Loading metrics
Open Access
Peer-reviewed
Research Article

A systematic review of hepatitis B virus (HBV) drug and vaccine escape mutations in Africa: A call for urgent action
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing
Affiliation Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Roles Data curation, Methodology, Writing – review & editing
Roles Visualization
Affiliation Oxford University Academic IT Department, Oxford, United Kingdom
Roles Writing – review & editing
Affiliation Institute of Infection and Global Health, University of Liverpool, Liverpool, United Kingdom
Affiliation Division of Virology, University of the Free State/National Health Laboratory Service, Bloemfontein, Republic of South Africa
Roles Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing
* E-mail: [email protected]
Affiliations Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom, Department of Microbiology and Infectious Diseases, Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Headington, Oxford, United Kingdom
- Jolynne Mokaya,
- Anna L. McNaughton,
- Martin J. Hadley,
- Apostolos Beloukas,
- Anna-Maria Geretti,
- Dominique Goedhals,
- Philippa C. Matthews

- Published: August 6, 2018
- https://doi.org/10.1371/journal.pntd.0006629
- See the preprint
- Reader Comments
International sustainable development goals for the elimination of viral hepatitis as a public health problem by 2030 highlight the pressing need to optimize strategies for prevention, diagnosis and treatment. Selected or transmitted resistance associated mutations (RAMs) and vaccine escape mutations (VEMs) in hepatitis B virus (HBV) may reduce the success of existing treatment and prevention strategies. These issues are particularly pertinent for many settings in Africa where there is high HBV prevalence and co-endemic HIV infection, but lack of robust epidemiological data and limited education, diagnostics and clinical care. The prevalence, distribution and impact of RAMs and VEMs in these populations are neglected in the current literature. We therefore set out to assimilate data for sub-Saharan Africa through a systematic literature review and analysis of published sequence data, and present these in an on-line database ( https://livedataoxford.shinyapps.io/1510659619-3Xkoe2NKkKJ7Drg/ ). The majority of the data were from HIV/HBV coinfected cohorts. The commonest RAM was rtM204I/V, either alone or in combination with associated mutations, and identified in both reportedly treatment-naïve and treatment-experienced adults. We also identified the suite of mutations rtM204V/I + rtL180M + rtV173L, that has been associated with vaccine escape, in over 1/3 of cohorts. Although tenofovir has a high genetic barrier to resistance, it is of concern that emerging data suggest polymorphisms that may be associated with resistance, although the precise clinical impact of these is unknown. Overall, there is an urgent need for improved diagnostic screening, enhanced laboratory assessment of HBV before and during therapy, and sustained roll out of tenofovir in preference to lamivudine alone. Further data are needed in order to inform population and individual approaches to HBV diagnosis, monitoring and therapy in these highly vulnerable settings.
Author summary
The Global Hepatitis Health Sector Strategy is aiming for the elimination of viral hepatitis as a public health threat by 2030. However, mutations associated with drug resistance and vaccine escape may reduce the success of existing treatment and prevention strategies. In the current literature, the prevalence, distribution and impact of hepatitis B virus (HBV) mutations in many settings in Africa are neglected, despite the high prevalence of HBV and co-endemic HIV infection. This systematic review describes the frequency, prevalence and co-occurrence of mutations associated with HBV drug resistance and vaccine escape mutations in Africa. The findings suggest a high prevalence of these mutations in some populations in sub-Saharan Africa. Scarce resources have contributed to the lack of HBV diagnostic screening, inconsistent supply of drugs, and poor access to clinical monitoring, all of which contribute to drug and vaccine resistance. Sustainable long-term investment is required to expand consistent drug and vaccine supply, to provide screening to diagnose infection and to detect drug resistance, and to provide appropriate targeted clinical monitoring for treated patients.
Citation: Mokaya J, McNaughton AL, Hadley MJ, Beloukas A, Geretti A-M, Goedhals D, et al. (2018) A systematic review of hepatitis B virus (HBV) drug and vaccine escape mutations in Africa: A call for urgent action. PLoS Negl Trop Dis 12(8): e0006629. https://doi.org/10.1371/journal.pntd.0006629
Editor: Manuel Schibler, University of Geneva Hospitals, SWITZERLAND
Received: February 8, 2018; Accepted: June 22, 2018; Published: August 6, 2018
Copyright: © 2018 Mokaya et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: JM is funded by a Leverhulme Mandela Rhodes Doctoral Scholarship. PCM is funded by a Wellcome Trust Intermediate Fellowship (grant number 110110), https://wellcome.ac.uk/ . The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
In 2015, the World Health Organisation (WHO) estimated that 3.5% of the world’s population (257 million people) were living with Hepatitis B virus (HBV) infection, resulting in 887,000 deaths each year, mostly from complications including cirrhosis and hepatocellular carcinoma (HCC) [ 1 ]. United Nations Sustainable Development Goals set out the challenge of elimination of viral hepatitis as a public health threat by the year 2030 [ 2 ]. One of the existing strategies in the elimination toolbox is use of antiviral drugs in the form of nucleos(t)ide analogues (NAs). Suppression of viraemia not only reduces inflammatory and fibrotic liver disease in the individual receiving treatment but also reduces the risk of transmission. However, the emergence of HBV resistance-associated mutations (RAMs) is a potentially significant concern for the success of this strategy.
Africa is the continent with the second largest number of individuals with chronic HBV (CHB) infection, with an estimated 6.1% of the adult population infected [ 1 ]. However, there is little commitment and resource invested into the burden of this disease, and many barriers are contributing to the epidemic [ 3 , 4 ]. Globally, less than 10% of the population with CHB are diagnosed, with an even smaller proportion on treatment [ 1 , 4 ]. This proportion is likely to be even lower in Africa. The situation in Africa is further complicated by the substantial public health challenge of coendemic human immunodeficiency virus (HIV) and HBV; coinfection worsens the prognosis in dually infected individuals [ 5 ]. There is also a lack of robust epidemiological data on HBV from Africa [ 3 , 4 ].
Widespread use of antiretroviral therapy (ART) for HIV, incorporating NAs that also have activity against HBV, may have an impact on HBV through improved rates of viraemic suppression, but also potentially by driving the selection of RAMs. The WHO recommends screening for Hepatitis B virus surface antigen (HBsAg) in all HIV-1 infected individuals prior to ART initiation, and for all pregnant women during antenatal visits, to improve the clinical outcomes of people living with CHB and to enhance interventions that reduce the incidence of new cases [ 6 ]. However, screening of HBsAg is not routinely performed in many settings in Africa, with lack of implementation at least partially driven by cost and lack of programmes for HBV treatment outside the setting of HIV coinfection. HBV infected patients either remain untreated (most typical in the setting of monoinfection), or are exposed to antiviral drugs without proper monitoring and often intermittently, putting them at risk of developing RAMs (more likely in the setting of HIV coinfection) [ 4 , 7 – 10 ].
HBV is a DNA virus that replicates via an RNA intermediate, with reverse transcriptase (RT) catalysing the transcription of RNA into DNA [ 7 ]. NAs that inhibit RT are therefore used to prevent HBV replication, including lamivudine (3TC), entecavir (ETV) and tenofovir (conventionally in the form of tenofovir disoproxil fumarate (TDF), but more recently available as the prodrug, tenofovir alafenamide fumarate (TAF)), with mostly historical use of other agents including telbivudine (LdT) and adefovir (ADV) [ 6 , 11 ]. Choice of TDF/TAF or ETV is determined by availability, cost, safety profile and barrier to resistance [ 4 ]. In Africa, the choice of agent is usually limited to 3TC or TDF. Emergence of mutations happens because the RT enzyme is error-prone and lacks the proofreading function required to repair errors during transcription [ 7 ]; when these mutations confer a selective advantage by allowing the virus to escape the effect of drug therapy, they will become amplified in the viral population. Some RAMs confer resistance to one agent only, while others are associated with resistance to several agents ( Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
HBV genes are shown in the coloured ovals. TDF = tenofovir, ETV = entecavir, 3TC = lamivudine. This figure incorporates data from eight studies; three were identified by the systematic review presented in this manuscript [ 12 – 14 ] and five from the wider literature [ 7 , 15 – 18 ].
https://doi.org/10.1371/journal.pntd.0006629.g001
3TC was originally seen as a major breakthrough in treating HBV [ 19 ]. However, it is now known to have a low genetic barrier to resistance and its long-term effectiveness is limited as a result of resistance mutations in the ‘YMDD’ motif (tyrosine, methionine, aspartate, aspartate; amino acids 203–206) in domain C of the viral polymerase (Pol). These occur with associated upstream mutations in Pol domains A, B and in the B-C interdomain [ 7 , 15 , 16 ]. Among chronic HBV monoinfected patients, incidence of HBV resistance to 3TC is as high as 20% per year. In HIV/HBV coinfected patients, this can reach 90% over 5 years of treatment, as development of resistance is accelerated in HIV coinfection [ 5 , 20 ]. 3TC has also been associated with the induction of cross-resistance to emtricitabine (FTC), LdT, and at least partially ETV, thus reducing the options for subsequent treatment [ 10 ].
TDF is widely used in treatment of both HIV and HBV and is generally well tolerated. TDF has a high genetic barrier to resistance and maintains effective suppression of HBV in both monoinfected and HIV/HBV coinfected individuals [ 5 , 7 , 10 , 21 , 22 ]. Although it has a recognised association with nephrotoxicity in HIV treatment, current literature suggests it may be better tolerated in HBV infection [ 11 ]. Conversely, African populations have a higher background of renal disease [ 23 ] and could be potentially more vulnerable to nephrotoxitiy from TDF [ 24 ]. TAF delivers equally potent viraemic suppression at lower plasma levels, and is therefore associated with reduced nephrotoxicity [ 25 ], but is not available in Africa at present. HBV resistance to TDF is not well characterised, but there are emerging data from in vitro studies associating Pol mutations rtA194T and rtN236T with decreased susceptibility [ 11 , 21 ]. Virological breakthrough on TDF therapy has been reported in two patients harbouring rtS78T/sC69 mutations [ 17 ], and in another patient with multi-site polymerase mutations; rtL80M, rtL180M, rtM204V/I, rtA200V, rtF221Y, rtS223A, rtT184A/L, rtR153Q, and rtV191I [ 26 ]. The significance of these mutations needs to be further explored in clinical studies.
First line ART treatment regimens for HIV in sSA now almost universally include TDF, and current guidelines also recommend TDF-based regimens in individuals with HBV/HIV coinfection [ 27 ]. Accordingly, in both HIV monoinfection and HBV/HIV coinfection, use of TDF has increased across much of Africa. Nevertheless, it remains the case that 3TC is used as the only HBV-active agent in some settings [ 7 , 8 ], as well as in second line regimens, exemplified by South Africa where second line ART substitutes Zidovudine (AZT) for TDF leaving only 3TC coverage for HBV [ 28 ]. Among HBV/HIV coinfected children in South Africa treated with regimens including 3TC and/or TDF, HBV viraemia has been demonstrated, highlighting potential underlying HBV drug resistance [ 29 ].
ETV is another active agent, and is safe and well tolerated. However it is not active against HIV and therefore has to be added to ART regimens rather than being part of the primary backbone, is not recommended in pregnancy, and is not routinely available in most African settings [ 30 ]. Resistance arises more commonly in the context of prior 3TC exposure [ 11 , 31 ], which may limit its future potential in Africa, particularly in HIV endemic populations.
As a component of the Expanded Programme on Immunization (EPI), HBV preventive vaccines have been rolled out in Africa since 1995 [ 4 ]. HBV vaccine is highly effective in prevention of mother to child transmission (PMTCT); when administered to infants within 24 hours of birth followed by a dose given at 6 and another at 14 weeks to complete the primary series, it reduces the rate of mother to child transmission by 85%–95% [ 32 , 33 ]. However, by 2016 only 11 countries in Africa had adopted birth dose HBV vaccination as part of the routine infant immunisation schedule [ 34 ]. Changes in the S protein can result in vaccine escape mutants (VEMs) [ 16 , 18 ], and also diagnostic escape mutations which result in false negative HBsAg testing [ 16 ]. Mutations in HBV Pol can also lead to amino acid changes in the Surface (S) protein due to overlapping reading frames (ORFs) in the genome [ 16 ]. Whilst the S protein mutation sG145R has been identified as the major VEM, recently other mutations in S protein have been associated with immune escape [ 16 ] Fig 1 . There are very few data for VEMs in Africa, but in other settings of high endemicity, VEMs can be common, as evidenced by a reported prevalence of 28% in vaccinated HBV-infected children in Taiwan [ 35 ].
To date, no systematic review has assessed the geography and prevalence of HBV RAMs and VEMs in Africa. An understanding of the extent to which these mutations circulate in Africa is essential to improving HBV therapy in patients with and without HIV coinfection. We therefore set out to describe the frequency, co-occurrence and distribution of RAMs and VEMs in Africa, and to suggest whether changes are needed in recommendations for laboratory diagnostics and/or approaches to drug therapy or vaccine deployment. This will underpin further research to identify and track relevant mutations in these populations.
Search strategy
Between October 2017 and January 2018, we searched the published literature, in MEDLINE (PubMed; https://www.ncbi.nlm.nih.gov/pubmed ), SCOPUS ( https://www.elsevier.com/solutions/scopus ) and EMBASE ( https://www.elsevier.com/en-gb/solutions/embase-biomedical-research ). Our search strategy is detailed in S1 Table (documenting use of PRISMA criteria and selection of studies) and S2 Table (listing our search criteria). The earliest paper we identified on HBV drug resistance in Africa was published in 2007. We reviewed the titles and abstracts matching the search terms and only included those relating to drug or vaccine resistance in HBV infection, including only those that presented original data and had undergone peer review. All retrieved articles were in English, therefore no exclusion in relation to language was required.
For each publication we recorded reference, publication year, study design, sample size, study population, proportion of participants who tested HBsAg+ or HBV DNA+, country, year(s) of specimen collection, genotype identified, antiviral treatment, sequencing method, gene sequenced, number of sequenced samples, participant recruitment site and sequence accession number. Data were curated using MS Excel software (Microsoft, Redmond, WA).
RAMs reported in published sequences not represented in primary studies
We expanded our search for evidence of RAMs by identifying publicly available HBV sequences from Africa, that had not been included in the results of our primary literature search. We used both the Hepatitis B Virus database ( https://hbvdb.ibcp.fr/ [ 36 ] and Hepatitis Virus Diversity Research Alignments database ( http://hvdr.bioinf.wits.ac.za/alignments/ ) [ 37 ].
In order to determine the prevalence of RAMs and VEMs, we first reported these using the denominator (total number of HBV positive patients) and numerator (total number of HBV positive patients with the specified mutation) as reported in published studies. We also pooled data by country in order to provide regional estimates. Downloaded sequences were managed using Sequence editor, database and analysis platform, SSE version 1.3, for analysis [ 38 ].
Data visualisation
We developed an R package, gene.alignment.tables, for the visualisation of the sequence data in this study; this is available on Github [ 39 ] and can be used for visualising generic gene sequence datasets. The package was developed by University of Oxford’s Interactive Data Network and a specific instance of the visualisation is hosted as a Shiny app which can be viewed here: https://livedataoxford.shinyapps.io/1510659619-3Xkoe2NKkKJ7Drg/ [ 40 ].
The initial search yielded 56 articles in MEDLINE, 150 in SCOPUS and 150 in EMBASE. Of these, 32, 136 and 119 were excluded from search results of MEDLINE, SCOPUS and EMBASE respectively, as they did not did not meet the inclusion criteria. After de-duplication, 37 articles were included. 27 articles identified from MEDLINE, SCOPUS and EMBASE were identical; five unique articles were included from EMBASE, four from SCOPUS and one from MEDLINE. A total of 37 articles were downloaded in full ( S1 Table (part II); S3 Table ).
Study characteristics
Epidemiological data for HBV represented by the 37 studies we identified are summarised in Table 1 . Studies included were from Southern Africa (Botswana, Mozambique, South Africa, Zambia and Zimbabwe), East Africa (Ethiopia, Kenya, Malawi, Sudan and Uganda), West Africa (Cote d’Ivoire, Gambia, Ghana, Guinea-Bissau and Nigeria) and Central Africa (Cameroon, Gabon). There was considerable heterogeneity in recruitment protocols and exposure to anti-viral treatment. Twenty-six studies recruited from hospitals, three studies recruited from the community [ 8 , 41 , 42 ] and eight studies did not specify where recruitment was undertaken [ 10 , 43 – 49 ]. All studies were observational.
https://doi.org/10.1371/journal.pntd.0006629.t001
Study populations were categorised as follows:
- HBV/HIV coinfected patients: (n = 28 studies), [ 8 – 10 , 12 – 14 , 20 , 43 – 48 , 50 – 56 , 58 – 62 , 64 , 67 , 70 ];
- HBV infected with and without HIV coinfection: (n = 8 studies), [ 41 – 43 , 57 , 63 , 65 , 66 , 68 ];
- Chronic HBV monoinfection: (n = 1 study), [ 69 ].
Antiviral treatment exposure varied as follows:
- Treatment-naïve: (n = 8 studies), [ 14 , 46 – 48 , 63 , 64 , 68 , 70 ];
- 3TC-based regimen only: (n = 10 studies), [ 8 , 9 , 20 , 44 , 45 , 49 , 53 , 59 , 61 , 69 ];
- Regimens including 3TC or TDF: (n = 6 studies), [ 10 , 13 , 51 , 52 , 55 , 65 ];
- Mixed regimen where some received 3TC, others TDF, while others left untreated; (n = 7 studies), [ 12 , 50 , 54 , 56 , 60 , 62 , 66 ];
- Treatment regimen not specified: (n = 6 studies), [ 41 – 43 , 57 , 58 , 67 ].
HBV amino acid polymorphisms were studied from within the following proteins;
- Pol only (n = 13 studies), [ 8 , 9 , 12 – 14 , 20 , 44 , 53 , 55 , 56 , 59 , 63 , 68 ]; only one of these used a deep sequencing method [ 20 ];
- S only (n = 3 studies), [ 43 , 57 , 65 ];
- Pol and S (n = 12 studies),[ 10 , 41 , 45 , 48 , 51 , 52 , 54 , 58 , 60 , 62 , 64 , 67 ];
- Pol, S and PC/BCP (n = 4 studies), [ 47 , 50 , 61 , 69 ];
- S and PC/BCP region (n = 3 studies), [ 42 , 46 , 70 ];
- Whole genome (n = 2 studies), [ 49 , 66 ].
All studies, except for two [ 53 , 68 ], specified the HBV genotype ( S3 Table & S2 Fig ).
Prevalence of HBsAg, HBeAg and HDV coinfection
The prevalence rates of HBsAg in these study cohorts ranged from 3%-26%; however, the populations included were highly selected and therefore not necessarily representative of the general population, particularly as a result of a strong bias towards HIV-infection ( Table 1 ). Only three studies included in this review reported on HDV prevalence: two studies did not detect any HDV antibodies [ 63 , 65 ], whereas the other study reported a HDV prevalence of 25% in Guinea-Bissau [ 56 ].
RAMs identified in African cohorts
The co-occurrence and distribution of HBV RAMs and VEMs are summarised according to the region where they were identified ( Fig 2 ). This illustrates the patchy and limited data that are available, with South Africa, Ghana and Cameroon best represented, but with large areas (especially in northern and central Africa) not represented at all in the literature.
Mutations identified from 33 studies of African cohorts published between 2007 and 2017 (inclusive). Four studies identified by our systematic literature review were not represented here as they did not report any RAMs. Full details of each citation can be found in Table 1 .
https://doi.org/10.1371/journal.pntd.0006629.g002
Although 35 studies specified the HBV genotype, it was only possible to group RAMs according to genotype in fourteen studies [ 8 , 9 , 13 , 14 , 44 , 46 , 47 , 50 , 51 , 56 , 60 – 62 , 69 ] ( S1 Fig ; S2 Fig ). The remaining 21 studies generally reported the genotypes identified, but did not specifically state the genotype of HBV within which RAMs were identified.
We have developed an interactive tool to display the genomic positions of RAMs identified through our literature review alongside relevant metadata. This can be accessed on-line here: https://livedataoxford.shinyapps.io/1510659619-3Xkoe2NKkKJ7Drg/ [ 40 ].
Overall, the most prevalent RAM was rtM204V/I in both treatment experienced and treatment naïve individuals, and occurring either alone or in combination with other polymorphisms rtL80I/V, rtV173L, rtL180M, rtA181S, rtT184S, rtA200V and/or rtS202S ( Fig 3 ); mutations among individuals with and without exposure to HBV therapy are listed in S4 Table and S5 Table , respectively). This mutation was present in 29 studies at a highly variable prevalence of between 0.4% [ 12 ] and 76% [ 69 ]. Across all cohorts, the mutation was present in 208/2569 (8%) of all individuals represented. The mutation, by itself, was most prevalent in South Africa; on pooling data for three studies from this setting, it was present in both treatment experienced and treatment naïve patients (n = 13/17, 76% [ 69 ] and n = 16/72, 22% [ 67 , 68 ] respectively). In addition to South Africa, rtM204I/V was also frequent in Malawi among treatment experienced patients (n = 24/154, 16% [ 20 , 45 ]) ( Fig 3 ), and in genotype non-A infection: in this setting, the mutation was detected in genotype C infection (n = 2/17, 12% [ 69 ]) ( S2 Fig ).
These data are derived from 27 studies of HBV drug resistance in Africa published between 2007 and 2017 (inclusive). The countries represented are listed in alphabetical order. A detailed summary of RAMs identified from each study is presented ( Fig 2 , S4 Table , S5 Table ). Prevalence of RAMs for a specific country was determined by grouping all studies from that country that reported a specific mutation. We used all individuals who tested HBsAg positive to generate a denominator in order to provide a conservative estimate of RAM prevalence, and the numerator was the total number of individuals with that specific mutation from these studies. A: treatment naïve; B: treatment experienced.
https://doi.org/10.1371/journal.pntd.0006629.g003
The rtM204I/V mutation by itself confers resistance to 3TC; in combination with A194T it may also be associated with reduced efficacy to TDF, and in combination with L180M and V173L with vaccine escape, through corresponding substitutions in the surface antigen sites targeted by neutralising antibodies. Although TDF has a high genetic barrier to resistance, and is associated with reliable suppression of HBV viraemia [ 7 , 10 , 21 , 22 ], mutations rtN236T and rtA194T, which have been linked with resistance to both TDF and ADV [ 7 ], have been identified in Southern Africa in both treatment naïve [ 14 ] and treatment experienced [ 10 ] patients.
WHO guidelines recommend a first-line regimen including TDF in HIV/HBV coinfected patients [ 6 ], and the South African Department of Health HIV/AIDS treatment guideline included TDF as first-line regimen from 2010 [ 71 ], however we found a minority of studies (9/37, 24%) reporting TDF-containing regimens for HIV/HBV coinfected individuals. As anticipated, most of the studies that did use TDF were carried out after 2010, whereas those that used 3TC were generally earlier ( S3 Table ).
From this dataset, it is difficult to ascertain whether RAMs are genuinely more prevalent in genotype A infection, or this simply reflects enrichment of genotype A in sub-Saharan African populations ( S2 Fig ). Interpreting RAMs according to sub-genotypes was difficult since most studies did not specify sub-genotype and others did not indicate which RAMs were identified in which genotype. Of concern is the detection of RAMs even in reportedly treatment naïve individuals ( Fig 3 & S4 Table ), suggesting that RAMs are being transmitted. A study in South Africa that recruited 3TC-naïve HBV infected adults with or without HIV, reported rtM204I in 13/35 (37%) individuals [ 68 ].
HBV RAMs in published sequences from Africa
We searched the Hepatitis B Virus database and GenBank to identify HBV sequences derived from Africa, from studies not already included in our review. We identified an additional 69 isolates: 23 had undergone full length genome sequencing whereas 46 isolates represented either the polymerase (n = 3) or S region (n = 43) of the HBV genome Table 2 . To avoid duplication of results, we excluded fourteen studies already identified by our literature review that had submitted their sequences to GenBank ( S3 Table ). RAMs in the additional 69 isolates were as follows:
- rtM204V in genotype A (2/69, 2.9% of sequences), this occurred in combination with rtL180M;
- rtM204V + rtL180M in genotype E (1/69, 1.5%);
- rt180M + rtA181V in genotype E (1/69 (1.5%);
- rtQ215S identified in genotype D (4/69, 5.8%).
All these mutations are associated with 3TC resistance; rtA181V has also been associated with reduced susceptibility to TDF [ 7 , 15 ].
In the S gene, the most prevalent mutations were:
- sD144A/E/G occurring in genotype A (6/69, 8.7%), D (10/69, 14.5%) and E (7/69, 10.1%) associated with VEM;
- sI110L occurring in genotype A (3/69, 4.3%), D (4/69, 5.8%) and E (11/69, 15.9%) associated with immunoglobulin resistance.
https://doi.org/10.1371/journal.pntd.0006629.t002
VEMs were identified in Central, East, West and Southern Africa ( Fig 2 ). However, it was not possible to ascertain whether individuals harbouring these mutations had been vaccinated against HBV infection. The most common VEM was the triple mutation rtV173L + rtL180M + rtM204I/V, found in the Pol gene. This suite of mutations was identified in 14 studies [ 12 , 13 , 20 , 44 , 50 – 55 , 58 – 60 , 62 ], at a pooled prevalence of 4% (57/1462). Another significant VEM, sG145K/R [ 16 ], was identified in six studies [ 12 , 42 , 47 , 57 , 60 , 62 ] and sM133L/T, associated with VEM, immunoglobulin and diagnostic escape mutation [ 12 , 48 ], was identified in seven studies [ 12 , 41 , 47 , 48 , 57 , 62 , 70 ] ( Fig 2 ).
To our knowledge, this is the first systematic review that assesses RAMs and VEMs for HBV in Africa. The high rates of HBV infection among HIV infected individuals in some locations including Cameroon [ 60 ] and South Africa [ 10 ] could be an indication that HBV infection has been previously under-reported, possibly due to lack of routine screening, poor awareness, stigma, high costs and limited clinical and laboratory infrastructure [ 4 , 8 – 10 , 45 , 53 ]. The literature suggests a widespread exposure of the HIV-infected population to 3TC-based treatment. This may be changing over time in line with current ART treatment recommendations (regimens for Africa summarised in S6 Table ), but the introduction of TDF-based regimens for HIV treatment has been inconsistent, and TDF monotherapy is not consistently available for HBV infection in the absence of HIV.
In keeping with other settings, the most common RAM identified here was rtM204I/V, either alone or in combination with compensatory mutations rtL180M ± rtV173L. Of concern, rtM204V/I was seen in 76% of treatment experienced patients [ 69 ] and 22% of treatment naïve patients [ 67 , 68 ] in South Africa. A review of worldwide incidence of RAMs among treatment naïve patients also described rtM204V/I as the most frequent, but with a much lower prevalence of 5% [ 72 ]. The contribution of unreported or undocumented 3TC exposure in the reportedly treatment naïve populations remains to be determined. A European study demonstrated that the most frequent primary mutation was rtM204V/I, found in 49% of treatment experienced patients [ 73 ], while in China rtM204I, rtN236T and rtL180M+rtM204V+rtV173L/rtS202G were also the most prevalent RAMs [ 74 ].
The triple mutation rtM204V + rtL180M + rtV173L has been identified in East, West and Central Africa [ 20 , 44 , 51 – 54 , 59 ]. This combination of polymorphisms is associated with both vaccine escape and resistance to 3TC and other L-nucleoside analogues [ 20 , 44 , 51 , 54 , 59 , 60 ]. Interestingly, this triple mutation has not been reported in the Southern African region to date, which is likely to reflect the composition of the study populations.
Clinical and public health significance of RAMs
Apart from the nature of drugs being used for HBV treatment, other reported predictors of HBV drug resistance include HBV viral load, HBV intra host heterogeneity, HBeAg status, host body mass index and serum alanine aminotransferase (ALT) activity [ 20 , 75 , 76 ]. Individuals with rtM204V/I plus compensatory mutations typically exhibit high HBV DNA levels [ 20 ] and are therefore highly infectious to others. The spread of RAMs may lead to a rise in drug resistance in treatment naïve chronic HBV infection, representing a substantial challenge for Africa and highlighting an imperative to ensure routine use of TDF in preference to, or in combination with, 3TC-based therapy.
Although these data provide a preliminary picture of the prevalence of RAMs in some settings, there are no recommendations to stipulate any specific prevalence threshold above which HBV drug resistance mutations represent a significant barrier to successful treatment at a population level, and/or RAM prevalence thresholds that should trigger a switch to alternative first-line therapy. For HIV, surveillance for transmission of RAMs is based on screening recently infected, treatment naive individuals, and classifies drug resistance using thresholds of <5%, 5–15%, and >15% to stratify the risk to public health [ 77 ]. Similar thresholds and recommendations for HBV could help to underpin the assimilation of epidemiological data and to unify treatment approaches.
TDF resistance
The identification of mutations associated with reduced TDF susceptibility are of concern, as they suggest the potential for increasing prevalence of polymorphisms that confer partial or complete viral escape from a drug that to date has not been widely associated with resistance. There is now potential for increasing selection of TDF resistance as this drug becomes more widely used. However, as a new first line single tablet option incorporating 3TC, TDF and Dolutegravir (DTG) (triple therapy abbreviated to ‘LTD’) emerges as a recommended option for HIV treatment in Africa, surveillance is needed to determine the clinical outcomes for HBV [ 78 ].
If clinically significant, TDF resistance mutations may still represent a particular problem for many African settings, as resource constraints make it unrealistic to provide baseline screening for RAMs, or to monitor patients on treatment with serial viral load measurements. Despite these potential concerns, it has been shown that TDF is effective even in the presence of RAMs and that there is comparable efficacy among 3TC-experienced and NA-naïve patients [ 79 ].
VEM were identified in 16 different countries in East, West, Central and Southern Africa. Information on vaccine exposure was not available, but there are two strands of evidence to support significant population exposure to HBV vaccination. First, vaccination has been progressively rolled out in most countries in sSA since the mid-1990’s; second, most HBsAg mutations reported by these studies are located within the common immunodominant B cell epitope (aa 124–147) in which selection of polymorphisms is associated with HBV vaccination [ 80 , 81 ].
VEM have been more robustly reported from Asia, in settings where the HBV infant vaccination programme is well established; for example, in Taiwan, VEM prevalence among vaccinated children increased from 7.8% to 23.1% within 15 years of the launch of the universal vaccine program, although the decline in VEM prevalence thereafter may be partly related to a smaller HBV carrier pool [ 80 ]. HBV infection despite immunoprophylaxis can occur either as a consequence of MTCT of pre-existing VEM, or as a result of de novo selection of escape mutations from vaccine- induced immune responses, particularly in the setting of delayed vaccination [ 80 , 81 ].The HBV genotype sequence used for vaccines may potentially have an influence on immunogenicity against non-vaccine genotypes, but there are limited data to support this [ 82 ]. Only 11 African countries recommend the first HBV vaccine dose at birth, in contrast to the majority of African countries in which HBV vaccination is delayed until 6 weeks of age [ 33 ]. It is likely that this delay not only provides a window of infection but also increases the possibility of transmitted VEM and/or emergence of new escape mutations.
High maternal HBV viral load and immunosuppression are other risk factors associated with VEM among infants [ 80 ]; both of these are pertinent for emergence of VEMs in Africa given that HBV viral load testing is not routinely available, and HIV is highly prevalent in some populations. Effective PMTCT strategies in Africa, including screening and treating antenatal women, increasing access to viral load monitoring, and introducing HBV birth dose vaccine will help to decrease the prevalence of VEM [ 4 , 33 , 83 ].
HDV/HBV coinfection
One study from our literature review reported a high HDV prevalence of 25%; however, in this cohort, RAMs occurred in individuals with HBV monoinfection [ 56 ]. Given that HDV is characteristically associated with decreased HBV replication [ 84 ], it is possible that emergence of HBV RAMs is altered in this setting. However, as the true prevalence and impact of HDV in sSA is not known [ 85 ], further studies are needed to determine the impact of HDV coinfection on HBV RAMs.
Limitations of current data
Screening for HBV infection is not routinely performed in many African settings and therefore the true prevalence and characteristics of HBV infection are not known [ 4 , 7 – 10 ]. We identified very few published studies; only a minority of patients had HBV sequencing undertaken, and there were no data from certain regions of Africa. This highlights the substantial problem of HBV neglect in Africa, and a specific blind-spot relating to sequence data [ 4 ]. Identifying the true prevalence of resistance mutations, and characterising the populations in which these are selected and enriched, is currently not possible due to sparse data and lack of clear descriptions of the denominator population. Most such studies do not perform a truly systematic assessment, but focus on high risk groups–particularly including those with HIV/HBV coinfection: of the 37 studies included here, only one exclusively reported on participants who were HBV mono-infected [ 69 ]. Although we have made every effort to assimilate the relevant data to build up a regional picture for Africa, the heterogeneity between studies makes it difficult to draw robust conclusions from pooled data. These findings are a reflection of the little attention paid towards the burden of this disease in Africa and the neglect in robust epidemiological data.
Only nine studies undertook a longitudinal approach to detection of drug resistance [ 8 – 10 , 20 , 44 , 45 , 49 , 52 , 53 ]. The results of the other 28 studies that undertook a cross-sectional approach could be skewed by the timing of recruitment of study participants, with a risk of under-representation of drug resistance if screening is undertaken only at baseline, and potentially an over-representation if screening is undertaken in patients with HIV coinfection, who are more at risk of advanced disease and prolonged drug exposure. As most of these studies recruited individuals from hospital settings, this raises the latter possibility.
Mutations across the whole genome might be relevant in determining resistance [ 86 ]. However, most of the included studies analysed only defined genes from within the HBV genome; only two sequenced the whole genome, and these determined consensus sequence. This potentially results in an under-representation of RAMs and VEMs that may be present as low numbers of quasispecies, but could become significant if selected out by exposure to drug or vaccine.
In studies that reported RAMs among treatment naïve individuals, the literature suggests that sequence analysis was performed prior to ART initiation. However, we cannot exclude the possibility that that some of these participants had prior ART exposure. Due to the nature of the cohorts that have been studied, most of the RAMs identified were from HIV/HBV coinfected individuals. It is possible that HIV increases the risk of HBV RAMs both in terms of drug exposure, and also as a function of increased HBV viral loads. A study from Malawi demonstrated the rapid emergence of 3TC resistance in HIV coinfection, with virtually all treatment naïve HBeAg positive individuals starting antiviral treatment showing emergence of rtM204I by six months. Likewise, a study carried out in Italy revealed that patients with HIV coinfection were more likely to harbour the rtM204V mutation and to show multiple mutations compared to HBV monoinfected patients [ 87 ]. It would be worth further exploration of this observation in Africa, as there are currently very limited data.
Challenges and opportunities for Africa
A major challenge for Africa is to improve coverage rates of infant vaccination, deploy catch-up vaccination programmes for older children and adults, adopt widespread screening and develop treatment programmes for HBV. While HBV vaccine is effective, gaps in vaccine coverage in Africa can be demonstrated by the high perinatal transmission rate of HBV in sSA (estimated at 38% among women with a high HBV viral load) and the observation that up to 1% of newborns in sSA are still infected with HBV [ 88 ]. Sustained efforts are required to build robust PMTCT programmes that deliver screening and treatment for antenatal women, and timely administration of HBV birth vaccine for their babies [ 33 , 83 ].
Although the WHO recommends monitoring for the development of drug resistance once on therapy [ 6 ], implementation remains challenging as viral load monitoring and sequencing are both rarely available [ 7 ]; despite the advancement and availability of HIV testing and monitoring, in many settings it remains uncommon to monitor HIV viral load after ART initiation [ 89 , 90 ]. Affordable, accessible and sustainable platforms for quantifying both HIV and HBV viral loads remain an important priority for many settings in Africa, given the lack of on-treatment monitoring in many settings. Given the simplicity and relative ease of collection, preparation and transport of dried-blood-spot (DBS) samples [ 91 ], adopting DBS testing could improve access to HBV diagnosis, viral load monitoring and linkage to care, especially in areas with limited access to laboratory facilities.
Development of a cheap, rapid test for the detection of the most frequently observed RAMs and VEMs should be considered as a potentially cost-effective strategy for Africa. Proof of principle for a rapid test for diagnosis and detection of resistance has been demonstrated by the GeneXpert MTB/RIF assay for Mycobacterium tuberculosis (MTB) [ 92 ]. A similar approach has been applied for HBV through use of a multiplex ligation-dependent probe real time PCR (MLP-RT-PCR) [ 93 ]. Although this assay is able to detect RAMs quickly and cheaply, there are still limitations as the test requires high viral load samples, is based on detection of known RAMs from within discrete regions of the genome, and may not identify RAMs that are present as minor quasispecies.
New metagenomic sequencing platforms, such as Illumina and Nanopore, provide the opportunity for whole deep genome sequencing, which can reveal the full landscape of HBV mutations in individual patients, quantify the prevalence of drug resistance mutations among HBV quasi-species, and determine the relationship between these polymorphisms and treatment outcomes [ 87 ]. Nanopore technology also has the potential to develop into an efficient point of care test that could detect viral infection and coinfection, as well as determining the presence of VEMs and RAMs [ 94 ], but is currently limited by cost and concerns about high error rates.
There have been few studies looking at the correlation between genotype, clinical outcomes of disease, response to antiviral therapy and RAMs/VEMs, but none from Africa. Studies outside Africa have shown that genotype A is more prone to immune/vaccine escape mutants, pre-S mutants associated with immune suppression, drug associated mutations and HCC in HIV/HBV coinfected participants [ 46 , 87 , 95 ]. Studies investigating the role of genotypes in predicting response to antiviral therapy and their association with various types of mutations are urgently needed in Africa, particularly in light of the high frequency of genotype A infection and high population exposure to antiviral agents that have been rolled out over the past two decades as a component of first-line ART.
Existing infrastructure for diagnosis, clinical monitoring and drug therapy for HIV represents an opportunity for linkage with HBV care. Particularly in settings of limited resource, joining up services for screening and management of blood-borne virus infection could be a cost-effective pathway to service improvements.
Conclusions
This review highlights the very limited data for HBV RAMs and VEMs that are available from Africa. Scarce resources resulting in lack of diagnostic screening, inconsistent supply of HBV drugs and vaccines, and poor access to clinical monitoring contribute to drug and vaccine resistance, potentially amplifying the risk of ongoing transmission and adding to the long-term burden of HBV morbidity and mortality in Africa. We call for urgent action to gather and analyse better data, particularly representing the HBV monoinfected population, and for improved access to TDF.
HBV RAMs and VEMs have been identified in several African countries among HIV/HBV coinfected and HBV monoinfected patients, before and during treatment with NAs but the data are currently insufficient to allow us to form a clear picture of the prevalence, distribution or clinical significance of these mutations. Overall, the data we describe suggest a significantly higher prevalence of drug resistance in some African populations than has been described elsewhere, and that is not confined only to drug-exposed populations, highlighting an urgent need for better population screening, assessment of HBV infection before and during therapy, and increasing roll out of TDF in preference to 3TC. At present, TDF accessibility is largely confined to HIV/HBV coinfected individuals; we now need to advocate to make monotherapy available for HBV monoinfected individuals. However, there are uncertainties as to whether its long-term use might result in nephrotoxicity, and potentially in an increase in selection of TDF RAMs.
We should ideally aim for the goals of a combined HBV test that includes diagnosis of infection, genotype and presence of RAMs/VEMs; new sequencing platforms such as Nanopore make this technically possible, although cost remains a significant barrier at present. Sustainable long-term investment is required to expand consistent drug and vaccine supply, to provide screening infection and for drug resistance, and to provide appropriate targeted clinical monitoring for treated patients.
Supporting information
S1 fig. hbv drug resistance associated mutations (rams) grouped according to genotype..
Data summarised from fourteen studies published between 2009–2017 (inclusive). 21 studies were not represented here as they did not specifically indicate which genotype individuals with RAMs belonged to. Available at https://doi.org/10.6084/m9.figshare.5774091 [ 96 ].
https://doi.org/10.1371/journal.pntd.0006629.s001
S2 Fig. Distribution of HBV genotypes and prevalence of HBV resistance associated mutations (RAMs) in Pol/RT proteins in geno-A and geno-non-A samples.
A: Distribution of HBV genotypes derived from 35 studies reporting resistance associated mutations (RAMs) in Africa published between 2009 to 2017 (inclusive); B: Prevalence of HBV resistance associated mutations (RAMs) in Pol/RT proteins in geno-A and geno-non-A samples. These data are derived from 14 studies of HBV drug resistance in Africa published between 2007 and 2017 (inclusive). 21 studies were not represented here as they did not specifically indicate which genotype individuals with RAMs belonged to. We had more geno-A samples represented than other samples, we therefore combined samples from other genotypes that had RAMs (B, C, D, E, D/E) to form geno-non-A samples. We then compared prevalence of Pol/RT mutation between geno-A samples to geno-non-A samples. Prevalence of RT/Pol mutations for a specific genotype(geno-A/geno-non-A) was determined by grouping all studies with geno-A/geno-non-A infection that reported a specific mutation; the denominator was the total number of individuals infected with geno-A/geno-non-A from these studies and the numerator was the total number of individuals infected with geno-A/geno-non-A with that specific mutation. Available at https://doi.org/10.6084/m9.figshare.5774091 [ 96 ].
https://doi.org/10.1371/journal.pntd.0006629.s002
S1 Table. PRISMA (preferred reporting items for systematic reviews and meta-analyses) criteria for a systematic review of hepatitis B virus (HBV) drug and vaccine escape mutations in Africa.
Available at https://doi.org/10.6084/m9.figshare.5774091 [ 96 ].
I. Checklist to demonstrate how PRISMA criteria (2009) have been met in this review;
II. Flow diagram illustrating identification and inclusion of studies for a systematic review of drug and vaccine resistance mutations in Africa.
https://doi.org/10.1371/journal.pntd.0006629.s003
S2 Table. Details of search strategy used to identify studies on HBV resistance associated mutations (RAMs) and vaccine escape mutations (VEMs) conducted in Africa.
A: PubMed database; B: SCOPUS and EMBASE database. Available at https://doi.org/10.6084/m9.figshare.5774091 [ 96 ].
https://doi.org/10.1371/journal.pntd.0006629.s004
S3 Table. Full details of 37 studies identified by a systematic literature search of HBV resistance associated mutations (RAMs) and vaccine escape mutations (VEMs) from African cohorts published between 2007 and 2017 (inclusive).
https://doi.org/10.1371/journal.pntd.0006629.s005
S4 Table. HBV Pol/RT mutations among treatment-naïve HBV infected patients in Africa from 12 studies published between 2007 and 2017 (inclusive).
https://doi.org/10.1371/journal.pntd.0006629.s006
S5 Table. HBV Pol/RT mutations among treatment-experienced HBV infected patients in Africa, from 25 studies published between 2009 and 2017 (inclusive).
https://doi.org/10.1371/journal.pntd.0006629.s007
S6 Table. First line ART regimen for adults in Africa, and overlap with HBV therapy.
Information derived from published ART guidelines in all cases where these are available in the public domain. This information was collated in May 2018. Available at https://doi.org/10.6084/m9.figshare.5774091 [ 96 ].
https://doi.org/10.1371/journal.pntd.0006629.s008
- 1. World Health Organization. Global Hepatitis Programme. Global hepatitis report, 2017. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
- 2. WHO Combating Hepatitis B and C to reach elimination by 2030. Advocacy brief. 2016. http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.04_eng.pdf
- View Article
- PubMed/NCBI
- Google Scholar
- 6. WHO Hepatitis B treatment guidelines. 2015 http://www.who.int/mediacentre/news/releases/2015/hepatitis-b-guideline/en/
- 7. Beloukas A, Geretti AM. Hepatitis B Virus Drug Resistance. In: Antimicrobial Drug Resistance; 2017.p.1227–42.
- 27. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach—Second edition http://www.who.int/hiv/pub/arv/chapter4.pdf?ua=1
- 28. World Health Organisation–South Africa HIV Country Profile: 2016 http://www.who.int/hiv/data/Country_profile_South_Africa.pdf
- 31. Locarnini SA. The Hepatitis B Virus and Antiviral Drug Resistance: Causes, Patterns and Mechanisms. In: Antimicrobial Drug Resistance; 2017.p. 565–77.
- 34. WHO UNICEF coverage estimates WHO World Health Organization: Immunization, Vaccines And Biologicals. Vaccine preventable diseases Vaccines monitoring system 2017 Global Summary Reference Time Series: HEPB3. http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragehepb3.html
- 39. Mokaya J, Hadley M, Matthews P. gene.alignment.tables. Figshare. 2017. https://doi.org/10.6084/m9.figshare.5729229
- 40. Mokaya J, Hadley M, Matthews P. On-line tool to visualise sites of drug and vaccine escape mutations within the HBV genome. 2018. https://livedataoxford.shinyapps.io/1510659619-3Xkoe2NKkKJ7Drg/
- 71. WHO Clinical guidelines for the management of HIV & AIDS in adults and adolescents. 2010. http://www.who.int/hiv/pub/guidelines/south_africa_art.pdf
- 77. WHO Surveillance of transmitted HIV drug resistance. 2013. http://www.who.int/hiv/topics/drugresistance/surveillance/en/
- 83. McNaughton A, Lourenco J, Hattingh L, Adland E, Daniels S, Zyl A van, et al. Can we meet global challenges for elimination of Hepatitis B Virus infection by 2030? Vaccine-mediated immunity in a South African cohort and a model of transmission and prevention. bioRxiv. 2017;162594.
- 94. Oxford Nanopore Technologies: https://nanoporetech.com/
ORIGINAL RESEARCH article
A study of knowledge, experience, and beliefs about hepatitis b virus (hbv) infection in south western uganda.

- 1 Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine, Entebbe, Uganda
- 2 Nuffield Department of Medicine, Medawar Building for Pathogen Research, University of Oxford, Oxford, United Kingdom
- 3 Department of Health Sciences, University of York, York, United Kingdom
- 4 Department of Infectious Diseases and Microbiology, Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Headington, Oxford, United Kingdom
- 5 Oxford Biomedical Research Centre, John Radcliffe Hospital, Oxford, United Kingdom
- 6 Department of Global Health and Development, London School of Hygiene and Tropical Medicine, London, United Kingdom
Introduction: United Nations sustainable development goals aim for the elimination of viral hepatitis as a public health threat by 2030, leading to efforts to upscale the availability and accessibility of hepatitis B virus (HBV) vaccination, diagnosis, and treatment globally. However, a variety of societal factors, including beliefs, traditions, and stigma, can be a major obstacle to all of these interventions. We therefore set out to investigate how HBV is understood and described in communities in Uganda, and whether there is evidence of potential stigma.
Method: We carried out a qualitative formative study in two sites in South Western Uganda: a village in Kalungu district (site A) and an area on the outskirts of Masaka town (site B). We undertook a rapid assessment to investigate how adults describe HBV infection and their perceptions about the infection. We collected data by conducting a transect walk, observations, community group discussions, and in-depth interviews, sampling a total of 131 individuals. We used inductive content analysis to extract key themes associated with HBV.
Results: There is no specific word for HBV infection in local languages, and knowledge about this infection is varied. While some individuals were completely unfamiliar with HBV infection, some had heard of HBV. Radio was a common source of information. There was awareness of HBV as a cause of liver disease, but limited knowledge regarding the cause, mode of transmission, and treatment. Stigma in HBV may be rare in this community due to limited understanding and experience of HBV.
Conclusion: There is an ongoing need to improve awareness and understanding of HBV in this community. Careful dissemination of accurate information is required to promote acceptance of interventions for prevention, diagnosis, and treatment.
Introduction
Globally, ~290 million people are chronically infected with hepatitis B virus (HBV), and more than 800,000 people consequently die each year from liver cirrhosis or liver cancer ( 1 ). In response to the United Nations Sustainable Development Goals, which aim to eliminate viral hepatitis as a public health threat by the year 2030 ( 2 ), HBV infection is receiving increasing recognition with efforts to upscale prevention, diagnosis, and treatment ( 1 , 3 ). However, there are complex barriers to the elimination of this infection in the African subcontinent, including a high prevalence of chronic infection (≥8% in many settings), >90% of those infected being undiagnosed, and poor awareness ( 4 , 5 ).
Stigma and discrimination are recognized in the setting of chronic viral hepatitis infections, but there is very limited research to determine the nature and impact of stigma in communities in Africa ( 4 ). To date, only three studies have been carried out in Africa exploring the social impact of HBV. In Zambia, stigma has been reported to be a barrier to HBV disclosure and referral of contacts for testing ( 6 ), whereas in Ghana, low levels of knowledge and pervasive misconceptions about HBV contribute toward psychological and social problems among individuals with chronic infection ( 7 , 8 ). There has been no previous work on stigma in HBV in Uganda, although HBV is highly endemic in some regions [prevalence estimates vary from 6 to 52% ( 9 – 11 )].
Given the high prevalence of HBV in Uganda, and the emerging evidence of stigma in HBV, we sought to investigate how adults in rural and peri-urban communities in Uganda describe HBV infection, their perceptions, beliefs and experiences about the infection, and whether there is evidence of stigma.
Study Setting
We conducted the study at two sites in South West Uganda:
i. Site A: This is a village location within the General Population Cohort (GPC). The GPC was established in a rural population in south-western Uganda in Kyamulibwa sub-county of Kalungu district in 1989, with the initial aim of studying the epidemiology of HIV/AIDS in a rural population, but subsequently also being a source of recruitment for other research studies ( 12 , 13 ). The GPC comprises 25 study villages with a total population of ~25,000 people. Most of the inhabitants practice small-scale farming. Local tribes are Baganda, Banyarwanda, Barundi, and Banyakole. A number of infectious diseases are prevalent, including HIV (prevalence ~10%), malaria, hepatitis B and C (HBV prevalence 3%; HCV prevalence <1%), sexually transmitted infections, and respiratory tract infections. Within this sub-county, there are a number of public and private health care facilities and traditional healers. We selected Site A for this study because a previous survey on HBV reported a higher prevalence within this village compared to elsewhere in the GPC (unpublished data).
ii. Site B: This is a peri-urban site in Masaka Municipality, located along the highway that connects Uganda to the Democratic Republic of Congo, Tanzania and Rwanda, with a high concentration of low-cost housing. Most of the inhabitants earn their living from small businesses including motorcycle transport, selling food, and running bars; crop farming is also undertaken locally. The tribes represented in this region are Baganda, Banyarwanda, Batoro, and Basoga. The HIV prevalence in this setting may be slightly higher than within the GPC, but accurate data are not available. The prevalence of viral hepatitis infection is also not known but is likely to be higher than the GPC, given the concentration of settlements in the area and many inhabitants from different areas of Uganda. There are several private and public health care facilities in this site.
Study Design
Between July and October 2018, we conducted a rapid qualitative assessment to investigate how HBV is described, the experiences and beliefs associated with HBV, and the nature of any associated stigma. These rapid methods included transect walks, observations, introductory group sessions, community group discussions, and in-depth interviews ( Table 1 ), with the intention that the different methods complemented each other. Fieldwork was conducted at Site A first, after which the field team moved to Site B.
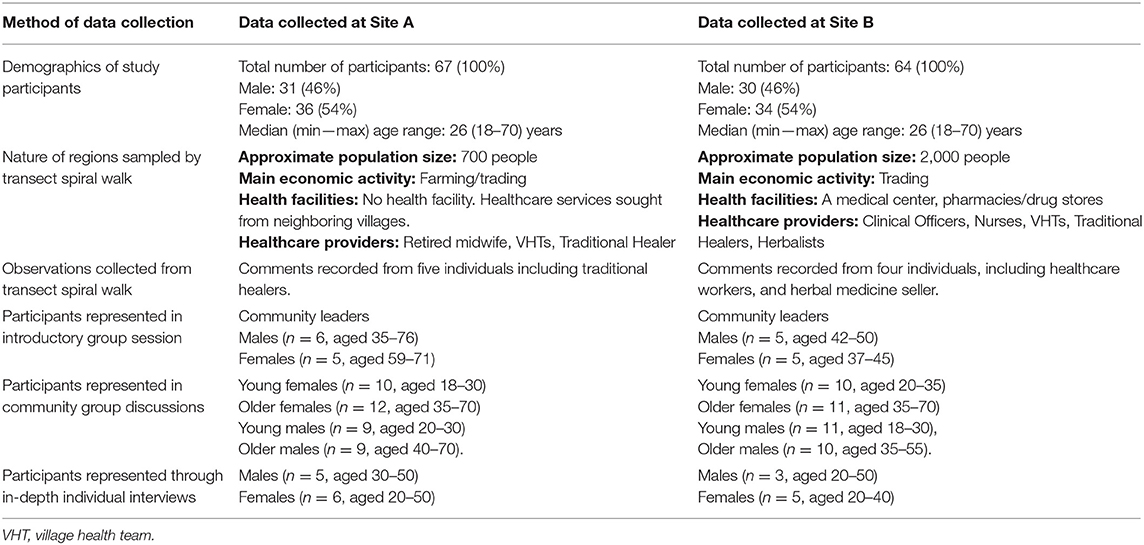
Table 1 . Description of study areas and characteristics of study participants.
Study Participants
Participants were recruited from the two sites using purposive sampling in an approach designed to collect qualitative data ( 14 , 15 ). We selected individuals who were influential people within the community such as community leaders, religious leaders, health care providers, traditional healers, village health teams. We also included people who had disclosed that they were living with hepatitis B virus infection. We set out to include participants representing both genders across a range of ages, stratifying as male or female, and younger (18–30 years) vs. older (>30 years). We recruited 131 people who met the above criteria. We had no refusals; all participants who were contacted at all stages of the study agreed to participate.
Data Collection
Data collection was done by two social science research assistants who have an understanding of these communities and are fluent in Luganda, the local language commonly used within the study setting. The research assistants were first trained on all aspects of the study and the use of a rapid qualitative assessment approach ( 16 ). Before visits to the study communities, the research team met with local leaders and explained the purpose of the study and sought permission to enter these communities. All the data were recorded using field notes that were expanded immediately after data collection.
i. Transect spiral walk: The aim was to observe, discuss, and find key features in the community that would inform our other methodologies such as identifying settlement patterns, health centers, churches, mosques, traditional healers, trading centers, bars, and any other places where people within communities concentrate. The transect walk was performed to understand the daily activities of people within the community in order to plan for the other assessment methods effectively. Two members of the research team first visited the local leader of each study site and explained the reasons of the activity within this community. With the help of the local leader, the team identified the center of each village that was used as a starting point. The team then split into two with each person taking a different direction. They moved in concentric circles until the whole village was covered. During the walk, observations were made in addition to talking to a few people.
ii. Observations: Informed by the transect walk, we visited particular places of interest to make observations. The team engaged with members of the community and healthcare providers [including traditional healers and village health teams (VHTs)], with the aim of better understanding the characteristics of individuals seeking health care, how and where they are treated, common illnesses, familiarity with HBV including local terms to describe it, its signs and symptoms, and any personal experience of individuals with HBV infection.
iii. Introductory group sessions: We met with ten influential people within each community including community leaders and local religious leaders, who had been identified based on information collected during the transect walks. The aim of these sessions was to understand perceptions and beliefs of diseases and illnesses in the community, and to explore understanding and experiences of HBV infection. The sessions were moderated by one interviewer, while the other was observing and taking notes.
iv. Community group discussions: We facilitated group discussions among adults to find out about perceptions and beliefs of disease within the community, specifically exploring understanding, and experiences of HBV infection. Four discussions were held at each study site with 10 participants in each group. These participants were identified after the first three visits within the study communities.
v. In-depth individual interviews: We interviewed a selected group of individuals who were willing to give information about their experiences, to allow deeper exploration of relevant issues established from the previous stages.
Data Management and Analysis
Researchers involved with data collection provided detailed descriptions of their observations and interviews in accounts written up from the notes. Two other researchers reviewed the transcripts prior to analysis to check the quality and accuracy, and thereafter independently coded the transcripts. We used thematic content analysis: classifying our data into themes and sub-themes relating to perceptions, beliefs, and experiences of HBV and the description and nature of associated stigma, based on narrative content, and research questions. Quotes used in the article are words of the interviewee used in the accounts.
Ethical Approvals
Approval was provided by Research Ethics Committee of the Uganda Virus Research Institute (ethics no GC/127/18/05/645) and UK Oxford Tropical Ethics Committee, OxTREC (ethics No. 516-18). All participants provided written informed consent for interviews and discussion groups. The local leaders provided verbal consent for the transect walks within their communities while health care providers, including traditional healers, consented for observations.
Between September and December 2018, we collected data from a total of 131 participants across Sites A and B. Table 1 summarizes the description of study areas and socio-demographic characteristics of study participants.
Knowledge and Awareness of HBV Infection
Awareness of HBV infection in these communities was varied. The commonest means of acquiring knowledge on HBV infection was through radio advertisements which encouraged members of the community to get tested and immunized (reported by 31 participants, age range 18–70 years). Others had personal experience of knowing someone perceived to be infected or diagnosed with HBV (reported by 30 individuals, age range 18–70 years). Among these respondents, 20 reported the death of individuals perceived to have HBV infection shortly after admission to a healthcare facility or as soon as they became symptomatic. One personal experience was reported by a teacher from site B: “ A neighbor who had gone to work at the lake shore came back when he was already infected. He was referred to a hospital in Kampala but died after 2 days.”
There were 10 participants (age range 19–55 years) who reported not having heard of HBV infection and therefore felt that it may not be a common illness, including both males and females from both settings. There is no specific name for HBV infection; naming of a disease in this community commonly depends on the symptoms of the disease. The local phrases used to describe liver disease are “ Obulwadde bwekibumba” and “endwadde y'ekibumba” which literally translate to “the liver is sick.”
Insights Into HBV as a Cause of Liver Disease
As the symptoms and signs of HBV are not distinct from other causes of liver disease, there may be poor understanding of the role of an infectious agent. This is illustrated from a comment recorded from the community group discussions at site A, “ We do not know about its mode of transmission, signs and causes so we cannot tell if the disease exists in the community .” Three participants specifically reported that they are not able to distinguish HBV from other illnesses.
More generally, some participants were able to list other causes of liver disease including excessive consumption of alcohol and eating fatty foods. Insect bites, exposure to pesticides/fertilizers and drinking dirty water were also suggested as causes of liver disease. For example, a man at Site B commented that: “ it could be caused through excessive drinking of waragi [local gin].…myrrh, khat drugs, herbal medicine, and fertilizers/pesticides can affect and make the liver sick,” while a woman of a similar age in Site A suggested that it was caused by “ walking through dirty swamp water which contains certain germs that cause liver disease.” Some of the symptoms that participants reported in association with HBV (or more generally with liver disease) included abdominal or whole body swelling, jaundice/yellow eyes, weight loss, and hair loss. Three participants mentioned that HBV was asymptomatic. Thirteen participants, mainly from Site A, were not able to suggest any signs or symptoms associated with HBV. Eight participants (aged 18–60 years) reported a similarity in signs and symptoms between HIV and HBV.
Perceptions of Mode of Transmission
A number of participants, predominantly from Site B, reported that they did not know how HBV is transmitted. Three people believed that transmission routes included sharing of public toilets/bathrooms, or sharing of utensils and clothing. A male at site B stated: “ When he shares any equipment—say clothing, cups, and forks with a person who looked malnourished—he would feel as if he was to contract the infection from him/her, not good to share anything with the patient.”
Of the eight healthcare professionals who took part in this study, only five knew that HBV is sexually transmitted and can also be transmitted through other exposures to infected body fluids. Five participants from Site A reported that anyone was at risk of contracting HBV. People who consumed alcohol or those who engage in risky sexual behaviors were considered to be at risk of contracting HBV infection. One participant interpreted the high-profile campaigns around HBV immunization for adults as suggesting that only adults were susceptible to infection.
Knowledge of, and Access to, HBV Treatment
Community members suggested possible management interventions, including modified diet and herbal medicine. Twelve participants, mostly from Site A (age range 18–70 years), did not know any treatment for HBV. Three young men from Site B reported that HBV has no cure. Others correctly recognized that antiretroviral drugs can be used in managing HBV infection, and that those infected are put-on long-term treatment, but some of the comments suggested that there is pessimism about outcomes, with participants suggesting poor chances of survival.
Potential Challenges Facing HBV Management
The asymptomatic nature of HBV can be a barrier to seeking healthcare services; some community members only seek treatment when the symptoms are noticeable and have worsened, and it may be common to stop taking medication in the absence of symptoms. Poverty, preference for traditional/herbal medicine and lack of awareness about a disease, can also contribute to delays in seeking healthcare and be barriers to seeking preventive, diagnostic and treatment services among members of the community. A description of a child unwell as a result of HBV infection was provided by a male participant at Site A: “ When the boy fell sick, he was taken to the health center for treatment. He was not admitted but they gave him some tablets which he didn't understand. He took the tablets and after a week the stomach normalized. About 3 weeks later, the stomach resumed swelling. He was taken to herbal clinic and was given 5 liters of herbal medicine and he was told to go back after one week. When the herbal was finished, he went back and still he was given the same drugs. By that time his eyes had a yellowish color, his urine was also yellow.”
Lack of diagnostic facilities, and limited access to healthcare professionals and medications contribute to the challenges facing HBV prevention, diagnosis, and therapy in this community. A female healthcare worker at site A explained: “ She can't even give that patient any drug, only refers him or her to Masaka hospital. She said that they don't have the machines that can diagnose it, so they have to refer the patient.” Among healthcare workers, poor knowledge can impede diagnosis and treatment for HBV infection. Three healthcare professionals reported not having received a complete course of HBV vaccination themselves and are therefore at increased risk of becoming infected.
Community Perceptions and Experience of HBV
We sought to understand the perception of community members about the occurrence and persistence of HBV infection in their community. Eleven participants, mostly from Site B reported that HBV was a new infection in their community because they had become aware of it only recently. For ten people who commented that HBV was a long-standing problem, their knowledge came from a community member known to them whom they believed had died of the infection. Others claimed that HBV was “an old sickness” which had been in the communities for a long time.
Emotional Responses to HBV Infection
Communities tend to fear diseases based on symptoms and outcomes associated with the disease. In particular, a disease can be feared because it is incurable and may be seen as leading inevitably to death ( 4 ). Stigma may stem from this fear, as well as from changes in body appearance that can limit interactions with other community members. Eight participants, primarily from Site B, reported that they would be scared or more cautious to take care of someone with an infectious disease. Ten participants, mostly men from Site B (age 18–50 years), reported a feeling of shame associated with sexually transmitted diseases. However, eleven participants, reported no fear or shame toward people infected with HBV, partly because it is a disease that is not well-known within the community.
With the recent increased attention regarding the urgent need to upscale prevention, diagnosis, and treatment of HBV infection globally, understanding the social impact of HBV infection in communities is important as this influences acceptability and impact of interventions. To date, there is very limited research on behavior and beliefs surrounding HBV infection in Africa.
Familiarity with HBV infection in the communities we investigated can be explained, in part, by the recent efforts by the Ministry of Health in Uganda to combat transmission of HBV by promoting vaccination among adolescents and young adults ( 17 ), particularly using radio messages to reach a wide audience. As a result, the syndrome of HBV infection is now recognized by many adults in this setting. However, even among healthcare workers, there is still a lack of in-depth understanding, in keeping with similar studies in Ghana and Zambia ( 4 , 6 – 8 ). The importance of education is highlighted by a study of HBV infection undertaken in Turkey ( 18 ).
Naming of a disease can be a reflection of how much it is recognized and understood ( 19 , 20 ). In our study population, local names used to describe diseases are based on the symptoms the sufferer bears. In part, this practice is encouraged by the WHO which states that a name of a disease should be based on the “symptoms, disease manifestation, severity and, if known, the pathogen causing the disease” ( 21 ). Naming HBV infection poses a challenge, since there is no specific symptom that distinguishes it from other liver diseases, and in many instances, it remains asymptomatic until the end-stages of infection. Although we identified local terms that broadly describe liver disease, gaps remain in understanding of HBV infection, particularly with regard to the infectious agent ( 21 ).
As a result of limited understanding and experience of HBV in our study population, stigma did not emerge as a strong theme. However, fear of symptoms and outcomes of a disease, as well as shame associated with mode of transmission of infection, can determine how those infected might be handled within their communities ( 22 , 23 ), and can lead on to stigma. Although sex is not the main route of transmission of HBV infection, this association could nevertheless lead to stigma and discrimination ( 24 ). Although HBV is asymptomatic in most cases, acute infection or advanced stages of the disease could result in visible manifestations, leading to isolation and lack of support from community members. Careful dissemination of accurate information on HBV is required to prevent possible stigma and misperceptions of personal risk.
Studies from Asia have reported stigma and limited knowledge among health practitioners as obstacles in the uptake of preventive, diagnostic, and treatment services among HBV patients ( 4 , 25 ). Delayed health seeking behavior has been widely reported in tuberculosis and is a result of several factors such as lack of symptoms, low patient knowledge, practicing self-medication, and the use of traditional healing methods ( 26 – 28 ). In our study, the asymptomatic nature of HBV, poverty and lack of knowledge (including among healthcare workers) were challenges in accessing timely and appropriate healthcare ( 29 ). While there are efforts to improve knowledge on HBV among healthcare professionals and community members in Uganda, studies are needed to evaluate the evidence of effectiveness of these interventions, and to evaluate availability, accessibility, and integration of HBV services.
Strength and Limitations of This Study
This is the first study to explore beliefs, understanding and stigma around HBV infection in Uganda. A strength of the study was that we sampled participants from both urban and rural settings, sampling men and women, and including a diverse range of ages which afforded a comparison for HBV description, experiences, and understanding. This approach provides some valuable reflections and insights into local understanding and beliefs about HBV infection, but represents only a small number of adults, who self-selected to participate.
Conclusions
Engaging local communities, understanding barriers, and providing appropriate and accessible information will be a crucial undertaking if diagnosis, treatment, and preventive strategies are to be rolled out to support progress toward the 2030 sustainable development goal targets for elimination of HBV infection as a public health threat. Although radio campaigns have raised some local awareness of HBV in the communities we studied in Uganda, there remain significant gaps in knowledge and understanding, and a risk of stigma associated with fear, especially in the context of physical signs of liver disease. Educating traditional healers and healthcare workers could have a positive impact on disseminating information. Further studies are needed to investigate the societal responses to HBV infection in other African populations, and to establish the best ways to engage with local populations to promote access to interventions.
Data Availability Statement
All datasets generated for this study are available online at https://doi.org/10.6084/m9.figshare.8340860.v1 .
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the Uganda Virus Research Institute (ethics no GC/127/18/05/645) and UK Oxford Tropical Ethics Committee, OxTREC (ethics no. 516-18). All participants provided written informed consent for interviews and discussion groups. The local leaders provided verbal consent for the transect walks within their communities while health care providers, including traditional healers, consented for observations. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
PM and JS conceived the study. JMu, JMo, DB, FS, and DM data collection. JMu, JMo, and DB analyzed the data. JMu, JMo, DB, PM, and JS wrote the manuscript. All authors revised the manuscript.
JMo was funded by Leverhulme Mandela Rhodes Doctoral Scholarship. PM was funded by the Wellcome Trust (Grant ref 110110). The SHEBA (Stigma in HEp B in Africa) project was supported by a project grant from the Medical Research Council Global Challenges Research Fund, GCRF (Principal Investigator Philippa Matthews). The MRC/UVRI and LSHTM is jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 programme supported by the European Union. The Uganda General Population Cohort (GPC) staff have received support from THRiVE ( https://thrive.or.ug/ ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AIDS, Acquired Immunodeficiency Syndrome; GPC, general population cohort, Uganda; HBV, hepatitis B virus; HIV, human immunodeficiency virus; sSA, sub Saharan Africa; UK MRC, United Kingdom Medical Research Council; WHO, World Health Organization.
1. World Health Organisation. Global Hepatitis Report, 2017 . WHO (2017).
PubMed Abstract | Google Scholar
2. World Health Organisation. Global Health Sector Strategy on Viral Hepatitis 2016–2021 .
Google Scholar
3. Cooke GS, Andrieux-Meyer I, Applegate TL, Atun R, Burry JR, Cheinquer H, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. (2019) 4:135–84. doi: 10.1016/S2468-1253(18)30270-X
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Mokaya J, McNaughton AL, Burbridge L, Maponga T, O'Hara G, Andersson M, et al. A blind spot? Confronting the stigma of hepatitis B virus (HBV) infection—A systematic review. Wellcome Open Res. (2018) 3:29. doi: 10.12688/wellcomeopenres.14273.1
5. O'Hara GA, McNaughton AL, Maponga T, Jooste P, Ocama P, Chilengi R, et al. Hepatitis B virus infection as a neglected tropical disease. PLoS Negl Trop Dis. (2017) 11:e0005842. doi: 10.1371/journal.pntd.0005842
6. Franklin S, Mouliom A, Sinkala E, Kanunga A, Helova A, Dionne-Odom J, et al. Hepatitis B virus contact disclosure and testing in Lusaka, Zambia: a mixed-methods study. BMJ Open. (2018) 8:e022522. doi: 10.1136/bmjopen-2018-022522
7. Mkandawire P, Richmond C, Dixon J, Luginaah IN, Tobias J. Hepatitis B in Ghana's upper west region: a hidden epidemic in need of national policy attention. Health Place. (2013) 23:89–96. doi: 10.1016/j.healthplace.2013.06.001
8. Adjei CA, Naab F, Donkor ES. Beyond the diagnosis: a qualitative exploration of the experiences of persons with hepatitis B in the Accra Metropolis, Ghana. BMJ Open. (2017) 7:e017665. doi: 10.1136/bmjopen-2017-017665
9. Ochola E, Ocama P, Orach CG, Nankinga ZK, Kalyango JN, McFarland W, et al. High burden of hepatitis B infection in Northern Uganda: results of a population-based survey. BMC Public Health. (2013) 13:727. doi: 10.1186/1471-2458-13-727
10. Bayo P, Ochola E, Oleo C, Mwaka AD. High prevalence of hepatitis B virus infection among pregnant women attending antenatal care: a cross-sectional study in two hospitals in northern Uganda. BMJ Open. (2014) 4:e005889. doi: 10.1136/bmjopen-2014-005889
11. Bwogi J, Braka F, Makumbi I, Mishra V, Bakamutumaho B, Nanyunja M, et al. Hepatitis B infection is highly endemic in Uganda: findings from a national serosurvey. Afr Health Sci. (2009) 9:98–108.
12. Asiki G, Murphy G, Nakiyingi-Miiro J, Seeley J, Nsubuga RN, Karabarinde A, et al. The general population cohort in rural south-western Uganda: a platform for communicable and non-communicable disease studies. Int J Epidemiol. (2013) 42:129–41. doi: 10.1093/ije/dys234
13. Asiki G, Murphy GAV, Baisley K, Nsubuga RN, Karabarinde A, Newton R, et al. Prevalence of dyslipidaemia and associated risk factors in a rural population in South-Western Uganda: a community based survey. PLoS ONE. (2015) 10:e0126166. doi: 10.1371/journal.pone.0126166
14. Marshall MN. Sampling for qualitative research. Fam Pract. (1996) 13:522–5. doi: 10.1093/fampra/13.6.522
15. Kielmann K, Cataldo F, Seeley J. Introduction to Qualitative Research Methodology: A Training Manual, Produced With the Support of the Department for International Development (DfID), UK, Under the Evidence for Action Research Programme Consortium on HIV Treatment and Care (2006–2011) (2012).
16. Bond V, Ngwenya F, Murray E, Ngwenya N, Viljoen L, Gumede D, et al. Value and limitations of broad brush surveys used in community-randomized trials in Southern Africa. Qual Health Res. (2019) 29:700–18. doi: 10.1177/1049732318809940
17. World Hepatitis Day. Press Statement on the Progress of Implementation of Hepatitis B Vaccination Program in Uganda. (2018). Available online at: https://reliefweb.int/report/uganda/world-hepatitis-day-2018-press-statement-progress-implementation-hepatitis-b (accessed February 21, 2019).
18. Tosun S, Aygün O, Özdemir HÖ, Korkmaz E, Özdemir D. The impact of economic and social factors on the prevalence of hepatitis B in Turkey. BMC Public Health. (2018) 18:649. doi: 10.1186/s12889-018-5575-6
19. Scully JL. What is a disease? EMBO Rep . (2004) 5:650–3. doi: 10.1038/sj.embor.7400195
20. Shim K. Expanding the definition of infectious disease. J Glob Health. (2015) 1.
21. World Health Organisation. WHO Issues Best Practices for Naming New Human Infectious Diseases.
22. Mahajan AP, Sayles JN, Patel VA, Remien RH, Sawires SR, Ortiz DJ, et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS . (2008)22 (Suppl. 2):S67–79. doi: 10.1097/01.aids.0000327438.13291.62
23. Halli SS, Khan CGH, Moses S, Blanchard J, Washington R, Shah I, et al. Family and community level stigma and discrimination among women living with HIV/AIDS in a high HIV prevalence district of India. J HIV AIDS Soc Serv. (2016) 16:1–16. doi: 10.1080/15381501.2015.1107798
CrossRef Full Text | Google Scholar
24. Blanas DA, Nichols K, Bekele M, Shankar H, Bekele S, Jandorf L, et al. Adapting the Andersen model to a francophone West African immigrant population: hepatitis B screening and linkage to care in New York City. J Community Health. (2015) 40:175–84. doi: 10.1007/s10900-014-9916-9
25. Lee H, Fawcett J, Kim D, Yang JH. Correlates of Hepatitis B virus-related stigmatization experienced by asians: a scoping review of literature. Asia-Pacific J Oncol Nurs. (2016) 3:324. doi: 10.4103/2347-5625.195896
26. Mbuthia GW, Olungah CO, Ondicho TG. Health-seeking pathway and factors leading to delays in tuberculosis diagnosis in West Pokot County, Kenya: a grounded theory study. PLoS ONE . (2018) 13:e0207995. doi: 10.1371/journal.pone.0207995
27. Chimbatata NBW, Zhou C-M, Chimbatata CM, Xu B. Post-2015, why delay to seek healthcare? Perceptions and field experiences from TB healthcare providers in northern Malawi: a qualitative study. Infect Dis Poverty. (2017) 6:60. doi: 10.1186/s40249-017-0279-1
28. Seid A, Metaferia Y. Factors associated with treatment delay among newly diagnosed tuberculosis patients in Dessie city and surroundings, Northern Central Ethiopia: a cross-sectional study. BMC Public Health. (2018) 18:931. doi: 10.1186/s12889-018-5823-9
29. Lemoine M, Thursz MR. Battlefield against hepatitis B infection and HCC in Africa. Hepatology. (2017) 66:645–54. doi: 10.1016/j.jhep.2016.10.013
Keywords: stigma, hepatitis, infection, Uganda, Africa, HBV
Citation: Mugisha J, Mokaya J, Bukenya D, Ssembajja F, Mayambala D, Newton R, Matthews PC and Seeley J (2019) A Study of Knowledge, Experience, and Beliefs About Hepatitis B Virus (HBV) Infection in South Western Uganda. Front. Public Health 7:304. doi: 10.3389/fpubh.2019.00304
Received: 31 July 2019; Accepted: 04 October 2019; Published: 25 October 2019.
Reviewed by:
Copyright © 2019 Mugisha, Mokaya, Bukenya, Ssembajja, Mayambala, Newton, Matthews and Seeley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philippa C. Matthews, philippa.matthews@ndm.ox.ac.uk
† These authors have contributed equally to this work as first authors
‡ These authors have contributed equally to this work as last authors
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Open access
- Published: 18 August 2020
Hepatitis B virus infections among health professional students in Mwanza city,Tanzania in 2016
- Mariam M. Mirambo 1 ,
- Emmanuel Mkumbo 1 ,
- Hadija Selega 1 ,
- Betrand Msemwa 2 ,
- Martha F. Mushi 1 ,
- Vitus Silago 1 ,
- Jeremiah Seni 1 ,
- Stephen E. Mshana ORCID: orcid.org/0000-0002-7526-6271 1 &
- Christa Kasang 3
Archives of Public Health volume 78 , Article number: 76 ( 2020 ) Cite this article
3516 Accesses
2 Citations
8 Altmetric
Metrics details
The World Health Organisation (WHO) recommends the vaccination against Hepatitis B virus in all infants and children up to the age of 18 years. In addition, adults in high-risk groups should also be vaccinated. This study investigated the prevalence and factors associated with Hepatitis B Virus (HBV) infections among health professional students in the city of Mwanza, Tanzania in order to provide data that can assist in devising prevention and control strategies in this special group.
A cross-sectional study involving health professional students of the Catholic University of health and Allied Sciences was conducted between April and July 2016. Hepatitis B surface antigen was detected using rapid antigen test while the anti-hepatitis B surface antibodies(anti-HBs) were quantified using Enzygnost Anti-HBs II assay and anti-HBV core antibodies tested using enzyme immunoassay.
A total of 1211 health professional students with median age of 22 interquartile range (IQR):21–24 years were enrolled. The slighlty majority (57.5%) of these students were males and 475(39.2%) were in clinical practices. Out of 1211 students, 37 (3.1%) were Hepatitis B surface antigen positive. Of 1174 students tested for anti-HBs, 258 (22%) had titres > 10 IU/L indicating HBV immunity. The median anti-HBs titres was 47.7 IU/L(IQR:16–3-113). A total of 230(89.2%) students among those who were positive for anti-HBs were also positive for HBV core antibodies indicating HBV natural infections. Male sex (adjusted odd ratio(AOR):1.77, p < 0.000), being married (AOR:1.82, p = 0.002) and being in clinical practices (AOR:1.39, p = 0.028) independenlty predicted anti-HBs positivity.
A significant proportion of health professional students was naturally immune to Hepatitis B virus. There is a need to measure anti-HBs in order to reduce the cost of unnecessary vaccination especially in the countries with high endemicity of HBV.
Peer Review reports
Approximately one third of the global population is infected by Hepatitis B virus(HBV) [ 1 ] with about 350–400 million people being chronically infected [ 2 ]. High endemicity of HBV is observed in the sub-Saharan Africa and East Asia whereby 5–10% of the adult population is chronically infected. Health professional students are among high risk groups of being infected with the HBV especially during early stage of their clinical practices [ 3 ]. Among health care workers the prevalence of chronic infection of 7.4% has been oberved in Tanzania [ 4 ] while in Cameroon the prevalence of chronic HBV infection was found to be 11% [ 5 ], indicating high endemicity in these countries.
A previous study done at Bugando Medical Centre among health care workers documented the prevalence of HBV natural antibodies of 36.5%, indicating high transmission of HBV infections [ 4 ]. However, there is limited information on the magnitude of anti-HBs among health professional students who are also considered as high-risk group that requires vaccination. In addition, the current vaccination practices among children below 5 year of age and among health care workes in Tanzania and in many low-icome countries do not consider the presence of natural antibodies leading to the possibility of unnecessary vaccination in a significant proportion of individuals [ 6 ]. Most of studies from low–income countries have estimated the magnitude of chronic HBV infections, with few studies documenting the magnitude of the immunity to HBV [ 4 , 7 , 8 ]. It should be noted that, the use of Hepatitis B surface antigen (HbsAg) does not indicate the true magnitude of HBV infections because the HbsAg indicates only those with chronic/acute infections and not those who have recovered from natural infections. Therefore, in order to combat HBV, the pathogen which has been mentioned in sustainable developmental goals (SDG) ‘Health Goal’, this study was done to estimate the prevalence of HBV infections among health professional students in order to produce data that can be used to formulate strategies to control HBV infections in this high risk population.
Study design, pupulation and area
This was a cross-sectional study which was conducted between April and July 2016 among undergraduate health professional students of the Catholic University of health and Allied Sciences. The Catholic University of Health and Allied sciences is the private University located in the city of Mwanza, Tanzania. It has about 2600 students in various field of health sciences. The study included students from medical laboratory sciences, nursing, medical doctors, radiology and pharmacy programmes.
Sample size, sampling technique and inclusion criteria
The sample size was estimated by Kish Leslie formula (1965) using the prevalence of 56.7% from previous study which was conducted at Bugando Medical Centre [ 9 ] among health care professionals. The minimum sample obtained was 377, however the study enrolled 1211 students. The study included all undergraduate students who consented to participate in the study with no history of HBV vaccination or known positivity of hepatitis B surface antigen. A recruitment centre was set at the University campus for 4 days and serial sampling was used to recruit students as they pass around the recruitment centre. The study did not exclude students on clinical practices because, the aim was establish the magnitude and factors associated with HBV infection. Clinical practices was treated as a factor to confirm what has been documented in previous studies.
Data collection
A pretested data collection tool was used to collect socio-demographic information and other related information from study participants. Data collected included age, sex, duration on clinical practices, marital status, histroy of unproteced sex etc.
Hepatitis B surface antigen, anti-hepatitis B surface antibodies(anti-HBs) and anti-hepatitis B core(anti-HBc) assays
About 5-ml of venous blood sample was collected in a plain vacutainer tube(BD, Nairobi, Kenya) under aseptic procedures. Samples were transported to the CUHAS microbiology laboratory whereby blood was centrifuged to obtain sera which was stored in cryovials at -80 °C until analysis. Hepatitis B surface antigen was detected using a qualitative, lateral flow immunochromotography assay test (Accu-Tell Rapid HBsAg Serum/Whole Blood Test, Beijing,101,300,China) following manufacturer instructions. The test has been found to have sensitivity and specificity of > 99.0 and 97.0%, respectively. The anti-HBs were detected using Sandwich ELISA test kit (SIEMENS Enzygnost, Germany) following manufacturer’s instructions, the assay has sensitivity and specificity of more than 95% while anti-HBc antibodies were detected using enzyme immunoassay (Elite Medical Company, India) as per manufacturer instructions, this assay has sensitivity and specificity of more than 95%.
Data analysis
Continuous variables were summarized as median with inter-quartile range while categorical variables were summarized as proportions. Univariable analysis was done followed by multivariable logistic regression analysis to determine the predictors of HBsAg positivity and the presence of anti-HBs. The titre of anti-HBs of ≥10 IU/L was considered as the presence of natural immunity to HBV. The medians were compared using the Wilcoxon rank-sum Mann Whitney test. Factors with a p -value of < 0.2 on univariable analysis were subjected into multivariable logistic regression analysis. All factors with p -values of < 0.05 at 95% confidence interval were considered statistically significant. Age was not subjected on the multivariable analysis due to its collinearity with being in clinical practices.
Sociodemographic characteristics of the study participants
A total of 1211 students were enrolled, representing 46% of the Catholic University of Health and Allied Sciences students’ population in 2016. The median age was 22[IQR:21–25] years with the slightly majority 57.5% (696/1211) of the students being male. Regarding marital status, the majority of the participants 86.1% (1043/1211) were single. Out of 1211 students, 475(39%) had started clinical practices(Table 1 ). None of these students had received HepB vaccination before.
Prevalence of hepatitis B surface antigen and anti-HBsAg
Out of 1211 students tested for hepatits B surface antigen(HbsAg), 37(3.1, 95%CI: 2.1–4.0) were HbsAg positive. All students (1174) who were HBsAg negative were tested for anti-HbsAg, of these, 258(22, 95%CI: 19.6–24.4) had titres > 10 IU/L, indicating immunity to HBV. Low, moderate and high anti-HBs titers were observed in 69.4%(179/258), 25.2% (65/258) and 5.4% (14/258) students, respectively (Table 2 ). The median anti-HBs titres was 47.7 IU/L(IQR:16–3-113). Out of 258 tested positive for anti-HBs, 230(89.2%) were positive for anti-HBc indicating natural infections.
Factors associated with HBV infections among health professional students
The median age of partcipants with HBV immunity was significantly higher than those with no immunity (23 IQR;22–26 vs. 22 IQR; 21–24, P < 0.001). Out of 502 females, 81(16.1%) were positive for anti-HBs compared to 177(26.3%) of 672 males, p < 0.001. Being in clinical practices was found to be associated with the presence of anti-HBs (27.2% vs. 18.7%, P = 0.001). By multivariable logistic regression analysis; male sex (OR:1.77, 95% CI:1.31–2.38), being married (OR:1.82, 95% CI:1.25–2.64) and being in clinical practices (OR:1.39, 95% CI:1.03–1.86) were found to predict anti-HBs positivity (Table 3 ). None of the factors studied (sex, age, clinical practices, marital status and practice unprotected sex) were found to be associated with HBsAg positivity.
The overall prevalence of HBsAg was 3.1% among CUHAS health professional students. The observed prevalence was significantly lower than what was reported in previous studies in Makerere Uganda (18%) and in Kenyatta National hospital(11%) among medical students [ 10 , 11 ]. The low prevalence of HBsAg could indicate high natural immunity in this population or high vaccine coverage, however this was not the case in this population because none of student had received HBV vaccination. In Tanzania, at the time of data collection the HBV immunization programme for children below 5 years of age was just 6 years old, therefore it was unlikely that these students were immunized before.
In this study, the overall prevalence of anti-HBs was found to be 22% with 89.2% of these students being positivite for anti-HBc indicating that, the majority of anti-HBs were due to natural infections. This was comparable with a previous study in South Africa [ 12 ] which reported prevalence of 18.8% among health care workers. However, when compared to the prevalence of immunity among the students who were in clinical practices the oberved prevalence of HBV immunty in the current study was significantly high indicating high transmission of HBV in this setting. The observation was further supported by the previous study in the same setting which observed the prevalence of natural immunity of 36.5% among health care workers. However, the observed immunity in the current study was comparable to 25.3% observed among febrile patients in two districts hospitals in Tanzania [ 13 ].
The difference between this study and that of South Africa could be explained by the fact that in South Africa the HBV vaccination among health care workers was introduced earlier than in Tanzania [ 5 , 14 ]. South Africa and Tanzania has similar endemicity status as per World Health Organization (WHO), this further supports the similarity in the general prevalence of HBV natural immunity.
In comparison to the previous study in China which reported the prevalence of 13%, the prevalence reported in this study is indeed high [ 15 ]. This could be due to the fact that in China HBV vaccination campaign has been there for many years [ 16 ].
None of the factors studied was found to predict HbsAg positivity, however male sex, being married and being in clinical practices were found to predict anti-HBs positivity in the present study. Previous studies [ 4 , 17 ] showed the association between HBV infection and the duration of employment/clinical practices, emphasizing the need to vaccinate health professional students to prevent subsequent infections.
In this study male sex was significantly associated with HBV immunity. This could be explained by the fact that in Tanzania boys tend to be more involved in high risk behaviours than girls [ 18 ]. Further multi -centre studies to asess the association between HBV infections and gender among students are recommended so that tartegted health education can be given. Furthermore, being married was found to predict HBV natural infections in this study confriming what was oberved in previous studies [ 19 , 20 ].
It should be noted that the 96 ELISA kit for for screening of the anti-HBs costs about 500USD(i.e. about 6USD per test) compared to the cost of single dose HBV vaccine of about 12.3USD [ 19 ]. Therefore, screening for anti-HBs should be recommnded in the countries with HBV high endemicity in order to avoid unnecessary costly vaccination. A previous study in China concluded that HepB vaccination campaign with screening provided more greater value than a vaccination without screening vaccination [ 19 ]. Furthermore, there is a need to invest on point of care anti-HBs assays that can be used in developing countries to asses the level of anti-HBs before vaccination.
In this study non significant association was observed between unprotected sexual intercourse and HBV infections. This could be contributed to the validity of the self-response regarding the issues of unprotected sexual intercourse. Self reports on the issue of sexual behaviours can be affected by both cognitive and situational factors in varying degrees [ 21 ]. As major limitation no attempt was made to validate the information given from these students due the design of this study.
A significant proportion of students undertaking health sciences courses were found to be naturally immune to HBV. The immunity was significanlty predicted by male sex, being in clinical practices and being married. This calls for the need to emphasize the screening for the presence of anti-HBs so that the vaccine can be given to those who are eligible to avoid waste vaccinations among those with natural immunity, this will ensure costeffective implementation of of HBV vaccination programme. If the implementation of universal screening for anti-HBs is considered as difficult option, it is recommended that in the Higher learning institutions for health in developing countries, married-male students in their clinical practices should mandatory be screened for anti-HBs before provision of HBV vaccination. This will significantly minimize the cost of HBV vaccination to the institutions and respective individual students.
Availability of data and materials
The data is available upon request and the request should be made to the Director of research and Innovation Catholic University of Health and allied Sciences.

Abbreviations
Catholic University of Health and Allied Sciences
Confidence interval
Enzyme-linked immunosorbent assay
Hepatitis B Virus
Hebatitis B surface antitigen
Hebatitis B core antigen
International Unit
Interquartile range
Hwang EW, Cheung R. Global epidemiology of hepatitis B virus (HBV) infection. N Am J Med Sci. 2011;4(1):7–13.
Google Scholar
McMahon BJ. Epidemiology and natural history of hepatitis B. In: Seminars in liver disease; 2004. p. 3–8.
Wang H, Fennie K, He G, Burgess J, Williams AB. A training programme for prevention of occupational exposure to bloodborne pathogens: impact on knowledge, behaviour and incidence of needle stick injuries among student nurses in Changsha, People's Republic of China. J Adv Nurs. 2003;41(2):187–94.
PubMed Google Scholar
Mueller A, Stoetter L, Kalluvya S, Stich A, Majinge C, Weissbrich B, Kasang C. Prevalence of hepatitis B virus infection among health care workers in a tertiary hospital in Tanzania. BMC Infect Dis. 2015;15(1):386.
CAS PubMed PubMed Central Google Scholar
Tatsilong HOP, Noubiap JJN, Nansseu JRN, Aminde LN, Bigna JJR, Ndze VN, Moyou RS. Hepatitis B infection awareness, vaccine perceptions and uptake, and serological profile of a group of health care workers in Yaoundé, Cameroon. BMC Public Health. 2016;16(1):706.
PubMed Central Google Scholar
Organization WH. Hepatitis B vaccines: WHO position paper, July 2017–recommendations. Vaccine. 2019;37(2):223–5.
Tandon B, Irshad M, Raju M, Mathur G, Rao M. Prevalence of HBsAg & anti-HBs in children & strategy suggested for immunisation in India. Indian J Med Res. 1991;93:337–9.
CAS PubMed Google Scholar
Soběslavský O. Prevalence of markers of hepatitis B virus infection in various countries: a WHO collaborative study. Bull World Health Organ. 1980;58(4):621.
PubMed PubMed Central Google Scholar
Mueller A, Stoetter L, Kalluvya S, Stich A, Majinge C, Weissbrich B, Kasang C. Prevalence of hepatitis B virus infection among health care workers in a tertiary hospital in Tanzania. BMC Infect Dis. 2015;15(1):1.
Pido B, Kagimu M. Prevalence of hepatitis B virus (HBV) infection among Makerere University medical students. Afr Health Sci. 2005;5(2):93–8.
Lule G, Okoth F, Ogutu E, Mwai S. HBV markers HBsAg, HBSAb, HBCAb in 160 medical students at Kenyatta National Hospital. East Afr Med J. 1989;66(5):315–8.
Sondlane TH, Mawela L, Razwiedani LL, Selabe SG, Lebelo RL, Rakgole JN, Mphahlele MJ, Dochez C, De Schryver A, Burnett RJ. High prevalence of active and occult hepatitis B virus infections in healthcare workers from two provinces of South Africa. Vaccine. 2016;34(33):3835–9.
Meschi S, Schepisi MS, Nicastri E, Bevilacqua N, Castilletti C, Sciarrone MR, Paglia MG, Fumakule R, Mohamed J, Kitwa A. The prevalence of antibodies to human herpesvirus 8 and hepatitis B virus in patients in two hospitals in Tanzania. J Med Virol. 2010;82(9):1569–75.
Burnett RJ, Kramvis A, Dochez C, Meheus A. An update after 16 years of hepatitis B vaccination in South Africa. Vaccine. 2012;30:C45–51.
Liao X-Y, Zhou Z-Z, Wei F-B, Qin H-N, Ling Y, Li R-C, Li Y-P, Nong Y, Sun K-X, Li J. Seroprevalence of hepatitis B and immune response to hepatitis B vaccination in Chinese college students mainly from the rural areas of western China and born before HBV vaccination integrated into expanded program of immunization. Human Vaccines Immunotherapeutics. 2014;10(1):224–31.
Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Reprint of: epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2013;31:J21–8.
Techasathit W, Ratanasuwan W, Sonjai A, Sangsiriwut K, Anekthananon T, Suwanagool S. Vaccination against hepatitis B virus: are Thai medical students sufficiently protected. J Med Assoc Thail. 2005;88(3):329–34.
Maswanya E, Moji K, Horiguchi I, Nagata K, Aoyagi K, Honda S, Takemoto T. Knowledge, risk perception of AIDS and reported sexual behaviour among students in secondary schools and colleges in Tanzania. Health Educ Res. 1999;14(2):185–96.
Tahan V, Karaca C, Yildirim B, Bozbas A, Ozaras R, Demir K, Avsar E, Mert A, Besisik F, Kaymakoglu S. Sexual transmission of HCV between spouses. Am J Gastroenterol. 2005;100(4):821.
Inaba N, Ohkawa R, Matsuura A, Kudoh J, Takamizawa H. Sexual transmission of hepatitis B surface antigen. Infection of husbands by HBsAg carrier-state wives. Sex Transm Infect. 1979;55(5):366–8.
CAS Google Scholar
Brener ND, Billy JO, Grady WR. Assessment of factors affecting the validity of self-reported health-risk behavior among adolescents: evidence from the scientific literature. J Adolesc Health. 2003;33(6):436–57.
Download references
Acknowledgements
The authors would like to acknowledge the technical support provided by Dr. Peter Maskini and Dr. Hysinta Jaka, Mr. Seif Abdu and all staff of the Bugando medical centre laboratory.
This research was supported by research grant from CUHAS to MMM and ELISA KITS from SIEMEN, GERMANY to CK.
Author information
Authors and affiliations.
Department of Microbiology and Immunology, Weill Bugando School of Medicine, Catholic University of Health and Allied sciences, P.O.Box 1464, Mwanza, Tanzania
Mariam M. Mirambo, Emmanuel Mkumbo, Hadija Selega, Martha F. Mushi, Vitus Silago, Jeremiah Seni & Stephen E. Mshana
Institute of Allied Health Sciences, Catholic University of Health and Allied sciences, P.O.Box 1464, Mwanza, Tanzania
Betrand Msemwa
Deutsche Lepra- und Tuberkulosehilfe e.V, Raiffeisenstr.3, 97080, Würzburg, Germany
Christa Kasang
You can also search for this author in PubMed Google Scholar
Contributions
MMM, CK and SEM participated in the design of the study. SEM, MMM, EM,HS, MFM, JS, VS and BM participated in the collection of specimens and data. EM, HS, MMM and SEM performed serological tests. MMM and SEM analysed and interpreted the data. MMM wrote the first draft of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Correspondence to Stephen E. Mshana .
Ethics declarations
Ethics approval and consent to participate.
The study obtained the ethical clearance from the joint CUHAS-BMC- research ethics and review committee (CREC) with ethical clearance number 303/2017. All participants were asked to sign informed consent prior enrollment.
Consent for publication
Not applicable.
Competing interests
None declared.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mirambo, M.M., Mkumbo, E., Selega, H. et al. Hepatitis B virus infections among health professional students in Mwanza city,Tanzania in 2016. Arch Public Health 78 , 76 (2020). https://doi.org/10.1186/s13690-020-00459-2
Download citation
Received : 02 March 2020
Accepted : 12 August 2020
Published : 18 August 2020
DOI : https://doi.org/10.1186/s13690-020-00459-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anti HBV-core
Archives of Public Health
ISSN: 2049-3258
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Microbiol Rev
- v.33(2); 2020 Apr

Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy
Mindie h. nguyen.
a Division of Gastroenterology and Hepatology, Stanford University Medical Center, Palo Alto, California, USA
b Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong
Edward Gane
c New Zealand Liver Transplant Unit, Auckland City Hospital, Auckland, New Zealand
d Department of Medicine, University of Auckland, Auckland, New Zealand
Jia-Horng Kao
e Division of Gastroenterology and Hepatology, Department of Internal Medicine, National Taiwan University College of Medicine and National Taiwan University Hospital, Taipei, Taiwan
f Graduate Institute of Clinical Medicine, National Taiwan University College of Medicine and National Taiwan University Hospital, Taipei, Taiwan
g Hepatitis Research Center, National Taiwan University College of Medicine and National Taiwan University Hospital, Taipei, Taiwan
Geoffrey Dusheiko
h UCL Medical School, Kings College Hospital, London, United Kingdom
Currently, despite the use of a preventive vaccine for several decades as well as the use of effective and well-tolerated viral suppressive medications since 1998, approximately 250 million people remain infected with the virus that causes hepatitis B worldwide. Hepatitis C virus (HCV) and hepatitis B virus (HBV) are the leading causes of liver cancer and overall mortality globally, surpassing malaria and tuberculosis. Linkage to care is estimated to be very poor both in developing countries and in high-income countries, such as the United States, countries in Western Europe, and Japan.
Currently, despite the use of a preventive vaccine for several decades as well as the use of effective and well-tolerated viral suppressive medications since 1998, approximately 250 million people remain infected with the virus that causes hepatitis B worldwide. Hepatitis C virus (HCV) and hepatitis B virus (HBV) are the leading causes of liver cancer and overall mortality globally, surpassing malaria and tuberculosis. Linkage to care is estimated to be very poor both in developing countries and in high-income countries, such as the United States, countries in Western Europe, and Japan. In the United States, by CDC estimates, only one-third of HBV-infected patients or less are aware of their infection. Some reasons for these low rates of surveillance, diagnosis, and treatment include the asymptomatic nature of chronic hepatitis B until the very late stages, a lack of curative therapy with a finite treatment duration, a complex natural history, and a lack of knowledge about the disease by both care providers and patients. In the last 5 years, more attention has been focused on the important topics of HBV screening, diagnosis of HBV infection, and appropriate linkage to care. There have also been rapid clinical developments toward a functional cure of HBV infection, with novel compounds currently being in various phases of progress. Despite this knowledge, many of the professional organizations provide guidelines focused only on specific questions related to the treatment of HBV infection. This focus leaves a gap for care providers on the other HBV-related issues, which include HBV’s epidemiological profile, its natural history, how it interacts with other viral hepatitis diseases, treatments, and the areas that still need to be addressed in order to achieve HBV elimination by 2030. Thus, to fill these gaps and provide a more comprehensive and relevant document to regions worldwide, we have taken a global approach by using the findings of global experts on HBV as well as citing major guidelines and their various approaches to addressing HBV and its disease burden.
INTRODUCTION
Chronic hepatitis B (CHB) virus infection is a well-known threat to global health ( 1 , 2 ). CHB is transmitted through exposure to infected blood and body secretions. Currently, the main route of transmission remains from mother to neonate (vertical transmission) or from mother to child or child to child (horizontal), but inadequate sterilization of health care instruments and the administration of contaminated blood products also remain major modes of transmission, especially in poorer countries. In addition, with the global opioid crisis, intravenous drug use has again become a more common mode of transmission. Finally, male-to-male sex and heterosexual sexual contact by an individual with many partners remain major modes of transmission ( 1 , 2 ) ( Table 1 ).
Geographic prevalence of hepatitis B surface antigen carriage in the general population and possible routes of transmission
Due to the various modes of transmission, the geographic prevalence of this infection varies widely as well and is categorized as high, intermediate, or low ( Table 1 ). Before the universal implementation of vaccination for hepatitis B, the prevalence of hepatitis B surface antigen (HBsAg) globally ranged from 2% to 20%. A review of published data from 161 countries that were reported between 1965 and 2013 estimated the worldwide prevalence of HBsAg to be 3.61%, with the highest rates being in Africa (8.83%) and the Western Pacific regions (5.26%). Within the World Health Organization (WHO) territories, the prevalence of the virus ranged from 0.20% (Mexico) to 13.55% (Haiti) in the Americas, 0.48% in the Seychelles, and 22.38% in the African (South Sudan) region ( 3 ). When combined, this prevalence corresponded to 248 million people globally in 2010.
In 2016, an updated estimate indicated that the total global hepatitis B virus (HBV) infection prevalence increased to 3.9% (95% confidence interval, 3.4 to 4.6%), corresponding to 292 million people globally, suggesting that the presence of HBV was not decreasing. Furthermore, only approximately 29 million (10%) were diagnosed with HBV infection. In addition, it was found that only 4.8 million (5%) of those eligible for treatment had actually been treated.
In the United States, as reported by a study using data from the National Health and Nutrition Examination Survey (NHANES; 1999 to 2016), there are considerable racial/ethnic disparities in HBV infection rates ( 4 ). From a total of 47,628 persons who underwent HBV serology testing, the overall prevalence of chronic hepatitis B (CHB) was 0.35%. However, the prevalence among Asians was 3.41%, while it was only 0.69% among non-Hispanic blacks and less than 0.2% among non-Asian, non-black individuals ( 4 ). These differences in CHB prevalence rates in the United States are attributed to the fact that the majority of CHB cases are considered imported ( 5 ), such that foreign-born Asians and blacks have a higher prevalence than their U.S.-born counterparts (3.85% versus 0.79%, respectively, for foreign-born Asians and 1.94% versus 0.52%, respectively, for blacks) ( 4 ). However, these numbers may still underestimate the number of persons infected with HBV due to the limitations of using NHANES data, which do not adequately account for institutionalized populations, such as those in prisons, or other populations at higher risk of HBV infection, such as immigrant populations ( Fig. 1 ).

Disease awareness and treatment care cascade of persons with HBV infection in the United States.
Other changes in the status of global CHB that have been noted were recently reported. A real-world study of 44,026 CHB patients from the United States found that the median age of the population with CHB had increased from 48 years in 2006 to 52 years in 2015. Alongside the increase in age, the number of non-liver-related comorbidities also increased, and these, combined with CHB, have also increased the disease burden of CHB ( 6 ). Other real-world studies have also reported findings similar to those described above on the increasing rates of liver comorbidities as well as the increased health care utilization by individuals with CHB and the cost of CHB ( 7 , 8 ).
Another factor that complicates the elimination of CHB is the difference in the distribution of HBV genotypes throughout the world ( 9 ). Currently, there are 10 HBV genotypes, identified by the letters A to J ( Table 2 ), which, through genetic mutations and the lack of proofreading in reverse transcriptase, have evolved over the long term, creating challenges to their elimination.
Global distribution of hepatitis B virus genotypes A to J
PREVENTION AND CURRENT LINKAGE TO CARE
In 2011, the World Health Organization passed a resolution recognizing viral hepatitis as a global health concern. In October 2015, that body released its first official strategy for the control of viral hepatitis, which aims to significantly reduce the considerable morbidity and mortality found in individuals with chronic hepatitis B and chronic hepatitis C (CHC) virus infections by the year 2030 ( 10 ). Governmental agencies ( n = 194 countries) have signed on to this strategy, which includes ambitious targets of a 90% reduction in the incidence of viral hepatitis, an 80% treatment uptake by eligible patients, and a 65% reduction in mortality.
The global elimination of HBV can become a reality thanks to the use of an effective and low-cost vaccine, which has been available for almost 30 years. The hepatitis B vaccine has been shown to prevent hepatocellular carcinoma (HCC) ( 11 , 12 ). Currently, most countries have implemented universal neonatal HBV vaccination programs, which are inexpensive and which may eradicate HBV infection within the next century ( 13 ). Improving the rates of vaccination at birth and providing prepartum therapy to highly viremic mothers to prevent transmission from mother to child could accelerate this eradication of hepatitis B.
Recent data demonstrate that immunoprophylaxis with hepatitis B immunoglobulin and the hepatitis B vaccine in newborns can reduce the rate of mother-to-child transmission (MTCT) from 90% to 10% ( 14 ). However, if the mother has an HBV DNA level of greater than 200,000 IU/ml, immunoprophylaxis has a failure rate of 10 to 30% in infants born to such mothers. Transmission can be effectively eliminated by the administration of antiviral therapy to the mother during the third trimester, as shown by a controlled and randomized study from China ( 15 , 16 ). The analysis showed that the transmission rate dropped from 7% in the control group to 0% in the treatment group at the 28th postpartum week. Thus, currently, major liver societies in the United States and Europe recommend that all pregnant women with HBV DNA levels greater than 200,000 IU/ml be considered for treatment with tenofovir disoproxil fumarate (TDF) starting toward the end of the second trimester and at the beginning of the third trimester (24 to 28 weeks of pregnancy). Importantly, breast-feeding and TDF treatment can continue postpartum in HBsAg-positive untreated women ( 17 ).
Unfortunately, universal neonatal vaccination and the elimination of transmission from mother to child do not affect the projected morbidity/mortality for the hundreds of millions of adults living with CHB, such that by 2030 there are expected to be 17 million deaths attributable to CHB. Currently, the cascade of care is poor, with only about 15% of the estimated 712,000 infected persons in the United States being aware of their infection and only 4.5% receiving antiviral therapies ( Fig. 1 ) ( 4 ).
The 2010, the United States Institute of Medicine (IOM) found many areas in which unmet needs regarding hepatitis existed, including health care providers’ education, vaccination, and prevention programs, as well as accurate diagnosis and treatment ( 18 ). Following publication of that report, in 2011 the U.S. Department of Health and Human Services (HHS) announced an action plan for the prevention, care, and treatment of viral hepatitis to assist with and accelerate the elimination of HBV in the United States. The strategic plan covers the following areas: the education of all affected parties to reduce health disparities among members of the community and the populations seen by health care providers, increase testing and treatment by increasing surveillance to detect transmission of the disease, increase the rate of vaccination for viral hepatitis, reduce drug use behaviors that contribute to the transmission of hepatitis, and prevent health care-associated viral hepatitis ( 19 ).
However, despite these programs, multiple surveys have shown that complete knowledge of care for CHB is lacking, even among primary care physicians and specialists, who sometimes do not know the most current management recommendations ( 20 , 21 ). In fact, one study found that patients who were evaluated by specialists were more likely to have a complete laboratory evaluation than those examined by only primary care providers, but even then, 40% of those who received specialty care did not receive a thorough evaluation that included continued follow-up of untreated patients to determine when or if treatment becomes necessary ( 22 , – 24 ).
The lack of appropriate patient education and cultural barriers are also variables that limit interaction with the health care setting. A recent study of 1,000 African immigrants to the United States found that culturally targeted patient navigators achieved very high rates of continued care (97%) and adherence ( 25 ). However, a major obstacle to the initiation and continuation of care for CHB, despite any extra efforts at patient engagement, was the lack of health insurance ( 26 ).
Due to treatment deferral, provider-requested further observation, patient loss to follow-up, and/or patient refusal of treatment, up to half of the patients in the United States eligible for treatment were not treated within the first year after their diagnosis ( 27 ). Specialists were more likely to initiate both treatment and treatment with the most appropriate drug therapy. However, even for university-based liver clinics, only 59% to 73% of eligible patients were treated as recommended by professional practice guidelines ( 28 ). Once treatment is initiated, adherence to treatment is often poor due to the asymptomatic nature of CHB during the pre-end-stage liver disease period. In one study, all the entecavir treatment failures were accounted for through nonadherence rates of 10% to 12% (over 4 years) ( 29 ).
Thus, trying to effectively screen, diagnose, and treat patients requires a multifaceted approach. Provider education, especially at the level of the primary care practitioner, is needed. Information technology is needed to obtain complete medical evaluations, improve the referral process, and improve the guidelines driving the delivery of patient care. Efforts need to be continued to raise awareness among the public as well as provide culturally sensitive and stigma-free education on HBV infection, its routes of transmission, and its prevention through vaccination. Interpreters and language-specific materials are other proven methods that can be used to improve rates of referral and follow-up with the health care system. Effective program delivery depends upon adequate financial support, accessibility, and promotion by community networks, all of which are vital to the meet the WHO goal of the elimination of viral hepatitis by 2030.
DIAGNOSIS AND CLINICAL EVALUATION
In order to accurately screen, diagnose, and then treat patients who are infected with HBV, an understanding of the current diagnostic tests is necessary. An indication of CHB virus infection is the presence of a positive HBsAg result for over 6 months. Seroclearance, identified by a qualitative test for HBsAg (i.e., the loss of HBsAg, which is equal to <0.05 IU/ml in serum) with or without the appearance of antibodies (anti-HBs), is regarded as a functional cure ( 17 , 30 , 31 ). Protective immunity is acknowledged when the anti-HBs level is greater than 10 IU/ml.
Serological, Molecular, and Genomic Testing
Quantitative hbsag..
HBsAg levels and the source of HBsAg production change over the different phases of CHB. In the immune-tolerant phase, HBsAg concentrations are high, while in the inactive phase they are low ( 32 , – 34 ). The source of HBsAg production also changes from being predominantly covalently closed circular DNA (cccDNA) transcription in the young hepatitis B e antigen (HBeAg)-positive patient to being from HBV integrants in the older HBeAg-negative patient ( 35 ). An HBsAg level of <100 IU/ml in Asian HBeAg-negative patients is predictive of spontaneous HBsAg seroclearance within 6 to 8 years ( 36 ). Meanwhile, higher HBsAg levels may mean a lower likelihood of spontaneous clearance; as a result, the HBsAg level has recently been incorporated into HCC risk scores ( 37 ).
The HBsAg level can also be useful when trying to predict and/or monitor a patient’s response to therapy with peginterferon (PEG-IFN) ( 38 ), such that in HBeAg-positive patients, an HBsAg level of >20,000 IU/ml at week 24 confers a 96% negative predictive value (NPV) for genotype A HBV and a 100% NPV for genotypes B, C, and D. A positive response to treatment for HBV infection is defined as the loss of HBeAg and an HBV DNA level of <2,000 IU/ml at 6 months posttreatment ( 39 ). The best-validated model for prediction of the impact of treatment in HBeAg-negative patients is to note a decline in both HBV DNA and HBsAg levels at week 12. The NPV for patients who fail to achieve a 2-log decline in the HBV DNA level and/or any decline in HBsAg is 95 to 100% when defined as an HBV DNA level of <2,000 IU/ml with a normal alanine aminotransferase (ALT) level at 24 weeks posttreatment ( 40 ). Nucleos(t)ide analogue (NA) therapy, which is associated with a very slow decrease in HBsAg levels, regardless of a strong suppression of HBV DNA, indicates that immune clearance is weak as well as the continued transcription of integrated viral genomes not affected by chain terminators ( 41 ). Studies from Hong Kong and Taiwan conducted with HBeAg-negative patients reported that a sustained response and HBsAg seroclearance after the cessation of lamivudine treatment can be predicted when the serum HBsAg level is <100 to 200 IU/ml ( 42 , 43 ).
Since the discovery of HBeAg in the early 1970s, it has long been seen as an indicator of viral replication and infectivity. With the introduction of HBV DNA testing, infection and replication are more effectively measured by the measurement of HBV DNA in serum. The definition of a patient’s phase in the history of chronic HBV infection is currently measured by the HBeAg level ( 17 , 44 ). The seroconversion of HBeAg indicates an important phase in immune clearance. In spite of this, some HBeAg-negative patients may contract active hepatitis with high HBV DNA concentrations. This state is often characterized as HBeAg-negative disease. Concomitant mutations in the basal core promoter and/or the precore stop codon can appear ( 45 ). Current international opinion includes recommendations regarding the status of HBeAg ( 17 , 30 , 31 ) (also see Treatment below).
HBeAg seroconversion can occur on peginterferon treatment, with a rapid decline in HBeAg levels. On the other hand, a high HBeAg level can be used to predict a nonresponse and terminate treatment early, when appropriate ( 46 ). Unfortunately, HBeAg quantification is not yet standardized, which hinders its application in clinical practice, while measurement of HBeAg levels is not recommended for patients who have HBeAg-negative HBV.
The core antibody to hepatitis B virus (anti-HBc) can be detected through immunoassays for total anti-HBc, which is able to detect both anti-HBc IgG and anti-HBc IgM. Anti-HBc IgM is the determinant of acute hepatitis B and is often the only sign that may be detected during the period of acute hepatitis B when HBsAg has become undetectable. Patients are also positive for anti-HBc when they have severe and acute flare-ups of chronic hepatitis B ( 47 ). However, anti-HBc IgG may indicate current or previous HBV infection. In patients with cryptogenic hepatocellular carcinoma (HCC), a negative result for HBsAg and a positive result for anti-HBc may indicate HBV infection or possible occult HBV infection ( 48 ). Patients receiving potent immunosuppression and cancer chemotherapy (for example, rituximab) may have a reactivation of occult HBV infection ( 49 ). The amount of HBV DNA detectable in patients with occult HBV infection is usually <200 IU/ml.
Current guidelines suggest antiviral therapy for patients with increased ALT levels and HBV DNA levels of 2,000 to 20,000 IU/ml, as well as in those with HBV DNA at any detectable levels in the presence of cirrhosis, since HBV DNA can be a marker of viral replication and is the main target of antiviral therapy ( 17 , 30 , 31 ). This tactic is based on data that demonstrate a strong relationship between high HBV DNA levels and the future development of cirrhosis and HCC ( 50 , 51 ). Suppression of HBV DNA to an undetectable level on testing is associated with a reduced risk of cirrhosis, HCC, and decompensation ( 52 , 53 ).
Patients on treatment with nucleos(t)ide analogs (NAs) need to be tested for HBV DNA to assess the treatment response and guide treatment. Failure to suppress HBV DNA to achieve undetectable levels by the 6th month of treatment with telbivudine and lamivudine or by 12 months of treatment with adefovir (all of these drugs have a low barrier to resistance) is associated with an increased risk of therapeutic resistance. Therefore, the use of a more potent agent to which the virus is not resistant is usually recommended ( 17 , 30 , 31 ). The baseline or on-treatment HBV DNA level also predicts the response to peginterferon therapy ( 40 , 54 ).
Hepatitis B core-related antigen (HBcrAg) is a new indicator that measures an amino acid sequence common to HBeAg and hepatitis B core antigen (HBcAg), as well as a putative 22-kDa precore protein. Since HBcrAg positivity correlates with intrahepatic HBV DNA and pregenomic RNA (pgRNA) levels among patients on NA treatment, HBcrAg may be a good serum marker of the active transcriptional activity of liver cccDNA ( 55 ). In patients treated or not treated with NA, higher HBcrAg levels may be associated with an increased risk of liver cancer ( 55 , 56 ). Therefore, among NA-treated subjects, the level of serum HBcrAg associates with the serum HBV DNA level but not the serum HBsAg level ( 57 ). The higher that the HBcrAg level is prior to stopping NA treatment, the higher the risk for hepatitis reactivation is, reflecting a possible role of HBcrAg in residual viral replication during NA therapy ( 58 , 59 ). Hence, HBcrAg may be an effective indicator of the presence of cccDNA in the liver and may be useful for surveillance and an indicator of the efficacy of treatment and the clinical course for CHB patients.
POC Diagnostics
A rapid point-of-care (POC) assay for HBsAg provides a plausible diagnostic strategy in low-resource areas. A recent meta-analysis summarizing the findings of 27 studies evaluated 49 rapid POC assays ( 60 ). Despite a robust specificity close to 100% (range, 90% to 100%), the reliability of individual testing varied greatly and was heterogeneous in a range of from 43.5% to 99.8%. Study location, reference standard, and study score were the three key factors most dependably aligned with the estimates and the heterogeneity ( 60 ). However, another study tested three POC tests for HBsAg in the field and in labs in the Western Africa country of the Gambia. They found that the sensitivity and specificity ranged from 88.5% to 90.0% and 99.8% to 100%, respectively, in the field and from 93.9% to 95.3% and 93.3% to 94.7%, respectively, in the laboratory setting ( 61 ). Though further research is indicated, POC tests for HBsAg may prove to be accurate, rapid, and less expensive choices than laboratory serological screening for HBV in the field.
Genomic and Novel Molecular Markers
The HBV genotype has been associated with both the response to antiviral therapy and disease outcomes ( 62 ). There are currently 10 HBV genotypes, with each genotype being classified by an 8% or more divergence in the nucleotide sequence of the genome ( 63 ). Among them, genotypes A to D are the four predominant genotypes ( 64 ). Genotypes B and C are the most common in eastern and southeastern Asia ( 65 , 66 ), while genotypes A and D are most commonly found in North America, Africa, and Europe ( 67 ). Genotype E has been reported from West Africa. Genotypes A and B appear to have a greater response to interferon therapy than genotypes C and D ( 68 ). In contrast, the various HBV genotypes do not have different responses to the nucleos(t)ide analogues. Delayed HBeAg seroconversion and a higher risk of reactivation in the HBeAg-negative phase are associated with genotype C, and hence, those infected with genotype C have more advanced fibrosis and more severe liver damage than those infected with genotype B ( 69 ). In a meta-analysis of 14,545 patients, a greater risk of HCC was seen in those infected with genotype C than in those infected with the other major genotypes ( 70 , 71 ). Patients with genotype C infection also account for more cases of fibrosis, cirrhosis, and liver cancer than those with genotype B infection. However, studies in Hong Kong and Taiwan have noted the development of HCC in young noncirrhotic genotype B-infected patients ( 72 , 73 ). In contrast, reports from different parts of the world have indicated that individuals infected with HBV genotypes C, D, and F are more likely to have a greater cumulative cirrhosis and HCC risk than those infected with genotypes A and B ( 74 ). In southern Africa, HCC occurs at a younger age in those infected with subtypes of genotype A.
In HBV, host genomic factors that interfere with the viral replication cycle could indicate additional targets for therapy to reduce viral loads ( 75 ). Interferon lambda 3 (IFN-λ3), formerly interleukin-28B (IL-28B), creates a polymorphism in the human leukocyte antigen (HLA) locus and thus is a promising single nucleotide polymorphism (SNP) that may indicate a virological response to peginterferon ( 76 ). HLA-DPA1, another HLA locus, is often linked to HBeAg seroconversion ( 77 ). Hepatic disease progression in Chinese Han hepatitis B patients has been found to be linked to the G-201A allele, in the promoter region of the interferon-inducible IP-10 gene, through the upregulation of IP-10 expression ( 78 ). This phenomenon should be studied in patients of other ethnicities.
Since HBV DNA is suppressed to low levels in most patients on NA therapy, the HBV DNA level does not accurately reflect the levels of viral cccDNA, RNA, or antigen production in the livers of treated patients. Other groups have studied the role of HBV RNA as a potential biomarker ( 79 ). Since mRNA exists in an unstable state in the serum, most HBV RNA found in the serum is thought to be pregenomic RNA. Previous studies have suggested that the measurement of HBV RNA may be helpful in predicting HBeAg seroconversion in patients on NA therapy and is deserving of further evaluation ( 80 ).
Recommendations for Screening and Diagnosis
The American Association for the Study of Liver Diseases (AASLD) currently suggests that all persons born in countries with a HBsAg seroprevalence of 2%, U.S.-born persons not vaccinated as infants whose parents were born in regions with high rates of HBV endemicity (8%), pregnant women, persons needing immunosuppressive therapy, and individuals at high risk for exposure to HBV (for example, blood recipients, blood donors, individuals with male-to-male sexual contact, prisoners, people with a history of liver disease; refer to the guidelines for a full list) be screened for HBV using tests for both HBsAg and anti-HBs. Screened persons who are anti-HBs negative should be vaccinated.
Other guidelines.
At present, the European Association for the Study of the Liver (EASL) does not have specific guidelines on who to screen and what tests to use for diagnosis. The guidelines of the Asian Pacific Association for the Study of the Liver (APASL) follow the AASLD recommendations on who would benefit the most from screening. Testing should include a serological assay for HBsAg (subgenotype A1), anti-HBs (subgenotype B2), and total anti-HBc (subgenotype B2). However, APASL emphasizes that screening should be linked to appropriate counseling and referral for further care, including clinical evaluation of the need for treatment and vaccination. The World Health Organization (WHO) also recognizes the same groups of people that need to be screened for HBV noted by AASLD. However, for testing, WHO recommends using a single quality-assured serological in vitro diagnostic test (IVD; i.e., either a laboratory-based immunoassay [an enzyme immunoassay or a chemiluminescence immunoassay] or rapid diagnostic test [RDT]) to detect HBsAg and hepatitis C virus (HCV) antibody. The RDTs used should meet minimum performance standards and should be delivered at the point of care to improve access and a linkage to care and treatment. When a person is found to have a reactive HBsAg serological test result, HBV DNA nucleic acid testing should be conducted to help further guide who to treat or not treat, if there is no evidence of cirrhosis, and to monitor for the treatment response.
Assessment of Liver Necroinflammation and Fibrosis
Serum alanine aminotransferase..
ALT concentrations generally correlate with hepatic necroinflammation in CHB patients. Cutoffs for ALT should be lowered to 30 IU/liter for males and 19 IU/liter for females ( 81 ), as high-normal ALT levels ranging from 40 to 70 IU/liter are linked to cirrhosis ( 82 ) and liver-related deaths ( 83 ). However, a current AASLD 2018 guidance update suggested that the ALT cutoffs should be 35 U/liter for males and 25 U/liter for females ( 84 ).
Assessment of hepatic fibrosis by invasive versus noninvasive tests.
Percutaneous biopsy of the liver is traditionally the best diagnostic measurement to use when assessing liver fibrosis in clinical trials ( 85 ), as well as for determination of patient prognosis and interventions ( 86 ). However, its use in routine clinical practice is limited due to its invasive nature, the potential for serious complications, difficulty with selection of the correct location for the biopsy, selection of a sample that is large enough, as well as heterogeneity among pathologist readings ( 86 ).
Recently, noninvasive tests for the staging of fibrosis have become available. Such tests can be classified as physical tests or tests for serum biomarkers, and each has its own advantages and disadvantages ( Table 3 ). There are two groups of physical tests: shear wave elastography (including transient, acoustic radiation force impulse, or multidimensional shear wave elastography) as well as magnetic resonance elastography (MRE). Among these tests, FibroScan transient elastography is one of the best applicable tools worldwide. The standard M probe transmits a shear wave followed by an ultrasound wave through a probe placed near the liver parenchyma to assess liver stiffness measurement (LSM) ( 87 ). Doppler calculates the velocity of the wave passing through the liver. This technique can discriminate between severe fibrosis, no or minimal fibrosis, and cirrhosis ( 88 ), and it is useful and accurate across liver diseases, such as CHB, CHC, and autoimmune hepatitis ( 87 ). However, measuring LSM by transient elastography is not as efficient in obese patients ( 89 ), unless an XL probe is used ( 90 ). On the other hand, MRE assesses liver stiffness with a phase-contrast imaging method that uses mechanical wave propagation ( 91 ). Generally, obesity or the severity of the ascites does not affect MRE, and it is not as operator dependent or prone to technical failure as other tests. MRE can be used to stage even mild fibrosis ( 92 ). However, MRE is less cost-effective, slower to perform, and less well tolerated than an ultrasound-based approach.
Different noninvasive approaches a
Serum biomarkers for liver fibrosis are often used in combination. The two most well-known indices which are based on routinely available laboratory markers are the aspartate aminotransferase (AST)-to-platelet ratio index (APRI) ( 93 ) and the Forns index ( 94 ), though caution is warranted if the APRI is used in patients from Africa, as its sensitivity is low for this group. Other tests include those that test for biochemical markers specifically related to fibrinolysis or fibrinogenesis ( 95 ). FibroTest (BioPredictive, Paris, France) and FibroSure (LabCorp, Burlington, NC, USA) test for gamma-glutamyltransferase (GGT), total bilirubin, α 2 -macroglobulin, apolipoprotein A1, and haptoglobin ( 96 , – 98 ). They have been validated for use in patients with CHB and are widely used. Other specific serum-based noninvasive fibrosis tests or measures are FibroMeter (Echosens, Paris, France) ( 99 ) and the enhanced liver fibrosis (ELF) score ( 100 , 101 ).
To enhance the accuracy of fibrosis assessment, different approaches combining physical tests and biochemical markers have been proposed. A significant proportion of patients can avoid liver biopsy if the ELF-LSM algorithm is used ( 100 ). An additional well-validated combination approach is the Forns index, which does not use the ALT level in the LSM algorithm ( 102 ).
PATHOGENESIS AND NATURAL HISTORY
Hbv life cycle and hbv cure targets.
HBV is a DNA virus of the Hepadnaviridae group. The virion contains a circular DNA genome of 3,200 bp. An RNA pregenome replicates DNA by reverse transcription within the capsid. The virion is internalized into the cell by attaching to a cellular receptor, sodium taurocholate cotransporting polypeptide (NTCP). The first replicative event is the change of the relaxed circular DNA (rcDNA) into a covalently closed circular DNA (cccDNA) minichromosome inside the hepatocytes. Conversion of the relaxed circular DNA to the closed circular DNA requires disassembly of the HBV capsid, deproteinization of the rcDNA, and the ligation of rcDNA to cccDNA. Notably, cccDNA is synthesized from rcDNA either from infecting virions or from subsequent intracellular nucleocapsids via a cccDNA shuttle amplification pathway, thus providing a critical mechanism for persistence as a minichromosome in the center of infected cells. The transcriptional template used for the transcription of major viral mRNA is cccDNA. HBsAg, HBcAg, HBeAg, the DNA polymerase, and the HBx protein are encoded by conserved open reading frames. HBsAg is also produced following the transcription of integrated viral DNA ( 103 ). Human RNA polymerase II mediates the transcription of cccDNA to generate pgRNA. This polymerase attaches to a secondary structure, epsilon, at the end of the pregenome. The capsid of the virus is constructed into core particles containing pgRNA and the viral polymerase (reverse transcriptase). HBV replication occurs within capsids and the nucleocapsid, where, through reverse transcription, the pgRNA forms incomplete rcDNA, where the HBV capsids become mature virus particles after being coated with HBsAg ( 104 ). The capsid-containing rcDNA alternatively shuttles to the nucleus to replenish cccDNA or becomes covered to form the infectious virions, which are then released from the cell ( 105 , 106 ).
Complete and incomplete viral particles are then secreted. Numerous empty non-DNA-containing virions are exuded; importantly, recent findings have shown that RNA-containing particles are released. As described above, inhibition of cccDNA synthesis within the nucleus is not directly affected by current nucleoside analogue therapy, as only minus- and plus-strand DNA synthesis within the cytoplasm is targeted. We know that HBx is needed to transcribe cccDNA via epigenetic regulation ( 107 , 108 ). We also know that fragments of HBV DNA become part of the genome of hepatocytes but that integration is not necessary for the replication of HBV. A soluble, dimeric protein, HBeAg, is secreted from hepatocytes. HBeAg is processed from the precore protein: the bulk of amino acids are shared with HBcAg, but HBeAg possesses an N-terminal extension of 10 amino acids and a C-terminal truncation of 34 amino acid residues. Both HBV and hepatitis D virus (HDV) have on their surfaces the large envelope glycoprotein, which plays an important role in the entry of the virus. The antigenic loop on the S protein mediates the attachment of heparin sulfate proteoglycans (HSPGs) to the cell surface ( 109 , 110 ). The entry receptor has recently been discovered to be the sodium taurocholate cotransporting polypeptide (NTCP) ( 111 , – 113 ). Based on nucleotide divergence, a sequence variation (variation of up to 12% of nucleotides) between isolates of HBV can produce up to 10 different genotypes (genotypes A to J) as a result of this divergence ( 111 , – 113 ).
Natural History
Since the pathophysiology of HBV is so complex, the history and phases of infection are still being studied. Most children infected in infancy or childhood will develop chronic HBV infection ( 114 , – 116 ). Different age-dependent phases of the disease have long been recognized and generally consist of an immunotolerant phase (or high-replication, low-inflammation phase), an immunoactive phase, an inactive carrier state (low replication levels and normal/nearly normal serum aminotransferase levels), and reactivated disease ( Table 4 ). These have been renamed in the recent EASL HBV clinical practice guidelines as HBeAg-positive infection, HBeAg-positive hepatitis, as well as HBeAg-negative infection and HBeAg-negative hepatitis ( 17 ).
Phases of chronic hepatitis B a
In children and young adults, the high-replication, low-inflammation phase is characterized by detectable serum HBsAg and HBeAg concentrations and high serum HBV DNA concentrations but only slightly increased serum ALT levels, and liver histology is often relatively benign (i.e., minimal to no inflammation or fibrosis). However, in this phase, disease is under way, with expansion of hepatocytes and HBV integration that may ultimately progress to active disease.
Progression to cirrhosis in HBeAg-positive patients occurs at a rate of 2 to 5.5% per year, becoming 8 to 20% in 5 years. The high-replication phase may be followed by active HBV infection with developing necroinflammation (also referred to as HBeAg hepatitis, or the immunoactive phase) or by HBeAg seroconversion and remission in a proportion of patients (HBeAg-negative infection, or the inactive carrier state). Inactive carriers characteristically exhibit normal aminotransferase levels, and the HBV DNA level is generally found to be less than 2,000 IU/ml. A small proportion of patients experience disease regression at a rate of from 0.5 to 2% per year. Anti-HBe early seroconversion before the onset of marked hepatic fibrosis may signal remission and may indicate a good prognosis, depending upon the degree of liver damage.
Conversely, HBeAg-negative anti-HBe-positive disease (also called HBeAg-negative hepatitis, or the reactivation phase) is a progressive stage of chronic disease. Though HBeAg is not apparent in these patients due to mutant precore HBV, HBcAg is detected in liver cells and evidence of active disease is still present. Patients with anti-HBe-positive CHB are usually older, have more continuing inflammatory changes, and experience variations in their liver disease course, with inconsistent serum aminotransferase levels and different HBV DNA concentrations. Anti-HBe-positive patients experience a quicker progression to cirrhosis at a yearly rate of 8 to 20%, and the levels of HBsAg and HBV DNA in these patients tend to be lower than those in patients who are HBeAg positive.
Patients with cirrhosis experience hepatic failure at a rate of 16% over 5 years. In 366 HBsAg-positive patients with compensated cirrhosis, the cumulative probability of survival was 84% and 68% at 5 and 10 years, respectively ( 117 , – 120 ). It was determined that risk factors for a high rate of progression and shorter survival were age, male sex, high liver enzyme levels, high HBV DNA levels, high HBsAg levels, infection with a genotype C strain, as well as basal core promoter expression. The REVEAL studies delineated the relationship between HCC risk and high HBV DNA concentrations ( 37 , 121 ).
The ultimate goals of treatment are to improve the patients’ quality of life and their survival. Therefore, treatment is geared toward the prevention of liver disease progression to cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, liver transplantation, and death. These goals can be achieved through the elimination of HBV replication, which results in the normalization of liver enzyme levels and the resolution of histologic necroinflammatory activity. Sustained viral suppression prevents fibrosis progression and leads to the regression of fibrosis even for patients with established cirrhosis. In the tenofovir registration study, at 5 years of follow-up, paired liver biopsy results demonstrated that 348 patients experienced an 87% regression of fibrosis, including 71 out of the 96 cirrhotic patients (74%) ( 122 ). Sustained viral suppression also reduced the risk for hepatocarcinogenesis ( 13 ). The following briefly describes the recommendations for initiating treatment per professional society guidelines and the current regimens available for the treatment of HBV infection ( Fig. 2 ).

Universal HBV screening and care action items for persons residing in and immigrants from areas where HBV is endemic (an HBsAg prevalence of 2% or higher). US, ultrasound; AFP, alpha-fetoprotein; HAV, hepatitis A virus; CBC, complete blood count; INR, international normalized ratio.
Summary of Current Recommendations for Initiating Antiviral Therapy
Treatment guidelines are regularly updated to incorporate the new developments in the field. The key features of the treatment recommendations from major guidelines available worldwide are found in Table 5 .
Summary of latest international guidelines for management of chronic hepatitis B a
EASL 2017 clinical practice guidelines.
One of the most frequently updated guidelines is from the European Association for the Study of the Liver (EASL), which appeared online in April 2017. The key features in the new EASL guidelines included updated definitions of the different CHB phases using the status of HBeAg, as well as the recommendation for the use of tenofovir alafenamide (TAF) as a first-line antiviral agent and discussion of the need for antiviral prophylaxis for patients with hepatitis B and C virus coinfection treated with direct-acting antivirals (DAAs) ( 17 ).
AASLD 2018 guidelines and ATA 2015.
There are two major practice guidelines or treatment algorithms in the United States ( 30 , 123 ). The American Association for the Study of Liver Diseases (AASLD) last updated is practice guidelines in 2018 ( 84 ). One major update was the increase in the ALT threshold for treatment (2× the upper limit of normal [ULN]), with the upper limit of normal being changed to 35 U/liter for males and 25 U/liter for females.
In addition, a very practical algorithm, comprehensive review, and recommendations on screening for HBV, pretreatment evaluation, treatment, and vaccination were published by a group of experts and are collectively known as the American treatment algorithm (ATA). The last update was in 2015 ( 123 ), and the ALT threshold treatment recommendation was 1× the upper limits of normal (30 U/liter males and 25 U/liter females).
APASL clinical practice guidelines 2015.
The practice guidelines developed by the Asian Pacific Association for the Study of the Liver (APASL) have had a major influence on the management of CHB in the Asia-Pacific region, which represents the area with the largest HBV disease burden ( 31 ). As NAs with low genetic barriers are still commonly used in some Asia-Pacific countries, a detailed drug resistance monitoring and management recommendation is presented along with a very detailed section on screening for hepatocellular carcinoma (HCC) and HCC risk factors.
The World Health Organization (WHO) issued its first guidelines for the management of CHB in March 2015. With a worldwide perspective and coverage (including Africa), the 2015 guidelines provided practical suggestions, with special attention being given to settings with limited resources. As an example, APRI was suggested to be the noninvasive test that should be used for assessment of the presence of cirrhosis, using a cutoff value of 2 for adults in monetarily limited areas. More advanced noninvasive tests, such as transient elastography or the FibroTest, were recommended for use in wealthier settings. An age greater than 30 years was also set as a condition to consider antiviral treatment, in particular, for patients with persistently abnormal ALT levels. Detailed recommendations about who should receive treatment, as well as when to end treatment and how to monitor and prevent hepatitis B virus infection, are described below. However, a recent publication from Ethiopia suggests a low performance of the WHO criteria in the correct selection of CHB patients who could benefit from therapy ( 124 , 125 ).
Current Treatment Regimens and Treatment Durations
Current therapies for the management of CHB include peginterferon (PEG-IFN) and orally administered nucleos(t)ide analogs (NAs). NAs are the most widely chosen option globally.
For NAs, all regional associations agree that first-line therapy should be with an oral antiviral with a strong barrier to resistance: either entecavir, TDF, or TAF ( 17 , 30 , 31 ). Short-term treatment with NAs is feasible for those HBeAg-positive patients who experience seroconversion to anti-HBe during treatment. After HBeAg seroconversion occurs, treatment should continue for at least 1 year and, it is hoped, an additional 3 years in order to achieve a long-lasting response once therapy is discontinued. Therapy continuation for at least 3 years lowers relapse rates to less than 30% and hastens the subsequent loss of HBsAg. Higher relapse rates following therapy discontinuation occur among older patients and those with HBV genotype C infection.
The recommended therapy cutoff criteria for NAs in HBeAg-negative patients vary by region. Given the very high rate of relapse following treatment withdrawal, AASLD recommends that NAs be withdrawn from HBeAg-negative patients only after confirmation of the loss of HBsAg, with or without seroconversion. Recent reports from Asian and European countries suggest that NAs may be stopped in HBeAg-negative patients who have undetectable HBV DNA at three different times when the testing times are 6 months apart, although the duration of on-therapy HBV DNA undetectability remains important ( 31 , 126 ). The disease remains inactive, defined as an HBV DNA level of <2,000 IU/ml and a normal ALT level, in approximately half of the patients at 3 years after therapy withdrawal. Treatment discontinuation is not recommended for patients with cirrhosis, due to the risk of life-threatening hepatitis flares following virological relapse ( 127 ). In the near future, newer markers, such as HBV RNA and HBcrAg, may guide treatment decisions.
In contrast to NA therapy, the use of peginterferon as primary therapy in patients with CHB has been limited by its poor efficacy and tolerability. However, unlike NA therapy, PEG-IFN has a finite duration. For HBeAg-positive and HBeAg-negative patients who have a good chance of HBeAg seroconversion, 48 weeks of PEG-IFN is recommended. In phase 3 clinical studies, only one third of HBeAg-positive patients and <10% of HBeAg-negative patients achieved a sustained response after 48 weeks of treatment with peginterferon. The efficacy seemed to be improved in those patients who had already developed an ostensible immune response against HBV, such as HBeAg-positive patients with an HBV DNA level of <9 log IU/ml and an ALT level >5× the ULN. Monitoring of an on-treatment HBsAg decline (at 12 or 24 weeks) identifies nonresponders, allowing the early cessation of treatment, which improves both the cost-effectiveness and the tolerability of this treatment ( 128 , 129 ). Effectiveness can also be found for HBeAg-negative patients, as it may be the only option that may produce an opportunity for a sustained response off treatment after a limited course of therapy.
Combinations of peginterferon with NAs or add-on or sequential therapy with NAs followed by peginterferon treatment have been demonstrated to be safe and to have improved seroconversion rates compared to those of single therapies ( 130 , 131 ). Larger studies of these options need to be performed before they can be recommended. It should be noted that peginterferon should not be used in patients with decompensation, but it can be used with caution in patients with compensated cirrhosis. In contrast, oral NA therapy improves and restores function even in patients with severe decompensated liver disease ( 132 ).
Disadvantages of Oral Antiviral Therapy and Future HBV Cure Therapies
Viral suppression difficulties..
Although maintained viral suppression improves the outcomes in most patients with CHB, long-term NA therapy has a number of disadvantages. First, a mere one-fifth of patients with CHB meet the current criteria for beginning treatment with antivirals. Second, the rate of seroclearance (functional cure) with oral antiviral therapies does not appear to be significantly higher than the spontaneous rate of about 1.0%; therefore, treatment is often lifelong ( 133 ). Also, with long-term therapy, the HBV transmission risk may decrease, but the risk is not completely eliminated. A recent in vitro study using new highly sensitive molecular assays for HBV DNA demonstrated a residual ability of HBV DNA to transmit HBV infection in an experimental model ( 134 ).
Development of liver cancer.
In addition, long-term therapy may reduce but not eliminate the risk of liver cancer ( 135 ). NAs prevent the formation of a covalent bond with the adjoining nucleotide by competing at the HBV catalytic site during the formation of nascent HBV, causing chain termination of the elongating DNA, but they do not act on cccDNA ( 136 ). The stable conservation and functioning of the cccDNA in the nuclei of HBV-infected cells in nucleoside analogue-treated patients provide a continual source of viral RNA transcripts but generally result in only minimal rates of HBsAg loss and prohibit cure in most patients. Integrated viral genomes are not directly affected by NA therapy ( 136 ).
Oral antiviral toxicity.
Although NAs have been proven to be safe and well tolerated, cumulative toxicity may develop in some patients after long-term use. Elderly patients and those with HIV infection have developed bone disease and renal tube injury with the use of tenofovir. Preclinical studies have shown carcinogenesis (cytotoxicity in human lymphocytes and tumors in rats) with the use of entecavir, and consequently, it is not indicated for use in women of childbearing age or in children. Finally, the risk of liver cancer may increase following sequential lamivudine-adefovir dipivoxil therapy, due to A181T variant selection and the development of the cytoplasmic accumulation of S proteins which have been truncated, causing activation of the c-Raf-1/mitogen-activated protein kinase pathway ( 137 ).
Future Directions for Therapy
Therefore, new therapies for HBV that can achieve sustained suppression and HBsAg loss after a limited course of therapy are of high interest. Such therapies will permit the discontinuation of NAs after short periods of therapy and provide a so-called HBV cure. There are three defined endpoints: (i) partial cure, which is HBV DNA suppression and normal ALT levels after the end of treatment without a loss of HBsAg; (ii) functional cure, which is HBV DNA suppression and ALT level normalization after the end of treatment with a loss of HBsAg but without cccDNA elimination (which does not retain the risk of late HBV reactivation during immunosuppression); and (iii) complete cure, which is permanent HBV DNA suppression and normal ALT levels after treatment with the loss of HBsAg and the elimination of cccDNA. None of these strategies will target integrated HBV DNA and, hence, will not prevent HCC unless they are administered early in the HBV infection process, before integration has occurred. Thus, HBV cure will require a combination of novel antivirals that will reduce the levels of HBV DNA (or the level of protein production) and immunomodulators which boost the exhausted natural immune responses found in chronic hepatitis B ( 137 , 138 ).
Therapeutic HBV vaccines.
The individual patient’s own immune response to HBV infection is important and foreshadows the clinical outcome following an acute HBV infection. The spontaneous clearance of HBV infection requires a vigorous, polyclonal antigen-specific, adaptive immune response against HBV proteins. A broad CD8 + T-cell response is also required for spontaneous clearance ( 138 ). Activation of antiviral immunity against HBV includes (i) the generation of new T cells via therapeutic vaccines; (ii) stimulation of antiviral effector cells, such as T cells, B cells, and dendritic cells; and (iii) reduction of the T-cell exhaustion that accompanies chronic HBV infection. To date, the therapeutic vaccines have been disappointing. Although immunogenic vaccines and T-cell peptide vaccines generate sustainable and protective HBV-specific B- and T-cell responses in HBV-naive patients and reduce hepatitis B virus replication in animals with chronic hepadnaviral infection, they have absolutely no efficacy in patients with chronic hepatitis B when they are used either alone or with oral antiviral therapy ( 139 , 140 ). A promising vaccine developed using the targeted molecular immunogen platform (the GS-4774 vaccine) uses recombinant Saccharomyces cerevisiae yeast to express surface, core, and X proteins. Weekly or monthly GS-4774 vaccines given to health volunteers produced HBV-specific T-cell-mediated responses in almost all the volunteers (90%), with two subjects developing low-level anti-HBs ( 141 ). GS-4774 was well tolerated in virally suppressed patients, but it did not produce significant reductions in HBsAg levels, even though almost half (40%) had an increase in HBV-specific T cells affecting HBV core and HBx proteins ( 142 ).
Pharmacological stimulation of the innate immune response.
There is also evidence that HBV proteins are recognized by pathogen recognition receptors, including Toll-like receptor 2 (TLR2), TLR4, and RIG-I ( 143 , – 145 ). The virus may have evolved mechanisms to inhibit pathogen recognition receptor pathway signaling. Pharmacological stimulation of the innate immune response with Toll-like receptor 7, 8, or 9 is being studied. Toll-like receptor 7 affects multiple arms of the immune system with its pattern recognition receptor, including both innate and adaptive effector cells and antiviral cytokine responses. Additionally, TLR7 is expressed by B lymphocytes, and upon activation, TLR7 results in polyclonal expansion and differentiation toward immunoglobulin-producing plasma cells, providing a humoral component to the adaptive immune response. Agonist-induced activation of TLR7 may provide a new treatment avenue for CHB patients through its effects on the innate immune effectors and HBV-specific T- and B-cell responses ( 143 , – 145 ).
Oral vesatolimod (GS-9620) is an oral TLR7 agonist being developed at the current time. The administration of vesatolimod to mammals for 1 to 2 months has led to HBV DNA suppression, the loss of HBsAg, and a reduced rate of development of HCC ( 143 , – 145 ). In two randomized controlled studies comparing virally suppressed and treatment-naive CHB patients, weekly vesatolimod was found to be safe, well tolerated, and associated with the production of peripheral interferon-stimulated gene 15 (ISG15) without significant systemic interferon alpha (IFN-α) levels or related symptoms but had no effect on HBsAg levels ( 146 , 147 ). This discrepancy between chimp and human responses may reflect the lower dosing used in humans because of dose-related toxicity. Several other potent TLR7 agonists are now entering clinical trials ( 148 ).
In contrast to TLR7, myeloid cells (myeloid dendritic cells, monocytes, and Kupffer cells) are where the expression of TLR8 receptors is found. Stimulation by TLR8 agonists should trigger the maturation of professional antigen cells, noted to be in gut lymphoid tissue or the liver. A variety of cytokines which can stimulate or rescue antigen-specific T-cell responses to improve anti-HBV activity can result from such stimulation. TLR8 agonism should produce a more effective antiviral immune response and lead to the functional cure of CHB. In animal models, TLR8 agonism can induce an innate immune response in circulating blood cells without inducing adverse systemic IFN-α. The first TLR8 agonist, GS-9688, has recently entered phase 1 clinical development ( 148 ).
Another innate immunity-related target is STING (stimulator of the IFN gene). STING is a pathogen recognition receptor that activates downstream signaling and the expression of interferons and is also the adapter protein of multiple cytoplasmic DNA receptors. Pharmacological activation of the innate immune response may use STING as a potential target ( 149 ). STING agonists are in preclinical development ( 150 , – 154 ).
The HBV polymerase may counteract IFN-β production in humans ( 155 ). RIG-I (retinoic acid-inducible protein) is a cytosolic sensor of RNA that can promote inflammatory signals through activation of interferon regulatory factor 3 (IRF-3) and NF-κB. Importantly, RIG-I has been proven to recognize and bind the epsilon stem-loop of HBV pgRNA and interfere with HBV replication, as well as induce IFN and cytokine production. Study data suggest that the mechanisms of action for RIG-I are 2-fold, whereby RIG-I interferes with the HBV polymerase in human liver cells and RIG-I performs as an HBV sensor that begins an innate signaling process ( 156 ). The experimental drug SB 9200, an oral prodrug relative of the dinucleotide SB 9000, then activates RIG-1 and nucleotide-binding oligomerization for protein 2, resulting in an IFN-mediated antiviral immune response in infected cells ( 157 ).
It is likely that to enhance the weakened innate and immune responses specific to HBV in patients with lifelong CHB, new immunomodulators need to be used along with other modalities to ameliorate T-cell exhaustion. The small interfering RNA (siRNA)/locked nucleic acid (LNA) approach (see below) could help reconstitute immune responses specific to HBV through the rapid and effective knockdown of hepatitis B proteins. However, due to the overexpression of T-cell receptors in patients with chronic hepatitis B, T-cell effector function is limited ( 158 ).
PD-1 and PD-1 inhibitors.
Among the T-cell receptors, PD-1 is expressed the most on HBV-specific T cells within the liver, while at the same time, PD-L1 expression is increased in hepatocytes ( 159 ). For this reason, PD-1 and PD-1 inhibitors are currently being studied as possible treatments for chronic hepatitis B. Experiments on woodchucks with chronic woodchuck hepatitis virus infection have shown that a blockade of PD-L1, in addition to DNA vaccination, proved to effectively control viremia ( 160 ). In the first clinical trial of anti-PD-1 in patients with CHB, a low dose (a single dose of 0.3 mg/kg of body weight; in comparison, monthly doses of 3 mg/kg are used in patients with melanoma) induced significant reductions of HBsAg, and a single patient achieved complete HBsAg seroconversion ( 161 ). Further studies will need to be cautious in determining the dosage to avoid stimulating autoimmune conditions, such as pneumonitis, colitis, and hepatitis, and immune-mediated HBV flares.
HBV life cycle.
In addition, the HBV life cycle provides many different access points for antiviral therapies, and clinical development is focused on small molecules that block HBV entry (myrcludex) ( 113 ), HBV protein synthesis (siRNAs ARC520 and ARC521, ALN-HBV, ARB-001467, and LNAs), core synthesis (NVR3-778, GLP-2, BAY41-410, AB-423, ABI-H0731), and finally, the release and formation of virions (REP-2139) ( 162 ). Researchers have discovered an allosteric agent, a synthesized derivative of the large HBsAg protein myrcludex B, that retards NTCP. The peptide irreversibly blocks the receptor to block the transport of bile salts and the introduction of HBV in cellular experiments. This protein is currently being studied in chronic hepatitis B and hepatitis D infections ( 163 ).
cccDNA target.
The hepatitis B core protein performs multiple crucial roles in virus replication, cccDNA maintenance, and downward progression of the host’s inborn immune response. As the core is preserved in all HBV genotypes, it is thus a promising target for inhibitors consisting of small molecules that attach to the core protein, causing allosteric modulation that prevents dimerization and assembly of the nucleocapsid ( 164 ). The use of small molecules that target capsid assembly represents a promising antiviral strategy. These molecules include heteroaryldihydropyrimidines, phenylpropenamides, pyridazinone derivatives, and sulfamoyl benzamides. As capsid formation is an indispensable step in the generation of HBV virions but also in the augmentation and persistence of cccDNA in the nucleus, targeting capsid assembly and disassembly is an attractive approach ( 165 ). Thus, inhibitors of either encapsidation or compounds that result in capsid disassembly hinder the entry of rcDNA into the nucleus, which impedes the transformation of rcDNA to cccDNA. The capsid assembly modulators (CpAMs) could have multiple effects on the HBV life cycle. Interruption of the assembly of HBcAg dimers into capsids will directly inhibit HBV replication. CpAMs could also prevent cccDNA formation (through the interruption of capsid disassembly) and cccDNA replenishment within the nucleus ( 165 ).
Finally, CpAMs could also restart the host innate immune response through the induction of interferon-stimulated gene (ISG) expression ( 166 , 167 ). In preclinical studies, CpAMs were found to diminish HBsAg and HBV DNA levels. The initial CpAM study demonstrated that over 28 days of NVR3-778 dosing was associated with 1- to 2-log reductions in HBV DNA levels, although there was no change in HBsAg levels ( 166 , 167 ). Studies of CpAMs in combination with pegylated interferon or entecavir are in progress. Several CpAMs in current preclinical studies show a significantly higher in vitro potency than NVR3-778 (GS4JHS, AL-034, ARB-423, AT-130, HAP12, HAP_R01, SBA_R01, JNJ-379, ABI-H0731, ABI-H2158, ABI-Nx). However, polymorphisms in the pocket region of the capsid significantly reduce susceptibility to CpAMs. Known signature mutations include T128I and T33N, which confer 20-fold and 80-fold reductions in susceptibility, respectively. CpAM resistance is likely to be against all agents in that class. Although the prevalence of these polymorphisms is low (0.03% for both mutations in untreated patients), they are likely to be rapidly selected by CpAM monotherapy. The clinical significance will be determined by their fitness. Strains with these polymorphisms are fully susceptible to oral antiviral therapy, so their selection would be prevented by combining CpAMs with oral antiviral therapy.
siRNA therapy.
siRNA therapy takes advantage of a natural process in which the body quiets unneeded genes to prevent the translation of unneeded proteins or enzymes. Researchers can use an intracellular RNA-induced silencing complex (RISC) to quiet any unneeded host, bacterial, or viral gene using synthetic siRNAs that complement the invasive viral mRNAs ( 168 , 169 ). The greatest problem barring therapeutic application of this methodology has been the type of administration and unneeded effects on other organs, such as the kidney. siRNA is digested rapidly in the gut, so it is necessary to administer the therapy parenterally. Infusion reactions can occur with intravenous doses, so dosing often requires medication with corticosteroids or antihistamines in advance of treatment. Subcutaneous injections that target the liver can now be administered. These injections have fewer side effects and require less frequent dosing, which improve patient tolerance. The host immune system is not activated by the metabolically stable siRNAs, so the reduction of off-target effects has been achieved through several techniques, including pairing of siRNA with N -acetylgalactosamine (GalNAc), which enhances liver cell uptake via the asialoglycoprotein receptor (ASGPR) ( 168 , 169 ).
As HBV is a compact genome with regional overlaps, a single siRNA can quiet the transcription of many genes, blocking the production of the core, surface, polymerase, and X proteins. By directly blocking HBV replication and indirectly facilitating HBV immunity, the expression of HBsAg and HBeAg (tolerogenic antigens) is reduced. Arrowhead’s siRNA ARC520 was the initial siRNA to be developed clinically ( 168 , 169 ). Different doses of ARC520 lowered HBsAg, HBeAg, HBcAg, and HBV DNA levels in both primates and CHB patients. However, the lowering of HBsAg occurred more often in HBeAg-negative chimpanzees and patients than in HBeAg-positive chimpanzees and patients. This happened because ARC520 targets cccDNA-derived pgRNA, the major source for HBsAg in young HBeAg-positive patients, and not integrated HBs (the major source of HBsAg in older HBeAg-negative patients). ARC250 had no effect on HBs transcriptions in HBV genomes, because the target sequence of ARC520 was upstream from the core promoter site and next to the site of insertion for integrated HBs sequences. To correct this problem, Arrowhead produced a second-generation siRNA that targets this region, which covers transcripts from both integrated and cccDNA-derived HBs. This effectively knocks down HBsAg in both HBeAg-positive and HBsAg-negative patients with chronic hepatitis B ( 35 ). Currently, multiple developers, such as Arrowhead and Alnylam, have active siRNA development programs.
LNA technology.
An alternative approach also being actively pursued to silence HBV transcripts and prevent HBV protein synthesis is the locked nucleic acid (LNA) technology, which uses liver-targeted single-strand oligodeoxyribonucleotides complementary to mRNAs derived from cccDNA. These more recent delivery systems for hepatic targeting should eliminate the problem of off-target toxicities due to antisense oligonucleotides ( 170 ).
CRISPR/Cas9.
The goal of siRNAs and core inhibitors is a functional cure, which is defined as the clearance of HBsAg with sustained suppression when the patient has discontinued treatment. However, if a patient is immunocompromised, these therapies do not address the lifelong risk of HBV reactivation in this circumstance. The deactivation or elimination of cccDNA is the only way to decrease this risk ( 171 ). Therapy could be produced via epigenetic modification of cccDNA using demethylation or deacetylase activation, but this approach is offset by the risk of off-target effects on host genes. Specific cccDNA cleavage via various nucleases and CRISPR/Cas9 would seem to be more attainable.
CRISPR/Cas9 occurs within bacteria, in which the Cas9 nuclease combines with a specific guide RNA (gRNA) that complements plasmid and phage DNA, causing destruction of the genetic material and ending in the acquisition of immunity to the bacteria. In many human diseases of heredity, gene editing via CRISPR/Cas9 has been studied. Most recently, through gene editing of the bone marrow, sickle cell disease was corrected ( 171 ). In vitro CRISPR/Cas9 experiments show deep and rapid reductions in the levels of cccDNA and HBV proteins encoded by genes carried by hepatitis B virus cccDNA ( 172 ). Combining CRISPR/Cas9 with either gene silencing (siRNA) or another gene editing tool (APOBEC3B) further enhances the disappearance of cccDNA ( 173 , 174 ).
However, several challenges need to be overcome before this technology can be safely applied to patients with CHB. A lack of cross-reactivity with human genetic material and the ability to specifically deliver the gene editing tool to the hepatocyte nucleus are required to ensure minimum off-target toxicities to host DNA. The lack of any standardized test for cccDNA limits the measurement of endpoints (changes in the levels of cccDNA transcripts and their proteins) ( 174 ). If researchers overcome these barriers, then such gene editing technology has the potential to eradicate cccDNA and achieve a complete HBV cure, thereby removing any long-term risk of reactivation. This will necessitate an efficient delivery system with a 100% hit rate. Even one residual cccDNA minichromosome could result in persistent infection. Finally, cccDNA elimination will not completely abrogate the lifelong risk of HCC attributed to integration. Although CRISPR could also be designed to specifically excise integrated HBV sequences from human DNA, any subsequent chromosomal translocation would have disastrous consequences ( 174 ).
Summary of new therapeutic targets.
The goal of HBV therapy will always remain complete cure. Functional cure may be attained within the next 5 to 10 years through the use of newly developed antivirals and immunomodulatory agents that will reduce the HBV viral load and build the host’s own immune response against disease. Complete cure will require gene editing approaches which are some years away from human trials. However, safe, well-tolerated, and cost-effective therapies must be the goal of the new therapies so that HBV elimination can be obtained in low-income countries that have the greatest HBV burden. To assist in meeting these goals, an international working group of professionals has come together to help coordinate, promote, and establish public-private partnerships to expedite the cure of CHB. This group, the International Coalition to Eliminate Hepatitis B (ICE-HBV), thus far has developed a global scientific strategy to cure HBV and is actively collaborating on a number of research protocols to cure HBV ( https://ice-hbv.org/about/about-ice-hbv/ ).
COINFECTION: EPIDEMIOLOGY, DIAGNOSIS, AND MANAGEMENT
Hepatitis d and b virus coinfection, introduction to natural history and epidemiology..
Hepatitis D virus (HDV), a unique liver-tropic human virusoid, is the causative agent of chronic hepatitis D but one that can survive only in persons infected with HBV ( 175 ). HDV is transmitted either at the same time as HBV or as a superinfection by an established HBV carrier as a result of it being a defective RNA virus.
The HDV virion contains the hepatitis delta antigen (HDAg) and HDV RNA. The virion contains a circular single-strand negative-sense genome. The viroid-like self-complementary genome encodes two varieties of a single protein (large and small), hepatitis D, or delta, antigen. HDV needs a concomitant HBV infection to organize and grow within the host, as the HDV complex requires an envelope made of the three HBV (small, middle, and large HBsAg) proteins during HDV assembly to assemble into an infectious HDV virion and to enter hepatocytes. HBsAg may also be provided by the HBsAg protein encoded by defective integrants of HBV ( 175 ).
HDV virions attach to cellular heparin sulfate proteoglycans, in which the penetration of liver cells is regulated by the irreversible attachment of the large HBsAg to the N-terminal pre-S1 region of the hepatocyte-specific human bile salt transporter sodium taurocholate cotransporting polypeptide (NTCP), mediating the liver tropism of both HBV and HDV. Subsequently, HDV RNA moves to the nucleus, where replication occurs by host DNA-dependent RNA polymerases. Replication via a rolling-circle mechanism generates multimeric linear transcripts of the genome and antigenome. These are subsequently cleaved and circularized into the infectious form by the HDV ribozyme.
The antigenomic RNA is edited by adenosine deaminase to catalyze a change in the small HDV RNA stop codon, thus leading to a 3′ elongation of the open reading frame. The subsequent mRNA produces large HDAg. The extension encompasses a binding motif for HBsAg through a prenylated location at Cys211 that is acted on by cellular farnesyltransferase. The identification of the NTCP receptor has enabled the adaptation of hepatically derived HCC cells that express the receptor, such as HepG2-NTCP and HuH7-NTCP cells, rendering these lines susceptible to both HBV and HDV ( 175 ).
Of the 240 million people with HBV infection, HDV is thought to affect about 15 million to 20 million. There are regional differences in the epidemiology of HDV. High prevalences have been reported in the Amazon region, central and eastern Africa, Turkey, Mongolia, Iran, and Pakistan. A prevalence of 25% has been reported in Romania. Several genotypes have been described: genotype 1 is prevalent worldwide; in Japan and Taiwan, genotype 2 is the most prevalent; the Amazon Basin harbors genotype 3; genotype 4 is found in Taiwan and Japan; and genotypes 5 to 8 are found in Africa. The disease has declined in Italy after universal HBV vaccination. The current residual reservoir of HDV in European comprises two principal cohorts: aging patients with advanced liver disease and young immigrants with active chronic hepatitis D from areas where HDV infection remains endemic. The increased HDV prevalence in the United Kingdom, France, and Germany reflects the prevalence in young individuals who have migrated from regions of high prevalence. Rates of 1 to 9% have been reported from China, and rates of 3 to 8% have been reported in the United States. The disease is predominantly acquired by horizontal rather than vertical transmission. The transmission of HDV occurs through the parental route through tainted blood and/or body fluids. There is some evidence of sexual transmission. Intrafamilial spread can occur, but perinatal transmission is rare.
Three to 4% of those coinfected contract fulminant hepatitis. The course of HDV infection is affected by the host immune response to HBV, but HDV with HBV causes a high rate of chronicity and the most serious and advancing form of chronic hepatitis, including a high rate of cirrhosis.
Diagnosis and testing.
The diagnosis of HDV infection requires testing of HBsAg-positive individuals for anti-HDV antibodies, and if these are found, the individuals should be tested for HDV RNA to confirm HDV viremia. Testing for HDV antigen in serum has poor sensitivity, and liver tissue staining is reserved for use for testing for HDV antigen ( 176 ). All HBsAg-positive individuals should be tested. Testing rates remain low in many countries, including in immigrants and intravenous drug users. Tests for HDV antigen and RNA require standardization.
The treatment of chronic HDV infection remains difficult. HDV may suppress HBV replication but cause progressive disease with a course more severe than that of HBV monoinfection ( 177 ). A new endpoint for the treatment of chronic HDV infection has recently been proposed: normal ALT levels and a decline of HDV RNA levels of at least 2 logs at the conclusion of treatment. The sole proven treatment is PEG-IFN ( 177 , 178 ). PEG-IFN administration is usually weekly for 12 to 18 months, even though HDV RNA may be undetectable in serum after 6 months of therapy in nearly 25% of patients ( 179 ). HDV is likely to relapse in patients who remain HBsAg positive. Unfortunately, treatment seldom achieves the endpoint of HBsAg and HDV RNA clearance. However, HDV RNA loss during the follow-up period, along with fewer liver-related complications and disease progression, is more frequent in those treated with IFN. If achieved, the lasting undetectability of HDV RNA is a solid stopping point for treatment with IFN ( 180 ).
A nucleoside analogue, in particular, tenofovir or entecavir, can be administered to patients with advancing disease and detectable HBV DNA. However, chain terminators do not directly inhibit HDV RNA replication. There are unconfirmed reports of declines in HDV RNA levels in HIV-positive patients treated with TDF ( 181 ). The combination of peginterferon and nucleoside analogues has been assessed. Peginterferon and adefovir for 48 weeks versus peginterferon or adefovir monotherapy was evaluated in the HIDIT-1 trial. HDV RNA became negative in 51%, 26%, and 0% of patients, respectively ( 182 ). A greater decline in the HBsAg concentration was observed in the combination arm. However, 56% of the patients relapsed after longer-term follow-up ( 183 ). Treatment with tenofovir and PEG-IFN together for 96 weeks offered no incremental benefit in the HIDIT-2 study ( 184 ).
There are a number of novel therapies for HDV infection ( 185 ). siRNA-mediated silencing of HDV RNA intermediates, in particular, mRNA of HDAg, is a theoretical possibility. Novel therapeutic agents are currently in clinical trials. Lonafarnib is a tricyclic derivative of carboxamide that prevents the prenylation of HDAg by reining in farnesyltransferase, which theoretically prevents the assembly and release of the ribonucleoprotein complex (prenylation is a posttranslational modification whereby prenyl lipids are added to proteins to render them lipophilic). The drug is administered orally ( 186 ). In an early-phase study in 12 patients, HDV RNA levels declined after administration of lonafarnib at 200 to 400 mg daily for 28 days. HBsAg concentrations did not change. Lonafarnib influences cell signaling pathways, and its efficacy may be limited by dose and tolerability. Dose-dependent reductions in HDV RNA concentrations were reported, but weight loss and gastrointestinal symptoms, including vomiting, occurred. The results may be improved by ritonavir boosting to effect higher concentrations of the drug in the liver ( 187 ). Studies with 25 mg, 50 mg, and 75 mg in combination with ritonavir and PEG-IFN are ongoing.
Myrcludex B (a 47-mer myristoylated pre-S1 lipopeptide) is a synthesized, acylated pre-S peptide derivative of the large HBsAg protein and is a subcutaneously administered allosteric inhibitor of NTCP. In experimental cells, the peptide irreversibly blocks the receptor and blocks the transport of bile salts and the entry of HBV. Currently, the use of myrcludex B in patients with chronic HDV and HBV infection is being explored ( 163 ). Myrcludex B in combination with nucleosides and interferon is being tested in phase 1 and 2 studies. In an open-label phase 2b multicenter clinical trial, interim results assessing the safety and efficacy of myrcludex B (bulevirtide) in combination with tenofovir as therapy for chronic HBV and HDV infection have recently been reported. Myrcludex led to reductions in HDV RNA levels and improvements to ALT levels, but after 24 weeks, despite a dose-related suppression of HDV RNA, no change in HBsAg levels was noted ( 188 ). In April 2019, the final results of a multicenter open-label phase 2 study of myrcludex with PEG-IFN-α2a were reported. Myrcludex monotherapy (2 mg once daily) led to a continuous HDV RNA decline and ALT reduction over 48 weeks. An HDV RNA response (to undetectable levels or a ≥2-log decline) was observed in 26.7% of patients at week 72. Myrcludex B and PEG-IFN-α in combination therapy resulted in HDV RNA becoming undetectable in 12/30 patients (40%) at week 72. An unexpected result was that for the 15 patients who received the drug combination of 2 mg myrcludex B plus PEG-IFN-α, HBsAg became undetectable in 4/15 patients (27%), and 3 of these 4 patients experienced HBsAg seroconversion. A long duration of therapy will be needed to clear HDV RNA, and thus, long-term studies are being planned ( 189 ). The compound inhibits the bile acid transporter function of NTCP to increase conjugated bile acid levels in treated subjects. These drugs have received fast track and prime eligibility status by the U.S. FDA after the need to improve the outlook for HDV infection was recognized.
Phosphorothioated oligonucleotides, such as nucleic acid polymers (NAPS), may prevent viral entry with heparin sulfate proteoglycans ( 190 ). In the duck model, the compound REP2055 apparently inhibited HBsAg secretion by inhibiting HBsAg assembly and egress ( 191 ). In a phase 2 study, the compound NAP 2139-Ca was studied in patients with HDV infection. Twelve patients were administered REP2139 at 500 mg intravenously once a week for 15 weeks, then received 250 mg intravenous REP2139 combined with PEG-IFN alfa-2a once a week for another 15 weeks, and then underwent monotherapy with PEG-IFN weekly for 8 months. By the conclusion of therapy, six patients had HBsAg levels of less than 50 IU/ml, which were maintained in five patients. Eleven became HDV RNA negative on treatment; seven remained HDV RNA negative after the first year of follow-up. Normalization of serum aminotransferase levels occurred in nine patients ( 192 ). Reports of longer follow-up add to the data, but further evidence from controlled trials is required.
Randomized, open-label studies with IFN-λ at 120 or 180 μg, administered by subcutaneous injections every week for 48 weeks, are being conducted in Pakistan, Israel, and New Zealand. Thirty-three patients have been randomized to receive IFN-λ at 180 μg ( n = 14) or 120 μg ( n = 19). IFN-λ at 180 μg showed antiviral activity comparable to that of IFN-α, suppressing HDV RNA by 2.3 log 10 units with better tolerability than historical data for IFN-α ( 193 ). An increase in bilirubin levels has been noted with IFN-λ. A study combining IFN-λ with lonafarnib is ongoing ( 194 , 195 ).
Thus, partial efficacy has been reported for these new strategies, which may complement IFN therapy. Generally, the higher response rates of treatment for chronic HDV can be found in younger and noncirrhotic patients, though there is a risk of relapse. The clearance of HDV RNA may not be sufficient, in the absence of the clearance of HBsAg, as the former does not guarantee that HDV has been eliminated and does not portend a cure. Integration of HBV may provide a significant proportion of the HBsAg that allows HDV to persist. Other strategies may be needed, including quieting of RNAs or other immunomodulatory therapies. The only treatment for those proceeding to end-stage liver disease, HCC, or fulminant hepatitis caused by coinfection or superinfection with HDV and HBV is liver transplantation.
Hepatitis B Virus and HIV Coinfection
Epidemiology and natural history..
Approximately 8% of HIV-infected persons are coinfected with HBV. In regions where HBV is highly endemic, such as sub-Saharan Africa, HBV infections occur during childhood, but HIV infection is usually a sexually transmitted disease acquired in adulthood ( 196 ). It is believed that in sub-Saharan Africa, there are 2.6 million people living with HIV and HBV coinfections. Both HBV and HIV can be passed from mother to baby, but infection may also occur via unsafe injections or inadequate blood safety programs.
HIV coinfection may exacerbate liver disease ( 197 , – 200 ). Liver-related deaths occur 3.73 times as frequently among those with hepatitis B as among those without; some subjects in some regions may have chronic hepatitis D. The added morbidity of HBV-HIV coinfection may be the result of increased replication, HBV reactivation, a greater likelihood of the chronicity of HBV, and accelerated progression to fibrosis or cirrhosis, which carries the risk of hepatocellular carcinoma at an age younger than that at which it would have occurred without HBV and HIV infections ( 201 ). There may also be a risk of hepatotoxicity from antiretroviral therapy (ART), particularly in resource-poor regions. HIV-induced immune deficiency may be aggravated in those with active HBV replication ( 202 , 203 ).
The diagnosis of coinfection requires assessment of risk factors and repeated testing. HIV-infected patients must undergo a thorough assessment and staging for any hepatic condition. The scores obtained by noninvasive tests, including the fibrosis 4 index (FIB-4) and the scores obtained by transient elastography, are useful in this group. All persons with HIV or HBV infection must be assessed for coinfection ( 204 ). Innovations to expand testing are required. These include innovations suggested by the WHO, which include strategies to improve the already existing infrastructure to identify high-risk patients while also providing to limited-resource areas and hard-to-reach populations near-patient or point-of-care assays for virological markers (nucleic acid testing). These include different serological and nucleic acid assays with dried blood spot specimens, multiplex and multidisease platforms to enable testing for multiple analytes/pathogens, as well as potential self-testing for viral hepatitis ( 205 , 206 ).
The first-line therapies used for both HBV and HIV are tenofovir and emtricitabine. These drugs, together with appropriate ART, are usually recommended for the treatment of HIV-HBV coinfection. Renal insufficiency may require a modification of treatment with tenofovir. Entecavir can be added to an appropriately potent suppressive ART regimen. Drug resistance testing is required where lamivudine has been utilized ( 207 ). Tenofovir alafenamide is an ART that offers effective activity with less renal or bone toxicity than other therapies ( 208 ). Its safety in pregnancy has not yet been established ( 209 ). The effective suppression of both viruses can limit the progression of hepatic fibrosis ( 210 , 211 ). Liver transplantation affords the opportunity of efficacious salvage treatment for those with HBV and HIV coinfection that have progressed to end-stage hepatic disease. The most effective tool in the prevention of HBV infection is vaccination; however, the responsiveness to vaccination is lower in HIV-infected children ( 212 ).
Several challenges to the elimination of HBV-HIV coinfection remain, particularly regarding diagnosis and access to treatment of HBV infection in regions where HIV treatment is selectively supported. In many regions, pregnant women are routinely screened for HIV but not HBV ( 213 ). Systematic surveillance is required in regions of endemicity ( 214 ). Low-cost generic medications have been crucial in expanding programs for the treatment of HIV infection, but their use requires extension to the treatment of HBV infection.
The commonalities between HIV and HBV infection and coinfection lend these diseases to a common strategic policy for elimination ( 215 , 216 ). Public awareness to increase the public’s understanding of HBV infection as well as HIV infection is growing but is still behind the curve necessary for elimination ( 217 ).
Hepatitis B and C Virus Coinfection
Epidemiology..
Because of potentially shared modes of transmission, coinfection with HBV and HCV is common in areas of high endemicity and for individuals with high-risk behaviors and exposure to parenteral methods of transmission. HBV-HCV coinfection is not carefully documented throughout the world and may be understated if occult HBV infection is included ( 218 , 219 ). The role of each virus in the progression of liver disease is not fully understood at this point.
There is, however, a considerable burden of coinfection in certain groups; coinfection is encountered among people living with AIDS ( 220 , – 222 ). As the main routes for the spread of HIV, HBV, and HCV are similar, HBV and HCV infections are prevalent in cohorts positive for HIV ( 223 ). In a pooled prevalence study in India, the prevalence of coinfection with HBV and HCV ranged from 0.02% to 3.2%, especially among patients with chronic liver disease, HIV-positive patients, and subjects who injected drugs or had chronic renal disease ( 224 ).
Anti-HBc-positive individuals and several high-risk groups (prisoners, intravenous drug users, and people living in regions where HBV is endemic) were reported to have a heightened prevalence of HCV coinfection ( 225 , 226 ). The prevalence differs in different regions and among HIV-positive cohorts. The TREAT Asia HIV Observational Database (TAHOD), a multicenter cohort of patients with HIV in the Asia-Pacific region, found an approximately 10% prevalence of HBV and HCV coinfection. Elevated alanine aminotransferase levels were found in individuals infected with both HBV and HCV. HIV-positive subjects in Rwanda were very commonly found to be infected with HBV and HCV ( 227 ). Coinfections with HIV-HBV, HIV-HCV, and HIV-HBV-HCV were noted in a reference center in southern Brazil at rates of 3.10%, 3.10%, and 0.16%, respectively ( 228 ).
Viral interference.
In the interaction between HCV and HBV, HCV is considered the more dominant infection, while HBV replication is inhibited, but this is not always the case. Some studies have shown that HCV is the major cause of active hepatitis in HBV- and HCV-coinfected patients ( 229 ). A U.S. study with matched analysis showed that HBV is usually the dominant virus in Asians, while HCV is dominant among non-Asians ( 230 ).
Past analyses of long-term outcomes confirm that patterns with viral coinfection may have dynamic profiles over time. Cells with replicating HBV may be infected with HCV, which is evidence against exclusion in superinfection. Also, replicating HBV does not interfere with the production of infectious HCV in cells ( 231 ). Genotype 1 HCV strains may have greater inhibitory activity on HBV than genotype 2 HCV strains ( 232 ). Chronic inflammatory activity is usually driven by HCV in patients with HCV-HBV coinfection, and the HBV DNA level may be either low or fluctuating. Despite an apparent reciprocal inhibition in plasma, HBV DNA and HCV RNA can be found to coexist in liver tissue, a condition which may explain reactivation in coinfected patients ( 233 , 234 ).
Natural history and comorbidity.
Acute hepatitis B and C virus coinfection is relatively rare; the kinetics of viremia may be affected. Superinfection and coinfection have been reported and may affect the outcome ( 235 ). Coinfection has been observed in patients with acute liver failure, and there are reports of nosocomial transmission.
Chen et al. reported coexisting acute HBV and HCV infections, showing a simultaneous elevation of ALT and bilirubin levels and subsequent continuing HCV infection with HBsAg clearance ( 236 ).
It has been suggested that the disease progression is enhanced in coinfected patients. Patients coinfected with HBV and HCV tend to have more serious liver injury and to have a greater likelihood of becoming cirrhotic and developing decompensated cirrhosis and HCC than monoinfected patients ( 237 ). Sagnelli et al. documented liver biopsy findings in 142 consecutive subjects with viral chronic hepatitis; those coinfected with HBV and HCV demonstrated a significantly higher fibrosis score. The 27 patients with detectable HBsAg and anti-HCV, despite having undetectable serum HCV RNA, showed histological activity indexes and fibrosis scores significantly higher than those found in the 17 HCV RNA-positive patients ( 238 ). Coinfection with any combination of HBV, HCV, or HDV has similarly been associated with a higher total Scheuer score, histological portal and lobular inflammation, and fibrosis ( 239 ). The level of regeneration of hepatocytes and the degree of inflammatory cell infiltration were significantly greater in HBV DNA-positive patients than in HBV DNA-negative patients.
A multivariate cause-specific proportional hazards model in Scotland indicated the risk of decompensated cirrhosis was increased in those who had previously been infected by HBV (HBsAg-negative but anti-HBc-positive individuals) ( 240 ). Patients identified with HBV coinfection (positive for HBsAg, HBV DNA, or HBeAg) were identified by the National Veterans Affairs HCV Clinical Case Registry. Of 99,548 HCV-infected patients, 1.4% had coinfection with HBV. Patients with HBV coinfection and detectable HBV DNA had a significantly greater chance of cirrhosis, HCC, and death (36.8, 6.9, and 41.7 per 1,000 person-years, respectively) than those infected only with HCV (17.4, 3.6, and 31.4 per 1,000 person-years, respectively) ( 241 ).
There may be a risk of undetected HBV infection, especially in HIV- and HCV-coinfected patients, although the clinical impact is uncertain ( 242 ). The chance of developing HCC was found to be greater in HBV DNA-positive patients than in HBV DNA-negative patients ( 243 ). The clinical outcomes of HIV coinfection with HCV and HBV may differ ( 244 ). In both Chinese and African populations and Western populations, HBV infection, HCV infection, or HBV-HCV coinfection may significantly increase the risk of developing HCC ( 245 , – 249 ). There are no hard data on the exact way in which tumors develop, although researchers have posited both direct and indirect roles for HBV and HCV.
Antiviral treatment of HCV and reactivation of HBV.
There have been instances of HBV reactivation in patients coinfected with HCV and HBV during both interferon-based and interferon-free DAA therapies, indicating the necessity of close monitoring of HBV coinfection, including infection that is either chronic, occult, or resolved ( 250 ). The presence of coinfection with HBV does not, however, appear to impact sustained virological response rates with DAA therapy but may play a role in reactivation ( 251 ). There appears to be a very slight risk of reactivation in anti-HBc-seropositive patients, which probably does not warrant prophylactic treatment ( 252 ).
Several systematic reviews have been conducted. Chen et al. compared the rate of reactivation of HBV in HCV-infected patients with HBsAg with that in patients with occult HBV infection (HBsAg-negative and HBV DNA-positive individuals) ( 253 ). The pooled rate of reactivation of HBV reactivation with obvious HBV infection rather than occult HBV infection was similar among patients who had IFN-based therapy, but reactivation occurred much earlier in those treated with DAAs. CHC patients with occult HBV infection had HBV reactivation, but much less frequently ( 253 ).
A study of 62,290 hepatitis C virus-infected veterans identified and characterized HBV reactivation among those who completed DAA treatment. HBV reactivation was characterized by a >1,000-IU/ml increase in the levels of HBV DNA or HBsAg detected in a person who was previously negative. Since this was a retrospective study, the investigators chose only patients who were considered to be at high risk for HBV reactivation (defined as baseline results for HBV infection showing HBsAg positivity and anti-HBc positivity prior to DAA initiation) to be evaluated to better control for normal fluctuations in HBV levels. In total, 9 of the 62,290 veterans demonstrated signs of HBV reactivation during treatment with DAAs. Eight of the cases occurred in HBsAg-positive patients ( 254 ).
Little is understood about the specific viral characteristics that facilitate reactivation, as the functional characterization of the reactivated HBV has been conducted only rarely ( 255 ). The U.S. FDA reviewed cases that also suggested that further investigation would possibly determine triggers for reactivation and refine recommendations for monitoring but concluded that DAAs continue to be safe and effective therapies for infection with HCV ( 256 ).
In one such study, among 14,695 HCV patients, 10,551 were tested for HBsAg and/or HBV DNA. One-hundred fifteen (1.1%) had a positive test result, while 3,836 (27.2%) had received DAA therapy. Only 4.0% of the quarter of the cohort of patients who received DAA therapy were identified as having a single raised ALT level during the 4 to 52 weeks after the start of therapy; no cases of DAA-associated reactivation were detected, but in this analysis, posttreatment HBsAg or HBV DNA testing was rarely performed ( 257 ).
A study pooling data from 17 observational investigations involving 1,621 patients with chronic ( n = 242) or resolved ( n = 1,379) HCV infection treated with different DAAs reported a reactivation rate of 24% in patients with chronic hepatitis B (HBsAg positive) versus a reactivation rate of 1.4% in those who had recovered from HBV infection (HBsAg-negative, anti-HBc-positive patients). HBV reactivation may also be more common in HCV-infected patients undergoing DAA therapy than in patients undergoing IFN-based therapy ( 258 ). As a result, nucleoside analogue prophylaxis is warranted in patients who are HBsAg positive, particularly patients with HBV DNA that is quantifiable, and persons who have recovered from HBV infection should be monitored ( 259 , – 262 ). An updated and comprehensive review by Loomba et al. summarizes HBV reactivation and nucleoside analogue prophylaxis in other settings of immunosuppression for HBsAg-positive patients and indicated that nucleoside analogue prophylaxis is generally indicated for isolated anti-HBc-positive patients mainly in the setting of stem cell transplantation or anti-CD20 therapies ( 263 ).
The long-term effects of hepatitis coinfection (HBV, HCV, or both) on HIV infection or deaths from hepatitis require monitoring, but fortunately, coinfection is readily treatable ( 264 ). Anti-HCV-positive individuals should receive vaccinations against hepatitis A and B.
CONCLUSIONS
In summary, an effective and safe vaccine is available to prevent HBV transmission. For those already infected, effective and safe antiviral therapies are also available. Persons infected with HBV as well as HDV, HCV, and/or HIV have a higher risk for progression of disease and complications and must have rigorous surveillance and treatment. Finally, to realize the goal of HBV elimination, improved linkage to care is needed, at-risk populations need to be vaccinated, infected populations need to be diagnosed and treated, and curative therapy with a finite treatment duration is needed. We suggest an algorithm for screening, diagnosis, and linkage to care that starts with universal screening of persons residing or coming from areas with a prevalence of HBV infection of 2% or higher; evaluation of infected persons by at least an assessment of liver enzyme, HBeAg, and HBV DNA levels; and, according to local guidelines and resources, the administration of antiviral therapies for persons at risk for disease progression ( Fig. 2 ) ( 3 ).
ACKNOWLEDGMENTS
This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We acknowledge the following members of the Mindie H. Nguyen lab at Stanford University: An Le for her assistance with our EndNote reference management and Karen Ely and Linda Henry for their editorial assistance. We thank Patrick Lane of ScEYEnce Studios for his assistance with the preparation of our figures.
We have the following conflicts of interest. Mindie H. Nguyen receives research support from Pfizer, Gilead Sciences, Janssen, the National Cancer Institute, and the B. K. Kee Foundation and is a consultant for or on the advisory boards of Anylam, Janssen, Intercept, Spring Banks, Bayer, Gilead, Dynavax, Eisai, Exact Sciences, and Novartis. Grace Wong receives research support from AbbVie and Gilead Sciences, is an advisory board member or consultant for Gilead Sciences and Janssen, and is a speaker for Abbott, Bristol-Myers Squibb, Echosens, Furui, Gilead Sciences, Janssen, and Roche. Edward Gane is a consultant for or on the advisory board of Abbvie, Arrowhead, Assembly, Gilead Sciences, Janssen, Roche, and VIR and is a speaker for Abbvie and Gilead Sciences. Jia-Horng Kao is a consultant for or on the advisory board of Abbvie, BMS, Gilead Sciences, and MSD and a speaker for Abbvie, Ascletis, BMS, Gilead Sciences, and MSD. Geoffrey Dusheiko is a consultant for or on the advisory board of Gilead Sciences, AbbVie, Abbott, BMS, Janssen, Merck, and Shionogi.
Biographies
Mindie H. Nguyen is a Professor of Medicine in the Division of Gastroenterology and Hepatology, Stanford Global Health Faculty, and a Fellow and Director for the Hepatology Fellowship and Clerkship at Stanford University, Stanford, CA, USA. After gaining her medical degree at the University of California, San Diego (UCSD), Dr. Nguyen trained in internal medicine at the UCSD Medical Center, where she was Chief Resident. Subsequently, she became Fellow and Chief Fellow in the Division of Gastroenterology and Hepatology at Stanford University before joining the faculty there in 2002. She obtained her master’s in Advanced Studies in Clinical Research at the University of California, San Francisco. Her main fields of scientific and clinical interest have been the epidemiology and management of chronic viral hepatitis, nonalcoholic fatty liver disease, and liver cancer for over 15 years. She is Chair of the American Association for the Study of Liver Diseases (AASLD) Hepatitis B Special Interest Group and a member of the Governing Council of the International Association for the Study of Liver Diseases (IASL). She has also served as an editorial board member for several journals, including The Lancet Gastroenterology and Hepatology , Gastroenterology , and Hepatology .
Grace Wong is a Professor at The Chinese University of Hong Kong. She has been in the field of hepatology research for 15 years. Her main research interest includes noninvasive assessment of liver fibrosis and chronic viral hepatitis and risk prediction for hepatocellular carcinoma. She has published over 250 articles in peer-reviewed journals, including Gastroenterology , Hepatology , and Gut . She is currently the Editor-in-Chief of Hepatology (Hong Kong edition) and the Associate Editor of Alimentary Pharmacology & Therapeutics . She was awarded the Young Investigator Award of the Asian Pacific Association for the Study of the Liver in 2009; the Distinguished Research Paper Award for Young Investigators of the Hong Kong College of Physicians in 2010, 2013, 2014, and 2015; the Ten Outstanding Young Persons (TOYP) of Hong Kong in 2014; the Sir David Todd Lectureship of the Hong Kong College of Physicians in 2016; and the JGHF Emerging Leader Lectureship in 2017.
Edward Gane is Professor of Medicine at the University of Auckland, Auckland, New Zealand, and Hepatologist and Deputy Director of the New Zealand Liver Unit at Auckland City Hospital. Dr. Gane trained in hepatology at the Institute of Liver Studies, King’s College School of Medicine, London, United Kingdom, where he completed his M.D. on the pathogenesis of hepatitis C-related liver injury. In 1998, Dr. Gane was appointed as Chief Physician for the first New Zealand Liver Unit at Auckland City Hospital, which provides a national transplant and HCC program. Dr. Gane helped set up the community-based national HBV Surveillance Programme, which is the largest in the world. He now chairs the Ministry of Health committee responsible for HCV elimination. Dr. Gane is an investigator for many international clinical trials, with particular interest in early-phase development of new direct-acting antiviral therapies against chronic hepatitis C and hepatitis B. He has published almost 300 papers in peer-reviewed journals, including The Lancet and The New England Journal of Medicine . In 2011, Dr. Gane was awarded Member of the Order of New Zealand for Services to Medicine and in 2017 was New Zealand Innovator of the Year for his work toward HCV elimination in New Zealand.
Jia-Horng Kao is the National Chair Professor of the Ministry of Education and Distinguished Professor at the Graduate Institute of Clinical Medicine, National Taiwan University College of Medicine. He serves as the President of the Taiwan Association for the Study of the Liver (TASL) and Advancing Clinical Treatment—Liver Disease (ACT-LD) and Chief of the Division of Hepatology/Gastroenterology at the National Taiwan University Hospital. Professor Kao’s main research interests include the prevention, natural history, molecular virology, pathogenesis, and treatment of chronic viral hepatitis and HCC. He has published more than 520 articles, with the sum of times that his articles have been cited being 18,296 and with an h-index of 63 by the Web of Science. He also contributed to 9 book chapters and edited a monograph on hepatitis B. Professor Kao is the Editor-in-Chief of the Journal of the Formosan Medical Association ( JFMA ) and sits on the editorial board of Gut , Alimentary Pharmacology & Therapeutics ( APT ), and the Journal of Infectious Diseases ( JID ). He also serves as the trustee of the JGH Foundation and is the founding member of AASLD Asia Pacific Regional Advisory Council.
Geoffrey Dusheiko is an Emeritus Professor of Medicine at the Royal Free Hospital and University College London School of Medicine as well as a Consultant Hepatologist at King’s College Hospital, London, United Kingdom. After graduating from the University of the Witwatersrand, he completed his residency at Johannesburg Hospital. His fellowships were conducted at the Johannesburg Hospital Liver Unit, the National Institutes of Health (MD, USA), and the University of Minnesota (MN, USA). Dr. Dusheiko’s research interests include the management and treatment of HCV, HBV, and small hepatocellular carcinoma. He has a special interest in research on viral hepatitis, particularly viral genotyping, applied molecular virology, the natural history of chronic viral hepatitis, and antiviral therapies. He served as educational councilor on the Governing Board of EASL for 4 years and was the recipient of the EASL Recognition Award in 2014. In addition to being on the editorial board of the Journal of Viral Eradication , he is Coeditor of Alimentary Pharmacology & Therapeutics , and he has previously served on the editorial boards for the Journal of Viral Hepatitis , Hepatology , Best Practice & Research: Clinical Gastroenterology , and Gut , among others. Professor Dusheiko has authored or coauthored more than 400 articles published in international peer-reviewed journals or books.

IMAGES
VIDEO
COMMENTS
B is built on three priority areas that are vital to developing a cure: 1. Understanding hepatitis B biology, public health challenges posed by HBV infection, a. 2. Developing tools and resources to advance HBV curative treatment will need to go hand in hand research, and 3.
Background and Aims. Hepatitis is an inflammation of the liver that can reason a variety of health problems and can be fatal. According to the most recent estimates of the Global Burden of Disease study and WHO, viral hepatitis is accountable for around 1.34 million deaths yearly, which is comparable to the yearly number of deaths from HIV/AIDS (1.3 million), malaria (0.9 million), and ...
Abstract and Figures. Background:Hepatitis B Virus (HBV) infection poses a grave public health problem worldwide. Over two billion people are infected and an estimated 387 million of these ...
The incidence of acute hepatitis C declined during the 1990's but has plateaued in recent years. While the incidence of acute hepatitis B has declined markedly since 1990, an estimated 1.25 million Americans remain chronically infected, with an estimated 3,000-5,000 chronic hepatitis B virus-related deaths per year. In the United States,
Wen-Juei Jeng, George V Papatheodoridis, Anna S F Lok. Hepatitis B virus (HBV) infection is a major public health problem, with an estimated 296 million people chronically infected and 820 000 deaths worldwide in 2019. Diagnosis of HBV infection requires serological testing for HBsAg and for acute infection additional testing for IgM hepatitis ...
10.1. Infant and neonatal hepatitis B vaccination 87 10.2. Prevention of mother-to-child HBV transmission using antiviral therapy 89 10.3. Prevention of hepatitis B transmission and measures to reduce disease progression in persons with chronic hepatitis B 94 10.4. Prevention of hepatitis B and C transmission in health-care settings 95 10.5.
Hepatitis B virus infection is a major global health burden accounting for 2.7% of all deaths globally. ... The result from this research work is comparable with a study conducted among medical and health science college students in Saudi ... EI, AM, and DG participated in the conception, design of the study, reviewing the proposal, data ...
Author summary The Global Hepatitis Health Sector Strategy is aiming for the elimination of viral hepatitis as a public health threat by 2030. However, mutations associated with drug resistance and vaccine escape may reduce the success of existing treatment and prevention strategies. In the current literature, the prevalence, distribution and impact of hepatitis B virus (HBV) mutations in many ...
The National Institutes of Health has updated its Strategic Plan for NIH Research to Cure Hepatitis B, a roadmap for ending the hepatitis B epidemic, focused on developing a cure as well as improved strategies for vaccination, screening and follow-up care. The revised plan incorporates lessons from the COVID-19 pandemic and recent advances in technology.
Original Research Introduction Hepatitis B virus (HBV) is a hepatotropic deoxyribonucleic acid (DNA) virus that occurs because of the immune-medi-ated killing of infected liver cells. 1 It is a major blood-borne and sexually transmitted infectious agent and poses a seri-ous global public health problem, which is approximately
Introduction. Globally, ~290 million people are chronically infected with hepatitis B virus (HBV), and more than 800,000 people consequently die each year from liver cirrhosis or liver cancer ().In response to the United Nations Sustainable Development Goals, which aim to eliminate viral hepatitis as a public health threat by the year 2030 (), HBV infection is receiving increasing recognition ...
Viral hepatitis is a group of infectious diseases that aff ects hun-dreds of millions of people worldwide, causing serious illness and death from acute hepatitis infection, liver cancer and liver cirrhosis. Although there are eff ective tools and strategies for the prevention and treatment of hepatitis, low awareness of
Chronic hepatitis B virus (HBV) infection is a major public health problem and cause of chronic liver disease and led to an estimated 1·1 million deaths in 2022, mainly due to cirrhosis and hepatocellular carcinoma.1 In 2022, WHO estimated that 254 million people were living with chronic hepatitis B, of whom 65% were in the African and Western ...
Hepatitis B virus (HBV) is one of the key etiological agents for liver diseases, including chronic hepatitis, liver cirrhosis, and liver cancer [1]. It is the second common- ... research article and then compared among the ex-posed and the unexposed to HBV. Finally, concerning the outcome, the central outcome was HBV infection ...
Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. Recommendations and Reports The MMWR series of publications is published by the Center for Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control and Prevention (CDC),
1. INTRODUCTION. Hepatitis B virus (HBV) infection is a life‐threatening viral infection leading to acute or chronic liver disease (World Health Organization, 2015a).Worldwide, infections with viral hepatitis B and C are the main causes of hepatocellular carcinoma (Giang, Selinger, & Lee, 2012).Chronic liver disease and cirrhosis are considered as the 12th leading cause of death in the USA ...
health research and have been reported to be the most commonly used study tools in health-seeking behaviour research [19]. KAP studies have been used to collect in-formation on what the participants know, believe and do in relation to a particular topic [17-20]. The knowledge refers to the understanding of any given topic [21, 22].
(HBsAg) in countries with low Hepatitis B endemicity (<2% HBsAg). Hepatitis B vaccine programs have been widely implemented over the past decades and were able to decrease the percentage of chronic Hepatitis B infections and liver cancer among children (Chang et al. 1997, Mast et al. 2004, see table 1.1). In fact, two regional offices of the ...
Indisputably, infection secondary to hepatitis B virus is the major public health problem globally, and is a leading cause of chronic hepatitis in low- and middle-income countries. 7, 8 Regarding the endemicity of the disease, the world has been classified into three sub-regions; high for >8%, intermediate for 2-8% and low for <2% prevalence ...
A plasma-derived Hepatitis B (HepB) vaccine was first licensed for use in the United States in 1981. The vaccine was safe and effective but was not well accepted, possibly because of unsubstantiated fears of transmission of live HBV and other blood-borne pathogens. Recombinant HepB vaccines replaced plasma-derived HepB vaccines beginning in 1986.
Hepatitis B is a serious and common infectious disease of the liver, affecting millions of people throughout the world.6, 10, 15, 23, 31. The severe pathological consequences of persistent HBV infections include the development of chronic hepatic insufficiency, cirrhosis, and hepatocellular carcinoma (HCC).
The World Health Organisation (WHO) recommends the vaccination against Hepatitis B virus in all infants and children up to the age of 18 years. In addition, adults in high-risk groups should also be vaccinated. This study investigated the prevalence and factors associated with Hepatitis B Virus (HBV) infections among health professional students in the city of Mwanza, Tanzania in order to ...
Sanjeet Bagcchi reports. On March 29, 2024, WHO brought out new guidelines for the prevention, diagnosis, and treatment of chronic hepatitis B infection, at the 2024 annual meeting of the Asian Pacific Association for the Study of the Liver (APASL), held in Kyoto, Japan. The new guidelines simplify chronic hepatitis B treatment criteria and ...
Recent data demonstrate that immunoprophylaxis with hepatitis B immunoglobulin and the hepatitis B vaccine in newborns can reduce the rate of mother-to-child transmission (MTCT) from 90% to 10% . However, if the mother has an HBV DNA level of greater than 200,000 IU/ml, immunoprophylaxis has a failure rate of 10 to 30% in infants born to such ...
Hawaiian, and Pacific Islander communities, are living with a chronic hepatitis B infection. The good news is that there is already a hepatitis B vaccine that can save lives. The Centers for Disease Control and Prevention urges all adults under 60 to be screened and vaccinated and for 30 years has recommended that children be vaccinated as well.