How to get through the MPharm dissertation

The MPharm dissertation is one of the longest pieces of writing we create as pharmacy students. I remember feeling quite anxious about it all. I wasn’t too sure how I was going to write 10,000 words on a topic which at the time I had no idea about, however once I got into it, I really enjoyed writing my dissertation.
The dissertation is usually a lengthy process composed of weeks filled with intense research, several draft versions, blog entries, and supervisory meetings. I was fortunate enough to get a research project in the area of pharmacy education. My project focused on student learning in integrated pharmacy curricular. At the time of receiving my title I wasn’t sure what to expect, but I was intrigued to see what developed once I started. I began my dissertation by conducting a literature search which allowed me to access the plethora of literature available within the field of pharmacy education. I made sure my literature search was as specific as possible to ensure the papers were relevant and accurate for the research topic. I remember when I would hear or read ‘buzz words’ like ‘integrated’ or ‘curriculum’ my ears would crop up and my eyes would become fixed on the particular article. It was also ironic that during the end of my dissertation the GPhC consultation on the initial education and training standards for pharmacists began. However, the MPharm dissertation is not always plain sailing and everyone’s experience varies, here I share four-tips which helped me get through the MPharm dissertation. I hope they will be useful for MPharm students starting their dissertation.
Firstly, organisation. Dissertation deadlines may seem like a long time away, but make sure you are aware of your deadline and start planning in advance. Within a qualitative research project, you may need to conduct interviews or focus groups with participants, and therefore ethical approval is necessary before you start data collection. This process can sometimes take a couple of weeks and slightly delay data collection, so it’s important to account for this. The best way I found to organise my time was by making lists and drafting a timeline or timetable. Aim to plan each week and write down what you want to get out from that week. It’s important to have a clear and coherent plan to keep on top of things, just make sure you know what your aiming for each week. Don’t worry too much if you don’t meet your plan or something else comes in the way that’s normal, but just getting into a habit of planning is really good. You can use post-it notes, diaries or the reminders app on your phone to maintain your progress.
Secondly, don’t panic. This is sometimes easier said than done. The dissertation can sometimes be stressful so try and be enthusiastic and remain positive. Speak to your peers, supervisor or module co-ordinator if you’re feeling overwhelmed, they will be more than happy to re-assure you that everything’s ok. There are also organisations like pharmacist support who can help you if you’re feeling like you just need to speak to someone anonymously.
Thirdly, work hard play hard. Make sure you give yourself at least one day of the week where you don’t think about your dissertation. For me personally this was Friday. I used this time to go out and see friends and visit the new restaurants and cafes that had opened in Newcastle. This was a great way for me to catch up with all my university friends who I would rarely see during the week. This becomes more important nearer to the end of your dissertation, as you will notice your stress levels increase, and you get a lot of encouragement from your peers to keep soldiering on.
Lastly, Enjoy it! Although the dissertation can sometimes feel overwhelming, the skills you learn from the MPharm dissertation are invaluable and the process is a real learning curve. You definitely strengthen your friendship groups during this time but also build good rapport with your supervisor. Once you have your dissertation printed and bound in your hands the surge of pride and achievement is just indescribable, and you soon realise that the good times at university are coming to an end.
About the author:
Ausaf Hayat Khan, recently completed his MPharm at Newcastle university. He will soon be pursuing his preregistration training within the hospital sector and will be presenting his dissertation at the 10th Biennial Monash Pharmacy Education Symposium 2019.

You may also be interested in
Artificial intelligence: act or be acted upon — staying relevant in the ai era, equality, diversity and inclusion: why true allyship must shift words into action, pharmacy needs to be at the forefront of shaping digital health — but how do we get there.
Login to your account
If you don't remember your password, you can reset it by entering your email address and clicking the Reset Password button. You will then receive an email that contains a secure link for resetting your password
If the address matches a valid account an email will be sent to __email__ with instructions for resetting your password
- AACP Member Login Submit
Current Issue Links
Articles in press.
- Past Issues
Submit Mobile
- Submit article
- Editorial Board
- Editorial Team
- Submit Article
- Aims and Scope
- For Authors
- Supports Open Access
- Events
- AACP
- Join AACP
Welcome to the new website for the American Journal of Pharmaceutical Education , the official journal of the American Association of Colleges of Pharmacy (AACP)! All current and archived articles back to Volume 72 are available here. Check our FAQ for further information.
Issue Highlights
A common language for competency-based education., an ongoing process: developing competency-based pharmacy education (cbpe) in the us.
A Cycle of Precepting Challenges and Ideas for Action in Experiential Settings
A cross-sectional study of vaccination-related education in pharmacy programs in the middle east, american journal of pharmaceutical education.

The American Journal of Pharmaceutical Education is an official scholarly publication of the American Association of Colleges of Pharmacy (AACP) . Its purpose is to document and advance pharmaceutical education in the United States and internationally. More

Most Read (Last 30 Days)
- Access for Developing Countries
- Articles & Issues
- Articles In Press
- Current Issue
- Journal Information
- About Open Access
- Aims & Scope
- History of AJPE
- Contact Information
- For Authors
- Guide for Authors
- Researcher Academy
- Rights & Permissions
- Submission Process
- Submit Article
- For Reviewers
- Reviewer Instructions
- Reviewer Frequently Asked Questions
The content on this site is intended for healthcare professionals.
- Privacy Policy
- Terms and Conditions
- Accessibility
- Help & Contact

Session Timeout (2:00)
Your session will expire shortly. If you are still working, click the ‘Keep Me Logged In’ button below. If you do not respond within the next minute, you will be automatically logged out.
- Search Menu
- Advance Articles
- Themed Collections
- Supplements
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- Call for Papers
- About International Journal of Pharmacy Practice
- About the Royal Pharmaceutical Society
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, declarations, acknowledgements.
- < Previous
Undergraduate pharmacy students’ perceptions of research in general and attitudes towards pharmacy practice research
- Article contents
- Figures & tables
- Supplementary Data
Vicky S Kritikos, Steven Carter, Rebekah J Moles, Ines Krass, Undergraduate pharmacy students’ perceptions of research in general and attitudes towards pharmacy practice research, International Journal of Pharmacy Practice , Volume 21, Issue 3, June 2013, Pages 192–201, https://doi.org/10.1111/j.2042-7174.2012.00241.x
- Permissions Icon Permissions
To investigate pharmacy students’ perceptions of research in general and attitudes towards pharmacy practice research (PPR) at each stage of the undergraduate programme and determine any relationship between perceptions and attitudes, and to validate a measure of attitudes towards PPR in an Australian cohort of pharmacy students.
A 23-item survey was administered to all students enrolled in each year of the 4-year pharmacy undergraduate programme, University of Sydney, Australia. Perceptions of research in general were measured with four items on a five-point semantic-differential scale and attitudes towards PPR with19 items on a five-point Likert scale.
In total 853 students responded to the survey (83% response rate). While students perceived research to be necessary, they found it difficult and were divided in their interests in pursuing research. Attitudes towards PPR were assessed within five identified domains: ‘role of PPR in the curriculum’, ‘engaging in PPR activities’, ‘confidence to do PPR’, ‘faculty involvement of students in PPR’ and ‘role of PPR in the profession’. Most participants agreed that PPR played an important part in the profession and curriculum but almost half of the cohort lacked confidence to undertake PPR, with very few holding positive attitudes towards all five domains. The PPR instrument was found to be valid and reliable. There were significant differences in perceptions and attitudes at various stages of the degree.
Future research should investigate changes in perceptions and attitudes in a single cohort over the 4-year degree, explore factors influencing attitudes and identify strategies for stimulating research interest.
Evidence-based practice is the new paradigm in healthcare professions. [ 1 ] Over the past two decades, the profession of pharmacy and the scope of its professional practice has undergone major changes. Today, pharmacists have extended their roles beyond the traditional services of dispensing and compounding, [ 2 ] to offer patient-centred cognitive services in both community pharmacy and hospital settings often in collaboration with other healthcare professionals. [ 3 ] These services range from patient education and counselling, medication and lifestyle management, health promotion, screening and prevention and chronic disease state management. [ 3–5 ] The changes in practice have come about in response to a number of drivers including increased complexity of medication regimens and the prevalence of preventable drug-related problems, [ 6 ] pressure on existing health services due to an ageing population, escalating costs associated with chronic and highly complex diseases and the pace of technological change. [ 7–9 ] With the introduction of new professional services, especially those involving government funding, there is a need to demonstrate evidence of clinical efficacy and cost-effectiveness.
Practice research plays a pivotal role in the development and establishment of a solid evidence base for potential new services and practices, and can both inform new policy and establish the value and/or feasibility of new roles and practices. [ 9 ] Only practices that are supported by a solid evidence base will be accepted by other healthcare providers and supported by consumers, who are becoming more informed and demanding of healthcare services. [ 9 ] Moreover, practice research is an essential driver for development and innovation in pharmacy professional practice. This requires the active participation of community pharmacists. At present, the level of involvement of community pharmacists in practice research is very limited and their reluctance to engage with healthcare researchers in new empirical studies has been reported. [ 10–13 ] Those practitioners who have engaged in research have been shown to have a particular interest in research, a belief that research is important and that it will benefit the patient. [ 10–13 ]
To sustain up-to-date clinical knowledge and keep professional practice at the cutting edge, a strong cohort of healthcare researchers is needed to serve as a critical link between high-quality research and innovations in healthcare practice. Trained jointly in patient care and research by academic educators, healthcare researchers perform research from a unique perspective and are a key component of evidence-based practice. In the field of pharmacy practice, a shortage of qualified academic educators projected by pharmacy schools in Australia and other countries could pose problems to the advancement of the profession. [ 14–16 ]
A profession's attitudes and values are formed by influences during undergraduate education and subsequent professional experience through socialisation. [ 17 , 18 ] Socialisation is a process whereby individuals selectively acquire not only the knowledge and skills but also the beliefs, perceptions, behaviours and values of their profession. [ 17 ] This is an ongoing process, which can occur via two mechanisms: professional socialisation (lay perspectives and knowledge are transformed into professional perspectives and knowledge) and developmental socialisation (professional perspectives mature and continue to develop). [ 18 ] Professional socialisation is the process whereby students learn about their professional role and the expectation of performance in that role. [ 2 , 19 ] The process is influenced by social interactions with faculty members, preceptors, peers, practitioner role models and other healthcare professionals, which shape students’ attitudes, perceptions and values. [ 17 ] Since professional socialisation is influenced by academic interactions and practice experiences, pharmacy education is an integral component of the process.
Pharmacy education has evolved rapidly worldwide in the last decade, with significant changes to the education curricula, pedagogical approaches and modes of delivery. [ 16 , 20–22 ] The latest revision to the mission of pharmacy education holds academic programmes responsible ‘to prepare graduates to conduct research and scholarly activity’. [ 23 ] In Australia at the University of Sydney, the goal of the Faculty of Pharmacy is to optimise the undergraduate learning experience of its students and to engage in research-led teaching throughout the undergraduate programme. A particular focus of the pharmacy practice discipline is to raise awareness about practice-based research conducted in the faculty at various stages throughout the 4-year undergraduate programme. In terms of research exposure, a limited number of opportunities are available for second and third-year students to engage in actual practice-based research activities through the summer vacation programme, and for selected fourth-year students to undertake a research training module and complete a research project through the honours programme. Students are also introduced to aspects of practice research in lectures throughout the pharmacy practice curriculum, although the research is infrequently an explicit focus. Given the key role pharmacy practice research (PPR) plays in evidence-based health care and advancing the profession, developing a cohort of students willing to embrace the challenge of practice-based research is important.
Numerous studies investigating students’ attitudes towards research have been published in the medical, nursing, physical therapy, occupational therapy, chiropractic and other allied health occupational literature. [ 24–32 ] Although pharmacy students’ attitudes towards postgraduate research have been investigated in the past, these early studies were conducted at a time when postgraduate education mainly involved laboratory-based research in pharmaceutics, pharmacology or medicinal chemistry. [ 33–36 ]
To date, no published study has investigated the attitudes of pharmacy students towards PPR at each stage of the undergraduate programme. This study coincided with the faculty's review of the undergraduate curriculum and aimed to gauge student views about research in general and PPR specifically. The specific objectives of this study were to: (1) investigate pharmacy students’ perceptions of research in general and their attitudes towards PPR at each stage of the undergraduate programme and determine any relationship between perceptions and attitudes; (2) validate a measure of attitudes towards PPR in an Australian cohort of pharmacy students. Future changes to the undergraduate curriculum can be underpinned by these research findings.
The study utilised a cross-sectional design and was carried out in October 2009. Ethics approval was obtained from the University of Sydney Human Research Ethics Committee prior to commencement of this study. All students enrolled in each year of the 4-year pharmacy undergraduate programme at the University of Sydney in Australia were invited to complete a voluntary, anonymous survey in the final week of the semester during tutorial time. Completion of the survey instrument implied informed consent.
The survey instrument
A 23-item survey instrument was used to assess students’ perceptions of research (or research-related activities) in general and attitudes towards PPR. This instrument was adapted from a measure originally developed by Winans and Madhavan [ 36 ] and included an additional item which assessed respondents’ attitudes towards the inclusion of PPR in experiential placements in community and hospital pharmacy. The survey comprised three sections:
Section 1 (perceptions of research in general): Students were asked to indicate their perceptions of research (or research-related activities) in general using four semantic-differential scales formed by bipolar adjectives. The bipolar adjectives anchoring a five-point scale from 0 to 4 were: (1) difficult–easy, (2) unnecessary–necessary, (3) repelling–attractive, (4) boring–attractive, where a rating of 2 was neutral.
Section 2 (attitudes towards PPR): Included a definition of PPR and contained 19 items covering students’ views about: engaging in PPR activities; their confidence in their abilities and knowledge related to understanding and/or conducting PPR; their intention to engage in PPR activities; the role of PPR in the profession; the inclusion of PPR in the undergraduate curriculum, externships, clinical placements; and faculty involvement of students in PPR, with responses on a five-point Likert scale (0 = strongly disagree, 2 = neutral and 4 = strongly agree).
Section 3 (student characteristics): Contained 15 questions covering age, gender, nationality, marital status, educational funding loan status, scholarship status and whether or not the respondent had: (1) a previous degree or intended to pursue another degree or a postgraduate research degree after graduation; (2) exposure to or involvement in any research during their education; (3) a mentor, a family member, or a friend involved in research; (4) an intention to practice pharmacy after completing their graduate year.
Data analysis
All data collected were analysed using Predictive Analytics SoftWare (PASW) Statistics (version 18) and AMOS software for confirmatory factor analyses. The factor structure of the attitudinal instrument (i.e. attitudes towards PPR – 19 items in Section 2) was initially investigated using exploratory factor analysis, using principal component extraction and varimax rotation. Assessment of the instrument's factor structure was considered necessary in view of supporting evidence for Winans and Madhavan's original five-factor solution. [ 36 ] The present study used additional criteria for the number of factors to be extracted, in order to more rigorously explore the instrument's factor structure, including the size of the eigenvalues, number of steps in the scree plot, and the proportion of total variance explained. Items that had poor factor loadings (<0.30) or cross-loaded on two or more factors were removed. [ 37 ] Reliability analyses of derived subscales were conducted using Cronbach's α.
Following exploratory factor analysis, the educed factor structure was formally evaluated using confirmatory factor analysis with maximum likelihood estimation. Model fit statistics for confirmatory analysis included: the χ 2 statistic, normal fit index (NFI), Tucker–Lewis index (TLI), and root mean square error of approximation (RMSEA), since these are the most frequently emphasised fit statistics. [ 38 ] Discriminant validity of the latent constructs was assessed through the use of the variance extracted test. [ 39 ] In this technique each pair of constructs within the model was compared. Discriminant validity was demonstrated if the variance extracted estimates for each construct were greater than the squared correlation between the constructs. To demonstrate that the factor structure was consistent over the 4 years of study, the technique of factorial invariance was employed. In this procedure, the χ 2 statistic of the model from each year of study was compared with the χ 2 statistic of a model of the combined group in which the measurement weights had been constrained to be equal across groups. A χ 2 statistic with P > 0.05 indicated a consistent pattern of measurement weights across groups. [ 40 ]
For all outcome variables, normality tests were conducted using the Kolmogorov–Smirnov test. For correlations, scatterplots were examined to ensure no violation of normality, linearity and homoscedasticity. Mean factor scores were calculated by summing responses for individual items within a factor and dividing by the number of items included in that factor. In addition to descriptive statistics, the Spearman's correlations were performed to examine the relationship between two outcome variables that were not normally distributed. Comparisons between independent groups were conducted using a one-way analysis of variance for normally distributed variables and the Kruskal–Wallis test and the Mann–Whitney U -test for variables that were not normally distributed. Factor scores derived by the regression method were used for correlations and group comparisons instead of mean factor scores. Proportional data were analysed using the χ 2 test. A two-tailed significance level of 0.05 was used for all analyses.
In total, 853 subjects were included in the survey. The overall response rate for undergraduates was 83% (853/1033): 84% (238/284) of year one, 83% (198/239) of year two, 80% (212/264) of year three and 83% (205/246) of year four.
Characteristics of respondents
The demographic and educational characteristics of the respondents in the total sample, and at each stage of the pharmacy programme, are summarised in Table 1 . At each stage of the degree, a significantly higher proportion of students had been exposed to research ( P < 0.001), had a mentor ( P < 0.001) or friends involved in research ( P < 0.001). In contrast to this, a significantly smaller proportion of students intended to pursue a postgraduate research degree ( P < 0.01) as they progressed through the degree course. Apart from age, there were no other significant differences in characteristics between the four cohorts.
Demographic and educational characteristics of respondents
BPharm, Bachelor of Pharmacy degree; SD, standard deviation.
Students’ perceptions of research in general (or research-related activities)
Mean scores on the ‘perceptions of research in general’ bipolar scales for the total samples are shown in Table 2 . Overall, 76% of students considered research necessary (18% neutral). In contrast, 45% found it difficult (34% neutral), only 31% found it attractive (41% neutral) and 36% found it interesting (32% neutral).
Mean scores on the ‘perceptions of research in general’ bipolar scales for the total sample ( n = 853)
Each pair of bipolar adjectives was used to anchor the ends of a five-point semantic-differential scale from 0 to 4, where a rating of 2 was neutral. Students were asked to respond to the question: ‘Please indicate using the following scales, how you feel toward research (or research related activities) in general. For each of the bipolar scales, circle the number which best represents your view.’
Exploratory factor analysis
In the exploratory factor analysis (EFA) of the 19-item PPR scale, three items: item five (factor loadings of 0.35 and 0.610); item six (factor loadings of 0.64 and 0.40); and item seven (factor loadings of 0.74 and 0.35) were removed due to cross-loading on two factors. For the 16 items, factor analysis yielded five primary factors with eigenvalues greater than unity, accounting for 65% of the total variance ( Table 3 ). Factor 1 was interpreted as ‘inclusion of PPR in the curriculum’; Factor 2 ‘engaging in PPR activities’; Factor 3 ‘confidence in abilities to do PPR’; Factor 4 ‘faculty involvement of students in PPR’ and Factor 5 ‘role of PPR in the profession’. Reliability analysis of the 16-item: ‘attitudes to PPR’ scale returned a Cronbach's α coefficient of 0.79, indicating homogeneity of items and good internal consistency ( Table 3 ).
Principal component estimates of the factor loadings for the ‘attitudes to PPR’ scale ( n = 853)
Varimax rotation used. PPR, pharmacy practice research.
Confirmatory factor analysis
The five-factor solution identified in EFA was formally evaluated using confirmatory factor analysis using the factors derived from the EFA. Model fit statistics indicated good fit for the data: the χ 2 statistic = 199.5 df = 95; P < 0.001; NFI = 0.95; TLI = 0.97; RMSEA (90% confidence intervals) = 0.036 (0.029, 0.043); and pclose = 1.00. All standardised factor loadings were statistically significant at the level P < 0.001 indicating convergent validity ( Table 4 ). Discriminant validity was affirmed by the variance extracted test. Factorial invariance of measurement weights across all 4 years of study was supported by four χ 2 differences tests with P > 0.05.
Factor loadings and measurement errors for completely standardised confirmatory factor analysis of the five-factor model of the ‘attitudes to PPR’ scale
PPR, pharmacy practice research; SE, standard error of unstandardised regression weights; SMC, squared multiple correlation; SRW, standardised regression weights; URW, unstandardised regression weights.
Students’ attitudes towards PPR
Mean factor scores on the ‘attitudes to PPR’ scale for the total sample are presented in Table 5 . Overall, the majority of students (86%) viewed PPR as an important part of the pharmacy profession (11% neutral), 71% agreed to the inclusion of PPR in the curriculum (11% neutral) and 67% felt that faculty involvement of students in PPR activities was satisfactory (15% neutral). In contrast, 55% enjoyed engaging in PPR activities (14% neutral) and 48% felt confident in their ability to carry out PPR (17% neutral). Overall, only 10% held positive views towards all five domains.
Mean factor scores on the ‘attitudes to PPR’ scale for the total sample ( n = 853)
Mean score of each item underlying the derived factor on a five-point Likert scale (0 = strongly disagree and 4 = strongly agree). PPR, pharmacy practice research; SD, standard deviation.
Relationship between students’ perceptions of research in general and attitudes towards PPR
Correlations between mean scores on the ‘perceptions of research in general’ bipolar scales and factor scores on the ‘attitudes to PPR’ scale for the total sample are shown in Table 6 . The perceived level of attractiveness of research was strongly associated with the level of interest in research. The more necessary research was considered to be, the more attractive and interesting it was perceived as being. There was a weak association between perceived level of the necessity of research in general and attitudes towards the role of PPR in the profession, and between perceived level of attractiveness of research or interest in research and attitudes towards the inclusion of PPR in the curriculum.
Correlations between mean scores on the ‘perceptions of research in general’ bipolar scales and factor scores on the ‘attitudes to PPR’ scale for the total sample ( n = 853)
P < 0.001. Spearman's ρ used to determine correlations between mean scores on the bipolar scales and factor scores derived by the regression method. PPR, pharmacy practice research. PPR, pharmacy practice research.
Perceptions of research in general at different stages of the pharmacy programme
Comparison of mean scores on the ‘unnecessary–necessary’ bipolar scale between individual groups showed that students in year three considered research more necessary than those in other years ( Table 7 ). There were no significant differences in mean scores on other bipolar scales between individual groups.
Mean scores on the ‘perceptions of research in general’ bipolar scales and factor scores on the ‘attitudes to PPR’ scale by curriculum year
Mean score of each item underlying the derived factor on a five-point Likert scale (0 = strongly disagree and 4 = strongly agree). b Kruskal–Wallis test used for group comparisons. c Factor scores derived by the regression method used for group comparisons. PPR, pharmacy practice research; SD, standard deviation.
Attitudes towards PPR at different stages of the pharmacy programme
Factor 1 scores (inclusion of PPR in the curriculum) were significantly more positive in years one and two than in other years ( P < 0.01); Factor 2 scores (engaging in PPR activities) were significantly more positive in year three than in years one and two ( P < 0.001); Factor 3 scores (confidence in abilities to do PPR) were significantly more positive in years two and four than in year three ( P < 0.05); and Factor 4 scores (faculty involvement of students in PPR) were significantly more positive in years two and three than in year one ( P < 0.05) ( Table 7 ). There were no significant differences in Factor 5 scores (role of PPR in the profession) between groups.
This study is the first to investigate pharmacy students’ perceptions of research in general and attitudes towards PPR, in particular across a 4-year pharmacy undergraduate programme. Students perceived research to be necessary, however they found it difficult and were divided in their opinions regarding the attractiveness of research and their interest in research. Attitudes towards PPR were assessed within five identified domains: ‘role of PPR in the curriculum’, ‘engaging in PPR activities’, ‘confidence in abilities to do PPR’, ‘faculty involvement of students in PPR’ and ‘role of PPR in the profession’. Most participants recognised the important role PPR played in the profession and curriculum but almost half of the cohort did not enjoy engaging in PPR and expressed a lack of confidence in their abilities to do PPR, with very few holding positive attitudes towards all five domains. The PPR instrument was found to be valid and reliable. Perceptions of research in general were inter-related and associated with attitudes towards two domains of PPR. There were significant differences in perceptions and attitudes at various stages of the degree.
The strengths of the study include the high response rate, the high internal consistency of responses, as indicated by the high Cronbach's α coefficient, and the high factor loadings for each of the identified factors. The sample was representative based on current national student population data [ 41 ] reflecting the increasing number of females entering the profession and the sample size was adequate for factor analysis and reliability analysis. The limitations of the study were associated with the cross-sectional design, as it did not allow us to infer causality. Furthermore, it only includes participation of students from one Australian undergraduate pharmacy programme; however, undergraduate pharmacy education throughout Australia is based on an indicative curriculum and national competency standards for pharmacists. [ 42 ] Thus, while there are some institutional variations, the education is essentially similar across the nation.
The perceptions of, and attitudes towards, research in general of the undergraduate pharmacy student cohort in this study are consistent with findings from a previous study using a similar instrument with fourth-year pharmacy students from the USA, which suggested that students found research difficult but necessary, and were ambivalent about the attractiveness of research and their interest in research. [ 36 ] Similar findings have also been reported by chiropractic undergraduates. [ 30 , 31 ] Among the most common reasons cited by pharmacy students for lack of interest in pursuing postgraduate research from previous studies were the ‘desire to practice pharmacy’, ‘desire not to spend additional time in pharmacy school’ and ‘no interest in a research career’. [ 35 , 36 ] A more recent survey assessing undergraduate students’ interest in postgraduate training and reasons for lack of interest in pursuing further studies, showed that almost 70% of respondents had not considered postgraduate training in a pharmacy-related field and the most common reason was a desire not to spend additional time for further study and a desire for patient contact and to practice pharmacy. [ 43 ]
Previous studies identifying factors that influence fourth-year pharmacy students’ perceptions and attitudes towards research in general suggest that students who had been involved in research projects and/or had mentors involved in research perceived research more favourably, had stronger intentions to do research, and were more confident in their abilities to research. [ 34 , 36 ] Given that only 39% of students indicated having been involved in research, our results suggest that perhaps the difficulty and ambivalence perceived by some students towards research may be perpetuated by insufficient ‘hands on’ exposure to research possibly owing to limited opportunities and resources available for research and could explain why a significantly smaller proportion of students intended to pursue a postgraduate research degree at each stage of the degree. This difficulty and ambivalence perceived by students during undergraduate education could have implications for their future professional practice. Pharmacists with an understanding of and an interest in research are more likely to endorse the importance of community research, while those who have not had experience of research are more likely to cite lack of time and remuneration as impediments to participation in research. [ 11 , 12 , 44 , 45 ]
This study showed that most participants recognised the important role PPR plays in the profession and curriculum but almost half of the cohort did not enjoy engaging in PPR activities and expressed a lack of confidence in their abilities to carry out PPR, with very few holding favourable attitudes towards all aspects of PPR. Possible explanations for lack of enjoyment, engagement and confidence expressed by students could relate to feeling intimidated by PPR, perceiving no value associated with research involvement, a belief that it is not part of ‘normal’ pharmacy practice and underestimation of their capabilities. Since students develop the ideology that underpins the profession and a ‘mind-set’ formed by an internalised set of attitudes and values regarding one's role through professional socialisation, a ‘mind-set’ formed by unfavourable attitudes towards PPR and undervalued capabilities of the profession has been shown to be a barrier to participation in practice-based research. [ 44 , 45 ] Given there is an identified need for practice-based research, [ 9 ] and the key role PPR plays in evidence-based health care and advancing the profession, unfavourable attitudes towards PPR nurtured during the professional socialisation process will have implications for the future of the profession. These findings will inform pharmacy educators in the development of structured programmes and/or strategies that cultivate more positive views, in light of the emphasis placed on PPR at the undergraduate level by the latest version (2010) of the National Competency Standards Framework for Pharmacists in Australia. [ 46 ]
An important contribution of our study has been the confirmation of the attitudinal instrument's factor structure in view of the evidence supporting Winans and Madhavan's original five-factor solution. [ 36 ] In that study, no justification for selection of a five-factor solution was presented. Details presented in the published study included only internal consistency assessments of the derived subscales and failed to include factor loadings and the proportion of variance explained, with the reader left to surmise that eigenvalues greater than unity was the most likely selection criterion to have been employed. The present study used additional criteria for the number of factors to be extracted, in order to more rigorously explore the instrument's factor structure. Implemented criteria include the size of the eigenvalues, number of steps in the scree plot, and the proportion of total variance explained. The five domains of the ‘attitudes to PPR’ scale are consistent with previous findings [ 36 ] and across all 4 years of the degree. The reliability ratings of the derived subscales were higher compared to previous ratings [ 36 ] and the reliability rating of the 16 items showed good internal consistency. Thus, a valid and reliable instrument is available (and accessed by contacting the authors) for other researchers to assess attitudes towards PPR within an undergraduate pharmacy student population in the Australian setting
An interesting finding from this study was that positive perceptions of research in general were inter-related and associated with positive attitudes towards some aspects of PPR. Promoting research as necessary, interesting, attractive and enjoyable during the early stages of the undergraduate programme could assist in cultivating positive perceptions and attitudes towards PPR and developing a cohort of students willing to embrace the challenge of practice-based research.
This study also explored the perceptions and attitudes of four sequential cohorts at different stages of the 4-year undergraduate programme. There was unequivocal agreement throughout all years that research in general was necessary and that PPR played an important role for the profession. Despite an increasing exposure to research, mentors or friends involved in research at each stage of the degree, students throughout all years continued to consider research difficult and remained ambivalent. Although significant differences were noted in students’ attitudes towards various aspects of PPR at different stages of the degree, our results differ from findings in previous research involving chiropractic students, which suggested that attitudes towards engaging in research, inclusion of research in the curriculum and confidence to do research increase significantly as students progressed through the course. This was thought to be a result of increasing exposure to research issues through the teaching of research modules and a general increase in knowledge of the professional and educational aspects of the profession. [ 30 ] It may be that insufficient exposure to PPR and its importance to professional innovation in pharmacy throughout the curriculum, limited opportunities to engage in research activities and a dearth of role models in the community of pharmacists engaged in research contribute to this ambivalence. It might also be the case that, as students in a professional degree programme approach graduation, they anticipate opportunities to enter the professional workforce and become economically independent and therefore lose interest in continuing their studies. A longitudinal study, which traces the same cohort over time, is necessary to clarify the true impact of progression through the new 4-year curriculum on students’ attitudes towards research in general and PPR specifically. Furthermore, curriculum changes incorporating structured programmes/strategies and more research-enhanced teaching may change student attitudes over time and should be monitored in the future.
In conclusion, the main contribution of this research is in understanding the perceptions and attitudes that undergraduate pharmacy students have of research, in particular PPR, and to make available a valid and reliable instrument for other researchers to assess attitudes towards PPR within an undergraduate student population in the Australian setting. The views expressed by participants suggest that they are not well-prepared to endorse the importance of community research or embrace the challenge of practice-based research in the near future. Structured programmes and strategies aimed at cultivating positive views must be developed and implemented, in light of the emphasis placed on PPR at the undergraduate level. Our investigations of students’ perceptions and attitudes have formed the basis for future work in this area, which will look at changes in perceptions and attitudes in a single cohort of students over the full 4 years of the degree. Further research into factors influencing students’ perceptions and attitudes towards PPR also needs to be investigated and strategies for stimulating research interest identified.
Conflict of interest
The Author(s) declare(s) that they have no conflicts of interest to disclose.
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors wish to thank all the undergraduate pharmacy students who participated in this study and Connie Van for her assistance with data analyses.
Sackett DL . Evidence-Based Medicine. How to Practice and Teach EBM , 2nd edn. New York : Churchill Livingstone , 1997 .
Google Scholar
Google Preview
Holland RW , Nimmo CM . Transitions, part 1: beyond pharmaceutical care . Am J Health Syst Pharm 1999 ; 56 : 1758 – 1764 .
Roberts AS et al. Implementing cognitive services in community pharmacy: a review of models and frameworks for change . Int J Pharm Pract 2006 ; 14 : 105 – 113 .
Tsuyuki RT , Schindel TJ . Changing pharmacy practice: the leadership challenge . CPJ/RPC 2008 ; 141 : 174 – 180 .
Reid LD , Posey LM . The changing face of pharmacy . J Am Pharm Assoc 2006 ; 46 : 320 – 321 .
Roughead EE . The nature and extent of drug-related hospitalisations in Australia . J Qual Clin Pract 1999 ; 19 : 19 – 22 .
The Pharmaceutical Society of Australia . Issues Paper on the Future of Pharmacy in Australia . Canberra : PSA , 2010 .
The Pharmacy Guild of Australia . The Roadmap: The Strategic Direction for Community Pharmacy . Canberra : PGA , 2010 .
Bond C . The need for pharmacy practice research . Int J Pharm Pract 2006 ; 14 : 1 – 2 .
Simpson SH et al. Practice-based research: lessons from community pharmacy participants . Pharmacotherapy 2001 ; 21 : 731 – 739 .
Saini B et al. Factors influencing Australian community pharmacists’ willingness to participate in research projects – an exploratory study . Int J Pharm 2006 ; 14 : 179 – 188 .
Peterson GM et al. Attitudes of Australian pharmacists towards practice-based research . J Clin Pharm Ther 2009 ; 34 : 397 – 405 .
Bond C . A science-based profession? Int J Pharm Pract 2010 ; 18 : 321 – 322 .
Taylor KMG et al. The implications of increasing student numbers for pharmacy education . Pharm Educ 2004 ; 4 : 33 – 39 .
Beardsley R et al. Factors influencing pharmacy faculty workforce . Am J Pharm Educ 2008 ; 72 : article 34.
Marriott JL et al. Pharmacy education in the context of Australian practice . Am J Pharm Educ 2008 ; 72 : article 131 .
Manasse HR et al. Inconsistent socialization in pharmacy- a pattern need of change . J Am Pharm Assoc 1975 ; 15 : 616 – 621 , 658.
Kritikos V et al. Pharmacy students’ perceptions of their profession relative to other health care professions . Int J Pharm Pract 2003 ; 11 : 121 – 129 .
Nimmo CM , Holland RW . Transitions in pharmacy practice, part 2: who does what and why . Am J Health Syst Pharm 1999 ; 56 : 1981 – 1987 .
Accreditation Council for Pharmacy Education . Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree . Chicago, IL : Accreditation Council for Pharmacy Education , 2011 .
Brazeau GA et al. Preparing pharmacy graduates for traditional and emerging career opportunities . Am J Pharm Educ 2009 ; 73 : article 157.
Blouin RA et al. Roles of innovation in education delivery . Am J Pharm Educ 2009 ; 73 : article 154.
Bradberry JC et al. Curricula then and now – an environmental scan and recommendations since the commission to implement change in pharmaceutical education: report of the 2006-2007 Academic Affairs Committee . Am J Pharm Educ 2007 ; 71 : article S10.
Delin CR . Research attitudes and involvement among medical students and students of allied health occupations . Med Teach 1994 ; 16 : 83 – 96 .
Burgoyne LN et al. Undergraduate medical research: the student perspective . Med Educ Online [online] 2010 ; 15 : 5212 . DOI: 10.3402/meo.v15i0.5212 . http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2939395/pdf/MEO-15-5212.pdf (accessed 19 August 2011).
Eckerling S et al. Perceptions and attitudes of academic nursing students to research . J Adv Nurs 1988 ; 13 : 759 – 767 .
Ehrenfeldt M , Eckerling S . Perceptions and attitudes of registered nurses to research: a comparison with a previous study . J Adv Nurs 1991 ; 16 : 224 – 232 .
Kamwendo K , Törnquist K . Do occupational therapy and physiotherapy students care about research? A survey of perceptions and attitudes to research . Scand J Caring Sci 2001 ; 15 : 295 – 302 .
Weber II KA , He X . Chiropractic students and research: assessing the research culture at a North American chiropractic college . J Chiropr Educ 2010 ; 24 : 35 – 45 .
Zhang JQ . Research attitudes among chiropractic college students . J Manipulative Phys Ther 1996 ; 19 : 205 – 211 .
Newell D , Cunliffe C . Attitudes toward research in undergraduate chiropractic students . Clin Chiropr 2003 ; 6 : 109 – 119 .
Wayne PM et al. Attitudes and interests toward research among students at two colleges of acupuncture and Oriental medicine . Explore 2010 ; 6 : 22 – 28 .
McElroy DL et al. Factors influencing pharmacy students’ interest in graduate study: a preliminary study . Am J Pharm Educ 1985 ; 49 : 163 – 167 .
Shepherd M et al. Examination of postgraduate educational plans for academically superior undergraduate pharmacy students . Am J Pharm Educ 1988 ; 52 : 1 – 9 .
Draugalis J et al. Attitudes of pharmacy students toward graduate education and research activities: suggestions for recruitment activities . Am J Pharm Educ 1989 ; 53 : 111 – 120 .
Winans KS , Madhavan S . Some factors influencing undergraduate pharmacy students’ perceptions of and attitudes toward research related activities . Am J Pharm Educ 1992 ; 56 : 29 – 35 .
Pett MA et al. Making Sense of Factor Analysis: The Use of Factor Analysis for Instrument Development in Health Care Research . Thousand Oaks, CA : Sage , 2003 .
Bagozzi RP et al. Assessing construct validity in organizational research . Adm Sci Q 1991 ; 36 : 421 – 458 .
Fornell C , Larcker DF . Evaluating structural equation models with unobservable variables and measurement error . JMR 1981 ; 18 : 39 – 50 .
Byrne BM . Testing for multigroup invariance using AMOS Graphics: a road less traveled . Struct Equation Model 2004 ; 11 : 272 – 300 .
Australian Institute of Health and Welfare (AIHW) . Pharmacy labour force to 2001 . AIHW cat. no. HWL 25. Canberra : AIHW (National Health Labour Force Series no. 25) , 2003 .
Australian Pharmacy Council . Accreditation Standards . December 2009 , Version 1.0 [online]. http://www.pharmacycouncil.org.au/PDF/Undergraduate%20and%20Graduate%20Accreditation%20Standards.pdf (accessed 14 January, 2012).
Taylor JG , Szmukier Z . Graduate studies as part of a career path for Saskatchewan pharmacy students . CPJ/RPC 2005 ; 138 : 43 – 49 .
Armour C et al. Pharmacists’ views on involvement in pharmacy practice research: strategies for facilitating participation . Pharm Prac 2007 ; 5 : 59 – 66 .
Cvijovic K et al. Pharmacists’ participation in research: a case of trying to find the time . Int J Pharm Pract 2010 ; 18 : 337 – 383 .
The Pharmaceutical Society of Australia . National Competency Standards Framework for Pharmacists in Australia . Canberra : The Pharmaceutical Society of Australia , 2010 .
Email alerts
Citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Service
- Journals Career Network
Affiliations
- Online ISSN 2042-7174
- Print ISSN 0961-7671
- Copyright © 2024 Royal Pharmaceutical Society
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- MedEdPublish (2016)
- PMC10939653

A three-year post-graduate Doctorate in Pharmacy course incorporating professional, experiential and research activities: A collaborative innovative approach
Janis vella.
1 University of Malta
Maresca Attard Pizzuto
Nicolette sammut bartolo, francesca wirth, louise grech, jennifer pham.
2 College of Pharmacy
Christina Mactal Haaf
Anthony serracino inglott, lilian m. azzopardi.
This article was migrated. The article was marked as recommended.
A three-year post-graduate international Doctorate in Pharmacy collaborative course, was launched by the Department of Pharmacy, University of Malta in collaboration with the College of Pharmacy, University of Illinois at Chicago.
Aim and rationale
To demonstrate that the professional Doctorate in Pharmacy (i) fits the requirements of a Level 8 degree according to the Bologna process, (ii) helps graduates develop competencies and attributes in proficiency in clinical and professional aspects, (iii) has a research component that provides the right level of abilities to participate in research initiatives and to interpret research outcomes, (iv) enables graduates to obtain leadership characteristics.
The unique characteristics of the course were evaluated through an outcomes result-oriented measurement. Leadership aspects were measured through policies and strategies presented by students and graduates.
i) course is in line with the Bologna declaration, ii) research work shown in the dissertation satisfied competencies required iii) research abilities have been examined through a third party and found to be compliant with acquiring of concepts in the design, carrying out, assessment of outcomes and interpretation of results of the research study carried out by each student, and iv) leadership characteristics were shown by the positions taken up by the graduates and early outcomes from these positions.
Learning activities enable development of professionals able to merge scientific and practice aspects in the evaluation of innovative therapies, the use of medicines and patient monitoring, and in pharmaceutical policy development and regulation. Leadership positions taken up by graduates point to the acquisition of leadership skills by graduates.
The authors are happy to extend collaboration for this model to be adapted by other institutions for the curricular development entailed in this programme to enhance and improve an innovative aspect in the evolvement of the pharmacy profession on the international scenario.
A three-year post-graduate international Doctorate in Pharmacy collaborative course incorporating research, experiential and professional components was launched six years ago by the Department of Pharmacy of the University of Malta in collaboration with the College of Pharmacy of the University of Illinois at Chicago (UIC). The course aims to empower students to further develop their knowledge, practice and research skills into leadership competencies ( Pham et al., 2019 ; Azzopardi and Serracino-Inglott, 2020 ; University of Malta, 2021 ). The course is a level 8 degree course in line with the Bologna declaration ( Pham et al., 2019 ).
The Bologna declaration helps in the harmonisation of quality and standards of higher-education qualifications between European countries. The aim of establishing the Bologna declaration was to introduce an efficient, transparent and homogenous development of professionals within the higher education system which can meet the demands of increasing globalisation ( Betlehem et al., 2009 ). Since the implementation of this process, student and staff mobility has been facilitated and higher education has been made more accessible, inclusive, attractive and competitive globally. A number of professionals have followed competence-based curricula as a way of increasing public trust and enhancing professional expertise since this process was established ( Davies, 2017 ).
The process brought along the development of tools such as the European Credit Transfer and Accumulation System (ECTS) to help improve transparency and international exchange ( Humar and Sansoni, 2017 ; Parviainen et al., 2018 ), and the inception of the European Qualifications Framework (EQF) to help relate the national qualification systems of a country to that of a common European framework ( Cedefop, 2020 ).
The EQF consists of eight reference levels, known as learning outcomes, which describe what the learner understands, knows and is able to do. The learning outcomes range from basic (Level 1) to advanced (Level 8) levels. Completion of Level 8 programmes allows learners to attain specialised techniques, skills and knowledge at the most advanced frontier of a field of study and become critical problem solvers in research and innovation ( Europa.eu, 2020 ). Examples of Level 8 programmes are the Doctor of Philosophy degree (PhD) and Professional Doctorate degrees.
Professional doctorate courses are designed to help students develop professional and research skills whilst supporting conduction of innovative research in relation to a professional practice. Professional Doctorate candidates are requested to make a substantial contribution to professional knowledge which has a potential to improve professional practices ( Council of Graduate Schools, 2007 ).
Appropriate interpretation and processing of evidence-based information and critical-thinking capability are necessary research skills which help in adequate medication management and pharmaceutical care ( Katajavuori, Hirvonent and Lindblom-Ylänne, 2003 ; Langley et al., 2007 ; Slack, Warholak and Murphy, 2015 ; Krajewski et al., 2013 ; Magwenzi, 2020 ; Smith-Gorvie et al., 2020 ). Leadership skills are given increasing interest in medical educational programmes and should be developed in postgraduate pharmacy education programmes since these skills positively impact the pharmacy profession and pharmaceutical care settings ( Janke, Traynor and Boyle, 2013 ; Black, Wilby and Jewesson, 2014 ; Fierke, Kading and Scott, 2014 ; Bowman and Raney, 2016 ; Barry et al., 2019 ; Arnold et al., 2019 ). Implementation of critical thinking skills is an ability acquired through complex and scientific problem solving and application of knowledge gained ( White et al., 2015 ).
The aim was to demonstrate that the professional Doctorate in Pharmacy with components of research, leadership and practice (i) fits the requirements of a Level 8 degree according to the Bologna declaration, (ii) helps graduates develop competencies and attributes in proficiency in the clinical and professional aspects, (iii) has a research component that provides the right level of abilities to participate in research initiatives and to interpret research outcomes and (iv) empowers graduates to obtain leadership characteristics through the development and evolvement of their abilities throughout all course components.
Key components of the degree which target the development and improvement of evidence-based research skills, empower students to improve clinical skills and take up leadership positions in their practice that will drive policies and service developments in clinical practice, use of medicines and service developments were examined.
The unique characteristics of the course, such as combination of the professional component and the robust research aspects with equal emphasis, were evaluated through an outcomes result-oriented measurement. Evaluation of the dissertation through a public presentation, peer-reviewed publications, viva examination and examination by an external assessor was carried out. Leadership aspects were measured through policies and strategies presented by students and graduates in the course. Present job opportunities availed of by the graduates were noted.
Features of the post-graduate Professional Doctorate allow learners to attain specialised techniques, skills and knowledge at the most advanced frontier of Pharmacy and allows students to become critical problem solvers in research and innovation.
Research Aspects
The development of evidence-based research skills is a focal point of the Doctorate in Pharmacy programme, and acquisition of these skills is attained through ‘Drug Information and Statistics’, an advanced taught study unit, journal club sessions, research seminars and a dissertation.
i. Drug Information and Statistics
Students start to develop and improve upon their research skills during the first year of the programme, where they follow a didactic study unit entitled ‘Drug Information and Statistics’, which has 8 ECTS credits and reviews drug information sources used in health systems ( University of Malta, 2021 ). Critical evaluation of literature is presented and the clinical application of statistical tools is explored. Topic discussions include ensuring patient safety, legal and ethical implications when responding to drug information requests. The use of evidence-based medical literature is emphasised and analysis of information and literature evaluation for formulary development and disease state management policies are conducted. Students learn how to design, develop and evaluate educational information and are exposed to methods of development and management of drug information services in different care settings. Comparative efficacy reports and health technology assessments, reviews and medication use evaluations are discussed and issues relating to pharmacovigilance and drug use monitoring processes are addressed.
Competencies developed by students following this study unit as shown in examination results, include the ability to develop and evaluate educational information, to assess legal and ethical considerations during drug information service provision, and to develop comparative efficacy reports, health technology assessments and medication use evaluations.
ii. Journal Club Sessions
During the first year of study, students participate in two formal journal club sessions, led by a pharmacist preceptor ( University of Malta, 2021 ). The journal club sessions focus on practice-based or translational research. An article from a peer-reviewed journal is selected by the preceptor, one for each session, for students to critically appraise. The students are requested to identify and analyse two additional articles related to the selected article. During the journal club session, the preceptor engages the students in reflective discussion and critical analysis of the articles and guides the students to acquire the necessary critical appraisal skills. Points of discussion include justification by the student on the rationale for selection of the two additional articles, discussion of similarities and differences between articles, comparison of methodologies and findings, and application of knowledge gained through research outcomes. Student participation in the discussion and critical analysis of the articles is evaluated by the preceptor using an assessment sheet. The preceptor assesses the students’ understanding of the background provided, whether the rationale for the study is comprehensive and ethical, and whether the objectives are reasonable, attainable and within the scope of the study described. The preceptor assesses the student’s ability to judge whether the study design and methodology chosen by the authors of the articles are appropriate and whether data and results are presented appropriately. The preceptor assesses the student’s ability to reflect on the consistency of the conclusion with study objectives and clinical importance of the study.
iii. Research Seminars
Seven 2-hour research seminars are delivered during the second year of the course to support the students in applying advanced pharmacy practice research skills to their dissertation. During each seminar, tasks for the student in relation to the topic covered are assigned. The topics covered during the seminars are: 1) Proposal writing : students are guided on how to develop a research proposal using the correct content, presentation and style of writing, 2) Referencing : different referencing styles in scientific research are discussed and students are guided on how to apply the required referencing style requested for their dissertation, 3) Applying for ethics approval : ethical issues in relation to different research scenarios are discussed and requirements for research ethics approval application are covered, 4) Writing scientific reports : appropriate styles and ways of presenting and writing scientific reports are discussed, 5) Editing scientific reports : students are guided on how to summarise important scientific information, highlighting the salient points and study findings and are guided on how to present data or information in a correct scientific manner, 6) Presentation skills : students are guided on how to present their research data in a clear, concise and effective way and on how to disseminate information efficiently.
iv. Dissertation
During the second and third year, students work on a dissertation (60 ECTS) which aims at enhancing critical analytical skills while exposing the student to international research communities ( University of Malta, 2021 ). Students evolve into independent researchers within an applied professional context by contributing to knowledge and putting forward original ideas that may lead to service development, safe use of medicines and improved pharmaceutical processes. Research areas covered by students who completed the programme include development of pharmaceutical care models and clinical pharmacy services, pharmacist intervention in chronic disease management, pharmacogenetics and precision medicine, use of and access to innovative therapies such as stem cell therapy, biosimilars, rare diseases and orphan medication, medication errors and development of innovative methods to improve patient safety, and research in patient-centered pharmaceutical regulatory sciences ( Table 1 ).
Students successfully completed their dissertation where knowledge on how to analyse and interpret data, critically appraise results and ability to contribute significantly to development of practice research was demonstrated. Knowledge and abilities were evaluated by an external assessor at the end of the third year of the course through a Viva examination.
Research Outcomes
Students participate in local and international research fora, including conferences, symposia and meetings, to disseminate their findings and discuss their research work. National research symposia are held annually, where students present progress in their research to fellow students, an inter-professional panel and interested stakeholders. Students successfully publish results of their research in international peer-reviewed journals ( Cilia et al., 2017 ; Vella et al., 2018 ; Abbas et al., 2019 ; Mifsud et al., 2019 ; Zuccarelli et al., 2020 ).
Research outcomes have led to the implementation of services within different sectors of pharmacy practice. Examples of such services established within an acute general teaching hospital in Malta include the development and implementation of a: (i) pharmaceutical care model for peadiatric-adolescent oncology treatment, (ii) patient-centered pharmacist-led discharge service, (iii) holistic pharmaceutical service within the emergency department, (iv) standard guidance for intravenous medication at ward level. Within the community pharmacy, outcomes of student research have: (i) changed the practice of community pharmacy regulatory audits by making them more patient oriented (ii) led to the development of point of care testing service and (iii) evaluated clinical pharmacist interventions in chronic disease management. Services which have been established in regulatory science settings include the development and implementation of (i) incident reporting forms for medical device use and (ii) a training programme in veterinary pharmaceutical sciences for pharmacists. The outcomes of the research overflowed to other European countries such as within a central hospital in Estonia where a new clinical pharmacist service was established.
Leadership Aspects
Students following the Doctorate in Pharmacy programme are empowered to take up leadership roles through reflection on pharmacoeconomic implications of pharmacotherapy and health systems models in the EU and USA and experiencing innovative advanced practice scenarios ( University of Malta, 2021 ).
i. Pharmacoeconomics
During the first year of study, students cover a taught unit in ‘Pharmacoeconomics’ (4 ECTS), in which advanced concepts of pharmacoeconomics such as advanced economic evaluation in healthcare, health policy management, pricing of medicinal products and reimbursement schemes are discussed ( University of Malta, 2021 ). Methodologies adopted in the economic approach of pharmaceutical services and drug therapy are considered and application of economic-based evaluation methods in pharmaceutical care services are discussed.
Competencies developed by students following this study unit as shown in examination results include the ability to: (i) develop decision-making models when considering access to new therapies, (ii) handle results of economic evaluations and (iii) evaluate the impact of drug therapy and professional services on patients’ quality of life and health outcomes.
ii. Health Systems in USA and Europe
Students follow a unit entitled ‘Health systems in USA and Europe’ (4 ECTS) during the first year of study, where the various healthcare systems, their associated regulations and international government regulations of healthcare are introduced and critically analysed ( University of Malta, 2021 ). Delivery systems and health financing models in different countries are taken as examples to appraise health systems and to propose models for developing health systems with rational and safe delivery.
Competencies developed by students following this study unit as shown in examination results include the ability to (i) appraise examples of health systems, (ii) assess strengths and weaknesses of different health systems and (iii) identify challenges and priorities with respect to service provision, workforce, technology, policy, leadership, advocacy and governance.
iii. Pharmacotherapeutics
Two study units, each of 16 ECTS, are covered during the two semesters of the first year ( University of Malta, 2021 ). These study units present an integration of scientific aspects of medicinal chemistry, toxicology, pharmacokinetics and drug action in disease state management and identification of limitations and benefits of applied evidence-based medicine is conducted. An advanced up-to-date overview of principles of pharmacotherapeutics in areas including cardiology, endocrinology, hepatology, infectious disease, fluid and electrolyte disorders and nutrition, nephrology, neurology, oncology, paediatrics, psychiatry and rheumatology is provided, and the development of an integrated approach of knowledge and skills required in decision-making for pharmacotherapeutic management are emphasised. Case discussion sessions are conducted by to illustrate and teach clinical skills and simulate thinking.
Competencies developed by students following this study unit as shown in examination results include the ability to: (i) manage medication knowledge, mitigate errors and support decision-making based on evidence-based sources, (ii) provide individualised treatment, (iii) support patient care and support practice of clinical pharmacy and therapeutics enabling seamless patient care.
iv. Experiential
The experiential study unit (4 ECTS) is followed by students in their first year and includes analysis of contemporary issues relating to innovative drugs and pharmaceutical services ( University of Malta, 2021 ). It relies on self-reflective development where students complete a workbook to discuss on contemporary and innovative pharmaceutical policies and new drug therapies. Each experience consists of 7 seminars (3 hours each) and 10 three-hour sessions in a practical scenario, namely a clinical pharmacy setting in a hospital or community pharmacy. Students are required to complete a workbook and present reflections on the experience. The experiential study unit provides the opportunity for students to evaluate patient case notes and prepare pharmacist interventions within a collaborative therapeutic management framework.
Competencies developed by students as shown in their workbook and reflections are the ability to: (i) identify opportunities for improvements of medication-use systems, (ii) design and implement quality improvement changes in a medication-use system, (iii) exercise leadership and practice management and (iv) demonstrate project management skills.
v. Practice Rotations
Practice rotations (68 ECTS) are a prominent feature of the programme ( University of Malta, 2021 ). These practical placements spread over 26 weeks, take place during each year of the programe and are undertaken in different pharmaceutical scenarios namely a rehabilitation hospital setting, an acute general hospital setting, community pharmacy, point-of-care testing, pharmacy health-systems and pharmacovigilance settings.
In the first year, students follow two practice rotations, each of four weeks duration. One rotation is a clinical rotation at a rehabilitation hospital where students are involved in inter-professional medication review strategies, clinical decision making and pharmaceutical care planning, and the other rotation is a practice rotation in a pharmacovigilance, point-of-care testing or in a community pharmacy setting.
During the second and third year, three practice rotations each of six weeks duration are undertaken. Students follow a compulsory rotation in an acute hospital setting, where they are involved in inter-professional medication review strategies, clinical decision making, optimisation of therapy and patient monitoring and drug information. Students have the opportunity to carry out the rotation in a hospital setting in Malta or in the USA- namely at the teaching hospital of the University of Illinois at Chicago or at the University of Florida. For the other two rotations, students can choose the hospital setting, the pharmaceutical regulatory sciences setting where they are involved in licensing and post-licensing operations, a pharmacy systems setting with an emphasis on healthcare management, medication safety and patient support in transition of care, or community pharmacy.
Skills in managing and improving medication-use processes are developed and opportunities for pharmacist intervention in patient care and in medication use at a population or individual patient level are identified. Experience in evidence-based, patient-centered medication therapy management within an interdisciplinary team is highlighted. During rotation periods, students produce a self-reflection portfolio and seminars are held in relation to these rotations to follow-up students and discuss topical issues in pharmaceutical services. For each rotation, the student is assigned a mentor who supports the student to develop the learning outcomes from the practice rotation.
Evaluation of skills acquired by the students during practice rotations is established. The evaluation method developed for these rotations identifies the competences and skills developed during such sessions.
A workbook including a (i) ‘Self-evaluation and planning form’ to reflect student experiences and a (ii) ‘Preceptor evaluation form’ is compiled and provided to students following the sessions.
a. The ‘Self-evaluation and planning form’ is completed by the student and consists of three sections:
- 1. Student characteristics - where the student lists strengths, areas for improvement and interests
- 2. Initial plan - which includes a list of scheduled activities to be followed
- 3. Self-improvement goals - which ranks goals in order of importance
The ‘self-evaluation and planning form’ is completed by the student at the start of the rotation and is discussed with the preceptor during the first session. The ‘student characteristics’ and ‘initial plan’ are discussed again by the student and preceptor during the third week of the rotation follow-up sessions. The ‘self-improvement goals’ are to be discussed again between the student and preceptor at the end of the rotation.
b. The ‘Preceptor evaluation form’ is completed by the preceptor and takes into consideration the criteria listed in Table 2 :
At the end of the rotations, students are asked to prepare two presentations on topics related to experiences encountered during the practice rotations and present them to pharmacy students and healthcare professionals.
Skills that students attained on completion of rotations included the ability to: (i) collect and critically assess clinically relevant data to facilitate monitoring and management of drug therapy plans, (ii) monitor and recommend adjustments to drug regimens to maximise therapeutic outcomes and (iii) efficiently collect, analyse and apply evidence-based literature for appropriate clinical management of patients.
Table 3 shows an overview of the units covered during the three-year programme.
Student and Graduate Cohort
The programme has captured students from nineteen different countries: Estonia, Finland, Germany, India, Ireland, Italy, Japan, Jordan, Latvia, Lebanon, Libya, Malta, Serbia, Spain, Turkey, Philippines, Sudan, Uganda and the United Kingdom. Sharing of knowledge and educational perspectives between international students enhances and enriches the programme as can be seen during activities such as class discussions and research seminars.
Fifty-four students have graduated since the course started in 2014. All students hold influential managerial roles in different pharmaceutical sectors. Table 4 shows examples of job positions held by 39 of the 54 graduates.
The postgraduate Professional Doctorate in Pharmacy programme fits the requirements of a level 8 degree. The learning activities undertaken in the programme enable development of professionals who are specialised in rational person-centered care and who are able to merge scientific and practice aspects in the evaluation of innovative therapies, the use of medicines and patient monitoring, and in pharmaceutical policy development and regulation. The leadership positions taken up by graduates from this programme point to the preparedness and acquisition of leadership skills by the graduates.
Take Home Messages
- • Students coming from different cultures enhance and enrich the programme
- • Positions taken up by graduates guide the requirements and improvements that should be adapted for a highly successful programme
- • There is a substantial interest in innovative programmes that, while moving from the traditional purely research-oriented doctorate programmes, entertain a combination of research, professionalism and leadership
- • Collaboration between institutions notwithstanding how geographically distant they are, are key to developing diverse and peer-reviewed curricula.
Notes On Contributors
Dr Janis Vella :B.Pharm.(Hons)(Melit.), M.Sc.(Melit.), Ph.D.(Melit.), M.R.Pharm.S. ORCiD: https://orcid.org/0000-0001-6061-3608
Phamacist and resident academic lecturer at the Department of Pharmacy, University of Malta, participates in running of described course and development and organisation of course content. Janis Vella is involved in tutorials, journal clubs, clinical rotations and supervision of PharmD students’ dissertations. Dr Vella Szijj is involved in the teaching of bioanalysis, pharmaceutical analysis, pharmaceutical chemistry, clinical pharmacy aspects and pharmaceutical technology. Dr Vella Szijj is also involved in the supervision of a number of undergraduate and postgraduate students.
Dr Maresca Attard Pizzuto: B.Pharm.(Hons)(Melit.), M.Sc.(Melit.), Ph.D.(Melit.). ORCiD: https://orcid.org/0000-0001-6221-386X
Pharmacist and resident academic lecturer at the Department of Pharmacy, University of Malta, participates in running of described course and development and organisation of course content. Maresca Attard Pizzuto is involved in tutorials, lectures, journal clubs and supervision of PharmD students’ dissertations. Dr Attard Pizzuto’s lecturing portfolio extends from applied pharmaceutical science to pharmaceutics, pharmaceutical regulatory science and drug information and statistics. She is also involved in the supervision of a number of undergraduate and postgraduate students.
Dr Nicolette Sammut Bartolo: B.Pharm.(Hons)(Melit.), M.Sc.(Melit.), Ph.D.(Melit.). ORCiD: https://orcid.org/0000-0002-9499-5875
Phamacist and resident academic lecturer at the Department of Pharmacy, University of Malta, participates in running of described course and development and organisation of course content. Nicolette Sammut Bartolo is involved in tutorials, journal clubs and supervision of PharmD students’ dissertations. Dr Sammut Bartolo is involved in teaching related to pharmaceutical technology and green practices and supervises a number of undergraduate and postgraduate students.
Dr Francesca Wirth: B.Pharm.(Hons)(Melit.), M.Phil.(Melit.), Ph.D.(Melit.), ARPharmS. ORCiD: https://orcid.org/0000-0002-3225-2363
Pharmacist and resident academic lecturer at the Department of Pharmacy, University of Malta, participates in running of described course and development and organisation of course content. Francesca Wirth is involved in tutorials, journal clubs, clinical rotations and supervision of PharmD students’ dissertations. Dr Wirth is actively involved in the pharmacy practice teaching and research group and the pharmacogenetics research group and supervises undergraduate projects and postgraduate dissertations in these areas.
Dr Louise Grech: B.Pharm.(Hons)(Melit.), M.Phil.(Glas.), Ph.D.(Melit.), M.R.Pharm.S. ORCiD: https://orcid.org/0000-0002-1644-4680
Pharmacist and resident academic lecturer at the Department of Pharmacy University of Malta, participates in running of described course and development and organisation of course content. Louise Grech is involved in tutorials, clinical rotations and supervision of PharmD students’ dissertations. Dr. Grech is involved in teaching clinical pharmacy aspects, pharmacy practice and terminology. She is the supervisor of a number of undergraduate and postgraduate students mainly in the area of clinical pharmacy and pharmaceutical care.
Dr Jennifer Pham: PharmD, BCPS, BCPPS. ORCiD: https://orcid.org/0000-0002-3791-2965
Pharmacist and resident academic lecturer at the College of Pharmacy, University of Illinois at Chicago, USA, participates in running of described course and development and organisation of course content. Jennifer Pham is involved in tutorials and clinical rotations of PharmD students. Jennifer Pham is a clinical pharmacist at the University of Illinois Hospital. Research interests of Dr Pham include neonatal and pediatric nutrition, infectious diseases/antimicrobial stewardship, and medication safety.
Dr Christina Mactal Haaf: PharmD, BCOP, BCPS. ORCiD: https://orcid.org/0000-0002-2008-3486
Pharmacist and resident academic lecturer at the College of Pharmacy, University of Illinois at Chicago, USA, participates in running of described course and development and organisation of course content. Christina Haaf is involved in tutorials and clinical rotations of PharmD students. Christina Mactal Haaf is a clinical pharmacist at the University of Illinois Hospital in the Medical Oncology / Hematology - Stem Cell Transplant units.
Dr Alan Lau: PharmD, FCCP. ORCiD: https://orcid.org/0000-0002-9245-6659
Pharmacist and director of International Clinical Pharmacy Education, University of Illinois at Chicago, coordinator of described course. Dr. Lau’s research interests include the application of clinical pharmacokinetic principles to various areas of pharmacotherapeutics, primarily in nephrology.
Prof Anthony Serracino Inglott: B.Pharm., Pharm.D.(Cinc.), M.A.C.C.P., M.R.Pharm.S. ORCiD: https://orcid.org/0000-0003-1196-0376
Pharmacist and resident academic lecturer at the Department of Pharmacy, University of Malta, participates in running of described course and development and organisation of course content. Anthony Serracino Inglott is involved in tutorials and supervision of PharmD students’ dissertations. He is currently chairperson of the Malta Medicines Authority and a member of the Management Board of the European Medicines Agency.
Prof Lilian M. Azzopardi: B.Pharm.(Hons), M.Phil., Ph.D., M.R.Pharm.S., F.F.I.P., F.E.S.C.P. ORCiD: https://orcid.org/0000-0002-3811-0761
Pharmacist and head of department of Pharmacy at the University of Malta, coordinator of described course. Professor Azzopardi’s areas of interest include quality systems and clinical pharmacy interventions. Professor Azzopardi was elected to the Executive Committee of the European Association of Faculties of Pharmacy, served as General Secretary and is currently President of the Association.
Acknowledgments
The authors would like to formally thank the University of Malta and the University of Illinios at Chicago.
[version 1; peer review: This article was migrated, the article was marked as recommended]
Declarations
The author has declared that there are no conflicts of interest.
Ethics Statement
This research did not require Ethics Board approval because it does not involve human or animal subjects.
External Funding
This article has not had any External Funding
Bibliography/References
- Abbas A. Vella Szijj J. Azzopardi L. M. and Serracino Inglott A.(2020) Orphan drug policies in different countries. JPHSR. 10 ( 3 ):295–302, 10.1111/jphs.12305 [ CrossRef ] [ Google Scholar ]
- Arnold L., Cuddy P., Hathaway S. B., Quaintance J. L., et al.(2019) Medical leaders identify personal characteristics and experiences that contribute to leadership success in medicine. MedEdPublish. 8 ( 3 ). 10.15694/mep.2019.000206.1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Azzopardi L. M. and Serracino Inglott A.(2020). Clinical pharmacy education and practice evolvement in Malta. JACCP. 3 ( 5 ):973–979, 10.1002/jac5.1280 [ CrossRef ] [ Google Scholar ]
- Barry E., Hudepohl N., Kleber H. G., McManigle J. E., et al.(2019) Leader and leadership education and development in medical education across the professional lifecycle. MedEdPublish. 8 ( 3 ). 10.15694/mep.2019.000218.1 [ CrossRef ] [ Google Scholar ]
- Betlehem J., Kukla A., Deutsch K., MartonSimora J., et al.(2009) The changing face of European healthcare education: the Hungarian experience. Nurse Educ.Today. 29 ( 2 ),pp.240–254. 10.1016/j.nedt.2008.08.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Black E. K. Wilby K. J. and Jewesson P. J.(2014) International exposure to pharmacy leadership, education and practice: the early Qatar experience. Pharmacy Education. 14 ( 1 ). 7075. Available at: https://pharmacyeducation.fip.org/pharmacyeducation/article/view/194/166 (Accessed: 29/03/21). [ Google Scholar ]
- Bowman B. J. and Raney E. C.(2016) Pilot implementation of a formal leadership development strategy within a student chapter of an American pharmacy organisation. Pharmacy Education. 16 ( 1 ),8487. Available at: https://pharmacyeducation.fip.org/pharmacyeducation/article/view/471/368 ( Accessed: 29/03/21). [ Google Scholar ]
- Cedefop (2020) European Qualifications Framework (EQF). Available at: https://www.cedefop.europa.eu/en/events-and-projects/projects/european-qualifications-framework-eqf ( Accessed: 29/03/21). [ Google Scholar ]
- Cilia M., Ruiz S., Richardson P., Salmonson. T. et al. (2017) Quality issues identified during the evaluation of biosimilars by the European Medicines Agency’s Committee for Medicinal Products for Human Use. AAPS PharmSciTech. 123 . 10.1208/s12249-017-0892-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Council of Graduate Schools.(2007) CGS Task force report on the Professional Doctorate. Available at: https://cgsnet.org/sites/default/files/task_force_on_professional_doctorate.pdf ( Accessed: 28/03/21). [ Google Scholar ]
- Davies H.(2017) Competence based curricula in the context of Bologna and EU higher education policy. Pharmacy. 5 ( 2 ): 17. 10.3390/pharmacy5020017 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Europa.eu. (2020) Description of the eight EQF levels. Available at: https://europa.eu/europass/en/description-eight-eqf-levels ( Accessed: 28/03/21). [ Google Scholar ]
- Fierke K. K. Kading M. L. and Scott D. R.(2014) Creating international opportunities in the classroom. Pharmacy Education. 14 ( 1 ). Available at: https://pharmacyeducation.fip.org/pharmacyeducation/article/view/181/171 ( Accessed: 02/04/21). [ Google Scholar ]
- Humar L. and Sansoni J.(2017) Bologna Process and Basic Nursing Education in 21 European countries. Ann Ing. 29 ( 6 ), pp.561571. [ PubMed ] [ Google Scholar ]
- Janke K. K. Traynor A. P. and Boyle C. J.(2013) Competencies for student leadership development in Doctor of Pharmacy curricula to assist curriculum committees and leadership instructions. American Journal of Pharmaceutical Education. 77 ( 10 ), Article 222. 10.5688/ajpe7710222 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Katajavuori N. Hirvonent J. and Lindblom Ylänne S.(2003) The development of excellence in pharmaceutical knowledge: New curriculum for the B.Sc. (Pharmacy) studies. Pharmacy Education. 3 ( 3 ),149–160. Available at: https://pharmacyeducation.fip.org/pharmacyeducation/article/view/45/31 ( Accessed: 30/03/21). [ Google Scholar ]
- Krajewski M. P., Doloresco F., Wrobel M. J., Chiarella D., et al.(2013) Literature searching skills of first and third professional year pharmacy students. Pharmacy Education. 13 ( 1 ),165171. Available at: https://pharmacyeducation.fip.org/pharmacyeducation/article/view/215/188 ( Accessed: 29/03/21). [ Google Scholar ]
- Langley C. A., Jesson J. K., Wilson K. A, Clarke L., et al.(2007) What purpose does the MPharm research project serve? Pharmacy Education. 7 ( 3 ),1992057. Available at: https://pharmacyeducation.fip.org/pharmacyeducation/article/view/158/133 ( Accessed: 29/03/21). [ Google Scholar ]
- Magwenzi M. M.(2020) Promoting evidence-based practice through peer teaching: Practical tips to run a departmental teaching program in a healthcare setting. MedEdPublish. 9 ( 1 ),223. 10.15694/mep.2020.000223.1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mifsud E., Wirth F., Camilleri L., Azzopardi L. M., et al.(2019) Pharmacist led medicine use review in community pharmacy for patients on warfarin. Int J Clin Pharm. 41 ( 3 ):741–750. 10.1007/s11096-019-00824-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Parviainen H. M., Halava H., Leinonen E. V., Kosunen E., et al.(2018) Successful curriculum change in health management and leadership studies for the specialist training programs in medicine in Finland. Front Public Health. 6 : 271 . 10.3389/fpubh.2018.00271 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pham J. T. Azzopardi L. M. Lau A. H. and Jarret J. B.(2019) Student perspectives on a collaborative international Doctorate of Pharmacy program. Pharmacy. 7 ( 3 ),8592. 10.3390/pharmacy7030085 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Slack M. K. Warholak T. and Murphy J. E.(2015) Writing a research proposal: A workshop course developed for PharmD students. Pharmacy Education. 15 ( 1 ),1017. Available at: https://pharmacyeducation.fip.org/pharmacyeducation/article/view/323/294 ( Accessed: 17/10/20). [ Google Scholar ]
- SmithGorvie T., Nyhof-Young J., Ng. J., D’Urzo T., et al.(2020) Medical student perceptions of research training on patient care during clerkship. MedEdPublish. 9 ( 1 ),107. 10.15694/mep.2020.000107.1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vella J. Wirth F. Azzopardi L. M. and Serracino Inglott A.(2018) Serum digoxin concentrations: A retrospective analysis. J Pharmacol Clin Toxicol. 6 ( 7 ): 1129. [ Google Scholar ]
- University of Malta.(2021) Course information: Doctorate in Pharmacy. Available at: https://www.um.edu.mt/courses/overview/PPDPHRFTT6-2021-2-O ( Accessed 30/03/21). [ Google Scholar ]
- White P. J., Larson I., Styles K., Yuriev E. et al. (2015) Using active learning strategies to shift student attitudes and behaviours about learning and teaching in a research intensive educational context. Pharmacy Education. 15 ( 1 ),62172. Available at: https://pharmacyeducation.fip.org/pharmacyeducation/article/view/373 ( Accessed: 17/10/20). [ Google Scholar ]
- Zuccarelli M. Vella Szijj J. Serracino Inglott A. and Borg J. J.(2020) Treatment of Leber’s Hereditary Optic Neuropathy: An overview of recent developments. Eur J Ophthalmol. 19 . https://doi.org/10.1177/1120672120936592 [ PubMed ] [ Google Scholar ]
- Version 1. MedEdPublish (2016). 2021; 10: 93.
Reviewer response for version 1
Hui meng er.
1 International Medical University
This review has been migrated. The reviewer awarded 4 stars out of 5 I would like to congratulate the team for a well thought out postgraduate pharmacy curriculum with many innovative features delivered through student-led activities, research and experiential learning. Students have ample hands-on opportunities to explore contemporary topics and issues that are relevant to pharmacy practice, and at the same time develop leadership skills that are much required in inter-professional practice. I am also interested to know if any feedback from employers and graduates have been obtained. Such evidence would further support the achievement of the program educational objectives. This will also provide the basis for curriculum improvement.
Reviewer Expertise:
No decision status is available
Mildred López
1 Tecnologico de Monterrey, School of Medicine and Health Sciences
This review has been migrated. The reviewer awarded 4 stars out of 5 I enjoyed reading about this collaborative innovative approach, I think other educational institutions would benefit from understanding this experience. I was left with some questions at the end, and these relate to the recommendations that I would like to make. I think it is important that the authors provide more information about the firsts impressions of the implementation. For example, the authors mentioned the workbook with the self-evaluation and planning form, but no more information on how it relates to the continuous improvement of the program. The authors also mentioned in the take-home messages that the different cultures are a strength of the program, and that might be the case because of the different nationalities that are represented by the program, but the paper does not provide information of how it is shown "during activities such as class discussions and research seminars". I think that the manuscript is valuable and could be enriched by including these matters.Best regards,

UKnowledge > College of Pharmacy > Theses & Dissertations
Theses and Dissertations--Pharmacy
Theses/dissertations from 2024 2024.
Investigating a New Drug Target in Alzheimer’s Disease: NOX2 , Tiffany Adams
Design of Kappa Opioid Receptor Agonists for Potential Treatment of Multiple Sclerosis , Lindsay Kornberger
Theses/Dissertations from 2023 2023
Self-Assembled Ternary Polypeptide Nanoparticles With Improved Biostability For Drug Delivery In Cancer Therapy , Preye Mike Agbana
Investigation of Folate-Poly(Glutamic Acid)/Polyethylenimine/DNA Complexes for in vitro Gene Delivery , Caleb Akers
POPULATION-BASED EVALUATION OF TREATMENT PATTERNS, DRUG-DRUG INTERACTIONS, AND CARDIOVASCULAR RISK IN PATIENTS WITH METASTATIC CASTRATION-RESISTANT PROSTATE CANCER , Yue Cheng
An Epidemiological and Pharmacokinetic-pharmacodynamic Investigation into the Impact of Carbapenem-resistant Enterobacterales , Justin Clark
STRIVING FOR APPROPRIATE ANTIBIOTIC USE: A BIOMARKER INITIATIVE, AND OUTCOMES ASSOCIATED WITH AZITHROMYCIN EXPOSURE , Amanda Gusovsky
New Tools for Biocatalysis: Studies on the Carminomycin 4-O-Methyltransferase DnrK , Elnaz Jalali
Optimization of Orally Bioavailable Inhibitors of Defective in Cullin Neddylation 1 (DCN-1) , Leah Kovalic
Genetic and Pharmacogenetics Associations of Cancer Disparities in Appalachia , Nan Lin
Design and Synthesis of Small Molecular Inhibitors of DCN1-UBE2M Interaction , Tucker J. Moseley
Effectiveness of a long-acting cocaine hydrolase in metabolizing cocaine and its physiologically active metabolites , Linyue Shang
Anti-Inflammatory and Analgesic Effects of Highly Selective Microsomal Prostaglandin E2 Synthase-1 (mPGES-1) Inhibitors , Madeline Stewart
INVESTIGATING THE USE OF mPGES-1 INHIBITORS FOR THE TREATMENT OF ABDOMINAL AORTIC ANEURYSMS , Lauren M. Weaver
DEVELOPING A BIOCATALYTIC TOOLBOX TO AID IN UNDERSTANDING NUCLEOSIDE ANTIBIOTICS , Jasmine Brianna Woods
BIOINFORMATIC ANALYSIS OF PROTEOMIC AND GENOMIC DATA FROM NSCLC TUMORS ON PROGNOSTIC AND PREDICTIVE FACTORS OF IMMUNOTHERAPY TREATMENT , Mark Wuenschel
Theses/Dissertations from 2022 2022
Response of Dopaminergic System to Cocaine Exposure, Recovery after Cocaine Abstinence, and Impact of a Long-acting Cocaine Hydrolase , Jing Deng
ANALYSIS OF POTENTIAL FACILITATORS TO USE OF HIV PRE-EXPOSURE PROPHYLAXIS (PrEP) IN A YOUNG TRANSGENDER POPULATION , Noah Dixon
Studies Toward the Development of an Improved Countermeasure for Synthetic Opioid Overdose , Sidnee L. Hedrick
Development of zafirlukast derivatives active against Porphyromonas gingivalis , Kaitlind C. Howard
Investigating the Physical Stability of Amorphous Pharmaceutical Formulations , Travis W. Jarrells
THE RELATIVE CONTRIBUTION OF LIVER AND INTESTINE IN REVERSE CHOLESTEROL TRANSPORT , Rupinder Kaur
LIPOSOMAL TECHNOLOGIES TO IMPROVE GENE DELIVERY , David Nardo Padron
DEVELOPMENT OF ACCURATE AND EFFICIENT COMPUTATIONAL METHODOLOGIES FOR PREDICTING PROTEIN-LIGAND AND PROTEIN-PROTEIN BINDING FREE ENERGIES , Alexander Hamilton Williams
BUILDING TOOLS FOR IMPROVED MODULATION OF THE HUMAN GABAA RECEPTOR, A CENTRAL NERVOUS SYSTEM TARGET FOR THE TREATMENT OF ANXIETY , Garrett Edward Zinck
Page 1 of 8
Advanced Search
- Notify me via email or RSS
Browse by Author
- Collections
- Disciplines
Author Corner
- Submit Research
New Title Here
Below. --> connect.
- Law Library
- Special Collections
- Copyright Resource Center
- Graduate School
- Scholars@UK

- We’d like your feedback
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
University of Kentucky ®
An Equal Opportunity University Accreditation Directory Email Privacy Policy Accessibility Disclosures

Home > USC Columbia > Pharmacy, College of > Pharmacy Theses and Dissertations
Pharmacy Theses and Dissertations
Theses/dissertations from 2023 2023.
Drug Repurposing: New Mechanisms and Applications of Existing Compounds , Biyun Celia Cui
The Mechanism of PDA-PEG/Copper Selectively Killing Cancer Cells and Its Application in Treating Triple-Negative Breast Cancer and Advanced Ovarian Cancer , Xiangxiang Hu
CDK8 AND CDK19 Mediator Kinases as Novel Targets in Advanced Prostate Cancer , Jing Li
Targeting of Mediator Kinases for Cancer Therapy and Resistance Prevention , Zachary Thomas Mack
PD-L1 Nano-Eraser: A Novel Intracellular PD-L1 Degradation Approach and Its Application in Cancer and Alzheimer’s Disease Therapy , Mingming Wang
Prevalence, Disparities, and Health Outcomes in Patients With Alzheimer’s Disease, Dementia, and Mild Cognitive Impairment , Xiaomo Xiong
Theses/Dissertations from 2022 2022
Discovery and Mechanistic Study of Novel PBD-Targeted Inhibitors of PLK1 for Prostate Cancer Treatment , Danda Pani Chapagai
Mediator Kinase Inhibitors for Breast Cancer Therapy , Xiaokai Ding
Peromyscus Maniculatus Bairdii and Peromyscus Maniculatus Sonoriensis Polymorphisms , Matthew Dixon Lucius
Changes in the Regulation of the Unfolded Protein Response During Aging in Peromyscus , Elham Soltanmohammadi
Development of Peptidomimetic Inhibitors Targeting the Polo Box Domain of PLK1 as Potential Cancer Therapeutics , Jessy Mariam Stafford
Genomic Analysis Workflows to Identify Mutational Signatures and Structural Variations in OVCAR8 Cells and Rad51d-deficient Mouse Embryonic Fibroblasts , Manli Yang
Theses/Dissertations from 2021 2021
The Association of Antidepressants Use with Healthcare Utilization, Patient-Reported Outcomes, and Alzheimer's Disease and Related Dementias (ADRD) Among Medicare Beneficiaries with Depression , Abdulrahman Abdullah A Alnijadi
Sex Differences and Neuroimmune Effects of Microglial Response in the Mesolimbic Reward Pathway in Nicotine Substance Use Disorder , Erin Leigh Anderson
Age-Dependent Increase in Tyrosine Level Depletes Tyrosyl-tRNA Synthetase and Causes Neuronal Oxidative DNA Damage in Alzheimer’s Disease , Megha Jhanji
Panaxynol’s Pharmacokinetic Properties and Its Mechanism of Action in Treating Ulcerative Colitis , Hossam Sami Tashkandi
The Development of Novel CDK8 and CDK19 Inhibitors and Degraders as Potential Anti-cancer Agents , Li Zhang
Variations in ER Stress and Unfolded Protein Response Predict Disease Susceptibility , Youwen Zhang
Theses/Dissertations from 2019 2019
Glia Responsivity in Nicotine Dependence , Adewale Oluwamuyiwa Adeluyi
Investigating the Neural Correlates of Nicotine Withdrawal Phenotypes in Mice: Involvement of CREB-dependent NRG3-ErbB4 Signaling in Mediating Anxiety-like Behavior , Miranda Fisher
Susceptibility to Metabolic Disease and Individual Variations in Unfolded Protein Response in Deer Mice ( Peromyscus Maniculatus ) , Amanda Rose Havighorst
Effects of Human Dopamine Transporter (DAT) Mutations and Novel Allosteric Modulatory Compounds in Disrupting HIV-1 Tat-DAT Protein Interaction , Pamela Marie P. Quizon
Theses/Dissertations from 2018 2018
Kv7 Channels Of The Urinary Bladder Smooth Muscle: Functional Roles And Therapeutic Potential , Aaron Provence
Theses/Dissertations from 2017 2017
Impact of Medicare Reimbursement Policy Change on the Utilization, Risks, and Costs Associated with Erythropoiesis-Stimulating Agents in Cancer Patients with Chemotherapy-Induced Anemia , Minghui Li
Impact Of Prenatal Exposure To Antidepressants On Adverse Birth Outcomes , Sudeepti Pahuja
Theses/Dissertations from 2016 2016
Selectivity Targeting Polo-Like Kinase 1 (PLK1) Using Novel Inhibitors of the Polo-Box Domain , Merissa Lynnea Baxter
Multifunctional Nanocarriers for Cancer Therapy , Bei Cheng
Stimuli Responsive Nanoparticle for Cancer Targeted Therapy , Huacheng He
The Effect Of Depression And Antidepressants On Cost, Survival And Adherence To Hormone Therapy In Breast Cancer , Virginia Noxon
High-Throughput Sequencing-Based Approaches for the Identification and Development of Molecular Targets for Cancer Therapy , David Joel Oliver
Chlor-Amidine, A Novel PAD Inhibitor, As An Effective Drug For The Treatment Of Ulcerative Colitis And Prevention Of Colorectal Cancer , Erin Witalison
Theses/Dissertations from 2015 2015
Molecular Mechanisms Underlying Environmental Enrichment-Induced Neuroprotection in Vulnerability to Nicotine Addiction , Adrian Michael Gomez
Theses/Dissertations from 2014 2014
Role of Micro RNA 148/152 Family in Cancer Progression , Anusha Chaparala
Design and Development of a Novel Class of Cell Cycle CDK Inhibitors Targeting the Cyclin Binding Groove Utilizing the Replace Strategy , Padmavathy Nandha Premnath
Theses/Dissertations from 2013 2013
K+ Channels and Beta3 Adrenergic Receptors as New Pharmacological Targets for Overactive Bladder Treatment , Serge Amani Yao Afeli
Effects of DNA Damage and CDK Inhibitors on the Nucleoli , Vineet Garg
A Novel Approach to the Design of Selective Inhibitors for Cell Cycle Cyclin Dependent Kinases , Tracy Perkins
Bioassay Guided Fractionation of American Ginseng. A Hexane Fraction of American Ginseng Suppresses Colitis and Associated Colon Cancer. Mechanism of Action. , DEEPAK POUDYAL
Theses/Dissertations from 2012 2012
Design of Non-ATP Competitive and Cell Cycle Specific CDK Inhibitors Targeting Cyclin Binding Groove , Shu Liu
Ca2+-Activated K+ Channels in Urinary Bladder Smooth Muscle: Functional Role and Potential as Therapeutic Targets for Overactive Bladder , Rupal Pandey Soder
Theses/Dissertations from 2011 2011
Development of Non-Atp Competitive, Plk1-Selective Inhibitors , Kara Michelle Estes
Model-Based Measures Of Interrater Agreement , Jie Gao
DNA Damage and Cell Death Mediated by Thymidylate Synthase Inhibitors: Role of UNG and SMUG1 DNA Glycosylase , Pratik K. Nagaria
Folate Deficiency and Its Effect On Dna Repair , Virginia Noxon
Computational and Pharmacological Approaches to Design Novel Allosteric Thymidylate Synthase Inhibitors , Jayanthi Repalli
Regulation of Rad51D In Homologous Recombination Dna Repair by Alternative Splicing and Ubiquitin Modification , Brian David Yard
Theses/Dissertations from 2010 2010
Interactions between Folate Deficiency and β-Nicotinamide Adenine Dinucleotide Deficiency and Conformational Study of Thymidylate Synthase and its Physiological Relevance , Beibei Luo
Homology-Directed Repair of Dna Lesions Generated by the Mismatch Repair Dependent Processing of Cytotoxic Guanine Adducts , Preeti Rajesh
Theses/Dissertations from 2009 2009
Molecular Mechanisms of American Ginseng For the Prevention and Treatment of Colitis and Colon Cancer Associated With Colitis , Yu Jin
Advanced Search
- Notify me via email or RSS
- Collections
- Disciplines
Submissions
- University Libraries
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
Strategies for Managing Pharmacy Experiential Education During COVID-19
Affiliation.
- 1 Department of Pharmacy Practice, 15478Nova Southeastern University College of Pharmacy, Fort Lauderdale, FL, USA.
- PMID: 33267726
- DOI: 10.1177/0897190020977730
In recent months, the coronavirus pandemic has significantly affected almost every industry in the United States, including health care and higher education. Faculty and students at colleges and schools of pharmacy nationwide have needed to quickly adapt as the delivery of curricula has shifted to primarily online format. Additionally, experiential rotations have been significantly affected as practice settings such as hospitals and outpatient clinics have limited students' interactions with patients or stopped allowing students on-site altogether. Our commentary will explore strategies that have been employed by experiential education coordinators and pharmacy preceptors from various settings to navigate experiential education during these difficult times while ensuring students successfully meet requirements for graduation. These will include descriptions of transitioning advanced pharmacy practice experiences (APPEs) to virtual format, how to safely involve students in the care of COVID-19 patients, and managing scheduling issues.
Keywords: COVID-19; experiential education; pharmacy education; virtual education.
- COVID-19 / epidemiology*
- Education, Distance / organization & administration*
- Education, Pharmacy / organization & administration*
- Preceptorship / organization & administration
- Problem-Based Learning / organization & administration*
- United States / epidemiology
Advertisement
Perceptions of Pharmacy Graduate Students Toward Research Ethics Education: A Cross-Sectional Study from a Developing Country
- Original Research/Scholarship
- Open access
- Published: 26 October 2022
- Volume 28 , article number 47 , ( 2022 )
Cite this article
You have full access to this open access article

- Wesam S. Ahmed ORCID: orcid.org/0000-0002-3441-2631 1 ,
- Amgad Ahmed 2 ,
- Karem H. Alzoubi ORCID: orcid.org/0000-0002-2808-5099 3 , 4 &
- Camille Nebeker ORCID: orcid.org/0000-0001-6819-1796 5
2084 Accesses
Explore all metrics
Despite the potential value of graduate-level research ethics training, most Middle East countries, including Jordan, do not routinely offer formal research ethics training. In students enrolled in Jordanian master’s level graduate program in pharmacy, the current study assessed: 1- differences in pre- and post-enrollment exposure to research ethics core themes, 2- whether this exposure was through a formal course or in an informal setting, and 3- student attitudes towards research ethics education and the need for integrating a dedicated research ethics course into pharmacy graduate programs. A 12-item on-line survey was developed by the authors and disseminated to a convenience sample of current and former master-level pharmacy students in Jordan. A total of 61 eligible respondents completed the survey. A minority of respondents (38%) acknowledged receiving research ethics training prior to enrollment into a postgraduate pharmacy program with nearly half (16%) describing this training as informal. In comparison, a larger percentage of the total respondents (56%) had received research ethics training during their postgraduate program enrollment, with nearly half of those (25%) indicating that this training was informal. A majority of respondents reported a strong need for integrating a formal research ethics course into postgraduate pharmacy curriculum (90%) to support their research training and thesis writing (89%). Overall, the study revealed a notable lack of research ethics education for graduate-level pharmacy students in Jordan.
Similar content being viewed by others

Knowledge, Awareness, Attitudes, and Practices towards Research Ethics and Research Ethics Committees among Myanmar Post-graduate Students

The Course on Research Ethics (CORE): Implications for SoTL

Knowledge and attitudes of Chinese medical postgraduates toward research ethics and research ethics committees: a cross-sectional study
Avoid common mistakes on your manuscript.

Introduction
Most countries of the Arab Middle East and North Africa (MENA) region lack national mandates requiring education in the responsible conduct of research (RCR). Increased cases of research misconduct in the 1970’s led the United States (US) National Institute of Health (NIH) to introduce a federal requirement for RCR education for trainees engaged in biomedical research. The regulation stated expectations for research organizations to foster research integrity by creating opportunities for the research community to be aware of accepted norms and social responsibilities (Albertsa et al., 2014 ; Antes et al., 2009 ; Kalichman, 2013 , 2016 ; Kalichman & Plemmons, 2018 ; Steneck & Bulger, 2007 ). The NIH was among the first research sponsors to call for RCR training in 1989 (NIH, 1989 ). These requirements were further refined through updated versions of the NIH training mandate that followed in 1992, 2000, and finally 2009 (NIH, 1992 , 2000 , 2009 ). The NIH defined RCR training expectations in its 2009 update as the application of ethical principles in the practice of all research-related activities dictated by relevant professional norms (NIH, 2009 ). Furthermore, the NIH requirements specified topics to be discussed including ethics of research involving humans and animals, conflict of interest, data management, research misconduct, and others (Kalichman, 2013 ). Additionally, The US federal Office of Research Integrity (ORI) published nine core areas Footnote 1 to be addressed in an RCR course. These core topic areas were further expanded in a Delphi consensus panel report that identified 53 topics in seven core areas that could be included in RCR teaching (DuBois & Dueker, 2009 ; NIH, 2009 ).
While the US was advancing RCR training through federal training mandates, researchers were beginning to explore RCR instructional goals and evaluating the potential value of RCR training (AlMahmoud et al., 2017 ; Antes et al., 2009 ; Mulhearn et al., 2017 ; Plemmons et al., 2006 ; Watts et al., 2017 ). While clearly a priority in the US, RCR training expectations remain nonextant in most Middle East countries including Jordan (Alahmad et al., 2012 ). Jordan is a country of the MENA region with a progressive clinical research agenda (Ahmed et al., 2020 ) and the first Arab country to enact clinical research regulations (Al-Omari & Al-Hussaini, 2017 ). The country has been chosen as a hub for the Research Ethics Program in Jordan, Footnote 2 an initiative that is supported by a research grant from the US NIH and targets early career researchers and senior graduate students from the Arab MENA region with the aim of promoting RE in the region (Al-Shami et al., 2022 ). Although Institutional Review Board (IRB) approval is required for all research involving human subjects, the country has no RCR educational expectations for students and researchers, regardless of the funding source. Training in RCR is an important aspect of postgraduate education, especially for those who are doing research as part of their postgraduate degree requirement (Peiffer et al., 2008 ). Similar to most undergraduate health science programs in the Middle East, undergraduate pharmacy programs do not require completion of a research project (Al-Wazaify et al., 2006 ). As such, it is safe to assume that newly enrolled graduate students will have little, if any, prior exposure to learning about RCR topics. It follows that there is a need for RCR training early in graduate education as part of the core curriculum. As many graduate students concurrently work as research assistants in research laboratories, RCR education becomes an important foundation for fostering awareness of accepted norms and conventions that support research integrity. Moreover, Jordan has a well-recognized pharmaceutical industry that leans on contract research organizations for drug development and post-marketing studies. In fact, this pharmaceutical sector exports about 80 percent of its production to more than 60 countries globally, making it the second largest exporting industry in the country (Sweis et al., 2015 ). Indeed, the research and development in this pharmaceutical industry relies on pharmacy graduates, which provides another reason why RCR education is important for this specific group.
In a recent content analysis study of pharmacy program course offerings in Jordan (Ahmed & Nebeker, 2021 ), 10 thesis-track master pharmacy programs were identified. Thesis-track master programs are programs that have both coursework and research completion requirements. These programs were distributed across seven universities in Jordan (Ahmed & Nebeker, 2021 ). Of the 10 programs, none reported having a standalone RCR course integrated within the curriculum and only four reported discussions of RCR topics integrated within one of the program’s courses. The RCR topics of focus were primarily human and animal research protections. Three programs were offered by Jordan University of Science and Technology (JUST), and one program by the University of Jordan (JU) (Ahmed & Nebeker, 2021 ). These two universities are the highest ranked universities in Jordan with high research output. Footnote 3 The University of Jordan is also the oldest university in Jordan (Tarboush et al., 2020 ). Our study was designed to assess exposure to RCR content by current students and recent graduates of pharmacy master programs at the aforementioned two universities. Specifically, we were interested in topics covered, whether exposure occurred through formal or informal settings, and whether there was a difference in exposure prior and post enrollment into a postgraduate program. In addition, we assessed participant attitudes towards RCR education and whether they believe there is a need for integrating a standalone RCR module into program curricula.
Questionnaire Design
The current study of pharmacy graduate students assessed: 1- differences in pre- and post-enrollment exposure to RCR core themes, 2- whether this exposure was through a formal course or in an informal setting, and, 3- student attitudes towards RCR education and the need for integrating a dedicated RCR course into pharmacy graduate programs. To achieve this, a 12-item survey (Appendix A ) was constructed and deployed online over a two-week period (Dec 1st to Dec 15, 2017) through “suveymonkey.com” website. The survey was administered in English, since English is the language used in graduate programs offered at JUST and JU. The survey utilized several response formats including multiple choice, “Yes”, “No”, or “I do not know”, multiple checkbox, and open-ended items. The survey consisted of three sections with the first section soliciting demographic information from participants, including the name of the postgraduate pharmacy program, the university at which it is offered, and the location of the university. Demographic questions also focused on whether the participant had recently completed the program or was currently enrolled and, if the latter was true, the academic year of current enrollment. Lastly, university and country where participants had received their undergraduate pharmacy education were requested.
The second section assessed participants’ prior and current exposure to RCR core areas as described by the US ORI Footnote 4 and NIH, 2009 updated mandates (NIH, 2009 ), whether this exposure was formal or informal, and the topics covered. RCR training was defined as training that addressed the ethical dimensions of planning, conducting, and reporting of research. Formal training was outlined as any course (online, face-to-face, or combination of the two) that the participant had enrolled in and completed. Informal training consisted of opportunities to discuss ethical dimensions of research with a supervisor, mentor or lab meeting or mentioned in a course but, not the primary focus of the course.
The last section of the questionnaire was designed to solicit the participants’ opinion towards RCR education as a tool in preparing trainees to conduct research and to write their thesis, as well as their thoughts towards implementing a standalone RCR course into pharmacy postgraduate programs.
The questionnaire was revised by a group of experts. Their feedback was then implemented in the revised questionnaire. The survey was piloted to a group of eligible participants (n = 7) (Connelly, 2008 ). Responses from the pilot study were not included in the final data analysis. The study was verified as exempt by the JUST IRB committee (ref. # 13/109/2017).
Study Participants
The survey was directed to current and former pharmacy graduate students at JU and JUST. The survey description and weblink was disseminated to potential participants through five social media groups (WhatsApp and Facebook) that included graduate pharmacy students or recent alumni of JUST or JU. A recruitment reminder was sent one week after deploying the survey and two days prior to closing the survey. The consent script was included in the introduction at the beginning of the questionnaire (Appendix A ) as well as in the recruitment invitation sent through the social media platforms.
Data Analysis and Figure Preparation
Questionnaires that were not fully completed or that included participants who did not meet the eligibility criteria (those who were not pharmacy master students/graduates, not studying in JU or JUST, or did not have a research completion degree requirement) were excluded from the data analysis. Data were analyzed using Microsoft Excel 13 and SPSS V21. Descriptive statistics were used to summarize the data. Answers to questions were reported as percentages out of the total. Chi square test was used for cross-tabulation of country of undergraduate education, postgraduate university, enrollment status, academic year, and master program with RE exposure prior or during enrollment, and with participants’ opinions about research ethics education. A P-value of less than 0.05 was considered statistically significant.
A priori power analysis was conducted using G*power v3.1.9.7 (Faul et al., 2009 ) to determine the minimum sample size required to test the study hypothesis. Results indicate the required sample size to achieve 80% power for detecting a large effect (w = 0.5) at a significant criterion of α = 0.05, assuming the highest degree of freedom used in the test (df = 8), was N = 61 for Chi-Squair test. The calculated critical Chi-Squair value and noncentrality parameter λ were 15.507 and 15.250, respectively. The actual (calculated) power was 80.718%. Figures were prepared using Microsoft Excel 13.
Respondents’ Demographics
The survey link was deployed to five social media groups. The social media groups collectively included 181 group members, 146 members saw the survey invitation post, which included the link to the survey, and 70 filled out the survey (response rate 48%). Incomplete responses and ineligible participants were excluded from data analysis, leaving a total of 61 eligible participants who completed the survey (completion rate of 87%). All participants had received (41%, n = 25) or were completing (59%, n = 36) their pharmacy master education at either JU or JUST. All had been enrolled in a thesis-track master program with a research completion requirement. The majority (89%, n = 54) received their undergraduate pharmacy education in Jordan, mostly in JU (30%, n = 18) and JUST (48%, n = 29). Most participants (59%, n = 36) were currently enrolled in a pharmacy master program, of whom the majority were in their third year (28%, n = 17). Most participants were enrolled in Clinical Pharmacy (38%, n = 23) and Pharmaceutical Sciences (36%, n = 22) master programs (Table 1 ).
Exposure to RCR Education Prior to Master’s Degree Enrollment
The majority of the participants (62%, n = 38) had not received RCR education prior to enrollment into a master’s degree program. Those who had received prior RCR training (38%, n = 23) reported mostly informal training (16%, n = 10). Cross tabulation of prior RCR training with undergraduate university, post-graduate university, enrollment status, current academic year, and master program, each separately, did not reveal a statistically significant difference between the groups ( P value > 0.05) (Table 2 ). Prior training content focused on data management (28%, n = 17), conflict of interest (26%, n = 16), and publication ethics (26%, n = 16). Respondents reported little prior training on mentor–mentee responsibility (8%, n = 5) or peer review ethics (10%, n = 6) (Fig. 1 A).
Research ethics core areas covered prior and during enrollment into a master’s degree program ( A ) and the percent change in participants’ exposure to these core areas after enrollment ( B ). N = 61
Exposure to RCR Education During Master’s Degree Enrollment
Results indicated that 56% (n = 34) of respondents received some form of RCR education during their enrollment, where 25% (n = 15) of this was informal training and 18% (n = 17) was formal education (Table 2 ). Cross tabulation of post-enrollment RCR education/training exposure with undergraduate university, post-graduate university, enrollment status, current academic year, and master program, each separately, revealed a statistically significant difference between post enrollment RCR exposure and post-graduate university ( P = 0.013) and master program ( P = 0.029). Conflict of interest (41%, n = 25), publication ethics (36%, n = 22), and human subjects protections (33%, n = 20) were the topics most frequently discussed, whereas mentor–mentee responsibility (12%, n = 7) and peer review (16%, n = 10) were the least discussed topics (Fig. 1 B).
Respondents’ Opinions
The majority of the respondents affirmed that receiving a formal RCR education would help in performing better in research projects and in thesis writing (89%, n = 54). Most respondents (90%, n = 55) believe that integrating a dedicated RCR module into the curriculum of the master programs is important (Table 2 ).
Participants who supported integrating a standalone RCR course justified their answer as follows:
“It helps to be more oriented”
“It helps in doing research more ethically”
“Because no course is found in the program to cover this part”
“It is a must”
“It is important to take into consideration the ethical issues while doing research”
“Ethics are important for doing good confidential research that helps society to grow”
“It facilitates the conduct of research and the completion of our research project”
“It is needed in our field of research”
“It would be very helpful”
Those who did not support the idea defended their point of view as follows:
“One comprehensive lecture or workshop is enough”
“I was fortunate enough to have been taught about RE by my supervisor, however, not everyone gets this opportunity, mainly due to the assumption that this was taught during the BSc degree, or because the subject “RE” is basic in one’s understanding. Having said that, I do not believe a course is needed, but a 1–2 hour workshop should be enough for postgrad students where the highlights are given to the students as well as a reference to read more about it”
“I think the research methodology course in our department is enough to cover the ethical issues of research”
The current study reveals a lack of exposure to RCR core themes prior and post-enrollment into graduate pharmacy programs, with pre-enrollment exposure being even less than post-enrollment. However, there was a relative increase in the exposure to all RCR core areas post-enrollment, with the highest increase in exposure noticed for topics that were formally discussed in mandatory courses (Ahmed & Nebeker, 2021 ), indicating the effectiveness of integrating formal RCR discussions in enhancing graduate student’s exposure to research ethics topics. Moreover, there was a consensus among the participants on the value formal RCR education offers (88.5%, n = 54), who also expressed the need for integrating a dedicated course into the programs curriculum that covers most core RCR areas relevant to their field of study (90.2%, n = 55).
Questionable research practices (QRPs) may occur during graduate education and can be difficult to resolve due to lack of clarity in definitions and limitations in student’s decision making. As such, to minimize the risk of both misconduct and QRPs, principal investigators need to address relevant scientific norms as well as what constitutes research misconduct with students (Resnik and Stewart Jr, 2012 ). In addition, underscoring the role of culture in adhering to the best ethical practices highlights the importance of early training in the RCR principles for both students and researchers (Davis, 2003 ). For that matter, it seems unethical conduct in research is a shared commodity in both developed and developing countries. In one study, research misconduct in low and middle income countries was reported to be as common there as in high income countries (Ana et al., 2013 ). Therefore, education in the RCR would seem a global language in responding to QRPs, research misconduct and in fostering research integrity and promoting positive attitudes in research.
The present study gives insight into the exposure and attitudes of pharmacy master students and graduates towards RCR education in the two major and top ranked universities in Jordan. The study revealed that only a minority (38%, n = 23) of the participants received some sort of RE training prior to their enrollment into a master’s degree program. This was regardless of whether they received their undergrad education inside or outside of Jordan. Almost half of this training (16%, n = 10) was informal. Undergraduate pharmacy programs in Jordan and most of the Middle East countries do not have a research project completion requirement (Al-Wazaify et al., 2006 ). Therefore, it is expected that most undergraduate students do not get exposed to formal RCR training during their undergraduate degree enrolment. Nonetheless, there are few optional research-oriented venues, such as students research clubs, where students can opt in to join. Therefore, it might be that those who reported prior-to-enrollment knowledge in some RCR topics have obtained such training through these venues. Importantly, data management (28%, n = 17), conflict of interest (26%, n = 16), and publication ethics (26%, n = 16) were the topics participants reported being most familiar.
Comparatively, more participants (56%, n = 34) reported receiving research ethics education during their enrollment into a graduate program, indicating an overall increase in the participants’ exposure to research ethics concepts post-enrollment. Analyzing the participants’ exposure to different RCR core areas revealed a post-enrollment increase in the exposure to a variable degree. The percent increase in exposure was the highest for such topics as human research protections (121% increase), animal research protections (113% increase) (Fig. 1 B). These topics were previously identified as the main focus of research ethics discussions in these master programs (Ahmed & Nebeker, 2021 ). Additionally, more modest, but relatively noticeable, exposure to other topics such as collaboration research ethics (75% increase), authorship ethics (70% increase), peer review (67% increase), and conflict of interest (56% increase) was observed. As many of these topics are not the main focus of the research ethics discussions in these master programs (Ahmed & Nebeker, 2021 ), it is possible that exposure to these core topics occurred through informal settings. In fact, a large percentage (25% of the total responses, n = 15) of post-enrollment RCR exposure was described as being purely informal. Participants may have been exposed to this informal training after engaging with their thesis projects, as 41% (n = 25) and 28% (n = 17) of the participants were graduates and third-year students, respectively. On the other hand, the least change in exposure was observed for such topics as mentor–mentee responsibility (40% increase), publication ethics (37% increase), social responsibility (20% increase), and data management (12% increase), indicating no large difference in exposure to these topics from pre-enrollment into the master programs (Fig. 1 B). More importantly, a cross-tabulation of post-enrollment overall exposure to RCR core areas with the graduate university where students were enrolled revealed a statistically significance difference between the two universities ( P = 0.013). This disparity can be explained as all master pharmacy programs offered by JUST (Clinical Pharmacy, Pharmaceutical Technology, and Medicinal Chemistry) were reported to integrate some discussions of RE themes into their “Research Methodology” mandatory course (Ahmed & Nebeker, 2021 ). However, the school of pharmacy at JU offers a “Clinical Pharmacy” master program that incorporates RE discussions into its “Clinical Research Methods and Statistics” course. Moreover, the same school also offers a “Pharmaceutical Sciences” master program that was reported to lack formal discussions of RE themes (Ahmed & Nebeker, 2021 ). For similar reasons, the cross tabulation with the type of master program revealed a statistically significant difference ( P = 0.029).
The majority of participants (89%, n = 54) think that a RCR course is important for doing research and aids in thesis writing. About 90% think that there is a need to integrate a standalone RCR course into pharmacy master programs. This perspective was regardless of the university, postgraduate program, enrollment status, or the master program in which the participants were enrolled ( P > 0.5). In fact, in a recent survey by Tarboush et al. ( 2020 ), the majority (87%) of health sciences faculty members in Jordan also supported integrating mandatory research ethics postgraduate course (Tarboush et al., 2020 ). This attitude of health science faculty members towards integrating RCR mandatory course also suggests that the lack of postgraduate training in research ethics in Jordan extends to graduate programs from health science disciplines other than pharmacy. In further support of this, Swedan et al. ( 2020 ) have reported in their multidisciplinary and multi-university survey that included graduate students performing human subjects research in Jordan that only 37% were exposed to RCR education (Swedan et al., 2020 ). Indeed, this type of RCR training was also deemed necessary for other research investigators, as previous studies in Jordan revealed that the majority (> 80%) of resident doctors, health sciences faculty members, and healthcare investigators have agreed that such training in research ethics is essential for clinical investigators, faculty affiliates, and IRB members in Jordan (Al Demour et al., 2019 ; Ayoub et al., 2019 ; Rababa'h et al., 2020 ; Tarboush et al., 2020 ), although graduate students reported more unfamiliarity and misconceptions regarding RCR topics (Rababa'h et al., 2020 ). Even though Jordan’s “Accreditation and Quality Assurance Commission for Higher Education Institutions” oversees that “minimal requirements” are being fulfilled in order to establish any new graduate program, research ethics education does not seem to be part of these “minimal requirements” (Ahmed & Nebeker, 2021 ). Therefore, integrating research ethics education into graduate pharmacy programs in Jordan is encouraged. Establishing a federal body in Jordan and other middle and low income countries that oversees research integrity—for instance, one with similar missions to the US federal Office of Research Integrity Footnote 5 and the Department of Supervision and Scientific Integrity of China’s Ministry of Science and Technology Footnote 6 may be a tremendous asset for implementing different aspects of research integrity into research institutions and research-based graduate programs and in handling research misconduct allegations (Frankel et al., 2016 ; Zeng & Resnik, 2010 ).
Overall, this study revealed a lack of appropriate training (both formal and informal) in core areas of RCR for graduate-level pharmacy students, who would like such training to be a required part of the curriculum. Although discussions in several RCR core areas were missing, our study highlights that integrating ‘formal’ research ethics discussions in a required course could be more efficient in providing more students’ exposure to these core topics compared to informal training. Therefore, given the potential role of RCR training in preserving research integrity (AlMahmoud et al., 2017 ; Antes et al., 2009 , 2010 ; Plemmons et al., 2006 ), integrating research ethics education material into pharmacy graduate programs is strongly recommended for programs that lack this training focus. Programs that have already integrated some formal RCR training are encouraged to expand the scope of related discussions to include more core areas and to consider RCR education as a standalone course. As research has shown, RCR programs conducted separately from the standard curricula were likely more successful than those imbedded into existing modules (Antes et al., 2009 ).
The approach we followed in this study can be extrapolated to graduate programs in Jordan from disciplines other than pharmacy. It can also be utilized and built on by academic institutions from other countries in the region to investigate what research ethics topics -regardless of modality- are covered in graduate programs, how this coverage compares to pre-enrollment exposure to these topics, which modality (formal vs informal) is more effective in promoting student’s exposure to the core themes, and in the assessment of students’ attitude and need for formally introducing a course that covers research ethics main themes.
Limitations and Future Directions
The current study provides insights based on students’ self-reported recall and may not accurately represent what RCR topics were actually covered, or the extent to which they were covered. A future study involving RCR training instructors may be useful in documenting what topics they cover, the depth of the material covered, and evidence that documents teaching and learning outcomes.
Generally, before enrolling into a research-based degree, RCR training should not be expected, nor it is necessarily needed for pharmacy undergraduate students in Jordan. This lack of expectation of training prior to graduate degree enrollment stems from lack of “research completion” requirement for these undergraduate programs. Nonetheless, our results show that few students have received some sort of research ethics training—mostly at informal settings—prior to graduate degree enrollment. Although we believe that the pre-enrollment, undergrad training might have occurred outside the standard curricula—for instance, through research-oriented student clubs -, there is still room for future investigations. For instance, what are the circumstances where this type of training occurred pre-enrollment, whether there was some aspect of undergraduate program(s) that specially emphasized research readiness, and what might be some reasons for differences in what is covered pre-enrolment vs post-enrollment.
Education in research ethics core topics is lacking for pharmacy graduate programs in Jordan. In this regard, integrating a formal and dedicated research ethics course into graduate programs in Jordan would seem an effective approach to enhance students’ exposure to these core topics. Informal training is often optional and may impose unequal exposure upon students. In fact, with respect to pharmacy master programs in Jordan, such informal training in the RCR is not a requirement under any circumstances and, in most instances, is not readily available outside the individual-to-individual context. Therefore, although this study revealed that a large percentage of student’s exposure was informal, such informal training may not be an adequate replacement to formal education. Evidence from other studies in Jordan suggest that such deficiency in research ethics training extends to medical residents, students from other disciplines, and even faculty and IRB members. Nonetheless, a comprehensive assessment that includes graduate students from other universities in Jordan and from other health science disciplines may be required to explore how RCR education deficiencies noted in the current study translate to other universities and graduate programs.
Data Availability
All relevant data are within the manuscript and its appendix.
https://ori.hhs.gov/rcr-objectives-introduction .
https://jordanrcrprogram.com .
https://www.topuniversities.com/university-rankings/world-university-rankings/2021 .
https://ori.hhs.gov/ .
http://en.most.gov.cn/organization/Departments/201809/t20180927_143072.htm .
Ahmed, W., Aburjai, T., Hudaib, M., & Al-Karablieh, N. (2020). Chemical composition of essential oils hydrodistilled from aerial parts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L.(Asteraceae) growing in Jordan. Journal of Essential Oil Bearing Plants, 23 (1), 15–25.
Article Google Scholar
Ahmed, W. S., & Nebeker, C. (2021). Assessment of research ethics education offerings of pharmacy master programs in an Arab nation relative to top programs worldwide: A qualitative content analysis. PLOS ONE, 16 (2), e0238755. https://doi.org/10.1371/journal.pone.0238755
Al-Omari, A., & Al-Hussaini, M. (2017). Research ethics governance in the Arab region: Jordan. In Henry Silverman (Ed.), Research ethics in the Arab region (pp. 221–228). Springer.
Al-Shami, K. M., Ahmed, W. S., & Alzoubi, K. H. (2022). Motivators and barriers towards clinical research participation: A population-based survey from an Arab MENA country. PLoS ONE, 17 (6), e0270300.
Al-Wazaify, M., Matowe, L., Albsoul-Younes, A., & Al-Omran, O. A. (2006). Pharmacy education in Jordan, Saudi Arabia, and Kuwait. American Journal of Pharmaceutical Education, 70 (1).
Al Demour, S., Alzoubi, K., Alabsi, A., Al Abdallat, S., & Alzayed, A. (2019). Knowledge, awareness, and attitudes about research ethics committees and informed consent among resident doctors. International Journal of General Medicine, 12 , 141. https://doi.org/10.2147/IJGM.S197511
Alahmad, G., Al-Jumah, M., & Dierickx, K. (2012). Review of national research ethics regulations and guidelines in Middle Eastern Arab countries. BMC Medical Ethics, 13 , 34. https://doi.org/10.1186/1472-6939-13-34
Albertsa, B., Kirschnerb, M. W., Tilghmanc, S., & Varmusd, H. (2014). Rescuing US biomedical research from its systemic flaws. Proceedings of the National Academy of Sciences, 111 (16), 5773–5777. https://doi.org/10.1073/pnas.1404402111
AlMahmoud, T., Hashim, M. J., Elzubeir, M. A., & Branicki, F. (2017). Ethics teaching in a medical education environment: Preferences for diversity of learning and assessment methods. Medical Education Online . https://doi.org/10.1080/10872981.2017.1328257
Ana, J., Koehlmoos, T., Smith, R., & Yan, L. L. (2013). Research misconduct in low-and middle-income countries. PLoS Medicine . https://doi.org/10.1371/journal.pmed.1001315
Antes, A. L., Murphy, S. T., Waples, E. P., Mumford, M. D., Brown, R. P., Connelly, S., & Devenport, L. D. (2009). A meta-analysis of ethics instruction effectiveness in the sciences. Ethics & Behavior, 19 (5), 379. https://doi.org/10.1080/10508420903035380
Antes, A. L., Wang, X., Mumford, M. D., Brown, R. P., Connelly, S., & Devenport, L. D. (2010). Evaluating the effects that existing instruction on responsible conduct of research has on ethical decision making. Academic Medicine, 85 (3), 519. https://doi.org/10.1097/ACM.0b013e3181cd1cc5
Ayoub, N., Qandil, A., & McCutchan, J. (2019). Knowledge, attitudes, and practice regarding research ethics committees among health care faculty at two public universities in Jordan. Journal of Empirical Research on Human Research Ethics, 14 (4), 372. https://doi.org/10.1177/1556264619851351
Connelly, L. (2008). Pilot studies. Medsurg Nursing: Official Journal of the Academy of Medical-Surgical Nurses, 17 (6), 411–412.
Google Scholar
Davis, M. S. (2003). The role of culture in research misconduct. Accountability in Research, 10 (3), 189–201. https://doi.org/10.1080/714906092
DuBois, J. M., & Dueker, J. M. (2009). Teaching and assessing the responsible conduct of research: A Delphi consensus panel report. The Journal of Research Administration, 40 (1), 49.
Faul, F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41 (4), 1149–1160.
Frankel, M. S., Leshner, A. I., & Yang, W. (2016). Research integrity: Perspectives from China and the United States. In Mark S. Frankel, Alan I. Leshner & Wei Yang (Eds), Handbook of academic integrity . Springer. https://doi.org/10.1007/978-981-287-098-8_65
Kalichman, M. (2013). A brief history of RCR education. Accountability in Research, 20 (5–6), 380. https://doi.org/10.1080/08989621.2013.822260
Kalichman, M. (2016). Responsible conduct of research education (What, why, and does it work?). Academic Medicine, 91 (12), e10. https://doi.org/10.1097/ACM.0000000000001442
Kalichman, M., & Plemmons, D. (2018). Intervention to promote responsible conduct of research mentoring. Science and Engineering Ethics, 24 (2), 699. https://doi.org/10.1007/s11948-017-9929-8
Mulhearn, T. J., Steele, L. M., Watts, L. L., Medeiros, K. E., Mumford, M. D., & Connelly, S. (2017). Review of instructional approaches in ethics education. Science and Engineering Ethics, 23 (3), 883–912. https://doi.org/10.1007/s11948-016-9803-0
NIH (1989). Requirement for programs on the responsible conduct of research in National Research Service Award Institutional Training Programs. NIH guide for grants and contracts, 18(45), 1. Available online at http://grants.nih.gov/grants/guide/historical/1989_12_22_Vol_18_No_45.pdf .
NIH (1992). Reminder and update: requirement for instruction in the responsible conduct of research in national research service award institutional training grants. NIH guide for grants and contracts, 21 (43), Available online at http://grants.nih.gov/grants/guide/notice-files/not92-236.html .
NIH (2000). PHS Policy on Instruction in the Responsible Conduct of Research (RCR) Announced December 5. Available online at http://grants.nih.gov/grants/guide/notice-files/NOT-OD-01-007.html .
NIH (2009). Update on the requirement for instruction in the Responsible Conduct of Research announced November 24. Available online at http://grants.nih.gov/grants/guide/notice-files/NOT-OD-10-019.html .
Peiffer, A., Laurienti, P., & Hugenschmidt, C. (2008). Fostering a culture of responsible lab conduct. Science, 322 (5905), 1186. https://doi.org/10.1126/science.322.5905.1186b
Plemmons, D. K., Brody, S. A., & Kalichman, M. W. (2006). Student perceptions of the effectiveness of education in the responsible conduct of research. Science and Engineering Ethics, 12 (3), 571–582. https://doi.org/10.1007/s11948-006-0055-2
Rababa’h, A., Alzoubi, K., Ababneh, M., & Khabour, O. (2020). Awareness of Jordanian investigators about the importance of ethics review committees: A pilot study. Science and Engineering Ethics, 26 (2), 821. https://doi.org/10.1007/s11948-019-00139-7
Resnik, D. B., & Stewart, C. N., Jr. (2012). Misconduct versus honest error and scientific disagreement. Accountability in Research, 19 (1), 56. https://doi.org/10.1080/08989621.2012.650948
Steneck, N., & Bulger, R. (2007). The history, purpose, and future of instruction in the responsible conduct of research. Academic Medicine, 82 (9), 829. https://doi.org/10.1097/ACM.0b013e31812f7d4d
Swedan, S., Khabour, O. F., Alzoubi, K. H., & Aljabali, A. A. (2020). Graduate students reported practices regarding the issue of informed consent and maintaining of data confidentiality in a developing country. Heliyon, 6 (9), e04940. https://doi.org/10.1016/j.heliyon.2020.e04940
Sweis, R. J., Al-Ghawi, H. J., AlSaleh, N. A.-A., Zu'bi, M., & Obeidat, B. Y. (2015). Benchmarking of TQM: The case of Hikma Pharmaceuticals company. Benchmarking: An International Journal .
Tarboush, N. A., Alkayed, Z., Alzoubi, K. H., & Al-Delaimy, W. K. (2020). The understanding of research ethics at health sciences schools in Jordan: A cross-sectional study. BMC Medical Education . https://doi.org/10.1186/s12909-020-02040-5
Watts, L. L., Medeiros, K. E., Mulhearn, T. J., Steele, L. M., Connelly, S., & Mumford, M. D. (2017). Are ethics training programs improving? A meta-analytic review of past and present ethics instruction in the sciences. Ethics & Behavior, 27 (5), 351–384. https://doi.org/10.1080/10508422.2016.1182025
Zeng, W., & Resnik, D. (2010). Research integrity in China: Problems and prospects. Developing World Bioethics, 10 (3), 164–171. https://doi.org/10.1111/j.1471-8847.2009.00263.x
Download references
Open Access funding provided by the Qatar National Library. Work on this project was supported by Grant # 5R25TW010026–02 from Fogarty International Center of the Unites States National Institutes of Health on behalf of the Research Ethics Program in Jordan. The research ethics program in Jordan had no role in study design, data collection and analysis, decision to publish, preparation of the manuscript, or funding of this publication.
Author information
Authors and affiliations.
College of Health and Life Sciences, Hamad Bin Khalifa University, Qatar Foundation, Doha, Qatar
Wesam S. Ahmed
Division of Orthodontics, Department of Preventive Dentistry, Faculty of Dentistry, Jordan University of Science and Technology, Irbid, Jordan
Amgad Ahmed
Department of Pharmacy Practice and Pharmacotherapeutics, College of Pharmacy, University of Sharjah, Sharjah, 27272, United Arab Emirates
Karem H. Alzoubi
Department of Clinical Pharmacy, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid, 22110, Jordan
Herbert Wertheim School of Public Health and Human Longevity Science, University of California San Diego, La Jolla, CA, USA
Camille Nebeker
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Wesam S. Ahmed .
Ethics declarations
Conflict of interest.
The authors declare having no conflicts of interest.
Ethical approval
The questionnaire and methodology for this study were approved by the Institutional Review Board of Jordan University of Science and Technology (ref. # 13/109/2017).
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A: Survey Questionnaire
Dear participant,
We invite you to participate in this quick survey. The survey will help us assess the research ethics education offerings in postgraduate pharmacy programs in Jordan. The target group of this survey is postgraduate pharmacy students who are currently receiving or have completed their postgraduate pharmacy education in Jordan. The information provided by you will be used for research purposes. The survey will take about 5 min to complete. All your answers are anonymous and confidential.
Thank you in advance for your participation.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Ahmed, W.S., Ahmed, A., Alzoubi, K.H. et al. Perceptions of Pharmacy Graduate Students Toward Research Ethics Education: A Cross-Sectional Study from a Developing Country. Sci Eng Ethics 28 , 47 (2022). https://doi.org/10.1007/s11948-022-00406-0
Download citation
Received : 31 March 2021
Accepted : 17 September 2022
Published : 26 October 2022
DOI : https://doi.org/10.1007/s11948-022-00406-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Middle East and North Africa (MENA)
- Research ethics
- Responsible conduct of research (RCR)
- Find a journal
- Publish with us
- Track your research
Completed Thesis Projects
Omokhodion Eriakha — Beliefs and Factors that Influence Intentions to Initiate Antihypertensive Medications
Saara Nasruddin — New Pharmacist Practitioner Experiences of Listening and Responding to Patient-Driven Misinformation Prachi Prajapati — Impact of Cardiovascular and Mental Health Comorbidities on All-Cause Healthcare Utilization among Older Adults with Type 2 Diabetes
Emily Gravlee — Naloxone Accessibility Across Mississippi
Saumil Jadhav — Acceptability and Feasibility of Healthcare Communication Consideration for Altering Perceptions toward Influenza Vaccine: A Qualitative Study
Shishir Maharjan — Opioid Tapering and Mental Health Crisis in Older Adults
Irene Nsiah — Factors Influencing Postpartum Contraception Uptake
Queenie Paltanwale — Understanding Attitudes Towards Deprescribing Among Older Adults with Osteoporosis
Wesley Sparkmon — Community Pharmacist Perceptions of Increased Technician Responsibility
Ian Freeman — The Association between Patient Characteristics and Use of Statin and Metformin among Elderly Women with Breast Cancer and Diabetes
Sonam Nair — Facilitators and Barriers to Biosimilar Adoption: A Systematic Review of the Global Stakeholder Perspective
David Allen III — Electronic Nicotine Delivery System (ENDS): Reasons for Use and Associated Factors in Self-Selected Nicotine Concentrations
Siddhi Korgaonkar — Comparative Effectiveness and Safety of Non-Vitamin K Antagonists Oral Anticoagulants and Warfarin in Elderly Patients with Non-Valvular Artial Fibrillation and Diabetes
Chukwuebuka Dibie — Predictors of Dental Opioid Analgesic Prescribing, Opioid Use and Dental Emergency Department Visits in the Mississippi Medicaid Population
Yiqiao Zhang — Opioid Use for Treatment of Acute Pain among Children and Adolescents enrolled in Mississippi Medicaid
Ashley Crumby — The Decision to Pursue Pharmacy Residency Training: Motivators, Barriers, and the Fear of Missing Out
Neeri Wahidullah — Predictors of Medication Nonadherence in Children/Adolescents with Attention Deficit Hyperactivity Disorder (ADHD) in Mississippi Medicaid Program
Kaustuv Bhattacharya — Burden of Depression among Irritable Bowel Syndrome Patients Enrolled in National Medicaid
Nicholas Keeling — Payer Perspectives on Preemptive Pharmacogenetic Testing
Sasikiran Nunna — Biological and Psychosocial Risk Factors of Stroke in African Americans Enrolled in the Jackson Heart Study (JHS)
Tristen Jackson — Primary Care Providers’ Provision of Therapeutic Lifestyle Change Counseling for Patients with Cardiovascular Risk
Sujith Ramachandran — Determining Physician and Patient Characteristics that Predict the Use of Atypical Antipsychotics Children with Mental Health Disorders
Divya Verma — Impact of Refill Synchronization Medication Adherence for Chronic Disease
James Parrett — Pharmacists’ Preparedness for Acute Medical Emergencies
Ruchitbhai Shah — Community Pharmacists’ Attitudes Toward an Expanded Class of Nonprescription Drugs
Sai Dharmarajan — Case-Mix Adjustment of Adherence Based Pharmacy Quality Indicator Scores
Namita Joshi — Factors Affecting Community Pharmacy Owners’ Attitudes toward and Likelihood to Adopt RxSync Service
Tasneem Lokhandwala — Do statins improve outcomes in patients with asthma on inhaled corticosteroid therapy? A retrospective analysis of the Mississippi Medicaid database 2002-2004
Hafiz Oko-Osi — Market Response to FDA Safety Warnings: A Case Study Using an Interrupted Time Series Analysis of the Medicare Database for 2006-2008
Tushar Padwal — An Examination of Factors Influencing the Program Choice of Graduate Students in the Pharmaceutical Sciences
Zainab Shahpurwala — Pharmacy-level Quality Measures and the Consumer: Preferences and Attitudes
Sumit Verma — The Strategic Value Driver Model: A Methodology For Examining Value Drivers For A New Pharmaceutical Product In Diabetes
Amod Athavale — The Measurement of Pharmacy Loyalty and its Use in the Development of Marketing Strategies for the RxSync Service™
Ram Sankar Basak — Willingness to Influence Indication-Based Off-Label Prescribing: An Investigation of Hospital Pharmacies
Krutika Jariwala — Factors that Physicians Find Encouraging and Discouraging about Electronic Prescribing: A Quantitative Study
Clive Mendonca — Product-Specific and Disease-Specific Direct-to-Consumer Drug Advertising (DTCA): An Investigation of Post-Exposure Information Search Behavior

Kyle Null — Consumer Acceptance of Health-Related Technologies: Incorporating Perceived Health Risk into the Technology Acceptance Model
Vennela Thumula — Type 2 Diabetes in Children: Estimates of Epidemiology, Quality of Care, Costs and Resource Utilization in a Medicaid Population
Leonardo Torres — Pharmacists’ Rating of Relevance of Available Information in Deciding the Validity of Opioid Medication Prescriptions
Philip Schwab — Cigarette Sales in Pharmacy: An Examination of the Relationship Value of Customers Who Purchase Cigarettes in Retail Pharmacies
Gayatri Gopal — Examining the Influence of Perceived Risk, Perceived Variability, and Confidence on Consumers Intentions to Seek OTC Medication Advice from Health Professionals
Su Bunniran — Examining Attributions of Blame and Consumer Trust Following Market Withdrawal of a Pharmaceutical Product
Nekshan Jalnawala — A Study of the Influence of Detail Message Characteristics on Physicians’ Beliefs about Medications and Credibility
Doug Paul — Pharmaceutical Marketers’ Perceptions of the Benefits and Drivers of the First-Mover Advantage in Pharmaceutical Markets: An Exploratory Study
Ravi Sadasivan — The Effect of Visual Images in Printed Direct-to-Consumer Advertising of Prescription Medications on External Information Search
William Lobb — The Effect of a School of Origin Variable on the Traditional Predictors and Prediction of Undergraduate Pharmacy Students’ Academic Performance
Mansi Shah — Effects of Direct-to-Consumer Advertising on the Quality of the Patient-Physician Relationship
Kornkanok Arntson — Determinants of Influence: An Investigation of Pharmacist Activity in the Pharmacy and Therapeutics Committee
James Blake Thompson — College Preparation for Pharmaceutical Sales Careers
Saurabh S. Sewak — Cybermarketing of Pharmaceuticals: A Case for Style Over Substance?
Vivek Kaisare — An Exploratory Investigation of the Potential for Direct-to-Consumer Advertising in the Type 2 Diabetes Market
Joseph Keith Bonnarens — Determining the Level of Patient Care Specialty Service Development and Entrepreneurial Characteristics Present in Independent Community Pharmacy
Donna Sue West — Information Technologies in Community Pharmacy Practice
Alicia Corrine Sanders Bouldin — Pharmacists’ Perceptions of Herbal Medicines: A Descriptive Study
John Paul Bentley — A Study of the Feasibility of the Utilization of Health Related Quality of Life Instruments in the Community Pharmacy Settings
Ram Mohan Chukkapalli — Consumer Self-Medication Behavior: the Influence of Different Factors on Consumer’s Purchase Decisions in the Selection of OTC Analgesics
For thesis project titles prior to 1995, please see the Department History page .

Pharmacy Administration Student Discusses His Academic Journey

Pharmacy (MPharm)
- Finding books
- Finding ebooks
- Your reading lists
- Finding journal articles
Dissertation Databases
- The Digital Library This link opens in a new window
- Referencing
- Study Skills This link opens in a new window
- Writing Development This link opens in a new window
- Systematic Reviews This link opens in a new window
- International students This link opens in a new window
- Decolonisation: what is it?
- Equality, Diversity and Inclusion: Diverse Library Resources
Dissertation eBooks
University of Lincoln Library Dissertation Showcase
- Library Dissertation Showcase The purpose of the dissertation showcase is to promote outstanding student work as exemplars of best practice. The dissertations in the showcase represent the “Top Ten” undergraduate and taught postgraduate dissertations selected by participating Schools within the University of Lincoln. These will normally have achieved a First Class degree and represent a range of subject areas.
The most comprehensive collection of dissertations and theses from around the world, spanning from 1743 to the present day and offering full text for graduate works added since 1997, along with selected full text for works written prior to 1997. It contains a significant amount of new international dissertations and theses both in citations and in full text. If you wish to access full text theses, please make sure the "Full text" option is ticked under the search bar.
Searchable index of UK theses with full text available to order.
Search and view open access theses and dissertations.
Information and a searchable index of international electronic theses and dissertations, including some open-access titles.
- << Previous: Finding journal articles
- Next: The Digital Library >>
- Last Updated: May 17, 2024 10:41 AM
- URL: https://guides.library.lincoln.ac.uk/pharmacy
- Admin login
- ICT Support Desk
- Policy Statement
- www.lincoln.ac.uk
- Accessibility
- Privacy & Disclaimer
- USC Libraries
- Research Guides
- Health Sciences
- Pharmacy Students: Year 3
- Literature Review
Pharmacy Students: Year 3: Literature Review
- Home and Help
- 567: Pharmacy Law
- 608: Therapeutics: Oncology and Immune Disorders
- 610: Therapeutics: Special Populations
- 611: Therapeutics: Infectious Diseases
- 619: Therapeutics: Cardiovascular System
- 620/621: Practice and Professionalism
- 622/623: Case Conference
- 633: Pharmacy Management and Economics
- 650: APPE Gateway
- Develop a Question
- Data Management
- IRB and CITI
- Make a Poster
- Cannabis User Safety (pharmacy elective)
- Community Pharmacy and Business Management (pharmacy elective)
- Geriatric Pharmacy (pharmacy elective)
- Global Pharmacy (pharmacy elective)
- Interprofessional Education (pharmacy electives)
- Marketing and Development in the Pharmaceutical Industry (pharmacy elective)
- Pharmaceutical Bioinformatics (pharmacy elective)
- Pharmaceutical Development (pharmacy elective)
- Pharmacy Education (pharmacy electives)
- Spanish for Pharmacists (pharmacy elective)
- Sports and Dietary Supplements (pharmacy elective)
- Toxicology and the Media (pharmacy elective)
- Research Resources
- Spanish for Health Care Professionals
- Career Resources
- Drug Information
Why do a literature review?
A literature review is an essential part of the research process. By conducting a review-- searching for and reading articles-- it helps you to understand who else has worked on your topic, methods that have been used to investigate this topic, and results/outcomes from similar studies, and develop your own research questions. When writing a literature review, you selective choose some of the readings you identified during your exploration, and write a short paragraph to prove to readers that your research question is necessary, important, and unanswered.
You do not need to conduct a Systematic Review. A Systematic Review is a research methodology where you attempt to gather all relevant literature describing a narrowly defined question. You may use a systematic technique to ensure you have searched relevant databases thoroughly, but your literature review does not need to be as extensive as a Systematic Review.
For a literature review, expect:
- That you will read two articles or book chapters for every one included in your final written review.
- You will need to search 2-6 subject-specific databases containing articles, book chapters, dissertations/theses, and other scholarly output. This typically happens at the start of the review.
- Your goal is to be thorough: expect to use both keywords and indexing terms to search databases
- You may need to use statistical sources, newspaper articles, articles from popular press magazines, reports from government organizations, or other types of unique sources in your review. These are unlikely to be a major part of your literature review, but can help to prove that a problem is widespread and deserving of research attention.
Process of a literature review
1. Define your question-- develop a hypothesis or research question. For example, "If we teach nurses to use asthma puffers correctly, does this increase patient-reported compliance with use of puffers and increase the rate of refills?"
2. Consider which databases would best provide information on your topic. Databases tend to focus on disciplines or subjects. If your research is about clinical use of drugs, you may only need to use clinically-focused databases. If your research concerns motivation for behavior, you may want to use psychology databases. If your research question is about economics of drugs, you may want to use business/economics-focused databases. Use this list of databases on this page or talk to a librarian to find the best databases for researching your question.
2. Break apart your question into concepts. With the question above, major concepts include nurses, asthma puffers, drug compliance, technique, refills.
3. For each concept, brainstorm synonyms. For example, puffer= inhaler, Flovent (or other brand names), etc.
3a. If using an indexed database like PubMed, look for the preferred indexing term for each concept and add it to your synonym list.
4. Combine the synonyms in your database. Use OR to connect synonyms. Use AND to connect different concepts. For example: (Nurse OR nurses) AND (inhaler OR puffer) AND (education OR training OR instruction).
4a. It is sometimes easier to look for research covering parts of your question rather than the whole. It is unlikely that there has been research on exactly your topic (nurses, and asthma puffers, and instruction, and compliance, and refills). Consider looking for research on nurses and inhalers; instruction with inhalers and if that affects patient compliance; use of puffers and refill rates; or other ways to break down the question into smaller parts.
5. Examine the results in your first database by reading the abstracts. Add to your list of synonyms by seeing what terms are used in the abstracts, and consider re-running the search with additional terms. Create a list of materials from this database that seem relevant. Next, download the full text of articles, book chapters, etc. on your list. Get older articles in print from the library's print collections. Repeat this process in your other databases.
6. Read the materials you found in step 5. Consider whether the information you have found allows you to demonstrate:
- That your topic is relevant and important, and deserves you and your group spending a year in investigating it.
- That you have found articles that cover a relevant timeframe and scope for your question.
- That you have seen any landmark studies, or major studies that have changed thinking on a topic. Not every topic area has landmark studies.
- That you can summarize the research methods that have been used to study this question/problem before and their results.
7. If you cannot demonstrate each of these, repeat steps 4 and 5 until you can.
8. Write your literature review, summarizing why your question/problem is important, other methods that have been used to investigate this problem and what they have found, and explain why your research question will help fill gaps in current knowledge. You can consider using the FINER criteria (Feasible, Interesting, Novel, Ethical, Relevant) to ensure you have covered the key aspects of the topic in enough depth. You will likely cite/reference about half the items you read. Your goal here is to select enough materials to demonstrate that you know this topic, without overwhelming your audience.
Help on literature reviews
- Bailey's Research for the Health Professional, 3rd ed ISBN: 9780803645134 Includes detailed help on literature reviews
Databases to use
USC subscribes to over 1300 databases, so only the primary databases you may use are listed here. If these do not cover your topic fully, examine the A-Z list of databases and read descriptions, or ask a librarian for help.
- Databases licensed by USC Libraries This link opens in a new window A-Z list of all databases licensed by USC Libraries.
Clinical databases
- CINAHL Complete This link opens in a new window Most comprehensive database of full-text for nursing & allied health journals from 1937 to present. Includes access to scholarly journal articles, dissertations, magazines, pamphlets, evidence-based care sheets, books, and research instruments.
- Embase This link opens in a new window Embase provides access to over more than 29 million citations for biomedical articles and conference proceedings. This version has been customized for USC users to find the full-text of articles from library holdings.
Basic sciences databases
- Biosis Citation Index This link opens in a new window Life sciences and biomedical research database covering pre-clinical and experimental research, methods and instrumentation, animal studies, and more.
- International Pharmaceutical Abstracts (Ovid) This link opens in a new window Worldwide coverage of pharmaceutical science and practice literature. Includes coverage of drug development, pharmacoeconomics, toxicity, regulation, and technology.
- SciFinder This link opens in a new window Articles, patents, and white papers describing chemicals. Includes information on structures, reactions, compounds, drug development, and manufacturing. Requires separate login. Go to USC's create an account page to register.
Social Sciences databases
- ERIC (ProQuest) This link opens in a new window Education focused database.
- Global Health This link opens in a new window Public health database, focus on international coverage.
- PsycINFO This link opens in a new window Abstract and citation database of scholarly literature in psychological, social, behavioral, and health sciences. Includes journal articles, books, reports, theses, and dissertations from 1806 to present.
- EconLit This link opens in a new window Literature in economics.
General Databases These databases include multiple subjects. They are useful databases for looking for landmark studies; determining if your topic is well-studied by another subject (as this would lead you to select a database that focuses on just that subject); and ensuring you did not miss research published in prominent scientific journals.
- Scopus This link opens in a new window Large abstract and citation database of peer-reviewed literature in science, technology, medicine, social sciences, and arts and humanities. Includes bibliometrics tools to track, analyze and visualize research.
- Web of Science This link opens in a new window
- Google Scholar This link opens in a new window Filtered Google Search finding scholarly journal articles, books, citations, and case law.
Writing & Citing Help
Use these handouts to get information on citing and writing; contact the Norris Medical Library with questions.
- Using Review Articles Review this handout to make sure you're using review articles appropriately.
- Quick Citing Reference- AMA 11th ed 2 page handout covering the basics of using AMA style, 11th edition
- << Previous: Develop a Question
- Next: Data Management >>
- Last Updated: Apr 12, 2024 2:21 PM
- URL: https://libguides.usc.edu/healthsciences/pharmyr3
- Open access
- Published: 08 May 2024
The digital transformation in pharmacy: embracing online platforms and the cosmeceutical paradigm shift
- Ahmad Almeman ORCID: orcid.org/0000-0002-6521-9463 1
Journal of Health, Population and Nutrition volume 43 , Article number: 60 ( 2024 ) Cite this article
726 Accesses
3 Altmetric
Metrics details
In the face of rapid technological advancement, the pharmacy sector is undergoing a significant digital transformation. This review explores the transformative impact of digitalization in the global pharmacy sector. We illustrated how advancements in technologies like artificial intelligence, blockchain, and online platforms are reshaping pharmacy services and education. The paper provides a comprehensive overview of the growth of online pharmacy platforms and the pivotal role of telepharmacy and telehealth during the COVID-19 pandemic. Additionally, it discusses the burgeoning cosmeceutical market within online pharmacies, the regulatory challenges faced globally, and the private sector’s influence on healthcare technology. Conclusively, the paper highlights future trends and technological innovations, underscoring the dynamic evolution of the pharmacy landscape in response to digital transformation.
Introduction
Digital technology is driving a massive shift in the worldwide pharmacy industry with the goal of improving productivity, efficiency, and flexibility in healthcare delivery. In the pharmacy industry, implementing digital technologies like automation, computerization, and robotics is essential to cutting expenses and enhancing service delivery [ 1 ]. With a predicted 14.42% annual growth rate, the digital pharmacy market is expanding significantly and is expected to reach a market volume of about $35.33 billion by 2026. This expansion reflects the pharmacy industry’s growing reliance on and promise for digital technologies [ 2 ].
Pharmacy services have always been focused on face-to-face communication and paper-based procedures. However, the drive for more effective, transparent, and patient-centered healthcare is clear evidence of the growing need for digital transformation. Breakthroughs like mobile communications, cloud computing, advanced analytics, and the Internet of Things (IoT) are reshaping the healthcare sector. These breakthroughs have the potential to greatly improve patient care and service delivery, as demonstrated in other industries including banking, retail, and media [ 3 ].
In the pharmacy industry, a number of significant factors are hastening this digital transition. Important concerns include the desire for cost-effectiveness, enhanced patient care, and more transparency and efficiency in medication development and manufacture. This change has been made even more rapid by the COVID-19 pandemic, which has highlighted the necessity for digital solutions to address the difficulties associated with providing healthcare in emergency situations [ 4 ].
In terms of specific technologies being adopted, artificial intelligence (AI) and machine learning are playing a pivotal role. The McKinsey Global Institute estimates that AI in the pharmaceutical industry could generate nearly $100 billion annually across the U.S. healthcare system. The use of AI and machine learning enhances decision-making, optimizes innovation, and improves the efficiency of research and clinical trials. This results in more effective patient care and a more streamlined drug development process [ 5 ].
The digital transformation in the pharmacy sector represents a pivotal shift in the delivery and experience of healthcare services. This evolution is more than a transient trend; it’s a fundamental alteration in the healthcare landscape [ 6 ]. The adoption of digital technologies is reshaping aspects of healthcare, including patient engagement and medication adherence, leading to enhanced healthcare outcomes. Research indicates that digital tools in pharmacy practices have resulted in more individualized and efficient patient care. Telehealth platforms, exemplified by companies like HealthTap, are being increasingly incorporated by pharmacies to augment patient care via technological solutions. The contribution of digital health technology to medication adherence is notable, employing a variety of tools such as SMS, mobile applications, and innovative devices like virtual pillboxes and intelligent pill bottles. These advancements are pivotal in addressing the critical issue of medication nonadherence in healthcare. Furthermore, digital health tools are empowering pharmacists with expanded clinical responsibilities, particularly in the management of chronic diseases like diabetes, where apps and smart devices provide essential features such as blood glucose tracking and medication reminders. This comprehensive integration of digital health into pharmacy practice signifies a transformative era in healthcare delivery and patient management [ 7 ].
Online platforms are being used increasingly by the pharmaceutical sector and educational institutions to improve efficiency, flexibility, and accessibility. The telepharmacy program at CVS Pharmacy is an example of how telepharmacy services, which provide remote counseling and prescription verification, bring pharmaceutical care to underprivileged communities [ 8 ]. Prescription accuracy and drug management are enhanced by e-prescribing software like Epic’s MyChart and digital health apps like Medisafe [ 9 ; 10 ]. Blockchain technology is also being investigated for transparent and safe supply chain management. Continuous learning and professional networking are made possible in education by Virtual Learning Environments (VLEs) like Moodle [ 11 ], simulation software like SimMan 3G Plus [ 12 ], Continuing Professional Development (CPD) platforms like the American Pharmacists Association [ 13 ], and online conference platforms, as shown in Fig. 1 . While these platforms offer significant benefits like enhanced access and cost-effectiveness, they also present challenges, including addressing the digital divide and ensuring the quality and credibility of online services to maintain professional standards and patient safety.
In this review, we summarized the digital transformation in the pharmacy sector, emphasizing the integration of online platforms and the emerging significance of cosmeceuticals. We discussed the global shift towards digital healthcare, including telehealth and online pharmacy services, and how these changes have been accelerated by the COVID-19 pandemic. The paper also examined the impact of digital technologies on pharmacy practice and education, with a focus on telepharmacy services, e-prescribing software, and digital health apps. Additionally, it addresses the challenges and opportunities presented by this transformation, including regulatory and safety concerns, and the need for continuous professional development in the digital era.
Comprehensive overview of different platforms in the pharmaceutical industry and education illustrating purposes and exemplary cases
The global impact of online pharmacy platforms
In recent years, the landscape of pharmacy practice and education has undergone a significant transformation, driven by technological advancements and catalyzed by the global COVID-19 pandemic. A study highlighting the increasing consumer trust in online medication purchases pre, during, and post-pandemic reveals a shift in consumer behavior towards online pharmacies [ 14 ]. This trend underscores a greater reliance on these platforms, where the perceived benefits significantly outweigh the perceived risks, indicating a positive reception and growing trust in digital healthcare solutions.
The adoption of telehealth, including telepharmacy, exemplifies this shift. In the United States, patient adoption of telehealth services surged from 11% in 2019 to 46%, with healthcare providers expanding their telehealth visits [ 15 ]. This shift is a reflection of how adaptable the healthcare sector is to digital platforms and how customer acceptance is increasing. The epidemic has also served as a catalyst, hastening the creation and uptake of online telepharmacy services throughout the world. The “new normal” has forced the addition of new platforms to support established sources of health information. The creation and evaluation of an online telepharmacy service in the Philippines during the pandemic serves as an example of this, demonstrating how quickly the global pharmacy industry adopted digital solutions. These services are essential for providing and elucidating pharmaceutical information within the context of primary healthcare delivery; they are not merely supplementary [ 16 ].
Simultaneously, pharmacist-led companies such as MedEssist and MedMehave, innovated digital platforms to facilitate services like flu shots or COVID-19 tests, reflecting a move towards customer-centric, digital-first services [ 17 ]. This transition enhances convenience and access to care but also introduces significant regulatory challenges. As the growth of online medicine sales disrupts traditional pharmacy markets, navigating these challenges becomes crucial for maintaining patient safety, quality standards, and fostering a trustworthy online healthcare environment [ 18 ].
Parallel to the practice changes, educational platforms for pharmacy have also evolved, especially under the impetus of the pandemic. These platforms have integrated a mix of traditional and student-centered teaching methodologies, including remote didactic lectures and on-site experiential training. The implementation of blended learning approaches, which combine remote lectures with on-site laboratory classes, reflects a broader educational trend towards hybrid models. This approach aims to leverage the advantages of both online and traditional methods, offering a more flexible and potentially more effective educational experience [ 19 ].
It takes more than just implementing new tools to integrate educational technology into pharmacy education, it also requires understanding how these technologies affect instruction and student learning. To effectively improve the educational experience, their utilization must have a purpose. There is an increasing amount of scholarly interest in this field, as evidenced by systematic reviews of the effects of new technologies on undergraduate pharmacy teaching and learning [ 20 ]. These digital platforms will probably become more significant in the future of pharmacy education, helping to mold the profession and guaranteeing that pharmacists are equipped to fulfill the ever-changing demands of the healthcare system. This development is indicative of a larger trend in the healthcare industry toward a more flexible, patient-focused, and technologically advanced environment [ 21 ].
Digital transformation in global healthcare
The recent advancements in digital transformation within global healthcare are significantly reshaping the landscape of healthcare and pharmacy services. These transformations are largely driven by the integration of digital technologies, which are redefining the tools and methods used in health, medicine, and biomedical science, ultimately aiming to create a healthier future for people worldwide [ 22 ]. In a 2018 report [ 23 ], Amazon’s potential entry into the $500 billion U.S. pharmacy market, the second-largest retail category, through mail-order and online pharmacies was highlighted as a significant industry disruptor. With licenses in at least 12 states in the US and a strategy focused on bypassing middlemen, Amazon’s historical success positions it to transform the pharmacy landscape, promising enhanced efficiency and cost savings for consumers.
One of the critical areas identified in recent research is the establishment of five priorities of e-health policy making: strategy, consensus-building, decision-making, implementation, and evaluation. These priorities emerged from stakeholders’ perceptions and are crucial for the effective integration and adoption of digital health technologies [ 24 ]. This holistic approach is increasingly relevant for scholars and practitioners, suggesting a focus on how multiple stakeholders implement digital technologies for management and business purposes in the healthcare sector [ 25 ]. The deployment of technological modalities, encompassed within five distinct clusters, can facilitate the development of a digital transformation model. This model ensures operational efficiency through several dimensions: enhanced operational efficacy by healthcare providers, the adoption of patient-centered methodologies, the integration of organizational factors and managerial implications, the refinement of workforce practices, and the consideration of socio-economic factors [ 25 ].
Studies focusing on value creation through digital means suggest healthcare as a consumer-centric realm ripe for center-edge transformations, characterized by self-service and feedback cycles. These transformations are vital in addressing inherent tensions between patients and physicians, steering the focus towards value co-creation and service-dominant logic [ 26 ]. Participatory design and decision-making approaches are emphasized for enhancing health information technology’s performance and institutional healthcare innovation. Such approaches are particularly crucial in developing national electronic medical record systems and improving chronic disease treatment through electronic health records. Additionally, telehealth research integrates patients’ perceptions, contributing to the understanding of technology, bureaucracy, and professionalism within healthcare [ 27 ].
The impact of health information technology (HIT) on operational efficiencies is profound. Empirical studies, such as those by Hong and Lee [ 28 ], Laurenza et al. [ 29 ], and Mazor et al. [ 30 ], demonstrate positive correlations between HIT and patient satisfaction, quality of care, and operational efficiency. However, challenges remain, as Rubbio et al. [ 31 ] highlight deficiencies in resilience-oriented practices for patient safety. Organizational and managerial factors in digital healthcare transformation also receive significant attention. Hikmet et al. [ 32 ] and Agarwal et al. [ 33 ] investigate the role of organizational variables and barriers in HIT adoption, whereas Cucciniello et al. [ 34 ] delve into the interdependence between implementing electronic medical records (EMR) systems and organizational conditions. Further, Eden et al. [ 35 ] and Huber and Gärtner [ 36 ] explore workforce adaptations and the implications of health information systems in hospitals that can increases transparency of work processes and accountability. Lastly, examining healthcare financialization and digital division provides an international perspective, contrasting the regulated environment in the EU with the US’s use of online medical crowdfunding as a potential solution to reduce bankruptcy [ 37 ; 38 ]. Collectively, these studies suggest a comprehensive model where stakeholders leverage digital transformation for management, enhancing operational efficiency in healthcare service providers.
Marques and Ferreira [ 39 ] performed a systematic literature review of digital transformation in healthcare, spanning the period from 1973 to 2018. Utilizing the SMARTER (Simple Multi-attribute Rating Technique Exploiting Ranks) method, 749 potential articles were analyzed, culminating in the prioritization and selection of 53 articles for detailed examination. The literature was organized into seven thematic areas: (1) Integrated management of IT in healthcare, (2) Medical images, (3) Electronic medical records, (4) IT and portable devices in healthcare, (5) Access to e-health, (6) Telemedicine, and (7) Privacy of medical data. It was observed that the predominant focus of research resides in the domains of integrated management, electronic medical records, and medical images. Concurrently, emerging trends were identified, notably the utilization of portable devices, the proliferation of virtual services, and the escalating concerns surrounding privacy. See Fig. 2 for visual representation of multifaceted digital transformation in healthcare.
Visual representation of multifaceted digital transformation in healthcare: a synthesis of provider-patient dynamics, HIT impact, and strategic management. HIT; health information technology, HC; healthcare, EMR; electronic medical records. IT; information technology, Pt.; patient
Telehealth and online pharmacy advancements in pandemic management
In the realm of online pharmacies and telehealth, digital health technologies have been instrumental in managing the COVID-19 pandemic through surveillance, contact tracing, diagnosis, treatment, and prevention. These technologies ensure that healthcare, including pharmacy services, is delivered more effectively, addressing the challenges of accessibility and timely care. The role of telemedicine and e-pharmacies, in particular, has been emphasized in improving access to care worldwide. By enabling remote consultations and drug delivery, these platforms are making healthcare more accessible, especially in regions where traditional healthcare infrastructure is limited or overstretched [ 40 ].
The Canadian Virtual Care Policy Framework advocates for the swift adoption and integration of virtual care, propelled by the COVID-19 pandemic. It emphasizes enhancing access and quality, ensuring equity and privacy, and devising appropriate remuneration models, employing a collaborative, patient-centered approach while addressing digital disparities. During the COVID-19 pandemic, Canadian provinces and territories rapidly adopted virtual health care, leading to 60% of visits being virtual by April 2020, up from 10 to 20% in 2019. However, these implementations were often temporary and not fully integrated into healthcare systems. By August 2020, virtual visits decreased to 40%, with variations across regions, while provinces and territories used temporary billing codes for these services. The framework’s “Diagnostique” provides a thorough analysis of policy enablers and strategies for virtual care, underscoring the need for comprehensive policy and partnership engagement [ 41 ]. In the context of digital transformation in pharmacy, the Hospital News article outlines the application and infrastructure of telepharmacy services in Canada, highlighting the geographical challenges and the early adoption of telepharmacy in certain regions since 2003. It notes the use of various technologies like Medication Order Management, Videoconferencing, and Remote Camera Verification. Although lacking specific quantitative data, the article underscores the necessity for expanded telepharmacy services to ensure uniform care quality across diverse locations [ 42 ].
Similarly, Telehealth offers extensive resources for patients and providers in the United States, emphasizing programs like the Affordable Connectivity Program and Lifeline to facilitate access. The Health Resources and Services Administration enhances telehealth through support services, research, and technical assistance, reflecting a significant outreach impact [ 43 ]. The Office for the Advancement of Telehealth (OAT) under Health Resources and Services Administration (HRSA) works to improve access to quality health care through integrated telehealth services in the US. It supports direct services, research, and technical assistance, with over 6,000 telehealth technical assistance requests sent to Telehealth Resource Centers and approximately 22,000 patients served [ 44 ].
Internationally, In the UK, the National Health Service (NHS) spearheads digital health and care, providing significant innovation opportunities through vast data management. Support for digital health spans various stages, from discovery with organizations like Biotechnology and Biological Sciences Research Council (BBSRC) and Intelligent Data Analysis (IDA) research group, to development with networks such as Catapults and CPRD, and delivery with entities like the Academic Health Science Networks (AHSNs) and DigitalHealth.London. Regulatory bodies like the Medicines and Healthcare products Regulatory Agency (MHRA) and NICE ensure safety and efficacy. The collaborative ecosystem involves academic, healthcare, and industry stakeholders, aiming to enhance health and care services through technology and innovation [ 45 ].
In Australia, the government’s investment of over $4 billion into COVID-19 telehealth measures has facilitated universal access to quality healthcare. This initiative has provided over 85 million telehealth services to more than 16 million patients, with approximately 89,000 healthcare providers engaging in this service delivery. From 1 January 2022, telehealth services, initially introduced in response to COVID-19, will become an ongoing part of Medicare. This will allow eligible patients across Australia continued access to general practice (GP), nursing, midwifery, and allied health services via telehealth, deemed clinically appropriate by the health professional [ 46 ].
European nations such as the Netherlands, Austria, and Italy are at the forefront of implementing cross-organizational patient records, significantly enhancing telehealth communication and facilitating cross-border healthcare. The role of strong government support in advancing telehealth is pivotal. Ursula von der Leyen, the President of the European Commission, has been a prominent advocate for eHealth. She proposed the establishment of a European Health Data Space to streamline health data exchange across member states. France, a leader in telehealth legislation for nearly a decade, has pioneered a public funding scheme for tele-expertise at a national scale. Despite these advancements, challenges like legislative barriers and the lack of consistent political direction continue to impede progress in the telehealth domain [ 47 ].
The Asia-Pacific region anticipates a surge in telehealth adoption driven by digital demand and pandemic-induced behavioral changes, while South East Asia exhibits widespread telehealth growth across healthcare aspects [ 48 ]. The telehealth adoption across the Asia-Pacific region has shown remarkable growth between 2019 and 2021 and is projected to continue rising by 2024. China’s adoption nearly doubled to 47% and is expected to reach 76%. Indonesia’s usage more than doubled to 51%, with a forecast of 72%. Malaysia and the Philippines both anticipate reaching a 70% adoption rate, increasing from 30% to 29%, respectively. India’s adoption is projected to more than double to 68%, while Singapore, which had a significant increase from 5 to 45%, is expected to achieve a 60% adoption rate. This trend indicates a robust uptake of telehealth services in the region [ 48 ].
Global telemedicine and E-pharmacy policy dynamics
In the context of telemedicine and e-pharmacy regulations within South East Asia, a notable distinction emerges with Singapore, Malaysia, and Indonesia being the only countries to have formalized legal frameworks governing both telemedicine practices and the dissemination of electronic information. In these countries, tele-consultation is restricted to patients already under the care of healthcare practitioners or as part of ongoing treatment, specifically in Singapore and Malaysia. Additionally, for scenarios requiring more intensive medical intervention, such as new referrals, emergency cases, or invasive procedures, both Malaysia and Indonesia mandate physical presence and face-to-face consultations, emphasizing a cautious and regulated approach to remote healthcare. In Malaysia, the regulations further stipulate that online prescriptions, excluding narcotics and psychotropic substances, are permissible solely under the continuation of care model, reflecting a judicious use of digital prescription services [ 49 ].
In Central and Eastern Europe (CEE), telemedicine has experienced substantial growth, primarily catalyzed by the COVID-19 pandemic, which necessitated rapid advancements in technology and alterations in healthcare practices. The region’s robust digital infrastructure, coupled with the innovative drive of local companies and the challenges posed by an aging demographic, has significantly contributed to this expansion. According to the European Commission’s Market Study on Telemedicine, the global telemedicine market was projected to grow annually by 14% by 2021, a rate that was likely surpassed due to the pandemic’s impact. More specifically, the Europe Telehealth Market, valued at US $6,185.4 million in 2019, is anticipated to witness an annual growth rate of 18.9% from 2020 to 2030. This trend underscores the increasing reliance on and potential of telemedicine in addressing healthcare needs in the CEE region [ 50 ].
In the Middle East, telehealth and telepharmacy, have seen varied degrees of adoption and progress. Despite attempts to reform healthcare delivery in the region, the progress of telemedicine has been somewhat slow, with certain expectations yet to be fully realized. However, there has been notable development in the use and adoption of these technologies [ 51 ]. In a survey comparing the utilization of digital-health applications in Saudi Arabia and the United Arab Emirates (UAE), it was observed that a higher percentage of Saudi participants have utilized online pharmacy services (48%) compared to the UAE (36%). Conversely, awareness of teleconsultation services without prior use was higher in the UAE (43%) than in Saudi Arabia (35%). Retention data indicates that a significant proportion of users in both countries continue to engage with these services, with 80% of Saudi participants and 71% of UAE participants using teleconsultations at varying frequencies. Notably, a substantial majority of users in Saudi Arabia reported regular use of online pharmacies (90%), slightly higher than the UAE (78%), reflecting robust ongoing engagement with these digital health modalities. Notably, consumer adoption of telehealth products is primarily driven by time savings (48%) and convenience (47%), with 24-hour accessibility and efficacy both influencing 34% of users. Affordability and personal recommendations are also notable factors, while a wide range of options and quality are lesser but relevant considerations [ 52 ].
In response to the COVID-19 pandemic, a cross-sectional study was conducted among 391 licensed community pharmacists in the United Arab Emirates to assess the adoption and impact of telepharmacy services. The study revealed a predominant use of telepharmacy services, particularly via phone (95.6%) and messaging applications (80.0%). The findings highlighted that pharmacies with more pharmacists and those operating as part of a group or chain were more likely to implement a diverse range of telepharmacy services. The study identified significant barriers to telepharmacy adoption in individual pharmacies, including limited time, inadequate training, and financial constraints. There was a noticeable shift in service provision during the lockdown, with an increased reliance on telepharmacy, especially among pharmacies serving 50–100 patients per day. However, a reduction in services such as managing mild diseases and selling health products was observed during the lockdown period. The study concluded that telepharmacy played a pivotal role in supporting community pharmacies during the pandemic, with its expansion facilitated by the UAE’s advanced internet infrastructure, supportive health policies, and widespread digital connectivity [ 53 ]. Collectively, these insights reflect a global shift towards integrating and enhancing telehealth services as a response to emerging healthcare needs and technological advancements.
Unni et al. [ 54 ] provided an extensive review of telepharmacy initiatives adopted globally during the COVID-19 pandemic. Predominantly, virtual consultations were utilized to enable at-risk patients and others to remotely access pharmacists, thereby monitoring chronic illnesses, optimizing medication usage, and providing educational support [ 55 ]. Home delivery of medicines was widely implemented to decrease the necessity for in-person visits and mitigate exposure risks [ 56 ]. Additionally, patient education was prioritized to ensure effective management of health conditions from a distance [ 57 ]. Notably, a network of hospitals in China developed cloud-pharmacy care, allowing patients to consult pharmacists via text and the internet, while Spain utilized information and communication technologies for remote pharmaceutical care [ 58 ; 59 ]. Zero-contact pharmaceutical care, introduced in China, facilitated online medication consultations, eliminating direct contact [ 60 ]. The Kingdom of Saudi Arabia and other regions adapted new e-tools and teleprescriptions to enhance service accessibility [ 61 ]. The U.S. focused on credentialing pharmacists for telehealth to ensure competent service provision, and New Zealand implemented hotline numbers for phone consultations to further reduce physical visits [ 62 ; 63 ]. These initiatives reflect a significant shift towards innovative, technology-driven solutions in pharmaceutical care during a global health crisis. Refer to Fig. 3 for a graphical depiction of the worldwide distribution and applications of telepharmacy initiatives.
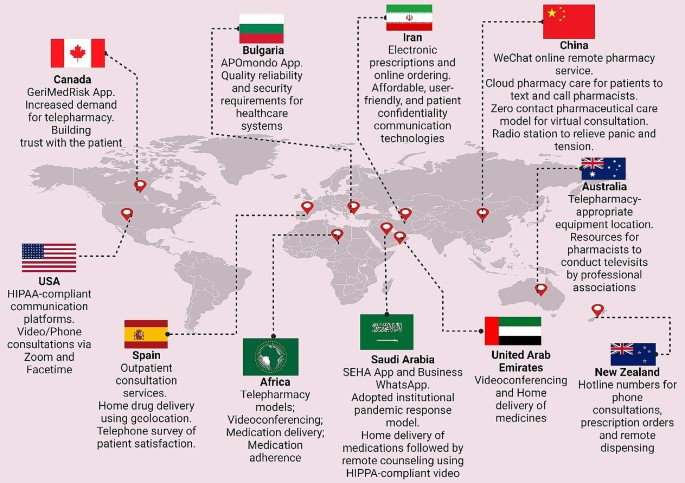
The global distribution of telepharmacy programs with an analysis of geographical distribution, technological applications, and associated benefits
Tracing the Private Sector’s Impact on Healthcare’s Technological Transformation
The role of the private sector in the fourth industrial revolution.
The World Economic Forum underscores the private sector’s leading role in digital inclusion and the acceleration of actions pertinent to the Fourth Industrial Revolution. This revolution affects economies, industries, and global issues profoundly, indicating the private sector’s critical role in driving technological advancements and digital platforms that deliver impactful healthcare solutions [ 64 ].
Mapping digital transformation in healthcare
A comprehensive analysis performed by Dal Mas et al. [ 65 ] meticulously maps the intricate terrain of digital transformation in healthcare, spotlighting the private sector’s instrumental role. Initially, the investigation encompassed an extensive array of diverse studies, leading to the identification of five main areas of digital technologies: smart health technologies, data-enabled and data collection technologies, Industry 4.0 tools and technologies, cognitive technologies, and drug & disease technologies. These domains frame the future research pathways, primarily steered by the private sector’s innovative drive. A significant proportion of the literature addresses healthcare broadly, suitable for both private and public sectors, yet a notable segment specifically focuses on the private sector’s endeavors, with a pronounced emphasis on the pharmaceutical domain [ 66 ; 67 ].
Public-private partnerships in healthcare delivery
The highlighted technologies, including digital platforms and telemedicine, exemplify the private sector’s trailblazing contributions to digital healthcare advancements. For instance, public-private partnerships (PPP) in India have emerged as a pivotal model for realizing universal healthcare (UHC), especially against the backdrop of acute healthcare shortages and urban-rural divides. Notably, mega PPP projects have successfully deployed technology-enabled remote healthcare (TeRHC), demonstrating its feasibility and impact in reaching isolated communities. These initiatives, overcoming various challenges, serve as a compelling example for global adoption, underscoring the transformative role of PPP in healthcare delivery [ 68 ].. Furthermore, a considerable majority of the literature in telemedicine underscores the necessity for profound research implications, yet a significant minority suggests policy implications [ 69 ; 70 ], reflecting a complex synergy between the private and public sectors in sculpting the digital healthcare framework [ 71 ]. This synthesis underscores the private sector’s critical influence in propelling the digital transformation in healthcare, charting a course that progressively fuses technological innovation with healthcare provision.
A study highlights Indonesia’s strategic initiatives to capitalize on telehealth business opportunities, driven by the Ministry of Research and Technology’s robust support for Technology-Based Start-up Company schemes [ 72 ]. With a demographic boon of 298 million from 2020 to 2024, escalating non-communicable diseases (71%), and a growing base of 222.4 million JKN participants, the stage is set for transformative growth. Despite a critical shortage of health workers (0.4 doctors per 1000 population), the enthusiasm for telemedicine is evident, with 71% satisfaction in hospital telemedicine and 32 million active telehealth users. The Ministry’s foresight in fostering technology start-ups, exemplified by the TEMENIN platform with its 11 health platforms, is steering Indonesia towards a future where high-quality healthcare is accessible and sustainable.
Lab@AOR: a model for PPPs in healthcare sector
The “Lab@AOR” initiative stands as a paradigmatic example of PPPs effectuating digital transformation within the healthcare sector. This strategic collaboration, between the University Hospital of Marche and Loccioni [ 73 ], a private entity, underscores the capacity of PPPs to navigate intricate challenges, stimulate international cooperation, and contribute to the development of sustainable, patient-centric healthcare solutions. Specifically, Lab@AOR was instituted to confront the nuanced challenges associated with the robotization of healthcare service delivery, highlighting the initiative’s role in fostering technological advancement through public and private sector synergy [ 74 ]. The project illustrates the evolution of Lab@AOR through three main phases: the pioneering stage, where groundwork for collaboration was laid; the nurturing stage, where collaborative exchanges were fostered; and the harvesting stage, wherein the potential of the PPP was fully unleashed. In the pioneering stage, Lab@AOR focused on a critical healthcare service component: the in-hospital preparation of medications for oncological patients. The University Hospital of Marche identified a need for innovation to improve service quality, efficiency, and safety, while Loccioni sought a real-life setting to test and refine its robotized system, APOTECAchemo [ 75 ]. This convergence of needs led to a symbiotic partnership aiming to enhance healthcare delivery through technological advancement.
During the nurturing stage, the partnership expanded the scope of APOTECAchemo to include non-oncological medications and developed additional tools like APOTECAps for manual preparation support. This phase was characterized by intensive collaboration, knowledge sharing, and continuous innovation, demonstrating the dynamic capability of the PPP to adapt and evolve in response to emerging healthcare challenges. The harvesting stage marked the international expansion of Lab@AOR, transforming it from a local initiative to an international community focused on leveraging digitalization and robotization to improve care quality and patient-centeredness. The PPP’s growth was catalyzed by its open perspective and inclusive approach, engaging entities from various cultural and institutional contexts, and fostering a network of 31 nodes across 19 countries and 3 continents.
Advancements in telehealth business models and frameworks
In their investigative study, Velayati et al. [ 76 ] delved into the articulation of emergent business models in telehealth and scrutinized the deployment of established frameworks across a variety of telehealth segments. The research spanned an extensive range of sectors, notably telemonitoring, telemedicine, mobile health, and telerehabilitation, alongside telehealth more broadly. The scope further extended to encompass niche areas such as assisted living technologies, sensor-based systems, and specific fields like mobile teledermoscopy, teleradiology, telecardiology, and teletreatment, presenting a thorough analysis of the telehealth landscape. Within the telemedicine and telehealth services sector, Barker et al. [ 77 ] introduced the Arizona Telemedicine Program (ATP) Model, a quintet-layer approach aimed at efficiently distributing telemedicine services throughout Arizona. Complementing this, Lee and Chang [ 78 ] proposed a four-component model specifically tailored for mobile health (mHealth) services pertaining to chronic kidney disease, focusing on offering a cost-effective platform for disease support and management. In the realm of telemonitoring, Dijkstra et al. [ 79 ] utilized the Freeband Business Blueprint Method (FBBM), which includes service, technological, organizational, and financial domains, to facilitate multiple telemonitoring services. Furthermore, the systemic and economic differences were explored in care coordination through Business to customer (B2C) and business (B2B) models for telemonitoring patients with chronic diseases, with the B2C model’s economic advantages were highlighted [ 80 ].
General telemedicine frameworks also received attention. Lin et al. [ 81 ] constructed a six-component framework analyzing major telemedicine projects in Taiwan, while Peters et al. [ 82 ] developed the CompBizMod Framework in Germany, encompassing value proposition, co-creation, communication and transfer, and value capture, designed to evaluate and enhance competitive advantages in telemedicine. In the specialized field of telecardiology, a comprehensive nine-component sustainable business model was crafted to facilitate mutual benefits for service providers and patients. This model emphasizes the importance of a holistic approach in ensuring the longevity and effectiveness of healthcare delivery within this domain [ 83 ]. Meanwhile, Mun et al. [ 84 ] presented a suite of five teleradiology business models aimed at providing effective, high-quality, and cost-efficient diagnoses.
The teletreatment sector saw innovative models from Kijl et al. [ 85 ], who designed a model for treating patients with chronic pain, focusing on the interrelation of components in the value network and the role of information technology. Complementarily, Fusco and Turchetti [ 86 ] introduced four models for telerehabilitation post-total knee replacement, emphasizing partnerships between care units and equipment suppliers to reduce costs and waiting lists. The mHealth and assisted living technology sector witnessed the introduction of a wearable biofeedback system model by Hidefjäll and Titkova [ 87 ], which employed Alexander Osterwalder’s Business Model Canvas and focused on a comprehensive commercialization process. Additionally, Oderanti and Li [ 88 ] presented a seven-component sustainable business model for assisted living technologies, aimed at encouraging older individuals to invest in eHealth services while reducing the pressure on health systems. These diverse clusters and models reflect the multifaceted nature of telehealth, each tailoring its approach to meet the unique demands of its domain. They collectively aim to optimize service delivery, stakeholder involvement, cost efficiency, and patient care quality, marking significant strides in the ongoing evolution of digital healthcare.
Challenges and biases in healthcare technology
One key aspect is the emergence of novel medical technologies and their potential biases. These biases are often a result of insufficient consideration of patient diversity in the development and testing phases. For example, disparities in the performance of medical devices like pulse oximeters among different racial groups have been observed, potentially due to a lack of diverse representation in clinical trials. This indicates a tendency for the development of healthcare technologies that may not adequately serve all patient populations [ 89 ]. A study on the profitability and risk-return comparison across health care industries highlights the use of return on equity (ROE) as a measure of profitability from a shareholder’s perspective. This measure combines profit margin, asset utilization, and financial leverage. The study analyzed financial data of publicly traded healthcare companies, providing insights into the financial dynamics of the healthcare sector. It revealed that while companies like Pfizer Inc. and UnitedHealth Group reported similar profitability, they had substantial differences in profit margin and asset utilization, indicating diverse financial strategies within the healthcare sector. This study underscores the complexity of financial performance in healthcare, where profitability measures need to be balanced with risk assessment and the broader impact on healthcare provision [ 90 ].
Additionally, an article discusses the benefits, pitfalls, and potential biases in healthcare AI. It emphasizes that as the healthcare industry adopts AI, machine learning, and other modeling techniques, it is seeing benefits for both patient outcomes and cost reduction. However, the industry must be mindful of managing the risks, including biases that may arise during the implementation of AI. Lessons from other industries can provide a framework for acknowledging and managing data, machine, and human biases in AI. This perspective is crucial in understanding how the integration of advanced technologies in healthcare can be influenced by the drive for profitability and efficiency, possibly at the expense of equitable and patient-centered care [ 91 ; 92 ].
Cosmeceuticals in the online pharmacy market
Cosmeceuticals, a term derived from the combination of cosmetics and pharmaceuticals, refer to a category of products that are formulated to provide both aesthetic improvements and therapeutic benefits. These products, typically applied topically, are designed to enhance the health and beauty of the skin, going beyond the mere cosmetic appearance. The exploration of cosmeceuticals in the online pharmacy market reveals a multifaceted and rapidly expanding industry. Bridging the gap between cosmetics and pharmaceuticals, they form a significant portion of the skincare industry. Cosmeceuticals are formulated from various ingredients, with their main categories being constantly discussed and analyzed in the scientific community [ 93 ]. They have taken a considerable share of the personal care industry globally, constituting a significant part of dermatologists’ prescriptions worldwide [ 94 ]. This surge is further fueled by increasing consumer demand for effective and safe products, including anti-aging skincare cosmeceuticals, a need which has been intensified by concerns over pollution, climate change, and the COVID-19 pandemic [ 95 ].
The global cosmeceuticals market is experiencing robust growth. Valued at USD 56.78 billion in 2022, it’s projected to expand to USD 95.75 billion by 2030, with a compound annual growth rate (CAGR) of 7.45%. This growth trajectory is propelled by the innovative integration of bioactive ingredients known for their medical benefits [ 96 ]. Another report confirms this upward trend, indicating the market was worth $45.56 billion in 2021 and is on a path of significant growth to USD 114 billion by 2030. The global disease burden is significantly impacted by various skin diseases, with dermatitis, psoriasis, and acne vulgaris among the most prevalent, contributing 0.38%, 0.19%, and 0.29% respectively. The pervasive nature of these conditions drives a substantial demand for effective treatments, propelling the integration of cosmeceuticals into the online pharmacy market. This integration not only offers convenient access to a range of therapeutic skincare products but also caters to the rising consumer inclination towards self-care and preventive healthcare. As a result, the online availability of cosmeceuticals is not just addressing the immediate needs of individuals suffering from skin conditions but is also reshaping the landscape of personal healthcare by making specialized treatments more accessible and customizable [ 97 ]. See Fig. 4 .
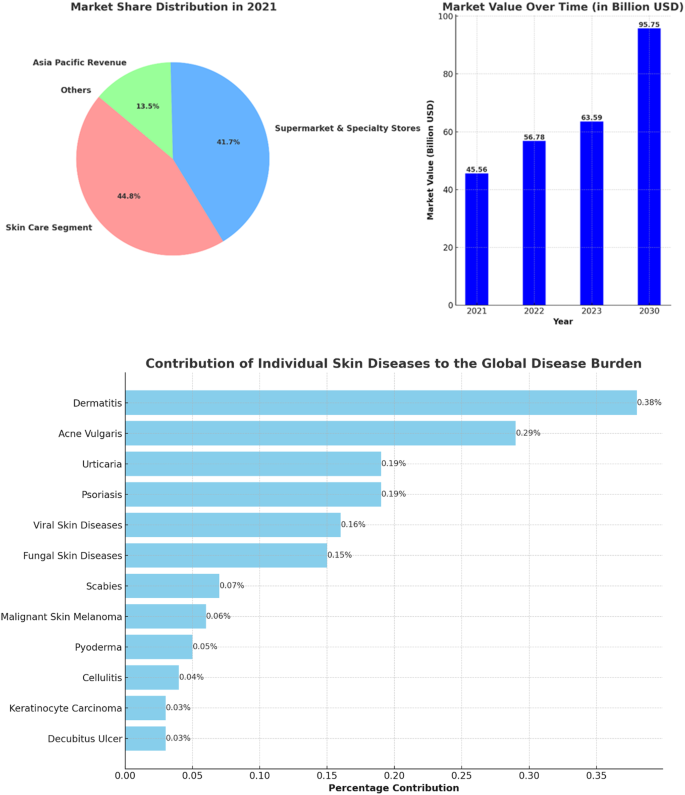
The left panel presents the market share distribution for key segments in the cosmeceuticals industry in 2021, including Skin Care Segment, and Supermarket & Specialty Stores, for Asia Pacific Revenue, with percentages for each category. The right panel displays the market value progression over time from 2021 to the projected value in 2030, with bold numbers indicating the value in billion USD for each year. The lower horizontal bar chart depicts the percentage contribution of various skin diseases to the global disease burden
Several factors are contributing to this expansion of the cosmeceuticals market. The market is driven by innovation in natural ingredients and a significant penetration of internet, smartphone, and social media applications, which attract potential consumer populations and reflect constantly changing consumer behavior [ 98 ]. The cosmeceuticals market’s robust CAGR and revenue share, especially in regions like Asia Pacific, further signify its burgeoning presence and potential within the global market [ 99 ]. Integration into online pharmacies is a key aspect of this market’s evolution, offering easier access to these products for a wider customer base. As the market continues to grow, it’s anticipated that the blend of cosmeceuticals with online pharmaceutical platforms will become increasingly seamless, offering consumers a diverse range of accessible, effective, and beneficial skincare and health products. This integration is likely to be driven by the growing trend of e-commerce and digitalization in healthcare and personal care sectors.
The landscape of online pharmacies, particularly concerning cosmeceuticals, is evolving. While the overall penetration for non-specialty drugs in mail-order and online pharmacies is low, they represent a significant portion of specialty prescription revenues at 37%. Despite this, only 13% of consumers consider these as their primary pharmacy choice, indicating a growing but still emerging market. Strategies are in place to enhance the market appeal of these pharmacies, focusing on speed, convenience, and personalized experiences, such as video telehealth visits, to attract a broader consumer base [ 100 ].
The dissertation “L’Oréal Portugal: A Digital Challenge for the Active Cosmetics Division” authored by Ascenso [ 101 ] provides an in-depth examination of the impact of digital evolution on the Portuguese cosmeceutical sector and its implications for L’Oréal, a significant cosmetics company. It posits that while L’Oréal has foundational digital competencies, the rapidly evolving digital landscape presents a broad spectrum of potential risks and opportunities. The study details the operations of L’Oréal’s Active Cosmetics Division, which manages brands predominantly sold in pharmacies and parapharmacies, and explores the potential repercussions of digitalization on L’Oréal Portugal’s strategic and operational frameworks. Furthermore, the thesis highlights the expanding role of e-pharmacies and the need for legal reforms to facilitate their operation. It discusses the prevalent trends in the cosmetic industry, such as the increasing demand for natural, male-focused, and environmentally friendly products. The dissertation scrutinizes L’Oréal’s strategic pillars, including innovation, acquisition, and regional growth, emphasizing the need for the company to integrate advanced technologies and recalibrate its business methodologies in light of digital progression [ 101 ]. Although L’Oréal has initiated some digital strategies targeting consumers and pharmacies, there’s a recognized need for an intensified focus on digital marketing aimed at clients. An exploratory attempt by L’Oréal to implement an online ordering platform for pharmacies did not meet success, indicating possible industry unreadiness for such advancements. This case study serves as a critical examination of how traditional companies in the pharmaceutical and cosmetics sectors must adapt to the digital age’s challenges and opportunities [ 101 ].
In a collaborative endeavor with L’Oréal, an associated digital agency provided a comprehensive suite of services that encompasses the full management of social media pages, the development of e-commerce websites, the establishment of Customer Relationship Management (CRM) platforms tailored for pharmacies, and the execution of digital campaigns leveraging QR codes, SMS marketing, and newsletters. These digital tools confer a competitive edge, facilitating a deeper comprehension of consumer behavior and the potential to augment value extraction from customer interactions. For the laboratories, particularly those associated with cosmetics, the advantages are twofold: an increase in sell-out figures, thereby enhancing direct sales to end consumers, and a boost in sell-in metrics, reflecting a rise in transactions to pharmacies or wholesalers. The online ordering feature, as noted by João Roma, a manager at La Roche-Posay, could result in a cacophony of processes if laboratories were to individually develop distinct methods. He advocates for the utilization of pre-existing platforms, such as the established e-learning infrastructure, to spearhead ventures into the online marketplace [ 101 ].
A survey conducted specifically for L’Oréal’s e-learning platform, cosmeticaactiva.pt [ 102 ], across the Portuguese landscape garnered responses from 324 participants, comprising 71% general pharmacists, 13% technical assistants, 8% directors, 7% individuals responsible for procurement from laboratories, and 2% beauty/cosmetic advisors. The findings from this survey underscore the pervasive adoption of digital tools within the pharmacy sector: 82% of respondents affirmed the presence of their pharmacies on social media platforms, 80% reported the use of basic management software, 64% indicated the deployment of advanced management systems, 61% were conversant with online ordering systems directed at laboratories, 38% utilized a store locator, 28% had an established website presence, and a smaller segment of 12% offered online shopping facilities.
Another survey conducted within this study to evaluate the significance of dermocosmetic products in pharmacies yielded a mean importance rating of 4.38 out of 5, indicating that a majority of pharmacists consider these products to be highly important to their business operations. Factors critical to the differentiation of a proficient laboratory/supplier were innovation and cost-effectiveness, with mean scores of 1.9 and 2.7 respectively, on a scale from 1 (most important) to 5 (least important). A substantial majority of pharmacists, amounting to 81.8%, perceive their pharmacies as beacons of innovation and modernity. Detailed interviews elucidated that digital tools are indispensable in augmenting sales for cosmeceutical products by catalyzing demand—a dynamic not feasible with medicinal products. These tools are paramount in managing customer loyalty, facilitating enhanced communication with existing clients via online and mobile channels. Despite the challenges posed by digitalization, particularly in the realms of logistics and human resources, the management at L’Oréal is well-equipped to swiftly adapt to the evolving business landscape, as evidenced by the proactive adoption and integration of these digital strategies [ 101 ] as illustrated in Fig. 5 .
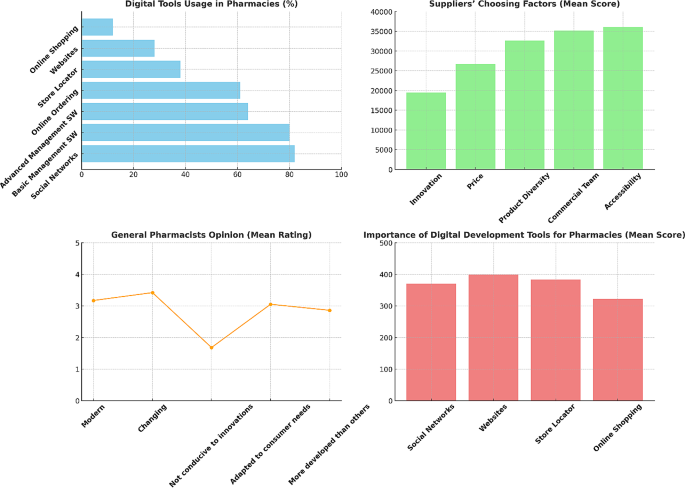
Results from Ascenso [ 101 ] survey assessing digital challenges for L’Oréal in the Portuguese cosmeceutical sector. Digital Tools Usage in Pharmacies (upper left) : the bar chart showing the percentage of respondents using various digital tools in pharmacies. Suppliers’ Choosing Factors (upper right) : the bar chart displaying the mean scores of factors that distinguish a good laboratory/supplier. General Pharmacists Opinion (lower left) : A line chart illustrating the mean ratings of pharmacists’ opinions on whether the pharmaceutical sector is modern, changing, conducive to innovations, adapted to consumer needs, and more developed than other sectors. Importance of Digital Development Tools for Pharmacies (lower right) : A vertical bar chart demonstrating the mean scores for the importance of different digital development tools for pharmacies
The digital transformation strategies, exemplified by companies like L’Oréal, extend beyond the mere targeting of end consumers, encompassing the perspectives of various stakeholders, including retailers. This broadened focus reflects a holistic and integrated approach to digital marketing and customer engagement, indicative of a larger trend within the market. The significance of digital channels in facilitating comprehensive customer interaction and brand development is increasingly recognized. The distinction of organizations such as L’Oréal in their digital initiatives highlights the competitive advantage that can be garnered through innovative digital strategies.
The receptiveness of industry professionals, such as pharmacists, to emerging digital trends, along with the readiness of companies to engage in non-face-to-face sales models, marks a paradigm shift in traditional sales and distribution methods. This shift is reflective of a broader market trend where digital platforms are becoming integral to the customer journey. Furthermore, the potential for online sales in specialized sectors, such as dermocosmetics, and the benefits that organizations derive from the technological advancement of their client base, underscore an escalating acknowledgment of e-commerce and digital tools as crucial elements of a business strategy. This trend, with L’Oréal as a prime example, emphasizes the broader market movement towards digital transformation, not merely as an option but as a necessity for maintaining relevance and competitiveness in an ever-evolving market landscape.
The global regulatory landscape for cosmeceuticals
Sophisticated regulatory legislation and enforcement mechanisms characterize many developed countries such as the USA, EU Member States, Canada, and Japan. These nations, along with influential organizations like the World Health Organization (WHO), significantly shape international market rules and regulations due to their market size and regulatory capacity [ 103 ]. The WHO is particularly noted for its crucial role in setting global standards, with a focus on developing and promoting international standards related to food, biological, pharmaceutical, and similar products [ 104 ]. In contrast to pharmaceuticals, the cosmetic industry necessitates a more advanced international regulatory framework due to consumers’ extensive exposure to these products. The distinction between cosmetics and pharmaceuticals varies significantly across different countries, with the USA employing a voluntary registration system for cosmetics and the EU and Japan requiring mandatory product filings prior to marketing [ 105 ]. Concerns over the safety of pharmaceutical and cosmetic products are highlighted, with an increasing consumer focus on “natural, ecological, and clean” products [ 106 ]. However, the lack of a regulatory framework for these categories underscores the need for more advanced regulations to mitigate health risks.
Intergovernmental cooperation is emphasized, with the US and EU portrayed as dominant players in the pharmaceutical and cosmetic industries, respectively. Regulatory capacity, which is essential for defining, implementing, and monitoring market rules, varies among countries and markets. This capacity depends on several factors, including staff expertise, statutory sanctioning authority, and the degree of centralization of regulatory authority [ 103 ]. The regulatory systems of the EU and US are explored, focusing on their unique approaches to medicine authorization and regulation. The European Medicines Agency (EMA) in the EU and the Food and Drug Administration (FDA) in the US serve as pivotal regulatory bodies [ 107 ; 108 ]. The EMA’s centralized procedure and the FDA’s premarket approval process are detailed, along with subsequent postmarket regulatory procedures. For instance, EU and US cosmetic regulations are compared, revealing differences in their approaches and the evolution of the EU’s regulatory landscape through various amendments and directives. In particular, directive 76/768/EC has been superseded by Regulation (EC) N° 1223/2009, serving as the principal regulatory framework for finished cosmetic products in the EU market. This regulation enhances product safety, optimizes the sector’s framework, and eases procedures to promote the internal cosmetic market. Incorporating recent technological advancements, including nanomaterials, it maintains an internationally acknowledged regime focused on product safety without altering existing animal testing prohibitions [ 109 ].
The Eurasian Economic Union’s (EAEU) regulatory framework for medicines and medical devices is detailed, including the legal framework established for regulating the circulation of these products. The conformity assessment methods, such as the EAC Declaration and the State Registration process, are required for manufacturers to demonstrate their products’ compliance with the standards [ 110 ]. Armenia is also part of the EAEU’s legal framework, which aims to unify regulations for the production and registration of pharmaceuticals and medical products by 2025. This unification is expected to reduce administrative costs for manufacturers and improve medicinal products for patients. Despite significant developments in the cosmetics industry, Armenia does not have an extensive regulatory framework for it. Prior to joining the EAEU, the only regulation concerning cosmetic products was the Order of the Minister of Health of the Republic of Armenia on “Hygiene Requirements of the Production and Safety of Perfume-Cosmetic Products.” Since joining the EAEU, Armenia has unified its national legislation with EAEU regulations, but there are challenges and gaps in the direct applicability of the EAEU’s technical regulations in the country [ 111 ].
In the context of the necessity for clear regulatory framework stems from two reasons. Firstly, cosmeceuticals - products straddling cosmetics and drugs - demand intensified regulatory attention. Examples include the 2007 FDA seizure of Jan Marini’s Age Intervention Eyelash, which contained the drug ingredient bimatoprost, and products boasting human stem cell cultured media, which claim rejuvenating effects but may pose safety risks due to minimal oversight [ 112 ]. A noted 1450% increase in FDA warnings (from 4 to 62 letters) between 2007 and 2011 and 2012–2017, with 8 targeting stem cell ingredient promotions, underscores the growing concern [ 113 ]. The FDA’s limited capacity to identify and assess potential drug-adulterated cosmetics raises concerns.
The second aspect focuses on the necessity for a more comprehensive and unbiased scientific and medical perspective in the FDA’s ingredient review process. The Personal Care Products Safety Act proposes a balanced committee formation including industry, consumer, and medical representatives, yet advocates for the inclusion of specialized professionals like chemists, dermatologists, toxicologists, and endocrinologists. Specific ingredients like diazolidinyl urea and quarternium-15, although effective antimicrobials, are flagged for potential skin allergy risks and formaldehyde release. The preservative 4-methylisothiazolinone, banned in Europe for rinse-off products, is noted for increasing allergic contact dermatitis cases in the US [ 114 ]. The lag in US cosmetic regulation compared to the EU is acknowledged, with the Personal Care Products Safety Act considered a significant advancement, albeit in need of further refinement [ 115 ].
The importance of consumer safety in the global regulatory landscape for cosmeceuticals, particularly for products that blur the line between cosmetics and pharmaceuticals, is a critical issue due to several key factors. Firstly, the cosmeceutical market is expanding rapidly, driven by new ingredients promising various skincare benefits like anti-aging and photoprotection. This growth necessitates clear regulatory guidelines to ensure that these products are safe and their claims are clinically proven. The FDA, for instance, differentiates between cosmetics and cosmeceuticals based on their intended use, particularly if a product is marketed as a cosmetic but functions in a way that affects the structure of the human body, classifying it as a cosmeceutical [ 116 ].
Secondly, the legal and regulatory distinctions between drugs and cosmetics are significant. Drugs are subject to FDA approval based on their intended use in treating diseases or affecting the body’s structure or function, whereas cosmetics are not. This difference becomes crucial when products are marketed with drug-like claims but are not regulated as drugs, potentially leading to consumer safety issues. For example, botanical cosmeceuticals, which contain natural ingredients like herbal extracts, need thorough evaluation to ensure consistency in therapeutic effects [ 117 ]. Additionally, cosmeceutical manufacturers must be careful with marketing and advertising claims to avoid legal implications. Misleading claims can lead to lawsuits and regulatory actions, as seen in past cases where companies faced consequences for unfounded product claims. Moreover, the FDA advises cosmeceutical manufacturers to follow Good Manufacturing Practices (GMP) to reduce the risk of misbranding or mislabeling. These guidelines include production practices and specific warning statement guidelines, emphasizing the importance of substantiating the safety of these products [ 118 ].
The global regulatory landscape for online pharmacy
Online pharmacies pose various risks to consumers, including the potential health hazards from counterfeit or substandard medications and the inappropriate use of prescription drugs. The regulatory landscape for these pharmacies varies significantly across nations, with some countries like the United States implementing specific laws, while others, such as France, have instituted outright bans [ 119 ]. The European Union, for instance, has implemented a mandate effective from 1 July 2015, which requires member states to adhere to legal provisions for a common logo specific to online pharmacies. This is coupled with an obligation for national regulatory authorities to maintain a registry of all registered online medicine retailers, as detailed by the European Medicines Agency [ 120 ]. Furthermore, the sale of certain medications online within the EU is permissible, contingent upon the registration of the pharmacy or retailer with respective national authorities [ 121 ]. Additionally, the Council of Europe’s MEDICRIME Convention introduces an international treaty that criminalizes the online sale of counterfeit medicinal products, enforcing prosecution irrespective of the country in which the crime is perpetrated [ 122 ].
Switzerland presents a unique stance, where Swissmedic strongly advises against the online purchase of medicines due to the high risk of illegal sourcing and poor quality. However, Swiss mail-order pharmacies with a valid cantonal license to operate a mail-order business are exempted from this advisory [ 123 ]. The Swiss Mail-Order Pharmacists Association and its affiliates, such as Zur Rose AG and MediService AG, actively advocate for a modern and equitable regulation of mail-order medicine sales [ 124 ]. The legislative framework is further bolstered by the Federal Act on Medicinal Products and Medical Devices, which regulates therapeutic products to guarantee their quality, safety, and efficacy [ 125 ]. In the Middle East, community pharmacy practice is predominantly governed by national Ministries of Public Health or equivalent governmental entities, with most community pharmacies being privately owned [ 126 ]. The region’s involvement in the Global Cooperation Group, which encompasses various international regulatory bodies like the EMA and USFDA, signifies a collaborative approach towards drug regulatory affairs in the MENA region [ 127 ]. Despite these advances in regulatory collaboration, it is notable that currently no specific regulations have been detected for online purchases from online pharmacies in the Middle East, highlighting a significant area for potential regulatory development. Furthermore, a notable transition is observed in pharmacy education across several Middle Eastern nations, with an inclination towards introducing Pharm.D degrees to replace traditional pharmacy degrees, reflective of evolving educational standards in the pharmaceutical field [ 128 ]. This shift in education parallels the need for updated regulatory frameworks, especially in the context of the burgeoning online pharmacy sector.
Furthermore, Australia permits the sale of both Prescription-Only Medicines (POMs) and Over-the-Counter (OTC) medications online, provided that brick-and-mortar pharmacies comply with all relevant laws and practice standards [ 129 ]. In contrast, South Korea maintains a stringent stance, prohibiting the online sale of both POMs and OTC medicines, with sales confined exclusively to physical stores registered with the Regulatory Authority (RA) [ 130 ]. China, Japan, Russia, Singapore, and Malaysia exhibit a more selective regulatory framework. China and Russia allow the online sale of OTC medicines only, with China imposing additional restrictions on third-party e-commerce platforms and Russia having introduced a draft law in December 2017 to formalize this practice [ 131 ; 132 ]. Japan permits the online sale of certain OTC medicines, explicitly excluding specific substances such as fexofenadine and loratadine [ 133 ]. Similarly, Singapore and Malaysia endorse the online sale of specific OTC medicines only, adopting a “buyers beware” approach to caution consumers about the associated risks [ 134 ; 135 ]. Lastly, the legal landscapes in India and Indonesia remain ambiguous. India’s RA has effectively banned the online sale of medicinal products, yet this prohibition lacks legislative backing. Indonesia, too, grapples with unclear regulations, leaving the legal status of online pharmacies indeterminate [ 136 ].
In response to these risks, several initiatives have been developed to guide and certify online pharmacies. In the United States, LegitScript offers certification to online pharmacies that comply with criteria such as appropriate licensing and registration [ 137 ]. Similarly, the Verified Internet Pharmacy Practice Sites (VIPPS) program, accredited by the National Association of Boards of Pharmacy, ensures pharmacies adhere to licensing requirements in the states where they dispense medications [ 138 ]. Internationally, the Health On the Net Foundation has introduced the HONcode, an ethical standard for health websites globally. This code certifies sites that provide transparent and qualified information. However, due to the absence of international harmonization, the HONcode’s certification is limited to US and Canadian pharmacies verified by VIPPS [ 139 ]. The lack of a harmonized international approach presents significant challenges. Consumers do not have access to a comprehensive, global repository of all certified pharmacies. The diverse certification schemes are not well articulated or interconnected, leading to consumer unawareness about their significance or existence. Moreover, enforcing standards across different legal jurisdictions is complex without a unified agreement. To enhance consumer protection, it is imperative to develop and promote a standardized, minimal international code of conduct for online pharmacies. Such a code would unify requirements and allow all initiatives to clarify their roles under a common framework. Adequate oversight in the borderless online pharmacy market can only be achieved through collaborative efforts. To visualize the infographic of the global regularity landscape for the online pharmacy see Fig. 6 .
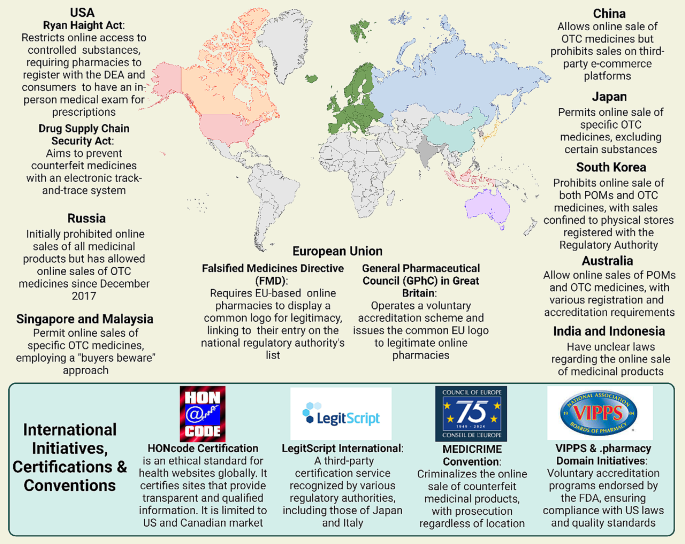
Comprehensive representation of the regulatory landscape for global online pharmacies, detailing international and national initiatives, certification programs, and conventions aimed at minimizing risks associated with the purchase of medications via online platforms
Technological innovations and Future trends in global pharmacy
The global pharmacy sector is undergoing a transformative shift, driven by the rapid advancement of technological innovations. As the world becomes increasingly digital, the integration of cutting-edge technologies like Artificial Intelligence (AI) and blockchain is setting the stage for a new era in pharmaceutical care and management. These advancements promise to revolutionize the industry by enhancing efficiency, accuracy, and security, ultimately leading to improved patient outcomes and a more streamlined healthcare experience [ 140 ].
Walgreens, in partnership with Medline, a telehealth firm, has developed a platform for patient interaction with healthcare professionals via video chat. AI’s role extends to inventory management in retail pharmacies, allowing pharmacists to predict patient needs, stock appropriately, and use personalized software for patient reminders. Although not all inventory management software in retail pharmacies utilizes AI, some, like Blue Yonder’s software developed for Otto group, demonstrate the potential of AI in predicting product sales with high accuracy, thus enhancing supply chain efficiency [ 141 ; 142 ]. At the University of California San Francisco (UCSF) Medical Center, robotic technology is employed to improve patient safety in medication preparation and tracking. This technology has prepared medication doses with a notable error-free record and surpasses human capabilities in accuracy and efficiency. It prepares both oral and injectable medicines, including chemotherapy drugs, freeing pharmacists and nurses to focus on direct patient care. The automated system at UCSF receives electronic medication orders, with robotics handling the picking, packaging, and dispensing of individual doses. This system also assembles medications on bar-coded rings for 12-hour patient intervals and prepares sterile preparations for chemotherapy and intravascular syringes [ 143 ].
In the realm of global pharmacy, blockchain technology emerges as a pivotal force, driving advancements across various facets of healthcare and pharmaceuticals. At the forefront of its application is the enhancement of supply chain transparency [ 144 ]. Blockchain’s immutable ledger ensures the provenance and legitimacy of medical commodities, offering an unprecedented level of visibility from manufacturing to distribution. This is particularly vital in areas plagued by counterfeit drugs, where systems like MediLedger are instrumental in verifying the legality and essential details of medicines [ 145 ].
The utility of blockchain extends to the implementation of smart contracts — scripts processed on the blockchain that bolster transparency in medical studies and secure patient data management [ 146 ]. These contracts find extensive use in advanced medical settings, as evidenced by a blockchain-based telemonitoring system for remote patients and Dermonet, an online platform for dermatological consultation [ 147 ].
Furthermore, blockchain is revolutionizing patient care through patient-centric Electronic Health Records (EHRs). By decentralizing EHR maintenance, blockchain empowers patients with secure access to their historical and current health records [ 148 ]. Prototypes like MedRec and systems such as MeD Share exemplify how blockchain can provide complete, permanent access to clinical documents and facilitate the sharing of medical data between untrusted parties, respectively, ensuring high information authenticity and minimal privacy risks [ 149 ; 150 ]. In verifying medical staff credentials, blockchain again proves invaluable. Systems like ProCredEx, based on the R3 Corda blockchain protocol, streamline the credentialing process, offering rapid verification while allowing healthcare entities to leverage their existing data for enhanced transparency and assurance about medical staff experience [ 151 ].
The integration of blockchain with Internet of Things devices for remote monitoring marks another leap forward, significantly bolstering data security. By safeguarding the integrity and privacy of patient data collected by these devices, blockchain mitigates the risk of tampering and ensures that only authorized parties can access sensitive information [ 152 ]. Besides, a blockchain-based drug supply chain initiative, PharmaChain, utilizes AI for approaches against drug counterfeit and ensures the drug supply chain is more traceable, visible, and secure. For online pharmacies, this means a more reliable supply chain and assurance of drug authenticity, crucial for maintaining trust and safety [ 153 ].
In response to the COVID-19 pandemic, the PharmaGo platform has emerged as an innovative solution in Sri Lanka, revolutionizing the delivery of pharmacy services. As traditional pharmacies grapple with the challenges of meeting all customer needs in one location, PharmaGo addresses this by providing a comprehensive online pharmaceutical service. It allows customers to access a wide range of medications through a single platform, reducing the need to visit multiple pharmacies. Utilizing image processing technology, pharmacy owners can accurately identify prescribed medicines, while the system’s predictive analytics forecasts future drug demands, enhancing stock management. Additionally, PharmaGo’s AI-powered medical chatbot offers real-time guidance, ensuring a seamless and efficient customer experience. This platform represents a significant advancement in healthcare accessibility and pharmacy service delivery in the pandemic era [ 154 ]. In the same context, ontology-based medicine information system, enhancing search relevance through a chatbot interface was presented by Amalia et al. [ 155 ]. Addressing conventional search engines’ limitations in interpreting data relationships, it employs semantic technology to represent metadata informatively. The ontology as a knowledge base effectively delineates disease-medicine relationships, with evaluations indicating a 90% response validity from the chatbot, offering a robust reference for medical information retrieval and its semantic associations.
Future trends for the digital transformation of in the pharmaceutical sector
Future trends for the digital transformation of pharmacies globally are heavily influenced by the transformative impact of digital technologies on healthcare delivery. The integration of telemedicine, electronic health records, and mobile health applications is pivotal in enhancing patient care. These technologies are instrumental in improving data sharing and collaboration among healthcare professionals, increasing the efficiency of healthcare services. Additionally, they offer significant potential for personalized medicine through data analytics and play a crucial role in patient engagement and self-management of health. The importance of these technologies in creating a more connected and efficient healthcare system is underscored, marking a significant shift in the global healthcare landscape [ 156 ].
In the pharmaceutical sector, the COVID-19 pandemic has catalyzed a significant shift towards Pharmaceutical Digital Marketing (PDM), particularly for over-the-counter drugs. This shift focuses on utilizing online pharmacies and digital platforms for targeted advertising, directly reaching consumers. The trend towards purchasing OTC drugs online has grown, driven by the convenience and efficiency of digital channels. While PDM faces challenges like regulatory constraints and the need for digital proficiency, it offers substantial opportunities in enhancing customer engagement and precise marketing. The future of PDM is poised to be more consumer-centric, integrating advanced technologies like AI, and emphasizing personalized marketing strategies to strengthen brand engagement and customer interaction [ 157 ].
Artificial intelligence holds immense potential to revolutionize the field of pharmacy, offering numerous benefits that can significantly enhance efficiency and patient care. One of the primary applications of AI in this sector is the automation of routine tasks. By utilizing AI, pharmacies can automate critical processes such as prescription processing, checking for drug interactions, and managing inventory. This automation not only streamlines operations but also minimizes the likelihood of human error, thereby increasing the overall efficiency of pharmacies [ 158 ].
Furthermore, AI can play a pivotal role in personalized medication management. This is particularly beneficial for patients with chronic conditions such as diabetes who require careful management of their insulin dosages, as fluctuations in blood sugar levels can lead to serious complications. AI systems can monitor patients continuously, provide timely reminders for medication intake, and dynamically adjust treatment plans based on individual health data. Such personalized management ensures that patients receive optimal care tailored to their specific needs, potentially improving treatment outcomes. Incorporation of AI into electronic health records presents another significant advancement. By integrating AI with EHRs, healthcare providers can access real-time patient data. This integration empowers healthcare professionals to make more informed care decisions, enhancing the quality of patient care. Moreover, it significantly reduces the likelihood of medication errors, a critical concern in healthcare.
Likewise, AI’s capability to analyze extensive patient data is invaluable. It can identify patterns and trends in medication adherence, detect potential drug interactions, and pinpoint adverse drug reactions. These insights are crucial for healthcare professionals and researchers. By understanding these patterns, they can develop more effective medication adherence strategies and support systems, contributing to better patient outcomes and advancing the overall field of pharmaceutical care.
In the expansive realm of chemical space, the pharmaceutical industry faces the continual challenge of identifying new active pharmaceutical ingredients (APIs) for diverse diseases [ 159 ]. High throughput screening (HTS), despite its advancements in recent decades, remains resource-intensive and often yields unsuitable hits for drug development. The failure rate of investigational compounds remains high, with a study citing only a 6.2% success rate for orphan drugs progressing from phase I to market approval [ 160 , 161 ].
Machine learning presents a transformative approach to this challenge. It offers an alternative to manual HTS through in silico methodologies. ML-driven drug discovery boasts several advantages: it operates continuously, surpasses the capacity of manual methods, reduces costs by decreasing the number of physical compounds tested, and early identifies negative characteristics of compounds, such as off-target effects and sex-dependent variability [ 162 ].
A substantial advancement in the realm of machine learning has emerged from major pharmaceutical entities, notably AstraZeneca, in conjunction with research institutions. This progress is evidenced by the development of an innovative algorithm that demonstrates both time efficiency and effectiveness in the sphere of drug discovery. The recent introduction of this algorithm significantly enhances the process of determining binding affinities between investigational compounds and therapeutic targets. It surpasses traditional in silico methods in terms of performance. The application of this algorithm underscores the remarkable potential of machine learning in accelerating the identification and development of novel therapeutic agents [ 163 ].
Moreover, the proficiency of machine learning in managing vast and intricate datasets has rendered it indispensable in research focused on cancer targets, utilizing diverse and extensive datasets. This approach is fundamental in numerous drug discovery initiatives, especially those targeting various forms of cancer. A wide array of ML techniques, ranging from supervised to unsupervised learning, are employed to discern chemical attributes that are indicative of potential therapeutic efficacy against a spectrum of cancer targets. This methodology is crucial in identifying novel compounds that could be effective in cancer treatment, leveraging the rich and complex data available in oncological research [ 164 ].
The digital transformation in the pharmacy sector is significantly reshaping healthcare delivery, driven by the integration of cutting-edge technologies like Artificial Intelligence and blockchain. This transformation is marked by a substantial growth in the digital pharmacy market, with a projected annual growth rate of 14.42%, leading to a market volume of approximately $35.33 billion by 2026.
One major aspect of this transformation is the growing reliance on online pharmacy platforms, largely influenced by the COVID-19 pandemic. Consumer trust in online medication purchases has significantly increased, indicating a shift towards digital healthcare solutions. The adoption of telehealth services, including telepharmacy, has surged, with patient adoption in the United States increasing from 11% in 2019 to 46%. This shift towards digital-first services enhances convenience and access to care but also introduces regulatory challenges, particularly in maintaining patient safety and quality standards in the rapidly evolving online healthcare environment.
The cosmeceuticals market, a segment within online pharmacies, is experiencing robust growth. Cosmeceuticals, which bridge the gap between cosmetics and pharmaceuticals, have become a significant part of the skincare industry. The market, valued at USD 56.78 billion in 2022, is projected to expand to USD 95.75 billion by 2030. This expansion is driven by factors like innovation in natural ingredients and significant penetration of internet, smartphone, and social media applications. Despite the growth, the overall penetration for non-specialty drugs in mail-order and online pharmacies remains low, representing a significant portion of specialty prescription revenues. The evolving landscape of online pharmacies in the cosmeceuticals sector reflects a trend towards more accessible and customizable personal healthcare solutions.
Technological innovations are setting the stage for a new era in pharmaceutical care and management. AI’s role extends to areas like inventory management in retail pharmacies, where it predicts patient needs and enhances supply chain efficiency. Blockchain technology enhances supply chain transparency and legitimizes medical commodities, especially crucial in areas affected by counterfeit drugs. Blockchain also plays a vital role in patient-centric Electronic Health Records and telemonitoring systems. For instance, PharmaGo, an innovative platform developed in response to the pandemic, provides a comprehensive online pharmaceutical service, demonstrating the significant advancements in healthcare accessibility and pharmacy service delivery.
These technological advancements are instrumental in improving data sharing and collaboration among healthcare professionals. They offer significant potential for personalized medicine through data analytics, playing a crucial role in patient engagement and self-management of health. The future trends in the pharmaceutical sector, particularly influenced by the COVID-19 pandemic, indicate a shift towards Pharmaceutical Digital Marketing (PDM) and a more consumer-centric approach. AI’s potential in revolutionizing pharmacy includes automation of routine tasks, personalized medication management, real-time patient data access, and the identification of patterns in medication adherence and potential drug interactions.
Data availability
No datasets were generated or analysed during the current study.
Hole G, Hole AS, McFalone-Shaw I. Digitalization in pharmaceutical industry: what to focus on under the digital implementation process? Int J Pharmaceutics: X. 2021;3:100095.
CAS Google Scholar
Shambhavi. Digital Pharmacy on the rise: transforming the Pharma Sector. Brillio.com; Oct. 2022.
Champagne HA, Leclerc D. O., The road to digital success in pharma [Internet]. https://www.mckinsey.com/industries/life-sciences/our-insights/the-road-to-digital-success-in-pharma# ~:text=Mobile%20communications%2 C%20the%20cloud%2 C%20advanced,media%2 C%20retail%2 C%20and%20banking%20industries, August 1, 2015.
COVID-19 accelerated. digital transformation of the pharma industry by five years: Poll, Available from: http://www.pharmaceutical-technology.com/news/covid-19-accelerated-digital-transformation-of-the-pharma-industry-by-five-years-poll/#:~:text=Digital%20transformation%20in%20the%20pharmaceutical,powerful%20analytics%20than%20ever%20before?cf-view&acf-closed , March 9, 2021.
Raza MA, Aziz S, Noreen M, Saeed A, Anjum I, Ahmed M, Raza SM. Artificial Intelligence (AI) in pharmacy: an overview of innovations. INNOVATIONS Pharm 13 (2022).
Zhang X. Investment analysis based on intrinsic value in the Information Technology and Pharmaceutical sectors for advancements in Digital Healthcare. Finance Econ 1 (2023).
Clark M, Clark T, Bhatti A, Aungst T. The rise of digital health and potential implications for pharmacy practice. J Contemp Pharm Pract. 2017;64:32–40.
Article Google Scholar
Health C, Pharmacy CVS. USA, 2023.
MyChart. Epic’s MyChart software system, USA, 2023.
Medisafe MD, Companion. USA, 2023.
Moodle. Moodle open source learning management system, USA, 2023.
PLUS SG. SimMan 3G PLUS advanced emergency care patient simulators, USA, 2023.
Association AP. American Pharmacists Association, USA, 2023.
Fittler A, Ambrus T, Serefko A, Smejkalová L, Kijewska A, Szopa A, Káplár M. Attitudes and behaviors regarding online pharmacies in the aftermath of COVID-19 pandemic: at the tipping point towards the new normal. Front Pharmacol. 2022;13:1070473.
Article PubMed PubMed Central Google Scholar
Hiskey O. The era of telehealth pharmacy practice. J Am Pharmacists Association. 2022;62:10–1.
Plantado ANR, de Guzman HJd, Mariano JEC, Salvan MRAR, Benosa CAC, Robles YR. Development of an online telepharmacy service in the Philippines and analysis of its usage during the COVID-19 pandemic. J Pharm Pract. 2023;36:227–37.
Article PubMed Google Scholar
Zhang PC. The future of pharmacy is intertwined with digital health innovation. Can Pharmacists Journal/Revue Des Pharmaciens Du Can. 2022;155:7–8.
Miller R, Wafula F, Onoka CA, Saligram P, Musiega A, Ogira D, Okpani I, Ejughemre U, Murthy S, Garimella S. When technology precedes regulation: the challenges and opportunities of e-pharmacy in low-income and middle-income countries. BMJ Global Health 6 (2021).
Shawaqfeh MS, Al Bekairy AM, Al-Azayzih A, Alkatheri AA, Qandil AM, Obaidat AA, Harbi SA, Muflih SM. Pharmacy students perceptions of their distance online learning experience during the COVID-19 pandemic: a cross-sectional survey study. J Med Educ Curric Dev. 2020;7:2382120520963039.
Lee CY, Lee SWH. Impact of the educational technology use in undergraduate pharmacy teaching and learning–A systematic review. Pharm Educ. 2021;21:159–68.
Strawbridge J, Hayden JC, Robson T, Flood M, Cullinan S, Lynch M, Morgan AT, O’Brien F, Reynolds R, Kerrigan SW. Educating pharmacy students through a pandemic: reflecting on our COVID-19 experience. Res Social Administrative Pharm. 2022;18:3204–9.
Lin B, Wu S. Digital transformation in personalized medicine with artificial intelligence and the internet of medical things. OMICS. 2022;26:77–81.
Article CAS PubMed Google Scholar
Company M. Trends disrupting pharmacy value pools and potential implications for the value chain, 2018.
Stoumpos AI, Kitsios F, Talias MA. Digital Transformation in Healthcare: Technology Acceptance and its applications. Int J Environ Res Public Health. 2023;20:3407.
Kraus S, Schiavone F, Pluzhnikova A, Invernizzi AC. Digital transformation in healthcare: analyzing the current state-of-research. J Bus Res. 2021;123:557–67.
Jefferies JG, Bishop S, Hibbert S. Customer boundary work to navigate institutional arrangements around service interactions: exploring the case of telehealth. J Bus Res. 2019;105:420–33.
Patrício L, Teixeira JG, Vink J. A service design approach to healthcare innovation: from decision-making to sense-making and institutional change. AMS Rev. 2019;9:115–20.
Hong KS, Lee D. Impact of operational innovations on customer loyalty in the healthcare sector. Service Bus. 2018;12:575–600.
Laurenza E, Quintano M, Schiavone F, Vrontis D. The effect of digital technologies adoption in healthcare industry: a case based analysis. Bus Process Manage J. 2018;24:1124–44.
Mazor I, Heart T, Even A. Simulating the impact of an online digital dashboard in emergency departments on patients length of stay. J Decis Syst. 2016;25:343–53.
Rubbio I, Bruccoleri M, Pietrosi A, Ragonese B. Digital health technology enhances resilient behaviour: evidence from the ward. Int J Oper Prod Manage. 2020;40:34–67.
Hikmet N, Bhattacherjee A, Menachemi N, Kayhan VO, Brooks RG. The role of organizational factors in the adoption of healthcare information technology in Florida hospitals. Health Care Manag Sci. 2008;11:1–9.
Agarwal R, Gao G, DesRoches C, Jha AK. Research commentary—the digital transformation of healthcare: current status and the road ahead. Inform Syst Res. 2010;21:796–809.
Cucciniello M, Lapsley I, Nasi G. Managing health care in the digital world: a comparative analysis. Health Serv Manage Res. 2016;29:132–42.
Eden R, Burton-Jones A, Casey V, Draheim M. Digital transformation requires workforce transformation. MIS Q Exec. 2019;18:1–17.
Google Scholar
Huber C, Gärtner C. Digital transformations in healthcare professionals’ work: Dynamics of autonomy, control and accountability. Manage Revue. 2018;29:139–61.
Burtch G, Chan J. Investigating the relationship between medical crowdfunding and personal bankruptcy in the United States: evidence of a digital divide. MIS Quarterly (Forthcoming; 2018.
Seddon JJJM, Currie WL. Healthcare financialisation and the digital divide in the European Union: narrative and numbers. Inf Manag. 2017;54:1084–96.
Marques IC, Ferreira JJ. Digital transformation in the area of health: systematic review of 45 years of evolution. Health Technol. 2020;10:575–86.
Naik N, Hameed B, Sooriyaperakasam N, Vinayahalingam S, Patil V, Smriti K, Saxena J, Shah M, Ibrahim S, Singh A. Transforming healthcare through a digital revolution: a review of digital healthcare technologies and solutions. Front Digit Health. 2022;4:919985.
Canada. Virtual care policy framework, Canada, 2023.
Dhaliwall S. Telepharmacy services in Canada. Canada: Hospital News; 2018.
Telehealth HHSgov. Telehealth services in the United States, USA, 2023.
HRSA. Health Resources & Services Administration, USA, 2023.
UK.digital. health, Digital health and care, UK, 2023.
Australia AT, Services. Australia, 2023.
Imenokhoeva M. Telehealth in the European Union: Improving Access to Healthcare, HIMSS, EU, 2020.
Lucy d’Arville, Boulton A, Kapur V. Asia Pacific, Telehealth Adoption is expected to soar through 2024. BIAN and Company; 2022.
Intan Sabrina M, Defi IR. Telemedicine guidelines in South East Asia—a scoping review. Front Neurol. 2021;11:581649.
Wolf.Theiss. Telemedicine– the future of healthcare in Central and Eastern Europe, 2022.
Al-Samarraie H, Ghazal S, Alzahrani AI, Moody L. Telemedicine in Middle Eastern countries: Progress, barriers, and policy recommendations. Int J Med Informatics. 2020;141:104232.
Mahdi AlBasri H, Elwan P, Georgiev, Ustun A. Growth opportunities for digital health in KSA and UAE. McKinsey: McKinsey; 2022.
Ibrahim OM, Ibrahim RM, Al Meslamani AZ, Al Mazrouei N. Role of telepharmacy in pharmacist counselling to coronavirus disease 2019 patients and medication dispensing errors. J Telemed Telecare. 2023;29:18–27.
Unni EJ, Patel K, Beazer IR. Hung, Telepharmacy during COVID-19: a scoping review. Pharmacy. 2021;9:183.
Leila Shafiee Hanjani JS, Bell, Freeman CR. Undertaking medication review by telehealth. Australian J Gen Pract. 2020;49:826–31.
Bejarano AP, Santos PV, Robustillo-Cortés MdlA, Gómez ES, Rubio MDS. Implementation of a novel home delivery service during pandemic. Eur J Hosp Pharm (2020) ejhpharm–2020.
Elson EC, Oermann C, Duehlmeyer S, Bledsoe S. Use of telemedicine to provide clinical pharmacy services during the SARS-CoV-2 pandemic. Am J Health-System Pharm. 2020;77:1005–6.
Li H, Zheng S, Liu F, Liu W, Zhao R. Fighting against COVID-19: innovative strategies for clinical pharmacists. Res Social Administrative Pharm. 2021;17:1813–8.
Margusino-Framiñán L, Illarro-Uranga A, Lorenzo-Lorenzo K, Monte-Boquet E, Márquez-Saavedra E, Fernández-Bargiela N, Gómez-Gómez D, Lago-Rivero N, Poveda-Andrés JL, Díaz-Acedo R, Hurtado-Bouza JL, Sánchez-Gundín J, Casanova-Martínez C. Morillo-Verdugo, Pharmaceutical care to hospital outpatients during the COVID-19 pandemic. Telepharmacy Farmacia Hospitalaria. 2020;44:61–5.
PubMed Google Scholar
Hua X, Gu M, Zeng F, Hu H, Zhou T, Zhang Y, Shi C. Pharmacy administration and pharmaceutical care practice in a module hospital during the COVID-19 epidemic. J Am Pharmacists Association. 2020;60:431–e4381.
Asseri AA, Manna MM, Yasin IM, Moustafa MM, Roubie FM, El-Anssasy SM, Baqawie SK. Implementation and evaluation of telepharmacy during COVID-19 pandemic in an academic medical city in the Kingdom of Saudi Arabia: paving the way for telepharmacy. World J Adv Res Reviews. 2020;7:218–26.
Segal EM, Alwan L, Pitney C, Taketa C, Indorf A, Held L, Lee KS, Son M, Chi M, Diamantides E, Gosser R. Establishing clinical pharmacist telehealth services during the COVID-19 pandemic. Am J Health-System Pharm. 2020;77:1403–8.
Bukhari N, Rasheed H, Nayyer B, Babar Z-U-D. Pharmacists at the frontline beating the COVID-19 pandemic. J Pharm Policy Pract. 2020;13:8.
Smith RF. The private sector is taking the lead on enabling digital inclusion. Here’s how. in: W.E. Forum, editor, World Economic Forum, 2021.
Dal Mas F, Massaro M, Rippa P, Secundo G. The challenges of digital transformation in healthcare: an interdisciplinary literature review, framework, and future research agenda. Technovation. 2023;123:102716.
Ianculescu M, Alexandru A. Microservices–A Catalyzer for Better Managing Healthcare Data Empowerment. Stud Inf Control. 2020;29:231–42.
López-Martínez F, Núñez-Valdez ER, García-Díaz V, Bursac Z. A case study for a big data and machine learning platform to improve medical decision support in population health management. Algorithms. 2020;13:102.
Ganapathy K, Reddy S. Technology enabled remote healthcare in public private partnership mode: A story from India. Telemedicine, Telehealth and Telepresence: Principles, Strategies, Applications, and New Directions (2021) 197–233.
Gleiss A, Kohlhagen M, Pousttchi K. An apple a day–how the platform economy impacts value creation in the healthcare market. Electron Markets. 2021;31:849–76.
Bonacina S, Koch S, Meneses I, Chronaki C. Can the European EHR exchange format support shared decision making and citizen-driven health science? Public Health and Informatics, IOS; 2021. pp. 1056–60.
Samuel L, TRANSFORMING THE HEALTHCARE SYSTEM: THE PUBLIC-PRIVATE HEALTHCARE DICHOTOMY IN INDIA IN THE ERA OF DIGITAL HEALTH., Proceedings of the International Conference on Public Health, 2020, pp. 18–31.
Antarsih NR, Setyawati SP, Ningsih S, Sulaiman E, Pujiastuti N. Telehealth Business Potential in Indonesia, International Conference on Social, Economics, Business, and Education (ICSEBE 2021), Atlantis Press, 2022, pp. 73–78.
Loccioni LG. Italy, 2023.
Casprini E, Palumbo R. Reaping the benefits of digital transformation through Public-Private Partnership: A service ecosystem view applied to healthcare. Global Public Policy Gov. 2022;2:453–76.
APOTECAchemo. APOTECAchemo Advanced Robotic Chemotherapy Drug Compounding Systems, Italy, 2023.
Velayati F, Ayatollahi H, Hemmat M, Dehghan R. Telehealth business models and their components: systematic review. J Med Internet Res. 2022;24:e33128.
Barker GP, Krupinski EA, McNeely RA, Holcomb MJ, Lopez AM, Weinstein RS. The Arizona telemedicine program business model. J Telemed Telecare. 2005;11:397–402.
Lee Y-L, Chang P. Modeling a mobile health management business model for chronic kidney disease, Nursing Informatics 2016, IOS Press, 2016, pp. 1047–1048.
Dijkstra SJ, Jurriëns JA, van der Mei RD. A business model for telemonitoring services, 14th Annual High Technology Small Firms Conference, HTSF 2006, University of Twente, 2006.
Grustam AS, Vrijhoef HJ, Poulikidis V, Koymans R, Severens JL. Extending the business-to-business (B2B) model towards a business-to-consumer (B2C) model for telemonitoring patients with chronic heart failure. J Bus Models. 2018;6:106–29.
Lin T-C, Chang H-J, Huang C-C. An analysis of telemedicine in Taiwan: a business model perspective. Int J Gerontol. 2011;5:189–92.
Peters C, Blohm I, Leimeister JM. Anatomy of successful business models for complex services: insights from the telemedicine field. J Manage Inform Syst. 2015;32:75–104.
Lin S-H, Liu J-H, Wei J, Yin W-H, Chen H-H, Chiu W-T. A business model analysis of telecardiology service. Telemedicine e-Health. 2010;16:1067–73.
Mun SK, Tohme WG, Platenberg RC, Choi I. Teleradiology and emerging business models. J Telemed Telecare. 2005;11:271.
Kijl B, Nieuwenhuis LJ, Veld RM, Hermens HJ. and M.M. Vollenbroek-Hutten, Deployment of e-health services–a business model engineering strategy. Journal of telemedicine and telecare 16 (2010) 344–353.
Fusco F, Turchetti G, Interactive Business Models for Telerehabilitation after Total Knee Replacement.: Preliminary Results from Tuscany, Bioinformatics and Biomedical Engineering: Third International Conference, IWBBIO 2015, Granada, Spain, April 15–17, 2015. Proceedings, Part II 3, Springer, 2015, pp. 502–511.
Hidefjäll P, Titkova D. Business model design for a wearable biofeedback system, pHealth, 2015, pp. 213–224.
Oderanti FO, Li F. A holistic review and framework for sustainable business models for assisted living technologies and services. Int J Healthc Technol Manage. 2016;15:273–307.
Valbuena VS, Seelye S, Sjoding MW, Valley TS, Dickson RP, Gay SE, Claar D, Prescott HC, Iwashyna TJ. Racial bias and reproducibility in pulse oximetry among medical and surgical inpatients in general care in the Veterans Health Administration 2013-19: multicenter, retrospective cohort study. BMJ 378 (2022).
Bai G, Rajgopal S, Srivastava A, Zhao R. Profitability and risk-return comparison across health care industries, evidence from publicly traded companies 2010–2019. PLoS ONE. 2022;17:e0275245.
Article CAS PubMed PubMed Central Google Scholar
McCall CJ, DeCaprio D, Gartner J. The Measurement and Mitigation of Algorithmic Bias and Unfairness in Healthcare AI Models Developed for the CMS AI Health Outcomes Challenge. medRxiv (2022) 2022.09. 29.22280537.
Hsueh P-YS. Ecosystem of patient-centered research and information System Design, Personal Health Informatics: patient participation in Precision Health. Springer; 2022. pp. 329–51.
Martin KI, Glaser DA. Cosmeceuticals: the new medicine of beauty. Mo Med. 2011;108:60.
PubMed PubMed Central Google Scholar
Pandey A, Jatana GK, Sonthalia S, Cosmeceuticals SP. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC., Treasure Island (FL) ineligible companies. Disclosure: Gurpoonam Jatana declares no relevant financial relationships with ineligible companies. Disclosure: Sidharth Sonthalia declares no relevant financial relationships with ineligible companies., 2023.
Morganti P, Morganti G, Gagliardini A, Lohani A. From cosmetics to innovative cosmeceuticals—Non-woven tissues as New Biodegradable Carriers. Cosmetics. 2021;8:65.
Article CAS Google Scholar
Market C. Fortune Business Insights., Cosmeceuticals Market, 2023.
LLP SMR. Global Cosmeceuticals Market, A $95.75 Billion Industry Opportunity by 2030., 2022.
R.a. Markets, Global Cosmeceuticals Market - Outlook & Forecast 2023–2028, 2023.
Research SM. Industry Report and Statistics (Facts & Figures) - Number of Users, Price, Demand & Sales Analysis by Product & Distribution Channel, 2021.
Company M. Meeting changing consumer needs: The US retail pharmacy of the future, 2023.
Ascenso FNS. L’Oréal Portugal: a digital challenge for the active cosmetics division, 2016.
cosmeticaactiva. L’Oreal E-Learning Platform in Portugal, 2023.
Bach D, Newman AL. Governing lipitor and lipstick: capacity, sequencing, and power in international pharmaceutical and cosmetics regulation. Rev Int Polit Econ. 2010;17:665–95.
Organization WH. Quality assurance of pharmaceuticals. a compendium of guidelines and related materials: Good manufacturing practices and inspection, 2007.
Rai S, Gupta A, Punetha V. Regulations of Cosmetics across the Globe. Applied Clinical Research. Clin Trials Regul Affairs. 2015;2:137–44.
Natural VLAMBERT. & Organic Living; Say ‘No’ To Chemicals For A Healthy Life. in: Raconteur, editor, 2016.
Agency EM. The European regulatory system for medicines, 2023.
FDA, Food and Drug Adimenstration in the United States of America, 2023.
Cosmetics I, Market. Industry, Entrepreneurship and SMEs, EU, European Commission 2023.
EEU EEU. TECHNICAL REGULATION ON THE SAFETY OF COSMETICS AND PERFUMERY, 2015.
Danghyan L. Armenian regulatory framework of the cosmetics and pharmaceutical industries, and its compliance with international regulations. Whether the existing regulatory framework of cosmetics and pharmaceutical industries complies with international best practice and ensures product safety and consumer protection. American University of Armenia; 2020.
TGA. Australian Public Assessment Report for Bimatoprost, 2017.
Kwa M, Welty LJ, Xu S. Adverse events reported to the US Food and Drug Administration for cosmetics and personal care products. JAMA Intern Med. 2017;177:1202–4.
DeKoven JG, Warshaw EM, Belsito DV, Sasseville D, Maibach HI, Taylor JS, Marks JG, Fowler JF, Mathias CT, DeLeo VA. North American contact dermatitis group patch test results 2013–2014. Dermatitis. 2017;28:33–46.
Janetos TM, Kwa M, Xu S. Regulation of cosmetics. JAMA Intern Med. 2018;178:1000–1.
Daum C. Self-Regulation in the Cosmetic Industry: A Necessary Reality or a Cosmetic Illusion? 2006.
Schaffer S, Reading Our Lips.: The History of Lipstick Regulation in Western Seats of Power, 2006.
Lincoln JE. Overview of the us fda gmps: good manufacturing practice (gmp)/quality system (qs) regulation (21 CFR part 820). J Validation Technol. 2012;18:17.
van der Heijden I, Pletneva N, Boyer C. How to protect consumers against the risks posed by the online pharmacy market. Swiss Med Inf 29 (2013).
Agency EM. Buying medicines online, 2015.
Union E. Buying medicine online, 2023.
ISPE. Regulating Online Pharmacies & Medicinal Product E-Commerce, 2019.
Swissmedic. Guideline on medicines and the Internet, 2020.
Kulali H. Online Pharmacy in Switzerland: A Full Guide. 2023, Pharmapidya, 2023.
Swissmedic. Current law, 2019.
El Hajj MS, Mekkawi R, Elkaffash R, Saleh R, Awaisi AE, Wilbur K. Public attitudes towards community pharmacy in Arabic speaking Middle Eastern countries: a systematic review. Res Social Administrative Pharm. 2021;17:1373–95.
DelveInsight. The Regulatory process for drug approval in the MENA region, DelveInsight, 2019.
Sallom H, Abdi A, Halboup AM, Başgut B. Evaluation of pharmaceutical care services in the Middle East Countries: a review of studies of 2013–2020. BMC Public Health. 2023;23:1364.
P.B.o. Australia, Guidelines for Dispensing of Medicines., 2019.
Center KLT. Statutes of the Republic of Korea. Pharmaceutical Affairs Act; 2016.
Goryachev I. Russia Federation: Telemedicine Law in Russia, Mondaq, 2018.
Jing M. China FDA stops online med sales, 2016.
L.a.W. o.J. Ministry of Health, List of Sales Sites for over-the-counter Drugs, 2020.
H.S.A.o, Singapore. Dangers of Buying Health Products Online, 2023.
M.o.H.o. Malaysia, Buying Medicines Online: Beware., 2023.
Ganapathy N. India’s pharmacies do battle with online rivals Straitimes, India, 2017.
LegitScript LS. Industry-Leading Certification, 2023.
NABP. The Verified Internet Pharmacy Practice Sites, 2012.
Boyer C, Baujard V, Geissbuhler A. Evolution of health web certification through the HONcode experience. Stud Health Technol Inf. 2011;169:53–7.
Tagde P, Tagde S, Bhattacharya T, Tagde P, Chopra H, Akter R, Kaushik D, Rahman MH. Blockchain and artificial intelligence technology in e-Health. Environ Sci Pollut Res. 2021;28:52810–31.
Walgreen. Convenient virtual care., 2023.
Group O. Otto Group, 2023.
U.o.C.S, Francisco, New UCSF. Robotic Pharmacy Aims to Improve Patient Safety, 2011.
Narayanaswami C, Nooyi R, Govindaswamy SR, Viswanathan R. Blockchain anchored supply chain automation. IBM J Res Dev. 2019;63:7: 1–7.
Khezr S, Moniruzzaman M, Yassine A, Benlamri R. Blockchain technology in healthcare: a comprehensive review and directions for future research. Appl Sci. 2019;9:1736.
Xia Q, Sifah EB, Asamoah KO, Gao J, Du X, Guizani M. MeDShare: Trust-less medical data sharing among cloud service providers via blockchain. IEEE access 5 (2017) 14757–14767.
Mannaro K, Pinna A, Marchesi M. Crypto-trading: Blockchain-oriented energy market, 2017 AEIT International Annual Conference, IEEE, 2017, pp. 1–5.
Rallapalli S, Minalkar A. Improving Healthcare-Big Data Analytics for. J Adv Inform Technol 7 (2016).
Wang H, Song Y. Secure cloud-based EHR system using attribute-based cryptosystem and blockchain. J Med Syst. 2018;42:152.
Cyran MA. Blockchain as a foundation for sharing healthcare data. Blockchain Healthc Today (2018).
Funk E, Riddell J, Ankel F, Cabrera D. Blockchain technology: a data framework to improve validity, trust, and accountability of information exchange in health professions education. Acad Med. 2018;93:1791–4.
Khan PW, Byun Y. A blockchain-based secure image encryption scheme for the industrial internet of things. Entropy. 2020;22:175.
Gomasta SS, Dhali A, Tahlil T, Anwar MM, Ali ABMS. PharmaChain: Blockchain-based drug supply chain provenance verification system. Heliyon. 2023;9:e17957.
Gamage RG, Bandara NS, Diyamullage DD, Senadeera KU, Abeywardena KY, Amarasena N. PharmaGo-An Online Pharmaceutical Ordering Platform, 2021 3rd International Conference on Advancements in Computing (ICAC), IEEE, 2021, pp. 365–370.
Amalia A, Sipahutar PYC, Elviwani E, Purnamasari F. Chatbot Implementation with Semantic Technology for Drugs Information Searching System. Journal of Physics: Conference Series 1566 (2020) 012077.
Trenfield SJ, Awad A, McCoubrey LE, Elbadawi M, Goyanes A, Gaisford S, Basit AW. Advancing pharmacy and healthcare with virtual digital technologies. Adv Drug Deliv Rev. 2022;182:114098.
Anis MS, Hassali MA. Pharmaceutical marketing of over-the-counter drugs in the current digital era: a review. Pharm Sci Asia 49 (2022).
Khan O, Parvez M, Kumari P, Parvez S, Ahmad S. The future of pharmacy: how AI is revolutionizing the industry. Intell Pharm. 2023;1:32–40.
Moshawih S, Hadikhani P, Fatima A, Goh HP, Kifli N, Kotra V, Goh KW, Ming LC. Comparative analysis of an anthraquinone and chalcone derivatives-based virtual combinatorial library. A cheminformatics proof-of-concept study. J Mol Graph Model. 2022;117:108307.
Moshawih S, Goh HP, Kifli N, Idris AC, Yassin H, Kotra V, Goh KW, Liew KB, Ming LC. Synergy between machine learning and Natural products Cheminformatics: application to the lead Discovery of anthraquinone derivatives. Chemical Biology & Drug Design; 2022.
Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20:273–86.
Moshawih S, Goh HP, Kifli N, Darwesh MAE, Ardianto C, Goh KW, Ming LC. Identification and optimization of TDP1 inhibitors from anthraquinone and chalcone derivatives: consensus scoring virtual screening and molecular simulations. J Biomol Struct Dynamics (2023) 1–25.
Moore JH, Margreitter C, Janet JP, Engkvist O, de Groot BL, Gapsys V. Automated relative binding free energy calculations from SMILES to ∆∆G. Commun Chem. 2023;6:82.
Moshawih S, Lim AF, Ardianto C, Goh KW, Kifli N, Goh HP, Jarrar Q, Ming LC. Target-based small Molecule Drug Discovery for Colorectal Cancer: a review of Molecular pathways and in Silico studies. Biomolecules. 2022;12:878.
Download references
Acknowledgements
The researcher would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
Deanship of Scientific Research, Qassim University
Author information
Authors and affiliations.
Department of Pharmacology, College of Medicine, Qassim University, Buraydah, Saudi Arabia
Ahmad Almeman
You can also search for this author in PubMed Google Scholar
Contributions
Conceived the conceptual framework, Wrote the paper, Designed search strategies, Critically reviewed the manuscript for important intellectual content: A.A.
Corresponding author
Correspondence to Ahmad Almeman .
Ethics declarations
Ethical approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The author declares no competing interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Almeman, A. The digital transformation in pharmacy: embracing online platforms and the cosmeceutical paradigm shift. J Health Popul Nutr 43 , 60 (2024). https://doi.org/10.1186/s41043-024-00550-2
Download citation
Received : 14 February 2024
Accepted : 09 April 2024
Published : 08 May 2024
DOI : https://doi.org/10.1186/s41043-024-00550-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Digital transformation
- Healthcare technology
- Cosmeceuticals
- Global health
- Patient care
- 2019 novel coronavirus
- Drug safety
Journal of Health, Population and Nutrition
ISSN: 2072-1315
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Hannah Hoffmann wins first place in Education Research Poster at ASPET 2024
BSPS and incoming PharmD student Hannah Hoffmann won first place in the División of Pharmacology Education Student Poster Competition at the American Society of Pharmacology and Experimental Therapeutics (ASPET) 2024 Annual Meeting May 16-19 in Arlington, Virginia.
Hoffman's research poster, "Equitable Impacts of Coaching Reports on Undergraduate Students Self-Directed Learning and Pharmaceutical Science Exam Performance," demonstrated that undergraduate students who received an individualized coaching report on the subjects and question types they struggled with on the exam were better informed about how to study for the next exam. Student exam scores further improved when exam difficulty gradually increased over time and included some questions from previous exams.
Hoffman's research was done during her ASPET summer research fellowship under the advisement of Dr. Nicholas Denton. She was recognized by ASPET with a travel award to present her research at the annual meeting.

- open search
From the latest big breakthrough to the most influential and inspiring figures on campus to Pitt in the community, Pittwire is your official source for what’s happening now.
- Health and Wellness
- Technology and Science
- Arts and Humanities
- Community Impact
- Diversity, Equity, and Inclusion
- Innovation and Research
- Our City/Our Campus
- Pitt Magazine
- Features & Articles
- Accolades & Honors
- Ones to Watch
- Announcements and Updates
- Life at Pitt
- Arts & Sciences
- Computing & Information
- Dental Medicine
- Engineering
- General Studies
- Health & Rehabilitation
- Honors College
- Public & Intl Affairs
- Public Health
- Social Work
- COVID-19 Response
- Sustainability
- Graduate and professional students
- Humanities Center

Subscribe to Pittwire Today
9 phd students were named 2024 humanities engage fellows.

Nine PhD students at the University of Pittsburgh have received research funding from the Pitt’s Humanities Engage project, which is committed to broadening and deepening the intellectual and professional development of all PhD candidates.
Six of the recipients pitched their own Summer Immersive Fellowships, which offer the chance to gain experiences with host organizations in collaborative, mission-focused project work drawing on their high-level skills as researchers and writers. They will be co-mentored by the host organization supervisors, a cohort of faculty mentors and the senior director for graduate advising and engagement for the humanities.
This year’s Summer Immersive Fellowship recipients are:
- Juwon Adenuga (Music)
- Monica Daniels (History of Art and Architecture)
- Luis Delgado (Music)
- Frederick Miller (Theatre Arts)
- Senjuti Mukherjee (Film and Media Studies)
- Ernest Owusu-Poku (Music)
The two-term Immersive Dissertation Research Fellowship supports Humanities dissertation projects that involve substantial professional development and will likely result in dissertation formats other than the conventional proto-monograph. The fellowship carries a competitive stipend, a tuition scholarship and professional development funds for its duration.
The Immersive Dissertation Research Fellowship awardees are:
- Rahul Kumar (Film and Media Studies)
- Apala Kundu (English)
- Warner Sabio (Music)
Pitt is updating its Campus Master Plan
Employees, benefits open enrollment is may 1-15, pitt is launching an office of sustainability in the health sciences.
Journal of Materials Chemistry B
Homologous-targeting biomimetic nanoparticles co-loaded with melittin and a photosensitizer for the combination therapy of triple negative breast cancer †.

* Corresponding authors
a Key Laboratory of Immune Microenvironment and Disease (Ministry of Education), The Province and Ministry Co-sponsored Collaborative Innovation Center for Medical Epigenetics, Tianjin Key Laboratory on Technologies Enabling Development of Clinical Therapeutics and Diagnostics (Theranostics), School of Pharmacy; Department of Genetics, School of Basic Medical Sciences, Tianjin Medical University, Tianjin 300070, China E-mail: [email protected] , [email protected]
b Department of Pharmacy, Tianjin First Central Hospital, Tianjin 300192, China
Melittin (Mel) is considered a promising candidate drug for the treatment of triple negative breast cancer (TNBC) due to its various antitumor effects. However, its clinical application is hampered by notable limitations, including hemolytic activity, rapid clearance, and a lack of tumor selectivity. Here, we designed novel biomimetic nanoparticles based on homologous tumor cell membranes and poly(lactic- co -glycolic acid) (PLGA)/poly(beta-aminoester) (PBAE), denoted MDM@TPP, which efficiently coloaded the cytolytic peptide Mel and the photosensitizer mTHPC. Both in vitro and in vivo , the MDM@TPP nanoparticles effectively mitigated the acute toxicity of melittin and exhibited strong TNBC targeting ability due to the homologous targeting effect of the tumor cell membrane. Under laser irradiation, the MDM@TPP nanoparticles showed excellent photodynamic performance and thus accelerated the release of Mel by disrupting cell membrane integrity. Moreover, Mel combined with photodynamic therapy (PDT) can synergistically kill tumor cells and induce significant immunogenic cell death, thereby stimulating the maturation of dendritic cells (DCs). In 4T1 tumor-bearing mice, MDM@TPP nanoparticles effectively inhibited the growth and metastasis of primary tumors and finally prevented tumor recurrence by improving the immune response.

Supplementary files
- Supplementary information PDF (1599K)
Article information
Download citation, permissions.
Homologous-targeting biomimetic nanoparticles co-loaded with melittin and a photosensitizer for the combination therapy of triple negative breast cancer
T. Zhang, L. Bai, R. You, M. Yang, Q. Chen, Y. Cheng, Z. Qian, Y. Wang and Y. Liu, J. Mater. Chem. B , 2024, Advance Article , DOI: 10.1039/D3TB02919K
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
- Skip to navigation
- Skip to content
- UMB Shuttle
University of Maryland, Baltimore
About UMB History, highlights, administration, news, fast facts
- Accountability and Compliance
- Administration and Finance
- Center for Information Technology Services
- Communications and Public Affairs
- Community Engagement
- Equity, Diversity, and Inclusion
- External Relations
- Government Affairs
- Philanthropy
- Office of the President
- Office of the Provost
- Research and Development
- University Counsel
- Administrative Officers
- Boards of Visitors
- Faculty Senate
- Staff Senate
- Center for Health and Homeland Security
- Council for the Arts & Culture
- Interprofessional Education
- Leaders in Education: Academy of Presidential Scholars
- Middle States Self-Study
- President's Council for Women
- President's Symposium and White Paper Project
- For the Media
- Steering Committee Roster
- Logistics Committee Roster
- UMB Police and Public Safety
- Graduation Celebration 2024
- Founders Week
- UMB Holiday Craft Fair
Academics Schools, policies, registration, educational technology
- School of Dentistry
- Graduate School
- School of Medicine
- School of Nursing
- School of Pharmacy
- School of Social Work
- Carey School of Law
- Health Sciences and Human Services Library
- Thurgood Marshall Law Library
Admissions Admissions at UMB are managed by individual schools.
- Carey School of Law Admissions
- Graduate School Admissions
- School of Dentistry Admissions
- School of Medicine Admissions
- School of Nursing Admissions
- School of Pharmacy Admissions
- School of Social Work Admissions
- Tuition and Fees
- Student Insurance
- Academic Calendar
- Financial Assistance for Prospective Students
- Financial Assistance for Current Students
- Financial Assistance for Graduating Students
Research Offices, contracts, investigators, UMB research profile
- Organized Research Centers and Institutes
- UMB Institute for Clinical & Translational Research
- Sponsored Programs Administration
- Sponsored Projects Accounting and Compliance (SPAC)
- Kuali Research
- Clinical Trials and Corporate Contracts
- CICERO Log-in
- Conflict of Interest
- Human Research Protections
- Environmental Health and Safety
- Export Compliance
- Effort Reporting
- Research Policies and Procedures
- Center for Innovative Biomedical Resources
- Baltimore Life Science Discovery Accelerator (UM-BILD)
- Find Funding
- File an Invention Disclosure
- Global Learning for Health Equity Network
- Manage Your Grant
- Research Computing
- UM Research HARBOR
- Center for Violence Prevention
- Office of Research and Development
- Center for Clinical Trials and Corporate Contracts
- Technology Transfer/UM Ventures
- Contact Research and Development
Services For students, faculty, and staff, international and on-campus
- Student Health Resources
- Educational Support and Disability Services
- Writing Center
- URecFit and Wellness
- Intercultural Leadership and Engagement
- Educational Technology
- Student Counseling Center
- UMB Scholars for Recovery
- UMB Student Affairs
- Human Resource Services
- Travel Services
- Strategic Sourcing and Acquisition Services
- Office of the Controller
- Office of the Ombuds
- Employee Assistance Program (EAP)
- Workplace Mediation Service
- Faculty Center for Teaching and Learning
- UMB Travel: Start Here
- International Students, Scholars, and Employees
- Center for Global Engagement
- International Travel SOS
- International Operations
- Parking and Transportation Services
- UMB shuttle
- SMC Campus Center Event Services
- Donaldson Brown Riverfront Event Center
- All-Gender Bathrooms
- Environmental Services
- Interprofessional Program for Academic Community Engagement
University Life Alerts, housing, dining, calendar, libraries, and recreation
- Emergency Reference Guide
- Campus Life Weekly with USGA
- Starting a New Universitywide Organization
- University Student Government Association
- Planned Closures
- Intramural Sports
- Safety Education
- About URecFit and Wellness
- How to Get Your One Card
- One Card Uses
- Lost One Card
- One Card Policies
- Photo Services
- One Card Forms
- One Card FAQs
- Office Hours and Directions
Give to UMB Sustain excellence and meet UMB's educational needs for today and tomorrow.

Thank You for Your Gift to UMB
The University of Maryland, Baltimore (UMB) is excited to share its new online giving page.
With enhanced searchability, a streamlined checkout process, and new ways to give such as Venmo, PayPal, Apple Pay, and Google Pay in addition to credit card, donors can support UMB quickly and securely.
- Ways to Give
- Where to Give
- Staying Connected: You and UMB
- The UMB Foundation
- Office of Philanthropy
- Maryland Charity Campaign

- Archived News
Search UMB News:
There are no reported emergencies on campus at this time. Sign up for UMB Alerts.
Graduate School Celebrates Student Perseverance, Excellence
May 20, 2024 | By Lorri Angelloz
“With this ceremony, we welcome you into this community of scholars, with all of its prestige, all of its privileges, and all of its responsibilities. You have earned this moment,” said University of Maryland, Baltimore (UMB) Vice Provost for Graduate Education and Graduate School Dean Kenneth Wong, PhD , to graduating students during the Graduate School’s Doctoral Hooding Ceremony.
Wong’s remarks came during two days of celebrations for students from the Graduate School. In addition to the Doctoral Hooding Ceremony on May 16, the school held a Master’s and Certificate Graduation Ceremony on May 15. Both events underscored the interprofessional and interdisciplinary nature of study at the Graduate School, as well as the impressive effort made by all students in their pursuit of advanced education.
During the May 16 event, Wong noted the importance of support systems throughout each student’s academic journey. That journey toward doctoral education, he stressed, is the pinnacle of personalized education, and it involves years of dedicated effort shaped by faculty and family support as well as the unique experiences of each student.

PhD graduate Todd Becker receives his hood from faculty mentor John Cagle, PhD, during the 2024 Graduate School Hooding Ceremony.
“It is clear that our mentors have created an environment where students can excel, and that our graduates are really at the top of their game,” he said. “In a world that is being transformed daily by machine learning and technology, doctoral research — research at this level — is still a very human and very personal endeavor.”
Each of the 81 graduates who received their PhD degree from the multidisciplinary Graduate School is prepared for a career in research in the health and social sciences, addressing many of the most pressing issues of our time. Over the course of the ceremony, graduates received their ceremonial hood from their faculty mentors, who hail from five of UMB’s six professional schools — dentistry, medicine, nursing, pharmacy, and social work. Each hood, hemmed in a bright or dark blue color signifying their PhD degree, is lined with black and gold to indicate the institution conferring the degree.
Before each hood was placed, written introductions by mentors extolling each graduate and their research were read by representatives of their professional school. Common themes that emerged during these remarks included the impressive research accomplishments of students and their strong work ethic and collaborative spirit. Mentors also pointed to unique personal qualities and circumstances that impacted their mentees’ work.
Luana Colloca, MD, PhD, MS , director of the Department of Pain and Translational Symptom Science and director of the Placebo Beyond Opinions Center at the University of Maryland School of Nursing, mentored Emily Werthman , and wrote, “It is wonderful to present this outstanding candidate as she reached this important milestone. Emily began her PhD during the COVID pandemic, meeting her mentors and classmates over Zoom while juggling homeschool needs for her two young children.”
Adding that Werthman also had to balance night shifts in the ICU and emergency room along with school and family responsibilities, Colloca stressed that her mentee excelled despite the demanding situation. Colloca wrote that as the pandemic waned, “Emily published her first article associated with her dissertation, a literature review of burn pain and adverse childhood experience. She also continued advancing her profession of burn nursing with an appointment to the Board of Certification for Emergency Nursing, and as a special advisor and subject matter expert to the Biomedical Advanced Research and Development Authority.”
Werthman, who discussed her research after the ceremony, explained, “I’m interested specifically in burn pain, how pain in general is impacted by diversity in childhood. So, my dissertation looked at chronic pain in people who are survivors of childhood adversity and how they use pain catastrophizing as a coping mechanism, and how that impacts their experience of pain in adulthood.”
She added, “It was challenging. I had a full-time job, a full-time family, and I was a full-time PhD student. But relationship building, not just with my mentor but with other people in the lab, was worthwhile. My mentor, Luana Colloca, has a multidisciplinary lab, so I was working with PhD students from neurology, neuroscience, and epidemiology. We were working on similar problems but from extraordinarily different viewpoints.”
Students in the May 15 ceremony earned master’s degrees or certificates in biomedical, health, and human service sciences. They will work in a variety of fields as they focus on some of society’s most challenging questions, fulfilling a mission to serve the public good.
In his remarks during that event, Wong emphasized the remarkable perseverance of graduating students.
“This is a major accomplishment and marks yet another impressive milestone on your quest for personal and professional fulfillment. I know that many of you have pursued this degree while working full time or part time, raising a family, and being caregivers in other ways,” Wong said. “We are exceptionally proud of you. We can’t wait to see what you do next.”
UMB Provost and Executive Vice President Roger J. Ward, EdD, JD, MSL, MPA , who attended both ceremonies to officially confer degrees and certificates, thanked families at the master’s and certificate celebration for supporting students throughout their academic journey. He also stressed the dedication faculty have to each student’s intellectual and personal growth.
“I want to also recognize the exceptional faculty here in the Graduate School at the University that continue to invest in you as students, support your growth, support you intellectually, challenge you, stimulate you, and frustrate you at times, I’m sure,” Ward said, adding that faculty do so because they “recognize that you are the future. And we hope, truly, that you appreciate, understand, and pay it forward as you go on to become the next generation of great leaders in whatever your discipline is.”
After the ceremony, students spoke about the motivating factors that led them to pursue study at the Graduate School and the rigor of the academic experience.
Gabriel Dang , who earned a Master of Science from the Physician Assistant Program, said a visit to the emergency room after an elbow fracture spurred him toward his course of study. “That experience made me realize that I wanted to go into a field where I could deliver care to people and just help them establish a sense of security and well-being during that kind of event,” he explained.
He spoke about the program’s demanding and immersive experience and said that forming friendships with his cohort was one of the best aspects of the program. “Being in a group willing to put in so much work — seeing the growth we’ve had has been phenomenal,” Dang said, and he summed up his time pursuing a master’s by adding, “It’s been intensive, it’s been formative, and it’s been really inspirational.”
The University of Maryland, Baltimore is the founding campus of the University System of Maryland. 620 W. Lexington St., Baltimore, MD 21201 | 410-706-3100 © 2023-2024 University of Maryland, Baltimore. All rights reserved.

Mourning the Loss of Dr. Bill Higuchi, 93
College of pharmacy, student tools, graduate studies, faculty resources, research departments, research centers, dr. william "bill" higuchi, former department chair, has passed on.
The College of Pharmacy mourns the recent passing of Dr. William “Bill” Higuchi on Friday, May 10th.
Higuchi was a renowned researcher and faculty member at the University of Utah for two and a half decades – devoting his life to advancements in pharmaceutics and pharmaceutical chemistry, higher education at large, and lifting those around him in the process.
At the College’s 2023 Convocation ceremony, Higuchi was recognized for receiving an honorary doctorate from the U. He served as Department Chair of Pharmaceutics and Pharmaceutical Chemistry (now Molecular Pharmaceutics) from 1982 – 1998.

From being forced into an internment camp during World War II, to receiving high honors in both the United States and Japan, Higuchi’s story is both an inspiration and testament to the power of determination and education:
He will be dearly missed, and we thank him for his years of service and endless love towards those closest to him and the Higuchi family.
Heart Mountain Wyoming Foundation: Full Press Release
William Iyeo Higuchi, who was incarcerated as an 11-year-old boy from San Jose at Heart Mountain and who became a pioneer in pharmaceutical sciences, died Friday, May 10, at his home in Salt Lake City, Utah. He was 93.
Born in San Jose, California, on March 16, 1931, Higuchi and his family were forced from their farm in San Jose and sent first to the Santa Anita Assembly Center and then to Heart Mountain, where they arrived on Sept. 13, 1942.
While a student in the camp’s high school, Higuchi first met his future wife, Setsuko Saito Higuchi. They later reconnected while students at the University of California at Berkeley, married and had four children, including Heart Mountain Wyoming Foundation Chair Shirley Ann Higuchi.
Higuchi built a storied career in pharmaceutical sciences and was a professor at the universities of Michigan and Utah, where he mentored hundreds of doctoral students from around the world. This support would lead to him being awarded the Order of the Rising Sun, Gold Ray with Neck Ribbon, from the Japanese government in 2012.
In his later years, Higuchi was an active and generous supporter of the Heart Mountain Wyoming Foundation.
“Like many Nisei of the Greatest Generation, my father never let his incarceration experience define him,” said Shirley Ann Higuchi. “When we began our work together to fulfill my mother’s dream of building ‘something’ at the Heart Mountain site, he told me that he’s ‘made it in life’ and now his job is to share his story so it never happens to anyone again.”
“Our hearts are heavy with the passing of William Higuchi, a man whose spirit will forever ripple throughout our work at Heart Mountain,” said Heart Mountain Executive Director Aura Sunada Newlin. “We extend our deepest sympathies to the Higuchi family as they grieve.”
The family requests that gifts be made in his memory to the Heart Mountain Wyoming Foundation, 1539 Road 19, Powell, WY 82435 or at heartmountain.org .
The Heart Mountain Wyoming Foundation, a Smithsonian Affiliate, preserves the site where some 14,000 Japanese Americans were unjustly incarcerated in Wyoming from 1942 through 1945. Their stories are told within the foundation’s museum, Heart Mountain Interpretive Center, located between Cody and Powell. For more information, call the center at (307) 754-8000 or email [email protected] .
^ Originally published as " Heart Mountain mourns the passing of Dr. William Higuchi " by the Heart Mountain Wyoming Foundation .
- Campus News /
MCW School of Pharmacy Celebrates Class of 2024 PharmD Graduates
May 17, 2024

On May 16, the Medical College of Wisconsin School of Pharmacy’s Hooding Ceremony formally marked the transition from student pharmacist to Doctor of Pharmacy (PharmD) graduate. Faculty members placed elongated hoods over the shoulders of 39 students at MCW’s T. Michael Bolger Auditorium, signifying the official start of their professional pharmacy career.
"Access to care is critical,” said George E. MacKinnon III, PhD, DMSc (Hon.), MS, RPh , founding dean and professor. “There are so many individuals that are underinsured and uninsured, and as a profession, we provide access throughout our communities. Society needs our profession, and they need you.”
In his keynote speech, Russell B. Melchert, PhD, RPh, dean and professor at the University of Missouri-Kansas City School of Pharmacy, highlighted the word “doctor” and its Latin roots meaning “teacher.”
“The term ‘doctor’ should be reserved as a reverent term for those who use their skills and knowledge to teach others how to live better, healthier, happier lives,” said Dr. Melchert. “So, if you dedicate yourself to teaching others how to do just that, then I will call you ‘doctor’ every time.”
Kimberly Bell, PharmD, chief of pharmacy at the Clement J. Zablocki VA Medical Center and assistant dean of the MCW School of Pharmacy , led graduating students in reciting the Oath of a Pharmacist. This promise states that pharmacists will devote themselves to a lifetime of service to others, including prioritizing relief of suffering and advocating for justice to advance health equity.
“Class of 2024, I am infinitely proud of all of you,” said class president, Matthew DeSchepper. “As we go our separate ways, I hope you remember our time together. I hope you strive to be that invigorating force, and I hope you share your life and your vitality with the world.”
During the Awards Ceremony, eleven students were recognized for outstanding performance in academics, on clinical rotations or in community service.
- American Society of Health-System Pharmacists (ASHP) Student Society – Officer Recognition Award
- American Pharmacists Association (APhA) Academy of Student Pharmacists – Senior Recognition Certificate
- Advanced Pharmacy Practice Experience (APPE) Certificate of Merit
- Diversity and Inclusion Pharmacy Student Award
- Pharmacy Society of Wisconsin – Student Achievement Award
- Interprofessional Education Student Award
- Friends of MCW Service Award
- Fostering Healthy Communities Award
- Clement J. Zablocki Veterans Affairs – Student Achievement Award
- United States Public Health Service (UHPS) Excellence in Public Health Pharmacy Award
- Froedtert and the Medical College of Wisconsin – Advancing Pharmacy Practice Award
- Viatris Excellence in Pharmacy Award
- Student Pharmacist Research Dissemination Award
A number of graduates are continuing their education through residencies and fellowships. Following the American Society of Health-System Pharmacists (ASHP) Match, 22 of 23 participating students obtained postgraduate year one (PGY1) positions, yielding a 96 percent PGY1 match rate. Overall, 64 percent of these graduates are continuing their training in Wisconsin. An additional student also secured a postdoctoral fellowship position in the pharmaceutical industry.
Congratulations to the entire Class of 2024!
To see where MCW School of Pharmacy graduates have matched, view our interactive map.

Elektrostal
City in moscow oblast, russia / from wikipedia, the free encyclopedia, dear wikiwand ai, let's keep it short by simply answering these key questions:.
Can you list the top facts and stats about Elektrostal?
Summarize this article for a 10 year old

COMMENTS
The dissertation is usually a lengthy process composed of weeks filled with intense research, several draft versions, blog entries, and supervisory meetings. I was fortunate enough to get a research project in the area of pharmacy education. My project focused on student learning in integrated pharmacy curricular.
In fact, some pharmacy education researchers included in the study expressed a need for such mentoring and suggested that increased exposure, training, and understanding of qualitative research may increase its value and acceptability in pharmacy education. This mentoring could be formed through schools and colleges of pharmacy partnering with ...
A dissertation submitted to the Graduate Faculty of . Auburn University . in partial fulfillment of the . requirements for the Degree of . Doctor of Philosophy . Auburn, Alabama . August 5, 2023 . Keywords: curricular integration, competency-based education, pharmacy education, transformational leadership, innovative mindset, qualitative case study
The mission of AACP is to lead and partner with our members in advancing pharmacy education, research, scholarship, practice and service to improve societal health. More . Metrics. Metrics. 3.3. 2022 Impact Factor. 3.1. 2022 5-Year Impact Factor. 4.2. 2022 CiteScore. 5,544. 2022 Total Citations. Most Read (Last 30 Days)
Dissertations Graduate School 5-2013 A Case Study on Pharmacy to Explore the Perceptions of Pharmacy Leaders and Policy Makers on the Benefits, Risks, and Alternatives of ... furtherance of pharmacy education that began with the four-year Bachelor's degree mandate in 1932 (Cohen, 2008). Throughout the 20th century, the AACP and other
This Dissertation is brought to you for free and open access by the Theses and Dissertations at DUNE: DigitalUNE. ... Pharmacy Education, 2010) and pharmacy and medical programs have required accreditation standards for interprofessional collaboration effective in 2016 (Zorek & Raehl, 2013).
Pharmacy education has evolved rapidly worldwide in the last decade, with significant changes to the education curricula, pedagogical approaches and modes of delivery. [16, 20-22] The latest revision to the mission of pharmacy education holds academic programmes responsible 'to prepare graduates to conduct research and scholarly activity'.
In 2005, the American College of Clinical Pharmacy (ACCP) defined clinical pharmacy as. a health science discipline in which pharmacists provide patient care that optimizes medication therapy and promotes health, wellness, and disease prevention. 1. The practice of clinical pharmacy embraces the philosophy of pharmaceutical care 2; it blends a ...
Linda S. White — Functional Analysis of Birth Certificate Data Collection Procedures in Mississippi Hospitals. For dissertation titles prior to 1990, please see Department History page. Get more information on the Department of Pharmacy Administration's Completed Dissertations. Learn more now.
The Center for the Advancement of Pharmacy Education (CAPE) and Accreditation Council for. Pharmacy Education (ACPE) recognized this need for improving student leadership development. (SLD) by incorporating it into their guidance documentation, CAPE 2013 and ACPE Standards. for Doctor of Pharmacy Programs 2016.
Evaluation of the dissertation through a public presentation, peer-reviewed publications, viva examination and examination by an external assessor was carried out. Leadership aspects were measured through policies and strategies presented by students and graduates in the course. ... Clinical pharmacy education and practice evolvement in Malta ...
Pharmacy Education in the Twenty First Century and Beyond, 2018, pp. 11-20. Derek Stewart, Donald E. Letendre. Evaluation of Video-enhanced Case-based Activities Guided by the Pharmacists' Patient Care Process. American Journal of Pharmaceutical Education, Volume 83, Issue 4, 2019, Article 6676.
Theses/Dissertations from 2022 PDF. Response of Dopaminergic System to Cocaine Exposure, Recovery after Cocaine Abstinence, and Impact of a Long-acting Cocaine Hydrolase, Jing Deng. PDF. ANALYSIS OF POTENTIAL FACILITATORS TO USE OF HIV PRE-EXPOSURE PROPHYLAXIS (PrEP) IN A YOUNG TRANSGENDER POPULATION, Noah Dixon. PDF
Theses/Dissertations from 2021. PDF. The Association of Antidepressants Use with Healthcare Utilization, Patient-Reported Outcomes, and Alzheimer's Disease and Related Dementias (ADRD) Among Medicare Beneficiaries with Depression, Abdulrahman Abdullah A Alnijadi. PDF.
Strategies for Managing Pharmacy Experiential Education During COVID-19. J Pharm Pract. 2021 Feb;34 (1):7-10. doi: 10.1177/0897190020977730. Epub 2020 Dec 3.
A majority of respondents reported a strong need for integrating a formal research ethics course into postgraduate pharmacy curriculum (90%) to support their research training and thesis writing (89%). Overall, the study revealed a notable lack of research ethics education for graduate-level pharmacy students in Jordan.
1995. Ram Mohan Chukkapalli — Consumer Self-Medication Behavior: the Influence of Different Factors on Consumer's Purchase Decisions in the Selection of OTC Analgesics. For thesis project titles prior to 1995, please see the Department History page. Get more information on the Department of Pharmacy Administration's Completed Theses.
The purpose of the dissertation showcase is to promote outstanding student work as exemplars of best practice. The dissertations in the showcase represent the "Top Ten" undergraduate and taught postgraduate dissertations selected by participating Schools within the University of Lincoln. These will normally have achieved a First Class ...
of (interprofessional) education, interdisciplinary work, and pharmacy history and status. Your commitment to "learning outside of the box" allowed me to take courses in other colleges, which became the impetus for my dissertation topic and research question. I also have much gratitude for the committee members for their expertise and ...
Pharmacy research : a how-to guide for students, residents, and new practitioners by Vellurattil. ISBN: 1582122415. Publication Date: 2015. Detailed info on conducting a lit review. Bailey's Research for the Health Professional, 3rd ed. ISBN: 9780803645134. Includes detailed help on literature reviews.
The University of Florida College of Pharmacy, the oldest college in the UF Health Science Center, was established in 1923. Today, the college is ranked among the top colleges and schools of pharmacy in the nation. In keeping with the University of Florida mission, the college is dedicated to excellence in pharmacy research, service, and educational programs enhanced through online technologies.
In the face of rapid technological advancement, the pharmacy sector is undergoing a significant digital transformation. This review explores the transformative impact of digitalization in the global pharmacy sector. We illustrated how advancements in technologies like artificial intelligence, blockchain, and online platforms are reshaping pharmacy services and education. The paper provides a ...
BSPS and incoming PharmD student Hannah Hoffmann won first place in the División of Pharmacology Education Student Poster Competition at the American Society of Pharmacology and Experimental Therapeutics (ASPET) 2024 Annual Meeting May 16-19 in Arlington, Virginia. ... College of Pharmacy. 217 Lloyd M. Parks Hall. 500 West 12th Ave. Columbus ...
May 20, 2024. Nine PhD students at the University of Pittsburgh have received research funding from the Pitt's Humanities Engage project, which is committed to broadening and deepening the intellectual and professional development of all PhD candidates. Six of the recipients pitched their own Summer Immersive Fellowships, which offer the ...
* Corresponding authors a Key Laboratory of Immune Microenvironment and Disease (Ministry of Education), The Province and Ministry Co-sponsored Collaborative Innovation Center for Medical Epigenetics, Tianjin Key Laboratory on Technologies Enabling Development of Clinical Therapeutics and Diagnostics (Theranostics), School of Pharmacy; Department of Genetics, School of Basic Medical Sciences ...
Wong's remarks came during two days of celebrations for students from the Graduate School. In addition to the Doctoral Hooding Ceremony on May 16, the school held a Master's and Certificate Graduation Ceremony on May 15. Both events underscored the interprofessional and interdisciplinary nature of study at the Graduate School, as well as ...
The College of Pharmacy mourns the recent passing of Dr. William "Bill" Higuchi on Friday, May 10th. Higuchi was a renowned researcher and faculty member at the University of Utah for two and a half decades - devoting his life to advancements in pharmaceutics and pharmaceutical chemistry, higher education at large, and lifting those around him in the process.
On May 16, the Medical College of Wisconsin School of Pharmacy's Hooding Ceremony formally marked the transition from student pharmacist to Doctor of Pharmacy (PharmD) graduate. Faculty members placed elongated hoods over the shoulders of 39 students at MCW's T. Michael Bolger Auditorium, signifying the official start of their professional ...
In 1938, it was granted town status. [citation needed]Administrative and municipal status. Within the framework of administrative divisions, it is incorporated as Elektrostal City Under Oblast Jurisdiction—an administrative unit with the status equal to that of the districts. As a municipal division, Elektrostal City Under Oblast Jurisdiction is incorporated as Elektrostal Urban Okrug.
Elektrostal , lit: Electric and Сталь , lit: Steel) is a city in Moscow Oblast, Russia, located 58 kilometers east of Moscow. Population: 155,196 ; 146,294 ...