Historical Evolution of India’s Patent Regime and Its Impact on Innovation in the Indian Pharmaceutical Industry
- Open Access
- First Online: 07 September 2019

Cite this chapter
You have full access to this open access chapter

- Uday S. Racherla 5
Part of the book series: ARCIALA Series on Intellectual Assets and Law in Asia ((ARCIALA))
39k Accesses
2 Citations
8 Altmetric
Article 21 of the Indian Constitution guarantees every person and citizen of India the right to life and the right to personal liberty . Further, Article 47 of the Indian Constitution declares that it is the duty and obligation of the Indian state to improve public health . In addition, Article 12 of the International Covenant on Economic, Social and Cultural Rights (ICESCR) adopted by India asserts that nations have an obligation to facilitate the right to health . Thus, the Indian government operates under the premise that medicines critical to the important healthcare needs of India’s population must be both available and affordable. Indeed, this paradigm is the foundational basis for India’s vision for the right to health under the Article 21 of the Indian Constitution . Thus, the Indian policy makers strive to meet India’s constitutional obligations for the right to health while promoting its innovation ecosystem and safeguarding the legitimate business interests of MNCs. Indeed, this powerful undercurrent has been shaping the evolution of the Indian patent regime since India’s independence in 1947, through the 1970s, the economic liberalization era initiated in the 1990s, through the membership of WTO and TRIPs Agreement in 1995, post-TRIPS in 2005 and all the way up to today. In this context, this chapter analyzes how the Indian patent regime has been leveraging the flexibilities afforded under the TRIPS Agreement for the prevention of evergreening , award of compulsory licenses , retention of pre-grant opposition , and introduction of post-grant opposition and discusses how these dynamic changes are having a global impact.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others

The Efficiency of India's Patent Layering Regime – Section 3(d) from Scientific Terminology to Patenting Implications

Patent Law and Access to Medicines in Ukraine

COVID-19 and the Issue of Affordable Access to Innovative Health Technologies: An Analysis of Compulsory Licensing of Patents as a Policy Option
- Innovation ecosystem
- Competitive advantage
- Sustainable economic growth
- Indian intellectual property rights regime
- Indian generic pharmaceutical industry
- Available and affordable medicines
- TRIPS flexibilities
- Evergreening
- Compulsory license
- Pre-grant opposition
- Post-grant opposition
1 Introduction
It has been recognized by industry, academia, and policy makers alike that innovation is pivotal to value creation , competitive advantage , and sustainable economic growth . As knowledge economy became the basis for globalization, innovation opportunities have been incessantly emerging around the world for value-added products, processes, and services to meet the ever-growing needs, wants, challenges, and opportunities of the world. As a result, today we find many individuals, companies, communities, and nations working relentlessly on innovation. Thus, policy makers, both nationally and internationally, have recognized that innovation either flourishes or suffers depending upon the innovation ecosystem . However, the innovation ecosystem of a nation depends upon three primary factors, namely, technology environment, business environment, and policy environment (Fig. 1 ). Footnote 1
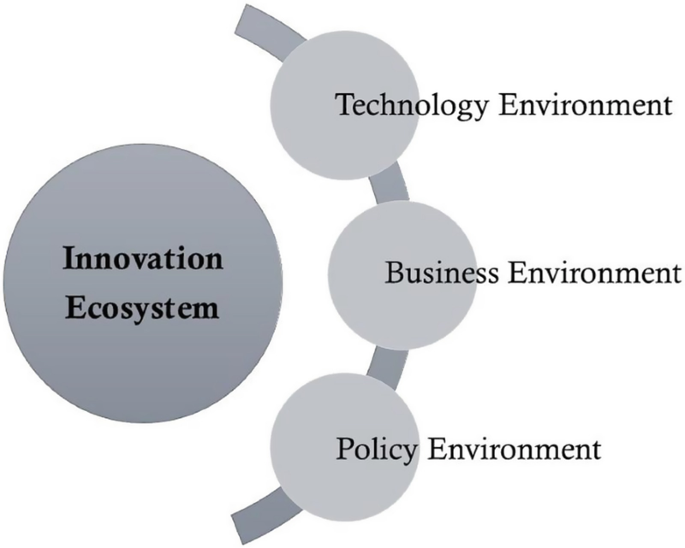
Innovation ecosystem and the factors governing it
Consequently, academia, industries, and governments around the world have been focused on strengthening and promoting the innovation ecosystem in order to meet the national priorities, as well as achieve competitive advantage , sustainable economic growth , and creation of employment in the global economy.
In this regard, it is important to note that the vision and strategies of a country’s patent regime play a crucial role in (a) advancing the goals of its innovation ecosystem (indigenously or as part of international agreements), (b) protecting its social and economic interests, and (c) safeguarding the legitimate business interests of competition.
Indeed, it is in this light that the historical evolution of the Indian patent regime and its impact on innovation in the Indian pharmaceutical industry must be analyzed and understood.
2 Overview of the Indian Pharmaceutical Industry
2.1 business achievements.
India is a unique global player in the pharmaceuticals business world. To start with, India has a large pool of well-trained scientists and engineers who have the potential to innovate and steer the industry to meet India’s vision, national needs, and future goals.
The Indian pharmaceutical industry, valued at US $33 billion in 2017, is currently the largest global supplier of cost-effective generic drugs . Thus, the drugs made in India are exported to more than 200 countries around the world, with the United States of America (USA) being India’s biggest market. According to India Brand Equity Foundation (IBEF), in 2016–2017, around 40.6% of India’s pharmaceutical exports (US$ 16.8 billion) were to the American continent, followed by a 19.7% to Europe, 19.1% to Africa, and 18.8% to the Asian continent. Footnote 2
The Indian pharmaceutical companies meet over 50% of the global demand for various vaccines, 40% of the generic demand in the USA, and 25% of demand of all the medicines in the UK. In addition, India supplies over 80% of the antiretroviral drugs needed globally for AIDS ( acquired immunodeficiency syndrome ). In 2017, the Indian pharmaceutical companies received 304 Abbreviated New Drug Application (ANDA) approvals from the US Food and Drug Administration (USFDA).
India is also emerging as a key player in the biotechnology industry. India’s biotechnology industry includes biopharmaceuticals, bio-services, bio-agriculture, bio-industry, and bioinformatics. This sub-sector of the Indian pharma industry is expected to grow at an average annual growth rate of around 30% and reach US$ 100 billion by 2025. In fact, the biopharma industry – comprising of vaccines, therapeutics, and diagnostics – contributes US$ 1.89 billion, which is a significant portion of the total industry revenues.
The Indian pharmaceutical market grew at a CAGR Footnote 3 of 5.6%, during FY Footnote 4 2011–2016, from US$ 20.95 billion in FY2011 to US$ 27.57 billion in FY2016. In FY2017 alone, the industry’s revenues grew by 7.4% and stood at US$ 33 billion. In March 2018, the market grew at 9.5% year-on-year with sales of US$ 1.56 billion. According to the industry analysts, India’s pharmaceutical sector is predicted to grow at a CAGR of 22.4% during FYs 2015–2020, to reach US$ 55 billion, as branded drugs worth US$ 55 billion will become off-patent during this period. By FY2020, India is expected to be among the top three pharmaceutical markets in the world by organic growth and sixth largest market in absolute size.
2.2 Investments, Mergers, and Acquisitions
The growing middle-class population in India, increasing demand for better access to healthcare, improving medical facilities, and better penetration of health insurance in the country point to lucrative investment opportunities in the Indian pharmaceutical sector. Not surprisingly, the Government of India amended its Foreign Direct Investment (FDI) policy in the pharmaceutical sector to automatically allow up to 100% FDI for the manufacture of medical devices subject to, of course, some guidelines.
Thus, according to the data released by the Department of Industrial Policy and Promotion (DIPP), the Indian pharmaceutical sector attracted US$ 15.59 billion worth of FDI in 17 years, between 2000 and 2017. In Q2 2018, the Indian pharmaceutical sector posted private equity and venture capital investments of US$ 396 million. Also, in 2017, India witnessed 46 mergers and acquisitions (M&As) – worth US$ 1.47 billion – in the pharmaceutical sector. Footnote 5
3 Goals and Priorities of the Indian Patent Regime
The Indian pharmaceutical industry is well aware that innovation is critical for wealth creation, competitive advantage , and sustainable growth . Innovation in the pharmaceutical industry is often a high-risk, high payoff gamble. While companies may deliver high returns on investment (ROI) when innovations are successful, innovation failures can threaten company’s survival itself. 2 Consequently, Indian pharmaceutical companies rely on successful innovations Footnote 6 to make high profits , deliver consistent ROI to shareholders , and achieve sustainable growth . On the other hand, the policy makers of the Indian government depend on the patent regime to ensure that pharmaceutical innovations deliver affordable medicines and accessible health care to all citizens . Therefore, successful pharma companies are those that can innovate to solve the healthcare needs, wants, and challenges of millions of people in India while also posting robust revenues, profits, market share, and growth. In other words, nations such as India aim to balance social goals (which aim to ensure affordable medicines and accessible healthcare to all citizens) against the economic goals (which are aligned with the interests of pharmaceutical companies). Figure 2 summarizes this dilemma of Indian policy makers.
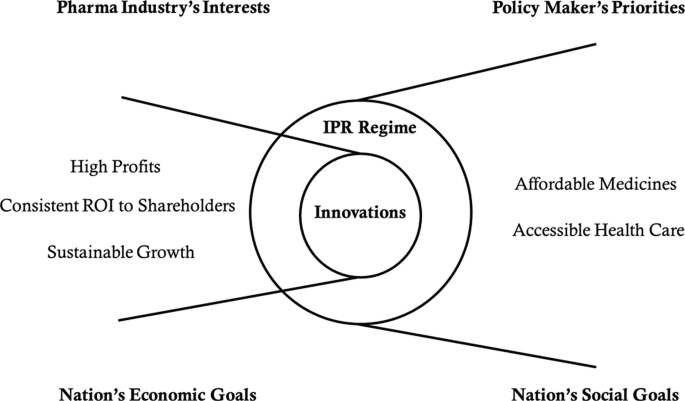
Dilemma of the Indian policy makers’ goals and priorities
Indeed, it is this powerful undercurrent that has been shaping the policies of the Indian patent regime since India’s independence in 1947, through the 1970s, the economic liberalization era that started in the 1990s, through the membership of WTO and TRIPS Agreement in 1995, post-TRIPS in 2005, and all the way up to today. Therefore, the historical evolution of the Indian patent regime , imperfect as it may seem, makes sense only when one understands how India tries to continually balance its social goals and priorities against the economic goals .
4 Brief Overview of the Indian Patent Regime’s History
It is important to note that a seminal paper in this area has been published by Janice Mueller. Footnote 7 Thus, the Indian patent regime reflects India’s journey in three different periods: colonization , post-independence, and globalization . During the “colonization” phase, the Indian Patents and Designs Act of 1911 – drafted by the British – enacted India’s first patent statutes. India gained its independence in 1947. However, during the “post-independence” phase, the British-imposed, foreigner-favoring patent laws stunted the development of the Indian pharmaceutical industry and forced independent India to import even basic medicines at unaffordable prices. Consequently, in 1949, the Indian government constituted a high-powered committee headed by an eminent jurist of the Lahore High Court, Bakshi Tek Chand, and sought an intensive review of the existing patent laws. The most significant finding of the Chand Committee was that the prevailing Indian patent laws offered asymmetrically strong protections to foreign multinational corporations (MNCs) while severely inhibiting the development of the domestic manufacturing sector. Further, according to the Controller General of Patents, Designs, and Trademarks Footnote 8 :
The committee also observed that the Patents Act should contain clear indication to ensure that food and medicine and surgical and curative devices are made available to the public at the cheapest price commensurate with giving reasonable compensation to the patentee .
In 1957, the Government of India appointed another committee led by the distinguished retired Justice of the Supreme Court of India, N. Justice Rajagopala Ayyangar, to examine the question of revising the Patents Act and advising government. This committee’s recommendations acted as a catalyst for changing the Indian patent law, which eventually led to India Patents Act of 1970. The India Patents Act of 1970 incorporated major provisions to reduce the social costs of the foreigner-owned patents. Thus, the Patents Act of 1970 (a) prohibited patents on products useful as medicines and food, (b) shortened the term of chemical process patents , and (c) significantly expanded the availability of compulsory licensing . This led to the birth and growth of the powerful Indian pharmaceutical generic drugs industry. The third “globalization” phase approximately spans the years 1986 up to the present. According to Mueller, during the “globalization” phase:
India’s participation in the debates over the inclusion of intellectual property within the GATT framework and its eventual entry into the World Trade Organization (WTO), along with its accession to the Paris Convention for the Protection of Industrial Property and the Patent Cooperation Treaty, have compelled significant strengthening of the nation’s patent laws . The implementation of those changes is ongoing, and their anticipated impact remains to be fully seen. Today India stands as a rising global power with a patent system still very much in flux .
5 The Historical Evolution of India’s Patent Regime and Its Impact
Now we can look at some of the important details in the evolution of India’s patent regime and its impact on innovation in the pharmaceutical industry. Footnote 9
5.1 1947–1970
India won its independence in 1947. In its Constitution, India declared itself as sovereign socialist secular democratic republic. Thus, from the beginning, Indian policy makers believed in the principle of distributive justice and government’s active role in curbing socioeconomic inequalities .
At the outset, the Indian government had to meet the needs of nearly 400 million people and confront simultaneous challenges such as food and water shortages, inadequate housing, illiteracy, unemployment, infant mortality, epidemics, inaccessible healthcare, and unaffordable medicines. Faced with the staggering healthcare needs and under the burden of British imposed, foreigner-favoring patent laws, India had no choice but to rely on importing even the most basic medicines such as insulin and penicillin manufactured by other nations, at some of the highest prices in the world.
The Bakshi Tek Chand Committee Report noted that the existing Indian patent law afforded “inequitably strong IP protection” to MNCs a situation that was blocking the Indian manufacturing industry in its infancy itself.
As reported in the Shodhganga – the digital repository of Indian Electronic Theses and Dissertations – setup by the INFLIBNET Centre Footnote 10 :
The Indian patent system has failed in its main purpose, namely to stimulate invention among Indians and to encourage the development and exploitation of new inventions for industrial purposes in the country so as to secure benefits thereof to the largest sections of the people. Footnote 11
Strong evidence for this had come just a few years afterward, when Hoechst pharmaceutical company won an injunction in Bombay High Court against Unichem Laboratories of India over infringement of its patent for the manufacture of a highly needed antidiabetic drug. Footnote 12
Thus, the Bakshi Tek Chand Committee Report recommended Footnote 13 :
The main provisions suggested by the committee among others include compulsory licensing , commercial working of patented inventions in India barring importations, setting up of appellate body in the form of an ad-hoc Special Tribunal nominated by the Central Government consisting of a sitting or retired judge of a High Court (as the President), and ensuring that food and medicines are available at cheapest rates to the public commensurate with giving reasonable compensation to the patentee etc.
This is the first instance where the Indian policy makers unequivocally articulated the need to balance India’s social goals against its economic goals.
In 1957, yet another committee was constituted under Rajagopala Ayyangar, for the purpose of building on the recommendations of Bakshi Tek Chand Committee Report and sculpting policies that will ensure India’s national goals and interests and kick-start the Indian industry. Thus, the new committee once again carefully examined the patent laws of India, in light of the successful public welfare models of several other nations.
Accordingly, the 1959 Ayyangar Committee Report Footnote 14 a noted the following:
It would be convenient to consider the two matters dealt with by this provision separately – (1) The precise degree and extent of patentability to be permitted in regard to inventions of chemical products in general ; and (2) the law determining the patentability of inventions relating to food and medicine .
In continuation, the Ayyangar Committee Report 14a recommended:
As regards inventions relating to chemical products, or products produced by chemical processes, I am clearly of the view that the interests of the country would be best served by confining patentability to the processes by which the products are obtained and to deny patents to the products either per se or in the qualified manner suggested in the Bill . The reasons for this recommendation are based on (1) the history of the law relating to patents regarding chemical inventions in Europe during the past nearly 100 years and the lessons to be derived therefrom; (2) the experience of other countries somewhat similarly situated like India; and (3) the disadvantages to an underdeveloped country of permitting product claims for such inventions .
Also notable are the arguments advanced in the Ayyangar Committee Report 14b on patent grant vis-à-vis economic benefits and social costs :
Where the patentee has no intention of working the invention in this country either because he considers that this is not profitable or because he prefers to expand the production in his home country so as to achieve there greater efficiency and more production or is otherwise not interested in working the invention in India, the grant of the Indian patent might tend to improve the economy of the patentee’s home country but offers little advantage to us . Unless therefore the law provides for measures to be taken to compel the patentees to work the invention within the country , and these measures are effective to achieve their purpose, the social cost involved in the grant of the patent is not offset by any benefit to the community [ by way of an increase of technical skill or of national wealth ].
Next, a joint select committee of the parliament and the parliament itself debated the findings and recommendations of the Bakshi Tek Chand Committee Report and the Ayyangar Committee Report . This resulted in the Patent Bill of 1965 which incorporated changes relating to patents for food, drug, and medicines. This bill was introduced first in the lower house of the Parliament on September 21, 1965. The bill was reintroduced with some changes in the Parliament in 1966 but could not be passed. The bill eventually lapsed with the dissolution of the Lok Sabha (the lower house of Parliament) in March 1967. However, the bill was finally passed by the Parliament, and the Patents Act 1970 Footnote 15 a came into force on April 20, 1972 along with Patent Rules 1972. Thus, the Patents Act 1970 repealed and replaced the 1911 Act while incorporating the recommendations of both the committees. The 1911 Act however continued to apply to designs.
5.2 1970–1995
In the Patents Act 1970, the following four articles and their intended purpose are noteworthy 15b :
Chapter II ( Inventions not Patentable ), Article 5 clarifies that only processes are patentable :
In the case of inventions – (a) claim.ing substances intended for use, or capable of being used, as food or as medicine or drug , or (b) relating to substances prepared or produced by chemical processes (including alloys, optical glass, semi- conductors and inter-metallic compounds), no patent shall be granted in respect of claims for the substances themselves , but claims for the methods or processes of manufacture shall be patentable .
Chapter VIII ( Grant and Sealing of Patents and Rights Conferred Thereby ), Article 53 states:
(1) Subject to the provisions of this Act, the term of every patent granted under this Act shall – (a) in respect of an invention claiming the method or process of manufacture of a substance , where the substance is intended for use, or is capable of being used, as food or as a medicine or drug, be five years from the date of sealing of the patent , or seven years from the date of the patent whichever period is shorter ; and (b) in respect of any other invention , be fourteen years from the date of the patent .
Finally, XVI ( Working of Patents, Compulsory Licences, Licences of Right and Revocation ), Article 83 clarifies the economic goals of the Indian policy makers:
Without prejudice to the other provisions contained in this Act, in exercising the powers conferred by this Chapter, regard shall be had to the following general considerations, namely; (a) that patents are granted to encourage inventions and to secure that the inventions are worked in India on a commercial scale and to the fullest extent that is reasonably practicable without undue delay; (b) that they are not granted merely to enable patentees to enjoy a monopoly for the importation of the patented article; (c) that the protection and enforcement of patent rights contribute to the promotion of technological innovation and to the transfer and dissemination of technology , to the mutual advantage of producers and users of technological knowledge and in a manner conducive to social and economic welfare, and to a balance of rights and obligation s; (d) that patents granted do not impede protection of public health and nutrition and should act as instrument to promote public interest specially in sectors of vital importance for socio-economic and technological development of India ; (e) that patents granted do not in any way prohibit Central Government in taking measures to protect public health ; (f) that the patent right is not abused by the patentee or person deriving title or interest on patent from the patentee, and the patentee or a person deriving title or interest on patent from the patentee does not resort to practices which unreasonably restrain trade or adversely affect the international transfer of technology ; and (g) that patents are granted to make the benefit of the patented invention available at reasonably affordable prices to the public .
Chapter XVI ( Working of Patents, Compulsory Licences, Licences of Right and Revocation ), Article 97 clarifies when compulsory licenses will be given:
(1) If the Central Government is satisfied in respect of any patent or class of patents in force that it is necessary or expedient in the public interest that compulsory licences should be granted at any time after the sealing thereof to work the invention or inventions, it may make a declaration to that effect in the Official Gazette , and thereupon the following provisions shall have effect, that is to say – (i) the Controller shall on application made at any time after the notification by any person interested grant to the applicant a licence under the patent on such terms as he thinks fit; (ii) (in settling the terms of a licence granted under this section, the Controller shall endeavour to secure that the articles manufactured under the patent shall be available to the public at the lowest prices consistent with the patentees deriving a reasonable advantage from their patent rights . …
Thus, the Patents Act 1970 (a) prohibited patenting of products and allowed patenting only on the methods/processes of manufacture useful as medicines and food (see Article 5), (b) shortened the term of chemical process patents (see Article 53), and (c) significantly expanded the grant of compulsory licenses (see Articles 83 and 97).
Indeed, the India Patents Act, 1970, was momentous in the history of the Indian pharmaceutical industry as it enabled domestic firms to replicate the drugs patented by MNCs, creating a booming generic pharmaceutical industry. As MNCs began to exit the Indian market due to significantly diminished IP protection, the Indian pharmaceutical companies began to fill the void and dominate the global business of reverse-engineered highly cost-efficient generics that sold at exceptionally cheaper prices compared to the counterparts marketed by MNCs. This is how the generic pharmaceutical industry of India was able to become one of the most prolific drug manufacturing industries in the world , ranking third globally in annual volume.
Thus, the Indian government was able to meet its social goals as well as economic goals (Fig. 2 ). Interestingly, however, there were advantages as well as disadvantages to the unexpected nearly complete exit of the western pharmaceutical companies from India. Thus, while the generics industry saw a rapid growth, creativity and new drug discovery took a hit.
In 1990s, the Indian government led by Prime Minister P. V. Narasimha Rao initiated the economic reforms and opened up the Indian economy to foreign investment . Footnote 16 While this created many opportunities for rapid growth of the economy, it also necessitated India to become a member of the international trade agreements. Indeed, this exposed the limitations of the Indian patent regime on investment, imports, as well as exports of medicines.
As the USA succeeded in the inclusion of patent rights in particular and intellectual property rights (IPRs) in general, in the General Agreement on Tariffs and Trade (GATT) negotiations, India faced new challenges.
Thus, India needed to figure out how to leverage the trade benefits of globalization against its obligation to afford stronger patent protections to MNCs under the TRIPS Agreement , which could threaten its high priority social goals and the interests of the domestic pharmaceutical industry. India feared that the stronger IP protection mechanisms under TRIPS could once again unduly favor the MNCs and may unravel the benefits achieved under the Patents Act of 1970.
Initially, India opposed the strong IP protections which are part of the TRIPS Agreement, leading a group of developing countries with similar reservations. However, India signed the Uruguay Round Agreements (along with 116 other nations) on April 15, 1994, and became a member of the WTO effective January 1, 1995, while continuing advocacy of more equitable provisions. Thus, India became obligated to modify its domestic intellectual property laws in order to comply with the TRIPS Agreement.
The TRIPS Agreement afforded a 10-year grace period to developing countries for configuring their judicial systems and economies, to fully comply with the TRIPS provisions.
5.3 1995–2005
To start with, the Indian government enacted the Patents (Amendments) Ordinance of 1994 to buy time, while statutory changes to the law were pursued in Parliament. Footnote 17 However, this ordinance expired on March 26, 1995, before a permanent legislative solution from the Indian Parliament was put in place for compliance of the TRIPS requirements. Unfortunately, the tenth Lok Sabha was itself dissolved later in 1995, throwing the Indian IPR regime into uncertainty. During this period of political chaos, India was taken to the dispute settlement panel of the WTO by the USA and EU separately that resulted in pronouncements against India. Under the impending threat of trade sanctions, the Indian Parliament then acted rapidly to pass the necessary laws.
Thus, changing the IP Laws for TRIPS Compliance was a big challenge for India once TRIPS Agreement came into force on January 1, 1995. To meet its obligations, India embarked on a substantive overhaul of its patent laws but chose to do so gradually and stagewise. This resulted in three separate patent amendment Acts in 1999, 2002, and 2005 that incrementally modify the Patents Act of 1970 to make it fully TRIPS compliant.
In this context, it is important to note that the Doha Declaration of November 2001 took into consideration the concerns expressed by several emerging and less-developed nations and strengthened the cause of flexibilities under TRIPS. Thus, these flexibilities enabled some WTO member states to alleviate hardships resulting from the need to modify patent laws to TRIPS standards.
The DOHA Declaration (2001): Key Articles
The DOHA Declaration recognized the serious concerns of the least developed countries. Footnote 18 a,b Some of the articles shown below exemplify this:
Ministerial Declaration
(3) We recognize the particular vulnerability of the least-developed countries and the special structural difficulties they face in the global economy . We are committed to addressing the marginalization of least-developed countries in international trade and to improving their effective participation in the multilateral trading system. We recall the commitments made by ministers at our meetings in Marrakesh, Singapore and Geneva, and by the international community at the Third UN Conference on Least-Developed Countries in Brussels, to help least-developed countries secure beneficial and meaningful integration into the multilateral trading system and the global economy. We are determined that the WTO will play its part in building effectively on these commitments under the Work Programme we are establishing. (5) We are aware that the challenges Members face in a rapidly changing international environment cannot be addressed through measures taken in the trade field alone . We shall continue to work with the Bretton Woods institutions for greater coherence in global economic policy-making.
Trade-Related Aspects of IPRs
(17) We stress the importance we attach to implementation and interpretation of the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement) in a manner supportive of public health, by promoting both access to existing medicines and research and development into new medicines and, in this connection , are adopting a separate declaration .
Special and Differential Treatment
(44) We reaffirm that provisions for special and differential treatment are an integral part of the WTO Agreements. We note the concerns expressed regarding their operation in addressing specific constraints faced by developing countries, particularly least-developed countries . In that connection, we also note that some Members have proposed a Framework Agreement on Special and Differential Treatment (WT/GC/W/442). We therefore agree that all special and differential treatment provisions shall be reviewed with a view to strengthening them and making them more precise, effective and operational . In this connection, we endorse the work programme on special and differential treatment set out in the Decision on Implementation-Related Issues and Concerns.
Finally, the India Patents Act 1970 was amended in 2005, so as to Footnote 19 :
Include products in the patentable subject matter category.
Make reverse-engineering or copying of patented drugs without requisite licensing from the patent holder illegal after January 1, 1995. One exception was that the Act did allow the manufacture of generic versions of drugs patented prior to 1995.
Provide a 20-year guaranteed term of protection to patents under Article 32 of TRIPS.
5.4 The Key Provisions That Gained Prominence After 2005
However, the India Patents Act, 1970 (2005), also needed to ensure that (Fig. 2 ):
The new policies did not adversely impact India’s social goals to provide affordable medicines and accessible healthcare to all its citizens ; that means preventing abuse by MNCs.
The interests of India’s generic pharmaceutical industry are considered, while India meets its social goals ; that means FDI and ensuring access to new markets outside India.
The innovation ecosystem is supported by the Indian government to facilitate the Indian pharma industry achieve long-term competitive advantage.
Accordingly, the Indian policy makers decided to (i) invoke 3(d) to prevent Evergreening by MNCs Footnote 20 and (ii) retain certain articles to allow Compulsory Licensing, Footnote 21 so as to ensure its social goals. In addition, India decided to (iii) retain pre-grant opposition and (iv) introduce post-grant opposition. The details are described below.
Prevent Evergreening
Simply stated, “Ever-greening” refers to the different means a pharmaceutical patent holder employs to exploit the legal loopholes of patenting to extend/fortify monopoly typically over blockbuster drugs Footnote 22 by either filing disguised or artful patents on previously patented invention just before the end of the term of the parent patent or employing other related regulatory policies.
According to Kumar and Nanda 22 :
Ever-greening is a strategy employed by the innovator companies to recover high costs incurred by them in Research and Development and as a means to legally protect any minor modifications that are intentionally made to the parent patent just to obtain multiple patents on the same drug and hence extend the overall term of the patent to enjoy monopoly for extended periods of time. In simple words, a company launches a drug product and obtains patent protection for it and just before the end of the term of that patent; the company files a new patent for a minor modification in the original molecule that extends the overall term of patent protection which ultimately contributes to their monopoly. Hence, extending the patent protection period delays or prevents the entry of the generic versions of the drug which can affect the budget for public health and finally the patient.
Companies often seek protection of the following for the purpose of evergreening :
Combinations of two or more drugs
Dosing regimen, dosing rate, and dosing route
Biological targets for a known compound
Delivery profiles, mechanisms of action
Isomeric forms and derivatives
Screening methods
Different treatment methods
Thus, the rationale and tactics for evergreening of blockbuster drugs by MNCs are the following 22 :
Pharmaceutical research and development is an expensive, time consuming and uncertain process that may take 8–10 years to complete. Patent clock starts much before a new drug is approved for marketing and significant amount of time may be lost in the review and approval process by regulatory bodies. So, in order to recoup the considerable time and resources invested in the drug development and approval process , the pharmaceutical companies depend on exclusivity provisions granted by the regulatory bodies . There are several official and unofficial methods to extend term of a patent beyond 20 years . Official methods include provisions by some regulatory bodies such as data exclusivity , orphan drug exclusivity , pediatric exclusivity , the 180-day exclusivity (Hatch Waxman Act, U.S. Food and Drug Administration), Footnote 23 and supplementary protection certificate (European Medical Agency), whereas unofficial methods include altering or reformulating the existing compound to obtain a new patent by utilising polymorphism, creating combinations, stereo-selective/chiral switches, conversion to NDDS, OTC switching, authorised generics , etc.
Evergreening , if unchecked, blocks the generic drugs market, undermines innovation, and prevents access to affordable medicines and accessible healthcare to citizens. Not surprisingly, this would be detrimental to India’s social goals as well as economic goals. Consequently, the following was substituted for clause (d) in Section 3 in the Patents (Amendment) Act, 2005, to discourage or prevent evergreening . Thus, the new Section 3(d) states Footnote 24 :
The mere discovery of a new form of a known substance which does not result in the enhancement of the known efficacy of that substance or the mere discovery of any new property or new use for a known substance or of the mere use of a known process, machine or apparatus unless such known process results in a new product or employs at least one new reactant . Explanation . For the purposes of this clause, salts, esters, ethers, polymorphs, metabolites, pure form, particle size, isomers, mixtures of isomers, complexes, combinations and other derivatives of known substance shall be considered to be the same substance , unless they differ significantly in properties with regard to efficacy .
Aleksandar Ristanić nicely articulated how India leveraged the flexibility afforded by Article 27(1) TRIPS to design 3(d) Footnote 25 a :
First and foremost, Article 27(1) TRIPS requires member states to make patents “ available for any inventions, whether products or processes, in all fields of technology, provided that they are new, involve an inventive step and are capable of industrial application ”. TRIPS thus simply enunciates these essential patent law concepts such as invention , novelty , inventiveness and industrial applicability without defining them , which leaves a considerable discretion to states with how to apply those requirements in their national laws . The way these key terms are defined, however, can be of the utmost importance for both innovation and access to medicine. If a drug is unpatented it is in the public domain and anyone can produce it. 25b An example of taking advantage of freedom and flexibilities under TRIPS is to be found in India’s 2005 amendment to its Patents Act 1970 . While finally allowing the patent protection for pharmaceutical products, India’s patent law, Section 3(d), in particular, limits the number of patents that can protect a drug, providing two important exclusions from the scope of that protection. It excludes from the scope of inventions mere discoveries of (1) new forms of known substances – unless there is an enhancement of the known efficacy of that substance, and (2) new uses for known substances. In addition, the amendment provides a list of substances that would be considered a new form of the same substance unless they differ significantly in properties with regard to efficacy .
Indeed, the US and the EU strongly oppose Section 3(d) of the Patents (Amendment) Act, 2005. However, many other countries – like Argentina, Brazil, China, Indonesia, Malaysia, the Philippines, South Africa, and Thailand – either emulate India’s patent reforms or strongly support them.
Thus, in 2008, the Philippines amended Section 22 of the Republic Act 8293, exactly along the lines of Section 3(d). Argentina revised and restricted the patentability of derivatives of pharmaceutical products, following the example set by 3(d), and going even tougher in certain respects. Mexico revised its patent law precisely adopting the language of 3(d). The same is true of many other countries mentioned. The Novartis vs. Union of India Case Study is instructive of how 3(d) set a precedent in India. Footnote 26 a
5.5 The Novartis vs. Union of India Case Study
Soon after Section 3(d) in the Patents (Amendment) Act, 2005, came into force, the statute was tested. Thus, in 2006, Novartis applied for an Indian patent on the beta crystalline form of imatinib mesylate . Footnote 27 The Madras Patent Office rejected the patent application, citing that imatinib mesylate was a known compound (a pre1990s molecule) and the beta crystalline form was merely a derivative of imatinib mesylate .
Novartis then appealed the Madras Patent Office’s decision to the Intellectual Property Appellate Board (IPAB). IPAB modified the decision of the Patent Office stating that ingredients for grant of patent novelty and nonobviousness may be present in the application but rejected the application on the ground that the drug is not a new substance but an amended version of a known compound. Novartis mounted a separate and concurrent litigation before the Madras High Court arguing that Section 3(d) has violated Article 14 of the Indian Constitution because the definition of “enhanced efficacy” was too vague and was in violation of India’s obligations under the TRIPs Agreement. The High Court ruled that the law was not vague and that the law complied with TRIPS. In upholding the constitutionality of Section 3(d), the Madras High Court noted that 26b : “India, being a welfare and a developing country, which is predominantly occupied by people below poverty line, has a constitutional duty to provide good health care to its citizens by giving them easy access to life saving drugs. In so doing, the Union of India would be right, it is argued, to take into account the various factual aspects prevailing in this big country and prevent ‘evergreening’ by allowing generic medicine to be available in the market.” Thus, it was evident that Novartis could not back up its claim of “enhanced efficacy” for imatinib mesylate over the parent molecule , according to the patentability standards laid down by Section 3(d).
Next, Novartis appealed IPAB’s decision to the Supreme Court of India. Footnote 28 However, the Indian Supreme Court agreed with the IPAB ruling that Novartis had not established the “enhanced therapeutic efficacy” over the parent compound , and thus failed to meet the requirements laid down by Section 3(d). In addition, the Indian Supreme Court opined that the constitutional validity of Section 3(d) was as per the flexibilities offered by TRIPS framework. Footnote 29
Analyzing the Novartis vs. Union of India case , Lukose Footnote 30 noted that the patented drug “Gleevec” by Novartis costed the patients Rs. 4115/per tablet, while its generic version was available at Rs. 30/per tablet, at 99% cost savings to the patient. The differential is even greater in annual costs. Thus, while the annual cost of the Gleevec to patients in India is Rs. 1,500,000, its generic versions costed just Rs. 10,000 annually, a whopping savings of Rs. 1,490,000. Typically, when a patent for a blockbuster drug expires, the price falls up to 95%. Footnote 31
Thus, the Novartis vs. Union of India case makes clear that India will not permit the evergreening of patents, risking its social and economic goals. Indian patent regime also sends a strong message to the world that an extended monopoly to salts, esters, ethers, polymorphs, metabolites, pure form, particle size, isomers, mixtures of isomers, complexes, combinations, and other derivatives of a known substance will not be possible unless they exhibit demonstrably high therapeutic efficacy over the known substance .
Allow Compulsory Licensing
The flexibilities afforded by the TRIPS Agreement (see Articles 30 and 31 below) Footnote 32 allow “compulsory licensing” – in the case of a national emergency, other circumstances of extreme urgency or public health use etc. Thus, compulsory licensing enables other companies to produce a patented product without the permission of the patent holder, under certain conditions .
Exceptions to Rights Conferred
Members may provide limited exceptions to the exclusive rights conferred by a patent , provided that such exceptions do not unreasonably conflict with a normal exploitation of the patent and do not unreasonably prejudice the legitimate interests of the patent owner, taking account of the legitimate interests of third parties.
Other Use Without Authorization of the Right Holder
Where the law of a Member allows for other use of the subject matter of a patent without the authorization of the right holder, including use by the government or third parties authorized by the government , the following provisions shall be respected:
authorization of such use shall be considered on its individual merits ;
such use may only be permitted if, prior to such use, the proposed user has made efforts to obtain authorization from the right holder on reasonable commercial terms and conditions and that such efforts have not been successful within a reasonable period of time. This requirement may be waived by a Member in the case of a national emergency or other circumstances of extreme urgency or in cases of public noncommercial use. In situations of national emergency or other circumstances of extreme urgency, the right holder shall, nevertheless, be notified as soon as reasonably practicable. In the case of public non-commercial use, where the government or contractor, without making a patent search, knows or has demonstrable grounds to know that a valid patent is or will be used by or for the government, the right holder shall be informed promptly;
the scope and duration of such use shall be limited to the purpose for which it was authorized , and in the case of semi-conductor technology shall only be for public noncommercial use or to remedy a practice determined after judicial or administrative process to be anti-competitive;
such use shall be non-exclusive ;
such use shall be non-assignable , except with that part of the enterprise or goodwill which enjoys such use;
any such use shall be authorized predominantly for the supply of the domestic market of the Member authorizing such use ; …. (continued)
TRIPS Agreement empowers individual nations to rightfully exercise the option of compultsory licensing under justifiable socioeconomic circumstances and legitimate needs . Therefore, a compulsory license would ensure much needed access to affordable medicines to all citizens, shielding them from the negative effects of the monopoly of patents. The Natco Pharma vs. Bayer Corporation case exemplifies how India awarded its first compulsory license in 2012. Footnote 33
5.6 The Natco Pharma vs. Bayer Corporation ( Nexavar Compulsory License) Case
Bayer first obtained a US patent (US8609854B2) on the drug sorafenib tosylate in 1999. Following further development, it launched sorafenib tosylate internationally in 2005, under the brand name “Nexavar,” an oncology drug useful for the treatment of advanced stage liver and kidney cancers (Fig. 3 ).
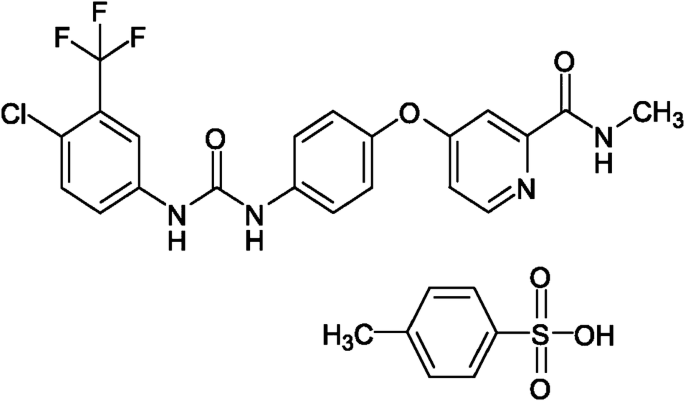
Sorafenib tosylate (“Nexavar”)
In 2008, Bayer obtained the Indian patent (Patent No. 215758) on sorafenib tosylate and launched the drug into the Indian market under the same trade name, “Nexavar,” selling it at Rs. 275,000 (US$ 5500) per patient per month in India. In 2010, Cipla, a well-known Indian generic drug manufacturer, which held one of the largest market shares in India, started selling the generic version of Nexavar at about Rs. 29,000 (US$ 580) per month, a price that is 90% cheaper. In 2011, Bayer sued Cipla for patent infringement. On a different front, the Indian generic drug manufacturer Natco Pharma Limited (“Natco”) applied for compulsory license in India on the sorafenib tosylate patent .
In 2012, the Controller General of Patents Designs and Trademarks of India granted the Indian generic drug manufacturer, Natco Pharma Limited, a compulsory license for Bayer AG’s drug, sorafenib tosylate (“Nexavar”), an oncology drug useful for the treatment of advanced stage liver and kidney cancers.
The compulsory license application of Natco was based on the grounds stated in Section 84(1) of the Indian Patents Act 1970, as amended by Act 15 (2005), Footnote 34 which reads as follows:
84. Compulsory licenses. – (1) At any time after the expiration of three years from the date of the grant of a patent , any person interested may make an application to the Controller for grant of compulsory license on patent on any of the following grounds, namely: (a) that the “reasonable requirements of the public” with respect to the patented invention have not been satisfied , or (b) that the patented invention is “not available to the public at a reasonably affordable price” , or (c) that the patented invention is “not worked in the territory of India .
In a landmark decision on 9 March 2012, the Controller granted the compulsory license to Natco and stripped Bayer of its exclusive right to the medicine in India, citing that the grounds (a)–(c) in Section 84(1) of the Indian Patents Act 1970 (2005) were individually met , though any one of them would have been sufficient for the grant of a compulsory license .
Further, the Controller reasoned that Footnote 35 :
Bayer’s drug was available only to a small percentage of eligible patients (slightly above 2%), which failed to meet the “reasonable requirements of the public.”
The 2012 price of Rs. 280,000 per month (approximately US$5600) to the patient does not meet the condition that the drug must be available to the public at a “reasonably affordable price.” This is based on the purchasing power of patients in India.
Bayer’s patented invention was “not being worked in India” as Nexavar was not manufactured within India and imported from the manufacturing facilities outside India. This did not satisfy the third mandatory requirement.
Natco may sell the drug to patients within India at a price of no more than Rs. 8800 (approximately US$176) per month, which is 97% cheaper than the Bayer’s price.
Natco was required to pay a 6% royalty to Bayer.
Next, Bayer then appealed the decision of the Controller to the IPAB. Pending appeal, Bayer also petitioned for stay of the Controller’s order, which was denied. About a year later, the IPAB upheld the Controller’s decision of granting the compulsory license to Natco with certain changes. Though both the Controller and the IPAB arrived at the same conclusion in the Natco Pharma vs. Bayer Corporation Case , their approaches differed from each other. Thus, while the Controller relied on the statistical data submitted by the parties for analyzing the substantive issues of the case, the IPAB analyzed the issues from the public health perspective in the context of the right to life under Article 21 of the Indian Constitution. Footnote 36
Then, Bayer tried to appeal the IPAB’s decision to the Bombay High Court, which simply refused to take up the case. On 12 December 2014, the Indian Supreme Court finally dismissed Bayer’s petition against the Bombay High Court and ruled in favor of the compulsory license of Nexavar to Natco. The Supreme Court’s judgment fits the established opinion that all the three grounds for compulsory license had been fully met . Once again, being the first of its kind in the history of the Indian patent regime, the Bayer vs. Natco ruling has set a clear precedence for seeking compulsory licenses by other generic pharmaceutical companies in India, as well as by the policy makers and patent regimes of other countries.
Naturally, MNCs strongly opposed the judgment in the Natco Pharma vs. Bayer Corporation and expressed many concerns. Footnote 37 On the other hand, the judgment was hailed by the champions for affordable access to drugs . The case is also notable because it is only the second time in the world that a nation issued a compulsory license for a drug used for treatment of a chronic rather than an infectious disease. Prior to this case, only Thailand awarded compulsory licenses to four drugs between 2006 and 2008. Footnote 38
Retain Pre-grant Opposition
Pre-grant opposition Footnote 39 a was already there in the India Patents Act 1911, 39b and it was retained in the India Patents Act, 1970 (2005). 39c India’s pre-grant opposition procedures in 1911 were modeled similar to the British patent laws in force. Footnote 40 However, during that time, the British patent system was not the only patent system that relied on pre-grant opposition ; many other countries also used a novelty-only examination system coupled with pre-grant opposition Footnote 41 to supplement the resources of the patent examiner.
Thus, Section 25(1) of the India Patents Act, 1970 (2005), provides for pre-grant opposition of pending patent applications. The pre-grant opposition may be based on any number of grounds, Footnote 42 including anticipation, lack of inventive step, non-invention under Section 3 of the Patents Act (including the anti-evergreening provisions of Section 3(d)), insufficient or unclear description of the invention in the specification, failure to disclose the source of biological material used for the invention, and inventions which are considered traditional knowledge .
Notably, Section 25(1) of the India Patents Act, 1970 (2005), does not impose any estoppel limitations on a party who first files a pre-grant opposition and later tries to challenge the patent’s validity in a court proceeding. The Act even mandates that the party filing the pre-grant opposition receive a hearing before the Controller if requested, which will delay the process even if patentability was affirmed over all objections.
The Indian patent regime views that pre-grant opposition is actually helpful to get all the prior art before the patent examiner. Hence, the same patent examiner who examines the initial patent application will also examine the pre-grant opponent’s submission . The Indian Patent Office does not agree that pre-grant opposition would delay the process of patent grants. It is important to note that the Indian generic pharmaceutical companies and the MNCs are on opposite sides of the pre-grant opposition debate. Thus, while the former favor pre-grant opposition , the latter strongly oppose it. Footnote 43
Mueller observes Footnote 44 that according to press reports in March 2006, approximately 100 pre-grant oppositions were pending in the four Indian Patent Office branches, including challenges filed by Indian generic firms against pending patent applications on Astra Zeneca’s cholesterol drug Rosuvastatin , Pfizer’s antifungal drug Voriconazole , Wockhardt’s antibacterial drug Nadifloxacin , Gilead-Roche’s bird-flu drug Oseltamivir , and Astra Aktiebolag’s formulation of ulcer drug Omeprazole . In addition, nongovernmental organizations and healthcare advocacy groups in India have also been using the pre-grant opposition as a powerful tool to challenge the grant of patents on essential medicines.
Introduce Post-grant Opposition
Unlike pre-grant opposition “which already existed” in the Indianpatent law in one form or another, post-grant opposition is a “new addition” to the Patents (Amendment) Act, No. 15 of 2005. The grounds for post-grant opposition are very similar to those of pre-grant opposition , including virtually all patentability criteria. Therefore, to understand the “new” grounds for post-grant opposition , see Section 25 (2) (a)–(k) below of the India Patents Act 1970 (2005) Footnote 45 :
25. Opposition to the patent . (2) At any time after the grant of patent but before the expiry of a period of one year from the date of publication of grant of a patent , any person interested may give notice of opposition to the Controller in the prescribed manner on any of the following grounds, namely – (a) that the patentee or the person under or through whom he claims, wrongfully obtained the invention or any part thereof from him or from a person under or through whom he claims; (b) that the invention so far as claimed in any claim of the complete specification has been published before the priority date of the claim – (i) in any specification filed in pursuance of an application for a patent made in India on or after the 1st day of January, 1912; or (ii) in India or elsewhere, in any other document: Provided that the ground specified in sub-clause (ii) shall not be available where such publication does not constitute an anticipation of the invention by virtue of sub-section (2) or sub-section (3) of section 29; (c) that the invention so far as claimed in any claim of the complete specification is claimed in a claim of a complete specification published on or after the priority date of the claim of the patentee and filed in pursuance of an application for a patent in India, being a claim of which the priority date is earlier than that of the claim of the patentee; (d) that the invention so far as claimed in any claim of the complete specification was publicly known or publicly used in India before the priority date of that claim. Explanation . For the purposes of this clause, an invention relating to a process for which a patent is granted shall be deemed to have been publicly known or publicly used in India before the priority date of the claim if a product made by that process had already been imported into India before that date except where such importation has been for the purpose of reasonable trial or experiment only; (e) that the invention so far as claimed in any claim of the complete specification is obvious and clearly does not involve any inventive step , having regard to the matter published as mentioned in clause (b) or having regard to what was used in India before the priority date of the claim; (f) that the subject of any claim of the complete specification is not an invention within the meaning of this Act , or is not patentable under this Act ; (g) that the complete specification does not sufficiently and clearly describe the invention or the method by which it is to be performed; (h) that the patentee has failed to disclose to the Controller the information required by section 8 or has furnished the information which in any material particular was false to his knowledge; (i) that in the case of a patent granted on a convention application, the application for patent was not made within twelve months from the date of the first application for protection for the invention made in a convention country or in India by the patentee or a person from whom he derives title; (j) that the complete specification does not disclose or wrongly mentions the source and geographical origin of biological material used for the invention; (k) that the invention so far as claimed in any claim of the complete specification was anticipated having regard to the knowledge, oral or otherwise , available within any local or indigenous community in India or elsewhere, but on no other ground.
Unlike pre-grant oppositions, the post-grant oppositions will be heard by a three-person Opposition Board that does not include the original patent examiner. Post-grant opposition is a very important aspect of European Patent Convention (not yet part of the US patent law). In 2017, of all the patents granted under the European Patent Convention, approximately 3.7% were subjected to post-grant oppositions . Footnote 46
It is important to mention that neither the Patents Act nor any other rules require the Controller to (a) notify (in the Official Journal) that a post-grant opposition has been initiated or (b) announce the decision taken and provide the underlying reasons. At this time, it is yet unclear how much the post-grant opposition procedure will be used in India.
6 Conclusion
India is a dominant player in the global generics market, with the largest number of USFDA-approved labs outside the USA, and holding a 30% share (by volume) of the US generics market. However, India has been witnessing increasing competition from other nations such as South Korea and China, who are also trying to establish themselves in the global generics market. Indeed, competition is always better for the patients seeking cheaper medicines and better access to healthcare. Footnote 47 In response to these dynamic market changes, the Indian generic pharmaceutical industry has been strategically repositioning itself. Some highlights are:
Gaining Proficiency in Complex Generics . According to Vijayaraghavan, Footnote 48 complex generics include, “ Complex injectable formulations (liposomal, microsphere-based depot formulations et al), inhalation drugs , topical products and transdermals .” Case and point: Sun Pharma could commence selling Lipodox , a pegylated liposomal doxorubicin formulation (generic of Janssen’s Doxil ), even prior to the patent expiry in the USA due to drug shortage and be the only generic on the market even after the Doxil patent expired.
Focusing on Specialty Portfolios . Indian pharma companies are increasingly focusing on specialty portfolios in specific therapeutic categories. In other words, Indian pharmaceutical companies are actively pursuing the Section 505(b)(2) of USFDA specialty drugs . Footnote 49 This is evident from industry examples such as :
Glenmark’s targeted focus on dermatology, oncology, and respiratory
Dr. Reddy’s focus on Dermatology – through its US subsidiary Promius Pharma
Lupin Pharma’s focus on pediatrics – proprietary portfolio of branded drugs such as Alinia ® and Locoid ® Lotion
Capitalizing on the Abuse Deterrent Opioids Footnote 50 Market . The USFDA’s Opioids Action Plan laid down the new draft guidance in March 2016, for regulation of the generic versions of approved opioids with abuse-deterrent formulations . Footnote 51 Indeed, this attracted the Indian pharmaceutical companies to do business in this space. Further, as the USA is a key market for Indian-made drugs, Indian pharma companies have been innovating to gain a strong foothold in this new market segment. This is evident in Zydus Cadila’s recent acquisition of Sentynl Therapeutics (USA) for $171 million. Currently, Sentynl holds the US market rights for “Abstral,” a unique sublingual abuse deterrent formulation of Fentanyl for cancer pain – a product that has no direct market competition.
Fortifying Domestic R&D and Fostering Innovation . The Indian pharmaceutical companies as well as the policy makers of India have long recognized the absolute need to strengthen domestic R&D and nurture innovation opportunities for creating sustainable competitive advantage . Significant innovation opportunities for India include Footnote 52 3D printing in medical applications across product development and commercial manufacturing, increasing pursuit of drug device combinations for life cycle management and competitive advantage, innovations in biomaterials expanding possibilities, pervasive use of robotics, artificial intelligence and machine learning for developing smart devices, and leveraging Internet of Things (IoT) to progress toward a more connected continuum of care.
Not surprisingly, therefore, India’s policy makers know that they have a critical role to play in fortifying the innovation ecosystem of India. Accordingly, the Government of India declared 2010–2020 as the “Decade of Innovation.” In 2013, the Ministry of Science and Technology unveiled a coherent vision for bringing the different pieces of the Indian innovation ecosystem together, which states Footnote 53 :
The guiding vision of aspiring Indian STI [Science, Technology, and Innovation] enterprise is to accelerate the pace of discovery and delivery of science-led solutions for faster, sustainable and inclusive growth. A strong and viable Science, Research and Innovation System for High Technology-led path for India (SRISHTI) is the goal of the new STI policy.
Therefore, India is embracing many important initiatives such as Make in India Footnote 54 and Startup India Footnote 55 and many others Footnote 56 shown below to meet its socioeconomic goals:
The National Health Protection Scheme (Ayushman Bharat, 2018) . This is the largest government funded healthcare program in the world, expected to benefit 100 million poor families in the country for secondary and tertiary care hospitalization – by providing a cover of up to US$ 7720 per family per year.
Single-window facility to provide consents, approvals, and other information . In March 2018, the Drug Controller General of India announced this to boost the Make in India initiative .
Electronic platform to regulate online pharmacies . The Government of India announced this initiative to stop any misuse of online pharmacies .
Drug Price Control Order and the National Pharmaceutical Pricing Authority . The Government of India introduced these initiatives to ensure the affordability and availability of medicines .
The Government of India’s “Pharma Vision 2020.” This is aimed at making India a global leader in end-to-end drug manufacture with reduced approval time for new facilities.
In support of this vision, the Government of India is striving to create a robust IPR regime that can serve as the bedrock of innovative and competitive India. Indeed, many countries are closely observing the evolution of Indian IPR regime to see how it further leverages the flexibilities offered by TRIPS to advance its own socioeconomic goals while simultaneously promoting its innovation ecosystem and protecting the legitimate business interests of MNCs. Thus, India’s patent reforms are having a global impact. Footnote 57
There are other secondary factors as well. Technology environment depends upon the quality of education, innovation support, and the training the citizens receive; business environment depends upon the investment and policy support for innovative growth of an existing business or new venture creation; policy environment depends upon the socioeconomic goals, science and technology vision, and commitment to innovation.
https://www.ibef.org/industry/pharmaceutical-india.aspx
CAGR, compound annual growth rate.
FY, fiscal year.
https://www.rdmag.com/article/2016/02/intellectual-property-and-indian-pharmaceutical-industry
Here the word “innovations” encompass not only new drug discoveries but also reverse engineering and remaking of old drugs for which patent rights have expired (known as generic drugs ) or frugal innovation of important drugs for which compulsory licenses have been given − in a highly competitive and cost-effective manner .
Mueller, J. M. “The Tiger Awakens: The Tumultuous Transformation of India’s Patent System and the Rise of the Indian Pharmaceutical Innovation,” University of Pittsburgh Law Review, 2006, 68, 491–641.
http://www.ipindia.nic.in/history-of-indian-patent-system.htm
(a) https://www.rdmag.com/article/2016/02/intellectual-property-and-indian-pharmaceutical-industry; (b) http://icrier.org/pdf/India’s_IPR_Regime.pdf ; (c) See Reference 7 as well.
http://shodhganga.inflibnet.ac.in/bitstream/10603/128146/14/07_chapter%202.pdf
Venkataramiah, E.S. Supra Note , 2 , pp. 23.
https://indiankanoon.org/doc/865758/
Draft Manual 2008. Supra Note , 1 , pp. 8 and 9.
a) https://spicyip.com/wp-content/uploads/2013/10/ayyangar_committee_report.pdf , page 23. (b) https://spicyip.com/wp-content/uploads/2013/10/ayyangar_committee_report.pdf , page 18.
(a) http://www.ipindia.nic.in/writereaddata/Portal/IPOAct/1_113_1_The_Patents_Act_1970_-_Updated_till_23_June_2017.pdf . (b) http://www.wipo.int/wipolex/en/text.jsp?file_id=128091
https://qz.com/india/799883/how-narasimha-rao-fixed-the-indian-economy-and-the-congress-party-only-to-be-forgotten/
See Reference 10.
(a) https://www.wto.org/english/tratop_e/dda_e/dohaexplained_e.htm; (b) https://www.wto.org/english/thewto_e/minist_e/min01_e/mindecl_e.pdf
For “Post-TRIPS experience of the generic pharmaceutical industry in India,” see next chapter by B. Dhar.
http://www.ipindia.nic.in/writereaddata/Portal/IPOAct/1_113_1_The_Patents_Act_1970_-_Updated_till_23_June_2017.pdf , page 8
http://www.ipindia.nic.in/writereaddata/Portal/IPOAct/1_113_1_The_Patents_Act_1970_-_Updated_till_23_June_2017.pdf , pages 64–73.
Kumar, A.; Nanda, A. “Ever-greening in Pharmaceuticals: Strategies, Consequences and Provisions for Prevention in USA, EU, India and Other Countries”, Pharmaceutical Regulatory Affairs: Open Access. 6(1), DOI: https://doi.org/10.4172/2167-7689.1000185 (2017).
The “Drug Price Competition and Patent Term Restoration Act of 1984,” also known as the Hatch-Waxman Amendments , established the approval pathway for generic drug products, under which applicants can submit an ANDA under section 505(j) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (see https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ucm613498.htm ).
http://www.ipindia.nic.in/writereaddata/Portal/IPOAct/1_113_1_The_Patents_Act_1970_-_Updated_till_23_June_2017.pdf
(a) http://lup.lub.lu.se/luur/download?func=downloadFile&recordOId=8894039&fileOId=8894040 , page 18. (b) Ho, Cynthia M., Access to Medicine in the Global Economy: International Agreements on Patents and Related Rights, (Oxford University Press, 2011), page 92.
(a) https://www.omicsonline.org/open-access/evergreening-in-pharmaceuticals-strategies-consequences-and-provisions-for-prevention-in-usa-eu-india-and-other-countries-2167-7689-1000185.pdf . (b) High Court of Judicature at Madras for W.P., Novartis AG and another v. Union of India and others, nos. 24759 and 24760 of 2006, 6 August 2007.
Imatinib mesylate is used to treat chronic myeloid leukemia and is marketed by Novartis as “Glivec” or “Gleevec.”
After IPAB rejected the patent application in 2009, Novartis appealed directly to the Supreme Court through a Special Leave Petition under Article 136 of the Indian Constitution. Under normal circumstances, an appeal from IPAB should have been before one of the High Courts before it could proceed to the Supreme Court. However, the patent if granted on appeal would expire by 2018 and thus any further appeal at that stage would be pointless. Considering this urgency and the need for an authoritative decision on Section 3(d), the Supreme Court granted special leave to bypass the High Court appeals process.
Banerjee R. “The Success of, and Response to, India’s Law against Patent Layering.” Harvard Int Law J. 2013, 54 : 205–232.
(a) Lukose, L. “Patent Evergreening and Ethics,” 7th International Conference on Information Law and Ethics , University of Pretoria, South Africa (2016). (b) Proceedings of the 7th International Conference on Information Law and Ethics ICIL, Bottis M. and Alexandropoulos E. (Eds) (2016), ISBN: 978-618-5196-25-7.
https://theconversation.com/explainer-evergreening-and-how-big-pharma-keeps-drug-prices-high-33623
https://www.wto.org/english/docs_e/legal_e/27-trips.pdf
http://lup.lub.lu.se/luur/download?func=downloadFile&recordOId=8894039&fileOId=8894040 , page 33
http://www.wipo.int/edocs/lexdocs/laws/en/in/in065en.pdf
https://www.whitecase.com/sites/whitecase/files/files/download/publications/alerts-indian-patent-office-grants-compulsory-license.pdf; See 84(1) above.
Sood, M. “Natco Pharma Ltd. v. Bayer Corporation and the Compulsory Licensing Regime in India,” NUJS Law Review , 99 (2013), p. 104.
http://lup.lub.lu.se/luur/download?func=downloadFile&recordOId=8894039&fileOId=8894040
https://www.reuters.com/article/us-india-drugs/analysis-india-cancer-ruling-opens-door-for-cheaper-drugs-idUSBRE82C0IN20120313
(a) See Reference 3. This author describes the pre- and post-grant opposition to patents in great detail. (b) Indian Patents and Designs Act, 1911. http://theindianlawyer.in/statutesnbareacts/acts/d42.html , Section 9. (c) See Reference 20, Chapter V, Section 25, page 25.
Janis, M. D. “Patent Abolitionism”, Berkeley Tech. L. J . 899, 903 (2002).
Vojacek, J. A Survey of the Principal National Patent Systems , 28 (1936).
See Section 25(1)(k) for details.
https://economictimes.indiatimes.com/news/economy/policy/what-is-patents-amendment-bill/articleshow/971708.cms (December 27, 2004).
https://core.ac.uk/download/pdf/129759353.pdf
http://www.ipindia.nic.in/writereaddata/Portal/IPOAct/1_113_1_The_Patents_Act_1970_-_Updated_till_23_June_2017.pdf , page 26.
https://www.epo.org/about-us/annual-reports-statistics/annual-report/2017/statistics/searches.html#tab4
https://www.sathguru.com/news/2017/05/03/innovation-in-indian-pharma-empowering-stronger-global-presence-but-fraught-with-challenges-for-serving-indian-market/
https://www.sathguru.com/Publication/download/Medcon-2017-Whitepaper.pdf , page 80. See full details in this reference.
For drugs approved under section 505(b)(2) of USFDA, an NDA must be filed but for which approval can be based in part on the safety and effectiveness of an already-approved drug.
Abuse deterrence is an emerging market segment in the global pharmaceutical industry for extended release and rapid release prescription control substances (opioids) .
See Reference 47, page 17.
See Reference 47, page 7.
https://timreview.ca/article/818
http://www.makeinindia.com/home
https://up.startupindia.gov.in/content/sih/en/home-page.html
http://www.nbr.org/downloads/pdfs/ETA/IIP_kapoor_sharma_workingpaper_070815.pdf
For understanding (a) how China’s patent legislation has impacted the patent protection for pharmaceuticals in China and (b) what China is interested in learning from the Indian example, see the previous chapter on “Indian Patent Law and Its Impact on Pharmaceutical Industry: What China can learn from India?” by Juan HE.
Banerjee, R. (2013). The success of, and response to, India’s law against patent layering. Harvard Int Law Journal, 54 , 205–232.
Google Scholar
Ho, C. M. (2011). Access to medicine in the global economy: International agreements on patents and related rights . New York: Oxford University Press.
Book Google Scholar
Janis, M. D. (2002). Patent abolitionism. Berkeley Tech. L. J., 899 , 903.
Juan, H. E. (2019). Chapter 11: Indian Patent Law and its impact on pharmaceutical industry: What China can learn from India? In Innovation, economic development, and intellectual property in India and China . Springer: Singapore.
Kumar, A., & Nanda, A. (2017). Ever-greening in pharmaceuticals: Strategies, consequences and provisions for prevention in USA, EU, India and other countries. Pharmaceutical Regulatory Affairs: Open Access, 6 (1), 1–6. https://doi.org/10.4172/2167-7689.1000185 .
Article Google Scholar
Lukose, L. (2016). Patent evergreening and ethics . In 7th international conference on information law and ethics, University of Pretoria, South Africa.
Mueller, J. M. (2006). The tiger awakens: The tumultuous transformation of India’s patent system and the rise of the Indian pharmaceutical innovation. University of Pittsburgh Law Review, 68 , 491–641.
Saha, M. (2005). Drug patent: A Viagra for Indian Pharmaceutical Industry. Deccan Herald (Bangalore, India) , April 4.
Sood, M. (2013). Natco Pharma Ltd. v. Bayer Corporation and the compulsory licensing regime in India. NUJS Law Review, 99 , 104.
Vojacek, J. (1936). A survey of the principal national patent systems (p. 28).
Download references
Author information
Authors and affiliations.
Indian Institute of Technology Kanpur, Kanpur, India
Uday S. Racherla
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Uday S. Racherla .
Editor information
Editors and affiliations.
Renmin University of China, Beijing, China
Kung-Chung Liu
Singapore Management University, Singapore, Singapore
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions
Copyright information
© 2019 The Author(s)
About this chapter
Racherla, U.S. (2019). Historical Evolution of India’s Patent Regime and Its Impact on Innovation in the Indian Pharmaceutical Industry. In: Liu, KC., Racherla, U.S. (eds) Innovation, Economic Development, and Intellectual Property in India and China. ARCIALA Series on Intellectual Assets and Law in Asia. Springer, Singapore. https://doi.org/10.1007/978-981-13-8102-7_12
Download citation
DOI : https://doi.org/10.1007/978-981-13-8102-7_12
Published : 07 September 2019
Publisher Name : Springer, Singapore
Print ISBN : 978-981-13-8101-0
Online ISBN : 978-981-13-8102-7
eBook Packages : Law and Criminology Law and Criminology (R0)

Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- My Shodhganga
- Receive email updates
- Edit Profile
Shodhganga : a reservoir of Indian theses @ INFLIBNET
- Shodhganga@INFLIBNET
- Swami Ramanand Teerth Marathwada University
- Department of Law
Items in Shodhganga are licensed under Creative Commons Licence Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0).

Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Arbitrability of Intellectual Property Disputes in India: A Critique

2020, NLS Business Law Review
Arbitration is increasingly becoming the default commercial dispute resolution mechanism across the world. Recognising this, many States are reserving lesser classes of disputes for resolution in courts and are enlarging the scope of arbitrable disputes. One such class of disputes is those concerning intellectual property (IP) rights. In India, the shift has not been taking place in a linear fashion. This paper surveys the law on the topic, explores the non-linear movement towards a liberalized arbitrability regime of IPR disputes, and critically evaluates the law on the subject.
Related Papers
Arnold Stanley
Bond Law Review
tania sebastian
Shivam Goel
With the advent of globalisation, the world has become a global village. Business organisations have expanded themselves beyond borders and hence, there has been a real time increase in cross-border transactions. Agreements and contracts executed between commercial organisations many a times go ugly, thus, giving rise to disputes which are not within the confines of municipal law of a particular country, because the transactions are ‘cross-border’ in nature. Adjudication of cross-border business disputes demand expertise of a different sort, especially when the organisations in dispute hail from nations following different legal systems, as for example common law system and civil law system. In situations like these, redressal of disputes qua 'arbitration' is the most plausible and non-arbitrary solution. If India is to progress in the area of International Commercial Arbitration, the law as laid down by the Parliament and the interpretation given to it by the Apex Court, must coincide. If such a thing doesn’t happen, cross-border investments (FDI) in India will continue to decline, with the countries world over doubting our international integrity, finding India, not “fine-tuning” but rather “musical-chairing” with the ‘interpretative skills’ in regards to legislation enacted; to arbitrarily promote what suits best to its national entities. That said, what else needs to be seen is that, there is no re-circulation back to the days of the 1940 Act, in regards to which the Supreme Court once observed, ‘let not arbitral proceedings be done in a way that will make the lawyers laugh and legal philosophers weep’.
Kanika Ahuja
ILE LAW LETTER
PRASANNA S. , LAVANYA P
The digital age has ushered in a new era of challenges for intellectual property rights (IPR) in India, necessitating a comprehensive re-evaluation of existing legal frameworks. This abstract delves into the multifaceted complexities faced by the Indian legal system in protecting intellectual property in the digital landscape. From issues related to online piracy and digital copyright infringement to the advent of artificial intelligence and its implications on patent law, this abstract highlights the crucial areas where Indian IPR law requires adaptation and innovation. Additionally, it explores the ethical considerations surrounding emerging technologies and the need for harmonizing international standards with India's unique sociocultural milieu. Through an in-depth analysis of case studies and legal precedents, this abstract provides valuable insights into the challenges posed by the digital age and suggests potential strategies to safeguard intellectual property rights in India.
IJAR Indexing
India is a large and a diverse country. It had opened its market in the early 1990s and has embraced the good and the bad of the globalisation process. The globalisation trends as well as the large population exert tremendous pressure on India?s resources and Institutions including the Judicial System. In this scenario the ADR mechanism especially arbitration has proved to be a success resort to dispute resolution. India has acknowledged this fact and has a specific legislation governing the arbitration regime called the Arbitration and Conciliation Act, 1996 which is based upon the UNCITRAL Model Law. India is also a signatory to the New York convention on Recognition and Enforcement of arbitral awards. Acutely conscious of the pace which India should have at international counterparts, it has amended the Arbitration and Conciliation Act, 2015. The amendments were much needed as India was at a cross road, pushing forward a permissive party autonomy regime where courts were to play a minimum interventionist role with a frame work of making, challenging and enforcing awards. Theoretically the system was workable but on a practical front it had become cumbersome. The changes brought by the new legislation are heartening and intents to make India a desired destination for International Commercial Arbitration. The Article will cover the major pro-arbitration changes brought in by the new amended legislation and a critical review of the gaps still left. The article would also try to analyse what efforts are yet to be undertaken to reach to the desired platform which is the shift from Ad-hocism towards Institutionalisation.
The many advantageous facets of international arbitration over litigation is manifested by the increasing use of this method to solve several types of disputes, including intellectual property (“IP”) disputes. Despite many known benefits of arbitration, business parties still hesitate to arbitrate intellectual property related conflicts because the consequent award is still subject to court review, thus susceptible to impediments therefrom. Among which, this paper focuses on the concern of objective arbitrability of disputes related to intellectual property rights (“IPRs”) in order to clear doubts regarding this particularly attractive method of dispute resolution.
Arbitration Brief
IOSR Journal of Humanities and Social Science
Dr. Rajesh Bahuguna
Deep J Francis
Intellectual property rights has emerged as one powerful institution we come across in our daily life. In the era of globalization this institutionhas been transferred throughout the globe through policies. State has been seen as an important driver of economy and regulating policy. Also, there have been numerous debates on diverse interests that back up a policy when introduced into a country. However, the issue becomes serious when it comes to infringement of the sovereignty of the country in decision making. There have been numerous pressures by WTO through the Special 301 report on countries to strengthen their IP regime. Though there have been debates of strength of IPR being diverse across the globe in terms of implementation, such institution transfer do carry along a set of values and interests which may differ across countries. This study attempts to analyze the discourses that have taken place while the patent bill •. was introduced India The study aims at identifying the various interests backing up the IPR policy in India.
RELATED PAPERS
saifulllah qohari
Josef Valenta
JualObatkuatEjakulasidinitahanlamaHajarjahanamdiprobolinggo
Jual obat kuat ejakulasi dini tahan lama hajar jahannam asli
Archives of Oral Biology
Andras Szakal
Nursing Outlook
Barbara Hatcher
Artery Research
Rahil Ahmed
TERDEKAT Cetak Buku Nota
jasa cetak buku
2008 IEEE International Conference on Fuzzy Systems (IEEE World Congress on Computational Intelligence)
DergiPark (Istanbul University)
özlem civelek
The Psychiatrist
Daniel Herlihy
RePEc: Research Papers in Economics
Tuna Mektupları
Aygün ÜLGEN
Circulation: Cardiovascular Imaging
J.-f. Avierinos
Pamela Chebii
Canadian Journal of Public Health
Monique Benoit
Annals of Forest Science
Donato Chiatante
Topoi. Revista de História
BJOG: An International Journal of Obstetrics & Gynaecology
Philippa Middleton
Baburname: stichi neizvestnych avtorov, 2-čast
Nilufar Khadimetova
Drug testing and analysis
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
An International Guide to Patent Case Management for Judges
6.1.2 the justice n rajagopala ayyangar committee report.
In 1957, the Government of India appointed a committee led by a distinguished retired Justice of the Supreme Court of India, Justice N Rajagopala Ayyangar, to examine the revision of the Patents Act and advise the Government in this respect.
The Justice N Rajagopala Ayyangar Committee report stated, in no uncertain terms, that the patent system was a quid pro quo system: the monopoly that a patentee obtains is only in exchange for the disclosure of the invention to the public, free to be used after the monopoly period is over. The quid pro quo, according to the report, also included the obligation on the part of the patentee to work the invention in India. The report also underscored, rather emphatically, that the patent system had failed in India because it had failed to spark the kind of innovation that it sought to encourage – underdeveloped countries could not yield the same result from the patent system as their more developed counterparts could. The patent system was recommended to be continued only because there was no better alternative to achieve better results – in their form at the time, patents were the lesser evil. The report was unequivocal in its apprehension that foreign patentees could misuse the patent system to capture large markets in India at the cost of domestic innovation while simultaneously not investing in the manufacture of the patented product.
The committee’s recommendations were a catalyst for wide changes in Indian patent law, eventually leading to the Patents Act of 1970, replacing the Indian Patents and Designs Act, 1911. The Patents Bill was introduced in 1965 and amended in 1967. The Patents Act, 1970, and Patents Rules, 1972 came into force on April 20, 1972.
Build your custom guide
To create your custom guide, select any combination of the topics and jurisdiction below and then press "Confirm selection".
1. Select topics
2. Select jurisdictions

Patent Law Dissertation Topics in India
30 patent law dissertation topics in India: Topics cover a wide range of issues related to patent law in India, from pharmaceuticals to technology, traditional knowledge to competition law. You can choose a topic that aligns with your interests and expertise.
30 Patent law dissertation topics in India
Sure, here are some content ideas based on the keyword “technology”:
- “The Future of Technology: 5 Emerging Trends to Watch”
- “How Technology is Transforming Industries in the Digital Age”
- “Exploring the Impact of Artificial Intelligence on Society and Technology”
- “The Role of Technology in Improving Healthcare and Patient Outcomes”
- “The Ethical Dilemmas of Technology Advancements: Privacy, Data Security, and More”
- “The Green Revolution: How Technology is Driving Sustainability”
- “The Rise of Blockchain Technology: Decentralization and Beyond”
- “Enhancing Business Productivity Through Automation and Technology Integration”
- “The Promise and Perils of Augmented Reality: Navigating a Virtual World”
- “Understanding the Internet of Things: Connecting Everyday Objects in a Smarter World”
Dissertation Topics
15. “Biopiracy and Protection of Traditional Knowledge in India: Legal Framework and Case Studies.” 16. “Patent Litigation Trends in India: A Comparative Analysis.” 17. “The Intersection of Competition Law and Patent Law in India.” 18. “Geographical Indications and Patent Protection: A Study of Indian Products.” 19. “Pharmaceutical Data Exclusivity in India: Balancing Innovation and Access.” 20. “Patent Valuation and Licensing Strategies in India.” 21. “The Role of Patent Prosecution in Maximizing IP Portfolio Value.” 22. “Plant Varieties Protection and Plant Patents in India: A Comparative Study.” 23. “Standard-Essential Patents (SEPs) and FRAND Licensing in India.” 24. “Patents and Public Health: A Study of Access to Medicines in India.” 25. “Intellectual Property and Startups in India: Challenges and Opportunities.” 26. “The Role of IP Clusters in Promoting Innovation in India.” 27. “AI and Machine Learning Patents in India: Legal and Ethical Aspects.” 28. “Patents and the Indian IT Industry: Trends and Strategies.” 29. “Blockchain Patents in India: Emerging Applications and Legal Issues.” 30. “Sustainable Development Goals (SDGs) and Patents: A Case for India.” These topics cover a wide range of issues related to patent law in India, from pharmaceuticals to technology, traditional knowledge to competition law. You can choose a topic that aligns with your interests and expertise.
Another 10 patent law dissertation topics in India:
31. “The Role of Intellectual Property Rights in the Indian Film and Entertainment Industry.” 32. “Patent Assertion Entities (PAEs) in India: Challenges and Regulatory Responses.” 33. “Blockchain and Intellectual Property Rights: Implications for India’s Digital Economy.” 34. “Patent Filing Strategies for Indian SMEs: A Comparative Analysis.” 35. “The Impact of Patent System Reforms on Innovation in India.” 36. “Antitrust Issues in Patent Licensing and Technology Transfer Agreements in India.” 37. “Patents and Agricultural Biotechnology in India: A Study of Genetically Modified Crops.” 38. “Patent Litigation and Enforcement in India: A Comprehensive Analysis.” 39. “Artificial Intelligence and Inventorship: Legal Implications in Indian Patent Law.” 40. “The Role of Intellectual Property Rights in India’s Clean Energy Transition.” These topics delve deeper into various aspects of patent law and intellectual property in India, including their intersection with entertainment, agriculture, technology, and innovation. You can further refine your choice based on your specific interests and research goals.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.

IMPACT OF PATENT LAW ON ECONOMIC GROWTH OF INDIA : A STUDY
- Dept. of Commerce and Management Studies AKN University, Rajamahendravaram.
- Department of Commerce, Sir C R Reddy College, Eluru.
- Cite This Article as
- Corresponding Author
Technology and knowledge have played a crucial role in the economic growth of world developed economies as well as emerging ones. Intellectual Property despite being clearly identified in most countries has not yet been recognized as an economic asset for most of the developing countries. Intellectual Property Rights protection has the ability to encourage innovation and the formation of a well functioning market system in developing countries, which leads to economic growth. In India, pharmaceutical patents were not granted prior to 1995, which changed after the TRIPS agreement came into effect and brought the amendment of 1995 in the Patent Act, 1970. Section-5 of the Patent Act, 1970 which stated that patent is granted only for methods or processes and not for products was repealed after the amendment of 2005. Today, patent is not only granted for methods or processes but also for pharmaceutical products which has a very huge impact on the growth of the economy. In the present paper examines on impact of Patent Law on economic development in India especially in pharmaceutical company.
- Intellectual Property Rights
- Pharmaceutical Industry
[ Ramesh Moturi and J. Madan Mohan (1970); IMPACT OF PATENT LAW ON ECONOMIC GROWTH OF INDIA : A STUDY Int. J. of Adv. Res. (Jan). 143-146] (ISSN 2320-5407). www.journalijar.com
Download Full Paper
Share this article.

- 한국어 ( Korean )
- 日本語 ( Japanese )
- 中文 ( Chinese (Simplified) )
Law.asia home
Follow law.asia.

- Streaming / Technology
Best Law Dissertation Topics Ideas for University Students
Choosing a dissertation topic is one of the most challenging yet crucial steps for university students pursuing a degree in law. A well-chosen topic not only reflects a student’s interest and passion but also sets the stage for their research journey. If you’re struggling to find the right topic, this blog will provide a comprehensive guide on the best law dissertation topics ideas, ensuring that your dissertation is both impactful and engaging. Furthermore, if you find yourself in need of professional assistance, dissertation writing services and research paper writing services can offer valuable support.
1. Introduction to Law Dissertation Topics
The field of law is vast, encompassing various specializations such as criminal law, international law, corporate law, human rights, environmental law, and more. Selecting a dissertation topic involves balancing personal interest, the scope of the research, and the availability of resources. Here are some tips to help you get started:
- Identify Your Interests: Your dissertation will demand a significant amount of time and effort, so choose a topic that genuinely interests you.
- Consider the Scope: Ensure the topic is neither too broad nor too narrow. A broad topic might be overwhelming, while a narrow topic might lack sufficient research material.
- Review Existing Literature: Familiarize yourself with existing research paper writing service to identify gaps that your dissertation can fill.
- Seek Guidance: Discuss potential topics with your advisors or professors to gain insights and refine your ideas.
Now, let’s delve into some specific law dissertation topics across various specializations.
2. Criminal Law Dissertation Topics
Criminal law is a popular choice among law students due to its dynamic nature and societal relevance. Here are some compelling topics in this field:
2.1 The Effectiveness of Rehabilitation Programs for Juvenile Offenders
This topic explores the impact of rehabilitation programs on juvenile offenders’ reintegration into society and recidivism rates.
2.2 Cybercrime and the Law: A Comparative Study of Legislation in Different Countries
Analyze how different countries address cybercrime, focusing on the effectiveness of their legal frameworks and international cooperation.
2.3 The Role of Forensic Evidence in Securing Convictions in Criminal Cases
Investigate the significance of forensic evidence in criminal trials, including its reliability and challenges.
2.4 The Death Penalty Debate: Ethical, Legal, and Social Perspectives
Examine the arguments for and against the death penalty, considering ethical, legal, and social dimensions.
2.5 Racial Bias in the Criminal Justice System
Study the presence and impact of racial bias in various stages of the criminal justice process, from arrest to sentencing.
3. International Law Dissertation Topics
International law governs the relationships between nations and is crucial in maintaining global order. Here are some intriguing topics in international law:
3.1 The Role of the International Criminal Court in Prosecuting War Crimes
Evaluate the effectiveness of the International Criminal Court (ICC) in prosecuting individuals for war crimes, genocide, and crimes against humanity.
3.2 Climate Change and International Law: Obligations and Challenges
Explore the legal obligations of states under international environmental agreements and the challenges in enforcing these obligations.
3.3 The Protection of Refugees Under International Law
Analyze the legal frameworks for refugee protection, focusing on the 1951 Refugee Convention and the role of UNHCR.
3.4 Maritime Disputes and the United Nations Convention on the Law of the Sea (UNCLOS)
Investigate how UNCLOS addresses maritime disputes, with case studies on specific conflicts.
3.5 Humanitarian Intervention and Sovereignty: A Legal Perspective
Examine the legality and ethical implications of humanitarian intervention in the internal affairs of sovereign states.
4. Corporate Law Dissertation Topics
Corporate law is essential for regulating business operations and ensuring corporate governance. Here are some significant topics in this area:
4.1 Corporate Social Responsibility (CSR) and Its Legal Implications
Study the legal requirements and voluntary practices of CSR, and their impact on corporate governance and stakeholder relations.
4.2 Mergers and Acquisitions: Legal Challenges and Solutions
Explore the legal complexities involved in mergers and acquisitions, including antitrust issues and shareholder rights.
4.3 Insider Trading Regulations: Effectiveness and Enforcement
Investigate the effectiveness of insider trading laws in preventing market manipulation and ensuring fair trading practices.
4.4 Corporate Governance and the Role of Board of Directors
Examine the legal responsibilities of board members in corporate governance and the measures to ensure accountability and transparency.
4.5 The Impact of Brexit on Corporate Law in the UK and EU
Analyze how Brexit has affected corporate law, focusing on regulatory changes and their implications for businesses.
5. Human Rights Law Dissertation Topics
Human rights law aims to protect the fundamental rights and freedoms of individuals. Here are some important topics in this field:
5.1 The Right to Privacy in the Digital Age
Examine the challenges to privacy rights posed by digital technologies and the effectiveness of legal protections.
5.2 Gender Equality and Human Rights: Legal Progress and Challenges
Analyze the progress made in achieving gender equality through legal reforms and the ongoing challenges.
5.3 Human Trafficking: Legal Frameworks and Enforcement Challenges
Study the effectiveness of national and international legal frameworks in combating human trafficking.
5.4 Freedom of Expression vs. Hate Speech: A Legal Dilemma
Explore the legal boundaries between protecting freedom of expression and preventing hate speech.
5.5 The Rights of Indigenous Peoples Under International Law
Investigate the protection of indigenous peoples’ rights under international law, focusing on case studies of specific communities.
6. Environmental Law Dissertation Topics
Environmental law addresses the legal aspects of environmental protection and sustainability. Here are some relevant topics in this domain:
6.1 The Legal Framework for Renewable Energy Development
Examine the laws and policies promoting renewable energy, and their effectiveness in achieving sustainable energy goals.
6.2 Environmental Impact Assessments: Legal Requirements and Practical Challenges
Analyze the role of environmental impact assessments (EIAs) in project planning and the challenges in their implementation.
6.3 Legal Measures to Combat Climate Change: A Comparative Study
Compare the climate change laws of different countries, focusing on their effectiveness and enforcement mechanisms.
6.4 The Role of International Environmental Agreements in Biodiversity Conservation
Study the impact of international agreements, such as the Convention on Biological Diversity, on biodiversity conservation efforts.
6.5 Environmental Justice: Legal Approaches to Addressing Environmental Inequality
Explore the concept of environmental justice and the legal measures to address environmental inequalities affecting marginalized communities.
7. Intellectual Property Law Dissertation Topics
Intellectual property (IP) law protects the creations of the mind, such as inventions, literary works, and trademarks. Here are some exciting topics in IP law:
7.1 The Protection of Traditional Knowledge Under Intellectual Property Law
Investigate the challenges and legal mechanisms for protecting traditional knowledge and cultural expressions.
7.2 Patent Law and Innovation: Balancing Protection and Access
Examine the role of patent law in promoting innovation while ensuring access to essential technologies.
7.3 Copyright in the Digital Age: Challenges and Solutions
Analyze the impact of digital technologies on copyright protection and the legal responses to address these challenges.
7.4 Trademark Law: The Protection of Brand Identity
Study the legal frameworks for trademark protection and their effectiveness in safeguarding brand identity.
7.5 Intellectual Property Rights and the Pharmaceutical Industry
Explore the interplay between intellectual property rights and the pharmaceutical industry, focusing on issues like drug patents and access to medicines.
8. Family Law Dissertation Topics
Family law deals with legal issues related to family relationships, such as marriage, divorce, and child custody. Here are some thought-provoking topics in family law:
8.1 The Legal Recognition of Same-Sex Marriages: Progress and Challenges
Examine the legal status of same-sex marriages in different jurisdictions and the challenges faced in achieving recognition.
8.2 Child Custody Disputes: Best Interests of the Child vs. Parental Rights
Study the legal principles guiding child custody decisions and the balance between the child’s best interests and parental rights.
8.3 Domestic Violence and Legal Protections: An International Perspective
Analyze the legal measures to protect victims of domestic violence and their effectiveness in different countries.
8.4 Surrogacy Laws: Ethical and Legal Considerations
Investigate the legal frameworks governing surrogacy and the ethical issues involved in surrogacy arrangements.
8.5 The Impact of Divorce Laws on Family Dynamics
Explore how different divorce laws affect family relationships and the well-being of family members.
9. Employment Law Dissertation Topics
Employment law regulates the relationship between employers and employees, addressing issues like workplace rights and discrimination. Here are some significant topics in employment law:
9.1 The Legal Protection of Gig Economy Workers
Examine the employment rights of gig economy workers and the legal challenges in ensuring their protection.
9.2 Workplace Discrimination: Legal Remedies and Effectiveness
Study the legal mechanisms to combat workplace discrimination and their effectiveness in promoting equality.
9.3 The Role of Trade Unions in Protecting Workers’ Rights
Analyze the legal status and functions of trade unions in advocating for workers’ rights and interests.
9.4 Occupational Health and Safety Laws: Implementation and Challenges
Investigate the legal requirements for workplace health and safety and the challenges in their enforcement.
9.5 Employee Privacy Rights in the Workplace
Explore the balance between employee privacy rights and employer interests, focusing on legal protections and limitations.
Guarding Secrets: Law Commission Proposal For Trade Secrets Bill 2024
Contributor
Those who forget history are condemned to repeat it, the Battle of Plassey fought in 1757 ,which was the turning point of our Indian history was lost because confidential information and war strategy were leaked to the British by the Indian Mughal Commander. With time, safeguarding and protecting trade secrets and information have become important. According to WIPO trade secrets are “Intellectual Property rights in confidential information which may be sold or licensed” 1 . However, keeping in mind the current economic scenario where everything is driven by technology, maintaining confidentiality with just NDAs and contracts seems difficult. Meeting the challenges head-on and understanding the dire need for a codified law that specifically protects Trade Secrets, the 22nd Law Commission of India headed by Hon'ble Justice Mr. Ritu Raj Awasthi in its 289th Report has proposed the Trade Secrets Bill 2024.
Trade Secrets Bill 2024
In the report Hon'ble Justice Ms. Prathiba M. Singh of the Delhi High Court in her comment highlighted the increase in the number of cases pertaining to Trade Secrets filed before the Delhi IP Division and the need to have a separate law for the same. 2 At present, India does not have a codified law yet that protects trade secrets. So when issues about trade secrets arise courts in the absence of an act have to rely on precedents, interpretation, and judge-made laws to address such problems.
According to Justice Singh, having a clear law on trade secrets would help companies and businesses keep their secrets safe and understand what remedies are available if someone tries to steal their trade secrets. It would also make businesses feel safe about sharing their technology with India. Lastly having a clear law on trade secrets can help India avoid problems when entering into trade agreements with other countries. 3
The Commission studied the TRIPS Agreement and the obligation of India arising out from it. The commission has also explored the best practices followed in other countries like the UK, USA, EU, and Germany, before proposing the Bill. 4
Highlights of the Bill
The newly recommended Bill under Section 2(f) defines Trade Secret as any information that is secret in the sense that it is not generally known among or readily accessible to people, derives commercial value on account of being secret, has been subject to reasonable steps under the circumstances by the holder of such information to keep it secret and disclosure of such information is likely to cause damage to the holder of such information
- Appropriate Jurisdiction
Section 8 of the bill states that all suits on misappropriation of trade secrets should be instituted at the Commercial Courts having jurisdiction.
- Rights of holder of Trade Secrets
Section 3 provides that the holder of the trade secret has the right to use and disclose his trade secrets. It gives the holder of the trade secret the right to institute suits in case of misappropriation or disclosure of the trade secret. Further, it states that if the right holder enters into any contract/agreement to safeguard its trade secret, restricts access, or prevents disclosure of such trade secret then he shall be governed by the provisions of the Indian Contract Act, of 1872.
Section 7 of the Act deals with the relief that a court may grant to the Plaintiff in any suit for misappropriation of Trade Secrets, the reliefs include injunction, damages/accounts of profit, destruction of documents, objects, materials, etc., and pecuniary remedies.
The Law Commission's effort to recommend the Trade Secrets Bill marks as the first step in safeguarding trade secrets through a codified law. If this Bill progresses and is enacted as an Act this would not only support large corporations but also small businesses like MSMEs and start-ups, leading to enhanced economic growth.
1. https://www.wipo.int/tradesecrets/en/
2. 22nd Law Commission of India 289th Report (Part 3) (Para. c)
3. 22nd Law Commission of India 289th Report (Part 3) (Para.d)
4. https://indianexpress.com/article/india/law-panel-head-bats-for-robust-legal-framework-in-india-to-protect-trade-secrets-9199005/
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

Intellectual Property
Mondaq uses cookies on this website. By using our website you agree to our use of cookies as set out in our Privacy Policy.

IMAGES
VIDEO
COMMENTS
The Bakshi Tek Chand Committee Report noted that the existing Indian patent law afforded "inequitably strong IP protection" to MNCs a situation that was blocking the Indian manufacturing industry in its infancy itself. As reported in the Shodhganga - the digital repository of Indian Electronic Theses and Dissertations - setup by the ...
Shodhganga: a reservoir of Indian theses @ INFLIBNET The Shodhganga@INFLIBNET Centre provides a platform for research students to deposit their Ph.D. theses and make it available to the entire scholarly community in open access.
The outlook of the research is a critical newlinestudy of Commercialisation of Patent in the Institutions of Higher Learning and newlinecontemporary conditions prevailing in the universities relating to the university owned newlineand inventor owned patents in and around the world, prominently in the universities newlineacross India.
Journal of Intellectual Property Rights Vol 20, July 2015, pp 210-222 Software Patent in India: A Comparative Judicial and Empirical Overview Ravindra Chingalea† and Srikrishna Deva Raob aNational Law University Delhi, Sector 14, Dwarka, New Delhi - 110078, India bNational Law University Odisha, Sector C - 13, CDA, Cuttack - 753015, Odisha, India
Shodhganga: a reservoir of Indian theses @ INFLIBNET The Shodhganga@INFLIBNET Centre provides a platform for research students to deposit their Ph.D. theses and make it available to the entire scholarly community in open access.
The main research question for this dissertation is "How does the patent law framework of India, the UK, and the USA address the patentability of AI-basedinventions?" The objectives of this study are to: 1. Examine the patent law framework of India, UK, and the USA, with a focus on the patentability of AI-based inventions. 2.
2 Department of Pharmacy, Royal College of Pharmacy, Tatibandh, Raipur, Chhattisgarh 492099, India. Received 9 September 2022 Revised 15 October 2022 Accepted 25 October 2022 Published online 8 ...
I declare that the thesis entitled "Interface between Competition Law and Intellectual Property Law: A study of United States, European Union and Indian Law" for the award of the degree of Doctor of Philosophy is the record of bonafide research carried out by me under the guidance and supervision of Prof. (Dr.) Mamata
The first legislation in India relating to patents was the Act VI of 1856. The Indian Patents and Design Act, 1911 (Act II of 1911) replaced all the previous Acts. The Act brought patent administration under the management of Controller of Patents for the first time. After Independence, it was felt that the Indian Patents & Designs Act, 1911 ...
The history of Patent law in India dates back to 1911 when the Indian Patents and Designs Act, 1911 was enacted. The present Patents Act, 1970 came into force in the year 1972, amending and ...
In a review of the patent and patent information scene in India over the last 25 years, the author highlights the key influences of the Indian Patents Act of 1970 and the accession of India to the ...
4 Kriti Singh, Critical analysis of section 3(d) of Indian patent act, 1970, 3 INT'L J. MULTIDISC. TRENDS, 72, 73-77 (2021) 5 Ms. Sheetal & M.Parakh, Analysis of Patent Law in India, 5 J. EMERGING TECH. & INNOVATIVE RES. (2018) 6 Palak Ratwani, From the Age of Antiquity to the Age of Modernization: An Overview of Patent History in India, 4
DISSERTATION TOPIC Bail and Judicial Discretion: A Critical Analysis Formula One: Trade Secret Infringement and the Controvers : A Critical Stud Patent Regime in India and its Effect on Generic Pharmaceuticals Market Ent : An Anal sis A Comparative Study of Trademark Laws in India and USA Status of Traditional Knowledge under the Patent Law
For a survey on the Indian law on the subject, see, Kshama A Loya and Gowree Gokhale, 'Arbitrability of Intellectual Property Disputes: A Perspective from India' (2019) 14 Journal of Intellectual Property Law & Practice 632-641; Utkarsh Srivastava, 'Putting the Jig Saw Pieces Together: An Analysis of the Arbitrability of Intellectual ...
The committee's recommendations were a catalyst for wide changes in Indian patent law, eventually leading to the Patents Act of 1970, replacing the Indian Patents and Designs Act, 1911. The Patents Bill was introduced in 1965 and amended in 1967. The Patents Act, 1970, and Patents Rules, 1972 came into force on April 20, 1972. The indian ...
30 Patent law dissertation topics in India. Sure, here are some content ideas based on the keyword "technology": "The Future of Technology: 5 Emerging Trends to Watch". "How Technology is Transforming Industries in the Digital Age". "Exploring the Impact of Artificial Intelligence on Society and Technology". "The Role of ...
dissertation submitted in partial fulfilment of requirements for the degree of masters of laws on the topic arbitration as a means to resolve intellectual property disputes under the guidance and supervision of prof. (dr.) mini s submitted by nanda surendran register no: lm0220003 ll.m batch of 2020-21 stream: international trade law
Nishith Desai Associates: Nishith Desai
Intellectual Property Rights protection has the ability to encourage innovation and the formation of a well functioning market system in developing countries, which leads to economic growth. In India, pharmaceutical patents were not granted prior to 1995, which changed after the TRIPS agreement came into effect and brought the amendment of 1995 ...
2301 words (9 pages) Law Dissertation Topic. 3rd Oct 2019 Law Dissertation Topic Reference this In-house law team ... Share this: Facebook Twitter Reddit LinkedIn WhatsApp Intellectual property law, sometimes known as IP Law, governs the ownership and accessibility of ideas and inventions. There are many different ways to protect these ideas ...
CCPIT Patent and Trademark Law Office. Beijing. Tel: +86 10 6604 6247. It is believed that the Supreme People's Court initially started to explore the possibility of introducing a precedent case system in 2005. In the following 10 years, the apex court continued this effort and issued several regulations on guiding cases.
Dissertation in llm dessertation on analysis of patent process in india submitted to pune university towards the fulfillment for the award of diploma in ... Intellectual Property Rights Law, conducted by Marathwada Mitra Mandal's ... In this dissertation an attempt is made by the researcher to throw light on the Role of Patent Act in society ...
Pun e. Abstract - Arbitrability refers to the question of whether a particular dispute can be submitted to arbitration or whether an. arbitral tribunal has jurisdictio n over the subject matter ...
Here are some intriguing topics in international law: 3.1 The Role of the International Criminal Court in Prosecuting War Crimes. Evaluate the effectiveness of the International Criminal Court (ICC) in prosecuting individuals for war crimes, genocide, and crimes against humanity. 3.2 Climate Change and International Law: Obligations and Challenges.
The Law Commission's effort to recommend the Trade Secrets Bill marks as the first step in safeguarding trade secrets through a codified law. If this Bill progresses and is enacted as an Act this would not only support large corporations but also small businesses like MSMEs and start-ups, leading to enhanced economic growth. Footnotes.