- Research article
- Open access
- Published: 04 June 2021

Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
16k Accesses
29 Citations
13 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
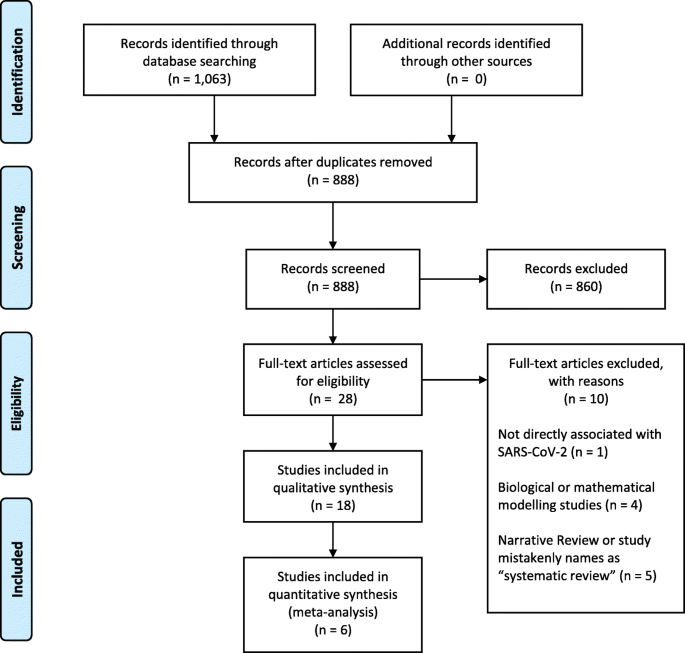
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
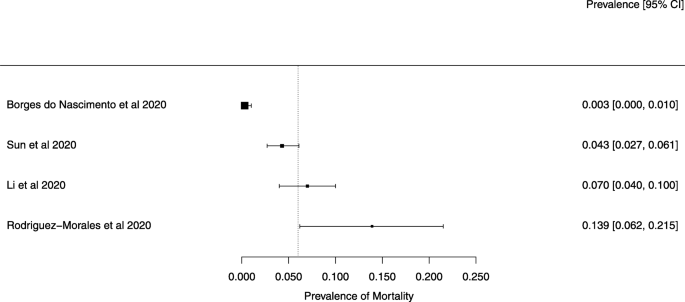
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
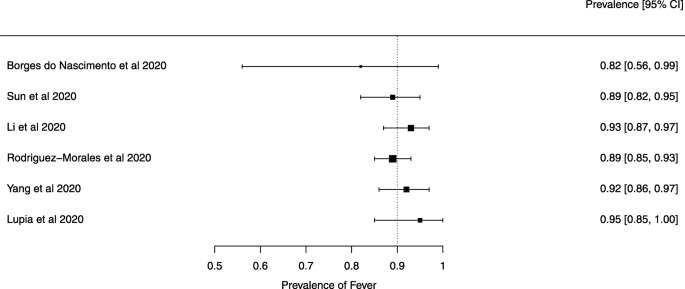
A meta-analysis of the prevalence of fever
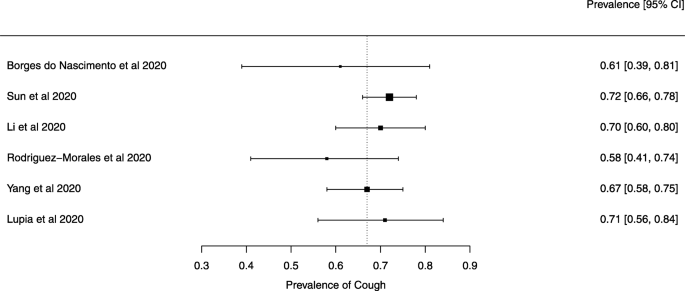
A meta-analysis of the prevalence of cough
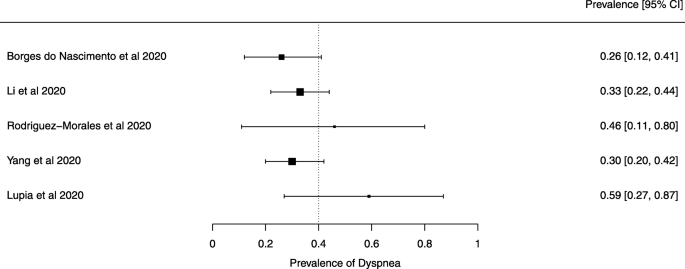
A meta-analysis of the prevalence of dyspnea
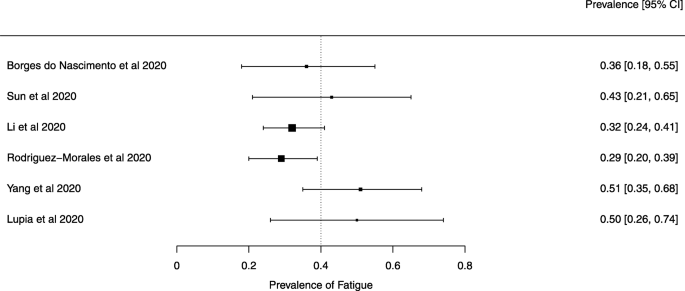
A meta-analysis of the prevalence of fatigue or myalgia
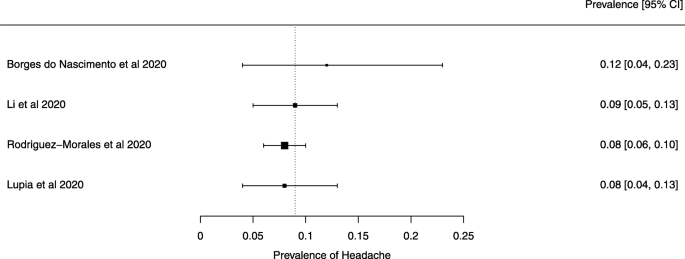
A meta-analysis of the prevalence of headache
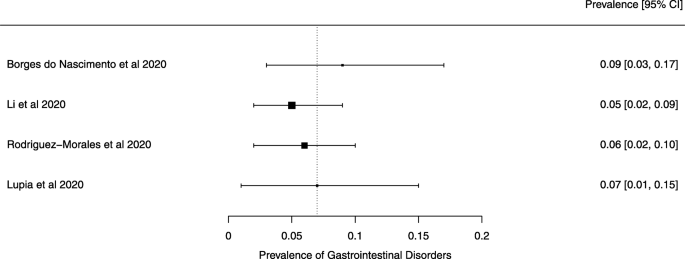
A meta-analysis of the prevalence of gastrointestinal disorders
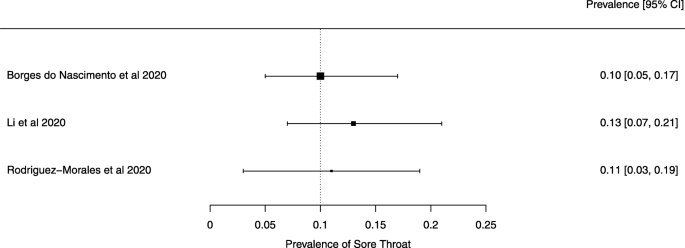
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Euro Surveill
- v.25(15); 2020 Apr 16
Coronavirus disease (COVID-19): a scoping review
1 School of Public Health, Lanzhou University, Lanzhou, China
2 These authors contributed equally to this work and share first authorship
3 Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China
Janne Estill
4 Institute of Global Health, University of Geneva, Geneva, Switzerland
5 Institute of Mathematical Statistics and Actuarial Science, University of Bern, Bern, Switzerland
Mengjuan Ren
Jianjian wang.
6 Department of Health Research Methods, Evidence and Impact, Faculty of Health Sciences, McMaster University, Hamilton, Canada
Xiaohui Wang
7 College of Medical Information Engineering, Chengdu University of Traditional Chinese Medicine, Chengdu, China
8 School of Public Health, Chengdu Medical College, Chengdu, China
9 Department of Respiratory Diseases, Children’s Hospital of Chongqing Medical University, Chongqing, China
10 Chongqing Key Laboratory of Pediatrics, Chongqing, China
Xianzhuo Zhang
11 The First School of Clinical Medicine, Lanzhou University, Lanzhou, China
12 The First Hospital of Lanzhou University, Lanzhou, China
Xiaolong Qi
Yangqin xun, yaolong chen.
13 World Health Organization (WHO) Collaborating Centre for Guideline Implementation and Knowledge Translation, Lanzhou, China
14 Guideline International Network Asia, Lanzhou, China
15 Key Laboratory of Evidence Based Medicine and Knowledge Translation of Gansu Province, Lanzhou University, Lanzhou, China
16 Lanzhou University, an affiliate of the Cochrane China Network, Lanzhou, China
on behalf of the COVID-19 evidence and recommendations working group
17 The study collaborators are acknowledged at the end of the article
Associated Data
In December 2019, a pneumonia caused by a novel coronavirus (SARS-CoV-2) emerged in Wuhan, China and has rapidly spread around the world since then.
This study aims to understand the research gaps related to COVID-19 and propose recommendations for future research.
We undertook a scoping review of COVID-19, comprehensively searching databases and other sources to identify literature on COVID-19 between 1 December 2019 and 6 February 2020. We analysed the sources, publication date, type and topic of the retrieved articles/studies.
We included 249 articles in this scoping review. More than half (59.0%) were conducted in China. Guidance/guidelines and consensuses statements (n = 56; 22.5%) were the most common. Most (n = 192; 77.1%) articles were published in peer-reviewed journals, 35 (14.1%) on preprint servers and 22 (8.8%) posted online. Ten genetic studies (4.0%) focused on the origin of SARS-CoV-2 while the topics of molecular studies varied. Nine of 22 epidemiological studies focused on estimating the basic reproduction number of COVID-19 infection (R 0 ). Of all identified guidance/guidelines (n = 35), only ten fulfilled the strict principles of evidence-based practice. The number of articles published per day increased rapidly until the end of January.
The number of articles on COVID-19 steadily increased before 6 February 2020. However, they lack diversity and are almost non-existent in some study fields, such as clinical research. The findings suggest that evidence for the development of clinical practice guidelines and public health policies will be improved when more results from clinical research becomes available.
Introduction
A new type of coronavirus (severe acute respiratory syndrome coronavirus 2; SARS-CoV-2) that began in Wuhan, China in late 2019 has spread across the world since then. The virus has caused an outbreak of viral pneumonia, which has been named Coronavirus disease (COVID-19). As of 24:00 on 6 February 2020, over 31,000 cases and 636 deaths had been confirmed in China [ 1 ]. Furthermore, more than 1,770,000 cases had been diagnosed in 213 countries, areas or territories as at 13 April 2020 [ 2 ]. On 23 January 2020, Chinese authorities imposed a lockdown of Wuhan [ 3 ]. On 30 January 2020, the World Health Organization (WHO) declared the outbreak a Public Health Emergency of International Concern (PHEIC) [ 4 ] and on 11 March 2020, a pandemic [ 5 ].
The WHO [ 6 - 9 ], the United States (US) Centers for Disease Control and Prevention (CDC) [ 10 , 11 ], the European Centre for Disease Prevention and Control (ECDC) [ 12 , 13 ] as well as Chinese researchers have issued several guidance documents or guidelines to help address the outbreaks. Meanwhile, many scientific journals have rapidly published a number of articles, comments, editorials and perspectives related to COVID-19. It may however be challenging for the global research community to find all the available evidence: many of the first studies on COVID-19 were published in Chinese, and because of the rapidly developing situation, the latest studies are often available on websites or preprint servers only [ 14 ].
Scoping reviews are regarded as a valid tool to map the available evidence on a given topic, to clarify the characteristics of body of literature, to organise the key concepts and their relationship and to analyse knowledge gaps [ 15 ]. The methodology continues to be developed, and a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRSIMA) extension for Scoping Reviews (PRISMA-SCR) including reporting guidance was published in 2018 [ 16 ]. Given the urgency of the COVID-19 epidemic and the need to understand and access information about it, a scoping review was considered suitable for the situation. We therefore conducted this scoping review to help identify research gaps related to this new viral disease and propose recommendations for future research on COVID-19.
Search strategy
We performed a systematic search of MEDLINE via PubMed, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Data and China Biology Medicine (CBM) on 27 February 2020 with the terms “COVID-19” OR “SARS-CoV-2” OR “2019 novel coronavirus” OR “2019-nCoV” OR “Wuhan coronavirus” OR “novel coronavirus” OR “Wuhan seafood market pneumonia virus” OR “Wuhan virus”, published between 1 December 2019 and 6 February 2020 (see Supplement S1 for details of search strategies). Because of potential delays in indexing of databases, we also searched selected infectious disease journals ( Supplementary Table S1 ). We also searched Google Scholar; the official websites of WHO ( https://www.who.int/ ), US CDC ( https://www.cdc.gov/ ), ECDC ( https://www.ecdc.europa.eu/en ), Public Health England (PHE) ( https://www.gov.uk/government/organisations/public-health-england ); some preprint servers, including BioRxiv ( https://www.biorxiv.org/ ), ChemRxiv ( https://chemrxiv.org/ ), medRxiv ( https://www.medrxiv.org/ ) and SSRN ( https://www.ssrn.com/index.cfm/en/ ); and reference lists of the identified articles to find reports of additional studies.
Inclusion and exclusion criteria
We included all literature related to COVID-19 published in English and Chinese between 1 December 2019 and 6 February 2020 without restrictions, including guidance/guidelines, reviews, clinical studies, basic research, epidemiological studies and comments. Documents and guidance/guidelines posted by international organisations, government institutions, associations and societies were also included. We excluded news reports that were not published in scientific journals, and articles where we failed to access full text despite contacting the authors.
Article selection and data extraction
Two reviewers (ML and XL) screened all titles, abstracts and full texts independently and solved disagreements by consensus or consultation with a third reviewer. Then the following information was extracted: (i) title, (ii) first author, (iii) whether peer-reviewed or not, (iv) journal, (v) publication or posted date, (vi) first author’s country (or international organisation), (vii) type of article/study and (viii) topic. The details are shown in Supplementary Table S2 .
Data analysis
We conducted a descriptive analysis of the characteristics of the included literature. We described the source where we found the article, publication date, type of article/study, and topic of article/study or guidance/guideline on COVID-19 to examine the existing gaps in research. We categorised the literature into guidance/guidelines and consensus statements, reviews, clinical studies (including randomised controlled trials and observational studies), basic research, epidemiological studies, editorial comments on COVID-19 and other categories if identified. We conducted this scoping review in accordance with the PRISMA-ScR Checklist [ 16 ] ( Supplementary Table S3 ).
Search results
We identified 1,511 records, 280 of which were excluded as duplicates. Title and abstract screening were conducted for the remaining 1,231 articles, 989 of which were excluded because of being unrelated to COVID-19. For two articles, we failed to access the full text after contacting the authors. We retrieved the full texts of the 242 remaining articles. After further screening and supplementary searching of articles published or posted between 31 January 2020 and 6 February 2020, we identified an additional 42 articles and a total of 249 articles were included in the review ( Figure 1 ).

Flowchart of selection process for the scoping review of coronavirus disease (COVID-19) articles/studies and results, 1 December 2019–6 February 2020
CBM: China Biology Medicine; CNKI: China National Knowledge Infrastructure.
Characteristics of included articles/studies
Of the 249 included articles/studies, 147 (59.0%) were from China. The article/study type varied vastly, which we broadly characterised into 11 types ( Table 1 ). Of these, guidance/guidelines and consensuses statements were the most common (n = 56; 22.5%).
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; WHO: World Health Organization.
a Includes the websites of WHO, United States Centers for Disease Control and Prevention (US CDC), European Centre for Disease Prevention and Control (ECDC) and Public Heath England (PHE), and preprint servers.
b Other than cross-sectional studies.
c Includes reviews and correspondence that discussed the characteristics of the virus in general.
d Other than traditional Chinese medicine.
Sources of articles/studies
Of all included articles/studies, 192 (77.1%) were published in peer-reviewed journals, 35 (14.1%) were posted on preprint servers and 22 (8.8%) were published on the official websites of public health organisations. The journal with the highest number of articles was The Lancet, with 13 (6.8%) published articles. Of preprint articles, most (n = 28) were posted on BioRxiv. Articles published on official websites were mainly COVID-19 guidance/guidelines, including 10 WHO interim guidance documents, nine US CDC interim guidelines/guidance documents, two ECDC guidance documents and one Communicable Diseases Network Australia (CNDA) guideline.
Publication date
Figure 2 shows the cumulative number of articles published daily between 10 January 2020 and 6 February 2020. As at 6 February 2020, the number of articles on COVID-19 had been steadily increasing. Of the 192 articles that were published in peer-reviewed journals, the highest number of journal publications on a single day was on 30 January, with 24 articles (12.5%). For the 35 preprints, the number posted per day rose steadily from 19 January 2020 to 6 February 2020.

Cumulative number of coronavirus disease (COVID-19)-related articles/studies included in the scoping review, 10 January–6 February 2020 (n = 249)
Type of article/study
The types of articles/studies published on each day are shown in Figure 3 . The daily number of guidance/guidelines peaked between 29 January and 3 February whereas the number of published reviews showed an increasing trend since 29 January 2020. Only one systematic review was identified [ 17 ]. We found no randomised controlled studies or cohort studies.

Number of coronavirus disease (COVID-19)-related articles/studies published per day according to type, 10 January–6 February 2020 (n = 249)
a Including cross-sectional studies.
The different types of articles/studies focused on different topics. The basic research could be divided broadly into two categories: 21 genetic studies and 12 molecular biology studies. Ten genetic studies traced the origin of SARS-CoV-2 and tried to determine the possible virus reservoir. Among these, most suggested that SARS-CoV-2 evolved from a bat-CoV, namely bat-SL-CoVZC45, bat-SL-CoVZXC21, bat-SL-CoVZX45 and bat-CoV-RaTG13 as potential candidates [ 18 - 26 ]. However, Ji et al. [ 18 ] found snakes to be the most probable reservoir for SARS-CoV-2 while Guo et al. [ 26 ] suggested mink could be a candidate reservoir. Of the molecular studies, five [ 27 - 31 ] showed that the key receptor of SARS-CoV-2 is angiotensin converting enzyme 2 (ACE2), which is highly expressed in lung type II alveolar cells (AT2) [ 27 ], positive cholangiocytes [ 29 ], upper oesophagus, stratified epithelial cells and absorptive enterocytes from ileum and colon [ 30 ]. The other studies included an assessment of the cross-reactivity of anti-SARS-CoV antibodies with SARS-CoV-2 spike protein [ 32 ], and SARS-CoV-2 main proteases [ 33 , 34 ].
The main topic of epidemiological studies was the estimation of the transmissibility of COVID-19. The value of the basic reproduction number (R 0 ) varied across studies [ 35 - 43 ], however, all estimated it to be higher than one, which indicates the potential for sustained human-to-human transmission. According to the nine articles [ 35 - 43 ], R 0 ranges between 2.2 and 3.9. Some studies showed that the transmissibility of SARS-CoV-2 is comparable to [ 37 , 44 ] or even higher [ 39 ] than SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV). In addition, studies focused on the disease burden associated with COVID-19 [ 45 ] and the global patterns of disease dispersion [ 46 , 47 ].
Most reviews on COVID-19 gave a brief summary of the clinical features [ 48 - 51 ] and the characteristics of SARS-CoV-2 [ 52 - 54 ], as well as recommendations on how to prevent and control [ 55 - 60 ] this novel pneumonia. A systematic review [ 17 ] explored the possibility of using lopinavir/ritonavir (LPV/r) to treat COVID-19, with the results supporting the use of LPV/r as a part of an experimental regimen for COVID-19 pneumonia treatment. Clinical features were reported in 21 studies [ 48 - 51 , 61 - 77 ]. The main symptoms of patients with COVID-19 at onset were found to be fever and cough, with a reduced lymphocyte count, which is similar to previous beta coronavirus infections [ 78 , 79 ].
Seventeen of the 56 editorials, comments and letters [ 80 - 96 ] were first reports or comments on the situation of the COVID-19 epidemic. Some [ 97 - 101 ] also briefly introduced the general information and characteristics of the new virus. The mapping of article/study type and topics, as well as associated gaps, is shown in Table 2 .
a Other than cross-sectional studies.
b Includes perspectives, case-control study and investigation protocols.
c Other than traditional Chinese medicine.
d Guidance/guideline or consensus statement: guidance for laboratory biosafety, caring and travellers, and national capacity review tools; review: reviews on human resources of healthcare, the causes and counter-measures of Wuhan ‘stigma’, and public health; letter: outbreak assessment; epidemiology study: studies on disease burden, the number of unreported cases, and infection fatality; editorial: journal’s opinion on matters related to COVID-19, and incidence rate estimation; cross-sectional study: hazard vulnerability analyses, epidemiology reports, and studies on public attitudes and perception; other: investigation protocol.
Guidance/guidelines and consensus statements
Of the 56 published guidance/guidelines and consensuses statements, 35 were guidance/guidelines. Nine of the 35 addressed the treatment and management of COVID-19 infection, eight addressed prevention and five addressed diagnostics. Ten of the guidance/guidelines were interim guidance documents issued by the WHO, including those on COVID-19 prevention, surveillance, assessment, care, management and mask use [ 6 - 9 , 102 - 107 ]. The US CDC published nine interim guidance/guidelines documents for evaluating, preventing and managing the new coronavirus [ 10 , 11 , 108 - 114 ]. In addition, ECDC published two guidance documents about COVID-19 patient care and the management of persons having had contact with SARS-CoV-2 cases [ 12 , 13 ]. Chinese researches also published 14 rapid-advice guidance/guidelines documents on diagnosis, prevention and management of COVID-19, all of which were interim guidance/guidelines documents developed by hospitals [ 115 - 128 ].
Only eight of the guidance documents/guidelines formed a guideline development group (GDG) [ 129 ]; the recommendations of 15 guidance documents/guidelines, including six developed by the WHO, were difficult to distinguish. Only ten guidance/guidelines fulfilled the strict principles of evidence-based practice and cited reference documents, which were mainly epidemic reports, government documents, and indirect evidence related to SARS-CoV or MERS-CoV [ 6 , 7 , 105 , 116 - 118 , 120 , 122 , 125 , 126 ]. Only two guidelines, both developed by Chinese researchers, were graded using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [ 116 , 117 ]. Among the 35 guidance/guidelines, one [ 115 ] was completely on Traditional Chinese medicine and one [ 116 ] covered Chinese medicine. One Australian guideline [ 130 ] was adapted from SARS-CoV guidelines.
Our scoping review shows that while the number of articles on COVID-19 has been constantly increasing, as at 6 February, there were still clear gaps in several study types and research fields. We identified that some study types, in particular randomised controlled trials and cohort studies, were still non-existent before 6 February. According to a preliminary search of the Cochrane Network database up to 10 April 2020, the number of randomised controlled trials (RCTs) (n = 8) and observational studies (n = 42) still remains low [ 131 ].
We also found that there were only a few studies on clinical practice, making it difficult to develop clinical practice guidelines and health policies. The reason for the gaps in this area may be the rapid development of the outbreak and limited understanding of the new virus and the disease caused by it. Moreover, it takes time to conduct clinical research. When facing a public health emergency with a previously unknown cause, researchers should conduct studies on whether some clinical practice and public health interventions from other public health emergencies can be used as indirect evidence. However, we identified no such studies in our review.
We found that 14% of the studies related to COVID-19 were posted on preprint servers. This approach of sharing research as quickly as possible is very reasonable, especially in the case of such public health emergency. Previous studies have shown that preprints can accelerate progress in handling outbreaks of infectious disease [ 132 , 133 ].
The research topics in different types of articles/studies had both similarities and differences. Basic research was mostly focused on exploring the origin and reservoirs of the new virus, while epidemiological studies mainly focused on its transmissibility. Reviews and reports provided more general information of the virus and the outbreak, while guidance/guidelines included recommendations on how to prevent and control it.
Clinical practice guidelines are statements that include recommendations intended to optimise patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options [ 134 ]. Clinical practice guidelines can inform healthcare workers' actions [ 134 ], and, especially when public health emergencies occur, rapid advice guidelines can guide clinicians in terms of how to perform related work [ 135 ]. After the outbreak of COVID-19, the WHO, US CDC and ECDC released guidance/guidelines as soon as possible, as did several Chinese institutions. However, most of these documents did not establish formal guideline development groups, and they did not fulfil the strict principles of evidence-based practice. For example, most guidance/guidelines did not grade the quality of evidence and strength of recommendations, and thus owed to the emerging crisis, such guidance/guidelines need to be considered with these limitations in mind. In 2007, the WHO published guidance about the process of developing rapid advice guidelines [ 129 ], stating that when a public health emergency occurs, a rapid review is needed and the development time should not exceed 6 months [ 135 ]. However, considering the limited time to set up panels, this could be a challenge for guidance/guideline developers. Nonetheless, we still expect guidance/guideline developers to establish formal development groups and fulfil the evidence-based practice principles.
Our scoping review can help researchers identify research gaps so as to conduct research to fill these gaps. For example, in the current situation, a systematic review to estimate the incubation period or research on new drugs or treatments, would be of great importance. This scoping review has several strengths. We performed a systematic search of a comprehensive set of sources, including databases, preprint servers, and official websites of international organisations and associations at the early stage of the pandemic. Furthermore, our large sample size is sufficient to illustrate the state of research and identify research gaps related to COVID-19 at the onset of the pandemic.
This study also has some limitations. Because of the delay in indexing, some articles published as at 6 February 2020 may not have been identified. Also, because our retrieval time was only until this date, articles published or posted after this date, of which there have been many, have not been included in the analysis. As some preprints, guidance/guidelines and disease control plans are constantly updated, the publication date we extracted may not be the time of their first publication time. Also, we did not assess the quality of the included literature because of diversity of the types of included articles. Another limitation of our study was that it only included articles published in English and Chinese, which could introduce publication bias. However, as the epidemic was most heavily affecting China until early February, it is reasonable to expect that literature published in English and Chinese up until this point in time covered the majority of the available knowledge. Finally, we were unable to access the full texts of two articles despite contacting the authors. However, compared with the total number of articles included in the review, we anticipate that the exclusion of these two articles is unlikely to have a major impact.
This scoping review shows the state of literature published or posted online related to COVID-19 as at 6 February 2020. The number of articles in this field has steadily increased since the outbreak became evident. However, the types of studies lacked diversity, especially clinical studies. More clinical research is needed, but in the rapidly evolving global pandemic, we encourage researchers to continuously review the latest literature, to take into account the latest available evidence and avoid overlapping work, and to improve evidence for the development of clinical practice guidelines and public health policies.
Acknowledgements
Funding statement: 2020 Key R & D project of Gansu Province; Special funding for prevention and control of emergency of COVID-19 from Key Laboratory of Evidence Based Medicine and Knowledge Translation of Gansu Province (No. GSEBMKT-2020YJ01).
The members of the COVID-19 evidence and recommendations working group: Xiao Liu (Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China); Nan Yang (Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China); Shuya Lu (Sichuan Provincial People’s hospital, Chengdu, China ); Peipei Du ( School of Public Health, Chengdu Medical College, Chengdu, China); Yanfang Ma (Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China); Zijun Wang (Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China); Qianling Shi (The First School of Clinical Medicine, Lanzhou University, Lanzhou, China); Hairong Zhang (Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China); Qiangqiang Guo (School of Public Health, ShanXi Medical University, Taiyuan,China); Yuting Yang (Children's Hospital of Chongqing Medical University, Chongqing, China); Bo Yang (Children's Hospital of Chongqing Medical University, Chongqing, China); Shouyuan Wu (School of Public Health, Lanzhou University, Lanzhou, China); Xiaoqin Wang (Michael G. DeGroote Institute for Pain Research and Care, McMaster University, Hamilton, Ontario, Canada).
Supplementary Data
Conflict of interest: None declared.
Authors’ contributions: All authors have read and agree to the published version of the manuscript. Conceptualisation, YC and XW; methodology, ML, XL and JE; software, YL, MR and JW; data extraction, QW, SZ, MR, XZ, LW, QZ and SY; formal analysis, XL and ML; resources, ML and WL; writing—original draft preparation, ML, XL, WM and XQ; writing—review and editing, YX, XY, YC, XW, SY, XF, WM, JE, EL and XQ; visualisation, ML and XL; supervision, YC and XW; project administration, YC; funding acquisition, YC.
Experience, Perceptions, and Recommendations Concerning COVID-19-Related Clinical Research Adjustments
Affiliations.
- 1 1Department of Internal Medicine, Division of Hematology-Oncology.
- 2 2Harold C. Simmons Comprehensive Cancer Center, and.
- 3 3Department of Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, Texas.
- PMID: 33027755
- PMCID: PMC8173586
- DOI: 10.6004/jnccn.2020.7643
Background: During the COVID-19 public health emergency, the FDA and NIH altered clinical trial requirements to protect participants and manage study conduct. Given their detailed knowledge of research protocols and regular contact with patients, clinicians, and sponsors, clinical research professionals offer important perspectives on these changes.
Methods: We developed and distributed an anonymous survey assessing COVID-19-related clinical trial adjustment experiences, perceptions, and recommendations to Clinical Research Office personnel at the Harold C. Simmons Comprehensive Cancer Center. Responses were compared using the Fisher exact test.
Results: A total of 94 of 109 contacted research personnel (87%) responded. Among these individuals, 58% had >5 years' professional experience in clinical research, and 56% had personal experience with a COVID-19-related change. Respondents perceived that these changes had a positive impact on patient safety; treatment efficacy; patient and staff experience; and communication with patients, investigators, and sponsors. More than 90% felt that positive changes should be continued after COVID-19. For remote consent, telehealth, therapy shipment, off-site diagnostics, and remote monitoring, individuals with personal experience with the specific change and individuals with >5 years' professional experience were numerically more likely to recommend continuing the adjustment, and these differences were significant for telehealth (P=.04) and therapy shipment (P=.02).
Conclusions: Clinical research professionals perceive that COVID-19-related clinical trial adjustments positively impact multiple aspects of study conduct. Those with greatest experience-both specific to COVID-19-related changes and more generally-are more likely to recommend that these adjustments continue in the future.
Publication types
- Research Support, N.I.H., Extramural
- Biomedical Research / standards*
- COVID-19 / prevention & control*
- COVID-19 / virology
- Delivery of Health Care / standards*
- Interdisciplinary Communication*
- Practice Guidelines as Topic / standards*
- SARS-CoV-2 / isolation & purification*
- Surveys and Questionnaires
- Telemedicine / methods*
Grants and funding
- UL1 TR001105/TR/NCATS NIH HHS/United States
- UG1 CA233302/CA/NCI NIH HHS/United States
- P30 CA142543/CA/NCI NIH HHS/United States
- K24 CA201543/CA/NCI NIH HHS/United States
- T32 CA124334/CA/NCI NIH HHS/United States
- NICE Guidance
- Conditions and diseases
- Antimicrobial stewardship
COVID-19 rapid guideline: managing COVID-19
NICE guideline [NG191] Published: 23 March 2021 Last updated: 08 May 2024
- Tools and resources
- 1 Communication and shared decision making
- 2 Assessment
- 3 Management
- 4 Therapeutics for COVID-19
- 5 Preventing and managing acute complications
- 6 Identifying and managing co-infections
- 7 Follow‑up and rehabilitation
- 8 Palliative care
- Terms used in the guideline
Recommendations for research
- Finding more information and expert advisory panel details
- Update information
1 Vitamin D for treating COVID-19
2 awake prone positioning, 3 antifungal treatments for covid-19-associated pulmonary aspergillosis (capa), 4 patient experience of capa diagnosis and management, 5 diagnosing capa, 6 outcomes for capa, 7 risk factors for capa, 8 budesonide for covid-19, 9 multidisciplinary team agreed approach to continuous positive airway pressure (cpap) weaning times, 10 high-flow nasal oxygen (hfno) for covid-19 and respiratory failure, 11 low molecular weight heparins (lmwhs) for venous thromboembolism (vte) prophylaxis, 12 extended pharmacological vte prophylaxis.
- 13 Standard- versus intermediate-dose VTE prophylaxis for COVID‑19
What is the clinical effectiveness and safety of vitamin D for treating COVID-19 in children, young people and adults?
For a short explanation of why the committee made this recommendation for research, see the rationale section on vitamin D .
Full details of the evidence and the committee's discussion are in evidence review N: vitamin D .
What is the effectiveness of awake body positioning in improving outcomes for people in hospital with COVID-19 who are not intubated and have higher oxygen needs?
For a short explanation of why the committee made this recommendation for research, see the rationale section on early treatment escalation planning for non-invasive respiratory support .
Full details of the evidence and the committee's discussion are in evidence review G: prone positioning .
What are the clinical and cost effectiveness, and the safety, of specific antifungal treatments for treating suspected or confirmed CAPA, and the optimal treatment duration? When should treatment be started, stopped or modified?
For a short explanation of why the committee made this recommendation for research, see the rationale section on treating CAPA .
Full details of the evidence and the committee's discussion are in evidence review K: CAPA – effectiveness and safety of treatments .
What are the views, preferences and experiences of people with CAPA, and their families or carers, on: available tests for diagnosing CAPA and available treatments for CAPA?
Full details of the evidence and the committee's discussion are in:
evidence review J: CAPA – diagnostics
evidence review K: CAPA – effectiveness and safety of treatments .
In people with suspected CAPA, what are the most accurate tests for diagnosing the infection and when should they be done?
For a short explanation of why the committee made this recommendation for research, see the rationale section on diagnosing CAPA .
Full details of the evidence and the committee's discussion are in evidence review J: CAPA – diagnostics .
What are the possible outcomes for people who are critically ill and have CAPA?
What risk factors in people who are critically ill and have, or have had, COVID-19 as part of their acute illness are associated with developing CAPA?
Full details of the evidence and the committee's discussion are in evidence review I: CAPA – risk factors and signs and symptoms .
What is the clinical and cost effectiveness of budesonide for treating COVID-19 in the community in adults, young people and children?
For a short explanation of why the committee made this recommendation for research, see the rationale section on budesonide (inhaled) .
Full details of the evidence and the committee's discussion are in evidence review E: inhaled budesonide .
Does a multidisciplinary team agreed approach to weaning from CPAP improve weaning times and result in stopping CPAP for people with COVID-19 and acute respiratory failure?
For a short explanation of why the committee made this recommendation for research, see the rationale section on delivering non-invasive respiratory support .
Full details of the evidence and the committee's discussion are in evidence review H: respiratory support strategies .
Is HFNO effective in reducing breathlessness compared with standard care or conventional oxygen therapy for people in hospital with COVID-19 and respiratory failure when it is agreed that treatment will not be escalated beyond non-invasive respiratory support or palliative care is needed?
What is the effectiveness and safety of a treatment dose with an LMWH compared with a standard prophylactic dose for VTE prophylaxis in young people under 18 years with COVID-19?
For a short explanation of why the committee made this recommendation for research, see the rationale section on in-hospital prevention and management of venous thromboembolism (VTE) prophylaxis .
Full details of the evidence and the committee's discussion are in evidence review D: VTE prevention .
What is the effectiveness and safety of extended pharmacological VTE prophylaxis for people who have been discharged after treatment for COVID-19?
13 Standard- versus intermediate-dose VTE prophylaxis for COVID‑19
What is the effectiveness and safety of standard-dose compared with intermediate-dose pharmacological VTE prophylaxis for people with COVID-19, with or without additional risk factors for VTE?
For a short explanation of why the committee made this recommendation for research, see the rationale section on people with COVID-19 and additional risk factors .
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
CDC updates and simplifies respiratory virus recommendations
Recommendations are easier to follow and help protect those most at risk
For Immediate Release: Friday, March 1, 2024 Contact: Media Relations (404) 639-3286
CDC released today updated recommendations for how people can protect themselves and their communities from respiratory viruses, including COVID-19. The new guidance brings a unified approach to addressing risks from a range of common respiratory viral illnesses, such as COVID-19, flu, and RSV, which can cause significant health impacts and strain on hospitals and health care workers. CDC is making updates to the recommendations now because the U.S. is seeing far fewer hospitalizations and deaths associated with COVID-19 and because we have more tools than ever to combat flu, COVID, and RSV.
“Today’s announcement reflects the progress we have made in protecting against severe illness from COVID-19,” said CDC Director Dr. Mandy Cohen. “However, we still must use the commonsense solutions we know work to protect ourselves and others from serious illness from respiratory viruses—this includes vaccination, treatment, and staying home when we get sick.”
As part of the guidance, CDC provides active recommendations on core prevention steps and strategies:
- Staying up to date with vaccination to protect people against serious illness, hospitalization, and death. This includes flu, COVID-19, and RSV if eligible.
- Practicing good hygiene by covering coughs and sneezes, washing or sanitizing hands often, and cleaning frequently touched surfaces.
- Taking steps for cleaner air , such as bringing in more fresh outside air, purifying indoor air, or gathering outdoors.
When people get sick with a respiratory virus, the updated guidance recommends that they stay home and away from others. For people with COVID-19 and influenza, treatment is available and can lessen symptoms and lower the risk of severe illness. The recommendations suggest returning to normal activities when, for at least 24 hours, symptoms are improving overall, and if a fever was present, it has been gone without use of a fever-reducing medication.
Once people resume normal activities, they are encouraged to take additional prevention strategies for the next 5 days to curb disease spread, such as taking more steps for cleaner air, enhancing hygiene practices, wearing a well-fitting mask, keeping a distance from others, and/or getting tested for respiratory viruses. Enhanced precautions are especially important to protect those most at risk for severe illness, including those over 65 and people with weakened immune systems. CDC’s updated guidance reflects how the circumstances around COVID-19 in particular have changed. While it remains a threat, today it is far less likely to cause severe illness because of widespread immunity and improved tools to prevent and treat the disease. Importantly, states and countries that have already adjusted recommended isolation times have not seen increased hospitalizations or deaths related to COVID-19.
While every respiratory virus does not act the same, adopting a unified approach to limiting disease spread makes recommendations easier to follow and thus more likely to be adopted and does not rely on individuals to test for illness, a practice that data indicates is uneven.
“The bottom line is that when people follow these actionable recommendations to avoid getting sick, and to protect themselves and others if they do get sick, it will help limit the spread of respiratory viruses, and that will mean fewer people who experience severe illness,” National Center for Immunization and Respiratory Diseases Director Dr. Demetre Daskalakis said. “That includes taking enhanced precautions that can help protect people who are at higher risk for getting seriously ill.”
The updated guidance also includes specific sections with additional considerations for people who are at higher risk of severe illness from respiratory viruses, including people who are immunocompromised, people with disabilities, people who are or were recently pregnant, young children, and older adults. Respiratory viruses remain a public health threat. CDC will continue to focus efforts on ensuring the public has the information and tools to lower their risk or respiratory illness by protecting themselves, families, and communities.
This updated guidance is intended for community settings. There are no changes to respiratory virus guidance for healthcare settings.
### U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
Whether diseases start at home or abroad, are curable or preventable, chronic or acute, or from human activity or deliberate attack, CDC’s world-leading experts protect lives and livelihoods, national security and the U.S. economy by providing timely, commonsense information, and rapidly identifying and responding to diseases, including outbreaks and illnesses. CDC drives science, public health research, and data innovation in communities across the country by investing in local initiatives to protect everyone’s health.
To receive email updates about this page, enter your email address:
- Data & Statistics
- Freedom of Information Act Office
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines

Coronavirus (COVID-19): What Do I Do If I Feel Sick?
Reviewed By:

Lisa Lockerd Maragakis, M.D., M.P.H.
If you have a cough, a fever or difficulty breathing, and you are worried that you may have COVID-19, here are recommendations from Lisa Maragakis, M.D., M.P.H., senior director of infection prevention at Johns Hopkins, on what to do, step by step.
Coronavirus: What do I do if I Feel Sick?

If you are concerned that you may have COVID-19, follow these steps to help protect your health and the health of others.
1. Stay home and call a health care provider
Unless it is an emergency, to reduce your risk of catching or spreading illness, stay home if you feel sick, even if your symptoms are mild. Do not go to work, school or public places, and avoid public transportation.
If your symptoms are severe or you feel like you need medical care, call before you go to your health care provider. Describe your symptoms over the phone.
If you have a medical emergency, call 911 and tell the dispatcher about your symptoms.
2. Answer questions to determine your risk
When you call a health care facility, you will be asked about your risks for COVID-19. Risk factors include being unvaccinated, attending indoor events, recent travel to certain areas or exposure to an infected person.
For instance, people calling Johns Hopkins Health System hospitals or clinics are asked:
- Have you had close contact with someone diagnosed with COVID-19, the disease caused by the new coronavirus? (Close contact means having been within 6 feet of that person for an extended time or being exposed to their cough or sneeze.)
- Do you have a cough, fever or chills, shortness of breath or difficulty breathing, muscle or body aches, sore throat, new loss of taste or smell, diarrhea, headache, new fatigue, nausea or vomiting, or congestion or runny nose?
- Has a public health officer said you were potentially exposed to COVID-19?
3. Follow your health care provider’s instructions
Based on your answers to these questions, the care provider will provide instructions over the phone. You will be told if you need to be evaluated, and if so, what to do next. Based on your risk for COVID-19, your health care provider may recommend that you:
- Continue to monitor your health and call back if you develop a fever or respiratory symptoms.
- Stay home and await further instructions.
- Report to a designated medical care facility for evaluation and treatment. It’s best to go alone to your appointment. Do not bring children or other family members unless you need assistance.
- Go to a clinic or emergency department if you have more severe symptoms, such as shortness of breath.
4. Practice hand hygiene and respiratory etiquette
- If you do leave your home to go to a care facility, wear a mask so droplets from your breath, coughs and sneezes are less likely to infect others.
- Wash your hands thoroughly (for at least 20 seconds) after sneezing, blowing your nose, coughing or using the bathroom, and before preparing or eating food.
- If you cough or sneeze, do so into the bend of your elbow, not your hand. Or use a tissue, and then throw it away immediately afterward.
- At home, clean often-touched surfaces such as doors and doorknobs, cabinet handles, bathroom hardware, tabletops, phones, tablets and keyboards regularly with disinfectant.
Learn other ways to protect yourself and others from COVID-19 .
5. Stay calm
The possibility of having a contagious illness can be scary, but doctors, nurses and other caregivers can help provide care to patients while avoiding spread of the illness in the community.
6. Consider being vaccinated for COVID-19
Once you feel better, talk to your doctor or health professional about getting vaccinated for the coronavirus. The COVID-19 vaccines are safe and effective, and the U.S. Centers for Disease Control and Prevention (CDC) recommends that people who have already had COVID-19 or tested positive should still get the COVID-19 vaccination. Studies show that vaccination provides a strong boost in protection in people who have recovered from COVID-19.
For Johns Hopkins Patients
Learn how we are adapting our care practices to avoid spread of COVID-19.

Trauma Team Puts an Athlete Back in the Saddle

Patient Safety Infographic
Coronavirus: Younger Adults Are at Risk, Too
Related Topics
- Open access
- Published: 14 May 2024
Rain, rain, go away, come again another day: do climate variations enhance the spread of COVID-19?
- Masha Menhat 1 ,
- Effi Helmy Ariffin ORCID: orcid.org/0000-0002-8534-0113 2 ,
- Wan Shiao Dong 3 ,
- Junainah Zakaria 2 ,
- Aminah Ismailluddin 3 ,
- Hayrol Azril Mohamed Shafril 4 ,
- Mahazan Muhammad 5 ,
- Ahmad Rosli Othman 6 ,
- Thavamaran Kanesan 7 ,
- Suzana Pil Ramli 8 ,
- Mohd Fadzil Akhir 2 &
- Amila Sandaruwan Ratnayake 9
Globalization and Health volume 20 , Article number: 43 ( 2024 ) Cite this article
114 Accesses
3 Altmetric
Metrics details
The spread of infectious diseases was further promoted due to busy cities, increased travel, and climate change, which led to outbreaks, epidemics, and even pandemics. The world experienced the severity of the 125 nm virus called the coronavirus disease 2019 (COVID-19), a pandemic declared by the World Health Organization (WHO) in 2019. Many investigations revealed a strong correlation between humidity and temperature relative to the kinetics of the virus’s spread into the hosts. This study aimed to solve the riddle of the correlation between environmental factors and COVID-19 by applying RepOrting standards for Systematic Evidence Syntheses (ROSES) with the designed research question. Five temperature and humidity-related themes were deduced via the review processes, namely 1) The link between solar activity and pandemic outbreaks, 2) Regional area, 3) Climate and weather, 4) Relationship between temperature and humidity, and 5) the Governmental disinfection actions and guidelines. A significant relationship between solar activities and pandemic outbreaks was reported throughout the review of past studies. The grand solar minima (1450-1830) and solar minima (1975-2020) coincided with the global pandemic. Meanwhile, the cooler, lower humidity, and low wind movement environment reported higher severity of cases. Moreover, COVID-19 confirmed cases and death cases were higher in countries located within the Northern Hemisphere. The Blackbox of COVID-19 was revealed through the work conducted in this paper that the virus thrives in cooler and low-humidity environments, with emphasis on potential treatments and government measures relative to temperature and humidity.
• The coronavirus disease 2019 (COIVD-19) is spreading faster in low temperatures and humid area.
• Weather and climate serve as environmental drivers in propagating COVID-19.
• Solar radiation influences the spreading of COVID-19.
• The correlation between weather and population as the factor in spreading of COVID-19.
Graphical abstract
Introduction
The revolution and rotation of the Earth and the Sun supply heat and create differential heating on earth. The movements and the 23.5° inclination of the Earth [ 1 ] separate the oblate-ellipsoid-shaped earth into northern and southern hemispheres. Consequently, the division results in various climatic zones at different latitudes and dissimilar local temperatures (see Fig. 1 ) and affects the seasons and length of a day and night in a particular region [ 2 ]. Global differential heating and climate variability occur due to varying solar radiation received by each region [ 3 ]. According to Trenberth and Fasullo [ 4 ] and Hauschild et al. [ 5 ] the new perspective on the issue of climate change can be affected relative to the changes in solar radiation patterns. Since the study by Trenberth and Fasullo [ 4 ] focused on climate model changes from 1950 to 2100, it was found that the role of changing clouds and trapped sunlight can lead to an opening of the aperture for solar radiation.
The annual average temperature data for 2021 in the northern and southern hemispheres ( Source: meteoblue.com ). Note: The black circles mark countries with high Coronavirus disease 2019 (COVID-19) infections
Furthermore, the heat from sunlight is essential to humans; several organisms could not survive without it. Conversely, the spread of any disease-carrying virus tends to increase with less sunlight exposure [ 6 ]. Historically, disease outbreaks that led to epidemic and pandemic eruptions were correlated to atmospheric changes. Pandemic diseases, such as the flu (1918), Asian flu (1956–1958), Hong Kong flu (1968), and recently, the coronavirus disease 2019 (COVID-19) (2019), recorded over a million death toll each during the winter season or minimum temperature conditions [ 7 ]. The total number of COVID-19 cases is illustrated in Fig. 2 .
A graphical representation of the total number of COVID-19 cases across various periods between 2020 and 2021. ( Source : www.worldometers.info ). Note: The black circles indicate countries with high numbers COVID-19-infections
In several previous outbreaks, investigations revealed a significant association between temperature and humidity with a particular focus on the transmission dynamics of the infection from the virus into the hosts [ 8 , 9 , 10 ]. Moreover, disease outbreaks tended to heighten in cold temperatures and low humidity [ 11 ]. Optimal temperature and sufficient relative humidity during evaporation are necessary for cloud formation, resulting in the precipitated liquid falling to the ground as rain, snow, or hail due to the activity of solar radiation balancing [ 4 ].
Consequently, the radiation balancing processes in the atmosphere are directly linked to the living beings on the earth, including plants and animals, and as well as viruses and bacterias. According to Carvalho et al. [ 12 ]‘s study, the survival rate of the Coronaviridae Family can decrease during summer seasons. Nevertheless, numerous diseases were also developed from specific viruses, such as influenza, malaria, and rubella, and in November 2019, a severe health threat originated from a 125 nm size of coronavirus, had resulted in numerous deaths worldwide.
Transmission and symptoms of COVID-19
The COVID-19, or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an infectious disease caused by a newly discovered pathogenic virus from the coronavirus family, the novel coronavirus (2019-nCoV) [ 13 ]. The first case was recorded in Wuhan, China, in December 2019 [ 14 ]. The pathogenic virus is transmitted among humans when they breathe in air contaminated with droplets and tiny airborne particles containing the virus [ 14 , 15 , 16 , 17 , 18 ].
According to the World Health Organization (WHO), the most common symptoms of COVID-19 infection include fever, dry cough, and tiredness. Nevertheless, older people and individuals with underlying health problems (lung and heart problems, high blood pressure, diabetes, or cancer) are at higher risk of becoming seriously ill and developing difficulty breathing [ 19 ]. The COVID-19 was initially only predominant in China but rapidly spread to other countries globally. The remarkably swift acceleration of the number of infections and mortality forced WHO to declare COVID-19 a global public health emergency on the 30th of January 2020, which was later declared as a pandemic on the 11th of March 2020 [ 20 ].
Since no vaccine was available then, WHO introduced the COVID-19 preventative measures to reduce the chances of virus transmission. The guideline for individual preventative included practising hand and respiratory hygiene by regularly cleaning hands with soap and water or alcohol-based sanitisers, wear a facemask and always maintaining at least a one-meter physical distance [ 21 ]. Nevertheless, the worldwide transmission of COVID-19 has resulted in fear and forced numerous countries to impose restrictions rules, such as lockdown, travel bans, closed country borders, restrictions on shipping activities, and movement limitations, to diminish the spread of COVID-19 [ 22 ].
According to WHO, by the 2nd of December 2020, 63,379,338 confirmed cases and 1,476,676 mortalities were recorded globally. On the 3rd of December 2021, 263,655,612 confirmed cases and deaths were recorded, reflecting increased COVID-19 infections compared to the previous year. The American and European regions documented the highest COVID-19 patients with 97,341,769 and 88,248,591 cases, respectively (see Fig. 2 ), followed by Southeast Asia with 44,607,287, Eastern Mediterranean accounted 16,822,791, Western Pacific recorded 6,322,034, and Africa reported the lowest number of cases at 6,322,034 [ 19 ].
Recently, an increasing number of studies are investigating the association between environmental factors (temperature and humidity) and the viability, transmission, and survival of the coronavirus [ 23 , 24 , 25 , 26 ]. The results primarily demonstrated that temperature was more significantly associated with the transmission of COVID-19 [ 27 , 28 , 29 ] and its survival period on the surfaces of objects [ 30 ]. Consequently, the disease was predominant in countries with low temperature and humidity [ 31 ], which was also proven by Diao et al. [ 32 ]‘s study demonstrating higher rates of COVID-19 transmission in China, England, Germany, and Japan.
A comprehensive systematic literature review (SLR) is still lacking despite numerous research on environmental factors linked to coronavirus. Accordingly, this article aimed to fill the gap in understanding and identifying the correlation between environmental factors and COVID-19 by analysing existing reports. Systematically reviewing existing literature is essential to contribute to the body of knowledge and provide beneficial information for public health policymakers.

Methodology
The present study reviewed the protocols, formulation of research questions, selection of studies, appraisal of quality, and data abstraction and analysis.
The protocol review
The present SLR was performed according to the reporting standards for systematic evidence syntheses (ROSES) and followed or adapted the guidelines as closely as possible. Thus, in this study, a systematic literature review was guided by the ROSES review protocol (Fig. 3 ). Compared to preferred reporting items for systematic review and meta-analysis (PRISMA), ROSES is a review protocol specifically designed for a systematic review in the conservation or environment management fields [ 33 ]. Compared to PRISMA, ROSES offers several advantages, as it is tailored to environmental systematic review, which reduces emphasis on quantitative synthesis (e.g. meta-analysis etc.) that is only reliable when used with appropriate data [ 34 ].
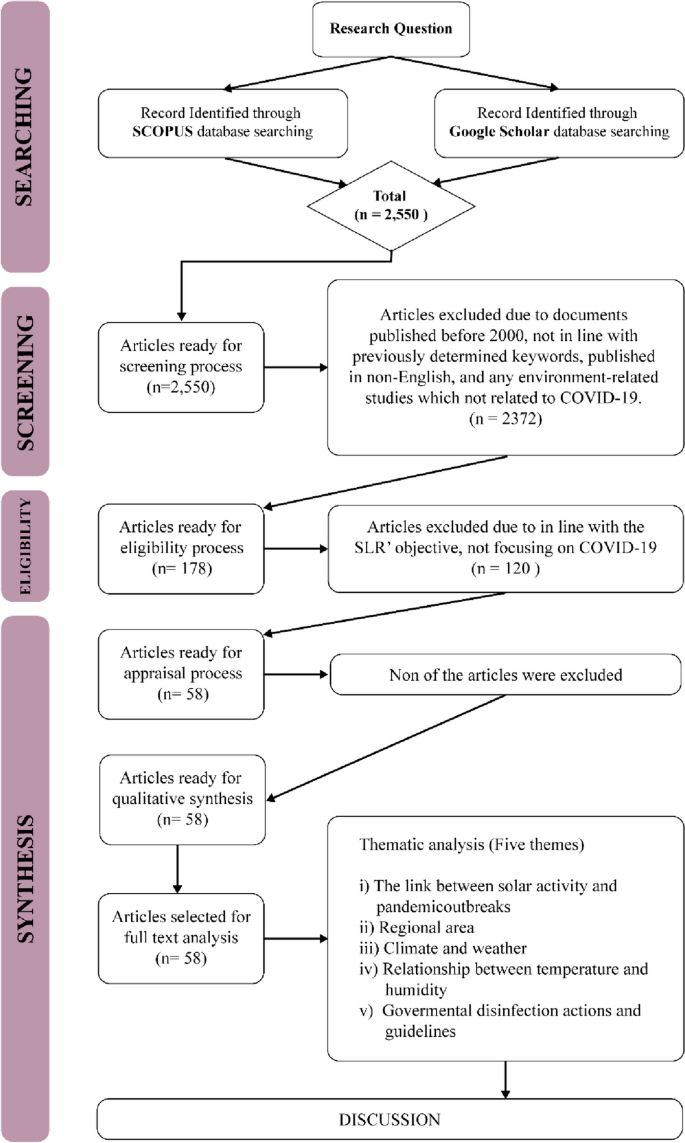
The flow diagram guide by ROSES protocol and Thematical Analysis
The current SLR started by determining the appropriate research questions, followed by the selection criteria, including the review, specifically on the keywords employed and the selection of journals database. Subsequently, the appraisal quality process and data abstraction and analysis were conducted.
Formulation of research questions
The entire process of this SLR was guided by the specific research questions, while sources to be reviewed and data abstraction and analysis were in line with the determined research question [ 35 , 36 ]. In the present article, a total of five research questions were formed, namely:
What the link between solar activity and COVID-19 pandemic outbreaks?
Which regions were more prone to COVID-19?
What were the temporal and spatial variabilities of high temperature and humidity during the spread of COVID-19?
What is the relationship between temperature and humidity in propagating COVID-19?
How did the government’s disinfection actions and guidelines can be reducing the spread of COVID-19?
Systematic searching strategies
Selection of studies.
In this stage of the study, the appropriate keywords to be employed in the searching process were determined. After referring to existing literature, six main keywords were chosen for the searching process, namely COVID-19, coronavirus, temperature, humidity, solar radiation and population density. The current study also utilised the boolean operators (OR, AND, AND NOT) and phrase searching.
Scopus was employed as the main database during the searching process, in line with the suggestion by Gusenbauer and Haddaway [ 37 ], who noted the strength of the database in terms of quality control and search and filtering functions. Furthermore, Google Scholar was selected as the supporting database. Although Halevi et al. [ 38 ] expressed concerns about its quality, Haddaway et al. [ 39 ] reported that due to its quantity, Google Scholar was suitable as a supporting database in SLR studies.
In the first stage of the search, 2550 articles were retrieved, which were then screened. The suitable criteria were also determined to control the quality of the articles reviewed [ 40 ]. The criteria are: any documents published between 2000 to 2022, documents that consist previously determined keywords, published in English, and any environment-related studies that focused on COVID-19. Based on these criteria, 2372 articles were excluded and 178 articles were proceeded to the next step namely eligibility. In the eligibility process, the title and the abstract of the articles were examined to ensure its relevancy to the SLR and in this process a total of 120 articles were excluded and only 58 articles were processed in the next stage.
Appraisal of the quality
The study ensured the rigor of the chosen articles based on best evidence synthesis. In the process, predefined inclusion criteria for the review were appraised by the systematic review team based on previously established guidelines and the studies were then judged as being scientifically admissible or not [ 40 ]. Hence, by controlling the quality based on the best evidence synthesis, the present SLR controls its quality by including articles that are in line with the inclusion criteria. It means that any article published within the timeline (in the year 2000 and above), composed of predetermined keywords, in English medium, and environment-related investigations focusing on COVID-19 are included in the review. Based on this process, all 58 articles fulfilled all the inclusion criteria and are considered of good quality and included in the review.
Data abstraction and analysis
The data abstraction process in this study was performed based on five research questions (please refer to 2.2, formulation of research questions). The data that was able to answer the questions were abstracted and placed in a table to ease the data analysis process. The primary data analysis technique employed in the current study was qualitative and relied on thematic analysis.
The thematic technique is a descriptive method that combines data flexibly with other information evaluation methods [ 41 ], aiming to identify the patterns in studies. Any similarities and relationships within the abstracted data emerge as patterns. Subsequently, suitable themes and sub-themes would be developed based on obtained patterns [ 42 ]. Following the thematic process, five themes were selected in this study.
Background of the selected articles
The current study selected 58 articles for the SLR. Five themes were developed based on the thematic analysis from the predetermined research questions: the link between solar activity and pandemic outbreaks, regional area, climate and weather, the relationship between temperature and humidity, and government disinfection action guidelines. Among the articles retrieved between 2000 and 2022; two were published in 2010, one in 2011, four in 2013, three in 2014, two in 2015, six in 2016 and 2017, respectively, one in 2018, six in 2019, twelve in 2020, eight in 2021, and seven in 2022.
Temperature- and humidity-related themes
The link between solar activity and pandemic outbreaks.
Numerous scientists have investigated the relationship between solar activities and pandemic outbreaks over the years ([ 43 ]; A [ 27 , 44 , 45 ].). Nuclear fusions from solar activities have resulted in minimum and maximum solar sunspots. Maximum solar activities are characterised by a high number of sunspots and elevated solar flare frequency and coronal mass injections. Minimum solar sunspot occurrences are identified by low interplanetary magnetic field values entering the earth [ 1 ].
A diminished magnetic field was suggested to be conducive for viruses and bacteria to mutate, hence the onset of pandemics. Nonetheless, Hoyle and Wickramasinghe [ 46 ] reported that the link between solar activity and pandemic outbreaks is only speculative. The literature noted that the data recorded between 1930 and 1970 demonstrated that virus transmissions and pandemic occurrences were coincidental. Moreover, no pandemic cases were reported in 1979, when minimum solar activity was recorded [ 47 ].
Chandra Wickramasinghe et al. [ 48 ] suggested a significant relationship between pandemic outbreaks and solar activities as several grand solar minima, including Sporer (1450–1550 AD), Mounder (1650–1700 AD), and Dalton (1800–1830) minimums, were recorded coinciding with global pandemics of diseases, such as smallpox, the English sweat, plague, and cholera pandemics. Furthermore, since the Dalton minimum, which recorded minimum sunspots, studies from 2002 to 2015 have documented the reappearance of previous pandemics. For example, influenza subtype H1N1 1918/1919 episodically returned in 2009, especially in India, China, and other Asian countries. Zika virus, which first appeared in 1950, flared and became endemic in 2015, transmitted sporadically, specifically in African countries. Similarly, SARS-CoV was first recorded in China in 2002 and emerged as an outbreak, MERS-CoV, in middle east countries a decade later, in 2012.
In 2020, the World Data Centre Sunspot Index and Long-term Solar Observations ( http://sidc.be ) confirmed that a new solar activity was initiated in December 2019, during which a novel coronavirus pandemic also occurred, and present a same as the previous hypothesis. Nevertheless, a higher number of pandemic outbreaks were documented during low minimum solar activities, including Ebola (1976), H5N1 (Nipah) (1967–1968), H1N1 (2009), and COVID-19 (2019–current). Furthermore, Wickramasinghe and Qu [ 49 ] reported that since 1918 or 1919, more devastating and recurrent pandemics tend to occur, particularly after a century. Consequently, within 100 years, a sudden surge of influenza was recorded, and novel influenza was hypothesised to emerge.
Figure 4 demonstrates that low minimum solar activity significantly reduced before 2020, hence substantiating the claim that pandemic events are closely related to solar activities. Moreover, numerous studies (i.e. [ 43 ], Chandra [ 46 , 47 , 48 ]) reported that during solar minimums, new viruses could penetrate the surfaces of the earth and high solar radiation would result in lower infection rates, supporting the hypothesis mentioned above.
The number of sunspots in the last 13 years. Note : The yellow curve indicates the daily sunspot number and the 2010–2021 delineated curve illustrates the minimum solar activity recorded (source: http://sidc.be/silso )
Regional area
In early December 2019, Wuhan, China, was reported as the centre of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak [ 50 ]. Chinese health authorities immediately investigated and controlled the spread of the disease. Nevertheless, by late January 2020, the WHO announced that COVID-19 was a global public health emergency. The upgrade was due to the rapid rise in confirmed cases, which were no longer limited to Wuhan [ 28 ]. The disease had spread to 24 other countries, which were mainly in the northern hemisphere, particularly the European and Western Pacific regions, such as France, United Kingdom, Spain, South Korea, Japan, Malaysia, and Indonesia [ 51 , 52 ]. The migration or movement of humans was the leading agent in the spread of COVID-19, resulting in an almost worldwide COVID-19 pandemic [ 53 ].
The first hotspots of the epidemic outspread introduced by the Asian and Western Pacific regions possessed similar winter climates with an average temperature and humidity rate of 5–11 °C and 47–79%. Consequently, several publications reviewed in the current study associated the COVID-19 outbreak with regional climates (i.e. [ 1 , 29 , 54 , 55 ]) instead of its close connection to China. This review also discussed the effects of a range of specific climatological variables on the transmission and epidemiology of COVID-19 in regional climatic conditions.
America and Europe documented the highest COVID-19 cases, outnumbering the number reported in Asia [ 19 ] and on the 2nd of December 2020, the United States of America (USA) reported the highest number of confirmed COVID-19 infections, with over 13,234,551 cases and 264,808 mortalities (Da S [ 56 ].). The cases in the USA began emerging in March 2020 and peaked in late November 2020, during the wintertime in the northern hemisphere (December to March) [ 53 ]. Figure 5 demonstrates the evolution of the COVID-19 pandemic in several country which represent comparison two phase of summer and one phase of winter. Most of these countries tend to increase of COVID cases close to winter season. Then, it can be worsening on phase two of summer due to do not under control of human movement although the normal trend it is presenting during winter phase.
The evolution of the COVID-19 pandemic from the 15th of February 2020 to the 2nd of December 2020 ( Source: https://www.worldometers.info/coronavirus )
The coronavirus spread aggressively across the European region, which recorded the second highest COVID-19 confirmed cases after America. At the end of 2020, WHO reported 19,071,275 Covid-19 cases in the area, where France documented 2,183,275 cases, the European country with the highest number of confirmed cases, followed by the United Kingdom (1,629,661 cases) and Spain (1,652,801 cases) [ 19 ]. Europe is also located in the northern hemisphere and possesses a temperate climate.
The spatial and temporal transmission patterns of coronavirus infection in the European region were similar to America and the Eastern Mediterranean, where the winter season increased COVID-19 cases. Typically, winter in Europe occurs at the beginning of October and ends in March. Hardy et al. [ 57 ] also stated that temperature commonly drops below freezing (approximately − 1 °C) when snow accumulates between December to mid-March, resulting in an extreme environment. Figure 5 indicates that COVID-19 cases peaked in October when the temperature became colder [ 21 ]. Similarly, the cases were the highest in the middle of the year in Australia and South Asian countries, such as India, that experience winter and monsoon, respectively, during the period.
In African regions, the outbreak of COVID-19 escalated rapidly from June to October before falling from October to March, as summer in South Africa generally occurs from November to March, while winter from June to August. Nevertheless, heavy rainfall generally transpires during summer, hence the warm and humid conditions in South Africa and Namibia during summer, while the opposite happens during winter (cold and dry). Consequently, the outbreak in the region recorded an increasing trend during winter and subsided during the summer, supporting the report by Gunthe et al. [ 58 ]. Novel coronavirus disease presents unique and grave challenges in Africa, as it has for the rest of the world. However, the infrastructure and resources have limitations for Africa countries facing COVID-19 pandemic and the threat of other diseases [ 59 ].
Conclusively, seasonal and regional climate patterns were associated with COVID-19 outbreaks globally. According to Kraemer et al. [ 60 ], they used real-time mobility data in Wuhan and early measurement presented a positive correlation between human mobility and spread of COVID-19 cases. However, after the implementation of control measures, this correlation dropped and growth rates became negative in most locations, although shifts in the demographics of reported cases were still indicative of local chains of transmission outside of Wuhan.
Climate and weather
The term “weather” represents the changes in the environment that occur daily and in a short period, while “climate” is defined as atmospheric changes happening over a long time (over 3 months) in specific regions. Consequently, different locations would experience varying climates. Numerous reports suggested climate and weather variabilities as the main drivers that sped or slowed the transmission of SARS-CoV-2 worldwide [ 44 , 61 , 62 , 63 ].
From a meteorological perspective, a favourable environment has led to the continued existence of the COVID-19 virus in the atmosphere [ 64 ]. Studies demonstrated that various meteorological conditions, such as the rate of relative humidity (i.e. [ 28 ]), precipitation (i.e. [ 65 ]), temperature (i.e. [ 66 ]), and wind speed factors (i.e. [ 54 ]), were the crucial components that contributed to the dynamic response of the pandemic, influencing either the mitigation or exacerbation of novel coronavirus transmission. In other words, the environment was considered the medium for spreading the disease when other health considerations were put aside. Consequently, new opinions, knowledge, and findings are published and shared to increase awareness, thus encouraging preventive measures within the public.
The coronavirus could survive in temperatures under 30 °C with a relative humidity of less than 80% [ 67 ], suggesting that high temperatures and lower relative humidity contributed to the elicitation of COVID-19 cases [ 18 , 51 , 58 , 68 ]. Lagtayi et al. [ 7 ] highlighted temperature as a critical factor, evidently from the increased transmission rate of MERS-Cov in African states with a warm and dry climate. Similarly, the highest COVID-19 cases were recorded in dry temperate regions, especially in western Europe (France and Spain), China, and the USA, while the countries nearer to the equator were less affected. Nevertheless, the temperature factor relative to viral infections depends on the protein available in the viruses. According to Chen and Shakhnovich [ 69 ], there is a good correlation between decreasing temperature and the growth of proteins in virus. Consequently, preventive measures that take advantage of conducive environments for specific viruses are challenging.
Precipitation also correlates with influenza [ 43 ]. A report demonstrated that regions with at least 150 mm of monthly precipitation threshold level experienced fewer cases than regions with lower precipitation rates. According to Martins et al. [ 70 ], influenza and COVID-19 can be affected by climate, where virus can be spread through the respiratory especially during rainfall season. The daily spread of Covid-19 cases in tropical countries, which receive high precipitation levels, are far less than in temperate countries [ 27 ]. Likewise, high cases of COVID-19 were reported during the monsoon season (mid-year) in India during which high rainfall is recorded [ 71 ]. Moreover, the majority of the population in these regions has lower vitamin D levels, which may contribute to weakened immune responses during certain seasons [ 27 ].
Rainfall increases the relative atmospheric humidity, which is unfavourable to the coronaviruses as its transmission requires dry and cold weather. Moreover, several reports hypothesised that rain could wash away viruses on object surfaces, which is still questioned. Most people prefer staying home on rainy days, allowing less transmission or close contact. Conversely, [ 72 ] exhibited that precipitation did not significantly impact COVID-19 infectiousness in Oslo, Norway due the location in northern hemisphere which are during winter season presenting so cold.
Coşkun et al. [ 54 ] and Wu et al. [ 29 ] claimed that wind could strongly correlate with the rate of COVID-19 transmission. Atmospheric instability (turbulent occurrences) leads to increased wind speed and reduces the dispersion of particulate matter (PM 2.5 and PM 10 ) in the environment and among humans. An investigation performed in 55 cities in Italy during the COVID-19 outbreak proved that the areas with low wind movement (stable atmospheric conditions) possessed a higher correlation coefficient and exceeded the threshold value of the safe level of PM 2.5 and PM 10 . Resultantly, more individuals were recorded infected with the disease in the regions. As mentioned in Martins et al. [ 70 ] the COVID-19 can be affected by climate and the virus can be spread through respiratory which is the virus moving in the wind movement.
The relationship between temperature and humidity
Climatic parameters, such as temperature and humidity, were investigated as the crucial factors in the epidemiology of the respiratory virus survival and transmission of COVID-19 ([ 61 ]; S [ 73 , 74 ].). The rising number of confirmed cases indicated the strong transmission ability of COVID-19 and was related to meteorological parameters. Furthermore, several studies found that the disease transmission was associated with the temperature and humidity of the environment [ 55 , 64 , 68 , 75 ], while other investigations have examined and reviewed environmental factors that could influence the epidemiological aspects of Covid-19.
Generally, increased COVID-19 cases and deaths corresponded with temperature, humidity, and viral transmission and mortality. Various studies reported that colder and dryer environments favoured COVID-19 epidemiologically [ 45 , 76 , 77 ]. As example tropical region, the observations indicated that the summer (middle of year) and rainy seasons (end of the year) could effectively diminish the transmission and mortality from COVID-19. High precipitation statistically increases relative air humidity, which is unfavourable for the survival of coronavirus, which prefers dry and cold conditions [ 32 , 34 , 78 , 79 ]. Consequently, warmer conditions could reduce COVID-19 transmission. A 1 °C increase in the temperature recorded a decrease in confirmed cases by 8% increase [ 45 ].
Several reports established that the minimum, maximum, and average temperature and humidity correlated with COVID-19 occurrence and mortality [ 55 , 80 , 81 ]. The lowest and highest temperatures of 24 and 27.3 °C and a humidity between 76 and 91% were conducive to spreading the virulence agents. The propagation of the disease peaked at the average temperature of 26 °C and humidity of 55% before gradually decreasing with elevated temperature and humidity [ 78 ].
Researchers are still divided on the effects of temperature and humidity on coronavirus transmission. Xu et al. [ 26 ] confirmed that COVID-19 cases gradually increased with higher temperature and lower humidity, indicating that the virus was actively transmitted in warm and dry conditions. Nevertheless, several reports stated that the spread of COVID-19 was negatively correlated with temperature and humidity [ 10 , 29 , 63 ]. The conflicting findings require further investigation. Moreover, other factors, such as population density, elderly population, cultural aspects, and health interventions, might potentially influence the epidemiology of the disease and necessitate research.
Governmental disinfection actions and guidelines
The COVID-19 is a severe health threat that is still spreading worldwide. The epidemiology of the SAR-CoV-2 virus might be affected by several factors, including meteorological conditions (temperature and humidity), population density, and healthcare quality, that permit it to spread rapidly [ 16 , 17 ]. Nevertheless, in 2020, no effective pharmaceutical interventions or vaccines were available for the diagnosis, treatment, and epidemic prevention against COVID-19 [ 73 , 82 ]. Consequently, after 2020 the governments globally have designed and executed non-pharmacological public health measures, such as lockdown, travel bans, social distancing, quarantine, public place closure, and public health actions, to curb the spread of COVID-19 infections and several studies have reported on the effects of these plans [ 13 , 83 ].
The COVID-19 is mainly spread via respiratory droplets from an infected person’s mouth or nose to another in close contact [ 84 ]. Accordingly, WHO and most governments worldwide have recommended wearing facemasks in public areas to curb the transmission of COVID-19. The facemasks would prevent individuals from breathing COVID-19-contaminated air [ 85 ]. Furthermore, the masks could hinder the transmission of the virus from an infected person as the exhaled air is trapped in droplets collected on the masks, suspending it in the atmosphere for longer. The WHO also recommended adopting a proper hand hygiene routine to prevent transmission and employing protective equipment, such as gloves and body covers, especially for health workers [ 86 ].
Besides wearing protective equipment, social distancing was also employed to control the Covid-19 outbreak [ 74 , 87 ]. Social distancing hinders the human-to-human transmission of the coronavirus in the form of droplets from the mouth and nose, as evidenced by the report from Sun and Zhai [ 88 ]. Conversely, Nair & Selvaraj [ 89 ] demonstrated that social distancing was less effective in communities and cultures where gatherings are the norm. Nonetheless, the issue could be addressed by educating the public and implementing social distancing policies, such as working from home and any form of plague treatment.
Infected persons, individuals who had contact with confirmed or suspected COVID-19 patients, and persons living in areas with high transmission rates were recommended to undergo quarantine by WHO. The quarantine could be implemented voluntarily or legally enforced by authorities and applicable to individuals, groups, or communities (community containment) [ 90 ]. A person under mandatory quarantine must stay in a place for a recommended 14-day period, based on the estimated incubation period of the SARS-CoV-2 [ 19 , 91 ]. According to Stasi et al. [ 92 ], 14-days period for mandatory quarantine it is presenting a clinical improvement after they found 5-day group and 10-day group can be decrease number of patient whose getting effect of COVID-19 from 64 to 54% respectively. This also proven by Ahmadi et al. [ 43 ] and Foad et al. [ 93 ], quarantining could reduce the transmission of COVID-19.
Lockdown and travel bans, especially in China, the centre of the coronavirus outbreak, reduced the infection rate and the correlation of domestic air traffic with COVID-19 cases [ 17 ]. The observations were supported by Sun & Zhai [ 88 ] and Sun et al. [ 94 ], who noted that travel restrictions diminished the number of COVID-19 reports by 75.70% compared to baseline scenarios without restrictions. Furthermore, example in Malaysia, lockdowns improved the air quality of polluted areas especially in primarily at main cities [ 95 ]. As additional, Martins et al. [ 70 ] measure the Human Development Index (HDI) with the specific of socio-economic variables as income, education and health. In their study, the income and education levels are the main relevant factors that affect the socio-economic.
A mandatory lockdown is an area under movement control as a preventive measure to stop the coronavirus from spreading to other areas. Numerous governments worldwide enforced the policy to restrict public movements outside their homes during the pandemic. Resultantly, human-to-human transmission of the virus was effectively reduced. The lockdown and movement control order were also suggested for individuals aged 80 and above or with low or compromised immunities, as these groups possess a higher risk of contracting the disease [ 44 ].
Governments still enforced movement orders even after the introduction of vaccines by Pfizer, Moderna, and Sinovac, as the vaccines only protect high-risk individuals from the worst effects of COVID-19. Consequently, in most countries, after receiving the first vaccine dose, individuals were allowed to resume life as normal but were still required to follow the standard operating procedures (SOP) outlined by the government.
The government attempted to balance preventing COVID-19 spread and recovering economic activities, for example, local businesses, maritime traders, shipping activities, oil and gas production and economic trades [ 22 , 96 ]. Nonetheless, the COVID-19 cases demonstrated an increasing trend during the summer due to the higher number of people travelling and on vacation, primarily to alleviate stress from lockdowns. Several new variants were discovered, including the Delta and Omicron strains, which spread in countries such as the USA and the United Kingdom. The high number of COVID-19 cases prompted the WHO to suggest booster doses to ensure full protection.
As mentioned in this manuscript, the COVID-19 still uncertain for any kind factors that can be affected on spreading of this virus. However, regarding many sources of COVID-19 study, the further assessment on this factor need to be continue to be sure, that we ready to facing probably in 10 years projection of solar minimum phase can be held in same situation for another pandemic.
The sun has an eleven-year cycle known as the solar cycle, related to its magnetic field, which controls the activities on its surface through sunspots. When the magnetic fields are active, numerous sunspots are formed on its surface, hence the sun produces more radiation energy emitted to the earth. The condition is termed solar maximum (see Fig. 6 , denoted by the yellow boxes). Alternatively, as the magnetic field of the sun weakens, the number of sunspots decreases, resulting in less radiation energy being emitted to the earth. The phenomenon is known as the solar minimum (see Fig. 6 , represented by the blue boxes).
The emergence and recurrence of pandemics every 5 years in relation to solar activities ( Source: www.swpc.noaa.gov/ ). Note: The yellow boxes indicate the solar maximum, while the blue boxes represent the solar minimum
The magnetic field of the sun protects the earth from cosmic or galactic cosmic rays emitted by supernova explosions, stars, and gamma-ray bursts [ 97 ]. Nevertheless, galactic cosmic rays could still reach the earth during the solar minimum, the least solar radiation energy period. In the 20th and early 21st centuries, several outbreaks of viral diseases that affected the respiratory system (pneumonia or influenza), namely the Spanish (1918–1919), Asian (1957–1958) and Hong Kong (1968) flu, were documented. Interestingly, the diseases that claimed numerous lives worldwide occurred at the peak of the solar maximum.
Figure 6 illustrates the correlation between the number of sunspots and disease outbreaks from 1975 to 2021, including COVID-19, that began to escalate in December 2019. Under the solar minimum conditions, the spread of Ebola (1976), H5N1 (1997–1998), H1N1 (2009), and COVID-19 (2019-2020) were documented, while the solar maximum phenomenon recorded SARS (2002) and H7N9 (2012–2013) or MERS outbreaks. Nonetheless, solar activity through the production of solar sunspots began to decline since the 22nd solar cycle. Accordingly, further studies are necessary to investigate the influence such solar variations could impart or not on pandemic development.
Despite the findings mentioned above, the sun and cosmic radiations could influence the distribution or outspread of disease-spreading viruses. The rays could kill the viruses via DNA destruction or influence their genetic mutations, which encourage growth and viral evolution. Nevertheless, the connection between radiation and the evolutionary process requires further study by specialists in the field it is become true or not.
The spread of viral diseases transpires naturally in our surroundings and occurs unnoticed by humans. According to records, the spread of pandemic diseases, including the Black Death (fourteenth century) and the Spanish flu (1919), was significantly influenced by the decline and peak of solar activities. Furthermore, in the past 20 years, various diseases related to the influenza virus have been recorded. According to the pattern observed, if all diseases were related to the solar cycle (solar maximum and minimum), the viral diseases would reoccur every 5 to 6 years since they first appeared between 1995 and 2020. Accordingly, the next pandemic might occur around 2024 or 2025 and need to have a proper study for prove these statements. Nonetheless, the activities on the surface of the sun have been weakening since the 23rd solar cycle and it can be proven later after the proper study can be make it.
The beginning of the COVID-19 spread, only several countries with the same winter climate with an average temperature of 5–11 °C and an average humidity rate of 47–79% located at latitudes 30–50 N reported cases. The areas included Wuhan distribution centres in China, the United Kingdom, France, Spain, South Korea, Japan, and the USA (see Fig. 5 ). Other than biological aspects, the higher number of confirmed cases recorded in colder environments was due to the human body secreting less lymphoproliferative hormone, leading to decreased immunogenicity effects and increased risk of infection [ 24 ]. Consequently, the virus could attack and rapidly infect humans during the period [ 1 , 54 ].
The lymphoproliferative response is a protective immune response that plays a vital role in protecting and eradicating infections and diseases. On the other hand, staying in warm conditions or being exposed to more sunlight would lower the risks of infection. According to Asyary and Veruswati [ 98 ], sunlight triggers vitamin D, which increases immunity and increases the recovery rates of infected individuals.
Researchers believe that viruses could survive in the environment for up to 3 to 4 years or even longer. The survival rate of the microorganisms is relatively high, which is related to their biological structures, adaptability on any surfaces, and transmission medium to spread diseases. Viruses possess simple protein structures, namely the spike, membrane, and envelope protein; therefore, when they enter living organisms (such as through the respiratory system), the viruses are easily transmitted.
Once they have entered a host, the viruses duplicate exponentially and swarm the lungs. Subsequently, after the targeted organs, such as the lungs, are invaded, the viruses attack the immune system and create confusion in protective cells to destroy healthy cells. The situation is still considered safe in younger and healthy individuals as their immune systems could differentiate and counter-attack the viruses, curing them. Nonetheless, in elders and individuals with several chronic diseases, most of their protective cells are dead, hence their immune system is forced to work hard to overcome the infection. Pneumonia and death tend to occur when the situation is overwhelming [ 85 ]. Consequently, the viruses are harmful to humans as they could multiply in a short period, enter the blood, and overrun the body.
The coronavirus could attach to surfaces without a host, including door knobs and steel and plastic materials. The microorganisms could survive alone, but virologists have yet to determine how long. If someone touches any surface with the virus, the individual would then be infected. The situation would worsen if the infected person contacted numerous people and became a super spreader. A super spreader does not exhibit any symptoms and continuously transmits the virus without realising it. An infected individual transmits the coronavirus via droplets from coughs or sneezes. Nevertheless, scientists have yet to determine if coronavirus is spread via airborne or droplets, hence requiring thorough evaluation [ 99 ].
The COVID-19 virus mutates over time, and it can be changing any times. Mutations alter the behaviour and genetic structure of the virus, resulting in a new strain. Numerous research have been conducted to procure vaccines and anti-viral medications, but mutations have led to evolutionary disadvantages. The novel strains are more infectious than the original ones. As of November 2020, approximately six new coronavirus strains have been detected, each displaying different transmission behaviours [ 100 ].
Recent studies demonstrated that the mutated viruses exhibit little variability, allowing scientists to produce viable vaccines [ 71 ]. Furthermore, different types of vaccines are manufactured by different countries, which could be advantageous. Currently, most countries also recommend booster doses to attain extra protection after receiving the mandatory two vaccine doses. In same time, the social and physical interactions between humans also necessitate to be aware.
The COVID-19 virus is primarily transmitted through droplets produced by an infected person. Accordingly, physical distancing, a one-metre minimum distance between individuals [ 19 ], and following the SOP might prevent or avoid spreading the disease. Moreover, self-quarantine, school closures, working from home, cancelling large events, limiting gatherings, and avoiding spending long periods in crowded places are essential strategies in enforcing physical distancing at a community level. The policies are essential precautions that could reduce the further spreading of coronavirus and break the chain of transmission.
Government support also need to control the spread of COVID-19 with the strict SOP. The SOP enforcement in public places would enhance adherence to the new practice among the public and the community, aiding in curbing disease transmission. Practising limited meetings and social gatherings, avoiding crowded places, workplace distancing, preventing non-necessary travels of high-risk family members, especially those with chronic disease, and adhering to the recommended SOP could reduce coronavirus outbreaks. Nonetheless, individual awareness is also necessary to achieve COVID-19 spread prevention.
Many researchers are focused on identifying the primary drivers of pandemic outbreaks. Seasonal, temperature, and humidity differences significantly impacted COVID-19 growth rate variations. It is crucial to highlight the potential link between the recurrence of pandemics every 5 years and solar activities, which can influence temperature and humidity variations. Notable variations in COVID-19 mortality rates were observed between northern and southern hemisphere countries, with the former having higher rates. One hypothesis suggests that populations in the northern hemisphere may receive insufficient sunlight to maintain optimal vitamin D levels during winter, possibly leading to higher mortality rates.
The first COVID-19 case was detected in Wuhan, China, which is in the northern hemisphere. The number of cases rapidly propagated in December during the winter season. At the time, the temperature in Wuhan was recorded at 13–18 °C. Accordingly, one theory proposes that the survival and transmission of the coronavirus were due to meteorological conditions, namely temperatures between 13 and 18 °C and 50–80% humidity.
Daily rainfall directly impacts humidity levels. The coronavirus exhibited superior survival rates in cold and dry conditions. Furthermore, transmissible gastroenteritis (TGEV) suspensions and possibly other coronaviruses remain viable longer in their airborne states, which are more reliably collected in low relative humidity than in high humidity. Consequently, summer rains would effectively reduce COVID-19 transmission in southern hemisphere regions.
In southern hemisphere regions, the summer seasons are accompanied by a high average temperature at the end and beginning of the year. Countries with temperatures exceeding 24 °C reported fewer infections. As temperatures rise from winter to summer, virus transmission is expected to decline. Nonetheless, the activities and transmission of the virus were expected to decrease during winter to summer transitions, when the countries would be warmer. The peak intensity of infections strongly depends on the level of seasonal transmissions.
Social distancing plays a critical role in preventing the overload of healthcare systems. Many respiratory pathogens, including those causing mild common cold-like syndromes, show seasonal fluctuations, often peaking in winter. This trend can be attributed to increased indoor crowding, school reopening, and climatic changes during autumn.
The spread of COVID-19 to neighbouring regions can be attributed to population interactions. Migration patterns, such as the movement from northern to southern regions during the warmer months, have significant epidemiological impacts. This trend mirrors the behavior of influenza pandemics where minor outbreaks in spring or summer are often followed by major waves in autumn or winter.
Availability of data and materials
Not applicable.
Abbreviations
Novel coronavirus
Coronavirus disease 2019
Deoxyribonucleic acid
Swine influenza
Influenza A virus subtype H5N1
Asian Lineage Avian Influenza A(H7N9) Virus
Middle East respiratory syndrome
Middle East respiratory syndrome Coronavirus
Particulate matter
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
RepOrting standards for Systematic Evidence Syntheses
Severe Acute Respiratory Syndrome
Severe Acute Respiratory Syndrome Coronavirus
Syndrome coronavirus 2
Systematic literature review
Standard operating procedure
Transmissible gastroenteritis Virus
United States of America
World Health Organization
Apanovich I. Climate and man. Opposition or natural stage of the earth’s evolution? Norwegian journal of development of the international. Science. 2019;26(25):12–27.
Google Scholar
Borah P, Singh MK, Mahapatra S. Estimation of degree-days for different climatic zones of north-East India. Sustain Cities Soc. 2015;14(1):70–81.
Article Google Scholar
Chen D, Chen HW. Using the Köppen classification to quantify climate variation and change: an example for 1901-2010. Environmental Development. 2013;6(1):69–79.
Trenberth KE, Fasullo JT. Global warming due to increasing absorbed solar radiation. Geophys Res Lett. 2009;36
Hauschild MZ, Huijbregts MAJ, Guinée L, Lane J, Fantke P, Zelm v R, et al. Life Cycle Impact Assessment – The Complete World of Life Cycle Assessment; 2015. p. 345.
Book Google Scholar
Nakada LYK, Urban RC. COVID-19 pandemic: environmental and social factors influencing the spread of SARS-CoV-2 in São Paulo. Brazil Environmental Science and Pollution Research. 2021;28(30):40322–8.
Article CAS PubMed Google Scholar
Lagtayi, R., Lairgi, L., Daya, A., & Khouya, A. (2021). The impact of the average temperature, humidity, wind speed, altitude and population density on daily COVID-19 infections’ evolution. January, 9094.
Majumder MS, Liu D, Poirier C, Mandl KD, Lipsitch M, The MS. The role of absolute humidity on transmission rates of the COVID-19 outbreak; 2020.
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA - Journal of the American Medical Association. 2020;324(8):782–93.
Zhang X, Maggioni V, Houser P, Xue Y, Mei Y. The impact of weather condition and social activity on COVID-19 transmission in the United States. J Environ Manag. 2022;302:114085.
Article CAS Google Scholar
Mäkinen TM, Juvonen R, Jokelainen J, Harju TH, Peitso A, Bloigu A, et al. Cold temperature and low humidity are associated with increased occurrence of respiratory tract infections. Respir Med. 2009;103(3):456–62.
Article PubMed Google Scholar
Carvalho FRS, Henriques DV, Correia O, Schmalwieser AW. Potential of solar UV radiation for inactivation of Coronaviridae family. Photochem Photobiol. 2021;97:213–20.
Ali I, Alharbi OM. COVID-19: disease, management, treatment, and social impact. Sci Total Environ. 2020;728:138861.
Article CAS PubMed PubMed Central Google Scholar
Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of covid-19. Viruses. 2020;12(4):1–17.
Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World health organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71–6.
Article PubMed PubMed Central Google Scholar
Wang J, Tang K, Feng K, Lv W. High temperature and high humidity reduce the transmission of COVID-19; 2020a.
Wang L, Duan Y, Zhang W, Liang J, Xu J, Zhang Y, et al. Epidemiologic and clinical characteristics of 26 cases of covid-19 arising from patient-to-patient transmission in Liaocheng, China. Clinical Epidemiology. 2020b;12:387–91.
Xie J, Zhu Y. Science of the Total environment association between ambient temperature and COVID-19 infection in 122 cities from China. Sci Total Environ. 2020;724:138201.
World Health Organization. (2020a). Director-General’s opening remarks at the media briefing on COVID-19-10April 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—10- april-2020.
Bostock B. South Korea is testing 200,000 members of a doomsday church linked to more than 60% of its coronavirus cases; 2020.
World Health Organization. (2020b). Health topics/coronavirus. https://www.who.int/health-topics/coronavirus#tab=tab_1.
Menhat M, Mohd Zaideen IM, Yusuf Y, Salleh NHM, Zamri MA, Jeevan J. The impact of Covid-19 pandemic: A review on maritime sectors in Malaysia. Ocean Coast Manag. 2021;209:105638.
Byun WS, Heo SW, Jo G, Kim JW, Kim S, Lee S, et al. Is coronavirus disease (COVID-19) seasonal? A critical analysis of empirical and epidemiological studies at global and local scales. Environ Res. 2021;196:110972.
Dhakal P, Pokhrel P, B. Seasonal variation and COVID-19 pandemic in Nepal. Nepal Medical Journal. 2020;3(2):77–80.
Mehmet Ş. Science of the Total environment impact of weather on COVID-19 pandemic in Turkey. 728; 2020.
Xu H, Yan C, Fu Q, Xiao K, Yu Y, Han D, et al. Science of the Total environment possible environmental effects on the spread of COVID-19 in China. Sci Total Environ. 2020;731:139211.
Rosario DKA, Mutz YS, Bernardes PC, Conte-Junior CA. Relationship between COVID-19 and weather: case study in a tropical country. Int J Hyg Environ Health. 2020;229:113587.
Wang J, Tang K, Feng K, Lin X, Lv W, Chen K, et al. Impact of temperature and relative humidity on the transmission of COVID-19: A modelling study in China and the United States. BMJ Open. 2021;11(2):1–16.
Wu Y, Jing W, Liu J, Ma Q, Yuan J, Wang Y, et al. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci Total Environ. 2020;729:1–7.
Casanova LM, Jeon S, Rutala WA, Weber DJ, Sobsey MD. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol. 2010;76(9):2712–7.
Islam N, Bukhari Q, Jameel Y, Shabnam S, Erzurumluoglu AM, Siddique MA, et al. COVID-19 and climatic factors: A global analysis. Environ Res. 2021;193:110355.
Diao Y, Kodera S, Anzai D, Gomez-Tames J, Rashed EA, Hirata A. Influence of population density, temperature, and absolute humidity on spread and decay durations of COVID-19: A comparative study of scenarios in China, England, Germany, and Japan. One Health. 2021;12:100203.
Haddaway NR, Macura B, Whaley P, Pullin AS. ROSES reporting standards for systematic evidence syntheses: pro forma, flow-diagram and descriptive summary of the plan and conduct of environmental systematic reviews and systematic maps. Environ Evid. 2018;7(1):4–11.
Sharif N, Dey SK. Impact of population density and weather on COVID-19 pandemic and SARS-CoV-2 mutation frequency in Bangladesh. Epidemiol Infect. 2021:1–10.
Kraus S, Breier Dasí-Rodríguez S. El arte de elaborar una revisión bibliográfica sistemática en la investigación sobre el espíritu empresarial. Int Entrep Manag J. 2020;16:1023–42.
Xiao Y, Watson M. Guidance on conducting a systematic literature review. J Plan Educ Res. 2019;39(1):93–112.
Gusenbauer M, Haddaway NR. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google scholar, PubMed, and 26 other resources. Res Synth Methods. 2020;11(2):181–217.
Halevi G, Moed H, Bar-Ilan J. Suitability of Google scholar as a source of scientific information and as a source of data for scientific evaluation—review of the literature. Journal of Informetrics. 2017;11(3):823–34.
Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of google scholar in evidence reviews and its applicability to grey literature searching. PLoS One. 2015;10(9):1–17.
Littlewood C, Chance-Larsen K, McLean S. Quality appraisal as a part of the systematic review. International Journal of Physiotherapy and Rehabilitation. 2010;1(1):53–8.
Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398–405.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101.
Ahmadi M, Sharifi A, Dorosti S, Jafarzadeh Ghoushchi S, Ghanbari N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Sci Total Environ. 2020;729
Gupta A, Banerjee S, Das S. Significance of geographical factors to the COVID-19 outbreak in India. Modeling Earth Systems and Environment. 2020;6(4):2645–53.
Pequeno P, Mendel B, Rosa C, Bosholn M, Souza JL, Baccaro F, et al. Air transportation, population density and temperature predict the spread of COVID-19 in Brazil. PeerJ. 2020;2020(6):1–15.
Hoyle F, Wickramasinghe NC. Sunspots and influenza [6]. Nature. 1990;343(6256):304.
Wickramasinghe NC, Rocca MC, Tokoro G, Temple R. Journal of infectious diseases. Scienctific Research and Community. 2020;1(4):1–10.
Wickramasinghe NC, Steele EJ, Wainwright M, Tokoro G, Fernando M, Qu J. Sunspot cycle minima and pandemics : A case for vigilance at the present time. Journal of Astrobiology & Outreach. 2017;5:2332–519.
Wickramasinghe NC, Qu J. Are we approaching a new influenza pandemic. Virol Curr Res. 2018;2(107):2.
Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Disease 2019 in China; 2020.
Bashir MF, Ma B, Bilal K, Bashir MA, Tan D, Bashir M. Correlation between climate indicators and COVID-19 pandemic in New York, USA. Sci Total Environ. 2020;728:138835.
Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–60.
PubMed PubMed Central Google Scholar
Lin C, Lau AKH, Fung JCH, Guo C, Chan JWM, Yeung DW, et al. A mechanism-based parameterisation scheme to investigate the association between transmission rate of COVID-19 and meteorological factors on plains in China. Sci Total Environ. 2020;737:140348.
Coşkun H, Yıldırım N, Gündüz S. The spread of COVID-19 virus through population density and wind in Turkey cities. Sci Total Environ. 2021;751
Yang HY, Lee JKW. The impact of temperature on the risk of covid-19: A multinational study. Int J Environ Res Public Health. 2021;18(8)
Candido DD, Watts A, Abade L, Kraemer MUG, Pybus OG, Croda J, et al. Routes for COVID-19 importation in Brazil. Journal of Travel Medicine. 2020;27(3):1–3.
Hardy JP, Groffman PM, Fitzhugh RD, Henry KS, Welman AT, Demers JD, et al. Snow depth manipulation and its influence on soil frost and water dynamics in a northern hardwood forest. Biogeochemistry. 2001;56(2):151–74.
Gunthe SS, Swain B, Patra SS, Amte A. On the global trends and spread of the COVID-19 outbreak: preliminary assessment of the potential relation between location-specific temperature and UV index. Journal of Public Health (Germany). 2020:1–10.
Rosenthal PJ, et al. COVID-19: shining the light on Africa. Am J Trop Med Hyg. 2020;102(6):1145–8.
Kraemer MUG, et al. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science. 2020;368:493–7.
Dalziel BD, Kissler S, Gog JR, Viboud C, Bjørnstad ON, Metcalf CJE, et al. Urbanization and humidity shape the intensity of influenza epidemics in U.S. cities. Science. 2018;362:75–9.
Sahoo PK, Powell MA, Mittal S, Garg VK. Is the transmission of novel coronavirus disease (COVID-19) weather dependent? J Air Waste Manage Assoc. 2020;70(11):1061–4.
Selcuk M, Gormus S, Guven M. Impact of weather parameters and population density on the COVID-19 transmission: evidence from 81 provinces of Turkey. Earth Syst Environ. 2021;5(1):87–100.
Abraham J, Turville C, Dowling K, Florentine S. Does climate play any role in covid-19 spreading?—an Australian perspective. Int J Environ Res Public Health. 2021;18(17)
Sehra ST, Salciccioli JD, Wiebe DJ, Fundin S, Baker JF. Maximum daily temperature, precipitation, ultraviolet light, and rates of transmission of severe acute respiratory syndrome coronavirus 2 in the United States. Clin Infect Dis. 2020;71(9):2482–7.
CAS PubMed Google Scholar
Rubin D, Huang J, Fisher BT, Gasparrini A, Tam V, Song L, et al. Association of Social Distancing, population density, and temperature with the instantaneous reproduction number of SARS-CoV-2 in counties across the United States. JAMA Netw Open. 2020;3(7):1–12.
Comunian S, Dongo D, Milani C, Palestini P. Air pollution and covid-19: The role of particulate matter in the spread and increase of covid-19’s morbidity and mortality. Int J Environ Res Public Health. 2020;17(12):1–22.
Tosepu R, Gunawan J, Effendy DS, Ahmad LOAI, Lestari H, Bahar H, et al. Correlation between weather and Covid-19 pandemic in Jakarta, Indonesia. Sci Total Environ. 2020;725
Chen and Shakhnovich. Thermal adaptation of viruses and Bacteria. Biophys J. 2010;98:1109–18.
Martins LD, da Silva I, Batista WV, Andrade MF, Freitas ED, Jorge Alberto Martins JA. How socio-economic and atmospheric variables impact COVID-19 and influenza outbreaks in tropical and subtropical regions of Brazil. Environ Res. 2020;191:110184.
Kulkarni H, Khandait H, Narlawar UW, Rathod P, Mamtani M. Independent association of meteorological characteristics with initial spread of Covid-19 in India. Sci Total Environ. 2021;764:142801.
Menebo MM. Science of the Total environment temperature and precipitation associate with Covid-19 new daily cases : A correlation study between weather and Covid-19 pandemic in. Sci Total Environ. 2020;737:139659.
Gupta S, Patel KK. Global Epidemiology of First 90 Days into COVID-19 Pandemic :Disease Incidence , Prevalence , Case Fatality Population Density, Urbanisation. J Health Manag. 2020;22(2):117–28.
Haque SE, Rahman M. Association between temperature, humidity, and COVID-19 outbreaks in Bangladesh. Environ Sci Pol. 2020;114:253–5.
Sharma P, Singh AK, Agrawal B, Sharma A. Correlation between weather and COVID-19 pandemic in India: an empirical investigation. J Public Aff. 2020;20(4)
Fu S, Wang B, Zhou J, Xu X, Liu J, Ma Y, et al. Meteorological factors, governmental responses and COVID-19: evidence from four European countries. Environ Res. 2021;194:110596.
Mecenas P, Bastos RT, Vallinoto AC, Normando D. Effects of temperature and humidity on the spread of COVID-19: A systematic review. PLoS One. 2020;15:1–21.
Malki Z, Atlam ES, Hassanien AE, Dagnew G, Elhosseini MA, Gad I. Association between weather data and COVID-19 pandemic predicting mortality rate: machine learning approaches. Chaos, Solitons Fractals. 2020;138:110137.
Sasikumar K, Nath D, Nath R, Chen W. Impact of extreme hot climate on COVID-19 outbreak in India. GeoHealth. 2020;4(12)
Kodera S, Rashed EA, Hirata A. Correlation between COVID-19 morbidity and mortality rates in Japan and local population density, temperature, and absolute humidity. Int J Environ Res Public Health. 2020;17(15):1–14.
Sobral MFF, Duarte GB, da Penha Sobral AIG, Marinho MLM, de Souza Melo A. Association between climate variables and global transmission oF SARS-CoV-2. Sci Total Environ. 2020;729:138997.
Patel SK, Pathak M, Tiwari R, Yatoo MI, Malik YS. A vaccine is not too far for COVID-19 coronavirus pandemic A vaccine is not too far for COVID-19. May; 2020.
Nicola M, Neill NO, Sohrabi C, Khan M, Agha M, Agha R. Evidence based management guideline for the COVID-19 pandemic - review article. Int J Surg. 2020;77:206–16.
Atangana E, Atangana A. Facemasks simple but powerful weapons to protect against COVID-19 spread: can they have sides effects? Results in Physics. 2020;19:103425.
Sarmadi M, Moghanddam VK, Dickerson AS, Martelletti L. Association of COVID-19 distribution with air quality, sociodemographic factors, and comorbidities: an ecological study of US states. Air Qual Atmos Health. 2021;14(4):455–65.
Chung CJ, Nazif TM, Wolbinski M, Hakemi E, Lebehn M, Brandwein R, et al. The restructuring of structural heart disease practice during The Covid-19 pandemic. J Am Coll Cardiol. 2020; InPress
Bukhari Q, Massaro JM, D’agostino RB, Khan S. Effects of weather on coronavirus pandemic. Int J Environ Res Public Health. 2020;17(15):1–12.
Sun C, Zhai Z. The efficacy of social distance and ventilation effectiveness in preventing COVID-19 transmission. Sustain Cities Soc. 2020;62:102390.
Nair N, Selvaraj P. Using a cultural and social identity lens to understand pandemic responses in the US and India. Int J Cross-cult Manag. 2021;21(3):545–68.
Cetron M, Landwirth J. Public health and ethical considerations in planning for quarantine. Yale J Biol Med. 2005;78(5):325–30.
Jernigan DB. Update: public health response to the coronavirus disease 2019 outbreak—United States, February 24, 2020. MMWR. Morbidity and mortality weekly report, 69. 2020.
Stasi C, Fallani S, Voller F, Silvestri C. Treatment for COVID-19: an overview. Eur J Pharmacol. 2020;889:173644.
Foad CAKK, Xun N, Pejman J, Nataraj RC. Nonlinear dynamic analysis of an epidemiological model for COVID-19 including public behavior and government action. Nonlinear Dynamics. 2020;101(3):1545–59.
Sun Z, Zhang H, Yang Y, Wan H, Wang Y. Science of the Total environment impacts of geographic factors and population density on the COVID-19 spreading under the lockdown policies of China. Sci Total Environ. 2020;746(666):141347.
Abdullah S, Mansor AA, Napi NNLM, Mansor WNW, Ahmed AN, Ismail M, et al. Air quality status during 2020 Malaysia movement control order (MCO) due to 2019 novel coronavirus (2019-nCoV) pandemic. Sci Total Environ. 2020;729:139022.
Menhat M, Yusuf Y. Factors influencing the choice of performance measures for the oil and gas supply chain - exploratory study. IOP Conference Series: Materials Science and Engineering. 2018;342(1)
Ćirkovića MM, Vukotića B. Long-term prospects: mitigation of supernova and gamma-ray burst threat to intelligent beings. Acta Astronautica. 2016;129:438–46.
Asyary A, Veruswati M. Science of the Total environment sunlight exposure increased Covid-19 recovery rates : A study in the central pandemic area of Indonesia. Sci Total Environ. 2020;729:139016.
Jayaweeraa M, Pererab H, Gunawardanaa B, Manatungea J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ Res. 2020;188:1–18.
Leung K, Shum MHH, Leung GM, Lam TTY, Wu JT. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill. 2020;26(1)
Download references
Acknowledgements
The authors would also like to acknowledge the Editors and an anonymous reviewer, who contributed immensely to improving the quality of this publication and a special thanks to Muhammad Hafiy Nauwal Effi Helmy, that contributed an excellent idea through singing during the COVID-19 lockdown period.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Faculty of Maritime Studies, Universiti Malaysia Terengganu, 21030, Kuala Nerus, Terengganu, Malaysia
Masha Menhat
Institute of Oceanography and Environment, Universiti Malaysia Terengganu, 21030, Kuala Nerus, Terengganu, Malaysia
Effi Helmy Ariffin, Junainah Zakaria & Mohd Fadzil Akhir
Faculty of Science and Marine Environment, Universiti Malaysia Terengganu, 21030, Kuala Nerus, Terengganu, Malaysia
Wan Shiao Dong & Aminah Ismailluddin
Institute for Social Science Studies, Universiti Putra Malaysia, 43400, Serdang, Selangor, Malaysia
Hayrol Azril Mohamed Shafril
Social, Environmental and Developmental Sustainability Research Center, Faculty of Social Sciences and Humanities, Universiti Kebangsaan Malaysia, 43600, Bangi, Selangor, Malaysia
Mahazan Muhammad
Institute of Geology Malaysia, Board of Geologists, 62100, Putrajaya, Malaysia
Ahmad Rosli Othman
Executive Office, Proofreading By A UK PhD, 51-1, Biz Avenue II, 63000, Cyberjaya, Malaysia
Thavamaran Kanesan
Faculty of Engineering, Universiti Malaya, 50603, Kuala Lumpur, Malaysia
Suzana Pil Ramli
Faculty of Applied Sciences, Uva Wellassa University, Badulla, 90000, Sri Lanka
Amila Sandaruwan Ratnayake
You can also search for this author in PubMed Google Scholar
Contributions
All authors have been involved in writing this editorial and contributing to the review of the manuscript. MM and EHA contribute to conceptualization. IA and ARO have made the figure.
Corresponding author
Correspondence to Effi Helmy Ariffin .
Ethics declarations
Ethics approval and consent to participate.
Not Applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Menhat, M., Ariffin, E.H., Dong, W.S. et al. Rain, rain, go away, come again another day: do climate variations enhance the spread of COVID-19?. Global Health 20 , 43 (2024). https://doi.org/10.1186/s12992-024-01044-w
Download citation
Received : 27 July 2023
Accepted : 22 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s12992-024-01044-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Solar radiation
- Temperature
- Social distancing
Globalization and Health
ISSN: 1744-8603
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 13 November 2021
Revisiting COVID-19 policies: 10 evidence-based recommendations for where to go from here
- Daniel T. Halperin ORCID: orcid.org/0000-0003-1557-5379 1 ,
- Norman Hearst ORCID: orcid.org/0000-0003-1218-9173 2 ,
- Stephen Hodgins ORCID: orcid.org/0000-0001-8365-3311 3 ,
- Robert C. Bailey ORCID: orcid.org/0000-0002-9202-1794 4 ,
- Jeffrey D. Klausner ORCID: orcid.org/0000-0002-6922-7364 5 ,
- Helen Jackson 6 ,
- Richard G. Wamai ORCID: orcid.org/0000-0001-6566-5159 7 , 8 ,
- Joseph A. Ladapo ORCID: orcid.org/0000-0002-8518-4800 9 ,
- Mead Over 10 ,
- Stefan Baral ORCID: orcid.org/0000-0002-5482-2419 11 ,
- Kevin Escandón ORCID: orcid.org/0000-0002-7173-7486 12 , 13 na1 &
- Monica Gandhi ORCID: orcid.org/0000-0002-7025-1994 14 na1
BMC Public Health volume 21 , Article number: 2084 ( 2021 ) Cite this article
40k Accesses
27 Citations
468 Altmetric
Metrics details
Strategies to control coronavirus 2019 disease (COVID-19) have often been based on preliminary and limited data and have tended to be slow to evolve as new evidence emerges. Yet knowledge about COVID-19 has grown exponentially, and the expanding rollout of vaccines presents further opportunity to reassess the response to the pandemic more broadly.
We review the latest evidence concerning 10 key COVID-19 policy and strategic areas, specifically addressing: 1) the expansion of equitable vaccine distribution, 2) the need to ease restrictions as hospitalization and mortality rates eventually fall, 3) the advantages of emphasizing educational and harm reduction approaches over coercive and punitive measures, 4) the need to encourage outdoor activities, 5) the imperative to reopen schools, 6) the far-reaching and long-term economic and psychosocial consequences of sustained lockdowns, 7) the excessive focus on surface disinfection and other ineffective measures, 8) the importance of reassessing testing policies and practices, 9) the need for increasing access to outpatient therapies and prophylactics, and 10) the necessity to better prepare for future pandemics.
Conclusions
While remarkably effective vaccines have engendered great hope, some widely held assumptions underlying current policy approaches call for an evidence-based reassessment. COVID-19 will require ongoing mitigation for the foreseeable future as it transforms from a pandemic into an endemic infection, but maintaining a constant state of emergency is not viable. A more realistic public health approach is to adjust current mitigation goals to be more data-driven and to minimize unintended harms associated with unfocused or ineffective control efforts. Based on the latest evidence, we therefore present recommendations for refining 10 key policy areas, and for applying lessons learned from COVID-19 to prevent and prepare for future pandemics.
Peer Review reports
The coronavirus disease 2019 (COVID-19) pandemic has caused devastating loss of life and disrupted healthcare systems and daily life globally. By late October 2021, over 245 million confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection cases and over 4.9 million related deaths had been reported globally [ 1 ]. As the international vaccination rollout continues to expand [ 2 ], we call for a reexamination of existing mitigation approaches to adapt to emerging evidence on effectiveness and to minimize unintended consequences. COVID-19 vaccines have proven to be highly effective at preventing severe disease and mortality and, to a lesser extent, milder symptomatic and asymptomatic cases. While vaccination has ushered in great hope, the time is ripe to revisit the assumptions underlying some current interventions and to implement more context-sensitive, evidence-based policies. Accordingly, we review the available evidence regarding 10 key policy areas for which we recommend modification or refinement (Fig. 1 ).

Evidence-based recommendations for 10 key COVID-19 policy and strategic areas. Figure designed by Karina Escandón
One limitation of this review is the paucity of data from randomized controlled trials (RCTs) to measure the efficacy and effectiveness of COVID-19 prevention interventions. Aside from vaccines and therapeutics [ 3 , 4 ], the only exceptions to date are two RCTs of masks [ 5 , 6 ]. A trial conducted in Denmark found no statistically significant difference in infection rates between the group provided with and urged to wear surgical masks and a control group [ 5 ]. Meanwhile, a cluster RCT in Bangladesh found a statistically significant 9% reduction in symptomatic seroprevalence in villages where surgical masks were provided and their use promoted [ 6 ]. In this study, no significant decrease in symptomatic seroprevalence was observed in villages where cloth masks were promoted. Moreover, some public health interventions can be difficult or even impossible to definitely study with RCTs [ 7 – 10 ]. We therefore rely mainly on the best available observational data, despite limitations and potential biases, to suggest refinements to current approaches and policies.
1: Accelerate vaccination rollout
Even with the continuing emergence of viral variants, widespread vaccination remains the quickest and most powerful way to reduce the toll from COVID-19 and continue returning toward a greater sense of normality. Maximizing global vaccine production and equitable distribution must be the highest priority, with innovative mechanisms of financing and licensing production as required. The wealthier countries should largely pay for this ongoing effort as a humanitarian imperative as well as from enlightened self-interest. This could be modeled on the experience with AIDS, in which antiretroviral drugs are provided to poorer countries by bilateral and multilateral donors at discounted prices and/or through low-cost international generic production via waived patents [ 11 , 12 ], while pharmaceutical companies continue to benefit financially in higher-income countries. Many countries have recently made encouraging promises in this regard, including at the June 2021 G7 Summit [ 13 ], but such promises will need to be kept if not exceeded.
Since vaccine supplies are still not adequate to meet the global population’s needs, they must be used as strategically and efficiently as possible. Such strategies include prioritizing vulnerable populations and healthcare workers (HCWs), and delaying doses for those with previous COVID-19 until those without prior immunity are vaccinated. Delaying the second dose of 2-dose vaccines for longer than the interval used in clinical trials may increase overall public health benefit by maximizing coverage with first doses more quickly and may also lead to greater immunogenicity [ 14 – 19 ]. The US Centers for Disease Control and Prevention (CDC) recommended that the second dose can be given up to 6 weeks following the first one [ 20 ], but implementing an even longer duration between doses, when necessary, is consistent with a population-health perspective. Several countries, such as Canada, have taken this approach of extending the duration between doses. Moreover, persons known to have been previously infected may defer vaccination for 6 months or perhaps even longer post-infection [ 21 , 22 ], and when they get vaccinated, appear to require only 1 dose of a 2-dose vaccine regimen [ 23 – 26 ].
Such approaches will require careful implementation and messaging to minimize the potential risk of persons not getting vaccinated in the unconfirmed belief that they have already been infected, or not returning for a second dose in the mistaken belief that they are fully protected by a single dose. Other challenges facing some countries involve choosing between rapid application of less effective vaccines or waiting for the availability of better ones. Generally, those approaches that offer the most people some protection as quickly as possible should be followed. Furthermore, higher-income countries should refrain from distributing booster shots more broadly or frequently than necessary (e.g., for the immunocompetent general population), as this appears currently unjustified both scientifically and ethically [ 27 , 28 ]. In late October 2021, it was estimated that globally about three times as many booster shots were being given per day compared to the total number of vaccine shots administered daily in lower-income countries [ 29 ].
Vaccination and other mitigation efforts must focus on protecting the most vulnerable through prioritizing the elderly, HCWs, and other essential workers. Additional criteria for determining which persons should be prioritized due to existing medical conditions must be evidence-based. Cardiometabolic comorbidities such as diabetes, chronic obstructive pulmonary disease, hypertension, and obesity are known risk factors strongly associated with increased COVID-19 severity and mortality [ 30 – 35 ]. But asthma, for example, turns out not to be a risk factor (and is probably even partially protective against death and other serious COVID-19 outcomes) [ 36 – 41 ]. In certain situations and particularly among groups at the highest risk of disease or exposure, vaccination mandates can be considered, e.g., for working in hospitals, nursing homes, prisons, or other high-risk settings [ 42 , 43 ]. While we applaud the US and other countries for having joined the World Health Organization (WHO) COVAX Initiative, we urge high-income countries to also unilaterally deploy their soon-to-expire as well as other doses overseas and to join the WHO COVID-19 Technology Access Pool, which would allow other countries to produce patented vaccines, thereby expanding their availability in low and middle-income countries [ 11 ]. International governance of vaccine distribution is essential to address vaccine inequity and to maximize outcomes globally.
2: Gradually ease restrictions as vaccination expands
Accumulating real-world evidence is documenting the large extent to which COVID-19 vaccines reduce severe disease, hospitalizations, and mortality. Although asymptomatic infection and symptomatic disease were both greatly reduced by the vaccines in the context of the Alpha variant and earlier D614G mutants [ 18 , 19 , 44 – 53 ], more recent data during the ascendency of the Delta variant indicate reduced effectiveness against asymptomatic or mild infections [ 54 – 60 ]. However, the vaccine-induced protection against severe disease from the Delta variant appears to be remarkably intact across multiple settings, at over 90% [ 55 , 59 ]. Declines in antibodies are expected over time following vaccination, but cellular memory (which enhances antibody production and protects against severe disease) appears to be much more durable [ 61 , 62 ].
Once vaccination has been made widely and equitably available and rates of hospitalization and mortality eventually fall, it becomes untenable to expect the vaccinated to follow all current restrictions imposed mainly to protect those who decline vaccination. The same can be said regarding immunity following infection. Given the rarity of reinfection [ 7 , 22 , 63 ] and the duration of immunity post-infection (at least 6–12 months ) [ 21 , 63 – 68 ], those with evidence of prior infection appear to be as immune as those who have been vaccinated [ 69 – 72 ].
Mass vaccination will accelerate achieving much greater pandemic control, allowing measures such as masking and physical distancing to be gradually relaxed [ 7 ]. It is critical to acknowledge the physical, psychological, sociopolitical, and other costs of enforcing restrictions and to begin easing them as hospitalization and death rates fall substantially, while remaining vigilant and ready to revisit such decisions if circumstances change significantly.
3: Emphasize education and harm reduction approaches over coercive and punitive measures
“Abstinence-only” approaches have not worked for AIDS or teen pregnancy prevention [ 73 ], nor have absolutist approaches worked well for preventing SARS-CoV-2 [ 74 , 75 ]. Instead, prevention measures should be founded on the provision of accurate information, sensitively communicated, and informed by harm reduction approaches that are more effective and sustainable in the longer term [ 7 , 74 – 76 ]. Harm reduction involves informing people how to assess and mitigate risk, while acknowledging the real-world conditions that may lead some persons to take calculated risks. One example of a successful mitigation campaign (prior to vaccines) is that of Japan’s 3 Cs, which generally did not shut down society, but instead advised the public to avoid c lose, sustained interactions in c rowded en c losed spaces [ 77 ]. Importantly, educating and motivating the public to adopt effective precautions, including vaccination, as opposed to coercive or punitive measures (e.g., shaming, fines or imprisonment, and even police violence) will be more effective and will help alleviate pandemic response fatigue [ 7 , 78 – 80 ]. Accordingly, any restrictions and mandates, including vaccinations passports [ 81 – 83 ], should focus on high-risk situations and consider a number of scientific and ethical questions. Most importantly, COVID-19 measures should be formulated and reassessed based on the latest information, levels of ongoing threat, and resource availability. As mentioned above, vaccine mandates should be carefully focused and should take into account prior SARS-CoV-2 infection [ 22 ].
4: Encourage outdoor activities
Current evidence on SARS-CoV-2 transmission dynamics must inform policy recommendations for mitigation strategies and restrictions [ 84 ]. Unfortunately, lower-risk activities, especially those conducted in outdoor environments (e.g., parks, beaches, hiking trails, playgrounds), have often been discouraged or even prohibited [ 85 – 90 ]. The risk of SARS-CoV-2 transmission outdoors is vastly lower than indoors, with most studies finding the proportion of new cases attributable to outdoor exposure to be < 1% [ 7 , 42 , 91 – 94 ]. Policies should reflect this enormous difference in risk, including allowing access to outdoor spaces even during periods of severe restrictions and reserving mask mandates for indoor (and very crowded outdoor) situations [ 7 ], as recommended by the WHO and CDC [ 95 – 97 ]. Strongly encouraging outdoor activities and including nuance in public health recommendations (such as discouraging outdoor gatherings from leading to crowded indoor situations) is more consistent with the previously discussed harm reduction-based approaches [ 7 , 98 ]. When weather or other factors preclude holding activities outdoors, windows should be kept open whenever possible, including in shared vehicles [ 99 ], and air ventilation (at least 4 air exchanges per hour) should be ensured to reduce the risk of transmission [ 100 – 102 ].
5: Reopen schools now
COVID-19 has caused by far the largest disruption to learning in recent history [ 103 ]. As the pandemic has unfolded, there is mounting evidence that the harm of keeping schools closed dwarfs any public health benefits [ 41 , 104 , 105 ]. By early 2020, most kindergarten-to-grade 12 (K-12) schools worldwide had closed for in-person instruction, and many remain shuttered over a year later [ 104 , 106 – 109 ]. As of September 2021, based on United Nations Educational, Scientific and Cultural Organization (UNESCO) data [ 109 ], over 100 million students remained affected and 18 countries still had nationwide closures. There is no good substitute for in-person schooling [ 108 ]. Remote learning further exacerbates inequities, especially among communities with low resources, not only related to education but also to safety, wellbeing, social support, and nutrition [ 105 , 108 , 110 – 112 ].
Schools have not been shown to be major drivers of SARS-CoV-2 transmission, when studied in a variety of settings employing a range of mitigation strategies and intensity [ 106 , 107 , 113 , 114 ]. However, their prolonged closure have had disastrous academic, psychosocial, and other harmful consequences on children, including access to essential services, especially in lower-income populations [ 41 , 111 , 115 , 116 ]. Furthermore, contact tracing studies worldwide have found children are less likely to infect adults or other children, and that most SARS-CoV-2 infections among children are mild and are contracted at home or in the community, not at school [ 106 , 107 , 117 – 119 ].
In the US state of North Carolina prior to vaccine availability, 11 school districts (many in regions with high SARS-CoV-2 incidence) implemented in-person instruction accompanied by mitigation plans, for > 90,000 children over 9 weeks [ 117 ]. Across the 11 school districts, there were 773 community-acquired SARS-CoV-2 infections documented by reverse transcriptase-polymerase chain reaction (RT-PCR) testing, of which only 32 were identified as secondary cases, with no cases of within-school transmission from children to teachers or other adults. Among 17 US schools in rural Wisconsin also conducting in-person learning, with a range of precautions, SARS-CoV-2 incidence among students, teachers, and other staff members was lower than in the surrounding communities overall [ 118 ]. During 13 weeks in late 2020, 191 cases were identified among students and staff, of which only 7 (3.7%) cases (all among students) were traced to in-school transmission. In Sweden, where schools generally remained open (and masks have not been required) [ 120 , 121 ], deaths of children aged 1–16 years were statistically similar in the 4 months before versus after COVID-19 arrived, and intensive care unit admission rates for teachers were comparable to those for other occupations [ 122 ]. Many other investigations, such as one among children aged 0 to 19 years in childcare facilities and schools in Baden-Württemberg, Germany, after the reopening of schools in May 2020, have also suggested that child-to-child transmission in school settings is uncommon [ 123 ]. To the extent that in-school transmission is an issue, especially given the continuing emergence of highly transmissible variants (e.g., Delta), vaccinating school staff is likely the most effective way to protect those at risk [ 124 – 126 ].
Also, after reviewing data indicating that 3 ft of physical distancing is sufficient [ 127 ], in March 2021 the CDC modified their guidelines accordingly, at least for elementary school settings [ 128 ]. A large-scale CDC study, comparing schools that mandated various interventions in late 2020 with ones that did not, found that while improving ventilation and requiring teachers and staff members to wear masks was associated with reduced SARS-CoV-2 incidence in schools, mandating students to wear masks was not [ 129 ]. Masking guidelines for children from major public health organizations differ, which has generated confusion. For instance, the CDC currently recommends that all children over age 2 wear masks indoors, while the WHO mask guidance applies to children over age 5, with a caveat that benefits from mask mandates at school may not outweigh the potential academic and psychosocial harms [ 130 ]. Despite the inconsistent data and guidelines, student masking in communities where rates of hospitalization and death remain high may be useful [ 113 ], if for no other reason than to help maintain the necessary consensus to keep schools open.
The emergence of variants does not warrant closing or delaying the reopening of schools unless compelling evidence unexpectedly indicates that a new mutation affects children in some substantially new way [ 131 ]. Reassuring data from high schools [ 106 , 107 , 117 , 118 , 122 , 123 ] suggest that in-person classes also can be safely conducted in colleges, especially if combined with interventions to prevent outside-the-classroom transmission. As endorsed by the United Nations Children’s Fund (UNICEF) [ 132 ], no effort should be spared to keep students in classes, and closing schools should be a measure of last resort.
6: Avoid lockdowns
The cumulative evidence suggests that “sledge-hammer” lockdown approaches, such as the closing of all non-essential workplaces and schools, should be avoided in favor of more effective, carefully targeted “scalpel” public health strategies [ 7 , 78 , 133 , 134 ]. Indiscriminate lockdowns have had far-reaching unintended consequences, disproportionately affecting socioeconomically disadvantaged and vulnerable populations. Other consequences include alarming increases in mental health problems (e.g., depression, anxiety, and social isolation), drug overdose, domestic violence, child abuse, weight gain, abuse by law enforcement in some places, and discontinuation of non-COVID-19 clinical services and prevention programs [ 41 , 78 , 110 , 115 , 134 – 139 ]. While substantial evidence highlights the deleterious impact of sustained lockdowns, the direct impact of SARS-CoV-2 transmission on disease outcomes, healthcare systems, and employment, particularly in the context of huge inequity, can also produce many of the same negative effects, even in the absence of official lockdowns [ 140 , 141 ].
Tailored, context-sensitive interventions involving fewer economic, societal, and quality-of-life costs than lockdowns are likely more effective and minimize harm [ 7 ]. Non-pharmaceutical interventions such as physical distancing, improved ventilation, and effective indoor mask wearing are also more sustainable than broad stay-at-home orders [ 142 – 146 ]. Although emerging genetic SARS-CoV-2 variants may pose additional challenges [ 147 ], the biological and epidemiological evidence suggests that the same interventions will work to reduce their transmission. When lockdowns, isolation, or quarantine measures are mandated, economic hardship should be considered and paid sick/quarantine leaves and other types of support must be provided to affected workers, especially those who are most economically vulnerable [ 7 ].
7: De-emphasize excessive surface disinfection and other ineffective measures
The evidence is consistent that indirect contact (fomite) transmission is not a significant driver of SARS-CoV-2 spread [ 148 – 151 ], as acknowledged by the CDC [ 152 ]. Many routine disinfection rituals, including the ubiquitous usage of alcohol-based hand sanitizers and the excessive use of strong cleaning products, are unnecessary [ 41 , 153 ]. Misuse of sanitizers, cleansers, and disinfectants has resulted in toxic reactions occasionally leading to hospitalization and even death [ 154 – 156 ]. Such hazardous disinfection practices include washing food products with bleach, applying household cleaning or disinfectant products to bare skin, mixing bleach solutions with vinegar or ammonia, and intentionally or accidentally inhaling or ingesting such products [ 155 , 156 ]. Beyond being ineffective and occasionally dangerous, excessive cleaning rituals divert important resources, time, and energy from much more useful forms of prevention [ 151 , 153 ]. There are also growing concerns about the potential longer-term impact on what many scientists have warned is the looming “next pandemic,” that of antimicrobial resistance [ 157 , 158 ]. Similarly to the misplaced focus on disinfection rituals, public health authorities and the media must do a much better job of educating the public how the coronavirus is—and is not—typically transmitted [ 159 , 160 ]. For example, fleeting encounters pose minimal risk, even from more transmissible variants 41 , 78 .
Another pervasive practice, temperature screening—especially when using non-contact handheld cutaneous infrared thermometers—is often inaccurate due to environmental factors (e.g., subject-to-sensor distance, ambient temperature, humidity), operator-dependent performance, device variability, and feature changes in target subjects [ 161 – 165 ]. Furthermore, fever is a poor differentiator of the presence or absence of SARS-CoV-2 infection (and the use of antipyretic drugs may mask fever). The ubiquitous use of thermometers for permitting entry to public establishments is thus ineffective. A systematic review of studies regarding exit and entry screening practices (e.g., symptom questionnaires, body temperature measurement) during previous epidemics of influenza A(H1N1), Ebola, and severe acute respiratory syndrome (SARS) found extremely low or no utility in differentiating infected from uninfected [ 166 ]. For COVID-19, similar findings have been reported, with only a very small proportion of SARS-CoV-2 infection cases detected during such screening practices [ 167 ]. Again, such measures divert resources and attention away from much more effective strategies to control infection.
Furthermore, travel-related restrictions have clearly had a considerable impact on global trade and economies as well as on other systems, including those for international humanitarian responses [ 145 ]. Other negative consequences include generating a false sense of security, discouraging travelers from engaging transparently with authorities, and potentially disincentivizing open disclosure by countries during future outbreaks [ 131 , 168 ]. Although a few countries (e.g., New Zealand, Australia, Taiwan, China), mainly island nations, have attempted SARS-CoV-2 elimination through use of robust quarantine and contact tracing measures [ 7 , 131 , 169 ], it makes little sense, from either an epidemiological or human rights perspective, to shut international land borders or require a negative RT-PCR test result for entry into countries where SARS-CoV-2 is already circulating widely. Similarly, the routine use of quarantine upon arrival and various other entrance screening procedures [ 164 ] are also largely ineffective. Such border controls are akin to confiscating matches after the forest is already ablaze. Experience, including lessons learned during this pandemic, suggests that imposition of travel restrictions also generally fails to prevent the spread of new genetic variants, as their discovery typically lags well behind their emergence, and local detection often depends more on which locations are conducting routine genomic surveillance than on where the new variants actually originate [ 131 ].
8: Reassess testing practices and policies
Experience suggests that choice of diagnostic technologies should be determined by the intended use, whether to detect infection in individuals with suspected clinical symptoms or to identify potentially infectious individuals to inform isolation recommendations and conduct contact tracing. RT-PCR-based assays have so far been the preferred method for most such purposes [ 170 ]. Rapid antigen tests, which are both cheaper and faster, can lead to false negatives, especially in pre-symptomatic carriers, and when conducted without adequate quality control procedures. However, if performed correctly in appropriate populations, they may be sufficiently sensitive and specific for detecting potential infectivity [ 171 ], thus suggesting that antigen tests should increasingly be utilized for public health screening. Moreover, further investigation is needed regarding the extent to which positive SARS-CoV-2 RT-PCR results do not always reflect actual infectiousness [ 172 – 174 ], particularly among vaccinated or asymptomatic persons. Finally, given that vaccination reduces symptomatic and asymptomatic SARS-CoV-2 infections and that vaccinated individuals are likely to be less infectious if infected [ 175 – 177 ], testing and quarantine of vaccinated (or previously infected) persons following exposure to someone with suspected or confirmed COVID-19 should in general only be needed if COVID-19 symptoms develop [ 178 ]. As we increasingly recognize that SARS-CoV-2 is gradually becoming an endemic virus, it is vital to deemphasize identification of new cases as the key outcome metric of mitigation measures and rather to assess mortality and hospitalization rates [ 179 ]. This is also relevant considering that the vaccines were developed to reduce severe and fatal outcomes from COVID-19 and not to fully prevent onward transmission and infection.
9: Expand access to outpatient therapies and prophylactics
As with vaccines, the pandemic has presented challenges in identifying effective therapeutics on a greatly accelerated timeline. Although vaccination remains the priority, some vaccinated individuals will still contract SARS-CoV-2, and some persons will remain unvaccinated. While some medications have been tentatively permitted (not without controversy) on a compassionate use basis in a few countries, approved outpatient therapies for COVID-19 have been limited in most places to intravenous monoclonal antibodies, which are cost-prohibitive in most settings globally and often pose other considerable challenges for widespread use. As evidence on treatment options evolves, policymakers should prioritize quick access to effective outpatient therapies in patients with risk factors for severe disease and to prophylactics for unvaccinated persons at high risk. Assessment of previously identified safe medications might be an efficient way to quickly identify new therapies [ 180 ]. In addition, more research is urgently needed regarding the prevalence, diagnosis, prognosis, and treatment options for longer-term (“long haul”) COVID-19 complications.
10: Prevent and prepare for future pandemics
COVID-19 is the second major respiratory viral pandemic in just over a decade and the third coronavirus pandemic within 2 decades. More pandemics are likely in the coming years, whether from new coronaviruses and/or from other pathogens. We clearly must do everything possible to prevent and be better prepared for future pandemics and other public health emergencies [ 181 , 182 ], and must learn and apply lessons from the recent experience with mitigating COVID-19.
Regarding prevention, policymakers need to take prudent actions immediately to reduce the likelihood of future pandemics, including addressing environmental destruction that brings different species into closer contact with humans, restricting the trafficking of animals, and strengthening biosecurity in laboratories that work with potential human pathogens.
Preparation for the next pandemics should include detailed plans by international organizations that are widely vetted and agreed upon. Lockdowns and quarantines, when (and only if) necessary, need to be designed equitably and to include protection, prioritization, and compensation for those most vulnerable [ 7 ], including the elderly, the poor, and workers in frontline and informal jobs. Effective mechanisms must also be established to address equity in access to treatments and vaccines, prioritizing those at highest risk. We certainly must avoid another situation where public health authorities and politicians are left to fly blind and then try to clean up the damage later. It would be a grave error to respond to a new pandemic without applying lessons from the current one.
Given the high transmissibility of SARS-CoV-2, its continuing widespread circulation in some regions, and the emergence of new viral variants [ 147 ], it is unlikely that SARS-CoV-2 will be eradicated. Therefore, we will need to continue focusing on mitigation strategies, particularly vaccination [ 131 ]. Although SARS-CoV-2 genetic variants will keep emerging, vaccines have so far largely retained their ability to prevent fatal and other severe COVID-19 outcomes [ 183 , 184 ]. Concerns that such variants will soon evade current vaccines may be overstated, as both the mRNA and adenovirus-DNA vaccines encode for the entire spike protein, providing robust and complex antibody-mediated as well as T-cell immune responses [ 17 , 21 , 185 , 186 ]. Furthermore, vaccines can be rapidly modified, if necessary, to adapt to future variants [ 183 , 184 ]. As previously noted, it is crucial to focus on the key public health objectives of preventing death and other severe disease outcomes, rather than continuing to use numbers of reported cases as the main metric. In any event, maintaining a constant state of emergency until the pandemic is over is not viable. Public health decision-making requires transparency and debate, which are often precluded by emergency orders. A more realistic public health goal is to adjust mitigation and treatment goals as the pandemic evolves, minimizing negative outcomes including the unintended harms associated with unfocused or irrelevant control efforts. The foregoing suggestions for refining our current approaches are presented as best practices that will nevertheless require continuous adjustment through reassessment of the latest evidence. We offer these in the reasonable hope of widespread vaccination helping to achieve far greater control of COVID-19, and also that the world will be better prepared for the next pandemic.
Availability of data and materials
Not applicable.
Abbreviations
US Centers for Disease Control and Prevention
Coronavirus disease 2019
Healthcare worker
Randomized controlled trial
Reverse transcriptase-polymerase chain reaction
Severe acute respiratory syndrome
Severe acute respiratory syndrome coronavirus 2
United Nations Educational, Scientific and Cultural Organization
United Nations Children’s Fund
World Health Organization
Johns Hopkins University. Coronavirus Resource Center. https://coronavirus.jhu.edu/ . Accessed 30 Oct 2021.
Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5(7):947–53. https://doi.org/10.1038/s41562-021-01122-8 .
Article PubMed Google Scholar
He Q, Mao Q, Zhang J, Bian L, Gao F, Wang J, et al. COVID-19 vaccines: current understanding on immunogenicity, safety, and further considerations. Front Immunol. 2021;12:669339. https://doi.org/10.3389/fimmu.2021.669339 .
Article CAS PubMed PubMed Central Google Scholar
Lai C-C, Chen I-T, Chao C-M, Lee P-I, Ko W-C, Hsueh P-R. COVID-19 vaccines: concerns beyond protective efficacy and safety. Expert Rev Vaccines. 2021;20(8):1013–25. https://doi.org/10.1080/14760584.2021.1949293 .
Article CAS PubMed Google Scholar
Bundgaard H, Bundgaard JS, Raaschou-Pedersen DET, von Buchwald C, Todsen T, Norsk JB, et al. Effectiveness of adding a mask recommendation to other public health measures to prevent SARS-CoV-2 infection in Danish mask wearers. Ann Intern Med. 2021;174(3):335–43. https://doi.org/10.7326/M20-6817 .
Abaluck J, Kwong LH, Styczynski A, Haque A, Kabir A, Bates-Jefferys E, et al. The impact of community masking on COVID-19: A cluster-randomized trial in Bangladesh. Preprint at poverty-action.org . 2021. https://www.poverty-action.org/publication/impact-community-masking-covid-19-cluster-randomized-trial-bangladesh , DOI: https://doi.org/10.1093/qje/qjab017 .
Escandón K, Rasmussen AL, Bogoch II, Murray EJ, Escandón K, Popescu SV, et al. COVID-19 false dichotomies and a comprehensive review of the evidence regarding public health, COVID-19 symptomatology, SARS-CoV-2 transmission, mask wearing, and reinfection. BMC Infect Dis. 2021;21(1):710. https://doi.org/10.1186/s12879-021-06357-4 .
Victora CG, Habicht J-P, Bryce J. Evidence-based public health: moving beyond randomized trials. Am J Public Health. 2004;94(3):400–5. https://doi.org/10.2105/AJPH.94.3.400 .
Article PubMed PubMed Central Google Scholar
Rutter H, Savona N, Glonti K, Bibby J, Cummins S, Finegood DT, et al. The need for a complex systems model of evidence for public health. Lancet. 2017;390(10112):2602–4. https://doi.org/10.1016/S0140-6736(17)31267-9 .
Greenhalgh T. Will COVID-19 be evidence-based medicine’s nemesis? PLoS Med. 2020;17(6):e1003266. https://doi.org/10.1371/journal.pmed.1003266 .
Iacobucci G. Covid-19: how will a waiver on vaccine patents affect global supply? BMJ. 2021;373:n1182. https://doi.org/10.1136/bmj.n1182 .
Gandhi M. The most important thing rich countries can do to help; 2021. https://time.com/6046096/india-covid-19-vaccine-patents/ . Accessed 22 Jun 2021.
Martuscelli C. G7 leaders pledge 870 million vaccines to developing world; 2021. Politico. https://www.politico.eu/article/g7-leaders-coronavirus-vaccines-developing-countries/ . Accessed 30 Jun 2021.
Parry H, Bruton R, Stephens C, Brown K, Amirthalingam G, Hallis B, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation. Preprint at medRxiv. 2021. https://doi.org/10.1101/2021.05.15.21257017 .
Parry H, Bruton R, Stephens C, Brown K, Amirthalingam G, Otter A, et al. Differential immunogenicity of BNT162b2 or ChAdOx1 vaccines after extended-interval homologous dual vaccination in older people. Immun Ageing. 2021;18(1):34. https://doi.org/10.1186/s12979-021-00246-9 .
Payne RP, Longet S, Austin JA, Skelly DT, Dejnirattisai W, Adele S, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021 (in press). https://doi.org/10.1016/j.cell.2021.10.011 .
Plotkin SA, Halsey N. Accelerate coronavirus disease 2019 (COVID-19) vaccine rollout by delaying the second dose of mRNA vaccines. Clin Infect Dis. 2021;73(7):1320–1. https://doi.org/10.1093/cid/ciab068 .
Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397(10277):875–7. https://doi.org/10.1016/S0140-6736(21)00448-7 .
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. https://doi.org/10.1016/S0140-6736(20)32661-1 .
US Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States; 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html . Accessed 28 Aug 2021.
Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. https://doi.org/10.1126/science.abf4063 .
Klausner J, Kojima N. Op-Ed: Quit ignoring natural COVID immunity; 2021. Medpage Today. https://www.medpagetoday.com/infectiousdisease/covid19/92836 . Accessed 20 Jun 2021.
Saadat S, Tehrani ZR, Logue J, Newman M, Frieman MB, Harris AD, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;383(24):2320–32. https://doi.org/10.1001/jama.2021.3341 .
Article CAS Google Scholar
Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021:27(6):981–4. https://doi.org/10.1038/s41591-021-01325-6 .
Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–8. https://doi.org/10.1016/S0140-6736(21)00501-8 .
Prendecki M, Clarke C, Brown J, Cox A, Gleeson S, Guckian M, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397(10280):1178–81. https://doi.org/10.1016/S0140-6736(21)00502-X .
Maxmen A. COVID boosters for wealthy nations spark outrage. Nature News. 2021. https://doi.org/10.1038/d41586-021-02109-1 .
Pai M. 10 images that illustrate the shameful global vaccine inequity, Nature Portfolio Microbiology Community. 2021. https://naturemicrobiologycommunity.nature.com/posts/10-images-illustrate-the-global-vaccine-inequity
Mancini DP, Burn-Murdoch D. Global Covid-19 death toll tops 5m but underestimates true figure, say experts. 2021. Financial times. https://www.ft.com/content/35a3d40a-f71f-4fca-893d-884fec5633d8 . Accessed 2 Nov 2021.
Brosseau LM, Escandón K, Ulrich AK, Rasmussen AL, Roy CJ, Bix GJ, et al. SARS-CoV-2 dose, infection, and disease outcomes for COVID-19 – a review. Clin Infect Dis. 2021 (Epub ahead of print). https://doi.org/10.1093/cid/ciab903 .
Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS One. 2020;15(8):e0238215. https://doi.org/10.1371/journal.pone.0238215 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Inf Secur. 2020;81(2):e16–25. https://doi.org/10.1016/j.jinf.2020.04.021 .
Fang X, Li S, Yu H, Wang P, Zhang Y, Chen Z, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. 2020;12(13):12493–503. https://doi.org/10.18632/aging.103579 .
Földi M, Farkas N, Kiss S, Zádori N, Váncsa S, Szakó L, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: a systematic review and meta-analysis. Obes Rev. 2020;21(10):e13095. https://doi.org/10.1111/obr.13095 .
Mendes NF, Jara CP, Mansour E, Araújo EP, Velloso LA. Asthma and COVID-19: a systematic review. Allergy, Asthma Clin Immunol. 2021;17(1):5. https://doi.org/10.1186/s13223-020-00509-y .
Halpin DMG, Faner R, Sibila O, Badia JR, Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8(5):436–8. https://doi.org/10.1016/S2213-2600(20)30167-3 .
Sunjaya AP, Allida SM, Di Tanna GL, Jenkins C. Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: systematic review and meta-analysis. J Asthma. 2021 (Epub ahead of print). https://doi.org/10.1080/02770903.2021.1888116 .
Wang Y, Chen J, Chen W, Liu L, Dong M, Ji J, et al. Does asthma increase the mortality of patients with COVID-19?: a systematic review and meta-analysis. Int Arch Allergy Immunol. 2021;182(1):76–82. https://doi.org/10.1159/000510953 .
Morais-Almeida M, Pité H, Aguiar R, Ansotegui I, Bousquet J. Asthma and the coronavirus disease 2019 pandemic: a literature review. Int Arch Allergy Immunol. 2020;181(9):680–8. https://doi.org/10.1159/000509057 .
Halperin DT. Facing COVID without panic: 12 common myths and 12 lesser known facts about the pandemic, clearly explained by an epidemiologist. Independently published; July 2020.
Leclerc QJ, Fuller NM, Knight LE, Funk S, Knight GM. What settings have been linked to SARS-CoV-2 transmission clusters? Wellcome Open Res. 2020;5:83. https://doi.org/10.12688/wellcomeopenres.15889.2 .
Althouse BM, Wenger EA, Miller JC, Scarpino SV, Allard A, Hébert-Dufresne L, et al. Superspreading events in the transmission dynamics of SARS-CoV-2: opportunities for interventions and control. PLoS Biol. 2020;18(11):e3000897. https://doi.org/10.1371/journal.pbio.3000897 .
Pawlowski C, Lenehan P, Puranik A, Agarwal V, Venkatakrishnan AJ, Niesen MJM, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med. 2021;2(8):979–992.e8. https://doi.org/10.1016/j.medj.2021.06.007 .
Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers — eight U.S. locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(13):495–500. https://doi.org/10.15585/mmwr.mm7013e3 .
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. https://doi.org/10.1056/NEJMoa2034577.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. https://doi.org/10.1056/NEJMoa2035389 .
Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–35. https://doi.org/10.1016/S0140-6736(21)00790-X .
Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397(10282):1351–62. https://doi.org/10.1016/S0140-6736(21)00628-0 .
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–29. https://doi.org/10.1016/S0140-6736(21)00947-8 .
Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–21. https://doi.org/10.1038/s41591-021-01446-y .
Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. https://doi.org/10.1136/bmj.n1088 .
Pritchard E, Matthews PC, Stoesser N, Eyre DW, Gethings O, Vihta K-D, et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med. 2021;27(8):1370–8. https://doi.org/10.1038/s41591-021-01410-w .
Rosenberg ES, Holtgrave DR, Dorabawila V, Conroy M, Greene D, Lutterloh E, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status — New York, May 3–July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1150–5. https://doi.org/10.15585/mmwr.mm7034e1 .
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–94. https://doi.org/10.1056/NEJMoa2108891 .
Fowlkes A, Gaglani M, Groover K, Thiese MS, Tyner H, Ellingson K. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) variant predominance — eight U.S. locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1167–9. https://doi.org/10.15585/mmwr.mm7034e4 .
Nanduri S, Pilishvili T, Derado G, Soe MM, Dollard P, Wu H, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1163–6. https://doi.org/10.15585/mmwr.mm7034e3 .
Tande AJ, Pollock BD, Shah ND, Binnicker M, Berbari EF. mRNA vaccine effectiveness against asymptomatic SARS-CoV-2 infection over a seven-month period. Infect Control Hosp Epidemiol. 2021 (Epub ahead of print). https://doi.org/10.1017/ice.2021.399 .
Seppälä E, Veneti L, Starrfelt J, Danielsen AS, Bragstad K, Hungnes O, et al. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Eurosurveillance. 2021;26(35):2100793. https://doi.org/10.2807/1560-7917.ES.2021.26.35.2100793 .
Article PubMed Central Google Scholar
Bian L, Gao Q, Gao F, Wang Q, He Q, Wu X, et al. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20(10):1201–9. https://doi.org/10.1080/14760584.2021.1976153 .
Noh JY, Jeong HW, Kim JH, Shin E. T cell-oriented strategies for controlling the COVID-19 pandemic. Nat Rev Immunol. 2021;21(11):687–8. https://doi.org/10.1038/s41577-021-00625-9 .
Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–80. https://doi.org/10.1016/j.cell.2021.01.007 .
Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384(6):533–40. https://doi.org/10.1056/NEJMoa2034545 .
Vitale J, Mumoli N, Clerici P, De Paschale M, Evangelista I, Cei M, et al. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy, Italy. JAMA Intern Med. 2021;384(6):533–40. https://doi.org/10.1001/jamainternmed.2021.2959 .
Article Google Scholar
Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet. 2021;397(10283):1459–69. https://doi.org/10.1016/S0140-6736(21)00675-9 .
Leidi A, Koegler F, Dumont R, Dubos R, Zaballa M-E, Piumatti G, et al. Risk of reinfection after seroconversion to SARS-CoV-2: a population-based propensity-score matched cohort study. Clin Infect Dis. 2021 (Epub ahead of print). https://doi.org/10.1093/cid/ciab495 .
Ripperger TJ, Uhrlaub JL, Watanabe M, Wong R, Castaneda Y, Pizzato HA, et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53(5):925–933.e4. https://doi.org/10.1016/j.immuni.2020.10.004 .
Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397(10280):1204–12. https://doi.org/10.1016/S0140-6736(21)00575-4 .
Gazit S, Shlezinger R, Perez G, Lotan R, Peretz A, Ben-Tov A, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. Preprint at medRxiv. 2021. https://doi.org/10.1101/2021.08.24.21262415 .
Skelly DT, Harding AC, Gilbert-Jaramillo J, Knight ML, Longet S, Brown A, et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat Commun. 2021;12(1):5061. https://doi.org/10.1038/s41467-021-25167-5 .
Greaney AJ, Loes AN, Gentles LE, Crawford KHD, Starr TN, Malone KD, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13(600):eabi9915. https://doi.org/10.1126/scitranslmed.abi9915 .
Block J. Vaccinating people who have had covid-19: why doesn’t natural immunity count in the US? BMJ. 2021;374:n2101. https://doi.org/10.1136/bmj.n2101 .
Potts M, Halperin DT, Kirby D, Swidler A, Marseille E, Klausner JD, et al. Public health. Reassessing HIV prevention. Science. 2008;320(5877):749–50. https://doi.org/10.1126/science.1153843 .
Kutscher E, Greene RE. A harm-reduction approach to coronavirus disease 2019 (COVID-19)—safer socializing. JAMA Health Forum. 2020;1(6):e200656. https://doi.org/10.1001/jamahealthforum.2020.0656 .
Marcus J. Quarantine fatigue is real; 2020. The Atlantic. https://www.theatlantic.com/ideas/archive/2020/05/quarantine-fatigue-real-and-shaming-people-wont-help/611482/ . Accessed 20 May 2020.
Barocas J, Gandhi M. Harm reduction principles can help us restore trust in public health messaging on covid-19. 2020. The BMJ Opinion. https://blogs.bmj.com/bmj/2020/12/15/harm-reduction-principles-can-help-us-restore-trust-in-public-health-messaging-on-covid-19/ . Accessed 8 Mar 2021.
Google Scholar
Normile D. Japan ends its COVID-19 state of emergency. 2020. Science. https://doi.org/10.1126/science.abd0092 .
Halperin DT. Coping with COVID-19: learning from past pandemics to avoid pitfalls and panic. Glob Heal Sci Pract. 2020;8(2):155–65. https://doi.org/10.9745/GHSP-D-20-00189 .
Barocas J, Gonsalves G. Make it easier to stay safe from COVID- 19, instead of shaming and punishing people. 2020. USA Today. https://www.usatoday.com/story/opinion/2020/12/07/stop-covid-shaming-punishing-give-incentives-to-stay-safe-column/3812823001/ . Accessed 31 Jan 2021.
Marcus J, Martin M. Epidemiologist on why “pandemic shaming” isn’t working; 2020. National Public Radio. https://www.npr.org/2020/12/19/948403401/epidemiologist-on-why-pandemic-shaming-isn-t-working . Accessed 1 Jan 2021.
Pavli A, Maltezou HC. COVID-19 vaccine passport for safe resumption of travel. J Travel Med. 2021;28(4):taab079. https://doi.org/10.1093/jtm/taab079 .
Sharun K, Tiwari R, Dhama K, Rabaan AA, Alhumaid S. COVID-19 vaccination passport: prospects, scientific feasibility, and ethical concerns. Hum Vaccin Immunother. 2021 (Epub ahead of print). https://doi.org/10.1080/21645515.2021.1953350 .
Dye C, Mills MC. COVID-19 vaccination passports. Science. 2021;371(6535):1184. https://doi.org/10.1126/science.abi5245 .
Cevik M, Marcus JL, Buckee C, Smith TC. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission dynamics should inform policy. Clin Infect Dis. 2021;73(S2):S170–6. https://doi.org/10.1093/cid/ciaa1442 .
Miller AM. Stop shaming people for going outside. The risks are generally low, and the benefits are endless. 2020. Business Insider. https://www.businessinsider.com/you-can-still-go-outside-while-quarantining-sheltering-in-place-2020-4 . Accessed 27 Jun 2020.
Popkin G. Don’t cancel the outdoors. We need it to stay sane. 2020. The Washington Post. https://www.washingtonpost.com/outlook/2020/03/24/dont-cancel-outdoors-we-need-them-stay-sane/ . Accessed 1 Jun 2020.
DeCosta-Klipa N. UMass Amherst is prohibiting outdoor exercise during its lockdown. But why? 2021. Boston.com . https://www.boston.com/news/coronavirus/2021/02/11/umass-amherst-lockdown-outdoor-exercise . Accessed 8 Mar 2021.
Bote J. Officers at dorms, outdoor exercise ban: UC Berkeley extends dorm lockdown with stricter mandates. 2021. SFGate. https://www.sfgate.com/education/article/Police-dorms-outdoor-exercise-UC-Berkeley-lockdown-15937294.php . Accessed 8 Mar 2021.
Tufekci Z. Keep the parks open; 2020. The Atlantic. https://www.theatlantic.com/health/archive/2020/04/closing-parks-ineffective-pandemic-theater/609580/ . Accessed 15 Jun 2020.
Tufekci Z. Scolding beachgoers isn’t helping; 2020. The Atlantic. https://www.theatlantic.com/health/archive/2020/07/it-okay-go-beach/613849/ . Accessed 6 Jul 2020.
Qian H, Miao T, Liu L, Zheng X, Luo D, Li Y. Indoor transmission of SARS-CoV-2. Indoor Air. 2021;31(3):639–45. https://doi.org/10.1111/ina.12766 .
McGreevy R. Outdoor transmission accounts for 0.1% of State’s Covid-19 cases; 2021. The Irish Times. https://www.irishtimes.com/news/ireland/irish-news/outdoor-transmission-accounts-for-0-1-of-state-s-covid-19-cases-1.4529036 . Accessed 30 Apr 2021.
Lakha F, Rudge JW, Holt H. Rapid synthesis of evidence on settings which have been associated with SARS-CoV-2 transmission clusters. 2020. https://superspreadingdatabase.github.io/Evidence_on_clusters_final.pdf . Accessed 10 Sept 2020.
Fouda B, Tram HPB, Makram OM, Abdalla AS, Singh T, Hung I-C, et al. Identifying SARS-CoV2 transmission cluster category: an analysis of country government database. J Infect Public Health. 2021;14(4):461–7. https://doi.org/10.1016/j.jiph.2021.01.006 .
US Centers for Disease Control and Prevention. Choosing safer activities; 2021. https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/participate-in-activities.html . Accessed 1 Jun 2021.
World Health Organization. Mask use in the context of COVID-19: Interim guidance. 2020. https://apps.who.int/iris/handle/10665/337199 . Accessed 30 Jan 2021.
US Centers for Disease Control and Prevention. Guidance for wearings masks; 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html . Accessed 30 Apr 2021.
Slater SJ, Christiana RW, Gustat J. Recommendations for keeping parks and green space accessible for mental and physical health during COVID-19 and other pandemics. Prev Chronic Dis. 2020;17(E59):200204. https://doi.org/10.5888/pcd17.200204 .
Mathai V, Das A, Bailey JA, Breuer K. Airflows inside passenger cars and implications for airborne disease transmission. Sci Adv. 2021;7(1):eabe0166. https://doi.org/10.1126/sciadv.abe0166 .
Allen JG, Ibrahim AM. Indoor air changes and potential implications for SARS-CoV-2 transmission. JAMA. 2021;325(20):2112. https://doi.org/10.1001/jama.2021.5053 .
Halperin D. A marshall plan for Covid-19. 2020. Real Clear Policy. https://www.realclearpolicy.com/articles/2020/11/05/a_marshall_plan_for_covid-19_583019.html . Accessed 5 Mar 2021.
World Health Organization. Roadmap to improve and ensure good indoor ventilation in the context of COVID-19; 2021. https://www.who.int/publications/i/item/9789240021280 . Accessed 30 Mar 2021.
United Nations Educational Scientific and Cultural Organization. One year into COVID: Prioritizing education recovery to avoid a generational catastrophe; 2021. https://unesdoc.unesco.org/ark:/48223/pf0000376984 .
Evans D, Hares S, Mendez Acosta A, Saintis C. It’s been a year since schools started to close due to COVID-19; 2021. https://www.cgdev.org/blog/its-been-year-schools-started-close-due-covid-19 . Accessed 9 Apr 2021.
Hawrilenko M, Kroshus E, Tandon P, Christakis D. The association between school cosures and child mental health during COVID-19. JAMA Netw Open. 2021;4(9):e2124092. https://doi.org/10.1001/jamanetworkopen.2021.24092 .
Honein MA, Barrios LC, Brooks JT. Data and policy to guide opening schools safely to limit the spread of SARS-CoV-2 infection. JAMA. 2021;325(9):823. https://doi.org/10.1001/jama.2021.0374 .
European Centre for Disease Prevention and Control. COVID-19 in children and the role of school settings in transmission–second update. 2020. https://www.ecdc.europa.eu/en/publications-data/children-and-school-settings-covid-19-transmission . Accessed 7 Aug 2021.
Reid M. Latin America’s silent tragedy of empty classrooms. De Economist. 2021; https://www.economist.com/the-americas/2021/06/19/latin-americas-silent-tragedy-of-empty-classrooms . Accessed 23 Jun 2021.
United Nations Educational Scientific and Cultural Organization. Education: From disruption to recovery. COVID-19 impact on education. https://en.unesco.org/covid19/educationresponse#schoolclosures . Accessed 16 Sep 2021.
Douglas M, Katikireddi SV, Taulbut M, McKee M, McCartney G. Mitigating the wider health effects of covid-19 pandemic response. BMJ. 2020;369:m1557. https://doi.org/10.1136/bmj.m1557 .
Mayurasakorn K, Pinsawas B, Mongkolsucharitkul P, Sranacharoenpong K, Damapong S. School closure, COVID-19 and lunch programme: unprecedented undernutrition crisis in low-middle income countries. J Paediatr Child Health. 2020;56(7):1013–7. https://doi.org/10.1111/jpc.15018 .
Cattan S, Farquharson C, Krutikova S, Phimister A, Salisbury A, Sevilla A. Inequalities in responses to school closures over the course of the first COVID-19 lockdown: Institute for Fiscal Studies; 2021. https://doi.org/10.1920/wp.ifs.2021.421 .
Christie A, Brooks JT, Hicks LA, Sauber-Schatz EK, Yoder JS, Honein MA. Guidance for implementing COVID-19 prevention strategies in the context of varying community transmission levels and vaccination coverage. MMWR Morb Mortal Wkly Rep. 2021;70(30):1044–7. https://doi.org/10.15585/mmwr.mm7030e2 .
Lessler J, Grabowski MK, Grantz KH, Badillo-Goicoechea E, Metcalf CJE, Lupton-Smith C, et al. Household COVID-19 risk and in-person schooling. Science. 2021;372(6546):1092–7. https://doi.org/10.1126/science.abh2939 .
Ghosh R, Dubey MJ, Chatterjee S, Dubey S. Impact of COVID −19 on children: special focus on the psychosocial aspect. Minerva Pediatr. 2020;72(3):226–35. https://doi.org/10.23736/S0026-4946.20.05887-9 .
Rundle AG, Park Y, Herbstman JB, Kinsey EW, Wang YC. COVID-19-related school closings and risk of weight gain among children. Obesity. 2020;28(6):1008–9. https://doi.org/10.1002/oby.22813 .
Zimmerman KO, Akinboyo IC, Brookhart MA, Boutzoukas AE, McGann KA, Smith MJ, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. 2021;147(4):e2020048090. https://doi.org/10.1542/peds.2020-048090 .
Falk A, Benda A, Falk P, Steffen S, Wallace Z, Høeg TB. COVID-19 cases and transmission in 17 K–12 schools — Wood County, Wisconsin, August 31–November 29, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(4):136–40. https://doi.org/10.15585/mmwr.mm7004e3 .
Soriano-Arandes A, Gatell A, Serrano P, Biosca M, Campillo F, Capdevila R, et al. Household severe acute respiratory syndrome coronavirus 2 transmission and children: a network prospective study. Clin Infect Dis. 2021;73(6):e1261–9. https://doi.org/10.1093/cid/ciab228 .
Baral S, Chandler R, Prieto RG, Gupta S, Mishra S, Kulldorff M. Leveraging epidemiological principles to evaluate Sweden’s COVID-19 response. Ann Epidemiol. 2021;54:21–6. https://doi.org/10.1016/j.annepidem.2020.11.005 .
Vlachos J, Hertegård EB, Svaleryd H. The effects of school closures on SARS-CoV-2 among parents and teachers. Proc Natl Acad Sci. 2021;118(9):e2020834118. https://doi.org/10.1073/pnas.2020834118 .
Ludvigsson JF, Engerström L, Nordenhäll C, Larsson E. Open schools, Covid-19, and child and teacher morbidity in Sweden. N Engl J Med. 2021;384(7):669–71. https://doi.org/10.1056/NEJMc2026670 .
Ehrhardt J, Ekinci A, Krehl H, Meincke M, Finci I, Klein J, et al. Transmission of SARS-CoV-2 in children aged 0 to 19 years in childcare facilities and schools after their reopening in may 2020, Baden-Württemberg, Germany. Eurosurveillance. 2020;25(36):4–7. https://doi.org/10.2807/1560-7917.ES.2020.25.36.2001587 .
US Centers for Disease Control and Prevention. Guidance for COVID-19 prevention in K-12 schools; 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/k-12-guidance.html . Accessed 1 Sept 2021.
Torjesen I. Covid-19: Delta variant is now UK’s most dominant strain and spreading through schools. BMJ. 2021;373:n1445. https://doi.org/10.1136/bmj.n1445 .
Lam-Hine T, McCurdy SA, Santora L, Duncan L, Corbett-Detig R, Kapusinszky B, et al. Outbreak associated with SARS-CoV-2 B.1.617.2 (Delta) variant in an elementary school — Marin County, California, May–June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(35):1214–9. https://doi.org/10.15585/mmwr.mm7035e2 .
van den Berg P, Schechter-Perkins EM, Jack RS, Epshtein I, Nelson R, Oster E, et al. Effectiveness of three versus six feet of physical distancing for controlling spread of COVID-19 among primary and secondary students and staff: a retrospective, state-wide cohort study. Clin Infect Dis. 2021 (Epub ahead of print). https://doi.org/10.1093/cid/ciab230 .
US Centers for Disease Control and Prevention. Operational strategy for K-12 schools through phased prevention; 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/operation-strategy.html . Accessed 30 May 2021.
Gettings J, Czarnik M, Morris E, Haller E, Thompson-Paul AM, Rasberry C, et al. Mask use and ventilation improvements to reduce COVID-19 incidence in elementary schools — Georgia, November 16–December 11, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(21):779–84. https://doi.org/10.15585/mmwr.mm7021e1 .
Gandhi M, Baral S. What does public health really mean? Lessons from covid-19. BMJ Opinion. 2021; https://blogs.bmj.com/bmj/2021/07/26/what-does-public-health-really-mean-lessons-from-covid-19/ .
Grubaugh ND, Hodcroft EB, Fauver JR, Phelan AL, Cevik M. Public health actions to control new SARS-CoV-2 variants. Cell. 2021;184(5):1127–32. https://doi.org/10.1016/j.cell.2021.01.044 .
United Nations Children’s Fund. UNICEF chief: Closing schools should be “measure of last resort”. 2021. United Nations News. https://news.un.org/en/story/2021/01/1081912 . Accessed 30 Jan 2021.
Hodgins S, Saad A. Will the higher-income country blueprint for COVID-19 work in low- and lower middle-income countries? Glob Heal Sci Pract. 2020;8(2):136–43. https://doi.org/10.9745/GHSP-D-20-00217 .
Bavli I, Sutton B, Galea S. Harms of public health interventions against covid-19 must not be ignored. BMJ. 2020;371:m4074. https://doi.org/10.1136/bmj.m4074 .
Gunnell D, Appleby L, Arensman E, Hawton K, John A, Kapur N, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry. 2020;7(6):468–71. https://doi.org/10.1016/S2215-0366(20)30171-1 .
Marques ES, de Moraes CL, Hasselmann MH, Deslandes SF, Reichenheim ME. Violence against women, children, and adolescents during the COVID-19 pandemic: overview, contributing factors, and mitigating measures. Cad Saude Publica. 2020;36(4):e00074420. https://doi.org/10.1590/0102-311X00074420 .
Baral S, Rao A, Twahirwa Rwema JO, Lyons C, Cevik M, Kågesten AE, et al. Competing health risks associated with the COVID-19 pandemic and response: a scoping review. Preprint at medRxiv. 2021. https://doi.org/10.1101/2021.01.07.21249419 .
Chang AY, Cullen MR, Harrington RA, Barry M. The impact of novel coronavirus COVID-19 on noncommunicable disease patients and health systems: a review. J Intern Med. 2021;289(4):450–62. https://doi.org/10.1111/joim.13184 .
Lin AL, Vittinghoff E, Olgin JE, Pletcher MJ, Marcus GM. Body weight changes during pandemic-related shelter-in-place in a longitudinal cohort study. JAMA Netw Open. 2021;4(3):e212536. https://doi.org/10.1001/jamanetworkopen.2021.2536 .
Aum S, Lee SY, Shin Y. COVID-19 doesn’t need lockdowns to destroy jobs: The effect of local outbreaks in Korea. Labour Econ. 2021;70:101993. https://doi.org/10.1016/j.labeco.2021.101993 .
Chetty R, Friedman JN, Hendren N, Stepner M. The economic impacts of COVID-19: Evidence from a new public database built using private sector data. National Bureau Econ Res. 2020. https://www.nber.org/papers/w27431
Honein MA, Christie A, Rose DA, Brooks JT, Meaney-Delman D, Cohn A, et al. Summary of guidance for public health strategies to address high levels of community transmission of SARS-CoV-2 and related deaths, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(49):1860–7. https://doi.org/10.15585/mmwr.mm6949e2 .
Escandón K, Martin GP, Kuppalli K, Escandón K. Appropriate usage of face masks to prevent SARS-CoV-2: sharpening the messaging amid the COVID-19 pandemic. Disaster Med Public Health Prep. 2021;15(4):e5–7. https://doi.org/10.1017/dmp.2020.336 .
Bo Y, Guo C, Lin C, Zeng Y, Li HB, Zhang Y, et al. Effectiveness of non-pharmaceutical interventions on COVID-19 transmission in 190 countries from 23 January to 13 April 2020. Int J Infect Dis. 2021;102:247–53. https://doi.org/10.1016/j.ijid.2020.10.066 .
Haug N, Geyrhofer L, Londei A, Dervic E, Desvars-Larrive A, Loreto V, et al. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat Hum Behav. 2020;4(12):1303–12. https://doi.org/10.1038/s41562-020-01009-0 .
Rasmussen AL, Escandón K, Popescu SV. Facial masking for Covid-19. N Engl J Med. 2020;383(21):2092. https://doi.org/10.1056/NEJMc2030886 .
World Health Organization. COVID-19 weekly epidemiological update. Edition 56. 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-7-september-2021 . Accessed 11 Sept 2021.
Mondelli MU, Colaneri M, Seminari EM, Baldanti F, Bruno R. Low risk of SARS-CoV-2 transmission by fomites in real-life conditions. Lancet Infect Dis. 2021;21(5):e112. https://doi.org/10.1016/S1473-3099(20)30678-2 .
Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect Dis. 2020;20(8):892–3. https://doi.org/10.1016/S1473-3099(20)30561-2 .
Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2021;174(1):69–79. https://doi.org/10.7326/M20-5008 .
Lewis D. COVID-19 rarely spreads through surfaces. So why are we still deep cleaning? Nature. 2021;590(7844):26–8. https://doi.org/10.1038/d41586-021-00251-4 .
US Centers for Disease Control and Prevention. Scientific brief: SARS-CoV-2 transmission. 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html . Accessed 8 May 2021.
Thompson D. Hygiene theater is still a huge waste of time; 2021. https://www.theatlantic.com/ideas/archive/2021/02/hygiene-theater-still-waste/617939/ . Accessed 8 Mar 2021.
Yip L, Bixler D, Brooks DE, Clarke KR, Datta SD, Dudley S, et al. Serious adverse health events, including death, associated with ingesting alcohol-based hand sanitizers containing methanol — Arizona and New Mexico, May–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1070–3. https://doi.org/10.15585/mmwr.mm6932e1 .
Gharpure R, Hunter CM, Schnall AH, Barrett CE, Kirby AE, Kunz J, et al. Knowledge and practices regarding safe household cleaning and disinfection for COVID-19 prevention — United States, May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):705–9. https://doi.org/10.15585/mmwr.mm6923e2 .
Chang A, Schnall AH, Law R, Bronstein AC, Marraffa JM, Spiller HA, et al. Cleaning and disinfectant chemical exposures and temporal associations with COVID-19 — National Poison Data System, United States, January 1, 2020–March 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(16):496–8. https://doi.org/10.15585/mmwr.mm6916e1 .
Rezasoltani S, Yadegar A, Hatami B, Asadzadeh Aghdaei H, Zali MR. Antimicrobial resistance as a hidden menace lurking behind the COVID-19 outbreak: the global impacts of too much hygiene on AMR. Front Microbiol. 2020;11:590683. https://doi.org/10.3389/fmicb.2020.590683 .
Makary M, Das I, Hashim F, Walsh C. The next pandemic is here. 2021. MedPage Today. https://www.medpagetoday.com/opinion/marty-makary/90795 . Accessed 8 Mar 2021.
Zhang XS, Duchaine C. SARS-CoV-2 and health care worker protection in low-risk settings: a review of modes of transmission and a novel airborne model involving inhalable particles. Clin Microbiol Rev. 2020;34(1):e00184–20. https://doi.org/10.1128/CMR.00184-20 .
Leung NHL. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol. 2021;19(8):528–45. https://doi.org/10.1038/s41579-021-00535-6 .
Wright WF, Mackowiak PA. Why temperature screening for coronavirus disease 2019 with noncontact infrared thermometers does not work. Open Forum Infect Dis. 2021;8(1):4–6. https://doi.org/10.1093/ofid/ofaa603 .
Aw J. The non-contact handheld cutaneous infra-red thermometer for fever screening during the COVID-19 global emergency. J Hosp Infect. 2020;104(4):451. https://doi.org/10.1016/j.jhin.2020.02.010 .
Dzien C, Halder W, Winner H, Lechleitner M. Covid-19 screening: are forehead temperature measurements during cold outdoor temperatures really helpful? Wien Klin Wochenschr. 2021;133(7–8):331–5. https://doi.org/10.1007/s00508-020-01754-2 .
Normile D. Airport screening is largely futile, research shows. Science. 2020;367(6483):1177–8. https://doi.org/10.1126/science.367.6483.1177 .
Kojima N, Klausner J. It’s time to ditch COVID-19 temperature checks. 2021. https://www.thedailybeast.com/its-time-to-ditch-covid-19-temperature-checks?source=email&via=desktop . Accessed 3 Jun 2021.
Mouchtouri, Christoforidou, an der Heiden, Lemos, Fanos, Rexroth, et al. Exit and entry screening practices for infectious diseases among travelers at points of entry: Looking for evidence on public health impact. Int J Environ Res Public Health. 2019;16(23):4638. https://doi.org/10.3390/ijerph16234638 .
Mouchtouri VA, Bogogiannidou Z, Dirksen-Fischer M, Tsiodras S, Hadjichristodoulou C. Detection of imported COVID-19 cases worldwide: early assessment of airport entry screening, 24 January until 17 February 2020. Trop Med Health. 2020;48(1):79. https://doi.org/10.1186/s41182-020-00260-5 .
Devi S. Travel restrictions hampering COVID-19 response. Lancet. 2020;395(10233):1331–2. https://doi.org/10.1016/S0140-6736(20)30967-3 .
Baker MG, Wilson N, Blakely T. Elimination could be the optimal response strategy for covid-19 and other emerging pandemic diseases. BMJ. 2020;371:m4907. https://doi.org/10.1136/bmj.m4907.
Binnicker MJ. Challenges and controversies to testing for COVID-19. J Clin Microbiol. 2020;58(11):e01695–20. https://doi.org/10.1128/JCM.01695-20 .
Mina MJ, Andersen KG. COVID-19 testing: one size does not fit all. Science. 2021;371(6525):126–7. https://doi.org/10.1126/science.abe9187 .
Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–22. https://doi.org/10.1016/S2666-5247(20)30172-5 .
Walsh KA, Jordan K, Clyne B, Rohde D, Drummond L, Byrne P, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Inf Secur. 2020;81(3):357–71. https://doi.org/10.1016/j.jinf.2020.06.067 .
Jefferson T, Spencer EA, Brassey J, Heneghan C. Viral cultures for COVID-19 infectious potential assessment – a systematic review. Clin Infect Dis. 2020 (Epub ahead of print). https://doi.org/10.1093/cid/ciaa1764 .
Levine-Tiefenbrun M, Yelin I, Katz R, Herzel E, Golan Z, Schreiber L, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27(5):790–2. https://doi.org/10.1038/s41591-021-01316-7 .
Petter E, Mor O, Zuckerman N, Oz-Levi D, Younger A, Aran D, et al. Initial real world evidence for lower viral load of individuals who have been vaccinated by BNT162b2. Preprint at medRxiv. https://doi.org/10.1101/2021.02.08.21251329 .
McEllistrem MC, Clancy CJ, Buehrle DJ, Lucas A, Decker BK. Single dose of an mRNA SARS-CoV-2 vaccine is associated with lower nasopharyngeal viral load among nursing home residents with asymptomatic COVID-19. Clin Infect Dis. 2021;73(6):e1365–7. https://doi.org/10.1093/cid/ciab263 .
US Centers for Disease Control and Prevention. COVID-19 interim public health recommendations for fully vaccinated people. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated-guidance.html . Accessed 28 May 2021.
Denzer O, Nienaber M. Germany drops incidence levels as key COVID yardstick. 2021. Reuters. https://www.reuters.com/world/europe/german-drop-incidence-levels-key-covid-yardstick-sources-2021-08-23/ . Accessed 1 Sept 2021.
Bugin K, Woodcock J. Trends in COVID-19 therapeutic clinical trials. Nat Rev Drug Discov. 2021;20(4):254–5. https://doi.org/10.1038/d41573-021-00037-3 .
Bedford J, Farrar J, Ihekweazu C, Kang G, Koopmans M, Nkengasong J. A new twenty-first century science for effective epidemic response. Nature. 2019;575(7781):130–6. https://doi.org/10.1038/s41586-019-1717-y .
Alam U, Nabyonga-Orem J, Mohammed A, Malac DR, Nkengasong JN, Moeti MR. Redesigning health systems for global heath security. Lancet Glob Heal. 2021;9(4):e393–4. https://doi.org/10.1016/S2214-109X(20)30545-3 .
Darby AC, Hiscox JA. Covid-19: variants and vaccination. BMJ. 2021:372n771. https://doi.org/10.1136/bmj.n771 .
Fontanet A, Autran B, Lina B, Kieny MP, Karim SSA, Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397(10278):952–4. https://doi.org/10.1016/S0140-6736(21)00370-6 .
Tarke A, Sidney J, Methot N, Yu ED, Zhang Y, Dan JM, et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Reports Med. 2021;2(7):100355. https://doi.org/10.1016/j.xcrm.2021.100355 .
Ledford H. How ‘killer’ T cells could boost COVID immunity in face of new variants. Nature. 2021;590(7846):374–5. https://doi.org/10.1038/d41586-021-00367-7 .
Download references
Acknowledgments
We thank Karina Escandón for designing the figure for this manuscript. We acknowledge Arthur Allen, Arlyne Beeche, Richard Cash, Julia Marcus, Malcolm Potts, Josh Sharfstein, Ann Swidler, Muhammad Usman, Zeynep Tufekci, and David Wolfson for their useful comments. This article was first preprinted in April 2021 at https://osf.io/nrvtf/ and was continuously updated until publication date.
This article did not receive any funding or sponsorship for publication.
Author information
Kevin Escandón and Monica Gandhi are co-senior authors.
Authors and Affiliations
Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, USA
Daniel T. Halperin
Department of Family and Community Medicine, School of Medicine, University of California, San Francisco, CA, USA
Norman Hearst
School of Public Health, University of Alberta, Edmonton, AB, Canada
Stephen Hodgins
School of Public Health, University of Illinois, Chicago, IL, USA
Robert C. Bailey
Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Jeffrey D. Klausner
Independent Consultant, Harare, Zimbabwe
Helen Jackson
Integrated Initiative for Global Health, Northeastern University, Boston, MA, USA
Richard G. Wamai
School of Public Health, University of Nairobi, Nairobi, Kenya
Division of General Internal Medicine and Health Services Research, David Geffen School of Medicine, University of California, Los Angeles, CA, USA
Joseph A. Ladapo
Center for Global Development, Washington, D.C, USA
Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore, MD, USA
Stefan Baral
School of Medicine, Universidad del Valle, Cali, Colombia
Kevin Escandón
Department of Microbiology, Universidad del Valle, Grupo de Investigación en Virus Emergentes VIREM, Cali, Colombia
Division of HIV, Infectious Diseases, and Global Medicine, Department of Medicine, University of California, San Francisco, CA, USA
Monica Gandhi
You can also search for this author in PubMed Google Scholar
Contributions
DTH initially conceptualized the article and led the manuscript development. All authors (DTH, NH, SH, RCB, JDK, HJ, RW, JAL, MO, SB, KE, and MG) contributed to the writing of the manuscript, critically revised subsequent versions, and agreed upon the final version of this manuscript prior to submission. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Kevin Escandón .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
Dr. Kevin Escandón and Dr. Stefan Baral are Senior Editorial Board Members for BMC Infectious Diseases. These authors were not involved in any of the decisions regarding review of the manuscript or its acceptance. Two in-house Editors for the BMC Series and two anonymous expert reviewers assessed this manuscript. Dr. Jeffrey D. Klausner serves as an independent medical director of Curative, Inc., a SARS-CoV-2 testing and vaccination company. The other authors declare no conflicts of interest. The authors confirm that they have read BMC’s guidance on competing interests. Views expressed here are solely those of the authors and do not represent the position or policy of any institution or organization.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Halperin, D.T., Hearst, N., Hodgins, S. et al. Revisiting COVID-19 policies: 10 evidence-based recommendations for where to go from here. BMC Public Health 21 , 2084 (2021). https://doi.org/10.1186/s12889-021-12082-z
Download citation
Received : 28 June 2021
Accepted : 22 October 2021
Published : 13 November 2021
DOI : https://doi.org/10.1186/s12889-021-12082-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Public health
- Harm reduction
- Outdoor transmission
- School closure
- Pandemic preparedness
- Evidence-based recommendations
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
This paper is in the following e-collection/theme issue:
Published on 16.5.2024 in Vol 26 (2024)
Implementation of Inpatient Electronic Consultations During the COVID-19 Crisis and Its Sustainability Beyond the Pandemic: Quality Improvement Study
Authors of this article:

Original Paper
- Anna S Aledia, BS ;
- Amish A Dangodara, MD ;
- Aanya A Amin ;
- Alpesh N Amin, MD, MBA
Department of Medicine & Hospital Medicine, University of California, Irvine, Orange, CA, United States
Corresponding Author:
Alpesh N Amin, MD, MBA
Department of Medicine & Hospital Medicine
University of California, Irvine
333 City Boulevard West
Orange, CA, 92868
United States
Phone: 1 714 456 3785
Fax:1 714 456 3871
Email: [email protected]
Background: Limiting in-person contact was a key strategy for controlling the spread of the highly infectious novel coronavirus (COVID-19). To protect patients and staff from the risk of infection while providing continued access to necessary health care services, we implemented a new electronic consultation (e-consult) service that allowed referring providers to receive subspecialty consultations for patients who are hospitalized and do not require in-person evaluation by the specialist.
Objective: We aimed to assess the impact of implementing e-consults in the inpatient setting to reduce avoidable face-to-face referrals during the COVID-19 pandemic.
Methods: This quality improvement study evaluated all inpatient e-consults ordered from July 2020 to December 2022 at the University of California Irvine Medical Center. The impact of e-consults was assessed by evaluating use (eg, number of e-consults ordered), e-consult response times, and outcome of the e-consult requests (eg, resolved electronically or converted to the in-person evaluation of patient).
Results: There were 1543 inpatient e-consults ordered across 11 participating specialties. A total of 53.5% (n=826) of requests were addressed electronically, without the need for a formal in-person evaluation of the patient. The median time between ordering an e-consult and a specialist documenting recommendations in an e-consult note was 3.7 (IQR 1.3-8.2) hours across all specialties, contrasted with 7.3 (IQR 3.6-22.0) hours when converted to an in-person consult ( P <.001). The monthly volume of e-consult requests increased, coinciding with surges of COVID-19 cases in California. After the peaks of the COVID-19 crisis subsided, the use of inpatient e-consults persisted at a rate well above the precrisis levels.
Conclusions: An inpatient e-consult service was successfully implemented, resulting in fewer unnecessary face-to-face consultations and significant reductions in the response times for consults requested on patients who are hospitalized and do not require an in-person evaluation. Thus, e-consults provided timely, efficient delivery of inpatient consultation services for appropriate problems while minimizing the risk of direct transmission of the COVID-19 virus between health care providers and patients. The service also demonstrated its value as a tool for effective inpatient care coordination beyond the peaks of the pandemic leading to the sustainability of service and value.
Introduction
When the novel coronavirus (COVID-19), the disease caused by SARS-CoV-2, began to quickly spread around the world, the high transmissibility of this disease urged health care systems to explore alternatives to face-to-face interactions that would reduce the risk of exposure for both the patient and the provider. Electronic consultations (e-consults) are asynchronous, non–face-to-face, provider-to-provider exchanges that have been shown to improve patient access to specialty care for appropriate referral problems that do not require an in-person evaluation of the patient by the specialist [ 1 - 3 ]. The rapid rise in COVID-19 cases induced a demand for the adoption of e-consult services and triggered an increase in the use of e-consults [ 4 ]. Although its use in the outpatient setting is well established [ 5 , 6 ], e-consults in the inpatient arena are relatively new.
As the only academic health system in the sixth largest county in the United States, University of California Irvine (UCI) Health has been a leader in the advancement of telehealth technologies that expand access to care and improve health care efficiency and resource use [ 7 ]. UCI already has a well-developed e-consults program in the ambulatory setting [ 8 ], and to complement this existing service, we expanded e-consults to patients who were hospitalized to further help reduce in-person contacts between consulting providers and patients, thereby minimizing disease transmission and conserving scarce personal protective equipment (PPE) during the COVID-19 crisis. Other health systems have implemented similar e-consult services for inpatients, but provider use of e-consults was temporary in response to the pandemic, favoring in-person consultative care instead [ 9 ]; inpatient e-consults were offered by only a single specialty consulting service [ 10 , 11 ]; and use cases involved early inpatient e-consult models [ 12 , 13 ]. In this study, we describe our rapid implementation of inpatient e-consults in multiple specialties and its sustained use beyond the peaks of the pandemic.
The inpatient e-consult service was implemented at the UCI Medical Center, a 478-bed acute care hospital providing tertiary and quaternary care, ambulatory and specialty medical clinics, behavioral health care, and rehabilitation services. Located in Orange County, California, it serves a diverse population of close to 4 million persons with broad health care needs. With more than 500 specialty and primary care physicians, UCI offers a full scope of acute and general care services. It is also the primary teaching location for UCI medical and nursing students, medical residents, and fellows, and it is home to Orange County’s only adult level 1 and pediatric level 2 trauma centers, a National Cancer Institute–designated comprehensive cancer center, a regional burn center, the county’s only hematopoietic stem cell and bone marrow transplant program, and the region’s only high-risk perinatal and neonatal program and maternal-fetal transport system. In winter 2020, UCI Medical Center opened a temporary mobile field hospital that added up to 50 acute care beds in response to a surge of patients with COVID-19.
Implementation
The design and implementation of inpatient e-consults were guided by a steering committee, which included the Chair of the Department of Medicine and Executive Director of Hospital Medicine (who was the lead to design and develop e-consults at the UCI), a clinical informaticist, specialty physician leads, an IT build team, representatives from the Compliance and Privacy Office and Physicians Billing Group, and a project manager. Early on, members of this committee engaged UCI leadership to affirm support for the new service and obtain the IT resources needed to build the inpatient e-consults workflow. Regular steering committee meetings were established to discuss the design of the inpatient e-consults workflow and develop a process for provider reimbursement or credit. Prior to the go-live, the inpatient e-consult service was publicized by members of the steering committee through email communications with house staff. Steering committee members also hosted Zoom training (Zoom Technologies) and orientation sessions with participating consulting services, and they distributed tip sheets summarizing the steps to complete the requesting provider and responding consultant workflows.
Our IT team was able to efficiently implement our inpatient e-consult service by designing workflows similar to those for traditional in-person consults. Thus, the processes for requesting and responding to inpatient e-consults were not unfamiliar to providers ( Figure 1 ). To request an e-consult, the inpatient service or team places a consult order in the electronic health record (EHR), indicating that the request is for an e-consult. The patient is then added to the physician e-consults system list of the appropriate specialty. A follow-up call or page is also sent to the specialty by the requesting team to alert the inpatient consulting team of the e-consult and, if necessary, provide them with any additional details. To respond to the e-consult request, the inpatient consulting team reviews the relevant clinical information available in the EHR and documents their assessment and recommendations in a consult note. If the case is deemed too complex to be addressed electronically, the consulting team converts the e-consult to a traditional in-person consultation and the patient is examined before documenting guidance in the EHR. The requesting provider and responding consultant are each credited with 0.7 work relative value units (a measure of the provider’s time and effort required to perform the service) for every completed e-consult that does not result in an in-person evaluation of the patient by the consulting service, while usual billing or relative value unit credit applies for in-person consultations.

Measurement and Analysis
We conducted a retrospective evaluation of all inpatient e-consults ordered at the UCI Medical Center from implementation in July 2020 to December 2022 to assess use, outcomes, and response times. Use was tracked by examining the volume of e-consults ordered per specialty over the 2.5-year period and comparing it with the volume of traditional in-person consults ordered for the specialties offering inpatient e-consults. To assess outcomes, we categorized the result of each e-consult order as either “resolved electronically” if the consulting team addressed the request without a face-to-face evaluation of the patient or “converted to in-person” if the consulting team deemed the case too complex and the patient required a physical examination. The response time was defined as the interval between the documented consult order in the EHR and the consulting team filing recommendations in a consult note. We calculated the median response time and the IQR in hours for each specialty and performed nonparametric Mann-Whitney U tests in SPSS (version 28; IBM Corp) to compare the median response times of requests resolved electronically and converted to in person. All P values were 2-tailed, and P <.05 was considered statistically significant.
Ethical Considerations
Our implementation and retrospective analysis of the inpatient e-consults service constituted as quality improvement activities and not human subjects research. Thus, our study did not require institutional review board review. This study followed the Standards for Quality Improvement Reporting Excellence guidelines.
UCI’s e-consults service was launched in 11 total specialties (allergy and immunology, cardiology, dermatology, endocrinology, infectious diseases, nephrology, palliative care, pediatric endocrinology, pulmonary and critical care, radiation oncology, and rheumatology). Over a 2.5-year period, 1543 e-consults were requested out of 14,974 total consult orders (e-consult and traditional in-person consults) across the 11 participating specialties ( Figure 2 ). Thus, the average proportion of consult orders requested as e-consults is 10.3%, although this proportion varied widely among participating specialties. The specialty with the lowest e-consult proportion was pulmonary and critical care, which had 1.5% (13/850) of total consult orders requested as e-consults, while the specialty with the highest e-consult proportion was pediatric endocrinology, which had 48% (12/25) of total consult orders requested as e-consults. However, with only 25 total consult orders, pediatric endocrinology had the fewest number of total consult orders of all participating specialties.
The most requested e-consult specialties were infectious diseases (which received 574/1543, 37.2% of the e-consult requests), cardiology (261/1543, 16.9% of the e-consult requests), endocrinology (229/1543, 14.8% of the e-consult requests), and dermatology (226/1543, 14.6% of the e-consult requests; Table 1 ). A total of 53.5% (826/1543) of e-consult requests across all participating specialties were addressed without the need for an in-person evaluation of the patient by the consulting team. The specialty with the fewest e-consult requests resolved electronically was pulmonary and critical care, which completed 0% (0/13) of requests electronically, while the specialty with the most e-consult requests resolved electronically was pediatric endocrinology, which completed 100% (12/12) of e-consult requests, without needing to physically examine the patient. However, both specialties had the smallest volumes of e-consult requests of all participating specialties.
We found that the overall median response time of e-consult requests resolved electronically was significantly lower than requests converted to an in-person consultation ( Figure 3 ). The median time between ordering an e-consult and a specialist documenting recommendations in a consult note was 3.7 (IQR 1.3-8.2) hours across all specialties when resolved electronically, contrasted with 7.3 (IQR 3.6-22.0) hours when converted to an in-person consult ( P <.001). Over half (6/11, 55%) of the participating specialties had significantly faster median e-consult response times for requests resolved electronically compared to requests converted to an in-person consultation. The specialties with the fastest e-consult response times were dermatology and radiation oncology, which had median response times of 1.3 (IQR 0.4-3.0) hours and 0.9 (IQR 0.3-1.5) hours when resolved electronically, respectively. However, radiation oncology had one of the smallest volumes of e-consult requests among participating specialties.
The overall response times of e-consult requests were much faster than the turnaround goal mandated by our institutional guidelines, which require a same-day response by 8 PM if the consult is ordered before noon or a response by the following morning if ordered after noon. For reference, the overall median response time for completion of a traditional in-person consult by the same 11 specialties during the same 2.5-year period is 25.8 (IQR 10.8-65.7) hours ( Multimedia Appendix 1 ). Thus, regardless of whether an e-consult request was resolved electronically or converted to an in-person consult, e-consults significantly improved the turnaround times for inpatient consultations.
The average volume of requests was 19 inpatient e-consults per month during the first 5 months that inpatient e-consults were live ( Figure 4 ). Then, California experienced surges of COVID-19 cases throughout the pandemic and we saw corresponding increases in inpatient e-consults use. During the winter 2020 surge, the average volume of requests increased to 52 inpatient e-consults per month. Then, the Delta variant wave arrived in summer 2021, and the average volume of requests increased to 61 inpatient e-consults per month. When the Omicron variant wave emerged in winter 2021, the average volume of requests peaked at 75 inpatient e-consults per month. During a sustained wave in spring-summer 2022 driven by Omicron subvariants, the average volume of requests was 62 inpatient e-consults per month. After these surges subsided and COVID-19 cases declined, the use of inpatient e-consults remained at a high-level baseline with an average of 53 inpatient e-consults per month. Interestingly, similar patterns of increased e-consults use were observed in the ambulatory setting.

Principal Findings
In response to the COVID-19 crisis, we successfully implemented an inpatient e-consult service that offered providers the option of requesting a subspecialty consultation for patients who are hospitalized and do not require an in-person evaluation by the specialist. Strong engagement by the clinical champions and technology partners in our steering committee, along with support from UCI’s leadership which provided us with dedicated IT, compliance, and billing teams, contributed to the successful design and implementation of our inpatient e-consults service. In addition, we were able to rapidly launch the service by leveraging our experiences with implementing e-consults in the ambulatory setting and capitalizing on existing infrastructure for inpatient consults. Instead of creating unique e-consult orders, configuring our existing inpatient consult order reduced the build components for our IT team, allowing us to quickly and effectively launch the inpatient e-consults service. Because we used workflows similar to those for traditional in-person consults, the processes for requesting and responding to inpatient e-consults were not new for providers. This strategy, along with provider familiarity with our well-established e-consults service in the ambulatory setting, likely helped to foster the adoption of inpatient e-consults. Although the COVID-19 crisis provided the key stimulus, these factors may have also contributed to the more rapid adoption of inpatient e-consults in comparison to the initial uptake of our ambulatory e-consults.
We found that the e-consult services helped to significantly reduce the response time for consults requested on patients who are hospitalized and do not require an in-person evaluation. In fact, the overall median response time of e-consult requests resolved electronically was approximately half of the response time for requests converted to an in-person consultation and nearly 7 times faster than the response time for traditional in-person consults. This time saving was critical during surges of COVID-19 cases when emergency departments and inpatient units were overwhelmed, leading to prolonged wait times for patients who were hospitalized to receive consultative care. e-Consults helped to streamline the inpatient consultation process and enabled the consulting team to promptly and efficiently provide recommendations on patients not needing a physical examination.
Although some diagnoses require in-person evaluation of the patient, lower complexity problems can be managed effectively using e-consults. Indeed, we found that over half of e-consult requests were addressed electronically without the need for an in-person evaluation of the patient by the consulting team. By reducing unnecessary in-person consultations, e-consults likely helped to limit the use of scarce PPE; minimize disease transmission; and free up specialists for other activities, such as examining patients with more complicated conditions and performing procedures. This improved resource use may also translate to potential cost savings associated with avoided in-person consultations and increased productivity. Future work should aim to analyze the cost-effectiveness of inpatient e-consults.
After the peaks of the COVID-19 crisis subsided in California, we discovered that provider use of inpatient e-consults persisted at a rate well above the precrisis levels. This sustained use implies positive provider experiences with the service and suggests a preference for e-consults when addressing lower complexity problems. Developing workflows for the inpatient e-consults service that were familiar to providers and significantly improving the turnaround times for inpatient consultations also likely helped to facilitate this sustainability. Thus, while case numbers and death rates associated with the COVID-19 pandemic have declined, e-consults continued to be an important part of our health care delivery.
Although relatively new, there have been a few reports of e-consults in the inpatient setting. The earliest examples involved the unexpected use of the ambulatory e-consult platform in the inpatient setting [ 13 ] and the design of an inpatient e-consult protocol that provided subspecialty consultations to inpatients at a remote hospital that lacked access to these clinical services [ 12 ]. Other reports described the feasibility and use of inpatient e-consults for only 1 specific specialty consulting service [ 10 , 11 ]. While 1 health system reported their implementation of an inpatient e-consult program in several specialties, provider adoption was temporary in response to the COVID-19 crisis [ 9 ]. Our experience with inpatient e-consults uniquely contrasts with these other health systems because we not only successfully implemented inpatient e-consults in multiple specialties but also demonstrated its sustained use beyond the pandemic.
Limitations
Although anecdotal provider feedback has been positive, limitations to this study include the absence of a formal assessment of user experiences with the inpatient e-consults service. In addition, the volumes of e-consult requests and total consult orders were low for some specialties; thus, caution must be applied in the interpretation of results from these low-volume specialties. Nevertheless, we believe our unique development of inpatient e-consults is easily translatable to other institutions interested in implementing it and will lead to a positive user experience and greater use since we fit the e-consult process into already existing and common workflows of requesting a consultation. Additionally, although the implementation of our inpatient e-consults service was in a single academic health system, we successfully demonstrated that the use of e-consults in the inpatient setting is a promising approach to expediting patient care and reporting our experience in designing and implementing inpatient e-consults may provide guidance to other health systems considering similar telehealth models.
Conclusions
Our implementation of e-consults in the inpatient setting highlighted an innovative use for e-consults in the era of COVID-19. It allowed for timely, efficient delivery of inpatient consultation services while reducing the unnecessary exposure of health care workers to potential infection. Consequently, inpatient e-consults likely helped to conserve precious PPE, minimize disease transmission, and enhance our ability to deal with surges in COVID-19 cases by expediting rapid assessment and management of lower complexity referrals. Although the COVID-19 emergency served as motivation to expand our ambulatory e-consults program to the inpatient setting, the service has become a vital component of our regular practices and will remain an essential part of our health care delivery, both in the ambulatory and inpatient settings, beyond the current pandemic, achieving sustainability and value.
Acknowledgments
The authors thank our steering committee members (Dr Byron Allen and Dr Nathan Rojek) and IT build team (Donna Jackson, Brian Lambertson, Elizabeth Burrows, Jaymee Zillgitt, and Tanya Sickles) for their contributions to the design and implementation of our inpatient e-consults. We also thank additional team members Kathy LaPierre, Jennifer Rios, and Debra Webb Torres for their guidance with compliance and billing issues.
Conflicts of Interest
ANA has been a principal investigator or coinvestigator of clinical trials sponsored by the National Institutes of Health/National Institute of Allergy and Infectious Diseases, NeuroRx Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, as well as a speaker and consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achogen LaJolla, Ferring, Seres, Spero, Eli Lilly, Gilead, Millenium, HeartRite, Aseptiscope, and Sprightly; these relationships were unrelated to the current work. ASA, AAD, and AAA have no conflicts of interest to report.
Median (IQR) response times by specialty for traditional in-person consults, compared with median (IQR) response times for e-consults converted to in-person and e-consults resolved electronically.
- Chen AH, Kushel MB, Grumbach K, Yee HF. Practice profile. A safety-net system gains efficiencies through 'eReferrals' to specialists. Health Aff (Millwood). 2010;29(5):969-971. [ CrossRef ] [ Medline ]
- Keely E, Liddy C, Afkham A. Utilization, benefits, and impact of an e-consultation service across diverse specialties and primary care providers. Telemed J E Health. 2013;19(10):733-738. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Malagrino GD, Chaudhry R, Gardner M, Kahn M, Speer L, Spurrier BR, et al. A study of 6,000 electronic specialty consultations for person-centered care at The Mayo Clinic. Int J Pers Cent Med. 2012;2(3):458-466. [ CrossRef ]
- Arora A, Fekieta R, Nouri Z, Carder D, Colgan MM, Fuhlbrigge A, et al. Trends in utilization of electronic consultations associated with patient payer and language among US academic medical centers during the COVID-19 pandemic. JAMA Netw Open. 2022;5(7):e2224628. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Liddy C, Moroz I, Mihan A, Nawar N, Keely E. A systematic review of asynchronous, provider-to-provider, electronic consultation services to improve access to specialty care available worldwide. Telemed J E Health. 2019;25(3):184-198. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Vimalananda VG, Gupte G, Seraj SM, Orlander J, Berlowitz D, Fincke BG, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare. 2015;21(6):323-330. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kuo S, Aledia A, O'Connell R, Rudkin S, Dangodara AA, Amin AN. Implementation and impact on length of stay of a post-discharge remote patient monitoring program for acutely hospitalized COVID-19 pneumonia patients. JAMIA Open. 2022;5(3):ooac060. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Amin AN, Aledia A. An eConsults program to improve patient access to specialty care in an academic health system. J Clin Outcomes Manag. 2020;27(3):115-122. [ CrossRef ]
- Rikin S, Epstein EJ, Gendlina I. Rapid implementation of inpatient eConsult programme addresses new challenges for patient care during COVID-19 pandemic. BMJ Innov. 2021;7(2):271-277. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mustafa SS, Staicu ML, Yang L, Baumeister T, Vadamalai K, Ramsey A. Inpatient electronic consultations (E-consults) in allergy/immunology. J Allergy Clin Immunol Pract. 2020;8(9):2968-2973. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Yagnik KJ, Saad HA, King HL, Bedimo RJ, Lehmann CU, Medford RJ. Characteristics and outcomes of infectious diseases electronic COVID-19 consultations at a multisite academic health system. Cureus. 2021;13(11):e19203. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Najafi N, Harrison JD, Duong J, Greenberg A, Cheng HQ. It all just clicks: development of an inpatient e-consult program. J Hosp Med. 2017;12(5):332-334. [ CrossRef ] [ Medline ]
- Gupte G, Vimalananda V, Simon SR, DeVito K, Clark J, Orlander JD. Disruptive innovation: implementation of electronic consultations in a veterans affairs health care system. JMIR Med Inform. 2016;4(1):e6. [ FREE Full text ] [ CrossRef ] [ Medline ]
Abbreviations
Edited by T de Azevedo Cardoso, S He; submitted 19.12.23; peer-reviewed by J Chen, I Moroz; comments to author 14.02.24; revised version received 06.03.24; accepted 27.03.24; published 16.05.24.
©Anna S Aledia, Amish A Dangodara, Aanya A Amin, Alpesh N Amin. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 16.05.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 15 February 2023
Coronavirus research: knowledge gaps and research priorities
- Stanley Perlman ORCID: orcid.org/0000-0003-4213-2354 1 &
- Malik Peiris ORCID: orcid.org/0000-0001-8217-5995 2 , 3
Nature Reviews Microbiology volume 21 , pages 125–126 ( 2023 ) Cite this article
5954 Accesses
5 Citations
16 Altmetric
Metrics details
- Policy and public health in microbiology
Decades of coronavirus research and intense studies of SARS-CoV-2 since the beginning of the COVID-19 pandemic have led to an unprecedented level of knowledge of coronavirus biology and pathogenesis, yet many outstanding questions remain. Here, we discuss knowledge gaps and research priorities in the field.
Introduction
The COVID-19 pandemic showed that, based on previous research efforts, we understood many aspects of coronavirus biology and pathogenesis, but also that there was much we did not know. In 2019, the worldwide number of coronavirus investigators was small, having increased after the severe acute respiratory syndrome coronavirus (SARS-CoV) outbreak in 2003 but decreasing thereafter. The influx of scientists with diverse expertise into the field after the pandemic onset contributed to an increased understanding of coronavirus replication, epidemiology, SARS-CoV-2 pathogenesis and immune responses in humans, to the development and characterization of experimentally infected animal models for COVID-19, and to SARS-CoV-2 vaccine and antiviral drug development. Here, as investigators who have studied coronaviruses for decades, we outline some of the outstanding research questions that we think need to be addressed.
SARS-CoV-2 emergence
Where did SARS-CoV-2 originate and how did it evolve to infect humans? The emergence of SARS-CoV-2 continues to be an area of controversy and has been, and is being, investigated by many national and international organizations, including the WHO (World Health Organization). It is almost certain that the virus originated in bats and crossed species to humans either directly or indirectly via intermediary hosts. There remains debate on whether the virus first infected humans from a zoonotic source or from a research laboratory, but, no matter what the answer to this question is, it is clear to us that in order to be prepared for the next pandemic, we need to further delineate the panoply of coronaviruses present in bats and possible intermediary hosts 1 . We need to better understand coronavirus circulation in hotspots, such as parts of China and Southeast Asia, where humans, wildlife gathered for food or medicinal purposes and bats are in close proximity. These investigations should include surveillance (virological and serological) of humans in close contact with bats and the game animal trade, with or without respiratory disease, for evidence of coronavirus infection. A related question, discussed below, is why coronaviruses are especially good at jumping species, to humans and other animals.
Zoonotic risk
Once coronaviruses in animal reservoirs are identified, can they be better risk assessed for threats for human spillover? Surveillance of bat reservoirs of sarbecoviruses (Sarbecovirus is the subgenus to which SARS-CoV-2 belongs) had previously found evidence of viruses with a capacity for infecting human cells using the angiotensin converting enzyme 2 (ACE2) receptor (reminiscent of SARS-CoV) 2 . Serological evidence of viral spillover to humans was demonstrated before the emergence of SARS-CoV-2 (ref. 3 ). Arguably, these signals together should have been triggers for action to develop countermeasures with greater urgency. The availability of human organoid cultures and ex vivo cultures of human respiratory tissue may enable the use of physiologically relevant systems for a more systematic risk assessment of animal coronaviruses in the future, analogous to ongoing risk assessments being carried out for animal influenza viruses 4 .
SARS-CoV-2 transmissibility
What explains the high transmissibility of SARS-CoV-2 compared with SARS-CoV or Middle East respiratory syndrome coronavirus (MERS-CoV)? A critical factor leading to the COVID-19 pandemic was the ability of SARS-CoV-2 to grow to high levels in the upper respiratory tract and therefore to readily transmit to other humans. Titres of SARS-CoV and MERS-CoV in the upper respiratory tract peak at later times after infection 5 , consistent with the ability to interrupt transmission with relevant public health infection-prevention methods. A second, related question is why SARS-CoV and a common cold coronavirus, HCoV-NL63, which both use the same receptor as SARS-CoV-2 (ACE2) 6 , have such different patterns of infection within the human respiratory tract. HCoV-NL63 rarely infects the lower respiratory tract, whereas SARS-CoV preferentially causes pneumonia. These different patterns of infection most likely relate to differences in cell entry, including differences in co-receptor usage, host protease usage or fusogenicity of the spike protein, but there are other possibilities. Understanding these differences will provide information on which coronavirus might be expected to be transmissible and to identify additional targets for therapeutic interventions. Further elucidation of the factors that contribute to virus spread will require additional experimental animal models of coronavirus transmission.
The SARS-CoV-2 outbreak also highlighted the lack of evidence-based data on the transmission of coronaviruses, or indeed respiratory viruses in in general, and on which non-pharmaceutical countermeasures (for example, social distancing and masks (surgical versus N99/FFP3 masks)) are effective or not. The SARS-CoV-2 outbreak demonstrated that the only effective control options available in the first months of the pandemic were non-pharmaceutical, but our understanding of the efficacy of specific measures is limited.
Coronavirus genome complexity
Why do coronavirus genomes encode so many more proteins than other RNA viruses? Coronavirus genomes are bigger than those of any other RNA virus, apart from those of related members of the Nidovirales order. The genomes are so large that they require genomic proofreading activity to avoid error catastrophe 7 . A large genome size may contribute to enhanced cross-species transmission, but, at present, this notion is speculative. In any case, an important question is to understand the function of the many non-structural proteins involved in virus replication. Development of a cell-free or entirely in vitro replication system would facilitate detailed probing of the role of individual proteins in replication and transmission. Efforts to develop such cell-free systems were initiated 40 years ago, but it is only in the past few years with the advent of cryo-electron microscopy and new biochemical approaches that progress has been made. These efforts are expected to complement studies in intact cells, which use high-resolution microscopy and related techniques to analyse macromolecular interactions and function at the subcellular level.
Related to the previous question, why do coronaviruses encode so many proteins with apparent immunoevasive function? Coronaviruses encode a variable number of accessory proteins, the genes of which are intermingled within the structural protein genes located at the 3′ end of the genome. For example, SARS-CoV-2 encodes at least six such proteins, with several other putative open reading frames in the genome hypothesized to be expressed and have immunoevasive properties 8 . Confusingly, these genes are often deleted in viruses isolated from infected animals, without apparent loss of virulence. This was shown most clearly in the case of MERS-CoV, in which diverse deletions and insertions in accessory genes were detected in some isolates obtained in Africa from camels, the primary host of the virus 9 . These genetic changes may have unpredictable consequences for virus transmissibility or pathogenesis. Deletion of these genes occasionally leads to increased virulence 10 . The variable and sometimes unexpectedly high numbers of these proteins suggest that they have redundant and, perhaps, additional functions. Such redundancy could contribute to cross-species transmission. The genetic instability of MERS-CoV camels in Africa therefore needs to be monitored and evidence for human spillover needs to be continually assessed.
Predictive evolution
Can coronavirus evolution in infected human or other animal hosts be predicted? Coronaviruses readily mutate and recombine as they adapt to a new host. This is well illustrated by the COVID-19 pandemic, in which ancestral strains of SARS-CoV-2 initially mutated to better infect humans, and later evolved to evade the human immune response, generating a series of variants of concern. Several studies have modelled SARS-CoV-2 evolution but so far it has not been possible to predict how the virus will evolve in the future. Such predictive modelling is recognized to be difficult, but would be very useful in the present pandemic as well as in future coronavirus outbreaks or pandemics for vaccine development, for anticipating clinical disease and pathogenesis, and for risk assessment of animal viruses with zoonotic potential.
Keusch, G. T. et al. Pandemic origins and a One Health approach to preparedness and prevention: solutions based on SARS-CoV-2 and other RNA viruses. Proc. Natl Acad. Sci. USA 119 , e2202871119 (2022).
Article CAS PubMed PubMed Central Google Scholar
Menachery, V. D. et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 21 , 1508–1513 (2015).
Li, H. et al. Human-animal interactions and bat coronavirus spillover potential among rural residents in Southern China. Biosaf. Health 1 , 84–90 (2019).
Article PubMed PubMed Central Google Scholar
Cox, N. J., Trock, S. C. & Burke, S. A. Pandemic preparedness and the Influenza Risk Assessment Tool (IRAT). Curr. Top. Microbiol. Immunol. 385 , 119–136 (2014).
PubMed Google Scholar
Cevik, M. et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2 , e13–e22 (2021).
Article CAS PubMed Google Scholar
Hofmann, H. et al. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl Acad. Sci. USA 102 , 7988–7993 (2005).
Eckerle, L. D., Lu, X., Sperry, S. M., Choi, L. & Denison, M. R. High fidelity of murine hepatitis virus replication is decreased in nsp14 exoribonuclease mutants. J. Virol. 81 , 12135–12144 (2007).
Lowery, S. A., Sariol, A. & Perlman, S. Innate immune and inflammatory responses to SARS-CoV-2: implications for COVID-19. Cell Host Microbe 29 , 1052–1062 (2021).
Chu, D. K. W. et al. MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity. Proc. Natl Acad. Sci. USA 115 , 3144–3149 (2018).
Gutierrez-Alvarez, J. et al. Middle East respiratory syndrome coronavirus gene 5 modulates pathogenesis in mice. J. Virol. 95 , e01172-20 (2021).
Download references
Author information
Authors and affiliations.
Department of Microbiology and Immunology, University of Iowa, Iowa City, IA, USA
Stanley Perlman
HKU-Pasteur Research Pole, The University of Hong Kong (HKU), Hong Kong Special Administrative Region, People’s Republic of China
Malik Peiris
School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong (HKU), Hong Kong Special Administrative Region, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Stanley Perlman .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Perlman, S., Peiris, M. Coronavirus research: knowledge gaps and research priorities. Nat Rev Microbiol 21 , 125–126 (2023). https://doi.org/10.1038/s41579-022-00837-3
Download citation
Published : 15 February 2023
Issue Date : March 2023
DOI : https://doi.org/10.1038/s41579-022-00837-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Sars-cov-2 and innate immunity: the good, the bad, and the “goldilocks”.
- Benjamin L. Sievers
- Mark T. K. Cheng
- Ravindra K. Gupta
Cellular & Molecular Immunology (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.

- Liberty University
- Jerry Falwell Library
- Special Collections
- < Previous
Home > ETD > Doctoral > 5557
Doctoral Dissertations and Projects
The experience of university academics with emergency remote teaching during the covid-19 pandemic of 2020: a phenomenological study.
Judith M. Peterson , Liberty University Follow
School of Education
Doctor of Philosophy in Education (PhD)
Susan Quindag
COVID-19, Emergency Remote Teaching, online education, online teaching, online pedagogy, higher education, crisis plan, burnout
Disciplines
Recommended citation.
Peterson, Judith M., "The Experience of University Academics with Emergency Remote Teaching during the COVID-19 Pandemic of 2020: A Phenomenological Study" (2024). Doctoral Dissertations and Projects . 5557. https://digitalcommons.liberty.edu/doctoral/5557
The purpose of this transcendental phenomenological study was to explore the experiences of academics at the university level with emergency remote teaching during the 2020-2021 COVID-19 pandemic. The theory guiding this study was Milheim, K. L. (2012) application of Maslow, A. H. (1943) hierarchy of needs. The central research question was: How did academics at the university level experience transitioning their course and teaching online during emergency remote teaching during the COVID-19 pandemic of 2020-2021? Eleven lecture academics were selected from six universities from the University of Wisconsin System who transitioned their residence courses to online during the pandemic. I used three methods to collect data: semi-structured individual videoconference interviews, e-journals, and videoconference focus group interviews to provide triangulation of evidence and validate data accuracy. The themes that emerged were overtime, relationships, burnout/stress, technical struggles, digital divide, and outliers. The study found that the universities and participants were not prepared to transition online causing academics to burnout. The first recommendation was to continue studying the experiences of university academics with emergency remote teaching during the COVID-19 pandemic 2020 from other parts of the United States. The second recommendation was to study the lived experiences of instructors who taught hands-on type of courses such as art, music, and science labs. The third recommendation was to study what consequences the COVID-19 pandemic had on higher education. The fourth recommendation is to study the phenomenon of students becoming quiet for two years after being in isolation and wearing masks during the COVID-19 pandemic.
Included in
Education Commons
- Collections
- Faculty Expert Gallery
- Theses and Dissertations
- Conferences and Events
- Open Educational Resources (OER)
- Explore Disciplines
Advanced Search
- Notify me via email or RSS .
Faculty Authors
- Submit Research
- Expert Gallery Login
Student Authors
- Undergraduate Submissions
- Graduate Submissions
- Honors Submissions
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
Advertisement
Supported by
U.S. Tightens Rules on Risky Virus Research
A long-awaited new policy broadens the type of regulated viruses, bacteria, fungi and toxins, including those that could threaten crops and livestock.
- Share full article

By Carl Zimmer and Benjamin Mueller
The White House has unveiled tighter rules for research on potentially dangerous microbes and toxins, in an effort to stave off laboratory accidents that could unleash a pandemic.
The new policy, published Monday evening, arrives after years of deliberations by an expert panel and a charged public debate over whether Covid arose from an animal market or a laboratory in China.
A number of researchers worried that the government had been too lax about lab safety in the past, with some even calling for the creation of an independent agency to make decisions about risky experiments that could allow viruses, bacteria or fungi to spread quickly between people or become more deadly. But others warned against creating restrictive rules that would stifle valuable research without making people safer.
The debate grew sharper during the pandemic, as politicians raised questions about the origin of Covid. Those who suggested it came from a lab raised concerns about studies that tweaked pathogens to make them more dangerous — sometimes known as “gain of function” research.
The new policy, which applies to research funded by the federal government, strengthens the government’s oversight by replacing a short list of dangerous pathogens with broad categories into which more pathogens might fall. The policy pays attention not only to human pathogens, but also those that could threaten crops and livestock. And it provides more details about the kinds of experiments that would draw the attention of government regulators.
The rules will take effect in a year, giving government agencies and departments time to update their guidance to meet the new requirements.
“It’s a big and important step forward,” said Dr. Tom Inglesby, the director of the Johns Hopkins Center for Health Security and a longtime proponent of stricter safety regulations. “I think this policy is what any reasonable member of the public would expect is in place in terms of oversight of the world’s most transmissible and lethal organisms.”
Still, the policy does not embrace the most aggressive proposals made by lab safety proponents, such as creating an independent regulatory agency. It also makes exemptions for certain types of research, including disease surveillance and vaccine development. And some parts of the policy are recommendations rather than government-enforced requirements.
“It’s a moderate shift in policy, with a number of more significant signals about how the White House expects the issue to be treated moving forward,” said Nicholas Evans, an ethicist at University of Massachusetts Lowell.
Experts have been waiting for the policy for more than a year. Still, some said they were surprised that it came out at such a politically fraught moment . “I wasn’t expecting anything, especially in an election year,” Dr. Evans said. “I’m pleasantly surprised.”
Under the new policy, scientists who want to carry out experiments will need to run their proposals past their universities or research institutions, which will to determine if the work poses a risk. Potentially dangerous proposals will then be reviewed by government agencies. The most scrutiny will go to experiments that could result in the most dangerous outcomes, such as those tweaking pathogens that could start a pandemic.
In a guidance document , the White House provided examples of research that would be expected to come under such scrutiny. In one case, they envisioned scientists trying to understand the evolutionary steps a pathogen needed to transmit more easily between humans. The researchers might try to produce a transmissible strain to study, for example, by repeatedly infecting human cells in petri dishes, allowing the pathogens to evolve more efficient ways to enter the cells.
Scientists who do not follow the new policy could become ineligible for federal funding for their work. Their entire institution may have its support for life science research cut off as well.
One of the weaknesses of existing policies is that they only apply to funding given out by the federal government. But for years , the National Institutes of Health and other government agencies have struggled with stagnant funding, leading some researchers to turn instead to private sources. In recent years, for example, crypto titans have poured money into pandemic prevention research.
The new policy does not give the government direct regulation of privately funded research. But it does say that research institutions that receive any federal money for life-science research should apply a similar oversight to scientists doing research with support from outside the government.
“This effectively limits them, as the N.I.H. does a lot of work everywhere in the world,” Dr. Evans said.
The new policy takes into account the advances in biotechnology that could lead to new risks. When pathogens become extinct, for example, they can be resurrected by recreating their genomes. Research on extinct pathogens will draw the highest levels of scrutiny.
Dr. Evans also noted that the new rules emphasize the risk that lab research can have on plants and animals. In the 20th century, the United States and Russia both carried out extensive research on crop-destroying pathogens such as wheat-killing fungi as part of their biological weapons programs. “It’s significant as a signal the White House is sending,” Dr. Evans said.
Marc Lipsitch, an epidemiologist at Harvard and a longtime critic of the government’s policy, gave the new one a grade of A minus. “I think it’s a lot clearer and more specific in many ways than the old guidance,” he said. But he was disappointed that the government will not provide detailed information to the public about the risky research it evaluates. “The transparency is far from transparent,” he said.
Scientists who have warned of the dangers of impeding useful virus research were also largely optimistic about the new rules.
Gigi Gronvall, a biosafety specialist at the Johns Hopkins Bloomberg School of Public Health, said the policy’s success would depend on how federal health officials interpreted it, but applauded the way it recognized the value of research needed during a crisis, such as the current bird flu outbreak .
“I was cautiously optimistic in reading through it,” she said of the policy. “It seems like the orientation is for it to be thoughtfully implemented so it doesn’t have a chilling effect on needed research.”
Anice Lowen, an influenza virologist at Emory University, said the expanded scope of the new policy was “reasonable.” She said, for instance, that the decision not to create an entirely new review body helped to alleviate concerns about how unwieldy the process might become.
Still, she said, ambiguities in the instructions for assessing risks in certain experiments made it difficult to know how different university and health officials would police them.
“I think there will be more reviews carried out, and more research will be slowed down because of it,” she said.
Carl Zimmer covers news about science for The Times and writes the Origins column . More about Carl Zimmer
Benjamin Mueller reports on health and medicine. He was previously a U.K. correspondent in London and a police reporter in New York. More about Benjamin Mueller

IMAGES
COMMENTS
Main text. We review the latest evidence concerning 10 key COVID-19 policy and strategic areas, specifically addressing: 1) the expansion of equitable vaccine distribution, 2) the need to ease restrictions as hospitalization and mortality rates eventually fall, 3) the advantages of emphasizing educational and harm reduction approaches over coercive and punitive measures, 4) the need to ...
The Choosing Wisely recommendations for the prevention, care and control of COVID-19 include the best evidence available at present, and address practices that are common, inefficacious, of low ...
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical ...
Long COVID is an often debilitating illness that occurs in at least 10% of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. More than 200 symptoms have been identified with ...
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular ...
For the present study, we surveyed a large group of clinical research professionals to determine their impressions of COVID-19-related clinical trial adjustments. To our knowledge, this report represents the first systematic assessment of experience, perceptions, and recommendations related to COVID-19 trial changes.
Read CFR's recommendations for how the world can improve pandemic readiness, prevention, detection and response in the wake of COVID-19. ... scientific research on emerging and zoonotic diseases ...
The WHO COVID-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). It contained citations with abstracts to scientific articles, reports, books, preprints, and clinical trials on COVID-19 and related literature. The WHO Covid-19 Research Database was maintained by the WHO ...
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
Given the urgency of the COVID-19 epidemic and the need to understand and access information about it, a scoping review was considered suitable for the situation. We therefore conducted this scoping review to help identify research gaps related to this new viral disease and propose recommendations for future research on COVID-19.
Background: During the COVID-19 public health emergency, the FDA and NIH altered clinical trial requirements to protect participants and manage study conduct. Given their detailed knowledge of research protocols and regular contact with patients, clinicians, and sponsors, clinical research professionals offer important perspectives on these changes.
Department of Health Research Methods, Evidence & Impact, McMaster University, Hamilton, Canada. Public Health Ontario, Toronto, Canada. ... Our systematic survey of antibiotic prescribing recommendations in COVID-19 identified a wide range in guideline and recommendation quality. There was inconsistency in the extent to which guidelines ...
While previous research has of... The COVID-19 pandemic has left a profound impact on higher education, prompting the need to assess its effects and provide guidance for future pandemics or disasters. ... The proactive measures and recommendations set forth by the investigated University in response to the challenges presented by the COVID-19 ...
Vaccination is critical to controlling the COVID-19 pandemic, and health care providers play an important role in achieving high vaccination coverage (1).To examine the prevalence of report of a provider recommendation for COVID-19 vaccination and its association with COVID-19 vaccination coverage and attitudes, CDC analyzed data among adults aged ≥18 years from the National Immunization ...
This guideline covers managing COVID-19 in babies, children, young people and adults in community and hospital settings. It includes recommendations on communication, assessment, therapeutics for COVID-19, non-invasive respiratory support, preventing and managing acute complications, and identifying and managing co-infections.
CDC released today updated recommendations for how people can protect themselves and their communities from respiratory viruses, including COVID-19. The new guidance brings a unified approach to addressing risks from a range of common respiratory viral illnesses, such as COVID-19, flu, and RSV, which can cause significant health impacts and strain on hospitals and health care workers.
3.1. Containment and / or Mitigation Measures. A mathematical model suggests that 80% of contacts would need to be identified to contain an epidemic that starts with 20 cases of COVID-19 (assuming an R0 of 2.5), within three months. However, in the case of COVID-19, containment measures will likely be insufficient in light of presymptomatic ...
If you are concerned that you may have COVID-19, follow these steps to help protect your health and the health of others. 1. Stay home and call a health care provider. Unless it is an emergency, to reduce your risk of catching or spreading illness, stay home if you feel sick, even if your symptoms are mild. Do not go to work, school or public ...
The spread of infectious diseases was further promoted due to busy cities, increased travel, and climate change, which led to outbreaks, epidemics, and even pandemics. The world experienced the severity of the 125 nm virus called the coronavirus disease 2019 (COVID-19), a pandemic declared by the World Health Organization (WHO) in 2019. Many investigations revealed a strong correlation between ...
Background Strategies to control coronavirus 2019 disease (COVID-19) have often been based on preliminary and limited data and have tended to be slow to evolve as new evidence emerges. Yet knowledge about COVID-19 has grown exponentially, and the expanding rollout of vaccines presents further opportunity to reassess the response to the pandemic more broadly. Main text We review the latest ...
The median time between ordering an e-consult and a specialist documenting recommendations in an e-consult note was 3.7 (IQR 1.3-8.2) hours across all specialties, contrasted with 7.3 (IQR 3.6-22.0) hours when converted to an in-person consult (P<.001). ... After the peaks of the COVID-19 crisis subsided, the use of inpatient e-consults ...
Cellular & Molecular Immunology (2023) Decades of coronavirus research and intense studies of SARS-CoV-2 since the beginning of the COVID-19 pandemic have led to an unprecedented level of ...
Abstract. Current situation in COVID-19 pandemic as well as the significant digital transformation, where the whole world is being forced to participate, are lead for a wide acceptance to use the mobile payments. The main objective for the current study is to focus on analysing the primary variable "intention to use" through the Apple ...
The first recommendation was to continue studying the experiences of university academics with emergency remote teaching during the COVID-19 pandemic 2020 from other parts of the United States. The second recommendation was to study the lived experiences of instructors who taught hands-on type of courses such as art, music, and science labs.
A new study reveals how COVID-19 vaccines prevent severe disease. This is based on research undertaken at the University of Oxford and it provides insights into the way that COVID-19 vaccines ...
Covid-19 Guidance. Symptoms and Treatment ... And some parts of the policy are recommendations rather than government-enforced requirements. ... Research on extinct pathogens will draw the highest ...