Studies Confirm COVID-19 mRNA Vaccines Safe, Effective for Pregnant Women
Posted on June 1st, 2021 by Dr. Francis Collins
Clinical trials have shown that COVID-19 vaccines are remarkably effective in protecting those age 12 and up against infection by the coronavirus SARS-CoV-2. The expectation was that they would work just as well to protect pregnant women. But because pregnant women were excluded from the initial clinical trials, hard data on their safety and efficacy in this important group has been limited.
So, I’m pleased to report results from two new studies showing that the two COVID-19 mRNA vaccines now available in the United States appear to be completely safe for pregnant women. The women had good responses to the vaccines, producing needed levels of neutralizing antibodies and immune cells known as memory T cells, which may offer more lasting protection . The research also indicates that the vaccines might offer protection to infants born to vaccinated mothers.
In one study, published in JAMA [1], an NIH-supported team led by Dan Barouch, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, wanted to learn whether vaccines would protect mother and baby. To find out, they enrolled 103 women, aged 18 to 45, who chose to get either the Pfizer/BioNTech or Moderna mRNA vaccines from December 2020 through March 2021.
The sample included 30 pregnant women,16 women who were breastfeeding, and 57 women who were neither pregnant nor breastfeeding. Pregnant women in the study got their first dose of vaccine during any trimester, although most got their shots in the second or third trimester. Overall, the vaccine was well tolerated, although some women in each group developed a transient fever after the second vaccine dose, a common side effect in all groups that have been studied.
After vaccination, women in all groups produced antibodies against SARS-CoV-2. Importantly, those antibodies neutralized SARS-CoV-2 variants of concern . The researchers also found those antibodies in infant cord blood and breast milk, suggesting that they were passed on to afford some protection to infants early in life.
The other NIH-supported study, published in the journal Obstetrics & Gynecology , was conducted by a team led by Jeffery Goldstein, Northwestern’s Feinberg School of Medicine, Chicago [2]. To explore any possible safety concerns for pregnant women, the team took a first look for any negative effects of vaccination on the placenta, the vital organ that sustains the fetus during gestation.
The researchers detected no signs that the vaccines led to any unexpected damage to the placenta in this study, which included 84 women who received COVID-19 mRNA vaccines during pregnancy, most in the third trimester. As in the other study, the team found that vaccinated pregnant women showed a robust response to the vaccine, producing needed levels of neutralizing antibodies.
Overall, both studies show that COVID-19 mRNA vaccines are safe and effective in pregnancy, with the potential to benefit both mother and baby. Pregnant women also are more likely than women who aren’t pregnant to become severely ill should they become infected with this devastating coronavirus [3]. While pregnant women are urged to consult with their obstetrician about vaccination, growing evidence suggests that the best way for women during pregnancy or while breastfeeding to protect themselves and their families against COVID-19 is to roll up their sleeves and get either one of the mRNA vaccines now authorized for emergency use.
References :
[1] Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women . Collier AY, McMahan K, Yu J, Tostanoski LH, Aguayo R, Ansel J, Chandrashekar A, Patel S, Apraku Bondzie E, Sellers D, Barrett J, Sanborn O, Wan H, Chang A, Anioke T, Nkolola J, Bradshaw C, Jacob-Dolan C, Feldman J, Gebre M, Borducchi EN, Liu J, Schmidt AG, Suscovich T, Linde C, Alter G, Hacker MR, Barouch DH. JAMA. 2021 May 13.
[2] Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: Measures of immunity and placental histopathology . Shanes ED, Otero S, Mithal LB, Mupanomunda CA, Miller ES, Goldstein JA. Obstet Gynecol. 2021 May 11.
[3] COVID-19 vaccines while pregnant or breastfeeding . Centers for Disease Control and Prevention.
COVID-19 Research (NIH)
Barouch Laboratory (Beth Israel Deaconess Medical Center and Harvard Medical School, Boston)
Jeffery Goldstein (Northwestern University Feinberg School of Medicine, Chicago)
NIH Support: National Institute of Allergy and Infectious Diseases; National Cancer Institute, National Institute of Child Health and Human Development; National Center for Advancing Translational Sciences; National Institute of Biomedical Imaging and Bioengineering

Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on X (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: News
Tags: breast milk , breastfeeding , cord blood , COVID-19 , COVID-19 vaccine , gynecology , infants , Moderna vaccine , mRNA vaccine , neutralizing antibodies , obstetrics , pandemic , Pfizer/BioNTech vaccine , placenta , pregnancy , pregnancy complications , SARS-CoV-19 variants , SARS-CoV-2 , T cells , women's health
27 Comments
How could they know that? There haven’t been any long-term studies and VAERS is backlogged with reports of adverse reactions. Slow down and focus on the elderly in our country and other countries.
Yes, effective in 12 year olds and older but myocarditis and pericarditis are being investigated currently with this age group. Statistically zero hospitalization and fatality rate for this age group. Risk-Benefit?
I’m an elderly person. Was vaccinated two months ago. My doctor ordered many routine blood test last week for my autoimmune conditions, including an antibody test, to see if I got enough protection and I don’t have any changes. No side effects. Feel great.
I agree! They should end public phase III trials, wait and sit back and really analyze the saers data and wait for the many independent studies to finalize.. Covid curve is down and this is pretty much aftermath. To many professionals warning against theae injections and we don’t know any real life long term effects either.
Go read the study. It explained how that came to believe this.
Agree 100%. They indicate it’s safe for the mother because there was “no damage to the placenta”, but how can they possibly know what the long-term effects will be on either the mother or the baby???
As for the article’s author, ” appear to be completely safe for pregnant women” – remind yourself to do that statistics refresher course.
As a layman American I want to thank everyone for all they did to fight the epidemic, BUT, I have to think – realizing hindsight is 20/20, but still – IF we had, after the first mRNA vaccines were prepared, given the makers of them a green light to vaccinate people at high risk of severe Covid, or if they were unwilling, FORCED them to make the vaccines in large quantities, to be bought by the government, then administered to high risk people on a voluntary basis, we might have kept serious illness way down. If this had been started in March and April, when the first testing of the two mRNA vaccines we are using now began, how many people could have been vaccinated before the midsummer surge, let alone the holiday peaks in states previously not much effected? I am going to say something I know will offend many medical people – I do not feel we learned from this epidemic – not very much – if we had another epidemic, and a vaccine was developed quickly, I believe it would be withheld, for, operationally, about a year, while the epidemic destroyed the country. The reasons given for withholding the vaccine will be, essentially, we’ve always done it this way and we can’t change that just because we have a once in a century pandemic. In the circumstances we faced – not solely the virus, which was very bad, but ultimately, shown to be mostly a threat to old folks, – but the REACTION to the virus, which was extremely destructive, generally – EVERY possible strategy should have been on the table. I see a massive failure I am very angry about, but worse, I think our enemies have seen the failures, and now know one more epidemic like this will destroy the US. We need to have a better plan for a future epidemic and I see no sign we will.
These studies do not address safety, they address effectiveness in pregnant and breast feeding women. The study from Chicago looked at placentas and at antibody levels. The authors assume the lack of placental abnormalities equals safety in the 33 (30% of the 80 enrolled) women whose placentas were examined. The study suffers from being underpowered and the populations were not controlled for. The JAMA paper enrolled a total of 103 women, 30 were vaccinated while pregnant. The results were entirely on immunogenicity with only a mention of “no severe adverse events or pregnancy or neonatal complications were observed.” An n of 30 is not an adequate study to infer anything. This study is way underpowered as well and too small to find any majority much less uncommon adverse events. For those who like to say they follow the science you need to read the papers Blogs like this one reference. There is virtually no meaningful safety data in either of these studies, despite what the blog states. Both studies do, however, give us effectiveness data.
Thank you for your call out on the lack of clarity on this study. I am a woman of child bearing age with uncertainty if I will choose to have another child or not. At this point, I want the raw data from the studies – vaccination prior to pregnancy and during and the outcomes of the vaccinated individuals and their offspring. Not the findings of the data as they’ve been extrapolated by an individual or a group of medical professionals with good intentions but an unconscious bias. I asked my OB for a place where I could start to collect the data as it comes in (given the fact that we are just beginning to see women who may have received the vaccine prior to pregnancy and the outcomes of the pregnancy) and she could not point me to any single data source. She could only “reassure” me that ACOG promotes the vaccine. Again, I don’t need an endorsement by well intended medical professionals. I need the data so I can make an informed, educated decision on my own.
E x a c t l y ‼️
What are your qualifications to evaluate this study?
She sounds pretty knowledgeable to me, but…
If you think she’s wrong, you should have no trouble pointing out why from the papers themselves, rather than questioning her qualifications, a far weaker argument.
There are many, many more pregnant women who have taken the vaccine without being part of a research study. Myself included. If just one had adverse effects so far, it would have made international news. Even if it wasn’t caused by the vaccine. Pregnant women are at increased risk of hospitalization/death from the virus and the general public is at risk of the formation of vaccine-resistant variants popping up due to unvaccinated people. It was a great relief to me to know I wouldn’t play a part in anyone else getting sick. And that my healthy and happy newborn is likely to have immunity. When the vaccines are approved for younger children, I will happily have my 3-1/2 year-old vaccinated!
“If just one had adverse effects so far, it would have made international news. Even if it wasn’t caused by the vaccine.” Are you sure about that…. since our news sources are so clearly biased in encouraging ‘the jab’. It must be easy for others to say “pregnant women should get the vaccine” when they are in fact not pregnant and faced with that difficult decision. There are soo many things pregnant women should not consume or inject in their bodies while pregnant, but others are quick to say a 1 year old vaccine that nobody really knows the long term effects of, is okay for pregnant women. 1 year ago did they predict a booster shot would be needed? No. I rest my case.
When gets the vaccine to the public?
Thank you very much for this very important post.
The value of trust in research studies and their publication. And summary write ups….is vitally important. When a write up over simplifies. Or let’s a casual reader draw am incorrect safety if efficacy conclusion. Beware. As trust. Is vital if this part of our county and world culture wants an vital and happily invited family member at our future crisis and holiday and every dinner table. Break trust. And the family member will be sat in the corner with the face to the wall. Trust and good data transparency and clear not deceptive summaries are vital. The choice to keep this family member at our dinner table. Or not. Is being asked by too many who see to many reasons not to trust. I think it is a vital member. But like a five year old. Needs to know the value of truth.
Spanish flu was a vaccine 60,000,000 dead. 1917 WW1
The first study only looked at antibody response, it was not a safety study. Why are you even mentioning it here? The second study only ensured that the vaccine didn’t cause an immune response to the placenta? That is important, but by itself is a very weak and limited outcome measure. They did not even follow through to see if there was any injury to the newborns. And really, it will take a few years before more subtle neurological damage would even be noticeable. And the sample size is tiny.
These are the studies on which the NIH is basing the claim that the vaccine does not cause reproductive harm? This is shocking and disgraceful. We need accountability.
We should just wait then, until we absolutely KNOW that everyone is SAFE from the vaccine. We can do a longitudinal study of say, 25 years? At that point, many of the infants exposed to the vaccine will be adults and you’ll have you definitive data. If everything looks good at that point, then you can get the vaccine for a virus that is 25 years old and likely doesn’t even resemble the original virus that caused the disease in the first place. Leave the vaccine out of it entirely until we KNOW for CERTAIN beyond ALL doubt that this thing is safe and take your chances on contracting the disease itself. I am glad my parents didn’t wait for the longitudinal Polio study to conclude, but, that being said, this sounds like a solid risk assessment strategy to me.
These studies are weak. Far too small and have not looked at the babies. If this is what we are basing the “safe and effective” mantra on then we are truly lost. Amazing double standard when compared with the kind of evidence they are demanding for early treatments such as ivermectin or fluvoxamine. Hard not to be a conspiracy theorist.
n June 25, the FDA added new warnings for both vaccine provider and recipients to their fact sheets on Pfizer and Moderna over rare cases of heart inflammation called myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart). The agency added the warnings just two days after the CDC’s Advisory Committee on Immunization Practices (ACIP) met and confirmed the “likely association” between myocarditis and pericarditis and the Pfizer and Moderna vaccines, both of which use mRNA. However, it’s rare, treatable, and usually mild, they say.
Looks like some very helpful information! I read the entire article, and I love the writing style and passion in your posts . . .
I received the first dose of fed Pfizer vaccine when I was only a few weeks pregnant. (I didn’t know that I was pregnant at the time.) I received the second dose the end of January. I carried my baby to term, and I’m happy to say I have a very healthy, happy baby!! I had zero complications during pregnancy and in labor.
Thanks for sharing, good luck with your newborn.
Thank you for sharing such an informative blog with us. I found this very useful for everyone . . .
Leave a Comment Cancel reply
@nihdirector on x, nih on social media.
Kendall Morgan, Ph.D.
Comments and Questions
If you have comments or questions not related to the current discussions, please direct them to Ask NIH .
You are encouraged to share your thoughts and ideas. Please review the NIH Comments Policy

- Visitor Information
- Privacy Notice
- Accessibility
- No Fear Act
- HHS Vulnerability Disclosure
- U.S. Department of Health and Human Services
- USA.gov – Government Made Easy
Discover more from NIH Director's Blog
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
COVID-19 Vaccines While Pregnant or Breastfeeding
- Everyone ages 6 months and older is recommended to get the updated COVID-19 vaccine, including people who are pregnant, breastfeeding a baby, trying to get pregnant now, or who might become pregnant in the future.
- COVID-19 vaccination during pregnancy is safe and effective.
- COVID-19 vaccines are not associated with fertility problems in women or men.
- Infants ages 6 months and older are recommended to get the updated COVID-19 vaccine even if born to people who were vaccinated or had COVID-19 before or during pregnancy.
- If you are pregnant or were recently pregnant, you are more likely to get very sick from COVID-19, compared to those who are not pregnant. Additionally, if you have COVID-19 during pregnancy, you are at increased risk of complications that can affect your pregnancy and your baby from serious illness from COVID-19.
People Who Are Pregnant
Safety and effectiveness of covid-19 vaccination during pregnancy, common questions about vaccination during pregnancy, people who are breastfeeding a baby, people who would like to have a baby, vaccine side effects.

If you are pregnant or were recently pregnant, you are:
- More likely to get very sick from COVID-19 compared to those who are not pregnant.
- More likely to need hospitalization, intensive care, or the use of a ventilator or special equipment to breathe if you do get sick from COVID-19. Severe COVID-19 illness can lead to death.
- At increased risk of complications that can affect your pregnancy and baby including, preterm birth or stillbirth.
COVID-19 vaccination remains the best protection against COVID-19-related hospitalization and death for you and your baby . CDC recommendations align with those from professional medical organizations including the American College of Obstetricians and Gynecologists , Society for Maternal Fetal Medicine, and American Society for Reproductive Medicine .

Studies including hundreds of thousands of people around the world show that COVID-19 vaccination before and during pregnancy is safe, effective, and beneficial to both the pregnant person and the baby. The benefits of receiving a COVID-19 vaccine outweigh any potential risks of vaccination during pregnancy. Data show:
- COVID-19 vaccines do not cause COVID-19, including in people who are pregnant or their babies. None of the COVID-19 vaccines contain live virus. They cannot make anyone sick with COVID-19, including people who are pregnant or their babies. Learn more about how vaccines work .
- It is safe to receive an mRNA COVID-19 vaccine (Moderna or Pfizer-BioNTech), before and during pregnancy. Both vaccines show no increased risk for complications like miscarriage, preterm delivery, stillbirth, or birth defects . 1,2
- mRNA COVID-19 vaccines during pregnancy are effective. They reduce the risk of severe illness and other health effects from COVID-19 for people who are pregnant. COVID-19 vaccination might help prevent stillbirths and preterm delivery. 1-4
- COVID-19 vaccination during pregnancy builds antibodies that can help protect the baby. 4,5
- Receiving mRNA COVID-19 vaccines during pregnancy can help protect babies younger than age 6 months from hospitalization due to COVID-19.
- Most babies hospitalized with COVID-19 were born to pregnant people who were not vaccinated during pregnancy. 6-8
Scientific studies to date have shown no safety concerns for babies born to people who were vaccinated against COVID-19 during pregnancy. Based on how these vaccines work in the body, experts believe they are unlikely to pose a risk for long-term health effects. CDC continues to monitor, analyze, and disseminate information from people vaccinated during all trimesters of pregnancy to better understand effects on pregnancy and babies.
CDC and professional medical organizations, including the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, recommend COVID-19 vaccination at any point in pregnancy . COVID-19 vaccination can protect you from getting very sick from COVID-19. Keeping yourself as healthy as possible during pregnancy is important for the health of your baby.
Pregnant people can choose which updated COVID-19 vaccine to get.
Children, teens, and adults, including pregnant people, may get a COVID-19 vaccine and other vaccines, including a flu vaccine, at the same time.
If you would like to speak to someone about the COVID-19 vaccination during pregnancy, you can talk to your healthcare provider. You can also contact MotherToBaby, whose experts are available to answer questions in English or Spanish by phone or chat. This service is free and confidential. To reach MotherToBaby:
- Call 1-866-626-6847
- Text 855-999-3525
- Chat Click the MotherToBaby Live Chat window
CDC recommends that people who are breastfeeding a baby, and infants 6 months of age and older, get vaccinated and stay up to date with their COVID-19 vaccines .
Vaccines are safe and effective at preventing COVID-19 in people who are breastfeeding a baby. Available data on the safety of COVID-19 vaccination while breastfeeding indicate no severe reactions after vaccination in the breastfeeding person or the breastfed child. 9 There has been no evidence to suggest that COVID-19 vaccines are harmful to either people who have received a vaccine and are breastfeeding or to their babies. 10
Studies have shown that people who are breastfeeding a baby and have received mRNA COVID-19 vaccines have antibodies in their breast milk, which could help protect their babies. 9,10
CDC also recommends COVID-19 vaccines for children aged 6 months and older .
CDC recommends that people who are trying to get pregnant now or might become pregnant in the future , as well as their partners, stay up to date and get the updated COVID-19 vaccine. COVID-19 vaccines are not associated with fertility problems in women or men. COVID-19 vaccines are not associated with fertility problems in women or men .
People who are pregnant have not reported different side effects from people who are not pregnant after vaccination with mRNA COVID-19 vaccines (Moderna and Pfizer-BioNTech vaccines). 1,2
- Fever during pregnancy, for any reason, has been associated with adverse pregnancy outcomes.
- Fever in pregnancy may be treated with acetaminophen as needed, in moderation, and in consultation with a healthcare provider.
- Learn more about possible side effects and rare severe allergic reactions after receiving a COVID-19 vaccine.
To find COVID-19 vaccine locations near you: Search vaccines.gov , text your ZIP code to 438829, or call 1-800-232-0233.
Related Pages
- Allergic Reactions
For Healthcare and Public Health
- Considerations for the Use of COVID-19 Vaccines in the United States
- COVID-19 Vaccination among Pregnant People
- Management of Anaphylaxis after COVID-19 Vaccination
- ACOG Vaccine Confidence Training
- ACOG Recommendations for Vaccinating Pregnant People
- ACOG video about COVID-19 vaccines for people who are pregnant
- COVID-19 Clinical and Professional Resources
- Clinic Poster: Protect yourself and your baby from COVID-19
- Clinic Poster: Protect yourself and your baby from COVID-19 (Español)
More Information
- Mother-To-Baby: Information for people who are pregnant or breastfeeding a baby
- Pregnant and Protected from COVID-19 | CDC Foundation
- Fleming-Dutra KE, Zauche LH, Roper LE, Ellington SR, Olson CK, Sharma AJ, Woodworth KR, Tepper N, Havers F, Oliver SE, Twentyman E, Jatlaoui TC. Safety and Effectiveness of Maternal COVID-19 Vaccines Among Pregnant People and Infants. Obstet Gynecol Clin North Am. 2023 Jun;50(2):279-297. https://www.doi.org/10.1016/j.ogc.2023.02.003
- Prasad, S., Kalafat, E., Blakeway, H. et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun 13, 2414 (2022). https://doi.org/10.1038/s41467-022-30052-w
- Schrag SJ, Verani JR, Dixon BE, et al. Estimation of COVID-19 mRNA Vaccine Effectiveness Against Medically Attended COVID-19 in Pregnancy During Periods of Delta and Omicron Variant Predominance in the United States. JAMA Netw Open. 2022;5(9):e2233273. doi:10.1001/jamanetworkopen.2022.33273
- Piekos SN, Price ND, Hood L, Hadlock JJ. The impact of maternal SARS-CoV-2 infection and COVID-19 vaccination on maternal-fetal outcomes. Reprod Toxicol. 2022;114:33-43. doi:10.1016/j.reprotox.2022.10.003
- Yang YJ, Murphy EA, Singh S, et al. Association of Gestational Age at Coronavirus Disease 2019 (COVID-19) Vaccination, History of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, and a Vaccine Booster Dose With Maternal and Umbilical Cord Antibody Levels at Delivery. Obstetrics & Gynecology: 2021. DOI: https://doi.org/10.1097/AOG.0000000000004693
- Halasa NB, Olson SM, Staat MA, et al. Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. N Engl J Med. 2022;387(2):109-119. doi:10.1056/NEJMoa2204399
- Hamid S, Woodworth K, Pham H, et al. COVID-19–Associated Hospitalizations Among U.S. Infants Aged <6 Months — COVID-NET, 13 States, June 2021–August 2022. MMWR Morb Mortal Wkly Rep 2022;71:1442–1448. DOI: http://dx.doi.org/10.15585/mmwr.mm7145a3
- Simeone RM, Zambrano LD, Halasa NB, et al. Effectiveness of Maternal mRNA COVID-19 Vaccination During Pregnancy Against COVID-19–Associated Hospitalizations in Infants Aged <6 Months During SARS-CoV-2 Omicron Predominance — 20 States, March 9, 2022–May 31, 2023. MMWR Morb Mortal Wkly Rep 2023;72:1057–1064. DOI: http://dx.doi.org/10.15585/mmwr.mm7239a3
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 18 March 2022
SARS-CoV-2 infection and COVID-19 vaccination in pregnancy
- Victoria Male ORCID: orcid.org/0000-0001-5654-5083 1
Nature Reviews Immunology volume 22 , pages 277–282 ( 2022 ) Cite this article
31k Accesses
95 Citations
1334 Altmetric
Metrics details
- Reproductive biology
SARS-CoV-2 infection poses increased risks of poor outcomes during pregnancy, including preterm birth and stillbirth. There is also developing concern over the effects of SARS-CoV-2 infection on the placenta, and these effects seem to vary between different viral variants. Despite these risks, many pregnant individuals have been reluctant to be vaccinated against the virus owing to safety concerns. We now have extensive data confirming the safety and effectiveness of COVID-19 vaccination during pregnancy, although it will also be necessary to determine the effectiveness of these vaccines specifically against newly emerging viral variants, including Omicron. In this Progress article, I cover recent developments in our understanding of the risks of SARS-CoV-2 infection in pregnancy, and how vaccination can reduce these.
Similar content being viewed by others

Proactive vaccination using multiviral Quartet Nanocages to elicit broad anti-coronavirus responses

Long COVID: major findings, mechanisms and recommendations

Imprinting of serum neutralizing antibodies by Wuhan-1 mRNA vaccines
Introduction.
Viruses that cause pneumonia, including severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), have long been known to be of particular concern during pregnancy 1 . So as the world entered the SARS-CoV-2 pandemic in early 2020, clinicians and scientists working in obstetrics knew that their patients were likely to be at increased risk. Initially, lockdowns and a tendency towards risk avoidance masked some of the increased risks associated with SARS-CoV-2 infection in pregnancy 2 , 3 , but with the passing of time the risks have become clearer. Although pregnant people were excluded from the first trials of COVID-19 vaccines, the pressing need to protect this group meant that the vaccines were rolled out to them in advance of the completion of clinical trials, and we now have extensive real-world data confirming the safety and effectiveness of the vaccines during pregnancy. In this Progress article, I cover recent developments in our understanding of the risks of SARS-CoV-2 infection that are specific to pregnancy, and how vaccination can safely reduce these.
SARS-CoV-2 infection in pregnancy
Obstetric outcomes following sars-cov-2 infection.
Pregnancy is associated with increased disease severity in those infected with SARS-CoV-2: a meta-analysis of 92 studies comparing outcomes for pregnant patients with COVID-19 with age and sex-matched non-pregnant patients with COVID-19 found that pregnancy increases the risk of needing intensive care (OR 2.13, 95% confidence interval (CI) 1.54–2.95), invasive ventilation (OR 2.59, CI 2.28–2.94) and extracorporeal membrane oxygenation (OR 2.02, CI 1.22–3.34), although the risk of all-cause mortality was not increased (OR 0.96, CI 0.79–1.18) 4 . A more recent meta-analysis of 111 studies, which compared outcomes for pregnant patients infected with SARS-CoV-2 with those who were not infected, found that infection significantly increased the odds of premature delivery (OR 1.48, 95% CI 1.22–1.8), pre-eclampsia (OR 1.6, CI 1.2–2.1), stillbirth (OR 2.36, CI 1.24–4.46), neonatal mortality (OR 3.35, CI 1.07–10.5) and maternal mortality (OR 3.08, CI 1.5–6.3) 5 .
Since the publication of these meta-analyses, further large studies have also found increased risks of maternal morbidity and mortality 6 , preterm birth (PTB) and perinatal death associated with SARS-CoV-2 infection in pregnancy 7 , 8 . There is also evidence that both maternal 9 and neonatal 10 outcomes were worse during the Delta wave of the SARS-CoV-2 pandemic than in preceding periods.
The increased risk of PTB associated with SARS-CoV-2 infection seems to be driven largely by iatrogenic PTBs, with doctors opting to deliver the infant to try to save the critically ill patient 11 . The increased risk of stillbirth and pre-eclampsia are more likely to be associated with inflammatory changes affecting the placenta (Fig. 1 ).
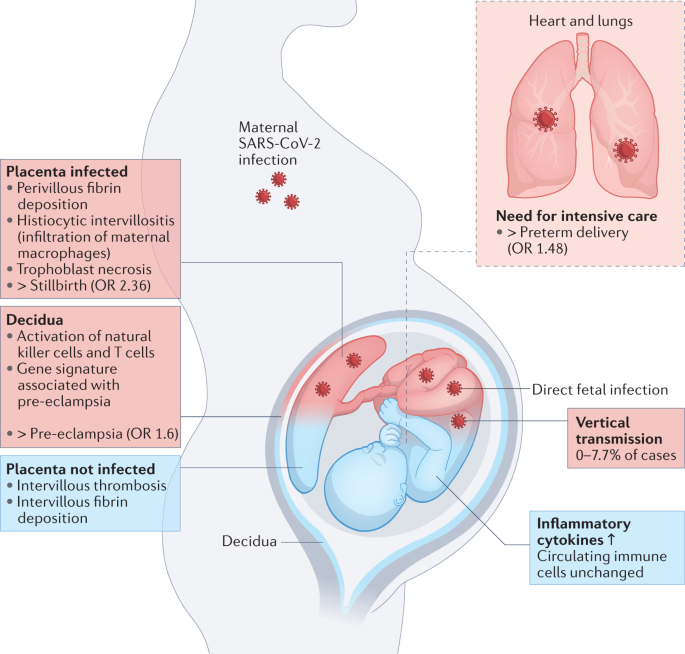
Maternal SARS-CoV-2 infection can impact pregnancy in numerous ways. The need for intensive care associated with severe disease can necessitate delivering the infant, causing an increased rate of preterm delivery. Placental infection can be associated with SARS-CoV-2 placentitis, which is associated with an increased risk of stillbirth. Even in the absence of placental infection, inflammatory changes are observed in the decidua and placenta, and these may be linked to the increased risk of pre-eclampsia associated with SARS-CoV-2 infection in pregnancy. SARS-CoV-2 can also be vertically transmitted to infect the fetus, although this is uncommon. Blue indicates indirect outcomes on the fetus and placenta associated with maternal infection with SARS-CoV-2, whereas red indicates outcomes associated with direct fetal infection.
SARS-CoV-2 and the placenta
The placenta expresses the cellular receptors for SARS-CoV-2, namely angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) 12 , 13 , 14 , 15 , and some patients with COVID-19 do become viraemic 16 , meaning there is the potential for SARS-CoV-2 infection of the placenta. However, SARS-CoV-2 viraemia in pregnancy seems to be uncommon 12 , and there is little placental co-expression of ACE2 and TMPRSS2, which is required for the canonical route of virus entry into the cell 12 , 13 , 14 . Moreover, placental expression of ACE2 declines over the course of pregnancy 15 .
In addition to the general defences the placenta has against viral infection 17 , these factors might be expected to protect the placenta from infection with SARS-CoV-2. Indeed, placental infection seems to be uncommon. However, SARS-CoV-2-associated coagulation and inflammation occur even in the absence of placental infection, most commonly manifesting as intervillous thrombosis and fibrin deposition 12 , 15 , 18 , 19 , 20 . The mucosal lining of the uterus is a maternal tissue into which the placenta implants and, in pregnancy, is called the decidua. Examination of the decidua in pregnancies affected by SARS-CoV-2 demonstrated local activation of maternal natural killer cells and T cells, including the expression of gene signatures associated with pre-eclampsia 15 , 21 .
A more severe inflammatory syndrome occurs when the placenta does become infected; namely, SARS-CoV-2 placentitis. This is characterized by histiocytic intervillositis, perivillous fibrin deposition and trophoblast necrosis, and is emerging as a risk factor for fetal distress or demise 22 , 23 , 24 , 25 , 26 . A series of 68 cases of SARS-CoV-2 placentitis associated with either stillbirth or neonatal death found that the causes of death were likely to be fetal hypoxic-ischaemic injury resulting from severe placental damage, rather than fetal infection with SARS-CoV-2. Indeed, placental infection with SARS-CoV-2 does not necessarily equate with fetal infection; in this case series, infection of the fetus was only confirmed in 2 of the 68 cases 26 .
SARS-CoV-2 and the fetus
Numerous studies have reported SARS-CoV-2 infection in infants born to infected individuals. The largest of these have examined infection by nasopharyngeal swab, finding the rate at which infants test positive for SARS-CoV-2 as between 0.9 and 2.8% 27 , 28 , 29 , 30 . However, infants who test positive in this way have not necessarily been infected in utero, as they may have been infected by horizontal transmission shortly after birth.
Numerous smaller studies have examined umbilical cord blood to more accurately identify those neonates infected by vertical transmission. Although the fetus begins producing both IgG and IgM between 12 and 20 weeks of gestation, maternal IgG can cross the placenta so only the presence of IgM signals fetal exposure to antigen. In pregnancies affected by SARS-CoV-2 infection, detection of Spike-specific IgM in cord blood has been reported in between 0 and 7.7% of cases 21 , 27 , 31 . A systematic review of studies examining the presence of the viral genome in cord blood found it in 2.9% of cases 27 , although since then a larger case series of 64 deliveries was unable to detect the viral genome in the umbilical cord blood of any infant 12 .
Increased levels of inflammatory cytokines have been observed in the cord blood of neonates, even in the absence of placental infection 21 , 32 . It is unclear whether these cytokines were produced locally by the fetus or reflect maternal cytokines that have crossed the placenta 33 . However, the findings that immune cells in cord blood show higher cytokine production if the pregnancy was affected by SARS-CoV-2 infection and that IL-8 concentrations are generally higher in cord blood than that in maternal blood suggest that at least some of these cytokines may be produced by the neonate 32 .
New variants, new outcomes?
One important caveat to much of the preceding data is that they were collected in earlier waves of the pandemic, in which the predominant variants of SARS-CoV-2 were different from those we face now. Of particular concern is that reports of SARS-CoV-2 placentitis were rare in the first wave, caused by the original strain of SARS-CoV-2, but became increasingly common in the Alpha and Delta variant waves of the pandemic 24 , 25 . This demonstrates that we cannot necessarily assume that obstetric outcomes will be the same in the current wave, or in future ones, as they have been previously. At the time of writing, we do not have solid data on how the Omicron wave has affected pregnant people.
Vaccine safety in pregnancy
Benefits of vaccination in pregnancy.
Vaccination in pregnancy to prevent maternal morbidity and mortality, or to confer passive immunity to the infant, has a long and successful history 34 . As early as 1879, it was noticed that infants born to individuals who received the smallpox vaccine during pregnancy were themselves protected, and similar observations were made for pertussis and tetanus vaccination in the middle of the twentieth century. Similar to SARS-CoV-2 infection, infection with influenza virus in pregnancy is associated with increased maternal morbidity and, as a result, influenza vaccination in pregnancy has been recommended in the United States since 1997, although it was not until 2005 that clinical trials formally demonstrated its benefits. In the United Kingdom, influenza and pertussis vaccination have been routinely offered in pregnancy since 2010 and 2012, respectively.
Safety of COVID-19 vaccination in pregnancy
The increased potential for severe consequences following SARS-CoV-2 infection in pregnancy makes COVID-19 vaccination of this population particularly attractive. However, pregnant patients naturally want to know whether vaccination is safe for them and their infants. Although we await clinical trial data in this population, pregnant people have been vaccinated against COVID-19 since December 2020, and we now have safety data from more than 185,000 individuals vaccinated during pregnancy (Table 1 ).
Because the first countries to offer the COVID-19 vaccine in pregnancy, namely the United States and Israel, were using the mRNA vaccines BNT162b2 (Pfizer) and mRNA-1273 (Moderna), the first data available were on these vaccines. As a result, when other countries later made the vaccines available in pregnancy, many preferentially offered mRNA vaccines to this group. Because of this, mRNA-based COVID-19 vaccines have been most widely used in pregnancy and, therefore, the majority of safety data come from these vaccines.
A key finding with regards to safety is that IgM is not detected in umbilical cord blood following vaccination in pregnancy 31 , 35 , 36 . This indicates that the vaccine has not elicited an immune response in the fetus, suggesting that it has not crossed the placental barrier. In line with this, one study that looked for SARS-CoV-2 Spike mRNA or protein in placenta and cord blood following vaccination was unable to detect it 36 . COVID-19 vaccination in pregnancy is also not associated with pathological changes to the placenta 37 . These findings indicate that a direct effect of vaccination on fetal development is unlikely. However, local and systemic immune reactions to COVID-19 vaccination do occur in pregnant people, at roughly the same rate at which they occur in the general population 38 , 39 , 40 , 41 . Therefore, it is important to consider the possibility that the immune response to COVID-19 vaccination could affect the placenta or fetus and undertake epidemiological studies to determine whether it could be associated with any poor obstetric outcome. Such studies have taken one of three broad approaches: registry studies, case–control studies and cohort studies (Table 1 ).
Registry studies
Registry studies recruit participants at the time of vaccination, determine the outcomes of their pregnancies and compare the rates at which adverse events occur in the registry population relative to those seen either in the general pregnant population or during pregnancy historically. The first such study used the v-safe pregnancy registry of the US Centers for Disease Control and Prevention (CDC). Among 713 people vaccinated in pregnancy who had given birth by 30 March 2021, the rates of adverse events were the same as have been reported historically 38 , and a follow-up study looking at 1,613 vaccinated people who had given birth by September 2021 continued to find a normal rate of adverse events 42 . Focusing only on those vaccinated before 20 weeks, there was also no increased risk of miscarriage following vaccination 43 .
The Better Outcomes Registry and Network (BORN) comprises 64,234 people vaccinated against COVID-19 during pregnancy in Ontario, Canada. Of the 31,343 individuals who had given birth by 31 October 2021, the rate of stillbirth, PTB or infants being born small for their gestational age was not increased, compared with either historical data or the background rate 44 . A study of 18,399 people vaccinated against COVID-19 during pregnancy in Scotland found no increased risk of PTB or neonatal mortality, compared with the general pregnant population 8 . An early registry study in Israel examined only 390 people vaccinated with BNT162b2 during pregnancy, but also found no increased risk of miscarriage, PTB or infants being born small for their gestational age 41 .
Case–control studies
Case–control studies identify individuals who experience a predefined adverse event and determine whether those people are more likely to have experienced a particular exposure than those who did not experience the event. Two such studies have been done in the US Vaccine Safety Datalink system, which includes 31,080 people vaccinated during pregnancy. One of these studies found no indication that COVID-19 vaccination is linked to stillbirth 45 ; the second found that people who experienced a miscarriage were no more likely to have been vaccinated in the preceding 28 days than those who did not miscarry 46 . A similar study carried out in Norway found that, among 18,447 pregnancies, those that ended in miscarriage were no more likely to have received a COVID-19 vaccine in either the preceding 3-week or 5-week period than those that continued 47 .
Cohort studies
Cohort studies examine the outcomes for those who are vaccinated against COVID-19 in pregnancy, compared with the outcomes of a contemporary cohort of unvaccinated people. Because the participants in these studies have not been randomized to vaccination, there are systematic differences between those who chose to receive a COVID-19 vaccine and those who declined, although the majority of studies attempt to control for these variables, either with multivariate analysis 48 , 49 , 50 or by identifying pairs matched for potential confounders 51 , 52 . Among seven cohort studies there was no increased risk of miscarriage 52 , pre-eclampsia 49 , 52 , 53 or any adverse outcome at the time of birth 48 , 49 , 50 , 51 , 52 , 53 , 54 associated with COVID-19 vaccination in pregnancy. One study that followed up infants after birth, to an average of 134 days, also found no increased risk of death or hospitalization in the first months of life in infants born following vaccination during pregnancy 50 .
Although each of these approaches to addressing the question of COVID-19 vaccine safety in pregnancy has its own weakness, the findings from each approach lend weight to the others. Together with the sheer number of participants in these studies, this gives us confidence that COVID-19 vaccination is not associated with adverse pregnancy outcomes.
Vaccine efficacy
Effectiveness of covid-19 vaccination in pregnancy.
Although it is untrue to say that the immune system is weakened in pregnancy, it does differ from the non-pregnant state, with a shift away from cell-mediated immune responses. This is demonstrated by the long-standing observation that cell-mediated autoimmune diseases tend to go into remission during pregnancy, whereas antibody-mediated diseases can flare 55 . It is therefore not unreasonable to ask whether COVID-19 vaccination is as effective at preventing disease during pregnancy as it is in the broader population.
Early attempts to answer this question looked at vaccine immunogenicity in pregnant participants, compared with age and sex-matched non-pregnant controls. Two reports found that the titres of anti-Spike, anti-RBD (receptor binding domain of the Spike protein) and SARS-CoV-2 neutralizing antibodies were the same in the two groups 39 , 56 . Importantly, both of these studies found higher virus-specific antibody titres associated with COVID-19 vaccination compared with SARS-CoV-2 infection, underlining the benefits of vaccination even in those who have already been infected. A third study, using systems serology, also found that overall titres of antibodies did not differ between the groups but further revealed that after only one dose of vaccine, antibody effector functions were induced with delayed kinetics in the pregnant group compared with the non-pregnant group: following the second dose, there was no significant difference between the groups 57 . Comparing vaccine responses across trimesters using this approach, the same team also found a subtle reduction in antibody effector functions following vaccination in the second trimester, compared with vaccination in the first or third trimesters, which might reflect trimester-specific immune alterations that are known to be associated with a somewhat more quiescent state in the second trimester 58 . Comparing T cell responses, it was shown that Spike-induced production of IFNγ by total and central memory CD4 + and CD8 + T cells following vaccination did not differ between pregnant and non-pregnant groups 56 .
More recently, two cohort studies from Israel have enabled an estimate of COVID-19 vaccine effectiveness during pregnancy, finding it to be roughly the same as in the general population 52 , 59 . UK surveillance data have not been used to model vaccine effectiveness, but nevertheless point to COVID-19 vaccination being effective in pregnancy, particularly against severe disease 60 . In Scotland, 68% of the pregnant population was unvaccinated by October 2021, but unvaccinated individuals accounted for 77.4% of all SARS-CoV-2 infections, 90.9% of COVID-19 hospitalizations and 98% of intensive care unit admissions among pregnant people; furthermore, all perinatal deaths following SARS-CoV-2 infection in pregnancy occurred in unvaccinated individuals 8 . These data sets were collected largely during the period when the Alpha variant was dominant, but with some contribution from the Delta wave. As the Omicron variant becomes prominent, it will be important to determine the effectiveness of vaccination specifically against this strain, how this varies following a booster dose and the extent to which protection wanes over time. It will also be necessary to determine the extent to which vaccination provides protection specifically against COVID-19-associated pregnancy complications.
Protection of infants by maternal COVID-19 vaccination
As expected, maternal IgG raised by vaccination during pregnancy crosses the placenta and is present in umbilical cord blood at birth 31 , 35 , 36 , 39 , 56 , 58 , 61 , 62 , 63 , remaining detectable in the blood of more than half of infants at 6 months 61 . Transplacental transfer of IgG following vaccination against tetanus and pertussis provides infants with protection against these diseases, and by analogy, COVID-19 vaccination in pregnancy may provide infants with some protection against COVID-19. Early estimates suggest that vaccination after 20 weeks of pregnancy is 80% effective (95% CI, 55–91%) and vaccination before 20 weeks is 32% effective (95% CI, 43–68%) at preventing hospitalization of infants younger than 6 months old with COVID-19 (ref. 64 ).
This increased protection of infants following vaccination later in pregnancy is in line with findings that maximum cord blood antibody titres are achieved when vaccination occurs in the late second to early third trimester 62 , 63 . This is likely to reflect higher maternal IgG titres at the time of birth when vaccination has occurred more recently, as the efficiency of Spike-specific IgG transfer across the placenta is highest following vaccination in the first trimester 58 . However, it is important to be clear that the main benefit of COVID-19 vaccination in pregnancy is the reduction in risk of disease during pregnancy. Therefore, the timing of vaccination should seek to optimize protection during pregnancy, rather than that of the infant after birth.
Notably, the titres of anti-Spike antibodies seen in cord blood are lower following SARS-CoV-2 infection in pregnancy compared with COVID-19 vaccination in pregnancy 56 , 61 . This is likely to be a result of both lower maternal IgG titres being elicited by infection compared with vaccination 56 , 61 and also because the transport of SARS-CoV-2-specific antibodies is compromised following SARS-CoV-2 infection in the third trimester 12 , 65 .
SARS-CoV-2 infection poses significant risks to pregnant people and their infants, but COVID-19 vaccination is safe in pregnancy. This underlies the recommendation that pregnant people receive the COVID-19 vaccine, which is now being made by public health bodies all over the world 66 , 67 . Despite these recommendations, in many countries uptake of the COVID-19 vaccine in pregnancy remains low, so it is essential that we continue to communicate this message to those who are making the decision about COVID-19 vaccination, for themselves and their infants. Although it is understandable that this group might feel cautious, the task has been made more difficult by the proliferation of misinformation about the safety of COVID-19 vaccination in pregnancy. Strong public health messaging is needed, but more importantly we must ensure that midwives and obstetricians are adequately equipped to counsel their patients on the benefits of COVID-19 vaccination.
Di Mascio, D. et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2 , 100107 (2020).
Article PubMed PubMed Central Google Scholar
Docherty, A. B. et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 369 , m1985 (2020).
Molteni, E. et al. Symptoms and syndromes associated with SARS-CoV-2 infection and severity in pregnant women from two community cohorts. Sci. Rep. 11 , 6928 (2021).
Article CAS PubMed PubMed Central Google Scholar
Allotey, J. et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 370 , m3320 (2020).
Article PubMed Google Scholar
Marchand, G. et al. Review and meta-analysis of COVID maternal and neonatal clinical features and pregnancy outcomes to June 3rd 2021. AJOG Glob. Rep. 3 , 100049 (2021).
Google Scholar
Metz, T. D. et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA 327 , 748–759 (2022).
Article CAS PubMed Google Scholar
Piekos, S. N. et al. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: a retrospective multicentre cohort study. Lancet Digit. Health 4 , e95–e104 (2022).
Stock, S. J. et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat. Med. https://doi.org/10.1038/s41591-021-01666-2 (2022).
Strid, P. et al. COVID-19 severity among women of reproductive age with symptomatic laboratory-confirmed SARS-CoV-2 by pregnancy status — United States, Jan 1, 2020–Sep 30, 2021. ResearchSquare https://doi.org/10.21203/rs.3.rs-1090075/v1 (2021).
Article Google Scholar
The National Perinatal Epidemiology Unit. Key information on COVID-19 in pregnancy. NPEU www.npeu.ox.ac.uk/assets/downloads/npeu-news/MBRRACE-UK_Rapid_COVID_19_DEC_2021_-__Infographic_v13.pdf (2021).
Vousden, N. et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS). PLoS ONE 16 , e0251123 (2021).
Edlow, A. G. et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open 3 , e2030455 (2020).
Pique-Regi, R. et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife 9 , e58716 (2021).
Ashary, N. et al. Single-cell RNA-seq identifies cell subsets in human placenta that highly expresses factors driving pathogenesis of SARS-CoV-2. Front. Cell Dev. Biol. 8 , 783 (2020).
Lu-Culligan, A. et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal–fetal interface. medRxiv 2 , 591–610 (2021).
Fajnzylber, J. et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 11 , 5493 (2020).
Delorme-Axford, E., Sadovsky, Y. & Coyne, C. B. The placenta as a barrier to viral infections. Ann. Rev. Virol. 1 , 133–146 (2014).
Article CAS Google Scholar
Mulvey, J. J., Magro, C. M., Ma, L. X., Nuovo, G. J. & Baergen, R. N. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann. Diagn. Pathol. 46 , 151530 (2020).
Prabhu, M. et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG 127 , 1548–1556 (2020).
Smithgall, M. C. et al. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology 77 , 994–999 (2020).
Garcia-Flores, V. et al. Maternal–fetal immune responses in pregnant women infected with SARS-CoV-2. Nat. Commun. 13 , 320 (2022).
Watkins, J. C., Torous, V. F. & Roberts, D. J. Defining severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placentitis. Arch. Pathol. Lab. Med. 145 , 1341–1349 (2021).
Linehan, L. et al. SARS-CoV-2 placentitis: an uncommon complication of maternal COVID-19. Placenta 104 , 261–266 (2021).
Shook, L. L. et al. SARS-CoV-2 placentitis associated with B.1.617.2 (Delta) variant and fetal distress or demise. J. Infect. Dis. 225 , 754–758 (2022).
Fitzgerald, B. et al. Fetal deaths in Ireland due to SARS-CoV-2 placentitis caused by SARS-CoV-2 Alpha. Arch. Pathol. Lab. Med. https://doi.org/10.5858/arpa.2021-0586-SA (2022).
Schwartz, D. A. et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury: a study of 68 cases with SARS-CoV-2 placentitis from 12 countries. Arch. Pathol. Lab. Med. https://doi.org/10.5858/arpa.2022-0029-SA (2022).
Kotlyar, A. M. et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 224 , 35–53 (2021).
Woodworth, K. R. et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy — SET-NET, 16 jurisdictions, March 29–October 14, 2020. MMWR 69 , 1635–1640 (2020).
CAS PubMed PubMed Central Google Scholar
Flaherman, V. J. et al. Infant outcomes following maternal infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): first report from the Pregnancy Coronavirus Outcomes Registry (PRIORITY) study. Clin. Infect. Dis. 73 , e2810–e2813 (2021).
Norman, M. et al. Association of maternal SARS-CoV-2 infection in pregnancy with neonatal outcomes. JAMA 325 , 2076–2086 (2021).
Beharier, O. et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Invest. 131 , e150319 (2021).
Article CAS PubMed Central Google Scholar
Gee, S. et al. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat. Immunol. 22 , 1490–1502 (2021).
Dahlgren, J. et al. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr. Res. 60 , 147–151 (2006).
Mackin, D. W. & Walker, S. P. The historical aspects of vaccination in pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 76 , 13–22 (2021).
Mithal, L. B., Otero, S., Shanes, E. D., Goldstein, J. A. & Miller, E. S. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am. J. Obstet. Gynecol. 225 , 192–194 (2021).
Prahl, M. et al. Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and early infancy. Preprint at medRxiv https://doi.org/10.1101/2021.12.09.21267423 (2021).
Shanes, E. D. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet. Gynecol. 138 , 281–283 (2021).
Shimabukuro, T. T. et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N. Engl. J. Med. 384 , 2273–2282 (2021).
Gray, K. J. et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am. J. Obstet. Gynecol. 225 , 303 (2021).
Article PubMed PubMed Central CAS Google Scholar
Kadali, R. A. K. et al. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: a cross-sectional study on healthcare workers with detailed self-reported symptoms. Am. J. Obstet. Gynecol. 225 , 458–460 (2021).
Bookstein Peretz, S. et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet. Gynecol. 58 , 450–456 (2021).
Olson, C. K. COVID-19 vaccine safety in pregnancy: updates from the v-safe COVID-19 Vaccine Pregnancy Registry . Centers for Disease Control and Prevention https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-09-22/09-COVID-Olson-508.pdf (2021).
Zauche, L. H. et al. Receipt of mRNA COVDID-19 vaccines and risk of spontaneous abortion. N. Engl. J. Med. 385 , 1533–1535 (2021).
BORN Ontario. COVID-19 vaccination during pregnancy in Ontario. BORN Ontario https://www.bornontario.ca/en/whats-happening/covid-19-vaccination-during-pregnancy-in-ontario.aspx (2021).
Kharbanda E. O. Safety of COVID-19 vaccination in pregnancy, interim data from the Vaccine Safety Datalink. Centers for Disease Control https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-09-22/10-COVID-Kharbanda-508.pdf (2021).
Kharbanda, E. O. et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA 326 , 1629–1631 (2021).
Magnus, M. C. et al. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N. Engl. J. Med. 385 , 2008–2010 (2021).
Lipkind, H. S. et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth — eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR 71 , 26–30 (2022).
Wainstock, T., Yoles, I., Sergienko, R. & Sheiner, E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine 39 , 6037–6040 (2021).
Goldshtein, I. et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. https://doi.org/10.1001/jamapediatrics.2022.0001 (2022).
Blakeway, H. et al. COVID-19 vaccination during pregnancy: coverage and safety. Am. J. Obstet. Gynecol. 10 , S0002–S9378 (2021).
Goldshtein, I. et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA 326 , 728–735 (2021).
Theiler, R. N. et al. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am. J. Obstet. Gynecol. MFM 3 , 100467 (2021).
UK Health Security Agency. COVID-19 vaccine surveillance report. Week 4. UK Health Security Agency https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050721/Vaccine-surveillance-report-week-4.pdf (2022).
Buyon, J. P. The effects of pregnancy on autoimmune diseases. J. Leukoc. Biol. 6 , 281–287 (1998).
Collier, A. Y. et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA 325 , 2370–2380 (2021).
Atyeo, C. et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci. Transl. Med. 13 , eabi8631 (2021).
Atyeo, C. et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Preprint at medRxiv https://doi.org/10.1101/2021.11.12.21266273 (2021).
Dagan, N. et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 27 , 1693–1695 (2021).
Vousden, N. et al. Impact of SARS-CoV-2 variant on the severity of maternal infection and perinatal outcomes: data from the UK Obstetric Surveillance System national cohort. Preprint at medRxiv https://doi.org/10.1101/2021.07.22.21261000 (2021).
Shook, L. L. et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA 327 , 1087–1089 (2022).
Rottenstreich, A. et al. Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study. Clin. Microbiol. Infect. 3 , S1198–S1743 (2021).
Yang, Y. J. et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery. Obstet. Gynecol. 139 , 373–380 (2021).
Halasa, N. B. et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months — 17 states, July 2021–January 2022. MMWR 71 , 264–270 (2022).
Atyeo, C. et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 184 , 628–642 (2021).
UK Health Security Agency. Pregnant women urged to come forward for COVID-19 vaccination. GOV.UK https://www.gov.uk/government/news/pregnant-women-urged-to-come-forward-for-covid-19-vaccination (2021).
Centers for Disease Control and Prevention. COVID-19 vaccine monitoring systems for pregnant people. CDC https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/monitoring-pregnant-people.html (2021).
Download references
Author information
Authors and affiliations.
Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Victoria Male
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Victoria Male .
Ethics declarations
Competing interests.
The author declared no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat Rev Immunol 22 , 277–282 (2022). https://doi.org/10.1038/s41577-022-00703-6
Download citation
Accepted : 28 February 2022
Published : 18 March 2022
Issue Date : May 2022
DOI : https://doi.org/10.1038/s41577-022-00703-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Sars-cov-2 vaccination may mitigate dysregulation of il-1/il-18 and gastrointestinal symptoms of the post-covid-19 condition.
- Claudia Fischer
- Edith Willscher
- Christoph Schultheiß
npj Vaccines (2024)
COVID-19 Vaccination and Reproductive Health: a Comprehensive Review for Healthcare Providers
- Yaima Valdes
- Braian Ledesma
- Himanshu Arora
Reproductive Sciences (2024)
The COVID-19 International Drug Pregnancy Registry (COVID-PR): Protocol Considerations
- Diego F. Wyszynski
- Aris T. Papageorghiou
- Sonia Hernández-Díaz
Drug Safety (2024)
Intrauterine transmission of SARS-CoV-2 to and prenatal ultrasound abnormal findings in the fetus of a pregnant woman with mild COVID-19
- Meixiang Zhang
- Liqiong Hou
- Yingchun Luo
BMC Pregnancy and Childbirth (2023)
Commentary: Predicting adverse outcomes in pregnant patients positive for SARS-CoV-2 by a machine learning approach
- Noemi Salmeri
- Massimo Candiani
- Paolo Ivo Cavoretto
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Wednesday, June 23, 2021
NIH begins study of COVID-19 vaccination during pregnancy and postpartum
Researchers will evaluate antibody responses in vaccinated participants and their infants.

A new observational study has begun to evaluate the immune responses generated by COVID-19 vaccines administered to pregnant or postpartum people. Researchers will measure the development and durability of antibodies against SARS-CoV-2, the virus that causes COVID-19, in people vaccinated during pregnancy or the first two postpartum months. Researchers also will assess vaccine safety and evaluate the transfer of vaccine-induced antibodies to infants across the placenta and through breast milk.
The study, called MOMI-VAX, is sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. MOMI-VAX is conducted by the NIAID-funded Infectious Diseases Clinical Research Consortium (IDCRC) .
“Tens of thousands of pregnant and breastfeeding people in the United States have chosen to receive the COVID-19 vaccines available under emergency use authorization. However, we lack robust, prospective clinical data on vaccination in these populations,” said NIAID Director Anthony S. Fauci, M.D., “The results of this study will fill gaps in our knowledge and help inform policy recommendations and personal decision-making on COVID-19 vaccination during pregnancy and in the postpartum period.”
Pregnant people with COVID-19 are more likely to be hospitalized, be admitted to the intensive care unit, require mechanical ventilation, and die from the illness than their non-pregnant peers. Severe COVID-19 during pregnancy also may put the infant at risk for complications such as preterm birth. Individuals who are pregnant or breastfeeding can choose to receive authorized COVID-19 vaccines, and studies to gather safety data in these populations are ongoing. So far, COVID-19 vaccines appear to be safe in these populations. The NIAID study will build on these studies by improving the understanding of antibody responses to COVID-19 vaccines among pregnant and postpartum people and the transfer of antibodies to their infants during pregnancy or through breast milk. Experience with other diseases suggests that the transfer of vaccine-induced antibodies from mother to baby could help protect newborns and infants from COVID-19 during early life.
Investigators will enroll up to 750 pregnant individuals and 250 postpartum individuals within two months of delivery who have received or will receive any COVID-19 vaccine authorized or licensed by the U.S. Food and Drug Administration. Their infants also will be enrolled in the study. Vaccines are not provided to participants as part of the study protocol. Currently, three COVID-19 vaccines are available in the United States under emergency use authorization: the Moderna and Pfizer-BioNTech mRNA vaccines and the Johnson & Johnson adenoviral vector vaccine. The study is designed to assess up to five types of FDA-licensed or authorized COVID-19 vaccines, should additional options become available.
Participants and their infants will be followed through the first year after delivery. To assess the development and durability of vaccine-induced antibodies overall and by vaccine type and vaccine platform, researchers will analyze blood samples collected from pregnant and postpartum participants. These samples will be collected at study enrollment; at delivery for participants who enrolled during pregnancy; and two, six, and 12 months after delivery. Pregnant participants enrolled in the study prior to receiving the vaccine will have blood drawn at enrollment as well as approximately one month after vaccination. To assess transfer of antibodies through the placenta and the levels and durability of antibodies in infants, researchers will perform antibody testing on samples from umbilical cord blood collected at delivery and blood samples collected from infants two and six months after delivery.
Investigators also will assess the potential effects on maternal immune responses and transfer of antibodies across the placenta according to the mother’s age, the trimester of pregnancy during which the vaccine was received, the mother’s health, and the mother’s COVID-19 risk status. Additionally, mothers will have the option of providing breast milk samples at approximately two weeks, two months, six months, and 12 months after delivery. The investigators will evaluate breast milk antibodies to assess the potential for protection against COVID-19 in breastfed infants. Study staff also will gather information on COVID-19 illnesses in pregnant and postpartum participants, birth and neonatal outcomes, and COVID-19 illnesses in infant participants.
The work is led by principal investigators Flor M. Munoz, M.D., of Baylor College of Medicine in Houston and Richard H. Beigi, M.D., of University of Pittsburgh Medical Center. The study will be conducted at up to 20 clinical research sites nationwide. More information about the study, including a list of sites, is available on the IDCRC website .
NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ann Med Surg (Lond)
- v.72; 2021 Dec

Covid-19 vaccine and its consequences in pregnancy: Brief review
Nang kham oo leik.
a Department of Obstetrics & Gynaecology, Faculty of Medicine & Health Sciences, Universiti Malaysia Sabah, Malaysia
Fatimah Ahmedy
b Department of Medical Education, Faculty of Medicine & Health Sciences, Universiti Malaysia Sabah, Malaysia
Rhanye Mac Guad
c Department of Biomedical Sciences, Faculty of Medicine & Health Sciences, Universiti Malaysia Sabah, Malaysia
Dg Marshitah Pg Baharuddin
Pregnancy is linked to a higher incidence of severe Covid-19. It's critical to find safe vaccinations that elicit protective pregnant and fetal immune responses. This review summarises the rate of COVID-19 infection, maternal antibodies responsiveness, placenta antibody transmission, and adverse events after COVID-19 vaccination in pregnancy studied in epidemiological studies evaluating mRNA vaccines. Potential COVID-19 infection in pregnant women can be prevented using mRNA-based vaccinations. Gestation, childbirth, and perinatal mortality were proven unaffected by COVID-19 vaccination. Injection-site discomfort, tiredness, and migraine are the most prevalent side effects, but these are temporary. After the first dosage of vaccinations, fast antibody responses were demonstrated. The adaptive immunity is found to be more significant after booster vaccination, and is linked to improved placental antigen transmission. Two vaccination doses are associated with more robust maternal and fetal antibody levels. Longer delays between the first immunization dosage and birth are linked to greater fetal IgG antibody levels with reduction in antigen transmission proportion. The mRNA vacciness are effective in reducing the severity of COVID-19 infection and these vaccinations are regarded to be safe options for pregnant women and their unborn fetus.
- • mRNA vacciness are effective and regarded safe for reducing the severity of COVID-19 infection in pregnancy.
- • Injection-site discomfort, tiredness, and migraine are the most prevalent side effects, but these are temporary.
- • Adaptive immunity is more significant after booster vaccination with improved placental antigen transmission.
- • Longer delay between first immunization and birth is linked to higher fetal IgG antibody level and lower antigen transmission.
1. Introduction
COVID-19 infection in pregnancy leads to expansion of the respiratory tract and increases the susceptibility of expectant mother for acquiring respiratory diseases [ 1 ]. A pro-inflammatory stage is more evident during the first trimester where embryonic and placental implantation occur, as well as within the third trimester for adaptation towards delivery [ 2 ]. In particular, cytokine outbursts production is linked to acute COVID-19. This pro-inflammatory stage of gestation throughout the first and third trimesters rendered pregnant women to be more susceptible for more severe presentations of COVID-19 infection. Although the majority of pregnant mothers experienced mild to moderate symptoms with COVID-19 infection, the illness is more severe in this group compared to non-pregnant females, with a higher risk of hospitalization. Most of the hospitalized expectant mothers with COVID-19 infection were asymptomatic, allowing the virus to spread unnoticed [ 3 ]. This demonstrates the importance of effective measure that halt the viral transmission from one person to another.
One of the most effective public health measures to counter the spread of communicable diseases is through vaccination. The main goal of nationwide vaccination programs is to accomplish the desired herd immunity, but only if high vaccination rate is achieved [ 4 ]. mRNA vaccine, Moderna and Pfizer–BioNTech, are proven to be effective in preventing and reducing the severity of COVID-19 infections. However, the evidence on mRNA vaccines’ safety profile and effectiveness during pregnancy are gradually emerging [ 5 ].
This brief review intends to summarise the rate of COVID-19 infection, maternal antibodies responsiveness, placenta antibody transmission, and adverse events after COVID-19 vaccination in pregnancy. The data involved the outcomes of epidemiological studies that evaluated two different mRNA vaccines; Pfizer–BioNTech vaccine and Moderna vaccine. The outcomes from this review should help to enhance the understanding on COVID-19 vaccination during pregnancy for assisting healthcare professionals on counselling expectant mothers.
1.1. COVID-19 infection after vaccination
After 14 days of receiving the Pfizer–BioNTech vaccine, 0.18% (4/2136) of expectant mothers had COVID-19 infection, while 0.51% (11/2136) acquired COVID-19 infection within 2 weeks of vaccination. Around 0.5% (9/1822) of pregnant women who received Moderna vaccine developed COVID-19 infection within 14 days of vaccination and 0.5% (9/1822) after 2 weeks of receiving this vaccine [ 6 ]. More than halved of these pregnant women who were diagnosed with COVID-19 within 14 days of vaccination were traced to acquire the virus before receiving their first dose of vaccine. mRNA vaccines substantially decreased the probability of acquiring COVID-19 infections in expectant mothers.
1.2. Antibody reaction in mothers
Antibodies responses are rapidly developed following vaccination, but such desired effect is not seen with natural infection as the latter tends to produce more gradual responses. This concept is applied for acquiring improved reactions with booster vaccine administration. In pregnancy, following vaccination, the rise in the concentration of IgG and IgM antibodies against COVID-19 were observed considerably [ 7 ]. In majority, IgG seroconversion was found to predominate in these pregnant women, but IgM seroconversion was seen, albeit in a much smaller proportion.
The IgG against spike (both S1 and S2) and RBD proteins are produced in response to COVID-19 vaccine, whereas IgG towards spike (both S1 and S2) RBD and neutralising proteins are generated after infected with COVID-19 [ 8 ]. In 72% of expectant mothers, COVID-19 vaccination has led to IgG and IgM antibodies production, in which 14% generated IgG only antibody with remaining 14% had immeasurable IgG or IgM antibody levels. After receiving the first dose of vaccine, the spike-IgG and RBD-IgG titers grew fast, but these levels are more significant with the second dose compared to the initial one.
S1-IgG and RBD-IgG levels are greater in expectant mothers after vaccinated [ 9 ]. On the other hand, infected pregnant women had greater titres of S2-IgG and neutralising-IgG antibodies. The spike-IgG level was 22.814.5AU in pregnant women who had flu-like symptoms compared to the spike-IgG level of 0.040.05 AU among expected mothers who were asymptomatic after COVID-19 vaccination. The median RBD-IgG levels were 27601 AU and 1321 AU respectively with neutralising-IgG antibody titres of 900 AU and 150 AU in the vaccinated and infected pregnant women correspondingly. On the other hand, among non-pregnant females, the median RBD-IgG titers were 38000 among those who were vaccinated but only 800 AU in infected individuals, with corresponding neutralising-IgG levels of 900 AU and 200 AU, respectively.
1.3. Transfer of antibodies
Following COVID-19 vaccination, the antibodies produced are transferred to the fetus. Maternal and fetal antibodies in blood were demonstrated to be almost comparable [ 10 ]. The blood plasma concentration of IgG antibodies was discovered at 1.31 U/mL among expectant mothers receiving the second dose of Pfizer–BioNTech vaccine and one dose of Moderna vaccine. The IgG antibodies were found in 98.5% of newborns born to mothers that had completed two doses of Pfizer–BioNTech vaccination. On contrary, 43.6% of newborns of mothers that had one dose of Pfizer–BioNTech vaccine demonstrated evidence of COVID-19 specific IgG antibodies in their blood [ 11 ].
Both RBD-IgG and neutralising-IgG antibodies were found in the fetal blood samples. The maternal and fetal (cord) blood plasma levels of RBD-IgG antibody were measured around 15000 AU and 20000 AU with neutralising-IgG antibody titre of 1000 AU and 300 AU correspondingly after COVID-19 vaccination [ 12 ].
Aside from the number of vaccine dosages, the time between the vaccination and birth was found to be associated to the levels of IgG antibodies titres and its transfer ratio [ 13 ]. A higher IgG transfer ratio is associated with longer duration of completed vaccination to childbirth. Transfer ratio, calculated by dividing the IgG antibody concentration in fetal cord blood with the IgG antibody concentration in maternal blood, of spike-IgG antibody was reported to be almost halved (transfer ratio of 0.45).
1.4. Adverse reactions
The safety of COVID-19 vaccination is the primary concern for both the expectant mothers and clinicians. According to a poll conducted in 16 countries, pregnant mothers were less inclined to accept vaccinations for themselves [ 14 ]. Despite the established report of COVID-19 vaccination delivering up to 90% effectiveness, approximately three-quarter of non-pregnant women agreed for vaccination, compared to around 50% of pregnant women. One significant predictor of vaccination uptake was the trust in the vaccination efficacy and safety. Surprisingly, vaccine safety was not considered as a significant contributing factor in both pregnant and non-pregnant women.
It should be noted that unpleasant responses and adverse events affect both expectant mothers and non-pregnant women [ 15 ]. In both the Moderna and Pfizer–BioNTech vaccinations, injection-site discomfort is the most prevalent complication in pregnant mothers. After the Pfizer–BioNTech vaccine, up to 84% and 89% of expectant mothers who received one and two doses respectively, have reported injection-site discomfort [ 16 ]. For the Moderna vaccination, 93% and 96% had injection-site discomfort after the first and subsequent doses, correspondingly. In another study, 88% of pregnant women complained of injection-site discomfort after the first dosage and 57% after the second dose. In contrast, following the first and second vaccination doses, 75% of non-pregnant females reported similar adverse event. Sore shoulders or discomfort were reported in 97% of expectant mothers and 90% of non-pregnant women after receiving the Moderna and Pfizer–BioNTech vaccinations.
The incidence of systemic adverse reactions increased following the second dose of vaccination in Moderna and Pfizer–BioNTech vaccinations [ 17 ]. Tiredness, migraines, shivers, malaise, rash, and vomiting were among the most commonly reported systemic side effects. In most cases, these were temporary and rarelt lasted beyond three days. Compared to the first dosage, the frequency for these systemic adverse reactions to occur was significantly higher following the second dosage. In terms of numbers, the Moderna vaccination group had more people who had these systemic side effects than the Pfizer-BioNTech category.
Vaccination does not affect the gestation or delivery when compared to unvaccinated expectant mothers. There were no demonstrable significant differences on the frequency of gestational hypertension or thrombosis between vaccinated and unvaccinated pregnant women [ 18 ]. Looking from the delivery perspective, there were no significant negative impact on the incidence of premature birth, endometrial break, or unexpected ICU hospitalization among vaccinated expectant mothers [ 19 ].
2. Limitations of review
This review, albeit its attempt to compile as much evidence as possible, is not without limitations. Firstly, the gathering of evidence for the review was conducted in the absence of systematic literature search and methodological quality assessment. Therefore, the review lacks its ability to provide clear recommendations as one would have expected with systematic review or scoping review articles. Nevertheless, the evidence included remained as the few available studies that were conducted among pregnant females which provide required safety and effectiveness profile on the mRNA vaccines for combating COVID-19. Secondly, the review covered several key topics of concern on the use of this vaccine in this particular vulnarable group. Although the review is not solely focusing on a single safety profile or exploring on a specific angle of vaccine's efficacy, the presented brief data has strong inclined towards the use of vaccine. Thirdly, the included literature are primarily observational studies performed in specific regions of the world. A major pondering question is for clinicians to consider and expect similar outcomes for recommending this vaccine to pregnancy women within countries having completely different genetic make-up and cultural beliefs. Even so, considering the grieve complications from COVID-19 infection in pregnancy, the evidence from this review could further heightened the needs for recommending this vaccine to expectant mothers.
3. Conclusion
As randomised controlled trials on COVID-19 vaccination in pregnancy is lacking, the resultant derived outcomes are purely observational from epidemiological studies evaluating mRNA vaccines. mRNA vaccines are proven to be beneficial in deterring COVID-19 in pregnant women and demonstrated the ability to induce antibody reactions in this vulnarable population and their unborn fetus. It is highly recommended for pregnant women to receive two doses of vaccination and achieve completion earlier aiming for higher levels of antibody titres and transfer ratio. The mRNA vaccine is primarily safe for expectant mothers and common adverse reactions are similar with non-pregnant individuals including fever and injection-site discomfort. There is no evidence that COVID-19 vaccination affects gestation, birth, or birth complications.
Ethical approval
The article did not require any ethical approval.
Sources of funding
The article did not receive any funding.
Author contribution
Nang Kham Oo Leik: First Author, writing the paper.
Fatimah Ahmedy: Corresponding author, review and revise the paper for submission.
Rhanye Mac Guad: revising the structure of the write up.
Dg Marshitah Pg Baharudin: data/evidence collection.
Acquiring consent from patients is not relevant for this article.
Registration of Research Studies
Registration of research studies is not relevant for this article.
Nang Kham Oo Leik.
Declaration of competing interest
No potential conflict of interest relevant to this article was reported.
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
COVID-19 Vaccines and Pregnancy
The ethics and safety of COVID vaccines for pregnant people
Q&A WITH RUTH FADEN, ELANA JAFFE, CARLEIGH KRUBINER, AND CHIZOBA WONODI
This article originally appeared on the Johns Hopkins University Coronavirus Resource Center .
Globally, over 200 million people are pregnant each year. Whether they should be offered the new COVID vaccines as they become available is an important public health policy decision. Whether pregnant people should seek vaccination is a deeply personal decision.
Are pregnant people at higher risk of developing severe COVID?
Evidence to date suggests that people who are pregnant face a higher risk of severe disease and death from COVID compared to people who are not pregnant. For instance, pregnant people are three times more likely to require admission to intensive care and to need invasive ventilation. The overall risk of death among pregnant people is low, but it is elevated compared to similar people who are not pregnant. Some studies suggest that COVID in pregnancy might be associated with increased rates of preterm birth.
Our understanding of the probability and severity of harms from SARS-CoV-2 infection in pregnancy is evolving. The pandemic has been ongoing for just over a year, which limits what can currently be known about the health risks of COVID for pregnant people, and especially their offspring. Whether SARS-CoV-2 infection in pregnancy poses risks to the developing fetus remains underdetermined. Current evidence suggests that transmission of SARS-CoV-2 to the fetus is rare. However, severe maternal illness can have serious implications for the fetus. For example, fevers during early pregnancy have in some studies been associated with increased risk for certain birth defects. Since the pandemic has only been with us for just over a year, there are no data yet on long-term childhood outcomes for offspring exposed in utero.
There are still significant unknowns: How do risks vary by trimester? What are the risks of asymptomatic infection? Further, most current information about COVID and pregnancy comes from high-income countries, limiting its global generalizability
Do we know if COVID vaccines are safe in pregnancy?
At this point, tens of thousands of pregnant people have received COVID vaccines globally, including in the U.S., Canada, the U.K., and Israel. Thus far, there have been no reports suggestive of concern. Additionally, none of the vaccines that have thus far been authorized for use in the U.S.—the Pfizer-BioNtech, Moderna, and Johnson & Johnson/Janssen vaccines, as well as the Oxford-AstraZeneca vaccine authorized in other countries—contain live or replication-competent viruses. Therefore, it is extremely unlikely that a vaccine virus could replicate, cross the placenta, and infect the fetus. However, more research is needed in order to better characterize the safety profile of each COVID vaccine in pregnancy.
Although there is not yet pregnancy-specific data about COVID vaccines from clinical trials, the vaccines have been studied in pregnant laboratory animals. Called developmental and reproductive toxicity (DART) studies, research with pregnant animals can provide reassurance about moving forward with vaccine research in pregnant people. There are no concerning signals from DART study data for the Pfizer-BioNtech, Moderna, Johnson & Johnson/Janssen, and preliminary DART data for the Oxford-AstraZeneca vaccines. Small numbers of participants in the research trials for these vaccines have become pregnant. No concerning risk signals in those pregnancies have been reported.
All three of these vaccines offer a very high level of protection against severe COVID. There is little reason to believe these vaccines will be less effective in pregnant people than they are in people of comparable age who are not pregnant.
What positions have different national and global authorities taken on pregnant people and COVID vaccines that are authorized for use?
The absence of pregnancy-specific data for COVID vaccines has made regulatory and public health decision-making complicated. Largely due to the absence of evidence, most public health agencies have held back on making explicit recommendations on COVID vaccine administration in pregnancy. In the U.S., Canada, the U.K., and several other countries, the position of the relevant public health authority is that pregnant people who otherwise qualify for an authorized vaccine—such as pregnant people who are health care workers or members of other prioritized essential workforces—should be permitted to make their own decisions about vaccination, based on their assessment of whether the prospect of benefit to them and their offspring outweighs the risks. This is also the position of the World Health Organization for the vaccines they have thus far evaluated. In Israel, the Ministry of Health and Vaccines Prioritization Committee recommended vaccination for pregnant people in their second or third trimester. Most jurisdictions in the U.S. are already offering the vaccine to pregnant people given higher COVID risk in pregnancy, including the District of Columbia, Pennsylvania, and Mississippi.
What do obstetricians say about COVID vaccines and pregnancy?
Professional societies, such as the American College of Obstetricians and Gynecologists , the Society for Maternal-Fetal-Medicine , and the Royal College of Obstetricians and Gynaecologists , all support COVID vaccination in pregnancy when the benefits outweigh the risks.
How should pregnant people think about the benefits and risks?
The major benefit of the Pfizer-BioNtech, Moderna, Johnson & Johnson/Janssen, and Oxford-AstraZeneca vaccines to all people, pregnant or not, is that being vaccinated provides a high level of protection against serious illness from COVID.
How important the protective benefit of COVID vaccination is to any individual pregnant person depends on how likely they are to get infected, and how likely they are to get seriously ill, if infected. Pregnant people differ in how likely they are to get infected. A person’s risk of becoming infected depends on at least three things: 1) whether their job puts them at risk of infection; 2) the rate of transmission in their community; and 3) who they live with, especially whether they live with people who are at increased risk because of their jobs, or in a crowded home or densely populated neighborhood. For example, people whose jobs require them to be in regular contact with many people are at higher risk of infection than people who can work from home. Similarly, people who live with other people who also work outside the home are at greater risk than people who live alone or only with others who also work or attend school from home.
Pregnant people also differ in how likely they are to get seriously ill with COVID, if they become infected. While pregnancy by itself is a risk factor for serious illness, some medical conditions like diabetes, heart disease, or being very overweight are even greater risk factors. People who are pregnant and also have high-risk medical conditions are more likely to develop severe COVID if they become infected than pregnant people who do not have those medical conditions.
Pregnant people should also consider whether they have access to alternative modes of protection from infection. Questions to ask include: Can they take a leave from work or be temporarily transferred to a lower-risk job; do they have access to high quality personal protective equipment; and, if someone in their household gets infected or exposed, is there a way for that person to safely isolate away from others?
Resources, including provider information sheets , conversation guides , and decision aids , have been developed to facilitate the values-driven and context-dependent calculations that pregnant people face in the coming months.
When are we likely to get data from pregnant people?
Some people prioritized for vaccination have received COVID vaccines while pregnant, and data about their pregnancies are being collected by public health agencies. Registries are being established in multiple countries to capture the experiences of pregnant people who are receiving COVID vaccines. At least one developer, Pfizer-BioNTech, has begun a pregnancy-specific trial for their vaccine, which will enroll 4,000 pregnant people across nine countries.
What is wrong with this picture?
The absence of pregnancy-specific data around COVID vaccines continues an unfair pattern in which evidence about safety of new vaccines for pregnant people lags behind . This unfairness is ethically problematic in at least two important ways.
First, people may be denied vaccine, or may face barriers in accessing vaccine, because they are pregnant. Public health agencies globally have struggled to determine the most ethical position regarding whether to allow pregnant people to receive COVID vaccines in the absence of pregnancy-specific data. While there is still limited evidence on the safety of currently authorized vaccines in pregnancy, with high vaccine efficacy, no risk signals from studies in pregnant animals, and few biologically plausible risks, the permissive approach that most health authorities have taken enabling individuals to decide for themselves is ethically appropriate.
However, in some settings—whether by policy guidance, local guidelines, or even individual provider reticence—a lack of evidence may mean that pregnant people will face unfair denial of highly effective vaccines from which they stand to benefit.
Second, even when pregnant people are eligible for vaccination, because public health authorities have not explicitly recommended COVID vaccines in pregnancy, the burden of making decisions about vaccination has shifted to pregnant people. Evidence gaps shift the responsibility for associated risk more squarely to pregnant people, where their nonpregnant peers have an evidence base and a public health recommendation to back up their vaccination decision. While endorsement from medical professional societies is helpful, without pregnancy-specific evidence or explicit pregnancy recommendations, there is also the risk that pregnant people’s decisions will be biased by the strong risk distortions that are known to be present in the context of pregnancy .
Hopefully, the evidence necessary for public health agencies to make clear, full-throated recommendations about the use of at least some COVID vaccines in pregnancy will be forthcoming in the coming months. Efforts are underway to encourage developers of vaccines not yet approved for use to move more quickly to conduct studies with pregnant people and otherwise undertake efforts to systematically generate evidence on the safety of their products in pregnancy. We will continue to update this brief, as new data and new policies become available, both for the vaccines discussed here and for additional vaccines that will shortly be evaluated for use in public health programs.
Ruth Faden, PhD, MPH , is the founder of the Johns Hopkins Berman Institute of Bioethics and was its director from 1995 until 2016. She is a professor in Health Policy and Management .
Carleigh Krubiner, PhD , is an associate faculty member at the Johns Hopkins Berman Institute of Bioethics .
Chizoba Wonodi, DrPh ’09, MPH ’04 , is an associate scientist in International Health and the Nigeria Country Director for the International Vaccine Access Center.
Related Content

What to Know About COVID FLiRT Variants

Amsterdam’s Struggle to Improve Sex Worker Health

Public Health On Call Special Series—The Consequences of Abortion Restriction


Rotavirus the Leading Cause of Diarrheal Deaths Among Children Under 5, New Analysis Finds

OB-GYN Training and Practice in Dobbs’ Shadow
Advertisement
Supported by
C.D.C. Recommends Covid Vaccines During Pregnancy
New research shows the shots do not increase risk of miscarriage, the agency said, but the risks of severe disease from a coronavirus infection while pregnant are serious.
- Share full article

By Roni Caryn Rabin
The Centers for Disease Control and Prevention on Wednesday strongly recommended that pregnant and breastfeeding women be vaccinated against Covid-19 , pointing to new safety data that officials hope will sway the many who have resisted despite mounting evidence that the coronavirus can pose grave risks to their health and increase the chance of a preterm birth.
The new guidance marked the first time the agency gave strong, unambiguous support to vaccination during pregnancy, bringing it in line with the advice of the American College of Obstetricians and Gynecologists and other medical specialty groups.
It comes amid a surge in coronavirus infections across the nation, driven by the highly contagious Delta variant, and renewed efforts by the Biden administration to push up vaccination rates to stem the virus’s spread. The Food and Drug Administration is expected to authorize a third vaccine dose for certain immunocompromised people as soon as Thursday.
“CDC encourages all pregnant people or people who are thinking about becoming pregnant and those breastfeeding to get vaccinated to protect themselves from Covid-19,” the C.D.C. director, Dr. Rochelle Walensky, said. “The vaccines are safe and effective, and it has never been more urgent to increase vaccinations.”
Only 23 percent of pregnant women have received even one dose of Covid vaccine in the United States, and, in recent weeks, physicians have reported seeing more pregnant patients becoming infected , C.D.C. officials said.
The C.D.C. said its new guidance applies not only to pregnant women but also to pregnant individuals who do not identify as women. (The surveillance data it reviewed, however, is based on participants who self reported as women.)
Pregnancy is on the C.D.C.’s list of health conditions that increase the risk of severe disease for people infected with the coronavirus. They are significantly more likely than patients who are not pregnant to require intensive care, to be connected to a heart-lung bypass machine, and to require mechanical ventilation, and they face a 70 percent increased risk of dying.
Contracting Covid can also increase the risk of the mother developing a dangerous condition called pre-eclampsia, and raise the risk of preterm births and stillbirths; severe Covid disease further elevates these risks, and has been linked to gestational diabetes and low birth weight. In rare cases, the virus can be transmitted to the fetus during gestation.
Dr. Walensky pointed to new safety data that found no increased risk of miscarriage in a study of nearly 2,500 pregnant people who were immunized with one of the mRNA vaccines (the shots by Moderna or Pfizer and BioNTech) during the first 20 weeks of gestation. Earlier research found similarly reassuring data for those vaccinated later in pregnancy.
Some women remained conflicted about the decision.
“I’m highly considering getting vaccinated, I feel like it’s the more responsible thing to do,” said Raeshel Contreras, 29, who lives in the Bay Area. She is 28 weeks pregnant and worries that the vaccine could disrupt fetal development, though there is no scientific evidence of this. “I don’t know what I would do if I got vaccinated and something happened to the baby.”
She has given herself two weeks to make a decision, she said, but the timing of the new C.D.C. directive, during a surge, concerned her.
“Why was this not recommended before?” she asked. “Now there’s this new variant, and now the C.D.C. is jumping, but it wasn’t recommended, I’m like ‘Why is that?’”
The data on birth outcomes is limited, considering the vaccine has only been available since December, but the small number of pregnancies of immunized obstetric patients followed to term have not identified any safety issues, according to Sascha R. Ellington, an epidemiologist who leads the emergency preparedness response team in the division of reproductive health at the C.D.C.
A study of 827 people who gave birth after being vaccinated found the rates of adverse events and poor outcomes were similar to prepandemic rates, but called for more long-term follow-up of such pregnancies. The study was published in April in the New England Journal of Medicine.
“At this time, the benefits of vaccination, and the known risks of Covid during pregnancy and the high rates of transmission right now, outweigh any theoretical risks of the vaccine,” Dr. Ellington said.
“I think it’s pretty clear with everything we’ve learned that you do have more severe disease in pregnancy, and there is concerning data from other countries that pregnant women are being more severely affected with the Delta variant, compared with the earlier strains,” said Dr. Denise Jamieson, an obstetrician at Emory University in Atlanta.
Still, many pregnant patients, reluctant to put any foreign substance in their bodies, want more long-term data and scientific evidence that the vaccines will not have an effect on the development of the fetus, said Dr. Adam Urato, a maternal-fetal medicine specialist in Framingham, Mass., who counsels patients about the vaccine.
“The one question my patients ask me all the time is, ‘Are we absolutely sure that these vaccines won’t affect my baby?’” he said.
Tista Banerjee, 32, who gave birth to twins at the end of June, said she chose not to be vaccinated until after her pregnancy.
“During pregnancy they say that if you don’t have to take external medicines, don’t, and that you should be particular about what you put in your body,” Ms. Banerjee said. The vaccine was still quite new in April when she was considering vaccination, she said, and she was fortunate that she was able to work remotely and avoid unnecessary exposure to the virus.
She was fully vaccinated in July, soon after she gave birth, she said.
Pregnant women, often excluded from medical studies, were not included in the clinical trials of the Covid vaccines, and the World Health Organization has been ambiguous in its guidance about vaccines, both for breastfeeding women, for whom it says there is no safety data, and for pregnant women.
In interim recommendations, issued in June, the global health organization said that it recommends vaccination “when the benefits of vaccination to the pregnant woman outweigh the potential risks.” The examples given were women who are at a high risk of being exposed to Covid, and those with chronic health conditions, like obesity or diabetes, that place them at higher risk for severe illness.
Sabrina Imbler contributed reporting.
- Follow us on Facebook
- Follow us on Twitter
- Criminal Justice
- Environment
- Politics & Government
- Race & Gender
Expert Commentary
COVID-19 vaccines during pregnancy: What research shows
We've summarized several academic papers that investigate outcomes of COVID-19 -- and the vaccines against it -- among pregnant individuals.
Republish this article

This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License .
by Naseem S. Miller, The Journalist's Resource September 29, 2021
This <a target="_blank" href="https://journalistsresource.org/home/covid19-vaccines-pregnancy-research/">article</a> first appeared on <a target="_blank" href="https://journalistsresource.org">The Journalist's Resource</a> and is republished here under a Creative Commons license.<img src="https://journalistsresource.org/wp-content/uploads/2020/11/cropped-jr-favicon-150x150.png" style="width:1em;height:1em;margin-left:10px;">
This piece was originally published on Aug. 22, 2021. It is regularly updated with new research on COVID-19 and pregnancy under the “More studies” subhead. It was last updated on August 16, 2022.
On Sept. 29, the Centers for Disease Control and Prevention issued a health advisory recommending “urgent action” to increase COVID-19 vaccination among people who are pregnant, trying to or might become pregnant or were pregnant recently, in order to prevent serious illness, death and adverse pregnancy outcomes due to the coronavirus infection.
“Pregnancy can be both a special time and also a stressful time – and pregnancy during a pandemic is an added concern for families,” said CDC Director Dr. Rochelle Walensky in a news release . “I strongly encourage those who are pregnant or considering pregnancy to talk with their healthcare provider about the protective benefits of the COVID-19 vaccine to keep their babies and themselves safe.”
The agency’s recommendation follows a growing body of research that shows benefits of COVID-19 vaccine before or during pregnancy far outweigh the potential risks for moms and babies.

It aims to improve vaccination rates among pregnant people in the U.S., only 31% of whom have received their COVID-19 shot. That’s compared with 56% of U.S. adults who have been fully vaccinated. And even though the overall gap in coronavirus vaccination disparities is narrowing, pregnant people who are Hispanic and Black continue to fall behind in getting their shots compared to their white counterparts, according to the CDC : 25% of Hispanic or Latino and 15.6% of Black individuals who are pregnant have received their COVID shots, compared with 33.8% of white adults. The highest vaccination rate among pregnant individuals by race and ethnicity is among Asian adults, CDC data shows , at 45.7%.
The advisory follows an Aug. 11 update in which the CDC recommended the COVID-19 vaccine for all people 12 years and older, including pregnant people, saying there’s no evidence that the vaccine leads to fertility problems, miscarriage or other health issues. At the time, the federal agency’s endorsement was prompted by an increase in the number of infections among pregnant individuals .
As of Sept. 27, more than 125,000 pregnant people in the U.S. had been diagnosed with COVID-19, more than 22,000 hospitalized and 161 had died since the pandemic started, according to the latest available data from the CDC, which is updated weekly.
As of Sept. 18, 189,986 pregnant people in the U.S. were fully vaccinated against COVID-19, according to the latest available data from the agency.
The agency isn’t alone in recommending the vaccine. The American College of Obstetricians and Gynecologists , or ACOG, and the Society for Maternal-Fetal Medicine — the two leading groups in the U.S. representing specialists providing health care for women — issued a joint statement in July, recommending that all pregnant people get vaccinated against COVID-19.
The World Health Organization recommends pregnant women get COVID-19 vaccines in consultation with their health-care provider. Also, in an interim guidance published in June, it recommends vaccination in pregnant women when the benefits outweigh potential risk, particularly for women who are at a high risk of exposure to COVID-19 and those whose comorbidities put them at a higher risk of developing severe COVID-19.
Even though the overall risk for severe COVID-19 infection remains low, studies have shown that pregnant people are at an increased risk for severe illness from COVID-19 compared with those who are not pregnant.
Underlying medical conditions such as asthma, diabetes, heart disease and obesity can increase the risk of complications even more for pregnant people. Pregnant individuals of color face higher risk of severe illness from COVID-19 due to persisting racial inequalities in the U.S., the CDC says.
In addition, pregnant people who were infected with COVID-19 during the third trimester are at an increased risk of having a preterm birth, which is delivery earlier than 37 weeks , compared with pregnant women who aren’t infected with the virus, according to a CDC study .
Studies are still underway and long-term safety data aren’t available yet, but relying on a growing body of evidence, the CDC says that at this point the benefits of receiving a COVID-19 vaccine outweigh any potential risks of vaccination during pregnancy.
But belief in misinformation continues to hamper vaccination efforts. A national survey by The COVID States Project, conducted between June and July, finds that 11% of Americans believe that COVID-19 vaccines can cause infertility, even though the claim has been debunked . About 20% of Americans also falsely believe that COVID-19 vaccines will alter people’s DNA, contain microchips to track people or contain the lung tissue of aborted fetuses, the survey shows. The COVID States Project was launched in March 2020 by a multi-university group of researchers, focusing on social behaviors and the impact of messaging on the pandemic.
In addition, pregnant people were excluded from the initial COVID-19 vaccine clinical trials , which also may have fueled vaccine hesitancy sentiments. Some COVID-19 vaccine makers have since planned to enroll pregnant people in their clinical trials.
We have summarized several academic papers that investigate outcomes of COVID-19 infection — and the vaccines against it — among pregnant individuals, to help journalists bolster their reporting with data. Please note that while the studies in this roundup have undergone the peer review process, the findings from most are still from a limited time frame, given that the pandemic arrived less than two years ago and the vaccines became available to the public less than a year ago. Periodically, we’ll update this roundup with new studies as new information comes to light.
Risk for Stillbirth Among Women With and Without COVID-19 at Delivery Hospitalization — United States, March 2020 – September 2021 Carla DeSisto; et. al. CDC’s Morbidity and Mortality Weekly Report, Nov. 2021
The study finds pregnant people with COVID-19 had a higher risk of experiencing a stillbirth compared with pregnant people who were not infected.
Among 8,154 stillbirths in U.S. hospitals between March 2020 and Sept. 2021, 1.26% occurred among those who had COVID-19, compared with 0.64% among those who weren’t infected. The risk of stillbirths increased with the arrival of the delta variant. In July to Sept. 2021, 2.7% of 1,171 stillbirths involved pregnant people with COVID-19, compared with 0.63% of those who were uninfected.
“This analysis adds to growing evidence of an association between COVID-19 in pregnancy and stillbirth, highlights that the risk for stillbirth associated with COVID-19 is affected by maternal morbidity, and demonstrates that the risk has increased during the Delta period,” the authors write.

It’s important to note that stillbirths are rare. Researchers looked at 1.25 million hospital deliveries in the study period, in which there were 8,154 stillbirths. That’s 0.65% of the deliveries.
Researchers also note that among deliveries with COVID-19, certain underlying medical conditions, such as chronic high blood pressure, and need for mechanical ventilation and ICU admission were associated with stillbirth.”
Additional studies are warranted to investigate the role of maternal complications from COVID-19 on the risk for stillbirth,” the authors write.
Researchers used the Premier Healthcare Database Special COVID-19 Release (PHD-SR), a large hospital-based administrative database for their study. Even though the database is large, it only includes hospitals that report their data and the findings may not be generalizable to the U.S. population, the authors write.
Characteristics and Outcomes of Women With COVID-19 Giving Birth at US Academic Centers During the COVID-19 Pandemic Justine Chinn; et al. JAMA Network Open, August 2021.
The study shows that pregnant women with COVID-19 had higher rates of preterm birth, ICU admission, respiratory failure requiring intubation, and death compared with pregnant women who didn’t have COVID-19.
Using a large national database without patient-identifying information, researchers looked at records for 869,079 women who gave birth at 499 U.S. medical centers between March 2020 and February 2021. Of those, 18,715 (2.2%) had COVID-19.
Researchers didn’t control for confounders like age, race and co-morbidities such as obesity when calculating outcomes.
The study finds that pregnant women with COVID-19 had a higher risk of preterm delivery — 16.4% versus 11.5%. That’s an increased risk of 42%.
The comparison of pregnant women with and without COVID-19 also shows that those with the infection were nearly six times more likely to be admitted to the ICU, 14 times more likely to require mechanical ventilation, and 15 more times likely to die in the hospital.
It’s important to note the risk of ICU admission, mechanical ventilation and death were still low in each group, relative to the total number of women in the sample. For instance, of the 18,715 pregnant women with COVID-19, 24 died. That’s about 13 deaths in 10,000. For pregnant women without COVID, there were 71 deaths in 850,364, or about 8 deaths in 10,000.
Researchers add that pregnant individuals with COVID-19 were more likely to be Hispanic or Black.
“This information is critically important given the ongoing issues surrounding health care disparities and race,” the authors write in the study. “When considering mortality during childbirth, it is important to understand that racial disparities have been well established preceding the COVID-19 pandemic; however, they have likely been augmented by the pandemic.”
They found no significant increase in C-sections among pregnant women with and without COVID-19.
Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons Dr. Tom Shimabukuro; et al. The New England Journal of Medicine, June 2021.
In this study, the authors use data from three U.S. vaccine monitoring systems and find no safety concerns among pregnant individuals who received COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, both of which are based on mRNA technology .
Researchers look at data for 35,691 pregnant individuals, using the V-safe Surveillance System and Pregnancy Registry, a new CDC smartphone-based surveillance app developed for the COVID-19 vaccination program, the V-safe Pregnancy Registry and the national Vaccine Adverse Event Reporting System, or VARES , between December 2020 and February 2021. They compared the data with published studies of pregnant populations before the pandemic.
They find that pregnant individuals didn’t report having any more severe reactions to the COVID-19 vaccines compared with women who were not pregnant — except for nausea and vomiting, which were reported slightly more frequently among vaccinated pregnant individuals after the second dose of the vaccine. Researchers also report similar pregnancy outcomes, including for preterm birth. No newborns, regardless of whether their mothers had received the COVID-19 vaccine, had birth defects.
The authors note that the registry data are preliminary and from a relatively small sample, describing pregnancy outcomes primarily from women who were vaccinated during the third trimester of pregnancy. Long-term studies with large numbers of women vaccinated earlier in pregnancy is needed to assess outcomes, the authors write.
They point out that the vaccines not only protect pregnant individuals from a severe COVID-19 infection, they may also provide some level of protection in newborn babies, although there’s a need for more research.
Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status — United States, January 22–October 3, 2020 Laura Zambrano; et al. CDC’s Morbidity and Mortality Weekly Report, November 2020
This study compares COVID-19 infection in women who are and aren’t pregnant, finding that pregnant women with COVID-19 are at a higher risk for severe health outcomes.
Researchers use data from the CDC’s National Notifiable Diseases Surveillance System for 409,462 women who had COVID-19 symptoms between January and October 2020. About 6%, or 23,434, of the study population were pregnant.
The authors adjusted for age, race and ethnicity and underlying medical conditions.
Their analysis shows that pregnant women with COVID-19 had three times the risk of being admitted to the ICU and requiring intubation, compared with women who were diagnosed with the infection but were not pregnant. They also had a 2.4 times higher risk of requiring extracorporeal membrane oxygenation, or ECMO , which is a heart-lung machine used in critically-ill patients. The pregnant women had a 70% increased risk of death.
Risk Factors for Illness Severity Among Pregnant Women With Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 Infection — Surveillance for Emerging Threats to Mothers and Babies Network, 22 State, Local, and Territorial Health Departments, 29 March 2020-5 March 2021 Dr. Romeo Galang; et al. Clinical Infectious Diseases, July 2021.
The aim of this study is to determine the risk factors associated with developing severe COVID-19 illness in pregnant women.
The authors used the CDC’s Surveillance for Emerging Threats to Mothers and Babies Network , or SET-NET, which collects information on pregnant individuals and their children for the first three years of their life from 31 state, local and territorial health departments in the U.S. They analyze data for 7,950 pregnant women who had a COVID-19 infection between March 2020 and March 2021.
They find that severe illness from COVID-19 in pregnant women is associated with being 25 years or older, having a job in the health care profession, obesity, chronic lung disease, chronic high blood pressure and diabetes. The severity of the COVID-related illness increased with the number of underlying medical conditions.
Having any underlying medical condition such as asthma or diabetes was associated with a 39% increased risk of severe or critical COVID-19 illness in pregnant women, while having three or more underlying conditions was associated with more than twice the risk, when compared with pregnant women who didn’t have any underlying medical conditions.
The authors note that the findings on risk factors are similar to what’s observed among nonpregnant adults.
“These data can help counsel pregnant women about their risk for moderate-to-severe or critical COVID-19 illness and guide their choice of prevention strategies, target public health messaging, and inform decisions around resource allocation,” the authors write.
The Coronavirus Disease 2019 Vaccine in Pregnancy: Risks, Benefits, and Recommendations Dr. Irene Stafford, Dr. Jacqueline Parchem and Dr. Baha Sibai. American Journal of Obstetrics and Gynecology, January 2021.
This study stands out because the authors look back at history to show that this is not the first pandemic to pose a higher risk of severe disease for pregnant individuals.
During the 2002 Severe Acute Respiratory Syndrome, or SARS, pandemic, 25% of infected pregnant women died and 57% had miscarriages, the authors write.
Meanwhile, studies of the 2009 H1N1 flu pandemic have shown higher rates of ICU admission and death among infected pregnant individuals than those who were not pregnant, they add.
The authors add that even though pregnant individuals were excluded from the initial mRNA COVID-19 vaccine trials, so far there’s no evidence that the vaccines pose a risk to them or their babies.
Additional research
SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland Sarah Stock; et. al. Nature Medicine, January 2022
This whole-population data from a national, prospective cohort in Scotland, shows that between December 2020 and October 2021, when the delta variant was dominant, severe complication association with COVID-19 during pregnancy, including critical care admission and death of babies, were more common in women who were unvaccinated when they were infected with the virus compared with women who were vaccinated. “Our data support the importance of women being vaccinated in pregnancy to prevent adverse outcomes associated with COVID-19,” the authors write.
The effect of maternal SARS-CoV-2 infection timing on birth outcomes: a retrospective multicentre cohort study Samantha Piekos; et. al. The Lancet Digital Health, January 2022
Researchers used clinical data from Providence St. Joseph Health electronic health records for pregnant people who delivered in the U.S. Between March 5, 2020, and July 4, 2021, 73,666 pregnant people delivered, including 882 people infected with SARS-CoV-2 during their pregnancy. The team compared the pregnancy outcomes of the 822 individuals, none of whom were vaccinated, with those who tested negative for the virus during pregnancy. Their analysis shows those who were infected with COVID-19 during pregnancy were more likely to have poor birth outcomes, including preterm birth and stillbirth. “These results suggest that pregnant people would benefit from increased monitoring and enhanced prenatal care after first or second trimester SARS-CoV-2 infection, regardless of acute COVID-19 severity,” the authors write.
Receipt of COVID-19 Vaccine During Pregnancy and Preterm or Small-for-Gestational-Age at Birth — Eight Integrated Health Care Organizations, United States, December 15, 2020–July 22, 2021 Heather Lipkind; et. al. CDC’s Morbidity and Mortality Weekly Report, January 2022
Among 46,079 pregnant women, 10,064 (21.8%) received one or more doses of the COVID-19 vaccine during pregnancy. Nearly all were vaccinated during the second or third trimester. Researchers find “vaccination during pregnancy is not associated with preterm birth or small-for-gestational-age at birth overall, stratified by trimester of vaccination, or number of vaccine doses received during pregnancy, compared with unvaccinated pregnant women.”
Obstetrical outcomes and maternal morbidities associated with COVID-19 in pregnant women in France: A national retrospective cohort study Sylvie Epelboin; et. al. PLOS Medicine, November 2021
Researchers investigated whether morbidities like preeclampsia, obesity, high blood pressure, and adverse outcomes like ICU admission and death were more common in pregnant women with COVID-19 compared with pregnant women without COVID-19 during the first wave of the pandemic, between January and June 2020. A total of 244,645 births were included from the French national hospitalization database, of which 874 (0.36%) were in the COVID-19 group. They found an association between maternal morbidities and diagnosis of COVID-19.
“Although causality cannot be determined from these associations, these results may be in line with recent recommendations in favor of vaccination for pregnant women,” researchers wrote.
COVID-19–Associated Deaths After SARS-CoV-2 Infection During Pregnancy — Mississippi, March 1, 2020 – October 6, 2021 Laurin Kasehagen; et. al. CDC’s Morbidity and Mortality Weekly Report, November 2021
The report describes 15 COVID-19-associated deaths during pregnancy between March 2020 and Oct. 2021 in Mississippi. There were 1,637 COVID-19 infections among pregnant women during the study period, 15 of whom died. The median age of the 15 women who died was 30 years, ranging from 23–40 years. Nine were Black women, three were Hispanic and three were white.
“Given existing disparities in vaccination rates among pregnant women, partnerships to address vaccine access, hesitancy, or other concerns about vaccination can enhance fair and just access to COVID-19 vaccination, including among Black persons and Hispanic persons,” the authors write.
Short-term Reactions Among Pregnant and Lactating Individuals in the First Wave of the COVID-19 Vaccine Rollout Dr. Alisa Kachikis; et al. JAMA Network Open, August 2021
This study is based on the first wave of vaccinations in the U.S. in March 2021. Researchers use data from the University of Washington COVID-19 Vaccine in Pregnancy and Lactation Registry to compare the experiences of COVID-19 vaccination among pregnant and lactating individuals with those who were neither pregnant nor lactating, but were planning pregnancy. The majority of the 17,525 participants were health-care workers and white. Researchers find that COVID-19 vaccines were well-tolerated among the three groups. Several study authors reported receiving grants from drug-makers including Merck, Pfizer and GlaxoSmithKline, but not for this published study.
The Differences in Clinical Presentation, Management, and Prognosis of Laboratory-Confirmed COVID-19 between Pregnant and Non-Pregnant Women: A Systematic Review and Meta-Analysis Durray Shahwar A. Khan; et al. International Journal of Environmental Research and Public Health, May 2021
This study shows that pregnant women with COVID-19 had twice the risk of ICU admission and requiring mechanical ventilation compared with pregnant women who didn’t have COVID-19.
The Impact of COVID-19 on Pregnancy Outcomes: A Systematic Review and Meta-Analysis Shu Qin Wei, Marianne Bilodeau-Bertrand, Shiliang Liu and Nathalie Auger. Canadian Medical Association Journal, April 2021
This study looks at 42 international studies involving a combined 438,548 pregnant people. It finds that COVID-19 during pregnancy was associated with an 82% increased risk of preterm birth and a 33% increased risk of preeclampsia, which is a complication of high blood pressure, compared with no COVID-19 during pregnancy. The infection was also associated with a two-fold increase in the risk of stillbirth.
More studies and data sources
- “ Risk of preterm birth, small for gestational age at birth, and stillbirth after covid-19 vaccination during pregnancy: population based retrospective cohort study ,” Deshayne Fell; et. al. The BMJ, August 2022.
- “ Comparison of Pregnancy and Birth Outcomes Before vs During the COVID-19 Pandemic ,” Rose Molina; et al. JAMA Network Open, August 2022.
- “ Safety of COVID-19 Vaccines in Pregnancy: a Canadian National Vaccine Safety (CANVAS) Network Cohort Study ,” Manish Sadarangani; et al. LANCET Infectious Diseases, August 2022.
- “ Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms ,” Caroline G. Atyeo; et. al. Nature Communications, June 2022.
- “ Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants .” Dr. Natasha B. Halasa; et. al. NEJM, June 2022.
- “ Association of COVID-19 Vaccination During Early Pregnancy With Risk of Congenital Fetal Anomalies ,” Dr. Rachel S. Ruderman; et al. JAMA Pediatrics, April 2022.
- CDC data on health outcomes among pregnant people diagnosed with COVID-19
- CDC data on birth and infant outcomes for people with COVID-19 during pregnancy
- CDC data on the percentage of pregnant people receiving at least one dose of a COVID-19 vaccine during pregnancy
- CDC COVID-19 toolkit for pregnant people and new parents
- American College of Obstetricians and Gynecologists’ practice advisories on COVID-19 and COVID-19 vaccination (also a good source for the latest clinical studies)
- Society for Maternal-Fetal Medicine’s COVID-19 clinical guidance
About The Author
Naseem S. Miller
- Introduction
- Article Information
Data are stratified by gestational age groups (6-8, 9-13, and 14-19 weeks), maternal age group, number of antenatal visits, race and ethnicity, and Vaccine Safety Datalink site. The dashed beige lines indicate time during pregnancy but outside the surveillance period; the amount of time represented varies by pregnancy and is not to scale with the figure. The solid blue lines represent pregnancy time during the surveillance period. LMP indicates last menstrual period.
Generalized estimating equation models included gestational age group, surveillance period, maternal age group, number of antenatal visits, site, and race and ethnicity factors and accounted for unique pregnancies that included multiple pregnancy periods. mRNA indicates messenger RNA.
eTable. Secondary Analyses, Adjusted Odds Ratios and 95% Confidence Intervals Among Pregnant People Eligible for a COVID-19 Booster Dose, by 28-Day and 42-Day Exposure Windows and by Manufacturer
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Kharbanda EO , Haapala J , Lipkind HS, et al. COVID-19 Booster Vaccination in Early Pregnancy and Surveillance for Spontaneous Abortion. JAMA Netw Open. 2023;6(5):e2314350. doi:10.1001/jamanetworkopen.2023.14350
Manage citations:
© 2024
- Permissions
COVID-19 Booster Vaccination in Early Pregnancy and Surveillance for Spontaneous Abortion
- 1 HealthPartners Institute, Minneapolis, Minnesota
- 2 Department of Obstetrics and Gynecology, Weill Cornell Medical College, New York, New York
- 3 Kaiser Permanente Center for Health Research, Portland, Oregon
- 4 Institute for Health Research, Kaiser Permanente Colorado, Denver
- 5 Marshfield Clinic Research Institute, Marshfield, Wisconsin
- 6 Kaiser Permanente Southern California, Pasadena
- 7 Denver Health, Denver, Colorado
- 8 Kaiser Permanente Vaccine Study Center, Oakland, California
- 9 Kaiser Permanente Washington Health Research Institute, Seattle, Washington
- 10 Centers for Disease Control and Prevention, Atlanta, Georgia
Question Is COVID-19 booster vaccination in early pregnancy associated with an increased risk of spontaneous abortion?
Findings In this case-control surveillance study of more than 100 000 pregnancies at 6 to 19 weeks’ gestation from 8 health systems in the Vaccine Safety Datalink, the odds of having received a COVID-19 booster vaccination in either a 28- or 42-day exposure window before spontaneous abortion were not increased compared with ongoing pregnancies.
Meaning These findings support the safety of COVID-19 booster vaccination in early pregnancy.
Importance Adherence to COVID-19 booster vaccine recommendations has lagged in pregnant and nonpregnant adult populations. One barrier to booster vaccination is uncertainty regarding the safety of booster doses among pregnant people.
Objective To evaluate whether there is an association between COVID-19 booster vaccination during pregnancy and spontaneous abortion.
Design, Setting, and Participants This observational, case-control, surveillance study evaluated people aged 16 to 49 years with pregnancies at 6 to 19 weeks’ gestation at 8 health systems in the Vaccine Safety Datalink from November 1, 2021, to June 12, 2022. Spontaneous abortion cases and ongoing pregnancy controls were evaluated during consecutive surveillance periods, defined by calendar time.
Exposure Primary exposure was receipt of a third messenger RNA (mRNA) COVID-19 vaccine dose within 28 days before spontaneous abortion or index date (midpoint of surveillance period in ongoing pregnancy controls). Secondary exposures were third mRNA vaccine doses in a 42-day window or any COVID-19 booster in 28- and 42-day windows.
Main Outcomes and Measures Spontaneous abortion cases and ongoing pregnancy controls were identified from electronic health data using a validated algorithm. Cases were assigned to a single surveillance period based on pregnancy outcome date. Eligible ongoing pregnancy time was assigned to 1 or more surveillance periods as an ongoing pregnancy-period control. Generalized estimating equations were used to estimate adjusted odds ratios (AOR) with gestational age, maternal age, antenatal visits, race and ethnicity, site, and surveillance period as covariates and robust variance estimates to account for inclusion of multiple pregnancy periods per unique pregnancy.
Results Among 112 718 unique pregnancies included in the study, the mean (SD) maternal age was 30.6 (5.5) years. Pregnant individuals were Asian, non-Hispanic (15.1%); Black, non-Hispanic (7.5%); Hispanic (35.6%); White, non-Hispanic (31.2%); and of other or unknown (10.6%); and 100% were female. Across eight 28-day surveillance periods, among 270 853 ongoing pregnancy-period controls, 11 095 (4.1%) had received a third mRNA COVID-19 vaccine in a 28-day window; among 14 226 cases, 553 (3.9%) had received a third mRNA COVID-19 vaccine within 28 days of the spontaneous abortion. Receipt of a third mRNA COVID-19 vaccine was not associated with spontaneous abortion in a 28-day window (AOR, 0.94; 95% CI, 0.86-1.03). Results were consistent when using a 42-day window (AOR, 0.97; 95% CI, 0.90-1.05) and for any COVID-19 booster in a 28-day (AOR, 0.94; 95% CI, 0.86-1.02) or 42-day (AOR, 0.96; 95% CI, 0.89-1.04) exposure window.
Conclusions and Relevance In this case-control surveillance study, COVID-19 booster vaccination in pregnancy was not associated with spontaneous abortion. These findings support the safety of recommendations for COVID-19 booster vaccination, including in pregnant populations.
As of March 2023, more than 682 million SARS-CoV-2 infections and more than 6.8 million COVID-19 deaths have been reported worldwide. 1 Although COVID-19 in otherwise healthy young adults is often mild or asymptomatic, infections during pregnancy are associated with increased risk of morbidity and adverse birth outcomes. 2 , 3 Vaccination has been shown to reduce the risks of severe disease during pregnancy and to provide additional protection from complications in newborns. 4 - 9
Because of waning immunity and the emergence of more contagious variants, starting in September 2021, booster doses of the messenger RNA (mRNA) vaccines were made available for populations in the United States who had completed the primary vaccine series and were at increased risk for severe illness due to COVID-19, including pregnant people. 10 Subsequently, all adolescents and adults were encouraged to receive a COVID-19 vaccine booster after primary vaccination. 11
Adherence to COVID-19 booster vaccine recommendations has lagged in pregnant and nonpregnant adult populations. 12 , 13 One barrier to booster vaccination is the uncertainty regarding the effectiveness and duration of protection of additional vaccine doses. Others may question whether a booster is needed given a prior history of SARS-CoV-2 infection. An additional concern among pregnant people is regarding the safety of booster doses. Previous studies 5 , 14 - 17 have demonstrated that receipt of 1 or 2 mRNA COVID-19 vaccine doses in pregnancy, as part of the primary vaccine series, was not associated with adverse pregnancy or birth outcomes, including spontaneous abortion, preterm birth, small-for-gestational-age birth, and infant neonatal care admissions. The aim of the current study was to evaluate potential associations between COVID-19 booster vaccination in early pregnancy and spontaneous abortion through adaptation of previously described COVID-19 vaccination in pregnancy safety surveillance. 15
The Vaccine Safety Datalink (VSD) is a collaborative effort between the Centers for Disease Control and Prevention’s (CDC’s) Immunization Safety Office and several large US integrated health care systems. The aim of the VSD is to monitor the safety of vaccines routinely administered in the United Sates. 18 In this observational, case-control, surveillance study conducted from November 1, 2021, to June 12, 2022, we used administrative and electronic health record (EHR) data from 8 sites participating in the VSD to evaluate potential associations between receipt of COVID-19 booster vaccine doses before 20 weeks’ gestation and spontaneous abortion. Primary analyses focused on receipt of a third mRNA COVID-19 vaccine dose in a 28-day exposure window. Secondary analyses evaluated receipt of a booster dose in 28-day or 42-day exposure windows in the full population and limited to the subset who had completed the primary vaccine series. This surveillance was approved by the institutional review boards of all participating sites and the CDC with a waiver of informed consent because this was a minimal risk, observational study and was conducted consistent with federal law and CDC policy. The analytic approach followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline for case-control designs. External researchers can request deidentified data from the VSD for conducting secondary analyses, as described on the VSD website. 19
Data for this case-control surveillance study came from 8 VSD sites (Kaiser Permanente: Washington, Northwest, Northern California, Southern California, and Colorado; Denver Health; HealthPartners; and Marshfield Clinic). People in the VSD population aged 16 to 49 years with a pregnancy 6 to 19 weeks’ gestation between November 1, 2021, and June 12, 2022, were identified using a validated algorithm applied to automated electronic health data. 20 The algorithm uses International Statistical Classification of Diseases, Tenth Revision, Clinical Modification ( ICD-10-CM ) and Current Procedural Terminology ( CPT ) codes from inpatient, outpatient, and emergency department visits, supplemented with clinical data, with updates on a weekly basis, to identify ongoing and completed pregnancies. Ectopic pregnancies, gestational trophoblastic disease, and pregnancies ending in therapeutic abortion were excluded. Pregnancies resulting from assisted reproduction, and thus at increased risk for having a medically attended spontaneous abortion, were also excluded. Exclusions were identified through diagnostic ( ICD-10-CM ) or procedure ( CPT ) codes.
Surveillance was conducted from November 2021 through the middle of June 2022. Data presented are from the final data extraction on August 3, 2022. For the primary analyses, evaluating a 28-day vaccine exposure window, eight 28-day surveillance periods were included. For secondary analyses, evaluating a 42-day exposure window, five 42-day surveillance periods were included. Midpoints of the surveillance periods were assigned as the index date for ongoing pregnancies. This index date was then used to assign a gestational age for an ongoing pregnancy period and to evaluate for receipt of a COVID-19 booster vaccination in the prior 28 or 42 days ( Figure 1 ). 21
Spontaneous abortion cases and ongoing pregnancy controls were identified using a validated algorithm, applied to automated electronic health data. In a previous validation, of 105 spontaneous abortions identified by the algorithm, agreement on pregnancy outcome was 95% and agreement on date of pregnancy outcome was 94%. 20 Consistent with prior work, 15 gestational age for both spontaneous abortions and ongoing pregnancies was based on the algorithm’s hierarchical approach to clinical data (last menstrual period and estimated delivery date), gestational age–specific ICD-10-CM codes (eg, Z3A.11 for 11 weeks’ gestation), or trimester-specific ICD-10-CM codes (eg, O13.2 for gestational hypertension without significant proteinuria, second trimester). Spontaneous abortion cases without gestational age available from these sources were assigned to the earliest gestational age category (6-8 weeks). Ongoing pregnancies with unknown gestational age were excluded.
Spontaneous abortions occurring between 6 and 19 weeks’ gestation were included as cases and assigned to a single surveillance period based on the pregnancy outcome date. 15 In the primary analyses, during each 28-day surveillance period, eligible ongoing pregnancies between 6 and 19 weeks’ gestation were included as ongoing pregnancy-period controls and assigned an index date equal to the midpoint (day 14) of the 28-day surveillance period. For secondary analyses, using a 42-day surveillance period, the index date was assigned at the midpoint (day 21) of the surveillance period. In both primary and secondary analyses, ongoing pregnancies could be included in more than 1 surveillance period, contributing data as 2 or more ongoing pregnancy-period controls; pregnancies ending in a spontaneous abortion case in 1 surveillance period could contribute data as an ongoing pregnancy-period control in 1 or more surveillance periods before the spontaneous abortion.
As previously described, most vaccines administered in the VSD pregnant population have been the mRNA COVID-19 vaccines, mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech). 14 , 22 A previous case-control surveillance study 15 of spontaneous abortion after receipt of the COVID-19 primary vaccine series in pregnancy used a 28-day exposure window, consistent with the presumed timing of the inflammatory response after COVID-19 vaccination. As such, primary analyses for the evaluation of booster vaccination in early pregnancy evaluated a third mRNA vaccine (mRNA-1273 or BNT162b2) dose occurring in a 28-day exposure window before the date for the spontaneous abortion (or index date in ongoing pregnancy controls).
In secondary analyses, we evaluated any COVID-19 vaccine booster (including a second dose of Ad26.COV.2.S [Janssen] or a second or third dose of an mRNA COVID-19 vaccine after Ad26.COV.2.S or a fourth or fifth mRNA COVID-19 vaccine dose) in a 28-day exposure window. In addition, we evaluated a third mRNA vaccine dose or any COVID-19 vaccine booster in a 42-day exposure window before spontaneous abortion (or index date in ongoing pregnancy controls). Secondary analyses also evaluated associations between COVID-19 booster vaccination and spontaneous abortion among those who had completed the primary vaccine series and thus were booster eligible.
COVID-19 vaccines administered from the start of the COVID-19 vaccine program (December 15, 2020) through June 12, 2022, in the eligible study population were identified from standardized VSD files. The VSD vaccine files include EHR data as well as medical and pharmacy claims and are supplemented through bidirectional communication with regional or state immunization information systems with standardized data quality checks and deduplication of vaccines from multiple sources. 23 Vaccines were then classified as first, second, third, or subsequent doses. To reduce the potential for vaccine data from different sources (eg, EHR, state immunization registry, and claims) to be counted as distinct doses, we required at least 14 days between dose 1 and dose 2 and at least 28 days between dose 2 and dose 3 or subsequent doses. The median (IQR) time between the second and third mRNA COVID-19 vaccine dose was 252 (220-289) days.
Covariates associated with likelihood of vaccination and risks for spontaneous abortion outcomes were included in models. Maternal age was categorized into the following groups: 16 to 24, 25 to 34, 35 to 39, and 40 to 49 years. Race and ethnicity were based on self-report as documented in the EHR and categorized as Asian, non-Hispanic; Black, non-Hispanic; Hispanic; White, non-Hispanic; or other or unknown (including Hawaiian or other Pacific Islander, Native American or Aleutian, and multiple races). Antenatal health care visits before the spontaneous abortion (or index date for ongoing pregnancy controls) came from EHR data and were classified as 1 or fewer or 2 or more. Gestational week of the spontaneous abortion or index date for ongoing pregnancy-period controls was categorized as 6 to 8, 9 to 13, or 14 to 19 weeks. The VSD site and surveillance period were also included as covariates.
In primary analyses, we calculated the odds of receiving a third mRNA COVID-19 vaccine in the 28 days before spontaneous abortion for cases compared with the odds of receiving a third mRNA COVID-19 vaccine in the 28 days before an index date for ongoing pregnancy-period controls. Generalized estimating equations with binomial distribution and logit link with robust variance estimates were used to account for unique pregnancies that contributed data in 2 or more surveillance periods and included covariates listed as main factors. Subgroup analyses by manufacturer (Moderna for mRNA-1273 and BioNTech-Pfizer for BNT162b2) were also conducted.
Using this same approach, we calculated adjusted odds ratios (AORs) and 95% CIs for receipt of any COVID-19 booster vaccine in the 28 days before spontaneous abortion or index date in ongoing pregnancy periods. In addition, we evaluated receipt of a third mRNA COVID-19 vaccine or any COVID-19 vaccine booster in the 42 days before spontaneous abortion or index date in ongoing pregnancy periods in secondary analyses. This same approach was applied in secondary analyses for the subset who were booster eligible, having completed the primary COVID-19 vaccine series. All analyses were performed using SAS/STAT software, version 9.4 (SAS Institute Inc).
A total of 112 718 unique pregnancies (mean [SD] maternal age, 30.6 [5.5] years; 100% women; 15.1% Asian, non-Hispanic; 7.5% Black, non-Hispanic; 35.6% Hispanic; 31.2% White, non-Hispanic; and 10.6% other or unknown race or ethnicity) were included in the study, with 14 226 pregnancies (12.6%) ending in a spontaneous abortion. The primary analyses included a total of 285 079 pregnancy periods (14 226 spontaneous abortion cases and 270 853 ongoing pregnancy-period controls), with 11 648 (4.1%) having received a third mRNA vaccine in a 28-day exposure window. Across both cases and controls, receipt of a third mRNA vaccine dose in a 28-day window varied by race and ethnicity (2775 [6.5%] in Asian, non-Hispanic people, 495 [2.3%] in Black, non-Hispanic people, 3276 [3.2%] in Hispanic people, and 4323 [4.9%] in White, non-Hispanic pregnant people) ( Table 1 ).
Of 270 853 ongoing pregnancy-period controls, 11 095 (4.1%) received a third mRNA COVID-19 vaccine dose within 28 days of their index date, whereas 553 of 14 226 cases (3.9%) ending in spontaneous abortion received a third mRNA COVID-19 vaccine dose within 28 days of the spontaneous abortion. Although third mRNA vaccine doses were noted in every surveillance period, most were during the first four 28-day surveillance periods ( Table 2 ).
In primary analyses, receipt of a third mRNA COVID-19 vaccine was not associated with spontaneous abortion (AOR, 0.94; 95% CI, 0.86-1.03) ( Table 3 ). Results were consistent when stratified by vaccine manufacturer (mRNA-1273: AOR, 0.93; 95% CI, 0.81-1.07; and BNT162b2: AOR, 0.95; 95% CI, 0.84-1.07) ( Figure 2 ).
Among 270 853 ongoing pregnancy-period controls, 11 952 (4.4%) received any COVID-19 booster dose within 28 days of their index date, whereas 592 (4.2%) of 14 226 cases received any COVID-19 vaccine booster dose in a 28-day window before the spontaneous abortion. In these analyses, among 12 544 booster doses administered to cases and controls, 11 701 (93.3%) were third doses, 698 (5.6%) were second doses, 135 (1.1%) were fourth doses, and 10 (0.1%) were fifth doses. Receipt of any COVID-19 booster vaccination within a 28-day window was not associated with spontaneous abortion (AOR, 0.94; 95% CI, 0.86-1.02) ( Table 3 ), with results consistent when stratified by vaccine manufacturer (mRNA-1273: AOR, 0.94; 95% CI, 0.82-1.07; and BNT1262b2: AOR, 0.93; 95% CI, 0.83-1.05) ( Figure 2 ).
Secondary analyses included 103 156 unique pregnancies across five 42-day surveillance periods from November 1, 2021, to May 29, 2022; 89 830 (87.1%) remained ongoing pregnancies and 13 326 (12.9%) ended in a spontaneous abortion. Secondary analyses using a 42-day surveillance period included a total of 182 025 pregnancy periods (13 326 cases and 168 699 ongoing pregnancy-period controls), with 11 669 (6.4%) having received a third mRNA vaccine and 12 494 (6.9%) having received any COVID-19 vaccine booster in a 42-day exposure window. Receipt of a third mRNA COVID-19 vaccine (AOR, 0.97; 95% CI, 0.90-1.05) or any COVID-19 vaccine booster (AOR, 0.96; 95% CI, 0.89-1.04) was not associated with spontaneous abortion ( Table 3 ). Results were consistent when stratified by vaccine manufacturer ( Figure 2 ). In secondary analyses limited to those who were booster eligible, there was no association between COVID-19 booster vaccination and spontaneous abortion, consistent with results for the full cohort (eTable in Supplement 1 ).
In this large case-control surveillance study of more than 100 000 unique pregnancies and analyses that included 285 079 pregnancy periods, receipt of a COVID-19 booster vaccine in early pregnancy was not associated with spontaneous abortion. Our primary analyses focused on the receipt of a third mRNA COVID-19 vaccine in a 28-day exposure window because this was the most common type of booster vaccination in our population. Our findings were consistent in secondary analyses that evaluated receipt of any COVID-19 booster, the 42-day exposure window, and associations in the subset who had completed the primary vaccine series.
The need to monitor the safety of booster vaccination in early pregnancy is clear, given the potential for repeat vaccine doses to be associated with local and systemic symptoms. 24 Furthermore, concerns regarding reactogenicity of booster vaccination may be contributing to vaccine hesitancy. 24 However, data to date have not demonstrated that COVID-19 booster vaccination is associated with an increase in acute reactions in pregnant or other adult populations. A prospective study 24 of more than 17 000 adults receiving a COVID-19 booster vaccine, including 2009 who were pregnant at the time of vaccination, found that the rates of self-reported local and systemic reactions after COVID-19 booster vaccination were similar to those following a second COVID-19 vaccine dose. As of March 24, 2022, the Vaccine Adverse Event Reporting System had received 323 reports of adverse events in pregnant people after a COVID-19 booster vaccine. However, the overall safety profile was similar to that after the initial COVID-19 vaccine series. 25 In addition, v-Safe, a voluntary after-vaccination health checker, found that individuals 18 years or older reported fewer injection site or systemic reactions after a first booster dose than after dose 1 or dose 2 of the primary series. 26 Similarly, our surveillance study of third mRNA vaccine doses and spontaneous abortion found an AOR of 0.94 (95% CI, 0.86-1.03), consistent with our findings following the first or second mRNA vaccine dose (AOR, 1.02; 95% CI, 0.96-1.08).
This study has several strengths, including the use of a large and diverse population-based sample, the availability of comprehensive data on COVID-19 vaccine exposures, 23 and the ability to monitor associations between vaccine exposures in early pregnancy and spontaneous abortion with data and analyses updated on a monthly basis. 27 However, several limitations to these analyses should be noted. First, spontaneous abortion cases were identified and assigned a gestational age based on automated electronic health data by using a validated algorithm. The algorithm shows substantial agreement with manual record review for distinguishing pregnancies ending in live birth from pregnancies ending in spontaneous abortion. However, for pregnancies ending in spontaneous abortion, the agreement between the algorithm assigned and record review gestational age might be lower. 20 Errors in algorithm-derived gestational age assignment could result in misclassification of vaccine exposure status, biasing results to the null, especially when applying a 28-day exposure window. It was reassuring that results were consistent in secondary analyses using a 42-day exposure window.
Second, all included spontaneous abortion cases were medically attended and estimated to have reached at least 6 weeks’ gestation. These requirements are consistent with definitions applied in prior maternal vaccine safety surveillance studies 15 , 28 , 29 and with American College of Obstetricians and Gynecologists case definitions. 30 Furthermore, multiple-gestation pregnancies with a single fetal demise could not be excluded. Alternate approaches would be needed (eg, prospective design and active participant enrollment) to analyze all cases of early pregnancy loss.
Third, although we included available covariates, including maternal age group and number of antenatal visits, as main factors in the analyses, we did not have data on other important confounders, such as maternal educational level, prior history of spontaneous abortion, maternal body mass index, or recent COVID-19 infection. Residual confounding is a potential limitation of observational studies of maternal vaccination. 27
Fourth, our primary analyses focused on a third mRNA vaccine dose (after 2 prior mRNA vaccines) because this was the most common booster vaccine type used in VSD during the surveillance period. We did not have sufficient mRNA COVID-19 vaccine doses administered in pregnant people after prior receipt of the Janssen vaccine or second doses of the Janssen vaccine to specifically evaluate these exposures, but they were included in the secondary analyses of any COVID-19 vaccine booster. Furthermore, these analyses predated availability of the bivalent COVID-19 booster vaccine.
In summary, compared with ongoing pregnancies, the odds of having received a COVID-19 booster vaccination in a 28- or 42-day exposure window before spontaneous abortion were not increased. These findings support the safety of recommendations for COVID-19 booster vaccination, including in pregnant populations. As of September 1, 2022, bivalent COVID-19 booster vaccines have been approved and recommended for use in the United States. 31 Studies of bivalent booster vaccine exposures in early pregnancy are ongoing and will be important.
Accepted for Publication: April 5, 2023.
Published: May 19, 2023. doi:10.1001/jamanetworkopen.2023.14350
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Kharbanda EO et al. JAMA Network Open .
Corresponding Author: Elyse O. Kharbanda, MD, MPH, HealthPartners Institute, Mail Stop 21112R, Minneapolis, MN 55440-1524 ( [email protected] ).
Author Contributions: Drs Kharbanda and Vazquez-Benitez had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Kharbanda, Lipkind, DeSilva, Daley, Weintraub, Vazquez-Benitez.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Kharbanda, Haapala, Vazquez-Benitez.
Critical revision of the manuscript for important intellectual content: Kharbanda, Lipkind, DeSilva, Zhu, Vesco, Daley, Donahue, Getahun, Hambidge, Irving, Klein, Nelson, Weintraub, Williams, Vazquez-Benitez.
Statistical analysis: Haapala, Nelson, Vazquez-Benitez.
Obtained funding: Kharbanda, Irving, Nelson.
Administrative, technical, or material support: Zhu, Daley, Donahue, Hambidge, Weintraub, Williams.
Supervision: Lipkind, Hambidge, Klein, Weintraub.
Conflict of Interest Disclosures: Dr Lipkind reported receiving personal fees from Pfizer outside the submitted work. Dr Vesco reported receiving grants from Pfizer outside the submitted work. Dr Klein reported receiving grants from Pfizer, Merck, GlaxoSmithKline, and Sanofi Pasteur outside the submitted work. Dr Nelson reported receiving personal fees from Elsevier outside the submitted work. Dr Vazquez-Benitez reported receiving grants from AbbVie and Sanofi Pasteur outside the submitted work. No other disclosures were reported.
Funding/Support: This study was funded by contract 200-2012-53526 from the Centers for Disease Control and Prevention.
Role of the Funder/Support: The CDC participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Mention of a product or company name is for identification purposes only and does not constitute endorsement by the Centers for Disease Control and Prevention.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: Leslie Kuckler, MPH, HealthPartners Institute; Erika Kiniry, MPH, and Rachel Burganowski, MS, Kaiser Washington; Kristin Goddard, MPH, and Pat Ross, BA, Kaiser Northern California; Hannah Berger, MPH, Kayla Hanson, MPH, Sai Sudha Medabalimi, M Pharm, and Erica Scotty, MS, Marshfield Clinic; JoAnn Shoup, PhD, Kaiser Colorado; and Brad Crane, MS, Kaiser Permanente Northwest assisted with data collection. These individuals were all compensated through CDC contracts for their work on the study.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Current Students
New study differentiates perinatal risks of COVID-19 infection from pandemic era societal changes
California preterm births declined during pandemic
- 2 min. read ▪ Published May 9
- Share on LinkedIn
- Share on Facebook
- Share on X (Twitter)
A new study has disentangled the risks to infants and birth parents from infection with SARS-CoV-2—the virus that causes COVID-19— from risks related to broader societal changes during the pandemic period.
Led by Dr. Shelley Jung , a UC Berkeley School of Public Health researcher, and published today in JAMA Network Open , the article shows that COVID-19 infection was associated with increased risk of preterm birth, hypertension, and severe maternal morbidity—which the CDC defines as “unexpected outcomes of labor and delivery that result in significant short- or long-term consequences to a woman’s health.” Interestingly, the pandemic period itself was associated with a lower risk of preterm birth, but a higher risk of hypertension and gestational diabetes.
This is the first study to separate the infant and birth parent risks linked to the pre-pandemic period, the societal changes of the pandemic period, and individual COVID-19 infection. “As far as we know, ours is the first within one coherent set of data to pull these three groups apart,” said Dr. Jennifer Ahern , a UC Berkeley epidemiology professor and the paper’s senior author.
The societal changes that may have affected health included differential access to care, economic strain, physical inactivity, and other stressors.
“It was an extremely stressful time for people for a variety of reasons,” said Ahern. “There were the economic impacts, which were pretty substantial, people losing jobs and sources of income.”
Jung noted that while COVID-19 infections had negative effects, the pandemic did also bring some positive impact.
“You’ve got maybe less commute stress, less physical stress during pregnancy,” she said. “It’s just a very complex set of changes that all coincided. It was exciting to dig into the net effect of the period on the people of California.” This study examined statewide California data, individually linking all birth and hospital discharge records for 2019 to 2020. “We linked the birth records to the hospital records,” Jung said. “Then we looked nine months back at the birth parent’s pregnancy and looked at all the hospital visits during those nine months.”
Ahern praised the state of California for coordinating data sources and making them available through a rigorous process that she said allows for valuable research while also protecting patient privacy.
The team will move on to look not just at the overall effect of the COVID pandemic period on the population, but how COVID may affect health disparities.
Additional authors include: Emily F. Liu, Mahasin S. Mujahid, and William H. Dow of UC Berkeley School of Public Health and Dana E. Goin and Kara E. Rudolph of Mailman School of Public Health at Columbia University.
This project was funded by grants from the National Institutes of Health.
People of BPH found in this article include:
- Jennifer Ahern Professor, Epidemiology
- William Dow Professor, Health Policy and Management
- Mahasin Mujahid Chair, Epidemiology Division
More in category “Research Highlights”:
Timing of puberty varies by up to 14 months among asian youth, surgery in a hospital doesn’t necessarily lead to better outcomes than surgery in a surgical center, collaboration is key to pioneering research with youth experiencing homelessness, exposure to wildfire smoke during pregnancy increases risk of preterm birth.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 20, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Uptake of flu, whooping cough, and COVID-19 vaccines remains low among pregnant women
by Kat Beauchamp, University of Warwick
A study conducted by researchers at the University of Warwick has unveiled crucial insights into the complex factors shaping vaccination decisions among pregnant women, particularly in the wake of the COVID-19 pandemic.
Pregnant women and their unborn babies face heightened risks of serious illness from infectious diseases such as Influenza (flu), Pertussis (whooping cough), and COVID-19. The research shows that despite the proven safety and efficacy of vaccinations during pregnancy, uptake remains alarmingly low, presenting a significant public health concern.
Despite the availability of free vaccinations for pregnant women in the U.K., of those who gave birth in England in October 2021, 29.4% had received two doses of the COVID-19 vaccine , compared to approximately 60.4% of the general population.
The study , titled "What factors influence the uptake of vaccinations amongst pregnant women following the Covid-19 pandemic: A qualitative study," published in Midwifery , interviewed pregnant women aged between 19 and 41 exploring their perceptions, experiences, and the factors influencing their decisions regarding vaccinations.
Dr. Jo Parsons from the University of Warwick who led the research said, "This research demonstrates the influence that the COVID-19 pandemic has had on pregnant women's views and uptake of recommended vaccinations and is further evident by the continuing decline in uptake since the pandemic.
"It is essential that pregnant women receive clear and consistent messaging, to allow them to make accurate and informed choices about vaccinating in pregnancy."
The findings are categorized into four main areas, each influencing pregnant women's vaccination decisions:
- Internal factors or beliefs: Including feelings about susceptibility to illness during pregnancy, perceived immunity, feelings of responsibility for the health and well-being of themselves, their unborn baby and other people, and fear of vaccinations.
- Vaccination related factors: Including perceived effectiveness, perceived safety, how available or accessible vaccinations were, and the preference to use other strategies of protection.
- External Factors: This included how visible the illness being vaccinated against was felt to be, and how much of the illness was felt to be around at the time.
- COVID-19 specific factors: Including doubts around the newness of the vaccination a
Commenting on the significance of the study, Dr. Jo Parsons said, "This research is vital to learn how pregnant women feel about accepting vaccinations following a pandemic , and how to address low uptake, to protect more pregnant women from largely preventable conditions. This research provides valuable insights and informs future interventions to be developed."
Explore further
Feedback to editors

Study shows mind wandering is associated with specific kind of brain activity linked to memory and sleep
2 hours ago

Ancient viral DNA in the human genome linked to major psychiatric disorders

Study: Newborns whose mother spoke in a mix of languages during pregnancy are more sensitive to a range of sound pitches
7 hours ago
Study finds ultraviolet radiation may affect subcutaneous fat regulation, could lead to new obesity treatments

Regular fish oil supplement use might increase first-time heart disease and stroke risk
12 hours ago

Pedestrians may be twice as likely to be hit by electric/hybrid cars as petrol/diesel ones
Study finds jaboticaba peel reduces inflammation and controls blood sugar in people with metabolic syndrome
13 hours ago

Study reveals how extremely rare immune cells predict how well treatments work for recurrent hives
15 hours ago

Researchers find connection between PFAS exposure in men and the health of their offspring

Researchers develop new tool for better classification of inherited disease-causing variants
Related stories.
Key barriers and solutions identified to increase vaccine uptake in pregnancy
Apr 18, 2024

Study reveals complexity of COVID-19 vaccine decision-making for pregnant people
Feb 20, 2024

First UK data on COVID-19 vaccine uptake and outcomes in pregnant women
Aug 12, 2021

What should I know about COVID-19 vaccines if I'm pregnant?
Jan 14, 2021

Urgent review needed on influenza and whooping cough vaccines during pregnancy
Jun 14, 2022

Pregnant this winter? Here's how to prepare for COVID and get vaccinated
Apr 14, 2023
Recommended for you
A boost for HIV vaccine research: Studies present comprehensive platform for validating next steps
21 hours ago

Researcher finds mothers live longer as child mortality declines

Study sheds light on bacteria associated with pre-term birth

Repeat COVID-19 vaccinations elicit antibodies that neutralize variants, other viruses
May 18, 2024

Trial HIV vaccine triggers elusive and essential antibodies, pointing the way toward a successful vaccine
May 17, 2024
Study opens the door to designing therapies to improve lung development in growth-restricted fetuses
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
COVID-19 linked to increased preterm birth, other problems in pregnancy

Findings from a large study in California, which distinguished the COVID-19 pandemic period from individual SARS-CoV-2 infections, suggest that SARS-CoV-2 infection is tied to increased preterm birth (PTB), high blood pressure during pregnancy, and severe maternal morbidity. The study is published as a research letter in JAMA Network Open .
Researchers looked at live birth data from California hospital discharge records for 2019 to 2020. They compared pregnant women with COVID-19 in 2020, pregnant women without COVID-19 in 2020, and prepandemic pregnant women in 2019.
Overall, parents with COVID-19 infections were more likely to be Hispanic, have lower education, receive public insurance, and live in lower income neighborhoods compared with the other groups, the authors said.
Infection tied to high blood pressure, severe outcomes
Birth outcomes noted included preterm birth (PTB), high blood during pregnancy, gestational diabetes, and severe maternal morbidity.
Compared to 2020 births without COVID-19 infections, there were higher burdens of PTB (2.8%; 95% confidence interval [CI], 2.1% to 3.5%), high blood pressure (3.3%; 95% CI, 2.4% to 4.1%), and SMM (2.3%; 95% CI, 1.9% to 2.7%) among women with COVID-19.
This study adds to understanding of the associations between COVID-19 and perinatal health in a large, diverse population.
"This study adds to understanding of the associations between COVID-19 and perinatal health in a large, diverse population by distinguishing the connections of SARS-CoV-2 infection from those of the COVID-19 pandemic period with PTB and birth parent conditions," the authors concluded.
COVID, other misinformation varies by topic, country on social media
PLOS One has published a study noting that the spread of COVID-related and other misinformation on social media varies by topic and by country in Europe.
The study was conducted by analyzing news activity on Twitter (now X) in France, Germany, Italy and the United Kingdom from 2019 to 2021, noting misinformation on major news topics including Brexit, coronavirus, and COVID-19 vaccines.
News sources cited were rated as either "reliable" or "questionable" based on their NewsGuard scores, which measure nine journalistic criteria, assigning outlets a reliability score out of 100.
Rate of questionable news highest in German
The authors found the United Kingdom maintained a relatively stable proportion between questionable and reliable retweets across different topics. Germany, on the other hand, had the highest ratio of questionable news retweets on each of the three topics analyzed, followed by France.
" Our findings indicated that reliable sources dominate the information landscape, but users consuming content mainly or exclusively from questionable news outlets were often present, " the authors concluded.
The authors also said monitoring news consumption by country rather than continental region would be useful for any efforts looking to combat misinformation.
Monitoring the information landscape at both national and European levels is indeed crucial to understanding the state of public discourse on contentious topics.
"Monitoring the information landscape at both national and European levels is indeed crucial to understanding the state of public discourse on contentious topics and detecting the emergence of new and divisive narratives within the European context," they said.
Report highlights role of socioeconomic, sociocultural factors in antimicrobial resistance

A policy brief published yesterday by the European Observatory on Health Systems and Policies suggests antimicrobial resistance (AMR) policies need to take socioeconomic and sociocultural factors into account.
The brief notes that while efforts to understand AMR have focused on the biomedical model, interactions between socioeconomic and sociocultural determinants of health and AMR, particularly in low- and middle-income countries, have not been studied extensively. Among the factors the authors highlight are gender, living situations, healthcare access, educational access, poor governance, mobility, conflict, and climate change.
Although how these factors contribute to the spread of AMR are complex, the authors say that understanding them could inform development of interventions. Such interventions could address, for example, why women are more likely than men to experience exposure to drug-resistant infections and be prescribed antibiotics, why people in urban and overcrowded environments are associated with a higher risk of AMR, how limited access to healthcare can result in more inappropriate antibiotic use, and how human mobility and conflict can lead to the introduction and spread of new strains of drug-resistant organisms.
"Policy that understands these and the way they interact with one another will be more likely to achieve its aims," the authors write.
A new policy framework
The brief suggests that a policy framework to respond to these socioeconomic and sociocultural factors should focus on antimicrobial stewardship, infection prevention and control, equitable access to diagnostics and effective treatments, and increased investment in incentives to stimulate research and development into new treatments. It should also be people-centered, multifactoral, and evidence-based and emphasize effective governance.
"There is increasing evidence of the critical role that socioeconomic and sociocultural factors play in driving AMR, shaping the health and economic impacts of AMR, and influencing the effectiveness of innovations and progress to tackle AMR at the individual, health system and societal level," the authors write. "It is essential that AMR policy takes these socioeconomic drivers and impacts into account moving forward."
Quick takes: Cholera vaccine shortages, pre-exposure COVID prevention, HPV screening specimen self-collection
- The critical shortage of oral cholera vaccine continues, due to a surge in requests from countries in multiple world regions, the World Health Organization (WHO) said today in an update on the disease. Since January 2023, there have been 82 million doses requested from 15 countries, almost double the 46 million doses produced over the same period. The global stockpile was depleted until early March 2024 and currently has 3.2 million doses, far short of the 5 million-dose goal. Compared to last year at this time, cases are 32% lower, but deaths are 14% higher. Since the WHO's last cholera update, a new outbreak has been reported in Mayotte, the French overseas territory in the Indian Ocean. The outbreak, which began in late April, includes people who arrived by boat from cholera-affected countries Comoros and Tanzania.
- Astra Zeneca today reported promising phase 3 findings its long-acting monoclonal antibody for pre-exposure prophylaxis (prevention) against COVID-19 in immunocompromised patients. The drug, called sipavibart, showed a statistically significant reduction in symptomatic COVID when compared to placebo. Also, the drug showed potential benefits that spanned evolving SARS-CoV-2 variants, given that several variants circulated over the course of the trial. The drug was well tolerated, with levels of adverse events similar in both the treatment and control groups. Astra Zeneca is currently in talks with drug regulators about possible approval or authorization pathways. In early 2023, the US Food and Drug Administration (FDA) pulled the emergency use authorization for Evusheld , an earlier pre-exposure prophylaxis monoclonal antibody, because it was unlikely to be effective against the latest SARS-CoV-2 variants.
- BD (Becton, Dickenson and Company) yesterday announced that the FDA has approved the use of self-collected vaginal specimens for human papillomavirus (HPV) screening in settings where cervical specimens can't be obtained. Settings could include nontraditional sites like retail pharmacies or mobile clinics. The company said the self-collection option could improve access in underserved areas or among patients who aren't comfortable with pelvic exams.
In case you missed it
This week's top reads, wastewater testing finds h5n1 avian flu in 9 texas cities.
Wastewater detections began in early March, and so far sequencing hasn't found any mutations linked to human adaptation.

CDC launches new influenza A wastewater dashboard; states report more H5N1 in dairy herds
The tracker will help with surveillance, but it doesn't distinguish the influenza A subtype or determine the source of the virus.

USDA experiments suggest H5N1 not viable in properly cooked ground beef
Federal officials said it's unclear if dairy cow outbreaks are peaking, and they haven't made much headway testing farm workers for avian flu virus.

Drug-resistant Trichophyton fungus represents emerging threat in US
Retrospective reviews suggest the earliest confirmed US isolate was from 2017.
Global meta-analysis estimates 43% rate of multidrug resistance in COVID patients
A total of 76% of patients were prescribed antibiotics.
Study: Before vaccines, 44% of COVID-19 patients in ICU died
The overall case-hospitalization rate among patients was 5.7%.

USDA confirms 3 more H5N1 outbreaks in dairy herds
Of the three newly confirmed outbreaks, two are in Michigan and one is in Idaho.
Michigan reports 3 more H5N1 outbreaks in dairy herds
In other developments, H5N1 has been detected in cats from South Dakota with no known links to poultry or dairy herd outbreaks.

Data: Heart-failure patients have 82% better odds of living longer if vaccinated against COVID
Vaccinated patients also had a 47% lower risk of hospitalization for heart failure and a 13% reduced risk of infection over 6 months.
Hospital COVID patients 35% more likely to die than flu patients last winter, study suggests
The results should be interpreted in the context of twice as many hospitalizations for COVID than flu during the study period, the authors say.

Our underwriters
Unrestricted financial support provided by.

- Antimicrobial Resistance
- Chronic Wasting Disease
- All Topics A-Z
- Resilient Drug Supply
- Influenza Vaccines Roadmap
- CIDRAP Leadership Forum
- Roadmap Development
- Coronavirus Vaccines Roadmap
- Antimicrobial Stewardship
- Osterholm Update
- Newsletters
- About CIDRAP
- CIDRAP in the News
- Our Director
- Osterholm in the Press
- Shop Merchandise
News & Events
Press releases.
Show all news items
Uptake of flu, whooping cough, and Covid-19 vaccines remains low among pregnant women, according to University of Warwick research
A study conducted by researchers at the University of Warwick has unveiled crucial insights into the complex factors shaping vaccination decisions among pregnant women, particularly in the wake of the Covid-19 pandemic. Pregnant women and their unborn babies face heightened risks of serious illness from infectious diseases such as Influenza (flu), Pertussis (whooping cough), and Covid-19. The research, funded by the National Institute for Health and Care Research (NIHR), shows that despite the proven safety and efficacy of vaccinations during pregnancy, uptake remains alarmingly low, presenting a significant public health concern. Despite the availability of free vaccinations for pregnant women in the UK, of those who gave birth in England in October 2021, 29.4% had received 2 doses of the Covid-19 vaccine, compared to approximately 60.4% of the general population. The study , titled "Factors Influencing Vaccination Uptake Amongst Pregnant Women Following the Covid-19 Pandemic: A Qualitative Study," interviewed pregnant women aged between 19 and 41 exploring their perceptions, experiences, and the factors influencing their decisions regarding vaccinations. Dr Jo Parsons from the University of Warwick who led the research said, “This research demonstrates the influence that the Covid-19 pandemic has had on pregnant women's views and uptake of recommended vaccinations and is further evident by the continuing decline in uptake since the pandemic. It is essential that pregnant women receive clear and consistent messaging, to allow them to make accurate and informed choices about vaccinating in pregnancy.” The findings are categorised into four main areas, each influencing pregnant women's vaccination decisions:
Internal factors or beliefs: Including feelings about susceptibility to illness during pregnancy, perceived immunity, feelings of responsibility for the health and wellbeing of themselves, their unborn baby and other people, and fear of vaccinations.
Vaccination related factors: Including perceived effectiveness, perceived safety, how available or accessible vaccinations were, and the preference to use other strategies of protection.
External Factors: This included how visible the illness being vaccinated against was felt to be, and how much of the illness was felt to be around at the time.
Covid-19 specific factors: Including doubts around the newness of the vaccination and, changing perceptions of the pandemic.
Commenting on the significance of the study, Dr Jo Parsons said, “This research is vital to learn how pregnant women feel about accepting vaccinations following a pandemic, and how to address low uptake, to protect more pregnant women from largely preventable conditions. This research provides valuable insights and informs future interventions to be developed. ENDS For media enquires please contact: Kat Beauchamp Communications Officer (Press Office) Marketing, Communications & Insight | The University of Warwick [email protected] |Mobile: 07880 175 408 Notes to Editors The University of Warwick is one of the UK’s leading universities with over twenty-eight thousand students from 147 countries. Ranked 9th in the UK by The Guardian University Guide, it has an acknowledged reputation for excellence in research and teaching, for innovation, and for links with business and industry. The recent Research Excellence Framework classed 92% or it’s research as ‘world leading’ or ‘internationally excellent’. The University of Warwick was awarded University of the Year for Teaching Quality by The Times and Sunday Times. About the National Institute for Health and Care Research (NIHR): The mission of the National Institute for Health and Care Research (NIHR) is to improve the health and wealth of the nation through research. We do this by:
Funding high quality, timely research that benefits the NHS, public health and social care;
Investing in world-class expertise, facilities and a skilled delivery workforce to translate discoveries into improved treatments and services;
Partnering with patients, service users, carers and communities, improving the relevance, quality and impact of our research;
Attracting, training and supporting the best researchers to tackle complex health and social care challenges;
Collaborating with other public funders, charities and industry to help shape a cohesive and globally competitive research system;
Funding applied global health research and training to meet the needs of the poorest people in low and middle income countries.
NIHR is funded by the Department of Health and Social Care. Its work in low and middle income countries is principally funded through UK international development funding from the UK government.

IMAGES
COMMENTS
It is notable that as of April 26, 2021, more than 100,000 pregnant women reported having received a Covid-19 vaccination and yet only a small fraction (4.7%) have enrolled in the v-safe pregnancy ...
Despite effective vaccination strategies for the general population, the evidence on the safety and efficacy of Coronavirus disease 2019 (COVID-19) vaccinations in pregnancy is limited due to a ...
SARS-CoV-2 infection during pregnancy is associated with increased risk for maternal morbidity and adverse birth outcomes. 1,2 COVID-19 vaccines are effective for preventing severe disease, including in pregnant populations. 3 Although more than 100 countries recommend COVID-19 vaccination during pregnancy, 4 COVID-19 vaccination in pregnant people has lagged behind that for age-matched ...
What. Receiving a COVID-19 mRNA vaccine or booster during pregnancy can benefit pregnant people and their newborn infants, according to findings recently published in Vaccine. The paper describes results from the Multisite Observational Maternal and Infant Study for COVID-19 (MOMI-VAX), which was funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National ...
Clinical trials have shown that COVID-19 vaccines are remarkably effective in protecting those age 12 and up against infection by the coronavirus SARS-CoV-2. The expectation was that they would work just as well to protect pregnant women. But because pregnant women were excluded from the initial clinical trials, hard data on their safety and ...
COVID-19 vaccination during pregnancy builds antibodies that can help protect the baby. 4,5; Receiving mRNA COVID-19 vaccines during pregnancy can help protect babies younger than age 6 months from hospitalization due to COVID-19. Most babies hospitalized with COVID-19 were born to pregnant people who were not vaccinated during pregnancy. 6-8
Conclusion. SARS-CoV-2 infection poses significant risks to pregnant people and their infants, but COVID-19 vaccination is safe in pregnancy. This underlies the recommendation that pregnant people ...
Answering Questions with Research "With outbreaks of new diseases like COVID-19, I worry about possible effects on pregnancy and whether pregnancy might worsen symptoms," Edlow said. "When the COVID-19 pandemic began, no one knew how the virus affects pregnancy." ... V-safe COVID-19 vaccine pregnancy registry. Retrieved September 10 ...
VOL. 385 NO. 16. mRNA Covid-19 Vaccines in Pregnant Women ( Editorial, N Engl J Med 2021;384:2342-2343 ). At the time of publication of preliminary findings in the Original Article related to this ...
Pregnant people with COVID-19 are at increased risk of severe illness and death compared with non-pregnant females of reproductive age (aged 15-49 years).1 Additionally, COVID-19 during pregnancy is associated with increased risk for adverse pregnancy outcomes, such as preterm birth and stillbirth.1 When mRNA COVID-19 vaccines first became available in December, 2020, safety data in ...
A new observational study has begun to evaluate the immune responses generated by COVID-19 vaccines administered to pregnant or postpartum people. Researchers will measure the development and durability of antibodies against SARS-CoV-2, the virus that causes COVID-19, in people vaccinated during pregnancy or the first two postpartum months.
A total of 13 observational studies with 48,039 pregnant women who received Covid-19 vaccines were included in this systematic review. Among these studies, 10 (6 cohort, 1 cross-sectional, 1 case series, and 2 case reports) were conducted in the USA, while the other three (all cohort studies) were carried out in Israel in 2021.
COVID-19 in pregnancy, during the first 6 months of omicron as the variant of concern, was associated with increased risk of severe maternal morbidity and mortality, especially among symptomatic and unvaccinated women. Women with complete or boosted vaccine doses had reduced risk for severe symptoms, complications, and death. Vaccination coverage among pregnant women remains a priority.
Potential COVID-19 infection in pregnant women can be prevented using mRNA-based vaccinations. Gestation, childbirth, and perinatal mortality were proven unaffected by COVID-19 vaccination. Injection-site discomfort, tiredness, and migraine are the most prevalent side effects, but these are temporary.
The overall risk of death among pregnant people is low, but it is elevated compared to similar people who are not pregnant. Some studies suggest that COVID in pregnancy might be associated with increased rates of preterm birth. Our understanding of the probability and severity of harms from SARS-CoV-2 infection in pregnancy is evolving.
As of Nov 4, 2021, 191 360 women aged 15-49 years with known pregnancy status had completed the first vaccine dose survey and 94 937 had completed the second dose survey. 180 388 received one dose and 94 262 received a second dose of an mRNA vaccine, with 5597 pregnant participants receiving dose one and 3108 receiving dose two, and 174 765 non-pregnant participants receiving dose one and 91 ...
Pregnant women have an increased risk of severe COVID-19 compared with their nonpregnant counterparts and COVID-19 during pregnancy has been associated with fetal and neonatal morbidity and mortality. 1,2 Vaccination is routinely recommended to protect pregnant women and their newborns from acute respiratory tract infections, such as influenza ...
Research shows that vaccines, including the Pfizer and Moderna mRNA vaccines, are safe and effective while pregnant or breastfeeding. Vaccination prevents COVID-19 and reduces the risk of severe disease. Vaccines are very effective against SARS-CoV-2 variants. The antibodies your body makes after vaccination can travel to the baby before birth ...
C.D.C. Recommends Covid Vaccines During Pregnancy. New research shows the shots do not increase risk of miscarriage, the agency said, but the risks of severe disease from a coronavirus infection ...
The agency's recommendation follows a growing body of research that shows benefits of COVID-19 vaccine before or during pregnancy far outweigh the potential risks for moms and babies. It aims to improve vaccination rates among pregnant people in the U.S., only 31% of whom have received their COVID-19 shot.
Key Points. Question Is COVID-19 booster vaccination in early pregnancy associated with an increased risk of spontaneous abortion?. Findings In this case-control surveillance study of more than 100 000 pregnancies at 6 to 19 weeks' gestation from 8 health systems in the Vaccine Safety Datalink, the odds of having received a COVID-19 booster vaccination in either a 28- or 42-day exposure ...
More than 75 years of transformational research and hands-on social impact for a better ... A new study has disentangled the risks to infants and birth parents from infection with SARS-CoV-2—the virus that causes COVID-19— from risks related to broader societal changes during the pandemic period. ... less physical stress during pregnancy ...
Pregnant women and their unborn babies face heightened risks of serious illness from infectious diseases such as Influenza (flu), Pertussis (whooping cough), and COVID-19. The research shows that ...
But pregnancy is a factor that raises the risk of severe COVID-19. That risk stays higher for at least a month after giving birth. And the risk continues to go up if a pregnant person has other ...
The study is published as a research letter in JAMA Network Open. Researchers looked at live birth data from California hospital discharge records for 2019 to 2020. They compared pregnant women with COVID-19 in 2020, pregnant women without COVID-19 in 2020, and prepandemic pregnant women in 2019. ... coronavirus, and COVID-19 vaccines. News ...
Pregnant women and their unborn babies face heightened risks of serious illness from infectious diseases such as Influenza (flu), Pertussis (whooping cough), and Covid-19. The research, funded by the National Institute for Health and Care Research (NIHR), shows that despite the proven safety and efficacy of vaccinations during pregnancy, uptake ...
U.S. Food and Drug Administration