein and RAGE-expressing microglia occur in AD, and correlate with disease severity. 101 Pericytes are crucial for regulating Aβ trafficking between the BBB and parenchyma via the LRP-1 and RAGE pathways described previously. They assist with Aβ clearance by phagocytosis and promote Aβ efflux from parenchyma via the LRP-1 pathway. 102 Despite this role, pericytes remain susceptible to Aβ toxicity. 103 In AD, the accumulation of Aβ within pericytes leads to dysmorphic remodeling 104 and apoptosis. In a transgenic model of pericyte-deficient viable mice overexpressing APP, degeneration and loss of pericytes resulted in elevated levels of Aβ40 and Aβ42 in the brain, as well as increased levels of tau not typically observed in this transgenic model. Reinforcement of this destructive cycle occurs when reduced cerebral blood flow leads to further synthesis and accelerating burden of Aβ, worsening pericyte apoptosis, progressive microvascular injury, and cognitive impairment. 102 Endothelin-1 (ET-1), which is a powerful vasoconstrictor that is secreted by endothelial cells, also appears to play an important role in Aβ-mediated vascular insult. Levels of ET-1 are elevated in individuals with AD, and are up-regulated by Aβ. 105 Subsequent research revealed that Aβ oligomers induced the release of ROS in pericytes, which enhances transcription and release of ET-1, 106 which then binds ET-1 type A (ETA) receptors. It is this ligand-receptor complex that causes pericytes to contract, which leads to capillary constriction, and results in hypoxic ischemia, local hypoglycemia, and neuronal loss in affected microregions. Constriction of capillaries worsens with increasing Aβ load. 107 Furthermore, BBB disruption is directly related to the influx of circulating Aβ into brain parenchyma. 94 The intramural deposition of Aβ within the walls of the cerebral vasculature that occurs in CAA is an important yet overlooked component of AD pathology. 108 Cultured human brain pericytes exposed in vitro to the Dutch-type mutation that affects the Aβ40 species is the predominant species that induces the formation of Aβ fibrils at the cell surface, which leads to pericyte degeneration. Insulin appears to inhibit Aβ fibril formation in a dose-dependent manner, further suggesting that insulin might be involved in the regulation of Aβ fibrillization and might mitigate Aβ-induced pericyte loss in AD. 109 In humans with CAA, dystrophic neurites are found within the perivascular spaces of capillaries in occipital association cortex, more so than in primary occipital cortex. In addition, the density of dystrophic neurites correlates with Aβ levels. 108 Relationships among insulin regulation, Aβ fibril formation, and the deposition of Aβ in intramural and perivascular spaces have implications for diabetes and the risk it poses for the development of sporadic AD. Aβ interferes directly with the integrity of endothelial tight junctions, which are regulated by pericytes and leads to an inappropriate increase in BBB permeability. 60 In addition, Aβ stabilizes fibrin clots; 110 increased fibrin deposition has been observed in vessels that are affected by CAA. 74 Of note, age-dependent vascular damage in pericyte-deficient mice precedes neuroinflammation, neurodegeneration, and consequent impairments in learning and memory. 80 This is consistent with cerebral hypometabolism outweighing the degree of volume loss among humans who are presymptomatic EOFAD mutation carriers. 111 Pericytes and tauIn addition to the deleterious influence of Aβ on the BBB that was identified recently, tau plays a significant but less understood role in the regulation of BBB integrity. In a transgenic mouse model, age-related increases in tau were associated with increased BBB permeability against erythrocytes, peripheral T lymphocytes, and immunoglobulin G. This effect was first observed in the hippocampus, 112 where the earliest tau aggregation occurs in AD. The migration of cells and molecules of the immune system, which is facilitated by a leaky BBB, helps to drive neuroinflammation. 113 However, detectable neuroinflammation and neurodegeneration do not occur until well after the initiation of BBB deterioration. 112 One mechanism through which tau might influence BBB permeability occurs through its interactions with tight junctions and adherens junctions between endothelial cells and their actin cytoskeleton proteins. Disrupting this association appears to result in tau-induced neurotoxicity. 114 Pericyte deficiency in a transgenic model of AD in mice led to increased phosphorylation of tau in the hippocampus and cortex. 102 Collectively, these processes could potentially cause a synergistic effect between pericyte loss, tau generation, Aβ accumulation, and neurodegeneration.
Based on recent and emerging evidence, pericytes present a novel opportunity to shift the focus of disease-modifying therapies away from failed efforts to break the link between amyloid deposition and cognitive decline. AD pathology appears to develop 15–20 years prior to symptom onset. One likely reason for the failure of available therapies could be that the development of pathology in AD has advanced too far to be amenable to treatment once individuals become symptomatic. Thus, the main thrust of AD research over the last decade has been to identify early markers of AD well in advance of symptom onset. 115 One potential approach entails a renewed focus on neuroinflammation. However, rather than using traditional NSAIDs, inflammation that is mediated by IL-1β, IL-6, and other pro-inflammatory cytokines could be specifically targeted in an attempt to block Aβ-mediated generation of ROS in pericytes. 116 The Aβ-RAGE-cyclophilin A-nuclear factor B-matrix metalloproteinase 9 cascade, which is an important mechanism of AD pathogenesis via BBB disruption might be a promising potential target for AD therapies. 60 Agents that inhibit the release of ET-1 or antagonize the ETA receptors on pericytes are worthy of consideration. In a murine model, C-type natriuretic peptide reversed the effects that were mediated by ET-1 and interrupted Aβ-provoked capillary constriction. 105 Depletion of fibrinogen reduces CAA and cognitive decline in transgenic AD mice, 110 and therefore, could represent an important therapeutic target. Finally, because of their unique properties, in particular, their ability to withstand hypoxic conditions, perictyes should be considered as a viable option for cell-based therapies. In one study, mesenchymal stem cells that had differentiated into pericytes were stereotactically injected into the brains of mice genetically modified as an animal model for AD. The mice showed improved microcirculation and reduced levels of insoluble cortical and hippocampal Aβ, 117 which suggested that pericyte implantation might provide a novel approach to the management of AD. Additional data indicate that pericytes harvested from temporal neocortex and cerebral ventricular zone proliferate readily in culture and are robust under storage. 118,119 In summary, research into the NVU, in particular, the so-called “forgotten” 120 cell—the pericyte—has yielded valuable insights into the pathogenesis of AD. With this knowledge, there is potential to develop promising avenues for treating what is perhaps the most relentless and refractory disease known to neuroscience.
Converging evidence has revealed that vascular pathology, rather than reflecting collateral or ancillary damage, is central to the pathophysiology of AD. Moreover, the markers for AD pathology that had been considered evidence of pure neurodegeneration, are mediators of vascular pathology. Pericytes, with their unique attributes as a locus for the interaction of multiple factors that contribute to neuronal integrity and stability, are perhaps positioned as a crucial nexus to resolve the pathophysiology of AD and establish a basis for the first effective disease-modifying interventions.
amyloid-beta amyloid cascade hypothesis Alzheimer disease advanced glycation end products apolipoprotein E amyloid precursor protein blood-brain barrier cerebral amyloid angiopathy cerebrovascular disease endothelin-1 interleukin low-density lipoprotein receptor-related protein-1 nicotinamide adenine dinucleotide phosphate oxidase neurovascular unit platelet-derived growth factor platelet-derived growth factor-beta receptor for advance glycation end products reactive oxygen species tumor necrosis factor
AcknowledgementConflict of interest. The author has no conflicts of interest to declare. Authors’ contributionsThis manuscript is the sole work of SPC.
Article Options
Table of ContentsCite this article. Cercy SP. Pericytes and the Neurovascular Unit: The Critical Nexus of Alzheimer Disease Pathogenesis? Explor Res Hypothesis Med . 2021;6(3):125-134. doi: 10.14218/ERHM.2020.00062. Copied to clipboard  Share this Article


share this! July 1, 2024 This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility: fact-checked Factors influencing extravasation of newborn intravenous infusionsby Xia & He Publishing Inc. 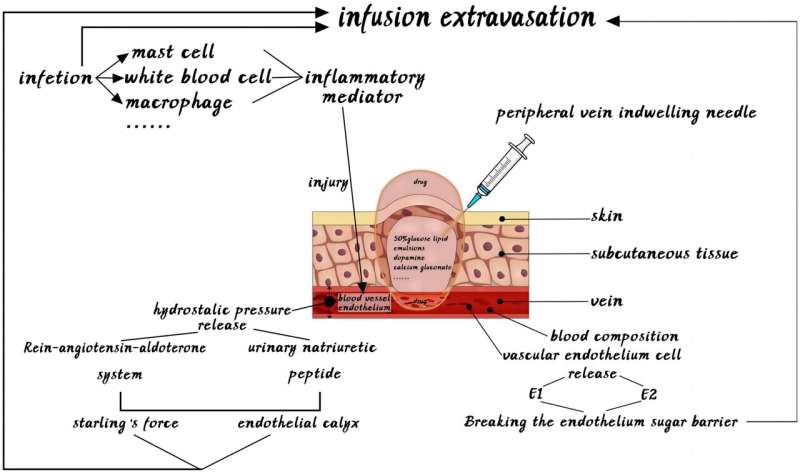 Newborns, particularly premature infants, are highly vulnerable during intravenous infusion treatments due to their low immunity, inability to express discomfort, and fragile veins. Extravasation, the leakage of infusion fluids into surrounding tissues, is a significant concern that can lead to severe complications, including tissue necrosis, infection, and even death. This essay reviews the intrinsic and extrinsic factors contributing to neonatal infusion extravasation, emphasizing the importance of early detection and intervention to prevent severe outcomes. The research is published in the journal Exploratory Research and Hypothesis in Medicine . Chemical stimulation of blood vessels due to intravenous drugs is a primary factor causing extravasation. Drugs with high osmotic pressure, such as 50% glucose and lipid emulsions, can lead to dehydration and necrosis of tissue cells, causing sterile inflammation. Vasoactive drugs like dopamine, adrenaline, and noradrenaline cause peripheral blood vessel constriction, leading to tissue ischemia and increased vascular permeability , which heightens the risk of extravasation. Furthermore, drugs like calcium gluconate, used to prevent metabolic bone disease in neonates, can cause lysosomal rupture, autolysis, and inflammatory changes, leading to tissue damage and necrosis. The infusion rate and temperature significantly affect the likelihood of extravasation. Rapid infusion rates can overwhelm local blood vessel capacity, causing phlebitis and extravasation. Long-term infusion of irritant solutions should be avoided, with continuous infusions limited to less than two hours. Additionally, maintaining an appropriate infusion temperature is crucial, as excessively low temperatures can cause local blood vessel spasms and increase vascular permeability, thereby raising the risk of extravasation. The type of infusion tools and the chosen site for infusion also influence the risk of extravasation. Peripheral intravenous catheters are preferred over disposable steel needles, as they cause less irritation to blood vessels and can remain in place for 3–4 days, reducing the need for repeated punctures. However, the risk of extravasation increases with prolonged catheter placement. Infants and young children are more prone to extravasation in the lower limbs than the upper limbs , and important veins are more sensitive to irritating drugs, increasing the likelihood of venous inflammation and extravasation. The proficiency of nurses in using peripheral intravenous catheters, their knowledge of intravenous infusion, and their clinical experience are critical in preventing extravasation. Inexperienced nurses may struggle with vein selection and control of puncture depth, leading to repeated punctures and mechanical damage to blood vessels. Factors such as a weak sense of responsibility, inadequate inspection of infusion sites, and insufficient inspection frequency can further contribute to extravasation risks. Emotional stability and psychological qualities of nurses, influenced by environmental factors like instrument alarms and infant crying, also play a significant role in the success rate of punctures. Certain neonatal diseases can increase the likelihood of intravenous fluid extravasation. Conditions such as acute respiratory distress syndrome , meconium aspiration pneumonia, neonatal asphyxia, and pulmonary hypertension can cause lesions in blood vessels or subcutaneous tissues, enhancing the risk of extravasation. These diseases involve inflammatory processes, changes in vascular permeability, and structural defects in skin proteins, all of which can contribute to the extravasation of intravenous fluids. Understanding the various factors influencing neonatal infusion extravasation is crucial for developing effective prevention and intervention strategies. By addressing chemical stimulation, infusion rates, temperature management, appropriate selection of infusion tools and sites, and improving nurse training and disease management, health care providers can significantly reduce the incidence of extravasation and improve outcomes for neonates undergoing intravenous therapy. Explore further Feedback to editors  New cancer treatment slows progression of aggressive neuroendocrine tumors, study finds4 hours ago  Why schizophrenia and apathy go hand in hand6 hours ago  Study: Women veterans at higher risk for repeat suicide attempts Childhood obesity tied to double the risk of dengue hospitalization7 hours ago  Financial incentives found to double smoking cessation rate for people with socioeconomic challenges Study discovers connection between between heart and brain in KBG syndrome8 hours ago  Clinical trial could lead to new 'gold standard' test for prostate cancer detection Serendipity reveals new method to fight cancer with T cells The path to Parkinson's disease: All roads lead to the nigrosome9 hours ago  Researchers discover a new face-detecting brain circuit10 hours ago Related Stories 3D bioprinting sheds light on why blood vessel curvature may foster brain cancer metastasisJan 19, 2024  Study uncovers mechanism behind primary graft dysfunctionNov 18, 2022  Optum joins FDA, manufacturer in recalling infusion pumps that killed one patientMay 31, 2024  Study shows amino acids reduce acute kidney injury after cardiac surgeryJun 18, 2024  Iron infusion before bowel surgery reduces need for blood transfusionNov 23, 2023  Nanomedicine research aims to transform treatment of aortic aneurysmsMar 4, 2024 Recommended for you Common respiratory infections may have protected children from COVID-19, study suggestsJul 1, 2024  Umbilical cord milking does not appear to increase risk of neurodevelopmental delay in non-vigorous infants Hormones associated with body composition during pregnancy linked to infants' mental health  Pandemic newborns in India more likely to have lower birth weight, shows study Kids given 'digital pacifiers' to calm tantrums fail to learn how to regulate emotions, study findsJun 28, 2024 Let us know if there is a problem with our contentUse this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ). Please select the most appropriate category to facilitate processing of your request Thank you for taking time to provide your feedback to the editors. Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages. E-mail the storyYour email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form. Newsletter sign upGet weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties. More information Privacy policy Donate and enjoy an ad-free experienceWe keep our content available to everyone. Consider supporting Science X's mission by getting a premium account. E-mail newsletter
Retinal peri-arteriolar versus peri-venular amyloidosis, hippocampal atrophy, and cognitive impairment: exploratory trial
Acta Neuropathologica Communications volume 12 , Article number: 109 ( 2024 ) Cite this article 110 Accesses 1 Altmetric Metrics details The relationship between amyloidosis and vasculature in cognitive impairment and Alzheimer’s disease (AD) pathogenesis is increasingly acknowledged. We conducted a quantitative and topographic assessment of retinal perivascular amyloid plaque (AP) distribution in individuals with both normal and impaired cognition. Using a retrospective dataset of scanning laser ophthalmoscopy fluorescence images from twenty-eight subjects with varying cognitive states, we developed a novel image processing method to examine retinal peri-arteriolar and peri-venular curcumin-positive AP burden. We further correlated retinal perivascular amyloidosis with neuroimaging measures and neurocognitive scores. Our study unveiled that peri-arteriolar AP counts surpassed peri-venular counts throughout the entire cohort ( P < 0.0001), irrespective of the primary, secondary, or tertiary vascular branch location, with a notable increase among cognitively impaired individuals. Moreover, secondary branch peri-venular AP count was elevated in the cognitively impaired ( P < 0.01). Significantly, peri-venular AP count, particularly in secondary and tertiary venules, exhibited a strong correlation with clinical dementia rating, Montreal cognitive assessment score, hippocampal volume, and white matter hyperintensity count. In conclusion, our exploratory analysis detected greater peri-arteriolar versus peri-venular amyloidosis and a marked elevation of amyloid deposition in secondary branch peri-venular regions among cognitively impaired subjects. These findings underscore the potential feasibility of retinal perivascular amyloid imaging in predicting cognitive decline and AD progression. Larger longitudinal studies encompassing diverse populations and AD-biomarker confirmation are warranted to delineate the temporal-spatial dynamics of retinal perivascular amyloid deposition in cognitive impairment and the AD continuum. The vascular contribution to cognitive impairment and Alzheimer’s disease (AD) is increasingly being recognized [ 1 ]. Multiple studies have emphasized the role of various vascular derangements in neurodegeneration [ 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 ], with disruption of the blood–brain barrier proposed as an early biomarker of cognitive dysfunction in AD [ 1 ]. Given the similar pathophysiology shared between the blood-retina barrier and the blood–brain barrier [ 11 , 12 , 13 , 14 ], and the feasibility of non-invasive and reproducible high-resolution imaging of the retina, multiple static and dynamic retinal vascular biomarkers were investigated across the AD spectrum [ 11 , 15 , 16 , 17 , 18 , 19 ]. Retinal vascular pathology in AD was characterized using techniques such as color and autofluorescence fundus photography, optical coherence tomography angiography [ 20 , 21 , 22 ], fluorescein angiography [ 23 , 24 ] in humans, and retinal pericyte imaging in animal models [ 25 ]. Fundus photography has revealed several vascular abnormalities in AD, including venular narrowing, diminished vascular branching, increased tortuosity, and decreased arterial fractal dimension [ 19 , 26 , 27 , 28 , 29 ], leading to the recent development of retinal photography-based deep learning algorithms for cost-effective AD screening in community settings [ 30 , 31 ]. Furthermore, it has been proposed that retinal vascular tortuosity could improve the detection of cerebral amyloid status as determined by 18F-florbetaben PET [ 32 ]. Amyloid β-protein (Aβ) is an early core biomarker of AD, a prerequisite for AD diagnosis, and is the target of AD-specific therapies [ 33 ], including the recently approved anti-Aβ monoclonal antibodies that bind with high affinity to Aβ plaque and/or soluble protofibrils [ 34 , 35 ]. These immune-based therapies have demonstrated efficacy in patients with mild cognitive impairment (MCI) and mild AD dementia [ 34 , 35 , 36 , 37 ]. Accurately detecting vascular-associated amyloid deposition in early AD remains an unmet need in current clinical practice. The cost-effective and non-invasive detection of retinal amyloid burden carries the potential for early AD identification and monitoring [ 2 , 38 , 39 , 40 ]. Thus, several retinal amyloid imaging methodologies have been recently developed [ 41 , 42 ], including scanning laser ophthalmoscopy (SLO) [ 17 , 39 , 42 , 43 , 44 , 45 , 46 ] and hyperspectral retinal imaging [ 13 , 39 , 40 , 41 ]. SLO fluorescence imaging following curcumin administration provides an opportunity to visualize and quantify not only the Aβ plaques (AP) but also the retinal arteries and veins [ 17 , 44 , 45 , 47 ]. We have previously reported the specificity of curcumin for retinal Aβ (especially for AD-linked Aβ 42 alloforms) and its optical signature when bound to retinal Aβ deposits, both ex vivo and in vivo [ 41 , 42 ]. Additionally, we have demonstrated that retinal venular tortuosity, combined with retinal mid-periphery AP count, can discriminate between patients with normal and impaired cognition [ 17 ]. Moreover, our group has identified significant accumulation of vascular and perivascular Aβ deposition in postmortem retinas of MCI (due to AD) and AD patients [ 16 , 42 , 48 ]. We further showed that increased histopathological arteriolar Aβ 40 deposition and tight junction loss in the inner blood-retinal barrier of prodromal and symptomatic AD patients are strongly correlated with the severity of cerebral amyloid angiopathy (CAA) and other AD-related brain neuropathological changes [ 49 ]. While various vascular alterations have been described in retinal imaging studies in AD, the specific AP localization in the vascular-adjacent zones (termed here as perivascular AP) during preclinical or minimally symptomatic AD stages, and how these evolve with AD progression, remain unclear. To elucidate the retinal perivascular AP deposition in early AD, we conducted a retrospective exploratory analysis of a cohort of subjects with normal or impaired cognition who underwent retinal curcumin fluorescence amyloid imaging with SLO. Our aim was to study the topographic relationship between perivascular AP burden and retinal vessel types, determining whether retinal AP distribution is predominantly peri-arteriolar or peri-venular, proximal to the first-order vessels or further distal following secondary or tertiary vessel bifurcation. We hypothesized that an increased perivascular retinal AP burden would correlate with cognitive decline, and similar to the AD brain, retinal AP deposits would be more abundant in the peri-arteriolar space than in the peri-venular space. We compared the perivascular AP distribution in subjects with and without cognitive impairment and assessed the correlation between retinal peri-arteriolar and peri-venular AP counts with neuroimaging markers of neurodegeneration. Study designThis is a retrospective retinal imaging investigation of a cohort study approved by the Cedars-Sinai Medical Center Ethics Committee and Institutional Review Board (IRB protocol number 00052349), which was conducted in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. The prospective parent study enrolled 34 subjects aged over 40, with subjective cognitive decline, that provided written informed consent prior to enrollment. All subjects underwent retinal imaging with a confocal scanning laser ophthalmoscope (SLO Retia™, CenterVue SpA; Fig. 1 A), utilizing blue light to excite curcumin emission for obtaining fluorescent images of the retina (Fig. 1 B), following a previously reported study design [ 17 , 42 , 44 ]. In addition, patients underwent brain magnetic resonance imaging (MRI), fludeoxyglucose-18 positron emission tomography (PET) and a comprehensive neuropsychometric evaluation (detailed below). Exclusion criteria for the parent retinal imaging study included a self-reported history of glaucoma (to avoid ocular dilation-triggered angle-closure), allergy to mydriatic eye drops, curcumin, or vitamin E. In the current retrospective study, we excluded subjects diagnosed with other neurodegenerative disorders (e.g. frontotemporal dementia) and subject with poor retinal image quality. Overall, this study included 28 subjects, with either normal cognition and normal neuroimaging (9), MCI (16; 6 amnestic MCI, 9 multidomain MCI, 1 non-amnestic MCI), or probable AD (3).  Retinal vascular amyloid imaging. A Pipeline of fluorescent imaging using Retia® SLO, Afina™ cloud storage, and a fully automated image processing and analyses. B Representative retinal fluorescent image of before and after image processing. C Representative retinal image of an AD patient demonstrating putative retinal amyloid deposits (white) along the blood vessels. D Representative histopathological images from a confirmed AD patient, that did not undergo retinal imaging pre-mortem. Postmortem retinal flatmount immunolabeled with anti-c monoclonal antibody (12F4; brown) and peroxidase-base 3,3′ diaminobenzidine (DAB) immunostaining. Typical Aβ plaque structures and Aβ deposition along and inside blood vessels (red arrows) and ‘plaque-free’ regions of retinal blood vessels (blue arrows). E High magnification images of perivascular and vascular Aβ deposits in retinal flatmounts of an AD patient that did not undergo retinal imaging pre-mortem Standard neuropsychological testing was administered by a licensed neuropsychologist (DS) and included the Montreal Cognitive Assessment (MOCA), global Clinical Dementia Rating (CDR), general cognitive (ACS-test of Premorbid Functioning), and specific cognitive domain assessments: attention and concentration (Wechsler Adult Intelligence Scale (WAIS)-IV); verbal memory (California Verbal Learning Test (CVLT) II, Wechsler Memory Scale (WMS)-IV, and Logical Memory II); non-verbal memory (Rey Complex Figure Test and Recall (RCFT) 30 min, and Brief Visuo-Spatial Memory Test Revised (BVMT-R) Delayed Recall); language [Fluency-Letter (FAS) and Fluency-category (animals)]; visuo-spatial ability (Rey Complex Figure Test and Recognition Trial (RCFT) Copy); speed of information processing (Trails A and B); and symptom validity and functional status (SF-36 Physical Component Score (PCS) and Mental Component Score (MCS)). The subject’s emotional status was assessed using the Beck Depression Inventory II, Geriatric Depression Scale, and Profile of Mood State/Total Mood Disturbance. All subjects underwent 3 Tesla non-contrast structural MRI. Brain volumetric analysis was conducted using automated NeuroQuant software [ 50 ], and the following parameters were collected: total intracranial volume (ICV) (cm 3 ), hippocampal volume (HV) (cm 3 ), and inferior lateral ventricle volume (ILVV) (cm 3 ). The number and volume of white matter hyperintensities (WMHI) were measured using SPIN Software (SpinTech, Bingham Farms, MI). Post-mortem human retinal flatmounts preparation and Aβ immunostainingTwo eyes from a deceased AD female patient, provided by the Rush University’s Alzheimer’s Disease Research Center (ADRC ORA# 18011111), were collected within 8 h postmortem. Eyes were punctured once at the limbus and then preserved in Optisol-GS media (Bausch & Lomb, 50006-OPT, Sterile) before storage at 4 °C for up to 24 h. Retinas were isolated from whole eyes and the vitreous was removed before flatmount preparation. The retinas then underwent antigen retrieval followed by immunostaining using anti-Aβ 42 antibodies (Abs) and peroxidase-base 3,3’ diaminobenzidine (DAB) labeling, according to a previously described method [ 13 , 42 , 51 ]. In detail, fresh eyes were dissected over ice, with removal of the cornea, anterior/posterior chamber, pupil, and lens to create the eyecup. The vitreous body was thoroughly removed manually throughout this process. Eyecups were then washed with 1X PBS solution and fixed in 2.5% paraformaldehyde (PFA). Retinas were dissected, separated from the choroid, and prepared into flatmounts by dividing them into four quadrants (superior, temporal, inferior, and nasal). Next, retinal flatmounts were washed in 1X PBS then treated with target retrieval solution at 97 °C for 1 h (pH 6.1; Dako #S1699) and washed one more time in 1X PBS. The tissues were next treated with 3% hydrogen peroxide (H 2 O 2 ) for 12 min and washed in 1X PBS. Thereafter, the tissues were immunostained using a Vectastain Elite ABC HRP kit (Vector Laboratories, USA #PK-6102, Peroxidase Mouse IgG), a sensitive avidin/biotin-based peroxidase enzyme system, according to the manufacturer’s instructions. Briefly, tissues were first treated with blocking solution and permeabilized with 0.2% Triton® X-100 (Sigma #T8787) for 30 min at room temperature. They were then incubated with primary mouse anti-human Aβ 42 monoclonal Abs (clone 12F4; recognizing and specific for the 42aa C-terminus of Aβ; 1:500 in PBS with 10% permeabilization/blocking solution; Biolegend #805501). Slides were covered with parafilm and incubated for 48 h at 4 °C. Following incubation, tissues were washed with 1X PBS, followed by incubation with a biotinylated secondary antibody for 30 min, and then ABC reagent incubation for another 30 min. Aβ 42 immunoreactivity was detected using the DAB plus substrate chromogen system (Dako #K3467). Afterward, tissues were mounted with Paramount aqueous mounting medium (Dako #S3025). Routine controls were processed using an identical protocol, omitting the primary antibody to assess nonspecific labeling. Bright-field images were acquired using a Carl Zeiss Axio Imager Z1 fluorescence microscope (Carl Zeiss MicroImaging, Inc.) equipped with ApoTome, AxioCam MRm, and AxioCam HRc cameras (Fig. 1 D–E; Supplementary Fig. 1). Histological studies were performed under the IRB protocol Pro00055802 at Cedars-Sinai Medical Center. Retinal image processing and analysisThe retinal AP imaging analysis was conducted on the left and right eyes following our previously reported methodology in a predominantly Caucasian population [ 44 ] which has recently been replicated in a Japanese population [ 45 ] and the A4 trial [ 39 ]. Noninvasive retinal amyloid images were acquired in predetermined geometrical regions using the Retia™ SLO, followed by fully automated Afina™-based cloud storage and NeuroVision imaging-based image processing output (Fig. 1 A). The researchers responsible for the retinal image processing and AP quantification were blinded to the patients’ clinical characteristics. Retinal curcumin fluorescence imaging revealed multiple diffuse retinal hyperfluorescence spots (APs), which became more discernable after image processing (Fig. 1 B, white dots). Quantification of perivascular AP (Fig. 1 C) was performed in retinal fluorescence images obtained from the supero-temporal quadrant of the left eye. To achieve this, retinal arteries and veins were manually identified by trained observers based on their vessel diameter (vein > artery [ 52 , 53 ]), location, and morphology, and traced using Adobe Photoshop CC 2020. Vessels originating from the optic disc were defined as the "primary (1°) main branch" for each venular and arteriolar network. After the defined main primary branch bifurcation, subsequent divisions were labeled as "secondary (2°) main branches" for all venules and arterioles. Following these main secondary arterioles and venules split, the subsequent divisions were termed "tertiary (3°) branches" (Fig. 2 C–D). Any protruding vessels from the main branches were categorized as 'small.' For example, a protruding vessel from the main primary branch was labeled "primary branch—small". Similarly, if they protruded from the main secondary branch, they were denoted as "secondary branch—small" (Fig. 2 C’–D’). Since tertiary vessels and their branches had relatively equal diameters, all vessels following the second bifurcation were grouped as tertiary branches. For analytical purposes, main branches along with protruding vessels were combined into one measurement, referred to as primary branches and secondary branches, encompassing both main and small primary and secondary branches, respectively.  Retinal peri-venular and peri-arteriolar amyloid plaque distribution. A – B Representative retinal fluorescent fundus image illustrating retinal curcumin-positive amyloid hyperfluorescent plaques in the left eye supero-temporal quadrant ( A , magnification B ). Illustration of the primary, secondary, and tertiary retinal venular ( C ) and arteriolar ( D ) branches. Magnifications of the peri-venular ( C’ ) and peri-arteriolar ( D’ ) area used for the amyloid plaque (AP) quantification; boundary zone delineated by dotted lines producing a perivascular area of one equivalent vessel diameter on either side. E Quantitative analysis of retinal perivascular area stratified by venules (V) and arterioles (A) showing no significant difference between the total area for each vessel type in the analyzed supero-temporal region. F – J Quantitative analyses of retinal perivascular AP count stratified by V versus A in the total branches ( F ), in females ( G ), males ( H ), individuals with normal cognition ( I ) and impaired cognition ( J ). Individual data points are shown. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 by paired two-tailed Student’s t test. NC, Normal cognition; IC, Impaired cognition; 1°V, primary venular branch; 2°V, secondary venular branch; 3°V tertiary venular branch; 1°A, primary arteriolar branch, 2°A, secondary arteriolar branch; 3°A, tertiary arteriolar branches. Color code vessel type: blue—peri-venular; red—peri-arteriolar Starting within the highlighted imaging field and nearest to the optic disc, we measured the diameter of the primary vessel at pre-set intervals along its length using the line segment tool. The diameter of each vessel was calculated by averaging these measurements from the pre-set intervals, as well as the most proximal and distal segments. Afterwards, we manually traced through the middle of the vessel using the curvature tool, prioritizing accuracy. The diameter of the drawn external perivascular line was set to automatically display three times the diameter of the vessel, producing a “perivascular area” equivalent to one vessel diameter on either side. We adjusted the line opacity to 40% to enable the detection of plaque positive signals within this perivascular area. Plaques that touched the border were counted as perivascular, even if their entire body was not within the boundary. These steps were repeated for all venules and arterioles to delineate all peri-arteriolar and peri-venular areas. We counted all hyperfluorescent AP positive signals falling within the boundaries of the perivascular areas, categorizing them by (1) vessel type (peri-venular versus peri-arteriolar), and (2) location (primary vessel, primary branch, secondary vessel, secondary branch, or tertiary vessel). Retinal images were independently analyzed by two graders (JD and MSM) to ensure reliability. Any discrepancies were discussed with a senior grader (OMD, YK, or MKH) for final clarification. Statistical analysisDescriptive statistics were performed, and continuous demographic and clinical data are presented as the mean ± standard deviation (SD) in the text and tables. Comparisons between peri-arteriolar and peri-venular AP counts utilized paired and unpaired, two-tailed tests (unadjusted P) or one-way analysis of variance (ANOVA), corrected P values after the Tukey’s multiple comparisons posttest. Subjects were divided into three groups according to the Clinical Dementia Rating (CDR) (0.5, questionable impairment; 1, mild cognitive impairment; and 2, moderate cognitive impairment) [ 54 ]. They were also dichotomized using a MOCA cut-off of 26 [ 55 ] and neuropsychometric diagnosis (normal cognition versus impaired cognitive performance). D'Agostino-Pearson and Shapiro–Wilk normality tests were conducted to assess whether continuous variables followed the normal distribution curve. Data set that passed at least one of the tests was considered to have a Gaussian distribution (Supplementary Table 1). Normally distributed variables were compared using two-tailed paired or unpaired Student’s t -test. For non-normally distributed independent variables, we utilized the two-tailed unpaired Mann–Whitney test. Fold changes (FC) and corresponding 95% confidence intervals (CI) were calculated (Tables 2 and 3 ). Differences in continuous variables among levels of CDR were examined through ANOVA with Tukey’s test applied to correct for multiple comparisons (Supplementary Table 4). Pearson’s r correlation analysis was conducted to investigate the relationship between retinal perivascular AP count and cognitive and brain imaging volumetric measures. The scatterplot graphs present the null hypothesis of a pair-wise Pearson’s r with the unadjusted P values that indicate the direction and strength of the linear relationship between two variables. Pearson’s correlations that remained significant after Holms-Bonferroni multiple comparison correction were indicated in Tables 4 and 5 . All statistical analyses were performed using GraphPad Prism 9 or 10. Significance was defined as a P-value less than 0.05. A total of 34 patients underwent retinal and brain imaging, as well as neuropsychometric evaluation. Figure 1 C depicts a human retinal curcumin fluorescence imaging revealing APs putatively located along retinal blood vessels. Additionally, a representative microscopic image of Aβ immunohistochemical analysis in postmortem retinal flatmounts from an 89-year-old female with AD is provided to demonstrate multiple abluminal and perivascular APs accumulating along and inside retinal blood vessels (Fig. 1 D-E). We compared total temporal AP counts between the left and the right eye and observed a very strong and positive inter-eye correlation (r = 0.82, P = 0.0002; Supplementary Fig. 2). Since no difference between the left and right retinal AP counts was noted, we have arbitrarily chosen to conduct further analyses of AP counts within the supero-temporal quadrant of the left eyes. This is also based on prior retinal pathological and amyloid imaging studies highlighting that the supero-temporal quadrant has the highest burden of retinal AP, which is also most predictive of cognitive loss and hippocampal atrophy [ 16 , 42 , 44 , 48 , 56 ]. Perivascular AP analyses included twenty-eight patients who had suitable left eye supero-temporal retinal images for vascular tracing and perivascular AP quantitative analysis. The mean (SD) age was 65 (7.4) years, with 50% being female. The mean (SD) MOCA score was 25 (5.6), with a median MOCA score of 27 (range 4–32). Among the subjects, 11 had a CDR of 0.5, 14 had a CDR of 1, and 3 had a CDR of 2. Based on formal neuropsychometric cognitive evaluation corroborated with neuroimaging findings, 9 (32.1%) patients had normal cognition (NC), while 19 (67.9%) had impaired cognition (IC; 6 amnestic MCI, 9 multidomain MCI, 1 non-amnestic MCI, and 3 probable AD). The cohorts with normal and impaired cognition were matched for age, sex, and years of education (Table 1 ). Despite finding no significant difference between the perivascular areas of retinal venules and arterioles (Fig. 2 E), the total retinal perivascular AP count was significantly higher in the peri-arteriolar (A) region compared to the peri-venular (V) region (Fig. 2 F; A: 51.96 ± 19.65 vs. V: 35.57 ± 12.66, P < 0.0001; detailed data for each vascular subtype in Supplementary Table 2). These findings remained consistent regardless of sex (Fig. 2 G-H, Supplementary Table 3) or the degree of cognitive impairment (F i g. 2 I–J). Notably, the increased retinal peri-arteriolar versus peri-venular AP count was statistically more significant in individuals with impaired cognition (IC) compared to those with normal cognition (NC) (F i g. 2 I versus 2J). The peri-venular AP count exceeded the peri-arteriolar plaque count ( P = 0.013; Supplementary Table 2) only in the retinal primary main branches. Following the initial bifurcation, retinal peri-arteriolar AP burden remained significantly greater than the peri-venular AP burden at all levels, except for a similar non-significant trend observed in the tertiary branches (Supplementary Figu. 3A–E, Supplementary Table 2). When stratified by cognitive status (Fig. 3 A–F; Table 2 ; extended data in Supplementary Fig. 3F–H), IC subjects demonstrated 1.3-fold greater non-perivascular amyloid count ( P = 0.018, Fig. 3 C) and significantly greater (2.3-fold) retinal perivascular AP burden in the secondary branches compared to NC subjects ( P = 0.0037). This difference was observed in both the total peri-venular ( P = 0.0011) and total peri-arteriolar areas ( P = 0.015), with a substantial increase (2.4–2.9-fold) noted in the secondary small branches (Fig. 3 A–F and Table 2 ; extended data in Supplementary Fig. 3F–H). Tertiary branches also exhibited significantly greater retinal AP burden in the peri-venular areas in the IC group ( P = 0.020, Supplementary Fig. 3H). Interestingly, there was a non-significant trend indicating greater perivascular AP burden in the main primary branches among NC subjects compared to IC subjects ( P = 0.08, Table 2 ).  Retinal peri-arteriolar and peri-venular amyloid plaque count stratified by cognitive status and MOCA scores. A – B Representative fundus images of retinal perivascular amyloid plaque (AP) showing their density and distribution along arterioles (red tracing) and venules (blue tracing), in individuals with normal cognition (NC; A ) or impaired cognition (IC; B ). C Quantitative analyses of retinal non-perivascular AP count stratified by cognitive status, NC versus IC. D – F Quantitative analyses of retinal AP count stratified by cognitive status, in perivascular ( D ), peri-arteriolar ( E ), and peri-venular ( F ), for the secondary (2°) small branches. G Quantitative analyses of retinal non-perivascular AP count stratified by MOCA scores of 26 or lower compared with greater than 26. H – J Quantitative analyses of AP count stratified by MOCA, in perivascular 2° branches ( H ), 2° small branches ( I ), and peri-venular 2° small branches ( J ). Violin plots are showing individual data points, median and interquartile range. Statistics: * P < 0.05, ** P < 0.01, **** P < 0.0001, by unpaired two-tailed Student’s t test or Mann–Whitney. M, male; MOCA, Montreal Cognitive Assessment; y, years. Color code for vessel type: purple—perivascular; blue—peri-venular; red—peri-arteriolar When compared to subjects with a MOCA score greater than 26 (Fig. 3 G–J and Table 3 ; detailed data in Supplementary Fig. 3I), subjects with MOCA scores of 26 or lower exhibited a significantly greater total perivascular AP count in the secondary branches ( P = 0.002), notably showing a substantial threefold increase in the peri-venular small branch areas (3.50 ± 3.13 vs. 10.46 ± 3.26, P < 0.0001). In contrast to the stratification based on cognitive status, no significant differences were noted when non-perivascular AP counts were compared between subjects with MOCA greater than 26 and those with MOCA 26 or lower ( P = 0.15, Fig. 3 G). Patients with high CDR scores (Supplementary Fig. 4 and Supplementary Table 4) exhibited a greater total retinal perivascular AP count in the secondary branches ( P = 0.020), along with significantly elevated values in the tertiary branch areas upon further topographic analysis ( P = 0.0058; Supplementary Fig. 4A–B). Total peri-venular AP burden, particularly in the tertiary branches, was significantly greater in subjects with higher CDR scores ( P = 0.0025 and P = 0.0007 respectively; Supplemental Fig. 4 C–D). Moreover, both the main and small branches of peri-arteriolar secondary regions exhibited a significantly greater AP count in subjects with a CDR of 2 compared to CDRs of 0.5 and 1 (Supplementary Fig. 4E and Supplementary Table 4).  Correlations between retinal perivascular amyloid plaque distribution with cognitive and neuroimaging measures. Pearson’s r correlation analyses between retinal perivascular AP count and CDR ( A ), MOCA ( B ), RCFT-copy registration ( C ), Trail A-paper and pencil ( D ), ACS-TOFF ( E ), CVLT-II Long delay ( F ), hippocampal volume ( G ) and white matter hyperintensities lesions ( H ). AP, Amyloid plaques; CDR, Clinical dementia rating; MOCA, Montreal cognitive assessment; RCFT, Rey Complex Figure Test and Recognition Trial; ACS-TOPF, test of Premorbid Functioning; CVLT, California Verbal Learning Test; HV, hippocampal volume, WMH, White matter hypertensity; 2° Br, secondary branches; 3° Br, tertiary branches. Color code for vessel type: purple—perivascular; blue—peri-venular; red—peri-arteriolar Pearson’s correlation analyses revealed a correlation between retinal perivascular AP count and CDR scores, with the most significant correlation in this cohort observed for the total peri-venular AP count (Fig. 4 A and Table 4 ; r = 0.66, P = 0.0001). Topographically, the tertiary branch peri-venular AP count ( r = 0.61, P = 0.0016) and the secondary branch peri-arteriolar AP count ( r = 0.51, P = 0.0055) showed significant correlations with CDR scores (Table 4 ). The secondary small branch peri-venular AP count showed the highest correlation with the MOCA score (Fig. 4 B; r = − 0.51, P = 0.0063). The evaluation of specific cognitive domains (Fig. 4 , Table 5 ; detailed data in Supplementary Fig. 5) revealed greater overall retinal peri-venular AP accumulation in subjects with lower visuo-spatial ability as assessed by the Rey Complex Figure Test and Recognition Trial (RCFT)-Copy registration (Fig. 4 C; r = − 0.45, P = 0.043) and speed of information processing (Trail A paper and pencil; Table 5 ; r = − 0.42, P = 0.039) Z-scores. Similarly, moderate correlations were found between lower speed of information processing (Trail A paper and pencil) Z-scores and retinal AP counts in peri-venular tertiary branches (Fig. 4 D; r = − 0.47, P = 0.037) and peri-arteriolar secondary branches (Table 5 ; r = − 0.44, P = 0.027). We also noted a higher retinal peri-venular AP count in distal secondary branches in subjects with lower Z-scores on the general cognitive Test of Premorbid Functioning ACS (Fig. 4 E; r = − 0.49, r = 0.015) and the California Verbal Learning Test (CVLT) II Long Delay (Fig. 4 F; r = − 0.51, P = 0.0091). Interestingly, positive correlations were found between peri-arteriolar primary branch AP count and RCFT recall at 20 min and CVLT-II Z-scores ( r = 0.41, P = 0.039 and r = 0.53, P = 0.0052 respectively; Supplementary Fig. 5A–B). Symptom validity and functional status (SF-36PCS) also positively correlated with retinal AP count in the primary venular branches only (Supplementary Fig. 5C). Correlation analyses conducted with neuroimaging measurements revealed inverse relationships between HV and retinal AP count in the total perivascular area (Fig. 4 G, left; r = − 0.47, P = 0.028), and moreover, in the tertiary branch area (Fig. 4 G, right; r = − 0.55, P = 0.0087). Inverse correlations were also observed between HV and AP count in both peri-venular total and tertiary branches ( r = − 0.51, P = 0.016 and r = − 0.51, P = 0.026, respectively), as well as AP count in peri-arteriolar secondary small and tertiary branches ( r = − 0.42, P = 0.049 and r = − 0.46, P = 0.033, respectively; Table 4 and Supplementary Fig. 5D). In contrast, the number of WMHI (Fig. 4 H, Table 4 , and Supplementary Fig. 5E) positively correlated with retinal AP count in the peri-arteriolar secondary branch ( r = 0.53, P = 0.016) and peri-venular secondary main and tertiary branches ( r = 0.56, P = 0.013 and r = 0.52, P = 0.032 respectively). None of the perivascular AP counts exhibited significant correlations with total intracranial volumes, but some showed associations with the volume of WMHI and subcortical Fazekas scores (Supplementary Fig. 5F–G). We describe the topographic distribution of retinal supero-temporal peri-arteriolar and peri-venular amyloid deposits in a noninvasive human retinal imaging study involving subjects with normal or impaired cognition (mostly amnestic MCI). To the best of our knowledge, this description has not been conducted previously. Our exploratory analysis of the topographical interaction between two retinal imaging biomarkers of AD, amyloid and vasculature, conveys novel insights into (1) the localization of perivascular retinal amyloid deposits, which, akin to AD brain patterns, were predominantly detected in the peri-arteriolar regions compared to the peri-venular regions, and (2) the relationship between the differentiated perivascular amyloidosis with neuroimaging markers of neurodegeneration and cognitive performance. We found that the secondary vascular branches have significantly higher perivascular amyloid burden in subjects with impaired cognition compared to normal cognition, irrespective of stratification by neuropsychometric diagnosis, MOCA or CDR, and this finding remained consistent across sex. Patients with even slightly lower MOCA scores and greater CDR scores had statistically significant greater peri-venular amyloid burden, which also showed significant negative correlation with HV. Moreover, secondary branch peri-arteriolar and peri-venular AP counts significantly and positively correlated with the WMHI count. These promising exploratory findings encourage future studies in larger and more diverse cohorts to assess the efficacy of monitoring AD progression, response to therapy, and the risk of amyloid-related imaging abnormalities (ARIA) through non-invasive retinal perivascular amyloid imaging. Pericyte loss in the brain [ 3 ] and retina [ 16 ] is linked to a rapid cascade of neurodegeneration and amyloid deposition, leading to increased vascular amyloidosis in both the retina and the brain. Abnormalities in the blood-retina barrier and vascular-associated retinal Aβ deposition have been reported in postmortem retinas of AD patients, occurring inside the blood vessel walls, as well as around and along the blood vessels [ 11 , 42 , 48 , 49 ]. More Aβ 40 was found to be accumulated in arterioles than in venules [ 49 ]. Furthermore, various blood-retinal barrier tight junction biomarkers were found to be deficient in the retinas of MCI and AD individuals and were associated with retinal and brain amyloid burden and cognitive status [ 49 ]. Apart from the well-characterized pericyte injury, it remains unclear whether perivascular amyloidosis affects arterioles and venules differentially at various stages of AD progression [ 57 , 58 , 59 , 60 ]. Similar to the retina, the cerebral vasculature has been studied to understand AD pathophysiology and identify potential specific therapeutic targets [ 61 , 62 , 63 ]. Increased cerebral capillary damage has been shown to correlate with the level of insoluble Aβ in AD [ 64 ], and pericyte downregulation in the deep white matter has been observed in both vascular dementia and AD [ 65 ]. Aβ deposition around cerebral blood vessels, predominantly arteries (CAA), is thought to be major contributor to vascular dysfunction in AD [ 66 , 67 , 68 , 69 ]. The two-hit vascular hypothesis of AD emphasizes the role of neurovascular dysfunction as an early factor that favors vascular Aβ aggregation and neurodegeneration [ 69 ]: vascular dysfunction (hit one) is followed by Aβ accumulation (hit two), which precedes and promotes neurodegeneration [ 70 ]. According to the vascular hypothesis of AD, alterations in the neurovascular unit could lead to vascular Aβ accumulation, which in turn promotes neuronal dysfunction, accelerating neurodegeneration and dementia. Insoluble vascular amyloid deposits trigger neurovascular unit dysfunction in the AD brain [ 71 ]. Whereas most human studies have focused on amyloid deposition in leptomeningeal and cortical arterioles, murine models of AD have also shown abnormalities in venules [ 72 ]. Additionally, the brain’s glymphatic system is impaired in AD, leading to insufficient amyloid clearance [ 73 ], hence the development of novel perivascular clearance system imaging techniques are underway [ 74 ]. Since the phenomenon of vascular amyloid clearance potentially leading to perivascular amyloidosis had not been inspected in the retina, we conducted this topographic analysis of retinal fluorescent images to assess the distribution of AP in relation to the primary, secondary, and tertiary retinal arteriolar and venular branches in subjects with normal and impaired cognition. In the supero-temporal quadrant of the retina, we noted a higher prevalence of perivascular amyloidosis in the peri-arteriolar region compared to the peri-venular region, both overall and across primary, secondary, and tertiary branches. Our discovery that vascular amyloid accumulation occurs more frequently along the arterioles in the retina is consistent with patterns observed in cerebral arterial amyloidosis [ 16 , 68 , 69 ]. We also noted a significant increase in retinal amyloidosis in the peri-venular and peri-arteriolar secondary and tertiary branch regions in subjects with CDR scores of 1 and 2, as well as MOCA scores lower than 26. Additionally, the perivascular amyloid burden around the secondary small and tertiary branches inversely correlated with HV. The secondary branch peri-arteriolar and peri-venular plaque burden positively correlated with the number of WMHI lesions. These findings generate further hypotheses. Venular narrowing and increased venular tortuosity were described to correlate with the cognitive status and AP burden in AD [ 15 , 17 , 26 , 29 , 75 ]. It is conceivable that the overall retinal peri-venular AP burden could emerge as a marker of cognitive impairment, while the burden of perivascular AP in secondary branches may correlate of neurodegeneration markers, such as hippocampal atrophy and WMHI burden. This suggests that although peri-arteriolar amyloid deposition may occur earlier, peri-venular amyloid accumulation becomes more apparent later in the disease process and could serve as an indicator of more advanced neurodegenerative disease progression. The association between WMHI and CAA is gaining increased recognition [ 76 ]. The deposition of Aβ 40 in the walls of cerebral arteries suggests an age-related failure of perivascular drainage of soluble Aβ from the brain. White matter pathology is proposed to be the link between blood–brain barrier leakage and the decline in information processing speed in older individuals, with and without cognitive impairment [ 77 ]. The negative association between white matter blood–brain barrier leakage and information processing speed performance was mediated by the WMHI volume, with no correlation observed with HV [ 77 ]. Disruption of the brain microcirculation not only contributes to amyloidopathy but also initiates a non-amyloidogenic pathway of vascular-mediated neuronal dysfunction and injury, characterized by increased permeability of blood vessels, leakage of blood-borne components into the brain, and, consequently, neurotoxicity. The diminished brain capillary flow leads to multiple focal ischemic or hypoxic microinjuries, diminished Aβ clearance, and the formation of neurotoxic oligomers, ultimately resulting in neuronal dysfunction [ 78 ]. There appears to be a greater prevalence of retinal amyloidosis around first-order branches in cognitively intact individuals, while significantly greater retinal amyloidosis is found around smaller branches in patients with impaired cognition. The reduced complexity of the retinal vascular branching network, as previously described in subjects with MCI and AD [ 15 , 26 , 29 , 79 ], may explain the preferential accumulation of perivascular amyloid in secondary branches in cognitively impaired individuals. Interestingly, an agnostic machine learning-based study identified the same secondary branch perivascular area on heat-maps derived from retinal photographs, discriminating between AD individuals and healthy controls with high accuracy [ 80 ]. Our results within small subgroups should be interpreted cautiously and require confirmation in larger well-powered studies, further exploring the clinical utility of perivascular amyloidosis topography and the underlying pathophysiology of peri-arteriolar and peri-venular amyloid deposition at various stages of AD progression. With this current methodology, we were unable to assess the retinal far periphery or determine the status of amyloid burden adjacent to capillaries, both of which warrant future investigation. Furthermore, automated protocols to analyze retinal perivascular amyloidosis in the whole fundus are needed. Other study limitations include the relatively small number of subjects with cognitive decline, predominantly of Caucasian race, and the lack of AD biomarker confirmation, which reduces the generalizability of our findings. ConclusionsIn this exploratory study of the topographical interaction between retinal vasculature and Aβ deposits, we discovered that increased supero-temporal retinal Aβ deposits in the distal peri-venular regions can differentiate between normal and impaired cognition and are inversely associated with hippocampal neurodegeneration. Our findings from both in vivo imaging and histopathological analyses reveal similar patterns of perivascular Aβ depositions. These data further support the hypothesis that the pathophysiology of AD in the retina and brain is analogous, evidenced by the preferential accumulation of amyloid in the peri-arterial regions. However, the relationship between retinal perivascular amyloidosis and cognitive performance is complex and necessitates further investigation through longitudinal prospective studies involving a larger and more diverse cohort with confirmation of cerebral amyloid status via amyloid-PET imaging. It is worthwhile to further explore and validate the potential of peri-arteriolar and peri-venular amyloid burden as biomarkers across various stages of AD and as potential risk for ARIA. Availability of data and materialsThe datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. AbbreviationsAlzheimer’s disease Amyloid β-protein Mild cognitive impairment Scanning laser ophthalmoscopy Cerebral amyloid angiopathy Institutional Review Board Montreal Cognitive Assessment Clinical Dementia Rating Wechsler Adult Intelligence Scale California Verbal Learning Test Wechsler Memory Scale Rey Complex Figure Test Brief Visuo-Spatial Memory Test Revised Physical Component Score Mental Component Score Magnetic resonance imaging Intracranial volume
Inferior lateral ventricle volume White matter hyperintensities One-way analysis of variance Impaired cognition Normal cognition Nation DA et al (2019) Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25(2):270–276 Article CAS PubMed PubMed Central Google Scholar Li M et al (2021) Increase of blood-brain barrier leakage is related to cognitive decline in vascular mild cognitive impairment. BMC Neurol 21(1):159 Article PubMed PubMed Central Google Scholar Nikolakopoulou AM et al (2019) Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci 22(7):1089–1098 Riphagen JM et al (2020) Linking APOE-epsilon4, blood-brain barrier dysfunction, and inflammation to Alzheimer’s pathology. Neurobiol Aging 85:96–103 Article PubMed Google Scholar Montagne A et al (2020) APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 581(7806):71–76 He JT et al (2020) Vascular risk factors and Alzheimer’s disease: blood-brain barrier disruption, metabolic syndromes, and molecular links. J Alzheimers Dis 73(1):39–58 Article CAS PubMed Google Scholar Sweeney MD et al (2019) Vascular dysfunction-The disregarded partner of Alzheimer’s disease. Alzheimers Dement 15(1):158–167 Mountjoy CQ, Tomlinson BE, Gibson PH (1982) Amyloid and senile plaques and cerebral blood vessels. A semi-quantitative investigation of a possible relationship. J Neurol Sci 57(1):89–103 Hartmann DA et al (2018) Does pathology of small venules contribute to cerebral microinfarcts and dementia? J Neurochem 144(5):517–526 Keith J et al (2017) Collagenosis of the deep medullary veins: an underrecognized pathologic correlate of white matter hyperintensities and periventricular infarction? J Neuropathol Exp Neurol 76(4):299–312 Shi H et al (2021) Retinal vasculopathy in Alzheimer’s disease. Front Neurosci 15:731614 Hart NJ et al (2016) Ocular indicators of Alzheimer’s: exploring disease in the retina. Acta Neuropathol 132(6):767–787 Doustar J et al (2017) Optical coherence tomography in Alzheimer’s disease and other neurodegenerative diseases. Front Neurol 8:701 Dumitrascu OM, Koronyo-Hamaoui M (2020) Retinal vessel changes in cerebrovascular disease. Curr Opin Neurol 33(1):87–92 Frost S et al (2013) Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl Psychiatry 3(2):e233 Shi H et al (2020) Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol 139(5):813–836 Dumitrascu OM et al (2021) Retinal venular tortuosity jointly with retinal amyloid burden correlates with verbal memory loss: a pilot study. Cells 10(11):2926 Jiang H et al (2021) Retinal microvascular alterations as the biomarkers for Alzheimer disease: are we there yet? J Neuroophthalmol 41(2):251–260 Dumitrascu OM et al (2018) Retinal microvascular abnormalities as surrogate markers of cerebrovascular ischemic disease: a meta-analysis. J Stroke Cerebrovasc Dis 27(7):1960–1968 Bulut M et al (2018) Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br J Ophthalmol 102(2):233–237 Jiang H et al (2018) Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J Neuroophthalmol 38(3):292–298 van de Kreeke JA et al (2020) Optical coherence tomography angiography in preclinical Alzheimer’s disease. Br J Ophthalmol 104(2):157–161 Marmor MF, Ravin JG (2011) Fluorescein angiography: insight and serendipity a half century ago. Arch Ophthalmol 129(7):943–948 Ruia S, Tripathy K (2022) Fluorescein angiography. StatPearls. StatPearls Publishing, Treasure Island Google Scholar Schallek J et al (2013) Morphology and topography of retinal pericytes in the living mouse retina using in vivo adaptive optics imaging and ex vivo characterization. Invest Ophthalmol Vis Sci 54(13):8237–8250 Cheung CY et al (2014) Retinal vascular fractal dimension is associated with cognitive dysfunction. J Stroke Cerebrovasc Dis 23(1):43–50 Csincsik L et al (2018) Peripheral retinal imaging biomarkers for Alzheimer’s disease: a pilot study. Ophthalmic Res 59(4):182–192 Frost S et al (2017) Modulation of retinal arteriolar central reflection by APOE genotype. Curr Alzheimer Res 14(9):916–923 Berisha F et al (2007) Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci 48(5):2285–2289 Cheung CY et al (2022) A deep learning model for detection of Alzheimer’s disease based on retinal photographs: a retrospective, multicentre case-control study. Lancet Digit Health 4(11):e806–e815 Wagner SK et al (2022) AlzEye: longitudinal record-level linkage of ophthalmic imaging and hospital admissions of 353 157 patients in London, UK. BMJ Open 12(3):e058552 Sharafi SM et al (2019) Vascular retinal biomarkers improves the detection of the likely cerebral amyloid status from hyperspectral retinal images. Alzheimers Dement 5:610–617 Article Google Scholar McDade E et al (2022) Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther 14(1):191 van Dyck CH et al (2023) Lecanemab in early Alzheimer’s disease. N Engl J Med 388(1):9–21 Sims JR et al (2023) Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330(6):512–527 Gandy S, Ehrlich ME (2023) Moving the needle on Alzheimer’s disease with an anti-oligomer antibody. N Engl J Med 388(1):80–81 Sperling RA et al (2023) Trial of solanezumab in preclinical Alzheimer’s disease. N Engl J Med 389(12):1096–1107 Mirzaei N et al (2020) Alzheimer’s retinopathy: seeing disease in the eyes. Front Neurosci 14:921 Ngolab J et al (2021) Feasibility study for detection of retinal amyloid in clinical trials: the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) trial. Alzheimers Dement Diagn Assess Dis Monit 13(1):e12199 Snyder PJ et al (2021) Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimers Dement 17(1):103–111 Koronyo-Hamaoui M et al (2011) Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 54(Suppl 1):S204–S217 Koronyo Y et al (2017) Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2(16):e93621 Rafii MS et al (2015) The down syndrome biomarker initiative (DSBI) pilot: proof of concept for deep phenotyping of Alzheimer’s disease biomarkers in down syndrome. Front Behav Neurosci 9:239 Dumitrascu OM et al (2020) Sectoral segmentation of retinal amyloid imaging in subjects with cognitive decline. Alzheimers Dement 12(1):e12109 Tadokoro K et al (2021) Retinal amyloid imaging for screening Alzheimer’s disease. J Alzheimers Dis 83(2):927–934 den Haan J et al (2022) No difference in retinal fluorescence after oral curcumin intake in amyloid-proven AD cases compared to controls. Alzheimers Dement 14(1):e12347 Lemmens S et al (2020) Combination of snapshot hyperspectral retinal imaging and optical coherence tomography to identify Alzheimer’s disease patients. Alzheimers Res Ther 12(1):144 La Morgia C et al (2016) Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol 79(1):90–109 Shi H et al (2023) Retinal arterial Abeta(40) deposition is linked with tight junction loss and cerebral amyloid angiopathy in MCI and AD patients. Alzheimers Dement 19:5185–5197 Engedal K et al (2012) Diagnosis of dementia–automatic quantification of brain structures. Tidsskr Nor Laegeforen 132(15):1747–1751 Koronyo Y et al (2023) Retinal pathological features and proteome signatures of Alzheimer’s disease. Acta Neuropathol 145(4):409–438 Knudtson MD et al (2003) Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 27(3):143–149 Ikram MK et al (2004) Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci 45(7):2129–2134 Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43(11):2412–2414 Nasreddine ZS et al (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699 Asanad S et al (2019) The retina in Alzheimer’s disease: histomorphometric analysis of an ophthalmologic biomarker. Invest Ophthalmol Vis Sci 60(5):1491–1500 Wu J et al (2020) Retinal microvascular attenuation in mental cognitive impairment and Alzheimer’s disease by optical coherence tomography angiography. Acta Ophthalmol 98(6):e781–e787 Lahme L et al (2018) Evaluation of ocular perfusion in Alzheimer’s disease using optical coherence tomography angiography. J Alzheimers Dis 66(4):1745–1752 Zhang YS et al (2019) Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer’s Disease on optical coherence tomography angiography. PLoS ONE 14(4):e0214685 Rifai OM et al (2021) The application of optical coherence tomography angiography in Alzheimer’s disease: a systematic review. Alzheimers Dement 13(1):e12149 Sweeney MD et al (2018) The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21(10):1318–1331 Govindpani K et al (2019) Vascular dysfunction in Alzheimer’s disease: a prelude to the pathological process or a consequence of it? J Clin Med 8(5):651 Govindpani K et al (2020) Vascular dysfunction in Alzheimer’s disease: a biomarker of disease progression and a potential therapeutic target. Neural Regen Res 15(6):1030–1032 Miners JS, Schulz I, Love S (2018) Differing associations between Abeta accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer’s disease. J Cereb Blood Flow Metab 38(1):103–115 Ding R et al (2020) Loss of capillary pericytes and the blood-brain barrier in white matter in poststroke and vascular dementias and Alzheimer’s disease. Brain Pathol 30(6):1087–1101 Zamolodchikov D, Strickland S (2016) A possible new role for Abeta in vascular and inflammatory dysfunction in Alzheimer’s disease. Thromb Res 141(Suppl 2):S59-61 Canobbio I et al (2015) Role of amyloid peptides in vascular dysfunction and platelet dysregulation in Alzheimer’s disease. Front Cell Neurosci 9:65 Falsetti L et al (2022) Shared molecular mechanisms among Alzheimer’s disease, neurovascular unit dysfunction and vascular risk factors: a narrative review. Biomedicines 10(2):439 Apatiga-Perez R et al (2022) Neurovascular dysfunction and vascular amyloid accumulation as early events in Alzheimer’s disease. Metab Brain Dis 37(1):39–50 Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12(12):723–738 Soto-Rojas LO et al (2021) Insoluble vascular amyloid deposits trigger disruption of the neurovascular unit in Alzheimer’s disease brains. Int J Mol Sci 22(7):3654 Lai AY et al (2015) Venular degeneration leads to vascular dysfunction in a transgenic model of Alzheimer’s disease. Brain 138(Pt 4):1046–1058 Municio C et al (2023) Choroid plexus aquaporins in CSF homeostasis and the glymphatic system: their relevance for Alzheimer’s disease. Int J Mol Sci 24(1):878 van der Thiel MM et al (2023) Novel developments in non-contrast enhanced MRI of the perivascular clearance system: what are the possibilities for Alzheimer’s disease research? Neurosci Biobehav Rev 144:104999 Feke GT et al (2015) Retinal blood flow in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement 1(2):144–151 Charidimou A et al (2022) The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol 21(8):714–725 Freeze WM et al (2020) White matter hyperintensities mediate the association between blood-brain barrier leakage and information processing speed. Neurobiol Aging 85:113–122 Janota C, Lemere CA, Brito MA (2016) Dissecting the contribution of vascular alterations and aging to Alzheimer’s disease. Mol Neurobiol 53(6):3793–3811 Cabrera DeBuc D et al (2018) Investigating multimodal diagnostic eye biomarkers of cognitive impairment by measuring vascular and neurogenic changes in the retina. Front Physiol 9:1721 Dumitrascu OM, Zhu W, Qiu P, Nandakumar K, Wang Y (2022) Automated retinal imaging analysis for Alzheimers disease screening. In: IEEE international symposium on biomedical imaging: from nano to macro (ISBI), 2022 Download references AcknowledgementsWe thank Elijiah Maxfield for assisting with manuscript editing. This work has been supported by the National Institutes of Health (NIH)/the National Institute on Aging (NIA) through the following Grants: R01AG056478, R01AG075998, R01AG055865, R41AG044897 (MK-H). This work is also supported by The Hertz Innovation Fund, and the Gordon, Wilstein, and Saban Private Foundations (MK-H). Author informationAuthors and affiliations. Departments of Neurology, Mayo Clinic, AZ, 13400 E. Shea Blvd, Scottsdale, AZ, 85259, USA Oana M. Dumitrascu Department of Neurosurgery, Cedars-Sinai Medical Center, Maxine Dunitz Neurosurgical Institute, 127 S. San Vicente Blvd., Los Angeles, CA, 90048, USA Jonah Doustar, Dieu-Trang Fuchs, Yosef Koronyo, Michelle Shizu Miller, Keith L. Black & Maya Koronyo-Hamaoui Department of Physical Medicine and Rehabilitation, Cedars-Sinai Medical Center, 127 S. San Vicente Blvd., Los Angeles, CA, 90048, USA Dale S. Sherman Department of Neurosurgery, Tulane University School of Medicine, 1415 Tulane Ave, New Orleans, LA, 70112, USA Michelle Shizu Miller NeuroVision Imaging LLC, 1395 Garden Hwy, Sacramento, CA, 95833, USA Kenneth O. Johnson & Steven R. Verdooner Department of Clinical Neuroanatomy, University of Southampton, University Road Southampton, Southampton, SO17 1BJ, UK Roxana O. Carare Department of Physiology and Neuroscience, Zilkha Neurogenetic Institute, Keck School of Medicine, University of Southern California, 1501 San Pablo St, Los Angeles, CA, 90033, USA Patrick D. Lyden Department of Pathology, Department of Neurological Sciences, Alzheimer’s Disease Research Center, Rush Medical College, Rush University, 600 S. Paulina St., Chicago, IL, 60612, USA Julie A. Schneider Department of Neurology, Cedars-Sinai Medical Center, 127 S. San Vicente Blvd., Los Angeles, CA, 90048, USA Maya Koronyo-Hamaoui Division of Applied Cell Biology and Physiology, Department of Biomedical Sciences, Cedars-Sinai Medical Center, 127 S. San Vicente Blvd., Los Angeles, CA, 90048, USA You can also search for this author in PubMed Google Scholar ContributionsOMD, JD, D-TF, YK, MSM, JS, MK-H: designed and performed experiments, collected, and analyzed data, created figures, drafted, and edited the manuscript. SRV, KOJ: provided the processed retinal images. OMD, DSS, PDL: cognitive and behavioral testing and imaging data. OMD, JD, D-TF, JAS, PDL, KLB, ROC, MK-H: data interpretation and discussion. OMD, MK-H: responsible for study conception, design, interpretation of data, supervision, and manuscript writing and editing. All authors have read and approved of this manuscript. Corresponding authorsCorrespondence to Oana M. Dumitrascu or Maya Koronyo-Hamaoui . Ethics declarationsEthics approval and consent to participate. The retrospective human cohort study was approved by the Cedars-Sinai Medical Center Institutional Review Board (IRB) and was performed in accordance with the ethical standards as established in the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. All human subjects provided written informed consent prior to enrollment. The eyes from deceased AD patients were obtained from Rush University’s Alzheimer’s Disease Research Center (ADRC ORA# 18011111). Histological studies were performed under the IRB protocol Pro00055802 at Cedars-Sinai Medical Center. Consent for publicationNot applicable. Competing interestsKoronyo-Hamaoui, Koronyo, Verdooner, and Black are co-founding members of NeuroVision Imaging Inc., 1395 Garden Highway, Suite 250, Sacramento, CA 95833, USA. Johnson and Verdooner are employed by NeuroVision Imaging Inc. NeuroVision’s sole involvement was to provide the processed retinal images. NeuroVision did not fund this study and was not involved in the development of the novel perivascular AP methodology or analysis. All authors declare that the research study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Additional informationPublisher's note. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Co-first authors: Oana M. Dumitrascu and Jonah Doustar. Supplementary InformationAdditional file1., rights and permissions. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data. Reprints and permissions About this articleCite this article. Dumitrascu, O.M., Doustar, J., Fuchs, DT. et al. Retinal peri-arteriolar versus peri-venular amyloidosis, hippocampal atrophy, and cognitive impairment: exploratory trial. acta neuropathol commun 12 , 109 (2024). https://doi.org/10.1186/s40478-024-01810-2 Download citation Received : 26 March 2024 Accepted : 02 June 2024 Published : 28 June 2024 DOI : https://doi.org/10.1186/s40478-024-01810-2 Share this articleAnyone you share the following link with will be able to read this content: Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative
Acta Neuropathologica CommunicationsISSN: 2051-5960
Modulatory Effect of Blood LDL Cholesterol on the Association between Cerebral Aβ and Tau Deposition in Older Adults
Cite this articleYou have full access to this open access article 
KBASE Research GroupThis study investigates the synergistic relationship between blood low-density lipoprotein cholesterol (LDL-C) and cerebral beta-amyloid (Aβ) in relation to tau deposition, a key factor in the pathology of Alzheimer’s disease (AD), in older adults across a diverse cognitive spectrum. To examine whether higher levels of LDL-C in the blood moderate the association of cerebral Aβ with tau deposition in older adults, including those with normal cognition, mild cognitive impairment, and Alzheimer’s disease dementia. Cross-sectional design. Setting: The study was conducted as a part of a prospective cohort study. All assessments were done at the Seoul National University Hospital, Seoul, South Korea. Participants: A total of 136 older adults (aged 60–85 years) with normal cognition, mild cognitive impairment or Alzheimer’s disease (AD) dementia were included. MeasurementsSerum lipid measurements, [11C] Pittsburgh Compound B-positron emission tomography (PET), [18F] AV-1451 PET, and magnetic resonance imaging were performed on all participants. There was a significant Aβ × LDL-C interaction effect on tau deposition indicating a synergistic moderation effect of LDL-C on the relationship between Aβ and tau deposition. Subsequent subgroup analysis showed that the positive association between Aβ and tau deposition was stronger in higher LDL-C group than in lower LDL-C group. In contrast, other lipids, such as total cholesterol, high-density lipoprotein cholesterol, and triglycerides, did not show a similar moderation effect on the relationship between Aβ deposition and tau deposition. Our findings suggest that blood LDL-C synergistically enhances the influence of Aβ deposition on tau pathology, emphasizing the need for greater attention to the role of LDL-C in AD progression. Avoid common mistakes on your manuscript. IntroductionH ypercholesterolemia in midlife and early late-life has been proposed as a potential risk factor for dementia ( 1 – 3 ). Higher blood low-density lipoprotein cholesterol (LDL-C) levels, in particular, have been repeatedly related to Alzheimer’s disease (AD) and related cognitive decline ( 4 , 5 ). Nevertheless, the underlying neuropathological links in the relationship between increased blood LDL-C and AD-related cognitive impairment are not clearly understood. A cell culture study suggested that LDL-C may increase not only beta-amyloid protein (Aβ) but also phosphorylated tau protein ( 6 ). Other preclinical studies also showed that apolipoprotein B containing LDL-C increased the accumulation of phosphorylated tau in brain by inhibiting the activity of cathepsin D in autophagotic lysosomes ( 7 ). Lysosomal enzyme cathepsin D is known to degrade tau protein ( 8 ). Given the well-established relationship between Aβ and tau deposition in AD ( 9 , 10 ) such influence of LDL-C on tau protein raises the possibility that LDL-C level may synergistically increase tau deposition with Aβ pathology. Although a couple of human studies reported that serum LDL-C levels were associated with an increase of brain Aβ accumulation ( 11 , 12 ), and cerebrospinal fluid (CSF) levels of apolipoprotein B (APOB), the primary apolipoprotein of LDL-C, were correlated with CSF tau levels or brain tau deposition ( 13 ) little is known about the synergistic interaction between blood LDL-C and Aβ for tau deposition in the human brain. In this context, we aimed to test the hypothesis that blood LDL-C moderates the association between cerebral Aβ and tau deposition in older adults with a diverse cognitive spectrum including cognitively normal (CN), mild cognitive impairment (MCI), and AD dementia. We also explored the associations of various blood lipids, including total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) with in vivo AD pathologies and cerebrovascular injury as measured by white matter hyperintensities (WMHs). ParticipantsThe current study was conducted for the part of older individuals who participated in the Korean Brain Aging Study for Early Diagnosis and Prediction of Alzheimer’s Disease (KBASE), a prospective cohort study which begun in 2014. The main purposes of the KBASE are to find new AD biomarkers and investigate how different life events and physical changes affect AD-related brain alterations ( 14 ). Participants were recruited from four sites in Seoul, Republic of Korea. Potential study candidates who attended memory clinics at two university hospitals in Seoul or participated in dementia screening programs at two public dementia prevention and management centers were informed about the study and invited to be evaluated for eligibility. Furthermore, community volunteers were recruited through personal referrals, posters and brochures, and online advertisements. The present study finally included 136 older adults (aged 55 to 90) who had undergone tau-PET scans as of August 22, 2018. They consisted of 69 cognitively normal (CN), 32 mild cognitive impairment (MCI), and 35 Alzheimer’s disease (AD) dementia individuals. Those without dementia and MCI diagnosis and with Clinical Dementia Rating (CDR)15 score of 0 were classified as CN. All MCI patients satisfied the commonly accepted criteria for amnestic MCI16 which encompass (1) subjective or informant-corroborated memory complaints; (2) demonstrated objective memory deficits; (3) intact overall cognitive function; (4) independence in daily activities; and (5) no dementia. For the second criterion, a z-score of 1.0 in at least one of the four episodic memory tests was applied. AD dementia was diagnosed according to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders17 and the National Institute on Aging and Alzheimer’s Association guidelines for probable AD.18 All participants with AD dementia also had a global CDR score of either 0.5 or 1. Exclusion criteria included a major psychiatric disorder and significant neurological or medical illnesses that could alter mental status. The use of investigational medications, significant vision or hearing impairments, illiteracy, severe communication or behavioral problems that might hinder clinical assessments or brain imaging were additional exclusion criteria. The study procedure received approval from the institutional review boards of Seoul National University Hospital (C-1401-027-547) and SNU-SMG Boramae Medical Center (26-2015-60), and adhered to the latest version of the Declaration of Helsinki. Participants or their authorized representatives gave their written consent after receiving comprehensive information about the study. Clinical assessmentsUsing the KBASE assessment procedure ( 14 ), which included and enhanced the Korean edition of the Consortium to Establish a Registry for Alzheimer’s Disease assessment package (CERAD-K), comprehensive clinical data were gathered from each participant ( 19 ). The presence of dyslipidemia and additional vascular risk factors including diabetes mellitus, hypertension, transient ischemic attack, coronary heart disease, and stroke was systematically evaluated based on information gathered by skilled nurses through systematic interviews with participants and their informants and medical record reviews. Participants were deemed to have dyslipidemia if they had received a dyslipidemia diagnosis at a medical facility or were already receiving medication for the condition at the time of enrollment. The total number of vascular risk factors other than dyslipidemia was regarded as a vascular risk score reflecting the burden of vascular risk aside from dyslipidemia (VRSnoDLP) ( 20 ). Measurement of cerebral Aβ depositionSimultaneous acquisition of three-dimensional [11C] Pittsburgh compound B (PiB)-positron emission tomography (PET) and T1-weighted MRI images was performed using a 3.0T Biograph mMR (PET-MR) scanner (Siemens; Washington DC, WC, USA) for all participants. The methods for PiB-PET image acquisition and preprocessing were detailed in a previous report.10 By employing automated anatomical labeling and a region-combining method ( 21 ), regions of interests (ROIs) were established to examine PiB retention in various brain regions, including the frontal, lateral temporal, lateral parietal, and posterior cingulate-precuneus areas. To calculate the standardized uptake value ratio (SUVR) for each ROI, the average voxel value within each ROI was divided by the corresponding mean cerebellar uptake value. A global Aβ deposition was calculated by dividing the average voxel value in the global cortical ROI, which consists of the 4 ROIs, by the mean cerebellar uptake value ( 21 ). Measurement of tau deposition[18F] AV-1451 PET scans were obtained for all subjects using a Siemens Biograph True Point 40 PET/CT scanner. [18F] AV-1451 PET imaging was performed on average 2.6 years (SD = 0.3) after the baseline assessment including clinical evaluations, other brain imaging including PiB-PET and blood sampling. The detailed methods for AV-1451 PET image acquisition and preprocessing were described in our earlier report.10 The AV-1451 SUVR value of «AD-signature regions» of tau deposition, which is compared of a size-weighted mean of partial volume-corrected uptake in the middle temporal, inferior temporal, fusiform, parahippocampal, entorhinal, and amygdala ROIs ( 22 ), was used as an outcome variable for global tau deposition. Measurement of WMHsFluid-attenuated inversion recovery images obtained with the same MRI scanner were used to estimate the volume of the cerebral WMHs. We used an automated process that had already been validated ( 23 ) with two changes. First, as it was more appropriate for our data, a threshold value of 70 rather than 65 from the original reference was applied. Second, because individuals with acute cerebral infarction were not included, diffusion-weighted imaging was not used in the process. Measurements of lipid profilesParticipants’ blood was drawn in the morning (8–9 a.m.) after an overnight fast and kept in a serum separator tube (Becton, Dickinson and Co., Franklin Lakes, NJ, USA) At room temperature, the tubes were centrifuged at 1300 x g for 10 minutes. The serum supernatants were then collected and stored at −80°C. Serum levels of TC, LDL-C, HDL-C, and TG were measured at Seoul Clinical Laboratories (SCL) with a colorimetric technique and an ADVIA 1800 Auto Analyzer (Siemens, USA). APOE genotypingGenomic DNA was isolated from venous blood samples. Apolipoprotein E (APOE) genotyping followed the protocol outlined by Wenham et al ( 24 ). Statistical analysisWe initially explored the association of each lipid with AD biomarkers and WMH volume through partial correlation analysis using age, education, gender, APOE ε4 positivity and VRSnoDLP as covariates. To test the main hypothesis that blood LDL-C moderates the association between cerebral Aβ and tau deposition, we analyzed the multiple linear regression model including Aβ deposition × LDL-C interaction term as well as Aβ deposition and LDL-C as independent variables with tau deposition as the dependent variable and age, gender, education, APOE ε4 positivity, and VRSnoDLP as covariates. When the interaction term was significant, we performed two further analyses for LDL-C subgroups, separately. First, The participants were divided into lower (<116 mg/dL) and higher (≥116 mg/dL) LDL-C subgroups according to the 2019 European Society of Cardiology (ESC) / European Atherosclerosis Society (EAS) guideline for the management of dyslipidemia ( 25 ). Next, we divided the subjects into three groups based on their LDL-C levels, and conducted same analysis for each group: the low-level group (n=45), the mid-level group (n=45), and the high-level group (n=46). We additionally performed exploratory analyses for the moderating effect of other lipids (TC, HDL-C, and TG) on the relationships between Aβ and tau deposition using a similar regression model. All analyses were conducted using IBM SPSS Statistics 25 software (IBM Corporation, Armonk, NY, USA) and a threshold of p < 0.05 was set to determine statistical significance for all the analyses. Data availabilityThe data can be accessed through the KBASE research group’s independent data sharing committee upon a reasonable request. Email requests for data access can be sent to the KBASE group’s administrative coordinator ([email protected]). Characteristics of ParticipantsDemographic and medical attributes of the participants are presented in Table 1 . As shown in Table 1 , lower (<116 mg/dL) LDL-C group had more common history of hypertension and dyslipidemia, higher VRSnoDLP, and more frequent use of statin, but lower LDL-C and TC, than higher LDL-C (≥116 mg/dL) group. Partial correlation between serum lipids and Aβ deposition, tau deposition and WMH volumeWe did not find any association between serum lipids and Aβ or tau deposition (Table 2 ). There was a significant but weak relationship between LDL-C and WMH volume, while other lipids did not relate with the volume. Moderation of LDL-C for the relationship between Aβ deposition and tau depositionThere was a significant Aβ deposition × LDL-C interaction effect on tau deposition, indicating the moderation effect of LDL-C on the relationship between Aβ and tau deposition (Table 3 ). Sensitivity analyses that controlled for statin use or the time gap between PiB PET and AV-1451 PET as an additional covariate revealed similar results (eTable 1 and eTable 2, respectively). Subsequent subgroup analyses demonstrated that while a significant association between Aβ and tau deposition was observed in both LDL-C subgroups, the association was stronger in higher LDL-C group (≥116 mg/dL) than in lower LDL-C group (<116 mg/dL) (Table 4 and Figure 1A ). When additional subgroup analyses were performed for the three tertile groups of LDL-C, the degree of association between Aβ and tau deposition gradually increased from low tertile group to high tertile one (Table 4 and Figure 1B ). For the purpose of demonstration, we also provided a figure (eFigure 1 ) that includes regression lines for the top 50% LDL-C and the bottom 50% LDL-C groups.  Multiple linear regression plots showing moderating effects of LDL-C on the relationships between Aβ and tau deposition Note: For the purpose of demonstration, participants were divided into (A) two and (B) three LDL-C subgroups. Multiple linear regression model included Aβ, LDL-cholesterol subgroup, and their interaction term as independent variables; tau retention as dependent variable; and age, gender, education, APOE ε4 positivity, and VRSnoDLP as covariates. Statistical significance was observed with the interaction term between Aβ deposition and LDL-C (p < 0.05), as detailed in the manuscript. Abbreviations: Aβ, beta-amyloid; APOE, apolipoprotein; LDL-C, low density lipoprotein cholesterol; VRSnoDLP, vascular risk score reflecting vascular risk burden other than dyslipidemia. Exploratory analyses for the moderation of other lipidsAny other lipids did not have any significant moderation effect for the relationship between Aβ and tau deposition (eTable 3). While there was no direct relationship of serum LDL-C with brain Aβ and tau deposition, we found significant LDL-C × brain Aβ synergistic interaction on tau deposition, which supported our hypothesis that blood LDL-C moderates the association between cerebral Aβ and tau deposition. Subsequent subgroup analyses demonstrated that the positive association between Aβ and tau deposition was stronger at higher LDL-C levels than at lower LDL-C levels. In contrast to LDL-C, none of the other lipids demonstrated a moderating effect on the Aβ-tau association, nor did they show a direct association with Aβ or tau deposition. The finding that higher LDL-C levels strengthened the association of brain Aβ with tau deposition is generally consistent with previous clinical reports on the relationship between blood LDL-C and AD-related cognitive decline ( 4 , 5 ). This finding also aligns with a report showing a reduced risk of AD dementia in users of statin drugs that lower blood LDL-C ( 2 , 6 ). Similarly, the association between statin use and reduced burden of neurofibrillary tangles at autopsy was reported ( 27 ). Although it is not easy to provide the exact mechanisms which underlie the moderation effect of LDL-C on the Aβ-tau relationship, some possible explanation can be made. As the brain is the most cholesterol-rich organ in the body, containing about 20% of the body’s total cholesterol ( 28 ), changes in cholesterol levels can lead to brain pathology ( 29 ). However, the presence of the blood-brain barrier (BBB) prevents blood cholesterol from entering the brain ( 30 ). Nevertheless, free radicals, formed under the influence of high blood cholesterol, can destroy the BBB and, as a result, increase brain cholesterol ( 30 , 31 ). Elevated brain LDL-C levels have been shown to promote neuroinflammatory responses ( 32 ). The induced neuroinflammatory response in turn influence tau pathogenesis ( 33 ). A study using an animal model reported that intra-cerebral administration of a potent inflammatory substance to myeloid receptor promoted tau hyper-phosphorylation and tangle formation ( 34 ). It was also reported that minocycline treatment reduced cortical tau phosphorylation through reduced inflammation in a mouse model of tauopathy ( 35 ). Other transgenic AD model study showed that inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway ( 36 ). Meanwhile, microglia, an important regulators of neuroinflammation, attaches to amyloid plaques and abundantly release cytokines that induce neuroinflammation, which potentially exacerbate tau pathology in the periphery of the amyloid plaques ( 37 ). Given all together, increased blood LDL-C may aggravate tau accumulation closely related with amyloid pathology ( 9 , 10 , 38 ) by elevating brain LDL-C and in turn promoting neuroinflammatory response. Additionally, recent studies have highlighted the role of apolipoprotein B (APOB), the primary apolipoprotein of LDL-C, in the neurobiology of AD. Several mutations in the APOB gene in familial cases with early onset AD have been identified, independent of the usual culprits (PEN1, PEN2, and APP) ( 39 ). Several studies on transgenic mice have shown that APOB can affect tau pathology, providing evidence for the role of APOB in AD ( 40 – 42 ). A recent human study also demonstrated that cerebrospinal fluid (CSF) APOB levels were elevated in AD patients, and correlated with CSF tau and brain tau PET binding in pre-symptomatic individuals, suggesting CSF APOB markedly associates with early tau dysregulation ( 13 ). Given the progressive deterioration of the BBB at the MCI and AD stages ( 40 , 41 ), which allows APOB and LDL-C to enter the brain ( 13 , 39 ), these findings are particularly relevant to our results regarding the contribution of blood LDL-C, an acolyte of APOB, on brain tau deposition. In contrast to the moderation effect of LDL-C, other lipids did not have any relationship with Aβ or tau accumulation in our study. Several studies have shown that high TC or low HDL-C levels are associated with clinical diagnosis of AD dementia or cognitive impairment ( 43 , 44 ). However, direct comparison between the findings of the previous studies and those of the current study is not easy because the considerable differences in study methodology. While we focused on the associations of lipids on in vivo AD pathologies, most previous studies did not measure the pathologies and just investigated the relationship with clinically defined AD dementia or cognitive impairment ( 43 , 44 ). Approximately 14–32% of clinically defined AD dementia cases ( 45 ) and 29–73% of MCI cases did not exhibit Aβ pathology in the brain.46 In addition, many of the previous studies investigated the relationship between lipid levels in midlife and dementia in late-life ( 43 , 44 ) while we measured lipid levels and in vivo brain pathologies in late-life. A study reported that high midlife TG levels were associated with increased brain Aβ and tau pathology after 20 years ( 11 ). Our exploratory analyses showed that serum LDL-C was positively associated WMH volume, but other lipids were not. This is consistent with the results of other studies: An observational study showed that high blood LDL-C was associated with increased WMHs ( 47 ), and another study also reported that LDL-C was related with periventricular WMHs, but other lipids, i.e., TC, HDL-C, and TG, were not ( 48 ). Since brain endothelial cells are sensitive to circulating LDL-C levels, impaired vascular responses are induced through oxidative stress and the secretion of inflammatory mediators caused by increased LDL-C ( 49 ). Our result that serum LDL-C has a moderating influence on brain Aβ-tau association in human is new. The present study does, however, include several limitations that need to be taken into account. Firstly, because this research was cross-sectional, no causal connections can be determined by the results. Additional prospective longitudinal research is necessary. Second, it may have been difficult to identify a direct link between higher serum LDL-C levels and each of Aβ and tau deposition because of the limited number of participants (n=48, 34.6%) with unusually high serum LDL-C levels (116mg/dL). Lastly, while amyloid PET and MRI scans were performed at baseline, tau PET scans were conducted on average 2.6 years (standard deviation = 0.3 years) after the baseline visit. The relationship between blood lipids and tau deposition may have been affected by this time gap. However, the results remained consistent when the temporal gap was adjusted as an extra covariate. Our findings suggest that blood LDL-C synergistically increases tau pathology with Aβ deposition. In terms of AD pathophysiology, more attention need to be paid to the role of LDL-C. Availability of data and materials: Data supporting the findings of this study are available from the Korean Brain Aging Study for Early Diagnosis and Prediction of Alzheimer’s Disease (KBASE) research group but are not publicly available. Data access requests can be made by contacting the KBASE group’s administrative coordinator via email ([email protected]). Abbreviations
apolipoprotein blood-brain barrier Clinical Dementia Rating Consortium to Establish a Registry for Alzheimer’s Disease assessment package cognitively normal high-density lipoprotein cholesterol Korean Brain Aging Study for Early Diagnosis and Prediction of Alzheimer’s Disease low density lipoprotein cholesterol mild cognitive impairment positron emission tomography Pittsburgh compound B regions of interest standardized uptake value ratio triglyceride; total cholesterol vascular risk score reflecting vascular risk burden other than dyslipidemia white matter hyperintensities Röhr S, Pabst A, Baber R, et al. Social determinants and lifestyle factors for brain health: implications for risk reduction of cognitive decline and dementia. Sci Rep. Jul 28 2022;12(1):12965. doi: https://doi.org/10.1038/s41598-022-16771-6 Article PubMed PubMed Central Google Scholar Olmastroni E, Molari G, De Beni N, et al. Statin use and risk of dementia or Alzheimer’s disease: a systematic review and meta-analysis of observational studies. Eur J Prev Cardiol. May 5 2022;29(5):804–814. doi: https://doi.org/10.1093/eurjpc/zwab208 Article PubMed Google Scholar Brain J, Greene L, Tang EYH, et al. Cardiovascular disease, associated risk factors, and risk of dementia: An umbrella review of meta-analyses. Front Epidemiol. 2023;3:1095236. doi: https://doi.org/10.3389/fepid.2023.1095236 Ma C, Yin Z, Zhu P, Luo J, Shi X, Gao X. Blood cholesterol in late-life and cognitive decline: a longitudinal study of the Chinese elderly. Mol Neurodegener. Mar 7 2017;12(1):24. doi: https://doi.org/10.1186/s13024-017-0167-y Pokharel Y, Mouhanna F, Nambi V, et al. ApoB, small-dense LDLC, Lp(a), LpPLA2 activity, and cognitive change. Neurology. May 28 2019;92(22):e2580–e2593. doi: https://doi.org/10.1212/WNL.0000000000007574 Article CAS PubMed PubMed Central Google Scholar Hui L, Chen X, Geiger JD. Endolysosome involvement in LDL cholesterol-induced Alzheimer’s disease-like pathology in primary cultured neurons. Life Sci. Dec 10 2012;91(23–24):1159–68. doi: https://doi.org/10.1016/j.lfs.2012.04.039 Boland B, Kumar A, Lee S, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. Jul 2 2008;28(27):6926–37. doi: https://doi.org/10.1523/JNEUROSCI.0800-08.2008 Hamano T, Gendron TF, Causevic E, et al. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. Mar 2008;27(5):1119–30. doi: https://doi.org/10.1111/j.1460-9568.2008.06084.x Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. Apr 2014;71(4):505–8. doi: https://doi.org/10.1001/jamaneurol.2013.5847 Park JC, Han SH, Yi D, et al. Plasma tau/amyloid-beta1-42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer’s disease. Brain. Mar 1 2019;142(3):771–786. doi: https://doi.org/10.1093/brain/awy347 Nägga K, Gustavsson AM, Stomrud E, et al. Increased midlife triglycerides predict brain β-amyloid and tau pathology 20 years later. Neurology. Jan 2 2018;90(1):e73–e81. doi: https://doi.org/10.1212/wnl.0000000000004749 Reed B, Villeneuve S, Mack W, DeCarli C, Chui HC, Jagust W. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol. Feb 2014;71(2):195–200. doi: https://doi.org/10.1001/jamaneurol.2013.5390 Picard C, Nilsson N, Labonté A, et al. Apolipoprotein B is a novel marker for early tau pathology in Alzheimer’s disease. Alzheimers Dement. May 2022;18(5):875–887. doi: https://doi.org/10.1002/alz.12442 Article CAS PubMed Google Scholar Byun MS, Yi D, Lee JH, et al. Korean Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer’s Disease: Methodology and Baseline Sample Characteristics. Psychiatry Investig. Nov 2017;14(6):851–863. doi: https://doi.org/10.4306/pi.2017.14.6.851 Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. Nov 1993;43(11):2412–4. doi: https://doi.org/10.1212/wnl.43.11.2412-a Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. Sep 2004;256(3):240–6. doi: https://doi.org/10.1111/j.1365-2796.2004.01380.x Article CAS Google Scholar American Psychiatric Association A, Association AP. Diagnostic and statistical manual of mental disorders: DSM-IV. vol 4. American psychiatric association Washington, DC; 1994. Frisoni GB, Winblad B, O’Brien JT. Revised NIA-AA criteria for the diagnosis of Alzheimer’s disease: a step forward but not yet ready for widespread clinical use. International Psychogeriatrics. 2011;23(8):1191–1196. doi: https://doi.org/10.1017/S1041610211001220 Lee JH, Lee KU, Lee DY, et al. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci. Jan 2002;57(1):P47–53. doi: https://doi.org/10.1093/geronb/57.1.p47 DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. Jul 27 2004;63(2):220–7. doi: https://doi.org/10.1212/01.wnl.0000130531.90205.ef Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. Apr 21 2009;106(16):6820–5. doi: https://doi.org/10.1073/pnas.0900345106 Jack CR, Jr., Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. Mar 2017;13(3):205–216. doi: https://doi.org/10.1016/j.jalz.2016.08.005 Tsai JZ, Peng SJ, Chen YW, et al. Automated segmentation and quantification of white matter hyperintensities in acute ischemic stroke patients with cerebral infarction. PLoS One. 2014;9(8):e104011. doi: https://doi.org/10.1371/journal.pone.0104011 Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet. May 11 1991;337(8750):1158–9. doi: https://doi.org/10.1016/0140-6736(91)92823-k Authors/Task Force M, Guidelines ESCCfP, Societies ESCNC. 2019 ESC/ EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. Nov 2019;290:140–205. doi: https://doi.org/10.1016/j.atherosclerosis.2019.08.014 Article Google Scholar Cramer C, Haan MN, Galea S, Langa KM, Kalbfleisch JD. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. Jul 29 2008;71(5):344–50. doi: https://doi.org/10.1212/01.wnl.0000319647.15752.7b Li G, Larson EB, Sonnen JA, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. Aug 28 2007;69(9):878–85. doi: https://doi.org/10.1212/01.wnl.0000277657.95487.1c Chen X, Hui L, Geiger JD. Role of LDL cholesterol and endolysosomes in amyloidogenesis and Alzheimer’s disease. J Neurol Neurophysiol. Oct 1 2014;5(5)doi: https://doi.org/10.4172/2155-9562.1000236 Yassine HN, Feng Q, Chiang J, et al. ABCA1-Mediated Cholesterol Efflux Capacity to Cerebrospinal Fluid Is Reduced in Patients With Mild Cognitive Impairment and Alzheimer’s Disease. J Am Heart Assoc. Feb 12 2016;5(2) doi: https://doi.org/10.1161/jaha.115.002886 Martins IJ, Hone E, Foster JK, et al. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry. Aug 2006;11(8):721–36. doi: https://doi.org/10.1038/sj.mp.4001854 Jeong W, Lee H, Cho S, Seo J. ApoE4-Induced Cholesterol Dysregulation and Its Brain Cell Type-Specific Implications in the Pathogenesis of Alzheimer’s Disease. Mol Cells. Nov 30 2019;42(11):739–746. doi: https://doi.org/10.14348/molcells.2019.0200 CAS PubMed PubMed Central Google Scholar Thirumangalakudi L, Prakasam A, Zhang R, et al. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem. Jul 2008;106(1):475–85. doi: https://doi.org/10.1111/j.1471-4159.2008.05415.x Laurent C, Buée L, Blum D. Tau and neuroinflammation: What impact for Alzheimer’s Disease and Tauopathies? Biomed J. Feb 2018;41(1):21–33. doi: https://doi.org/10.1016/j.bj.2018.01.003 Lee DC, Rizer J, Selenica ML, et al. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J Neuroinflammation. Sep 16 2010;7:56. doi: https://doi.org/10.1186/1742-2094-7-56 Garwood CJ, Cooper JD, Hanger DP, Noble W. Anti-inflammatory impact of minocycline in a mouse model of tauopathy. Front Psychiatry. 2010;1:136. doi: https://doi.org/10.3389/fpsyt.2010.00136 Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. Sep 28 2005;25(39):8843–53. doi: https://doi.org/10.1523/jneurosci.2868-05.2005 Bolmont T, Haiss F, Eicke D, et al. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. Apr 16 2008;28(16):4283–92. doi: https://doi.org/10.1523/jneurosci.4814-07.2008 Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. Oct 2006;112(4):389–404. doi: https://doi.org/10.1007/s00401-006-0127-z Wingo TS, Cutler DJ, Wingo AP, et al. Association of Early-Onset Alzheimer Disease With Elevated Low-Density Lipoprotein Cholesterol Levels and Rare Genetic Coding Variants of APOB. JAMA Neurol. Jul 1 2019;76(7):809–817. doi: https://doi.org/10.1001/jamaneurol.2019.0648 Chan RB, Oliveira TG, Cortes EP, et al. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem. Jan 20 2012;287(4):2678–88. doi: https://doi.org/10.1074/jbc.M111.274142 Liu Q, Trotter J, Zhang J, et al. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J Neurosci. Dec 15 2010;30(50):17068–78. doi: https://doi.org/10.1523/jneurosci.4067-10.2010 Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. Aug 13 2009;63(3):287–303. doi: https://doi.org/10.1016/j.neuron.2009.06.026 Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75–80. doi: https://doi.org/10.1159/000231980 Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. Jan 25 2005;64(2):277–81. doi: https://doi.org/10.1212/01.WNL.0000149519.47454.F2 Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. Jama. May 19 2015;313(19):1939–49. doi: https://doi.org/10.1001/jama.2015.4669 Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. Jama. May 19 2015;313(19):1924–38. doi: https://doi.org/10.1001/jama.2015.4668 Smit RA, Trompet S, Sabayan B, et al. Higher Visit-to-Visit Low-Density Lipoprotein Cholesterol Variability Is Associated With Lower Cognitive Performance, Lower Cerebral Blood Flow, and Greater White Matter Hyperintensity Load in Older Subjects. Circulation. Jul 19 2016;134(3):212–21. doi: https://doi.org/10.1161/CIRCULATIONAHA.115.020627 Duan D, Shen L, Cui C, Shu T, Zheng J. Association between Low-density lipoprotein cholesterol and occipital periventricular hyperintensities in a group of Chinese patients: an observational study. Lipids Health Dis. Feb 27 2017;16(1):48. doi: https://doi.org/10.1186/s12944-017-0436-3 Dias HK, Brown CL, Polidori MC, Lip GY, Griffiths HR. LDL-lipids from patients with hypercholesterolaemia and Alzheimer’s disease are inflammatory to microvascular endothelial cells: mitigation by statin intervention. Clin Sci (Lond). Dec 2015;129(12):1195–206. doi: https://doi.org/10.1042/CS20150351 Download references AcknowledgementsWe thank the KBASE study participants and their caregivers. A complete list of KBASE research group members can be found at http://kbase.kr/eng/about/research.php . We also thank AVID Radiopharmaceuticals for providing the precursor of [18F] AV-1451. Funding: This study was supported by a grant from the Ministry of Science and ICT, Republic of Korea (grant No: 2014M3C7A1046042 and RS-2022-00165636), a grant from the Ministry of Health & Welfare, Republic of Korea (HI18C0630, HI19C0149, and HU23C0140), a grant from the Seoul National University Hospital, Republic of Korea (No. 3020200030) and a grant from the National Institute of Aging, United States of America (U01AG072177). The funding source had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit it for publication. Author informationAuthors and affiliations. Department of Psychiatry, Seoul National University College of Medicine, Seoul, Republic of Korea S. M. Han, M. S. Byun & Dong Young Lee Department of Neuropsychiatry, Seoul National University Hospital, 101, Daehak-ro, Jongno-gu, Seoul, Republic of Korea, 03080 M. S. Byun, G. J. Jung, J.-Y. Lee & Dong Young Lee Institute of Human Behavioral Medicine, Medical Research Center, Seoul National University, Seoul, Republic of Korea D. Yi & Dong Young Lee Department of Neuropsychiatry, Chungbuk National University Hospital, Cheongju, Republic of Korea Department of Psychiatry, Keimyung University Dongsan Hospital, Daegu, Republic of Korea Department of Psychiatry, Sanggye Paik Hospital, College of Medicine, Inje University, Seoul, Republic of Korea Y. Y. Chang Department of Neuropsychiatry, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Republic of Korea Department of Neuropsychiatry, SMG-SNU Boramae Medical Center, Seoul, Republic of Korea Department of Nuclear Medicine, Seoul National University Hospital, Seoul, Republic of Korea Department of Nuclear Medecine, SMG-SNU Boramae Medical Center, Seoul, Republic of Korea Department of Radiology, Seoul National University Hospital, Seoul, Republic of Korea K. M. Kang & C.-H. Sohn Interdisciplinary program of cognitive science, Seoul National University College of Humanities, Seoul, Republic of Korea Dong Young Lee You can also search for this author in PubMed Google Scholar ContributionsAuthors’ contributions: Seung Min Han: Study concept and design; acquisition of data, analysis and interpretation of data; drafting and critical revision of manuscript for intellectual content. Min Soo Byun: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Dahyun Yi: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Joon Hyung Jung: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Nayeong Kong: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Yoonyoung Chang: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Musung Keum: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Gi Jung Jung: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Jun-Young Lee: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Yun-Sang Lee: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Yu Kyeong Kim,: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Koung Mi Kang: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Chul-Ho Sohn: Acquisition of data; drafting and critical revision of manuscript for intellectual content. Dong Young Lee: Study concept and design; acquisition of data; analysis and interpretation of data; drafting and critical revision of manuscript for intellectual content. Corresponding authorCorrespondence to Dong Young Lee . Ethics declarationsEthics approval and consent to participate: The study was approved by the Institutional Review Boards of Seoul National University Hospital (IRB No: C-1401-027-547) and SNU-SMG Boramae Medical Center (IRB No: 26-2015-60). All procedures were in accordance with the latest version of the Declaration of Helsinki. Written informed consent was obtained from all individual participants included in the study or their authorized representatives. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests. Data Statement: The data has not been previously presented orally or by poster at scientific meetings. Electronic supplementary materialSupplementary material, approximately 52 kb., rights and permissions. Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. Reprints and permissions About this articleHan, S.M., Byun, M.S., Yi, D. et al. Modulatory Effect of Blood LDL Cholesterol on the Association between Cerebral Aβ and Tau Deposition in Older Adults. J Prev Alzheimers Dis (2024). https://doi.org/10.14283/jpad.2024.131 Download citation Received : 26 January 2024 Accepted : 12 June 2024 Published : 02 July 2024 DOI : https://doi.org/10.14283/jpad.2024.131 Share this articleAnyone you share the following link with will be able to read this content: Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative
 |
IMAGES
VIDEO
COMMENTS
ERHM publishes original articles and reviews on novel findings and hypotheses in medicine, with an emphasis on epidemiology, infectious diseases, cancers, and artificial intelligence. The journal covers a wide range of topics, such as new methods, discoveries, risk factors, and technologies in medicine.
Exploratory Research and Hypothesis in Medicine (ERHM) publishes original exploratory research articles and state-of-the-art reviews that focus on novel findings and the most recent scientific advances that support new hypotheses in medicine, with an emphasis on epidemiology, infectious diseases, cancers, cardiovascular diseases, artificial intelligence, and machine learning.
ERHM publishes original exploratory research articles and state-of-the-art reviews that focus on novel findings and the most recent scientific advances that support new hypotheses in medicine. The journal covers topics such as epidemiology, infectious diseases, cancers, cardiovascular diseases, artificial intelligence, and machine learning.
Exploratory research and hypothesis in medicine 5 (2), 1. , 2020. 97. 2020. Associations of stay-at-home order and face-masking recommendation with trends in daily new cases and deaths of laboratory-confirmed COVID-19 in the United States. J Xu, S Hussain, G Lu, K Zheng, S Wei, W Bao, L Zhang.
INTRODUCTION. Scientific research is usually initiated by posing evidenced-based research questions which are then explicitly restated as hypotheses.1,2 The hypotheses provide directions to guide the study, solutions, explanations, and expected results.3,4 Both research questions and hypotheses are essentially formulated based on conventional theories and real-world processes, which allow the ...
ISSN 2472-0712 (Online) | Exploratory research and hypothesis in medicine. Skip to main content. Leave this field blank . Log In; Automatic login IP; PUBLISHERS' AREA DISCOVER ISSN SERVICES SEARCH OPEN ACCESS RESOURCES ... Exploratory research and hypothesis in medicine. Identifiers. ISSN : 2472-0712. Linking ISSN (ISSN-L): 2472-0712. Resource ...
Exploratory Research and Hypothesis in Medicine 2019;4(1):1-11. doi: 10.14218/ ERHM.2018.00024. Introduction The rapid global growth in our ageing population has resulted in an increase in neurodegenerative conditions, such as dementia, which results in cognitive decline, loss of functional capacity and reduced
ATG7, ATG16L1, etc.are associated with the pathogenesis of vari- ous cancers.1,3. In the current issue of Exploratory Research and Hypothesis in Medicine, Shawn Gurwara et al. has reported that dysregula- tion of autophagy pathways could be a leading mechanism in the carcinogenesis of human colorectal cancer.
The researcher conducts exploratory research by reviewing medical records and interviewing patients and their families to identify potential risk factors. ... Useful for hypothesis generation: Exploratory research can be useful for generating hypotheses and testable propositions that can be further explored through more rigorous research methods.
The research hypothesis is remarkably like a research question except for the fact that in a hypothesis the researcher assumes either positively or negatively about a causality, relation, correlation, and association. ... ethics in medical research and practice assumes increased significance as most health-related research is undertaken to find ...
EXPLORATORY RESEARCH AND HYPOTHESIS IN MEDICINE CONTENTS 2018 3(1):1-19 Editorials ... the molecular features of disseminated tumor cells from various biopsies can aid in future control and . . Exploratory Research and Hypothesis in Medicine 2018 vol. * 1.. 20 * Hypothesis. 1 3 10.
Learn how to prepare and submit your manuscript to ERHM, an open access journal that publishes exploratory and hypothesis-driven studies in biomedicine. Find out the article types, formatting guidelines, ethics policies, and peer review process of ERHM.
Abstract. While hypotheses frame explanatory studies and provide guidance for measurement and statistical tests, deductive, exploratory research does not have a framing device like the hypothesis. To this purpose, this article examines the landscape of deductive, exploratory research and offers the working hypothesis as a flexible, useful ...
Exploratory Research and Hypothesis in Medicine 2021 vol. 6(4) | 205-206 DOI: 10.14218/ERHM.2021.000RA Reviewer Acknowledgement 2021 Reviewer Acknowledgement Editorial Office of Exploratory Research and Hypothesis in Medicine Piero Amodio Italy John J. Araujo Colombia Muthuswamy Balasubramanyam India Andre Gustavo Bonavita Brazil Yulong Cao ...
Aim & Scope. Exploratory Research and Hypothesis in Medicine (ERHM) publishes original exploratory research articles and state-of-the-art reviews that focus on novel findings and the most recent scientific advances that support new hypotheses in medicine. The journal accepts a wide range of topics, including innovative diagnostic and ...
Exploratory Research and Hypothesis in Medicine 2022 vol. 7(4) | 277-278 DOI: 10.14218/ERHM.2022.000RA Reviewer Acknowledgement 2022 Reviewer Acknowledgement Editorial Office of Exploratory Research and Hypothesis in Medicine Bamidele Adebayo Nigeria Abel Nosereme Agbon Nigeria Vijender Anand India Maurizio Aricò Italy Anda Băicuș Romania
A journal that publishes original research and hypotheses in various fields of medicine. Browse the latest documents on topics such as COVID-19, diabetes, heel pain, and more.
eISSN 2472-0712 A Biomedical Open Access Journal EXPLORATORY RESEARCH AND HYPOTHESIS IN MEDICINE Volume 5 Issue 1, March 2020 XIA & HE ... Exploratory Research and Hypothesis in Medicine 2020;5(1):27-37. conditions, including hyperplasia and dysplasia, and a reduced doi: 10.14218/ERHM.2019.00029. rate of proliferation, which can lead to the ...
Exploratory Research and Hypothesis in Medicine 2021; 6 (3): 125-134. doi: 10.14218/ERHM.2020.00062. Abstract. Alzheimer disease (AD) has been viewed as the quintessential neurodegenerative disorder, and has defied decades of extensive research to find safe and effective disease-modifying treatment approaches. ... Exploratory Research and ...
A paper published in the journal Exploratory Research and Hypothesis in Medicine reviews the current state of immunotherapy in CRC, focusing on its use as first-line treatment, challenges with ...
low coronary flow (SCF).1995.41 It results from a naturally occurring point replacement of guanine (G) to thymine (T) at nucleotide 1917 in. exon 7 of the NOS3 gene. The consequence of this is a further replacement of glutamic acid by aspartic acid at codon 298, known as Glu298Asp (also commonly.
The research is published in the journal Exploratory Research and Hypothesis in Medicine. Chemical stimulation of blood vessels due to intravenous drugs is a primary factor causing extravasation ...
The relationship between amyloidosis and vasculature in cognitive impairment and Alzheimer's disease (AD) pathogenesis is increasingly acknowledged. We conducted a quantitative and topographic assessment of retinal perivascular amyloid plaque (AP) distribution in individuals with both normal and impaired cognition. Using a retrospective dataset of scanning laser ophthalmoscopy fluorescence ...
DOI: 10.14218/ERHM.2017.00004 | Volume 3 Issue 2, June 2018 25 Ali A. et al:HSP 90 and LQTS Explor Res Hypothesis Med 65.04 at p≤ 0.00001 was obtained, indicating that the "CG" geno- type carriers might have bad/poor prognosis in their subsequent generation due to segregation of the recessive alleles (Table 3).
Background This study investigates the synergistic relationship between blood low-density lipoprotein cholesterol (LDL-C) and cerebral beta-amyloid (Aβ) in relation to tau deposition, a key factor in the pathology of Alzheimer's disease (AD), in older adults across a diverse cognitive spectrum. Objectives To examine whether higher levels of LDL-C in the blood moderate the association of ...