

68 Best Chemistry Experiments: Learn About Chemical Reactions
Whether you’re a student eager to explore the wonders of chemical reactions or a teacher seeking to inspire and engage your students, we’ve compiled a curated list of the top 68 chemistry experiments so you can learn about chemical reactions.
While the theories and laws governing chemistry can sometimes feel abstract, experiments bridge the gap between these concepts and their tangible manifestations. These experiments provide hands-on experiences illuminating the intricacies of chemical reactions, molecular structures, and elemental properties.
1. Covalent Bonds

By engaging in activities that demonstrate the formation and properties of covalent bonds, students can grasp the significance of these bonds in holding atoms together and shaping the world around us.
Learn more: Covalent Bonds
2. Sulfuric Acid and Sugar Demonstration
Through this experiment, students can develop a deeper understanding of chemical properties, appreciate the power of chemical reactions, and ignite their passion for scientific exploration.
3. Make Hot Ice at Home
Making hot ice at home is a fascinating chemistry experiment that allows students to witness the captivating transformation of a liquid into a solid with a surprising twist.
4. Make a Bouncing Polymer Ball

This hands-on activity not only allows students to explore the fascinating properties of polymers but also encourages experimentation and creativity.
Learn more: Thought Co
5. Diffusion Watercolor Art

This experiment offers a wonderful opportunity for students to explore the properties of pigments, observe how they interact with water, and discover the mesmerizing patterns and textures that emerge.
Learn more: Diffusion Watercolor Art
6. Exploding Baggie

The exploding baggie experiment is a captivating and dynamic demonstration that students should engage in with caution and under the supervision of a qualified instructor.
Learn more: Exploding Baggie
7. Color Changing Chemistry Clock

This experiment not only engages students in the world of chemical kinetics but also introduces them to the concept of a chemical clock, where the color change acts as a timekeeping mechanism.
Learn more: Color Changing Chemistry Clock
8. Pipe Cleaner Crystal Trees

By adjusting the concentration of the Borax solution or experimenting with different pipe cleaner arrangements, students can customize their crystal trees and observe how it affects the growth patterns.
Learn more: Pipe Cleaner Crystal Trees
9. How To Make Ice Sculptures

Through this experiment, students gain a deeper understanding of the physical and chemical changes that occur when water freezes and melts.
Learn more: Ice Sculpture
10. How to Make Paper

Through this hands-on activity, students gain a deeper understanding of the properties of cellulose fibers and the transformative power of chemical reactions.
Learn more: How to Make Paper
11. Color Changing Chemistry
Color changing chemistry is an enchanting experiment that offers a captivating blend of science and art. Students should embark on this colorful journey to witness the mesmerizing transformations of chemicals and explore the principles of chemical reactions.
12. Gassy Banana
The gassy banana experiment is a fun and interactive way for students to explore the principles of chemical reactions and gas production.
Learn more: Gassy Banana
13. Gingerbread Man Chemistry Experiment

This hands-on activity not only introduces students to the concepts of chemical leavening and heat-induced reactions but also allows for creativity in decorating and personalizing their gingerbread creations.
Learn more: Gingerbread Man Chemistry Experiment
14. Make Amortentia Potion

While the love potion is fictional, this activity offers a chance to explore the art of potion-making and the chemistry behind it.
Learn more: How to Make Amortentia Potion
15. Strawberry DNA Extraction
This hands-on experiment offers a unique opportunity to observe DNA, the building blocks of life, up close and learn about its structure and properties.
16. Melting Snowman

The melting snowman experiment is a fun and whimsical activity that allows students to explore the principles of heat transfer and phase changes.
Learn more: Melting Snowman
17. Acid Base Cabbage Juice

The acid-base cabbage juice experiment is an engaging and colorful activity that allows students to explore the pH scale and the properties of acids and bases.
By extracting the purple pigment from red cabbage leaves and creating cabbage juice, students can use this natural indicator to identify and differentiate between acidic and basic substances.
Learn more: Acid Base Cabbage Juice
18. Magic Milk

The magic milk experiment is a mesmerizing and educational activity that allows students to explore the concepts of surface tension and chemical reactions.
By adding drops of different food colors to a dish of milk and then introducing a small amount of dish soap, students can witness a captivating display of swirling colors and patterns.
Learn more: Magic Milk
19. Melting Ice with Salt and Water

Through this hands-on activity, students can gain a deeper understanding of the science behind de-icing and how different substances can influence the physical properties of water.
Learn more: Melting Ice with Salt and Water
20. Barking Dog Chemistry Demonstration

The barking dog chemistry demonstration is an exciting and visually captivating experiment that showcases the principles of combustion and gas production.
21. How to Make Egg Geodes

Making egg geodes is a fascinating and creative chemistry experiment that students should try. By using common materials like eggshells, salt, and food coloring, students can create their own beautiful geode-like crystals.
Learn more: How to Make Egg Geodes
22. Make Sherbet

This experiment not only engages the taste buds but also introduces concepts of acidity, solubility, and the chemical reactions that occur when the sherbet comes into contact with moisture.
Learn more: Make Sherbet
23. Hatch a Baking Soda Dinosaur Egg

As the baking soda dries and hardens around the toy, it forms a “shell” resembling a dinosaur egg. To hatch the egg, students can pour vinegar onto the shell, causing a chemical reaction that produces carbon dioxide gas.
Learn more: Steam Powered Family
24. Chromatography Flowers

By analyzing the resulting patterns, students can gain insights into the different pigments present in flowers and the science behind their colors.
Learn more: Chromatography Flowers
25. Turn Juice Into Solid

Turning juice into a solid through gelification is an engaging and educational chemistry experiment that students should try. By exploring the transformation of a liquid into a solid, students can gain insights of chemical reactions and molecular interactions.
Learn more: Turn Juice into Solid
26. Bouncy Balls
Making bouncy balls allows students to explore the fascinating properties of polymers, such as their ability to stretch and rebound.
27. Make a Lemon Battery
Creating a lemon battery is a captivating and hands-on experiment that allows students to explore the fundamentals of electricity and chemical reactions.
28. Mentos and Soda Project
The Mentos and soda project is a thrilling and explosive experiment that students should try. By dropping Mentos candies into a bottle of carbonated soda, an exciting eruption occurs.
29. Alkali Metal in Water
The reaction of alkali metals with water is a fascinating and visually captivating chemistry demonstration.
30. Rainbow Flame
The rainbow flame experiment is a captivating and visually stunning chemistry demonstration that students should explore.
31. Sugar Yeast Experiment
This experiment not only introduces students to the concept of fermentation but also allows them to witness the effects of a living organism, yeast, on the sugar substrate.
32. The Thermite Reaction
The thermite reaction is a highly energetic and visually striking chemical reaction that students can explore with caution and under proper supervision.
This experiment showcases the principles of exothermic reactions, oxidation-reduction, and the high temperatures that can be achieved through chemical reactions.
33. Polishing Pennies
Polishing pennies is a simple and enjoyable chemistry experiment that allows students to explore the concepts of oxidation and cleaning methods.
34. Elephant Toothpaste
The elephant toothpaste experiment is a thrilling and visually captivating chemistry demonstration that students should try with caution and under the guidance of a knowledgeable instructor.
35. Magic Potion
Creating a magic potion is an exciting and imaginative activity that allows students to explore their creativity while learning about the principles of chemistry.
36. Color Changing Acid-Base Experiment

Through the color changing acid-base experiment, students can gain a deeper understanding of chemical reactions and the role of pH in our daily lives.
Learn more: Color Changing Acid-Base Experiment
37. Fill up a Balloon
Filling up a balloon is a simple and enjoyable physics experiment that demonstrates the properties of air pressure. By blowing air into a balloon, you can observe how the balloon expands and becomes inflated.
38. Jello and Vinegar

The combination of Jello and vinegar is a fascinating and tasty chemistry experiment that demonstrates the effects of acid on a gelatin-based substance.
Learn more: Jello and Vinegar
39. Vinegar and Steel Wool Reaction

This experiment not only provides a visual demonstration of the oxidation process but also introduces students to the concept of corrosion and the role of acids in accelerating the process.
Learn more: Vinegar and Steel Wool Reaction
40. Dancing Rice

The dancing rice experiment is a captivating and educational demonstration that showcases the principles of density and buoyancy.
By pouring a small amount of uncooked rice into a clear container filled with water, students can witness the rice grains moving and “dancing” in the water.
Learn more: Dancing Rice
41. Soil Testing Garden Science

Soil testing is a valuable and informative experiment that allows students to assess the composition and properties of soil.
By collecting soil samples from different locations and analyzing them, students can gain insights into the nutrient content, pH level, and texture of the soil.
Learn more: Soil Testing Garden Science
42. Heat Sensitive Color Changing Slime

Creating heat-sensitive color-changing slime is a captivating and playful chemistry experiment that students should try.
Learn more: Left Brain Craft Brain
43. Experimenting with Viscosity

Experimenting with viscosity is an engaging and hands-on activity that allows students to explore the flow properties of liquids.
Viscosity refers to a liquid’s resistance to flow, and this experiment enables students to investigate how different factors affect viscosity.
Learn more: Experimenting with Viscosity
44. Rock Candy Science

Rock candy science is a delightful and educational chemistry experiment that students should try. By growing their own rock candy crystals, students can learn about crystal formation and explore the principles of solubility and saturation.
Learn more: Rock Candy Science
45. Baking Soda vs Baking Powder

Baking soda and baking powder have distinct properties that influence the leavening process in different ways.
This hands-on experiment provides a practical understanding of how these ingredients interact with acids and moisture to create carbon dioxide gas.
46. Endothermic and Exothermic Reactions Experiment

The endothermic and exothermic reactions experiment is an exciting and informative chemistry exploration that students should try.
By observing and comparing the heat changes in different reactions, students can gain a deeper understanding of energy transfer and the concepts of endothermic and exothermic processes.
Learn more: Education.com
47. Diaper Chemistry

By dissecting a diaper and examining its components, students can uncover the chemical processes that make diapers so effective at absorbing and retaining liquids.
Learn more: Diaper Chemistry
48. Candle Chemical Reaction
The “Flame out” experiment is an intriguing and educational chemistry demonstration that students should try. By exploring the effects of a chemical reaction on a burning candle, students can witness the captivating moment when the flame is extinguished.
49. Make Curds and Whey

This experiment not only introduces students to the concept of acid-base reactions but also offers an opportunity to explore the science behind cheese-making.
Learn more: Tinkerlab
50. Grow Crystals Overnight

By creating a supersaturated solution using substances like epsom salt, sugar, or borax, students can observe the fascinating process of crystal growth. This experiment allows students to explore the principles of solubility, saturation, and nucleation.
Learn more: Grow Crystals Overnight
51. Measure Electrolytes in Sports Drinks
The “Measure Electrolytes in Sports Drinks” experiment is an informative and practical chemistry activity that students should try.
By using simple tools like a multimeter or conductivity probe, students can measure the electrical conductivity of different sports drinks to determine their electrolyte content.
52. Oxygen and Fire Experiment
The oxygen and fire experiment is a captivating and educational chemistry demonstration that students should try. By observing the effects of oxygen on a controlled fire, students can witness the essential role of oxygen in supporting combustion.
53. Electrolysis Of Water
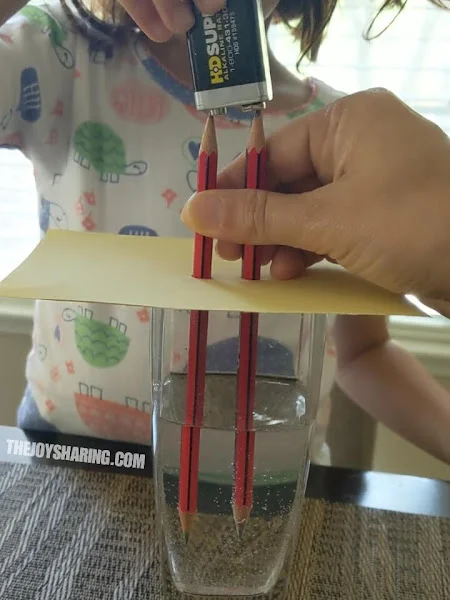
The electrolysis of water experiment is a captivating and educational chemistry demonstration that students should try.
Learn more: Electrolysis Of Water
54. Expanding Ivory Soap

The expanding Ivory Soap experiment is a fun and interactive chemistry activity that students should try. By placing a bar of Ivory soap in a microwave, students can witness the remarkable expansion of the soap as it heats up.
Learn more: Little Bins Little Hands
55. Glowing Fireworks

This experiment not only introduces students to the principles of pyrotechnics and combustion but also encourages observation, critical thinking, and an appreciation for the physics and chemistry behind.
Learn more: Glowing Fireworks
56. Colorful Polymer Chemistry

Colorful polymer chemistry is an exciting and vibrant experiment that students should try to explore polymers and colorants.
By combining different types of polymers with various colorants, such as food coloring or pigments, students can create a kaleidoscope of colors in their polymer creations.
Learn more: Colorful Polymer Chemistry
57. Sulfur Hexafluoride- Deep Voice Gas
This experiment provides a firsthand experience of how the density and composition of gases can influence sound transmission.
It encourages scientific curiosity, observation, and a sense of wonder as students witness the surprising transformation of their voices.
58. Liquid Nitrogen Ice Cream

Liquid nitrogen ice cream is a thrilling and delicious chemistry experiment that students should try. By combining cream, sugar, and flavorings with liquid nitrogen, students can create ice cream with a unique and creamy texture.
59. White Smoke Chemistry Demonstration

The White Smoke Chemistry Demonstration provides an engaging and visually captivating experience for students to explore chemical reactions and gases. By combining hydrochloric acid and ammonia solutions, students can witness the mesmerizing formation of white smoke.
60. Nitrogen Triiodide Chemistry Demonstration

The nitrogen triiodide chemistry demonstration is a remarkable and attention-grabbing experiment that students should try under the guidance of a knowledgeable instructor.
By reacting iodine crystals with concentrated ammonia, students can precipitate nitrogen triiodide (NI3), a highly sensitive compound.
61. Make a Plastic- Milk And Vinegar Reaction Experiment

Through the “Make a Plastic – Milk and Vinegar Reaction” experiment, students can gain a deeper understanding of the chemistry behind plastics, environmental sustainability, and the potential of biodegradable materials.
Learn more: Rookie Parenting
62. Eno and Water Experiment
This experiment not only introduces students to acid-base reactions but also engages their senses as they witness the visible and audible effects of the reaction.
63. The Eternal Kettle Experiment
By filling a kettle with alcohol and igniting it, students can investigate the behavior of the alcohol flame and its sustainability.
64. Coke and Chlorine Bombs
Engaging in this experiment allows students to experience the wonders of chemistry firsthand, making it an ideal choice to ignite their curiosity and passion for scientific exploration.
65. Set your Hand on Fire
This experiment showcases the fascinating nature of combustion and the science behind fire.
By carefully following proper procedures and safety guidelines, students can witness firsthand how the sanitizer’s high alcohol content interacts with an open flame, resulting in a brief but captivating display of controlled combustion.
66. Instant Ice Experiments
The Instant Ice Experiment offers an engaging and captivating opportunity for students to explore the wonders of chemistry and phase changes.
By using simple household ingredients, students can witness the fascinating phenomenon of rapid ice formation in just a matter of seconds.
67. Coke Cans in Acid and Base
Engaging in this experiment allows students to gain a deeper understanding of the chemical properties of substances and the importance of safety protocols in scientific investigations.
68. Color Changing Invisible Ink

The Color Changing Invisible Ink experiment offers an intriguing and fun opportunity for students to explore chemistry and learn about the concept of chemical reactions.
Learn more: Research Parent
Similar Posts:
- Top 100 Fine Motor Skills Activities for Toddlers and Preschoolers
- 37 Water Science Experiments: Fun & Easy
- Top 40 Fun LEGO Science Experiments
Leave a Comment Cancel reply
Save my name and email in this browser for the next time I comment.

Chemistry at Home: Exploring the Ingredients in Everyday Products
Hand cream, detergent, shower gel, toothpaste, toilet cleaner, air freshener, lipstick, perfume, low-fat spread, painkiller, diet drink, insect repellent… hundreds of everyday products that make our lives so much better than those of our forebears. And yet most of us know little about the ingredients they contain and why they deliver the benefits we enjoy.
Some people find it worrying when they examine the list of ingredients on a packaging label, because all they read may be unintelligible names or E numbers. It appears to be just chemicals, chemicals, chemicals. The aim of this book is to examine the ingredients more closely and explain the reasons for their being used.
Start reading and stop worrying.
Chemistry at Home has been written by award-winning popular science writer and chemist, John Emsley, using non-technical language. The book has 12 chapters, each devoted to the kinds of products we are likely to find around the home, including in the garage and the garden shed. Chemistry at Home also includes a glossary which gives more technical information about the molecules mentioned in the book.
- Cite Icon Cite
J. Emsley, Chemistry at Home: Exploring the Ingredients in Everyday Products, The Royal Society of Chemistry, 2015.
Download citation file:
- Ris (Zotero)
- Reference Manager
Digital access
Print format, table of contents.
- Front Matter
- Disclaimers
- Acknowledgements
- Technical Words You May Need to Help You Understand the Text
- The Medicine Cabinet p1-29 Abstract Open the PDF Link PDF for The Medicine Cabinet in another window
- Chapter 2: The Utility Room p30-52 Abstract Open the PDF Link PDF for Chapter 2: The Utility Room in another window
- Chapter 3: The Bathroom p53-110 Abstract Open the PDF Link PDF for Chapter 3: The Bathroom in another window
- Chapter 4: The Desk p111-126 Abstract Open the PDF Link PDF for Chapter 4: The Desk in another window
- Chapter 5: The Toilet p127-150 Abstract Open the PDF Link PDF for Chapter 5: The Toilet in another window
- Chapter 6: The Cupboard Under the Stairs p151-167 Abstract Open the PDF Link PDF for Chapter 6: The Cupboard Under the Stairs in another window
- Chapter 7: The Bedroom p168-202 Abstract Open the PDF Link PDF for Chapter 7: The Bedroom in another window
- Chapter 8: The Kitchen p203-230 Abstract Open the PDF Link PDF for Chapter 8: The Kitchen in another window
- Chapter 9: The Dining Room/Food and Drink p231-267 Abstract Open the PDF Link PDF for Chapter 9: The Dining Room/Food and Drink in another window
- Chapter 10: The Living Room p268-284 Abstract Open the PDF Link PDF for Chapter 10: The Living Room in another window
- Chapter 11: The Garage and the Car p285-299 Abstract Open the PDF Link PDF for Chapter 11: The Garage and the Car in another window
- Chapter 12: The Garden Shed p300-320 Abstract Open the PDF Link PDF for Chapter 12: The Garden Shed in another window
- Glossary p321-377 Open the PDF Link PDF for Glossary in another window
- Sources and Web Sites p378-381 Open the PDF Link PDF for Sources and Web Sites in another window
- Subject Index p382-395 Open the PDF Link PDF for Subject Index in another window
Advertisement
- Campaigning and outreach
- News and events
- Awards and funding
- Privacy policy
- Journals and databases
- Locations and contacts
- Membership and professional community
- Teaching and learning
- Help and legal
- Cookie policy
- Terms and conditions
- Get Adobe Acrobat Reader
- Registered charity number: 207890
- © Royal Society of Chemistry 2023
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Chemistry Essay Examples and Topics
Aldol synthesis of dibenzalacetone.
- Words: 1136
The Synthesis of Aspirin and Determination of Product Purity
- Words: 1650
Solubility of Potassium Nitrate
- Words: 1140
Potassium Iodide and Iron (III) Chloride Chemical Reaction
- Words: 1683
Acids, Bases and Buffers in Real-Life Applications
Methyl salicylate preparation using esterification.
- Words: 2059
Hydrated Copper (II) Sulphate Experiment
- Words: 1066
Primary Salt Effect in Kinetic of Ionic Reactions
- Words: 1114
Activation Energy of the Oxalic Acid/Potassium Permanganate Solution
Plastic impact on humans and planet.
- Words: 1079
Bomb Calorimetry: Theory and Experiment
- Words: 1595
Quantitative Analysis of Aspirin Tablets by Spectrophotometry
Concentration as a factor affecting the rate of chemical reactions, chemistry application in daily life.
- Words: 1435
Functional Group Identification
- Words: 1390
Nanofabrication. Bottom-Up vs. Top-Down Method
Chemistry: expansion processes of a perfect gas, spectrophotometric determination of the pka of an acid-base indicator.
- Words: 1592
Production of Acetone from isopropyl alcohol
- Words: 2271
Pinacol Rearrangement Laboratory Experiment
- Words: 3066
KHT Molar Solubility Experiment
- Words: 1322
The Solubility of Potassium Nitrate
- Words: 1738
Buffer Solution and Determination Changes in pH
The rate and order of a chemical reaction, rate law for iodine-acetone reaction, methamphetamine (meth), organic chemistry: combinatorial synthesis of azo dyes, oxidation states of manganese ion, interrelationship of metabolic pathways.
- Words: 1417
Thin Layer Chromatography
- Words: 1218
Retinol: Physical and Chemical Properties, Sources, Benefits, and Adverse Effects
The effect of ph on water holding capacity of chicken, preparation and recrystallization of acetanilide, liquid crystals: fundamental properties, and the effects of external factors.
- Words: 2803
Chemical Laser: Definition, Environment, Process, and Types
- Words: 2769
Synthesis of 4, 4’ -di(t-butyl)biphenyl by the Friedel-Crafts reaction
- Words: 2286
The Experiment on Substitution Reactions of Alcohols
Food dye and bleach reaction experiment.
- Words: 1353
Methyl Salicylate: Carboxylic Acids and Esters
- Words: 2741
P-Nitroacetanilide: Measurements and Calculations
Determination of quinine in tonic water with fluorescence spectroscopy.
- Words: 1400
Organic Chemistry – Contributions and in Life
Effect of ph on protein solubility, hydrolysis of tert-butyl chloride: kinetics lab, micro method determination of boiling point of hydrocarbons, determination of the molecular weight and pka for an unknown weak acid by titration.
- Words: 1497
Potassium Chloride, Magnesium Sulphate and Glucose Effects
- Words: 1841
Materials Used for Ethanol Production
- Words: 2849
Extraction of Sucrose, Acetylsalicylic Acid, and Acetanilide From Phensuprin
- Words: 2894
Titration of a Strong and Weak Acid
- Words: 1233
The Heat of Dissolution and Neutralization Reaction
- Words: 1152
Formula of a Complex With the Slope-Ratio Method
Fractional distillation lab report, hair care products: components and effects.
- Words: 1383
Amperometry: Chemical Analysis
- Words: 3681
Using Solar (PV) Energy to Generate Hydrogen Gas for Fuel Cells
Carboxylic acids and esters: preparation of methyl salicylate.
- Words: 2859
Diffusion in Polymer Solutions
- Words: 5561
UV & VIS Spectroscopy Experiment
The effect of acid on enzyme activity, types of saturation indices: calcium carbonate.
- Words: 1698
Analysis of Lab: Heat of Fusion of Water
Effect of potassium chloride concentration on the rate of mung seed germination.
- Words: 1395
The Ideal Gas Law in a Practical Experiment
Organic compounds at home and at the workplace, electrochemical methods of analysis, seawater vs. brackish water reverse osmosis, chromatography in the determination of amino acids, contribution of amedeo avogadro to chemistry, long residue processing in oil refineries.
- Words: 1096
Conversion of Tyrosine to Serotonin, Dopamine, and Norepinephrine
Determination of the enthalpy of an acid-base reaction, the pinacol rearrangement organic reaction.
- Words: 1660
Cooper and Silver: Physical and Chemical Properties
- Words: 1976
Iron: Properties, Occurrence, and Uses
The scope of use of chromatography, alkanes and alkenes: structure and reactions.
- Words: 1697
Aspects of Chemistry of Oxygen
Aqueous solution definition in chemistry.
- Words: 1760
Acid Extraction by Acid-Base-Coupled Extractants
- Words: 3105
Acetone: Physical and Chemical Properties
- Words: 2200
Sugar Results: Experiment on Chocolate
Application of catalyst and energy production.
- Words: 8961
Sodium Chloride – Science of Salt
Chemical raising agent in bread in lab experiment.
- Words: 1703
Chemical and Physical Properties of Ethane
Massive leak of liquified chlorine gas.
- Words: 2169
Pinacol Rearrangement Experiment
- Words: 1230
Green Chemistry for Consumer Products
- Words: 2195
Chemical Processes: The Diels-Alder Reaction
Intrinsic viscosity: chain linkage in polyvinyl alcohol, percent composition of calcium in salt: gravimetric analysis.
- Words: 1126
Stoichiometry and Process Calculations
Recent chemistry developments and their influence on society, substitution reactions of alcohols: practical experimentation.
- Words: 2290
Biochemical Metabolism: Foreign DNA Molecule
- Words: 1272
Benadryl: Structure, Properties, Interesting Facts
Nucleophilic substitution reactions of alcohols.
- Words: 2137
The Production of Aluminum
- Words: 1101
Oxidation-Reduction (Redox) Reactions
- Words: 1201
The Chemistry Behind Mineral Make-Up
- Words: 1398
Aqueous HCl Solution Preparation
Research of amorphous solids in world.
- Words: 2570
Indirect Definition of the Unit of a Penny Weight
Nylons: production, characteristics and applications.
- Words: 4161
Polypropylene Concept in Chemistry
- Words: 5166
Lubricating Oil Extraction Methods
- Words: 2147
Eggs: The Osmosis Process Investigation
Water properties as a solvent: an experiment lab, the physical and chemical properties of water, mixtures and compounds in chemistry.
- Words: 1284
Identifying a Weak Acid by Titrimetry
The solventless reaction: the aldol reaction condensation, biogeochemical cycle: nitrogen, acid effects on starch gels in food preparation, the pinacol rearrangement as a dehydration process.
- Words: 1575
Lubricants Oil Production in Refineries
- Words: 2245
Gold’s Production and Processing
- Words: 1634
Mechanism of Formation of Benzoic Acid
Texas safety standards in the science classroom.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation
- hot-topics Extras
- Newsletters
- Reading room
Tell us what you think. Take part in our reader survey
Celebrating twenty years
- Back to parent navigation item
- Collections
- Water and the environment
- Chemical bonding
- Antimicrobial resistance
- Energy storage and batteries
- AI and automation
- Sustainability
- Research culture
- Nobel prize
- Food science and cookery
- Plastics and polymers
- Periodic table
- Coronavirus
- More from navigation items
Chemistry at home
By Simon Cotton 2015-11-05T00:00:00+00:00
- No comments
John Emsley RSC 2015 | 394pp | £21.99 ISBN 9781849739405

I am sure that one reason for some misconceptions about chemicals is that little is taught in schools about their everyday applications and uses. That’s not the fault of the teacher in the classroom – they have a specification to teach and a lot to fit in. Of course, all packaging carries lists of ingredients that may provide some limited insight, but it is sometimes difficult to know what they all do.
For many years, the go-to resource was Ben Selinger’s Chemistry in the marketplace , while John Emsley has provided much cogent material in books like The consumer’s good chemical guide and Vanity, vitality and virility: the science behind the products you love to buy .
The latest edition of Selinger’s book first appeared in 1997, so it’s time for a spring clean of the literature, and that is what we are given in Emsley’s Chemistry at home . Before you get to grips with all of the different consumer products described in the book, there’s an alphabetical list of technical words. The chemicals are grouped by the part of the home in which they are found – the medicine cabinet; the utility room; the bathroom; the desk; the toilet; the cupboard under the stairs; the bedroom; the kitchen; the dining room; the living room; the garage; and the garden shed. Ingredient lists are given for well over 150 different products, a representative selection of what is on the market, and for each product there is a breakdown giving the purpose of each ingredient.
So, taking Savlon as an example, we are informed that cetrimide, the active ingredient, enters the microbial cell membrane and causes cell death by interfering with vital proteins. There are three other antibacterials involved and it also contains compounds with emollient and thickening properties, plus an unnamed fragrance and sterile water.
If you, as a chemist, want to find out more about a particular chemical, simply look in the alphabetical glossary at the end of the book, which supplies further information, usually including a structure, but keeping it separate for the benefit of readers who are fazed by this sort of thing.
This book is highly recommended, not least to teachers, and is an antidote to smear stories in the media. We should all have a copy on our bookshelves – it is very browsable, and you never know when some of the many bits of information it contains will come in handy. As the back cover says, ‘start reading and stop worrying’.
Purchase Chemistry at home from Amazon.co.uk
- Culture and people
Related articles

Using XRF to uncover the secrets of three Irish chalices
2024-06-07T13:30:00Z
By Raychelle Burks

Cryptic chemistry crossword #041
2024-06-07T13:03:00Z
By Paul Board
Chemistry wordoku #047
2024-06-07T13:02:00Z
By Hamish Kidd
Quick chemistry crossword #041
2024-06-07T13:00:00Z

Everyone belongs in the chemical sciences
2024-06-06T08:52:00Z
By Ollie Thomas

20 years. 20 chemists. 20 stories.
2024-06-04T11:30:00Z
By Phillip Broadwith
No comments yet
Only registered users can comment on this article., more from culture.

June 2024 puzzles
2024-06-03T09:30:00Z

Gregory Robinson: ‘We were members of the last generation to attend segregated schools’
2024-05-24T08:34:00Z
By Rebecca Trager

Catherine Ngila: ‘I am very passionate about empowering my students’
2024-05-03T13:00:00Z
By Jamie Durrani

May 2024 puzzles
2024-05-01T09:30:00Z

April 2024 puzzles
2024-04-01T09:30:00Z

Jani Ingram: ‘We have seen wells with uranium levels higher than the drinking water standard’
2024-03-25T14:05:00Z
- Contributors
- Terms of use
- Accessibility
- Permissions
- This website collects cookies to deliver a better user experience. See how this site uses cookies .
- This website collects cookies to deliver a better user experience. Do not sell my personal data .
- Este site coleta cookies para oferecer uma melhor experiência ao usuário. Veja como este site usa cookies .
Site powered by Webvision Cloud
Examples of Chemical Reactions in Everyday Life
ThoughtCo / Emily Roberts
- Chemistry In Everyday Life
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Chemistry happens in the world around you, not just in a lab. Matter interacts to form new products through a process called a chemical reaction or chemical change. Every time you cook or clean, it's chemistry in action. Your body lives and grows thanks to chemical reactions. There are reactions when you take medications, light a match, and draw a breath.
These examples of chemical reactions from everyday life are a small sampling of the hundreds of thousands of reactions you experience as you go about your day.
Key Takeaways: Chemical Reactions in Everyday Life
- Chemical reactions are common in daily life, but you may not recognize them.
- Look for signs of a reaction. Chemical reactions often involve color changes, temperature changes, gas production, or precipitant formation.
- Simple examples of everyday reactions include digestion, combustion, and cooking.
- What Is a Chemical Reaction?
A chemical change , often called a chemical reaction , occurs when substances transform into new and distinct substances. Essentially, it involves the rearrangement of atoms. Generally, chemical changes can be identified by temperature changes, light emission, bubble formation, precipitate formation, color changes, and odor release. These effects signify a change in composition, but they may not always be immediately apparent.
Usually, chemical changes are permanent, so they cannot be undone. Conversely, physical changes do not create new substances and can be reversed. Understanding these distinctions is fundamental to the study of chemistry .
Photosynthesis
Frank Krahmer / Getty Images
Plants apply a chemical reaction called photosynthesis to convert carbon dioxide and water into food (glucose) and oxygen. It's one of the most common everyday chemical reactions and also one of the most important because this is how plants produce food for themselves (and animals) and convert carbon dioxide into oxygen. The equation for the reaction is:
6 CO 2 + 6 H 2 O + light → C 6 H 12 O 6 + 6 O 2
Aerobic Cellular Respiration
Kateryna Kon/Science Photo Library / Getty Images
Aerobic cellular respiration is the opposite process of photosynthesis in that energy molecules are combined with the oxygen we breathe to release the energy needed by our cells plus carbon dioxide and water. Energy used by cells is chemical energy in the form of ATP, or adenosine triphosphate .
Here is the overall equation for aerobic cellular respiration:
C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O + energy (36 ATPs)
Anaerobic Respiration
Tastyart Ltd Rob White / Getty Images
Anaerobic respiration is a set of chemical reactions that allows cells to gain energy from complex molecules without oxygen. Your muscle cells perform anaerobic respiration whenever you exhaust the oxygen being delivered to them, such as during intense or prolonged exercise. Anaerobic respiration by yeast and bacteria is harnessed for fermentation to produce ethanol, carbon dioxide, and other chemicals that make cheese, wine, beer, yogurt, bread, and many other common products.
The overall chemical equation for one form of anaerobic respiration is:
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 + energy
Every time you strike a match, burn a candle, build a fire, or light a grill, you see the combustion reaction. Combustion combines energetic molecules with oxygen to produce carbon dioxide and water.
For example, the equation for the combustion reaction of propane, found in gas grills and some fireplaces, is:
C 3 H 8 + 5O 2 → 4H 2 O + 3CO 2 + energy
Alex Dowden/EyeEm / Getty Images
Over time, iron develops a red, flaky coating called rust. This is an example of an oxidation reaction . Other everyday examples include the formation of verdigris on copper and the tarnishing of silver.
Here is the chemical equation for the rusting of iron:
Fe + O 2 + H 2 O → Fe 2 O 3 . XH 2 O
If you combine vinegar and baking soda for a chemical volcano or milk with baking powder in a recipe, you experience a double displacement , or metathesis reaction (plus some others.) The ingredients recombine to produce carbon dioxide gas and water. The carbon dioxide forms bubbles in the volcano and helps baked goods rise .
These reactions seem simple in practice but often consist of multiple steps. Here is the overall chemical equation for the reaction between baking soda and vinegar:
HC 2 H 3 O 2 (aq) + NaHCO 3 (aq) → NaC 2 H 3 O 2 (aq) + H 2 O(l) + CO 2 (g)
Electrochemistry
Batteries use electrochemical or redox reactions to convert chemical energy into electrical energy. Spontaneous redox reactions occur in galvanic cells , while nonspontaneous chemical reactions take place in electrolytic cells .
Peter Dazeley/Photographer's Choice / Getty Images
Thousands of chemical reactions take place during digestion. As soon as you put food in your mouth, an enzyme in your saliva called amylase starts to break down sugars and other carbohydrates into simpler forms your body can absorb. Hydrochloric acid in your stomach reacts with food to further break it down, while enzymes cleave proteins and fats so they can be absorbed into your bloodstream through the walls of the intestines.
Acid-Base Reactions
Lumina Imaging / Getty Images
Whenever you combine an acid (e.g., vinegar, lemon juice, sulfuric acid , or muriatic acid ) with a base (e.g., baking soda , soap, ammonia, or acetone), you are performing an acid-base reaction. These reactions neutralize the acid and base to yield salt and water.
Sodium chloride isn't the only salt that can be formed. For example, here is the chemical equation for an acid-base reaction that produces potassium chloride, a common table salt substitute:
HCl + KOH → KCl + H 2 O
Soap and Detergent Reactions
JGI/Jamie Grill / Getty Images
Soaps and detergents clean by way of chemical reactions . Soap emulsifies grime, which means oily stains bind to the soap so they can be lifted away with water. Detergents act as surfactants, lowering the surface tension of water so it can interact with oils, isolate them, and rinse them away.
Cooking uses heat to cause chemical changes in food. For example, when you hard boil an egg, the hydrogen sulfide produced by heating the egg white can react with iron from the egg yolk to form a grayish-green ring around the yolk . When you brown meat or baked goods, the Maillard reaction between amino acids and sugars produces a brown color and a desirable flavor.
More Examples of Chemistry in Everyday Life
Chemical reactions are everywhere, and in a way, chemistry really makes up everything. From the emotions you feel to peculiar questions such as, "Can bottled water go bad?" Here are some examples of chemistry in everyday life.
- Examples of Physical Changes and Chemical Changes
- Simple Chemical Reactions
- Types of Chemical Reactions
- What Are the Products of Photosynthesis?
- Chemical Change Examples
- 10 Fascinating Photosynthesis Facts
- Chemical Change Definition in Chemistry
- Equation for the Reaction Between Baking Soda and Vinegar
- Understanding Endothermic and Exothermic Reactions
- An Introduction to Types of Respiration
- Calvin Cycle Steps and Diagram
- The Balanced Chemical Equation for Photosynthesis
- 12 Examples of Chemical Energy
- Combustion Definition in Chemistry
- How Many Types of Chemical Reactions Are There?

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
What Are Some Examples of Chemistry in Daily Life?

You encounter chemistry every day, yet might have trouble recognizing it, especially if you are asked as part of an assignment! What are some examples of chemistry in daily life?
Examples of Chemistry in the Real World
There are many examples of chemistry in daily life, showing how common and important it is.
- Digestion relies on chemical reactions between food and acids and enzymes to break down molecules into nutrients the body can absorb and use.
- Vitamins and minerals are everyday chemicals we need for survival.
- Hormones are chemicals that help us grow, heal, sense the world around us, and find love.
- Soaps and detergents act as emulsifiers that surround dirt and grime so it can be washed away from clothing, dishes, and our bodies.
- Fire is combustion, which is a chemical reaction. It cooks food, heats homes, and runs vehicles.
- Drugs work because of chemistry. The chemical compounds may fit into the binding site for natural chemicals in our body (e.g., block pain receptors) or may attack chemicals found in pathogens, but not human cells (e.g., antibiotics).
- Cooking is a chemical change that makes food more palatable, kills dangerous microorganisms, and makes it more digestible. The heat of cooking denature proteins, promotes chemical reactions between ingredients, carmelizes sugars , etc.
- Cosmetics are chemicals we use that improve our skin and change our appearance.
- Insecticides, herbicides, and pesticides are examples of chemicals that control pesky plants and animals.
- Synthetic fabrics and plastics come from chemical reactions, usually starting from petroleum.
- Paint is a complex mixture of colorful chemicals.
- Perfume is a set of chemicals that change the way we smell.
Reader Submissions: Chemistry in Everyday Life
Here are some examples of chemistry, from readers just like you.
- Medicines are the best example of chemistry. They save us from diseases and helps us live. They contain chemical compounds. —aim
- Everything is the product of chemistry. In the morning, we use toothpaste, which is a chemistry product. At night when we go to bed we burn a coil which contains chemicals that keep mosquitoes far from us. —Animesh
- You apply chemistry in your daily life to make sure any drugs you use aren’t compromised or overly strengthened by the foods you eat. For example, alcohol affects many drugs. Some medications are negated by eating something as seemingly harmless as grapefruit! Others contain caffeine as an active ingredient, so if you take the medicine with coffee or cola, you’re increasing your dosage. —gemdragon
- Colors in clothes come from azo dyes, which are organic compounds. —RG veena
- Chips in computers come from silicon. Electricity comes from chemistry in the form of electrons. —P Katual
- Our food is not tasty without salt. Salt is a chemical compound. —Tarun Omer
- Think of living without water or your favorite snacks. We are nothing without chemistry! —swati
- Chemicals make our clothes clean. We use chemicals when washing utensils. —swetha
- Fertilizers are one of the best examples of chemistry in everyday life. —savita
- Food is all about chemistry. The ingredients are chemicals. Cooking is a set of chemical reactions. Even the spoon you use is a chemical. —Satya ranjan jena
- The cement and other materials that we use in construction of houses (paints, plaster) are products of chemistry. —Hamna Riaz
- Cosmetics are chemicals we use that make us more attractive. —shikhar
- Water is an everyday chemical. It contains elements and hydrogen bonds and participates in chemical reactions. —junaid bangash
Do you have more examples of chemistry in daily life? Post a comment!
Related Posts
Leave a reply cancel reply.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

8 Easy Chemistry Experiments At Home (Get a Great Reaction!)

If you want to have some fun with chemistry at home, there are three main ways to go about it. You can buy a chemistry set, subscribe to a subscription box, or find some instructions and use household items. However you go about it, chemistry is a great way to get kids excited about chemistry and science in general.
Related post: Best STEM Subscription Box for Kids (Ultimate Guide 2024)
Chemistry Sets and Subscription Boxes
Before we dive into easy experiments, you can do with things you’ll probably have at home. I just wanted to talk a little bit about your other options.
Chemistry sets can be an excellent investment. They come with equipment that you can reuse over and over. It’s a lot cheaper than having to replace your drinking glasses and measuring jugs because the kids keep using them for chemistry experiments! They also come with instructions on a range of experiments that you can try. If you’ve done a few of the experiments below and are looking for something more, a chemistry set can be a really good option. There are ones aimed at young kids all the way up to teenagers .
Another great option to consider is subscription boxes. These are great for extending learning and keeping kids entertained and engaged for a more extended period. There are loads of great options to choose from. But, when it comes to chemistry, you really can’t go wrong with MEL Science . They have two subscription levels, so you can get a big box or a small one each month. Because everything is in the box, it really takes all the planning and hunting for ingredients out of the equation.
Experiments Using Household Items
If you don’t have a science kit on hand and are looking for something quick and easy to try at home, then these are the experiments you should try. Most of these use items that you will probably have at home, although a few might require you to get a little creative or grab the odd thing the next time you go to the shops.
Chromatography
Chromatography is a technique used in chemistry. It lets you find out what’s inside chemicals. In this version from Fizzics Education, you’ll see what colors are mixed up inside felt tip pens. It’s a straightforward experiment to carry out. All you need is some paper towels, felt tips, and a glass of water.
This is a simple version of this experiment, but there are some easy ways to make it more interesting or scientific. One way to extend this experiment is to try the same technique but using your favorite sweets’ colorings.
For instance, sweets like M&Ms, Skittles, and Smarties all have food coloring on their outside. You can get a sample of this coloring by sitting the candy in a small amount of water. Then you use the colored water in the same way as the felt tip pens.
To add a bit of rigor and math to the experiment, you need a pencil and a ruler. Instead of drawing a line of felt tip, you draw a line with a pencil. Then put a spot of the felt tip on the pencil line. When you take the paper out, you mark a second pencil line to show how high the water went.
By measuring the distance, each of the colors went and the distance the water went, you can calculate something called the retention factor. The retention factor will be unique for different dyes. To find the retention factor, you take the distance your sample travels and divide it by the length the solvent (water in this case) traveled. You can use this number to see if the same dye is used in different pens.
Pop Rockets
This is one of my favorite chemistry experiments for kids. It does get a little messy, so make sure you have some cloths on hand. Alternatively, you can do it outside to make it a little easier to clean up. Steve Spangler Science has some great instructions to follow.
In their version, they use an old film canister. But these can be a bit hard to get hold of these days since everything is digital. Some good alternatives that work well include empty glue stick containers. It’s also worth keeping your eye out for any food containers with push-on lids, as these can work well. There are always a lot of good options around Halloween, Christmas, and Easter – the snack size containers tend to be pretty good options.
The reason I love this experiment is that it’s a lot of fun. There’s the excitement of the pop and watching the canister fly. But, there are also a lot of opportunities to turn this into a real investigation. You can try changing the volumes of liquid or the type of liquid. You can find the best mix to make the biggest noise, the loudest pop, or the perfect mix to make it pop in precisely 8 seconds.
Make Oobleck Dance!

Oobleck is the name that’s been given to an awesome type of slime that you can make at home. If it sounds like something out of Dr. Suess, that’s because it is. This slime is just a mix of cornstarch and water, so it’s pretty easy to make. These instructions from Housing A Forest are pretty good.
What’s cool about Oobleck is that it’s a Non-Newtonian solid. That means that it behaves a little differently than you might expect. For instance, when you try to stir it quickly, it gets hard and almost solid. If you run your fingers through it slowly, it flows like runny syrup.
Now just playing with this stuff is fun, but if you have a speaker to hand, you can do something even cooler. In the guide from Housing A Forest, they suggest using a subwoofer and a cookie sheet. The speaker’s vibrations make the Oobleck bounce around and switch from a solid to a liquid to the beat of the music.
If you have an old speaker that you don’t mind breaking, you can wrap the speaker in saran wrap and put the Oobleck straight onto that. It works a lot better, but if you don’t cover the speaker correctly, it can break.
Make Rubber Eggs
Eggs are an excellent ingredient for science experiments. This experiment from 3P Learning lets you turn a hardboiled egg into a bouncy rubber one. To do this, all you need to do is soak it in vinegar for a day or so. This will dissolve the calcium carbonate of the eggshell. When it’s done, you’ll be able to rub off the tough outer shell.
Without the shell, you’ll be left with the membrane that lines the shell. This membrane helps hold the egg together. This membrane is strong enough to drop the egg onto a surface from a reasonable height, and it will bounce back without falling apart.
The harder your egg is, the less it will bounce. If you want a mix between bounciness and minimal potential for mess, then you’ll want to aim for a soft boiled egg. But, if you don’t mind the mess, try a raw egg. You remove the shell in the same way. When it comes off, you get a peek inside the raw egg. Because it’s raw, it’s squishy and bounces better. Of course, if you drop it from high enough, it will break. When you do this, you’ll find the stretchy membrane, which is pretty cool to feel.
Lemony Eruption
I’m sure you’ve all done the classic volcano eruption with baking soda and vinegar. This is a twist on that experiment. It takes advantage of the fact that lemons are already full of natural citric acid. Here are some great instructions from Babble Dabble Do. They have some handy tips on how to make the most out of each lemon. The great thing about this version is that your room will smell lovely and lemony for the rest of the day.
If you want to extend this, you try investigating which other fruits this would work with. You could explore a whole citric family of volcanoes.
Concoct Some Invisible Ink
Write Secret Messages With Invisible Ink! by Science Buddies
Making invisible ink is really easy. There is some fun chemistry behind how it works. As a bonus, once your kids get the hang of it, they’ll have loads of fun sending coded messages. It’s a great way to keep them entertained.
This great recipe from Thoughtco can be revealed using two different methods. If you have a safe and controllable heat source, you can hold the paper up to that. Ironing the paper works as well, although that’s best left to adults. Otherwise, you can use purple grape juice to reveal the message. If you paint over the page with grape juice, the message will show up in a different color.
If sending secret messages isn’t appealing to your kids, you could challenge them to create something artistic with this technique. The only limit is their imagination.
If you’ve done any chemistry experiments for kids, then you probably know that an acid + baking soda makes for an awesome fizzy experiment. I’ve seen this used in many different ways, but this version from STEAM powered family is one of the best.
In this experiment/activity, you encase small dinosaur toys in a paste made of baking soda and water. By adding food coloring, you can create multicolored eggs. You can even hide glitter inside for an added surprise. When made, you freeze the eggs for about an hour, so they are set hard.
To hatch the eggs, you give your kids syringes and a cup of vinegar. They can then apply the vinegar wherever they want to discover what’s hiding inside the eggs. Just remember to place the eggs in an easy to wash container with reasonably high sides.
Fireworks Alternative
I love fireworks, but I feel guilty about enjoying them because they are an environmental disaster. So, whenever bonfire night rolls around, I always set this up to have some fun and color in our home without having to damage the environment. It’s not quite as good as fireworks, but it’s pretty cool never-the-less.
All you need is:
- Oil – any sort will do. Cheap vegetable oil is just fine
- Food coloring (The liquid kind, not gel)
- Droppers (medicine syringes work well too)
- A clear, tall jar – a mason jat is perfect
To get the magic going, all you need to do is fill your jar ¾ full with warm water. Then add a good layer of oil on top. About an inch deep is plenty. Then you use your dropper to drip food coloring into the jar.
At first, the food coloring will sit at the interface between the oil and water. Then all of a sudden, it will drop through, leaving a trail of color behind it in the water. It looks impressive if you do lots of drops of different colors and then sit back and watch as they drop through the liquids.

Eventually, your water will turn a muddy, muddle color. But, this is such an easy experiment that you can wash out your jar and try again.
Just a little not to say that if you can’t get hold of a dropper or syringe then you can just drip the food coloring from the bottle. As long as you only put in small amounts at a time it does still work.
If you want to extend the fun, keep the oil and some of the colored water when you pour out the container. You want to have more oil than water this time, so I suggest moving them to a second smaller bottle. Then if you add an Alka-Seltzer tablet, you’ve got a homemade lava lamp. To get the best effect, stand your lava lamp bottle on top of a light.
Frequently Asked Questions
What are the most useful household ingredients for chemistry experiments.
When it comes to chemistry experiments, having the right ingredients makes all the difference. If you like explosions, then you’ll probably want to have a good supply of baking soda and vinegar on hand. Other common ingredients include ice, food coloring, citric acid, cornflour, and borax.
If you’re planning on doing lots of experiments, you might want to have a clear measuring jug and a few clear bowls of glasses that you don’t mind sacrificing.
Is cooking chemistry?
Absolutely! There is loads of chemistry behind making food taste great. If you like a perfectly browned steak, then you’re a fan of the Maillard reaction. If you like sweets and desserts, then you’re benefiting from the careful balance of ingredients and use of temperature needed to create the textures and flavor you love.
There is a whole field of science called food science, which is a specific field of chemistry. A great introduction to this field is to experiment with the ratio of ingredients in a simple recipe. You’ll learn what effect the different ingredients have on the outcome. Alternatively, you could make a sourdough starter.
What is the easiest science project?
All of the experiments on this list are pretty easy to try. The chromatography experiment is probably the one that has the most common equipment and is pretty hard to mess up. The fireworks alternative is also an easy experiment that looks great.

Sandy is an experienced STEM educator, having spent a decade teaching Physics. She also loves to volunteer at local STEM fairs to show kids, especially girls, how awesome it is. She is so passionate about science that one science degree wasn’t enough and she decided to complete a second part-time, while working.
Editor’s Picks

7 Best LEGO Star Wars Sets | Our Top Picks of All Time!

Best LEGO Creator Sets – Take Your Pick From These 7 Gems!

How to Use a Metal Detector: 8 Essential Tips to Get the Most of It

Best Metal Detector for Kids: 5 Top Picks (+ Buying Guide)

Best 2+ Player Cooperative Board Games (Top 6 in 2024)

MEL Chemistry Review: Is Your Child the Next Bill Nye?

Click Here for Answer
Chemistry@Home

LEARNING CHEMISTRY OUTSIDE OF THE CLASSROOM
Your resource for exploring chemistry in your home!
Experiment Center

Activity Location (Inside or Outside)
Age Group (Kids or Adults)
Experiment Duration (Short or Long)
Equipment Required
(Needed Materials)
Parental Supervision
Heat Needed
Babble Dabble Do
50 Chemistry Projects That Will Amaze Kids!
February 26, 2019 by Ana Dziengel 5 Comments
Chemistry projects feel like magic , do they not? If you think about some of your favorite science projects, the ones you love to try with your kids or the ones that amazed YOU as a kid, more likely than not most of them involved chemistry.
Now I know a lot of us associate chemistry with lab coats, beakers and specialty ingredients but the reality is there are so many chemistry projects you can do using very simple, easy to find ingredients, often found in your own pantry. And since these types of simple chemistry projects use relatively safe ingredients, they are perfect to try with younger kids, ie. preschool and elementary aged children! In fact I think it’s so important for young kids to have a positive association with chemistry from a young age that fosters a love of this branch of science.
When most children are finally exposed to chemistry in school, it is at the high school level where the subject turns complex quickly; hopefully giving kids a chance to have fun at young age mixing up concoctions and watching chemical reactions will help carry their interest through the more complicated days of study ahead.
This post is a GIANT compilation of chemistry projects that would be great for the science fair, classroom demos, or at home science with your kids.
Before we get started let’s talk a little bit about what chemistry is and for parents I also included a section covering How to Do Chemistry Projects at Home. If you are a classroom teacher you can skip this section and head right to the projects here.
What is chemistry?
Chemistry is the branch of science that studies matter (anything that has mass and takes up space) and its properties, and how different substances (especially molecules and their atoms) interact, combine, and change to form new substances.
Here are some important definitions to know when working on chemistry projects:
- Element A substance that cannot be separated into any further substances. There are 120 known elements.
- Atom The smallest particle of an element
- Molecule Groups of atoms held together by a chemical bond.
- Ion An atom or molecule that has an electric charge
While most people think of chemistry purely in terms of chemical reactions, chemistry also covers the study of the states of matter as well as the density of substances.
The five branches of chemistry are:
- Analytical chemistry
- Physical chemistry
- Organic chemistry
- Inorganic chemistry
- Biochemistry
Read more about what each branch covers here.
How to Do Chemistry Projects at Home
Many chemistry projects can be done at home using simple materials and are a great way to foster a love of science in kids! I wholeheartedly believe that a wow factor in a project engages and inspires kids to learn more. If you want to try chemistry projects at home here are some suggestions and precautions:
Safety First
Even though most of the projects in this list use safe, easy to find materials they should be used with safety precautions and under adult supervision. Why? Sometimes the chemical reaction that ensues can irritate the skin or eye, can be harmful if swallowed, or is just plain sticky or messy and adults should be on hand to supervise use . Also be advised that there are a few projects on this list that do use materials that are unsafe for kids to handle. These projects are meant to be demonstrations only and are labeled accordingly.
- Use household items for chemistry The classic chemistry project that never fails to impress is the reaction of baking soda (sodium bicarbonate) and vinegar (look for a number of variations on this classic in our Acids and Bases section) but there are lots of other great ingredients for chemistry to find in your kitchen including sugar, salt, yeast, lemons, dish soap, milk, Kool- Aid, cabbage, gelatin, and food coloring to name a few…before you order any materials online, try some projects with pantry essentials.
- Safety Goggles
- Large plastic beakers
- Prepare for mess Since a lot of chemistry involves reactions and the ensuing mess, be sure to choose a place in your home that you can easily clean up and where you won’t worry about getting dirty. A patio, breakfast area, or the garage are great choices.
- Generous work area Be sure to have a large table available so everyone has plenty of room to work and/or view projects without bumping into each other.
- Access to Water Clean up is always easier with water at the ready! Choose a location near a hose or shop sink.
Managing Messes
- Hose it down Depending on the project I suggest doing super messy chemical reactions outside. That way spills can be hosed down easily.
- Painter’s Tarp & Trays If you cannot go outside a large plastic painter’s tarp is a great way to contain spills and mess. I also highly recommend doing projects on trays or cookie sheets. The raised edges help contain bubbly brews and are easy to dump out and wash.
- Dump station Have a bucket nearby to act as dump station for liquid reactions. Bring it around a table and dump at each station.
- Think about disposal Vinegar kills grass! Slime bits clogs drains! Be sure to consider where you can dump out the liquids safely.

Chemistry Projects for Kids
The following chemistry projects for kids are sorted by topic: Chemical Reactions, Acids and Bases, Carbon Reactions, Chromatography, Colloids & Solutions, Polymers, and Crystals.
Please note that many if these projects could fit in two or more categories in this post as they demonstrate various scientific and chemical processes. I only classified them once on this list.
Chemistry Projects with Chemical Reactions
What is a chemical reaction.
Chemical reactions occur when the chemical bonds in a substance are either destroyed or created. In other words the bonds in a molecule are broken during a chemical reaction and the atoms rearranged to create new molecules. Interestingly enough the number of original atoms does not change during the reaction, they are simply reconfigured.
An easy way to explain chemical reactions to kids is to use this analogy: Atoms are like letters, molecules are like words. Chemistry is like taking apart words and rearranging the letters to form a new word.
Read more about chemical reactions here.
Chemical Reactions Projects:
1. milk painting, 2. citrus battery, 3. elephant toothpaste.
<span data-mce-type=”bookmark” style=”display: inline-block; width: 0px; overflow: hidden; line-height: 0;” class=”mce_SELRES_start”></span>
4. Density Lava Lamps
To make a density lava lamp fill a plastic bottle with the following liquids: Clear corn syrup, water with a few drops of food coloring, and layer of vegetable oil. Be sure to leave a space at the top of the bottle. Wait until the liquids settle then add in a tablet of extra strength alka seltzer. Watch as the alka seltzer and water react and bubble up through the oil layer. To see this in a step by step video check out this video (Pssst this is one of our students!!!)
5. Plastic Milk and Curds & Whey Experiment
6 . color mixing.
Pour water into three clear plastic cups, then add blue, red, and yellow food coloring to each. Have an additional cup full of uncolored water available as well. Give your child an empty ice cube tray and pipettes and let them create different colors by mixing different ratios of two different primary colors in each ice cube compartment. The secondary colors are new colors created from two primary colors. This is a simple visual of how chemical reactions work.
7. Chemistry Clock
8. blow balloons with yeast and sugar, 9. shiny pennies.
- Collect dirty tarnished pennies.
- Pour different acidic liquids into shallow containers. Try vinegar, salsa, lemon & lime juice.
- Add a teaspoon of salt to each container and stir to combine.
- Place a handful of pennies in each container and soak for 5 minutes.
- Remove them from the solution and rinse in soapy water. Let dry on separate paper towels.
- Compare the results! Which ones are shiniest? Which are dull? Did any turn green?
Acids are corrosive and sour tasting. Liquids such as vinegar, lemon juice, and tomato juice are acids. Pennies are made from copper which tarnishes (turns dark) when exposed to oxygen over time. Placing the copper pennies in an acid will clean the copper oxide off them and make them shiny again.
Learn about Acids and Bases
Most liquids are either an acid or a base. Liquids with lots of hydrogen ions in them are considered acids. Liquids with many hydroxide ions are bases. Scientists use a scale called the ph scale to measure how acidic or basic a liquid is. The more hydrogen ions in a liquid the more acidic it is and ranks low on the ph scale. The more hydroxide ions in a liquid the more basic it is and ranks high on the ph scale. You can see what that looks like here.
When acids and bases are mixed chemical reactions occur and the solution becomes neutralized.
Acid and Bases Projects:
1. baking soda & vinegar volcano, 2. lemon volcano, 3. the colorful cabbage juice science experiment and acid base experiment with cabbage, 4. dancing rice, 5. green eggs & ham, 6. bubbly citric acid brew , 7. baking soda vs baking powder science experiment, 8. exploding bags, 9. rainbow rubber eggs , 10. surprise eggs , 11. rainbow wizard’s brew, chemistry projects with fire (carbon reactions).
Carbon is the most important element for life. Chemicals that contain carbon are called organic compounds. Carbon has two main forms: The first is in the hard form of diamonds and graphite, and the second is the impure form found in charcoal, coal and soot.
SAFTEY WARNING: Carbon reactions are always fascinating to watch however the presence of fire means that these experiments must be supervised by adults at all times!
Carbon Reactions Projects:
1. smoking fingers, 2. fire snake, 3. silver egg, 4. invisible ink, chromatography.
Chromatography is the process of separating mixtures. We usually think of it in terms of color hence the prefix -chroma, however in chemistry is means simply a method of separating mixtures by letting them slowly move past each other. It applies to both liquids and gasses. This is wonderful in-depth explanation of chromatography.
Chromatography Projects:
1. chromatography .
In this project you will separate the color black into other colors. Fold a coffee filter in half. Fold in half two more times until you have a triangular shape. Color the tip of the coffee filter with washable black marker. Get a good coat of ink on the filter. Add a small amount of water to a plastic cup. Place the black tip of the coffee filter in the cup Wait and observe. Come back to the filter after an hour or two and see what happens to the ink. As the coffee filter absorbs water through capillary action, the black ink moves through the filter and is separated by the water into other colors. You should see blue, green and even red as the water separates the ink.
2. Chromatography Flowers
3. chromatography art, 4. chromatography bags, colloids and solutions/solubility.
Colloids and Solutions are two types of homogenous mixtures.
- Colloids are mixtures in which a small particles of a substance are suspended throughout another substance but not chemically bonded. They are stable though and do not separate. Examples of colloids are gelatin, butter, mayonnaise, fog and smoke.
- Solutions are mixtures in which the particles of one substance are completely dissolved in another substance. The solute is the substance being dissolved and the solvent is the substance doing the dissolving. An example of a solution is saltwater.
If you want a more in-depth primer on solutions and colloids hop over here .
Colloid Projects:
1. colloid examples, 2. oobleck , 3. make butter , 4. gelatin streaking, solutions/solubility projects:, 5. ice sculptures , 6. ice cream in a bag.
A printable of the science facts at play here
7. Skittles Science
8. magical water blossoms <span data-mce-type=”bookmark” style=”display: inline-block; width: 0px; overflow: hidden; line-height: 0;” class=”mce_selres_start”></span>, 9. diffusion art, 10. paint solubility , 11. bleeding blossoms .
A polymer is a substance made up of a long chain of molecules. Polymers are typically flexible materials like plastic or gum.
The classic polymer kids LOVE to make is slime! Glue is already a polymer but when combined with sodium tetraborate (borax ) the protein molecules of the glue and the borate ions crosslink, making it harder for the molecules to move and forming the gooey, sticky, substance we know as slime.
Other polymers you are probably familiar with are plastic bags, balloons, instant snow, and even the powdery substance found in diapers that expands when wet.
Polymer Projects:
1. best basic slime .
Bonus: Get the Science Behind Slime printable here
2. Heat Sensitive Slime
3. diy bouncy balls, 4. magic plastic bag experiment, 5. instant terrariums, 6. how to make paper, 7. skewer through balloon , 8. dry erase figure and dry erase drawings, 9. recycled plastic flowers.
Crystals are a type of material that is formed by patterns of repeating molecules. There are four types of chemical bonds in crystals and therefore four categories of crystals. These are: Covalent, Molecular, Metallic, and Ionic Crystals. You can grow crystals by mixing up a super saturated solution (usually with a type of salt and water) and letting it settle over time so crystals will form. Check out the various types of easy to grow crystal below and go here to read more about the science of crystals .
Crystals Projects:
1. classic borax crystals , 2. overnight crystal garden, 3. egg geodes, 4. crystal wind catchers, 5. crystal landscapes, 6. candy geodes , 7. salt crystals, conclusion & more.
Alright you guys, do you feel like you have some good project ideas for exploring chemistry with kids? Many of these will make greats science fair projects. Be sure to start with them as a topic then start asking questions, form a hypotheses, and do some experiments.
Now I have to admit that I really fell in love with chemistry projects as an adult. Working with kids in camp, after school, and with my own kids at home I’ve had the chance to try fun chemistry projects and discovered that I love watching chemical reactions AND the reactions on the faces of kids and bystanders during demonstration or project!
If you have kids who fall in love with this branch of science please do check out the incredible book series Elements , Molecules , and Reactions by Theodore Gray (see the series in our Amazon science ideas list here ) The books are stunning, informative, easy to understand and, wait for it…funny!
Another valuable resource for kids who love chemistry is Mel Science’s Chemistry subscription box. They send you a starter kit for free with all the materials you’ll need and then each month you get a new chemistry experiment delivered to your door! This is great product because a lot of specialty chemistry ingredients are hard to find and these kits simplify getting the materials you need! Check it out here:
Are you passionate about raising creative kids?
Join over 22,179 parents and educators who want connect with kids and nurture their creative process through magical, easy projects you can do TOGETHER.
Subscribe to our email list to receive project ideas as well as offers for some our creative products.
If you want to read our privacy policy before subscribing, hop over here.
February 26, 2019 at 10:31 pm
These were some really awesome projects. I really liked the Citrus Battery projects. It seems simple and easy for a young kid. I wish schools should give more focus to such experiments instead of shoving down the theoretical knowledge down the throat of young kids.
Carol Biggs says
March 1, 2019 at 4:58 am
Is all of this info available on book form?
Ana Dziengel says
March 6, 2019 at 5:49 am
Not at this time but that’s a great idea!
Betsy Mitten says
March 5, 2019 at 10:43 am
Thank you for making this fantastic collection of experiments with clear directions and easy to understand explanations of the science behind the fun! I know I’ll refer to this list often. I especially appreciate the way the experiments are classified/organized. I teach art with science connections and we are already planning on chalkboard and magnetic slime :). I’ll be sure to tag Babble Dabble Do when I post photos of work inspired by this on target collection!
Kyra Rodriguez says
March 5, 2019 at 10:54 pm
These are all great ideas! I’m pretty sure the kids will have fun and love this activities
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Pin It on Pinterest
- You are here:
- American Chemical Society
- Students & Educators
- Educational Resources
- Resources by Topic
Chemistry Education Resources by Topic
Chemistry at Home
The ACS is making educational resources available by topic to aid parents and teachers in student enrichment during this time of distance learning. We have bundled materials from the Reactions Video series, C&EN, our magazines within the Education Division and our portfolio of hands-on activities for students in grades K–12 and beyond. These resources focus on five topics — The Earth, Water, Food, Health & Your Body, and The Periodic Table — and put a spotlight on the connection between chemistry and everyday life.

Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society

110 Awesome Chemistry Experiments For All Ages
Sharing is caring!

Chemistry experiments are a great way to get kids excited about studying chemistry even at a young age. I mean, what child doesn’t think about creating bubbling potions or sending secret messages?
The study of chemistry has a scary connotation for many people. Chemistry has this stigma of being only for really, really smart students who want a career in the sciences. The truth is that, like all science, chemistry is everywhere.
In fact, chemistry experiments for kids can be bubbly and full of fungi! Check out the video below of our Making Peeps Blow Up a Balloon chemistry activity.
It is in the way water freezes into ice . It is in the way apples turn brown when you leave their flesh exposed to the air. Chemistry is in the way sugar dissolves in water .
How does chemistry apply to our bodies? Check out our version of the egg with vinegar experiment . We added a little twist that makes an excellent connection between chemistry and our dental health. We have a 25+ page printable pack to go with it for just $2.95 .
Showing how chemistry is involved in everyday life can take that scary factor out of studying chemistry for students. When it comes time to study chemistry, they will be more excited about jumping in.

Chemistry Experiments For All Ages
I wanted to create a resource for you to be able to find the perfect chemistry experiments for your students no matter their age or interests. This post contains 100 chemistry experiments for students from preschool age through high school. I have divided them into 3 age ranges.
- Preschool and Primary
- Middle and High School
Here are a few disclaimers to my divisions of the experiments.
I realize that all students are different and are ready for different levels of experiments. For instance, some students in the elementary age group might be ready for more advanced experiments found in the Middle and High School section, while others need something more basic like those experiments found in the Preschool and Primary section.
Some may question why I put certain experiments in certain sections. First, I looked at the level of maturity I felt needed to conduct the experiment and if parental help was necessary. Next, I looked at the level of understanding the child would need to learn from the experiment.
Some experiments could teach something at different levels or could be done with parental help or independently and still be successful. When this was the case, I put the experiment in the lowest recommended age level.
With all that being said, these are just guidelines. Feel free to try experiments in sections that differ from your students’ age range if you think they would work.
For chemistry experiments, lesson ideas, and resources, check out my Homeschool Chemistry Pinterest board.
First, download the STEM Resource Guide
We have put together a FREE resource for parents and teachers that includes STEM activities , links to no-cost or low-cost coding, math, engineering, an robotics resources. You’ll find everything from preschool worksheets to high school apprenticeship information. Plus, there are articles to help you get your kids interested in STEM activities or ready for a career in STEM. Our contributors include The STEMKids , a mechanical engineer, and a biologist.
Preschool Science Experiments
Color Changing Flowers – includes free printable
Make butter! This comes with a free printable lesson that covers a wide range of ages. This is a preschool favorite!
Glow Stick Experiment – this one is especially easy for preschoolers – includes their own little observation sheet and coloring pages
Making Fizzy Moon Rocks (and learning about Moon rocks)
Puffy Paint turned Slime activity (This is also good for older students who are studying polymers.)
Pumpkin Candy Experiment
Dissovling Candy Corn – In this pumpkin and candy-themed printable , your little scientist will enjoying dissolving candy corn (or any candy for that matter) and recording what they observed. Includes three science activities, preschool/kindergarten math resources, and coloring and puzzle pages.
Make Crystal Snowflakes with Borax
Experiment with Yeast and Sugar – Making Peeps Blow Up a Balloon – Includes a free printable pack. Peeps make for interesting chemistry experiments. Whether you use the Peep snowmen or Peep chicks, you can make them blow up a balloon! Includes a free printable pack.
Make Crystals with Borax – This works every time and is exciting to watch throughout a 24-hour period. Obviously, adult supervision is needed when using the Borax, but your littlest scientists can twist their chenille sticks and make their own crazy creations! Plus, stirring the Borax and water will make them feel like real chemists! The activity can be adapted to make snowflake shapes in winter, hearts for Mother’s Day or Valentine’s Day. Flowers for spring or summer.
Baking Soda Fizz Experiment
Another Baking Soda Fizz Experiment
Diet Coke and Mentos Explosion
Dripping Slime Experiment
Lava Lamp Experiment
Rainbow Walking Water
Ice Cream in a Bag
Primary Science Experiments
Make a “Stained Glass” window
6 Experiments with Oranges
Make butter! This comes with a free printable lesson that covers a wide range of ages. This is a family favorite!
Glow Stick Experiment – learn about chemiluminescence, chemical reactions, and kinetic energy.
Dissolving candy experiment with printables

A fun TWIST on the egg with vinegar experiment. This activity helps children see the chemical reactions that go on in our mouth! Free printable.

Do Some Soil Testing – This is important life skills information too!
Color Changing Flowers – learn about capillary action in plants
Make Your Own Snowflakes
Polishing Pennies Experiment

Vitamin C And Apple Experiment
Homemade Butter Experiment
Secret Messages Science Experiment
120 Kitchen Chemistry & Culinary Science Resources – This is a very comprehensive list. If you want to also get some ideas for teaching your children about chemistry while cooking, this is a good place to look too!
Make Plastic From Milk
Fun Bubbles Experiment
Solubility Experiment
Bending Candy Canes
Experimenting With Viscosity And Sensory Bottles

Sudsy Bubble Experiment
Taffy Slime Chemistry
Dissolving Egg Shell Experiment
Make Ice Grow
Skittles Rainbow Science Experiment
Chromatography Butterflies
Erupting Lemon Volcano Chemistry
Make A Lava Lamp
Rock Candy Experiment
Make Heat Changing Color Sensitive Slime

Elementary School Science Experiments
Glow Stick Experiment – learn about chemiluminescence, chemical reactions, and kinetic energy. The printable is definitely geared towards elementary and middle school
Easy Science Experiments with Oranges – The Homeschool Scientist
Making Fizzy Moon Craters turned out to be a fun chemistry study and a lesson in realy Moon rocks!
Testing for vitamin C with iodine. We used a pumpkin, cranberries, oj, lemons, and more! It’s a lot of fun!

Oxidation And Reduction Experiment
Make a “Stained Glass” window – a lesson about states of matter and crystallization
Make butter
Making Peeps Candies Blow Up A Balloon – lesson with printable sheets
Add the dental health printable pack we have to go with the egg in vinegar chemistry activity for $2.95

Make A Polymer Ball
Enzyme Experiment
Red Cabbage Litmus Experiment
Harry Potter Potions Experiment
Peeps Science Experiment
Baking Powder vs. Baking Soda Experiment
Charcoal Water Purifying Experiment

Kitchen Chemistry: Cake Experiment
Polymer Science: Homemade Fruit Gummies
Food Chemistry: Turn Juice Into A Solid
Endothermic Chemical Reactions
Egg Float Science Experiment
Eggshell Geodes Science Experiment
Density Experiment
Forensic Chemistry Experiment
Kitchen Chemistry Experiments
Mentos and Soda Eruption
Make Invisible Ink
Make Quicksand with Engineering Emily and her children
Glow Stick Reactions
Using Lemons To Make Batteries
Make A Potato Battery
Diaper Chemistry
Candle Chemical Reaction
Melting Ice With Salt
Viscosity Experiment
Melting Ice Experiment
Ice Experiments
Chemiluminesence
Non-Newtonian Fluids
Explore An Unknown Material
Poke but Don’t Soak – a material science activity from the American Chemistry Society
The Science Of Jello
Kitchen Chemistry – 2 projects
Make Curds And Whey
Making Hot Ice
The Science Behind Edible Glass

Grow A Crystal Garden
Sugary Drinks And Teeth
Big Hero 6 Chemistry Concoctions
Compare The Electrolytes In Sports Drinks
Measure Glucose In Your Food

Charged Atoms Experiment
Gummy Bears Osmosis Experiment
Milk Polarity Experiment
Simple Digestion Experiment
Disappearing Color Experiment
Middle and High School Science Experiments
Parents of middle and high school students .

What Happens to the pH and temperature of a solvent when you add candy corn??
Testing for Vitamin C with Iodine (We used a pumpkin, cranberries, oj, lemons, and more! It’s a lot of fun!)
Peeps Science: Change In Mass Experiment
Peeps Science Experiment: Blowing Up a Balloon with Peeps
Chemical Reaction Experiment

Oxygen And Fire Experiment
Make Poinsettia pH Paper
Make Elephant Toothpaste
Make A Rainbow Of Colored Flames
Make Green Fire Pinecones
Copper Plating Ornaments
Make Colored Fire

Check out ChemistryTalk.org. This is a charity non-profit (all of their content is free) whose mission is to make chemistry fun and easy. They have tutorials, experiments, videos, a podcast, and many resources to help your teen understand and enjoy chemistry.
Make A Black Fire Snake
Three Station Gas Lab
Solubility Of Gases In Water
Salt Formation From Chemical Reactions
Check out ChemistryTalk.org. This is a charity non-profit (all of their content is free) whose mission is to make chemistry fun and easy. They have tutorials, experiments, videos, a podcast, and many resources to help your teen understand and enjoy chemistry
Make A Silver Egg
Water Content Lab

Water Quality Experiment
Make A Balloon Egg
Separating Sand And Salt
Rate Of Evaporation
Create A Compound Of Two Elements
Melting And Freezing Experiment
Soft Water Experiment
Make Homemade Root Beer
Desalinization Experiment
Need 120 MORE Kitchen Chemistry Experiments and Culinary Science Ideas?

I hold a master’s degree in child development and early education and am working on a post-baccalaureate in biology. I spent 15 years working for a biotechnology company developing IT systems in DNA testing laboratories across the US. I taught K4 in a private school, homeschooled my children, and have taught on the mission field in southern Asia. For 4 years, I served on our state’s FIRST Lego League tournament Board and served as the Judging Director. I own thehomeschoolscientist and also write a regular science column for Homeschooling Today Magazine. You’ll also find my writings on the CTCMath blog. Through this site, I have authored over 50 math and science resources.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 05 June 2024
How we talk about harmful chemicals in the environment
- Shira Joudan 1 na1
Nature Chemistry ( 2024 ) Cite this article
85 Accesses
10 Altmetric
Metrics details
- Environmental monitoring
Environmental contamination is in the news more than ever. Shira Joudan introduces key concepts to talk about what happens to chemicals in the environment and what chemists should consider in their day-to-day lives, both at work and at home.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Cousins, I. T., Ng, C. A., Wang, Z. & Scheringer, M. Environ. Sci. Process. Impacts 21 , 781–792 (2019).
Article CAS PubMed Google Scholar
Tian, Z. et al. Science. 371 , 185–189 (2020).
Article PubMed Google Scholar
Wiemeyer, S. N. & Porter, R. D. Nature 227 , 737–738 (1970).
Anastas, P. T. & Warner, J. C. Green Chemistry: Theory and Practice (Oxford Univ. Press, 1998).
Download references
Acknowledgements
The author acknowledges S. Baskaran for useful discussions.
Author information
Twitter/X: @ShiraJoudan
Authors and Affiliations
Department of Chemistry at University of Alberta, Edmonton, Alberta, Canada
Shira Joudan
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Shira Joudan .
Ethics declarations
Competing interests.
The author declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Joudan, S. How we talk about harmful chemicals in the environment. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01554-5
Download citation
Published : 05 June 2024
DOI : https://doi.org/10.1038/s41557-024-01554-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Grades 6-12
- School Leaders
NEW: Classroom Clean-Up/Set-Up Email Course! 🧽
72 Easy Science Experiments Using Materials You Already Have On Hand
Because science doesn’t have to be complicated.

If there is one thing that is guaranteed to get your students excited, it’s a good science experiment! While some experiments require expensive lab equipment or dangerous chemicals, there are plenty of cool projects you can do with regular household items. We’ve rounded up a big collection of easy science experiments that anybody can try, and kids are going to love them!
Easy Chemistry Science Experiments
Easy physics science experiments, easy biology and environmental science experiments, easy engineering experiments and stem challenges.

1. Taste the Rainbow
Teach your students about diffusion while creating a beautiful and tasty rainbow! Tip: Have extra Skittles on hand so your class can eat a few!
Learn more: Skittles Diffusion

2. Crystallize sweet treats
Crystal science experiments teach kids about supersaturated solutions. This one is easy to do at home, and the results are absolutely delicious!
Learn more: Candy Crystals
3. Make a volcano erupt
This classic experiment demonstrates a chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid), which produces carbon dioxide gas, water, and sodium acetate.
Learn more: Best Volcano Experiments
4. Make elephant toothpaste
This fun project uses yeast and a hydrogen peroxide solution to create overflowing “elephant toothpaste.” Tip: Add an extra fun layer by having kids create toothpaste wrappers for plastic bottles.
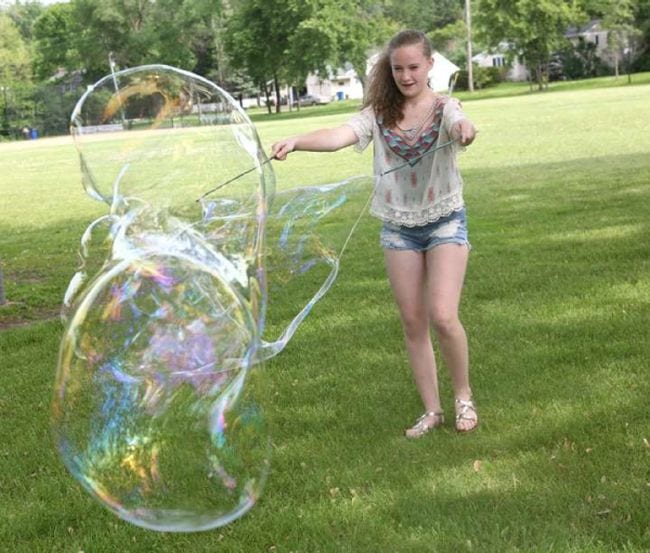
5. Blow the biggest bubbles you can
Add a few simple ingredients to dish soap solution to create the largest bubbles you’ve ever seen! Kids learn about surface tension as they engineer these bubble-blowing wands.
Learn more: Giant Soap Bubbles

6. Demonstrate the “magic” leakproof bag
All you need is a zip-top plastic bag, sharp pencils, and water to blow your kids’ minds. Once they’re suitably impressed, teach them how the “trick” works by explaining the chemistry of polymers.
Learn more: Leakproof Bag

7. Use apple slices to learn about oxidation
Have students make predictions about what will happen to apple slices when immersed in different liquids, then put those predictions to the test. Have them record their observations.
Learn more: Apple Oxidation
8. Float a marker man
Their eyes will pop out of their heads when you “levitate” a stick figure right off the table! This experiment works due to the insolubility of dry-erase marker ink in water, combined with the lighter density of the ink.
Learn more: Floating Marker Man

9. Discover density with hot and cold water
There are a lot of easy science experiments you can do with density. This one is extremely simple, involving only hot and cold water and food coloring, but the visuals make it appealing and fun.
Learn more: Layered Water

10. Layer more liquids
This density demo is a little more complicated, but the effects are spectacular. Slowly layer liquids like honey, dish soap, water, and rubbing alcohol in a glass. Kids will be amazed when the liquids float one on top of the other like magic (except it is really science).
Learn more: Layered Liquids

11. Grow a carbon sugar snake
Easy science experiments can still have impressive results! This eye-popping chemical reaction demonstration only requires simple supplies like sugar, baking soda, and sand.
Learn more: Carbon Sugar Snake
12. Mix up some slime
Tell kids you’re going to make slime at home, and watch their eyes light up! There are a variety of ways to make slime, so try a few different recipes to find the one you like best.

13. Make homemade bouncy balls
These homemade bouncy balls are easy to make since all you need is glue, food coloring, borax powder, cornstarch, and warm water. You’ll want to store them inside a container like a plastic egg because they will flatten out over time.
Learn more: Make Your Own Bouncy Balls

14. Create eggshell chalk
Eggshells contain calcium, the same material that makes chalk. Grind them up and mix them with flour, water, and food coloring to make your very own sidewalk chalk.
Learn more: Eggshell Chalk

15. Make naked eggs
This is so cool! Use vinegar to dissolve the calcium carbonate in an eggshell to discover the membrane underneath that holds the egg together. Then, use the “naked” egg for another easy science experiment that demonstrates osmosis .
Learn more: Naked Egg Experiment
16. Turn milk into plastic
This sounds a lot more complicated than it is, but don’t be afraid to give it a try. Use simple kitchen supplies to create plastic polymers from plain old milk. Sculpt them into cool shapes when you’re done!

17. Test pH using cabbage
Teach kids about acids and bases without needing pH test strips! Simply boil some red cabbage and use the resulting water to test various substances—acids turn red and bases turn green.
Learn more: Cabbage pH

18. Clean some old coins
Use common household items to make old oxidized coins clean and shiny again in this simple chemistry experiment. Ask kids to predict (hypothesize) which will work best, then expand the learning by doing some research to explain the results.
Learn more: Cleaning Coins

19. Pull an egg into a bottle
This classic easy science experiment never fails to delight. Use the power of air pressure to suck a hard-boiled egg into a jar, no hands required.
Learn more: Egg in a Bottle
20. Blow up a balloon (without blowing)
Chances are good you probably did easy science experiments like this when you were in school. The baking soda and vinegar balloon experiment demonstrates the reactions between acids and bases when you fill a bottle with vinegar and a balloon with baking soda.
21 Assemble a DIY lava lamp
This 1970s trend is back—as an easy science experiment! This activity combines acid-base reactions with density for a totally groovy result.

22. Explore how sugary drinks affect teeth
The calcium content of eggshells makes them a great stand-in for teeth. Use eggs to explore how soda and juice can stain teeth and wear down the enamel. Expand your learning by trying different toothpaste-and-toothbrush combinations to see how effective they are.
Learn more: Sugar and Teeth Experiment
23. Mummify a hot dog
If your kids are fascinated by the Egyptians, they’ll love learning to mummify a hot dog! No need for canopic jars , just grab some baking soda and get started.
24. Extinguish flames with carbon dioxide
This is a fiery twist on acid-base experiments. Light a candle and talk about what fire needs in order to survive. Then, create an acid-base reaction and “pour” the carbon dioxide to extinguish the flame. The CO2 gas acts like a liquid, suffocating the fire.

25. Send secret messages with invisible ink
Turn your kids into secret agents! Write messages with a paintbrush dipped in lemon juice, then hold the paper over a heat source and watch the invisible become visible as oxidation goes to work.
Learn more: Invisible Ink
26. Create dancing popcorn
This is a fun version of the classic baking soda and vinegar experiment, perfect for the younger crowd. The bubbly mixture causes popcorn to dance around in the water.

27. Shoot a soda geyser sky-high
You’ve always wondered if this really works, so it’s time to find out for yourself! Kids will marvel at the chemical reaction that sends diet soda shooting high in the air when Mentos are added.
Learn more: Soda Explosion

28. Send a teabag flying
Hot air rises, and this experiment can prove it! You’ll want to supervise kids with fire, of course. For more safety, try this one outside.
Learn more: Flying Tea Bags

29. Create magic milk
This fun and easy science experiment demonstrates principles related to surface tension, molecular interactions, and fluid dynamics.
Learn more: Magic Milk Experiment
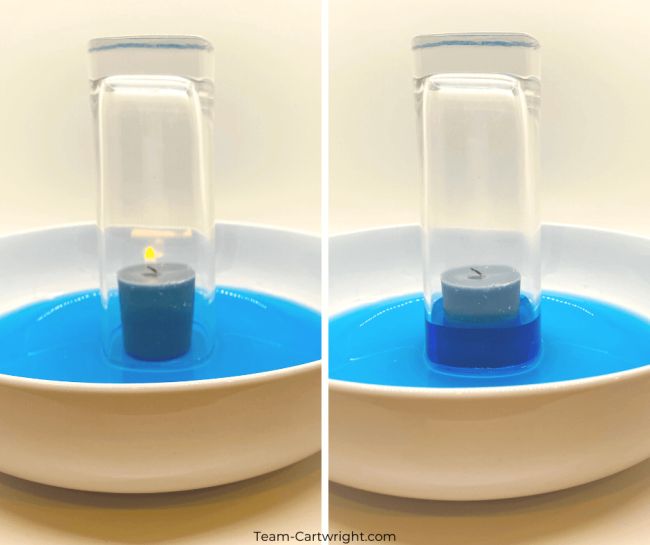
30. Watch the water rise
Learn about Charles’s Law with this simple experiment. As the candle burns, using up oxygen and heating the air in the glass, the water rises as if by magic.
Learn more: Rising Water

31. Learn about capillary action
Kids will be amazed as they watch the colored water move from glass to glass, and you’ll love the easy and inexpensive setup. Gather some water, paper towels, and food coloring to teach the scientific magic of capillary action.
Learn more: Capillary Action

32. Give a balloon a beard
Equally educational and fun, this experiment will teach kids about static electricity using everyday materials. Kids will undoubtedly get a kick out of creating beards on their balloon person!
Learn more: Static Electricity
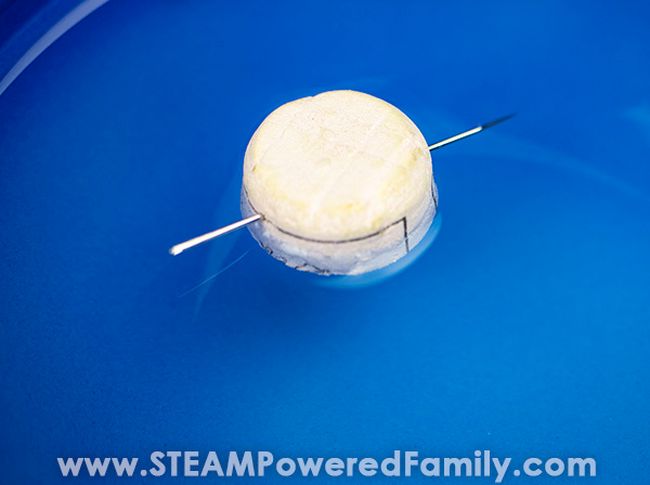
33. Find your way with a DIY compass
Here’s an old classic that never fails to impress. Magnetize a needle, float it on the water’s surface, and it will always point north.
Learn more: DIY Compass
34. Crush a can using air pressure
Sure, it’s easy to crush a soda can with your bare hands, but what if you could do it without touching it at all? That’s the power of air pressure!

35. Tell time using the sun
While people use clocks or even phones to tell time today, there was a time when a sundial was the best means to do that. Kids will certainly get a kick out of creating their own sundials using everyday materials like cardboard and pencils.
Learn more: Make Your Own Sundial
36. Launch a balloon rocket
Grab balloons, string, straws, and tape, and launch rockets to learn about the laws of motion.

37. Make sparks with steel wool
All you need is steel wool and a 9-volt battery to perform this science demo that’s bound to make their eyes light up! Kids learn about chain reactions, chemical changes, and more.
Learn more: Steel Wool Electricity
38. Levitate a Ping-Pong ball
Kids will get a kick out of this experiment, which is really all about Bernoulli’s principle. You only need plastic bottles, bendy straws, and Ping-Pong balls to make the science magic happen.

39. Whip up a tornado in a bottle
There are plenty of versions of this classic experiment out there, but we love this one because it sparkles! Kids learn about a vortex and what it takes to create one.
Learn more: Tornado in a Bottle

40. Monitor air pressure with a DIY barometer
This simple but effective DIY science project teaches kids about air pressure and meteorology. They’ll have fun tracking and predicting the weather with their very own barometer.
Learn more: DIY Barometer

41. Peer through an ice magnifying glass
Students will certainly get a thrill out of seeing how an everyday object like a piece of ice can be used as a magnifying glass. Be sure to use purified or distilled water since tap water will have impurities in it that will cause distortion.
Learn more: Ice Magnifying Glass

42. String up some sticky ice
Can you lift an ice cube using just a piece of string? This quick experiment teaches you how. Use a little salt to melt the ice and then refreeze the ice with the string attached.
Learn more: Sticky Ice
43. “Flip” a drawing with water
Light refraction causes some really cool effects, and there are multiple easy science experiments you can do with it. This one uses refraction to “flip” a drawing; you can also try the famous “disappearing penny” trick .
Learn more: Light Refraction With Water
44. Color some flowers
We love how simple this project is to re-create since all you’ll need are some white carnations, food coloring, glasses, and water. The end result is just so beautiful!

45. Use glitter to fight germs
Everyone knows that glitter is just like germs—it gets everywhere and is so hard to get rid of! Use that to your advantage and show kids how soap fights glitter and germs.
Learn more: Glitter Germs

46. Re-create the water cycle in a bag
You can do so many easy science experiments with a simple zip-top bag. Fill one partway with water and set it on a sunny windowsill to see how the water evaporates up and eventually “rains” down.
Learn more: Water Cycle

47. Learn about plant transpiration
Your backyard is a terrific place for easy science experiments. Grab a plastic bag and rubber band to learn how plants get rid of excess water they don’t need, a process known as transpiration.
Learn more: Plant Transpiration

48. Clean up an oil spill
Before conducting this experiment, teach your students about engineers who solve environmental problems like oil spills. Then, have your students use provided materials to clean the oil spill from their oceans.
Learn more: Oil Spill

49. Construct a pair of model lungs
Kids get a better understanding of the respiratory system when they build model lungs using a plastic water bottle and some balloons. You can modify the experiment to demonstrate the effects of smoking too.
Learn more: Model Lungs

50. Experiment with limestone rocks
Kids love to collect rocks, and there are plenty of easy science experiments you can do with them. In this one, pour vinegar over a rock to see if it bubbles. If it does, you’ve found limestone!
Learn more: Limestone Experiments

51. Turn a bottle into a rain gauge
All you need is a plastic bottle, a ruler, and a permanent marker to make your own rain gauge. Monitor your measurements and see how they stack up against meteorology reports in your area.
Learn more: DIY Rain Gauge

52. Build up towel mountains
This clever demonstration helps kids understand how some landforms are created. Use layers of towels to represent rock layers and boxes for continents. Then pu-u-u-sh and see what happens!
Learn more: Towel Mountains

53. Take a play dough core sample
Learn about the layers of the earth by building them out of Play-Doh, then take a core sample with a straw. ( Love Play-Doh? Get more learning ideas here. )
Learn more: Play Dough Core Sampling

54. Project the stars on your ceiling
Use the video lesson in the link below to learn why stars are only visible at night. Then create a DIY star projector to explore the concept hands-on.
Learn more: DIY Star Projector

55. Make it rain
Use shaving cream and food coloring to simulate clouds and rain. This is an easy science experiment little ones will beg to do over and over.
Learn more: Shaving Cream Rain
56. Blow up your fingerprint
This is such a cool (and easy!) way to look at fingerprint patterns. Inflate a balloon a bit, use some ink to put a fingerprint on it, then blow it up big to see your fingerprint in detail.

57. Snack on a DNA model
Twizzlers, gumdrops, and a few toothpicks are all you need to make this super-fun (and yummy!) DNA model.
Learn more: Edible DNA Model
58. Dissect a flower
Take a nature walk and find a flower or two. Then bring them home and take them apart to discover all the different parts of flowers.

59. Craft smartphone speakers
No Bluetooth speaker? No problem! Put together your own from paper cups and toilet paper tubes.
Learn more: Smartphone Speakers

60. Race a balloon-powered car
Kids will be amazed when they learn they can put together this awesome racer using cardboard and bottle-cap wheels. The balloon-powered “engine” is so much fun too.
Learn more: Balloon-Powered Car

61. Build a Ferris wheel
You’ve probably ridden on a Ferris wheel, but can you build one? Stock up on wood craft sticks and find out! Play around with different designs to see which one works best.
Learn more: Craft Stick Ferris Wheel
62. Design a phone stand
There are lots of ways to craft a DIY phone stand, which makes this a perfect creative-thinking STEM challenge.
63. Conduct an egg drop
Put all their engineering skills to the test with an egg drop! Challenge kids to build a container from stuff they find around the house that will protect an egg from a long fall (this is especially fun to do from upper-story windows).
Learn more: Egg Drop Challenge Ideas

64. Engineer a drinking-straw roller coaster
STEM challenges are always a hit with kids. We love this one, which only requires basic supplies like drinking straws.
Learn more: Straw Roller Coaster

65. Build a solar oven
Explore the power of the sun when you build your own solar ovens and use them to cook some yummy treats. This experiment takes a little more time and effort, but the results are always impressive. The link below has complete instructions.
Learn more: Solar Oven
66. Build a Da Vinci bridge
There are plenty of bridge-building experiments out there, but this one is unique. It’s inspired by Leonardo da Vinci’s 500-year-old self-supporting wooden bridge. Learn how to build it at the link, and expand your learning by exploring more about Da Vinci himself.
Learn more: Da Vinci Bridge
67. Step through an index card
This is one easy science experiment that never fails to astonish. With carefully placed scissor cuts on an index card, you can make a loop large enough to fit a (small) human body through! Kids will be wowed as they learn about surface area.

68. Stand on a pile of paper cups
Combine physics and engineering and challenge kids to create a paper cup structure that can support their weight. This is a cool project for aspiring architects.
Learn more: Paper Cup Stack

69. Test out parachutes
Gather a variety of materials (try tissues, handkerchiefs, plastic bags, etc.) and see which ones make the best parachutes. You can also find out how they’re affected by windy days or find out which ones work in the rain.
Learn more: Parachute Drop

70. Recycle newspapers into an engineering challenge
It’s amazing how a stack of newspapers can spark such creative engineering. Challenge kids to build a tower, support a book, or even build a chair using only newspaper and tape!
Learn more: Newspaper STEM Challenge

71. Use rubber bands to sound out acoustics
Explore the ways that sound waves are affected by what’s around them using a simple rubber band “guitar.” (Kids absolutely love playing with these!)
Learn more: Rubber Band Guitar

72. Assemble a better umbrella
Challenge students to engineer the best possible umbrella from various household supplies. Encourage them to plan, draw blueprints, and test their creations using the scientific method.
Learn more: Umbrella STEM Challenge
Plus, sign up for our newsletters to get all the latest learning ideas straight to your inbox.

You Might Also Like

Magic Milk Experiment: How-To Plus Free Worksheet
This classic experiment teaches kids about basic chemistry and physics. Continue Reading
Copyright © 2024. All rights reserved. 5335 Gate Parkway, Jacksonville, FL 32256
Get Your ALL ACCESS Shop Pass here →

65 Amazing Chemistry Experiments for Kids
Chemistry is so much fun, and we have tons of cool chemistry experiments to share with you. Like our awesome physics experiments , we decided we needed to put together a list of fun chemistry projects kids can do at home or in the classroom. Check out these examples of easy chemical reactions below!

65 Chemistry Experiments You Want To Try
We have divided our chemistry experiments below into chemical reactions, acids and bases, chromatography, solutions, polymers, and crystals. Some chemistry experiments also explore concepts in physics .

Chemical Reactions
A chemical reaction is a process where two or more substances react together to form a new chemical substance. This might look like a gas formed, cooking or baking, milk souring, etc.
Sometimes a physical change occurs, like our popcorn experiment or melting crayons, rather than a chemical change . However, these experiments below are all great examples of chemical change, where a new substance is formed.
CHECK OUT: Examples Of Physical Change and Chemical Change Examples
Can chemical reactions happen safely at home or in the classroom? Absolutely! This is one of the most fun parts of chemistry for kids, and you will find lots of ideas below for safe chemical reactions you can do with your junior scientists.

Acids And Bases
Acids and bases are important for many chemical processes in everyday life. An acid has hydrogen ions and can donate protons. Acids taste sour and have a pH from 0 to 7. Vinegar and citric acid are examples of acids.
Bases are molecules that can accept hydrogen ions. They have a pH higher than seven and can taste bitter. Sodium bicarbonate or baking soda and ammonia are examples of bases. Learn more about the pH scale.
Vinegar and baking soda experiments are classic acid-base reactions. You’ll also find experiments that use an acid such as vinegar or lemon juice. We have so many fun variations that your kids will love to try! Check out these acid-base chemistry experiments below.

Chromatography
Chromatography is a technique that involves the separation of a mixture into its parts so you can see each one individually.
This marker and paper chromatography lab uses chromatography to separate the pigments in a black marker. Or set up a leaf chromatography experiment to find the hidden pigments in the leaves in your backyard!

A solution is a mixture of 2 or more solutes dissolved in a solvent up to its solubility limit. It most often refers to liquids, but solutions, gases, and solids are also possible.
A solution will have its components evenly distributed throughout the mixture.
Chemistry experiments involving solutions are great for kids. Gather liquids you commonly find in your kitchen, oil, water, detergent, etc., and explore what dissolves.

A polymer is a huge molecule made of many smaller molecules layered together in repeating patterns called monomers. Putty, slime, and cornstarch are all examples of polymers. Learn more about the science of slime polymers .
Making slime is great for at-home chemistry! It’s also a classic middle school science demonstration for the classroom. Here are a few of our favorite slime recipes to get you started.

Non-newtonian Fluids
Learn how to make oobleck ! Explore polymers with a simple cornstarch and water mixture. Check out these fun variations of the oobleck recipe below.

A crystal is a solid material with a highly ordered internal structure of atoms, molecules, or ions held together by chemical bonds.
Grow crystals and observe them by mixing a super-saturated solution and leaving it for several days to let the crystals form.
Simple to grow and taste-safe, a sugar crystals experiment is more accessible for younger kids, but you can also try growing borax crystals for older kids.
Check out our fun theme variations of growing crystals too!

Chemistry For Preschoolers
Let’s keep it basic for our younger or junior scientists! Chemistry is all about how different materials are put together and what they are made up of, like atoms and molecules.
What can you do with your youngest scientists? While working 1-1 or in a very small group is ideal, you can explore chemistry in a few fun ways that don’t require a lengthy setup or a lot of directions to follow. Do NOT overcomplicate the ideas!
Take, for example, our very first baking soda science experiment (age 3). So simple to set up, but so lovely to watch the amazement on my son’s face.
Check out these fun ways for preschoolers to explore science…
- Make liquid mixtures! Mix water and oil in a jar, let it rest, and observe what happens.
- Make solid mixtures! Mix two solid items and observe the changes!
- Mix a solid and a liquid! Add ice to a drink and observe the changes!
- Make a reaction! Set up a tray with baking soda in small cups and colored vinegar in small cups with pipettes. Mix and observe!
- Make oobleck ! Mix cornstarch and water for a weird and messy science activity.
- Explore characteristics of things! Use new science words to describe how different materials feel. Explore squishy, hard, rough, smooth, wet, etc…
Much of preschool science is about you sharing new experiences with them that are relatable and simple. A sk questions, share new words, and offer verbal prompts to get them to communicate with you about what they see!
Chemistry Science Fair Projects
Science projects are excellent tools for older kids to demonstrate their knowledge of science. They can also be used in various environments, including classrooms, homeschools, and groups.
Kids can take everything they have learned about using the scientific method , stating a hypothesis, choosing variables , and analyzing and presenting data.
Want to turn one of these fun chemistry experiments into a science project? Then you will want to check out these helpful resources.
- Easy Science Fair Projects
- Science Project Tips From A Teacher
- Science Fair Board Ideas
More Helpful Science Resources
Here are a few resources that will help you introduce science more effectively to your kiddos or students and feel confident yourself when presenting materials. You’ll find helpful free printables throughout.
- Best Science Practices (as it relates to the scientific method)
- Science Vocabulary
- 8 Science Books for Kids
- All About Scientists
- Free Science Worksheets
- Science Supplies List
- Science Tools for Kids
- Scientific Method for Kids
- Citizen Science Guide
- Join us in the Club
Printable Science Projects For Kids
If you’re looking to grab all of our printable science projects in one convenient place plus exclusive worksheets and bonuses like a STEAM Project pack, our Science Project Pack is what you need! Over 300+ Pages!
- 90+ classic science activities with journal pages, supply lists, set up and process, and science information. NEW! Activity-specific observation pages!
- Best science practices posters and our original science method process folders for extra alternatives!
- Be a Collector activities pack introduces kids to the world of making collections through the eyes of a scientist. What will they collect first?
- Know the Words Science vocabulary pack includes flashcards, crosswords, and word searches that illuminate keywords in the experiments!
- My science journal writing prompts explore what it means to be a scientist!!
- Bonus STEAM Project Pack: Art meets science with doable projects!
- Bonus Quick Grab Packs for Biology, Earth Science, Chemistry, and Physics

17 Comments
- Pingback: Homemade Sand Slime Recipe for Kids Summer Science Activity
- Pingback: Magic Milk Classic Science Experiment Kids Science
- Pingback: Children to Leaders Foundation | 18 Great Online Resources to Get Your Child into Science
- Pingback: Balloon Baking Soda Vinegar Science Experiment for Kids
- Pingback: Erupting Apple Science and Apple Volcano Chemistry Activity for Kids
- Pingback: Simple Physics Activities Science Experiments STEM Ideas for Kids
- Pingback: How to Get Slime Out of Clothes (2 Methods to Try!)
- Pingback: How to Incorporate Chemistry at Home
- Pingback: Winter Magic Milk Science Project for Snowman Science Activities
- Pingback: Coffee Filter Flowers Science and STEAM Activity for Kids
- Pingback: Crystal Flowers Spring Science Experiment and Craft for Mother Day
- Pingback: Erupting Lemon Volcano Chemistry for Kids Science Activities
- Pingback: Grow Sugar Crystals for Edible Rock Candy Chemistry Experiment
- Pingback: 187Great Online Resources to Get Your Child into Science
- Pingback: The BEST Very Simple Science Experiments for Kids to Try Anywhere
- Pingback: Simple Ways To Take STEAM Outdoors This Summer
- Pingback: Geometric Bubble STEM Activity for Kids Summer Science
Comments are closed.

Subscribe to receive a free 5-Day STEM Challenge Guide
~ projects to try now ~.

You are using an outdated browser. Please upgrade your browser to improve your experience.
8 Simple Chemistry Experiments That Your Kids Can Do at Home

Chemistry is a fascinating subject. And what better way to learn than through science experiments?
Here are 8 hands-on science experiments for kids over the school holiday. These experiments are great for older children, or with assistance from mum or dad. They can be done at home with ingredients you already have on hand.
So pick an experiment, and grab your lab coats to get started!
1. Cabbage chemistry
2. lolly fountain, 3. bath bombs, 5. rubber egg, 6. crystals, 8. snot slime, cabbage chemistry.
Follow these instructions to learn about acids and bases using red cabbage.

Safety: This activity requires the use of a knife, poisonous chemicals and hot water. Ask an adult to assist you. Always follow the safety advice on the products you are using.
You will need
- fresh red cabbage
- sharp knife
- cutting board
- hot tap water
- 7 clear plastic disposable cups
- 7 plastic spoons
- large plastic bottle
- strongly acidic, e.g. powdered toilet cleaner
- acidic, e.g. vinegar, lemon juice, white wine, lemonade or citric acid
- weakly acidic, e.g. cream of tartar
- neutral, e.g. pure water, shampoo or baby shampoo
- slightly basic, e.g. bicarbonate soda
- basic, e.g. milk of magnesia, washing soda or floor cleaner
- strongly basic, e.g. dishwasher liquid or powder
- Using a sharp knife and cutting board, finely slice three or four red cabbage leaves.
- Place the cabbage leaves in the plastic bottle, half fill the bottle with hot water and screw the lid on tightly.
- Shake the bottle for a few minutes until the water becomes a deep purple colour. Leave the solution to cool.
- Strain the solution and add sufficient water to the solution to make about 1 L.
- In each of the cups, place a small amount of one of the above household substances in the following order: strongly acidic; acidic; slightly acidic; neutral; slightly basic; basic and strongly basic.
- Now half fill each cup with the red cabbage water and stir the solution. If arranged in order, the jars should display a spectrum of colours from cherry red (strongly acidic), pink-red (acidic), lilac (slightly acidic), purple (neutral), blue (slightly basic), green (basic) and yellow (strongly basic).
What’s happening
The things we eat and drink are all acidic, and the things we use for cleaning are basic. This is because basic substances taste unpleasant, but a cleaning agent usually needs to be basic to remove dirt and grease.
Substances that are acidic or basic make the eyes sting, so baby shampoo is made neutral.
Acids are a very common group of chemical compounds, many of which occur naturally. Acids can be strong or weak.
Citric acid, which occurs naturally in lemons, is a weak acid. Hydrochloric acid (used for soldering) and sulfuric acid (battery acid) are very strong acids.
Bases (often called alkalis) are another group of chemical compounds that have different chemical properties from acids. When bases and acids are added together, they will neutralise each other’s properties.
We describe whether things are acidic, basic or neutral by using a scale called the pH scale. The pH scale ranges from zero to 14. A substance with a pH of:
- 0 is a very strong acid
- 3 – 5 is a weak acid
- 7 is neutral
- 8 – 9 is a weak base
- 13 – 14 is a very strong base.
Pure water has a pH of seven and is regarded as neutral.
Acids and bases can be detected by a group of chemical compounds called acid-base indicators. One of the first known naturally occurring indicators was a type of lichen called litmus. (Lichens are plant-like growths that are often found on rocks and tree bark.) Litmus turns red in the presence of an acid or blue with a base.
Most indicators used today to detect acids and bases are man-made. However, many plant pigments, such as the red cabbage you used, contain chemicals that act as acid-base indicators.

Looking for more science resources?
There are over 120 science e-books in the reading eggs library to read and explore., lolly/candy fountain.
Learn more about gases by creating a soft drink fountain using lollies/sweets. What a sweet way to find out more about chemistry!
- Roll of lollies/sweets (mint lollies/sweets work well)
- 2 L bottle of soft drink
- Piece of paper or a tube for the lollies/sweets
- Outdoor area
Do this activity in an outdoor area.
- Open the bottle of soft drink and place the bottle on the ground so it will not tip over.
- Roll up the paper into a cylinder that’s just wide enough for the lollies to slide through.
- Put your finger over the bottom of the roll and ask your friend to put the lollies into the paper tube.
- Hold the tube of lollies just above the bottle and remove your finger so all the lollies drop straight in. You need to drop all the lollies into the bottle at the same time.
- As soon as you have done that, move away from the bottle as quickly as possible.
- Diet soft drink works just as well and is less sticky to clean up as it contains no sugar.
- Orange soft drink doesn’t always work. Neither does Solo as it is light on fizz.
- Experiment with different types of lollies – Kool Mints were used in this activity. Try Mentos or other sugar coated lollies.
- Experiment with the soft drink at room temperature or from the fridge.
What’s happening?
Soft drink is bubbly because carbon dioxide gas has been forced into the bottle under pressure.
Until you open the bottle, the gas mostly stays dissolved in the liquid and cannot expand to form bubbles, which the gas will do when not under pressure.
If you shake the bottle and then open it, the gas escapes with a whoosh, taking some of the soft drink along with it. Adding anything to a soft drink enables more bubbles to form and escape.
Try stirring soft drink with a spoon – it gets less fizzy.
The lollies provide lots of surface area very quickly, which means the bubbles of gas form very rapidly in huge numbers.
You need non-smooth surfaces to enable the gas to form.
Both sand and sugar have the same effect when dropped in soft drink.
When you look at a glass of soft drink, there are normally just a few streams of bubbles coming off specific points on the glass where the surface is uneven.
Sometimes you see a stream of bubbles coming from the middle and if you look carefully you can often see a piece of dust with bubbles coming off its end.
The place where the bubbles start to form is called the centre of nucleation.
As the lolly dissolves, it forms hundreds of nucleation points which are tiny pits on the surface of the lolly where more carbon dioxide bubbles can form.
When all this gas is released, it thrusts the entire contents of the bottle skyward, in an incredible soft drink blast.
Make your own bath bombs
Follow these instructions to make your own bath bombs and learn about science while having fun in the bath!

- food colouring/coloring
- flower petals or body glitter
- sweet almond oil
- scented oil such as lavender oil
- 10 tablespoons of bicarbonate of soda
- 3 tablespoons of citric acid
- 2 large mixing bowls
- 1 large muffin tray
- 1 small glass jar
- rubber gloves
- Grease the sides and bases of a large muffin tray with a small amount of almond oil.
- Place the citric acid and bicarbonate of soda into a large bowl. Mix the ingredients together well, to form the base mixture.
- Scoop out about half a cup of this mixture and put it in into another bowl. This will make about one or two bath bombs (depending on the size of the holes in your muffin tray). You could also use old plastic containers or anything that will hold a shape.
- Add the flower petals or body glitter to the base mixture.
- In the small glass jar, mix together 6 drops of your scented oil, 5 teaspoons of sweet almond oil and about 10 drops of food colouring.
- Gradually pour the oil mixture into the half cup of the base mixture. While wearing rubber gloves, quickly mix it all together. The mixture is ready when it stays together in your hands without crumbling too much.
- Spoon the mixture into the muffin tray. Press it down firmly.
- You can use the rest of the mixture with other types of scented oil or food colouring to make more bath bombs.
- Leave the bombs in the tray to set for a few days.
- Carefully up-end your bath bombs to remove them from the moulds.
- Run a bath, hop in and drop a bomb. Watch it fizzzzzz!
What’s happening?
When the bath bomb dissolves in water, there is a chemical reaction between the citric acid and the sodium bicarbonate. The result is called sodium citrate. During the reaction, carbon dioxide is released. This causes the ‘fizzing’ that you see, similar to that in carbonated water.
The sweet almond oil is released during this reaction. It will form a thin layer on your skin which can help to moisturise/moisturize it. The lavender oil is for fragrance.

Wait, there’s more science experiment books in the Reading Eggs Library!
How to make sherbet.
Follow these instructions to create an acid-base reaction in your mouth!

- icing sugar
- citric acid
- bicarbonate soda
- flavored/flavoured jelly crystals
- dessert spoon
- small mixing bowl
- small snap lock bag.
To make sherbet you will need to:
- add 1 level teaspoon of citric acid crystals to the bowl
- add 1 level teaspoon of bicarbonate soda to the bowl
- now add 3 heaped dessert spoons of icing sugar
- add at least 2 level dessert spoons of jelly crystals (or more to taste)
- place a small amount, about half a teaspoon on your tongue
- after tasting you may need to vary the ingredients. If it is too bitter add more sugar, if there isn’t enough fizz you may need to add either bicarbonate soda or citric acid. Make sure you add only in small amounts, remember you can always add more but it is very hard to remove some.
You have just created an acid-base reaction in your mouth. When you combine an acid (in this activity the citric acid) and an alkaline (the bicarbonate soda) with saliva they mix together to create a gas in the form of lots of tiny bubbles.
This is called an acid-based reaction and it’s what gives sherbet its fizz. You are actually feeling the sensation of carbon dioxide bubbles on your tongue. These are the same bubbles that are in fizzy drinks.
The icing sugar is needed to add sweetness as the citric acid and bicarbonate soda are quite sour. Citric acid is one of the acids found in lemons, oranges and limes. That is why they are called ‘citric fruit’.
The other acid in lemons and other citric fruit is called ascorbic acid. This is commonly known as vitamin C. The jelly crystals simply add flavour.
Follow these instructions to make an egg bounce while learning about chemical reactions.

- hard-boiled egg, with shell on
- glass of vinegar.
To make your eggs bounce you will need to:
- Put the egg into the vinegar – you should see bubbles start to form on the egg.
- Leave the egg undisturbed for at least a day. You should see some wonderful scum form.
- Take the egg out of the vinegar and rinse it with water. The shell will rub off.
- Give the egg a poke with your finger and squeeze it gently.
Vinegar, or dilute acetic acid, ‘eats up’ the calcium carbonate in the egg shell, just leaving the inner membrane, or skin, of the egg behind. As the calcium carbonate is responsible for making the shell hard, the vinegar soaked egg feels soft and rubbery.
When calcium carbonate (the egg shell) and acetic acid (the vinegar) combine, a chemical reaction takes place and carbon dioxide gas is released. That’s why you see the bubbles.
The chemical reaction keeps happening for about a day until all of the calcium carbonate in the egg is used up. Calcium carbonate is in eggshells, seashells, limestone, and many other materials.
Let’s have a closer look at the chemical reaction. Calcium carbonate’s formula is CaCO 3 and acetic acid is CH 2 COOH.
So the reaction is: CaCO 3 + CH 2 COOH -> Ca 2+ (in the form of a salt) + H 2 O + 2CO 2 .
The calcium ions (Ca 2+ ) float free in the solution. Ions are atoms or molecules that have an electric charge due to the loss or gain of electrons.
Applications
Limestone is a sedimentary rock that is largely made of calcium carbonate. It is ordinarily white, but may be coloured by impurities; iron oxide making it brown, yellow, or red and carbon making it blue, black, or grey. The texture varies from coarse to fine.
Most limestones are formed over thousands of years from the skeletons of marine invertebrates. Among the important varieties of limestone are marl, chalk, oolite, travertine, dolomite, and marble.
Acid rain causes reactions like the ones in this activity. One kind of acid rain can come from air pollution caused by burning fuels that have sulfur atoms, which when burnt produce sulphur dioxide gas.
When the sulfur dioxide mixes in with rain, it turns to weak sulfuric acid. When the acid rain hits the limestone it slowly makes it fall apart, like the egg shell did. People use limestone in buildings and statues.
This is why over time, buildings and statues are being damaged by acid rain.
If you collect small rock samples and drop them in vinegar, you may see bubbles appear, like they did on the egg. The presence of bubbles indicates that calcium carbonate may be present in the sample.
Calcium carbonate reacts with acids to produce carbon dioxide gas, which we observe as bubbles. This is called the ‘acid test’. The ‘acid test’ is one of many tests that geologists use to determine the identity of a rock sample.
Creating Crystals

- Bicarbonate soda
- 3 eye-droppers
- 3 plastic containers or bowls
- Measuring cup
- 3 small plastic cups
- Label the containers ‘sugar’, ‘salt’ and ‘bi-carb’.
- Pour half a cup of warm water into the container labelled ‘sugar’.
- Add a spoonful of sugar to the water and stir until dissolved. Keep adding sugar until no more will dissolve.
- Repeat Steps 2 and 3, but with the salt instead of sugar.
- Again repeat Steps 2 and 3, but this time with bi-carb soda instead of sugar or salt.
- Label the small plastic cups ‘sugar’, ‘salt’ and ‘bi-carb’.
- Use separate eye-droppers to put a few drops of each container’s solution into the matching cup.
- Place the cups in a warm, sunny place and leave them until the liquid has evaporated. What do you see?
You can try this activity with other crystalline substances as well.
When a solid (or ‘solute’) is dissolved in the water until no more dissolves, the solution is ‘saturated’. The amount of substance that dissolves in water increases with temperature. As the solution cools back down to room temperature, there is now more solute in the water than would normally be the case – the solution is ‘supersaturated’.
As the water evaporates, the solute precipitates out of solution in the form of crystals. This is an example of crystallisation. You will notice that each precipitate forms slightly different crystals: they might be different in size and shape. The size and shape of a crystal depend on a number of factors including chemical formula, temperature and pressure. In general, crystals that form slowly tend to be larger than crystals that form quickly.
- Food colouring
- Small mixing bowl
- Plastic spoon
- Pour some cornflour into a mixing bowl.
- Stir in small amounts of water until the cornflour has become a very thick paste.
- To make the slime the colour of your choice, thoroughly stir about five drops of food colouring into the mixture.
- Stir your slime REALLY slowly. This shouldn’t be hard to do.
- Stir your slime REALLY fast. This should be almost impossible.
- Now punch your slime REALLY hard and fast. It should feel like you’re punching a solid.
You can keep your cornflour and water mixture covered in a fridge for several days. If the cornflour settles, you need to stir it to make it work well again.
Anything that flows is called a fluid. This means that both gases and liquids are fluids.
Fluids like water which flow easily are said to have low viscosity, whereas fluids like cold honey which do not flow so easily are said to have a high viscosity.
Cornflour slime is a special type of fluid that doesn’t follow the usual rules of fluid behaviour. When a pressure is applied to slime, its viscosity increases and the cornflour slime becomes thicker.
At a certain point, slime actually seems to lose its flow and behave like a solid. Cornflour slime is an example of a shear-thickening fluid.
The opposite happens in shear-thinning fluids; they get runnier when you stir them or shake them up. For example, when toothpaste is sitting on a toothbrush it is pretty thick, so you can turn the toothbrush upside down and the toothpaste doesn’t fall off.
But if it was that thick when you tried to squeeze it out of the tube, there is no way you could manage it. Fortunately, toothpaste gets runnier when you are squeezing it out of the tube. Other shear-thinning fluids include:
- ballpoint pen ink
- nail polish
Although there are lots of shear-thinning and shear-thickening fluids, nobody has a really good idea why they behave the way they do.
The interactions between atoms in the fluids are so complicated that even the world’s most powerful supercomputers can not model what is happening. This can be a real problem for people who design machinery that involves shear-thinning fluids, because it makes it hard to be sure if they will work.
- 1 tablespoon of unflavoured gelatine (from supermarkets)
- ½ cup golden syrup or glucose
- 1 tablespoon of salt
- Heat-proof bowl
- Place the gelatine and salt in your bowl.
- Add ½ cup of syrup.
- Add ½ cup of hot water. Now is the time to add food colouring if you want icky green or yellow coloured snot.
- Mix every thing together and cool in a fridge for 30 minutes.
- Run a fork through the snotty mixture to see what it looks like. Your mucus will get thicker and thicker as it cools, if it is too thick, you can add more water.
You have just made a realistic model of your very own snot. Mucus is composed of water, epithelial (surface) cells, dead leukocytes (white blood cells), mucins (large proteins), and inorganic salts. Your home made mucus contains water, salt and proteins (gelatine is animal protein, usually made from beef or pig skin and hooves), almost like real mucus.
The gelatine dissolves in hot water making a thick solution, but is insoluble (won’t dissolve) in cold water. When cooled, the particles swell to make jelly-like goo.
Mucus has an important role to play in your body. In your nose it traps dust and anything else unwanted in the air. Mucus dries around particles which harden and this means it can take a quick exit out of your body when you blow your nose.
It’s your mucous membrane that makes snot, and this lines the inside of your nose and respiratory system. The outermost cells of this membrane produce the thick mucus fluid.
You may think that mucus is only found in your nose, but did you know that you also find it in your mouth, lungs, stomach and intestines!
When you get a common cold, an infection in your upper respiratory tract, your body produces loads more mucus than normal to carry away waste material. When sick, your mucus can change colour/color to yellow or green because of trapped bacteria, virus particles and white blood cells – the causalities of your body fighting the viral or bacterial infection.
Reference: These experiments are from our partners at CSIRO.

Looking to boost your child’s literacy or mathematics confidence and skills?
Explore reading eggs , mathletics and mathseeds (for younger learners), you might like....

- Customer Reviews
- Extended Essays
- IB Internal Assessment
- Theory of Knowledge
- Literature Review
- Dissertations
- Essay Writing
- Research Writing
- Assignment Help
- Capstone Projects
- College Application
- Online Class
Chemistry Extended Essay Topics: 30+ Ideas to Get You Started
by Antony W
September 2, 2022
Have you searched for the best IB Chemistry Extended Essay topics but you haven’t found any that’s useful for further investigation?
Or maybe you don’t know how to choose a topic for the subject and you need guidance?
Continue reading this guide to learn more.
What is an Extended Essay in Chemistry?
An extended essay in chemistry gives you the chance to study a specific area of our environment's components.
Within a more broad set of research standards, the essay should highlight a particular chemical aspect.
The result of the study should be a logical and organized piece of writing that effectively tackles a certain subject or research question and reaches a specific, and ideally personal, conclusion.
Chemistry Extended Essay Writing Help
Are you struggling with topic selection for your Chemistry EE? Or maybe you have no idea how to start your preliminary research to develop your research issue?
You can contact Help for Assessment right now for professional help.
If you’re asking yourself who can write my Extended Essay for me , you can be sure that our platform has the writing team that you’re looking for. We can help you with IB Chemistry EE topic selection, conduct preliminary research, develop a relevant research issue, and get the writing done.
It doesn’t matter whether you’ve run out of time or your grasp of Chemistry is weak.
We connect you with professional writers with experience in the subject, so you never have to worry about quality and relevance of the work you get from us.
Take advantage of this writing service, and let us help you score top grades in your Chemistry EE.
How to Choose Chemistry Extended Essay Topics
Below are some points that will help you to choose the best Chemistry extended essay topics:
1. Pick a Topic Focused on Chemistry
It is essential that the emphasis of the extended essay be on chemistry, and not on another subject.
Chemistry is the study of the composition, classification, and change of substances.
Therefore, a long essay in chemistry should include chemical principles and theory and underline the core character of chemistry, which is the study of matter and its transformations.
2. Pick a Topic for Which You Can Provide a Chemistry-based Approach
Although the same evaluation criteria apply to all extended essays, the topic chosen for a chemistry extended essay must provide a different chemistry-based approach.
Whereas you can handle a topic from several perspectives, you have to treat everything from a chemical standpoint.
For instance, if registered as a chemistry extended essay, a biochemistry extended essay will be evaluated based on its chemical content, not its biological content.
3. Pay Attention to the Assessment Criteria
The topic's breadth and accompanying research should allow for the consideration of all criteria.
An excellent subject is one in which the single research question is focused and may be adequately addressed within the word limit.
Perhaps the most crucial component is your ability to provide an in-depth analysis of the issue.
Broad or complex survey topics (such as investigations into health problems caused by water pollution, chemotherapy for cancer treatment, or the use of spectroscopy for chemical analysis) will not allow you to discuss opposing ideas and theories or produce an in-depth personal analysis within the word limit.
4. Avoid Topics that Raise Safety Concerns
Some topics may not be acceptable for examination due to safety concerns.
You should not experiment using poisonous or hazardous chemicals, carcinogenic compounds, or radioactive materials, for instance, unless suitable safety equipment and competent supervision are available.
5. Avoid Topics Whose Outcomes are Already Documented in Textbooks
Other topics may not be appropriate since the outcome is well documented in standard textbooks and the student may not be able to demonstrate personal involvement.
Example: a study of the interactions of alkali metals with water, which is previously covered in the curriculum.
However, you must use caution when determining if a topic is appropriate; for instance, the study of the allotropes of carbon may have been considered trivial in the past, but this is no longer the case.
Related Reading
- Chemistry Extended Essay Complete Guide
- Physics Extended Essay Guide for IB Students
- Computer Science Extended Essay
IB Chemistry Extended Essay Topic Ideas
The following are some Chemistry Extended Essay topic ideas that you can start exploring right away – or just use for inspiration.
- How does the flower come into being?
- Why do plants grow while submerged in water?
- The influence that shifts in climate have on the reproductive processes of plants
- Sugar and cocaine are both very addictive.
- Why do some individuals consume more food but still manage to seem thin?
- What causes the pollen tube to develop, and how does pollination take place in isolated areas?
- The several kinds of eucalyptus trees
- The impact that rising temperatures are having on plant life.
- What is the one item that is absolutely necessary for the development of plants?
- The process of photosynthesis that takes place in the absence of sunlight
- The connection between the brain and the rest of the body The health and safety regulations regarding the preservation of cow milk for commercial purposes
- What distinguishes plants that grow in water from those that grow on land?
- The effectiveness of pollination by different species
- The possibility that medications used to treat pain and other similar substances might be harmful to the human brain.
- How can plants be used as a kind of treatment?
- Is it feasible for plants growing in the same environment to act in a variety of ways?
- What kind of an impact does a shift in habitat have on the touch-me-not plant?
- The phases of development that occur in a fetus
- Ultrasound's influence on the ratio of elimination to substitution yields in the reaction of halogenoalkanes with sodium hydroxide.
- How does the equilibrium change when you replace hydrogen in ethanoic acid with groups that pull electrons and release them?
- Diaper gel (sodium polyacrylate) absorbs liquids, but how much sodium chloride (as well as the solution's pH) depends on how much water is present.
- A Green Chemistry approach is used to studying the synthesis of vanillyl alcohol from vanillin and sodium borohydride.
- Raw milk's accessible calcium is studied in relation to the temperature at which it was heated, and lab-treated milk is compared to commercially-processed milk.
- Thanaka powder and traditional sunscreens are compared for their thermal stability and photostability.
- Analysis of catalase denaturation during the breakdown of hydrogen peroxide
- Visible light absorption by 1,x-dihydroxyanthraquinone as a function of the location of the second hydroxyl group.
- Temperature's impact on the fading kinetics of a photochromic dye was studied using spectrophotometry.
Final Thoughts
There are many IB Chemistry Extended Essay topics, but these ones should be good for a start, especially if you’re already struggling to find topics in the first place.
About the author
Antony W is a professional writer and coach at Help for Assessment. He spends countless hours every day researching and writing great content filled with expert advice on how to write engaging essays, research papers, and assignments.

Essay on Chemistry Importance In Daily Life
Students are often asked to write an essay on Chemistry Importance In Daily Life in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Chemistry Importance In Daily Life
What is chemistry.
Chemistry is like a secret recipe that makes everything around us. It is the study of matter, which means anything that takes up space and has weight. From the air we breathe to the food we eat, chemistry is a big part of our everyday lives.
Chemistry in Cooking
When we cook, we use chemistry. Mixing ingredients causes reactions that make our food taste good. For example, baking a cake changes liquid batter into a fluffy solid because of heat causing a chemical change.
Medicine and Health
Chemistry keeps us healthy. Doctors use medicines to fight sickness, and these medicines are made using chemistry. Even our bodies use chemistry to turn food into energy, which keeps us going every day.
Cleaning Our Homes
Cleaning products are full of chemicals that help us get rid of dirt and germs. Soaps and detergents break down grease and stains, making our clothes and homes clean thanks to chemistry.
Environment and Recycling
Chemistry helps us protect the environment. It helps us understand pollution and create ways to recycle materials. This way, we use less of Earth’s resources and take better care of our planet.
250 Words Essay on Chemistry Importance In Daily Life
Chemistry is like a secret recipe that explains everything around us. It is the science that tells us what things are made of and how they work together. Every time we cook, clean, or even breathe, we are part of a big chemistry experiment.
When we cook food, chemistry is at play. For example, when bread rises, it’s because of a chemical reaction. Heat changes the food, making it taste different and easier to digest. Without chemistry, we wouldn’t have bread, cheese, or even yummy chocolate.
Cleaning products are full of chemicals. Soap helps wash away dirt because it can stick to both water and grease. It’s like a magnet that pulls the dirt off our clothes and dishes. Chemistry helps us keep our homes and ourselves clean and healthy.
Medicines and Health
Medicines are chemicals that help our bodies fight sickness. When we are hurt or ill, the medicine makes the pain less or helps us get better. Chemistry is behind the vitamins we take to stay strong and the vaccines that protect us from diseases.
Every Breath We Take
Every time we take a breath, we are living chemistry. Air is a mix of gases, and breathing is a chemical process that gives our body the oxygen it needs. Plants use chemistry to turn sunlight into food, which is a process called photosynthesis. This is how plants help make the air clean for us to breathe.
In short, chemistry is part of everything we do. From the moment we wake up until we go to sleep, it makes our lives easier, safer, and more fun.
500 Words Essay on Chemistry Importance In Daily Life
Chemistry is like a secret language of everything we see, touch, and feel. It’s a part of science that tells us what stuff is made of and how different things work together. Imagine being a detective, but instead of solving mysteries, you’re figuring out the secrets of the world around you. That’s what chemists do. They study substances and how they change when they mix.
Chemistry in the Kitchen
Every day, we use chemistry when we cook. Have you ever baked a cake? The ingredients like flour, sugar, and eggs are mixed and heated to make something delicious. This is because of chemical reactions, which are like tiny events where the ingredients change and become a cake. When you cook eggs, they change from liquid to solid, and that’s chemistry at work too!
Cleaning Made Easy
When we clean, we’re using chemistry to help us. Soaps and detergents are made from chemicals that can grab onto dirt and wash it away with water. Even when you brush your teeth, the toothpaste is a kind of chemical that cleans and protects your teeth from germs.
Chemistry is super important for keeping us healthy. Medicines are chemicals that can fix or prevent health problems. For example, if you have a headache, you might take a painkiller. This medicine is made from chemicals that stop the pain signals in your body. Vaccines, which protect us from diseases, are also made thanks to chemistry.
Breathing and Living
Did you know that even breathing is a chemical process? When we breathe in, we take in oxygen, and when we breathe out, we release carbon dioxide. Plants do the opposite; they take in carbon dioxide and give out oxygen. This exchange is all because of chemical reactions that are essential for life on our planet.
Clothes and Materials
The clothes we wear are often made from materials like cotton, nylon, or polyester. These materials are created through chemical processes. For example, nylon is made in a factory where chemicals are combined to make this strong, stretchy fabric. Even the colors of our clothes come from dyes, which are chemicals that add colors to fabrics.
Technology and Gadgets
Our phones, computers, and TVs all work because of chemistry. The batteries that power them have chemicals that store electricity. The screens use chemicals to light up and show us pictures and videos. Without chemistry, none of these gadgets would work!
So, you see, chemistry is everywhere! It’s not just something that scientists think about in labs. It’s part of our everyday lives, helping us cook, clean, stay healthy, breathe, dress, and even use technology. Understanding chemistry helps us know more about the world and how to make our lives better. Next time you see something happening, like a cake rising in the oven or a bubble popping, remember, it’s all chemistry!
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Chef Career
- Essay on Cheating
- Essay on Challenges Of Prefectship
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

Jennifer Aniston Shared Some Insight Into “Uncomfortable” Auditions Where She Was Asked To “Create Chemistry” And “Make Out With A Complete Stranger”
“If you’re a nervous auditioner to begin with, to then say, ‘Now let’s have you make out with a complete stranger’... Put some music on or something.”

BuzzFeed Staff
Some of Hollywood’s biggest stars have weighed in on intimate chemistry tests between actors — and whether they’re even necessary.

For the Hollywood Reporter’s Drama Actress Roundtable , Anna Sawai, Jennifer Aniston , Naomi Watts , Nicole Kidman, Jodie Foster , Brie Larson , and Sofía Vergara came together to discuss a range of topics relating to their careers. At one point, they were asked to comment on some of Anne Hathaway’s comments about chemistry tests during the movie audition process.

If you missed it, Anne told V magazine in April that in the 2000s it was “considered normal to ask an actor to make out with other actors to test for chemistry.”

“I was told, ‘We have ten guys coming today and you’re cast. Aren’t you excited to make out with all of them?’ And I thought, ‘Is there something wrong with me?’ because I wasn’t excited. I thought it sounded gross,” she said.
Despite finding the whole thing very uncomfortable, Anne admitted that she always went along with such situations out of fear of being perceived as “difficult” to work with.
“I was so young and terribly aware how easy it was to lose everything by being labeled ‘difficult,’ so I just pretended I was excited and got on with it. It wasn’t a power play; no one was trying to be awful or hurt me. It was just a very different time, and now we know better,” she said.
Discussing these quotes and how times have changed in the industry, all the actors at the roundtable agreed that they’d found themselves in similarly awkward situations during their acting careers. What’s more, Jennifer and Nicole suggested that chemistry tests aren’t even necessary to make an onscreen partnership believable.

“You can not have chemistry, and onscreen, it’s made. There’s a way you can shoot things,” Nicole said. “I think just relying on chemistry is lazy. There’s the writing. There’s the interaction. You can literally be directed through it.”
Still on the topic of chemistry tests, Jennifer said that the pressure can often dilute any natural chemistry between actors anyway, saying: “When you’re in an audition room, you’re already at a disadvantage.”

“Maybe you’d have chemistry with this person if you were in a different environment and not, like, ‘Create chemistry. Ready? Go!’” she continued.
Jen also described herself as a “terrible auditioner,” noting that her nerves would make chemistry tests all the more difficult.

“So, if you’re a nervous auditioner, to begin with, to then say, ‘Now let’s have you make out with a complete stranger,’ it’s very uncomfortable,” she told her fellow actors. “Put some music on or something.”
Although intimacy coordinators are widely used on movie and TV sets to make intimate scenes more comfortable for actors in situations like this, Jen has said previously that she’s been in the industry long enough to “figure” it out herself.

For context, an intimacy coordinator is a professional who comes on to set when actors are shooting sex scenes and anything else that could be potentially compromising, like scenes involving drug use or nudity. The role involves not only supporting the actors but also assisting in choreography and ensuring that the cast and crew feel comfortable as well.
Speaking about her first-ever sex scene on The Morning Show late last year, Jen told Variety : “They asked us if we wanted an intimacy coordinator. I’m from the olden days, so I was like, ‘What does that mean?’ They said, ‘Where someone asks you if you’re OK,’ and I’m like, ‘Please, this is awkward enough!’”

In response, Jennifer assured producers that she and her costar, Jon Hamm , were “seasoned” pros and therefore didn’t need any support. “We can figure this one out,” she recalled saying.

“I never felt uncomfortable,” she added. “Jon was such a gentleman, always — I mean every move, every cut, ‘You OK?’ It was also very choreographed.”
At the time, Jennifer was criticized online for her seemingly “ flippant ” attitude, which fans thought minimized the importance of intimacy coordinators’ work and how they keep actors safe on set.

“‘Where someone asks if you’re okay’ is such a minimizing and disrespectful description of what an intimacy coordinator actually does,” one person wrote on X in response to her Variety interview, amassing more than 45,000 likes.
“Having seen firsthand the kind of work an intimacy coordinator actually does, it actually deflates the awkwardness and helps actors build boundaries and trust with one another,” another person agreed . “If it’s not for her that’s fine but it’s legitimate work that can be effective for performance.”
You can find the Hollywood Reporter’s full Drama Actress Roundtable here.
Topics in this article.
- Jennifer Aniston
- Nicole Kidman
- Anne Hathaway

The Fun and Easy Way to Teach Your Kids Chemistry at Home
Believe it or not, teaching your kids chemistry at home can be both fun and easy and it can definitely be worthwhile!
Learning chemistry at a young age can also have numerous benefits, including developing critical thinking skills and fostering a curiosity about the world around them.
So today, let's talk about some ways to make learning about chemistry (from the comfort of one's own home) both easy and fun.
*Post contains affiliate links. Full disclosure can be viewed here .
Why Teach Your Kids Chemistry at Home?
First things first, why should you even bother exploring chemistry with your kids at home?
Well, chemistry is an integral part of everyday life. Understanding basic chemical principles can help kids make sense of the world around them and help them develop critical thinking skills.
For example, they can learn why certain foods taste sour or how a particular soap works to clean dirty dishes. They can even learn why certain substances make fires grow while others extinguish them.
Basically, by teaching chemistry at home, you can provide your kids with a solid foundation in this particular area of science. These concepts can be used to their advantage throughout life.
You may even help them discover a new interest or career path .
The Benefits of Using MEL Science Kits for Your Chemistry Experiments
MEL Science Kits are a monthly subscription box explicitly designed to make learning about science fun and engaging for kids. They offer a hands-on approach allowing children to explore chemical reactions safely and in a controlled environment.
These kits also include the vast majority of all the necessary materials (for some experiments you need to provide things like water) and detailed instructions, making it easy for parents to guide their children through these mind-blowing experiments .
They also provide video lessons to make things even easier!
Fun Experiments to Explore Various Areas of Science Using MEL Science Kits
- Create a spring force racecar ( STEM )
- Build a device to show how infinity may look ( Math )
- Make sand that never gets wet ( Physics )
- Make incisions in artificial skin using a real scalpel ( Medicine )
- Use a lemon to light up a diode ( Chemistry )
How to Get Started with MEL Science Kits
To order MEL Science Kits and get started with teaching chemistry to your kids at home, follow these simple steps:
1. Visit the MEL Science website HERE .
2. Browse the available subject matters and choose the ones that suit your child’s interests.
3. Select a subscription option: monthly, quarterly, 6-month, or yearly.
4. Provide the necessary shipping and payment information to checkout!
5. Wait for your monthly kits to arrive at your doorstep!
Final Thoughts
You can easily teach kids chemistry at home in an exciting way using MEL Science Kits. These kits provide an engaging and hands-on learning experience that sparks curiosity and truly makes learning fun.
Ultimately, by conducting experiments and following step-by-step instructions, children develop critical thinking, problem-solving, and scientific reasoning skills.
The post The Fun and Easy Way to Teach Your Kids Chemistry at Home appeared first on Major League Mommy .

- Share full article
Advertisement
Supported by
Guest Essay
A Federal Judge Wonders: How Could Alito Have Been So Foolish?

By Michael Ponsor
Judge Ponsor is a senior judge on the U.S. District Court for the District of Massachusetts. He was appointed by President Bill Clinton in 1994 after serving 10 years as a federal magistrate judge.
The controversy about the decision to fly an upside-down American flag outside the home of Justice Samuel Alito recalls St. Paul’s admonition that while some things may be lawful, “not all things are helpful.”
I can offer no opinion as to whether the flag display at the justice’s house was unlawful. I won’t even opine whether my flying the flag upside-down at my house would have constituted a violation of the code of ethics that binds me and all federal judges — except the justices.
To me, the flag issue is much simpler. The fact is that, regardless of its legality, displaying the flag in that way, at that time, shouldn’t have happened. To put it bluntly, any judge with reasonable ethical instincts would have realized immediately that flying the flag then and in that way was improper. And dumb.
The same goes for the flying of an “Appeal to Heaven” flag at Justice Alito’s vacation house along the New Jersey shore. Like the upside-down flag, this flag is viewed by a great many people as a banner of allegiance on partisan issues that are or could be before the court.
Courts work because people trust judges. Taking sides in this way erodes that trust.
In four decades as a federal judge, I have known scores, possibly hundreds, of federal trial and appellate judges pretty well. I can’t think of a single one, no matter who appointed her or him, who has engaged or would engage in conduct like that. You just don’t do that sort of thing, whether it may be considered over the line, or just edging up to the margin. Flying those flags was tantamount to sticking a “Stop the steal” bumper sticker on your car. You just don’t do it.
Assuming it is true that it was Justice Alito’s wife who raised the inverted American flag, apparently in response to some provocative behavior from a neighbor, I do sympathize. (How the “Appeal to Heaven” flag came to be flown at his house is not known.) Being a judge’s spouse is not easy. On the one hand, a person should not have to forfeit the right to free expression at the marriage altar. On the other hand, it is not unreasonable to expect a spouse to avoid embarrassing a loved one or complicating his or her professional life. This is true not only for Supreme Court justices but also for people in many walks of life.
Let me offer an example. About 25 years ago, I presided over a death penalty case involving a nurse charged with murdering several of her patients at a Veterans Affairs hospital in Western Massachusetts. It was a tough case, regularly on the front pages of our local papers. Let’s say my wife was strongly opposed to the death penalty and wished to speak out publicly against it. I’m not saying this is true, but let’s imagine it. The primary emotional current in our marriage is, of course, deep and passionate love, but right next to that is equally deep and passionate respect. We would have had a problem, and we would have needed to talk.
In this hypothetical situation, I hope that my wife would have held off making any public statements about capital punishment, and restrained herself from talking about the issue with me, while the trial unfolded. On the other hand, if my wife had felt strongly that she needed to espouse her viewpoint publicly, I would have had to recuse myself from presiding over the case, based on the appearance of partiality.
However this issue came out, by the way, I certainly would not have had the temerity to claim that my wife and I never discussed the problem. Any protestation of this sort would have provoked raucous laughter from our circle of friends. They know very well that we talk about everything.
Did Justice Alito and his wife discuss the issue of the upside-down flag before it went up? I don’t know, of course. But I do know they should have. And I know that some other method should have been found to express the couple’s unhappiness with their neighbor’s possibly crummy conduct.
The court recently adopted an ethics code to “guide the conduct” of the justices. One of its canons states that a justice should “act at all times in a manner that promotes public confidence in the integrity and impartiality of the judiciary.” That’s all very well. But basic ethical behavior should not rely on laws or regulations. It should be folded into a judge’s DNA. That didn’t happen here. The flag display may or may not have been unlawful, but as far as the public’s perception of the court’s integrity, it certainly was not helpful.
Michael Ponsor is a senior judge on the U.S. District Court for the District of Massachusetts. He was appointed by President Bill Clinton in 1994 after serving 10 years as a federal magistrate judge.
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , WhatsApp , X and Threads .
Journal of Materials Chemistry A
Pore-size tuning of hard carbon to optimize its wettability for efficient na + storage †.

* Corresponding authors
a State Key Lab of Physical Chemistry of Solid Surfaces, College of Materials, Xiamen University, Xiamen 361005, China E-mail: [email protected]
b Key Laboratory of Advanced Metallic Materials of Jiangsu Province, School of Materials Science and Engineering, Southeast University, Nanjing, People's Republic of China
c Fujian Key Laboratory of Surface and Interface Engineering for High Performance Materials (Xiamen University), Xiamen Key Laboratory of High Performance Metals and Materials (Xiamen University), China
Hard carbons hold great promise as the anode materials for sodium-ion batteries (SIBs), but the limited capacities and sluggish Na + transfer kinetics still hinder their practical applications. These issues can be addressed from the perspective of improving the wettability of hard carbons with electrolyte. Herein, we demonstrate that the wettability of hard carbons can be regulated by tuning their pore size, so as to optimize their electrochemical properties. A series of N-doped hollow mesoporous carbon nanotubes (HMCNTs) with the average pore sizes in the range of 2–8 nm are synthesized. Among them, the HMCNTs with an average pore size of 6.1 nm (HMCNTs-6.1) exhibits the most improved wettability and reaction kinetics, which delivers a high reversible capacity (415.5 mA h g −1 after 100 cycles at 0.1 A g −1 ), superior long-term cycle stability and rate capability. In addition, as visualized by in situ transmission electron microscopy, the HMCNTs-6.1 with relatively large mesopores is more favorable for the mass transport across the carbon shells than the HMCNTs with smaller pore sizes. This work provides new insight for understanding the relationship between pore size, electrolyte wettability and electrochemical performance of porous hard carbon, which can help to design the high-performance SIB anodes.

- This article is part of the themed collections: Journal of Materials Chemistry A HOT Papers and Design and characterization of flexible electrode materials
Supplementary files
- Supplementary information PDF (2100K)
- Supplementary movie MP4 (3722K)
- Supplementary movie MP4 (2661K)
Article information
Download citation, permissions.
Pore-size tuning of hard carbon to optimize its wettability for efficient Na + storage
L. Guo, M. Huang, W. Liu, H. Zhu, Y. Cheng and M. Wang, J. Mater. Chem. A , 2024, Advance Article , DOI: 10.1039/D4TA02303J
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements

IMAGES
VIDEO
COMMENTS
Chemistry is a big part of your everyday life. You find chemistry in foods, the air, cleaning chemicals, your emotions, and literally every object you can see or touch. Here are 10 examples of everyday chemistry. Some common chemistry might be obvious, but other examples might surprise you. 01.
Anne Helmenstine. Making a borax snowflake is a crystal-growing project that is safe and easy enough for kids. You can make shapes other than snowflakes, and you can color the crystals. The snowflakes sparkle really nicely. If you use these as Christmas decorations and store them, the borax is a natural insecticide and will help keep your long ...
25. Turn Juice Into Solid. Turning juice into a solid through gelification is an engaging and educational chemistry experiment that students should try. By exploring the transformation of a liquid into a solid, students can gain insights of chemical reactions and molecular interactions.
Chemistry at Home has been written by award-winning popular science writer and chemist, John Emsley, using non-technical language. The book has 12 chapters, each devoted to the kinds of products we are likely to find around the home, including in the garage and the garden shed.
Check our 100% free chemistry essay, research paper examples. Find inspiration and ideas Best topics Daily updates. Stuck with your chemistry paper? Check our 100% free chemistry essay, research paper examples. ... Home > Free Essays > Sciences > 1 hour! We'll deliver . a 100% original paper. this fast Learn More. Similar Subjects
Chemistry at home. By Simon Cotton 4 November 2015. John Emsley. RSC. 2015 | 394pp | £21.99. ISBN 9781849739405. I am sure that one reason for some misconceptions about chemicals is that little ...
Fig. 1: Science experiments at home. Clockwise from bottom left, soap on water: a simple way to measure molecular dimensions — inspired by Irving Langmuir — using talcum powder sprinkled on ...
Alex Dowden/EyeEm / Getty Images. Over time, iron develops a red, flaky coating called rust. This is an example of an oxidation reaction. Other everyday examples include the formation of verdigris on copper and the tarnishing of silver. Here is the chemical equation for the rusting of iron: Fe + O 2 + H 2 O → Fe 2 O 3.
Extinguish flames with carbon dioxide. This is a fiery twist on acid-base experiments. Light a candle and talk about what fire needs in order to survive. Then, create an acid-base reaction and "pour" the carbon dioxide to extinguish the flame. The CO2 gas acts like a liquid, suffocating the fire.
Chemicals make our clothes clean. We use chemicals when washing utensils. —swetha. Fertilizers are one of the best examples of chemistry in everyday life. —savita. Food is all about chemistry. The ingredients are chemicals. Cooking is a set of chemical reactions. Even the spoon you use is a chemical. —Satya ranjan jena.
Then add a good layer of oil on top. About an inch deep is plenty. Then you use your dropper to drip food coloring into the jar. At first, the food coloring will sit at the interface between the oil and water. Then all of a sudden, it will drop through, leaving a trail of color behind it in the water.
Chemistry@Home describes and demonstrates simple and complex chemistry experiments that can be completed by kids and adults using only items that can be found in your home. It also highlights where elements of the periodic table can be found in their home.
Chemistry Projects for Kids. The following chemistry projects for kids are sorted by topic: Chemical Reactions, Acids and Bases, Carbon Reactions, Chromatography, Colloids & Solutions, Polymers, and Crystals. Please note that many if these projects could fit in two or more categories in this post as they demonstrate various scientific and ...
Chemistry at Home. The ACS is making educational resources available by topic to aid parents and teachers in student enrichment during this time of distance learning. We have bundled materials from the Reactions Video series, C&EN, our magazines within the Education Division and our portfolio of hands-on activities for students in grades K-12 ...
Continue reading. The Homeschool Scientist. 8. Experiment with Yeast and Sugar - Making Peeps Blow Up a Balloon - Includes a free printable pack. Peeps make for interesting chemistry experiments. Whether you use the Peep snowmen or Peep chicks, you can make them blow up a balloon! Includes a free printable pack.
3. Consider whether your new technology requires the usage of 'harmful chemicals'. If you can identify that it does not, then it is beneficial for the market and society at large, as we move ...
43. "Flip" a drawing with water. Light refraction causes some really cool effects, and there are multiple easy science experiments you can do with it. This one uses refraction to "flip" a drawing; you can also try the famous "disappearing penny" trick. Learn more: Light Refraction With Water.
Make a Hygrometer to Measure Humidity - STEM activity. Design and Make Automata. Uncover the inner workings of reactions, mixtures, and chemical phenomena through exciting experiments. Explore classic and cutting-edge high school science experiments in this collection of top-quality science investigations.
Chemical Reactions. A chemical reaction is a process where two or more substances react together to form a new chemical substance. This might look like a gas formed, cooking or baking, milk souring, etc. Sometimes a physical change occurs, like our popcorn experiment or melting crayons, rather than a chemical change.However, these experiments below are all great examples of chemical change ...
These experiments are great for older children, or with assistance from mum or dad. They can be done at home with ingredients you already have on hand. So pick an experiment, and grab your lab coats to get started! 1. Cabbage chemistry. 2. Lolly fountain. 3. Bath bombs.
Chemistry is the study of the composition, classification, and change of substances. Therefore, a long essay in chemistry should include chemical principles and theory and underline the core character of chemistry, which is the study of matter and its transformations. 2. Pick a Topic for Which You Can Provide a Chemistry-based Approach.
Chemistry is the branch of science that deals with the identification of the substances of which matter is composed. Also the investigation of their properties and the ways in which they interact, combine and change. Matter is based on the physical and the chemical structure and matter is made up of atoms. There are three states of matter and ...
Chemistry - Free Essay Examples and Topic Ideas. Chemistry is a natural science that deals with the composition and behavior of matter. It focuses on the study of atoms, molecules, and their interactions with one another. Chemistry is an essential field of science that advances our understanding of the world around us and enables us to develop ...
Coating metal structures with a protective material is a popular strategy to prevent their deterioration by corrosion. However, maintaining the barrier properties of coatings after their mechanical damage is challenging. Herein, we prepare multifunctional coatings with a self-healing ability so that anticorr Journal of Materials Chemistry B HOT Papers
Chemistry is super important for keeping us healthy. Medicines are chemicals that can fix or prevent health problems. For example, if you have a headache, you might take a painkiller. This medicine is made from chemicals that stop the pain signals in your body.
Jen also described herself as a "terrible auditioner," noting that her nerves would make chemistry tests all the more difficult. "So, if you're a nervous auditioner, to begin with, to then say, 'Now let's have you make out with a complete stranger,' it's very uncomfortable," she told her fellow actors. "Put some music on or ...
Microwave absorbing materials applied in ocean environments should not only hold a strong wave absorption performance but also be capable of resisting corrosion in seawater. Here, a polypyrrole@NiFe-layered double hydroxide (PPy@NiFe-LDH) composite was fabricated an electrodeposition method and tried to use Journal of Materials Chemistry C HOT Papers
To order MEL Science Kits and get started with teaching chemistry to your kids at home, follow these simple steps: 1. Visit the MEL Science website HERE. 2. Browse the available subject matters ...
The controversy about the decision to fly an upside-down American flag outside the home of Justice Samuel Alito recalls St. Paul's admonition that while some things may be lawful, "not all ...
Hard carbons hold great promise as the anode materials for sodium-ion batteries (SIBs), but the limited capacities and sluggish Na+ transfer kinetics still hinder their practical applications. These issues can be addressed from the perspective of improving the wettability of hard carbons with electrolyte. Herein, w Journal of Materials Chemistry A HOT Papers Design and characterization of ...