- Patient Care & Health Information
- Diseases & Conditions
- Hepatitis B
Hepatitis B is a serious liver infection caused by the hepatitis B virus (HBV). For most people, hepatitis B is short term, also called acute, and lasts less than six months. But for others, the infection becomes chronic, meaning it lasts more than six months. Having chronic hepatitis B increases your risk of developing liver failure, liver cancer or cirrhosis — a condition that permanently scars the liver.
Most adults with hepatitis B recover fully, even if their symptoms are severe. Infants and children are more likely to develop a long-lasting hepatitis B infection. This is known as a chronic infection.
A vaccine can prevent hepatitis B, but there's no cure if you have the condition. If you're infected, taking certain precautions can help prevent spreading the virus to others.
Symptoms of acute hepatitis B range from mild to severe. They usually appear about 1 to 4 months after you've been infected, although you could see them as early as two weeks after you're infected. Some people, usually young children, may not have any symptoms.
Hepatitis B signs and symptoms may include:
- Abdominal pain
- Loss of appetite
- Nausea and vomiting
- Weakness and fatigue
- Yellowing of the skin and the whites of the eyes, also called jaundice

When to see a doctor
If you know you've been exposed to hepatitis B, contact your health care provider immediately. A preventive treatment may reduce your risk of infection if you receive the treatment within 24 hours of exposure to the virus.
If you think you have symptoms of hepatitis B, contact your health care provider.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get the latest health information from Mayo Clinic delivered to your inbox.
Subscribe for free and receive your in-depth guide to digestive health, plus the latest on health innovations and news. You can unsubscribe at any time. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth digestive health guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest health news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Hepatitis B infection is caused by the hepatitis B virus (HBV). The virus is passed from person to person through blood, semen or other body fluids. It does not spread by sneezing or coughing.
Common ways that HBV can spread are:
- Sexual contact. You may get hepatitis B if you have unprotected sex with someone who is infected. The virus can pass to you if the person's blood, saliva, semen or vaginal secretions enter your body.
- Sharing of needles. HBV easily spreads through needles and syringes contaminated with infected blood. Sharing IV drug paraphernalia puts you at high risk of hepatitis B.
- Accidental needle sticks. Hepatitis B is a concern for health care workers and anyone else who comes in contact with human blood.
- Mother to child. Pregnant women infected with HBV can pass the virus to their babies during childbirth. However, the newborn can be vaccinated to avoid getting infected in almost all cases. Talk to your provider about being tested for hepatitis B if you are pregnant or want to become pregnant.
Acute vs. chronic hepatitis B
Hepatitis B infection may be short-lived, also called acute. Or it might last a long time, also known as chronic.
- Acute hepatitis B infection lasts less than six months. Your immune system likely can clear acute hepatitis B from your body, and you should recover completely within a few months. Most people who get hepatitis B as adults have an acute infection, but it can lead to chronic infection.
- Chronic hepatitis B infection lasts six months or longer. It lingers because your immune system can't fight off the infection. Chronic hepatitis B infection may last a lifetime, possibly leading to serious illnesses such as cirrhosis and liver cancer. Some people with chronic hepatitis B may have no symptoms at all. Some may have ongoing fatigue and mild symptoms of acute hepatitis.
The younger you are when you get hepatitis B — particularly newborns or children younger than 5 — the higher your risk of the infection becoming chronic. Chronic infection may go undetected for decades until a person becomes seriously ill from liver disease.
Risk factors
Hepatitis B spreads through contact with blood, semen or other body fluids from an infected person. Your risk of hepatitis B infection increases if you:
- Have unprotected sex with multiple sex partners or with someone who's infected with HBV
- Share needles during IV drug use
- Are a man who has sex with other men
- Live with someone who has a chronic HBV infection
- Are an infant born to an infected mother
- Have a job that exposes you to human blood
- Travel to regions with high infection rates of HBV , such as Asia, the Pacific Islands, Africa and Eastern Europe
Complications
Having a chronic HBV infection can lead to serious complications, such as:
- Scarring of the liver (cirrhosis). The inflammation associated with a hepatitis B infection can lead to extensive liver scarring (cirrhosis), which may impair the liver's ability to function.
- Liver cancer. People with chronic hepatitis B infection have an increased risk of liver cancer.
- Liver failure. Acute liver failure is a condition in which the vital functions of the liver shut down. When that occurs, a liver transplant is necessary to stay alive.
- Reactivation of the hepatitis B virus. People with chronic hepatitis B who have suppression of their immune system are prone to reactivation of the hepatitis B virus. This can lead to significant liver damage or even liver failure. This includes people on immunosuppressive medications, such as high-dose corticosteroids or chemotherapy. Before taking these medications, you should be tested for hepatitis B. If you test positive for hepatitis B, you should be seen by a liver specialist (hepatologist) before starting these therapies.
- Other conditions. People with chronic hepatitis B may develop kidney disease or inflammation of blood vessels.
The hepatitis B vaccine is typically given as two injections separated by a month or three or four injections over six months, depending on which vaccine is given. You can't get hepatitis B from the vaccine. The hepatitis B vaccine is recommended by the United States Advisory Committee on Immunization Practices for adults 19 to 59 years of age who do not have a contraindication to the vaccine.
The hepatitis B vaccine is also strongly recommended for:
- Children and adolescents not vaccinated at birth
- Those who work or live in a center for people who are developmentally disabled
- People who live with someone who has hepatitis B
- Health care workers, emergency workers and other people who come into contact with blood
- Anyone who has a sexually transmitted infection, including HIV
- Men who have sex with men
- People who have multiple sexual partners
- Sexual partners of someone who has hepatitis B
- People who inject illegal drugs or share needles and syringes
- People with chronic liver disease
- People with end-stage kidney disease
- Travelers planning to go to an area of the world with a high hepatitis B infection rate
Take precautions to avoid HBV
Other ways to reduce your risk of HBV include:
- Know the HBV status of any sexual partner. Don't engage in unprotected sex unless you're absolutely certain your partner isn't infected with HBV or any other sexually transmitted infection.
- Use a new latex or polyurethane condom every time you have sex if you don't know the health status of your partner. Remember that although condoms can reduce your risk of contracting HBV , they don't eliminate the risk.
- Don't use illegal drugs. If you use illicit drugs, get help to stop. If you can't stop, use a sterile needle each time you inject illicit drugs. Never share needles.
- Be cautious about body piercing and tattooing. If you get a piercing or tattoo, look for a reputable shop. Ask about how the equipment is cleaned. Make sure the employees use sterile needles. If you can't get answers, look for another shop.
- Ask about the hepatitis B vaccine before you travel. If you're traveling to a region where hepatitis B is common, ask your provider about the hepatitis B vaccine in advance. It's usually given in a series of three injections over a six-month period.
Living with hepatitis b?
Connect with others like you for support and answers to your questions in the Transplants support group on Mayo Clinic Connect, a patient community.
Transplants Discussions

1588 Replies Thu, Jun 06, 2024

21 Replies Tue, Jun 04, 2024

42 Replies Thu, May 23, 2024
- Hepatitis B. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/liver-disease/viral-hepatitis/hepatitis-b. Accessed Aug. 15, 2022.
- Feldman M, et al., eds. Hepatitis B. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Aug. 16, 2022.
- Kellerman RD, et al. Hepatitis A, B, D, and E. In: Conn's Current Therapy 2022. Elsevier; 2022. https://www.clinicalkey.com. Accessed Aug. 16, 2022.
- Lok AS. Hepatitis B virus: Clinical manifestations and natural history. https://www.uptodate.com/contents/search. Accessed Aug. 16, 2022.
- Eng-Kiong T, et al. Epidemiology, transmission, and prevention of hepatitis B virus infection. https://www.uptodate.com/contents/search. Accessed Aug. 16, 2022.
- Picco MF (expert opinion). Mayo Clinic. Aug. 22, 2022.
- Weng MK, et al. Universal hepatitis B vaccination in adults aged 19–59 years: Updated recommendations of the advisory committee on immunization practices — United States, 2022. MMWR [Morbidity and Mortality Weekly Report; Recommendations and Reports; Surveillance Summaries; or Supplements]. 2022; doi:10.15585/mmwr.mm7113a1.
Associated Procedures
- Liver biopsy
- Liver function tests
- Liver transplant
News from Mayo Clinic
- Hepatitis B vaccine: What to know to protect yourself Jan. 22, 2024, 03:30 p.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
We’re transforming healthcare
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year.
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
This topic review will discuss the characteristics of the hepatitis B virus and the pathogenesis of HBV-related liver disease. The immune response to HBV contributes to the hepatic injury, helps control the infection, and provides the means for establishing the serologic diagnosis of HBV infection. (See "Hepatitis B virus: Screening and diagnosis in adults" .)
CHARACTERISTICS OF THE VIRUS
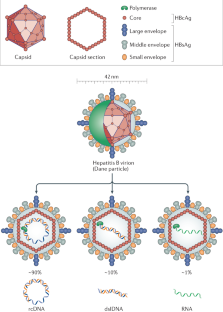
● An envelope composed of viral-encoded proteins and host-derived lipid components
● A core particle made up of the nucleocapsid protein, the viral genome, and the polymerase protein

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Hepatitis B Basic Information
- Hepatitis B is a vaccine-preventable liver infection caused by the hepatitis B virus (HBV) that can lead to chronic infection causing cirrhosis, liver cancer and death.
- All medically stable infants weighing ≥2,000 grams are recommended to receive the hepatitis B vaccine within the first 24 hours following birth.
- All adults aged 19 through 59 years and adults ≥60 years with risk factors for hepatitis B or without identified risk factors but seeking protection are recommended to receive the hepatitis B vaccine.
- All adults aged 18 years and older are recommended to be screened at least once in their lifetime using a triple panel test.
- There is no cure for hepatitis B but there are treatments that can reduce the chance of developing serious liver disease and liver cancer.
- Progress toward hepatitis B elimination has stalled. Since 2012, the rate of reported acute hepatitis B cases has ranged from 0.9 to 1.1 per 100,000 population.
- New hepatitis B infections are highest among people aged 30-59 years because many people at risk in this group have not been vaccinated as recommended.
Topics on this page : What Is Hepatitis B? | How Many People Have Hepatitis B? | Who Is Most Affected? | HIV and HBV Coinfection | How Is Hepatitis B Transmitted? | HBV Prevention | Testing | Treatment | Help Raise Awareness About Hepatitis B | Learn More About Hepatitis B
What Is Hepatitis B?
Hepatitis B is a liver infection caused by the hepatitis B virus (HBV). HBV infection causes inflammation of the liver. When the liver is inflamed or damaged, its function can be affected.
- The best way to prevent HBV infection is by getting vaccinated. Safe and effective vaccines are available and covered as a preventive service by most health plans.
- Hepatitis B is transmitted when blood, semen, or another body fluid from a person infected with HBV enters the body of someone who is not infected. This can happen through sexual contact; sharing needles, syringes, or other drug-injection equipment; or from mother to baby at birth.
- For some people, HBV infection is an acute, or short-term, illness; for others, it can become a long-term, chronic infection. Risk for chronic infection is related to age at infection: approximately 90% of infected infants become chronically infected, compared with 2-6% of adults.
- Chronic hepatitis B can lead to cirrhosis, liver cancer, liver failure, and premature death.
- Hepatitis B is diagnosed with a simple blood test that can detect HBV infection years before symptoms develop and the virus has caused liver damage.
- There is no cure for hepatitis B, but there are several FDA-approved medications that treat HBV infection. People with chronic hepatitis B should be monitored regularly for signs of liver disease and evaluated for possible treatment.
How Many People Have Hepatitis B?
In the United States, an estimated 880,000 to 1.89 million people are chronically infected with HBV. New cases of HBV infection in the United States had been decreasing until 2012. Since that time, reported cases of acute hepatitis B have been fluctuating around 3,000 cases per year. In 2020, 2,157 cases of acute hepatitis B were reported; however, because of low case detection and reporting, the Centers for Disease Control and Prevention (CDC) estimates that there were 14,000 acute hepatitis B infections. The rate of acute cases of HBV decreased by 32% after 2019 which may be related to the disruptions of the COVID-19 pandemic. For the most recent surveillance data visit CDC Viral Hepatitis Surveillance .
Globally, HBV is the most common blood-borne infection with an estimated 296 million people infected according to the World Health Organization .
Who Is Most Affected?
In the United States, rates of new HBV infections are highest among adults aged 30-59 years, reflecting low hepatitis B vaccination coverage among adults at risk. The most common risk factor among people with new HBV infections is injecting drugs, related to the opioid crisis and other drug use.
The highest rates of chronic hepatitis B infection in the United States occur among foreign-born individuals, especially people born in Asia, the Pacific Islands, and Africa. Approximately 70% of cases in the United States are among people who were born outside of the United States. CDC developed this map of the geographic distribution of hepatitis B around the world - PDF . Other groups who have higher rates of chronic HBV infection include people who inject drugs and men who have sex with men.
HIV and HBV Coinfection
About 2% of people with HIV in the United States are coinfected with HBV; both infections have similar routes of transmission. People with HIV are at greater risk for complications and death from HBV infection. All people with HIV are recommended to be tested for HBV, and if susceptible, are further recommended to receive the hepatitis B vaccination or, if chronically infected, evaluated for treatment to prevent liver disease and liver cancer. For more information about HIV and HBV coinfection, visit HIV.gov’s pages about hepatitis B and HIV coinfection .
How Is Hepatitis B Transmitted?
Hepatitis B is spread in several distinct ways: sexual contact; sharing needles, syringes, or other drug-injection equipment; or from mother-to-child at birth.
In the United States, in 2018, injection drug use was the most common risk factor reported among people with an acute HBV infection, followed by having multiple sex partners. Less commonly reported risk factors included accidental needle sticks, surgery, transfusions, and household contact with a person with HBV infection. In the United States, healthcare-related transmission of HBV is rare.
Mother-to-child transmission of HBV is especially concerning, because it is preventable. An estimated 25,000 infants are born to mothers diagnosed with HBV each year in the United States, and approximately 1,000 mothers transmit HBV to their infants. Without appropriate medical care and vaccinations, 90% of HBV-infected newborns will develop chronic infection, remaining infected throughout their lives. Up to 25% of people infected at birth will die prematurely of HBV-related causes. For this reason, the standard of care for pregnant women includes an HBV test during each pregnancy so that the appropriate steps can be taken to prevent HBV-positive mothers from transmitting the disease to her infant.
Globally, mother-to-child transmission and inadequate infection control in health care settings represent significant modes of viral hepatitis transmission. That is why immigrants from many countries are recommended to be tested for HBV as well as hepatitis C virus (HCV).
Hepatitis B Prevention
Hepatitis B is a vaccine-preventable disease. The best way to prevent hepatitis B is to get vaccinate. The hepatitis B vaccine is safe and effective.
Hepatitis B vaccine is recommended for the following people:
- All infants
- Unvaccinated children aged <19 years
- Adults aged 19 through 59 years
- Adults aged 60 years and older with risk factors for hepatitis B
The following groups may receive hepatitis B vaccination:
- Adults aged 60 years and older without known risk factors for hepatitis B
To receive protection against hepatitis B, universal hepatitis B vaccination within 24 hours of birth for all medically stable infants weighing ≥2,000 grams, followed by completion of the series is recommended.
Three doses are required to complete the vaccine series.
Two, three, or four doses are required. The two-dose vaccine is given over 30 days, which increases protection among adults more rapidly with fewer medical visits. There is also a combination vaccine that protects people from both hepatitis A and hepatitis B. The combined vaccine is usually given as 3 shots over a 6-month period. These tools may support increased vaccination in settings such as jails, prisons, substance use disorder prevention and treatment facilities, sexually transmitted disease treatment facilities, and HIV testing and treatment facilities.
The Centers for Disease Control and Prevention (CDC) and the Advisory Committee on Immunization Practices (ACIP) published additional guidance on the hepatitis B vaccine for adults aged 19 through 59 years in 2022.
Immunization programs for infants and adolescents that started in 1991 have resulted in substantial declines in the incidence of hepatitis B virus (HBV) infection in young people. The hepatitis B vaccine is a covered preventive service for those for whom it is recommended under many health plans.
Hepatitis B can also be prevented by avoiding contact with contaminated blood and unprotected sexual exposure. Using condoms has also been shown to reduce the chance of sexually transmitted infections.
Mother-to-child HBV transmission can be prevented by identifying pregnant women who are chronically infected and providing the infant with hepatitis B vaccine and hepatitis B immunoglobulin at birth. Recently updated guidelines also recommend that pregnant women with chronic HBV be referred to a specialist and considered for HBV treatment to further reduce the chance of transmitting the virus.
Screening & Testing
The CDC estimates that 68% of people with chronic hepatitis B are unaware of their infection. The only way to find out if you have hepatitis B is to get tested. Hepatitis B testing is a covered preventive service under many health plans.
Being aware of your hepatitis B status is important because treatments are available that reduce the chance of developing liver disease and liver cancer. If you are diagnosed with hepatitis B, you can also protect your family members by getting them vaccinated.
CDC recently published updated recommendations for hepatitis B screening and testing
All adults aged 18 years and older are recommended to receive screening for hepatitis B at least once in their lifetime using a triple panel test. To ensure increased access to testing, anyone who requests HBV testing should receive it regardless of disclosure of risk. Many people might be reluctant to disclose stigmatizing risks.
CDC recommends HBV screening for hepatitis B surface antigen (HBsAg) for all pregnant people during each pregnancy, preferably in the first trimester, regardless of vaccination status or history of testing. Pregnant people with a history of appropriately timed triple panel screening without subsequent risk for exposure to HBV (i.e., no new HBV exposures since triple panel screening) only need HBsAg screening.
Persons at increased risk for HBV exposure or with symptoms for hepatitis B should receive HBV testing. Persons at increased risk, regardless of age, should receive periodic testing while risk for exposure is ongoing.
People at increased risk:
- People with a history of sexually transmitted infections or multiple sex partners
- People with hepatitis C infection or a history of hepatitis C virus infection
- People incarcerated or formerly incarcerated in a jail, prison, or other detention setting
- People born in countries with an HBV prevalence of ≥2%
- People born in the United States not vaccinated as infants whose parents were born in regions with high rates of HBV infections (HBsAg prevalence ≥8%)
- Men who have sex with men
- People who inject drugs or have a history with injection drug use
- Needle-sharing or sexual contacts of people with known HBV infection
- People with HIV
- Household and sexual contacts of HBV-infected people
- People requiring immunosuppressive therapy
- People with end-stage renal disease (including hemodialysis patients)
- Blood and tissue donors
- People with elevated alanine aminotransferase levels (≥19 IU/L for women and ≥30 IU/L for men)
- Infants born to HBV-infected mothers
There are several antiviral treatments available for chronic hepatitis B. Everyone with chronic hepatitis B should be linked to care, considered for treatment, and regularly checked for liver damage and liver cancer. Hepatitis B treatments reduce the amount of virus in the body and reduce the chance of developing serious liver disease and liver cancer. There is no cure for hepatitis B and treatment is recommended to continue for years if not for life. Research is ongoing for more effective treatments and a cure for HBV.
Take action! These online tools help consumers understand and locate recommended hepatitis B and hepatitis C preventive and screening services .
Help Raise Awareness About Hepatitis B
Know Hepatitis B – CDC’s Hepatitis B Education Campaign for Asian Americans, Pacific Islanders, and others at risk
Learn More About Hepatitis B
Centers for Disease Control and Prevention, Division of Viral Hepatitis
- Hepatitis B information
National Institutes of Health
- Hepatitis B
Find additional learning opportunities for both the public and healthcare providers .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 17 May 2021
Immunobiology and pathogenesis of hepatitis B virus infection
- Matteo Iannacone ORCID: orcid.org/0000-0002-9370-2671 1 , 2 , 3 &
- Luca G. Guidotti ORCID: orcid.org/0000-0002-0205-2678 1 , 2
Nature Reviews Immunology volume 22 , pages 19–32 ( 2022 ) Cite this article
17k Accesses
203 Citations
43 Altmetric
Metrics details
- Hepatitis B
- Immunological surveillance
- Viral host response
- Viral pathogenesis
Hepatitis B virus (HBV) is a non-cytopathic, hepatotropic virus with the potential to cause a persistent infection, ultimately leading to cirrhosis and hepatocellular carcinoma. Over the past four decades, the basic principles of HBV gene expression and replication as well as the viral and host determinants governing infection outcome have been largely uncovered. Whereas HBV appears to induce little or no innate immune activation, the adaptive immune response mediates both viral clearance as well as liver disease. Here, we review our current knowledge on the immunobiology and pathogenesis of HBV infection, focusing in particular on the role of CD8 + T cells and on several recent breakthroughs that challenge current dogmas. For example, we now trust that HBV integration into the host genome often serves as a relevant source of hepatitis B surface antigen (HBsAg) expression during chronic infection, possibly triggering dysfunctional T cell responses and favouring detrimental immunopathology. Further, the unique haemodynamics and anatomy of the liver — and the changes they frequently endure during disease progression to liver fibrosis and cirrhosis — profoundly influence T cell priming, differentiation and function. We also discuss why therapeutic approaches that limit the intrahepatic inflammatory processes triggered by HBV-specific T cells might be surprisingly beneficial for patients with chronic infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Vaccination induces broadly neutralizing antibody precursors to HIV gp41
An IFNγ-dependent immune–endocrine circuit lowers blood glucose to potentiate the innate antiviral immune response
Perinatal thymic-derived CD8αβ-expressing γδ T cells are innate IFN-γ producers that expand in IL-7R–STAT5B-driven neoplasms
Guidotti, L. G. & Chisari, F. V. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. Mech. Dis. 1 , 23–61 (2006).
Article CAS Google Scholar
Locarnini, S., Hatzakis, A., Chen, D.-S. & Lok, A. Strategies to control hepatitis B: public policy, epidemiology, vaccine and drugs. J. Hepatol. 62 , S76–S86 (2015).
Article PubMed Google Scholar
Yuen, M.-F. et al. Hepatitis B virus infection. Nat. Rev. Dis. Primers 4 , 18035 (2018).
Revill, P. A. et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol. Hepatol. 4 , 545–558 (2019).
Article PubMed PubMed Central Google Scholar
Udompap, P. & Kim, W. R. Development of hepatocellular carcinoma in patients with suppressed viral replication: changes in risk over time. Clin. Liver Dis. 15 , 85–90 (2020).
Article Google Scholar
Levrero, M., Testoni, B. & Zoulim, F. HBV cure: why, how, when? Curr. Opin. Virol. 18 , 135–143 (2016).
Fanning, G. C., Zoulim, F., Hou, J. & Bertoletti, A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat. Rev. Drug Discov. 18 , 827–844 (2019).
Article CAS PubMed Google Scholar
Rehermann, B., Ferrari, C., Pasquinelli, C. & Chisari, F. V. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat. Med. 2 , 1104–1108 (1996).
Kim, C. Y. & Tilles, J. G. Purification and biophysical characterization of hepatitis B antigen. J. Clin. Invest. 52 , 1176–1186 (1973).
Article CAS PubMed PubMed Central Google Scholar
Seeger, C. & Mason, W. S. Molecular biology of hepatitis B virus infection. Virology 479 , 672–686 (2015).
Article PubMed CAS Google Scholar
Bertoletti, A. & Ferrari, C. Adaptive immunity in HBV infection. J. Hepatol. 64 , S71–S83 (2016).
Guidotti, L. G., Isogawa, M. & Chisari, F. V. Host–virus interactions in hepatitis B virus infection. Curr. Opin. Immunol. 36 , 61–66 (2015).
Tu, T. et al. Integration occurs early in the viral life cycle in an in vitro infection model via sodium taurocholate cotransporting polypeptide-dependent uptake of enveloped virus particles. J. Virol. 92 , e02007–e02017 (2018). This study shows that HBV DNA integration occurs early upon infection in an in vitro infection model .
Summers, J. et al. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc. Natl Acad. Sci. USA 100 , 11652–11659 (2003).
Yang, W. & Summers, J. Integration of hepadnavirus DNA in infected liver: evidence for a linear precursor. J. Virol. 73 , 9710–9717 (1999).
Wooddell, C. I. et al. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci. Transl Med. 9 , eaan0241 (2017). This paper reveals integrated HBV DNA as a relevant source of HBsAg in patients and chimpanzees with chronic infection .
Article PubMed PubMed Central CAS Google Scholar
Simon, T. G. et al. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N. Engl. J. Med. 382 , 1018–1028 (2020). This manuscript represents one of a large number of meta-analyses describing an association between low-dose aspirin treatment and reduced HCC incidence .
Sitia, G. et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc. Natl Acad. Sci. USA 109 , E2165–E2172 (2012). This preclinical study shows that anti-platelet therapy reduces liver fibrosis and prevents HCC in mouse models of CHB .
Iannacone, M., Sitia, G., Narvaiza, I., Ruggeri, Z. M. & Guidotti, L. G. Antiplatelet drug therapy moderates immune-mediated liver disease and inhibits viral clearance in mice infected with a replication-deficient adenovirus. Clin. Vaccine Immunol. 14 , 1532–1535 (2007).
Jilbert, A. R., Miller, D. S., Scougall, C. A., Turnbull, H. & Burrell, C. J. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus DNA genome is infectious in neonatal ducks. Virology 226 , 338–345 (1996).
Asabe, S. et al. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J. Virol. 83 , 9652–9662 (2009).
Wisse, E., Jacobs, F., Topal, B., Frederik, P. & Geest, B. D. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 15 , 1193–1199 (2008).
Vollmar, B. & Menger, M. D. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol. Rev. 89 , 1269–1339 (2009).
Whalley, S. A. et al. Kinetics of acute hepatitis B virus infection in humans. J. Exp. Med. 193 , 847–854 (2001).
Guidotti, L. G., Matzke, B., Schaller, H. & Chisari, F. V. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69 , 6158–6169 (1995).
Guidotti, L. G. et al. Viral clearance without destruction of infected cells during acute HBV infection. Science 284 , 825–829 (1999).
Wieland, S. F., Spangenberg, H. C., Thimme, R., Purcell, R. H. & Chisari, F. V. Expansion and contraction of the hepatitis B virus transcriptional template in infected chimpanzees. Proc. Natl Acad. Sci. USA 101 , 2129–2134 (2004).
Wieland, S., Thimme, R., Purcell, R. H. & Chisari, F. V. Genomic analysis of the host response to hepatitis B virus infection. Proc. Natl Acad. Sci. USA 101 , 6669–6674 (2004).
Suslov, A. et al. Virus does not interfere with innate immune responses in the human liver. Gastroenterology 154 , 1778–1790 (2018).
Tsui, L. V., Guidotti, L. G., Ishikawa, T. & Chisari, F. V. Posttranscriptional clearance of hepatitis B virus RNA by cytotoxic T lymphocyte-activated hepatocytes. Proc. Natl Acad. Sci. USA 92 , 12398–12402 (1995).
Heise, T., Guidotti, L. G., Cavanaugh, V. J. & Chisari, F. V. Hepatitis B virus RNA-binding proteins associated with cytokine-induced clearance of viral RNA from the liver of transgenic mice. J. Virol. 73 , 474–481 (1999).
Heise, T., Guidotti, L. G. & Chisari, F. V. La autoantigen specifically recognizes a predicted stem-loop in hepatitis B virus RNA. J. Virol. 73 , 5767–5776 (1999).
McClary, H., Koch, R., Chisari, F. V. & Guidotti, L. G. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J. Virol. 74 , 2255–2264 (2000).
Wieland, S. F., Guidotti, L. G. & Chisari, F. V. Intrahepatic induction of α/β interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J. Virol. 74 , 4165–4173 (2000).
Kimura, K., Kakimi, K., Wieland, S., Guidotti, L. G. & Chisari, F. V. Activated intrahepatic antigen-presenting cells inhibit hepatitis B virus replication in the liver of transgenic mice. J. Immunol. 169 , 5188–5195 (2002).
Vilarinho, S., Ogasawara, K., Nishimura, S., Lanier, L. L. & Baron, J. L. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc. Natl Acad. Sci. USA 104 , 18187–18192 (2007).
Isogawa, M., Robek, M. D., Furuichi, Y. & Chisari, F. V. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J. Virol. 79 , 7269–7272 (2005).
Suslov, A., Wieland, S. & Menne, S. Modulators of innate immunity as novel therapeutics for treatment of chronic hepatitis B. Curr. Opin. Virol. 30 , 9–17 (2018).
Iwasaki, A. A virological view of innate immune recognition. Annu. Rev. Microbiol. 66 , 177–196 (2012).
Webster, G. J. M. et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 32 , 1117–1124 (2000).
Thimme, R. et al. CD8 + T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77 , 68–76 (2003).
Hoofnagle, J. H., Gerety, R. J. & Barker, L. F. Antibody to hepatitis-B-virus core in man. Lancet 302 , 869–873 (1973).
Maini, M. K. & Burton, A. R. Restoring, releasing or replacing adaptive immunity in chronic hepatitis B. Nat. Rev. Gastroenterol. Hepatol. 16 , 662–675 (2019).
Guidotti, L. G. et al. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc. Natl Acad. Sci. USA 91 , 3764–3768 (1994).
Guidotti, L. G. et al. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4 , 25–36 (1996).
Wong, Y. C., Tay, S. S., McCaughan, G. W., Bowen, D. G. & Bertolino, P. Immune outcomes in the liver: is CD8 T cell fate determined by the environment? J. Hepatol. 63 , 1005–1014 (2015).
Isogawa et al. CD40 activation rescues antiviral CD8 + T cells from PD-1-mediated exhaustion. PLoS Pathog. 9 , e1003490 (2013).
Bénéchet, A. P. et al. Dynamics and genomic landscape of CD8 + T cells undergoing hepatic priming. Nature 574 , 200–205 (2019). This paper reveals that hepatocellular priming leads to a T cell dysfunction that is refractory to checkpoint inhibition but responds to IL-2 .
Bertolino, P. et al. Death by neglect as a deletional mechanism of peripheral tolerance. Int. Immunol. 11 , 1225–1238 (1999).
Pol et al. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med. 217 , 2261 (2020).
Blattman, J. N. et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 9 , 540–547 (2003).
West, E. E. et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J. Clin. Invest. 123 , 2604–2615 (2013).
Kuipery, A., Gehring, A. J. & Isogawa, M. Mechanisms of HBV immune evasion. Antivir. Res. 179 , 104816 (2020).
Kennedy, P. T. F. et al. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 143 , 637–645 (2012).
Shimizu, Y., Guidotti, L. G., Fowler, P. & Chisari, F. V. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J. Immunol. 161 , 4520–4529 (1998).
Kakimi, K., Isogawa, M., Chung, J., Sette, A. & Chisari, F. V. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J. Virol. 76 , 8609–8620 (2002).
Ishak, K. et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 22 , 696–699 (1995).
Fisicaro, P. et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat. Med. 23 , 327–336 (2017). This article suggests a central role for reactive oxygen species in T cell exhaustion during CHB, thus providing novel potential therapeutic targets .
Wieland, S. F. The chimpanzee model for hepatitis B virus infection. CSH Perspect. Med. 5 , a021469 (2015).
Google Scholar
Chen, M. T. et al. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc. Natl Acad. Sci. USA 101 , 14913–14918 (2004).
Chen, M. et al. Immune tolerance split between hepatitis B virus precore and core proteins. J. Virol. 79 , 3016–3027 (2005).
Tian, Y., Kuo, C., Akbari, O. & Ou, J. J. Maternal-derived hepatitis B virus e antigen alters macrophage function in offspring to drive viral persistence after vertical transmission. Immunity 44 , 1204–1214 (2016).
Publicover, J. et al. Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J. Clin. Invest. 123 , 3728–3739 (2013).
Brunetto, M. R. et al. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc. Natl Acad. Sci. USA 88 , 4186–4190 (1991).
Rivino, L. et al. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J. Clin. Invest. 128 , 668–681 (2018).
Schuch, A. et al. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8 + T cells in chronically HBV-infected patients with low viral load. Gut 68 , 905–915 (2019).
Fumagalli, V. et al. Serum HBsAg clearance has minimal impact on CD8 + T cell responses in mouse models of HBV infection. J. Exp. Med. 217 , e20200298 (2020). This study shows that circulating HBsAg clearance does not improve HBV-specific CD8 + T cell responses .
Bert, N. L. et al. Effects of hepatitis B surface antigen on virus-specific and global T cells in patients with chronic hepatitis B virus infection. Gastroenterology 159 , 652–664 (2020).
Li et al. A potent human neutralizing antibody Fc-dependently reduces established HBV infections. eLife 6 , e26738 (2017).
Zhang, T.-Y. et al. Prolonged suppression of HBV in mice by a novel antibody that targets a unique epitope on hepatitis B surface antigen. Gut 65 , 658 (2015).
Neumann et al. Novel mechanism of antibodies to hepatitis B virus in blocking viral particle release from cells. Hepatology 52 , 875–885 (2010).
Galun, E. et al. Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hepatology 35 , 673–679 (2002).
Bertoletti, A. et al. Cytotoxic T lymphocyte response to a wild type hepatitis B virus epitope in patients chronically infected by variant viruses carrying substitutions within the epitope. J. Exp. Med. 180 , 933–943 (1994).
Bertoletti, A. et al. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature 369 , 407–410 (1994).
Maini, M. K. et al. T cell receptor usage of virus-specific CD8 cells and recognition of viral mutations during acute and persistent hepatitis B virus infection. Eur. J. Immunol. 30 , 3067–3078 (2000).
Bertoletti, A. & Kennedy, P. T. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell Mol. Immunol. 12 , 258–263 (2015).
Fisicaro, P. et al. Pathogenetic mechanisms of T cell dysfunction in chronic HBV infection and related therapeutic approaches. Front. Immunol. 11 , 849 (2020).
Burton, A. R. et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J. Clin. Invest. 128 , 4588–4603 (2018).
Salimzadeh, L. et al. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J. Clin. Invest. 128 , 4573–4587 (2018). Together with Burton et al. (2018), this paper detects and characterizes dysfunctional HBsAg-specific B cell responses in patients with chronic HBV infection .
Tian, C. et al. Use of ELISpot assay to study HBs-specific B cell responses in vaccinated and HBV infected humans. Emerg. Microbes Infec 7 , 16 (2018).
Xu, X. et al. Reversal of B-cell hyperactivation and functional impairment is associated with HBsAg seroconversion in chronic hepatitis B patients. Cell Mol. Immunol. 12 , 309–316 (2015).
Bert, N. L. et al. Comparative characterization of B cells specific for HBV nucleocapsid and envelope proteins in patients with chronic hepatitis B. J. Hepatol. 72 , 34–44 (2019).
Vanwolleghem, T. et al. Hepatitis B core-specific memory B cell responses associate with clinical parameters in patients with chronic HBV. J. Hepatol. 73 , 52–61 (2020).
Milich, D. & McLachlan, A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science 234 , 1398–1401 (1986).
Guidotti, L. G. & Iannacone, M. Effector CD8 T cell trafficking within the liver. Mol. Immunol. 55 , 94–99 (2013).
Iannacone, M. Hepatic effector CD8 + T-cell dynamics. Cell Mol. Immunol. 12 , 269–272 (2015).
Inverso, D. & Iannacone, M. Spatiotemporal dynamics of effector CD8 + T cell responses within the liver. J. Leukoc. Biol. 99 , 51–55 (2016).
Benechet, A. P. & Iannacone, M. Determinants of hepatic effector CD8 + T cell dynamics. J. Hepatol. 66 , 228–233 (2017).
Guidotti, L. G. et al. Immunosurveillance of the liver by intravascular effector CD8 + T cells. Cell 161 , 486–500 (2015). This manuscript reports that effector CD8 + T cells can recognize and kill antigen-expressing hepatocytes without extravasating by extending cytoplasmic protrusions through endothelial fenestration .
Sironi, L. et al. In vivo flow mapping in complex vessel networks by single image correlation. Sci. Rep. 4 , 7341 (2014).
Warren, A. et al. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology 44 , 1182–1190 (2006).
Guidotti, L. G. The role of cytotoxic T cells and cytokines in the control of hepatitis B virus infection. Vaccine 20 , A80–A82 (2002).
Fioravanti, J. et al. Effector CD8 + T cell-derived interleukin-10 enhances acute liver immunopathology. J. Hepatol. 67 , 543–548 (2017).
Iannacone, M. & Guidotti, L. G. Mouse models of hepatitis B virus pathogenesis. CSH Perspect. Med. 5 , a021477 (2015).
Guidotti, L. G., McClary, H., Loudis, J. M. & Chisari, F. V. Nitric oxide inhibits hepatitis b virus replication in the livers of transgenic mice. J. Exp. Med. 191 , 1247–1252 (2000).
Wieland, S. F., Eustaquio, A., Whitten-Bauer, C., Boyd, B. & Chisari, F. V. Interferon prevents formation of replication-competent hepatitis B virus RNA-containing nucleocapsids. Proc. Natl Acad. Sci. USA 102 , 9913–9917 (2005).
Robek, M. D., Wieland, S. F. & Chisari, F. V. Inhibition of hepatitis B virus replication by interferon requires proteasome activity. J. Virol. 76 , 3570–3574 (2002).
Xia, Y. et al. Interferon-γ and tumor necrosis factor-α produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology 150 , 194–205 (2016).
Michalak, T. I., Pasquinelli, C., Guilhot, S. & Chisari, F. V. Hepatitis B virus persistence after recovery from acute viral hepatitis. J. Clin. Invest. 93 , 230–239 (1994).
Pallett, L. J. et al. IL-2 high tissue-resident T cells in the human liver: sentinels for hepatotropic infection. J. Exp. Med. 214 , 1567–1580 (2017). This paper characterizes tissue-resident memory T cells in the liver of patients chronically infected by HBV .
Ando, K. et al. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J. Immunol. 152 , 3245–3253 (1994).
Nakamoto, Y., Guidotti, L. G., Pasquetto, V., Schreiber, R. D. & Chisari, F. V. Differential target cell sensitivity to CTL-activated death pathways in hepatitis B virus transgenic mice. J. Immunol. 158 , 5692–5697 (1997).
Sitia, G. et al. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLoS Pathog. 7 , e1002061 (2011).
Sitia et al. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J. Leukoc. Biol. 81 , 100–107 (2007).
Sitia, G. et al. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc. Natl Acad. Sci. USA 99 , 13717–13722 (2002).
Sitia, G. et al. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J. Clin. Invest. 113 , 1158–1167 (2004).
Kakimi, K. et al. Blocking chemokine responsive to γ-2/interferon (IFN)-γ inducible protein and monokine induced by IFN-γ activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J. Exp. Med. 194 , 1755–1766 (2001).
Maini, M. K. et al. The role of virus-specific CD8 + cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191 , 1269–1280 (2000).
Reignat, S. et al. Escaping high viral load exhaustion CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J. Exp. Med. 195 , 1089–1101 (2002).
Webster, G. J. M. et al. Longitudinal analysis of CD8 + T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J. Virol. 78 , 5707–5719 (2004).
Boni, C. et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81 , 4215–4225 (2007).
Hoogeveen, R. C. et al. Phenotype and function of HBV-specific T cells is determined by the targeted epitope in addition to the stage of infection. Gut 68 , 893–904 (2018).
Nakamoto, Y., Guidotti, L. G., Kuhlen, C. V., Fowler, P. & Chisari, F. V. Immune pathogenesis of hepatocellular carcinoma. J. Exp. Med. 188 , 341–350 (1998).
Isogawa, M., Furuichi, Y. & Chisari, F. V. Oscillating CD8 + T cell effector functions after antigen recognition in the liver. Immunity 23 , 53–63 (2005).
Khakpoor, A. et al. Spatiotemporal differences in presentation of CD8 T cell epitopes during hepatitis B virus infection. J. Virol . 93 , e01457-18 (2018).
Nakamoto, Y., Suda, T., Momoi, T. & Kaneko, S. Different procarcinogenic potentials of lymphocyte subsets in a transgenic mouse model of chronic hepatitis B. Cancer Res. 64 , 3326–3333 (2004).
Tang, L. S. Y., Covert, E., Wilson, E. & Kottilil, S. Chronic hepatitis B infection: a review. JAMA 319 , 1802–1813 (2018).
Buendia, M.-A. & Neuveut, C. Hepatocellular carcinoma. CSH Perspect. Med. 5 , a021444 (2015).
Levrero, M. & Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 64 , S84–S101 (2016).
Bisceglie, A. M. D. Hepatitis B and hepatocellular carcinoma. Hepatology 49 , S56–S60 (2009).
Schuppan, D. & Afdhal, N. H. Liver cirrhosis. Lancet 371 , 838–851 (2008).
Bataller, R. & Brenner, D. A. Liver fibrosis. J. Clin. Invest. 115 , 209–218 (2005).
Friedman, S. L. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat. Clin. Pract. Gastr 1 , 98–105 (2004).
Iannacone, M. et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat. Med. 11 , 1167–1169 (2005). This study establishes platelets as critical mediators of liver damage through their capacity to promote liver homing of effector CD8 + T cells .
Ornelas, A. et al. Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metast Rev. 36 , 289–303 (2017).
Haemmerle, M., Stone, R. L., Menter, D. G., Afshar-Kharghan, V. & Sood, A. K. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell 33 , 965–983 (2018).
Lee, P.-C. et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann. Surg. Oncol. 23 , 874–883 (2016).
Hwang, I. C., Chang, J., Kim, K. & Park, S. M. Aspirin use and risk of hepatocellular carcinoma in a national cohort study of Korean adults. Sci. Rep. 8 , 4968 (2018).
Simon, T. G. et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 4 , 1683 (2018).
Lee, T.-Y. et al. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern. Med. 179 , 633–640 (2019).
Wang, S. et al. Association of aspirin therapy with risk of hepatocellular carcinoma: a systematic review and dose–response analysis of cohort studies with 2.5 million participants. Pharmacol. Res. 151 , 104585 (2019).
Liao, Y.-H. et al. Aspirin decreases hepatocellular carcinoma risk in hepatitis C virus carriers: a nationwide cohort study. BMC Gastroenterol. 20 , 6 (2020).
Bosetti, C., Santucci, C., Gallus, S., Martinetti, M. & Vecchia, C. L. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann. Oncol. 31 , 558–568 (2020).
Hayashi, T. et al. Antiplatelet therapy improves the prognosis of patients with hepatocellular carcinoma. Cancers 12 , 3215 (2020).
Article CAS PubMed Central Google Scholar
Guidotti, L. G., Vecchia, C. L. & Colombo, M. Is it time to recommend low-dose aspirin treatment for the prevention of hepatocellular carcinoma? Gastroenterology 159 , 1988–1990 (2020).
Martinez, M. G., Villeret, F., Testoni, B. & Zoulim, F. Can we cure hepatitis B virus with novel direct-acting antivirals? Liver Int. 40 , 27–34 (2020).
Hillis, W. D. Viral hepatitis associated with sub-human primates. Transfusion 3 , 445–454 (1963).
Walter, E., Keist, R., Niederöst, B., Pult, I. & Blum, H. E. Hepatitis B virus infection of tupaia hepatocytes in vitro and in vivo. Hepatology 24 , 1–5 (1996).
CAS PubMed Google Scholar
Schulze, A., Gripon, P. & Urban, S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 46 , 1759–1768 (2007).
Sureau, C. & Salisse, J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus A-determinant. Hepatology 57 , 985–994 (2013).
Roskams, T. et al. Heparan sulfate proteoglycan expression in normal human liver. Hepatology 21 , 950–958 (1995).
Yan, H. et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1 , e00049 (2012).
Döring, B., Lütteke, T., Geyer, J. & Petzinger, E. The SLC10 carrier family: transport functions and molecular structure. Curr. Top. Membr. 70 , 105–168 (2012).
Hu, J. & Liu, K. Complete and incomplete hepatitis B virus particles: formation, function, and application. Viruses 9 , 56 (2017).
Article PubMed Central CAS Google Scholar
Seitz, S., Habjanič, J., Schütz, A. K. & Bartenschlager, R. The hepatitis B virus envelope proteins: molecular gymnastics throughout the viral life cycle. Ann. Rev. Virol. 7 , 1–26 (2020).
CAS Google Scholar
Wisse, E., de Zanger, R. B., Charels, K., Van Der Smissen, P. & McCuskey, R. S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of disse. Hepatology 5 , 683–692 (1985).
Ficht, X. & Iannacone, M. Immune surveillance of the liver by T cells. Sci. Immunol. 5 , eaba2351 (2020).
Iwakiri, Y. The lymphatic system: a new frontier in hepatology. Hepatology 64 , 706–707 (2016).
Jenne, C. N. & Kubes, P. Immune surveillance by the liver. Nat. Immunol. 14 , 996–1006 (2013).
Horst, A. K., Neumann, K., Diehl, L. & Tiegs, G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol. Immunol. 13 , 277–292 (2016).
Wong, Y. C., McCaughan, G. W., Bowen, D. G. & Bertolino, P. The CD8 T-cell response during tolerance induction in liver transplantation. Clin. Transl Immunol. 5 , e102 (2016).
Mason, W. S. et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology 151 , 986–998.e4 (2016).
Tu, T., Budzinska, M. A., Shackel, N. A. & Urban, S. HBV DNA integration: molecular mechanisms and clinical implications. Viruses 9 , 75 (2017).
Budzinska, M. A., Shackel, N. A., Urban, S. & Tu, T. Cellular genomic sites of hepatitis B virus DNA integration. Genes 9 , 365 (2018).
Huang, Z. M. & Yen, T. S. Dysregulated surface gene expression from disrupted hepatitis B virus genomes. J. Virol. 67 , 7032–7040 (1993).
Dienes, H. P. et al. Hepatic expression patterns of the large and middle hepatitis B virus surface proteins in viremic and nonviremic chronic hepatitis B. Gastroenterology 98 , 1017–1023 (1990).
Chisari, F. V. et al. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell 59 , 1145–1156 (1989).
Su, I., Wang, H., Wu, H. & Huang, W. Ground glass hepatocytes contain pre-S mutants and represent preneoplastic lesions in chronic hepatitis B virus infection. J. Gastroen Hepatol. 23 , 1169–1174 (2008).
Hadziyannis, S., Gerber, M. A., Vissoulis, C. & Popper, H. Cytoplasmic hepatitis B antigen in “ground-glass” hepatocytes of carriers. Arch. Pathol. 96 , 327–330 (1973).
Tu, T. et al. Clonal expansion of hepatocytes with a selective advantage occurs during all stages of chronic hepatitis B virus infection. J. Viral Hepat. 22 , 737–753 (2015).
Download references
Acknowledgements
The authors thank M. Silva for secretarial assistance, F. Andreata for help with figure preparation and the members of the Iannacone and Guidotti laboratories for helpful discussions. They apologize to all authors whose work they could not cite due to space constraints. M.I. is supported by the European Research Council (ERC) Consolidator Grant 725038, ERC Proof of Concept Grant 957502, Italian Association for Cancer Research (AIRC) Grants 19891 and 22737, Italian Ministry of Health (MoH) Grants RF-2018-12365801 and COVID-2020-12371617, Lombardy Foundation for Biomedical Research (FRRB) Grant 2015-0010, the European Molecular Biology Organization Young Investigator Program and a Funded Research Agreement from Gilead Sciences. L.G.G. is supported by the AIRC Grant 22737, Lombardy Open Innovation Grant 229452, PRIN Grant 2017MPCWPY from the Italian Ministry of Education, University and Research, and Funded Research Agreements from Gilead Sciences, Avalia Therapeutics and CNCCS SCARL.
Author information
Authors and affiliations.
Division of Immunology, Transplantation and Infectious Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy
Matteo Iannacone & Luca G. Guidotti
Vita-Salute San Raffaele University, Milan, Italy
Experimental Imaging Center, IRCCS San Raffaele Scientific Institute, Milan, Italy
Matteo Iannacone
You can also search for this author in PubMed Google Scholar
Contributions
M.I. and L.G.G. contributed equally to this work.
Corresponding authors
Correspondence to Matteo Iannacone or Luca G. Guidotti .
Ethics declarations
Competing interests.
M.I. participates in advisory boards/consultancies for Gilead Sciences, Roche, Third Rock Ventures, Amgen, Asher Bio and Allovir. L.G.G is a member of the board of directors at Genenta Science and Epsilon Bio and participates in advisory boards/consultancies for Gilead Sciences, Roche and Arbutus Biopharma. M.I. and L.G.G. are inventors on patents filed, owned and managed by San Raffaele Scientific Institute, Vita-Salute San Raffaele University and Telethon Foundation on technology related to work discussed in this manuscript (WO2020/016434, WO2020/016427, WO2020/030781, WO2020/234483, EU patent applications n. 19211249.8 and n. 20156716.1, and UK patent application n. 1907493.9).
Additional information
Peer review information.
Nature Reviews Immunology thanks Anna Lok, Antonio Bertoletti and Mala Maini for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The final stage of fibrosis in which fibrous septa surrounding nodules of regenerating hepatocytes induce profound architectural distortion of the liver and functional insufficiency.
A functional outcome of cross-presentation (the presentation of extracellular antigens on MHC class I molecules), whereby antigen-specific naive CD8 + T cells are activated by antigen-presenting cells to become effector cells.
A form of cancer immunotherapy targeting immune checkpoints (for example, PD1, CTLA4).
T cell-induced cytokines such as IFNγ and TNF have been shown to induce the post-transcriptional downregulation of hepatitis B virus (HBV) RNAs in vivo. This process appears to rely on the degradation of the full-length SSB/La protein, which normally functions as a HBV RNA stabilizer in the nucleus of the hepatocyte.
The serum concentrations of the liver enzyme alanine aminotransferase. Commonly measured clinically as a biomarker for liver damage.
(Also referred to as perisinusoidal space). The space that lies between the hepatocytes and the sinusoids.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Iannacone, M., Guidotti, L.G. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol 22 , 19–32 (2022). https://doi.org/10.1038/s41577-021-00549-4
Download citation
Accepted : 01 April 2021
Published : 17 May 2021
Issue Date : January 2022
DOI : https://doi.org/10.1038/s41577-021-00549-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The potential and promise for clinical application of adoptive t cell therapy in cancer.
- Yeteng Zheng
Journal of Translational Medicine (2024)
The combination of Schisandrin C and Luteolin synergistically attenuates hepatitis B virus infection via repressing HBV replication and promoting cGAS-STING pathway activation in macrophages
- Xiaomei Zhao
- Xiaohe Xiao
Chinese Medicine (2024)
Noninvasive models for the prediction of liver fibrosis in patients with chronic hepatitis B
- Juanxia Wang
BMC Gastroenterology (2024)
Phosphorylation of RGS16 at Tyr168 promote HBeAg-mediated macrophage activation by ERK pathway to accelerate liver injury
- Miaomiao Tian
Journal of Molecular Medicine (2024)
Insights into the impact of hepatitis B virus on hepatic stellate cell activation
- Hongjuan You
- Renxian Tang
Cell Communication and Signaling (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Mount Sinai Health System
- Find a Doctor
- Request an Appointment

What Is the Difference Between Hepatitis C and Hepatitis B?
May 2, 2024 | Featured , Your Health

Hepatitis is inflammation of the liver—an organ we depend on to digest nutrients, filter blood, and overcome infection. There are many different types of hepatitis, including hepatitis A, B, C, D, and E, with symptoms that include fever, abdominal pain, nausea, jaundice (yellowing of the skin and eyes), and fatigue.
However, most people with chronic viral hepatitis do not experience any symptoms and often do not know they have the infection even while it silently damages their liver. Hepatitis B and C are among the most common types of hepatitis. While they both affect the liver, they are very different.

Douglas Dieterich, MD
In this Q&A, Douglas Dieterich , MD, Professor of Medicine (Liver Diseases) and Director of the Institute for Liver Medicine at the Icahn School of Medicine at Mount Sinai, explains the differences between hepatitis C and B, how they are transmitted and treated, who is at risk, and more.
What is the difference between hepatitis C and B?
Hepatitis C virus (HCV) and hepatitis B virus (HBV) are vastly different viruses. Hepatitis B is highly contagious through sex, using drugs with shared straws and needles, blood transfusions, and even saliva, which can put people living in the same household at risk. The good news is hepatitis B is entirely preventable with a vaccine, which has been around since 1991. The Centers for Disease Control and Prevention now recommends universal vaccination for hepatitis B for all adults under 60 who did not get vaccinated by their pediatrician starting in 1991. People over 60 can also request the vaccine and should, especially if they have ongoing risk factors. If people do get hepatitis B, there are very good drugs to control it and to suppress the virus down to zero so it doesn’t do any damage or infect others. We also have exciting clinical trials happening to study medications that can cure Hepatitis B.
Currently, there is no vaccine for hepatitis C, which is a different class of virus. It actually belongs to a class that you may have heard of—West Nile virus, dengue fever, yellow fever, and Zika, which has been in the news the last few years. None of those become chronic, however, while hepatitis C does. Over time, it can cause the same liver damage that hepatitis B can, including liver cancer, which can lead to death. The good news is, it’s now easily curable. We have fantastic new drugs for hepatitis C—most patients need to take only 8 to 12 weeks of easy-to-take pills with virtually no side effects and a 99 percent cure rate. It’s absolutely important to find out if you have hepatitis C or B because we can cure hepatitis C and control hepatitis B.
What do I need to know about hepatitis D?
Hepatitis D, also known as hepatitis Delta virus (HDV), is the most severe form of viral hepatitis. This is a type of hepatitis that can only infect people who have hepatitis B. Approximately 70 percent of people who have hepatitis Delta will develop cirrhosis (liver scarring) within 5 to 10 years of infection. This is a much higher and faster progression than for most people with hepatitis C and hepatitis B.
Hepatitis Delta can only function in a body that is also infected with hepatitis B. Not everyone with hepatitis B has hepatitis Delta, but everyone with hepatitis Delta also has hepatitis B. That’s why we recommend everyone with hepatitis B get screened for hepatitis Delta too.
New effective treatments for hepatitis Delta are coming soon and are already available to some patients, depending on their specific health situation. Our providers can screen you for hepatitis Delta and help get you onto treatment if needed.
Who is at risk for contracting hepatitis B and C, and who should get screened?
The CDC recommends all adults be screened for hepatitis B and C at least once in their life, even if they don’t think they have any risk factors. Many people have been exposed but don’t know it. The major method of transmission for hepatitis B, globally, is from mother to infant at birth. Other people who are at risk are those who have never been vaccinated—primarily people born before 1991—and we see that happening now. When people born before 1991 come in contact with people who have hepatitis B, they can catch it quite easily. Hepatitis C is more difficult to catch. The major risks for hepatitis C are having had a transfusion of blood or blood products, such as gamma globulin, before 1992, or using IV drugs or intranasal drugs. Just snorting drugs with a straw is enough to spread Hepatitis C. People who have unprotected sex—especially men who have sex with men—are also at risk for hepatitis C. It’s very important to get diagnosed early so you can get treated and cured. If you know you have ongoing risk factors, you should be screened at least once a year.
Why is hepatitis more common in New York City?
About 48 percent of the people who live in New York City were born outside of the United States. Many of those people come from countries where hepatitis B or C is endemic, and that’s the major risk factor for hepatitis B. Endemic means that a high percentage of people in an area have the disease and therefore the risk of getting the disease is high. The New York City Department of Health and Mental Hygiene estimates that 243,000 New Yorkers, or 2.9 percent of the population, have chronic hepatitis B. The Department also estimates that approximately 86,000 New Yorkers, or 1 percent of the population, have chronic hepatitis C. If we catch viral hepatitis early, we can help you prevent liver scarring and liver cancer.
What is the best way to prevent hepatitis B and C?
The best way to prevent hepatitis B is to get vaccinated for hepatitis B. The CDC now recommends everyone aged 18 to 59 be vaccinated for hepatitis B. If you weren’t vaccinated as a kid, it’s easy to check if you have antibodies to hepatitis B, or if you have hepatitis B, we can treat that. Ask your doctor about testing and vaccination.
Hepatitis C is mostly spread blood to blood. Shared needles—if you’re using IV drugs, and shared straws if you’re using intranasal drugs—things like that—are really high risk for spreading hepatitis C. Getting a tattoo or piercing from an unlicensed technician may also put you at risk if they are not properly cleaning their needles. If you are using drugs, don’t share needles, don’t share straws. And get tested for hepatitis C, because if you have it, we can cure it. Once cured, you can become reinfected with hepatitis C, so it’s very important to continue avoiding infection after getting cured, which means not sharing needles or straws and practicing safe sex, and only getting tattoos and piercings from licensed technicians.
What resources are available at Mount Sinai for screening and treatment of hepatitis?
We have numerous resources dedicated to screening and treatment of hepatitis B and hepatitis C at Mount Sinai. We’re the largest independent liver program in the country. We have liver clinics all over Manhattan and the metropolitan area—from Long Island to Westchester. Our care coordinators will support you from screening through treatment and cure, working closely with your provider to ensure you get the best care.
Related Posts
- What You Need to Know About Cataract Surgery and Choosing the Right Replacement Lens
- TelePrEP? PrEP on Demand? Here’s the Latest on Pre-Exposure Prophylaxis for HIV.
- Why It’s Important for AAPI Communities to Be Vigilant About Breast and Colon Cancer Screening
- Celebrating Asian/Pacific American Heritage Month: Why Diversity Matters in Health Care
- Bird Flu: What You Need to Know Now
Pin It on Pinterest
Share this post with your friends!
- Print Friendly
- Open access
- Published: 04 June 2024
Alpha-fetoprotein and APRI as predictive markers for patients with Type C hepatitis B-related acute-on-chronic liver failure: a retrospective study
- Chunyan Li 1 ,
- Chengzhi Bai 1 ,
- Huaqian Xu 1 ,
- Lin Liu 1 &
- Shanhong Tang 1
BMC Gastroenterology volume 24 , Article number: 191 ( 2024 ) Cite this article
75 Accesses
Metrics details
Type C hepatitis B-related acute-on-chronic liver failure (HBV-ACLF), which is based on decompensated cirrhosis, has different laboratory tests, precipitating events, organ failure and clinical outcomes. The predictors of prognosis for type C HBV-ACLF patients are different from those for other subgroups. This study aimed to construct a novel, short-term prognostic score that applied serological indicators of hepatic regeneration and noninvasive assessment of liver fibrosis to predict outcomes in patients with type C HBV-ACLF.
Patients with type C HBV-ACLF were observed for 90 days. Demographic information, clinical examination, and laboratory test results of the enrolled patients were collected. Univariate and multivariate logistic regression were performed to identify independent prognostic factors and develop a novel prognostic scoring system. A receiver operating characteristic (ROC) curve was used to analyse the performance of the model.
A total of 224 patients with type C HBV-ACLF were finally included. The overall survival rate within 90 days was 47.77%. Age, total bilirubin (TBil), international normalized ratio (INR), alpha-fetoprotein (AFP), white blood cell (WBC), serum sodium (Na), and aspartate aminotransferase/platelet ratio index (APRI) were found to be independent prognostic factors. According to the results of the logistic regression analysis, a new prognostic model (named the A3Twin score) was established. The area under the curve (AUC) of the receiver operating characteristic curve (ROC) was 0.851 [95% CI (0.801-0.901)], the sensitivity was 78.8%, and the specificity was 71.8%, which were significantly higher than those of the MELD, IMELD, MELD-Na, TACIA and COSSH‐ACLF II scores (all P < 0.001). Patients with lower A3Twin scores (<-9.07) survived longer.
Conclusions
A new prognostic scoring system for patients with type C HBV-ACLF based on seven routine indices was established in our study and can accurately predict short-term mortality and might be used to guide clinical management.
Peer Review reports
Acute-on-chronic liver failure (ACLF) is a life-threatening clinical syndrome with rapid progression of hepatic injury based on chronic liver diseases, accompanied by liver and/or extrahepatic organ failure [ 1 ]. In the West, the most common aetiologies of ACLF are alcoholic liver disease and hepatitis C virus infection. In the East, hepatitis B virus (HBV) infections dominate [ 2 ], and HBV infection is the major cause of ACLF in China, accounting for more than 50% of cases [ 3 ]. ACLF can be induced by acute intrahepatic (e.g, alcoholic hepatitis or hepatitis B virus reactivation) or extrahepatic insults (e.g, bacterial infection, gastrointestinal haemorrhage). The ACLF insults also differ in Eastern and Western populations. Due to the differences in aetiology and inducement, ACLF has regional phenotypic specificities, and there are different definitions for ACLF in different geographic regions. One of the major differences is that the Asian Pacific Association for the Study of the Liver (APASL) includes noncirrhotic chronic liver disease (CLD) and compensated cirrhosis to represent “chronic”, whereas the European Association for the Study of the Liver (EASL) includes only cirrhosis, either compensated or decompensated, to define CLD [ 4 ]. Attempting to cover all ACLF patients diagnosed in the East and West, the WGO defined ACLF into three categories in 2014[ 5 ]: patients with CLD but no cirrhosis (type A), compensated cirrhosis (type B), and decompensated cirrhosis (type C).
Type-C ACLF patients with prior decompensated cirrhosis have the lowest baseline hepatic reserve, heaviest liver fibrosis, and highest portal hypertension (PH) [ 6 ]. Decompensated cirrhosis is associated with the development of disease-related complications, such as ascites, oesophageal variceal bleeding, and hepatic encephalopathy, usually with HVPG >10 mmHg. PH has strong positive implications for the patient’s disease course and prognosis [ 7 ]. Type C patients have higher mortalities than either type A or type B patients [ 8 ]. Except for liver transplantation, the current therapeutic methods are limited. Thus, prognostic models could play an essential role in type C ACLF management.
At present, there are many prognostic scoring models for ACLF, including the MELD score [ 9 ], CLIF-C ACLF score [ 10 ], AARC score [ 11 ], COSSH-ACLF score [ 12 ], and COSSH-ACLF II score [ 13 ]. The predictive accuracy of MELD is limited [ 14 ]. Based on the complicated assessment of organ failure, the CLIF-C ACLF and COSSH-ACLF scores should be further simplified. The AARC score and COSSH-ACLF II score contain subjective indicators. They rarely focus on liver fibrosis/portal hypertension and liver regeneration. This study aimed to construct a new, short-term prognosis model that considers the combination of noninvasive assessment of liver fibrosis and liver regeneration to provide a simple and accurate prognosis of type C HBV-ACLF.
Patient management
Standard medical treatment was obtained, including bed rest, liver-protective treatment, and energy supplements. Patients also received plasma and albumin infusion, water-electrolyte maintenance, and complication-preventing treatment. All patients received antiviral therapy.
Study population
Patients were retrospectively screened and enrolled from January 2015 to January 2023 in the General Hospital of Western Theater Command and followed up for 90 days from the date of ACLF diagnosis. The endpoint of follow-up was death or liver transplantation. In this retrospective analysis, a total of 302 type C ACLF patients were initially screened. The diagnostic criteria for HBV-ACLF should include the following: (1) The cause of ACLF was hepatitis B virus (HBV). The diagnosis of chronic hepatitis B was hepatitis B surface antigen positivity or hepatitis B virus deoxyribonucleic acid level (HBV-DNA) positivity for > 6 months. (2) The patients were those who were previously diagnosed with hepatitis B virus-associated decompensated cirrhosis. Liver cirrhosis was diagnosed based on previous liver biopsy findings, ultrasonography, computed tomography, or magnetic resonance imaging findings. Decompensated cirrhosis was diagnosed based on cirrhosis with portal hypertension complications (ascites, hepatic encephalopathy (HE), gastrointestinal haemorrhage, bacterial infection, and hepatorenal syndrome, or any combination of these and/or hypo-hepatic function). (3) ACLF was diagnosed according to the Asian Pacific Association for the Study of the Liver (APASL) [ 15 ]: ACLF is an acute hepatic insult manifesting as jaundice (serum bilirubin ≥ 5 mg/dL (85 umol/L) and coagulopathy (INR ≥ 1.5 or prothrombin activity < 40%) complicated within 4 weeks by clinical ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease/cirrhosis, and is associated with a high 28-day mortality. The exclusion criteria were as follows: (1) age below 18 or over 80 years; (2) coinfection with other viruses (hepatitis A, hepatitis C, hepatitis D or E, or HIV); (3) other causes of ACLF, such as autoimmune liver disease, drug-induced hepatitis, alcoholic liver injury, etc ; (4) hepatocellular carcinoma (HCC); and (5) clinical data missing or patients lost to follow-up. Finally, 224 patients were included in this study (Fig. 1 ).

Inclusion and exclusion criteria of this research. HBV-ACLF: hepatitis B-related acute-on-chronic liver failure; HCC: hepatocellular carcinoma
Data collection
Patient demographics (age, sex) and laboratory data at the time of admission, including routine blood tests, liver and kidney function, coagulation parameters, blood lipids, serum tumour markers, HBV-DNA and hepatitis B e antigen (HBeAg), were collected. Complications such as ascites, gastrointestinal haemorrhage, hepatic encephalopathy (HE), acute kidney injury (AKI), and bacterial infections were also recorded and analysed. The aspartate aminotransferase/platelet ratio index (APRI) and Fibrosis-4 (FIB4) were used to assess the degree of liver fibrosis or cirrhosis. APRI=[(AST/ULN)×100]/PLT; FIB-4=(age ×AST)/(PLT×*ALT 1/2 . Prognostic models including the MELD, MELD-Na, iMELD, COSSH-ACLF II scores, and TACIA scores were recorded as tools of condition assessment. The MELD score was calculated by the following formula: MELD = 3.78 × ln [TBil (mg/dL)] + 11.2 × ln (INR) + 9.57 × ln [serum creatinine (mg/dL)] + 6.4 [ 16 ]. MELD Na = MELD – Na- [0.025 × MELD × (140 - Na)] + 140 [ 17 ]. iMELD=MELD+ (0.3×age)-(0.7× Na) +100 [ 18 ]. COSSH-ACLF II =1.649 × ln (INR) + 0.457 × HE score (HE grade: 0/1, 1-2/2 and 3-4/3) + 0.425 × ln (neutrophil) (109/L) + 0.396 × ln (TBil) (µmol/L) + 0.576 × ln (serum urea) (mmol/L) + 0.033 × age [ 13 ]. TACIA= 0.003 × TBil (µmol/L) + 0.036 × age + 0.009 × Cre (μmol/L) + 0.525 × INR – 0.003 × AFP (ng/mL) [ 19 ].
Statistical analyses
SPSS version 25.0 software (IBM Corp, Armonk, NY, USA) was used for statistical processing. Continuous data were expressed as the means ± SDs or medians with an appropriate interquartile range. Those variables were compared by using Student's t test or the nonparametric Mann‒Whitney U test. Percentages were used to present categorical data, which were compared by the chi-squared test or Fisher's exact test. Binary logistic regression with forward elimination was employed to demonstrate the independent predictors for the 90-day mortality rate of patients with type C HBV-ACLF and establish a new prognostic scoring system. The Spearman correlation test was conducted to examine the correlation between AFP and other biochemical parameters. The area under the receiver operating characteristic curve (ROC) was used for model discrimination and calibration. Survival analysis was performed using Kaplan–Meier analysis, and differentiation analysis was evaluated by the log-rank test. The Youden index was used to identify the optimal cut-off point. Statistical significance was considered when P ≤ 0.05.
Characteristics and outcomes of type C HBV-ACLF patients
There were 224 patients included in our study. The baseline characteristics of type C HBV-ACLF patients are shown in Table 1 . The AFP (64.22 (16.10-178.61) vs. 24.80 (10.50-76.45), p < 0.05) of the survival group was significantly higher than that of the death or transplanted group, whereas the APRI (8.00 (4.37-14.91) vs. 10.90 (4.72-25.08), p < 0.05) was significantly lower than that of the death or transplanted group. The age, INR, TBil, WBC, neutrophils, Na, Cre, and complications of the survival group were significantly lower than those of the death or transplanted group ( p < 0.05). Furthermore, there were no significant differences between the groups in terms of Alb, PLT, AST, ALT and HBV-DNA. During a 90-day follow-up, one hundred and seventeen patients (52.23%) were deceased or received a liver transplant, and the liver transplant-free survival rate was 47.77% (107/224).
Independent prognostic factors and development of a new predictive model
First, the clinical variables were included in the univariate logistic analysis. The results of the analysis showed that age, TBil, INR, AFP, WBC, Cre, Na, NLR, and APRI showed significant associations with 90-day survival ( p < 0.05). Then, the choice of variables for the multivariable analysis was based on the results of the univariable analysis, as shown in Table 2 . Age[OR1.05, 95% CI(1.00-1.09), p = 0.019], TBil[OR 1.01, 95% CI(1.00-1.01), p ≤0.001] , INR[OR 3.76, 95% CI (1.23-10.89), p =0.015], AFP[OR 1.00, 95% CI (0.99-1.00), p =0.026], WBC[OR 1.16, 95% CI (1.01-1.32), p =0.03], Na[OR 0.89, 95% CI(0.82-0.97), p =0.009]and APRI[OR 1.02, 95% CI (1.00-1.04), p =0.047] were independently associated with the prognosis of HBV-ACLF over 90 days. Then, a new prognostic model (we named it the A3Twin score) according to the β determination formula in the regression equation was established as the following mathematical formula: A3Twin=0.047×age+0.005×TBil+ 1.325×INR+0.147×WBC+0.019×APRI-0.111×Na-0.003×AFP-1.878.
Correlations of AFP with biochemical parameters in patients with type C HBV-ACLF
We evaluated the correlations between AFP and clinical parameters in patients with type C HBV-ACLF. The results showed that the AFP level was significantly positively correlated with PLT (r = 0.198, P = 0.003), Pre-Alb (r = 0.146, P = 0.035), and ALT (r = 0.280, P <0.001) and negatively correlated with MLR (r = -0.152, P = 0.023) and FIB-4 (r = -0.270, P <0.001). However, there were no significant correlations among APF and TBil, AST, and APRI. (Table 3 )
Performance of the new model
The ROC curves for AFP, the other models, and the new model are shown in Fig. 2 . We compared the efficiency of the A3Twin score with the other formulas in predicting short-term prognosis. The ROC analysis showed that A3Twin has good accuracy in predicting mortality [AUC= 0.851, 95% CI (0.801–0.901), p < 0.001], followed by the COSSH-ACLF II score [AUC = 0.836, 95% CI (0.784–0.888), p < 0.001], TACIA score [AUC = 0.792, 95% CI (0.731–0.837), p < 0.001], iMELD score [AUC =0.775, 95% CI (0.713–0.837), p < 0.001], MELD score [AUC =0.760, 95% CI (0.697–0.824), p < 0.001], and MELD-Na score [AUC =0.723, 95% CI (0.655–0.790), p < 0.001]. The results illustrated that the A3Twin score was superior to those models mentioned above. More details are displayed in Table 4 . In addition, the A3Twin score was positively associated with the MELD scores (r = 0.651, P <0.001) but more strongly associated with the iMEID, COSSH‐ACLF II scores, and TACIA (r = 0.763, 0.745, and 0.803, respectively; all P < 0.001; Fig. 3 ).

Efficacy of receiver operating characteristic curves (ROC) in predicting the outcome. (a)ROC curve of AFP. (b) ROC curve for the prognostic model. AFP: alpha-fetoprotein; MELD: a model for end-stage liver disease; MELD-Na: MELD-sodium; iMELD: integrated MELD; COSSH-ACLF II: Chinese Group on the Study of Severe Hepatitis B-ACLF II score

Correlations between the A3Twin score and other prognostic scores. MELDs: a model for end-stage liver disease score; iMELD: integrated MELD score; MELD-Na: MELD-sodium score; COSSH-ACLF II: Chinese Group on the Study of Severe Hepatitis B-ACLF II score
The applicability of the newly found A3Twin score for predicting a poor prognosis within 90 days in type C HBV-ACLF was shown. A cut-off point of the A3Twin score ≥ -9.07 was suggested to indicate a poor outcome with 78.8% sensitivity and 78.1% specificity. Thus, we further analysed patient survival according to their A3Twin scores (Fig. 4 ). The transplant-free survival rate at 90 days was 22.40% (28/125) versus 77.80% (79/99) ( P < 0.001) in the groups of patients with A3Twin scores ≥-9.07 and <-9.07.
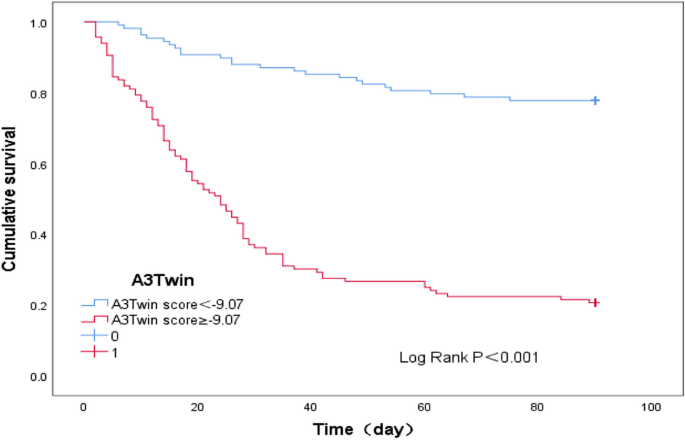
Kaplan‒Meier curve of A3Twin score<-9.07 and A3Twin score ≥-9.07
ACLF in patients with basic liver disease of different severities has different pathophysiologies, clinical manifestations, and prognoses [ 20 , 21 ]. Unlike chronic hepatitis, which is more reversible even if it deteriorates into ACLF after an insult, cirrhosis, especially decompensated cirrhosis, is more likely to progress to multiple organ failure, with an “end-stage” irreversible state [ 22 , 23 ]. In our study, patients with type C HBV-ACLF had a worse prognosis, and the mortality rate reached 52.23%. Consequently, the early identification of patients with type C HBV-ACLF who may not recover from conservative therapies is critical for clinical decisions.
The existing assessment models are based on organ failure and do not account for indicators related to liver regeneration. The secretion of AFP is minimal in an adult liver. Reactivation of AFP production in adults occurs during liver regeneration and hepatic carcinoma genesis [ 24 ]. Studies [ 19 , 25 ] have indicated that elevated AFP levels could predict a better prognosis for HBV-ACLF and acute liver failure with chronic HBV infection. However, a combination of cirrhosis of the liver, acute hepatic insult, and bacterial infection induces severe liver injury with high mortality, recapitulating some features of clinical type C ACLF [ 8 ]. Xiang et al. [ 26 ] found that chronic liver fibrosis and bacterial infection can suppress liver regeneration due to the shift from the activation of proregenerative IL-6/STAT3 to the antiregenerative IFN-γ/STAT1 pathway in animal experiments. Studies on liver regeneration in type C ACLF are still lacking; therefore, our study explored the prognostic value of liver regeneration in patients with type C HBV-ACLF. We found that the AFP level was significantly negatively correlated with FIB-4 and MLR. From clinical aspects, we demonstrated that acute hepatic injury-induced liver regeneration was markedly suppressed in fibrotic livers, which was also inhibited by inflammation. Whether in clinical studies or animal experiments, PLT has been demonstrated to be able to protect the liver and promote hepatic regeneration [ 27 , 28 ]. In the present study, we verified that AFP levels were significantly positively correlated with PLTs. We also found that AFP was significantly different between the survival group and the death or transplantation group, which can also be considered a prognostic indicator of type C HBV-ACLF.
Wang et al. [ 29 ] showed that liver cirrhosis at admission was an independent risk factor for both short-term and long-term outcomes in HBV-ACLF patients. The severity of liver fibrosis or the degree of portal hypertension plays an important role in the prognosis of type C ACLF. Liver biopsy has been considered the "gold standard" for the diagnosis and grading of liver fibrosis. Measurement of the hepatic venous pressure gradient (HVPG) is currently the best available method and is considered the gold standard for portal hypertension assessment. However, liver biopsy and HVPG measurement are invasive and are only routinely available and/or performed with adequate standards in expert centres. Verma et al. [ 30 ] verified that the APRI, which has been proposed as a good noninvasive estimator of hepatic fibrosis, correlates fairly well with the hepatic venous pressure gradient (HVPG) in patients with cirrhosis. Recent studies have detected that noninvasive assessment of liver fibrosis predicted long-term outcomes in patients with chronic HBV and predicted accurately regarding progression to ACLF in patients with AE and SAE [ 31 , 32 ]. However, the roles of APRI in HBV-ACLF are unknown. In the present study, we found that APRI and FIB-4 were significantly different between the survival group and the death or transplanted group, and logistic regression revealed that APRI was an independent risk factor for prognosis of type C HBV-ACLF.
The other five independent risk factors (TB, INR, age, WBC, Na) were selected, and we developed a simplified A3Twin score for patients with type C HBV-ACLF. Among these factors, TBil and INR were associated with liver and coagulation, respectively, and have been commonly used in previous scores. Livers of the elderly show increased nonparenchymal cell senescence, decreased liver regenerative capacity, altered metabolism functions, and immune response dysfunction, making these patients more susceptible to developing chronic liver diseases, and the prognosis of liver disease is worse [ 33 ]. We found that age is closely related to the prognosis of type C HBV-ACLF patients. Additionally, age was significantly associated with the severity of ACLF in both the iMELD, COSH-ACLF, and COSS-ACLF II groups. Systemic inflammation is a major driver of HBV-associated ACLF [ 34 ]. Infections are among the most frequent (>30% of patients) precipitating events of ACLF [ 35 ], and more than 50% of patients with ACLF develop an infection during their hospital stay, increasing their death rate from 34% to 71% [ 36 ]. As an inflammatory factor, WBC was also used in the CLIF-C ACLF score. Diluted hyponatremia is attributed to the impairment of the kidneys to eliminate solute-free water, resulting in a disproportionate accumulation of water with sodium [ 37 ]. Ruf et al. [ 38 ] found that hyponatraemia is an excellent predictor of outcome in patients with advanced cirrhosis and significantly increases the efficacy of MELD to predict waitlist mortality, establishing MELD-Na. Previous research has expounded that hyponatraemia is a poor prognostic predictor in those with acute-on-chronic liver disease (AoCLD) and ACLF [ 39 , 40 ]. Hyponatraemia was used in both iMELD and United Kingdom Model for end-stage liver disease (UKELD) scores. Thus, these five independent risk factors reflect type C HBV-ACLF pathophysiology.
Any prognostic score should use objective and accessible clinical indicators to simply and accurately predict the disease outcome for clinical application. The prognostic value for type C HBV-ACLF of our novel scoring system, which was backed by pathogenesis and included seven routine indices, was showed and had an advantage over MELD, iMELD, MELD-Na, COSSH-ACLF II, and TACIA. Patients who have lower A3Tiwn scores (<-9.07) might survive longer than those who have a higher A3Tiwn score (≥-9.07). The results indicate that patients with high A3Twin scores might have serious liver dysfunction, inflammation, and liver cirrhosis, and the capability of hepatic regeneration would be diminished.
Our study has several limitations. First, we retrospectively investigated type C HBV-ACLF patients in a single centre, and the sample size was not large enough to establish a validation cohort. Second, the accuracy of serum markers of liver fibrosis for the degree of liver cirrhosis was potentially affected by severe liver inflammation. The potential benefits of incorporating the APRI into the prognostic model should be further confirmed in another independent and larger study. Third, due to the retrospective study, some indicators could not be collected (such as arterial partial pressure of oxygen and lactic acid). Our new model cannot be compared with the other models (CLIF-C ACLF、 AARC、COSSH-ACLF). Finally, due to regional differences, the etiology of acute-on-chronic liver failure is different in the East and the West, and the definition of chronic liver disease is different. Whether the new model can predict patients with other causes of acute-on-chronic liver failure needs further verification. Type-C HBV-ACLF patients is associated with higher levels of inflammation, fibrosis, and poorer liver regeneration than type A/B HBV-ACLF [ 8 , 21 ]. Whether the new model which based on liver function, fibrosis, inflammation level, and liver regeneration can predict type A/B HBV-ACLF needs further verification. Large-scale, multicenter, prospective studies are needed to evaluate the usability of this novel prognostic model, especially in patients with other causes or other types of acute-on-chronic liver failure.
Alpha-fetoprotein and APRI can be used as prognostic indices of patients with type C HBV-ACLF. The novel model, which is comprised of seven routine indices, could effectively predict the prognosis of type C HBV-ACLF. A lower A3Twin score could indicate a better outcome. The results of our research might be helpful in the management of type C HBV-ACLF in clinics.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
Acute-on-chronic liver failure
World Gastroenterology Organization
Hepatitis B-related acute-on-chronic liver failure
Receiver operating characteristic
Hepatocellular carcinoma
Pre-albumin
Neutrophil to lymphocyte ratio
Monocyte to lymphocyte ratio
Hepatic encephalopathy
Hepatitis B viral
Hepatitis B virus deoxyribonucleic acid
Acute kidney injury
Total bilirubin
International normalized ratio
- Alpha-fetoprotein
White blood cell
serum sodium
Aspartate aminotransferase/platelet ratio index
Area under the curve
Asian Pacific Association for the Study of the Liver
Chronic liver disease
European Association for the Study of the Liver
Model for end-stage liver disease
MELD-sodium
Integrated MELD
Chinese Group on the Study of Severe Hepatitis B-ACLF
Upper limit of normal
Confidence interval
Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353–90. https://doi.org/10.1007/s12072-019-09946-3 .
Article PubMed Google Scholar
Mikolasevic I, Milic S, Radic M, et al. Clinical profile, natural history, and predictors of mortality in patients with acute-on-chronic liver failure (ACLF). Wien Klin Wochenschr. 2015;127:283–9. https://doi.org/10.1007/s00508-015-0707-9 .
Article CAS PubMed Google Scholar
Yan Y, Lyu C, Zhou X, et al. The predictive value of liver failure-related etiology for clinical outcomes. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022;34:172–7. https://doi.org/10.3760/cma.j.cn121430-20210705-01006 .
Moreau R, Gao B, Papp M, et al. Acute-on-chronic liver failure: A distinct clinical syndrome. J Hepatol. 2021;75(Suppl 1):S27–35. https://doi.org/10.1016/j.jhep.2020.11.047 .
Jalan R, Yurdaydin C, Bajaj JS, et al. Toward an Improved Definition of Acute-on-Chronic Liver Failure. Gastroenterology. 2014;147:4–10. https://doi.org/10.1053/j.gastro.2014.05.005 .
D’Amico G, Morabito A, D’Amico M, et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563–76. https://doi.org/10.1016/j.jhep.2017.10.020 .
Engelmann C, Clària J, Szabo G, et al. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75:S49–66. https://doi.org/10.1016/j.jhep.2021.01.002 .
Article CAS PubMed PubMed Central Google Scholar
Tang X, Qi T, Li B, et al. Tri-typing of hepatitis B-related acute-on-chronic liver failure defined by the World Gastroenterology Organization. J Gastroenterol Hepatol. 2021;36:208–16. https://doi.org/10.1111/jgh.15113 .
Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. https://doi.org/10.1053/jhep.2001.22172 .
Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–47. https://doi.org/10.1016/j.jhep.2014.06.012 .
Choudhury A, Jindal A, Maiwall R, et al. Liver failure determines the outcome in patients of acute-on-chronic liver failure (ACLF): comparison of APASL ACLF research consortium (AARC) and CLIF-SOFA models. Hepatol Int. 2017;11:461–71. https://doi.org/10.1007/s12072-017-9816-z .
Wu T, Li J, Shao L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181–91. https://doi.org/10.1136/gutjnl-2017-314641 .
Li J, Liang X, You S, et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75:1104–15. https://doi.org/10.1016/j.jhep.2021.05.026 .
Wu D, Sun Z, Liu X, et al. HINT: a novel prognostic model for patients with hepatitis B virus-related acute-on-chronic liver failure. Aliment Pharmacol Ther. 2018;48:750–60. https://doi.org/10.1111/apt.14927 .
Liver Failure and Artificial Liver Group CSoID, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure. Chin J Hepatol. 2019;27(1):18–26. https://doi.org/10.3760/cma.j.issn.1000-6680.2019.01.001 .
Article Google Scholar
Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797–805. https://doi.org/10.1002/hep.21563 .
Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–26. https://doi.org/10.1056/NEJMoa0801209 .
Luca A, Angermayr B, Bertolini G, et al. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13:1174–80. https://doi.org/10.1002/lt.21197 .
Wang X, Sun M, Yang X, et al. Value of Liver Regeneration in Predicting Short-Term Prognosis for Patients with Hepatitis B-Related Acute-on-Chronic Liver Failure. BioMed Res Int. 2020;2020:1–7. https://doi.org/10.1155/2020/5062873 .
Article CAS Google Scholar
Shi Y, Zheng MH, Yang Y, et al. Increased delayed mortality in patients with acute-on-chronic liver failure who have prior decompensation. J Gastroenterol Hepatol. 2015;30:712–8. https://doi.org/10.1111/jgh.12787 .
Mu X, Tong J, Xu X, et al. World Gastroenterology Organisation classification and a new type-based prognostic model for hepatitis B virus-related acute-on-chronic liver failure. Clin Res Hepatol Gastroenterol. 2021;45:101548. https://doi.org/10.1016/j.clinre.2020.09.009 .
Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care. 2011;17:165–9. https://doi.org/10.1097/MCC.0b013e328344b42d .
Zhao RH, Shi Y, Zhao H, et al. Acute-on-chronic liver failure in chronic hepatitis B: an update. Expert Rev Gastroenterol Hepatol. 2018;12:341–50. https://doi.org/10.1080/17474124.2018.1426459 .
Takikawa Y, Suzuki K. Is AFP a new reliable marker of liver regeneration in acute hepatic failure? J Gastroenterol. 2002;37:681–2. https://doi.org/10.1007/s005350200111 .
Yang SSCK, Lai Y. Decreasing serum alpha-fetoprotein levels in predicting poor prognosis of acute hepatic failure in patients with chronic hepatitis B. J Gastroenterol Hepatol. 2002;37(8):626–32. https://doi.org/10.1007/s005350200111 .
Xiang X, Feng D, Hwang S, et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J Hepatol. 2020;72:736–45. https://doi.org/10.1016/j.jhep.2019.11.013 .
Matsuo R, Nakano Y, Ohkohchi N. Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Ann Surg. 2011;253:759–63. https://doi.org/10.1097/SLA.0b013e318211caf8 .
Xu X, Hou Z, Xu Y, et al. The dynamic of platelet count as a novel and valuable predictor for 90-day survival of hepatitis B virus-related acute-on-chronic liver failure patients. Clin Res Hepatol Gastroenterol. 2021;45:101482. https://doi.org/10.1016/j.clinre.2020.06.008 .
Wang L, Xu W, Li X, et al. Long-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure: a retrospective study. BMC Gastroenterol. 2022;22:162. https://doi.org/10.1186/s12876-022-02239-4 .
Verma V, Sarin SK, Sharma P, et al. Correlation of aspartate aminotransferase/platelet ratio index with hepatic venous pressure gradient in cirrhosis. United European Gastroenterol J. 2014;2:226–31. https://doi.org/10.1177/2050640614527084 .
Yang F, Liu Y, Zeng B, et al. Noninvasive assessment of liver fibrosis for predicting acute-on-chronic liver failure in patients with chronic hepatitis B. Hepatol Int. 2021;15:593–601. https://doi.org/10.1007/s12072-020-10106-1 .
Wong GL, Chan HL, Yu Z, et al. Noninvasive assessments of liver fibrosis with transient elastography and Hui index predict survival in patients with chronic hepatitis B. J Gastroenterol Hepatol. 2015;30:582–90. https://doi.org/10.1111/jgh.12779 .
Allaire M, Gilgenkrantz H. The aged liver: Beyond cellular senescence. Clin Res Hepatol Gastroenterol. 2020;44:6–11. https://doi.org/10.1016/j.clinre.2019.07.011 .
Wu W, Yan H, Zhao H, et al. Characteristics of systemic inflammation in hepatitis B-precipitated ACLF: Differentiate it from No-ACLF. Liver Int. 2018;38:248–57. https://doi.org/10.1111/liv.13504 .
Moreau R. Role of Infections in Acute-on-Chronic Liver Failure. Dig Dis. 2015;33:577–81. https://doi.org/10.1159/000375356 .
Mucke MM, Rumyantseva T, Mucke VT, et al. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018;38:645–53. https://doi.org/10.1111/liv.13568 .
Alukal JJ, John S, Thuluvath PJ. Hyponatremia in Cirrhosis: An Update. Am J Gastroenterol. 2020;115:1775–85. https://doi.org/10.14309/ajg.0000000000000786 .
Ruf AE, Kremers WK, Chavez LL, et al. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–43. https://doi.org/10.1002/lt.20329 .
Pereira G, Baldin C, Piedade J, et al. Combination and sequential evaluation of acute-on-chronic liver failure (ACLF) and hyponatremia and prognosis in cirrhotic patients. Dig Liver Dis. 2020;52:91–7. https://doi.org/10.1016/j.dld.2019.08.013 .
Mei X, Li H, Deng G, et al. Prevalence and clinical significance of serum sodium variability in patients with acute-on-chronic liver diseases: a prospective multicenter study in China. Hepatol Int. 2022;16:183–94. https://doi.org/10.1007/s12072-021-10282-8 .
Download references
Acknowledgements
Not applicable.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Gastroenterology, The General Hospital of Western Theater Command, Chengdu, 610083, Sichuan, China
Chunyan Li, Chengzhi Bai, Huaqian Xu, Lin Liu & Shanhong Tang
Endoscopy Center and Endoscopy Research Institute, Shanghai Collaborative Innovation Center of Endoscopy, Zhongshan Hospital, Fudan University, Shanghai, 200032, China
You can also search for this author in PubMed Google Scholar
Contributions
Shanhong Tang designed the study and carried it out. Chunyan Li conducted the statistical analysis and drafted the manuscript. Lin Liu helped to collect data. Hao Hu , Chengzhi Bai and Huaqian Xu helped to finalize the manuscript. All the authors read and approved the manuscript.
Corresponding author
Correspondence to Shanhong Tang .
Ethics declarations
Ethics approval and consent to participate.
This study was conducted following the principles of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of the General Hospital of Western Theater Command (No: 2020ky031). All study participants provided oral informed consent.
Consent for publication
Competing interests.
The authors declare no competing interests.

Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Li, C., Hu, H., Bai, C. et al. Alpha-fetoprotein and APRI as predictive markers for patients with Type C hepatitis B-related acute-on-chronic liver failure: a retrospective study. BMC Gastroenterol 24 , 191 (2024). https://doi.org/10.1186/s12876-024-03276-x
Download citation
Received : 09 June 2023
Accepted : 23 May 2024
Published : 04 June 2024
DOI : https://doi.org/10.1186/s12876-024-03276-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Liver fibrosis
- Type C HBV-ACLF
- Prognostic score
BMC Gastroenterology
ISSN: 1471-230X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
HBeAg induces neutrophils activation impairing NK cells function in patients with chronic hepatitis B
- Original Article
- Published: 03 June 2024
Cite this article

- Zhiqian Feng 2 na1 ,
- Junliang Fu 1 na1 ,
- Lili Tang 1 ,
- Chunmei Bao 1 ,
- Honghong Liu 1 ,
- Kai Liu 1 ,
- Tao Yang 1 ,
- Jin-Hong Yuan 1 ,
- Chun-Bao Zhou 1 ,
- Chao Zhang 1 ,
- Ruonan Xu ORCID: orcid.org/0000-0002-4180-7754 1 , 2 &
- Fu-Sheng Wang 1 , 2
1 Altmetric
The role of neutrophils in hepatitis B virus (HBV) infection has been a subject of debate due to their involvement in antiviral responses and immune regulation. This study aimed to elucidate the neutrophil characteristics in patients with chronic hepatitis B (CHB).
Through flow cytometry and ribonucleic acid-sequencing analysis, the phenotypes and counts of neutrophils were analyzed in patients with CHB. Moreover, the effects of HBeAg on neutrophils and the corresponding pattern recognition receptors were identified. Simultaneously, the cross-talk between neutrophils and natural killer (NK) cells was investigated.
Neutrophils were activated in patients with CHB, characterized by higher expression levels of programmed death-ligand 1 (PD-L1), cluster of differentiation 86, and interleukin-8, and lower levels of CXC motif chemokine receptor (CXCR) 1 and CXCR2. Hepatitis B e antigen (HBeAg) partially induces neutrophil activation through the Toll-like receptor 2 (TLR2). A consistent upregulation of the TLR2 and HBeAg expression was observed in patients with CHB. Notably, the genes encoding molecules pivotal for NK-cell function upon NK receptor engagement enriched in neutrophils after HBeAg activation. The HBeAg-activated neutrophils demonstrated the ability to decrease the production of interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) in NK cells, while the PD-1 and PD-L1 pathways partially mediated the immunosuppression.
Conclusions
The immunosuppression of neutrophils induced by HBeAg suggests a novel pathogenic mechanism contributing to immune tolerance in patients with CHB.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price excludes VAT (USA) Tax calculation will be finalised during checkout.
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Differential expression of viral pathogen-associated molecular pattern receptors mRNA in Egyptian chronic hepatitis C virus patients

NKG2D modulates aggravation of liver inflammation by activating NK cells in HBV infection
Host–virus interactions in hepatitis b and hepatitis c infection, data availability.
The data that support the findings of the current study are available from the corresponding author upon reasonable request.
Abbreviations
Hepatitis B virus
Peg-interferon
Toll-like-receptors
Reactive oxygen species
Neutrophil extracellular traps
Interleukin
Hepatitis B e antigen.
Hepatitis B surface antigen
Natural killer
Tumor necrosis factor
Programmed cell death protein 1
Programmed Cell Death-Ligand 1
Healthy controls
- Chronic hepatitis B
HBeAg + chronic infection
HBeAg + chronic hepatitis
HBeAg- chronic infection
Peripheral blood mononuclear cells
RNA integrity number
Gene ontology
Kyoto encyclopedia of genes and genomes
Differentially expressed genes
RNA-sequencing
Pattern recognition receptors
NOD-like receptors
Hutin Y, Nasrullah M, Easterbrook P, Nguimfack BD, Burrone E, Averhoff F, et al. Access to treatment for hepatitis B virus infection—worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(28):773–777. https://doi.org/10.15585/mmwr.mm6728a2
Article PubMed PubMed Central Google Scholar
Spyrou E, Smith CI, Ghany MG. Hepatitis b: current status of therapy and future therapies. Gastroenterol Clin North Am. 2020;49(2):215–238. https://doi.org/10.1016/j.gtc.2020.01.003
Khanam A, Chua JV, Kottilil S. Immunopathology of chronic hepatitis b infection: role of innate and adaptive immune response in disease progression. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22115497
Yang G, Wan P, Zhang Y, Tan Q, Qudus MS, Yue Z, et al. Innate immunity, inflammation, and intervention in HBV infection. Viruses. 2022. https://doi.org/10.3390/v14102275
Maini MK, Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol. 2016;64(1 Suppl):S60–S70. https://doi.org/10.1016/j.jhep.2016.01.028
Article CAS PubMed Google Scholar
Liu K, Wang FS, Xu R. Neutrophils in liver diseases: Pathogenesis and therapeutic targets. Cell Mol Immunol. 2021;18(1):38–44. https://doi.org/10.1038/s41423-020-00560-0
Galani IE, Andreakos E. Neutrophils in viral infections: current concepts and caveats. J Leukoc Biol. 2015;98(4):557–564. https://doi.org/10.1189/jlb.4VMR1114-555R
Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102(7):2660–2669. https://doi.org/10.1182/blood-2003-04-1078
Zhou R, Liu L, Wang Y. Viral proteins recognized by different TLRs. J Med Virol. 2021;93(11):6116–6123. https://doi.org/10.1002/jmv.27265
Hoar DI, Bowen T, Matheson D, Poon MC. Hepatitis B virus DNA is enriched in polymorphonuclear leukocytes. Blood. 1985;66(6):1251–1253
Leu CM, Lu YC, Peng WL, Chu HT, Hu CP. The hepatitis B virus e antigen suppresses the respiratory burst and mobility of human monocytes and neutrophils. Immunobiology. 2014;219(11):880–887. https://doi.org/10.1016/j.imbio.2014.07.008
Hu S, Liu X, Gao Y, Zhou R, Wei M, Dong J, et al. Hepatitis b virus inhibits neutrophil extracellular trap release by modulating reactive oxygen species production and autophagy. J Immunol. 2019;202(3):805–815. https://doi.org/10.4049/jimmunol.1800871
Mitra B, Wang J, Kim ES, Mao R, Dong M, Liu Y, et al. Hepatitis b virus precore protein p22 inhibits alpha interferon signaling by blocking STAT nuclear translocation. J Virol. 2019. https://doi.org/10.1128/JVI.00196-19
Block TM, Guo H, Guo JT. Molecular virology of hepatitis B virus for clinicians. Clin Liver Dis. 2007;11(4):685–706. https://doi.org/10.1016/j.cld.2007.08.002
Park JJ, Wong DK, Wahed AS, Lee WM, Feld JJ, Terrault N, et al. Hepatitis b virus-specific and global T-Cell dysfunction in chronic hepatitis b. Gastroenterology. 2016;150(3):684-695.e5. https://doi.org/10.1053/j.gastro.2015.11.050
Yang F, Yu X, Zhou C, Mao R, Zhu M, Zhu H, et al. Hepatitis B e antigen induces the expansion of monocytic myeloid-derived suppressor cells to dampen T-cell function in chronic hepatitis B virus infection. Plos Pathog. 2019;15(4):e1007690. https://doi.org/10.1371/journal.ppat.1007690
Article CAS PubMed PubMed Central Google Scholar
de Groen RA, Hou J, van Oord GW, Groothuismink Z, van der Heide M, de Knegt RJ, et al. NK cell phenotypic and functional shifts coincide with specific clinical phases in the natural history of chronic HBV infection. Antiviral Res. 2017;140:18–24. https://doi.org/10.1016/j.antiviral.2017.01.007
Kayesh M, Kohara M, Tsukiyama-Kohara K. Toll-Like receptor response to hepatitis b virus infection and potential of TLR agonists as immunomodulators for treating chronic hepatitis b: an overview. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms221910462
Lang T, Lo C, Skinner N, Locarnini S, Visvanathan K, Mansell A. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll-like receptor signaling pathway. J Hepatol. 2011;55(4):762–769. https://doi.org/10.1016/j.jhep.2010.12.042
Xie X, Lv H, Liu C, Su X, Yu Z, Song S, et al. HBeAg mediates inflammatory functions of macrophages by TLR2 contributing to hepatic fibrosis. Bmc Med. 2021;19(1):247. https://doi.org/10.1186/s12916-021-02085-3
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int. 2016;10(1):1–98. https://doi.org/10.1007/s12072-015-9675-4
Liu K, Huang HH, Yang T, Jiao YM, Zhang C, Song JW, et al. Increased neutrophil aging contributes to t cell immune suppression by PD-L1 and arginase-1 in HIV-1 treatment naïve patients. Front Immunol. 2021;12:670616. https://doi.org/10.3389/fimmu.2021.670616
Zhang LX, Jiao YM, Zhang C, Song JW, Fan X, Xu RN, et al. HIV reservoir decay and CD4 recovery associated with high CD8 counts in immune restored patients on long-term ART. Front Immunol. 2020;11:1541. https://doi.org/10.3389/fimmu.2020.01541
Zhao R, Wang TZ, Kong D, Zhang L, Meng HX, Jiang Y, et al. Hepatoma cell line HepG2.2.15 demonstrates distinct biological features compared with parental HepG2. World J Gastroenterol. 2011;17(9):1152–9. https://doi.org/10.3748/wjg.v17.i9.1152
Zhang J, Zheng H, Diao Y. Natural killer cells and current applications of chimeric antigen Receptor-Modified NK-92 cells in tumor immunotherapy. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20020317
Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. https://doi.org/10.1111/j.1600-065X.2006.00457.x
Alfarra H, Weir J, Grieve S, Reiman T. Targeting NK cell inhibitory receptors for precision multiple myeloma immunotherapy. Front Immunol. 2020;11:575609. https://doi.org/10.3389/fimmu.2020.575609
Gehring AJ, Protzer U. Targeting innate and adaptive immune responses to cure chronic HBV infection. Gastroenterology. 2019;156(2):325–337. https://doi.org/10.1053/j.gastro.2018.10.032
George ST, Lai J, Ma J, Stacey HD, Miller MS, Mullarkey CE. Neutrophils and influenza: a thin line between helpful and harmful. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060597
Article PubMed Google Scholar
Naumenko V, Turk M, Jenne CN, Kim SJ. Neutrophils in viral infection. Cell Tissue Res. 2018;371(3):505–516. https://doi.org/10.1007/s00441-017-2763-0
Xu Y, Zhang Q, Zhao Y. The functional diversity of neutrophils and clustered polarization of immunity. Cell Mol Immunol. 2020;17(11):1212–1214. https://doi.org/10.1038/s41423-020-0378-y
Goh JG, Ravikumar S, Win MS, Cao Q, Tan AL, Lim J, et al. Neutrophils differentially attenuate immune response to Aspergillus infection through complement receptor 3 and induction of myeloperoxidase. Cell Microbiol. 2018. https://doi.org/10.1111/cmi.12798
Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122(1):327–336. https://doi.org/10.1172/JCI57990
Xu R, Lin F, Bao C, Huang H, Ji C, Wang S, et al. Complement 5a receptor-mediated neutrophil dysfunction is associated with a poor outcome in sepsis. Cell Mol Immunol. 2016;13(1):103–109. https://doi.org/10.1038/cmi.2014.136
Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003;38(5):1075–1086. https://doi.org/10.1053/jhep.2003.50453
Tsai KN, Ou JJ. Hepatitis B virus e antigen and viral persistence. Curr Opin Virol. 2021;51:158–163. https://doi.org/10.1016/j.coviro.2021.10.003
Milich DR, Jones JE, Hughes JL, Price J, Raney AK, Mclachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A. 1990;87(17):6599–6603. https://doi.org/10.1073/pnas.87.17.6599
Tsai SL, Chen PJ, Lai MY, Yang PM, Sung JL, Huang JH, et al. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens Implications for hepatitis B e antigen seroconversion. J Clin Invest. 1992;89(1):87–96. https://doi.org/10.1172/JCI115590
Zhu SS, Dong Y, Xu ZQ, Wang LM, Chen DW, Gan Y, et al. a retrospective study on HBsAg clearance rate after antiviral therapy in children with HBeAg-positive chronic hepatitis B aged 1–7 years. Zhonghua Gan Zang Bing Za Zhi. 2016;24(10):738–743. https://doi.org/10.3760/cma.j.issn.1007-3418.2016.10.005
Yang Y, Han Q, Zhang C, Xiao M, Zhang J. Hepatitis B virus antigens impair NK cell function. Int Immunopharmacol. 2016;38:291–297. https://doi.org/10.1016/j.intimp.2016.06.015
Download references
This study is supported by National Natural Science Foundation of China (No. 82171732), (No. 82272311), (81721002), National Key Research and Development Program of China (2023YFC2306804) and Natural Science Foundation of Shandong Province (ZR2022QH329). Figure 6 was created by BioRender software.
Author information
Zhiqian Feng and Junliang Fu contribute equally to this work.
Authors and Affiliations
Senior Department of Infectious Diseases, The Fifth Medical Center of PLA General Hospital, National Clinical Research Center for Infectious Diseases, Beijing, China
Junliang Fu, Lili Tang, Chunmei Bao, Honghong Liu, Kai Liu, Tao Yang, Jin-Hong Yuan, Chun-Bao Zhou, Chao Zhang, Ruonan Xu & Fu-Sheng Wang
The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
Zhiqian Feng, Ruonan Xu & Fu-Sheng Wang
You can also search for this author in PubMed Google Scholar
Contributions
Fu-Sheng Wang and Ruonan Xu conceived the study, wrote the manuscript, and constructed the figures with Zhiqian Feng and Junliang Fu. Junliang Fu, Chunmei Bao and Honghong Liu recruited participants and provided samples. jin-Hong Yuan and Chun-Bao Zhou performed flow cytometry. Lili Tang, Kai Liu and Chao Zhang contributed to scientific planning. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Ruonan Xu or Fu-Sheng Wang .
Ethics declarations
Conflict of interest.
The authors Zhiqian Feng, Junliang Fu, Lili Tang, Chunmei Bao, Honghong Liu, Kai Liu, Tao Yang, Jin-Hong Yuan, Chun-Bao Zhou, Chao Zhang, Ruonan Xu, Fu-Sheng Wang declare no competing interests.
Ethical approval
The study was approved by the Ethics Committee of the Fifth Medical Center of Chinese PLA General Hospital (ethics number: 2020053D). All participants signed informed consent for the study.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 724 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Feng, Z., Fu, J., Tang, L. et al. HBeAg induces neutrophils activation impairing NK cells function in patients with chronic hepatitis B. Hepatol Int (2024). https://doi.org/10.1007/s12072-024-10689-z
Download citation
Received : 04 February 2024
Accepted : 21 April 2024
Published : 03 June 2024
DOI : https://doi.org/10.1007/s12072-024-10689-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Neutrophils
- Natural killer cells
- Immunosuppression
- Innate immunity
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 24 May 2023
Efficacy and safety of PD-1/PD-L1 inhibitors combined with anti-angiogenic therapy for the unresectable hepatocellular carcinoma and the benefit for hepatitis B virus etiology subgroup: a systematic review and meta-analysis of randomized controlled trials
- Danxue Huang 1 ,
- Liyuan Ke 1 ,
- Hongxia Cui 1 &
BMC Cancer volume 23 , Article number: 474 ( 2023 ) Cite this article
1913 Accesses
2 Citations
1 Altmetric
Metrics details
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death, worldwide. The predominant causative factor for HCC is hepatitis B virus (HBV) infection. We conducted a meta-analysis to estimate the efficacy and safety of PD-1/PD-L1 inhibitors combined with anti-angiogenic therapy for the first-line treatment of the unresectable HCC and to evaluate the benefits of different geographic regions and etiology stratifications.
Randomized clinical trials published up to 12th November 2022 were searched by online databases. Moreover, effects of hazard ratio (HR) for overall survival (OS) and progression-free survival (PFS) were extracted from included studies. Pooled odds ratio (OR) and 95% CI for objective response rate (ORR), disease control rate (DCR), and treatment-related adverse events (TRAEs) were calculated.
A total of 3057 patients from five phase III randomized clinical trials were collected and reviewed for this meta-analysis. The pooled HR of OS (HR = 0.71; 95% CI: 0.60–0.85) and PFS (HR = 0.64; 95% CI: 0.53–0.77) demonstrated significantly better benefit in PD-1/PD-L1 inhibitors combination group than targeted monotherapy to treat unresectable HCC. In addition, combination therapy showed better ORR and DCR, with ORs of 3.29 (95% CI: 1.92–5.62) and 1.88 (95% CI: 1.35–2.61), respectively. The subgroup analysis indicated that PD-1/PD-L1 inhibitors combination therapy was significantly superior to anti-angiogenic monotherapy for HBV-related HCC in terms of OS (HR = 0.64; 95% CI: 0.55–0.74) and PFS (HR = 0.53; 95% CI:0.47–0.59), while there was no significant difference in patients with HCV (OS, HR = 0.81, p = 0.1) or non-viral (OS, HR = 0.91, p = 0.37; PFS, HR = 0.77, p = 0.05).
Conclusions
Meta-analysis revealed for the first-time that PD-1/PD-L1 inhibitors combination therapy for unresectable HCC was associated with better clinical outcomes than anti-angiogenic monotherapy, especially for HBV infection and Asian population.
Peer Review reports
Introduction
Hepatocellular carcinoma (HCC) is the major type of primary liver cancer and the third leading cause of cancer-related death worldwide [ 1 ]. Although early-stage tumor can be curable by surgical resection, ablation, or liver transplantation [ 2 ], the vast majority of patients had advanced unresectable disease at time of initial diagnosis with a relatively poor prognosis owing to the absence of early clinical symptoms and effective screening methods. Hepatitis B virus (HBV) is the leading cause of incident cases of HCC and deaths worldwide (33%), followed by alcohol (30%), hepatitis C virus (HCV) (21%) and other reasons (16%) [ 3 , 4 ].
The previous standard first-line systemic treatments for HCC were only lenvatinib and sorafenib [ 5 , 6 , 7 , 8 , 9 , 10 ]. However, targeted agents only conferred limited survival benefits [ 11 , 12 , 13 ]. In addition, the efficacy of sorafenib in patients with HBV-related HCC was revealed to be inferior to that in patients without HBV infection [ 11 , 13 ]. Recently, immunotherapy is changing treatment strategies for many malignant tumors, and increasing evidence suggests that patients with HCC may benefit from these new therapies [ 14 , 15 ]. Single-agent immune checkpoint inhibitors (ICIs) represented by programmed cell death 1(PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors have been recently evaluated in HCC patients, and the results of clinical trials were disappointed [ 16 , 17 , 18 ]. A combination of ICIs and vascular endothelial growth factor (VEGF) inhibitors might promote an immune permissive environment and enhance ICI response [ 19 , 20 ]. Therefore, immune-based combinations have been more striking [ 21 ]. In 2020, the IMbrave-150 trial demonstrated for the first-time that atezolizumab combined with bevacizumab is superior to sorafenib in the treatment of unresectable HCC and obtained clinically meaningful improvement in overall survival (OS) and progression-free survival (PFS), leading to its global approval [ 22 ]. Similar result has also been found in ORIENT-32 trial [ 23 ]. COSMIC-312 study reported that atezolizumab plus cabozantinib achieved a lack of improvement in OS compared to sorafenib [ 24 ]. In 2022, European society of medical oncology (ESMO) congress which updated the latest progress of first-line treatment regimens for HCC published primary results from Leap-002 and SHR-1210-III-310 studies [ 25 , 26 ]. Leap-002 as a multicenter phase III study did not meet pre-specified statistical significance for primary endpoints of OS and PFS between lenvatinib plus pembrolizumab and lenvatinib in advanced HCC. Correspondingly, SHR-1210-III-310 study showed positive findings, that the combination of camrelizumab and apatinib in patients with advanced liver cancer demonstrated significant clinical benefits in terms of OS and PFS at the common primary endpoint.
Although some studies have displayed that combination therapy achieved better survival benefits than alone [ 27 , 28 ]. Leap-002 and SHR-1210-III-310 study, global multicenter phase III clinical trials reported in ESMO 2022 have not been included in the previous research. Considering a large number of immunotherapy studies and new combination therapies for HCC exhibiting different clinical outcomes, we conducted this systematic review and meta-analysis aimed to overcome the limitations of individual research to better estimate the efficacy and safety of ICIs-combined anti-angiogenic therapy in treatment of unresectable HCC. Simultaneously, whether subgroups provided better OS and PFS outcomes were also explored to screen advantageous populations and determine the best therapy regimen.
Materials and methods
This systematic review and meta-analysis were in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). This study was registered with the International Prospective Register of Systematic Reviews (PROSPERO CRD42023400568).
Data source and search strategy
The databases, including Cochrane Library, PubMed, Embase and Web of Science were searched for eligible studies. The search time was from inception to November 2022. The main search therapy-related retrieval fields included (anti-angiogenic OR molecular targeted therapy OR targeted therapy) AND (PD-1 inhibitors OR programmed death ligand 1 OR PD-L1 inhibitors OR programmed death 1 receptor OR immunotherapy OR immune checkpoint inhibitors). The disease-related retrieval fields included hepatocellular carcinoma OR liver cell carcinoma OR Liver cancer. In addition, the reference lists of all relevant articles as well as conference abstracts published in main international oncological meetings (such as American Society of Clinical Oncology (ASCO), ASCO gastrointestinal cancer Symposium (ASCO-GI) and ESMO) were also searched to identify additional relevant studies.
Study selection
Potential trials, with the exception of reviews (including meta-analysis), editorials, fundamental studies, animal studies, comments and case reports, were eligible to be included in this meta-analysis if all of the following criteria apply: (1) prospective phase III randomized controlled trials (RCTs); (2) diagnosis of unresectable HCC; (3) comparison with PD-1/PD-L1 inhibitors plus anti-angiogenic drugs and anti-angiogenic therapy alone; (4) clinical outcomes of the study including OS, PFS, objective response rate (ORR), disease control rate (DCR) and treatment-related adverse events (TRAEs); (5) English as study language.
Data extraction and quality assessment
The following contents were extracted for each eligible study: (1) study general information (study name, first author, publication year, trial phase, study design, sample size); (2) basic information about the patients (age, male, etiology, geographical region); (3) interventions and control group. The main outcomes are PFS, OS, ORR, DCR and TRAEs. Both Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) and hepatocellular carcinoma-specific modified RECIST (mRECIST) criteria were used in the study. The risk of bias to verify methodological quality was evaluated based on the Cochrane Collaboration’s tool for randomized control trials by the Review Manager 5.4 (RevMan5.4).
Statistical analysis
The statistical analysis was conducted using Stata 14.0 and RevMan5.4. The pooled hazard ratios (HRs) and 95% confidence interval (CI) for OS and PFS were calculated, as well as the pooled odds ratios (ORs) and 95% CI for ORR, DCR, any grade TRAEs and grade 3–5 TRAEs. Heterogeneity among studies was quantified by the I 2 test, and I 2 > 50% and p < 0.05 was considered statistically significant heterogeneity [ 29 ]. When heterogeneity was significant, a random-effects model was used to calculate the pooled HR and OR; otherwise, the fixed-effects model was adopted.
Through subgroup analysis, publication bias assessment and sensitivity analysis, the origin of the heterogeneity was further explored. Begg’s test and Egger’s test were conducted to evaluate publication bias. The publication bias was absent with p > 0.05 in Begg’s test and Egger’s test [ 30 ]. We performed a sensitivity analysis by removing each study to observe changes in pooled HR. Region (Asia vs non-Asia), macrovascular invasion (MVI) or extra hepatic spread (EHS) (presence vs absence), alphafetoprotein (AFP) level (< 400 vs ≥ 400 ng per milliliter), etiology of HCC (HBV vs HCV vs Non-viral) and Barcelona Clinic Liver Cancer (BCLC) stage (B vs C) were considered in subgroup analysis.
Study selection and characteristics
A total of 2996 potential relevant reports were collected through two authors’ independent evaluation. After excluding duplicate and irrelevant studies, the initial search identified 1190 articles and abstracts. Finally, five studies were included among the 203 eligible full-text articles and conference abstracts (Fig. 1 ) [ 22 , 23 , 24 , 25 , 26 ].
Flow diagram of the screening and selection process
The studies’ general information, baseline characteristics of patients and therapeutic regimen were recorded in Table 1 . All studies were prospective phase III RCTs, with two reported in conference abstracts and three in articles. The studies were published between 2020 and 2022. There were up to 3057 patients available for the meta-analysis with a mean age around 61 years old.
Risk of bias
Four studies were judged as having high risk for blinding participants and personnel blinding bias. One study was rated as unclear risk for the blinding of outcome assessment. The others were rated as low risk (Fig. S 1 ).
Meta-analysis of OS and PFS
For all five trials, the pooled effects of HR for OS and PFS were available. The results revealed that combination therapy with PD-1/PD-L1 inhibitors and anti-angiogenic drugs had significantly better pooled OS than anti-angiogenic monotherapy (HR = 0.71; 95% CI: 0.60–0.85, p < 0.0001) (Fig. 2 ). Compared with anti-angiogenic monotherapy, combination therapy resulted in a significant improvement in PFS (HR = 0.64; 95% CI: 0.53–0.77, p < 0.00001) (Fig. 2 ). In addition, OS and PFS results showed a high degree of heterogeneities among the included studies ( I 2 = 62% and 73%, respectively). We performed subgroup analyses to determine the origin of heterogeneities among different studies.
Forest plots of OS ( A ) and PFS ( B ) of combination therapy with ICIs and anti-angiogenic drugs
Meta-analysis of ORR and DCR
Four studies used both RECIST 1.1 and mRECIST methodologies to assess ORR, and one study used RECIST 1.1 alone. Interestingly, the combination therapy group generated respectable ORRs ((OR = 3.29; 95%CI: 1.92–5.62, p < 0.0001) and (OR = 2.88; 95%CI: 1.48–5.63, p = 0.002)) according to RECIST 1.1 and mRECIST, respectively (Fig. 3 ). The DCR was also assessed similarly to ORR. The pooled analysis revealed higher DCRs ((OR = 1.88; 95%CI: 1.35–2.61, p = 0.0002) and (OR = 1.79; 95%CI: 1.19–2.70, p = 0.005)) according to RECIST 1.1 and mRECIST in the combination therapy compared with anti-angiogenic monotherapy (Fig. 3 ).
Forest plots of ORR by RECIST 1.1 ( A ), ORR by mRECIST ( B ), DCR by RECIST 1.1 ( C ) and DCR by mRECIST ( D )
Subgroup analysis for OS
Subgroup analyses were performed for OS based on stratification factors (geographical region, presence of MVI or EHS, AFP level, etiology and BCLC Stage). The results were exhibited in Table 2 and Fig. S 2 . In MVI or EHS, AFP level and BCLC Stage subgroups, significant benefits of OS were observed in patients with combination treatment, while no significant reduction of heterogeneity. In terms of the HBV subgroup, the combination therapy displayed more benefits of OS (HR = 0.64; 95% CI: 0.55–0.74, p < 0.00001), however, there was no statistically significant difference between the combination treatment group and anti-angiogenic group in HCV (HR = 0.81; 95% CI: 0.64–1.04, p = 0.1) and non-viral subgroups (HR = 0.91; 95% CI: 0.75–1.11, p = 0.37). Meanwhile, the heterogeneities of the region subgroup and etiology subgroup were significantly reduced through subgroup analysis. A better OS benefit was demonstrated in Asian population and hepatitis B-positive population.
Subgroup analysis for PFS
The results of the subgroup analysis for PFS were depicted in Table 3 and Fig. S 3 . The geographical region, presence of MVI or EHS, AFP level, etiology and BCLC Stage as subgroups revealed that patients treated with combination therapy showed better PFS than anti-angiogenic therapy. Only the non-viral subgroup showed no significant difference between the combination treatment group and the anti-angiogenic group (HR = 0.77, 95% CI: 0.59–1.0, p = 0.05). The heterogeneity of each subgroup was drastically reduced through subgroup analysis, especially the geographical region subgroup and etiology subgroup ( I 2 = 0%). This also suggested that hepatitis B-positive people have significantly more PFS benefit.
Meta-analysis of TRAEs
All included trials recorded the incidences of any grade and grade 3–5 TRAEs. Combination therapy was associated with significantly higher incidences compared with anti-angiogenic monotherapy for both any grade TRAEs (OR = 2.66; 95% CI: 1.80–3.93, p < 0.00001) and grade 3–5 TRAEs (OR = 1.80; 95% CI: 1.15–2.83, p = 0.01) (Fig. 4 ).

Forest plots of any grade ( A ) and grade 3–5 TRAEs ( B )
Sensitivity analysis and publication bias
Microvariation was observed in sensitivity analysis for the pooled effects by removing each trial in turn (Fig. S 4 ). Publication biases were absent by Begg’s test (OS, p = 0.462; PFS, p = 0.462) and Egger’s test (OS, p = 0.315; PFS, p = 0.318).
Unresectable HCC accounts for approximately 75–85% of primary liver cancers, and treatment options are limited due to poor prognosis [ 31 ]. Finding appropriate treatment is necessary to improve patient survival [ 32 ]. Combination immunotherapy had a higher chance of being the most effective therapy than targeted monotherapy [ 22 ]. Recently, several ICI combination strategies for unresectable HCC have reported the encouraging results [ 22 , 23 , 26 ], but other results have been disappointing [ 24 , 25 ]. Therefore, screening advantageous populations and determining the best combination therapy regimen has become a major challenge for HCC immunotherapy. We sought to find biomarkers or specific populations associated with immunoefficacy to stratify patients with HCC, distinguish between responders and non-responders, and recommend alternative therapies for patients who are not expected to respond to immunotherapy to avoid unnecessary toxicity. We conducted a meta-analysis which included five randomized controlled phase III trials of first-line therapies for unresectable HCC to show significantly better OS, PFS, ORR and DCR outcomes with PD-1/PD-L1 inhibitors in combination with anti-angiogenic drugs compared with anti-angiogenic drugs alone. Moreover, heterogeneities were revealed among the included studies for both OS and PFS. Subgroup analyses were performed to assess differences in outcomes and screen out the dominant population.
Theoretically, PD-L1 expression is the most direct marker for predicting the efficacy of PD-1/PD-L1 inhibitors, but unlike other malignancies, HCC is often accompanied by hepatitis or cirrhosis, which makes the tumor microenvironment of HCC more complex. The more complex classification of PD-L1 in HCC tissues and higher levels of spatial and cellular heterogeneity may affect the reliability and reproducibility of PD-L1 as a predictor of ICIs efficacy [ 33 ]. In the CheckMate 040 study [ 34 ], the ORR of PD-L1-positive patients was 26% in PD-L1-positive patients and 19% in negative patients, suggesting that negative expression of PD-L1 on tumor cells had no significant difference in the ORR against PD-1 therapy compared with PD-L1-positive patients. A phase II clinical trial of pembrolizumab in patients with unresectable advanced HCC suggested that there was no significant correlation between PD-L1 positivity and treatment response [ 35 ]. However, Zhou et al. reported a meta-analysis study showing that positive PD-L1 expression is better associated with ORR in patients with advanced liver cancer treated with anti-PD-1/PD-L1 [ 36 ]. Therefore, the expression of PD-L1 is currently controversial in predicting the efficacy of HCC. All included clinical trials in this meta-analysis lacked clinical outcomes in PD-L1-positive people. Therefore, PD-L1 expression was not included in the subgroup analysis in this study. Subgroup analyses were conducted according to the baseline characteristics of patients (geographical region, presence of MVI or EHS, AFP level, etiology and BCLC Stage).
In subgroup analysis, COSMIC-312 [ 24 ], IMbrave150 [ 22 ], SHR-1210-III-310 [ 26 ] and Leap-002 [ 25 ] were stratified for OS according to etiology (HBV, HCV and non-viral). The HRs of OS and PFS were stratified based on the factors of HBV status (positive vs negative) in the ORIENT-32 study [ 23 ]. Therefore, ORIENT-32 study was only included in the HBV subgroup. Subgroup analysis for PFS included COSMIC-312, IMbrave150 and SHR-1210-III-310. Leap-002 was not included due to the lack of data for each interested subgroup for PFS. It is found that combination therapy has no significant impact on the reduction of the risk of death compared with anti-angiogenic drugs in HCV patients. In the non-virus subgroup, there did not appear to be a difference between the combination therapy and anti-angiogenic treatment for OS and PFS. When considering only HBV-infected patients, combination therapies of all studies were confirmed to substantially reduce the risk of death compared to monotherapy, and the heterogeneity decreased substantially ( I 2 = 0%). Additionally, most patients in ORIENT-32 (94%) and SHR-1210-III-310 (76.8%) studies had HBV-related HCC, compared with less than 50% of participants in the Leap-002, IMbrave 150 and COSMIC-312 studies. This may be the reason for the better clinical outcomes of the ORIENT-32 and SHR-1210-III-310 studies. Basic studies have discovered that chronic HBV infection results in virus-specific T cell exhaustion and the PD-1/PD-L1 axis is a crucial inhibitor of HBV-specific CD8 + T cell activity [ 37 ]. Therefore, PD-1/PD-L1 inhibitors blocking could partially restore effective HBV-specific T-cell responses to viral proteins, which could theoretically affect the efficacy of ICIs [ 38 , 39 ]. By contrast, non-viral HCC as a heterogeneous population that includes hepatic steatosis might be less responsive to immunotherapy compared with other etiologies of HCC [ 40 ]. HCV patients have wide geographical variations that exhibit different regional characteristics, such as metabolic syndrome and alcohol consumption, as well as anti-cancer treatments which might influence survival through both hepatic and extra hepatic effects or through follow-up therapy [ 41 , 42 ]. Unlike previous meta-analyses that have not highlighted the characteristics of population, the present meta-analysis exclusively focused on differences in efficacy in subgroups of HBV, HCV and non-viral patients. Observations were extended to new combinations of therapeutic that were not covered in previous work.
COSMIC-312 [ 24 ], IMbrave150 [ 22 ], SHR-1210-III-310 [ 26 ] and Leap-002 [ 25 ] were included in subgroup analysis based on geographical region for the primary endpoints (OS). ORIENT-32 [ 23 ] study which was done for the Chinese population was only included in the Asian subgroup. Because of the lack of information for each subgroup of interest for PFS, Leap-002 was not included. The findings showed that combination therapy was significantly superior to monotherapy for OS in Asia, whereas there was no advantage benefit in patients with HCC of non-Asian population. For PFS, that combination therapy was significantly superior to monotherapy in both Asian and non-Asian population, however, pooled HR value was lower in Asia. In Africa and East Asia, the largest proportion of the population is attributable to be cause by HBV (60%); however, only 20% of cases in the Western world can be attributed to HBV infection, and chronic HCV is the most common potential liver disease etiology [ 4 , 43 ]. Therefore, the perfect clinical outcomes of OS and PFS in the Asian population were also attributed to HBV infection being the dominant immunotherapy population. ORIENT-32 and SHR-1210-III-310 studies which had more patients in Asia (100% and 83.0%) differed from those in IMbrave150 (40%) study, COSMIC-312 (28%) study and Leap-002 (30.6%) study. This may induce the ORIENT-32 and SHR-1210-III-310 studies to have a lower HR value with positive outcomes.
Combination immunotherapy producing better clinical outcomes in patients with HBV-positive patients and Asian patients was discussed. However, there are still some patients who do not benefit from immunotherapy. Exosomes are closely related to viral hepatitis, cirrhosis and HCC. As an important intercellular communication mediator in the tumor immune microenvironment, exosomes may play a unique role in the immune response of HCC, thereby affecting the efficiency of immunotherapy. Exosomes exhibit the dual characteristics of tumor promotion and inhibition. On the one hand, they can mediate immunotherapy resistance by affecting the PD-1/PD-L1 axis or the anti-tumor function of immune cells in the tumor microenvironment. On the other hand, exosomes can carry drugs to downregulate PD-L1 expression on the surface of immune cells to improve the efficacy of ICI [ 44 ]. Unfortunately, however, there were no RCTs on exosome treatment under our search strategy. The relationship between exosome therapy and liver cancer (including HBV-related HCC) will be further explored in the future.
The mRECIST measured only the viable tumor, which is defined as the contrast-enhanced portion of the tumor on hepatic arterial phase images. However, RECIST 1.1 measured the whole lesion, which is not enough to evaluate therapy induced intratumoural necrosis [ 45 , 46 ]. ESMO guidelines indicated the application of mRECIST or RECIST 1.1 in patients with HCC treated with anti-angiogenic targeted therapies [ 47 ]. However, National Comprehensive Cancer Network (NCCN) guidelines suggested that mRECIST and RECIST 1.1 are needed to assess tumor response of molecular targeted drugs [ 48 ]. The efficacies (ORR and DCR) of combination therapy compared with anti-angiogenic monotherapy for HCC were assessed according to both RECIST 1.1 and mRECIST in this meta-analysis.
The analysis results of TRAEs showed that compared with anti-angiogenic therapy, the combination therapy appears to have a significantly higher incidence of TRAEs. For these five trials, the most common TRAEs from combination therapies were hypertension, increased alanine aminotransferase, increased aspartate aminotransferase, proteinuria, diarrhea, fatigue, etc. Most of the TRAEs were concentrated in grade 1–2 indicating that the adverse events could be manageable. Grade 3–5 TRAEs occurred more frequently with camrelizumab plus apatinib in the SHR-1210-III-310 study (OR = 3.98; 95% CI: 2.70–5.86). The most common serious TRAEs were hypertension, increased alanine aminotransferase and increased aspartate aminotransferase.
There may be some possible limitations in this meta-analysis. Firstly, the RCTs selected in this meta-analysis involved various types of therapeutic drugs and diverse baseline characteristics, which may cause significant heterogeneities in data analysis in the aspect of the dissimilar clinical therapeutic effects and TRAEs. Therefore, the subgroup analyses were conducted attempting to stratify by baseline characteristics to mitigate the impact of heterogeneities. The efficacy and safety of the combination therapy can be further investigated through network meta-analysis in the future. Secondly, the present study included only five RCTs to compare PD-1/PD-L1 inhibitors combination therapy with anti-angiogenic monotherapy in patients with unresectable HCC. Further clinical trials would provide more reliable data for analysis, which may be included in future studies. Thirdly, there were inadequate cost-effective analyses for HCC in these trials, which might prove to be important for individual therapy. More cost-effective analyses are warranted due to the higher cost of the combination therapy than anti-angiogenic monotherapy. Lastly, there are inadequate mechanism reports of HBV response and resistance of immunotherapy. Future research will be critical for demonstrating the relationship between HBV infection and efficacy of ICIs.
PD-1/PD-L1 inhibitors combination therapy for unresectable HCC was associated with better OS, PFS, ORR, and DCR than anti-angiogenic monotherapy, especially for the first-time discovery of better survival benefits for HBV infection and Asian population. Responses achieved with combination therapy may not have been more clinically meaningful to HCV infection and non-viral patients with unresectable HCC. The incidences of any grade and grade 3–5 TRAEs were significantly higher in patients receiving combination therapy, but the safety was manageable. This meta-analysis provides new treatment options for unresectable HCC patients, especially for those with HBV-associated HCC.
Availability of data and materials
The original datasets for this study are included in the article/Supplementary Material.
Abbreviations
- Hepatocellular carcinoma
Hepatitis B virus
Immune checkpoint inhibitor
Hazard ratio
Confidence intervals
Overall survival
Progression-free survival
Objective response rate
Disease control rate
Treatment-related adverse events
Hepatitis C virus
Vascular endothelial growth factor
Programmed cell death 1
Programmed cell death ligand 1
American Society of Clinical Oncology
European society of medical oncology
American Society of Clinical Oncology gastrointestinal cancer Symposium
Random control trials
Response Evaluation Criteria in Solid Tumors version 1.1
Hepatocellular carcinoma-specific modified RECIST
Macrovascular invasion
Extra hepatic spread
Alphafetoprotein
Barcelona Clinic Liver Cancer
National Comprehensive Cancer Network
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Article Google Scholar
Lau WY, Leung TW, Lai BS, Liew CT, Ho SK, Yu SC, et al. Preoperative systemic chemoimmunotherapy and sequential resection for unresectable hepatocellular carcinoma. Ann Surg. 2001;233(2):236–41.
Article CAS PubMed PubMed Central Google Scholar
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604.
Article PubMed PubMed Central Google Scholar
Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72(2):250–61.
Cheng AL, Kang YK, Chen ZD, Tsao CJ, Qin SK, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34.
Article CAS PubMed Google Scholar
Kudo M, Finn RS, Qin SK, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):238–55.
Song YX, Fu Y, Xie Q, Zhu B, Wang J, Zhang BC. Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol. 1956;2020:11.
Google Scholar
Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis. 2017;20(2):185–204.
Matteis SD, Ghetti M, Gramantieri L, Marisi G, Casadei-Gardini A. Sorafenib in the treatment of virus-related HCC: differences between HCV and HBV. Onco Targets Ther. 2021;14:4305–8.
Zhang X, Wang F, Gu G, Wu Q. High HBV load weakens predictive effect of serum miR-122 on response to sorafenib in hepatocellular carcinoma patients. J Oncol. 2021;2021:9938207.
PubMed PubMed Central Google Scholar
Choi NR, Kim JY, Hong JH, Hur MH, Cho H, Park MK, et al. Comparison of the outcomes between sorafenib and lenvatinib as the first-line systemic treatment for HBV-associated hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol. 2022;22(1):135.
Rizzo A, Ricci AD, Gadaleta-Caldarola G, Brandi G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: current management and future challenges. Expert Rev Gastroenterol Hepatol. 2021;15(11):1245–51.
Makuku R, Khalili N, Razi S, Keshavarz-Fathi M, Rezaei N. Current and future perspectives of PD-1/PDL-1 blockade in cancer immunotherapy. J Immunol Res. 2021;2021:1–15.
Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind Phase III Trial. J Clin Oncol. 2020;38(3):193–202.
Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90.
Sperandio RC, Pestana RC, Miyamura BV, Kaseb AO. Hepatocellular carcinoma immunotherapy. Annu Rev Med. 2022;73:267–78.
Article PubMed Google Scholar
Fu YJ, Liu SS, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):396.
Yang J, Yan J, Liu BR. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9(978):1–9.
Kudo M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers (Basel). 2020;12(5):1089.
Finn RS, Qin SK, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905.
Ren ZG, Xu JM, Bai YX, Xu AB, Cang SD, Du CY, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3study. Lancet Oncol. 2021;22(7):977–90.
Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin SK, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995–1008.
Finn RS, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, et al. LBA34 primary resultsfrom the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinibasfirst-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33:S1401. https://doi.org/10.1016/j.annonc.2022.08.031 .
Qin S, Chan LS, Gu S, Bai Y, Ren Z, Lin X, et al. LBA35 camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Ann Oncol. 2022;33:1401–2. https://doi.org/10.1016/j.annonc.2022.08.032 .
Yang TK, Yu YF, Tsai CL, Li HJ, Yang PS, Huang KW, et al. Efficacy and safety of combined targeted therapy and immunotherapy versus targeted monotherapy in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. BMC Cancer. 2022;22(1):1085.
Zhong Y, Huo H, Dai S, Li S. Efficacy and safety of immune checkpoint inhibitors-combined antiangiogenic drugs in the treatment of hepatocellular carcinoma: a systematic review and meta analysis. Front Oncol. 2022;12: 964779.
Rhodes KM, Turner RM, Savović J, Jones HE, Mawdsley D, Higgins JPT. Between-trial heterogeneity in meta-analyses may be partially explained by reported design characteristics. J Clin Epidemiol. 2018;95:45–54.
Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74(3):785–94.
Gilles HC, Garbutt T, Landrum J. Hepatocellular Carcinoma. Crit Care Nurs Clin North Am. 2022;34(3):289–301.
Anwanwana D, Singha SK, Singhb S, Saikamc V, Singha R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1): 188314.
Pinato DJ, Mauri FA, Spina P, Cain O, Siddique A, Goldin R, et al. Clinical implications of heterogeneity in PD-L1 immunohistochemical detection in hepatocellular carcinoma: the Blueprint-HCC study. Br J Cancer. 2019;120(11):1033–6.
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502.
Feun LG, Li YY, Wu C, Wangpaichitr M, Jones PD, Richman SP, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer. 2019;125(20):3603–14.
Zhou X, Cao J, Topatana W, Xie T, Chen T, Hu J, et al. Evaluation of PD-L1 as a biomarker for immunotherapy for hepatocellular carcinoma: systematic review and meta-analysis. Immunotherapy. 2023;15(5):353–65.
Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6(3):1694.
Raziorrouh B, Schraut W, Gerlach T, Nowack D, Grüner NH, Ulsenheimer A, et al. The immuneregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology. 2010;52(6):1934–47.
Zhao J, Zhang YH, Qin SY, Zou BW, Wang YS. Hepatitis B virus reactivation in cancer patients undergoing immune checkpoint inhibitors therapy: a systematic review. J Cancer. 2022;13(14):3539–53.
Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–6.
El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–73.
Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–91.
Torimura T, Iwamoto H. Treatment and the prognosis of hepatocellular carcinoma in Asia. Liver Int. 2022;42(9):2042–54.
Tian BW, Han CL, Dong ZR, Tan SY, Wang DX, Li T. Role of exosomes in immunotherapy of hepatocellular carcinoma. Cancers (Basel). 2022;14(16):4036.
Ronot M, Bouattour M, Wassermann J, Bruno O, Dreyer C, Larroque B, et al. Alternative response criteria (Choi, European association for the study of the liver, and modified response evaluation criteria in solid tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014;19(4):394–402.
Takada J, Hidaka H, Nakazawa T, Kondo M, Numata K, Tanaka K, et al. Modified response evaluation criteria in solid tumors is superior to response evaluation criteria in solid tumors for assessment of responses to sorafenib in patients with advanced hepatocellular carcinoma. BMC Res Notes. 2015;8:609.
Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Correction to: “Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up.” Ann Oncol. 2019;30(5):871–3.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
Download references
Acknowledgements
Not applicable.
Disclosure statement
The authors have nothing to disclose.
There was no funding source for this study.
Author information
Authors and affiliations.
Department of Pharmacy, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, China
Danxue Huang, Liyuan Ke, Hongxia Cui & Su Li
You can also search for this author in PubMed Google Scholar
Contributions
HDX and KLY contributed the study concept and design. HDX and LS contributed to the data acquisition. HDX and CHX were responsible for data analysis and editing the manuscript. LS and CHX contributed to critical revision of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Correspondence to Danxue Huang .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: figure s1. .
Risk of bias summary (A) and risk of bias graph (B). Figure S2. Forest plots of HRs comparison of OS between PD-1/PD-L1 inhibitors plus anti-angiogenic group and anti-angiogenic group in subgroup analysis (A) region, (B) MVI or EHS, (C) AFP Level, (D) Etiology, (E) BCLC stage. Figure S3. Forest plots of HRs comparison of PFS between PD-1/PD-L1 inhibitors plus anti-angiogenic group and anti-angiogenic group in subgroup analysis (A) region, (B) MVI or EHS, (C) AFP Level, (D) Etiology, (E) BCLC stage. Figure S4. Pooled HRs of OS (A) and PFS (B) in sensitivity analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Huang, D., Ke, L., Cui, H. et al. Efficacy and safety of PD-1/PD-L1 inhibitors combined with anti-angiogenic therapy for the unresectable hepatocellular carcinoma and the benefit for hepatitis B virus etiology subgroup: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 23 , 474 (2023). https://doi.org/10.1186/s12885-023-10960-w
Download citation
Received : 22 February 2023
Accepted : 14 May 2023
Published : 24 May 2023
DOI : https://doi.org/10.1186/s12885-023-10960-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- PD-1 inhibitor
- PD-L1 inhibitor
- Anti-angiogenic
- Hepatitis B Virus
- Meta-analysis
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

The Structural Biology of Hepatitis B Virus: Form and Function
Baruch Blumberg discovered the Australia antigen when searching for immunological evidence of genetic polymorphisms. His hypothesis was that antibodies to blood proteins could be used to investigate human genetic diversity. The experiment was to look for antibodies in serum from patients who had been exposed to diverse blood sources via repeated transfusions. Blumberg and co-workers discovered a “protein with which they react [that] is very rare in populations of Western origin, but it is not uncommon in Micronesians, Vietnamese, Formosans and Australian aborigines” ( 1 , 2 ). By 1967, Blumberg and others recognized that Australia antigen was correlated with hepatitis. In 1970 Australia antigen was identified as a structural component of the hepatitis B virus (HBV). That year Dane and co-workers published electron micrographs of 17–22 nm spherical HBV surface antigen (HBsAg) particles, 17–22 nm diameter filamentous HBsAg particles, and 45 nm diameter virions comprised of a HBsAg envelope and a 36 nm diameter core ( 3 ). HBV virions are known as Dane particles.
These early studies identified the salient features of the HBV virion and its epidemiology. The virus is outwardly simple but endemic and persistent. HBV is predominantly a virus of Southeast Asia, sub-Saharan Africa, and populations such as aboriginal Australians and Inuit. Worldwide, an estimated 240 million people have chronic HBV ( 4 ). This prevalence is consistent with predominantly vertical transmission of infection from mother to child. In young children HBV has a high probability of establishing chronic infection. HBV contributes to about 780,000 deaths each year, predominantly through hepatocellular carcinoma, cirrhosis, and liver failure ( 4 ). The HBV vaccine, now based on yeast-expressed HBsAg, the Australia antigen, is very effective and is changing the demographics of HBV disease ( 5 ), but cannot help those who are already infected.
Mature HBV is an enveloped, icosahedral virus. It packages a circular dsDNA genome with gaps in both strands and a reverse transcriptase (usually referred to as ‘P’) covalently bound to the 5′ end of the antisense strand; it also packages a number of host proteins. A description of the HBV lifecycle introduces the structural components of the virus and the basis for chronic infection.
To initiate infection, a Dane particle binds to the Na + -Taurocholate Co-transporter Polypeptide (NTCP) ( 6 , 7 ). The virion is endocytically transferred to the cytoplasm; the mechanism of escape from the endocytic vesicle is not understood – release of duck HBV from endosomes appears to be insensitive to pH ( 8 ). The naked, DNA-filled core binds to importin α-importin β complexes via nuclear localization signals on the core protein (HBcAg) ( 9 , 10 ). Core dissociation and genome release are associated with the nuclear pore complex ( 11 ). In the nucleus, P is removed from the DNA and the DNA “repaired” ( 12 , 13 ). The resulting covalently closed circular DNA (cccDNA) becomes nucleosome-decorated and appears to function like host DNA. Two HBV proteins, HBcAg and the X protein, have been localized to cccDNA and may have regulatory roles ( 14 – 17 ).
In an infected cell, production of new virus starts with RNAs transcribed from cccDNA, presumably by Pol2. A terminally redundant, unspliced copy of the cccDNA (pregenomic RNA or pgRNA) is the messenger for HBcAg, an HBcAg variant known as the “e” antigen (HBeAg), and P protein. A set of sub-genomic RNAs encode the large, medium, and small variants of the surface antigens (L-, M-, and S-HBsAg). A final subgenomic RNA encodes the X protein; X is a non-structural protein that affects cccDNA persistence, cccDNA transcription, and mitochondrial Ca ++ flux ( 15 , 18 – 20 ). In the cytoplasm, a complex of P protein and pgRNA appears to nucleate assembly of the immature RNA-filled HBV core ( 21 ). A substantial number of empty capsids are also formed ( 22 ) (a capsid refers to the protein shell of the HBV core). Reverse transcription takes place within the core while it is resident in the cytoplasm, leading to synthesis of a relaxed circular dsDNA product from the linear pgRNA ( 23 ); large pores in the capsid surface allow nucleotides to diffuse in and out ( 24 ). The mature capsid, and also the empty capsid, carries signals that allow it to bind the L-HBsAg (possibly from late endoplasmic reticulum (ER) membranes) for envelopment and secretion or to bind importin proteins for nuclear transport to generate more cccDNA.
The goal of this review is to describe the structural biology of the HBV virion. This necessarily ignores much of the interplay between virus and host that is critical for infection. We also concentrate on human HBV, the type member of the hepadnavirus family. However, we should note that related hepadnaviruses, Woodchuck Hepatitis Virus (WHV) ( 25 ) and duck Hepatitis B Virus (dHBV) ( 26 ) have made great contributions to our understanding of hepadnavirus biology and pathology.
The Dane particle
As little as a single virion, measured in terms of genomes, can lead to chronic HBV infection ( 27 ). Yet examination of electron micrographs reveals a startling heterogeneity. Particles typically show an enveloped core containing the three co-terminal HBsAg proteins – S, M and L – embedded in the membrane. However, the envelope may have a filamentous extension or the enclosed core may have a smaller diameter, 32 nm, or an aberrant shape ( 28 ). As many as 90% of secreted particles are actually empty ( 22 ). This suggests that cores containing correctly processed nucleic acid and empty cores both display a secretion signal ( 22 ). Efforts to apply cryo-electron microscopy (cryo-EM) image reconstruction to Dane particles revealed that the envelope and core do not share symmetry ( 29 , 30 ). A composite map was built when the core and the envelope were independently reconstructed (by masking the other) and these reconstructions were then superposed ( Figure 1 ). It was shown that HBsAg spikes, 40Å protrusions from the envelope, are arranged in a trigonal lattice such that positions of HBsAg and HBcAg did not necessarily coincide. Capsids were structurally indistinguishable from other cryo-EM reconstructions of capsid alone; there was also limited order in the encapsidated DNA due in part to icosahedral averaging during reconstruction. Packaged DNA was densest under fivefold and quasi-sixfold vertices, where HBcAg nucleic-acid binding domains are located ( 29 ) ( Figure 1 ). Notably, in some Dane particles the envelope was only loosely associated with the core ( 30 ), this may be a function of the distribution of the different forms of the HBsAg. The capsid does not firmly constrain the distribution of HBsAg units in the envelope but provides a generalized template for packing ( 30 ).

A composite cryo-EM reconstruction of a dane particle. (a) Cut away views of a composite model of the HBV virion comprised of an icosahedral capsid (blue) containing packaged DNA (red) and an outer envelope (gold) with protein projections spaced 60Å apart. Views are cross-sections (left), and two cut aways (b) X-ray crystal structure of recombinant capsid ( 36 ) docked into the cryo-EM density map of the virion capsid (left). The tips of the core spikes are in close apposition but do not penetrate the envelope. Additional details and cartoon of interpretation (right). The surface protein projections are ascribed to HBsAg and are designated as large (L), medium (M), and small (S) arbitrarily. These figures reproduced with permission from Dryden et al. ( 29 ).
Dimer structure
The basic soluble unit of HBcAg is a dimer ( Figure 2a ). A HBcAg monomer is 183 residues long (for in vitro studies we refer to it as core protein 183 or Cp183). The first 149 residues form the predominantly α-helical assembly domain (Cp149). The remaining 34 residues are the arginine-rich RNA-binding, C-Terminal Domain (CTD) ( 31 , 32 ). This domain is located inside the capsid and has been identified to be intrinsically disordered based on sequence and Cryo-EM studies ( 33 ). The assembly domain alone is sufficient for assembly of morphologically regular empty capsids ( 32 ). The assembly domain has 5 α-helices connected by loops ( 34 – 36 ) ( Figure 2a ). Helices 1, 2 and 5 are part the chassis of the capsid. A tight proline-rich loop connects helix 5 to the CTD ( 37 ). Holding together a dimer, helices 3 and 4 from one half-dimer form a 4-helix motif with corresponding helices from the other half-dimer. A total of about 3200 Å 2 of hydrophobic surface is buried by this interaction. This motif is flanked by salt bridges and hydrogen bonds that stabilize the hydrophobic interface. Helix 4 is kinked in the middle and is sometimes referred to as helices 4a and 4b. A disulfide bond can form over time between highly conserved C61 residues in helix 3, which contributes to the dimer interface ( 38 – 40 ).

X-ray crystal structures of the assembly domain. (a) A HBcAg dimer in the context of a capsid. The helices in HBcAg are named 1–5 from N to C-terminus. The dimer interface comprises of a four-helix bundle created by two helices from each monomer. (b) a superposition of an HBcAg dimer in the context of a capsid (grey) on a free dimer (Y132A mutant) (blue). The free dimer is less compact than the dimer in the context of a capsid (c) An HBeAg dimer. The dimeric interface is drastically altered and stabilized by disulfide bonds (d) An HBcAg T=4 capsid with the asymmetric unit in color. The individual subunits are A (blue), B (red), C (green) and D (yellow) or AB and CD dimers.
Despite its conserved sequence and α-helical state, there is a degree of variability in the structure of the dimer. Dimers in the context of capsids are more compact than free dimers in solution ( Figure 2b ) ( 36 , 41 ). Crystal structures of an assembly-incompetent mutant (Y132A) of HBcAg ( 41 – 43 ) show substantial variability, mainly at the spike tips and the C-termini. This variability in structure is thought to be indicative of the variety in functional roles for HBcAg in the viral life cycle. Regulating the structural state of HBcAg would induce/stabilize specific conformational states that match specific functional states. For example, an oxidized C61–C61 dimer results in weaker dimer-dimer interactions and slower capsid assembly rates in comparison to the reduced form ( 38 ); thus, the oxidized dimer favors a conformation (or conformations) that are unfavorable for capsid assembly. While the cytoplasm (where capsid assembly takes place during an infection) is a reducing environment, the proximity of the C61 residues is high may be sufficient to overcome the reducing potential. Data suggest that structural dynamics that allow HBcAg to transition between these states are an important key to its function ( 38 , 41 , 44 , 45 ).
HBeAg is a secreted protein expressed by every member of the Hepadnaviridae family though its expression is not required to maintain infection. HBeAg is transcribed from the C gene using the first of the two start codons, HBcAg uses the second. The HBeAg pre-protein has 29 residues upstream of HBcAg ( 46 ). The first 19 residues, a signal peptide, trafficks nascent HBeAg to the ER where the signal and the CTD are proteolytically removed ( 47 , 48 ). The remaining 10 residues upstream of HBcAg including a Cys at position -7. This C-7 forms an intramolecular disulfide with C61 to stabilize the structure ( 40 ). HBeAg is thought to have an immune-modulatory role that allows for humoral and cell-mediated immune evasion ( 49 , 50 ). Paradoxically, loss of e antigen is correlated with clearance of acute HBV infection and establishment of chronic infection ( 51 ). In a similar vein, loss of HBeAg in culture correlates with increased HBV expression while in humans it correlates with decreased viremia ( 52 ).
HBeAg and HBcAg show important physical differences: the thermal melting temperature of HBeAg is 51°C while that of HBcAg dimer is 63°C ( 53 ). Reducing the C-7–C61 disulfide or mutating C-7 to Ala residue increased the stability of the HBeAg to near HBcAg levels ( 54 ). While oxidized HBeAg does not assemble into capsids, sedimentation analysis and electron microscopy showed that reduced HBeAg dimers could ( 53 , 54 ). These results suggest that HBeAg could adopt a conformation similar to that of HBcAg ( 40 , 53 ). Thus, it is possible that both HBeAg- and HBcAg-associated dimer conformations may be accessible to both proteins in solution. Assembly studies with reduced HBeAg dimers indicate that their capsid-like products are less regular than HBcAg-assembled capsids ( 53 ). This would suggest that co-assembly of HBcAg with HBeAg would have a negative impact on regular capsid assembly, both from a kinetic and thermodynamic point of view.
The 10-residue peptide upstream of HBcAg makes HBcAg and HBeAg serologically distinct, though there is also substantial cross reactivity ( 35 , 55 , 56 ). In the HBeAg crystal structure ( Figure 2c ) ( 54 ), the overall fold of an HBeAg monomer was the same as an HBcAg monomer, with some differences at the spike tips and C-termini. The dimer interface, however, was heavily altered; one half-dimer was rotated by 140°, so that the HBeAg four-helix bundle is almost antiparallel relative to the motif in HBcAg ( Figure 2c ). Thus, the tip of the spike must have evolved to support both parallel and antiparallel interaction. This gross change was stabilized by the disulfide from C-7 in the peptide to C61 of helix 3 (one per monomer), which replaces the HBcAg C61–C61 disulfide ( 36 ). The structure is also stabilized by hydrophobic contacts between the peptide and helix 3 and 4. The peptide covers some of the exposed hydrophobic surface at the base of helix 3, creating a new interface ( 54 ).
Capsid structure
HBcAg can self-assemble to form the icosahedral virus capsid. A 120-dimer T=4 capsid ( Figure 2d ) is the major assembly product (~95%) with a small amount T=3 capsid observed in infected human livers, recombinant E. coli capsids and in vitro assembled recombinant HBcAg subunits ( 24 , 28 , 57 – 59 ). Structures for T=4 and T=3 capsids have been determined by Cryo-EM ( 24 , 34 , 35 , 37 , 60 , 61 ). Crystal structures have been determined for T=4 capsids and assembly-incompetent HBcAg dimers ( 36 , 41 , 42 , 62 ).
Icosahedra are comprised of 60 asymmetric units. A T=4 asymmetric unit contains contains 4 HBcAg monomers, A through D, (or two dimers, AB and CD) that form the icosahedral asymmetric unit ( Figure 2d ). The subunits are in “quasi-equivalent” environments. The 4-helix motifs from the dimers are oriented perpendicular to the surface of the capsid, forming the spikes that punctuate the capsid. By convention, A monomers form the fivefold vertices and two sets of B, C and D monomers form the quasi-sixfold vertices ( Figure 2d ). A T=3 asymmetric unit has 3 quasi-equivalent monomers resulting in AB dimers and twofold symmetric CC dimers. Large holes fenestrate the capsid surface and provide a means for nucleotides and other small molecules to diffuse in and out of the particle.
Contacts between dimers are mediated by burial of about 1700 Å 2 of hydrophobic surface. This is a tongue-and-groove contact where the groove is located near the junction where helix 5 emerges from the four-helix bundle at the “intradimer” contact. This groove is filled by the helix-turn-extended structure of an incoming subunit ( 36 ). Tyrosine 132 from the incoming subunit contributes about 10% of the buried surface are of this interaction ( 36 ); a Y132A mutant is assembly-incompetent ( 63 ) and has been the basis of HBcAg dimer structures ( 41 – 43 ). A peculiar pocket formed at this interface is the basis of assembly-directed antivirals, which will be discussed later in this review.
The HBcAg capsid is itself highly immunogenic and reported to induce both B- and T-cell responses ( 64 ), though these responses are not protective against HBV infection. Peptide mapping studies identified the spike tip, around residue 80, as the major epitope ( 56 , 65 ) In a series of cryo-EM studies antibodies to capsids were shown to bind not only to a linear epitope on the spike tip but to conformational epitopes on the sides of the spike and its base ( 35 , 66 – 68 ). Other epitopes have been observed at interdimer contacts ( 65 , 68 ). Because of its immunogenicity, HBV capsids have been used as a carrier for epitopes ( 69 , 70 ). To facilitate insertion of large immunogens into the spike tip while avoiding steric clashes, investigators have used proteolytically processed “split cores” or a tandem fusion of HBcAg that allows modification of one half dimer at a time ( 71 , 72 ).
HBcAg C-terminal Domain, the CTD
The 34-residue (or 36 residue, depending on genotype) arginine-rich HBcAg CTD ( Figure 3a ) is localized to the interior of the capsid ( Figure 3b ), though it can transiently be exposed on the capsid exterior ( 37 , 73 – 75 ). CTDs regulate RNA packaging, reverse transcription, and binding to host proteins. The CTD can be considered a sequence of four arginine-rich repeats ( Figure 3 ). The CTD has 7 conserved serines and a threonine which can be phosphorylated ( 168 , 169 , 79 , 148 ) ( Figure 3a ). Several host protein kinases have been implicated, including protein kinase C, serine/arginine protein kinase (SRPK) and cyclin dependent kinase 2 (Cdk2) ( 76 – 78 ). These kinases may act on HBcAg prior to assembly or in the context of capsid. One or more types of kinase also gets packaged in cores, with strong evidence supporting identification as Cdk2 ( 78 ). Phosphorylation of S155, S162, and S170 is critical for packaging RNA ( Figure 3a ) – substitution of these residues with alanine to mimic the unphosphorylated state suppresses pgRNA packaging ( 76 , 79 – 81 ).

The HBcAg CTD. (a) A schematic of the assembly domain and the CTD including the sequence of the CTD. S155, S162 and S170 are deemed to be critical for pgRNA packaging ( 173 , 174 ) (b) cut-away view of a cryo-EM reconstruction of an empty Cp183 capsid. The density in color corresponds to the CTD based on the fitting of an X-ray crystal structure of a Cp149 capsid in the reconstruction. CTD density is prominent beneath the fivefold and quasi-sixfolds (c) A cryo-EM reconstruction of empty Cp183 capsids bound to SRPK molecules. SRPK (red) binds to transiently exposed CTDs at the quasi-sixfolds. These figures reproduced with permission from Selzer et al. ( 75 ) and Chen et al. ( 74 ).
Interaction of Cp183 with SRPK has been used to demonstrate flexibility of the CTD and a possible mechanism for regulating assembly. In vitro, SRPK binds Cp183 dimers and empty capsids ( 74 ). However, the affinity for dimer is 50-fold stronger suggesting a thermodynamic basis for stalling assembly; SRPK also is large enough that it would occlude assembly of bound dimers. However, assembly of SRPK-bound Cp183 can be reactivated by addition of ATP to initiate phosphorylation and release the bound SRPK. Thus, hypothetically SRPK can acts as a non-canonical chaperone that gates assembly. A cryo-EM reconstruction of pre-assembled empty Cp183 capsids bound to SRPK revealed that SRPK trapped CTDs that were transiently exposed to the capsid surface at twofold (quasi-sixfold) vertices ( Figure 3c ) ( 74 ). Transient exposure of CTDs in empty Cp183 capsids has also been documented by trypsin digestion studies ( 75 ). Mass spectrometry of trypsin-digested empty capsids showed ready accessibility as far as residue 157. Cryo-EM reconstructions of these products verified the cleavage and loss of CTD density underneath quasi-sixfold vertices ( Figure 3b ) ( 75 ).
There is a strong correlation between the positive charge of a viral capsid and its nucleic acid content ( 82 , 83 ); this correlation extends to HBV ( 84 – 86 ). In vivo , a capsid with fully phosphorylated CTDs packages about 3400 nucleotides of pgRNA with its polyA tail, a ratio of 1.77 bases per arginine, and a fully dephosphorylated DNA-filled core has about 6300 bases, a ratio of 1.75 bases per arginine. The number of positively charged arginines in the CTD correlates with the amount of encapsidated RNA ( 84 , 86 ). Decreasing the amount of positive charge in an HBV expression system, by truncating the CTD ( 85 ) or by replacing blocks of arginines with alanines ( 87 ), decreases the amount of pgRNA encapsidated in cell culture expression of HBV. Surprisingly, the decreased RNA content correlates with decreased representation of 3′ ends compared to 5′ ends, suggesting that RNA is packaged so that the 3′ end is particularly vulnerable to nucleases or that it is packaged in a stepwise process 3′ end last.
Phosphorylation appears to modulate structural interactions between CTDs that affect capsid stability and RNA organization within the capsid. In empty Cp183 capsids, external exposure of CTDs is decreased and capsid stability is strengthened by an S155E, S162E, S170E triple mutation, mimicking phosphorylation of the three critical serines ( 75 ). In a virus expression system, the triple mutant assembles without packaging RNA ( 85 ). Image reconstructions comparing this triple mutant to wild type capsids show that mutant CTDs, particularly around the fivefold vertices, cluster together, which was suggested to be a result of electrostatic interactions between “phosphorylations” on one CTD with arginines on an adjacent CTD ( 33 ).
The triple mutant phosphorylation mimic also modified the organization of packaged pgRNA in in vitro assembled particles ( 33 ). In vitro , Cp183 can be assembled on (single-stranded DNA) ssDNA and single-stranded RNA (ssRNA) but not on double-stranded (dsDNA) ( 88 ). The pgRNA in capsids with unphosphorylated CTDs formed an icosahedral cage of thick RNA ropes ( Figure 4a )( 33 ). In the phosphorylation-mimic mutant the RNA formed a more diffuse mesh-like network ( Figure 4b ) of thin and short stretches of electron density ( 33 ). Consistent with the ability of CTD modification to change the disposition of packaged RNA, the CTD has been shown to have RNA chaperone activity in the context of a capsid ( 89 ).

RNA containing capsids. Icosahedral reconstructions of (a) wildtype Cp183 and (b) a phosphorylation mimic mutant with in vitro packaged pgRNA (blue and gold respectively). The pgRNA in the former forms an icosahedral cage while the same in the latter is more mesh-like (c) An asymmetric reconstruction of an RNA-containing virion shows density for pgRNA (gold), P protein (red) and other unassigned content. These figures reproduced with permission from Wang et al. ( 33 ) and Wang et al. ( 94 ).
Though CTDs are ostensibly on the capsid interior, in empty capsids and DNA-filled capsids they are accessible to the capsid exterior ( 74 – 76 ). While in RNA-filled capsids, the CTD is trapped on the capsid interior. External exposure of CTDs is biologically critical as the CTD has a role in nuclear translocation. Mature cores are imported into the nucleus through nuclear pores in a process that involves the binding of an Importins α-Importin β complex ( 11 ). Importin α binds to nuclear localization signals (NLSs), a sequence of three or four to six consecutive basic amino acids. When bound to an NLS, importin α exposes an importin β binding domain, a motif of 13 basic residues spread over 39 residues ( 90 , 91 ). Emphasizing a fundamental difference between CTD exposure of DNA-filled versus empty capsids, in vitro empty capsids and free dimers bind Importin β without Importin α mediation ( 73 ). Furthermore, importin β appears to bind dimer and destabilize empty capsids. These observations suggest that intracellular transport of free dimer, empty capsids, and mature capsids use different mechanisms. Efforts to clarify the specific NLS sites have been complicated by the fact that they may overlap. Furthermore, the same sequences in the CTD have been shown to act as nuclear export signals, presumably for Cp183 dimer; this has been suggested as a mechanism for export of viral RNA from the nucleus ( 92 ).
Reverse Transcription
Reverse transcription (reviewed in ( 23 , 93 , 94 )) is the target of most anti-HBV therapeutics now available. Capsid assembly is nucleated by a pgRNA-reverse transcriptase (P protein) complex, with P bound to the ε stem loop near the 5′ end of pgRNA. The P protein has four domains, a terminal priming domain, a linker, a polymerase domain, and an RNaseH. The polymerase, though it is monomeric, has been modeled based on the HIV reverse transcriptase ( 95 ). The RNAseH shares little sequence identity with other RNaseH proteins but enough to allow identification of active site residues and evaluate potential inhibitors ( 96 ). Within the capsid, using the loop in ε as its first template, a tyrosine in P’s N-terminal domain primes minus strand DNA synthesis, leaving the nascent chain covalently bound to the protein. After 3 bases, P switches template to a sequence (direct repeat 1, DR1) near the 3′ end of pgRNA and then completes minus strand DNA synthesis. The carboxy-terminal RNaseH domain of P digest the template pgRNA leaving only about 17 bases of the original RNA. ssDNA-filled capsids accumulate in cytoplasm, suggesting that the next step in DNA synthesis has a high kinetic barrier. The remaining RNA with the P-protein (covalently bound to minus strand DNA) now makes a second template switch to a region overlapping a copy of the DR1 sequence, DR2, near the 5′ end the minus strand. Synthesis of the plus strand begins there, using the RNA as a primer. After running out of template, the P complex with the growing plus strand switches template for the third time. Successful maturation of the HBV virion requires three template switches in which P and associated nucleic acids jump from one end of the template nucleic acid to the other. These template switches are facilitated by complementary sequences at the 5′ and 3′ ends of the nucleic acid ( 97 , 98 ). For these to anneal, the encapsidated P protein and pgRNA adopt a specific quaternary arrangement.
There were two hypotheses to explain the process of reverse transcription: (i) The RNA lines the interior wall of the capsid allowing the P protein to travel on a nucleic acid track where 5′ and 3′ ends are topographically close to one another; (ii) The polymerase is directly bound to the inner wall of the capsid, requiring the nucleic acid to move through the enzyme like a conveyer belt. An asymmetric Cryo-EM reconstruction of RNA-filled cores with packaged P protein, determined to 16 Å resolution ( Figure 4c ), strongly supports the first hypothesis ( 94 ). Mutation of the priming tyrosine in P supported pgRNA packaging but arrested the core in the RNA-filled state ( 99 ). RNA density was irregular but lined the interior surface of the capsid, similar to in vitro -assembled RNA-filled capsids ( Figure 4c ). The RNA density was not resolved enough to fit individual strands. Internal to the RNA, a “donut-shaped” density was adsorbed to the RNA shell interior (under a threefold) consistent in size and shape to a polymerase ( Figure 4c ). Other unassigned internal density was observed and could be other packaged protein or could be an artifact arising from irregularity in the capsids and the difficulty of determining asymmetric orientation in an icosahedral particle.
The shell of the capsid as well as its interior surface impact reverse transcription. Mutation of conserved Val124 at the inter-dimer interface affects capsid assembly in a predictable way, changing capsid stability proportional to the change in buried surface ( 100 ). These mutations lead to defects in packaging of pgRNA, suggesting a competition between assembly of RNA-filled capsids with assembly of empty/defective ones. Reverse transcription was also profoundly affected ( 100 ). While first strand synthesis was proportional to the amount of pgRNA packaged, second strand synthesis was abolished ( 100 , 101 ). This suggests that the capsid is not just an inert carrier of genomic material and its structural properties affect seemingly unrelated processes.
Interaction of HBV cores with Surface proteins
The HBV Surface antigen has distinct roles in the virus lifecycle. Intracellularly, HBsAg binds cores, attenuating core transport to the nucleus and effectively regulating cccDNA copy number ( 102 ). HBsAg is secreted as subviral particles that attenuate immune response to the virus ( 103 ); indeed, yeast-expressed S-HBsAg is the basis of the HBV vaccine. HBsAg is responsible for binding cores during assembly and receptor during infection. HBsAg is also a structural component of Hepatitis Delta Virus, a satellite of HBV that drastically increases morbidity and mortality ( 104 ).
The structure of HBsAg is known to low resolution only. At a basic level, HBsAg is a glycosylated integral membrane protein that has been localized to ER, pre-Golgi, and late endosomal membranes ( 105 – 107 ). HBsAg comes in three sizes, small, medium, and large, referred to as S-HBsAg, M-HBsAg, and L-HBsAg. The L-HBsAg has three domains, in order, pre-S1 (108 or 119 residues), pre-S2 (55 residues), and the S domain (226 residues). M-HBsAg, which is not required for infection, lacks pre-S1. S-HBsAg is comprised of only the S domain which is composed of four trans-membrane helices ( 108 ). The S domain forms disulfide crosslinked dimers, allowing formation of homo- and heterodimers.
Subviral particles are heterogeneous in chemical makeup and structure. Spherical subviral particles are enriched for S-HBsAg with only trace amounts of L-HBsAg whereas filaments have a 1:1:4 ratio of L-, M-, and S-HBsAg ( 109 ). Micrographs of subviral particles show serrations indicative of regular protrusions ( Figure 5a, b ). However, spherical particles appear to be heterogeneous in size and geometry – they are not icosahedral – frustrating efforts to generate image reconstructions ( 110 ). Filamentous particles also are heterogeneous but can be classified based on helical parameters (eg. one-, two-, and three-start helices) allowing successful reconstruction to 20Å resolution. Spikes in the reconstruction are similar in size to those in the micrograph. They project 40Å from the membrane and are separated by about 50Å. The volume of a given spike corresponds well with the expected volume for four S domains, suggesting the biologically relevant complex is a dimer of dimers ( 110 ). In a Dane particle reconstruction ( 29 ), HBsAg spikes have a similar spacing but are arranged with approximately trigonal geometry on the membrane surface; notably, the HBsAg lattice does not align with the core’s icosahedral lattice.

HBsAg subviral particles. (a) A cryo-micrograph of a mixture of ~22nm spherical and filamentous particles. Note how some filaments vary in their diameter. The scale bars correspond to 100nm. (b) A helical real-space reconstruction of a self-consistent data set. This two-start helix has a subunit twist of 35° and rise of 9.8Å. (c) A HBcAg dimer highlighting residues that affect secretion of Dane particles but do not affect core assembly ( 117 ). Residues from different monomers are in magenta and orange, respectively. Residues that are partially obscured by interdimer interfaces are highlighted in muted colors. Panels (a) and (b) are reproduced with permission from Short et al. ( 110 ).
In most viruses, internal features of the envelope protein(s) interact with the virus core and external features mediate interaction between the virion and its receptor(s). In HBV, both of these activities are functions of the pre-S domains of L-HBsAg, a peptide that can change its localization over time ( 111 ) and possibly in response to external triggers. The N-terminus of S-HBsAg and the pre-S2 domain of newly translated M-HBsAg are in the ER lumen, presumably due to an internal signal sequence ( 112 ). In L-HBsAg pre-S1, which is myristoylated, and pre-S2 are largely cytoplasmic, based on proteolytic sensitivity and the absence of pre-S2 glycosylation. This places the pre-S domains where they can interact with newly matured HBV cores. Conversely, in Dane particles the pre-S1 and pre-S2 domains are accessible to external proteases.
Dane particles appear to first interact with host cells through a relatively weak electrostatic interaction to highly sulfated proteoglycan (HSPG); this interaction is a necessary precursor to infection ( 113 , 114 ). In vitro other anionic glycans, e.g. heparin sulfate, can bind HBsAg. This interaction is modulated by a disulfide-stabilized motif on the S domain ( 115 , 116 ). The virions then bind to NTCP ( 6 , 7 ) in an interaction mediated specifically by a highly conserved N-terminal sequence of pre-S1, residues 2–48 ( 117 ). A myristoylated peptide based on this sequence, named MyrcludexB, is the basis for an HBV entry inhibitor that that displays an 80 picomolar IC 50 ( 118 , 119 ). Thus it is likely that binding to HSPG induces a conformational change exposing the N-terminal region of pre-S1 ( 116 ) – antibodies to this segment of pre-S1 do not precipitate Dane particles ( 120 ).
Interaction of cores with L-HBsAg is sensitive to the nucleic acid content of the core via a poorly characterized switch ( 22 )( 121 ). We have a rudimentary understanding of the structural basis of interaction of the pre-S domains with the HBV core that are associated with virion assembly and egress. Peptides based on the C-terminus of pre-S1 shows notable affinity for capsids based on binding of sequential peptides to capsids ( 122 ) and the ability of a series of L-HBsAg mutants with substitutions in the Pre-S1 and S2 sequences to support virion secretion ( 123 ). Additional determinants of interaction are associated with the S domain ( 122 , 124 ). Ponsel and Bruss conducted a rigorous alanine mapping of HBcAg by evaluating the ability of the HBcAg mutants to support capsid formation and virion secretion ( 125 ). The amino acids identified by that study run down the solvent-exposed face of HBcAg helix 4 ( Figure 5c ). Using an alternative approach, phage display, a hydrophobic peptide with sequence similarity to the first 20 amino acids of pre-S1 was identified that bound at the core spike tips ( 126 ).
Helix 4 is particularly attractive as regulatory element for core-envelope interaction. Its conformation is responsive to assembly state and bound small molecules ( Fig 5c ) ( 41 , 127 ). In a cryo-EM comparison of DNA-filled cores from HBV-expressing cells with RNA-filled capsids from E. coli, Roseman and Crowther observed small structural changes in the protruding HBcAg spike centering on a hydrophobic cavity bounded by residues K96 and F97 from one half-dimer and L60 from the other half ( 121 ) ( Figure 5c ). K96 and F97 are on the back face of helix 4.
Notably, mutation F97L (or I97L, depending on genotype) results in a core that is secreted in the immature ssDNA state while a L60V mutation inhibits secretion altogether ( 128 – 130 ). One cannot tell whether the pocket mutations affect the helix or vice versa. It is also possible that both the helix and pocket are involved in binding pre-S or that these mutations affect a dynamic state rather than a static structure. In Dane particle structures ( 29 , 30 ), the spike tip is in contact with the envelope. Direct interaction with protein could not be discerned in these structures due to the relative disorder of the envelope. However, the spike tip has been suggested as a binding site for HBsAg ( 126 , 131 ).
HBcAg spontaneously assembles into a mixture of T=4 and T=3 particles ( 58 ). Self-association of the assembly domain, the first 149 residues of HBcAg (Cp149), has been investigated extensively. The assembly reaction can be summarized by two equations ( 132 – 134 ). The kinetics of assembly can be described as a rate-limiting nucleation step followed by rapid elongation by addition of one dimer at a time:
This simple equations can result in complex behavior ( 135 , 136 ). While HBV is relatively simple, virus assembly reactions can be much more complex involving an array of metastable intermediates ( 137 , 138 ). The importance of the nucleation step is that it prevents formation of kinetic traps, reactions where incomplete capsids accumulate but there are not enough subunits to complete them. To prevent kinetic traps, nuclei must be relatively rare either because they form slowly compared to elongation or because they are relatively unstable ( 132 , 139 ). Assembly models predict a high energy barrier to disassembly (i.e. hysteresis to dissociation) that has been observed in experimental studies ( 140 – 142 ). Simulations suggest an array of intermediates during assembly ( 135 , 136 ), biased towards smaller sizes, which have now been observed by ion-mobility mass spectroscopy ( 143 ). An implication of this kinetic model is that allostery between assembly incompetent and competent states can contribute to regulation of nucleation and elongation ( 144 ), an implication borne out by structural differences between free and capsid-bound HBV dimers ( 41 , 44 , 45 ). Hydrogen-deuterium exchange ( 45 ) and protease sensitivity ( 44 , 75 ) indicate that HBV Cp149 is extremely dynamic, prone to conformational fluctuations and local melting of secondary structure.
At equilibrium, the assembly reaction reduces to a simple equilibrium expression:
The huge exponent in equation 2 leads to a pseudo-critical concentration (pcc), where the maximal concentration of free Cp149 dimer in solution is approximately equal to the pcc and all addition Cp149 is in the form of capsid and sometimes non-capsid polymer. Unlike a true critical concentration, equation 2 predicts assembly will occur at low Cp149 concentrations; this has now been observed using nanofluidic devices sensitive to very low protein concentrations ( 145 ). The term K capsid is a function of the pairwise interaction energy between two dimers, ΔG contact ( 139 ). For HBV, and most other viruses, ΔG contact is remarkably weak and assembly at physiological concentrations is only possible because subunits are multivalent. Weak association energy prevents entrapment of defects by favoring dissociation of misassembled components. Consistent with the hydrophobic inter-dimer contacts observed in capsids ( 36 ), assembly is entropy-driven ( 134 ). Kinetic traps can form when Cp149 association energy is strengthened by solution conditions; this was not evident until recent developments in charge detection mass spectrometry, a single particle technique ( 146 ). The same approach was used to show that Woodchuck Hepatitis Virus has a much greater tendency to form aberrant capsids ( 147 , 148 ).
In vitro assembly on nucleic acid is much more complicated because of the combination of protein-protein and protein nucleic acid interactions ( 149 , 150 ). In vitro assembly of Cp183 on ssRNA and ssDNA is fast and, based on the paucity of intermediates, highly cooperative ( 88 ). Assembly on dsDNA is problematic. The protein binds dsDNA with apparently high affinity. However, the assembly products are heterogeneous as if protein-protein interactions are insufficient bend and enclose the relatively rigid dsDNA substrate ( 88 , 150 ) leading to a calculation that a dsDNA could destabilize capsids ( 88 , 150 ). Consistent with this calculation, a growing body of evidence indicates that dsDNA-filled cores are not particularly stable – they are sensitive to proteases and nucleases and show alter biophysical properties ( 151 , 152 ).
Capsids and capsid-directed antiviral strategies
HBcAg is an attractive antiviral target because dimer and capsid participate in several steps of the virus life cycle ( 153 , 154 ). Misdirection of in vitro assembly by altering temperature and ionic strength can lead to formation of kinetically trapped intermediates or aberrant non-capsid structures. Forming these products under physiological conditions may be an effective antiviral strategy ( 155 , 156 ).
In cell-based screens, ostensibly for non-nucleoside reverse transcriptase inhibitors, two enigmatic classes of molecules were discovered: the phenylpropenamides (PPAs) and the heteroaryldihydropyrimidines (HAPs) ( 157 , 158 ). In both cases it was discovered the mode of action focused on the core protein, not the reverse transcriptase. PPAs led to loss of cytoplasmic RNA-filled cores and accumulation of empty capsids ( 159 ). HAPs led to loss of core protein ( 158 , 160 ). Unsurprisingly, these molecules were insensitive to nucleoside analog-resistant mutants of HBV reverse transcriptase ( 161 – 163 ).
In vitro assembly experiments uncovered important clues to mechanism of action. HAPs and PPAs speed up assembly and stabilize protein-protein interaction ( 162 , 164 – 166 ). HAPs, in concentrations that can saturate available HBcAg, lead to assembly of aberrant non-icosahedral complexes ( 165 – 167 ). On examination, HAPs favored formation of hexagonal repeats of core protein at the expense of fivefolds ( 165 ). Quantification of the effect of HAPs and PPAs on assembly shows that they strengthen dimer-dimer association energy, decreasing the pseudo-critical concentration of assembly from ca. 15μM down to ca. 30nM, and increasing the apparent rate of assembly by more than two orders of magnitude. Some of this effect may be due to simply filling a hydrophobic pocket – stabilizing intermediates will affect the observed rate of assembly as well as the yield of product ( 168 ). However, some the kinetic effect has been attributed to allosterically modulating HBcAg conformation; hence this molecules that drive assembly may be though of as Core protein Allosteric Modulators (CpAMs).
HAP1 led to substantial quaternary structural differences, an expansion at fivefold axes, when compared to an unliganded apo capsid structure ( 62 ). Any changes in tertiary structure were negligible at the observed 5Å resolution. CpAMs were found to wedge between dimers at the interfacial HAP pocket, which explained the strengthening of inter-dimer interactions. The site was made by a C-shaped four helix motif with helices 2, 4 and 5 forming a pocket and helix 5 from the interfacial monomer forming a cap over the pocket. Strong electron density was only observed in the pocket associated with C monomers that interfaced with a D monomer from a neighboring dimer; weak density was observed in the B pocket.
A crystal structure of capsid bound to AT130 showed similar quaternary structural changes but substantial tertiary structural changes in C and D monomers ( 127 ). Electron density for the CpAM was found in the HAP pocket at the B–C inter-dimer interface with weak density at the C–D interfacial pocket. The findings from this structure supported the hypothesis that CpAMs drive aggressive assembly towards misdirection by affecting the quaternary arrangement of dimers.
A third structure of capsid bound to HAP18, a derivative of HAP1, determined to 4Å resolution, surprisingly showed no quaternary structural changes and minimal changes in tertiary structure ( 169 ). HAP18 had been previously shown to affect assembly in vitro (leading to microns long 100 nm diameter tubes) and in vivo ( 162 ). Equally strong HAP18 density in both B–C and C–D sites though there were substantial differences between the different binding modes. Thus, HBV capsids have diverse structures and structural responses to CpAMs.
Efforts to obtain higher resolution structures have been based on the assembly-deficient Y132A mutant, with an apo-structure determined to 2.25Å resolution. The structure of a HAP1 derivative (NVR10-001E2) soaked into crystals of the mutant was determined to 1.95Å ( 43 ). The CpAM bound in HAP pocket in a similar orientation as CpAMs observed in capsids. However, the lack of a capsid-like environment and quasi-equivalence in the quaternary arrangement of the monomers in this structure limit its use in rational drug design, despite the resolution. The structure, however, does serve to validate the orientation of similar molecules in other low-resolution structures.
The range of quaternary structure changes in the presence and absence of CpAMs suggest that the altering the quaternary structure may not be a basis for CpAM mechanism. Changes in structure, rates of crystallization, effects of CpAM-emulating mutants ( 100 , 101 ), the effect of antibodies damping global HD exchange ( 170 ) – the variety of structural responses – leads to the hypothesis there may be dynamic basis to CpAM activity. Damping capsid dynamics as an antiviral mechanism has been demonstrated previously in studies with picornaviruses ( 171 , 172 ). This would have important implications in future CpAM design. For HBV this would also have larger implications for the mechanism of CpAM action in the viral life cycle because affecting dynamics would affect the role of the capsid in processes upstream and downstream of assembly.
Closing comments
There is no known cure for HBV infection. Eliminating or silencing the cccDNA in cells is essential to clearing infection permanently. Direct acting antivirals may be the key to cccDNA. The virus capsid is one such target. Reverse transcriptase is the primary target for available antiviral therapeutics, however even the best of these methods lead to only about 5% clearance of infection after five years of treatment ( 51 ). Clearly other targets are needed, perhaps in combination. The RNaseH domain of the reverse transcriptase is an independent and unutilized target with opportunity for synergism ( 96 ). Another direct target is the cellular entry process which has shown its value in protecting liver transplants ( 118 , 119 ). The virus may also be blocked by interfering RNAs that block viral products ( 174 ). Recent advances indicate that HBV X protein has a direct role in preventing the host cell from silencing cccDNA, but do not suggest a mechanism for targeting it ( 175 ).
HBV is one of the smallest human pathogens, based on a 3200 bp genome encoding only four open reading frames. Yet structurally the virus shows a remarkable diversity of structural features, often with the same proteins. In part this is the parsimony of viruses, where a minimal number of proteins performs a wide variety of functions. However, a more important them is that weak interactions between components and irregular interactions between components lead to a highly dynamic system. In HBV this is manifested as a virion where the envelope proteins have multiple structures, the envelope capsid interaction is irregular, the capsid is a dynamic metabolic compartment that actively participates in structural changes to its encapsidated genome. Thus, direct acting antivirals have a limited repertoire of targets, but have the opportunity to be highly specific.

CpAMs alter the structure and dynamics of the capsid. (a) Overlays of CpAM bound capsids (magenta) on apo-capsid (cyan) when viewed down the fivefold reveal systematic differences in capsid structure in the exterior (upper panels) and interior (lower panels). This figure reproduced with permission from Venkatakrishnan et al. (b) Superpositions of Cα traces of individual dimers from CpAM bound structures (red) on the apo structures (grey).
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Tools and Resources
- Clinical Overview
- Clinical Signs and Symptoms
- Clinical Testing and Screening
- Clinical Care and Treatment
- Guidelines for Health Care Personnel Exposed to Hepatitis C Virus
Related Topics:
- Show All Home
- Viral Hepatitis
- Hepatitis A
- Hepatitis B
- Viral Hepatitis Statistics & Surveillance
Clinical Signs and Symptoms of Hepatitis C
- Many people with acute or chronic hepatitis C don't look or feel sick and therefore don't know they are infected.
- Most people with chronic hepatitis C will not have specific symptoms for 20 years or more.
- If a patient does have symptoms, they are usually non-specific complaints like fatigue or depression.
- Without treatment, chronic hepatitis C infection can progress to chronic liver disease.

Disease presentation
Most often, clinicians will not recognize hepatitis C virus (HCV) infection until the patient is tested.
Without early diagnosis and treatment, patients with hepatitis C can develop chronic liver disease, which can range from mild to severe. The disease can progress silently and slowly over several decades. In some cases, a routine examination of the patient may show elevated alanine aminotransferase (ALT) enzyme levels either during acute or advanced HCV infection.
For the public
If symptoms of acute HCV infection do occur, they can include:
- Abdominal pain, nausea, and/or vomiting
- Dark urine or clay-colored stools
- Loss of appetite
For those who do develop symptoms, they typically appear 2–12 weeks after exposure, but the window can range from 2–26 weeks. 1 2
Symptoms of chronic HCV infection
Most people with chronic HCV infection experience non-specific symptoms — such as chronic fatigue and depression — or have no symptoms at all. Many eventually develop severe chronic liver disease, including cirrhosis and liver cancer.
Extrahepatic manifestations
Some patients can develop medical conditions from HCV infection not related to the liver. These include:
- Diabetes mellitus
- Glomerulonephritis
- Essential mixed cryoglobulinemia
- Porphyria cutanea tarda
- Non-Hodgkin's lymphoma
Clinical assessment
CDC recommends universal HCV screening for all adults 18 and older at least once in a lifetime and all pregnant people during each pregnancy, except in settings where the prevalence of HCV infection is less than 0.1%.
Any person who requests hepatitis C testing should receive it, regardless of disclosure of risk, because many persons may be reluctant to disclose stigmatizing risks.
CDC also recommends routine periodic testing for people with ongoing risk factors including:
- People who inject drugs.
- People who share needles, syringes, etc.
- People who have received maintenance hemodialysis.
Clinicians should test people with risk factors regardless of setting prevalence.
For detailed guidance on testing and determining hepatitis C prevalence, keep reading .
- National Institute of Diabetes and Digestive and Kidney Diseases. Definition and facts of liver transplant. Available at: https://www.niddk.nih.gov/health-information/liver-disease/liver-transplant/definition-facts .
- Organ Procurement and Transplantation Network. Health Resources and Services Administration, US Department of Health and Human Services. National data website. Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/build-advanced .
Hepatitis C
Hepatitis C is a liver infection caused by the hepatitis C virus (HCV). HCV information for both the public and health professionals.
For Everyone
Health care providers.

IMAGES
VIDEO
COMMENTS
Hepatitis B is a serious liver infection caused by the hepatitis B virus (HBV). For most people, hepatitis B is short term, also called acute, and lasts less than six months. But for others, the infection becomes chronic, meaning it lasts more than six months.
Hepatitis B is a vaccine-preventable liver infection caused by HBV. HBV is transmitted when blood, semen, or another body fluid from a person infected with the virus enters the body of someone who is uninfected. Hepatitis B can range from a mild, short-term, acute illness lasting a few weeks to a serious, long-term, chronic infection.
CHARACTERISTICS OF THE VIRUS. Hepatitis B virus belongs to the family of hepadnaviruses, which include duck hepatitis virus, woodchuck hepatitis virus, and ground squirrel hepatitis virus. The complete virion or Dane particle is 42 nm in diameter. It consists of: An envelope composed of viral-encoded proteins and host-derived lipid components.
Hepatitis B is an infection of the liver caused by the hepatitis B virus. The infection can be acute (short and severe) or chronic (long term). Hepatitis B can cause a chronic infection and puts people at high risk of death from cirrhosis and liver cancer. It can spread through contact with infected body fluids like blood, saliva, vaginal ...
Hepatitis B virus belongs to the family of hepadnaviruses, which include duck hepatitis virus, woodchuck hepatitis virus, and ground squirrel hepatitis virus. The complete virion or Dane particle is 42 nm in diameter. It consists of: An envelope composed of viral-encoded proteins and host-derived lipid components.
Hepatitis B is a viral infection that affects your liver. It causes inflammation in your liver tissues, which is what " hepatitis " means. It begins as an acute infection that's usually short-lived. But in some people, it turns into a chronic infection that never goes away. Long-term inflammation does serious damage to your liver over time.
Hepatitis B is a liver infection caused by the hepatitis B virus (HBV). HBV infection causes inflammation of the liver. When the liver is inflamed or damaged, its function can be affected. The best way to prevent HBV infection is by getting vaccinated. Safe and effective vaccines are available and covered as a preventive service by most health ...
Hepatitis B can be a serious liver disease that results from infection with the Hepatitis B virus. Acute Hepatitis B refers to a short-term infection that occurs within the first 6 months after someone is infected with the virus. The infection can range in severity from a mild illness with few or no symptoms to a serious condition requiring ...
Hepatitis B virus is classified in the genus Orthohepadnavirus, which contains 11 other species. [3] The genus is classified as part of the Hepadnaviridae family, which contains four other genera, Avihepadnavirus, Herpetohepadnavirus, Metahepadnavirus and Parahepadnavirus. [3] This family of viruses is the only member of the viral order ...
Hepatitis B viral infection is a serious global healthcare problem. It is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). It is often transmitted via body fluids like blood, semen, and vaginal secretions. The majority (more than 95%) of immunocompetent adults infected with HBV can clear the infection spontaneously. Patients can present with acute ...
Hepatitis B is an infectious disease caused by the Hepatitis B virus (HBV) that affects the liver; it is a type of viral hepatitis. It can cause both acute and chronic infection.. Many people have no symptoms during an initial infection. For others, symptoms may appear 30 to 180 days after becoming infected and can include a rapid onset of sickness with nausea, vomiting, yellowish skin ...
Hepatitis B. Hepatitis is defined as inflammation of the liver. Viral hepatitis is one of the various forms of hepatitis and refers to infections caused by viruses that affect the liver. Viral hepatitis includes five distinct diseases, caused by five different viruses. The different viruses are each called by a letter name:
Hepatitis B Clinical Features. Incubation period 60 to 90 days. Clinical signs and symptoms more common in adults. Prodromal phase lasts 3 to 10 days; abrupt onset of fever, malaise, anorexia, nausea, abdominal discomfort, and dark urine before jaundice.
Studies to identify the characteristics of patients at risk have been limited by the long duration of follow-up required. ... The hepatitis B vaccine should be given to all HIV-positive persons who are negative for HBV seromarkers. The vaccine should be given when CD4 cell counts are ≥200/μL, as response is poor below this level.
The hepatitis B virus (HBV) is commonly transmitted via body fluids such as blood, semen, and vaginal secretions. [ 1] The hematoxylin and eosin (H&E) stain below depicts "ground-glass" cells seen in approximately 50-75% of livers affected by chronic HBV infection. Hepatitis B. Under higher-power magnification, ground-glass cells may be visible ...
Abstract. Hepatitis B virus (HBV) is a non-cytopathic, hepatotropic virus with the potential to cause a persistent infection, ultimately leading to cirrhosis and hepatocellular carcinoma. Over the ...
Hepatitis B virus (HBV) infects more than 300 million people worldwide and is a common cause of liver disease and liver cancer. HBV, a member of the Hepadnaviridae family, is a small DNA virus with unusual features similar to retroviruses. HBV replicates through an RNA intermediate and can integrate into the host genome.
Hepatitis C virus (HCV) and hepatitis B virus (HBV) are vastly different viruses. Hepatitis B is highly contagious through sex, using drugs with shared straws and needles, blood transfusions, and even saliva, which can put people living in the same household at risk. The good news is hepatitis B is entirely preventable with a vaccine, which has ...
Type C hepatitis B-related acute-on-chronic liver failure (HBV-ACLF), which is based on decompensated cirrhosis, has different laboratory tests, precipitating events, organ failure and clinical outcomes. The predictors of prognosis for type C HBV-ACLF patients are different from those for other subgroups. This study aimed to construct a novel, short-term prognostic score that applied ...
Hepatitis means inflammation of the liver. The liver is a vital organ that processes nutrients, filters the blood, and fights infections. When the liver is inflamed or damaged, its function can be affected. Heavy alcohol use, toxins, some medications, and certain medical conditions can cause hepatitis. However, hepatitis is often caused by a virus.
Hepatitis B virus (HBV) is a hepatotropic virus and an important human pathogen. There are an estimated 296 million people in the world that are chronically infected by this virus, and many of them will develop severe liver diseases including hepatitis, cirrhosis and hepatocellular carcinoma (HCC). HBV is a small DNA virus that replicates via ...
Background The role of neutrophils in hepatitis B virus (HBV) infection has been a subject of debate due to their involvement in antiviral responses and immune regulation. This study aimed to elucidate the neutrophil characteristics in patients with chronic hepatitis B (CHB). Methods Through flow cytometry and ribonucleic acid-sequencing analysis, the phenotypes and counts of neutrophils were ...
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death, worldwide. The predominant causative factor for HCC is hepatitis B virus (HBV) infection. We conducted a meta-analysis to estimate the efficacy and safety of PD-1/PD-L1 inhibitors combined with anti-angiogenic therapy for the first-line treatment of the unresectable HCC and to evaluate the benefits of different ...
Viral hepatitis has emerged as a major public health problem throughout the world affecting several hundreds of millions of people. Viral hepatitis is a cause of considerable morbidity and mortality in the human population, both from acute infection and chronic sequelae which include, in the case of hepatitis B, C and D, chronic active hepatitis and cirrhosis.
This is backed by the finding that 1301B7 utilizes a VH1-69 heavy chain, which has been demonstrated to be produced in response to diverse viruses, such as influenza, hepatitis, HIV-1, and SARS-CoV-2.
HBV virions are known as Dane particles. These early studies identified the salient features of the HBV virion and its epidemiology. The virus is outwardly simple but endemic and persistent. HBV is predominantly a virus of Southeast Asia, sub-Saharan Africa, and populations such as aboriginal Australians and Inuit.
If symptoms of acute HCV infection do occur, they can include: Abdominal pain, nausea, and/or vomiting. Dark urine or clay-colored stools. Fatigue. Fever. Jaundice. Joint pain. Loss of appetite. For those who do develop symptoms, they typically appear 2-12 weeks after exposure, but the window can range from 2-26 weeks. 1 2.