Loading metrics
Open Access
Peer-reviewed
Research Article

Systematic review and meta-analysis of Tuberculosis and COVID-19 Co-infection: Prevalence, fatality, and treatment considerations
Roles Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing
¶ ‡ QW, YC, and XL are joint first authors of this paper and they contributed equally.
Affiliations School of Public Health, Peking University, Beijing, China, Brown School, Washington University in St Louis, St Louis, Missouri, United States of America
Roles Data curation, Formal analysis, Investigation, Validation
Affiliation Jinan Municipal Center for Disease Control and Prevention, Jinan, Shandong Province, China
Roles Data curation, Formal analysis, Investigation, Validation, Writing – review & editing
Roles Writing – review & editing
Affiliation School of Public Health, Peking University, Beijing, China
Affiliation Centre for Global Health Economics, University College London, London, United Kingdom
Affiliation Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada
Roles Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing
* E-mail: [email protected]
- Quan Wang,
- Yanmin Cao,
- Xinyu Liu,
- Yaqun Fu,
- Jiawei Zhang,
- Yeqing Zhang,
- Lanyue Zhang,
- Xiaolin Wei,

- Published: May 13, 2024
- https://doi.org/10.1371/journal.pntd.0012136
- Reader Comments
Tuberculosis (TB) and COVID-19 co-infection poses a significant global health challenge with increased fatality rates and adverse outcomes. However, the existing evidence on the epidemiology and treatment of TB-COVID co-infection remains limited.
This updated systematic review aimed to investigate the prevalence, fatality rates, and treatment outcomes of TB-COVID co-infection. A comprehensive search across six electronic databases spanning November 1, 2019, to January 24, 2023, was conducted. The Joanna Briggs Institute Critical Appraisal Checklist assessed risk of bias of included studies, and meta-analysis estimated co-infection fatality rates and relative risk.
From 5,095 studies screened, 17 were included. TB-COVID co-infection prevalence was reported in 38 countries or regions, spanning both high and low TB prevalence areas. Prevalence estimates were approximately 0.06% in West Cape Province, South Africa, and 0.02% in California, USA. Treatment approaches for TB-COVID co-infection displayed minimal evolution since 2021. Converging findings from diverse studies underscored increased hospitalization risks, extended recovery periods, and accelerated mortality compared to single COVID-19 cases. The pooled fatality rate among co-infected patients was 7.1% (95%CI: 4.0% ~ 10.8%), slightly lower than previous estimates. In-hospital co-infected patients faced a mean fatality rate of 11.4% (95%CI: 5.6% ~ 18.8%). The pooled relative risk of in-hospital fatality was 0.8 (95% CI, 0.18–3.68) for TB-COVID patients versus single COVID patients.
TB-COVID co-infection is increasingly prevalent worldwide, with fatality rates gradually declining but remaining higher than COVID-19 alone. This underscores the urgency of continued research to understand and address the challenges posed by TB-COVID co-infection.
Author summary
Tuberculosis (TB) and COVID-19, both highly infectious diseases, have posed significant global health challenges, particularly in low/middle-income countries (LMICs) with limited medical resources. Our research highlights that TB-COVID co-infection remains a substantial concern, impacting regions with varying TB burdens. The predominant treatment approach for TB-COVID co-infection has not notably evolved since our earlier study in 2021. It typically involves a combination of the recommended TB regimen and standard COVID-19 treatment. Our analysis consistently shows that individuals with TB-COVID co-infection are at heightened risk of hospitalization, protracted recovery periods, and accelerated mortality compared to those with sole COVID-19 infections. Remarkably, we found limited information on the post-COVID-19 condition of co-infected patients. One study indicated a higher prevalence of anxiety symptoms, highlighting the potential psychological toll of TB-COVID co-infection. Although the fatality rate has gradually decreased, it remains notably higher than that of COVID-19 alone. Our findings underscore the urgent need for global collaboration to address the complex challenges posed by TB-COVID co-infection, particularly in countries with limited medical resources.
Citation: Wang Q, Cao Y, Liu X, Fu Y, Zhang J, Zhang Y, et al. (2024) Systematic review and meta-analysis of Tuberculosis and COVID-19 Co-infection: Prevalence, fatality, and treatment considerations. PLoS Negl Trop Dis 18(5): e0012136. https://doi.org/10.1371/journal.pntd.0012136
Editor: Dileepa Ediriweera, University of Kelaniya Faculty of Medicine, SRI LANKA
Received: September 5, 2023; Accepted: April 5, 2024; Published: May 13, 2024
Copyright: © 2024 Wang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting information files.
Funding: LY was supported by grant from National Natural Science Foundation of China [72174010] and Natural Science Foundation of Beijing Municipality [M22033]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The ongoing COVID-19 pandemic has created an unprecedented healthcare crisis, especially in low/middle-income countries (LMICs) where medical resources are severely limited [ 1 , 2 ]. Unfortunately, these countries are also heavily burdened by tuberculosis (TB), with their populations being the main victims of this disease [ 3 ]. World Health Organization (WHO) has emphasized that the COVID-19 pandemic has not only disrupted TB services and response but also reversed years of progress made in the fight against tuberculosis [ 4 , 5 ]. Consequently, more people have fallen ill with TB and experienced higher mortality rates, timely diagnosis rates have decreased, and global spending on essential TB services has significantly declined [ 6 ].
A systematic review, encompassing evidence from 2019 to mid-2021, revealed a consistent upward trend in the absolute number of co-infected patients. Furthermore, an increasing number of countries reported co-infected patients, including both high-income countries and LMICs [ 7 ]. TB, as one of the world’s deadliest infectious diseases, comes second only to COVID-19 in terms of its impact[ 8 ]. Some experts believe that TB-COVID co-infection is associated with a poorer prognosis and a higher risk of mortality[ 9 , 10 ]. It is crucial to note that despite an exhaustive review, we did not encounter a universally accepted definition for TB–COVID co-infection. In this context, our systematic analysis provides a preliminary characterization, defining TB–COVID co-infection as a state arising from both ongoing and past infections involving M . tuberculosis and SARS-CoV-2. It’s essential to emphasize that while latent TB infection and TB disease (or active TB) present significant clinical distinctions, our usage of ’TB’ in this study encompasses all forms of M . tuberculosis infection, spanning latent, active, cured, and current states.
While there have been studies that have synthesized evidence on co-infection, they have primarily relied on case reports and case series, providing relatively weak support for epidemiology and treatment [ 11 , 12 ]. Consequently, there remains a dearth of information regarding the treatment and outcomes of TB-COVID co-infection, and a lack of consensus regarding its epidemiological status. This study serves as an update to our previous systematic review, which collected and pooled evidence as of the middle of 2021[ 7 ]. In this updated systematic review, we aim to summarize the latest epidemiological data on TB-COVID co-infection, discuss fatality rates, and explore possible clinical outcomes.
This systematic review follows the PRISMA guidelines ( S1 Table ) [ 13 ]. The study was registered in PROSPERO’s database with the registration number CRD42021253660.
Search strategy
We conducted a comprehensive search using six electronic databases: MEDLINE, Web of Science, ProQuest, Scopus, Cochrane database, and Embase. To maximize the scope of our search, we also employed the Grey Matters Checklist to identify relevant grey literature [ 14 ]. The literature search was conducted until January 24, 2023. Medical Subject Heading (MeSH) terms, title/abstract, topic, or subject words were used in the selected databases. The search formula included the terms "TB" AND "COVID-19". For "TB," key terms such as "tuberculosis," "TB," "tuberculos*," "mycobacterium tuberculosis," and "m.tuberculosis" were used. For "COVID-19," the key terms used were "COVID-19" and "SARS-COV-2".
Eligibility criteria of included studies
This systematic review included epidemiological and fatality data on TB-COVID co-infection from cohort studies, cross-sectional studies, and experimental research, excluding case reports, series, reviews, editorials, and clinical guidelines. Studies with sample sizes less than 20 were also excluded to reduce potential bias. Two reviewers (QW and XL) independently screened and selected studies using Covidence. Non-English and non-Chinese articles were translated to English using TranslateGo (Hangzhou Qingxun Science and Technology Co., China). Manual reference screening ensured study inclusivity. Conflicts were resolved by a third author (LY), and duplicates were managed across similar studies. We would like to stress that, unlike our previous work in 2021, we did not include case reports or case series in this study. Building on the insights from our earlier research, we found that these study types contributed little to our understanding of the topic, and they did not provide sufficient data for estimating fatality rates, prevalence status, or determining best practices in treatment.
Data extraction, quality assessment, and analysis
Relevant data, including authors, publication dates, study design, location, sample size, settings, epidemiological and treatment information, and clinical outcomes, were extracted. Prevalence rates of co-infection were prioritized for epidemiological data, along with total and hospitalized fatality rates. The total fatality rate represents the proportion of patients documented as deceased among all TB-COVID co-infected individuals, irrespective of whether they received treatment. On the other hand, the hospitalized fatality rate pertains to the proportion of patients documented as deceased among all TB-COVID co-infected individuals who underwent hospitalization. Treatment details, including drugs and ICU utilization, were also collected. The quality of included studies was evaluated using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Study Reporting Prevalence Data [ 15 ].
Location data from all studies identified the reporting countries and regions of TB-COVID co-infection cases. Prevalence and fatality rates were chronologically listed for temporal trends analysis.
Random-effects meta-analysis calculated pooled fatality rates and relative risks (RR) of fatality between TB-COVID co-infection and single COVID-19 patients. Forest plots displayed point estimates and 95% confidence intervals (CIs), while I 2 assessed heterogeneity. P values < 0.05 indicated statistical significance.
Egger’s tests assessed publication bias, and sensitivity analyses assessed robustness by omitting studies one at a time. Subgroup analyses explored LMICs vs. high-income countries and active TB vs. previous TB status. Stata 17 (StataCorp LLC, USA) performed calculations.
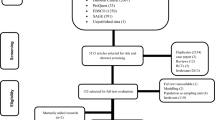
A comprehensive search strategy utilizing the building blocks approach was executed to identify pertinent studies. After an extensive search, we retrieved 1,792 records from MEDLINE, 2,863 from Web of Science, 2,404 from ProQuest, 2,928 from Scopus, 1,314 from the Cochrane database, 1,962 from Embase, and 61 from Grey Matters Checklist (refer to S2 Table for details). Upon importing these records into Covidence, 8,229 duplicate records were identified and subsequently removed, resulting in 5,095 records available for title and abstract screening. In this phase, 4,391 records were excluded. The remaining 704 records entered the full-text review process, during which 38 potentially relevant records were identified. Ultimately, 689 out of 704 records and 36 out of 38 records were excluded, and 17 retrospective studies were included for analysis; no experimental studies were identified in the search. The entire process is visually presented in Fig 1 .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pntd.0012136.g001
As of the search date, our analysis identified TB-COVID co-infection cases reported in 38 countries or regions, including Argentina, Belarus, Belgium, Brazil, Chile, China, France, Republic of Guinea, India, Italy, Mexico, Niger, Pakistan, Panama, Peru, Philippines, Portugal, Romania, Russia, Singapore, Spain, Switzerland, UK, Australia, Canada, Colombia, Greece, Honduras, Lithuania, the Netherlands, Oman, Paraguay, Serbia, Slovakia, South Africa, Turkey, Thailand, and USA. Among the studies included, there was one notable study conducted by the TB/COVID-19 Global Study Group in 2022, which involved TB-COVID patients from 172 centers in 34 countries. The remaining 16 studies reported patients within a single region or country [ 16 ].
Regarding the prevalence rate of TB-COVID co-infection, two studies provided information. The first study, conducted by the Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, analyzed data from the Western Cape Provincial Health Data Centre. They found a prevalence rate of approximately 0.04% among individuals aged 20 years or above in the Western Cape Province until 1 June 2020. After testing criteria changed, the prevalence rate increased to approximately 0.06% until 9 June 2020 [ 17 ]. The second study, led by Nabity in 2021, identified 6371 co-infected patients among all California residents between September 3, 2019, and December 31, 2020, resulting in a prevalence rate of approximately 0.02% [ 18 ]. The S3 Table provides detailed information on these two studies.
Among the studies included in our analysis, only a limited number of studies provided information on the treatment of TB-COVID co-infection. Upon comparing our findings with our previous study conducted in 2021, we did not identify any new treatments that have emerged. The most commonly utilized treatment approach involved the use of first-line anti-TB treatment (ATT) drugs, including rifampicin, isoniazid, ethambutol, and pyrazinamide, which were administered in the majority of cases. In terms of antiviral drugs, lopinavir, ritonavir, and arbidol were the three most frequently prescribed medications. Notably, the use of hydroxychloroquine (HCQ) has become limited, as it has been demonstrated to have no benefit in the treatment of TB-COVID co-infection [ 19 ]. Three studies included in our review highlighted the utilization of Intensive Care Units (ICUs) in the management of TB-COVID co-infection. The reported ICU admission rates varied from 1.3% to 31.8% [ 20 – 22 ]. Additionally, Wang discussed the usage of Paxlovid, an antiviral therapeutic for COVID-19 treatment, and emphasized its contraindication in patients receiving rifampicin, one of the first-line agents for TB treatment, due to drug interactions as Paxlovid is a strong cytochrome P450 3A4 inhibitor. Consequently, Paxlovid was not deemed suitable for treating patients with active TB-COVID co-infection undergoing ATT [ 21 ].
Several studies have indicated that TB-COVID co-infected patients face increased risks of hospitalization, longer time-to-recovery in elderly patients, and shorter time-to-death compared to individuals with single COVID-19 infection [ 21 , 23 – 25 ]. Parolina’s study highlighted various factors associated with an increased risk of developing severe COVID-19 in TB patients, including female gender, smoking, fever, dyspnea, disseminated TB, having three or more co-morbidities, and patient age[ 26 ]. Wang emphasized that despite the milder nature of infections with the Omicron variant compared to earlier variants, patients with TB-COVID co-infection do not exhibit the mild disease course observed in the general population [ 21 ]. Notably, the majority of patients in Wang’s study, 142 out of 153 co-infected individuals, were classified as nonsevere, with 10 being asymptomatic [ 21 ]. This may be attributed to lung parenchyma damage resulting from pulmonary remodeling due to persistent cavitation, fibrosis, or bronchiectasis, which is present in approximately 50% of cured TB patients and may increase susceptibility to COVID-19 and mortality rates [ 25 ]. The presence of dual lung damage following both TB and COVID-19 necessitates careful follow-up of patients with post-tuberculosis lung disease who have experienced COVID-19 pneumonia [ 25 ]. These findings underscore the complex interactions and challenges associated with TB-COVID co-infection. The coexistence of two lung diseases can lead to heightened severity and poorer outcomes, warranting specialized management approaches and continued monitoring of affected individuals. For more detailed information, please refer to S4 Table .
Fatality rate
A total of 17studies were included in our analysis, reporting data on the fatality rate of TB-COVID co-infection. The reported fatality rates among the total patient population varied widely, ranging from 0% to 23.6%. Similarly, the in-hospital fatality rates also showed considerable variation, ranging from 0% to 27.3% ( Table 1 ).
https://doi.org/10.1371/journal.pntd.0012136.t001
To further explore the impact of active TB and previous TB on fatality rates, we collected and analyzed information specific to these subgroups ( Table 2 ). Among co-infected patients with concurrent TB disease (active TB), the reported fatality rates ranged from 7.6% to 23.6% for the total patient population, and for hospitalized active TB-COVID patients, the fatality rates ranged from 0% to 27.3%. Regarding previous TB-COVID patients, the fatality rates ranged from 4.9% to 14.5% for the total patient population, and for hospitalized patients, the fatality rates ranged from 0% to 24.0%. Please refer to S4 Table and S5 Table , and S6 Table for comprehensive and detailed information about the included studies.
https://doi.org/10.1371/journal.pntd.0012136.t002
Quality assessment of included studies
We employed the JBI Critical Appraisal Checklist for Study Reporting Prevalence Data as a tool to assess the quality of the 17 included studies. The checklist consisted of 9 questions covering various aspects such as sampling method, sample size, study subjects and setting, analysis method, and participant response. Each question was evaluated using one of the four options: yes, no, unclear, or not applicable. In total, 10 studies reached more than 70% of ‘yes’ scores, 6 studies reached from 50% to 69% of ‘yes’ scores, and 1 study was below 50%. Upon further analysis, it was identified that the sample frame, sampling method, and sample size were the areas most frequently identified as having a higher risk of bias within the included studies. Check S7 Table and S1 Fig for assessment result of each study.
Meta-analysis of fatality rates
Among all included studies, the pooled fatality rate of TB-COVID co-infection among total patients was estimated to be 7.1% (95% CI, 4.0%-10.8%). However, when examining the results by country income status, significant variations were observed. In high-income countries (HICs), the pooled fatality rate was higher, with a result of 10.2% (95% CI, 9.4%-10.9%) based on two studies that included a total of 6,569 individuals. On the other hand, in low- and middle-income countries, the pooled fatality rate was lower at 5.8% (95% CI, 2.0%-11.3%), based on five studies involving 2,888 individuals ( Fig 2 ). The GTN’s study provided three cohorts: total co-infected patients, co-infected patients in Europe, and co-infected patients outside of Europe. Considering that most included countries in Europe are HICs and most countries outside of Europe are LMICs, we placed these two cohorts in the HICs and LMICs subgroups, respectively. The results of Egger’s test indicated no evidence of publication bias across all the included study groups, as well as within the low- and middle-income countries subgroup ( S8 Table and S2 Fig ). To assess the robustness of our pooled results, we performed sensitivity analyses by systematically omitting one study at a time. These analyses consistently demonstrated the stability and reliability of our pooled estimates ( S9 Table and S3 Fig ).
https://doi.org/10.1371/journal.pntd.0012136.g002
The estimated fatality rate among hospitalized patients with TB-COVID co-infection was 11.4% (95% CI, 5.6%-18.8%). It is important to note that significant heterogeneity was detected among the studies and groups analyzed. Unlike the total fatality rate, the results for low- and middle-income countries (LMICs) were similar to those of high-income countries (HICs) in terms of fatality rate among hospitalized patients. The pooled result for LMICs was 11.1% (95% CI, 4.0%-20.9%) based on eight studies involving 985 individuals. In comparison, the pooled result for HICs was 10.9% (95% CI, 5.9%-17.1%) based on four studies involving 148 individuals. These findings suggest a comparable fatality rate among hospitalized TB-COVID co-infection patients in both LMICs and HICs. For detailed results, please refer to Fig 3 . Based on the results of Egger’s tests, publication bias was observed in all included study groups. However, no evidence of publication bias was found within the 2 subgroups ( S10 Table and S4 Fig ). Furthermore, the sensitivity analysis, which involved systematically omitting one study at a time, demonstrated that the exclusion of any particular study did not significantly alter the pooled results. This finding supports the robustness and reliability of our study findings ( S11 Table and S5 Fig ).
https://doi.org/10.1371/journal.pntd.0012136.g003
In our subgroup analysis based on TB status (active/previous), the pooled results revealed significant differences in the fatality rates between active TB-COVID infection and previous TB- COVID infection ( Fig 4 ). For total fatality rate, the pooled estimate for active TB-COVID infection was 10.6% (95% CI, 7.9%-13.6%), which was higher compared to previous TB-COVID infection with a pooled estimate of 5.7% (95% CI, 4.7%-6.7%). Regarding in-hospital fatality rate, the estimated pooled result for active TB-COVID infection was 9.8% (95% CI, 2.8%-19.8%) based on eight studies involving 739 individuals. In contrast, the in- hospital fatality rate for previous TB-COVID infection was higher, with a pooled estimate of 21.0% (95% CI, 16.7%-25.6%). Furthermore, Egger’s tests were conducted to assess publication bias, and the results can be found in S12 Table and S6 Fig . Additionally, sensitivity analyses were performed, and the results demonstrated the stability and robustness of the study findings (refer to S13 Table and S7 Fig ).
A: Total active TB-COVID co-infection patients; B: Total previous TB-COVID co-infection patients; C: Hospitalized active TB-COVID co-infection patients; D: Hospitalized previous TB-COVID co-infection patients.
https://doi.org/10.1371/journal.pntd.0012136.g004
Meta-analysis of relative risk
Three studies included in our analysis provided results on the relative risk (RR) of in-hospital fatality between TB-COVID patients and single COVID patients. The pooled analysis, which involved a total of 1285 patients, suggested that TB infection might potentially reduce the fatality risk with a relative risk estimate of 0.8 (95% CI, 0.18–3.68) ( S8 Fig ). Regarding publication bias, the results of Egger’s test indicated no evidence of publication bias in the included studies ( S9 Fig ). However, the sensitivity analysis revealed some instability in the pooled results, suggesting the need for caution in interpreting these findings ( S14 Table and S10 Fig ).
This updated systematic review collected relevant studies up until January 24, 2023, and included a total of 17 studies. In comparison to a previous study that identified co-infection cases in 12 countries or regions based on population studies, our review expanded the scope and identified an additional 18 countries or regions reporting TB-COVID co-infection. This finding suggests that despite COVID-19 no longer being classified as a Public Health Emergency of International Concern by the WHO, the prevalence of TB-COVID co-infection remains significant in both high and low TB-burden countries or regions.
A notable contribution to the field is a large-scale study led by Nabity in 2021, which provided an updated prevalence rate estimate of approximately 0.02% in California, USA [ 18 ]. In contrast, an earlier study conducted in West Cape Province, South Africa in early 2020 reported a prevalence rate of 0.06%. The discrepancy in prevalence rates between these two regions suggests that the burden of TB-COVID co-infection may vary across different geographic locations. Factors such as differences in TB prevalence, COVID-19 incidence, and the effectiveness of TB and COVID-19 control measures implemented in each region may contribute to these variations.
In terms of treatment, our analysis revealed that the treatment approach for TB-COVID co-infection has not undergone significant changes since our previous study in 2021. The predominant strategy employed in the included studies involved the administration of first-line anti-TB drugs, which is in accordance with the established standard treatment protocol for TB. Despite our comprehensive review of the available literature, we did not identify any experimental studies that could provide specific guidance on the best practices for managing TB-COVID co-infection. Only a limited number of studies made any mention of adjustments to treatment regimens based on the unique characteristics of co-infected patients. As a result, the current approach to treatment for TB-COVID co-infection appears to be a combination of the recommended TB regimen and the standard treatment for COVID-19.
The studies that reported ICU utilization in the context of TB-COVID co-infection provided insights into the severity of the disease and the clinical management required. The wide range of reported ICU admission rates, ranging from 1.3% to 31.8%, highlights the heterogeneity in disease presentation and underscores the need for specialized care for individuals with severe forms of co-infection. These findings emphasize the importance of tailored management approaches that address the complex interactions between TB and COVID-19. Consistent findings across multiple studies indicate that individuals with TB-COVID co-infection face a higher risk of hospital admission, longer time-to-recovery, and shorter time-to-death compared to individuals with single COVID-19 infection [ 21 , 23 – 25 ]. These observations underscore the unique challenges posed by the coexistence of TB and COVID-19 and emphasize the necessity for tailored management strategies that effectively address both diseases.
Another important aspect to consider in the context of TB-COVID co-infection is the potential development of Post-COVID-19 condition (PCC), commonly known as long COVID [ 34 ]. PCC refers to a range of persistent symptoms and health issues that can affect individuals even after recovering from acute COVID-19 infection [ 35 ]. It has been observed that PCC can significantly impact a person’s daily functioning, employability, and overall well-being. Moreover, it has been associated with an increased risk of developing new health conditions and the utilization of healthcare services, which can further strain the individual’s financial stability [ 34 ]. However, it is worth noting that the current evidence regarding PCC specifically in the context of TB-COVID co-infection is scarce. We only identified one study that mentioned the proportion of individuals experiencing long-lasting symptoms after COVID-19 infection in conjunction with previous tuberculosis (PTB) treatment [ 36 ]. This study reported that over time, the proportion of individuals with persistent symptoms decreased, although a significant proportion, approximately one in six, still experienced ongoing symptoms. Furthermore, this group exhibited a higher prevalence of anxiety symptoms, underscoring the potential psychological impact of TB-COVID co-infection. The recurrence of pulmonary tuberculosis and the need for psychological support for individuals with a history of both COVID-19 and pulmonary TB after discharge warrant additional attention and investigation [ 36 ].
The meta-analyses conducted on the overall fatality rate of TB-COVID co-infection revealed an estimated rate of 7.1%, which is lower than our previous study’s estimate of 13.9%. This difference could potentially be attributed to the emergence of new SARS-CoV-2 variants that may exhibit milder clinical manifestations. However, it is important to note that the fatality rate of TB-COVID co-infection remains higher than that of COVID-19 alone, which was estimated at 0.68% by mid of 2020[ 37 ]. Subgroup analyses based on high-income countries and low- and middle-income countries showed a higher fatality rate in high-income countries (10.2%) compared to LMICs (5.8%). It is crucial to recognize that multiple confounding factors may contribute to this observed discrepancy. For instance, lower vigilance and delayed time-to-diagnosis in outpatient clinics, particularly in higher-income countries with traditionally lower TB burdens, could play a role. Another potential factor is the higher frequency of COVID-19 testing in high-income countries, which might dilute the numbers of identified active TB-COVID infection. Additionally, the average age of co-infected patients tends to be higher in HICs, and given that age is a proven risk factor for COVID-19 mortality, this demographic difference could contribute to the observed higher fatality rate. These findings underline the importance of considering various contextual factors when interpreting fatality rates and emphasize the need for further research to elucidate the complex dynamics at play. In terms of in-hospital fatality rates, the results were similar between high-income countries (11.1%) and LMICs (10.9%), further supporting the assumption mentioned above.
Our subgroup analysis based on TB status (active/previous) revealed significant differences in the fatality rates between active TB-COVID infection and previous TB-COVID infection. These findings highlight the differential risks and outcomes associated with active and previous TB in the context of COVID-19 co-infection. The reasons for these differences may be multifactorial. Active TB-COVID infection may impose a greater burden on the immune system and respiratory function, leading to increased susceptibility to severe COVID-19 illness and poorer outcomes. In contrast, individuals with previous TB may have partially developed immunity or residual lung damage, which could potentially confer some level of protection or adaptation against severe COVID-19. We acknowledge the variability in the status of TB infection extracted from the included origin studies, as there was no uniform standard criterion across different studies. Active TB is a complex disease with a lengthy treatment regimen, which is commonly defined as disease that occurs in someone infected with Mycobacterium tuberculosis . It is characterized by signs or symptoms of active disease, or both, and is distinct from latent tuberculosis infection, which occurs without signs or symptoms of active disease [ 38 ]. The absence of consistent definitions or criteria may have contributed to the heterogeneity observed in the meta-analysis.
An intriguing trend in current TB-COVID research centers around a significant focus on the pandemic’s impact on TB care services. Global studies have demonstrated a substantial adverse effect on the delivery, accessibility, and utilization of TB care services [ 39 ]. Comparing 2020 to 2019, there was an 18% reduction in global tuberculosis case detection, dropping from 7.1 million to 5.8 million cases, with up to a 24% decrease in the ten worst-affected countries with a high tuberculosis burden [ 5 ]. This service disruption in TB care has led to a consequential increase in additional tuberculosis-related deaths. From a critical thinking perspective, we posit that this impact might contribute to an augmentation in our estimated TB-COVID fatality rate in two crucial ways. Firstly, the reduction in tuberculosis case detection may result in fewer identified TB-COVID co-infected patients. This is particularly significant as COVID-related deaths are usually more rigorously recorded in many countries, and during this process, the TB infection can also be documented. Secondly, the disruption in TB care services might result in insufficient treatment for numerous co-infected individuals, potentially contributing to preventable deaths. This concern is particularly pronounced in LMICs, where healthcare services are often limited and of lower quality [ 40 , 41 ]. Additionally, the decrease in discovered cases of TB could contribute to a lower total number of identified co-infected patients.
In our analysis, we observed a relative risk (RR) value suggesting that TB-COVID co-infection might reduce the fatality risk compared to single COVID-19 infection. This finding may initially seem counterintuitive given that TB is a known risk factor for severe respiratory illness and mortality. It’s essential to emphasize that the groups with TB-COVID co-infection and those with single COVID-19 infection did not exhibit precisely homogeneous patient characteristics, including differences in age, gender, comorbidities, and treatment modalities. For instance, studies by Parolina and Sereda reported a higher proportion of male patients in the TB-COVID co-infection group compared to the single COVID-19 infection group [ 26 , 31 ]. Also of note is that Sy’s 2020 study, employing propensity score matched sampling, suggested that co-infected patients experienced higher fatality rates [ 24 ]. However, due to the limited information available regarding the specific details of the included patient groups, we cannot deduce the underlying reasons for this counterintuitive RR. Therefore, readers are advised to approach this finding with caution and interpret it within the acknowledged limitations we have outlined.
As a systematic review focused on TB-COVID co-infection, understanding how TB impacts COVID-19 is as crucial as comprehending how COVID-19 impacts TB. However, given the prominence of COVID-19 as a research topic, many studies at the individual level tend to emphasize the perspective of COVID-19 infection. While we did encounter studies exploring how COVID-19 impacts TB, these primarily delved into microbiological mechanisms or the pandemic’s disruption of TB service delivery. Immunologically, a shared dysregulation of immune responses in COVID-19 and TB has been identified, indicating a dual risk posed by co-infection in worsening COVID-19 severity and favoring TB disease progression [ 42 , 43 ]. Notably, for some severe COVID-19 patients, corticosteroid use can induce immunosuppression [ 44 ], significantly increasing the risk of new secondary infections and/or reactivation of existing quiescent TB infections [ 45 , 46 ]. From the TB service perspective, the COVID-19 pandemic has substantially impacted the normal delivery of TB services, exerting a negative influence on TB patients [ 39 ]. However, some studies suggest a potential reduction in Mycobacterium tuberculosis transmission during the pandemic, potentially lowering TB fatality rates [ 47 , 48 ]. Unfortunately, the current evidence is limited, and the impact of the pandemic on TB remains conflicting and inconclusive. We cautiously posit that COVID-19 exerts a negative influence on individuals already carrying Mycobacterium tuberculosis .
In our assessment of study quality, two critical bias factors emerged: insufficient sample size and unappreciated sample frame. Insufficient sample size refers to studies with limited participants, hampering findings’ generalizability. With relatively lower prevalence for TB-COVID co-infection compared to individual TB or COVID-19, obtaining a sizeable co-infected cohort, especially where TB and COVID-19 are rarer, becomes challenging. Limited sample size may curtail statistical power and precision, potentially biasing prevalence estimates. Unappreciated sample frame denotes studies unintentionally selecting populations misrepresenting the target group. Poorly described sampling or inclusion criteria misaligned with intended population characteristics can lead to biases. In TB-COVID co-infection, ensuring representation of individuals with both conditions, not biased subgroups, is vital. Incorrect sample framing may introduce biases and limit findings’ applicability.
While we recognize that a randomized controlled trial (RCT) stands as the gold standard for investigating treatments or risk factors, we contend that diverse study designs can offer valuable contributions to this field. In light of our current findings, we advocate for the consideration of a comparable sampling frame, such as the utilization of propensity score matched sampling in future studies. This approach allows for the creation of balanced groups, resembling the random assignment achieved in an RCT, thus minimizing selection bias and improving the internal validity of observational studies. Furthermore, we propose a more comprehensive description of patients’ baseline conditions and treatment regimens in subsequent research endeavors. This detailed information holds the potential to mitigate bias significantly. A thorough account of patients’ characteristics and treatment variables enhances the ability to control for confounding factors, providing a clearer understanding of the associations under investigation. Employing such strategies not only bolsters the robustness of observational studies but also facilitates the comparability of findings across different research designs.
Several limitations should be acknowledged in the interpretation of our findings. First, we did not include “comorbidity” as a keyword and MeSH term in the searching process, which might have resulted in the omission of relevant studies taking TB as a kind of comorbidity of COVID-19 patients. Second, the observational design precludes establishing causation, and although we employed rigorous statistical methods to control for confounding factors, residual confounders may persist. Third, the generalizability of our results may be influenced by the predominantly retrospective and multicentric nature of the included studies. Variability in healthcare settings, patient populations, diagnostic criteria, and treatment approaches across different regions and countries could impact the external validity of our findings. Additionally, the lack of uniformity in reporting across studies may have introduced inconsistencies in our data synthesis. Furthermore, the limited availability of detailed information on certain variables, such as socioeconomic status, comorbidities, and M . tuberculosis infection status, restricted our ability to conduct more granular subgroup analyses. As mentioned earlier, distinctions exist among latent, active, cured, and current M . tuberculosis infections. However, due to insufficient details, we faced considerable challenges in differentiating between these states. Finally, the evolving landscape of the COVID-19 pandemic and variations in healthcare infrastructure over time may have influenced treatment strategies and outcomes. Despite these limitations, our study provides valuable insights into the landscape of TB-COVID co-infection, emphasizing the need for further research to address these complexities comprehensively.
In conclusion, the fatality rate of co-infection declined gradually and still stayed higher than COVID-19 alone, underscoring the heightened vulnerability in co-infected individuals. Addressing this challenge requires targeted measures such as heightened awareness campaigns, improved screening strategies for TB infection, and the provision of comprehensive long COVID care for co-infected patients. Collaboration on a global scale may be beneficial in addressing the challenges posed by TB-COVID co-infection, particularly in regions with limited medical resources.
Supporting information
S1 table. the preferred reporting items for systematic reviews and meta-analyses (prisma) 2020 checklist..
https://doi.org/10.1371/journal.pntd.0012136.s001
S2 Table. Search strategies to identify studies reporting the prevalence status, treatment and outcomes of tuberculosis and COVID-19.
https://doi.org/10.1371/journal.pntd.0012136.s002
S3 Table. Studies reported prevalence rate (n = 2).
https://doi.org/10.1371/journal.pntd.0012136.s003
S4 Table. Detailed basic information of included studies (n = 17).
Detailed basic information of included case reports (n = 17).
https://doi.org/10.1371/journal.pntd.0012136.s004
S5 Table. The fatality rates of active and previous TB-COVID co-infection (n = 11).
The fatality rates of active TB-COVID co-infection (n = 11).
https://doi.org/10.1371/journal.pntd.0012136.s005
S6 Table. The fatality rates of previous TB-COVID co-infection (n = 3).
https://doi.org/10.1371/journal.pntd.0012136.s006
S7 Table. Quality assessment of each included study.
https://doi.org/10.1371/journal.pntd.0012136.s007
S8 Table. Egger’s test on total fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s008
S9 Table. Sensitives analysis on MA of total fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s009
S10 Table. Egger’s test on MA of In-hospital fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s010
S11 Table. Sensitives analysis on MA of In-hospital fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s011
S12 Table. Egger’s test on MA of active/previous TB-COVID co-infection fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s012
S13 Table. Sensitives analysis on MA of In-hospital fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s013
S14 Table. Sensitives analysis on RR of in-hospital fatality between TB-COVID patients and single COVID patients.
https://doi.org/10.1371/journal.pntd.0012136.s014
S1 Fig. Quality assessment of included studies (N = 17).
https://doi.org/10.1371/journal.pntd.0012136.s015
S2 Fig. Egger’s test on MA of total fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s016
S3 Fig. Sensitives analysis on MA of total fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s017
S4 Fig. Egger’s test on MA of In-hospital fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s018
S5 Fig. Sensitives analysis on MA of In-hospital fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s019
S6 Fig. Egger’s test on MA of hospitalized Active TB-COVID co-infection patients fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s020
S7 Fig. Sensitives analysis on MA of hospitalized Active TB-COVID co-infection patients fatality rate.
https://doi.org/10.1371/journal.pntd.0012136.s021
S8 Fig. Relative risk of in-hospital Fatality between TB-COVID co-infection and Single COVID-19 co-infection.
https://doi.org/10.1371/journal.pntd.0012136.s022
S9 Fig. Egger’s test on RR of in-hospital fatality between TB-COVID patients and single COVID patients.
https://doi.org/10.1371/journal.pntd.0012136.s023
S10 Fig. Sensitives analysis on RR of in-hospital fatality between TB-COVID patients and single COVID patients.
https://doi.org/10.1371/journal.pntd.0012136.s024
Acknowledgments
We would like to express our sincere thanks to Dr. Lusine Abrahamyan at University of Toronto for her kind help. We also want to present our best wishes to the front-line medical worker all over the world, and we believe their work of integrity and selflessness is key to ending the COVID-19 pandemic.
- View Article
- PubMed/NCBI
- Google Scholar
- 4. World Health Organization. Global tuberculosis report 2022. Geneva: World Health organization; 2022.
- 8. European Centre for Disease Prevention and Control. Tuberculosis remains one of the deadliest infectious diseases worldwide, warns new report 2022 [cited 2023 27, Jan]. Available from: https://www.ecdc.europa.eu/en/news-events/tuberculosis-remains-one-deadliest-infectious-diseases-worldwide-warns-new-report .
- 14. CADTH. Grey Matters: a practical tool for searching health-related grey literature Ottawa2018 [cited 2022 16, July]. Available from: https://www.cadth.ca/grey-matters-practical-tool-searching-health-related-grey-literature .
- 15. The JBI. CRITICAL APPRAISAL TOOLS 2022 [cited 2023 28, Jan]. Available from: https://jbi.global/critical-appraisal-tools .
- 38. World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations Geneva: World Health Organization,; 2013 [cited 2024 19, Feb]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK294076/ .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 16 May 2024
Catastrophic costs incurred by tuberculosis affected households from Thailand’s first national tuberculosis patient cost survey
- Sitaporn Youngkong 1 , 2 ,
- Phalin Kamolwat 3 ,
- Phichet Wongrot 4 ,
- Montarat Thavorncharoensap 1 , 2 ,
- Usa Chaikledkaew 1 , 2 ,
- Sriprapa Nateniyom 3 ,
- Petchawan Pungrassami 3 ,
- Naiyana Praditsitthikorn 5 ,
- Surakameth Mahasirimongkol 6 ,
- Jiraphun Jittikoon 7 ,
- Nobuyuki Nishikiori 8 ,
- Ines Garcia Baena 8 &
- Takuya Yamanaka 10 , 8 , 9
Scientific Reports volume 14 , Article number: 11205 ( 2024 ) Cite this article
1 Altmetric
Metrics details
- Health care economics
- Tuberculosis
Tuberculosis (TB) causes an economic impact on the patients and their households. Although Thailand has expanded the national health benefit package for TB treatment, there was no data on out-of-pocket payments and income losses due to TB from patients and their household perspectives. This national TB patient cost survey was conducted to examine the TB-related economic burden, and assess the proportion of TB patients and their households facing catastrophic total costs because of TB disease. A cross-sectional TB patient cost survey was employed following WHO methods. Structured interviews with a paper-based questionnaire were conducted from October 2019 to July 2021. Both direct and indirect costs incurred from the patient and their household perspective were valued in 2021 and estimated throughout pre- and post-TB diagnosis episodes. We assessed the proportion of TB-affected households facing costs > 20% of household expenditure due to TB. We analyzed 1400 patients including 1382 TB (first-line treatment) and 18 drug-resistant TB patients (DR-TB). The mean total costs per TB episode for all study participants were 903 USD (95% confident interval; CI 771–1034 USD). Of these, total direct non-medical costs were the highest costs (mean, 402 USD, and 95%CI 334–470 USD) incurred per TB-affected household followed by total indirect costs (mean, 393 USD, and 95%CI 315–472 USD) and total direct medical costs (mean, 107 USD, and 95%CI 81–133 USD, respectively. The proportion of TB-affected households facing catastrophic costs was 29.5% (95%CI 25.1–34.0%) for TB (first-line), 61.1% (95%CI 29.6–88.1%) for DR-TB and 29.9% (95%CI 25.6–34.4%) overall. This first national survey highlighted the economic burden on TB-affected households. Travel, food/nutritional supplementation, and indirect costs contribute to a high proportion of catastrophic total costs. These suggest the need to enhance financial and social protection mechanisms to mitigate the financial burden of TB-affected households.
Similar content being viewed by others
A systematic review and meta-analysis of the catastrophic costs incurred by tuberculosis patients
Economic burden of multidrug-resistant tuberculosis on patients and households: a global systematic review and meta-analysis
A sequential explanatory mixed-methods study on costs incurred by patients with tuberculosis comorbid with diabetes in Bhavnagar, western India
Introduction.
Tuberculosis (TB) causes a significant economic impact on the patients and their households 1 , 2 . Although most high TB-burden countries have offered diagnosis and treatment free of charge, patients and their households still incur substantial cost including the direct medical cost (during pre-treatment phase), direct non-medical cost (i.e., transportation, accommodation, and food), as well as indirect costs from job loss and productivity loss. Therefore, TB-affected household are still facing the risk of catastrophic costs, defined as the total costs related to TB management exceeding 20% of annual household income or expenditures 3 , leading to poor treatment access, adherence, and worsening health outcome 1 , 2 , 4 . Hence, to achieve the End TB Strategy introduced by the Sustainable Development Goals (SDGs) 5 , one of the World Health Organization (WHO)’s strategies 6 was to eliminate the catastrophic costs among TB-affected households by 2020. According to the WHO’s global monitoring of the End TB indicators reports 7 , 8 , which covered the findings from the national TB patient cost survey data of the 27 countries, one in two patients (48%, 95%CI 36–67%) faces catastrophic costs. Recent modelling that produced estimates for countries that had not yet been able to complete survey 9 shows that estimated proportions of TB-affected households experiencing catastrophic total costs were 54.9% (47.0–63.2%) overall. According to the recent meta-analysis 10 , the pooled proportion of patients faced catastrophic costs (95% Confident Interval) from the existing 29 studies was 43% (34–51%) while the main predictors of the catastrophic costs included country, drug sensitivity, and Human immune-deficiency virus (HIV) co-infection.
Thailand, an upper-middle-income country, has high TB-burden with an incidence (new TB cases per year) of 105,000 (79,000–134,000) in 2020 8 . At present, almost all necessary diagnostic and TB treatments have been covered by public health insurance schemes. As of 2019, there was no data on economic burden due to TB from patients and their household perspectives. To achieve the goal of zero catastrophic costs due to TB as one of the three targets of the WHO End TB Strategy, the current situation must be investigated. This paper is the first study aiming to estimate the prevalence of catastrophic costs due to TB from the patient and their household perspective. Factors affecting catastrophic costs were also explored. The findings could provide important evidences to guide the development of policies/strategies to protect TB patients from risk of financial crisis, hence, improving the treatment outcomes leading to the achievement of end TB target.
Study design
The national cross-sectional survey design and methodology were in line with WHO recommendations in their handbook for TB patient cost surveys 11 . The cost components included direct medical costs (i.e., out-of-pocket spent on diagnostic tests, medication, outpatient and inpatient care, and doctor fees), direct non-medical costs (i.e., out-of-pocket spent on transportation, food, and accommodation), and indirect costs (i.e., productivity loss due to TB) based on hourly wage computed individually from reported.
Sample size and sampling method
We calculated the sample size based on an estimated proportion of households experiencing catastrophic costs (p) at 50%, a design effect (D.E.) of 2.0 and 4% precision level (e) with the following standard formula 12 .
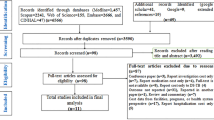
where n is the total number of TB notifications registered in 2017 13 was as 67,971; and 15% adjustment of data incompleteness, the required sample size was 1400. A stratified multi-stage cluster sampling was used to sample TB patients for the interview to ensure balance in the economic status and healthcare services accessibility of each locality that can be nationally representation in this case. Firstly, the health facilities with TB clinics were stratified into 2 groups (i.e., low- and high-poverty area) according to the poverty level (i.e., the proportion of number of individuals with income below the per capita poverty thresholds to the total number of individuals of each province compared to the national poverty proportion of 7.87 14 ). Then, health facilities in each poverty level were further stratified into secondary and tertiary level, resulting in 4 stratums. The total of 40 clusters were, then, randomly selected from the 4 stratums. The number of clusters for each stratum were calculated using proportional to size approach. For each cluster, 35 patients were recruited. These resulted in 420 patients recruited from 12 clusters of tertiary hospitals in low-poverty incidence areas, 280 patients recruited from 8 clusters of tertiary hospitals in high-poverty incidence areas, 315 patients recruited from 9 clusters of secondary hospitals in low-poverty incidence area, and 385 patients recruited from 11 clusters of secondary hospitals in high-poverty incidence area). The patients were eligible if they (1) were registered for TB treatment enrolled in the National Tuberculosis Control Programs (NTPs) from October 2019 to July 2021 at sampled facility, and (2) were on treatment for a minimum of 14 days either in intensive or continuation phase. Eligible patients were selected randomly from database of each facility, and then were asked for their consent to face-to-face interview.
Data collection
Structured face-to-face interviews with a paper-based questionnaire were conducted by the 60 trained interviewers who were the employees of the 12 Regional office of Disease Prevention and Control covering the 40 clusters of this survey. One-day training on the interview approach with the survey questionnaire was provided to all interviewers prior to data collection. Questionnaires were adapted to Thai contexts and translated into Thai language (and were pre-tested to ensure the clarity and understandability) from a generic data collection tool provided by the WHO handbook for TB patient cost surveys 11 comprising four sections: (1) informed consent; (2) patient information (including patient and clinical characteristics, employment, household composition, healthcare utilization, time spent and income lost while seeking and receiving care); (3) costs (i.e., direct medical, direct non-medical, and indirect costs), and time loss before/during the current TB treatment; and (4) coping mechanisms during the treatment phase 15 .
Data analysis
To estimate direct costs per month, the cost per visit were multiplied by the number of visits per month. The number of visits including outpatient visits, facility-based directly observed therapy (DOT), follow-up, and drug pick-up, of each treatment phase was derived from the national TB control guidelines while direct cost per visit included direct medical cost and direct non-medical cost.
Indirect costs were estimated using a human capital approach. We selected this approach because the proportion of the patients with informal employment in the survey was much higher than other sectors, and this was the better way to present socioeconomic status of the patients based on the Thai context as the consensus from the Thai expert’s consultation. This approach included time lost due to traveling to health facilities and waiting time lost during healthcare consultations of both patients and their household members. The self-reported total time spent on those activities was multiplied by the estimated income per person per minute.
To estimate costs in the remainder of the patient’s current treatment phase (i.e., intensive or continuous phase), extrapolation of the patient's costs in that treatment phase to date was done according to WHO methods 11 . In the case that the costs were estimated for different treatment phases, the mean and median reported costs and number of hours from other patients who were sampled in that treatment phase were used.
Total cost was, then, calculated as the summation of direct medical cost, direct non-medical costs, and indirect costs and was reported for the following treatment stages: pre-diagnosis (from the onset of symptoms to the first visit to a health facility), and post-diagnosis (from first visit to end of treatment).
All cost data were calculated in 2021 value and then converted to USD using the average UN operational rates of exchange during the data collection period (October 2019 to July 2021) of 1 USD = 31.07 THB 16 .
Descriptive statistics were used to describe the participated patients’ characteristics (i.e., genders, age, education level, insurance status, and household size), clinical characteristics (i.e., treatment phase, treatment category, HIV status, type of TB, diagnostic delay, modality of TB treatment, and hospitalization), household economic status (i.e., incomes, expenditures, and impoverishment), costs incurred in TB-affected households, coping strategies, social consequences, social support and perceived financial impact. The proportion of TB-affected household facing catastrophic costs, TB-related total costs (direct and indirect) exceeding 20% of the annual household expenditure as per definition by WHO 11 and global monitoring 8 was estimated. Annualized self-reported household expenditure was used as the primary method for determining household ability to pay. In addition, we evaluated pre-disease household poverty levels by comparing daily income (calculated from self-reported household monthly income) against the international poverty threshold of 1.90 USD purchasing power parity 11 adjusted dollars (converted to PPP by using the PPP conversion factor of 12.34 for Thailand in 2020 17 ).
Pearson's chi-square test was applied to compare between patients with first line treatment and patient with drug resistance. Univariate logistic regression analysis was conducted to identify variables associated with facing catastrophic costs due to TB. The variables explored in the univariate analysis included age, sex, employment status, household expenditure quintile, household size, education level, insurance status, HIV status, drug resistance status, TB history, hospitalization during TB episode, mode of TB treatment. Multivariate backward stepwise logistic regression was performed to identify factors affecting catastrophic cost. Adjusted odds ratios (OR) and 95%CI was reported.

Ethical issues
Prior to the primary data collection of this study, ethical clearance was approved by the Institute for the Development of Human Research Protections (IHRP) (COA No.IHRP2019081 and IHRP No.073-2562), and the Ethical Committee for human research at the Faculty of Dentistry and Faculty of Pharmacy, Mahidol University, Bangkok, Thailand (COA.No.MU-DT/PY-IRB 2018/068.0711 for the initial approval and COA.No.MU-DT/PY-IRB 2020/029.0206 for changes in the sample size). All respondents received a written and oral explanation of the study, and each of them signed an informed consent form before participating in the interview. All methods were performed in accordance with the relevant guidelines and regulations.
Patients characteristics
One thousand and four hundred patients (1382 first-line treatment TB and 18 drug-resistant TB, DR-TB patients) in total participated in the costing survey. Table 1 shows the demographic and clinical data for those participants included in the analysis. Most patients were male (68.9%), aged older than 45 years (69.3%) including one quarter over 65 years, had attended pre/primary school education (60.1%), and had public health insurance (98.0%). The median of their household size was three members (range 1–17). The patients who participated in this survey were in any of the two treatment phases with similar proportions (46.1% were in the intensive phase and 53.9% were in the continuation phase). Most patients were new TB (94.4%) without HIV infection (88.2%). Around 31.7% of the patients in the intensive phase experienced a long diagnostic delayed (> 4 weeks). For modality of TB treatment, most patients (75.4%) self-administered their medications, 18.0% of them had home-based directly observed therapy (DOT), and few of them (6.6%) received facility-based DOT. Only 6.6% were hospitalized during their current TB episode, and almost half of them (47.6%) previously hospitalized in their current treatment phase.
Socio-economic characteristics and the changes in income among TB-affected households
The average monthly income of survey participants and that of their households before the onset of TB symptoms was 355 USD (95%CI 321–388 USD), and 1152 USD (95%CI 708–1597 USD), respectively (Table 2 ). Almost half of TB patients (48.3%) were the primary income earner. The average monthly household expenditure was 640 USD (95%CI 459–822 USD). While at the interview, the average monthly income of the patient and household decreased to 220 USD (95%CI 193–246 USD), and 643 USD (95%CI 572–714 USD), respectively.
Before the onset of TB symptoms, 2.2% of the participant households faced impoverishment (their incomes were below the poverty line—poverty headcount ratio at USD 1.90 per day at 2011 PPP), and it was increased from 2.2 to 11.1% due to TB (Table 2 ). The differences in the percentage of impoverishment of TB-affected households before and during TB episodes among the different household income quintile groups are demonstrated in Supplementary (Fig. S1 ). Our findings show that TB has affected the patients and their households in terms of income loss. The proportion of TB-affected households living below the poverty line was substantially higher among those in lower quintiles.
Costs of TB-affected households
The mean total costs per TB episode for all study participants (n = 1400) were 903 USD per patient (95%CI 771–1034 USD), and median total costs per episode were 412 USD per patient (IQR 184–879 USD) (Table 3 ). Of these, total direct non-medical costs were the highest costs (mean, 402 USD, and 95%CI 334–470 USD) incurred per TB-affected households followed by total indirect costs (mean, 393 USD, and 95%CI 315–472 USD) and total direct medical costs (mean, 107 USD, and 95%CI 81–133 USD, respectively. The mean total costs per episode among TB first-line treatment patients (n = 1382) and DR-TB patients (n = 18) were 848 USD (95%CI 725–971 USD) and 4987 USD (95%CI 2884–7090 USD), respectively (Fig. S2 in the Supplementary).
For the pre-TB diagnosis episode, the mean direct costs (37 USD with 95%CI 33–42 USD) were the highest costs incurred by the patients. The mean total costs incurred during pre-TB diagnosis episode were less than those incurred during post-TB diagnosis episode. Whereas the post-TB diagnosis episode, the mean direct non-medical costs (384 USD with 95%CI 98–191 USD) and the mean indirect costs (381 USD with 95%CI 303–458 USD) were the two highest costs incurred by the patients and their households. This reflects travel, food, and time costs (or productivity lost) by the patients and their caregivers during the TB treatment due to the many facility visits and hour lost (Table 4 ). In terms of number of facility visits, patients involved in facility-based DOT made 125.8 visits (ranged 114.6–137.0 visits) mainly during their treatment, followed by medical follow-up 9.4 visits (ranged 8.5–10.2 visits). Of these visits, DR-TB patients had significantly higher total number of visits than those of TB patients. Hours lost by DR-TB patients (743.4 h with ranged 350.3–1136.4 h) were also significantly much higher than the lost by TB patients (142.0 h with ranged 122.7–161.3 h). Although hours lost by caregivers were not statistically significant different between TB and DR-TB patients, total lost time of DR-TB caregivers were around four times of those of TB caregivers (372.9 h vs. 85.9 h, respectively).
Catastrophic total costs
Figure 1 illustrates the percentage of TB-affected households facing catastrophic total costs. At the 20% threshold, the percentage of catastrophic total costs was 29.5% (95%CI 25.1–34.0%) for TB and 61.1% (95%CI 29.6–88.1%) for DR-TB patients; this reflects 29.9% of TB-affected households facing catastrophic costs for overall TB participants of this study.
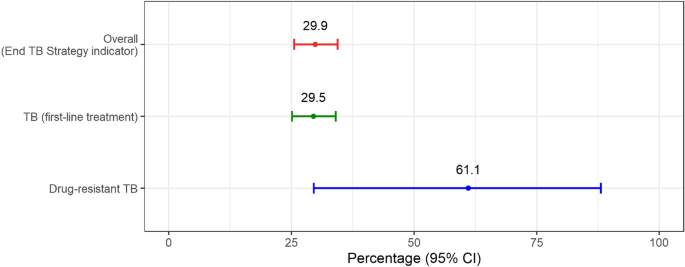
Percentage of TB-affected households facing catastrophic costs. CI confidence interval, TB tuberculosis. *Error bars represent 95% confidence interval.
Coping mechanisms and social consequences
The patients reported the use of loan as the main coping strategy (19.1%) to face costs incurred with very little social support; 2.2% and 1.0% of survey participants reported receipt of social assistance and vouchers from NTP (Table 5 ). Getting TB infection causes social consequences, i.e., their working days loss (41.9%), job loss (34.6%), and social exclusion (27.8%). Overall, those proportions of social consequences were significantly higher among DR-TB patients. The proportion of patients who became unemployed more than doubled when comparing the employment status before TB episode to the status during TB episode (at the time of interview) (16.0–42.0%) (Fig. 2 ). While the proportion of employment in the informal and formal sector decreased from 69.0% and 11.0% to 46.0% and 8.5%, respectively, when comparing the same time periods. More than half of the patients (52.0%) did not perceive any change in the financial impact, while 38.2% of them perceived they were poorer and 8.5% felt they were much poorer than in the past.
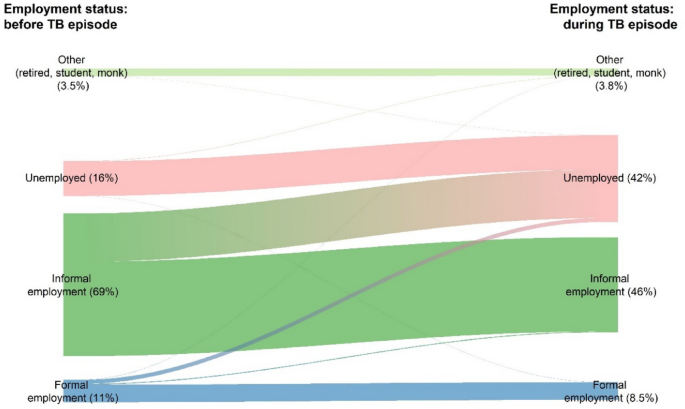
Changes in employment status before and during TB episode.
Factors affecting catastrophic costs
Figure 3 presents the selected final model with adjusted odd ratio (OR) of the risk factors that had a significant association with the probability of facing catastrophic costs due to TB. Households with lower expenditure quintiles (for the first 3 quintiles) had a significantly higher incidence of facing catastrophic costs compared to those in the highest expenditure quintile (the lowest expenditure quintile: OR 54.6, 95%CI 29.0–103.0; the second lowest expenditure quintile: OR 8.1, 95%CI 4.6–14.0, and the third expenditure quintile: OR 3.6, 95%CI 1.8–7.0). The other significant factors associated with the catastrophic costs include experiencing hospitalization (OR 9.4, 95%CI 6.0–15.0, compared to not hospitalizing), being DR-TB patient (OR 5.3, 95%CI 1.4–20.0, compared to those with first-line treatment), patients who do not have health insurance (OR 5.0, 95%CI 1.3–19, compared to those with health insurance), patients with extrapulmonary TB (OR 3.0, 95%CI 1.1–8.4, compared to those with pulmonary TB), and patients who received the facility-based directly observed therapy as their treatment support (OR 1.7, 95%CI 1.1–2.6, compared to those with self-administration).
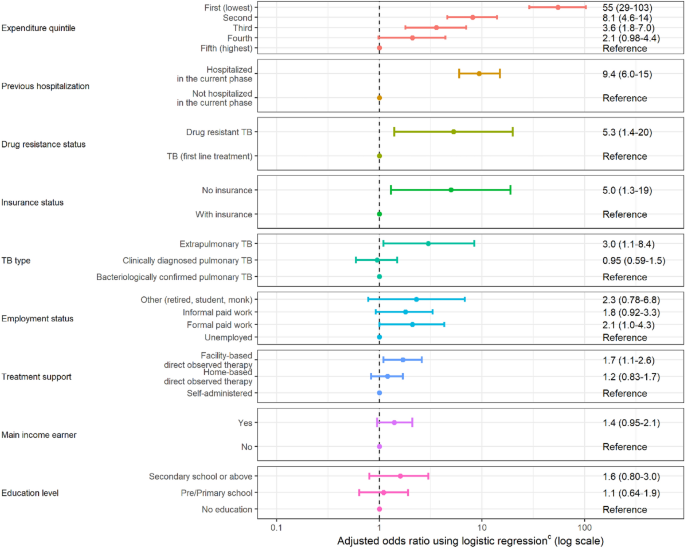
Risk factors for TB-affected households facing costs > 20% of household expenditure due to TB. *Error bars represent 95% confidence interval.
Our findings illustrated that 29.9% of TB-affected households face catastrophic total costs, a lower proportion compared to the global pooled average of 48% (95%CI 36–61%) with 27 countries with published survey data 8 and also lower than the global pooled average of 135 low- and middle-income countries with meta-regression estimates 54.9% (47.0–63.2%) overall 9 .
The largest cost driver to the economic burden supported by TB-affected households were travel, food, and nutritional supplementation, in the form of direct non-medical costs (44.5% of total costs), and patient (and their caregivers) productivity loss, in the form of indirect costs (43.6% of total costs).
On the other hand, overall out-of-pocket expenses associated with direct medical costs accounted for only 12% of total costs. Thus, our findings also confirm that most of the direct medical costs have been covered by the Thai public health insurance 18 . Although, almost all direct medical costs during the treatment phase were covered by public health insurance, this study showed that most direct medical costs incurred before TB diagnosis episode were disbursed by patients (pre-diagnosis out-of-pocket expenses represent 2.1% of total episode costs). The patient might seek care by going to the private sector, such as drug store. This could increase out-of-pocket expenses. Thus, increasing proactive access to early TB diagnosis can help early detection of people with TB and bring them to be covered under the public health insurance schemes. Although this has been already included in the Thailand operational plan to end TB for 2017–2021 19 , this finding encourages the Ministry of Public Health to continue this strategy for the next plan to end TB. Moreover, refining benefit packages in all public health insurance schemes to include standard TB care, including diagnosis, treatment and social support is recommended. This can ensure that all presumptive TB cases have access to standard TB treatment.
Although the Thai UHC provides free TB treatment and other medical services, this does not cover traveling and productivity loss incurred from the facility-visits due to TB treatment. Enhancing patient-centered care in the Thai TB treatment guidelines or strengthen all primary health care services may reduce the time required for those facility visits and then decrease the direct non-medical costs and income losses of the patients. Moreover, this has led to another issue of social protection policies that required attention from national policymakers. Social protection policies beyond free medical services, e.g., financial incentives for cost of living, should be strengthened by the national and local government. Only 2.2% (95%CI 1.4–3.3) of survey respondents (Table 5 ) were accessing social assistance and 1% (95%CI 0.2–2.5) accessed vouchers. For TB patients who are in formal employment, the government should strengthen the policy by securing their jobs. Nevertheless, this issue is not solely the responsibility of government organizations in the health sector, but it also requires cooperation among the health and non-health sectors. Cooperation between The Ministry of Public Health and the Ministry of Labour, the Ministry of Social Development and Human Security, or non-government agencies is required to support TB patients in developing social support mechanism, such as enabling patients to take sick leave or be compensated in case of dismissal, especially for the patients with lower expenditure quintiles. This can mitigate the economic burden and reduce the proportion of households that experience catastrophic costs in Thailand.
Despite the free TB treatment policy under the UHC in Thailand, the percentage of TB-affected households living below the international poverty line 11 among the TB-affected households increased during TB treatment compared to the pre-TB episode (from 2.2 to 11%). The disease does not affect only to the poor households (percentage living below international poverty line rose from 11 and 0% to 22% and 15% in the 1st and 2nd household income quintiles, respectively) but it also impacts on the richer households (percentage living below international poverty line increased from 0 to 4.7% in the 5th household income quintile). This requires policy actions beyond the strictly medical and into social protection especially for those who are poorer. In addition to the free medical services during TB treatment, income replacement during TB treatment and the post-TB socioeconomic recovery strategies (e.g., maintain their formal employment, looking for a new job, and re-employment) are also key to protect the patients and their households against financial hardship due to TB.
It is also noteworthy that the mean monthly individual incomes reported by TB (first-line treatment) patients is significantly higher than that reported by patients with DR-TB. In fact, the mean total costs incurred by DR-TB cases were almost 6 times of the costs incurred by TB (first-line treatment) patients, even though Thailand has started shorter DR-TB regimen 20 . This highlights the serious socioeconomic impact of DR-TB on their households.
To our knowledge, this is the first national TB patient cost survey in Thailand using the standardized methodology for cross-sectional survey in TB-affected countries developed by WHO 11 . Our findings do not only deliver the significant indicator of catastrophic costs status due to TB in Thailand to achieve the end TB strategies, but we also provide insights that there were gaps in TB policy implementation that needed to improve.
This study has limitations that have led to some concerns. First, we started the survey in 2019 and data collection was ongoing as COVID-19 pandemic hit. This brought an obstacle to the interview process and many of the related health facilities did not allow the interviewers to go to the field. This may cause recall biases due to the delay of the interview appointment. Moreover, the number of health facility visits and income losses may have been interrupted by the pandemic. These might cause under-reported number of the facility visits and the income losses might be resulted from the pandemic. Second, there were missing income data reported from the patients, especially the ones working in informal sector, even though the interviewers tried to ask them to estimate. This might affect the indirect cost estimation. For those missing ones, the estimations of their individual incomes were based on ascribing a proportion of the household annual income to the individual of the reported one. Third, we did not specifically sample for DR-TB, and randomly selected DR-TB in the random clusters; therefore, our findings due to DR-TB cases may not represent the DR-TB patients in Thailand. Although the costs calculation for DR-TB patients were referred to the national standard practice guideline of the DR-TB, its sample size was small and we did not design our data collection of the DR-TB patients for this survey. However, our findings can highlight the higher economic burden of DR-TB than those incurred by TB patients. Thus, we strongly suggest the further study focusing only on DR-TB patients to examine economic burden and catastrophic total costs incurred in DR-TB patients that can be representative of this specific groups of TB patients in Thailand.
This study is the first national TB patient cost survey in Thailand. Our findings highlight the economic burden on TB patients and their households and of their falling into deeper poverty and greater unemployment. Travel costs, food/nutritional supplementation, and productivity costs drive total TB episode costs in Thailand and a significant proportion of TB-affected households incur in costs > 20% of household expenditure (i.e. catastrophic total costs). Such evidence suggests financial and social protection mechanisms to mitigate the economic burden of the TB-affected households.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request and with permission of the Health System Research Institute.
Laurence, Y. V., Griffiths, U. K. & Vassall, A. Costs to health services and the patient of treating tuberculosis: A systematic literature review. Pharmacoeconomics 33 , 939–955 (2015).
Article PubMed PubMed Central Google Scholar
Tanimura, T., Jaramillo, F., Weil, D., Raviglione, M. & Lönnroth, K. Financial burden for tuberculosis patients in low- and middle-income countries: A systematic review. Eur. Respir. J. 43 , 1763–1775 (2014).
World Health Organization. The end TB Strategy . http://who.int/tb/post2015_TBstrategy.pdf?ua=1 (2015).
Getahun, B., Wubie, M., Dejenu, G. & Manyazewal, T. Tuberculosis care strategies and their economic consequences for patients: The missing link to end tuberculosis. Infect. Dis. Poverty 5 , 93 (2016).
United Nations. Draft outcome document of the United Nations summit for the adoption of the post-2015 development agenda. In Sixty-ninth session of the General Assembly of the United Nations (United Nation, New York, 2015).
World Health Organization. The End TB Strategy . https://www.who.int/teams/global-tuberculosis-programme/the-end-tb-strategy (2021).
World Health Organization. National Surveys of Costs Faced by TB Patients and Their Households, 2015–2021 (2023).
World Health Organization. Global Tuberculosis Report 2022 (World Health Organization, 2022).
Google Scholar
Portnoy, A. et al. Costs incurred by people receiving tuberculosis treatment in low-income and middle-income countries: A meta-regression analysis. Lancet Glob. Health 11 , e1640-1647 (2023).
Article CAS PubMed PubMed Central Google Scholar
Ghazy, R. M. et al. A systematic review and meta-analysis on catastrophic cost incurred by tuberculosis patients and their households. Sci. Rep. 12 , 558. https://doi.org/10.1038/s41598-021-04345-x (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
World Health Organization. Tuberculosis Patient Cost Surveys: A Handbook (World Health Organization, 2017).
Krejcie, R. V. & Morgan, D. W. Determining sample size for research activities. Educ. Psychol. Meas. 30 , 607–610 (1970).
Article Google Scholar
National Tuberculosis Information Program (2018).
Office of the National Economic and Social Development Council. Report on the Poverty Situation and Inequity in Thailand 2007 (Office of the National Economic and Social Development Council, Bangkok, 2008).
Division of Tuberculosis, Department of Disease Control & Faculty of Pharmacy, M. U. Workshop Document to Prepare Research Interviewers (Division of Tuberculosis, Department of Disease Control, Ministry of Public Health, Bangkok, 2019).
United Nations. UN Operational Rates of Exchange . https://treasury.un.org/operationalrates/OperationalRates.php (2022).
World Bank Group. PPP Conversion Factor, GDP (LCU per international $)—Thailand . https://data.worldbank.org/indicator/PA.NUS.PPP?locations=TH (2021).
Tangcharoensathien, V., Witthayapipopsakul, W., Panichkriangkrai, W., Patcharanarumol, W. & Mills, A. Health systems development in Thailand: A solid platform for successful implementation of universal health coverage. Lancet 391 , 1205–1223 (2018).
Article PubMed Google Scholar
Division of Tuberculosis, Department of Disease Control, Ministry of Public Health. (Ministry of Public Health, Nonthaburi, 2017).
Division of Tuberculosis, Department of Disease Control. National Tuberculosis Control Programme Guideline 2018 (Aksorn Graphic and Design Publishing Limited, 2018).
Download references
Acknowledgements
We gratefully acknowledge the contribution of our study participants; the research team from both Faculty of Pharmacy, Mahidol University, and the Division of Tuberculosis, Department of Disease Control, Ministry of Public Health, Thailand. This research was funded by the Health system Research Institute (HSRI). The findings, interpretations and conclusions expressed in this article do not necessarily reflect the views of the aforementioned funding agencies. Also, we would like to thank Dr.Viroj Tangcharoensathien and his team at the International Health Policy Program, Thailand, for their support in data validation and verification in Thailand context.
This research was funded by the Health system research Institute (HSRI) under Grant HSRI 64-019.
Author information
Authors and affiliations.
Mahidol University Health Technology Assessment (MUHTA) Graduate Program, Mahidol University, Bangkok, Thailand
Sitaporn Youngkong, Montarat Thavorncharoensap & Usa Chaikledkaew
Social and Administrative Pharmacy Division, Department of Pharmacy, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand
Division of Tuberculosis, Department of Disease Control, Ministry of Public Health, Bangkok, Thailand
Phalin Kamolwat, Sriprapa Nateniyom & Petchawan Pungrassami
Faculty of Nursing, Mahidol University, Nakhon Pathom, Thailand
Phichet Wongrot
Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand
Naiyana Praditsitthikorn
Department of Medical Sciences, Ministry of Public Health, Nonthaburi, Thailand
Surakameth Mahasirimongkol
Department of Biochemistry, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand
Jiraphun Jittikoon
World Health Organization Global Tuberculosis Programme, Geneva, Switzerland
Nobuyuki Nishikiori, Ines Garcia Baena & Takuya Yamanaka
Department of Global Health and Development, London School of Hygiene and Tropical Medicine, London, UK
Takuya Yamanaka
School of Tropical Medicine and Global Health, Nagasaki University, Nagasaki, Japan
You can also search for this author in PubMed Google Scholar
Contributions
All authors conceived and designed the work. S.Y., P.K., P.W., M.T. and U.C. supervised and monitored the survey. S.Y., P.W. and T.Y. did the analysis. S.Y. wrote the first draft of the manuscript with input from other authors. All authors interpreted the data, provided critical revision for important intellectual content and approved the final version to be published.
Corresponding author
Correspondence to Sitaporn Youngkong .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Youngkong, S., Kamolwat, P., Wongrot, P. et al. Catastrophic costs incurred by tuberculosis affected households from Thailand’s first national tuberculosis patient cost survey. Sci Rep 14 , 11205 (2024). https://doi.org/10.1038/s41598-024-56594-1
Download citation
Received : 04 January 2024
Accepted : 08 March 2024
Published : 16 May 2024
DOI : https://doi.org/10.1038/s41598-024-56594-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Catastrophic total cost
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Tuberculosis infection and disease among persons seeking social services in New York City
Affiliation.
- 1 Department of Medicine, New York University School of Medicine, USA.
- PMID: 9441056
Setting: A large public hospital in New York City.
Objective: To determine the prevalence of tuberculosis infection and disease in a cohort of indigent persons in New York.
Design: Persons seeking social services at any of five community-based organizations in New York City were screened for tuberculosis infection using tuberculin skin testing and a symptom questionnaire. Skin test or symptom positive persons were referred to the Bellevue Hospital Chest Clinic for a chest radiograph and medical evaluation. After this evaluation, patients were classified into a diagnostic category (e.g. tuberculosis infection, tuberculosis disease, no evidence of tuberculosis infection or disease).
Results: Of 651 persons screened, 591 (91%) completed the initial evaluation. The tuberculosis infection prevalence for the entire cohort was 41% (95% Confidence Interval [CI], 37% to 45%). Risk factors for infection included residence in a congregate setting, drug use, and birth outside the United States. Human immunodeficiency virus (HIV) infection was not a risk factor for infection. Eleven cases of active tuberculosis were also detected (disease prevalence of 1.7%, 95% CI, 0.85% to 3%). Most of the patients with active tuberculosis had documented HIV infection or clear risk factors for HIV.
Conclusion: We conclude that tuberculosis infection and disease remain common in populations characterized by poor housing conditions, drug use, and HIV infection. Linking a major medical provider with community-based organizations is an effective means to provide highly targeted screening services to a population at serious risk for disease acquisition and transmission.
Publication types
- Research Support, Non-U.S. Gov't
- Research Support, U.S. Gov't, P.H.S.
- Age Distribution
- Cohort Studies
- Community Health Services / statistics & numerical data*
- Confidence Intervals
- Mass Screening
- Medical Indigency / statistics & numerical data*
- Middle Aged
- Multivariate Analysis
- New York City / epidemiology
- Risk Factors
- Sex Distribution
- Social Work / statistics & numerical data*
- Tuberculosis / epidemiology*
- Tuberculosis / prevention & control
Grants and funding
- K07 HL03030/HL/NHLBI NIH HHS/United States

- Report Highlights
- EU-Summaries

- About the publications
Drug-resistant Tuberculosis
- Level 1: Summary
- Level 2: Details
7. Conclusions
- 7.1 Magnitude and trends of drug-resistant tuberculosis
- 7.2 Extensively drug-resistant tuberculosis (XDR-TB)
- 7.3 Drug-resistant tuberculosis and HIV
- 7.4 Survey coverage and methods
- 7.5 Tuberculosis control and drug-resistant tuberculosis
In 2006, approximately half a million new cases of multidrug resistant tuberculosis ( MDR-TB ) emerged in the world. China and India are estimated to carry 50% of the global burden of cases, and the Russian Federation is estimated to carry a further 7%.
Globally, MDR-TB makes up 4.6% of all cases of tuberculosis but, in some parts of the former Soviet Union, this proportion exceeds 35%. The patients in these countries have forms of tuberculosis that are resistant to a wide range of drugs, with the highest rates of extensively drug resistant tuberculosis ( XDR-TB ) in the world.
China has the second highest proportion of MDR-TB among TB cases but, in absolute terms, it has the highest number of MDR-TB cases in the world. The high proportion of drug-resistant TB among new cases in China suggests a concerning level of transmission of drug-resistant forms of TB.
In most countries where cases of TB are relatively few, the absolute numbers of cases of drug-resistant tuberculosis as well as the proportions of resistance are stable. Trend results are good in Hong Kong where MDR-TB is falling faster than tuberculosis. In Peru and in South Korea, tuberculosis is declining but MDR-TB is increasing. In Peru this could be due to a weakening in the control of the disease but in South Korea it may be due to changes in the surveillance method and not reflect a true worsening of the situation.
In the Baltic countries, tuberculosis is declining and levels of MDR-TB are relatively stable. However, in parts of the Russian Federation drug-resistance is rising rapidly, both in absolute numbers and in terms of proportion among new TB cases. Tuberculosis control is improving but there is a large pool of long-term cases that continues to fuel the epidemic . Current efforts to control the disease will have to be accelerated to have any impact in what appears to be a growing epidemic of drug-resistant tuberculosis.
Extensively drug-resistant tuberculosis ( XDR-TB ) can only be treated with a handful of drugs and these are more expensive and have worse side-effects than those used to treat multidrug-resistant tuberculosis ( MDR-TB ).
Extensively drug-resistant tuberculosis is widespread and 45 countries have reported at least one case. There is a significant problem within countries of the former Soviet Union, where cases of XDR-TB are high both in absolute and in relative terms. Levels of resistance to second-line drugs are also high in Japan and South Korea, and moderate in South Africa.
Elsewhere, in Africa, levels of extensively drug-resistant tuberculosis seem to be low. XDR-TB is likely to emerge as a result of inappropriate use of second-line anti-tuberculosis drugs , but these drugs are not yet widely used in the region
In order to understand the extent and the pattern of extensively drug-resistant tuberculosis throughout the world, all countries need to increase their efforts to measure resistance to second-line anti-tuberculosis drugs .
There is a significant association between HIV and multidrug-resistant tuberculosis ( MDR-TB ). A major reason for this association might be environmental: people become infected with both HIV and MDR-TB in places where patients are in close contact with each other such as health care facilities and prisons. Improving infection control in these settings may be critical to reducing the number of people infected with both HIV and multidrug-resistant tuberculosis .
People who have both infections at the same time are likely to die from TB unless they are diagnosed and treated quickly. This is a great concern for countries without sufficient testing facilities.
It is extremely important to develop methods that can detect drug-resistant tuberculosis quickly, particularly for HIV infected patients.
Monitoring of drug resistance should be part of routine surveillance, but this requires culture and drug susceptibility testing to be the standard of diagnosis. Since many countries do not yet have these facilities, surveys are important to determine the extent of the drug resistance problem. Survey coverage and reliability of data are increasing, but major gaps remain. For instance, it is very difficult to determine trends in most high burden countries.
The largest obstacle is the lack of laboratory capacity. Testing for resistance to second-line drugs is not available in most countries and it has been difficult to introduce HIV testing as part of the general care for tuberculosis . Supranational reference laboratories will continue to provide testing for resistance while countries develop their own national facilities.
New methods to detect and monitor drug-resistant tuberculosis are being developed. Special studies are necessary to answer questions such as the risk factors for acquiring drug-resistant tuberculosis, or how the disease is transmitted in different populations .
The main priority for all countries is to prevent the development of drug resistant tuberculosis but all cases that emerge have to be treated properly.
Some countries need to develop ways of detecting and treating drug-resistant cases quickly. This is particularly important in countries with high proportions of anti- tuberculosis drug resistance , countries with high absolute numbers of multidrug-resistant tuberculosis ( MDR-TB ), and countries with a TB population heavily co- infected with HIV .
New drugs to treat multidrug-resistant tuberculosis are urgently needed.
To control MDR-TB there needs to be a coordinated effort from all countries. The three priority areas include improvements in infection control measures to prevent transmission, expansion of testing services to detect cases quickly, and community involvement to ensure patients get tested and take all their drugs regularly. Most importantly, all patients must be registered in a suitable treatment programme.
- 1. What is tuberculosis and why is it a concern?
- 2. What is the Global Project on Anti-tuberculosis Drug Resistance Surveillance?
- 3. What are current trends in drug-resistant tuberculosis?
- 4. Why do HIV and tuberculosis form a lethal combination?
- 5. What is the status of drug-resistant tuberculosis in the different WHO regions?
- 6. Why is it difficult to gather information on drug-resistant tuberculosis?
- Accidental poisoning
- Acrylamide in food
- Acupuncture
- Agriculture
- Aids Epidemic
- Air Pollution Europe
- Air quality in Europe
- Allergenic fragrances
- Aluminium exposure
- Animal testing
- Antibiotic resistance
- Antibiotics Research
- Antimicrobial resistance
- Aquatic environment
- Arctic Climate Change
- Artificial Light
- Artificial Light and Health
- Aspartame Reevaluation
- Aspirin & Cancer
- Benzodiazepines
- Biodiversity
- Biological Diversity
- Biosecurity
- Bisphenol A
- CO 2 Capture & Storage
- Cancer rates and mortality, types and causes
- Chemical Mixtures
- Children & Screens
- Chlorine Sodium Hypochlorite
- Chlorpyrifos pesticide
- Chronic Diseases on Labour Practices
- Circular Economy
- Climate Change
- Climate Change Mitigation
- Climate impact of shale gas
- Climate impacts adaptation
- Dental Amalgams
- Dental Fillings
- Desertification
- Diet & Nutrition
- Ecosystem Change
- Effects of cannabis
- Electromagnetic Fields
- Electronic Cigarettes
- Endocrine Disruptors
- Endocrine disrupting properties of pesticides
- Endocrine disruptors risks
- Energy Saving Lamps
- Energy Technologies
- Epidemic diseases
- Estrogen-progestogen cancer risk
- Europe Green Deal
- Evaluation of endocrine disruptors
- Exposure to chemical mixtures
- Fisheries and aquaculture
- Fluorinated gases
- Food & Agriculture
- Food Wastage
- Forests & Energy
- Forests & agriculture land use
- Fukushima Consequences
- Fukushima accident
- Genetically Modified Crops
- Geothermal Energy
- Global Biodiversity Outlook 4
- Global Public Health Threats
- Global Warming
- Gluten intolerance
- Glyphosate and cancer
- Hazardous chemicals
- Health Effects of Electromagnetic Fields
- Health Environment Management
- Illicit drugs in Europe
- Impacts of a 4°C global warming
- India Millennium Development Goals
- Indonesian forests
- Indoor Air Quality
- Land Degradation and Desertification
- Lyme Disease
- Marine Litter
- Marine litter
- Mercury from dental amalgam
- Mercury in CFL
- Metal-on-Metal hip implants
- Methylene glycol
- Mineral extraction risks
- Multiple vaccinations
- Nano-silica
- Nanomaterials
- Nanotechnologies
- Neonicotinoids
- Nitrogen Dioxide
- Non-human primates
- Organic Food
- Ozone layer depletion
- Parabens used in cosmetics
- Particulate Matter
- Perfluorooctanoic acid (PFOA)
- Personal Music Players & Hearing
- Pesticides occupational risks
- Pharmaceuticals environment
- Phosphate resources
- Phthalates Comparison
- Phthalates in school supplies
- Poly brominated flame retardant decaBDE
- Power lines
- Psychoactive Drugs
- Radiological nuclear emergency
- Respiratory Diseases
- Safety of Cosmetics
- Safety of sunscreens
- Sand Extraction
- Security Scanners
- Silver Nanoparticles
- Single-use plastics
- Soils degradation
- Solar Energy
- State of the European Environment
- Static Fields
- Substitution of harmful chemicals
- Sulfaxoflor Pesticide
- Sunbeds & UV radiation
- Sustainable oceans
- Synthetic Biology
- Thorium nuclear fuel
- Tidal Energy
- Titanium dioxide nanoparticles
- Tooth Whiteners
- Transgenic salmon
- Tuberculosis
- Wastewater management
- Water Disinfectants
- Water Resources
- Water Resources Assessments
- Water resources
- Wind Resources
- X-Ray Full-Body Scanners

Get involved!
This summary is free and ad-free, as is all of our content. You can help us remain free and independant as well as to develop new ways to communicate science by becoming a Patron!
- Terms & Conditions

ORIGINAL RESEARCH article
This article is part of the research topic.
Building the Future of Education Together: Innovation, Complexity, Sustainability, Interdisciplinary Research and Open Science
Developing the Skills for Complex Thinking Research: A Case Study Using Social Robotics to Produce Scientific Papers Provisionally Accepted

- 1 Institute for the Future of Education, Monterrey Institute of Technology and Higher Education (ITESM), Mexico
- 2 University of Cienfuegos, Cuba
The final, formatted version of the article will be published soon.
The development of university students' skills to successfully produce scientific documents has been a recurring topic of study in academia. This paper analyzes the implementation of a training experience using a digital environment mediated by video content materials starring humanoid robots. The research aimed to scale complex thinking and its subcompetencies as a hinge to strengthen basic academic research skills. Students from Colombia, Ecuador, and Mexico committed to preparing a scientific document as part of their professional training participated. A pretest to know their initial level of perception, a posttest to evaluate if there was a change, and a scientific document the students delivered at the end of the training experience comprised the methodology to demonstrate the improvement of their skills. The results indicated students' perceived improvement in the sub-competencies of systemic, creative, scientific, and innovative thinking; however, their perceptions did not align with that of the tutor who reviewed the delivered scientific product. The conclusion was that although the training experience helped strengthen the students' skills, variables that are determinants for a student to develop the knowledge necessary to prepare scientific documents and their derived products remain to be analyzed.
Keywords: higher education, research skills, Educational innovation, complex thinking, scientific thinking, Critical Thinking, Innovative thinking, social robotics
Received: 16 Oct 2023; Accepted: 17 May 2024.
Copyright: © 2024 Lopez-Caudana, George-Reyes and Avello-Martínez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Dr. Edgar O. Lopez-Caudana, Institute for the Future of Education, Monterrey Institute of Technology and Higher Education (ITESM), Monterrey, Mexico
People also looked at
Are Markups Driving the Ups and Downs of Inflation?

Download PDF (158 KB)
FRBSF Economic Letter 2024-12 | May 13, 2024
How much impact have price markups for goods and services had on the recent surge and the subsequent decline of inflation? Since 2021, markups have risen substantially in a few industries such as motor vehicles and petroleum. However, aggregate markups—which are more relevant for overall inflation—have generally remained flat, in line with previous economic recoveries over the past three decades. These patterns suggest that markup fluctuations have not been a main driver of the ups and downs of inflation during the post-pandemic recovery.
In the recovery from the pandemic, U.S. inflation surged to a peak of over 7% in June 2022 and has since declined to 2.7% in March 2024, as measured by the 12-month change in the personal consumption expenditures (PCE) price index. What factors have been driving the ups and downs of inflation? Production costs are traditionally considered a main contributor, particularly costs stemming from fluctuations in demand for and supply of goods and services. As demand for their products rises, companies need to hire more workers and buy more intermediate goods, pushing up production costs. Supply chain disruptions can also push up the cost of production. Firms may pass on all or part of the cost increases to consumers by raising prices. Thus, an important theoretical linkage runs from cost increases to inflation. Likewise, decreases in costs should lead to disinflation.
Labor costs are an important factor of production costs and are often useful for gauging inflationary pressures. However, during the post-pandemic surge in inflation, nominal wages rose more slowly than prices, such that real labor costs were falling until early 2023. By contrast, disruptions to global supply chains pushed up intermediate goods costs, contributing to the surge in inflation (see, for example, Liu and Nguyen 2023). However, supply chains have more direct impacts on goods inflation than on services inflation, which also rose substantially.
In this Economic Letter , we consider another factor that might drive inflation fluctuations: changes in firms’ pricing power and markups. An increase in pricing power would be reflected in price-cost markups, leading to higher inflation; likewise, a decline in pricing power and markups could alleviate inflation pressures. We use industry-level measures of markups to trace their evolving impact on inflation during the current expansion. We find that markups rose substantially in some sectors, such as the motor vehicles industry. However, the aggregate markup across all sectors of the economy, which is more relevant for inflation, has stayed essentially flat during the post-pandemic recovery. This is broadly in line with patterns during previous business cycle recoveries. Overall, our analysis suggests that fluctuations in markups were not a main driver of the post-pandemic surge in inflation, nor of the recent disinflation that started in mid-2022.
Potential drivers of inflation: Production costs and markups
To support households and businesses during the pandemic, the Federal Reserve lowered the federal funds rate target to essentially zero, and the federal government provided large fiscal transfers and increased unemployment benefits. These policies boosted demand for goods and services, especially as the economy recovered from the depth of the pandemic.
The increase in overall demand, combined with supply shortages, boosted the costs of production, contributing to the surge in inflation during the post-pandemic recovery. Although labor costs account for a large part of firms’ total production costs, real labor costs were falling between early 2021 and mid-2022 such that the increases in prices outpaced those in nominal wages. This makes it unlikely that labor costs were driving the surge in inflation.
Instead, we focus on another potential alternative driver of inflation that resulted from firms’ ability to adjust prices, known as pricing power. As demand for goods surged early in the post-pandemic recovery, companies may have had a greater ability to raise their prices above their production costs, a gap known as markups. Following a sharp drop in spending at the height of the pandemic, people may have become eager to resume normal spending patterns and hence more tolerant to price increases than in the past. In fact, growth of nonfinancial corporate profits accelerated in the early part of the recovery (see Figure 1), suggesting that companies had increased pricing power. Some studies have pointed to the strong growth in nonfinancial corporate profits in 2021 as evidence that increased markups have contributed to inflation (see, for example, Weber and Wasmer 2023). However, the figure also shows that growth in corporate profits is typically volatile. Corporate profits tend to rise in the early stages of economic recoveries. Data for the current recovery show that the increase in corporate profits is not particularly pronounced compared with previous recoveries.
Figure 1 Profit growth for nonfinancial businesses
More importantly, corporate profits are an imperfect measure of a firm’s pricing power because several other factors can drive changes in profitability. For instance, much of the recent rise in corporate profits can be attributed to lower business taxes and higher subsidies from pandemic-related government support, as well as lower net interest payments due to monetary policy accommodation (Pallazzo 2023).
Instead of relying on profits as a measure of pricing power, we construct direct measures of markups based on standard economic models. Theory suggests that companies set prices as a markup over variable production costs, and that markup can be inferred from the share of a firm’s revenue spent on a given variable production factor, such as labor or intermediate goods. Over the period of data we use, we assume that the specific proportion of a company’s production costs going toward inputs does not change. If the share of a firm’s revenue used for inputs falls, it would imply a rise in the firm’s price-cost margin or markup. In our main analysis, we use industry-level data from the Bureau of Economic Analysis (BEA) to compute markups based on the share of revenue spent on intermediate inputs. Our results are similar if we instead use the share of revenue going toward labor costs.
We compare the evolution of markups to that of prices, as measured by the PCE price index, since the recovery from the pandemic. In constructing this price index, the BEA takes into account changes in product characteristics (for instance, size) that could otherwise bias the inflation measure by comparing the prices of inherently different products over time. Similarly, based upon standard economic theory, our markup measure implicitly captures changes in those characteristics (see, for example, Aghion et al. 2023).
The post-pandemic evolution of markups
We examine the evolution of markups in each industry since the third quarter of 2020, the start of the post-pandemic recovery. Figure 2 shows that some sectors, such as the motor vehicles and petroleum industries, experienced large cumulative increases in markups during the recovery. Markups also rose substantially in general merchandise, such as department stores, and for other services, such as repair and maintenance, personal care, and laundry services. Since the start of the expansion, markups in those industries rose by over 10%—comparable in size to the cumulative increases over the same period in the core PCE price index, which excludes volatile food and energy components. However, the surge in inflation through June 2022 was broad based, with prices also rising substantially outside of these sectors. Thus, understanding the importance of markups for driving inflation requires a macroeconomic perspective that examines the evolution of aggregate markups across all sectors of the economy.
Figure 2 Cumulative changes in markups for salient industries
The role of aggregate markups in the economy
To assess how much markup changes contribute to movements in inflation more broadly, we use our industry-level measurements to calculate an aggregate markup at the macroeconomic level. We aggregate the cumulative changes in industry markups, applying two different weighting methods, as displayed in Figure 3. In the first method (green line), we match our industry categories to the spending categories in the core PCE price index for ease of comparison; we then use the PCE weights for each category to compute the aggregate markup. Alternatively, we use each industry’s cost weights to compute the aggregate markup (blue line). Regardless of the weighting method, Figure 3 shows that aggregate markups have stayed essentially flat since the start of the recovery, while the core PCE price index (gray line) rose by more than 10%. Thus, changes in markups are not likely to be the main driver of inflation during the recovery, which aligns with results from Glover, Mustre-del-Río, and von Ende-Becker (2023) and Hornstein (2023) using different methodologies or data. Markups also have not played much of a role in the slowing of inflation since the summer of 2022.
Figure 3 Cumulative changes in aggregate markups and prices
Moreover, the path of aggregate markups over the past three years is not unusual compared with previous recoveries. Figure 4 shows the cumulative changes in aggregate markups since the start of the current recovery (dark blue line), alongside aggregate markups following the 1991 (green line), 2001 (yellow line), and 2008 (light blue line) recessions. Aggregate markups have stayed roughly constant throughout all four recoveries.
Figure 4 Cumulative changes of aggregate markups in recoveries
Firms’ pricing power may change over time, resulting in markup fluctuations. In this Letter , we examine whether increases in markups played an important role during the inflation surge between early 2021 and mid-2022 and if declines in markups have contributed to disinflation since then. Using industry-level data, we show that markups did rise substantially in a few important sectors, such as motor vehicles and petroleum products. However, aggregate markups—the more relevant measure for overall inflation—have stayed essentially flat since the start of the recovery. As such, rising markups have not been a main driver of the recent surge and subsequent decline in inflation during the current recovery.
Aghion, Philippe, Antonin Bergeaud, Timo Boppart, Peter J. Klenow, and Huiyu Li. 2023. “A Theory of Falling Growth and Rising Rents.” Review of Economic Studies 90(6), pp.2,675-2,702.
Glover, Andrew, José Mustre-del-Río, and Alice von Ende-Becker. 2023. “ How Much Have Record Corporate Profits Contributed to Recent Inflation? ” FRB Kansas City Economic Review 108(1).
Hornstein, Andreas. 2023. “ Profits and Inflation in the Time of Covid .” FRB Richmond Economic Brief 23-38 (November).
Liu, Zheng, and Thuy Lan Nguyen. 2023. “ Global Supply Chain Pressures and U.S. Inflation .” FRBSF Economic Letter 2023-14 (June 20).
Palazzo, Berardino. 2023. “ Corporate Profits in the Aftermath of COVID-19 .” FEDS Notes , Federal Reserve Board of Governors, September 8.
Weber, Isabella M. and Evan Wasner. 2023. “Sellers’ Inflation, Profits and Conflict: Why Can Large Firms Hike Prices in an Emergency?” Review of Keynesian Economics 11(2), pp. 183-213.
Opinions expressed in FRBSF Economic Letter do not necessarily reflect the views of the management of the Federal Reserve Bank of San Francisco or of the Board of Governors of the Federal Reserve System. This publication is edited by Anita Todd and Karen Barnes. Permission to reprint portions of articles or whole articles must be obtained in writing. Please send editorial comments and requests for reprint permission to [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Research Questions and Priorities for Tuberculosis: A Survey of Published Systematic Reviews and Meta-Analyses
Ioana nicolau.
1 McGill University, Montreal, Quebec, Canada
Daphne Ling
2 Emory University, Atlanta, Georgia, United States of America
Christian Lienhardt
3 Stop TB Partnership, World Health Organization, Geneva, Switzerland
Madhukar Pai
Conceived and designed the experiments: IN CL MP. Performed the experiments: IN DL LT. Analyzed the data: IN CL MP. Wrote the paper: IN MP.
Systematic reviews are increasingly informing policies in tuberculosis (TB) care and control. They may also be a source of questions for future research. As part of the process of developing the International Roadmap for TB Research, we did a systematic review of published systematic reviews on TB, to identify research priorities that are most frequently suggested in reviews.
Methodology/Principal Findings
We searched EMBASE, MEDLINE, Web of Science, and the Cochrane Library for systematic reviews and meta-analyses on any aspect of TB published between 2005 and 2010. One reviewer extracted data and a second reviewer independently extracted data from a random subset of included studies. In total, 137 systematic reviews, with 141 research questions, were included in this review. We used the UK Health Research Classification System (HRCS) to help us classify the research questions and priorities. The three most common research topics were in the area of detection, screening and diagnosis of TB (32.6%), development and evaluation of treatments and therapeutic interventions (23.4%), and TB aetiology and risk factors (19.9%). The research priorities determined were mainly focused on the discovery and evaluation of bacteriological TB tests and drug-resistant TB tests and immunological tests. Other important topics of future research were genetic susceptibility linked to TB and disease determinants attributed to HIV/TB. Evaluation of drug treatments for TB, drug-resistant TB and HIV/TB were also frequently proposed research topics.
Conclusions
Systematic reviews are a good source of key research priorities. Findings from our survey have informed the development of the International Roadmap for TB Research by the TB Research Movement.
Introduction
Tuberculosis (TB) continues to pose a major threat to global health [1] , and research is a key component of the Global Plan to Stop TB2011-2015 [2] . Research is particularly critical for developing new tools and approaches needed for eliminating TB by 2050 [3] . Recognizing this, the Stop TB Partnership and the World Health Organization's (WHO) Stop TB Department have launched the TB Research Movement, with the aim of boosting TB research and accelerating progress in TB control towards international targets [4] . One of the main outputs of the TB Research Movement in 2011 was the publication of the International Roadmap for Tuberculosis Research [5] in October of 2011. This roadmap outlines all priority areas for investment in TB research and is intended to promote coordination and harmonization of scientific work on TB. Research priorities are identified in the areas of epidemiology; fundamental research; R&D of new diagnostics, drugs and vaccines; and operational and public health research. The ultimate goal is to reach all populations, including people with TB/HIV co-infection or MDR-TB and children, with new and better methods of prevention, diagnosis and treatment [5] .
The process for developing this roadmap has been recently described by Lienhardt and colleagues [4] . Briefly, the research roadmap was developed through a priority ranking exercise conducted by a multidisciplinary group of 50 research experts, a multidisciplinary Delphi consultation, a series of systematic reviews and an open web-based survey [4] .Among the systematic reviews that were commissioned, one was focused on all the TB research agendas that have been published from 1998 to 2010 [6] . As a next step, we were commissioned to review all the published systematic reviews and meta-analyses on TB (in all areas, including drugs, vaccines, diagnostics), to assess what research priorities have been identified in these reviews. The objectives of our systematic review were as follows: (1) to identify all systematic reviews and meta-analyses pertaining to any aspect of tuberculosis from 2005 to 2010, and (2) to assess, compile and rank the research priorities that were identified.
MEDLINE, EMBASE, Web of Science, and the Cochrane Library were searched for systematic reviews and meta-analyses on TB. The search strategy was developed in consultation with a medical librarian. The search was limited to systematic reviews and meta-analyses published between January 1, 2005 and July 1, 2010, in order to focus on contemporary TB literature and identify research priorities of greatest relevance to current TB control.
The search strategy included the following keywords and MeSH terms: [‘tuberculosis’ (explode) OR ‘ Mycobacterium tuberculosis ’(explode) OR ‘tuberculosis’.ti,ab. OR ‘tuberculos*’.tw] AND [‘meta analysis’ (explode) OR ‘meta analyses’.ti,ab OR ‘meta-analyses’.ti,ab OR ‘meta-analysis’.ti,ab OR ‘metanalys*’.ti,ab OR ‘systematic review’.tw]. The search was not limited to English and the last search was performed on August 18, 2010.
Studies were included if they focused on any aspect of tuberculosis. We included systematic reviews and meta-analyses published in English, French, Spanish, and Italian. The languages included were based on the skill set of our research team. We included systematic review and meta-analyses that had focused on tuberculosis or on a tuberculosis related topic (e.g. BCG), in the title or abstract. We considered a study to be a systematic review or meta-analysis if the authors identified the study as such, or if the title or abstract contained the words “systematic review” or “meta-analysis”. Moreover, studies were regarded as systematic reviews if the authors reported a systematic, explicit approach to identify, select, and synthesize the available evidence.
The first screening of the titles and abstracts obtained following the electronic search was done by one reviewer (IN). Subsequently, the same reviewer (IN) screened the full text articles, determined the eligibility, and decided on the final inclusion of studies in the systematic review. Further, a second reviewer (MP) independently searched, screened and identified studies for the inclusion in the review.
Data abstraction
We developed a data extraction form which was pilot-tested by two reviewers (IN and DL). The reviewers independently piloted the forms until there were no major disagreements in the data extraction process. One reviewer (IN) extracted the data from all the included studies and the second reviewer (DL) extracted data in duplicate for a random subset of 15% of the total number of included articles. Additionally, a third reviewer (LT) independently extracted data for all included studies on the study characteristics section of the data extraction form. Disagreements between the three reviewers were resolved by consensus.
Study characteristics
We extracted data from the text or online supplement of each included systematic review or meta-analysis. Information was collected on two main points: i) the main focus of the systematic review, and ii) questions and priorities identified for future research. The UK Health Research Classification System (HRCS) [7] , developed by the UK Clinical Research Collaboration for the classification and analysis of all types of health research, was used to determine the focus of the included studies as well as the focus of the research questions/priorities. In particular, the HRCS Research Activity Codes [7] were used to assign a category for the main focus of the studies and the research questions/priorities.
The main focus of each included systematic review was determined by extracting keywords from the title and abstract and matching them with the criteria developed by the HRCS. The Codes were divided into eight major categories: (1) Underpinning research; (2) Aetiology; (3) Prevention of disease and conditions, and promotion of well-being; (4) Detection, screening and diagnosis; (5) Development of treatments and therapeutic interventions; (6) Evaluation of treatments and therapeutic interventions; (7) Management of diseases and conditions; and (8) Health and social care services research (see Table 1 for full description). These research categories were used in Tables 2 and and3, 3 , to provide an overarching framework for grouping TB research.
In the HRCS, each of the eight major categories is further subdivided into five to nine subcategories with definitions for the type of research that belonged to that subcategory. For instance, “(1)Underpinning research” includes five subcategories: (1.1)studies of normal biological development and functioning, including gene, gene products, biological pathways, molecular and cellular structures, and development and characterization of model systems; (1.2) studies that do not address health directly but cover issues such as psychological and socioeconomic processes, individual or group characteristics and behaviours, and social and cultural beliefs; (1.3) research in chemical and physical sciences that may lead to the future development of diagnostic tools or treatments; (1.4) studies that target the development of novel methodologies and measurements including the development of statistical methods, and the development of mapping methodologies; and (1.5)research involving the development and/or distribution of resources for use by the research community, and infrastructure to support research networks. Using the main categories and the subdivisions within each category, we mapped the corresponding TB research areas found in the literature search (refer to Tables 1 and and2 2 ).
Quantitative data synthesis
Study characteristics were summarized using descriptive statistics. Measures such as total count, frequency, and proportion, were used to summarize data. Data analyses were performed using STATA Version 11.0.
There were a total of 973 records identified through the electronic database search ( Figure 1 ). The first screening of titles and abstracts was done on 680 records. Following the first screening process, 528 records were excluded. The reasons for exclusion are listed in Figure 1 . The full text screening of articles was performed on 152 records. Overall, there were 137 systematic reviews included in our analysis [8] – [144] .

Characteristics of included TB systematic reviews
The 137 reviews were published in 61 different journals. The majority of reviews (39.4%) were published in journals with impact factors of five or less, and only six (4.3%) reviews were published in journals with a high impact factor (>15). However, a large proportion of the reviews (38.6%) were published in journals that did not have an impact factor. In addition, approximately 24% of the main authors were from the United States and 41% were from four other countries (China, UK, Canada, and Italy). The remaining 34.1% of authors were from 26 different countries.
Out of the 137 reviews, 131 (95.6%) self identified as a systematic review or meta-analysis, which means that they used the term “systematic review” or “meta-analysis” in the title or abstract. Approximately 91% (124) of all reviews were not Cochrane reviews. Among the 13 Cochrane reviews, 9 of them focused on “evaluation of treatments and therapeutic interventions”.
Half of the reviews (67 [48.9%]) reported having a funding source, whereas only 15 reviews (11.0%) reported not being funded and 55 reviews (40.1%) did not report funding status. Most of the reviews (109 [79.6%]) included less than 50 studies in their review and within those reviews, the majority had between 1,000 and 10,000 participants (34/109[31.2%]).
Focus of TB systematic reviews
The main focus of each review was determined using the HRCS as described in the Methods section. The classification categories were subdivided into major tuberculosis research areas as described in Table 2 . The three most common review categories, in decreasing order, were “Detection, screening and diagnosis” with 46/141(32.6%) systematic reviews, “Development and evaluation of treatments and therapeutic interventions” with 33/141(23.4%) systematic reviews and “Aetiology” with 28/141(19.9%) systematic reviews.
Within the category of “Detection, screening and diagnosis”, 17/46 (37%) of the reviews focused on bacteriological diagnostics for active TB, such as improving processing methods of sputum smear microscopy, and assessing the use of nucleic acid amplification tests (NAATs). The two other most common TB research aims were bacteriological diagnostics for MDR-TB (9/46[20%]) and immunological diagnostics (9/46[20%]). More specifically, bacteriological diagnostics for MDR-TB included tests such as line-probe assays, bacteriophage based assays, and colorimetric redox assays. Immunological diagnostics were focused mainly on testing and evaluating interferon-gamma release assays (IGRAs).
In the category “Development and evaluation of treatments and therapeutic interventions”, 10/33 (30%) studies focused on drug resistant tuberculosis treatment, 9/33 (27.3%) studies on evaluating different regimen combinations for tuberculosis treatment, and 6/33(18.2%) on treatment of latent tuberculosis infection (LTBI).
In the category “Aetiology”, 11/28 (39.3%) systematic reviews focused on biological/genetic risk factors such as genetic susceptibility and gene targets,11/28 (39.3%) studies targeted surveillance and distribution of TB/HIV co-infection, MDRTB and HIV, and diabetes and TB, and 5/28(17.9%) focused on travel risk for LTBI and nosocomial TB exposure.
Research priorities
Out of 137 reviews, 103 (75%) identified at least one research question or a research priority. Of these, 48 (46.6%) identified only one research priority, 33 (32.0%) two research priorities, 7 (6.8%) three, 7 (6.8%) four, and 8 (7.8%) five research priorities. None of the reviews identified more than five research priorities.
Table 3 shows the summary of research priorities by category, subdivision, and TB-specific research priority. The three major categories of research priorities/questions were “Detection, screening and diagnosis” responsible for 50/191 (26.2%) of all the identified research priorities, “Aetiology” with 42/191 (22.0%), and “Evaluation of treatments and therapeutic interventions” with 37/191 (19.4%).
In the most common category, “Detection, screening and diagnosis”, the top research priority was the evaluation of bacteriological TB diagnostic tests in 14/50 (28.0%) reviews. Other frequently cited TB research priorities were: evaluation of immunological TB diagnostic tests (6/50 [12.0%]); discovery and development of new TB diagnostic tests (5/50 [10.2%]); and development of new bacteriological MDR-TB diagnostics (5/50 [10.2%]). Two priorities had almost equal importance and were highly prevalent in TB literature. The main priority in that category was to investigate the detection, screening and diagnosis of drug-resistant TB and MDR-TB. Studies called for the need to develop studies that detect resistance from smear positive specimens, determine the accuracy of colorimetric methods, line-probe assays, phage-based assays for rapid screening and nitrate reductase assay (NRA), and find the clinical usefulness of rapid diagnosis of rifampicin-resistant TB. Another frequency priority was to address unresolved research questions on interferon-gamma release assays (IGRAs), discover new antigens with immunodiagnostic potential, and test IGRAs in various populations and settings to establish test reproducibility. Evaluating sputum processing methods and smear microscopy, assessing nucleic acid amplification tests (NAATs), and evaluating tests for extrapulmonary TB (e.g. adenosine deaminase for pleural TB) were commonly cited priorities.
Within the “Aetiology” category, the main TB research priorities were: development of new research methods; better study designs or statistical tools for studying drug resistant TB, MDR-TB, links between HIV and MDR-TB; comparison of diagnostic tests (17/42 [40.5%]); identification of biological and genetic risk factors (15/42 [35.7%]); and evaluation of the role of risk factors such as tobacco and air pollutants (7/42 [16.7%]). The most frequent priority was to examine gene and gene products in relation to TB disease and susceptibility to disease. Key genes such as vitamin D receptor polymorphisms, IL10 gene, and drug-metabolizing enzyme (DME) gene polymorphisms were commonly mentioned for future research. The second most frequent research priority on TB/HIV included recommendations to conduct studies investigating XDR-TB and HIV co-infection, identifying a comprehensive definition of IRIS (immune reconstitution inflammatory syndrome), and investigating sputum processing methods with direct smears in settings with high and low HIV prevalence.
The category “Evaluation of treatments and therapeutic interventions” was the third most frequent. It focused on TB/HIV drug treatments (12/37 [32.4%]), drug-resistant TB treatments (11/37 [29.7%]), new TB drugs and active tuberculosis regimens (8/37[21.6%]). Implementing studies that evaluate new treatments and therapeutic interventions for drug-resistant TB, MDR-TB, and XDR-TB, was a prominent research priority. Such studies would need to examine methods to improve treatment outcomes for patients with XDR TB such as using later-generation fluoroquinolones, discovering methods to tailor treatment regimens for various forms of TB drug resistance, and investigating the use of quality-controlled laboratory testing for all first and second-line drugs that define XDR-TB. Another frequently cited priority was designing trials to evaluate the optimal duration of TB treatment, the influence of level of immunosuppression on effectiveness of TB drugs, and the combination of anti-TB chemoprophylaxis with antiretroviral therapy.
Systematic reviews and meta-analyses are widely acknowledged as a key component of the policy and guideline development process [145] . A large number of systematic reviews have been published in the area of TB diagnostics [146] , and these are increasingly being used for developing guidelines [147] . To this end, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool has increasingly been adopted by policy makers and guideline developers to provide an explicit, comprehensive and transparent process for moving from evidence to recommendations [145] .
Systematic reviews often conclude by making suggestions for the direction of future research, and thus could be a good source for identifying the most important questions for TB research. Our survey collected descriptive information from all eligible systematic reviews and meta-analyses that were subsequently used to generate a list of research priorities in TB which were used for developing the International Roadmap for Tuberculosis Research [5] .
Our systematic search showed that a fairly high number of systematic reviews were published on TB during the period of 2005 to 2010. The findings of our review need to be interpreted along with a recent systematic review by Rylance and colleagues [6] on 33 articles with research agendas on TB. These authors found that the top priority areas for research were new TB drug development (28 articles), diagnosis and diagnostic tests (27), epidemiology (20), health services research (16), basic research (13), and vaccine development and use (13).
In our review of 137 TB systematic reviews, the top three categories for the focus of the research priorities/questions were “Detection, screening and diagnosis” “Aetiology” and “Evaluation of treatments and therapeutic interventions.” TB diagnosis and treatment were among the most important research priorities in both reviews. One possible reason of why TB diagnosis research ranked high on our list could be that our review focused on years 2005 to 2010, a period when major advances have been made in TB diagnostics, especially with IGRAs becoming a very popular subject of research [148] . Also, this time period saw the introduction of several WHO policies on TB diagnostics. Further, the emphasis on new tools in the Global Plan to Stop TB 2006–2015 [149] , along with the creation of product development partnerships such as the Foundation for Innovative New Diagnostics (FIND), AERAS, and Global Alliance for TB Drug Development, may have inspired research on new diagnostics and drugs.
The research priorities determined were mainly focused on the discovery and evaluation of bacteriological TB tests, drug-resistant TB tests and immunological tests, with special focus on IGRA tests. Also, tests for extra-pulmonary TB came up as a frequently cited priority in the Detection of TB category. Other important topics of future research were genetic susceptibility to TB and disease determinants attributed to HIV/TB. Evaluation of drug treatments for TB, drug-resistant TB and HIV/TB were also frequently proposed research topics. Many articles cited the need for improved and tailored treatment methods for MDR-TB and XDR-TB.
Although several systematic reviews identified areas for further research, the questions themselves were often framed in a generic way, rather than in a highly focused manner with specific recommendation for action. Future TB systematic reviews will need to be more focused, and propose highly specific, answerable questions that are amenable to well-designed research studies.
Our study has several limitations. Due to the poor overall quality of reporting of the systematic reviews, the findings may not be representative of the general output from the TB research community [150] . The inclusion of eligible studies was limited by the fact that we only reviewed articles in three other languages besides English. We were also unable to search ‘grey’ literature, contact authors, or hand search journals. The review also did not include any unpublished literature. Due to its overarching and generic nature, the Health Research Classification System categories were at times non-specific and difficult to match with specific areas of TB research. Furthermore, it was difficult to classify research priorities into narrow subdivisions since some research priorities could qualify for more than one subdivision. By categorizing research priorities into larger, predefined categories, we lost detailed information on individual research priorities. To remedy this, we condensed each priority and extracted the topic words from it. The topic words were then grouped together to form the summary of repeated priorities/questions and calculate the frequency.
There has been a lot of recent attention and focus on childhood TB, but because our search was last performed in 2010, our analysis may have missed research priorities in this important area.
In summary, our systematic review of published systematic reviews on TB helped identify several key priorities for future TB research. This exercise was useful to describe the landscape of TB research and the overarching TB research themes arising from systematic reviews and meta-analyses conducted over the last 5 years. Their scope is, however, limited, since systematic reviews themselves are influenced by current hot topics or new technologies. They are nevertheless useful in indicating research priorities on areas that receive high attention, either due to recent scientific developments or increasing questions surrounding advancement of knowledge in these very areas. They bring useful additional arguments and information to the broader, deeper and more rigorously conducted process of international research agenda development.
Funding Statement
This work was supported in part by the Stop TB Partnership and World Health Organization. Dr Christian Lienhardt from the Stop TB Partnership and WHO provided input in study design and interpretation and revised the manuscript for intellectual content. Additional funding was provided by the European-Developing Countries Clinical Trials Programme (EDCTP; TB-NEAT grant). EDCTP had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.

IMAGES
VIDEO
COMMENTS
Abstract. Tuberculosis (TB) remains one of the deadliest infectious diseases responsible for millions of deaths annually across the world. In this paper we present a general overview of TB ...
Introduction. Tuberculosis (TB) represents a major global health threat that, despite being preventable and treatable, is the 13th leading cause of death worldwide and the second leading infectious killer after coronavirus disease 2019 (COVID-19) [1, 2].In the past decades, the TB burden has been slowly decreasing; however, with the emergence of COVID-19 and the current political conflicts ...
INTRODUCTION. Tuberculosis (TB) is one of the most ancient diseases of mankind and has co-evolved with humans for many thousands of years or perhaps for several million years.[] The oldest known molecular evidence of TB was detected in a fossil of an extinct bison (Pleistocene bison), which was radiocarbon dated at 17,870±230 years[] ; and in 9000, year old human remains which were recovered ...
Conclusion. The repercussions of ... Fox, W., Ellard, G. A. & Mitchison, D. A. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946 ...
the biggest challenge to ending tuberculosis has been insufficient case finding and diagnosis. An analysis from 2019 indicates that a country's capacity to screen and diagnose tuberculosis is the most substantial gap in the cascade from incident tuberculosis disease to successful completion of treatment. 14. Although COVID-19 has shown
Tuberculosis (TB) is an airborne disease caused by Mycobacterium tuberculosis (MTB) that usually affects the lungs leading to severe coughing, fever, and chest pains. Although current research in the past four years has provided valuable insight into TB transmission, diagnosis, and treatment, much remains to be discovered to effectively decrease the incidence of and eventually eradicate TB.
Introduction. Tuberculosis (TB) remains an important public health problem .With close to 10 million new cases per year, and a pool of two billion latently infected individuals, control efforts are struggling in many parts of the world (Figure 1).Nevertheless, the renewed interest in research and improved funding for TB give reasons for optimism.
Tuberculosis (TB), a preventable and curable disease, is claimed as the second largest number of fatalities, and there are 9,025 cases reported in the United States in 2018. Many researchers have done a lot of research and achieved remarkable results, but TB is still a severe problem for human beings. The study is a further exploration of the prevention and control of tuberculosis.
Author summary Tuberculosis (TB) and COVID-19, both highly infectious diseases, have posed significant global health challenges, particularly in low/middle-income countries (LMICs) with limited medical resources. Our research highlights that TB-COVID co-infection remains a substantial concern, impacting regions with varying TB burdens. The predominant treatment approach for TB-COVID co ...
Tuberculosis (TB) is a communicable infectious disease affecting around one quarter of the world's population [].The 'BRICS' countries of Brazil , Russia, India, China, and South Africa account for 47% of the total number of TB cases annually [1,2,3].Caused by the bacillus Mycobacterium tuberculosis, around 5-10% of those infected will develop active disease.
Abstract and Figures. Tuberculosis Is a major bluster to humanity resist to progress in health-care systems and the widespread weapon of TB control programs. The World Health Organization (WHO ...
Tuberculosis is highly prevalent among the low socioeconomic section of the population and marginalized sections of the community. In India, National strategic plan (2017-2025) has a national goal of elimination of tuberculosis by 2025. It requires increased awareness and understanding of Tuberculosis. In this review article history, taxonomy ...
Tuberculosis (TB) is the second leading infectious killer after COVID-19, causing 10 million new cases and claiming the lives of more than 1.5 million people every year []; furthermore, a growing number of multidrug-resistant TB strains constitute a major health threat.During long co-evolution, Mycobacterium tuberculosis has developed a plethora of molecular mechanisms that successfully bypass ...
Worldwide, tuberculosis (TB) is the leading cause of death from a single infectious disease agent (1) and the leading cause of death among persons living with human immunodeficiency virus (HIV ...
Tuberculosis (TB) causes a significant economic impact on the patients and their households 1,2.Although most high TB-burden countries have offered diagnosis and treatment free of charge, patients ...
Tuberculosis remains prominent in international statistics of ill health mainly because it kills young adults. More than 80% of the burden of tuberculosis, as measured in terms of disability-adjusted life years (DALYs) lost, is due to premature death rather than illness. About 1·7 million people died of tuberculosis in 2004, including 264 000 ...
This qualitative market research study was conducted between July 2020 and February 2021. Eight TB patients from each country (n = 40) completed health questionnaires, video/telephone interviews, and diaries regarding their experiences of TB. Additionally, 52 household members were interviewed.
Tuberculosis Research and Treatment publishes original research articles and review articles related to all aspects of tuberculosis, from the immunological basis of disease to translational and clinical research. ... Conclusion. The antituberculosis drug nonadherence is high. Marital status, educational status, drug side effects, HIV screening ...
1. Introduction. Tuberculosis (TB) is still a major global health problem as the global incidence in 2018 was estimated to be 10.0 millions, and the mortality 1.2 millions .The World Health Organization (WHO) and the United Nations' Millennium Development Goals (MDG) together with the Stop TB Strategy have developed strategies for eliminating TB, which have led to a decline in absolute ...
Tuberculosis (TB) is a progressive granulomatous infectious disease caused by the gram positive, acid fast bacilli classified under the genus Mycobacterium. Tuberculosis in human is mostly by Mycobacterium tuberculosis and primarily affects lungs causing pulmonary tuberculosis. It can also affect intestine, meninges, bones, joints, lymph nodes ...
Conclusion: We conclude that tuberculosis infection and disease remain common in populations characterized by poor housing conditions, drug use, and HIV infection. Linking a major medical provider with community-based organizations is an effective means to provide highly targeted screening services to a population at serious risk for disease ...
Conclusions. 7.1 Magnitude and trends of drug-resistant tuberculosis. 7.2 Extensively drug-resistant tuberculosis (XDR-TB) 7.3 Drug-resistant tuberculosis and HIV. 7.4 Survey coverage and methods. 7.5 Tuberculosis control and drug-resistant tuberculosis.
1 Introduction. Tuberculosis (TB) caused by drug resistant (DR) strains of the Mycobacterium tuberculosis complex (MTBC) is called DR-TB whose treatment requires the use of relatively more toxic drugs for extended period of time with comparatively low success rate (1-4).Over the years, TB has been the leading cause of mortality by a single infectious disease until the recent COVID-19 pandemic.
Tuberculosis (TB) continues to be one of the most significant infectious diseases, representing a serious threat to global public health. In 2022, ~7.5 million new TB cases were reported worldwide, marking the highest number since the World Health Organization (WHO) began its global TB monitoring in 1995 [].In the same year, TB was responsible for ~1.3 million deaths globally [].
Tuberculosis (TB) encompasses a vast amount of information about a common disease that is challenging to diagnose, treat, and prevent. The following quote by S.T. Cole provides a perspective on the importance of this disease: "More human lives have been lost to tuberculosis than to any other disease."[1] Before the SARS-CoV-2 pandemic, Mycobacterium tuberculosis (Mtb) was the most ...
The development of university students' skills to successfully produce scientific documents has been a recurring topic of study in academia. This paper analyzes the implementation of a training experience using a digital environment mediated by video content materials starring humanoid robots. The research aimed to scale complex thinking and its subcompetencies as a hinge to strengthen basic ...
The 2019 Lancet Commission on Tuberculosis laid out an optimistic vision for how to build a tuberculosis-free world through smart investments based on sound science and shared responsibility.1 Since then, several major strides have been made towards ending tuberculosis, including substantive improvements in treatment outcomes for people with drug-resistant disease.2,3 Although COVID-19 has ...
Conclusion. Firms' pricing power may change over time, resulting in markup fluctuations. In this Letter, we examine whether increases in markups played an important role during the inflation surge between early 2021 and mid-2022 and if declines in markups have contributed to disinflation since then. Using industry-level data, we show that ...
Introduction. Tuberculosis (TB) continues to pose a major threat to global health , and research is a key component of the Global Plan to Stop TB2011-2015 .Research is particularly critical for developing new tools and approaches needed for eliminating TB by 2050 .Recognizing this, the Stop TB Partnership and the World Health Organization's (WHO) Stop TB Department have launched the TB ...