An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Educ Health Promot

Impact of knowledge, attitude, and practices of Type 2 diabetic patients: A study in the locality in Vietnam
Nghiep ke le.
Department of Public Health, Faculty of Public Health, Mahasarakham University, Mahasarakham, Thailand
Niruwan Turnbull
Cuong van dam.
1 University Administrators, Faculty of Medicine, Can Tho University of Medicine and Pharmacy, Can Tho Province, Vietnam
Santisith Khiewkhern
Surasak thiabrithi, background:.
Disease knowledge, appropriate attitude, and proper practices play an important role in disease control and reduction of diabetes-related complications and deaths. This study aims to investigate the impact of knowledge, attitude, and practices (KAPs) of Type 2 diabetic patients' outcomes.
MATERIALS AND METHODS:
A cross-sectional research was conducted on a group of 102 Type 2 diabetic participants in 17 communities in Tam Binh District, Vinh Long Province, Vietnam. The research tool employed the KAP questionnaire using IBM SPSS 22 to analyze the data.
The participants' average age was 57.02 ± 6.323 years. The proportion of women was 76.5% (three times higher than men). The knowledge score of the participants was low (30.04 ± 12.823), the attitude toward score of diabetics was moderate (61.544 ± 29.99), and the practice of self-care score was low (50.59 ± 14.881). There were also some significant relationships between KAPs with ethnicity, marital status, diabetic duration, location, employment status, and treatment method. In addition, there were only significant differences between the self-care practice groups and patients' attitude toward Type 2 diabetes.
CONCLUSION:
There is a significant relationship between KAP with some participants' characteristics. The KAPs of the diabetic patients in Tam Binh district are still low. This result showed that although the patient's attitude towards disease was good, it was not enough for them to practice good self-control due to poor knowledge.
Introduction
Type 2 diabetes mellitus (T2DM) is a long-term metabolic confusion disease that is related to a high rate of complication and mortality in a population.[ 1 , 2 ] The worldwide prevalence of diabetes was 177 million in 2000,[ 3 ] which increased to 422 million in 2014,[ 4 ] and it will be reaching 592 million by 2035.[ 5 ] In 2015, there were over 3.5 million Vietnamese adults living with diabetes. Particularly, T2DM is the most common type, with the incidence doubling in the previous decade (2.7% in 2002–5.4% in 2012).[ 6 , 7 ]
Diabetic treatment is a lifelong process, so self-motivation of the patient is needed. Therefore, patients need a basic knowledge of diabetes, and if they have knowledge about the disease, they will be more positive about the attitude and better practice.[ 8 , 9 ] It can help early disease detection and complication reduction.[ 10 , 11 ] Some authors have assessed the knowledge, attitude, and practice (KAP) of diabetes using the KAP questionnaire and promoted them for better cognizance of how to manage risk factors including program intervention of the diabetes.[ 12 ] They also indicated that diabetes knowledge, attitudes toward disease, and practices of the diabetic self-management are associated with a greater understanding of the prevention, diagnosis, and control of risk factors.[ 13 ] This study assessed the impact of knowledge, attitude toward diabetes, and practice of self-care management of T2DM patients. In spite of that, the knowledge related to diabetic control has globally been realized to be scanty.[ 9 ] Especially, no studies have been conducted on the general population in Tam Binh district, Vinh Long province, Vietnam, to assess the KAP of T2DM.
Therefore, this study aims to ascertain the impact of the knowledge, attitude toward diabetes, and practices of T2DM in Tam Binh district, Vinh Long province, Vietnam, which will further identify the relationship between KAPs in participants.
Materials and Methods
The participants.
This cross-sectional research was conducted on one group including 102 participants at 17 communes (six participants per commune) in Tam Binh district, Vinh Long province, Vietnam, from July to August 2019. The participants were randomly selected based on each local diabetic management list. Sampling criteria were patients aged 35–65 years with T2DM; diabetic duration from 6 months or more; those who were not hospitalized in the past 3 months; and those who did not have neurological abnormalities and malformations.
The knowledge, attitude, practice questionnaire
The KAP questionnaire was created by the researcher in both Vietnamese and English to suit Vietnamese culture [ Supplement Table 1 ]. The KAP questionnaire consists of four parts including (1) the demographic of the participants, (2) the knowledge of individuals with diabetes, (3) participants' attitude toward diabetics, and (4) participants' self-care management of diabetes. The knowledge part contains ten multiple choices with 1 score for each correct answer.

The practice of self-care section has ten questions about diabetic self-management. For a question that is divided into several subtleties, if the participant gives an incorrect answer any of details, the question was considered wrong. Each correct answer is scored “1;” on the other hand, an incorrect answer is scored “0.”
The scores are divided into three levels, namely, low level (<60% of the total points), moderate level (60%–79% of the total points), and high level (≥80% of the total points).[ 16 ]
Data collection
The questionnaire was reviewed by five experts with a doctoral or higher degree in Can Tho University of Medicine and Pharmacy, with an item objective congruence = 1 [ Supplement Table 2 ]. Then, the questionnaire was administered to ten participants in Tam Binh District Health Centre center with Cronbach's alpha = 0.738 [ Supplement Table 3 ]. The questionnaire was sent directly to each patient. The staffs would guide how to answer but they had absolutely no hint of the answer.
Supplement Table 2
The item objective congruence index
IOCI=Item objective congruence index
Supplement Table 3
The reliability and validity of the knowledge, attitude, and practice questionnaire
Statistical analysis
All collected data were coded before they were analyzed by IBM SPSS software version 22, IBM corporation. The descriptive statistics including frequency, mean, and standard deviation were used for evaluating participant characteristics and KAP score. Correlation between variables was assessed using Pearson's correlation coefficients. The relation between knowledge, practice, and attitude sections was analyzed by regression correlation. The significance level for all tests was fixed at α < 0.05.
Besides, age was separated into two groups as Group 1 from 35 to 49 years and Group 2 from 50 to 65 years. In addition, the duration of T2DM was divided into four groups as Group 1 under 10 years, Group 2 from 10 to 20 years, Group 3 from 20 to 30 years, and Group 4 over 30 years. Furthermore, the glycemic levels diverged into three groups such as group 1 under 3.9 mmol/L, Group 2 from 3.9 to 6.4 mmol/L, and Group 3 above 6.4 mmol/L. In addition, the HbA1c levels were divided into three groups as Group 1 below 4%, Group 2 from 4% to 6%, and Group 3 above 6%.
Participant demographic data
All the study patients (102) had an average age of 57.02 ± 6.32 years. The proportion of women accounted for 76.5% (more than three times of men, 23.5%). The ethnicity was Kinh who suffered the most from diabetes, 96.1%; 101 participants (99%) were married and are living with small families for 1–2 generations (73.5%), while 26.5% of the participants are living in large families over three generations. Most of the participants had primary to higher education (94.1%); only 5.9% of them were illiterate. Nearly 76.5% of the patients had jobs, both part time and full time, and the remaining (23.5%) did not work including retirement and unemployment. The majority of participants had a high monthly income of 82.4% (84 participants). The average duration of the diabetics was 4.33 ± 4.56 years, the longest was 22 years, the shortest was 0.5 years. The blood glucose level and HbA1c level of the participants were 9.60 ± 3.77 mmol/L and 7.40 ± 2.46%, respectively [ Table 1 ].
The demographic data and knowledge, attitudes, and practices of the participants
SD=Standard deviation, KAP=Knowledge, attitude, and practice
The participants' knowledge, attitudes, and practices
All patients completed the KAP questionnaire, in which the score was low (50.057 ± 10.644). Specifically, their knowledge score was low (30.04 ± 12.823). In particular, the majority of participants (97 people) had a low knowledge level of 95.1% [ Table 1 ]. Despite this, some knowledge had a quite high patient rate such as: “how many types of diabetes” were 71.6%; “the concept of type 2 diabetes” had 53.9%; “the symptoms of hypoglycemic” occupied 66.7%. However, their attitude score was moderate (61.544 ± 29.99). Among them, those with low attitudes accounted for more than half of the 52% (53 people), followed by those with an average attitude of 25.5% (26 patients), and those with high attitude22.5% (23 participants) [ Table 1 ]. In addition, the practice score was low at 50.59 ± 14.881. In this section, the practice was recorded as an average with 8.8% (14 people), six times lower than patients with a low level of practice of 86.3% (88 people). However, only 5.9% of the people with diabetes practiced high level of practice [ Table 1 ].
Regarding diabetic self-management practice, the highest percentage of patients treated with oral medication constituted 77.5% (77 participants), followed by insulin injections with 6.9% (7 patients) and diet therapy with 5.9% (6 participants); in addition, patients without treatment accounted for 11.8% (12 patients). The majority of patients using one type of drug to treat diabetes each day accounted for 56.9%. Two patients (2%) used six tables of diabetic drug per day. Patients in the study injected the insulin into the abdomen and shoulders [ Table 2 ].
The proportion of the components of practice section
The relation between participants' characteristics and knowledge, attitude, and practice
Table 3 describes the relation between patients' KAP and their characteristics such as age, gender, ethnicity, location, marital status, type of family, education level, employment status, monthly income, diabetic duration, diabetic information, glycemic level, HbA1c status, glycemic checking place, other disease, treatment method, hypoglycemia, smoking history, and drinking history. It showed a significant relationship in diabetic knowledge between Kinh and Khmer ethnic groups, as well as between groups of patients with different diabetic duration ( P = 0.000 and 0.043) [ Table 3 ]. Moreover, the results also described a statistically significant relationship between the patients' attitude to diabetes and different patient groups in terms of location ( P = 0.003) [ Table 3 ], employment status ( P = 0.000), treatment method, hypo-glycemia and diabetic duration. On the other hand, the research results also found a significant association between marital status and diabetic duration with patients' daily disease self-management practices [ Table 3 ].
The relation between patients’ characteristics and knowledge, attitudes, and practices by one-way ANOVA
The relation between knowledge, attitude, and practice
Table 4 shows the difference in knowledge and attitude of Type 2 diabetic patients between the different practice groups. In this relationship, only the difference in the practice of the attitude groups was statistically significant ( P = 0.014). There were also differences in knowledge between practice groups, but this was not statistically significant.
The relation between patients’ knowledge, attitude, and practice
Diabetes is a chronic metabolic disorder with many different complications.[ 5 ] Therefore, in order to control the disease effectively, patients need to have the right KAP about diabetes.[ 9 ] This study assessed diabetic patients' KAP of diabetes management. It also explored the relationship between KAPs of Type 2 diabetic patients.
The study was conducted on individuals aged between 35 and 65 years because at this age diabetes had been seem to be highly prevalent in Vietnam according to the 2002 National Statistical Survey ,[7 ] and it is also an age group of cognitive maturity. The median age of the patients in this study was 57.02 years, which is consistent with the study of Ng et al .[ 1 ] and Le Roux et al .[ 9 ] Like many other studies, this study had a higher proportion of women with Type 2 diabetes than men.[ 3 , 6 , 9 ] However, some studies report that diabetes is more common in men than in women,[ 5 , 17 ] but the difference was not significant.
Furthermore, Salem et al . also reported that the patients in their study were highly educated from high school and above.[ 13 ] Simultaneously, the study of Saengtipbovorn et al . reported that 76.5% of their participants had completed primary school education.[ 2 ] Similarly, this study found that most patients had primary or higher level of education (93%). Nevertheless, a study in Iran by Mohammadi et al . found that nearly 27 illiterate patients, but the majority (41%) of the study participants, were not attending primary school.[ 18 ] The low levels of education were also found in the study by Al-Maskari et al . with 46% illiteracy.[ 19 ]
Most patients had a job, so their income was high. Concurrently, a study by Saengtipbovorn et al . showed that 37.1% of the study participants earned <1500 baht per month.[ 2 ] In addition, a study by Mohammadi et al . found that only 27% of the patients had jobs and their monthly income was <8,000,000 Rials.[ 18 ] The average duration of diabetes in the study by Al-Maskari et al . was 9 years.[ 19 ] Rahaman et al . also showed that the average duration of diabetes was 9.16 ± 6.03 years.[ 20 ] However, patients in the current study had a significantly lower duration of Type 2 diabetes than the previous two studies (4.33 ± 4.56 years). More than half of the patients have received information about diabetes. However, Rahaman et al . reported that only 38.6% of the patients participated in a diabetes-related education program.[ 20 ] About one-quarter (26%) of the patients in the study by Magbanua and Lim-Alba participated in the diabetes education.[ 21 ]
Most patients had at least one other condition related to diabetes (95.1%) such as hypertension, hypercholesterolemia, heart disease, vision problems, neurological problems, poor sexual desire, and kidney problems. These issues were also found in the study by Mohammadi et al . in Iran.[ 18 ] Participants' blood sugar and HbA1c levels were quite high. High levels of HbA1c were also found in the study by Al-Maskari et al .[ 19 ] and Rahaman et al .[ 20 ] Rahaman et al . also showed that blood glucose levels were also high, although participants tested their own blood glucose levels at home and in the hospital.[ 20 ] However, patients in this study did not self-test their blood glucose and HbA1c level; most of them checked it at government hospitals and a few did at private clinics. Moreover, the results of this study showed that patients with poor glycemic control have a relatively high rate of hypoglycemia (59.8%).
Similar to the research by Karaoui et al .,[ 22 ] most patients in the present study have used oral medications to control the disease. In addition, this result was similar to those of Salem et al .,[ 13 ] with high smoking denial rates. Similar results were found in the study of Saengtipbovorn et al . with the rate of never smokers up to 87.1%.[ 2 ] In contrast, Karaoui et al . reported that more than half of the smoking patients participated in the study.[ 22 ] Correspondingly, the alcohol consumption rate in this study was low.
The related of knowledge within people with diabetes
The analysis showed that participants' knowledge of diabetes was still low. This was because patients had not been provided with basic information about Type 2 diabetes. This problem had also been reported by Cao My Phuong et al .[ 23 ] Nhung and Dao showed that knowledge about diabetes treatment and complications of the patients was low.[ 24 ] In addition, a research by Karaoui et al . showed that the knowledge base of diabetes in the research population was still low.[ 22 ] Indeed, Rahaman et al . reported a lack of diabetic knowledge in the research community.[ 20 ] Indeed, the study by Quang et al . also indicated that the number of participants without knowledge about diabetes was quite high.[ 7 ]
Attitude toward diabetes in Vietnamese culture
Al-Maskari et al . concluded that although patients have poor knowledge, a positive attitude was an important issue in the care and practice of diabetes.[ 19 ] Meanwhile, Salem et al . stated that, although most patients have the knowledge of diabetes, it was not at a high level, and their attitude and practice were not satisfactory.[ 13 ] Similarly, this study also showed that participants had an average attitude level toward diabetes.
Practice of self-care management
The participants' diabetes management practices were generally poor. This showed that a medium attitude score is not enough; it requires good knowledge to lead to the right practices to control diabetes. Ng et al . concluded that factors of proper knowledge and attitude led to good disease control practices.[ 1 ] Saadia et al . also confirmed that the participants' knowledge of diabetes in research was good, but their attitude and practice were poor.[ 25 ]
The relation of participants' components and knowledge, attitude, and practice
Our research shows that most of the relationships between participants' characteristics and their KAPs had a negligible difference. However, there were some significant relational characteristics, such as race and blood sugar that differed significantly in knowledge about Type 2 diabetes; marital status and family type were statistically significantly related to the patient's attitude toward the disease. Moreover, gender, marital status, education, and monthly income were significantly related to diabetes control practices. Similarly, Ghannadi et al . also showed that the relationship between sex and marital status with KAP was not statistically significant.[ 17 ] However, Salem et al . reported that there was a significant relationship between KAP scores and different categories such as location, gender, and education.[ 13 ] Moreover, Ng et al . showed a significant inverse correlation between KAP scores and HbA1c.[ 1 ]
The relation of knowledge and attitude with practice
The results of this study showed that the relationship between patient attitude groups and practical components was statistically significant. However, this was not found in the relationship between knowledge and attitude of diabetic patients. This was due to the culture of the Vietnamese people. Indeed, the study of Al-Maskari et al . also found that there was a significant relationship between practice and attitude of patients, but the authors also reported more meaningful results between attitude and knowledge.[ 19 ] Meanwhile, the study by Ghannadi et al . showed that higher knowledge was significantly correlated with higher attitudes and practices.[ 17 ]
Although KAP of self-control in diabetes are important contributions to the good treatment of the disease, patients in the study had low scores for these issues. Despite the average attitude about Type 2 diabetes, limited knowledge about the disease is not sufficient, the lack of which leads to poor practices of care and control. However, the results showed that there was only significant difference between attitude and practice in patients with Type 2 diabetes. Furthermore, the relationship between KAP with patients' characteristics had different significance.
Financial support and sponsorship
This article is a part of my thesis “The development of health-related quality of life programme among type 2 diabetic patients in Tam Binh District, Vinh Long Province, Vietnam,” which is accepted by the ethical committee for the fieldwork of Mahasarakham University; with the certificate of approval number of 071/2019.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the participants and the local Government from Tam Binh District, Vinh Long Province, Vietnam, and Dr. Ngo Van Truyen PhD, MD, Dean of Faculty of Medicine; Dr. Le Van Minh PhD, MD, Vice Dean of Faculty of Medicine and Deputy Head of the Department of Interventional Cardiology-Neurology; Dr. Tran Kim Son PhD, MD, Department of Internal Medicine; Dr. Vo Pham Minh Thu PhD, MD, Head of the Personal Department and Dean of Department of General Medicine; Dr. Nguyen Thi Diem PhD, MD, Faculty of Medicine and ethics committee and public health faculty of Mahasarakham University, Thailand, who had made the study possible, and the health commune staffs and the research sampling groups.
Supplement Table 1: Knowledge, Attitude, and Practice Questionnaire

MAHASARAKHAM UNIVERSITY
DIABETIC KNOWLEDGE, ATTITUDE, PRACTICE
Participant Number (Office use): ___________
Date: ___________________
A. PARTICIPANT INFORMATION
- Full name: ______________________________________________________
- Birth year: __________________
- Gender: □ Male □ Female
- Address: _______________________________________________________
- Glycemia: ______________ mmol/L
- HbA 1 C: ________ %
B. DIABETIC KNOWLEDGE
Please circle in the letter that you think is the best.
- Diabetes is a chronic metabolic disorder characterized by hyperglycemia
- Diabetes is a chronic metabolic disorder with a manifestation of hypoglycemia
- Diabetes is a disease spread in the community
- Because the body produces lack or does not produce insulin
- Because the body is resistant to insulin (usually occurs in obese people and >40 years old)
- Occurs in pregnant women (no previous diabetes)
- People who are obese, sedentary, eat a lot of fat, sweet, starch, alcohol, tobacco, family history of diabetes
- Muscular people, exercise regularly, eat well, do not smoke, do not drink alcohol
- Thin people, eat normally, have no family history of diabetes
- Eat a lot, drink a lot, lose weight a lot, urinate a lot
- Eating normally, losing little weight, moderate urination
- Eat less, lose weight, urinate often
- One type: acute complications
- Two types: acute complications and chronic complications
- Three types: acute complication, subacute complication and chronic complication
- Hyperglycemia and foot ulcer
- Insomnia, anxiety and weight loss
- Hypoglycemia and coma due to hyperglycemia, ketoacidosis and lactic infections
- Hypoglycemia and coma
- Cardiovascular complications, decreased vision, kidney failure, impotence, foot ulcers
- Insomnia, anxiety, difficulty breathing
- Routine blood glucose testing, prescription medication, reasonable eating, proper exercise
- There is no need for routine blood glucose testing, no need for food, no medication, and limited movement
- Test whenever you want, just taking the medicine is enough without don't need the well eating and exercise
- High fever, cold shaking
- Uncomfortable, sweating, dizziness
- Abdominal pain, difficulty breathing
C. DIABETIC ATTITUDE
Please circle the answer you choose
1. Do you agree that blood glucose testing for you and your family is necessary?

2. Do you agree that diabetes can be well controlled?
3. Do you agree that blood sugar can be controlled by exercise, sports and medicine?
4. Do you agree with a reasonable diet that can control blood sugar?
5. Do you agree with the need to have regular medical checkups and blood sugar checks?
6. Do you agree that complications of diabetes are a very serious problem?
7. Do you agree that prevention of complications is important in treating diabetes?
8. Do you agree that daily exercise can control diabetes complications?
9. Do you agree about worrying about hypoglycemic complications?
10. Do you agree with taking care of your feet while treating diabetes?
D. DIABETIC PRACTICE
Please answer all the questions below
1. Which method do you treat diabetes with?
□ Oral medicine. How many tablets per day? ____ tablets. How many times
per day? ____ times
□ Insulin injection. How many times of injection? ____________ times.
Injection site? ___________________
2. Do you have regular blood sugar tests? ___ yes ___ no
Where do you check? ______________________ How often? ____________
3. Do you have an HbA1C test? _____ has _____ no
Where do you check? ______________________ How often? _________
4. Do you exercise regularly? ______ yes _______ no
How long is a day? ___________ How many days per week? ____________
Which method do you exercise? ___________________________________
Do you know exercise can lower blood sugar? ___ yes ___ no
5. How many meals do you eat a day? _______________________________
Should you skip meals? ______ yes _______ no
6. What kind of foods do you need to limit or reduce?
______________________________________________________________
7. Do you smoke cigarettes? _______ has ________ no
How many cigarettes per day? _________________ cigarettes
How long have you smoked? __________________________
8. Do you drink alcohol? ________ yes ________ no
If yes, what is the level of drinking? _______________________________
9. Have you ever had hypoglycemia? _____ has _______ not yet
If so, how did you handle it? __________________________________
10. How do you take care of your feet?
THANK YOU FOR YOUR ANSWERS!
- How it works

Useful Links
How much will your dissertation cost?
Have an expert academic write your dissertation paper!
Dissertation Services

Get unlimited topic ideas and a dissertation plan for just £45.00
Order topics and plan
Get 1 free topic in your area of study with aim and justification
Yes I want the free topic

45 of the Best Diabetes Dissertation Topics
Published by Owen Ingram at January 2nd, 2023 , Revised On August 16, 2023
The prevalence of diabetes among the world’s population has been increasing steadily over the last few decades, thanks to the growing consumption of fast food and an increasingly comfortable lifestyle. With the field of diabetes evolving rapidly, it is essential to base your dissertation on a trending diabetes dissertation topic that fills a gap in research.
Finding a perfect research topic is one of the most challenging aspects of dissertation writing in any discipline . Several resources are available to students on the internet to help them conduct research and brainstorm to develop their topic selection, but this can take a significant amount of time. So, we decided to provide a list of well-researched, unique and intriguing diabetes research topics and ideas to help you get started.
Other Subject Links:
- Evidence-based Practice Nursing Dissertation Topics
- Child Health Nursing Dissertation Topics
- Adult Nursing Dissertation Topics
- Critical Care Nursing Dissertation Topics
- Palliative Care Nursing Dissertation Topics
- Mental Health Nursing Dissertation Topics
- Nursing Dissertation Topics
- Coronavirus (COVID-19) Nursing Dissertation Topics
List of Diabetes Dissertation Topics
- Why do people recently diagnosed with diabetes have such difficulty accepting reality and controlling their health?
- What are the reactions of children who have recently been diagnosed with diabetes? What can be done to improve their grasp of how to treat the disease?
- In long-term research, people getting intensive therapy for the condition had a worse quality of life. What role should health professionals have in mitigating this effect?
- Why do so many individuals experience severe depression the months after their diagnosis despite displaying no other signs of deteriorating health?
- Discuss some of the advantages of a low-carbohydrate, high-fat diet for people with diabetes
- Discuss the notion of diabetes in paediatrics and why it is necessary to do this research regularly.
- Explain the current threat and difficulty of childhood obesity and diabetes, stressing some areas where parents are failing in their position as guardians to avoid the situation
- Explain some of the difficulties that persons with diabetes have, particularly when obtaining the necessary information and medical treatment
- Explain some of the most frequent problems that people with diabetes face, as well as how they affect the prevalence of the disease. Put out steps that can be implemented to help the problem.
- Discuss the diabetes problem among Asian American teens
- Even though it is a worldwide disease, particular ethnic groups are more likely to be diagnosed as a function of nutrition and culture. What can be done to improve their health literacy?
- Explain how self-management may be beneficial in coping with diabetes, particularly for people unable to get prompt treatment for their illness
- Discuss the possibility of better management for those with diabetes who are hospitalized
- What current therapies have had the most influence on reducing the number of short-term problems in patients’ bodies?
- How have various types of steroids altered the way the body responds in people with hypoglycemia more frequently than usual?
- What effects do type 1, and type 2 diabetes have on the kidneys? How do the most widely used monitoring approaches influence this?
- Is it true that people from specific ethnic groups are more likely to acquire heart disease or eye illness due to their diabetes diagnosis?
- How has the new a1c test helped to reduce the detrimental consequences of diabetes on the body by detecting the condition early?
- Explain the difficulty of uncontrolled diabetes and how it can eventually harm the kidneys and the heart
- Discuss how the diabetic genetic strain may be handed down from generation to generation
- What difficulties do diabetic people have while attempting to check their glucose levels and keep a balanced food plan?
- How have some individuals with type 1 or type 2 diabetes managed to live better lives than others with the disease?
- Is it true that eating too much sugar causes diabetes, cavities, acne, hyperactivity, and weight gain?
- What effect does insulin treatment have on type 2 diabetes?
- How does diabetes contribute to depression?
- What impact does snap participation have on diabetes rates?
- Why has the number of persons who perform blood glucose self-tests decreased? Could other variables, such as social or environmental, have contributed to this decrease?
- Why do patients in the United States struggle to obtain the treatment they require to monitor and maintain appropriate glucose levels? Is this due to increased healthcare costs?
- Nutrition is critical to a healthy lifestyle, yet many diabetic patients are unaware of what they should consume. Discuss
- Why have injuries and diabetes been designated as national health priorities?
- What factors contribute to the growing prevalence of type ii diabetes in adolescents?
- Does socioeconomic status influence the prevalence of diabetes?
- Alzheimer’s disease and type 2 diabetes: a critical assessment of the shared pathological traits
- What are the effects and consequences of diabetes on peripheral blood vessels?
- What is the link between genetic predisposition, obesity, and type 2 diabetes development?
- Diabetes modifies the activation and repression of pro- and anti-inflammatory signalling pathways in the vascular system.
- Understanding autoimmune diabetes through the tri-molecular complex prism
- Does economic status influence the regional variation of diabetes caused by malnutrition?
- What evidence is there for using traditional Chinese medicine and natural products to treat depression in people who also have diabetes?
- Why was the qualitative method used to evaluate diabetes programs?
- Investigate the most common symptoms of undiagnosed diabetes
- How can artificial intelligence help diabetes patients?
- What effect does the palaeolithic diet have on type 2 diabetes?
- What are the most common diabetes causes and treatments?
- What causes diabetes mellitus, and how does it affect the United Kingdom?
Hire an Expert Writer
Orders completed by our expert writers are
- Formally drafted in an academic style
- Free Amendments and 100% Plagiarism Free – or your money back!
- 100% Confidential and Timely Delivery!
- Free anti-plagiarism report
- Appreciated by thousands of clients. Check client reviews
You can contact our 24/7 customer service for a bespoke list of customized diabetes dissertation topics , proposals, or essays written by our experienced writers . Each of our professionals is accredited and well-trained to provide excellent content on a wide range of topics. Getting a good grade on your dissertation course is our priority, and we make sure that happens. Find out more here .
Free Dissertation Topic
Phone Number
Academic Level Select Academic Level Undergraduate Graduate PHD
Academic Subject
Area of Research
Frequently Asked Questions
How to find diabetes dissertation topics.
To find diabetes dissertation topics:
- Study recent research in diabetes.
- Focus on emerging trends.
- Explore prevention, treatment, tech, etc.
- Consider cultural or demographic aspects.
- Consult experts or professors.
- Select a niche that resonates with you.
You May Also Like
Diplomacy dissertation is an interesting and important academic pursuit, especially given the current global climate.
Are you passionate about helping others, especially those facing mental health challenges? If this is the case for you, then mental health nursing is a career choice you may not want to pursue.
If you are writing a research paper on criminal psychology, you can discuss any relevant topic. Do psychopaths have an innate or developed nature? Is there a way to help criminals get better?
USEFUL LINKS
LEARNING RESOURCES

COMPANY DETAILS

- How It Works
- Open access
- Published: 08 May 2024
Advances and challenges of the cell-based therapies among diabetic patients
- Ramin Raoufinia 1 , 2 ,
- Hamid Reza Rahimi 2 ,
- Ehsan Saburi 2 &
- Meysam Moghbeli ORCID: orcid.org/0000-0001-9680-0309 2
Journal of Translational Medicine volume 22 , Article number: 435 ( 2024 ) Cite this article
361 Accesses
Metrics details
Diabetes mellitus is a significant global public health challenge, with a rising prevalence and associated morbidity and mortality. Cell therapy has evolved over time and holds great potential in diabetes treatment. In the present review, we discussed the recent progresses in cell-based therapies for diabetes that provides an overview of islet and stem cell transplantation technologies used in clinical settings, highlighting their strengths and limitations. We also discussed immunomodulatory strategies employed in cell therapies. Therefore, this review highlights key progresses that pave the way to design transformative treatments to improve the life quality among diabetic patients.
Diabetes mellitus poses a formidable global public health challenge due to its rapid growing prevalence and associated morbidity, disability, and mortality [ 1 ]. According to the International Diabetes Federation, over 537 million adults aged 20–79 had diabetes worldwide in 2021 that is expected to rise to around 783 million cases by 2045 [ 2 ]. Obesity, unhealthy diets, physical inactivity as well as genetic and epigenetic predispositions are important risk factors of diabetes [ 3 , 4 , 5 ]. Diabetes is typically classified into type 1 diabetes mellitus (T1DM), gestational diabetes mellitus (GDM), and type 2 diabetes mellitus (T2DM) [ 2 ]. T1DM primarily arises from autoimmune-related damage of insulin-secreting beta cells, resulting in severe hyperglycemia and ketoacidosis [ 6 ]. In contrast, T2DM generally has a more gradual onset characterized by insulin resistance along with diminished compensatory insulin secretion from pancreatic beta cell dysfunction [ 7 ]. Diabetes is associated with macrovascular complications such as heart disease and stroke, as well as microvascular issues in eyes, kidneys, and nervous system [ 8 ]. Cancer is also a leading cause of diabetes-related death, and dementia-associated mortality has risen in recent decades [ 9 , 10 , 11 , 12 ]. Cell therapy involves transferring autologous or allogenic cellular material into patients [ 13 ]. The global market size of cell therapy is estimated to grow from $9.5 billion in 2021 to $23 billion by 2028 [ 14 ]. It combines stem and non-stem cell therapies consisting of unicellular or multicellular preparations. Cell therapies typically use autologous or allogenic cells via injection and infusion [ 15 ]. In the present review, we discussed the recent advances in cell-based therapy of diabetes, from foundational islet transplantation to regenerative strategies to highlight key developments that improve the effective treatments for diabetic patients.
Cell replacement therapy for diabetes
Pancreatic transplantation was firstly used in 1966 to treat type 1 diabetes using whole organ transplants. During the 1970s–80s, segmental pancreatic grafts were combined with techniques to divert digestive secretions away from transplanted cells. Three main techniques emerged; simultaneous pancreas-kidney transplants, pancreas transplants following kidney transplants, and pancreatic transplants. International collaboration on tracking outcomes began in 1980 with the formation of several pancreatic transplant registries and associations. However, whole organ transplantation was faced with several challenges including organ rejection, vascular complications, limited organ availability, and the effects of lifelong immunosuppression [ 16 , 17 ]. Islet cell transplantation was explored as an alternative, however isolating and transplanting pancreatic islets proved difficult due to donor availability, rejection, and immunosuppression side effects. Recent research has focused on stem cell sources that could reconstitute immune tolerance and preserve beta cell function such as mesenchymal stem cells, bone marrow cells, and embryonic stem cells [ 18 ]. A novel stem cell therapy called VX-880 was developed using proprietary technology to grow insulin-producing beta cells from allogeneic stem cells. Clinical trials began in 2021 after FDA approval to deliver the cells intrahepatically under immune suppression. A second approach called VX-264 encapsulates the same cells, avoiding immunosuppression but requiring surgical implantation [ 17 ]. In 2023, FDA approved the first allogeneic pancreatic islet cell therapy called Lantidra for adults with type 1 diabetes experiencing severe hypoglycemia. Approval was based on two studies where 21–30% of participants no longer required insulin one year post-treatment, with benefits lasting over five years in some cases. However, this treatment have mild and serious adverse events that are associated with treatment dose and the methods of islet cell infusion [ 19 , 20 ].
Emerging strategies for cell delivery via microencapsulation and biological devices in clinical trials
Alginate capsules as cell delivery systems.
A seminal investigation conducted in 1994 demonstrated the successful transplantation of alginate-encapsulated islets into the peritoneum of kidney transplant patients who were receiving immunosuppression therapy. Remarkably, these patients achieved insulin independence for up to nine months [ 21 ]. However, subsequent trials conducted without immunosuppression yielded inconsistent outcomes. In a study conducted in 2006, islets were encapsulated in triple-layer alginate capsules and implanted intraperitoneally in type 1 diabetes (T1D) patients. There was a positive correlation between the encapsulation and insulin production that reduced exogenous insulin requirements during one year. Despite this progress, the entry of cytokines remained a potential concern [ 22 ]. Another study employed the single-layer barium-alginate capsules that sustained insulin production for up to 2.5 years [ 23 ]. It has been reported that the microneedle, comprising a calcium alginate frame with polydopamine-coated poly-lactic-co-glycolic acid microspheres encapsulating insulin, enables light-triggered insulin release. Microneedle provided a suitable insulin dose to maintain blood glucose levels in line with daily fluctuations. These results established the efficacy and safety of the developed microneedle for diabetes treatment [ 24 ]. Another therapeutic approach explored the encapsulation of pancreatic islets with mesenchymal stem cells (MSCs) and decellularized pancreatic extracellular matrix (ECM). ECM derived from the pancreas supported islet cell growth and maintenance to enhance insulin expression [ 25 ]. Sodium alginate and hyaluronic acid were incorporated due to their roles in collagen production, wound healing, and physical crosslinking. The 3D porous membranes allowed optimal water and oxygen transfer while diverting excess exudate from diabetic wounds. Hydrogel accelerated re-epithelization, while decreased inflammation, indicating potential as the diabetic wound dressings [ 26 ]. Additionally, the incorporation of specific ECM components, such as collagen IV and RGD, into alginate-based microcapsules significantly improved the survival, insulin secretion, and longevity of microencapsulated islets [ 27 ].
Encaptra® device from ViaCyte
In contrast to microencapsulation techniques, ViaCyte developed a semipermeable pouch method named Encaptra, which contains pancreatic precursor cells derived from the embryonic stem cells [ 28 ]. In the initial trial conducted in 2014, the “VC-01” device was implanted in T1D individuals without the use of immunosuppression [ 29 ]. The trial confirmed the safety of the device; however, the occurrence of hypoxia induced cellular necrosis [ 30 ]. The device was modified as “VC-02” with larger pores, and two trials (NCT03162926, NCT03163511) demonstrated promising outcomes, including increased fasting C-peptide levels and a 20% reduction in insulin requirements during one year in the majority of participants [ 31 ]. In order to eliminate the necessity for immunosuppressants, ViaCyte collaborated with Gore to develop an expanded polytetrafluoroethylene (ePTFE) device with both immuno-isolating and pro-angiogenic properties [ 32 ]. This device (NCT04678557) aimed to prevent immune cell attachment and T-cell activation [ 33 ]. Additionally, ViaCyte is exploring the integration of CRISPR technology to modify stem cells, specifically by eliminating β2-microglobulin expression and PD-L1 up regulation. It is hypothesized that these genetic modifications will further hinder immune cell attachment and T-cell activation [ 30 , 34 ].
Semipermeable device from Semma therapeutics
Semma Therapeutics, which has been acquired by Vertex, pioneered the utilization of differentiated stem cell-derived islet cell clusters in clinical trials. Semma houses these cells between two semipermeable polyvinylidene fluoride membranes and is designed for subcutaneous implantation (NCT04786262) [ 31 , 35 ]. Vertex reported a significant breakthrough by infusing differentiated beta cells via the portal vein in a participant who was receiving immunosuppressants. This approach led to substantial C-peptide production and improved glycemic control during 90 days [ 36 ].

βAir device from Beta O2
Beta O2’s innovative βAir device utilizes an alginate-PTFE membrane complex to encapsulate islets, providing partial immunoisolation while ensuring a continuous supply of oxygen, which is crucial for optimal islet function [ 37 , 38 ]. The βAir device that was seeded with human islets was subcutaneously implanted in T1D individuals (NCT02064309). Although, low insulin levels were produced for up to eight weeks, there was not any reduction in the required exogenous insulin [ 37 ]. While, increasing the number of islets could potentially enhance their function, it is important to note that the continuous reliance on oxygen poses a risk of infection, despite efforts to optimize the survival of encapsulated islets [ 39 , 40 ].
Cell pouch™ device from Sernova
Sernova has developed the Cell Pouch device, which offers pre-vascularized polypropylene chambers for islet transplantation without the need for immunoprotection. The device consists of multiple cylindrical chambers that are prefilled with PTFE plugs, which are then removed after implantation to create the empty space [ 41 ]. In a 2012 trial (NCT01652911), islets were placed in the vascularized pouches of three recipients who were also receiving immunosuppression that resulted in a transient increase in C-peptide levels [ 41 ]. In a 2018 trial (NCT03513939), immunosuppression was administered after implantation and islet introduction. This trial reported sustained C-peptide production for up to nine months in two recipients, along with improved glycemic control [ 42 ]. Regarding the limitations of immunosuppression, Sernova is exploring the possibility of encapsulating islets in hydrogel as an alternative approach [ 43 ].
Shielded living therapeutics™ from Sigilon Therapeutics
Sigilon has developed the Shielded Living Therapeutics sphere, which consists of cell clusters enclosed within an alginate-TMTD coating [ 44 ]. Preclinical studies demonstrated that murine islet transplants encapsulated within these spheres maintained normoglycemia for a period of six months [ 45 ]. In a 2020 trial conducted for hemophilia (NCT04541628), the spheres were evaluated for their ability to express Factor VIII [ 46 ]. However, the trial was paused due to the development of antibodies in the third recipient receiving the highest cell doses. While, preclinical studies have shown promising efficacy, there are safety concerns regarding the TMTD coating that need to be addressed before these spheres can be used for human islet transplantation as a treatment for diabetes [ 31 ]. Emerging technologies have been investigated in clinical trials for delivering insulin-producing islets or stem cell-derived beta cells via microencapsulation or use of implantable biological devices (Table 1). Optimizing encapsulation and developing alternative implantable devices moves the field toward delivering safe and effective islet replacement without chronic immunosuppression dependency that represented an important new frontier for the cell-based treatment of diabetes. However, continued refining will be required to fully realize this promising vision and using these preclinical concepts in clinic.
Immunoengineering strategies: biomaterials for modulating immune responses
Islet encapsulation aims to prevent immune responses toward transplant antigens. However, foreign body response (FBR) against biomaterials induces inflammation around encapsulated islets that obstructs oxygen/nutrient access and causes graft failure [ 31 ]. Extensive research revealed biomaterial properties profoundly influence FBR severity, with high purity/biocompatibility moderating inflammation [ 47 ]. Deeper understanding of biomaterial immunobiology enabled developing immune-modulating constructs to steer host interactions. By altering topology/chemistry to hinder nonspecific binding and cell adhesion, these “immune-evasive biomaterials” intended to attenuate xenograft rejection at inception [ 44 ]. Both innate and adaptive immune responses have crucial roles in the context of pancreatic islet transplantation. These responses encompass the activation of tissue macrophages and neutrophils following injury, leading to the release of inflammatory cytokines that subsequently activate antigen-presenting cells (APCs), CD8 + T cells, CD4 + T cells, and cytotoxic T lymphocytes (Fig. 1 ). Zwitterionic polymers conferred anti-fouling attributes but crosslinking limitations constrained their application [ 48 ]. Novel mild zwitterionization introduced alginate modifications that prolonged prevention of fibrotic overgrowth by mitigating initial responses [ 49 , 50 , 51 ]. The prevention of graft rejection following islet cell transplantation necessitates the systemic administration of immunosuppressive agents. While, these agents effectively suppress immune responses, their continuous use exposes patients to an increased risk of infection and cancer. To mitigate these concerns, an alternative approach involving the localized delivery of immunosuppressants at the transplantation site has emerged. This localized delivery system offers several advantages, including targeted drug delivery, reduced systemic exposure, and potentially reduces the immunosuppressants doses [ 52 ]. Polymeric carriers dispersed cyclosporine A continuously at the graft site to dynamically tamp down proinflammatory cascades and T-cell activation [ 53 , 54 ]. TGF-β/IL-10 co-delivery at the microencapsulation interface hindered innate antigen presentation, obstructing adaptive response priming [ 55 , 56 ]. Regulatory T-cells emerged as the potent immunomodulators when coated on islets to improve insulin production in vitro [ 57 ]. Similarly, recombinant Jagged-1 surface patterning increased regulatory lymphocytes in vitro while enhancing glycemic oversight in vivo [ 58 ]. Targeting proinflammatory effector T-cells or presenting their Fas ligand death receptor improved long-term viability when combined with rapamycin prophylaxis [ 52 , 59 ]. Immobilizing thrombomodulin or urokinase mitigated local inflammation, with the latter conferring lifelong xenotransplant survival [ 60 ]. Peptides recognizing IL-1 receptors provided robust protection from destabilizing proinflammatory cytokines [ 61 ]. Leukemia inhibiting factor improved islet performance over polyethylene glycol encapsulation alone by inducing regulatory T-cell lineages [ 62 ]. Silk scaffolds facilitated IL-4/dexamethasone emancipation that meaningfully decreased immune reactions to grafts [ 63 ]. Therefore, the localized delivery of immunosuppressants at the transplantation site represents a promising strategy for islet cell transplantation. Compared to systemic administration, local delivery can achieve targeted immune modulation only at the graft location while reducing drug exposure throughout the body. This localized approach aims to sufficiently suppress the immune response to prevent rejection, while limiting negative side effects that may occur from systemic immunosuppression. A variety of biomaterials and surface modification strategies have been developed and investigated for the local delivery of immunosuppressive agents and immunomodulatory cytokines [ 64 , 65 , 66 ]. Understanding how biomaterial properties influence the immune response is critical to design biomaterials that can modulate inflammation and improve islet graft survival through localized immunomodulation.
Cell-based therapy through the integration of additive manufacturing techniques
Additive manufacturing utilizes computer modeling to fabricate complex 3D structures on-site with minimal post-processing. Common methods for the biomedical application are fused filament fabrication (FFF), stereolithography (SLA), and bioprinting [ 67 ]. FFF is a layer-by-layer technique that extrudes heated thermoplastics [ 68 ]. Commonly used feedstocks include acrylonitrile butadiene styrene (ABS) and polylactic acid (PLA). Other thermoplastics that have been utilized with FDM include thermoplastic polyurethane (TPU), polycarbonate (PC), polystyrene (PS), polyetherimide (PEI), polycaprolactone (PCL), polyaryletherketone (PAEK), and polyetheretherketone (PEEK), with the latter demonstrating high strength and heat tolerance. A major advantage of FDM is its ability to fabricate multi-material objects through continuous printing and alteration of the build material. In addition to typical polymers like PC and polystyrene (PS), FDM can print composites reinforced with glass, metals, ceramics, and bioresorbable polymers via integration of the constituent powders with a binding matrix. This enables enhanced control over the experimental component fabrication. While, ceramic and metal filaments traditionally contain the corresponding powder mixed with a binder, FDM provides versatility in the functional prototype construction from a wide range of thermoplastic feedstocks using precise and additive layer manufacture [ 68 , 69 , 70 , 71 , 72 ]. It provides geometric reproducibility and reduced variability compared to traditional techniques. FFF prints served as scaffolds for the transplanted cells [ 67 ]. However, minimum feature size is limited to ? ∼ 250 μm by nozzle diameter [ 68 ]. SLA employs light-curable liquid resins and achieves higher 50–150 μm resolution than FFF but with restricted material choices. Bone grafts and surgical guides are common applications [ 67 ]. Incorporating biomaterials like hydroxyapatite has expanded utility, though processing is required to mitigate cytotoxicity. Additive manufacturing can address limitations in oxygen transport, cell/material placement control and vasculature formation, and clinically translatable insulin-secreting implants [ 67 ]. Therefore, additive manufacturing technologies have the potential to enhance various aspects of the cell-based transplant design, from improving nutrient transport through optimized implant geometry to achieving precision integration of therapeutic agents (Table 2).
Enhancing nutrient transport through optimization of implant geometry
Tissue engineering for the islet transplantation requires maximizing nutrient transport [ 73 , 74 ]. Traditional scaffold fabrication introduces macroporosity but lacks precision that results in inflammation [ 67 ]. Cell encapsulation provides immunoprotection by limiting interactions between transplanted cells and the host immune system. However, this protective barrier also poses challenges for the efficient transport of essential nutrients, including oxygen, to the encapsulated cells. Modifying the geometries of encapsulation devices using conventional methods to enhance oxygen delivery has proven to be inconsistently challenging [ 67 ], so that novel approaches are required to address these challenges. Additive manufacturing allows customizing biomaterial scaffolds with defined geometries and micropore sizes to improve transport [ 75 , 76 , 77 , 78 , 79 ]. The 3D printed PLA scaffolds with islets have successful vascularization and cellular survival after subcutaneous transplantation [ 80 , 81 ]. Interlocking toroidal hydrogel-elastomer constructs also increased surface area and cell viability [ 82 , 83 , 84 ].
Enhancing vascularization and engraftment
Rich host vascularization of transplant devices is essential to support long-term islet survival through efficient nutrient delivery and insulin kinetics. Early platforms modified bulk material properties to promote vessel infiltration and anastomoses [ 85 , 86 , 87 , 88 , 89 ]. Additive manufacturing can further optimize microscale geometry to both accelerate host vessel connections and control intra-device vasculature homogeneity beyond traditional fabrication. Initial work reproduced macroscale vessels but scales were diverged from cell-based therapies [ 73 , 90 , 91 , 92 ]. Leveraging Additive manufacturing designed structures guided vessel formation in vitro and in vivo [ 80 , 89 , 93 ]. Shifting to bioprinting complex branching conduits in supportive hydrogels facilitated clinical translation for diverse cell therapies [ 94 , 95 , 96 , 97 , 98 ]. Researchers focused on developing a 3D scaffold platform to improve the transplantation outcomes of islet cells in T1D. The scaffold featured a heparinized surface and immobilized vascular endothelial growth factor (VEGF) to enhance vascularization. Scaffold effectively promoted angiogenesis and facilitated the growth of new blood vessels. Additionally, encapsulated islets within the scaffold had functional responses to glucose stimuli. These findings suggested that the developed scaffold platform holds potential for successful extra-hepatic islet transplantation, offering new possibilities for T1D treatment [ 99 ]. Research on vascularization of islets via additive manufacturing techniques has primarily focused on the fundamental discoveries. In one study, engineered pseudo islets (EPIs) were created by combining the mouse insulin-secreting beta cells with rat heart microvascular endothelial cells. EPIs demonstrated extensive outgrowth of capillaries into the surrounding matrix. Although, EPIs containing both cell types that underwent capillarization maintained viability and function over time in culture, non-vascularized EPIs lacking endothelial cells could not sustain viability or functionality long-term. This supported the potential for inducing angiogenesis within bioengineered islet constructs. Future work may combine patient-specific stem cell-derived human beta cells with endothelial cells using this approach to promote long-term graft survival for treating type 1 diabetes [ 98 ]. While, large-scale 3D printed vascularized structures are currently limited for the islet transplantation, advancements in leveraging additive manufacturing for the optimization vascularization conditions through the pore sizes and material choices, may facilitate translation to β-cell therapy in type 1 diabetes.
Precision placement of cells and matrix for enhanced control
Beyond distributing biomaterials, additive manufacturing enables micro-level cell and protein control. For islet transplantation, optimal cellular distribution and supportive extracellular matrix niche reduce rapid dysfunction and apoptosis [ 100 , 101 , 102 ]. Traditional techniques heterogeneously load cells after fabrication or struggle with incomplete encapsulation [ 103 , 104 ]. Bioprinting allows in situ encapsulation and printing of multiple cell types and matrix components while dictating 3D placement and dimensions [ 105 , 106 ]. Islet transplant research prints hydrogel-encapsulated clusters surrounded by supportive cells and doped with immune modulators to improve the transplant environment [ 107 ]. Progress in bioprinting offers consistency and defines physical/chemical graft properties beyond traditional fabrication.
Achieving controlled integration of therapeutic agents for enhanced efficacy
In addition to the cell and matrix placement, additive manufacturing enables precision therapeutic integration. Incorporating therapeutics aims to recapitulate the in vivo environment through angiogenesis, islet health promotion, and immunomodulation [ 67 , 108 ]. Growth factors promote vessel formation and insulin secretion while decrease apoptosis [ 108 , 109 , 110 , 111 ]. Local immunomodulators regulate the immune system in a specific site of the body. They decrease inflammation and promote the successful integration of transplanted cells or tissues by minimizing the need for widespread immune suppression in whole body [ 67 ]. Traditional homogeneous delivery methods restrict the ability to customize the spatial distribution of substances and pose a risk of harmful effects on transplants or hosts [ 112 ]. The use of discreet gradients in bioprinting can offer precise physiological signals. By combining traditional drug release methods with AM, it becomes possible to create tissues that exhibit distinct therapeutic localization. Bioprinted composites have the ability to release factors with gradients throughout the entire construct that enables a more comprehensive and targeted approach in tissue engineering [ 112 , 113 , 114 ].
Cell based gene therapy
Gene therapy holds great promise for diabetes management, offering innovative approaches to deliver and manipulate the insulin gene in various tissues. Viral methods, such as lentivirus, adenovirus, and adeno-associated virus (AAV), along with non-viral techniques like liposomes and naked DNA, have been utilized to deliver the insulin gene to target tissues [ 115 ]. This section aims to provide an overview of important studies in the field of gene therapy for diabetes management, emphasizing advancements in insulin gene delivery and manipulation (Table 3).
Enteroendocrine K-cells and pancreatic β-cells
Enteroendocrine K-cells in the intestines and pancreatic β-cells share similarities in their production of glucose-dependent insulinotropic polypeptide (GIP) and their regulatory mechanisms. Understanding these similarities offers insights into T2D management and improving glucose homeostasis. However, attempts to reverse diabetes effectively through K-cell transplantation have been unsuccessful. Nevertheless, research on gene editing techniques has shown promising results in management of the diabetes mellitus [ 116 , 117 ]. AAV vectors have been employed to co-express insulin and glucokinase genes in skeletal muscles, demonstrating long-term effectiveness in achieving normo-glycemia without exogenous insulin [ 118 , 119 ].
Gene editing techniques
Gene editing techniques using AAV vectors effectively improved normo-glycemia in animal models. Co-expression of insulin and glucokinase in transgenic mice increased glucose absorption and regulated insulin production. Duodenal homeobox 1 (PDX1) gene transfer via AAV2 in a humanized liver mouse model also led to insulin secretion and glycemic control [ 120 ]. Adenovirus-mediated transfection of hepatic cells with neurogenin 3 (NGN3) resulted in insulin production and trans-differentiation of oval cell populations [ 121 , 122 ]. Targeting specific promoters in liver cells such as phosphoenolpyruvate carboxykinase (PEPCK), glucose 6-phosphatase (G6Pase), albumin, and insulin-like growth factor binding protein-1 (IGFBP-1) enhanced hepatic insulin gene therapy [ 123 , 124 ]. AAV-mediated overexpression of SIRT1 reduced inflammation, hypoxia, apoptosis and improved neural function in the retina of diabetic db/db mice [ 125 ]. Another study developed a plasmid expressing a single-strand insulin analogue for intramuscular injection using a specialized gene delivery technique. A single administration provided sustained insulin expression for 1.5 months and effectively regulated blood glucose levels without immune responses or tissue damage in diabetic mice.
Non-viral gene delivery methods
Non-viral approaches have also key roles in achieving glycemic control. The combination of insulin fragments with DNA plasmid, administered via intravenous injection improved normo-glycemia for extended periods. DNA transposon facilitated gene integration into the host chromosome that addressed the short-term liver expression. Additionally, the co-injection of DNA plasmid containing insulin with furin significantly enhanced insulin production within muscles [ 126 ]. Non-viral plasmids were engineered to carry proinsulin and pancreatic regenerating genes to ameliorate streptozotocin-induced T1DM [ 127 ]. The pVAX plasmid vectors prolonged therapeutic effects in achieving normo-glycemia without the need for further treatment [ 127 ]. Bioreducible cationic polymers, such as poly-(cystamine bisacrylamide-diamino hexane) (p(CBA-DAH)), have been employed to deliver RAE-1 to pancreatic islets, resulting in improved insulin levels [ 128 ]. Furthermore, ex vivo gene transfer and autologous grafts have shown promising outcomes in animal models. The introduction of the human insulin gene into pancreatic or liver cells followed by autologous grafts improved insulin secretion, glycemic control, and alleviated the diabetic complications in pigs. However, gene silencing eventually occurred, necessitating a deeper understanding of the underlying mechanisms [ 128 , 129 ].
Stem cell based therapy in diabetes
Efforts are ongoing to develop standardized processes for donor and recipient selection/allocation to increase pancreas utilization [ 130 , 131 , 132 , 133 ]. Techniques for isolating pancreatic islets are being optimized to become more standardized and consistent. Noninvasive imaging technologies allow the monitoring of the transplanted islets without surgery [ 134 , 135 ]. Biomarkers could also evaluate how immunomodulation strategies are working [ 136 , 137 , 138 ]. Researchers are also exploring alternative transplant sites in the body beyond just the liver, to see if the other locations may better support islet graft survival and function. Together, these areas of refinement aim to improve the safety and reliability of islet transplantation procedures as a potential therapy for diabetes [ 139 ]. Bioengineering approaches are being developed to optimize the islet transplantation microenvironment using biomaterials which enhance islet engraftment and function through engineered extracellular niches [ 140 , 141 ]. For example, encapsulation techniques aim to protect pancreatic islets against immune reponse by enclosing them within semipermeable hydrogel polymer capsules [ 142 , 143 ]. This localized immunoisolation strategy utilizes biomaterials like alginate to create a physical barrier preventing immune cell contact while still allowing nutrient and oxygen diffusion. Researchers concurrently seek alternative unlimited cellular sources to address limited islet availability. Mesenchymal stem cells possess immunomodulatory properties and their adjuvant delivery, either early in disease onset or simultaneously with islet transplantation, has shown promising signs of improving outcomes in preclinical investigations. By dampening inflammatory responses and favoring regenerative processes, stem cells may help to establish a more tolerogenic transplant environment. These bioengineering and cell therapy approaches offer potential pathways towards eliminating the exogenous insulin requirement [ 144 , 145 ]. A variety of stem cell types have therapeutic potential for diabetes (Fig. 2 ). Pluripotent stem cells possess immense promise for overcoming the limitations of islet transplantation. Human embryonic stem cells and induced pluripotent stem cells are especially attractive candidates due to their unique ability to both self-renew indefinitely and differentiate into any cell type. This makes them an ideal source of replacement pancreatic beta cells. Significant research effort across academic and industrial laboratories has led to advancement in differentiation protocols that can convert pluripotent stem cells into functional beta-like cells in vitro. However, establishing consistent, well-characterized cellular production methods that comply with stringent safety and efficacy standards remains a priority for clinical translation. Ongoing work aims to generate therapeutic stem cell-derived beta cell replacements exhibiting stable, glucose-responsive insulin secretion comparable to primary islets. Although, technological and regulatory hurdles still must be cleared, pluripotent stem cells have the greatest potential to finally solve the problem of limited cell availability and provide an unlimited source of transplantable tissue suitable for widespread treatment of diabetes [ 145 , 146 , 147 , 148 ]. There are currently six registered clinical trials evaluating the use of human pluripotent stem cells for the T1D treatment. All trials except one use PEC-01 cells, which consist of a mixture of pancreatic endoderm and polyhormonal cell population derived from CyT49 stem cells that are fully committed to endocrine differentiation upon implantation [ 149 ]. The initial trial implanted PEC-01 cells within an encapsulation device, hypothesizing no need for immunosuppression. While, well-tolerated with minor adverse effects, insufficient engraftment occurred due to foreign body responses that eliminated the cells [ 150 ]. The trial transitioned in 2017 to use an open encapsulation device that required immunosuppression. Subcutaneous engraftment, differentiation of cells into islet-like clusters, and glucose-responsive insulin production provided the first evidence that pancreatic progenitor cells can survive, mature, and function as the endocrine cells in humans. Potential benefits on stimulated C-peptide levels and glycemic control were observed in one patient [ 151 , 152 ]. Two reports in late 2021 described results in 17 patients receiving PEC-01 cells in an open device. Engraftment and insulin expression occurred in the majority, glucose-responsive secretion in over one-third, and various glycemic improvements were observed at six months. Explanted tissues contained heterogeneous pancreatic compositions including mature beta cells, with no teratoma formation and mild adverse effects related to surgery/immunosuppression. VX-880 uses fully differentiated insulin-producing stem cell-derived islet cells in phase 1/2 trial evaluating portal infusion and different doses requiring immunosuppression. Preliminary results suggest early engraftment and insulin secretion. The manin challenge was controlling immune rejection without systemic immunosuppression [ 149 ]. Several strategies are being explored to address the challenges of immune rejection in stem cell therapies for diabetes. They include generating stem cell lines that are universally compatible through HLA silencing, developing milder regimens of immunosuppression, and refining encapsulation and containment approaches to protect transplanted cells toward immune response. Establishing standardized stem cell banks is also an area of investigation [ 153 , 154 ]. Xenotransplantation using gene-edited porcine islets remains an exciting avenue of research given advances to improve engraftment and reduce immunogenicity in preclinical studies [ 155 ]. Novel approaches continue to emerge as well, such as decellularization techniques, 3D bioprinting of tissue constructs, and creating interspecies chimeras. Rapid evolution of cell-based therapies across both academic and commercial sectors is promising to restore normoglycemic control in diabetic cases. Refinement of existing methods and development of new strategies hold potential to perform a safe and effective cell replacement without reliance on systemic immunosuppression. Stem cell and regenerative therapies may ultimately manage diabetes through restored endogenous insulin production [ 156 ]. Recently a meta analysis evaluated the safety and efficacy of MSC-based therapy for diabetes in humans. This comprehensive analysis was conducted on 262 patients across six trials that met the inclusion criteria within the last five years. The results reveal that treatment with MSCs significantly reduced the dosage of anti-diabetic drugs over a 12-months. Following treatment, HbAc1 levels decreased by an average of 32%, fasting blood glucose levels decreased by an average of 45%, and C-peptide levels showed a decrease of 38% in two trials and an increase of 36% in four trials. Notably, no severe adverse events were reported across all trials. Therefore, it can be concluded that MSC therapy for type 2 diabetes is safe and effective [ 157 ].
Advances in islet transplantation and stem cell-derived Beta cells
Limited number of the islet transplantation donors highlights the importance of cell therapy in diabetes. Although, higher islet numbers from multiple donors increase the success, limited pancreas availability restricts widespread use [ 158 ]. Using multiple donors also increases rejection risk, while isolation of the islets can cause tissue damage [ 159 ]. To overcome these challenges, researchers have explored the differentiation of stem cells into beta cells in vitro to generate an unlimited supply of insulin-producing cells with standardized and characterized products. Genetic engineering techniques have also been investigated to confer advantages such as stress resistance or immune evasion [ 158 ]. ViaCyte has developed a stem cell-derived pancreatic progenitor called PEC-01, which has the ability to mature into endocrine cells in rodent models. To protect the transplanted cells from immune response, retrieval encapsulation devices were also created [ 160 , 161 , 162 ]. In an initial human clinical trial conducted in 2014 (NCT02239354), the Encaptra device was utilized with the aim of providing complete immunoprotection of transplanted cells through the use of a cell-impermeable membrane. Although, the PEC-Encap product showed reliable tolerance and minimal adverse effects, the trial was stopped due to the inadequate engraftment of functional products. While, a few endocrine cells were observed, fibrosis around the capsule led to graft loss and supression of the insulin secretion. To address this challenge, a more recent development called the PEC-Direct device was introduced, which featured openings in the membrane to facilitate vascularization, thereby improving nutrient exchange and supporting cell viability. However, since host cells could infiltrate the device, immunosuppression was necessary following the transplantation [ 163 , 164 , 165 ]. Protocols were developed to generate clusters of stem cell-derived beta cells that secreted glucose-responsive insulin. These clusters, referred to SC-islets, also contained other endocrine cells, including glucagon-producing cells. SC-islets improved glycemic control in diabetic mice and nonhuman primates [ 146 , 166 , 167 , 168 ]. In a trial conducted in 2017 (NCT03163511), the transplantation of progenitor cells resulted in the maturation of endocrine cells, and glucose-responsive C-peptide secretion was observed 6–9 months post-transplantation. Notably, the majority of these mature endocrine cells exhibited glucagon-positive characteristics. The porous regions housing the endocrine cells allowed for the infiltration of host vessels to facilitate vascularization. However, non-cellular regions were isolated by the presence of fibrosis [ 164 , 165 ]. Although, there was not a sufficient levels of circulating C-peptide in these trials, the findings underscored the significance of promoting vascularization and minimizing fibrotic reactions [ 164 , 169 ]. Vertex conducted a human trial in 2021 (NCT04786262) involving the transplantation of half-dose VX-880 cells (SC-islets) without a device to avoid previous problems, which necessitated immunosuppression. Preliminary results reported improved glycemic control, although it took longer to achieve the same outcome compared to rodent models [ 158 ]. Overall, progresses in islet transplantation and stem cell-derived beta cells pave the way for overcoming the limitations of traditional approaches. Further research and refinements are also required to achieve consistent and clinically significant outcomes in the treatment of diabetes.
Chalenges and limitations
Cell-based therapies have been significantly progressed for diabetes; however, there are still several challenges that need to be overcome. Clinical trials investigating encapsulation devices and islet transplantation techniques have provided valuable insights but face several obstacles including oxygenation, host immune responses, and insufficient long-term engraftment success. Immunoengineering of biomaterials and additive manufacturing for the development of 3D islet structures aim to modulate inflammation and promote graft revascularization. Nevertheless, achieving consistent normalization of blood glucose levels without exogenous insulin remains a challenge in human studies. In the field of gene therapy and stem cell differentiation, research focuses on genetically-modified or progenitor-derived insulin-secreting β-like cells to optimize protocols that ensure safety and functionality. The main challenge is to establish stable and functional cells capable of permanently restoring normoglycemia without the need for external intervention. One major barrier is the immune response, which targets allogeneic and xenogeneic islet grafts. Although, local immunotherapy minimizes the systemic effects, evading graft destruction through biomaterials without the requirement of immune suppression remains a significant challenge. The translation of precision 3D islet constructs and genetically reprogrammed cells also necessitates scalable manufacturing processes to ensure consistent function and long-term safety across batches. When critically appraising progress in the field of cell-based diabetes treatments, it is imperative to consider the regulatory, ethical, economic, and safety factors that shape translational applications. At the regulatory level, oversight bodies play a pivotal role in establishing standards to ensure patient welfare while enabling therapeutic innovation. FDA oversees clinical trials and product approvals in the United States (US), while in Europe the EMA provides parallel regulatory guidance. Within the US, organizations like the United Network for Organ Sharing (UNOS) and Organ Procurement and Transplantation Network (OPTN) govern organ and cell allocation protocols [ 17 , 170 ]. However, as regenerative approaches diverge from traditional organ transplantation, regulatory pathways require ongoing harmonization between the agencies and jurisdictions. Continual dialogue between researchers, oversight boards, and policymakers will be crucial to streamline guidelines in a patient-centric manner that balances safety, efficacy, and timely access to cutting-edge therapies. For instance, as stem cell-derived beta cells and 3D bioprinted tissue constructs emerge, traditional drug and device frameworks may not adequately address product characterization and manufacturing complexities for these advanced therapeutic products [ 67 ]. Within clinics, maintaining compliance with evolving regulations impacts research directives and ultimately patients’ access to the novel treatments. Addressing informed consent, clinical trial design, and privacy protections for sensitive health data are also paramount from an ethical perspective [ 128 , 129 ]. Autonomy and agency of research participants in decision-making related to experimental therapies demand prudency. Equitable accessibility of new treatment options also warrants attention to avoid certain populations facing undue barriers. Cell sourcing presents ethical issues depending on derivation from embryonic, fetal or adult tissues. Logistical matters like shipping and processing stem cell-derived islets prior to transplantation necessitate scrutiny. Tumorigenic potential of the undifferentiated pluripotent stem cells should be optimized through rigorous preclinical testing. Transitioning therapies between animal and early human investigations necessitates well-characterized cellular products showing consistent safety and glucose-responsive insulin secretion profiles comparable to pancreatic islets. Long-term animal model data substantiating lack of malignant transformation following transplantation aids allaying ethical safety concerns as the therapies progress clinically. Researchers carefully screen new concepts to prevent side effects in participants while pursuing curative goals. In terms of economic costs, islet and stem cell transplant procedures remain prohibitively expensive for broad applicability despite promising clinical signals. The field requires sustained study to validate techniques, track long-term outcomes, assess healthcare costs offsets from mitigating diabetes’ debilitating complications, and establish cost-benefit ratios for national reimbursement paradigms. Public-private partnerships may accelerate large, interventional trials and longitudinal research to precisely quantify the cellular therapies’ safety profiles and real-world efficacies compared to intensive management versus costs of intensive diabetes care. Ongoing developments like 3D bioprinting offer catalytic manufacturing potential fundamentally recalibrating economics by enhancing yields, standardizing procedures, and reducing costs through scale. By thoroughly and sensitively examining regulatory frameworks, informed consent processes, risks and benefits, as well as financial considerations at both micro and macro levels, researchers, oversight boards and broader stakeholder networks can advance cell-based therapies towards delivering life-changing benefits for all communities. A multidisciplinary, conscientious approach balances progress against patient welfare. A combination of multiple strategies may help to overcome these limitations. For instance, gene-modified islets integrated within vascularized biomaterial implants or sequenced therapies have promising results to prime grafts in pro-regenerative environments before transplantation. Collaboration across disciplines offers hope that refined individualized therapies may eventually achieve durable insulin independence through functional pancreatic cell or tissue engraftment, not only for diabetes but also for chronic pancreatitis. Regarding, ongoing progresses in unraveling these barriers, cell replacement approaches have the potential to improve diabetes management.
Conclusions
This review provides a comprehensive overview of the advances, challenges, and future directions in various cell-based therapeutic approaches for the treatment of diabetes. Significant progresses have been achieved in microencapsulation design, immunomodulation, tissue constructs, genetic and cellular reprogramming techniques, as well as initial clinical translation. However, the complete restoration of normoglycemia without the need for lifelong immunosuppression is still considered as a significant therapeutic challenge. Therefore, addressing the transplant environment of the hostile nature, developing minimally invasive delivery methods, and overcoming limitations in engraftment efficiency and longevity are crucial issues for the future researches. Through the sustained multidisciplinary efforts for the improvement of existing strategies and establishing novel paradigms, achieving durable insulin independence can be a realistic goal for all diabetic cases through the personalized cell replacement or regeneration.
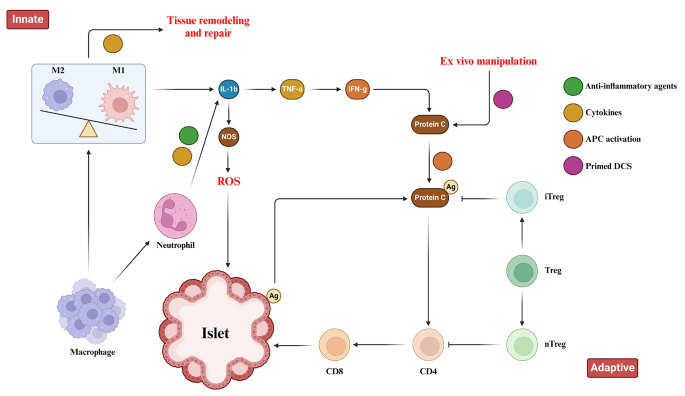
Immune Responses toward pancreatic islets following transplantation. This figure illustrates the immune responses, including the innate and adaptive immunity that are triggered upon pancreatic islet transplantation. Immune response begins with the activation of tissue macrophages and neutrophils in response to injury. Subsequent, release of inflammatory cytokines stimulates antigen-presenting cells (APCs), CD4 + T cells, CD8 + T cells, and cytotoxic T lymphocytes to orchestrate the immune response
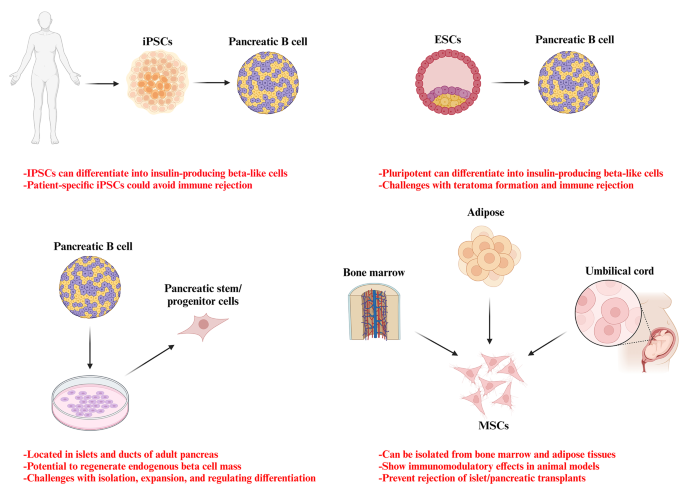
Potential stem cell sources for the treatment of diabetes
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Acrylonitrile butadiene styrene
Activate antigen-presenting cells
Adeno-associated virus
Duodenal homeobox 1
Engineered pseudo islets
Expanded polytetrafluoroethylene
Extracellular matrix
Foreign body response
Fused filament fabrication
Gestational diabetes mellitus
Glucose 6-phosphatase
Insulin-like growth factor binding protein-1
Mesenchymal stem cells
Neurogenin 3
Organ Procurement and Transplantation Network
Phosphoenolpyruvate carboxykinase
Polyaryletherketone
Polycaprolactone
Polycarbonate
Polyetheretherketone
Polyetherimide
Poly-lactic acid
Polystyrene
Stereolithography
Thermoplastic polyurethane
Type 1 diabetes
Type 1 diabetes mellitus
Type 2 diabetes mellitus
United Network for Organ Sharing
United States
Vascular endothelial growth factor
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
Article Google Scholar
Cho NH, Shaw J, Karuranga S, Huang Y, da Rocha Fernandes J, Ohlrogge A, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Article CAS PubMed Google Scholar
Moghbeli M, Naghibzadeh B, Ghahraman M, Fatemi S, Taghavi M, Vakili R, et al. Mutations in HNF1A gene are not a Common cause of familial young-onset diabetes in Iran. Indian J Clin Biochem. 2018;33(1):91–5.
Akhlaghipour I, Bina AR, Mogharrabi MR, Fanoodi A, Ebrahimian AR, Khojasteh Kaffash S, et al. Single-nucleotide polymorphisms as important risk factors of diabetes among Middle East population. Hum Genomics. 2022;16(1):11.
Article CAS PubMed PubMed Central Google Scholar
Moghbeli M, Khedmatgozar H, Yadegari M, Avan A, Ferns GA, Ghayour Mobarhan M. Cytokines and the immune response in obesity-related disorders. Adv Clin Chem. 2021;101:135–68.
Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Reviews Endocrinol. 2020;16(7):349–62.
Article CAS Google Scholar
Siqueira ISLd, Alves Guimarães R, Mamed SN, Santos TAP, Rocha SD, Pagotto V, et al. Prevalence and risk factors for self-report diabetes mellitus: a population-based study. Int J Environ Res Public Health. 2020;17(18):6497.
Free radical research.
Zhu B, Qu S. The relationship between diabetes mellitus and cancers and its underlying mechanisms. Front Endocrinol. 2022;13:800995.
Mojarrad M, Moghbeli M. Genetic and molecular biology of bladder cancer among Iranian patients. Mol Genet Genomic Med. 2020;8(6):e1233.
Article PubMed PubMed Central Google Scholar
Moghbeli M. Genetic and molecular biology of breast cancer among Iranian patients. J Transl Med. 2019;17(1):218.
Abbaszadegan MR, Moghbeli M. Genetic and molecular origins of colorectal Cancer among the iranians: an update. Diagn Pathol. 2018;13(1):97.
Kim I. A brief overview of cell therapy and its product. J Korean Association Oral Maxillofacial Surg. 2013;39(5):201.
Mount NM, Ward SJ, Kefalas P, Hyllner J. Cell-based therapy technology classifications and translational challenges. Philosophical Trans Royal Soc B: Biol Sci. 2015;370(1680):20150017.
El-Kadiry AE-H, Rafei M, Shammaa R. Cell therapy: types, regulation, and clinical benefits. Front Med. 2021;8:756029.
Squifflet J-P, Gruessner R, Sutherland D. The history of pancreas transplantation: past, present and future. Acta Chir Belg. 2008;108(3):367–78.
Article PubMed Google Scholar
Parums DV. First Regulatory approval for allogeneic pancreatic islet Beta cell infusion for adult patients with type 1 diabetes Mellitus. Med Sci Monitor: Int Med J Experimental Clin Res. 2023;29:e941918–1.
Yang L, Hu Z-M, Jiang F-X, Wang W. Stem cell therapy for insulin-dependent diabetes: are we still on the road? World J Stem Cells. 2022;14(7):503.
Affan M, Dar MS. Donislecel-the first approved pancreatic islet cell therapy medication for type 1 diabetes: a letter to the editor. Ir J Med Sci (1971-). 2023:1–2.
Harris E. FDA greenlights first cell therapy for adults with type 1 diabetes. JAMA. 2023.
Soon-Shiong P, Heintz R, Merideth N, Yao Q, Yao Z, Zheng T, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet (London England). 1994;343(8903):950–1.
Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29(1):137–8.
Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32(10):1887–9.
Weng L, Wang X, Liu H, Yu Z, Liu S. Light-responsive microneedle array with tunable insulin release function for painless and on-demand anti-diabetic therapy. Mater Lett. 2023:135684.
Okcu A, Yazir Y, Şimşek T, Mert S, Duruksu G, Öztürk A, et al. Investigation of the effect of pancreatic decellularized matrix on encapsulated islets of Langerhans with mesenchymal stem cells. Tissue Cell. 2023;82:102110.
Khaliq T, Sohail M, Minhas MU, Mahmood A, Munir A, Qalawlus AHM, et al. Hyaluronic acid/alginate-based biomimetic hydrogel membranes for accelerated diabetic wound repair. Int J Pharm. 2023;643:123244.
Kuwabara R, Qin T, Llacua LA, Hu S, Boekschoten MV, de Haan BJ, et al. Extracellular matrix inclusion in immunoisolating alginate-based microcapsules promotes longevity, reduces fibrosis, and supports function of islet allografts in vivo. Acta Biomater. 2023;158:151–62.
Kirk K, Hao E, Lahmy R, Itkin-Ansari P. Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem cell Res. 2014;12(3):807–14.
Dufrane D, van Steenberghe M, Goebbels R-M, Saliez A, Guiot Y, Gianello P. The influence of implantation site on the biocompatibility and survival of alginate encapsulated pig islets in rats. Biomaterials. 2006;27(17):3201–8.
Pullen LC. Stem cell–derived pancreatic progenitor cells have now been transplanted into patients: report from IPITA 2018. Wiley Online Library; 2018. pp. 1581–2.
Dang HP, Chen H, Dargaville TR, Tuch BE. Cell delivery systems: toward the next generation of cell therapies for type 1 diabetes. J Cell Mol Med. 2022;26(18):4756–67.
Viacyte. ViaCyte and gore enter clinical phase agreement based on novel membrane technology for PEC-encap product candidate. 2020.
Viacyte. viacyte announces initiation of phase 2 study of encapsulated cell therapy for type 1 diabetes patients 2021 2021. https://viacyte.com/press-releases/viacyte‐announces‐initiation‐of‐phase‐2‐study‐of‐encapsulated‐cell‐ther‐apy‐for‐type‐1‐diabetes‐patients/ .
Hodgson J. Drug pipeline 3Q23—ERT, bispecifics and CRISPR in sickle cell disease. Nat Biotechnol. 2023;41(11):1498–500.
Pagliuca F. Pre-clinical proof-of-Concept in two lead programs in type 1 diabetes. International Socety for Stem Cell Research; 2019.
Jones PM, Persaud SJ. β-cell replacement therapy for type 1 diabetes: closer and closer. Diabet Med. 2022;39(6).
Carlsson P-O, Espes D, Sedigh A, Rotem A, Zimerman B, Grinberg H, et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am J Transplant. 2018;18(7):1735–44.
Ludwig B, Zimerman B, Steffen A, Yavriants K, Azarov D, Reichel A, et al. A novel device for islet transplantation providing immune protection and oxygen supply. Horm Metab Res. 2010;42(13):918–22.
Evron Y, Colton CK, Ludwig B, Weir GC, Zimermann B, Maimon S, et al. Long-term viability and function of transplanted islets macroencapsulated at high density are achieved by enhanced oxygen supply. Sci Rep. 2018;8(1):6508.
Cao R, Avgoustiniatos E, Papas K, de Vos P, Lakey JR. Mathematical predictions of oxygen availability in micro-and macro‐encapsulated human and porcine pancreatic islets. J Biomedical Mater Res Part B: Appl Biomaterials. 2020;108(2):343–52.
Gala-Lopez B, Pepper A, Dinyari P, Malcolm A, Kin T, Pawlick L, et al. Subcutaneous clinical islet transplantation in a prevascularized subcutaneous pouch–preliminary experience. CellR4. 2016;4(5):e2132.
Google Scholar
Sernova Corp Presents Positive Preliminary. Safety and Efficacy Data in its Phase I/II Clinical Trial for Type-1 Diabetes: Biospace. https://www.biospace.com/article/sernova‐corp‐presents‐positive‐preliminary‐safety‐and‐efficacy‐data‐in‐its‐phase‐i‐ii‐clinical‐trial‐for‐type‐1‐diabetes/ .
Bachul PJ, Perez-Gutierrez A, Juengel B, Golab K, Basto L, Perea L et al. 306-OR: modified approach for improved isllotransplantation into prevascularized sernova cell pouch device: preliminary results of the phase i/ii clinical trial at University of Chicago. Diabetes. 2022;71(Supplement_1).
Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol. 2016;34(3):345–52.
Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, et al. Long-term glycemic control using polymer-encapsulated human stem cell–derived beta cells in immune-competent mice. Nat Med. 2016;22(3):306–11.
Shapiro AD, Konkle BA, Croteau SE, Miesbach WA, Hay CRM, Kazmi R, et al. First-in-human phase 1/2 clinical trial of SIG-001, an innovative shielded cell therapy platform, for hemophilia Α. Blood. 2020;136:8.
Taraballi F, Sushnitha M, Tsao C, Bauza G, Liverani C, Shi A, et al. Biomimetic tissue engineering: tuning the immune and inflammatory response to implantable biomaterials. Adv Healthc Mater. 2018;7(17):1800490.
Yesilyurt V, Veiseh O, Doloff JC, Li J, Bose S, Xie X, et al. A facile and versatile method to endow biomaterial devices with zwitterionic surface coatings. Adv Healthc Mater. 2017;6(4):1601091.
Liu Q, Chiu A, Wang L-H, An D, Zhong M, Smink AM, et al. Zwitterionically modified alginates mitigate cellular overgrowth for cell encapsulation. Nat Commun. 2019;10(1):5262.
Noverraz F, Montanari E, Pimenta J, Szabó L, Ortiz D, Gonelle-Gispert C, et al. Antifibrotic effect of ketoprofen-grafted alginate microcapsules in the transplantation of insulin producing cells. Bioconjug Chem. 2018;29(6):1932–41.
Jeon SI, Jeong J-H, Kim JE, Haque MR, Kim J, Byun Y, et al. Synthesis of PEG-dendron for surface modification of pancreatic islets and suppression of the immune response. J Mater Chem B. 2021;9(11):2631–40.
Derakhshankhah H, Sajadimajd S, Jahanshahi F, Samsonchi Z, Karimi H, Hajizadeh-Saffar E, et al. Immunoengineering Biomaterials in Cell-based therapy for type 1 diabetes. Tissue Eng Part B: Reviews. 2022;28(5):1053–66.
Piemonti L, Maffi P, Nano R, Bertuzzi F, Melzi R, Mercalli A, et al. Treating diabetes with islet transplantation: lessons from the Milan experience. Transplantation, Bioengineering, and regeneration of the endocrine pancreas. Elsevier; 2020. pp. 645–58.
Azzi J, Tang L, Moore R, Tong R, El Haddad N, Akiyoshi T, et al. Polylactide-cyclosporin A nanoparticles for targeted immunosuppression. FASEB J. 2010;24(10):3927.
Chen X, Liu H, Li H, Cheng Y, Yang L, Liu Y. In vitro expansion and differentiation of rat pancreatic duct-derived stem cells into insulin secreting cells using a dynamic three-dimensional cell culture system. Genet Mol Res. 2016;15(2).
Becker MW, Simonovich JA, Phelps EA. Engineered microenvironments and microdevices for modeling the pathophysiology of type 1 diabetes. Biomaterials. 2019;198:49–62.
Graham JG, Zhang X, Goodman A, Pothoven K, Houlihan J, Wang S, et al. PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng Part A. 2013;19(11–12):1465–75.
Izadi Z, Hajizadeh-Saffar E, Hadjati J, Habibi-Anbouhi M, Ghanian MH, Sadeghi-Abandansari H, et al. Tolerance induction by surface immobilization of Jagged-1 for immunoprotection of pancreatic islets. Biomaterials. 2018;182:191–201.
McHugh MD, Park J, Uhrich R, Gao W, Horwitz DA, Fahmy TM. Paracrine co-delivery of TGF-β and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells. Biomaterials. 2015;59:172–81.
Chen H, Teramura Y, Iwata H. Co-immobilization of urokinase and thrombomodulin on islet surfaces by poly (ethylene glycol)-conjugated phospholipid. J Controlled Release. 2011;150(2):229–34.
Su J, Hu B-H, Lowe WL Jr, Kaufman DB, Messersmith PB. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31(2):308–14.
Dong H, Fahmy TM, Metcalfe SM, Morton SL, Dong X, Inverardi L, et al. Immuno-isolation of pancreatic islet allografts using pegylated nanotherapy leads to long-term normoglycemia in full MHC mismatch recipient mice. PLoS ONE. 2012;7(12):e50265.
Kumar M, Nandi SK, Kaplan DL, Mandal BB. Localized immunomodulatory silk macrocapsules for islet-like spheroid formation and sustained insulin production. ACS Biomaterials Sci Eng. 2017;3(10):2443–56.
Hotaling NA, Tang L, Irvine DJ, Babensee JE. Biomaterial Strategies for Immunomodulation. Annu Rev Biomed Eng. 2015;17:317–49.
Shi Y, Zhao YZ, Jiang Z, Wang Z, Wang Q, Kou L, et al. Immune-Protective formulations and process strategies for improved survival and function of transplanted islets. Front Immunol. 2022;13:923241.
Zhang S, Yang H, Wang M, Mantovani D, Yang K, Witte F, et al. Immunomodulatory biomaterials against bacterial infections: Progress, challenges, and future perspectives. Innovation. 2023;4(6):100503.
CAS PubMed PubMed Central Google Scholar
Accolla RP, Simmons AM, Stabler CL. Integrating Additive Manufacturing techniques to improve cell-based implants for the treatment of type 1 diabetes. Adv Healthc Mater. 2022;11(13):e2200243.
Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. ACS; 2014.
Bol RJ, Šavija B. Micromechanical models for FDM 3D-Printed polymers: a review. Polymers. 2023;15(23):4497.
Paul S. Finite element analysis in fused deposition modeling research: a literature review. Measurement. 2021;178:109320.
Monaldo E, Ricci M, Marfia S. Mechanical properties of 3D printed polylactic acid elements: experimental and numerical insights. Mech Mater. 2023;177:104551.
Anoop M, Senthil P. Microscale representative volume element based numerical analysis on mechanical properties of fused deposition modelling components. Materials Today: Proceedings. 2021;39:563 – 71.
McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proceedings of the National Academy of Sciences. 2006;103(31):11461-6.
Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proceedings of the National Academy of Sciences. 2012;109(11):4245-50.
Espona-Noguera A, Ciriza J, Cañibano-Hernández A, Orive G, Hernández RM, del Saenz L, et al. Review of advanced hydrogel-based cell encapsulation systems for insulin delivery in type 1 diabetes mellitus. Pharmaceutics. 2019;11(11):597.
Dimitrioglou N, Kanelli M, Papageorgiou E, Karatzas T, Hatziavramidis D. Paving the way for successful islet encapsulation. Drug Discovery Today. 2019;24(3):737–48.
Omer A, Duvivier-Kali V, Fernandes J, Tchipashvili V, Colton CK, Weir GC. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation. 2005;79(1):52–8.
Song S, Roy S. Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: cells, biomaterials, and devices. Biotechnol Bioeng. 2016;113(7):1381–402.
Zhi ZL, Kerby A, King AJF, Jones PM, Pickup JC. Nano-scale encapsulation enhances allograft survival and function of islets transplanted in a mouse model of diabetes. Diabetologia. 2012;55(4):1081–90.
Farina M, Chua CYX, Ballerini A, Thekkedath U, Alexander JF, Rhudy JR, et al. Transcutaneously refillable, 3D-printed biopolymeric encapsulation system for the transplantation of endocrine cells. Biomaterials. 2018;177:125–38.
Farina M, Ballerini A, Fraga DW, Nicolov E, Hogan M, Demarchi D et al. 3D printed vascularized device for Subcutaneous Transplantation of Human islets. Biotechnol J. 2017;12(9).
Lei D, Yang Y, Liu Z, Yang B, Gong W, Chen S, et al. 3D printing of biomimetic vasculature for tissue regeneration. Mater Horiz. 2019;6(6):1197–206.
Melchels FP, Domingos MA, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW. Additive manufacturing of tissues and organs. Prog Polym Sci. 2012;37(8):1079–104.
Ernst AU, Wang LH, Ma M. Interconnected toroidal hydrogels for islet encapsulation. Adv Healthc Mater. 2019;8(12):1900423.
Liang J-P, Accolla RP, Jiang K, Li Y, Stabler CL. Controlled release of anti-inflammatory and proangiogenic factors from macroporous scaffolds. Tissue Eng Part A. 2021;27(19–20):1275–89.
Pedraza E, Brady A-C, Fraker CA, Molano RD, Sukert S, Berman DM, et al. Macroporous three-dimensional PDMS scaffolds for extrahepatic islet transplantation. Cell Transplant. 2013;22(7):1123–35.
Chiu Y-C, Cheng M-H, Engel H, Kao S-W, Larson JC, Gupta S, et al. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials. 2011;32(26):6045–51.
Kuss MA, Wu S, Wang Y, Untrauer JB, Li W, Lim JY, et al. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J Biomedical Mater Res Part B: Appl Biomaterials. 2018;106(5):1788–98.
Liu X, Jakus AE, Kural M, Qian H, Engler A, Ghaedi M, et al. Vascularization of natural and synthetic bone scaffolds. Cell Transplant. 2018;27(8):1269–80.
Costa-Almeida R, Gomez-Lazaro M, Ramalho C, Granja PL, Soares R, Guerreiro SG. Fibroblast-endothelial partners for vascularization strategies in tissue engineering. Tissue Eng Part A. 2015;21(5–6):1055–65.
Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell. 2011;22(20):3791–800.
Vlahos AE, Cober N, Sefton MV. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proceedings of the National Academy of Sciences. 2017;114(35):9337-42.
Farina M, Ballerini A, Fraga DW, Nicolov E, Hogan M, Demarchi D, et al. 3D printed vascularized device for subcutaneous transplantation of human islets. Biotechnol J. 2017;12(9):1700169.
Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14(13):2202–11.
Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68.
Gao Q, Liu Z, Lin Z, Qiu J, Liu Y, Liu A, et al. 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomaterials Sci Eng. 2017;3(3):399–408.
Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci. 2019;6(11):1900344.
Hospodiuk M, Dey M, Ayan B, Sosnoski D, Moncal KK, Wu Y, et al. Sprouting angiogenesis in engineered pseudo islets. Biofabrication. 2018;10(3):035003.
Marchioli G, Luca AD, de Koning E, Engelse M, Van Blitterswijk CA, Karperien M, et al. Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de novo vessel formation for the transplantation of islets of Langerhans. Adv Healthc Mater. 2016;5(13):1606–16.
Dionne KE, Colton CK, Lyarmush M. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12–21.
de Groot M, Schuurs TA, Keizer PP, Fekken S, Leuvenink HG, Van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12(8):867–75.
Thomas F, Wu J, Contreras JL, Smyth C, Bilbao G, He J, et al. A tripartite anoikis-like mechanism causes early isolated islet apoptosis. Surgery. 2001;130(2):333–8.
Barkai U, Rotem A, de Vos P. Survival of encapsulated islets: more than a membrane story. World J Transplantation. 2016;6(1):69.
Jiang K, Chaimov D, Patel SN, Liang JP, Wiggins SC, Samojlik MM, et al. 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials. 2019;198:37–48.
Pati F, Jang J, Ha D, Won Kim S, Rhie J, Shim J, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935.
Kim BS, Kwon YW, Kong J-S, Park GT, Gao G, Han W, et al. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53.
Hu S, Martinez-Garcia FD, Moeun BN, Burgess JK, Harmsen MC, Hoesli C, et al. An immune regulatory 3D-printed alginate-pectin construct for immunoisolation of insulin producing β-cells. Mater Sci Engineering: C. 2021;123:112009.
Phelps EA, Templeman KL, Thulé PM, García AJ. Engineered VEGF-releasing PEG–MAL hydrogel for pancreatic islet vascularization. Drug Delivery Translational Res. 2015;5:125–36.
Kooptiwut S, Kaewin S, Semprasert N, Sujjitjoon J, Junking M, Suksri K, et al. Estradiol prevents high glucose-induced β-cell apoptosis by decreased BTG2 expression. Sci Rep. 2018;8(1):12256.
Dang TT, Thai AV, Cohen J, Slosberg JE, Siniakowicz K, Doloff JC, et al. Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug. Biomaterials. 2013;34(23):5792–801.
Wang Y, He D, Ni C, Zhou H, Wu S, Xue Z, et al. Vitamin D induces autophagy of pancreatic β-cells and enhances insulin secretion. Mol Med Rep. 2016;14(3):2644–50.
Tarafder S, Koch A, Jun Y, Chou C, Awadallah MR, Lee CH. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. 2016;8(2):025003.
Liu YY, Yu HC, Liu Y, Liang G, Zhang T, Hu QX. Dual drug spatiotemporal release from functional gradient scaffolds prepared using 3 D bioprinting and electrospinning. Polym Eng Sci. 2016;56(2):170–7.
Freeman FE, Pitacco P, van Dommelen LH, Nulty J, Browe DC, Shin J-Y, et al. 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci Adv. 2020;6(33):eabb5093.
Wong MS, Hawthorne WJ, Manolios N. Gene therapy in diabetes. Self Nonself. 2010;1(3):165.
Ahmad Z, Rasouli M, Azman AZF, Omar AR. Evaluation of insulin expression and secretion in genetically engineered gut K and L-cells. BMC Biotechnol. 2012;12:1–9.
Tudurí E, Bruin JE, Kieffer TJ. Restoring insulin production for type 1 diabetes. J Diabetes. 2012;4(4):319–31.
Romer AI, Sussel L. Pancreatic islet cell development and regeneration. Current opinion in endocrinology, diabetes, and obesity. 2015;22(4):255.
Jaén ML, Vilà L, Elias I, Jimenez V, Rodó J, Maggioni L, et al. Long-term efficacy and safety of insulin and glucokinase gene therapy for diabetes: 8-year follow-up in dogs. Mol therapy-methods Clin Dev. 2017;6:1–7.
Li H, Li X, Lam KS, Tam S, Xiao W, Xu R. Adeno-associated virus-mediated pancreatic and duodenal homeobox gene-1 expression enhanced differentiation of hepatic oval stem cells to insulin-producing cells in diabetic rats. J Biomed Sci. 2008;15:487–97.
Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127(16):3533–42.
Abed A, Critchlow C, Flatt PR, McClenaghan NH, Kelly C. Directed differentiation of progenitor cells towards an islet-cell phenotype. Am J Stem Cells. 2012;1(3):196.
PubMed PubMed Central Google Scholar
Zhao M, Amiel SA, Ajami S, Jiang J, Rela M, Heaton N, et al. Amelioration of streptozotocin-induced diabetes in mice with cells derived from human marrow stromal cells. PLoS ONE. 2008;3(7):e2666.
Handorf AM, Sollinger HW, Alam T. Genetic engineering of surrogate β cells for treatment of type 1 diabetes mellitus. J Diabetes Mellitus. 2015;5(04):295–312.
Grant MB, Adu-Agyeiwaah Y, Vieira CP, Asare-Bediako B, Hammer SS, Calzi SL, et al. Intravitreal administration of AAV2-SIRT1 reverses diabetic retinopathy (DR) in a murine model of type 2 diabetes (T2D). Investig Ophthalmol Vis Sci. 2022;63(7):2310.
Yoon J-W, Jun H-S. Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol Med. 2002;8(2):62–8.
Hou W-R, Xie S-N, Wang H-J, Su Y-Y, Lu J-L, Li L-L, et al. Intramuscular delivery of a naked DNA plasmid encoding proinsulin and pancreatic regenerating III protein ameliorates type 1 diabetes mellitus. Pharmacol Res. 2011;63(4):320–7.
Joo WS, Jeong JH, Nam K, Blevins KS, Salama ME, Kim SW. Polymeric delivery of therapeutic RAE-1 plasmid to the pancreatic islets for the prevention of type 1 diabetes. J Controlled Release. 2012;162(3):606–11.
Dezashibi HM, Shabani A. A Mini-review of Current Treatment approaches and Gene Therapy as potential interventions for diabetes Mellitus types 1. Adv Biomed Res. 2023;12:219.
Vantyghem M-C, de Koning EJ, Pattou F, Rickels MR. Advances in β-cell replacement therapy for the treatment of type 1 diabetes. Lancet. 2019;394(10205):1274–85.
Hudson A, Bradbury L, Johnson R, Fuggle S, Shaw J, Casey J, et al. The UK pancreas allocation scheme for whole organ and islet transplantation. Am J Transplant. 2015;15(9):2443–55.
Cornateanu SM, O’Neill S, Dholakia S, Counter CJ, Sherif AE, Casey JJ, et al. Pancreas utilization rates in the UK–an 11-year analysis. Transpl Int. 2021;34(7):1306–18.
Nordheim E, Lindahl JP, Carlsen RK, Åsberg A, Birkeland KI, Horneland R, et al. Patient selection for islet or solid organ pancreas transplantation: experiences from a multidisciplinary outpatient-clinic approach. Endocr Connections. 2021;10(2):230–9.
Arifin DR, Bulte JW. In vivo imaging of pancreatic islet grafts in diabetes treatment. Front Endocrinol. 2021;12:640117.
Murakami T, Fujimoto H, Inagaki N. Non-invasive beta-cell imaging: visualization, quantification, and beyond. Front Endocrinol. 2021;12:714348.
Piemonti L, Everly MJ, Maffi P, Scavini M, Poli F, Nano R, et al. Alloantibody and autoantibody monitoring predicts islet transplantation outcome in human type 1 diabetes. Diabetes. 2013;62(5):1656–64.
Anteby R, Lucander A, Bachul PJ, Pyda J, Grybowski D, Basto L, et al. Evaluating the prognostic value of islet autoantibody monitoring in islet transplant recipients with long-standing type 1 diabetes mellitus. J Clin Med. 2021;10(12):2708.
Buron F, Reffet S, Badet L, Morelon E, Thaunat O. Immunological monitoring in beta cell replacement: towards a pathophysiology-guided implementation of biomarkers. Curr Diab Rep. 2021;21:1–11.
Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep. 2011;11:364–74.
Tremmel DM, Odorico JS. Rebuilding a better home for transplanted islets. Organogenesis. 2018;14(4):163–8.
Citro A, Moser PT, Dugnani E, Rajab TK, Ren X, Evangelista-Leite D, et al. Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials. 2019;199:40–51.
Basta G, Montanucci P, Calafiore R. Microencapsulation of cells and molecular therapy of type 1 diabetes mellitus: the actual state and future perspectives between promise and progress. J Diabetes Invest. 2021;12(3):301–9.
Samojlik MM, Stabler CL. Designing biomaterials for the modulation of allogeneic and autoimmune responses to cellular implants in type 1 diabetes. Acta Biomater. 2021;133:87–101.
Carlsson P-O, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587–92.
Madani S, Setudeh A, Aghayan HR, Alavi-Moghadam S, Rouhifard M, Rezaei N, et al. Placenta derived mesenchymal stem cells transplantation in type 1 diabetes: preliminary report of phase 1 clinical trial. J Diabetes Metabolic Disorders. 2021;20:1179–89.
Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–39.
Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34(13):1759–72.
Sambathkumar R, Migliorini A, Nostro MC. Pluripotent stem cell-derived pancreatic progenitors and β-like cells for type 1 diabetes treatment. Physiology. 2018;33(6):394–402.
Sordi V, Monaco L, Piemonti L. Cell therapy for type 1 diabetes: from islet transplantation to stem cells. Hormone Res Paediatrics. 2022;96(6):658–69.
Henry RR, Pettus J, Wilensky J, SHAPIRO AJ, Senior PA, Roep B et al. Initial clinical evaluation of VC-01TM combination product—a stem cell–derived islet replacement for type 1 diabetes (T1D). Diabetes. 2018;67(Supplement_1).
Shapiro A, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus JH et al. Insulin expression and glucose-responsive circulating C-peptide in type 1 diabetes patients implanted subcutaneously with pluripotent stem cell-derived pancreatic endoderm cells in a macro-device. David and Donner, Thomas W and Bellin, Melena D and Hsueh, Willa and Pettus, Jeremy H and Wilensky, Jon S and Daniels, Mark and Wang, Richard M and Kroon, Evert J and Brandon, Eugene Paul and D’Amour, Kevin A and Foyt, Howard, Insulin Expression and Glucose-Responsive Circulating C-Peptide in Type. 2019;1.
Keymeulen B, Jacobs-Tulleneers-Thevissen D, Kroon EJ, Jaiman MS, Daniels M, Wang R et al. 196-LB: stem cell–derived islet replacement therapy (VC-02) demonstrates production of C-peptide in patients with type 1 diabetes (T1D) and hypoglycemia unawareness. Diabetes. 2021;70(Supplement_1).
Piemonti L. Felix dies natalis, insulin… ceterum autem censeo beta is better. Acta Diabetol. 2021;58(10):1287–306.
Sordi V, Pellegrini S, Piemonti L. Immunological issues after stem cell-based β cell replacement. Curr Diab Rep. 2017;17:1–8.
Coe TM, Markmann JF, Rickert CG. Current status of porcine islet xenotransplantation. Curr Opin Organ Transpl. 2020;25(5):449–56.
Edgar L, Pu T, Porter B, Aziz J, La Pointe C, Asthana A, et al. Regenerative medicine, organ bioengineering and transplantation. J Br Surg. 2020;107(7):793–800.
Mathur A, Taurin S, Alshammary S. The safety and efficacy of mesenchymal stem cells in the treatment of type 2 Diabetes- A literature review. Diabetes Metab Syndr Obes. 2023;16:769–77.
Hogrebe NJ, Ishahak M, Millman JR. Developments in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell. 2023;30(5):530–48.
Paraskevas S, Maysinger D, Wang R, Duguid WP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20(3):270–6.
Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29(8):750–6.
Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, et al. Maturation of human embryonic stem cell–derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–29.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52.
Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, et al. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Translational Med. 2015;4(10):1214–22.
Ramzy A, Thompson DM, Ward-Hartstonge KA, Ivison S, Cook L, Garcia RV, et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell. 2021;28(12):2047–61. e5.
Dolgin E, Diabetes. Encapsulating the problem. Nature. 2016;540(7632):S60–2.
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–33.
Hogrebe NJ, Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat Biotechnol. 2020;38(4):460–70.
Nair GG, Liu JS, Russ HA, Tran S, Saxton MS, Chen R, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat Cell Biol. 2019;21(2):263–74.
Shapiro AJ, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med. 2021;2(12).
Witkowski P, Anteby R, Olaitan OK, Forbes RC, Niederhaus S, Ricordi C, et al. Pancreatic islets Quality and Potency cannot be verified as required for drugs: reflection on the FDA Review of a biological license application for human islets. Transplantation. 2021;105(12):e409–10.
Download references
Acknowledgements
Author information, authors and affiliations.
Noncommunicable Diseases Research Center, Neyshabur University of Medical Sciences, Neyshabur, Iran
Ramin Raoufinia
Department of Medical Genetics and Molecular Medicine, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Ramin Raoufinia, Hamid Reza Rahimi, Ehsan Saburi & Meysam Moghbeli
You can also search for this author in PubMed Google Scholar
Contributions
RR, HRR, and ES were involved in search strategy and drafting. MM revised, designed, and supervised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Meysam Moghbeli .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Raoufinia, R., Rahimi, H.R., Saburi, E. et al. Advances and challenges of the cell-based therapies among diabetic patients. J Transl Med 22 , 435 (2024). https://doi.org/10.1186/s12967-024-05226-3
Download citation
Received : 14 February 2024
Accepted : 22 April 2024
Published : 08 May 2024
DOI : https://doi.org/10.1186/s12967-024-05226-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cell therapy
- Translplantation
- Immunosuppression
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Lab on a Chip
Omef biochip for evaluating red blood cell deformability using dielectrophoresis as a diagnostic tool for type 2 diabetes mellitus †.

* Corresponding authors
a Division of Engineering, New York University Abu Dhabi (NYUAD), Abu Dhabi, United Arab Emirates E-mail: [email protected]
b Department of Mechanical and Aerospace Engineering, New York University, New York, USA
c Cleveland Clinic Abu Dhabi (CCAD), Abu Dhabi, United Arab Emirates
d The Malta College of Arts, Science & Technology, Paola, Malta
e Department of Biomedical Engineering, New York University, New York, USA
Type 2 diabetes mellitus (T2DM) is a prevalent and debilitating disease with numerous health risks, including cardiovascular diseases, kidney dysfunction, and nerve damage. One important aspect of T2DM is its association with the abnormal morphology of red blood cells (RBCs), which leads to increased blood viscosity and impaired blood flow. Therefore, evaluating the mechanical properties of RBCs is crucial for understanding the role of T2DM in cellular deformability. This provides valuable insights into disease progression and potential diagnostic applications. In this study, we developed an open micro-electro-fluidic (OMEF) biochip technology based on dielectrophoresis (DEP) to assess the deformability of RBCs in T2DM. The biochip facilitates high-throughput single-cell RBC stretching experiments, enabling quantitative measurements of the cell size, strain, stretch factor, and post-stretching relaxation time. Our results confirm the significant impact of T2DM on the deformability of RBCs. Compared to their healthy counterparts, diabetic RBCs exhibit ∼27% increased size and ∼29% reduced stretch factor, suggesting potential biomarkers for monitoring T2DM. The observed dynamic behaviors emphasize the contrast between the mechanical characteristics, where healthy RBCs demonstrate notable elasticity and diabetic RBCs exhibit plastic behavior. These differences highlight the significance of mechanical characteristics in understanding the implications for RBCs in T2DM. With its ∼90% sensitivity and rapid readout (ultimately within a few minutes), the OMEF biochip holds potential as an effective point-of-care diagnostic tool for evaluating the deformability of RBCs in individuals with T2DM and tracking disease progression.

Supplementary files
- Supplementary information PDF (826K)
- Supplementary information PDF (138K)
- Supplementary movie MP4 (173K)
- Supplementary movie MP4 (465K)
- Supplementary movie MP4 (690K)
Article information
Download citation, permissions.
OMEF biochip for evaluating red blood cell deformability using dielectrophoresis as a diagnostic tool for type 2 diabetes mellitus
D. S. Ali, S. O. Sofela, M. Deliorman, P. Sukumar, M. Abdulhamid, S. Yakubu, C. Rooney, R. Garrod, A. Menachery, R. Hijazi, H. Saadi and M. A. Qasaimeh, Lab Chip , 2024, Advance Article , DOI: 10.1039/D3LC01016C
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
SYSTEMATIC REVIEW article
The unmet drug-related needs of patients with diabetes in ethiopia: a systematic review and meta-analysis.

- 1 Wollo University, Dessie, Ethiopia
- 2 Addis Ababa University, Addis Ababa, Addis Ababa, Ethiopia
The final, formatted version of the article will be published soon.
Select one of your emails
You have multiple emails registered with Frontiers:
Notify me on publication
Please enter your email address:
If you already have an account, please login
You don't have a Frontiers account ? You can register here
Background: Diabetes is a major health concern globally and in Ethiopia. Ensuring optimal diabetes management through minimizing drug therapy problems is important for improving patient outcomes. However, data on the prevalence and factors associated with unmet drug-related needs in patients with diabetes in Ethiopia is limited. This systematic review and meta-analysis aims to provide a comprehensive analysis of the prevalence of unmet drug-related needs among patients with diabetes mellitus in Ethiopia.Methods: A thorough exploration of databases, including PubMed, Scopus, Hinari, and Embase and Google Scholar, was conducted to identify pertinent studies. Inclusion criteria involved observational studies that reported the prevalence of unmet drug-related needs in Ethiopian patients with diabetes. The quality of the studies was assessed using Joanna Briggs Institute (JBI) checklists.A random-effects meta-analysis was employed to amalgamate data on study characteristics and prevalence estimates, followed by subsequent subgroup and sensitivity analyses. Graphical and statistical assessments were employed to evaluate publication bias.Results: Analysis of twelve studies involving 4,017 patients revealed a pooled prevalence of unmet drug-related needs at 74% (95% CI 63-83%). On average, each patient had 1.45 unmet drug-related needs. The most prevalent type of unmet need was ineffective drug therapy, 35% (95% CI 20-50).Type 2 diabetes, retrospective study designs, and studies from the Harari Region were associated with a higher prevalence. Frequently reported factors associated with the unmet drug-related needs includes multiple comorbidities, older age, and polypharmacy. Notably, the results indicated significant heterogeneity (I 2 = 99.0%; p value < 0.001), and Egger's regression test revealed publication bias with p<0.001.The prevalence of unmet drug-related needs among diabetes patients with diabetes in Ethiopia is high with the most prevalent issue being ineffective drug therapy. Targeted interventions are needed; especially patients on multiple medications, advanced age, with comorbidities, and prolonged illness duration to improve diabetes management and outcomes.
Keywords: Meta-analysis, Diabetes Mellitus, Ethiopia, systematic reviews, Unmet drug-related need
Received: 12 Mar 2024; Accepted: 13 May 2024.
Copyright: © 2024 Gobezie, Tesfaye, Solomon, Demessie, Fentie, Belayneh, Baye and Hassen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Mengistie Yirsaw Gobezie, Wollo University, Dessie, Ethiopia
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
THESIS TYPE II DIABETES MELLITUS SELF-MANAGEMENT: RELATING DIABETES DISTRESS, SOCIAL SUPPORT, SELF-EFFICACY, AND PERFORMANCE OF DIABETES SELF-CARE ACTIVITIES ... Type II Diabetes Mellitus (T2DM) is a chronic disease affecting approximately 30 million individuals in the United States, or 9.4% of the population (Centers for Disease Control ...
Diabetes mellitus (DM) is commonest endocrine disorder that affects more than 100 million people. worldwide (6% po pulation). It is caused b y deficiency or ineffective production of insulin by ...
hyperglycemic non-ketotic state, diabetes can lead to a number of micro- and macrovascular sequelae, including retinopathy, nephropa-thy, coronary artery disease (CAD) and cerebrovascular accident (CVA).3,6 Type 2 diabetes mellitus (T2DM) is characterized by a combination of increased insulin resistance and a decline in beta cell function.7,8
The second type of diabetes is Type II diabetes, also known as diabetes mellitus or insulin resistance. Type II diabetes is seen more in adults; however, the incidence of Type II diabetes among children and younger adults has also increased globally due to obesity and physical inactivity (International Diabetes Federation [IDF], 2019; WHO, 2019).
Type 2 Diabetes Mellitus (T2DM) is a chronic condition wherein the beta cells. in the body do not produce enough insulin - a hormone that regulates blood. sugar - or the body does not use insulin well enough (also called insulin. resistance), or there is complete absence of insulin production.
The International Diabetes Federation has estimated that there were 3.72 million diagnosed. cases of T2DM in the Philippines with a 6.2% prevalence rate in adults in 2017 (104) and also it. is reported that around 1.76 million (2%) people with T2DM remain undiagnosed in 2014 (105). 3.
Diabetes can be treated and its complications can be reduced by maintaining diet, physical activity, and proper medication and by regular monitoring of the complications [15,16,22-25]. Table 1. Genes behind monogenic diabetes and their clinical features. Features of Diabetes Gene Involved Clinical Outcome MODY GCK [26]
This Dissertation is brought to you for free and open access by the Walden Dissertations and Doctoral Studies Collection at ScholarWorks. It has been ... Type 1 diabetes mellitus (T1DM) is one of the most frequently diagnosed endocrine and metabolic conditions that occur during childhood. This research study utilized a
Diabetes mellitus is a metabolic disease characterized by high blood glucose levels and a range of other symptoms that last for a long period of time. It has a rapid prevalence globally, and there is a possibility of the statistic doubling in a few years. This review is aimed at evaluating the mechanism, pathophysiology, and pharmacology of ...
Self-management of diabetes is the "basis of diabetes care" (Jalilian, Motlagh, Solhi, & Gharibnavaz, 2014, p. 1). The cost of diabetes care can be drastically reduced by. creating awareness of risk factors and symptoms through education on diet, exercise, blood sugar monitoring, and medication adherence.
Type 1, Type 2, and the Gestational diabetes are the three major types of diabetes mellitus, although there is a collection of other specific types. The Type 1 diabetes results from the failure of ...
Thesis title: Barriers to self-management in type II diabetes. Conducted at The University of Manchester by Emily Bland for the award of Master of Philosophy (MPhil) ... Background: Type II diabetes is both a worldwide and national healthcare. Certain self-management practices can help people with diabetes to control the condition, these ...
Type 2 diabetes mellitus (T2DM) is a long-term metabolic confusion disease that is related to a high rate of complication and mortality in a population. ... This article is a part of my thesis "The development of health-related quality of life programme among type 2 diabetic patients in Tam Binh District, Vinh Long Province, Vietnam," which ...
CERTIFICATE This is to certify that the dissertation titled "CLINICAL FEATURES OF TYPE 1 DIABETES MELLITUS AND PREVALENCE OF ITS COMPLICATIONS" is the original work done by Dr.M.RAJKUMAR, Postgraduate in Institute of Internal Medicine, Madras Medical College, Government General Hospital, Chennai - 600 003 to be submitted to
Bachelor's thesis Published Autumn 2020 Number of pages 43 Title of publication Primary prevention of type 2 diabetes mellitus Descriptive literature review Name of Degree Bachelor of Nursing Abstract The current prevalence of type 2 diabetes mellitus is critical and a vast number of peo-ple have a high risk for its onset.
Diabetes mellitus (DM) also known as simply diabetes, is a group of metabolic. diseases in which there are high blood sugar levels over a prolonged period This high. blood sugar produces the ...
This Dissertation is brought to you for free and open access by the Walden Dissertations and Doctoral Studies Collection at ScholarWorks. It has been ... Type two diabetes mellitus (T2DM) is a chronic, progressive health condition that presents a need for ongoing assessment, identification of risks, interventions, education
45 of the Best Diabetes Dissertation Topics. Published by Owen Ingram at January 2nd, 2023 , Revised On August 16, 2023. The prevalence of diabetes among the world's population has been increasing steadily over the last few decades, thanks to the growing consumption of fast food and an increasingly comfortable lifestyle.
UBIRA ETheses - University of Birmingham eData Repository
Diabetes mellitus is a common endocrine disorder, and affects more than 100 million people worldwide (World Health Organization, 1994). It is recognized as being a syndrome, a collection of disorders that have hyperglycaemia and glucose intolerance as a hallmark, due either to insulin deficiency or to impaired effectiveness of insulin's ...
Diabetes mellitus is a significant global public health challenge, with a rising prevalence and associated morbidity and mortality. Cell therapy has evolved over time and holds great potential in diabetes treatment. In the present review, we discussed the recent progresses in cell-based therapies for diabetes that provides an overview of islet and stem cell transplantation technologies used in ...
Type 2 diabetes mellitus (T2DM) is a global epidemic, impacting the economy and quality of life of affected individuals. The treatment and management of the disease often rely heavily on self-efficacy and provider guidance. Adherence to provider treatment regimens can help to prevent T2DM-related complications. However, non-adherence to
Type 2 diabetes mellitus (T2DM) is a prevalent and debilitating disease with numerous health risks, including cardiovascular diseases, kidney dysfunction, and nerve damage. ... If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the ...
Diabetes self-management involves several behaviors to prevent complications and ensure a good quality of life. Several studies addressed how the COVID-19 lockdown impacted diabetes self-management practices worldwide, yet little was known about self-management experiences in Grenada and the Caribbean region. The purpose of this
This systematic review and meta-analysis aims to provide a comprehensive analysis of the prevalence of unmet drug-related needs among patients with diabetes mellitus in Ethiopia.Methods: A thorough exploration of databases, including PubMed, Scopus, Hinari, and Embase and Google Scholar, was conducted to identify pertinent studies.
recognizing populace knowledge gaps and behavior regarding pre-diabetes and obesity, which would facilitate the development of diabetes and obesity management initiatives. Healthcare providers and practitioners may find the results of this project significant to. use in handling people with pre-diabetes and obesity.